Abstract
Background
We aimed to assess whether recurrence patterns affect survival and to use a multi-state model to predict the prognosis of early stage non-small cell lung cancer in patients who underwent surgical resection.
Methods
Patients with early stage non-small cell lung cancer who underwent surgical resection at two tertiary medical centers between 2010 and 2015 were retrospectively analyzed. A multi-state model was employed with one initial state (surgery), two intermediate states (locoregional recurrence, distant metastasis), and one absorbing state (death), comprising five transitions: surgery to locoregional recurrence, surgery to distant metastasis, surgery to death without recurrence, locoregional recurrence to death, and distant metastasis to death. Cox proportional hazards models stratified for these transitions were performed with the risk factors; transition probabilities for each patient were predicted.
Results
A total of 949 patients were identified [median age: 67 years, male: 614 (64.6%)]. Recurrence occurred in 194 (20.4%) patients (locoregional recurrence: 7.3%, distant metastasis: 13.1%). Hazard ratios for distant metastasis after surgery were higher for older age (hazard ratio: 1.03, 95% confidence interval: 1.01–1.06) and adenocarcinoma (hazard ratio: 1.67, 95% confidence interval: 1.06–2.61). Lower lobe location exhibited a higher hazard ratio for death after surgery without recurrence (hazard ratio: 1.59, 95% confidence interval: 1.00–2.53). Stage IIB lung cancer showed a higher probability of transition to distant metastasis after surgery than other stages. Cumulative transition hazards rapidly increased in both recurrence patterns until approximately two years after surgery (locoregional recurrence: 0.052; distant metastasis: 0.104). Patients with distant metastasis were more likely to die within 5 years of surgery than those with locoregional recurrence (6.8% and 2.6%, respectively).
Conclusions
With the multi-state model, risk factors and post-relapse survival probabilities differed between locoregional recurrence and distant metastasis. These findings may enable clinicians to establish personalized follow-up strategies for patients undergoing curative resection for early stage non-small cell lung cancer.
Keywords: Early stage non-small cell lung cancer, multi-state model, prognosis, treatment failure
Introduction
Early stage non-small cell lung cancer (NSCLC) accounts for approximately 40% of all NSCLC stages (1). With the development of low-dose computed tomography (CT) and widespread application of lung cancer screening, staging shifts have increased detection of early stage lung cancers (2). Earlier diagnosis leads to a survival gain of 33% and 60% for regional and local cancer stages, respectively (3). Surgery is the mainstay treatment for early stage NSCLC (4); however, treatment failure in patients undergoing surgery for early stage NSCLC is quite high (30–55%) (5). Treatment failure may result in locoregional recurrence and distant metastasis and is the most common cause of death in patients with lung cancer (6). The prognosis is worse in patients presenting with distant metastasis as a recurrence pattern than in those presenting with locoregional recurrence (7). Since the incidence of early stage NSCLC is gradually increasing and patient survival is stratified according to the recurrence pattern that is commonly encountered after surgery, the need for a more strategic and detailed management is emerging.
In traditional survival analysis, the disease process is expressed only in the ‘initial’ and ‘final’ states. However, events other than death such as recurrence or metastasis, which are called intermediate events, can occur during the disease process. It is expected that the outcome of patients who have experienced these intermediate events would be worse; therefore, it is desirable to use a model that considers intermediate events when predicting survival after surgery or identifying risk factors for death. In this study, we propose a multi-state model in which the ‘locoregional recurrence’ and ‘distant metastasis’ states are inserted between the initial and final states to provide a more detailed description of the disease pathway of lung cancer surgery patients (8).
Therefore, we aimed to comprehensively analyze the risk factors and survival according to recurrence patterns to establish a personalized follow-up strategy in patients who underwent surgical resection for early stage NSCLC using a multi-state model. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-148/rc).
Methods
Study population
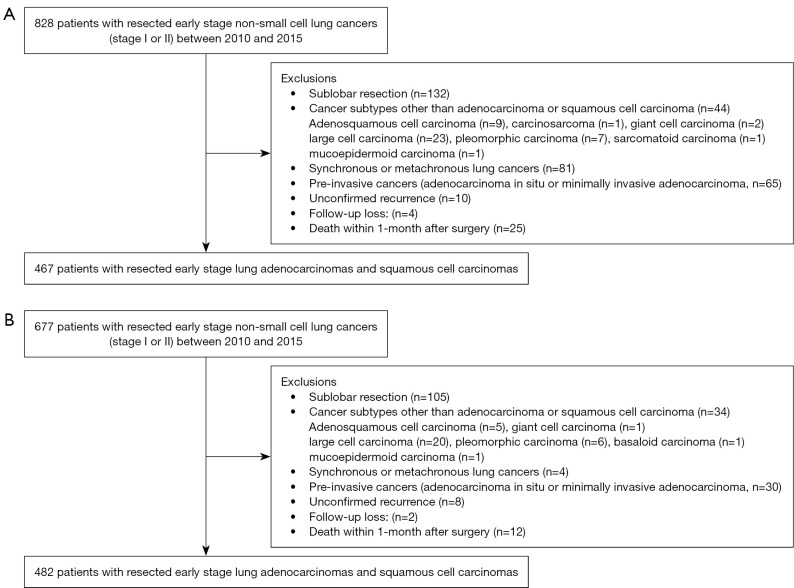
Data of patients who underwent curative resection for early stage NSCLCs between 2010 and 2015 were retrospectively collected. The end date of the study is December 31, 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (Approval No. CNUHH-2021-248) and Jeonbuk National University Hospital (Approval No. CUH-2020-11-010). The requirement for informed consent was waived owing to the retrospective study design. A total of 828 patients were identified in center I (Chonnam National University Hwasun Hospital) and 677 patients were identified in center II (Jeonbuk National University Hospital). The exclusion criteria were as follows: (I) sublobar resection, (II) cancer subtypes other than adenocarcinoma or squamous cell carcinoma, (III) synchronous or metachronous lung cancer, and (IV) pre-invasive lung cancer (adenocarcinoma in situ or minimally invasive adenocarcinoma). Additionally, patients with unconfirmed recurrence, follow-up loss, and deaths within 1-month after surgery were excluded. The final study population comprised 467 and 482 patients in centers I and II, respectively (Figure 1).
