Abstract
Substantial evidence suggests physical exercise may sustain cognitive function and perhaps prevent Alzheimer’s Disease(1, 2). Current public health recommendations call for older adults to do at least 150 minutes a week of aerobic exercise (e.g. walking) and twice a week resistance exercise (e.g. weight lifting) for physical health. Yet, much remains unknown about how these exercise modalities support brain health independently or in combination. The COMbined Exercise Trial (COMET) is designed to test the combined and independent effects of aerobic and resistance training specifically focusing on exercise-related changes in 1) cognitive performance, 2) regional brain volume, 3) physical function, and 4) blood-based factors. To explore these questions, we will enroll 280 cognitively normal older adults, age 65–80 years, into a 52-week community-based exercise program. Participants will be randomized into one of four arms: 1) flexibility/toning- control 2) 150 minutes of aerobic exercise only, 3) progressive resistance training only, or 4) combined aerobic and progressive resistance training. Outcomes assessed include a comprehensive cognitive battery, blood biomarkers, brain magnetic resonance imaging, physiological biomarkers, cardiorespiratory fitness, physical function, and battery of psychosocial questionnaires is assessed at baseline, 6 and 12-months. COMET will provide rigorous randomized controlled trial data to understand the effects of the most common exercise modalities, and their combination (i.e., the standard public health recommendation), on brain health.
Keywords: Alzheimer’s Disease, Exercise, Cognition, Brain Structure, Resistance Training, Aerobic Activity, Fitness
1.0. Introduction
The societal and economic burden of aging-related cognitive and functional decline underscores the need to develop interventions to maximize successful aging, independence, and health(3–7). Physical activity has a biologically plausible and temporal relationship with coronary heart disease, atherosclerosis, stroke, type 2 diabetes, some cancers, and all-cause mortality(3, 4). Regular aerobic and resistance training decreases age-related morbidity and mortality, improves risk factors for chronic disease, and helps maintain independent functioning(3, 4, 7). Accumulating data suggest exercise may attenuate age-related cognitive and functional decline(1, 2, 8, 9). Expert consensus panels (NIH, CDC, ACSM) have concluded, however, that the available clinical trial evidence is insufficient to demonstrate that exercise prevents cognitive decline or dementia(5–7). Ongoing research designed to definitively determine the effect of aerobic exercise on brain health in cognitively normal older adults will provide key insights (i.e., IGNITE, NCT02875301 (10)). Yet, trials exploring cognitive and brain effect of other exercise modalities and the potential interactions of modalities, key to sustaining function, are still lacking.
We have designed the COMbined Exercise Trial (COMET; R01 AG070036, NCT04848038) to provide rigorous randomized controlled trial data to understand the effects of the most common exercise modalities (aerobic and resistance exercise) independently, and their combination (i.e., the standard public health recommendation), on brain health and cognition. When completed, COMET will provide key information for: 1) patients who need guidance on how much and what types of exercise are necessary to impact brain health, 2) healthcare providers who provide front-line advice and exercise prescription to their patients, 3) healthcare systems in need of definitive proof before major efforts are undertaken to invest in preventative health programs and infrastructure. Moreover, if aerobic and resistance training are both necessary to promote optimal brain health or delay dementia, realizing the benefits would require additional public health efforts to support adoption by older adults. A more precise understanding of the role and impact of current exercise recommendations on cognitive function is essential knowledge for our healthcare force and for convincing the millions of older adults in the United States who do not currently meet all aspects of the recommendations.
1.2. Exercise and Brain Health
1.2.1. Exercise and Brain Health
Aerobic exercise consists of prolonged physical exertion with energy requirements supplied primarily by aerobic metabolism. Public health recommendations from the World Health Organization (WHO), Centers for Disease Control (CDC), American College of Sports Medicine (ACSM), and others, recommend that older adults do at least 150 minutes of moderate intensity aerobic exercise per week, like walking or swimming, as part of a regular exercise regimen to maintain health and fitness(7). Though results of prior trials have been mixed, the overall evidence suggests that aerobic exercise in healthy, older adults may have a beneficial impact on cognitive performance(2, 11), brain plasticity(11), and hippocampal atrophy while improving visual attention and memory(11). One meta-analysis(8) examined 18 aerobic intervention studies of varying quality and found a moderate effect for combined exercise programs across all cognitive outcome measures (effect size=0.6).
Resistance training is also considered an important component of a complete exercise program for older adults(12). It uses muscular contraction against resistance to mitigate the effects of aging on neuromuscular function and functional capacity(13). It also has the potential to improve muscle strength, mass and output(14). Older adults retain the ability to benefit from resistance exercise to a similar extent as younger adults(7). In addition to aerobic exercise, public health recommendations suggest that older adults perform resistance training at least two days per week to maintain function, health, and fitness(15). Few large, well-designed randomized controlled trials assessing resistance training on brain health outcomes have been conducted, although the available literature has proved promising(12, 16, 17).
1.2.2. Brain Health Mechanisms of Exercise
A wealth of animal research suggests that exercise positively impacts brain health(18–27). Exercise appears to stimulate neurogenesis(18) as evidenced by increased counts of new neurons in adult animals on an exercise regimen. Exercise is associated with enhanced neuronal survival(21), resistance to brain insults(19), and increased synaptic development and plasticity(22). Exercise promotes brain vascularization(23), increases learning(18), mobilizes gene expression profiles predicted to benefit brain plasticity(24), and maintains cognitive function(25). Additionally, exercise in cognitively normal older adults is associated with evidence of lower cerebral amyloid-beta, Aβ, deposition(26, 27). Exercise modulates vascular risk factors for dementia, decreases systemic inflammatory markers, increases levels of endogenously-produced, neuroprotective proteins such as brain derived neurotrophic factor (BDNF) that support neuronal growth and survival(28). Exercise also positively affects energy balance and glucose metabolism via actions on AMP kinase and insulin signaling, processes that have been suggested to increase Aβ trafficking and clearance(29).
