Abstract
There is substantial evidence linking the prefrontal cortex (PFC) to a variety of cognitive abilities, with adolescence being a critical period in its development. In the current study, we investigated the neural basis of differences in learning in pre-adolescent common marmosets. At 8 months old, marmosets were given anatomical and resting state MRI scans (n=24). At 9 months old, association learning and inhibitory control was tested using a ‘go/no go’ visual discrimination (VD) task. Marmosets were grouped into ‘learners’ (n=12) and ‘non-learners’ (n=12), and associations between cognitive performance and sub-regional PFC volumes, as well as PFC connectivity patterns, were investigated. ‘Learners’ had significantly (p<0.05) larger volumes of areas 11, 25, 47 and 32 than non-learners, although ‘non-learners’ had significantly larger volumes of areas 24a and 8v than ‘learners’. There was also a significant correlation between average % correct responses to the ‘punished’ stimulus and volume of area 47. Further, non-learners had significantly greater global PFC connections, as well as significantly greater numbers of connections between the PFC and basal ganglia, cerebellum and hippocampus, compared to non-learners. These results suggest that larger sub-regions of the orbitofrontal cortex and ventromedial PFC, as well more refined PFC connectivity patterns to other brain regions associated with learning, may be important in successful response inhibition. This study therefore offers new information on the neurodevelopment of individual differences in cognition during pre-adolescence in non-human primates.
Keywords: prefrontal cortex, neuroanatomy, learning, marmoset, development
1. Introduction
1.1. Development of the brain and cognition
The study of normal brain development and cognitive function in childhood and adolescence has gained increased attention, due to the importance of understanding neurodevelopmental disorders. The common marmoset is uniquely suited for such research (Fukushima et al, 2019), due to their sophisticated cognitive abilities (Saito, 2015), as well as similar brain organization (Chaplin et al, 2013) and patterns of aging to humans (Workman et al, 2019; Sawaik et al, 2018). Like humans, marmosets have a highly developed prefrontal cortex (PFC) (Paxinos et al, 2012; Roberts et al, 2007) and can successfully perform a range of prefrontal dependent tasks (eg. LaClair & Lacreuse, 2016), including those that require cognitive control, ie. goal-directed, rather than habitual responding (Balleine and O’Doherty, 2010), which can be assessed with asymmetric ‘go/no-go’ tasks (Iversen and Mishkin, 1970).
Adolescence, the period of physical and psychological maturation between childhood and adulthood (Sisk and Foster, 2004), is accompanied by significant structural and functional reorganization of the brain (review: Blakemore et al, 2010; Sisk and Foster, 2004; Resnick et al, 2007). Neuroimaging studies have found that grey matter volumes follow a U-shape trajectory, with widespread cell proliferation early in development (Tanaka et al, 2012). Volumes then start declining in adolescence, due to pruning of the extensive connectivity acquired in childhood (Anderson et al, 1995). Cognition also changes considerably at this time, including reward processing (van Duijvenvoorde et al, 2016) and inhibitory control (marmosets: Ash et al, 2020), with impulsivity showing an inverted U-shaped trajectory, peaking at the time of pubertal maturation (Steinberg and Monahan, 2007). Non-invasive MRI, combined with performance in tasks outside the scanner, provides the opportunity to investigate neural substrates of such behaviour during development (Poirier et al, 2021).
1.2. Sex differences in the brain and cognition
Similar brain maturational patterns and PFC volumes have been found in males and females (marmosets: Sawaik et al, 2018; Seki et al, 2017). However, grey matter volume has been found to peak earlier in females than males (rhesus macaques: Knickmeyer et al, 2010), which may relate to the timing of puberty (Lenroot et al, 2007). Steroid hormones could also promote sex differences in the brain and cognitive abilities (Goldman et al, 1974; Goldman, 1975), with studies in rhesus macaques finding that male infants develop reversal learning earlier than female infants. However, there were no sex differences in juveniles, suggesting that the OFC matures earlier in males than females, possibly due to higher androgen levels (review: Bachevalier and Hagger, 1991; Hagger et al, 1987).
1.3. PFC sub-regions and cognitive ability
Many studies have highlighed the crucial role of the PFC in learning (D’Esposito et al., 1995; Williams and Goldman-Rakic, 1995; Dias et al., 1996; Owen et al., 1996; review: Alvarez and Emory, 2006), with several studies in humans finding positive associations between PFC volume and cognitive functioning (Gunning-Dixon and Raz, 2003; Colom et al, 2013). Although there have been differences in results between studies of regional volume (Yuan and Raz, 2014), research has found consistent developmental correlations between PFC activation and cognitive performance in children (Moriguchi & Hiraki, 2009; 2011; 2013).
The PFC consists of multiple cortical areas that support different aspects of behaviour (Yuan and Raz, 2014), with lateral areas involved in higher-order rule-based behaviour, and ventral/medial areas involved in lower-order reinforcement learning (Murray et al, 2000), including suppression of irrelevant responses (Mazzola-Pomietto et al, 2009; Matthews et al, 2005). The orbitofrontal cortex (OFC) is particularly important for changing an established behaviour following unexpected outcomes (review: Schoenbaum et al, 2009; marmosets: Roberts, 2006; Rolls et al, 1994), and is most often associated with motivational control of goal-directed behaviour in non-human primates (Wallis and Miller, 2003; Gottfried et al, 2003; O’Doherty et al, 2002; Hikosaka and Watanabe, 2000; Hikosaka and Watanabe, 2004; Padoa-Schioppa and Assad, 2006). Human neuroimaging studies also implicate the dorsolateral PFC and anterior cingulate cortex (ACC) in instrumental approach-avoidance paradigms (Aupperle et al, 2015; Schlund et al, 2016; Croxson et al, 2009). However, relationships between cognition and specific sub-regions can be unclear (Roberts and Clarke, 2019).
1.3. PFC connectivity and cognitive ability
The PFC is highly interconnected to the rest of the cortex (Fuster, 2001), and so strong projections to other brain areas could promote cognitive functions. Connectedness with other regions associated with motivation, learning and memory, including limbic (ie. amygdala, hippocampus, thalamus, hypothalamus) and basal ganglia structures (review: Barbas, 2000), as well as the cerebellum (Van Overwalle et al, 2020), may therefore be better predictors of individual cognitive control than volume of isolated areas (review: Friedman and Robbins, 2022; Voss et al, 2010a,b; Barbas, 1992).
Resting state functional connectivity (rsFC) is a useful non-invasive tool to investigate whole-brain circuitry (Belcher et al, 2013), with brain networks underlying cognitive control thought to have especially high connectivity (Cole et al, 2010; Miller and Cohen, 2001). Studies have found that specific connections (eg. Jung and Haier, 2007), global connectivity (eg. van den Heuvel et al, 2009) and PFC sub-region global connectivity (Cole et al, 2012) are associated with intelligence. However, children and adolescents have broader, less focal activation patterns than adults, suggesting that there is more effective recruitment of neural resources with age, as activity decreases in regions other than those needed for the task (Casey et al, 2010). Adolescents also have heightened activity in limbic areas (eg. Galvan et al, 2006), and so immature connections between the PFC and limbic system may therefore underlie greater impulsivity in the peri-puberty period (Casey et al, 2008). However, functional connectivity patterns have not been examined in young (under 1 year old) marmosets in relation to learning.
In the current study, we aimed to determine the relationship between performance in a ‘go-no go’ visual discrimination task, requiring association learning and response inhibition (Iversen and Mishkin, 1970), and both PFC structure and connectivity in 8–9-month-old marmosets (equivalent to a 6–10 year old child), as well as look at sex differences. Based on previous research, we predicted that successful learners would have larger volumes of PFC sub-regions, particularly those in the OFC, than those who failed to learn the task, with possible sex differences due to earlier male development. We also predicted that successful learners would have more efficient connectivity patterns between the PFC and other brain regions, compared to non-learners.
2. Method
2.1. Subjects
Twenty-four common marmosets (11 male, 13 female) housed at the Wisconsin National Primate Research Centre (WNPRC) were studied. All were born into the colony at the WNPRC, and were family reared from birth. The animals were housed in their natal group, with group sizes ranging from 3–9. Each had results from cognitive testing at approx. 9 months old to compare with anatomical and resting state data at approx. 8 months old. Twelve marmosets learned the task, while twelve did not learn the task in the 6-week time period (including 2 marmosets who didn’t progress onto visual discrimination (VD) learning, as they failed to perform to criteria on the initial familiarization task- see section 2.4.2). Mean age at cognitive testing was not significantly different between males and females (mean M=9.71, mean F=9.84: t=−0.756, P=0.458), nor between learners and non-learners (mean L=9.74, mean nL=9.82: t=−0.453, P=0.655). Mean age at imaging was not significantly different between males and females (mean M=8.99, mean F=8.96: t=0.143, P=0.887), nor between learners and non-learners (mean L=8.93, mean nL=9.02: t=−0.576, P=0.571). Table 1 shows our study sample, split by sex and VD learning ability.
Table 1:
Subjects by sex (male/female) and visual discrimination task learning ability (learner/non-learner).
| Learner | Non-learner | Total | |
|---|---|---|---|
| Sample size | |||
| Sex | |||
| Male | 8 | 3 | 11 |
| Mean age at cognitive | |||
| testing | 9.7 months | 9.6 months | |
| Mean age at imaging | 9.0 months | 8.9 months | |
| Female | 4 | 9 | 13 |
| Mean age at cognitive | |||
| testing | 9.7 months | 9.9 months | |
| Mean age at imaging | 8.7 months | 9.1 months | |
| Total | 12 | 12 | 24 |
7 excluded from resting state data analysis (3 males and 4 females; 4 learners and 3 non-learners)
2.2. Housing and husbandry
Marmosets were housed in aluminium and wire mesh cages, containing a variety of cage furnishings, including a nest box, platforms, wooden perches and rope ladders, to encourage species-typical behaviour. Novel hanging objects were introduced on a rotational basis. The monkeys had auditory and olfactory contact with conspecifics, although visual contact was limited with a partition. Single cages measured 0.61m × 0.91m × 1.83m (containing families of 3); double cages measured 1.22m × 0.91m × 1.83m (containing families of 4 or more). The marmosets received 2 daily feedings: at approximately 8:00 they were given primate diet (Mazuri 5MI6, Land O’Lakes, Arden Hills, MN) and at 13:00 they were given a variety of fruit and vegetables. Extra protein, including yoghurt, eggs or nuts, were provided between these times, as well as other enrichment, such as gum or small parcels of fruit. To encourage participation during cognitive testing periods, the marmosets received their primate diet as normal, but extra protein and enrichment items were withheld until directly after testing. Water was provided ad libitum. Lighting was maintained on a 12:12 light/dark cycle (lights on at 6:30 to 18:30), with ambient temperature at approximately 27°C and humidity at approximately 50%. Every 2 weeks cages were changed and cleaned, and animals were weighed.
As part of an ongoing longitudinal study following individuals from infancy to young adulthood to look at the effect of dietary fat on development of adolescent depression, each marmoset was assigned to a diet condition at 6 months of age (prior to which all were given the standard low-fat colony diet). However, previous research has found no effects on metabolic parameters until at least 3 months after the onset of the new diet (Zeigler et al, 2013), and no diet differences in cognition were found at 9 months in the current study.
2.3. MRI scanning at 8 months old
2.3.1. Apparatus
We used the novel equipment and imaging technique fully described in Ziegler et al (2020) to obtain MRI scans of awake 8-month-old marmosets. A quadrature transmit/receive (T/R) volume coil was developed with an ID of 52 mm and length of 120 mm and integrated into a unique head and body restraining system. A semi-flexible head cradle, which could be slid onto the marmoset’s head, was anchored into the coil by side screws. A jacket was put on the marmoset, which secured them into the body tube with Velcro. To reduce noise, earplugs were pressed over the ear canal of the marmoset. The head coil and body tube were then mounted onto a chassis with a built-in shield, which was positioned in the magnet and locked into place. The entire procedure took approximately 15 minutes, following acclimation to the apparatus.
