Background:
In a post hoc analysis, the frequency of occurrence of an early decline (dip) in estimated glomerular filtration rate (eGFR) after initiation of dapagliflozin and its association with outcomes were evaluated in patients with heart failure and reduced ejection fraction randomized in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial.
Methods:
Patients with heart failure with reduced ejection fraction with or without type 2 diabetes and an eGFR ≥30 mL·min−1·1.73 m−2 were randomized to placebo or dapagliflozin 10 mg daily. The primary outcome was the composite of worsening heart failure or cardiovascular death. The extent of the dip in eGFR between baseline and 2 weeks, patient characteristics associated with a >10% decline, and cardiovascular outcomes and eGFR slopes in participants experiencing this decline were investigated. Time-to-event outcomes were assessed in Cox regression from 14 days; eGFR slopes were assessed with repeated-measures mixed-effect models.
Results:
The mean change in eGFR between day 0 and 14 was −1.1 mL·min−1·1.73 m−2 (95% CI, −1.5 to −0.7) with placebo and −4.2 mL·min−1·1.73 m−2 (95% CI, −4.6 to −3.9) with dapagliflozin, giving a between-treatment difference of 3.1 mL·min−1·1.73 m−2 (95% CI, 2.6–3.7). The proportions of patients randomized to dapagliflozin experiencing a >10%, >20%, and >30% decline in eGFR were 38.2%, 12.6%, and 3.4%, respectively; for placebo, they were 21.0%, 6.4%, and 1.3%, respectively. The odds ratio for a >10% early decline in eGFR with dapagliflozin compared with placebo was 2.36 (95% CI, 2.07–2.69; P<0.001). Baseline characteristics associated with a >10% decline in eGFR on dapagliflozin were older age, lower eGFR, higher ejection fraction, and type 2 diabetes. The hazard ratio for the primary outcome in patients in the placebo group experiencing a >10% decline in eGFR compared with ≤10% decline in eGFR was 1.45 (95% CI, 1.19–1.78). The corresponding hazard ratio in the dapagliflozin group was 0.73 (95% CI, 0.59–0.91; Pinteraction<0.001). A >10% initial decline in eGFR was not associated with greater long-term decline in eGFR or more adverse events.
Conclusions:
The average dip in eGFR after dapagliflozin was started was small and associated with better clinical outcomes compared with a similar decline on placebo in patients with heart failure with reduced ejection fraction. Large declines in eGFR were uncommon with dapagliflozin.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03036124.
Keywords: glomerular filtration rate; heart failure; kidney; renal insufficiency, chronic; sodium-glucose transporter 2 inhibitors
Clinical Perspective.
What Is New?
The placebo-corrected early decline in estimated glomerular filtration rate (eGFR) after initiation of dapagliflozin is similar across the range of eGFR.
Patients randomized to dapagliflozin who had an initial decline (dip) in eGFR had better outcomes than those who did not, without safety concerns.
What Are the Clinical Implications?
Although a decline in eGFR is generally associated with a poorer prognosis in most situations, an initial decline in eGFR with a sodium-glucose cotransporter 2 inhibitor was instead associated with better cardiovascular outcomes and a slower rate of decline in kidney function.
An initial decline in eGFR after initiation of a sodium-glucose cotransporter 2 inhibitor should not usually lead to discontinuation of treatment.
Editorial, see p 463
Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of worsening heart failure (HF) and death attributable to cardiovascular causes in patients with HF with reduced ejection fraction (HFrEF).1,2 These agents also reduce the long-term rate of decline in estimated glomerular filtration rate (eGFR) and development of end-stage kidney disease in patients with HFrEF and patients with chronic kidney disease, with or without type 2 diabetes.3,4 However, SGLT2 inhibitors cause an initial decline (dip) in eGFR, which has caused some clinical concern, particularly in patients with a reduced baseline eGFR. One concern is that the initial decline, if substantial, might lead to discontinuation of existing evidence-based and lifesaving treatments such as renin-angiotensin system blockers or a mineralocorticoid receptor antagonist or even to consideration of renal replacement therapy. Physicians may also associate an acute decline in eGFR with a risk of progressive, chronic worsening of kidney function and poor outcomes, and concern about reducing eGFR may lead to underuse of SGLT2 inhibitors in patients with HFrEF.5 Although there have been several analyses of the early decline in eGFR with SGLT2 inhibitors, these have all been in patients with type 2 diabetes, in whom the renal pathophysiology may be different from that of individuals with HFrEF.6–8 Moreover, patients with HFrEF are universally treated with diuretics, renin-angiotensin system blockers, and often mineralocorticoid receptor antagonists, all of which also affect eGFR. In contrast to the participants in the other trials, generally with normal or elevated blood pressure, patients with HFrEF often have low blood pressure and fluctuations in plasma volume that may reduce glomerular filtration, especially in the setting of renin-angiotensin system blockade causing efferent arteriolar dilatation.
It is therefore important to understand the frequency and extent of an early decline in eGFR after initiation of an SGLT2 inhibitor, its predictors, and its association with subsequent clinical outcomes in patients with HFrEF, including those without type 2 diabetes and low eGFR at baseline. We have explored these questions in the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure).1
Methods
DAPA-HF was a randomized, double-blind, placebo-controlled, event-driven trial in patients with HFrEF with or without type 2 diabetes. The design, baseline characteristics, and primary results have been published.1,9,10 Ethics committees for the 410 participating institutions in 20 countries approved the protocol, and all patients gave written informed consent. The first authors had full access to the data in the study and take responsibility for the integrity of the data and the data analysis. The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Patients and Treatment
Patients in New York Heart Association functional class II to IV with a left ventricular ejection fraction (LVEF) ≤40% and an elevated NT-proBNP (N-terminal pro-B-type natriuretic peptide) concentration were eligible if receiving standard pharmacological and device therapy. The key exclusion criteria were type 1 diabetes, symptomatic hypotension/systolic blood pressure (SBP) <95 mm Hg, and an eGFR <30 mL·min−1·1.73 m−2. Dapagliflozin 10 mg was compared with matching placebo taken once daily in addition to standard treatment.
In the event of an unexpected decline in renal function of concern, investigators were advised to check for other causes, including the use of drugs causing renal dysfunction, and to stop them if nonessential. It was recommended that essential medications for HF were not discontinued. If kidney function did not improve with other measures, the dose of randomized therapy could be reduced to 5 mg/d or stopped, with advice to restart or uptitrate later if possible.
Measurement of Kidney Function and eGFR Subgroup Analysis
Blood samples were taken at randomization; 14 days later; at 2, 4, 8, and 12 months; and every 4 months thereafter. Serum creatinine was measured in a central laboratory, and eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration 2009 equation.
