Abstract
Rationale:
Insufficient sleep is associated with a number of negative health outcomes; as most adolescents obtain <7 hours of sleep per night, it is important to understand how sleep impacts asthma among adolescents.
Objectives:
To examine the impact of sleep opportunity on asthma in adolescents.
Methods:
Fifty-four adolescents with asthma (12–17 years, 69% female, 65% Caucasian) participated in a randomized, cross-over sleep manipulation trial, including a sleep stabilization week, 5 nights of a “Short” sleep opportunity (time in bed: 6.5 hours/night), and 5 nights of a “Long” sleep opportunity (time in bed: 9.5 hours/night). Wake times were consistent across all three study weeks. Primary outcomes were lung function (daily peak expiratory flow rate, weekly spirometry) and functional asthma outcomes (daily asthma symptoms, Asthma Control Questionnaire, PROMIS Asthma Impact Scale). Markers of inflammation were also explored.
Measurements and Main Results:
Compared to the Long sleep week, during the Short sleep week, morning FEV1 was lower (p=0.006), while asthma symptoms and albuterol use was higher (p<0.05), and asthma showed a trend towards greater negative impact on daily life (p=0.07). No differences were found for weekly measures of lung function or inflammation.
Conclusions:
An insufficient sleep opportunity negatively impacts objective and subjective daily symptoms of asthma in adolescents, as well as health related quality of life. As most adolescents are significantly sleep deprived, it is important to target sleep health in the treatment of asthma.
Keywords: adolescence, pediatrics, quality of life, sleep extension, sleep restriction
Introduction
Asthma prevalence among adolescents has increased since 2001,1 resulting in hospitalization, emergency/urgent care visits, and missed school days.2–5 Along with the medical management of asthma, modifiable health behaviors such as sleep opportunity should be considered. Most studies of adolescents with asthma have focused on the associations between asthma and sleep quality or sleep-disordered breathing.6–10 Yet associations cannot provide information on causality. Thus, the purpose of the current study was to use an experimental manipulation of sleep opportunity to examine the causal impact of sleep opportunity on lung function and functional outcomes in adolescents with asthma.
The nocturnal worsening of asthma has been well established and attributed to multiple factors, including circadian rhythms and sleep posture.11–16 Early studies with adults with asthma found that acute total sleep deprivation (i.e., 1–2 nights with no sleep) decreased peak expiratory flow rate (PEFR).17;18 However, acute total sleep deprivation among adolescents is rare, while chronic partial sleep restriction (routinely sleeping less than required for optimal functioning) is quite common.
Although adolescents have an average biological need of over 9 hours of sleep per night,19;20 only 25% of adolescents in the United States obtain at least 8 hours of sleep on an average school night,21 resulting in chronic partial sleep restriction (i.e., insufficient sleep). Insufficient sleep among adolescents is so significant that increasing adolescent sleep duration is one of the Healthy People 2020 primary Sleep Health outcomes.22 Known consequences of insufficient adolescent sleep include increased daytime sleepiness and poor school performance;23–26 inattention, poor impulse control, and mood problems (e.g., depression);27–30 more risk-taking behaviors;31;32 and increased risk for automobile crashes.33–35 Studies also suggest that insufficient adolescent sleep is a risk factor for hypertension, hypercholesterolemia, insulin resistance, and obesity.36–40 However, only one small pilot study (n=10) used an experimental protocol to examine the role of chronic partial sleep restriction on asthma in adolescents, finding that compared to a week of a sufficient sleep opportunity, during a week of partial sleep restriction adolescents had a significant decrease in overnight PEFR and reported greater asthma interference with daytime functioning.41
The current study extends that preliminary work with a larger sample and more comprehensive outcome measures. Using a similar sleep manipulation protocol, we hypothesized that compared to 5 nights of sufficient sleep opportunity (9.5 hours in bed/night), adolescents with asthma would have worse lung function (i.e., daily peak expiratory flow rate, weekly spirometry) and report worse functional asthma outcomes (i.e., daily asthma symptoms, subjective asthma control, poorer health related quality of life) after 5 nights of partial sleep restriction (6.5 hours in bed/night). Finally, there are known associations between insufficient sleep and inflammatory markers associated with asthma (i.e., FeNO, CRP, IL-6, TNF-α),42;43 as well as known associations between asthma and obstructive sleep apnea due to overlap in airway inflammation.44;45 However, no study has considered the association between sleep, inflammation, and asthma in adolescents without obstructive sleep apnea. Thus, we also explored the relationship between sleep opportunity and inflammatory markers.
Methods
This study was approved by the Institutional Review Board at National Jewish Health (NJH, HS# 2860). Informed parental consent and adolescent assent were obtained for all participants.
Participants
Adolescents (ages 12–17 years) with asthma were recruited from either a tertiary medical center or community advertisements (e.g., radio, Facebook) between May 2015 and July 2017. An experienced Clinical Research Coordinator with specialized training in pediatric asthma screened for inclusion criteria and verification of asthma diagnosis, symptoms, and medications. Inclusion criteria were (a) age 12.0 – 17.9 years at time of summer participation; (b) evidence of active asthma in the last year, defined as presence of recurrent daytime OR nighttime symptoms (cough, wheeze, chest tightness, shortness of breath) OR asthma exacerbations that required an urgent care visit, hospitalization or systemic corticosteroids in the past year, OR the use of inhaled or oral medications for asthma; (c) average weekday sleep opportunity (bedtime to wake time) of 7.5 to 9 hours. Asthma diagnoses were confirmed through an in-person evaluation with an allergist, pulmonologist, or physician assistant with extensive experience with pediatric asthma.
Exclusion criteria were (a) presence of another significant chronic illness or diagnosed sleep disorder; (b) parent report of professionally diagnosed psychiatric disorder or developmental disorder (e.g., autism, ADHD); (c) suspicion of recurrent illegal substance use, (d) a history of neurologic illness or injury; (e) currently pregnant; (f) use of a medication or supplement (e.g., melatonin) with known effects on sleep or daytime alertness; g) obligations that would require a bedtime later than 10 p.m. or waking prior to 6 a.m. during the study; (h) obligations that would require driving, using heavy machinery, or otherwise engaging in activities that would be dangerous during the deficient sleep opportunity week; (i) daily consumption of more than 1 coffee or “energy drink” or more than 2 caffeinated sodas; (j) obesity (BMI > 98th percentile); and (k) cigarette smoking or smokeless tobacco product use in the past year.
