Abstract
Osteosarcoma is a primary malignant cancer of the bone identified by the direct formation of osteoid tissue or immature bone by cancer cells. The liver and kidneys represent two major secondary organs to which osteosarcoma metastasizes. In this study, we assessed Shilajit, a phytomineral diffusion traditionally used in Ayurvedic medicine, for its possible protective effects against metastasis induced liver and kidney damages in an osteosarcoma rat model. Osteosarcoma rats displayed typical dysregulation of serum levels of hepatic and renal functional markers (p < 0.05) including aspartate aminotransferase (AST)* and alanine aminotransferase (ALT), alkaline phosphatase (ALP), total proteins, albumin, bilirubin, creatinine, urea, and uric acid. Changes in functional markers were also positively correlated with marked histopathological alterations in liver and kidney tissues. Whereas Shilajit's treatment of osteosarcoma rates in combination with CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) chemotherapy drug cocktail significantly (p < 0.05) reversed the studied functional markers to their near-normal levels. Co-treatment of shilajit and drug cocktails also markedly alleviated histopathological changes in liver and kidney tissues. Correlation co-efficient analysis of hepatic and renal functional markers revealed a significant inter-association among these markers. Collectively, present data indicate that shilajit may potentiate the effects of chemotherapy drugs and mitigate the metastasis-induced liver and kidney damage in osteosarcoma. Thus, the findings of this study substantiate the beneficial health effects of shilajit and promote its regular consumption.
Keywords: Shilajit, Osteosarcoma, Liver, Kidney, Histology, Metastasis
Abbreviations: CMF, cyclophosphamide, methotrexate and 5, fluorouracil; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; OS, osteosarcoma; LDS, low dose shilajit; HDS, high dose shilajit
1. Introduction
Osteosarcoma (OS), also named osteogenic sarcoma, is one of the commonest primary tumors of the skeleton recognized by osteogenic progenitor cells or mesenchymal cells producing osteoid and immature bone (Bielack et al., 2009). Worldwide, 2–4/million OS cases per year are reported to occur with the first increase in incidence between 15 and 19 years which is followed by a second minor peak in people above 60 years (Mirabello et al., 2009, Whelan et al., 2012). The OS most commonly originates in the metaphysis of the proximal tibia, distal femur, proximal humerus and proximal femur, while it is rarely found in the axial skeleton. In localized OS, 60 %–70 % survival rate is reported to reach a plateau in 5 years since the commencement of systematic chemotherapy (Stiller et al., 2001). However, in metastasis cases, a 20 % survival rate is still unexpected (Bielack et al., 2002, Kager et al., 2003, Mialou et al., 2005). Importantly, subclinically, most OS patients are likely to have micrometastatic lesions at the time of diagnosis. However dismal 15 %–20 % of OS cases which are newly diagnosed, are successfully diagnosed with metastasis(Kaste et al., 1999). The high propensity of OS to metastasize is the major cause of poor prognosis and failed treatments. OS is heterogeneous when it comes to its metastasis. For instance, tumors may grow concomitantly with the primary tumor while the treatment is on or soon or after a long gap or after the end of the treatment regimen. Interestingly, metastasized tumors are identical in histology to that of the primary tumor or display a variation in a different differentiation pathway (Kager et al., 2003, Odri et al., 2022). The metastatic osteosarcoma cells go through a series of crucial stages to populate and grow in the secondary site and eventually advance to clinically identifiable tumors. Additionally, the biology of metastasis differs from the primary tumor with regard to karyotype, cell cycle, metabolism, surrounding microenvironment, and differentiation. These variations are reported to be caused due to differences in the expression of relevant genes, changes in molecule profiles as well as interaction with tumor surroundings (Gorlick et al., 2003, PosthumaDeBoer et al., 2011). Several recent and previous studies reported that osteosarcoma could metastasize to extraskeletal organs such as the liver and kidney, rendering the disease treatment more complex and difficult (Dalinka et al., 1971, Daw et al., 2001, Hagay et al., 1986, Sigdel et al., 2021). Chemotherapy remained the primary choice for the treatment of metastasized OS. However, the outcome of chemotherapy in most cases is unsatisfactory with lower than expected recovery, relapse of tumor and the well-known side effects of chemotherapy. Chemotherapy-linked shortcomings led to renewed interest in research finding dietary intervention with minimal or no side effects and high potency in the treatment of OS (Leite et al., 2021, Rabelo et al., 2022).
