Abstract
Currently, the coronavirus disease 2019 (COVID-19) pandemic is ravaging the world, causing serious crisis in economy and human health. The top priority is the detection and drug development of the novel coronavirus. The gold standard for real-time diagnosis of coronavirus disease is the reverse transcription-polymerase chain reaction (RT-PCR), which is usually operatively complex and time-consuming. Biosensors are known for their low cost and rapid detection, which are developing rapidly in detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The current study showed that the spike protein of SARS-CoV-2 will bind to angiotensin-converting hormone 2 (ACE2) to mediate the entry of the virus into cells. Interestingly, the affinity between ACE2 and SARS-CoV-2 spike protein increases with the mutation of the virus. Using ACE2 as a biosensor recognition receptor to detect SARS-CoV-2 will effectively avoid the decline of detection accuracy and false negative caused by variants. In fact, due to the variation of the virus, it may even lead to enhanced detection performance. In addition, ACE2-specific drugs to prevent SARS-CoV-2 from entering cells will be effectively evaluated using the biosensors even with virus mutations. Here, we reviewed the biosensors for rapid detection of SARS-CoV-2 by ACE2 and discussed the advantages of ACE2 as an antibody for the detection of SARS-CoV-2 variants. The review also discussed the value of ACE2-based biosensors for screening for drugs that modulate the interaction between ACE2 and SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, ACE2, Detection, Biosensors
Graphical abstract
1. Introduction
In December 2019, a public health emergency of international concern caused by the novel coronavirus occurred in Wuhan, China [1]. It is known to be caused by SARS-CoV-2, which causes severe acute respiratory illness and a host of complications [2]. The novel coronavirus disease also known as COVID-19, which is highly contagious and spreads rapidly around the world, causing a huge impact on human life and the global economy [3,4]. Up to May 2022, 515,192,979 confirmed cases of COVID-19 including 6,254,140 deaths have been reported to the World Health Organization (WHO). Despite the development and use of multiple vaccines against the virus, the global epidemic has not been effectively controlled due to high mutations in SARS-CoV-2 and low vaccination rates [5].
Genome sequencing of SARS-CoV-2 virus extracted from multiple clinical patients showed 80% similarity to SARS-CoV [[6], [7], [8]]. As shown in Fig. 1 , SARS-CoV-2 is a single-stranded β coronavirus RNA virus, which mainly contains four structural proteins: nucleocapsid (N), membrane (M), envelope (E) and spike (S) proteins [[8], [9], [10]]. The S-protein binds to the receptor ACE2 on the surface of human cells, mediating subsequent viral uptake and fusion, and then SARS-CoV-2 multiplies rapidly in the body tissues [[11], [12], [13], [14], [15], [16]]. The SARS-CoV-2 multiplies rapidly in the body tissues. Typically, symptoms of COVID-19 may appear after exposure to SARS-CoV-2 for 2–14 days [17]. Cough, shortness of breath and fever are the most common clinical symptoms that provide some basis for determining whether you have COVID-19 [[18], [19], [20], [21], [22]]. However, the emergence of a large number of asymptomatic infected persons makes it difficult for SARS-CoV-2 to guard against.
Fig. 1.
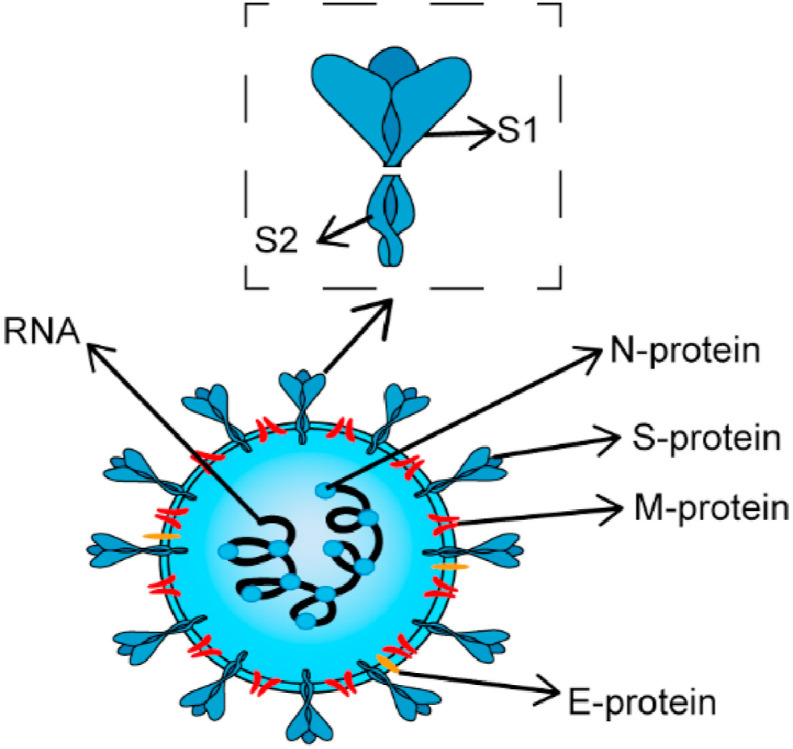
Basic structure diagram of SARS-CoV-2.
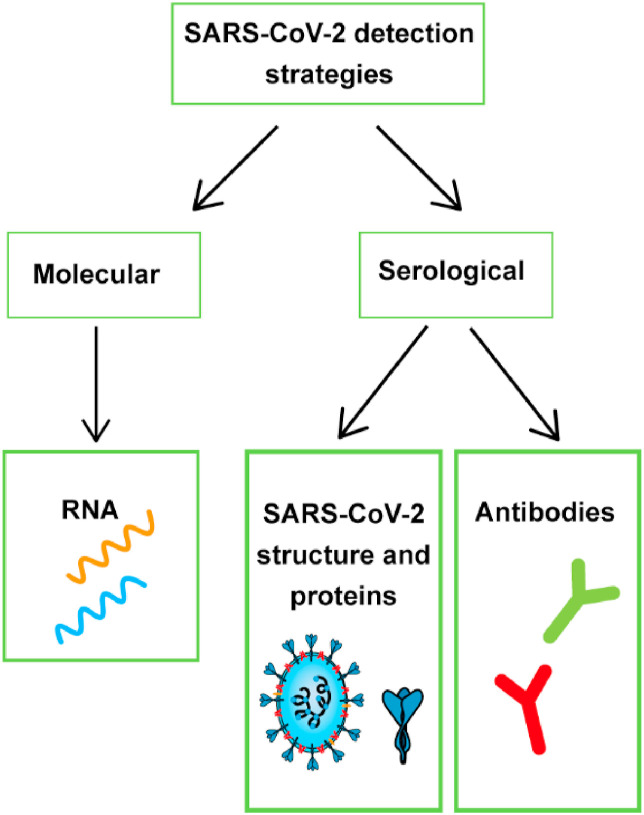
At present, in the absence of specific drugs, the best way to control the epidemic is to isolate the source of infection through testing and stop the spread of SARS-CoV-2. Therefore, it is necessary to establish a detection method that has the advantages of being cheap, fast, accurate and capable of large-scale testing to enable frequent and extensive virus testing. As shown in Fig. 2 , based on the structure of SARS-CoV-2, the most used biomarkers for detecting SARS-CoV-2 are single-stranded RNA, nucleocapsid and spike proteins [23,24]. In addition, antibody detection can also be carried out, but the generation of antibodies needs a certain period [25], antibody detection is generally only used as a means of clinical confirmation [26,27].
Fig. 2.
SARS-CoV-2 detection strategy.
Currently, the gold standard for SARS-CoV-2 testing is RT-PCR [28]. But RT-PCR is a time-consuming test that can take two to 4 h per test and requires a professional to perform [29]. With the continuous mutation of SARS-CoV-2, false negative results may occur in RT-PCR detection, and it is difficult to determine the mutant strains, requiring further gene sequencing [30,31]. The appearance of false-negative patients is catastrophic and can hamper the control of the outbreak [32,33]. Large-scale nucleic acid testing has placed a huge burden on medical services with the further development of the epidemic.
Biosensors realize the detection of viruses in biological samples cheaper, simpler, faster, and without using professionals. Nowadays, many biosensors have been developed to detect SARS-CoV-2 using RNA or proteins as markers for detection [34]. SARS-CoV-2 has evolved to adapt to human hosts in a variety of ways, including single nucleotide mutations and structural mutations, which have a significant impact on genome structure and protein function [35]. This may lead to a decrease in the accuracy of detection methods based on genes and marker proteins. The advantage of the biosensors based on ACE2 is the accuracy will not be affected by S-protein mutation which is specifically combined with ACE2. The affinity between ACE2 and S-protein increases is conducive to detecting after the virus mutation. The purpose of this review is to introduce the current research progress of biosensors for detecting SARS-CoV-2 using viral receptor ACE2. In addition, this review also discussed the role of such biosensors in drug screening and neutralizing antibodies.
