Abstract
Objective:
To report substance and polysubstance use at the time of delivery.
Study Design:
A cross-sectional study was performed on mothers consented for universal drug testing (99%) during hospital admission at six delivery hospitals in Cincinnati, Ohio. Mass spectrometry urinalysis detected positivity rates of 46 substances. Rates of positive drug tests for individual and common co-occurring substances measured were reported.
Results:
2 531 maternal samples were tested (88%) and 33% contained cotinine, 11.3% THC, 7.2% opioids, 3.8% cocaine, and 1.9% methamphetamines. Polysubstance use prevalence was as high as 15%. Among mothers testing positive for methadone or buprenorphine, 93% also tested positive for cotinine and 39% tested positive for a third substance in addition to cotinine.
Conclusions:
Substance use at delivery is more prevalent than previously reported. Many mothers testing positive for opioids also test positive for other substances which may increase overdose risk and exacerbate neonatal opioid withdrawal syndrome (NOWS).
INTRODUCTION
Substance use during pregnancy is associated with a myriad of adverse maternal and infant outcomes. Use of tobacco or other drugs during pregnancy is linked to low birthweight, increased rates of stillbirth, preterm birth, infant mortality, and other complications 1-6. Infants exposed prenatally to substances, particularly opioids, may have impaired neurodevelopment and frequently display long-term behavioral and cognitive deficits 1,7-10. Polysubstance use can exacerbate the negative effects of prenatal drug exposure on the neonate and is particularly problematic in combination with opioid use 11,12. As the opioid epidemic continues to worsen, neonatal opioid withdrawal syndrome (NOWS) remains a major public health burden and polysubstance exposed infants are often more difficult to treat 13-17.
Previous work at a single site in 2012-2013 established that universal maternal testing at delivery was superior to risk-based screening in detecting opioid exposure, where 20% of opioid-positive mothers identified through urine testing did not otherwise have screening risk factors 18. Late/third trimester exposure to opioids increases the risk of NOWS compared to early/first-second trimester exposure17, emphasizing the importance of late detection. Our previous studies have also found that universal testing was superior to self-report for cotinine exposure with cotinine-positive urine tests occurring at more than two times greater prevalence than estimated by self-report of smoking during pregnancy 19,20.
Pregnant women frequently avoid disclosing substance use, and maternal overdose is a leading cause of maternal mortality during the first year after delivery 21-24. Women may delay prenatal care out of fear of judgement and punitive actions, often making the delivery hospitalization the first opportunity to engage mothers in evidence-based treatment for a substance use disorder 21,22,25.
Our goal of this study was to use a cross-sectional study of mothers who consented to routine universal drug testing to assess the prevalence of maternal drug exposures, rates of polysubstance exposures, and combinations of exposures at the time of delivery in Hamilton County, Ohio, the urban county encompassing the city of Cincinnati.
SUBJECTS AND METHODS
Study design
We conducted a two-month cross-sectional study and descriptive analysis from August through September 2019 within Hamilton County, Ohio. In all six birth hospitals within Hamilton County, urine was collected at the time of delivery from consenting mothers. Mothers are consented to universal drug testing at delivery as part of the standard hospital admission process. This written consent for urine testing is described specifically on the labor and delivery consent form and mothers may opt out of the urine drug test portion of the consent. Universal drug testing has been adopted by Cincinnati area hospitals since 2013, based on data demonstrating the efficacy of universal testing compared to risk-based screening for identifying infants at risk for NOWS in an endemic area18. After 6 years, this practice has been accepted as a part of standard care by the community. Mothers provide written consent specific to obstetrics prior to any testing. Hospitals in the study included private, safety net, community, and academic centers. Median Medicaid coverage within the six sites was 26.2, 32.8, 33.1, 34.5, 38.7, 39.4, and 73.5%. A more detailed description of these hospitals can be found in our previous study and is summarized in Supplemental Table 1 20. All women who consented to an admission urine drug test during the study period were eligible, and there were no exclusion criteria. Mass spectrometry laboratory analysis was performed to measure late pregnancy exposures to 46 substances. The Cincinnati Children's Hospital IRB approved the study, including a waiver of consent with the condition that all urine samples were de-identified prior to testing or analysis.
