Abstract
Introduction
The impact of nicotine, the addictive component of both traditional cigarettes and e-cigarettes, on many physiological processes remains poorly understood. To date, there have been few investigations into the impact of nicotine on the gut microbiome, and these studies utilized oral administration rather than inhalation. This study aimed to establish if inhaled nicotine alters the gut microbiome and the effect of sex as a biological variable.
Methods
Female (n = 8 air; n = 10 nicotine) and male (n = 10 air; n = 10 nicotine) C57BL6/J mice were exposed to air (control) or nicotine vapor (12 hour/day) for 13 weeks. A fecal sample was collected from each mouse at the time of sacrifice, and the gut microbiome was analyzed by 16S rRNA gene sequencing. QIIME2, PICRUSt, and STAMP were used to detect gut bacterial differences and functional metabolic pathways.
Results
Sex-specific differences were observed in both alpha and beta diversities in the absence of nicotine. While nicotine alters microbial community structure in both male and female mice as revealed by the beta diversity metric, nicotine significantly reduced alpha diversity only in female mice. A total of 42 bacterial taxa from phylum to species were found to be significantly different among the treatment groups. Finally, analysis for functional genes revealed significant differences in twelve metabolic pathways in female mice and ten in male mice exposed to nicotine compared to air controls.
Conclusions
Nicotine inhalation alters the gut microbiome and reduces bacterial diversity in a sex-specific manner, which may contribute to the overall adverse health impact of nicotine.
Implications
The gut microbiota plays a fundamental role in the well-being of the host, and traditional cigarette smoking has been shown to affect the gut microbiome. The effects of nicotine alone, however, remain largely uncharacterized. Our study demonstrates that nicotine inhalation alters the gut microbiome in a sex-specific manner, which may contribute to the adverse health consequences of inhaled nicotine. This study points to the importance of more detailed investigations into the influence of inhaled nicotine on the gut microbiota.
Introduction
Cigarette smoking is the leading cause of preventable disease and disability in the United States and contributes to about 1 in 5 deaths.1 As of 2018, 13.7% of all adults (15.6% of men and 12.0% of women) in the US were current cigarette smokers.2 Marketed as a safer alternative to cigarette smoking, in 2007, electronic cigarettes (e-cigs) became available in the United States.3 By 2014, e-cigs were the most popular tobacco product among young people; by 2020, 1 in 5 high school students and 3.6 million youth were using e-cigs.4 Nicotine is the addictive component of both tobacco cigarettes and e-cigs; however, its effects extend beyond the central nervous system. Nicotine is rapidly absorbed from the oral mucosa and respiratory tract,5 and it alone has been associated with increased risk of cardiovascular, respiratory, and gastrointestinal related disorders.6
To date, there have been few investigations into nicotine’s effects on the gut microbiome. The human gut contains trillions of microorganisms, including bacteria, archaea, fungi, eukaryotes, and viruses. Collectively, these microbes are referred to as the gut microbiota and their genes as the microbiome. Much of the gut microbiome research has focused on the bacteria kingdom.7 The activity of gut microflora is considered essential to human health: these organisms protect gut epithelial cells from injury, form biofilms facilitating nutrient exchange and host innate immunity,8 compete for resources with harmful organisms,9 and ferment waste products into nutritional substrates for gut epithelial cells and the host.10
The composition of the gut microbiota can be influenced by diet, illness, medications, sleep, and physical activity.11 Imbalance of gut microorganisms, termed dysbiosis, is associated with adverse effects on gut health, including disruptions in the intestinal barrier11 and inflammatory bowel disease (IBD)9 as well as neuropsychiatric disorders such as autism spectrum disorder, Alzheimer’s disease, depression, and Parkinson’s disease.12 In addition, studies have suggested a potential role of gut dysbiosis in the pathogenesis of substance use disorders.13
The effects of cigarette smoking on the gut microbiome are not clearly known, and limited rodent and human studies suggest that smoking changes gut microbiota composition. Generally, bacteria from the phyla Proteobacteria and Bacteroidetes and genera Clostridium, Bacteroides, and Prevotella are increased by smoking, whereas phyla Actinobacteria and Firmicutes and genera Bifidobacteria and Lactococcus are decreased.14 In addition, smoking has been shown to decrease gut microbial diversity.14 In a study of 758 men, however, Lee et al. found lower proportions of Proteobacteria in the guts of smokers compared to never smokers.15 More recently, a cross-sectional study in Bangladesh found high relative abundances of Erysipelotrichi-to-Catenibacterium lineage taxa and a greater presence of Alphaproteobacteria in smokers as compared to never-smokers.16 Compared to cigarette smoking, the effects of nicotine on the gut microbiome are even less understood. To date, only two studies in mice examined the effects of nicotine on the gut microbiota, and in both studies, nicotine was administered orally instead of through inhalational route as in human smokers or e-cig users.17,18
This study aims to determine if inhaled nicotine alters the gut microbiome and if an animal’s sex can influence those changes. Female and male mice were exposed to air (control) or nicotine vapor (daily, 12 hours on/12 hours off) for 13 weeks. Differences in alpha and beta diversity, microbial species, and metabolic pathway predictions were analyzed.
