Abstract
Hepatic specification and functional maturation are tightly controlled throughout development. N6-methyladenosine (m6A) is the most abundant RNA modification of eukaryotic mRNAs and is involved in various physiological and pathological processes. However, the function of m6A in liver development remains elusive. Here we dissect the role of Mettl3-mediated m6A modification in postnatal liver development and homeostasis. Knocking out Mettl3 perinatally with Alb-Cre (Mettl3 cKO) induces apoptosis and steatosis of hepatocytes, results in severe liver injury, and finally leads to postnatal lethality within 7 weeks. m6A-RIP sequencing and RNA-sequencing reveal that mRNAs of a series of crucial liver-enriched transcription factors are modified by m6A, including Hnf4a, a master regulator for hepatic parenchymal formation. Deleting Mettl3 reduces m6A modification on Hnf4a, decreases its transcript stability in an Igf2bp1-dependent manner, and down-regulates Hnf4a expression, while overexpressing Hnf4a with AAV8 alleviates the liver injury and prolongs the lifespan of Mettl3 cKO mice. However, knocking out Mettl3 in adults using Alb-CreERT2 does not affect liver homeostasis. Our study identifies a dynamic role of Mettl3-mediated RNA m6A modification in liver development.
Subject terms: Development, Transcriptional regulatory elements, Hepatocytes
m6A is the most abundant RNA modification of eukaryotic mRNAs and is involved in various physiological and pathological processes. Here the authors show a role for Mettl3-mediated RNA m6A modification in postnatal liver development by regulating the Hnf4a-centered transcriptional network
Introduction
The liver is the primary organ responsible for metabolism, lipid transportation, drug detoxification, and hormone secretion1. Hepatic specification and the dramatic functional transition from haematopoiesis to metabolism of the liver are tightly controlled by the intricate crosstalk among extracellular signals, transcription factors, and epigenetic regulators. Previous reports showed that coordination of liver-enriched transcription factors, histone modifications, and DNA methylation orchestrates the hepatic differentiation program during development and maintains liver homeostasis in adults2,3.
N6-methyladenosine (m6A) modification has been identified as the most abundant modification in eukaryotic mRNAs since its discovery in the 1970s4–6. Dynamic deposition and removal of m6A is catalyzed by the methyltransferase complex containing methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14)7,8, and Wilms tumor 1-associating protein (WTAP)9, along with other co-factors and demethylases, including ALKB homolog 5 (ALKBH5)10 and fat mass and obesity-associated protein (FTO)11, respectively. m6A modification is recognized by YTH family members which embrace the domain of YT521-B homology (YTHDF1-3, YTHDC1-2)12–16 and insulin-like growth factor-2 mRNA-binding proteins (IGF2BP1/2/3)17, and are thus involved in various steps of RNA metabolism, such as stability, translation, nuclear exportation, splicing of mRNAs, and biogenesis and maturation of miRNAs18.
In this work, we generate hepatic-specific Mettl3 knockout (Mettl3 cKO) mice by crossing Mettl3flox/flox mice with Albumin (Alb)-enhancer/promoter driven-Cre transgenic mice to investigate the role of m6A modification in liver development. Hepatic perinatal loss of Mettl3 causes severe liver damage, including steatosis, apoptosis, and fibrosis, and finally results in lethality within 7 weeks. Using m6A-RNA immunoprecipitation (m6A-RIP) sequencing and RNA-sequencing, we identify that crucial liver-enriched transcription factors, including Hnf4a, are modified by m6A in liver development. Loss of Mettl3 induces depletion of m6A on Hnf4a transcripts, decreases its transcript stability in an Igf2bp1-dependent manner, and down-regulates Hnf4a expression, while overexpressing Hnf4a with AAV8 alleviates the liver injury and prolongs lifespan of Mettl3 cKO mice. However, deletion of Mettl3 in adult mouse livers using Albumin-enhancer/promoter-driven CreERT2 shows minimal effects on liver homeostasis. In conclusion, we elucidate a dynamic role of Mettl3-mediated RNA m6A modification during mouse postnatal liver development and decipher a novel function of epitranscriptomic control of liver organogenesis.
Results
Generation of hepatic specific Mettl3 knockout mice
To study the role of m6A modification in liver development, we first tested the expression level of critical subunits of the m6A methyltransferase complex, Mettl3 and Mettl147. Both components showed shallow protein levels in mouse neonates (within one day after birth), increased gradually, and peaked at 2-3 weeks, and then decreased from 4 weeks onwards (Supplementary Fig. 1a). A similar trend was observed in human livers with high expression in children and a subsequent decline with age (Supplementary Fig. 1b). These results indicated that m6A is dynamically regulated in postnatal liver development. Global knockout of either Mettl3 or Mettl14 results in embryonic lethality caused by gastrulation defects19–21. Thus, to study the role of m6A modification, we generated mice with hepatic specific knockout of the catalytic subunit of the m6A methyltransferase complex, Mettl3, by crossing Mettl3flox/flox mice (with loxP sites flanking exons 2 and 4) with Alb-enhancer/promoter-driven Cre transgenic mice (Supplementary Fig. 1c, d). The specific knockout of Mettl3 in the liver was confirmed by genomic PCR, quantitative real-time PCR (RT-qPCR), western blot, and immunochemistry (Fig. 1a-d and Supplementary Fig. 1e-j). Genomic PCR and RT-qPCR showed that efficient knockout of Mettl3 started from day 1 after birth (Fig. 1b and Supplementary Fig. 1k), along with Cre expression (Supplementary Fig. 1i). As expected, livers from Mettl3 cKO mice showed a significant decrease in mRNA m6A levels compared to control mice (Supplementary Fig. 1l). In addition, we also observed that knocking out Mettl3 led to disruption of Mettl14 (Supplementary Fig. 1m), which is in accordance with previous reports19.
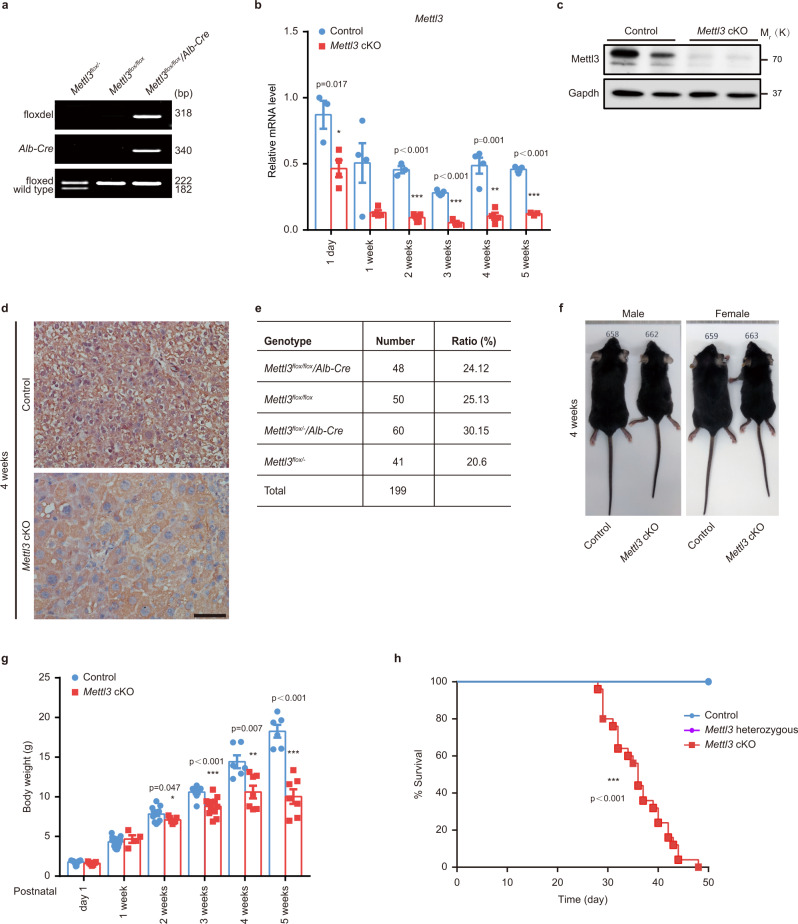
Fig. 1. Hepatic Mettl3 knockout in mice results in postnatal lethality.
a Genomic PCR characterization for tail and liver tissues (the top lane for liver tissues and the rest for tails) from 2 weeks old mouse of the indicated genotype. The top lane (floxdel) showed the exon 2–4 deleted alleles (amplified using F1 and R2 primer shown in (Supplementary Fig. 1c)) that could be detected only in Mettl3flox/flox/Alb-Cre (Mettl3 cKO) mouse livers. The middle lane (Alb-Cre) showed the effective insertion of Albumin enhancer/promoter-driven Cre into the “genomic safe harbor” Hipp11 (H11) locus. The bottom lane displayed genotyping of heterozygous (Mettl3flox/-) or homozygous (Mettl3flox/flox) flox flanking alleles (amplified by F1 and R1 primer shown in (Supplementary Fig. 1c)) (3 experiments were repeated independently with similar results). b Quantitation of Mettl3 mRNA expression in livers of wild-type (WT) control (Control, also hereafter in similar experiments) and Mettl3 cKO mice at different time points postnatally via RT-qPCR (n = 3 for 1 day Control and 3 weeks cKO group; n = 5 for 3 weeks Control group; n = 4 for other groups). c Western blot for Mettl3 in Control and Mettl3 cKO mouse liver tissues at two weeks after birth (6 experiments were repeated independently with similar results). Gapdh was used as a loading control (also hereafter in similar experiments). d Immunohistochemistry staining of Mettl3 in 4 weeks old Control and Mettl3 cKO mouse livers (6 experiments were repeated independently with similar results). Scale bar = 50 μm. e The number of offspring with different genotypes from intercrossing Mettl3flox/flox and Mettl3flox/-/Alb-Cre mice. f Representative appearance of sex-matched Control mice and Mettl3 cKO littermates at 4 weeks after birth. g Body weight of male Control and Mettl3 cKO littermates at different time points after birth (n = 4 for 1 week cKO group; n = 6 for 4 weeks and 5 weeks Control groups; n = 9 for 3 weeks Control group; n = 12 for 3 weeks cKO group; n = 13 for 2 weeks Control group; n = 15 for day 1 and 1 week Control group; n = 7 for other groups). h Survival curves of Control, Mettl3 cKO, and Mettl3 heterozygous (Mettl3flox/-/Alb-Cre) littermates (n = 25 for each group). Data in b and g were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001; two-tailed student’s t-test). Data in (h) were analyzed by Log-rank (Mantel-Cox) test with the indicated significance (***P < 0.001). Source data are provided as a Source Data file.
