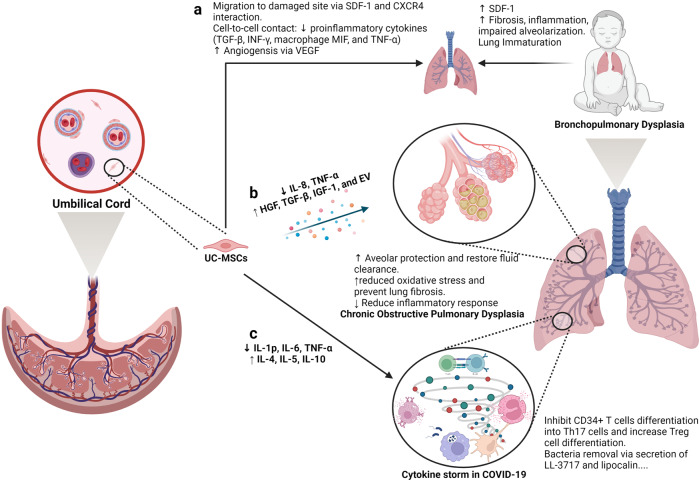
Abstract
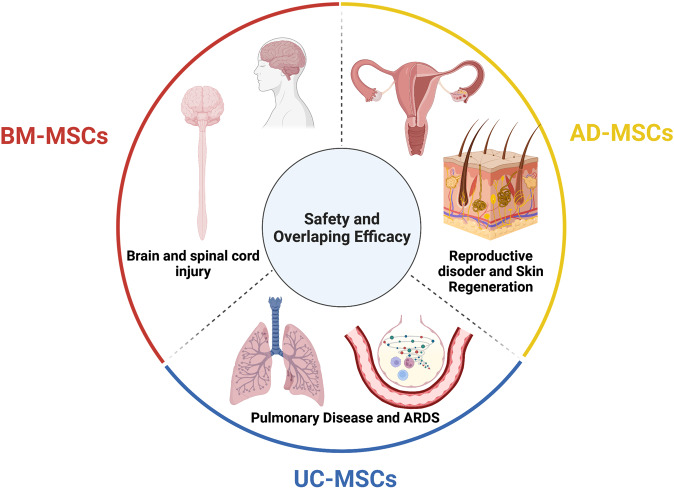
Recent advancements in stem cell technology open a new door for patients suffering from diseases and disorders that have yet to be treated. Stem cell-based therapy, including human pluripotent stem cells (hPSCs) and multipotent mesenchymal stem cells (MSCs), has recently emerged as a key player in regenerative medicine. hPSCs are defined as self-renewable cell types conferring the ability to differentiate into various cellular phenotypes of the human body, including three germ layers. MSCs are multipotent progenitor cells possessing self-renewal ability (limited in vitro) and differentiation potential into mesenchymal lineages, according to the International Society for Cell and Gene Therapy (ISCT). This review provides an update on recent clinical applications using either hPSCs or MSCs derived from bone marrow (BM), adipose tissue (AT), or the umbilical cord (UC) for the treatment of human diseases, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and cardiovascular conditions. Moreover, we discuss our own clinical trial experiences on targeted therapies using MSCs in a clinical setting, and we propose and discuss the MSC tissue origin concept and how MSC origin may contribute to the role of MSCs in downstream applications, with the ultimate objective of facilitating translational research in regenerative medicine into clinical applications. The mechanisms discussed here support the proposed hypothesis that BM-MSCs are potentially good candidates for brain and spinal cord injury treatment, AT-MSCs are potentially good candidates for reproductive disorder treatment and skin regeneration, and UC-MSCs are potentially good candidates for pulmonary disease and acute respiratory distress syndrome treatment.
Subject terms: Stem-cell research, Mesenchymal stem cells
Introduction
The successful approval of cancer immunotherapies in the US and mesenchymal stem cell (MSC)-based therapies in Europe have turned the wheel of regenerative medicine to become prominent treatment modalities.1–3 Cell-based therapy, especially stem cells, provides new hope for patients suffering from incurable diseases where treatment approaches focus on management of the disease not treat it. Stem cell-based therapy is an important branch of regenerative medicine with the ultimate goal of enhancing the body repair machinery via stimulation, modulation, and regulation of the endogenous stem cell population and/or replenishing the cell pool toward tissue homeostasis and regeneration.4 Since the stem cell definition was introduced with their unique properties of self-renewal and differentiation, they have been subjected to numerous basic research and clinical studies and are defined as potential therapeutic agents. As the main agenda of regenerative medicine is related to tissue regeneration and cellular replacement and to achieve these targets, different types of stem cells have been used, including human pluripotent stem cells (hPSCs), multipotent stem cells and progenitor cells.5 However, the emergence of private and unproven clinics that claim the effectiveness of stem cell therapy as “magic cells” has raised highly publicized concerns about the safety of stem cell therapy. The most notable case involved the injection of a cell population derived from fractionated lipoaspirate into the eyes of three patients diagnosed with macular degeneration, resulting in the loss of vision for these patients.6 Thus, as regenerative medicine continues to progress and evolve and to clear the myth of the “magic” cells, this review provides a brief overview of stem cell-based therapy for the treatment of human diseases.
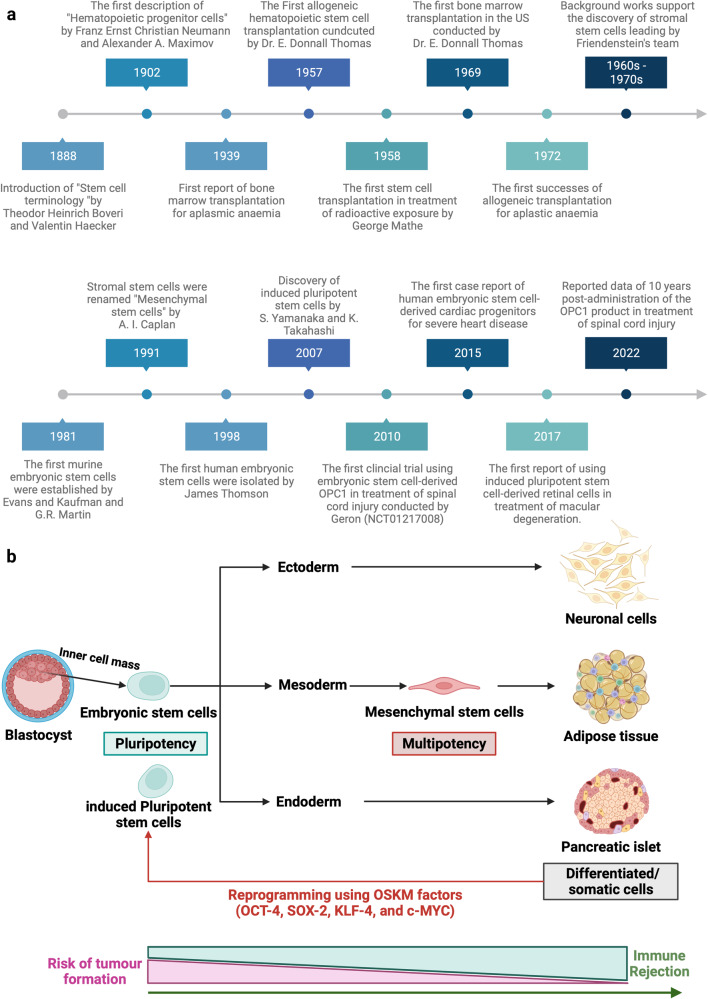
Stem cell therapy is a novel therapeutic approach that utilizes the unique properties of stem cells, including self-renewal and differentiation, to regenerate damaged cells and tissues in the human body or replace these cells with new, healthy and fully functional cells by delivering exogenous cells into a patient.7 Stem cells for cell-based therapy can be of (1) autologous, also known as self-to-self therapy, an approach using the patient’s own cells, and (2) allogeneic sources, which use cells from a healthy donor for the treatment.8 The term “stem cell” were first used by the eminent German biologist Ernst Haeckel to describe the properties of fertilized egg to give rise to all cells of the organism in 1868.9 The history of stem cell therapy started in 1888, when the definition of stem cell was first coined by two German zoologists Theodor Heinrich Boveri and Valentin Haecker,9 who set out to identify the distinct cell population in the embryo capable of differentiating to more specialized cells (Fig. 1a). In 1902, studies carried out by the histologist Franz Ernst Christian Neumann, who was working on bone marrow research, and Alexander Alexandrowitsch Maximov demonstrated the presence of common progenitor cells that give rise to mature blood cells, a process also known as haematopoiesis.10 From this study, Maximov proposed the concept of polyblasts, which later were named stem cells based on their proliferation and differentiation by Ernst Haeckel.11 Maximov described a hematopoietic population presented in the bone marrow. In 1939, the first case report described the transplantation of human bone marrow for a patient diagnosed with aplastic anemia. Twenty years later, in 1958, the first stem cell transplantation was performed by the French oncologist George Mathe to treat six nuclear researchers who were accidentally exposed to radioactive substances using bone marrow transplantation.12 Another study by George Mathe in 1963 shed light on the scientific community, as he successfully conducted bone marrow transplantation in a patient with leukemia. The first allogeneic hematopoietic stem cell transplantation (HSCT) was pioneered by Dr. E. Donnall Thomas in 1957.13 In this initial study, all six patients died, and only two patients showed evidence of transient engraftment due to the unknown quantities and potential hazards of bone marrow transplantation at that time. In 1969, Dr. E. Donnall Thomas conducted the first bone marrow transplantation in the US, although the success of the allogeneic treatment remained exclusive. In 1972, the year marked the discovery of cyclosporine (the immune suppressive drug),14 the first successes of allogeneic transplantation for aplastic anemia and acute myeloid leukemia were reported in a 16-year-old girl.15 From the 1960s to the 1970s, series of works conducted by Friendenstein and coworkers on bone marrow aspirates demonstrated the relationship between osteogenic differentiation and a minor subpopulation of cells derived from bone marrow.16 These cells were later proven to be distinguishable from the hematopoietic population and to be able to proliferate rapidly as adherent cells in tissue culture vessels. Another important breakthrough from Friendenstein’s team was the discovery that these cells could form the colony-forming unit when bone marrow was seeded as suspension culture following by differentiation into osteoblasts, adipocytes, and chondrocytes, suggesting that these cells confer the ability to proliferate and differentiate into different cell types.17 In 1991, combined with the discovery of human embryonic stem cells (hESCs), which will be discussed in the next section, the term “mesenchymal stem cells”, previously known as stromal stem cells or “osteogenic” stem cells, was first coined in Caplan and widely used to date.18 Starting with bone marrow transplantation 60 years ago, the journey of stem cell therapy has developed throughout the years to become a novel therapeutic agent of regenerative medicine to treat numerous incurable diseases, which will be reviewed and discussed in this review, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and cardiovascular conditions).
Fig. 1.
Stem cell-based therapy: the history and cell source. a The timeline of major discoveries and advances in basic research and clinical applications of stem cell-based therapy. The term “stem cells” was first described in 1888, setting the first milestone in regenerative medicine. The hematopoietic progenitor cells were first discovered in 1902. In 1939, the first bone marrow transplantation was conducted in the treatment of aplasmic anemia. Since then, the translation of basic research to preclinical studies to clinical trials has driven the development of stem cell-based therapy by many discoveries and milestones. The isolations of “mesenchymal stem cells” in 1991 following by the discovery of human pluripotent stem cells have recently contributed to the progress of stem cell-based therapy in the treatment of human diseases. b Schematic of the different cell sources that can be used in stem cell-based therapy. (1) Human pluripotent stem cells, including embryonic stem cells (derived from inner cell mass of blastocyst) and induced pluripotent stem cells confer the ability to proliferate indefinitely in vitro and differentiate into numerous cell types of the human body, including three germ layers. (2) Mesenchymal stem cells are multipotent stem cells derived from mesoderm possessing self-renewal ability (limited in vitro) and differentiation potential into mesenchymal lineages. The differentiated/somatic cells can be reprogrammed back to the pluripotent stage using OSKM factors to generate induced pluripotent stem cells. It is important to note that stem cells show a relatively higher risk of tumor formation and lower risk of immune rejection (in the case of mesenchymal stem cells) when compared to that of somatic cells. The figure was created with BioRender.com
In this review, we described the different types of stem cell-based therapies (Fig. 1b), including hPSCs and MSCs, and provided an overview of their definition, history, and outstanding clinical applications. In addition, we further created the first literature portfolio for the “targeted therapy” of MSCs based on their origin, delineating their different tissue origins and downstream applications with an in-depth discussion of their mechanism of action. Finally, we provide our perspective on why the tissue origin of MSCs could contribute greatly to their downstream applications as a proposed hypothesis that needs to be proven or disproven in the future to further enhance the safety and effectiveness of stem cell-based therapy.
Stem cell-based therapy: an overview of current clinical applications
Cardiovascular diseases
The clinical applications of stem cell-based therapies for heart diseases have been recently discussed comprehensively in the reviews19,20 and therefore will be elaborated in this study as the focus discussions related to hPSCs and MSCs in the following sections. In general, the safety profiles of stem cell-based therapies are supported by a large body of preclinical and clinical studies, especially adult stem cell therapy (such as MSC-based products). However, clinical trials have not yet yielded data supporting the efficacy of the treatment, as numerous studies have shown paradoxical results and no statistically significant differences in infarct size, cardiac function, or clinical outcomes, even in phase III trials.21 The results of a meta-analysis showed that stem cells derived from different sources did not exhibit any therapeutic effects on the improvement of myocardial contractility, cardiovascular remodeling, or clinical outcomes.22 The disappointing results obtained from the clinical trials thus far could be explained by the fact that the administered cells may exert their therapeutic effects via an immune modulation rather than regenerative function. Thus, well-designed, randomized and placebo-controlled phase III trials with appropriate cell-preparation methods, patient selection, follow-up schedules and suitable clinical measurements need to be conducted to determine the efficacy of the treatments. In addition, concerns related to optimum cell source and dose, delivery route and timing of administration, cell distribution post administration and the mechanism of action also need to be addressed. In the following section of this review, we present clinical trials related to MSC-based therapy in cardiovascular disease with the aim of discussing the contradictory results of these trials and analyzing the potential challenges underlying the current approaches.
Digestive system diseases
Gastrointestinal diseases are among the most diagnosed conditions in the developed world, altering the life of one-third of individuals in Western countries. The gastrointestinal tract is protected from adverse substances in the gut environment by a single layer of epithelial cells that are known to have great regenerative ability in response to injuries and normal cell turnover.23 These epithelial cells have a rapid turnover rate of every 2–7 days under normal conditions and even more rapidly following tissue damage and inflammation. This rapid proliferation ability is possible owing to the presence of a specific stem cell population that is strictly compartmentalized in the intestinal crypts.24 The gastrointestinal tract is highly vulnerable to damage, tissue inflammation and diseases once the degradation of the mucosal lining layer occurs. The exposure of intestinal stem cells to the surrounding environment of the gut might result in the direct destruction of the stem cell layer or disruption of intestinal functions and lead to overt clinical symptoms.25 In addition, the accumulation of stem cell defects as well as the presence of chronic inflammation and stress also contributes to the reduction of intestinal stem cell quality.
In terms of digestive disorders, Crohn’s disease (CD) and ulcerative colitis are the two major forms of inflammatory bowel disease (IBD) and represent a significant burden on the healthcare system. The former is a chronic, uncontrolled inflammatory condition of the intestinal mucosa characterized by segmental transmural mucosal inflammation and granulomatous changes.26 The latter is a chronic inflammatory bowel disease affecting the colon and rectum, characterized by mucosal inflammation initiating in the rectum and extending proximal to the colon in a continuous fashion.27 Cellular therapy in the treatment of CD can be divided into haematopoietic stem cell-based therapy and MSC-based therapy. The indication and recommendation of using HSCs for the treatment of IBD were proposed in 1995 by an international committee with four important criteria: (1) refractory to immunosuppressive treatment; (2) persistence of the disease conditions indicated via endoscopy, colonoscopy or magnetic resonance enterography; (3) patients who underwent an imminent surgical procedure with a high risk of short bowel syndromes or refractory colonic disease; and (4) patients who refused to treat persistent perianal lesions using coloproctectomy with a definitive stroma implant.28 In the standard operation procedure, patents’ HSCs were recruited using cyclophosphamide, which is associated with granulocyte colony-stimulating factor (G-CSF), at two different administered concentrations (4 g/m2 and 2 g/m2). Recently, it was reported that high doses of cyclophosphamide do not improve the number of recruited HSCs but increase the risk of cardiac and bladder toxicity. An interest in using HSCTs in CD originated from case reports that autologous HSCTs can induce sustained disease remission in some29,30 but not all patients31–33 with CD. The first phase I trial was conducted in Chicago and recruited 12 patients with active moderate to severe CD refractory to conventional therapies. Eleven of 12 patients demonstrated sustained remission after a median follow-up of 18.5 months, and one patient developed recurrence of active CD.31 The ASTIC trial (the Autologous Stem Cell Transplantation International Crohn Disease) was the first randomized clinical trial with the largest cohort of patients undergoing HSCT for refractory CD in 2015.34 The early report of the trial showed no statistically significant improvement in clinical outcomes of mobilization and autologous HSCT compared with mobilization followed by conventional therapy. In addition, the procedure was associated with significant toxicity, leading to the suggestion that HSCT for patients with refractory CD should not be widespread. Interestingly, by using conventional assessments for clinical trials for CD, a group reassessed the outcomes of patients enrolled in the ASTIC trial showing clinical and endoscopic benefits, although a high number of adverse events were also detected.35 A recent systematic review evaluated 18 human studies including 360 patients diagnosed with CD and showed that eleven studies confirmed the improvement of Crohn’s disease activity index between HSCT groups compared to the control group.36 Towards the cell sources, HSCs are the better sources as they afforded more stable outcomes when compared to that of MSC-based therapy.37 Moreover, autologous stem cells were better than their allogeneic counterparts.36 The safety of stem cell-based therapy in the treatment of CD has attracted our attention, as the risk of infection in patients with CD was relatively higher than that in those undergoing administration to treat cancer or other diseases. During the stem cell mobilization process, patient immunity is significantly compromised, leading to a high risk of infection, and requires carefully nursed and suitable antibiotic treatment to reduce the development of adverse events. Taken together, stem cell-based therapy for digestive disease reduced inflammation and improved the patient’s quality of life as well as bowel functions, although the high risk of adverse events needs to be carefully monitored to further improve patient safety and treatment outcomes.
Liver diseases
The liver is the largest vital organ in the human body and performs essential biological functions, including detoxification of the organism, metabolism, supporting digestion, vitamin storage, and other functions.38 The disruption of liver homeostasis and function might lead to the development of pathological conditions such as liver failure, cirrhosis, cancer, alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), and autoimmune liver disease (ALD). Orthotropic liver transplantation is the only effective treatment for severe liver diseases, but the number of available and suitable donor organs is very limited. Currently, stem cell-based therapies in the treatment of liver disease are associated with HSCs, MSCs, hPSCs, and liver progenitor cells.
Liver failure is a critical condition characterized by severe liver dysfunctions or decompensation caused by numerous factors with a relatively high mortality rate. Stem cell-based therapy is a novel alternative approach in the treatment of liver failure, as it is believed to participate in the enhancement of liver regeneration and recovery. The results of a meta-analysis including four randomized controlled trials and six nonrandomized controlled trials in the treatment of acute-on-chronic liver failure (ACLF) demonstrated that clinical outcomes of stem cell therapy were achieved in the short term, requiring multiple doses of stem cells to prolong the therapeutic effects.39,40 Interestingly, although MSC-based therapies improved liver functions, including the model of end-stage liver disease score, albumin level, total bilirubin, and coagulation, beneficial effects on survival rate and aminotransferase level were not observed.41 A randomized controlled trial illustrated the improvement of liver functions and reduction of severe infections in patients with hepatitis B virus-related ACLF receiving allogeneic bone marrow-derived MSCs (BM-MSCs) via peripheral infusion.42 HSCs from peripheral blood after the G-CSF mobilization process were used in a phase I clinical trial and exhibited an improvement in serum bilirubin and albumin in patients with chronic liver failure without any specific adverse events related to the administration.43 Taken together, an overview of stem cell-based therapy in the treatment of liver failure indicates the potential therapeutic effects on liver functions with a strong safety profile, although larger randomized controlled trials are still needed to assure the conclusions.
Liver cirrhosis is one of the major causes of morbidity and mortality worldwide and is characterized by diffuse nodular regeneration with dense fibrotic septa and subsequent parenchymal extinction leading to the collapse of liver vascular structure.44 In fact, liver cirrhosis is considered the end-stage of liver disease that eventually leads to death unless liver transplantation is performed. Stem cell-based therapy, especially MSCs, currently emerges as a potential treatment with encouraging results for treating liver cirrhosis. In a clinical trial using umbilical cord-derived MSCs (UC-MSCs), 45 chronic hepatitis B patients with decompensated liver cirrhosis were divided into two groups: the MSC group (n = 30) and the control group (n = 15).45 The results showed a significant reduction in ascites volume in the MSC group compared with the control. Liver function was also significantly improved in the MSC groups, as indicated by the increase in serum albumin concentration, reduction in total serum bilirubin levels, and decrease in the sodium model for end-stage liver disease score.45 Similar results were also reported from a phase II trial using BM-MSCs in 25 patients with HCV-induced liver cirrhosis.46 Consistent with these studies, three other clinical trials targeting liver cirrhosis caused by hepatitis B and alcoholic cirrhosis were conducted and confirmed that MSC administration enhanced and recovered liver functions.47–49 With the large cohort study as the clinical trial conducted by Fang, the safety and potential therapeutic effects of MSC-based therapies could be further strengthened and confirmed the feasibility of the treatment in virus-related liver cirrhosis.49 In terms of delivery route, a randomized controlled phase 2 trial suggested that systemic delivery of BM-MSCs does not show therapeutic effects on patients with liver cirrhosis.50 MSCs are not the only cell source for liver cirrhosis. Recently, an open-label clinical trial conducted in 19 children with liver cirrhosis due to biliary atresia after the Kasai operation illustrated the safety and feasibility of the approach by showing the improvement of liver function after bone marrow mononuclear cell (BMNC) administration assessed by biochemical tests and pediatric end-stage liver disease (PELD) scores.51 Another study using BMNCs in 32 decompensated liver cirrhosis patients illustrated the safety and effectiveness of BMNC administration in comparison with the control group.52 Recently, a long-term analysis of patients receiving peripheral blood-derived stem cells indicated a significant improvement in the long-term survival rate when compared to the control group, and the risk of hepatocellular carcinoma formation did not increase.53 CD133+ HSC infusion was performed in a multicentre, open, randomized controlled phase 2 trial in patients with liver cirrhosis; the results did not support the improvement of liver conditions, and cirrhosis persisted.54 Notably, these results are in line with a previous randomized controlled study, which also reported that G-CSF and bone marrow-derived stem cells delivered via the hepatic artery did not introduce therapeutic potential as expected.55 Thus, stem cell-based therapy for liver cirrhosis is still in its immature stage and requires larger trials with well-designed experiments to confirm the efficacy of the treatment.
Nonalcoholic fatty liver disease (NAFLD) is the most common medical condition caused by genetic and lifestyle factors and results in a severe liver condition and increased cardiovascular risk.56 NAFLD is the hidden enemy, as most patients are asymptomatic for a long time, and their routine life is unaffected. Thus, the detection, identification, and management of NAFLD conditions are challenging tasks, as patients diagnosed with NAFLD often develop nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.57 Although preclinical studies have shown that stem cell administration could enhance liver function in NAFLD models, a limited number of clinical trials were performed in human subjects. Recently, a multi-institutional clinical trial using freshly isolated autologous adipose tissue-derived regenerative cells was performed in Japan to treat seven NAFLD patients.58 The results illustrated the improvement in the serum albumin level of six patients and prothrombin activity of five patients, and no treatment-related adverse events or severe adverse events were observed. This study illustrates the therapeutic potential of stem cell-based therapy in the treatment of NAFLD.
Autoimmune liver disease (ALD) is a severe liver condition affecting children and adults worldwide, with a female predominance.59 The condition occurs in genetically predisposed patients when a stimulator, such as virus infection, leads to a T-cell-mediated autoimmune response directed against liver autoantigens. As a result, patients with ALD might develop liver cirrhosis, hepatocellular carcinoma, and, in severe cases, death. To date, HSCT and bone marrow transplantation are the two common stem cell-based therapies exhibiting therapeutic potential for ALD in clinical trials. An interesting report illustrated that haploidentical HSCTs could cure ALD in patients with sickle cells.60 This report is particularly important, as it illustrates the potential therapeutic approach of using haploidentical HSCTs to treat patients with both sickle cells and ALD. Another case report described a 19-year-old man with a 4-year history of ALD who developed acute lymphoblastic leukemia and required allogeneic bone marrow transplantation from this wholesome brother.61 The clinical data showed that immunosuppressive therapy for transplantation generated ALD remission in the patient.62 However, the data also provided valid information related to the sustained remission and the normalization of ASGPR-specific suppressor-inducer T-cell activity following bone marrow transplantation, suggesting that these suppressor functions originated from donor T cells.61 Thus, it was suggested that if standard immunosuppressive treatment fails, alternative cellular immunotherapy would be a viable option for patients with ALD. Primary biliary cholangitis (PBC), usually known as primary biliary cirrhosis, is a type of ALD characterized by a slow, progressive destruction of small bile ducts of the liver leading to the formation of cirrhosis and accumulation of bile and other toxins in the liver. A pilot, single-arm trial from China recruited seven patents with PBC who had a suboptimal response to ursodeoxycholic acid (UDCA) treatment.63 These patients received UDCA treatment in combination with three rounds of allogeneic UC-MSCs at 4-week intervals with a dose of 0.5 × 106 cells/kg of patient body weight via the peripheral vein. No treatment-related adverse events or severe adverse events were observed throughout the course of the study. The clinical data indicated significant improvement in liver function, including reduction of serum ALP and gamma-glutamyltransferase (GGT) at 48 weeks post administration. The common symptoms of PBC, including fatigue, pruritus, and hypogastric ascites volume, were also reduced, supporting the feasibility of MSC-based therapy in the treatment of PBC, although major limitations of the study were nonrandomized, no control group and small sample size. Another study was conducted in China with ten PBC patients who underwent incompetent UDCA treatment for more than 1 year. These patients received a range of 3–5 allogeneic BM-MSCs/kg body weight by intravenous infusion.64 Although these early studies have several limitations, such as small sample size, nonrandomization, and no control group, their preliminary data related to safety and efficacy herald the prospects and support the feasibility of stem cell-based therapy in the treatment of ALD.
In summary, the current number of trials for liver disease using stem cell-based therapy has provided fundamental data supporting the safety and potential therapeutic effects in various liver diseases. Unfortunately, due to the small number of trials, several obstacles need to be overcome to prove the effectiveness of the treatments, including (1) stem cell source and dose, (2) administration route, (3) time of intervention, and (4) clinical assessments during the follow-up period. Only by addressing these challenges we will be able to prove, facilitate and promote stem cell-based therapy as a mainstream treatment for liver diseases.
Arthritis
Arthritis is a general term describing cartilage conditions that cause pain and inflammation of the joints. Osteoarthritis (OA) is the most common form of arthritis caused by persistent degeneration and poor recovery of articular cartilage.65 OA affects one or several diarthrodial joints, such as small joints at the hand and large joints at the knee and hips, leading to severe pain and subsequent reduction in the mobility of patients. There are two types of OA: (1) primary OA or idiopathic OA and secondary OA caused by causative factors such as trauma, surgery, and abnormal joint development at birth.66 As conventional treatments for OA are not consistent in their effectiveness and might cause unbearable pain as well as long-term rehabilitation (in the case of joint replacement), there is a need for a more reliable, less painful, and curative therapy targeting the root of OA.67 Thus, stem cell therapy has recently emerged as an alternative approach for OA and has drawn great attention in the regenerative field.
The administration of HSCs has been proven to reduce bone lesions, enhance bone regeneration and stimulate the vascularization process in degenerative cartilage. Attempts were made to evaluate the efficacy of peripheral blood stem cells in ten OA patients by three intraarticular injections. Post-administration analysis indicated a reduction in the WOMAC index with a significant reduction in all parameters. All patients completed 6-min walk tests with an increase of more than 54 meters. MRI analysis indicated an improvement in cartilage thickness, suggesting that cartilage degeneration was reduced post administration. To further enhance the therapeutic potential of HSCT, CD34+ stem cells were proposed to be used in combination with the rehabilitation algorithm, which included three stages: preoperative, hospitalization and outpatient periods.68 Currently, a large wave of studies has been directed to MSC-based therapy for the treatment of OA due to their immunoregulatory functions and anti-inflammatory characteristics. MSCs have been used as the main cell source in several multiple and small-scale trials, proving their safety profile and potential effectiveness in alleviating pain, reducing cartilage degeneration, and enhancing the regeneration of cartilage structure and morphology in some cases. However, the best source of MSCs, whether from bone marrow, adipose tissue, or umbilical cord, for the management of OA is still a great question to be answered. A systematic review investigating over sixty-one of 3172 articles with approximately 2390 OA patients supported the positive effects of MSC-based therapy on OA patients, although the study also pointed out the fact that these therapeutic potentials were based on limited high-quality evidence and long-term follow-up.69 Moreover, the study found no obvious evidence supporting the most effective source of MSCs for treating OA. Another systematic review covering 36 clinical trials, of which 14 studies were randomized trials, provides an interesting view in terms of the efficacy of autologous MSC-based therapy in the treatment of OA.70 In terms of BM-MSCs, 14 clinical trials reported the clinical outcomes at the 1-year follow-up, in which 57% of trials reported clinical outcomes that were significantly better in comparison with the control group. However, strength analysis of the data set showed that outcomes from six trials were low, whereas the outcomes of the remaining eight trials were extremely low. Moreover, the positive evidence obtained from MRI analysis was low to very low strength of evidence after 1-year post administration.70 Similar results were also found in the outcome analysis of autologous adipose tissue-derived MSCs (AT-MSCs). Thus, the review indicated low quality of evidence for the therapeutic potential of MSC therapy on clinical outcomes and MRI analysis. The low quality of clinical outcomes could be explained by the differences in interventions (including cell sources, cell doses, and administration routes), combination treatments (with hyaluronic acid,71 peripheral blood plasma,72 etc.), control treatments and clinical outcome measurements between randomized clinical trials.73 In addition, the data of the systematic analysis could not prove the better source of MSCs for OA treatment. Taken together, although stem cell-based therapy has been shown to be safe and feasible in the management of OA, the authors support the notion that stem cell-based therapy could be considered an alternative treatment for OA when first-line treatments, such as education, exercise, and body weight management, have failed.
