Abstract
This study investigates the sounds and the anatomy of the sound‐producing organ in the male and female sand‐dwelling cusk‐eel Parophidion vassali. Although both sexes have similar external phenotype, they can be distinguished by their sonic apparatus and sounds. As in many Ophioidei, Parophidion vassali presents a panel of highly derived characters. Fish possess three pairs of sonic muscles, and males have mineralized swimbladder caps on which inserts the ventral sonic muscle, a neural arch that pivots, a stretchable swimbladder fenestra, an osseous swimbladder plate and a rounded pressure‐release membrane in the caudal swimbladder. Females, however, do not possess anterior swimbladder caps, a swimbladder fenestra and the caudal rounded membrane. Males possess the unusual ability to produce sounds starting with a set of low amplitude pulses followed by a second set with higher amplitudes clearly dividing each sound unit into two parts. Females do not vary their sound amplitude in this way: they produce shorter sounds and pulse periods but with a higher peak frequency. Morphology and sound features support the sound‐producing mechanism is based on a rebound system (i.e. quick backward snap of the anterior swimbladder). Based on features of the sounds from tank recordings, we have putatively identified the sound of male Parophidion vassali at sea. As these species are ecologically cryptic, we hope this work will allow assessment and clarify the distribution of their populations.
Keywords: acoustic, cusk‐eel, gonads, ophidiiform, sonic muscles
This study investigates the sounds and the anatomy of the sound‐producing organ in the dwelling cusk‐eel. Males produce sounds starting with a set of low amplitude pulses followed by a second set with higher amplitudes clearly dividing each sound unit into two parts. Females produce shorter sounds and pulse periods but with a higher peak frequency. Differences in sounds correspond to differences in sound‐producing mechanisms.

1. INTRODUCTION
Among vocal teleost species, the Ophidiiformes are particularly interesting because most species have extensive anatomical modifications related to sound production in both sexes (Howes, 1992). Modifications include anterior vertebrae and associated bony structures such as epineurals, the development of two to three pairs of sonic muscles and various specialisations in swimbladder anatomy (Parmentier & Diogo, 2006). Their swimbladder wall possesses two main layers: a thin tunica interna and a thick tunica externa that can be further modified. Different species, for example, possess a swimbladder fenestra, a small thin dorsolateral band deprived of the tunica externa (Parmentier, Gennotte, et al., 2003; Parmentier, Vandewalle, & Lagardère, 2003). The fenestra is consequently more flexible and allows independent movements between regions of the bladder enabling sound production. Different species also have modifications of the tunica externa related to the insertion of sound‐producing muscles on the swimbladder. These include thickening of the tunica externa in Carapus spp. (Parmentier et al., 2002), sclerification of delineated zones in front of the swimbladder in Echiodon spp. (Markle & Olney, 1990), development of “cartilaginous” caps in Ophidion marginatum and the development of a skeletal piece called the rocker bone in different Ophidion (Kéver, Boyle, et al., 2014; Parmentier, Compère, et al., 2008; Rose, 1961) and Onuxodon species (Courtenay & McKittrick, 1970; Kéver, Colleye, et al., 2014; Parmentier et al., 2002; Tyler, 1970). Furthermore, a remarkable sexual dimorphism of the sound‐producing apparatus contrasts with the absence of sexual dimorphism of the external phenotype in different Ophidiiformes. For example, the rocker bone in different Ophidion sp is found only in males (Casadevall et al., 1996; Parmentier, Fontenelle, et al., 2006; Rose, 1961), and males of Lepophidium sp possess larger sonic muscles than females (Fine et al., 2007, 2018). As a result, important differences between acoustic features of social sounds can be found between males and females (Kéver et al., 2012).
These morphological features clearly demonstrate that Ophidiiforme species invest massively in acoustic communication. They produce highly species‐specific sounds that possess a high‐discrimination potential and could be helpful for both conspecifics and for studies using passive acoustic monitoring (Kéver et al., 2016; Picciulin et al., 2019). The selective pressures leading to deep modifications of the sonic phenotypes likely relate to their ecology which severely impair other forms of intraspecific communication to identify species presence and distribution (e.g. visual cues). These include nocturnal and sand‐dwelling habits in coastal areas and the colonisation of the aphotic zone, where this taxon is extremely successful and ubiquitous (Marshall, 1967; Merret & Haedrich, 1997; Priede, 2017). These ways of living impede observation and access to specimens, impairing scientific understanding of these species, known mostly from museum specimens (Nielsen et al., 1999). Therefore, sounds have been recorded and described in a small number of Ophioidei species (e.g. Mann et al., 1997; Mooney et al., 2016; Parmentier et al., 2016; Parmentier, Bahri, et al., 2018; Parmentier, Fine, et al., 2018; Parmentier, Vandewalle, & Lagardère, 2003), that is seven Carapidae, two Ophidion (Ophidiidae) and two Genypterus (Ophidiidae). Sound‐producing abilities in other species were deduced from their internal anatomy (Ali et al., 2016; Fine et al., 2007; Howes, 1992; Nguyen et al., 2008; Parmentier, Fine, et al., 2018).
The ophidiid Parophidion vassali (Risso, 1810) is an eel‐like Mediterranean species found in coastal areas in Posidonia oceanica meadows and shallow rocky‐sandy habitats (Capape et al., 2016; Pergent et al., 2012). It is, however, suspected to inhabit deeper waters up to 600 m (Matallanas & Casadevall, 1990). Similarly, the vocal Ophidion rochei and Ophidion marginatum, they are nocturnal carnivores (Stergiou & Karpouzi, 2002), hiding in the sand during the day and becoming active at night. These nocturnal activities and an intimate association with the substrate likely relate to the development of barbels derived from the pelvic fins. During locomotion, these barbels continuously sweep the seafloor, allowing the fish to follow the bottom topography (Codina et al., 2012). In Parophidion species, the swimbladder has been described as “a short, thick air bladder with a large posterior opening” (Moods et al., 1951; Tortonese, 1954), similar to O. marginatum (Courtenay, 1971). Although there are differences in the sound‐producing mechanism, O. rochei possesses also this large rounded special structure on the caudal edge of its elongated swimbladder (Casadevall et al., 1996) as do male Hoplobrotula armata (Ali et al., 2016) and Dicrolene intronigra (Fine et al., 2018). These common anatomical characteristics, the similarity in their way of life and their close phylogenetic position, suggest that Parophidion vassali relies on acoustic communication. In this study, we describe the sound‐producing apparatus in Parophidion vassali males and females and we characterise their sounds. This first study provides more information on the fish biology and helps identify the species in passive acoustic recordings.
2. MATERIAL AND METHODS
All procedures were approved by the ethical commission of the University of Liège (ethics case 1759).
2.1. Recordings in controlled conditions
Parophidion vassali specimens (total length = 125–165 mm), caught with a small dredge in La Ciotat Bay in France (43°10′19”N, 5°38′39″E), were kept in tanks with running sea water before being sent to the laboratory in Liège. This study used different lots for a total of 50 individuals: ten specimens in 2018, 15 in 2019, 4 in 2020 and 21 in 2021. For each lot, fish were caught in February, sent to Liège in March / April and recorded between April and June.
Sound Recording. In Liège, fish were housed in a glass community tank (0.8 m × 0.3 m × 0.3 m) filled with seawater (31 mg/L), and with a 10 cm sandy bottom, and were maintained at 21 ± 2°C with a 12–12 h light–dark cycle. Fish were fed ad libitum with living artemia and small amphipods.
Parophidion vassali spent the day buried in the sand and started to emerge ca. 1 h after the lights were turned off. Fish were recorded between 7 pm and midnight.
In the community tank, the hydrophone was placed in the middle of the tank, 10 cm below the surface. In 2018, recording continued over 10 days (n = 1–5 specimens in the tank) from March to August; in 2019, 6 days from March to May (n = 10–15 specimens); in 2020, 6 days in March, (n = 4 specimens) and in 2021, 3 days in April (n = 1–7 specimens). Because of the night recordings and the lack of external sexual dimorphism, identification of sound‐emitting individuals is arduous in the community tank. However, we took advantage from the fact that P. vassali also emit sound when alone in the tank to overcome these issues. Fish were successively isolated in a tank (1 m × 0.4 m × 0.4 m) 24 h before recordings. One isolated fish was recorded in 2018 and eight isolated individuals were recorded in 2021. The nine fish were subsequently euthanized and dissected for sex identification. In each case, recordings in the community and isolated tanks lasted from 19:00 to 22:30. The resonant frequency of each recording tank was calculated (3.6 and 2.7 kHz) using the formula of Akamatsu (Akamatsu et al., 2002).
2.2. Sound recording devices
In 2018, 2019 and 2021, we used a calibrated Aquarian H2a hydrophone (sensitivity: ‐ 180 dB re 1 V μPa−1; flat frequency response range 10 Hz–100 kHz; Aquarian Audio, USA) connected to a Handy H1 portable audio recorder (sampling frequency: 44.1 kHz, 24‐bit resolution; Zoom, UK). In 2020 and 2021, we also used an HTI‐96‐Min hydrophone (sensitivity: −163.9 dB re 1 V μPa−1; flat frequency response range 2 Hz–30 kHz; High Tech, Inc.) connected to a TASCAM DR‐05 portable audio recorder (sampling frequency: 44.1 kHz, 16‐bit resolution; TEAC).
2.3. Field recordings
Passive acoustic recordings were made in different locations in Posidonia meadows around the bay of La Ciotat (France). Two types of autonomous acoustic recorders (i.e. SNAP and EA‐SDA14) were used. SNAP recorders (Loggerhead Instruments), connected to an HTI96‐min hydrophone (sensitivity −170 re 1 V μPa−1; flat frequency response 2 Hz‐30 kHz; High Tech Inc.), were deployed i) in Figuerolle Bay, France (5 m depth, 43°09′55.3”N, 5°35′46.1″E) in 2015, from August 22nd to 27th, and ii) in La Ciotat Bay, France (22 m depth, 43°10′19”N, 5°38′39″E) in 2018, from August 27th to 30th. Systems were programmed to record for 10 min every 60 min (sampling frequency of 44.1 kHz, 16‐bit resolution).
Two EA‐SDA14 recorders (RTSYS; Caudan, France), connected to an HTI‐92‐WB hydrophone (sensitivity −155 re 1 V μPa−1, flat frequency response 5 Hz‐50 kHz; High Tech Inc.) were deployed in La Ciotat Bay in 2016, from June 2nd to 3rd). The first was placed at a depth of 33 m (43°10′25.4”N, 5°39′47.6″E) and the second at a depth of 15 m (43°10′43.3”N, 5°39′51.8″E). Recording were continuous (sampling frequency of 78 kHz, 24‐bit resolution).
2.4. Sound analysis
Sounds were analysed using the software Avisoft SAS‐Lab Pro 4.5, version 5.2.14 (Avisoft Bioacoustics©). Sound files were filtered (2 kHz) to avoid high‐frequency reverberations of aquarium walls (Akamatsu et al., 2002). Temporal and amplitude features were measured from oscillograms and spectral features from power spectra (FFT size = 512, Hamming). Measured acoustic features included: sound duration (ms), number of pulses, pulse period (peak‐to‐peak interval between two consecutive pulses, ms), pulse dominant frequency of each single pulse (Hz). Relative amplitude was also measured for each pulse allowing within sound comparisons. Since the sounds were seemingly very loud, the maximal SPL was also calculated for sound recorded in captivity. For the calculation of the SPL we used a recording chain made of H2a hydrophone connected to a ZOOM H1 digital recorder). During a previous study, a comparative calibration of different recording chains, including the one used in this present study, was carried out. This comparative calibration has an error of ±3 dB. SPL was calculated in Matlab thanks to a custom‐build script (Bolgan et al., 2019). In this study, we used the same custom‐built script and global sensitivity factor; in particular, SPL was calculated as root mean square (rms) and as peak to peak (p–p) in the frequency range 100–900 Hz, from the start to the end of each sound, and by using a sample rate of 4 kHz and FFT size of 256.
