This randomized clinical trial assesses whether practicing early time-restricted eating is more effective for weight loss, fat loss, and cardiometabolic health in adults with obesity than eating over a period of 12 or more hours.
Key Points
Question
Is early time-restricted eating more effective than eating over a period of 12 or more hours for losing weight and body fat?
Findings
In a randomized clinical weight-loss trial involving 90 adults with obesity, early time-restricted eating was more effective for losing weight (−6.3 kg) than eating over a window of 12 or more hours (−4.0 kg) but not for losing body fat (−4.7 vs −3.4 kg). In a secondary analysis of completers, early time-restricted eating was more effective for losing weight and body fat.
Meaning
Early time-restricted eating was more effective for weight loss than eating over a window of 12 or more hours; larger studies are needed on fat loss.
Abstract
Importance
It is unclear how effective intermittent fasting is for losing weight and body fat, and the effects may depend on the timing of the eating window. This randomized trial compared time-restricted eating (TRE) with eating over a period of 12 or more hours while matching weight-loss counseling across groups.
Objective
To determine whether practicing TRE by eating early in the day (eTRE) is more effective for weight loss, fat loss, and cardiometabolic health than eating over a period of 12 or more hours.
Design, Setting, and Participants
The study was a 14-week, parallel-arm, randomized clinical trial conducted between August 2018 and April 2020. Participants were adults aged 25 to 75 years with obesity and who received weight-loss treatment through the Weight Loss Medicine Clinic at the University of Alabama at Birmingham Hospital.
Interventions
All participants received weight-loss treatment (energy restriction [ER]) and were randomized to eTRE plus ER (8-hour eating window from 7:00 to 15:00) or control eating (CON) plus ER (≥12-hour window).
Main Outcomes and Measures
The co–primary outcomes were weight loss and fat loss. Secondary outcomes included blood pressure, heart rate, glucose levels, insulin levels, and plasma lipid levels.
Results
Ninety participants were enrolled (mean [SD] body mass index, 39.6 [6.7]; age, 43 [11] years; 72 [80%] female). The eTRE+ER group adhered 6.0 (0.8) days per week. The eTRE+ER intervention was more effective for losing weight (−2.3 kg; 95% CI, −3.7 to −0.9 kg; P = .002) but did not affect body fat (−1.4 kg; 95% CI, −2.9 to 0.2 kg; P = .09) or the ratio of fat loss to weight loss (−4.2%; 95% CI, −14.9 to 6.5%; P = .43). The effects of eTRE+ER were equivalent to reducing calorie intake by an additional 214 kcal/d. The eTRE+ER intervention also improved diastolic blood pressure (−4 mm Hg; 95% CI, −8 to 0 mm Hg; P = .04) and mood disturbances, including fatigue-inertia, vigor-activity, and depression-dejection. All other cardiometabolic risk factors, food intake, physical activity, and sleep outcomes were similar between groups. In a secondary analysis of 59 completers, eTRE+ER was also more effective for losing body fat and trunk fat than CON+ER.
Conclusions and Relevance
In this randomized clinical trial, eTRE was more effective for losing weight and improving diastolic blood pressure and mood than eating over a window of 12 or more hours at 14 weeks.
Trial Registration
ClinicalTrials.gov Identifier: NCT03459703
Introduction
Intermittent fasting (IF) is the practice of alternating eating and extended fasting. In recent years, IF has been touted for losing weight and body fat. Indeed, IF can decrease body fat and preserve lean mass in animals and humans.1,2,3,4,5,6,7,8,9,10,11 Moreover, a few clinical trials have reported that IF is better for losing weight and/or body fat than continuous energy restriction in the short term.3,6,12,13,14,15,16 However, the literature is mixed, and there is no definitive evidence that IF selectively burns body fat while sparing muscle tissue.
One form of intermittent fasting that is particularly promising is time-restricted eating (TRE), which we define as eating within a consistent window of 10 hours or less and fasting for the rest of the day.17 Studies have shown that TRE prevents and reverses diet-induced obesity in rodents.18,19,20 Adults who adhere to TRE typically lose 1% to 4% of their body weight within several weeks.21,22,23,24,25,26,27,28,29,30,31,32 Further, TRE increases fat oxidation33 and can improve cardiometabolic end points, such as insulin sensitivity and blood pressure, even when calorie intake is matched to the control group.17,33,34,35,36
Although promising, most trials on TRE are small or single arm or used a weak control group. Therefore, we conducted a weight-loss randomized clinical trial comparing TRE with eating over a window of 12 or more hours, where both groups received identical weight-loss counseling. We tested a version of TRE called early TRE (eTRE), which involves stopping eating in the afternoon and fasting for the rest of the day. Because key circadian rhythms in metabolism—such as insulin sensitivity and the thermic effect of food—peak in the morning, eTRE may confer additional benefits relative to other forms of TRE.37 We hypothesized a priori that eTRE would be more effective for losing weight and body fat and improving cardiometabolic health than eating over a window of 12 or more hours and that participants would adhere to eTRE about 5 days per week.
Methods
Participants
New patients with obesity at the Weight Loss Medicine Clinic of the University of Alabama at Birmingham (UAB) Hospital were recruited between August 2018 and December 2019 by direct email, clinic newsletter, and physician referral. Applicants were eligible if they were aged 25 to 75 years, had a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30.0 and 60.0, and did not have diabetes or a severe or unstable chronic medical condition. Additional eligibility criteria are listed in the eMethods in Supplement 1. Participants self-reported their race, ethnicity, and sex. All participants provided written informed consent, and the study was approved by UAB’s Institutional Review Board (IRB-300001207). The trial protocol and statistical analysis plan appear in Supplement 2 and Supplement 3, respectively.
Intervention Groups and Randomization
The study was a 14-week parallel-arm, randomized controlled weight-loss trial. Participants were randomized to follow eTRE (8-hour eating window between 7:00 and 15:00) or a control (CON) eating schedule (a self-selected ≥12-hour window), which was designed to mimic US median meal timing habits.38 Participants were instructed to follow their assigned eating schedule at least 6 days per week. Aside from when participants ate, all other intervention components were matched across groups. Randomization was performed by the statistician in a 1:1 allocation ratio, with stratification by sex, race (Black vs not Black), and baseline physical activity level (≤2 days/week vs ≥3 days/week of exercise of any duration or intensity), using block sizes of 2 in the software program R.