2. Virus receptor-ACE2
2.1. ACE2
ACE2, a type I integral membrane protein with transmembrane domain and extracellular domain, was discovered independently by two different teams in 2000 [36,37]. The extracellular domain of ACE2 contains a zinc metallopeptidase domain, which is a single catalytic domain [[36], [37], [38]]. ACE2 exists in two forms, one is the lack of transmembrane domain, which exists in body fluids and blood; the other is anchored on the cell membrane through the transmembrane domain, and widely distributed in ACE2 in the heart, kidney, liver, intestine, upper respiratory tract and alveolar (type II) pulmonary vascular epithelial cells [36].
ACE2 is known as a regulator of the renin-angiotensin system (RAS) at primary, whose main function is to convert angiotensin I and angiotensin II into angiotensin 1-9 and angiotensin 1-7 [39,40]. During the SARS outbreak in 2003, researchers discovered that ACE2 was a functional receptor for SARS-CoV, mediating virus invasion into host cells [[41], [42], [43]] and this finding was later confirmed in animal experiments [44]. Due to the genetic similarity between SARS-CoV-2 and SARS-CoV, it was speculated and confirmed that SARS-CoV and SARS-CoV-2 have the same receptor ACE2 [8,[45], [46], [47]].
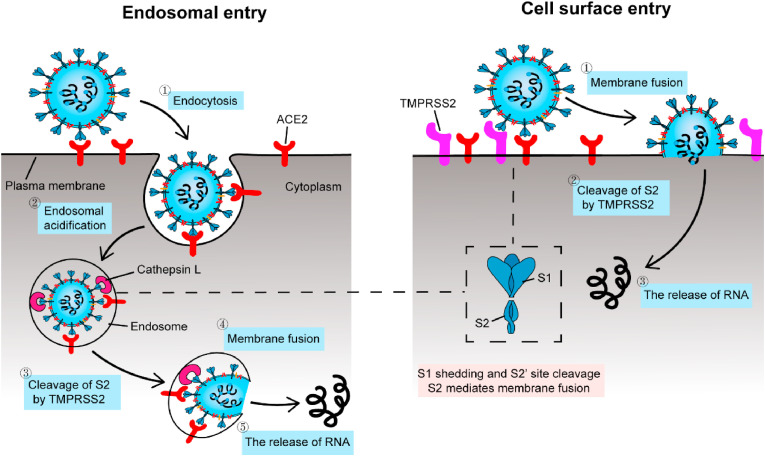
SARS-CoV-2 S-protein, which is a homotrimer protruding from the viral surface and includes the S1 subunit and S2 subunit responsible for binding to host cell receptors and fusing viral and cell membrane, respectively [48,49]. The entry of SARS-CoV-2 into host cells is a complex process that requires the synergistic action of ACE2 and proteolytic enzymes to facilitate the mutual fusion of virus and cell [50]. As shown in Fig. 3 , SARS-CoV-2 has two ways of entering cells and the binding of ACE2 to the receptor binding domain (RBD) domain on the S1 subunit is well-defined [[51], [52], [53]]. Viruses must rely on ACE2 to enter host cells, so drugs targeting ACE2 are under development. Studies have shown that the antibody neutralization reaction is targeted at RBD and inhibits the binding of RBD to ACE2 [51]. The specific binding of ACE2 to S-protein has led many researchers to design and develop biosensors for SARS-CoV-2 detection. Since ACE2 is the receptor for viruses to enter cells, this biosensor can also be used to screen or validate drugs that block the binding of coronavirus to ACE2.
Fig. 3.
Two distinct SARS-CoV-2 entry pathways. Left: when transmembrane protease, serine 2 (TMPRSS2, SARS-CoV-2 uses it to activate their entry glycoproteins) is absent on the cell surface or the SARS-CoV-2-ACE2 complex does not bind TMPRSS2, the SARS-CoV-2-ACE2 complex is internalized into lysosomes through endocytosis. Under the action of Cathepsin L (A soluble cysteine proteinase involved in antigen processing in lysosomes), S-protein is cleaved, exposing the S2 site, mediating membrane fusion and releasing viral RNA. Right: when TMPRSS2 is present on the cell surface, the S-protein is cleaved on the cell surface and the S2 site is exposed, mediating membrane fusion and releasing RNA. Adapted from Ref. [52].
2.2. The advantage of ACE2
Since the beginning of the COVID-19 pandemic, SARS-CoV-2 has been widely circulating for a long time, with the accumulation of gene mutations and significant changes in the viral gene sequence leading to corresponding changes in acupuncture (S) proteins, viral transmission and antigenicity [53,54]. For example, the rapid spread of Omicron around the world has increased uncertainty in the fight against COVID-19 and is another blow to the already troubled global economy [[55], [56], [57], [58]].
Changes in genes and expressed proteins of SARS-CoV-2 may affect the accuracy of currently available diagnostic methods. False-negative results occur from time to time in molecular tests, and now that the virus genome has mutated, the probability of false-negative results will be much greater. It has been found that the deletion of the N gene may reduce the sensitivity of the detection of SARS-CoV-2 [59]. Most of the current antigen tests used to detect SARS-CoV-2 are based on antibodies against SARS-CoV-2. However, with the rapid variation of SARS-CoV-2, the development cycle of biosensors based on antibodies against SARS-CoV-2 has greatly extended due to a series of problems such as cost, sensitivity, accuracy and stability [54]. For various reasons above, the development of biosensors is slower than the rate of virus mutation, resulting in a significant decline in its application value. The safest way to detect SARS-CoV-2 is to find a constant substance or relationship that does not change with the variants of the virus. This is the advantage of ACE2.
Studies have shown that variants in SARS-CoV-2 can replace the RBD amino acid sequence of spike protein, and the affinity between S-protein and ACE2 may be affected by the substitution of amino acid caused by virus variants [60]. Moreover, studies on the affinity between RBD and ACE2 of mutant strains showed that the affinity between ACE2 and RBD increased rather than decreased with the evolution of the spike protein RBD region which explained the reasons that SARS-CoV-2 variants were more infectious and spread more quickly [[61], [62], [63], [64]]. The research also demonstrated that the incidence of RBD mutation in the population was positively correlated with the increase of ACE2 binding affinity [65]. Therefore, the RBD amino acid of S-protein will change with mutation, but the affinity between ACE2 and RBD is stronger after mutation, which makes the method of detecting SARS-CoV-2 using ACE2 more advantageous in the face of virus mutation. Detection with ACE2 will ignore the impact of virus mutations and focus more on other aspects, such as improving detection accuracy, low cost, shortening detection time, etc. If the antibody used to detect SARS-CoV-2 biosensor antibodies is standardized, it will be conducive to the further commercialization of biosensors and greatly improve their application value. The ease of operation of using biosensors is conducive to reducing the workload of medical staff or professionals. Those who are tested can test by themselves, making it easier to prevent and control the epidemic.
3. ACE2-based biosensors for SARS-CoV-2 detection
A biosensor is an analytical tool which has three main parts, including the bio-recognition component, sensor and signal analysis system [66]. The bio-recognition component is the basis of biosensor selectivity which mainly uses biomolecules to identify the objects to be tested and cause signal changes. The commonly molecular recognition components including antibodies, nucleic acids and aptamers will convert the signal changes caused by recognition into measurable physical or chemical signals for perform operational analysis [67,68]. The basic composition and principle of biosensor was displayed in Fig. 4 .
Fig. 4.
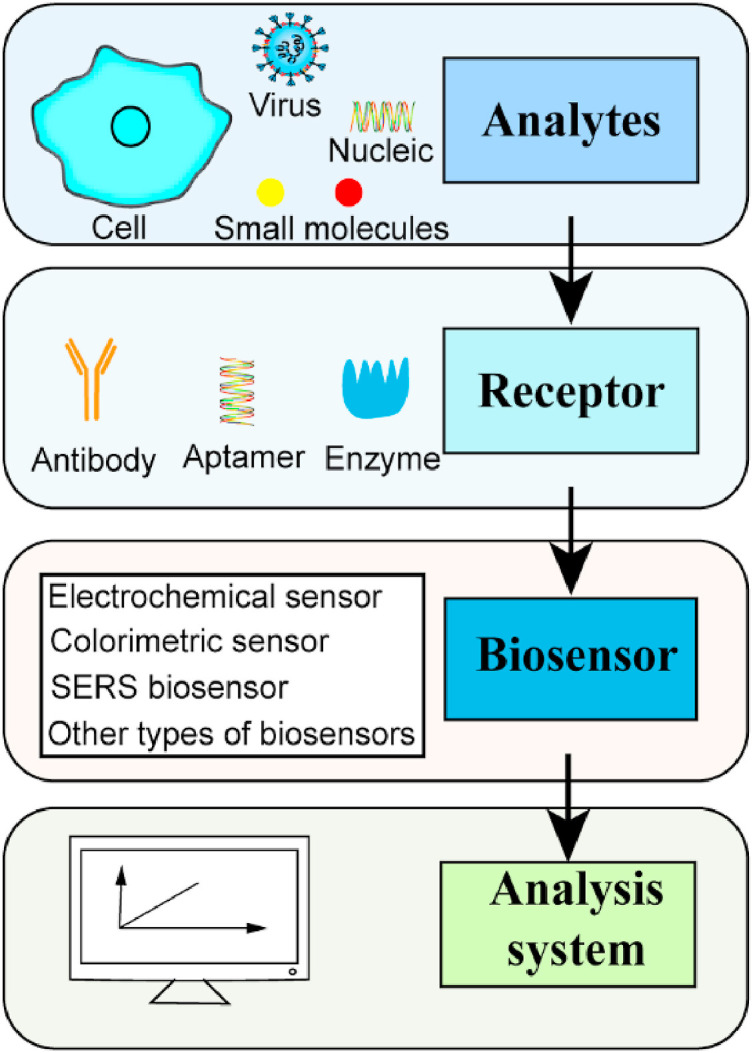
The basic composition and principle of biosensor.