Figure 1.
Flow diagram of patient inclusions and exclusions. (A) Center I (Chonnam National University Hwasun Hospital); (B) Center II (Jeonbuk National University Hospital).
Data processing
Demographic data, including age, sex, smoking status (current or former vs. never), and history other than lung cancer, were investigated. Tumor characteristics were investigated as follows: location (non-lower lobe vs. lower lobe), tumor-node-metastasis (TNM) staging, and histology (adenocarcinoma or squamous cell carcinoma). The right middle lobe was regarded as a non-lower lobe. The pathological TNM staging was re-evaluated according to the eighth edition of the American Joint Commission on Cancer staging system for lung cancer (9). Surgery was categorized into lobectomy, bilobectomy, and pneumonectomy. Finally, we investigated whether adjuvant treatment had been administered.
Postoperative surveillance was performed with chest CT every 3–6 months for 2 years, then every 6–12 months until 5 years. In addition, annual or biannual surveillance with chest CT was performed in high-risk patients after 5 years. Two centers followed a similar surveillance protocol. Adjuvant treatment was administered in margin-positive cancers, high-risk IB lung cancers, and stage II cancers according to National Comprehensive Cancer Network (NCCN) guidelines (10). Recurrences were detected on routine surveillance studies in asymptomatic patients. When patient presented specific symptoms, recurrences were confirmed with imaging studies [CT, magnetic resonance imaging (MRI), 18F-labeled fluorodeoxyglucose positron emission tomography (18F-FDG PET), bone scan] or pathologic exams. A total of 112 recurrences were confirmed pathologically (112/194, 57.7%). Most of the pathologically unconfirmed recurrences were as follows: (I) small brain metastasis, (II) not able to obtain tissue due to the location, and (III) pathologically unconfirmed cases.
Treatment failure was divided into locoregional and distant metastases. Locoregional recurrence was defined as recurrent cancers in the ipsilateral lung or from 1 to 14 thoracic lymph node stations. Any new lesion beyond the criteria for locoregional recurrence was defined as a distant metastasis (6). The coexistence of locoregional recurrence and distant metastasis was considered distant metastasis. The dates and causes of death were investigated using the Korean Statistical Information Service.
Statistical analysis
The multi-state model composes of nodes and arrows (11). Nodes denote the states and are usually drawn as rectangles, and the arrows denote transitions and describe the disease pathways. The model considered in this study was a multi-state model as shown in Figure 2. A node with only outgoing arrows like ‘surgery’ is called the initial state, a node with both incoming and outgoing arrows like ‘locoregional recurrence’ or ‘distant metastasis’ is called the intermediate state, and a node with only incoming arrows like ‘death’ is called the final or absorbing state. Our model of interest in this study is a multi-state model with one initial state, two intermediate states, and one absorbing state. For notational simplicity, we have denoted ‘surgery’, ‘locoregional recurrence’, ‘distant metastasis’, and ‘death’ states by 0, 1, 2, and 3, respectively. In state 0, a patient is exposed to three competing events such as ‘locoregional recurrence’, ‘distant metastasis’, and ‘death’, but in states 1 and 2, it is exposed only to the ‘death’ event. Thus, patients undergoing lung cancer surgery experience one of six different disease processes: first, after experiencing locoregional recurrence after surgery, either survive until the end of the study (PW1) or die during the study period (PW2); second, after experiencing distant metastasis after surgery, either survive until the end of the study (PW3) or die during the study period (PW4); third, after surgery, either survive until the end of the study (PW5) or die during the study period (PW6).
Figure 2.
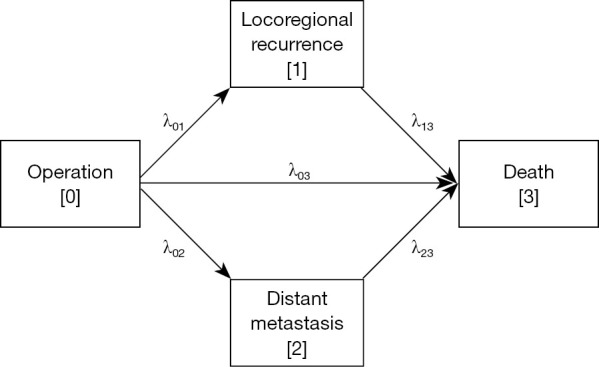
Multi-state model of disease course from surgery to death with locoregional recurrence and distant metastasis as intermediate events.
Changes in the disease process can be examined using transition hazards or probabilities. The former refers to the instantaneous probability of transitioning from one state to another at a time point, and the latter refers to the probability of transitioning from one state to another during the time interval. In Figure 2, λ01, λ02, λ03, λ13, and λ23 represent the hazard of transition from state 0 to state 1 (Transition 1), state 0 to state 2 (Transition 2), state 0 to state 3 (Transition 3), state 1 to state 3 (Transition 4), and state 2 to state 3 (Transition 5), respectively. The cumulative transition hazard is more widely used than the transition hazard, which is defined as the cumulative hazard of the transition from the start of the study to the time point. The cumulative transition hazard for each transition is estimated using the Nelson-Aalen estimator (12,13), and the transition probability is estimated using the Aalen-Johansen estimator (14). The former makes it possible to compare the changes in the hazard of recurrence and death after surgery and the changes in the hazard of death according to the presence or absence of recurrence. The latter can be used to compare the probabilities of being in states 0, 1, 2, and 3 until the time point after surgery. Note that because state 0 is the initial state, the transition probability monotonically decreases as time increases, and because states 1 and 2 are intermediate states, the transition probabilities can be up or down; however, because state 3 is the final state, the transition probability monotonically increases. To quantify the effects of covariates on the transition hazards, a Cox proportional hazards model was fitted for each of the five transitions, λ01, λ02, λ03, λ13, and λ23 (8). Univariate analysis was performed for each risk factor after controlling for the hospital effect. Subsequently, multivariate analysis was performed with only the risk factors that passed through the univariate analysis at a significance level of 10%. In addition, the transition probabilities for each patient were predicted using a model estimated by multivariate analysis.
