Abstract
Background
Elevated blood pressure, or hypertension, is the leading cause of preventable deaths globally. Diets high in sodium (predominantly sodium chloride) and low in potassium contribute to elevated blood pressure. The WHO recommends decreasing mean population sodium intake through effective and safe strategies to reduce hypertension and its associated disease burden. Incorporating low‐sodium salt substitutes (LSSS) into population strategies has increasingly been recognised as a possible sodium reduction strategy, particularly in populations where a substantial proportion of overall sodium intake comes from discretionary salt. The LSSS contain lower concentrations of sodium through its displacement with potassium predominantly, or other minerals. Potassium‐containing LSSS can potentially simultaneously decrease sodium intake and increase potassium intake. Benefits of LSSS include their potential blood pressure‐lowering effect and relatively low cost. However, there are concerns about potential adverse effects of LSSS, such as hyperkalaemia, particularly in people at risk, for example, those with chronic kidney disease (CKD) or taking medications that impair potassium excretion.
Objectives
To assess the effects and safety of replacing salt with LSSS to reduce sodium intake on cardiovascular health in adults, pregnant women and children.
Search methods
We searched MEDLINE (PubMed), Embase (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection (Clarivate Analytics), Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCOhost), ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP) up to 18 August 2021, and screened reference lists of included trials and relevant systematic reviews. No language or publication restrictions were applied.
Selection criteria
We included randomised controlled trials (RCTs) and prospective analytical cohort studies in participants of any age in the general population, from any setting in any country. This included participants with non‐communicable diseases and those taking medications that impair potassium excretion. Studies had to compare any type and method of implementation of LSSS with the use of regular salt, or no active intervention, at an individual, household or community level, for any duration.
Data collection and analysis
Two review authors independently screened titles, abstracts and full‐text articles to determine eligibility; and extracted data, assessed risk of bias (RoB) using the Cochrane RoB tool, and assessed the certainty of the evidence using GRADE. We stratified analyses by adults, children (≤ 18 years) and pregnant women. Primary effectiveness outcomes were change in diastolic and systolic blood pressure (DBP and SBP), hypertension and blood pressure control; cardiovascular events and cardiovascular mortality were additionally assessed as primary effectiveness outcomes in adults. Primary safety outcomes were change in blood potassium, hyperkalaemia and hypokalaemia.
Main results
We included 26 RCTs, 16 randomising individual participants and 10 randomising clusters (families, households or villages). A total of 34,961 adult participants and 92 children were randomised to either LSSS or regular salt, with the smallest trial including 10 and the largest including 20,995 participants. No studies in pregnant women were identified. Studies included only participants with hypertension (11/26), normal blood pressure (1/26), pre‐hypertension (1/26), or participants with and without hypertension (11/26). This was unknown in the remaining studies. The largest study included only participants with an elevated risk of stroke at baseline. Seven studies included adult participants possibly at risk of hyperkalaemia. All 26 trials specifically excluded participants in whom an increased potassium intake is known to be potentially harmful. The majority of trials were conducted in rural or suburban settings, with more than half (14/26) conducted in low‐ and middle‐income countries.
The proportion of sodium chloride replacement in the LSSS interventions varied from approximately 3% to 77%. The majority of trials (23/26) investigated LSSS where potassium‐containing salts were used to substitute sodium. In most trials, LSSS implementation was discretionary (22/26). Trial duration ranged from two months to nearly five years.
We assessed the overall risk of bias as high in six trials and unclear in 12 trials.
LSSS compared to regular salt in adults: LSSS compared to regular salt probably reduce DBP on average (mean difference (MD) ‐2.43 mmHg, 95% confidence interval (CI) ‐3.50 to ‐1.36; 20,830 participants, 19 RCTs, moderate‐certainty evidence) and SBP (MD ‐4.76 mmHg, 95% CI ‐6.01 to ‐3.50; 21,414 participants, 20 RCTs, moderate‐certainty evidence) slightly.
On average, LSSS probably reduce non‐fatal stroke (absolute effect (AE) 20 fewer/100,000 person‐years, 95% CI ‐40 to 2; 21,250 participants, 3 RCTs, moderate‐certainty evidence), non‐fatal acute coronary syndrome (AE 150 fewer/100,000 person‐years, 95% CI ‐250 to ‐30; 20,995 participants, 1 RCT, moderate‐certainty evidence) and cardiovascular mortality (AE 180 fewer/100,000 person‐years, 95% CI ‐310 to 0; 23,200 participants, 3 RCTs, moderate‐certainty evidence) slightly, and probably increase blood potassium slightly (MD 0.12 mmol/L, 95% CI 0.07 to 0.18; 784 participants, 6 RCTs, moderate‐certainty evidence), compared to regular salt.
LSSS may result in little to no difference, on average, in hypertension (AE 17 fewer/1000, 95% CI ‐58 to 17; 2566 participants, 1 RCT, low‐certainty evidence) and hyperkalaemia (AE 4 more/100,000, 95% CI ‐47 to 121; 22,849 participants, 5 RCTs, moderate‐certainty evidence) compared to regular salt. The evidence is very uncertain about the effects of LSSS on blood pressure control, various cardiovascular events, stroke mortality, hypokalaemia, and other adverse events (very‐low certainty evidence).
LSSS compared to regular salt in children: The evidence is very uncertain about the effects of LSSS on DBP and SBP in children. We found no evidence about the effects of LSSS on hypertension, blood pressure control, blood potassium, hyperkalaemia and hypokalaemia in children.
Authors' conclusions
When compared to regular salt, LSSS probably reduce blood pressure, non‐fatal cardiovascular events and cardiovascular mortality slightly in adults. However, LSSS also probably increase blood potassium slightly in adults. These small effects may be important when LSSS interventions are implemented at the population level. Evidence is limited for adults without elevated blood pressure, and there is a lack of evidence in pregnant women and people in whom an increased potassium intake is known to be potentially harmful, limiting conclusions on the safety of LSSS in the general population. We also cannot draw firm conclusions about effects of non‐discretionary LSSS implementations. The evidence is very uncertain about the effects of LSSS on blood pressure in children.
Keywords: Adult; Child; Female; Humans; Pregnancy; Hyperkalemia; Hypertension; Hypertension/drug therapy; Hypokalemia; Potassium; Potassium/therapeutic use; Pregnant Women; Randomized Controlled Trials as Topic; Sodium; Sodium Chloride; Sodium Chloride/therapeutic use; Sodium Chloride, Dietary; Sodium Chloride, Dietary/adverse effects; Stroke
Plain language summary
Does using low‐sodium salt substitutes (LSSS) instead of regular salt reduce blood pressure and heart disease risks, and is it safe?
Key messages
• In adults, using LSSS instead of regular salt in food probably lowers blood pressure slightly. Adults using LSSS instead of regular salt probably have a slightly lower risk of non‐fatal heart conditions, such as stroke or a sudden reduced blood flow to the heart, and death from heart disease.
• Using LSSS instead of regular salt probably also slightly increases the level of blood potassium (a mineral that keeps your heart beating at the right pace) in adults. This could be harmful for people who cannot effectively regulate the potassium in their bodies. Other evidence on safety is very limited.
• We are not certain about effects of using LSSS instead of regular salt on blood pressure in children, or whether using LSSS is safe in children.
• This evidence may not directly apply to people known to be at risk of high blood potassium, such as people with kidney problems or on certain medications.
What are low‐sodium salt substitutes (LSSS)?
LSSS are products with less sodium than regular salt. Amounts of sodium in LSSS are lowered by replacing some of the sodium with potassium or other minerals. LSSS may help lower risks of using regular salt, since eating lots of sodium and not enough potassium contributes to high blood pressure. Globally, high blood pressure is the largest cause of preventable deaths, mainly because it causes stroke, acute coronary syndrome (ACS; where less blood flows to the heart), and kidney problems.
However, LSSS also has potential health risks. Using LSSS may lead to higher than normal blood potassium (hyperkalaemia), which causes problems with the heartbeat speed and rhythm, or can cause the heart to stop. These risks are greater in certain people, for example, those whose kidneys do not work properly to remove potassium.
What did we want to find out?
We wanted to find out what the effects of using LSSS instead of regular salt are on blood pressure as well as on events (stroke and ACS) and heart disease death. We also wanted to know if using LSSS instead of regular salt is safe, both in the general population and in people who are known to be at risk of high blood potassium levels.
We wanted to find this out for adults, children and pregnant women.
What did we do? We searched five electronic databases and trial registries for studies that compared using LSSS with using regular salt. We compared and summarised the results of the studies and rated our confidence in the combined evidence, based on factors such as study methods and sizes.
What did we find?
We found 26 trials* involving 34,961 adults and 92 children. No studies in pregnant women were found. Most trials were undertaken in rural or suburban areas, with more than half done in low‐ and middle‐income countries. Most trials included some people with high blood pressure (22); the largest included only people with a high risk of stroke. Seven trials were done in people at possible risk of high blood potassium. All trials excluded people where high potassium intake is known to be harmful, such as people with kidney problems or on certain medications. Nearly all trials (23) examined LSSS types where some sodium was replaced with potassium. The amount of sodium replaced in the various LSSS used in the trials ranged from very small (3%) to large (77%).
*Trials are types of studies in which participants are assigned randomly to two or more treatment groups. This is the best way to ensure similar groups of participants.
Main results
In adults, LSSS probably lowers blood pressure (diastolic and systolic) slightly when compared to regular salt. Using LSSS also probably lowers risk of non‐fatal stroke, non‐fatal ACS and heart disease death slightly when compared to regular salt.
However, using LSSS instead of regular salt probably also slightly increases the level of potassium in the blood.
Compared to regular salt, LSSS may result in little to no difference in high blood pressure and hyperkalaemia.
We could not draw any conclusions about effects of LSSS on blood pressure control, various heart disease events, death caused by stroke, lower than normal blood potassium (hypokalaemia), and other adverse events.
We could not draw any conclusions about the effects or safety of using LSSS instead of regular salt in children.
What are the limitations of the evidence?
We are moderately confident in the evidence. Our confidence was lowered mainly because of concerns about how some trials were conducted, and whether the results apply to the general population. We are not sure about the effects and safety of LSSS in children, pregnant women, people known to have a risk of high blood potassium, or those who do not have high blood pressure. We are also unsure about the effects of LSSS when used in foods not prepared at home. Further research may change these results.
How up to date is this evidence?
The evidence is up‐to‐date to August 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ LSSS intervention compared to regular salt in adults (≥ 18 years) in the general population.
| LSSS intervention compared to regular salt in adults (≥ 18 years) in the general population | ||||||
| Patient or population: adults (≥ 18 years) in the general population Setting: any setting in any country Intervention: LSSS intervention Comparison: regular salt | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with regular salt | Risk with LSSS intervention | |||||
| Change in DBP (mmHg) follow‐up: range 56 days to 3 years | The mean change in DBP (mmHg) was ‐0.74 mmHg | MD 2.43 mmHg lower (3.5 lower to 1.36 lower) | ‐ | 20830 (19 RCTs) | ⊕⊕⊕⊝ Moderatea | LSSS interventions probably reduce DBP (mmHg) slightly. |
| Change in SBP (mmHg) follow‐up: range 56 days to 3 years | The mean change in SBP (mmHg) was ‐1.32 mmHg | MD 4.76 mmHg lower (6.01 lower to 3.5 lower) | ‐ | 21414 (20 RCTs) | ⊕⊕⊕⊝ Moderateb | LSSS interventions probably reduce SBP (mmHg) slightly. |
| Hypertension follow‐up: 18 months | 580 per 1000 | 563 per 1000 (522 to 598) | RR 0.97 (0.90 to 1.03) | 2566 (1 RCT) | ⊕⊕⊝⊝ Lowc,d | LSSS interventions may result in little to no difference in hypertension. |
| Blood pressure control follow‐up: range 8 weeks to 3 months | 128 per 1000 | 271 per 1000 (169 to 436) | RR 2.12 (1.32 to 3.41) | 253 (2 RCTs) | ⊕⊝⊝⊝ Very lowe,f,g | The evidence is very uncertain about the effect of LSSS interventions on blood pressure control. |
| Cardiovascular events: various follow‐up: range ≤ 3 to > 3‐12 months | 1623 per 100,000 | 1980 per 100,000 (795 to 4933) | RR 1.22 (0.49 to 3.04) | 982 (5 RCTs) | ⊕⊝⊝⊝ Very lowh,i | The evidence is very uncertain about the effect of LSSS interventions on various other cardiovascular events. |
| Cardiovascular events: non‐fatal stroke follow‐up: range ≤ 3 to > 12 months | 198 per 100,000 | 178 per 100,000 (158 to 200) | RR 0.90 (0.80 to 1.01) | 21250 (3 RCTs) | ⊕⊕⊕⊝ Moderatej | LSSS interventions probably reduce non‐fatal stroke events slightly. |
| Cardiovascular events: non‐fatal acute coronary syndrome (events per 100,000 person‐years) follow‐up: mean 4.75 years | 512 per 100,000 | 358 per 100,000 (266 to 481) | Rate ratio 0.70 (0.52 to 0.94) | 20995 (1 RCT) | ⊕⊕⊕⊝ Moderatej | LSSS interventions probably reduce non‐fatal acute coronary syndrome events slightly. |
| Cardiovascular mortality (events per 100,000 person‐years) follow‐up: range mean 2.6 to 13 years | 786 per 100,000 | 605 per 100,000 (472 to 786) | Rate ratio 0.77 (0.60 to 1.00) | 23200 (3 RCTs) | ⊕⊕⊕⊝ Moderatej | LSSS interventions probably reduce cardiovascular mortality slightly. |
| Stroke mortality (events per 100,000 person‐years) follow‐up: range mean 4.75 to 13 years | 405 per 100,000 | 259 per 100,000 (134 to 506) | Rate ratio 0.64 (0.33 to 1.25) | 21423 (2 RCTs) | ⊕⊝⊝⊝ Very lowj,k | The evidence is very uncertain about the effect of LSSS interventions on stroke mortality. |
| Change in blood potassium (mmol/L) follow‐up: range 56 days to 1.5 years | The mean change in blood potassium (mmol/L) was 0.01 mmol/L | MD 0.12 mmol/L higher (0.07 higher to 0.18 higher) | ‐ | 784 (6 RCTs) | ⊕⊕⊕⊝ Moderatel | LSSS interventions probably increase blood potassium (mmol/L) slightly. |
| Hyperkalaemia follow‐up: range 3 months to mean 4.75 years | 88 per 100,000 | 91 per 100,000 (40 to 209) | RR 1.04 (0.46 to 2.38) | 22849 (5 RCTs) | ⊕⊕⊕⊝ Moderatem | LSSS interventions likely result in little to no difference in hyperkalaemia. |
| Hypokalaemia follow‐up: 12 weeks | One small trial in younger, hypertensive participants receiving potassium supplementation due to the use of potassium‐depleting diuretics reported no hypokalaemia events in the intervention (n = 12) or control (n = 10) group. | 22 (1 RCT) | ⊕⊝⊝⊝ Very lowc,n | The evidence is very uncertain about the effect of LSSS interventions on hypokalaemia. | ||
| Adverse events: other follow‐up: range ≤ 3 to > 12 months | Eight trials reported other adverse events, with a total of 25/1094 (2.3%) and 14/1015 (1.4%) diverse adverse events reported across studies in the intervention and control groups, respectively (not pooled). | 2109 (8 RCTs) | ⊕⊝⊝⊝ Very lowl,o,p | The evidence is very uncertain about the effect of LSSS interventions on other adverse events. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431386681611398744. | ||||||
a Serious inconsistency: Substantial heterogeneity (I^2 = 88%), not explained by subgroup analyses (study duration, ethnicity, BP status, type of LSSS, baseline Na excretion) or meta‐regression (type of LSSS, baseline Na excretion, overall risk of bias) b Serious inconsistency: Substantial heterogeneity (I^2 = 78%), not explained by subgroup analyses (study duration, ethnicity, BP status, type of LSSS, baseline Na excretion) or meta‐regression (type of LSSS, baseline Na excretion, overall risk of bias) c Serious risk of bias: All information is from a study at unclear overall risk of bias d Serious imprecision: 95%CI is consistent with the possibility of important benefit and unimportant harm (minimally important threshold: 5000 per 100,000) e Serious risk of bias: The majority of information is from a study at unclear overall risk of bias f Serious indirectness: majority of information is from a study using an LSSS with 97% NaCl (table salt) g Serious imprecision: 95% CI was consistent with the possibility of unimportant and important benefit (minimally important threshold 5000 per 100,000) h Serious indirectness: Pooled effect is driven by a large study in high‐risk individuals (participants selected based on high risk of future vascular disease) that is less likely to be directly applicable to the general population i Very serious imprecision: Using the OIS approach, the ratio of the upper to the lower boundary of the 95% CI is more than 3 (RR); 18 events in total j Serious indirectness: Pooled effect is driven by a large secondary prevention trial (73% of participants with previous stroke) that is less likely to be directly applicable to the general population, and that reported limited data on safety outcomes k Very serious imprecision: The ratio of the upper to the lower boundary of the 95% CI is more than 3 l Serious risk of bias: The majority of information is from studies at high or unclear overall risk of bias m Serious risk of bias: The majority of information is from a study at high overall risk of bias n Very serious indirectness: All information is from a study including younger hypertensive participants with a different rationale for administering LSSS (potassium supplementation due to the use of potassium‐depleting diuretics) o Serious inconsistency: Other adverse event outcomes were too diverse to pool p Serious imprecision: 39 events in total, OIS not met (not rare events)
Summary of findings 2. Summary of findings table ‐ LSSS intervention compared to regular salt in children (2 to < 18 years) in the general population.
| LSSS intervention compared to regular salt in children (2 to < 18 years) in the general population | ||||||
| Patient or population: children (2 to < 18 years) in the general population Setting: any setting in any country Intervention: LSSS intervention Comparison: regular salt | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with regular salt | Risk with LSSS intervention | |||||
| Change in DBP (mmHg) follow‐up: 4 months | The mean change in DBP (mmHg) was ‐5.87 mmHg | MD 1.28 mmHg higher (1.56 lower to 4.12 higher) | ‐ | 92 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of LSSS interventions on change in DBP (mmHg). |
| Change in SBP (mmHg) follow‐up: 4 months | The mean change in SBP (mmHg) was ‐6.05 mmHg | MD 0.12 mmHg higher (4.41 lower to 4.64 higher) | ‐ | 92 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of LSSS intervention on change in SBP (mmHg). |
| Hypertension ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies in children reported on this outcome. |
| Blood pressure control ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies in children reported on this outcome. |
| Change in blood potassium (mmol/L) ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies in children reported on this outcome. |
| Hyperkalaemia ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies in children reported on this outcome. |
| Hypokalaemia ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | No studies in children reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431387925381133163. | ||||||
a Serious risk of bias: All information is from a study at unclear overall risk of bias b Serious indirectness: LSSS was delivered in bread only (non‐discretionary) for 4 months c Serious imprecision: Wide 95% CI including both reductions and increases in blood pressure
Background
Description of the condition
High blood pressure is the leading cause of preventable deaths worldwide, contributing to more than 10 million deaths and 211 million disability‐adjusted life years annually, mainly due to acute coronary syndrome (formerly called ischaemic heart disease) and stroke (Forouzanfar 2017).
Hypertension is typically defined by a diastolic blood pressure (DBP) ≥ 90 mmHg and a systolic blood pressure (SBP) ≥ 140 mmHg, although recent guidelines define stage 1 hypertension as a SBP ranging from 130 to 139 mmHg and a DBP ranging from 80 to 89 mmHg to reflect current blood‐pressure lowering targets (Arnett 2019). After 50 years of age, SBP increases disproportionately to DBP in many individuals due to factors such as reduced arterial stiffness (Franklin 2011; Lee 2010), with an elevated SBP being a prominent risk factor for cardiovascular events in older people (Staessen 2000). In 2015, an estimated 874 million adults had a SBP of 140 mmHg or higher (Forouzanfar 2017).
High sodium together with insufficient potassium intake contribute to hypertension, thereby increasing the risk of cardiovascular disease and stroke. Current global estimates of sodium intake are 3950 mg (172 mmol) per person per day (Powles 2013), which equates to nearly ten grams of salt (sodium chloride) per person per day. For sodium, current World Health Organization (WHO) guidelines strongly recommend reducing intake in adults to < 2 g/day sodium (equating to about 5 g salt per day) and a downward adjusted intake in children (WHO 2012a). Global estimates of potassium intake for all ages, education levels, residences and sexes in 2018 are 2.3 grams per person per day (Global Dietary Database 2022), which equates to an intake of 59 mmol per person per day. For potassium, WHO conditionally recommends an intake in adults of at least 90 mmol/day (3150 mg/day) and a downward adjusted intake in children (WHO 2012b). Although antihypertensive drug therapy is an effective method for controlling blood pressure, poor adherence to antihypertensive therapy substantially increases the near‐ and long‐term risk of stroke among patients with hypertension (Herttua 2013), and access to health care such as blood‐pressure lowering medication is not universally available.
Hypertension is also a major contributor to the development and progression of chronic kidney disease (CKD). Adequate blood pressure control has been shown to be effective in slowing the progression of CKD to end stage renal disease (ESRD). In addition, adequate treatment of diabetes and cardiovascular risk factors such as dyslipidemia, are also linked to lower rates of progression to ESRD, and associated with significant reductions in cardiovascular morbidity and mortality (Couser 2011).
Description of the intervention
The WHO target of a 30% relative reduction in mean population salt/sodium intake by 2025 requires effective and safe strategies to reduce population intake. One of several existing salt reduction strategies is using salt products with lower concentrations of sodium ‐ usually replaced by potassium or other minerals, or both. These low‐sodium salt substitutes (LSSS) vary widely in their formulations and are available in high‐income as well as low‐ and middle‐income countries. In many LSSS, a proportion of sodium chloride (NaCl) is replaced with potassium chloride (KCl), which shares many properties with NaCl but also has unwanted relatively offensive side tastes (bitter, acrid, and metallic). A recent narrative review (Cepanec 2017) described the many various formulations of KCl‐based LSSS, which include the use of numerous taste‐improving agents (TIAs) and formulation concepts. Authors concluded that “within the great number of various compositions of KCl‐based salt substitutes, presumably the most effective ones are based on well‐balanced mixtures of KCl and NaCl, maintaining a sodium reduction range from −25% to −50% (relative to NaCl), which always include certain percentages of one or more TIAs. A typical formulation of a KCl‐based salt substitute with 50% in sodium reduction is 50% NaCl + 30‐45% KCl + 5‐20% taste‐improving agents.” Incorporating salt substitutes into population strategies to reduce sodium intake has increasingly been recognised by health authorities and public health organisations (Greer 2020), especially in countries where the majority of sodium intake comes from the discretionary use of salt by households.
How the intervention might work
The dose‐response relationship between reduced dietary sodium and blood pressure change was examined in a recent systematic review (133 studies with 12,197 participants). Authors showed that in diverse populations, lower sodium intakes resulted in blood pressure reductions, with greater reductions in sodium intake producing greater reductions in BP (Huang 2020). Additionally, older and non‐white populations (for SBP), as well as those with higher baseline blood pressure (for SBP and DBP) achieved greater blood pressure reductions from the same amount of sodium reduction (Huang 2020). Reductions in blood pressure, such as a reduction of 5% in SBP, translate to important reductions (10%) in the risk of major cardiovascular events (e.g. fatal or non‐fatal stroke or myocardial infarction), as demonstrated by a recent meta‐analysis of individual participant‐level data from 48 trials of blood‐pressure lowering medication (Rahimi 2021). Observational studies have demonstrated that stroke risk is inversely associated with dietary potassium intake (Vinceti 2016). In addition, data from randomised clinical trials have shown that potassium supplements have a blood‐pressure lowering effect in people with hypertension, particularly those with a high sodium intake (Filippini 2017). As described in the Description of the condition section, global estimates of potassium intake are lower than what is currently recommended by the WHO. The low dietary intake of potassium, in addition to high dietary sodium intake, contributes to hypertension. Therefore, interventions or strategies promoting the use of a potassium‐enriched LSSS could aid in reducing sodium intake, while concurrently increasing potassium intake, at the population level.
Reduction in sodium intakes ‐ either through reduction of dietary salt intake, salt substitution, or a combination of these ‐ may also be a practical choice for patients with hypertension who are resistant to antihypertensive medications or who experience side effects from medications. It may also play an important role as an adjunctive therapy in the management of hypertensive individuals by potentially lowering the doses of antihypertensive medication required. In cases where the behavioural changes required to reduce dietary salt intake are very difficult or unfeasible, salt substitutes may offer convenience and practicality. Therefore, salt substitution as a cost‐effective strategy could result in reductions in health‐care costs associated with non‐communicable diseases at a population level. However, it should be noted that if foods with high levels of non‐discretionary sodium chloride are regularly consumed, the discretionary use of LSSS may not result in a sufficient reduction in sodium intake to be beneficial.