Methods
Animals
Adult male and female C57BL6/J mice (5 months of age) were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in a temperature- and humidity-controlled facility and kept under a 12-hour dark/light cycle. The mice were fed standard mouse chow (iOS Teklab Extruded Rodent Diet 2019S; Envigo, Indianapolis, Indiana) and water ad libitum. Our animal care facility is routinely tested for and is free of the following mouse pathogens: Mycoplasma pulmonis, Ectromelia, Epizootic diarrhea of infant mice (EDIM), Lymphocytic choriomeningitis virus (LCMV), Mouse hepatitis virus (MHV), Mouse parvovirus (MPV), Minute virus of mice (MVM), Pneumonia virus of mice (PVM), Reovirus type 3 (REO3), Sendai, and Theiler’s murine encephalomyelitis virus (TMEV). All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee.
Chronic Nicotine Inhalation Model
Male and female mice were randomly assigned to air or nicotine vapor treatments (females, n = 8 air and n = 10 nicotine; males, n = 10 air and n = 10 nicotine) and were housed five mice/cage. The chronic nicotine inhalation model was carried out as described by Oakes et al.19 Briefly, mice in the nicotine vapor group were housed in a nicotine inhalation chamber (La Jolla Alcohol Research, La Jolla, CA). Nicotine vapor was produced by bubbling air at a flow rate of 3 L/minute through a solution of pure nicotine (free base; Sigma-Aldrich, St. Louis, MO). This highly concentrated nicotine vapor was then diluted by adding 60 L/min of fresh air before distribution to the nicotine chamber at a final flow rate of 7–8 L/min. Mice were exposed to nicotine on a 12 hours on/12 hours off schedule for a total of 13 weeks, with the nicotine exposure (9:00 pm to 9:00 am) overlapping with the dark cycle (6:00 pm to 6:00 am). The exhaust from the nicotine inhalation chamber was filtered through an activated carbon drum canister (Carbtrol with 200 lbs of carbon) connected to the exhaust line of the research building, so no nicotine leaked into the animal housing room. Before commencement of nicotine vapor exposure, mice in the nicotine group were acclimated to the nicotine chamber for one week with air flow on only. Mice in the air-exposed group were housed in the same room, outside the nicotine inhalation chamber, and breathed room air.
Blood Sampling and ELISA
Serum cotinine levels were monitored biweekly. Blood for cotinine level analysis was obtained by submandibular vein puncture, and samples were collected within 15 minutes following the end of nicotine exposure (i.e., by 9:15 am on the day of collection). The whole blood was allowed to coagulate undisturbed at room temperature for 30 min before centrifugation at 1500 g for 10 min at 4°C. Cotinine levels in the serum (supernatant) were then measured by enzyme-linked immunosorbent assay (ELISA; CO096D-100, Calbiotech, El Cajon, CA) per the manufacturer’s instructions.
Fecal Sample Collection and DNA Isolation
Each animal was placed in a sterile cage and allowed to defecate. The stool sample was collected using a sterile pipette tip, placed into a sterile microcentrifuge tube, immediately frozen in liquid nitrogen, and then stored at –80°C. DNA was extracted using the QIAamp DNA Stool Mini Kits (Qiagen, Germantown, MD, USA) modified to include bead-beating and RNase A treatment. A negative control was set for checking any potential bacterial DNA existing in chemicals or involving during the DNA extraction process. Purity and quantity were determined using a Thermo Scientific NanoDrop spectrophotometer (ThermoFisher Scientific, Waltham, MA).
