Abstract
Research on cell death mechanisms gets a lot of attention. This is understandable as it underlies biology in general, as well as the insight in pathological conditions and the development of opportunities for therapeutic intervention. Over the last years a steady rise in the number of scientific reports and in the impact of this literature on the different mechanisms of programmed cell death can be observed. A number of new concepts are highlighted.
Over the last few years, a rise in the impact of Apoptosis was observed and Clarivate announce the 2015 Impact Factor to be 5.561. This was due to the increased submission of high-impact and high-quality manuscripts. We, as editors of Apoptosis are in service as of 2016 and we are determined to improve the quality of the journal. Over the last years we celebrated adulthood [1] and the quarter-century anniversary of Apoptosis [2]. We will continue to maintain the rigorous, fair and fast peer-review process of submitted manuscripts. We also aim to continue inviting reviews on trending topics in the field of programmed cell death, as well as ensuring that high-quality original research articles continue to be published in Apoptosis. We would like to thank the members of our editorial board and the outside reviewers for their invaluable support in selecting and improving the submitted manuscripts for publication in Apoptosis. Similarly, we thank the contributing authors for their trust in the journal to submit their best and most original research. Last but not least, we would like to thank our publisher Springer-Nature for the opportunity to serve the scientific community with the publication of Apoptosis.
Five years ago, we decided to select new cover art related to programmed cell death for every volume of Apoptosis [1]. Last year’s cover was adorned with an image from a paper of co-Editor-in-Chief Dr. Nowak-Sliwinska on the process of spindle pole clustering [2, 3]. For the coming year we selected an image from the best-cited 2021 original research paper published in Apoptosis [4]. It shows an A375 human melanoma cell that is exposed to the vitamin E derivative δ-Tocotrienol (δ-TT) (Fig. 1). This treatment induces cytoplasmic vacuolation, dilated endoplasmic reticulum and swollen mitochondria, specific features of cells undergoing paraptosis, a non-apoptotic form of programmed cell death [5, 6]. Apoptosis is the well-known programmed cell death mediating tumor growth suppression induced by standard anti-cancer therapies. However, over the years, it has become increasingly clear that additional forms of programmed cell deaths are involved in the anti-tumor activity of different synthetic and natural compounds [7, 8]. Specifically, paraptosis is a type of non-canonical programmed cell death characterized by a peculiar cytoplasmic vacuolation, associated with endoplasmic reticulum (ER) dilatation and mitochondrial swelling [9]. It was previously demonstrated that the vitamin E derivative δ-tocotrienol (δ-TT) triggers ER stress-mediated apoptosis in human melanoma cells [10]. This article presents that paraptosis might also be involved in the anti-cancer activity of this natural compound. Interestingly, by taking advantage of the TEM analysis technique, it was observed that, in human A375 and BLM melanoma cells, δ-TT induces cell death by promoting an intense cytoplasmic vacuolation, associated with dilated ER cisternae, swollen mitochondria with rare cristae and an enlarged nuclear envelope. It was also observed that these structural organelle alterations were strictly related to ER proteostasis disruption and mitochondrial dysfunction due to the accumulation of ER-derived Ca2 + and ROS overproduction. These data support that paraptosis is deeply involved in the anti-cancer activity of the natural compound δ-TT in human melanoma cells.
Fig. 1.
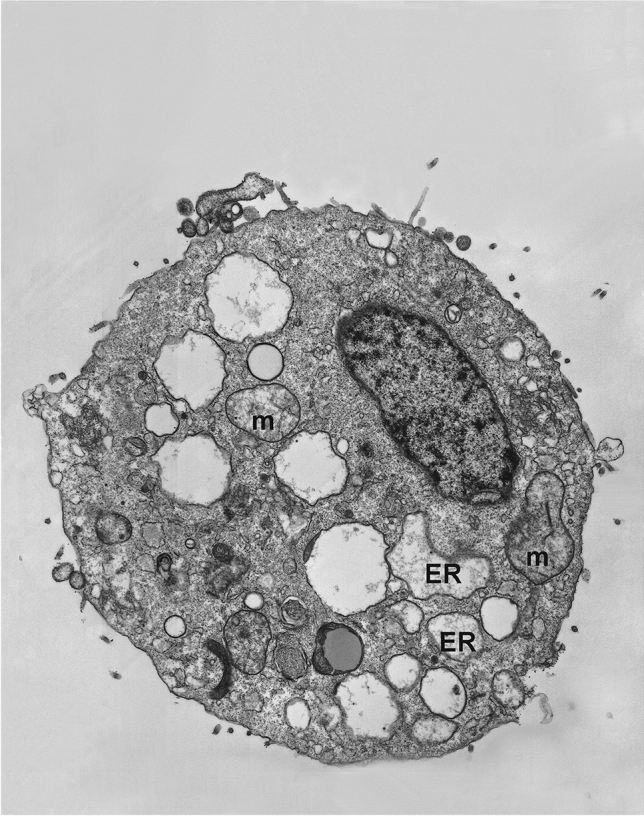
Melanoma cell undergoing paraptosis after exposure to the vitamin E derivative δ-Tocotrienol (δ-TT). This TEM image shows that A375 human melanoma cells treated with δ-TT exhibit vacuolation, dilated endoplasmic reticulum (ER) cisternae and swollen mitochondria (m), as specific features of cells undergoing paraptosis. This image is taken from Raimondi M et al. Apoptosis 26(5–6):277–92, 2021 [4]
While science, as well as almost every other sector of society, suffered significantly from the COVID-19 pandemic, Apoptosis received several excellent submissions on the role of SARS-CoV-2 mediated disease. It is known that COVID-19 is primarily a vascular disease, affecting next to the (upper) airway system many other organs as well [11–13]. We have published on the disruptive effects of SARS-CoV-2 on spermatogenesis through the induction of apoptosis [14]. Another submission that we published in 2021 was on the relationship between apoptosis and COVID-19 severity, which induced the discussion whether it is possible to target the virus-induced apoptosis as a therapeutic strategy [15].
