Abstract
Purpose
This study aims to evaluate the correlations between the severity of the disease and serum steroid levels by analyzing the serum steroid levels in COVID-19 patients with different levels of disease progression and the control group.
Methods
Morning serum Aldosterone, 11-deoxycortisol, Androstenedione, 17-hydroxyprogesterone, Dihydrotestosterone (DHT), Dehydroepiandrosterone (DHEA), Corticosterone, Dehydroepiandrosterone sulfate (DHEAS), Estrone, Estradiol, Progesterone, 11-deoxycorticosterone, Cortisol, Corticosterone, Androsterone, Pregnenolone, 17-hydroxypregnenolone and 21-deoxycortisol levels were measured in 153 consecutive patients were grouped as mild, moderate, and severe based on the WHO COVID-19 disease severity classification and the control group. Steroid hormone levels were analyzed at once with a liquid chromatography-tandem mass spectrometric method (LC-MS/MS).
Results
In our study, nearly all steroids were statistically significantly higher in the patients’ group than in the control group (p < 0.001). Also, DHEA was an independent indicator of the disease severity with COVID-19
Conclusions
Our study reveals that the alteration in steroid hormone levels was correlated with disease severity. Also, steroid hormone levels should be followed up during COVID-19 disease management.
Keywords: COVID-19, Steroids, Adrenal Insufficiency, Tandem Mass Spectrometry
Introduction
Coronavirus disease (COVID-19), caused by Coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, in December 2019 and spread to other countries and even continents rapidly within a short period. At present, it has affected over 500,000,000 individuals, and more than 6,200,000 lives in over 200 countries worldwide [1].
The adrenal gland is a multifunctional organ that produces steroid hormones and neuropeptides. Control of adrenal steroid hormone synthesis is complex, including ACTH and renin-angiotensin-aldosterone system (RAAS). SARS-CoV-2 has the potential to activate RAAS and the secretion of aldosterone and also expresses some amino acids that mimic the host adrenocortical hormone (ACTH1-39) [2]. The first 24 amino acids (ACTH1-24) are preserved in different mammalian cells. But 26th, 29th, 31st, 33rd, 37th, and 39th amino acids of mammalians’ ACTH have antigenic properties. SARS-CoV-2 has amino acids that resemble these significant ACTH amino acid residues. Due to these amino acids, antibodies against the SARS-CoV-2 destroy the ACTH of the host and thus preventing mainly the increase in cortisol levels [3]. From this point of view, the dynamics of adrenal steroidogenesis including glucocorticoids, mineralocorticoids, and androgens might be affected in patients with SARS-CoV-2.
In critical illness conditions including COVID-19, serum cortisol levels increase by activating the hypothalamic-pituitary-adrenal (HPA) axis. And also cortisol metabolism and cortisol binding protein levels decrease [4–6]. Systemic (i.e., intravenous or oral) corticosteroid therapy is strongly recommended for 7 to 10 days in patients with severe and critical COVID-19 in the guideline released by the World Health Organisation (WHO) on Sep 2, 2020 [7]. Cytokine storm emerging by COVID-19 in the host is also prevented by using corticosteroids [2, 8]
Sex steroids are produced mainly by the gonads and also in the adrenal glands. It is shown that there are differences between males and females in terms of COVID-19 morbidity and mortality. Male patients have poor prognosis than females. This might be attributed to differences in levels of sex steroid hormones. Estrogen and progesterone hormones may protect females against COVID-19 through their immunomodulatory and cardioprotective roles [9].
In the light of this information, the blood levels of steroids might be a quite complex situation, especially in COVID-19, which can show a critically serious course. According to our literature review, there were no studies in which all of the steroids used in clinical diagnosis and treatment in COVID-19 patients were analyzed simultaneously with a liquid chromatography-tandem mass spectrometric method (LC-MS/MS). And also, in most studies, steroids were analyzed by non-extraction methods. These methods have poor sensitivity, and specificity, and are susceptible to interference. These problems have been overcome with LC-MS/MS method to obtain more reliable results for this study.
