Abstract
Objectives:
Persons living with dementia (PLWD), particularly those with higher levels of functional impairment, are at increased risk of hospitalization and higher hospital-associated health care costs. Our objective was to provide a nuanced description of reasons for hospitalizations over a 12-month period among community living persons with dementia taking part in a dementia care coordination study using caregiver-reported data and to describe how reasons varied by disease stage.
Design:
Retrospective descriptive analysis of pooled data from two concurrent studies of PLWD receiving the MIND at Home dementia care coordination program.
Setting and Participants:
494 community-living PLWD with a family caregiver in the Greater Baltimore and Central Maryland region, 2015–2019.
Methods:
PLWD sociodemographic, clinical, functional, cognitive, and behavioral characteristics were assessed during an in-home baseline visit. Caregiver-reported hospitalizations and primary reasons for events were recorded every 4.5 months by research staff and by memory care coordinators during program delivery for a 12-month period. Hospitalization event data were subsequently reviewed, reconciled, and coded by a trained investigator.
Results:
One hundred and seventy PLWD (34.4%) had at least one hospitalization within 12 months of enrollment, with 316 separate events. The most common primary reason for hospitalization according to caregivers was infection (22.4%), falls (16.5%), and cardiovascular/pulmonary (12.4%). Top reasons for hospitalization were falls among persons with mild and moderate functional impairment (17.7% and 21.9% respectively) and infection among PLWD with severe impairment (30.3%).
Conclusions and Implications:
Infections and falls were the most common caregiver-reported reasons for hospitalization in PLWD receiving dementia care coordination. Reasons for hospitalization varied based on severity of functional impairment. Greater understanding of reasons for hospitalization among PLWD receiving dementia care management interventions, from multiple important perspectives, may help programs more effectively address and prevent hospitalization.
Keywords: Hospitalization, Dementia, Care coordination
Brief summary:
Infections and falls were the most common caregiver-reported causes of hospitalization in older adults with dementia receiving care coordination. Causes of hospitalization varied by functional impairment severity.
Introduction
Alzheimer’s disease and related dementias pose a significant burden on patients and families, affecting an estimated 6.2 million Americans.1 Dementia is associated with greater healthcare utilization: persons living with dementia (PLWD) experience more skilled nursing facility stays, all-cause hospitalizations, and emergency room use.2–4 PLWD are at particularly high risk for fall-related healthcare use and potentially preventable admissions due to ambulatory care-sensitive conditions (e.g. congestive heart failure, diabetes, pneumonia).3,5 Once hospitalized, PLWD are also at increased risk for worse hospitalization outcomes including longer lengths of stay, unnecessary interventions, and functional decline;6 it is therefore important to understand the initial event that results in hospitalization for community-dwelling PLWD. Functional impairment, a proxy for disease stage, is a consistent and strong risk factor for hospitalization among PLWD.7, 8
Reducing hospitalizations in PLWD is an important patient-centered and public health outcome. Healthy People 2030 specifically includes an aim to decrease the proportion of preventable hospitalizations among PLWD with strategies including increased ambulatory care and care coordination.5 Several dementia care management interventions that have improved other outcomes, such as nursing home placement, have had limited success in addressing hospitalization.9, 10 Understanding reasons for hospitalization in PLWD is essential to preventing hospitalization though few studies have examined this issue,3,11,12 and most have used administrative claims data, which provides a singular, narrow perspective.3,11 Examining reasons for hospitalization from the caregiver’s perspective provides a different, important perspective. Caregiver factors are associated with hospitalization among PLWD,13 and caregivers are integral in their medical care and support, monitoring symptoms, managing chronic diseases and medical visits, and making decisions regarding need for urgent or hospital-level care. Caregiver perspectives on hospitalization may provide key insight into more effective ways to target dementia care coordination interventions.
The objective of this study was to provide a description of caregiver-reported reasons for hospitalization over a 12-month period among community living PLWD receiving the Maximizing Independence (MIND) at Home dementia care coordination program and to describe how reasons varied by functional impairment.