LSSS may offer a potential solution for the food industry to develop lower sodium food products without compromising on taste or safety, particularly in countries where non‐discretionary sodium intake contributes significantly to the overall population intake of sodium. However, because KCl costs more than NaCl, significant consumer demand, industry‐targeted subsidies or taxes on high sodium content foods will likely be required before the food industry will absorb the costs of product reformulation. Therefore, the application of LSSS strategies at population level to reduce sodium consumption is dependent on several factors, including its main uses within a population, as well as its effects on food taste and cost (Greer 2020).
The greatest risk with potassium‐based LSSS is the potential for adverse effects resulting from hyperkalaemia, particularly the increased risk of arrhythmias and sudden cardiac death. The risk of adverse events is greater at higher levels of serum potassium. There is no absolute threshold at which these adverse events occur, however a serum potassium level of ≥ 6.0 mmol/L is commonly considered to be a clinically significant threshold above which the most serious manifestations of hyperkalaemia occur (Ahee 2000; Hollander‐Rodriguez 2006). Multiple factors influence the occurrence of these adverse events, such as the underlying cause of hyperkalaemia and the rate at which serum potassium increases. High intakes of dietary potassium have not been linked to adverse effects in healthy adults and children with normal kidney function. However, the effects of high dietary potassium intakes on the risk of adverse effects are a key concern among people with impaired potassium excretion, such as those with chronic kidney disease or taking medications that impair potassium excretion (Greer 2020; Kovesdy 2018).
A reduction of dietary sodium intake through the population‐level implementation of LSSS may also result in hyponatraemia in people with impaired renal function (Sahay 2014), including older people and people treated with thiazide diuretics (Upadhyay 2009).
Why it is important to do this review
If the best available evidence on replacing salt with LSSS shows adequate effectiveness and safety for important outcomes, it could be recommended as a population‐level intervention for reducing cardiovascular disease risk. However, concerns exist about potential adverse effects of LSSS, such as hyperkalaemia, particularly in those at risk, such as people with chronic kidney disease or on medications that impair potassium excretion.
The WHO is currently developing a guideline on the use of LSSS in adults and children. This review was commissioned by the WHO Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health in order to inform and contribute to the development of a WHO recommendation on the use of LSSS for this guideline. The results of this review, including Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessments, were discussed and reviewed by the WHO NUGAG Subgroup on Diet and Health as part of their guideline development process.
Objectives
To assess the effects and safety of replacing salt with LSSS to reduce sodium intake on cardiovascular health in adults, pregnant women and children.
Methods
Criteria for considering studies for this review
Types of studies
The Populations, Intervention, Comparison, and Outcomes (PICO) were agreed by the WHO NUGAG Subgroup on Diet and Health, who ranked the outcomes and also agreed on subgroups and study designs to be included. As per these agreements and our prospective registration on the international prospective register of systematic reviews (PROSPERO 2020 CRD42020180162; available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=180162), we included individually randomised controlled trials (RCTs) and cluster‐randomised controlled trials (cluster‐RCTs) with true randomisation methods, regardless of the unit of allocation. We also planned to include prospective analytical cohort studies, where LSSS intake/exposure was assessed at baseline and related to any of the prespecified outcomes at a later time point using empirical data. We excluded RCTs with a cross‐over trial design if data for the first phase per group were unavailable, due to the possible period and carry‐over effects that would arise with the eligible dietary interventions/exposures and outcomes not being easily reversible, as required for a valid cross‐over design (Younge 2015). We additionally excluded cluster‐RCTs with fewer than two intervention and two control clusters.
Types of participants
We included studies in the general population, from any setting in any country, including participants with the following condition(s) and/or risk factors: hypertension, cardiovascular disease (CVD), diabetes mellitus, renal impairment and those taking medications that impair potassium excretion.
In accordance with the WHO NUGAG PICO conceptualisations and agreements, the following three comparisons were planned if data allowed:
LSSS versus regular salt or no active intervention in adults (aged 18 years and older)
LSSS versus regular salt or no active intervention in children aged 2 to < 18 years
LSSS versus regular salt or no active intervention in pregnant women
Types of interventions
We included studies that assessed the health effects associated with the use of LSSS at an individual, household or community level. LSSS interventions/exposures of any type or duration were included, provided they aimed to replace the dietary intake of any amount of sodium with another mineral or compound. Studies investigating either discretionary (i.e. salt on table or added during cooking) or non‐discretionary use of LSSS (i.e. included during food manufacturing), or both, were included.
Eligible comparators/controls included the use of regular salt (NaCl) or no active intervention to reduce salt intake. Studies where the control group received only basic information on sodium reduction at baseline, were included. Studies with multi‐component interventions were included if effects of LSSS could be isolated from the multifactorial design.
We excluded studies with multi‐component interventions if the additional intervention components were not aimed primarily at promoting LSSS use by participants or communities, but were instead focussed more broadly on reducing sodium intake (e.g. changing lifestyle and dietary behaviour of which LSSS use is only one component) or aimed at improving health in general (e.g. counselling for exercise or smoking cessation), such that LSSS effects could not be isolated.
Types of outcome measures
We did not exclude studies on the basis of outcomes measured. However, we did exclude studies measuring only sensory or organoleptic outcomes (e.g. taste of or preference for LSSS).
Primary outcomes
Table 3, Table 4 and Table 5 detail the prespecified primary outcomes for each comparison, with outcome ranking by the WHO NUGAG Subgroup on Diet and Health indicated as follows: criticalc, importanti and not important ni. The following primary outcomes were regarded as safety outcomes related to the intake of LSSS with potassium: change in blood potassium, hyperkalaemia and hypokalaemia.
1. Primary and secondary outcomes for the comparison in adults.
| Primary clinical outcomes* | Primary laboratory outcomes* | Secondary clinical outcomes* | Secondary laboratory outcomes* |
| Change in diastolic blood pressure (DBP, mmHg)c | Change in blood potassium (mmol/L)c | All‐cause mortalityi | Renal function (e.g. serum creatinine, albuminuria, urinary albumin‐to‐creatinine ratio (uACR), glomerular filtration rate (GFR))i |
| Change in systolic blood pressure (SBP, mmHg)c |
Hyperkalaemia (e.g. number of adults with serum potassium concentration > 5.5 mmol/L, or as reported by study authors)c |
Adverse events (other), excluding those that overlap with other outcomes, such as electrolyte disturbances and cardiac arrhythmias (e.g. nausea, vomiting)i | Hyponatraemia (e.g. number of adults with serum sodium concentration < 135 mmol/L, or as reported by study authors)i |
| Hypertension (e.g. number of adults with SBP > 140 mmHg or DBP > 85 mmHg, or as reported by study authors)c | Hypokalaemia (e.g. number of adults with serum potassium concentration < 3.5 mmol/L, or as reported by study authorsc | Antihypertensive medication usei | Change in fasting blood glucose (mmol/L)i |
| Blood pressure control (e.g. number of adults achieving blood pressure threshold or blood pressure under "control", or as prespecified by study authors)c | Diabetes mellitus diagnosis (as reported by study authors)i |
Change in blood triglycerides (mmol/L)i |
|
| Cardiovascular events (as reported by study authors, such as stroke, myocardial infarction, dysrhythmia)c | Change in body mass index (BMI) (kg/m2)i | Change in total blood cholesterol (mmol/L)i |
|
| Cardiovascular mortalityc | Change in 24‐hour urinary sodium excretion (mmol/24‐hours)** | ||
| Change in 24‐hour urinary potassium excretion (mmol/24‐hours)** |
Outcome ranking by WHO NUGAG ‐ Subgroup on Diet and Health: criticalc, importanti and not importantni
* Outcomes measured at longest follow‐up
** Additional outcomes added by WHO NUGAG during the guideline development process; measured using 24‐h urine samples only (spot samples excluded) Abbreviations: BMI: body mass index DBP: diastolic blood pressure GFR: glomerular filtration rate NUGAG: Nutrition Guidance Expert Advisory Group SBP: systolic blood pressure uACR: urine albumin‐to‐creatinine ratio
2. Primary and secondary outcomes for the comparison in pregnant women.
| Primary clinical outcomes* | Primary laboratory outcomes* | Secondary clinical outcomes in women* | Secondary clinical outcomes in newborns* | Secondary laboratory outcomes* |
| Pre‐eclampsia (e.g. number of women meeting the following diagnostic criteria: SBP > 140 mmHg or DBP > 90 mmHg after 20 weeks of pregnancy, with proteinuria and/or other maternal organ dysfunction such as renal, liver neurological or haematological abnormalities, or uteroplacental dysfunction, or as reported by study authors)c | Change in blood potassium (mmol/L)c | All‐cause mortalityi | Pre‐term infant (i.e. number of infants born < 37 weeks gestation)i | Renal function (e.g. serum creatinine, albuminuria, urinary albumin‐to‐creatinine ratio (uACR), glomerular filtration rate (GFR))i |
| Eclampsia (e.g. number of women with pre‐eclampsia who present with convulsions, or as reported by study authors)c | Hyperkalaemia (e.g. number of women with serum potassium concentration > 5.5 mmol/L, or as reported by study authors)c | Cardiovascular mortalityi | Intra‐uterine growth restriction (IUGR) (e.g. number of small‐for‐gestational age (SGA) infants, defined as those with a birthweight < 2 SD below the reference standard or < 10th percentile, or as reported by study authors)i | Change in fasting blood glucose (mmol/L)i |
| Change in diastolic blood pressure (DBP, mmHg)c | Hypokalaemia (e.g. number of women with serum potassium concentration < 3.5 mmol/L, or as reported by study authors)c | Adverse events (other), excluding those that overlap with other outcomes, such as electrolyte disturbances and cardiac arrhythmias (e.g. nausea, vomiting)i | Birthweight (g)i | Change in blood triglycerides (mmol/L)i |
| Change in systolic blood pressure (SBP, mmHg)c | Antihypertensive medication usei | Change in total blood cholesterol (mmol/L)i | ||
| Hypertension (e.g. number of women with SBP > 140 mmHg or DBP > 85 mmHg, or as reported by study authors)c | Gestational diabetes diagnosisi (e.g. number of women meeting one of the following diagnostic criteria: fasting plasma glucose 5.1–6.9 mmol/L; 1‐hour plasma glucose 10.0 mmol/L, or 2‐hour plasma glucose 8.5–11.0 mmol/L following a 75 g oral glucose load, or as reported by study authors) | Change in 24‐hour urinary sodium excretion (mmol/24‐hours)** | ||
| Blood pressure control (e.g. number of women achieving blood pressure threshold or blood pressure under "control", or as prespecified by study authors)c | Diabetes mellitus diagnosis (as reported by study authors)i | Change in 24‐hour urinary potassium excretion (mmol/24‐hours)** | ||
| Cardiovascular events (as reported by study authors, such as strokei, myocardial infarction, dysrhythmiac) |
Outcome ranking by WHO NUGAG ‐ Subgroup on Diet and Health: criticalc, importanti and not importantni
* Outcomes measured at longest follow‐up
** Additional outcomes added by WHO NUGAG during the guideline development process; measured using 24‐h urine samples only (spot samples excluded)
Abbreviations: DBP: diastolic blood pressure GFR: glomerular filtration rate IUGR: intra‐uterine growth restriction NUGAG: Nutrition Guidance Expert Advisory Group SBP: systolic blood pressure SD: standard deviation SGA: small‐for‐gestational age uACR: urine albumin‐to‐creatinine ratio
3. Primary and secondary outcomes for the comparison in children.
| Primary clinical outcomes* | Primary laboratory outcomes* | Secondary clinical outcomes* | Secondary laboratory outcomes* |
| Change in diastolic blood pressure (DBP, mmHg)c | Change in blood potassium (mmol/L)c | Growth changes (e.g. z‐scores for height‐ or length‐for‐age (HAZ or LAZ), weight‐for‐height (WHZ), weight‐for age (WAZ), BMI‐for‐age)i | Renal function (e.g. serum creatinine, albuminuria, urinary albumin‐to‐creatinine ratio (uACR), glomerular filtration rate (GFR))i |
| Change in systolic blood pressure (SBP, mmHg)c | Hyperkalaemia (e.g. number of children with serum potassium concentration > 5.5 mmol/L, or as reported by study authors)c | Adverse events (other), excluding those that overlap with other outcomes, such as electrolyte disturbances and cardiac arrhythmias (e.g. nausea, vomiting)i | Bone health (e.g. serum alkaline phosphatase (ALP) in mmol/L)i |
| Hypertension (e.g. as average systolic BP (SBP) and/or diastolic BP (DBP) that is ≥ 95th percentile for gender, age, and height on ≥ 3 occasions, or as reported by study authors)c | Hypokalaemia (e.g. number of children with serum potassium concentration < 3.5 mmol/L, or as reported by study authors)c | Cardiovascular events (as reported by study author, such as stroke, myocardial infarction, dysrhythmia)i | Hyponatraemia (e.g. number of children with serum sodium concentration < 135 mmol/L, or as reported by study authors)i |
| Blood pressure control (e.g. number of children achieving blood pressure threshold or blood pressure under "control", or as prespecified by study authors)c | Antihypertensive medication usei | Changes in fasting blood glucose (mmol/L)i |
|
| All‐cause mortalityi | Changes in blood triglycerides (mmol/L)i | ||
| Cardiovascular mortalityi | Changes in total blood cholesterol (mmol/L)i | ||
| Bone densitometry measures (e.g. bone mineral density changes)i |
Change in 24‐hour urinary sodium excretion (mmol/24‐hours)** | ||
| Change in 24‐hour urinary potassium excretion (mmol/24‐hours)** |
Outcome ranking by WHO NUGAG ‐ Subgroup on Diet and Health: criticalc, importanti and not importantni
* Outcomes measured at longest follow‐up
** Additional outcomes added by WHO NUGAG during the guideline development process; measured using 24‐h urine samples only (spot samples excluded)
Abbreviations: ALP: alkaline phosphatase BMI: body mass index BP: blood pressure DBP: diastolic blood pressure GFR: glomerular filtration rate HAZ: height‐for‐age z‐score LAZ: length‐for‐age z‐score NUGAG: Nutrition Guidance Expert Advisory Group SBP: systolic blood pressure uACR: urine albumin‐to‐creatinine ratio WAZ: weight‐for‐age z‐score WHZ: weight‐for‐height z‐score
Secondary outcomes
Table 3, Table 4 and Table 5 detail the prespecified secondary outcomes for each comparison, with outcome ranking by the WHO NUGAG Subgroup on Diet and Health indicated as follows: criticalc, importanti and not important ni. The following secondary outcomes were regarded as safety outcomes related to the intake of LSSS with potassium: adverse events, renal function and hyponatraemia.
Search methods for identification of studies
The search strategy was developed, peer‐reviewed and implemented by Cochrane information specialists in consultation with the review team. We used a comprehensive search strategy aiming to identify all eligible studies regardless of language, publication type or publication status. Publication date restrictions were not imposed, except for conference abstracts identified through Embase which covered only those published in the past two years. With this, we specifically aimed to find recent proceedings of studies that may not yet have been published as full articles at the time of the search. We used filters for trials (Lefebvre 2022), cohort studies (Li 2019) and adverse effects (Golder 2006; Golder 2012) to inform our search strategy.
Electronic searches
We aimed to identify RCTs and prospective analytical cohort studies through systematic searches of the following bibliographic databases:
MEDLINE (PubMed, from 1946 to 18 August 2021)
Embase (Ovid, from 1947 to 18 August 2021)
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (Issue 8 of 12, 2021)
Web of Science Core Collection with Indexes SCI‐Expanded, SSCi, CPCI‐S (Clarivate Analytics, from 1970 to 18 August 2021)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost, from 1937 to 18 August 2021)
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing and unpublished trials (https://trialsearch.who.int/). The date of the last searches here was also 18 August 2021. Search strategies per database/registry searched are detailed in Appendix 1.
Searching other resources
To identify any additional eligible records, two reviewers also screened the reference lists of three recent systematic reviews evaluating the effects of LSSS use (Hernandez 2019; Jafarnejad 2020; Jin 2020), as well as the bibliographies of all studies included in this review.
Data collection and analysis
Selection of studies
After de‐duplication of search records, titles and abstracts were screened independently by two reviewers using Covidence (Covidence). Full‐text articles for all records identified as potentially eligible for inclusion were then screened by two reviewers independently to determine final eligibility. Records where we could not obtain the full text or more details of the study in order to determine eligibility, were classified as ‘Studies awaiting classification’. We resolved any disagreements between reviewers at any stage of the eligibility assessment process through discussion and consultation with a third reviewer, where necessary.
Data extraction and management
Two reviewers independently extracted data onto forms designed and piloted for the review, and we resolved any disagreements during the data extraction and management process through discussion and consultation with a third reviewer, where necessary. Where necessary, translations of records in non‐English were obtained. We extracted data on the following:
Study details, including author details, conflict of interest declaration, funding source, setting
Methods, including design, aim, dates, limitations as reported by authors, sample size calculation, participants, including eligibility criteria; method of recruitment; number of clusters per trial arm and how authors accounted for the effect of clustering; participant flow details such as number assessed for eligibility, number randomised; baseline characteristic such as demographic and lifestyle characteristics, health status and intake of sodium and potassium; and any differences in these characteristics by trial arm
Interventions/exposures, including description, delivery/use, addition of fortificants, duration, co‐interventions, integrity of delivery
Comparators, including description, delivery, duration, co‐interventions, integrity of delivery
Outcomes, including numeric data relevant to all primary and secondary outcomes according to the following time point ranges, when available: baseline to 3 months, > 3 to 12 months and > 12 months, except for cardiovascular events, all‐cause mortality, cardiovascular mortality and adverse events, for which data were extracted for the duration of the study. When outcome data were reported at more than one point, we extracted data from the latest point available. For studies that did not use the International System of Units (SI) to report outcomes, we converted values to SI units, where possible. For trials, we extracted change data (change in the outcome from baseline to outcome assessment) with relevant data on variance for intervention and control groups (along with numbers of participants at the time point). Where change data were not available, we extracted end‐values at the time point, along with the variance and numbers of participants for each group, or mean differences (MDs) and measures of variance per group. Where outcome data were only reported per subgroup of the total sample of study participants (e.g. participants with hypertension and participants with normal blood pressure), we extracted these data and calculated the combined mean and standard deviation (SD) for the total sample according to the guidance by (Higgins 2020a), where possible. We preferentially extracted and used supine over standing blood pressure measurements, 24‐hour measurements over measurements done at a single time point, and ambulatory measurements over those conducted in a clinic setting. For cohort studies, we planned to extract the most adjusted odds ratio, risk ratio, mean change or mean end values per group, when comparing the most exposed group of participants with the least exposed group, and the most adjusted regression outputs when LSSS intake was assessed at baseline and related to an outcome measure later.
Assessment of risk of bias in included studies
We assessed the risk of bias in RCTs and cluster‐RCTs using the Cochrane tool for assessment of risk of bias (Higgins 2017). Two reviewers conducted these assessments independently for each included study. We resolved disagreements by discussion or through consultation with a third reviewer. We assessed the risk of bias for RCTs according to the following domains:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other bias
We also assessed the risk of bias for cluster‐RCTs according to the following domains (Higgins 2017):
Recruitment bias (selection bias)
Comparability with RCTs
Baseline imbalance (selection bias)
Loss of clusters (attrition bias)
Incorrect analysis
For cohort studies, we planned to use the following domains to assess risk of bias (Naude 2018):
Were adequate outcome data available?
Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome, or were relevant statistical adjustments done?
Did the exposures between groups differ in components other than only LSSS exposure?
Could we be confident in the assessment of outcomes?
Could we be confident in the assessment of exposure?
Could we be confident in the assessment of presence or absence of prognostic factors?
Was selection of less‐exposed and more‐exposed groups from the same population?
Overall risk of bias assessment
As this review addressed mainly objective outcomes (e.g. blood pressure measurements, laboratory‐determined electrolyte values), we did not regard blinding to be of key importance for informing judgements on overall bias. Consequently, we judged overall risk of bias for each included study using two key domains for RCTs and four key domains for cluster‐RCTs, as follows:
RCTs: allocation concealment (selection bias) and incomplete outcome data (attrition bias), and
Cluster‐RCTs: baseline imbalance (selection bias), recruitment bias (selection bias), incomplete outcome data (attrition bias) and loss of clusters (attrition bias).
We assessed the overall risk of bias of each included study as follows:
low risk (low risk of bias for all key domains);
high risk (high risk of bias for one or more key domains); or
unclear risk (unclear risk of bias for one or more key domains).
For cohort studies, we planned to consider domains relevant to confounding to inform judgements of the overall risk of bias.
Measures of treatment effect
For dichotomous outcomes, we presented proportions; for two‐group comparisons where numbers of events and participants were provided, we presented results as risk ratios (RRs) with 95% confidence intervals (CIs). Where event rates were reported per person‐years followed in separate groups, we calculated incidence rate ratios (IRRs) with 95% CIs to enable meta‐analysis of these studies with studies reporting rate ratios for the same outcomes. Rate ratios were calculated by dividing the rate in the intervention group by the rate in the control group. The 95% confidence interval (95% CI) of these rate ratios were calculated by taking the antilogarithm of the natural log of the rate ratio (log(IRR)), plus or minus 1.96 times the standard error of the log(IRR) (Boston University School of Public Health 2018). Briefly, the standard error was calculated as the square root of the sum of the inverse of events in the intervention and control group.
Where hazard ratios (HRs) were reported for incident hypertension in the stepped‐wedge trial (Bernabe‐Ortiz 2014), we presented these results with 95% CIs. Due to the different way of analysing these data and the unique design of this trial, these measures were not combined with other data reporting on hypertension.
For continuous outcomes, we used the mean difference (MD) with 95% CIs if outcomes were measured in the same way between trials. Where continuous data were reported using different units across included studies, we planned to calculate and present the standardised mean difference (SMD).
Unit of analysis issues
Studies with more than two intervention groups
For the single study with more than two intervention groups (Pan 2017), we combined event outcome data reported separately for both intervention groups (LSSS < 50% KCl and LSSS ≥ 50% KCl) in our meta‐analyses. These intervention groups were combined using the methods set out in the Cochrane Handbook (Higgins 2020a). Another study randomised participants to receive LSSS or continue with their usual practice, after which intervention participants were again randomised to receive LSSS with or without price subsidy (Li 2016). As the LSSS intervention was the same in both these arms, we only extracted and used data for the overall LSSS group (both with and without subsidy) and the usual practice group.
Cluster‐RCTs
Four included cluster‐RCTs did not report sufficient information on adjustment for clustering in the statistical analysis or results section of either the full text (Hu 2018; Li 2014; Zhou 2013), or conference abstract (Zhang 2015). We calculated the effective sample sizes for these trials by calculating the design effect (DE), which is 1 + (c ‐ 1) x ICC, where c is the average cluster size. Our calculations were based on an estimated intra‐cluster correlation coefficient (ICC) of 0.04, reported by a study conducted in similar trial settings in Northern China (Neal 2017). For continuous data (e.g. DBP, SBP), we adjusted for the sample size only; while for dichotomous outcomes (e.g. cardiovascular events) we divided both the sample size and the number of people who experienced the event by the design effect. Where cluster‐RCTs reporting rates did not account for the effect of clustering in its analyses, we adjusted for clustering by inflating the standard errors by multiplying the standard error of the log(IRR) by the square root of the DE (Higgins 2011). All the estimates from cluster‐RCTs were combined with those from RCTs that had individual group assignment in our meta‐analyses (Higgins 2020b).
Dealing with missing data
We contacted study authors to request any missing or unreported data, such as group means, SDs, details of attrition, or details of the type of analysis conducted (e.g. intention‐to‐treat).
In cases where there were missing data due to attrition, we used the data available to conduct available case (modified intention‐to‐treat) meta‐analyses. We assessed the extent and impact of missing data and attrition for each included study during the Risk of bias assessment.
Assessment of heterogeneity
For each meta‐analysis, we examined the forest plots visually to determine whether heterogeneity of the size and direction of treatment effect was present between studies. We used the I² statistic, Tau², and the Chi² test to estimate the level of heterogeneity among the studies in each analysis. We defined substantial heterogeneity as Tau² > 0, and either I² > 50% or a low P value (< 0.10) in the Chi² test. Where substantial heterogeneity was found, we noted this in the text and explored it by conducting prespecified subgroup analyses to account for potential sources of clinical heterogeneity (see section: Subgroup analysis and investigation of heterogeneity). We also considered other potential sources of heterogeneity, for example, differences in the nature of the interventions delivered. In addition, we explored methodological sources of heterogeneity by examining studies with different levels of risk of bias in a sensitivity analysis (see section: Sensitivity analysis). We used caution in the interpretation of results with high levels of unexplained heterogeneity. We did not perform a meta‐analysis if the I² statistic was 90% or higher (considerable heterogeneity) (Deeks 2020).