Cancer treatment
Stem cell therapy in the treatment of cancer is a sensitive term and needs to be used and discussed with caution. Clinicians and researchers should protect patients with cancer from expensive and potentially dangerous or ineffective stem cell-based therapy and patients without a cancer diagnosis from the risk of malignancy development. In general, unproven stem cell clinics employed three cell-based therapies for cancer management, including autologous HSCTs, stromal vascular fraction (SVF), and multipotent stem cells, such as MSCs. Allogeneic HSCTs confer the ability to generate donor lymphocytes that contribute to the suppression and regression of hematological malignancies and select solid tumors, a specific condition known as “graft-versus-tumor effects”.74 However, stem cell clinics provide allogeneic cell-based therapy for the treatment of solid malignancies despite limited scientific evidence supporting the safety and efficacy of the treatment. High-quality evidence from the Cochrane library shows that marrow transplantation via autologous HSCTs in combination with high-dose chemotherapy does not improve the overall survival of women with metastatic breast cancer. In addition, a study including more than 41,000 breast cancer patients demonstrated no significant difference in survival benefits between patients who received HSCTs following high-dose chemotherapy and patients who underwent conventional treatment.75 Thus, the use of autologous T-cell transplants as monotherapy and advertising stem cell-based therapies as if they are medically approved or preferred treatment of solid tumors is considered untrue statements and needs to be alerted to cancer patients.76
Over the past decades, many preclinical studies have demonstrated the potential of MSC-based therapy in cancer treatment due to their unique properties. They confer the ability to migrate toward damaged sites via inherent tropism controlled by growth factors, chemokines, and cytokines. MSCs express specific C–X–C chemokine receptor type 4 (CXCR4) and other chemokine receptors (including CCR1, CCR2, CCR4, CCR7, etc.) that are essential to respond to the surrounding signals.77 In addition, specific adherent proteins, including CD49d, CD44, CD54, CD102, and CD106, are also expressed on the MSC surface, allowing them to attach, rotate, migrate, and penetrate the blood vessel lumen to infiltrate the damaged tissue.78 Similar to damaged tissues, tumors secrete a wide range of chemoattractant that also attract MSC migration via the CXCL12/CXCR4 axis. Previous studies also found that MSC migration toward the cancer site is tightly controlled by diffusible cytokines such as interleukin 8 (IL-8) and growth factors including transforming growth factor-beta 1 (TGF-β1),79 platelet-derived growth factor (PDGF),80 fibroblast growth factor 2 (FGF-2),81 vascular endothelial growth factor (VEGF),81 and extracellular matrix molecules such as matrix metalloproteinase-2 (MMP-2).82 Once MSCs migrate successfully to cancerous tissue, accumulating evidence demonstrates the interaction between MSCs and cancer cells to exhibit their protumour and antitumour effects, which are the major concerns of MSC-based therapy. MSCs are well-known for their regenerative effects that regulate tissue repair and recovery. This unique ability is also attributed to the protumour functions of these cells. A previous study reported that breast cancer cells induce MSC secretion of chemokine (C–C motif) ligand 5 (CCL-5), which regulates the tumor invasion process.83,84 Other studies also found that MSCs secrete a wide range of growth factors (VEGF, basic FGF, HGF, PDGF, etc.) that inhibits apoptosis of cancer cells.85 Moreover, MSCs also respond to signals released from cancer cells, such as TGF-β,86 to transform into cancer-associated fibroblasts, a specific cell type residing within the tumor microenvironment capable of promoting tumorigenesis.87 Although MSCs have been proven to be involved in protumour activities, they also have potent tumor suppression abilities that have been used to develop cancer treatments. It has been suggested that MSCs exhibit their tumor inhibitory effects by inhibiting the Wnt and AKT signaling pathways,88 reducing the angiogenesis process,89 stimulating inflammatory cell infiltration,90 and inducing tumor cell cycle arrest and apoptosis.91 To date, the exact functions of MSCs in both protumour and antitumor activities are still a controversial issue across the stem cell field. Other approaches exploit gene editing and tissue engineering to convert MSCs into “a Trojan horse” that could exhibit antitumor functions. In addition, MSCs can also be modified to express specific anticancer miRNAs exhibiting tumor-suppressive behaviors.92 However, genetically modified MSCs are still underdeveloped and require intensive investigation in the clinical setting.
To date, ~25 clinical trials have been registered on ClinicalTrials.gov aimed at using MSCs as a therapeutic treatment for cancer.93 These trials are mostly phase 1 and 2 studies focusing on evaluating the safety and efficacy of the treatment. Studies exploiting MSC-based therapy have combined MSCs with an oncolytic virus approach. Oncolytic viruses are specific types of viruses that can be genetically engineered or naturally present, conferring the ability to selectively infect cancer cells and kill them without damaging the surrounding healthy cells.94 A completed phase I/II study using BM-MSCs infected with the oncolytic adenovirus ICOVIR5 in the treatment of metastatic and refractory solid tumors in children and adult patients demonstrated the safety of the treatment and provided preliminary data supporting their therapeutic potential.95 The same group also reported a complete disappearance of all signs of cancer in response to MSC-based therapy in one pediatric case three years post administration.96 A reported study in 2019 claimed that adipose-derived MSCs infected with vaccinia virus have the potential to eradicate resistant tumor cells via the combination of potent virus amplification and senitization of the tumor cells to virus infection.97 However, in a recently published review, a valid question was posed regarding the 2019 study that “do these reported data merit inclusion in the publication record when they were collected by such groups using a dubious therapeutic that was eventually confiscated by US Marshals?”76
Taken together, cancer research and therapy have entered an innovative and fascinating era with advancements in traditional therapies such as chemotherapy, radiotherapy, and surgery on one hand and stem cell-based therapy on the other hand. Although stem cell-based therapy has been considered a novel and attractive therapeutic approach for cancer treatment, it has been hampered by contradictory results describing the protumour and antitumour effects in preclinical studies. Despite this contradictory reality, the use of stem cell-based therapy, especially MSCs, offers new hope to cancer patients by providing a new and more effective tool in personalized medicine. The authors support the use of MSC-based therapy as a Trojan horse to deliver specific anticancer functions toward cancer cells to suppress their proliferation, eradicate cancer cells, or limit the vascularization process of cancerous tissue to improve the clinical safety and efficacy of the treatment.
Human pluripotent stem cell-based therapy: a growing giant
The discovery of hPSCs, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), has revolutionized stem cell research and cell-based therapy.98 hESCs were first isolated from blastocyst-stage embryos in 1998,99 followed by breakthrough reprogramming research that converted somatic cells into hiPSCs using just four genetic factors.100,101 Methods have been developed to maintain these cells long-term in vitro and initiate their differentiation into a wide variety of cell types, opening a new era in regenerative medicine, particularly cell therapy to replace lost or damaged tissues.
History of hPSCs
hPSCs are defined as self-renewable cell types that confer the ability to differentiate into various cellular phenotypes of the human body, including three germ layers.102 Historically, the first pluripotent cell lines to be generated were embryonic carcinoma (EC) cell lines established from human germ cell tumors103 and murine undifferentiated compartments.104 Although EC cells are a powerful tool in vitro, these cells are not suitable for clinical applications due to their cancer-derived origin and aneuploidy genotype.105 The first murine ESCs were established in 1981 based on the culture techniques obtained from EC research.106 Murine ESCs are derived from the inner cell mass (ICM) of the pre-implantation blastocyst, a unique biological structure that contains outer trophoblast layers that give rise to the placenta and ICM.107 In vivo ESCs only exist for a short period during the embryo’s development, and they can be isolated and maintained indefinitely in vitro in an undifferentiated state. The discovery of murine ESCs has dramatically changed the field of biomedical research and regenerative medicine over the last 40 years. Since then, enormous investigations have been made to isolate and culture ESCs from other species, including hESCs, in 1998.99 The success of Thomson et al. in 1998 triggered the great controversy in media and ethical research boards across the globe, with particularly strong objections being raised to the use of human embryos for research purposes.108 Several studies using hESCs have been conducted demonstrating their therapeutic potential in the clinical setting. However, the use of hESCs is limited due to (1) the ethical barrier related to the destruction of human embryos and (2) the potential risk of immunological rejection, as hESCs are isolated from pre-implantation blastocysts, which are not autologous in origin. To overcome these two great obstacles, several research groups have been trying to develop technology to generate hESCs, including nuclear transfer technology, the well-known strategy that creates Dolly sheep, although the generation of human nuclear transfer ESCs remains technically challenging.109 Taking a different approach, in 2006, Yamanaka and Takahashi generated artificial PSCs from adult and embryonic mouse somatic cells using four transcription factors (Oct-3/4, Sox2, Klf4, and c-Myc, called OSKM) reduced from 24 factors.100 Thereafter, in 2007, Takahashi and colleagues successfully generated the first hiPSCs exhibiting molecular and biological features similar to those of hESCs using the same OSKM factors.101 Since then, hiPSCs have been widely studied to expand our knowledge of the pathogenesis of numerous diseases and aid in developing new cell-based therapies as well as personalized medicine.
Clinical applications of hPSCs
Since its beginning 24 years ago, hPSC research has evolved momentously toward applications in regenerative medicine, disease modeling, drug screening and discovery, and stem cell-based therapy. In clinical trial settings, the uses of hESCs are restricted by ethical concerns and tight regulation, and the limited preclinical data support their therapeutic potential. However, it is important to acknowledge several successful outcomes of hESC-based therapies in treating human diseases. In 2012, Steven Schwartz and his team reported the first clinical evidence of using hESC-derived retinal pigment epithelium (RPE) in the treatment of Stargardt’s macular dystrophy, the most common pediatric macular degeneration, and an individual with dry age-related macular degeneration.110,111 With a differentiation efficiency of RPE greater than 99%, 5 × 104 RPEs were injected into the subretinal space of one eye in each patient. As the hESC source of RPE differentiation was exposed to mouse embryonic stem cells, it was considered a xenotransplantation product and required a lower dose of immunosuppression treatment. This study showed that hESCs improved the vision of patients by differentiating into functional RPE without any severe adverse events. The trial was then expanded into two open-label, phase I/II studies with the published results in 2015 supporting the primary findings.112 In these trials, patients were divided into three groups receiving three different doses of hESC-derived RPE, including 10 × 104, 15 × 104 and 50 × 104 RPE cells per eye. After 22 months of follow-up, 19 patients showed improvement in eyesight, seven patients exhibited no improvement, and one patient experienced a further loss of eyesight. The technical challenge of hESC-derived RPE engraftment was an unbalanced proliferation of RPE post administration, which was observed in 72% of treated patients. A similar approach was also conducted in two South Korean patients diagnosed with age-induced macular degeneration and two patients with Stargardt macular dystrophy.113 The results supported the safety of hESC-derived RPE cells and illustrated an improvement in visual acuity in three patients. Recently, clinical-graded hESC-derived RPE cells were also developed by Chinese researchers under xeno-free culture conditions to treat patients with wet age-related degeneration.114 As hESC development is still associated with ethical concerns and immunological complications related to allogeneic administration, hiPSC-derived RPE cells have emerged as a potential cell source for macular degeneration. Although RPE differentiation protocols have been developed and optimized to improve the efficacy of hiPSC-derived RPE cells, they are still insufficient, time-consuming and labor intensive.115,116 For clinical application, an efficient differentiation of “primed” to “naïve” state hiPSCs toward the RPE was developed using feeder-free culture conditions utilizing the transient inhibition of the FGF/MAPK signaling pathway.117 Overexpression of specific transcription factors in hiPSCs throughout the differentiation process is also an interesting approach to generate a large number of RPE cells for clinical use. In a recent study, overexpression of three eye-field transcription factors, including OTX2, PAX6, and MITF, stimulated RPE differentiation in hiPSCs and generated functional RPE cells suitable for transplantation.118 To date, although reported data from phase I/II clinical trials have been produced enough to support the safety of hESC-derived RPE cells, the treatment is still in its immature stage. Thus, future studies should focus on the development of the cellular manufacturing process of RPE and the subretinal administration route to further improve the outcomes of RPE fabrication and engraftment into the patient’s retina (recommended review119).
Numerous studies have demonstrated that hESC-derived cardiomyocytes exhibit cardiac transcription factors and display a cardiomyocyte phenotype and immature electrical phenotype. In addition, using hPSC-derived cardiomyocytes could provide a large number of cells required for true remuscularization and transplantation. Thus, these cells can be a promising novel therapeutic approach for the treatment of human cardiovascular diseases. In a case report, hESC-derived cardiomyocytes showed potential therapeutic effects in patients with severe heart failure without any subsequent complications.120 This study was a phase I trial (ESCORT [Transplantation of Human Embryonic Stem Cell-derived Progenitors in Severe Heart Failure] trial) to evaluate the safety of cardiomyocyte progenitor cells derived from hESCs seeded in fibrin gel scaffolds for 10 patients with severe heart failure (NCT02057900). The encouraging results from this study demonstrated the feasibility of producing hESC-derived cardiomyocyte progenitor cells toward clinical-grade standards and combining them with a tissue-engineered scaffold to treat severe heart disease (the first patient of this trial has already reached the 7-year follow-up in October 2021).121 Currently, the two ongoing clinical trials using hPSC-derived cardiomyocytes have drawn great attention, as their results would pave the way to lift the bar for approving therapies for commercial use. The first trial was conducted by a team led by cardiac surgeon Yoshiki Sawa at Osaka University using hiPSC-derived cardiomyocytes embedded in a cell sheet for engraftment (jRCT2052190081). The trials started first with three patients followed by ten patients to assess the safety of the approach. Once safety is met, the treatment can be sold commercially under Japan’s fast-track system for regenerative medicine.122 Another trial used a collagen-based construct called BioVAT-HF to contain hiPSC-derived cardiomyocytes. The trial was divided into two parts to evaluate the cell dose: (Part A) recruiting 18 patients and (Part B) recruiting 35 patients to test a broad range of engineered human myocardium (EHM) doses. The expected results from this study will provide the “proof-of-concept” for the use of EHM in the stimulation of heart remuscularization in humans. To date, no adverse events or severe adverse events have been reported from these trials, supporting the safety of the procedure. However, as the number of treated patients was relatively small, limitations in drawing conclusions regarding efficacy are not yet possible.21,123
One of the first clinical trials using hPSC-based therapy was conducted by Geron Corporation in 2010 using hESC-derived oligodendrocyte progenitor cells (OPC1) to treat spinal cord injury (SCI). The results confirmed the safety one year post administration in five participants, and magnetic resonance imaging demonstrated improvement of spinal cord deterioration in four participants.124 Asterias Biotherapeutic (AST) continued the Geron study by conducting the SCiStar Phase I/IIa study to evaluate the therapeutic effects of AST-OPC1 (NCT02302157). The trial’s results published in clinicaltrials.gov demonstrated significant improvement in running speed, forelimb stride length, forelimb longitudinal deviations, and rear stride frequency. Interestingly, the recently published data of a phase 1, multicentre, nonrandomized, single-group assignment, interventional trial illustrated no evidence of neurological decline, enlarging masses, further spinal cord damage, or syrinx formation in patients 10 years post administration of the OPC1 product.125 This data set provides solid evidence supporting the safety of OPC1 with an event-free period of up to 10 years, which strengthens the safety profile of the SCiStar trial.
Analysis of the global trends in clinical trials using hPSC-based therapy showed that 77.1% of studies were observational (no cells were administered into patient), and only 22.9% of studies used hPSC-derived cells as interventional treatment.126 The number of studies using hiPSCs was relatively higher than that using hESCs, which was 74.8% compared to 25.2%, respectively. The majority of observational studies were performed in developed countries, including the USA (41.6%) and France (16.8%), whereas interventional studies were conducted in Asian countries, including China (36.7%), Japan (13.3%), and South Korea (10%). The trends in therapeutic studies were also clear in terms of targeted diseases. The three most studied diseases were ophthalmological conditions, circulatory disorders, and nervous systems.127 However, it is surprising that the clinical applications of hPSCs have achieved little progress since the first hESCs were discovered worldwide. The relatively low number of clinical trials focusing on using iPSCs as therapeutic agents to administer into patients could be ascribed to the unstable genome of hiPSCs,128 immunological rejection,129 and the potential for tumor formation.130
Mesenchymal stem/stromal cell-based therapy: is it time to consider their origin toward targeted therapy?
Approximately 55 years ago, fibroblast-like, plastic-adherent cells, later named mesenchymal stem cells (MSCs) by Arnold L. Caplan,18 were discovered for the first time in mouse bone marrow (BM) and were later demonstrated to be able to form colony-like structures, proliferate, and differentiate into bone/reticular tissue, cartilage, and fat.131 Protocols were subsequently established to directly culture this subpopulation of stromal cells from BM in vitro and to stimulate their differentiation into adipocytes, chondroblasts, and osteoblasts.132 Since then, MSCs have been found in and derived from different human tissue sources, including adipose tissue (AT), the umbilical cord (UC), UC blood, the placenta, dental pulp, amniotic fluid, etc.133 To standardize and define MSCs, the International Society for Cell and Gene Therapy (ISCT) set minimal identification criteria for MSCs derived from multiple tissue sources.134 Among them, MSCs derived from AT, BM, and UC are the most commonly studied MSCs in human clinical trials,135 and they constitute the three major tissue sources of MSCs that will be discussed in this review.
The discovery of MSCs opened an era during which preclinical studies and clinical trials have been performed to assess the safety and efficacy of MSCs in the treatment of various diseases. The major conclusion of these studies and trials is that MSC-based therapy is safe, although the outcomes have usually been either neutral or at best marginally positive in terms of the clinically relevant endpoints regardless of MSC tissue origin, route of infusion, dose, administration duration, and preconditioning.136 It is important to note that a solid background of knowledge has been generated from all these studies that has fueled the recent translational research in MSC-based therapy. As MSCs have been intensively studied over the last 55 years and have become the subject of multiple reviews, systematic reviews, and meta-analyses, the objective of this paper is not to duplicate these publications. Rather, we will discuss the questions that both clinicians and researchers are currently exploring with regard to MSC-based therapy, diligently seeking answers to the following:
“With a solid body of data supporting their safety profiles derived from both preclinical and clinical studies, does the tissue origin of MSCs also play a role in their downstream clinical applications in the treatment of different human diseases?”
“Do MSCs derived from AT, BM, and UC exhibit similar efficacy in the treatment of neurological diseases, metabolic/endocrine-related disorders, reproductive dysfunction, skin burns, lung fibrosis, pulmonary disease, and cardiovascular conditions?”
To answer these questions, we will first focus on the most recently published clinical data regarding these targeted conditions, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and heart-related diseases, to analyze the potential efficacy of MSCs derived from AT, BM, and UC. Based on the level of clinical improvement observed in each trial, we analyzed the potential efficacy of MSCs derived from each source to visualize the correlation between patient improvement and MSC sources. We will then address recent trends in the exclusive use of MSC-based products, focusing on the efficacy of treatment with MSCs from each of the abovementioned sources, and we will analyze the relationship between the respective efficacies of MSCs from these sources in relation to the targeted disease conditions. Finally, we propose a hypothesis and mechanism to achieve the currently still unmet objective of evaluating the use of MSCs from AT, BM, and UC in regenerative medicine.
An overview of MSC tissue origins and therapeutic potential
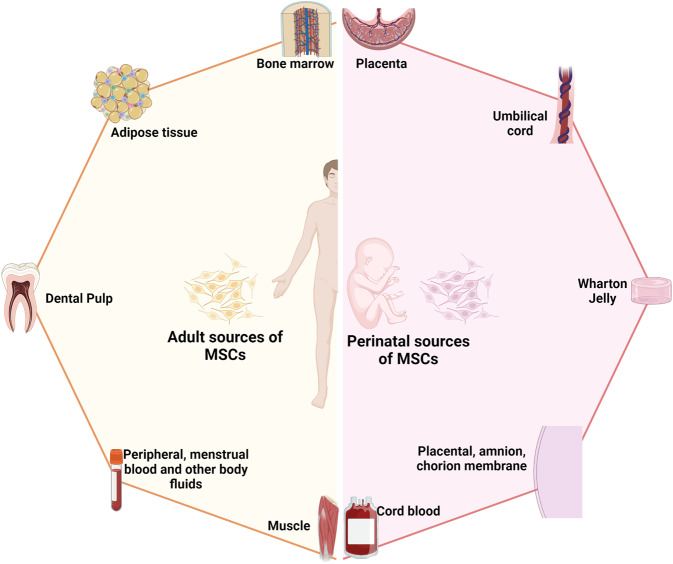
In general, MSCs are reported to be isolated from numerous tissue types, but all of these types can be organized into two major sources: adult137 and perinatal sources138 (Fig. 2). Adult sources of MSCs are defined as tissues that can be harvested or obtained from an individual, such as dental pulp,139 BM, peripheral blood,140 AT,141 lungs,142 hair,143 or the heart.144 Adult MSCs usually reside in specialized structures called stem cell niches, which provide the microenvironment, growth factors, cell-to-cell contacts and external signals necessary for maintaining stemness and differentiation ability.145 BM was the first adult source of MSCs discovered by Friedenstein131 and has become one of the most documented and largely used MSC sources to date, followed by AT. BM-MSCs are isolated and cultured in vitro from BM aspirates using a Ficoll gradient-centrifugation method146 or a red blood cell lysate buffer to collect BM mononuclear cell populations, whereas AT-MSCs are obtained from stromal vascular fractions of enzymatically digested AT obtained through liposuction,141 lipoplasty, or lipectomy procedures.147 These tissue collection procedures are invasive and painful for the patient and are accompanied by a risk of infection, although BM aspiration and adipose liposuction are considered safe procedures for BM and AT biopsies. The number of MSCs that can be isolated from these adult tissues varies significantly in a tissue-dependent manner. The percentage of MSCs in BM mononuclear cells ranges from 0.001 to 0.01% following gradient centrifugation.132 The number of MSCs in AT is at least 500 times higher than that in BM, with approximately 5,000 MSCs per 1 g of AT. Perinatal sources of MSCs consist of UC-derived components, such as UC, Wharton’s jelly, and UC blood, and placental structures, such as the placental membrane, amnion, chorion membrane, and amniotic fluid.138 The collection of perinatal MSCs, such as UC-MSCs, is noninvasive, as the placenta, UC, UC blood, and amnion are considered waste products that are usually discarded after birth (with no ethical barriers).148 Although MSCs represent only 10−7% the cells found in UC, their higher proliferation rate and rapid population doubling time allow these cells to rapidly replicate and increase in number during in vitro culture.149 Under standardized xeno-free and serum-free culture platforms, AT-MSCs show a faster proliferation rate and a higher number of colony-forming units than BM-MSCs.149 UC-MSCs have the fastest population doubling time compared to AT-MSCs and BM-MSCs in both conventional culture conditions and xeno- and serum-free environments.149 MSCs extracted from AT, BM and UC exhibit all minimal criteria listed by the ISCT, including morphology (plastic adherence and spindle shape), MSC surface markers (95% positive for CD73, CD90 and CD105; less than 2% negative for CD11, CD13, CD19, CD34, CD45, and HLR-DR) and differentiation ability into chondrocytes, osteocytes, and adipocytes.150
Fig. 2.
The two major sources of MSCs: adult and perinatal sources. The adult sources of MSCs are specific tissue in human body where MSCs could be isolated, including bone marrow, adipose tissue, dental pulp, peripheral blood, menstrual blood, muscle, etc. The perinatal sources of MSCs consist of umbilical cord-derived components, such as umbilical cord, Wharton’s jelly, umbilical cord blood, and placental structures, such as placental membrane, amnion, chorion membrane, amniotic fluid, etc. The figure was created with BioRender.com
In fact, although MSCs derived from either adult or perinatal sources exhibit similar morphology and the basic characteristics of MSCs, studies have demonstrated that these cells also differ from each other. Regarding immunophenotyping, AT-MSCs express high levels of CD49d and low levels of Stro-1. An analysis of the expression of CD49d and CD106 showed that the former is strongly expressed in AT-MSCs, in contrast to BM-MSCs, whereas CD106 is expressed in BM-MSCs but not in AT-MSCs.151 Increased expression of CD133, which is associated with stem cell regeneration, differentiation, and metabolic functions,152 was observed in BM-MSCs compared to MSCs from other sources.153 A recent study showed that CD146 expression in UC-MSCs was higher than that in AT- and BM-MSCs,153 supporting the observation that UC-MSCs have a stronger attachment and a higher proliferation rate than MSCs from other sources, as CD146 is a key cell adhesion protein in vascular and endothelial cell types.154 In terms of differentiation ability, donor-matched BM-MSCs exhibit a higher ability to differentiate into chondrogenic and osteogenic cell types than AT-MSCs, whereas AT-MSCs show a stronger capacity toward the adipogenic lineage.150 The findings from an in vitro differentiation study indicated that BM-MSCs are prone to osteogenic differentiation, whereas AT-MSCs possess stronger adipogenic differentiation ability, which can be explained by the fact that the epigenetic memory obtained from either BM or AT drives the favored MSC differentiation along an osteoblastic or adipocytic lineage.155 Interestingly, although UC-MSCs have the ability to differentiate into adipocytes, osteocytes, or chondrocytes, their osteogenic differentiation ability has been proven to be stronger than that of BM-MSCs.156 The most interesting characteristic of MSCs is their immunoregulatory functions, which are speculated to be related to either cell-to-cell contact or growth factor and cytokine secretion in response to environmental/microenvironmental stimuli. MSCs from different sources almost completely inhibit the proliferation of peripheral blood mononuclear cells (PBMCs) at PBMC:MSC ratios of 1:1 and 10:1.149 At a higher ratio, BM-MSCs showed a significantly higher inhibitory effect than AT- or UC-MSCs.153 Direct analysis of the immunosuppressive effects of BM- and UC-MSCs has revealed that these cells exert similar inhibitory effects in vitro with different mechanisms involved.157 With these conflicting data, the mechanism of action related to the immune response of MSCs from different sources is still poorly understood, and long-term investigations both in preclinical studies and in clinical trial settings are needed to shed light on this complex immunomodulation function.
The great concern in MSC-based therapy is the fate of these cells post administration, especially through different delivery routes, including systemic administration via an intravenous (IV) route or tissue-specific administration, such as dorsal pancreatic administration. It is important to understand the distribution of these cells after injection to expand our understanding of the underlying mechanisms of action of treatments; in addition, this knowledge is required by authorized bodies (the Food and Drug Administration (FDA) in the United States or the regulation of advanced-therapy medicinal products in Europe, No. 1394/2007) prior to using these cells in clinical trials. The preclinical data using various labeling techniques provide important information demonstrating that MSCs do not have unwanted homing that could lead to the incorrect differentiation of the cells or inappropriate tumor formation. In a mouse model, human BM-MSCs and AT-MSCs delivered via an IV route are rapidly trapped in the lungs and then recirculate through the body after the first embolization process, with a small number of infused cells found mainly in the liver after the second embolization.158 Using the technetium-99 m labeling method, intravenously infused human cells showed long-term persistence up to 13 months in the bone, BM compartment, spleen, muscle, and cartilage.159 A similar result was reported in baboons, confirming the long-term homing of human MSCs in various tissues post administration.160 Although the retainment of MSCs in the lungs might potentially reduce their systemic therapeutic effects,161 it provides a strong advantage when these cells are used in the treatment of respiratory diseases. Local injection of MSCs also revealed their tissue-specific homing, as an injection of MSCs via the renal artery route resulted in the majority of the injected cells being found in the renal cortex.162 Numerous studies have been conducted to track the migration of administered MSCs in human subjects. Henriksson and his team used MSCs labeled with iron sucrose in the treatment of intervertebral disc degeneration.163 Their study showed that chondrocytes differentiated from infused MSCs could be detected at the injured intervertebral discs at 8 months but not at 28 months. A study conducted in a patient with hemophilia A using In-oxine-labeled MSCs showed that the majority of the cells were trapped in the lungs and liver 1 h post administration, followed by a reduction in the lungs and an increase in the number of cells in the liver after 6 days.164 Interestingly, a small proportion of infused MSCs were found in the hemarthrosis site at the right ankle after 24 h, suggesting that MSCs are attracted and migrate to the injured site. The distribution of MSCs was also reported in the treatment of 21 patients diagnosed with type 2 diabetes using 18-FDG-tagged MSCs and visualized using positron emission tomography (PET).165 The results illustrated that local delivery of MSCs via an intraarterial route is more effective than delivery via an IV route, as MSCs home to the pancreatic head (pancreaticoduodenal artery) or body (splenic artery). Therefore, although the available data related to the biodistribution of infused MSCs are still limited, the results obtained from both preclinical and clinical studies illustrate a comparable set of data supporting results on homing, migration to the injured site, and the major organs where infused MSCs are located. The following comprehensive and interesting reviews are highly recommended.166–168
To date, 1426 registered clinical trials spanning different trial phases have used MSCs for therapeutic purposes, which is four times the number reported in 2013.169,170 As supported by a large body of preclinical studies and advancements in conducting clinical trials, MSCs have been proven to be effective in the treatment of numerous diseases, including nervous system and brain disorders, pulmonary diseases,171 cardiovascular conditions,172 wound healing, etc. The outcomes of MSC-based therapy have been the subject of many intensive reviews and systematic analyses with the solid conclusion that these cells exhibit strong safety profiles and positive outcomes in most tested conditions.173–175 In addition, the available data have revealed several potential mechanisms that could explain the beneficial effects of MSCs, including their homing efficiency, differentiation potential, production of trophic factors (including cytokines, chemokines, and growth factors), and immunomodulatory abilities. However, it is still not known which MSC types should be used for which diseases, as it seems to be that MSCs exhibit beneficial effects regardless of their sources.169
Acquired brain and spinal cord injury treatment: BM-MSCs have emerged as key players
The theory that brain cells can never regenerate has been challenged by the discovery of newly formed neurons in the human adult hippocampus or the migration of stem cells in the brain in animal models.176 These observations have triggered hope for regeneration in the context of neuronal diseases by using exogenous stem cell sources to replenish or boost the stem cell population in the brain. Moreover, the limited regenerative capacity of the brain and spinal cord is an obstacle for traditional treatments of neurodegenerative diseases, such as autism, cerebral palsy, stroke, and spinal cord injury (SCI). As current treatments cannot halt the progression of these diseases, studies throughout the world have sought to exploit cell-based therapies to treat neurodegenerative diseases on the basis of advances in the understanding and development of stem cell technology, including the use of MSCs. Successful stem cell therapy for treating brain disease requires therapeutic cells to reach the injured sites, where they can repair, replace, or at least prevent the deteriorative effects of neuronal damage.177 Hence, the gold standard of cell-based therapy is to deliver the cells to the target site, stimulate the tissue repair machinery, and regulate immunological responses via either cell-to-cell contact or paracrine effects.178 Among 315 registered clinical trials using stem cells for the treatment of brain diseases, MSCs and hematopoietic stem cells (HSCs; CD34+ cells isolated from either BM aspirate or UC blood) are the two main cell types investigated, whereas approximately 102 clinical trials used MSCs and 62 trials used HSCs for the treatment of brain disease (main search data from clinicaltrial.gov). MSCs are widely used in almost all clinical trials targeting different neuronal diseases, including multiple sclerosis,179 stroke,180 SCI,181 cerebral palsy,182 hypoxic-ischemic encephalopathy,183 autism,184 Parkinson’s disease,185 Alzheimer’s disease185 and ataxia. Among these trials in which MSCs were the major cells used, nearly two-thirds were for stroke, SCI, or multiple sclerosis. MSCs have been widely used in 29 registered clinical trials for stroke, with BM-MSCs being used in 16 of these trials. With 26 registered clinical trials, SCI is the second most common indication for using MSCs, with 16 of these trials using mainly expanded BM-MSCs. For multiple sclerosis, 15 trials employed BM-MSCs among a total of 23 trials conducted for the treatment of this disease. Hence, it is important to note that in neuronal diseases and disorders, BM-MSCs have emerged as the most commonly used therapeutic cells among other MSCs, such as AT-MSCs and UC-MSCs.
