Abstract
Background
A comprehensive examination of the incidence and mortality of subsequent primary cancers (SPCs) among adolescent and young adult (AYA) cancer survivors in the United States is lacking.
Methods
Cancer incidence and mortality among 170 404 cancer survivors of 5 or more years who were aged 15-39 years at first primary cancer diagnosis during 1975-2013 in 9 Surveillance, Epidemiology, and End Results registries were compared with those in the general population using standardized incidence ratio (SIR), absolute excess incidence (AEI), standardized mortality ratio (SMR), and absolute excess mortality (AEM).
Results
During a mean follow-up of 14.6 years, 13 420 SPC cases and 5008 SPC deaths occurred among survivors (excluding the same site as index cancer), corresponding to 25% higher incidence (95% confidence interval [CI] = 1.23 to 1.27, AEI = 10.8 per 10 000) and 84% higher mortality (95% CI = 1.79 to 1.89, AEM = 9.2 per 10 000) than that in the general population. Overall, SPC risk was statistically significantly higher for 20 of 29 index cancers for incidence and 26 for mortality, with the highest SIR among female Hodgkin lymphoma survivors (SIR = 3.05, 95% CI = 2.88 to 3.24, AEI = 73.0 per 10 000) and the highest SMR among small intestine cancer survivors (SMR = 6.97, 95% CI = 4.80 to 9.79, AEM = 64.1 per 10 000). Type-specific SPC risks varied substantially by index cancers; however, SPCs of the female breast, lung, and colorectum combined constituted 36% of all SPC cases and 39% of all SPC deaths, with lung cancer alone representing 11% and 24% of all cases and deaths, respectively.
Conclusion
AYA cancer survivors are almost twice as likely to die from a new primary cancer as the general population, highlighting the need for primary care clinicians to prioritize cancer prevention and targeted surveillance strategies in these individuals.
The number of survivors of adolescent and young adult (AYA) cancer continues to increase in the United States, reflecting improved survival, growth of the population, and increasing incidence of several cancers (1-3). AYA cancer survivors are at higher risk of long-term adverse health outcomes (4), including new malignancies, as late effects of cancer treatment (5-7). Despite unique medical challenges and opportunities for risk reduction and early detection (8), few studies have comprehensively examined the risk of subsequent primary cancer (SPC) diagnosis and death among AYA cancer survivors. Previous studies mainly focused on survivors of a specific type of AYA cancer (6,7,9-17) or on a specific type of SPC (16,18-22). Recent reports have been more expansive but only investigated incidence of SPC and did not examine mortality (23-25). One study of survivors of AYA cancer in southern California reported that those who developed SPC had more than sevenfold higher all-cause mortality than survivors who did not develop SPC, although cause-specific mortality was not considered (5). A comprehensive and concurrent examination of SPC incidence and mortality across wide-ranging survivor groups is essential to establish an integrated strategy for quality surveillance and care for AYA cancer survivors. Herein, we follow up on a previous study of subsequent malignancies among adult-onset cancer survivors (26) by exploring young-onset cancer survivors in detail. Specifically, we estimate the overall and type-specific risk of developing and dying from SPC after diagnosis of 29 AYA cancers in the United States.
Method
Study Participants
We used population-based cancer incidence (27) and mortality (28) data from 9 Surveillance, Epidemiology, and End Results Program (SEER) registries. We identified cancer survivors of 5 or more years whose first primary (index) malignant cancer was diagnosed at ages 15-39 years during 1975-2013. We included 29 index cancers (Supplementary Table 1, available online), coded per the International Classification of Diseases for Oncology (ICD), 3rd edition, and then categorized according to SEER Site Recode International Classification of Diseases for Oncology (ICD), 3rd edition–World Health Organization 2008 definition (29), with 300 or more survivors to permit adequate numbers of events for type-specific risk assessment. We used a 5-year cut-off to provide risk estimates among long-term survivors, minimize bias from heightened medical surveillance and incidental finding, and permit comparison of findings with published results (23-25,30).
SPC Ascertainment
Follow-up began 5 years from initial cancer diagnosis and continued until censoring at death, loss to follow-up, or December 31, 2018, whichever came first. SPCs that occurred at the same site of index cancers (Supplementary Table 2, available online) were excluded to minimize bias from potential misclassification of recurrence, as was nonmelanoma skin cancer. Ascertainment of SPC death was based on the SEER cause-specific death classification (31). SPC death calculations excluded deaths from nonmelanoma skin cancer, same-type SPC, and miscellaneous malignant cancer. Second and higher-order cancers were counted for comparability between the observed and expected numbers of SPCs because the expected numbers, based on population-based cancer registrations in SEER, count multiple primary cancers in an individual.
Statistical Analysis
Cumulative incidence and cumulative mortality of SPCs were calculated at 35 years after index cancer diagnosis with deaths from any cause and non-SPC deaths treated as competing risks, respectively (Supplementary Methods, available online). The excess risk of developing SPCs among survivors beyond that expected from the general population was quantified using standardized incidence ratio (SIR) and absolute excess incidence (AEI) (32). SIRs were calculated as the observed divided by the expected number of SPCs, and AEIs were calculated as the observed minus the expected number of SPCs, divided by person-years of follow-up and expressed per 10 000 person-years. The expected number of SPCs was computed by multiplying age-, sex-, race-, and year-specific incidence rates in the 9 SEER registries by stratum-specific follow-up years. Similarly, the excess risk of dying from SPC among survivors beyond that expected in the general population was estimated using standardized mortality ratio (SMR) and absolute excess mortality (AEM).
For each index cancer, risks of developing or dying from any SPCs (overall risk) were calculated with and without stratification by time since initial diagnosis (5-9, 10-19, 20-29, ≥30 years). To further characterize type-specific risks of developing SPC, SIRs and AEIs for select SPCs were further stratified by stage at SPC diagnosis (localized, regional, distant, unstaged) (33), treatment era (1975-1986, 1987-1999, 2000-2013), and the first course of treatment (radiotherapy alone, chemotherapy alone, both radiotherapy and chemotherapy, neither [no or unknown radiotherapy or chemotherapy]) (Supplementary Methods, available online). Sex-combined analyses were used to provide more stable risk estimates, except Hodgkin lymphoma (HL) (because of known sex differences in the risk) (24), female breast (breast hereafter), and male Kaposi sarcoma. Analyses were performed using SEER*Stat 8.3.9, Stata 13, and SAS 9.4. A 2-sided P value of less than .05 was considered statistically significant.
Results
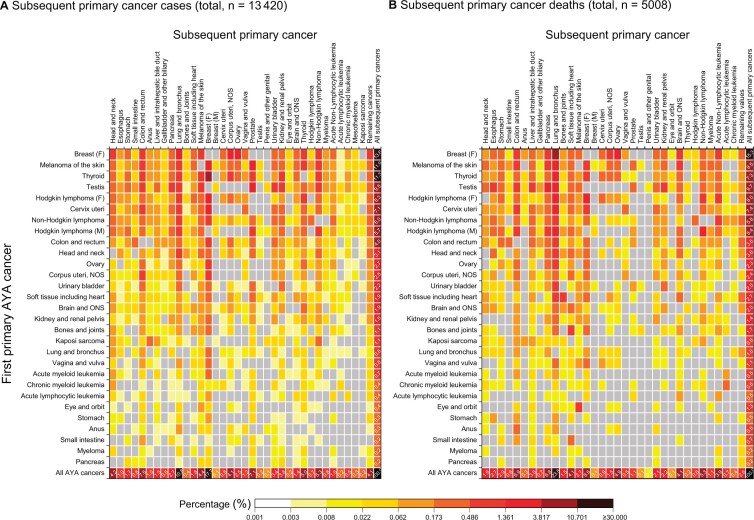
Among 170 404 survivors, 62.6% were females and 64.3% were in their 30s at first cancer diagnosis (Table 1). The most prevalent AYA cancer diagnoses were breast, followed by melanoma of the skin, thyroid, and testicular cancers (Table 2). During a mean follow-up of 14.6 years, 13 420 SPC (53.8 per 10 000) cases and 5008 (20.4 per 10 000) SPC deaths occurred (excluding the same site as index cancer), with a 35-year cumulative incidence of 13.7% and cumulative mortality of 6.2%. Breast cancer accounted for 17.8% of SPCs, followed by lung (10.8%), colorectum (7.6%), and prostate (7.1%) (Figure 1, A). Lung cancer was the leading cause of SPC death (23.7%), followed by breast (8.6%), colorectal (6.9%), and pancreatic (6.8%) cancers (Figure 1, B).
Table 1.