2.5. Morphology and histology
Forty Parophidion vassali specimens were euthanized using an overdose of tricaine methanesulfonate solution (CAS: 886–86‐2). Specimens were fixed in a 5% formalin solution for 2 days and then transferred to 70% ethyl alcohol. Five males (TL 12.5–16 cm) and three females (TL: 13.8–14.1 cm) were dissected and examined using a Wild M10 binocular microscope (Leica Microsystems GmbH, Germany) equipped with a camera lucida. After formalin fixation, two additional males (TL: 14.2 and 15.7 cm) were dehydrated in butanol, decalcified, embedded in paraffin and sectioned serially at 10 μm (Reichert microtome). The first specimen was serially cut from the mesethmoid to the first half of the swimbladder (500 cross sections) and stained with haematoxylin and eosin. In a second specimen, cross sections were taken only at the swimbladder level and were stained using Gill III haematoxylin. Gonads were extracted from four fish (three males from 110 to 150 mm‐ and one female of 115 mm), which had been recorded in isolation in 2021 and stained with haematoxylin and eosin stain to identify the sex. We follow the gonad terminology of previous studies on Ophidiidae Casadevall et al., 1993, 1987; Hernández et al., 2005). Histological sections were observed with a polarising Olympus BX50 binocular microscope coupled with an Olympus OM‐4 Ti camera.
Three‐dimensional reconstructions of skeleton and swimbladder were performed at the Centre for Microtomography of the University of Poitiers (France) to compare males (n = 1) and females (n = 2) sonic apparata. X‐ray microtomography was performed on all specimens according to the protocols reported by Boistel et al. (Boistel et al., 2011) and Zanette et al. (Zanette et al., 2014) using an RXsolutions (Annecy, France) EasyTom XL Duo microtomograph. However, these specimens were not bathed with phosphomolybdic acid. All specimens were scanned simultaneously, generating 2073 images with a voxel size of 24 μm. The reconstruction was performed using the software XAct (RX solution) with the FDK algorithms. Three‐dimensional (3D) images were produced in 16‐bit and subsequently converted into 8‐bit voxels using ImageJ (Abramoff et al., 2014). Three‐dimensional processing and rendering according to the protocols reported by Zanette et al. (Zanette et al., 2014) were obtained after semi‐automatic segmentation of the body structures using ‘generated surface.’ Direct volume renderings (iso‐surface reconstruction) were used to visualise the subset of selected voxels of the skull and the part of the sound‐producing mechanism connected to the vertebral column in AMIRA 2019.2.
2.6. Statistical analysis
Statistical analyses were carried out in R 4.0.2. As it was not possible to identify the caller, all sounds from the community tank were pooled together. Acoustic features and muscle diameters were assessed for normality using Shapiro–Wilk tests; non‐parametric tests were chosen because both failed the normality assumption. Fibre diameters of muscles were compared using Kruskal–Wallis H test followed by subsequent Dunn's multiple comparison test for pairwise comparisons. To characterise the pulse variability within individual sounds, Wilcoxon signed‐rank tests were used for comparing pulse dominant frequency, pulse period and relative pulse amplitude measured in subsequent pulses of the same sound of a specific sound type recorded in males (N sounds = 98). Spearman's rank‐order tests were used to determine the relationships between low amplitude and high amplitude pulses occurring within the same sound.
3. RESULTS
3.1. Sounds
Sounds were recorded in the community tank from 2018 to 2021. In 2021, 8 individual fish were isolated in a tank and were consequently recorded separately. Sound emission in captivity began 60 to 90 min after the lights were turned off. Fish made sounds when still buried in the sand and while swimming in the tank. Moreover, as isolated fish vocalise when no other fish is present, sounds could at least be intended to signal the caller presence and/or identity to conspecifics (advertisement). Dissections and histological examination (see later) after the recording sessions revealed that 8 specimens were males, and 1 specimen was a female allowing examination of sexual differences. Three sound types were recorded (type A1, A2 and B). Both type A was produced by males and type B was produced by the female.
Type A1 calls, the most common, start with low amplitude pulses and change abruptly into high‐amplitude ones, A2 calls have only high amplitude pulses, and type B, calls have low and high amplitude pulses that vary in order randomly. Type A1 call was recorded during each session each year and is particularly characteristic (Figures 1, 2; Table 1). Recordings of isolated specimens indicate these sounds were produced by males. Type A1 sounds can be emitted alone or in series of 2 to 3 units. Each unit of type A1 is made of multiple pulses. Trains of pulses can be clearly divided into two parts (Figure 1b). The first part contains 1 to 9 low amplitude pulses, and the second part contains 2–28 pulses that are three to ten times greater in voltage (Figure 1, Table 2; n = 20, Wilcoxon: Z = 0, p < 0.001), equivalent to a 9.5–20 dB increase. Additionally, the second and or third pulse of the first part is always of higher amplitude than other initial pulses (Figure 1a). Comparison of pulses from different specimens were consistent and showed major differences in waveform shape (Figure 2). These differences could indicate individual signatures although this requires additional work to rule out tank reverberation. Within isolated specimens, fish #5 can be distinguished with its quite high dominant frequency. It can, however, be noted that frequency appears to be the H4 of a fundamental frequency around 430 Hz.
FIGURE 1.
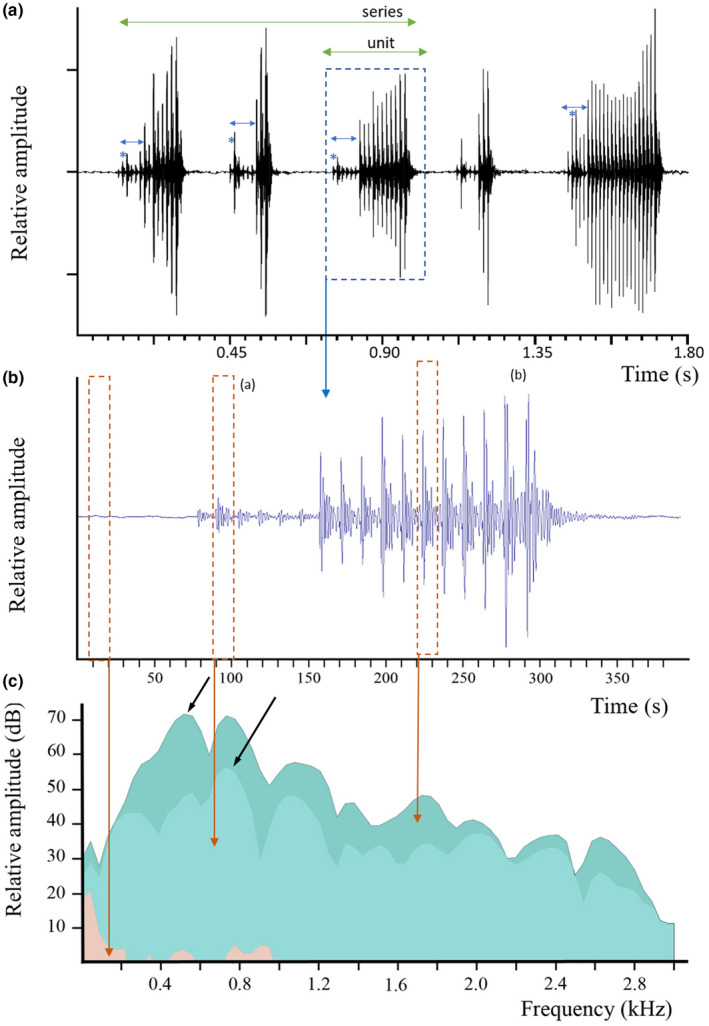
Parophidion vassali type A1 sound. (a) Oscillogram of different sounds. Five different units were placed on the same graph to show the shape variability. (b) Enlargement of the third unit of panel a to show the different pulses; note the amplitude difference between pulses. (c) Power spectrum (FFT size = 512, hamming) of low amplitude (a) and high amplitude (b) pulses. Black arrows indicate the dominant frequency. The roughly similar contour suggests that pulses are made in the same way. Salmon colour corresponds to background noise. Double blue arrows in a show low amplitude pulse always starting sound type A1; * the second pulse is usually louder than other low amplitude pulses.
FIGURE 2.
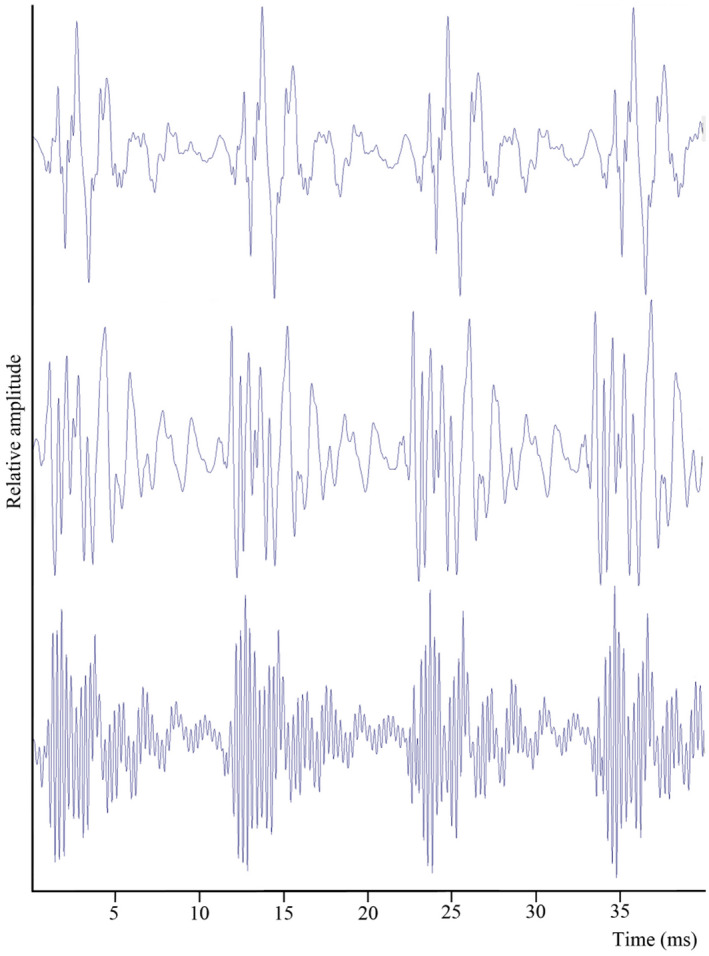
Parophidion vassali type A1 sounds. A. Enlarged oscillogram of few pulses produced by different specimens, all recorded in the same tank with the same hydrophone.
TABLE 1.
Mean (±SD) of acoustic features characterising sounds in Parophidion vassali. Isolated specimens allow to distinguish sounds from males (M) and from females (F) in captivity. Fish 1, 2, 3, 5, 6 and 9 were recorded while they were alone in the tank. N refers to the number of sounds (units)
| Sound type | Dominant frequency (Hz) | Pulse period (ms) | Sound duration (ms) | Number of pulses |
|---|---|---|---|---|
| Type A1 (n = 106) | 503 ± 82 | 12.3 ± 0.95 | 159.0 ± 71.5 | 14 ± 6 |
| Fish #1 (n = 7) M | 368 ± 161 | 13.7 ± 0.54 | 137.5 ± 88.7 | 19 ± 11 |
| Fish #2 (n = 6) M | 423 ± 86 | 13.8 ± 0.47 | 241 ± 93 | 17 ± 6 |
| Fish #3 (n = 6) M | 437 | 12.6 ± 0.58 | 238 ± 88 | 18 ± 7 |
| Fish #5 (n = 37) M | 1734 ± 14 | 12.8 ± 0.47 | 201 ± 103 | 14 ± 8 |
| Fish #6 (n = 6) M | 470 ± 87 | 12.1 ± 0.92 | 149 ± 52 | 12 ± 4 |
| Fish #7 (n = 4) M | 457 ± 39 | 13.6 ± 0.54 | 300 ± 67 | 21 ± 4 |
| Type A2 (n = 4) M | 424 ± 238 | 35 ± 16 | 258 ± 136 | 9 ± 4 |
| Type B (n = 63) | 648 ± 109 | 7.8 ± 0.6 | 41 ± 16 | 6 ± 2 |
| Fish #9 (n = 5) F | 700 ± 100 | 8.1 ± 0.1 | 26 ± 5 | 4 ± 1 |
TABLE 2.
Comparison of the acoustic features between low (LA) and high amplitude (HA) pulses belonging to the same sounds (type A1) in Parophidion vassali (Figure 1)
| (n = 98) | # LA | # HA | LA – PF (Hz) | HA – PF (Hz) | LA – PP (ms) | HA PP (ms) |
|---|---|---|---|---|---|---|
| Mean | 5 | 8 | 501 | 506 | 12.3 | 12.3 |
| Min | 1 | 1 | 345 | 345 | 9.8 | 10.6 |
| Max | 10 | 26 | 705 | 705 | 14.0 | 14.0 |
Note: #Number of pulses; PF, dominant frequency; PP, pulse period.