Methods
Study Design
This study included a pooled sample of 494 PLWD who received the MIND at Home intervention14 (http://www.mindathome.org/) for up to 18 months through a randomized controlled trial (RCT, PLWD randomized to the intervention arm)15 or a CMS Health Care Innovation Award demonstration project.16 Participants in both the RCT and demonstration project consisted of English-speaking patients diagnosed with all-cause dementia living privately in the greater Baltimore, Maryland region with a reliable caregiver and without end-stage disease (Supplementary Figure 1 displays participant flow diagram). The two studies occurred simultaneously in Maryland from 2015 to 2019 and used nearly identical assessments and home-based dementia care coordination interventions previously described in detail.15,16 Primary outcomes were time to nursing home placement or death, with secondary outcomes of unmet care needs, neuropsychiatric symptoms, quality of life, caregiver burden, and healthcare costs. 15,16 Both studies were approved and monitored by the Johns Hopkins Medicine Institutional Review Board.
Following an initial baseline visit and needs assessment, intervention participants and their study partner (usually their primary caregiver) received a comprehensive in-person home-based needs assessment followed by individualized care planning and care coordination led by a trained, non-clinical memory care coordinator. Care coordinators were integrated into a collaborative interdisciplinary team that included registered nurses and geriatric psychiatrists. Weekly case round meetings were held to review cases and provide clinical support. Unmet needs and patient/family priorities guided protocolized, individualized care planning, which included dementia education, direct care facilitation, referrals to services, emotional support and informal coaching, and care monitoring. Participants and caregivers had direct encounters, phone or in-person, with care coordinators every 30 days at a minimum and averaged about 2.5 encounters per month. When indicated, video visits were conducted with team clinicians. The intervention also facilitated communication with primary care and specialty care providers.
Study procedures and measures
Study measurements were collected at scheduled timepoints (baseline, 4.5, 9, 13.5, and 18 months for the RCT; baseline, 9, and 18 months for the demonstration project) through self-report by the study partner.15 Baseline data collected included demographic information, medications, comorbidities, and measures of function (Psychogeriatric Dependency Rating Scales [PGDRS-b] behavioral subscale), and cognition (Mini Mental State Examination [MMSE]). The PGDRS-b subscale consists of 16 items on a 3-point scale for a maximum score of 48; higher scores reflect worse mental and behavioral dysfunction and greater functional impairment.17,18 MMSE is measured out of 30 with higher scores reflecting better cognitive function.19
The MIND at Home studies defined Serious Adverse Events (SAE) as hospitalization, long-term care placement, or death. Hospitalization, the focus of this study, was identified either at the time of hospitalization by caregiver self-report to the memory care coordinator or at scheduled assessment intervals, during which caregivers were asked whether any hospitalizations had occurred in the interval between the current and last visit date. For each SAE, the date of onset, cause, duration, discharge disposition, and a written narrative were recorded by trained staff.15
Data analysis
From 316 hospitalization-related SAEs occurring among study participants, one rater (YL) coded the reason for first hospitalization from baseline to 12-months for participants by reviewing individual SAE forms. Forty-nine unique codes were initially generated from the narrative description of the events leading up to the hospitalization. One primary cause for each hospitalization was coded. The 49 initial codes were stepwise consolidated into fifteen and then nine categories (Supplementary Table 1) based on clinical reasoning, prior literature, incidence, and emerging data.2, 3, 11, 12, 20 A second rater (HA) independently coded a randomly selected 10% of events into 15 categories with strong interrater reliability with the codes generated by YL (kappa statistic 0.89 95% CI [0.78, 1.00]).
We first described reasons for hospitalization for the entire cohort and then based on dementia severity, using tertiles of the PGDRS-b score as a marker of dementia severity. The resulting PGDRS-b tertiles, including all intervention participants regardless of hospitalization, were mildly-impaired (PGDRS-b 0–5, n=175, 35.3%), moderately-impaired (PGDRS-b 6–14, n=152, 30.6%), and severely-impaired (PGDRS-b 15–35, n=145, 29.2%) for this study. PGDRS-b was selected over MMSE as the proxy for dementia severity because it was the strongest and most consistent predictor of hospitalization in multivariable logistic regression models examining any hospitalization in this cohort. Participants mildly-impaired by PGDRS score had mean MMSE score of 21, moderately-impaired participants had mean MMSE 18, and severely-impaired participants had mean MMSE 13.