Outcomes
The primary trial outcome was the composite of worsening HF (HF hospitalization or urgent visit for HF requiring intravenous therapy) or cardiovascular death, whichever occurred first. Prespecified secondary end points included HF hospitalization or cardiovascular death; HF hospitalizations (first and recurrent) and cardiovascular deaths; change from baseline to 8 months in Kansas City Cardiomyopathy Questionnaire total symptom score; worsening kidney function (sustained decline in eGFR ≥50%, end-stage kidney disease, sustained dialysis, renal transplantation, or renal death); and all-cause mortality.
All outcomes were examined in the current study except worsening kidney function and change in Kansas City Cardiomyopathy Questionnaire total symptom score. Worsening kidney function was not analyzed because of the small number of events overall; instead, we calculated the eGFR slope, as described later and reported previously.11 Change in Kansas City Cardiomyopathy Questionnaire total symptom score was not examined because it was not measured at 2 weeks and therefore could not be used in the landmark analysis from 14 days.
Statistical Analysis
Baseline Characteristics
Baseline characteristics were summarized as means (SDs), medians (interquartile ranges), or percentages. Groups were defined by percent change in eGFR at day 14 (no decline, up to 10% decline, and >10% decline). The Cochran-Armitage test was used to test for trend across groups for binary variables, and the Jonckheere-Terpstra test was used for continuous variables. Nonordered multiple categories were compared with the χ2 test. Baseline characteristics were also summarized within each randomized treatment group.
Change in eGFR
Change in eGFR at 2 weeks was compared between treatment arms with medians. A repeated-measures mixed-effect model including all visits was used to calculate adjusted mean change and between-treatment differences at 14 days. Repeated-measures mixed-effect models were adjusted for baseline eGFR, randomized treatment, study visit, and the interaction between study visit and randomized treatment with intercepts and slopes allowed to vary randomly between patients, with patient and visit as random effects with an unstructured covariance structure. Analysis was repeated in each eGFR subgroup at baseline (≥75, <75 to ≥60, <60 to ≥45, <45 mL·min−1·1.73 m−2). An interaction between baseline eGFR and randomized treatment on the change in eGFR at 14 days was tested in a mixed model including all patients. Repeated-measures mixed-effect models were repeated for percentage change and absolute change in eGFR.
Median, quartiles, and probability density curves (violin plots) were used to visualize change in eGFR in each treatment arm at 14, 60, and 120 days from randomization.12 The percentage of patients with a 10%, 20%, or 30% decline in eGFR from baseline at days 14, 60, and 120 in each treatment arm was calculated, and logistic regression adjusted for baseline eGFR was applied to give an odds ratio (OR) for occurrence of the degree of eGFR dip (10%, 20%, and 30% decline) with dapagliflozin over placebo at each time point. Analysis was also repeated for different commonly used definitions of worsening renal function, including ≥0.3-mg/dL (26.5-μmol/L) increase in creatinine, ≥25% increase in creatinine, >0.5-mg/dL (44.2-μmol/L) change in creatinine, and ≥5– and ≥10–mL·min−1·1.73 m−2 decrease in eGFR.13
The odds of a dip in eGFR of >10% in the whole population by continuous eGFR at baseline was examined with a restricted cubic spline. The odds of an eGFR dip with dapagliflozin compared with placebo over continuous eGFR at baseline was examined with a fractional polynomial.
Outcomes
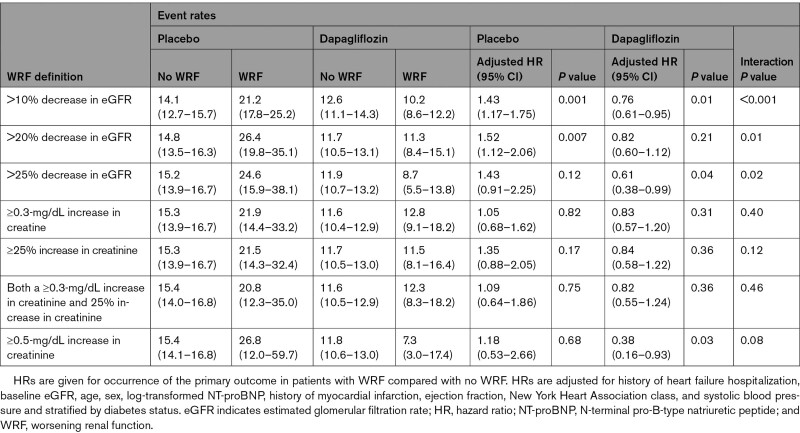
Landmark analysis from 14 days was carried out for the main outcomes to assess the effect of any dip in renal function over the first 2 weeks and distal outcomes. Patients were included if alive at 14 days with follow-up time restarting at 14 days. For example, for the primary outcome, if the patient experienced hospitalization for HF in the first 14 days, they were included with a new event censor for the next worsening HF event or cardiovascular death. Hazard ratios (HRs) for a 10% decline versus ≤10% decline/no change/improvement were assessed for the outcomes described with a Cox proportional hazards regression for time to first event outcomes and Lin-Wei-Yang-Ying method for recurrent event outcome with an interaction between randomized treatment and eGFR dip assessed.14 For each outcome, the interaction P value was significant; therefore, the HR for the occurrence of an eGFR dip on the distal outcomes was reported within each treatment arm separately. All models included stratification for diabetes status and adjustment for baseline eGFR and history of HF hospitalization (apart from all-cause mortality). HRs are given for unadjusted analysis and analysis adjusted for baseline clinical characteristics (age, sex, [log-transformed] NT-proBNP, history of myocardial infarction, LVEF, New York Heart Association class, and systolic blood pressure). Other cutoffs for change in renal function and their relationship with the primary outcome were examined in the same manner.
The relationship between change in eGFR at 14 days as a continuous variable and risk of the primary outcome was modeled as a restricted cubic spline within each randomized treatment arm.
The interaction between eGFR dip group and randomized treatment on the occurrence of the prespecified safety outcomes was tested in a logistic regression model. eGFR dip category was entered into the model as a categorical variable, with nested models with and without an interaction between treatment and eGFR dip group compared with a likelihood ratio test.
eGFR Slopes and Renal Outcome
Because few patients (n=67) experienced the renal composite outcome in the trial overall, we did not perform subgroup analysis. When we plotted the results from the repeated mixed-effect model of eGFR by treatment group, there were 2 clear phases to the slope of eGFR: an initial decline with rebound increase followed by a slower decline. To explore long-term trajectories depending on the early change in eGFR (no decline, up to 10% decline, and >10% decline), the mean change in eGFR in these subgroups was plotted within each treatment arm. Mean change in eGFR and eGFR slopes (expressed as mL·min−1·1.73 m−2 per year) were calculated from days 0 to 14, 14 to 60, and 60 to 720 for each eGFR change subgroup within randomized treatment arms.