Biosensors can be classified according to molecular recognition elements, such as enzyme-sensor, immunosensor, organall-sensor and so on. It can also be classified according to the type of detection signal, such as optical sensor, electrochemical sensor, etc. Due to the rapid development of biosensors and the emergence of various biosensors, the classification of biosensors should consider all aspects [69].
With its obvious advantages of simplicity, cheapness and low cost, biosensors are widely used in food safety testing, ecological environment monitoring, medical hygiene and other fields, and have full potential [70]. There are already many biosensors for the detection of SARS-CoV-2. The use of biosensors to detect viruses can not only meet the needs of poor areas and individuals for detection but also play a major role in the control of the epidemic [71]. There are many biosensors using viral RNA and antibodies. However, they have obvious shortcomings in the face of SARS-CoV-2 variants. At present, mutations of SARS-CoV-2 affect various characteristics of the virus, including infectivity, antigenicity and lead to a lower sensitivity of SARS-CoV-2 antigen tests. ACE2-based biosensors have unique advantages in detecting SARS-CoV-2, especially in the face of mutants (see Table 1 ). Next, we will briefly introduce various biosensors based on ACE2 detection of SARS-CoV-2.
Table 1.
Comparison of ACE2 - based biosensors.
| Type of Biosensor | Testing Sample | Assay Time (min) | LOD | Ref. |
|---|---|---|---|---|
| Electrochemical | saliva | 6.5 | 229 fg mL−1 | [80] |
| Electrochemical | saliva | 4 | 2.8 fg mL−1 | [81] |
| Electrochemical | saliva | 60 | 0.35 ag mL−1 | [82] |
| Colorimetric | NP/OP | 5 | 0.154 pg mL−1 | [83] |
| Colorimetricr | NP/OP | 30 | 4.98 ng mL−1 | [84] |
| Colorimetric | NP/OP | 5 | 100 pfu mL−1 | [85] |
| SERS | – | – | 300 nM | [89] |
| SERS | urines | 5 | 80 copies mL−1 | [90] |
| Nano | biofluids | 0.08 | 12.6 nM | [93] |
| FET | PBS | 20 | 165 copies mL−1 | [94] |
3.1. Electrochemical sensor for the detection of SARS-CoV-2
Electrochemical sensor is an important branch of sensors. Owing to its advantages such as good selectivity, high sensitivity, low cost, simple operation and easy miniaturization, it has become an important analytical tool and is widely used in the food industry, pharmaceutical industry, environmental analysis, clinical detection and other fields [72,73]. The interaction between the recognition system of the electrochemical sensor and the analyte is converted into a certain signal to the transduction system, which is presented in the form of the electrical signal to analyze the concentration of the analyte [74]. At present, the most used electrochemical sensor is a three-electrode system, including a counter electrode (CE), a reference electrode (RE) and a working electrode (WE). In general, the selection and fixation of receptors (aptamer, enzyme, or antibodies) fixed on the surface of WE are the core part of electrochemical sensor construction, which is of great help to improve selectivity and specificity.
With the development of electrochemical sensors, they have been applied to virus detection and showed good development potential [75]. Electrochemical sensors for detecting SARS-CoV-2 are under rapid development. Currently, electrochemical sensors based on SARS-CoV-2 genes [76,77] and antigen detection are available [78,79]. However, most of them have inherent defects in the face of viral variants and cannot detect the variant strains. The advantages of using ACE2 to detect viruses have been introduced previously.
De Lima, L.F. et al. designed a low-cost electrochemical sensor for rapid diagnosis of SARS-CoV-2 [80]. In this electrochemical sensor, a traditional three-electrode mode was adopted, and gold nanoparticles (AuNPs) were fixed to the electrode surface by crosslinking aldehyde group of glutaraldehyde with amine group of cysteamine on the graphite working electrode. Subsequently, ACE2 was added to a solution containing the pre-prepared reaction intermediates N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) to obtain EDC–NHS–ACE2. AuNPs-Cys and EDC–NHS–ACE2 were incubated at 37 °C for 30 min to generate ACE2-AuNPs-Cys. The specific binding between ACE2 and S-protein prevented the redox probe [Fe (CN)6 −3/−4] from entering the working electrode surface, which improved the sensitivity of square wave voltammetry (SWV) and enhanced the detection of SARS-CoV-2. The sensor can detect SARS-CoV-2 in 6.5 min, which not only cost less overall ($1.50) but also was much faster than traditional RT-PCR detection. The limit of detection (LOD) of the sensor in saliva samples was 229 fg mL−1. When clinical saliva was used for testing, the accuracy was 100% compared with RT-PCR results, indicating that the sensor has excellent performance.
Due to its low cost and short time, high-frequency testing can be widely used in various groups of people and provide opportunities for people in remote areas to increase detection opportunities. In addition, because ACE2 was used to detect the virus, the sensor was also responsive to the mutant strain, and analysis of the infectious SARS-CoV-2 B.1.1.7 UK variant showed a higher interaction between the S-protein and ACE2 than SARS-CoV-2 [80]. This sensor was a good example of the advantages of electrochemical sensors and had a great advantage in the face of mutant strains. Today, mutant strains are prevalent around the world and are still mutating. Therefore, this type of biosensor will have great potential in the future to play a role in the detection of SARS-CoV-2 variants.
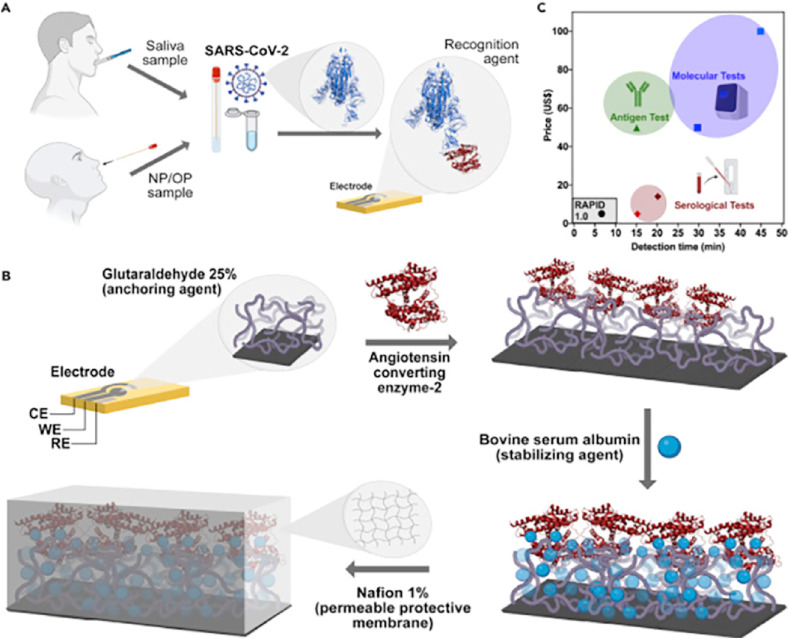
Marcelo D.T. Torres and his team prepared another electrochemical biosensor (Fig. 5 ) that used a 1% Nafion solution to create a protective polymer film that enhances the robustness and sensitivity of the biosensor. The biosensor was able to detect SARS-CoV-2 using 10 μL samples in 4 min by electrochemical impedance spectroscopy. The lowest concentration detected is 2.8 fg mL−1 and the cost is only $4.67. The sensitivity and specificity of the biosensor to nasopharyngeal swabs were 85.3% and 100%, respectively. The team also tested the highly contagious SARS-CoV-2 UK B.1.1.7 variant with good results [81].
Fig. 5.
(A) Diagnosis using saliva and nasopharyngeal/oropharyngeal (NP/OP) swab samples. (B) Schematic for the preparation of the electrodes. (C) Comparison of cost and testing time of various testing methods. Reproduced from Ref. [81] with permission from Elsevier, copyright 2021.
Nascimento et al. developed an ultra-sensitive magnetic assay using magnetic beads to capture and detect SARS-CoV-2 spike proteins in human saliva. Magnetic beads and gold nanoparticles were connected to ACE2 and mixed with saliva. The magnetic beads and gold nanoparticles were connected to SARS-CoV-2 through the affinity of ACE2-S-protein. SARS-CoV-2 was captured by magnetic force and then dropped onto a disposable electrochemical device containing eight screen-printed carbon electrodes. S-protein was detected by differential pulse voltammetry (DPV) with HCl as an auxiliary electrolyte. The sensor allowed simultaneous analysis of up to eight samples, the detection limit was 0.35 ag mL−1. In terms of efficiency, the specificity was 93.7%, which showed good similarity with RT-PCR. The results indicate that this method has simple, low-cost application potential for salivary based magnetic analysis and for immediate diagnosis of COVID-19. The advantages of this magnetic method are short analysis time (about 60 min), simple sample preparation, low cost, and no long and tedious steps [82].
