Abstract
Objectives: To explore the possible mechanism of human umbilical cord mesenchymal stem cell (hUC-MSC) transplantation in mice after spinal cord hemisection. Methods: Thoracic spinal cord hemisection injuries were performed on adult female Kunming mice. The mice with spinal cord injury (SCI) were injected with hUC-MSCs suspended in normal saline, while the control mice received an equal volume of normal saline. The histological HE staining and Nissl staining were performed 4 and 8 weeks after hUC-MSC transplantation in SCI mice. The Basso-Beattie-Bresnahan (BBB) locomotor rating scale was used to assess functional recovery after SCI. Western blotting was performed to determine the protein expressions. Results: hUC-MSCs transplantation decreased cavitation and tissue loss and increased the number of Nissl bodies in the damaged areas of the spinal cord after 4 and 8 weeks. The BBB locomotor performance of the transplanted mice was significantly improved (P<0.01). The wet weight of the injured side of the gastrocnemius muscle was significantly higher in the transplant group than that in the control group. Western blotting showed that TUJ1 and Olig2 expressions were significantly higher in hUC-MSC-grafted mice than those in vehicle controls. Three days after hUC-MSC transplantation, the expressions of TNF-α and NF-κB were higher in MSC-grafted mice than those in vehicle controls. However, 4 weeks after stem cell transplantation, the expressions of these two factors decreased in hUC-MSC-grafted mice compared with those in the vehicle controls. At 8 weeks after hUC-MSC transplantation, the expression of PTBP-1 was decreased in hUC-MSC-grafted mice compared with that in vehicle controls. Conclusions: hUC-MSC transplantation can protect neuron survival, promote myelin repair, and control glial scar formation in SCI mice.
Keywords: Mouse model of spinal cord hemisection, TNF-α, NF-κB pathway, PTBP-1, hUC-MSC transplantation
Introduction
Spinal cord injury (SCI) is a severe disease of the nervous system, with an annual worldwide incidence of approximately 22 in 100 million people [1]. Current treatments for SCI include surgical decompression and fixation, supportive medications and rehabilitation therapy. However, the therapeutic effect is not yet satisfactory. Most patients with SCI have permanent disabilities, including loss of bowel and bladder faculties, intractable pain, and sensory and motor functions impairment [2].
In recent years, stem cells have received extensive attention as seed cells for SCI treatment. Human umbilical cord mesenchymal stem cells (hUC-MSCs) are easy to isolate and culture, with strong proliferation capacity, low immunogenicity, multi-directional differentiation potential, and no tumorigenicity. Besides, these cells are safe and have no ethical and moral disputes, making hUC-MSCs the best seed cells for the treatment of SCI. But stem cell therapy has not been approved for the clinical treatment of SCI [3,4].
Polypyrimidine tract-binding proteins (PTBPs) are RNA-binding proteins that regulate several post-transcriptional events. Human PTBP-1 transitions between the nucleus and cytoplasm are believed to be able to regulate RNA processes [5]. The main functions of PTBP-1 include splicing regulation, terminal processing, and internal ribosome entry site mediated translation, localization, and mRNA stability. As a transcription factor, PTBP-1 plays an important role in embryonic development, particularly in neuronal differentiation [6,7]. The PTBP-1 knockout embryo sac could not establish a stem cell line, which completely prevented blastocyst implantation [8]. PTBP-1 depletion promotes the transdifferentiation of fibroblasts into neurons [9]. Astrocytes proliferate abnormally after SCI. Glial scars are mainly formed by reactive astrocytes with high expression of the glial fibrillary acidic protein (GFAP) around the cavity of SCI. A study published in 2020 showed that astrocytes could transform into mature neurons by single PTBP-1 knockdown in mice and humans [10].
Inflammation is an important pathophysiological process in SCI, and TNF-α and NF-κB are involved in this process. However, the role of TNF-α/NF-κB in the repair mechanisms of SCI and whether hUC-MSCs inhibit glial scar formation and promote neuronal regeneration by regulating the expression of PTBP-1 have not yet been determined. This study aimed to assess the safety and neurological effects of allogeneic hUC-MSC transplantation in mice after spinal cord hemisection at the First Affiliated Hospital of the Dalian Medical University in China. This preliminary study also explored the mechanisms of inflammation and astrocyte scar regulation during the hUC-MSC treatment for SCI mice. Our results demonstrated that direct injection of hUC-MSCs into thoracic SCI models is safe, well-tolerated, and of modest benefit neurologically. PTBP-1 and TNF-α/NF-κB are involved in the repair mechanisms of hUC-MSC transplantation in SCI mice.
Materials and methods
Preparation of the hUC-MSCs and cell quality control
Before hUC-MSC collection, the identity and information of the donor were confirmed. The donor completed the “informed consent for collection and preservation of samples” from the donor organization. Immediately after childbirth, the umbilical cord was clamped and cut 5-7 cm from the umbilicus. The tissue was cut into 2-3 cm segments under aseptic conditions. hUC-MSCs were isolated and cultured in the laboratory following good manufacturing practice (GMP). Cultured cells were passaged when cell fusion reached 80-90%. After one to four passages, we tested the quality of the P1 cells (both the cells and media) by flow cytometry. Thereafter, all of the hUC-MSCs were stored in liquid nitrogen until use. The differentiation of hUC-MSCs into osteoblasts, chondrocytes, and adipocytes was identified. Before releasing hUC-MSCs for transplantation, inprocess quality testing of the cells at each passage was performed for cellular endotoxins, mycoplasmas, bacteria, fungi, and viruses. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of the Dalian Medical University (registration number: LCKY2016-58).
Establishment of SCI mouse model
Thoracic spinal cord hemisection injuries were performed on adult female Kunming mice. The mice were housed in groups of 4-5 under a 12-h light/12-h dark cycle at 22°C, fed ad libitum, and maintained in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Spinal cord hemisection was performed under 10% chloral hydrate (40 mg/kg) intraperitoneal injection anesthesia and prophylactic administration of penicillin (40,000 units per mouse). A dorsal laminectomy was performed on T10 to expose the spinal cord. Hemisection injury was induced using the No. 11 surgical blade at the T10 level of the right half of the spinal cord with the mouse. After hemisection, the deep and superficial muscle layers were sutured. SCI mice received manual bladder expression twice daily and were inspected for weight loss, dehydration, and distress with veterinary care. This study was performed under a protocol approved by the Institutional Animal Care and Use Committee of Medical University.