2.3.2. Acclimation sessions
Before the imaging session, monkeys were acclimated to the apparatus over 4 weeks, following the procedure detailed in Ziegler et al (2020). Briefly, all marmosets were given 2 acclimation sessions per week, lasting around 30–45 mins each. Marmosets were first introduced to the jacket and plexiglass restraint tube, then allowed to habituate to these for longer periods of time. They were then introduced to the head restraint and coil, before being restrained in the entire system of jacket, tube, head holder, ear plugs and coil for increasing periods of time. Finally, MRI noises were also added. The marmosets were given a favoured drink (Ensure ®, Abbott Laboratories) to reward their cooperative behaviour during acclimation (and in imaging sessions). A behavioural rating scale (from 1: quiet throughout restraint, to 8: agitated throughout restraint) was used to assess the individual animals’ tolerance to the restraint procedure, with a score of 4 and below considered acclimated for imaging, while animals with a score of 5 or above were not imaged. All subjects in the current study received a score of 2–4, and so were imaged.
On the day of the MRI session, the marmosets were removed from their families and transported to the Wisconsin Institute of Medical Research (WIMR), an approximately 5-minute drive from their housing location. Each marmoset was fitted into the restraint system while awake and positioned into the bore of the magnet.
2.3.3. Anatomical and resting state functional connectivity
All MR images were acquired on a 4.7 T Agilent (Agilent Technologies, Santa Clara, CA USA) small animal imaging scanner. High resolution T2 weighted anatomical scans of the full brain (34 slices; 1 mm thick; FOV 4.5 cm; matrix size 256×256; TR 2665 ms, TE 38 ms, TE 38 ms) were acquired using FSEMS pulse sequence. A single shot spin echo EPI resting state functional connectivity scan (34 slices; 1 mm thick; FOV 4.5 cm; matrix size 96 × 96; TR 3.0 s, TE 60 ms) was then acquired for 10 minutes.
A pre-defined MRI Marmoset Brain Atlas containing 234 brain regions was used for segmentation of different regions of the brain and labelled accordingly. Each subject was normalized for total brain volume for each area, and so values are based on percent of total brain volume (raw data was measured in mm^3). Fifteen areas were identified as sub-regions comprising the marmoset PFC, based on the Marmoset Brain Atlas (see Ziegler et al, 2020). Nomenclature for the areas of the PFC and their division within the PFC were based on Paxinos et al, 2000 (rhesus macaque atlas), looking at axial sections at the level of the genu of the corpus collosum and anterior to that (frontal pole). These areas generally correspond to those in humans (eg. Fukushima et al, 2019), and are associated with cognitive function in marmosets (eg. Roberts and Clarke, 2019). Table 2 shows each PFC area used in analysis, including the general PFC division they lie within, and figure 2 shows a dorsal view of each PFC area used in analysis.
Table 2:
Areas of the PFC and their division within the PFC (based on Paxinos et al, 2000).
| Area | PFC division |
|---|---|
| Area 10 | Orbitofrontal/ frontal pole/ventromedial/lateral |
| Area 11 | Orbitofrontal/medial |
| Area 13 | Orbitofrontal |
| Area 14c | Orbitofrontal/ventromedial/medial |
| Area 45 | Ventrolateral/lateral |
| Area 47 | Ventrolateral/Orbitofrontal |
| Area 24a | Anterior cingulate cortex/ventromedial |
| Area 25 | Anterior cingulate cortex/ventromedial |
| Area 32 | Medial/ventromedial |
| Area 8 | Dorsolateral |
| Area 8c | Dorsolateral |
| Area 8d | Dorsolateral |
| Area 8v | Dorsolateral |
| Area 9 | Dorsolateral/lateral |
| Area 46 | Dorsolateral/lateral |
Fig 2:
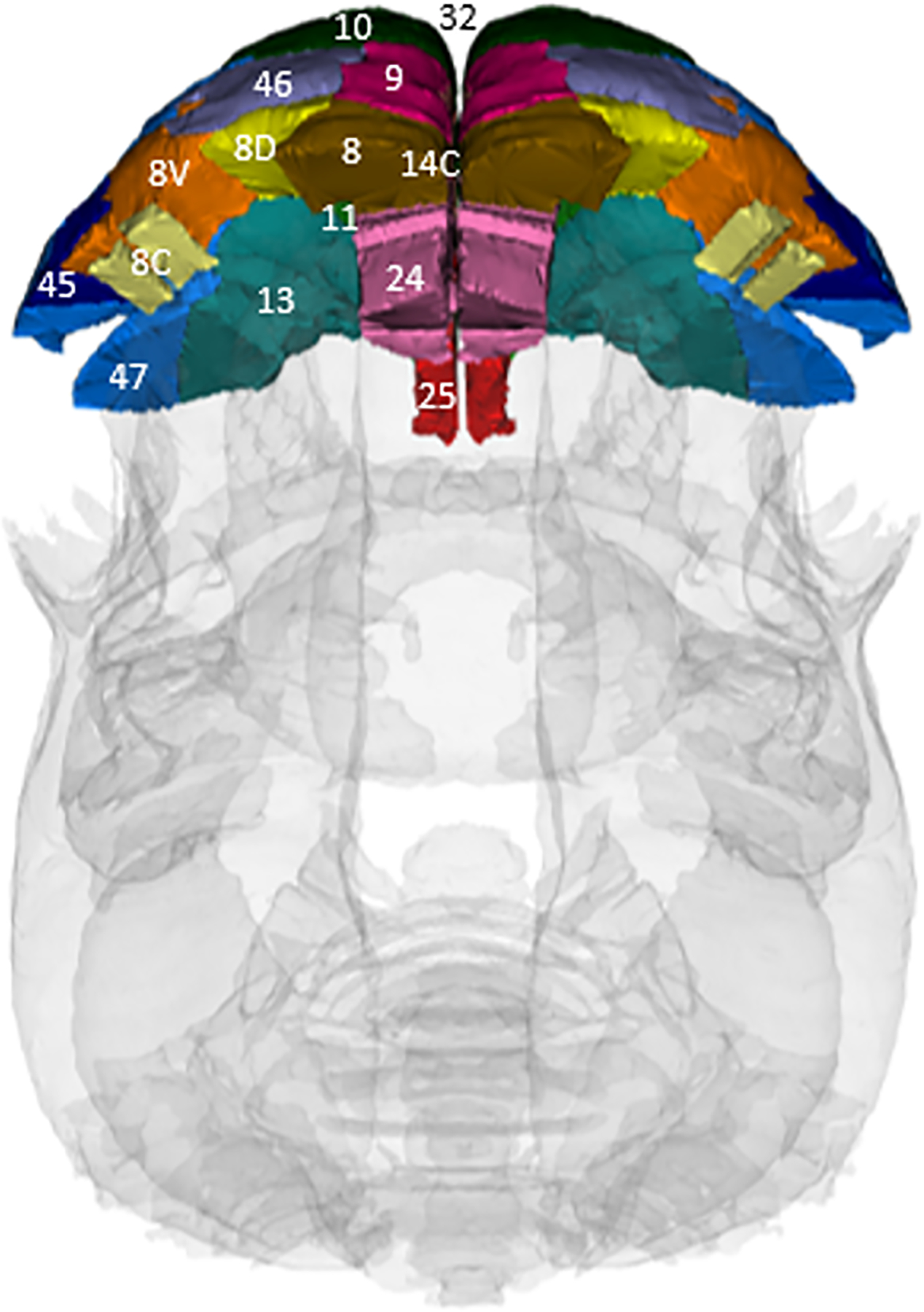
Dorsal view of the marmoset brain, showing each PFC area used in analysis, based on the Marmoset Brain Atlas.
2.3.4. rsFC image processing and analyses
Software used in the pre-processing of rsFC data include Analysis of Functional NeuroImages (AFNI_18.3.16), Advanced Normalization Tools (ANTS_3.0.0.0), MINC Toolkit (MINC_1.9.16) and FMRIB Software library (FSL_6.0.3). Matlab was used for the seed-to-seed functional connectivity analysis.
Functional data were first denoised due to the presence of motion spikes, which was followed by slice timing correction from an interleaved slice acquisition order. A 2-step affine motion correction procedure was applied using the first volume as a reference. To improve registration, a common template was constructed to realign each subject, and affine transformations were then applied to transform each subject to this new common template. The realigned subjects were registered to the MRI Marmoset Brain Atlas (Ekam Solutions LLC, Boston, MA USA) using 3 rotation factors, 3 translation factors and 3 scale factors. Seven marmosets were excluded (1 due to a corrupt file and 6 due to poor registration to the atlas), resulting in a sample size of 8 learners (5 male, 3 female) and 9 non-learners (3 male, 6 female)- see Table 1. Masking and skull-stripping was subsequently applied, using the same atlas as a reference. Each subject was then detrended and spatially smoothed (FWHM = 1.5mm) and passed through band-pass filtering (0.01 Hz ~ 0.1 Hz) to reduce the effects of low-frequency drift.
For each subject, the mean time series was computed for each brain region and then normalized across all volumes. Ten of these brain regions were not captured due to insufficient resolution. The subjects remaining were grouped into Learners and Non-learners and within group comparisons were carried out. The Pearson’s correlation coefficient was computed across all 224×224 pairs of brain regions remaining to determine the pairwise temporal-spatial correlations in spontaneous BOLD fluctuations. The Fisher’s Z transformation was first applied to the Pearson’s correlation coefficients to enforce normality. A two tailed t-test (alpha=0.05) was carried out to assess whether the mean correlation was significantly different from 0. The Benjamini-Hochberg correction was applied (alpha=0.05) to control the FDR. To assess the presence of clusters of functional networks, the k-nearest neighbors method was performed to each group’s Z-score matrix. A threshold of Z=2.3 was applied to avoid any spurious or weak node connections (Worsley et al, 1992) and improve statistical power.
2.3.5. Graph theory analysis: Degree centrality
Computation of graph theory network analysis was calculated via Gephi, an open-source network and visualization software (M.B, S.H, M.J. 2009). For both learners and non-learners, the absolute values of their respective symmetric connectivity matrices were loaded as undirected networks. The measurement of degree centrality (binary) examines the number of connections a specific node has to the network. Degree centrality is defined as:
where n is the number of rows in the matrix in the adjacency matrix A and the elements of the matrix are given by Aij, the number of edges between nodes i and j.
2.4. Cognitive testing at 9 months old
2.4.1. Apparatus
A custom-made testing box was designed, which easily attached to the front of the marmosets’ homecage (see Ash et al, 2020). Stimuli were presented on a tray, which was attached to the outside of the test box. Grey plastic horizontal rectangular blocks were used during testing (Fig 1). Reference blocks (rewarded S+ and unrewarded S−) were 2cm and 10cm long (both 2cm high × 2cm wide). In order to counterbalance the rewarded and unrewarded stimuli, half the animals were allocated the largest block and the other half were allocated the smallest block as S+ (Bethell et al, 2012). Prior preference testing using dichotomous choice tests revealed that banana cereal or animal crackers would be suitable, low calorie rewards that the marmosets were willing to work for. A small piece of the preferred food item was placed under each stimulus (S+, S−), to avoid olfactory cues.
Fig 1:

A 9-month-old marmoset performing the visual discrimination task inside the homecage testing box (insert: stimuli presented). He was required to touch the small block for a food reward and refrain from touching the large block.