At present, electrochemical sensors are modified with nanomaterials to improve their sensitivity. However, due to the outbreak and short time, the above sensors did not use new materials to modify the electrochemical sensor, but still showed a good response to virus detection. Although electrochemical sensors using ACE2 for detection are still very limited, they have great application potential in future detection of SARS-CoV-2 and need to be further developed and optimized.
3.2. Colorimetric sensor for the detection of SARS-CoV-2
It is attractive to use biosensors to enable direct and simple detection of entire virus particles or their corresponding surface antigen epitopes. This strategy could be used to develop diagnostic or screening tools for COVID-19. The most straightforward and simplest detection is the color response, which can be detected by the naked eye rather than through complicated procedures. SARS-CoV-2 can be detected directly and easily by a colorimetric method, greatly reducing the detection time. If ACE2 can be effectively fixed to cotton swab-like objects and SARS-CoV-2 can be detected through specific color reactions, it will bring a victory to the fight against SARS. This will directly reduce the cost of testing and operation difficulty, which is beneficial to epidemic prevention and control in poor and remote areas and areas lacking medical resources.
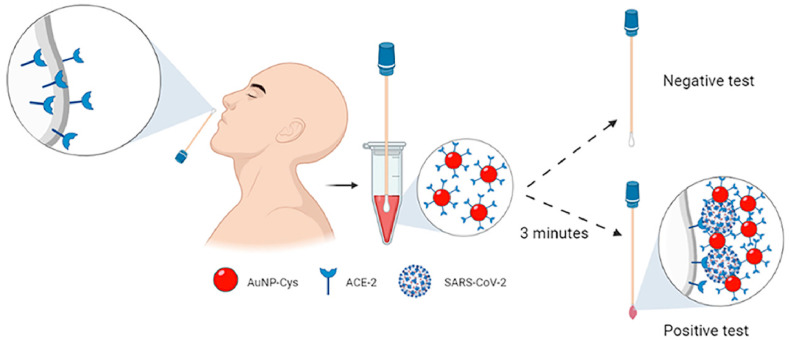
As shown in Fig. 6 , Ferreira, A.L. et al. developed a low-cost colorimetric biosensor for rapid detection. First, they used tetrachlorauric acid, AuNPs and cysteine (Cys) to synthesize AuNPs-Cys nanomaterial, and then AuNPs-Cys was added to the mixture containing ACE2 and EDC/NHS for incubation, forming the structure of AuNPs-Cys-ACE2. A cotton swab containing ACE2 was inserted into SARS-CoV-2 containing saliva and the AuNPs-Cys-ACE2 structure was added. If SARS-CoV-2 is present in saliva, a sandwich structure of (ACE2) -(SARS-CoV-2) -(AuNPs-Cys-ACE2) will be formed. When AuNPs were attached to the surface of the virus, the plasma effect was produced and the color changes from red to purple, resulting in a color change visible to the naked eye. In the presence of SARS-CoV-2, the sensor produced a color change from white to purple in the swab within 3 min. This colorimetric biosensor ensured selective and rapid identification of SARS-CoV-2 S-protein using ACE2, which is fixed directly to cotton. The detection limit of the sensor is 0.154 pg mL−1, and the cost is only 15 cents. The sensitivity, specificity and accuracy of 100 nasopharyngeal/oropharyngeal clinical samples were 96%, 84% and 90%, respectively [83].
Fig. 6.
A low-cost colorimetric biosensor. Reproduced from Ref. [83] with permission from American Chemical Society, copyright 2021.
Buyuksunetci, Y.T. et al. synthesized magnetic γ-Fe2O3 nanoparticles with peroxidase activity, under which 3,3′,5,5′-Tetramethylbenzidine (TMB) changed from colorless state to blue TMB (Ox) state. They found that the blue TMB (Ox) was converted to colorless TMB during the interaction of the S-protein with ACE2. They used this feature to design a colorimetric sensor. During the design process, the redox reaction was studied by spectroscopic and electrochemical techniques and the experimental parameters were optimized. Theoretically, the detection limit can be achieved 4.98 ng mL−1. Finally, real samples were detected and compared with RT-PCR, and the results were good [84]. Alhadrami, H.A.et al. fixed lactoferrin on cotton swabs as the trapping agent and used ACE2, which was decorated with orange polymer nanoparticles, as the labeled antibody. When SARS-CoV-2 was present, ACE2 bound to S-protein and aggregated on cotton swabs to produce color change. A colorimetric change from white to orange in the swab test area confirmed the positive result. The test time was only 5 min, and the visual detection limit was 100 pfu mL−1 [85].
For SARS-CoV-2 variants, Lee, J.H. et al. designed an ACE2-based biosensor to detect SARS-CoV-2 variants and neutralize antibodies. This biosensor can detect not only the α (500 pg mL−1) and β (10 ng mL−1) of SARS-CoV-2 variants and S1-protein of wild-type S1-protein (10 ng mL−1), but also distinguish SARS-CoV-2 variants by color, which is a major advance for other sensors. In addition, the biosensor has a binding mode for detecting SARS-CoV-2 and s blocking mode for detecting neutralizing antibodies [53]. Rapid and accurate measurement of SARS-CoV-2 neutral antibodies may be helpful to understanding human immunity to SARS-CoV-2, and to making an accurate judgment and evaluation of vaccine efficacy. Therefore, biosensors for detecting neutralizing antibodies are also very important at present [86]. Colorimetric sensors are the simplest and most promising method for large-scale application, but limited specific color reactions, storage costs, mass production and other problems still have a long way to go before large-scale application.
3.3. SERS sensor for the detection of SARS-CoV-2
Surface-enhanced Raman scattering (SERS) has developed rapidly and become a mature spectroscopic technique. It is more and more widely used in detection. In particular, the development of the design and manufacture of biosensors based on SERS has brought great development to biological and biomedical sensing applications. The chemical specificity and signal amplification ability of SERS is beneficial to the quantitative analysis of SARS-CoV-2 and improve the speed and accuracy of existing detection methods.
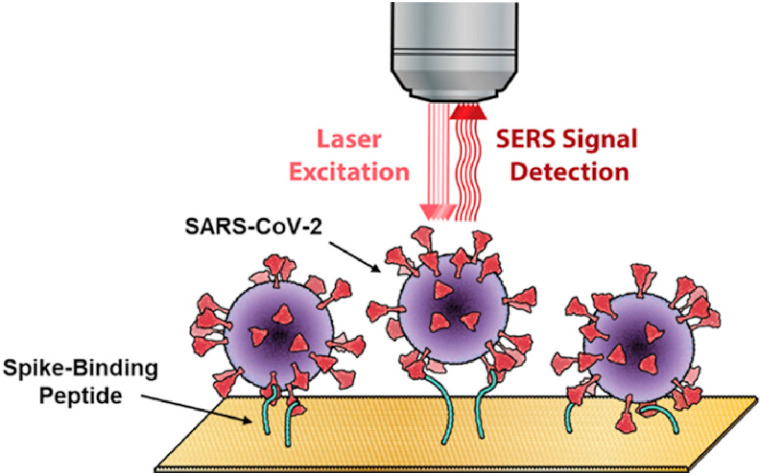
SERS can be used for highly sensitive, rapid and accurate detection of single biomolecules such as DNA and protein. However, other abundant biomolecules in the sample will generate interference signals, which is the main limiting factor for SERS application in detection [87,88]. In order to improve the selectivity in complex biological environment, Payne, T.D. et al. developed a SARS-CoV-2 SERS sensor based on using ACE2 as a viral protein capture probe as shown in Fig. 7 . A multivariable calibration model was used to identify and quantify the unique vibration characteristics of the surface modified by S-protein binding peptide [89]. The sensor showed a detection limit of 300 nM.
Fig. 7.
A SARS-CoV-2 SERS sensor based on ACE2. Reproduced from Ref. [89]with permission from American Chemical Society, copyright 2021.
A gold “virus trap” nanostructure functionalized by ACE2 was proposed as a highly sensitive biosensor for SERS, which can selectively capture and rapidly detect coronavirus expressing S-protein. ACE2 can specifically capture viruses within the 10 nm strong electromagnetic field enhancement region on the nanowire surface. This SERS sensor greatly enhanced the SERS signal by enriching viruses, and the detection limit could reach the level of a single virus. Through the SERS signal recognition standard was established which could quickly identify simulated SARS-CoV-2 from urines with a low viral load (80 copies mL−1) within 5 min [90].
Many other biosensors were prepared to detect SARS-CoV-2 genes using aptamers or directly detect S-protein based on SERS technology [91,92]. However, in the face of SARS-CoV-2 variants, ACE2-based biosensors reflect unique advantages, and the above two biosensors have directly or indirectly detected and verified variants. It proves the universality of detecting SARS-CoV-2 and variants based on ACE2.