All analyses were performed in R 4.1.2, and P<0.05 was considered statistically significant. The cumulative transition hazard was estimated using the ‘mvna’ function of the R package ‘etm’ (15) and the transition probability was estimated using the ‘transprob’ function of the R package ‘mstate’ (16). In addition, the function ‘coxph’ of the R package ‘survival’ (17) was employed for univariate and multivariate analyses.
Results
Patient characteristics
The median age of the patients was 67 years (25th to 75th percentile, 60 to 72 years). Among the 949 patients included in the analysis, 614 were male (64.7%) and 520 were current or former smokers (54.8%). A total of 134 patients had a history of malignancies other than lung cancer (14.1%). Postoperative pathological staging was as follows: IA =467 (49.2%), IB =231 (24.3%), IIA =65 (6.9%); and IIB =186 (19.6%). A total of 414 patients (43.6%) had lung cancer of the lower lobe. A total of 638 patients had adenocarcinoma (67.2%), and 311 patients had squamous cell carcinoma (32.8%). Adjuvant treatment was administered to 259 patients (27.3%). Most of the patients underwent lobectomy (93.8%), and the remaining patients underwent bilobectomy or pneumonectomy (6.2%). In total, 244 deaths were reported (25.7%). Of these, 186 patients who died from lung cancer were included (19.6%), and the remaining 58 patients who died from a cause other than lung cancer were classified as censored at the time of death (6.1%) (Table 1).
Table 1. Demographics of the study population.
| Variable | Number of patients (%) |
|---|---|
| Age (year), median (25th of 75th percentile) | 67 (60 to 72)* |
| Sex | |
| Female | 335 (35.3) |
| Male | 614 (64.7) |
| Smoking | |
| Never smoker | 429 (45.2) |
| Current or former smoker | 520 (54.8) |
| History of malignancy other than lung cancer | |
| Absent | 815 (85.9) |
| Present | 134 (14.1) |
| Stage | |
| IA | 467 (49.2) |
| IB | 231 (24.3) |
| IIA | 65 (6.9) |
| IIB | 186 (19.6) |
| Location | |
| Non-lower lobe | 535 (56.4) |
| Lower lobe | 414 (43.6) |
| Histology | |
| Squamous cell carcinoma | 311 (32.8) |
| Adenocarcinoma | 638 (67.2) |
| Adjuvant treatment | |
| Performed | 259 (27.3) |
| No | 690 (72.7) |
| Surgery method | |
| Lobectomy | 890 (93.8) |
| Bilobectomy or pneumonectomy | 59 (6.2) |
| Recurrence | |
| No | 755 (79.6) |
| Locoregional recurrence | 69 (7.3) |
| Distant metastasis | 125 (13.1) |
| Death | |
| Alive | 705 (74.3) |
| Death (lung cancer) | 186 (19.6) |
| Death (with a cause other than lung cancer)** | 58 (6.1) |
*, median (25th to 75th percentile); **, patients were classified as censored at the time of death.
Among 69 (7.3%) patients who experienced locoregional recurrence, 35 survived until the end of the study, 4 were censored (PW1, 4.1%), and 30 died during the study period (PW2, 3.2%). Among 125 patients (13.1%) who experienced distant metastasis, 40 survived, 9 were censored (PW3, 5.2%), and 76 died (PW4, 8%). A total of 630 patients survived without recurrence until the end of the study, 45 patients were censored (PW5, 71.1%), and 80 patients died without recurrence (PW6, 8.4%). Table 2 shows the number of patients in each pathway according to the stage.
Table 2. Number (%) of patients corresponding to each of the six progression pathways by stage.
| Stage | Progression pathway | Total | |||||
|---|---|---|---|---|---|---|---|
| PW1 | PW2 | PW3 | PW4 | PW5 | PW6 | ||
| IA | 17 (3.6) | 7 (1.5) | 16 (3.4) | 17 (3.6) | 381 (81.6) | 29 (6.2) | 467 |
| IB | 9 (3.9) | 4 (1.7) | 11 (4.8) | 20 (8.7) | 167 (72.3) | 20 (8.7) | 231 |
| IIA | 3 (4.6) | 7 (10.8) | 4 (6.2) | 8 (12.3) | 37 (56.9) | 6 (9.2) | 65 |
| IIB | 10 (5.4) | 12 (6.5) | 18 (9.7) | 31 (16.7) | 90 (48.4) | 25 (13.4) | 186 |
| Total | 39 (4.1) | 30 (3.2) | 49 (5.2) | 76 (8.0) | 675 (71.1) | 80 (8.4) | 949 |
PW1: alive or censored with locoregional recurrence; PW2: dead with locoregional recurrence; PW3: alive or censored with distant metastasis; PW4: dead with distant metastasis; PW5: alive or censored without recurrence; PW6: dead without recurrence.
Estimating the cumulative transition hazards and transition probabilities: Nonparametric approaches
To analyze the multi-state model, first, one-row data in the time-oriented format or state-oriented format must be transformed into a transition-oriented format or stacked format to generate multi-row data (8).