In addition to qualitative analysis, we examined characteristics of the cohort by whether or not they experienced a hospitalization over 12 months using chi-square or t-tests. We used p<0.05 to represent statistical significance. Statistical analyses were conducted using Stata v. 15.1 (Stata Corp, College Station, TX).
Results
Among 494 PLWD who received MIND at Home, 170 (34.4%) reported at least one hospitalization within the first 12 months of enrollment. Table 1 displays participant characteristics by hospitalization status. The mean age overall was 80.4 years (SD 9.7) and 70.5% were female. Participants who were hospitalized were older than the non-hospitalized group. Hospitalized participants took more routine medications at baseline and had more baseline medical comorbidities, though only cardiovascular disease was statistically significant. Baseline PGDRS-b score was significantly associated with hospitalization (p<0.001), with mean score of 13.2 for hospitalized versus 9.7 for those not hospitalized.
Table 1.
Baseline characteristics of persons living with dementia receiving the MIND at Home intervention
| Overall intervention (N=494) |
Hospitalized (N=170) |
Not hospitalized (N=324) |
p-value | |
|---|---|---|---|---|
| Age, mean (SD) | 80.4 (9.7) | 81.7 (10.0) | 79.8 (9.5) | 0.04 |
| Female, N (%) | 348 (70.5) | 104 (61.2) | 244 (75.3) | 0.001 |
| White, N (%) | 195 (39.6) | 69 (40.6) | 126 (39.0) | 0.73 |
| Living with caregiver, N (%) | 384 (77.9) | 132 (77.7) | 252 (78.0) | 0.93 |
| Education, years, mean (SD) | 11.9 (3.8) | 11.9 (3.6) | 12.0 (4.0) | 0.85 |
| Dementia-related medication use | ||||
| Cholinesterase inhibitors | 144 (29.2) | 41 (24.1) | 103 (31.8) | 0.08 |
| Memantine | 96 (19.4) | 29 (17.1) | 67 (20.7) | 0.33 |
| Antidepressants | 180 (36.4) | 54 (31.8) | 126 (38.9) | 0.12 |
| Antipsychotics | 48 (9.7) | 21 (12.4) | 27 (8.3) | 0.15 |
| Benzodiazepines | 35 (7.1) | 14 (8.2) | 21 (6.5) | 0.47 |
| Routine medications, mean (SD) | 8.4 (4.3) | 8.9 (4.5) | 8.2 (4.2) | 0.08 |
| Cardiovascular disease, N (%) * | 394 (79.8) | 145 (85.3) | 249 (76.9) | 0.03 |
| Pulmonary disease, N (%) † | 50 (10.1) | 19 (11.2) | 31 (9.6) | 0.57 |
| Endocrine disease, N (%) ‡ | 227 (46.0) | 83 (48.8) | 144 (44.4) | 0.35 |
| Other neurologic disease, N (%) § | 123 (24.9) | 50 (29.4) | 73 (22.5) | 0.09 |
| Prior falls with injury | 169 (34.2) | 64 (37.7) | 105 (32.4) | 0.24 |
| PGDRS-b score, mean (SD) | 10.9 (8.8) | 13.2 (9.3) | 9.7 (8.3) | <0.001 |
| PGDRS 0–5, N (%) | 175 (37.2) | 44 (27.0) | 131 (42.5) | 0.001 |