The outcomes of the use of BM-MSCs in the treatment of neuronal diseases have been widely reported in various clinical trial types. A review by Zheng et al. indicated that although the treatments appeared to be safe in patients diagnosed with stroke, there is a need for well-designed phase II multicentre studies to confirm the outcomes.173 One of the earliest trials using autologous BM-MSCs was conducted by Bang et al. in five patients diagnosed with stroke in 2005. The results supported the safety and showed an improved Barthel index (BI) in MSC-treated patients.186 In a 2-year follow-up clinical trial, 16 patients with stroke received BM-MSC infusions, and the results showed that the treatment was safe and improved clinical outcomes, such as motor impairment scale scores.187 A study conducted in 12 patients with ischemic stroke showed that autologous BM-MSCs expanded in vitro using autologous serum improved the patient’s modified Rankin Scale (mRS), with a mean lesion volume reduced by 20% at 1 week post cell infusion.188 In 2011, a modest increase in the Fugl Meyer and modified BI scores was observed after autologous administration of BM-MSCs in patients with chronic stroke.189 More recently, a prospective, open-label, randomized controlled trial with blinded outcome evaluation was conducted, with 39 patients and 15 patients in the BM-MSC administration and control groups, respectively. The results of this study indicated that autologous BM-MSCs with autologous serum administration were safe, but the treatment led to no improvements at 3 months in modified Rankin Scale (mRS) scores, although leg motor improvement was observed.180 Researchers explored whether the efficacy of BM-MSC administration was maintained over time in a 5-year follow-up clinical trial. Patients (85) were randomly assigned to either the MSC group or the control group, and follow-ups on safety and efficacy were performed for 5 years, with 52 patients being examined at the end of the study. The MSC group exhibited a significant improvement in terms of decreased mRS scores, whereas the number of patients with an mRS score increase of 0–3 was statistically significant.187 Although autologous BM-MSCs did not improve the Basel index, mRS, or National Institutes of Health Stroke Scale (NIHSS) score 2 years post infusion, patients who received BM-MSC therapy showed improvement in their motor function score.190 In addition, a prospective, open-label, randomized controlled trial by Lee et al. showed that autologous BM-MSCs primed with autologous “ischemic” serum significantly improved motor functions in the MSC-treated group. Neuroimaging analysis also illustrated a significant increase in interhemispheric connectivity and ipsilesional connectivity in the MSC group.191 Recently, a single intravenous infection of allogeneic BM-MSCs has been proven to be safe and feasible in patients with chronic stroke with a significant improvement in BI score and NIHSS score.192
In two systematic reviews using MSCs for the treatment of SCI, BM-MSCs (n = 16) and UC-MSCs (n = 5) were reported to be safe and well-tolerated.193,194 The results indicated significant improvements in the stem cell administration groups compared with the control groups in terms of a composite of the American Spinal Injury Association (ASIA) impairment scale (AIS) grade, AIS grade A, and ASIA sensory scores and bladder function (Table 1). However, larger experimental groups with a randomized and multicentre design are needed for further confirmation of these findings. For multiple sclerosis, several early-phase (phase I/II) registered clinical studies have used BM-MSCs. A study compared the potential efficacy of BM-MSC and BM mononuclear cell (BMMNC) transplantation in 105 patients with spastic cerebral palsy.195 The results showed that the GMFM (gross motor function measure) and the FMFM (fine motor function measure) scores of the BM-MSC transplant group were higher than those of the BMNNC transplant group at 3, 6, and 12 months of assessment. In terms of autism spectrum disorder, a review of 254 children after BMMNC transplantation found that over 90% of patients’ ISAA (Indian Scale for Assessment of Autism) and CARS (Childhood Autism Rating Scale) scores improved. Young patients and those in whom autism spectrum disorder was detected early generally showed better improvement.196
Table 1.
The reported clinical trials using MSCs from AT, BM, and UC in the treatment of brain-related injuries and neurological disorders
| Year | Disease | MSC source | No. of MSC-treated patients | Efficacy |
|---|---|---|---|---|
| 2022206 | Acute ischemic stroke | AT-MSC | 4 | No significant improvement compared to placebo in mRS and NIHSS score. |
| 2014461 | Acute ischemic stroke | AT-MSC | 10 | Potential efficacy of intravenous administration of allogeneic AT-MSCs within the first 2 weeks of stroke. |
| 2020196 | Autism spectrum disorders | BM-MSC | 254 | After transplantation, 94.48% patients showed a positive change on ISAA (Indian Scale for Assessment of Autism) and 95.27% of patients showed an improved score on CARS (Childhood Autism Rating Scale) and 86 (86/86) patients showed improved brain activity through the FDG-PET CT scan |
| 2020201 | Autism spectrum disorders | UC-MSC | 12 | Six of 12 participants demonstrated improvement in at least two ASD-specific measures |
| 2017195 | Cerebral palsy | BM-MSC | 35 | Scores of A, B, C, D, E and total scores of GMFM and FMFM significant improvement compared to before transplantation and control group |
| 2020199 | Cerebral palsy | UC-MSC | 19 | The ADL, CFA, and GMFM-88 scores significant improvement compared to before transplantation and control group |
| 2011189 | Chronic stroke | BM-MSCs | 12 | A modest increase in Fugl Meyer and modified Barthel index score. |
| Increase the number of cluster activation of Brodmann areas 4 and 6 after MSC infusion. | ||||
| 2019192 | Chronic stroke | Allogeneic BM-MSCs | 36 | The treatment was safe and well-tolerated based on serial exams, electrograms, laboratory tests, and computed tomography scans of chest/abdomen/pelvis. |
| All behavioral endpoints showed significant improvement over 12 months of follow-up. | ||||
| 2005186 | Ischemic stroke | BM-MSCs | 5 | Improve motor functions in the MSC-treated group during the follow-up period with no statistical significance. |
| 2010187 | Ischemic stroke | BM-MSC | 16 | mRS score significant improvement over the control group |
| 2011188 | Ischemic stroke | BM-MSCs | 12 | Slight change in NIHSS and mean lesion volume after the first week of infusion. |
| Slight improvement in mRS score. | ||||
| 2021180 | Ischemic stroke | BM-MSC | 39 | lower extremity motor function significant improvement over the control group |
| 2020190 | Ischemic stroke | BM-MSC | 16 | Did not improve the Basel index, mRS, and NIHSS after 2 years post infusion. |
| MSC-based therapy might improve motor performance and task-related primary motor cortex activity. | ||||
| 2022191 | Ischemic stroke | BM-MSC | 31 | Significant improvement in motor functions in MSC group. |
| In neuroimaging analysis, corticospinal tract and posterior limb of the internal capsule fractional anisotrophy did not reduced in the MSC group but significantly decreased in the control group 90 days post infusion. | ||||
| Interhemispheric connectivity and ipsilesional connectivity significantly increased in the MSC group. | ||||
| 2018462 | Ischemic stroke | Allogeneic UC-MSC | 10 | A slight improvement in mRS and NIHSS score relative to baseline. |
| 2013203 | Spinal cord injury | UC-MSC | 22 | Treatment was effective in 13 of 22 patients in ASIA, and IANR-SCIFRS scores |
| 2021202 | Spinal cord injury | UC-MSC | 41 | Significant improvement compared to before transplantation in ASIA total score, pinprick score and light touch, IANR-SCIFRS total score and sphincter score |
| 2009463 | Spinal cord injury | BM-MSC | 10 | Improvement in ASIA score, SEP and EMG. |
| 2012464 | Spinal cord injury | BM-MSC | 10 | 6/10 patients showed improvement of motor power of the upper extremities at a 6-month follow-up |
| 3/10 patients showed gradual improvement in activities of daily living. | ||||
| MRI showed reduction in cavity size and the presence of fiber-like slow signal intensity steaks. | ||||
| 2012465 | Spinal cord injury | BM-MSC | 5 | Significant improvement was observed in patients with AIS grade B and C. |
| 2013466 | Spinal cord injury | BM-MSC | 50 | BM-MSC-treated patients combined with physical therapy showed functional improvement over the control group. |
| At 18-month follow-up, 23/50 MSC-treated cases (46%) maintained functional improvement. | ||||
| 2012467 | Spinal cord injury | BM-MSC | 11 | 5/11 (45%) MSC-infused cases showed remarkable recovery in AIS grade from A to C compared to 3/20 (15%) patients in the control group. |
| 2009468 | Spinal cord injury | BM-MSC | 30 | No changes in AIS grade. |
| No significant changes in SEP, MEP, and NCV. | ||||
| 2014469 | Spinal cord injury | BM-MSC | 14 | 7 MSC-infused patients improved AIS score. |
| 2018469,470 | Spinal cord injury | BM-MSC | 11 | 10 patients showed improvement in their neurological dysfunction after MSC infusion, although there was no statistical significance. |
| 2013471 | Spinal cord injury | BM-MSC | 20 | The clinical symptoms were improved in 10 patients with total effective rate 50% (improved AIS grade A, B, and C). |
| BM-MSCs can effectively improve the neurologic dysfunction associated with complete and chronic cervical spinal cord injury. | ||||
| 2013472 | Spinal cord injury | BM-MSC | 20 | 30 days post administration, 15 patients showed improvement, including 4/8 patients with SCI grade A, 3/4 patients with SCI grade B, and 8 patients with SCI grade C. |
| 2015473 | Spinal cord injury | BM-MSC | 1 | Improvement in AIS grade. |
| 2018474 | Cerebral palsy | UC-MSC | 27 | The significant improvement compared to the control group in GMFM-88, CFA score |
| 2010475 | Multiple sclerosis | BM-MSC | 15 | - EDSS score improved from 6.7 (1.0) to 5.9 (1.6). |
| - 24 h after MSC transplantation: increase in the proportion of CD4+CD25+ regulatory T cells, a decrease in the proliferative responses of lymphocytes, and the expression of CD40+, CD83+, CD86+, and HLA-DR on myeloid dendritic cells | ||||
| 2010476 | Multiple sclerosis | BM-MSC | 10 | - The EDSS improvement in 5/7 patients |
| - Vision and low contrast sensitivity testing at 3 months showed improvement in 5/6 | ||||
| 2011477 | Multiple sclerosis | BM-MSC | 7 | The expression of the FOXP3 was significantly increased compared to before transplantation (P<0.005) |
| 2011478 | Multiple sclerosis | BM-MSC | 8 | The improvement of 0.5-1 point on EDSS was seen in 6/8, stabilization in 1/8, progression in 1/8 |
| 2012479 2013480 | Multiple sclerosis | BM-MSC | 25 | - No statistically significant variations in gene expression and serum level of cytokines after a 1-year follow-up of MSC-treated MS patients |
| - No significant improvement compared to before transplantation in EDSS score and MRI evaluation | ||||
| 2014481 | Multiple sclerosis | BM-MSC | 9 | GEL had a trend to lower mean cumulative number |
| 2016482 | Multiple sclerosis | BM-MSC | 6 | 4/6 patients showed a measurable clinical improvement following MSC-NP treatment. |
| 2017483 | Multiple sclerosis | BM-MSC | 10 | The overall trend of improvement in EDSS and secondary clinical test included (25 WFT, 9-PHT), cognitive (MMSE), and ophthalmology (OCT, VEP) |
| 2018484 | Multiple sclerosis | UC-MSC | 2 | Improvement of physical and mental problems in both patients |
| 2018485 | Multiple sclerosis | AD-MSC | 34 | Measures of treatment effect showed an inconclusive trend of efficacy include EDSS and magnetic resonance-imaging |
25 WFT Timed 25‐Foot Walk, 9-PHT 9 Hole Peg Test, ADL Activities of Daily Living, AIS American Spinal Cord Injury Association (ASIA) Impairment Scale, CARS Childhood Autism Rating Scale CFA Comprehensive functional assessment, EDSS The Expanded Disability Status Scale, EMG electromyography, FMFM Fine motor function measurement, FOXP3 forkhead box P3, also known as scurfin, GMFM Gross motor function measurement, GMFM-88 Gross motor function measurement-88, IANR-SCIFRS International Association of Neurorestoratology-Spinal Cord Injury Functional Rating Scale, ISAA Indian Scale for Assessment of Autism, MMSE Mini‐Mental Status Examination, MRI Magnetic Resonance Imaging, mRS modified Rankin Scale, NCV nerve conduction velocity, NIHSS National Institutes of Health Stroke Scale, OCT Optical Coherence Tomography, SCI Spinal Cord Injury, SER somatosensory evoked potentials, VEP Visual Evoked Potential
One of the biggest limitations when using BM-MSCs is the bone marrow aspiration process, as it is an invasive procedure that can introduce a risk of complications, especially in pediatric and elderly patients.197 Therefore, UC-MSCs have been suggested as an alternative to BM-MSCs and are being studied in clinical trials for the treatment of neurological diseases in approximately 1550 patients throughout the world; however, only three studies have been completed, with data published recently.198 A recent study showed that UC-MSC administration improved both gross motor function and cognitive skills, assessed using the Activities of Daily Living (ADL), Comprehensive Function Assessment (CFA), and GMFM, in patients diagnosed with cerebral palsy. The improvements peaked 6 months post administration and lasted for 12 months after the first transplantation.199 In a single-targeted phase I/II clinical trial using UC-MSCs for the treatment of autism, Riordan et al. reported decreases in Autism Treatment Evaluation Checklist (ATEC) and CARS scores for eight patients, but the paper has been retracted due to a violation of the journal’s guidelines.200 In an open-label, phase I study, UC-MSCs were used as the main cells to treat 12 patients with autism spectrum disorder via IV infusions. It is important to note that five participants developed new class I anti-human leukocyte antigen in response to the specific lot of manufactured UC-MSCs, although these responses did not exhibit any immunological response or clinical manifestations. Only 50% of participants showed improvements in at least two autism-specific measurements.201 Although not as widely used as BM-MSCs, these trials have demonstrated the efficacy of using UC-MSCs in the treatment of SCIs. In a pilot clinical study, Yang et al. showed that the use of UC-MSCs has the potential to improve disease status through an increase in total ASIA and SCI Functional Rating Scale of the International Association of Neurorestoratology (IANR-SCIFRS) scores, as well as an improvement in pinprick, light touch, motor and sphincter scores.202 A study of 22 patients with SCIs showed a potential therapeutic effect in 13 patients post UC-MSC infusion.203 AT-MSCs were also used to treat SCI, with a single case report indicating an improvement in neurological and motor functions in a domestic ferret patient.204 However, a result obtained from another phase I trial using AT-MSCs showed mild improvements in neurological function in a small number of patients.205 A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial using AT-MSCs in the treatment of acute ischemic stroke published a data set that supports the safety of the therapy, although patients who received AT-MSCs showed a nonsignificant improvement after 24 months of follow-up.206 In all of the above studies, the safety of using either AT-MSCs or UC-MSCs was evaluated, and no significant reactions were reported after infusion.
Therefore, based on the number of recovered patients post-transplantation and the number of recruited patients in large-scale trials using BM-MSCs, it seems that BM-MSCs are the prominent cells in regard to treating neurodegenerative disease with potentially good outcomes (Table 1). It is important to note that we do not negate the fact that AT- and UC-MSCs also show positive outcomes in the treatment of neuronal diseases, with numerous ongoing large-scale, multicentre, randomized, and placebo-control trials,207,208 but we suggest alternative and thoughtful decisions regarding which sources of MSCs are best for the treatment of neuronal diseases and degenerative disorders.
Respiratory disease and lung fibrosis: clinical data support UC as a good source of MSCs
In the last decade, significant increases in respiratory disease incidence due to air pollution, smoking behavior, population aging, and recently, respiratory virus infections such as coronavirus disease 2019 (COVID-19)209 have been observed, leading to substantial burdens on public health and healthcare systems worldwide. Respiratory inflammatory diseases, including bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), and acute respiratory distress syndrome (ARDS), have recently emerged as three prevalent pulmonary diseases in children and adults. These conditions are usually associated with inflammatory cell infiltration, a disruption of alveolar structural integrity, a reduction in alveolar fluid clearance ability, cytokine release and associated cytokine storms, airway remodeling, and the development of pulmonary fibrosis. Traditional treatments are focused on relieving symptoms and preventing disease progression using surfactants, artificial respiratory support, mechanical ventilation, and antibiotic/anti-inflammatory drugs, with limited effects on the damaged airway, alveolar fluid clearance, and other detrimental effects caused by the inflammatory response. MSCs are known for their immunomodulatory abilities, showing potential in injury reduction and aiding lung recovery after injury. According to ClinicalTrials.gov, from 2017 to date, there have been 159 studies testing the application of MSCs in the treatment of pulmonary diseases, including but not limited to BPD, COPD, and ARDS, suggesting a trend in the use of MSCs as an alternative approach for the treatment of respiratory diseases, especially MSCs from UC as an “off-the-shelf” and allogeneic source.
Extremely premature infants are born with arrested lung development at the canalicular-saccular phases prior to alveolarization and before pulmonary maturation occurs, which results in the development of BPD.210 These infants require intensive care during the first three months of life using postnatal interventions, including positive pressure mechanical ventilation, external oxygen support, and surfactant infusions, and the newborns have recurrent infections that further compromise normal lung development.211 To date, 13 clinical trials have been proposed to use UC-MSCs in the treatment of BPD, recruiting ~566 premature infants throughout the world, including Vietnam, Korea, the United States, Spain, Australia, and China. The majority of these trials use UC-derived stem cells for phases I and II, focusing on evaluating the safety and efficacy of stem cell-based therapy.212 Human UC tissue and its derivative components are considered the most attractive cell sources for MSCs in the treatment of BPD due to the ease of obtaining them, being readily available, with no ethical concerns, low antigenicity, a high cell proliferation rate, and superior regenerative potential. Chang et al. used MSCs derived from UC blood in a phase I dose-escalation clinical trial to treat 9 preterm infants via intratracheal administration to prevent the development of BPD.213 All 9 preterm infants survived, and only three developed BPD; these infants had significantly decreased BPD severity compared with the historically matched control group. A follow-up study of the same patients after 24 months indicated that only one infant had an E. cloacae infection after discharge at 4 months, with subsequent disseminated intravascular coagulation, which was later proven to be unrelated to the intervention. The remaining eight patients survived with normal pulmonary development and function, suggesting that the therapy was safe. MSCs from UC blood were also used for the treatment of 12 extremely low birthweight preterm patients using the same administration route, which further confirmed the safety of the therapy in the treatment of BPD, although ten of 12 infants still developed severe BPD at 36 weeks.214 Our group also reported the safety and potential efficacy of using UC-MSCs in the treatment of four preterm infants, and the results supported the safety of UC-MSCs and demonstrated that patients could be weaned from oxygen supply and develop normal lung structure and function.215 A phase II clinical trial of 66 infants born at 23–28 weeks with a birthweight of 500–1250 g who were recruited and randomized into an MSC-administration group and a control group was conducted. Although the results supported the safety of MSC administration in preterm infants, the efficacy of the treatment was not supported by statistical analysis, potentially due to the small sample size. Subgroup analysis showed that patients with severe BPD born at 23–24 weeks showed a significant improvement in BPD severity, but those born at 25–28 weeks did not.216 Hence, it is important to conduct controlled phase II clinical trials with larger cohort sizes to further substantiate the efficacy of UC blood-derived MSCs in the treatment of infants with BPD.
With more than 65 million patients worldwide, COPD was the third-leading cause of death in 2020, according to World Health Organization records. COPD is classified as a chronic inflammatory and destructive pulmonary disease characterized by a progressive reduction in lung function. Averyanov et al. performed a randomized, placebo-controlled phase I/IIa study in 20 patients with mild to moderate idiopathic pulmonary fibrosis (IPF). Treatment group patients received two IV doses of allogeneic MSCs (2 × 108 cells) every 3 months, and the second group received a placebo.217 Evaluation tests were performed at weeks 13, 26, 39, and 52. The 6-min walking test distance (6MWTD) results showed that patient fitness improved from week 13 onwards and was maintained until up to the 52nd week. Pulmonary function indicators improved markedly before and after treatment in the treated group but did not change significantly in the placebo group. The goal of MSC therapy in the treatment of COPD is to promote the regeneration of parenchymal cells and alveolar structure and the restoration of lung function. Based on the results of a phase I trial of a commercial BM-MSC product, ProchymalTM, which led to improvements in pulmonary function in treated patients, a multicentre, double-blind, placebo-controlled phase II trial was conducted in 62 patients diagnosed with COPD to determine the safety and potential efficacy of the product. Although the results supported the safety of BM-MSCs, their effectiveness in the treatment of COPD was not assured. No statistically significant differences in FEV1 or FEV1%, total lung capacity, or carbon monoxide diffusing capacity were detected after 2 years of follow-up between the two treatment groups. To date, there have been five clinical trials using BM-MSCs as the main stem cells for the treatment of COPD, but the overall clinical outcomes did not demonstrate the potential therapeutic effects of the treatment.218–222 In clinical trial NCT001110252, the results showed that there was an overall reduction in the process of COPD pathological development 3 years after the administration of BM-MSCs, although the trial had a phase I design, with no control group, and evaluated only a small cohort (four patients).219 To alleviate local inflammatory progression in COPD, Oliveira et al. studied the combination treatment of one-way endobronchial valve (EBV) and BM-MSC intubation.223 Ten GOLD (Global Initiative for Obstructive Lung Disease) stage C or D patients were equally divided into 2 groups: one group received a dose of 108 cells before valve insertion, and the other group received a normal saline infusion. The follow-up time was 90 days. Inflammation was significantly improved as assessed by the CRP (C-reactive protein) index at 30 and 90 days after infusion. In addition, improvements in St. George’s Respiratory Questionnaire (SGRQ) scores indicated improved patient quality of life. Furthermore, an investigation into the homing ability of MSCs in vivo was performed on 9 GOLD patients, from stage A to stage D. Each patient received two 2 × 106 BM-MSC/kg IV infusions 1-week apart.224 The marking of MSCs with indium-111 showed that MSCs were retained in the pulmonary vasculature longer in patients with mild COPD and that the levels of inflammatory mediators improved after 7 days of treatment. The results of the evaluation survey conducted after 1 year showed that the number of COPD exacerbations decreased to six times/year compared to 11 times/year before treatment. In addition, AT-MSCs present in the stromal vascular fraction were used to treat patients with COPD, and no adverse events were observed after 12 months of follow-up, but the clinical improvements post administration were not clear.225 The results from a phase I clinical trial using AT-MSCs in eight patients with COPD also reported no significant change in pulmonary function test parameters.226 A study evaluating the use of AT-MSCs as adjunctive therapy for COPD in 12 patients was performed.227 AT was obtained using standard liposuction, MSCs were isolated, and 150–300 million cells were intravenously infused. The patients showed improvements in quality of life, with improved SGRQ scores after 3 and 6 months of treatment. Recently, UC-MSCs have emerged as potential allogeneic stem cell candidates for the treatment of COPD.228 In a pilot clinical study, it was demonstrated that allogeneic administration of UC-MSCs in the treatment of COPD was safe and potentially effective.229 In one study, 20 patients, including 9 at stage C and 11 at stage D per the GOLD classification, with histories of smoking were recruited and received cell-based therapy. The patients who received UC-MSC treatment showed significant reductions in Modified Medical Research Council scores, COPD assessment test scores, and the number of pulmonary exacerbations 6 months post administration. The results of the second trial using UC-MSCs showed that the mean FEV1/FVC ratios were increased along with improvements in SGRQ scores and 6MWTDs at three months post administration.230 Although thorough assessments of the effectiveness of UC-MSCs are still in the early stages, the number of trials using UC-MSCs for the treatment of COPD is increasing steadily, with larger sample sizes and stronger designs (randomized or matched case–control studies), providing a data set strongly supporting the future applications of UC-MSCs.231
The ongoing pandemic of the 21st century, the COVID-19 pandemic, emerged as a major pulmonary health problem worldwide, with a relatively high mortality rate. Numerous studies, reviews, and systematic analyses have been conducted to discuss and expand our knowledge of the virus and propose different mechanisms by which the virus could alter the immune system.232 One of the most critical mechanisms is the generation of cytokine storms, which result from the initiation of hyperreactions of the adaptive immune response to viral infection.233 These cytokine storms are formed by the establishment of waves of hypercytokinaemia generated from overreactive immune cells, which enhance their expression of TNF-α, IL-6, and IL-10, preventing T-lymphocyte recruitment and proliferation and culminating in T-lymphocyte apoptosis and T-cell exhaustion. In COVID-19, once a cytokine storm is formed, it spreads from an initial focal area through the body via circulation, which has been discussed in a comprehensive review by Jamilloux et al.234 At the time of writing this review, there were 74 clinical trials using MSCs from UC (29 trials; including WJ-derived MSCs (WJ-MSCs) and placenta-derived MSCs (PL-MSCs)), AT (15 trials), and BM (11 trials) (comprehensive review171,235). Hence, UC-MSCs have emerged as the most common MSCs for the treatment of COVID-19, with a total of 1047 patients participating in these trials. Among these trials, 15 completed trials using UC-MSCs (including WJ- and PL-MSCs) have been reported, with clinical data from approximately 600 recruited patients.232 Eight of these 15 studies used allogenic UC-MSC transplantation to treat critically ill patients.236 A list of case reports using UC-MSCs showed that the treatments were safe and well-tolerated in 14 patients with COVID-19, with the primary outcomes including increased percentages and numbers of T cells,237,238 improved respiratory and renal functions,239 reductions in inflammatory biomarker levels,240 and positive outcomes in the PaO2/FiO2 ratio.240 In a pilot study conducted in ten patients with severe COVID-19, a single dose of UC-MSCs was safe and improved clinical outcomes, although the study did not investigate whether multiple doses of UC-MSCs could further improve the outcomes.241 Two trials without a control group were conducted in 47 patients, and the results indicated that UC-MSCs were safe and feasible for the treatment of patients with COVID-19.235,242 A single-center, open-label, individually randomized, standard treatment-controlled trial was performed in 41 patients (12 patients assigned to the UC-MSC group), and the results showed that significant improvements in C-reactive protein levels, IL-6 levels, oxygen indices, and lymphocyte numbers were found in the MSC groups. Chest computed tomography (CT) illustrated significant reductions in lung inflammatory responses as reflected by CT findings, the number of lobes involved, and pulmonary consolidation.238 In a phase I trial conducted in 18 hospitalized patients with COVID-19, UC-MSCs were administered via an IV route in nine patients (five patients with moderate COVID-19 and 4 patients with severe COVID-19) at days 0, 3, and 6, with no treatment-related adverse events or severe adverse events.243 Only one patient in the UC-MSC group required mechanical ventilation, compared to four patients in the control group. However, the clinical outcomes, such as COVID-19 symptoms, laboratory test results, CT findings of lung damage, and pulmonary function test parameters, were improved in both groups. Interestingly, a 1-year follow-up of the same sample revealed that the patients who received UC-MSC administration improved in terms of whole-lung lesion volume compared to the control group.244 Moreover, chest CT at 12 months showed significant regeneration of lung tissue in the MSC-administered groups, whereas lung fibrosis was found in all patients in the control group. This finding is of interest because it indicates that a long time is needed to detect the regenerative functions of MSC-based therapy, as the biological process to enhance lung tissue regeneration occurs relatively slowly and requires multiple steps. The effects of UC-MSCs in the attenuation and prevention of the development of cytokine storms were illustrated in an interventional, prospective, three-parallel arm study with two control arms conducted in 30 patients in moderate and critical clinical conditions.245 The results indicated a significant decrease in proinflammatory cytokines (IFNγ, IL-6, IL-17A, IL-2, and IL-12) and an increase in anti-inflammatory cytokines (IL-10, IL-13, and IL-1ra), suggesting that UC-MSCs might participate in the prevention of cytokine storm development. Lanzoni et al. performed a double-blind, randomized, controlled trial and found that UC-MSC infusions significantly decreased cytokine levels at day 6 and improved survival in patients with COVID-19 with ARDS. In this trial, 24 patients were randomized and assigned 1:1 to receive either MSCs or placebo.246 MSC treatment was associated with a significant improvement in the survival rate without serious adverse events. To date, other trials conducted using UC-MSCs as the main MSCs provide a solid data set on their safety and efficacy in preventing the development of cytokine storms, reducing the inflammatory response, improving pulmonary function, reducing intensive care unit (ICU) stay duration, enhancing lung tissue regeneration, and reducing lung fibrosis progression.240,247–249 In two large cohort studies (phase I with 210 patients and phase II with 100 patients), the volume of lung lesions and solid component injuries of patients’ lungs were reduced significantly after the administration of UC-MSCs,250 and clinical symptoms and inflammatory levels were improved.251 Of the 26 reported clinical trials for the treatment of COVID-19 with MSCs, 1 study used AT-MSCs as the main MSCs.236 Thirteen COVID-19 adult patients under invasive mechanical ventilation who had received previous antiviral and/or anti-inflammatory treatments (including steroids, lopinavir/ritonavir, hydroxychloroquine, and/or tocilizumab, among others) were treated with allogeneic AT-MSCs. With a mean follow-up time of 16 days after infusion, 9/13 patients’ clinical symptoms improved, and 7/13 patients were intubated. A decrease in inflammatory cytokines and an increase in immunoregulatory cells were also observed in patients, especially in the group of patients with overall clinical improvement. Although there is a lack of clinical efficacy data supporting the use of AT-MSCs in the treatment of patients with COVID-19, AT-MSCs are still potential candidates for inhibiting COVID-19 due to their high secretory activity, strong immune-modulatory effects, and homing ability.252–254
For ARDS, in a phase IIa trial, 60 patients with moderate to severe disease were randomized into 2 groups. A group of 40 patients received a single infusion of BM-MSCs at a dose of 1 × 106 cells/kg body weight, and another 20 patients received a placebo.255 After 6 and 24 h of infusion, the decrease in plasma inflammatory cytokine levels in the MSC group was significantly greater than that in the placebo group. For severe pulmonary hypertension (PH) associated with BPD (BPD-PH), in a small trial, two preterm infants born at 26–27 weeks of age were intravenously administered heterologous BM-MSCs at a dose of 5 × 106 cells per kg of body weight; the treatment reduced oxygen requirements and supported respiration in the infants.256 The administration of allogeneic AT-MSCs in the treatment of ARDS appeared to be safe and well-tolerated in 12 adult patients, but clinical outcomes were not observed.257 The results of two patients who received BM-MSCs showed that both patients had improved respiratory function and hemodynamic function and a reduction in multiorgan failure.258 Although the safety of BM-MSCs was confirmed in a multicentre, open-label, dose-escalation, phase I clinical trial (The Stem cells for ARDS treatment—START trial),259 no significant improvements were found in a phase II trial, including in respiratory function and ARDS conditions.260 The safety profile of UC-MSCs is also supported by the findings of a previous phase I clinical trial conducted in 9 patients, which showed that a single IV administration of UC-MSCs was safe and led to positive outcomes in terms of respiratory function and a reduction in the inflammatory response.261 The findings of this study were also supported by those of the REALIST (Repair of Acute Respiratory Distress with Stromal Cell Administration) trial, which further confirmed the maximum tolerated dose of allogeneic UC-MSCs in patients with moderate to severe ARDS.262
Although AT- and BM-MSCs have demonstrated therapeutic potential with similar mechanisms of action, UC-MSCs have emerged as potential candidates in the treatment of pulmonary diseases due to their ease of production as “off-the-shelf” products, rapid proliferation, noninvasive isolation methods, and supreme immunological regulation as well as anti-inflammatory effects.263 However, it is important to note that there is a need to conduct phase III clinical trials with larger cohorts and trials with at least two sources of MSCs in the treatment of pulmonary conditions to further confirm this speculation.264 Table 2 summarizes several clinical trials with published results discussed in this review.