Hepatic Mettl3 knockout results in postnatal lethality
Hepatic Mettl3 knockout mice were born at almost expected Mendelian frequencies (Fig. 1e), excluding the possibility of prenatal lethality. However, both male and female knockout mice were smaller in body size than their age and sex-matched wild-type (WT) control (Control, also hereafter in similar experiments) littermates (Fig. 1f). This difference appeared at 2 weeks after birth and gradually became more pronounced at 4 and 5 weeks (Fig. 1g and Supplementary Fig. 1n, o). Moreover, all the Mettl3 cKO mice died within 7 weeks after birth, while heterozygous knockout individuals were fertile and survived for over 12 months without discernible defects in development (Fig. 1h), indicating that one allele of Mettl3 was sufficient to maintain normal development and function of mouse livers. These results demonstrate that Mettl3 is critical for postnatal liver development, especially during the highly proliferative stages from 0 to 4 weeks after birth.
Hepatic Mettl3 deletion causes liver injury
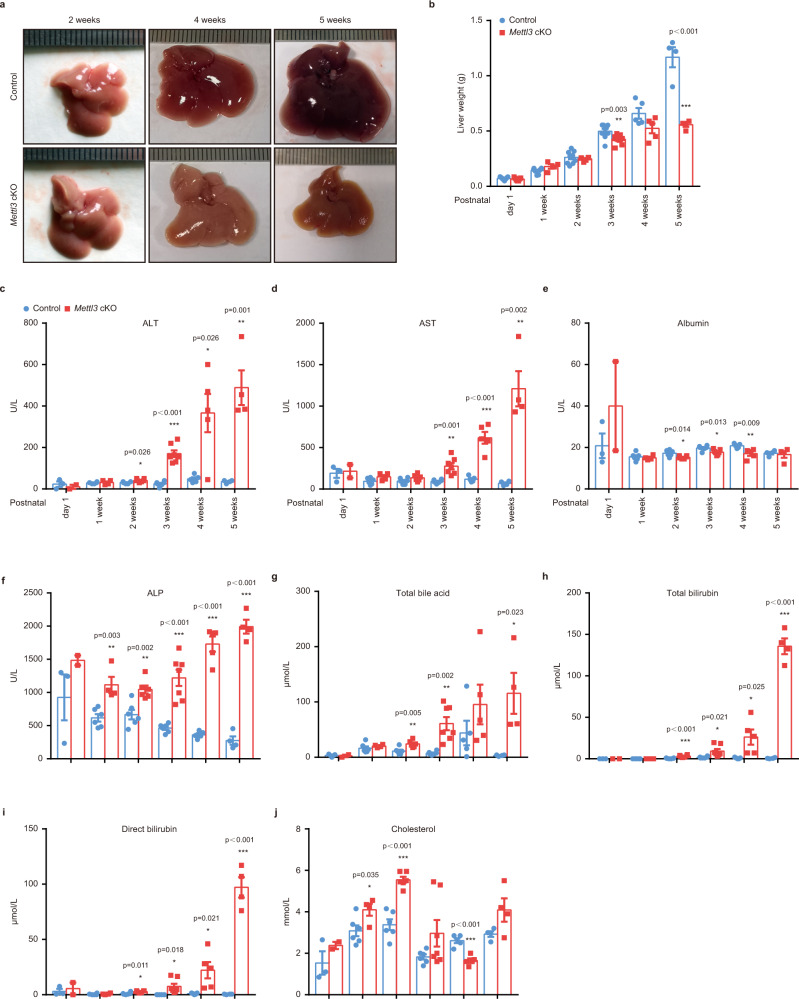
To delineate the exact role of Mettl3 in liver organogenesis, we dissected the livers of Control and Mettl3 cKO mice at different time points after birth. Grossly, an obvious mottled appearance, which is an indication of lipid deposition, was observed in the livers of Mettl3 cKO mice 2 weeks after birth (Fig. 2a). The livers of 4-week-old Mettl3 cKO mice were yellow, smaller, and stiffer than the WT Control. These differences became more significant at 5 weeks (Fig. 2a). The liver weight of Mettl3 cKO mice decreased since 3 weeks after birth (Fig. 2b), while the liver weight to body weight ratio was slightly increased (Supplementary Fig. 2a). Serum indicators of liver function showed that Mettl3 deficiency caused defects in metabolism, detoxification, protein synthesis, and secretion functions in the liver from 1-2 weeks after birth, indicating progressive liver damage (Fig. 2c-j and Supplementary Fig. 2b).
Fig. 2. Hepatic Mettl3 knockout in mice causes liver injury.
a Representative gross appearance of livers from Control and Mettl3 cKO mice at 2 weeks, 4 weeks, and 5 weeks postnatally. b Liver weight at different time points postnatally (n = 4 for 1 week cKO group and 5 weeks groups; n = 5 for 4 weeks groups; n = 6 for 2 weeks cKO group; n = 7 for day 1 cKO group; n = 8 for 1 week Control group; n = 9 for 3 weeks Control group; n = 10 for 2 weeks Control group; n = 12 for 3 weeks cKO group; n = 15 for day 1 Control group). c–j Serum levels of ALT c, AST d, Albumin e, ALP f, Total bile acid g, Total bilirubin h, Direct bilirubin i, and Cholesterol j of Control and Mettl3 cKO mice at different time points postnatally (n = 2 for day 1 cKO group; n = 3 for day 1 Control group; n = 4 for 1 week cKO and 5 weeks groups; n = 5 for 4 weeks groups; n = 6 for 1 week Control group, 2 weeks groups, and 3 weeks Control group; n = 7 for 3 weeks cKO group). Data in b–j were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001, two-tailed student’s t-test). Source data are provided as a Source Data file.
Mettl3 deletion results in apoptosis, steatosis, fibrosis, and activation of hepatic progenitors
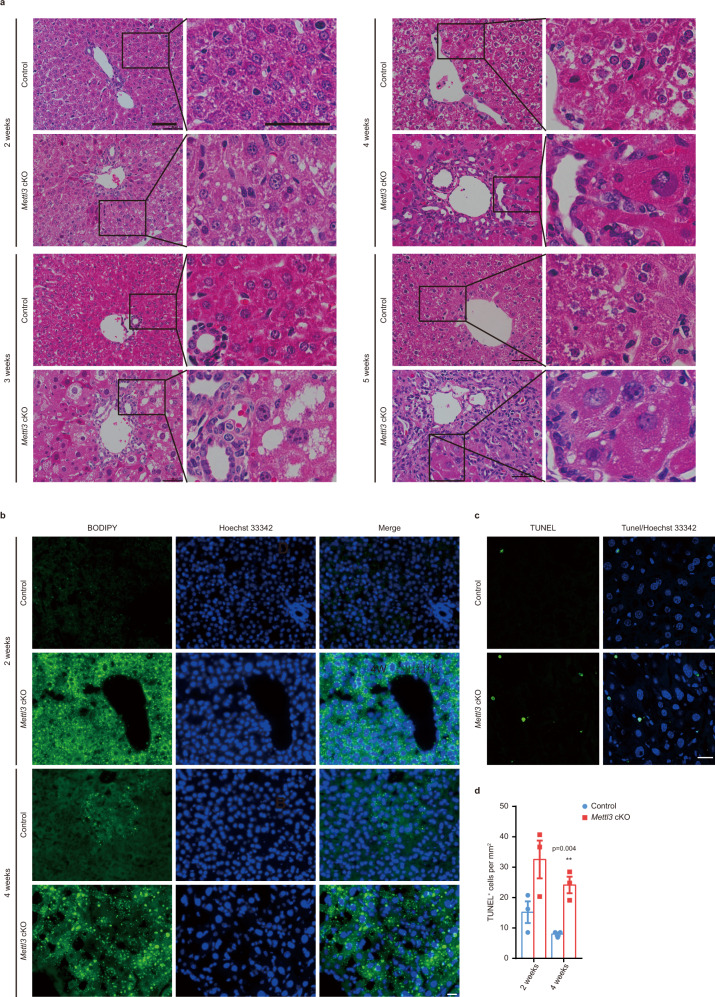
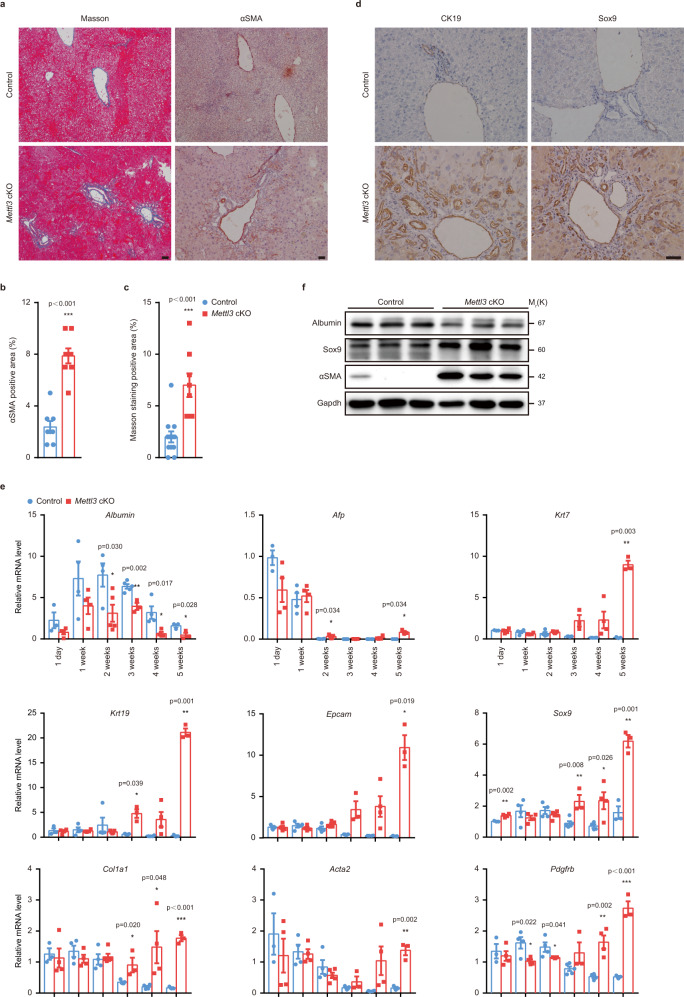
Although histologic analysis showed no apparent differences between the Control and Mettl3 cKO mice at 1 day and 1 week after birth (Supplementary Fig. 3a, b), marked pathological lesions were observed in Mettl3 cKO mice since 2 weeks old (Fig. 3a). Increased lipid droplet deposition in Mettl3 cKO livers was observed 2 weeks after birth, confirmed by BODIPY staining and Oil Red O staining for both frozen liver tissues (Fig. 3b and Supplementary Fig. 3c, d) and primary hepatocytes (Supplementary Fig. 3e). We also observed enlarged cell size, enlarged nucleus, and increased apoptosis of hepatocytes in Mettl3 cKO mice starting from 2 weeks and expansion of ductular cells at 3 weeks after birth (Fig. 3a-d and Supplementary Fig. 3f, g). In addition, prominent fibrosis in Mettl3 cKO livers was seen at 4 weeks and became more pronounced at 5 weeks (Fig. 4a-c and Supplementary Fig. 4a, b). We did not observe any abnormalities of heterozygous cKO livers by histological analysis (Supplementary Fig. 4c). Consistent with the expansion of ductular cells, we detected marked increases of Sox9, CK19, and Ki67 positive cells in Mettl3 cKO livers (Fig. 4d and Supplementary Fig. 4d). Meanwhile, RT-qPCR of liver tissues collected at different time points showed that hepatocyte markers (Albumin (Alb)) decreased, while hepatic progenitor markers (Afp, Krt7, Krt19, Epcam, and Sox9) and fibrosis markers (Col1a1, Acta2, and Pdgfrb) increased in Mettl3 cKO individuals (Fig. 4e). These changes were confirmed by western blot (Fig. 4f). The above results demonstrate that Mettl3 deletion in hepatocytes perinatally leads to hepatocyte injury, activation of progenitor cells, and fibrosis.