Laboratory data
A sample was collected from each consenting woman, even in the case of multifetal deliveries. Laboratory technicians split the urine samples (1-2 mL) collected as part of the standard universal testing process. One sample was used as standard clinical care, and the second sample was de-identified using a numerical code. All study staff were blinded, permitting access only to the numerical code and birth hospital without identifiers. Samples were transported to Cincinnati Children’s Hospital Medical Center (CCHMC) for mass spectrometry analysis. The birth hospital was the only identifier included with the split samples, but the hospital information was anonymized in this study. We quantified positive exposure rates, exposure levels, and the type and frequency of polysubstance exposures in the population of each detected substance.
Maternal drug analysis by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
Forty-six drugs were quantified in maternal urine by LC-MS/MS in a CAP and CLIA certified laboratory. Supplemental Table 2 shows the lower limit of quantification (LLOQ) for 46 drugs analyzed by LC-MS/MS. Drugs levels greater than lower limit of quantification are reported as detected. The drugs and their conjugates were extracted from urine using a Hamilton Robotics MicroSTARlet paired with a Tecan SP IP8 automated SPE (solid phase extraction) processor. Urine samples were hydrolyzed with β-glucuronidase enzyme to obtain free (non-conjugated) drugs in the presence of the stable-labeled internal standards for each analyte, and solid phase extraction was then performed. The drugs were detected and quantified by LC-MS/MS with multiple reaction monitoring (MRM). All samples were analyzed with the LC20AD HPLC system (Shimadzu) coupled to the SCIEX QTRAP 4500 mass spectrometer (Sciex, Concord, Canada). Data acquisition on the mass spectrometer was controlled by Analyst 1.6.2 software (Sciex, Concord, Canada). Data processing and quantification were performed with MultiQuant software version 3.0 (Sciex, Concord, Canada).
Opioids
Any opioid was defined as a positive test for morphine, 6-monoacetylmorphine (6-MAM), codeine, fentanyl, norfentanyl, hydrocodone, hydromorphone, oxycodone, oxymorphone, meperidine, normeperidine, sufentanil, tramadol, methadone, EDDP, buprenorphine, norbuprenorphine, naloxone, or naltrexone. Short acting opioids were defined as a positive test for: morphine, 6-MAM, codeine, fentanyl, norfentanyl, hydrocodone, hydromorphone, oxycodone, oxymorphone, meperidine, normeperidine, tramadol, or sufentanil. Poppy seed consumption can cause detectable concentrations of morphine in the urine 26. While poppy seed consumption was not assessed in the women who provided urine samples, a more stringent morphine cutoff of 40 ng/mL was also assessed in case of detection from poppy seed consumption. 27
Statistics
We assessed associations between substances using Chi-square tests or Fisher’s exact test (for n < 5 28) and report associations as odds ratios with 95% confidence intervals (CI) calculated via the Baptista-Pike method. Odds ratios reflect unadjusted values, since samples were de-identified and unable to be linked to demographic data. P values were not adjusted for multiple comparisons because all comparisons were planned, reported, and relatively few in number 29,30. For exposure ranges and population estimates, skewed data (> 0.5 or < −0.05) were log transformed to calculate mean and 95% CI, which are reported as geomean and converted back into the original units. If the data remained skewed after log transformation, median and CI of the median were reported. GraphPad Prism 9.0 (GraphPad Software, La Jolla CA USA) was used for data analysis.
RESULTS
Of the 2,876 mothers who delivered infants in six Hamilton County delivery hospitals and consented to universal drug testing during August and September 2019, a total of 2 531 urine samples were tested. This represents a capture rate of 88% during our two-month study period (2 531/2 876 mothers)20. Across all centers in the study, 1% of women did not give consent for urine drug testing and 12% of urine samples were not tested due to medical circumstances (precipitous labor) or human error (shortage in testing supplies). The median age range for mothers across all 6 birth hospitals was 27-30 years. Medicaid insurance at hospitals ranged from 26.2 – 73.5%.With respect to race and ethnicity, 0.4 – 18.4% of mothers identified as Hispanic, 0.4 – 44.4% as Non-Hispanic Black, and 33.6 – 93.5% as Non-Hispanic White.
Of the 2 531 samples, 38% (n = 969) tested positive for at least one substance. Thirty-three percent were positive for nicotine. We found that approximately half (n = 396) of the nicotine-positive mothers also tested positive for another substance, with a 15.6% prevalence of testing positive for nicotine plus a second substance across the entire study. (Table 1). There was a significant positive association between nicotine exposure and the detection of another substance with 11.1 times greater odds of testing positive for another substance (47.1%; 398/844) compared to samples that did not test positive for cotinine, the biomarker for nicotine exposure (7.4%; 125/1687 (X2 = 543.0, 1; OR, 11.11; 95% CI 8.87 – 13.96; p < 0.0001) (Figure 1A). Exposure ranges are presented in Table 2 and prevalence of individual substances by maternity hospital is in Supplemental Table 3.