3.4. Other types of biosensors for the detection of SARS-CoV-2
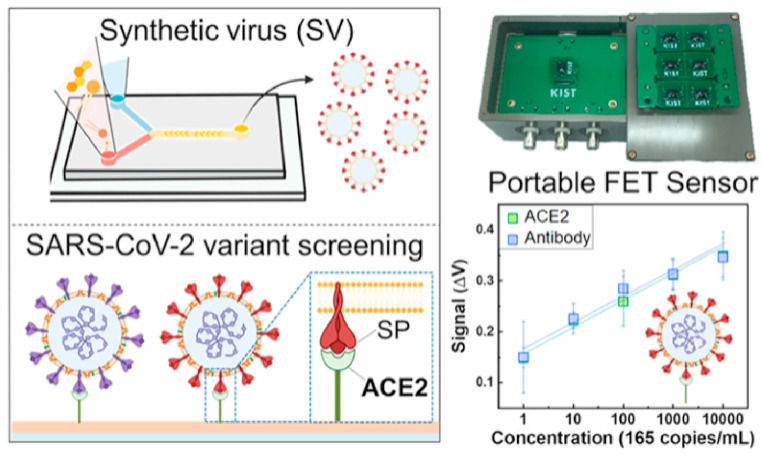
In addition to the types of sensors mentioned above, there are several other sensors not mentioned. Pinals, R.L.et al. constructed a sensor by connecting ACE2 to SWCNTs in a non-covalent manner, which could protect the surface lattice of SWCNT. The binding of the S-protein to ACE2-functionalized SWCNTs resulted in the quenching of the inherent near-infrared fluorescence of SWCNT. The sensor could achieve LOD of 12.6 nM and rapidly detect S-protein or virus-like SARS-CoV-2 virus in seconds [93]. In addition, Park, S.et al. introduced a highly sensitive, portable ACE2-based field-effect transistor (FET) biosensor as shown in Fig. 8 . They synthesized a SARS-CoV-2 analog virus and optimized the FET biosensor. The biosensor can be detected in 20 min with sensitivity comparable to molecular diagnostic tests (165 copies mL−1) [94].
Fig. 8.
Portable ACE2-based field-effect transistor (FET) biosensor. Reproduced from Ref. [94] with permission from American Chemical Society, copyright 2022.
ACE2 is used for ultra-fast and ultra-sensitive detection of SARS-CoV-2 as a novel biometric element in cell biosensor [95]. The results of various sensors indicate the potential of viral receptor ACE2 as a recognition element for screening variation in biosensors. ACE2-based viruses could be a useful tool for screening for upcoming SARS-CoV-2 variants.
4. Drug screening sensors based on ACE2
As mentioned above, biosensors can take advantage of the affinity between antigen and antibody for detection. If inhibitors or specific drugs are added to the solution under test, they interfere with the interaction between antigen and antibody, resulting in changes in physical and chemical properties that can be converted into measurable signals [96]. Today, many tailored biosensors are an integral part of the drug discovery platform. These tailored biosensors are designed for drug discovery and are limited by specific drug goals, which speed up the evaluation of potential drug candidates for efficacy and toxicity and enable drugs to be screened for challenging targets such as protein-protein interactions [97].
The development of a drug for the treatment of SARS-CoV-2 has become an urgent priority in the wake of COVID-19 in 2019. Although many vaccines have been developed and widely vaccinated, with the rapid variation of SARS-CoV-2, the effectiveness of the vaccine has decreased. Drug development for SARS-CoV-2 can be based on the inhibition of ACE2 or S-protein to develop inhibitory antibodies, short peptides, small molecules, etc. [[98], [99], [100], [101], [102], [103], [104]].
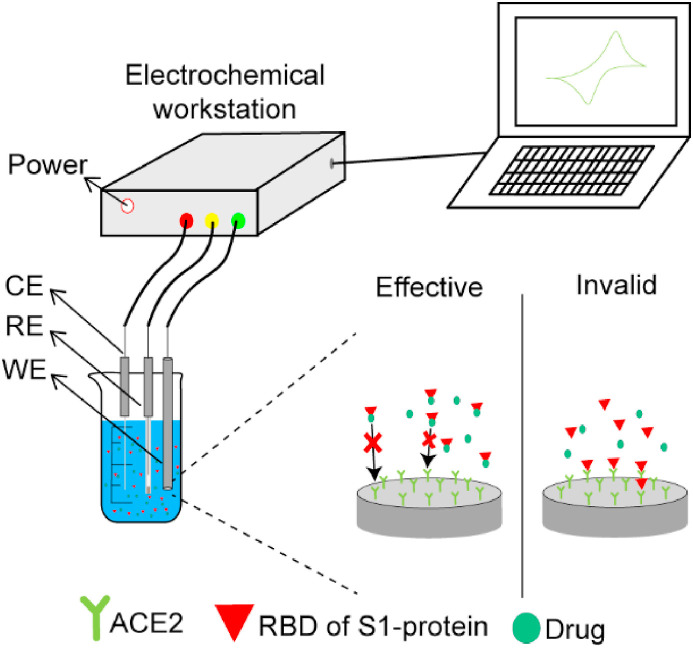
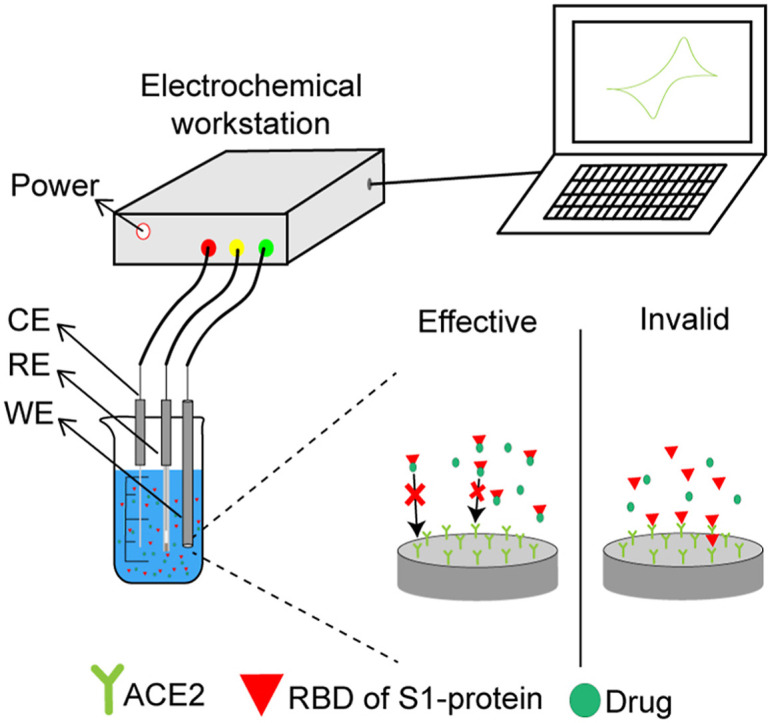
Now it's clear during the early stages of the SARS-CoV-2 infection cycle, the RBD of S1-protein binds to host cells by binding ACE2 receptor [105]. Thus, inhibition of the S1-ACE2 interaction may prevent the virus from entering the host cell, effectively limiting transmission of the virus in vivo. Strategies for screening and evaluating drugs against COVID-19 using electrochemical sensors are shown in Fig. 9 . ACE2 was fixed to the working electrode, the RBD of S1-protein and drug were added to the solution to be tested. If the drug has an effect, it will prevent ACE2 from binding to S-protein, then there is no signal; If the drug doesn't work, ACE2 binds to the S-protein and responds.
Fig. 9.
Strategies for screening and evaluating drugs against COVID-19 by electrochemical sensor.
Kiew, L. V. et al. constructed a biosensing platform based on electrochemical impedance spectroscopy (EIS), using recombinant palladium nanomembrane electrode and ACE2 coating as the core sensing elements to screen potential S1-ACE2 binding inhibitors. The platform can detect the interference of small analytes on S1-ACE2 binding at low concentrations and in small volumes, and determine some potential of inhibitors to S1-ACE2 binding within 21 min [106]. Roth, S. et al. developed a rapid and sensitive inhibitor screening tool using fluorescence and magnetic modulated biosensor (MMBS) which could screen small molecules that inhibit the interaction between S1 and ACE2. The assay based on MMBS has high sensitivity, minimal non-specific binding, and was much faster than the commonly used ELISA [107]. Han, Y.X. et al. designed the calculation of SARS-CoV-2 peptide inhibitors by changing the ACE2 structure [108].
Specific drugs for SARS-CoV-2 are being developed, and biosensors for drug screening can play an important role in reducing the time and efficacy validation. Because it is based on the interaction between ACE2 and S-protein, the use of biosensors can also verify whether the drug works on the SARS-CoV-2 variants. Such biosensors could be useful in the future as the focus shifts from vaccines to drugs.