Prediction of eGFR Dip
A logistic regression model was used to estimate the odds of a dip of >10% in eGFR at 2 weeks with dapagliflozin compared with placebo in the whole population and in subgroups considered clinically to be potential predictors of eGFR dip (sex; age; race; type 2 diabetes; New York Heart Association class; LVEF; body mass index; HF type; eGFR at baseline; NT-proBNP; history of HF hospitalization; hypertension; use of a mineralocorticoid receptor antagonist, diuretic, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, or angiotensin receptor-neprilysin inhibitor) in univariable analysis. Interactions were tested between randomized treatment and each subgroup on the occurrence of eGFR dip.
Predictions were also assessed in multivariable models with the same variables simultaneously and testing for an interaction between randomized treatment and subgroup in turn.
Given the strong predictive effect of randomized treatment on the occurrence of eGFR dip and the presence of several interactions between treatment allocation and subgroup, multivariable logistic regression for eGFR dip was repeated in each treatment group separately with the same variables that were used as subgroups in the above univariable analysis.
Continuation of Concurrent Renin-Angiotensin-Aldosterone System Antagonists
Patients were considered to be on treatment with a renin-angiotensin-aldosterone system antagonist if they were on an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor or mineralocorticoid receptor antagonist. The number of patients who stopped and restarted treatment during the trial was calculated.
All analyses were conducted with Stata version 17 (College Station, TX) or R (version 3.6.1). A value of P<0.05 was considered statistically significant.
Results
Overall, 4618 participants (97%) had data available to calculate the change in eGFR from baseline to 14 days, 4498 (95%) had data at 60 days, and 4416 (93%) had data at 120 days. The mean and median eGFRs at baseline in the placebo group were 65.5 mL·min−1·1.73 m−2 (SD, 19.3 mL·min−1·1.73 m−2) and 64 mL·min−1·1.73 m−2 (interquartile range, 51–79 mL·min−1·1.73 m−2), respectively; the corresponding values in the dapagliflozin groups were 66.0 mL·min−1·1.73 m−2 (SD, 19.6 mL·min−1·1.73 m−2) and 64 mL·min−1·1.73 m−2 (interquartile range, 51–80 mL·min−1·1.73 m−2).
Mean Change in eGFR Early After Initiation of Randomized Treatment
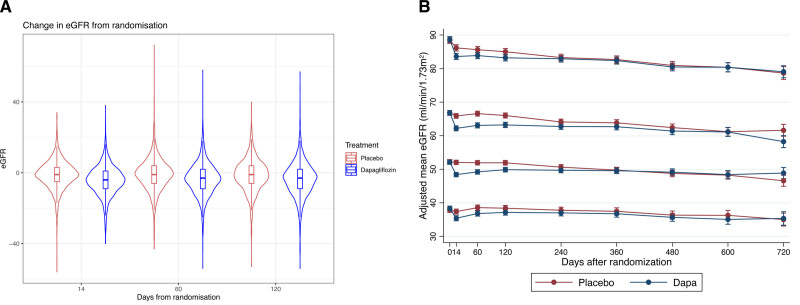
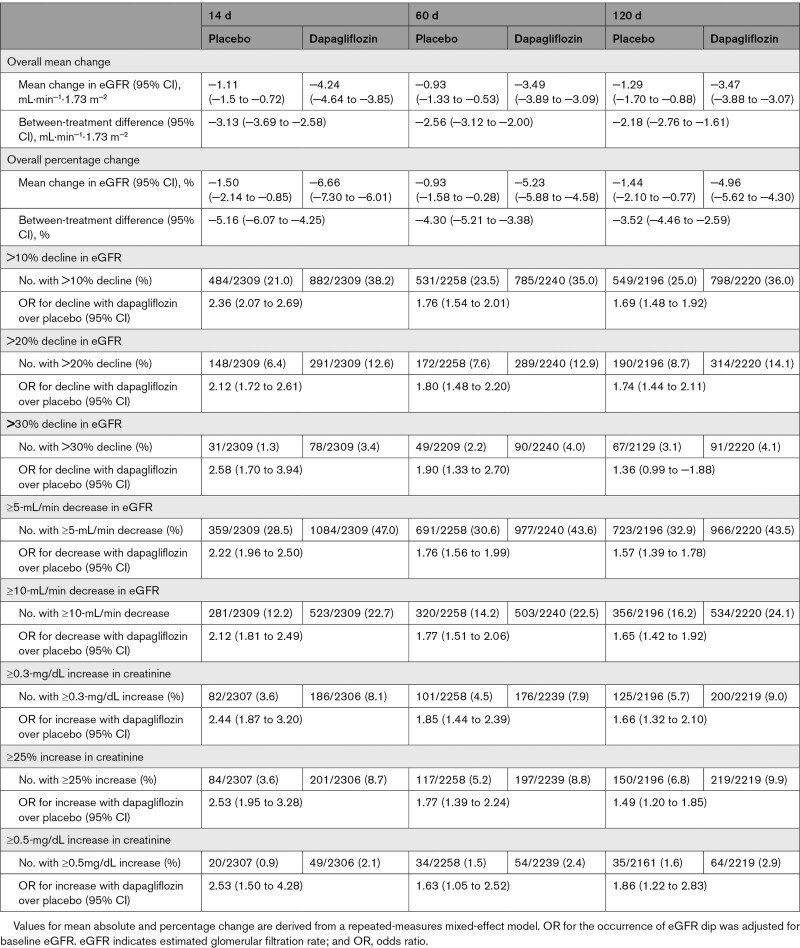
The median change in eGFR at 14 days was −4 mL·min−1·1.73 m−2 (interquartile range, −9 to 1 mL·min−1·1.73 m−2) with dapagliflozin and −1 mL·min−1·1.73 m−2 (interquartile range, −5 to 3 mL·min−1·1.73 m−2) with placebo (Figure 1A). The adjusted mean change in eGFR at 14 days was −4.2 mL·min−1·1.73 m−2 (95% CI, −4.6 to −3.9) and −1.1 mL·min−1·1.73 m−2 (95% CI, −1.5 to −0.7), respectively, giving a placebo-corrected difference of −3.1 mL·min−1·1.73 m−2 (95% CI, −3.7 to −2.6; Table 1). There was no interaction between baseline eGFR (analyzed as a continuous variable) and the effect of dapagliflozin on absolute change in eGFR at 14 days (Pinteraction=0.81).
Figure 1.