2.4.2. Visual discrimination test
A single researcher conducted all cognitive testing sessions in the homecage, so animals remained in their family environment. Each marmoset received habitation to the test box and stimuli at 3 months old (see Ash et al, 2020 for full details). At 9 months old, the marmosets were allowed to re-familiarize themselves with the testing box and stimuli, during which time they were encouraged to reach out of the testing box to touch the S+ for a food reward (which was manually uncovered for access), until they obtained criterion (80% correct trials on 3 consecutive days). Correct S+ responses was therefore a test of motivation to perform the task.
Sessions were carried out once a day, 4 days a week (Monday, Tuesday, Thursday, Friday) between 10:00–13:00 (between am. and pm. feedings). If there were persistent escape attempts for more than 10 seconds, the marmoset was immediately allowed to leave the testing box, given a break and encouraged to complete the session once calm. If the marmoset continued their escape attempts, the session was repeated at a later date. All sessions lasted for 15 minutes, or if the marmoset had earned the maximum number of rewards (10 pieces).
Visual discrimination (VD) training sessions then involved ‘go/no go’ trials, in which single stimuli were presented (Burman et al, 2008), with a 2 sec inter-trial interval for responses. Correct ‘go’ responses to S+ (a touch of the stimulus) were rewarded with access to a treat on a 100% fixed ratio schedule. If there was no response within the 2 sec period, the next trial was presented. Correct ‘no go’ responses to S− (no touch of the stimulus) were not rewarded with a treat (ie. it was not uncovered for access). Incorrect ‘go’ responses to S− were followed by a 5 sec time-out ‘punishment’ (following Pryce et al, 2004). Correct S− responses was therefore a test of inhibition. As touching the S− would result in a timeout, the ‘reward’ for correctly with-holding a response was a quicker inter-trial period.
Twenty trials were evenly divided between S+ and S− sizes (10 rewarded, 10 unrewarded) on a pseudorandom schedule. No more than 2 rewarded or unrewarded sizes were presented consecutively (Burman e al, 2008), and the first and last trials were always rewarded. The marmosets were considered to have learned the visual discrimination task when they were responding correctly on 80% of S+ trials and 80% of S− trials (Bethell et al, 2012) on 3 consecutive sessions. A 6-week learning period was imposed.
Responses were video recorded and scored following the session. The number of sessions to criterion (from first to last day), cumulative number of errors to reach criterion (sum of incorrect go/no-go responses over all sessions), and average % correct responses to S+ and S− were recorded as an indication of learning ability. Although we made every effort to encourage each individual to complete the task, they were never forced to perform. Those that did perform (ie. reached criteria on the familiarization task and continued to respond during VD testing, although failed to learn the task in the time) were given a ceiling value of 24 (maximum number of sessions available). Figure 1 shows a pre-adolescent marmoset performing the cognitive task inside the testing box (inset: stimuli presented during the task).
2.5. Statistical analysis
2.5.1. Sex and learner differences in learning measures
As sample sizes were too small when split by sex and learning group, learning measures were assessed separately for males/females and learners/non-learners. Due to non-normality of data, Mann-Whitney U tests were conducted to look at differences between males and females, as well as differences between learners and non-learners, in the 4 learning measures.
2.5.2. Effect of sex and learning ability on PFC total and sub-regional volumes
As PFC volume data was normally distributed (assessed using Kolmogorov Smirnov tests), independent samples t tests were conducted to examine sex (male/female) and learning ability (learner/ non-learner) effects on whole PFC normalized volume.
A repeated measures ANOVA with between subject of sex and within subject of PFC sub-region was conducted to examine main effects and interactions. A repeated measures ANOVA with between subject of learning ability and within subject of PFC region was also conducted to examine main effects and interactions. Follow up independent samples t tests were carried out to understand any interaction effects.
2.5.3. Associations between learning measures and sub-regional PFC volumes
Each learning parameter was also assessed separately, as individuals may differ in their motivation (eg. average % correct s+) or impulse control (eg. average % correct s−). Due to non-normality of learning parameters, we used Spearman’s rank correlations to assess the relationship between normalized volume of PFC sub-regions and number of sessions to criterion, cumulative number of errors to reach criterion, and average % correct responses to S+ and S−. Statistical analyses were conducted in SPSS, and level of significance was ≤0.05. Although multiple tests were carried out, Bonferroni adjustments were not made, to allow maximum information to be extracted from the data, and independent assessment of the validity of results (Caldwell et al., 2005).
2.5.4. Effect of learning ability on PFC rs functional connectivity
Statistical analysis of the graph theory methods was calculated via GraphPad Prism version 9.0.0 for MacOS, GraphPad Software, San Diego, California USA, www.graphpad.com. Normality tests of learner and non-learner neural regions were performed to examine if parametric or non-parametric assumptions were required. Shapiro-Wilk’s tests were performed to examine normality assumption. Regional degree centrality p-values that were greater than 0.05 were assumed to be normal. After assumptions of normality were validated, unpaired t tests were used to compare degree centrality differences between groups. When necessary, a nonparametric Mann-Whitney test was performed if there was evidence against the normality assumption.
Global degree centrality analysis measured nodal connections to the entire network, while local analysis (sparcification) measured degree centrality exclusively between regions. Global and isolated PFC connections were examined, as well as local PFC connections to the limbic system (amygdala, hippocampus, thalamus and hypothalamus), basal ganglia and cerebellum. Choice to look at these particular regions was driven by evidence in the literature. A list of the nodes of these regions are detailed in Supplementary Material 1.
2.6. Ethics statement
Housing conditions were approved by the Institutional Animal Care and Use Committee (IACUC) of the Office of Vice Chancellor for Research and Graduate Education at the University of Wisconsin, Madison. The facility meets ALAAC approval and USDA standards. The current study meets the standards and approval of the IACUC, with funding from NIH HD086057, and complies with legal and ethical requirements in the USA. The study adheres to the American Society of Primatologists Principles for the Ethical Treatment of Non-human Primates, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
3. Results
3.1. Sex and learner differences in learning measures
Number of sessions to criterion was significantly higher in females than males (U=26.5, P=0.022), although there were no significant differences between males in females in total number of errors (U=31.50, P=0.060), average % correct s+ (U=35.00, P=0.099) and average % correct s− (U=44.00, P=0.291).
Significant differences were found between learners and non-learners in all 4 measures examined. Sessions to criterion and cumulative errors to criterion were significantly higher for non-learners than learners (U=2.00, P<0.001; U=4.00, P<0.001). Average % correct s+ and average % correct s− were significantly lower for non-learners than learners (U=9.00, P<0.001; U=17.50, P=0.005). Table 3 shows descriptive statistics for the 4 learning measures for males and females, as well as learners and non-learners.
Table 3:
Descriptive statistics for males and females, as well as learners and non-learners for all 4 learning measures examined (*highlights where groups are significantly different).
| Males | Females | Learners | Non-learners | |
|---|---|---|---|---|
| Sessions to criterion | ||||
| Mean | 16.30 | 20.75 | 14.83 | 23.40 |
| SD | 4.85 | 4.25 | 2.79 | 1.90 |
| Median | 14.00 | 24.00 | 14.00 | 24.00 |
| Min | 11.00 | 14.00 | 11.00 | 18.00 |
| Max | 24.00 | 24.00 | 21.00 | 24.00 |
| Errors to criterion | ||||
| Mean | 112.00 | 147.50 | 90.33 | 180.60 |
| SD | 62.26 | 51.69 | 25.94 | 46.32 |
| Median | 84.50 | 132.50 | 84.50 | 183.00 |
| Min | 61.00 | 79.00 | 61.00 | 107.00 |
| Max | 246.00 | 240.00 | 156.00 | 246.00 |
| Average % correct s+ (motivation/attention) | ||||
| Mean | 78.29 | 66.44 | 87.40 | 53.15 |
| SD | 23.59 | 25.52 | 7.12 | 25.78 |
| Median | 86.93 | 78.71 | 88.07 | 53.76 |
| Min | 22.00 | 20.83 | 72.00 | 20.83 |
| Max | 98.18 | 88.13 | 98.18 | 86.67 |
| Average % correct s− (inhibition) | ||||
| Mean | 47.26 | 39.35 | 52.16 | 31.90 |
| SD | 13.53 | 17.68 | 5.17 | 17.93 |
| Median | 50.35 | 47.23 | 52.00 | 33.32 |
| Min | 14.00 | 5.00 | 43.57 | 5.00 |
| Max | 61.88 | 61.67 | 61.88 | 61.67 |
3.2. Effect of sex and learning ability on PFC sub-regional volumes
Independent samples t tests found no significant sex effect (t=1.442, P=0.163) nor any significant learner effect (t=1.599, P=0.124) on normalized whole PFC volume.
A repeated measures ANOVA with between subject effect of sex and within subject effect of PFC found a significant effect of PFC region (F(14)=769.29, P<0.001), but no effect of sex (F(1)=2.08, P=0.163) and no PFC region × sex interaction (F(14)=0.910, P=0.548). No follow-up tests were therefore conducted.
A repeated measures ANOVA with between subject effect of learning ability and within subject effect of PFC region found a significant effect of PFC region (F(14)=830.823, p<0.001). There was also a significant PFC region × learning group interaction (F(14)=2.66, P=0.001), suggesting that specific PFC volumes depend on learning group. There was no individual effect of learning ability (F(1)=2.56, P=0.124).
Independent samples t tests were therefore conducted on each normalized PFC sub-regional volume to find which were significantly different between learning groups. Significant differences were found between learners and non-learners for 6 sub-regional volumes. Volumes of area 11 (t=2.877, P=0.009), area 25 (t=2.121, P=0.045), area 47 (t=2.197, P=0.039) and area 32 medial PFC (t=2.233, P=0.036) were significantly larger for learners than non-learners. Volumes of area 24 anterior cingulate cortex (t=−2.336, P=0.029) and area 8v (t=−2.159, P=0.042) were significantly larger for non-learners than learners.
No significant differences were found between learners and non-learners for volumes of area 10 (t=−0.383, P=0.767), area 13 (t=0.377, P=0.709), area 14c (t=−0.997, P=0.330), area 45 (t=1.093, P=0.286), area 46 (t=−0.809, P=0.427), area 8 (t=−1.586, P=0.127), area 8c (t=1.407, P=0.173), area 8d (t=0.189, P=0.852) and area 9 (t=1.109, P=0.279). Figure 3 displays the PFC sub-regions with significantly different volumes between VD task learners and non-learners, and Table 4 shows descriptive statistics for total PFC volume and sub-regional volumes for learners and non-learners, as well as males and females.