In a series, the first sound unit is always longer than the second that is always longer than the third. However, single units are usually longer than ones emitted in a series. Type A1 has a clearly distinguishable signature allowing its identification in the field: it consists of between 6 and 36 pulses for a total duration between 58 and 574 ms. The pulse period was between 10.3 and 15.4 ms and the dominant frequency was about 503 Hz (Table 1). The peak to peak SPL ranged from 119 to 137 dB re 1 μPa (n = 30 from 3 specimens), the maximum distance between the hydrophone and sound being about 35 cm.
In type A1 sounds, a comparison of pulses having low and high amplitude (Figure 1b) shows that their dominant frequency (Wilcoxon: Z = 295, p = 0.362) and their pulse period (Wilcoxon: Z = 1700, p = 0.313) are not significantly different (Table 2). Also, no correlation was found between the number of low amplitude and high amplitude pulses within the same sound (Rs = 0.03). Parophidion vassali can thus modify their sound amplitude without altering other features. This implies the same mechanism is used for the production of all pulses.
In 2021, a second type of sound produced by an isolated male (type A2) was recorded (Figure 3). This sound type differed from type A1 because of the absence of low‐amplitude pulses, and a longer pulse period, lasting from 20 to 83 ms (n = 4), with mean values of 35 ± 16 ms (n = 33). Type A2 calls were composed of 4 to 13 (n = 4) pulses with a duration extending from 114 to 410 ms (n = 4) and a dominant frequency of 424 ± 238 Hz (n = 33).
FIGURE 3.
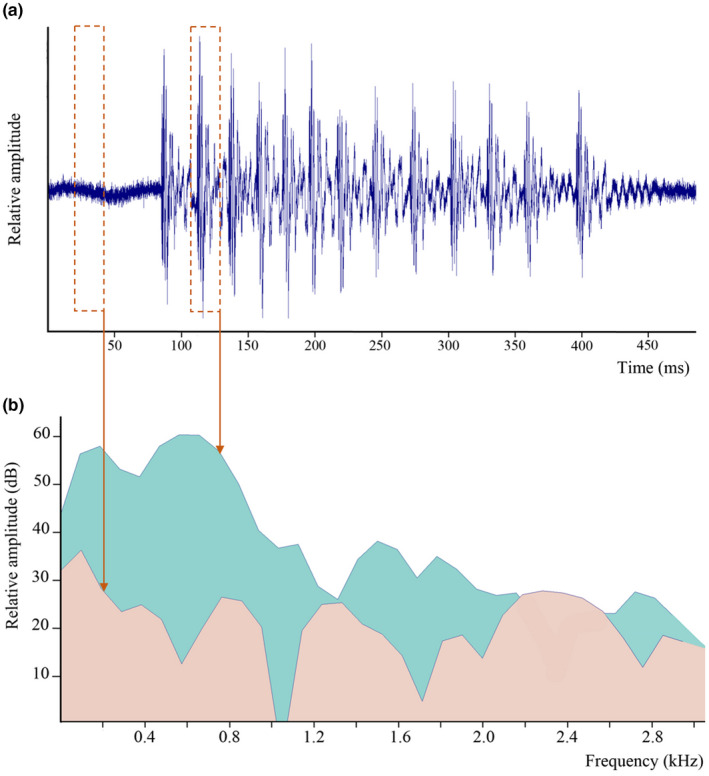
Parophidion vassali type A2 sound. (a) Oscillogram. (b) Power spectrum (FFT size = 512, hamming) of one pulse (green) and background noise (salmon).
A third sound type (type B) was also recorded each year. Contrarily to type A1 calls, where low and high‐amplitude pulses are separated clearly within a unit, low and high‐amplitude pulses of type B calls cannot be separated into two distinct groups (Figure 4). Compared to type A1 calls, the envelope of type B calls is not step‐like but often present a rhomboid shape: lower amplitude pulses gradually increase in amplitude, reaching a maximum and subsequently decreasing. These sounds are shorter (41 ± 16 ms; n = 63) and show shorter pulse periods (7.8 ± 0.6 ms; n = 63), shorter pulses (Figure 4) and a higher main frequency (648 ± 109 Hz, n = 63) than type A. This kind of sound has been recorded in a single isolated female, isolated males never produced this sound.
FIGURE 4.
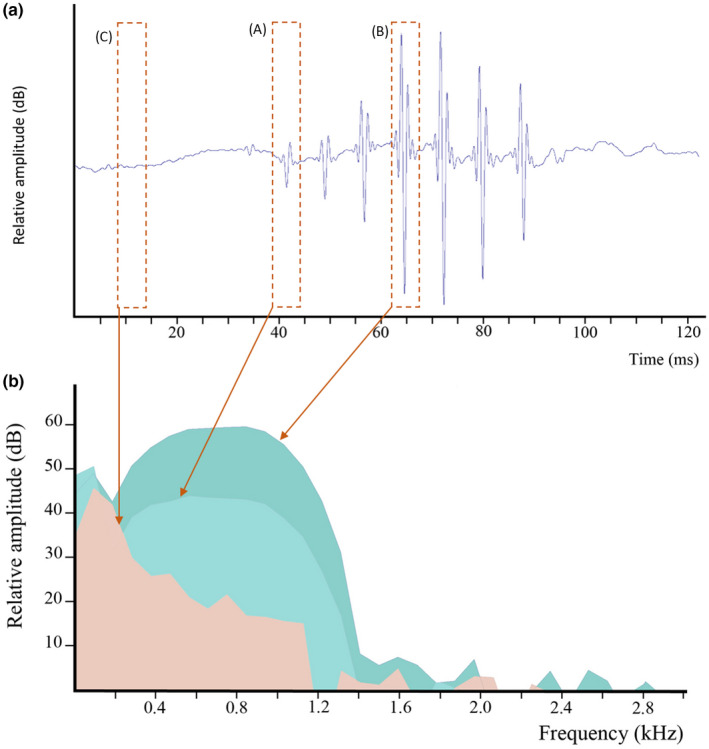
Parophidion vassali type B sound. (a) Oscillogram. Es. (b) Power spectrum (FFT size = 512, hamming) showing low amplitude (A) and high amplitude (B) pulses, and background noise (C). Salmon area corresponds to background noise.
The characterisation of Parophidion vassali sounds recorded in captivity allowed the identification of sounds emitted by this species in the field. As sounds in captivity were emitted from 19:00 to midnight, analysis of field‐collected data focused on this period. A total of 8 sounds could be assigned to P. vassali in 2015 recordings from La Ciotat. Low amplitude initial pulses support they correspond to type A sound (Figure 5). These in situ recorded sounds were 74 ± 28 ms long and composed of 8.5 ± 2.6 pulses (1.2 ± 0.3 low‐amplitude pulses and 5.2 ± 1.3 high‐amplitude pulses). The pulse period was 9 ± 1 ms, and the dominant frequency was 499 ± 2 Hz (Figure 6). No sounds corresponding to the ones recorded in the tank were found from 2016 to 2018. It suggests these fish are rare or could be more common in deeper water. Additional sounds are, however, required to better describe in situ recorded sounds and to make more accurate comparisons with sounds recorded in the tank.
FIGURE 5.
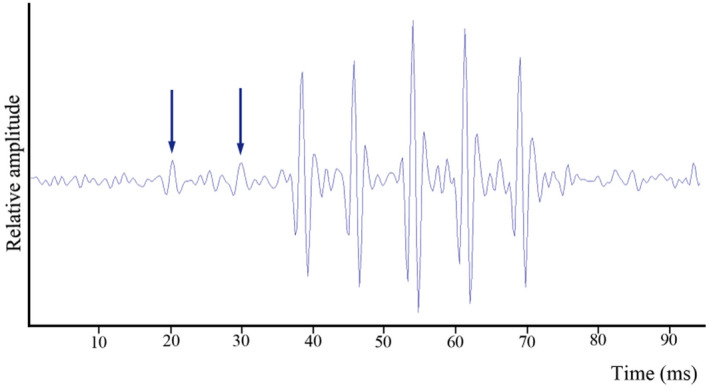
Oscillogram of a field recorded sound attributed to Parophidion vassali. Arrows indicate the low amplitude pulses at the beginning of the sound.
FIGURE 6.
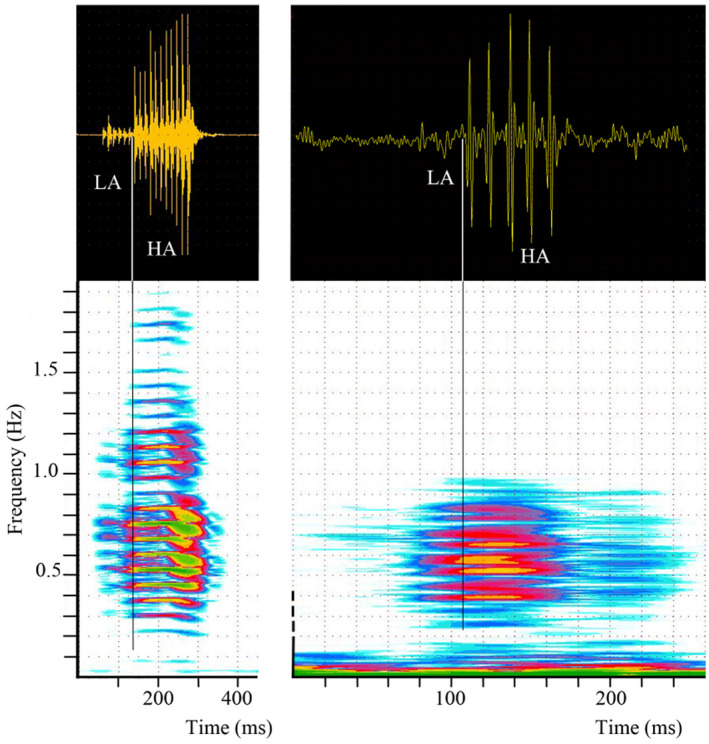
Comparison of type A1 sounds of Parophidion vassali recorded in the tank (left) and in the field (right) with spectrograms (lower panel) aligned to corresponding oscillograms (upper panel). Sound in the tank is here three times longer than sound in the field because they are more pulses. HA, High amplitude pulses; LA, Low amplitude pulses.
3.2. Gonad anatomy
Since the external phenotype is similar between sexes, we provide a brief description of the gonads to help in future studies. In females, a single‐lobed ovary (Figure 7a) is found in the posterior part of the abdominal cavity, dorsal to the digestive tract. It is externally oblong and shows a smooth surface with various dark spots randomly distributed below its surface. A band of external dorsal vessel divides the ovary into two parts. The rounded ovisac wall is made of smooth muscles and blood vessels that covers a germinal (cuboidal) epithelium (Figure 7b). Within the ovisac, different ovarian lamellae house oocytes at different stages of development (Figure 7c).
FIGURE 7.