Shilajit, which is known by different names such as salajeet, mumie, mumijo, mummiyo, and moomiyo, is a phytomineral diffusion found in sedimentary rocks and appears blackish-brown colored semi-solid matter with a sharp odor and pungent tang, formed due to the extended humification process of many plants (Agarwal et al., 2007, Carrasco-Gallardo et al., 2012a, Carrasco-Gallardo et al., 2012b). The chemical composition of shilajit is well characterized and found to contain 60–70 % humus (Agarwal et al., 2007, Aldakheel et al., 2021, Ghosal et al., 1976, Schepetkin et al., 2003). Shilajit has been used extensively in Indian traditional medicine Ayurveda to treat chronic diseases and a number of ailments due to its medicinal properties (Jafari et al., 2019, Schepetkin et al., 2003). Shilajit is shown to act as a chemotherapeutic agent against urinary bladder cancer and to induce apoptosis in human breast cancer cells Kloskowski et al., 2021, Rahmani Barouji et al., 2020b). Wound-induced inflammation and aspirin-induced gastric lesions were found to be abrogated in rats by shilajit treatment (Ghasemkhani et al., 2021, Kim et al., 2021). Shilajit activated peritoneal macrophages and splenocytes in tumor bearing murine during different tumor growth stages (Rahmani Barouji et al., 2020a). Shilajit is also attributed to a number of beneficial effects against neurological disorders including Alzheimer’s disease (Bhattarai et al., 2016, Calfio et al., 2020, Carrasco-Gallardo et al., 2012a, Carrasco-Gallardo et al., 2012b). Importantly, shilajit has been shown to prevent liver damage in high-fat diet-induced non-alcoholic fatty liver disease and induce apoptosis in hepatic cancer cells k(Ghezelbash et al., 2020, Pant et al., 2016). The prognosis of metastatic OS cases remains off the mark and despite progress in research leading to the discovery of several metastasis-predicting and promoting factors, there is a lack of proven treatment strategy to minimize the OS cases progressing into metastasis except for surgery. Therefore, the rationale of this study was to evaluate the possible potentiation capacity of shilajit in combination with chemotherapeutic drugs against metastasis induced liver and kidney damage in the osteosarcoma rat model.
2. Materials and methods
2.1. Chemicals and reagents
Shilajit was bought from Natural Spirit Trading Est, Riyadh., Saudi Arabia. 4 T1 cells were obtained from ATCC (US). Chemotherapy drugs, cyclophosphamide, methotrexate and 5- fluorouracil were purchased locally. All other used reagents and chemicals were of standard grade.
2.2. Animals
Thirty-five male adult albino Sprague –Dawley rats weighing about 220 g were sourced from the College of Pharmacy, King Abdulaziz University, Jeddah and kept in an animal care facility to acclimatize to the environment. Animals had free access to water and a standard diet. The ethics committee approved guidelines were followed to carry out animal experiments. The study was approved by the ethical committee at the College of Science, King Abdulaziz University, Jeddah, Saudi Arabia (No. 845–19-2019).
2.3. Preparation of extract
Shilajit extract was prepared by dissolving it in boiling water at 100° C and placed in a 70° C incubator for 30 h to allow it to dry completely. After drying, shilajit extract was dissolved in HBSS and filtrated through a 0.2-mm filter before administering to animals. (Das et al., 2016).
2.4. Cell line
The 4 T1 cells (ATCC, USA) were cultured in RPMI medium supplemented with 1 % penicillin/streptomycin and10% FBS.
2.5. Induction of osteosarcoma
For bone cancer induction, rats were anesthetized with chloral hydrate and a superficial carving of nearly 1 cm size was made just over the knee that overlaid the patella. Gentle pressure was put to find condyles of the distal femur and using a 30-gauge needle a hole was made in the right femoral medullary cavity., which was replaced with a 10-μL syringe. Four microliters of PBS containing 1.0 × 104 4 T1 tumor cells were injected into the intramedullary space of the femur. The syringe was kept in place for one extra minute to avoid 4 T1 cells from backing up through the injection track, a syringe was left in its place for one more minute. The injection site was locked with a gelatin sponge and washed multiple times with normal saline. The skin was sutured with 5–0 Ethicon Vicryl rapid suture. Following the procedure, animals were left to recover for 3 days prior to using them for further experiments (Lelekakis et al., 1999, Rosol et al., 2003).
2.6. Treatment with CMF cocktail of chemotherapy drugs
The cyclophosphamide, methotrexate and 5-fluorouracil (CMF) cocktail of drugs was prepared by mixing 10 mg/kg body weight. of cyclophosphamide, 1 mg/kg body weight. Methotrexate, and 10 mg/kg body weight of 5-Fluorouracil dissolved in normal saline. The CMF cocktail was intravenously administered on days 1 and 8 for 6 rounds with a gap of 14 days between each round. The above mentioned dosages of drugs were selected from previously published studies on rats (Tomoda et al., 2005, Witzel et al., 1992).
2.7. Experimental design
The OS rats were randomly divided into 5 groups designated as control, OS, OS + CMF, OS + CMF + LDS (low dose shilajit) and OS + CMF + HDS (high dose shilajit) with each group consisting of 7 animals. Control group rats received 0.9 % normal saline and served as normal healthy rats, OS group rats received 4 T1 cancer cells and served as osteosarcoma positive rats, OS + CMF group rats received a CMF cocktail of drugs, OS + CMF + LDS group rats received a low dose of shilajit (150 mg/kg body weight) and OS + CMF + HDS group rats received a high dose of shilajit (250 mg/kg body weight). Osteosarcoma was induced in rats as described above. Osteosarcoma rats were treated with a CMF cocktail of chemotherapy drugs with or without shilajit as detailed above. Osteosarcoma rats were treated with either a low dose (150 mg/ml) or a high dose (250 mg/ml) of shilajit to assess dose dependent effects.
Control and all the treatment group rats had free access to food and water. Animals were examined daily for any change in their behavior and food and water intake. Throughout the experimental period, animals were maintained in ambient temperature and relative humidity and allowed to have free access to food and water.