Transplantation of hUC-MSCs in the SCI mice and preparation of the samples
Cell or vehicle injection occurred simultaneously with the induction of SCI. A total of 30 µL of hUC-MSCs (1×104 cells/µL or 2×104 cells/µL, 13 mice in each group) suspended in normal saline was injected subpially along the injured spinal cord at the lesion epicenter using a 50 µL microsyringe with injection speed of 5 μL/min. Control animals (13 mice) received an equal volume of normal saline at the same injection rate. Animals were sacrificed, perfusion fixed, and removed for spinal cord 3 days or 4 weeks after hUC-MSC transplantation to evaluate TNF-α and NF-κB expressions, or 8 weeks after the operation to determine PTBP-1 expression and conduct other tests. Fixation was accomplished using 4% paraformaldehyde (PFA; Sigma) in 0.1 M PIPES buffer, pH 6.9, for 24 h, followed by immersion in PBS containing 30% sucrose. Spinal cords were cut into 5 µm thick frozen longitudinal sections, and then we performed histological HE staining and Nissl staining 4 and 8 weeks after hUC-MSC transplantation in SCI mice.
Hematoxylin-eosin (H&E) staining and Nissl staining
First, the slices were immersed in hematoxylin for 1 h, rinsed in distilled water for 2 min, separated in 1% hydrochloric acid for 5 s, and returned to hematoxylin for 5 s in ammonia water. Next, the slices were immersed in eosin for 1 min and serially dehydrated in increasing concentrations of ethanol: 60%, 75%, 80%, 95%, and 100% for 5 s each. This was followed by clearing for 2×2 min in xylene. Finally, the samples were coverslipped using Permount (Fisher Scientific).
For Nissl staining, the slices were stained with 1% toluidine blue in an oven at 60°C for 1 h and then rinsed in distilled water for 5 s. Then, the slices were separated in a special color separation solution (anhydrous ethanol: chloroform: ether =1:1:1) for 5 s and serially dehydrated in increasing concentrations of ethanol: 50%, 75%, 80%, 95%, and 100% for 5 s each. This was followed by clearing for 2×2 min in xylene. Finally, the samples were coverslipped using Permount (Fisher Scientific).
Behavioral test and wet weight of skeletal muscle that loses innervation
The Basso-Beattie-Bresnahan (BBB) locomotor rating scale was used to assess functional recovery after SCI. Each mouse was individually assessed for 2 min and scored by two independent observers in an open field [11]. Testing was performed 1 day and 3 days after SCI and continued weekly for 8 weeks. The mean group scores for the hind limb ipsilateral to the T10 hemisectioned SCI mice were assessed. The dissected spinal cords were sectioned into axial sections.
At 8 weeks post-injury, mice were anesthetized by intraperitoneal injection of 10% chloral hydrate. The skin and subcutaneous tissue of the right hind limb were cut, the gastrocnemius muscles of each group were completely removed, and the connective tissue on the surface was carefully removed. The wet weight of the muscles was weighed using an electronic analytical balance (index value 0.001 mg), and the average value of the skeletal muscle wet weight of each group of SCI mice was recorded.
Immunofluorescence analysis and western blot
Eight weeks after hUC-MSC intervention in SCI mice, we collected frozen sections of the spinal cord of SCI mice (see section 2.3). The slices were fixed in cold acetone at 4°C for 30 min and then repaired in citrate buffer for 3 min. Thereafter, the slices were incubated with 0.5% Triton X100 (phosphate buffer) for 20 min at 37°C to destroy the cell membrane and incubated for 30 min in goat serum or 5% BSA to block non-specific antibody binding. The sections were then incubated overnight at 4°C with primary antibodies against β-tubulin isotype III (TUJ1, mouse from Sigma-Aldrich, 1:800) to label neurons, oligodendrocyte transcription factor (OLIG2, rabbit from Proteintech, 1:300) to label oligodendrocytes, GFAP (goat from Abcam, 1:1000) to label astrocytes, and polypyrimidine tract-binding protein 1 protein (PTBP-1, rabbit form Abcam, 1:2000). After the primary incubation, the sections were washed and incubated at 37°C for 2 h with Alexa 488 or 594 conjugated donkey secondary antibodies (1:200). The slices were stained with nuclear stain DAPI (1:100) at room temperature. Thereafter, the sections were washed, mounted on slides, and covered with Fluoromount G (Solarbio).
Samples were collected, and the tissue was lysed in a mixed extraction reagent containing protease and phosphatase inhibitors, and the lysate was centrifuged (12,000 rpm, 5 min, 4°C). The supernatant was collected and preserved at 80°C. Protein concentrations were determined using the Bradford assay. Samples were electrophoresed on a 12% SDS-PAGE gel and transferred to nitrocellulose membranes. After blocking with 5% skim milk in PBS containing 0.1% Tween20 (PBST), the membranes were incubated with the following antibodies overnight at 4°C: mouse anti-β tubulin III (TUJ1, Sigma-Aldrich, 1:1,000), rabbit anti-OLIG2 (Proteintech, 1:400), and goat anti-GFAP (Abcam, 1:1000) 8 weeks after hUC-MSC transplantation in SCI mice. For the evaluation of TNF-α and NF-κB expressions, we utilized rabbit anti-TNF-α (Proteintech, 1:600) and rabbit anti-NF-κB (Abcam, 1:1000) antibodies for western blotting 3 days and 4 weeks after hUC-MSC transplantation in SCI mice, and the membrane was washed three times with PBST at room temperature for 10 min. Thereafter, the membranes were incubated with peroxidase-conjugated antibodies (Proteintech, 1:8000) at 37°C for 1 h, and then washed three times with PBST for 10 min each time. The membrane was washed once with PBS for 10 min. Electrochemiluminescence (ECL) was then developed, and GAPDH and β-actin were used as internal references. Image J software performed grayscale analysis using the gray value of the target protein and the corresponding GAPDH and β-actin band gray values as the relative expression of the protein after setting the control group to 1.
Statistical analyses
All data were analyzed using a one-way repeated measure analysis of variance. All differences were considered significant at P<0.05. Statistical comparisons were analyzed using the SPSS19.0 software (Chicago, IL, USA).