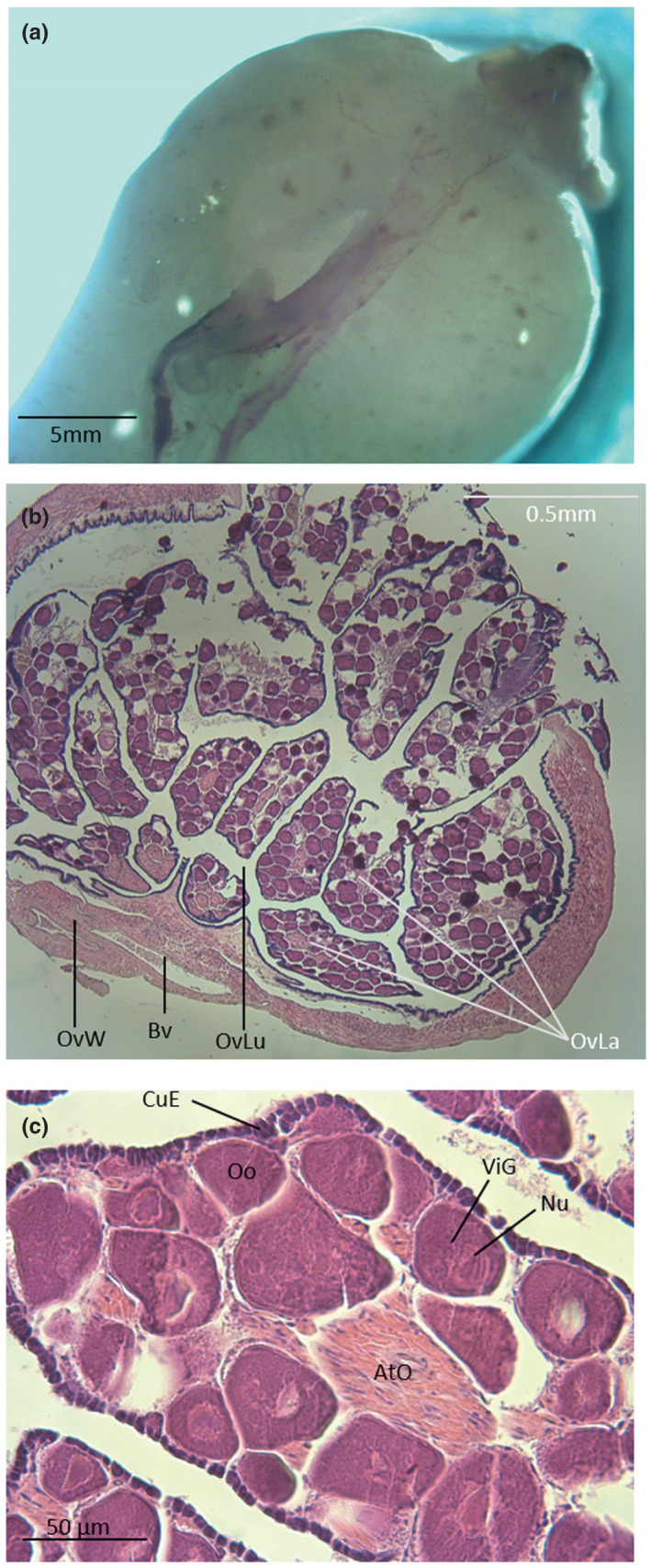
Dorsal view (a) and cross sections (b and c) of an ovary in Parophidion vassali. Ato, Atretic oocyte; Bv, Blood vessel; CuE, Cubic epithelium; nu, Nucleolis; Oo, Oocyte; OvLa, Ovarian lamella; OvLu, Ovisac lumen; OvW, Ovisac wall; ViG, Vitelline granule.
The testes (Figure 8) of P. vassali form an elongated structure located at the posterior end of the abdominal cavity, above the digestive tract. Testes are externally oblong with dorsal blood vessels dividing the length into two equal parts that show inner compartments having a transverse orientation (Figure 8c). Dark spot (one/lobule) can be observed. Histological sections show these multiple lobules converge towards the central part where they empty into the sperm duct. Lobules shelter different male germ cells at various stages including spermatogonia, spermatocytes, spermatids and a few spermatozoa.
FIGURE 8.
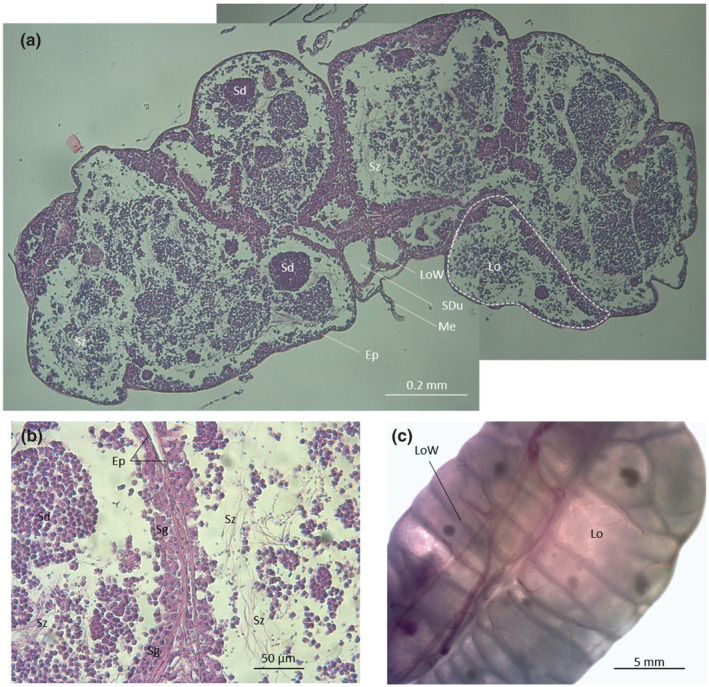
Cross sections (a and b, c) and anterodorsal view (c) and testes in Parophidion vassali. Ep, Epithelium; lo, Lobule; LoW, lobule wall; me, Mesentery; Sd, Spermatid; Sdu, Spermiduct; sg, Spermatogonia, Sz, Spermatozoon.
3.3. Sound‐producing mechanism
The sound‐producing mechanism of Parophidion vassali shares many features with other Ophidion species. For consistency, we follow the description made in O. rochei (Parmentier et al., 2010) for depicting the P. vassali sound‐producing mechanism.
3.3.1. Males
The sound‐producing mechanism is made of modified vertebrae, three pairs of sonic muscles, a highly modified swimbladder possessing anterior mineralized structures (swimbladder caps), a swimbladder fenestra and a rounded thin wall surrounded by an elevated tube on its posterior edge.
3.3.2. Vertebral modifications
The first neural arch, or neural rocker, has no neural spine (Figure 9). The neural rocker is shaped like a horseshoe above the vertebra; both its vertical branches articulate with the vertebral body so that it can pivot in the anteroposterior plane. The vertical branches of the neural rocker are also firmly attached to the first epineurals, originally called the wing‐like process (Fine et al., 2007), thanks to connective fibres. The wing‐like processes are located in front of the swimbladder (Figures 9, 10) and possess a triangular blade‐like shape (Figure 10a). Their horizontal edge is connected to the neural arch, and their vertical edge is perpendicular to the backbone. The neural rocker, and consequently the wing‐like process, can swing back and forth: forward swinging of the neural rocker drives backward displacement of the wing‐like process (Figure 9b). The second epineurals possess a rod‐like shape, articulate on the second vertebra and do not contact the swimbladder. The third vertebra has an osseous stem which articulates proximally with the vertebral body; its distal part expands into a widespread convex bony swimbladder plate. The plate margins are embedded within the tissues (tunica externa) of the swimbladder. Dorsally, a large band of ligamentous tissue can be found between epineurals 1st and 2nd and another one between the 2nd and 3rd. These ligaments should prevent important forward displacement of epineurals 1st and 2nd. The fourth and fifth vertebra possess a pair of curved rod‐like epineurals each, which do not contact the swimbladder and are probably not involved in the sound‐producing mechanism.
FIGURE 9.
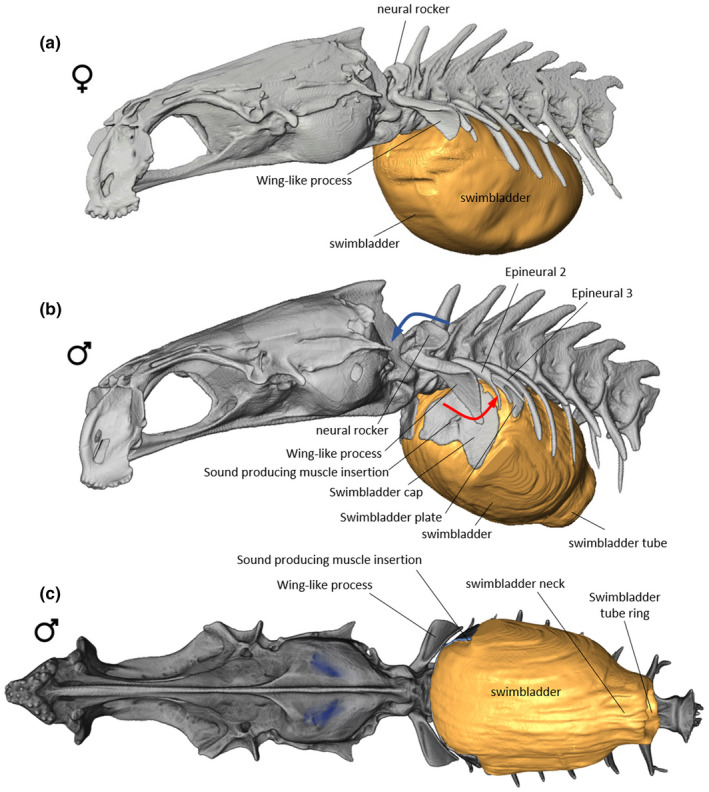
Left lateral view of the anterior part of vertebral column and skull in female (a) of Parophidion vassali. Left lateral (b) and ventral (c) views of the anterior part of vertebral column and skull in male of Parophidion vassali. Rocker forward movement (blue arrow) of the neural rocker drive the ventral tip of the wing‐like process backwards (red arrow).
FIGURE 10.
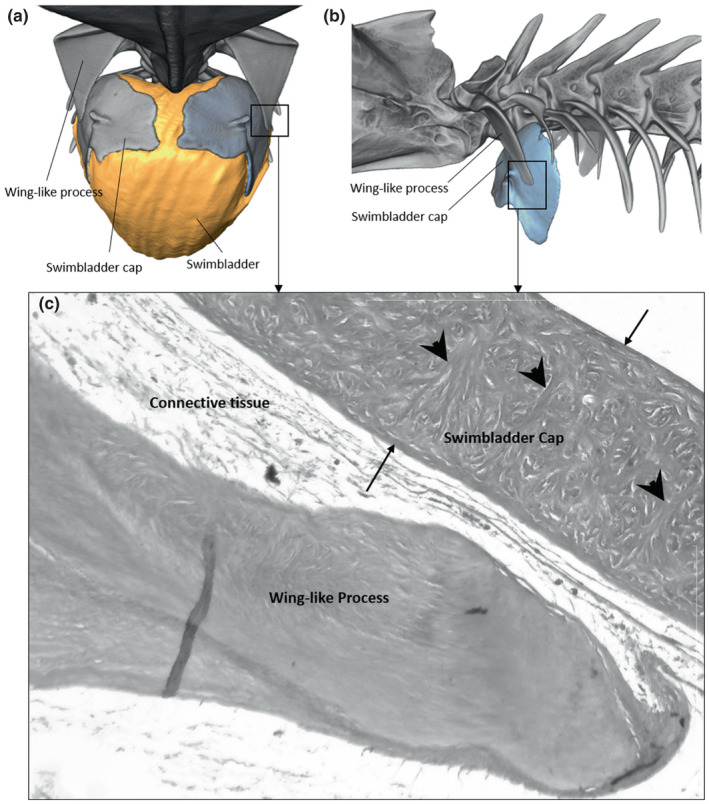
Frontal (a) and left lateral views (b) of the swimbladder cap in Parophidion vassali. Histological cross section (c) showing the swimbladder cap and distal tip of the wing‐like process. The swimbladder cap is composed of two external wall (arrow) of fibres that are connected by transversal fibres (arrowhead). Between the fibres are found mineralised items (white spaces).
3.3.3. Swimbladder
The swimbladder is elliptical and quite short (Figure 9), extending from the first to the eighth vertebral body. The swimbladder is firmly attached to the vertebral column at the level of vertebrae 4, 5 and 6. Internally, the swimbladder has a single chamber. The white swimbladder walls are rather thick (up to 150 μm), rigid and relatively resistant to deformation (Figure 11). These features are mainly due to the tunica externa, which is composed of a thick layer of interlaced elastic and collagen fibres. The fibre orientation in the tunica externa is not uniform. On the lateral and ventral sides, fibres of the tunica externa show different kinds of organisation with an inner layer of circular fibres and an external layer of longitudinal fibres, plus a helicoidal plywood patterns or orthogonal plywood pattern (Figure 11). These fibres orientation patterns suggest that the swimbladder can resist the deformation of its walls.
FIGURE 11.
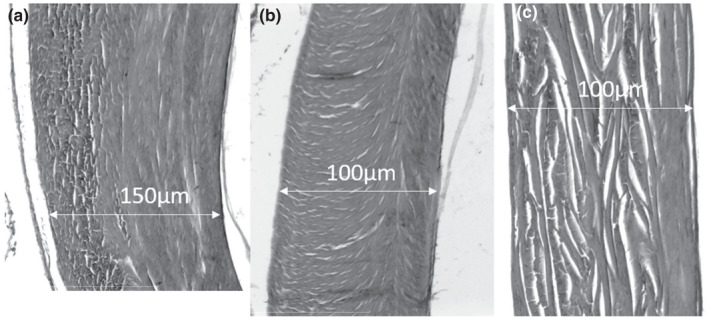
Cross section in the Parophidion vassali swimbladder wall showing the different organisation of the fibres within the tunica externa: Inner layer of circular fibres and external layer of longitudinal fibres (a), helicoidal plywood pattern (b) and orthogonal plywood lateral (c). On each picture, the inner side is on the right.
