Abstract
Background and objective: Research on allergic rhinitis (AR) immunotherapy has increased in recent decades. This study conducted a bibliometric and visualization analysis of studies related to AR immunotherapy to identify research trends and highlight current research foci. Methods: Relevant original publications were obtained from the Science Citation Index-Expanded and Social Sciences Citation Index in the Web of Science Core Collection databases between 2002 and 2021. CiteSpace and VOSviewer software were used to identify and analyze the research foci and emerging trends in the field of AR immunotherapy. Results: Over the last two decades, the number of publications related to AR immunotherapy has increased markedly. With regard to publications and access to collaborative networks, the leading country was the USA. Inspection of keyword bursts suggested that “subcutaneous immunotherapy”, “quality of life”, “prevalence”, “rhino-conjunctivitis”, and “mechanism” are emerging research hotspots. The timeline of the co-cited references cluster diagram revealed that the mechanism of allergen immunotherapy has emerged as a main topic in AR immunotherapy. Conclusion: Over the past 20 years, scholars have significantly improved their understanding of AR immunotherapy. The current research hotspots of AR immunotherapy in the health promotion domain lie in “subcutaneous immunotherapy”, “quality of life”, and “rhino-conjunctivitis”. In addition, the mechanism of allergen immunotherapy has emerged as a frontier and focus of this field.
Keywords: Allergic rhinitis, immunotherapy, bibliometric analysis, CiteSpace, VOSviewer
Introduction
Allergic rhinitis (AR) refers to an IgE-mediated inflammatory disease of the nasal mucosa following exposure of the body to environmental allergens [1]. Typical symptoms of AR include nasal congestion, sneezing, rhinorrhea, and rhinocnesmus. According to estimates produced by epidemiological survey research, AR affects approximately 10-40% of the global population [2]. Alarmingly, the prevalence of AR has risen globally in recent years. A recent large retrospective cohort study in the USA showed that the prevalence of AR was 19.9% [3]. In Europe, the prevalence of AR in the Danish adult population has increased by 16% in the last three decades [4]. An epidemiological survey from China showed that the prevalence of AR jumped from 11.1% in 2005 to 17.6% in 2011 in 18 major cities across the country [5]. Nasal allergies can lead to decreased productivity, learning difficulties, deterioration of social functioning, and even sleep disturbances [6,7]. Thus, AR has a serious negative influence on the quality of life. Poorly controlled AR can lead to complications, such as otitis media, chronic sinusitis, and conjunctivitis [8]. AR is also a key risk factor for asthma. In addition, AR is closely related to mental health problems. Patients with AR are at significantly higher risk of developing psychiatric disorders, such as anxiety, depression, and cognitive dysfunction, compared with the normal population [9,10]. AR not only severely damages the physical and mental health of individual patients, but also imposes a heavy financial burden on the healthcare system. In the USA, the medical expenditure for AR alone reached $11.2 billion in 2005 and is growing rapidly [11]. A European Union survey estimated the total annual cost of lost productivity caused by AR to be as high as 100 billion Euros [12]. Numerous pieces of evidence highlight AR as a serious global health problem and economic burden. Therefore, the search for an effective treatment is urgent.
Currently, drug therapy remains the cornerstone of AR treatment [8]. Common first-line agents for AR include intranasal glucocorticoids, oral and intranasal H1-antihistamine, and leukotriene receptor antagonists. These medications work by anti-inflammatory and anti-allergic effects to control symptoms and prevent worsening of the disease. Unfortunately, pharmacotherapy only controls the condition for the duration of use, and does not provide sustained efficacy after drug discontinuation [13]. In addition, these medications only partially control the symptoms. It is estimated that 1/3 of pediatric patients and 2/3 of adult patients do not achieve adequate symptom relief using pharmacotherapy [7]. Worries about the financial costs, side effects, and efficacy of long-term drug therapy have driven people to consider new treatments.
Allergen immunotherapy (AIT) is the etiologic treatment of type I allergic diseases caused by specific allergens, which is achieved by improving the body’s immune tolerance to the allergens [14]. Since 1998, the World Health Organization has recommended AIT as the only etiologic treatment for allergic diseases. The latest AIT guidelines developed by the European Academy of Allergy and Clinical Immunology [15] and the most recent Allergic Rhinitis and its Impact on Asthma guidelines [8] have all included AIT as a first-line treatment for AR. The main treatment modalities for AIT are subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). There is a large body of high-quality evidence demonstrating that AIT is effective in improving symptoms and decreasing the need for medication in patients with AR [16,17]. Currently, AIT is also the only treatment modality that can provide long-term efficacy after treatment has been stopped. More importantly, AIT not only provides good control of AR symptoms, but also modifies the natural course of AR and prevents the development of new allergic diseases [8].
Bibliometric analysis is a pioneering tool to quickly explore structures and trends of a subject through visualization and statistical methods [18]. It allows quantitative assessment of the impact of research literature on a selected research area, countries/regions, research collaboration, journals, institutions, and authors over a given period [19]. Bibliometric analysis has been shown to influence various research domains. Compared to conventional systematic reviews and meta-analyses, bibliometric analysis can reveal critical issues and developments in the field of interest more systematically and visually, and can guide future research [20]. CiteSpace is a visualized analysis software demonstrating the structure, pattern, and distribution of research fields [21]. VOSviewer software is used effectively for knowledge domain mapping [22]. VOSviewer and CiteSpace can reflect directly the development of a research field by presenting numerous data, including the productivity of authors and institutions, the geographical distribution by regions, and the results of collaborative relationships. VOSviewer and CiteSpace are widely used in various fields of application [22,23].
To the best of our knowledge, research conducting an overview of AR immunotherapy utilizing bibliometric and visualization methods to investigate the longitudinal and cross-sectional characteristics, trends, and multiple ramifications of this topic has not been published. Thus, we endeavored to identify collaborative networks among countries, institutions, and authors in this field. This work also aimed to explore key contributors to the field over the last two decades, and to identify hotspots and research trends in various aspects. The findings of the present study depicted a historical and promising perspective. Our results also provide new insights to global research teams, assist them in drafting and administering their scientific studies, and will help rhinologists to gain a broad grasp of macro and micro perspectives across the whole field of AR knowledge.
Materials and methods
Sources of data and strategies for searching
The Science Citation Index-Expanded and Social Sciences Citation Index from the Web of Science Core Collection have been considered as the most appropriate database for bibliometric analysis. Thus, this study was conducted using this database. The search terms were Topic = ((allergic rhinitis) AND (immunotherapy OR immunological therapy)). We searched the database broadly for relevant publications between 2002 and 2021, including only original research articles and review articles. The language was limited to English. Other document types and non-English publications were excluded. To avoid the bias induced by regular updating of the databases, all publication searches and data downloads were performed on 1 January 2022. The detailed search procedure for this study is shown in Figure 1. Two researchers examined these data individually. Controversial points were resolved through discussion or by seeking the assistance of other experts.
Figure 1.