Assessment of reporting biases
Where more than 10 included studies addressed a primary outcome, we used funnel plots to assess the possibility of small‐study effects and, in the case of asymmetry, intended to consider various explanations such as publication bias, poor study design and the effect of study size (Sterne 2017).
Data synthesis
All syntheses were conducted using Review Manager Web 2021 (RevMan Web 2021). We used a random‐effects meta‐analysis to combine data across more than one study, as we anticipated that there may be natural heterogeneity between studies, attributable to the different study settings, intervention strategies, or both. If a study only reported an MD and variance per group for an outcome, we first calculated MDs and 95% CI for the other studies reporting on that outcome, and then combined MDs and 95% CIs from all studies in a meta‐analysis using generic inverse variance (GIV). If a study reported rate ratios or events per person‐years, from which rate ratios could be calculated (see section: Measures of treatment effect), we also combined these rate ratios and 95% CIs in a meta‐analysis using GIV. Where studies reported event outcomes as rate ratios and risk ratios, these were combined in a meta‐analysis using GIV by using rate ratios as approximations for risk ratios.
We sought to only generate pooled estimates where data from separate studies were similar enough to be combined (see section: Assessment of heterogeneity). Data not suitable for pooling (defined as considerable heterogeneity, I2 ≥ 90%) in meta‐analyses were presented in forest plots without the pooled estimate, or in tables, as appropriate. Data from peer‐reviewed publications and conference abstracts were eligible for inclusion in meta‐analysis. Data from conference abstracts were identified in forest plots using footnotes. If needed, we also planned to conduct a narrative synthesis, by adopting a systematic approach to presentation, guided by the reporting guideline, Synthesis Without Meta‐analysis (SWiM) in systematic reviews (Campbell 2020).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses where data allowed using a test for interaction (i.e. heterogeneity across subgroups rather than across studies), calculating summary effect sizes for each subgroup in a univariate analysis for prespecified subgroups provided by WHO NUGAG, as follows.
All comparisons:
Study duration: short‐term (≤ 3 months) versus medium‐term (> 3 to 12 months) versus long‐term (> 12 months)
Gender: male versus female versus mixed versus unknown
Ethnicity: African versus Asian versus European versus mixed versus conducted in one setting (e.g. Europe), but ethnicity unspecified
Blood pressure status (as defined by study authors): hypertensive versus normotensive versus hypotensive versus mixed versus unknown
Baseline potassium intake: lower (urinary 24‐hour [24‐h] potassium excretion < 59 mmol [2.3 g] per day) versus higher (urinary 24‐h potassium excretion ≥ 59 mmol [2.3 g] per day) versus unknown or not reported as 24‐h excretion; based on global potassium intake estimates (Global Dietary Database 2022)
Baseline sodium intake: lower (urinary 24‐h sodium excretion < 172 mmol [3.95 g sodium or 9.88 g sodium chloride] per day) versus higher (urinary 24‐h sodium excretion ≥ 172 mmol [3.95 g sodium or 9.88 g sodium chloride] per day) versus unknown or not reported as 24‐h excretion; based on global sodium intake estimates (Powles 2013)
Iodine status (as defined by study authors): within normal ranges versus insufficient or deficient versus mixed versus unknown
Type of LSSS: based on proportion of potassium chloride: ≥ 30% KCl versus < 30% KCl versus unknown versus non‐potassium containing LSSS (based on the description of a 'typical' formulation of potassium‐based salt substitutes with a 50% sodium chloride reduction in Cepanec 2017)
LSSS implementation: discretionary only (through added LSSS in cooking and at table) versus non‐discretionary only (through consumption of manufactured products) versus discretionary and non‐discretionary
Salt as fortification vehicle: using salt as fortification vehicle versus not or unknown
All comparisons (only safety outcomes):
Possible risk of hyperkalaemia versus not at risk or unclear risk of hyperkalaemia (according to the criteria and assessment in Table 6), regardless of heterogeneity, only in the primary analyses of the following safety outcomes: change in blood potassium, hyperkalaemia, hypokalaemia and adverse events. The WHO NUGAG made the decision to limit this subgrouping to the safety outcomes since there are no clinical justifications to expect differences in the effects of LSSS on the effectiveness outcomes in populations possibly at risk of hyperkalaemia.
4. Summary of criteria and assessments applied to classify the hyperkalaemia risk of participants in the included studies .
| Study | Renal function reported at baseline (e.g. serum creatinine concentration, GFR) | Renal impairment at screening as exclusion criterion (e.g. or serum creatinine concentration, GFR) | Blood Pressure status reported at baseline | Use of antihypertensive medication reported at baseline | Use of antihypertensive medication at screening as exclusion criterion | Use of potassium‐sparing medications at screening as exclusion criterion | Assessment of hyperkalaemia risk |
| Allaert 2013 | NR | NR | Hypertensive, % (n/N): 100 (40/40) | No medication | Yes | No | Not at risk |
| Allaert 2017 | NR | Kidney failure or disease (not defined) | Pre‐hypertensive, % (n/N): 100 (22/22); 100 (19/19) | No medication | Yes | No | Not at risk |
| Arzilli 1986 | NR | NR | Hypertensive, % (n/N); 100 (10/10) | NR | NR | NR | Unclear risk a |
| Bernabe‐Ortiz 2014 | NR | A history of terminal or severe chronic kidney disease (receiving any form of dialysis) | (Hypertensive, % (n/N): 18.3 (428/2342) [village A 17.1 (91/534); village B 20.5 (90/449); village C 18.2 (59/329); village D 13.6 (56/414); village E 24.8 (79/328); village F 16.9 (53/322)] | NR | No | Yes | Not at risk |
| Chang 2006 | NR | Serum creatinine ≥ 3.5 mg/dL (>= 309 µmol/L) | Hypertensive, % (n/N): 40.2 (309/768); 40.4 (490/1213) | NR | No | No | Possibly at risk |
| CSSS Collaborative Group 2007 | Serum creatinine in µmol/L, mean (SD): 74.0 (20.0); 74.5 (19.1) | Abnormal serum creatinine concentrations | Hypertensive, % (n/N): 57 (173/306); 57 (172/302) |
Any antihypertensive medication, % (n): 61 (185/306); diuretic, % (n): 6 (19/306); ACE inhibitor or ARB, % (n): 10 (31/306); beta‐blocker, % (n): 6 (17/306); calcium antagonist, % (n): 23 (70/306) | No | Yes | Not at risk |
| Geleijnse 1994 | NR | Serum creatinine > 200 µmol/L | Hypertensive, % (n/N): 100 (49/49); 100 (51/51) | No medication | Yes | No | Possibly at risk |
| Gilleran 1996 | NR | Hypertensive nephropathy (persistent proteinuria or serum creatinine > 130 µmol/L) | Hypertensive, % (n/N): 100 (20/20); 100 (20/20) |
Stopped one month prior | Yes | No | Not at risk |
|
Hu 2018 (hypertensive participants) |
NR | Serum creatinine > 177 µmol/L | Hypertensive, % (n/N):100 (110/110); 100 (110/110) |
Antihypertensive medication, % (n/N): 71.8 (79/110); 77.3 (85/110) | No | Yes | Possibly at risk b |
|
Hu 2018 (family members) |
NR | Serum creatinine > 177 µmol/L | Family members: Hypertensive, % (n/N): 31.6 (59/187); 24.2 (45/186) | No medication | No | Yes | Possibly at risk b |
| Kawasaki 1998 | NR | NR | Hypertensive, % (n/N): 47.6 (10/21); 40.0 (4/20) | Beta‐blockers, calcium channel blockers or both, n/N: 19.0 (4/21); 20.0 (4/20) | No | No | Not at risk |
| Li 2014 | NR | Serious kidney diseases (not defined) | NR | NR | No | Yes | Not at risk |
| Li 2016 c | NR | Microalbuminuria, % (n/N) 6.6 (64/969); 9.2 (84/916); macroalbuminuria, % (n/N): 0.01 (5/975); 0.011 (10/928). | Hypertensive, %: 56.5 (731/1294); 58 (738/1272) | Antihypertensive medication use, % (n/N): 19 (246/1294); 21 (267/1272) | NR | NR | Unclear risk |
| Mu 2003 | NR | NR | Hypertensive, % (n/N): 100 (110/110); 100 (110/110) | NR | No | No | Unclear risk a |
|
Mu 2009 (participants with hypertension and family members) |
NR | Serum creatinine above normal range | Hypertensive, % (n/N): 100 (101/101); 100 (114/114) |
NR | No | Yes | Not at risk |
| Neal 2021 | NR | Serious kidney disease (not defined) | Hypertensive (uncontrolled), % (n/N): 59.4 (6240/10505) , 59.2 (6211/10491) | Any antihypertension medication use g, % (n/N): 79.9 (8393/10505), 78.7 (8256/10491); ACE inhibitor or ARB, % (n/N): 23.1 (2427/10505), 23.0 (2413/10491) | No | Yes | Possibly at risk |
| Omvik 1995 | NR | Serum creatinine above normal range |
Hypertensive, % (n/N): 100 (20/20); 100 (20/20) | No medication | No | No | Not at risk |
| Pan 2017 | NR | GFR < 60ml/min |
Hypertensive, %(n/N): 56.7 (55/97); 68.4 (62/95) | NR | No | Yes | Not at risk |
| Pereira 2005 | Serum creatinine in µmol/L, mean (SD): 81.35 (8.84); 80.46 (8.84) | Kidney disease (not defined) | Hypertensive, % (n/N): 100 (28/28) | Chlorthalidone 25 mg, % (n/N): 68 (15/22); on hydrochlorothiazide 25 mg, % (n/N): 32 (7/22) | Use of other antihypertensives other than those specified. |
Yes | Not at risk |
| Sarkkinen 2011 | NR | Abnormal kidney function (not defined) | Hypertensive, % (n/N): 100 (25/25); 100 (25/25) | No medication | Yes | Yes (NSAIDs, cyclosporine, tacrolimus) | Not at risk |
| Suppa 1988 | NR | Serum creatinine ≥ 1.5 mg/dL (133 µmol/L) |
Hypertensive % (n/N): 100 (163/163); 100 (159/159) | Beta‐blocker monotherapy (metoprolol), % (n/N): 100 (163/163); 100 (159/159) |
No | No | Not at risk |
| Toft 2020 | NR | NR | Normotensive | No medication | Yes | No | Not at risk |
| Yu 2021 | NR | History of acute or chronic kidney disease (CKD) g | Hypertensive, % (n/N) 100 (252/252); 100 (250/250) | Antihypertensive medication use, % (n/N): 97.2 (245/252); 94.4 (236/250); ACE inhibitors or ARB, % (n/N): 27.8 (70/252); 32.0 (80/250) | No | Yes | Possibly at risk |
| Zhang 2015 | Serum creatinine in µmol/L, mean (SD mean (SD): 69 (20.4); 69 (18.7) | NR | NR | NR | NR | NR | Unclear risk d |
| Zhao 2014 | NR | History of kidney disease | Hypertensive, % (n/N): 100 (141/141); 100 (141/141) | Antihypertensive use in the past month, % (n/N): 47.0 (61/141); 50.7 (71/141); average number of antihypertensive medicines taken, mean (SD): 0.4 (0.5); 0.5 (0.5) | No | No | Possibly at risk e |
| Zhou 2009 (hypertensive participants) | Serum creatinine, in µmol/L, mean (SD): 78.5 (18.5); 76.8 (19.0) | Impaired renal function (not defined) | Hypertensive, % (n/N): 100 (62/62); 100 (64/64) | Antihypertensive medication use, % (n/N): 53.2 (33/62); 54.7 (35/64) | No | Yes | Not at risk |
| Zhou 2009 (normotensive participants) | Serum creatinine, in µmol/L, mean (SD): 75.6 (21.2); 77.5 (18.9) | Impaired renal function (not defined) | Normotensive, % (n/N): 100 (57/57); 100 (65/65) | No medication | No | Yes | Not at risk |
| Zhou 2013 | NR | Significant renal impairment (not defined) | History of hypertension, % (n/N): 75 (169/224); 74 (176/238) | Captopril, nifedipine or compound reserpine, % (n/N): 41.07 (92/224); 40 (94/238) | No | Yes | Possibly at risk f |
Abbreviations: ACE: angiotensin‐converting enzyme ARB: angiotensin receptor blocker CKD: chronic kidney disease GFR: glomerular filtration rate NR: not reported NSAID: non‐steroidal anti‐inflammatory drug SD: standard deviation a Medication use and renal function unclear b Even though participants on potassium‐sparing medications were excluded, serum creatinine cut‐off used could still indicate sub‐optimal kidney function and possible risk of hyperkalaemia c Baseline data not collected. Values reflect data collected during the endline survey d Medication use not reported e Some of participants judged to be at risk due to antihypertensive medication use f Use of captopril g From Levey 2013
Additionally, for adults:
Age: adults younger than 65 years versus 65 years and older versus mixed ages versus unknown ages
Body mass index (BMI): underweight (< 18.5 kg/m2) versus normal weight (18.5 to 24.9 kg/m2) versus overweight (25 to 29.9 kg/m2) versus obese (≥ 30 kg/m2) for non‐Asian adults, or underweight (< 18.5 kg/m2) versus normal weight (18.5 to 22.9 kg/m2) versus overweight (23 to 24.9 kg/m2) versus obese (≥ 25 kg/m2) for Asian adults (WHO 2000)
Additionally, for children:
Age at start of study: 2 to 5 years versus 6 to 12 years versus 13 to 18 years versus mixed versus unknown
We also planned the following additional subgroup analyses, but available data did not allow these:
Term of pregnancy at start of study: first trimester versus second trimester versus third trimester versus mixed versus unknown (in comparison of pregnant women);
Conditions and risk factors: renal impairment versus other NCDs versus medication use that impair potassium excretion versus mixed versus unknown (in comparison of pregnant women).
Sensitivity analysis
We conducted sensitivity analyses for primary outcomes if we had three or more studies per meta‐analysis, assessing the impact of:
Risk of bias: removing studies with a high risk of overall bias (see section: Assessment of risk of bias in included studies);
Study design: removing cluster‐RCTs
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to judge the certainty of the evidence as it relates to the studies contributing data to the meta‐analyses for the main outcomes, using GRADEprofiler (GRADEpro) software (GRADEpro GDT). The GRADE approach assesses certainty as high, moderate, low, or very low according to five criteria, namely, risk of bias, inconsistency of results, indirectness, imprecision and publication bias (Schünemann 2020).
For the following outcomes, we presented assessments in a GRADE Evidence Profile for Comparisons 1 and 3 (i.e. per type of population included in this review): DBP, SBP, hypertension, blood pressure control, cardiovascular events, cardiovascular mortality, blood potassium, hyperkalaemia, hypokalaemia and adverse events (other). We could not compile GRADE Evidence Profiles for the comparison in pregnant women as no eligible studies reported on outcomes in pregnant women. The effects of interventions on the outcomes included in the GRADE Evidence Profiles were interpreted according to magnitude of effect and certainty of the evidence, using GRADE guidance on informative statements to combine size and certainty of an effect (Santesso 2020).
We used the approaches described below for each domain to guide our ratings and included explanations as footnotes in the GRADE Evidence Profiles.
Risk of bias
We considered downgrading if the majority (> 50%) of the weighted outcome data in a meta‐analysis were from studies at a high or unclear overall risk of bias.
Inconsistency
We considered downgrading due to either unexplained considerable (defined as I² ≥ 90%) or substantial heterogeneity (defined as I² between 50% to < 90%). We explored heterogeneity using prespecified subgroup analysis, and examined sensitivity analyses of study design and quality (overall risk of bias).
Indirectness
We considered downgrading based on population characteristics such as age, ethnicity, blood pressure status; or due to intervention characteristics (e.g. purpose of intervention, LSSS type), comparator, direct comparison and outcome.
Imprecision
Number of events or participants
We considered downgrading based on an insufficient number of events (i.e. 300 events) for dichotomous outcomes or sample size not meeting the optimal information size (OIS) (i.e. 400 people providing outcome measures) for continuous outcomes.
Minimally contextualised approach to GRADE ratings
In line with recent GRADE guidance (Hultcrantz 2017; Zeng 2021), we selected a minimally contextualised approach that required us to specify thresholds for minimally important differences for key outcomes. The upper and lower limits of the 95% CIs were assessed in the same way to determine if they included the possibility of a small, trivial or no effect and an important benefit or harm (Zeng 2021).
Applying this approach to rating the certainty of evidence using GRADE in relation to thresholds (other than no effect) ideally requires the use of absolute numbers (Zeng 2021). To further support decision‐making by the WHO NUGAG Subgroup on Diet and Health about a population‐level intervention, we generated estimated population impacts for effect estimates and variation (95% CIs) for key clinical effectiveness outcomes when LSSS use was compared to regular salt use (Verbeek 2021). This was applied to the following key clinical effectiveness outcomes in adults: change in DBP, change in SBP, cardiovascular events: non‐fatal stroke, cardiovascular events: non‐fatal acute coronary syndrome, cardiovascular mortality and stroke mortality. We used a simplified model to estimate absolute numbers from relative cardiovascular measures, as well as the absolute numbers of stroke deaths prevented or caused by changes in blood pressure, as a surrogate outcome (Verbeek 2021). More detail on this simplified modelling approach can be found in Appendix 2.
Results
Description of studies
For detailed information, see Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
The study selection flowchart is available in Figure 1. We screened the titles and abstracts of 6511 de‐duplicated records identified through searching electronic databases, as well as 14 records identified through handsearching of three relevant systematic reviews. We assessed the full texts of 161 records against our eligibility criteria, of which four were in Chinese and one in Portuguese; we obtained language translation assistance for assessment of these. We included 26 studies reported in 74 full‐text records (Included studies), of which one did not provide data that could be used in the quantitative syntheses (meta‐analyses) (Arzilli 1986). Eight studies were identified as ongoing. We placed three studies under awaiting classification because we were unable to obtain further study details or data from the study authors in order to assess their eligibility for inclusion. We excluded a total of 75 full‐text records, of which 42 were duplicates (Excluded studies).
1.
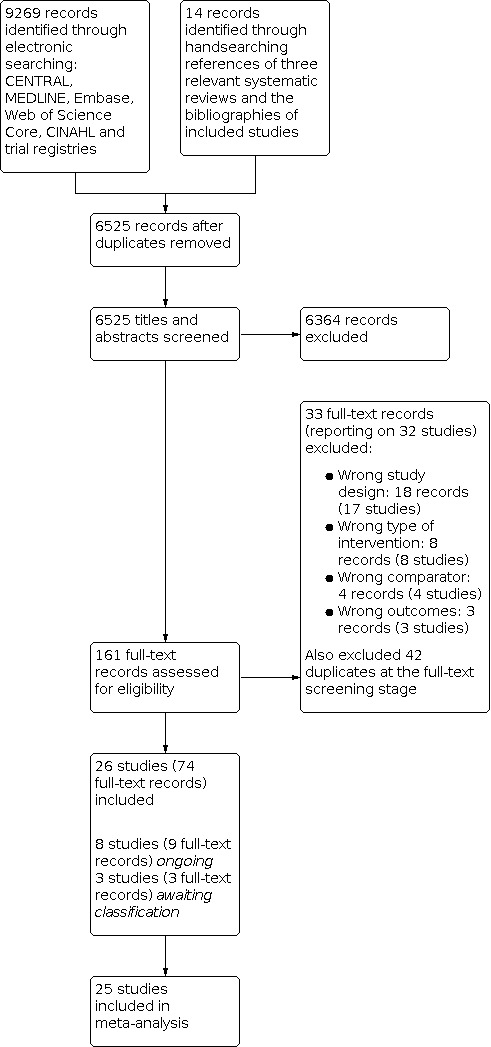
PRISMA flow diagram
Included studies
Study designs
We included 26 eligible RCTs and did not identify any eligible prospective analytical cohort studies. The details of the included studies are summarised in Characteristics of included studies. Of the included trials, 16 were individually randomised trials (Allaert 2013; Allaert 2017; Arzilli 1986; CSSS Collaborative Group 2007; Geleijnse 1994; Gilleran 1996; Kawasaki 1998; Mu 2003; Omvik 1995; Pan 2017; Pereira 2005; Sarkkinen 2011; Suppa 1988; Yu 2021; Zhao 2014; Zhou 2009) and 10 were cluster‐RCTs (Bernabe‐Ortiz 2014; Chang 2006; Hu 2018; Li 2014; Li 2016; Mu 2009; Neal 2021; Toft 2020; Zhang 2015; Zhou 2013), including one stepped‐wedge cluster‐RCT (Bernabe‐Ortiz 2014). One RCT reported a cross‐over design (Allaert 2013) for which we only used first‐phase data. Twenty‐five of the eligible trials were published in peer‐reviewed journals; one cluster‐RCT was published in three separate conference abstracts and one trial as an abstract in a journal supplement. Full‐text publications of these could not be sourced after numerous attempts to contact authors.
Sample sizes and follow‐up
Nine of the 16 RCTs randomised ≤ 100 participants while seven were larger, randomising > 100 participants up to a maximum of 608 participants (CSSS Collaborative Group 2007; Mu 2003; Pan 2017; Suppa 1988; Yu 2021; Zhao 2014; Zhou 2009). Fewer than half of these RCTs (n = 7) reported sample size calculations, based on expected changes of between 3 and 10 mmHg in SBP (Allaert 2013; Allaert 2017; CSSS Collaborative Group 2007; Geleijnse 1994; Yu 2021; Zhao 2014; Zhou 2009); of these, two trials additionally based sample size calculations on expected changes of between 1.7 and 4 mmHg in DBP (CSSS Collaborative Group 2007; Geleijnse 1994).
Of the cluster‐RCTs, five randomised families or households (Hu 2018; Li 2014; Mu 2009; Toft 2020; Zhou 2013), ranging between 89 and 325 households, including 309 to 659 individual participants. Three cluster‐RCTs randomised villages; one conducted in Peru (N = 6 clusters; 2376 participants; Bernabe‐Ortiz 2014) and two conducted in China (N = 120 clusters; 2566 participants; Li 2016 and N = 600; 20,995 participants; Neal 2021). The cluster‐RCT by Zhang 2015 randomised nursing homes (N = 30); another randomised kitchens within a retirement home (N = 5; 2764 participants) (Chang 2006). Of the ten cluster‐RCTs, only four reported appropriate sample size calculations (including an ICC) based on expected changes in blood pressure, 24‐h sodium excretion, relative reduction in stroke and sodium intake reduction (Bernabe‐Ortiz 2014; Li 2016; Neal 2021; Toft 2020, respectively); two cluster‐RCTs (Chang 2006; Mu 2009) did not report a sample size calculation but did adjust for the effect of clustering in their analyses. One cluster‐RCT (Hu 2018) reported a sample size calculation based on an expected change in SBP, but did not report incorporating an ICC in this calculation.
Eleven (Allaert 2013; Allaert 2017; Arzilli 1986; CSSS Collaborative Group 2007; Gilleran 1996; Kawasaki 1998; Mu 2003; Omvik 1995; Sarkkinen 2011; Suppa 1988; Zhou 2009) of the 16 RCTs included a run‐in period, ranging between five days and six weeks. Ten RCTs tested an active LSSS intervention for a period of up to three months (Allaert 2013; Allaert 2017; Arzilli 1986; Geleijnse 1994; Kawasaki 1998; Pereira 2005; Sarkkinen 2011; Suppa 1988; Yu 2021; Zhao 2014), and five for between three and 12 months (CSSS Collaborative Group 2007; Gilleran 1996; Omvik 1995; Pan 2017; Zhou 2009). One RCT implemented a LSSS intervention for longer than 12 months (Mu 2003). For the 10 cluster‐RCTs, the duration of the LSSS intervention was two months in one trial (Li 2014), four months in another (Toft 2020), and ranged between one and five years in the other eight cluster‐RCTs.
Settings
Four of the 16 RCTs were conducted in northern China or Tibet; most were done in rural or suburban households (CSSS Collaborative Group 2007; Mu 2003; Zhao 2014; Zhou 2009). The remaining individually randomised trials from Brazil (Pereira 2005), France (Allaert 2013; Allaert 2017), Finland (Sarkkinen 2011), India (Yu 2021); Italy (Suppa 1988), Japan (Kawasaki 1998), Netherlands (Geleijnse 1994), Norway (Omvik 1995), Taiwan (Pan 2017) and the UK (Gilleran 1996) were conducted at household level, except for one trial in a European hospital setting (Arzilli 1986). Seven cluster‐RCTs were conducted in northern China or Tibet; most were done in rural or suburban households or communities (Hu 2018; Li 2014; Li 2016; Mu 2009; Neal 2021; Zhou 2013), with one cluster‐RCT having been conducted in nursing homes (Zhang 2015). One included cluster‐RCT from Taiwan (Chang 2006) was also conducted in a nursing home setting. In Peru, one stepped‐wedge cluster‐RCT was conducted in rural villages and households (Bernabe‐Ortiz 2014); a cluster‐RCT in Southwestern Denmark was conducted in families (Toft 2020).