Characteristics of survivors of 5 or more years of adolescent and young adult (AYA) cancer with first cancer diagnoses in 1975-2013 in 9 registries of the Surveillance, Epidemiology, and End Results Program (SEER)
| Characteristics | No. (%) | Total follow-up years (beginning 5 years after index cancer diagnosis) | Mean follow-up years (beginning 5 years after index cancer diagnosis) |
|---|---|---|---|
| Total | 170 404 (100.0) | 2 492 830 | 14.6 |
| Sex | |||
| Female | 106 622 (62.6) | 1 565 914 | 14.7 |
| Male | 63 782 (37.4) | 926 916 | 14.5 |
| Age at index cancer diagnosis, y | |||
| 15-19 | 10 775 (6.3) | 169 424 | 15.7 |
| 20-24 | 18 722 (11.0) | 292 356 | 15.6 |
| 25-29 | 31 255 (18.3) | 480 099 | 15.4 |
| 30-34 | 45 437 (26.7) | 668 032 | 14.7 |
| 35-39 | 64 215 (37.7) | 882 919 | 13.7 |
| Calendar year of index cancer diagnosis | |||
| 1975-1986 | 39 725 (23.3) | 1 011 451 | 25.5 |
| 1987-1999 | 58 366 (34.3) | 1 020 898 | 17.5 |
| 2000-2013 | 72 313 (42.4) | 460 482 | 6.4 |
| Total follow-up time since index cancer diagnosis, y | |||
| 5-9 | 170 404 (100) | 749 459 | 4.4 |
| 10-19 | 131 778 (77.3) | 1 021 438 | 7.8 |
| 20-29 | 75 376 (44.2) | 530 849 | 7.0 |
| ≥30 | 33 041 (19.4) | 191 084 | 5.8 |
| Race | |||
| Black | 14 134 (8.3) | 180 071 | 12.7 |
| Other (American Indian, Asian, or Pacific Islander) | 12 993 (7.6) | 158 462 | 12.2 |
| White | 141 142 (82.8) | 2 127 072 | 15.1 |
| Unknowna | 2135 (1.3) | 27 225 | 12.8 |
| Registry | |||
| Seattle | 27 435 (16.1) | 391 281 | 14.3 |
| San Francisco-Oakland | 26 558 (15.6) | 402 178 | 15.1 |
| Detroit | 25 405 (14.9) | 381 972 | 15.0 |
| Connecticut | 22 755 (13.4) | 342 379 | 15.0 |
| Iowa | 18 628 (10.9) | 287 024 | 15.4 |
| Atlanta | 18 019 (10.6) | 248 822 | 13.8 |
| Utah | 14 317 (8.4) | 187 910 | 13.1 |
| New Mexico | 9543 (5.6) | 138 261 | 14.5 |
| Hawaii | 7744 (4.5) | 113 003 | 14.6 |
| Stage of index cancersb | |||
| Localized | 90 894 (63.5) | 1 414 807 | 15.6 |
| Regional | 32 558 (22.7) | 438 266 | 13.5 |
| Distant | 5668 (4.0) | 74 410 | 13.1 |
| Unstaged | 13 309 (9.3) | 177 202 | 13.3 |
| Blank(s)c | 770 (0.5) | 12 457 | 16.2 |
| Reasons for the exit of the study | |||
| End of study | 125 484 (73.6) | — | — |
| Death | 29 851 (17.5) | — | — |
| Lost to follow-up | 15 069 (8.8) | — | — |
| Number of subsequent primary cancers per survivord | |||
| 0 | 158 609 (93.1) | — | — |
| 1 | 10 411 (6.1) | — | — |
| 2 | 1178 (0.7) | — | — |
| ≥3 | 206 (0.1) | — | — |
Because populations are not available for unknown race, these cases were grouped with Whites when calculating risks. — = Not calculated.
Restricted to solid cancers and classified based on by SEER historic stage A. Hematologic cancer cases accounted for 16.0% (n = 27 205) of all cases, accruing 375 689 (mean = 13.8 ) follow-up years.
Cases with certain site and/or year combinations that were not covered by SEER historic stage A are coded as “blank(s),” including 451 lung cancer cases from 1975 to 1987; 302 head and neck cancer cases for certain subsites from 1975 to 1982 and 2004 to 2013; and 17 vaginal cancer cases from 2004 to 2013. Refer to the SEER Variable and Recode Definitions for details (https://seer.cancer.gov/seerstat/variables/seer/yr1973_2009/lrd_stage/index.html).
Includes second and later primaries and encompasses all cancer sites that were diagnosed 5 or more years since the first primary cancer diagnosis, except for nonmelanoma skin and same-type cancer as the index AYA cancer.
Table 2.
Risks of developing or dying from subsequent primary cancers (SPCs)a by first primary index cancer types among 5 or more years AYA cancer survivors with first cancer diagnosis in 1975-2013 in 9 SEER registries
| First AYA cancer | Survivors, n (%) | Person-years, total (mean) | SPC cases |
SPC death |
Mean age |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35-year cumulative incidence, % (95% CI) | Observed/Expectedb | SIR (95% CI)c | AEI per 10 000d | 35-year cumulative mortality, % (95% CI) | Observed/Expectedb | SMR (95% CI)c | AEM per 10 000d | First cancer | SPC diagnosis | SPC death | |||
| Totale | 170 404 (100) | 2 492 830 (14.6) | 13.7 (13.4 to 14.0) | 13 420/10 721 | 1.25 (1.23 to 1.27) | 10.8 | 6.2 (6.0 to 6.5) | 5008/2719 | 1.84 (1.79 to 1.89) | 9.2 | 31.6 | 53.5 | 54.7 |
| Breast (F)f | 27 176 (15.9) | 369 017 (13.6) | 10.8 (10.2 to 11.5) | 1821/1394 | 1.31 (1.25 to 1.37) | 11.6 | 5.9 (5.5 to 6.5) | 768/430 | 1.79 (1.66 to 1.92) | 9.2 | 35.4 | 54.9 | 57.7 |
| Melanoma of the skin | 24 000 (14.1) | 375 984 (15.7) | 11.6 (10.9 to 12.3) | 1594/1826 | 0.87 (0.83 to 0.92) | −6.2 | 4.0 (3.6 to 4.5) | 427/461 | 0.93 (0.84 to 1.02) | −0.9 | 31.7 | 54.9 | 56.5 |
| Thyroid | 23 408 (13.7) | 336 131 (14.4) | 13.0 (12.2 to 13.8) | 1514/1513 | 1 (0.95 to 1.05) | 0 | 3.7 (3.3 to 4.2) | 365/361 | 1.01 (0.91 to 1.12) | 0.1 | 31 | 53.3 | 55.5 |
| Testis | 16 501 (9.7) | 263 825 (16) | 15.1 (14.1 to 16.1) | 1280/1061 | 1.21 (1.14 to 1.27) | 8.3 | 7.2 (6.5 to 8.0) | 484/278 | 1.74 (1.59 to 1.9) | 7.8 | 29.6 | 55.1 | 56.7 |
| Cervix uteri | 10 314 (6.1) | 184 623 (17.9) | 13.4 (12.5 to 14.5) | 950/904 | 1.05 (0.99 to 1.12) | 2.5 | 6.1 (5.4 to 6.9) | 369/220 | 1.68 (1.51 to 1.86) | 8.1 | 32.8 | 55.5 | 57.2 |
| Non-Hodgkin lymphomag | 9474 (5.6) | 122 395 (12.9) | 17.9 (16.3 to 19.5) | 803/490 | 1.64 (1.53 to 1.76) | 25.6 | 7.5 (6.48 to 8.71) | 291/120 | 2.43 (2.16 to 2.72) | 14 | 31.2 | 53.1 | 52.5 |
| Hodgkin lymphoma (M) | 6854 (4.0) | 104 855 (15.3) | 18.8 (17.3 to 20.3) | 769/343 | 2.24 (2.09 to 2.41) | 40.6 | 12.3 (11.0 to 13.6) | 422/87 | 4.84 (4.39 to 5.32) | 31.9 | 27.4 | 51.8 | 51.9 |
| Hodgkin lymphoma (F) | 6550 (3.8) | 102 533 (15.7) | 25.9 (24.2 to 27.6) | 1113/365 | 3.05 (2.88 to 3.24) | 73 | 13.0 (11.6 to 14.5) | 399/72 | 5.54 (5.01 to 6.11) | 31.9 | 26.7 | 47.8 | 50.4 |
| Brain and other nervous system cancer | 6499 (3.8) | 73 731 (11.3) | 6.56 (5.57 to 7.65) | 250/226 | 1.11 (0.97 to 1.25) | 3.3 | 2.8 (2.17 to 3.5) | 100/48 | 2.08 (1.69 to 2.53) | 7 | 28.5 | 48.6 | 47.5 |
| Colon and rectum | 5843 (3.4) | 77 069 (13.2) | 15.8 (14.2 to 17.5) | 536/394 | 1.36 (1.25 to 1.48) | 18.5 | 6.9 (5.75 to 8.2) | 178/101 | 1.76 (1.51 to 2.04) | 10 | 33.9 | 54.6 | 54.2 |
| Head and neck | 4683 (2.7) | 70 464 (15) | 13.6 (12.1 to 15.1) | 412/348 | 1.18 (1.07 to 1.3) | 9.1 | 7.2 (6.07 to 8.4) | 181/90 | 2.01 (1.73 to 2.32) | 12.9 | 32.1 | 54 | 53.7 |
| Ovary | 4066 (2.4) | 69 792 (17.2) | 12.2 (10.8 to 13.8) | 357/299 | 1.19 (1.07 to 1.33) | 8.3 | 4.5 (3.6 to 5.6) | 115/70 | 1.65 (1.36 to 1.98) | 6.5 | 30.3 | 53.2 | 56.1 |