Figure 3 displays the Nelson-Aalen estimates of the cumulative transition hazards along with the 95% confidence intervals for all the five transitions. Note that the slope of each curve reflects the transition hazard that transitions to the subsequent state. As seen in the enlarged image in Figure 3, the transition hazard of death without recurrence remains constant after surgery, but the transition hazards of recurrence tend to be constant until approximately 2 years after surgery and then decrease. For example, at 2 years after surgery, the cumulative transition hazard of having experienced locoregional recurrence and distant metastasis was 0.502 (95% CI: 0.039–0.070) and 0.104 (95% CI: 0.085–0.128), respectively. Moreover, patients with recurrence have a much higher risk of death than patients without recurrence, and between the two types of recurrence, ‘distant metastasis’ was more exposed to death than ‘locoregional recurrence’. For example, at 5 years after surgery, the cumulative transition hazards of death without recurrence, death with locoregional recurrence, and death with distant metastasis were 0.088 (95% CI: 0.068–0.112), 0.678 (95% CI: 0.436–1.053), and 1.09 (95% CI: 0.846–1.404), respectively.
Figure 3.
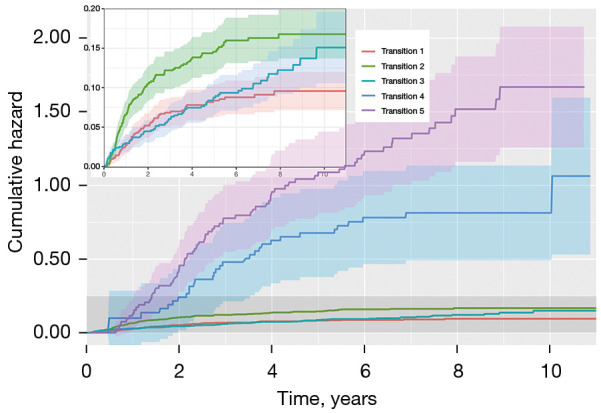
Nelson-Aalen estimates of the cumulative transition hazard for all five transitions. Transition 1: surgery to locoregional recurrence; Transition 2: surgery to distant metastasis; Transition 3: surgery to death; Transition 4: locoregional recurrence to death; Transition 5: distant metastasis to death.
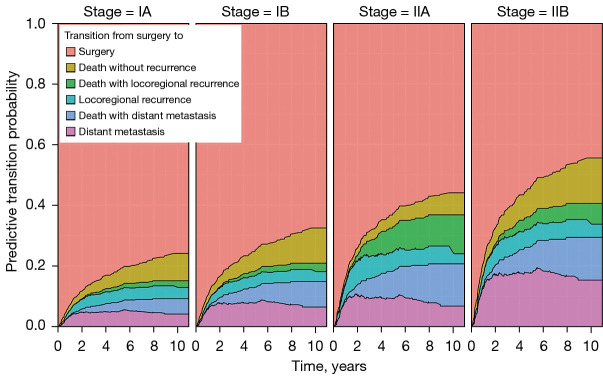
In Figure 4, the height of each colored region corresponds to Aalen–Johansen’s estimated probability of being in the relevant state at the time point, and the sum of the heights at any time point is equal to 1. Note that the estimated transition probability to the state ‘death’ is partitioned into three regions depending on the pathways of death: i.e., death without recurrence, death with locoregional recurrence, and death with distant metastasis. As expected, the estimated transition probabilities corresponding to the states, ‘locoregional recurrence’ and ‘distant metastasis’, were up or down, while that to the state ‘surgery’ was monotone decreasing and that to the state ‘death’ was monotone increasing. For example, the probability of death within 5 years for a patient who underwent lung cancer surgery was estimated to be 16.8%, the probability of locoregional recurrence was 4.6% (95% CI: 3.4–6.1%), and the probability of distant metastasis was 5.8% (95% CI: 4.5–7.7%). Among the 16.8% probability of death, the estimated probability of death without recurrence was 7.4% (95% CI: 5.9–9.4%) and that of death after recurrence was 9.4%. In addition, among 9.4% of deaths after recurrence, the probabilities of death after locoregional recurrence and distant metastasis were 2.6% (95% CI: 1.7–3.8%) and 6.8% (95% CI: 5.3–8.6%), respectively. The supplementary forest plots contain the 95% confidence intervals along with the point estimates of the transition probability for each of the five transitions at 1, 2, 3, and 5 year(s) since surgery (Figure S1).
Figure 4.
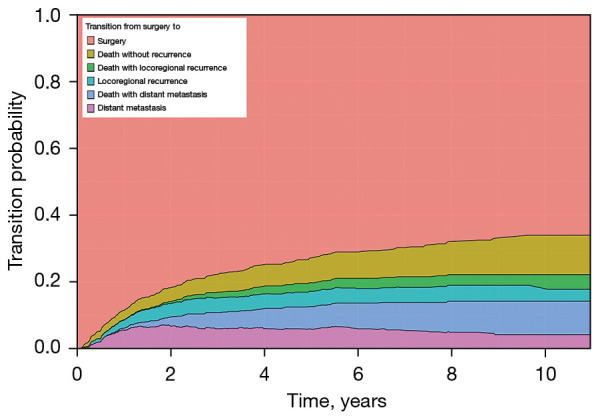
Aalen-Johansen estimates of transition probabilities from surgery to surgery, locoregional recurrence, distant metastasis or death in a stacked display format.