Absolute change in eGFR from baseline to 14, 60, and 120 days in each treatment arm and change in eGFR with placebo and dapagliflozin according to baseline eGFR category (from top to bottom: ≥75, <75–≥60-, <60–≥45, <45 mL·min−1·1.73 m−2). A, Violin plot illustrating the change in estimated glomerular filtration rate (eGFR) from baseline. Rectangle in the middle of the violin shows the median and interquartile range. Lines show the smoothed probability density at different values; therefore, the width of the violin corresponds to the distribution of the data. B, Change in eGFR over time in each treatment arm and each baseline eGFR category from a repeated-measures mixed-effect model. Dapa indicates dapagliflozin.
Table 1.
Mean Change in eGFR From Baseline and Number of Patients Meeting Different Renal Function Thresholds at 14, 60, and 120 days
The percent change in eGFR at 14 days was −6.7% (95% CI, −7.3 to −6.0) with dapagliflozin and −1.5% (95% CI, −2.1 to −0.9) with placebo, giving a difference of −5.2% (95% CI, −6.1 to −4.3; Table 1).
The placebo-corrected absolute and percent changes in eGFR at 2 weeks according to baseline eGFR category (≥75, <75–≥60, <60–≥45, <45 mL·min−1·1.73 m−2) are shown in Figure 1B and Table S1. The OR for a 10% dip in eGFR with dapagliflozin compared with placebo over the range of baseline eGFR as a continuous variable modeled with a fractional polynomial is given in Figure S1 (Pinteraction=0.07).
Proportions of Patients With Different Threshold Changes at 2 Weeks
Any Decline in eGFR
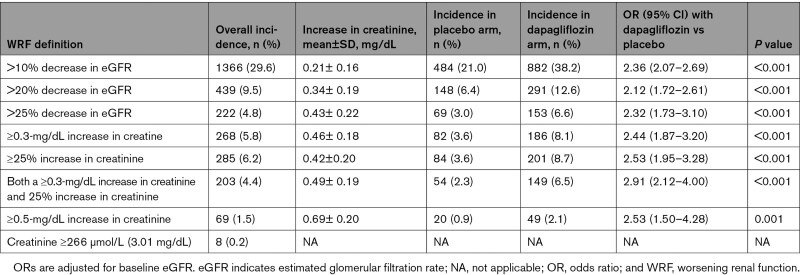
Among patients assigned to dapagliflozin, 69.4% had some decline in eGFR between baseline and day 14 compared with 52.7% of patients in the placebo group. The OR for any decline in eGFR with dapagliflozin compared with placebo was 2.03 (95% CI, 1.80–2.29; P<0.001).
Decline in eGFR of >10%, >20%, and >30%
Overall, 38.2% of patients treated with dapagliflozin had a decline in eGFR of >10%; the proportion in patients assigned to placebo with this change was 21.0% (OR, 2.36 [95% CI, 2.07–2.69]; P<0.001; Table 1). Because the absolute decrease in eGFR was similar across eGFR categories, the proportion with a >10% relative decline in eGFR was greater in patients starting with a lower eGFR (Figure S2).
The proportions and ORs for a 20% and 30% decline in eGFR are shown in Table 1. A >30% decline was uncommon, occurring in only 3.4% of patients on dapagliflozin and 1.3% of patients on placebo at day 14.
A ≥5– or ≥10–mL·min−1·1.73 m−2 Decrease in eGFR
In the dapagliflozin group, 1084 patients (47.0%) had a decrease in eGFR of ≥5 mL·min−1·1.73 m−2 between baseline and 14 days; this number in the placebo group was 359 (28.5%; P=0.001). Among patients randomized to dapagliflozin, 523 (22.7%) had a decrease in eGFR of ≥10 mL·min−1·1.73 m−2 compared with 281 (12.2%) in the placebo group (Table 1). The proportions of patients having these changes in eGFR according to the baseline eGFR category are shown in Table S1.
eGFR Reaching a Threshold of ≤20 mL·min−1·1.73 m−2
The number of patients reaching an eGFR of ≤20 mL·min−1·1.73 m−2 at 14 days was 5 (0.22%) in the dapagliflozin group and 0 in the placebo group (all of these patients had a baseline eGFR <45 mL·min−1·1.73 m−2).
The proportions of patients meeting other definitions of worsening kidney function are shown in Tables 1 and 2.
Table 2.
Incidence of Different Changes in eGFR at 14 Days and Odds of a Dip in Renal Function With Dapagliflozin Over Placebo
Baseline Characteristics and Early Change in eGFR
Baseline characteristics according to treatment assignment and eGFR change from baseline to 14 days are given in Table S2.
In univariable models, several baseline characteristics showed a significant interaction with treatment for the occurrence of eGFR dip >10% (Figure S3); an eGFR dip was more likely with dapagliflozin in older patients, male patients, and those with diabetes, a higher LVEF, and a lower eGFR. In a multivariable logistic regression model, significant interactions with treatment remained for age, diabetes, LVEF, and eGFR. In an exploratory analysis, dapagliflozin was found to reduce systolic blood pressure significantly more in patients with an LVEF ≥30% compared with patients with an LVEF <30%; the placebo-corrected difference was −3.2 mm Hg (95% CI, −4.1 to −2.3) versus −1.6 mm Hg (95% CI, −2.5 to −0.7), respectively (P=0.02; Table S3).
Given these interactions, multivariable logistic regression analyses were performed in each treatment arm separately. In these, female sex and lower eGFR were the only significant predictors of an eGFR dip in the placebo group, and the significant predictors in the dapagliflozin group were older age and diabetes.