Randomized clinical trials have examined the effects of resistance training on cognitive function and have found that participation results in improvements in executive function(30), memory(31), verbal fluency(31), and global cognition(31, 32). However, results have been inconsistent in showing that resistance training can prevent cognitive decline and AD(8).
The field has not directly assessed whether public health recommendations provide independent or combined effects on cognition in older adults. Conclusions from prior work are limited by design:
10 studies comparing resistance or combined exercise to a non-exercise control (16, 30, 33–36);
Variability in aerobic exercise: walking, circuit training, running(37), swimming/aqua aerobics(38), etc.(8, 11);
Variability in resistance training parameters including modality, weekly sessions, and progression(16, 30, 33–36).
1.2.3. Rationale for Studying Combined Exercise
Despite widespread recommendation for combined exercise, no studies have directly compared the effects of aerobic vs. resistance or combined training on cognition, although studies have assessed the differential impact of these exercise modalities on body weight and composition(39), insulin resistance(39–41), inflammation(42), and functional limitations(40, 41, 43). The results of these studies suggest that combining aerobic and resistance training is optimal for effects on insulin resistance(41) and physical function(41) but does not offer advantages for altering adiposity(44).
Resistance and aerobic training elicit different physiologic adaptations to cardiovascular, muscular, bioenergetic, and neuroendocrine systems(17, 36). Resistance training relies preferentially on anaerobic metabolism during the short but intense bouts of training. This improves muscle strength and quality while increasing high energy phosphate (ATP and creatine phosphate) availability, mitochondrial density, and oxidative capacity(7), effects that are generally not observed with aerobic exercise. In contrast, aerobic exercise training increases the capacity of muscle to generate energy though increased myoglobin content in muscle and increased efficiency of oxygen extraction and carbohydrate oxidation. Despite some concern that combined aerobic and resistance training will result in an “interference effect” where the development of strength during the same period might influence the development of aerobic capacity, and vice versa, several studies have found no evidence of this possible effect(42).
1.3. Hypotheses
Our guiding scientific premise is that standard public health recommendations will have benefits for brain health specific to the type of exercise performed. Specifically, we hypothesize that combined aerobic and resistance exercise will be associated with cognitive benefits, our primary outcome, and combining modalities will have additional benefits over either one alone. We hypothesize similar combined benefits for our secondary and ancillary outcomes of hippocampal volume, maximal oxygen consumption, maximal strength, and functional fitness.
2.0. Methods
This is a randomized controlled trial testing the effects of 52 weeks of aerobic, weight training or combined aerobic and weight training on cognition and brain structure in 280 cognitively normal older adults aged 65 to 80 years. Participants are equally randomized into one of four treatment groups:
Core and Fusion Training (CFT)
Resistance training (RT)
Aerobic training (AT)
Combined weight and aerobic training (COMBO)
Figure 1 provides an overview of the screening and study events. In addition, the ongoing IGNITE trial (NCT02875301 (10)) a 12-month, randomized dose-response exercise trial in 639 cognitively normal adults between 65 and 80 years of age to definitively addressing whether aerobic exercise influences cognitive and brain health in cognitively normal older adults helped to inform the methods of the COMET trials. During the IGNITE study, participants are randomized to (1) a moderate intensity aerobic exercise (3–6 METs) condition of 150 min/week (N = 213), (2) a moderate intensity aerobic exercise condition at 225 min/week (N = 213), or (3) a light intensity core and fusion control condition for 150 min/week (N = 213). Participants are engaged in 3 days/week of supervised exercise and two more days per week of unsupervised exercise for 12 months. A comprehensive cognitive battery, blood biomarkers and battery of psychosocial questionnaires is assessed at baseline, 6 and 12-months. In addition, brain magnetic resonance imaging, physiological biomarkers, cardiorespiratory fitness, physical function, and positron emission tomography of amyloid deposition are assessed at baseline and at the 12-month follow-up. While this trial does not explore the influence of resistance training or combination (COMBO) on cognitive and brain health, it does provide infrastructure that was leveraged to investigate the present studies aims.
Figure 1.
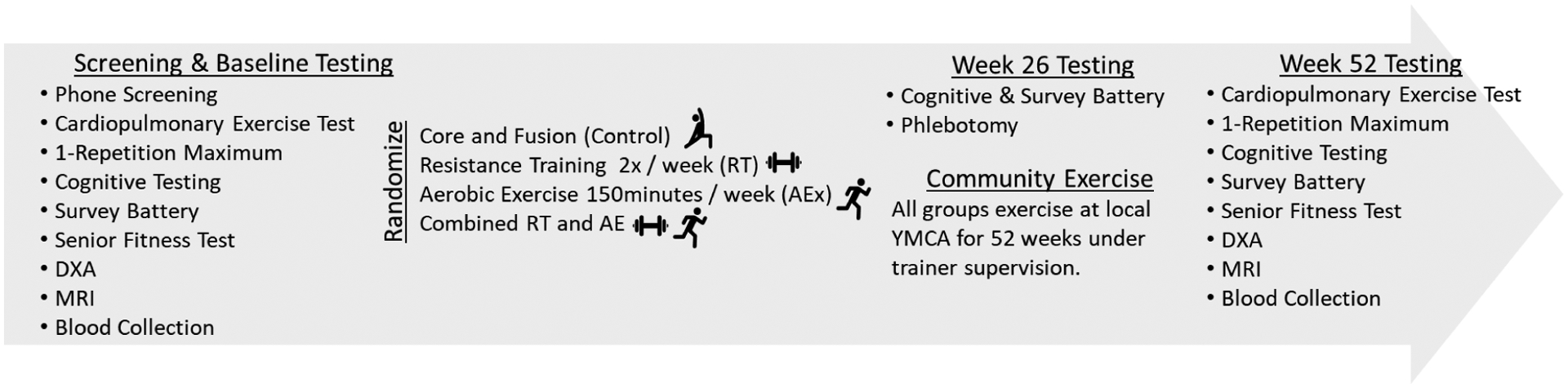
Study Flow and Schedule of Testing
2.1. Screening
2.1.1. Participant Recruitment
The KU ADRC is a national leader in developing and testing recruitment strategies for brain aging studies. COMET leverages the research recruitment infrastructure of the KU ADRC, reducing study staff load. Research inquiries are returned in a timely manner and all contacts are recorded to create continuity and track timely interactions managed with an in-house “participant relationship management” system we developed using REDCap (45) and Shiny (shiny.rstudio.com) (46). This system allows the team to manage interactions with potential participants, improve communication among staff and across teams, and reliably manage handoffs of participants from staff to staff. Referral source, enrollment progress, and recruitment strategy success are monitored in near real time.