Weight Loss Treatment
All participants received weight-loss counseling involving energy restriction (ER) at the UAB Weight Loss Medicine Clinic. In brief, participants received one-on-one counseling from a registered dietitian at baseline (60-minute session) and at weeks 2, 6, and 10 (30-minute sessions). Participants were counseled to follow a hypocaloric diet (500 kcal/d below their resting energy expenditure) and exercise 75 to 150 minutes per week, depending on their baseline physical activity. Participants were also instructed to attend at least 10 group classes. See eMethods in Supplement 1 for more details.
Outcome Measures
The co–primary outcomes were weight loss and fat loss. The secondary outcomes were fasting cardiometabolic risk factors. Additional outcomes included adherence, satisfaction with the eating windows, food intake, physical activity, mood, and sleep. All week 0 and 14 outcomes except adherence and food intake were measured in the morning following a water-only fast of at least 12 hours. In addition, we measured body weight in the nonfasting state in the clinic every 2 weeks throughout the trial.
Body Composition
Body composition was measured using dual x-ray absorptiometry (DEXA [iDXA; GE-Lunar Radiation Corporation]) and analyzed using enCORE software, version 15 (GE Healthcare). Fat loss was assessed in 2 ways: as the ratio of fat loss to weight loss (primary fat loss end point) and as the absolute change in fat mass (secondary fat loss end point). The former was used to test the hypothesis that IF selectively burns more body fat and less lean tissue per kilogram of weight lost (as a proxy for “selective fat loss”), while the latter was used to represent total fat loss. To accurately assess the former end point, we limited the analysis to completers who lost at least 3.6 kg (see eMethods in Supplement 1).
Cardiometabolic Risk Factors
Fasting blood pressure, glucose levels, insulin levels, homeostatic model assessment for insulin resistance (HOMA-IR), HOMA for β-cell function (HOMA-β), hemoglobin A1c level, and plasma lipid levels were measured using standard procedures (see eMethods in Supplement 1).
Adherence
Participants reported when they started and stopped eating daily through surveys administered via REDCap (Research Electronic Data Capture) software.39,40 Participants were classified as adherent if they followed their assigned eating window within 30 minutes. Days with missing surveys were considered nonadherent.
Food Intake, Physical Activity, Mood, Sleep, and Satisfaction
Energy intake and macronutrient composition were measured by 3-day food record using the Remote Food Photography Method.41 We also conducted a post hoc analysis of energy intake using weight-loss modeling techniques (see eMethods in Supplement 1). We measured physical activity, mood, sleep, and satisfaction with the eating window using the Baecke Physical Activity Questionnaire, the Profile of Moods–Short Form (POMS-SF), the Patient Health Questionnaire-9 (PHQ-9), the Munich Chronotype Questionnaire (MCTQ), the Pittsburgh Sleep Quality Index (PSQI), and a 5-point Likert scale, respectively (see eMethods in Supplement 1).
Statistical Analysis
The trial was statistically powered to detect a 10.0 plus or minus 13.4% difference in the ratio of fat loss to weight loss. We decided to assess the ratio of fat loss to weight loss only in completers who lost at least 3.6 kg because otherwise technical errors associated with DEXA can produce spurious values greater than 100% to 200% or less than 20% when participants lose marginal amounts of fat and/or fat-free mass (see eMethods in Supplement 1). Assuming 30% of participants would drop out or not lose at least 3.6 kg (8 lb), an estimated 86 enrollees were needed to have 60 completers who lost sufficient weight.
Analyses were performed in R, version 4.0.3 (R Foundation for Statistical Computing) using 2-sided tests with α = .05. All analyses were intention-to-treat, except that the ratio of fat loss to weight loss and questionnaire data were analyzed in completers only. End points with 3 or more repeated measures included body weight and adherence and were analyzed using linear mixed models. All other end points were analyzed using multiple imputation by chained equations, followed by linear regression. Between-group analyses were adjusted for age, race (Black vs non-Black), and sex (male vs female), while baseline data and within-group changes were analyzed using independent t tests. Following our preregistered statistical plan, we also performed a secondary analysis in completers using the same statistical methods. See eMethods in Supplement 1 for more statistical details.
Results
Participant Characteristics and Attrition
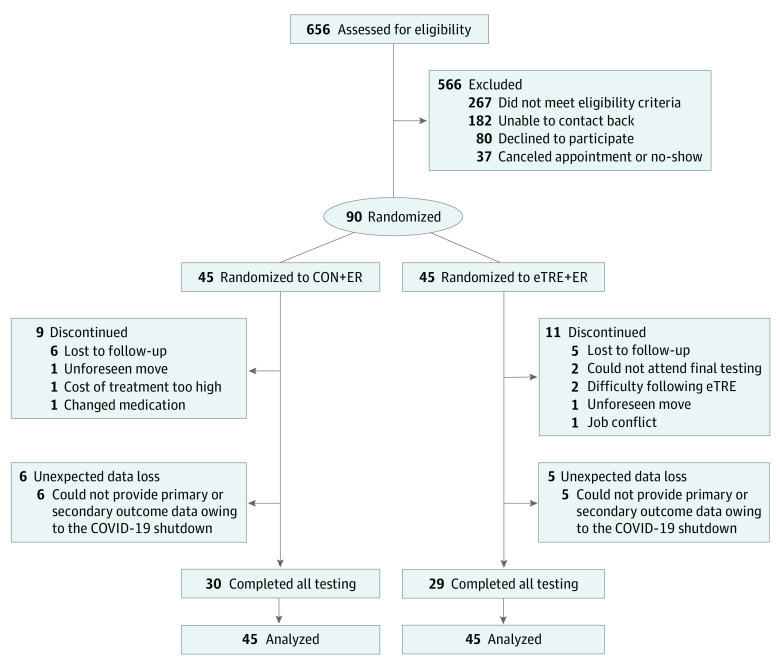
We screened 656 people and enrolled 90 participants (Figure 1). Participants had a mean (SD) BMI of 39.6 (6.7) and mean age of 43 (11) years. Seventy-two participants (80%) were female; 2 (2%) were Asian, 30 (33%) were Black, 56 (62%) were White, and 2 (2%) reported being of more than 1 race; and 2 (2%) were of Hispanic ethnicity, 85 (94%) were not of Hispanic ethnicity, and 3 (3%) had unknown or not reported ethnicity (Table 1). The attrition rate was similar between groups: 9 (20%) and 11 (24%) participants dropped out of the CON+ER and eTRE+ER groups, respectively (P = .80). Two participants in the eTRE+ER group withdrew owing to difficulty following the eating schedule, whereas none in the CON+ER group withdrew. Adverse events in both groups were mild (see eAppendix in Supplement 1). Unfortunately, because of the COVID-19 pandemic, we were unable to collect postintervention data on primary and secondary outcomes in 11 participants (see eMethods in Supplement 1). As a result, 59 participants (66%) completed all aspects of the study (see eTable 1 in Supplement 1 for a comparison of completers vs noncompleters).