2.8. Blood sampling
Following the end of treatment duration, rats were anesthetized by diethyl ether and sacrificed by cervical dislocation. Blood was collected without anticoagulants. Coagulated blood was centrifuged at 300 rpm for 10 min and serum was separated and stored until used. Liver and kidney were resected and immediately frozen in liquid nitrogen and part of the organs were also stored in formalin solution for histopathological examinations.
2.9. Determination of serum liver and kidney functional markers
Liver and kidney functional markers were measured using standard methods or commercial kits. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzyme activities were quantified according to the methods described by (Pappas, 1989, Reichling and Kaplan, 1988). Serum bilirubin (Cat#MAk126), total protein (Cat#TPO100), and albumin (Cat#MAK125) were quantified using commercially available kits following the instructions of the manufacturer (MERCK, Germany). Serum creatinine, uric acid and urea were enzymatically measured based on the procedure detailed earlier (Fossati et al., 1980, Taussky, 1956). Serum alkaline phosphatase (ALP) (Cat#MAK447) levels were measured by commercial kits (MERCK, Germany).
2.10. Histopathological study
Liver and kidney specimens were collected and immediately fixed with10% formalin and dehydrated in increasing concentrations (70, 80 and 90 %) of ethanol. Specimen were further processed in xylene and finally paraffin embedded. About 4-µm sections were cut and stained with hematoxylin and eosin (H&E) dye. The H&E stained slides were visualized under a light microscope.
2.11. Statistical methods
SPSS Statistical software was used for statistical analysis (SPSS, Inc, Chicago, IL, USA). Data collected from different experiments were expressed as the mean ± SD. Spearman correlation was carried out to examine the correlation among liver and kidney functional markers. Significance of variance between the means was obtained using the Student’s t-test or by one-way analysis of variance (ANOVA). Significance was set at p < 0.05.
3. Results
3.1. Body weight change
Changes in body weights of control and treatment groups are presented in Table 1. Body weights of osteosarcoma rats were found to be significantly lower than those of control. Body weights of osteosarcoma rats treated with CMF cocktail showed significant improvement and further increased in response to co-treatment of rats with CMF cocktail and shilajit. Shilajit at 250 mg/kg dose exhibited a better effect on body weight than 150 mg/kg dose.
Table 1.
Body weight change in control and different groups of rats.
| Groups | Initial weight (g) (N = 7) |
Final weight (g) (N = 7) |
|---|---|---|
| Control | 220.34 ± 8.11* | 284.45 ± 7.23* |
| OS | 221.15 ± 8.09# | 96.74 ± 5.36# |
| OS + CMF | 221.61 ± 9.27*# | 106.91 ± 6.74*# |
| OS + CMF_LDS | 224.24 ± 4.12*# | 126.22 ± 5.28*# |
| OS + CMF + HDS | 221.58 ± 5.92*# | 161.05 ± 5.86*# |
OS, osteosarcoma; CMF, cocktail of chemotherapy drugs; LDS, low dose shilajit; HDS, high dose shilajit. Data are expressed as mean ± SD, *p < 0.05 as compared to control ans #p < 0.05 compared to OS group.
3.2. Liver markers
Levels of liver functional markers in serum are presented in Table 2. Serum levels of ALT, ALP and AST were significantly augmented in osteosarcoma rats as compared to those in control. Whereas albumin total protein and bilirubin were significantly declined in osteosarcoma rats as against in control. Osteosarcoma rats treated with CMF cocktail displayed a significant decrease in ALT, AST and ALP levels and a significant spike in albumin, total protein and bilirubin as compared to those in untreated osteosarcoma rats. Co-treatment of CMF cocktail and shilajit at 150 mg/kg or 250 mg/kg concentrations led to a further significant decline in ALT, AST and ALP and a significant increase in albumin, total protein and bilirubin compared to those in osteosarcoma rats treated only with CMF cocktail. Importantly, Shilajit at 250 mg/kg concentration had significantly more positive effects on the studied functional markers as matched to that with 150 mg/kg concentration of shilajit.
Table 2.
Liver functional parameters in the serum of control and different groups of rats.
| Groups | Parameters |
|||||
|---|---|---|---|---|---|---|
| Bilirubin (mg/dl) n (7) |
TP (g/dl) n (7) |
Albumin (g/dl) n (7) |
AST (U/L) n (7) |
ALT (U/L) n (7) |
ALP mcg/L n (7) |
|
| Control | 0.43 ± 0.020# | 7.64 ± 0.15# | 4.92 ± 0.298# | 19.85 ± 2.91# | 19.28 ± 3.03# | 223.28 ± 26.47# |
| OS | 2.20 ± 0.22* | 3.35 ± 0.25* | 1.54 ± 0.222* | 100.28 ± 5.76* | 99.85 ± 5.87* | 698.00 ± 9.00* |
| OS + CMF | 1.64 ± 0.198*# | 4.88 ± 0.27*# | 2.01 ± 0.307*# | 81.42 ± 3.20*# | 80.71 ± 4.71*# | 593.00 ± 30.5*# |
| OS + CMF_LDS | 0.89 ± 0.028*# | 5.71 ± 0.22*# | 3.04 ± 0.171*# | 66.42 ± 4.68*# | 64.00 ± 1.82*# | 432.28 ± 16.5*# |
| OS + CMF + HDS | 0.67 ± 0.026*# | 6.45 ± 0.17*# | 4.10 ± 0.230*# | 56.71 ± 2.28*# | 49.57 ± 1.71*# | 350.71 ± 40.5*# |
OS, osteosarcoma; CMF, cocktail of chemotherapy drugs; LDS, low dose shilajit; HDS, high dose shilajit; TP, total protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALT, alkaline phosphatase. Data are expressed as mean ± SD, *p < 0.05 as compared to control ans #p < 0.05 compared to OS group.