Fig 3:
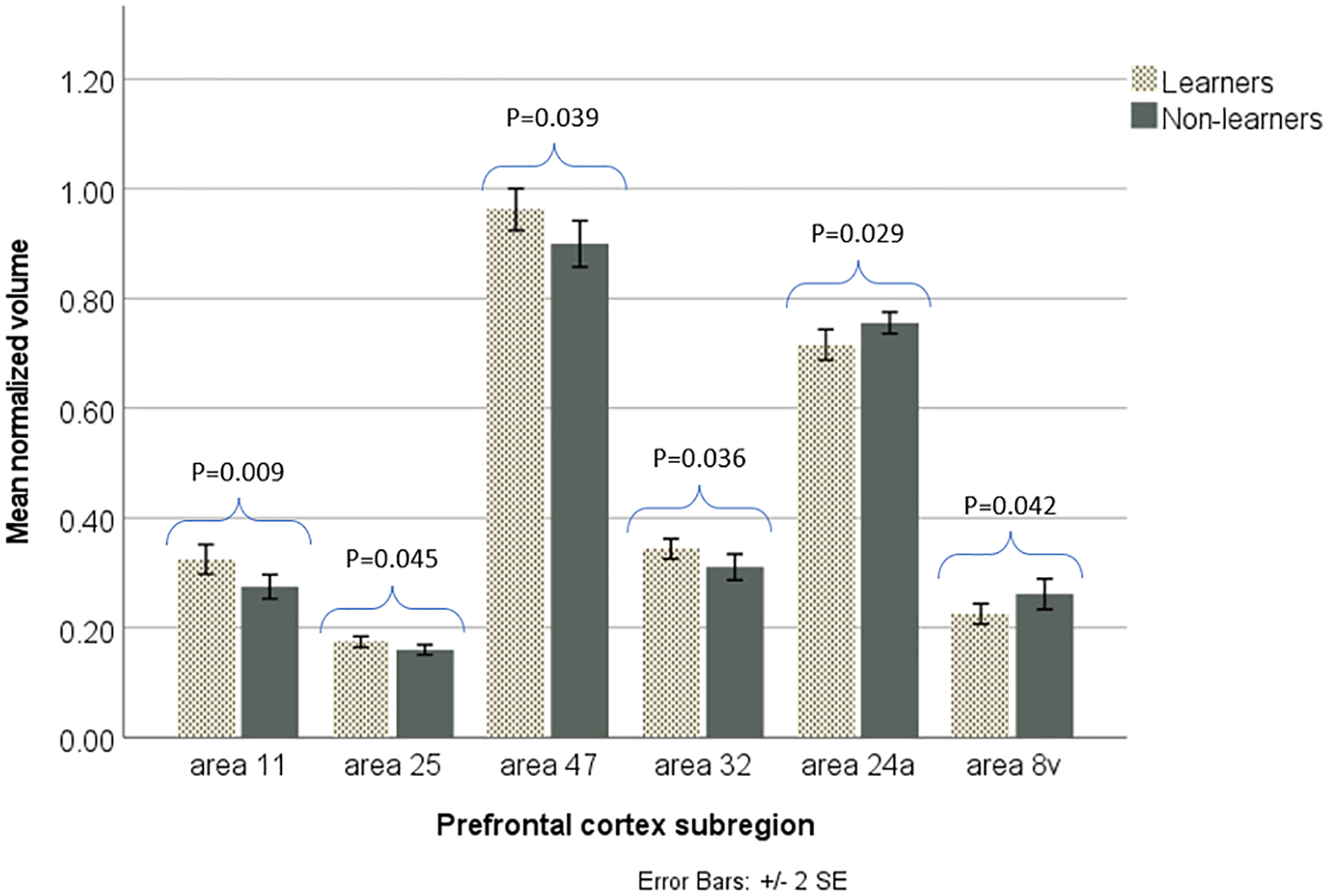
Significant effect (p<0.05) of learning ability on normalized volumes of area 11 (an orbitofrontal region), area 25 (a ventromedial PFC region), area 47 (an orbitofrontal/ventrolateral region), area 32 (medial PFC), area 24a (anterior cingulate cortex) and area 8v (a dorsolateral region) (n=24; 12 learners, 12 non-learners). Learners had significantly larger volumes of areas 11, 25, 47 and 32 than non-learners, while non-learners had a significantly larger volumes of areas 24a and 8v than learners.
Table 4:
Means and SDs for total PFC volume and each sub-regional PFC volume for males and females, as well as learners and non-learners (*highlights where groups are significantly different).
| Males | Females | Learners | Non-learners | |
|---|---|---|---|---|
| Whole PFC | ||||
| Mean | 5.564 | 5.510 | 5.564 | 5.505 |
| SD | 0.096 | 0.088 | 0.091 | 0.090 |
| Area 10 | ||||
| Mean | 0.771 | 0.762 | 0.761 | 0.771 |
| SD | 0.068 | 0.092 | 0.064 | 0.097 |
| Area 11 | ||||
| Mean | 0.306 | 0.294 | 0.325 | 0.275 |
| SD | 0.049 | 0.050 | 0.047 | 0.038 |
| Area 13 | ||||
| Mean | 0.558 | 0.546 | 0.556 | 0.548 |
| SD | 0.046 | 0.052 | 0.037 | 0.059 |
| Area 14c | ||||
| Mean | 0.256 | 0.273 | 0.258 | 0.272 |
| SD | 0.029 | 0.034 | 0.035 | 0.029 |
| Area 45 | ||||
| Mean | 0.166 | 0.173 | 0.176 | 0.164 |
| SD | 0.022 | 0.033 | 0.030 | 0.025 |
| Area 47 | ||||
| Mean | 0.959 | 0.908 | 0.962 | 0.900 |
| SD | 0.070 | 0.074 | 0.066 | 0.073 |
| Area 24a | ||||
| Mean | 0.739 | 0.733 | 0.716 | 0.756 |
| SD | 0.044 | 0.049 | 0.048 | 0.034 |
| Area 25 | ||||
| Mean | 0.167 | 0.168 | 0.175 | 0.160 |
| SD | 0.017 | 0.019 | 0.017 | 0.016 |
| Area 32 | ||||
| Mean | 0.340 | 0.317 | 0.344 | 0.311 |
| SD | 0.024 | 0.048 | 0.032 | 0.041 |
| Area 8 | ||||
| Mean | 0.255 | 0.268 | 0.248 | 0.277 |
| SD | 0.048 | 0.046 | 0.044 | 0.046 |
| Area 8c | ||||
| Mean | 0.034 | 0.034 | 0.040 | 0.028 |
| SD | 0.022 | 0.020 | 0.019 | 0.021 |
| Area 8d | ||||
| Mean | 0.125 | 0.129 | 0.128 | 0.126 |
| SD | 0.022 | 0.025 | 0.022 | 0.025 |
| Area 8v | ||||
| Mean | 0.233 | 0.253 | 0.225 | 0.262 |
| SD | 0.040 | 0.047 | 0.032 | 0.048 |
| Area 9 | ||||
| Mean | 0.271 | 0.259 | 0.269 | 0.260 |
| SD | 0.015 | 0.020 | 0.013 | 0.022 |
| Area 46 | ||||
| Mean | 0.383 | 0.393 | 0.381 | 0.396 |
| SD | 0.042 | 0.047 | 0.041 | 0.047 |
3.3. Correlations between learning and sub-regional PFC volumes
Spearman’s rank correlations revealed a significant moderate positive association between average % of correct responses to S− and normalized volume of area 47 (r= 0.429, p=0.046, p=22) (see Fig 4). N=22, due to 2 marmosets not progressing onto the VD task from the initial familiarization task. There were no other significant correlations between any learning measure and either PFC sub-regional or total PFC volumes. Table 5 shows all correlations between the PFC (whole and sub-regional volumes) and the 4 learning measures examined in the VD task.
Fig 4:
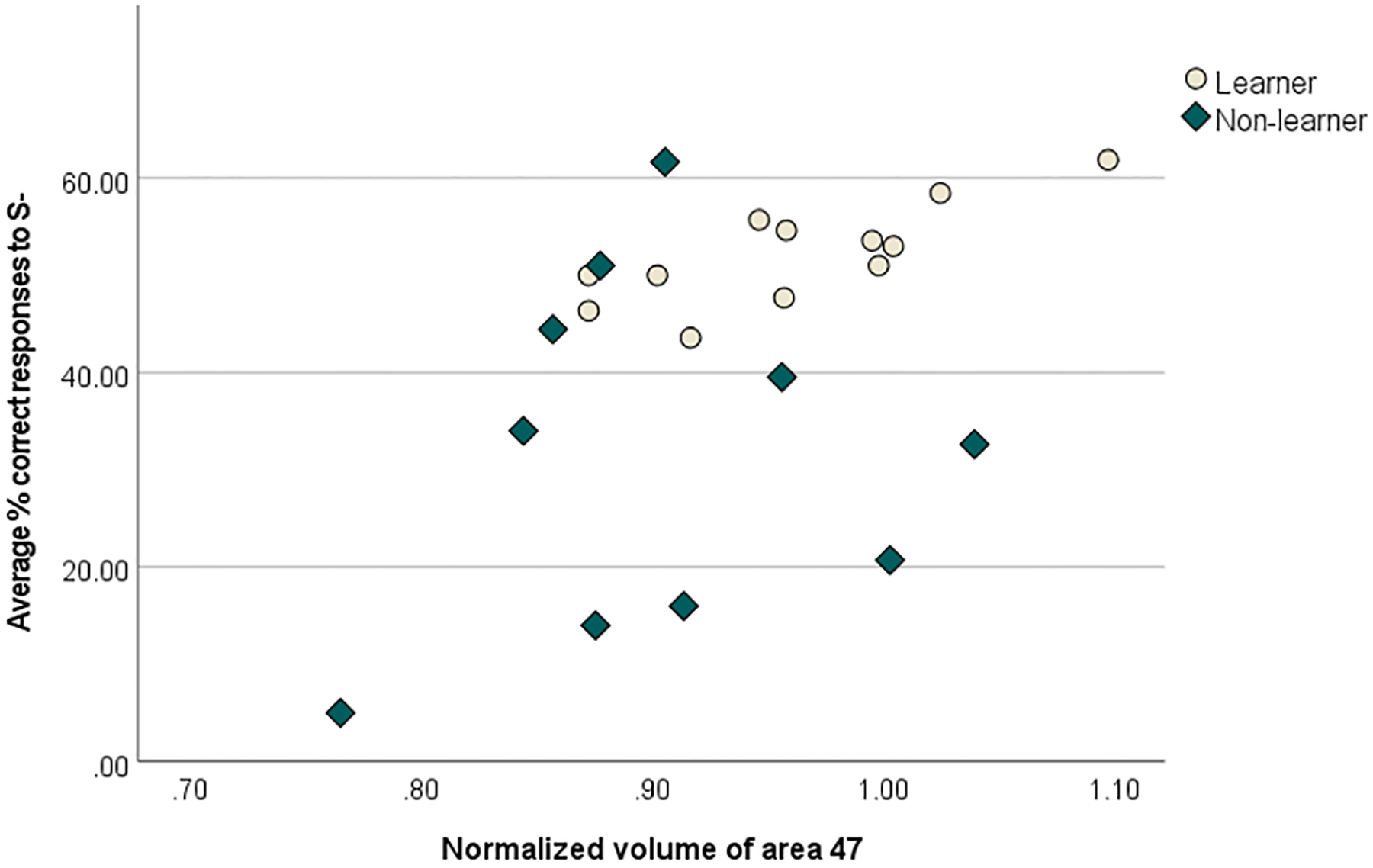
Significant (p<0.05) positive Spearman’s rank correlation between average % correct responses to S− (the ‘punished’ stimulus) in a visual discrimination task and normalized volume of area 47 (an orbitofrontal/ventrolateral region) (n=22; 12 learners, 10 non-learners). Larger volumes were associated with greater average correct responses to S−.