Table 2.
The reported clinical trials using MSCs from AT, BM, and UC in the treatment of respiratory diseases
| Year | Disease | MSC source | No. of MSC-treated patients | Efficacy |
|---|---|---|---|---|
| 2015258 | ARDS | BM | 2 | - Failed to improve after both standard life support measures, including mechanical ventilation, and additional measures, including extracorporeal ventilation |
| 2015259 | ARDS | BM | 9 | - Safety evaluation without secondary outcomes |
| 2019260 | ARDS | BM | 60 | - Increase Acute Physiology and Chronic Health Evaluation III, minute ventilation, and PEEP |
| - No difference in mortality between the treatment and control group. | ||||
| 2020261 | ARDS | UC | 9 | - Reduction of inflammation |
| - Increase in immune cells | ||||
| 2021262 | ARDS | UC | 9 | - Reduction of inflammation |
| - Increase clinical outcomes | ||||
| 2018486 | BPD | BM | 2 | - No improvement after the treatment |
| 2014213 | BPD | UC | 9 | - Reduction in inflammation |
| - Improvement in respiratory severity score | ||||
| 2020215 | BPD | UC | 4 | - Reduction in fibrosis |
| - All treated patients recovered | ||||
| 2021216 | BPD | UC | 12 | - Reduction in inflammation |
| - Improvement in secondary outcomes | ||||
| 2021216 | BPD | UCB | 33 | - No significant improvement in the primary outcomes between the two groups. |
| - Severe BPD patients were significantly improved with MSC transplantation. | ||||
| 2015226 | COPD | AD | 8 | - Safety and feasibility |
| 2017225 | COPD | AD | 12 | - Safety |
| 2018224 | COPD | BM | 9 | - Fail to migrate to areas of emphysematous remodeling in the lung |
| - Reduction of systemic immunological response | ||||
| 2011218 | COPD | BM | 4 | - Improvement in Psychological condition and quality of life, and clinical condition |
| 2013219 | COPD | BM | 4 | - Improvement in FVC, 6MWD, and DLCO |
| 2013220 | COPD | BM | 30 | - Improvement in FVC, FEV1, 6MWD, and – Reduction in inflammation |
| 2017221 | COPD | BM | 5 | - Improvement in FVC, TLC, 6MWD, and DLCO |
| 2020230 | COPD | UC | 5 | - Improvement in SGRQ symptom, activity, and impact scores |
| 2020263 | COPD | UC | 20 | - Improvement in FEV1, FCV, 6MWT, but not significant |
| - Improvement in Modified Medical Research Council and COPD assessment test in all patients | ||||
| 2021175 | COVID-19 | UC | 65 | - Decrease in lung lesion proportion |
| 2020235 | COVID-19 | UC | 16 | - Improvement in oxygenation index |
| - Increase in the number of lymphocytes | ||||
| - Reduction in inflammation | ||||
| 2020238 | COVID-19 | UC | 12 | - Clinical improvement |
| - Increase the number of lymphocytes | ||||
| - Decrease in inflammatory cytokines and CRP | ||||
| - Improvement in CT score | ||||
| 2020239 | COVID-19 | UC | 1 | - Improvement in renal function |
| - Increase in the number of lymphocytes | ||||
| 2020243 | COVID-19 | UC | 9 | - Improvement in PaO2/FiO2 and CT score |
| - Decrease inflammatory cytokine levels | ||||
| 2020248 | COVID-19 | UC | 6 | - Reduction in dyspnea, serum inflammatory cytokines |
| - Increase in Sp02 | ||||
| - Improvement in CT score | ||||
| 2021242 | COVID-19 | UC | 31 | - Reduction in inflammation |
| - Improvement in CRP and CT score | ||||
| 2021247 | COVID-19 | UC | 12 | - Increase patients ‘survival |
| - Reduction in inflammatory cytokines | ||||
| 2021249 | COVID-19 | UC | 29 | - Maintenance of SARS-CoV-2-specific antibodies and immune homeostasis |
| - Reduction in CRP, proinflammatory cytokines, neutrophil extracellular traps | ||||
| 2021250 | COVID-19 | UC | 65 | - Reduction in lung lesion |
| - Increase 6MWD | ||||
| - Improve CT score | ||||
| 2021251 | COVID-19 | UC | 210 | - Increase high survival |
| - Improvement in SaO2 | ||||
| 2020237 | COVID-19 | WJ | 1 | - Decrease in inflammatory cytokines and CRP |
| - Increase in number of CD3+, CD4+, CD8+ | ||||
| 2021240 | COVID-19 | WJ | 5 | - Reduction in inflammation |
| - Improvement in CRP and CT score | ||||
| 2021245 | COVID-19 | WJ | 10 | - Improvement in CRP |
| - Decrease in inflammation | ||||
| 2019487 | Idiopathic pulmonary fibrosis | BM | 10 | - Improvement in FVC, 6MWD, and DLCO |
| 2016222 | LVES | BM | 7 | - Improvement in FEV1 |
| - Increase in number of CD3+ in alveolar septa and CD31+ in the alveolar septum, |
6MWD 6-min walk distance, ARDS acute respiratory distress syndrome, BPD bronchopulmonary dysplasia, COPD chronic obstructive pulmonary dysplasia, CRP C-reactive protein, CT computed tomography scan, DLCO diffusing capacity for CO, FCV flow-controlled ventilation, FEV1 forced expiratory volume in 1 s, FiO2 fraction of inspired oxygen, FVC forced vital capacity, PaO2 partial pressure of oxygen, PEEP positive end-expiratory pressure, SGRQ St George’s Respiratory Questionnaire, TLC total lung capacity
Endocrine disorders, infertility/reproductive function recovery, and skin burns: should we consider AT-MSCs as the main MSCs based on their origin?
Endocrine disorders
The human body maintains function and homeostatic regulation via a complex network of endocrine glands that synthesize and release a wide range of hormones. The endocrine system regulates body functions, including heartbeat, bone regeneration, sexual function, and metabolic activity. Endocrine system dysregulation plays a vital role in the development of diabetes, thyroid disease, growth disorder, sexual dysfunction, reproductive malfunction, and other metabolic disorders. The central dogma of regenerative medicine is the use of adult stem cells as a footprint for tissue regeneration and organ renewal. The functions of these stem cells are tightly regulated by microenvironmental stimuli from the nervous system (rapid response) and endocrine signals via hormones, growth factors, and cytokines. This harmonized and orchestrated system creates a symphony of signals that directly regulate tissue homeostasis and repair after injury. The disruption of these complex networks results in an imbalance of tissue homeostasis and regeneration that can lead to the development of endocrine disorders in humans, such as diabetes, sexual hormone deficiency, premature ovarian failure (POF), and Asherman syndrome.
In recent years, obesity and diabetes (type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM)) have been the two biggest challenges in endocrinology research, and the application of MSCs has emerged as a novel approach for therapeutic consideration. T1DM is characterized by the autoimmune destruction of pancreatic β-cells, whereas T2DM is defined as a combination of insulin resistance and pancreatic insulin-producing cell dysfunction. Regenerative medicine seeks to provide an exogenous cell source for replacing damaged or lost β-cells to achieve the goal of stabilizing patients’ blood glucose levels. To date, there are 28 clinical trials using MSCs in the treatment of T1DM (http://www.clinicaltrials.gov, searched in October 2021), among which three trials were completed using autologous BM-MSCs (NCT01068951), allogeneic BM-MSCs (NCT00690066), and allogeneic AT-MSCs (NCT03920397). Interestingly, UC-MSCs were the most favored MSCs for the remaining trials. All published studies confirmed the safety of MSC therapy in the treatment of T1DM with no adverse events. The first study using autologous BM-MSCs showed that patients who were randomized into the MSC-administration group showed an increase in C-peptide levels in response to a mixed-meal tolerance test (MMTT) in comparison to the control group.265 Unfortunately, there was no significant improvement in C-peptide levels, HbA1C or insulin requirements. The use of autologous AT-MSCs in combination with vitamin D was safe and improved HbA1C levels 6 months post administration.266 WJ-MSCs were used as the main MSCs for the treatment of new-onset T1DM, which showed a significant improvement in both HbA1C and C-peptide levels when compared to those of the control group at three and six months post administration.267,268 The combination of allogeneic WJ-MSCs with autologous BM-derived mononuclear cells improved insulin secretion and reduced insulin requirements in patients with T1DM.269 In terms of T2DM, 23 studies were registered on clinicaltrials.gov (searched in October 2021), with six completed studies (three studies used BM-MSCs and three studies used allogeneic UC-MSCs). Although the number of studies using MSCs for the treatment of T2DM is small, their findings support the safety of MSCs, with no severe adverse events observed during the course of these studies.270 It was confirmed that MSC therapy potentially reduced fasting blood glucose and HbA1C levels and increased C-peptide levels. However, these effects were short-term, and multiple doses were required to maintain the MSC effects. Interestingly, the autologous MSC approach in the treatment of patients with diabetes in general is hampered, as both BM-MSCs and AT-MSCs isolated from patients with diabetes showed reduced stemness and functional characteristics.271,272 In addition, the durations of diabetes and obesity are strongly associated with autologous BM-MSC metabolic function, especially mitochondrial respiration, and the accumulation of mitochondrial DNA, which directly interfere with the functions of BM-MSCs and reduce the effectiveness of the therapy.271 Therefore, the allogeneic approach using MSCs from healthy donors provides an alternative approach for stem cell therapy in the treatment of patients with diabetes.
Infertility and reproductive function recovery
Modern society is increasingly facing the problem of infertility, which is defined as the inability to become pregnant after more than 1 year of unprotected intercourse.273 This problem has emerged as an important worldwide health issue and social burden. Assisted reproductive techniques and in vitro fertilization technology have recently become the most effective methods for the treatment of infertility in humans, but the use of these approaches is limited, as they cannot be applied in patients with no sperm or those who are unable to support implantation during pregnancy, they are associated with complications, they are time-consuming and expensive, and they are associated with ethical issues in certain territories.274 Numerous conditions are related to infertility, including POF, nonobstructive azoospermia, endometrial dysfunction, and Asherman syndrome. Recent progress has been illustrated in preclinical studies for the potential applications of stem cell-based therapy for reproductive function recovery, especially recent studies in the field of MSCs, which provide new hope for patients with infertility and reproductive disorders.275
POF is characterized by a loss of ovarian activity during middle age (before 40 years old) and affects 1–2% of women of reproductive age.276 Patients diagnosed with POF exhibit oligo-/amenorrhea for at least 4 months, with increased levels of follicle-stimulating hormone (FSH) (>25 IU/L) on two occasions more than 1 month apart.277 Diverse factors, such as genetic backgrounds, autoimmune disorders, environmental conditions, and iatrogenic and idiopathic situations, have been reported to be the cause of POF.278 POF can be treated with limited effectiveness via psychosocial support, hormone replacement intervention, and fertility management.279 MSCs from AT, BM, and UC have been used in the treatment of POF, with improvements in ovarian function in preclinical studies using chemotherapy-induced POF animal models. The early published POF study using BM-MSCs as the main cell source is a single case report in which a perimenopausal woman showed an improvement in follicular regeneration, and increased AMH levels resulted in a successful pregnancy followed by delivery of a healthy infant.280 A report using autologous BM-MSCs in two women with POF illustrated an increase in baseline estrogen levels and the volume of the treated ovaries along with amelioration of menopausal symptoms.281 The clinical procedures used in this early trial were invasive, as patients underwent two operations: (1) BM aspiration and (2) laparoscopy. A similar approach was used in two trials conducted in 10 women with POF (age range from 26–33 years old) and 30 patients (age from 18 to 40 years old).282 A later study investigated two different routes of cell delivery, including laparoscopy and the ovarian artery, but the results have not been reported at this time.282 Based on the positive outcomes of the mouse model, an autologous stem cell ovarian transplantation (ASCOT) trial was deployed using BM-derived stem cells with encouraging observations of improved ovarian function, as determined by elevated levels of AMH and AFC in 81.3% of participants, six pregnancies, and the successful delivery of three healthy babies.283 A randomized trial (NCT03535480) was conducted in 20 patients with POF aged less than 39 years to further elaborate on the results of the ASCOT trial.284 To date, there are no completed trials using AT-MSCs or UC-MSCs in the treatment of patients with POF, limiting the evaluation of these MSCs in the treatment of POF. The speculated reason is that POF is a rare disease, affecting 1% of women younger than 40 years, and with improvements in assisted productive technology, patients have several alternative options to enhance the recovery of reproductive function.285
Wound healing and skin burns
Burns are the fourth most common injury worldwide, affecting ~11 million people, and are a major cause of death (180,000 patients annually). The severity of burns is defined based on the percentage of surface area burned, burn depth, burn location and patient age, and burns are usually classified into first-, second-, third-, and fourth-degree burns on the basis of their severity.286 Postburn recovery depends on the severity of the burn and the effectiveness of treatment. Rapid healing may occur over weeks, while alternatively, healing can take months, with the ultimate result being scar formation and disability in patients with severe burns. Different from mechanical injury, burn injury is an invasive progression of damage to tissue at the burn site, including both mechanical damage to the skin surface and biological damage caused by natural apoptosis that prolongs excessive inflammation, oxidative stress, and impaired tissue perfusion.287 To date, completely reversing the devastating damage of severe burns remains unachievable in medicine, and stem cell therapy provides an alternative option for patients with burn injury. The first case report of the use of BM-MSCs to treat a 45-year-old patient with burns on 40% of their body demonstrated the safety of the therapy and showed partial improvements in vascularization at the wound site and reduced coarse cicatrices.288,289 Later, patients with second- and third-degree burns as well as deep burns were treated using either autologous BM-MSCs or allogeneic BM-MSCs by spraying the MSCs onto the burn sites or adding MSCs over a dermal matrix sheet to cover the wound. The results in these case reports revealed the potential efficacy of MSC-based therapy, which not only enhanced the speed of wound recovery but also reduced pain and improved blood supply without introducing infection.288,290,291 In 2017, a study conducted in 60 patients with 10–25% of their total body surface areas burned treated with either autologous BM-MSCs or UC-MSCs showed that both MSC types improved the rate of healing and reduced the hospitalization period.292 The drawback of BM-MSCs in the treatment of burns is the invasive harvesting method, which causes pain and possible complications in patients. Hence, treatment with allogeneic MSCs obtained from healthy donors is the method of choice, and AT- and UC-MSCs are two suitable candidates for this option. To date, a limited number of clinical trials have been conducted using MSC therapy. These trials have several limitations in trial design, such as a lack of a negative control group and blinding, small sample sizes, and the use of standardized measurement tools for burn injury and wound healing. Currently, AT-MSCs are being used in seven ongoing phase I and II trials in the treatment of burns. Hence, it is important to note that among the most widely studied MSCs, AT-MSCs have advantages over BM-MSCs when obtained from an allogeneic source, while their abilities in burn treatment remain to be determined. The main MSCs that should be used in the regeneration of burn tissue remain undefined (Table 3), and we observed the trend that AT-MSCs are more suitable candidates due to their biological nature, which contributes to the generation of keratinocytes and secretion profiles that strongly enhance the skin regeneration process.293–296
Table 3.
The reported clinical trials using MSCs from AT, BM, and UC in the treatment of the endocrinological disorder, reproductive disease, and skin healing
| Year | Type of disease | Cell source | No. of treated patients | Efficacy |
|---|---|---|---|---|
| 2014265 | Type 1 diabetes | BM | 9 | - No significant improvement compared to control group in HbA1c, insulin doses per kilogram, fasting C-peptide |
| - 3/9 MSC-treated patients decreased their peak C-peptide or AUC response to the MMTT while 8/9 patients decreased in peak C-peptide, and 7/9 decreased in AUC response in the control group | ||||
| 2021266 | Type 1 diabetes | AD | 7 | Significant improvement compared to before transplantation in basal C-peptide and HbA1C |
| 2013268 | Type 1 diabetes | WJ | 15 | Significant improvement over the control group in HbA1c and fasting C-peptide |
| 2015269 | Type 1 diabetes | WJ + BM | 21 | The metabolic measures improved in treated patients: |
| + AUC C-Pep increased 105.7% (P = 0.00012); | ||||
| + insulin area under the curve increased 49.3% (P = 0.01) | ||||
| + HbA1c decreased 12.6% (P < 0.01) | ||||
| + Fasting glycemia decreased 24.4% (P < 0.002) | ||||
| + Daily insulin requirements decreased 29.2% (P = 0.001) | ||||
| 2021271 | Type 2 diabetes | BM | 25 | A slight reduction in HbA1c levels was observed in the first 3 months after administration, but the level returned to normal after 6 months and even increased |
| 2005288 | Skin burns | BM | 1 | The improvement in vascularization at the wound site and reduced coarse cicatrices |
| 2012290 | Skin burns | BM | 1 | The areas treated with autologous BM-MSCs combined with transplantation of split skin were less likely to have contraction of the skin grafts. |
| 2008291 | Skin wounds | BM | 20 | The wound mostly healed in 18 of the 20 patients showed the BM-MSCs transplantation effectively |
| 2017292 | Skin burns | BM-MSC & UC-MSC | 40 | The significantly improved rate of healing in both BM-MSC and UC-MSC groups as compared to traditionally treated group in percent of burn extent (%), hospitalization time. |
| 2018280 | Premature ovarian insufficiency | BM-MSC | 1 | - The AMH level improved from 0.4 to 0.9 ng/mL |
| - The improvement of follicular regeneration resulted in a successful pregnancy followed by the delivery of a healthy infant | ||||
| 2020281 | Premature ovarian failure | BM-MSC | 2 | The increase in baseline estrogen levels and amelioration of menopausal symptoms |
| 2018422 | Premature ovarian insufficiency | UC-MSC | 14 | The elevated estradiol concentrations, improved follicular development, and increased number of antral follicles |
| 2016488 | Premature ovarian insufficiency | BM-MSC | 10 | The improvement in Edessy ovarian reserve score (EORS) and increased pregnancy capacity |
| 2016489 | Premature ovarian insufficiency | BM-MSC | 30 | 86.7% of patients showed a fall in FSH levels and a rise in estrogen and AMH levels after 4 weeks of injection |
| 2018283 | Premature ovarian insufficiency | BM-MSC | 15 | - The significant improvement in AFC and AMH after treatment. |
| - Increased the number of stimulable antral follicles and oocytes | ||||
| - Ovarian function improved in 81.3% of women |
AFC antral follicle count, AMH anti-Müllerian hormone, AUC area under the curve (oral glucose tolerance test), FSH follicle-stimulating hormone, HbA1C hemoglobin A1C, MMTT mixed-meal tolerance test
MSC applications in cardiovascular disease: a promising but still controversial field
In the last two decades, great advancements have been achieved in the development of novel regenerative medicine and cardiovascular research, especially stem cell technology.297 The discovery of human embryonic stem cells and human induced pluripotent stem cells (hiPSCs) opened a new door for basic research and therapeutic investigation of the use of these cells to treat different diseases.298 However, the clinical path of hiPSCs and hiPSC-derived cardiomyocytes in the treatment of cardiovascular diseases is limited due to the potential for teratoma formation with hiPSCs and the immaturity of hiPSC-derived cardiomyocytes, which might pose a risk of cancer formation,299 arrhythmia, and cardiac arrest to patients.300 A recently emerged stem cell type is adult stem cells/progenitor cells, including MSCs, which can stimulate myocardial repair post administration due to their paracrine effects. Promising results of MSC-based therapy obtained from preclinical studies of cardiac diseases enhance the knowledge and strengthen the clinical research to investigate the safety and efficacy in a clinical trial setting. There are papers that discuss the importance of MSC therapy in the treatment of cardiovascular diseases, with the following references being highly recommended.301–306 To date, 36 trials have evaluated the therapeutic potential of MSCs in different pathological conditions, with the most prevalent types being BM-MSCs (25 trials), followed by UC-MSCs (7 trials) and AT-MSCs (4 trials).303 However, the reported results are contradictory and create controversy about the efficacy of the treatments.
One of the first trials using MSCs in the treatment of chronic heart failure was the Cardiopoietic Stem Cell Therapy in Heart Failure (C-CURE) trial, a multicentre, randomized clinical trial that recruited 47 patients. The trial findings supported the safety of BM-MSC therapy and provided a data set that demonstrated improvements in cardiovascular scores along with New York Heart Association functional class, quality of life, and general physical health.307 Despite these encouraging results in the phase I trial, the treatment failed to achieve the primary outcomes in the phase II/III trial (CHART-1 trial), including no significant improvements in cardiac structure or function or patient quality of life.308 A positive outcome was also found in a phase I/II, randomized pilot study called the POSEIDON trial, which was the first trial to demonstrate the superior effectiveness of the administration of allogeneic BM-MSCs compared to allogeneic MSCs from other sources.309,310 Published results from the MSC-HF study, with 4 years of follow-up results,311,312 and the TRIDENT study313 illustrated the positive outcomes of BM-MSCs in the treatment of heart failure. However, a contradictory result from the recently published CONCERT-HF trial demonstrated that the administration of autologous BM-MSCs to patients diagnosed with chronic ischemic heart failure did not improve left ventricular function or reduce scar size at 12 months post administration, but the patient’s quality of life was improved.314 This observation is similar to that of the TAC-HFT trial315 but completely different from the reported results of the MSC-HF trial. A comprehensive investigation is still needed to determine the reasons behind these contradictory results. The largest clinical trial to date using BM-MSCs is the DREAM-HF study, which was a randomized, double-blind, placebo-controlled, phase III trial that was conducted at 55 sites across North America and recruited a total of 565 patients with ischemic and nonischaemic heart failure.172 Although recent reports from the sponsor confirmed that the trial missed its primary endpoint (a reduction in recurrent heart failure-related hospitalization), other prespecified endpoints were met, such as a reduction in overall major adverse cardiac events (including death, myocardial infarction, and stroke).306 Thus, a complete report from the DREAM-HF trial will provide pivotal data supporting the therapeutic potential of BM-MSCs in the treatment of heart failure and open a new path for the FDA to approve cell-based therapy for cardiovascular diseases.
The early trial using AT-derived cells was the PRECISE trial, which was a phase I, randomized, placebo-controlled, double-blind study that examined the safety and efficacy of adipose-derived regenerative cells (ADRCs) in the treatment of chronic ischemic cardiomyopathy.316 ADRCs are a homogenous population of cells obtained from the vascular stromal fraction of AT, which contains a small proportion of AT-MSCs.317 Although the study supported the safety of ADRC administration and illustrated a preserved functional capacity (peak VO2) in the treated group and improvements in heart wall motion, neither poor left ventricle (LV) volume nor poor left ventricular ejection fraction (LVEF) was ameliorated. The follow-up trial of the PRECISE trial, called the ATHENA trial, was conducted in 31 patients, although the study was terminated prematurely because two cerebrovascular events occurred, which were not related to the cell product itself.318 The results of the study illustrated increases in functional capacity, hospitalization rate, and MLHFQ scores, but the LV volume and LVEF were not significantly different between the two groups. Kastrup and colleagues conducted the first in vitro expanded AT-MSC trial in ten patients with ischemic heart disease and ischemic heart failure in 2017. The results confirmed that ready-to-use AT-MSCs were well-tolerated and potentially effective in the treatment of ischemic heart disease and heart failure.319 Comparable results of AT-MSCs were also reported from the MyStromalCell Trial, which was a randomized placebo-controlled study. In this trial, 61 patients were randomized at a 2:1 ratio into two groups, with the results showing no significant difference in the primary endpoint, which was a change in the maximal bicycle exercise tolerance test (ETT) score from baseline to 6 months post administration.320 A 3-year follow-up report from the MyStromalCell Trial confirmed that patients who received AT-MSC administration maintained their preserved exercise capacity and their cardiac symptoms improved, whereas the control group experienced a significant reduction in exercise performance and a worsened cardiovascular condition.321
UC-MSCs are potential allogeneic cells for the treatment of cardiovascular disease, as they are “ready to use” and easy to isolate, they rapidly proliferate, and they secrete hepatocyte growth factors,322 which are involved in cardioprotection and cardiovascular regeneration.323 The pilot study using UC-MSCs in 30 patients with heart failure, called the RIMECARD trial, was the first reported trial for which the results supported the effectiveness of UC-MSCs, as seen in the improved ejection fraction, left ventricular function, functional status, and quality of life in patients administered UC-MSCs.324 Encouraging results reported from a phase I/II HUC-HEART trial325 showed improvements in LVEF and reductions in the size of the injured area of the myocardium. However, the opposite observations were also reported from a recently published phase I randomized trial using a combination of UC-MSCs and a collagen scaffold in patients with ischemic heart conditions, in which the size of fibrotic scar tissue was not significantly reduced.326
Although MSCs from AT, BM, and UC have proven to be safe and feasible in the treatment of cardiovascular diseases, the correlation between the MSC types and their therapeutic potentials is still uncertain because different results have been reported from different clinical trials (Table 4). The mechanisms by which MSCs participate in recovery and enhance myocardial regeneration have been discussed comprehensively in a recently published review;305,327 therefore, they will not be discussed in this review. In fact, the challenges of MSC-based therapy in cardiovascular diseases have been clearly described previously,328 including (1) the lack of an in vitro evaluation of the transdifferentiation potential of MSCs to functional cardiac and endothelial cells,329 (2) the uncontrollable differentiation of MSCs to undesirable cell types post administration,330 and (3) the undistinguishable nature of MSCs derived from different sources with various levels of differentiation potential.331 Therefore, the applications of MSC-based therapy in cardiovascular disease are still in their immature stage, with potential benefits to patients. Thus, there is a need to conduct large-scale, well-designed randomized clinical trials not only to confirm the therapeutic potential of MSCs from various sources but also to enhance our knowledge of cardiovascular regeneration post administration.
Table 4.
The reported clinical trials using MSCs from AT, BM, and UC in the treatment of cardiovascular diseases
| Year | Disease | MSC source | No. of treated patients | Efficacy |
|---|---|---|---|---|
| 2013307 | Heart failure | BM | 21 | - Improvement in LVEF and MLHFQ |
| - Decrease in LVESV and LVEDV | ||||
| - Increase in 6MWT | ||||
| - No evidence of increased cardiac or systemic toxicity | ||||
| 2017308 | Ischemic heart failure | BM | 120 | - Improvement in LVEF, ESV, LEVDV, and MLHFQ |
| 2012309 | ischemic cardiomyopathy | BM | 30 | - Functional improvement (change in 6MWT, MLHFQ, and NYHA classification) |
| 2017310 | Nonischemic Dilated Cardiomyopathy | BM | 34 | - Increase in 6MWT, ejection fraction |
| - Improvement in MLHFQ | ||||
| - Reduction in inflammatory cytokine, TNF-a | ||||
| 2015311 | Severe ischemic heart failure | BM | 60 | - Significant Improvement in LVEF, ESV, stroke volume, and myocardial mass. No difference in NYHA class, 6MWT, and Kansas City cardiomyopathy questionnaire after 6-month follow-up |
| 2020312 | Severe ischemic heart failure | BM | 60 | - Significant improvements in LVEF, LVESV, stroke volume and myocardial mass. Significant reduction in the amount of scar tissue and quality of life score after 12 months’ of follow-up. |
| - Significantly fewer hospitalizations for angina after 4 years of follow-up | ||||
| 2017313 | Ischemic Cardiomyopathy | BM | 30 | - Reduction in scar size |
| - Increase in ejection fraction when using 100 million dose | ||||
| 2021314 | Ischemic heart failure | BM | 25 | - Improvement in clinical outcomes, including MACE and quality of life |
| - No improvement in LVEF, left ventricular, scar size, 6MWT, and oxy peak oxygen consumption did not differ between groups | ||||
| 2013315 | ischemic cardiomyopathy | BM | 19 | - Improvement in MLHF, 6MWT, regional myocardial function |
| - No change in ejection fraction and left ventricular chamber volume | ||||
| 2014316 | Chronic ischemic cardiomyopathy | AD | 21 | - No significant change in LVEF, SPECT, and rest total severity score |
| - Increase in LV total mass | ||||
| - Improvement in WMSI | ||||
| - Preserved MVO2 and METs | ||||
| 2017318 | Chronic myocardial ischemia | AD | 15 | - Significant improvement in LVEF, ESV, LEVDV, and MLHFQ |
| 2017319 | Ischemic heart failure | AD | 10 | - Improvement in LVEF, LVSEV, 6MWT, NYHA class |
| - No difference in KKCQ scores and CCS class | ||||
| 2017320 | Chronic Ischemic Heart Disease | AD | 41 | - Improvement in Exercise capacity compared to placebo but not significant |
| 2019321 | Refractory angina | AD | 41 | - Improvement in cardiac symptoms but no change in exercise capacity |
| 2017324 | Heart failure | UC | 15 | - Improvements in LVEF compared to baseline at 3 months’follow-up |
| - No changes in left ventricular volumes | ||||
| 2020325 | Chronic ischemic cardiomyopathy | UC | 26 | - Improvement in LVEF, 6MWT, and NYHA |
| 2020326 | Chronic ischemic heart disease | UC | 32 | - Improvement in LVEF, MLHF, and NYHA |
| - Decrease infarct size |
6MWT 6-min walk test, CCS Canadian Cardiovascular Society, ESV end-systolic volume, LVEDV left ventricular end-diastolic chamber volume, LVEF left ventricular ejection fraction, LVESV left ventricular end-systolic volume, MACE major adverse cardiovascular events, MET metabolic equivalents, MLHF Minnesota Living with Heart Failure, MLHFQ Minnesota Living with Heart Failure Questionnaire, MVO2 maximal oxygen consumption, NYHA New York Heart Association, SPECT single photon emission computed tomography, TNF-α tumor necrosis factor alpha, WMSI wall motion score index
Proposed mechanism of BM-MSCs in the treatment of acquired brain and spinal injury
Bones are complex structures constituting a part of the vertebrate skeleton, and they play a vital role in the production of blood cells from HSCs. Similar to the functions of most vertebrate organs, bone function is tightly regulated by its constituents and by long-range signaling from AT and the adrenal glands, parathyroid glands, and nervous system.332 The central nervous system (CNS) orchestrates the voluntary and involuntary input transmitted by a network of peripheral nerves, which act as the bridge between the nervous system and target organs. The CNS controls involuntary responses via the autonomic nervous system (ANS), consisting of the sympathetic nervous system and the parasympathetic nervous system, and voluntary responses via the somatic nervous system. The ANS penetrates deep into the BM cavity, reaching the regions of hematopoietic activity to deliver neurotransmitters that tightly regulate BM stem cell niches.333 The BM microenvironment consists of various cell types that participate in the maintenance of HSC niches, which are composed of specialized cells, including BM-MSCs (Fig. 3a). The release of a specific neurotransmitter, circadian norepinephrine, from the sympathetic nervous system at nerve terminals leads to a reduction in the circadian expression of C–X-C chemokine ligand 12 (CXCL12, which is also known as stromal cell-derived factor-1 (SDF-1)) by Nestin+/NG22+ BM-MSCs, resulting in the secretion of HSCs into the peripheral bloodstream.334,335 In fact, BM-MSCs play a significant role in the regulation of HSC quiescence and are closely associated with arterioles and sympathetic nervous system nerve fibers. Nestin-expressing BM-MSCs have been shown to express high levels of SDF-1, stem cell factor (SCF), angiopoietin-1 (Ang-1), interleukin-7, vascular cell adhesion molecule 1 (VCAM-1), and osteopontin (OPN), which are directly involved in the regulation and maintenance of HSC quiescence.336 The depletion of BM-MSCs in BM leads to the mobilization of HSCs into the peripheral bloodstream and spleen. The findings from a previous study demonstrated that reduced SDF-1 expression in norepinephrine-treated BM-MSCs resulted in the mobilization of CXCR4+ HSCs into circulation.337 The ability of BM-MSCs to produce SDF-1 is tightly related to their neuronal protective functions.338 SDF-1 is a member of a chemokine subfamily that orchestrates an enormous diversity of pathways and functions in the CNS, such as neuronal survival and proliferation. The chemokine has two receptors, CXCR4 and CXCR7, that are involved in the pathogenic development of neurodegenerative and neuroinflammatory diseases.339 In the damaged brain, SDF-1 functions as a stem cell homing signal, and in acquired immune deficiency syndrome (AIDS), SDF-1 has been reported to be involved in the protection of damaged neurons by preventing apoptosis. In a traumatic brain injury model, SDF-1 was found to function as an inhibitor of the caspase-3 pathway by upregulating the Bcl-2/Bax ratio, which in turn protects neurons from apoptosis.340 Moreover, the release of SDF-1 also facilitates cell recruitment, cell migration, and the homing of neuronal precursor cells in the adult CNS by activating the CXCR4 receptor.341,342 Existing data support that SDF-1 acts as the guiding signal for the regeneration of axon growth in damaged neurons and enhances spinal nerve regeneration.343,344 Hence, the ability of BM-MSCs to express SDF-1 in response to the neuronal environment provides a unique neuronal protective effect that could explain the potential therapeutic efficacy of BM-MSCs in the treatment of neurodegenerative diseases (Fig. 3b).
Fig. 3.
The nature of the “stem niche” of bone marrow-derived mesenchymal stem cells (BM-MSCs) supports their therapeutic potential in neuron-related diseases. a Bone marrow is a complex stem cell niche regulated directly by the central nervous system to maintain bone marrow homeostasis and haematopoietic stem cell (HSC) functions. MSCs in bone marrow respond to the environmental changes through the release of norepinephrine (NE) from the sympathetic nerves that regulate the synthesis of SDF-1 and the migration of HSCs through the sinusoids. The secretion of stem cell factors (SCFs), VCAM-1 and angiotensin-1 from MSCs also plays a significant role in the maintenance of HSCs. b BM-MSCs have the ability to produce and release SDF-1, which directly contributes to neuroprotective functions at the damaged site through interaction with its receptors CXCR4/7, located on the neuronal membrane. c Neuronal protection and the functional remyelination induced by BM-MSCs are also modulated by the release of a wide range of growth factors, including VEGF, BDNF, and NGF, by the BM-MSCs. d BM-MSCs also have the ability to regulate neuronal immune responses by direct interaction or paracrine communication with microglia. Figure was created with BioRender.com
The migration of exogenous MSCs after systemic administration to the brain is limited by the physical blood–brain barrier (BBB), which is a selective barrier formed by CNS endothelial cells to restrict the passage of molecules and cells. The mechanism of molecular movement across the BBB is well established, but how stem cells can bypass the BBB and home to the brain remains unclear. Recent studies have reported that MSCs are able to migrate through endothelial cell sheets by paracellular or transcellular transport followed by migration to the injured or inflammatory site of the brain.345,346 During certain injuries or ischemic events, such as brain injury, stroke, or cerebral palsy, the integrity and efficiency of BBB protection is compromised, which allows MSC migration across the BBB via paracellular transport through the transient formation of interendothelial gaps.347 CD24 expression has been detected in human BM-MSCs, which are regulated by TGF-β3,348 allowing them to interact with activated endothelial cells via P-selectin and initiate the tethering and rolling steps of MSCs.349 Additionally, BM-MSCs express high levels of CXCR4 or CXCR7,350,351 which bind to integrin receptors, such as VLA-4, to activate the integrin-binding process and allow the cells to anchor to endothelial cells, followed by the migration of MSCs through the endothelial cell layer and basement membrane in a process called transmigration.352 This process is facilitated by the secretion of matrix metalloproteinases (MMPs), which degrade the endothelial basement membrane, allowing BM-MSCs to enter the brain environment.353,354 BM-MSCs can also regulate the integrity of the BBB via the secretion of tissue inhibitor of matrix metalloproteinase-3 (TIMP3), which has been shown to ameliorate the effects of a compromised BBB in traumatic brain injury.355 The secretion of TIMP3 from MSCs directly blocked vascular endothelial growth factor a (VEGF-a)-induced breakdown of endothelial cell adherent junctions, demonstrating the potential mechanism of BM-MSCs in the regulation of BBB integrity.
The therapeutic applications of BM-MSCs in neurodegenerative conditions have been significantly increased by the demonstration of BM-MSC involvement in axonal and functional remyelination processes. Remyelination is a spontaneous regenerative process occurring in the human CNS to protect oligodendrocytes, neurons, and myelin sheaths from neuronal degenerative diseases.356 Remyelination is considered a neuroprotective process that limits axonal degeneration by demyelination and neuronal damage. The first mechanism of action of BM-MSCs related to remyelination is the activation of the JAK/STAT3 pathway to regulate dorsal root ganglia development.357 It was reported that BM-MSCs secrete vascular endothelial growth factor-A (VEGF-A),358 brain-derived neurotrophic factor (BDNF), interleukin-6, and leukemia inhibitor factor (LIF), which directly function in neurogenesis and neurite growth.357 VEGF-A is a key regulator of hemangiogenesis during development and bone homeostasis. Postnatally, osteoblast- and MSC-derived VEGF plays a critical role in maintaining and regulating bone homeostasis by stimulating MSC differentiation into osteoblasts and suppressing their adipogenic differentiation.359–361 To balance osteoblast and adipogenic differentiation, VEGF forms a functional link with the nuclear envelope protein laminin A, which in turn directly regulates the osteoblast and adipocyte transcription factors Runx2 and PPARγ, respectively.361,362 In the brain, VEGF is a potent growth factor mediating angiogenesis, neural migration, and neuroprotection. VEGF-A, secreted from BM-MSCs under in vitro xeno- and serum-free culture conditions, is the most studied member of the VEGF family and is suggested to play a protective role against cognitive impairment, such as in the context of Alzheimer’s disease pathology or stroke.363–365 Recently, it was reported that the neurotrophic and neuroprotective function of VEGF is mediated through VEGFR2/Flk-1 receptors, which are expressed in the neuroproliferative zones and extend to astroglia and endothelial cells.366 In animal models of intracerebral hemorrhage and cerebral ischemia, the transfusion of Flk-1-positive BM-MSCs promotes behavioral recovery and anti-inflammatory and angiogenic effects.367,368 Moreover, supplementation with VEGF-A in neuronal disorders enhances intraneural angiogenesis, improves nerve regeneration, and promotes neurotrophic capacities, which in turn increase myelin thickness via the activation of the prosurvival transcription factor nuclear factor-kappa B (NF-kB). This activation, together with the downregulation of Mdm2 and increased expression of the pro-apoptotic transcription factor p53, is considered to be the neuroprotective process associated with an increased VEGF-A level.369–371 An analysis of microRNA (miRNA) in extracellular vesicles (EVs) secreted from BM-MSCs revealed that BM-MSCs release substantial amounts of miRNA133b, which suppresses the expression of connective tissue growth factor (CTGF) and protects hippocampal neurons from apoptosis and inflammatory injury372–374 (Fig. 3c).
In terms of immunoregulatory functions, the administration of human BM-MSCs into immunocompetent mice subjected to SCI or brain ischemia showed that BM-MSCs exhibited a short-term neuronal protective function against neurological damage (Fig. 3d). Further investigation demonstrated the ability of BM-MSCs to directly communicate with host microglia/macrophages and convert them from phenotypic polarization into alternative activated microglia/macrophages (AAMs), which are key players in axonal extension and the reconstruction of neuronal networks.375 Other studies have also illustrated that the administration of AAMs directly to the injured spinal cord induced axonal regrowth and functional improvement.376 The mechanism by which BM-MSCs activate the conversion of microglia/macrophages occurs through two representative macrophage-related chemokine axes, CCL2/CCR2 and CCL-5/CCR5, both of which exhibit acute or chronic elevation following brain injury or SCI.377 The CCL2/CCR2 axis contributed to the enhancement of inflammatory function, and BM-MSC-mediated induction of CCL2 did not alter the total granulocyte number (Fig. 3d). Although the chemokine-mediated mechanism of BM-MSCs in the activation of AAMs and enhanced axonal regeneration at the damage sites is evident, the direct mechanism by which the communication between BM-MSCs and the target cells results in these phenomena remains unclear, and further investigation is needed.
BM-MSCs also confer the ability to regulate the inflammatory regulation of the immune cells present in the brain by (1) promoting the polarization of macrophages toward the M2 type, (2) suppressing T-lymphocyte activities, (3) stimulating the proliferation and differentiation of regulatory T cells (Tregs), and (4) inhibiting the activation of natural killer (NK) cells. BM-MSCs secrete glial cell line-derived neurotrophic factor (GDNF), a specific growth factor that contributes directly to the transition of the microglial destructive M1 phenotype into the regenerative M2 phenotype during the neuroinflammatory process.378 A similar result was also found in AT-379 and UC-MSCs380 under neuroinflammation-associated conditions, suggesting that AT-, BM-, and UC-MSCs share the same mechanism in promoting macrophage polarization. In terms of T-lymphocyte suppression, compared to MSCs from AT and BM, UC-MSCs show the strongest potential to inhibit the proliferation of T-lymphocytes by promoting cell cycle arrest (G0/G1 phase) and apoptosis.381 In addition, UC-MSCs have been proven to be more effective in promoting the proliferation of Tregs382 and inhibiting NK activation.383 Although MSCs are well-known for their inflammatory regulatory ability, the mechanism is not exclusive to BM-MSCs, especially in neurological disorders.384
Proposed mechanism of UC-MSCs in the treatment of pulmonary diseases and lung fibrosis
In contrast to AT-MSCs and BM-MSCs, UC-MSCs have lower expression of major histocompatibility complex I (MHC I) and no expression of MHC II, which prevents the complications of immune rejection.385 Moreover, as UC is considered a waste product after birth, with the option of noninvasive collection, UC-MSCs are easier to obtain and culture than AD- and BM-MSCs.386 These advantages of UC-MSCs have contributed to their use in the treatment of pulmonary diseases, especially during the rampant COVID-19 pandemic, as “off-the-shelf” products. Numerous pulmonary diseases have been the subject of applications of UC-MSCs, including BPD, COPD, ARDS, and COVID-19-induced ARDS. In BPD, premature infants are born before the alveolarization process, resulting in arrested lung development and alveolar maturation. Upon administration via an IV route, the majority of exogenous UC-MSCs reach the immature lung and directly interact with immune cells to exert their immunomodulatory properties via cell-to-cell interaction mechanisms (Fig. 4a). UC-MSCs interact with T cells via the PD-L1 ligand, which binds to the PD-1 inhibitory molecule on T cells, resulting in the suppression of CD3+ T-cell proliferation and effector T-cell responses.387 In addition, UC-MSCs also express CD54 (ICAM-1), which plays a crucial role in the immunomodulatory functions of T cells.388 Direct contact between UC-MSCs and macrophages via CD54 expression on UC-MSCs promotes the immune regulation of UC-MSCs via the regulation of phagocytosis by monocytes.389 Moreover, the contact of UC-MSCs with macrophages during proinflammatory responses increases the secretion of TSG-6 by UC-MSCs, which in turn promotes the inhibitory regulation of CD3+ T cells, macrophages, and monocytes by MSCs.390 Recently, upregulation of SDF-1 was described in neonatal lung injury, especially in layers of the respiratory epithelium.391 SDF-1 has been shown to participate in the migration and initiation of the homing process of MSCs via the CXCR4 receptors on their surface.392 It was reported that UC-MSCs express low levels of CXCR4, allowing them to induce SDF-1-associated migration processes via the Akt, ERK, and p38 signal transduction pathways.393 Hence, in BPD, the upregulation of SDF-1 together with the homing ability of UC-MSCs strongly supports the therapeutic effects of UC-MSCs in the treatment of BPD. Furthermore, UC-MSCs have the ability to communicate with immune cells via cell-to-cell contact to reduce proinflammatory responses and the production of proinflammatory cytokines (such as TGF-β, INF-γ, macrophage MIF, and TNF-α). The modulation of the human innate immune system by UC-MSCs is mediated by cell–cell interactions via CD54-LFA-1 that switch macrophage polarization processes, promoting the proliferation of M2 macrophages, which in turn reduce inflammatory responses in the immature lung.394 Moreover, UC-MSCs also have the ability to produce VEGF and hepatocyte growth factors (HGFs), promoting angiogenesis and enhancing lung maturation.395
Fig. 4.
Adipose tissue-derived mesenchymal stem cells (AT-MSCs) and the nature of their tissue of origin support their use in therapeutic applications. a Adipose tissue is considered an endocrine organ, supporting and regulating various functions, including appetite regulation, immune regulation, sex hormone and glucocorticoid metabolism, energy production, the orchestration of reproduction, the control of vascularization, and blood flow, the regulation of coagulation, and angiogenesis and skin regeneration. b In terms of metabolic disorders, such as type 2 diabetes mellitus (T2DM), as adipose tissue is directly involved in the metabolism of glucose and lipids and the regulation of appetite, the detrimental effects of T2DM also alter the functions of AT-MSCs, which in turn, hampers their therapeutic effects. Hence, the use of autologous AT-MSCs is not recommended for the treatment of metabolic disorders, including T2DM, suggesting that allogeneic AT-MSCs from healthy donors could be a better alternative approach. c AT-MSCs are suitable for the treatment of reproductive disorders due to their unique ability to mobilize and home to the thecal layer of the injured ovary, enhance the regeneration and maturation of thecal cells, increase the structure and function of damaged ovaries via exosome-activated SMAD, decrease oxidative stress and autophagy, and increase the proliferation of granulosa cells via PI3K/AKT pathways. These functions are regulated specifically by growth hormones produced by AT-MSCs in response to the surrounding environment, including HGF, TGF-β, IGF-1, and EGF. d AT-MSCs are also good candidates for skin healing and regeneration as their growth factors strongly support neovascularization and angiogenesis by reducing PLL4, increase anti-apoptosis via the activation of PI3K/AKT pathways, regulate inflammation by downregulating NADPH oxidase isoform 1, and increase immunoregulation through the inhibition of NF-κB activation. The figure was created with BioRender.com
COPD is characterized by an increase in hyperinflammatory reactions in the lung, compromising lung function and increasing the development of lung fibrosis. The mechanism by which UC-MSCs contribute to the response to COPD is inflammatory regulation (Fig. 4b). The administration of UC-MSCs prevented the infiltration of inflammatory cells in peribronchiolar, perivascular, and alveolar septa and switched macrophage polarization to M2.396 A significant reduction in proinflammatory cytokines, including IL-1β, TNF-α, and IL-8, was also observed following UC-MSC administration.224 MSCs, including UC-MSCs, have been reported to trigger the production of secretory leukocyte protease inhibitors in epithelial cells through the secretion of HGF and epidermal growth factor (EGF), which is believed to have beneficial effects on COPD.397,398 In addition to their inflammatory regulation ability, UC-MSCs exhibit antimicrobial effects through the inhibition of bacterial growth and the alleviation of antibiotic resistance during Pseudomonas aeruginosa infection.399 The combination of the regulation of the host immune response and the antimicrobial effects of UC-MSCs may be relevant for the prevention and treatment of COPD exacerbations, as inflammation and bacterial infections are important risk factors that significantly contribute to the morbidity and mortality of patients with COPD. In terms of regenerative functions, UC-MSCs were reported to be able to differentiate into type 2 alveolar epithelial cells in vitro and alleviate the development of pulmonary fibrosis via β-catenin-regulated cell apoptosis.400 Furthermore, UC-MSCs enhanced alveolar epithelial cell migration and proliferation by increasing matrix metalloproteinase-2 levels and reduced their endogenous inhibitors, tissue inhibitors of matrix metalloproteinases, providing a potential mechanism underlying their anti-pulmonary-fibrosis effects.401,402
In ARDS, especially that associated with COVID-19, the proinflammatory state is initiated by increases in plasma concentrations of proinflammatory cytokines, such as IL-1 beta, IL-7, IL-8, IL-9, IL-10, bFGF, granulocyte colony-stimulating factor (G-CSF), GM-CSF, IFN-γ, and TNF-α. The significant increases in the concentrations of these cytokines in patient plasma suggest the development of a cytokine storm, which is a leading cause of COVID-induced mortality. In addition to the immunomodulatory functions regulated via cell-to-cell interactions between UC-MSCs and immune cells, such as macrophages, monocytes, and T cells, UC-MSCs exert their functions via paracrine effects through the secretion of growth factors, cytokines, and exosomes (Fig. 4c). The most relevant immunomodulatory function of UC-MSCs is considered to be their inhibition of effector T cells via the induction of T-cell apoptosis and cell cycle arrest by the production of indoleamine 2,3- dioxygenase (IDO), prostaglandin E2 (PGE-2), and TGF-β. Elevated levels of PGE-2 in patients with COVID-19 are reported to be a crucial factor in the initiation of inflammatory regulation by UC-MSCs post administration and prevent the development of cytokine storms by direct inhibition of T- and B lymphocytes.403 UC-MSCs exert these inhibitory activities through a PGE-2-dependent mechanism.404 It was reported that UC-MSCs confer the ability to secrete tolerogenic mediators, including TGF-β1, PGE-2, nitric oxide (NO), and TNF-α, which are directly involved in their immunoregulatory mechanism. The secretion of NO from UC-MSCs is reported to be associated with the desensitization of T cells via the IFN-inducible nitric oxide synthase (iNOS) pathways and to stimulate the migration of T cells in close proximity to MSCs that subsequently suppress T-cell sensitivities via NO.405 Lung infection with viruses usually leads to impairments in alveolar fluid clearance and protein permeability. The administration of UC-MSCs enhances alveolar protection and restores fluid clearance in patients with COVID-19. UC-MSCs secrete growth factors associated with angiogenesis and the regeneration of pulmonary blood vessels and micronetworks, including angiotensin-1, VEGF, and HGF, which also reduce oxidative stress and prevent fibrosis formation in the lungs. These trophic factors have been identified as key players in the modulation of the microenvironment and promote pulmonary repair. Additionally, UC-MSCs are more effective than BM-MSCs in the restoration of impaired alveolar fluid clearance and the permeability of airways in vitro, supporting the use of UC-MSCs in the treatment of patients with pulmonary pneumonia.406 In the context of pulmonary regeneration, UC-MSCs were shown to inhibit apoptosis and fibrosis in pulmonary tissue by activating the PI3K/AKT/mTOR pathways via the secretion of HGF, which also acts as an inhibitory stimulus that blocks alveolar epithelial-to-mesenchymal transition.407,408 Moreover, UC-MSCs can reverse the process of fibrosis via enhanced expression of macrophage matrix-metallopeptidase-9 for collagen degradation and facilitate alveolar regeneration via Toll-like receptor-4 signaling pathways.409 UC-MSCs were shown to communicate with CD4+ T cells through HGF induction not only to inhibit their differentiation into Th17 cells, reducing the secretion of IL-17 and IL-22 but also to switch their differentiation into regulatory T cells.410,411 In addition, UC-MSCs conferred the ability to facilitate the number of M2 macrophages and reduce M1 cells via the control of the macrophage polarization process.412
There are several potential mechanisms of UC-MSCs in the treatment of patients with pulmonary diseases and pneumonia, including the regulation of immune cell function, immunomodulation, the enhancement of alveolar fluid clearance and protein permeability, the modulation of endoplasmic reticulum stress, and the attenuation of pulmonary fibrosis. Hence, based on these discussions, UC-MSCs are recommended as suitable candidates for the treatment of pulmonary disease both in pediatric and adult patients.
Proposed mechanism of AT-MSCs in the treatment of endocrinological diseases, reproductive disorders, and skin burns
Human AT was first viewed as a passive reservoir for energy storage and later as a major site for sex hormone metabolism, the production of endocrine factors (such as adipsin and leptin), and a secretion source of bioactive peptides known as adipokines.413 It is now clear that AT functions as a complex and highly active metabolic and endocrine organ, orchestrating numerous different biological features414 (Fig. 5a). In addition to adipocytes, AT contains hematopoietic-derived progenitor cells, connective tissue, nerve tissue, stromal cells, endothelial cells, MSCs, and pericytes. AT-MSCs and pericytes mobilize from their perivascular locations to aid in healing and tissue regeneration throughout the body. As AT is involved directly in energy storage and metabolism, AT-MSCs are also mediated and regulated by growth factors related to these pathways. In particular, interleukin-6 (IL-6), IL-33, and leptin regulate the maintenance of metabolic activities by increasing insulin sensitivity and preserving homeostasis related to AT. Nevertheless, in the development of obesity and diabetes, omental and subcutaneous AT maintains a low-grade state of inflammation, resulting in the impairment of glucose metabolism and potentially contributing to the development of insulin resistance.415 In normal AT, direct regulation of Pre-B-cell leukemia homeobox (Pbx)-regulating protein-1 (PREP1) by leptin and thyroid growth factor-beta 1 (TGF-β1) in AT-MSCs and mature adipocytes is involved in the protective function and maintenance of AT homeostasis. However, under diabetic conditions, the balance between the expression of leptin and the secretion of TGF-β1 is compromised, resulting in the malfunction of AT-MSC metabolic activity and the proliferation, differentiation, and maturation of adipocytes. Therefore, the use of autologous AT-MSCs in the treatment of diabetic conditions is not a suitable option, as the functions of AT-MSCs are directly altered by diabetic conditions, which reduces their effectiveness in cell-based therapy (Fig. 5b).
Fig. 5.
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are good candidates for the treatment of pulmonary diseases. a Lung immaturity and fibrosis are the major problems of patients with bronchopulmonary dysplasia and lead to increased levels of SDF-1, the development of fibrosis, the induction of the inflammatory response, and the impairment of alveolarization. UC-MSCs are attracted to the damaged lung via the chemoattractant SDF-1, which is constantly released from the immature lung via SDF-1 and CXCR4 communication. Moreover, UC-MSCs reduce the level of proinflammatory cytokines (TGF-β, INF-γ, macrophage MIF, and TNF-α) via a cell-to-cell contact mechanism. The ability of UC-MSCs to produce and secrete VEGF also involves in the regeneration of the immature lung through enhanced angiogenesis. b Upon an exacerbation of chronic obstructive pulmonary disease (COPD), UC-MSCs respond to the surrounding stimuli by reducing IL-8 and TNF-α levels, resulting in the inhibition of the inflammatory response but an increase in the secretion of growth factors participating in the protection of alveoli, fluid clearance and reduced oxidative stress and lung fibrosis, including HGF, TGF-β, IGF-1, and exosomes. c In a similar manner, UC-MSCs prevent the formation of cytokine storms in coronavirus disease 2019 (COVID-19) by inhibiting CD34+ T-cell differentiation into Th17 cells and enhancing the number of regulatory T cells. Moreover, UC-MSCs also have antibacterial activity by secreting LL-3717 and lipocalin. Figure was created with BioRender.com
Preclinical studies and clinical trials have revealed the therapeutic effects of MSCs, in general, and AT-MSCs, in particular, in the management of POF, with relatively high efficacy and enhanced regeneration of the ovaries. Understanding the molecular and cellular mechanisms underlying these effects is the first step in the development of suitable MSC-based therapies for POF. One of the mechanisms by which MSCs exert their therapeutic effects is their ability to migrate to sites of injury, a process known as “homing”. Studies have shown that MSCs from different sources have the ability to migrate to different compartments of the injured ovary. For example, BM-MSCs administered through IV routes migrated mostly to the ovarian hilum and medulla,416 whereas a significant number of UC-MSCs were found in the medulla.417 Interestingly, AT-MSCs were found to be engrafted in the theca layers of the ovary but not in the follicles, where they acted as supportive cells to promote follicular growth and the regeneration of thecal layers.418 The structure and function of the thecal layer have a great impact on fertility, which has been reviewed elsewhere.419 In brief, the thecal layer consists of two distinct parts, the theca interna, which contains endocrine cells, and the theca externa, which is an outer fibrous layer. The thecal layer contains not only endocrine-derived cells but also vascular- and immune-derived cells, whose functions are to maintain the structural integrity of the follicles, transport nutrients to the inner compartment of the ovary and produce key reproductive hormones such as androgens (testosterone and dihydrotestosterone) and growth factors (morphogenic proteins, e.g., BMPs and TGF-β).420 As AT-MSCs originate from an endocrine organ, their ability to sense signals and migrate to the thecal layer is anticipated. Additionally, secretome analysis of AT-MSCs showed a wide range of growth factors, including HGF, TBG-β, VEGF, insulin-like growth factor-1 (IGF-1), and EGF,421 that are directly involved in the restoration of the structure and function of damaged ovaries by stimulating cell proliferation and reducing the aging process of oocytes via the activation of the SIRT1/FOXO1 pathway, a key regulator of vascular endothelial homeostasis.422,423 In POF pathology, autophagy and its correlated oxidative stress contribute to the development of POF throughout a patient’s life. Recently, AT-MSCs were shown to be able to improve the structure and function of mouse ovaries by reducing oxidative stress and inflammation, providing essential data supporting the mechanism of AT-MSCs in the treatment of POF.424 Several studies have illustrated that AT-MSCs secrete biologically active EVs that regulate the proliferation of ovarian granulosa cells via the PI3K/AKT pathway, resulting in the enhancement of ovarian function.425 Direct regulation of ovarian cell proliferation modulates the state of these cells, which in turn restores the ovarian reserve.426 Other mechanisms supporting the effectiveness of MSCs have been carefully reviewed, confirming the therapeutic potential of MSCs derived from different sources426 (Fig. 5c).
In the last decade, the number of clinical trials using AT-MSCs in the treatment of chronic skin wounds and skin regeneration has exponentially increased, with data supporting the enhancement of the skin healing processes, the reduction of scar formation, and improvements in skin structure and quality. Several mechanisms are directly linked to the origin of AT-MSCs, including differentiation ability, neovascularization, anti-apoptosis, and immunological regulation. AT is a connective and supportive tissue positioned just beneath the skin layers. AT-MSCs have a strong ability to differentiate into adipocytes, endothelial cells,427 epithelial cells428 and muscle cells.429 The adipogenic differentiation of AT-MSCs is one of the three mesoderm lineages that defines MSC features, and AT-MSCs are likely to be the best MSC type harboring this ability compared to BM- and UC-MSCs. Recent reports detailed that AT-MSCs accelerated diabetic wound tissue closure through the recruitment and differentiation of endothelial cell progenitor cells into endothelial cells mediated by the VEGF-PLCγ-ERK1/ERK2 pathway.430 Upon injury, the skin must be healed as quickly as possible to prevent inflammation and excessive blood loss. The reparation process occurs through distinct overlapping phases and involves various cell types and processes, including endothelial cells, keratinocyte proliferation, stem cell differentiation, and the restoration of skin homeostasis.431 Hence, the differentiation ability of AT-MSCs plays a critical role in their therapeutic effect on skin wound regeneration and healing processes. AT-MSCs accelerate wound healing via the production of exosomes that serve as paracrine factors. It was reported that AT-MSCs responded to skin wound injury stimuli by increasing their expression of the lncRNA H19 exosome, which upregulated SOX9 expression via miR-19b, resulting in the acceleration of human skin fibroblast proliferation, migration, and invasion.432 In addition, the engraftment of AT-MSCs supported wound bed blood flow and epithelialization processes.433 Anti-apoptosis plays a critical role in AT-MSC-based therapy, as without a microvascular supply network established within 4 days post injury, adipocytes undergo apoptosis and degenerate. Exogenous sources of AT-MSCs mediate anti-apoptosis via IGF-1 and exosome secretion by triggering the activation of PI3K signaling pathways.434 Another mechanism supporting the therapeutic potential of AT-MSCs is their anti-inflammatory function, which results in the reduction of proinflammatory factors, such as tumor necrosis factor (TNF) and interferon-γ (IFN-γ), and increases the production of the anti-inflammatory factors IL-10 and IL-4. Exosomes from AT-MSCs in response to a wound environment were found to contain high levels of Nrf2, which downregulated wound NADPH oxidase isoform 1 (NOX1), NADPH oxidase isoform 4 (NOX4), IL-1β, IL-6, and TNF-α expression. The anti-inflammatory functions of AT-MSCs are also regulated by their immunomodulatory ability, partially through the inhibition of NF-κB activation in T cells via the PD-L1/PD-1 and Gal-9/TIM-3 pathways, providing a novel target for the acceleration of wound healing435 (Fig. 5d).
Therefore, as an endocrine organ in the human body, AT and its derivative stem cells, including AT-MSCs, have shown great potential in the treatment of reproductive disorders and skin diseases. Their potential is supported by mechanisms that are directly related to the nature of AT-MSCs in the maintenance of tissue homeostasis, angiogenesis, anti-apoptosis, and the regulation of inflammatory responses.
The current challenges for MSC-based therapies
Over the past decades, MSC-based research and therapy have made tremendous advancements due to their advantages, including immune evasion, diverse tissue sources for harvesting, ease of isolation, rapid expansion, and cryopreservation as “off-the-shelf” products. However, several important challenges have to be addressed to further enhance the safety profile and efficacy of MSC-based therapy. In our opinion, the most important challenge of MSC-based therapy is the fate of these cells post administration, especially the long-term survival of allogeneic cells in the treatment of certain diseases. Although reported data confirm that the majority of MSCs are trapped in the lung and rapidly removed from the circulation, caution has been raised related to the occurrence of embolism events post infusion, which was proven to be related to MSC-induced innate immune attack (called instant blood-mediated inflammatory reaction).436 Another related challenge is the homing ability of infused cells, as successful homing at targeted tissue might result in long-term benefits to patients. Other concerns related to MSC-based therapy are the number of dead cells infused into the patients. An interesting study reported that dead MSCs alone still exerted the same immunomodulatory property as live MSCs by releasing phosphatidylserine.437 This is an interesting observation, as there is always a certain number of dead cells present in the cell-based product, and concerns are always raised related to their effects on the patient’s health. Finally, the hypothesis presented in this review is also a great challenge of the field, which has been proposed for future studies to answer the question: “What is the impact of MSC sources on their downstream application?”. Tables 5 and 6 illustrate the comparative studies that were conducted in preclinical and clinical settings to address the MSC source challenge. Other challenges of MSC-based therapies have been discussed in several reviews and systematic studies,135,185,438,439 which are highly recommended.
Table 5.
Comparative analysis of the effectiveness of MSC sources in a preclinical setting
| Study name | Time | Disease | Intervention | Treatment outcomes | Effective cell sources | ||
|---|---|---|---|---|---|---|---|
| Source | Dose | Route | |||||
| Comparison of Transplantation of Bone Marrow Stromal Cells (BMSC) and Stem Cell Mobilization by Granulocyte Colony-Stimulating Factor after Traumatic Brain Injury in Rat.490 | 2010 | Traumatic Brain Injury | BM-MSCs and bone marrow hematopoietic stem cell mobilization, induced by granulocyte colony-stimulating factor (G-CSF) | 2 × 106 cells | IV | Significant improvement in functional outcome. The recovery of motor and sensory score was faster and more tangible after injection of BMSC and G-CSF. | No significant difference between two sources. |
| Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury491 | 2013 | Spinal cord injury | Human BM-MSCs vs. AT-MSCs | 2 × 105 cells | Inject into injured spinal cord |
Increase BDNF levels in the injured spinal cord, reduce lesion cavity volume and microglia/macrophage infiltration Induce angiogenesis, axonal regeneration Improve behavioral performance. |
Both AT- and BM-MSCs migrated to the injured spinal cord without differentiating into glial or neuronal cells. AT-MSCs significantly increases BDNF levels more than in BM-MSCs group. |
| Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model.492 | 2016 | Spinal cord injury | Human BM-MSCs vs. UC-MSCs | 1 × 106 cells | Dorsal horn of the spinal cord injection | MSC administration resulted in improving functional recovery, allodynia, and hyperalgesia. |
BM-MSCs and UC-MSCs show similar effectiveness in alleviating the symptoms of neuropathic pain and improve motor function. Survival rate and electrophysiological findings of UC-MSC were significantly better than BM-MSC. |
| Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury.493 | 2018 | Spinal cord injury | Mice AT-MSCs vs. BM-MSCs | 1 × 105 cells | T9-T10 total laminectomy |
Promoted axonal regeneration and blood vessel formation in injury epicenter. Improved the motor function. |
Survival rate of AT-MSCs was higher than BM-MSCs. AT-MSCs transplantation protected neuronal/vascular better than BM-MSCs The motor function was equally improved following moderate spinal cord injury in both groups. No significant improvement in the severe spinal cord in either group. |
| Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis.494 | 2017 | Liver fibrosis | Rat AT-MSCs vs. BM-MSCs | 5 × 106 cells | portal vein | Attenuate liver fibrosis by inhibiting the activation and proliferation of Hepatic stellate cells (HSCs), as well as promoting the apoptosis of HSCs. | No significant difference between two sources. |
| Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure.495 | 2018 | Acute liver failure | Murine AT-MSCs vs. BM-MSCs. | 1 × 106 cells | IV | Improved histopathological score of liver tissue, reduced serum concentration of ALT and AST, and led to increase in survival rate. | AT-MSCs were more effective in preventing hepatic enzyme rise (lower level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT)). |
| Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction.496 | 2010 | Myocardial infarction | Human BM-MSCs vs. Human CD34+ Hematopoietic stem cells. |
1.2 × 106 BM-MSCs 6 × 105 CD34+ cells |
IV | Both cell types induced an improvement in LV cardiac function and increased tissue cell proliferation in myocardial tissue and neoangiogenesis. However, MSC were more effective for the reduction of infarct size and prevention of ventricular remodeling. | Mesenchymal stem cells might be more effective than CD34+cells for the healing of the infarct |
| Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration497 | 2011 | Myocardial infarction |
AT-MSCs BM-MSCs UC-MSCs |
4 × 105 cells/animal | intramyocardial injection | The findings suggests that hMSC originating from different sources showed a different healing performance in cardiac regeneration. | CD105+ hMSC exhibited a favorable survival pattern in infarcted hearts, which translates into a more robust preservation of cardiac function |
| Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction498 | 2011 | Myocardial infarction |
human umbilical cord perivascular cells vs. hBM-MSCs |
2 × 106 cells | IV | In the presence of either cell types, overall macrophage/monocyte levels were reduced, including proinflammatory M1-type macrophages, while the proportion of alternatively activated M2-type macrophages was significantly increased in the circulation and heart but not the BM. Moreover, we found decreased expression of IL-1β and IL-6, increased IL-10 expression, and fewer apoptotic cardiomyocytes without changes in angiogenesis in the infarct area. Fractional shortening was enhanced 2 weeks after cell infusion but was similar to medium controls 16 weeks after MI. | No significant difference between two sources. |
| Wharton’s jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: a preliminary report499 | 2013 | Myocardial infarction |
Mouse Wharton’s jelly MSCs vs. Mouse BM-MSCs. |
4–10 × 106 cells | IV | MSCs administered at 24–48 hr after MI have a significant and durable beneficial effect more than 25 weeks after MI and that MSC treatment can home to damaged tissue and improve heart function after intravenous infusion 24–48 hrs after MI, and that WJCs may be a useful source for off-the-shelf cellular therapy for MI. | WJCs might be more advantageous than BM-MSCs as an allogeneic cell source. |
| Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model.500 | 2014 | Myocardial infarction |
AT-MSCs BM-MSCs |
1 × 106 cells | intramyocardial injection | After 4 weeks, left ventricular ejection fraction (LVEF) was improved in the adipose-derived stem cell group, and scar wall thickness was greater compared with the saline group. Adipose-derived, as well as bone marrow-derived mesenchymal stem cells, prevented left ventricular end-diastolic dilation | Adipose-derived stem cells from a human ischemic patient preserved cardiac function following MI, whereas this could not be demonstrated for bone marrow-derived mesenchymal stem cells, with only adipose-derived stem cells leading to an improvement in LVEF. |
| Mechanical and Chemical Predifferentiation of Mesenchymal Stem Cells Into Cardiomyocytes and Their Effectiveness on Acute Myocardial Infarction501 | 2018 | Myocardial infarction |
AT-MSCs BM-MSCs |
1 × 106 cells | intramyocardial injection | The results demonstrated significant scar size reduction and greater recovery of left ventricle ejection fraction after transplantation of predifferentiated cells, although the differences were not significant when comparing mechanically with chemically predifferentiated MSCs. | No significant difference between two sources. |
| Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells Derived from Adipose Tissue and Bone Marrow on Acute Myocardial Infarction Model502 | 2019 | Myocardial infarction |
AT-MSCs BM-MSCs |
2 × 106 cells | intramyocardial injection | MSC therapy repaired cardiac functions shown by the restoration of ST segment, QT and QRS intervals in the ECG when compared to the AMI group. Infarct area was significantly decreased, and cardiac tissue regeneration signs were shown on histopathological examination. | No significant difference between two sources. |
Table 6.
Clinical trials comparing the efficacy of MSCs derived from different sources in the treatment of pulmonary diseases and cardiovascular conditions
| Study name | Time | Disease | Intervention | Treatment outcomes | Effective cell sources | ||
|---|---|---|---|---|---|---|---|
| Source | Dose | Route | |||||
|
Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial315 |
2014 | Ischemic Heart Failure |
BM-MSCs Vs. Autologous bone marrow mononuclear cells (aBMNCs) |
2 × 108 cells | Transendocardial (TE) injection | TE injection of BM-MSCs or aBMNCs appeared to be safe for patients with chronic cardiomyopathy and left ventricular dysfunction. | No significant difference between two approaches. |
|
Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial310 |
2017 | Nonischemic Dilated Cardiomyopathy (NIDCM) |
Autologous BM-MSCs vs. Allogeneic BM-MSCs. |
1 × 108 cells | Transendocardial (TE) injection | The results support the safety profiles of BM-MSCs in the treatment of NIDCM patients. | Allogeneic BM-MSCs |
|
Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial)325 |
2020 | Myocardial Infarction |
Allogeneic UC-MSCs vs. bone marrow mononuclear cells (aBMNCs) |
2 × 107 UC-MSCs 20–25 × 107 aBMNCs |
intramyocardial injection | Significant results were observed in the intramyocardial delivery of UC-MSCs justified their efficacy in chronic ischemic cardiomyopathy. | UC-MSCs |
|
Autologous Infusion of Bone Marrow and Mesenchymal Stromal Cells in Patients with Chronic Obstructive Pulmonary Disease: Phase I Randomized Clinical Trial315 |
2021 | Chronic Obstructive Pulmonary Disease |
Autologous bone marrow mononuclear cells (aBMNCs) vs. Coinfusion of BMNCs and AT-MSCs. |
1 × 108 cells | IV |
Safety: No adverse events Efficiency: - BMMC group showed an increase in forced expiratory volume (FEV1) and diffusing capacity for carbon monoxide (DLCO). - Coinfusion group showed a DLCO, and gas exchange improvement and a better quality of life. |
No significant difference between two approaches. |
Limitations of the current hypothesis
The proposed hypothesis presented in this review was made based on (1) the calculated number of recovered patients from published clinical trials; (2) the empirical experience of the authors in the treatment of brain-related diseases,440 pulmonary disorders,215 and endocrinological conditions;271,441 and (3) the proposed mechanisms by which each type of MSC exhibits its best potential for downstream applications. The authors understand that the approach that we used has a certain level of research bias, as a comprehensive meta-analysis is needed to first confirm the correlation between the origins of MSCs and their downstream clinical outcomes before a complete hypothesis can be made. However, to date, a limited number of clinical trials have been conducted to directly compare the efficacy of MSCs from different sources in treating the same disease, which in turn dampened our analysis to prove this hypothesis. In addition, MSC-based therapy is still in its early stages, as controversy and arguments are still present in the field, including (1) the name of MSCs (medicinal signaling cells vs. MSCs or mesenchymal stromal cells),442,443 (2) the existence of “magic cells” (one cell type for the treatment of all diseases),444,445 (3) the conflicting results from large-scale clinical trials,135 and (4) the dangerous issues of unauthorized, unproven stem cell therapies and clinics.446,447 Therefore, our hypothesis is proposed at this time to encourage active researchers and clinicians to either prove or disprove it so that future research can strengthen the uses of MSC-based therapies with solid mechanistic study results and clarify results for “one cell type for the treatment of all diseases”.
Another limitation is the knowledge coverage in the field of MSC-based regenerative medicine, as discussed in this study. First, the abovementioned diseases were narrowed to four major disease categories for which MSC-based therapy is widely applied, including neuronal, pulmonary, cardiovascular, and endocrinological conditions. In fact, other diseases also receive great benefits from MSC therapy, including liver cirrhosis,448 bone regeneration,360 plastic surgery,449 autoimmune disease,450 etc., which are not fully discussed in this review and included in our hypothesis. Recently, the secretome profile of MSCs and its potential application in clinical settings have emerged as a new player in the field, with a recently published comprehensive review including MSC-derived exosomes.451,452 To date, the therapeutic potential of MSCs is believed to be strongly influenced by their secretomes, including growth factors, cytokines, chemokines, and exosomes.453 However, this body of knowledge is also not fully included in our discussion, as this review focuses on the function and potency of MSCs as a whole with considerations derived from published clinical data. Therefore, the authors believe in and support the future applications of the secreted components derived from MSCs, including exosomes, in the treatment of human diseases. In fact, this potential approach could elevate the uses of MSCs to the next level, where the sources of MSCs could be neglected with advancements in the development of protocols that allow strict control of the secretome profiles of MSCs under specific conditions.454–456 Finally, strategies that could potentially enhance the therapeutic outcomes of MSC-based therapy, such as the “priming” process, are not discussed in this review. The idea of “priming” MSCs is based on the nature of MSCs, which is similar to the immune cells,457 that MSCs have proven to be able to “remember” the stimulus from the surrounding environment.458,459 Thus, activating or priming MSCs using certain conditions, such as hypoxia, matrix mechanics, 3D environment, hormones, or inflammatory cytokines, could trigger the memory mechanism of the MSCs in vitro so that these cells are ready to function towards specific therapeutic activities without the need for in vivo activation.3,460
Conclusion
From a cellular and molecular perspective and from our own experience in a clinical trial setting, AD-, BM- and UC-MSCs exhibit different functional activities and treatment effectiveness across a wide range of human diseases. In this paper, we have provided up-to-date data from the most recently published clinical trials conducted in neuronal diseases, endocrine and reproductive disorders, skin regeneration, pulmonary dysplasia, and cardiovascular diseases. The implications of the results and discussions presented in this review and in a very large body of comprehensive and excellent reviews as well as systematic analyses in the literature provide a different aspect and perspective on the use of MSCs from different sources in the treatment of human diseases. We strongly believe that the field of regenerative medicine and MSC-based therapy will benefit from active discussion, which in turn will significantly advance our knowledge of MSCs. Based on the proposed mechanisms presented in this review, we suggest several key mechanistic issues and questions that need to be addressed in the future:
The confirmation and demonstration of the mechanism of action prove that tissue origin plays a significant role in the downstream applications of the originated MSCs.
Is it required that MSCs derived from particular cell sources need to have certain functionalities that are unique to or superior in the original tissue sources?
As mechanisms may rely on the secretion of factors from MSCs, it is important to identify the specific stimuli from the wound environments to understand how MSCs from different sources can exhibit similar functions in the same disease and whether or not MSCs derived from a particular source have stronger effects than their counterparts derived from other tissue sources.
Should we create “universal” MSCs that could be functionally equal in the treatment of all diseases regardless of their origin by modeling their genetic materials?
Can new sources of MSCs from either perinatal or adult tissues better stimulate the innate mechanisms of specific cell types in our body, providing a better tool for MSC-based treatment?
A potential ‘priming’ protocol that allows priming, activating, and switching the potency of MSCs from one source to another with a more appropriate clinical phenotype to treat certain diseases. This idea is potentially relevant to our suggestion that each MSC type could be more beneficial in downstream applications, and the development of such a “priming” protocol would allow us to expand the bioavailability of specific MSC types.
From our clinical perspective, the underlying proposal in our review is to no longer use MSCs for applications while disregarding their sources but rather to match the MSC tissue source to the application, shifting from one cell type for the treatment of all diseases to cell source-specific disease treatments. Whether the application of MSCs from different sources still shows their effectiveness to a certain extent in the treatment of diseases or not, the transplantation of MSCs derived from different sources for each particular disease needs to be further investigated, and protocols need to be established via multicentre, randomized, placebo-controlled phase II and III clinical trials (Fig. 6).
Fig. 6.
The tissue sources of mesenchymal stem cells (MSCs) contribute greatly to their therapeutic potential, as all MSC types share safety profiles and overlapping efficacy. Although a large body of data and their review and systematic analysis indicated the shared safety and potential efficacy of MSCs derived from different tissue sources, targeted therapies considering MSC origin as an important factor are imperative to enhance the downstream therapeutic effects of MSCs. We suggest that bone marrow-derived MSCs (BM-MSCs) are good candidates for the treatment of brain and spinal cord injury, adipose tissue-derived MSCs (AT-MSCs) are suitable for the treatment of reproductive disorders and skin regeneration, and umbilical cord-derived MSCs (UC-MSCs) could be alternatives for the treatment of pulmonary diseases and acute respiratory distress syndrome (ARDS). Figure was created with BioRender.com
Acknowledgements
The authors would like to thank the Vingroup Scientific Research and Clinical Application Fund (grant number: PRO. 19.47) for supporting this work. All figures were created with Biorender.com. This work is supported by the Vingroup Scientific Research and Clinical Application Fund (Grant number: PRO.19.47).
Author contributions
D.M.H.: conception and design, manuscript writing, administrative support, data analysis and interpretation, and final approval of the manuscript. P.T.P.: manuscript writing (BM- and UC-MSC sections) and data analysis and interpretation. T.Q.B.: manuscript writing (BM- and UC-MSC sections) and data analysis and interpretation. A.T.L.N.: manuscript writing (UC-MSC section), figure presentation, and data analysis and interpretation. Q.T.N., T.T.K.P., G.H.N., P.T.T.L., and V.T.H.: manuscript writing and data analysis and interpretation. N.R.F. and M.H.: manuscript writing and editing and data analysis and interpretation. L.T.N.: manuscript writing, administrative support, and final approval of the manuscript. All authors have read and approved the article.
Data availability
All data generated or analyzed in this study are included in this published article.
Competing interests
The authors declare no competing interests.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front. Immunol. 2012;3:253. doi: 10.3389/fimmu.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 2019;3:90–104. doi: 10.1038/s41551-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien T, Barry FP. Stem cell therapy and regenerative medicine. Mayo Clin. Proc. 2009;84:859–861. doi: 10.4065/84.10.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mousaei Ghasroldasht, M., Seok, J., Park, H. S., Liakath Ali, F. B. & Al-Hendy, A. Stem cell therapy: from idea to clinical practice. Int. J. Mol. Sci. 23, 2850 (2022). [DOI] [PMC free article] [PubMed]
- 6.Kuriyan AE, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N. Engl. J. Med. 2017;376:1047–1053. doi: 10.1056/NEJMoa1609583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biehl JK, Russell B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009;24:98–103. doi: 10.1097/JCN.0b013e318197a6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srijaya TC, Ramasamy TS, Kasim NH. Advancing stem cell therapy from bench to bedside: lessons from drug therapies. J. Transl. Med. 2014;12:243. doi: 10.1186/s12967-014-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell”. Cell Stem Cell. 2007;1:35–38. doi: 10.1016/j.stem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinov IE. In search of Alexander A. Maximow: the man behind the unitarian theory of hematopoiesis. Perspect. Biol. Med. 2000;43:269–276. doi: 10.1353/pbm.2000.0006. [DOI] [PubMed] [Google Scholar]
- 11.Droscher A. Images of cell trees, cell lines, and cell fates: the legacy of Ernst Haeckel and August Weismann in stem cell research. Hist. Philos. Life Sci. 2014;36:157–186. doi: 10.1007/s40656-014-0028-8. [DOI] [PubMed] [Google Scholar]
- 12.Jansen J. The first successful allogeneic bone-marrow transplant: Georges Mathe. Transfus. Med. Rev. 2005;19:246–248. doi: 10.1016/j.tmrv.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Blume KG, Weissman ILE. Donnall Thomas (1920-2012) Proc. Natl Acad. Sci. USA. 2012;109:20777–20778. doi: 10.1073/pnas.1218913109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng M. Hartmann Stahelin (1925-2011) and the contested history of cyclosporin A. Clin. Transpl. 2013;27:326–329. doi: 10.1111/ctr.12072. [DOI] [PubMed] [Google Scholar]
- 15.Thomas ED, et al. Aplastic anaemia treated by marrow transplantation. Lancet. 1972;1:284–289. doi: 10.1016/S0140-6736(72)90292-9. [DOI] [PubMed] [Google Scholar]
- 16.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Caplan AI. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 19.Bolli R, Tang XL, Guo Y, Li Q. After the storm: an objective appraisal of the efficacy of c-kit+ cardiac progenitor cells in preclinical models of heart disease. Can. J. Physiol. Pharm. 2021;99:129–139. doi: 10.1139/cjpp-2020-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Han D, Liang P, Li Y, Cao F. The current dilemma and breakthrough of stem cell therapy in ischemic heart disease. Front. Cell Dev. Biol. 2021;9:636136. doi: 10.3389/fcell.2021.636136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, et al. Basic and translational research in cardiac repair and regeneration: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;78:2092–2105. doi: 10.1016/j.jacc.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyongyosi M, Wojakowski W, Navarese EP, Moye LA, Investigators A. Meta-analyses of human cell-based cardiac regeneration therapies: controversies in meta-analyses results on cardiac cell-based regenerative studies. Circ. Res. 2016;118:1254–1263. doi: 10.1161/CIRCRESAHA.115.307347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum. Cell. 2006;19:71–75. doi: 10.1111/j.1749-0774.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- 24.Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- 25.Santos AJM, Lo YH, Mah AT, Kuo CJ. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 2018;28:1062–1078. doi: 10.1016/j.tcb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roda G, et al. Crohn’s disease. Nat. Rev. Dis. Prim. 2020;6:22. doi: 10.1038/s41572-020-0156-2. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, et al. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 28.Gratwohl A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transpl. 2005;35:869–879. doi: 10.1038/sj.bmt.1704892. [DOI] [PubMed] [Google Scholar]
- 29.Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Br. J. Haematol. 1998;103:651–652. doi: 10.1046/j.1365-2141.1998.01059.x. [DOI] [PubMed] [Google Scholar]
- 30.Hurley JM, Lee SG, Andrews RE, Jr., Klowden MJ, Bulla LA., Jr. Separation of the cytolytic and mosquitocidal proteins of Bacillus thuringiensis subsp. israelensis. Biochem Biophys. Res. Commun. 1985;126:961–965. doi: 10.1016/0006-291X(85)90279-7. [DOI] [PubMed] [Google Scholar]
- 31.Oyama Y, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552–563. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 32.Burt RK, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123–6132. doi: 10.1182/blood-2010-06-292391. [DOI] [PubMed] [Google Scholar]
- 33.Hasselblatt P, et al. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharm. Ther. 2012;36:725–735. doi: 10.1111/apt.12032. [DOI] [PubMed] [Google Scholar]
- 34.Hawkey CJ, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. J. Am. Med. Assoc. 2015;314:2524–2534. doi: 10.1001/jama.2015.16700. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay JO, et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2017;2:399–406. doi: 10.1016/S2468-1253(17)30056-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, et al. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12:463. doi: 10.1186/s13287-021-02533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawkey CJ. Hematopoietic stem cell transplantation in Crohn’s disease: state-of-the-art treatment. Dig. Dis. 2017;35:107–114. doi: 10.1159/000449090. [DOI] [PubMed] [Google Scholar]
- 38.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Xue R, et al. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: a systematic review and meta-analysis. J. Transl. Med. 2018;16:126. doi: 10.1186/s12967-018-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi M, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl. Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Dong Y, Wu X, Xu X, Niu J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 2022;13:204. doi: 10.1186/s13287-022-02882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin BL, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 43.Gordon MY, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 44.Arroyo V, et al. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Prim. 2016;2:16041. doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 46.El-Ansary M, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. Rep. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 47.Xu L, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J. Gastroenterol. Hepatol. 2014;29:1620–1628. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 48.Suk KT, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 49.Fang X, et al. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J. Gastroenterol. Hepatol. 2018;33:774–780. doi: 10.1111/jgh.14081. [DOI] [PubMed] [Google Scholar]
- 50.Mohamadnejad M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen TL, et al. Autologous bone marrow mononuclear cell infusion for liver cirrhosis after the Kasai operation in children with biliary atresia. Stem Cell Res. Ther. 2022;13:108. doi: 10.1186/s13287-022-02762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai YQ, et al. Outcomes of autologous bone marrow mononuclear cell transplantation in decompensated liver cirrhosis. World J. Gastroenterol. 2014;20:8660–8666. doi: 10.3748/wjg.v20.i26.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo C, et al. Long-term outcomes of autologous peripheral blood stem cell transplantation in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2019;17:1175–1182 e1172. doi: 10.1016/j.cgh.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 54.Newsome PN, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2018;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spahr L, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS ONE. 2013;8:e53719. doi: 10.1371/journal.pone.0053719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin. Med. 2018;18:245–250. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2020;50:1038–1047. doi: 10.1111/imj.14709. [DOI] [PubMed] [Google Scholar]
- 58.Sakai Y, et al. Clinical trial of autologous adipose tissue-derived regenerative (stem) cells therapy for exploration of its safety and efficacy. Regen. Ther. 2021;18:97–101. doi: 10.1016/j.reth.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mieli-Vergani G, et al. Autoimmune hepatitis. Nat. Rev. Dis. Primers. 2018;4:18018. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 60.Calore E, et al. Haploidentical stem cell transplantation cures autoimmune hepatitis and cerebrovascular disease in a patient with sickle cell disease. Bone Marrow Transpl. 2018;53:644–646. doi: 10.1038/s41409-017-0065-5. [DOI] [PubMed] [Google Scholar]
- 61.Vento S, Cainelli F, Renzini C, Ghironzi G, Concia E. Resolution of autoimmune hepatitis after bone-marrow transplantation. Lancet. 1996;348:544–545. doi: 10.1016/S0140-6736(05)64700-9. [DOI] [PubMed] [Google Scholar]
- 62.Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol. Immunol. 2022;19:158–176. doi: 10.1038/s41423-021-00768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 2013;28(Suppl 1):85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 65.Martel-Pelletier J, et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 66.Olsson S, Akbarian E, Lind A, Razavian AS, Gordon M. Automating classification of osteoarthritis according to Kellgren-Lawrence in the knee using deep learning in an unfiltered adult population. BMC Musculoskelet. Disord. 2021;22:844. doi: 10.1186/s12891-021-04722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early-stage symptomatic osteoarthritis of the knee—time for action. Nat. Rev. Rheumatol. 2021;17:621–632. doi: 10.1038/s41584-021-00673-4. [DOI] [PubMed] [Google Scholar]
- 68.Kubsik-Gidlewska A, et al. CD34+ stem cell treatment for knee osteoarthritis: a treatment and rehabilitation algorithm. J. Rehabil. Med Clin. Commun. 2018;3:1000012. doi: 10.2340/20030711-1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jevotovsky DS, Alfonso AR, Einhorn TA, Chiu ES. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthr. Cartil. 2018;26:711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- 70.Wiggers TG, Winters M, Van den Boom NA, Haisma HJ, Moen MH. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br. J. Sports Med. 2021;55:1161–1169. doi: 10.1136/bjsports-2020-103671. [DOI] [PubMed] [Google Scholar]
- 71.Han SB, Seo IW, Shin YS. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a network meta-analysis. Arthroscopy. 2021;37:292–306. doi: 10.1016/j.arthro.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 72.Bastos R, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:3342–3350. doi: 10.1007/s00167-018-4883-9. [DOI] [PubMed] [Google Scholar]
- 73.Molnar, V. et al. Mesenchymal stem cell mechanisms of action and clinical effects in osteoarthritis: a narrative review. Genes13, 949 (2022). [DOI] [PMC free article] [PubMed]
- 74.Barisic S, Childs RW. Graft-versus-solid-tumor effect: from hematopoietic stem cell transplantation to adoptive cell therapies. Stem Cells. 2022;40:556–563. doi: 10.1093/stmcls/sxac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mello MM, Brennan TA. The controversy over high-dose chemotherapy with autologous bone marrow transplant for breast cancer. Health Aff. (Millwood) 2001;20:101–117. doi: 10.1377/hlthaff.20.5.101. [DOI] [PubMed] [Google Scholar]
- 76.Sissung TM, Figg WD. Stem cell clinics: risk of proliferation. Lancet Oncol. 2020;21:205–206. doi: 10.1016/S1470-2045(19)30787-9. [DOI] [PubMed] [Google Scholar]
- 77.Fu, X. et al. Mesenchymal stem cell migration and tissue repair. Cells8, 784 (2019). [DOI] [PMC free article] [PubMed]
- 78.Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Araujo Farias V, Carrillo-Galvez AB, Martin F, Anderson P. TGF-beta and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018;43:25–37. doi: 10.1016/j.cytogfr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Ding W, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ritter E, et al. Breast cancer cell-derived fibroblast growth factor 2 and vascular endothelial growth factor are chemoattractants for bone marrow stromal stem cells. Ann. Surg. 2008;247:310–314. doi: 10.1097/SLA.0b013e31816401d5. [DOI] [PubMed] [Google Scholar]
- 82.Cronwright G, et al. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207–2215. doi: 10.1158/0008-5472.CAN-04-1882. [DOI] [PubMed] [Google Scholar]
- 83.Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers12, 1765 (2020). [DOI] [PMC free article] [PubMed]
- 84.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 85.Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol. Cancer. 2010;9:129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmohl KA, Muller AM, Nelson PJ, Spitzweg C. Thyroid hormone effects on mesenchymal stem cell biology in the tumour microenvironment. Exp. Clin. Endocrinol. Diabetes. 2020;128:462–468. doi: 10.1055/a-1022-9874. [DOI] [PubMed] [Google Scholar]
- 87.Mishra PJ, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J, Han G, Liu H, Qin C. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS ONE. 2013;8:e62844. doi: 10.1371/journal.pone.0062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho IA, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31:146–155. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- 90.Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rhee KJ, Lee JI, Eom YW. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int. J. Mol. Sci. 2015;16:30015–30033. doi: 10.3390/ijms161226215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang W, et al. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 2021;26:3. doi: 10.1186/s11658-020-00246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao GD, et al. The oncolytic virus in cancer diagnosis and treatment. Front. Oncol. 2020;10:1786. doi: 10.3389/fonc.2020.01786. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Melen GJ, et al. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016;371:161–170. doi: 10.1016/j.canlet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Castro J, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 2010;17:476–483. doi: 10.1038/cgt.2010.4. [DOI] [PubMed] [Google Scholar]
- 97.Draganov DD, et al. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J. Transl. Med. 2019;17:100. doi: 10.1186/s12967-019-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cyranoski D. How human embryonic stem cells sparked a revolution. Nature. 2018;555:428–430. doi: 10.1038/d41586-018-03268-4. [DOI] [PubMed] [Google Scholar]
- 99.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 102.Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ. Res. 2002;91:866–876. doi: 10.1161/01.RES.0000041435.95082.84. [DOI] [PubMed] [Google Scholar]
- 103.Andrews PW. From teratocarcinomas to embryonic stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:405–417. doi: 10.1098/rstb.2002.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Finch BW, Ephrussi B. Retention of multiple developmental potentialities by cells of a mouse testicular teratocarcinoma during prolonged culture in vitro and their extinction upon hybridization with cells of permanent lines. Proc. Natl Acad. Sci. USA. 1967;57:615–621. doi: 10.1073/pnas.57.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ried T, et al. The consequences of chromosomal aneuploidy on the transcriptome of cancer cells. Biochim Biophys. Acta. 2012;1819:784–793. doi: 10.1016/j.bbagrm.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 107.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo B, Parham L. Ethical issues in stem cell research. Endocr. Rev. 2009;30:204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 111.Atala A. Human embryonic stem cells: early hints on safety and efficacy. Lancet. 2012;379:689–690. doi: 10.1016/S0140-6736(12)60118-4. [DOI] [PubMed] [Google Scholar]
- 112.Schwartz SD, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 113.Song WK, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y, et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018;4:50. doi: 10.1038/s41421-018-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Limnios IJ, Chau YQ, Skabo SJ, Surrao DC, O’Neill HC. Efficient differentiation of human embryonic stem cells to retinal pigment epithelium under defined conditions. Stem Cell Res. Ther. 2021;12:248. doi: 10.1186/s13287-021-02316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foltz, L. P. & Clegg, D. O. Rapid, directed differentiation of retinal pigment epithelial cells from human embryonic or induced pluripotent stem cells. J. Vis. Exp.128, e56274 (2017). [DOI] [PMC free article] [PubMed]
- 117.Kuroda T, Ando S, Takeno Y, Kishino A, Kimura T. Robust induction of retinal pigment epithelium cells from human induced pluripotent stem cells by inhibiting FGF/MAPK signaling. Stem Cell Res. 2019;39:101514. doi: 10.1016/j.scr.2019.101514. [DOI] [PubMed] [Google Scholar]
- 118.Dewell TE, et al. Transcription factor overexpression drives reliable differentiation of retinal pigment epithelium from human induced pluripotent stem cells. Stem Cell Res. 2021;53:102368. doi: 10.1016/j.scr.2021.102368. [DOI] [PubMed] [Google Scholar]
- 119.Dehghan S, Mirshahi R, Shoae-Hassani A, Naseripour M. Human-induced pluripotent stem cells-derived retinal pigmented epithelium, a new horizon for cells-based therapies for age-related macular degeneration. Stem Cell Res. Ther. 2022;13:217. doi: 10.1186/s13287-022-02894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Menasche P, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 121.Menasche P, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 122.Cyranoski D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature. 2018;557:619–620. doi: 10.1038/d41586-018-05278-8. [DOI] [PubMed] [Google Scholar]
- 123.Povsic, T. J. & Gersh, B. J. Stem cells in cardiovascular diseases: 30,000-foot view. Cells10, 600 (2021). [DOI] [PMC free article] [PubMed]
- 124.Romito A, Cobellis G. Pluripotent stem cells: current understanding and future directions. Stem Cells Int. 2016;2016:9451492. doi: 10.1155/2016/9451492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKenna, S. L. et al. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J. Neurosurg. Spine1, 1–10 (2022). [DOI] [PubMed]
- 126.Deinsberger J, Reisinger D, Weber B. Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. NPJ Regen. Med. 2020;5:15. doi: 10.1038/s41536-020-00100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim JY, Nam Y, Rim YA, Ju JH. Review of the current trends in clinical trials involving induced pluripotent stem cells. Stem Cell Rev. Rep. 2022;18:142–154. doi: 10.1007/s12015-021-10262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ji P, Manupipatpong S, Xie N, Li Y. Induced pluripotent stem cells: generation strategy and epigenetic mystery behind reprogramming. Stem Cells Int. 2016;2016:8415010. doi: 10.1155/2016/8415010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu X. The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol. Immunol. 2014;11:14–16. doi: 10.1038/cmi.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 132.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 133.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 135.Zhou T, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Witkowska-Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton’s jelly, amnion and chorion. Cell Mol. Biol. Lett. 2011;16:493–514. doi: 10.2478/s11658-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alkhalil M, Smajilagic A, Redzic A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med. Glas. (Zenica) 2015;12:27–32. [PubMed] [Google Scholar]
- 140.Ouryazdanpanah N, Dabiri S, Derakhshani A, Vahidi R, Farsinejad A. Peripheral blood-derived mesenchymal stem cells: growth factor-free isolation, molecular characterization and differentiation. Iran. J. Pathol. 2018;13:461–466. [PMC free article] [PubMed] [Google Scholar]
- 141.Francis MP, Sachs PC, Elmore LW, Holt SE. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis. 2010;6:11–14. doi: 10.4161/org.6.1.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gong X, et al. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol. Int. 2014;38:405–411. doi: 10.1002/cbin.10240. [DOI] [PubMed] [Google Scholar]
- 143.Wang B, et al. Human hair follicle-derived mesenchymal stem cells: Isolation, expansion, and differentiation. World J. Stem Cells. 2020;12:462–470. doi: 10.4252/wjsc.v12.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pilato, C. A. et al. Isolation and characterization of cardiac mesenchymal stromal cells from endomyocardial bioptic samples of arrhythmogenic cardiomyopathy patients. J. Vis. Exp. 132, e57263 (2018). [DOI] [PMC free article] [PubMed]
- 145.Mannino G, et al. Adult stem cell niches for tissue homeostasis. J. Cell Physiol. 2022;237:239–257. doi: 10.1002/jcp.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pavlushina SV, Orlova TG, Tabagari DZ. [Isolation of mononuclear cells from the bone marrow of patients with hemoblastoses using one-step ficoll-verographin density gradient separation] Eksp. Onkol. 1984;6:68–70. [PubMed] [Google Scholar]
- 147.Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med Res. 2017;22:17. doi: 10.1186/s40001-017-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Torre, P. & Flores, A. I. Current status and future prospects of perinatal stem cells. Genes12, 6 (2020). [DOI] [PMC free article] [PubMed]
- 149.Hoang, V. T. et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy23, 88–99 (2020). [DOI] [PubMed]
- 150.Mohamed-Ahmed S, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Z. CD133: a stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013;2:17. doi: 10.1186/2162-3619-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Petrenko Y, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci. Rep. 2020;10:4290. doi: 10.1038/s41598-020-61167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 155.Xu L, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8:275. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han I, Kwon BS, Park HK, Kim KS. Differentiation potential of mesenchymal stem cells is related to their intrinsic mechanical properties. Int. Neurourol. J. 2017;21:S24–S31. doi: 10.5213/inj.1734856.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song Y, et al. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J. Stem Cells. 2020;12:1032–1049. doi: 10.4252/wjsc.v12.i9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Allers C, et al. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78:503–508. doi: 10.1097/01.TP.0000128334.93343.B3. [DOI] [PubMed] [Google Scholar]
- 160.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 161.Fischer UM, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sierra-Parraga JM, et al. Mesenchymal stromal cells are retained in the porcine renal cortex independently of their metabolic state after renal intra-arterial infusion. Stem Cells Dev. 2019;28:1224–1235. doi: 10.1089/scd.2019.0105. [DOI] [PubMed] [Google Scholar]
- 163.Henriksson HB, et al. The traceability of mesenchymal stromal cells after injection into degenerated discs in patients with low back pain. Stem Cells Dev. 2019;28:1203–1211. doi: 10.1089/scd.2019.0074. [DOI] [PubMed] [Google Scholar]
- 164.Sokal EM, et al. Biodistribution of liver-derived mesenchymal stem cells after peripheral injection in a hemophilia A patient. Transplantation. 2017;101:1845–1851. doi: 10.1097/TP.0000000000001773. [DOI] [PubMed] [Google Scholar]
- 165.Sood V, et al. Biodistribution of 18F-FDG-labeled autologous bone marrow-derived stem cells in patients with type 2 diabetes mellitus: exploring targeted and intravenous routes of delivery. Clin. Nucl. Med. 2015;40:697–700. doi: 10.1097/RLU.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 166.Sanchez-Diaz, M. et al. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: a systematic review. J. Clin. Med.10, 2925 (2021). [DOI] [PMC free article] [PubMed]
- 167.Sensebe L, Fleury-Cappellesso S. Biodistribution of mesenchymal stem/stromal cells in a preclinical setting. Stem Cells Int. 2013;2013:678063. doi: 10.1155/2013/678063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhuang WZ, et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021;28:28. doi: 10.1186/s12929-021-00725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wei X, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharm. Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kouchakian MR, et al. The clinical trials of mesenchymal stromal cells therapy. Stem Cells Int. 2021;2021:1634782. doi: 10.1155/2021/1634782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen L, et al. Mesenchymal stem cell-based treatments for COVID-19: status and future perspectives for clinical applications. Cell Mol. Life Sci. 2022;79:142. doi: 10.1007/s00018-021-04096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ. Res. 2019;125:265–281. doi: 10.1161/CIRCRESAHA.119.314951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zheng H, et al. Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transpl. 2018;27:1723–1730. doi: 10.1177/0963689718806846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rodriguez-Fuentes DE, et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch. Med. Res. 2021;52:93–101. doi: 10.1016/j.arcmed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 175.Shi L, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct. Target Ther. 2021;6:339. doi: 10.1038/s41392-021-00754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carney BJ, Shah K. Migration and fate of therapeutic stem cells in different brain disease models. Neuroscience. 2011;197:37–47. doi: 10.1016/j.neuroscience.2011.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yao P, Zhou L, Zhu L, Zhou B, Yu Q. Mesenchymal stem cells: a potential therapeutic strategy for neurodegenerative diseases. Eur. Neurol. 2020;83:235–241. doi: 10.1159/000509268. [DOI] [PubMed] [Google Scholar]
- 178.Bonaventura, G. et al. Stem cells: innovative therapeutic options for neurodegenerative diseases? Cells10, 1992 (2021). [DOI] [PMC free article] [PubMed]
- 179.Mansoor SR, Zabihi E, Ghasemi-Kasman M. The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci. 2019;235:116830. doi: 10.1016/j.lfs.2019.116830. [DOI] [PubMed] [Google Scholar]
- 180.Chung JW, et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. doi: 10.1212/WNL.0000000000011440. [DOI] [PubMed] [Google Scholar]
- 181.Yamazaki, K., Kawabori, M., Seki, T. & Houkin, K. Clinical trials of stem cell treatment for spinal cord injury. Int. J. Mol. Sci. 21, 3994 (2020). [DOI] [PMC free article] [PubMed]
- 182.Xie B, Chen M, Hu R, Han W, Ding S. Therapeutic evidence of human mesenchymal stem cell transplantation for cerebral palsy: a meta-analysis of randomized controlled trials. Stem Cells Int. 2020;2020:5701920. doi: 10.1155/2020/5701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McDonald, C. A. et al. Intranasal delivery of mesenchymal stromal cells protects against neonatal hypoxic(-)ischemic brain injury. Int. J. Mol. Sci. 20, 2449 (2019). [DOI] [PMC free article] [PubMed]
- 184.Liu Q, et al. Rational use of mesenchymal stem cells in the treatment of autism spectrum disorders. World J. Stem Cells. 2019;11:55–72. doi: 10.4252/wjsc.v11.i2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fricova D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. npj Regen. Med. 2020;5:20. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 187.Lee JS, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 188.Honmou O, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bhasin A, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc. Dis. Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jaillard A, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl. Stroke Res. 2020;11:910–923. doi: 10.1007/s12975-020-00787-z. [DOI] [PubMed] [Google Scholar]
- 191.Lee J, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke. 2022;53:20–28. doi: 10.1161/STROKEAHA.121.034505. [DOI] [PubMed] [Google Scholar]
- 192.Levy ML, et al. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50:2835–2841. doi: 10.1161/STROKEAHA.119.026318. [DOI] [PubMed] [Google Scholar]
- 193.Xu P, Yang X. The efficacy and safety of mesenchymal stem cell transplantation for spinal cord injury patients: a meta-analysis and systematic review. Cell Transpl. 2019;28:36–46. doi: 10.1177/0963689718808471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liau LL, et al. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:112. doi: 10.1186/s13578-020-00475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu, X. et al. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med.15, 1–9 (2017). [DOI] [PMC free article] [PubMed]
- 196.Sharma, A. K. et al. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: a clinical study. Am J. Stem Cells9, 89 (2020). [PMC free article] [PubMed]
- 197.Ballen K, Kurtzberg J. Exploring new therapies for children with autism: “Do no harm” does not mean do not try. Stem Cells Transl. Med. 2021;10:823–825. doi: 10.1002/sctm.20-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reyhani S, Abbaspanah B, Mousavi SH. Umbilical cord-derived mesenchymal stem cells in neurodegenerative disorders: from literature to clinical practice. Regen. Med. 2020;15:1561–1578. doi: 10.2217/rme-2019-0119. [DOI] [PubMed] [Google Scholar]
- 199.Gu J, et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther. 2020;11:43. doi: 10.1186/s13287-019-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Retraction. Stem Cells Transl. Med.10, 1717 (2021). [DOI] [PMC free article] [PubMed]
- 201.Sun JM, et al. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl. Med. 2020;9:1137–1146. doi: 10.1002/sctm.19-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang, Y. et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy23, 57–64 (2021). [DOI] [PubMed]
- 203.Liu J, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15:185–191. doi: 10.1016/j.jcyt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 204.Przekora A, Juszkiewicz L. The effect of autologous adipose tissue-derived mesenchymal stem cells’ therapy in the treatment of chronic posttraumatic spinal cord injury in a domestic ferret patient. Cell Transpl. 2020;29:963689720928982. doi: 10.1177/0963689720928982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hur JW, et al. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J. Spinal Cord. Med. 2016;39:655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.de Celis-Ruiz E, et al. Final results of allogeneic adipose tissue-derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): a phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transpl. 2022;31:9636897221083863. doi: 10.1177/09636897221083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yang Y, et al. Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial. Neural Regen. Res. 2020;15:1532–1538. doi: 10.4103/1673-5374.274347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.de Celis-Ruiz E, et al. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open. 2021;11:e051790. doi: 10.1136/bmjopen-2021-051790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399:417–419. doi: 10.1016/S0140-6736(22)00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Thebaud B, et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019;5:78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mohamed T, Abdul-Hafez A, Gewolb IH, Uhal BD. Oxygen injury in neonates: which is worse? hyperoxia, hypoxia, or alternating hyperoxia/hypoxia. J. Lung Pulm. Respir. Res. 2020;7:4–13. [PMC free article] [PubMed] [Google Scholar]
- 212.Omar, S. A. et al. Stem-cell therapy for bronchopulmonary dysplasia (BPD) in newborns. Cells11, 1275 (2022). [DOI] [PMC free article] [PubMed]
- 213.Chang YS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 2014;164:966–972 e966. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 214.Powell SB, Silvestri JM. Safety of intratracheal administration of human umbilical cord blood derived mesenchymal stromal cells in extremely low birth weight preterm infants. J. Pediatr. 2019;210:209–213 e202. doi: 10.1016/j.jpeds.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 215.Nguyen LT, et al. Allogeneic administration of human umbilical cord-derived mesenchymal stem/stromal cells for bronchopulmonary dysplasia: preliminary outcomes in four Vietnamese infants. J. Transl. Med. 2020;18:398. doi: 10.1186/s12967-020-02568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ahn SY, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl. Med. 2021;10:1129–1137. doi: 10.1002/sctm.20-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Averyanov, A. et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med.9, 6–16 (2020). [DOI] [PMC free article] [PubMed]
- 218.Ribeiro-Paes JT, et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int. J. Chron. Obstruct Pulmon Dis. 2011;6:63–71. doi: 10.2147/COPD.S15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stessuk T, et al. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev. Bras. Hematol. Hemoter. 2013;35:352–357. doi: 10.5581/1516-8484.20130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.de Oliveira HG, et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl. Med. 2017;6:962–969. doi: 10.1002/sctm.16-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stolk J, et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM. 2016;109:331–336. doi: 10.1093/qjmed/hcw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.de Oliveira, H. G. et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl. Med.6, 962–969 (2017). [DOI] [PMC free article] [PubMed]
- 224.Armitage J, et al. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur. Respir. J. 2018;51:1702369. doi: 10.1183/13993003.02369-2017. [DOI] [PubMed] [Google Scholar]
- 225.Comella K, et al. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: a phase I trial of safety and tolerability. J. Clin. Med. Res. 2017;9:701–708. doi: 10.14740/jocmr3072w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tzilas, V. et al. Prospective phase 1 open clinical trial to study the safety of adipose derived mesenchymal stem cells (ADMSCs) in COPD and combined pulmonary fibrosis and emphysema (CPFE). Eur. Respir. J.46, (2015).