| PGDRS 6–14, N (%) | 152 (32.3) | 55 (33.7) | 97 (31.5) | |
| PGDRS 15–35, N (%) | 144 (30.6) | 64 (39.3) | 80 (26.0) | |
| ≥1 hospitalization in prior year, N (%) | 186 (37.7) | 73 (42.9) | 113 (34.9) | 0.08 |
| MMSE score, mean (SD) | 17.3 (7.6) | 16.5 (7.6) | 17.7 (7.5) | 0.09 |
Includes history of myocardial infarction, atrial fibrillation, angioplasty, cardiac bypass surgery, pacemaker, congestive heart failure, hypertension, hypercholesterolemia
Includes history of chronic obstructive pulmonary diseases
Includes history of diabetes, vitamin B12 deficiency, thyroid disease
Includes history of stroke, transient ischemic attack, Parkinson’s disease, other Parkinsonian disorder, seizure, traumatic brain injury
SD=Standard Deviation; PGDRS-b=Psychogeriatric Dependency Ratings Behavior Subscale; MMSE=Mini-Mental State Exam
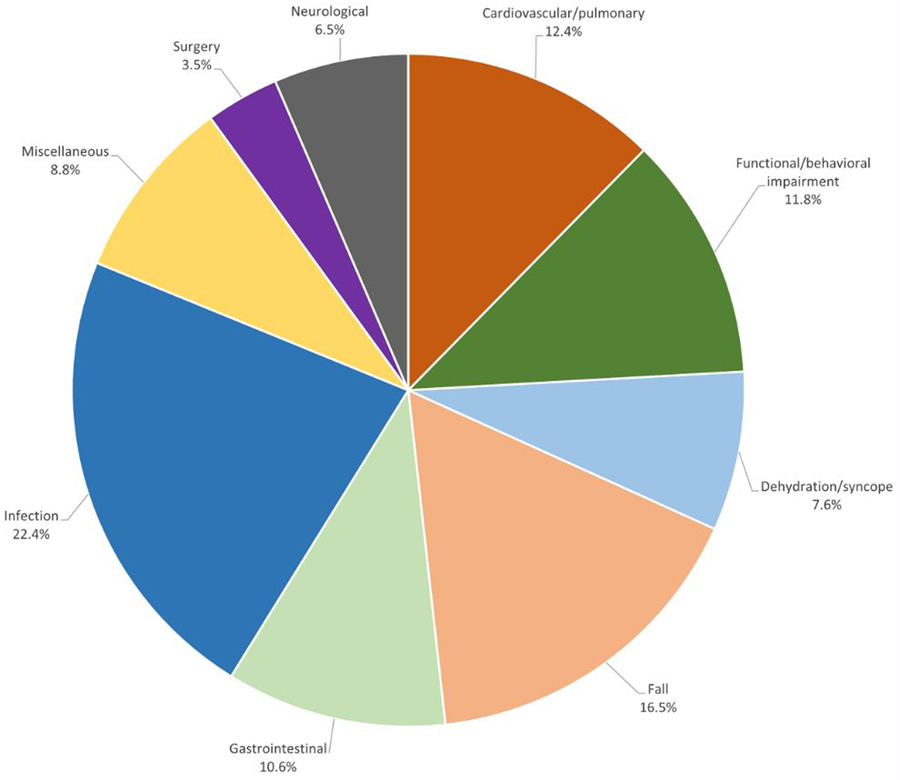
Reasons for first hospitalization within 12 months are shown in Figure 1. The most common reason was infection, reported in 22.4% of hospitalized participants. The most common subset of infection was urinary tract infection (UTI), cited 18 out of 44 times. Pneumonia accounted for 12/44; less common causes of infection included cellulitis and viral/influenza. Falls ranked second (16.5%) overall, cardiovascular/pulmonary reasons third (12.4%), and behavioral/functional impairment, largely consisting of behavioral or neuropsychiatric symptoms, accounted for 11.8% of hospitalizations.
Figure 1. Reason for first hospitalization among persons living with dementia receiving MIND at Home intervention.
Reasons for hospitalization over 12 months shown as percent of all hospitalizations among intervention participants.
Figure 2 displays each reason for hospitalization stratified by functional impairment tertile. Among the 170 hospitalized participants, 44 (25.9%) were in the mildly-impaired tertile, 55 (32.4%) in the moderate, and 64 (37.6%) in the severe. The severely-impaired group accounted for the largest proportions of hospitalizations for infection and dehydration/syncope (52.2% and 48.1% respectively). Mild/moderately-impaired participants made up larger proportions of hospitalizations for behavioral/functional impairments and falls. Mildly-impaired participants disproportionately experienced surgical or neurological hospitalizations though these were less common causes overall.
Figure 2. Proportion of hospitalization type, by severity of functional impairment, in persons living with dementia receiving MIND at Home intervention.