This study aims to evaluate the correlations between the severity of the disease and serum steroid levels by analyzing the serum steroid levels in COVID-19 patients with different levels of disease progression and the control group. In this way, we aim to contribute to the literature by obtaining data for therapeutic strategies for SARS-CoV-2 related endocrinopathies and especially COVID-19, which has a severe clinical course.
Material and methods
Subjects
The institutional review board (Ethics Board, Ankara City Hospital) approved the study on the basis that it complied with the declaration of Helsinki and that the protocol followed existing good clinical practice guidelines (No. E1-22-2296). COVID-19 patients who were followed by Ankara City Hospital Infectious Diseases Clinic, with confirmed diagnosis by detecting SARS-CoV-2 RNA in oro-nasopharyngeal swab samples, included in this study. These patients were grouped as mild, moderate, and severe based on the WHO COVID-19 disease severity classification. According to this classification, patients without evidence of pneumonia or hypoxia were included in the mild group, while patients with signs and symptoms of pneumonia but no signs of severe pneumonia were considered as moderate. Patients with pneumonia and any of the following; >30 breaths/min; severe respiratory distress; or SpO2 < 90% at room were included in the severe patient group. In addition, blood samples taken from healthy volunteers were used as a control group. There were no gender discrimination and no age limit for the people included in the study None of the female patients were under hormone replacement therapy and has premature menopause history.
For the patient group, those who had negative SARS-CoV-2 RNA test, those who had been diagnosed with hypoadrenalism or endocrine disease before COVID 19, and those who received systemic glucocorticoid and diuretic therapy; for comparison of sex steroids, those who were after menopause; for the control group, those who have a diagnosed chronic disease and those who do not volunteer to participate in the study were not included in this study. We intentionally excluded patients admitted to the ICU to avoid the many confounding factors that may affect the adrenal function in the ICU setting.
Sample collection and laboratory analysis
Blood samples were collected at 08:00 h on the first morning after hospitalization with confirmed COVID-19 diagnosis and before the COVID-19 treatment for hospitalized patients based on the COVID-19 Guide of the Turkish Ministry of Health by venipuncture into blood collection tubes for Aldosterone, 11-deoxycortisol, 21-deoxycortisol, Androstenedione, 17-hydroxyprogesterone, Testosterone, Dihydrotestosterone (DHT), Dehydroepiandrosterone (DHEA), Corticosterone, Dehydroepiandrosterone sulfate (DHEAS), Estrone, Estradiol, Progesterone, 11-deoxycorticosterone, Cortisol, Androsterone, Pregnenolone, 17-hydroxypregnenolone analysis. After centrifugation, serum aliquots were separated, frozen, and stored at −80 °C until the analysis day. Laboratory tests were carried out using LC-MS/MS method.