Table 5:
All Spearman’s rank correlational results between the 4 learning measures and PFC total/ subregional volumes (*highlights where results are significant).
| Sessions to criterion | Total errors | Average % correct s+ | Average % correct s− | |
|---|---|---|---|---|
| Whole PFC | ||||
| r | −0.14, | −0.207, | 0.224, | 0.275, |
| p | 0.44 | 0.356 | 0.316 | 0.215 |
| Area 10 | ||||
| r | 0.132 | 0.022 | 0.018 | −0.220 |
| p | 0.559 | 0.922 | 0.938 | 0.324 |
| Area 11 | ||||
| r | −0.277 | −0.246 | 0.179 | 0.283 |
| p | 0.213 | 0.270 | 0.425 | 0.202 |
| Area 13 | ||||
| r | −0.308 | −0.244 | 0.228 | 0.288 |
| p | 0.163 | 0.274 | 0.308 | 0.194 |
| Area 14c | ||||
| r | 0.104 | 0.243 | −0.375 | −0.119 |
| p | 0.645 | 0.275 | 0.085 | 0.597 |
| Area 45 | ||||
| r | 0.085 | −0.024 | 0.170 | −0.005 |
| p | 0.708 | 0.915 | 0.450 | 0.982 |
| Area 47 | ||||
| r | −0.221 | −0.261 | 0.249 | 0.429 |
| p | 0.332 | 0.241 | 0.264 | 0.046 |
| Area 24a | ||||
| r | 0.236 | 0.282 | −0.207 | −0.338 |
| p | 0.291 | 0.204 | 0.355 | 0.123 |
| Area 25 | ||||
| r | −0.151 | −0.141 | 0.112 | 0.241 |
| p | 0.502 | 0.531 | 0.619 | 0.279 |
| Area 32 | ||||
| r | −0.380 | −0.351 | 0.290 | 0.249 |
| p | 0.081 | 0.109 | 0.191 | 0.265 |
| Area 8 | ||||
| r | 0.176 | 0.071 | 0.085 | −0.111 |
| p | 0.434 | 0.753 | 0.706 | 0.624 |
| Area 8c | ||||
| r | −0.404 | −0.278 | 0.264 | 0.353 |
| p | 0.062 | 0.210 | 0.235 | 0.107 |
| Area 8d | ||||
| r | 0.183 | 0.094 | −0.134 | 0.134 |
| p | 0.414 | 0.676 | 0.553 | 0.551 |
| Area 8v | ||||
| r | 0.373 | 0.279 | −0.273 | −0.225 |
| p | 0.087 | 0.209 | 0.219 | 0.314 |
| Area 9 | ||||
| r | −0.157 | −0.138 | 0.018 | 0.008 |
| p | 0.484 | 0.541 | 0.938 | 0.972 |
| Area 46 | ||||
| r | 0.040 | 0.106 | −0.077 | −0.140 |
| p | 0.860 | 0.638 | 0.732 | 0.534 |
2 non-learners did not reach criterion on the initial familiarization task, and so were not included in correlations involving visual discrimination learning measures.
3.4. PFC isolated and global connections between learning groups
Mann Whitney U tests found no significant difference in degree centrality within the PFC between learners and non-learners (U=106.5, P=0.807), although there was a significant difference in PFC global degree centrality (u=35.50, P=0.0009), with non-learners having greater connectivity than learners. Figure 5 displays the isolated and global degree centrality of the PFC for learners and non-learners.
Fig 5:
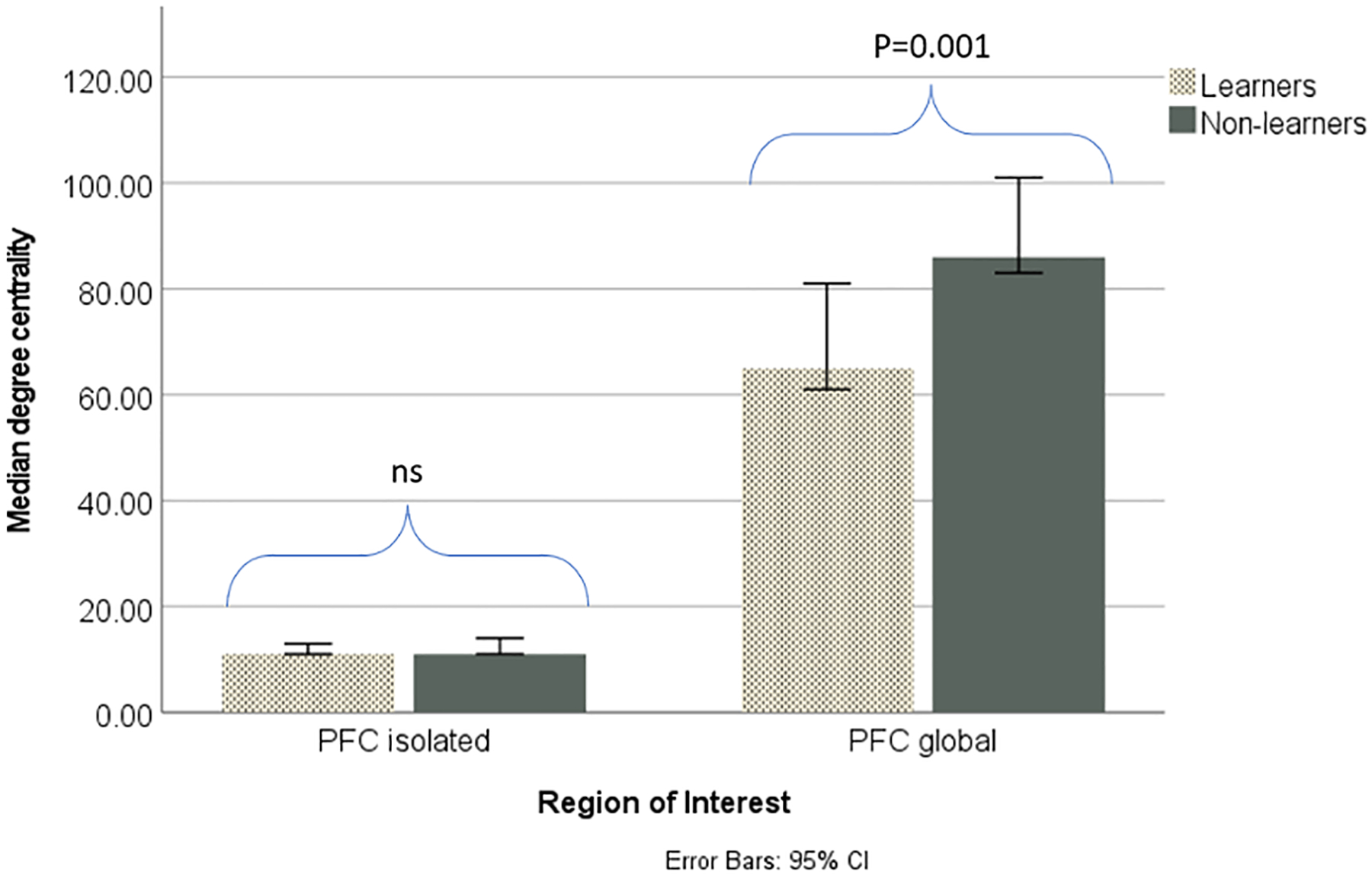
Isolated and global PFC connectivity in learners and non-learners. There was no significant difference between the groups in isolated PFC degree centrality, although non-learners had significantly (p<0.05) greater global PFC degree centrality than learners.
3.5. PFC connectivity to the limbic system, basal ganglia and cerebellum
Non-learners had a significantly greater number of connections between the PFC and basal ganglia (t=2.601, P=0.0118), PFC and hippocampus (U=135.5, P=0.0303), and PFC and cerebellum (t=2.501, P=0.0148) than non-learners. There were no significant differences in degree centrality of the PFC and amygdala (t=1.826, P=0.0746), PFC and hypothalamus (U=213, P=0.0516), nor PFC and thalamus (U=696, P=0.1804). Figure 6a and b displays the degree centrality of the PFC and other brain regions for learners and non-learners.
Fig 6:
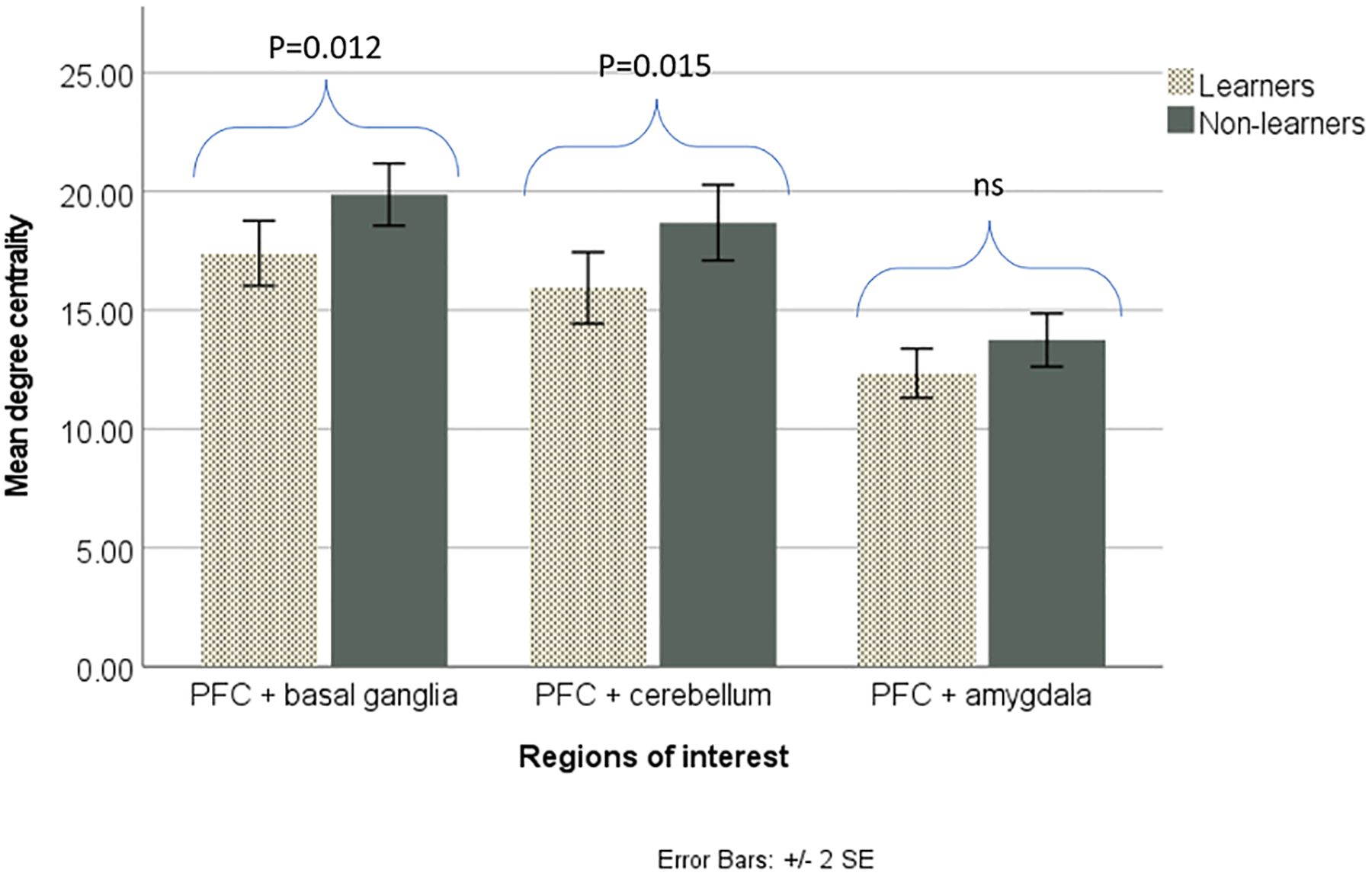
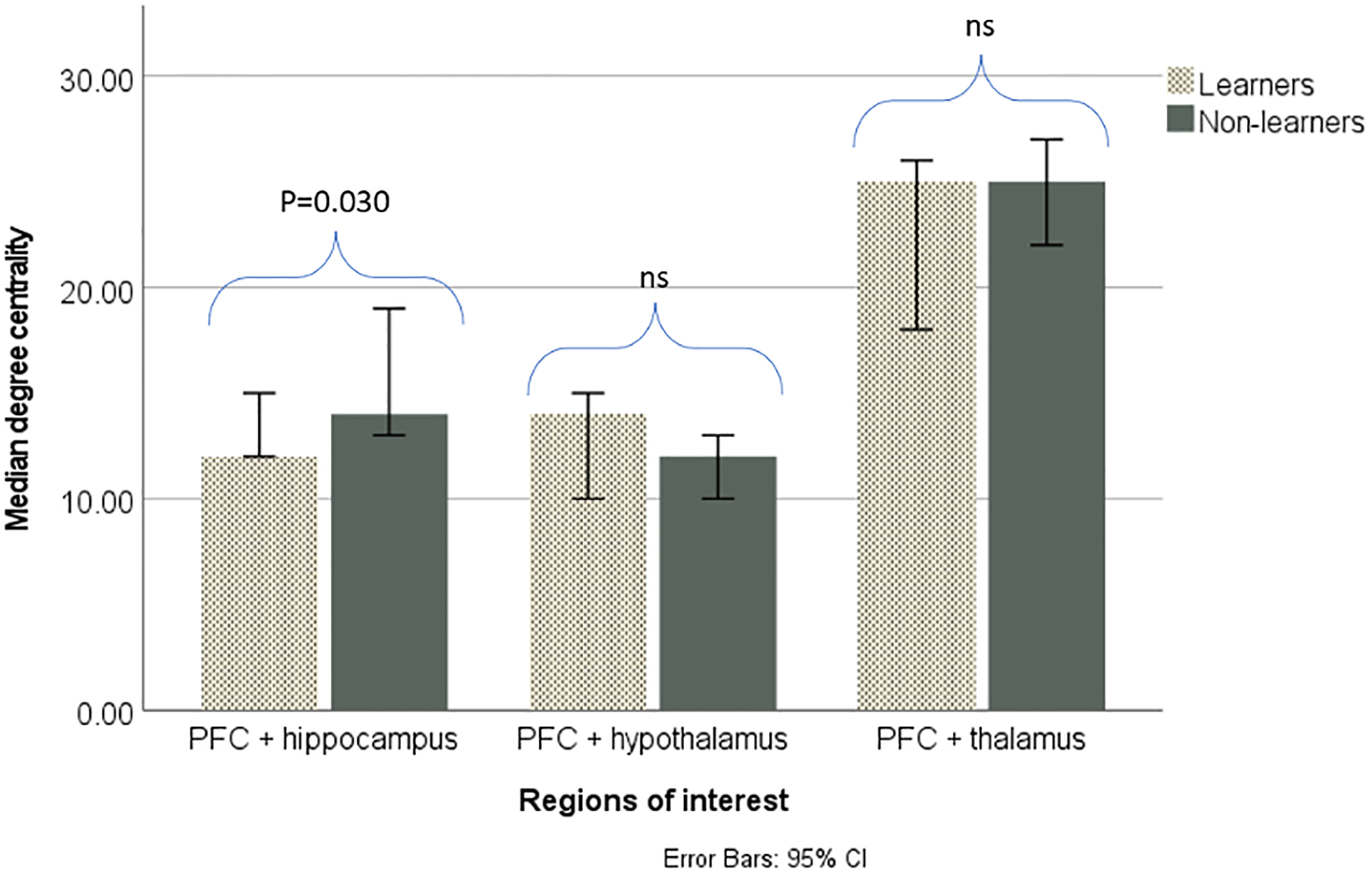
PFC connectivity with other brain regions of interest in learners and non-learners.