Figure 1. Participant Flow Diagram.
CON+ER indicates control eating window plus energy restriction group; eTRE+ER, early time-restricted eating plus energy restriction group.
Table 1. Baseline Characteristics.
| Characteristic or risk factor | No. (%) | ||
|---|---|---|---|
| All participants (n = 90) | CON+ER group (n = 45) | eTRE+ER group (n = 45) | |
| Demographic characteristics | |||
| Age, mean (SD), y | 43 (11) | 43 (11) | 43 (10) |
| Sex | |||
| Female | 72 (80) | 37 (82) | 35 (78) |
| Male | 18 (20) | 8 (18) | 10 (22) |
| Race | |||
| Asian | 2 (2) | 1 (2) | 1 (2) |
| Black | 30 (33) | 15 (33) | 15 (33) |
| White | 56 (62) | 28 (62) | 28 (62) |
| >1 Race | 2 (2) | 1 (2) | 1 (2) |
| Ethnicity | |||
| Hispanic | 2 (2) | 1 (2) | 1 (2) |
| Not Hispanic | 85 (94) | 41 (91) | 44 (98) |
| Unknown or not reported | 3 (3) | 3 (7) | 0 |
| Body composition | |||
| Height, mean (SD), cm | 165.5 (8.7) | 163.8 (7.2) | 167.3 (9.7) |
| Weight, mean (SD), kg | 108.8 (20.6) | 105.3 (20.7) | 112.3 (20.1) |
| Fat mass | 52.5 (14.4) | 50.6 (14.8) | 54.4 (13.9) |
| Fat-free mass | 56.3 (11.1) | 54.8 (10.7) | 57.8 (11.4) |
| BMI, mean (SD) | 39.6 (6.7) | 39.2 (6.8) | 40.1 (6.6) |
| Cardiometabolic risk factors | |||
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 124 (13) | 122 (11) | 126 (14) |
| Diastolic | 81 (9) | 80 (8) | 82 (9) |
| Heart rate, mean (SD), beats/mina | 74 (10) | 74 (12) | 74 (8) |
| Glucose, mean (SD), mg/dL | 105 (16) | 103 (14) | 107 (17) |
| Insulin, mean (SD), µIU/mLa | 19.6 (14.1) | 17.2 (9.5) | 22.0 (17.2) |
| HOMA-IR, mean (SD) | 5.24 (4.40) | 4.44 (2.88) | 6.02 (5.41) |
| HOMA-β, mean (SD) | 181 (118) | 170 (97) | 192 (135) |
| HbA1c, mean (SD), % | 5.5 (0.4) | 5.5 (0.4) | 5.5 (0.4) |
| Cholesterol, mean (SD), mg/dL | |||
| Total | 203 (38) | 202 (36) | 204 (41) |
| LDL | 118 (28) | 117 (26) | 119 (29) |
| HDL | 62 (15) | 61 (16) | 62 (14) |
| Triglycerides, mean (SD), mg/dL | 117 (64) | 118 (62) | 117 (67) |
| Eating habits, mean (SD) | |||
| Eating duration, h/d | 12.6 (1.5) | 12.8 (1.6) | 12.4 (1.4) |
| Eating start time, h:m | 7:29 (1:05) | 7:27 (1:10) | 7:31 (1:00) |
| Eating end time, h:m | 20:06 (1:21) | 20:14 (1:35) | 19:57 (1:03) |
| Baecke Global Physical Activity Index | 8.3 (1.0) | 8.4 (1.0) | 8.1 (0.9) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CON+ER, control eating schedule plus energy restriction; eTRE+ER, early time-restricted eating plus energy restriction; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-β, Homeostasis Model Assessment for β-cell Function, calculated as 360 × insulin/[glucose – 63], where the unit of measure for insulin is µIU/mL and the unit of measure for glucose is mg/dL; HOMA-IR, Homeostasis Model Assessment for Insulin Resistance, calculated as insulin × glucose/405, where the unit of measure for insulin is µIU/mL and the unit of measure for glucose is mg/dL; LDL, low-density lipoprotein.
SI conversion factors: To convert cholesterol mg/dL to mmol/L, multiply by 0.0259; glucose mg/dL to mmol/L, multiply by 0.0555; insulin µIU/mL to pmol/L, multiply by 6.945; triglycerides mg/dL to mmol/L, multiply by 0.0113.
One baseline value for heart rate in the eTRE+ER group and for insulin in the CON+ER group were excluded. See eMethods in Supplement 1.