Results
Identification and quality control of hUC-MSCs
Before releasing hUC-MSCs for transplantation, in-process quality testing of the cells at each passage was performed for cellular endotoxins, mycoplasmas, bacteria, fungi or viruses. All of the above were determined to be negative for preclinical use. For each passage, to analyze the cellular composition of the cells, we conducted flow cytometry. The results showed that the majority of cells (~99%) expressed CD13+, CD44+, CD73+, CD90+, and CD105+, with CD14-, CD34-, CD45-, and HLA-DR-. These results indicate that the transplanted cells consisted mostly of MSCs. After 14 days of lipogenesis and 21 days of osteogenesis and chondrogenesis, hUC-MSCs of different generations (P3, P5, P7, P11) were differentiated into fat, osteogenesis, and chondrogenesis (Figure 1).
Figure 1.
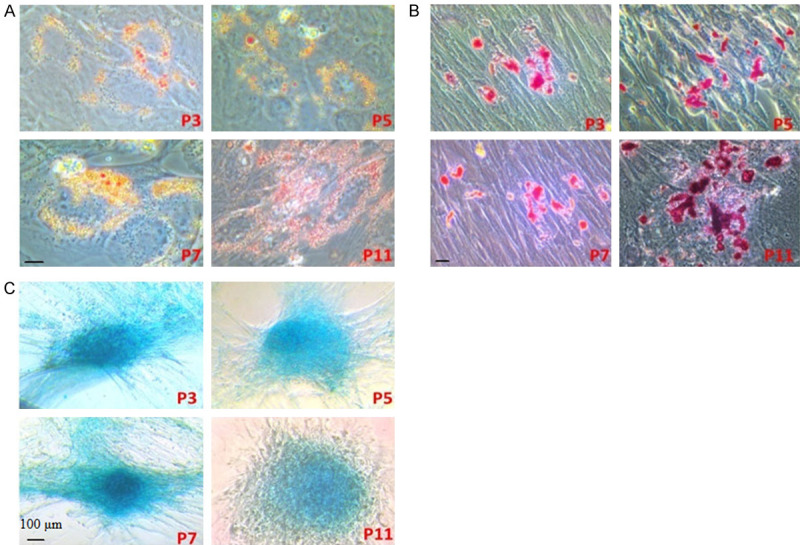
Adipogenic, osteogenic, and chondrogenic differentiation of different passages of hUC-MSCs cultured in vitro. A. Adipogenic differentiation of different passages of hUC-MSCs cultured in vitro. After 14 days of lipogenesis, Oil Red O staining was performed on hUC-MSCs of different generations (P3, P5, P7, P11), showing reddish-orange lipid droplets. B. Osteogenic differentiation of different passages of hUC-MSCs cultured in vitro. After 21 days of osteogenesis, Alizarin red S staining was performed on hUC-MSCs of different generations (P3, P5, P7, P11), showing mineralized matrix in red staining. C. Chondrogenic differentiation of different passages of hUC-MSCs cultured in vitro. After 21 days of chondrogenesis, toluidine blue staining was performed on hUC-MSCs of different generations (P3, P5, P7, P11), showing metachromasy in cartilage matrix (black scale bars: 100 μm).
Establishment of hemisection SCI mouse model
The spinal cord of female mice was exposed to T10 (Figure 2A). The right half spinal cord of the mouse was transected, with obvious bleeding. After complete hemostasis (Figure 2B), the muscles and skin were sutured layer by layer, and the right lower limb of the mouse with hemisection of the spinal cord was completely paralyzed without joint activity (Figure 2C).
Figure 2.
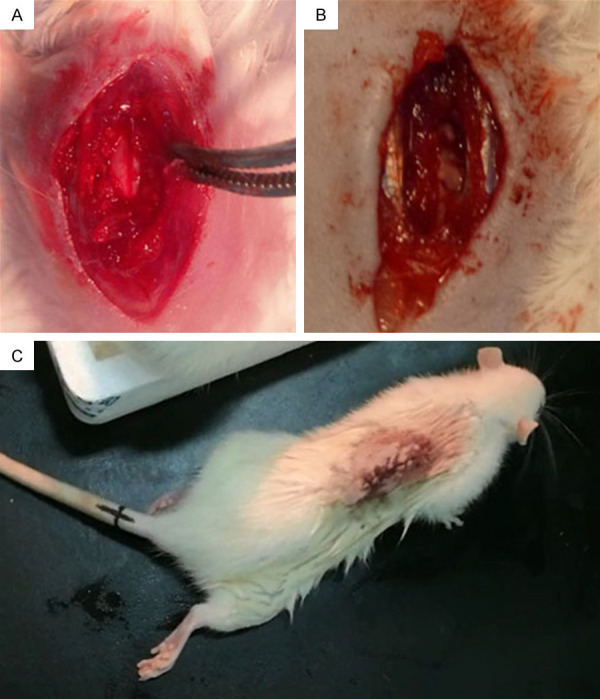
Spinal cord of a female mouse was exposed at T10 level (A). The right side of spinal cord was transected, and bleeding was obvious (B). After the T10 hemisection operation, the right lower limb of the spinal cord injury mouse was completely paralyzed without joint movement (C).
hUC-MSC transplantation promotes locomotor recovery, decreases motor neuron loss and increases wet weight of denervated skeletal muscle in SCI mice
To investigate whether hUC-MSC transplantation contributes to long-term locomotor recovery in SCI mice, we assessed the BBB locomotor rate scale 1 day, 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 7 weeks and 8 weeks after transplantation. Moreover, we found that BBB locomotor performance was significantly improved in 2×107 MSC-grafted mice compared with that in vehicle controls and 1×107 MSC-grafted mice 7 weeks and 8 weeks after SCI (Figure 3). At 8 weeks post-transplantation (termination of the study), the average BBB scores were 1.625±2.722 in 2×107 MSC-grafted mice (n=13), 1.750±2.375 in 1×107 MSC-grafted mice (n=13) and 0.286±0.756 in vehicle-treated animals (n=13). These results suggest that hUC-MSC transplantation significantly improves locomotor recovery in hemisectioned SCI mice.
Figure 3.