3.3. Kidney functional markers
Kidney functional markers in the serum of control and treated rats are provided in Table 3. Compared to control, osteosarcoma rats exhibited a significant increase in creatinine, uric acid (UA) and urea. Treatment of osteosarcoma rats with CMF cocktail resulted in a significant reduction in all the studied markers matched to those in untreated osteosarcoma rats. On the other hand, combined treatment of CMF cocktail and shilajit at 150 or 250 mg/kg concentration further lowered creatinine, urea, and uric acid as compared to CMF cocktail alone treatment. Shilajit at 250 mg/kg dose exerted a superior effect in reducing these kidney markers than 150 mg/kg dose as shown in Table 4.
Table 3.
Kidney functional parameters in serum of control and different groups of rats.
| Groups | Parameters |
||
|---|---|---|---|
| Uric acid (mg/dl)n (7) |
Urea mg/dLn (7) |
Creatinine (mg/dl)n (7) |
|
| Control | 0.51 ± 0.035# | 20.85 ± 2.11# | 4.04 ± 0.097# |
| OS | 2.40 ± 0.23* | 73.14 ± 2.26* | 7.41 ± 0.22* |
| OS + CMF | 1.97 ± 0.13*# | 61.71 ± 3.14*# | 7.00 ± 0.31*# |
| OS + CMF_LDS | 1.62 ± 0.11*# | 54.14 ± 3.1*# | 6.17 ± 0.075*# |
| OS + CMF + HDS | 0.87 ± 0.067*# | 38.42 ± 2.87*# | 4.98 ± 0.106*# |
OS, osteosarcoma; CMF, cocktail of chemotherapy drugs; LDS, low dose shilajit; HDS, high dose shilajit; Data are expressed as mean ± SD, *p < 0.05 as compared to control ans #p < 0.05 compared to OS group.
Table 4.
Correlation co-efficient (r) among the studied liver and kidney functional markers.
| ALT | AST | UA | Creatinine | Alp | |
|---|---|---|---|---|---|
| ALT | 0.985** | 0.972** | 0.961* | 0.967* | |
| AST | 0.985** | 0.955** | 0.939* | 0.953* | |
| UA | 0.972** | 0.955* | 0.973** | 0.964** | |
| Creatinine | 0.961** | 0.939* | 0.973** | 0.948* | |
| Alp | 0.967** | 0.953* | 0.964** | 0.948* |
TP, total protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALT, alkaline phosphatase. Data are expressed as mean ± SD, *p < 0.05 as compared to control ans #p < 0.05 compared to OS group. *p < 0.05; **p < 0.01.
3.4. Correlations among the liver and kidney functional markers
Correlation coefficient values among the serum liver and kidney functional indices are shown in Table 2. It was found that liver and kidney functional markers included ALT, AST, and ALP. Uric acid and creatinine were significantly and positively correlated with each other. Whereas no significant correlations were found among other studied functional indices of liver and kidney.
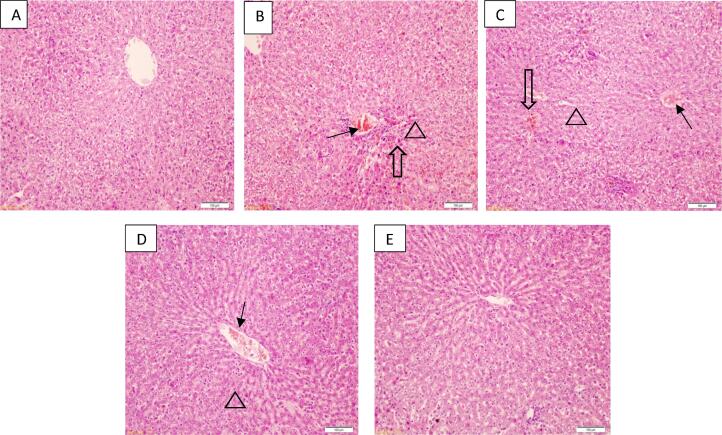
3.5. Histopathology of liver tissue
Histopathological changes in liver tissue of control and treated groups are presented in Fig. 1. Hepatocytes, central vein, and sinusoids demonstrated different morphological changes in liver tissue of osteosarcoma than those in the control negative group. The distinct differences such as ballooning degeneration of hepatocytes, moderate inflammation, and central vein and sinusoidal congestion were noticed in osteosarcoma liver (Fig. 1 B), whereas the normal histology was seen in hepatic tissue of control rats (Fig. 1 A). Histology of liver tissue from osteosarcoma rats treated with CMF cocktail showed a reduced inflammation as compared to that in CMF untreated osteosarcoma rats (Fig. 1 C). While rats treated with CMF cocktail and shilajit at 150 mg/kg concentration displayed further inhibition of the hepatic inflammation and hepatocytes, and blood sinusoids were more similar to that of the control group (Fig. 1D). Liver histology of rats treated with CMF cocktail and shilajit at 250 mg/kg body weight revealed that inhibition of the inflammatory reactions and significant improvement in the histological changes when compared to that in osteosarcoma induced rats and other treated groups and the histological features of hepatocytes, and blood sinusoids were more identical to that of the control than any other group (Fig. 1E).