5. Perspectives and conclusions
Since the outbreak, the impact of COVID-19 on human life and economic development has been enormous. With the further development of the epidemic, the number of infected people keeps expanding and SARS-CoV-2 keeps mutating in the transmission process, which makes the epidemic prevention and control more difficult and even leads to the decrease of the effectiveness of the vaccine. With vaccination and mutation of the virus, the severity of the disease has decreased, and it may even coexist with humans in the future. RT-PCR is a relatively good method for detecting SARS-CoV-2, but its use in resource-poor areas will be hampered by the need for specialized personnel and equipment. With the deepening of the research on SARS-CoV-2, the detection methods of SARS-CoV-2 are becoming more and more abundant, and the biosensor represented by SARS-CoV-2 is developing particularly rapidly.
It is not realistic for biosensors to completely replace RT-PCR. Biosensors are still in development and are some ways from being commercially available. However, it is not difficult to find from current studies that biosensors have the advantages of short time consumption, relatively low cost and simple operation. Biosensors for detecting SARS-CoV-2 based on virus receptor ACE2 introduced in this paper not only have the traditional advantages of biosensors but also have unmatched advantages in detecting SARS-CoV-2 variants. For several virus variants found so far, their affinity for ACE2 increases with variation. This means that using ACE2 as the receptor can prevent the loss of sensitivity caused by the mutation of the virus. In addition, ACE2 can also be used for drug screening. Effective use of biosensors could shorten the development cycle of anti-coronavirus drugs which is of great significance in the fight against the epidemic.
Due to the specific binding of ACE2 and SARS-CoV-2 S-protein, biosensors have a good selectivity. However, the accuracy is still lower compared with RT-PCR and stability has always been a headache for biosensors. In the future, the anti-interference and stability of biosensors should be addressed. Many biosensors combined with the new nanomaterials to improve sensitivity and anti-interference, but there is a balance to be struck because of the increased cost of using nanomaterials.
In this review, biosensors using ACE2 to detect SARS-CoV-2 were reviewed and their advantages were highlighted. In addition, the use of ACE2 for drug screening was briefly discussed. The ACE2-based biosensor has been developed to avoid the effects of SARS-CoV-2 variants. It is hoped that this review will be helpful to the development of the biosensor for the detection of SARS-CoV-2, and more biosensors targeting variants could be developed based on ACE2 to end the epidemic as soon as possible.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The financial supports from National Natural Science Foundation of China (Grant nos. 32170734 and 32170921) and the Young Talent of Lifting engineering for Science and Technology in Shandong of China (SDAST2021qt07) are acknowledged.
Data availability
Data will be made available on request.
References
- 1.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y.X., Xu Y.H., Wang X., Sun C., Guo Y., Qiu S., Ma K.W. Advances in SARS-CoV-2: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 2020;24(17):9208–9215. doi: 10.26355/eurrev_202009_22873. [DOI] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., Zhao X., Huang B.Y., Shi W.F., Lu R.J., Niu P.H., Zhan F.X., Ma X.J., Wang D.Y., Xu W.B., Wu G.Z., Gao G.G.F., Tan W.J., China Novel C. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee B., Thakur S.S. Diverse vaccine platforms safeguarding against SARS-CoV-2 and its variants. Expet Rev. Vaccine. 2022;21(1):47–67. doi: 10.1080/14760584.2022.1997601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., Singh K.P., Chaicumpa W., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020;33(4) doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R.J., Zhao X., Li J., Niu P.H., Yang B., Wu H.L., Wang W.L., Song H., Huang B.Y., Zhu N., Bi Y.H., Ma X.J., Zhan F.X., Wang L., Hu T., Zhou H., Hu Z.H., Zhou W.M., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J.Y., Xie Z.H., Ma J.M., Liu W.J., Wang D.Y., Xu W.B., Holmes E.C., Gao G.F., Wu G.Z., Chen W.J., Shi W.F., Tan W.J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., Nakane T., Zivanov J., Neufeldt C.J., Cerikan B., Lu J.M., Peukes J., Xiong X., Krausslich H.G., Scheres S.H.W., Bartenschlager R., Briggs J.A.G. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292 e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904 e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–+. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z.Y., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA, J. Am. Med. Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.F., Duan Q., Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao D.H., Yao F.F., Wang L.J., Zheng L., Gao Y.J., Ye J., Guo F., Zhao H., Gao R.B. A comparative study on the clinical features of coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin. Infect. Dis. 2020;71(15):756–761. doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) A review. JAMA, J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 21.Chan J.F.W., Yuan S.F., Kok K.H., To K.K.W., Chu H., Yang J., Xing F.F., Liu J.L., Yip C.C.Y., Poon R.W.S., Tsoi H.W., Lo S.K.F., Chan K.H., Poon V.K.M., Chan W.M., Ip J.D., Cai J.P., Cheng V.C.C., Chen H.L., Hui C.K.M., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate covid-19. N. Engl. J. Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Guo H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv Biomark Sci Technol. 2020;2:1–23. doi: 10.1016/j.abst.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asif M., Ajmal M., Ashraf G., Muhammad N., Aziz A., Iftikhar T., Wang J., Liu H. The role of biosensors in coronavirus disease-2019 outbreak. Curr Opin Electrochem. 2020;23:174–184. doi: 10.1016/j.coelec.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–+. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J.J., Yuan Q., Wang H.Y., Liu W., Liao X.J., Su Y.Y., Wang X., Yuan J., Li T.D., Li J.X., Qian S., Hong C.M., Wang F.X., Liu Y.X., Wang Z.Q., He Q., Li Z.Y., He B., Zhang T.Y., Fu Y., Ge S.X., Liu L., Zhang J., Xia N.S., Zhang Z. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.To K.K.W., Tsang O.T.Y., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C.Y., Cai J.P., Chan J.M.C., Chik T.S.H., Lau D.P.L., Choi C.Y.C., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C.K., Poon R.W.S., Luo C.T., Cheng V.C.C., Chan J.F.W., Hung I.F.N., Chen Z.W., Chen H.L., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim W.Y., Lan B.L., Ramakrishnan N. Emerging biosensors to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review. Biosensors. 2021;11(11) doi: 10.3390/bios11110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konka A., Lejawa M., Gazdzicka J., Bochenek A., Fronczek M., Strzelczyk J.K. RT-PCR detection of SARS-CoV-2 among individuals from the upper silesian region-analysis of 108,516 tests. Diagnostics. 2022;12(1) doi: 10.3390/diagnostics12010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrera-Avalos C., Luraschi R., Vallejos-Vidal E., Mella-Torres A., Hernandez F., Figueroa M., Rioseco C., Valdes D., Imarai M., Acuna-Castillo C., Reyes-Lopez F.E., Sandino A.M. The rapid antigen detection test for SARS-CoV-2 underestimates the identification of COVID-19 positive cases and compromises the diagnosis of the SARS-CoV-2 (K417N/T, E484K, and N501Y) variants. Front. Public Health. 2022;9 doi: 10.3389/fpubh.2021.780801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020;20(5):453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., Li Y., Wu B., Hou Y., Bao J., Deng X. A patient with COVID-19 presenting a false-negative reverse transcriptase polymerase chain reaction result. Korean J. Radiol. 2020;21(5):623–624. doi: 10.3348/kjr.2020.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aquino A., Paschoalin V.M.F., Tessaro L.L.G., Raymundo-Pereira P.A., Conte C.A. Updating the use of nano-biosensors as promising devices for the diagnosis of coronavirus family members: a systematic review. J. Pharmaceut. Biomed. Anal. 2022;211 doi: 10.1016/j.jpba.2022.114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng S., Zhou H., Ji C., Li L., Han N., Yang R., Shang J., Wu A. Conserved pattern and potential role of recurrent deletions in SARS-CoV-2 evolution. Microbiol. Spectr. 2022 doi: 10.1128/spectrum.02191-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme - cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 37.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 38.Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gheblawi M., Wang K.M., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Escobar A., Jimenez-Valero S., Galeote G., Jurado-Roman A., Garcia-Rodriguez J., Moreno R. The soluble catalytic ectodomain of ACE2 a biomarker of cardiac remodelling: new insights for heart failure and COVID19. Heart Fail. Rev. 2021;26(4):961–971. doi: 10.1007/s10741-020-10066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W.H., Moore M.J., Vasilieva N., Sui J.H., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell. Mol. Life Sci. 2004;61(21):2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F., Li W.H., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 44.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyerstedt S., Casaro E.B., Rangel E.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–+. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ke Z.L., Oton J.Q., Qu K., Cortese M., Zila V., McKeane L., Nakane T., Zivanov J., Neufeldt C.J., Cerikan B., Lu J.M., Peukes J., Xiong X.L., Krausslich H.G., Scheres S.H.W., Bartenschlager R., Briggs J.A.G. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–+. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Djomkam A.L.Z., Olwal C.O., Sala T.B., Paemka L. Commentary: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–+. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., Acton O.J., Jaconi S., Guarino B., Minola A., Zatta F., Sprugasci N., Bassi J., Peter A., De Marco A., Nix J.C., Mele F., Jovic S., Rodriguez B.F., Gupta S.V., Jin F., Piumatti G., Lo Presti G., Pellanda A.F., Biggiogero M., Tarkowski M., Pizzuto M.S., Cameroni E., Havenar-Daughton C., Smithey M., Hong D., Lepori V., Albanese E., Ceschi A., Bernasconi E., Elzi L., Ferrari P., Garzoni C., Riva A., Snell G., Sallusto F., Fink K., Virgin H.W., Lanzavecchia A., Corti D., Veesler D. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–+. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J.H., Lee Y., Lee S.K., Kim J., Lee C.S., Kim N.H., Kim H.G. Versatile role of ACE2-based biosensors for detection of SARS-CoV-2 variants and neutralizing antibodies. Biosens. Bioelectron. 2022;203 doi: 10.1016/j.bios.2022.114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemp S.A., Collier D.A., Datir R.P., Ferreira I., Gayed S., Jahun A., Hosmillo M., Rees-Spear C., Mlcochova P., Lumb I.U., Roberts D.J., Chandra A., Temperton N., Sharrocks K., Blane E., Modis Y., Leigh K.E., Briggs J.A.G., van Gils M.J., Smith K.G.C., Bradley J.R., Smith C., Doffinger R., Ceron-Gutierrez L., Barcenas-Morales G., Pollock D.D., Goldstein R.A., Smielewska A., Skittrall J.P., Gouliouris T., Goodfellow I.G., Gkrania-Klotsas E., Illingworth C.J.R., McCoy L.E., Gupta R.K., The CITIID-NIHR BioResource COVID-19 Collaboration, The COVID-19 Genomics UK (COG-UK) Consortium SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–+. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren S.Y., Wang W.B., Gao R.D., Zhou A.M. Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance. World Journal of Clinical Cases. 2022;10(1) doi: 10.12998/wjcc.v10.i1.1. 1-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., Anyaneji U.J., Bester P.A., Boni M.F., Chand M., Choga W.T., Colquhoun R., Davids M., Deforche K., Doolabh D., du Plessis L., Engelbrecht S., Everatt J., Giandhari J., Giovanetti M., Hardie D., Hill V., Hsiao N.Y., Iranzadeh A., Ismail A., Joseph C., Joseph R., Koopile L., Pond S.K.L., Kraemer M.U.G., Kuate-Lere L., Laguda-Akingba O., Lesetedi-Mafoko O., Lessells R.J., Lockman S., Lucaci A.G., Maharaj A., Mahlangu B., Maponga T., Mahlakwane K., Makatini Z., Marais G., Maruapula D., Masupu K., Matshaba M., Mayaphi S., Mbhele N., Mbulawa M.B., Mendes A., Mlisana K., Mnguni A., Mohale T., Moir M., Moruisi K., Mosepele M., Motsatsi G., Motswaledi M.S., Mphoyakgosi T., Msomi N., Mwangi P.N., Naidoo Y., Ntuli N., Nyaga M., Olubayo L., Pillay S., Radibe B., Ramphal Y., Ramphal U., San J.M.E., Scott L., Shapiro R., Singh L., Smith-Lawrence P., Stevens W., Strydom A., Subramoney K., Tebeila N., Tshiabuila D., Tsui J.S., van Wyk S., Weaver S., Wibmer C.K., Wilkinson E., Wolter N., Zarebski A.E., Zuze B., Goedhals D., Preiser W., Treurnicht F., Venter M., Williamson C., Pybus O.G., Bhiman J., Glass A., Martin D.P., Rambaut A., Gaseitsiwe S., von Gottberg A., de Oliveira T. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–+. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grabowski F., Kochanczyk M., Lipniacki T. The spread of SARS-CoV-2 variant Omicron with a doubling time of 2.0-3.3 Days can Be explained by immune evasion. Viruses-Basel. 2022;14(2) doi: 10.3390/v14020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulliam J.R.C., van Schalkwyk C., Govender N., von Gottberg A., Cohen C., Groome M.J., Dushoff J., Mlisana K., Moultrie H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science (New York, N.Y.) 2022;376(6593) doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H., Jean S., Wilson S.A., Lucyshyn J.M., McGrath S., Wilson R.K., Magrini V., Leber A.L. A deletion in the N gene of SARS-CoV-2 may reduce test sensitivity for detection of SARS-CoV-2. Diagn. Microbiol. Infect. Dis. 2022;102(4) doi: 10.1016/j.diagmicrobio.2021.115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., Huang B., Li D., Zhang R., Tian M., Fu L., Gao Y., Zhao X., Liu K., Qi J., Gao G.F., Wang P. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185(4):630–640. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramanathan M., Ferguson I.D., Miao W., Khavari P.A. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect. Dis. 2021;21(8):1070. doi: 10.1016/S1473-3099(21)00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang T.J., Yu P.Y., Chang Y.C., Liang K.H., Tso H.C., Ho M.R., Chen W.Y., Lin H.T., Wu H.C., Hsu S.D. Effect of SARS-CoV-2 B.1.1.7 mutations on spike protein structure and function. Nat. Struct. Mol. Biol. 2021;28(9):731–739. doi: 10.1038/s41594-021-00652-z. [DOI] [PubMed] [Google Scholar]
- 63.Ozono S., Zhang Y., Ode H., Sano K., Tan T.S., Imai K., Miyoshi K., Kishigami S., Ueno T., Iwatani Y., Suzuki T., Tokunaga K. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat. Commun. 2021;12(1):848. doi: 10.1038/s41467-021-21118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motozono C., Toyoda M., Zahradnik J., Saito A., Nasser H., Tan T.S., Ngare I., Kimura I., Uriu K., Kosugi Y., Yue Y., Shimizu R., Ito J., Torii S., Yonekawa A., Shimono N., Nagasaki Y., Minami R., Toya T., Sekiya N., Fukuhara T., Matsuura Y., Schreiber G., Ikeda T., Nakagawa S., Ueno T., Sato K., J G.P. Genotype Phenotype Japan, SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7):1124. doi: 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahradnik J., Marciano S., Shemesh M., Zoler E., Harari D., Chiaravalli J., Meyer B., Rudich Y., Li C., Marton I., Dym O., Elad N., Lewis M.G., Andersen H., Gagne M., Seder R.A., Douek D.C., Schreiber G. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat Microbiol. 2021;6(9):1188–1198. doi: 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- 66.Charoenkitamorn K., Yakoh A., Jampasa S., Chaiyo S., Chailapakul O. Electrochemical and optical biosensors for biological sensing applications. Sci. Asia. 2020;46(3):245–253. [Google Scholar]
- 67.Mohankumar P., Ajayan J., Mohanraj T., Yasodharan R. Recent developments in biosensors for healthcare and biomedical applications: a review. Measurement. 2021;167 [Google Scholar]
- 68.Bhalla N., Jolly P., Formisano N., Estrela P. Introduction to biosensors. Essays Biochem. 2016;60(1):1–8. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song S., Xu H., Fan C.H. Potential diagnostic applications of biosensors: current and future directions. Int. J. Nanomed. 2006;1(4):433–440. doi: 10.2147/nano.2006.1.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du X., Zhang Z., Zheng X., Zhang H., Dong D., Zhang Z., Liu M., Zhou J. An electrochemical biosensor for the detection of epithelial-mesenchymal transition. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-019-14037-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L., Li D., Ramadan S., Li Y., Klein N. Facile biosensors for rapid detection of COVID-19. Biosens. Bioelectron. 2020;170 doi: 10.1016/j.bios.2020.112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maduraiveeran G., Sasidharan M., Ganesan V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018;103:113–129. doi: 10.1016/j.bios.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Hughes G., Westmacott K., Honeychurch K.C., Crew A., Pemberton R.M., Hart J.P. Recent advances in the fabrication and application of screen-printed electrochemical (Bio)Sensors based on carbon materials for biomedical. Agri-Food and Environmental Analyses, Biosensors. 2016;6(4) doi: 10.3390/bios6040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thevenot D.R., Toth K., Durst R.A., Wilson G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001;16(1–2):121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 75.Khan M.Z.H., Hasan M.R., Hossain S.I., Ahommed M.S., Daizy M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: state of the art. Biosens. Bioelectron. 