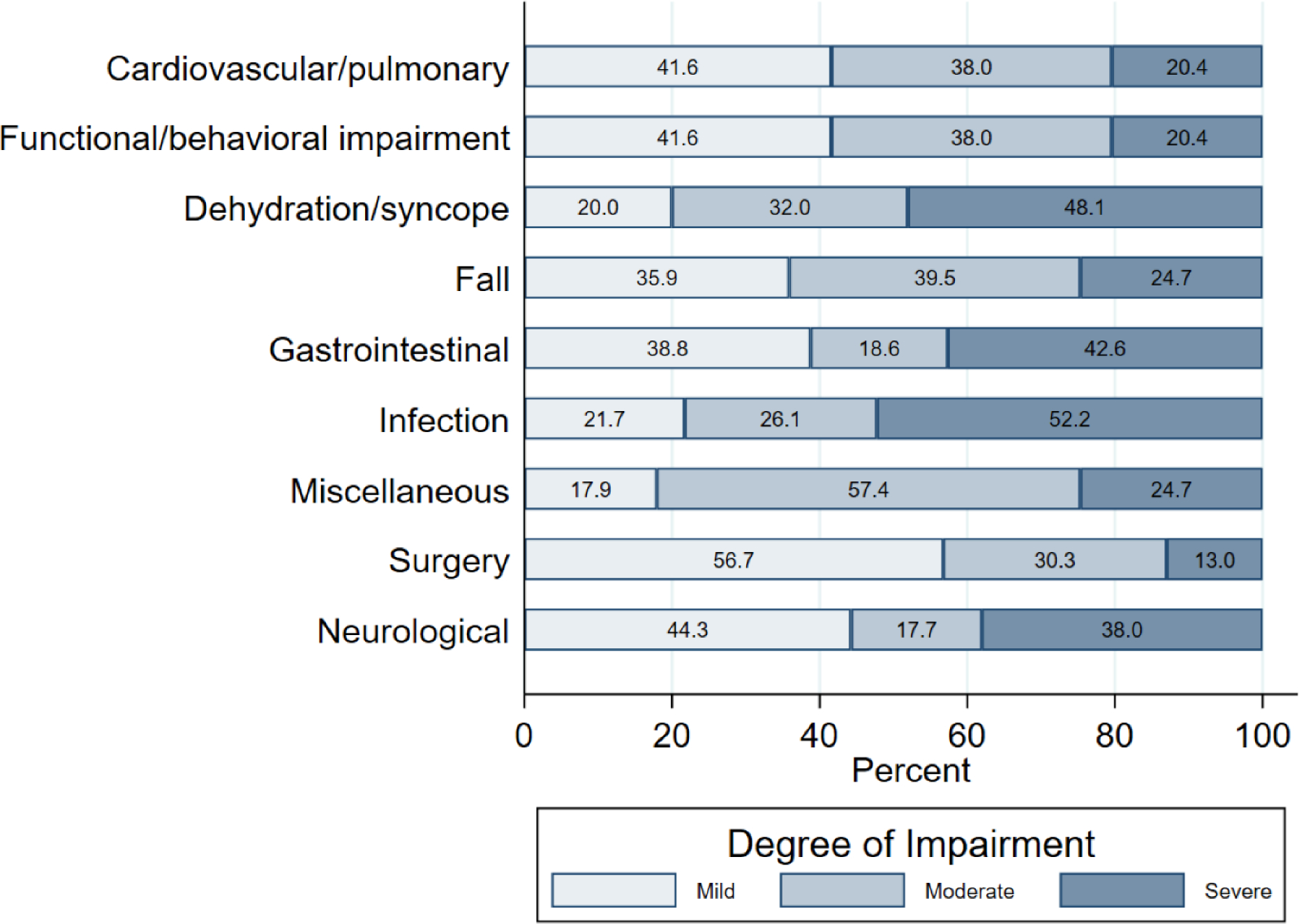
Proportion of each type of hospitalization by functional impairment severity is shown.
Among mildly-impaired participants, the most common reason for hospitalization was fall (17.7%). Other common reasons for hospitalization in this group included functional/behavioral impairments or cardiovascular/pulmonary, accounting for 14.5% of hospitalizations each, and infections, accounting for 12.9% of hospitalizations. Moderately-impaired participants were most likely to be hospitalized for falls (21.9%), miscellaneous—pain, renal, and unknown/unclear causes (16.4%), and cardiovascular/pulmonary and functional/behavioral impairments (15.1%). Among severely-impaired participants, infection was the most common reason for hospitalization (30.3%), followed by gastrointestinal (15.8%) and falls (10.5%).