Table 1:
Prevalence of individual substances among all mothers (n = 2531)
| Prevalence of substances among all mothers | ||
|---|---|---|
| Substance | % | n / 2531 |
| Any substance | 38.0 | 963 |
| Nicotine | 33.0 | 836 |
| Nicotine and another substance | 15.6 | 396 |
| Tetrahydrocannabinol (THC) | 11.3 | 287 |
| Active THC | 8.1 | 204 |
| Opioids | 7.2 | 182 |
| Short acting opioids | 5.0 | 126 |
| Long acting opioids | 2.8 | 71 |
| Cocaine | 3.8 | 95 |
| Methamphetamine | 1.9 | 48 |
| Amphetamine | 0.4 | 10 |
| Barbiturates | 1.3 | 33 |
| Benzodiazepines | 0.6 | 16 |
| Phencyclidine (PCP) | 0 | 0 |
Figure 1:
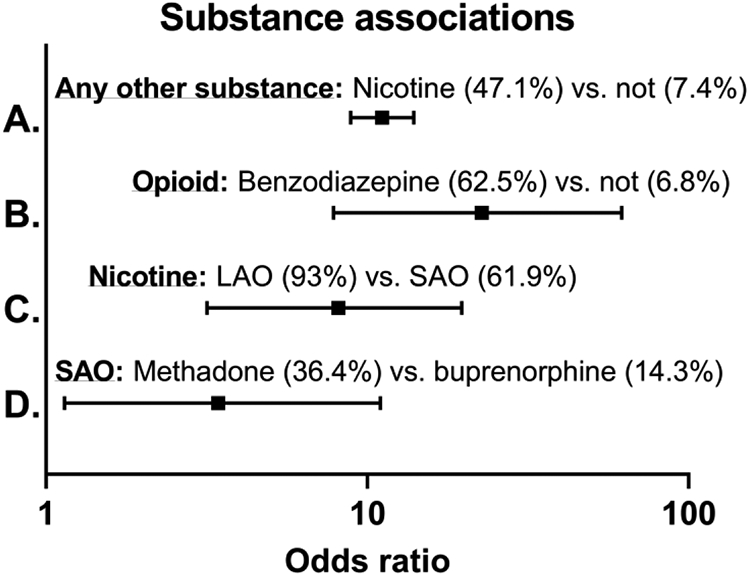
Odds ratios depicting for A.) The 11.1 times increased odds of testing positive for another substance if a mother tests positive for nicotine. B.) 22.7 times increased odds of testing positive for an opioid if a mother tests positive for a benzodiazepine C.) 8.1 times increased odds of testing positive for nicotine if a mother tests positive for a long-acting opioid (LAO) compared to a short-acting opioid (SAO) and D.) 3.4 times increased odds of testing positive for a SAO if a mother tests positive for methadone compared to buprenorphine.
Table 2:
The range and mean/median exposures for the most prevalent substances (n = 21 – 836)
| Exposure levels | ||||
|---|---|---|---|---|
| Substance | n | Geomean or median* (ng/mL) |
95% CI | Range (ng/mL) |
| Cotinine | 836 | 144.4 | 124.6 – 167.3 | 4.0 – 4157.0 |
| THCCOOH | 286 | 59.3 | 49.4 – 73.7 | 3.3 – 2547.9 |
| THCOH | 152 | 96.5 * | 79.4 – 121.0 | 20.2 – 3601.6 |
| Benzoylecgonine | 95 | 7.5 * | 6.2 – 10.8 | 2.0 – 2971.4 |
| Methamphetamine | 48 | 59.3 | 32.7 – 107.7 | 4.2 – 1904.6 |
| Morphine | 50 | 218.6 | 121.3 – 393.9 | 8.90 – 9222.6 |
| Fentanyl | 42 | 20.4 | 10.3 – 40.4 | 0.8 – 846 |
| Norfentanyl | 56 | 118.4 | 58.6 – 239.4 | 4.3 – 9265.1 |
| Methadone | 22 | 357.7 * | 307.2 – 420.7 | 3.8 – 491.1 |
| EDDP | 21 | 1078.6 * | 706.1 – 1565.1 | 48.7 – 4799.6 |
| Buprenorphine | 46 | 121.5 | 79.8 – 185.1 | 6.1 – 1419.9 |
| Norbuprenorphine | 49 | 607.1 * | 340.7 – 879.6 | 17.1 – 5105.9 |
A total of 11.3% (n = 287) of the samples were positive for tetrahydrocannabinol (THC) metabolites (11-hydroxy- tetrahydrocannabinol (THCOH) and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH)). To reduce the contribution of passive exposure, a threshold of 15 ng/mL THCCOOH was assessed, and 8.1% (n = 204) of mothers tested above this cutoff indicating active THC exposure.