All participants had a single night of in-lab polysomnography (PSG) at National Jewish Health to ensure there was no obstructive sleep apnea or periodic limb movements in sleep. The PSG included a standard montage recommended by a Task Force of the American Academy of Sleep Medicine, and was scored using the recommended pediatric scoring criteria.46;47
Sleep Manipulation Protocol
The study protocol was similar to that of Meltzer and colleagues.41 Participants were asked to select a consistent wake time for all three study weeks that would limit within-subject circadian variation and allow them to attend their weekly morning appointment. Participants were also instructed not to nap during the study. During the sleep stabilization week (and on weekends), participants were allowed to self-select a bedtime that allowed 7.5 to 8.5 hours in bed per night trying to sleep (“time in bed” or TIB), allowing for the stabilization of their sleep schedule, and to ensure both an increase and decrease in sleep opportunity. For the insufficient or “Short” sleep week, participant bedtime was set to restrict their time in bed to 6.5 hours per night, while during the sufficient or “Long” sleep week, participant bedtime was set to allow for 9.5 hours in bed per night trying to sleep (TIB). The 3 hour gap between Short and Long sleep opportunities was determined based on the recommended sleep amount and the actual sleep amount obtained by adolescents, including adolescents with asthma.7;48 Each study week was 5 nights, simulating a typical school week. Following successful completion of the sleep stabilization week, adolescents were randomized to either the Short or Long sleep week first. Block randomization was used, with a computer-generated randomized number list. Only the study PI had access to the random allocation sequence that was used to assign participations to the Long or Short sleep week first. The full trial protocol can be obtained from the first author (LJM).
Measurements – Daily Assessments
Actigraphy.
To ensure adherence to the study protocol (i.e., following the required bedtimes, required wake times, and ensure no napping), participants wore a Motionlogger Sleep Watch (Ambulatory-Monitoring, Inc., Ardsley, NY) on the non-dominant wrist for the full three weeks of the study, removing the devices for bathing, swimming, or if the watch could be damaged (e.g., sports practice). An event marker, in combination with the daily sleep diary, provided required information for scoring the actigraphy. Study outcomes included time in bed (reported bedtime to reported wake time), sleep period (actigraphic sleep onset to actigraphic sleep offset), total sleep time (number of minutes of actigraphy identified sleep between reported bedtime and reported wake time), and sleep efficiency (total sleep time/time in bed, expressed as a percent).
Daily Sleep and Asthma Diary.
A daily diary was sent via email through REDCap (Research Electronic Database Capture) at the University of Colorado Denver, a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.49 Participants were asked to complete the diary within one hour of waking. If the diary was not completed by 10:00 a.m., a member of the study team contacted the participant to remind them to complete the diary.
Diary questions for sleep included bedtime (tried to fall asleep), wake time (time of final waking in the morning), subjective sleep quality, napping, caffeine use, and subjective daytime sleepiness. Asthma symptoms were assessed with the Pediatric Asthma Diary.50;51 Specifically, participants were asked “Yesterday did you have any coughing, wheezing, shortness of breath, or chest tightness?” (daytime asthma symptoms); “How many puffs of albuterol did you use yesterday” (daytime albuterol use); “Did you have any coughing, wheezing, shortness of breath, or chest tightness last night?” (nighttime asthma symptoms); and “How many puffs of albuterol did you use during the night last night?” (nighttime albuterol use). Finally participants were asked if both morning and evening peak flow meter assessments had been completed.
Peak Expiratory Flow Meter.
Participants used an electronic peak flow meter (PF100, Microlife, Clearwater, FL) every morning and evening to measure peak expiratory flow (PEF) and FEV1 (forced expiratory volume-1 second). Participants were instructed to complete their measurements before scheduled or rescue inhaler, and within one hour of waking and approximately 12 hours later. Data were downloaded at each of the weekly visits and reviewed for adherence.
Weekly Objective Measures.
At the end of each study week participants had a set visit time (to limit within-subject circadian variance) at NJH. During these visits adolescents completed spirometry (using ATS Standardization of Spirometry 1994 Guidelines) and exhaled nitric oxide (with the NiOx Mino or NiOx Vero, Circassia Pharmaceuticals Inc., Morrisville, NC) assessments. In addition, venous blood sampling was performed by a trained phlebotomist. Cytokine levels (IL-1β, TNF-α, and IL-6) were analyzed by ELISA and CRP by the latex-enhanced turbidimetric immunoassay method.
Asthma Control Questionnaire (ACQ).
The ACQ52 is one of two asthma control instruments designated as core measures of NIH-initiated clinical research.53 The ACQ includes 6 questions that assess symptom frequency, rescue therapy use, and activity limitation, as well as clinically measured FEV1. The ACQ was completed by participants at each of the weekly visits. A score of ≤ 0.75 was considered well-controlled asthma, and a score of ≥ 1.5 was considered inadequately controlled asthma.54
PROMIS Asthma Impact Scale (PAIS).
The PAIS addressed how a child’s life is affected by asthma.55 The PAIS is a self-report measure with 8 items and a 7-day recall period, and was completed by participants at each of the weekly visits.
Data Analysis
For weekly measures, Short and Long sleep weeks were compared using paired t-tests, pairing within subject based on the crossover design. For daily measures (actigraphy, measures from peak flow meters, symptoms), linear mixed models (LMM) were used for continuous measures, and generalized linear mixed models (GzLMM) were used for binary outcomes (logistic regression) or counts (Poisson regression). For both modeling approaches, a random intercept was included for subjects in addition to a spatial power structure for errors. Pseudo-likelihood methods were employed for the GzLMM’s to allow fitting of both components. Predictors in the longitudinal models included treatment (Short, Long), day, and the treatment*day interaction. When appropriate, covariates were added in some preliminary models, such as when testing for adequacy of randomization by inclusion of sequence or other demographic variables. Sample sizes were selected based on our pilot data41 that suggested 49 participants would provide adequate (at least 90%) power to detect mean differences between the Short and Long sleep weeks for peak flow variables.