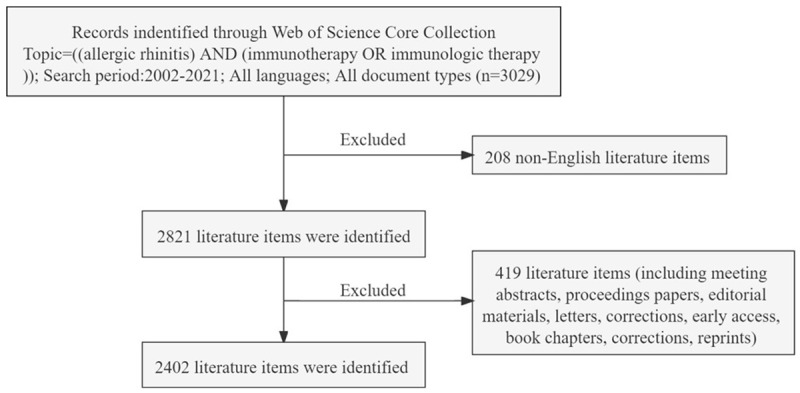
Flow frame diagram of the included publications. The diagram displays the detailed selection criteria for AR immunotherapy-related publications in the database and the steps of the bibliometric analysis.
Bibliometric analysis
Data were converted to text documents before being uploaded into the bibliometric analysis software. CiteSpace 5.8. R3, 64-bit (Drexel University, Philadelphia, PA, USA), VOSviewer 1.6.16 (Leiden University, Leiden, the Netherlands), and a bibliometric online analysis platform (http://bibliometric.com/) were applied to locate co-cited references, keywords, countries, institutions, authors, journals, and network characteristics of “keyword bursts”, as well as to demonstrate the results visually. We queried the Journal Citation Report 2020, including its H-index, impact factor category quartile, and category rank. The H-index is considered a vital indicator to determine the scientific impact of a journal, author, institution, or country [24].
VOSviewer can be utilized to construct “scientific knowledge networks” that portray the evolution of research domains, institutional collaborations, and predict future research hotspots. In this work, we used VOSviewer to estimate visually the co-occurrence of terms and to build density diagrams. The co-occurrence analysis function in VOSviewer can be used to categorize keywords into different clusters, which are denoted by different colors. Clustering analysis of study hotspots can be visualized, and the keyword co-occurrence network can predict growth trends.
CiteSpace was utilized to perform a series of analyses of publications to identify research hotspots for AR immunotherapy. The publishing institution, co-cited references, and relevant keywords were included in this analysis. In the constructed network visualization diagram, the nodes reflected the observed items, with larger nodes representing more frequently occurring items. In addition, we used CiteSpace to analyze centrality, which is an index that defines the importance of a network node, where more prominent nodes represent higher centrality [25]. Centrality is employed to measure the significance of a node’s position in the network. The higher the centrality, the higher the number of connections through that node in the network.
Results
General overview of publications
From 2002 to 2021, a total of 2402 original articles related to AR immunotherapy were published. In past two decades, research output linked to AR immunotherapy has exhibited an overall increasing trend (Figure 2A). The number of publications on AR immunotherapy has steadily increased over the past two decades, with > 4-times as many published in 2021 as were published in 2002. From 2016 to 2021, AR immunotherapy research activity peaked, with 846 papers being published in 5 years, accounting collectively for 35.2% of the overall number of papers. In terms of the number of publications annually, the largest number of articles waspublished in 2020 (192, 8.0%).
Figure 2.
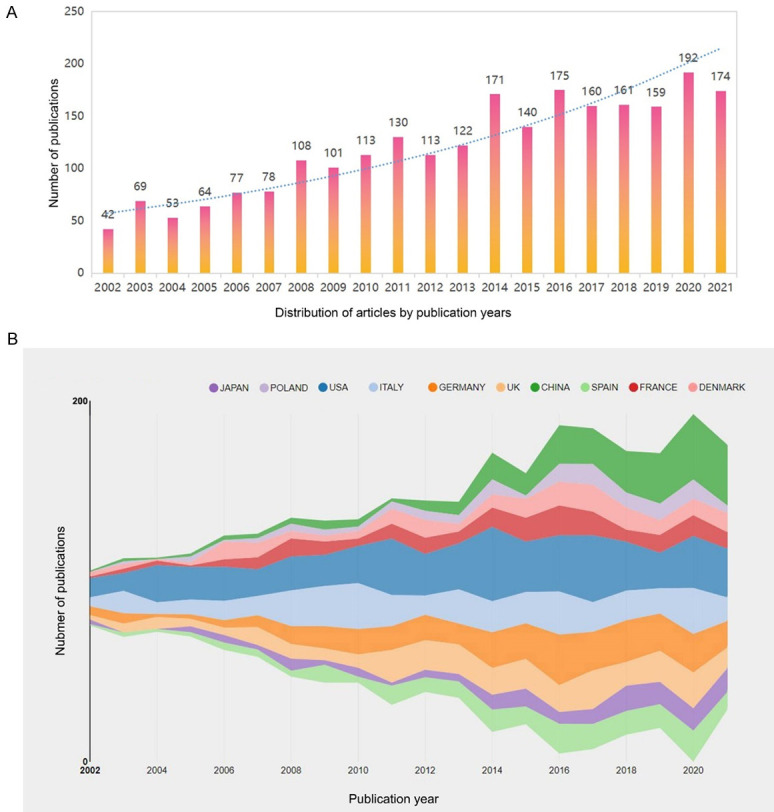
The annual publication trend (A) and the top 10 countries/regions (B) performing research into AR-immunotherapy (2002-2021).
Distribution of countries/regions and institutions
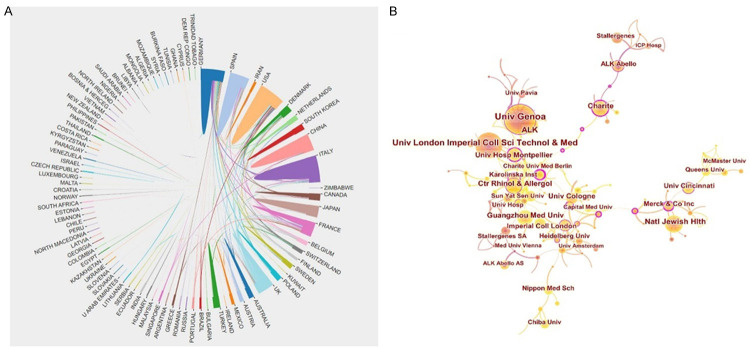
The top 10 contributing countries are shown in Table 1 (Figure 2B). The country-collaboration network of research into AR immunotherapy is shown in Figure 3A. Most of the publications were produced from the USA (581), followed by Italy (382), Germany (330), the UK (281), and China (256). Among the top 10 countries, the USA was the most prolific producer of AR research, publishing 24.2% of articles. Notably, the number of publications from China has increased markedly over the past 20 years, rising to fifth place overall. Higher centrality in a collaborative network correlates with more frequent cooperation. The low density of the country-based research-network map indicated largely independent research teams, which underlined the need for further collaboration. The result of centrality analysis showed that the USA (0.56) was at the network core, followed by Italy (0.16), and Germany (0.14).
Table 1.