Microbial Community Analysis
Two amplification steps were performed to prepare a sequencing library using the AccuPrime Taq high fidelity DNA polymerase system (Invitrogen, Carlsbad, CA). A negative control with the control from DNA extraction and a positive control of Microbial Mock Community HM-276D (BEI Resources, Manassas, VA) were set during amplicon library preparation. 16S ribosomal DNA hypervariable region V4 was amplified using genomic DNA and the gene-specific primers with Illumina adaptors: forward 5ʹ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA 3ʹ, and reverse 5ʹ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT 3ʹ. PCR products were purified using AMPure XP beads, and the purified amplicon DNA was amplified using the primers with different molecular barcodes: forward 5ʹ AATGATACGGCGACCACCGAGATCTACAC [i5] TCGTCGGCAGCGTC 3ʹ; reverse 5ʹ CAAGCAGAAGAC GGCATACGAGAT [i7] GTCTCGTGGGCTCGG 3ʹ. The indexed amplicon libraries purified using AMPure XP beads and quantified using Quant-iT PicoGreen (Invitrogen) were normalized and pooled. The pooled library was quantified using KAPA Library Quantification Kit (Kapa Biosystems), diluted, and denatured according to Illumina’s sequencing library preparation guidelines. PhiX (10%) was added to the sequencing library as an internal control and increased 16S RNA amplicon library diversity. The paired-end sequencing was performed on an Illumina MiSeq (Illumina, San Diego, CA) using the 2 × 250 bp V2 sequencing kit.
Raw fastq files were processed using QIIME2 with the DADA2 plugin.20 Forward and reverse reads were truncated to a uniform length of 240 bp and 20 bp were trimmed off of the front of each read to remove the primer. Amplicon sequence variants (ASVs) identified by DADA2 were merged and any that fell out of the expected 250 bp–255 bp length were discarded. Contingency-based filtering was performed to remove any ASVs that appeared in only one sample and the consensus method was used to remove chimeric ASVs. A phylogenetic tree for diversity analysis was built by aligning remaining ASVs using mafft21 and fasttree.22 Taxonomic classification was performed using Greengenes v13.8.23 After primary data analysis, remaining reads were analyzed using QIIME2 (Quantitative Insights Into Microbial Ecology).24
Thirty-eight samples were included in the QIIME analysis with read counts ranging from 14 628 to 90 465, with an average read count per sample of 56 041. Alpha rarefaction was performed at a level of 14 600 reads to include all samples.
Prediction of Metabolic Profile
Potential microbial functions were identified from the 16S sequencing data. The raw data was formatted and imported into QIIME2. The dereplicated feature table and representative sequences were then used for closed-reference clustering against the Greengenes 13_5 97% OTUs reference database. The closed-reference OTU table was used as input into the PICRUSt25 pipeline, and the resulting PICRUSt metagenome data were further analyzed using STAMP (Statistical Analysis of Metagenomic Profiles).26 Several pathways were not classified at Level 1, which causes an error in STAMP, so the pathways were labeled at Level 2. From this, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways were compared between 1) air and nicotine, 2) male air and male nicotine, and 3) female air and female nicotine.
Statistical Analysis
Data were expressed as mean ± SEM, and, where appropriate, statistical significance was corrected for multiple comparisons. p < .05 was considered statistically significant. Using GraphPad Prism 8 or higher (GraphPad Software, San Diego, CA), differences in serum cotinine and weight change were determined by Student’s t-test. One-way ANOVA and Tukey’s multiple comparisons test were used to determine significant differences in the ratio of Firmicutes to Bacteroidetes (F/B) among the four groups. Statistically significant differences within alpha diversity were determined using Kruskal-Wallis non-parametric test (pairwise) within QIIME2 to tell which groups were significantly different. Principal coordinate analysis was used to graph beta diversity and permutational multivariate analysis of variance using distance matrices (PERMANOVA) was used to determine if the communities were significantly different. Significant differences in bacterial species were determined using Linear Discriminant Analysis (LDA) effect size (LefSe)27 which was also used to plot the data. Statistical differences in functional pathways were determined between groups using STAMP, which was also used to generate post hoc (Tukey-Kramer) plots for each KEGG pathway significantly different between treatment groups.