Opioids were detected in 7.2% (n = 182) of the samples. Short acting opioids (including metabolites of heroin, fentanyl and prescription pain medications) were detected in 5% (n = 126) of all samples, and long-acting opioids (buprenorphine and methadone) were detected in 2.8% (n = 71) of all samples. These long-acting opioids are traditionally prescribed as medications for opioid use disorder (MOUD), but our study was unable to determine intended use. After excluding 14 samples testing positive for morphine below 40 ng/mL with potential poppy seed consumption 26,27, 6.6% of samples remained positive for opioids. No samples tested positive for sufentanil or naltrexone and all naloxone positive samples were also positive for another opioid.
Of the stimulants, 3.8% (n = 95) of samples tested positive for cocaine, 1.9% (n = 48) for methamphetamines, and an additional 0.4% (n = 10) were positive for amphetamines. Finally, 1.3% (n = 33) of samples were positive for barbiturates, 0.6% (n = 16) for benzodiazepines, and 0% for PCP, MDMA, MDA, MDEA, carisoprodol, and meprobamate. Benzodiazepine-positive samples tested positive for an opioid at 22 times increased odds compared to a benzodiazepine-negative samples (62.5%; 10/16 vs. 6.8%; 172/2 515) (Fisher’s exact test; OR = 22.7; 95% CI 7.8 – 61.9; p < 0.0001) (Figure 1B).
The prevalence of additional substances with cotinine and THC exposure are reported in Table 3. Nicotine was detected in 88.5% of THC positive samples, with THC being found in 30.4% of nicotine samples. Among nicotine positive samples, 9.4% also tested positive for cocaine, 15.6% for an opioid, and 5.6% for methamphetamine. Among THC positive samples, 11.8% also tested positive for cocaine, 10.1% for an opioid, and 6.6% for methamphetamine. Among all samples, nicotine and THC were the most common co-exposure combination, detected in 10% of all samples (n = 254/2 531). Nicotine and opioids were detected in 5.1% of all samples (n = 130/2 531). Furthermore, high nicotine exposure (> 100 ng/mL) combined with opioids was detected in 4.3% of all samples (n = 108/2531).
Table 3:
Prevalence of polysubstance exposure among mothers testing positive for cotinine (n = 836) and THC (n = 287)
| Polysubstance exposure within nicotine and THC | ||
|---|---|---|
| Primary substance (n) | Additional substances (n) | % Primary |
| Nicotine (836) | THC (254) | 30.4 |
| Nicotine (836) | Cocaine (79) | 9.4 |
| Nicotine (836) | Opioids (130) | 15.6 |
| Nicotine (836) | Methamphetamine (47) | 5.6 |
| THC (287) | Nicotine (254) | 88.5 |
| THC (287) | Cocaine (34) | 11.8 |
| THC (287) | Opioids (29) | 10.1 |
| THC (287) | Methamphetamine (19) | 6.6 |
Table 4 reports the prevalence of the most common substances present in addition to short- and long-acting opioids. Among the long vs. short-acting opioid positive samples, nicotine was detected in 93% (66/71) vs. 61.9% (78/126) (Fisher’s exact test; OR = 8.12; 95% CI 3.17 – 19.7; p < 0.0001) (Figure 1C), indicating that mothers positive for a long-acting opioid had an 8 times greater odds of testing positive for nicotine in comparison to mothers positive for a short-acting opioid. THC was detected in 16.7% of mothers positive for a short-acting opioid vs. 12.7% among those positive for a long-acting opioid (X2; p > 0.05), cocaine in 19.8% vs. 12.7% (X2; p > 0.05), methamphetamine in 17.5% vs. 14.1% (X2; p > 0.05), and benzodiazepines in 6.3% vs. 4.2% (Fisher’s exact test; p > 0.05). Of the samples that tested positive for long-acting opioids (buprenorphine or methadone) 14.1% also tested positive for fentanyl, 9.9% for morphine, and 8.5% had both fentanyl and methamphetamine. At least 3-5% of samples positive for short acting opioids were also positive for combinations of 2 or more non-nicotine substances. Among long-acting opioid samples tested, 39% were positive for nicotine plus another substance. Among mothers testing positive for long-acting opioids, methadone positive mothers were significantly more likely to test positive for a short acting opioid (36.4%; 8/14) in comparison to mothers testing positive for buprenorphine (14.3%; 7/42) (X2 = 4.44, 1; OR, 3.43; 95% CI 1.14 – 10.98; p > 0.05) (Figure 1D).