a. Non-learners had significantly (p<0.05) greater degree centrality between the PFC and basal ganglia, as well as between the PFC and cerebellum. There was no significant difference between the groups in degree centrality between the PFC and amygdala.
b. Non-learners had significantly (p<0.05) greater degree centrality between the PFC and hippocampus. There was no significant difference between the groups in degree centrality between the PFC and hypothalamus, nor between the PFC and thalamus.
4. Discussion
Using a non-invasive MRI method, we investigated whether PFC structural and functional differences were related to performance of a visual discrimination task in 8–9-month-old marmosets. Significant differences between learners and non-learners were found in sub-regional PFC volumes, as well as in degree centrality of PFC connections.
4.1. Sex differences in learning and PFC volume
In the current study, females had significantly more sessions to criterion than males, with more male learners than female learners in our pre-adolescent sample. Previous research in adult marmosets has found that males are better at PFC dependent tasks than females, which may be because female marmosets have enhanced habit formation, while male marmosets are more sensitive to punishment and so have greater inhibitory control (reversal tasks: LaClair and Lacreuse, 2016; LaClair et al, 2019). An even split between male and female learners and non-learners would however have been more ideal in our sample. Sex differences may be due to early influences of testosterone on OFC development (Bachevalier and Hagger, 1991; Hagger et al, 1987), which we will investigate in upcoming studies looking at the influence of hormone levels on learning ability.
There were no significant sex differences in total PFC volume or any PFC sub-regional volumes in the current study, which supports previous studies of cortical development in marmosets (Seki et al, 2017). There may however be differences in maturational rates between male and female monkeys in earlier stages of development, which were not seen in pre-adolescence (eg. infancy: Bachevalier and Hagger, 1991; Hagger et al, 1987).
4.2. Relationship between learning and PFC sub-regional volumes in adolescence
In the current study, learners had significantly less sessions and errors and more average % correct S+ and S− compared to non-learners. As half of our sample learnt the task and half failed to learn within the allotted time, this appears to be an important stage for the development of inhibitory control (eg. Diamond, 1990) and reward processing (van Duijvenvoorde et al, 2016). In marmosets, maximal PFC volume is reached during the pre-adolescent period (5–7 months) (Sawaik et al, 2018), which is also likely to be a critical period of experience dependent moulding of cortical architecture (Power et al, 2010).
Although we found no significant differences in total PFC volume between learning groups in the current study, distinct sub-regions appeared to play important roles (Roberts and Wallis, 2000). Learners in the current study had significantly larger volumes of area 11 (an orbitofrontal region), area 25 (a ventromedial region), area 32 (medial PFC) and area 47 (an OFC/ventrolateral region) than non-learners. Larger volumes of area 47 were also associated with greater average % correct responses to S− (the ‘punished’ stimulus).
Area 11 may play an important role in learning, with studies finding that neurons in this region must be active to make advantageous behavioural choices (macaques: Murray et al, 2015; marmosets: Jackson et al, 2016). Other sub-regions, including area 25, appear to have distinct roles in goal-directed behaviour (Duan et al, 2021), including sensitivity to punishment (Wallis et al, 2017). lPFC excitotoxic lesions that included area 47 in marmosets have been found to lead to a failure to disengage from an immediately preceding response (Walker et al, 2009; humans: Owen et al, 1996), while medial PFC lesions have been found to produce prolonged responses during extinction of a food-rewarded response (marmosets: Roberts and Wallis, 2000), suggesting these areas are involved in attentional control and knowledge of stimulus-reward relationships respectively. Therefore, individuals with larger volumes of these areas may be more sensitive to punishment, as well as better able to track actions and understand contingencies, and so are more successful in VD learning involving a speeded touch to ‘go’ stimuli and inhibition of responding to ‘no go’ stimuli.
Contrary to prediction, non-learners had significantly larger volumes of area 24a (anterior cingulate cortex- ACC) and area 8v (a dorsolateral area). Previous research has found that the ACC is necessary for detecting and acting upon changes in instrumental action-outcome contingencies (Tang et al, 2019; Pearson et al, 2011; Mansouri et al, 2009; Chudasama et al, 2013; Duan et al, 2021). It is possible that non-learners in the current study had an overactive ACC, which impaired their VD learning, or that the ACC is not as important in this task. For example, ACC activation was not associated with motor response inhibition in go/no go tasks in healthy subjects (eg. Watanabe et al, 2002). In the marmoset brain, area 8aV appears to be functionally dissociated from other area 8 regions (Fukushima et al, 2019). While 8b is associated with higher cognitive functions (Eradath et al, 2015), areas 8aD and 8aV are more associated with eye movements (Bruce et al, 1985).
4.3. Relationship between learning and PFC connectivity patterns in adolescence
Complex cognitive behaviours emerge from dynamic interactions of many distributed neural systems (eg. Barton, 2012), with considerable reorganisation of functional connectivity during maturation (Power et al, 2010). In the current study, spatial maps representing the strongest and most consistent coactivation between the PFC and other brain regions were examined. There was no significant difference in within (isolated) PFC connectivity between learners and non-learners. However, non-learners had significantly greater global PFC connectivity than learners. When looking at particular regions, non-learners had a significantly greater number of connections between the PFC and basal ganglia, PFC and hippocampus, and PFC and cerebellum than non-learners, suggesting that projections to and from these areas may be important in inhibitory control and association learning. The hippocampus is associated with long-term memory and has robust connections to the OFC (review: Amaral et al, 1990; Barbas et al, 1991). There are also indirect projections of basal ganglia to all prefrontal cortices (Joel and Weiner, 1994), an area which is involved in habit (stimulus-response) behaviour (Rolls, 2015). The cerebellum is involved in a range of cognitive processes, including associative learning (Cheng et al, 2008), with damage leading to disinhibition (Torrens et al, 2008), and impairments in sustained attention (Adel and Bergman, 2005) and executive function (review: O’Halloran et al, 2012).
Results of the current study therefore suggest that in learners, more extensive pruning during adolescence (Anderson et al, 1995) has led to more focussed PFC networks, which were associated with greater success in the cognitive task than non-learners, who appeared to have broader PFC connectivity patterns. Results are consistent with behavioural evidence in humans showing developmental differences in the brain depending on task success (eg. Carlson and Moses, 2001; Moriguchi & Hiraki, 2009; 2011; 2013). For example, in a go-no go task, adults have been found to be faster and more accurate than children, which corresponded to more refined activation patterns (Carlson & Moses, 2001). As well as the PFC, changes in the limbic system have been found during adolescence (Nunez et al, 2003, Hebbard et al, 2003), which may facilitate various cognitive processes. Further, research into ADHD, characterised by inattention, hyperactivity and impulsivity, have found associations between increased symptom severity and increased functional connectivity in certain pathways (Oldehinkel et al, 2016; Soros et al, 2019), including the cortico-striatal network (Sanefuji et al, 2016).
Larger PFC sub-regional volumes, as well as more refined PFC connectivity, in learners may therefore reflect greater development of inhibitory control. Although mechanisms underlying associations between cognitive ability and brain volume is unclear, larger areas have more neurones, which may increase cognitive capacity (Pakkenberg and Gundersen, 1997). While smaller volumes in non-learners could be due to greater pruning or increased myelination, the greater connectivity in this group supports the theory that non-learners may have delayed development, as the increased connectivity appears to be due to brain function, rather than being an epiphenomenon of greater grey matter.
4.5. Limitations and future research
Although differences were found between learners and non-learners in the current study, scanning at 8 months may have missed the peak of PFC development at approximately 6 months old. While studies in younger monkeys would be beneficial, we deemed the effects of handling and separation from the family too much of a risk, and it would have been difficult to securely restrain such small animals in the apparatus. As well as this, to demonstrate if a particular area of the PFC is involved in VD would require task-based fMRI, but it is difficult to give such manual tasks to a marmoset while in the scanner. However, as imaging took place before cognitive testing, MRI differences could be predictors of subsequent learning.
Head motion was common in our 8–9 month old marmosets, as it is in younger human subjects (eg. De Lacy et al (2019). However, scan time was kept short to reduce the chance of movement, and extensive quality control was performed (ie. elimination of any data that couldn’t be corrected). Other factors could also mediate brain-behaviour relationships, such as stress levels (Hanson et al, 2012), distractibility (Chadick et al, 2014) and behavioural traits (Schwartz et al, 2010; Ash et al, 2020). Future work could therefore measure and control for such factors.
As we looked at a single anatomical scan at one developmental time point, non-learners may have delayed development, although not permanent differences (Hanson et al, 2012). Using the techniques developed previously in Zeigler et al (2020), we plan to continue with-in subject longitudinal studies to characterize normal learning and brain development in marmosets, including whether certain individuals develop cognitive skills later than others or remain slower learners throughout life. This may be particularly useful in increasing knowledge of the role of puberty in determining brain changes (Knickmeyer et al, 2010), with imaging studies such as this allowing awake marmosets to be scanned repeatedly and non-invasively (Ferris et al, 2004).