Results
Serum Cotinine Levels Differ Between Male and Female Mice
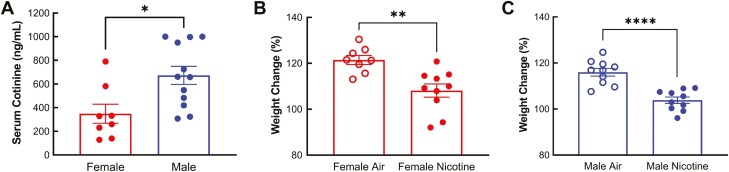
Cotinine is the primary nicotine metabolite, and biological sex affects the metabolism of nicotine and cotinine in humans and animals.28–31 We monitored serum cotinine levels biweekly and found that levels remained consistent within the same sex throughout the study. Cotinine levels in males and females obtained at different time points were thus combined and shown in Figure 1A. The average serum cotinine level in males (672.9 ± 76.36 ng/mL) was significantly higher than in females (348.9 ± 80.59 ng/mL, p = .0111, Figure 1A), which suggests that female mice metabolize nicotine faster than male mice.
Figure 1.
Nicotine metabolism and nicotine-induced weight changes in male and female mice. A. Serum cotinine (the primary nicotine metabolite) levels were measured biweekly by ELISA. Average serum cotinine levels in males were significantly higher than those in females. B and C. The mice were weighed before and after the 13-week nicotine exposure period. Weight gain over the 13 weeks was significantly lower in both females (B, n = 8 air and n = 10 nicotine) and males (C, n = 10/group) exposed to nicotine vapor. *p < .05, **p < .01, ****p < .0001, Student’s t-test.
Chronic Nicotine Inhalation Results in Reduced Weight Gain in Both Male and Female Mice
Prior to nicotine exposure, the bodyweight of mice assigned to receive nicotine vapor treatment was similar to mice assigned as air controls in both females (average of all females: 23.52 ± 0.27 g) and males (average of all males: 30.91 ± 0.48 g). However, bodyweight was significantly lower after chronic nicotine exposure in both females (Air = 28.36 ± 0.64 g, Nicotine vapor = 25.50 ± 0.53 g, p = .0030) and males (Air = 36.07 ± 1.09 g, Nicotine vapor = 31.92 ± 0.86 g, p = .0080). Calculated as the percentage of initial bodyweight, the percent weight increase over the 13 weeks was significantly lower in both females (Air = 121.5 ± 1.97 %, Nicotine vapor = 108.1 ± 2.89 %, p = .0023) and males (Air = 116.0 ± 1.67 %, Nicotine vapor = 103.8 ± 1.41 %, p < .0001) exposed to nicotine vapor (Figure 1B and C). These results indicate that nicotine causes a decrease in weight gain over time in both male and female mice.
Chronic Nicotine Inhalation Reduces Gut Microbiome Alpha Diversity in Female Mice
Measurements of alpha diversity are composite indices indicative of the diversity within a community. In this study, we examined two measures of alpha diversity, the Shannon refraction index, which reflects diversity of the observed taxa, and the Chao 1 index, which reflects the abundance. Two major findings were revealed by our analysis. First, in both alpha diversity indices, there were sex differences in the absence of nicotine, with female air controls displaying increased alpha diversity compared to male air controls (Figure 2A). Second, nicotine significantly reduced alpha diversity in female mice (both the Shannon refraction and the Chao 1 indices) but not in male mice (Figure 2A). In addition, the Shannon refraction index remained higher in female mice exposed to nicotine compared to either male group (Figure 2A). However, the Chao 1 index of female mice exposed to nicotine was not significantly different from male mice exposed to nicotine or male air controls (Figure 2A).
Figure 2.
Sex-dependent effects of nicotine on alpha and beta diversity indices of the gut microbiome. A. Alpha diversity of the gut microbiome as assessed by the Shannon index (left, which reflects the diversity of the observed taxa) and the Chao 1 index (right, which reflects the abundance of taxa). Statistical significance among groups was determined by QIIME2 which uses Kruskal-Wallis (pairwise). *p < .05, **p < .01, ***p < .001. B. Beta diversity is a measure of the dissimilarity between communities as clusters. Bray-Curtis beta diversity metric of gut microbial diversity was plotted using principal coordinate analysis. Permutational multivariate analysis of variance using distance matrices (PERMANOVA) was used to test differences. F-value = 10.8423, p-value = .001.