Association Between an Early Decline in eGFR and Subsequent Cardiovascular Outcomes
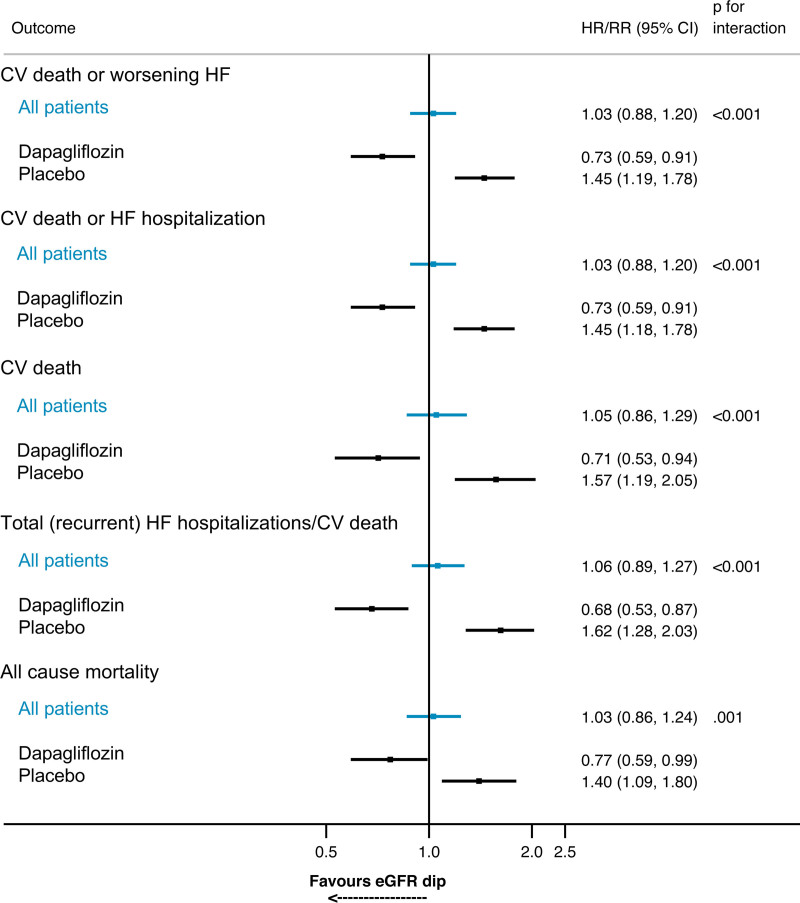
In a landmark analysis, with survival analysis time starting at 14 days, the HR for the primary composite outcome (worsening HF or cardiovascular death) in patients in the placebo group experiencing a >10% decline in eGFR between baseline and 14 days compared with the remainder of participants (≤10% decline/no change/increase in eGFR) was 1.45 (95% CI, 1.19–1.78). In contrast, the corresponding HR in the dapagliflozin group was 0.73 (95% CI, 0.59–0.91; Pinteraction<0.001). The same pattern was seen for cardiovascular death, total (recurrent) HF hospitalizations, and cardiovascular death and all-cause mortality (Figure 2). Using different eGFR and creatinine thresholds for change in renal function gave consistent results (Table 3).
Figure 2.
Risk of prespecified outcomes for patients experiencing a >10% decline in eGFR between baseline and day 14 compared with patients not experiencing a >10% decline in eGFR within each randomized treatment group. Follow-up is for outcomes occurring after 14 days (landmark analysis). Models are stratified by diabetes status and adjusted for baseline estimated glomerular filtration rate (eGFR) and history of heart failure (HF) hospitalization (HF hospitalization for each outcome apart from all-cause mortality). Analysis for all patients includes adjustment for randomized treatment. For example, between day 14 and the end of the study, the risk of the primary outcome (cardiovascular [CV] death or worsening HF) was 45% higher in patients randomized to placebo who experienced a >10% decline in eGFR between baseline and day 14 compared with placebo-treated patients who did not experience this decline. Conversely, the risk of the primary outcome was 27% lower among patients randomized to dapagliflozin who experienced a >10% decline in eGFR compared with dapagliflozin-treated patients who did not experience a >10% decline in eGFR between day 0 and 14. HR indicates hazard ratio; and RR, rate ratio.
Table 3.
Rates and HRs in Each Treatment Arm for the Primary Outcome in a Landmark Analysis From 14 Days in Subgroups Defined by Change in Renal Function at 14 Days
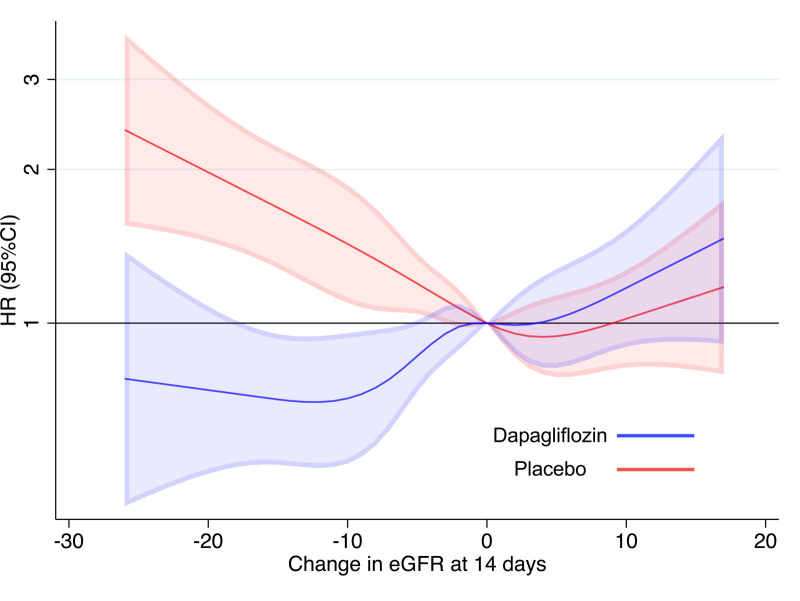
Analyzing change in eGFR from baseline to 14 days as a continuous variable with the use of restricted cubic splines also showed that a decline in eGFR with dapagliflozin was associated with a reduction in the hazard of the primary outcome and cardiovascular death, whereas a decline in eGFR on placebo was associated with an increase in risk (Figure 3).
Figure 3.
Risk of the primary outcome occurring after 14 days (landmark analysis) according to change in eGFR between baseline and day 14 within each randomized treatment group. Reference point 0 (no change). eGFR indicates estimated glomerular filtration rate; and HR, hazard ratio.
Later Changes in eGFR: Rebound and Chronic Slope
Change in eGFR at 14, 60, and 120 days After Initiation of Randomized Treatment
The OR for a >10% decline in eGFR was greatest at 14 days (OR, 2.36 [95% CI, 2.07–2.69]) and was less at both 60 days (OR, 1.76 [95% CI, 1.54–2.01]) and 120 days (OR, 1.69 [95% CI, 1.48–1.92; Figure 1A and Table 1).
In patients with a drop in eGFR of >10% between baseline and 14 days, there was a partial reversal of this dip between days 14 and 60 in both treatment groups (Figure 1B and Figure S4). The mean decrease in eGFR from day 0 to 14 was 4.2 mL·min−1·1.73 m−2 (95% CI, 3.8–4.6) with dapagliflozin and 1.1 mL·min−1·1.73 m−2 (95% CI, 0.7–1.5) with placebo. From day 14 to 60, the mean increase in eGFR was 0.7 mL·min−1·1.73 m−2 (95% CI, 0.3–1.1) with dapagliflozin and 0.2 mL·min−1·1.73 m−2 (95% CI, 0.2 decrease–0.6 increase) with placebo. Patients with an initial >10% decline had a greater early drop in eGFR but also a greater rebound between days 14 and 60. Within this group of patients, the mean decrease from day 0 to 14 was 11.8 mL·min−1·1.73 m−2 (95% CI, 11.2–12.5) with dapagliflozin and 11.6 mL·min−1·1.73 m−2 (95% CI, 10.8–12.5) with placebo. The mean increase from day 14 to 60 was 4.9 mL·min−1·1.73 m−2 (95% CI, 4.2–5.5) with dapagliflozin and 6.8 mL·min−1·1.73 m−2 (95% CI, 5.8–7.7) with placebo (Figure S4).