Participants
We did not find any eligible studies in pregnant women. Trial participants were adults with a mean age ranging from 20 to 75.21 years and children with a mean age ranging from 8.4 to 9.5 years. Most of the included trials (15/26) were conducted in populations living in Asian countries. Eleven studies specifically included only participants with hypertension (Allaert 2013; Arzilli 1986; Geleijnse 1994; Gilleran 1996; Mu 2003; Omvik 1995; Pereira 2005; Sarkkinen 2011; Suppa 1988; Yu 2021; Zhao 2014), 11 included participants with and without hypertension (Bernabe‐Ortiz 2014; Chang 2006; CSSS Collaborative Group 2007; Hu 2018; Kawasaki 1998; Li 2014; Mu 2009; Neal 2021; Pan 2017; Zhou 2009; Zhou 2013), one each included only participants with normal blood pressure (Toft 2020) and participants who were pre‐hypertensive (Allaert 2017), and blood pressure status at baseline in the remaining studies were unknown (Li 2016; Zhang 2015). The largest trial (Neal 2021) included participants with an elevated risk of stroke and approximately 70% of participants in intervention and control groups had a history of stroke at baseline. Fifteen studies reported outcome data separately in participants with hypertension (Allaert 2013; Arzilli 1986; Geleijnse 1994; Gilleran 1996; Hu 2018; Kawasaki 1998; Mu 2003; Mu 2009; Omvik 1995; Pereira 2005; Sarkkinen 2011; Suppa 1988; Yu 2021; Zhao 2014; Zhou 2009). Twelve of these studies specifically included only participants with hypertension. Three of these studies included participants with hypertension and their family members (Hu 2018; Mu 2009; Zhou 2009) and one study included clinically healthy middle‐aged and elderly volunteers (Kawasaki 1998); these studies additionally reported outcome data in participants with normal blood pressure separately.
One cluster‐RCT compared LSSS to regular salt in 92 children (numbers analysed) (Toft 2020) by randomising families to LSSS or regular salt in bread. Seven studies included participants possibly at risk of hyperkalaemia (Chang 2006; Geleijnse 1994; Hu 2018; Neal 2021; Yu 2021; Zhao 2014; Zhou 2013), four studies included participants at unclear risk of hyperkalaemia (Arzilli 1986; Li 2016; Mu 2003; Zhang 2015) and the remaining trials included participants considered not to be at risk of hyperkalaemia. The criteria and assessments applied to classify the hyperkalaemia risk of participants per included study are summarised in Table 6. All 26 included trials excluded participants in whom an increased intake of potassium is known to be potentially harmful, for example, people with chronic kidney disease, type 1 or 2 diabetes mellitus, impaired renal function, or those using potassium‐sparing medications.
Interventions
In 23 of the 26 studies, combinations of potassium and/or magnesium and/or calcium salts were used as sodium substitutes in the LSSS interventions, with two studies assessing a LSSS intervention consisting of NaCl combined with 3% chitosan (Allaert 2013; Allaert 2017) and the remaining study assessing a LSSS intervention ‘naturally low in sodium’ in bread (Toft 2020). Product characteristics of the latter, obtained through author correspondence (Toft 2020), showed that the compound contained trace amounts of potassium (approximately 0.1 to 0.2%). Two RCTs (Arzilli 1986; Mu 2003) and one cluster‐RCT (Mu 2009) assessed LSSS with an unknown KCl content. Four cluster‐RCTs (Chang 2006; Li 2014; Neal 2021; Zhang 2015) and six RCTs (Geleijnse 1994; Gilleran 1996; Pan 2017; Pereira 2005; Yu 2021; Zhou 2009) assessed the effects of a LSSS intervention containing ≥ 30% KCl, while the remainder of the trials used LSSS interventions containing < 30% KCl. One RCT included two LSSS intervention arms, both including ≥ 30% KCl (Pan 2017).
Most trials (22/26) administered the LSSS intervention as a discretionary intervention (at the individual, household, institution or salt supply chain level). Of these, most trials replaced the supply of regular salt with LSSS within each household, to be used at the table and during food preparation (Bernabe‐Ortiz 2014; CSSS Collaborative Group 2007; Gilleran 1996; Hu 2018; Li 2014; Li 2016; Mu 2003; Mu 2009; Neal 2021; Omvik 1995; Pan 2017; Pereira 2005; Yu 2021; Zhao 2014; Zhou 2009; Zhou 2013). Two trials were conducted in nursing homes where LSSS was used during food preparation in the intervention kitchens of one trial (Chang 2006), while the specific implementation of the LSSS was unclear in the other trial (Zhang 2015). LSSS was administered as ‘added salt’ in four trials (Allaert 2013; Allaert 2017; Arzilli 1986; Suppa 1988).
A cluster‐RCT from Peru replaced the supply of regular salt in each village with LSSS in the salt supply chain, including households, food vendors, bakeries, community kitchens and restaurants. A social marketing/education strategy promoting LSSS in each village was aimed at women who were responsible for household food preparation (Bernabe‐Ortiz 2014). Another cluster‐RCT from northern China provided LSSS via the local food supply chain. LSSS was available for purchase at local village shops at either a subsidised price (same as regular salt) in half of the intervention villages, or at a regular price (approximately double that of regular salt). A community‐based health education programme to promote the use of LSSS was implemented via public announcement systems, bulletin boards, and specially developed promotional materials (Li 2016).
Three RCTs incorporated LSSS into prepared test foods, such as processed main dishes, bread, cheese, luncheon meats, soups or smoked sausage (Geleijnse 1994; Sarkkinen 2011), or seasonings containing LSSS, such as miso and soy sauce (Kawasaki 1998). These trials also provided trial participants with LSSS as salt for household food preparation and for use as table salt. A fourth cluster‐RCT used bread as the exclusive method of LSSS implementation by incrementally replacing normal salt with LSSS in bread over a period of five to six weeks, with participants followed up for four months in total (Toft 2020).
Trial participants in four studies were instructed not to change their dietary habits during the study period (Allaert 2017; Geleijnse 1994; Hu 2018; Kawasaki 1998), whereas participants from two trials were advised to either reduce their salt intake (Omvik 1995), or avoid salt‐rich foods (Sarkkinen 2011). Two trials reported co‐interventions such as lifestyle advice about eating less fat and sugar and doing more physical exercise (Allaert 2013), or a hypocaloric diet with increased physical exercise (Pereira 2005).
Outcome measures
Outcomes were regarded as clinical effectiveness outcomes, or safety outcomes related to the intake of LSSS with potassium (as guided by the WHO NUGAG Subgroup on Diet and Health).
Clinical effectiveness outcomes for comparisons in both adults and children were change in DBP, change in SBP, hypertension, blood pressure control, cardiovascular events, cardiovascular mortality, all‐cause mortality, antihypertensive medication use, change in fasting blood glucose, change in blood triglycerides, change in total blood cholesterol, and change in 24‐h urinary sodium and potassium excretion. In addition, clinical effectiveness outcomes in adults only were diabetes mellitus diagnosis and change in BMI; and in children only were growth changes, bone densitometry and bone health.
Safety outcomes for comparisons in both adults and children were change in blood potassium, hyperkalaemia, hypokalaemia, adverse events, renal function and hyponatraemia.
Primary outcomes
Only one RCT (Pan 2017) and one cluster‐RCT (Chang 2006) did not report on changes in DBP and SBP. However, we were also unable to use relevant outcome data from four trials, due to blood pressure data being reported in a figure (CSSS Collaborative Group 2007 (DBP only); Kawasaki 1998 (DBP and SBP)) or study authors providing insufficient information on the number of participants per treatment group (Arzilli 1986; Mu 2009). Three RCTs reported two types of DBP and SBP outcome data, i.e. ambulatory and clinic BP measurements (Allaert 2017; Omvik 1995; Pereira 2005). In order to minimise potential heterogeneity between studies, we included only the clinic BP measurements from these trials in our meta‐analyses.
Two cluster‐RCTs reported on the outcome hypertension; defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or the use of blood‐pressure lowering therapy in the last two weeks (Li 2016) or SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, a self‐reported physician diagnosis or current treatment for hypertension (Bernabe‐Ortiz 2014). Two RCTs reported on blood pressure control, defined as achieving SBP ≤ 140 mmHg and DBP ≤ 90 mmHg in both trials (Allaert 2013; Zhao 2014).
For the outcome cardiovascular events, we extracted data related to events such as stroke (Gilleran 1996; Hu 2018; Neal 2021; Pan 2017; Zhao 2014), myocardial infarction (Hu 2018) or acute coronary syndrome (Neal 2021), coronary heart disease or heart failure (Li 2016), hypotension (Li 2016), angina (Allaert 2017; Omvik 1995), bradycardia (Suppa 1988), as well as composite outcomes such as cardiovascular events (CSSS Collaborative Group 2007; Zhou 2009) or cardiovascular symptoms (Sarkkinen 2011). Three trials reported insufficient data on the number of events per group (Li 2016; Suppa 1988) as described in Table 7. Cardiovascular mortality was reported by one RCT (Zhao 2014) and three cluster‐RCTs (Chang 2006; Neal 2021; Zhou 2013); stroke mortality was reported by two cluster‐RCTs (Neal 2021; Zhou 2013).
5. Trials without usable data for outcomes in Summary of Findings tables in Comparison 1.
| Outcomes included in Summary of Findings tables | Included studies that are believed to have measured the outcome, but did not report it in a usable format |
| Change in DBP (mmHg) |
Arzilli 1986: only between‐group P values reported CSSS Collaborative Group 2007: reported in a figure Kawasaki 1998: reported mean change in intervention group, but not control group Mu 2009: reported only mean change with no SDs or participant numbers |
| Change in SBP (mmHg) |
Arzilli 1986: only between‐group P values reported Kawasaki 1998: reported mean change in intervention group, but not control group Mu 2009: reported only mean change with no SDs or participant numbers |
| Hypertension | None |
| Blood pressure control | None |
| Cardiovascular events: various |
Li 2016: reported non‐significant difference between groups (not exact P value), mean difference not reported Suppa 1988: numbers of events per group not reported |
| Cardiovascular events: non‐fatal stroke | None |
| Cardiovascular events: non‐fatal ACS | None |
| Cardiovascular mortality | None |
| Stroke mortality | None |
| Change in blood potassium (mmol/L) | Sarkkinen 2011: reported change and significance of change in control group only |
| Hyperkalaemia | Li 2016: reported non‐significant difference between groups (not exact P value), mean difference not reported |
| Hypokalaemia | None |
| Adverse events: other | Suppa 1988: numbers of events per group not reported |
Abbreviations: ACS: acute coronary syndrome DBP: diastolic blood pressure SBP: systolic blood pressure SD: standard deviation
Six trials reported on hyperkalaemia events; two trials (CSSS Collaborative Group 2007; Mu 2009) did not explicitly define criteria for hyperkalaemia, and one assessed self‐reported hyperkalaemia without defined criteria (Li 2016). Yu 2021 and Zhang 2015 defined the outcome as a serum potassium level more than 6.5 mmol/L and 5.5 mmol/L, respectively. Neal 2021 reported on definite, probable, possible and unlikely hyperkalaemia ‐ only data on definite and probable events, defined as elevated serum potassium > 5.5 mmol/L and typical electrocardiogram (ECG) changes documented in medical notes, were extracted; events that were possible (self‐reported serum potassium > 5.5 mmol/L or ECG changes but no supporting documentation to verify) and unlikely (clinical history and documentation suggest minimal indication for the diagnosis) were excluded from our review. Insufficient information was available from one trial (Li 2016) as described in Table 7. Only one RCT reported on hypokalaemia events (Pereira 2005), though criteria for the condition were not explicitly defined.
Seven trials reported changes in serum (Allaert 2017; Geleijnse 1994; Kawasaki 1998; Pereira 2005; Zhang 2015) or plasma concentrations of potassium (Omvik 1995; Sarkkinen 2011) between intervention and control groups. Data could not be extracted from one study (Sarkkinen 2011), as described in Table 7.
Secondary outcomes
Four RCTs (CSSS Collaborative Group 2007; Pan 2017; Zhao 2014; Zhou 2013), and three cluster‐RCTs (Chang 2006; Neal 2021; Zhang 2015) reported on all‐cause mortality.
Three trials reported on the occurrence of serious adverse events (not defined) during the study period (Bernabe‐Ortiz 2014; CSSS Collaborative Group 2007; Pan 2017). Other adverse event outcomes extracted included gastrointestinal symptoms (stomach ache or abdominal distension) (Sarkkinen 2011; Zhao 2014), hypercalcemia and renal calculi (Mu 2009), appendicitis, nephritis, nephrosis (Hu 2018), influenza (Allaert 2017), respiratory symptoms (Sarkkinen 2011) and dorsalgia (Allaert 2017). Suppa 1988 included self‐reported adverse events including asthenia, bradycardia, drowsiness, insomnia, decreased libido and depression, but did not report the number of events per group. None of the included trials reported adverse events such as nausea or vomiting.
Five trials reported on changes in antihypertensive medication use (Bernabe‐Ortiz 2014; Hu 2018; Li 2016; Zhao 2014; Zhou 2013). Four trials reported the number of participants using antihypertensive medications: two trials reported on this outcome in participants with hypertension (Hu 2018 included participants with hypertension and their family members, but only reported on the former; Zhao 2014 included only participants with hypertension) and one trial each in participants with unknown hypertensive status (Li 2016) and participants using hypotensive medication at baseline (Zhou 2013). Bernabe‐Ortiz 2014 assessed participant‐reported changes in medication use for hypertension and type 2 diabetes mellitus combined.
Three trials reported changes in serum creatinine (Omvik 1995; Pereira 2005; Zhang 2015). One cluster‐RCT (Li 2016) reported the mean urinary albumin‐to‐creatinine ratios of participants in the intervention and control groups, as well as the proportion of participants with albuminuria (including micro‐ and macro‐albuminuria) in both groups.
Four studies reported changes in BMI (Li 2016; Pereira 2005; Sarkkinen 2011; Toft 2020), while two reported changes in fasting blood glucose concentrations (Toft 2020; Zhou 2013). Five studies reported changes in blood triglycerides (Gilleran 1996; Kawasaki 1998; Pereira 2005; Toft 2020; Zhou 2009) and total blood cholesterol (Geleijnse 1994; Gilleran 1996; Kawasaki 1998; Toft 2020; Zhou 2009), respectively.
A total of 12 studies collected 24‐h urine samples and reported on 24‐h urinary sodium excretion (Bernabe‐Ortiz 2014; Geleijnse 1994; Gilleran 1996; Kawasaki 1998; Li 2016; Neal 2021; Omvik 1995; Sarkkinen 2011; Suppa 1988; Toft 2020; Yu 2021; Zhou 2009); the same studies reported on 24‐h urinary potassium excretion from 24‐h urine samples.
None of the included studies reported on the outcomes of diabetes mellitus diagnosis or hyponatremia.
Funding sources and conflicts of interest
Nine of the 26 studies did not disclose their funding source(s). The remaining studies were funded as follows:
nine public/non‐commercial funding only, including government bodies and research institutions (Bernabe‐Ortiz 2014; CSSS Collaborative Group 2007; Li 2014; Li 2016; Mu 2009; Pan 2017; Toft 2020; Zhao 2014; Zhou 2013);
four public/non‐commercial funding plus LSSS provided for the trial by LSSS manufacturer (Geleijnse 1994; Neal 2021; Omvik 1995; Yu 2021);
two commercial funding by LSSS manufacturers plus LSSS provided for the trial (Chang 2006; Sarkkinen 2011);
one commercial funding for the study from food industry research fund plus LSSS provided for the trial by LSSS manufacturer (Hu 2018); and
one LSSS provided for the trial by the LSSS manufacturer (Kawasaki 1998).
The authors of 13 included studies did not report on potential conflicts of interest (COI), whereas those from 13 studies did. Of these 13 studies, authors of eleven declared that they had no potential COI (Bernabe‐Ortiz 2014; Chang 2006; CSSS Collaborative Group 2007; Mu 2009; Pan 2017; Pereira 2005; Toft 2020; Yu 2021; Zhao 2014; Zhou 2009; Zhou 2013), whereas the author of one study declared a potential conflict of interest as the chair of the Australian Division of World Action on Salt and Health (Li 2016), and some members of the author team of a large cluster‐RCT declared potential conflicts of interest, while the remaining members declared no conflict (Neal 2021).
Excluded studies
We contacted nine corresponding authors for further information to assist with study inclusion. We excluded 32 studies (33 full‐text records) due to the following reasons:
Wrong study design (single‐arm trial): 5 Wrong study design (commentary/letter): 3 Wrong study design (case report/study): 2 Wrong study design (case series): 2 Wrong study design (non‐randomised trial): 2 Wrong study design (quasi‐randomised trial): 2 Wrong study design (cross‐over with first phase data not available): 1 Wrong type of intervention (multifactorial): 4 Wrong type of intervention (dietary): 2 Wrong type of intervention (LSSS administered as supplement): 1 Wrong type of intervention (salt restriction education): 1 Wrong comparator: 4 Wrong outcome (sensory/organoleptic): 2 Wrong outcome (sodium concentration of homemade food): 1
The Characteristics of excluded studies section illustrates these 32 studies with reasons for exclusion. We also excluded 42 duplicates at the full‐text screening stage. The remaining references where we could not reach the authors or information provided was not sufficient to make a clear judgement (n = 3) were included as Studies awaiting classification. The eight ongoing studies are detailed in Characteristics of ongoing studies.
Risk of bias in included studies
The Characteristics of included studies provides details of the judgements for each risk of bias domain per study. Figure 2 presents a summary of the risk of bias judgements for each included study and Figure 3 the summary of the judgements per risk of bias domain.
2.

Summary of the risk of bias judgements for each included study
3.

Summary of the judgements per risk of bias domain
Allocation
Random sequence generation
Fifteen trials described adequate methods of random sequence generation and were at low risk of selection bias (Bernabe‐Ortiz 2014; Chang 2006; CSSS Collaborative Group 2007; Geleijnse 1994; Hu 2018; Li 2014; Li 2016; Mu 2009; Neal 2021; Pan 2017; Toft 2020; Yu 2021; Zhao 2014; Zhou 2009; Zhou 2013). Eleven trials did not report how the random sequence had been generated and were at unclear risk of selection bias.
Allocation concealment
Eleven trials described methods of allocation concealment judged to be at low risk of selection bias (Allaert 2017; Bernabe‐Ortiz 2014; CSSS Collaborative Group 2007; Hu 2018; Li 2016; Neal 2021; Pan 2017; Toft 2020; Yu 2021; Zhao 2014; Zhou 2009). The remaining fifteen did not report sufficient information on allocation concealment, and were at unclear risk of selection bias.
Blinding
Blinding of participants and personnel (performance bias)
Performance bias was assessed as unlikely in fifteen trials, while eleven trials had an unclear risk of bias, mainly due to insufficient information on blinding of either participants or personnel (Allaert 2013; Arzilli 1986; Bernabe‐Ortiz 2014; Chang 2006; Kawasaki 1998; Li 2014; Li 2016; Mu 2003; Mu 2009; Suppa 1988; Zhang 2015).
Blinding of outcome assessment (detection bias)
Nineteen trials were assessed to have a low risk of detection bias, while seven had an unclear risk mainly due to insufficient information on blinding of outcome assessors (Li 2016; Mu 2003; Mu 2009; Pereira 2005; Suppa 1988; Zhang 2015; Zhou 2013). Some of these trials reported the measurement of blood pressure with non‐automatic devices, increasing the likelihood of detection bias (Mu 2003; Pereira 2005; Suppa 1988).
Incomplete outcome data
Four trials were at a high risk of bias due to high overall or differential attrition (≥ 10%) (Geleijnse 1994; Gilleran 1996; Hu 2018; Mu 2003). Fourteen studies were at low risk because they reported low overall or differential attrition (Allaert 2013; Allaert 2017; Chang 2006; CSSS Collaborative Group 2007; Kawasaki 1998; Li 2014; Neal 2021; Omvik 1995; Sarkkinen 2011; Suppa 1988; Toft 2020; Yu 2021; Zhou 2009; Zhou 2013). Two studies reported high attrition; however intention‐to‐treat analyses (ITT) were conducted using multiple imputation in one study (Zhao 2014) and the last‐observation‐carried‐forward method in the other study (Pan 2017); therefore, these were judged as being at low risk. High attrition was reported at some time points in the stepped‐wedge cluster‐RCT; however, it was unclear whether any data were imputed and therefore this study had an unclear risk (Bernabe‐Ortiz 2014). Five additional studies were at unclear risk of bias since insufficient information on attrition was provided.
Selective reporting
Two trials were assessed as being at high risk of bias. Substudies of both trials reported outcomes not prespecified in the study protocol (CSSS Collaborative Group 2007; Li 2016). Two trials at low risk of bias reported outcomes prespecified in the study protocol; the remaining studies had an unclear risk due to inadequate reporting of all prespecified outcomes, or the unavailability of the study protocol.
Other potential sources of bias
One trial was assessed as being high risk for misclassification bias due to limited information for adjudication of clinical outcome events, as well as self‐reported potential hyperkalaemia events (Neal 2021). Two trials were assessed as being at unclear risk. In the stepped‐wedge trial, a reduction in blood pressure was observed in some clusters (villages) before the intervention (Bernabe‐Ortiz 2014) while, in another cluster‐RCT, there was a considerable risk of contamination in the intervention group due to the unlimited availability of condiments and spices such as soy sauce and monosodium glutamate (Chang 2006). No potential sources of bias were identified in the remaining studies.
Additional domains assessed for cluster‐RCTs
Recruitment bias
One trial had a high risk for recruitment bias since a number of participants were recruited after the clusters (kitchens) were randomised (Chang 2006). Six trials reported recruitment of participants before randomisation of clusters and were therefore considered at low risk (Bernabe‐Ortiz 2014; Hu 2018; Li 2014; Li 2016; Neal 2021; Toft 2020). Three trials did not provide sufficient information regarding the timing of recruitment.
Comparability with individually randomised trials (RCTs)
Six trials at low risk of bias reported effect estimates comparable to those reported by similar RCTs (Bernabe‐Ortiz 2014; Chang 2006; Hu 2018; Li 2014; Zhang 2015; Zhou 2013), whereas it was not possible to compare the effect estimates in four trials.
Baseline imbalance
Three trials reported no differences in baseline characteristics of the participants in the intervention and control groups (Hu 2018; Li 2014; Neal 2021), while two adjusted for baseline differences and were therefore also considered to be at low risk (Bernabe‐Ortiz 2014; Zhou 2013). Five studies that reported insufficient information regarding baseline characteristics were at unclear risk of bias.
Loss of clusters
One trial reported an analysis without outcome data from almost one‐third of their included clusters, thus was considered at high risk of attrition bias (Zhang 2015). Seven trials had a low risk (Bernabe‐Ortiz 2014; Chang 2006 ; Hu 2018; Li 2014; Li 2016; Neal 2021; Toft 2020) while two did not provide sufficient information on the number of clusters (families) lost to follow‐up (Mu 2009), or the reasons for loss to follow‐up (Zhou 2013).
Incorrect analysis
Three trials did not report adjustment for clustering in their analysis, thus were considered at high risk of bias (Hu 2018; Li 2014; Zhou 2013). Six trials were at low risk; five reported statistical adjustment for clustering (Chang 2006; Li 2016; Mu 2009; Neal 2021; Toft 2020) and one also accounted for time trends in their analysis (Bernabe‐Ortiz 2014). One trial that reported insufficient information regarding statistical adjustment for clusters was considered at unclear risk.
Overall risk of bias
Three RCTs and three cluster‐RCTs had a high overall risk of bias due to attrition (Geleijnse 1994; Gilleran 1996; Hu 2018; Mu 2003; Zhang 2015) or recruitment bias (Chang 2006). Six RCTs had a low overall risk (Allaert 2017; CSSS Collaborative Group 2007; Pan 2017; Yu 2021; Zhao 2014; Zhou 2009) and two cluster‐RCTs (Li 2014; Neal 2021) had a low overall risk, while the remaining seven RCTs and five cluster‐RCTs had an unclear risk mainly due to uncertainty about selection, attrition and recruitment bias (Allaert 2013; Arzilli 1986; Bernabe‐Ortiz 2014; Kawasaki 1998; Li 2016; Mu 2009; Omvik 1995; Pereira 2005; Sarkkinen 2011; Suppa 1988; Toft 2020; Zhou 2013).