2.1.2. Initial Eligibility
After initial interest screening by KU ADRC recruitment staff, the COMET study team phone screens potential participants prior to scheduling an in-person baseline evaluation. Screening occurs in two parts, basic study explanation, and medical history. We collect demographic information on those who express interest after hearing a basic explanation of the study. The medical history portion of the phone screen assesses inclusion and exclusion criteria (Table 1). Screening questions are asked in reverse order of likelihood(47) to capture the maximum amount of information from each screener. Source of referral is also captured for recruitment return-on-investment analyses.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2. Baseline Evaluation
Baseline evaluation consists of approximately 3 visits to the KU ADC within a time window of 35 days between consent initiation of exercise, for those who do not screen fail on the phone.
2.2.1. Cognitive testing
In consultation with the IGNITE study team, we have constructed a comparable battery, emphasizing key domains while reducing the overall time required for testing (Table 2). The battery captures global cognitive function while focusing on executive, attentive and visuospatial aspects of cognition specifically, as they appear to be most responsive to exercise(10). The same battery is administered at baseline, 6- and 12-month visits, with changes in form versions of tests as available. Cognitive testing is performed by a trained psychometrists after standardized training, who undergo annual fidelity checks. Testing takes approximately 2 hours to complete during 1 visit. Participants have the right to refuse to complete any test, however doing so may result in ineligibility.
Table 2.
Cognitive Testing Battery
| Task | Time | Description |
|---|---|---|
| WECHSLER TEST OF ADULT READING(58) | 3 min | This test asks participants to pronounce a list of words that are recorded. It is a proxy of IQ. This will be administered only at baseline. |
| HOPKINS VERBAL LEARNING TEST-REVISED(59) | 10 min | This is a word list learning and memory task that measures immediate and short-term and longer-term retention. It consists of three presentations with recall of a 12-word list, a delayed recall trial (20 minutes after Trial 3), and a delayed recognition trial. It is recorded for review. |
| DIMENSIONAL CARD SORT- NIH TOOLBOX(60) | 4 min | Two target pictures are presented that vary along two dimensions (e.g. shape and color). Participants are asked to match a series of test pictures (e.g. yellow balls and blue trucks) to the target pictures, first according to one dimension (e.g. color) and then, after several trials, according to the other dimension (e.g. shape). |
| ORAL SYMBOL DIGIT TEST-NIH TOOLBOX(61) | 3 min | Symbols on the screen are associated with a number, then presented with symbols without numbers. |
| FLANKER-NIH TOOLBOX(60) | 3 min | The task requires the participant to focus on a particular stimulus while inhibiting attention to the stimuli flanking. |
| LOGICAL MEMORY-VCAP(62) | 10 min | Participants are read stories and asked to recite as much as they remember at two different time points during testing. |
| SPATIAL RELATIONS-VCAP(63) | 15 min | Participants are asked to determine the correspondence between a 3-D figure and alternative 2-D figures. |
| MATRIX REASONING-VCAP(64) | 15 min | Participant determines which pattern best completes the missing cell in a matrix. |
| TASK SWITCHING(10) | 15 min | This task asks participants to alternate between attention to stimuli presented on a computer display. Participants are asked to response to whether the number is greater than or less than 5 (task 1) or to respond to whether the number is odd or even (task 2). |
| LETTER COMPARISON-VCAP(65) | 5 min | In this task participants are asked to determine whether a series of letters is the same as another series of letters. They are asked to do this as quickly as they can. |
| SPATIAL WORKING MEMORY(11) | 15 min | This Task measures spatial memory functions. It requires that participants attend to and retain the location of several dots presented simultaneously on a computer display. They are requested to press buttons on a keyboard that correspond to whether a probe dot appeared in one of the same locations as the previous dots. |
| STROOP TASK(10) | 10 min | This task measures selective attention, cognitive flexibility and processing speed and is used as a tool in the evaluation of executive function. This task is administered on a computer and the participants are asked to respond to the colors of the ink for printed words on the display as quickly as they can while ignoring the meaning of the word. |
2.2.1.2. Study Adjudication
A clinician interprets the baseline neuropsychological data and determines exclusion based on possible/probable cognitive impairment in the context of age, sex, race, ethnicity, reported schooling, and any other factors the medical monitor may consider important. A potential participant must be successfully adjudicated to be randomized.
2.2.2. Health and Activity Surveys
Health surveys focus on quality of life, psychological state, and self-reported activity (Table 3). The questionnaires are completed either during a study visit or done at home by the participants on paper or on computer via REDCap Survey. The battery takes about 1 hour to complete. The surveys are administered at baseline and 52 weeks.
Table 3.