Identifying risk factors to recurrence and/or death and predicting transition probabilities: Semi-parametric approaches
A multi-state Cox proportional hazards model, stratified for transition, was fitted to estimate the appropriate contribution of each variable to the risk of recurrence or death. We included all potential predictors at baseline (age, sex, smoking status, history other than lung cancer, tumor location, stage, histology, surgery method, and adjuvant treatment). The transition hazard rates in the univariate analyses, after adjusting for the hospital, are presented in Table 3. No significant role of a history other than lung cancer emerged in any transition. All other covariates had significant univariate effects on recurrence or death, at a significance level of 0.1. Therefore, we set up a multivariate Cox regression model with all covariates considered for univariate analysis, except for a history of other malignancies (Table 4). In multivariate analysis, when controlling for hospital, higher stage (HR: 3.16; 95% CI: 1.43–6.98 for stage IIA and HR: 2.38; 95% CI: 1.08–5.21 for stage IIB, both compared to stage IA) was a risk factor for locoregional recurrence after surgery (Transition 1). Older age (HR: 1.03; 95% CI: 1.01–1.06) and higher stage (HR: 1.69; 95% CI: 1.00–2.85 for stage IB, HR: 2.58; 95% CI: 1.29–5.18 for stage IIA, and HR: 3.96; 95% CI: 2.20–7.11 for stage IIB, compared to stage IA) were significantly associated with a higher risk of distant metastasis after surgery (Transition 2). Additionally, adenocarcinoma had a higher hazard ratio to transition 2 (HR: 1.67; 95% CI: 1.06–2.61). Risk factors associated with death without recurrence (Transition 3) were older age (HR: 1.09; 95% CI: 1.05–1.13), stage IIB (HR: 2.30; 95% CI: 1.16–4.54), and lower lobe (HR: 1.59; 95% CI: 1.00–2.53). In addition, older age (HR: 1.04; 95% CI: 1.01–1.07) had a higher hazard ratio to transition 5 (death after distant metastasis). No significant covariate was found for death after locoregional recurrence (Transition 4) in the model.
Table 3. Results of univariate analysis after adjusting for the hospital effect.
| Variable | Transition 1 | Transition 2 | Transition 3 | Transition 4 | Transition 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age | 0.99 (0.97, 1.02) | 0.585 | 1.03 (1.01, 1.05) | 0.004 | 1.11 (1.07, 1.14) | <0.001 | 1.03 (1.00, 1.08) | 0.085 | 1.04 (1.01, 1.07) | 0.019 | ||||
| Male (reference: female) | 1.77 (1.04, 3.04) | 0.037 | 1.30 (0.89, 1.89) | 0.175 | 2.91 (1.63, 5.18) | <0.001 | 2.36 (0.90, 6.20) | 0.081 | 1.05 (0.64, 1.71) | 0.852 | ||||
| Current or former smoker (reference: never smoker) | 1.87 (1.13, 3.11) | 0.016 | 1.26 (0.88, 1.80) | 0.208 | 2.03 (1.26, 3.26) | 0.004 | 1.91 (0.82, 4.46) | 0.134 | 1.25 (0.79, 1.99) | 0.344 | ||||
| History of malignancy other than lung cancer (reference: no) | 0.65 (0.28, 1.50) | 0.311 | 1.24 (0.77, 2.00) | 0.387 | 1.54 (0.88, 2.71) | 0.131 | 1.85 (0.68, 5.04) | 0.228 | 1.39 (0.75, 2.56) | 0.297 | ||||
| Stage (reference: IA) | ||||||||||||||
| IB | 1.17 (0.60, 2.31) | 0.640 | 2.06 (1.26, 3.36) | 0.004 | 1.56 (0.88, 2.75) | 0.128 | 1.00 (0.29, 3.45) | 1.000 | 1.25 (0.64, 2.42) | 0.512 | ||||
| IIA | 3.43 (1.64, 7.19) | 0.001 | 3.07 (1.58, 5.94) | 0.001 | 1.79 (0.74, 4.32) | 0.195 | 2.77 (0.95, 8.07) | 0.062 | 1.82 (0.78, 4.25) | 0.165 | ||||
| IIB | 3.14 (1.76, 5.61) | <0.001 | 4.91 (3.16, 7.65) | <0.001 | 2.96 (1.73, 5.05) | <0.001 | 1.98 (0.77, 5.07) | 0.157 | 1.13 (0.62, 2.06) | 0.683 | ||||
| Lower lobe location (reference: non-lower lobe) | 0.87 (0.54, 1.42) | 0.585 | 0.90 (0.63, 1.29) | 0.569 | 1.88 (1.20, 2.94) | 0.005 | 0.62 (0.29, 1.34) | 0.223 | 1.11 (0.69, 1.78) | 0.680 | ||||
| Adenocarcinoma (reference: squamous cell carcinoma) | 0.73 (0.45, 1.19) | 0.211 | 0.91 (0.63, 1.32) | 0.619 | 0.32 (0.21, 0.50) | <0.001 | 0.43 (0.21, 0.89) | 0.023 | 0.68 (0.43, 1.09) | 0.110 | ||||
| No adjuvant treatment (reference: performed adjuvant treatment) | 0.41 (0.25, 0.68) | <0.001 | 0.35 (0.24, 0.51) | <0.001 | 1.02 (0.61, 1.73) | 0.929 | 0.80 (0.37, 1.77) | 0.585 | 0.92 (0.56, 1.52) | 0.759 | ||||
| Bilobectomy or pneumonectomy (reference: lobectomy) | 1.79 (0.82, 3.94) | 0.144 | 1.77 (0.97, 3.22) | 0.061 | 1.94 (0.93, 4.05) | 0.076 | 2.29 (0.85, 6.20) | 0.103 | 1.02 (0.46, 2.25) | 0.963 | ||||
Transition 1: surgery to locoregional recurrence; Transition 2: surgery to distant metastasis; Transition 3: surgery to death without recurrence; Transition 4: locoregional recurrence to death; Transition 5: distant metastasis to death.