Effects of interventions
Comparison 1. Low‐sodium salt substitutes versus regular salt or no active intervention in adults
Table 1 presents the effects of LSSS compared to regular salt or no active intervention in adult participants on changes in DBP and SBP; as well as the number of participants per group with hypertension, blood pressure control, cardiovascular events (various events and non‐fatal stroke); the rate ratio of participants in the intervention group with non‐fatal acute coronary syndrome (ACS), cardiovascular mortality and stroke mortality; changes in blood potassium; and the number of participants per group with hyperkalaemia, hypokalaemia and other adverse events.
A total of 26 RCTs, 16 randomising individual participants and 10 randomising clusters, reporting on 34,961 adult participants, were included in this comparison. Key details about studies in this comparison, including study design, setting and overall risk of bias; characteristics of the intervention, comparator, population and outcomes; method of synthesis, and time points of measurement are included in the Overview of Synthesis and Included Studies (OSIS) table (Table 8).
6. Comparison 1: Overview of Synthesis and Included Studies (OSIS).
| Study name (year) | Study design (individual vs stepped‐wedge vs cluster‐randomised [and unit of randomisation, if applicable]) | Country of conduct | Overall risk of bias (arranged low to high) | Key details of intervention (% KCl, LSSS (implementation [discretionary, non‐discretionary, both], quantity, provided or purchased, co‐interventions [education, advice]) | Key details of the comparator (implementation [discretionary, non‐discretionary, both], quantity, provided or household supply, co‐interventions [education, advice]) | Population (No. of participants randomised [intervention/control], age, gender, hypertensive status, antihypertensive medication use, BMI) | Outcome domains with available data (synthesis method/metric) | Specific outcomes measure | Time point of measurement |
| Allaert 2017 | Individually randomised | France | Low | No KCl content in LSSS (97% NaCl and 3% chitosan) Discretionary use to a maximum of 3 g per day 300 g provided Participants not to change their dietary, physical activity, smoking habits during study period |
Regular salt Discretionary use to a maximum of 3 g per day 300 g provided Participants not to change their dietary, physical activity, smoking habits during study period |
22/19 51 (16) years Male, %: 51.2 Pre‐hypertensive None NR |
Change in blood pressure Cardiovascular events Change in blood potassium Adverse events |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Change in blood potassium 5. Various other adverse events |
1. 56 days 2. 56 days 3. ≤ 3 months 4. 56 days 5. ≤ 3 months |
| CSSS Collaborative Group 2007 | Individually randomised | China | Low | 25% KCl LSSS (with 65% NaCl and 10% MgSO4) Discretionary use Up to 3 kg per month provided for household use Co‐interventions NR |
Regular salt Discretionary use Up to 3 kg per month provided for household use Co‐interventions NR |
306/302 59 (10)/61 (9.7) years Female, %: 54/58 Hypertensive, %: 57/57 Use of any antihypertensive medication, %: 61/61 26 (3.6)/25(3.9) kg/m2 |
Change in blood pressure Cardiovascular events Hyperkalaemia Mortality Adverse events |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Hyperkalaemia 5. All‐cause mortality 6. Various other adverse events |
1. See Table 7 2. 12 months 3. > 3 to 12 months 4. 12 months 5. > 3 to 12 months 6. > 3 to 12 months |
| Li 2014 | Cluster‐randomised (households) | China | Low | 30% KCl LSSS (KCl 30.0 ± 10.0 %; NaCl 70.0 ± 10.0%;) Discretionary use 350 g provided (frequency NR) Co‐interventions NR |
Regular salt Discretionary use Own household supply Co‐interventions NR |
253/263 59.3 (11.7)/59.2 (8.7) years Female, %: 50.9/48.7 NR NR NR |
Change in blood pressure |
1. Change in DBP 2. Change in SBP |
1. 2 months 2. 2 months |
| Neal 2021 | Cluster‐randomised (villages) | China | Low | 30 ± 10% KCl (with 70 ± 10% NaCl ) Discretionary use at average intake of 20 g per person per day Provided regular supply of LSSS to households (up to 20 kg per year for a household with 3 members) Co‐interventions NR |
Regular salt Discretionary use Own household supply Advice about reducing salt intake given at study commencement. |
10504/10491 65.2 (8.5)/ 65.5 (8.5) years Female, %: 49.7 Hypertensive, %: 59.4/59.2 Any antihypertensive medication, %: 79.9/78.7 24.8 (3.6)/24.9 (3.7) kg/m2 |
Change in blood pressure Cardiovascular events Mortality Hyperkalaemia 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Cardiovascular events: non‐fatal stroke 4. Cardiovascular events: non‐fatal acute coronary syndrome 5. Cardiovascular mortality 6. Stroke mortality 7. All‐cause mortality 8. Hyperkalaemia 9. 24‐h urinary sodium excretion 10. 24‐h urinary potassium excretion |
1. 60 months 2. 60 months 3. 4.75 years mean follow‐up 4. 4.75 years mean follow‐up 5. 4.75 years mean follow‐up 6. 4.75 years mean follow‐up 7. 4.75 years mean follow‐up 8. 4.75 years mean follow‐up 9. 60 months 10. 60 months |
| Pan 2017 | Individually randomised | Taiwan | Low | Two intervention arms: 50% KCl LSSS (with NaCl 50%) or 42.85% KCl LSSS, (with 42.85% NaCl, 14.3% MgSO4) Discretionary use at approx. 6.5 g per person day 1 kg provided at study entry and 3 months, for household use Co‐interventions NR |
Regular salt Discretionary use at approx. 6.5 g per person per day 1 kg provided at study entry and 3 months, for household use Co‐interventions NR |
97/95/99 64.4 (9.8)/64.7 (9.9)/ 64.8 (10.3) years Female, %: 42.3/34.7/32.3 Hypertensive, %: 56.7/68.4/50.5 NR NR |
Cardiovascular events Mortality Adverse events |
1. Cardiovascular events: non‐fatal stroke 2. All‐cause mortality 3. Various other adverse events |
1. > 3 to 12 months 2. > 3 to 12 months 3. > 3 to 12 months |
| Yu 2021 | Individually randomised | India | Low | 30% KCl (with 70% NaCl) Discretionary use of 20 g per person per day. Provided up to 5 kg every 3 months for household use Co‐interventions NR |
Regular salt Discretionary use of 20 g per person per day Provided up to 5 kg every 3 months for household use Co‐interventions NR |
252/252 61.5 (11.1)/ 61.7 (12.9) years Female, %: 58.3/59.2 Hypertensive Any hypertensive medication use, %: 97.2/94.4 23.1 (4.7)/23.6 (4.2) kg/m2 |
Change in blood pressure Hyperkalaemia Adverse events 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Hyperkalaemia 4. Various other adverse events 5. 24‐h urinary sodium excretion 6. 24‐h urinary potassium excretion |
1. 3 months 2. 3 months 3. 3 months 4. ≤ 3 months 5. 3 months 6. 3 months |
| Zhao 2014 | Individually randomised | Tibet | Low | 25% KCl LSSS (with 65% NaCl and 10% MgSO4) Discretionary use Provided in sufficient amounts for household use Patients with pre‐existing antihypertensive medications not alter their prior regimen |
Regular salt Discretionary use Provided in sufficient amounts for household use Patients with pre‐existing antihypertensive medications not alter their prior regimen |
141/141 62.8 (11.1)/ 63.5 (11.3) years Female, %: 60.3/57.4 Hypertensive Antihypertensive use in the past month, %: 47.0/50.7 23.7 (3.1)/23.6 (3.4) kg/m2 |
Change in blood pressure Blood pressure control Adverse events |
1. Change in DBP 2. Change in SBP 3. Blood pressure control 4. Antihypertensive medication use 5. Various other adverse events |
1. 3 months 2. 3 months 3. 3 months 4. 3 months 5. ≤ 3 months |
|
Zhou 2009 |
Individually randomised | China | Low | 30% KCl LSSS (with 65% NaCl, calcium, folic acid) Discretionary use 3 kg per month provided for household use Co‐interventions NR |
Regular salt Discretionary use 3 kg per month provided for household use Co‐interventions NR |
62/64 67.5 (5.2)/ 65.7 (6.3) years Female, %: 56.5/57.8 Hypertensive Any antihypertensive medication use, %: 53.2/54.7 25.2 (3.5)/24.9 (3.7) kg/m2 57/65 68.1 (8.3)/65.4 (4.5) years Female, %: 50.9/55.4 Normotensive N/A 23.9 (3.2)/23.7 (3.3) kg/m2 |
Change in blood pressure Cardiovascular events Change in blood glucose Change in blood lipids 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Change in fasting blood glucose 5. Change in blood triglycerides 6. Change in total blood cholesterol 7. 24‐h urinary sodium excretion 8. 24‐h urinary potassium excretion |
1. 6 months 2. 6 months 3 > 3 to 12 months 4. 6 months 5. 6 months 6. 6 months 7. 6 months 8. 6 months |
| Allaert 2013 | Individually randomised | France | Unclear | No KCl content in LSSS (97% NaCl and 3% chitosan) Discretionary use to a maximum of 3 g per day Quantity provided NR Lifestyle advice (eating less fat and sugar, avoidance of liquorice) and physical exercise |
Sea salt Discretionary use to a maximum of 3 g per day Quantity provided NR Lifestyle advice (eating less fat and sugar, avoidance of liquorice) and physical exercise |
21/19 59.1 (11.6)/ 58.0 (12.7) years Female, %: 61.9/57.9 Hypertensive None 25.1 (3.8)/27.7 (5.8) kg/m2 |
Change in blood pressure Blood pressure control |
1. Change in DBP 2. Change in SBP 3. Blood pressure control |
1. 8 weeks 2. 8 weeks 3. 8 weeks |
| Arzilli 1986 | Individually randomised | Italy | Unclear | Unknown KCl LSSS Discretionary use 2 g twice daily provided Hospital diet containing 20 mmol Na per day provided Co‐interventions NR |
Regular salt Discretionary use 2 g twice daily provided Hospital diet containing 20 mmol Na per day provided Co‐interventions NR |
10/10 28 to 53 years Female, %: 40 Hypertensive NR NR |
Change in blood pressure |
1. Change in DBP 2. Change in SBP |
1. See Table 7 2. See Table 7 |
| Bernabe‐Ortiz 2014 | Stepped‐wedge (villages) | Peru | Unclear | 25% KCl LSSS (with 75% NaCl) Discretionary use Provision of LSSS via salt supply chain in each village. Social marketing strategy promoting LSSS use |
Regular salt Discretionary use Normal salt supply chain |
2376 (total) 43.3 (17.2) years Female, %: 50.4 Hypertensive, %: 18.3 NR 27.2 (4.6) kg/m2 |
Change in blood pressure Blood pressure control 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Hypertension 4. 24‐h urinary sodium excretion 5. 24‐h urinary potassium excretion |
1. 30 months 2. 30 months 3. 30 months 4. 30 months 5. 30 months |
| Kawasaki 1998 | Individually randomised | Japan | Unclear | 10.1% K LSSS (with 22.9 % Na; 1.2% Mg) Discretionary and non‐discretionary use (soy sauce, miso) Participants to refrain from dining out and were not to change their lifestyle during the study period. |
Regular salt Discretionary and non‐discretionary use (regular soy sauce, miso) Participants to refrain from dining out and were not to change their lifestyle during the study period. |
21/20 65.9 (7.4)/65.8 (7.6) years Female, %: 47.6/ 50 Hypertensive, %: 47.6/40.0 Antihypertensive medication use, %: 19.0/20.0 22.9 (2.7)/23.3 (2.3) kg/m2 |
Change in blood pressure Change in blood potassium Change in blood lipids 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Change in blood potassium 4. Change in blood triglycerides 5. Change in total blood cholesterol 6. 24‐h urinary sodium excretion 7. 24‐h urinary potassium excretion |
1. See Table 7 2. See Table 7 3. 5 weeks 4. 5 weeks 5. 5 weeks 6. 5 weeks 7. 5 weeks |
| Li 2016 | Cluster‐randomised (villages) | China | Unclear | 20.0% KCl (up to 35.0%) (with 70.0% ± 10.0% NaCl, iodine) Discretionary use LSSS for purchase at village shops (subsidised LSSS or non‐subsidised LSSS) Community‐based health education programme on salt reduction 50% of villages included in a concurrent trial (primary‐care‐based high cardiovascular risk management package delivered by village doctors) |
Regular salt Discretionary use Regular salt for purchase at village shops 50% of villages included in a concurrent trial (primary‐care‐based high cardiovascular risk management package delivered by village doctors) |
1268/1253/1272 Age NR Gender NR Hypertensive status NR NR NR |
Change in blood pressure Blood pressure control Cardiovascular events Hyperkalaemia Change in BMI Renal function 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Hypertension 4. Antihypertensive medication use 5. Various other cardiovascular events 6. Hyperkalaemia 7. Change in BMI 8. Microalbuminuria 9. Macroalbuminuria 10. Change in uACR 11. 24‐h urinary sodium excretion 12. 24‐h urinary potassium excretion |
1. 18 months 2. 18 months 3. 18 months 4. 18 months 5. See Table 7 6. See Table 7 7. 18 months 8. 18 months 9. 18 months 10. 18 months 11. 18 months 12. 18 months |
| Mu 2009 | Cluster‐randomised (families) | China | Unclear | Unknown KCl LSSS Discretionary use Provided for household use (quantity NR) Co‐interventions NR |
Regular salt Discretionary use Provided for household use (quantity NR) Co‐interventions NR |
101/114 20.3 (3.1)/21.4 (3.9) years Female, %: 55.5/47.4 Hypertensive NR 23.6 (2.0)/23.8 (2.1) kg/m2 |
Change in blood pressure Hyperkalaemia Adverse events |
1. Change in DBP 2. Change in SBP 3. Hyperkalaemia 4. Various other adverse events |
1. See Table 7 2. See Table 7 3. 2 years 4. > 12 months |
| Omvik 1995 | Individually randomised | Norway | Unclear | KCl 28% LSSS (with NaCl 57%; MgSO4 12%; lysine 2%). Discretionary use 500 g provided for household use (frequency NR) Participants instructed re. salt‐restricted diet |
Regular salt Discretionary use 500 g provided for household use (frequency NR) Participants instructed re.salt‐restricted diet |
20/20 45.9/42.7 years Female, %: 30/35 Hypertensive None NR |
Change in blood pressure Cardiovascular events Change in blood potassium Renal function 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Change in blood potassium 5. Change in serum creatinine 6. 24‐h urinary sodium excretion 7. 24‐h urinary potassium excretion |
1. 6 months 2. 6 months 3. > 3 to 12 months 4. 6 months 5. 6 months 6. 6 months 7. 6 months |
| Pereira 2005 | Individually randomised | Brazil | Unclear | 50% KCl LSSS (with 50% NaCl) Discretionary use 1 kg provided for household use Individualised hypocaloric diet and increased physical activity |
Regular salt Discretionary use 1 kg provided for household use Individualised hypocaloric diet and increased physical activity |
15/13 45.4 (13.2)/ Gender NR Hypertensive All participants on thiazide diuretics 32.5 (13.2)/30.2 (2.7) kg/m2 |
Change in blood pressure Change in blood potassium Hypokalaemia Change in BMI Renal function Change in blood lipids |
1. Change in DBP 2. Change in SBP 3. Change in blood potassium 4. Hypokalaemia 5. Change in BMI 6. Change in serum creatinine 7. Change in blood triglycerides 8. Change in total blood cholesterol |
1. 12 weeks 2. 12 weeks 3. 12 weeks 4. 12 weeks 5. 12 weeks 6. 12 weeks 7. 12 weeks 8. 12 weeks |
| Sarkkinen 2011 | Individually randomised | Finland | Unclear | 25% KCl LSSS (with 50% NaCl; 25% Mg) Discretionary use (quantity provided NR) Non‐discretionary use (processed main dishes, bread, sausage/cold cuts and Edam cheese; to replace 60% of usual sodium intake) Participants instructed to avoid salt‐rich products, products containing bioactive peptides, licorice, ammonium chloride products and any food supplements that may affect BP |
Regular salt Discretionary use Non‐discretionary use (processed main dishes, bread, sausage/cold cuts and Edam cheese with regular salt content) Participants instructed to avoid salt‐rich products, products containing bioactive peptides, licorice, ammonium chloride products and any food supplements that may affect BP |
22/23 57 (12)/54 (11) years Female, %: 59/39 Hypertensive None 28 (3)/28 (3) kg/m2 |
Change in blood pressure Cardiovascular events Adverse events Change in blood potassium Change in BMI 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Various other adverse events 5. Change in blood potassium 6. Change in BMI 7. 24‐h urinary sodium excretion 8. 24‐h urinary potassium excretion |
1. 8 weeks 2. 8 weeks 3. ≤ 3 months 4. ≤ 3 months 5. See Table 7 6. 8 weeks 7. 8 weeks 8. 8 weeks |
| Suppa 1988 | Individually randomised | Italy | Unclear | KCl 25% (with 50% NaCl and 15% K3C6H5O7) Discretionary use 2 g provided twice daily Co‐interventions NR |
Regular salt Discretionary use 2 g provided twice daily Co‐interventions NR |
163/159 47.1 (9.8)/47.8 (10.1) years Female, %: 35.6/39 Hypertensive All participants on β‐blocker monotherapy (Metoprolol) NR |
Change in blood pressure Cardiovascular events Adverse events 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Various other cardiovascular events 4. Various other adverse events 5. 24‐h urinary sodium excretion 6. 24‐h urinary potassium excretion |
1. 4 weeks 2. 4 weeks 3. See Table 7 4. See Table 7 5. 4 weeks 6. 4 weeks |
| Toft 2020 | Cluster‐randomised (families) | Denmark | Unclear | < 30% KCl LSSS (Per 100g: approximately 8000 mg sodium; 870 mg Mg and 100 mg‐200 mg K estimated from the technical data sheet) Non‐discretionary use (LSSS bread products to replace usual consumption), provided to families twice a week Co‐interventions NR |
Regular salt Non‐discretionary use (regular wholegrain bread products for usual consumption), provided to families twice a week Co‐interventions NR |
81/101 41.5 (9.5)/ 40.9 (8.0) years Female, %: 47.5/53.1 Normotensive N/A 25.8 (3.8)/24.8 (4.1) kg/m2 |
Change in blood pressure Change in BMI Change in blood glucose Change in blood lipids 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Change in BMI 4. Change in fasting blood glucose 5. Change in blood triglycerides 6. Change in total blood cholesterol 7. 24‐h urinary sodium excretion 8. 24‐h urinary potassium excretion |
1. 4 months 2. 4 months 3. 4 months 4. 4 months 5. 4 months 6. 4 months 7. 4 months 8. 4 months |
| Zhou 2013 | Cluster‐randomised (families) | China | Unclear | 25% KCl LSSS (with 65% NaCl, 10% MgSO4). Discretionary use Estimated amount (based upon baseline salt intake) provided every 3 months for household use Co‐interventions NR |
Regular salt Discretionary use Estimated amount (based upon baseline salt intake) provided every 3 months for household use Co‐interventions NR |
224/238 45.63 (13.72)/ 47.05 (13.46) years Female, %: 50.45/50.84 NR Any antihypertensive use, %: 41.07/40.0 25.94 (3.82)/26.66 (4.27) kg/m2 |
Change in blood pressure Mortality Blood pressure control |
1. Change in DBP 2. Change in SBP 3. Cardiovascular mortality 4. Stroke mortality 5. All‐cause mortality 6. Antihypertensive medication use |
1. 36 months 2. 36 months 3. 13 years 4. 13 years 5. 13 years 6. 36 months |
| Chang 2006 | Cluster‐randomised (Retirement home kitchens) | Taiwan | High | 49% KCl LSSS (with 49% NaCl, 2% other additives) Discretionary use LSSS gradually replaced regular salt in the kitchens within a 4‐week period. Frequency and quantity provided NR Regular condiments and spices e.g. soy sauce and MSG, not limited |
Regular salt Discretionary use Frequency and quantity provided NR Regular condiments and spices e.g. soy sauce and MSG, not limited |
768/1213 75.21 (7.37)/74.67 years Male Hypertensive, % (n/N): 40.2/40.4 NR 23.3 (3.5) kg/m2 |
Mortality |
1. Cardiovascular mortality 2. All‐cause mortality |
1. 2.6 years mean follow‐up 2. 2.6 years mean follow‐up |
| Geleijnse 1994 | Individually randomised | Netherlands | High | 41% KCl LSSS (with 41% NaCl, 17% Mg) Discretionary use Non‐discretionary use (use of LSSS in bread, cheese, luncheon meats, canned and instant soups, smoked sausage ‐ replacement of approx. 57% of usual salt intake) Quantity provided and frequency NR Participants were instructed not to change dietary and lifestyle habits. |
Regular salt Discretionary use Non‐discretionary use (use of regular bread, cheese, luncheon meats, canned and instant soups, smoked sausage) Quantity provided and frequency NR Participants were instructed not to change dietary and lifestyle habits. |
49/51 65.7 (4.6)/ 67.1 (4.5) years Female, %: 47/51 Hypertensive None 27.1 (3.4)/27.2 (3.2) kg/m2 |
Change in blood pressure Change in blood potassium Change in blood lipids 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Change in blood potassium 4. Change in total blood cholesterol 5. 24‐h urinary sodium excretion 6. 24‐h urinary potassium excretion |
1. 24 weeks 2. 24 weeks 3. 24 weeks 4. 24 weeks 5. 24 weeks 6. 24 weeks |
| Gilleran 1996 | Individually randomised | United Kingdom | High | 40% KCl LSSS (with 50% NaCl and 10% MgSO4). Discretionary use 680 g provided monthly for household use Co‐interventions NR |
Regular salt Discretionary use 680 g provided monthly for household use Co‐interventions NR |
20/20 62.5 (7.8)/ 59.2 (10.8) years Female, %: 40/40 Hypertensive None 28.1 (4.6)/28.6 (3.7) kg/m2 |
Change in blood pressure Cardiovascular events Change in blood lipids 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Cardiovascular events: non‐fatal stroke 4. Change in blood triglycerides 5. Change in total blood cholesterol 6. 24‐h urinary sodium excretion 7. 24‐h urinary potassium excretion |
1. 9 months 2. 9 months 3. ≤ 3 months 4. 9 months 5. 9 months 6. 9 months 7. 9 months |
| Hu 2018 | Cluster‐randomised (families) | China | High | 25% KCl LSSS (with 65% NaCl, 10% MgSO4). Discretionary use 1 kg bags provided for household use (frequency NR) Participants instructed to avoid any changes in dietary or lifestyle habits. |
Regular salt Discretionary use 1 kg bags provided for household use (frequency NR) Participants instructed to avoid any changes in dietary or lifestyle habits. |
110/110 57.1 (10.9)/ 57.6 (10.1) years Female, %: 33.6/60.0 Hypertensive Antihypertensive medication use, %: 71.8/77.3 27.6 (3.3)/28.3 (3.5) kg/m2 187/186 family members 45.5 (17.5)/45.7 (17.4) years Female, %: 45.5/47.4 Hypertensive, % (n/N): 31.6/24.2 None 24.9 (3.8)/25.2 (4.3) kg/m2 |
Change in blood pressure Adverse events Blood pressure control |
1. Change in DBP 2. Change in SBP 3. Various other adverse events 4. Antihypertensive medication use |
1. 12 months 2. 12 months 3. > 3 to 12 months 4. 12 months |
| Mu 2003 | Individually randomised | China | High | Unknown KCl LSSS Discretionary use Provided for household use monthly (quantity NR) Co‐interventions: None |
Regular salt Discretionary use Provided for household use monthly (quantity NR) Co‐interventions: None |
110/110 20.7(2.0)/20.4 (2.2) years Female; %: 47.3/49 Hypertensive NR 21.9 (2.9)/22.2 (3.1) kg/m2 |
Change in blood pressure |
1. Change in DBP 2. Change in SBP |
1. 2 years 2. 2 years |
| Zhang 2015 | Cluster‐randomised (nursing homes) | China | High | 50% KCl (with 50% NaCl) Discretionary use of up to 10 g per person per day LSSS provided every 3 months Co‐interventions NR |
Regular salt Discretionary use of up to 10 g per person per day Regular salt provided every 3 months Co‐interventions NR |
NR 65 years Gender NR NR NR NR |
Change in blood pressure Change in blood potassium Hyperkalaemia Renal function |
1. Change in DBP 2. Change in SBP 3. Change in blood potassium 4. Hyperkalaemia 5. Change in serum creatinine 6. Microalbuminuria |
1. 3 years 2. 3 years 3. 1 to 1.5 years 4. 1 to 1.5 years 5. 1 to 1.5 years 6. 3 years |
Abbreviations: 24‐h: 24‐hour BMI: body mass index BP: blood pressure DBP: diastolic blood pressure K3C6H507: potassium citrate K(Cl): potassium (chloride) LSSS: low‐sodium salt substitutes Mg(SO4): magnesium (sulphate) MSG: monosodium glutamate N/A: not applicable Na(Cl): sodium (chloride) NR: not reported SBP: systolic blood pressure uACR: urinary albumin‐to‐creatinine ratio
Primary outcomes
Change in diastolic blood pressure (DBP, mmHg)
GRADE assessment suggests that LSSS probably reduce DBP slightly, on average, compared to regular salt in adults (moderate‐certainty evidence, downgraded once for inconsistency).