Fig. 3. Hepatic deletion of Mettl3 in mice induces steatosis and apoptosis.
a Representative H&E staining photographs of liver sections from Control and Mettl3 cKO mice at indicated time points postnatally (10 experiments were repeated independently with similar results). Scale bar = 50 μm. b Representative BODIPY staining fluorescent photographs of frozen liver sections from Control and Mettl3 cKO mice at 2 weeks and 4 weeks after birth (6 experiments were repeated independently with similar results). Cell nuclei were counterstained with Hoechst 33342 (also hereafter in similar experiments). Scale bar = 20 μm. c Representative TUNEL staining fluorescent photographs for frozen liver sections from Control and Mettl3 cKO mice 4 weeks after birth (6 experiments were repeated independently with similar results). Scale bar = 20 μm. d Quantification of TUNEL+ cells/Hoechst 33342+ cells ratio in liver sections from Control and Mettl3 cKO mice at 2 weeks and 4 weeks after birth (n = 3 for each group). Data in d were shown as mean ± SEM with the indicated significance (**P < 0.01; two-tailed student’s t-test). Source data are provided as a Source Data file.
Fig. 4. Hepatic deletion of Mettl3 in mice induces fibrosis and activation of hepatic progenitors.
a Representative Masson’s trichrome staining and αSMA immunohistochemistry staining photographs of liver sections from Control and Mettl3 cKO mice at 4 weeks after birth (10 experiments were repeated independently with similar results). Scale bar = 50 μm. b Quantification of the αSMA positive area (n = 8 for each group). c Quantification of the Masson’s trichrome staining positive area (n = 8 for cKO group; n = 12 for Control group). d CK19 and Sox9 immunohistochemistry staining of liver sections from Control and Mettl3 cKO mice at 4 weeks after birth (6 experiments were repeated independently with similar results). Scale bar = 50 μm. e RT-qPCR analysis of hepatocyte markers, hepatic progenitor markers, and fibrosis markers for liver tissues from Control and Mettl3 cKO mice at different time points postnatally (n = 3 for 1 day Control group, 3 weeks cKO group, and 5 weeks groups; n = 5 for 3 weeks Control group; n = 4 for other groups). f Western blot for Albumin, Sox9, and αSMA of Control and Mettl3 cKO mouse liver tissues at 4 weeks after birth (3 experiments were repeated independently with similar results). Data in b, c, and e were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001; two-tailed student’s t-test). Source data are provided as a Source Data file.
Transcriptome-wide m6A-RIP sequencing to identify potential targets of Mettl3
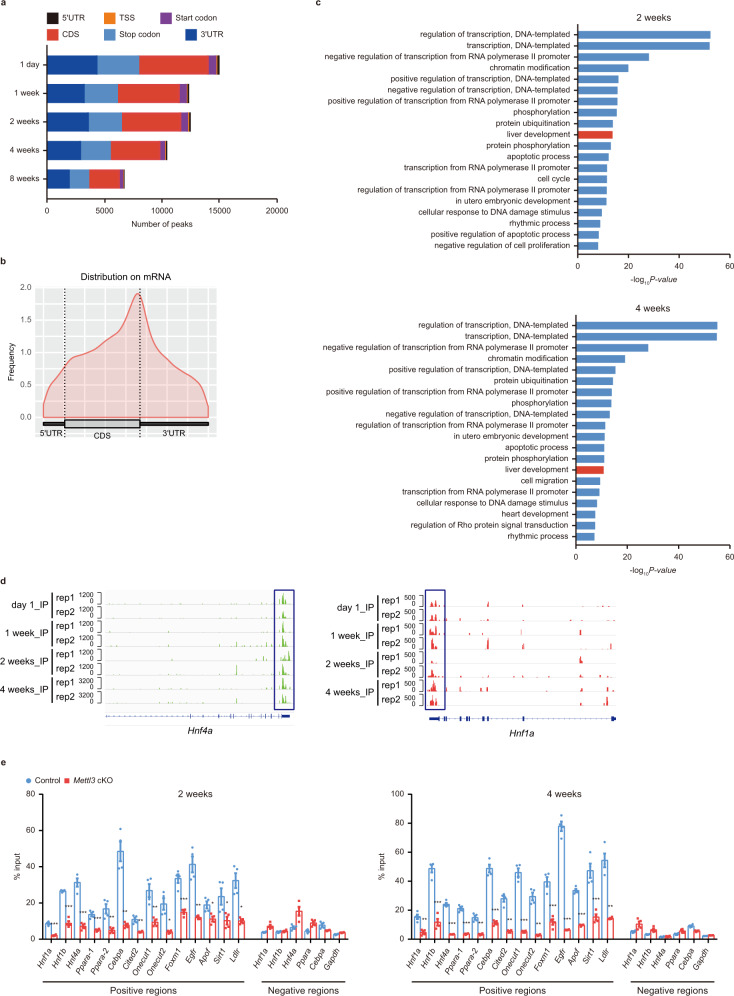
As Mettl3 is the key catalytic subunit of the m6A methyltransferase machinery, to gain a comprehensive insight into the molecular mechanisms underlying Mettl3 regulating postnatal liver development, we first quantified the m6A levels on mRNAs of the liver tissues from different developmental stages using LC-MS/MS. Global mRNA m6A levels of postnatal livers increased after birth, peaked at 2 weeks, and decreased then (Supplementary Fig. 5a). Next, we profiled the genome-wide m6A methylation distribution using m6A-RIP sequencing of RNAs from five developmental time points (1 day and 1, 2, 4, and 8 weeks after birth) of mouse liver tissues. The distribution of m6A modifications was dynamically regulated during different stages of postnatal liver development (Fig. 5a and Supplementary Fig. 5a-d). We identified 15139, 12483, 12615, 10561, and 6806 m6A peaks, corresponding to 6330, 5522, 5515, 4856, and 3445 genes from the above five groups, respectively (Supplementary Fig. 5b and Supplementary Dataset 1). Global m6A peak enrichment peaked 2 weeks after birth, along with patterns of bulk m6A levels during mouse liver development (Supplementary Fig. 5a, d). In line with previous reports, m6A peaks were significantly enriched in the vicinity of the stop codon (Fig. 5a, b), and the consensus motif “GGAC” was most commonly enriched in peaks from all samples (Supplementary Fig. 5e). Interestingly, “liver development” was one of the most significantly enriched terms of m6A modified genes by Gene Ontology (GO) analysis at all time points (Fig. 5c and Supplementary Dataset 2). mRNAs of several key liver-enriched transcription factors were highly methylated by m6A in liver tissues, including Hnf4a, Hnf1a, Ppara, and Cebpa (Fig. 5d and Supplementary Fig. 5f). These factors play essential roles in both liver development in vivo and hepatocyte differentiation in vitro2. Next, we used gene-specific m6A-RIP-qPCR assays to confirm the authentic deposition of RNA m6A modifications by Mettl3 on liver-enriched factors using liver tissues from Control and Mettl3 cKO mice. We observed a significant decrease of m6A deposition on Hnf1a, Hnf4a, Ppara, Cebpa et al. in Mettl3 cKO mice at both 2 weeks and 4 weeks (Fig. 5e). These results indicate that Mettl3-mediated m6A modification is dynamically regulated during liver development and modifies crucial transcription factors controlling liver specification and function.
Fig. 5. Mettl3-mediated m6A modification regulates postnatal liver development dynamically.
a Summary of peak numbers and the distribution of m6A peaks in the 5′UTR, TSS, start codon, CDS, stop codon, and 3′UTR regions across the entire set of mRNA transcripts. b Metagene distribution of m6A-immunoprecipitated reads across the length of mRNA transcript of livers from mice at postnatal 2 weeks. c Gene ontology (GO) analysis of m6A modified genes in mouse liver tissues at 2 and 4 weeks after birth identified by m6A-RIP sequencing. d Genome Browser screenshots of m6A-RIP sequencing read density signals under 4 different time points after birth at Hnf4a and Hnf1a loci. Two replicates were contained at each time point. e Gene-specific m6A-RIP-qPCR for Control and Mettl3 cKO mouse liver tissues at 2 and 4 weeks after birth (n = 4 for each group over 3 independent experiments). m6A positive and negative regions were selected based on m6A-RIP sequencing. Genes were marked by serial number if two or more m6A positive regions were found in an individual gene (e.g., Ppara−1 and Ppara−2 meant two separate m6A positive regions in Ppara mRNA). Exact P-value for each gene between Control and cKO in 2 weeks positive regions were listed here: Hnf1a (<0.001), Hnf1b (<0.001), Hnf4a (<0.001), Ppara-1 (<0.001), Ppara-2 (0.005), Cebpa (0.004), Cited2 (0.009), Onecut1 (0.004), Onecut2 (0.016), Foxm1 (<0.001), Egfr (0.007), Apof (0.018), Sirt1 (0.040), Ldlr (0.011). Exact P-value for each gene between Control and cKO in 4 weeks positive regions were listed here: Hnf1a (0.001), Hnf1b (<0.001), Hnf4a (<0.001), Ppara-1 (<0.001), Ppara-2 (0.003), Cebpa (<0.001), Cited2 (0.001), Onecut1 (<0.001), Onecut2 (0.002), Foxm1 (<0.001), Egfr (<0.001), Apof (<0.001), Sirt1 (<0.001), Ldlr (0.003). Data in (e) were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001; two-tailed student’s t-test). Source data are provided as a Source Data file.