A number of excellent papers were recently published on the different mechanisms of programmed cell death. An original research paper investigated ferroptosis, a process different from apoptosis as it is known to result from iron-dependent accumulation of lipid peroxides rather than caspase activation. This study demonstrates that the BAX-associated mitochondria-dependent pathway plays a pivotal role in the interplay between ferroptosis and apoptosis [16–19]. An overview on the role of ferroptosis in cancer was provided in two comprehensive reviews [5, 20]. An original research article reported on the induction of pyroptosis, an inflammatory form of programmed cell death often associated with infection [21, 22], after ischemia reperfusion injury in fatty liver disease [23]. Necroptosis, another caspase independent mechanism of cell death [24], was investigated in infectious diseases. It was suggested that intervention in necroptosis may be helpful for combatting pathogens, prevention of lesion formation and support the remodeling of tissues [24]. Many submissions were received on the process of autophagy. Two original research papers reported on autophagy mechanisms in cancer. Berberine and icotinib synergized at induction of autophagic cell death in lung cancer [25]. A second report identified autophagy as a mechanism for therapy of acute myeloid leukemia [26]. Several comprehensive reviews on the interaction between autophagy and apoptosis [27], as well as its role in cancer were published in 2021 [28, 29].
The team of editors and publishers highly values the excellent contributions of our editorial board and external reviewers. We can never express enough gratitude to these experts, whose work is an absolute requirement for the quality of a scientific journal. Of course, we are also grateful to the support of authors who submitted their manuscripts to Apoptosis. We highly encourage researchers to submit their exciting research to Apoptosis and communicate new ideas for invited reviews and special issues to further improve the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arjan W. Griffioen, Email: a.griffioen@amsterdamumc.nl
Patrycja Nowak-Sliwinska, Email: Patrycja.Nowak-Sliwinska@unige.ch.
References
- 1.Griffioen AW, Nowak-Sliwinska P. Apoptosis turns 21. Apoptosis. 2017;22(12):1485–1486. doi: 10.1007/s10495-017-1430-y. [DOI] [PubMed] [Google Scholar]
- 2.Griffioen AW, Nowak-Sliwinska P. A quarter century of apoptosis. Apoptosis. 2021;26(5–6):233–234. doi: 10.1007/s10495-021-01672-2. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Le Roux-Bourdieu M, Zoetemelk M, et al. Identification of a synergistic multi-drug combination active in cancer cells via the prevention of spindle pole clustering. Cancers (Basel) 2019 doi: 10.3390/cancers11101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondi M, Fontana F, Marzagalli M, et al. Ca(2+) overload- and ROS-associated mitochondrial dysfunction contributes to delta-tocotrienol-mediated paraptosis in melanoma cells. Apoptosis. 2021;26(5–6):277–292. doi: 10.1007/s10495-021-01668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Zhou L, Yuan H, Wu S. Interconnections among major forms of regulated cell death. Apoptosis. 2020;25(9–10):616–624. doi: 10.1007/s10495-020-01632-2. [DOI] [PubMed] [Google Scholar]
- 6.Kessel D. Photodynamic therapy: apoptosis, paraptosis and beyond. Apoptosis. 2020;25(9–10):611–615. doi: 10.1007/s10495-020-01634-0. [DOI] [PubMed] [Google Scholar]
- 7.Fontana F, Moretti RM, Raimondi M, et al. delta-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019;52(3):e12576. doi: 10.1111/cpr.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana F, Raimondi M, Marzagalli M, Di Domizio A, Limonta P. The emerging role of paraptosis in tumor cell biology: perspectives for cancer prevention and therapy with natural compounds. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188338. doi: 10.1016/j.bbcan.2020.188338. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Gu Y, Bian Y, et al. Honokiol induces paraptosis-like cell death of acute promyelocytic leukemia via mTOR & MAPK signaling pathways activation. Apoptosis. 