Chronic Nicotine Inhalation Alters Gut Microbiome Community Structure in Both Male and Female Mice
Beta diversity is a measure of the dissimilarity between communities as clusters. Here, we measured beta diversity based on the Bray-Curtis beta diversity metric. Similar to our findings for alpha diversity, we observed sex-specific clusters in the absence of nicotine (Figure 2B). However, different from the alpha diversity, nicotine clearly altered community structures in both male and female mice (Figure 2B). In addition, nicotine decreases the dissimilarity between the sexes, as indicated by the overlapping clusters between female nicotine and male nicotine groups (Figure 2B).
Chronic Nicotine Inhalation Leads to Changes in Microbial Species
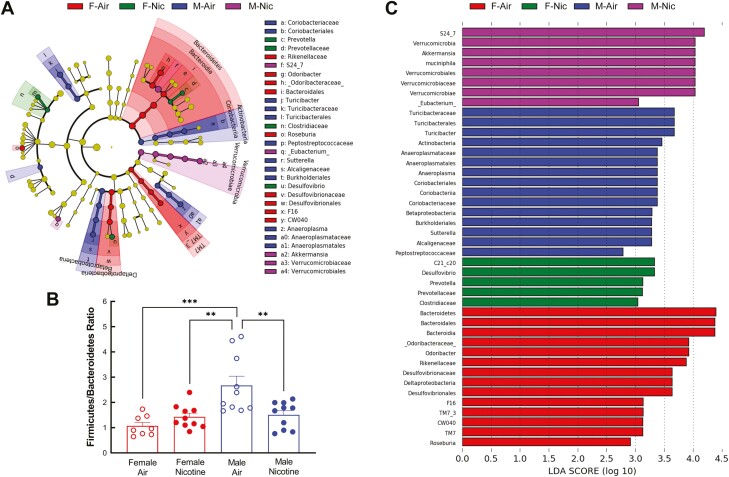
Differences in taxa among the four treatment groups are illustrated in the cladogram (Figure 3A). The Bacteroidetes phylum showed the most changes compared to the other phyla, and female mice breathing air had the greatest differences compared to other groups (Figure 3A).
Figure 3.
Effects of sex and nicotine on microbial taxa. Linear Discriminant Analysis (LDA) effect size (LefSe) analysis was used to determine microbial taxa with significant differences among the treatment groups. A. Taxa cladogram. Taxa with no significant differences are represented as yellow nodes. Taxa classification at the level of kingdom, phylum, class, order, family, genus, and species are shown from the inside to the outside of the cladogram. B. Firmicutes to Bacteroidetes (F/B) ratio. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. **p < .01, ***p < .001. C. Bar graph of the 42 taxa with significant differences. The length of the histogram reflects the LDA score, representing the degree of influence of the taxa between different groups. F-Air, female air; F-Nic, female nicotine; M-Air, male air; and M-Nic, male nicotine.
Details of the specific taxa are shown in Figure 3C. The taxa significantly different among the four groups include four phyla: Bacteroidetes, Actinobacteria, Verrucomicrobia, and TM7 (Saccharibacteria); six classes: Coriobacteriia, Bacteroidia, Betaproteobacteria, Deltaproteobacteria, TM7-3, and Verrucomicrobiae; eight orders: Coriobacteriales, Bacteroidales, Turicibacterales, Burkholderiales, Desulfovibrionales, Anaeroplasmatales, CW040, and Verrucomicrobiales; thirteen families: Coriobacteriaceae, Odoribacteraceae, Prevotellaceae, Rikenellaceae, S24-7, Turicibacteraceae, Clostridiaceae, Peptostreptococcaceae, Alcaligenaceae, Desulfovibrionaceae, Anaeroplasmataceae, F16, and Verrucomicrobiaceae; nine genera: Odoribacter, Turicibacter, Roseburia, Eubacterium, Sutterella, Desulfovibrio, Anaeroplasma, Prevotella, and Akkermansia; and two species: C21_c20 and muciniphila.
The most dominant gut bacterial phyla in both humans and rodents are Bacteroidetes and Firmicutes,32 and they are often expressed as a ratio. In the absence of nicotine, male mice had a higher F/B ratio than female mice (Figure 3B). Following nicotine exposure, F/B ratio was decreased in male mice, but not significantly altered in female mice (Figure 3B).