The top 10 countries contributing to publications related to AR immunotherapy between 2002 and 2021
| Rank | Article counts | Centrality score | Country |
|---|---|---|---|
| 1 | 581 | 0.56 | USA |
| 2 | 382 | 0.16 | Italy |
| 3 | 330 | 0.14 | Germany |
| 4 | 281 | 0.03 | UK |
| 5 | 256 | 0.00 | China |
| 6 | 206 | 0.00 | Spain |
| 7 | 188 | 0.01 | France |
| 8 | 179 | 0.00 | Denmark |
| 9 | 147 | 0.01 | Japan |
| 10 | 118 | 0.02 | Poland |
Figure 3.
The distribution of countries/regions and institutions engaged in research on AR immunotherapy (2002-2021). A. Maps visualizing the contributions of countries/regions to publications regarding research into AR-immunotherapy. B. Maps visualizing the contributions of institutions to publications regarding research into AR-immunotherapy. The size of node represents the number of articles published by the institution. The link represents their collaboration.
The institution-collaboration network (Figure 3B) revealed the top-10 institutions, including the USA institutions, National Jewish Health (46) and University of Cincinnati (36) (Table 2). The universities with the highest centrality score were Charité Universitatsmedizin Berlin (0.22), University of Montpellier (0.21), and University of Genoa (0.16).
Table 2.
The top 10 institutions that have collaborated the most on AR immunotherapy between 2002 and 2021
| Rank | Article counts | Institution | Country | Centrality score |
|---|---|---|---|---|
| 1 | 117 | University of Genoa | Italy | 0.16 |
| 2 | 81 | Imperial College London | UK | 0.03 |
| 3 | 50 | Charité Universitatsmedizin Berlin | Germany | 0.22 |
| 4 | 48 | Allergologisk Laboratorium Kbenhavn | Denmark | 0.01 |
| 5 | 46 | National Jewish Health | USA | 0.03 |
| 6 | 40 | Center for Rhinology and Allergology | Germany | 0.13 |
| 7 | 40 | Guangzhou Medical University | China | 0.03 |
| 8 | 37 | University of Montpellier | France | 0.21 |
| 9 | 37 | University of Pavia | Italy | 0.06 |
| 10 | 36 | University of Cincinnati | USA | 0.11 |
Author network analysis
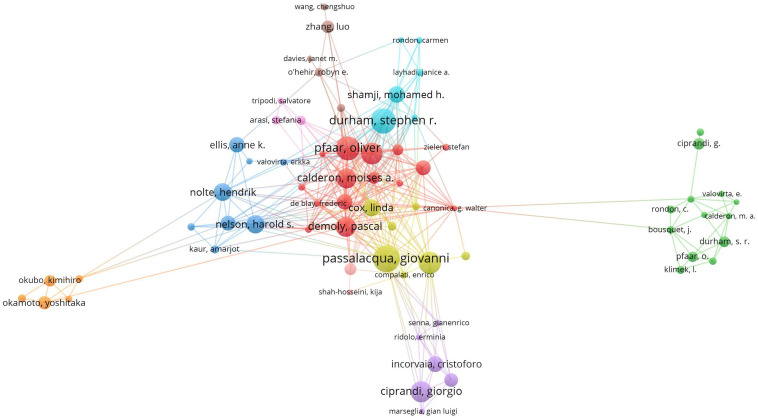
A visualization map of co-authorship may be used to establish analysis organizations with the greatest impact and potential collaborators, and could facilitate researchers form cooperative ties. Authors with ≥10 publications and ≥500 citations were visualized using VOSviewer (Figure 4). The presence of overlapping names in the diagram means that some names might not be visible. The circles represent “active” authors with strong research collaborations. We identified 8,758 authors who contributed articles on the topic of AR immunotherapy from 2002 to 2021. Table 3 lists the top-10 most prolific authors during the research period. G. Passalacqua of University of Genoa (46 publications; 2310 citations) published the most articles, followed by S. R. Durham of Imperial College London (43 publications; 3397 citations). O. Pfaar of University of Marburg ranked first with respect to the centrality score (0.09).
Figure 4.
Map visualizing the most productive authors performing research into ARimmunotherapy (2002-2021). Each colored circle represents an author. The size of each colored circle is proportional to the total number of articles published by the author.
Table 3.
The top 10 most prolific authors in the field of AR-immunotherapy between 2002 and 2021
| Rank | Article counts | Author | Country | Centrality score | Citations |
|---|---|---|---|---|---|
| 1 | 46 | Passalacqua G | Italy | 0.03 | 2310 |
| 2 | 43 | Durham SR | UK | 0.07 | 3397 |
| 3 | 41 | Pfaar O | Germany | 0.09 | 837 |
| 4 | 38 | Canonica GW | Italy | 0.01 | 1527 |
| 5 | 37 | Klimek L | Germany | 0.04 | 948 |
| 6 | 36 | Ciprandi G | Germany | 0.03 | 593 |
| 7 | 34 | Calderon MA | UK | 0.04 | 1946 |
| 8 | 34 | Demoly P | France | 0.04 | 1247 |
| 9 | 32 | Nelson HS | USA | 0.03 | 1301 |
| 10 | 31 | Nolte H | USA | 0.01 | 1626 |
Distribution of journals
Table 4 shows the characteristics of the top 10 most prolific journals. More than half of the publishers of these periodicals were located in the USA. The largest number of articles related to AR immunotherapy was published by Allergy (178), Journal of Allergy And Clinical Immunology (153), Clinical And Experimental Allergy (113), International Archives of Allergy And Immunology (102), and Annals of Allergy Asthma & Immunology (90). Multiple high-impact-factor articles on AR immunotherapy were published in Journal of Allergy and Clinical Immunology (impact factor =10.793) and allergy (impact factor =13.146). The highest average number of citations (118.96) and H-index (76) were achieved by Journal of Allergy and Clinical Immunology. The Journal Citation Report quartile Q1 included Journal of Allergy and Clinical Immunology, allergy, Pediatric Allergy And Immunology, and International Forum of Allergy & Rhinology. Q2 contained Clinical And Experimental Allergy, Annals of Allergy Asthma & Immunology, and Current Allergy And Asthma Reports. International Archives of Allergy And Immunology, Allergy And Asthma Proceedings, and Current Opinion In Allergy And Clinical Immunology were listed as Q3.
Table 4.