| Corpus uteri (including not otherwise specified) | 3608 (2.1) | 53 474 (14.8) | 15.4 (13.5 to 17.5) | 332/278 | 1.19 (1.07 to 1.33) | 10.1 | 6.6 (5.3 to 8.1) | 118/68 | 1.73 (1.43 to 2.07) | 9.3 | 34.8 | 55.7 | 57.2 |
| Soft tissue including heart | 3207 (1.9) | 47 565 (14.8) | 14.0 (12.0 to 16.1) | 257/204 | 1.26 (1.11 to 1.43) | 11.2 | 6.8 (5.5 to 8.3) | 134/50 | 2.69 (2.25 to 3.18) | 17.7 | 29 | 51.6 | 46.3 |
| Kidney and renal pelvis | 2916 (1.7) | 33 780 (11.6) | 16.4 (13.6 to 19.4) | 205/162 | 1.26 (1.1 to 1.45) | 12.6 | 6.9 (5.1 to 9.1) | 70/41 | 1.72 (1.34 to 2.18) | 8.7 | 34 | 53.6 | 54.4 |
| Urinary bladder | 2709 (1.6) | 50 379 (18.6) | 16.5 (14.4 to 18.7) | 296/300 | 0.99 (0.88 to 1.1) | −0.9 | 6.6 (5.3 to 8.1) | 102/83 | 1.23 (1 to 1.49) | 3.7 | 33.3 | 58 | 59.1 |
| Bones and joints | 1978 (1.2) | 29 386 (14.9) | 11.5 (9.3 to 13.9) | 146/98 | 1.48 (1.25 to 1.74) | 16.2 | 8.5 (6.5 to 10.8) | 85/23 | 3.73 (2.98 to 4.61) | 21.2 | 25.6 | 48 | 45.9 |
| Kaposi sarcoma (M)f | 1561 (0.9) | 15 256 (9.8) | 13.7 (11.1 to 16.7) | 145/56 | 2.58 (2.18 to 3.03) | 58.2 | 5.1 (3.4 to 7.3) | 42/14 | 2.96 (2.14 to 4.01) | 18.2 | 33.5 | 47.6 | 48.9 |
| Acute myeloid leukemia | 1477 (0.9) | 17 141 (11.6) | 16.8 (12.6 to 21.6) | 93/55 | 1.68 (1.36 to 2.06) | 22 | 5.7 (3.3 to 9.1) | 27/12 | 2.28 (1.51 to 3.32) | 8.9 | 28.6 | 49.7 | 47.6 |
| Lung and bronchus | 1456 (0.9) | 20 821 (14.3) | 10.4 (8.2 to 12.9) | 105/99 | 1.06 (0.87 to 1.28) | 2.9 | 6.9 (5.1 to 9.1) | 50/20 | 2.52 (1.87 to 3.32) | 14.5 | 32.7 | 54.2 | 51.9 |
| Chronic myeloid leukemia | 1259 (0.7) | 11 184 (8.9) | 13.2 (9.3 to 17.7) | 59/36 | 1.65 (1.26 to 2.13) | 20.8 | 5.9 (3.3 to 9.5) | 29/8 | 3.78 (2.53 to 5.43) | 19.1 | 31 | 48.8 | 45.7 |
| Vagina and vulva | 1123 (0.7) | 19 471 (17.3) | 10.7 (8.3 to 14.0) | 102/94 | 1.09 (0.89 to 1.32) | 4.2 | 8.2 (6.1 to 10.7) | 56/22 | 2.57 (1.94 to 3.34) | 17.6 | 30.7 | 53.2 | 51.7 |
| Acute lymphocytic leukemia | 1113 (0.7) | 13 750 (12.4) | 8.1 (5.6 to 11.1) | 55/31 | 1.77 (1.33 to 2.3) | 17.4 | 5.3 (3.1 to 8.3) | 23/6 | 3.65 (2.31 to 5.47) | 12.1 | 23.4 | 41.8 | 39.6 |
| Stomach | 566 (0.3) | 6829 (12.1) | 10.0 (6.5 to 14.5) | 45/34 | 1.34 (0.98 to 1.79) | 16.7 | 8.6 (4.9 to 13.6) | 24/8 | 2.89 (1.85 to 4.3) | 23 | 33.9 | 51.6 | 52.1 |
| Eye and orbit | 562 (0.3) | 8143 (14.5) | 11.1 (7.7 to 15.1) | 48/41 | 1.16 (0.85 to 1.54) | 8 | 17.6 (13.4 to 22.3)h | 67/10 | 6.53 (5.06 to 8.29)h | 69.7h | 31.7 | 53 | 46.8 |
| Myeloma | 478 (0.3) | 3832 (8) | 14.5 (9.1 to 21.1) | 32/16 | 1.95 (1.34 to 2.76) | 40.8 | 4.7 (2.1 to 8.8) | 44504 | 2.67 (1.33 to 4.77) | 18 | 35.1 | 52.1 | 48.3 |
| Small intestine | 390 (0.2) | 4408 (11.3) | 17.7 (9.3 to 28.3) | 33/20 | 1.67 (1.15 to 2.35) | 30 | 11.2 (7.0 to 16.5) | 33/5 | 6.97 (4.8 to 9.79) | 64.1 | 33.5 | 52.4 | 48.1 |
| Pancreas | 333 (0.2) | 3230 (9.7) | 13.4 (8.0 to 20.2) | 28/14 | 2.04 (1.35 to 2.94) | 44.1 | 4.5 (3.6 to 5.6) | 9/3 | 2.81 (1.28 to 5.33) | 17.9 | 31.8 | 47.8 | 47.9 |
| Anus | 300 (0.2) | 3766 (12.6) | 20.2 (13.0 to 28.5) | 40/19 | 2.14 (1.53 to 2.91) | 56.5 | 22.8 (13.8 to 33.1)i | 29/5 | 6.34 (4.25 to 9.11)i | 64.9i | 35.1 | 54.7 | 53.5 |
Includes second and later primaries and encompasses all cancer sites, except for nonmelanoma skin and same-type cancer as the index AYA cancer. Refer to Supplementary Table 2 (available online) for the definition of same-type cancer. AEI = absolute excess incidence; AEM = absolute excess mortality; AYA = adolescent and young adult; CI = confidence interval; F = female; ICD = International Classification of Diseases for Oncology; M = male; SEER = Surveillance, Epidemiology, and End Results Program; SIR = standardized incidence ratio; SMR = standardized mortality ratio; SPC = subsequent primary cancers.
The expected number of SPC cases and deaths from subsequent primary cancers were extrapolated from the incidence and mortality rates of 9 SEER areas, respectively, with a comparable distribution of race, sex, age, and calendar year. The expected risk of SPC deaths can be interpreted as the risk of dying from newly diagnosed cancer if survivors experienced the same cancer death rates as persons in the general reference population.
Calculated as the observed divided by the expected. The SIR and SMR are generally considered a measure of the strength of an association between the first and subsequent primary cancers.
Calculated as the observed minus the expected, divided by the total person-years of follow-up, and multiplied by 10 000. The modest or small relative excess risk (SIR or SMR) can result in high absolute excess risk (AEI or AEM) when the cancer rates in the general population are high.
Sum of 29 types of index AYA cancers.
Male breast cancer survivors were excluded because they accounted for only 0.2% (n = 60) of breast cancer survivors, as were female Kaposi sarcoma survivors because they accounted for only 1.6% (n = 25) of all Kaposi sarcoma survivors. For all other excluded cancers, refer to Supplementary Table 1 (available online).
Includes chronic lymphocytic leukemia (ICD-O-3 histology 9823 and site codes C420, C421, C424) and acute lymphoblastic lymphoma (ICD-O-3 histology codes 9811-9818, 9837 and all site codes except C024, C098-C099, C111, C142, C379, C420-C422, C424, C770-C779).
When excluding death from subsequent melanoma (n = 53), 35-year cumulative mortality, SMR (95% CI) and AEM were 3.7% (1.7-6.8) and 1.40 (0.77-2.35), and 4.9 per 10 000, respectively.
When excluding death from subsequent colorectal cancer (n = 11), 35-year cumulative mortality, SMR (95% CI) and AEM were 17.8% (9.5- 28.2) and 4.37 (2.59- 6.90), and 36.9 per 10 000, respectively.
Figure 1.
Perce ntage contribution of each first primary (index) cancer and subsequent primary cancer (SPC) combination to the total number of subsequent primary cancer cases (A) and deaths (B) among AYA cancer survivors of 5 or more years. Calculated by dividing the observed number of SPC cases or deaths of each cell by the total number of observed SPC cases or deaths. The percentages on the bottom row indicate proportions of all SPCs that are accounted for by each type of SPC and add up 100%, and the percentages on the far-right column indicate proportions of all SPCs that are contributed by each first primary cancer survivor group and add up 100%. Gray cells indicate zero events. AYA = adolescent and young adult; F = female; M = male; NOS = not otherwise specified; ONS = other nervous system.