Chronic Nicotine Inhalation Alters Gut Microbiome Metabolic Profiles
Functional gene predictions of metabolic pathways were performed using STAMP. First, comparing the effect of nicotine versus air controls regardless of sex, we found significant differences in three characterized pathways: Circulatory System (corrected p-value = 6.493e-3), Environmental Adaption (corrected p-value = .042), and Transport and Catabolism (corrected p-value = .044). We then specifically compared male air to male nicotine and female air to female nicotine (Figure 4). Significant differences were observed in twelve metabolic pathways in female mice and ten in male mice exposed to nicotine compared to their corresponding air controls. Among these pathways, “Metabolism of Cofactors and Vitamins” was the only pathway significantly altered in both sexes, and interestingly, this pathway was downregulated in females but upregulated in males by nicotine.
Figure 4.
Chronic nicotine inhalation alters gut microbiome metabolic profiles. Functional gene predictions of metabolic pathways were performed, and the mean proportion (%) and the difference in mean proportions (%) of different metabolic pathways between female air and female nicotine (Top) and between male air and male nicotine (Bottom) were shown. These data were generated using PICRUST analysis, and STAMP was used to generate significant differences and the figures.
Discussion
This study examined the effects of chronic nicotine inhalation on the gut microbiome. For the first time, our study demonstrates that nicotine inhalation alone alters gut microbial community structure and decreases gut microbiome diversity in a sex-dependent manner. Furthermore, the gut microbiome metabolic profiles are altered in both male and female mice exposed to nicotine vapor.
Our finding that female mice have a lower serum cotinine level is in agreement with published studies in human subjects, further supporting the validity of our model. Published studies have demonstrated higher metabolism and faster clearance of nicotine and cotinine in women compared to men, in women who use oral contraceptives compared to non-users, and in women during pregnancy compared to postpartum.28,31 The effect appears to be dependent on levels of estrogen and progesterone. The activity of CYP2A6, the liver enzyme that metabolizes nicotine, has been shown to be induced by female sex hormones.33 In postmenopausal women, circulating estrogen may be regulated in part by the gut microbiome, which secretes β-glucuronidase, an enzyme that deconjugates estrogen.34
Cigarette smoking and nicotine exposure have been shown to impact energy homeostasis resulting in reduced body weight.35,36 Our observation that nicotine inhalational exposure results in reduced weight gain in both male and female mice is consistent with published studies. Despite a reduction in body weight, an increase in abdominal obesity is observed in humans who smoke 20 cigarettes/day compared to never-smokers.37 Interestingly, changes in body composition associated with cigarette smoking appear to be driven primarily by nicotine signaling.36 In addition, nicotine directly alters glucose homeostasis, peripheral insulin sensitivity, and pancreatic β cell function, contributing to type 2 diabetes associated with cigarette smoking.35 Whether nicotine-induced changes in the gut microbiome play a role in nicotine-associated metabolic dysfunction is unknown and warrants further investigation.
In this study, we first examined the gut microbiome diversity using both the alpha and beta metrics. Our data reveal several important findings. In terms of alpha diversity measured by both Shannon refraction and Chao 1 indices, which assesses microbial diversity within a community, we found that females have higher alpha diversity compared to males in the absence of nicotine (air control groups), and nicotine exposure reduces alpha diversity in females but not in males. In terms of beta diversity which measures microbial diversity between communities, female and male mice exhibit distinct microbial clusters in the absence of nicotine, and nicotine exposure significantly shifted microbial community structures in both sexes. While the overall influence of gut microbiota diversity on human health remains poorly understood,38 reduced diversity has been associated with disease states, such as recurrent Clostridium difficile infection,39 IBD,40 and liver cirrhosis.41 Decreased microbiome diversity has been observed with traditional cigarette smoking.14 Using first principles basic graph theory, Larsen and Claassen showed that increased diversity in systems like the gut microbiome leads to more efficient and resilient systems, proposing a mechanism by which decreased gut microbiota diversity adversely influences health status.42 Our results suggest that nicotine’s impact on the gut microbiome diversity could contribute to nicotine’s overall negative health effect.