Average reductions in DBP ranged from 0.6 mmHg to 11.33 mmHg with LSSS and from a reduction of 7 mmHg to an increase of 2.6 mmHg with regular salt in the 19 trials that reported this outcome. Two trials did not have average changes per group available: Li 2016 reported end values and no baseline measures; Neal 2021 reported only mean differences between groups. The meta‐analysis showed small, important effects on DBP on average between LSSS and regular salt groups (MD ‐2.43 mmHg, 95% confidence interval (CI) ‐3.50 to ‐1.36, I2 = 88%, 20,830 participants, 19 RCTs, moderate‐certainty evidence, Analysis 1.1). Follow‐up ranged from four weeks to 60 months.
1.1. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 1: Change in DBP (mmHg)
This small yet important effect was confirmed by sensitivity analyses, including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.12) and including only trials randomising participants at the individual level, i.e. excluding cluster‐RCTs (Analysis 1.13). This direction of effect was also seen in a large stepped‐wedge cluster trial following 2376 participants over 30 months (unclear overall risk of bias), though the magnitude of the effect was considerably diminished (Analysis 1.14).
1.12. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 12: Change in DBP (mmHg); sensitivity analysis: study quality
1.13. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 13: Change in DBP (mmHg); sensitivity analysis: study design
1.14. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 14: Change in DBP (mmHg) stepped‐wedge trial
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary meta‐analysis (Analysis 1.1) corresponded to an estimated 60 (ranging from 35 to 83) stroke deaths prevented per 100,000 persons, aged 50 years and older, per year.
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by study duration (Analysis 1.2) suggests there may be no important differences in average effects between subgroups. Subgrouping participants by age (Analysis 1.3), gender (Analysis 1.4), ethnicity (Analysis 1.5), BMI (Analysis 1.6), blood pressure status (Analysis 1.7) or baseline 24‐h urinary sodium (Analysis 1.10) or potassium (Analysis 1.11) excretion further suggested there may be no important clinical differences in average effects between subgroups. Subgrouping by intervention characteristics also suggests there may be no important differences in average effects between the manner of LSSS implementation (Analysis 1.8) or the type of LSSS (Analysis 1.9).
1.2. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 2: Change in DBP (mmHg); subgroup study duration
1.3. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 3: Change in DBP (mmHg); subgroup age
1.4. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 4: Change in DBP (mmHg); subgroup gender
1.5. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 5: Change in DBP (mmHg); subgroup ethnicity
1.6. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 6: Change in DBP (mmHg); subgroup BMI
1.7. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 7: Change in DBP (mmHg); subgroup blood pressure status
1.10. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 10: Change in DBP (mmHg); subgroup baseline sodium excretion (mmol/24‐h)
1.11. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 11: Change in DBP (mmHg); subgroup baseline potassium excretion (mmol/24‐h)
1.8. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 8: Change in DBP (mmHg); subgroup LSSS implementation
1.9. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 9: Change in DBP (mmHg); subgroup type of LSSS
Five trials reported this outcome in an unusable format (e.g. reported only in a figure from which we could not extract exact values, or did not report standard deviations and participant numbers along with mean change), and usable data were not provided when requested from authors (Table 7). The funnel plot (Figure 4) shows that most trials had similar effect sizes despite varying inter‐trial standard errors, thereby limiting the ability to assess asymmetry. The mean difference in the fixed‐effect model, an analytical approach that gives less weight to small studies, for the primary analysis (‐2.27 mmHg, 95% CI ‐2.56 to ‐1.98) was similar to the mean difference when using the random‐effects model, which gives more weight to smaller studies, for the primary analysis (‐2.43 mmHg, 95% CI ‐3.50 to ‐1.36, Analysis 1.1). This suggests that any small‐study effects have little impact on the intervention effect estimate.
4.

Funnel plot for change in DBP (Analysis 1.1) in comparison 1
Change in systolic blood pressure (SBP, mmHg)
GRADE assessment suggests that LSSS probably reduce SBP slightly, on average, compared to regular salt in adults (moderate‐certainty evidence, downgraded once for inconsistency).
Average reductions in SBP ranged from 1.5 mmHg to 15.25 mmHg with LSSS and from a reduction of 6.8 mmHg to an increase of 4 mmHg with regular salt in the 20 trials that reported this outcome. Three trials did not have average changes per group available: Li 2016 reported end values and no baseline measures; CSSS Collaborative Group 2007 and Neal 2021 reported only mean differences between groups. The meta‐analysis showed small, important effects on SBP on average between LSSS and regular salt groups (MD ‐4.76 mmHg, 95% CI ‐6.01 to ‐3.50, I2 = 78%, 21,414 participants, 20 RCTs, moderate‐certainty evidence, Analysis 1.15). Follow‐up ranged from four weeks to 60 months.
1.15. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 15: Change in SBP (mmHg)
This small yet important effect was confirmed by sensitivity analyses, including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.26) and including only trials randomising participants at the individual level, i.e. excluding cluster‐RCTs (Analysis 1.27). This direction of effect was also seen in the stepped‐wedge cluster trial that followed 2376 participants over 30 months (unclear overall risk of bias), though the magnitude of the effect was considerably diminished (Analysis 1.28).
1.26. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 26: Change in SBP (mmHg); sensitivity analysis: study quality
1.27. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 27: Change in SBP (mmHg); sensitivity analysis: study design
1.28. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 28: Change in SBP (mmHg), stepped‐wedge trial
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary meta‐analysis (Analysis 1.15) corresponded to an estimated 53 (ranging from 40 to 65) stroke deaths prevented per 100,000 persons, aged 50 years and older, per year.
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by study duration (Analysis 1.16), and subgrouping participants by age (Analysis 1.17), gender (Analysis 1.18), ethnicity (Analysis 1.19), BMI (Analysis 1.20), blood pressure status (Analysis 1.21) or baseline 24‐h urinary sodium (Analysis 1.24) or potassium (Analysis 1.25) excretion suggests there may be no important clinical differences in average effects between subgroups. Subgrouping by intervention characteristics also suggested there may be no important differences in average effects between the manner of LSSS implementation (Analysis 1.22) or the type of LSSS (Analysis 1.23).
1.16. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 16: Change in SBP (mmHg); subgroup study duration
1.17. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 17: Change in SBP (mmHg); subgroup age
1.18. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 18: Change in SBP (mmHg); subgroup gender
1.19. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 19: Change in SBP (mmHg); subgroup ethnicity
1.20. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 20: Change in SBP (mmHg); subgroup BMI
1.21. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 21: Change in SBP (mmHg); subgroup blood pressure status
1.24. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 24: Change in SBP (mmHg); subgroup baseline sodium excretion (mmol/24‐h)
1.25. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 25: Change in SBP (mmHg); subgroup baseline potassium excretion (mmol/24‐h)
1.22. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 22: Change in SBP (mmHg); subgroup LSSS implementation
1.23. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 23: Change in SBP (mmHg); subgroup type of LSSS
Four trials reported this outcome in an unusable format (e.g. reported only between‐group P values or did not report standard deviations and participant numbers along with mean change), and usable data were not provided when requested from authors (Table 7). The funnel plot (Figure 5) shows that most trials had similar effect sizes and standard errors, with some outliers, thereby limiting the ability to assess asymmetry. The mean difference in the fixed‐effect model for the primary analysis (‐3.92 mmHg, 95% CI ‐4.37 to ‐3.47) was similar to the mean difference when using the random‐effects model for the primary analysis (‐4.76 mmHg, 95% CI ‐6.01 to ‐3.50, Analysis 1.15). This suggests that any small‐study effects have little impact on the intervention effect estimate.
5.

Funnel plot for change in SBP (Analysis 1.14) in comparison 1
Hypertension
GRADE assessment suggests that, on average, LSSS may result in little to no difference in hypertension in the adult population, when compared to regular salt (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
One study following participants for 18 months reported on this outcome (risk ratio (RR) 0.97, 95% CI 0.90 to 1.03, 2566 participants, 1 RCT, low‐certainty evidence, Analysis 1.29), with 725 participants in the LSSS group and 738 participants in the regular salt group having prevalent hypertension at the end of the study. The absolute effect for hypertension was 17 fewer per 1000 (95% CI 58 fewer to 17 more). The stepped‐wedge cluster trial (unclear overall risk of bias) reported on incident hypertension in 1914 participants represented by 2712.3 person‐years at risk in the LSSS group and 1961.1 person‐years at risk in the regular salt group, and found a reduction in hypertension with LSSS compared to regular salt (Analysis 1.30).
1.29. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 29: Hypertension (as reported, or SBP > 140 mmHg or DBP > 85 mmHg)
1.30. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 30: Hypertension (as reported, or SBP > 140 mmHg or DBP > 85 mmHg), stepped‐wedge trial
Blood pressure control
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS on blood pressure control in adults, when compared to regular salt (very low‐certainty evidence, downgraded once for risk of bias, once for indirectness and once for imprecision).
Two small studies reported on this outcome (RR 2.12, 95% CI 1.32 to 3.41, I2 = 0%, 253 participants, 2 RCTs, very low‐certainty evidence, Analysis 1.31), with the number of participants in the LSSS group achieving blood pressure control ranging from 16 to 19 and participants in the regular salt group achieving blood pressure control ranging from seven to 10. The absolute effect for blood pressure control was 143 more per 1000 (95% CI 41 more to 308 more). The two trials reporting this outcome had follow‐up at eight weeks and three months.
1.31. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 31: Blood pressure control (achieving blood pressure threshold or 'control' as prespecified)
Cardiovascular events: various
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS interventions on various other cardiovascular events when compared to regular salt in the adult population (very low‐certainty evidence, downgraded once for indirectness and twice for imprecision).
It should be noted that a very small number of participants presented with various other cardiovascular events in both groups for the five trials reporting this outcome. Event numbers ranged from zero to eight with LSSS and from zero to five with regular salt. The meta‐analysis of the RR was 1.22 (95% CI 0.49 to 3.04, I2 = 0%, 982 participants, 5 RCTs, very low‐certainty evidence, Analysis 1.32) when comparing LSSS and regular salt. The absolute effect for various other cardiovascular events was 357 more per 100,000 (95% CI 828 fewer to 3310 more). Two trials reporting this outcome had follow‐up at ≤ 3 months, while three followed participants for > 3 to 12 months.
1.32. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 32: Cardiovascular events: various events
Two trials reported this outcome in an unusable format (i.e. numbers of events per group and numbers of participants per group not reported), and usable data were not provided when requested from authors (Table 7).
Cardiovascular events: non‐fatal stroke
GRADE assessment for this outcome suggests that, on average, LSSS probably reduce non‐fatal stroke events slightly in adults, when compared to regular salt (moderate‐certainty evidence, downgraded once for indirectness).
The number of participants with non‐fatal stroke in two small trials (Gilleran 1996; Pan 2017) reporting on this outcome at ≤ 3 months and > 3 to 12 months, respectively, ranged from zero to four with LSSS and one participant each with regular salt. The third, a large cluster‐RCT (Neal 2021) that followed participants for a mean of 4.75 years, reported rates of events: 22.36 events per 1000 person‐years in the LSSS group and 24.86 events per 1000 person‐years in the regular salt group (rate ratio 0.90, 95% CI 0.80 to 1.01). The meta‐analysis combining these data as risk ratios resulted in an RR of 0.90 (95% CI 0.80 to 1.01, I2 = 0%, 21,250 participants, 3 RCTs, moderate‐certainty evidence, Analysis 1.33) when comparing LSSS with regular salt. This result translates to an absolute effect for non‐fatal stroke of 20 fewer per 100,000 (95% CI 40 fewer to 2 more). Since this pooled effect was driven by a large secondary prevention trial with a preponderance of participants with previous stroke, we downgraded once for indirectness.
1.33. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 33: Cardiovascular events: non‐fatal stroke
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary meta‐analysis (Analysis 1.33) corresponded to an estimated 10 non‐fatal strokes prevented (ranging from 21 prevented to 1 caused) per 100,000 persons per year.
Sensitivity analyses, including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.34) and including only trials randomising participants at the individual level, i.e. excluding cluster‐RCTs (Analysis 1.35), did not reflect this benefit with LSSS; instead showing highly imprecise effects of little to no effect, or harm.
1.34. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 34: Cardiovascular events: non‐fatal stroke; sensitivity analysis: study quality
1.35. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 35: Cardiovascular events: non‐fatal stroke; sensitivity analysis: study design
One trial reported this outcome in an unusable format (i.e. numbers of participants per group not reported), and usable data were not provided when requested from authors (Table 7).
Cardiovascular events: non‐fatal acute coronary syndrome
GRADE assessment suggests that LSSS probably reduce non‐fatal ACS events slightly, on average, when compared to regular salt in adults (moderate‐certainty evidence, downgraded once for indirectness).
A single large cluster‐RCT contributed data to this outcome at a mean follow‐up of 4.75 years, reporting rates of 3.79 events per 1000 person‐years in the LSSS group and 5.12 events per 1000 person‐years in the regular salt group. The rate ratio was 0.70 (95% CI 0.52 to 0.94, 20,995 participants, 1 RCT, moderate‐certainty evidence, Analysis 1.36) and the absolute effect for non‐fatal acute coronary syndrome was 150 fewer per 100,000 person‐years (95% CI 250 fewer to 30 fewer), when comparing LSSS with regular salt in this large secondary prevention trial in which most of the participants had a history of previous stroke. This setting limited generalisability to the general adult population, and the evidence was consequently downgraded once for indirectness.
1.36. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 36: Cardiovascular events: non‐fatal acute coronary syndrome
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary analysis (Analysis 1.36) corresponded to an estimated 50 (ranging from 10 to 80) non‐fatal ACS events prevented per 100,000 persons per year.
Cardiovascular mortality
GRADE assessment of this outcome suggests that LSSS probably reduce cardiovascular mortality slightly, on average, in adults when compared to regular salt (moderate‐certainty evidence, downgraded once for indirectness).
The number of cardiovascular mortality events per 1000 person‐years in the three trials reporting on this outcome ranged from 4.53 to 22.94 in the LSSS groups and 7.81 to 26.30 in the regular salt groups. The meta‐analysis comparing LSSS with regular salt resulted in a rate ratio of 0.77 (95% CI 0.60 to 1.00, I2 = 35%, 23,200 participants, 3 RCTs, moderate‐certainty evidence, Analysis 1.37). The absolute effect for cardiovascular mortality was 180 fewer per 100,000 person‐years (95% CI 310 fewer to 0 fewer). We downgraded this finding once for indirectness since the pooled effect was driven by the secondary prevention trial including a large proportion of participants with previous stroke (Neal 2021). Two trials reporting on this outcome had a mean follow‐up of between 2.6 and 4.75 years (Chang 2006; Neal 2021) while a third (Zhou 2013) reported on this outcome following three years of active intervention and ten years follow‐up.
1.37. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 37: Cardiovascular mortality
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary meta‐analysis (Analysis 1.37) corresponded to an estimated 53 cardiovascular deaths prevented (ranging from 92 prevented to none prevented or caused) per 100,000 persons per year.
A sensitivity analysis including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.38) confirmed this effect.
1.38. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 38: Cardiovascular mortality; sensitivity analysis: study quality
Stroke mortality
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS on stroke mortality in adults, when compared to regular salt (very low‐certainty evidence, downgraded once for indirectness and twice for imprecision).
The number of stroke mortality events per 1000 person‐years in the two trials reporting on this outcome ranged from 2.01 to 6.78 in the LSSS groups and 5.85 to 8.79 in the regular salt groups. The meta‐analysis comparing LSSS with regular salt resulted in a rate ratio 0.64 (95% CI 0.33 to 1.25, I2 = 45%, 21,423 participants, 2 RCTs, very low‐certainty evidence, Analysis 1.39). The absolute effect for stroke mortality was 145 fewer per 100,000 person‐years (95% CI 270 fewer to 100 more). The pooled effect was driven to a considerable extent by a large secondary prevention trial including a large proportion of participants with previous stroke, resulting in limited generalisability to the general adult population. This trial (Neal 2021) had a mean follow‐up of 4.75 years, while the second trial reporting on this outcome (Zhou 2013) had a follow‐up of three years of active intervention and ten years thereafter.
1.39. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 39: Stroke mortality
The estimated population impact, as described in Appendix 2, indicated that the effect of the primary meta‐analysis (Analysis 1.39) corresponded to an estimated 28 stroke deaths prevented (ranging from 53 prevented to 20 caused) per 100,000 persons per year.
Change in blood potassium (mmol/L)
GRADE assessment suggests that, on average, LSSS probably increase blood potassium slightly compared to regular salt in the adult population (moderate‐certainty evidence, downgraded once for risk of bias).
Average changes in blood potassium ranged from a reduction of 0.2 mmol/L to an increase of 0.38 mmol/L with LSSS and from a reduction of 0.2 mmol/L to an increase of 0.3 mmol/L with regular salt in the six trials that reported this outcome. The meta‐analysis showed small, important effects on blood potassium on average between LSSS and regular salt groups (MD 0.12, 95% CI 0.07 to 0.18, I2 = 0%, 784 participants, 6 RCTs, moderate‐certainty evidence, Analysis 1.40).
1.40. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 40: Change in blood potassium (mmol/L)
This small yet important effect was confirmed by sensitivity analyses, including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.42) and including only trials randomising participants at the individual level, i.e. excluding cluster‐RCTs (Analysis 1.43). The trials reporting on this outcome reported results at 56 days, five weeks, 12 weeks and between one and 1.5 years each, while two trials reported results at approximately six months.
1.42. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 42: Change in blood potassium (mmol/L); sensitivity analysis: study quality
1.43. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 43: Change in blood potassium (mmol/L); sensitivity analysis: study design
Subgroup analyses were undertaken for this outcome to explore whether there were differences in effects in subgroups based on hyperkalaemia risk. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping participants by risk of hyperkalaemia as per Table 6 suggests there may be no important clinical differences in average effects between participants not at risk, at unclear risk and those at possible risk of hyperkalaemia (Analysis 1.41).
1.41. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 41: Change in blood potassium (mmol/L); subgroup risk of hyperkalaemia
One trial reported this outcome in an unusable format (i.e. reported change and significance of change in the control group only), and usable data were not provided when requested from authors (Table 7).
Hyperkalaemia
GRADE assessment suggests that, on average, LSSS likely result in little to no difference in hyperkalaemia in the adult population when compared to regular salt (moderate‐certainty evidence, downgraded once for risk of bias).
It should be noted, however, that a very small number of participants presented with hyperkalaemia in both groups across the trials that reported this outcome. The number of participants with hyperkalaemia in the five trials reporting this outcome ranged from zero to 11 with LSSS and from zero to nine with regular salt. The meta‐analysis of the RR was 1.04 (95% CI 0.46 to 2.38, I2 = 0%, 22,849 participants, 5 RCTs, moderate‐certainty evidence, Analysis 1.44) when comparing LSSS to regular salt. The absolute effect for hyperkalaemia was 4 more per 100,000 (95% CI 47 fewer to 121 more).
1.44. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 44: Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors)
A sensitivity analysis including only trials with 'low' or 'unclear' overall risk of bias (Analysis 1.46) confirmed this effect, though this result was highly imprecise. A sensitivity analysis including only trials randomising participants at the individual level, i.e. excluding cluster‐RCTs (Analysis 1.47) was not informative due to zero events in both trial arms. These five trials reported results after three months, 12 months, one to 1.5 years, 2 years and a mean of 4.75 years follow‐up.
1.46. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 46: Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); sensitivity analysis: study quality
1.47. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 47: Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); sensitivity analysis: study design
Subgroup analyses were undertaken for this outcome to explore whether there were differences in effects in subgroups based on hyperkalaemia risk. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping participants by risk of hyperkalaemia as per Table 6 suggests there may be no important clinical differences in average effects between participants not at risk, at unclear risk and those at possible risk, of hyperkalaemia (Analysis 1.45).
1.45. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 45: Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); subgroup risk of hyperkalaemia
Hypokalaemia
GRADE assessment of this outcome suggests that the evidence is very uncertain about the effects of LSSS on hypokalaemia when compared to regular salt in the adult population (very low‐certainty evidence, downgraded once for risk of bias and twice for indirectness).
A single, small trial (Pereira 2005) reported no hypokalaemia events in either trial arm comparing LSSS and regular salt in young participants with hypertension requiring potassium supplementation due to the use of potassium‐depleting diuretics (RR and 95% CI not estimable, 22 participants, 1 RCT, very low‐certainty evidence, Analysis 1.48). This study reported outcomes at 12 weeks.
1.48. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 48: Hypokalaemia (< 3.5 mmol/L, or as reported by study authors)
Secondary outcomes
For comparison 1, no studies reported on diabetes mellitus diagnosis or hyponatraemia.
All‐cause mortality
The number of all‐cause mortality events in two trials (CSSS Collaborative Group 2007; Pan 2017) reporting on this outcome at > 3 to 12 months ranged from three to four with LSSS and one to four with regular salt. Three additional trials (Chang 2006; Neal 2021; Zhou 2013) reported rates of events and rate ratios; these ranged from 11.08 to 93.45 events per 1000 person‐years in the LSSS groups and 13.66 to 101.29 events per 1000 person‐years in the regular salt groups and corresponded to rate ratios (95% CIs) of 0.92 (0.77 to 1.10), 0.88 (0.82 to 0.95) and 0.81 (0.46 to 1.42), respectively. The meta‐analysis combining these data as risk ratios resulted in an RR of 0.89 (95% CI 0.83 to 0.95, I2 = 0%, 24,005 participants, 5 RCTs, Analysis 1.49) when comparing LSSS with regular salt.
1.49. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 49: All‐cause mortality
Adverse events (other)
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS on other adverse events when compared to regular salt in adults (very low‐certainty evidence, downgraded once for risk of bias, once for inconsistency and once for imprecision).
The number of participants with other adverse events in the eight trials reporting this outcome ranged from zero to 17 with LSSS and from zero to seven with regular salt. The events reported were highly diverse and not suitable for pooling in a meta‐analysis (2109 participants, 8 RCTs, very low‐certainty evidence, Analysis 1.50). Four trials reporting on other adverse events reported these at ≤ 3 months, three reported on this outcome at > 3 to 12 months and one trial reported other adverse events at > 12 months.
1.50. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 50: Adverse events: other
Subgroup analyses were undertaken for this outcome to explore whether there were differences in effects in subgroups based on hyperkalaemia risk. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping participants by risk of hyperkalaemia as per Table 6 suggests there may be no important clinical differences in average effects between participants not at risk, and those at possible risk, of hyperkalaemia (Analysis 1.51).
1.51. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 51: Adverse events: other; subgroup risk of hyperkalaemia
One trial reported this outcome in an unusable format (i.e. numbers of events per group not reported), and usable data were not provided when requested from authors (Table 7).
Antihypertensive medication use
The number of participants using antihypertensive medication across the four trials reporting this outcome ranged from 34 to 246 with LSSS and from 52 to 267 with regular salt. The meta‐analysis of the RR of the number of participants using antihypertensive medication was 0.80 (95% CI 0.67 to 0.95, I2 = 53%, 3301 participants, 4 RCTs, Analysis 1.52) when comparing LSSS and regular salt groups. The one individually randomised trial that reported this outcome had follow‐up at three months; the three cluster trials reporting this outcome had follow‐up at 12, 18 and 36 months. The stepped‐wedge cluster trial (unclear overall risk of bias) reported no changes in medication use, with 10.5% at baseline and 10.1% (P = 0.73) at the end of the study three years later; this was for hypertension and type 2 diabetes mellitus medication use combined.
1.52. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 52: Antihypertensive medication use
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by duration of study (Analysis 1.53) as well as age (Analysis 1.54), gender (Analysis 1.55), BMI (Analysis 1.56) or hypertensive status (Analysis 1.57) of participants at baseline suggests there may be no important clinical differences in average effects between subgroups. Subgrouping by ethnicity, type and implementation of LSSS, 24‐h sodium excretion at baseline and 24‐h potassium excretion at baseline could not be used to explore clinical differences, as all studies were categorised into the same subgroup.