Adherence, Satisfaction, and Acceptability
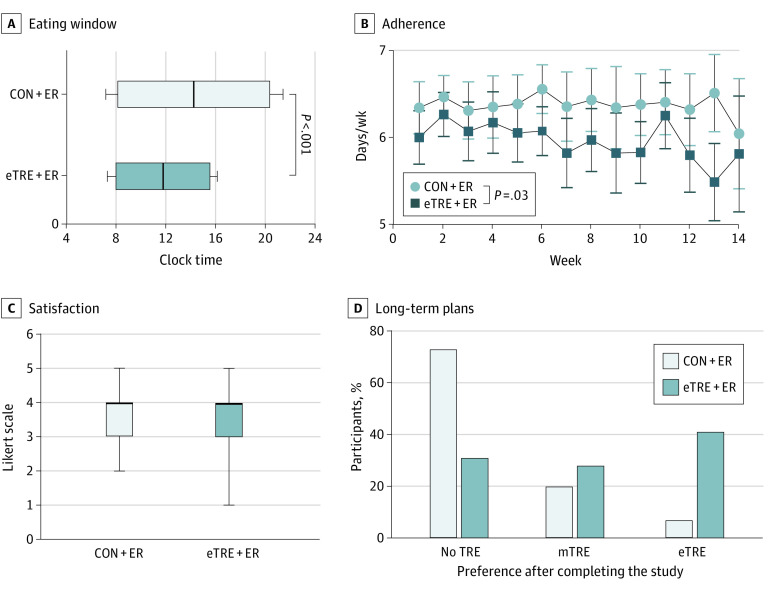
During the intervention, the eTRE+ER group ate within a mean (SD) time period of 7.6 (0.8) hours, while the CON+ER group ate over a mean (SD) time period of 12.3 (0.8) hours—a difference of about 4.8 hours (P < .001). Both groups ate breakfast at a similar time, but the eTRE+ER group finished eating at mean (SD) 15:35 (0:34) vs 20:25 (1:02) in the CON+ER group (P < .001; Figure 2A). The eTRE+ER group adhered a mean (SD) of 6.0 (0.8) days per week, which was lower than the CON+ER group (6.3 [0.8] days/week; P = .03), and adherence in the eTRE+ER group declined by 0.4 days per week over the 14-week intervention (P = .001; Figure 2B). Satisfaction with the eating window was similar between groups (−0.3; 95% CI, −0.8 to 0.2; P = .18; Figure 2C). After completing the intervention, 12 (41%) of the eTRE+ER group wanted to continue eTRE (vs 2 [7%] in the CON+ER group). Eight (28%) planned to eat within the originally prescribed 7:00 to 15:00 window, while the remainder wanted to use a different eTRE window (Figure 2D; see eMethods in Supplement 1).
Figure 2. Adherence, Satisfaction, and Acceptability.
A, Shown are the times of day (mean [SD]) that participants started eating (left end of box and left whisker) and stopped eating (right end of box and right whisker) in each group. The vertical line within the boxes indicates the median time of the eating window (averaged across all participants). The early time-restricted eating plus energy restriction (eTRE+ER) group ate over a 4.8-hour shorter window than the control plus energy restriction (CON+ER) group. B, The eTRE+ER group adhered a mean (SD) of 6.0 (0.8) days per week and was less adherent to the prescribed eating window than the CON+ER group. Data shown are raw means with 95% CIs. C, Among completers (n = 59), satisfaction with the prescribed eating window was similar between groups. Data shown are unadjusted means (boxes) with 95% CIs (whiskers), and the horizontal line within the boxes indicates the median. D, After completing the intervention, 12 participants (41%) in the eTRE+ER group planned to continue practicing eTRE, with 8 (28%) preferring to keep the original 7:00 to 15:00 schedule, and the remainder preferring to modify the window in a manner consistent with eTRE (defined as eating over a ≤10-hour window ending by 17:00). The other 8 (28%) and 9 (31%) participants wanted to practice midday TRE (mTRE; defined as eating over a ≤10-hour period ending after 17:00) or not practice TRE (defined as eating over a >10-hour period), respectively.
Weight Loss and Body Composition
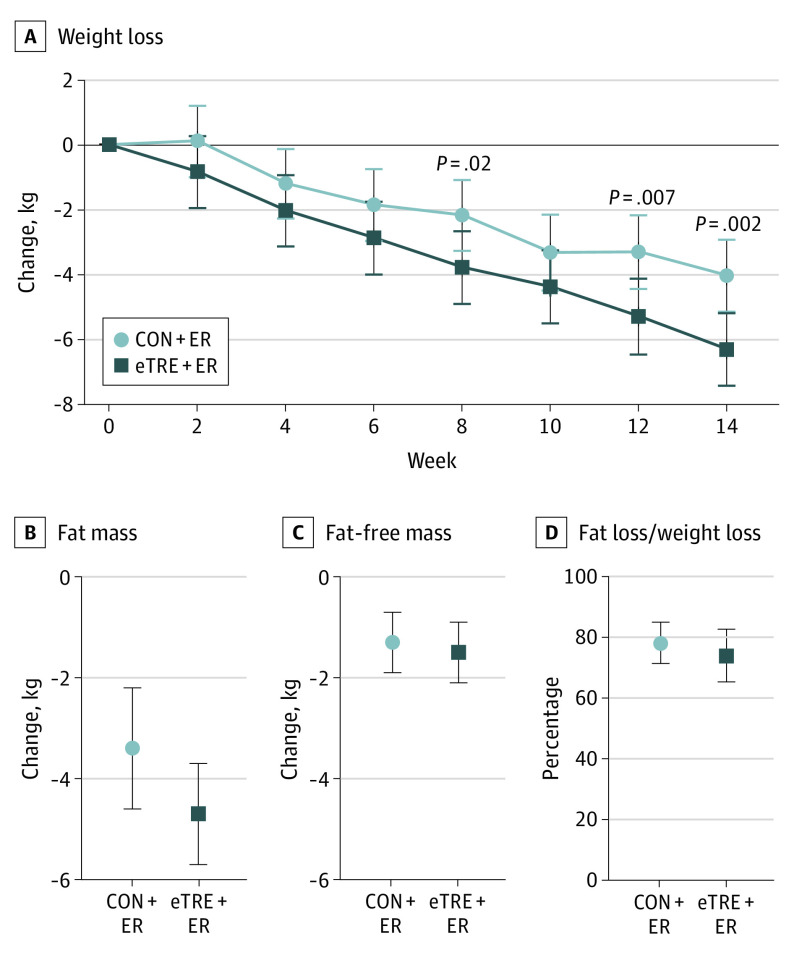
Both the eTRE+ER group (−6.3 kg [−5.7%]; 95% CI, −7.4 to −5.2 kg; P < .001) and CON+ER group (−4.0 kg [−4.2%]; 95% CI, −5.1 to −2.9 kg; P < .001) achieved clinically meaningful weight loss (Figure 3). The eTRE+ER group lost an additional 2.3 kg of body weight (95% CI, −3.7 to −0.9 kg; P = .002) relative to the CON+ER group. However, there were no statistically significant differences in absolute fat loss (−1.4 kg; 95% CI, −2.9 to 0.2 kg; P = .09) or the ratio of fat loss to weight loss (n = 41; −4.2%; 95% CI, −14.9 to 6.5%; P = .43). There were also no statistically significant differences in the changes in fat-free mass, trunk fat, visceral fat, waist circumference, or appendicular lean mass (Table 2).
Figure 3. Weight Loss and Body Composition.