Table 4:
Prevalence of polysubstance exposure among mothers testing positive for short acting (SAO, n = 126) and long acting (LAO, n = 71) opioids
| Polysubstance exposure within opioid subtypes | ||
|---|---|---|
| Primary substance (n) | Additional substances (n) | % Primary |
| SAO (126) | Nicotine (78) | 61.9 |
| SAO (126) | Nicotine and another substance (49) | 38.9 |
| SAO (126) | THC (21) | 16.7 |
| SAO (126) | Cocaine (25) | 19.8 |
| SAO (126) | Methamphetamine (22) | 17.5 |
| SAO (126) | Methamphetamine and cocaine (16) | 12.7 |
| SAO (126) | Benzodiazepines (8) | 6.3 |
| SAO (126) | Methamphetamine and THC (6) | 4.8 |
| SAO (126) | THC and cocaine (5) | 4.0 |
| SAO (126) | THC, cocaine, and methamphetamine (4) | 3.2 |
| SAO (126) | Methamphetamine and benzodiazepines (2) | 1.6 |
| LAO (71) | Nicotine (66) | 93.0 |
| LAO (71) | Nicotine and another substance (28) | 39.4 |
| LAO (71) | Fentanyl (10) | 14.1 |
| LAO (71) | Methamphetamine (10) | 14.1 |
| LAO (71) | Cocaine (9) | 12.7 |
| LAO (71) | THC (9) | 12.7 |
| LAO (71) | Morphine (7) | 9.9 |
| LAO (71) | Fentanyl and methamphetamine (6) | 8.5 |
| LAO (71) | Hydromorphone (3) | 4.2 |
| LAO (71) | Benzodiazepines (3) | 4.2 |
| LAO (71) | Naloxone (3) | 4.2 |
| LAO (71) | Oxycodone (3) | 4.2 |
| LAO (71) | Tramadol (2) | 2.8 |
DISCUSSION
In this 2019 multicenter cross-sectional study in southwest Ohio, we found that 38% of pregnant women tested positive for at least one substance at the time of delivery. Many substances were detected with higher prevalence than previously reported in recent literature, and polysubstance use was pervasive. Nicotine was the most common exposure, and nearly half of the cotinine positive samples also tested positive for another substance. Mothers who tested positive for an opioid frequently tested positive for additional substances known to exacerbate NOWS 11,12.
The 8-11% THC exposure rate was higher than the 5.2% found by testing a smaller cohort in the same region via mass spectrometry in 2014-2015 19. This is also greater than the recent national self-reported rates of 5-7% during pregnancy and 2-4% in the third trimester 31-33. Increased use of cannabis during pregnancy may reflect that 70% of pregnant women view it as safe for use 33. This may also be specific to our region since having small quantities of cannabis has long been decriminalized in Ohio, with recent removal of penalties in the county of this study. Despite this safe perception and decriminalization, cannabis use during pregnancy is associated with an increased risk of neonatal complications and long-term cognitive or psychological problems, particularly with exposure to additional substances 2,5,6,34-36.
The opioid exposure rate of 6.6 – 7.2% found in our study was nearly double the 3.7% estimate based on billing records in the same region from 2014 – 2016 37,38. Mothers who tested positive for an opioid had prevalent polysubstance exposures with substances that may increase neonatal complications and NOWS severity, such as nicotine and benzodiazepines 1,2,16,17,3-6,11,13-15. Notably, women positive for benzodiazepines had 22 times increased odds of testing positive for an opioid, a combination that is strongly associated with increased NOWS severity 11.