The top 10 journals according to the number of articles published on AR-immunotherapy between 2002 and 2021
| Rank | Journal | Article counts | Country | Journal citation reports (2020) | Impact factors (2020) | Total number of citations | Mean number of citations | H-index |
|---|---|---|---|---|---|---|---|---|
| 1 | Allergy | 178 | UK | Q1 | 13.146 | 12377 | 69.53 | 52 |
| 2 | Journal of Allergy And Clinical Immunology | 153 | USA | Q1 | 10.793 | 18201 | 118.96 | 76 |
| 3 | Clinical And Experimental Allergy | 113 | UK | Q2 | 5.018 | 4469 | 41.32 | 40 |
| 4 | International Archives of Allergy And Immunology | 102 | Switzerland | Q3 | 2.749 | 1888 | 18.51 | 25 |
| 5 | Annals of Allergy Asthma & Immunology | 90 | USA | Q2 | 6.347 | 2619 | 29.10 | 31 |
| 6 | Allergy And Asthma Proceedings | 85 | USA | Q3 | 2.587 | 1463 | 17.21 | 20 |
| 7 | Current Opinion In Allergy And Clinical Immunology | 61 | USA | Q3 | 3.142 | 1155 | 18.93 | 21 |
| 8 | Pediatric Allergy And Immunology | 57 | Denmark | Q1 | 6.377 | 1596 | 28.00 | 22 |
| 9 | Current Allergy And Asthma Reports | 51 | USA | Q2 | 4.806 | 716 | 14.04 | 16 |
| 10 | International Forum of Allergy & Rhinology | 49 | USA | Q1 | 3.858 | 621 | 12.67 | 12 |
Analysis of keywords
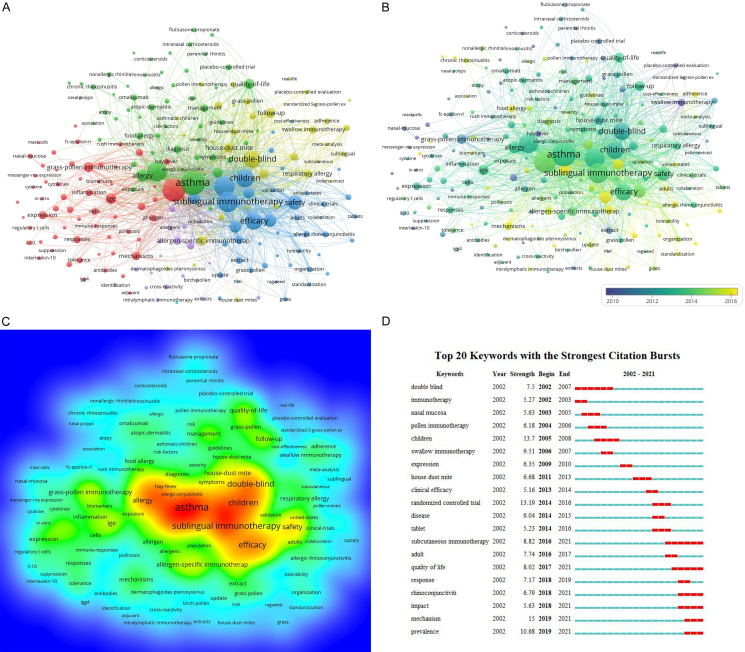
VOSviewer software was used to search the titles and abstracts of the 2402 retrieved publications for keywords. This produced a map of 212 terms (5630 in total) with ≥20 occurrences each, grouped into five clusters (Figure 5A). Among the high-frequency keywords in the map were “asthma” (1122), “AR” (1005), “sublingual immunotherapy” (790), “sinusitis” (778), “immunotherapy” (736), “children” (611), “efficacy” (565), “double-blind” (561), “safety” (384), and “rhino-conjunctivitis” (289). Study subjects with similar terms were combined in the same catalog, with five main clusters: clinical features, pathogenesis, molecular mechanisms, treatment, and pathophysiology of AR. The distribution of keywords in order of occurrence was visualized using VOSviewer software (Figure 5B). The number of appearances of a keyword is defined by the color of the region. Before 2010, the majority of studies concentrated on the topics “clinical trial” and “safety of immunotherapy”, while the latest identified research trends suggested that “mechanism of immunotherapy” and “standardization of immunotherapy” will likely be the focus of future research emphasis. VOSviewer was also utilized to measure the frequency of keywords to calculate their density, which was presented in a density map (Figure 5C). The “warmer” the hue (toward yellow), the higher the density. In a particular field, research hotspots tend to form at higher grayscale values.
Figure 5.
Keywords analysis in publications regarding research into to AR-immunotherapy worldwide (2002-2021). A. Keyword mapping in the field of research. B. Keyword distribution displayed chronologically in order of appearance. C. Keyword distribution ordered by their average frequency of appearance. D. AR-immunotherapy research-related keywords with the strongest citation bursts.
Detection of keyword bursts
Keyword bursts between 2002 and 2021 were detected on the basis of examination of 2402 articles included in the Web of Science Core Collection database (Figure 5D). In the figure, the chronology is shown by a blue line that crosses one year. The burst period is displayed as a red reflection line, which marks the start and end of the year, as well as the timespan of the citation burst. We eliminated terms that had little to no research value so that we could focus on representing the research trends in AR immunotherapy. From 2002 to 2011, “children” had the highest burst strength (13.70). Between 2012 and 2021, “randomized controlled trial” had the highest burst strength (13.19), followed by “prevalence” (10.68) and “subcutaneous immunotherapy” (8.82).
Analyses of co-cited references
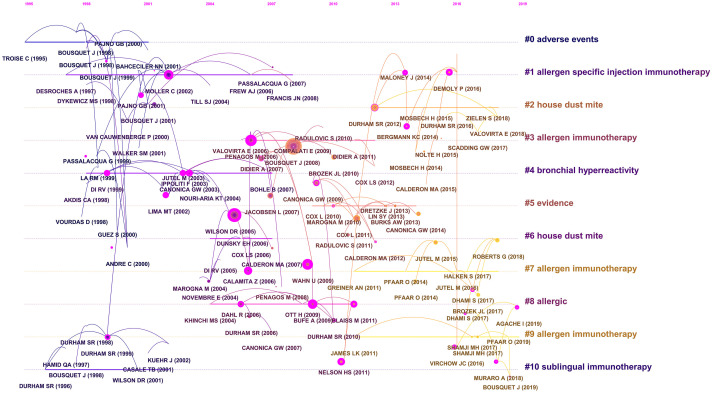
From 2402 articles, 29,432 cited references were presented to analyze the relevance of co-citations, and a cluster network map was established according to the results. Figure 6A presents the visualized network of the co-cited articles, with 119 nodes and 118 links. Each node represents a cited reference. The links between nodes show the frequency of the same article being cited. The node diameter is proportional to the total number of co-citations of the article. These nodes (surrounded by a thick purple ring) can be employed to connect the growth stages of a field. An “explosion” of citations is indicated by a red ring. Then, by creating a hierarchical order of the co-cited articles created in the co-citation network, research hotspots can be identified. “Adverse events”, “allergen specific injection immunotherapy”, “house dust mite”, “bronchial hyperreactivity”, and “sublingual immunotherapy” were among the critical clusters of co-cited references (Figure 6B).
Figure 6.
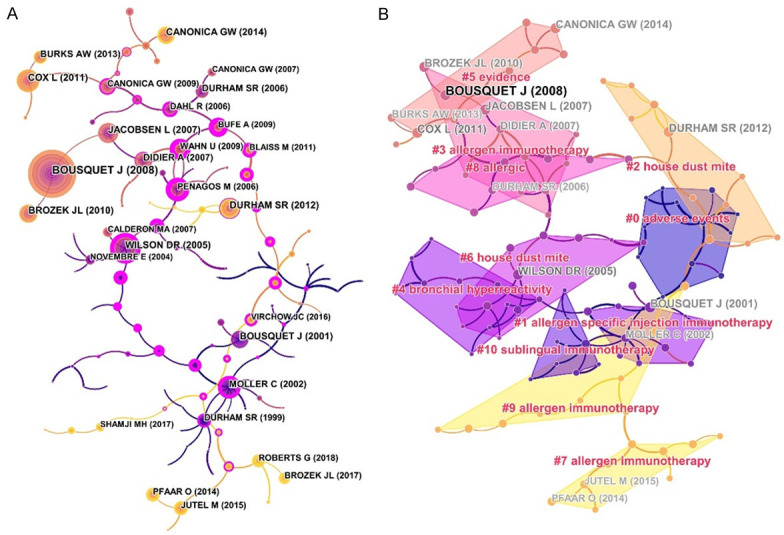
Map of co-cited references (A) and a map of the clustered network of co-cited references (B) related to research on AR-immunotherapy (2002-2021). In the map, the nodes represent the analyzed co-cited references. The color and thickness in the inner circle of the node indicated cited frequency of different time periods.