Results
Participant Characteristics
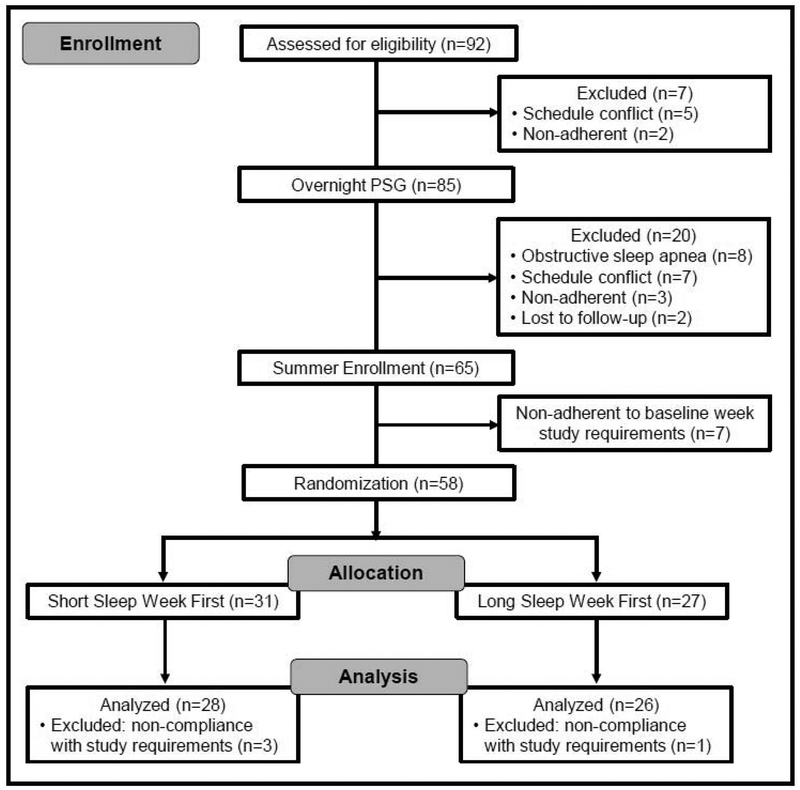
Ninety-two adolescents consented to participate in the study, with 54 completing the full three-week study protocol (Figure 1). Eight adolescents were diagnosed with obstructive sleep apnea (OSA) by overnight polysomnography (PSG), 19 dropped out prior to summer enrollment (i.e., before PSG, before start of summer), 7 were non-compliant prior to randomization, and 4 were non-compliant after randomization. There were no significant differences between adolescents who completed the study and those who did not in terms of age, gender, grade, race, or ethnicity; adolescents diagnosed with OSA had a higher body mass index percentile (mean=90.0, SD=10.8) compared to adolescents who completed (mean=62.0, SD=27.5), were non-compliant (mean=37.0, SD=22.6), or who dropped out of the study (mean=58.3, SD=30.0), F(3,88)=4.51, p=0.005.
Figure 1:
CONSORT flow diagram of participant recruitment, randomization, and analysis.
Table 1 provides demographic information for adolescents who completed the study; the study sample included more females than males, but was more racially diverse than the Colorado population.56 There were no significant demographic differences by randomization group, with similarities in gender, race, age, grade or BMI between adolescents randomized first to the Short or Long sleep. PSG variables are also presented in Table 1; means for these variables were similar between randomization groups. There were no significant associations between any PSG variable and outcome variables, including actigraphy and lung function.
Table 1.
Demographic characteristics [mean (SD)] of participants who completed study by randomization sequence
| Total (n=54) | Short Sleep First (n=28) | Lona Sleep First (n=26) | |
|---|---|---|---|
| Female: % (n) | 68.5 (37) | 60.7 (17) | 76.9 (20) |
| White/Caucasian: % (n) | 64.8 (35) | 57.1 (16) | 73.1 (19) |
| Black/African American: % (n) | 14.5 (8) | 17.9 (5) | 11.5 (3) |
| Multi-racial: % (n) | 14.5 (8) | 21.4 (6) | 7.7 (2) |
| Other: % (n) | 5.5 (3) | 3.6 (1) | 7.6 (2) |
| Hispanic: % (n) | 26.9 (14) | 21.4 (6) | 32.0 (8) |
| Age: mean (SD) | 14.7 (1.6) | 14.7 (1.5) | 14.7 (1.8) |
| Grade: mean (SD) | 10.0 (1.7) | 10.0 (1.5) | 10.0 (1.9) |
| BMI: mean (SD) | 22.3 (4.1) | 22.8 (4.8) | 21.7 (3.1) |
| BMI Percentile: mean (SD) | 62.0 (27.5) | 62.3 (30.0) | 61.8 (25.0) |
| Baseline ACQ Well Controlled: % (n) | 72.2 (39) | 82.1 (23) | 61.5 (16) |
| Baseline ACQ Inadequate Control: % (n) | 9.3 (5) | 7.1 (2) | 11.5 (3) |
| Medications: % (n) | |||
| Short-Acting Bronchodilator | 70.4 (38) | 75.0 (21) | 65.4 (17) |
| Inhaled Corticoid Steroid | 35.2 (19) | 35.7 (10) | 34.6 (9) |
| Combination (ICS plus LABA) | 16.7 (9) | 17.9 (5) | 15.4 (4) |
| Leukotriene Modifier | 9.3 (5) | 7.1 (2) | 11.5 (3) |
| Biologics | 1.9 (1) | 0.0 (0) | 3.8 (1) |
| Antihistamines | 22.2 (12) | 25.0 (7) | 19.2 (5) |
| Polysomnography: mean (SD) | |||
| Sleep Efficiency | 89.7 (8.2) | 89.95 (6.5) | 89.5 (9.8) |
| Percent of N1 Sleep | 4.9 (3.6) | 4.7 (3.2) | 5.1 (4.1) |
| Percent of N2 Sleep | 57.9 (8.1) | 56.0 (8.9) | 60.0 (6.7) |
| Percent of N3 Sleep | 21.6 (8.7) | 23.5 (9.4) | 19.4 (7.6) |
| Percent of REM Sleep | 15.6 (4.3) | 15.7 (4.7) | 15.4 (3.9) |
| Obstructive Apnea Index | 1.1 (0.9) | 1.3 (1.1) | 0.9 (0.6) |
| Total RDI* | 2.1 (1.6) | 2.4 (2.0) | 1.9 (1.1) |
| SpO2 | 92.9 (1.2) | 92.8 (1.4) | 93.0 (1.1) |
1 subject with missing value; ACQ: Asthma Control Questionnaire
Adherence to Sleep Schedule
Table 2 provides mean values for the sleep characteristics of study completers by randomization sequence. No significant differences were found between the two randomization sequences for sleep opportunity, sleep period, total sleep time, sleep efficiency, bedtime or wake time. In addition, participants were adherent to the required sleep conditions, averaging a sleep opportunity of 8.2 hours during the sleep stabilization week, 6.7 hours during the Short sleep week, and 9.4 hours during the Long sleep week, with a rise time close to 7:30 a.m. for all three study weeks.