The 3.8% prevalence of cocaine was higher than previously estimated 1.3-3.1% 39 and methamphetamine prevalence of 1.9% was much greater than 0.1% previously reported 19,40. However, methamphetamine prevalence was within the range of 0.7 – 4.8% of endemic areas 41,42. These increased exposure rates likely demonstrate underestimation by billing data or self-report, as well as a true increase in maternal substance use since exposure has been on the rise 43.
We found a higher prevalence of maternal polysubstance exposure than previously reported 43, which was especially high in cotinine positive samples. Nicotine and THC exposure (88% of THC-positive samples) was almost twice as high as previously reported, 6 and nearly all methadone or buprenorphine positive samples also contained cotinine (93%), consistent with the high prevalence of nicotine use disorder in the maternal population on MOUD 44,45.
In the samples containing methadone or buprenorphine, nearly 40% tested positive for a third substance in addition to nicotine. While we were not able to verify that the women positive for buprenorphine or methadone were receiving these medications as part of a MOUD program versus illicit use, the 10-14% positive rate for short acting opioids is consistent with the 9-15% positive rate previously found for women in a MOUD program46. Methadone was associated with a 3.4 times greater odds of testing positive for a short acting opioid compared to buprenorphine, which may be an important consideration for MOUD choice and requires further study.
Women with substance use disorders are less likely to receive prenatal care and are often distrustful of providers, especially regarding guidance for substance use 21,22,47. Furthermore, their infants are more likely to be admitted to the neonatal intensive care unit 48. Therefore, an unbiased and non-punitive patient-focused testing at the time of delivery could be an important opportunity to detect high risk infants, along with identifying women with substance use disorders and providing referrals for treatment. This is timely because maternal overdose is now a leading cause of maternal mortality, and having an unrecognized substance use disorder and polysubstance use are risk factors for maternal overdose 23,24. Confirmatory tests for polysubstance use at the time of delivery even after self-report of a single substance may enhance the effectiveness of evidence-based treatment programs to help prevent postpartum overdose and aide in caring for the exposed infant. Maternal overdose is detrimental for child mental health, as it is associated with an increase in childhood mental health service use 49. Nicotine use was strongly associated with additional substance use, but use of other substances was still observed in mothers who tested negative for nicotine. Overall, the very high rate of cotinine detection at delivery is alarming and more education about the risk of nicotine use during pregnancy is needed due to the tremendous public health burden of tobacco use on maternal, infant, and women’s health outcomes. Focus should include co-treatment of nicotine use disorder with opioid use disorder in the maternal population 50.
Limitations
A limitation of this study is that we did not screen for alcohol exposure so results underestimate the true prevalence of substance use at the time of delivery. The cross-sectional design prevented distinguishing exposure level and time since the last use of the substance. Across all centers in the study, 1% of mothers opted out of urine testing, and 12% of mothers were not tested due to precipitous delivery or human error. Because samples were de-identified to protect subject privacy, we were unable control for demographic factors, do not have information the 1% of mothers who opted out of universal testing, do not have information on prescribed drug use, and do not have self-report data matched to urine testing results. The site with the lowest capture rate had a three-day shortage in collection materials leading to less samples obtained. One site captured samples from 105% of women, due to a site-specific triage system with testing prior to admission that made it possible for a woman to be tested twice if she was not admitted on the first visit. However, these capture rate deviations most likely do not bias the study results as they occurred randomly. We were also unable to determine if any substances were prescribed, which would be a future focus for studying MOUD.
Supplementary Material
Acknowledgements
We would like to acknowledge financial support from Chiesi Farmaceutici S.p.A and Amgis Foundation, Inc.
Funding Information
In addition to financial support from Chiesi Farmaceutici S.p.A and Amgis Foundation, Inc., BLS is funded by the NIH/NIDA: K99DA049908.
Footnotes
Conflict of Interest
We disclose that financial support for this work was provided by a grant from Chiesi Farmaceutici and a gift from Amgis Foundation, Inc. However, neither organization participated in study design, data collection, data analysis and interpretation, manuscript preparation, or the decision for manuscript submission. Dr. Wexelblatt has been a member of the speaker bureau for Abbott Nutrition in the past two years.