Figure 7 shows the timeline of the clustering plot, which aids the identification of emerging research hotspots in AR immunotherapy. The top-10 co-cited articles are listed in Table 5. The study by Bousquet et al. [26] had the most citations in Allergy (2241 citations), followed by Brozek et al. [27] in Journal of Allergy And Clinical Immunology (1028 citations), and Moller et al. [28] in Journal of Allergy And Clinical Immunology (739 citations). Figure 8 indicates the top-20 references with the strongest citation bursts. Most references with citation bursts were derived from publications on allergology or immunology, suggesting that allergology and immunology are core issues in AR immunotherapy.
Figure 7.
Cluster-labeled co-cited references displayed on a timeline from 2002 to 2021. This cluster analysis revealed that the high-frequency co-cited references related to AR-immunotherapy research were mainly clustered into eleven categories. This diagram clearly shows the differences in the appearance time point and time span of those clusters.
Table 5.
The top 10 most co-cited references related to AR immunotherapy between 2002 and 2021
| Rank | Title | Author | Year | Journal | Citation frequency |
|---|---|---|---|---|---|
| 1 | Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) | Bousquet J | 2008 | Allergy | 2241 |
| 2 | Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 Revision | Brozek JL | 2010 | Journal of Allergy And Clinical Immunology | 1028 |
| 3 | Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-Study) | Moller C | 2002 | Journal of Allergy And Clinical Immunology | 739 |
| 4 | IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy | Jutel M | 2003 | European Journal of Immunology | 690 |
| 5 | Allergen immunotherapy: A practice parameter third update | Cox L | 2011 | Journal of Allergy And Clinical Immunology | 686 |
| 6 | Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study | Jacobsen L | 2007 | Allergy | 652 |
| 7 | Clinical efficacy and immune regulation with peanut oral immunotherapy | Jones SM | 2009 | Journal of Allergy And Clinical Immunology | 467 |
| 8 | Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis | Creticos PS | 2006 | New England Journal of Medicine | 441 |
| 9 | Sublingual immunotherapy with once-daily grass allergen tablets: A randomized controlled trial in seasonal allergic rhinoconjunctivitis | Durham SR | 2006 | Journal of Allergy And Clinical Immunology | 429 |
| 10 | Allergic rhinitis | Greiner AN | 2011 | Lancet | 420 |
Figure 8.
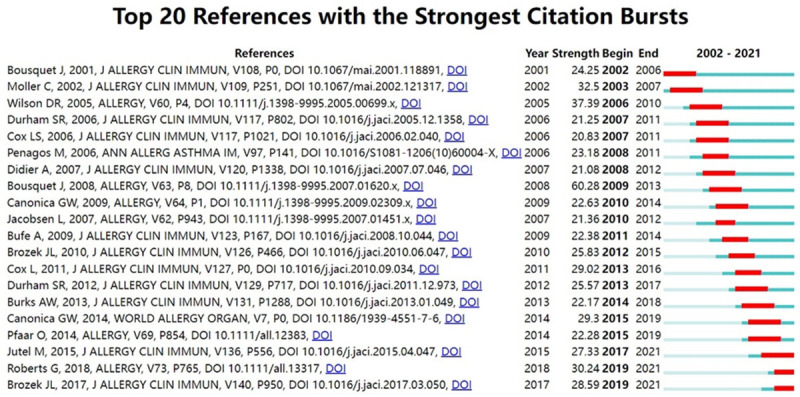
Top 20 co-cited references with the strongest bursts of citation (2002-2021). The red thick line represents frequently cited references during this time period. The green bar means that the reference was not frequently cited in this time period.
The distribution of links among journals is shown in a dual-map overlay of journals (Figure 9), with citing journals on the left and cited journals on the right. The relationships described are indicated by the colored routes connecting them. These labels stand for the topics encompassed by the journal. In Figure 9, the primary citation paths appear as two green paths and one orange path. The green path implies studies published in Medicine/Medical/Clinical journals are generally cited by Molecular/Biology/Genetics and Health/Nursing/Medicine journals. The orange route indicates that research published by Molecular/Biology/immunology journals is cited by research in Molecular/Biology/Genetics journals.
Figure 9.
The dual-map overlay of journals publishing papers related to AR immunotherapy from 2002 to 2021. Cited journals are on the right, citing journals are on the left, and the line paths represent citation relationships.
Discussion
In recent years, the world’s spectrum of diseases has undergone a dramatic shift as a result of factors such as changes in lifestyle, environmental pollution, and industrialization. Allergic diseases have become one of the most common diseases in the 21st century, among which AR affects the largest population. Although treatment of AR has made great progress in recent decades, it still presents a high recurrence rate, mainly because current traditional drug treatment cannot cure the root cause and it is difficult to effectively control AR symptoms. Worryingly, the recurrent symptoms seriously affect the physical and mental health of patients. AIT is recognized as the only etiological treatment option for AR. There are many advantages for AIT, such as effective control of allergy symptoms, modification of the natural course of AR, and reduction of new allergies. Currently, AIT is gaining increasing attention worldwide, and related research is increasing year by year. Therefore, a general overview of current trends in global AIT research is particularly important. This study analyzed the bibliometric output of publications in the global AR immunotherapy field and revealed the major research hotpots and trends between 2002 and 2021. According to the growth curve, we speculated that increasing numbers of scholars are interested in AR immunotherapy. We predict that this topic will remain a hot research topic in the next decade, and the number of related publications is forecast to continue to grow.
The USA leads the way by contributing > 20% of papers on AR immunotherapy. Most of the top 10 institutions are in the USA and Europe, which has stimulated advances in research related to AR immunotherapy. On the one hand, this trend reflects the mature environment of medical research and health in these countries/regions; however, on the other hand, it reflects an urgent demand for efficacious AR immunotherapy. In addition, population sizes and national economic differences might significantly affect the number of publications. Moreover, the USA had the highest centrality score and most active cooperation with other countries. Many publications were provided by the UK, Denmark, and Japan; however, they might have fewer collaborations with other countries, as evidenced by their lower centrality score. We suggest that countries with a lower centrality score should strengthen international exchange and cooperation with other countries to establish a good partnership, particularly with the pioneering countries in AR research, which will accelerate their progress in this field. Charité Universitatsmedizin Berlin, University of Montpellier, and University of Genoa have the most collaborations with other institutions, which is a worthwhile lesson for those institutions that rarely interact with each other. G. Passalacqua had the most publications in the field of AR immunotherapy. S. R. Durham, O. Pfaar, G. W. Canonica, and L. Klimek were the most productive authors in the past two decades. A distinct geographical pattern of global investigators in AR immunotherapy became evident. Most scholars were working in Europe and the USA, and these authors were working mainly in the allergy or otolaryngology departments of their university-affiliated hospital.