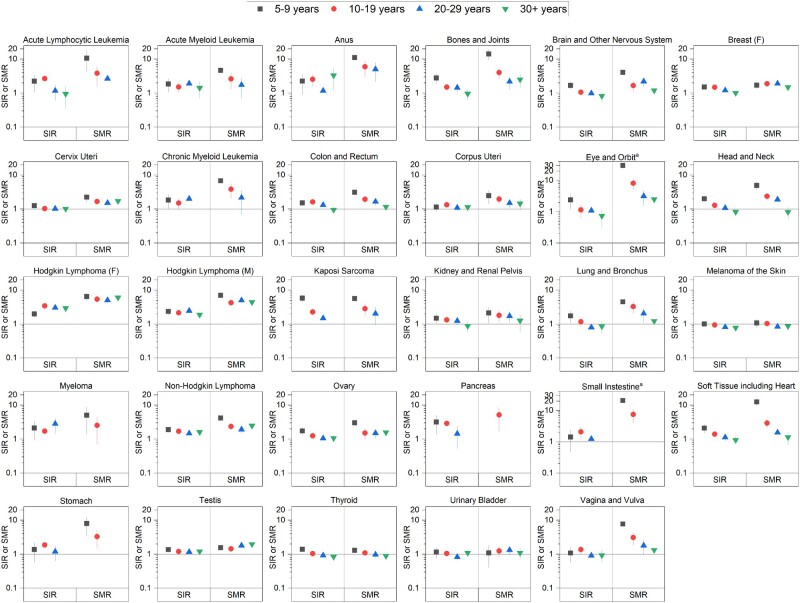
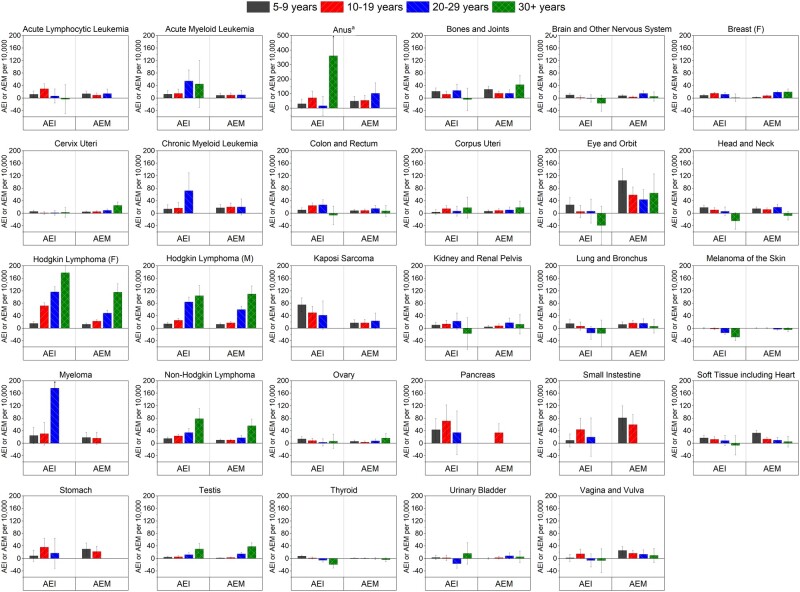
Survivors overall had a 25% higher risk of developing SPCs and an 84% higher risk of dying from SPCs than the expected risks in the general population, corresponding to 10.8 excess cases and 9.2 excess deaths per 10 000, respectively (Table 2). The overall SPC incidence was statistically significantly higher for 20 of 29 index cancers, with the highest SIRs after female HL (SIR = 3.05, AEI = 73.0 per 10 000), male Kaposi sarcoma (SIR = 2.58, AEI = 58.2 per 10 000), and male HL (SIR = 2.24, AEI = 40.6 per 10 000), with a 35-year cumulative incidence of 25.9%, 13.7%, and 18.8%, respectively. The mortality from any SPC was statistically significantly higher for 26 index cancers, with the highest SMRs after small intestine (SMR = 6.97, AEM = 64.1 per 10 000), eye (SMR = 6.53, AEM = 69.7 per 10 000), and anal (SMR = 6.34, AEM = 64.9 per 10 000) cancers. Although SIRs and SMRs generally attenuated with a longer-term follow-up, the elevated risk remained statistically significant 20 or more years postdiagnosis for 9 index cancers for incidence and 20 for mortality (Supplementary Figure 1, available online) and 35 or more years postdiagnosis for lymphomas and testicular cancer for both incidence and mortality (Figure 2), with corresponding AEIs and AEMs increasing over time (Figure 3).
Figure 2.
Standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) for overall risk of subsequent primary cancers among AYA cancer survivors of 5 or more years by time since index cancer diagnosis. Estimates are not shown for tests based on less than 5 observed events. AYA = adolescent and young adult; F = female; M = male. aA different scale from the other graphs in figure is used.
Figure 3.
Absolute excess incidences (AEIs) and absolute excess mortalities (AEMs) for overall risk of subsequent primary cancers among AYA cancer survivors of 5 or more years by time since index cancer diagnosis. Estimates are not shown for tests based on less than 5 observed events. AYA = adolescent and young adult; F = female; M = male. aA different scale from the other graphs in figure is used.
As for the 4 most common index cancers among survivors, the risk of developing SPCs compared with the general population was statistically significantly elevated among breast (SIR = 1.31, AEI = 12.1 per 10 000) and testicular (SIR = 1.21, AEI = 7.9 per 10 000) cancer survivors, not different among thyroid cancer survivors, and statistically significantly lower among melanoma survivors (SIR = 0.87, AEI = -6.2 per 10 000) (Table 2). Generally similar to incidence, the risks of dying from SPCs were statistically significantly elevated among breast (SMR = 1.79, AEM = 9.2 per 10 000) and testicular (SMR = 1.74, AEM = 7.8 per 10 000) cancer survivors and were not different among thyroid cancer and melanoma survivors.
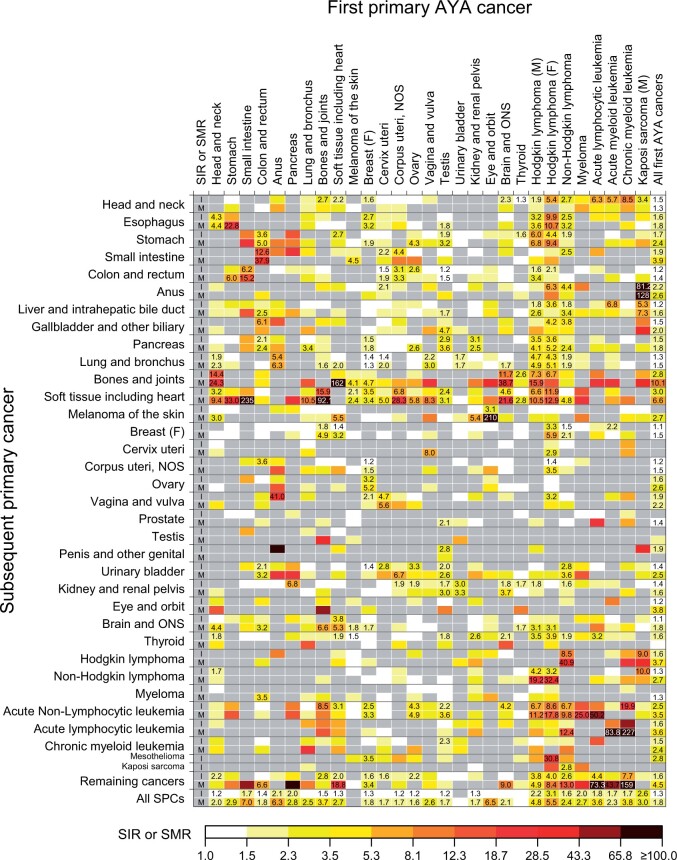
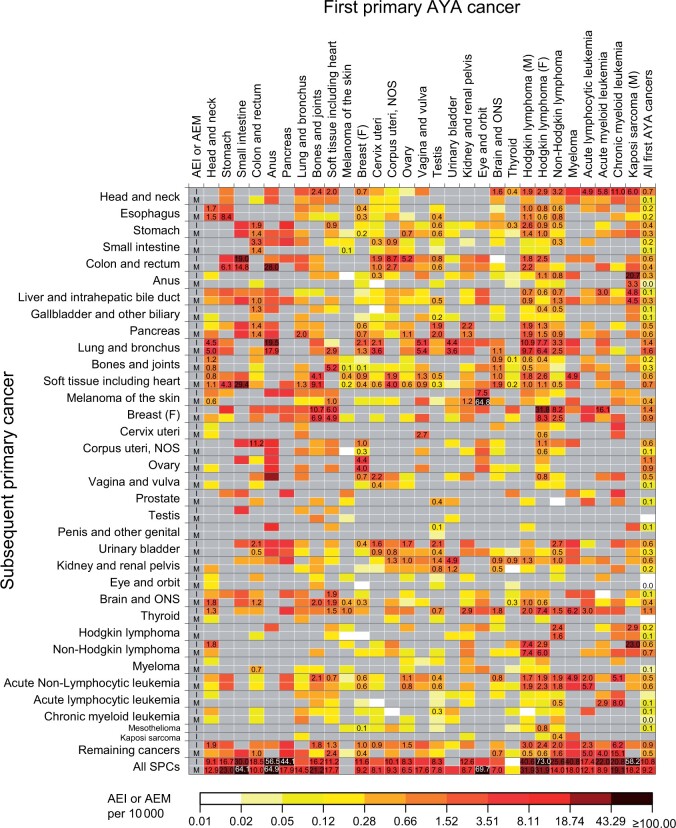
Figure 4 presents higher-than-expected SIRs and SMRs for type-specific SPC risks, with corresponding AEIs and AEMs shown in Figure 5 (Supplementary Table 3, available online for point estimates and 95% confidence intervals [CIs]). Among 689 eligible tests for incidence, the risk was statistically significantly higher than expected for 134 tests with the highest SIR for anal cancer following male Kaposi sarcoma (SIR = 81.2, 95% CI = 55.5 to 114.6) and the highest AEI for breast cancer following HL (AEI = 31.8 per 10 000, 95% CI = 27.8 to 36.2). Of the 523 tests for mortality, the risk was statistically significantly higher for 115 tests, with the highest SMR for soft tissue sarcoma following small intestine cancer (SMR = 235.3, 95% CI = 125.3 to 402.3) and the highest AEM for melanoma following eye cancer (AEM = 64.8 per 10 000, 95% CI = 48.4 to 84.8).