REFERENCES
- 1.Forray A. Substance use during pregnancy. F1000Research 2016; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res 2012; 71: 215–219. [DOI] [PubMed] [Google Scholar]
- 3.Fried PA, O’Connell CM. A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis and caffeine on birth size and subsequent growth. Neurotoxicol Teratol 1987; 9: 79–85. [DOI] [PubMed] [Google Scholar]
- 4.Varner MW, Silver RM, Hogue CJR, Willinger M, Parker CB, Thorsten VR et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol 2014; 123: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C et al. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman-cowger VH, Oga E, Mark K. Cannabis and Tobacco Cigarettes during Pregnancy. 2019; : 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall ES, Mcallister JM, Wexelblatt SL. Developmental Disorders and Medical Complications Among Infants with Subclinical Intrauterine Opioid Exposures. Popul Health Manag 2019; 22: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daseking M, Petermann F, Tischler T, Waldmann HC. Smoking during pregnancy is a risk factor for executive function deficits in preschool-aged children. Geburtshilfe Frauenheilkd 2015; 75: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nygaard E, Slinning K, Moe V, Walhovd KB. Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child Neuropsychol 2017; 23: 159–187. [DOI] [PubMed] [Google Scholar]
- 10.Yeoh SL, Eastwood J, Wright IM, Morton R, Melhuish E, Ward M et al. Cognitive and Motor Outcomes of Children with Prenatal Opioid Exposure: A Systematic Review and Meta-analysis. JAMA Netw Open 2019; 2: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanlorenzo LA, Cooper WO, Dudley JA, Stratton S, Maalouf FI, Patrick SW. Increased Severity of Neonatal Abstinence Syndrome Associated With Concomitant Antenatal Opioid and Benzodiazepine Exposure. Hosp Pediatr 2019; 9: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachman EM, Newby PK, Vreeland J, Byun J, Bonganzi A, Bauchner H et al. The relationship between maternal opioid agonists and psychiatric medications on length of hospitalization for neonatal abstinence syndrome. J Addict Med 2011; 5: 293–299. [DOI] [PubMed] [Google Scholar]
- 13.Jansson LM, Di Pietro JA, Elko A, Williams EL, Milio L, Velez M. Pregnancies exposed to methadone, methadone and other illicit substances, and poly-drugs without methadone: A comparison of fetal neurobehaviors and infant outcomes. Drug Alcohol Depend 2012; 122: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltenbach K, Holbrook AM, Coyle MG, Heil SH, Salisbury AL, Stine SM et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction 2012; 107: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansson LM, Velez ML, McConnell K, Spencer N, Tuten M, Jones H et al. Maternal buprenorphine treatment and infant outcome. Drug Alcohol Depend 2017; 180: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend 2004; 75: 253–260. [DOI] [PubMed] [Google Scholar]
- 17.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ 2015; 350: h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wexelblatt SL, Ward LP, Torok K, Tisdale E, Meinzen-derr JK, Greenberg JM. Universal Maternal Drug Testing in a High-Prevalence Region of Prescription Opiate Abuse. J Pediatr 2015; 166: 582–586. [DOI] [PubMed] [Google Scholar]
- 19.Hall ES, Wexelblatt SL, Greenberg JM. Self-reported and laboratory evaluation of late pregnancy nicotine exposure and drugs of abuse. J Perinatol 2016; 36: 814–818. [DOI] [PubMed] [Google Scholar]
- 20.Hall ES, Mcallister JM, Kelly EA, Setchell KD, Megaraj V, Jimenez KL et al. Regional comparison of self-reported late pregnancy cigarette smoking to mass spectrometry analysis. J Perinatol 2021. doi: 10.1038/s41372-021-01045-2. [DOI] [PubMed] [Google Scholar]
- 21.Roberts SCM, Nuru-Jeter A. Women’s perspectives on screening for alcohol and drug use in prenatal care. Womens Heal Issues 2010; 20: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone R. Pregnant women and substance use: fear, stigma, and barriers to care. Heal Justice 2015; 3. doi: 10.1186/s40352-015-0015-5. [DOI] [Google Scholar]
- 23.Campbell J, Matoff-Stepp S, Velez ML, Cox HH, Laughon K. Pregnancy-Associated Deaths from Homicide, Suicide, and Drug Overdose: Review of Research and the Intersection with Intimate Partner Violence. J Women’s Heal 2021; 30: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. Am J Obstet Gynecol 2019; 221: 489.e1–489.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts SCM, Pies C. Complex calculations: How drug use during pregnancy becomes a barrier to prenatal care. Matern Child Health J 2011; 15: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith ML, Nichols DC, Underwood P, Fuller Z, Moser MA, LoDico C et al. Morphine and codeine concentrations in human urine following controlled poppy seeds administration of known opiate content. Forensic Sci Int 2014; 241: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrig TP, Moore C. The determination of morphine in urine and oral fluid following ingestion of poppy seeds. J Anal Toxicol 2003; 27: 449–452. [DOI] [PubMed] [Google Scholar]