2020;166 doi: 10.1016/j.bios.2020.112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramirez-Chavarria R.G., Castillo-Villanueva E., Alvarez-Serna B.E., Carrillo-Reyes J., Ramirez-Zamora R.M., Buitron G., Alvarez-Icaza L. Loop-mediated isothermal amplification-based electrochemical sensor for detecting SARS-CoV-2 in wastewater samples. J. Environ. Chem. Eng. 2022;10(3) doi: 10.1016/j.jece.2022.107488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alafeef M., Dighe K., Moitra P., Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020;14(12):17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raziq A., Kidakova A., Boroznjak R., Reut J., Opik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yakoh A., Pimpitak U., Rengpipat S., Hirankarn N., Chailapakul O., Chaiyo S. Paper-based electrochemical biosensor for diagnosing COVID-19: detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021;176 doi: 10.1016/j.bios.2020.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Lima L.F., Ferreira A.L., Torres M.D.T., de Araujo W.R., de la Fuente-Nunez C. Minute-scale detection of SARS-CoV-2 using a low-cost biosensor composed of pencil graphite electrodes. P Natl Acad Sci USA. 2021;118(30) doi: 10.1073/pnas.2106724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torres M.D.T., de Araujo W.R., de Lima L.F., Ferreira A.L., de la Fuente-Nunez C. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point of care. Matter-Us. 2021;4(7):2403–2416. doi: 10.1016/j.matt.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nascimento E.D., Fonseca W.T., de Oliveira T.R., de Correia C.R.S.T.B., Faca V.M., de Morais B.P., Silvestrini V.C., Pott-Junior H., Teixeira F.R., Faria R.C. COVID-19 diagnosis by SARS-CoV-2 Spike protein detection in saliva using an ultrasensitive magneto-assay based on disposable electrochemical sensor. Sensor. Actuator. B Chem. 2022;353 doi: 10.1016/j.snb.2021.131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferreira A.L., de Lima L.F., Torres M.D.T., de Araujo W.R., de la Fuente-Nunez C. Low-cost optodiagnostic for minute-time scale detection of SARS-CoV-2. ACS Nano. 2021;15(11):17453–17462. doi: 10.1021/acsnano.1c03236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buyuksunetci Y.T., Citil B.E., Tapan U., Anik U. Development and application of a SARS-CoV-2 colorimetric biosensor based on the peroxidase-mimic activity of gamma-Fe2O3 nanoparticles. Microchim. Acta. 2021;188(10) doi: 10.1007/s00604-021-04989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alhadrami H.A., Suaifan G., Zourob M.M. A portable nanoprobe for rapid and sensitive detection of SARS-CoV-2 S1 protein. Biosens. Bioelectron. 2022;12(4) doi: 10.3390/bios12040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broccolo F., Fabris S., Ciccozzi M., Plebani M. Clinical Chemistry and Laboratory Medicine; 2022. A Rapid Semi-quantitative Test for Determination of SARS-CoV-2 Antibody Levels. [DOI] [PubMed] [Google Scholar]
- 87.Zong C., Xu M., Xu L.J., Wei T., Ma X., Zheng X.S., Hu R., Ren B. Surface-enhanced Raman spectroscopy for bioanalysis: reliability and challenges. Chem. Rev. 2018;118(10):4946–4980. doi: 10.1021/acs.chemrev.7b00668. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y., Zhen Y.R., Neumann O., Day J.K., Nordlander P., Halas N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance. Nat. Commun. 2014;5 doi: 10.1038/ncomms5424. [DOI] [PubMed] [Google Scholar]
- 89.Payne T.D., Klawa S.J., Jian T., Kim S.H., Papanikolas M.J., Freeman R., Schultz Z.D. Catching COVID: engineering peptide-modified surface-enhanced Raman spectroscopy sensors for SARS-CoV-2. ACS Sens. 2021;6(9):3436–3444. doi: 10.1021/acssensors.1c01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Y., Peng Y.S., Lin C.L., Long L., Hu J.Y., He J., Zeng H., Huang Z.R., Li Z.Y., Tanemura M., Shi J.L., Lombardi J.R., Luo X.Y. Human ACE2-functionalized gold "Virus-Trap" nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Lett. 2021;13(1) doi: 10.1007/s40820-021-00620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zavyalova E., Ambartsumyan O., Zhdanov G., Gribanyov D., Gushchin V., Tkachuk A., Rudakova E., Nikiforova M., Kuznetsova N., Popova L., Verdiev B., Alatyrev A., Burtseva E., Ignatieva A., Iliukhina A., Dolzhikova I., Arutyunyan A., Gambaryan A., Kukushkin V. SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials-Basel. 2021;11(6) doi: 10.3390/nano11061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang M.L., Li X.D., Pan J.L., Zhang Y.L., Zhang L., Wang C.G., Yan X., Liu X.M., Lu G.Y. Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosens. Bioelectron. 2021;190 doi: 10.1016/j.bios.2021.113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinals R.L., Ledesma F., Yang D.W., Navarro N., Jeong S., Pak J.E., Kuo L.L., Chuang Y.C., Cheng Y.W., Sun H.Y., Landry M.P. Rapid SARS-CoV-2 spike protein detection by carbon nanotube-based near-infrared nanosensors. Nano Lett. 2021;21(5):2272–2280. doi: 10.1021/acs.nanolett.1c00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S., Kim H., Woo K., Kim J.M., Jo H.J., Jeong Y., Lee K.H. SARS-CoV-2 variant screening using a virus-receptor-based electrical biosensor. Nano Lett. 2022;22:50–57. doi: 10.1021/acs.nanolett.1c03108. [DOI] [PubMed] [Google Scholar]
- 95.Mavrikou S., Tsekouras V., Hatziagapiou K., Tsalidou A., Bakakos P., Rovina N., Koutsoukou A., Michos A., Nikola O., Koniari E., Papaparaskevas J., Chrousos G.P., Kanaka-Gantenbein C., Kintzios S. Angiotensin-converting enzyme 2 (ACE2) as a novel biorecognition element in A cell-based biosensor for the ultra-rapid, ultra-sensitive detection of the SARS-CoV-2 S1 spike protein antigen. Chemosensors. 2021;9(12) [Google Scholar]
- 96.Wongkaew N., Simsek M., Griesche C., Baeumner A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: recent progress, applications, and future perspective. Chem. Rev. 2019;119(1):120–194. doi: 10.1021/acs.chemrev.8b00172. [DOI] [PubMed] [Google Scholar]
- 97.Scott D.E., Bayly A.R., Abell C., Skidmore J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016;15(8):533–550. doi: 10.1038/nrd.2016.29. [DOI] [PubMed] [Google Scholar]
- 98.Wang C.Y., Li W.T., Drabek D., Okba N.M.A., van R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan J.K., Xing S.H., Ding L.F., Wang Y.H., Gu C.J., Wu Y.L., Rong B.W., Li C., Wang S.Q., Chen K., He C.X., Zhu D.D., Yuan S.H., Qiu C.L., Zhao C., Nie L., Gao Z.Z., Jiao J.Y., Zhang X.Y., Wang X.X., Ying T.L., Wang H.B., Xie Y.H., Lu Y.A., Xu J.Q., Lan F. Human-IgG-neutralizing monoclonal antibodies block the SARS-CoV-2 infection. Cell Rep. 2020;32(3) doi: 10.1016/j.celrep.2020.107918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiwari V., Beer J.C., Sankaranarayanan N.V., Swanson-Mungerson M., Desai U.R. Discovering small-molecule therapeutics against SARS-CoV-2. Drug Discov. Today. 2020;25(8):1535–1544. doi: 10.1016/j.drudis.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25(4):668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olaleye O.A., Kaur M., Onyenaka C., Adebusuyi T. Discovery of clioquinol and analogues as novel inhibitors of severe acute respiratory syndrome coronavirus 2 infection, ACE2 and ACE2 - spike protein interaction in vitro. Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Magro G. COVID-19: review on latest available drugs and therapies against SARS- CoV-2. Coagulation and inflammation cross-talking. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou P., Yuan M., Song G., Beutler N., Shaabani N., Huang D., He W.-T., Zhu X., Callaghan S., Yong P., Anzanello F., Peng L., Ricketts J., Parren M., Garcia E., Rawlings S.A., Smith D.M., Nemazee D., Teijaro J.R., Rogers T.F., Wilson I.A., Burton D.R., Andrabi R. 2022. A Human Antibody Reveals a Conserved Site on Beta-Coronavirus Spike Proteins and Confers Protection against SARS-CoV-2 Infection, bioRxiv : the Preprint Server for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown E.E.F., Rezaei R., Jamieson T.R., Dave J., Martin N.T., Singaravelu R., Crupi M.J.F., Boulton S., Tucker S., Duong J., Poutou J., Pelin A., Yasavoli-Sharahi H., Taha Z., Arulanandam R., Surendran A., Ghahremani M., Austin B., Matar C., Diallo J.S., Bell J.C., Ilkow C.S., Azad T. Characterization of critical determinants of ACE2-SARS CoV-2 RBD interaction. Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiew L.-V., Chang C.-Y., Huang S.-Y., Wang P.-W., Heh C.-H., Liu C.-T., Cheng C.-H., Lu Y.-X., Chen Y.-C., Huang Y.-X., Chang S.-Y., Tsai H.-Y., Kung Y.-A., Huang P.-N., Hsu M.-H., Leo B.-F., Foo Y.-Y., Su C.-H., Hsu K.-C., Huang P.-H., Ng C.-J., Kamarulzaman A., Yuan C.-J., Shieh D.-B., Shih S.-R., Chung L.-Y., Chang C.-C. Development of flexible electrochemical impedance spectroscopy-based biosensing platform for rapid screening of SARS-CoV-2 inhibitors. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roth S., Danielli A. Rapid and sensitive inhibitor screening using magnetically modulated biosensors. Sensors. 2021;21(14) doi: 10.3390/s21144814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han Y.X., Kral P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14(4):5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.