Health and Activity Surveys
| CONSTRUCT | SURVEY |
|---|---|
| DEMOGRAPHICS AND HEALTH HISTORY | Cumulative Illness Rating Scale (CIRS), at in-person screening)(66) |
| Medication List | |
| MacArthur Scale of Subjective Social Status(67) | |
| ACTIVITY | Florida Cognitive Activities Scale(68) |
| Godin Exercise Leisure-time Questionnaire(69) | |
| SUBJECTIVE COGNITION AND PSYCHOLOGICAL DISTRESS | Cognitive Function Index(70) |
| PROMIS Applied Cognition: General Concern(71) | |
| PROMIS Anxiety(72) | |
| PROMIS Depression(72) | |
| Perceived Stress Scale (PSS-10)(73) | |
| DIET AND NUTRITION | NHANES DSQ(74) |
| QUALITY OF LIFE | PROMIS: Life Satisfaction(75) |
| EQ-5D-5L(76) | |
| PHYSICAL AND SOCIAL ROLES | PROMIS Physical Function(77) |
| PROMIS Ability to Participate in Social Roles and Activities(78) | |
| SELF-EFFICACY AND SELF-REGULATION (AT BASELINE, 3, 26, AND 52 WEEKS) | Barriers Self-Efficacy Scale (BARSE)(79) |
| Exercise Self-Efficacy (EXSE)(80) | |
| Lifestyle Self-Efficacy Scale (LSE)(81) | |
| Physical Activity Self-Regulation (PASR-12)(82) | |
| SLEEP/CIRCADIAN | Pittsburgh Sleep Quality Index (PSQI)(83) |
2.2.3. Physical Assessment and Blood Collection
If a participant passes medical monitor adjudication of cognitive testing, they are scheduled for the fasting blood collection, anthropometry, and physical function assessment. Height, mass, and dual x-ray absorptiometry are performed to estimate body composition, wearing a standardized hospital gown. The Senior Fitness Test(48) is performed. One-repetition maximum leg press and chest press are used to index strength, following ACSM guidelines for administration(49). Finally, a graded maximal exercise test is performed, holding speed constant, and increasing grade by 2% each 2-minute stage, like a Modified Balke protocol(49, 50). Initial speed finding is performed by a blinded exercise test leader based on heart rate response and rating of perceived exertion after the Senior Fitness test. Blood collection is performed prior to the Senior Fitness Test and approximately 20 minutes after the end of the exercise test. The visit lasts approximately 2 hours and is performed at baseline and 52 weeks (Supplemental Material 1).
2.2.4. Brain MRI
Participants undergo an MRI at baseline and 52 weeks. While willingness to attempt MRI is required, if a participant is consented and cannot complete the MRI due to discomfort, they are not excluded from the study. Standard structural, functional, and cerebral blood flow protocols are followed to maximize comparison with IGNITE(10). Data collected consist of images of brain structure, cerebral blood flow, and function. To assess brain function, the n-Back task is used to index working memory(10). Before entering the MRI machine, participants the n-Back task that they will be performing. MRI Sequences include:
T1-weighted magnetization prepared rapid acquisition gradient echo structural: sagittal, 0.8 mm isotropic resolution, TE/TI/TR = 2.31/1060/2400 ms, field of view 256 mm, 224 slices
High-resolution Hippocampus: 0.4×0.4×2 mm, TE/TR = 78/8830 ms, aligned perpendicular to hippocampus
Resting fMRI with eyes open (2.5×2.5×2.5 mm, TE/TR = 40/1000 ms, multiband factor = 8 (CMRR EPI sequence60–63), 64 slices, 480 measurements)
fMRI n-back task Resolution: 2.5×2.5×2.5 mm, TE/TR = 40/2000 ms, Multiband factor = 4, 64 slices, 183 measurements
Pseudo-continuous arterial spin labeling: 3D gradient spin echo, sequence 64,65, 3.1×3.1×2.5 mm, TE/TR = 22.08/4300 ms, 48 slices, post-label delay 2s, Background Suppression, 10 measurements for labeling and control, 4 segment readout
The visit lasts approximately 75 minutes to complete.
2.3. Week 3 Assessments
The activity-specific questionnaires of the home survey battery are completed again during the third week of the intervention.
2.4. Week 26 Evaluation
At 26 weeks after intervention start (+/− 2 week), participants return for an in-person evaluation to repeat the cognitive testing (Table 2). Health surveys and self-efficacy assessments are administered (Table 3).
2.5. Week 52 Evaluation
At 52 weeks after intervention start (+/− 2 weeks), participants return for an in-person evaluation to repeat the cognitive testing (Table 2), physical function testing, MRI, and health surveys (Table 3) are administered.
2.6. Telephone Checks
Participants are contacted by phone for formal review of medication changes, medical history, and adverse events in the first week of initiating the exercise intervention and again at Weeks 6, 13, 19, 32, 39, and 45 during the active intervention. Additionally, the telephone checks facilitate communication between the study team and the participants and encourage compliance with the intervention, supplementing regular supervised training session.
2.7. Randomization and Blinding
2.7.1. Randomization
Upon successful completion of baseline screening and testing, participants are randomized to 1 of 4 study arms. Intervention arms include:
core and fusion training (CF) condition,
150 minutes of aerobic training (AT) only,
2 days a week of progressive weight training (RT) only,
150 minutes of AT and RT.
We use the REDCap randomization module including a block randomization strategy to balance two factors: (1) age at study entry (=<72, >72), and (2) gender. We allow co-randomization for a limited number of couples (married, domestic partner etc.) with the intent of increasing male enrollment in the study(51). If a couple passes screening, they are randomized as one to the same group. A limited number of unblinded study staff can reveal the group assignment and none have access to the randomization table created by the study statistician prior to study start. Also, at the time of randomization, a staff member organizes date, day, time, and location of the participant’s first exercise session.
2.7.2. Blinding
Raters (psychometrician, exercise physiologist) who perform outcome assessments are blinded to the participant’s intervention group. The study medical monitor is unblinded to assist with safety assessments and address safety concerns or adverse events.
The MPIs are blinded to primary and secondary outcomes identified in ClinicalTrials.gov. The MPI team may have access to group assignments. This may be needed in the event exercise modification is needed by a physical therapist (i.e., PI Vidoni). However, any interventionist interactions or exercise prescription changes by the MPI team must be recorded.
2.8. Exercise Intervention Arms
This study tests aerobic and resistance exercise as they form the core of the current consensus recommendations for general health benefits(52, 53). Our intervention plans follow current public health recommendations. All participants, regardless of intervention assignment complete both supervised and unsupervised exercise during the study. Table 4 provides planned number of exercise visits.
Table 4.