Chronic eGFR Slope (Day 60–720)
Between days 60 and 720, eGFR declined in both treatment groups regardless of the initial eGFR dip. For patients with an initial decline in eGFR, treatment with dapagliflozin was associated with a slower long-term decline in eGFR. For patients with an initial decline up to 10%, the day 60 to 720 slope was −1.7 mL·min−1·1.73 m−2 per year for dapagliflozin and −3.5 mL·min−1·1.73 m−2 per year for placebo (P for the difference in slopes <0.001). For those with a >10% initial decline, the subsequent slopes were −0.7 and −2.3 mL·min−1·1.73 m−2 per year, respectively (P for difference in slopes=0.002). In patients with a stable eGFR or an increase in eGFR over the first 14 days of follow-up, the longer-term difference in slopes was not significant; the slope between 60 and 720 days was −2.3 mL·min−1·1.73 m−2 per year with dapagliflozin and −3.0 mL·min−1·1.73 m−2 per year with placebo (P for the difference in slopes=0.07; Figure S4).
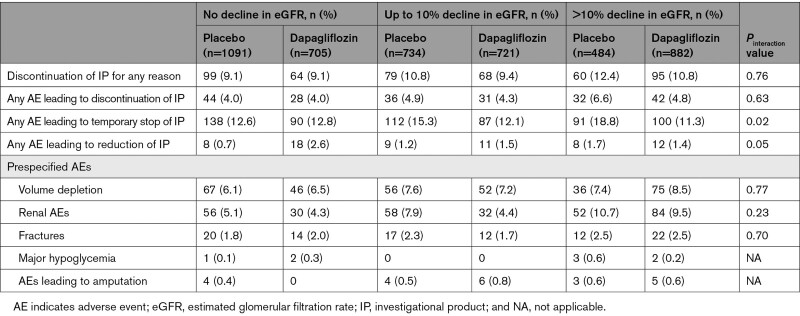
Safety and Study Drug Discontinuation
Placebo-treated patients with an initial decline (to 14 days) in eGFR had more adverse events than those without any decline in eGFR (Table 4). However, there was no significant difference in the risk of these events between dapagliflozin and placebo treatment in any eGFR change group. Of the 4618 patients with eGFR available at 14 days, 38 of 2309 patients (1.6%) assigned to dapagliflozin discontinued treatment between days 14 and 60 compared with 31 of 2309 (1.3%) assigned to placebo (P for the difference between groups=0.4).
Table 4.
Adverse Events and Treatment Discontinuation
Continuation of Concurrent Renin-Angiotensin-Aldosterone System Antagonists
Among patients assigned to placebo with analyzable data, 25 of 1091 (2.3%) of those with no decrease in eGFR by 14 days subsequently stopped treatment with a renin-angiotensin-aldosterone system antagonist permanently; of those with any decline in eGFR, this number was 51 of 1218 (4.2%). The corresponding numbers in the dapagliflozin group were 21 of 706 (3.0%) and 46 of 1603 (2.9%), respectively (between-treatment difference in patients with any decline, P=0.06; Table S4A). Further information on patients stopping temporarily and restarting is given in Table S4B. Patients randomly assigned to dapagliflozin tended to be more likely to restart treatment than those assigned to placebo.
Discussion
The key findings of the present study were that an early decline in eGFR occurred more commonly after starting dapagliflozin than after starting placebo in patients with HFrEF, but this decline was, on average, small. A decline in eGFR with dapagliflozin was associated with better cardiovascular outcomes compared with no decline in eGFR, whereas the opposite was observed with placebo. Evaluation of the eGFR slope showed that the initial dip in eGFR in patients randomized to an SGLT2 inhibitor was linked to a slower long-term rate of decline in eGFR, with no increase in renal adverse events.
SGLT2 inhibitors are thought to cause an initial decrease in eGFR by augmenting tubuloglomerular feedback.15 The average decline in DAPA-HF (≈3 mL·min−1·1.73 m−2 or 5%) was similar to the early placebo-corrected changes in EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; median decrease, 2.64 mL·min−1·1.73 m−2 at 4 weeks), VERTIS-CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial; mean decrease, 2.79 mL·min−1·1.73 m−2 at 6 weeks), and CREDENCE (Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation; mean decrease, 7.0% at 3 weeks).6–8 Overall, about a third of patients treated with dapagliflozin in DAPA-HF had no reduction in eGFR within 14 days of starting treatment, a third had a ≤10% reduction in eGFR, and a third had a decrease >10%. However, few patients experienced a large (>30%) reduction in eGFR with dapagliflozin (3.4% versus 1.3% on placebo), and the eGFR fell to a low level (≤20 mL·min−1·1.73 m−2) in a very small proportion of participants (0.22% of patients on dapagliflozin).
Several patient characteristics were associated with a greater likelihood of an early decline in eGFR >10%. These included older age, baseline eGFR <60 mL·min−1·1.73 m−2, higher LVEF, and type 2 diabetes. However, the interaction between baseline eGFR <60 and ≥60 mL·min−1·1.73 m−2 was partly artifactual because the absolute decrease in eGFR was similar in these 2 groups. Consequently, more patients with a low starting eGFR experienced a >10% decrease in eGFR than those with a higher eGFR. Important findings were that the average absolute placebo-corrected decrease in eGFR among patients in the lowest baseline eGFR category (30–45 mL·min−1·1.73 m−2) was only 2.4 mL·min−1·1.73 m−2 (−3.5 to −1.3) and that the proportion experiencing an eGFR decline of ≥10 mL·min−1·1.73 m−2 was 8.9% on dapagliflozin compared with 5.4% on placebo, with only 5 patients (0.22%) receiving dapagliflozin and none taking placebo having an eGFR decline to ≤20 mL·min−1·1.73 m−2.