A, Early time-restricted eating plus energy restriction (eTRE+ER) was more effective than the control eating schedule plus energy restriction (CON+ER) for losing weight. Data shown are least-squares means and 95% CIs from linear mixed modeling with adjustment for age, sex, and race. There were no statistically significant differences between groups for body fat (B), fat-free mass (C), or the ratio of fat loss to weight loss (n = 41) (D). Data shown in panels B-D are means and 95% CIs from multiple imputation by chained equations, followed by linear regression with adjustment for age, sex, and race.
Table 2. Body Composition and Cardiometabolic Risk Factors.
| Outcome | Change in the CON+ER group (n = 45)a | Change in the eTRE+ER group (n = 45)a | Difference between groupsb | ||||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | P value | Mean (95% CI) | P value | Mean (95% CI) | P value | Cohen d | |
| Weight, kgc | −4.0 (−5.1 to −2.9) | <.001 | −6.3 (−7.4 to −5.2) | <.001 | −2.3 (−3.7 to −0.9) | .002 | .33d |
| Weight loss, %c | −4.2 (−5.3 to −3.2) | <.001 | −5.7 (−6.7 to −4.7) | <.001 | −1.5 (−2.7 to −0.2) | .02 | .25d |
| Fat loss:weight loss, %e | 78.2 (71.4 to 85.0) | NA | 74.0 (65.3 to 82.7) | NA | −4.2 (−14.9 to 6.5) | .43 | .23 |
| Fat mass, kg | −3.4 (−4.6 to −2.2) | <.001 | −4.7 (−5.7 to −3.7) | <.001 | −1.4 (−2.9 to 0.2) | .09 | .34 |
| Fat-free mass, kg | −1.3 (−1.9 to −0.7) | <.001 | −1.5 (−2.1 to −0.9) | <.001 | −0.1 (−0.9 to 0.7) | .75 | .06 |
| Trunk fat, kg | −1.9 (−2.5 to −1.3) | <.001 | −2.8 (−3.4 to −2.2) | <.001 | −0.9 (−1.8 to 0.1) | .07 | .39 |
| Visceral fat, kgf | −0.2 (−0.4 to −0.1) | .002 | −0.3 (−0.5 to −0.1) | <.001 | −0.1 (−0.2 to 0.1) | .37 | .22 |
| Waist circumference, cm | −4.1 (−5.9 to −2.3) | <.001 | −5.3 (−7.1 to −3.5) | <.001 | −1.5 (−3.9 to 0.9) | .23 | .20 |
| Appendicular lean mass, kg | −0.8 (−1.2 to −0.4) | <.001 | −0.9 (−1.3 to −0.5) | <.001 | −0.1 (−0.6 to 0.5) | .78 | .07 |
| Blood pressure, mm Hg | |||||||
| Systolic | −3 (−7 to 1) | .07 | −8 (−12 to −4) | <.001 | −4 (−9 to 1) | .09 | .32 |
| Diastolic | −1 (−3 to 1) | .37 | −5 (−7 to −3) | <.001 | −4 (−8 to 0) | .04 | .45 |
| Heart rate, bpmg | −3 (−7 to 1) | .10 | −5 (−9 to −1) | .01 | −1 (−6 to 3) | .55 | .15 |
| Glucose, mg/dL | −6 (−10 to −2) | .01 | −8 (−12 to −4) | <.001 | −2 (−9 to 5) | .53 | .13 |
| Insulin, µIU/mLg | −1.8 (−6.4 to 2.8) | .44 | −6.4 (−10.8 to −2.0) | .006 | −4.2 (−10.7 to 2.2) | .20 | .30 |
| HOMA-IRg | −0.74 (−2.02 to 0.54) | .25 | −2.00 (−3.24 to −0.76) | .002 | −1.10 (−2.90 to 0.70) | .23 | .30 |
| HOMA-βg | 11 (−45 to 67) | .69 | −46 (−102 to 10) | .10 | −57 (−137 to 22) | .16 | .31 |
| HbA1c, % | −0.1 (−0.3 to 0.1) | .24 | −0.1 (−0.3 to 0.1) | .31 | 0.0 (−0.2 to 0.2) | .98 | .01 |
| Cholesterol, mg/dL | |||||||
| Total | −15 (−27 to −3) | .02 | −9 (−21 to 3) | .15 | 6 (−10 to 22) | .48 | .15 |
| LDL | −9 (−17 to −1) | .04 | −5 (−13 to 3) | .30 | 5 (−8 to 18) | .45 | .15 |
| HDL | −4 (−8 to 0) | .02 | −4 (−6 to −2) | .01 | 0 (−4 to 4) | .96 | .01 |
| Triglycerides, mg/dL | −9 (−27 to 9) | .32 | −3 (−21 to 15) | .78 | 6 (−17 to 30) | .61 | .10 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CON+ER, control eating schedule plus energy restriction; eTRE+ER, early time-restricted eating plus energy restriction; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-β, Homeostasis Model Assessment for β-cell Function, calculated as 360 × insulin/[glucose – 63], where the unit of measure for insulin is µIU/mL and the unit of measure for glucose is mg/dL; HOMA-IR, Homeostasis Model Assessment for Insulin Resistance, calculated as insulin × glucose/405, where the unit of measure for insulin is µIU/mL and the unit of measure for glucose is mg/dL; LDL, low-density lipoprotein; NA, not applicable.
SI conversion factors: To convert cholesterol mg/dL to mmol/L, multiply by 0.0259; glucose mg/dL to mmol/L, multiply by 0.0555; insulin µIU/mL to pmol/L, multiply by 6.945; triglycerides mg/dL to mmol/L, multiply by 0.0113.
Within-group changes were unadjusted and were based 90 observations at week 0 and 59 observations at week 14, respectively, except where otherwise noted.
Between-group differences were adjusted for sex, race, and age and were based on 90 observations at week 0 and 59 observations at week 14, respectively, except where otherwise noted.
For body weight, there were 90, 61, 69, 56, 60, 50, 48, and 59 observations at weeks 0, 2, 4, 6, 8, 10, 12, and 14, respectively.
Since there is no well-established method for calculating Cohen d from linear mixed models, we calculated the unadjusted Cohen d values using only week 0 and 14 data.
For the ratio of fat loss to weight loss, there were 41 observations. See eMethods in Supplement 1.