m6A regulates pathways of liver development and metabolism by controlling mRNA stability of the core transcription factor Hnf4a
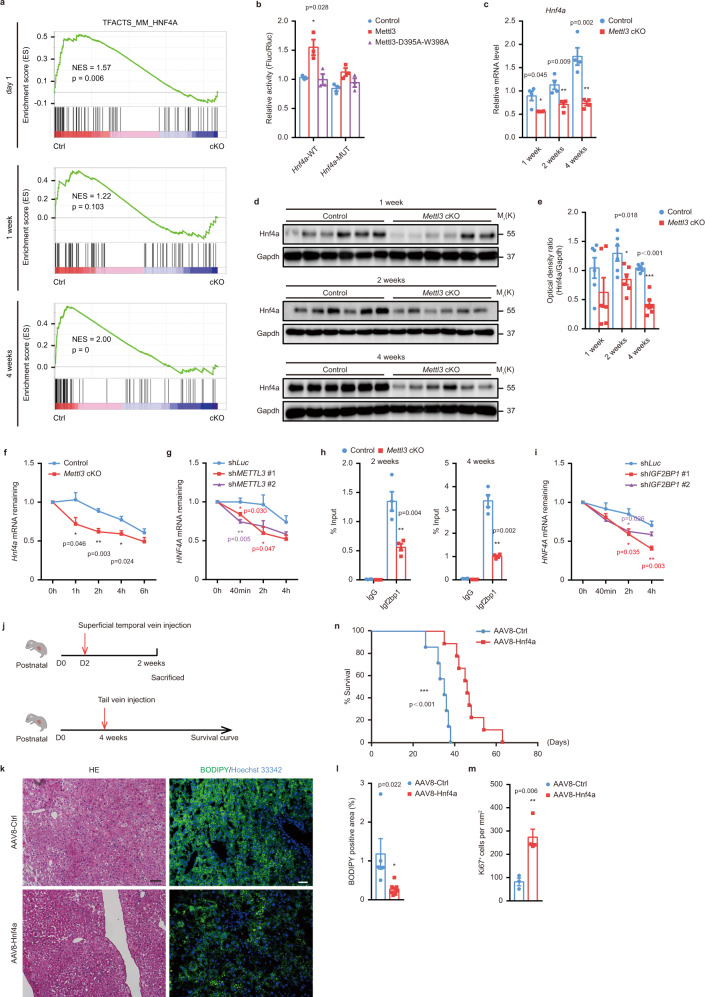
To gain further insights into the mechanism of Mettl3 regulating liver development, we conducted RNA-sequencing for liver tissues from Control and Mettl3 cKO mice at 1 day, 1 week, 2 weeks, and 4 weeks after birth. There were much more differentially regulated genes (DEGs) between Control and Mettl3 cKO mice at later time points (Supplementary Fig. 6a, Supplementary Dataset 3), which is consistent with our observations that Mettl3 cKO mice showed progressive severe liver damage 2 weeks after birth onward (Fig. 3a). Gene set enrichment analysis (GSEA) showed that targets of Hnf4a and Hnf1a were significantly repressed in Mettl3 cKO livers even at 1 day postnatally (Fig. 6a and Supplementary Fig. 6b). Dual-luciferase reporter assay and mutagenesis assay (Fig. 6b and Supplementary Fig. 6c, d) showed that co-transfection with WT, but not catalytic mutant Mettl322–24, significantly promoted luciferase activity in reporters carrying WT Hnf4a and Hnf1a fragments, while such increases were abolished when the m6A consensus motifs were mutated, confirming that the regulation of Hnf4a and Hnf1a by Mettl3 was indeed relying on m6A methylation of their transcripts. Both RT-qPCR and western blot confirmed that Hnf4a was downregulated in Mettl3 cKO livers at different time points postnatally (Fig. 6c-e). Although the RNA level of Hnf1a was downregulated at all time points (Supplementary Fig. 6e), we observed a dramatic decrease of Hnf1a protein with age and only observed a difference between Control and Mettl3 cKO mouse livers 1 week after birth (Supplementary Fig. 6f), indicating a less essential role of Hnf1a in the maturation of hepatocytes, which is consistent with previous studies25. Since Hnf4a is a master transcription factor required for liver development in both foetuses and adults and controls most aspects of mature hepatocyte function26,27, we mainly focused on Hnf4a for further studies. RNA-sequencing data showed that along with the downregulation of Hnf4a, most Hnf4a target genes, such as Apoa2, Apoc3, Cyp8b1, and Mttp, were repressed in Mettl3 cKO individuals (Supplementary Fig. 6g, Supplementary Dataset 3), which was validated by RT-qPCR (Supplementary Fig. 6h). We also noticed that Smad signaling, the central mediator of fibrosis28, was significantly enriched in Mettl3 cKO mouse liver tissues at 4 weeks (Supplementary Fig. 7a), supporting the phenomenon that massive liver fibrosis was induced in Mettl3 cKO animals (Fig. 4). These results indicate that Mettl3-mediated m6A controls the expression of crucial liver developmental genes during liver development.
Fig. 6. Mettl3 modulates the Hnf4a-centered regulatory network by controlling Hnf4a mRNA stability.
a Gene set enrichment analysis (GSEA) for Hnf4a pathways in RNA-sequencing data of Control (Ctrl) and Mettl3 cKO (cKO) mouse liver tissues at 1 day, 1 week, and 4 weeks after birth. b Dual-luciferase reporter assays showing the effects of Mettl3 on Hnf4a reporters with either WT or mutated m6A-modified sites (n = 3 for each group over 3 independent experiments). c RT-qPCR for mRNA levels of Hnf4a at different time points in Control and Mettl3 cKO mouse liver tissues (n = 4 for each group over 3 independent experiments). d Western blot for Hnf4α from Control and Mettl3 cKO mouse liver tissues at 1 week, 2 weeks, and 4 weeks after birth. e Densitometry analysis of western blot for Hnf4a from Control and Mettl3 cKO mouse liver tissues at indicated time points (n = 6 for each group). f RT-qPCR analysis of Hnf4a mRNA levels in primary hepatocytes isolated from Control and Mettl3 cKO mouse liver at different time points after 5 μM actinomycin D treatment (n = 4 for each group over 3 independent experiments). g RT-qPCR analysis of HNF4A mRNA levels in METTL3 knockdown HepG2 cells at different time points after 5 μM actinomycin D treatment (n = 4 for each group over 3 independent experiments). h RIP-qPCR detecting the binding of Igf2bp1 to Hnf4a in Control and Mettl3 cKO mouse liver tissues at 2 and 4 weeks after birth (n = 4 for each group). i RT-qPCR analysis of HNF4A mRNA levels in IGF2BP1 knockdown HepG2 cells at different time points after 5 μM actinomycin D treatment (n = 4 for each group). j Schematic diagram showing two rescue strategies with AAV8-Hnf4a. k Representative H&E staining and BODIPY staining photographs of 2-weeks-old liver sections from Mettl3 cKO mice intravenous injected with AAV8-Ctrl and AAV8-Hnf4a at day two after birth (6 experiments were repeated independently with similar results). Scale bar = 20 μm. l Statistical histogram of BODIPY staining (n = 5 for AAV8-Ctrl group; n = 7 for AAV8-Hnf4a group). m Statistical histogram of Ki67 immunohistochemical staining (n = 3 for AAV8-Ctrl group; n = 4 for AAV8-Hnf4a group). n Survival curves of Mettl3 cKO mice intravenous injected with AAV8-Ctrl and AAV8-Hnf4a (n = 8 for AAV8-Ctrl group; n = 9 for AAV8-Hnf4a group). Data in b, c, e–i, and l–m were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001; two-tailed student’s t-test). Data in n were analyzed by Log-rank (Mantel-Cox) test with the indicated significance (***P < 0.001). Source data are provided as a Source Data file.
m6A modification is involved in various aspects of RNA metabolism, including transcription, splicing, nuclear transportation, stability, and translation. Because we observed decreased expression of Hnf4a at both mRNA and protein levels, we determined the alternative splicing, nucleus-cytoplasm transportation, and mRNA stability of Hnf4a mRNA. Alternative splicing analysis showed no differences on Hnf4a transcripts in RNA-sequencing data from Control and Mettl3 cKO livers (Supplementary Dataset 4). The distribution of Hnf4a mRNA in nuclear and cytoplasm was also not affected by Mettl3 knockout (Supplementary Fig. 7b-d). Only mRNA stability showed significant changes in primary hepatocytes and the HepG2 cells with Mettl3 inhibition (Fig. 6f, g, and Supplementary Fig. 7e, f). Cells with Mettl3 deletion showed a shorter half-life of Hnf4a transcript, suggesting that Mettl3-mediated m6A controls the expression of Hnf4a at least partly by regulating its mRNA stability. To compare the global changes of mRNA stability after Mettl3 knockout, we subjected actinomycin D-treated hepatocytes from Control and Mettl3 cKO mice for RNA-sequencing (Supplementary Dataset 5). Consistent with previous reports15,19, knockout of Mettl3 enhanced mRNA stability globally, especially for m6A-modified genes (Supplementary Fig. 7g, h). Among genes involved in liver development (defined by Gene Ontology Resource, GO:0001889), only Hnf4a and another 10 genes showed decreased mRNA half-life when Mettl3 was knocked out, while most genes (including Cited2, Cebpa, Notch2, Dbp, et al.) were more stable or unchanged (Supplementary Fig. 7i and Supplementary Dataset 5). These results demonstrated that Mettl3 deficiency downregulated Hnf4a expression by reducing the half-life of Hnf4a mRNA.
m6A modification controls RNA fate mainly through “reader” proteins recognizing and binding to m6A-containing transcripts. Among identified m6A “readers”, insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs, including IGF2BP1/2/3) are known to promote the stability of their target mRNAs17. To further delineate the mechanism of m6A controlling Hnf4a expression, we checked previous publications and found that deletion of IGF2BP1 leads to destabilization of HNF4A mRNA in HepG2 cells while interfering with the other two members did not affect HNF4A mRNA degradation (Supplementary Fig. 7j)17, indicating that IGF2BP1 may directly recognize m6A on HNF4A mRNA and maintain its levels in the liver context. Thus, we tested Igf2bp1 binding on Hnf4a mRNA with RIP experiments. The results showed that Igf2bp1 could efficiently bind to Hnf4a transcripts in mouse livers of both 2 weeks and 4 weeks, and the enrichment significantly decreased after Mettl3 knockout (Fig. 6h). Accordingly, knocking down IGF2BP1 with small Hairpin RNA (shRNA) in HepG2 cells also decreased HNF4A mRNA half-life, similar to Mettl3 disruption (Fig. 6i and Supplementary Fig. 7k, l). These data demonstrated that Mettl3-mediated m6A controls the expression of Hnf4a by regulating its mRNA stability in an IGF2BP1-dependent manner.