The journals with the maximum number of publications on AR immunotherapy were Allergy, Journal of Allergy And Clinical Immunology, Clinical And Experimental Allergy, International Archives of Allergy And Immunology, and Annals of Allergy Asthma & Immunology. These publications are world leaders in the field of otolaryngology and allergy. This trend suggests that AR immunotherapy is one of the core topics of otolaryngology and allergology. The top-10 co-cited references for the period 2002-2021 demonstrated that scholars were paying more attention to the clinical administration of AR. Notably, the “Allergic rhinitis and its impact on asthma (ARIA) 2008 update” published by Bousquet et al. [26] was recognized as a treatment guideline by rhinologists and allergists worldwide. The 2008 version adds articles published after 2000 to the 2001 version, and analyzes and classifies them using the new method of classification of clinical evidence recommended by the World Health Organization and Shekelle et al. [29] published in the British Medical Journal. A dual-map overlay provides a macroscopic view of the evolution of research content at the discipline level. In Figure 9, the dual-map overlay of journals indicates the disciplinary distribution of academic journals. Immunology, molecular biology, and genetics are the fundamental and core subjects of AR immunotherapy. As observed from the three main pathways in the visualization map, research on AR immunotherapy has begun to translate from a single discipline to a multidisciplinary one.
The top-10 high-frequency keywords in co-occurrence cluster analyses demonstrated that the potential pathophysiological mechanism, optimal treatments, and outcome evaluation of multi-treatment regimens continue to be hot topics. Burst keywords indicate emerging trends and research frontiers. Five frontiers of AR immunotherapy were identified: “SCIT” (2016-2021), “quality of life” (2017-2021), “prevalence” (2019-2021), “rhinoconjunctivitis” (2018-2021), and “mechanism” (2019-2021). Recently, scholars around the world have sought AR treatments with better clinical efficacy. Numerous studies have shown that AIT has tremendous benefits for early intervention in respiratory allergic diseases. A recent systematic review showed that AIT can significantly improve nasal and ocular symptoms of AR and reduce drug use [30]. In addition, AIT can improve the quality of life of patients with AR, prevent further progression of AR to asthma, reduce the occurrence of new allergies, and provide long-term treatment effects [31]. All of these advantages are unmatched by drug therapy. Since 1911, when Noon first inoculated grass pollen extracts into patients with hay fever, SCIT has evolved into a well-established and effective treatment for AR and has been the dominant mode of administration of AIT for a long time [32]. In the last 20 years, however, SLIT has been increasingly advocated and widely used in clinical practice. In Europe, 45% of patients receiving AIT are treated with SLIT [33]. In addition, SLIT is now the predominant treatment modality for AIT in some European countries. Part of the reason for this is that the safety of SCIT has been questioned. Some cases of fatal adverse reactions to SCIT were reported in the 1980s [34]. In a 4-year safety study of SCIT, the probability of systemic adverse reactions was 0.1% for a total of 23.3 million injections, of which 97% were mild to moderate systemic adverse reactions and the incidence of fatal serious systemic adverse reactions was 1 in 1 million [35]. SCIT requires that patients must attend the hospital regularly to receive injections, which is impractical in many cases, particularly during epidemics.
SLIT is the delivery of allergen vaccines in tablet or droplet form by the sublingual route. There is clear evidence for the efficacy of SLIT in suppressing the progression of AR and in maintaining efficacy after discontinuation of the drug. Three randomized, double-blind, placebo-controlled trials all had 3 years of continuous SLIT treatment and followed patients for 1 to 2 years after the end of treatment. The results showed that patients in the SLIT group had lower symptom scores than those in the placebo-treated group [36-38]. It is generally accepted that SLIT lasting for 3 years or more can achieve a more satisfactory long-term outcome [39]. A prospective study by Marogna et al. showed that after 3 to 4 years of maintenance of house dust mite SLIT, patients with AR achieved 7 to 8 years of symptomatic remission [40]. The lack of direct controlled studies of SCIT and SLIT, means that currently, there is no consensus on the merits of the efficacy of the two modalities. A meta-analysis of dust mite AIT by Huang et al. showed no significant difference in the efficacy of SCIT and SLIT in patients with AR [40]. A network meta-analysis showed good efficacy of SCIT and SLIT for grass pollen allergies, with no significant difference between them [41]. In contrast to SCIT, where fatalities have been reported, there have been no reports in the literature of SLIT resulting in fatal adverse effects. SLIT has a lower incidence of systemic adverse reactions and the main adverse effects of SCIT are local oral mucosal pruritus, discomfort, and gastrointestinal reactions [42]. The vast majority of cases resolve spontaneously without medical intervention. A review summarizing 104 SLIT studies showed that SLIT-related systemic adverse reactions accounted for 0.056% of the total number of doses administered, while serious adverse reactions were even rarer, at about 0.014% [43]. However, it is important to educate the patient on how to manage any adverse reactions that occur. In summary, SLIT facilitates patient self-management without medical monitoring and has a low incidence of serious systemic adverse effects. SLIT can be used as a safe and effective alternative to SCIT. In the future, we recommend that SLIT should be one of the important directions of AIT research.
The timeline of the co-cited references cluster diagram indicates a growing interest in the mechanism of action of AIT. Although AIT has been used for many years, its molecular mechanism is still not fully understood. Recent studies have shown that AIT modulates the immune system through several pathways to induce immune tolerance to allergens [44]. A T helper cell (Th)1/Th2 imbalance is one of the most critical immunological features of AR, which is characterized by an increased allergen Th2-type immune response and a relatively weakened Th1-type response [45]. Several clinical studies have found that the Th1/Th2 balance is restored in patients with AR after AIT, as evidenced by a decrease in the production of Th2-type cytokines (e.g., interleukin (IL)-4, IL-5, and IL-13) in peripheral blood, and an increase in the proportion of Th1 cells and interferon gamma (IFN-γ) levels [46,47]. T regulatory cells (Tregs) play a key regulatory role in maintaining the body’s immune tolerance by secreting various suppressive cytokines such as IL-10, IL-35, and transforming growth factor beta (TGF-β) [48]. Several studies have further explored the molecular mechanisms by which AIT affects Tregs. Current studies show that AIT can induce an increase in Tregs in peripheral blood [47,49]. A clinical trial conducted by Gómez et al. [50] showed that a 1-year period of AIT increased allergen-specific Th1/Treg frequencies. Several studies have further explored the molecular mechanisms by which AIT affects Tregs. A recent study showed that AIT can regulate the TNF/TNFR2 signaling cascade to enhance function of Tregs [51]. Datta et al. [52] reported that AIT enhances the anti-apoptotic ability of Tregs, which results in an increase in the number of peripheral Tregs.