Discussion
Approximately one third of PLWD receiving a dementia care coordination intervention (MIND at Home) were hospitalized over 12 months. Infection and falls were the most common caregiver-reported causes of hospitalization. Hospitalizations due to reported cardiovascular/pulmonary problems and behavioral impairment were also common. These results are significant as some hospitalizations within these categories are likely preventable. Further, identifying differences in reasons for hospitalization by severity of dementia may facilitate targeted interventions to prevent hospitalization.
Infection was common overall, but most common among PLWD with the greatest functional impairment. Training caregivers to monitor for signs of infection among PLWD who may have difficulty communicating is one potential strategy to prevent hospitalization. UTI accounted for almost half of all infections leading to hospitalization, notable given concurrent concerns of overtreatment of asymptomatic and nonspecific bacteriuria in older adults.21 For PLWD admitted for dehydration/syncope, about half (48.1%) were in the severely-impaired group. Signs of dehydration may be unrecognized especially in severely-impaired PLWD requiring significant assistance to maintain hydration. In this area, caregiver training could also be beneficial. In severely-impaired PLWD at risk of infection and dehydration, proactively addressing goals of care can prevent hospitalization if hospitalization is not consistent with patient values.
Results pertaining to falls, behavioral impairment, and surgery are also notable. Falls often rank as the top reason for hospitalization for PLWD in other studies.11, 12, 20 In this study, falls were also found to be common causes of hospitalization for PLWD particularly for mild to moderately-impaired individuals. This finding may reflect that these populations have increased activity compared to those severely-impaired but with gait or balance impairment. Indeed, emerging research shows that dementia is associated with gait impairment and fall risk.22 At the same time, studies also show that exercise or balance training programs improve gait stability in PLWD.23, 24 Mild to moderately-impaired PLWD also accounted for about 80% of hospitalizations for behavioral impairment. Careful evaluation of neuropsychiatric symptoms and access to appropriate non-pharmacologic and pharmacologic treatment is important in these groups. Hospitalization for surgery decreased with increasing functional impairment. This finding suggests that risks versus benefits of surgery are being appropriately weighed in persons with moderate to severe impairment.
Studies of hospitalization in other cohorts of PLWD using administrative claims or medical records have reported similar findings for the top causes: neuropsychiatric, falls, infections, cardiovascular, and GI.3, 11, 12, 20, 25 This study adds to existing literature by considering caregiver reported perspectives on causes of hospitalization as well as presenting the data in the context of functional impairment. Caregiver perspectives are important to understanding the context in which hospitalization occurred. Though novel in studying hospitalization in PLWD, caregiver report has been used in the study of healthcare utilization events26 and reasons for institutionalization in dementia.27 Self or caregiver report has also been used in conditions such as heart failure28 and stroke29 and patient-reported reasons for hospitalization has shown concordance with medical records in younger populations.30 Focusing on preventing specific types of hospitalization, while considering risk depending on level of impairment, may allow interventions to more successfully address hospitalizations. Hospitalization information gathered from MIND at Home and other dementia care intervention studies should be further analyzed to promote innovative intervention strategies to prevent hospitalizations among PLWD.
Study limitations include lack of medical records to validate the clinical diagnoses for hospitalization. We relied on caregiver report to capture the reason for hospitalization. While this approach offers novel insights as the caregiver often initiates the path to hospitalization, there is the possibility of incorrectly or incompletely identifying the reason for hospitalization without medical records. Future research that links caregiver-reported perspectives on hospitalizations with medical record or, ideally, clinician perspectives is an important next step to build upon this study. Fully understanding preventability of hospitalizations will require both caregiver and clinician perspectives.31 Hospitalization frequency is similar to other studies32 but there is possibility of missed hospitalizations despite self-reporting and frequent scheduled visits and assessments. Lastly, our findings directly apply only to PLWD with a caregiver receiving care coordination services, limiting generalizability. Given increasing efforts to test and implement dementia care interventions, it is important to have nuanced understanding of rates and reasons for hospitalizations within intervention cohorts. Given the limited scope of this analysis, we cannot draw conclusions as to whether intervention recipients had differential rates or reasons for hospitalizations compared to PLWD in other community-based cohorts, particularly those not receiving care coordination. Further research is warranted to determine whether and how dementia care coordination affects hospitalization.