Hepatic Hnf4a overexpression alleviated liver injury caused by Mettl3 knockout
To further strengthen our conclusion that Hnf4a is the primary mediator of Mettl3 function in liver development, we conducted rescue experiments using AAV serotype 8 (AAV8) to express Hnf4a under the control of a liver-specific promoter (thyroxine-binding globulin, TBG) (AAV8-TBG-Hnf4a) on Mettl3 cKO mice (Fig. 6j). Injection of AAV8-TBG-Hnf4a by superficial temporal vein on day two after birth successfully overexpressed Hnf4a in the liver (Supplementary Fig. 7m) and alleviated liver damage caused by hepatic Mettl3 knockout compared to AAV8-Ctrl at two weeks, evidenced by an increased number of Ki67+ proliferating hepatocytes and reduced hepatic steatosis (Fig. 6k-m). However, we did not see long-term benefits on mortality. This may attribute to the rapid dilution of AAV caused by the vigorous hepatocyte division within four weeks after birth29. Then we overexpressed Hnf4a by AAV-TBG-Hnf4a through tail vein injection at four-week-old Mettl3 cKO mice and found that Hnf4a overexpression significantly prolonged the life span of Mettl3 cKO mice (Fig. 6n). These results further demonstrated that Hnf4a is the primary factor mediating the function of Mettl3 in liver development.
Mettl3 is dispensable for homeostasis of adult liver
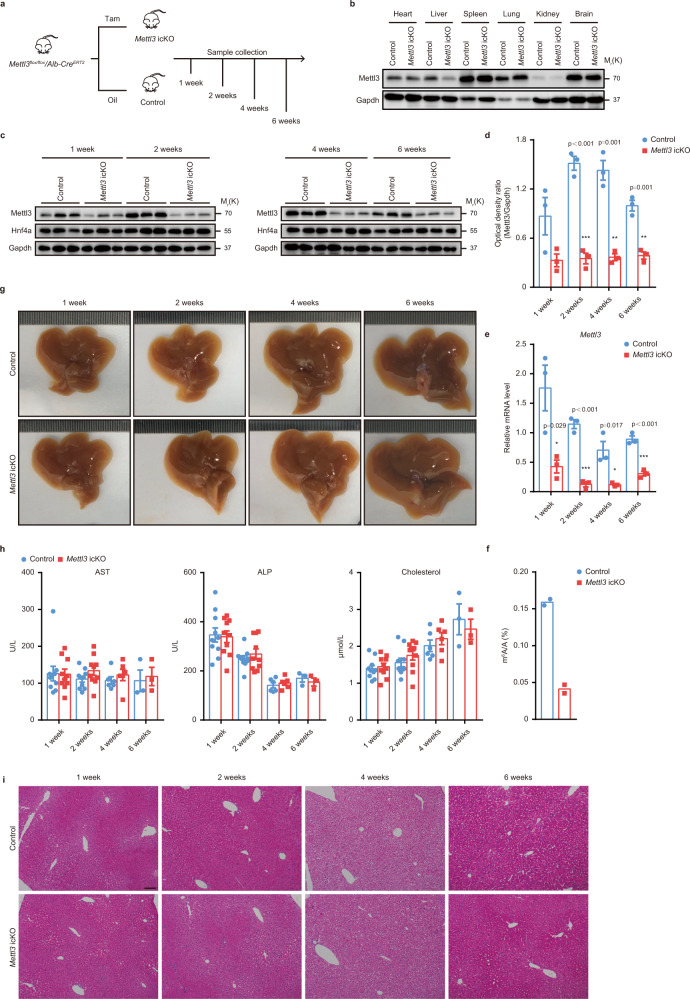
Given the lethality of Mettl3 cKO mice within 7 weeks, we generated conditional inducible Mettl3 knockout mice (Mettl3 icKO) by crossing Mettl3flox/flox mice with Alb-CreERT2 mice. Mettl3 could be deleted in adult mice by intraperitoneal (IP) injection of tamoxifen30. Genomic PCR, RT-qPCR, and western blot confirmed efficient and specific depletion of Mettl3 1 week after tamoxifen administration (Fig. 7a-e and Supplementary Fig. 8a). LC-MS/MS results also showed a significant decrease in bulk m6A modification of Mettl3 icKO mouse liver mRNAs (Fig. 7f). However, we did not observe any visible abnormalities in these Mettl3 icKO mice (Fig. 7g and Supplementary Fig. 8b, c). Serological and histologic examinations showed minimal liver damage (Fig. 7h, i, and Supplementary Fig. 8d). Both mRNAs of Hnf4a and downstream targets of Hnf4a showed no differences between Control and Mettl3 icKO livers (Supplementary Fig. 8e-k). These results indicate that although Mettl3 is essential for the early postnatal development of the liver, it is not crucial for the homeostasis of adult liver (Supplementary Fig. 9).
Fig. 7. Mettl3 is dispensable for homeostasis of adult liver.
a Schematic diagram of tamoxifen-induced liver-specific Mettl3 knockout (Mettl3 icKO) mouse generation. 4 weeks old Mettl3flox/flox/Alb-CreERT2 mice were treated with tamoxifen (Tam) or olive oil (Oil) for 5 consecutive days and then labeled with 0 week. b Western blot for Mettl3 in indicated tissues from Control and Mettl3 icKO mice at 1 week after tamoxifen treatment (3 experiments were repeated independently with similar results). c Western blot for Mettl3 and Hnf4a in Control and Mettl3 icKO mouse liver tissues at different time points after tamoxifen treatment (3 experiments were repeated independently with similar results). d Densitometry analysis of western blot for Mettl3 from Control and Mettl3 icKO mouse liver tissues at different time points after tamoxifen treatment (n = 3 for each group). e RT-qPCR for Mettl3 expression in liver tissues at different time points after tamoxifen treatment (n = 3 for each group over 3 independent experiments). f LC–MS/MS analysis of m6A/A ration in Control and Mettl3 icKO mouse livers 2 weeks after tamoxifen treatment (n = 2 for each group). g Representative gross appearance of livers from Control and Mettl3 icKO mice at 1 week, 2 weeks, 4 weeks, and 6 weeks after tamoxifen treatment (3 experiments were repeated independently with similar results). h Serum levels of AST, ALP, and Cholesterol of Control and Mettl3 icKO mice at different time points after tamoxifen treatment (n = 3 for 6 weeks group; n = 6 for 4 weeks group; n = 10 for other groups). i Representative H&E staining photographs of Control and Mettl3 icKO mouse liver sections at indicated time points (3 experiments were repeated independently with similar results). Scar bar = 100 μm. Data in d–f and h were shown as mean ± SEM with the indicated significance (*P < 0.05, **P < 0.01, ***P < 0.001; two-tailed student’s t-test). Source data are provided as a Source Data file.
Discussion
Previous reports had profiled transcriptome-wide m6A in porcine liver at three postnatal stages31 and supplied a roadmap of m6A modification across human and mouse livers32, indicating dynamic changes of m6A modification during liver development. This study demonstrated a vital role of Mettl3-mediated m6A modification in mouse postnatal liver development. Using Mettl3flox/flox/Alb-Cre mice, we found that Mettl3 cKO mice got steatosis at about 2 weeks after birth, liver fibrosis at 4–5 weeks, and finally died before 7 weeks due to severe liver injury.
The liver experiences abrupt functional changes from intra- to the extra-uterine environment, corresponding to a functional shift from haematopoiesis to metabolism and immunity33. In general, fetal liver haematopoiesis is characterized by initiation (E11.5), peak (E14.5), recession (E15.5), and disappearance (3 days after birth), while the neonatal liver rapidly evolves into a vital organ for immunosurveillance and metabolism. Histological analysis showed that haematopoietic cells disappeared rapidly, and parenchymal cells occupied in hepatic constituent in the first week after birth, consistent with previous reports34. Mettl3 was barely expressed in neonatal mouse livers, while highly expressed at both fetal livers35 and 1 week after birth at protein levels (even though the mRNA level only slightly changed during this process). The mechanism of how Mettl3 is tightly regulated during this perinatal period remains elusive and is worth considering. Given the functional transition of livers perinatally, the dynamic regulation and function of Mettl3 in this period might be involved in both haematopoietic and hepatic aspects. A series of studies have investigated the role of Mettl3-mediated m6A in haematopoietic system36,37, including early haematopoietic stem cell (HSC) development in the fetal liver35, revealing an essential role for m6A in both specification and homeostasis of haematopoietic system. However, the function of Mettl3 and m6A in hepatic lineage specification in prenatal development is currently not clear and worthy to be studied further.
Interestingly, the expression of Mettl3 and Mettl14 decreased synchronously in the liver after 4 weeks old, raising the possibility of a less critical role of m6A modification in adulthood and elder age. Indeed, we found that Mettl3 was not essential for liver homeostasis in adults. Previous studies also showed higher expression levels of the methyltransferase complex in early stages of differentiation or progenitors, such as in neurogenesis38,39 and haematopoiesis40. The level of global m6A modification and methyltransferase expression is reduced in premature mesenchymal stem cells41, replicative senescent cells42, and peripheral blood mononuclear cells from old cohorts43. Aging is always accompanied by a progressive decline in regenerative capacity, especially in the liver44, and thus it will be intriguing to delineate whether the age-related decline of regeneration capacity is elicited by decreased m6A dynamics in older individuals. Although overexpressing RNA methyltransferases attenuates the senescence phenotype41–43, increased methyltransferase expression is related to aggravated liver metabolic disorders45 and carcinogenesis progression46, including hepatocellular carcinoma47, indicating that fine-tuning regulation of m6A machinery is essential for physiological homeostasis.