Table 4. Results of multivariate analysis after adjusting for the hospital effect.
| Variable | Transition 1* | Transition 2* | Transition 3* | Transition 4* | Transition 5* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age | 0.99 (0.97, 1.02) | 0.536 | 1.03 (1.01, 1.06) | 0.005 | 1.09 (1.05, 1.13) | <0.001 | 1.03 (0.98, 1.07) | 0.247 | 1.04 (1.01, 1.07) | 0.014 | ||||
| Male (reference: female) | 1.20 (0.53, 2.68) | 0.663 | 1.16 (0.67, 2.02) | 0.592 | 1.94 (0.90, 4.19) | 0.093 | 2.42 (0.37, 15.7) | 0.354 | 0.66 (0.31, 1.41) | 0.286 | ||||
| Current or former smoker (reference: never smoker) | 1.42 (0.65, 3.08) | 0.376 | 1.03 (0.61, 1.76) | 0.908 | 1.04 (0.54, 1.98) | 0.91 | 0.66 (0.11, 3.78) | 0.639 | 1.43 (0.66, 3.09) | 0.367 | ||||
| Stage (reference: IA) | ||||||||||||||
| IB | 1.07 (0.53, 2.20) | 0.845 | 1.69 (1.00, 2.85) | 0.049 | 1.38 (0.76, 2.53) | 0.29 | 1.28 (0.31, 5.25) | 0.736 | 1.03 (0.51, 2.08) | 0.930 | ||||
| IIA | 3.16 (1.43, 6.98) | 0.004 | 2.58 (1.29, 5.18) | 0.008 | 0.99 (0.39, 2.49) | 0.983 | 3.16 (0.97, 10.3) | 0.057 | 1.38 (0.56, 3.39) | 0.486 | ||||
| IIB | 2.38 (1.08, 5.21) | 0.031 | 3.96 (2.20, 7.11) | <0.001 | 2.30 (1.16, 4.54) | 0.016 | 1.89 (0.46, 7.73) | 0.373 | 0.77 (0.37, 1.62) | 0.493 | ||||
| Lower lobe location (reference: non-lower lobe) | 0.83 (0.51, 1.34) | 0.436 | 0.82 (0.57, 1.17) | 0.278 | 1.59 (1.00, 2.53) | 0.048 | 0.75 (0.31, 1.79) | 0.515 | 1.14 (0.68, 1.92) | 0.620 | ||||
| Adenocarcinoma (reference: squamous cell carcinoma) | 1.31 (0.73, 2.36) | 0.362 | 1.67 (1.06, 2.61) | 0.026 | 0.61 (0.35, 1.05) | 0.075 | 0.56 (0.20, 1.54) | 0.263 | 0.69 (0.37, 1.28) | 0.241 | ||||
| No adjuvant treatment (reference: performed adjuvant treatment) | 0.69 (0.36, 1.32) | 0.264 | 0.65 (0.41, 1.05) | 0.076 | 1.60 (0.84, 3.03) | 0.151 | 0.65 (0.19, 2.25) | 0.501 | 0.66 (0.36, 1.23) | 0.194 | ||||
| Bilobectomy or pneumonectomy (reference: lobectomy) | 1.13 (0.49, 2.60) | 0.780 | 1.22 (0.65, 2.29) | 0.529 | 1.09 (0.51, 2.36) | 0.822 | 2.17 (0.72, 6.52) | 0.169 | 0.80 (0.33, 1.94) | 0.615 | ||||
*, Transition 1: surgery to locoregional recurrence, Transition 2: surgery to distant metastasis, Transition 3: surgery to death without recurrence, Transition 4: locoregional recurrence to death, Transition 5: distant metastasis to death.
Figure 5 illustrates predictive transition probabilities from surgery to surgery, locoregional recurrence, distant metastasis, or death for an illustrative patient according to the pathological TNM stages of IA, IB, IIA, and IIB. This patient’s risk factors were set to age =67, histology = adenocarcinoma, sex = male, adjuvant treatment = no, smoking = current or former smoker, location = non-lower lobes, and surgery method = lobectomy. The height from the baseline to the fourth graph represents the probability of recurrence after surgery. The graph located at the bottom shows the probability of distant metastasis after surgery. Patients with higher stage lung cancer tended to have a high probability of recurrence; for example, at 5 years since surgery, the estimated transition probabilities of recurrence were 13.3%, 18.4%, 32.9%, and 36.6% for each of the pathological TNM stages of IA, IB, IIA, and IIB. In particular, patients with stage IIB lung cancer had a higher probability of distant metastasis after surgery than those with other stage lung cancers; for example, at 5 years since surgery, the transition probabilities of distant metastasis after surgery were 4.9% (95% CI: 2.5–9.7%), 7.8% (95% CI: 3.6–16.9%), 9.2 (95% CI: 3.3–25.8%), and 17.6 (95% CI: 8.5–36.5%) for each of the pathological TNM stages of IA, IB, IIA, and IIB. The supplementary forest plots contain the 95% confidence intervals along with the point estimates of the transition probability for each of the five transitions at 1, 2, 3, and 5 year(s) since surgery by the pathological TNM stages of IA, IB, IIA, and IIB (Figure S2).
Figure 5.
Predictive transition probabilities from surgery to surgery, locoregional recurrence, distant metastasis or death for a patient according to the pathological TNM, tumor-node-metastasis. Stage of IA, IB, IIA, and IIB.
Discussion
Our study revealed the following: (I) among 20.4% of recurrences, locoregional recurrence and distant metastasis accounted for 7.3 and 13.1%, respectively. (II) A multi-state model was useful for identifying risk factors according to recurrence patterns. (III) The estimated probabilities of death within 5 years after surgery were 2.6 and 6.8% for death after locoregional recurrence and distant metastasis, respectively. (IV) The transition hazards rapidly increased in both recurrence patterns until approximately 2 years after surgery.