Other observations about patients exhibiting an early dip in eGFR are also clinically relevant. Among participants experiencing a >10% decline in eGFR, there was no excess premature discontinuation of dapagliflozin or greater risk of adverse events. More important, patients experiencing a >10% early decrease in eGFR on dapagliflozin had significantly better clinical outcomes, including the primary end point and rate of decline in eGFR, than participants assigned to dapagliflozin with an eGFR decline ≤10%. Conversely, those experiencing the same initial decline in eGFR during treatment with placebo had worse outcomes than those with an eGFR decline ≤10% on placebo. Similar findings were observed when different definitions of worsening kidney function were used. Given this, we believe that physicians should not withdraw an SGLT2 inhibitor in a patient with HFrEF if eGFR declines after this treatment is initiated except in the few patients in whom kidney function declines to an unacceptably low level. There is as yet no consensus on what the unacceptable lower limit is, but we suggest that 20 mL·min−1·1.73 m−2 is reasonable given that SGLT2 inhibitor trials have included patients with an eGFR down to this threshold.16 The risk of a patient with HFrEF developing an eGFR as low as 20 mL·min−1·1.73 m−2 with an SGLT2 inhibition is probably very small, even if initial kidney function is poor. For example, if a patient had an initial eGFR of 30 mL·min−1·1.73 m−2, a 30% reduction would result in an eGFR of 21 mL·min−1·1.73 m−2, and as described earlier, such a large drop in eGFR is uncommon. Moreover, in DAPA-HF, as in other SGLT2 inhibitor trials, the initial dip in eGFR partially reversed over the subsequent 6 to 8 weeks; that is, in this hypothetical patient, 21 mL·min−1·1.73 m−2 is likely to be the nadir in eGFR. It is important to note that patients with a larger initial decline in eGFR showed a greater rebound between days 14 and 60. Thus, rechecking eGFR may reveal improvement sufficient to avoid discontinuation of treatment. However, given that large declines in eGFR are unusual with SGLT2 inhibitors, a decrease of >30% should prompt consideration of alternative causes of worsening kidney function, including progression of HF.
The partial reversal of eGFR is an interesting phenomenon that has not been explained, and it may reflect compensatory responses in the distal nephron, resetting of tubuloglomerular feedback, or both.15 It is important to note that in previous research markers of kidney injury were not increased despite the decline in eGFR and that eGFR returns to the pretreatment level on discontinuation of an SGLT2 inhibitor.17–20
Limitations
Among the limitations of this study were its post hoc nature and the exclusion of patients with an eGFR <30 mL·min−1·1.73 m−2 from enrollment in DAPA-HF. Some of our analyses may be subject to postrandomization confounding. Participants were also hemodynamically stable outpatients, and the renal effects of administration of an SGLT2 inhibitor in other patient groups might be different, particularly in very elderly and multimorbid patients, especially if exposed to nephrotoxic agents or overdiuresis. We did not measure albuminuria and other markers of kidney injury, which would have given additional insight into the importance of the early dip in eGFR after treatment with an SGLT2 inhibitor.
Conclusions
In stable outpatients with HFrEF, treatment with an SGLT2 inhibitor caused a small average decrease in eGFR, and few patients experienced a substantial decline in kidney function. This early decrease in eGFR with an SGLT2 inhibitor was not associated with more adverse events or study drug discontinuation and was associated with improved HF and renal outcomes. We suggest that physicians should not discontinue SGLT2 inhibitors unless the eGFR decreases by >30% (and not <20 mL·min−1·1.73 m−2). Physicians should also recognize that the nadir in eGFR occurs early after an SGLT2 inhibitor is started and that the initial dip in eGFR partially reverses over the subsequent 6 to 8 weeks.
Article Information
Sources of Funding
The DAPA-HF trial was funded by AstraZeneca. Dr McMurray and C. Adamson are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Disclosures
K.F. Docherty received funding for the University of Glasgow, UK, from AstraZeneca for DAPA-HF and has received honoraria for lectures from AstraZeneca and Eli Lilly. Dr Heerspink reports grant funding and honoraria for consultancy as a member of the steering committee of the DAPA-CKD trial paid to his institution from AstraZeneca; research grants paid to his employer from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk for clinical trials; consulting fees paid to his employer from Abbvie, Boehringer Ingelheim, Travere Pharmaceuticals, and Novo Nordisk; fees for steering committee membership paid to his employer from Bayer, Chinook, CSL Pharma, Janssen, and Gilead; honoraria for lectures from AstraZeneca and Mitsubishi Tanabe; and honoraria for advisory board participation from Merck (paid to his employer), Mitsubishi Tanabe, and Mundipharma. Dr de Boer reports grants from Cardior Pharmaceuticals GmbH to other; compensation for other services from Novartis, AstraZeneca, Roche Diagnostics GmbH, Bayer, and Abbott Fund; and grants from AstraZeneca, Ionis Pharmaceuticals, Inc, Roche Diagnostics GmbH, Boehringer Ingelheim, Novo Nordisk, and Abbott Fund to other. Dr Damman has received consultancy fees from Abbott. Dr Inzucchi received funding from AstraZeneca for participating in the steering committee for DAPA-HF; has received consultancy fees from Abbott, VTV Therapeutics, Esperion, Pfizer, and Merck; and reports fees for clinical trial committee participation, advisory roles, and travel costs from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk, as well as honoraria for lectures from Merck and AstraZeneca. Dr Køber reports speaker honoraria from Novo Nordisk, Novartis, AstraZeneca, and Boehringer Ingleheim; support from AstraZeneca; and personal fees from Novartis and Bristol Myers Squibb as a speaker. Dr Kosiborod reports payment to his institution for participation in DAPA-HF; has received grant payment to his institution from Boehringer Ingelheim; has received personal fees, fees to his institution, or both for consultancy from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Vifor Pharma; has received personal honoraria and honoraria to his institution for lectures from AstraZeneca, Boeringer Ingelheim, and Novo Nordisk; has received personal honoraria and honoraria to his institution from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Vifor Pharma for participation on Data Safety Monitoring Boards; and has received study drug for a clinical trial from AstraZeneca and Boehringer Ingelheim. Dr Martinez reports personal fees from AstraZeneca. Dr Petrie reports compensation from Bayer, Boehringer Ingelheim, Takeda California, Inc, and Novo Nordisk for end point review committee services; compensation from Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Novartis, and SQ Innovation for consultant services; grants from Boehringer Ingelheim; and grants from Novartis, SQ, and AstraZeneca to other. Dr Ponikowski reports personal fees for consultancy and speaker bureau from AstraZeneca, Boehringer Ingelheim, Vifor Pharma, Servier, Bayer, Bristol Myers Squibb, Respocardia, Berlin-Chemie, Cibiem, Novartis, and RenalGuard; other support for participation in clinical trials from Boehringer Ingelheim, Amgen, Vifor Pharma, Bayer, Bristol Myers Squibb, Cibiem, Novartis, and RenalGuard; and research grants to his institution from Vifor Pharma. Dr Sabatine reports grants from Bayer, Daiichi Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda; grants and personal fees from Amgen, AstraZeneca, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, and Novartis; and personal fees from Anthos Therapeutics, Bristol Myers Squibb, CVS Caremark, DalCor, Dyrnamix, Esperion, IFM Therapeutics, and Ionis. Dr Sabatine received an institutional research grant from AstraZeneca for DAPA-HF and received institutional research grants from Abbott, Amgen, Anthos Therapeutics, Bayer, Daiichi-Sankyo, Eisai, Intarcia, IONIS, The Medicines Company, MedImmune, Merck, Novartis, Pfizer, and Quark Pharmaceuticals; received consulting fees from Althera, Amgen, Anthos Therapeutics, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, DalCor, Dr Reddy’s Laboratories, Fibrogen, IFM Therapeutics, Intarcia, MedImmune, Merck, Moderna, and Novo Nordisk. Dr Sabatine is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from Regeneron, Roche, and Zora Biosciences. Dr Schou reports no conflicts. Dr Solomon received payment to his institution for participation in DAPA-HF; received grants to his institution from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, IONIS, Lilly, Mesoblast, MyoKardia, National Institutes of Health National Heart, Lung, and Blood Institute, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; received fees for consultancy from Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, and Sarepta; and received honoraria for lectures from Novartis and AstraZeneca. Dr Verma has received research and/or speaking honoraria from Amgen, Amarin, AstraZeneca, Bayer, CMS, Janssen, HLS, Sanofi, Novo Nordisk, Novartis, Merck, and PhaseBio. He is also the president of the Canadian Medical and Surgical Knowledge Translation Research Group and holds the Tier 1 Canada Research Chair in Cardiovascular Surgery. Drs Bengtsson, Langkilde, and Sjöstrand are employees and stockholders of AstraZeneca. Dr Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (National Institutes of Health National Center for Advancing Translational Sciences Award UL 1TR002541); serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa; and has participated on clinical end point committees for studies sponsored by Novartis (including the PARAGON-HF trial) and the National Institutes of Health. Dr Jhund reports payment to the University of Glasgow by AstraZeneca for his time working on the DAPA-HF and DELIVER trials, from Novartis for work on the PARADIGM-HF and PARAGON-HF trials, and Novo Nordisk; reports speaker and advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; and reports research funding from Boehringer Ingelheim and Analog Devices. Dr McMurray reports payment to Glasgow University by AstraZeneca for time spent as principal investigator of DAPA-HF and coprincipal investigator of DELIVER and DETERMINE in HF and meetings and other activities related to these trials; AstraZeneca has also paid travel and accommodation for these meetings. These payments were made through a consultancy with Glasgow University, and he did not receive personal payments in relation to this trial or drug. He has received personal lecture fees from Abbott, Alkem Metabolics, Eris Lifesciences, Hikma, Lupin, Sun Pharmaceuticals, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Servier, and the Corpus; and reports steering committee and travel fees from Cytokinetics and Amgen, advisor and travel fees from KBP Biosciences, steering committee fees from Bayer, DalCor, and Bristol Myers Squibb, investigator and travel fees from Theracos, consultancy and travel fees from IONIS, investigator, steering committee, and travel fees from Novartis and GlaxoSmithKline, consultancy fees from Boehringer Ingelheim, and advisory board fees from Cardurion and Alnylam, all paid to the University of Glasgow. C. Adamson reports no conflicts.
Supplemental Material
Tables S1–S4
Figures S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- DAPA-HF
- Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure
- eGFR
- estimated glomerular filtration rate
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- HR
- hazard ratio
- LVEF
- left ventricular ejection fraction
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- OR
- odds ratio
- SGLT2
- sodium-glucose cotransporter 2
C. Adamson and K.F. Docherty contributed equally.
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.121.058910.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 448.
Contributor Information
Carly Adamson, Email: carly.adamson@glasgow.ac.uk.
Kieran F. Docherty, Email: kfdocherty@gmail.com.
Hiddo J.L. Heerspink, Email: h.j.lambers.heerspink@umcg.nl.
Rudolf A. de Boer, Email: r.a.de.boer@umcg.nl.
Kevin Damman, Email: k.damman@umcg.nl.
Silvio E. Inzucchi, Email: silvio.inzucchi@yale.edu.
Lars Køber, Email: Lars.koeber.01@regionh.dk.
Mikhail N. Kosiborod, Email: mkosiborod@saint-lukes.org.
Felipe A. Martinez, Email: Dr.martinez@usa.net.
Mark C. Petrie, Email: mark.petrie@glasgow.ac.uk.
Piotr Ponikowski, Email: piotr.ponikowski@umed.wroc.pl.
Marc S. Sabatine, Email: msabatine@partners.org.
Morten Schou, Email: morten.schou.04@regionh.dk.
Scott D. Solomon, Email: ssolomon@bwh.harvard.edu.
Subodh Verma, Email: Subodh.Verma@unityhealth.to.
Olof Bengtsson, Email: Olof.Bengtsson@astrazeneca.com.
Anna Maria Langkilde, Email: annamaria.langkilde@astrazeneca.com.
Mikaela Sjöstrand, Email: Mikaela.Sjostrand@astrazeneca.com.
Muthiah Vaduganathan, Email: mvaduganathan@bwh.harvard.edu.
Pardeep S. Jhund, Email: pardeep.jhund@glasgow.ac.uk.
References
- 1.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi:10.1056/nejmoa2024816. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 5.Damman K, Masson S, Lucci D, Gorini M, Urso R, Maggioni AP, Tavazzi L, Tarantini L, Tognoni G, Voors A, et al. Progression of renal impairment and chronic kidney disease in chronic heart failure: an analysis from GISSI-HF. J Card Fail. 2017;23:2–9. doi: 10.1016/j.cardfail.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Levin A, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021;99:999–1009. doi: 10.1016/j.kint.2020.10.042 [DOI] [PubMed] [Google Scholar]
- 7.Cherney DZI, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, Shih WJ, Frederich R, Maldonado M, Pong A, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99:750–762. doi: 10.1016/j.kint.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. A trial to evaluate the effect of the sodium–glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019;21:665–675. doi: 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019;21:1402–1411. doi: 10.1002/ejhf.1548 [DOI] [PubMed] [Google Scholar]
- 11.Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, BÃhm M, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143:298–309. doi: 10.1161/CIRCULATIONAHA.120.050391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; 2016. [Google Scholar]
- 13.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62:711–730. doi: 10.1111/1467-9868.00259 [Google Scholar]
- 15.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:422–434. doi: 10.1016/j.jacc.2019.11.031 [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 17.Sen T, Li J, Neuen BL, Neal B, Arnott C, Parikh CR, Coca SG, Perkovic V, Mahaffey KW, Gavin Y, et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia. 2021;64:2147–2158. doi: 10.1007/s00125-021-05512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opingari E. The Effect of Empagliflozin on Kidney Injury Markers in Subjects With Type 2 Diabetes and Cardiovascular Disease: A Sub-Analysis of the EMPA-HEART CardioLink-6 Trial [thesis]. Institute of Medical Science, University of Toronto; 2019. [Google Scholar]
- 19.Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle H-J, Broedl UC, von Eynatten M, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018;29:2755–2769. doi: 10.1681/ASN.2018010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Husker SJ, Brueckmann M, Pfarr E, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021;143:310–321. doi: 10.1161/circulationaha.120.051685 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.