Among our four experimental groups, a total of 42 bacterial taxa from phylum to species were found to be significantly different. The biological significance of these differences, however, is largely unknown. The most dominant phyla in the gut of both humans and rodents are Firmicutes and Bacteroidetes, and alterations in the F/B ratio have been associated with disease states.43 Our study shows that chronic nicotine inhalation significantly decreases the F/B ratio in male but not in female mice. At the genus level, nicotine decreases Odoribacter in female but not in male mice (Figure 3C), and decreased Odoribacter has been associated with high systemic blood pressure in rats.44 Interestingly, metabolic pathway prediction analysis revealed that nicotine alters genes associated with the circulatory system in female mice, but these changes were absent in male mice (Figure 4). Considering that women who smoke have a 25% greater relative risk of coronary heart disease compared to male smokers,45 sex-dependent effects of nicotine on the gut microbiome may contribute to sex differences in the cardiovascular risk of smoking.
Further analysis of metabolic pathways of functional genes in the gut microbiota revealed significant differences in twelve metabolic pathways in female mice and ten in male mice exposed to nicotine compared to air controls. Surprisingly, “Metabolism of Cofactors and Vitamins” is the only pathway significantly impacted by nicotine in both sexes. Much remains to be known about sex-specific differences in the gut microbiota,46 however, the stark difference in gut microbiota functional pathways between males and females highlights the importance of sex as a biological variable in nicotine/smoking/vaping-related gut microbiota research.
To date, there are very limited studies on the effects of nicotine on the gut microbiome, and two published studies in the literature investigated orally administered nicotine instead of inhaled nicotine. In one study, C57BL/6J mice were treated with nicotine-containing drinking water (60 mg/L) ad libitum for 13 weeks.17 Consistent with our study, sex-specific differences in gut microbiome composition (beta diversity) were observed both at baseline and following exposure to nicotine. In addition, nine bacteria families were altered by oral nicotine and three of which (Turicibacteraceae, Anaeroplasmataceae, and F16) were consistent with our findings following nicotine inhalation exposure. In contrast to our study, however, nicotine in drinking water perturbed the carbohydrate metabolism pathways of gut microbiota in a sex-specific manner and decreased body weight gain only in male but not in female mice.17 In a separate study, Wang et al. investigated the effect of nicotine administered by oral gavage (2 mg/kg daily) for 4 weeks in male C57BL/6 mice, and found that nicotine perturbed bacterial diversity and community composition of gut microbiota more pronouncedly in mice fed a high-fat diet than in mice fed standard chow.18 In mice fed standard chow, opposite to our findings with inhaled nicotine, oral nicotine actually increased the number of observed bacterial species (Chao 1). The above studies indicate that the effects of nicotine on gut microbiome depends on the route of nicotine exposure,47 and our nicotine inhalation model more closely mimics human smokers or e-cig users. Furthermore, our model allows the mice to receive nicotine vapor in their home cages without restraint or stress, which is known to alter metabolic and physiological processes.48
In summary, we provide novel evidence that inhaled nicotine alters gut microbiota community structure and decreases microbial diversity in a sex-dependent manner, which are coupled with alterations in the metabolic pathway profiles. The contribution of nicotine-induced changes in gut microbiome to nicotine’s overall negative health impact, especially sex-dependent health risks, warrants further investigation.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We would like to thank Dr. Eric Lazartigues (Professor of Pharmacology at Louisiana State University Health Sciences Center) for insightful discussion throughout the course of this study.
Contributor Information
Anna K Whitehead, Department of Physiology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Margaret C Meyers, Career Alternative Registered Nurse Education Program, School of Nursing, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Christopher M Taylor, Department of Microbiology, Immunology and Parasitology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Meng Luo, Department of Microbiology, Immunology and Parasitology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Scot E Dowd, Molecular Research LP, Shallowater, TX, USA.
Xinping Yue, Department of Physiology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Lauri O Byerley, Department of Physiology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Funding
This study was supported in part by research grants from the National Institute of Health (HL135635 to XY, 2P20GM103424 and UL1TR003096 to CMT, and F30HL160071 to AKW) and the American Heart Association (AHA Predoctoral Fellowship 829761 to AKW).
Declaration of Interests
None declared.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ.. Tobacco product use among adults—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2017;1394(1):5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- 5. National Center for Biotechnology Information. PubChem Annotation Record for NICOTINE, Source: Hazardous Substances Data Bank (HSDB). National Center for Biotechnology Information 2021; https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1107. Accessed February 22, 2021. [Google Scholar]
- 6. Mishra A, Chaturvedi P, Datta S, et al. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allaband C, McDonald D, Vazquez-Baeza Y, et al. Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol. 2019;17(2):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonnenburg JL, Angenent LT, Gordon JI.. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5(6):569–573. [DOI] [PubMed] [Google Scholar]
- 9. Zuo T, Ng SC.. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang B, Yao M, Lv L, Ling Z, Li L.. The human microbiota in health and disease. Engineering. 2017;3(1):71–82. [Google Scholar]
- 11. Lobionda S, Sittipo P, Kwon HY, Lee YK.. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019;7(8):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kho ZY, Lal SK.. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meckel KR, Kiraly DD.. A potential role for the gut microbiome in substance use disorders. Psychopharmacology (Berl). 2019;236(5):1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savin Z, Kivity S, Yonath H, Yehuda S.. Smoking and the intestinal microbiome. Arch Microbiol. 2018;200(5):677–684. [DOI] [PubMed] [Google Scholar]
- 15. Lee SH, Yun Y, Kim SJ, et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med 2018;7(9):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nolan-Kenney R, Wu F, Hu J, et al. The association between smoking and gut microbiome in bangladesh. Nicotine Tob Res. 2020;22(8):1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chi L, Mahbub R, Gao B, et al. Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem Res Toxicol. 2017;30(12):2110–2119. [DOI] [PubMed] [Google Scholar]
- 18. Wang R, Li S, Jin L, et al. Four-week administration of nicotine moderately impacts blood metabolic profile and gut microbiota in a diet-dependent manner. Biomed Pharmacother. 2019;115:108945. [DOI] [PubMed] [Google Scholar]
- 19. Oakes JM, Xu J, Morris TM, et al. Effects of chronic nicotine inhalation on systemic and pulmonary blood pressure and right ventricular remodeling in mice. Hypertension. 2020;75(5):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katoh K, Standley DM.. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price MN, Dehal PS, Arkin AP.. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6(3):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parks DH, Beiko RG.. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26(6):715–721. [DOI] [PubMed] [Google Scholar]
- 27. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benowitz NL, Lessov-Schlaggar CN, Swan GE, JacobP, 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen K, Kanamori K, Shin CS, Hamid A, Lutfy K.. The impact of sex on changes in plasma corticosterone and cotinine levels induced by nicotine in C57BL/6J Mice. Brain Sci. 2020;10(10):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen DR, Norris KJ, Thompson JA.. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12(6):725–731. [PubMed] [Google Scholar]
- 31. Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL.. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol. 2007;165(8):901–910. [DOI] [PubMed] [Google Scholar]
- 32. Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz NL, Hukkanen J, JacobP, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009(192):29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM.. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. [DOI] [PubMed] [Google Scholar]
- 35. Maddatu J, Anderson-Baucum E, Evans-Molina C.. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zoli M, Picciotto MR.. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14(11):1270–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yun JE, Kimm H, Choi YJ, Jee SH, Huh KB.. Smoking is associated with abdominal obesity, not overall obesity, in men with type 2 diabetes. J Prev Med Public Health. 2012;45(5):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reese AT, Dunn RR.. Drivers of microbiome biodiversity: a review of general rules, feces, and ignorance. mBio. 2018;9(4):e01294–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–438. [DOI] [PubMed] [Google Scholar]
- 40. Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. [DOI] [PubMed] [Google Scholar]
- 41. Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. [DOI] [PubMed] [Google Scholar]
- 42. Larsen OFA, Claassen E.. The mechanistic link between health and gut microbiota diversity. Sci Rep. 2018;8(1):2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clemente JC, Ursell LK, Parfrey LW, Knight R.. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toral M, Robles-Vera I, de la Visitacion N, et al. Critical role of the interaction gut microbiota—sympathetic nervous system in the regulation of blood pressure. Front Physiol. 2019;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huxley RR, Woodward M.. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305. [DOI] [PubMed] [Google Scholar]
- 46. Kim YS, Unno T, Kim BY, Park MS.. Sex differences in gut microbiota. World J Mens Health. 2020;38(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benowitz NL. Pharmacokinetic considerations in understanding nicotine dependence. Ciba Found Symp. 1990;152:186–200; discussion 200-189. [DOI] [PubMed] [Google Scholar]
- 48. De Palma G, Collins SM, Bercik P, Verdu EF.. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol. 2014;592(14):2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.