1.53. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 53: Antihypertensive medication use; subgroup study duration
1.54. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 54: Antihypertensive medication use; subgroup age
1.55. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 55: Antihypertensive medication use; subgroup gender
1.56. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 56: Antihypertensive medication use; subgroup BMI
1.57. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 57: Antihypertensive medication use; subgroup blood pressure status
Change in BMI (kg/m2)
Average change in BMI ranged from a reduction of 1.6 kg/m2 to no change with LSSS and from a reduction of 1.0 kg/m2 to no change with regular salt in the four trials that reported this outcome. We did not pool these results into an overall effect estimate due to considerable heterogeneity (I² = 96%, Tau² = 0.80, Chi² = 70.15, df = 3 (P < 0.00001)), but rather presented the individual effect sizes between LSSS and regular salt per study (2060 participants, 4 RCTs, Analysis 1.58). Trials reporting on this outcome followed participants for eight weeks, 12 weeks, four months and 18 months.
1.58. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 58: Change in BMI (kg/m2)
Change in serum creatinine (µmol/L)
Average change in serum creatinine ranged from a reduction of 0.8 µmol/L to an increase of 2 µmol/L with LSSS and from a reduction of 1.0 µmol/L to an increase of 3.54 µmol/L with regular salt in the three trials that reported this outcome. The mean difference in serum creatinine, on average, between LSSS and regular salt groups was 2.56 µmol/L (95% CI ‐0.59 to 5.71, I2 = 0%, 616 participants, 3 RCTs, Analysis 1.59). Trials reporting on this outcome followed participants for 12 weeks, six months and between one and 1.5 years.
1.59. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 59: Change in serum creatinine (µmol/L)
Subgroup analyses were undertaken for this outcome to explore whether there are differences in effects in subgroups based on hyperkalaemia risk. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping participants by risk of hyperkalaemia as per Table 6 suggests there may be no important clinical differences in average effects between participants not at risk, and those at possible risk, of hyperkalaemia (Analysis 1.60).
1.60. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 60: Change in serum creatinine (µmol/L); subgroup risk of hyperkalaemia
Microalbuminuria
Two studies reported on this outcome (RR 0.67, 95% CI 0.53 to 0.84, I2 = 0%, 2382 participants, 2 RCTs, Analysis 1.61), with the number of participants in the LSSS group with microalbuminuria ranging from 45 to 64, and participants in the regular salt group with microalbuminuria ranging from 58 to 84 across trials. The two trials reporting this outcome had follow‐up at 18 months and three years.
1.61. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 61: Microalbuminuria
Subgrouping participants by risk of hyperkalaemia could not be conducted as participants in both trials were at unclear risk of hyperkalaemia as per Table 6.
Macroalbuminuria
One trial reported on this outcome at 18 months (RR 0.48, 95% CI 0.16 to 1.39, 1903 participants, 1 RCT, Analysis 1.62), with five participants in the LSSS group experiencing macroalbuminuria events and 10 participants in the regular salt group experiencing macroalbuminuria events.
1.62. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 62: Macroalbuminuria
Change in urinary albumin‐to‐creatinine ratio (uACR)
One trial reported end values for this outcome at 18 months (MD ‐1.68, 95% CI ‐2.87 to ‐0.49, 1903 participants, 1 RCT, Analysis 1.63).
1.63. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 63: Change in urinary albumin‐to‐creatinine ratio (uACR)
Change in fasting blood glucose (mmol/L)
Two trials reported on this outcome for this comparison. Average increases in fasting blood glucose ranged from 0.1 mmol/L to 0.22 mmol/L with LSSS, while average changes ranged from no change to an increase of 0.10 mmol/L in fasting blood glucose with regular salt, across the two trials. We did not pool these results into an overall effect estimate due to considerable heterogeneity (I² = 94%, Tau² = 0.56, Chi² = 17.06, df = 1 (P < 0.0001)), but rather presented the individual effect sizes between LSSS and regular salt per study (338 participants, 2 RCTs, Analysis 1.64). The two trials reporting on the outcome followed up participants for four and six months each.
1.64. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 64: Change in fasting blood glucose (mmol/L)
Change in blood triglycerides (mmol/L)
Five trials reported on change in blood triglycerides for this comparison. Average changes in blood triglycerides ranged from a reduction of 0.7 mmol/L to an increase of 0.15 mmol/L with LSSS and from a reduction of 0.01 mmol/L to an increase of 0.9 mmol/L in blood triglycerides with regular salt across the five trials. The change in blood triglycerides, on average, between LSSS and regular salt groups was ‐0.11 mmol/L (95% CI ‐0.91 to 0.69, I2 = 81%, 420 participants, 5 RCTs, Analysis 1.65). Trials reporting on the outcome followed up participants for five weeks, 12 weeks, four months, six months and nine months each.
1.65. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 65: Change in blood triglycerides (mmol/L)
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by study duration (Analysis 1.66), age (Analysis 1.67), ethnicity (Analysis 1.68), BMI (Analysis 1.69), blood pressure status (Analysis 1.70), type of LSSS (Analysis 1.72), baseline sodium excretion (Analysis 1.73) and baseline potassium excretion (Analysis 1.74) suggests there may be no important differences in average effects between these subgroups. Subgrouping by the method of implementation of LSSS yielded a few studies in each subgroup, resulting in an analysis that was not powered to detect any differences in effect (Analysis 1.71). Subgrouping by gender could not be used to explore clinical differences as all studies were categorised into the same subgroup.
1.66. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 66: Change in blood triglycerides (mmol/L); subgroup study duration
1.67. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 67: Change in blood triglycerides (mmol/L); subgroup age
1.68. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 68: Change in blood triglycerides (mmol/L); subgroup ethnicity
1.69. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 69: Change in blood triglycerides (mmol/L); subgroup BMI
1.70. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 70: Change in blood triglycerides (mmol/L); subgroup blood pressure status
1.72. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 72: Change in blood triglycerides (mmol/L); subgroup type of LSSS
1.73. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 73: Change in blood triglycerides (mmol/L); subgroup baseline sodium excretion (mmol/24‐h)
1.74. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 74: Change in blood triglycerides (mmol/L); subgroup baseline potassium excretion (mmol/24‐h)
1.71. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 71: Change in blood triglycerides (mmol/L); subgroup LSSS implementation
Change in total blood cholesterol (mmol/L)
Six trials reported on change in total blood cholesterol for this comparison. One trial each followed up participants for five weeks, 12 weeks and four months; two trials reporting on this outcome had follow‐up of approximately six months and one trial followed up participants for nine months. Across the trials, average changes in total blood cholesterol ranged from a reduction of 0.7 mmol/L to an increase of 0.16 mmol/L with LSSS and from a reduction of 0.63 mmol/L to an increase of 0.24 mmol/L with regular salt. When LSSS were compared to regular salt, the mean difference in total cholesterol change on average was ‐0.31 mmol/L (95% CI ‐0.74 to 0.12, I2 = 85%, 509 participants, 6 RCTs, Analysis 1.75).
1.75. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 75: Change in total blood cholesterol (mmol/L)
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by study duration (Analysis 1.76), age (Analysis 1.77), ethnicity (Analysis 1.78), BMI (Analysis 1.79), blood pressure status (Analysis 1.80), implementation of LSSS (Analysis 1.81), type of LSSS (Analysis 1.82), baseline sodium excretion (Analysis 1.83) and baseline potassium excretion (Analysis 1.84) suggests there may be no important differences in average effects between these subgroups. Subgrouping by gender could not be used to explore clinical differences as all studies were categorised into the same subgroup.
1.76. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 76: Change in total blood cholesterol (mmol/L); subgroup study duration
1.77. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 77: Change in total blood cholesterol (mmol/L); subgroup age
1.78. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 78: Change in total blood cholesterol (mmol/L); subgroup ethnicity
1.79. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 79: Change in total blood cholesterol (mmol/L); subgroup BMI
1.80. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 80: Change in total blood cholesterol (mmol/L); subgroup blood pressure status
1.81. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 81: Change in total blood cholesterol (mmol/L); subgroup LSSS implementation
1.82. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 82: Change in total blood cholesterol (mmol/L); subgroup type of LSSS
1.83. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 83: Change in total blood cholesterol (mmol/L); subgroup baseline sodium excretion (mmol/24‐h)
1.84. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 84: Change in total blood cholesterol (mmol/L); subgroup baseline potassium excretion (mmol/24‐h)
Change in 24‐h urinary sodium excretion (mmol/24‐h)
Eleven trials reported on change in 24‐h urinary sodium excretion for this comparison. Average changes in this outcome ranged from a reduction of 75.5 mmol (1730 mg) sodium/24‐h to an increase of 20.2 mmol (460 mg) sodium/24‐h with LSSS and from a reduction of 31 mmol (710 mg) sodium/24‐h to an increase of 11 mmol (250 mg) sodium/24‐h with regular salt across the trials. We did not pool these results into an overall effect estimate due to considerable heterogeneity (I² = 91%, Tau² = 595.13, Chi² = 107.72, df = 10 (P < 0.00001)), but rather presented the individual effect sizes between LSSS and regular salt per study (3885 participants, 11 RCTs, Analysis 1.85). Three trials reporting on this outcome followed up participants for four, five, and eight weeks; five trials followed up participants for three, four, nine, 18 and 60 months each; three trials reported on the outcome at approximately six months.
1.85. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 85: Change in 24‐h urinary sodium excretion (mmol/24‐h)
The stepped‐wedge cluster trial (unclear overall risk of bias) reporting on 605 participants reported little to no difference between a LSSS and regular salt for this outcome (Analysis 1.86).
1.86. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 86: Change in 24‐h urinary sodium excretion (mmol/24‐h) stepped‐wedge trial
Change in 24‐h urinary potassium excretion (mmol/24‐h)
Eleven trials reported on change in 24‐h urinary potassium excretion for this comparison. Average changes in this outcome ranged from a reduction of 4.4 mmol (170 mg) potassium/24‐h to an increase of 18.5 mmol (720 mg) potassium/24‐h with LSSS and from a reduction of 16 mmol (630 mg) potassium/24‐h to an increase of 4.6 mmol (180 mg) potassium/24‐h with regular salt across the trials. The meta‐analysis showed a difference, on average, favouring LSSS when compared to regular salt in the effect on 24‐h urinary potassium excretion between LSSS and regular salt groups (MD 11.44 mmol (450 mg) potassium/24‐h, 95% CI 7.62 to 15.26 mmol/24‐h [298 to 597 mg/24‐h], I2 = 82%, 3885 participants, 11 RCTs, Analysis 1.87). Three trials reporting on the outcome followed up participants for four, five, and eight weeks, five trials followed up participants for three, four, nine, 18 and 60 months each; three trials reported on the outcome at approximately six months.
1.87. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 87: Change in 24‐h urinary potassium excretion (mmol/24‐h)
The stepped‐wedge cluster trial (unclear overall risk of bias) reporting on 605 participants found a similar direction of effect, though it was far smaller (Analysis 1.98).
1.98. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 98: Change in 24‐h urinary potassium excretion (mmol/24‐h) stepped‐wedge trial
Subgroup analyses were undertaken for this outcome due to the presence of substantial heterogeneity. In line with Cochrane guidance (Deeks 2020) detailing the limitations of subgroup analyses, caution was taken in the interpretation of findings from these subgroup analyses. Subgrouping by study duration (Analysis 1.88), age (Analysis 1.89), gender (Analysis 1.90), ethnicity (Analysis 1.91), BMI (Analysis 1.92), type of LSSS (Analysis 1.95), baseline sodium excretion (Analysis 1.96) and baseline potassium excretion (Analysis 1.97) suggests there may be no important differences in average effects between these subgroups.
1.88. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 88: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup study duration
1.89. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 89: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup age
1.90. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 90: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup gender
1.91. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 91: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup ethnicity
1.92. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 92: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup BMI
1.95. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 95: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup type of LSSS
1.96. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 96: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup baseline sodium excretion (mmol/24‐h)
1.97. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 97: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup baseline potassium excretion (mmol/24‐h)
Subgrouping by blood pressure status suggested some differences between subgroups (Analysis 1.93), but this was driven mainly by a large subgroup of participants of mixed hypertensive status from one study (Neal 2021). Therefore, the observed difference in effect was not considered to be attributable to hypertensive status. Subgrouping by the method of implementation of LSSS yielded a few studies in two of the three subgroups, resulting in an analysis that was not powered to detect any differences in effect (Analysis 1.94).
1.93. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 93: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup blood pressure status
1.94. Analysis.

Comparison 1: Low‐sodium salt substitutes versus regular salt or no active intervention in adults, Outcome 94: Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup LSSS implementation
Comparison 2. Low‐sodium salt substitutes versus regular salt or no active intervention in children
Table 2 presents the effects of LSSS compared to regular salt in children on changes in DBP and SBP.
A single RCT randomising families as clusters and reporting on 92 children was included in this comparison. Key details about the study in this comparison, including study design, setting and overall risk of bias; characteristics of the intervention, comparator, population and outcomes; method of synthesis, and time points of measurement are included in the Overview of Synthesis and Included Studies (OSIS) table (Table 9).
7. Comparison 2: Overview of Synthesis and Included Studies (OSIS).
| Study name (year) | Study design (individual vs stepped‐wedge vs cluster‐randomised [and unit of randomisation, if applicable]) | Country of conduct | Overall risk of bias (arranged low to high) | Key details of intervention (% KCl, LSSS implementation [discretionary, non‐discretionary, both], quantity, provided or purchased, co‐interventions [education, advice]) | Key details of the comparator (implementation [discretionary, non‐discretionary, both], quantity, provided or household supply, co‐interventions [education, advice]) |
Population (No. of participants randomised [intervention/control], age, gender, hypertensive status, anti‐hypertensive medication use, BMI) |
Outcome domains with available data (synthesis method/metric) | Specific outcomes measure | Time point of measurement |
| Toft 2020 | Cluster‐randomised (families) | Denmark | Unclear | < 30% KCl LSSS (per 100 g: approximately 8000 mg sodium; 870 mg Mg and 100 mg‐200 mg K estimated from the technical data sheet) Non‐discretionary use (LSSS bread products to replace usual consumption), provided to families twice a week Co‐interventions NR |
Regular salt Non‐discretionary use (regular wholegrain bread products for usual consumption), provided to families twice a week Co‐interventions NR |
40/52 9.5 (4.2)/8.4 (3.5) years Female, %: 47.5/48.1 Normotensive N/A 18.0 (2.9)/16.9 (2.8) kg/m2 |
Change in blood pressure Change in BMI 24‐h urinary excretion |
1. Change in DBP 2. Change in SBP 3. Change in BMI 4. 24‐h urinary sodium excretion 5. 24‐h urinary potassium excretion |
1. 4 months 2. 4 months 3. 4 months 4. 4 months 5. 4 months |
Abbreviations: 24‐h: 24‐hour BMI: body mass index DBP: diastolic blood pressure K(Cl): potassium (chloride) LSSS: low‐sodium salt substitute Mg: magnesium N/A: not applicable NR: not reported
Primary outcomes
For comparison 2, no studies reported on hypertension, blood pressure control, change in blood potassium, hyperkalaemia or hypokalaemia.
Change in diastolic blood pressure (DBP, mmHg)
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS on changes in DBP, when compared to regular salt, in children (very low‐certainty evidence, downgraded once risk of bias, once for indirectness and once for imprecision).
The average change in DBP was a reduction of 2.1 mmHg in the group that ate bread containing LSSS and a reduction of 5.87 mmHg in the group that ate bread containing regular salt for the single cluster‐RCT that reported this outcome at four months follow‐up. The mean difference, when comparing these groups, was 1.28 mmHg (95% CI ‐1.56 to 4.12, 92 participants, 1 RCT, very low‐certainty evidence, Analysis 2.1).
2.1. Analysis.

Comparison 2: Low‐sodium salt substitutes versus regular salt or no active intervention in children, Outcome 1: Change in DBP (mmHg) at > 3 to 12 months
Change in systolic blood pressure (SBP, mmHg)
GRADE assessment suggests that the evidence is very uncertain about the effect of LSSS on changes in SBP, when compared to regular salt, in children (very low‐certainty evidence, downgraded once risk of bias, once for indirectness and once for imprecision).
The average change in SBP was a reduction of 5.1 mmHg in the group that ate bread containing LSSS and a reduction of 6.05 mmHg in the group that ate bread containing regular salt for the single cluster‐RCT that reported this outcome at four months follow‐up. The mean difference when comparing these groups was 0.12 mmHg (95% CI ‐4.41 to 4.64, 92 participants, 1 RCT, very low‐certainty evidence, Analysis 2.2).
2.2. Analysis.

Comparison 2: Low‐sodium salt substitutes versus regular salt or no active intervention in children, Outcome 2: Change in SBP (mmHg) at > 3 to 12 months
Secondary outcomes
For comparison 2, no studies reported on adverse events (other), cardiovascular events, antihypertensive medication use, all‐cause mortality, cardiovascular mortality, bone densitometry measures, renal function, bone health, hyponatraemia, changes in fasting blood glucose, changes in blood triglycerides or changes in total blood cholesterol.
Growth changes (e.g. z‐scores for height‐ or length‐for‐age (HAZ or LAZ), weight‐for‐height (WHZ), weight‐for‐age (WAZ), BMI‐for‐age)
In the trial reporting on BMI changes in children, the unadjusted average reductions in BMI were 1.62 kg/m2 with bread containing LSSS and 1.50 kg/m2 with bread containing regular salt. The mean difference in BMI, on average, was 0.94 kg/m2 (95% CI 0.85 to 1.03, 92 participants, 1 RCT, Analysis 2.3) when comparing these groups at four months.
2.3. Analysis.

Comparison 2: Low‐sodium salt substitutes versus regular salt or no active intervention in children, Outcome 3: Change in BMI (kg/m2) at > 3 to 12 months
Change in 24‐h urinary sodium excretion (mmol/24‐h)
The average change in 24‐h urinary sodium excretion was an increase of 11.4 mmol (262 mg) sodium/24‐h in the group that ate bread containing LSSS and a reduction of 3.2 mmol (74 mg) sodium/24‐h in the group that ate bread containing regular salt for the single cluster‐RCT that reported this outcome at four months follow‐up. The mean difference when comparing these groups was 14.60 mmol (336 mg) sodium/24‐h (95% CI ‐11.22 to 40.42 mmol/24‐h [‐258 to 929 mg/24‐h], 92 participants, 1 RCT, Analysis 2.4).
2.4. Analysis.

Comparison 2: Low‐sodium salt substitutes versus regular salt or no active intervention in children, Outcome 4: Change in 24‐h urinary sodium (mmol/24‐h)
Change in 24‐h urinary potassium excretion (mmol/24‐h)
The average change in 24‐h urinary potassium excretion was a reduction of 1.6 mmol (64 mg) potassium/24‐h in the group that ate bread containing LSSS and a reduction of 5.7 mmol (223 mg) potassium/24‐h in the group that ate bread containing regular salt for the single cluster‐RCT that reported this outcome at four months follow‐up. The mean difference when comparing these groups was 4.10 mmol (160 mg) potassium/24‐h (95% CI ‐5.13 to 13.33 mmol/24‐h [‐201 to 521 mg/24‐h], 92 participants, 1 RCT, Analysis 2.5).
2.5. Analysis.

Comparison 2: Low‐sodium salt substitutes versus regular salt or no active intervention in children, Outcome 5: Change in 24‐h urinary potassium (mmol/24‐h)
Comparison 3. Low‐sodium salt substitutes versus regular salt or no active intervention in pregnant women
No eligible studies in pregnant women were found.
Discussion
Summary of main results
This review examined the effects and safety of LSSS compared to regular salt or no active intervention on blood pressure and cardiovascular health in adults, children and pregnant women. We included 16 RCTs and ten cluster‐RCTs (n = 26) conducted in adults from a range of different settings, including nursing homes, hospitals, rural and suburban households and communities, as well as rural villages. Importantly, these 26 trials included various clinical subpopulations, with nearly two‐thirds of trials conducted in people with existing hypertension. We also included one cluster‐RCT including healthy children. The proportion of sodium chloride replacement in the LSSS interventions varied from approximately 3% to 77% with 24 trials replacing the sodium chloride with some potassium chloride (more details in the Characteristics of included studies section). We did not find any eligible studies in pregnant women. We also did not find any eligible prospective analytical cohort studies.
Low‐sodium salt substitutes versus regular salt or no active intervention in adults
Adult participants in groups allocated to LSSS had lowered DBP and SBP (range of reductions: 0.6 to 11.33 mmHg and 1.5 to 15.25 mmHg, respectively) on average, while those allocated to regular salt had smaller reductions and more variable results for both DBP (range of change: 7 mmHg reduction to 2.6 mmHg increase) and SBP (range of change: 6.8 mmHg reduction to 4 mmHg increase) on average.
In adult participants, meta‐analysis showed that LSSS probably reduce DBP slightly at up to 60 months. The 95% CIs of the pooled mean differences did not include clinically meaningful benefit (ranging from 1.36 to 3.50 mmHg lower), as we considered changes in DBP of greater than 5 mmHg to be clinically meaningful due to a 'significant' reduction in stroke risk of approximately 60% in high risk individuals (Thomopoulos 2017). However, small mean reductions for an entire population are more beneficial than very large reductions in only those at high risk (Verbeek 2021). Since the review focussed on the population‐level substitution of regular salt with LSSS, we applied a population perspective and derived a simplified population impact estimate (as described in Appendix 2) of the reduction in DBP observed in our meta‐analysis. This estimate suggested that the observed reduction in DBP corresponded to an estimated 60 (ranging from 35 to 83) stroke deaths prevented per 100,000 persons aged 50 years and older, per year. The observed small, important mean difference in DBP between LSSS and regular salt was confirmed by sensitivity analyses. Substantial heterogeneity was, however, detected in the pooled analysis for this outcome: subgrouping by various characteristics of included studies, participants and interventions suggests there may be no important clinical differences in average effects between the various subgroups.
In adult participants, meta‐analysis showed that LSSS probably reduce SBP slightly at up to 60 months. The 95% CIs of the pooled mean differences did not include clinically meaningful benefit (ranging from 3.50 to 6.01 mmHg lower), as we considered changes of at least 10 mmHg in SBP to be clinically meaningful. This cut‐off was informed by a systematic review with meta‐regression, quantifying the effects of blood pressure reduction on cardiovascular outcomes and death from large‐scale blood‐pressure lowering trials, which indicated that relative risk reductions are proportional to the magnitude of blood‐pressure reduction, with every 10 mmHg reduction in SBP significantly reducing the risk of major cardiovascular disease events (Ettehad 2016). In addition, the overview and meta‐analysis by Thomopoulos 2017, investigating the effects of blood‐pressure lowering treatment and stratifying participants by total cardiovascular risk, reported a 'significant' stroke reduction of 60% with a 10 mmHg reduction in SBP in individuals at high risk. Our simplified population impact estimate suggested that the observed reduction in SBP with LSSS compared to regular salt corresponds to an estimated 53 (ranging from 40 to 65) stroke deaths prevented per 100,000 persons aged 50 years and older, per year. The observed small, important mean difference in SBP between LSSS and regular salt was broadly confirmed by sensitivity analyses. We explored the substantial heterogeneity detected in the pooled analysis for SBP using subgroup analyses, which suggest there may be no important clinical differences in average effects between the various subgroups.
For the presence of hypertension at 18 months, one trial showed little to no difference between the effects of LSSS and regular salt in adult participants. A stepped‐wedge cluster trial measuring incident hypertension indicated a more pronounced difference, with the hazard ratio for this outcome favouring LSSS.
We do not know whether there is a difference in the number of adult participants per group who achieve blood pressure control as the 95% CI limits of the pooled effect were consistent with the possibility for unimportant and important benefit, and most of the information for this outcome came from a study at unclear overall risk of bias. Furthermore, this study had limited generalisability as it investigated the effect of LSSS comprising 97% sodium chloride (regular salt) and therefore did not represent the composition of the majority of LSSS formulations on the market, most of which contain 65% or less sodium chloride.
We also do not know whether there is a difference in the number of adult participants per group who experience various cardiovascular events. Only 18 of these events were reported in total, including angina, serious cardiovascular events and cardiovascular symptoms, resulting in very imprecise 95% CI limits around the pooled effect. In addition, the pooled effect was driven by a large study in individuals at high risk of future vascular disease, thereby limiting the generalisability of the findings.
In adult participants, meta‐analysis showed that LSSS probably reduce non‐fatal stroke slightly at up to 60 months. The 95% CIs of the pooled risk and rate ratios included unimportant benefit and unimportant harm or no effect (ranging from a RR of 0.80 to 1.01), as we considered relative measures of less than 0.75 or greater than 1.25 to be important or 'appreciable' (Guyatt 2011). The simplified population impact estimate we derived (as described in Appendix 2) suggested that the observed relative risk when LSSS was compared to regular salt corresponds to an estimated 10 non‐fatal strokes prevented (ranging from 21 prevented to 1 caused) per 100,000 persons per year. The observed benefit with LSSS was not reflected in sensitivity analyses; with these instead showing highly imprecise effects of little to no effect, or harm.