In males, the swimbladder shows at least three notable features.
Its anterior end contains two hard convex rounded plates, the swimbladder caps (Courtenay, 1971) each having a rostral tubercle for muscle insertion (Figures 9, 10). These caps respond to μCT scans similarly to skeleton or otoliths, which suggest that they are mineralized. Histological sections show that the swimbladder caps are made of three main layers of fibres; two thin outer layers are connected to a thick inner wall made of circular fibres by mean of transverse and oblique bundles of fibres (Figure 10c). Moreover, clear holes are found between these transverse fibres. The holes could correspond to the positioning of mineral bodies that allows the detection of the swimbladders caps by μCT scan. However, these bodies were destroyed during the decalcification process used in histological preparations (Figure 10c). The mineralisation appears more concentrated in the centre of the swimbladder caps. There is a clear continuity between fibres found in the caps and fibres of the swimbladder wall, supporting that these are derived from the swimbladder wall. The connection between the swimbladder caps and the surrounding tissue is not homogenous. The lower part is thicker than the dorsal part, which allows for independent swimbladder caps' movements. The swimbladder caps are found at the back of the vertical part of the wing‐like process (Figure 10), meaning that their forward displacement is probably restricted by the wing‐like process.
The swimbladder fenestra is a delineated, thinner area of the swimbladder. In Parophidion vassali, the swimbladder fenestra is dorsal and forms a band between swimbladder plates and the posterior margin of the swimbladder cap. The thickness of the fenestra is less than 10 μm, whereas the rest of the swimbladder wall is between 100 and 150 μm thick (Figure 11). Therefore, the thin and elastic swimbladder fenestra allows for the displacement of the anterior part of the swimbladder with minimal or no movement of the posterior swimbladder. Manual handling of the sound‐production system confirmed this assumption.
A large, rounded tube is found in the posteroventral region of the swimbladder, and, in accordance with Courtenay (Courtenay, 1971), is shaped like a doughnut (Figures 9, 12). The centre of this tube is deprived of the thick tunica externa. The swimbladder projection is covered by a plug of mucus surrounded by connective tissue that lengthens the swimbladder (Figure 13). Gentle pressures on the swimbladder wall or on the swimbladder caps push the inner gas of the swimbladder to form a bubble penetrating into the plug of mucus (Figure 14). This structure could act as a pressure‐release membrane.
FIGURE 12.
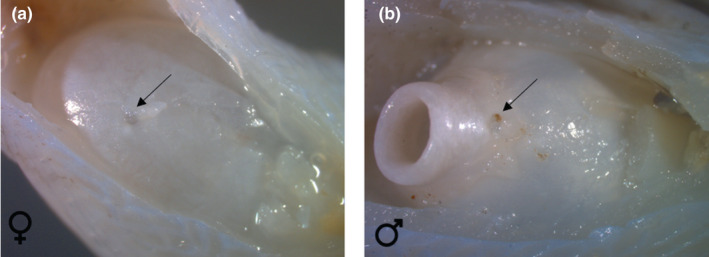
Ventral view of the posterior part of the swimbladder in Parophidion vassali female (a) and male (b). In males, a surrounding layer of connective tissue was removed to show the swimbladder tube. The arrow shows the swimbladder foramen for blood vessels.
FIGURE 13.
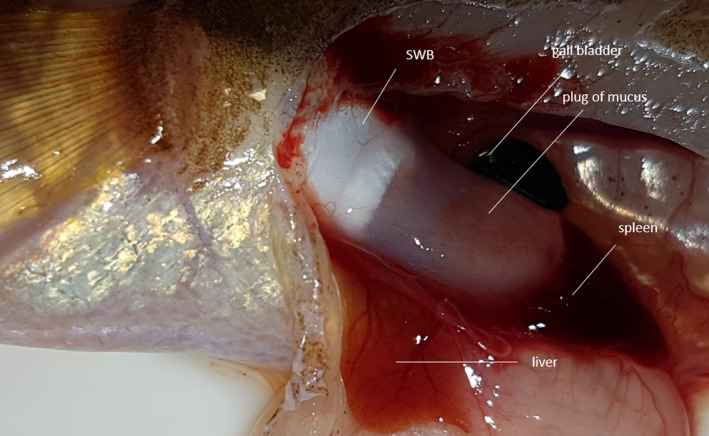
Left lateral view of the posterior part of the swimbladder in Parophidion vassali male showing the plug of mucus extending the swimbladder. The epithelium surrounding the plug cannot be distinguished.
FIGURE 14.
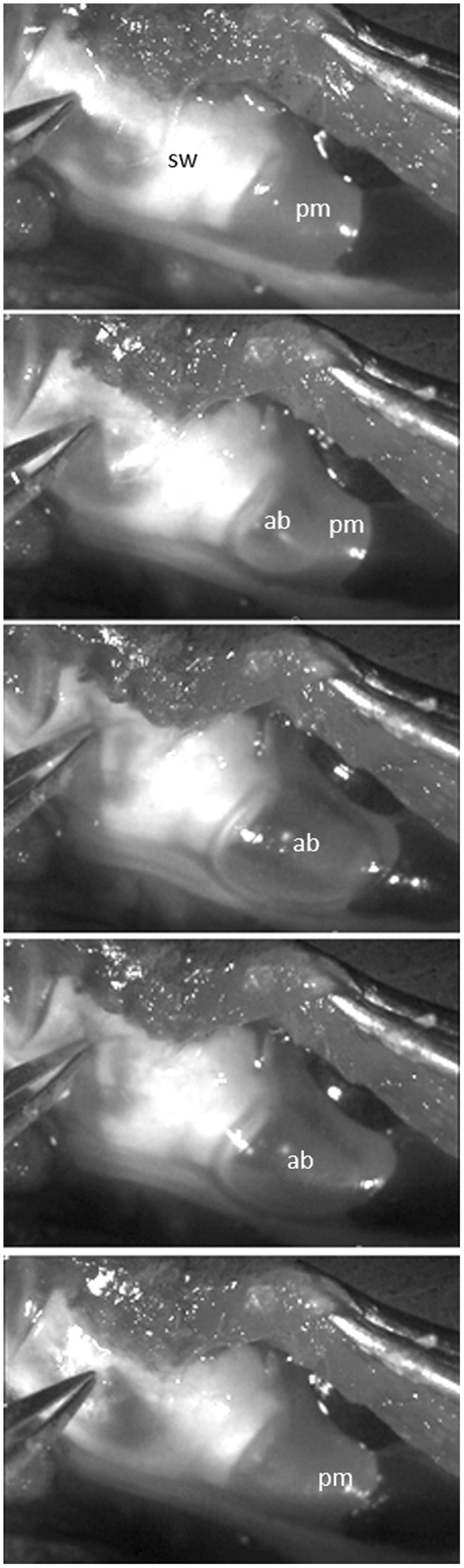
Left lateral view of the posterior part of the swimbladder (sw) in Parophidion vassali male. Pictures show the air bubble (ab) penetrating the plug of mucus (pm) once a pressure is applied on the swimbladder wall. This would correspond to the snap back of the swimbladder cap.
3.3.4. Sonic muscles
Three pairs of muscles are associated with the vertebral modifications and the swimbladder (Figures 15, 16). (1) The thick, dorsal sound‐producing muscle (DSM) originates on the back of the neurocranium from the supraoccipital‐exoccipital region and inserts dorsally on the neural rocker. (2) The intermediate sound‐producing muscle originates on the lower part of the exoccipital, surrounds Baudelot's ligament and inserts on the horizontal edge of the wing‐like process. (3) The ventral sound‐producing muscle (VSM) is unusually long (Figure 15d). This muscle has a tendinous attachment to the posterior margin of the vomer, follows ventrally the length of the neurocranium and inserts directly and tendinously on the process of the anterior bony plate. The muscle is pennate in part and is enlarged at the level of the otic region (Figure 15d). A thick ligament (=cap's ligament) connects the distal tip of the wing‐like process with the cap of the swimbladder, just behind the insertion of the ventral sonic muscle on the cap (Figure 15c).
FIGURE 15.
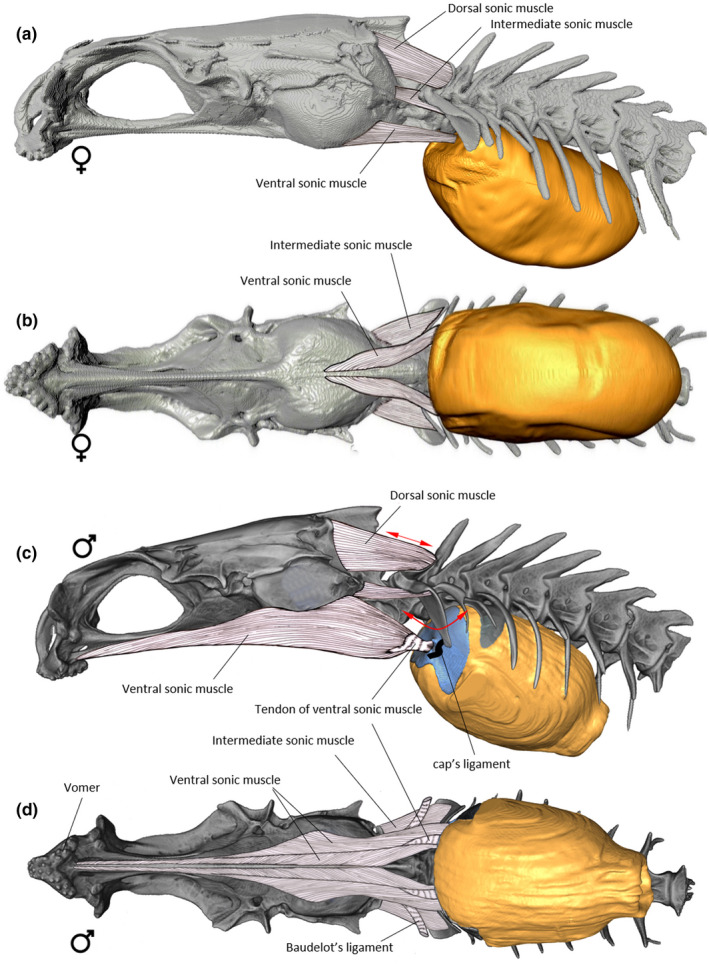
Left lateral (a) and ventral (b) views of the sound‐producing apparatus in female of Parophidion vassali and left lateral (c) and ventral (d) views of the sound‐producing apparatus in males of Parophidion vassali.
FIGURE 16.
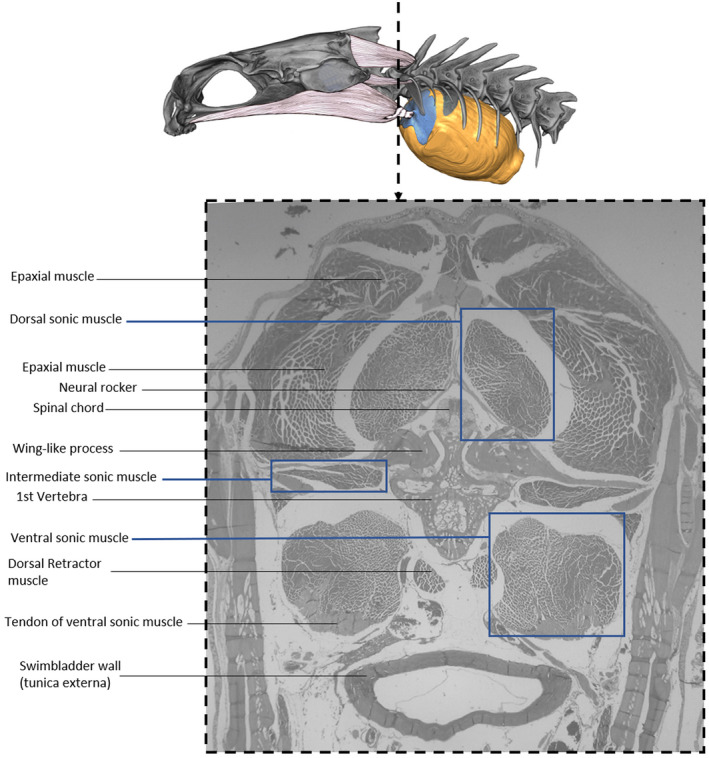
Cross section in a Parophidion vassali male showing the three pairs of sound‐producing muscles.