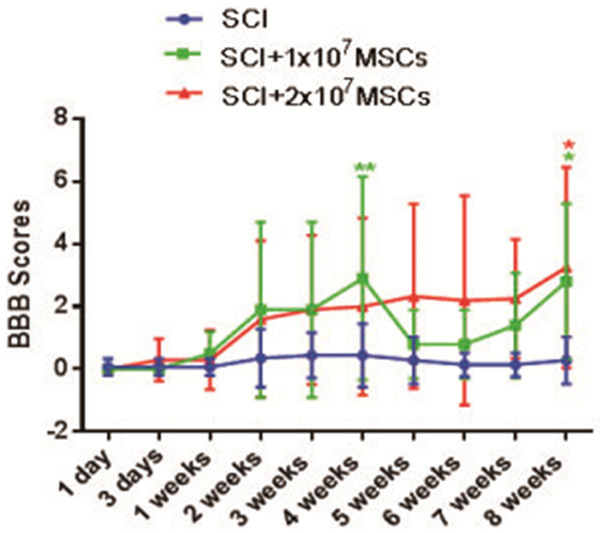
Basso-Beattie-Bresnahan (BBB) score of hemisectioned spinal cord injury (SCI) mice with or without hUC-MSC transplantation (P<0.01).
Morphometric analysis by HE staining indicated that transplantation of hUC-MSCs 4 and 8 weeks after SCI decreased cavitation and tissue loss (Figure 4). The effect of hUC-MSC transplantation on the distribution of neurons in SCI by Nissl staining showed that neurons were significantly increased in the injured spinal cord in 2×107 MSC-grafted mice compared to those in the vehicle control group after SCI (Figure 5). These results strengthened the hypothesis that hUC-MSC transplantation has neuroprotective effects on motor neurons in SCI mice and contributes to long-term motor recovery in SCI mice.
Figure 4.
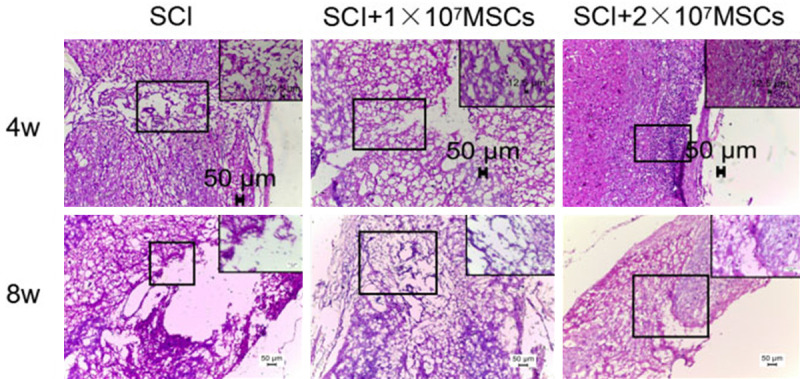
Spinal cord HE staining at the injury site 4 weeks and 8 weeks after hUC-MSC transplantation of spinal cord injury (SCI) mice (10×, enlarged image: 40×, black scale bars: 50 μm).
Figure 5.
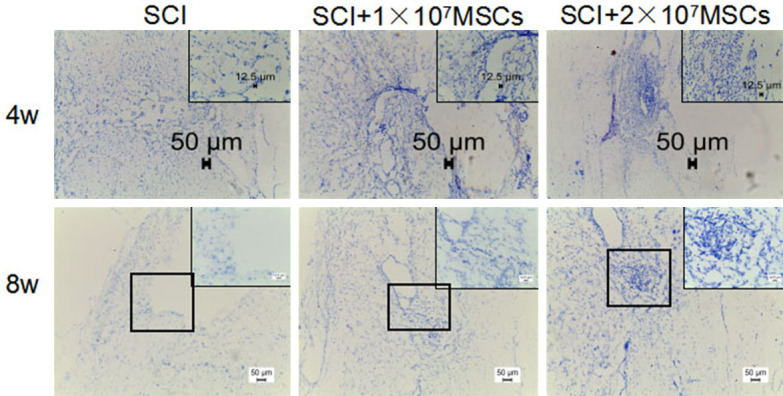
Distribution of Nissl bodies 4 and 8 weeks after hUC-MSC transplantation of spinal cord injury (SCI) mice (10×, enlarged image: 40×, black scale bars: 50 μm).
The average value of the skeletal muscle wet weight of each animal group is an important assessment value. In the preclinical study, the wet weight of the right leg gastrocnemius muscle 8 weeks after hUC-MSC transplantation was recorded. The results showed that the wet weight of the right leg gastrocnemius muscle in the 2×107 MSC-grafted mice was significantly higher than that in the control group or in 1×107 MSC-grafted mice (P<0.05, Figure 6). This suggests that hUC-MSC transplantation protects muscle tissue by protecting damaged spinal motor neurons and improves motor function in SCI mice.
Figure 6.
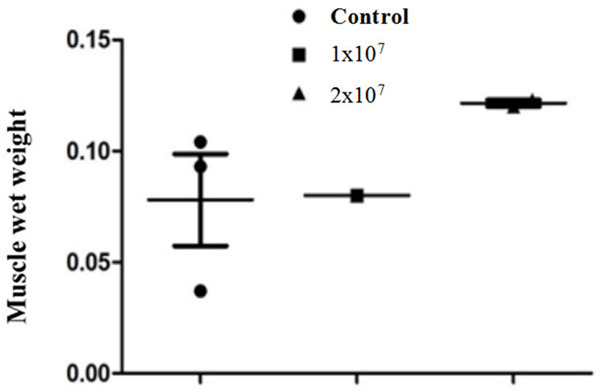
Comparison of wet weight of gastrocnemius muscle of right leg after 8 weeks of hUC-MSC transplantation for spinal cord injury (SCI) mice (P<0.05).
hUC-MSC transplantation protects neurons survival, promotes myelin repairment, and controls glial scar formation in SCI mice
To investigate how the hemisectioned SCI environment influenced the engrafted hUC-MSCs, we assessed SCI mice with different type of cells in the injured spinal cord area by immunofluorescence and western blot analysis after coronal section. At 8 weeks post-transplantation, we observed TUJ1 positive, GFAP-positive, Olig2 positive at the epicenter of the SCI area in the MSC-grafted mice (Figures 5, 6 and 7). Western blot analysis showed that 8 weeks after hUC-MSC transplantation, TUJ1 and Olig2 expressions were significantly higher in hUC-MSC-grafted mice than those in vehicle and sham controls (Figures 7 and 8). GFAP expression was lower in hUC-MSC-grafted mice than that in vehicle and sham controls (Figure 9). These results indicate that hUC-MSC transplantation could protect neuron survival, promote myelin repair, and control glial scar formation in SCI mice.