4.5. Conclusion
The current study aimed to identify neural correlates of cognitive performance in pre-adolescent common marmosets, with results suggesting that successful learners have greater volumes of certain PFC sub-regions compared to non-learners, as well as more focussed connectivity between the PFC and other brain regions, including the limbic system. Findings also lend support to previous studies showing that there are specializations within regions of the orbitofrontal cortex and ventromedial PFC which guide decision making. The study therefore sheds new light on the origins of individual differences in cognition during pre-adolescence and provides the basis for future MRI work using marmosets as a model to examine factors that may have a long-term impact on neurodevelopment.
Supplementary Material
Acknowledgements
We thank the NIH for their funding and support of the project (R01 HD079493 to TEZ and RJC). This publication was made possible in part by NCRR/ORIP grants P51RR000167/ P51OD011106 to the Wisconsin National Primate Research Center (WNPRC), University of Wisconsin-Madison. We are grateful to Bruce Pape for manufacturing the cognitive testing apparatus, as well as all the staff at the WNPRC colony for their assistance and care of the marmosets, particularly Megan Sosa. We also thank Kirsten Solonika, Natalie Dukes, Taylor Haag and Gabriela de Faria Oliveira for their help with the habituation and imaging sessions.
Footnotes
Declaration of interest: none.
Data availability statement:
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
References
- Adel KA & Bergman R (2005). Functional neuroanatomy (2nd ed.). New York, NY: McGraw-Hill. [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E & Hof P (2001). The anterior cingulate cortex. The evolution of an interface between emotioɱn and cognition. Annals of the New York Academy of Science, 935, 107–117. [PubMed] [Google Scholar]
- Alvarez JA & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review, 16, 17–42. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Zola-Morgan S, Squire LR, Suzuki WA (1990). The perirhinal and parahippocampal cortices and medial temporal lobe memory function. In: Iwai E (Ed). Vision, memory, and the temporal lobe. New York: Elsevier Science; 149–161. [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS & Lewis DA (1995). Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience, 67, 7–22. [DOI] [PubMed] [Google Scholar]
- Ash H, Zeigler TE & Colman RJ (2020). Early learning in the common marmoset (Callithrix jacchus): Behavior in the family group is related to preadolescent cognitive performance. American Journal of Primatology, 82, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RI, Melrose AJ, Francisco A, Paulus MP & Stein MB (2015). Neural substrates of approach-avoidance conflict decision-making. Human Brain Mapping, 36, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J & Hagger C (1991). Sex differences in the development of learning abilities in primates. Psychoneuroendocrinology, 16, 177–188. [DOI] [PubMed] [Google Scholar]
- Balasundaram P & Avulakunta ID (2021). Human growth and development. StatPearls Publishing LLC, Florida. [PubMed] [Google Scholar]
- Balleine BW & O’Doherty JP (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35, 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H (2000). Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin, 52, 319–330. [DOI] [PubMed] [Google Scholar]
- Barbas H & De Olmos J (1990). Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology, 301, 1–23. [DOI] [PubMed] [Google Scholar]
- Barbas H & Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus, 5, 511–533. [DOI] [PubMed] [Google Scholar]
- Barbas H, Henion TH & Dermon CR (1991). Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology, 313, 65–94. [DOI] [PubMed] [Google Scholar]
- Barbas H (1992). Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. In: Chauvel P; Delgado-Escueta AV; Halgren E; Bancaud J (Eds). Advances in neurology, Vol. 57. New York: Raven Press, pp 91–115. [PubMed] [Google Scholar]
- Barton RA (2012). Embodied cognitive evolution and the cerebellum. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 367, 2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H & Damasio AR (1996). Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex, 6, 215–225. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, Silva AC & Stein EA (2013). Large-scale brain networks in the awake, truly resting marmoset monkey. The Journal of Neuroscience, 33, 16796–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y & Farol P (1994): Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry, 51, 477–484. [DOI] [PubMed] [Google Scholar]
- Benevento LA & Fallon JH (1975). The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). Journal of Comparative Neurology, 160, 339–362. [DOI] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A & Semple S (2012). Cognitive bias in a non-human primate: Husbandry procedures influence cognitive indicators of psychological well being in captive rhesus macaques. Animal Welfare, 21, 185–195. [Google Scholar]
- Blakemore S-J, Burnett S & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC & Stanton GB (1985). Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. Journal of Neurophysiology, 54, 714–734. [DOI] [PubMed] [Google Scholar]
- Burman OH, Parker R, Paul ES & Mendl M (2008). A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus. Animal Behaviour, 76, 801–809. [Google Scholar]
- Caldwell CA, Ruxton GD & Colegrave N (2005). Multiple test corrections. In Plowman A (Ed.) Zoo Research Guidelines: Statistics for typical zoo datasets. British and Irish Association of Zoos and Aquariums, London. [Google Scholar]
- Carlson SM & Moses LJ (2001). Individual differences in inhibitory control and children’s theory of mind. Child Development, 72, 1032–1053. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz A & Galvan A (2008). The adolescent brain. Developmental Review, 28, 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG & Cohen AO (2019). Development of the emotional brain. Neuroscience Letters, 693, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S & Cohen MM (2010). Adolescence: what do transmission, transition, and translation have to do with it? Neuron, 67, 749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick JZ, Zanto TP & Gazzaley A (2014). Structural and functional differences in medial prefrontal cortex underlie distractibility and suppression deficits in ageing. Nature Communications, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TA, Yi H, Soares JGM, Gattass R & Rosa MGP (2013). A conserved pattern of differential expansion of cortical areas in simian primates. The Journal of Neuroscience, 33, 15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA & Desmond JE (2008). Neural substrates underlying human delay and trace eyeblink conditioning. Proceedings of the National Academy of Sciences U.S.A. 105, 8108–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH & Murray EA (2013). The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cerebral Cortex, 23, 2884–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S & Schneider W (2010) Identifying the brain’s most globally connected regions. Neuroimage, 49, 3132–3148. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A & Braver TS (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. The Journal of Neuroscience, 32, 8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Burgaleta M, Roman FJ, Karama S, Alvarez-Linera J, Abad FJ, Martinez K, Quiroga MA & Haier RJ (2013). Neuroanatomic overlap between intelligence and cognitive factors: morphometry methods provide support for the key role of the frontal lobes. Neuroimage, 72, 143–152. [DOI] [PubMed] [Google Scholar]
- Croxson PI, Walton ME, O’Reilly JX, Behrens TEJ & Rushworth MFS (2009). Effort based cost-benefit valuation and the human brain. The Journal of Neuroscience, 29, 4531–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E & Rubia K (2012). A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex, 48, 194–215. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1994). Descartes error: emotion, reason and the human brain. Grosset/Putnum, New York. [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S & Grossman M (1995). The neural basis of the central executive system of working memory. Nature, 378, 279–281. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW & Roberts AC (1996). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380, 69–72. [DOI] [PubMed] [Google Scholar]
- Diamond A (1990). Developmental time course in human infants and infant monkeys, and the neural basis of inhibitory control in reaching. Annals of the New York Academy of Sciences, 608, 637–676. [DOI] [PubMed] [Google Scholar]
- Duan LY., Horst NK, Cranmore SA, Horiguchi N, Cardinal RN, Roberts AC & Robbins TW (2021). Controlling one’s world: identification of sub-regions of primate PFC underlying goal-directed behavior. Neuron, 109, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN & Emslie H (2000). A neural basis for general intelligence. Science, 289, 457–460. [DOI] [PubMed] [Google Scholar]
- Eradath MK, Matsumoto M, Matsumoto K, Tanaka K & Ichinohe N (2015). Anatomical inputs to sulcal portions of areas 9m and 8Bm in the macaque monkey. Frontiers in Neuroanatomy, 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Snowdon CT, King JA, Sullivan JM, Ziegler TE, Olson DP, Schulz-Darken NJ, Tannenbaum PL, Ludwig R, Wu Z, Einspanier A, Vaughan T & Duong T (2004). Activation of neural pathways associated with sexual arousal in non-human primates. Journal of Magnetic Resonance Imaging, 19, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP & Robbins TW (2022). The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology, 47, 72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Ichinohe N & Okano H (2019). Chapter 3: Neuroanatomy of the marmoset. In: Marini R, Wachtman L, Tardif S, Mansfield K & Fox J (eds). The Common Marmoset in Captivity and Biomedical Research. Academic Press, London. pp. 43–62. [Google Scholar]
- Fuster JM (2001). The prefrontal cortex- an update: Time is of the essence. Neuron, 30, 319–333. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G & Casey BJ (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26, 6885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Crawford t-I.T, Stoke,s LP, Galkin TW & Rosvold HE (1974). Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkeys. Science, 186, 540–542. [DOI] [PubMed] [Google Scholar]
- Goldman PS (1975). Age, sex, and experience related to the neural basis of cognitive development. In: Buchwald NA, Brazier MAB (Eds). Brain Mechanisms in Mental Retardation. Academic Press, New York, pp 379–392. [Google Scholar]
- Gottfried JA, O’Doherty J & Dolan RJ (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–1107. [DOI] [PubMed] [Google Scholar]
- Groman SM,. James AS, Seu E, Crawford MA, Harpster SN & Jentsch JD (2013). Monoamine levels within the orbitofrontal cortex and putamen interact to predict reversal learning performance. Biological psychiatry, 73, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM & Raz N (2003). Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia, 41, 1929–1941. [DOI] [PubMed] [Google Scholar]
- Hagger C, Bachevalier J & Bercu BB (1987). Sexual dimorphism in the development of habit formation: effects of perinatal steroid gonadal hormones. Neuroscience, 22, S520. [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ & Pollak SD (2012). Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. The Journal of Neuroscience, 32, 7917–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW & Harley CW (2003): Two organisational effects of pubertal testosterone in male rats: Transient social memory and a shift away from LTP following a tetanus in hippocampal CA1. Experimental Neurology, 182, 470–475. [DOI] [PubMed] [Google Scholar]
- Hikosaka K & Watanabe M (2000). Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex, 10, 263–271. [DOI] [PubMed] [Google Scholar]
- Hikosaka K & Watanabe M (2004). Long- and short-range reward expectancy in the primate orbitofrontal cortex. European Journal of Neuroscience, 19, 1046–1054. [DOI] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Fornito A, Park S, Yang YH, Park MH, Song IC, Sohn CH, Shin MS, Kim BN, Cho SC, Han DH, Cheong JH & Kim JW (2014). Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biological Psychiatry, 76, 656–63. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LS, van Hulzen KJ, Medland SE, Shumskaya E, Jahanshad N, Zeeuw P, Szekely E, Sudre G, Wolfers T, Onnink AM, Dammers JT, Mostert JC, Vives-Gilabert Y, Kohls G, Oberwelland E, Seitz J, Schulte-Rüther M, Ambrosino S, Doyle AE, Høvik MF, Dramsdahl M, Tamm L, van Erp TG, Dale A, Schork A, Conzelmann A, Zierhut K, Baur R, McCarthy H, Yoncheva YN, Cubillo A, Chantiluke K, Mehta MA, Paloyelis Y, Hohmann S, Baumeister S, Bramati I, Mattos P, Tovar-Moll F, Douglas P, Banaschewski T, Brandeis D, Kuntsi J, Asherson P, Rubia K, Kelly C, Martino AD, Milham MP, Castellanos FX, Frodl T, Zentis M, Lesch KP, Reif A, Pauli P, Jernigan TL, Haavik J, Plessen KJ, Lundervold AJ, Hugdahl K, Seidman LJ, Biederman J, Rommelse N, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Oosterlaan J, Polier GV, Konrad K, Vilarroya O, Ramos-Quiroga JA, Soliva JC, Durston S, Buitelaar JK, Faraone SV, Shaw P, Thompson PM & Franke B (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry, 4, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD & Mishkin M (1970). Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Research, 11, 376–386. [DOI] [PubMed] [Google Scholar]