Steroid hormone analysis by LC-MS/MS
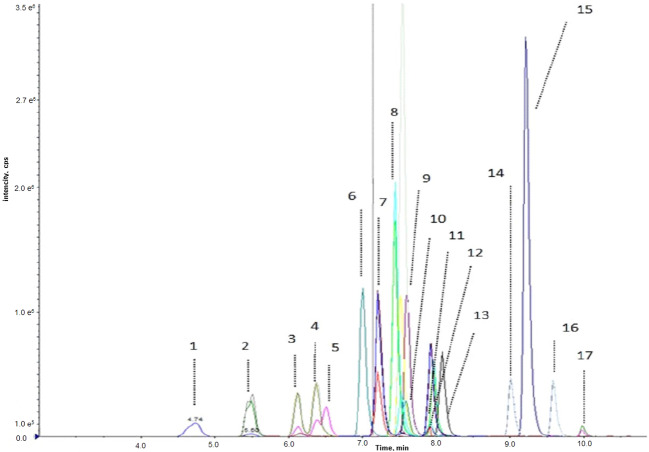
Steroid hormone levels were measured using the Eureka LC-MS/MS kit (Eureka Lab Division, Chiaravalle, Ancona, Italy) with AB Sciex 6500 QTRAP™ (Sciex, Concord, ON, Canada). In this method briefly, the sample was centrifuged at 14.000 rpm for 10 min after adding 1000 µl of deproteinization solution containing the internal standard. The supernatant was removed and dried by an evaporator followed by a 100 µL diluting solution addition centrifugation was done at 14.000 rpm for 5 min. The transferred supernatant into the vial was injected into the LC system. Agilent Zorbax RRHD Eclipse Plus C 18 (50 × 2.1 mm. 1.8 um) was used as an analytical column. 6 levels of calibrators, 2 levels of controls, and the internal standard solution containing Aldosterone-d7, Cortisol-d4, and Testosterone-d3 were used. The limit of quantification (LoQ) was 0.005 ng/mL for 17-hydroxyprogesterone, Androstenedione and Cortisol; 0.05 ng/mL for DHEA, Corticosterone and Androsterone; 0.007 ng/mL for 11- deoxycortisol and Deoxycorticosterone; 0.01 ng/mL for Aldosterone and Estradiol; 0.03 ng/mL for Dihydrotestosterone and Estrone; 0.02 ng/mL for Pregnenolone and 17- hydroxypregnenolone; 0.002 ng/mL for Testosterone and Progesterone; 10 ng/mL for DHEAS. Figure 1 depicted LC-MS/MS chromatogram for steroid hormones in a mixed solution.
Fig. 1.
LC-MS/MS chromatogram for steroid hormones in mix solution. Numbers indicate the following metabolites. 1: Aldosterone; 2: Cortisol; 3: 21-deoxycortisol, 4: Corticosterone, 5: 11-deoxycortisol, 6: Androstenedione, 7: Estrone, 8: 11-deoxycorticosterone, 9: Testosterone 10: Estradiol, 11: Dehydroepiandrosterone, 12: 17-hydroxyprogesterone, 13: 17-hydroxypPregnenolone, 14: Dihydrotestosterone, 15: Progesterone, 16: Androsterone, 17: Pregnenolone
Statistical analysis
Statistical analyses were performed using the SPSS 26.0. The Kolmogorov–Smirnov test was performed to check the normality of the variables. Descriptive analysis was presented using median (IQR) for normally and non-normally distributed variables. Laboratory data were compared between the groups using the Kruskal–Wallis test for nonparametric variables and the ANOVA test for parametric variables. Comparisons for categorical variables were executed using the chi-square test. For the multivariate analysis, the possible factors identified with univariate analyses were further entered into the logistic regression analysis to determine independent predictors of the severity of the disease. The Hosmer-Lemeshow goodness of fit test was used. The odds ratio (OR) was calculated for significantly associated variables. Statistical significance was defined as p < 0.05.
Results
A total of 153 patients were included in this study. Demographic characteristics and quick sequential organ failure assessment scores (qSOFA) of COVID-19 patients as indicators of disease severity were shown in Table 1. The qSOFA score considers (a) a Glasgow coma scale value smaller than 15, (b) a respiratory rate ≥22/min, and (c) systolic blood pressure of ≤100 mmHg. The highest score (max. 3 points) is associated with a poor prognosis. The median age was 39 (min-max, 5–84) years, and the male: female ratio was 86:67. Forty-three patients (28.1%) were in the severe group, sixty patients (39.2%) were in the moderate patient group, and fifty patients (32.7%) were in the mild group. There were 5 males and 28 females in the control group. In the patient group, 11 patients had hypertension, 5 patients had diabetes mellitus (DM), 6 patients had DM and hypertension, 1 patient had lung disease and hypertension, 2 patients had coronary artery disease, DM, and hypertension, 1 patient had hypertension and renal disease, 1 patient lung disease and hypertension, chronic kidney disease, 1 patient coronary artery disease, and hypertension.