Figure 7.
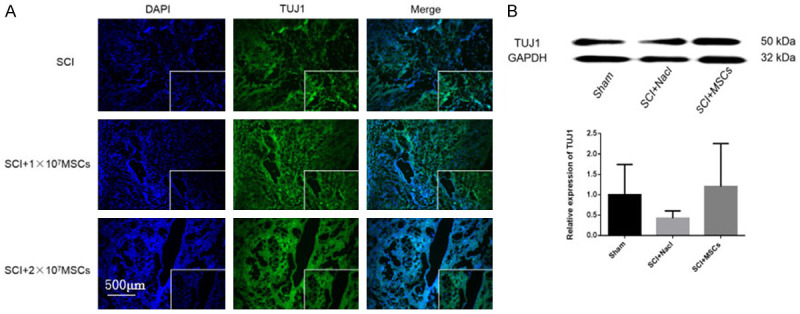
Immunofluorescence of neurons and expression of TUJ1 8 weeks after hUC-MSC transplantation in SCI mice. A. Immunofluorescence of TUJ1 8 weeks after hUC-MSC transplantation in SCI mice. Magnification: 100×. B. Western blot and quantitative analysis of TUJ1 protein in injured spinal cord 8 weeks after hUC-MSC transplantation.
Figure 8.
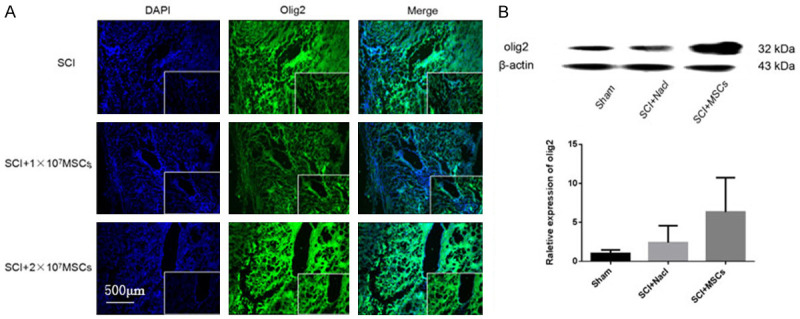
Immunofluorescence of oligodendrocytes and the expression of OLIG2 8 weeks after hUC-MSC transplantation in spinal cord injury (SCI) mice. A. Immunofluorescence of OLIG2 8 weeks after hUC-MSC transplantation in SCI mice. Magnification: 100×. B. Western blot and quantitative analysis of OLIG2 protein in injured spinal cord 8 weeks after hUC-MSC transplantation.
Figure 9.
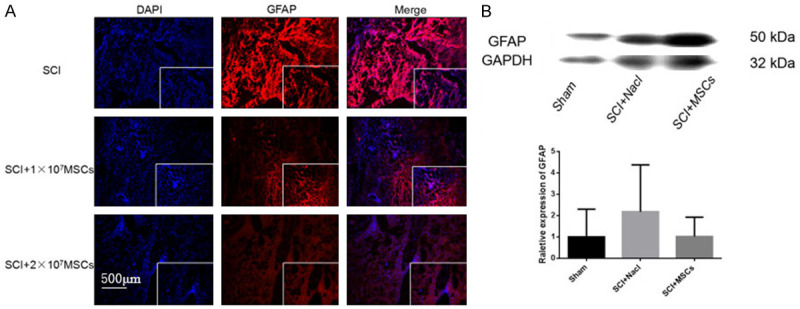
Immunofluorescence of astrocytes and expression of astrocyte marker GFAP protein 8 weeks after hUC-MSC transplantation in spinal cord injury (SCI) mice. A. Immunofluorescence of GFAP 8 weeks after hUC-MSC transplantation in SCI mice. Magnification: 100×. B. Western blot and quantitative analysis of GFAP protein in injured spinal cord 8 weeks after hUC-MSC transplantation.
hUC-MSC transplantation regulates TNF-α expression by NF-κB signaling pathway in SCI mice
To explore the expression of inflammatory factors and signaling pathway proteins in hUC-MSC transplantation after SCI, we assessed TNF-α and NF-κB levels by western blotting. Three days after hUC-MSC transplantation, the expressions of TNF-α and NF-κB were increased in 2×107 hMSC-grafted mice compared with those in vehicle controls. However, 4 weeks after hUC-MSC transplantation, the two expressions were decreased in hMSC-grafted mice compared with those of vehicle controls (Figures 10 and 11). Thus, hUC-MSCs may play a protective role in SCI mice by regulating the NF-κB pathway and TNF-α expressions.
Figure 10.
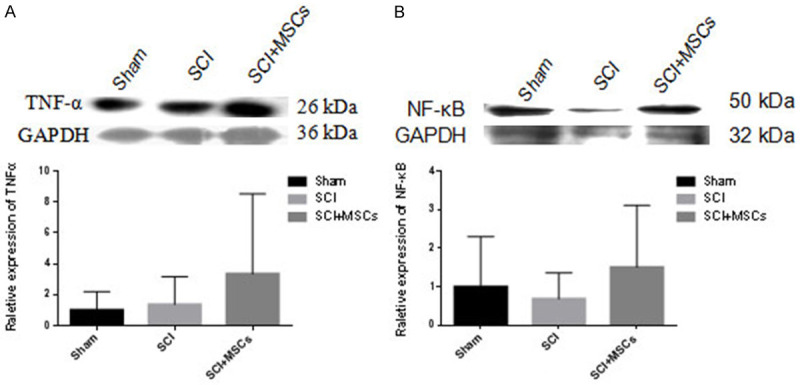
Inflammatory factor TNF-α and inflammatory pathway protein NF-κB expression in injured spinal cord 3 days after hUC-MSC transplantation in spinal cord injury (SCI) mice. A. Western blot and quantitative analysis of TNF-α protein in injured spinal cord 3 days after hUC-MSC transplantation. B. Western blot and quantitative analysis of NF-κB protein in injured spinal cord 3 days after hUC-MSC transplantation.
Figure 11.