2021;26(3–4):195–208. doi: 10.1007/s10495-020-01655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montagnani Marelli M, Marzagalli M, Moretti RM, et al. Vitamin E delta-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci Rep. 2016;6:30502. doi: 10.1038/srep30502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smadja DM, Mentzer SJ, Fontenay M, et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021;24(4):755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry BM, de Oliveira MHS, Cheruiyot I, et al. Circulating level of angiopoietin-2 is associated with acute kidney injury in coronavirus disease 2019 (COVID-19) Angiogenesis. 2021;24(3):403–406. doi: 10.1007/s10456-021-09782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovas A, Osiaevi I, Buscher K, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghimi N, Eslami Farsani B, Ghadipasha M, et al. COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis. 2021;26(7–8):415–430. doi: 10.1007/s10495-021-01680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donia A, Bokhari H. Apoptosis induced by SARS-CoV-2: can we target it? Apoptosis. 2021;26(1–2):7–8. doi: 10.1007/s10495-021-01656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YS, Kalimuthu K, Park YS, et al. BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. Apoptosis. 2020;25(9–10):625–631. doi: 10.1007/s10495-020-01627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghio AJ. Letter to the editor: iron, apoptosis, and ferroptosis. Apoptosis. 2020;25(9–10):605–606. doi: 10.1007/s10495-020-01628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ. The interplay between apoptosis and ferroptosis mediated by ER stress. Apoptosis. 2020;25(11–12):783. doi: 10.1007/s10495-020-01642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida GJ. The interplay between apoptosis and ferroptosis mediated by ER stress. Apoptosis. 2020;25(11–12):784–785. doi: 10.1007/s10495-020-01641-1. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wei Z, Pan K, Li J, Chen Q. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25(11–12):786–798. doi: 10.1007/s10495-020-01638-w. [DOI] [PubMed] [Google Scholar]
- 21.Murao A, Aziz M, Wang H, Brenner M, Wang P. Release mechanisms of major DAMPs. Apoptosis. 2021;26(3–4):152–162. doi: 10.1007/s10495-021-01663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowak-Sliwinska P, Griffioen AW. Programmed death, cells on the last train to glory. Apoptosis. 2020;25(3–4):151–153. doi: 10.1007/s10495-020-01598-1. [DOI] [PubMed] [Google Scholar]
- 23.Kolachala VL, Lopez C, Shen M, Shayakhmetov D, Gupta NA. Ischemia reperfusion injury induces pyroptosis and mediates injury in steatotic liver thorough caspase 1 activation. Apoptosis. 2021;26(5–6):361–370. doi: 10.1007/s10495-021-01673-1. [DOI] [PubMed] [Google Scholar]
- 24.Xia X, Lei L, Wang S, Hu J, Zhang G. Necroptosis and its role in infectious diseases. Apoptosis. 2020;25(3–4):169–178. doi: 10.1007/s10495-019-01589-x. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Dai CH, Shi ZH, et al. Synergistic inhibitory effect of berberine and icotinib on non-small cell lung cancer cells via inducing autophagic cell death and apoptosis. Apoptosis. 2021;26(11–12):639–656. doi: 10.1007/s10495-021-01694-w. [DOI] [PubMed] [Google Scholar]
- 26.Li YL, Zhou DJ, Cui ZG, et al. The molecular mechanism of a novel derivative of BTO-956 induced apoptosis in human myelomonocytic lymphoma cells. Apoptosis. 2021;26(3–4):219–231. doi: 10.1007/s10495-021-01664-2. [DOI] [PubMed] [Google Scholar]
- 27.Das S, Shukla N, Singh SS, Kushwaha S, Shrivastava R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis. 2021;26(9–10):512–533. doi: 10.1007/s10495-021-01687-9. [DOI] [PubMed] [Google Scholar]
- 28.Manea AJ, Ray SK. Regulation of autophagy as a therapeutic option in glioblastoma. Apoptosis. 2021;26(11–12):574–599. doi: 10.1007/s10495-021-01691-z. [DOI] [PubMed] [Google Scholar]
- 29.El-Baba C, Baassiri A, Kiriako G, et al. Terpenoids' anti-cancer effects: focus on autophagy. Apoptosis. 2021;26(9–10):491–511. doi: 10.1007/s10495-021-01684-y. [DOI] [PubMed] [Google Scholar]