Table 1.
Demographic characteristics, qSOFA score and steroid levels of COVID-19 patients
| Severe group (n = 43) | Moderate group (n = 60) | Mild group (n = 50) | Control group (n = 33) | *p | |
|---|---|---|---|---|---|
| AGE | 57 (22) | 15 (21) | 30 (14) | 27 (14) | <0.001a,b,d,e,f |
| Male (N;%) | 31 (34.1) | 39 (42.9) | 16 (17.6) | 5 (5.5) | <0.001a,b,c,d,e,f |
| Smoking (N;%) | 9 (21) | 6 (10) | 8 (16) | 0 | >0.05 |
| Patients without morbidity (N;%) | 28 (65.1) | 50 (83.3) | 47 (94) | – | <0.001d,e,f |
| qSOFA 0-1 (N;%) | 37 (86) | 59 (98) | 50 (100) | – | 0.016d,e,f |
| ALDOSTERONE pg/mL | 84.5 (16.4) | 89.9 (46) | 110.5 (75.3) | 7.74 (16.4) | <0.001a,b,c |
| 11 DEOXYCORTISOL** | 93.9 (224) | 497 (538) | 489 (512) | 13.5 (14.4) | <0.001a,b,c |
| 17- OH- PROGESTERONE** | 315 (542) | 625 (649) | 532 (417) | 26 (49.2) | <0.001a,b,c,e |
| CORTISOL ug/dL | 79.3 (154) | 149 (204) | 46.6 (73.7) | 10.4 (9.66) | <0.001a,b,c,e,f |
| CORTICOSTERONE** | 760 (1423) | 3382 (3769) | 2052 (1834) | 168 (243) | <0.001a,b,c,d,e |
| 11-DEOXYCORTICOSTERONE** | 66.5 (81) | 542 (697) | 747 (652) | 3.06 (3.81) | <0.001a,b,c,d,e |
| PREGNENOLENE** | 329 (561) | 510 (267) | 531 (462) | 111 (134) | <0.001a,b,c |
| 17-OH-PREGNENOLONE** | 14.4 (22.7) | 35.6 (43.8) | 41.6 (100) | 75.9 (132) | <0.001a,b,d,e |
| 21-DEOXYCORTISOL** | 35 (13) | 40 (17) | 42 (17) | 1.34 (1.73) | <0.001a,b,c,d |
Data are median (IQR); **, ng/dL
IQR interquartile rage
*Statistical significance was defined as p < 0.05
aSignificant difference between severe group and control group
bSignificant difference between moderate group and control group
cSignificant difference between mild group and control group
dSignificant difference between severe group and mild group
eSignificant difference between severe group and moderate group
fSignificant difference between moderate group and mild group
In our study, Corticosterone, 11- deoxycorticosterone, and 21- deoxycortisol levels were significantly lower in the severe disease group compared to the mild disease group (p < 0.001). Cortisol, corticosterone, and 11- deoxycorticosterone levels were significantly lower in the severe disease group compared to the moderate disease group (p < 0.001). Cortisol levels were significantly higher in the moderate disease group compared to the mild disease group (p < 0.001) 17- OH progesterone and pregnenolone levels in the control group were lower than in all of the disease groups (Table 1).
Sex steroid hormones were compared in each gender group separately. Estradiol, estrone, and progesterone levels in the control group were lower than in all of the disease groups (p < 0.001). Although estrone and progesterone levels in COVID-19 patients were higher than the levels obtained in the control group, this difference was statistically important only in the mild and moderate group in comparison to the control group (p < 0.001) (Table 2). There were only five men in the control group. Therefore, comparisons were done among the disease groups for male patients. DHT, Androstenedione, and Androsterone levels were not significantly different among all male patients group. Testosterone and DHEAS levels in the moderate disease group were the highest among all groups for these patients. DHEA levels in the severe group were the lowest among all groups for male COVID-19 patients (Table 3).