Planned exercise visits per week by intervention group
| Design Overview | Intervention groups | |||||||||||
| CFT | RT | AT | COMBO | |||||||||
| Weeks | Weeks | Weeks | Weeks | |||||||||
| Supervised Exercise | 1–26 | 27–36 | 37–52 | 1–26 | 27–36 | 37–52 | 1–26 | 27–36 | 37–52 | 1–26 | 27–36 | 37–52 |
| RT w/Trainer | 2 | 1 | 1 | 2 | 1 | 1 | ||||||
| Core and Fusion Exercise w/Trainer | 3 | 2 | 1 | 1 | 1 | 0 | ||||||
| AT w/Trainer | 3 | 2 | 1 | 1 | 1 | 0 | ||||||
| Independent Exercise | ||||||||||||
| RT | 1 | 1 | 1 | |||||||||
| Core and Fusion | 2 | 3 | 4 | 2 | 2 | 3 | ||||||
| AT | 2 | 3 | 4 | 2 | 3 | 4 | ||||||
| Total Exercise Sessions | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
CFT= Core and Fusion Training; RT= Weight Training; AT= Aerobic Training; COMBO= RT+AT
Aerobic training (AT): moderate-intensity aerobic training (150 minutes/week over 3–5 days). Exercise duration is increased from 60 minutes in the first week to 150 minutes in the seventh week. Intensity is dosed by heart rate reserve (HRR) using the resting maximum heart rate achieved during the Physical Assessment visit. Target intensity is 40–50% HRR in Weeks 1–12, 45–55% HRR in Weeks 13–39, and 50–60% HRR in Weeks 40–52.
Progressive weight training (RT): 2 days/week, non-consecutive, of 2 sets (10 – 15 repetitions) of 10 exercises (~75 minutes/week). Resistance progression is based on repetition completion, and increases from 60–75% of estimated 1-repetition maximum of each exercise. In addition, 3 days of core and fusion control exercise is also recommended for a total of about 150 minutes a week of exercise.
Combined training (COMBO): 150 minutes/week of aerobic training and 2 days/week of resistance exercise for a total training duration of ~225 minutes/week).
Core and Fusion (CFT): ~150 minutes/week of core and fusion exercise as implemented in IGNITE, previously(10).
Additional details regarding these conditions can be found in Supplemental Material 2.
2.9. Exercise Compliance
Participants in the study complete part of the exercise under supervised conditions and part of the exercise under unsupervised conditions. Monitoring compliance during the supervised exercise is assisted by trainers monitoring attendance, duration of exercise, perceived exertion, mode of activity, and heart rate intensity. However, compliance during the unsupervised sessions is inherently more challenging. COMET takes a two-faceted approach for monitoring compliance during the unsupervised periods. First, participants summarize their weekly exercise via a REDCap survey or paper log. These summaries are a natural component of training since training involves the identification of barriers and approaches to overcoming non-compliance while keeping individuals engaged and continuing to return for the sessions. We provide feedback to participants to assist with compliance by helping them see exercise activity increases.
In addition to the self-reported exercise summaries, participants are encouraged to wear their Fitbits’ daily throughout the 52-week exercise period. Fitbit data are gathered nightly through the Fitbit application programming interface. Compliance with the exercise prescription is based on the information gathered from the weekly summary, the supervised exercise sessions, and the accelerometer recording.
Participants are provided with email correspondence about their weekly performance and upcoming exercise prescription. Emails include name, historical information on their exercise and performance, and their upcoming prescription. Participants who do not have email receive paper correspondence with the same information via their personal trainer.
2.10. Outcomes and Analysis plan
The primary outcome is Global Cognition, a composite score of cognition test scores. Global Cognition will be calculated as a latent variable of the results across the cognitive test battery. We will use a second order confirmatory factor analysis (CFA) to estimate the factor loadings between the observed indicators and the first order factors and between the first and second order factors. The CFA will use baseline data to avoid any potential intervention related biases. The primary outcome will be constructed by summing the weighted average (by the estimated first and second order loadings) of the standardized observed scores across the fourteen tests. Global Cognition will be analyzed with a linear mixed model (LMM) with intent-to-treat using baseline, 26- and 52-week measures while adjusting for measurements obtained at baseline (i.e., demographics). Since there is only one primary outcome, we will test with α = 0.05. Secondary outcomes including whole brain volume, fitness and strength will be analyzed using similar strategies. Post-hoc analyses will include linear contrasts for resistance training vs control and endurance exercise vs control. With 280 patients and 15% attrition we expect to be able to detect a significant benefit of combined training for an effect size of 0.60 with >80% power.
We will employ an intent-to-treat analysis where all participants, regardless of whether they complete all sessions, are invited to return for follow-up assessments. However, there will likely be some participants who refuse to return for follow-up assessments or miss a mid-point assessment (cognitive and questionnaires are collected at the 6-month period). We will assess the missingness of data consistent with the assumptions of the modeling approach described above. We will determine if the proportion of participants lost to follow-up differs by treatment and demographic characteristics between completers and non-completers. If missing data are related to treatment and/or demographic characteristics (e.g., sex, baseline weight, etc. with p< 0.05) then we will assume the data is missing at random (MAR) and we will use model-based imputation since multiple imputation will only provide unbiased estimates if enough variables predictive of missingness are included in the model(54). If missingness is not related to these variables we will not use multiple imputation since linear mixed models do not require complete cases to provide unbiased estimates assuming the data is missing completely at random (MCAR) and neither mixed models nor imputation models are guaranteed to provide unbiased estimated if the data is not missing at random. Additional information can be found in supplemental material 3.