Table 2.
Sleep characteristics [mean (SD)] of study participants by randomization sequence
| Total (n=54*) | Short Sleep First (n=28*) | Long Sleep First (n=26) | |
|---|---|---|---|
| Sleep Stabilization Week | |||
| Time in bed (TIB), hours† | 8.21 (0.47) | 8.16 (0.53) | 8.26 (0.39) |
| Sleep period, hours† | 7.79 (0.63) | 7.65 (0.63) | 7.94 (0.60) |
| Total sleep time (TST), hours | 7.20 (0.78) | 7.06 (0.86) | 7.35 (0.67) |
| Efficiency (TIB/TST) | 0.88 (0.07) | 0.86 (0.07) | 0.89 (0.06) |
| Bedtime, time of day‡ | 11:22 pm (1.01) | 11:32 pm (1.21) | 11:11 pm (0.75) |
| Wake time, time of day‡ | 7:34 am (0.95) | 7:41 am (1.11) | 7:26 am (0.75) |
| Short Sleep Week | |||
| Time in bed (TIB), hours† | 6.71 (0.34) | 6.69 (0.32) | 6.73 (0.37) |
| Sleep period, hours† | 6.32 (0.51) | 6.32 (0.41) | 6.31 (0.61) |
| Total sleep time (TST), hours | 5.99 (0.50) | 5.97 (0.46) | 6.00 (0.55) |
| Efficiency (TIB/TST) | 0.89 (0.06) | 0.89 (0.05) | 0.89 (0.06) |
| Bedtime, time of day‡ | 12:55 am (0.88) | 1:05 am (0.99) | 12:45 am (0.72) |
| Wake time, time of day‡ | 7:37am (0.95) | 7:46 am (1.11) | 7:28 am (0.72) |
| Long Sleep Week | |||
| Time in bed (TIB), hours† | 9.38 (0.24) | 9.35 (0.23) | 9.41 (0.25) |
| Sleep period, hours† | 8.57 (0.59) | 8.53 (0.58) | 8.61 (0.61) |
| Total sleep time (TST), hours | 7.78 (0.81) | 7.75 (0.85) | 7.82 (0.77) |
| Efficiency (TIB/TST) | 0.83 (0.08) | 0.83 (0.08) | 0.83 (0.08) |
| Bedtime, time of day‡ | 10:07pm (0.89) | 10:17pm (1.03) | 9:58 pm (0.69) |
| Wake time, time of day‡ | 7:29 am (0.91) | 7:36 am (1.04) | 7:21 am (0.76) |
For Sleep Stabilization week, n=27
Time in bed is scored from reported bedtime to wake time, sleep period is scored from actigraphic sleep onset to actigraphic sleep offset
SD in hours
Comparison of Weekly Outcomes
Mean values for both objective and subjective weekly outcomes can be found in Table 3. No significant differences were found between the Short and Long sleep weeks on objective measures of lung functioning, including FEV1 (p=0.70), FVC (p=0.70), FEV1/FVC (p=0.73), or exhaled nitric oxide (p=0.27). Similarly, no significant differences were found between the Short and Long sleep weeks for any of the serum cytokines (all p’s > 0.25). For subjectively reported asthma control, no differences were found between the Short and Long sleep weeks (p=0.32), however during the Short sleep week adolescents reported that asthma had a greater impact on their daily functioning, with marginal significance (PAIS average T-score=41.82 in Short week, 40.37 in Long; p=0.07).
Table 3.
Percentiles and means for variables during Long and Short sleep weeks (Q1=25th percentile, Q2=50th percentile, Q3=75th percentile), and mean comparisons using paired t-tests.
| Long | Short | L vs S | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Q1 | Q2 | Q3 | Max | Mean | Min | Q1 | Q2 | Q3 | Max | p ‡ | |
| Questionnaires* | |||||||||||||
| ACQ, Total Score | 0.66 | 0 | 0 | 0.33 | 1.00 | 2.67 | 0.74 | 0 | 0.17 | 0.50 | 1.33 | 2.83 | 0.32 |
| PAIS, T-Score | 40.37 | 31.5 | 31.5 | 37.7 | 46.2 | 64.0 | 41.82 | 31.5 | 31.5 | 39.7 | 48.4 | 65.2 | 0.07 |
| Spirometry (pre-albuterol) | |||||||||||||
| FEV1, % pred. | 96.8 | 57.3 | 89.4 | 97.3 | 106.7 | 122.7 | 96.9 | 67.7 | 90.9 | 97.5 | 104.9 | 120.3 | 0.91 |
| FVC, % pred. | 102.6 | 74.0 | 94.8 | 101.0 | 110.2 | 131.1 | 102.7 | 80.2 | 95.1 | 101.9 | 110.8 | 130.4 | 0.83 |
| FEV1 / FVC ×100 | 82.7 | 61.0 | 78.0 | 84.0 | 89.0 | 98.0 | 82.9 | 56.0 | 80.0 | 85.0 | 88.0 | 94.0 | 0.73 |
| eNO, ppb | 34.6 | 6.0 | 14.0 | 26.0 | 43.0 | 130.0 | 37.1 | 5.0 | 13.0 | 23.0 | 45.0 | 153.0 | 0.21 |
| Cytokines† | |||||||||||||
| IL-1, fg/ml | 9.3 | 0.45 | 4.0 | 5.0 | 10.4 | 63.3 | 10.0 | 0.7 | 4.1 | 6.5 | 12.0 | 56.4 | 0.52 |
| IL-6, pg/ml | 0.20 | 0.05 | 0.11 | 0.18 | 0.24 | 0.67 | 0.21 | 0.03 | 0.11 | 0.14 | 0.21 | 1.30 | 0.51 |
| CRP, μg/ml | 1.04 | 0.01 | 0.13 | 0.38 | 1.13 | 5.85 | 0.86 | 0.02 | 0.12 | 0.30 | 0.89 | 6.52 | 0.20 |
| TNF-alpha, pg/ml | 1.58 | 0.8 | 1.3 | 1.5 | 1.8 | 2.6 | 1.59 | 0.9 | 1.3 | 1.6 | 1.8 | 2.7 | 0.69 |
ACQ: Asthma Control Questionnaire; PAIS: PROMIS Asthma Impact Scale
fg: femtograms; pg: picograms; μg: micrograms
Based on paired t-tests; for right-skewed cytokine variables (IL-1, IL-6, CRP) paired t-tests were performed on differences of natural-logged variables.