For visceral fat, there were 73 observations at week 0 and 47 at week 14. See eMethods in Supplement 1.
One baseline value was excluded. See eMethods in Supplement 1.
Cardiometabolic Risk Factors
The eTRE+ER intervention lowered diastolic blood pressure by an additional 4 mm Hg (95% CI, −8 to 0 mm Hg; P = .04) relative to CON+ER (Table 2). There were no statistically significant differences in systolic blood pressure, heart rate, glucose levels, insulin levels, HOMA-IR, HOMA-β, hemoglobin A1c level, or plasma lipid levels (Table 2).
Food Intake and Physical Activity
There were no between-group differences in self-reported physical activity, energy intake (1 kcal/d; 95% CI, −251 to 253 kcal/d; P = .99), or macronutrient composition (eTable 2 in Supplement 1). However, weight-loss modeling42 of all participants with at least 2 weight measurements (n = 77) indicated that eTRE reduced energy intake by an additional 214 kcal/d (95% CI, −416 to −12 kcal/d; P = .04) relative to the control eating window.
Mood and Sleep
The eTRE+ER intervention was more effective at improving total mood disturbances, as well as mood subscores for vigor-activity, fatigue-inertia, and depression-dejection (eFigure 1 in Supplement 1). All other mood and sleep end points were similar between groups (eFigures 1 and 2 in Supplement 1).
Completers-Only Analysis
In a preregistered secondary analysis of completers (eTable 3 in Supplement 1), eTRE was more effective for losing weight (−2.3 kg; 95% CI, −3.9 to −0.7 kg; P = .006), body fat (−1.8 kg; 95% CI, −3.6 to 0.0 kg; P = .047), and trunk fat (−1.2 kg; 95% CI, −2.2 to −0.1 kg; P = .03). Among secondary outcomes, eTRE+ER was more effective at lowering diastolic blood pressure (−5 mm Hg; 95% CI, −9 to −1 mm Hg; P = .01). All other primary and secondary outcomes were similar between groups (eTable 3 in Supplement 1).
Discussion
We conducted a randomized weight-loss trial comparing TRE with eating over a period of 12 or more hours where both groups received the same weight-loss counseling. Our data suggest that eTRE is feasible, as participants adhered 6.0 days per week on average, and most participants adhered at least 5 days per week. Despite the challenges of navigating evening social activities and occupational schedules, adherence to eTRE was similar to that of other TRE interventions (approximately 5.0-6.4 days/week),21,22,23,25,26,43,44,45 and satisfaction was similar between groups. Importantly, participants in the eTRE+ER group experienced greater improvements in mood—including fatigue, vigor, and feelings of depression/dejection—which may have helped them adhere to eTRE. Furthermore, we found that eTRE was acceptable for many patients. About 41% of completers in the eTRE+ER group planned to continue practicing eTRE after the study concluded.
The key finding of this study is that eTRE was more effective for losing weight than eating over a period of 12 or more hours. In our trial, the eTRE group lost an additional 2.3 kg relative to the control group, an approximately 50% improvement in weight loss. For comparison, prior studies are about evenly divided on whether TRE reduces body weight15,16,21,23,25,43,44,46,47,48,49,50,51,52,53 and are mixed for body fat,9,11,16,23,34,43,46,49,50,51,53,54,55,56 while studies that shift food intake to the morning and/or earlier in the daytime have more consistently reported weight loss.57,58,59,60,61,62 Of note, a somewhat larger study recently published in the New England Journal of Medicine by Liu et al53 reported that eTRE was not better for losing weight. However, our study had better post hoc statistical power owing to less variability in weight loss. Our 95% CI was narrower than and wholly contained within the other trial’s 95% CI. Therefore, our results are not incompatible. Furthermore, our eTRE group extended their daily fasting by twice as much, fasting an extra 4.8 hours per day vs only a modest 2.3-hour change in the Liu et al53 study. The magnitude of the weight-loss effect we observed was equivalent to reducing energy intake by an additional 214 kcal/d and is in line with 2 recent meta-analyses reporting that TRE has modest to moderate effects on body weight.31,32 Though we could not trace this energy deficit to changes in physical activity or food intake, we suspect that eTRE did reduce energy intake, but we were unable to detect it owing to the well-known limitations of accurately assessing food intake via self-report. Most previous studies report that TRE reduces energy intake and does not affect physical activity.21,24,25,27,51,52,55
On the other hand, we found no evidence of selective fat loss, as measured by the ratio of fat loss to weight loss. Also, total fat loss was not statistically significant in the main intention-to-treat analysis. Our finding of a difference in weight loss but not fat loss was likely due to lower statistical power because DEXA scans were performed only twice (whereas body weight was measured 8 times) and using a conservative imputation approach. In a secondary analysis of completers, eTRE was indeed better for losing body fat and trunk fat than eating over a window of 12 or more hours. The eTRE intervention increased fat loss by an additional 1.8 kg or 18 g/d. Our finding is consistent with a study reporting that participants burned an extra 15 g/d of fat when they ate 4.5 hours earlier in the day.63 Importantly, we found no evidence that extending the daily fasting period negatively affected lean mass, which is consistent with most studies on TRE.9,11,34,46,54,55 Although a large randomized clinical trial published in JAMA Internal Medicine by Lowe et al43 found that practicing TRE by skipping breakfast decreased appendicular lean mass, we had a larger sample size of DEXA scans and did not observe any deleterious effects.
The eTRE intervention was also more effective than eating over a period of 12 or more hours for lowering diastolic blood pressure. The effects were clinically significant and on par with those of the DASH (Dietary Approaches to Stop Hypertension) diet64 and endurance exercise.65 Although the effect was identical for systolic blood pressure, it was not statistically significant owing to the larger variance, suggesting that larger studies are needed on systolic blood pressure. For comparison, 1 previous controlled feeding study reported that eTRE reduces blood pressure,17 while other TRE studies are mixed but lean null.11,16,17,21,22,23,25,27,28,34,43,50,66,67 Studies reporting improvements tend to be longer and/or involve eating earlier in the day relative to the control group. Indeed, blood pressure has a pronounced circadian rhythm,68 and circadian misalignment elevates blood pressure in humans.69 Though other factors—such as fasting natriuresis or changes in sympathetic tone—could also explain these effects.