- 28.Mchugh ML. The Chi-square test of independence Lessons in biostatistics. Biochem Medica 2013; 23: 143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman K. No adjustments are needed for multiple comparisons. Epidemiology 1990; Jan: 43–6. [PubMed] [Google Scholar]
- 30.Saville D. Multiple Comparison Procedures: The Practical Solution. Am Stat 1990; 44: 174–180. [Google Scholar]
- 31.Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported Medical and Nonmedical Cannabis Use Among PregnantWomen in the United States. JAMA - J Am Med Assoc 2019; 322: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crume TL, Juhl AL, Brooks-Russell A, Hall KE, Wymore E, Borgelt LM. Cannabis Use During the Perinatal Period in a State With Legalized Recreational and Medical Marijuana: The Association Between Maternal Characteristics, Breastfeeding Patterns, and Neonatal Outcomes. J Pediatr 2018; 197: 90–96. [DOI] [PubMed] [Google Scholar]
- 33.Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol 2015; 213: 201.e1–201.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S et al. Association between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA - J Am Med Assoc 2019; 322: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicol Teratol 2016; 58: 5–14. [DOI] [PubMed] [Google Scholar]
- 36.Roncero C, Valriberas-Herrero I, Mezzatesta-Gava M, Villegas JL, Aguilar L, Grau-López L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health 2020; 17: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall ES, Wexelblatt SL, Greenberg JM. Surveillance of Intrauterine Opioid Exposures. 2018; 21: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Percy Z, Brokamp C, Mcallister JM, Ryan P, Wexelblatt SL, Hall ES. Subclinical and Overt Newborn Opioid Exposure: Prevalence and First-Year Healthcare Utilization. J Pediatr 2017; 222: 52–58.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azadi A, Dildy GA. Universal screening for substance abuse at the time of parturition. Am J Obstet Gynecol 2008; 198: 30–32. [DOI] [PubMed] [Google Scholar]
- 40.John WS, Wu L-T. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend 2017; 180: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arria AM, Derauf C, LaGasse LL, Grant P, Shah R, Smith L et al. Methamphetamine and other substance use during pregnancy: Preliminary estimates from the infant development, environment, and lifestyle (IDEAL) study. Matern Child Health J 2006; 10: 293–302. [DOI] [PubMed] [Google Scholar]
- 42.Wright TE, Schuetter R, Tellei J, Sauvage L. Methamphetamines and pregnancy outcomes. J Addict Med 2015; 9: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yossuck P, Tacker DH. Drug Positivity Findings from a Universal Umbilical Cord Tissue Drug Analysis Program in Appalachia. J Appl Lab Med 2020; : 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chisolm MS, Fitzsimons H, Leoutsakos JMS, Acquavita SP, Heil SH, Wilson-Murphy M et al. A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. Nicotine Tob Res 2013; 15: 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones HE, Heil SH, O’Grady KE, Martin PR, Kaltenbach K, Coyle MG et al. Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant patient samples. Am J Drug Alcohol Abuse 2009; 35: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR et al. Buprenorphine treatment of opioid-dependent pregnant women: A comprehensive review. Addiction 2012; 107: 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maupin R, Lyman R, Fatsis J, Prystowiski E, Nguyen A, Wright C et al. Characteristics of women who deliver with no prenatal care. J Matern Neonatal Med 2004; 16: 45–50. [DOI] [PubMed] [Google Scholar]
- 48.Friedman SH, Heneghan A, Rosenthal M. Disposition and health outcomes among infants born to mothers with no prenatal care. Child Abus Negl 2009; 33: 116–122. [DOI] [PubMed] [Google Scholar]
- 49.Hulsey EG, Li Y, Hacker K, Williams K, Collins K, Dalton E. Potential Emerging Risks Among Children Following Parental Opioid-Related Overdose Death. JAMA - J Am Med Assoc 2020; 174: 503–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris CD, Garver-Apgar CE. Nicotine and Opioids: a Call for Co-treatment as the Standard of Care. J Behav Heal Serv Res 2020; 47: 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