Figure 4.
Standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) for a specific type of subsequent primary cancers among AYA cancer survivors of 5 or more years. Point estimates are indicated in the cell when there was a statistically significant association between the index cancer and the subsequent primary cancer with higher-than-expected risks from tests based on 5 or more observed events (P <.05). All point estimates (95% confidence intervals) can be found in Supplementary Table 3 (available online). Gray cells indicate associations with lower-than-expected risks (SIR or SMR <1) or associations not tested because of zero events. AYA = adolescent and young adult; F = female; M = male; NOS = not otherwise specified; ONS = other nervous system.
Figure 5.
Absolute excess incidences (AEIs) and absolute excess mortalities (AEMs) for a specific type of subsequent primary cancers among AYA cancer survivors of 5 or more years. Point estimates are indicated in the cell when there was a statistically significant association between the index cancer and the subsequent primary cancer with higher-than-expected risks from tests based on 5 or more observed events (P <.05). All point estimates (95% confidence intervals) can be found in Supplementary Table 3 (available online). Gray cells indicate associations with lower-than-expected risks (AEI or AEM < 0) or associations not tested because of zero events. AYA = adolescent and young adult; F = female; M = male; NOS = not otherwise specified; ONS = other nervous system.
Despite considerable variation in type-specific SPC risks, subsequent breast cancer constituted 12.1% of total excess cases among all survivors, followed by lung (11.6%), thyroid (7.7%), colorectal (5.5%), and head and neck (5.4%) cancers, whereas lung cancer constituted 17.5% of all excess deaths, followed by soft tissue sarcoma (6.8%), non-HL (6.7%), and pancreatic cancer (6.6%) (“All first AYA cancers” column in Figure 5 for AEIs and AEMs; Supplementary Figure 2, available online for percentages). Stratifications by stage at SPC diagnosis revealed that statistically significant excess incidences among survivors were confined to localized- and regional-stage cancers of the thyroid, colorectum, and head and neck (Table 3). For breast and lung cancers, however, the risk was also statistically significantly elevated for distant-stage disease.
Table 3.
Risks of developing stage-specific subsequent primary cancers (SPCs) among 5 year or more AYA cancer survivorsa
| Subsequent primary cancer, stage | Observed, No. (%) | Expected, No. (%) | Standardized incidence ratio (95% CI) | Absolute excess incidence (95% CI) per 10 000 person-years | Survivors, No.b | Mean person-years at risk |
|---|---|---|---|---|---|---|
| Female breast cancer | ||||||
| Localized | 1206 (62.6) | 1098.9 (61.3) | 1.10 (1.04 to 1.16) | 0.89 (0.33 to 1.48) | 79 554c | 15.1 |
| Regional | 565 (29.3) | 562.6 (31.4) | 1.00 (0.92 to 1.09) | 0.02 (−0.36 to 0.43) | 79 554c | 15.1 |
| Distant | 128 (6.6) | 105.7 (5.9) | 1.21 (1.01 to 1.44) | 0.19 (0.01 to 0.39) | 79 554c | 15.1 |
| Unstaged | 27 (1.4) | 25.0 (1.4) | 1.08 (0.71 to 1.57) | 0.02 (−0.06 to 0.12) | 79 554c | 15.1 |
| Colon and rectum | ||||||
| Localized | 371 (44.0) | 281.4 (40.5) | 1.32 (1.19 to 1.46) | 0.37 (0.22 to 0.53) | 165 011 | 14.7 |
| Regional | 298 (35.3) | 241.2 (34.7) | 1.24 (1.1 to 1.38) | 0.24 (0.1 to 0.38) | 165 011 | 14.7 |
| Distant | 153 (18.1) | 149.7 (21.6) | 1.02 (0.87 to 1.2) | 0.01 (−0.08 to 0.12) | 165 011 | 14.7 |
| Unstaged | 21 (2.5) | 22.1 (3.2) | 0.95 (0.59 to 1.45) | −0.01 (−0.04 to 0.04) | 165 011 | 14.7 |
| Lung and bronchus | ||||||
| Localized | 231 (20.6) | 158.4 (18.8) | 1.46 (1.28 to 1.66) | 0.29 (0.18 to 0.42) | 169 398 | 14.6 |
| Regional | 271 (24.2) | 202.4 (24.0) | 1.34 (1.18 to 1.51) | 0.28 (0.15 to 0.42) | 169 398 | 14.6 |
| Distant | 578 (51.5) | 452.3 (53.6) | 1.28 (1.18 to 1.39) | 0.51 (0.32 to 0.71) | 169 398 | 14.6 |
| Unstaged | 42 (3.7) | 31.1 (3.7) | 1.35 (0.97 to 1.83) | 0.04 (0 to 0.1) | 169 398 | 14.6 |
| Thyroid | ||||||
| Localized | 336 (62.1) | 227.3 (65.2) | 1.48 (1.32 to 1.65) | 0.5 (0.34 to 0.68) | 147 446 | 14.7 |
| Regional | 186 (34.4) | 106.4 (30.5) | 1.75 (1.51 to 2.02) | 0.37 (0.25 to 0.5) | 147 446 | 14.7 |
| Distant | 6 (1.1) | 8.7 (2.5) | 0.69 (0.25 to 1.5) | −0.01 (−0.03 to 0.02) | 147 446 | 14.7 |
| Unstaged | 13 (2.4) | 5.9 (1.7) | 2.20 (1.17 to 3.76) | 0.03 (0 to 0.08) | 147 446 | 14.7 |
| Head and neck | ||||||
| Localized | 196 (47.9) | 91.0 (34.7) | 2.15 (1.86 to 2.48) | 0.43 (0.32 to 0.55) | 166 171 | 14.6 |
| Regional | 153 (37.4) | 127.0 (48.4) | 1.20 (1.02 to 1.41) | 0.11 (0.01 to 0.22) | 166 171 | 14.6 |
| Distant | 45 (11.0) | 33.9 (12.9) | 1.33 (0.97 to 1.78) | 0.05 (0 to 0.11) | 166 171 | 14.6 |
| Unstaged | 15 (3.7) | 10.5 (4.0) | 1.43 (0.8 to 2.36) | 0.02 (−0.01 to 0.06) | 166 171 | 14.6 |
Top 5 most common subsequent primary cancers that accounted for the highest proportions of all excess cases among all survivors combined. AYA = adolescent and young adult; CI = confidence interval.
All survivors excluding those whose index cancer was diagnosed at the same site as SPC in question.
All female survivors excluding breast cancer survivors.
Concerning the top 5 SPCs representing the largest proportions of the excess cases, the risks of developing and dying from lung cancer were statistically significantly elevated following lymphomas and cancers of the breast, anus, vagina or vulva, head and neck, urinary bladder, and cervix, with the greatest excess risks among survivors of anal cancer (SIR = 5.40, 95% CI = 2.47 to 10.24; SMR = 6.34, 95% CI = 2.74 to 12.49), male HL (SIR = 4.69, 95% CI = 3.96 to 5.52; SMR = 4.92, 95% CI = 4.10 to 5.85), and female HL (SIR = 4.32, 95% CI = 3.52 to 5.24; SMR = 5.08, 95% CI = 4.04 to 6.31) (Figure 4). The risks of developing and dying from breast cancer were statistically significantly higher among survivors of lymphomas and sarcomas, with the greatest excess risk following HL (SIR = 3.34, 95% CI = 3.04 to 3.66; SMR = 5.87, 95% CI = 4.79 to 7.13). The risk of developing colorectal cancer was statistically significantly higher among small intestine, corpus uteri, cervix, testis, ovary, and HL cancer survivors, with excess mortality statistically significant for all but ovarian cancer and female HL. The incidence of head and neck cancers was statistically significantly higher following leukemias, lymphomas, myeloma, sarcomas, and cancers of the brain, breast, and thyroid, with the greatest excess risk after chronic myeloid leukemia; however, no statistically significant excess death was observed. Similarly, thyroid cancer incidence was elevated following 10 index cancers, but the excess mortality was not statistically significant for any AYA cancer survivors.