The eTRE intervention was not more effective for improving other fasting cardiometabolic end points. Our results are at odds with most studies on eTRE17,35,36,70 or eating earlier in the daytime,57,58,61,62,71,72,73 which usually report improvements in postprandial or 24-hour glucose levels, insulin sensitivity, and/or fasting insulin levels. However, studies on other versions of TRE report more mixed results.23,34,35,51,52,74,75 We acknowledge the limitation that we did not measure glycemic end points in the postprandial state, which are more responsive to dietary interventions. We also had larger variability in fasting insulin level relative to our previous trial.17 For plasma lipid levels, our results are consistent with most studies on TRE, which report no effects.9,11,17,21,23,25,26,27,28,34,35,43,47,48,52,54,66,70,74
Limitations
Our study has a few limitations, including being modest in duration, enrolling mostly women, and not achieving our intended sample size, partly owing to the COVID-19 pandemic. Also, we measured physical activity by self-report, not by accelerometry, which may have limited our ability to detect differences in physical activity between groups. Finally, we measured cardiometabolic end points only in the fasting state. Future research should investigate glycemic end points in the postprandial state or over a 24-hour period.
Conclusions
In this randomized clinical trial, eTRE was more effective for losing weight and lowering diastolic blood pressure than eating over a period of 12 or more hours at 14 weeks. The eTRE intervention may therefore be an effective treatment for both obesity and hypertension. It also improves mood by decreasing fatigue and feelings of depression-dejection and increasing vigor, and those who can stick with eTRE lose more body fat and trunk fat. However, eTRE did not affect most fasting cardiometabolic risk factors in the main intention-to-treat analysis.
This trial also lays important groundwork for future IF research. In this trial, eTRE produced clinically meaningful improvements in body fat, trunk fat, and systolic blood pressure that fell within the statistical trend range, with modest to moderate effect sizes (Cohen d = 0.32-0.39). Therefore, future clinical trials will need to enroll much larger sample sizes—up to approximately 300 participants—to determine whether IF affects body composition and cardiometabolic health. Future studies should investigate whether the timing and duration of the eating window affect these results, as well as determine who can adhere to eTRE vs who cannot and would instead benefit from other meal-timing interventions. The eTRE intervention should be further tested as a low-cost, easy-to-implement approach to improve health and treat disease.
eMethods.
eAppendix. Adverse Events
eTable 1. Baseline Characteristics of Completers Versus Non-Completers
eTable 2. Food Intake and Physical Activity
eTable 3. Completers-Only Analysis of Primary and Secondary Outcomes
eFigure 1. Mood
eFigure 2. Sleep
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Smyers ME, Koch LG, Britton SL, Wagner JG, Novak CM. Enhanced weight and fat loss from long-term intermittent fasting in obesity-prone, low-fitness rats. Physiol Behav. 2021;230:113280. doi: 10.1016/j.physbeh.2020.113280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotthardt JD, Verpeut JL, Yeomans BL, et al. Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology. 2016;157(2):679-691. doi: 10.1210/en.2015-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchison AT, Liu B, Wood RE, et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity (Silver Spring). 2019;27(1):50-58. doi: 10.1002/oby.22345 [DOI] [PubMed] [Google Scholar]
- 4.Byrne NM, Sainsbury A, King NA, Hills AP, Wood RE. Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obes (Lond). 2018;42(2):129-138. doi: 10.1038/ijo.2017.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catenacci VA, Pan Z, Ostendorf D, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring). 2016;24(9):1874-1883. doi: 10.1002/oby.21581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534-1547. doi: 10.1017/S0007114513000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keenan S, Cooke MB, Belski R. The effects of intermittent fasting combined with resistance training on lean body mass: a systematic review of human studies. Nutrients. 2020;12(8):E2349. doi: 10.3390/nu12082349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler CS, Stange R, Schlenkermann M, et al. A nonrandomized controlled clinical pilot trial on 8 wk of intermittent fasting (24 h/wk). Nutrition. 2018;46:143-152.e2. doi: 10.1016/j.nut.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 9.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razavi R, Parvaresh A, Abbasi B, et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. 2021;91(3-4):242-250. doi: 10.1024/0300-9831/a000623 [DOI] [PubMed] [Google Scholar]
- 11.Tinsley GM, Moore ML, Graybeal AJ, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628-640. doi: 10.1093/ajcn/nqz126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schübel R, Nattenmüller J, Sookthai D, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):933-945. doi: 10.1093/ajcn/nqy196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoni R, Johnston KL, Steele C, Carter D, Robertson MD, Capehorn MS. Efficacy of an intermittent energy restriction diet in a primary care setting. Eur J Nutr. 2020;59(6):2805-2812. doi: 10.1007/s00394-019-02098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davoodi SH, Ajami M, Ayatollahi SA, Dowlatshahi K, Javedan G, Pazoki-Toroudi HR. Calorie shifting diet versus calorie restriction diet: a comparative clinical trial study. Int J Prev Med. 2014;5(4):447-456. [PMC free article] [PubMed] [Google Scholar]
- 15.Cai H, Qin Y-L, Shi Z-Y, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. doi: 10.1186/s12876-019-1132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YJ, Wang YT, Chan LC, Chu NF. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition. 2022;93:111504. doi: 10.1016/j.nut.2021.111504 [DOI] [PubMed] [Google Scholar]
- 17.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221.e3. doi: 10.1016/j.cmet.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. doi: 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. doi: 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26(8):3493-3502. doi: 10.1096/fj.12-208868 [DOI] [PubMed] [Google Scholar]
- 21.Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345-353. doi: 10.3233/NHA-170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton SD, Lee SA, Donahoo WT, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):E1500. doi: 10.3390/nu11071500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow LS, Manoogian ENC, Alvear A, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860-869. doi: 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. doi: 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366-378.e3. doi: 10.1016/j.cmet.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11(12):2854. doi: 10.3390/nu11122854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104.e5. doi: 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. 2020;75:32-43. doi: 10.1016/j.nutres.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond). 2021;18(1):88. doi: 10.1186/s12986-021-00613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adafer R, Messaadi W, Meddahi M, et al. Food timing, circadian rhythm and chrononutrition: a systematic review of time-restricted eating’s effects on human health. Nutrients. 2020;12(12):E3770. doi: 10.3390/nu12123770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JH, Lu LW, Ge Q, et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. Published online September 23, 2021. doi: 10.1080/10408398.2021.1974335 [DOI] [PubMed] [Google Scholar]