B cells promote allergic reactions through the production of IgE and the secretion of various cytokines. AIT reduces the pathological IgE+ B cell subset and promotes the production of blocking antibodies by antigen-specific B cells [53]. IgG4 is the primary blocking antibody induced by AIT, which competes with IgE to bind allergenic epitopes on effector cells, such as mast cells and basophils, to inhibit allergic reactions [48]. A randomized, double-blind, placebo-controlled study by James et al. demonstrated that grass pollen AIT induces allergen-specific IgG production that was sustained after treatment discontinuation [54]. Another study showed that one year of house dust mite-SCIT was able to downregulate CD23 (a low-affinity IgE receptor) on memory B cells, thereby impairing IgE synthesis and antigen presentation [55]. A growing body of evidence underscores the importance of B regulatory cells (Bregs) for the induction of allergen tolerance by AIT. Bregs are considered one of the major producers of peripheral IgG4 during AIT [14]. Many studies on inhaled allergens have found that AIT induces increased IgG4 and peripheral Breg frequencies. Boonpiyathad et al. reported a significant increase in peripheral blood IL-10+ B cell frequency in patients after 2 years of house dust mite Der p1-AIT and that this increased frequency correlated with improvement in the patients’ clinical symptoms [56]. The ability of AIT to induce IgG4 production and promote IL-10 secretion by Bregs was also observed in a study of grass pollen AIT [53]. In addition, the frequency of circulating IL-10+ Breg subsets is a good predictive biomarker for the success of AIT treatment [57]. In summary, AIT can treat AR by altering allergen-specific effector T cell and B cell response patterns, enhancing the function of regulatory T and B cells, and modifying antibody responses. However, the mechanism of action of AIT still requires further investigation.
Limitations
Some limitations of the present study should be addressed. The Web of Science Core Collection database is regarded as the most vital source of data for bibliometric analysis, thus it was searched as the sole database in this research. Some research might have been missed. Besides, only articles and reviews, and English publications were used for analysis, which may have led to some bias. In addition, the affiliations of the authors could not be fully determined; thus, some of them could be honorary or part-time. It is possible that some authors might have duplicate names or have the same author from different institutions. Nevertheless, this study has established a foundation for academics to quickly identify the research foci and emerging trends in AR immunotherapy.
Conclusion
This bibliometric research was the first to analyze publications on AR immunotherapy worldwide. Over the past two decades, there has been an overall upward trend in the number of publications in the field of AR immunotherapy. The USA and European countries publish the vast majority of the world’s papers in the field of AR immunotherapy. It is necessary to strengthen cooperation and communication between countries, institutions, and teams. “Subcutaneous immunotherapy”, “quality of life”, and “rhino-conjunctivitis” are academic foci in this area. In recent years, scholars have focused on the mechanisms and safety of immunotherapy. In future studies, the mechanism of AR immunotherapy still deserves in-depth investigation.
Acknowledgements
This work was supported by the Science and Technology Foundation of Guizhou Province (D2011160), National Natural Science Foundation Cultivation Project of Affiliated Hospital of Guizhou Medical University (gyfynsfc [2020]-7), and Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwkj2022-155).
Disclosure of conflict of interest
None.
References
- 1.Meng YF, Wang CS, Zhang L. Advances and novel developments in allergic rhinitis. Allergy. 2020;75:3069–3076. doi: 10.1111/all.14586. [DOI] [PubMed] [Google Scholar]
- 2.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378:2112–2122. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 3.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leth-Møller KB, Skaaby T, Linneberg A. Allergic rhinitis and allergic sensitisation are still increasing among Danish adults. Allergy. 2020;75:660–668. doi: 10.1111/all.14046. [DOI] [PubMed] [Google Scholar]
- 5.Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, Ge SQ, Zhang N, Zhang L, Bachert C. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36:235–248. doi: 10.1016/j.iac.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA, Boyle JM. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(Suppl):S43–70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Han XR, Krempski JW, Nadeau K. Advances and novel developments in mechanisms of allergic inflammation. Allergy. 2020;75:3100–3111. doi: 10.1111/all.14632. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues J, Franco-Pego F, Sousa-Pinto B, Bousquet J, Raemdonck K, Vaz R. Anxiety and depression risk in patients with allergic rhinitis: a systematic review and meta-analysis. Rhinology. 2021;59:360–373. doi: 10.4193/Rhin21.087. [DOI] [PubMed] [Google Scholar]
- 10.Papapostolou G, Kiotseridis H, Romberg K, Dahl Å, Bjermer L, Lindgren M, Aronsson D, Tunsäter A, Tufvesson E. Cognitive dysfunction and quality of life during pollen season in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2021;32:67–76. doi: 10.1111/pai.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox L. Pharmacoeconomics of allergy immunotherapy versus pharmacotherapy. Expert Rev Clin Immunol. 2021;17:255–268. doi: 10.1080/1744666X.2021.1886079. [DOI] [PubMed] [Google Scholar]
- 12.Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA (2) LEN review. Allergy. 2014;69:1275–1279. doi: 10.1111/all.12470. [DOI] [PubMed] [Google Scholar]
- 13.Nelson HS. The evolution of allergy immunotherapy. Ann Allergy Asthma Immunol. 2021;126:357–366. doi: 10.1016/j.anai.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep. 2020;21:2. doi: 10.1007/s11882-020-00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scadding G. Real world evidence of long-term benefits from allergen-specific immunotherapy (AIT) Lancet Reg Health Eur. 2021;13:100283. doi: 10.1016/j.lanepe.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339–349. doi: 10.1016/j.jaci.2015.12.1298. [DOI] [PubMed] [Google Scholar]
- 17.Hossenbaccus L, Linton S, Garvey S, Ellis AK. Towards definitive management of allergic rhinitis: best use of new and established therapies. Allergy Asthma Clin Immunol. 2020;16:39. doi: 10.1186/s13223-020-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D, Guan BY, Song LX, Liu QY, Fan YX, Zhao L, Wang TX, Zhang ZH, Gao ZY, Li SM, Xu H. A bibliometric analysis of exosomes in cardiovascular diseases from 2001 to 2021. Front Cardiovasc Med. 2021;8:734514. doi: 10.3389/fcvm.2021.734514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma CQ, Su H, Li HJ. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study. Front Oncol. 2021;10:627891. doi: 10.3389/fonc.2020.627891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JH, Zhang YX, Hu LY, Huang XX, Liu YF, Li JY, Hu QM, Xu JP, Yu HB. Global trends and performances of magnetic resonance imaging studies on acupuncture: a bibliometric analysis. Front Neurosci. 2021;14:620555. doi: 10.3389/fnins.2020.620555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Zhou RY, Wu Q. Global research hotspots and research trends on idiopathic pulmonary fibrosis: a bibliometric and visualization analysis. Ann Palliat Med. 2021;10:9057–9068. doi: 10.21037/apm-21-1836. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Li Y, Guo LM, Hong J, Zhao WJ, Hu XM, Chang CC, Liu W, Xiong K. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front Pharmacol. 