We found that SIRs differed statistically significantly by treatment group for lung cancer among survivors of HL and breast cancer with greater SIRs following radiotherapy alone and in combination with chemotherapy and among testicular cancer survivors following chemotherapy alone (Supplementary Table 4, available online). Additionally, SIRs also varied by treatment group for breast cancer following HL and colorectal cancer following uterine corpus and cervical cancers. For these associations, statistically significant declines in the SIRs across treatment eras from 1975-1986 to 2000-2013 were observed only among HL survivors, from 4.80 (95% CI = 4.11 to 5.58) to 2.53 (95% CI = 0.82 to 5.90) for lung cancer and from 3.81 (95% CI = 3.36 to 4.30) to 1.99 (95% CI = 1.31 to 2.89) for breast cancer (Supplementary Figure 3, available online).
Discussion
In this large population-based cohort, AYA cancer survivors had a 25% higher risk of developing cancer and an 84% higher risk of dying from a new primary cancer compared with the expected risks in the general population. The overall risk of developing or dying from SPC was statistically significantly higher for 20 and 26 of 29 index cancers, respectively, and remained elevated for 20 or more years postdiagnosis for 9 cancers for incidence and 20 cancers for mortality. Leading sites of SPCs were lung, breast, and colorectum, comprising 36% of all SPC cases and 39% of all SPC deaths, highlighting prevention priorities through intensifying health promotion and effective surveillance as part of routine survivorship care (34).
Many studies have examined the risks of developing SPCs among AYA cancer survivors (5-7,9-25), however, there is a paucity of data on the risks of SPC deaths, with prior studies investigating mainly overall (5) or subsequent breast cancer death (20,35-37). To our knowledge, this is the first study to present the overall and type-specific risks of incidence and mortality among AYA cancer survivors concurrently. Although the leading causes of SPC deaths among AYA cancer survivors were the same as those in the general population, AYA cancer survivors were almost twice as likely to die from new cancer than the general population, reflecting both higher incidence and lower survival. The overall relative risk of dying from SPCs was substantially smaller than the risk reported for survivors of childhood (12- to 15-fold) (30,38) or early adolescent and young adult (15-20 years at diagnosis; eightfold) cancer (30); however, the absolute risks were comparable (20 per 10 000 vs 17-27 per 10 000) likely because of higher baseline cancer rates among the AYA population, underscoring the importance of age-appropriate and risk-based follow-up and surveillance care. Studies have shown that SPCs are independently associated with poorer outcomes than first primary cancers after accounting for age, stage at diagnosis (20,35,36,39), and treatment (36) and that this disadvantage is more pronounced among AYA cancer survivors vs those first diagnosed at a younger or older age (39). Limited therapeutic options, multiple comorbidities (40,41), and concerns regarding additional treatment exposures may contribute to poor outcomes among individuals with a history of cancer, although factors specific to the disproportionate influence on AYA cancer survivors require further investigation. Additional research on tumor characteristics (42) and treatment patterns of SPCs occurring among AYA cancer survivors may provide insight into the outcomes of SPCs.
Lung cancer alone constituted approximately 1 in 4 SPC deaths. The elevated risks following diagnoses of smoking-related cancers (head and neck, cervix, urinary bladder, anus) point to smoking as a shared etiology (43,44), whereas the varying degrees of lung cancer risk by treatment group found among breast, male HL, and testicular cancer survivors support the well-documented late-effect of chest radiotherapy and chemotherapy as a contributing factor (32,45-47). Our study was unable to examine the contribution of smoking, alone or in combination with treatment, to lung cancer risk, and contributions of individual risk factors are unknown. Nevertheless, given the known synergistic effect of smoking with cytotoxic cancer therapy (48-50), the benefits of smoking cessation after a cancer diagnosis (51,52) and higher smoking prevalence among AYA cancer survivors than their general population counterparts (26% vs 16% in 2014-2018) (53), interventions for smoking cessation should be an integral part of survivorship care (54,55). Findings of the excess lung cancer risk following multiple major AYA cancers underscore the importance of future analytical studies to identify risk factors specific to the survivor cohort. Additionally, studies on the effectiveness of lung cancer screening among cancer survivors may merit a comprehensive examination of survivors at increased risk beyond survivors of HL and head and neck cancers (56-58).
Breast cancer was the most commonly diagnosed SPC (18%) and ranked second in SPC deaths (9%) excluding subsequent breast cancers among breast cancer survivors. The elevated risk following treatment of HL was greatest for those treated with radiotherapy alone, consistent with previous findings (59). We were not able to evaluate the effects of specific chemotherapeutic agents because of lack of data; however, the increasingly common use of anthracyclines, with or without chest radiotherapy, has been shown to increase breast cancer risk and may partly explain the elevated risks among survivors of lymphomas and sarcomas (60,61). Contrary to other major SPCs, the magnitude of SIR was slightly greater for distant- than earlier-stage disease for subsequent breast cancer (1.21 vs 1.00-1.10), which is counterintuitive given the increased contact with a health-care system expected among cancer survivors (62). Together with the excess breast cancer mortality among survivors, this finding underscores the importance of early detection to mitigate the excess deaths from subsequent breast cancer among AYA cancer survivors (63,64).
Colorectal cancer was the third most common SPC (8%) and the third cause of SPC deaths (7%) with the highest risks following small intestine, uterine corpus, and ovarian cancers. Among colorectal cancer survivors, risks of these cancers were also increased, suggesting a role of shared behavioral risk factors and genetic factors, such as Lynch syndrome (65), for which clinician referral of high-risk survivors to genetic risk assessment may allow opportunities for early detection and prevention of SPCs (66). Shared lifestyle factors relevant to observed associations include obesity and metabolic factors (ovary, uterine corpus, small intestine), alcohol consumption (small intestine), and smoking (small intestine, stomach, cervix) (67,68), warranting future analytical studies on the associations of these factors with the risk of subsequent colorectal cancer. Further, evidence suggests that abdominopelvic radiotherapy and possibly alkylating-based chemotherapy increase colorectal cancer risk (7,69-71), which may partly explain the increased risk observed among survivors of the uterine corpus, cervical, testicular cancers, and male HL with treatment history.
Increased risks of developing head and neck cancers were seen following 12 index cancers. Human papillomavirus (HPV) infectious status, an important risk factor, was not available in SEER, however, the increased risks following hematologic cancers are consistent with prior studies (21,32) and may relate to the increased risk of HPV infection because of prolonged immunosuppression among survivors who receive bone marrow transplantation, a curative treatment for a variety of hematologic disorders (72,73). Reasons remain unclear for the elevated risk after breast, thyroid, and brain cancers and soft tissue sarcoma, although the late effect of treatment and heightened medical scrutiny have been suspected (32). A study of childhood cancer survivors linked exposure to head and neck radiotherapy or platinum chemotherapy to a borderline increase in oropharyngeal cancer risk (74); however, studies are limited among AYA cancer survivors. Prevention efforts should continue to increase the uptake of HPV vaccination to mitigate increased risk for HPV-related cancers in AYA cancer survivors (21,75,76), given the substantially lower uptake among young cancer survivors vs the general population (23.8% vs 40.5% for at least 1 dose, ages 15-26 years) (77).
This study has several limitations. Even with long-term follow-up, events were rare, if any, for some SPCs, as reflected in the lack of estimates or wide confidence intervals. Restricting the analysis to SPCs that occurred 5 or more years after the initial diagnosis likely underestimated the risk for some SPCs, particularly treatment-related acute myeloid leukemia or myelodysplastic syndromes, which typically arise after a short-term latency (78). Additionally, underestimation is also possible because of outmigration from the 9 SEER areas of persons diagnosed with SPC (79) and exclusion of same-site SPCs that may include multicentric or multifocal tumors, new primaries at the other side of paired organs, and those with different histology and because the referent group general population includes persons with a history of cancer. Conversely, excess deaths from some SPCs may result from misclassified SPCs (eg, melanoma death after eye cancer and colorectal cancer death after small intestine and anal cancers) as the accuracy of cancer mortality data among individuals with multiple primary cancers remains to be determined (80). We caution overinterpretation of the presence or lack of treatment association with SPC risk because of limitations inherent in SEER treatment data—underascertainment, lack of detailed treatment regimen, no distinction between “no” and “unknown” indicated for chemotherapy or radiotherapy receipt, and lack of information beyond the first course of treatment (81)—that likely result in misclassification and residual confounding. Further, the lack of genetic and lifestyle risk factors, such as smoking and HPV infection, precluded the investigation of their role in SPC occurrence but was discussed as potentially contributing factors based on previous studies. Cancer classification was primarily based on topography code and did not use the classification scheme developed for AYA cancers (82) to maintain consistency between index and SPCs, which may have concealed heterogeneous risk patterns by tumor subtypes. Finally, some statistically significant associations may have occurred spuriously because of multiple tests, thus results should be interpreted based on biological and clinical plausibility.