Fig. 1.
H&E stained liver tissue sections from control (A), OS (B), OS + CMF (C), OS + CMF + LDS (D), OS + CMF + HDS (E) rats. Histology of osteosarcoma rats (OS group) displaying ballooning degeneration of hepatocytes (indicated by triangle), sinusoid congestion (indicated by hallow arrow) and inflammation (arrow) as against normal histology seen in the control group. Tissue sections from OS + CMF groups showed regeneration of hepatocytes but lesser sinusoid congestion and moderate inflammation, Rats from OS + CMF + LDS group demonstrated reduced degeneration, no sinusoid congestion and mild inflammation in liver tissues, whereas near normal histology was observed in tissue sections from OS + CMF + HDS group rats.
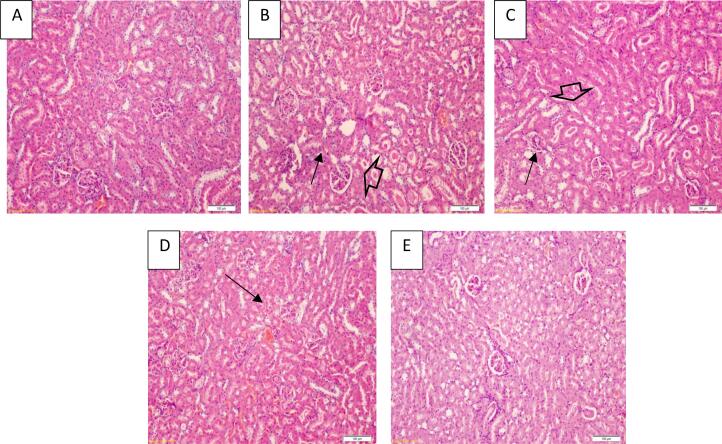
3.6. Histopathology of kidney tissue
Histopathological changes in kidney tissue of control and different treatment groups are presented in Fig. 2. Kidney tissue of osteosarcoma rats demonstrated shrinkage of glomerular tuft and tubular atrophy when compared with the normal renal tissue (Fig. 2 A &B). On other hand, mild tubular atrophy and slightly congested glomeruli were found in osteosarcoma rats treated with CMF cocktail as compared to untreated osteosarcoma rats (Fig. 2 C). A further improvement was noted in tubular atrophy and glomeruli of the kidney of rats treated with CMF cocktail and shilajit at 150 mg/kg dose (Fig. 2 D). Histology of kidney tissue of rats treated with CMF cocktail and shilajit at 250 mg/kg concentration displayed near normal features that were comparable to a normal control group (Fig. 2E).
Fig. 2.
H&E stained kidney tissue sections from control (A), OS (B), OS + CMF (C), OS + CMF + LDS (D), OS + CMF + HDS (E) rats. Histology of osteosarcoma rats (OS group) displaying shrinking of glomerular tuft (arrow) and tubular atrophy (hallow arrow) as compared to normal histology seen in the control group. Tissue sections from OS + CMF groups showed slightly congested glomeruli and moderate tubular atrophy. Rats from OS + CMF + LDS group demonstrated no glomeruli congestion and mild tubular atrophy, whereas no apparent histological changes were noted in kidney tissue sections from OS + CMF + HDS group rats.
4. Discussion
In the present study, we assessed the possible beneficial effects of shilajit in combination with chemotherapeutic drugs in mitigating the detrimental effects of metastasis of osteosarcoma on the liver and kidney tissue in a rat model. Effects of shilajit were measured at two different doses; one at a higher concentration and the other at a lower concentration. The effect of shilajit on these tissues was evaluated by measuring their key functional components as well as by examining histopathological changes. Shilajit is a well-known medicinal plant based product that has been used in traditional medicine such as Ayurveda (Jafari et al., 2019, Schepetkin et al., 2003). The medicinal properties of shilajit are mainly attributed to its antioxidant properties (Schepetkin et al., 2003).
Osteosarcoma is the commonest primary bone tumor found in adolescents and children. OS is characterized by osteogenic progenitor cells or mesenchymal cells producing immature bone and osteoid (Bielack et al., 2009). Recent investigations have shown that osteosarcoma can metastasize to the liver and kidney (Dalinka et al., 1971. Rats in which osteosarcoma was induced displayed decreased body weight, and altered levels of liver and kidney functional markers. Besides these unfavorable changes to functional markers, osteosarcoma rats also demonstrated marked histological changes in the liver and kidney tissues. These findings clearly indicate the pathological features of metastasis of osteosarcoma to the liver and kidney. Treatment of these osteosarcoma rats with shilajit at low and high doses in combination with CMF cocktail drug significantly increased the body weight, lowered the liver functional markers such as ALT, ALP, AST and bilirubin and significantly augmented albumin and total protein. Importantly shilajit at a higher dose exerted superior activity than a lower dose in restoring the levels of these functional markers. Similarly, shilajit treatment of osteosarcoma rats at low and high doses along with CMF cocktails led to a marked decrease in osteosarcoma induced levels of urea, creatinine and uric acid. As in the case of its effect on liver functional markers, shilajit at higher concentration showed improved action in lowering kidney functional markers than at lower concentration.