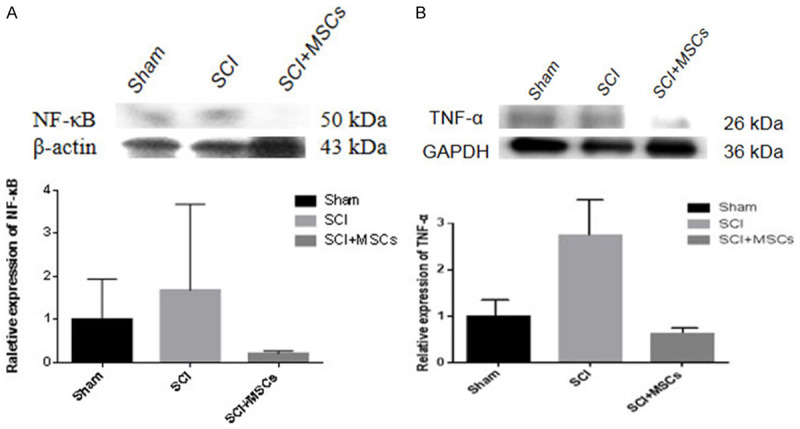
Inflammatory factor TNF-α and inflammatory pathway protein NF-κB expression in injured spinal cord 4 weeks after hUC-MSC transplantation in spinal cord injury (SCI) mice. A. Western blot and quantitative analysis of TNF-α protein in injured spinal cord 4 weeks after hUC-MSC transplantation. B. Western blot and quantitative analysis of NF-κB protein in injured spinal cord 4 weeks after hUC-MSC transplantation.
PTBP-1 involves in repair mechanisms of hUC-MSC transplantation in SCI mice
PTBP-1 acts as an alternative splicing factor in the nucleus and shuttles between the nucleus and the cytoplasm. PTBP-1 is thought to be involved in post-transcriptional regulation that requires cap-independent translational control, RNA localization, or changes in mRNA stability [12,13]. To determine the characteristics of PTBP-1 in the repair mechanism of hUC-MSC transplantation in SCI mice, we assessed PTBP-1 levels by immunofluorescence analysis, which showed that 8 weeks after hUC-MSC transplantation, the expression of PTBP-1 was decreased in hUC-MSC-grafted mice compared with that in SCI vehicle controls, and PTBP-1 was mainly distributed in the nucleus of astrocytes but not neurons (Figure 12). The above results revealed that PTBP-1 may play an inhibitory role in transforming astrocytes into neurons during SCI repair.
Figure 12.
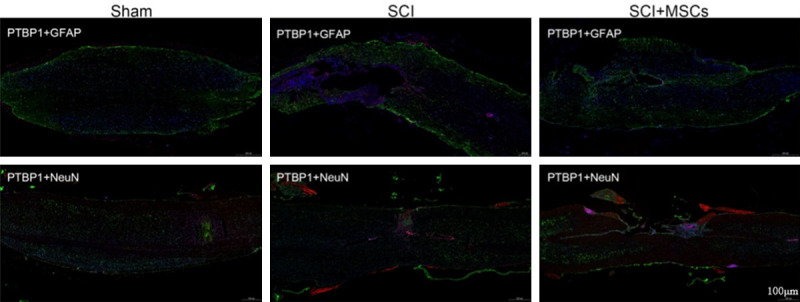
Immunofluorescence of PTBP-1 expression in injured spinal cord 8 weeks after hUC-MSC transplantation in spinal cord injury (SCI) mice. PTBP-1 may be upregulated in the periphery of spinal cord injury area and co-localized with astrocytes, except neurons after SCI, and downregulated after hUC-MSC intervention.
Discussion
SCI treatment faces three major dilemmas: first, injury and loss of local nerve tissue in the injured spinal cord, vacuolization, and lack of growth-enhancing ability of injured neurons; second, physical scars formed by astrocytes inhibiting spinal cord regeneration; third, reduced secretion of neurotrophic factors and persistent inflammatory response in the injured spinal cord [14]. In recent years, with the development of stem cell biology research, stem cell transplantation has provided new perspective for nerve repair and regeneration after SCI. Moreover, the use of hUC-MSCs [15] has become a research hotspot. At present, the efficacy of hUC-MSCs appears to be particularly related to their paracrine activity, not to the mechanism of cell replacement [16]. Under culture conditions, hUC-MSCs release extracellular vesicles (MSC-EVs) containing proteins, trophic factors, cytokines [17,18], mRNA [17,19], and microRNA [17,20,21]. These factors exert immunomodulatory, anti-inflammatory, neurotrophic, neuroprotective and angiogenic effects, which are extremely important for SCI repair [22-24].
Different experimental SCI mouse models have different advantages and disadvantages. SCI from contusion has a faster recovery of motor function after injury, but with poor consistency and many interference factors affecting the successful rate. Surgical spinal cord transection is simple, and the hind limbs of mice are completely paralyzed immediately after the operation. However, these mice have a high postoperative mortality because of surgical trauma, and the bleeding after surgery is also more severe. In the mouse model of spinal cord hemi-transection, the immediate recovery of cerebrospinal fluid circulation after surgery can ensure the supply of nutrients, with faster recovery of fecal function and higher survival rate than other SCI models. Additionally, the histological cell morphology of the injured and control sides of the spinal cord can be compared at the same time after hemisection. More importantly, these SCI mice were more consistent with clinical SCI cases. Therefore, we selected spinal cord hemi-transection mice as the objects of our study.
Previous studies have shown that the route of stem cell transplantation has certain effects on SCI molecular repair. At present, the most commonly used cell transplantation route for SCI is the local injection of stem cells into the injured spinal cord. Although this method can effectively deliver cells to the spinal cord parenchyma, the invasiveness of this method limits the frequency of injections, and this transplantation route cannot achieve widespread interventions across the spinal cord. In this study, hUC-MSCs were directly injected under the soft membrane of the semi-transected spinal cord right after SCI modeling. It is a single-dose, sufficient, non-invasive, and safe method of stem cell transplantation. Marsala et al. [25] also proposed the use of human neural stem cells under the soft spinal membrane of the spinal cord (between the protective layer surrounding the spine (the soft membrane layer) and the surface layer of the spinal cord) in immunodeficient rats. It has been demonstrated that this injection route allows a single injection to deliver a large number of cells, which then migrate into the spinal cord parenchyma and accumulate in multiple spinal segments and the brainstem over time. Stem cells transplanted into the white and gray matters were mainly differentiated into astrocytes and oligodendrocytes. This is consistent with our research on cell transplantation routes.