As the largest digestive and metabolic organ in adults, the liver is responsible for transforming protein, glycogen, cholesterol, fatty acid, and many other complex molecules into elementary molecules. The proper development and function of the liver were maintained by a series of liver-enriched transcription factors and comprehensive regulatory networks among them2,48. Here we found that plenty of liver-enriched transcription factor transcripts were modified by Mettl3-mediated m6A modification, including Hnf1a, Hnf1β, Hnf4a, Ppara, Cebpa, Onecut1, Onecut2, Cited2, et al. Hnf4a seemed to be the most critical downstream mediator of Mettl3 in postnatal liver development. Furthermore, we observed a decreased Hnf4a at different time points on both mRNA and protein levels in Mettl3 cKO mouse livers compared with Control livers and significant dysregulation of its downstream targets.
Hnf4a is a master transcription factor required for mouse livers in both foetuses and adults26. It directly binds to almost half of the actively transcribed genes in adult livers and serves as a high-level transcription factor in hepatic transcriptional hierarchies49,50. Most of the abnormalities we found in Mettl3 cKO mice (including lipid deposition in the liver, increased bile acids in serum, liver injury, and lethality in young adults) phenocopied that of hepatic Hnf4a knockout mice26. However, Mettl3 cKO mice showed more severe liver injury than hepatic Hnf4a knockout mice. This might be explained by the fact that multiple targets were regulated by Mettl3-mediated m6A modification, and auto-regulatory and cross-regulatory circuits among them may further accelerate the collapse of liver development and function49,51,52. Among others, accumulating evidence showed profound crosstalk between m6A and histone/DNA epigenetic modifications53, which added another layer of complexity to explain the role of Mettl3 in liver development and function. Recent studies showed that m6A directly regulates heterochromatin organization54,55. Coordinated remodeling of heterochromatin is essential for liver development56, and disturbed heterochromatin landscape contributes to impaired hepatic function and tumorigenesis57. Therefore, it will be interesting to see whether an abnormal chromatin state accounts for Mettl3 cKO-induced liver injury. Besides, Mettl3 knockout may also lead to liver development defects by controlling other aspects of RNA metabolism, such as mRNA transportation, translation, and splicing of other regulators involved in liver development and biogenesis of miRNAs critical for hepatogenesis.
In summary, our study demonstrated a novel regulatory function of epitranscriptomics by Mettl3-mediated m6A modification on liver postnatal development and homeostasis, expanding our understanding of the regulatory network in mammalian liver development and function.
Methods
Ethics statement
Animals in this study were used in accordance with the Guide for Care and Use of Laboratory Animals of the National Institute of Health, and protocols were approved by the Institutional Animal Care and Use Committee of The Third Affiliated Hospital of Sun Yat-sen University. For human specimens used in this study, informed consent was obtained from all individuals and recorded in the electronic database. Research Board protocols were conducted under the guidelines set out by the Medical Ethical Committees of the Third Affiliated Hospital of Sun Yat-sen University.
Animal experiments
Mettl3flox/flox mice were kindly gifted from Professor Qi Zhou58. Alb-Cre mice were purchased from the GemPharmatech Co. Ltd (Nanjing, China). Alb-CreERT2 mice were purchased from Beijing Biocytogen Co., Ltd. (Beijing, China). All the mice were C57BL/6 J background and housed in a specific pathogen-free facility under 12 h light/dark cycle, a temperature of 24 ± 2 °C, humidity between 30 and 70%, with access to food and water ad libitum. Mettl3flox/flox/Alb-Cre mice (Mettl3 cKO mice) were generated by crossing Mettl3flox/flox mice with heterozygous Alb-Cre mice. Mettl3flox/flox/Alb-CreERT2 mice were generated by crossing Mettl3flox/flox mice with Alb-CreERT2 mice. For liver-specific inducible knockout of Mettl3 (Mettl3 icKO) in adults, 4-5 weeks old Mettl3flox/flox/Alb-CreERT2 mice were treated with tamoxifen (Sigma-Aldrich, T5648) at 1 mg/mouse for 5 consecutive days by intraperitoneal injection, and control mice were treated with the same volume of olive oil (MACKLIN, O815211). Tamoxifen was dissolved in olive oil at a concentration of 10 mg/mL by shaking overnight at 37 °C. Control and Mettl3 cKO or Mettl3 icKO mice were littermates and cage mates. All the Alb-Cre or Alb-CreERT2 mice in this paper were heterozygous for Cre.
Genomic PCR for mouse genotyping
Mice were genotyped with tail and tissue DNA. Tail and tissue lysates were prepared using a Mouse Direct PCR kit (ApexBio Technology, k1025). Two pairs of primers were used to identify floxed alleles: Mettl3-F1 and Mettl3-R1, or Mettl3-F2 and Mettl3-R2 (shown in Supplementary Fig. 1c). Mettl3-F1 and Mettl3-R1 primer (shown in Supplementary Fig. 1c) were used to distinguish WT (182 bp) or floxed allele (222 bp) with mouse tails. Mettl3-F2 and Mettl3-R2 primer (shown in Supplementary Fig. 1c) were used to distinguish WT (295 bp) or floxed allele (335 bp) with mouse tails. Alb-Cre-F and Alb-Cre-R primer were used to detect the Alb-Cre allele with mouse tails (340 bp for Alb-Cre+/− and none for Alb-Cre−/−). Alb-CreERT-F and Alb-CreERT-R primer were used to detect the Alb-CreERT allele with mouse tails (788 bp for Alb-CreERT2+/− and none for Alb-CreERT2 −/−). For floxdel detection in different tissues, Mettl3-F1 and Mettl3-R2 primers were used. A 318 bp band of floxdel could be only observed in tissues with successful deletion of Mettl3, accompanied by a much thinner WT band at about 2500 bp. Uncropped gels were provided in the Source Data file. Detailed primer sequences were listed in Supplementary Table 1.
AAV virus preparation and in vivo transduction
Serotype 8 AAV (AAV8) was used in this study. Hnf4a coding sequencing was cloned into an AAV8 vector under the control of TBG promoter to achieve liver-specific overexpression of Hnf4a protein. The virus was packaged by Packgene Biotech Co., Ltd (Guangzhou, China) with a final titer larger than 2.0 × 1013 viral genomes/mL. For AAV transduction in vivo, we used two strategies for rescue experiments. In the first strategy, each Mettl3 cKO mouse received 1.0 × 1011 viral genomes of AAV8-Ctrl or AAV8-Hnf4a through the superficial temporal vein on day 2 after birth. Mice were sacrificed at 2 weeks old for further analysis. In another strategy, 1.0 × 1011 viral genomes/mouse of AAV8-Ctrl or AAV8-Hnf4a was injected into Mettl3 cKO mice through the tail vein at 4 weeks old for further survival rate analysis.
Serum analysis
Serum levels of liver function indicators (alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, cholesterol, triglyceride, total bile acid, total bilirubin, and direct bilirubin) were detected using Hitachi 7020 automatic biochemical analyser (Hitachi, Tokyo, Japan).
Human specimens
Human liver tissues were obtained from donation after cardiac death (DCD) during liver transplantation in the Third Affiliated Hospital of Sun Yat-sen University. The study has been approved by the Medical Ethical Committees of the Third Affiliated Hospital of Sun Yat-sen University. The study design and conduct complied with all relevant regulations regarding the use of human study participants and was conducted following the criteria set by the Declaration of Helsinki.
Cell culture
HEK293T and HepG2 cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM-high glucose medium (Thermo Scientific, C11995500BT) supplemented with 10% fetal bovine serum (FBS) (PAN, P30-3302). Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
Primary hepatocyte isolation
Primary hepatocytes were isolated according to traditional two-step collagenase perfusion methods59. Briefly, mice were perfused through portal vein cannulation by EGTA buffer (8000 mg/L NaCl, 400 mg/L KCl, 76.67 mg/L NaH2PO4, 120.45 mg/L Na2HPO4, 2380 mg/L HEPES, 350 mg/L NaHCO3, 190 mg/L EGTA, and 900 mg/L Glucose, PH = 7.35-7.4) at 8 mL/min for 2 min, followed by enzyme buffer (8000 mg/L NaCl, 400 mg/L KCl, 76.67 mg/L NaH2PO4, 120.45 mg/L Na2HPO4, 2380 mg/L HEPES, 350 mg/L NaHCO3, and 481.8 mg/L CaCl2, PH = 7.35-7.4) containing 100 U/mL collagenase IV (Sigma-Aldrich, C5138) at 8 mL/min for 8 min. The cell suspensions were filtered through a 70 μm cell strainer (Sorfa, 251200). Cell pellets were collected by centrifugation at 50 g for 1 min at 4 °C after three washes. Then cells were resuspended in Williams’ Medium E (GIBCO, 12551032) supplemented with 10% FBS and 1% penicillin/streptomycin (KeyGEN Biotech, KGY0023) and seeded in Type I Collagen (Invitrogen, A048301) pre-coated culture plates and cultured at 37 °C in 5% CO2 incubator. The culture medium was changed at 2 hours of incubation. Cells were changed to serum-free medium 6 hours later and cultured overnight before use.
Plasmid construction and virus transduction
shRNA targeting human METTL3 (shMETTL3), human IGF2BP1 (shIGF2BP1), and luciferase (shLuc) were cloned into pLKO.1 lentiviral vector (Addgene, 10878). All constructs were confirmed by Sanger sequencing. Lentivirus transfections were conducted using polyethylenimine (PEI, Polysciences, 23966) according to the manufacturer’s protocol. Briefly, HEK293T cell was seeded into 6 cm dish, and transfection experiments were performed at 80% confluence. For each well, 335 μl of pre-warmed Opti-MEM (Invitrogen) was mixed with 8 μg of plasmids (target plasmid: psPAX2: pMD2.G = 4:2:1), 36 μl of PEI (1 μg/ml), then incubated for 14 min at room temperature and added to the dish. The culture medium was changed 10 hours later. 48 h after transfection, the supernatants were collected and filtered through a 0.45 μm filter. HepG2 cells were infected with lentivirus-containing supernatants generated in HEK293T supplemented with 1 μg/ml polybrene (Sigma, H9268) for 8 h. Cells were then selected with 1.5 μg/ml puromycin (Thermo Scientific, A1113803) for two consecutive days. TRC lentiviral vectors encoding shRNAs against human METTL3 and human IGF2BP1 were listed in Supplementary Table 1.
Western Blot
Tissue samples were lysed with RIPA buffer (50 mM Tris-HCl (PH 7.4), 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, and 2 mM EDTA (PH 8.0)) containing protease inhibitor (Roche, 04693132001) and phosphatase inhibitor (Roche, 04906837001). For cells, samples were counted, washed twice with ice-cold PBS, and lysed the same as tissues. Then the lysates were separated with SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with TBS containing 5% (v/w) non-fat milk and 0.1% Tween-20 (Sigma-Aldrich, P1379) and incubated with primary and secondary antibodies sequentially. Protein bands were detected using Immobilon ECL Ultra Western HRP Substrate (Millipore, WBULS500) according to the manufacturer’s instructions. Gapdh or β-actin was used as the loading control. Uncropped blots were supplied in the Source Data file. Antibodies used for western blot were listed in Supplementary Table 2.