Table 2.
Comparison of sex steroids in females
| Severe group (n = 8) | Moderate group (n = 32) | Mild group (n = 41) | Control (n = 26) | *p | |
|---|---|---|---|---|---|
| ESTRONE pg/mL | 237 (286) | 602 (447) | 542 (386) | 55.6 (71.3) | <0001b,c |
| ESTRADIOL pg/mL | 580 (397) | 557 (418) | 400 (314) | 66.1 (52.2) | <0001a,b,c |
| PROGESTERONE ug/L | 1.05 (1.42) | 1.20 (2.02) | 1.31 (2.82) | 0.12 (1.55) | <0001b,c |
Data are median (IQR); **, ng/dL
IQR interquartile rage
*Statistical significance was defined as p < 0.05
aSignificant difference between severe group and control group
bSignificant difference between moderate group and control group
cSignificant difference between mild group and control group
Table 3.
Comparison of sex steroids between male patients groups
| Severe group (n = 31) | Moderate group (n = 39) | Mild group (n = 16) | *p | |
|---|---|---|---|---|
| TESTOSTERONE ng/dL | 1114 (1781) | 3555 (3133) | 1708 (148) | <0.001a.b |
| DIHYDROTESTOSTERONE pg/mL | 345 (301) | 342 (226) | 359 (236) | 0.722 |
| ANDROSTENEDIONE ng/dL | 935 (1422) | 1156 (1040) | 1021 (957) | 0.498 |
| DHEA ug/L | 1.29 (2.80) | 5.98 (3.62) | 5.66 (3.99) | <0.001a,c |
| ANDROSTERONE ug/L | 468 (323) | 492 (368) | 465 (274) | 0.671 |
| DHEAS ug/dL | 144 (499) | 466 (791) | 263 (268) | <0.001a,b,c |
DHEA Dehydroepiandrosterone, DHEAS Dehydroepiandrosterone sulfate, IQR interquartile range
Data are median (IQR)
*Statistical significance was defined as p < 0.05
aSignificant difference between severe group and moderate group
bSignificant difference between moderate group and mild group
cSignificant difference between and severe group and mild group
The binary logistic regression model included DHEA, 11-deoxycorticosterone. It was statistically significant with χ2 = 4.946; p < 0.001. The model correctly classified 95.7% of the cases. These increased parameters, especially DHEA, were independent indicators of the disease severity with the likelihood ratios shown in Table 4. Figures 2 and 3 depicted distributions of DHEA and 11-Deoxycorticostrone levels between groups.
Table 4.
Odds ratios and coefficients of binary logistic regression analysis of factors associated with disease severity
| 95% C.I.for EXP(B) | |||||||
|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | Sig | Exp(B) | Lower | Upper | |
| DHEA (ug/L) | 0.321 | 0.131 | 5.967 | 0.015 | 1.379 | 1.065 | 1.784 |
| 11-DEOXYCORTICOSTERONE (ng/L) | 0.023 | 0.007 | 11.553 | 0.001 | 1.023 | 1.010 | 1.037 |
Hosmer Lemeshow GFT: p = 0.763; Model: χ2 = 4.946; p < 0.001; Percentage Correct = 95.7%
Fig. 2.
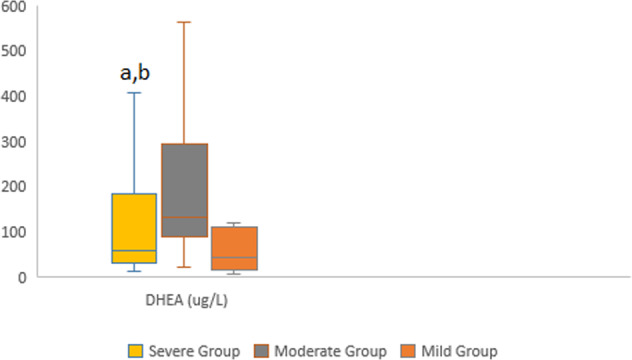
Distributions of Dehydroepiandrosterone male patients groups a Statistically significant than moderate group b Statistically significant than mild group
Fig. 3.