Inflammation is the most important pathological process after SCI, and it plays a vital role in the prognosis of SCI, leading to progressive deterioration of spinal anterior horn motor neurons [26]. After SCI, some cytotoxic cytokines, such as TNF-α, IL-1β, and IL-6, are produced. Therefore, these cytokines may be potential targets for SCI treatment [27-29]. NF-κB is essential for the survival of neurons against oxidative stress and ischemic degeneration, but it also contributes to inflammation and apoptosis after central nervous system injury [30]. TNF-α increases immediately after CNS-related trauma (such as SCI) [31,32]. TNF-α is synthesized by various cell types, including neurons, glial cells, T cells, astrocytes, activated macrophages and Schwann cells [33]. TNF-α is considered a biomarker for monitoring and predicting pro-inflammatory states and plays an important role in the occurrence and development of chronic inflammatory diseases [34,35]. Chi et al. argued that TNF-α might play a dual role in SCI, and its role might depend on when TNF-α is produced after SCI [36]. In this study, NF-κB and TNF-α were upregulated in the hUC-MSC transplantation group 3 days after SCI compared with those in vehicle controls. However, NF-κB and TNF-α were downregulated in the hUC-MSC transplantation group 4 weeks after SCI compared with those in the control group. These results indicate that the NF-κB pathway is related to the potential anti-inflammatory mechanism of hUC-MSCs in SCI repair. It is speculated that up-regulation of TNF-α and its upstream pathway protein NF-κB in the transplantation group in the acute phase after SCI is a protective factor for SCI mice, which protects neurons in the damaged area, and promotes the removal of damaged tissue cell debris. However, in the chronic phase of SCI, the over-activated inflammatory response can aggravate SCI tissue damage. Subsequently, the expressions of TNF-α and NF-κB were downregulated in the hUC-MSC transplantation group, indicating that hUC-MSCs could inhibit the over-activated inflammatory response in the chronic phase of SCI.
PTBP-1 is a 57 kDa protein comprising four RNA recognition motifs (RRMs) with a bipartite nuclear localization domain and a nuclear export signal at the N-terminus of the protein [37-39]. PTBP-1 mRNAs have been found to affect the levels of many additional alternative splicing events, possibly modulating the timing of transitions in the production of neural progenitors and mature neurons to affect brain morphology and complexity [40]. A study published in 2020 showed that astrocytes could be transformed into neurons by PTBP-1 knockout alone in mice and humans [10]. It was revealed that PTBP-1 plays an important role in regulating the transformation of astrocytes into mature neurons. However, in the process of SCI repair, the most important thing is to break through the physical barrier of the glial scar and promote the regeneration of neurons. The main component of the glial scar is reactive astrocytes proliferating around the SCI lesion. This study showed that PTBP-1 was upregulated and co-localized with GFAP positive astrocytes after SCI. After hUC-MSC intervention, PTBP-1 was downregulated. At the same time, Western blotting showed that 8 weeks after hUC-MSC transplantation, TUJ1 expression were significantly higher in hUC-MSC-grafted mice compared with that in vehicle and sham controls. However, the expression of GFAP was lower in hUC-MSC-grafted mice compared with that in vehicle and sham controls. These results indicate that PTBP-1 promotes inhibition of the transformation of astrocytes into neurons after SCI. The mechanism of hUC-MSC transplantation in repairing SCI may involve downregulation of the expression of PTBP-1 in GFAP-positive astrocytes and relieving its inhibitory effect on neuronal transformation, thus playing a vital role in the repair mechanism after SCI.
Conclusion
The possible mechanisms of hUC-MSC transplantation in repairing SCI in our study were: 1. through promoting the survival of neurons and oligodendrocytes in the damaged area of SCI to protect the material basis of nerve conduction pathways and myelin sheaths; 2. through reducing the formation of scar tissue and spinal cavity in the injured spinal cord and increasing the integrity of the spinal cord parenchyma; 3. through regulating the secondary immune-inflammatory response and PTBP-1 expression in the injured spinal cord to regulate scar formation and neuron regeneration, promoting functional recovery in SCI mice (Figure 13).
Figure 13.
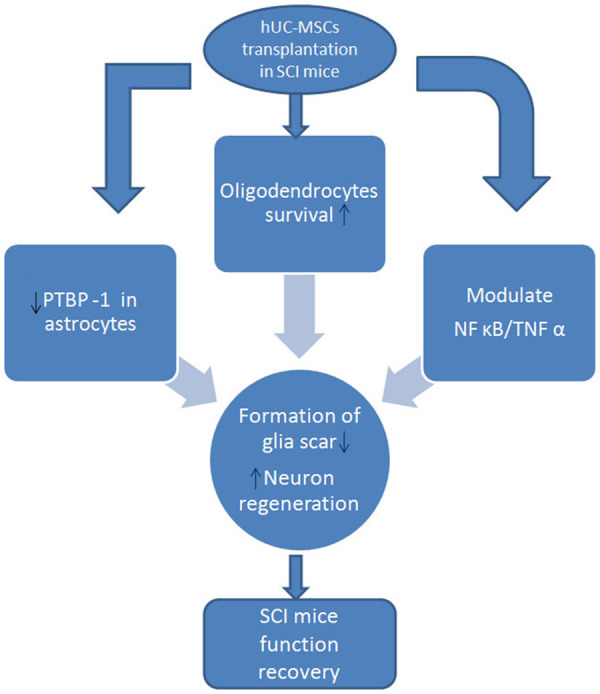
Possible repair mechanisms of hUC-MSC transplantation in SCI mice.
Prospects
Further studies are needed to explore the specific pathway of PTBP-1 after SCI and hUC-MSC transplantation. How can the repair effect of hUC-MSCs be enhanced by exogenous PTBP-1 intervention? What other mechanisms are involved in SCI repair? There is still a long way to go to improve the repair and treatment of SCI.
Acknowledgements
We are grateful for the support by Professor GuoHui Su’s team on the study conception and design. The project was funded by the National Natural Science Foundation of China (Grant No. 81471308), The Stem Cell Clinical Research Project (Grant No. CMR-20161129-1003), and Dalian Innovation Technology Funding (Grant No. 2018J11CY025).
Disclosure of conflict of interest
None.