- 32.Moon S, Kang J, Kim SH, et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12(5):E1267. doi: 10.3390/nu12051267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring). 2019;27(8):1244-1254. doi: 10.1002/oby.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens CR, Rossman MJ, Mazzo MR, et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience. 2020;42(2):667-686. doi: 10.1007/s11357-020-00156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchison AT, Regmi P, Manoogian ENC, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724-732. doi: 10.1002/oby.22449 [DOI] [PubMed] [Google Scholar]
- 36.Jones R, Pabla P, Mallinson J, et al. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr. 2020;112(4):1015-1028. doi: 10.1093/ajcn/nqaa192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11-27. doi: 10.1016/j.metabol.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marinac CR, Nelson SH, Breen CI, et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2(8):1049-1055. doi: 10.1001/jamaoncol.2016.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C. Measuring food intake with digital photography. J Hum Nutr Diet. 2014;27(suppl 1):72-81. doi: 10.1111/jhn.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826-837. doi: 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491-1499. doi: 10.1001/jamainternmed.2020.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesztyüs D, Vorwieger E, Schönsteiner D, Gulich M, Kesztyüs T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: results of a pilot study in a pre-post design. Ger Med Sci. 2021;19:Doc04. doi: 10.3205/000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PLoS One. 2021;16(1):e0246186. doi: 10.1371/journal.pone.0246186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domaszewski P, Konieczny M, Pakosz P, Bączkowicz D, Sadowska-Krępa E. Effect of a six-week intermittent fasting intervention program on the composition of the human body in women over 60 years of age. Int J Environ Res Public Health. 2020;17(11):E4138. doi: 10.3390/ijerph17114138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7:e22. doi: 10.1017/jns.2018.13 [DOI] [Google Scholar]
- 48.Karras SN, Koufakis T, Adamidou L, et al. Similar late effects of a 7-week orthodox religious fasting and a time restricted eating pattern on anthropometric and metabolic profiles of overweight adults. Int J Food Sci Nutr. 2021;72(2):248-258. doi: 10.1080/09637486.2020.1787959 [DOI] [PubMed] [Google Scholar]
- 49.Stratton MT, Tinsley GM, Alesi MG, et al. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients. 2020;12(4):1126. doi: 10.3390/nu12041126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotarsky CJ, Johnson NR, Mahoney SJ, et al. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9(10):e14868. doi: 10.14814/phy2.14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A. Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Med Sci Sports Exerc. 2021;53(12):2577-2585. doi: 10.1249/MSS.0000000000002738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady AJ, Langton HM, Mulligan M, Egan B. Effects of 8 wk of 16:8 time-restricted eating in male middle- and long-distance runners. Med Sci Sports Exerc. 2021;53(3):633-642. doi: 10.1249/MSS.0000000000002488 [DOI] [PubMed] [Google Scholar]
- 53.Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495-1504. doi: 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 54.Moro T, Tinsley G, Longo G, et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr. 2020;17(1):65. doi: 10.1186/s12970-020-00396-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tinsley GM, Forsse JS, Butler NK, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200-207. [DOI] [PubMed] [Google Scholar]
- 56.Tovar AP, Richardson CE, Keim NL, Van Loan MD, Davis BA, Casazza GA. Four weeks of 16/8 time restrictive feeding in endurance trained male runners decreases fat mass, without affecting exercise performance. Nutrients. 2021;13(9):2941. doi: 10.3390/nu13092941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504-2512. doi: 10.1002/oby.20460 [DOI] [PubMed] [Google Scholar]
- 58.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects of consuming later evening meal v. earlier evening meal on weight loss during a weight loss diet: a randomised clinical trial. Br J Nutr. 2021;126(4):632-640. doi: 10.1017/S0007114520004456 [DOI] [PubMed] [Google Scholar]
- 59.Dashti HS, Gómez-Abellán P, Qian J, et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2020;nqaa264. doi: 10.1093/ajcn/nqaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127(1):75-82. doi: 10.1093/jn/127.1.75 [DOI] [PubMed] [Google Scholar]
- 61.Lombardo M, Bellia A, Padua E, et al. Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr. 2014;33(3):198-205. doi: 10.1080/07315724.2013.863169 [DOI] [PubMed] [Google Scholar]
- 62.Allison KC, Hopkins CM, Ruggieri M, et al. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr Biol. 2021;31(3):650-657.e3. doi: 10.1016/j.cub.2020.10.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly KP, McGuinness OP, Buchowski M, et al. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020;18(2):e3000622. doi: 10.1371/journal.pbio.3000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Appel LJ, Moore TJ, Obarzanek E, et al. ; DASH Collaborative Research Group . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117-1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 65.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(1):e004473. doi: 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnason TG, Bowen MW, Mansell KD. Effects of intermittent fasting on health markers in those with type 2 diabetes: a pilot study. World J Diabetes. 2017;8(4):154-164. doi: 10.4239/wjd.v8.i4.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shea SA, Hilton MF, Hu K, Scheer FAJL. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980-984. doi: 10.1161/CIRCRESAHA.110.233668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453-4458. doi: 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):E1234. doi: 10.3390/nu11061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jakubowicz D, Wainstein J, Ahrén B, et al. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58(5):912-919. doi: 10.1007/s00125-015-3524-9 [DOI] [PubMed] [Google Scholar]
- 72.Jakubowicz D, Barnea M, Wainstein J, Froy O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin Sci (Lond). 2013;125(9):423-432. doi: 10.1042/CS20130071 [DOI] [PubMed] [Google Scholar]
- 73.Nakamura K, Tajiri E, Hatamoto Y, Ando T, Shimoda S, Yoshimura E. Eating dinner early improves 24-h blood glucose levels and boosts lipid metabolism after breakfast the next day: a randomized cross-over trial. Nutrients. 2021;13(7):2424. doi: 10.3390/nu13072424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients. 2020;12(2):E505. doi: 10.3390/nu12020505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729-1734. doi: 10.1016/j.metabol.2007.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eAppendix. Adverse Events
eTable 1. Baseline Characteristics of Completers Versus Non-Completers
eTable 2. Food Intake and Physical Activity
eTable 3. Completers-Only Analysis of Primary and Secondary Outcomes
eFigure 1. Mood
eFigure 2. Sleep
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement