Summary
Background
Previous reviews reported that the effects of CoQ10 on glycemic control were inconsistent. There is no review exploring the optimal intake of CoQ10 for glycemic control. We aimed to investigate the efficacy of CoQ10 on glycemic control and evaluate the dose–response relationship via integrating the existing evidence from randomized control trials (RCTs).
Methods
Databases (PubMed, Embase, and Cochrane Library) were searched to identify RCTs for investigating the efficacy of CoQ10 on fasting glucose, fasting insulin, HbA1c, and HOMA-IR up to March 12, 2022. We performed a meta-analysis on 40 RCTs of CoQ10. Weighted mean difference (WMD) and 95% confidence intervals (CIs) were calculated for net changes. Evidence certainty was assessed using GRADE. Dose-response relationships were evaluated using 1-stage restricted cubic spline regression model. The protocol was registered in PROSPERO (CRD42021252933).
Findings
Forty studies (n = 2,424 participants) were included in this meta-analysis. CoQ10 significantly reduced fasting glucose (WMD: -5.22 [95% CI: -8.33, -2.11] mg/dl; P <0.001; I2=95.10%), fasting insulin (-1.32 [-2.06, -0.58] μIU/ml; P < 0.001; I2=78.86%), HbA1c (-0.12% [-0.23, -0.01]; P =0.04; I2=49.10%), and HOMA-IR (-0.69 [-1.00, -0.38]; P <0.001; I2=88.80%). The effect of CoQ10 on outcomes was greater in diabetes with lower heterogeneity. A “U” shape dose-response relationship curve revealed that 100-200 mg/day of CoQ10 largely decreased fasting glucose (χ2 = 12.08, Pnonlinearity =0.002), fasting insulin (χ2 = 9.73, Pnonlinearity =0.008), HbA1c (χ2 = 6.00, Pnonlinearity =0.049), HOMA-IR (χ2 = 25.89, Pnonlinearity <0.001).
Interpretation
CoQ10 supplementation has beneficial effects on glycemic control, especially in diabetes, and 100-200 mg/day of CoQ10 could achieve the greatest benefit, which could provide a basis for the dietary guidelines of CoQ10 in patients with glycemic disorders.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82030098, 81872617 and 81730090), Shenzhen Science, Technology, and Innovation Commission (No. JCYJ20180307153228190), CNS Research Fund for DRI, and National innovation and entrepreneurship training program for undergraduate student (No. 202210558161).
Keywords: Coenzyme Q10, Dose-response, GRADE, Glycemic control, Meta-analysis
Research in context.
Evidence before this study
We searched PubMed for manuscripts published in English from inception and until November 30, 2021 with “coenzyme Q10” in combination with “glycemic control”, “dose-response effects” and “meta-analysis”. We found zero meta-analysis assessing the dose-response effects of CoQ10 supplementation for glycemic control in adults. Previous meta-analysis of the effects of CoQ10 in diabetes have reported inconsistent results. Only one meta-analysis reported that low dose of CoQ10 could reduce fasting glucose and HbA1c, without mentioning the optimal supplementary dose.
Added value of this study
In this updated meta-analysis of 40 randomized controlled trials, CoQ10 supplementation has beneficial effects on glycemic control, especially in diabetes patients. Taking 100-200 mg/day of CoQ10 could achieve the greatest benefit for glycemic control.
Implications of all the available evidence
These findings add new information about the beneficial effects of CoQ10 supplementation on glycemic control, and are conducive to setting up nutrition guidelines for recommended daily intake of CoQ10 in patients with glycemic disorders.
Alt-text: Unlabelled box
Introduction
Over the past few decades, cardiovascular disease (CVD) remains the leading cause of global deaths and disability, and the number of CVD deaths steadily increased year by year.1 In 2019, about 523 million adults were suffering from CVD, about twice as many as in 1990.1 The development of CVD is mainly driven by cardiometabolic risk factors, such as glucose metabolism disorder. Patients with disorder of glucose metabolism are at high risk of developing hyperglycemia-related CVD and metabolic diseases, including diabetes, obesity, dyslipidemia.2, 3, 4 There is mounting evidence that glycemic control can effectively reduce the risk of CVD and metabolic disease.5,6 In this point, early intervention in the management of glycemic level is an important target to reduce the risk of CVD and other metabolic diseases.7
Coenzyme Q10 (CoQ10), also known as ubiquinone, is a lipid-soluble benzoquinone similar to vitamins. CoQ10 has a wide distribution in plant and animal tissues, especially in meat, fish, nuts, and some oils. Under normal physiological conditions, endogenous synthesis was thought to be the main source. However, with the growth of age, the synthetic ability of CoQ10 decreased, which could not meet the needs of healthy adults.8 In addition, average dietary intake of CoQ10 was only 3-6 mg per day, which was far less than the demand for CoQ10. Supplementation of CoQ10 could increase the level of CoQ10 in vivo to some extent. CoQ10, as a nutritional supplement, has a wide range of biological effects, including antioxidation, maintenance of normal blood pressure and cholesterol concentrations, maintenance of normal cognitive function, and improving insulin resistance.9,10 Clinical trials of the effects of supplementary CoQ10 on glycemic control have reported inconsistent results. Previous meta-analysis of randomized control trials (RCTs) have focused on the effect of CoQ10 on glycemic control for specific populations, such as type 2 diabetes11,12 and diabetic kidney disease.13 However, these populations did not focus on other hyperglycemia-related diseases. While the prior meta-analysis included many relevant studies, the newly published studies in recent years still need to be updated. In addition, the current reviews are lack of an analysis of the optimal intake dose of CoQ10 supplement, and there is not enough evidence to set up nutrition guidelines for recommended daily intake of CoQ10. Therefore, further evaluation of the evidence quality is also needed to ascertain the efficacy of CoQ10 in glycemic control.
The aim of this meta-analysis is to 1) investigate the efficacy of CoQ10 supplementation in improving glycemic control in adult with hyperglycemia-related diseases, 2) update the latest published studies, 3) assess evidence certainty according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methods, and 4) conducted dose–response meta-analysis using a 1-stage restricted cubic spline regression model, so as to provide a basis for nutrition guidelines of recommended CoQ10 intake in patients with hyperglycemia-related diseases.
Methods
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement14 and have been prospectively registered at the International Prospective Register of Systematic Reviews (PROSPERO) with the identifier CRD42021252933.
Search strategy
Two investigators (YL and DZ) independently searched the PubMed, Embase, and Cochrane Library for RCTs concerning the effects of CoQ10 supplementation on outcomes of glycemic control, including fasting glucose, HbA1c, fasting insulin, and HOMA-IR up to March 12, 2022. In addition, we also searched relevant review and meta-analysis articles for eligible studies. Specific search strategies are presented in Supplemental Table S1.
Study inclusion and exclusion
Only studies that satisfied the following inclusion criteria were included: 1) the participants were over 18 years old; 2) the studies used CoQ10 as the intervention approach with duration more than 2 weeks; 3) the trials used placebo or other suitable controls; 4) baseline and follow-up mean for fasting glucose, fasting insulin, HbA1c, or HOMA-IR were reported (or could be calculated); 5) the studies were randomized controlled trials of either parallel or crossover design, with no limits on the language of publication. Studies were excluded if it: 1) was an acute feeding trial; 2) recruited pregnant or lactating women; 3) had a multifactorial study design so that the effects of CoQ10 cannot be isolated; 4) did not provide adequate data to estimate the effect sizes of CoQ10.
The selection of the studies was performed independently by the investigators (YL and DZ), screening on the titles and abstracts. For studies that could not be determined, full texts were evaluated. Any discrepancies during selection of studies were resolved by a third reviewer (ZT).
Data extraction
Two investigators (YL and QJ) independently extracted the following items from each eligible study: first author's name, year of publication, country where the study was performed, source of funding, study design and duration, sample size, intervention approach, dose of CoQ10, control approach, subject characteristics, and changes in the glycemic control outcomes aforementioned.
Mean changes and standard deviations (SDs) from baseline to the end of follow-up in both intervention and control groups were used to estimate the effect size of CoQ10. If the mean changes were not provided directly, the effect values before and after the intervention were extracted and converted into the mean changes and SDs as follows:
assuming a correlation coefficient (R) = 0.5. When standard errors of the mean (SEMs) rather than SDs were reported, the SDs were estimated using the following formula:
where n was the number of subjects. When the outcome measures were reported as means and 95% confidence intervals (CIs), the SDs were estimated using the following formula:
where n was the number of subjects. When studies were reported with median and range, we estimated SD as
provided by Cochrane Handbook recommendation.15 For multi-armed parallel trials, we treated each intervention group and corresponding control as an independent comparison. Crossover trials were regarded similarly to parallel trials, with separate CoQ10 and control arms.
For data extraction, two investigators (YL and QJ) processed articles independently. Inconsistency in particular information between researchers was resolved by discussion or negotiation with a third researcher (ZT).
Quality assessment
The Cochrane risk-of-bias tool (Review manager version 5.4)16 was used to evaluate the quality assessment of included studies, including domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Other biases, including study design rationality and compliance with treatment, were also assessed. We rated studies that satisfies four or more of seven low-risk domains of bias as low risk with the rest as high risk. Two investigators (YL and ML) evaluated the risk of biases independently, with any discrepancies adjudicated by a third researcher (DZ).
Statistical analysis
Stata software (version 16.0) was used to calculate pooled effect size estimates, which were expressed as weighed mean difference (WMD) with 95% CIs. Inter-study heterogeneity was assessed using Cochran Q test and I2 statistics. Given that we did not work with just one population, pooled estimates and 95% CIs of effect sizes were calculated using random-effects modeling with DerSimonian-Laird methods.17 Forest plots were generated to visually evaluate the pooled effect size estimates. P-values < 0.05 were considered statistically significant for all statistical test used.
To explore potential sources of confounding and heterogeneity, we conducted subgroup analysis on outcome measures. We prespecified subgroup stratified by study design (parallel vs. crossover), study duration (shorter term <12 weeks vs. longer term ≥12 weeks), the dosage of CoQ10 (lower dose < 200 mg/day, ≥200 mg/day and < 300 mg/day, higher dose ≥ 300 mg/day), corporate sponsorship, overall study risk of bias (high risk vs. low risk), and diseases. We evaluated the robustness of pooled estimates via leave-one-out sensitivity analysis.