- 227.Comella, K. et al. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: a phase I trial of safety and tolerability. J. Clin. Med. Res.9, 701–708 (2017). [DOI] [PMC free article] [PubMed]
- 228.Glassberg MK, Csete I, Simonet E, Elliot SJ. Stem cell therapy for COPD: hope and exploitation. Chest. 2021;160:1271–1281. doi: 10.1016/j.chest.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 229.Le Thi Bich, P. et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res. Ther.60, 11 (2020). [DOI] [PMC free article] [PubMed]
- 230.Karaoz E, Kalemci S, Ece F. Improving effects of mesenchymal stem cells on symptoms of chronic obstructive pulmonary disease. Bratisl. Lek. Listy. 2020;121:188–191. doi: 10.4149/BLL_2020_028. [DOI] [PubMed] [Google Scholar]
- 231.Hoang DM, Nguyen KT, Nguyen AH, Nguyen BN, Nguyen LT. Allogeneic human umbilical cord-derived mesenchymal stem/stromal cells for chronic obstructive pulmonary disease (COPD): study protocol for a matched case-control, phase I/II trial. BMJ Open. 2021;11:e045788. doi: 10.1136/bmjopen-2020-045788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xu R, Feng Z, Wang FS. Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 2022;77:103920. doi: 10.1016/j.ebiom.2022.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khoury M, et al. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 2020;55:2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jamilloux Y, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Feng Y, et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 2020;53:e12947. doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Primorac, D. et al. Mesenchymal stromal cells: potential option for COVID-19 treatment. Pharmaceutic13, 1481 (2021). [DOI] [PMC free article] [PubMed]
- 237.Zhang Y, et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shu L, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tao J, et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J. Infect. Dev. Ctries. 2020;14:1138–1145. doi: 10.3855/jidc.13081. [DOI] [PubMed] [Google Scholar]
- 240.Saleh M, et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res. Ther. 2021;12:410. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Leng Z, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Guo Z, et al. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care. 2020;24:420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Meng F, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct. Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shi L, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2021;75:103789. doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Adas G, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transpl. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shi, L. et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Targeted Ther.6, 58 (2021). [DOI] [PMC free article] [PubMed]
- 247.Lanzoni G, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hashemian SR, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhu R, et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021;31:1244–1262. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shi L, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.N OE, Pekkoc-Uyanik KC, Alpaydin N, Gulay GR, Simsek M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev. Rep. 2021;17:1917–1925. doi: 10.1007/s12015-021-10214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gentile P, Sterodimas A, Pizzicannella J, Calabrese C, Garcovich S. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVID-19 disease. Aging Dis. 2020;11:1191–1201. doi: 10.14336/AD.2020.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Copcu HE. Potential using of fat-derived stromal cells in the treatment of active disease, and also, in both pre- and post-periods in COVID-19. Aging Dis. 2020;11:730–736. doi: 10.14336/AD.2020.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gentile P, Sterodimas A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin. Biol. Ther. 2020;20:711–716. doi: 10.1080/14712598.2020.1761322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Matthay, M. A. et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med.7, 154–162 (2019). [DOI] [PMC free article] [PubMed]
- 256.Álvarez-Fuente, M. et al. Off-label mesenchymal stromal cell treatment in two infants with severe bronchopulmonary dysplasia: clinical course and biomarkers profile. Cytotherapy20, 1337–1344 (2018). [DOI] [PubMed]
- 257.Zheng G, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Simonson OE, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 2015;4:1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wilson JG, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Matthay MA, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yip HK, et al. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit. Care Med. 2020;48:e391–e399. doi: 10.1097/CCM.0000000000004285. [DOI] [PubMed] [Google Scholar]
- 262.Gorman E, et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine. 2021;41:101167. doi: 10.1016/j.eclinm.2021.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Le Thi Bich P, et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res. Ther. 2020;11:60. doi: 10.1186/s13287-020-1583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang MY, et al. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm. 2021;2:351–380. doi: 10.1002/mco2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 266.Dantas JR, et al. Adipose tissue-derived stromal/stem cells + cholecalciferol: a pilot study in recent-onset type 1 diabetes patients. Arch. Endocrinol. Metab. 2021;65:342–351. doi: 10.20945/2359-3997000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Joseph UA, Jhingran SG. Technetium-99m labeled RBC imaging in gastrointestinal bleeding from gastric leiomyoma. Clin. Nucl. Med. 1988;13:23–25. doi: 10.1097/00003072-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 268.Hu J, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J. 2013;60:347–357. doi: 10.1507/endocrj.EJ12-0343. [DOI] [PubMed] [Google Scholar]
- 269.Cai J, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39:149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 270.Huang Q, Huang Y, Liu J. Mesenchymal stem cells: an excellent candidate for the treatment of diabetes mellitus. Int. J. Endocrinol. 2021;2021:9938658. doi: 10.1155/2021/9938658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nguyen, L. T. et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl. Med.10, 1266–1278 (2021). [DOI] [PMC free article] [PubMed]
- 272.Alicka, M., Major, P., Wysocki, M. & Marycz, K. Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J. Clin. Med.8, 765 (2019). [DOI] [PMC free article] [PubMed]
- 273.Agarwal A, et al. Male infertility. Lancet. 2021;397:319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 274.Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane reviews. Cochrane Database Syst. Rev. 2018;8:CD010537. doi: 10.1002/14651858.CD010537.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chang Z, et al. Mesenchymal stem cells in preclinical infertility cytotherapy: a retrospective review. Stem Cells Int. 2021;2021:8882368. doi: 10.1155/2021/8882368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fenton AJ. Premature ovarian insufficiency: pathogenesis and management. J. Midlife Health. 2015;6:147–153. doi: 10.4103/0976-7800.172292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Coulam CB. Premature gonadal failure. Fertil. Steril. 1982;38:645–655. doi: 10.1016/S0015-0282(16)46688-4. [DOI] [PubMed] [Google Scholar]
- 278.Huhtaniemi I, et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol. Metab. 2018;29:400–419. doi: 10.1016/j.tem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 279.Torrealday S, Kodaman P, Pal L. Premature ovarian Insufficiency—an update on recent advances in understanding and management. F1000Res. 2017;6:2069. doi: 10.12688/f1000research.11948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gupta S, Lodha P, Karthick MS, Tandulwadkar SR. Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: review of literature and a case report of world’s first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J. Hum. Reprod. Sci. 2018;11:125–130. doi: 10.4103/jhrs.JHRS_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Igboeli P, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J. Med. Case Rep. 2020;14:108. doi: 10.1186/s13256-020-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ulin M, et al. Human mesenchymal stem cell therapy and other novel treatment approaches for premature ovarian insufficiency. Reprod. Sci. 2021;28:1688–1696. doi: 10.1007/s43032-021-00528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Herraiz S, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 2018;110:496–505 e491. doi: 10.1016/j.fertnstert.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 284.Ding L, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China Life Sci. 2018;61:1554–1565. doi: 10.1007/s11427-017-9272-2. [DOI] [PubMed] [Google Scholar]
- 285.Wang MY, Wang YX, Li-Ling J, Xie HQ. Adult stem cell therapy for premature ovarian failure: from bench to bedside. Tissue Eng. Part B Rev. 2022;28:63–78. doi: 10.1089/ten.teb.2020.0205. [DOI] [PubMed] [Google Scholar]
- 286.Kaddoura I, Abu-Sittah G, Ibrahim A, Karamanoukian R, Papazian N. Burn injury: review of pathophysiology and therapeutic modalities in major burns. Ann. Burns Fire Disasters. 2017;30:95–102. [PMC free article] [PubMed] [Google Scholar]
- 287.Jeschke MG, et al. Burn injury. Nat. Rev. Dis. Prim. 2020;6:11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rasulov MF, et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005;139:141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 289.Mansilla E, et al. Cadaveric bone marrow mesenchymal stem cells: first experience treating a patient with large severe burns. Burns Trauma. 2015;3:17. doi: 10.1186/s41038-015-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Xu Y, Huang S, Fu X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: a promising therapeutic strategy for prevention of skin-graft contraction. Clin. Exp. Dermatol. 2012;37:497–500. doi: 10.1111/j.1365-2230.2011.04260.x. [DOI] [PubMed] [Google Scholar]
- 291.Yoshikawa T, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 292.Abo-Elkheir W, et al. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study. Am. J. Stem Cells. 2017;6:23–35. [PMC free article] [PubMed] [Google Scholar]
- 293.Li, L. et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int. J. Mol. Sci.21, 49 (2019). [DOI] [PMC free article] [PubMed]
- 294.Lotfi, M. et al. Adipose tissue-derived mesenchymal stem cells and keratinocytes co-culture on gelatin/chitosan/beta-glycerol phosphate nanoscaffold in skin regeneration. Cell Biol. Int.43, 1365–1378 (2019). [DOI] [PubMed]
- 295.Yang JA, Chung HM, Won CH, Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin. Biol. Ther. 2010;10:495–503. doi: 10.1517/14712591003610598. [DOI] [PubMed] [Google Scholar]
- 296.Zhou Y, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2021;12:257. doi: 10.1186/s13287-021-02287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Arjmand B, et al. Regenerative medicine for the treatment of ischemic heart disease; status and future perspectives. Front. Cell Dev. Biol. 2021;9:704903. doi: 10.3389/fcell.2021.704903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Denning C, et al. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim Biophys. Acta. 2016;1863:1728–1748. doi: 10.1016/j.bbamcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wu R, Hu X, Wang J. Concise review: optimized strategies for stem cell-based therapy in myocardial repair: clinical translatability and potential limitation. Stem Cells. 2018;36:482–500. doi: 10.1002/stem.2778. [DOI] [PubMed] [Google Scholar]
- 300.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol. Ther. 2018;26:1610–1623. doi: 10.1016/j.ymthe.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Demurtas J, et al. Stem cells for treatment of cardiovascular diseases: an umbrella review of randomized controlled trials. Ageing Res. Rev. 2021;67:101257. doi: 10.1016/j.arr.2021.101257. [DOI] [PubMed] [Google Scholar]
- 303.Gubert, F. et al. Mesenchymal stem cells therapies on fibrotic heart diseases. Int. J. Mol. Sci.22, 7447 (2021). [DOI] [PMC free article] [PubMed]
- 304.da Silva, J. S. et al. Mesenchymal stem cell therapy in diabetic cardiomyopathy. Cells11, 240 (2022). [DOI] [PMC free article] [PubMed]
- 305.He X, et al. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target Ther. 2022;7:134. doi: 10.1038/s41392-022-00972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bolli R, Solankhi M, Tang XL, Kahlon A. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res. 2022;118:951–976. doi: 10.1093/cvr/cvab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bartunek J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 308.Bartunek J, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2017;38:648–660. doi: 10.1093/eurheartj/ehx300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hare JM, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. J. Am. Med. Assoc. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hare JM, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J. Am. Coll. Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mathiasen AB, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur. Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 312.Mathiasen AB, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020;22:884–892. doi: 10.1002/ejhf.1700. [DOI] [PubMed] [Google Scholar]
- 313.Florea V, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study) Circ. Res. 2017;121:1279–1290. doi: 10.1161/CIRCRESAHA.117.311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Bolli R, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur. J. Heart Fail. 2021;23:661–674. doi: 10.1002/ejhf.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Heldman AW, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. J. Am. Med. Assoc. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Perin EC, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE trial. Am. Heart J. 2014;168:88–95 e82. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 317.Han S, Sun HM, Hwang KC, Kim SW. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015;25:145–152. doi: 10.1615/CritRevEukaryotGeneExpr.2015013057. [DOI] [PubMed] [Google Scholar]
- 318.Henry TD, et al. The Athena trials: autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Inter. 2017;89:169–177. doi: 10.1002/ccd.26601. [DOI] [PubMed] [Google Scholar]
- 319.Kastrup J, et al. Cryopreserved off-the-shelf allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart failure—a safety study. Stem Cells Transl. Med. 2017;6:1963–1971. doi: 10.1002/sctm.17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Qayyum AA, et al. Adipose-derived stromal cells for treatment of patients with chronic ischemic heart disease (MyStromalCell Trial): a randomized placebo-controlled study. Stem Cells Int. 2017;2017:5237063. doi: 10.1155/2017/5237063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Qayyum AA, et al. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 2019;17:360. doi: 10.1186/s12967-019-2110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ngo ATL, et al. Clinically relevant preservation conditions for mesenchymal stem/stromal cells derived from perinatal and adult tissue sources. J. Cell Mol. Med. 2021;25:10747–10760. doi: 10.1111/jcmm.17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Madonna R, Cevik C, Nasser M, De Caterina R. Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb. Haemost. 2012;107:656–661. doi: 10.1160/TH11-10-0711. [DOI] [PubMed] [Google Scholar]
- 324.Bartolucci J, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]) Circ. Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ulus AT, et al. Intramyocardial transplantation of umbilical cord mesenchymal stromal cells in chronic ischemic cardiomyopathy: a controlled, randomized clinical trial (HUC-HEART trial) Int. J. Stem Cells. 2020;13:364–376. doi: 10.15283/ijsc20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.He X, et al. Effect of intramyocardial grafting collagen scaffold with mesenchymal stromal cells in patients with chronic ischemic heart disease: a randomized clinical trial. JAMA Netw. Open. 2020;3:e2016236. doi: 10.1001/jamanetworkopen.2020.16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang Q, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target Ther. 2022;7:78. doi: 10.1038/s41392-022-00925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Poomani MS, et al. Mesenchymal stem cell (MSCs) therapy for ischemic heart disease: a promising frontier. Glob. Heart. 2022;17:19. doi: 10.5334/gh.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Xu W, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 330.Jeong JO, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Denu RA, et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 2016;136:85–97. doi: 10.1159/000445096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann. N. Y Acad. Sci. 2016;1370:82–96. doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ono N, et al. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev. Cell. 2014;29:330–339. doi: 10.1016/j.devcel.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 336.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Golan K, Kollet O, Markus RP, Lapidot T. Daily light and darkness onset and circadian rhythms metabolically synchronize hematopoietic stem cell differentiation and maintenance: the role of bone marrow norepinephrine, tumor necrosis factor, and melatonin cycles. Exp. Hematol. 2019;78:1–10. doi: 10.1016/j.exphem.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 338.Cheng X, et al. The role of SDF-1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front. Neurosci. 2017;11:590. doi: 10.3389/fnins.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 340.Mao W, Yi X, Qin J, Tian M, Jin G. CXCL12 inhibits cortical neuron apoptosis by increasing the ratio of Bcl-2/Bax after traumatic brain injury. Int. J. Neurosci. 2014;124:281–290. doi: 10.3109/00207454.2013.838236. [DOI] [PubMed] [Google Scholar]
- 341.Wang Q, et al. Stromal cell-derived factor 1alpha decreases beta-amyloid deposition in Alzheimer’s disease mouse model. Brain Res. 2012;1459:15–26. doi: 10.1016/j.brainres.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 342.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013;2:300. doi: 10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Li J, et al. CXCL12 promotes spinal nerve regeneration and functional recovery after spinal cord injury. Neuroreport. 2021;32:450–457. doi: 10.1097/WNR.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 344.Gensel JC, Kigerl KA, Mandrekar-Colucci SS, Gaudet AD, Popovich PG. Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 2012;349:201–213. doi: 10.1007/s00441-012-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Matsushita T, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 2011;502:41–45. doi: 10.1016/j.neulet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 346.Schmidt A, et al. Mesenchymal stem cells transmigrate over the endothelial barrier. Eur. J. Cell Biol. 2006;85:1179–1188. doi: 10.1016/j.ejcb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 347.Yarygin, K. N. et al. Cell therapy of stroke: do the intra-arterially transplanted mesenchymal stem cells cross the blood-brain barrier? Cells10, 2997 (2021). [DOI] [PMC free article] [PubMed]
- 348.Schack LM, et al. Expression of CD24 in human bone marrow-derived mesenchymal stromal cells is regulated by TGFbeta3 and induces a myofibroblast-like genotype. Stem Cells Int. 2016;2016:1319578. doi: 10.1155/2016/1319578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ruster B, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 350.Pluchino N, et al. CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking. J. Cell Mol. Med. 2020;24:2464–2474. doi: 10.1111/jcmm.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kowalski K, et al. Stem cells migration during skeletal muscle regeneration—the role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adh. Migr. 2017;11:384–398. doi: 10.1080/19336918.2016.1227911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Liu L, et al. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093. doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell Physiol. 2011;226:385–396. doi: 10.1002/jcp.22344. [DOI] [PubMed] [Google Scholar]
- 354.Lozito TP, Jackson WM, Nesti LJ, Tuan RS. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014;34:132–143. doi: 10.1016/j.matbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 355.Menge T, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin—from mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017;18:753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 357.Brick RM, Sun AX, Tuan RS. Neurotrophically induced mesenchymal progenitor cells derived from induced pluripotent stem cells enhance neuritogenesis via neurotrophin and cytokine production. Stem Cells Transl. Med. 2018;7:45–58. doi: 10.1002/sctm.17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zupanc HRH, Alexander PG, Tuan RS. Neurotrophic support by traumatized muscle-derived multipotent progenitor cells: role of endothelial cells and vascular endothelial growth factor-A. Stem Cell Res. Ther. 2017;8:226. doi: 10.1186/s13287-017-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Liu Y, Olsen BR. Distinct VEGF functions during bone development and homeostasis. Arch. Immunol. Ther. Exp. 2014;62:363–368. doi: 10.1007/s00005-014-0285-y. [DOI] [PubMed] [Google Scholar]
- 360.Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020;11:492. doi: 10.1186/s13287-020-02001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Liu Y, et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Investig. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Berendsen AD, Olsen BR. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J. Histochem Cytochem. 2014;62:103–108. doi: 10.1369/0022155413516347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Garcia KO, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front. Aging Neurosci. 2014;6:30. doi: 10.3389/fnagi.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hohman TJ, Bell SP, Jefferson AL, Alzheimer’s Disease Neuroimaging, I. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:520–529. doi: 10.1001/jamaneurol.2014.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhang W, et al. Neuroprotective effects of SOX5 against ischemic stroke by regulating VEGF/PI3K/AKT pathway. Gene. 2021;767:145148. doi: 10.1016/j.gene.2020.145148. [DOI] [PubMed] [Google Scholar]
- 366.Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Bao XJ, et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and anti-inflammatory and angiogenesis effects in an intracerebral hemorrhage rat model. Int. J. Mol. Med. 2013;31:1087–1096. doi: 10.3892/ijmm.2013.1290. [DOI] [PubMed] [Google Scholar]
- 368.Bao X, et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur. J. Neurosci. 2011;34:87–98. doi: 10.1111/j.1460-9568.2011.07733.x. [DOI] [PubMed] [Google Scholar]
- 369.Pelletier J, et al. VEGF-A promotes both pro-angiogenic and neurotrophic capacities for nerve recovery after compressive neuropathy in rats. Mol. Neurobiol. 2015;51:240–251. doi: 10.1007/s12035-014-8754-1. [DOI] [PubMed] [Google Scholar]
- 370.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000;197(Pt 4):591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Hayakawa K, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J. Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Pei G, Xu L, Huang W, Yin J. The protective role of microRNA-133b in restricting hippocampal neurons apoptosis and inflammatory injury in rats with depression by suppressing CTGF. Int. Immunopharmacol. 2020;78:106076. doi: 10.1016/j.intimp.2019.106076. [DOI] [PubMed] [Google Scholar]
- 373.Xu H, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/beta-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019;10:381. doi: 10.1186/s13287-019-1446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Xin H, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Knoller N, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J. Neurosurg. Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 377.Yagura K, et al. The enhancement of CCL2 and CCL5 by human bone marrow-derived mesenchymal stem/stromal cells might contribute to inflammatory suppression and axonal extension after spinal cord injury. PLoS ONE. 2020;15:e0230080. doi: 10.1371/journal.pone.0230080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhong Z, et al. Bone marrow mesenchymal stem cells upregulate PI3K/AKT pathway and down-regulate NF-kappaB pathway by secreting glial cell-derived neurotrophic factors to regulate microglial polarization and alleviate deafferentation pain in rats. Neurobiol. Dis. 2020;143:104945. doi: 10.1016/j.nbd.2020.104945. [DOI] [PubMed] [Google Scholar]
- 379.Zhong Z, et al. Adipose-derived stem cells modulate BV2 microglial M1/M2 polarization by producing GDNF. Stem Cells Dev. 2020;29:714–727. doi: 10.1089/scd.2019.0235. [DOI] [PubMed] [Google Scholar]
- 380.Dong B, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021;12:204. doi: 10.1186/s13287-021-02244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Li X, et al. Umbilical cord tissue-derived mesenchymal stem cells induce T lymphocyte apoptosis and cell cycle arrest by expression of indoleamine 2, 3-dioxygenase. Stem Cells Int. 2016;2016:7495135. doi: 10.1155/2016/7495135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wang, A. Y. L. et al. Human Wharton’s jelly mesenchymal stem cell-mediated sciatic nerve recovery is associated with the upregulation of regulatory T cells. Int. J. Mol. Sci.21, 6310 (2020). [DOI] [PMC free article] [PubMed]
- 383.Noone C, Kihm A, English K, O’Dea S, Mahon BP. IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013;22:3003–3014. doi: 10.1089/scd.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Li X, et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022;13:18. doi: 10.1186/s13287-021-02690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shang Y, Guan H, Zhou F. Biological characteristics of umbilical cord mesenchymal stem cells and its therapeutic potential for hematological disorders. Front. Cell Dev. Biol. 2021;9:570179. doi: 10.3389/fcell.2021.570179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Mennan C, et al. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed. Res. Int. 2013;2013:916136. doi: 10.1155/2013/916136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.D’Addio F, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J. Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014;5:53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.de Witte SFH, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 390.Li Y, et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol. Immunol. 2019;16:908–920. doi: 10.1038/s41423-019-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.De Paepe ME, Wong T, Chu S, Mao Q. Stromal cell-derived factor-1 (SDF-1) expression in very preterm human lungs: potential relevance for stem cell therapy for bronchopulmonary dysplasia. Exp. Lung Res. 2020;46:146–156. doi: 10.1080/01902148.2020.1751899. [DOI] [PubMed] [Google Scholar]
- 392.Wynn RF, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 393.Ryu CH, et al. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys. Res. Commun. 2010;398:105–110. doi: 10.1016/j.bbrc.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 394.Yang C, et al. The biological changes of umbilical cord mesenchymal stem cells in inflammatory environment induced by different cytokines. Mol. Cell Biochem. 2018;446:171–184. doi: 10.1007/s11010-018-3284-1. [DOI] [PubMed] [Google Scholar]
- 395.Seedorf G, et al. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L1098–L1110. doi: 10.1152/ajplung.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Chen XY, et al. Therapeutic potential of human umbilical cord-derived mesenchymal stem cells in recovering from murine pulmonary emphysema under cigarette smoke exposure. Front. Med. 2021;8:713824. doi: 10.3389/fmed.2021.713824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Katsha AM, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol. Ther. 2011;19:196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Kyurkchiev D, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ren Z, et al. Human umbilical-cord mesenchymal stem cells inhibit bacterial growth and alleviate antibiotic resistance in neonatal imipenem-resistant Pseudomonas aeruginosa infection. Innate Immun. 2020;26:215–221. doi: 10.1177/1753425919883932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Liu J, et al. Type 2 alveolar epithelial cells differentiated from human umbilical cord mesenchymal stem cells alleviate mouse pulmonary fibrosis through beta-catenin-regulated cell apoptosis. Stem Cells Dev. 2021;30:660–670. doi: 10.1089/scd.2020.0208. [DOI] [PubMed] [Google Scholar]
- 401.Moodley Y, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Li DY, Li RF, Sun DX, Pu DD, Zhang YH. Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies. Stem Cell Res. Ther. 2021;12:461. doi: 10.1186/s13287-021-02496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Lam G, Zhou Y, Wang JX, Tsui YP. Targeting mesenchymal stem cell therapy for severe pneumonia patients. World J. Stem Cells. 2021;13:139–154. doi: 10.4252/wjsc.v13.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Chen K, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin. Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 405.Ren G, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 406.Loy H, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J. Infect. Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gazdhar A, et al. Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats. Hum. Gene Ther. 2013;24:105–116. doi: 10.1089/hum.2012.098. [DOI] [PubMed] [Google Scholar]
- 408.Wang W, et al. Therapeutic mechanisms of mesenchymal stem cells in acute respiratory distress syndrome reveal potentials for Covid-19 treatment. J. Transl. Med. 2021;19:198. doi: 10.1186/s12967-021-02862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Chu KA, et al. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics. 2019;9:6646–6664. doi: 10.7150/thno.33741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Chen QH, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res. Ther. 2020;11:91. doi: 10.1186/s13287-020-01612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Li L, et al. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology. 2013;92:257–264. doi: 10.1159/000354883. [DOI] [PubMed] [Google Scholar]
- 412.Zheng L, Wang S, Yang H, Lyu X. [Research progress of mesenchymal stem cells attenuating acute respiratory distress syndrome by regulating the balance of M1/M2 macrophage polarization] Zhonghua Wei Zhong Bing. Ji Jiu Yi Xue. 2021;33:509–512. doi: 10.3760/cma.j.cn121430-20200426-00334. [DOI] [PubMed] [Google Scholar]
- 413.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharm. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 414.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 415.Kurylowicz, A. & Kozniewski, K. Anti-inflammatory strategies targeting metaflammation in type 2 diabetes. Molecules25, 2224 (2020). [DOI] [PMC free article] [PubMed]
- 416.Liu J, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol. Cells. 2014;37:865–872. doi: 10.14348/molcells.2014.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Jalalie L, et al. Distribution of the CM-Dil-labeled human umbilical cord vein mesenchymal stem cells migrated to the cyclophosphamide-injured ovaries in C57BL/6 mice. Iran. Biomed. J. 2019;23:200–208. doi: 10.29252/ibj.23.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Takehara Y, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab. Investig. 2013;93:181–193. doi: 10.1038/labinvest.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 2018;39:1–20. doi: 10.1210/er.2017-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- 421.Trzyna, A. & Banas-Zabczyk, A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy”. Biomolecules11, 878 (2021). [DOI] [PMC free article] [PubMed]
- 422.Ding C, et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res. Ther. 2018;9:55. doi: 10.1186/s13287-018-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kedenko L, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med. Genet. 2014;15:112. doi: 10.1186/s12881-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Shojafar E, Soleimani Mehranjani M, Shariatzadeh SMA. Adipose derived mesenchymal stem cells improve the structure and function of autografted mice ovaries through reducing oxidative stress and inflammation: a stereological and biochemical analysis. Tissue Cell. 2019;56:23–30. doi: 10.1016/j.tice.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 425.Liu M, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2020;11:3. doi: 10.1186/s13287-019-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Li Z, Zhang M, Tian Y, Li Q, Huang X. Mesenchymal stem cells in premature ovarian insufficiency: mechanisms and prospects. Front. Cell Dev. Biol. 2021;9:718192. doi: 10.3389/fcell.2021.718192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Forghani A, et al. Differentiation of adipose tissue-derived CD34+/CD31- cells into endothelial cells in vitro. Regen. Eng. Transl. Med. 2020;6:101–110. doi: 10.1007/s40883-019-00093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Baer PC. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 2011;20:1805–1816. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- 429.Wang C, et al. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng. Part A. 2010;16:1201–1213. doi: 10.1089/ten.tea.2009.0303. [DOI] [PubMed] [Google Scholar]
- 430.Chen L, et al. Adipose-derived stem cells promote diabetic wound healing via the recruitment and differentiation of endothelial progenitor cells into endothelial cells mediated by the VEGF-PLCgamma-ERK pathway. Arch. Biochem Biophys. 2020;692:108531. doi: 10.1016/j.abb.2020.108531. [DOI] [PubMed] [Google Scholar]
- 431.Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019;21:18–24. doi: 10.1038/s41556-018-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab. Investig. 2021;101:1254–1266. doi: 10.1038/s41374-021-00611-8. [DOI] [PubMed] [Google Scholar]
- 433.Fujiwara O, et al. Adipose-derived stem cells improve grafted burn wound healing by promoting wound bed blood flow. Burns Trauma. 2020;8:tkaa009. doi: 10.1093/burnst/tkaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Chen T, et al. Efficient and sustained IGF-1 expression in the adipose tissue-derived stem cells mediated via a lentiviral vector. J. Mol. Histol. 2015;46:1–11. doi: 10.1007/s10735-014-9599-7. [DOI] [PubMed] [Google Scholar]
- 435.Zhou K, et al. Immunosuppression of human adipose-derived stem cells on T cell subsets via the reduction of NF-kappaB activation mediated by PD-L1/PD-1 and Gal-9/TIM-3 pathways. Stem Cells Dev. 2018;27:1191–1202. doi: 10.1089/scd.2018.0033. [DOI] [PubMed] [Google Scholar]
- 436.Moll G, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol. Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 437.He X, et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct. Target Ther. 2021;6:270. doi: 10.1038/s41392-021-00688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Lukomska B, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Li C, Zhao H, Wang B. Challenges for mesenchymal stem cell-based therapy for COVID-19. Drug Des. Devel Ther. 2020;14:3995–4001. doi: 10.2147/DDDT.S269407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Nguyen Thanh, L. et al. Outcomes of bone marrow mononuclear cell transplantation combined with interventional education for autism spectrum disorder. Stem Cells Transl. Med.10, 14–26 (2020). [DOI] [PMC free article] [PubMed]
- 441.Nguyen Thanh L, et al. Can autologous adipose-derived mesenchymal stem cell transplantation improve sexual function in people with sexual functional deficiency? Stem Cell Rev. Rep. 2021;17:2153–2163. doi: 10.1007/s12015-021-10196-w. [DOI] [PubMed] [Google Scholar]
- 442.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.de Windt TS, Vonk LA, Saris DBF. Response to: Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 2017;6:1747–1748. doi: 10.1002/sctm.17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Boregowda SV, Booker CN, Phinney DG. Mesenchymal stem cells: the moniker fits the science. Stem Cells. 2018;36:7–10. doi: 10.1002/stem.2713. [DOI] [PubMed] [Google Scholar]
- 445.Masterson C, O’Toole D. The mesenchymal stromal cell magic bullet finds yet another target. Stem Cell Res. Ther. 2014;5:82. doi: 10.1186/scrt471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Murray IR, et al. Rogue stem cell clinics. Bone Jt. J. 2020;102-B:148–154. doi: 10.1302/0301-620X.102B2.BJJ-2019-1104.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Lyons S, Salgaonkar S, Flaherty GT. International stem cell tourism: a critical literature review and evidence-based recommendations. Int. Health. 2022;14:132–141. doi: 10.1093/inthealth/ihab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.He C, et al. Mesenchymal stem cell-based treatment in autoimmune liver diseases: underlying roles, advantages and challenges. Ther. Adv. Chronic Dis. 2021;12:2040622321993442. doi: 10.1177/2040622321993442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Bertheuil N, et al. Adipose mesenchymal stromal cells: definition, immunomodulatory properties, mechanical isolation and interest for plastic surgery. Ann. Chir. Plast. Esthet. 2019;64:1–10. doi: 10.1016/j.anplas.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 450.Chen Y, Yu Q, Hu Y, Shi Y. Current research and use of mesenchymal stem cells in the therapy of autoimmune diseases. Curr. Stem Cell Res. Ther. 2019;14:579–582. doi: 10.2174/1574888X14666190429141421. [DOI] [PubMed] [Google Scholar]
- 451.Han Y, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target Ther. 2022;7:92. doi: 10.1038/s41392-022-00932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Rahmani A, et al. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res. Rev. 2020;62:101106. doi: 10.1016/j.arr.2020.101106. [DOI] [PubMed] [Google Scholar]
- 453.Zhou W, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am. J. Sports Med. 2019;47:1722–1733. doi: 10.1177/0363546519848678. [DOI] [PubMed] [Google Scholar]
- 454.Pachler K, et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19:458–472. doi: 10.1016/j.jcyt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 455.Borger V, Staubach S, Dittrich R, Stambouli O, Giebel B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr. Protoc. Stem Cell Biol. 2020;55:e128. doi: 10.1002/cpsc.128. [DOI] [PubMed] [Google Scholar]
- 456.Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J. Transl. Med. 2020;18:449. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Monticelli S, Natoli G. Short-term memory of danger signals and environmental stimuli in immune cells. Nat. Immunol. 2013;14:777–784. doi: 10.1038/ni.2636. [DOI] [PubMed] [Google Scholar]
- 458.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 459.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 460.Liu GY, et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol. Immunol. 2016;13:369–378. doi: 10.1038/cmi.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Diez-Tejedor E, et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014;23:2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 462.Laskowitz DT, et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl. Med. 2018;7:521–529. doi: 10.1002/sctm.18-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Jeon SR, et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng. Regenerative Med. 2010;7:316–322. [Google Scholar]
- 464.Park JH, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238–1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 465.Saito F, et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor. Neurol. Neurosci. 2012;30:127–136. doi: 10.3233/RNN-2011-0629. [DOI] [PubMed] [Google Scholar]
- 466.El-Kheir WA, et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 467.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012;114:935–939. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 468.Pal R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 469.Mendonca MV, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Vaquero J, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20:806–819. doi: 10.1016/j.jcyt.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 471.Dai G, et al. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 472.Jiang PC, et al. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 2013;6:140–146. doi: 10.3892/etm.2013.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Jarocha D, Milczarek O, Wedrychowicz A, Kwiatkowski S, Majka M. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transpl. 2015;24:661–672. doi: 10.3727/096368915X687796. [DOI] [PubMed] [Google Scholar]
- 474.Huang L, et al. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transpl. 2018;27:325–334. doi: 10.1177/0963689717729379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Karussis D, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Yamout B, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 477.Mohajeri M, Farazmand A, Mohyeddin Bonab M, Nikbin B, Minagar A. FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran. J. Allergy Asthma Immunol. 2011;10:155–161. [PubMed] [Google Scholar]
- 478.Odinak MM, et al. [Transplantation of mesenchymal stem cells in multiple sclerosis] Zh . Nevrol. Psikhiatr Im. S S Korsakova. 2011;111:72–76. [PubMed] [Google Scholar]
- 479.Bonab MM, et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr. Stem Cell Res Ther. 2012;7:407–414. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- 480.Mohyeddin Bonab M, et al. Evaluation of cytokines in multiple sclerosis patients treated with mesenchymal stem cells. Arch. Med Res. 2013;44:266–272. doi: 10.1016/j.arcmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 481.Llufriu S, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE. 2014;9:e113936. doi: 10.1371/journal.pone.0113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18:1476–1482. doi: 10.1016/j.jcyt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 483.Dahbour S, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017;23:866–874. doi: 10.1111/cns.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Meng M, et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 2018;10:212–223. [PMC free article] [PubMed] [Google Scholar]
- 485.Fernandez O, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: a triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. 2018;13:e0195891. doi: 10.1371/journal.pone.0195891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Alvarez-Fuente M, et al. Off-label mesenchymal stromal cell treatment in two infants with severe bronchopulmonary dysplasia: clinical course and biomarkers profile. Cytotherapy. 2018;20:1337–1344. doi: 10.1016/j.jcyt.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 487.Averyanov A, et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 2020;9:6–16. doi: 10.1002/sctm.19-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Edessy M, et al. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Med. Int. 2016;3:19–23. doi: 10.5530/ami.2016.1.7. [DOI] [Google Scholar]
- 489.Gabr, H., Elkheir, W. & El-Gazzar, A. Autologous stem cell transplantation in patients with idiopathic premature ovarian failure. J. Tissue Sci. Eng.7, 27 (2016).
- 490.Bakhtiary M, et al. Comparison of transplantation of bone marrow stromal cells (BMSC) and stem cell mobilization by granulocyte colony stimulating factor after traumatic brain injury in rat. Iran. Biomed. J. 2010;14:142–149. [PMC free article] [PubMed] [Google Scholar]
- 491.Zhou Z, et al. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15:434–448. doi: 10.1016/j.jcyt.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 492.Yousefifard M, et al. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res. Ther. 2016;7:36. doi: 10.1186/s13287-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Takahashi A, et al. Comparison of mesenchymal stromal cells isolated from murine adipose tissue and bone marrow in the treatment of spinal cord injury. Cell Transpl. 2018;27:1126–1139. doi: 10.1177/0963689718780309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Hao T, et al. Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp. Ther. Med. 2017;14:5956–5964. doi: 10.3892/etm.2017.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zare H, Jamshidi S, Dehghan MM, Saheli M, Piryaei A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J. Cell Biochem. 2018;119:5834–5842. doi: 10.1002/jcb.26772. [DOI] [PubMed] [Google Scholar]
- 496.Arminan A, et al. Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J. Am. Coll. Cardiol. 2010;55:2244–2253. doi: 10.1016/j.jacc.2009.08.092. [DOI] [PubMed] [Google Scholar]
- 497.Gaebel R, et al. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS ONE. 2011;6:e15652. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Dayan V, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 499.Lopez Y, et al. Wharton’s jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: a preliminary report. Curr. Stem Cell Res. Ther. 2013;8:46–59. doi: 10.2174/1574888X11308010007. [DOI] [PubMed] [Google Scholar]
- 500.Rasmussen JG, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transpl. 2014;23:195–206. doi: 10.3727/096368912X659871. [DOI] [PubMed] [Google Scholar]
- 501.Abd Emami B, et al. Mechanical and chemical predifferentiation of mesenchymal stem cells into cardiomyocytes and their effectiveness on acute myocardial infarction. Artif. Organs. 2018;42:E114–E126. doi: 10.1111/aor.13091. [DOI] [PubMed] [Google Scholar]
- 502.Omar AM, Meleis AE, Arfa SA, Zahran NM, Mehanna RA. Comparative study of the therapeutic potential of mesenchymal stem cells derived from adipose tissue and bone marrow on acute myocardial infarction model. Oman Med. J. 2019;34:534–543. doi: 10.5001/omj.2019.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this published article.