2.11. Community-Based Exercise
2.11.1. Intervention Supervision
Participants can exercise at any study approved exercise facilities for which we have established relationships. We have previously reported on our community exercise protocols(50) which predominantly use the YMCA network. Each YMCA employs certified personal trainers who conduct the exercise session. Direct supervision of participants by personal trainers occurs throughout the 52-week intervention. We introduce more flexibility in scheduling exercise after Week 26 if the participant is consistently and safely exercising by allowing unsupervised exercise sessions at the YMCA at times when personal trainers may not be available (i.e. early morning, nights, and weekends). Participants are required to have at least one directly supervised exercise session per week to maintain contact with program staff and encourage adherence to the program. This titration procedure has been successful in several completed (R01AG033673, R01AG034614) and ongoing studies (R01AG052954, R01AG49749, R01AG043962). YMCA trainers are asked to report anyone who has missed two weeks or more of supervised exercise to study staff. Study staff will then follow-up with the participant. Additional information on study safety can be found in Supplemental Material 4.
Trainers are oriented to the COMET protocol and are monitored during an annual fidelity check-off with study staff. Additionally, all participants are observed by study staff quarterly, providing additional opportunities to assess protocol fidelity and remediate deviations.
2.12. Data Management
Behavioral data are stored and linked on a HIPAA secure server (REDcap)(45). Imaging data are archived and stored using XNAT. Both REDcap and XNAT allow investigators to access data and coordinate analyses. Fitbit activity monitor data are harvested nightly from the Fitbit server and stored on protected university servers, but these data also exist on the Fitbit company server. To provide additional security, no protected health information is entered as part of Fitbit account creation. Additional information on quality control and data curation is available in Supplemental Methods 5.
3.0. Conclusion
Regular aerobic and resistance training decreases age-related morbidity and mortality, improves risk factors for chronic disease, and helps maintain independent functioning(3, 4, 7). Yet, trials exploring cognitive and brain effect of other exercise modalities and the potential interactions of modalities, key to sustaining function, are still lacking. Thus, a more precise understanding of the role and impact of current exercise recommendations on cognitive function is essential knowledge for our healthcare force and for convincing the millions of older adults in the US who do not currently meet all aspects of the recommendations. The COMET Study is vital to providing clinicians, researchers, public health organizations, and policy makers information of the independent and combined importance of aerobic and resistance exercise treatments on cognitive and physiological health of older adults. This study is among the first and likely the largest, exercise-specific randomized controlled trial to assess the independent and combined effect of aerobic training and resistance training on cognition in older adults withing the context of the public health recommendations(5–7, 15). The COMET study will provide rigorous randomized controlled trial data to understand the effects of the most common exercise modalities (AT and RT) more precisely, and their combination (i.e., public health recommendation), on brain health. In addition, the design selected, is potentially deployable as is uses community partners (i.e., YMCA and other local exercise facilities) to deliver the exercise prescription.
Supplementary Material
Funding:
R01AG070036
Abbreviations:
- NIH
National Institutes of Health
- CDC
Centers for Disease Control and Prevention
- ACSM
American College of Sports Medicine
- AD
Alzheimer’s Disease
- Aβ
Amyloid Beta
- BDNF
Brain Derived Neurotrophic Factor
- WHO
World Health Organization
- ATP
Adenosine triphosphate
- COMET
Combined Exercise Trial
- KU ADRC
University of Kansas Alzheimer’s Disease Research Center
- fMRI
functional Magnetic Resonance Imaging
- DXA
Dual-energy x-ray absorptiometry
- SFT
Senior Fitness Test
- 1RM
1-Repetition Maximum
- CPET
Cardiopulmonary Exercise Test
- RPE
Rating of Perceived Exertion
- PCASL
Pseudo-Continuous Arterial Spin Labeling
- fMRI
Functional Magnetic Resonance Imaging
- CF
Core and Fusion
- RT
Resistance Training
- AT
Aerobic Training
- MCAR
Missing completely at random
- MAR
Missing at random
- QC
Quality Control
- LMM
Linear Mixed Model
- OLS
Ordinary least squares
- IGF-1
Insulin Growth Factor-1
- DSMC
Data and Safety Monitoring Committee
Footnotes
NCT Registration: NCT02913664
References
- 1.Kramer AF, Colcombe S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study—Revisited. Perspectives on Psychological Science. 2018;13(2):213–7. doi: 10.1177/1745691617707316. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–9. [DOI] [PubMed] [Google Scholar]
- 3.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical Fitness as a Predictor of Mortality among Healthy, Middle-Aged Norwegian Men. The New England Journal of Medicine. 1993;328(8):533–7. [DOI] [PubMed] [Google Scholar]
- 4.Laukkanen JA, Lakka TA, Rauramaa R, Kuhanen R, Venäläinen JM, Salonen R, Salonen JT. Cardiovascular Fitness as a Predictor of Mortality in Men. Archives of Internal Medicine. 2001;161(6):825–31. doi: 10.1001/archinte.161.6.825. [DOI] [PubMed] [Google Scholar]
- 5.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES Jr., Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176–81. Epub 2010/06/16. doi: 0003–4819-153–3-201008030–00260 [pii] 10.1059/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 6.Snowden M, Steinman L, Mochan K, Grodstein F, Prohaska TR, Thurman DJ, Brown DR, Laditka JN, Soares J, Zweiback DJ, Little D, Anderson LA. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704–16. Epub 2011/03/29. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- 7.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30. Epub 2009/06/12. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 8.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–30. [DOI] [PubMed] [Google Scholar]
- 9.Gu MO, Conn VS. Meta-analysis of the effects of exercise interventions on functional status in older adults. Res Nurs Health. 2008;31(6):594–603. Epub 2008/06/12. doi: 10.1002/nur.20290. [DOI] [PubMed] [Google Scholar]
- 10.Erickson KI, Grove GA, Burns JM, Hillman CH, Kramer AF, McAuley E, Vidoni ED, Becker JT, Butters MA, Gray K, Huang H, Jakicic JM, Kamboh MI, Kang C, Klunk WE, Lee P, Marsland AL, Mettenburg J, Rogers RJ, Stillman CM, Sutton BP, Szabo-Reed A, Verstynen TD, Watt JC, Weinstein AM, Wollam ME. Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE): Protocol. Contemporary Clinical Trials. 2019;85:105832. doi: 10.1016/j.cct.2019.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. Epub 2011/02/02. doi: 1015950108 [pii] 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD, Ryan ED. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. The Journal of Strength & Conditioning Research. 2019;33(8). [DOI] [PubMed] [Google Scholar]
- 13.Borde R, Hortobágyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015;45(12):1693–720. Epub 2015/10/01. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985). 1998;84(4):1341–9. Epub 1998/05/09. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- 15.Elsawy B, Higgins KE. Physical activity guidelines for older adults. American family physician. 2010;81(1):55–9. Epub 2010/01/08. [PubMed] [Google Scholar]
- 16.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–8. Epub 2010/01/27. doi: 170/2/170 [pii] 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401–7. Epub 2007/09/01. doi: 10.1249/mss.0b013e318060111f 00005768–200708000-00024 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2001;21(15):5678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, Boldogh I. Exercise plays a preventive role against Alzheimer’s disease. J Alzheimers Dis. 2010;20(3):777–83. Epub 2010/02/26. doi: 10.3233/jad-2010-091531. [DOI] [PubMed] [Google Scholar]