References
- 1.Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang PC, Xiong WP, Wang G, Ma C, Yao WQ, Kendell SF, Mehling BM, Yuan XH, Wu DC. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp Ther Med. 2013;6:140–146. doi: 10.3892/etm.2013.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010;6:363–372. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arake de Tacca LM, Pulos-Holmes MC, Floor SN, Cate JHD. PTBP1 mRNA isoforms and regulation of their translation. RNA. 2019;25:1324–1336. doi: 10.1261/rna.070193.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutz PL, Stoilov P, Li Q, Lin CH, Chawla G, Ostrow K, Shiue L, Ares M Jr, Black DL. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 2007;21:1636–1652. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makeyev EV, Zhang JW, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JM, Kohn MJ, Bruinsma MW, Vech C, Intine RV, Fuhrmann S, Grinberg A, Mukherjee I, Love PE, Ko MS, DePamphilis ML, Maraia RJ. The multifunctional RNA-binding protein La is required for mouse development and for the establishment of embryonic stem cells. Mol Cell Biol. 2006;26:1445–1451. doi: 10.1128/MCB.26.4.1445-1451.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue YC, Ouyang KF, Huang J, Zhou Y, Ouyang H, Li HR, Wang GJ, Wu QL, Wei CL, Bi YZ, Jiang L, Cai ZQ, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian H, Kang XJ, Hu J, Zhang DY, Liang ZY, Meng F, Zhang X, Xue YC, Maimon R, Dowdy SF, Devaraj NK, Zhou Z, Mobley WC, Cleveland DW, Fu XD. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature. 2020;582:550–556. doi: 10.1038/s41586-020-2388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 12.Romanelli MG, Diani E, Lievens PM. New insights into functional roles of the polypyrimidine tract-binding protein. Int J Mol Sci. 2013;14:22906–22932. doi: 10.3390/ijms141122906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath RV, Leary DJ, Huang S. Nucleocytoplasmic shuttling of polypyrimidine tract-binding protein is uncoupled from RNA export. Mol Biol Cell. 2001;12:3808–3820. doi: 10.1091/mbc.12.12.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendonca MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83:214–222. doi: 10.1038/pr.2017.249. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Gao X, Wang JB. Human adipose-derived mesenchymal stem cell-conditioned media suppresses inflammatory bone loss in a lipopolysaccharide-induced murine model. Exp Ther Med. 2018;15:1839–1846. doi: 10.3892/etm.2017.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY, Chang YS. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12:e0174303. doi: 10.1371/journal.pone.0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nargesi AA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther. 2017;17:29–42. doi: 10.2174/1566523217666170412110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Zhao YM, Ma YS, Ge LH. Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev. 2016;25:499–508. doi: 10.1089/scd.2015.0298. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szekiova E, Slovinska L, Blasko J, Plsikova J, Cizkova D. The neuroprotective effect of rat adipose tissue-derived mesenchymal stem cell-conditioned medium on cortical neurons using an in vitro model of SCI inflammation. Neurol Res. 2018;40:258–267. doi: 10.1080/01616412.2018.1432266. [DOI] [PubMed] [Google Scholar]
- 25.Marsala M, Kamizato K, Tadokoro T, Navarro M, Juhas S, Juhasova J, Marsala S, Studenovska H, Proks V, Hazel T, Johe K, Kakinohana M, Driscoll S, Glenn T, Pfaff S, Ciacci J. Spinal parenchymal occupation by neural stem cells after subpial delivery in adult immunodeficient rats. Stem Cells Transl Med. 2020;9:177–188. doi: 10.1002/sctm.19-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015;38:609–620. doi: 10.1016/j.tins.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E, Bramanti P, Cuzzocrea S. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- 28.Gadani SP, Walsh JT, Smirnov I, Zheng JJ, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Papa S, Caron I, Erba E, Panini N, De Paola M, Mariani A, Colombo C, Ferrari R, Pozzer D, Zanier ER, Pischiutta F, Lucchetti J, Bassi A, Valentini G, Simonutti G, Rossi F, Moscatelli D, Forloni G, Veglianese P. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Zhan YT, Wang YB, Feuerstein GZ, Wang XK. Recombinant adenoviral expression of dominant negative IkappaBalpha protects brain from cerebral ischemic injury. Biochem Biophys Res Commun. 2002;299:14–17. doi: 10.1016/s0006-291x(02)02573-1. [DOI] [PubMed] [Google Scholar]
- 31.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 32.Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res. 2009;1290:102–110. doi: 10.1016/j.brainres.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Vidal PM, Lemmens E, Geboes L, Vangansewinkel T, Nelissen S, Hendrix S. Late blocking of peripheral TNF-alpha is ineffective after spinal cord injury in mice. Immunobiology. 2013;218:281–284. doi: 10.1016/j.imbio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Campion J, Milagro FI, Goyenechea E, Martinez JA. TNF-alpha promoter methylation as a predictive biomarker for weight-loss response. Obesity (Silver Spring) 2009;17:1293–1297. doi: 10.1038/oby.2008.679. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci. 2010;1193:153–159. doi: 10.1111/j.1749-6632.2009.05287.x. [DOI] [PubMed] [Google Scholar]
- 36.Chi LY, Yu J, Zhu H, Li XG, Zhu SG, Kindy MS. The dual role of tumor necrosis factor-alpha in the pathophysiology of spinal cord injury. Neurosci Lett. 2008;438:174–179. doi: 10.1016/j.neulet.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Yen TS. Characterization of the nuclear export signal of polypyrimidine tract-binding protein. J Biol Chem. 2002;277:10306–10314. doi: 10.1074/jbc.M109686200. [DOI] [PubMed] [Google Scholar]
- 38.Perez I, McAfee JG, Patton JG. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry. 1997;36:11881–11890. doi: 10.1021/bi9711745. [DOI] [PubMed] [Google Scholar]
- 39.Wollerton MC, Gooding C, Robinson F, Brown EC, Jackson RJ, Smith CW. Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB) RNA. 2001;7:819–832. doi: 10.1017/s1355838201010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gueroussov S, Gonatopoulos-Pournatzis T, Irimia M, Raj B, Lin ZY, Gingras AC, Blencowe BJ. An alternative splicing event amplifies evolutionary differences between vertebrates. Science. 2015;349:868–873. doi: 10.1126/science.aaa8381. [DOI] [PubMed] [Google Scholar]