Comparison of Daily Outcomes
Table 4 shows mean estimates for Short and Long sleep weeks from lung function longitudinal models averaged over days within treatment weeks, i.e., main effects for treatment. Although greater overnight drops occurred during the Short sleep week for both FEV1 and PEF compared to the Long sleep week, differences were not significant. Morning mean FEV1 was significantly lower in the Short sleep week than Long (2.66L vs. 2.73L, respectively; p=0.006). Both subjectively reported daily and nightly asthma symptoms were greater during the Short sleep week (p=0.002 and p=0.04, respectively), as was nighttime albuterol usage (p<0.0001).
Table 4.
Estimates for outcomes in Long and Short sleep weeks from longitudinal models. Values are composite estimates (i.e., main effects) for treatment, averaged over days, with 95% confidence intervals. [Peak Flow Meter variables are continuous, symptoms is binary, and albuterol use is a count; consequently estimates are odds for symptoms and means for other variables (see text for more detail on the methods).]
| Long Sleep | Short Sleep | L vs. S, p | |
|---|---|---|---|
| Peak Flow Meter | |||
| FEV1, mean overnight change, L | −0.04 (−0.09, 0.02) | −0.07 (−0.13, −0.01) | 0.31 |
| FEV1, morning mean, L | 2.73 (2.53, 2.94) | 2.66 (2.46, 2.87) | 0.006 |
| PEF, mean overnight change, L/min | −16.2 (−24.0, −8.3) | −20.1 (−28.3, −12.0) | 0.30 |
| PEF, morning mean, L/min | 400.2 (370.9, 429.6) | 397.1 (367.8, 426.5) | 0.40 |
| Odds of Symptoms | |||
| Daytime | 0.11 (0.06, 0.22) | 0.26 (0.14, 0.48) | 0.002 |
| Nighttime | 0.033 (0.017, 0.067) | 0.060 (0.031, 0.113) | 0.004 |
| Mean albuterol use | |||
| Daytime | 0.17 (0.10, 0.30) | 0.20 (0.11, 0.34) | 0.23 |
| Nighttime | 0.004 (0.002, 0.012) | 0.009 (0.004, 0.24) | <0.0001 |
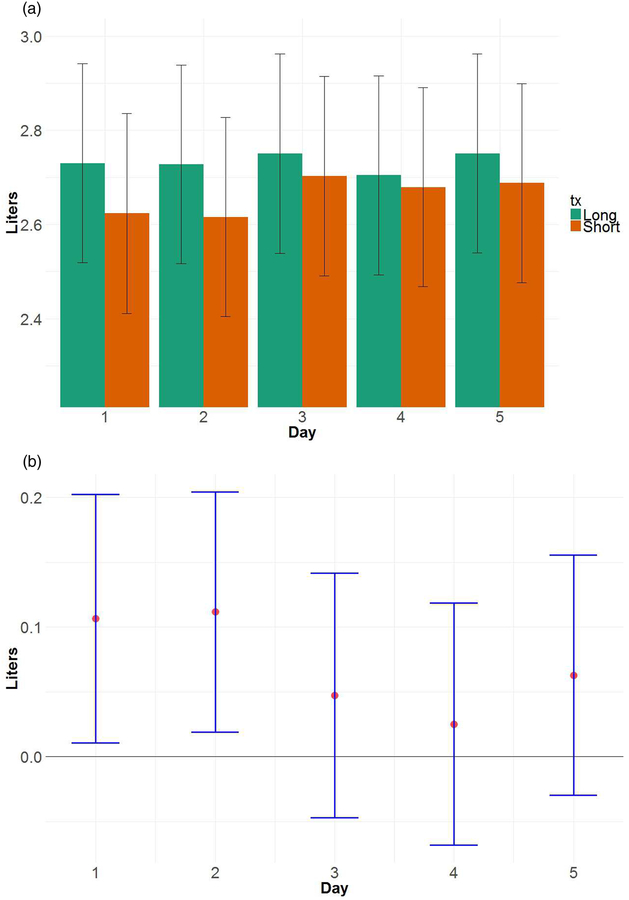
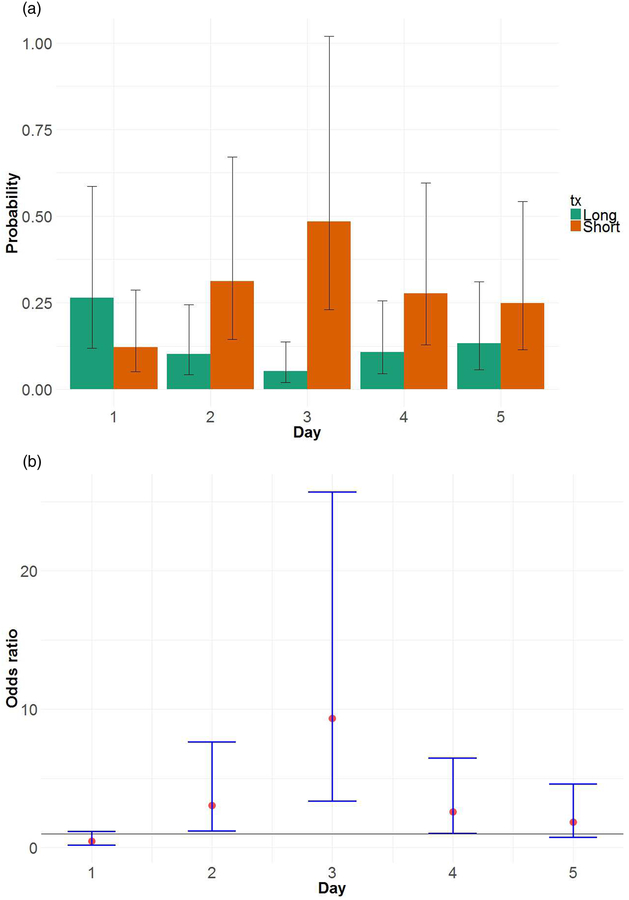
Figure 2 shows estimates of average FEV1 by day in Short and Long sleep weeks, which demonstrates the greatest differences between Short and Long sleep early in the week (mean=2.73 L for Long sleep on both Day 1 and 2; mean=2.62 L for Short sleep on both Day 1 and 2; p=0.03 and p=0.02 for differences on Day 1 and 2, respectively). For daytime asthma symptoms (Figure 3), the greatest difference in probability of symptoms between Short and Long sleep weeks occurred on Day 3 (probability=0.48 for Short, 0.05 for Long; p<0.0001 for difference); on Days 2 and 4 differences were marginally significant. As with lung function, differences between the Short and Long sleep weeks for daytime symptoms started to drop later in the week.
Figure 2:
(a) Mean morning FEV1 by treatment and day (bars), with 95% confidence intervals (error bars). Estimates were derived from longitudinal models discussed in the text. (b) Mean difference in morning FEV1, Long versus Short, by day, with 95% confidence intervals. Results show that the biggest mean differences occurred on Days 1 and 2.
Figure 3:
(a) Probability of daytime asthma symptoms by treatment and day (bars), with 95% confidence intervals (error bars). Estimates were derived from longitudinal models discussed in the text. (b) Estimated odds ratios for Short versus Long, by day, with 95% confidence intervals. Results show that the biggest mean difference occurred on Day 3.
Sensitivity and Per Protocol Analyses
To help assess adequacy of randomization, models for sleep duration and sleep efficiency were fit including predictors for randomization sequence (Short sleep first or Long sleep first), treatment*sequence, as well as subject height, weight, age, gender and race. As expected, the added predictors were not significant in these models and were thus dropped from further analyses.