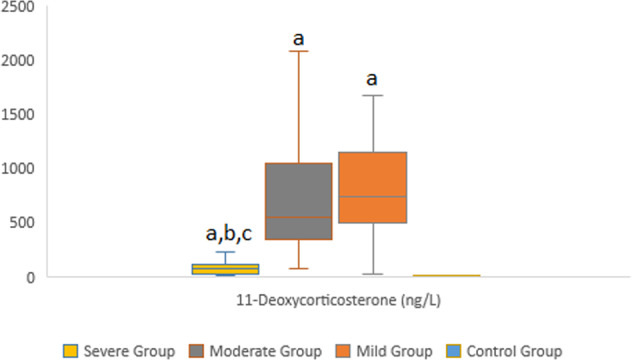
Distributions of 11-Deoxycorticosterone levels between groups a Statistically significant than control group b Statistically significant than mild group. c Statistically significant than moderate group
Discussion
COVID-19, the ongoing pandemic, has still issues that need to be clarified to manage the disease and its consequences despite extensive research and several large studies describing the clinical, biochemical, and radiological features. Steroid hormones are commonly classified as glucocorticoids, mineralocorticoids, and sex steroid hormones. Analyzing and monitoring these hormones provides useful information in terms of diagnosis, follow-up, and prognosis of diseases [10]. For this purpose, we analyzed sex steroids, glucocorticoids, and mineralocorticoids simultaneously with LC-MS/MS, a sensitive and specific method, in COVID-19 patients with different disease severity and healthy individuals. According to our literature review, these hormones were evaluated simultaneously in COVID-19 for the first time. Analyzing steroids, whose production is related to each other, in all individuals at the same time, allowed the hormones to be evaluated together and to obtain more reliable data.
SARS-CoV-2 viral entry requires two host proteins: the angiotensin-converting enzyme-2 (ACE2) and the transmembrane protease, serine2 (TMPRSS2). SARS-CoV-2 enters many organs, including endocrine organs, through ACE2 receptors and creates a clinical picture in various spectrums that can change depending on the individual’s immune system, age, gender, concomitant diseases, and other unexplained reasons, from mild to severe, and even cause death. Transcription of the TMPRSS2 gene and ACE2 receptor activation is positively regulated by androgens. It is accepted that this situation contributes to the more common and worse prognosis of COVID 19 in men [11, 12]. In our study, we found that the highest androgen levels were in patients with moderate COVID-19. However, we found that as the severity of the disease increased, steroid levels decreased. We attributed this situation to the possibility of high viral load interfering with hormone production, as in the study of Bermejo-Martin et al. in which they compared plasma viral load with disease severity and serum parameters [13].
There are also opinions supporting that the differences in the severity and mortality of COVID-19 between the sexes are due to immunological and especially hormonal differences, apart from the entry of SARS-CoV-2 into the cell [9, 11]. It has been observed that COVID-19 alters spermatogenesis and testosterone production. In a study by Montaño et al., a negative correlation was found between the severity of COVID-19 and testosterone levels [11]. In our study, we also found low testosterone levels in severely ill COVID-19 patients, but we did not find dihydrotestosterone (DHT) levels differently. DHT is a metabolite that results from the reduction of testosterone by 5α-reductase. DHT is a more potent agonist of androgen receptors than testosterone. But its half-life in plasma is shorter than that of testosterone and its levels reflect the local expression of 5α-reductase. Therefore, we think that we could not find DHT levels different in patients with different clinical courses. Due to these differences in studies, there are opinions supporting that innate immunity and genetic errors are more important than the sex steroid levels of individuals in the critical course of COVID-19 [14].