Conclusions and Implications
In this large community-based cohort of PLWD who received dementia care coordination, infections, falls, cardiovascular/pulmonary problems, and functional/behavioral impairments were the most common caregiver-reported causes of hospitalization. Reasons for hospitalization varied by severity of dementia. Evidence-based care management approaches and interventions that target these specific complications or illnesses may reduce hospitalizations in a vulnerable population.
Supplementary Material
Supplementary Figure 1. Flowchart of MIND at Home studies referrals and participation
Supplementary Table 1. Nine reasons for hospitalization codes and examples of reasons within each code
Acknowledgements:
I certify that everyone who has contributed significantly to this work is listed as an author. The sponsor had no role in the design, methods, subject recruitment, data collection, analysis and preparation of this paper.
Funding sources:
This work was supported by the National Institute on Aging (R01 AG047859, P30 AG06650, K23 AG064036, T35 AG026758); and the Centers for Medicare and Medicaid Services (CMS Health Care Innovation Award 1C1CMS331332).
Conflict of Interest:
YL has no conflicts to disclose. DJ, CL, QS, and HA have grant funding from the NIA. Related to evaluation and implementation of MIND at Home, DJ also has grant funding from the BrightFocus Foundation, Baltimore Behavioral Health System, and Centene Corporation/Superior Health Plan LTSS Initiative, and QS has grant funding from the BrightFocus Foundation, Centers for Medicare & Medicaid Services, and the Sibley Memorial Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.2021 Alzheimer’s disease facts and figures. Alzheimers Dement 2021;17(3):327–406. [DOI] [PubMed] [Google Scholar]
- 2.Beydoun MA, Beydoun HA, Gamaldo AA et al. Nationwide inpatient prevalence, predictors, and outcomes of alzheimer’s disease among older adults in the United States, 2002–2012. J Alzheimers Dis 2015; 48(2):361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelan EA, Borson S, Grothaus L, Balch S et al. Association of incident dementia with hospitalizations. JAMA 2012; 307(2):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacNeil-Vroomen JL, Nagurney JM, Allore HG. Comorbid conditions and emergency department treat and release utilization in multimorbid persons with cognitive impairment. Am J Emerg Med 2020; 38(1):127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dementias, Including Alzheimer’s Disease. Healthy People 2030, Office of Disease Prevention and Health Promotion, US Department of Health and Human Services (online). Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/dementias. Accessed April 15, 2021.
- 7.Andrieu S Reynish E, Nourhashemi F et al. Predictive factors of acute hospitalization in 134 patients with Alzheimer’s disease: A one year prospective study. Int J Geriatr Psychiatry 2002; 17(5):422–426. [DOI] [PubMed] [Google Scholar]
- 8.Voisin T, Andrieu S, Cantet C, Vellas B et al. Predictive factors of hospitalizations in Alzheimer’s disease: A two-year prospective study in 686 patients of the REAL.FR study. J Nutr Health Aging. 2010; 14(4):288–291. [DOI] [PubMed] [Google Scholar]
- 9.Phelan EA, Debnam KJ, Anderson LA, Owens SB. A systematic review of intervention studies to prevent hospitalizations of community-dwelling older adults with dementia. Med Care 2015; 53(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godard-Sebillote C, Le Berre M, Schuster T, Trottier M et al. Impact of health service interventions on acute hospital use in community-dwelling persons with dementia: A systematic literature review and meta-analysis. PLoS One 2019; 14(6):e0218426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph JL, Zanin NM, Jones RN et al. Hospitalization in community-dwelling persons with Alzheimer’s disease: frequency and causes. J Am Geriatr Soc 2010; 58(8):1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voisin T, Sourdet S, Cantet C, Andrieu S et al. Descriptive analysis of hospitalizations of patients with Alzheimer’s disease: A two-year prospective study of 686 patients from the REAL.FR study. J Nutr Health Aging 2009; 13(10):890–892. [DOI] [PubMed] [Google Scholar]
- 13.Amjad H, Mulcahy J, Kasper JD, Burgdorf J et al. Do Caregiving Factors Affect Hospitalization Risk Among Disabled Older Adults? J Am Geriatr Soc. 2021; 69(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samus QM, Johnston D, Black BS, Hess E et al. A multidementional home-based care coordination intervention for elders with memory disorders: the maximizing independence at home (MIND) pilot randomized trial. Am J Geriatr Psychiatry 2014; 22(4):398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samus QM, Black BS, Reuland M, Leoutsakos JS et al. MIND at Home-Streamlined: Study protocol for a randomized trial of home-based care coordination for persons with dementia and their caregivers. Contemp Clin Trials 2018; 71:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samus QM, Davis K, Willink A, Black BS et al. Comprehensive home-based care coordination for vulnerable elders with dementia: Maximizing Independence at Home-Plus-Study protocol. Int J Care Coord 2017; 20(4):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edell WS, Tunis SL. Antipsychotic treatment of behavioral and psychological symptoms of dementia in geropsychiatric inpatients. Am J Geriatr Psychiatry 2001; 9(3):289–297. [PubMed] [Google Scholar]
- 18.Wilkinson IM, Graham-White J. Psychogeriatric dependency rating scales (PGDRS): A method of assessment for use by nurses. Br J Psychiatry 1980; 137:558–565. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 20.Russ TC, Parra MA, Lim AE, Law E et al. Prediction of general hospital admission in people with dementia: Cohort study. Br J Psychiatry 2015; 206(2):153–159. [DOI] [PubMed] [Google Scholar]
- 21.Nicolle LE, Kalpana G, Bradley SF, Colgan R et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68(10):1611–1615. [DOI] [PubMed] [Google Scholar]
- 22.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012; 60(11):2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia: A randomized controlled trial. Neurology 2010; 74(24):1961–1968. [DOI] [PubMed] [Google Scholar]
- 24.Plummer P, Zukowski LA, Giuliana C, Hall AM et al. Effects of physical exercise interventions on gait-related dual-task interference in older adults: A systematic review and meta-analysis. Gerontology 2015; 62(1):94–117. [DOI] [PubMed] [Google Scholar]
- 25.Lyketsos CG, Sheppard JM, Rabins PV. Dementia in elderly persons in a general hospital. Am J Psychiatry 2000; 157(5):704–707. [DOI] [PubMed] [Google Scholar]
- 26.Amjad H, Wong SK, Roth DL, Huang J et al. Health services utilization in older adults with dementia receiving care coordination: the Maximizing Independence (MIND) at Home trial. Health Serv Res; 2018; 53(1): 556–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afram B, Stephan A, Verbeek H, et al. Reasons for institutionalization of people with dementia: Informal caregiver reports from 8 European countries. J Am Med Dir Assoc 2014; 15(2):108–116. [DOI] [PubMed] [Google Scholar]
- 28.Annema C, Luttik ML, Jaarsma T. Reasons for readmission in heart failure: Perspectives of patients, caregivers, cardiologists, and heart failure nurses. Heart Lung; Sep-Oct 2009;38(5):427–34. doi: 10.1016/j.hrtlng.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Jackson CA, Mishra GD, Tooth L, Byles J et al. Moderate agreement between self-reported stroke and hospital-recorded stroke in two cohorts of Australian women: a validation study. BMC Med Res Methodol 15, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman A, Gibney L, Person AD, Williams OD et al. Validity of self-reports of reasons for hospitalization by young adults and risk factors for discordance with medical records: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol;. 2005. Sep 1;162(5):491–8. [DOI] [PubMed] [Google Scholar]
- 31.Patel KK, Vakharia N, Pile J, Howell EH et al. Preventable admissions on a general medicine service: Prevalence, causes and comparison with AHRQ prevention quality indicators: A cross-sectional analysis. J Gen Intern Med. 2016;31(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu CW, Cosentino S, Ornstein K, Gu Y et al. Use and cost of hospitalization in dementia: longitudinal results from a community-based study. Int J Geriatr Psychiatry;. 2015. Aug;30(8):833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flowchart of MIND at Home studies referrals and participation
Supplementary Table 1. Nine reasons for hospitalization codes and examples of reasons within each code