Additional models were fit for subsets of subjects that did not meet protocol or with unusual sleep characteristics. Specifically, 3 subjects averaged greater than 7.5 hours of duration in bed during the Short sleep week (7.67, 7.76, and 8.01), and 6 subjects randomized to the Short sleep week first had relatively short sleep amounts in the weekend preceding the Short sleep week (range 6.13 to 7.20 hours). Models were fit without these subjects in two separate analyses, and results were similar to the main results reported, and thus not included here.
Observational models
Additional models were also fit that were more observational in nature, predicting average changes in outcomes based on increases or decreases in sleep amount, regardless of treatment. We found that an hour increase in sleep was associated with 24 mL average increase in FEV1 the following morning (p=0.008).
Discussion
This study is one of the first to examine the impact of sleep opportunity on daily and weekly asthma outcomes in a relatively large sample of adolescents. While previous studies have established an association between nocturnal asthma and sleep quality, few studies have used an experimental, prospective study design that is able to directly compare five nights of partial sleep restriction (6.5 hours) to five nights of a healthy sleep opportunity (9.5 hours). In sum, this study found that insufficient sleep has a negative impact on both objective and subjective daily symptoms of asthma, as well as a negative impact on health related quality of life (i.e., impact of asthma on daily functioning). However, no differences were found in weekly measures of lung function or inflammation. The following will review these different findings, and propose some areas for future study.
The findings from this study replicated our previously published pilot study.41 However, the current study had a much larger sample (n=54 vs n=10), utilized validated subjective measures of asthma severity and asthma quality of life, and explored the role of serum cytokines. The current study found lower average daily morning FEV1 during the Short sleep week (measured by home peak flow), suggesting that insufficient sleep resulted in poorer lung function. However, sleep opportunity did not significantly impact spirometry or exhaled nitric oxide. One possible explanation for this finding is that daily peak flow and spirometry are effort dependent measures. If sleepy, an adolescent may not give his or her best effort on the home peak flow meters, whereas in a clinical setting, there will be someone who is pushing the youth to full effort. However, these findings should not be discounted, as some asthma treatment decisions may be based on peak flow readings, which could potentially lead to unnecessary treatment.
Another possible explanation for average daily, but not weekly, differences is found in the observational results. Daily FEV1 was significantly worse on the first two days of the Short sleep week, but then began to improve, with smaller differences between the Short and Long sleep week found for the last three days. This suggests that insufficient sleep may cause an immediate negative response in lung function, with compensation or adjustment to the sleep deprivation occurring over the last three days. More research is needed to clarify this potential dose dependent response.
While objective symptoms of asthma increased over the first two days, it was notable that subjective asthma symptoms also began to increase on day two, with a very significant difference found on the third day. This parallel response in subjective symptoms to the changes in FEV1 provides further evidence for a dose-dependent response to sleep loss rather than simply poor effort following Short sleep. In addition, the impact of asthma on quality of life was greater in the Short week. As the PROMIS Asthma Impact Scale uses a 7-day recall period, this finding may be a reflection of the changes over the week of insufficient sleep.