RNA extraction and RT-qPCR
Total RNAs were extracted from tissues or cells using TRIzol (Ambion) according to the manufacturer’s instructions and quantified by UV spectrophotometry. Reverse transcription was conducted using PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, RR047B). RT-qPCR was then performed in triplicates on Light Cycler 480 II (Roche) using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711-03). Gapdh was used as the internal control. The primers used for RT-qPCR were listed in Supplementary Table 1.
H&E, Masson’s trichrome, and Immunohistochemistry staining
Liver samples were fixed in 4% paraformaldehyde and embedded with paraffin. Samples were sliced into 8 μm in thickness and then subjected to hematoxylin and eosin (H&E) staining or Masson’s trichrome staining. For immunohistochemistry, sections were dewaxed, rehydrated, and then incubated in EDTA antigen retrieval buffer (ZSGB-BIO, ZLI-9072) for 5 min at 100 °C. Slices were then incubated with 3% H2O2 for 10 min, washed three times with PBS containing 0.02% TritonTM X-100 (Sigma-Aldrich, T8787), followed by incubation with primary antibodies overnight at 4 °C. Horseradish peroxidase-conjugated antibody was used as the secondary antibody and incubated at 37 °C for 1 h. The color was developed by incubation with Dako RealTM kit (Dako, K5007). Sections were counterstained with hematoxylin (Baso, BA4041) and checked under the microscope (Nikon). Primary antibodies used in the immunohistochemistry staining were listed in Supplementary Table 2. Quantification for Masson’s trichrome staining positive area and αSMA immunohistochemistry staining positive area was conducted with 5-8 random fields (10*) each mouse using ImageJ software (version 1.8.0).
TUNEL assay
Liver tissues were fixed, embedded with OCT compound (Servicebio, G6059), and sliced into 8 μm in thickness, then permeated with PBS containing 0.25% TritonTM X-100 (Sigma-Aldrich, T8787) and stained with In Situ Cell Death Detection Kit (Roche, 11684795910) following the manufacturer’s instructions. Hoechst 33342 (Beyotime, C1022) was used to counterstain nuclei. Sections were visualized under the confocal microscope (ZEISS, LSM 880), and images were analyzed by ZEN 2012 software. Quantification for TUNEL+ cells/Hoechst 33342+ cells ratio and average nuclear diameter was conducted using ImageJ software (version 1.8.0).
PI staining
PI staining was performed on primary hepatocytes. The culture medium was removed, and cells were washed twice with pre-warmed PBS. Then cells were stained with propidium iodide solution (BD, 556547) for 15 min at 37 °C. Hoechst 33342 (Beyotime, C1022) was used to counterstain nuclei. Cells were detected under the microscope (Zeiss, Axio Observer Z1), and images were analyzed by ZEN 2012 software.
Oil Red O staining
Liver tissues were fixed, embedded with OCT compound, and sliced into 8 μm in thickness. Oil Red O (Sigma-Aldrich, O0625) powder was dissolved in isopropanol at 0.7 g/100 mL concentration and then diluted with water at the volume ratio of 3:2 to get the Oil Red O solution. Pre-warmed tissue sections were washed twice with PBS and stained with Oil Red O solution for 20 min, then washed with 60% isopropanol for 10 s three times, followed by counterstaining with hematoxylin (Baso, BA4041), and then viewed under the microscope (Nikon). Oil Red O staining positive area was quantified with 5-8 random fields (10*) for each mouse using ImageJ software (version 1.8.0).
BODIPY staining
BODIPY staining was performed on frozen liver sections and primary hepatocytes. For frozen liver tissue sections, liver tissues were fixed, embedded with OCT compound, sliced into 8 μm in thickness, and then stained with BODIPY (Invitrogen, D3922) at 7.6 μM for 30 min. Hoechst 33342 (Beyotime, C1022) was used to counterstain nuclei. Sections were mounted and scanned under the confocal microscope (ZEISS, LSM 880), and images were analyzed by ZEN 2012 software. For primary hepatocytes, the culture medium was removed, and the cells were washed twice with pre-warmed PBS. Then the cells were stained the same as liver tissues and checked under the microscope (Zeiss, Axio Observer Z1).
mRNA stability assay
mRNA stability assay was performed on primary hepatocytes and HepG2 cell line. Primary hepatocytes were isolated from Control and hepatic Mettl3 cKO mice. Cells were cultured overnight and then treated with 5 μM actinomycin D (Sigma-Aldrich, A1410) for 0 h, 1 h, 2 h, 4 h, and 6 h. HepG2 cells were cultured and treated with 5 μM actinomycin D for 0 h, 40 min, 2 h, and 4 h. For RT-qPCR analysis, total RNAs were isolated and subjected to reverse transcription, and the mRNA levels of genes of interest were detected by RT-qPCR. For RNA stability RNA-sequencing, total RNAs were sent for RNA-sequencing.
RNA-sequencing and m6A-RIP sequencing
RNA-sequencing and m6A-RIP sequencing for liver tissues were conducted by Guangzhou Epibiotek Co., Ltd. Briefly, for liver tissue RNA-sequencing, total RNA was isolated from Control and Mettl3 cKO mouse livers at day 1, 1 week, 2 weeks, 4 weeks, and 8 weeks postnatally (2 individuals for each time point). Sequence reads were aligned to the mouse genome version mm10 with HISAT2.1.060, and differentially expressed genes (DEGs) were calculated by DESeq261 under the following criteria: |log2FC | > 1 and P value < 0.05. Gene set enrichment analysis (GSEA) was conducted using the GSEA software (https://www.broadinstitute.org/gsea/)62. For mouse primary hepatocyte RNA stability RNA-sequencing, total RNA was isolated from Control and Mettl3 cKO primary hepatocyte at 0 h, 2 h, and 6 h treatment with 5 μM actinomycin D and sequenced by BerryGenomics Company (http://www.berrygenomics.com/, Beijing, China). Reads were mapped to the mouse mm10 genome with STAR v2.5.3a63, and the uniquely mapped reads with q score no less than 250 were kept. Gene counting was performed by featureCounts v2.0.1 with exon features documented in the Gencode mouse annotation gtf file64. Differentially expressed genes between WT and KO at different time points were identified by edgeR package65. For m6A-RIP sequencing, total RNA was isolated from WT mouse livers at day 1, 1 week, 2 weeks, 4 weeks, and 8 weeks postnatally (2 individuals for each time point) using TRIZOL reagent and fragmented. m6A-modified RNA was enriched by m6A antibody and rRNA was removed. The library was prepared by smart-seq method and sequenced. Sequence reads were aligned to the mouse genome version mm10 with HISAT2.1.0. Differential m6A-modified peaks between RIP and input samples were identified using exomePeak66. The longest isoform was retained if a gene had more than one isoform. Motif search was conducted with HOMER67.
LC-MS/MS for m6A detection and quantification
Bulk mRNA m6A modification quantification through LC-MS/MS was conducted by Wuhan Metware Co., Ltd. Briefly, 1 μg purified mRNA was sufficiently digested to nucleosides with S1 nuclease, phosphodiesterase, and alkaline phosphatase in 37 °C, then extracted by chloroform to get prepared solution samples. The samples were analyzed using a UPLC-ESI-MS/MS system (UPLC, ExionLCTM AD, https://sciex.com.cn/; MS, Applied Biosystems 6500 Triple Quadrupole, https://sciex.com.cn/). The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS. Linear ion trap (LIT) and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP) equipped with an ESI Turbo Ion-Spray interface, then operated in a positive ion mode and controlled by Analyst 1.6.3 software (Sciex). RNA modifications were analyzed using scheduled multiple reaction monitoring (MRM). Data acquisitions were performed using Analyst 1.6.3 software (Sciex). RNA modification contents were detected by MetWare (http://www.metware.cn/) based on the AB Sciex QTRAP 6500 LC-MS/MS platform.
m6A-RIP-qPCR
m6A-RIP-qRCR was performed according to previous reports68,69. Briefly, total RNAs were extracted from 2 weeks or 4 weeks old Control and Mettl3 cKO mice. Poly(A) mRNAs were separated using an mRNA purification kit (Sigma-Aldrich, GenEluteTM mRNA Miniprep Kit MRN10). Two rounds of purification process were conducted in each experiment to sufficiently remove rRNA contamination according to manufacturer’s instruction. 5 μg mRNAs were fragmented into 200-300 nt segments by incubation at 94 °C for 30 s in fragmentation buffer (10 mM ZnCl2, 10 mM Tris-HCl (PH 7.0)), then stopped with 50 mM EDTA, and purified by ethanol precipitation. Fragmented mRNAs and 2 μg anti-m6A antibody (Synaptic Systems, 202003) or mouse IgG (Beyotime, A7028) were added in 600 μL RIP buffer (150 mM NaCl, 0.1% Igepal CA-630, 10 mM Tris-HCl (PH 7.4)) and incubated at 4 °C for 2 h. Then 15 μL Dynabeads® Protein A beads (Thermo, 100-02D) and 15 μL Dynabeads® Protein G beads (Thermo, 100-04D) were added to the mixture and incubated at 4 °C for another 2 h. Beads were washed with RIP buffer 5 times. RNase inhibitor (Promega, N2611) was added throughout the entire process. Fragmented mRNAs were eluted by 100 μL 0.3 μg/μL Proteinase K (Thermo Scientific, AM2546) at 55 °C for 1 h, and followed by phenol-chloroform extraction and ethanol precipitation purification procedure. The precipitated mRNAs were reverse transcribed, and enrichment was determined by RT-qPCR as mentioned above. RT-qPCR primers for m6A positive regions were marked as “positive-m6A-RT” (e.g., primers for two separate m6A positive regions in Ppara were listed as Ppara-positive-m6A-1-RT and Ppara-positive-m6A-2-RT), and primers for m6A negative regions were marked as “negative-m6A-RT” (e.g., the primer for m6A negative regions in Ppara were listed as Ppara-negative-m6A-RT). Primers used for m6A-RIP-qPCR were listed in Supplementary Table 1.