In summary, using population-based data with a long-term follow-up, this study provides the risks of developing and dying from SPCs for a comprehensive list of AYA cancers. AYA cancer survivors are almost twice as likely to die from a new primary cancer as the general population, underscoring the critical role of primary care providers in the provision of high-quality posttreatment survivorship care to reduce the risks of developing SPCs or facilitate early detection of disease for successful treatment. Consolidated surveillance guidelines by the International Guideline Harmonization Group exist for subsequent breast (63) and thyroid (83) cancers and are under development for colorectal cancer (84,85), based on evidence primarily generated from studies of pediatric oncology and AYA survivors of HL and testicular cancer (16,47,86-89). Our findings highlight the need to expand the emphasis on SPC surveillance to include AYA as well as childhood cancer survivors and to develop age-specific and risk-appropriate surveillance strategies for SPCs in this growing population of survivors.
Funding
This work was supported by the Surveillance and Health Equity Science Department of the American Cancer Society (HS, RLS, KRY, KDM, AJ) and Institutional Research Grant from the American Cancer Society and the Medical College of Wisconsin Cancer Center (IRG #16-183-31, NH).
Notes
Role of the funder: The American Cancer Society had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: KRY serves on the Flatiron Health Equity Advisory Board. The other authors declare no competing interests. KRY, who is a JNCI Associate Editor and coauthor on this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: Conceptualization: HS, AJ; Data curation: HS; Methodology: HS and NH; Formal analysis: HS and NH; Writing—original draft: HS; Writing—review & editing: All authors; Supervision: AJ.
Prior presentations: This manuscript was published in abstract form (general poster session) at the 2021 American Society of Clinical Oncology Annual Meeting.
Data Availability
The database is publicly available and can be obtained upon user authentication from the Surveillance, Epidemiology, and End Results (SEER) Program in National Cancer Institute (https://seer.cancer.gov/data/).
Supplementary Material
Contributor Information
Hyuna Sung, Surveillance and Health Equity Science, American Cancer Society, Kennesaw, GA, USA.
Rebecca L Siegel, Surveillance and Health Equity Science, American Cancer Society, Kennesaw, GA, USA.
Noorie Hyun, Division of Biostatistics, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
Kimberly D Miller, Surveillance and Health Equity Science, American Cancer Society, Kennesaw, GA, USA.
K Robin Yabroff, Surveillance and Health Equity Science, American Cancer Society, Kennesaw, GA, USA.
Ahmedin Jemal, Surveillance and Health Equity Science, American Cancer Society, Kennesaw, GA, USA.
References
- 1. Trama A, Botta L, Foschi R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17(7):896-906. [DOI] [PubMed] [Google Scholar]
- 2. Berkman AM, Livingston JA, Merriman K, et al. Long-term survival among 5-year survivors of adolescent and young adult cancer. Cancer. 2020;126(16):3708-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137-e147. [DOI] [PubMed] [Google Scholar]
- 4. Chao C, Bhatia S, Xu L, et al. Chronic comorbidities among survivors of adolescent and young adult cancer. J Clin Oncol. 2020;38(27):3161-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao C, Bhatia S, Xu L, et al. Incidence, risk factors, and mortality associated with second malignant neoplasms among survivors of adolescent and young adult cancer. JAMA Netw Open. 2019;2(6):e195536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a National Cohort Study. J Clin Oncol. 2012;30(22):2745-2752. [DOI] [PubMed] [Google Scholar]
- 7. van Eggermond AM, Schaapveld M, Janus CP, et al. Infradiaphragmatic irradiation and high procarbazine doses increase colorectal cancer risk in Hodgkin lymphoma survivors. Br J Cancer. 2017;117(3):306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganz PA, Casillas JN.. Incorporating the risk for subsequent primary cancers into the care of adult cancer survivors: moving beyond 5-year survival. JAMA. 2020;324(24):2493-2495. [DOI] [PubMed] [Google Scholar]
- 9. Bhuller KS, Zhang Y, Li D, et al. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: report of the Childhood/Adolescent/Young Adult Cancer Survivors research program and the BC Cancer Agency Centre for Lymphoid Cancer. Br J Haematol. 2016;172(5):757-768. [DOI] [PubMed] [Google Scholar]
- 10. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373(26):2499-2511. [DOI] [PubMed] [Google Scholar]
- 11. Goldfarb M, Freyer DR.. Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer. 2014;120(8):1155-1161. [DOI] [PubMed] [Google Scholar]
- 12. Abrahao R, Li QW, Malogolowkin MH, et al. Chronic medical conditions and late effects following non-Hodgkin lymphoma in HIV-uninfected and HIV-infected adolescents and young adults: a population-based study. Br J Haematol. 2020;190(3):371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gingrich AA, Sauder CAM, Goldfarb M, et al. Disparities in the occurrence of late effects following treatment among adolescent and young adult melanoma survivors. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2195-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muffly L, Maguire FB, Li Q, et al. Late effects in survivors of adolescent and young adult acute lymphoblastic leukemia. JNCI Cancer Spectr. 2020;4(4):pkaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowers DC, Verbruggen LC, Kremer LCM, et al. Surveillance for subsequent neoplasms of the CNS for childhood, adolescent, and young adult cancer survivors: a systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021;22:e196-e206. doi: 10.1016/S1470-2045(20)30688-4. [DOI] [PubMed] [Google Scholar]
- 16. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-1437. [DOI] [PubMed] [Google Scholar]
- 17. Lee JS, DuBois SG, Boscardin WJ, et al. Secondary malignant neoplasms among children, adolescents, and young adults with osteosarcoma. Cancer. 2014;120(24):3987-3993. [DOI] [PubMed] [Google Scholar]
- 18. Schonfeld SJ, Merino DM, Curtis RE, et al. Risk of second primary bone and soft-tissue sarcomas among young adulthood cancer survivors. JNCI Cancer Spectr. 2019;3(3):pkz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fidler MM, Reulen RC, Winter DL, et al. Risk of subsequent bone cancers among 69 460 five-year survivors of childhood and adolescent cancer in Europe. J Natl Cancer Inst. 2018;110(2):183–194. [DOI] [PubMed] [Google Scholar]
- 20. Sauder CAM, Li Q, Othieno A, et al. Characteristics and outcomes for secondary breast cancer in childhood, adolescent, and young adult cancer survivors treated with radiation. Cancer Epidemiol Biomarkers Prev. 2020;29(9):1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ojha RP, Tota JE, Offutt-Powell TN, et al. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8(8):e70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teng A, Nelson DW, Dehal A, et al. Colon cancer as a subsequent malignant neoplasm in young adults. Cancer. 2019;125(21):3749-3754. [DOI] [PubMed] [Google Scholar]
- 23. Lee JS, DuBois SG, Coccia PF, et al. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122(1):116-123. [DOI] [PubMed] [Google Scholar]
- 24. Bright CJ, Reulen RC, Winter DL, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20(4):531-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trama A, Tittarelli A, Barigelletti G, et al. Excess risk of subsequent malignant neoplasms in adolescent and young adult cancer survivors: results from the first Italian population-based cohort. Cancer. 2022;128(2):364-372. [DOI] [PubMed] [Google Scholar]
- 26. Sung H, Hyun N, Leach CR, et al. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA. 2020;324(24):2521-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Research Plus Data, 9 Registries, Nov 2020 Sub (1975-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties. National Cancer Institute, DCCPS, Surveillance Research Program; 2021. http://www.seer.cancer.gov.
- 28.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Research Plus Data, 9 Registries, Nov 2020 Sub (1975-2018) for SMRs - Linked To County Attributes - Total U.S., 1969-2019 Counties. National Cancer Institute, DCCPS, Surveillance Research Program; 2021. http://www.seer.cancer.gov.
- 29.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Documentation for SEER data, variable & recode definitions, incidence site recode variables, site recode, site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html. Accessed March 7, 2022.
- 30. Suh E, Stratton KL, Leisenring WM, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21:421–435. doi: 10.1016/S1470-2045(19)30800-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. SEER cause-specific death classification. https://seer.cancer.gov/causespecific/. Accessed April 26, 2021.
- 32. Curtis RE, Freedman DM, Ron E, et al. , eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute, NIH Publ. No. 05-5302. Bethesda, MD, 2006. [Google Scholar]
- 33.National Cancer Institute Surveillance, Epidemiology, and End Results Program Variable and Recode Definitions on the SEER Historic Stage A. https://seer.cancer.gov/seerstat/variables/seer/yr1973_2009/lrd_stage/index.html. Accessed May 13, 2022.
- 34. Coccia PF, Pappo AS, Beaupin L, et al. Adolescent and young adult oncology, version 2. 2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(1):66-97. [DOI] [PubMed] [Google Scholar]
- 35. Sadler C, Goldfarb M.. Comparison of primary and secondary breast cancers in adolescents and young adults. Cancer. 2015;121(8):1295-1302. [DOI] [PubMed] [Google Scholar]
- 36. Moskowitz CS, Chou JF, Neglia JP, et al. Mortality after breast cancer among survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2019;37(24):2120-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang C, Hu K, Deng L, et al. Increased risk of breast cancer-specific mortality among cancer survivors who developed breast cancer as a second malignancy. BMC Cancer. 2021;21(1):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keegan THM, Bleyer A, Rosenberg AS, et al. Second primary malignant neoplasms and survival in adolescent and young adult cancer survivors. JAMA Oncol. 2017;3(11):1554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderson C, Gapstur SM, Leach CR, et al. Medical conditions and physical function deficits among multiple primary cancer survivors. J Cancer Surviv. 2020;14(4):518-526. [DOI] [PubMed] [Google Scholar]
- 41. Anderson C, Lund JL, Weaver MA, et al. Disparities in mortality from noncancer causes among adolescents and young adults with cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bychkovsky BL, Lo MT, Yussuf A, et al. Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer. 2022;128(6):1275-1283. [DOI] [PubMed] [Google Scholar]
- 43. Shiels MS, Gibson T, Sampson J, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park SM, Lim MK, Jung KW, et al. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007;25(30):4835-4843. [DOI] [PubMed] [Google Scholar]
- 45. Lorigan P, Radford J, Howell A, et al. Lung cancer after treatment for Hodgkin’s lymphoma: a systematic review. Lancet Oncol. 2005;6(10):773-779. [DOI] [PubMed] [Google Scholar]
- 46. Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94(3):182-192. [DOI] [PubMed] [Google Scholar]
- 47. Groot HJ, Lubberts S, de Wit R, et al. Risk of solid cancer after treatment of testicular germ cell cancer in the platinum era. J Clin Oncol. 2018;36(24):2504-2513. [DOI] [PubMed] [Google Scholar]
- 48. Grantzau T, Thomsen MS, Væth M, et al. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol. 2014;111(3):366-373. [DOI] [PubMed] [Google Scholar]
- 49. Reiner AS, Watt GP, John EM, et al. Smoking, radiation therapy, and contralateral breast cancer risk in young women. J Natl Cancer Inst. 2022;114:631–634. doi: 10.1093/jnci/djab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DiMarzio P, Peila R, Dowling O, et al. Smoking and alcohol drinking effect on radiotherapy associated risk of second primary cancer and mortality among breast cancer patients. Cancer Epidemiol. 2018;57:97-103. [DOI] [PubMed] [Google Scholar]
- 51. Aredo JV, Luo SJ, Gardner RM, et al. Tobacco smoking and risk of second primary lung cancer. J Thorac Oncol. 2021;16(6):968-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sitas F, Weber MF, Egger S, et al. Smoking cessation after cancer. J Clin Oncol. 2014;32(32):3593-3595. [DOI] [PubMed] [Google Scholar]
- 53.Cancer Trends Progress Report National Cancer Institute. Life after diagnosis. https://www.progressreport.cancer.gov/after. Published March 2015. Accessed August 15, 2020.
- 54. Shields PG, Herbst RS, Arenberg D, et al. Smoking cessation, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(11):1430-1468. [DOI] [PubMed] [Google Scholar]
- 55. Croyle RT, Morgan GD, Fiore MC.. Addressing a core gap in cancer care - the NCI moonshot program to help oncology patients stop smoking. N Engl J Med. 2019;380(6):512-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Henderson LM, Durham DD, Tammemagi MC, et al. Lung cancer screening with low dose computed tomography in patients with and without prior history of cancer in the national lung screening trial. J Thorac Oncol. 2021;16(6):980-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Massa ST, Gallogly JA, Walker RJ.. Most survivors of head and neck cancer should be offered lung cancer screening. JAMA Otolaryngol Head Neck Surg. 2021;147(12):1078. [DOI] [PubMed] [Google Scholar]
- 58. Wattson DA, Hunink MG, DiPiro PJ, et al. Low-dose chest computed tomography for lung cancer screening among Hodgkin lymphoma survivors: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys. 2014;90(2):344-353. [DOI] [PubMed] [Google Scholar]
- 59. Hodgson D, van Leeuwen F, Ng A, et al. Breast cancer after childhood, adolescent, and young adult cancer: it’s not just about chest radiation. Am Soc Clin Oncol Educ Book. 2017;37:736-745. [DOI] [PubMed] [Google Scholar]
- 60. Veiga LH, Curtis RE, Morton LM, et al. Association of breast cancer risk after childhood cancer with radiation dose to the breast and anthracycline use: a report from the childhood cancer survivor study. JAMA Pediatr. 2019;173(12):1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: a report from the childhood cancer survivor study. J Clin Oncol. 2016;34(9):910-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corkum M, Hayden JA, Kephart G, et al. Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7(3):455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mulder RL, Hudson MM, Bhatia S, et al. Updated breast cancer surveillance recommendations for female survivors of childhood, adolescent, and young adult cancer from the international guideline harmonization group. J Clin Oncol. 2020;38(35):4194-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yeh JM, Lowry KP, Schechter CB, et al. Breast cancer screening among childhood cancer survivors treated without chest radiation: clinical benefits and cost-effectiveness. J Natl Cancer Inst. 2022;114:235–244. doi: 10.1093/jnci/djab149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst. 2012;104(18):1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17(9):1032-1041. [DOI] [PubMed] [Google Scholar]
- 67. Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bennett CM, Coleman HG, Veal PG, et al. Lifestyle factors and small intestine adenocarcinoma risk: a systematic review and meta-analysis. Cancer Epidemiol. 2015;39(3):265-273. [DOI] [PubMed] [Google Scholar]
- 69. Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99(21):1634-1643. [DOI] [PubMed] [Google Scholar]
- 70. Milano MT, Dinh PC, Yang H, et al. Solid and hematologic neoplasms after testicular cancer: a US population-based study of 24 900 survivors. JNCI Cancer Spectr. 2020;4(3):pkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nottage K, McFarlane J, Krasin MJ, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30(20):2552-2558. [DOI] [PubMed] [Google Scholar]
- 72. Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464-471. [DOI] [PubMed] [Google Scholar]
- 73. Adhikari J, Sharma P, Bhatt VR.. Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future Oncol. 2015;11(23):3175-3185. [DOI] [PubMed] [Google Scholar]
- 74. Henderson TO, Fowler BW, Hamann HA, et al. Subsequent malignant neoplasms in the Childhood Cancer Survivor Study: occurrence of cancer types in which human papillomavirus is an established etiologic risk factor. Cancer. 2022;128(2):373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Temkin SM, Seibel NL.. Are we missing an opportunity for cancer prevention? Human papillomavirus vaccination for survivors of pediatric and young adult cancers. Cancer. 2015;121(19):3395-3402. [DOI] [PubMed] [Google Scholar]
- 76. Cherven B, Castellino SM, Chen Y, et al. Intent and subsequent initiation of human papillomavirus vaccine among young cancer survivors. Cancer. 2019;125(21):3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klosky JL, Hudson MM, Chen Y, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35(31):3582-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morton LM, Swerdlow AJ, Schaapveld M, et al. Current knowledge and future research directions in treatment-related second primary malignancies. EJC Suppl. 2014;12(1):5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kovalchik SA, Pfeiffer RM.. Re: Assessment of impact of outmigration on incidence of second primary neoplasms in childhood cancer survivors estimated from SEER data. J Natl Cancer Inst. 2012;104(19):1517-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35(2):126-131. [DOI] [PubMed] [Google Scholar]
- 81. Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54(9):e55-64-e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barr RD, Holowaty EJ, Birch JM.. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425-1430. [DOI] [PubMed] [Google Scholar]
- 83. Clement SC, Kremer LCM, Verburg FA, et al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of Childhood, adolescent and young adult cancer: recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat Rev. 2018;63:28-39. [DOI] [PubMed] [Google Scholar]
- 84.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 5.0. Monrovia, CA: Children’s Oncology Group; 2018. http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed January 18, 2022.
- 85. Teepen JC, Ronckers CM, Kremer LCM.. Colorectal cancer screening in childhood cancer survivors. J Natl Cancer Inst. 2019;111(11):1114-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465-475. [DOI] [PubMed] [Google Scholar]
- 87. De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27(26):4239-4246. [DOI] [PubMed] [Google Scholar]
- 88. van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95(13):971-980. [DOI] [PubMed] [Google Scholar]
- 89. Richiardi L, Scelo G, Boffetta P, et al. Second malignancies among survivors of germ-cell testicular cancer: a pooled analysis between 13 cancer registries. Int J Cancer. 2007;120(3):623-631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The database is publicly available and can be obtained upon user authentication from the Surveillance, Epidemiology, and End Results (SEER) Program in National Cancer Institute (https://seer.cancer.gov/data/).