- Jackson SA, Horst NK, Pears A, Robbins TW & Roberts AC (2016). Role of the perigenual anterior cingulate and orbitofrontal cortex in contingency learning in the marmoset. Cerebral Cortex, 26, 3273–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D & Weiner I (1994). The organization of the basal ganglia-thalamocortical circuits: Open interconnected rather than closed segregated. Neuroscience, 63, 363–379. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2011). Interactive specialization: a domain-general framework for human functional brain development? Developmental Cognitive Neuroscience, 1, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE & Haier RJ (2007). The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 30, 135–154, discussion 154–187. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL & Gilmore JH (2010). Maturational trajectories of cortical brain development through the pubertal transition: Unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cerebral Cortex, 20, 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaClair M & Lacreuse A (2016). Reversal learning in gonadectomized marmosets with and without hormone replacement: are males more sensitive to punishment? Animal Cognition, 19, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaClair M, Febo M, Nephew NJ, Gervais G, Poirier G, Workman K, Chumachenko S, Payne L, Moore MC, King JA & Lacreuse A (2019). Sex differences in cognitive flexibility and resting brain network states in middle-aged marmosets. Cognition and Behavior, 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM & Toga AW (2009). Neuroanatomical correlates of intelligence. Intelligence, 37, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM & Giedd JN (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage, 36, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yen CC, Szczupak D, Ye FQ, Leopold DA & Silva AC (2019). Anatomical and functional investigation of the marmoset default mode network. Nature Communications, 10, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K & Buckley MJ (2009) Conflict-induced behavioral adjustment: a clue to the executive functions of the prefrontal cortex. Nature Reviews Neuroscience, 10, 141–152. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Arce E & Paulus MP (2005). Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. NeuroReport, 16, 755–60. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin J-M, Anton J-L & Jeanningros R (2009). Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. Journal of Psychiatric Research, 43, 432–441. [DOI] [PubMed] [Google Scholar]
- M. B, S. H, M. J. (2009). Gephi : An Open Source Software for Exploring and Manipulating Networks. Presented at the International AAAI Conference on Weblogs and Social Media. [Google Scholar]
- McDaniel MA (2005). Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence, 33, 337–346. [Google Scholar]
- McEnaney KW & Butter CM (1969). Perseveration of responding and nonresponding in monkeys with orbital frontal ablations. Journal of Comparative and Physiological Psychology, 68, 558–561. [DOI] [PubMed] [Google Scholar]
- Miller EK & Cohen JD (2001). An integrative theory of prefrontal cortex function. Reviews in Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Miller EK & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y & Hiraki K (2009). Neural origin of cognitive shifting in young children. Proceedings of the National Academy of Sciences USA, 106, 6017–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y & Hiraki K (2011). Longitudinal development of prefrontal function during early childhood. Developmental Cognitive Neuroscience, 1, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y & Hiraki K (2013). Prefrontal cortex and executive function in young children: a review of NIRS studies. Frontiers in Human Neuroscience, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Moylan EJ, Saleem KS, Basile BM & Turchi J (2015). Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. eLife, 4, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ & Wise SP (2000). Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Experimental Brain Research, 133, 114–129. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K & Mataix-Cols D (2011). Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry, 168, 1154–63. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Huppenbauer CB, McAbee MD, Jurasaka JM & Don Carlos LL (2003). Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. Journal of Neurobiology, 56, 293–302. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Deichmann R, Critchley HD & Dolan RJ (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33, 815–826. [DOI] [PubMed] [Google Scholar]
- O’Halloran CJ Kinsella GJ & Storey E (2012). The cerebellum and neuropsychological functioning: A critical review. Journal of Clinical and Experimental Neuropsychology, 34, 35–56. [DOI] [PubMed] [Google Scholar]
- Oldehinkel M, Beckmann CF, Pruim RH, van Oort ES, Franke B, Hartman CA, Hoekstra PJ, Oosterlaan J, Heslenfeld D, Buitelaar JK & Mennes M (2016). Attention deficit/hyperactivity disorder symptoms coincide with altered striatal connectivity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 1, 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE & Robbins TW (1996). Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain, 119, 1597–1615. [DOI] [PubMed] [Google Scholar]
- Owen A, Doyon J, Petrides M & Evans A (1996). Planning and spatial working memory: a positron emission tomography study in humans. European Journal of Neuroscience, 8, 353–364. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C & Assad JA (2006). Neurons in orbitofrontal cortex encode economic value. Nature, 441, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B & Gundersen HJ (1997). Neocortical neuron number in humans: Effects of sex and age. The Journal of Comparative Neurology, 384, 312–320. [PubMed] [Google Scholar]
- Paxinos G, Huang X-F & Toga AW (2000). The rhesus monkey brain in stereotaxic coordinates. Academic Press, London. [Google Scholar]
- Paxinos G, Watson C, Petrides M, Rosa M & Tokuno H (2012). The marmoset brain in stereotaxic coordinates. Academic Press, London. [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY & Platt ML (2011) Posterior cingulate cortex: adapting behavior to a changing world. Trends in Cognitive Science, 15, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C, Hamed SB, Garcia-Saldivar P, Kwok SC, Meguerditchian A, Merchant H, Rogers J, Wells S & Fox AS (2021). Beyond MRI: on the scientific value of combining non-human primate neuroimaging with metadata. Neuroimage, 228, 117679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL & Petersen SE (2010). The development of human functional brain networks. Neuron, 67, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR & Feldon J (2004). Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry, 56, 72–79. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Lamar M & Driscoll I (2007). Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Annals of the New York Academy of Sciences, 1121, 562–575. [DOI] [PubMed] [Google Scholar]
- Roberts AC (2006). Primate orbitofrontal cortex and adaptive behaviour. Trends in Cognitive Science, 10, 83–90. [DOI] [PubMed] [Google Scholar]
- Roberts AC & Wallis JD (2000). Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cerebral Cortex, 10,252–262. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW & Everitt BJ (2007). Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. Journal of Comparative Neurology, 502, 86–112. [DOI] [PubMed] [Google Scholar]
- Roberts AC & Clarke HF (2019). Why we need nonhuman primates to study the role of ventromedial prefrontal cortex in the regulation of threat- and reward-elicited responses. PNAS, 116, 26297–26304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D & McGrath J (1994). Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry, 57, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2015). Limbic systems for emotion and for memory, but no single limbic system. Cortex, 62, 119–157. [DOI] [PubMed] [Google Scholar]
- Saito A (2015). The marmoset as a model for the study of primate parental behavior. Neuroscience Research, 93, 99–109. [DOI] [PubMed] [Google Scholar]
- Sanefuji M, Craig M, Parlatini V, Mehta MA, Murphy DG, Catani M, Cerliani L & Thiebaut de Schotten M (2016). Double-dissociation between the mechanism leading to impulsivity and inattention in attention deficit hyperactivity disorder: a resting-state functional connectivity study. Cortex, 86, 290–302. [DOI] [PubMed] [Google Scholar]
- Sawaik SJ, Shiba Y, Oikonomidis L, Windle CP, Santangelo AM, Grydeland H, Cockcroft G, Bullmore ET & Roberts AC (2018). Trajectories and milestones of cortical and subcortical development of the marmoset brain from infancy to adulthood. Cerebral Cortex, 28, 4440–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund MW, Brewer AT, Magee SK, Richman DM, Solomon S, Ludlum M & Dymond S (2016). The tipping point: Value differences and parallel dorsal-ventral frontal circuits gating human approach-avoidance behavior. Neuroimage, 136, 94–105. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA & Takahashi YK (2009). A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Perspectives, 10, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Moran LR, Viner JC, Covino JM, Kagan J, Stewart E, Snidman NC, Vangel MG & Wallace SR (2010). Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Archives of General Psychiatry, 67, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki F, Hikishima K, Komaki Y, Hata J, Uematsu A, Okahara N, Yamamoto M, Shinohara H, Sasaki E & Okano H (2017). Developmental trajectories of macroanatomical structures in common marmoset brain. Neuroscience, 364, 143–56. [DOI] [PubMed] [Google Scholar]
- Sisk CL & Foster DL (2004). The neural basis of puberty and adolescence. Nature Neuroscience, 7, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Siugzdaite R, Bathelt J, Holmes J & Astle DE (2020). Transdiagnostic brain mapping in developmental disorders. Current Biology, 30, 1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Hoxhaj E, Borel P, Sadohara C, Feige B, Matthies S, Muller HHO, Bachmann K, Schulze M & Philipsen A (2019). Hyperactivity/restlessness is associated with increased functional connectivity in adults with ADHD: a dimensional analysis of resting state fMRI. BMC Psychiatry, 19, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L & Monahan K (2007). Age differences in resistance to peer influence. Developmental Psychology, 43, 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P & Walhovd KB (2010). Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex, 20, 534–548. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Matsui M, Uematsu A, Noguchi K & Miyawaki T (2012). Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Developmental Neuroscience, 34, 477–487. [DOI] [PubMed] [Google Scholar]
- Tang W, Jbabdi S, Zhu Z, Cottaar M, Grisot G, Lehman JF, Yendiki A & Haber SN (2019). A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. eLife, 8, e43761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens L, Burns E, Stone J, Graham C, Wright H, Summers D, et al. (2008). Spinocerebellar ataxia type 8 in Scotland: Frequency, neurological, neuropsychological and neuropsychiatric findings. Acta Neurologica Scandinavica, 117, 41–48. [DOI] [PubMed] [Google Scholar]
- Tremblay L & Schultz W (1999). Relative reward preference in primate orbitofrontal cortex. Nature, 398, 704–708. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS & Hulshoff Pol HE (2009). Efficiency of functional brain networks and intellectual performance. Journal of Neuroscience, 29, 7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Peters S, Braams BR & Crone EA (2016). What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience and Biobehavioral Reviews, 70, 135–147. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JD, Michelutti M (2020). Consensus paper: Cerebellum and social cognition. The Cerebellum, 1e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wojcicki TR, Hu L, Szabo A, Klamm E, McAuley E & Kramer AF (2010a). Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia, 48, 1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF (2010b). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience, 2, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD & Miller EK (2003). Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience, 18, 2069–2081. [DOI] [PubMed] [Google Scholar]
- Wallis CU., Cardinal RN, Alexander L, Roberts AC & Clarke HF (2017). Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proceedings of the National Academy of Sciences, USA, 114, E4075–E4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Robbins TW & Roberts AC (2009). Response disengagement on a spatial self-ordered sequencing task: effects of regionally selective excitotoxic lesions and serotonin depletion within the prefrontal cortex. The Journal of Neuroscience, 29, 6033–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H & Kawashima R (2002). The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage, 17, 1207–1216. [DOI] [PubMed] [Google Scholar]
- Williams GV & Goldman-Rakic PS (1995). Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature, 376, 572–575. [DOI] [PubMed] [Google Scholar]
- Workman KP, Healey B, Carlotto A & Lacreuse A (2019). One-year change in cognitive flexibility and fine motor function in middle-aged male and female marmosets (Callithrix jacchus). American Journal of Primatology, 81, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S & Neelin P (1992). A Three-Dimensional Statistical Analysis for CBF Activation Studies in Human Brain. Journal of Cerebral Blood Flow and Metabolism, 12, 900–918. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Sosa ME, Peterson LJ & Colman RJ (2013). Using snacks high in fat and protein to improve glucoregulatory function in adolescent male marmosets (Callithrix jacchus). Journal of the American Association for Laboratory Animal Science, 52, 756–762. [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Kulkarni P, Ash H, Cai X, Mayerand ME, Rauch B & Ferris C (2020). Novel imaging technology and procedures for studying brain function in preadolescent awake marmosets. Journal of Neuroscience Methods, 343, 108823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P & Raz N (2014). Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neuroscience and Biobehavioral Reviews, 42, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author on reasonable request.