2021;11:626502. doi: 10.3389/fphar.2020.626502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YH, Liu QR, Chen YC, Qian YL, Pan B, Ge L, Wang Q, Ding GW, Wang JC. Global trends and future prospects of child nutrition: a bibliometric analysis of highly cited papers. Front Pediatr. 2021;9:633525. doi: 10.3389/fped.2021.633525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rad AE, Brinjikji W, Cloft HJ, Kallmes DF. The H-index in academic radiology. Acad Radiol. 2010;17:817–821. doi: 10.1016/j.acra.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Dong Q, Liang QC, Chen Y, Li JH, Lu LH, Huang XQ, Zhou Q. Bibliometric and visual analysis of vascular calcification research. Front Pharmacol. 2021;12:690392. doi: 10.3389/fphar.2021.690392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O’Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 27.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T, Schünemann HJ Global Allergy and Asthma European Network; Grading of Recommendations Assessment, Development and Evaluation Working Group. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, Urbanek R, Valovirta E, Wahn U. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 29.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318:593–596. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox L. Approach to patients with allergic rhinitis: testing and treatment. Med Clin North Am. 2020;104:77–94. doi: 10.1016/j.mcna.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Klimek L, Pfaar O, Bousquet J, Senti G, Kündig T. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897–906. doi: 10.1080/1744666X.2017.1333423. [DOI] [PubMed] [Google Scholar]
- 32.Pfaar O, Bousquet J, Durham SR, Kleine-Tebbe J, Larché M, Roberts G, Shamji MH, Gerth van Wijk R. One hundred and ten years of allergen immunotherapy: a journey from empiric observation to evidence. Allergy. 2022;77:454–468. doi: 10.1111/all.15023. [DOI] [PubMed] [Google Scholar]
- 33.James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17:55–59. doi: 10.1097/ACI.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche AM, Wise SK. Subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S51–54. doi: 10.1002/alr.21382. [DOI] [PubMed] [Google Scholar]
- 35.Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014;2:161–167. doi: 10.1016/j.jaip.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Ott H, Sieber J, Brehler R, Fölster-Holst R, Kapp A, Klimek L, Pfaar O, Merk H. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:179–186. doi: 10.1111/j.1398-9995.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 37.Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, Wurtzen PA, Andersen JS, Tholstrup B, Riis B, Dahl R. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 38.Didier A, Malling HJ, Worm M, Horak F, Sussman G, Melac M, Soulié S, Zeldin RK. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy. 2013;43:568–577. doi: 10.1111/cea.12100. [DOI] [PubMed] [Google Scholar]
- 39.Passalacqua G, Canonica GW, Bagnasco D. Benefit of SLIT and SCIT for allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2016;16:88. doi: 10.1007/s11882-016-0666-x. [DOI] [PubMed] [Google Scholar]
- 40.Huang YR, Wang CS, Wang XD, Zhang L, Lou HF. Efficacy and safety of subcutaneous immunotherapy with house dust mite for allergic rhinitis: a meta-analysis of randomized controlled trials. Allergy. 2019;74:189–192. doi: 10.1111/all.13583. [DOI] [PubMed] [Google Scholar]
- 41.Nelson H, Cartier S, Allen-Ramey F, Lawton S, Calderon MA. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256–266. e3. doi: 10.1016/j.jaip.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–1035. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 43.Calderón MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–311. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 44.Lam HY, Tergaonkar V, Ahn KS. Mechanisms of allergen-specific immunotherapy for allergic rhinitis and food allergies. Biosci Rep. 2020;40:BSR20200256. doi: 10.1042/BSR20200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016;36:261–278. doi: 10.1016/j.iac.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Mattson L, Lentini A, Gawel DR, Badam TV, Benson M, Ledin T, Nestor CE, Gustafsson M, Serra-Musach J, Bjorkander J, Xiang Z, Zhang H. Potential involvement of type I interferon signaling in immunotherapy in seasonal allergic rhinitis. J Immunol Res. 2016;2016:5153184. doi: 10.1155/2016/5153184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou HF, Huang YR, Ouyang YH, Zhang Y, Xi L, Chu XH, Wang Y, Wang CS, Zhang L. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: a randomized controlled trial. Allergy. 2020;75:2026–2036. doi: 10.1111/all.14218. [DOI] [PubMed] [Google Scholar]
- 48.Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, Akdis CA. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140:1250–1267. doi: 10.1016/j.jaci.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez M, Doña I, Palomares F, Campo P, Rodriguez MJ, Rondon C, Gomez F, Fernandez TD, Perkins JR, Escribese MM, Torres MJ, Mayorga C. Dermatophagoides pteronyssinus immunotherapy changes the T-regulatory cell activity. Sci Rep. 2017;7:11949. doi: 10.1038/s41598-017-12261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gómez E, Fernández TD, Doña I, Rondon C, Campo P, Gomez F, Salas M, Gonzalez M, Perkins JR, Palomares F, Blanca M, Torres MJ, Mayorga C. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin Exp Allergy. 2015;45:1542–1553. doi: 10.1111/cea.12578. [DOI] [PubMed] [Google Scholar]
- 51.Leonard C, Montamat G, Davril C, Domingues O, Hunewald O, Revets D, Guerin C, Blank S, Heckendorn J, Jardon G, Hentges F, Ollert M. Comprehensive mapping of immune tolerance yields a regulatory TNF receptor 2 signature in a murine model of successful Fel d 1-specific immunotherapy using high-dose CpG adjuvant. Allergy. 2021;76:2153–2165. doi: 10.1111/all.14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datta A, Moitra S, Das PK, Mondal S, Omar Faruk SM, Hazra I, Tripathi SK, Chaudhuri S. Allergen immunotherapy modulates sensitivity of Treg cells to apoptosis in a rat model of allergic asthma. Immunotherapy. 2017;9:1239–1251. doi: 10.2217/imt-2017-0038. [DOI] [PubMed] [Google Scholar]
- 53.Groh N, von Loetzen CS, Subbarayal B, Möbs C, Vogel L, Hoffmann A, Fötisch K, Koutsouridou A, Randow S, Völker E, Seutter von Loetzen A, Rösch P, Vieths S, Pfützner W, Bohle B, Schiller D. IgE and allergen-specific immunotherapy-induced IgG4 recognize similar epitopes of Betv1, the major allergen of birch pollen. Clin Exp Allergy. 2017;47:693–703. doi: 10.1111/cea.12835. [DOI] [PubMed] [Google Scholar]
- 54.Heeringa JJ, McKenzie CI, Varese N, Hew M, Bakx ATCM, Aui PM, Rolland JM, O’Hehir RE, van Zelm MC. Induction of IgG2 and IgG4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75:1121–1132. doi: 10.1111/all.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Y, Wang N, Chen CL, Pan L, Wang ZC, Yunis J, Chen ZA, Zhang Y, Hu ST, Xu XY, Zhu RF, Yu D, Liu Z. CD23 expression on switched memory B cells bridges T-B cell interaction in allergic rhinitis. Allergy. 2020;75:2599–2612. doi: 10.1111/all.14288. [DOI] [PubMed] [Google Scholar]
- 56.Boonpiyathad T, van de Veen W, Wirz O, Sokolowska M, Rückert B, Tan G, Sangasapaviliya A, Pradubpongsa P, Fuengthong R, Thantiworasit P, Sirivichayakul S, Ruxrungtham K, Akdis CA, Akdis M. Role of Der p 1-specific B cells in immune tolerance during 2 years of house dust mite-specific immunotherapy. J Allergy Clin Immunol. 2019;143:1077–1086. doi: 10.1016/j.jaci.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 57.Zissler UM, Jakwerth CA, Guerth FM, Pechtold L, Aguilar-Pimentel JA, Dietz K, Suttner K, Piontek G, Haller B, Hajdu Z, Schiemann M, Schmidt-Weber CB, Chaker AM. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three years grass-pollen AIT. EBioMedicine. 2018;36:475–488. doi: 10.1016/j.ebiom.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]