It is assumed that female sex hormones are potent immunomodulators, and therefore, COVID-19 has a milder clinical course in women [15]. However, in our study, we found lower sex steroid hormone levels in patients with a more severe COVID-19 clinical course. In the comparisons we made according to genders, sex steroids were lower in severe cases than in other patient groups. This may be due to the down-regulation of the gonadal axis by cytokines in patients with acute, severe disease [16]. The results of our study also support this situation.
In an autopsy study conducted on patients with SARS, it was shown that the virus reached the hypothalamus directly through the hematogenous route or directly through the cribriform plate and was destroyed by the identification of the SARS genome in the hypothalamus [3]. In a postmortem study of COVID-19 patients, no significant pathology in adrenal glands was found but areas of necrosis/infarction were seen in one out of the nineteen examined pituitaries [17]. In addition, the hypothalamic-pituitary-testicular axis is also suppressed in acute diseases [12]. In the study of Alzahrani et al., in which they investigated the effect of COVID-19 on the HPA axis, they found no difference between the median values of cortisol in patients with COVID-19 infection of different severity but found low cortisol levels in patients with severe infection [18]. In our study, there were more patients in each COVID-19 patient group than in the study of Alzahrani et al. We found significant differences between the median levels of cortisol and many other steroids between the groups at each severity level, but in severe cases, our steroid levels were low like the mentioned study. In an autopsy study conducted on patients with COVID-19, they observed signs of necrosis, cortical lipid degeneration, hemorrhage, and focal gland inflammation in the adrenal glands associated with infection [19]. This explains the low level of adrenal steroids in patients with severe COVID-19, which we demonstrated in our study.
DHEA is synthesized by zona reticularis of the adrenal cortex from 17 α-hydroxypregnenolone on ACTH stimulation. This androgenic steroid is sulfated to form dehydroepiandrosterone sulfate in the adrenals and peripheral tissues. DHEAS has an immunostimulatory effect. DHEA is more sensitive to HPA axis stimulation than cortisol [20, 21]. However, its relationship with the severity of COVID-19 has not been evaluated. As shown in Table 3, we found that DHEA and DHEAS levels were higher in the moderate group due to the immunostimulatory effect, while they decreased as the severity of the disease increased due to suppression of the HPA axis as the disease progressed. With logistic regression, we determined that with each unit increase in DHEA level, there may be a 1.379 (1.065–1.784)-fold risk increase for COVID-19 disease severity.
Both the cytopathic effect of the SARS-CoV-2 virus on the adrenal cortex and secondary adrenal insufficiency caused by ACTH destruction due to the homology of ACTH with viral RNA by antibodies against SARS-CoV-2 have been shown in COVID-19 patients. In addition, stress-induced cortisol increase may be prevented by inadvertent degradation of ACTH by antibodies formed against viral particles [3, 12]. We found that with each unit increase in 11- Deoxycorticosterone level, there may be a 1.023 (1.010–1.037)-fold risk increase for COVID-19 disease severity by logistic regression. So the mechanism underlying this finding may be the secretion of deoxycorticosterone (DOC), unlike that of aldosterone, which is not in response to sodium deficiency or volume depletion but is more affected by ACTH levels. In contrast to aldosterone, ACTH primarily regulates DOC secretion [22]. Because of the alteration of ACTH levels, we may have found an association between disease severity and level 11- deoxycorticosterone.
In a study by Gonen et al., they found the frequency of adrenal insufficiency to be 8.2%. They found Antipituitary antibodies (APA) in 3 of these patients who developed adrenal insufficiency and antihypothalamic antibodies (AHA) in 1 of them. They found the cortisol level to be high regardless of the severity of the COVID-19 disease [23]. In our study, cortisol levels were high in patients with moderate severity, but cortisol levels decreased in the group with severe COVID-19 based on the WHO classification for COVID-19. In addition, in many other publications such as Gonen et al., cortisol level was analyzed by electrochemiluminescence immunoassay (ECLIA). We think that one of the reasons for the inconsistency with our results may be the difference in the analysis method.