Using a multi-state model analysis, we were able to specify the risk factors in each transition. Transitions in distant metastasis demonstrated the following unique risk factors: the histology of adenocarcinoma was revealed as having significance in the transition from surgery to distant metastasis. This result is consistent with those of previous studies, which reported that distant metastasis is more common in adenocarcinoma (18,19). Patients with stage IIB lung cancer have a higher probability of distant metastasis after surgery than those with other lung cancer stages. This can be partly explained by the inclusion of lymph node metastasis in stage IIB lung cancer. Tian et al. reported that lymph node metastasis was associated with distant metastasis in patients with resected early-stage NSCLC (20). Older age was revealed as a risk factor for transitions of surgery to distant metastasis, surgery to death, and distant metastasis to death. Zhu et al. reported that older patients (≥65 years) have a higher hazard rate of recurrence than the younger group among patients with resected NSCLC (21). In addition, our study demonstrated that older age is a specific risk factor for distant metastasis after surgery rather than locoregional recurrence. The lower lobe location is generally regarded as an adverse prognostic factor for lung cancer (22); additionally, it was a specific risk factor for the transition from surgery to death without recurrence in our study. Lee et al. reported that the lower lobe location was adversely associated with 5-year survival in patients with stage I-III NSCLC (22). A high probability of upstaging of the T and N stages of lower lobe lung cancers is one of the most frequently mentioned pathomechanisms of poor prognosis (23). It is noteworthy that, in our study, the lower lobe location was only significantly associated with surgery to death but not associated with surgery to locoregional recurrence or distant metastasis. Chronic lung disease such as pulmonary fibrosis, which predominates in the lower lobe, may have mediated these outcomes (24). As we only conducted analysis with early stage NSCLC, different results may be obtained when advanced stage NSCLCs are included in the context of recurrence after surgery. Knowledge of these risk factors according to the transitions will aid clinicians in establishing a more customized follow-up strategy.
None of the covariates were statistically significant in the transitions of relapse to death, except age, which was a significant risk factor for the transition of distant metastasis to death in our study. In addition, the initial TNM staging was found to have no significant effect on survival after relapse. Overall survival is known to be associated with post-relapse survival rather than relapse-free survival in patients who undergo surgical resection of NSCLC (25). Imai et al. recently reported that good performance status at relapse and the use of tyrosine kinase inhibitors after relapse are favorable for post-relapse survival (26), and the administration of immune checkpoint inhibitors and radiotherapy for oligo-recurrences are favorable for post-relapse survival in driver gene mutation/translocation-negative or unknown type NSCLC (27). A study on the disease course and overall survival after relapse with the addition of relevant factors at the time of relapse needs to be conducted.
Our study also has implications for the management of resected early stage NSCLC, particularly regarding the timing of recurrence. Our results revealed that both locoregional recurrence and distant metastasis rapidly increased within 2 years after surgery, which is consistent with a previous report (28). Based on the results of our study, close follow-up is required until 2 years after surgery, which is in agreement with the American Society of Clinical Oncology (ASCO) guidelines that recommend follow-up once every 6 months for 2 years after surgery (29). Our study additionally justifies performing surveillance CT until 3 years after surgery, as the probability of recurrence significantly increased until 3 years. The NCCN also recommends performing surveillance CT every 6 months for 2–3 years (10). Our study also has clinical implications as a basis for personalized follow-up strategies during the follow-up period. 18F-labeled fluorodeoxyglucose positron emission tomography (18F-FDG PET) or brain MRI are not recommended as a routine surveillance tool in ASCO guideline (evidence quality: low; strength of recommendation: moderate) (29). Considering that distant metastasis is followed by a worse prognosis compared to locoregional recurrence, as in our study, using whole-body imaging after surgery may be emphasized for the early application of systemic chemotherapy. Torok et al. also reported that distant metastasis detected by surveillance imaging showed favorable overall survival compared with symptomatic distant metastasis (30). Our suggestion for early adjuvant chemotherapy is consistent with a previous meta-analysis by Burdett et al., which concluded that the 5-year survival can be improved with adjuvant chemotherapy following surgery and postoperative radiotherapy (31). Margin-positive cancers, high-risk stage IB lung cancers, and stage II lung cancers are the current indications for adjuvant treatment (10). In particular, the immune signature can be utilized in the selection of high-risk patients for guiding adjuvant treatment. Immune-related genes were significant prognostic biomarkers for estimating overall survival in early-stage lung adenocarcinomas (32,33). Furthermore, whole-body imaging and application of adjuvant treatment can be actively considered in patients with a histology indicative of adenocarcinoma, older age, and stage IIB lung cancers, as our study revealed that these characteristics were specifically associated with distant metastasis in multi-state models. Although we recommended a regular intensive follow-up strategy for improving patients’ prognosis, that does have the disadvantage of reduced cost-effectiveness (34). Further research is needed to formulate a balanced follow-up strategy keeping in mind clinical and economic aspects.
Our study had several limitations. First, this was a multicenter, retrospective study. Second, we did not include covariates that affect post-relapse survival. Further prospective studies, including covariates related to the time of recurrence, are required to confirm our results. Third, we excluded patients who underwent sublobar resection because lobectomy with lymph node dissection is considered the standard treatment for early stage disease (35). Finally, patients with second primary lung cancer were excluded. Surviving patients with primary lung cancer are at risk of developing second primary lung cancer (36). ASCO also recommends performing annual CT after 2 years of surveillance in patients with resected NSCLC to detect second primary lung cancers (29).
With the multi-state model, the risk factors and post-relapse survival probabilities differed between locoregional recurrence and distant metastasis. This will enable clinicians to establish personalized follow-up strategies for patients who undergo curative resection for early stage NSCLC.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. NRF-2020R1F1A1A01048397) (Jinheum Kim) and by an NRF grant funded by the Korean government (MSIT) (No. NRF-2021R1C1C1009818) (Kum Ju Chae).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (Approval No. CNUHH-2021-248) and Jeonbuk National University Hospital (Approval No. CUH-2020-11-010). The requirement for informed consent was waived owing to the retrospective study design.
Footnotes
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-148/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-148/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-148/coif). The authors have no conflicts of interest to declare.
References
- 1.Lee JG, Kim HC, Choi CM. Recent Trends of Lung Cancer in Korea. Tuberc Respir Dis (Seoul) 2021;84:89-95. 10.4046/trd.2020.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021;325:971-87. 10.1001/jama.2021.0377 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 4.Herrera LJ, Wherley EM, Agyabeng-Dadzie K, et al. 500 Consecutive Robotic Lobectomies for Non-Small Cell Lung Cancer: Perioperative and Oncologic Outcomes. Innovations (Phila) 2021;16:441-7. 10.1177/15569845211030917 [DOI] [PubMed] [Google Scholar]
- 5.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81; discussion 81-2. 10.1016/j.jtcvs.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 7.Consonni D, Pierobon M, Gail MH, et al. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst 2015;107:djv059. 10.1093/jnci/djv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geskus RB. Data analysis with competing risks and intermediate states. CRC Press Boca Raton; 2016. [Google Scholar]
- 9.Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger D, Wood D, Aisner D, et al. Non-small cell lung cancer. NCCN clinical Practice Guidelines in Oncology, Version 3. 2020. J Natl Compr Cancer Netw. 2020;3. [Google Scholar]
- 11.Beyersmann J, Allignol A, Schumacher M. Competing risks and multistate models with R. Springer Science & Business Media; 2011. [Google Scholar]
- 12.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics 1972;14:945-66. 10.1080/00401706.1972.10488991 [DOI] [Google Scholar]
- 13.Aalen O. Nonparametric inference for a family of counting processes. Ann Statist 1978;6:701-26. 10.1214/aos/1176344247 [DOI] [Google Scholar]
- 14.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Statist 1978;5:141-50. [Google Scholar]
- 15.Allignol A, Schumacher M, Beyersmann J. Empirical transition matrix of multi-state models: the etm package. Journal of Statistical Software 2011;38:1-15. 10.18637/jss.v038.i04 [DOI] [Google Scholar]
- 16.de Wreede LC, Fiocco M, Putter H. mstate: an R package for the analysis of competing risks and multi-state models. Journal of Statistical Software 2011;38:1-30. 10.18637/jss.v038.i07 [DOI] [Google Scholar]
- 17.Therneau T. A Package for Survival Analysis in R. R Package Version 3.2-13. 2021.
- 18.Hung JJ, Jeng WJ, Hsu WH, et al. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012;7:1115-23. 10.1097/JTO.0b013e31824cbad8 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Wang Z, Pan J, et al. Patterns of Extrathoracic Metastases in Different Histological Types of Lung Cancer. Front Oncol 2020;10:715. 10.3389/fonc.2020.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Y, He Y, Li X, et al. Novel nomograms to predict lymph node metastasis and distant metastasis in resected patients with early-stage non-small cell lung cancer. Ann Palliat Med 2021;10:2548-66. 10.21037/apm-20-1756 [DOI] [PubMed] [Google Scholar]
- 21.Zhu JF, Feng XY, Zhang XW, et al. Time-varying pattern of postoperative recurrence risk of early-stage (T1a-T2bN0M0) non-small cell lung cancer (NSCLC): results of a single-center study of 994 Chinese patients. PLoS One 2014;9:e106668. 10.1371/journal.pone.0106668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HW, Lee CH, Park YS. Location of stage I-III non-small cell lung cancer and survival rate: Systematic review and meta-analysis. Thorac Cancer 2018;9:1614-22. 10.1111/1759-7714.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha AT, McCormack M, Montana G, et al. Association between lower lobe location and upstaging for early-stage non-small cell lung cancer. Chest 2004;125:1424-30. 10.1378/chest.125.4.1424 [DOI] [PubMed] [Google Scholar]
- 24.Lee HW, Park YS, Park S, et al. Poor prognosis of NSCLC located in lower lobe is partly mediated by lower frequency of EGFR mutations. Sci Rep 2020;10:14933. 10.1038/s41598-020-71996-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai H, Takahashi T, Mori K, et al. Individual-level data on the relationships of progression-free survival, post-progression survival, and tumor response with overall survival in patients with advanced non-squamous non-small cell lung cancer. Neoplasma 2014;61:233-40. 10.4149/neo_2014_030 [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Onozato R, Kaira K, et al. Course of postoperative relapse in non-small cell lung cancer is strongly associated with post-progression survival. Thorac Cancer 2021;12:2740-8. 10.1111/1759-7714.14119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai H, Onozato R, Kaira K, et al. Post-Progression Survival Highly Influences Overall Survival in Driver Gene Mutation/Translocation Negative or Unknown Type of Non-Small Cell Lung Cancer. Oncology 2022;100:89-100. 10.1159/000521141 [DOI] [PubMed] [Google Scholar]
- 28.Lou F, Sima CS, Rusch VW, et al. Differences in patterns of recurrence in early-stage versus locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;98:1755-60; discussion 1760-1. 10.1016/j.athoracsur.2014.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider BJ, Ismaila N, Aerts J, et al. Lung Cancer Surveillance After Definitive Curative-Intent Therapy: ASCO Guideline. J Clin Oncol 2020;38:753-66. 10.1200/JCO.19.02748 [DOI] [PubMed] [Google Scholar]
- 30.Torok JA, Gu L, Tandberg DJ, et al. Patterns of Distant Metastases After Surgical Management of Non-Small-cell Lung Cancer. Clin Lung Cancer 2017;18:e57-70. 10.1016/j.cllc.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 31.Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015;(3):CD011430. 10.1002/14651858.CD011430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Cui Y, Diehn M, et al. Development and Validation of an Individualized Immune Prognostic Signature in Early-Stage Nonsquamous Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1529-37. 10.1001/jamaoncol.2017.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu P, Zheng Y, Wang Y, et al. Development and validation of a robust immune-related prognostic signature in early-stage lung adenocarcinoma. J Transl Med 2020;18:380. 10.1186/s12967-020-02545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egermann U, Jaeggi K, Habicht JM, et al. Regular follow-up after curative resection of nonsmall cell lung cancer: a real benefit for patients? Eur Respir J 2002;19:464-8. 10.1183/09031936.02.00231802 [DOI] [PubMed] [Google Scholar]
- 35.Adachi H, Maehara T, Nakayama H, et al. Mediastinal lymph node dissection in surgical treatment for early stage non-small-cell lung cancer: lobe-specific or systematic? J Thorac Dis 2017;9:2728-31. 10.21037/jtd.2017.07.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surapaneni R, Singh P, Rajagopalan K, et al. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol 2012;7:1252-6. 10.1097/JTO.0b013e3182582a79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as