Although several early studies in adults reported an association between deficient sleep and low-level systemic inflammation,42 and animal models have demonstrated that sleep loss may lead to non-eosinophilic/Th1 like airway inflammation,57;58 this study did not find any differences in systemic inflammation (serum cytokines), airway eosinophilic/Th2 (exhaled nitric oxide), or neutrophilic/Th1 inflammation (TNF-α). Our findings are more consistent with a systematic review (published after this study began) that found an association between inflammatory markers and both sleep disturbance and long sleep duration, but not short sleep duration.43 Other potential reasons for the lack of findings in the current study was the single weekly assessment of these outcomes, rather than serial assessments over a 24-hour period. In addition, although the weekly assessments were conducted at the same time for individual participants (e.g., 10:00 a.m. all three weeks), the samples were collected between 8:00 a.m. and 12:00 p.m. across participants. Finally, this study was powered to detect differences in peak flow variables, and may have been underpowered for the serum cytokines.
Although this study provides novel insights into the relationship between sleep duration and asthma in adolescents, there are several limitations that should be noted. First, overall the study sample had good asthma control, thus changes to asthma may not have been as noticeable as in a population with more variable asthma control. Second, we were unable to collect data during the school year due to ethical concerns about the known impact of deficient sleep on school performance; thus the study was conducted over the summer, when asthma control tends to be better. Additional research is needed to examine the impact of insufficient sleep on adolescents with asthma during the winter. Third, although results were consistent regardless of first sleep condition (Short vs. Long), it is possible that the two nights of recovery after the Short sleep week was not sufficient, attenuating differences between the two sleep weeks. Fourth, daytime activity could impact asthma, and as the study was conducted during the summer it is possible participants were engaging in a higher (or lower) than average level of activity. This is an important area for follow-up in future research. Fifth, although there were no significant differences in participants who completed the study and those who did not, the study sample may not be representative of all adolescents with asthma. Finally, as participants were not blinded to condition, their subjective reports and possibly even their peak flow effort may have been biased.
Despite these limitations, sleep duration is an important variable to consider in the management of asthma, especially as the majority of adolescents in the United States are chronically sleep deprived. It is thus important to optimize the sleep opportunity of adolescents with asthma and increase education for patients and families about the importance of sleep duration in health outcomes.
Supplementary Material
Highlights.
Daily lung function is worse when adolescents with asthma only have 6.5 h to sleep
A short sleep opportunity results in more reported daily asthma symptoms
Adolescents with asthma report poor health-related quality of life with short sleep
Acknowledgments
The authors thank the participants and their families for their time and effort; Ronina Covar, J. Tod Olin, and Gayle Spears for their assistance with medical evaluations; Joseph Ruybal for his assistance with recruitment and data collection; and Larissa Dixon for her review of the manuscript. This study was supported by funding from the National Institutes of Health (R01 HL119441), as well as the NIH/NCRR Colorado CTSI (UL1 RR025780).
Reference List
- 1.Akinbami LJ, Simon AE, and Rossen LM. Changing trends in asthma prevalence among children. Pediatrics 2016;137: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education - United States 2001–2009. Morb Mortal Wkly Rep Surveill Summ 2011;60:546–552. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, and Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 20111–14. [PubMed] [Google Scholar]
- 4.American Lung Association. Trends in asthma morbidity and mortality. American Lung Association, -26. 2012. [Google Scholar]
- 5.Moonie SA, Sterling DA, Figgs L, and Castro M. Asthma status and severity affects missed school days. J Sch Health 2006;76:18–24. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Huang IC, Thompson L, Tuli S, Huang SW, DeWalt D, Revicki D, and Shenkman E. The relationships between asthma control, daytime sleepiness, and quality of life among children with asthma: a path analysis. Sleep Med 2013;14:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer LJ, Ullrich M, and Szefler SJ. Sleep duration, sleep hygiene, and insomnia in adolescents with asthma. J Allergy Clin Immunol Pract 2014;2:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garden M, O’Callaghan M, Suresh S, Mamum AA, and Najman JM. Asthma and sleep disturbance in adolescents and young adults: A cohort study. J Paediatr Child Health 2016 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Thompson LA, Gross HE, Shenkman EA, Reeve BB, DeWalt DA, and Huang IC. Longitudinal associations among asthma control, sleep problems, and health-related quality of life in children with asthma: a report from the PROMIS((R)) Pediatric Asthma Study. Sleep Med 2016;20:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SR, Boergers J, Kopel SJ, McQuaid EL, Seifer R, LeBourgeois M, Klein RB, Esteban CA, Fritz GK, and Koinis-Mitchell D. Sleep hygiene and sleep outcomes in a sample of urban children with and without asthma. J Pediatr Psychol 2017;42:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RJ, Cicutto LC, Smith HR, Ballard RD, and Szefler SJ. Airways inflammation in nocturnal asthma. Am Rev Respir Dis 1991;143:351–357. [DOI] [PubMed] [Google Scholar]
- 12.Ballard RD, Saathoff MC, Patel DK, Kelly PL, and Martin RJ. Effect of sleep on nocturnal bronchoconstriction and ventilatory patterns in asthmatics. J Appl Physiol (1985) 1989;67:243–249. [DOI] [PubMed] [Google Scholar]
- 13.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, and White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol (1985) 1990;68:2034–2041. [DOI] [PubMed] [Google Scholar]
- 14.Ballard RD, Pak J, and White DP. Influence of posture and sustained loss of lung volume on pulmonary function in awake asthmatic subjects. Am Rev Respir Dis 1991;144:499–503. [DOI] [PubMed] [Google Scholar]
- 15.Ballard RD. Sleep, respiratory physiology, and nocturnal asthma. Chronobiol Int 1999;16:565–580. [DOI] [PubMed] [Google Scholar]
- 16.Atanasov ST and Calhoun WJ. The relationship between sleep and asthma. Sleep Medicine Clinics 20079–18. [Google Scholar]
- 17.Hetzel MR and Clark TJ. Does sleep cause nocturnal asthma? Thorax 1979;34:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall JR, Rhind GB, Stewart IC, Whyte KF, Shapiro CM, and Douglas NJ. Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax 1986;41:676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carskadon MA, Orav EJ, and Dement WC. Evolution of sleep and daytime sleepiness in adolescents. In: Guilleminault C and Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology, and long-term evolution New York; Raven Press; 1983, p. 201–216. [Google Scholar]