Fibre diameters of the sound‐producing muscles were compared with white epaxial fibres. Mean diameters were significantly different for all muscles (n = 50 for each muscle): 28.8 ± 2.5 μm for ventral sound‐producing muscles, 45.1 ± 7.3 μm for dorsal sound‐producing muscles, 94.7 ± 13.2 μm for intermediate sound‐producing muscles and 79.3 ± 8.9 μm (n = 50) for epaxial muscles (Figure 17, Kruskal‐Wallis: χ2 = 176.2, df = 3, p < 0.001; Dunn: Z de −7.93 to 12.25 and p‐adjusted all <0.05).
FIGURE 17.
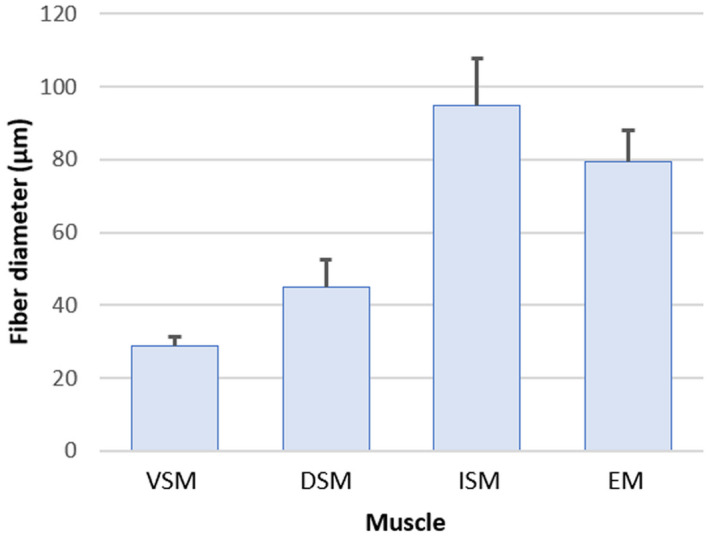
Histogram (mean ± SD) on the mean fibre diameters of different muscles in Parophidion vassali: Ventral (VSM), dorsal (DSM) and intermediate (ISM) sound‐producing muscles, white epaxial muscles (ESM).
3.4. Females
Females present a number of differences compared to males. The female swimbladder is elliptical and has a larger insertion on the vertebral column, from the 3rd to the 7th vertebral body. Its sound‐producing apparatus does not possess i) anterior swimbladder caps (Figure 7); ii) a swimbladder fenestra or iii) the pressure‐release membrane on the posterior tip of the swimbladder (Figure 12a). Females possess the neural rocker and the wing‐like process, but these are differently shaped than those of males (Figure 9). The neural rocker has a narrower surface, supporting a smaller dorsal sound‐producing muscle than in the male. The wing‐like process does not have a vertical blade, a characteristic which greatly reduces its total size. The intermediate muscle that originates on the lower part of the exoccipital and inserts on the wing‐like process is larger than in males, and its fibres completely surround the Baudelot's ligament (Figure 15). The third epineural does not have a swimbladder plate, but it possesses a rod‐like shape, as it is the case for the 2nd epineural (Figure 8). The ventral sonic muscle is shorter than in the male, originating on the basioccipital and inserting directly on the anterior wall of the swimbladder, adjacent to the insertion of the ligament of the wing‐like process (Figure 15). At the level of the muscle insertion, the swimbladder wall is thicker and sends connective tissue to the tip of the wing‐like process and the second epineural.
4. DISCUSSION
This study presents the first description of sound production in P. vassali, with the characterisation of at least three different sound types. As is often the case in ophidiids, the sound‐producing mechanism is highly specialised, presenting several distinctive features and a high level of sexual dimorphism. Although both sexes are capable of emitting sounds, sexual differences in morphology appear to underlie sexual differences in sound features (Courtenay, 1971; Howes, 1992; Kéver et al., 2012; Parmentier et al., 2010; Parmentier, Fontenelle, et al., 2006; Rose, 1961). As other known Ophidiids (Kéver et al., 2016; Mooney et al., 2016; Rountree & Bowers‐Altman, 2002; Sprague & Luczkovich, 2001), P. vassali began to produce sounds around 1 h after sunset, mainly when specimens emerged from the sand and became active. Since isolated fish emitted sounds in the tank, their calls may serve to identify or find conspecifics (i.e. advertisement calls). Sound emissions with different specimens in a tank indicate calls from an individual elicit sound production among conspecifics, but additional behavioural studies are required to assess the function of the recorded sounds. Importantly, P. vassali can modify the amplitude of their sounds dramatically (magnitude changes about five times between pulses from a single call unit).
The reason for this variation remains to be determined, but this ability could be important for the species because background noise can be problematic for acoustically communicating organisms due to masking of acoustic signals (Codarin et al., 2009; Ladich, 2019; Popper & Hawkins, 2019). Birds, mammals and frogs may increase the amplitude of their signals in order to elevate the signal‐to‐noise ratio of their call relative to background noise, a phenomenon referred to as the Lombard effect (Harlan & Bernard, 1971; Lopez et al., 1988; Oliveira & Canário, 2000). This ability is known in few fish species only (Brown et al., 2021; Holt & Johnston, 2014; Luczkovich et al., 2016). Although P. vassali are quite small (no more than 20 cm and 15 gr), they produce loud sounds, up to 137 dB, indicating that their sounds should be easily detected in the soundscape.
The presence of a very short swimbladder with a posterior pressure‐release membrane, and the lack of a rocker bone in Parophidion vassali are features in common with Ophidion marginatum. Moreover, both species possess a hardened part at the anterior end of the swimbladder, that is the “swimbladder cap” (Courtenay, 1971). In P. vassali, there is one cap for each ventral sonic muscle, while a single cap is present in O. marginatum. Caps or rocker bones are not found in all Ophidiidae, in which some species simply possess thicker swimbladder walls at the sonic muscle insertion (Fine et al., 2007; Howes, 1992). The reason for which some species only possess hardened swimbladder walls' is not known. However, it most likely impacts the resulting sounds since stiffer bladder might be more efficient at generating sound energy with smaller movements. In O. rochei, the development of the rocker bone appears during sexual maturation and corresponds to the development of secondary sexual characters (Kéver et al., 2012). Description of the rocker bone in Ophidion barbatum reveals a new kind of mineralised tissue (Parmentier, Compère, et al., 2008) that could be a response to the mechanical stress caused by the ventral sound‐producing muscles (Parmentier & Diogo, 2006). Deeper studies are required to describe the tissue that constitutes the swimbladder caps in P. vassali, but our preliminary observations support, at least, that these mineralised caps are derived from the swimbladder wall. The swimbladder cap could be an intermediate between the simple swimbladder wall and the rocker bone.
In Parophidion vassali, major sexual differences are found between the male and female sound‐producing apparatus. In comparison with males, females possess thinner and shorter sonic muscles, smaller neural rocker and wing‐like processes, and are deprived of swimbladder plate, cap and pressure‐release membrane. As in other ophidiid species, the sexual dimorphism of the sonic phenotype is significantly greater than that of the external phenotype, which highlights the importance of sound production for species' fitness. The gross morphology of Parophidion vassali sound‐producing apparatus is like that described in other Ophidion species, particularly to that of O. marginatum (Courtenay, 1971). This gross anatomical similarity suggests a similar functioning of their sound‐producing mechanisms.
In different carapid species, the lack of relationship between pulse period and dominant frequency (Eric Parmentier, Fine, et al., 2018; Parmentier, Lagardère, et al., 2008; Parmentier, Vandewalle, & Lagardère, 2003) supports that spectral features are not dictated by the contraction dynamics of superfast sonic muscles (Fine & Parmentier, 2015). Moreover, physiological studies conducted on Carapus acus showed that the cycle of contraction/relaxation in the ventral sonic muscle was surprisingly slow, that is below 20 Hz (Parmentier, Lagardère, et al., 2006). The sound‐producing mechanism of this species is currently called a “rebound system”: muscle contraction drives the forward displacement of the swimbladder, and sounds are produced when the swimbladder snaps back. The sound radiation could be aided by the vibration of the swimbladder plates surrounding the anterior part of the swimbladder, which would increase the coupling efficiency of muscle contraction to bladder movement. Additional studies are, however, required for a better understanding of the relationship between mechanisms and sound features. For example, all carapids possess a second pair of sonic muscles called “the secondary sound‐producing muscles” which are probably homologous to the intermediate muscles in the Ophidiidae. These muscles could affect the tension in the swimbladder walls.
The rebound system can be more complex in different Ophidiidae. Three pairs of sonic muscles (dorsal, intermediate and ventral) are involved in their sound production mechanism (Courtenay & McKittrick, 1970; Parmentier et al., 2010; Parmentier, Lagardère, et al., 2006). In male Ophidion rochei, following the “bow hypothesis”, the DSM is contracted for the whole duration of the sound to generate tension in the swimbladder (Parmentier et al., 2010). Simultaneously, contractions of the VSM provoke forward movements of the rocker bone. Once the VSM relaxes, the sustained contraction of the DSM causes a rapid reverse movement of the rocker bone, which likely corresponds to the rebound system and could create a sound pulse (Kéver, Boyle, et al., 2014). Physiological studies conducted in female O. rochei support this hypothesis since electromyograms suggest that the DSM contracts continuously, while the VSM sustains a series of rhythmic contractions. An alternative (or complementary) hypothesis of the DSM function would be that of preventing fish jack‐knifing when the VSM contract (Rose, 1961).
Rocker‐bone movements are an intrinsic characteristic of the rebound mechanism. Ophidion rochei females do not possess a rocker bone. Furthermore, DSM, VSM, wing‐like process and swimbladder plate are less developed, suggesting that sounds are produced differently than in males. In this species, while the pulse period of male's sounds is more than 80 ms, that of female's sounds is only ca. 4 ms; this supports that females sound‐producing mechanism is based on the activity of high‐speed muscles (Kéver, Boyle, et al., 2014). In other words, the interval between two contraction cycles is much shorter in females and it corresponds to the call dominant frequency. The longer pulse period in males does not correspond to the dominant frequency. Difference between both sexes could be a modification in the activation pattern (rate of action potential descending the nerve). It does not change males would also need fast contraction‐relaxations cycles since fast relaxation may be important to achieve the rebound. In this case, males and females could need fast contraction‐relaxation of their VSM but show a different periodicity in muscle activation. Important sexual dimorphism at the level of the sound‐producing apparatus has also been described in Ophidion holbrooki (Rose, 1961), Ophidion marginatum (Courtenay, 1971), Ophidion barbatum (Casadevall et al., 1996) and Lepophidium profundorum (Fine et al., 2007). The difference between male and female sounds remains unknown in O. marginatum (Mann et al., 1997; Mooney et al., 2016; Sprague & Luczkovich, 2001) and, unfortunately, sounds of O. holbrooki and O. barbatum were never recorded in either sex. In the carapid Onuxodon fowleri, both sexes possess a rocker bone, and smaller morphological differences are present between males and females. In the case of O. fowleri, the sound production mechanism would also correspond to the rebound system in both sexes (Kéver, Colleye, et al., 2014).
Sound types A and B differ in their (i) overall sound envelop, (ii) number of low and high amplitude pulses, (iii) pulse period, (iv) sound duration and (v) dominant frequency. The Sound type A2 requires more data for comparison. It suggests that P. vassali vocal repertoire might be more diverse than reported here.
Type A sounds are characterised by slower pulse periods (i.e. type A = 12 ms or 83 Hz; type B = 8 ms or 128 Hz) and lower dominant frequency (i.e. type A = 500 Hz; type B = 680 Hz). However, in both sound types, the pulse period does not mirror the dominant frequency, supporting muscle contraction dynamics do not dictate sound fundamental frequency. This statement is reinforced by the high variability in the dominant frequency (16%) versus the reduced variability of pulse periods (8%) found within each of these two sound types. As it is the case in C. acus (Parmentier, Lagardère, et al., 2006), O. fowleri (Kéver, Colleye, et al., 2014) and male O. rochei (Kéver, Boyle, et al., 2014; Parmentier et al., 2010)we hypothesise that both sound types emitted by P. vassali result from a «rebound system», in which sudden movements of the anterior part of the swimbladder might initiate the production of pulses.
The pattern of the sonic muscle diameters (Figure 17) is in complete accordance with previous observations in closely related species. Like O. barbatum (Parmentier, Fontenelle, et al., 2006) and O. rochei (Kéver, Boyle, et al., 2014) that also possess three different sonic muscles, Parophidion vassali have the smallest fibre diameter in the VSM, intermediate in the DSM whereas it is the largest, and close to the white epaxial muscles, in the ISM. In Carapidae (Carapus acus, Onuxodon fowleri and Encheliophis gracilis) that possess a sonic muscle homologous to VSM, the fibre diameter is also smaller than in the EM (Kéver, Colleye, et al., 2014; Parmentier, Gennotte, et al., 2003; Parmentier, Lagardère, et al., 2008). Il all previously studied species in Ophioidei, it has been suggested that the pulse period is dictated by the VSM contraction rate. The pulse period of 12 ms in males and of 8 ms in females should result from the contraction of high‐speed muscles. This is in complete accordance with the small diameter of VSM fibres since this a feature usually related to high‐speed muscles in different non closely related taxa (Appelt et al., 1991; Fine et al., 1990; Millot & Parmentier, 2014; Parmentier et al., 2020, 2014). The lower pulse rate in males could be related to the inertia of swimbladder caps. In order to move the swimbladder caps, males probably increase the VSM strength by adding fibres (Kéver, Boyle, et al., 2014). This is consistent with the larger and longer VSM in males than in female (Figure 4). In the P. vassali system, the back and forth displacement of the swimbladder caps generates variations of pressure. The flexible terminal thin membrane in the back of the swimbladder should undergo a greatly reduced load compared to the thicker and stiffer swimbladder walls. It would therefore act as a pressure release mechanism. Its effect on sound generation needs to be investigated experimentally. Despite its small size, it could serve as a sound radiator. However, sound transmission across an air/water interface is known to be ineffective due to a large acoustic impedance. Large acoustic impedance mismatch between the gas swimbladder and the body tissue means that an acoustic flow encounters resistance as it passes through a medium and an important part of the sounds is not transmitted (Urick, 1975).
In P. vassali, we hypothesise the gel‐like mucus structure in contact with the posterior bladder could function as an impedance matching structure that would increase transmission between the mediums. Note mucus has a density between air and water. This system could potentially facilitate the transmission of some frequencies but damp other frequencies, also explaining why the power spectra are quite smooth. However, the pressure release function of the posterior thin membrane could also attenuate pressure from swimbladder cap movements, preventing structural damages to swimbladder. Similar terminal membranes backed by a mucus structure have also been found in male neobythitine cusk‐eels Hoplobrotula armata and Dicrolene intronigra (Ali et al., 2016; Fine et al., 2018).
In both O. rochei male sounds and O. marginatum sounds, a sort of “warm up” period can be observed at the beginning of the sound. It corresponds to a changing pulse period duration between the initial pulses and an increasing pulse amplitude (Kéver et al., 2015; Parmentier et al., 2010; Picciulin et al., 2019). In Parophidion vassali type A, this warm up is not observed in the temporal scale but is rather visible in the sound envelope. As amplitude is the only acoustic feature that differs between pulses of the same sound, we hypothesise that all the pulses within a sound are made using a common mechanism. In the second part of type A sound, we hypothesise an increase in the muscle power should be related to a higher sound amplitude.
Moreover, this amplitude related feature could be useful to identify Parophidion vassali in the field. Together with pulse period and dominant frequency, this amplitude‐related characteristic of type A calls allowed the assignment of 8 sounds (Figure 4) recorded in the field in La Ciotat (2015) to P. vassali. Recordings in the same area in 2016 and 2018 did not include P. vassali type sounds although the initial low‐amplitude pulses could be masked by the background noise. Additional recordings at sea are required to fully assess acoustic features since tank walls could distort in part the sounds (Akamatsu et al., 2002; Parmentier et al., 2014).
Monitoring spatial and temporal fish biodiversity dynamics is a global priority, but a difficult challenge in the marine environment that is difficult to access (Desiderà et al., 2019). We, however, need to assess biodiversity to support management and conservation (Di Iorio et al., 2021). In passive acoustic studies, specific identifications of sound are rare meaning sonic classifications, based on acoustic properties, refer simply to sound type categories (Bolgan et al., 2020; Desiderà et al., 2019; Di Iorio et al., 2021). The present study provides a specific name for these sound types. Moreover, acoustic detection in the field is particularly important for species like Parophidion vassali since these sand‐dwelling fish are likely to go undetected by traditional visual monitoring techniques commonly used to survey biodiversity (Picciulin et al., 2019). The acoustic description we provide should help in future identifications.
AUTHOR CONTRIBUTIONS
EP and MB conceived and designed the experiments, performed the experiments and analysed the data. EP and RB performed the CT scan and 3D constructions. Ep analysed the histological sections and realised the dissections. LK and LDI collected and analysed field data. SG performed recording and sound analysis. EP, MF and MB MF drafted the manuscript; LK, LDI and SG, reviewed drafts of the paper, and approved the final draft.
ACKNOWLEDGEMENTS
David and Gérard Carrodano (http://poissons‐vivants.com/) kindly helped with specimen fishing. Prof T. Jauniaux, Mrs S. Smeets, Mrs J. Piret, Mr M. Sarlet and Mrs A. Tromme kindly helped in the microscopic study and L. Béranger for the deployment of hydrophones during fieldwork. Dr Margarida Casadevall was helpful in the description of the fish gonads and Dr Timothy Camron greatly help in the hypothesis of swimbladder mechanism.
Eric, P. , Gaëlle, S. , Renaud, B. , Fine, M.L. , Loïc, K., Lucia, D.I., et al (2022) Sound production and mechanism in the cryptic cusk‐eel Parophidion vassali . Journal of Anatomy, 241, 581–600. Available from: 10.1111/joa.13691
DATA AVAILABILITY STATEMENT
N/A
REFERENCES
- Abramoff, M.D. , Magalhaes, P.J. & Ram, S.J. (2014) Image processing with ImageJ. Biophotonics International, 11, 36–42. [Google Scholar]
- Akamatsu, T. , Okumura, T. , Novarini, N. & Yan, H.Y. (2002) Empirical refinements applicable to the recording of fish sounds in small tanks. The Journal of the Acoustical Society of America, 112, 3073–3082. [DOI] [PubMed] [Google Scholar]
- Ali, H.A. , Mok, H.‐K. & Fine, M.L. (2016) Development and sexual dimorphism of the sonic system in Deep Sea neobythitine fishes: the upper continental slope. Deep Sea Research Part I: Oceanographic Research Papers, 115, 293–308. [Google Scholar]
- Appelt, D. , Shen, V. & Franzini‐Armstrong, C. (1991) Quantitation of ca ATPase, feet and mitochondria in superfast muscle fibres from the toadfish, Opsanus tau. Journal of Muscle Research and Cell Motility, 12, 543–552. [DOI] [PubMed] [Google Scholar]
- Boistel, R. , Swoger, J. , Krzic, U. , Fernandez, V. , Gillet, B. & Reynaud, E.G. (2011) The future of three‐dimensional microscopic imaging in marine biology. Marine Ecology, 32, 438–452. [Google Scholar]
- Bolgan, M. , Gervaise, C. , Di Iorio, L. , Lossent, J. , Lejeune, P. , Raick, X. et al. (2020) Fish biophony in a Mediterranean submarine canyon. The Journal of the Acoustical Society of America, 147, 2466–2477. [DOI] [PubMed] [Google Scholar]
- Bolgan, M. , Soulard, J. , Di Iorio, L. , Gervaise, C. , Lejeune, P. , Gobert, S. et al. (2019) The sea chordophones make the mysterious /Kwa/: emitter identification of the dominating fish sound in Mediterranean seagrass meadows. The Journal of Experimental Biology, jeb.196931, 1–11. [DOI] [PubMed] [Google Scholar]
- Brown, N.A.W. , Halliday, W.D. , Balshine, S. & Juanes, F. (2021) Low‐amplitude noise elicits the Lombard effect in plainfin midshipman mating vocalizations in the wild. Animal Behaviour, 181, 29–39. [Google Scholar]
- Capape, C. , Rafrari‐Nouira, S. , El Kamel‐Moutalbi, O. , Boumaiza, M. & Casadevall, M. (2016) First record from the Tunisian coast of the rare species, snake blenny Parophidion vassali (Osteichthyes: Ophidiidae). Cahiers de Biologie Marine, 67, 89–91. [Google Scholar]
- Casadevall, M. , Bonet, S. & Matallanas, J. (1993) Description of different stages of oogenesis in Ophidion barbatum (Pisces, Ophidiidae). Environmental Biology of Fishes, 36, 127–133. [Google Scholar]
- Casadevall, M. , Matallanas, J. & Bonet, S. (1987) Anatomia macroscopia i microscopia de l'aparell genital femini d'Ophidion barbatum (L.) (Pisces, Ophidiidae). Science Gerudensis, 13, 23–33. [Google Scholar]
- Casadevall, M. , Matallanas, J. , Carrasson, M. & Munoz, M. (1996) Morphometric, meristic and anatomical differences between Ophidion barbatum L., 1758 and O. rochei muller, 1845 (Pisces, Ophidiidae). Publicaciones Espec. ‐ Inst. Esp. Oceanogr., 21, 45–61. [Google Scholar]
- Codarin, A. , Wysocki, L.E. , Ladich, F. & Picciulin, M. (2009) Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare, Italy). Marine Pollution Bulletin, 58, 1880–1887. [DOI] [PubMed] [Google Scholar]
- Codina, E. , Loïc, K. , Compère, P. , Dragičević, B. , Dulčić, J. & Parmentier, E. (2012) The barbel‐like specialization of the pelvic fins in Ophidion rochei (Ophidiidae). Journal of Morphology, 273, 1367–1376. [DOI] [PubMed] [Google Scholar]
- Courtenay, W.R. & McKittrick, F.A. (1970) Sound‐producing mechanisms in carapid fishes, with notes on phylogenetic implications. Marine Biology, 7, 131–137. [Google Scholar]
- Courtenay, W.R.J. (1971) Sexual dimorphism of the sound producing mechanism of the striped cusk‐eel, Rissola marginata (Pisces: Ophidiidae). Copeia, 1971, 259–268. [Google Scholar]
- Desiderà, E. , Guidetti, P. , Panzalis, P. , Navone, A. , CA, V.‐P. , Boissery, P. et al. (2019) Acoustic fish communities: sound diversity of rocky habitats reflects fish species diversity. Marine Ecology Progress Series, 608, 183–197. [Google Scholar]
- Di Iorio, L. , Audax, M. , Deter, J. , Holon, F. , Lossent, J. , Gervaise, C. et al. (2021) Biogeography of acoustic biodiversity of NW Mediterranean coralligenous reefs. Scientific Reports, 11, 16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, M.L. , Ali, H.A. , Nguyen, T.K. , Mok, H.‐K. & Parmentier, E. (2018) Development and sexual dimorphism of the sonic system in three deep‐sea neobythitine fishes: mid and lower continental slope. Deep Sea Research Part I: Oceanographic Research Papers, 131, 41–53. [Google Scholar]
- Fine, M.L. , Burns, N.M. & Harris, T.M. (1990) Ontogeny and sexual dimorphism of sonic muscle in the oyster toadfish. Canadian Journal of Zoology, 68, 1374–1381. [Google Scholar]
- Fine, M.L. , Lin, H. , Nguyen, B.B. , Rountree, R.A. , Cameron, T.M. & Parmentier, E. (2007) Functional morphology of the sonic apparatus in the fawn cusk‐eel Lepophidium profundorum (gill, 1863). Journal of Morphology, 268, 953–966. [DOI] [PubMed] [Google Scholar]
- Fine, M.L. & Parmentier, E. (2015) Mechanisms of sound production. In: Ladich, F. (Ed.) Sound communication in fishes. Wien: Springer, pp. 77–126. [Google Scholar]
- Harlan, L. & Bernard, T. (1971) The Lombard sign and the role of hearing in speech. Journal of Speech and Hearing Research, 14, 677–709. [Google Scholar]
- Hernández, M.R. , Sàbat, M. , Muñoz, M. & Casadevall, M. (2005) Semicystic spermatogenesis and reproductive strategy in Ophidion barbatum (Pisces, Ophidiidae). Acta Zoologica, 86, 295–300. [Google Scholar]
- Holt, D.E. & Johnston, C.E. (2014) Sound production and associated behaviours in blacktail shiner Cyprinella venusta: a comparison between field and lab. Environmental Biology of Fishes, 97, 1207–1219. [Google Scholar]
- Howes, G.J. , 1992. Notes on the anatomy and classification of Ophidiiform fishes with particular reference to the abyssal genus Acanthonus Gunther, 1878. Bull Bristish Museum (Nat Hist. 58, 95–131.
- Kéver, L. , Boyle, K.S. , Dragičević, B. , Dulčić, J. , Casadevall, M. & Parmentier, E. (2012) Sexual dimorphism of sonic apparatus and extreme intersexual variation of sounds in Ophidion rochei (Ophidiidae): first evidence of a tight relationship between morphology and sound characteristics in Ophidiidae. Frontiers in Zoology, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéver, L. , Boyle, K.S. , Dragičević, B. , Dulčić, J. & Parmentier, E. (2014) A superfast muscle in the complex sonic apparatus of Ophidion rochei (Ophidiiformes): histological and physiological approaches. The Journal of Experimental Biology, 217, 3432–3440. [DOI] [PubMed] [Google Scholar]
- Kéver, L. , Boyle, K.S. & Parmentier, E. (2015) Effects of seawater temperature on sound characteristics in Ophidion rochei Müller 1845 (Ophidiidae). Journal of Fish Biology, 85, 502–509. [DOI] [PubMed] [Google Scholar]
- Kéver, L. , Colleye, O. , Lugli, M. , Lecchini, D. , Lerouvreur, F. , Herrel, A. et al. (2014) Sound production in Onuxodon fowleri (Carapidae) and its amplification by the host shell. The Journal of Experimental Biology, 217, 4283–4294. [DOI] [PubMed] [Google Scholar]
- Kéver, L. , Lejeune, P. , Michel, L.N. & Parmentier, E. (2016) Passive acoustic recording of Ophidion rochei calling activity in Calvi Bay (France). Marine Ecology, 37, 1315–1324. [Google Scholar]
- Ladich, F. (2019) Ecology of sound communication in fishes. Fish and Fisheries, 20, 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, P.T. , Narins, P.M. , Lewis, E.R. & Moore, S.W. (1988) Acoustically induced call modification in the white‐lipped frog, Leptodactylus albilabris . Animal Behaviour, 36, 1295–1308. [Google Scholar]
- Luczkovich, J.J. , Krahforst, C.S. , Kelly, K.E. & Sprague, M.W. (2016) The Lombard effect in fishes: how boat noise impacts oyster toadfish vocalization amplitudes in natural experiments. Proceedings of Meetings on Acoustics, 27, 10035. [Google Scholar]
- Mann, D.A. , Bowers‐Altman, J. & Rountree, R.A. (1997) Sounds produced by the striped cusk‐eel Ophidion marginatum (Ophidiidae) during courtship and spawning. Copeia, 3, 610–612. [Google Scholar]
- Markle, D.F. & Olney, J.E. (1990) Systematics of the pearlfishes (Pisces:Carapidae). Bulletin of Marine Science, 47, 269–410. [Google Scholar]
- Marshall, N.B. (1967) Sound‐producing mechanisms and the biology of deep‐sea fishes. In: Tavolga, W.N. (Ed.) Marine bio‐acoustics. Oxford: Pergamon, pp. 123–133. [Google Scholar]
- Matallanas, J. & Casadevall, M. (1990) Parophidion vassali (Risso, 1810) (Pisces: Ophidiiformes) a species new to the Atlantic Ocean. Bocagiana, 134, 1–4. [Google Scholar]
- Merret, N.R. , Haedrich, R.L. , 1997. Deep‐sea demersal fish and fisheries (vol. 23). Springer Science & Business Media., fish & Fis. Ed. springer Netherlands.
- Millot, S. & Parmentier, E. (2014) Development of the ultrastructure of sonic muscles: a kind of neoteny ? BMC Evol. O Biologico, 14, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moods, L.P. , Kanazawa, R.H. , Woods, L.P. & Kanazawa, R.H. (1951) New species and new records of fishes from Bermuda. Fieldiana Zool., 31, 629–644. [Google Scholar]
- Mooney, T.A. , Kaplan, M.B. , Izzi, A. , Lamoni, L. & Sayigh, L. (2016) Temporal trends in cusk eel sound production at a proposed US wind farm site. Aquatic Biology, 24, 201–210. [Google Scholar]
- Nguyen, T.K. , Lin, H. , Parmentier, E. & Fine, M.L. (2008) Seasonal variation in sonic muscles in the fawn cusk‐eel Lepophidium profundorum . Biology Letters, 4, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J.G. , Cohen, D.M. , Markle, D.F. & Robins, C.R. (1999) FAO species catalogue. Ophidiiform fishes of the world (order Ophidiiformes): an annotated and illustrated catalogue of pearlfishes, cusk‐eels, brotulas and other ophidiiform fishes known to date. FAO Fisheries Synopsis No. 125, 18, 178. [Google Scholar]
- Oliveira, R.F. & Canário, A.V.M. (2000) Hormones and social behaviour in cichlid fishes: a case study in the Mozambique tilapia. Journal of Aquariculture and Aquatic Sciences, 9, 109–129. [Google Scholar]
- Parmentier, E. , Bahri, M.A. , Plenevaux, A. , Fine, M.L. & Estrada, J.M. (2018) Sound production and sonic apparatus in deep‐living cusk‐eels (Genypterus chilensis and Genypterus maculatus). Deep Sea Research Part I: Oceanographic Research Papers, 141, 83–92. [Google Scholar]
- Parmentier, E. , Bouillac, G. , Dragicevic, B. , Dulcic, J. & Fine, M. (2010) Call properties and morphology of the sound‐producing organ in Ophidion rochei (Ophidiidae). The Journal of Experimental Biology, 213, 3230–3236. [DOI] [PubMed] [Google Scholar]
- Parmentier, E. , Chardon, M. & Vandewalle, P. (2002) Preliminary study on the ecomorphological signification of the sound‐producing complex in Carapidae. In: Aerts, P. , D'Août, K. , Herrel, A. & Van Damme, R. (Eds.) Functional and ecological Vertabrate morphology. Maastricht: Shaker Publishing, pp. 139–151. [Google Scholar]
- Parmentier, E. , Colleye, O. & Lecchini, D. (2016) New insights into sound production in Carapus mourlani (Carapidae). Bulletin of Marine Science, 92, 335–342. [Google Scholar]
- Parmentier, E. , Compère, P. , Casadevall, M. , Fontenelle, N. , Cloots, R. & Henrist, C. (2008) The rocker bone: a new kind of mineralised tissue? Cell and Tissue Research, 334, 67–79. [DOI] [PubMed] [Google Scholar]
- Parmentier, E. & Diogo, R. (2006) Evolutionary trends of swimbladder sound mechanisms in some teleost fishes. In: Ladich, F. , Collin, S.P. , Moller, P. & Kapoor, B.G. (Eds.) Communication in fishes. Enfield, NH: Science Publishers, pp. 45–70. [Google Scholar]
- Parmentier, E. , Fontenelle, N. , Fine, M.L. , Vandewalle, P. & Henrist, C. (2006) Functional morphology of the sonic apparatus in Ophidion barbatum (Teleostei, Ophidiidae). Journal of Morphology, 267, 1461–1468. [DOI] [PubMed] [Google Scholar]
- Parmentier, E. , Gennotte, V. , Focant, B. , Goffinet, G. & Vandewalle, P. (2003) Characterization of the primary sonic muscles in Carapus acus (Carapidae): a multidisciplinary approach. Proceedings of the Royal Society of London Biological Sciences, 270, 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier, E. , Lagardère, J.‐P. , Braquegnier, J.‐B. , Vandewalle, P. & Fine, M.L. (2006) Sound production mechanism in carapid fish: first example with a slow sonic muscle. The Journal of Experimental Biology, 209, 2952–2960. [DOI] [PubMed] [Google Scholar]
- Parmentier, E. , Lagardère, J.P. , Chancerelle, Y. , Dufrane, D. & Eeckhaut, I. (2008) Variations in sound‐producing mechanism in the pearlfish Carapini (Carapidae). Journal of Zoology, 276, 266–275. [Google Scholar]
- Parmentier, E. , Marucco Fuentes, E. , Millot, M. , Raick, X. & Thiry, M. (2020) Sound production, hearing sensitivity, and in‐depth study of the sound‐producing muscles in the cowfish (Lactoria cornuta). Journal of Anatomy, 238, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier, E. , Tock, J. , Falguière, J.‐C. & Beauchaud, M. (2014) Sound production in Sciaenops ocellatus: preliminary study for the development of acoustic cues in aquaculture. Aquaculture, 432, 204–211. [Google Scholar]
- Parmentier, E. , Vandewalle, P. & Lagardère, J.P. (2003) Sound‐producing mechanisms and recordings in Carapini species (Teleostei, Pisces). Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 189, 283–292. [DOI] [PubMed] [Google Scholar]
- Parmentier, E. , Fine, M.L. , Berthe, C. & Lecchini, D. (2018) Taxonomic validation of Encheliophis chardewalli with description of calling abilities. Journal of Morphology, 279, 864–870. [DOI] [PubMed] [Google Scholar]
- Pergent, G. , Bazairi, H. , Bianchi, C. , Bouduresque, C. , Buia, M.C. , Clabaut, P. , Harmelin‐Vivien, M. , Mateo, M.A. , Montefalcone, M. , Morri, C. , Orfanidis, S. , Pergent‐Martini, C. , Semroud, R. , Serrano, O. , Verlaque, M. , 2012. Les herbiers de Magnoliophytes marines de Méditerranée: résilience et contribution à l'atténuation des changements climatiques. Gland, Suisse et Malaga, Espagne: IUCN. 80 pages. IUCN, Gland, Suisse et Malaga, Espagne.
- Picciulin, M. , Kéver, L. , Parmentier, E. & Bolgan, M. (2019) Listening to the unseen: passive acoustic monitoring reveals the presence of a cryptic fish species. Aquatic Conservation: Marine and Freshwater Ecosystems, 29, 202–210. [Google Scholar]
- Popper, A.N. & Hawkins, A.D. (2019) An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. Journal of Fish Biology, 94, 692–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priede, I.G. (2017) Deep‐sea fishes: biology, diversity, ecology and fisheries. Cambridge, UK: University Press. [Google Scholar]
- Rose, J.A. (1961) Anatomy and sexual dimorphism of the swim bladder and vertebral column in Ophidion holbrooki (Pisces: Ophidiidae). Bulletin of Marine Science, 11, 280–308. [Google Scholar]
- Rountree, R.A. & Bowers‐Altman, J. (2002) Soniferous behavior of the striped cusk‐eel, Ophidion marginatum . Bioacoustics, 12, 242–244. [Google Scholar]
- Sprague, M.W. & Luczkovich, J.J. (2001) Do striped cusk‐eels Ophidion marginatum (Ophidiidae) produce the “chatter” sound attributed to weakfish Cynoscion regalis (Sciaenidae)? Copeia, 3, 854–859. [Google Scholar]
- Stergiou, K.I. & Karpouzi, V.S. (2002) Feeding habits and trophic levels of Mediterranean fish. Reviews in Fish Biology and Fisheries, 11, 217–254. [Google Scholar]
- Tortonese, E. (1954) On Ophidion vassali Risso, type of a new genus of ophidiid fishes (Parophidion). Pubblicazioni della Stazione zoologica di Napoli, 25, 372–379. [Google Scholar]
- Tyler, J.C. (1970) A redescription of the inquiline carapid fish Onuxodon parvibrachium, with a discussion of the skull structure and the host. Bulletin of Marine Science, 20, 148–164. [Google Scholar]
- Urick, R.J. (1975) Principles of underwater sound. New York: McGraw‐Hill. [Google Scholar]
- Zanette, I. , Daghfous, G. , Weitkamp, T. , Gillet, B. , Adriaens, D. , Langer, M. et al. (2013). Looking inside marine organisms with magnetic resonance and X‐ray imaging. In: Reynaud, E.G. (Ed.) Imaging marine life. Available from: 10.1002/9783527675418.ch7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A