RNA immunoprecipitation (RIP)-qPCR
RIP was performed according to previously published protocols with modifications69,70. Briefly, 200 mg liver tissues were homogenized with 600 μL homogenizer buffer (100 mM KCl, 5 mM MgCl2, 10 mM HEPES, pH 7.0, 0.5% Nonidet P-40, 1 mM DTT, and 100 U/mL RNase inhibitor (Promega, N2611), then incubated on ice for 5 min, followed by centrifuging to obtain supernatant. 2 μg Igf2bp1 antibody was added to 15 μL Dynabeads® Protein A beads (Thermo, 100-02D) and 15 μL Dynabeads® Protein G beads (Thermo, 100-04D), then rotated at 4 °C for 2 h. Antibody-beads slurry was then incubated with homogenized supernatant and rotated at 4 °C for another 2 h. Beads were washed 5 times with Washing Buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM MgCl2, and 0.05% NP40), then extracted RNA using Trizol for RNA extraction and further analysis for RT-qPCR.
Dual-luciferase reporter assays
To construct the Mettl3 overexpression vector, the full-length coding sequences of mouse Mettl3 were amplified by PCR with Phanta Max DNA Polymerase (Vazyme, P505) using Mettl3-PKD-F and Mettl3-PKD-R primer, then cloned into a lentiviral vector PKD-EF1 using ClonExpress II One Step Cloning kit (Vazyme, C112). Mettl3-D395A-W398A catalytic mutant (DPPW/APPA) vector was constructed using the site-directed mutagenesis method (using PCR-based method with Mettl3 overexpression vector as template and Mettl3-AWWA-F and Mettl3-AWWA-R as primers). PKD-EF1 vector expressing EGFP served as a control in transfection. DNA fragments of Hnf1a and Hnf4a containing the WT m6A motifs and mutant motifs (Supplementary Fig. 6c) were synthesized by Shanghai Generay Biotech Co., LTD., and then subcloned into pMIR-REPORT firefly luciferase reporter vector (Ambion, AM5795) between Mlu I and Sac I sites. All sequences were confirmed by Sanger sequencing. 50 ng Mettl3 vectors (Mettl3 overexpressing vector or control), 40 ng firefly luciferase reporter vectors with WT or mutated fragments, and 10 ng pRL Renilla Luciferase Control Reporter Vector (Promega, E2231) were co-transfected into HEK293T cells in triplicates in 96-well plates. Fluc and Rluc activities were measured 24 h later with the Dual-Luciferase Reporter Assay System (Promega, E1910) according to the manufacturer’s instructions. The relative luciferase activity was calculated through dividing Fluc activity by individual Rluc activity and then normalizing to control of each assay. The sequences of PCR primers were listed in Supplementary Table 1.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We show our full respect and gratitude to all members of Qi Zhang and Yan Xu’s lab for their discussion and technical assistance. This work was supported by the National Key Research and Development Program of China (No. 2017YFA0106100, X.Y.), National Natural Science Foundation of China (No. 81970537, X.Y.; No. 31601184, X.Y.; No. 81870449, Z.Q.; No. 82170674, Z.Q.), Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515011385, X.Y.), Guangzhou Science and Technology Plan Project (No. 202206010072, Z.Q.), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (No. 22ykqb02, X.Y.), and Guangdong Science and Technology Program (2020B1212060019).
Source data
Author contributions
Q.Z. and Y.X. conceived the idea, supervised the study, analyzed data, and acquired funding. Y.X. drafted the manuscript with the help of Z.Z. and X.K. Q.Z. critically revised the manuscript. Z.Z. and X.K. contributed to the experimental design and conducted most experiments with the help of L.P., C.L., X.L., J.C., S.D., Y.L., Q.L., Y.S., and S.Y. The manuscript was read and approved by all authors.
Peer review
Peer review information
Nature Communications thanks Hua-Bing Li and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Data availability
Source data are provided with this paper. RNA-sequencing and m6A-RIP sequencing raw data and processed expression matrix are uploaded to GEO DataSets under accession code GSE197564. The sequencing reads were mapped to the mouse mm10 genome. All other data analyzed or generated in this study are provided along with the article. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yan Xu, Zhuowei Zhou, Xinmei Kang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32169-4.
References
- 1.Ober EA, Lemaigre FP. Development of the liver: Insights into organ and tissue morphogenesis. J. Hepatol. 2018;68:1049–1062. doi: 10.1016/j.jhep.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Lau HH, et al. The molecular functions of hepatocyte nuclear factors - In and beyond the liver. J. Hepatol. 2018;68:1033–1048. doi: 10.1016/j.jhep.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Macchi F, Sadler KC. Unraveling the epigenetic basis of liver development, regeneration and disease. Trends Genet. 2020;36:587–597. doi: 10.1016/j.tig.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Adams JM, Cory S. Modified nucleosides and bizarre 5’-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 5.Desrosiers R, et al. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry RP, et al. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5’ terminus. Cell. 1975;4:387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ping XL, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li A, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao W, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao BS, et al. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geula S, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 20.Meng TG, et al. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J. 2019;33:1179–1187. doi: 10.1096/fj.201800719R. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci. 2018;21:195–206. doi: 10.1038/s41593-017-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HB, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P, et al. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 25.Watt AJ, et al. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249–1253. doi: 10.1053/jhep.2003.50273. [DOI] [PubMed] [Google Scholar]
- 26.Hayhurst GP, et al. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parviz F, et al. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- 28.Meng XM, et al. TGF-beta: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 29.Haber B, et al. Coexpression of liver-specific and growth-induced genes in perinatal and regenerating liver: attainment and maintenance of the differentiated state during rapid proliferation. Hepatology. 1995;22:906–914. [PubMed] [Google Scholar]
- 30.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 31.He S, et al. mRNA N6-methyladenosine methylation of postnatal liver development in pig. PLoS ONE. 2017;12:e0173421. doi: 10.1371/journal.pone.0173421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, et al. Landscape and regulation of m(6)A and m(6)Am methylome across human and mouse tissues. Mol. Cell. 2020;77:426–440.e426. doi: 10.1016/j.molcel.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Nakagaki BN, et al. Immune and metabolic shifts during neonatal development reprogram liver identity and function. J. Hepatol. 2018;69:1294–1307. doi: 10.1016/j.jhep.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Si-Tayeb K, et al. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, et al. m(6)A modification prevents formation of endogenous double-stranded rnas and deleterious innate immune responses during hematopoietic development. Immunity. 2020;52:1007–1021.e1008. doi: 10.1016/j.immuni.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, et al. Stage-specific requirement for Mettl3-dependent m(6)A mRNA methylation during haematopoietic stem cell differentiation. Nat. Cell Biol. 2019;21:700–709. doi: 10.1038/s41556-019-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 38.Ma C, et al. RNA m(6)A methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biol. 2018;19:68. doi: 10.1186/s13059-018-1435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon KJ, et al. Temporal control of mammalian cortical neurogenesis by m(6)A Methylation. Cell. 2017;171:877–889.e817. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng H, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell. 2018;22:191–205.e199. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, et al. METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Res. 2020;48:11083–11096. doi: 10.1093/nar/gkaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, et al. Lamin A safeguards the m(6) A methylase METTL14 nuclear speckle reservoir to prevent cellular senescence. Aging Cell. 2020;19:e13215. doi: 10.1111/acel.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min KW, et al. Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell. 2018;17:e12753. doi: 10.1111/acel.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timchenko NA. Aging and liver regeneration. Trends Endocrinol. Metab. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Xie W, et al. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem. Biophys. Res. Commun. 2019;518:120–126. doi: 10.1016/j.bbrc.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Huang H, et al. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Z, et al. Epitranscriptomics in liver disease: basic concepts and therapeutic potential. J. Hepatol. 2020;73:664–679. doi: 10.1016/j.jhep.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Schrem H, et al. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol. Rev. 2002;54:129–158. doi: 10.1124/pr.54.1.129. [DOI] [PubMed] [Google Scholar]
- 49.Kuo CJ, et al. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 50.Odom DT, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura N, Tanaka K. Analysis of the rat hepatocyte nuclear factor (HNF) 1 gene promoter: synergistic activation by HNF4 and HNF1 proteins. Nucleic Acids Res. 1993;21:3731–3736. doi: 10.1093/nar/21.16.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian JM, Schibler U. Tissue-specific expression of the gene encoding hepatocyte nuclear factor 1 may involve hepatocyte nuclear factor 4. Genes Dev. 1991;5:2225–2234. doi: 10.1101/gad.5.12a.2225. [DOI] [PubMed] [Google Scholar]
- 53.Kan RL, et al. Crosstalk between epitranscriptomic and epigenetic mechanisms in gene regulation. Trends Genet. 2022;38:182–193. doi: 10.1016/j.tig.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, et al. The RNA m(6)A reader YTHDC1 silences retrotransposons and guards ES cell identity. Nature. 2021;591:322–326. doi: 10.1038/s41586-021-03313-9. [DOI] [PubMed] [Google Scholar]
- 55.Xu W, et al. METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature. 2021;591:317–321. doi: 10.1038/s41586-021-03210-1. [DOI] [PubMed] [Google Scholar]
- 56.Fonseca TL, et al. Neonatal thyroxine activation modifies epigenetic programming of the liver. Nat. Commun. 2021;12:4446. doi: 10.1038/s41467-021-24748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan DN, et al. Histone lysine methyltransferase, suppressor of variegation 3-9 homolog 1, promotes hepatocellular carcinoma progression and is negatively regulated by microRNA-125b. Hepatology. 2013;57:637–647. doi: 10.1002/hep.26083. [DOI] [PubMed] [Google Scholar]
- 58.Xu K, et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klaunig JE, et al. Mouse liver cell culture. I. Hepatocyte isolation. Vitro. 1981;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- 60.Kim D, et al. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Love MI, et al. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao Y, et al. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 65.Robinson MD, et al. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng J, et al. A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods. 2014;69:274–281. doi: 10.1016/j.ymeth.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim T, et al. Octopus-toolkit: a workflow to automate mining of public epigenomic and transcriptomic next-generation sequencing data. Nucleic Acids Res. 2018;46:e53. doi: 10.1093/nar/gky083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dominissini D, et al. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013;8:176–189. doi: 10.1038/nprot.2012.148. [DOI] [PubMed] [Google Scholar]
- 69.Peritz T, et al. Immunoprecipitation of mRNA-protein complexes. Nat. Protoc. 2006;1:577–580. doi: 10.1038/nprot.2006.82. [DOI] [PubMed] [Google Scholar]
- 70.Keene JD, et al. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Source data are provided with this paper. RNA-sequencing and m6A-RIP sequencing raw data and processed expression matrix are uploaded to GEO DataSets under accession code GSE197564. The sequencing reads were mapped to the mouse mm10 genome. All other data analyzed or generated in this study are provided along with the article. Source data are provided with this paper.