- 20.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am 2011;58:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Queen B, Lowry R, Chyen D, Whittle L, Thornton J, Lim C, Bradford D, Yamakawa Y, Leon M, Brener N, and Ethier KA. Youth Risk Behavior Surveillance - United States, 2017. MMWR Surveill Summ 2018;67:1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healthy People 2020. ODPHP Publication No. B0132. 2010. Department of Health and Human Services. [Google Scholar]
- 23.Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am 2011;58:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, and Pietro B. The pediatric daytime sleepiness scale (PDSS): Sleep habits and school outcomes in middle-school children. Sleep 2003;26:455–458. [PubMed] [Google Scholar]
- 25.Fallone G, Owens JA, and Deane J. Sleepiness in children and adolescents: Clinical implications. Sleep Med Rev 2002;6:287–306. [DOI] [PubMed] [Google Scholar]
- 26.Wolfson AR and Carskadon MA. Understanding adolescents’ sleep patterns and school performance: a critical appraisal. Sleep Med Rev 2003;7:491–506. [DOI] [PubMed] [Google Scholar]
- 27.Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, and Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep 2010;33:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfson AR and Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev 1998;69:875–887. [PubMed] [Google Scholar]
- 29.Fredriksen K, Rhodes J, Reddy R, and Way N. Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Dev 2004;75:84–95. [DOI] [PubMed] [Google Scholar]
- 30.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Ancoli-Israel S, and Redline S. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Pediatr Psychol 2009;34:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, and Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med 2011;53:271–273. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien EM and Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med 2005;3:113–133. [DOI] [PubMed] [Google Scholar]
- 33.Danner F and Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. J Clin Sleep Med 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- 34.Vorona RD, Szklo-Coxe M, Wu A, Dubik M, Zhao Y, and Ware JC. Dissimilar teen crash rates in two neighboring southeastern Virginia cities with different high school start times. J Clin Sleep Med 2011;7:145–151. [PMC free article] [PubMed] [Google Scholar]
- 35.Pizza F, Contardi S, Antognini AB, Zagoraiou M, Borrotti M, Mostacci B, Mondini S, and Cirignotta F. Sleep quality and motor vehicle crashes in adolescents. J Clin Sleep Med 2010;6:41–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Turner JB, Zammit GK, and Ginsberg HN. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep 2010;33:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaheri S, Storfer-Isser A, Rosen CL, and Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation 2008;118:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javaheri S, Storfer-Isser A, Rosen CL, and Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr 2011;158:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta NK, Mueller WH, Chan W, and Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol 2002;14:762–768. [DOI] [PubMed] [Google Scholar]
- 40.Lytle LA, Pasch KE, and Farbakhsh K. The relationship between sleep and weight in a sample of adolescents. Obesity (Silver Spring) 2011;19:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meltzer LJ, Faino A, Szefler SJ, Strand M, Gelfand EW, and Beebe DW. Experimentally manipulated sleep duration in adolescents with asthma: Feasibility and preliminary findings. Pediatr Pulmonol 2015;50:1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullington JM, Simpson NS, Meier-Ewert HK, and Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 2010;24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin MR, Olmstead R, and Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castro-Rodriguez JA, Brockmann PE, and Marcus CL. Relation between asthma and sleep disordered breathing in children: is the association causal? Paediatr Respir Rev 2017;22:72–75. [DOI] [PubMed] [Google Scholar]
- 45.Rogers VE, Bollinger ME, Tulapurkar ME, Zhu S, Hasday JD, Pereira KD, and Scharf SM. Inflammation and asthma control in children with comorbid obstructive sleep apnea. Pediatr Pulmonol 2018;53:1200–1207. [DOI] [PubMed] [Google Scholar]
- 46.Iber C, Ancoli-Israel S, Chesson AL Jr., Quan SF, and for the American Academy of Sleep Medicine. 2007. The AASM manual for the scoring of sleep and associated events American Academy of Sleep Medicine, Westchester, IL. [Google Scholar]
- 47.Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, Bista SR, Casey KR, Chowdhuri S, Kristo DA, and Ramar K. Practice parameters for the respiratory indications for polysomnography in children. Sleep 2011;34:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eaton DK, McNight-Eily LR, Lowry R, Perry GS, Presley-Cantrell L, and Crift JB. Prevalence of insufficient, borderline, and optimal hours of sleep among high school students - United States, 2007. J Adolesc Health 2010;46:399–401. [DOI] [PubMed] [Google Scholar]
- 49.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santanello NC, Davies G, Galant SP, Pedinoff A, Sveum R, Seltzer J, Seidenberg BC, and Knorr BA. Validation of an asthma symptom diary for interventional studies. Arch Dis Child 1999;80:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnan JA, Lemanske RF Jr., Canino GJ, Elward KS, Kattan M, Matsui EC, Mitchell H, Sutherland ER, and Minnicozzi M. Asthma outcomes: symptoms. J Allergy Clin Immunol 2012;129:S124–S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, and King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–907. [DOI] [PubMed] [Google Scholar]
- 53.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, Sheller J, Sorkness C, Stoloff S, and Gergen P. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol 2012;129:S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juniper EF, Bousquet J, Abetz L, and Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006;100:616–621. [DOI] [PubMed] [Google Scholar]
- 55.Yeatts KB, Stucky B, Thissen D, Irwin D, Varni JW, Dewitt EM, Lai JS, and DeWalt DA. Construction of the Pediatric Asthma Impact Scale (PAIS) for the Patient-Reported Outcomes Measurement Information System (PROMIS). J Asthma 2010;47:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.U.S.Census Bureau. Colorado population estimates 2017. 2017. 9-1-0018.
- 57.Nunes JOF, Apostolico JS, Andrade DAG, Ruiz FS, Fernandes ER, Andersen ML, Keller AC, and Rosa DS. Sleep deprivation predisposes allergic mice to neutrophilic lung inflammation. J Allergy Clin Immunol 2018;141:1018–1027. [DOI] [PubMed] [Google Scholar]
- 58.Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, Koya AK, Eldridge M, and Teodorescu M. Effects of chronic intermittent hypoxia on allergen-induced airway inflammation in rats. Am J Respir Cell Mol Biol 2015;52:162–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.