We performed a random-effects dose–response meta-analysis assessing the relationship between CoQ10 and fasting glucose, fasting insulin, HbA1c, and HOMA-IR respectively using the 1-stage restricted cubic spline regression model with Rstudio (version 1.4) based on the dosresmeta package.18, 19, 20
The GRADE method was used to assess the level of evidence for major outcome indicators on the basis of risk of bias, inconsistency, indirectness, imprecision, and publication bias with GRADEpro GDT software (version 3.6).21 Quality was appraised as ‘‘very low,’’ ‘‘low,’’ ‘‘moderate,’’ or ‘‘high’’ based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. Finally, we assessed the publication bias using funnel plots as well as the Egger's tests. The trim and fill methods were used to adjust theoretically missing studies and correct for funnel plot asymmetry, if any.
Role of the funding source
The funder of the study had no role in accessing the raw data, study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
Overall, a total of 3697 studies were retrieved from a search of databases, and after title and abstract screening, 2701 were excluded. Evaluation of 81 full-text reports resulted in identification of 40 randomized controlled trials articles22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 that could be included in the analysis. Reasons for exclusion included duration <2 weeks (n = 16), inappropriate placebo in control group (n = 10), intervention included CoQ10 and other factors (n = 12), and article cannot be obtained (n = 3). The PRISMA literature search flow diagram is presented in Figure 1.
Figure 1.
Flow diagram of studies search for trials, published through March 12, 2022.
The 40 studies, which were published between 1994 and 2020, included 2,424 participants (Table 1). The age of participants ranged 19 from 70 years. Included trials conducted in Europe (n = 8), Oceania (n = 5), North America (n = 2), Asia (n = 27). All but one study was crossover trial(33). Among the parallel studies, thirty-five trials were single-armed while the other five were multi-armed parallel trials. The number of participants were fanged from 23 to 101. The studies included subjects with CVD (n = 3), diabetes (n = 25), dyslipidemia (n = 6), obesity (n = 2), non-alcoholic fatty liver disease (n = 2), polycystic ovary syndrome (n = 2), and others (n = 4). Across trials, the dose intake of CoQ10 ranged from 100 to 900 mg, and the treatment and follow-up duration ranged from 4 weeks to 6 months.
Table 1.
Study characteristics of the 40 trials included in the analysis.
| Study/Country | Study design | Sample size (Intervention/Control) | Gender Male/female | Intervention |
CoQ10 form | Mean age (years) | Duration | Population | Received industry funding | |
|---|---|---|---|---|---|---|---|---|---|---|
| CoQ10 intake, mg/day | Control | |||||||||
| Akbari Fakhrabadi et al. 201439/Iran | Parallel | 62(32/30) | QG: 10/22 PG: 6/24 |
200 | placebo | Ubiquinone | QG: 56.7 ± 6.4 PG: 54.8 ± 6.7 |
12w | type 2 diabetes | no |
| Andersen et al. 199723/Danmark | Parallel | 34(17/17) | QG: 10/7 PG: 9/8 |
100 | placebo | Ubiquinone | QG: 33.5 ± 2.0 PG: 35.3 ± 2.4 |
12w | insulin dependent diabetes mellitus | yes |
| Bargossi et al. 199422/Italy | Parallel | 30(15/15) | QG: 10/5 PG: 11/4 |
100 | simvastatin | ubiquinone | QG: 53.7 ± 10.1 PG: 52.8 ± 10.8 |
3m | primary hyper-cholesterol | no |
| Chew et al. 2008 (Ⅰ)31/Australia | Parallel | 36(16/20) | QG: 13/3 PG: 14/6 |
200 | placebo | Ubiquinone | QG: 61.3 ± 4.1 PG: 62.4 ± 8.8 |
6m | type 2 diabetes | yes |
| Chew et al. 2008 (Ⅱ)31/Australia | Parallel | 38(19/19) | QG: 13/6 PG: 13/6 |
200 | fenofibrate | Ubiquinone | QG: 63.0 ± 9.4 PG: 64.8 ± 7.3 |
6m | type 2 diabetes | yes |
| Dai et al. 201135/China | Parallel | 56(28/28) | QG: 27/1 PG: 25/3 |
300 | placebo | Ubiquinone | QG: 67.7 ± 9.4 PG: 70.1 ± 9.8 |
8w | ischemic left ventricular systolic dysfunction | yes |
| Eriksson et al. 199924/ Finland | Parallel | 23(12/11) | NA | 100 | placebo | Ubiquinone | QG: 65.0 ± 5.0 PG: 64.0 ± 7.0 |
6m | type 2 diabetes | yes |
| Fallah et al. 201851/Iran | Parallel | 60(30/30) | QG: 22/8 PG: 18/12 |
120 | placebo | Ubiquinone | QG: 59.4 ± 12.2 PG: 64.8 ± 11.5 |
12w | diabetic hemodialysis | no |
| Farhangi et al. 201440/Iran | Parallel | 41(20/21) | QG: 15/5 PG: 16/5 |
100 | placebo | Ubiquinone | QG: 42.7 ± 10.8 PG: 42.2 ± 10.8 |
4w | Non-alcoholic fatty liver disease | no |
| Gholami et al. 201852/Iran | Parallel | 68(34/34) | QG: 0/34 PG: 0/34 |
100 | placebo | Ubiquinone | QG: 53.1 ± 6.2 PG: 53.3 ± 6.6 |
12w | type 2 diabetes | no |
| Gholami et al. 201958/Iran | Parallel | 70(35/35) | QG: 0/35 PG: 0/35 |
100 | placebo | Ubiquinone | QG: 53.0 ± 1.0 PG: 53.7 ± 1.1 |
12w | type 2 diabetes | no |
| Gholnari et al. 201853/Iran | Parallel | 50(25/25) | QG: 8/17 PG: 8/17 |
100 | placebo | Ubiquinone | QG: 61.1 ± 11.3 PG: 61.6 ± 10.0 |
12w | diabetic nephropathy | no |
| Hamilton et al. 200933/Australia | crossover | 46(23/23) | NA | 200 | placebo | Ubiquinone | 68.0 ± 6.0 | 12w | type 2 diabetes | yes |
| Henriksen et al. 199925/Danmark | Parallel | 34(17/17) | QG: 10/7 PG: 9/8 |
100 | placebo | Ubiquinone | QG: 35.5 ± 8.2 PG: 35.3 ± 10.0 |
3m | type 1 diabetes | yes |
| Hernandez-Ojeda et al. 201237/Mexico | Parallel | 49(24/25) | QG: 5/19 PG: 6/19 |
400 | placebo | Ubiquinone | QG: 55.3 ± 8.4 PG: 57.0 ± 8.9 |
12w | diabetic polyneuropathy | yes |
| Ho et al. 202061/China | Parallel | 29(15/14) | QG: 8/7 PG: 12/2 |
300 | placebo | Ubiquinone | QG: 19.9 ± 1.3 PG: 19.6 ± 1.3 |
12w | healthy | no |
| Hodgson et al. 2002 (Ⅰ)27/Australia | Parallel | 37(19/18) | QG: 14/5 PG: 14/4 |
200 | Fenofibrate | Ubiquinone | QG: 51.7 ± 7.0 PG: 53.6 ± 10.2 |
12w | type 2 diabetes and dyslipidemia | no |
| Hodgson et al. 2002 (Ⅱ)27/Australia | Parallel | 37(19/18) | QG: 17/2 PG: 13/5 |
200 | placebo | Ubiquinone | QG: 52.3 ± 6.1 PG: 55.2 ± 9.8 |
12w | type 2 diabetes and dyslipidemia | no |
| Hosseinzadeh-Attar et al. 201544/Iran | Parallel | 64(31/33) | QG: 19/12 PG: 18/15 |
200 | placebo | Ubiquinone | QG: 45.2 ± 7.6 PG: 47.1 ± 8.3 |
12w | type 2 diabetes | no |
| Ikematsu et al. 200629/Japan | Parallel | 85(PG:20 QG1:21 QG2:22 QG3:22) |
PG: 9/11 QG1: 11/10 QG2: 11/11 QG3: 22/0 |
QG1: 300 QG2: 600 QG3: 900 |
placebo | Ubiquinone | Male: 20.0∼60.0 Female: 24.0∼55.0 |
4w | healthy | yes |
| Izadi et al. 2019(Ⅰ)59/Iran | Parallel | 43(21/22) | QG: 0/21 PG: 0/22 |
200 | vitamin E | Ubiquinone | QG: 27.2 ± 5.8 PG: 28.3 ± 5.5 |
8w | polycystic ovary syndrome | no |
| Izadi et al. 2019(Ⅱ)59/Iran | Parallel | 43(22/21) | QG: 0/22 PG: 0/21 |
200 | placebo | Ubiquinone | QG: 27.6 ± 5.2 PG: 26.0 ± 4.5 |
8w | polycystic ovary syndrome | no |
| Kolahdouz Mohammadi et al. 201338/Iran | Parallel | 64(31/33) | QG: 19/12 PG: 18/15 |
200 | placebo | Ubiquinone | QG: 45.2 ± 7.6 PG: 47.2 ± 8.3 |
12w | type 2 diabetes | no |
| Kuhlman et al. 201960/Danmark | Parallel | 35(18/17) | QG: 14/4 PG: 8/9 |
400 | placebo | Ubiquinone | QG: 62.0 ± 1.0 PG: 64.0 ± 2.0 |
8w | patient in primary prevention with simvastatin ≥40 mg/d | yes |
| Lee et al. 201136/Korea | Parallel | 36(17/19) | QG: 11/15 PG: 10/15 |
200 | placebo | Ubiquinone | QG: 42.7 ± 11.3 PG: 42.5 ± 11.2 |
12w | obesity | no |
| Lim et al. 200832/Korea | Parallel | 80(40/40) | QG: 17/23 PG: 22/18 |
200 | placebo | Ubiquinone | QG: 54.0 ± 9.0 PG: 53.0 ± 9.0 |
12w | type 2 diabetes | yes |
| Majid Mohammadshahi et al. 201441/Iran | Parallel | 41(20/21) | NA | 100 | placebo | Ubiquinone | 19.0∼54.0 | 12w | non-alcoholic fatty liver disease | no |
| Mehrdadi et al. 201748/Iran | Parallel | 56(26/30) | QG: 17/9 PG: 15/15 |
200 | placebo | Ubiquinone | QG: 46.0 ± 7.0 PG: 48.0 ± 8.0 |
12w | type 2 diabetes | no |
| Moazen et al. 201545/Iran | Parallel | 52(26/26) | QG: 16/10 PG: 12/14 |
100 | placebo | Ubiquinone | QG: 50.7 ± 7.0 PG: 52.8 ± 7.7 |
8w | type 2 diabetes | yes |
| Mohammed-Jawad et al. 201442/Iran | Parallel | 38(19/19) | QG: 10/9 PG: 8/11 |
150 | placebo | Ubiquinone | QG: 49.4 ± 6.6 PG: 51.6 ± 8.1 |
8w | type 2 diabetes | no |
| Mori et al. 2009(Ⅰ)34/Australia | Parallel | 38(18/20) | QG: 17/1 PG: 12/8 |
200 | omega-3 PUFA | Ubiquinone | QG: 56.9 ± 16.5 PG: 53.3 ± 14.3 |
8w | chronic kidney disease | yes |
| Mori et al. 2009(Ⅱ)34/Australia | Parallel | 36(21/15) | QG: 17/4 PG: 8/7 |
200 | placebo | Ubiquinone | QG: 55.4 ± 12.4 PG: 58.6 ± 10.1 |
8w | chronic kidney disease | yes |
| Nuku et al. 200730/Japan | Parallel | 46(23/23) | QG: 12/11 PG: 11/12 |
900 | placebo | Ubiquinone | QG: 40.0 ± 13.0 PG: 38.0 ± 11.0 |
4w | healthy | no |
| Playford et al. 2003(Ⅰ)28/Australia | Parallel | 40(20/20) | QG: 14/6 PG: 14/6 |
200 | Fenofibrate | Ubiquinone | QG: 52.7 ± 8.0 PG: 53.5 ± 9.8 |
12w | type 2 diabetes and dyslipidemia | no |
| Playford et al. 2003(Ⅱ)28/Australia | Parallel | 40(20/20) | QG: 18/2 PG: 15/5 |
200 | placebo | Ubiquinone | QG: 52.7 ± 6.3 PG: 54.7 ± 9.4 |
12w | type 2 diabetes and dyslipidemia | no |
| Raygan et al. 201646/Iran | Parallel | 60(30/30) | NA | 100 | placebo | Ubiquinone | QG: 65.9 ± 12.5 PG: 59.9 ± 13.1 |
8w | obesity, type 2 diabetes and coronary heart disease | no |
| Rodriguez-Carrizalez et al. 201647/Mexico | Parallel | 40(20/20) | QG: 11/9 PG: 9/11 |
400 | Placebo | Ubiquinone | QG: 28.2 ± 3.7 PG: 29.3 ± 0.8 |
6 m | diabetic retinopathy | no |
| Samimi et al. 201749/Iran | Parallel | 60(30/30) | QG: 0/30 PG: 0/30 |
100 | placebo | Ubiquinone | QG: 24·5 ± 4·3 PG: 25·3 ± 5·7 |
12w | polycystic ovary syndrome | no |
| Singh and Niaz 199926/India | Parallel | 47(25/22) | QG: 19/6 PG: 18/4 |
120 | placebo | Ubiquinone | QG: 48.4 ± 0.5 PG: 47.6 ± 0.3 |
4w | acute myocardial infarction, unstable angina, angina pectoris | yes |
| Tóth et al. 201750/Slovakia | Parallel | 70(35/35) | QG: 17/18 PG: 18/17 |
200 | omega-3 PUFA | Ubiquinone | QG: 58.4 ± 13.8 PG: 62.0 ± 12.2 |
3m | dyslipidemia | no |
| Yen et al. 201854/China | Parallel | 47(24/23) | QG: 17/7 PG: 14/9 |
100 | placebo | Ubiquinol | QG: 61.5 ± 10.2 PG: 59.6 ± 11.7 |
12w | type 2 diabetes | yes |
| Yoo and Yum 201855/Korea | Parallel | 78(39/39) | QG: 29/10 PG: 28/11 |
200 | placebo | Ubiquinone | QG: 49.8 ± 8.4 PG: 52.4 ± 6.9 |
8w | impaired glucose tolerance | yes |
| Zahedi et al. 201443/Iran | Parallel | 40(20/20) | QG: 11/9 PG: 8/12 |
150 | placebo | Ubiquinone | QG: 53.5 ± 9.7 PG: 58.8 ± 9.6 |
12w | type 2 diabetes | no |
| Zarei et al. 201856/Iran | Parallel | 68(34/34) | QG: 0/34 PG: 0/34 |
100 | placebo | Ubiquinone | QG: 53.1 ± 6.2 PG: 53.3 ± 6.6 |
12w | type 2 diabetes | no |
| Zhang et al. 201857/China | Parallel | 101(51/50) | QG: 14/37 PG: 18/32 |
120 | placebo | Ubiquinone | QG: 51.8 ± 8.9 PG: 50.0 ± 10.9 |
24w | dyslipidemia | no |
Abbreviations: CoQ10, coenzyme Q10; QG, CoQ10 group; PG, control group; m, month; w, week; PUFA, polyunsaturated fatty acid; NA, not applicable.
Forty studies were evaluated for risk of bias in accordance with the Cochrane risk-of-bias tool (Supplemental Figure S1). Twenty-seven studies were rated as low risk in at least four of seven Cochrane risk-of-bias domains. Only 5 studies were rated as high risk in incomplete outcome data (n = 3)31,50,58 and blinding of participants and personnel (n = 2).26,42
The overall quality of evidence among the four primary outcomes ranged from very low to moderate for the RCTs in accordance with the GRADE evidence profiles (Table 2). HbA1c was found to have a moderate certainty of decreasing statistically with CoQ10 supplementation due to the degradation of indirectness. Fasting insulin was evaluated as a low evidence certainty for the downgrade of inconsistency and indirectness. Fasting glucose and HOMA-IR were found to have a very low certainty of decreasing statistically with CoQ10 supplementation because the inconsistency, indirectness and publication bias were downgraded.
Table 2.
GRADE Evidence Profile for effect of CoQ10 supplementation on glycemic control.
| Quality assessment |
No of patients |
Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CoQ10 | Placebo | Absolute (95% CI) | ||
| Fasting glucose (follow-up 4 to 24 weeks; Better indicated by lower values) | |||||||||||
| 38 | randomised trials | no serious risk of bias | seriousa | seriousb | no serious imprecision | reporting biasc | 1081 | 1076 | MD 5.22 lower (8.33 lower to 2.11 lower) | ÅOOO VERY LOW |
CRITICAL |
| Fasting insulin (follow-up 4 to 24 weeks; Better indicated by lower values) | |||||||||||
| 21 | randomised trials | no serious risk of bias | seriousa | seriousb | no serious imprecision | none | 619 | 615 | MD 1.32 lower (2.06 lower to 0.58 lower) | ÅÅOO LOW |
CRITICAL |
| HbA1c (follow-up 4 to 24 weeks; Better indicated by lower values) | |||||||||||
| 28 | randomised trials | no serious risk of bias | no serious inconsistency | seriousb | no serious imprecision | none | 752 | 753 | MD 0.12 lower (0.23 lower to 0.01 lower) | ÅÅÅO MODERATE |
CRITICAL |
| Homeostasis model assessment of insulin resistance (follow-up 4 to 24 weeks; Better indicated by lower values) | |||||||||||
| 16 | randomised trials | no serious risk of bias | seriousa | seriousb | no serious imprecision | reporting biasc | 496 | 492 | MD 0.69 lower (1 lower to 0.38 lower) | ÅOOO VERY LOW |
CRITICAL |
Significant heterogeneity in meta-analysis (I² >50%).
Surrogate outcome measure, not patient-important endpoint.
P-value of Egger's tests <0.05.
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Abbreviations: CoQ10, coenzyme Q10; CI, confidence interval.
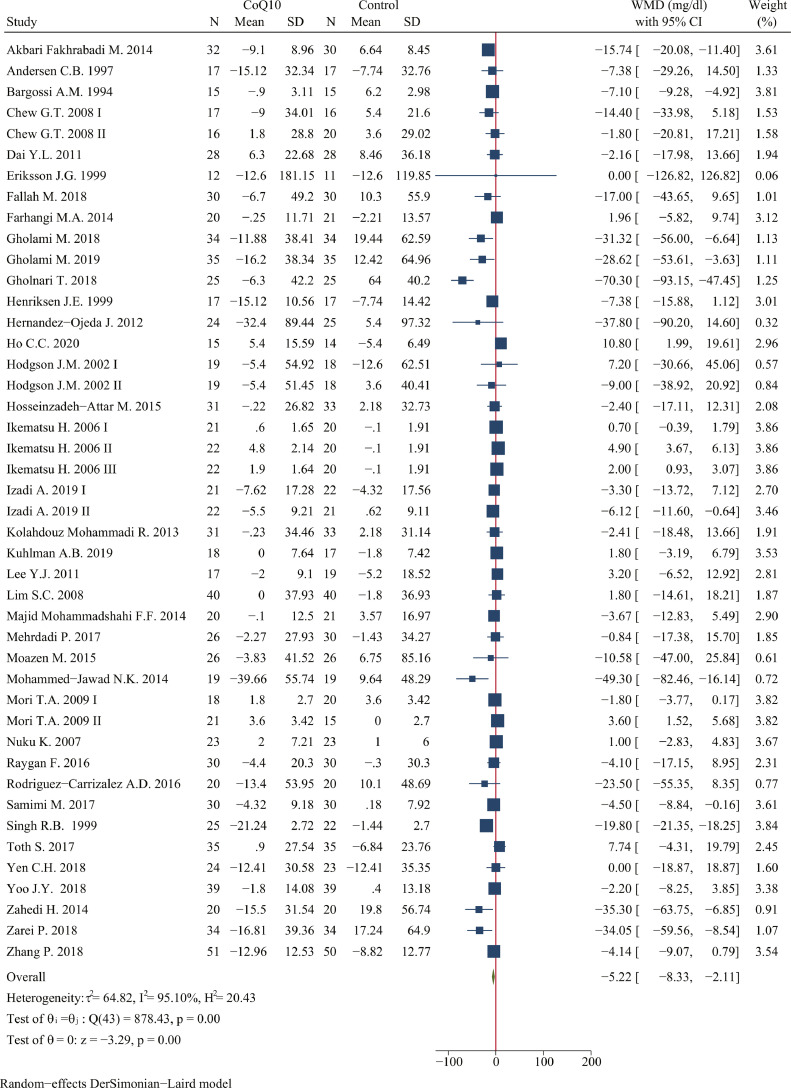
Meta-analysis of data from 44 treatment arms suggested a significant reduction in fasting glucose level following CoQ10 supplementation (WMD: -5.22 mg/dl; 95% CI: -8.33, -2.11 mg/dl; P < 0.001; n = 2157 in 38 studies; I2=95.10%) (Figure 2). Subgroup analysis of potentially modifying factors revealed that the most prominent effects on efficacy and heterogeneity were due to CoQ10 dosage, duration, the type of control, risk of bias, and industry funding (Table 3). The impact of CoQ10 on fasting glucose was greater at supplemental doses < 200 mg/day (WMD: -13.21 mg/dl, 95% CI: -18.43, -7.98 mg/dl, P < 0.001) compared with remaining two groups. With respect to treatment duration, the effect of CoQ10 on reducing fasting glucose level was better in the subsets of duration ≥ 12 weeks (WMD: -7.59 mg/dl, 95% CI: -11.66, -3.52 mg/dl, P < 0.001) compared with duration < 12 weeks. As for the type of control, the reduction on fasting glucose was greater at placebo group (WMD: -6.02 mg/dl, 95% CI: -9.56, -2.47 mg/dl, P < 0.001) rather than using other controls. CoQ10 had better efficacy in reducing fasting glucose level in the low risk of bias (WMD: -6.70 mg/dl, 95% CI: -11.28, -2.13 mg/dl, P < 0.001) and not receiving industry funding (WMD: -6.84 mg/dl, 95% CI: -10.70, -2.98 mg/dl, P < 0.001).
Figure 2.
Forest plots of effect of coenzyme Q10 supplementation on fasting glucose. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis; The horizontal line across each blue box reflects the 95% confidence intervals of the study. Abbreviations: CoQ10, coenzyme Q10; WMD, weighted mean difference; CI, confidence interval; SD, standard error.
Table 3.
Subgroup analysis of included randomized controlled trials for the effect of CoQ10 supplementation on fasting glucose.
| Group | No. of trials (participates) | WMD (95% CI) , mg/dl | Pdifferencea | I2, % | Pheterogeneityb | Pc for between subgroup heterogeneity |
|---|---|---|---|---|---|---|
| Overall | 44 (2157) | −5.22 (−8.33, −2.11) | <0.001 | 95.10 | <0.001 | |
| Study design | ||||||
| Parallel | 44 (2157) | −5.22 (−8.33, −2.11) | <0.001 | 95.10 | <0.001 | |
| Duration (weeks) | ||||||
| <12 | 16 (738) | −2.41 (−6.87, 2.06) | 0.29 | 97.93 | <0.001 | 0.09 |
| ≥12 | 28 (1419) | −7.59 (−11.66, −3.52) | <0.001 | 71.78 | <0.001 | |
| CoQ10 dosage | ||||||
| <200 mg/day | 20 (1026) | −13.21 (−18.43, −7.98) | <0.001 | 89.99 | <0.001 | <0.001 |
| ≥200 mg/day and <300 mg/day | 15 (751) | −0.71 (−3.42, 1.99) | 0.61 | 44.31 | 0.03 | |
| ≥300 mg/day | 9 (380) | 2.37 (0.38, 4.36) | 0.02 | 77.02 | <0.001 | |
| Control group | ||||||
| Placebo | 39 (1940) | −6.02 (−9.56, −2.47) | <0.001 | 75.39 | <0.001 | 0.30 |
| Other | 5 (217) | −2.97 (−7.32, 1.38) | 0.18 | 95.52 | <0.001 | |
| Risk of bias | ||||||
| High | 14 (544) | −1.18 (−4.44, 2.08) | 0.47 | 88.55 | <0.001 | 0.05 |
| Low | 29 (1613) | −6.70 (−11.28, −2.13) | <0.001 | 94.30 | <0.001 | |
| Received industry funding? | ||||||
| Yes | 18 (803) | −2.70 (−7.46, 2.06) | 0.27 | 97.63 | <0.001 | 0.19 |
| No | 26 (1354) | −6.84 (−10.70, −2.98) | <0.001 | 78.62 | <0.001 |
Dersimonian-Laird random effect model was used to calculate the effect size and P-value.
Cochrane Q test was used to detect the heterogeneity between studies.
Cochrane Q test was used to detect the subgroup heterogeneity.
Abbreviations: WMD, weighted mean difference; CI, confidence interval; CoQ10, coenzyme Q10.
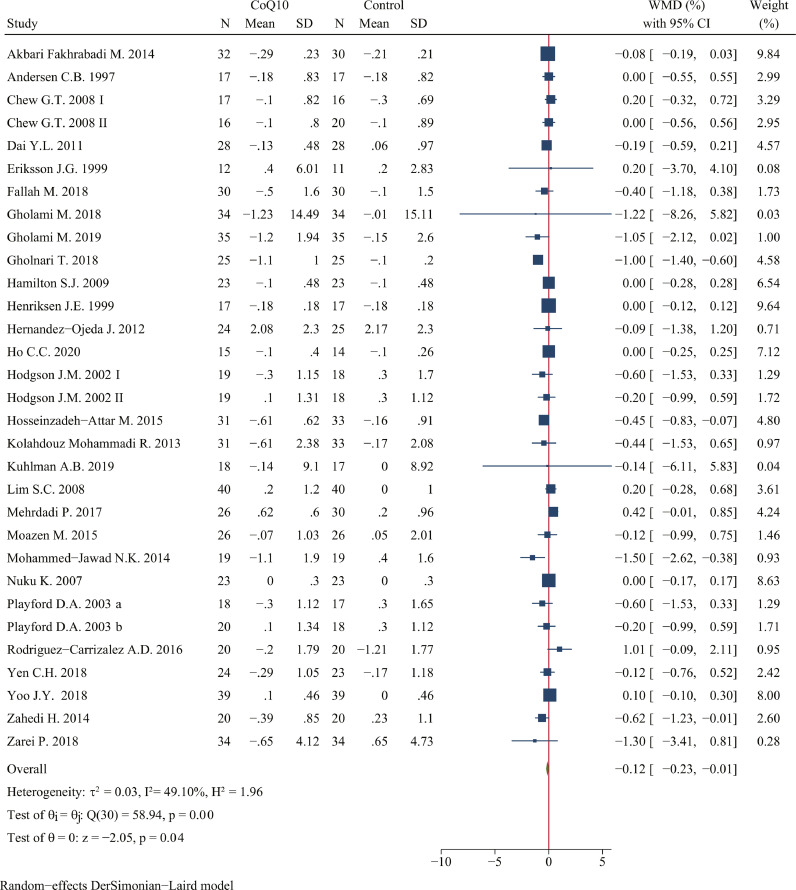
CoQ10 supplementation decreased HbA1c to a statistically significant degree (WMD: -0.12%; 95% CI: -0.23, -0.01%; P = 0.04; n = 1505 in 31 studies; I2=49.10%) (Figure 3). Subgroup analysis found that the effect of CoQ10 supplement on reducing HbA1c has a border statistical significance at duration ≥ 12 weeks (WMD: -0.14%; 95% CI: -0.27, 0.00%; P = 0.05) and placebo as control group (WMD: -0.12%; 95% CI: -0.23, 0.00%; P = 0.05). Besides, consuming less than 200 mg of CoQ10 per day (WMD: -0.47%; 95% CI: -0.83, -0.12%; P < 0.001) and not receiving industry funding (WMD: -0.28%; 95% CI: -0.48, -0.08%; P < 0.001) had a better efficacy in reducing HbA1c level (Table 4).
Figure 3.
Forest plots of effect of coenzyme Q10 supplementation on HbA1c. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis; The horizontal line across each blue box reflects the 95% confidence intervals of the study. Abbreviations: CoQ10, coenzyme Q10; WMD, weighted mean difference; CI, confidence interval; SD, standard error.
Table 4.
Subgroup analysis of included randomized controlled trials for the effect of CoQ10 supplementation on HbA1c.
| Group | No. of trials (participates) | WMD (95% CI) , % | Pdifferencea | I2, % | Pheterogeneityb | Pc for between subgroup heterogeneity |
|---|---|---|---|---|---|---|
| Overall | 31 (1505) | −0.12(−0.23, −0.01) | 0.04 | 49.10 | <0.001 | |
| Study design | ||||||
| Parallel | 30 (1459) | −0.13(−0.25, −0.01) | 0.04 | 50.64 | <0.001 | 0.40 |
| Crossover | 1 (46) | 0.00(−0.28, 0.28) | 1.00 | - | - | |
| Duration (week) | ||||||
| <12 | 6 (305) | −0.06(−0.28, 0.16) | 0.59 | 43.10 | 0.12 | 0.56 |
| ≥12 | 25 (1200) | −0.14(−0.27, 0.00) | 0.05 | 51.10 | <0.001 | |
| CoQ10 dosage | ||||||
| <200 mg/day | 12 (584) | −0.47(−0.83, −0.12) | <0.001 | 69.17 | 0.00 | 0.05 |
| ≥200 mg/day and <300 mg/day | 13 (666) | −0.03(−0.15, 0.10) | 0.70 | 27.85 | 0.16 | |
| ≥300 mg/day | 6 (255) | −0.01(−0.14, 0.12) | 0.92 | 0.00 | 0.54 | |
| Control group | ||||||
| Placebo | 29 (1434) | −0.12 (−0.23, 0.00) | 0.05 | 51.39 | <0.001 | 0.82 |
| Other | 2 (71) | −0.18 (−0.72, 0.36) | 0.52 | 14.87 | 0.29 | |
| Quality of study | ||||||
| High | 11 (397) | −0.18 (−0.41, 0.04) | 0.12 | 23.84 | 0.22 | 0.53 |
| Low | 20 (1108) | −0.10 (−0.23, 0.03) | 0.15 | 57.92 | <0.001 | |
| Received industry funding? | ||||||
| Yes | 13 (603) | 0.02(−0.07, 0.11) | 0.69 | 0.00 | 0.99 | <0.001 |
| No | 18 (902) | −0.28(−0.48, −0.08) | <0.001 | 66.30 | <0.001 |
Abbreviations: WMD, weighted mean difference; CI, confidence interval; CoQ10, coenzyme Q10.
Dersimonian–Laird random effect model was used to calculate the effect size and P-value.
Cochrane Q test was used to detect the heterogeneity between studies.
Cochrane Q test was used to detect the subgroup heterogeneity.
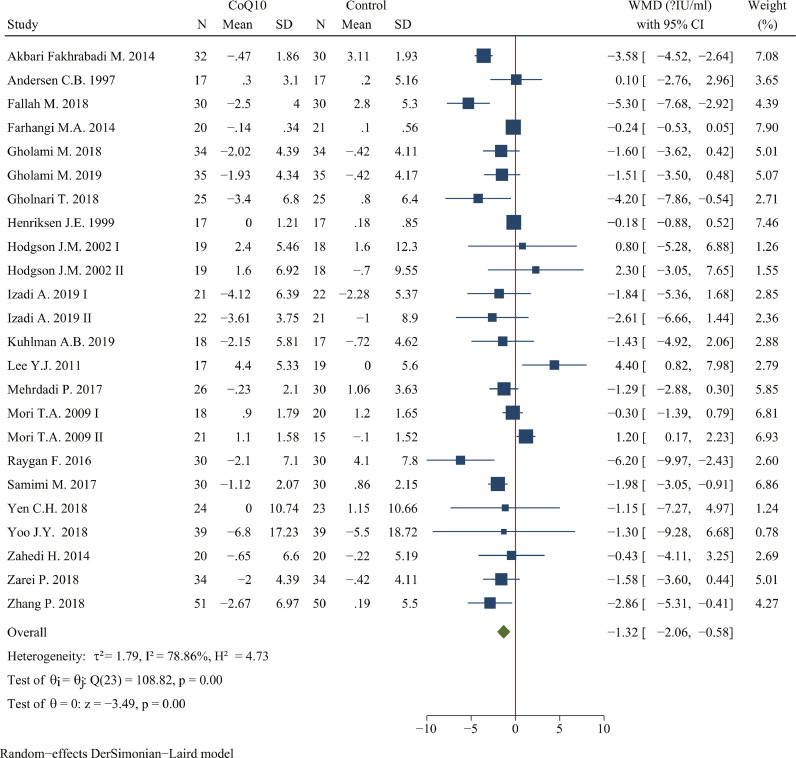
CoQ10 supplementation reduced fasting insulin to a statistically significant degree (WMD: -1.32 μIU/ml; 95% CI: -2.06, -0.58 μIU/ml; P <0.001; n=1234 in 24 studies; I2=78.86%) compared with control group (Figure 4). Subgroup analysis suggested that lower CoQ10 dosage, longer duration, low risk of bias, and placebo as control group were potential factors. The impact of CoQ10 on fasting insulin was greater at supplemental doses < 200 mg/day (WMD: -1.71 μIU/ml, 95% CI: -2.57, -0.85 μIU/ml, P < 0.001) compared with remaining groups. As for the treatment duration, the effect of CoQ10 on reducing fasting insulin was better at duration ≥ 12 weeks (WMD: -1.51 μIU/ml, 95% CI: -2.52, -0.50 μIU/ml, P < 0.001) compared with duration < 12 weeks. With respect to the risk of bias and industry funding, the reduction on fasting insulin was greater at low risk of bias (WMD: -1.50 μIU/ml, 95% CI: -2.29, -0.71 μIU/ml, P < 0.001) and not receiving industry funding (Table 5).
Figure 4.
Forest plots of effect of coenzyme Q10 supplementation on fasting insulin. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis; The horizontal line across each blue box reflects the 95% confidence intervals of the study. Abbreviations: CoQ10, coenzyme Q10; WMD, weighted mean difference; CI, confidence interval; SD, standard error.
Table 5.
Subgroup analysis of included randomized controlled trials for the effect of CoQ10 supplementation on fasting insulin.
| Group | No. of trials (participates) | WMD (95% CI) , μIU/ml | Pdifferencea | I2, % | Pheterogeneityb | Pc for between subgroup heterogeneity |
|---|---|---|---|---|---|---|
| Overall | 24 (1234) | −1.32(−2.06, −0.58) | <0.001 | 78.86 | <0.001 | |
| Study design | ||||||
| Parallel | 24 (1234) | −1.32(−2.06, −0.58) | <0.001 | 78.86 | <0.001 | |
| Duration (week) | ||||||
| <12 | 8 (374) | −0.59(−1.59, 0.42) | 0.26 | 64.37 | <0.001 | 0.20 |
| ≥12 | 16 (860) | −1.51(−2.52, −0.50) | <0.001 | 75.24 | <0.001 | |
| CoQ10 dosage | ||||||
| <200 mg/day | 13 (733) | −1.71(−2.57, −0.85) | <0.001 | 74.31 | <0.001 | 0.41 |
| ≥200 mg/day and <300 mg/day | 10 (466) | −0.43(−2.12, 1.27) | 0.62 | 84.82 | <0.001 | |
| ≥300 mg/day | 1 (35) | −1.43(−4.92, 2.06) | 0.42 | - | - | |
| Control group | ||||||
| Placebo | 22 (1153) | −1.39 (−2.20, −0.57) | <0.001 | 0.00 | 0.41 | 0.16 |
| Other | 2 (81) | −0.44 (−1.48, 0.61) | 0.41 | 80.57 | <0.001 | |
| Quality of study | ||||||
| High | 4 (148) | 0.32 (−1.65, 2.29) | 0.76 | 0.00 | 0.87 | 0.09 |
| Low | 20 (1086) | −1.50 (−2.29, −0.71) | <0.001 | 82.28 | <0.001 | |
| Received industry funding? | ||||||
| Yes | 7 (302) | 0.10(−0.46, 0.66) | 0.72 | 7.87 | 0.37 | <0.001 |
| No | 17 (932) | −1.84(−2.89, −0.80) | <0.001 | 82.84 | <0.001 |
Abbreviations: WMD, weighted mean difference; CI, confidence interval; CoQ10, coenzyme Q10.
Dersimonian–Laird random effect model was used to calculate the effect size and P-value.
Cochrane Q test was used to detect the heterogeneity between studies.
Cochrane Q test was used to detect the subgroup heterogeneity.
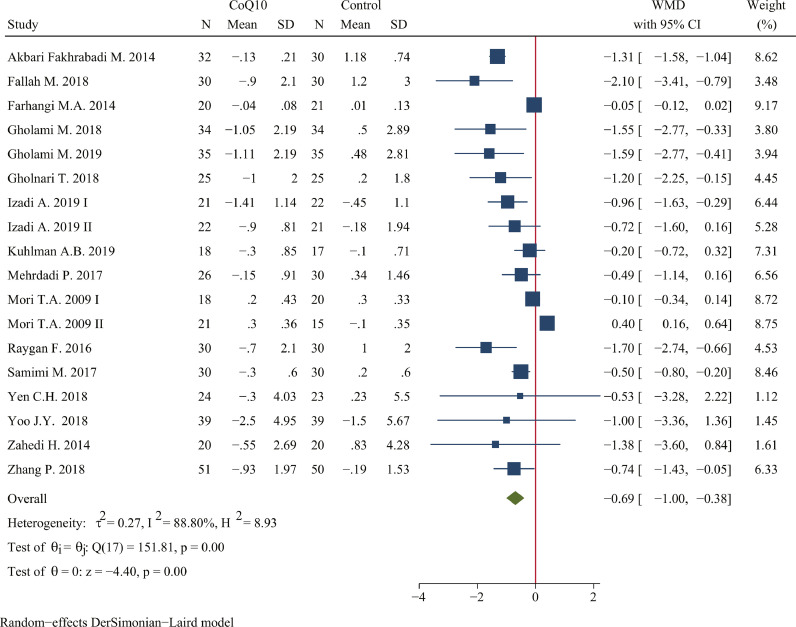
The pooled estimate of the effect of CoQ10 supplementation on HOMA-IR was -0.69 (95% CI: -1.00, -0.38; P <0.001; n=988 in 18 studies; I2=88.80%) (Figure 5). Subgroup analysis suggested that consumption of CoQ10 less than 200 mg/day can greatly reduce HOMA-IR (WMD: -0.97, 95% CI: -1.44, -0.50; P <0.001), and the same effect could be achieved in the subsets of duration ≥ 12 weeks (WMD: -1.03, 95% CI: -1.40, -0.65; P <0.001), low risk of bias (WMD: -0.68, 95% CI: -1.00, -0.37; P <0.001), placebo as control group (WMD: -0.76, 95% CI: -1.13, -0.39; P <0.001), and not receiving industry funding (WMD: -0.99, 95% CI: -1.42, -0.55; P <0.001) (Table 6).
Figure 5.
Forest plots of effect of coenzyme Q10 supplementation on HOMA-IR. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis; The horizontal line across each blue box reflects the 95% confidence intervals of the study. Abbreviations: CoQ10, coenzyme Q10; WMD, weighted mean difference; CI, confidence interval; SD, standard error.
Table 6.
Subgroup analysis of included randomized controlled trials for the effect of CoQ10 supplementation on HOMA-IR.
| Group | No. of trials (participates) | WMD (95% CI) | Pdifferencea | I2, % | Pheterogeneityb | Pc for between subgroup heterogeneity |
|---|---|---|---|---|---|---|
| Overall | 18 (988) | −0.69(−1.00, −0.38) | <0.001 | 88.80 | <0.001 | |
| Study design | ||||||
| Parallel | 18 (988) | −0.69(−1.00, −0.38) | <0.001 | 88.80 | <0.001 | |
| Duration (week) | ||||||
| <12 | 8 (374) | −0.24(−0.52, 0.05) | 0.11 | 79.45 | 0.00 | <0.001 |
| ≥12 | 10 (614) | −1.03(−1.40, −0.65) | <0.001 | 61.13 | 0.01 | |
| CoQ10 dosage | ||||||
| <200 mg/day | 10 (597) | −0.97(−1.44, −0.50) | <0.001 | 80.99 | <0.001 | 0.10 |
| ≥200 mg/day and <300 mg/day | 7 (356) | −0.54(−1.17, 0.10) | 0.10 | 93.79 | <0.001 | |
| ≥300 mg/day | 1 (35) | −0.20(−0.72, 0.32) | 0.45 | - | - | |
| Control group | ||||||
| Placebo | 16 (907) | −0.76 (−1.13, −0.39) | <0.001 | 89.72 | <0.001 | 0.53 |
| Other | 2 (81) | −0.47 (−1.31, 0.36) | 0.27 | 82.16 | 0.02 | |
| Quality of study | ||||||
| High | 1 (40) | −1.38 (−3.60, 0.84) | 0.22 | - | - | 0.54 |
| Low | 17 (948) | −0.68 (−1.00, −0.37) | <0.001 | 89.38 | <0.001 | |
| Received industry funding? | ||||||
| Yes | 5 (234) | 0.03(−0.33, 0.40) | 0.86 | 63.99 | 0.03 | <0.001 |
| No | 13 (754) | −0.99(−1.42, −0.55) | <0.001 | 90.72 | <0.001 |
Abbreviations: WMD, weighted mean difference; CI, confidence interval; CoQ10, coenzyme Q10.
Dersimonian–Laird random effect model was used to calculate the effect size and P-value.
Cochrane Q test was used to detect the heterogeneity between studies.
Cochrane Q test was used to detect the subgroup heterogeneity.
Considering an existing difference of subjects among included studies, we further performed subgroup analysis of diseases (Supplemental Table S2). Subgroup analysis of fasting glucose suggested that CoQ10 supplementation had the best effect on patients with diabetes (WMD: -13.12 mg/dl; 95% CI: -18.91, -7.32 mg/dl; P <0.001; I2=64.32%) (Supplemental Figure S2), compared with CVD (WMD: -10.28 mg/dl; 95% CI: -23.67, 3.11 mg/dl; P = 0.13), and dyslipidemia (WMD: -2.12 mg/dl; 95% CI: -7.18, 2.94 mg/dl; P = 0.41). Supplementation of CoQ10 could statistically reduce HbA1c in diabetic patients (WMD: -0.15%; 95% CI: -0.29, -0.01 %; P = 0.04; I2=55.00%) (Supplemental Figure S3), but there was no statistical difference in patients with CVD and dyslipidemia.
CoQ10 significantly lowered fasting insulin level in population with diabetes (WMD: -1.90 μIU/ml; 95% CI: -3.04, -0.76 μIU/ml; P < 0.001; I2=74.12%) (Supplemental Figure S4), compare with other diseases. Only one trial, concerning CVD, showed that fasting insulin levels have been decreased by - 6.20 μIU/ml on average after CoQ10 treatment.
The reduction in HOMA-IR was significant in diabetic patient (WMD: -1.26; 95% CI: -1.48, -1.04; P < 0.001; I2=0.00%) (Supplemental Figure S5), compare with dyslipidemia (WMD: -0.42; 95% CI: -0.94, 0.10; P = 0.11).
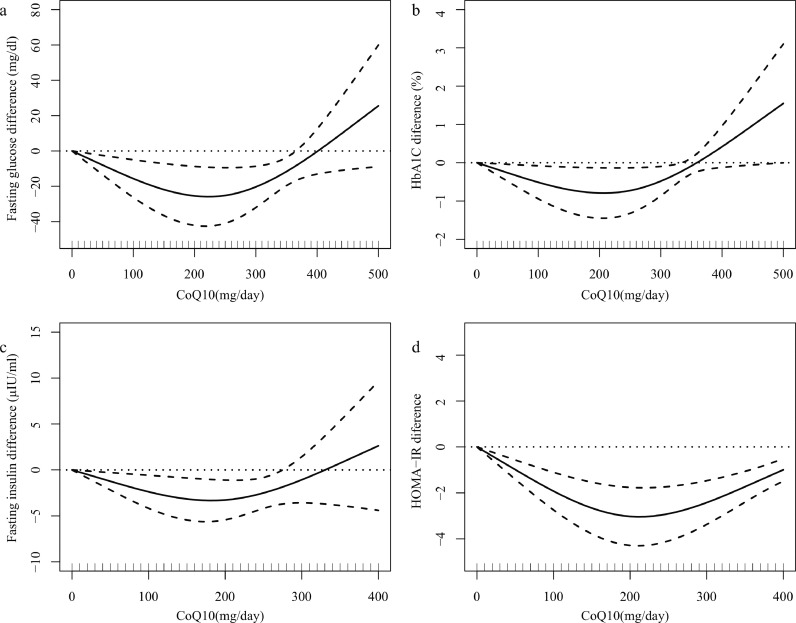
In the dose–response assessment of the effect of CoQ10 intake on glycemic control, we used a 1-stage restricted cubic spline regression model (Figure 6). Figure 6 shows a “U” shape dose-response curve of CoQ10 dosage and outcome indicators of glycemic control respectively in included studies. Considering the dosage subgroup analysis and dose-response curve, CoQ10 dose of 100-200 mg/day has better efficacy in improving fasting glucose (χ2 = 12.08, Pnonlinearity =0.002), fasting insulin (χ2 = 9.73, Pnonlinearity =0.008), HbA1c (χ2 = 6.00, Pnonlinearity =0.049), and HOMA-IR (χ2 = 25.89, Pnonlinearity <0.001).
Figure 6.
Dose–response meta-analysis of changes in glycemic control according to CoQ10 in the treatment and control groups at the end of the trials. (a) fasting glucose, (b) HbA1c, (c) fasting insulin, (d) HOMA-IR. The average curve (solid line) with 95% confidence limits (dotted lines) was estimated with a 1-stage random-effects restricted cubic spline model, using 0 mg/day as referent. Abbreviations: CoQ10, coenzyme Q10.
The pooled effect size was robust and remained significant in the leave-one-out sensitivity analysis. For fasting insulin and HbA1c, visual inspection of funnel plot did not suggest a significant potential publication bias. This observation was confirmed by the results of Egger's linear regression. For fasting glucose and HOMA-IR, funnel plot and Egger's linear regression suggested a significant potential publication bias (Supplemental Figure S6). After adjustment of effect size for potential publication bias using the ‘trim and fill’ correction, it yielded similar results to the overall pooled effect size estimates.
Discussion
This study was a meta-analysis that regarded the effects of CoQ10 in the improvement of glycemic control. We synthesized the results from 40 RCTs involving 2427 participants to draw an overall conclusion. The major findings of meta-analysis showed that CoQ10 supplementation statistically reduced fasting glucose, fasting insulin, HbA1c, and HOMA-IR. This means that CoQ10 supplementation might have beneficial effects in glycemic control. In addition, our results also show a “U” shape dose-response curve of CoQ10 dosage and outcome indicators of glycemic control, thus indicating that 100-200mg / day of CoQ10 has better efficacy on attenuating the level of fasting blood glucose, fasting insulin, HbA1c and HOMA-IR.
This meta-analysis showed that CoQ10 supplementation could significantly reduce the level of fasting glucose, HbA1c, fasting insulin, HOMA-IR by an average of 5.22 mg/dl (95%CI: -8.33, -2.11 mg/dl), -0.12% (95%CI: -0.23, -0.01%), -1.32 μIU/ml (95%CI: -2.06, -0.58 μIU/ml), -0.69 (95%CI: -1.00, -0.38), respectively. The results of prior meta-analysis on glycemic control were controversial. Part of the meta-analysis conducted in patients with diabetes revealed that CoQ10 could significantly decrease fasting glucose level (-11.21 mg/dl) and HbA1c (-0.29%),10 while some reported the opposite results (standard mean difference of fasting glucose: 0.17, HbA1c: 0.3, fasting insulin: 0.09 μIU/ml).10,62,63 The difference between our meta-analyses and the latter one is the fact that the publication for included studies62 only limited from 2015 to 2018. In our meta-analysis, we synthesized all the available studies concerning on the glycemic control in hyperglycemia-related diseases ranging from 1997 to 2021. Notably, our review contained 28 additional studies for these outcomes that were published since that prior reviews.10,62 Given the moderate level of evidence certainty for HbA1c findings, one of the strongest beneficial effects of CoQ10 supplementation might be a reduction in HbA1c.
Because of the significant heterogeneity, subgroup analysis was performed, indicating that longer intervention duration (>12 weeks), placebo as control group, low risk of bias, and not receiving industry funding were potential modifying factors in terms of treatment efficacy in glycemic control. Contrary to previous meta-analysis,63 we found that longer intervention duration can be beneficial in the reduction of the blood glucose level, because our review contained additional studies with longer duration that were published since that prior reviews. What's more, some animal experiments64,65 found that chronic ingestion of CoQ10 has been shown to increase the concentration of CoQ10 in plasma in rodent models. In addition, a study66 found that the average blood CoQ10 concentration increased by times after 90mg CoQ10 supplementation for 3 and 9 months in healthy subjects. Therefore, long-term intervention can significantly increase the concentration of plasma CoQ10, so as to improve glycemic control.
In contrast to the prior meta-analysis,10,62 our meta-analysis concerned on the glycemic control in difference diseases, which might be a potential modifying factor of heterogeneity. Thus, we created a subgroup analyses, revealing greater effects of CoQ10 for diabetes patients, but not for other diseases such as CVD, obesity and dyslipidemia. We also found a larger effect size in diabetes patients than did that prior review10 (-13.12 mg/dl vs. -11.21 mg/dl), because our review further contained 14 additional studies. The main reason might be that the mean fasting baseline blood glucose level in diabetes was higher than 6.1mmol/l (109.8 mg/dl), which is considered as the upper threshold for “normal” blood glucose level, thus suggesting CoQ10 as a potent compound for blood glucose reduction. Glucose metabolism disorder also plays an important role in CVD. In our meta-analysis, only three literatures26,35,46 had reported partial glucose outcomes in patients with CVD, which could not be analyzed. Among these literatures, CoQ10 supplementation decreased fasting glucose,26,46 fasting insulin,46 HbA1c,35 and HOMA-IR.46 Thus, further studies investigations in CVD are still required to evaluate effects of CoQ10 on glycemic control.
We synthesized data from included studies, finding that the dosage of CoQ10 reported in these studies was used in both higher and lower doses, ranging from 100 mg to 900 mg. Thus, we further analyzed the relationship between dosage of CoQ10 and glycemic control outcomes in order to explore the optimal intake of CoQ10. Through the dosage subgroup analysis of glycemic control, we found that the low-dose group (100 to 200 mg/day) can significantly improve glycemic control, while the medium (200 to 300 mg/day) and high-dose groups (>300 mg/day) have no significant statistical difference. This result was consistent with the results reported in previous meta-analysis.63 However, prior meta-analysis reached this conclusion only through subgroup analysis, and could not find the optimal dose of CoQ10 intervention. Thus, in order to explore the optimal intake dose of CoQ10, we further analyzed the dose-response relationship according to the data in the included studies. We newly used a 1-stage restricted cubic spline regression model to matching the data. We found that 100-200 mg/day was sufficient to beneficially improve glycemic control including fasting glucose, fasting insulin, HbA1c and HOMA-IR, which could be conducive to set up nutrition guidelines of daily recommendation in patients with hyperglycemia-related diseases. Reasons for the disappearance of glycemic control effect of high dose CoQ10 might be related to the decrease of intestinal absorption and utilization.67 CoQ10 was a lipid-soluble substance with a complex active transport process absorption in the gastrointestinal tract. Some studies found a non-linear or zero-order absorption process, suggesting that CoQ10 plasma concentration decreases as dosage is increased.68
Potential antihyperglycemic mechanisms of CoQ10 action might plausibly include antioxidant and anti-inflammatory effects of CoQ10 that promote improved insulin sensitivity. Tarry-Adkins et.al demonstrated that CoQ10 could prevented the programmed reduction in insulin receptor substrate-1 and p110-β and the programmed increased in IL-6.69 In addition, supplementation of CoQ10 could increase the activity of tyrosine kinase, phosphatidylinositol kinase, and adiponectin receptors in diabetes mice, and decrease the activity of insulin receptor isoforms and glucose transporters.70 In vitro studies showed that CoQ10 could improve the apoptosis of mouse pancreatic beta cells line MIN6 induced by staurosporine, improving the mitochondrial stress of pancreatic beta cells.71
CoQ10 widely exists in various natural foods, but the content of different kinds of foods varies greatly. The main food intake comes from meat, and a small amount comes from grains, fruits and vegetables. While our study only investigated effects of supplemental CoQ10 on outcomes, it is possible that regular high dietary intakes of CoQ10 could yield similar outcomes. However, there is a serious lack of survey data on CoQ10 dietary intake assessment. In a study published in the 1990s,72 based on the food consumption data of the Danish dietary survey and the detection data of CoQ10 content in specific foods, it was estimated that the average dietary intake of CoQ10 was 3-5 mg/day, which was far less than the results of this review. However, since it had been published for a long time, it is necessary to further update the content of CoQ10 in daily diet, which is our next work to calculate the intake of CoQ10 in daily diet according to the data of some databases.
Our findings, which are based largely on short-term studies with low evidence quality, suggested that CoQ10 supplementation may be potentially effective for improving glycemic control. Compared with previous meta-analyses,10,62 the strength of the present study was that we comprehensively analyzed the glycemic control effect of CoQ10 in different hyperglycemia-related diseases. As a result, we concluded that CoQ10 could significantly improve the glycemic control level of diabetic patients with minor heterogeneity. Furthermore, we newly used a 1-stage restricted cubic spline regression model to analyze the dose-response relationship between CoQ10 dose and glycemic control, so as to achieve the greatest benefit for glycemic control.
However, a limitation of this review was the absence of exploratory subgroup and meta regression analysis of outcomes based on plasma CoQ10 status as most studies in the review did not measure participants' CoQ10 concentrations. Therefore, it remains unclear whether baseline CoQ10 status might affect the outcomes explored in our review. Because of heterogeneity between studies, indirectness of outcomes, and publication bias, evidence certainty is mostly low across these measures. A limitation of studies included was that they were predominantly short term (<6 months) with a relatively small participant number (n < 50). Further investigations using larger sample sizes and longer supplementation durations are required to confirm these potential glycemic control benefits.
Studies have shown that different molecular might have an impact on the bioavailability of CoQ10. At present, it is still controversial whether there is a difference in bioavailability between ubiquinone and ubiquinol. Some found that there is no significant difference in bioavailability between ubiquinone and ubiquinol in healthy elderly people. These two molecules mainly existed in the form of ubiquinol in blood.73 Others suggested that ubiquinol was superior to ubiquinone to enhance CoQ10 status in older men.74 Besides, the formulation of CoQ10 supplements can also affect the bioavailability to some extent.75 The matrix used to dissolve CoQ10 could affect the bioavailability of CoQ10, such as nanoemulsions, cyclodextrin complexes.76,77 In our meta-analysis, we previously considered subgroup analysis of CoQ10 in different molecular forms, but we found that only one study54 used ubiquinol for glycemic control, and the rest used ubiquinone. Therefore, it is hard to conduct subgroup analysis to compare the effect between these two forms of CoQ10 on glycemic control. Based on this, our group decided to carry out a randomized controlled trial of ubiquinol intervention in the future to further explore the biological effects of ubiquinone and ubiquinol.
Our results found that CoQ10 supplementation might have beneficial effects on glycemic control, especially in diabetic patients. Taking 100-200 mg/day of CoQ10 could achieve the greatest benefit for glycemic control. These findings add new information about the beneficial effects of CoQ10 supplementation on glycemic control, and are conducive to setting up nutrition guidelines for recommended daily intake of CoQ10 in patients with glycemic disorders.
Contributors
YY designed research; YL, DZ, QJ, and ML extracted the data independently; ZT resolved any discrepancies; SD, SH, and ZL reviewed data; YL and DZ performed statistical analysis; YL wrote the manuscript; YY, YM, ZT and DZ contributed to the discussion and reviewed the manuscript; YY had primary responsibility for final content. All authors read and approved the final manuscript.
Data sharing statement
Corresponding authors have accessed and verified the data, and were responsible for the decision to submit the manuscript. All data, study protocol, and statistical analysis plan will be made available upon reasonable request via email to corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82030098, 81872617 and 81730090), Shenzhen Science, Technology, and Innovation Commission (No. JCYJ20180307153228190), CNS Research Fund for DRI, and National innovation and entrepreneurship training program for undergraduate student (No. 202210558161).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101602.
Contributor Information
Zezhong Tian, Email: tianzzh3@mail.sysu.edu.cn.
Yan Yang, Email: yangyan3@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sattar N, Gill JMR, Alazawi W. Improving prevention strategies for cardiometabolic disease. Nat Med. 2020;26(3):320–325. doi: 10.1038/s41591-020-0786-7. [DOI] [PubMed] [Google Scholar]
- 8.Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50(4):269–280. doi: 10.1080/10408390902773037. [DOI] [PubMed] [Google Scholar]
- 9.Arenas-Jal M, Suñé-Negre JM, García-Montoya E. Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf. 2020;19(2):574–594. doi: 10.1111/1541-4337.12539. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SY, Yang KL, Zeng LT, Wu XH, Huang HY. Effectiveness of coenzyme Q10 supplementation for type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Endocrinol. 2018;2018 doi: 10.1155/2018/6484839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S-Y, Yang K-L, Zeng L-T, Wu X-H, Huang H-Y. Effectiveness of coenzyme Q10 supplementation for type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Endocrinol. 2018;2018 doi: 10.1155/2018/6484839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40(4):413–418. doi: 10.1111/jcpt.12280. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Shi Z, Liu Q, Quan H, Cheng X. Effects of coenzyme Q10 intervention on diabetic kidney disease: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98(24):e15850. doi: 10.1097/MD.0000000000015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022) Cochrane; 2022. [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason SA, Keske MA, Wadley GD. Effects of Vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44(2):618–630. doi: 10.2337/dc20-1893. [DOI] [PubMed] [Google Scholar]
- 18.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–1596. doi: 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- 19.Vinceti M, Filippini T, Malavolti M, et al. Dose-response relationships in health risk assessment of nutritional and toxicological factors in foods: development and application of novel biostatistical methods. EFSA Support Publications. 2020;17(7):1899E. [Google Scholar]
- 20.Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. J Statis Softw, Code Snippets. 2016;72(1):1–15. [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Bargossi AM, Battino M, Gaddi A, et al. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Int J Clin Lab Res. 1994;24(3):171–176. doi: 10.1007/BF02592449. [DOI] [PubMed] [Google Scholar]
- 23.Andersen CB, Henriksen JE, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. The effect of coenzyme Q10 on blood glucose and insulin requirement in patients with insulin dependent diabetes mellitus. Mol Aspects Med. 1997;18(suppl):S307–S309. doi: 10.1016/s0098-2997(97)00010-1. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson JG, Forsen TJ, Mortensen SA, Rohde M. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors. 1999;9(2-4):315–318. doi: 10.1002/biof.5520090229. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen JE, Andersen CB, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with type 1 diabetes mellitus. Diabet Med. 1999;16(4):312–318. doi: 10.1046/j.1464-5491.1999.00064.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh RB, Niaz MA. Serum concentration of lipoprotein(a) decreases on treatment with hydrosoluble coenzyme Q10 in patients with coronary artery disease: discovery of a new role. Int J Cardiol. 1999;68(1):23–29. doi: 10.1016/s0167-5273(98)00323-4. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56(11):1137–1142. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- 28.Playford DA, Watts GF, Croft KD, Burke V. Combined effect of coenzyme Q10 and fenofibrate on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168(1):169–179. doi: 10.1016/s0021-9150(02)00417-3. [DOI] [PubMed] [Google Scholar]
- 29.Ikematsu H, Nakamura K, Harashima S, Fujii K, Fukutomi N. Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regul Toxicol Pharmacol. 2006;44(3):212–218. doi: 10.1016/j.yrtph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Nuku K, Matsuoka Y, Yamagishi T, Miyawaki H, Sato K. Safety assessment of PureSorb-Q40 in healthy subjects and serum coenzyme Q10 level in excessive dosing. J Nutr Sci Vitaminol (Tokyo) 2007;53(3):198–206. doi: 10.3177/jnsv.53.198. [DOI] [PubMed] [Google Scholar]
- 31.Chew GT, Watts GF, Davis TM, et al. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31(8):1502–1509. doi: 10.2337/dc08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SC, Lekshminarayanan R, Goh SK, et al. The effect of coenzyme Q10 on microcirculatory endothelial function of subjects with type 2 diabetes mellitus. Atherosclerosis. 2008;196(2):966–969. doi: 10.1016/j.atherosclerosis.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori TA, Burke V, Puddey I, et al. The effects of [omega]3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: a randomized controlled trial. J Hypertens. 2009;27(9):1863–1872. doi: 10.1097/hjh.0b013e32832e1bd9. [DOI] [PubMed] [Google Scholar]
- 35.Dai YL, Luk TH, Yiu KH, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011;216(2):395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Lee YJ, Cho WJ, Kim JK, Lee DC. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double-blind randomized controlled study. J Med Food. 2011;14(4):386–390. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Ojeda J, Cardona-Muñoz EG, Román-Pintos LM, et al. The effect of ubiquinone in diabetic polyneuropathy: a randomized double-blind placebo-controlled study. J Diabetes Complications. 2012;26(4):352–358. doi: 10.1016/j.jdiacomp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Kolahdouz Mohammadi R, Hosseinzadeh-Attar MJ, Eshraghian MR, Nakhjavani M, Khorami E, Esteghamati A. The effect of coenzyme Q10 supplementation on metabolic status of type 2 diabetic patients. Minerva Gastroenterol Dietol. 2013;59(2):231–236. [PubMed] [Google Scholar]
- 39.Akbari Fakhrabadi M, Zeinali Ghotrom A, Mozaffari-Khosravi H, Hadi Nodoushan H, Nadjarzadeh A. Effect of coenzyme Q10 on oxidative stress, glycemic control and inflammation in diabetic neuropathy: a double blind randomized clinical trial. Int J Vitam Nutr Res. 2014;84(5-6):252–260. doi: 10.1024/0300-9831/a000211. [DOI] [PubMed] [Google Scholar]
- 40.Farhangi MA, Alipour B, Jafarvand E, Khoshbaten M. Oral coenzyme Q10 supplementation in patients with nonalcoholic fatty liver disease: effects on serum vaspin, chemerin, pentraxin 3, insulin resistance and oxidative stress. Arch Med Res. 2014;45(7):589–595. doi: 10.1016/j.arcmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Mohammadshahi M, Farsi F, Nejad PA, Hajiani E, Zarei M, Engali KA. The coenzyme Q10 supplementation effects on lipid profile, fasting blood sugar, blood pressure and oxidative stress status among non-alcoholic fatty liver disease patients: a randomized, placebo-controlled, pilot study. J Gastroenterol Hepatol Res. 2014;3(6):1108–1113. [Google Scholar]
- 42.Mohammed-Jawad NK, Al-Sabbagh M, AL-Jezaeri KA. Role of L-carnitine and coenzyme Q10 as adjuvant therapy in patients with type 2 diabetes mellitus. Am J Pharmacol Sci. 2014;2(5):82–86. [Google Scholar]
- 43.Zahedi H, Eghtesadi S, Seifirad S, et al. Effects of CoQ10 supplementation on lipid profiles and glycemic control in patients with type 2 diabetes: a randomized, double blind, placebo-controlled trial. J Diabetes Metab Disord. 2014;13:81. doi: 10.1186/s40200-014-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosseinzadeh-Attar M, Kolahdouz Mohammadi R, Eshraghian M, et al. Reduction in asymmetric dimethylarginine plasma levels by coenzyme Q10 supplementation in patients with type 2 diabetes mellitus. Minerva Endocrinol. 2015;40(4):259–266. [PubMed] [Google Scholar]
- 45.Moazen M, Mazloom Z, Ahmadi A, Dabbaghmanesh MH, Roosta S. Effect of coenzyme Q10 on glycaemic control, oxidative stress and adiponectin in type 2 diabetes. J Pak Med Assoc. 2015;65(4):404–408. [PubMed] [Google Scholar]
- 46.Raygan F, Rezavandi Z, Dadkhah Tehrani S, Farrokhian A, Asemi Z. The effects of coenzyme Q10 administration on glucose homeostasis parameters, lipid profiles, biomarkers of inflammation and oxidative stress in patients with metabolic syndrome. Eur J Nutr. 2016;55(8):2357–2364. doi: 10.1007/s00394-015-1042-7. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, et al. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: a phase IIa, randomized, double-blind, and placebo-controlled study. Redox Report. 2016;21(4):155–163. doi: 10.1179/1351000215Y.0000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehrdadi P, Kolahdouz Mohammadi R, Alipoor E, Eshraghian MR, Esteghamati A, Hosseinzadeh-Attar MJ. The effect of coenzyme Q10 supplementation on circulating levels of novel adipokine adipolin/CTRP12 in overweight and obese patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2017;125(3):156–162. doi: 10.1055/s-0042-110570. [DOI] [PubMed] [Google Scholar]
- 49.Samimi M, Zarezade Mehrizi M, Foroozanfard F, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2017;86(4):560–566. doi: 10.1111/cen.13288. [DOI] [PubMed] [Google Scholar]
- 50.Tóth Š, Šajty M, Pekárová T, et al. Addition of omega-3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J Basic Clin Physiol Pharmacol. 2017;28(4):327–336. doi: 10.1515/jbcpp-2016-0149. [DOI] [PubMed] [Google Scholar]
- 51.Fallah M, Askari G, Soleimani A, Feizi A, Asemi Z. Clinical trial of the effects of coenzyme Q10 supplementation on glycemic control and markers of lipid profiles in diabetic hemodialysis patients. Int Urol Nephrol. 2018;50(11):2073–2079. doi: 10.1007/s11255-018-1973-z. [DOI] [PubMed] [Google Scholar]
- 52.Gholami M, Zarei P, Sadeghi Sedeh B, Rafiei F, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of adiponectin, leptin, 8-isoprostane and malondialdehyde in women with type 2 diabetes. Gynecol Endocrinol. 2018;34(12):1059–1063. doi: 10.1080/09513590.2018.1481944. [DOI] [PubMed] [Google Scholar]
- 53.Gholnari T, Aghadavod E, Soleimani A, Hamidi GA, Sharifi N, Asemi Z. The effects of coenzyme Q10 supplementation on glucose metabolism, lipid profiles, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2018;37(3):188–193. doi: 10.1080/07315724.2017.1386140. [DOI] [PubMed] [Google Scholar]
- 54.Yen CH, Chu YJ, Lee BJ, Lin YC, Lin PT. Effect of liquid ubiquinol supplementation on glucose, lipids and antioxidant capacity in type 2 diabetes patients: a double-blind, randomised, placebo-controlled trial. Br J Nutr. 2018;120(1):57–63. doi: 10.1017/S0007114518001241. [DOI] [PubMed] [Google Scholar]
- 55.Yoo JY, Yum KS. Effect of coenzyme Q(10) on insulin resistance in korean patients with prediabetes: a pilot single-center, randomized, double-blind, placebo-controlled study. BioMed Res Int. 2018;2018 doi: 10.1155/2018/1613247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zarei P, Rezvanfar MR, Ansarihadipour H, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q(10) supplementation on the serum levels of amylase, adenosine deaminase, catalase, and total antioxidant capacity in women with type 2 diabetes mellitus: a randomized, double-blind placebo-controlled trial. J Res Med Sci. 2018;23:91. doi: 10.4103/jrms.JRMS_970_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Yang C, Guo H, et al. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J Clin Lipidol. 2018;12(2):417–427.e5. doi: 10.1016/j.jacl.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Gholami M, Rezvanfar MR, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of gamma-glutamyl transferase, pseudocholinesterase, bilirubin, ferritin, and high-sensitivity C-reactive protein in women with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2019;127(5):311–319. doi: 10.1055/s-0043-124183. [DOI] [PubMed] [Google Scholar]
- 59.Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(2):319–327. doi: 10.1210/jc.2018-01221. [DOI] [PubMed] [Google Scholar]
- 60.Kuhlman AB, Morville T, Dohlmann TL, et al. Coenzyme Q10 does not improve peripheral insulin sensitivity in statin-treated men and women: the LIFESTAT study. Appl Physiol Nutr Metab. 2019;44(5):485–492. doi: 10.1139/apnm-2018-0488. [DOI] [PubMed] [Google Scholar]
- 61.Ho CC, Chang PS, Chen HW, et al. Ubiquinone supplementation with 300 mg on glycemic control and antioxidant status in athletes: a randomized, double-blinded, placebo-controlled trial. Antioxidants (Basel) 2020;9(9) doi: 10.3390/antiox9090823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dludla PV, Nyambuya TM, Orlando P, et al. The impact of coenzyme Q(10) on metabolic and cardiovascular disease profiles in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Endocrinol Diabetes Metab. 2020;3(2):e00118. doi: 10.1002/edm2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stojanović M, Radenković M. A meta-analysis of randomized and placebo-controlled clinical trials suggests that coenzyme Q10 at low dose improves glucose and HbA1c levels. Nutr Res. 2017;38:1–12. doi: 10.1016/j.nutres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Kwong LK, Kamzalov S, Rebrin I, et al. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Rad Biol Med. 2002;33(5):627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 65.Kamzalov S, Sumien N, Forster MJ, Sohal RS. Coenzyme Q intake elevates the mitochondrial and tissue levels of coenzyme Q and alpha-tocopherol in young mice. J Nutr. 2003;133(10):3175–3180. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]
- 66.Folkers K, Moesgaard S, Morita M. A one year bioavailability study of coenzyme Q10 with 3 months withdrawal period. Molecular Aspects Med. 1994;15(suppl):s281–s285. doi: 10.1016/0098-2997(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 67.Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7(suppl):S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007;47(1):19–28. doi: 10.1016/j.yrtph.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Tarry-Adkins JL, Fernandez-Twinn DS, Madsen R, et al. Coenzyme Q10 prevents insulin signaling dysregulation and inflammation prior to development of insulin resistance in male offspring of a rat model of poor maternal nutrition and accelerated postnatal growth. Endocrinology. 2015;156(10):3528–3537. doi: 10.1210/en.2015-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amin MM, Asaad GF, Abdel Salam RM, El-Abhar HS, Arbid MS. Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, tyrosine kinase, PI3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats. PLoS One. 2014;9(2):e89169. doi: 10.1371/journal.pone.0089169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sumi K, Okura T, Fujioka Y, et al. Coenzyme Q10 suppresses apoptosis of mouse pancreatic beta-cell line MIN6. Diabetol Metab Syndr. 2018;10:47. doi: 10.1186/s13098-018-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber C, Bysted A, Hłlmer G. The coenzyme Q10 content of the average Danish diet. Int J Vitam Nutr Res. 1997;67(2):123–129. [PubMed] [Google Scholar]
- 73.Pravst I, Rodríguez Aguilera JC, Cortes Rodriguez AB, et al. Comparative bioavailability of different coenzyme Q10 formulations in healthy elderly individuals. Nutrients. 2020;12(3) doi: 10.3390/nu12030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Liu J, Chen XQ, Oliver Chen CY. Ubiquinol is superior to ubiquinone to enhance Coenzyme Q10 status in older men. Food Funct. 2018;9(11):5653–5659. doi: 10.1039/c8fo00971f. [DOI] [PubMed] [Google Scholar]
- 75.López-Lluch G, Del Pozo-Cruz J, Sánchez-Cuesta A, Cortés-Rodríguez AB, Navas P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133–140. doi: 10.1016/j.nut.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 76.Barakat A, Shegokar R, Dittgen M, Müller RH. Coenzyme Q10 oral bioavailability: effect of formulation type. J Pharmaceut Investigat. 2013;43(6):431–451. [Google Scholar]
- 77.Schulz C, Obermüller-Jevic UC, Hasselwander O, Bernhardt J, Biesalski HK. Comparison of the relative bioavailability of different coenzyme Q10 formulations with a novel solubilizate (Solu Q10) Int J Food Sci Nutr. 2006;57(7-8):546–555. doi: 10.1080/09637480601058320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.