The strength of our study is that the method we used for hormone analysis is sensitive and specific in particularly measuring low hormone concentrations. However, the inability to perform menstrual cycle questioning in female patients is a limitation of our study. In addition, the control group was not matched with the patient group in terms of gender.
In conclusion, this research paper reveals that the alteration in steroid hormone levels was correlated with disease severity by simultaneous measurement of steroid hormone levels with LC-MS/MS. By analyzing the steroid hormone levels with sensitive and specific methods for especially patient follow-up, more reliable data will be obtained, and a positive contribution can be made to patient management with early diagnosis and treatment of the pathology that may occur.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Meter. Corona Virus Update (Live). Available from: https://www.worldometers.info/coronavirus/. [Accessed 23 APRIL 2022]
- 2.M. Wiegand et al. Unquantifiably low aldosterone concentrations are prevalent in hospitalised COVID-19 patients but may not be revealed by chemiluminescent immunoassay. medRxiv 2022 [DOI] [PMC free article] [PubMed]
- 3.Pal R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine. 2020;68(2):251–252. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal R, Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J. Endocrinological Investig. 2020;43(7):1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leow MKS, et al. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin. Endocrinol. 2005;63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan T, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation. Corticosteroids for COVID-19. Living guidance 2 September 2020
- 8.Almeida MQ, Mendonca BB. Adrenal insufficiency and glucocorticoid use during the COVID-19 pandemic. Clinics. 2020;75:e2022. doi: 10.6061/clinics/2020/e2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsa A, Prabhu JS. Gender disparity in COVID-19: Role of sex steroid hormones. Asian Pac. J. tropical Med. 2021;14(1):5. doi: 10.4103/1995-7645.304293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karashima S, Osaka I. Rapidity and precision of steroid hormone measurement. J. Clin. Med. 2022;11(4):956. doi: 10.3390/jcm11040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaño LM, et al. Could lower testosterone in older men explain higher COVID-19 morbidity and mortalities? Int. J. Mol. Sci. 2022;23(2):935. doi: 10.3390/ijms23020935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermejo-Martin JF, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care. 2020;24(1):1–13. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traish AM. Sex steroids and COVID-19 mortality in women. Trends Endocrinol. Metab. 2021;32(8):533–536. doi: 10.1016/j.tem.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinna G. Sex and COVID-19: a protective role for reproductive steroids. Trends Endocrinol. Metab. 2021;32(1):3–6. doi: 10.1016/j.tem.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Zeggeren IE, et al. Sex steroid hormones are associated with mortality in COVID-19 patients: Level of sex hormones in severe COVID-19. Medicine. 2021;100:34. doi: 10.1097/MD.0000000000027072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryce C, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod. Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzahrani AS, et al. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr. Pract. 2021;27(2):83–89. doi: 10.1016/j.eprac.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santana MF, et al. Case report: adrenal pathology findings in severe COVID-19: an autopsy study. The. Am. J. tropical Med. Hyg. 2020;103(4):1604. doi: 10.4269/ajtmh.20-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomo S, et al. Does dehydroepiandrosterone sulfate have a role in COVID-19 prognosis and treatment? Endocr. Regul. 2021;55(3):174–181. doi: 10.2478/enr-2021-0019. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliadi DA, et al. Pituitary–adrenal responses and glucocorticoid receptor expression in critically Ill patients with COVID-19. Int. J. Mol. Sci. 2021;22(21):11473. doi: 10.3390/ijms222111473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertagna X. Effects of chronic ACTH excess on human adrenal cortex. Front. Endocrinol. 2017;8:43. doi: 10.3389/fendo.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonen MS, et al. Assessment of neuroendocrine changes and hypothalamo-pituitary autoimmunity in patients with COVID-19. Horm. Metab. Res. 2022;54(03):153–161. doi: 10.1055/a-1764-1260. [DOI] [PubMed] [Google Scholar]