Histological analysis of liver and kidney tissues from osteosarcoma rats treated with shilajit and CMF cocktail drug revealed a significant restoration of osteosarcoma induced histopathological changes in these tissues. Collectively above data underscore the protective and favorable effects of shilajit against metastasis of osteosarcoma to the liver and kidney. Besides, superior effects of shilajit at a higher dose than a lower dose lend support to its efficacy and potential as a treatment drug. Importantly, shilajit further improved the effectiveness of the CMF cocktail drug, a widely used chemotherapy drug, on liver and kidney functions and the architecture of these tissues. Correlation coefficient analysis of studied functional markers of liver and kidney revealed a positive correlation among them, which suggests an inter-relationship among the functional markers.
To our understanding, this is the first study to report the protective effects of shilajit against metastasis induced damage to liver and kidney in osteosarcoma in addition to the effect of chemotherapy drugs. Shilajit, however, has been shown to exert multiple beneficial effects under different pathological conditions. For instance, shilajit induced apoptosis and inhibited hepatic cancer cells through modulating oxygen species (Pant et al., 2016). Shilajit was able to impart a protective effect and prevent histopathological damage to liver tissue in high fat-diet induced non-alcoholic fatty liver disease (NAFLD) in rats (Ghezelbash et al., 2020). Shilajit also favorably altered serum levels of adipocytokines and insulin resistance via modulating inflammation in a NAFLD rat model (Ghezelbash et al., 2022). Besides its protective effects on the liver and kidney, shilajit has also been shown to possess potential chemotherapeutic effects against other cancer types. For example, shilajit caused cell cycle arrest and induced apoptosis in urinary bladder cancer (Kloskowski et al., 2021). In human breast cancer cells, shilajit induced apoptosis through the inhibition of epithelial-mesenchymal transition (Rahmani Barouji et al., 2020b). In this study, the body weights of rats treated with shilajit were also found to be increased as matched to untreated osteosarcoma rats. This effect could possibly be attributed to improved function of the liver after shilajit treatment. In contrast to our finding on body weight, in a study, no significant change in body weight was noticed in humans after 45-day consumption of shilajit (Sharma et al., 2003). A possible mechanism underlying the beneficial effects of shilajit may include its suppressive action on tumor-promoting genes and concomitant activation of tumor-suppressive genes. Shilajit may have also directly or indirectly sensitized the biomolecules targeted by chemotherapeutic drugs thereby increasing their effect on salvaging the liver and kidney functions. To conclude, this study showed that shilajit possesses potentiating properties to augment the effects of chemotherapeutic drugs to abrogate the effect of metastasis on liver and kidney functions and tissue architecture.
Contribution
FAA-Conceptualized the study, data analysis and review of the manuscript, EJJ- carried out the experiments and manuscript preparation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors are grateful to the Deanship of Scientific Research. Project No. (KEP-PhD-47-130-38).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ebtihaj J. Jambi, Email: ejjambi@kau.edu.sa.
Fawzia Abdulaziz Alshubaily, Email: falshibli@kau.edu.sa.
References
- Agarwal S.P., Khanna R., Karmarkar R., Anwer M.K., Khar R.K. Shilajit: a review. Phytother. Res. 2007;21:401–405. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- Aldakheel R.K., Gondal M.A., Alsayed H.N., Almessiere M.A., Nasr M.M., Shemsi A.M. Rapid Determination and Quantification of Nutritional and Poisonous Metals in Vastly Consumed Ayurvedic Herbal Medicine (Rejuvenator Shilajit) by Humans Using Three Advanced Analytical Techniques. Biol. Trace Elem. Res. 2021 doi: 10.1007/s12011-021-03014-4. [DOI] [PubMed] [Google Scholar]
- Bhattarai J.P., Cho D.H., Han S.K. Activation of Strychnine-Sensitive Glycine Receptors by Shilajit on Preoptic Hypothalamic Neurons of Juvenile Mice. Chin. J. Physiol. 2016;59:39–45. doi: 10.4077/CJP.2016.BAE361. [DOI] [PubMed] [Google Scholar]
- Bielack S., Jurgens H., Jundt G., Kevric M., Kuhne T., Reichardt P., Zoubek A., Werner M., Winkelmann W., Kotz R. Osteosarcoma: the COSS experience. Cancer Treat. Res. 2009;152:289–308. doi: 10.1007/978-1-4419-0284-9_15. [DOI] [PubMed] [Google Scholar]
- Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., Zoubek A., Jurgens H., Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- Calfio C., Gonzalez A., Singh S.K., Rojo L.E., Maccioni R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimers Dis. 2020;77:33–51. doi: 10.3233/JAD-200443. [DOI] [PubMed] [Google Scholar]
- Carrasco-Gallardo C., Farias G.A., Fuentes P., Crespo F., Maccioni R.B. Can nutraceuticals prevent Alzheimer’s disease? Potential therapeutic role of a formulation containing shilajit and complex B vitamins. Arch. Med. Res. 2012;43:699–704. doi: 10.1016/j.arcmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Carrasco-Gallardo C., Guzmán L., Maccioni R.B. Shilajit: a natural phytocomplex with potential procognitive activity. Int J Alzheimers Dis. 2012;2012:1–4. doi: 10.1155/2012/674142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalinka M.K., Fiveash A.E., Aston J.K. Metastatic extraosseous osteosarcoma to the liver: a case demonstrated by 85 Sr and 99m Tc-colloid scanning. J. Nucl. Med. 1971;12:754–755. [PubMed] [Google Scholar]
- Das A., Datta S., Rhea B., Sinha M., Veeraragavan M., Gordillo G., Roy S. The Human Skeletal Muscle Transcriptome in Response to Oral Shilajit Supplementation. J. Med. Food. 2016;19:701–709. doi: 10.1089/jmf.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N.C., Kaste S.C., Hill D.A., Kun L.E., Pratt C.B. Metastatic osteosarcoma to the liver after treatment for synovial sarcoma: a case report. Pediatr. Hematol. Oncol. 2001;18:123–128. doi: 10.1080/088800101300002955. [DOI] [PubMed] [Google Scholar]
- Fossati P., Prencipe L., Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980;26:227–231. [PubMed] [Google Scholar]
- Ghasemkhani N., Tabrizi A.S., Namazi F., Nazifi S. Treatment effects of Shilajit on aspirin-induced gastric lesions in rats. Physiol Rep. 2021;9:e14822. doi: 10.14814/phy2.14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezelbash B., Shahrokhi N., Khaksari M., Ghaderi-Pakdel F., Asadikaram G. Hepatoprotective effects of Shilajit on high fat-diet induced non-alcoholic fatty liver disease (NAFLD) in rats. Horm Mol Biol Clin Investig. 2020;41 doi: 10.1515/hmbci-2019-0040. [DOI] [PubMed] [Google Scholar]
- Ghezelbash B., Shahrokhi N., Khaksari M., Asadikaram G., Shahrokhi M., Shirazpour S. Protective Roles of Shilajit in Modulating Resistin, Adiponectin, and Cytokines in Rats with Non-alcoholic Fatty Liver Disease. Chin J Integr Med. 2022;28(6):531–537. doi: 10.1007/s11655-022-3307-3. [DOI] [PubMed] [Google Scholar]
- Ghosal S., Reddy J.P., Lal V.K. Shilajit I: chemical constituents. J. Pharm. Sci. 1976;65:772–773. doi: 10.1002/jps.2600650545. [DOI] [PubMed] [Google Scholar]
- Gorlick R., Anderson P., Andrulis I., Arndt C., Beardsley G.P., Bernstein M., Bridge J., Cheung N.K., Dome J.S., Ebb D., Gardner T., Gebhardt M., Grier H., Hansen M., Healey J., Helman L., Hock J., Houghton J., Houghton P., Huvos A., Khanna C., Kieran M., Kleinerman E., Ladanyi M., Lau C., Malkin D., Marina N., Meltzer P., Meyers P., Schofield D., Schwartz C., Smith M.A., Toretsky J., Tsokos M., Wexler L., Wigginton J., Withrow S., Schoenfeldt M., Anderson B. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin. Cancer Res. 2003;9:5442–5453. [PubMed] [Google Scholar]
- Hagay Z.J., Zirkin H.J., Moses M., Khodadadi J. Ewing’s sarcoma metastatic to focal nodular hyperplasia of liver. J. Surg. Oncol. 1986;32:100–105. doi: 10.1002/jso.2930320212. [DOI] [PubMed] [Google Scholar]
- Jafari M., Forootanfar H., Ameri A., Foroutanfar A., Adeli-Sardou M., Rahimi H.R., Najafi A., Zangiabadi N., Shakibaie M. Antioxidant, cytotoxic and hyperalgesia-suppressing activity of a native Shilajit obtained from Bahr Aseman mountains. Pak J Pharm Sci. 2019;32:2167–2173. [PubMed] [Google Scholar]
- Kager L., Zoubek A., Pötschger U., Kastner U., Flege S., Kempf-Bielack B., Branscheid D., Kotz R., Salzer-Kuntschik M., Winkelmann W., Jundt G., Kabisch H., Reichardt P., Jürgens H., Gadner H., Bielack S.S. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003;21(10):2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- Kaste S.C., Pratt C.B., Cain A.M., Jones-Wallace D.J., Rao B.N. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86:1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Jung J.H., Chung W.S., Lee C.H., Jang H.J. Ferulic Acid Induces Keratin 6alpha via Inhibition of Nuclear beta-Catenin Accumulation and Activation of Nrf2 in Wound-Induced Inflammation. Biomedicines. 2021;9 doi: 10.3390/biomedicines9050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloskowski T., Szeliski K., Krzeszowiak K., Fekner Z., Kazimierski L., Jundzill A., Drewa T., Pokrywczynska M. Mumio (Shilajit) as a potential chemotherapeutic for the urinary bladder cancer treatment. Sci. Rep. 2021;11:22614. doi: 10.1038/s41598-021-01996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite T.C., Watters R.J., Weiss K.R., Intini G. Avenues of research in dietary interventions to target tumor metabolism in osteosarcoma. Journal of Translational Medicine. 2021;19 doi: 10.1186/s12967-021-03122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelekakis M., Moseley J.M., Martin T.J., Hards D., Williams E., Ho P., Lowen D., Javni J., Miller F.R., Slavin J., Anderson R.L. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis. 1999;17:163–170. doi: 10.1023/A:1006689719505. [DOI] [PubMed] [Google Scholar]
- Mialou V., Philip T., Kalifa C., Perol D., Gentet J.C., Marec-Berard P., Pacquement H., Chastagner P., Defaschelles A.S., Hartmann O. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome–the French pediatric experience. Cancer. 2005;104:1100–1109. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odri G.A., Tchicaya-Bouanga J., Yoon D.J.Y., Modrowski D. Metastatic Progression of Osteosarcomas: A Review of Current Knowledge of Environmental versus Oncogenic Drivers. Cancers (Basel) 2022;14(2):360. doi: 10.3390/cancers14020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant K., Gupta P., Damania P., Yadav A.K., Gupta A., Ashraf A., Venugopal S.K. Mineral pitch induces apoptosis and inhibits proliferation via modulating reactive oxygen species in hepatic cancer cells. BMC Complement Altern Med. 2016;16:148. doi: 10.1186/s12906-016-1131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas N.J. Enhanced cardiac enzyme profile. Clin. Lab. Med. 1989;9(4):689–716. [PubMed] [Google Scholar]
- PosthumaDeBoer J., Witlox M.A., Kaspers G.J., van Royen B.J. Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature. Clin. Exp. Metastasis. 2011;28:493–503. doi: 10.1007/s10585-011-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo A.C.S., Borghesi J., Noratto G.D. The role of dietary polyphenols in osteosarcoma: A possible clue about the molecular mechanisms involved in a process that is just in its infancy. J. Food Biochem. 2022;46 doi: 10.1111/JFBC.14026. [DOI] [PubMed] [Google Scholar]
- Rahmani Barouji S., Saber A., Torbati M., Fazljou S.M.B., Yari Khosroushahi A. Health Beneficial Effects of Moomiaii in Traditional Medicine. Galen Med J. 2020;9:e1743. doi: 10.31661/gmj.v9i0.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Barouji S., Shahabi A., Torbati M., Fazljou S.M.B., Yari Khosroushahi A. Mummy Induces Apoptosis Through Inhibiting of Epithelial-Mesenchymal Transition (EMT) in Human Breast Cancer Cells. Galen Med J. 2020;9:e1812. doi: 10.31661/gmj.v9i0.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling J.J., Kaplan M.M. Clinical use of serum enzymes in liver disease. Dig. Dis. Sci. 1988;33:1601–1614. doi: 10.1007/BF01535953. [DOI] [PubMed] [Google Scholar]
- Rosol T.J., Tannehill-Gregg S.H., LeRoy B.E., Mandl S., Contag C.H. Animal models of bone metastasis. Cancer. 2003;97:748–757. doi: 10.1002/cncr.11150. [DOI] [PubMed] [Google Scholar]
- Schepetkin I.A., Khlebnikov A.I., Ah S.Y., Woo S.B., Jeong C.S., Klubachuk O.N., Kwon B.S. Characterization and biological activities of humic substances from mumie. J. Agric. Food Chem. 2003;51:5245–5254. doi: 10.1021/jf021101e. [DOI] [PubMed] [Google Scholar]
- Sharma P., Jha J., Shrinivas V., Dwivedi L.K., Suresh P., Sinha M. Shilajit: evalution of its effects on blood chemistry of normal human subjects. Anc Sci Life. 2003;23:114–119. [PMC free article] [PubMed] [Google Scholar]
- Sigdel P.R., Gnyawali D., Parajuli P., Chudal S., Pandit D., Guragain B., Pradhan M.M., Poudyal S., Chapagain S., Luitel B.R., Chalise P.R., Sharma U.K., Goel A. Renal Metastasis of Osteosarcoma with IVC Thrombus. Case Rep Urol. 2021;2021:1–3. doi: 10.1155/2021/8882593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller C.A., Craft A.W., Corazziari I. Survival of children with bone sarcoma in Europe since 1978: results from the EUROCARE study. Eur. J. Cancer. 2001;37(6):760–766. doi: 10.1016/s0959-8049(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Taussky H.H. A procedure increasing the specificity of the Jaffe reaction for the determination of creatine and creatinine in urine and plasma. Clin. Chim. Acta. 1956;1:210–224. doi: 10.1016/0009-8981(56)90067-5. [DOI] [PubMed] [Google Scholar]
- Tomoda R., Seto M., Hioki Y., Sonoda J., Matsumine A., Kusuzaki K., Uchida A. Low-dose methotrexate inhibits lung metastasis and lengthens survival in rat osteosarcoma. Clin. Exp. Metastasis. 2005;22:559–564. doi: 10.1007/s10585-005-5377-y. [DOI] [PubMed] [Google Scholar]
- Whelan J., McTiernan A., Cooper N., Wong Y.K., Francis M., Vernon S., Strauss S.J. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int. J. Cancer. 2012;131:E508–E517. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- Witzel J.G., Prescher A., Weisser H. Experimental animal model for the evaluation of chemotherapeutical effects on osteosarcoma. Chemotherapy. 1992;38:251–260. doi: 10.1159/000239008. [DOI] [PubMed] [Google Scholar]