- 21.Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. ProgClin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 22.Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58(1):76–87. [PubMed] [Google Scholar]
- 23.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. Journal Of Cerebral Blood Flow And Metabolism: Official Journal Of The International Society Of Cerebral Blood Flow And Metabolism. 1992;12(1):110–9. [DOI] [PubMed] [Google Scholar]
- 24.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. Epub 2002/06/28. doi: S0166223602021434 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Escorihuela RM, Tobena A, Fernandez-Teruel A. Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman low-avoidance) rats. Learning & Memory (Cold Spring Harbor, NY: ). 1995;2(1):40–8. [DOI] [PubMed] [Google Scholar]
- 26.Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68(3):311–8. Epub 2010/09/08. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2012;69(5):636–43. Epub 2012/01/11. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–94. Epub 2009/06/30. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106(6):1971–6. Epub 2009/02/04. doi: 0809158106 [pii] 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu-Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J, Lord SR, Khan KM. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc. 2008;56(10):1821–30. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, Uemura K, Lee S, Park H. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128. Epub 2012/11/02. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, Salvador-Pascual A, Rodriguez-Mañas L, Viña J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J Am Med Dir Assoc. 2016;17(5):426–33. Epub 2016/03/08. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Coetsee C, Terblanche E. The effect of three different exercise training modalities on cognitive and physical function in a healthy older population. European review of aging and physical activity : official journal of the European Group for Research into Elderly and Physical Activity. 2017;14:13-. doi: 10.1186/s11556-017-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iuliano E, di Cagno A, Aquino G, Fiorilli G, Mignogna P, Calcagno G, Di Costanzo A. Effects of different types of physical activity on the cognitive functions and attention in older people: A randomized controlled study. Exp Gerontol. 2015;70:105–10. Epub 2015/07/18. doi: 10.1016/j.exger.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Kimura K, Obuchi S, Arai T, Nagasawa H, Shiba Y, Watanabe S, Kojima M. The influence of short-term strength training on health-related quality of life and executive cognitive function. J Physiol Anthropol. 2010;29(3):95–101. Epub 2010/06/19. doi: 10.2114/jpa2.29.95. [DOI] [PubMed] [Google Scholar]
- 36.Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiology of Aging. 2012;33(8):1690–8. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Albinet CT, Boucard G, Bouquet CA, Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol. 2010;109(4):617–24. Epub 2010/02/27. doi: 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- 38.Albinet CT, Abou-Dest A, André N, Audiffren M. Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biol Psychol. 2016;115:69–77. Epub 2016/01/27. doi: 10.1016/j.biopsycho.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Donges CE, Duffield R, Guelfi KJ, Smith GC, Adams DR, Edge JA. Comparative effects of single-mode vs. duration-matched concurrent exercise training on body composition, low-grade inflammation, and glucose regulation in sedentary, overweight, middle-aged men. Appl Physiol Nutr Metab. 2013;38(7):779–88. Epub 2013/08/29. doi: 10.1139/apnm-2012-0443. [DOI] [PubMed] [Google Scholar]
- 40.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69. Epub 2007/09/19. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 41.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122–31. Epub 2009/01/28. doi: 169/2/122 [pii] 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 42.Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MP. Effect of resistance, endurance, and concurrent training on TNF-α, IL-6, and CRP. Med Sci Sports Exerc. 2012;44(1):50–6. Epub 2011/06/24. doi: 10.1249/MSS.0b013e318229d2e9. [DOI] [PubMed] [Google Scholar]
- 43.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. Jama. 2010;304(20):2253–62. Epub 2010/11/26. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68–91. Epub 2011/09/29. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. Epub 2008/10/22. doi: S1532–0464(08)00122–6 [pii] 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidoni ED, Bothwell RJ, Burns JM, Dwyer JR. Novel recruitment models will drive Alzheimer’s trial success. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14(1):117–9. Epub 2017/11/20. doi: 10.1016/j.jalz.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidoni ED, Szabo-Reed A, Kang C, Perales-Puchalt J, Shaw AR, Grove G, Hamill M, Henry D, Burns JM, Hillman C, Kramer AF, McAuley E, Erickson KI. The IGNITE Trial: Participant Recruitment Lessons Prior to SARS-CoV-2. medRxiv. 2020:2020.05.19.20107458. doi: 10.1101/2020.05.19.20107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rikli R, Jones J. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–61. [Google Scholar]
- 49.Gary Liguori ACoSM. ACSMs guidelines for exercise testing and prescription: Eleventh edition. Philadelphia: : Lippincott Williams; 2021. [Google Scholar]
- 50.Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Donnelly JE, Burns JM. Dose-Response of Aerobic Exercise on Cognition: A Community-Based, Pilot Randomized Controlled Trial. PLOS ONE. 2015;10(7):e0131647. doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bracken K, Askie L, Keech AC, Hague W, Wittert G. Recruitment strategies in randomised controlled trials of men aged 50 years and older: a systematic review. BMJ Open. 2019;9(4):e025580. Epub 2019/04/06. doi: 10.1136/bmjopen-2018-025580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–45. Epub 2007/09/01. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 53.Rodgers AB. Exercise & physical activity [electronic resource] : your everyday guide from the National Institute on Aging. Rodgers AB, Pocinki KM, National Institute on A, editors. [Gaithersburg, MD: ]: National Institute on Aging; 2009. [Google Scholar]
- 54.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111–7. [Google Scholar]
- 56.Desmond DW, Tatemichi TK, Hanzawa L. The Telephone Interview for Cognitive Status (TICS): Reliability and validity in a stroke sample. International Journal of Geriatric Psychiatry. 1994;9(10):803–7. doi: 10.1002/gps.930091006. [DOI] [Google Scholar]
- 57.Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Prev Chronic Dis. 2008;5(1):A24. Epub 2007/12/18. doi: A24 [pii]. [PMC free article] [PubMed] [Google Scholar]
- 58.Wechsler D Wechsler adult intelligence scale. Archives of Clinical Neuropsychology. 1955. [Google Scholar]
- 59.Brandt J The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The clinical neuropsychologist. 1991;5(2):125–42. [Google Scholar]
- 60.Zelazo PD, Anderson JE, Richler J, Wallner‐Allen K, Beaumont JL, Weintraub S II. NIH Toolbox Cognition Battery (CB): Measuring executive function and attention. Monographs of the Society for Research in Child Development. 2013;78(4):16–33. [DOI] [PubMed] [Google Scholar]
- 61.Scott EP, Sorrell A, Benitez A. Psychometric properties of the NIH toolbox cognition battery in healthy older adults: Reliability, validity, and agreement with standard neuropsychological tests. Journal of the International Neuropsychological Society. 2019;25(8):857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wechsler D Wechsler memory scale. Psychological Corporation. 1945.
- 63.Bennett GK, Seashore HG, Wesman AG. Differential aptitude tests1947.
- 64.Raven JC, Raven JC, Court JH. Advanced progressive matrices: HK Lewis London; 1962. [Google Scholar]
- 65.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental psychology. 1991;27(5):763. [Google Scholar]
- 66.Linn BS, Linn MW, Gurel L. Cumulative Illness Rating Scale Journal of the American Geriatrics Society. 1968;16(5):622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 67.Giatti L, Camelo LdV, Rodrigues JFdC, Barreto SM. Reliability of the MacArthur scale of subjective social status - Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). BMC Public Health. 2012;12(1):1096. doi: 10.1186/1471-2458-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schinka JA, McBride A, Vanderploeg RD, Tennyson K, Borenstein AR, Mortimer JA. Florida Cognitive Activities Scale: initial development and validation. J Int Neuropsychol Soc. 2005;11(1):108–16. Epub 2005/02/03. doi: 10.1017/s1355617705050125. [DOI] [PubMed] [Google Scholar]
- 69.Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Medical Research Methodology. 2015;15(1):60. doi: 10.1186/s12874-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C, Neugroschl J, Luo X, Zhu C, Aisen P, Ferris S, Sano M. The Utility of the Cognitive Function Instrument (CFI) to Detect Cognitive Decline in Non-Demented Older Adults. J Alzheimers Dis. 2017;60(2):427–37. doi: 10.3233/JAD-161294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fieo R, Ocepek-Welikson K, Kleinman M, Eimicke JP, Crane PK, Celia D, Teresi JA. Measurement equivalence of the Patient Reported Outcomes Measurement Information System® (PROMIS®) Applied Cognition—General Concerns, short forms in ethnically diverse groups. Psychological Test and Assessment Modeling. 2016;58(2):255–307. [PMC free article] [PubMed] [Google Scholar]
- 72.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–83. Epub 2011/06/24. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. Epub 1983/12/01. [PubMed] [Google Scholar]
- 74.Thompson FE, Midthune D, Kahle L, Dodd KW. Development and Evaluation of the National Cancer Institute’s Dietary Screener Questionnaire Scoring Algorithms. J Nutr. 2017;147(6):1226–33. Epub 2017/05/12. doi: 10.3945/jn.116.246058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaughan B, Mulcahy J, Fitzgerald K. PROMIS® General Life Satisfaction scale: construct validity in musculoskeletal pain patients. Chiropractic & Manual Therapies. 2020;28(1):27. doi: 10.1186/s12998-020-00320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D–5L: South Australian population norms. Health and Quality of Life Outcomes. 2016;14(1):133. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crins MHP, van der Wees PJ, Klausch T, van Dulmen SA, Roorda LD, Terwee CB. Psychometric properties of the PROMIS Physical Function item bank in patients receiving physical therapy. PLOS ONE. 2018;13(2):e0192187. doi: 10.1371/journal.pone.0192187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamminga SJ, van Vree FM, Volker G, Roorda LD, Terwee CB, Goossens PH, Vliet Vlieland TPM. Changes in the ability to participate in and satisfaction with social roles and activities in patients in outpatient rehabilitation. Journal of Patient-Reported Outcomes. 2020;4(1):73. doi: 10.1186/s41687-020-00236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McAuley E The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. Journal of behavioral medicine. 1992;15(1):65–88. [DOI] [PubMed] [Google Scholar]
- 80.McAuley E, Lox C, Duncan TE. Long-term maintenance of exercise, self-efficacy, and physiological change in older adults. Journal of gerontology. 1993;48(4):P218–P24. [DOI] [PubMed] [Google Scholar]
- 81.McAuley E, Mullen SP, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe NP, Olson EA, Voss M, Erickson K. Self-regulatory processes and exercise adherence in older adults: executive function and self-efficacy effects. American journal of preventive medicine. 2011;41(3):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Umstattd MR, Motl R, Wilcox S, Saunders R, Watford M. Measuring physical activity self-regulation strategies in older adults. Journal of Physical Activity and Health. 2009;6(s1):S105–S12. [DOI] [PubMed] [Google Scholar]
- 83.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 84.Borg GAV. Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise. 1982;14(5). [PubMed] [Google Scholar]
- 85.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90–2. Epub 2020/09/07. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