Meta‐analysis in adult participants showed that LSSS probably reduce non‐fatal ACS slightly at up to 60 months. The 95% CIs of this effect included important and unimportant benefit (ranging from a rate ratio of 0.52 to 0.94), as we considered relative measures of less than 0.75 or greater than 1.25 to be important. The simplified population impact estimate we derived suggested that the observed relative risk when LSSS was compared to regular salt corresponds to an estimated 50 non‐fatal ACS events prevented (ranging from 10 to 80 prevented) per 100,000 persons per year.
In adult participants, meta‐analysis showed that LSSS probably reduce cardiovascular mortality slightly at up to 60 months. The 95% CIs of the pooled rate ratios included important benefit and no effect (ranging from a rate ratio of 0.60 to 1.00), as we considered relative measures of less than 0.75 or greater than 1.25 to be important. The simplified population impact estimate we derived suggested that the observed relative risk when LSSS was compared to regular salt corresponds to an estimated 53 cardiovascular deaths prevented (ranging from 92 prevented to none caused or prevented) per 100,000 persons per year. The observed relative effect between LSSS and regular salt was confirmed by sensitivity analysis excluding trials at high risk of overall bias.
We do not know whether there is a difference in the number of stroke deaths per group in adults as the 95% CI limits of the pooled effect were consistent with the possibility for important harm and important benefit. Furthermore, the generalisability of the results to the general population was limited as the pooled effect was driven by a large secondary prevention trial in which 73% of included participants had previously had a stroke.
In adult participants, meta‐analysis showed that LSSS probably increase blood potassium slightly at up to one and a half years. The 95% CIs of the pooled mean differences did not include clinically meaningful changes (ranging from 0.07 to 0.18 mmol/L higher), as we considered changes in blood potassium of greater than 1.0 mmol/L to be clinically meaningful. This is based on the variations around the 'normal' blood potassium levels of 3.6 to 5.0 mmol/L (Cohn 2000), which can cause moderate hyperkalaemia (defined as 6.0 to 6.9 mmol/L; Hollander‐Rodriguez 2006 and Ahee 2000, respectively).
For the presence of hyperkalaemia at up to 60 months, some trials reported no events while others reported hyperkalaemic events in both the LSSS and regular salt groups. In adult participants, meta‐analyses showed that LSSS likely result in little to no difference in hyperkalaemia. The 95% CIs of the pooled risk ratios included important benefit and important harm (ranging from a RR of 0.46 to 2.38), as we considered relative measures of less than 0.75 or greater than 1.25 to be important. Only five trials reported on this outcome, though all information included in the meta‐analysis came from two trials in participants judged to be at possible and unclear risk of hyperkalaemia.
We do not know whether there is a difference in the number of adult participants per group who experience hypokalaemia events as one small trial reporting on this outcome reported zero events in both groups. In addition, this trial was at unclear overall risk of bias, and included only younger participants with hypertension treated with potassium‐sparing diuretics. Consequently, as the rationale for the administration of LSSS was potassium supplementation, we considered the generalisability of the evidence to be limited.
We also do not know whether there is a difference in the number of adult participants per group who experience other adverse events. Most of the information for this outcome was from studies at high or unclear overall risk of bias, events were very sparsely reported (39 in total), and outcomes were too diverse to pool.
Low‐sodium salt substitutes versus regular salt or no active intervention in children
Children (mean age 9.5 (SD 4.2) years) allocated to bread containing LSSS had lowered DBP and SBP (reduction of 2.1 mmHg and 5.1 mmHg, respectively) on average. Children (mean age 8.4 (SD 3.5) years) allocated to bread containing regular salt had larger reductions for both DBP and SBP (reduction of 5.87 mmHg and 6.05 mmHg, respectively) on average.
In all participants aged 18 or younger, results from a single included trial showed that the evidence is very uncertain about the effect of LSSS compared to regular salt on change in DBP as well as SBP at four months. The trial contributing to these outcomes was at unclear overall risk of bias, and reported effects with wide 95% CIs including both reductions and increases in DBP and SBP. Furthermore, the intervention was delivered in bread only, thereby limiting generalisability to discretionary use settings.
Overall completeness and applicability of evidence
Our review made use of a comprehensive search strategy with no language or date restrictions to identify all RCTs and prospective analytical cohort studies assessing the effect of LSSS on cardiovascular health in adults, children and pregnant women in the general population. We searched multiple sources of information for all studies and handsearched three relevant systematic reviews to identify additional studies. We also contacted study authors in cases where we required additional data or information.
We found only one trial, included under Comparison 1 for the effect of LSSS on adults, which additionally reported certain outcomes in children. The sparse evidence in children may be due to the relatively low prevalence of elevated blood pressure in children, with two large systematic reviews conducted in low‐ and middle‐income settings reporting pooled prevalence of 5.5% and 9.8% in children and adolescents from Africa and China, respectively (Noubiap 2017; Wang 2019). A large systematic review and meta‐regression conducted in 122,000 adolescents further indicated that this prevalence was disproportionately affecting adolescents in low‐ and middle‐income countries (De Moraes 2014). Consequently, maximal population‐level effects would not be achieved through targeting children, but rather adults; a group in which the global prevalence of hypertension (defined as a SBP ≥ 140 mmHg and DBP ≥ 90 mmHg) was approximately 32.5% for adults aged 30 years and older in 2019 (NCD Risk Factor Collaboration 2021).
We found no studies assessing the effect of LSSS in pregnant women. While chronic hypertension is a known significant risk factor for pre‐eclampsia (Bilano 2014), mean arterial pressure (MAP) in the first and second trimester has been suggested as a better predictor of pre‐eclampsia than SBP and DBP (Cnossen 2008). This study additionally reported that high MAP before pregnancy can be used as a predictor of pre‐eclampsia (Cnossen 2008) suggesting that the timing of the management of blood pressure is important, and that interventions to lower blood pressure prior to pregnancy might have the most success in avoiding complications for the mother and infant. In addition, pre‐eclampsia is a complex disease involving multiple organ systems (Palei 2013) and risk factors (Bilano 2014), and there is a paucity of evidence to support lifestyle interventions, such as reducing dietary sodium intake, for preventing pre‐eclampsia (Thangaratinam 2011).
Though our review included a reasonable distribution of studies from low‐ and middle‐income (n = 15) and high‐income countries (n = 12), the majority of trials (n = 15) were conducted in Asian populations. Furthermore, only one included study was conducted in South America; no eligible studies conducted in Africa, Oceania or North America were found. As different populations may use different quantities of discretionary salt, as a proportion of total salt intake, this may have an impact on the degree to which substitution with discretionary LSSS will alter sodium and potassium intakes. Subgroup analyses suggest there may be no important clinical differences in average effects on blood pressure between subgroups by ethnicity, although these analyses were limited. This suggested that the mix of ethnicities included in the review may not systematically bias our pooled estimates to Asian populations. It is more difficult to judge whether evidence from other countries and regions not represented in the review may have changed our pooled estimates. Such potential systematic differences could likely be categorised as biological and behavioural; such categories might plausibly include differences in baseline prevalence, and extent, of hypertension and differences in adherence to LSSS, respectively. Our review included populations with diverse baseline risks of hypertension and diverse baseline 24‐h urinary excretion of sodium and potassium, but subgroup analyses of these factors suggest there may be no important clinical differences in average effects on blood pressure.
Importantly, the findings of subgroup analyses should always be interpreted with caution as these can often be misleading (Deeks 2020). The likelihood of false negative and positive results increase rapidly when numerous subgroup analyses are undertaken and statistical power to find significant differences between subgroups is often lacking (Cuijpers 2021; Deeks 2020). In our review in particular, subgroup analyses were often limited by very few studies or participants contributing information to certain subgroups. As a result, findings from the subgroup analyses in our review may not all be sufficiently robust and should be interpreted with caution and with consideration of the described limitations.
Most of the trials included in our review assessed the effects of LSSS in participants with elevated blood pressure at enrolment. Due to limited data, we could not adequately examine effect modification for the relationship between LSSS use and outcomes by hypertension status.
All trials included in the review specifically excluded participants in whom it is known that an increased intake of potassium could cause harm, for example, people with CKD, type 1 or 2 diabetes mellitus, impaired renal function or those using potassium‐sparing medications. This limits the generalisability of our findings regarding the effects and safety of LSSS to these subpopulations, as well as to settings where a considerable proportion of the population may have undiagnosed conditions rendering increased potassium intake as potentially harmful.
Furthermore, the majority of included trials investigated the implementation of LSSS as a discretionary intervention. This limits the generalisability of our findings to non‐discretionary applications of LSSS, particularly use in condiments, and in manufactured food products or foods sold in restaurants, markets, cafeterias and street vendors.
We did not find evidence for a number of prespecified outcomes in the review. Across comparisons, no studies reported on diabetes mellitus (DM) diagnosis or hyponatraemia. The absence of evidence on hyponatraemia represents a gap in the evidence related to the safety of LSSS. While global sodium intakes are approximately double the WHO recommendation at present, thereby lowering the overall likelihood of hyponatraemia events, the individual risk of this event remains ‐ particularly in older people and those using thiazide diuretics (Filippatos 2017; Upadhyay 2009).
In Comparison 2, a single study reporting on the effects of LSSS in children did not report on hypertension, blood pressure control, hyper‐ or hypokalaemia, changes in blood potassium or adverse events. The paucity of evidence for these outcomes in children is likely due to the low general prevalence of hypertension as well as conditions and risk factors related to blood potassium imbalances.
Quality of the evidence
The interpretation of many of the trials included in the review is constrained by small sample sizes and considerable loss to follow‐up. Lack of baseline exposure and dietary status, as well as adherence to the allocated intervention, have been identified as factors that undermine the translation of dietary clinical trials into practice (Mirmiran 2021); these were generally sparsely and diversely reported by the included trials in the review. Nine trials included in the review were judged as having low risk of bias overall; 12 were at unclear overall risk of bias.
The pooled estimates of the effect of LSSS interventions compared to regular salt on hypertension, blood pressure control, and stroke mortality were downgraded for imprecision in line with the minimally contextualised approach we used. Imprecision was also identified for the outcomes: various cardiovascular events and other adverse events. These were composite outcomes and studies reporting on them were not designed or powered to detect differences between LSSS and regular salt groups.
The evidence for blood pressure control was considered indirect because it is questionable whether the intervention for the study contributing the most data was sufficiently lower in sodium compared to regular salt (only 3% replacement).
The evidence for hypokalaemia was downgraded for indirectness since the participants included in the single study reporting on this outcome were not sufficiently generalisable, being younger hypertensive adults on potassium‐depleting diuretics.
Indirectness was also identified for all outcomes relating to cardiovascular events and mortality. This was due to most, or all, of the information for these outcomes coming from studies including participants at high risk of cardiovascular disease, or individuals who had already experienced a cardiovascular event; thereby limiting the generalisability of the findings to the general population.
Furthermore, the certainty in pooled estimates for changes in blood pressure was affected by unexplained substantial heterogeneity. This may be due to clinical heterogeneity related to the various ways in which blood pressure measurements are collected in practice; various studies have shown variability between single measures and the mean of consecutive measurements (Burkard 2018), between blood pressure measured in a clinical setting and those obtained from daytime ambulatory measurements (Banegas 2017), and between measurements obtained from aneroid (inflatable cuff) and electronic sphygmomanometers (Shahbabu 2016).
Potential biases in the review process
The review may be affected by non‐reporting bias. For the unknowns that we are aware of ('known unknowns'), i.e. particular results from a trial not reported in a usable format, we contacted trial authors to request the data in a usable format. In cases where we did not obtain these data and they were consequently excluded, this is a limitation, as we cannot be certain how the inclusion of these data would have affected the pooled estimates. Despite this, we do observe agreements in the pooled mean differences in blood pressure changes reported in other similar reviews on this topic; though the estimated reductions are typically more conservative in our review. As a result, we think it is unlikely that these missing data would have meaningfully changed our pooled estimates. A number of studies (Li 2014; Li 2016; Mu 2009; Zhou 2013) reported allocating entire villages or households to LSSS or regular salt without explicit exclusion of children, though only one study reported separate data for the effect of the intervention in children. It is possible that data from family members aged 18 years or younger who were included as part of a randomised village or household may have changed our pooled estimates for children, though we anticipate that these data would be from a small subset of participants included in these trials. We are unsure whether these missing data would have meaningfully changed our pooled estimates of effect in children.
It is more difficult for us to judge the effect of 'unknown unknowns', i.e. entire eligible studies not detected by our comprehensive search strategy. We acknowledge the possibility that small, unpublished studies may not have been identified and included in the review as the interpretation of funnel plot asymmetry could not definitively rule this out. This was due to similar effect sizes despite varying inter‐trial standard errors for DBP and similar effect sizes as well as standard errors between trials for SBP.
We did not exclude any studies based on the duration of intervention, the formulation of LSSS, or participant characteristics. We did, however, exclude studies with multifactorial designs where the effect of LSSS could not be isolated, though it may have been relevant to the review question. The reason for excluding studies with such multi‐component interventions was that any observed changes in outcomes of interest could not be attributed to LSSS alone.
Agreements and disagreements with other studies or reviews
Pooled mean differences in blood pressure in our review are in line with previous systematic reviews on the effects of LSSS use in adults. These recent systematic reviews reported reductions in DBP and SBP ranging from 2.00 to 4.04 mmHg and 7.81 to 8.87 mmHg, respectively (Hernandez 2019; Jafarnejad 2020; Jin 2020).
However, it is important to take note of differences between these reviews and our review. One of the reviews included only studies conducted in Chinese study participants (Jin 2020), while another restricted studies to participants with stage 2 hypertension (Jafarnejad 2020). Both of these can be considered limited in their ability to generalise to guidelines for the general population: the review by Jin 2020, through its restriction to an ethno‐geographic group with, according to a recent publication from the China Hypertension Survey, a high prevalence of hypertension (Wang 2018); the review by Jafarnejad 2020, for including only studies in participants with progressive disease (Giles 2009). The inclusion criteria for these reviews consequently resulted in enriched populations in respect of hypertension, which may have influenced treatment effect through an increased number of participants with resistant hypertension (Yaxley 2015). Jafarnejad 2020 reported stratified analyses by several effect modifiers, demonstrating that LSSS use resulted in reductions in SBP and DBP in hypertensive adults of all ages, though reductions in SBP and DBP were slightly more pronounced in hypertensive adults younger than 65 years. Though these results were numerically very similar in the subgroup analyses conducted as part of our review, they were not fully reflected since we found greater reductions in SBP and smaller reductions in DBP in younger participants compared to older participants in the general population. As discussed previously, these differences may be due to differences in the included populations. A large review investigating the effect of antihypertensive medication (therefore including participants with overt hypertension) reported larger reductions in DBP in younger participants when compared to older and very old participants (Guang Wang 2005).
Only one review evaluated the certainty of evidence and presented low‐certainty evidence for reductions of 3.96 mmHg and 7.81 mmHg, in DBP and SBP respectively, at any length of follow‐up (Hernandez 2019). Low‐certainty evidence was also presented for the effect on triglycerides. For other outcomes, Hernandez 2019 concluded, on the basis of moderate‐certainty evidence, that LSSS use probably has little or no effect on mortality, while its effects on detected hypertension, total blood cholesterol, glucose, as well as urinary sodium and potassium excretion were very uncertain. The pooled effect estimates of these outcomes were similar between the review by Hernandez 2019 and our review, despite slight differences in included studies and the exact definitions of outcomes.
Authors' conclusions
Implications for practice.
In an adult population, the small pooled mean differences in DBP and SBP favoured LSSS when compared to regular salt. Though these were statistically significant, they were not considered to be clinically important at the individual level (moderate‐certainty evidence). However, small mean reductions from a population‐level intervention can be more beneficial than larger reductions in at‐risk patients (Verbeek 2021). When taking this perspective and considering the general population for which the WHO NUGAG Subgroup on Diet and Health is formulating the guidance, we considered the observed reductions in DBP and SBP when comparing the use of LSSS to regular salt to be small but important from a population perspective. While maximum follow‐up for these outcomes was at 60 months, the majority of trials reporting on blood pressure outcomes followed participants for six months or less.
We observed a slight reduction in the risk ratio for non‐fatal stroke, non‐fatal ACS and cardiovascular mortality, favouring LSSS when compared to regular salt, in our review. Maximal follow‐up for these outcomes was at 60 months. The reduction in non‐fatal stroke and cardiovascular mortality was not considered to be clinically important at the individual level (both moderate‐certainty evidence). The reduction in non‐fatal ACS was considered to be clinically important at the individual level (moderate‐certainty evidence), though variation around the point estimate showed that both clinically important and unimportant benefits are possible. However, these benefits were all considered to be small but important from a population perspective.
Importantly, many of the trials included in our review restricted participants to those with elevated blood pressure or hypertension at enrolment. Therefore, the use of this evidence to inform population‐based public health guidance requires careful consideration as offered by evidence‐informed approaches to guideline development (e.g. the GRADE approach (Zhang 2019)). Our systematic review failed to show that LSSS meaningfully reduces prevalent hypertension when compared to regular salt, with little or no difference in this outcome between the two groups at maximal follow‐up of 18 months; though a large stepped‐wedge cluster trial showed a more pronounced effect on incident hypertension over 30 months. Evidence on blood pressure control, various cardiovascular events, and stroke mortality was limited and we could not draw any conclusions about these outcomes.
Furthermore, our systematic review found no meaningful increase in hyperkalaemia with LSSS when compared to regular salt, with little or no difference in effect for this important safety outcome at maximal follow‐up of five years. While global estimates of potassium intakes (Global Dietary Database 2022) are currently less than levels conditionally recommended by the WHO (WHO 2012b), thereby potentially delaying the onset of hyperkalaemia events, we consider five years likely to be sufficient follow‐up time to detect an event which usually develops over the course of weeks (Hollander‐Rodriguez 2006). It should be noted, however, that the evidence on hyperkalaemia presented in this review has several limitations. Very few studies reported on this important safety outcome, and studies that did report on hyperkalaemia also used variable criteria to define the condition (see Included studies). Most studies included in our review also had strict inclusion and exclusion criteria with regard to hyperkalaemia risk factors. All included trials specifically excluded participants in whom it is known that an increased intake of potassium could cause harm (e.g. people with chronic kidney disease). Only seven trials included participants judged to be at possible risk of hyperkalaemia, and four trials included participants at unclear risk of hyperkalaemia due mostly to limited reporting of the criteria assessed (Table 6). Though only five trials reported on this outcome, two including participants judged to be at possible risk and one including participants at unclear risk, all information in the meta‐analysis came from participants at possible or unclear risk. Therefore, caution should be taken in directly applying the results of our review to the general population, which would likely consist of some people in whom it is known that an increased intake of potassium could cause harm, as well as proportions of people with risk factors for hyperkalaemia, both diagnosed and undiagnosed.
A small pooled mean difference in blood potassium between LSSS and regular salt indicated a small increase in this outcome for the LSSS group; this was not considered to be clinically important (moderate‐certainty evidence) given its impact on the upper end of a 'normal' blood potassium level would not reach levels indicative of moderate hyperkalaemia. Again, these findings should be interpreted with caution given the limited number of studies reporting on this outcome, and the strict exclusion by all studies of participants in whom it is known that an increased intake of potassium could cause harm. Evidence on hypokalaemia events and various adverse effects was limited and we could not draw any conclusions about these safety outcomes.
Nearly all the trials in our review investigated the effects of LSSS implemented as a discretionary intervention. This restricts the generalisability of our findings to discretionary LSSS implementation, and we are unable to draw firm conclusions about non‐discretionary LSSS implementations (for example, where LSSS is added to manufactured foods). Furthermore, the contribution of discretionary salt to total sodium intake varies considerably across countries and settings. This variation is an important consideration when decisions are made about the implementation of LSSS since the absolute intakes of LSSS may vary across settings, thereby creating the possibility of variable impacts on both effectiveness and safety.
It should also be noted that the majority of trials included in our review (24/27) investigated the effects of potassium‐containing LSSS, therefore, we are unable to draw firm conclusions about the effects and safety of LSSS that do not displace sodium with potassium. Variable impacts on effectiveness and safety might also be expected as a result of the potassium content of the LSSS used. In our review, potassium content in potassium‐containing products ranged from 10.1% to 50%. The selection of potassium‐containing LSSS based on potassium content is another important implementation consideration.
There was sparse evidence for the effect and safety of LSSS in children, with no studies assessing the discretionary use of LSSS in this population group. Given the limited evidence, we could not draw any conclusions about the effect of LSSS on DBP and SBP in children. We could not draw any conclusions about the safety of the use of LSSS in children given that no studies reported on safety outcomes. Given that no studies were found in pregnant women, we also could not draw any conclusions about the effect and safety of LSSS in pregnant women.
Importantly, multi‐component and multi‐sector strategies are usually used to reduce sodium intake, blood pressure and cardiovascular disease risk in populations, and the use of LSSS to reduce sodium intake would be regarded as one of the approaches within these overall strategies.
Implications for research.
Given the sparse evidence available for the safety and effect of LSSS in children, and the lack of evidence in pregnant women, studies assessing the benefits and potential harms of LSSS in these groups are needed as a priority. In addition, the lack of information on hyponatraemia events in our review indicates that studies assessing this outcome in the context of LSSS use are needed, particularly since older people and those using certain classes of medication used to treat hypertension are disproportionately at risk.
Given the limitations of the evidence relating to hyperkalaemia, robust studies with explicitly defined measures of this outcome are needed to better understand the safety implications of widespread LSSS use (discretionary and non‐discretionary). In addition, highly monitored trials including participants who may be at risk of hyperkalaemia, or evidence from prospective cohort studies including participants that are representative of the general population, would provide evidence that is more generalisable to widespread population‐level LSSS implementation. Similarly, robust studies assessing a participant population that is representative of the general population in terms of blood pressure status are required to better understand the effectiveness and safety of LSSS in people with normal blood pressure.
Important evidence is still needed to answer the question of whether LSSS use sustainably decreases overall sodium intake, or whether it results in dietary compensation through behavioural modifications; such as, for example, an increased intake of products that are sources of non‐discretionary salt. Studies using reliable measures of dietary sodium and potassium intake, such as 24‐h urinary excretion are needed to assess the extent to which LSSS use reduces sodium intake, and increases potassium intake (when potassium‐containing LSSS are used) over the longer term. Other measures of dietary intake, such as dietary recalls, food frequency questionnaires and spot urine tests, have recently been shown to exhibit poor accuracy in individuals at high cardiovascular risk (Tsirimiagkou 2022). Evidence on the use of LSSS in manufactured and processed foods, particularly sauces and condiments, which represent a large proportion of dietary sodium intake in some regions of the world is also required to understand the extent to which population‐level exclusive replacement of discretionary salt would change global sodium intakes. Finally, studies assessing sodium intakes in populations and quantifying the level and extent of iodisation in LSSS are needed to ensure proactive recalibration of iodisation levels in salt in the context of increased LSSS use. Robust studies examining the effectiveness of multi‐component, multi‐sectoral strategies that include LSSS could further inform decision‐making to reduce sodium intake and cardiovascular disease risk.
The quality and utility of future research on these questions would be optimised by prospective registration of studies, as well as publication of protocols and detailed data analysis plans of future studies. The use of appropriate reporting guidelines (e.g. CONSORT) would also support in the production of high‐quality evidence of maximum utility.
Robust evidence linked to the resource implications of LSSS use is needed to better inform considerations related to population‐level implementation.
Acknowledgements
The World Health Organization (WHO) provided funding to Stellenbosch University towards the cost of carrying out this systematic review. AB, MV, AS and CN are partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
This Cochrane Review is associated with the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
We thank the following people:
Vittoria Lutje, Information Specialist for Cochrane Infectious Diseases Group, for the Embase searches.
Robin Featherstone, Information Specialist for Cochrane, for guidance in terms of the search strategy.
Ms Nina Robertson, for assistance with screening and data extraction.
Ms Yuan Chi, Information Specialist for Cochrane Campbell Global Ageing Partnership for Chinese translation, screening and data extraction of papers in Chinese.
Dr Marty Chaplin, Senior Research Associate, Liverpool School of Tropical Medicine, UK and Statistical Editor, Cochrane Infectious Diseases for guidance related to the stepped‐wedge cluster‐randomised controlled trial.
Prof Alfred Musekiwa, Associate Professor in Biostatistics, University of Pretoria, South Africa for providing guidance on meta‐analytic approach for rate ratios and conversion of incidence rates to rate ratios.
Prof Razeen Davids, Head of the Division of Nephrology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, South Africa, for providing guidance on criteria for the assessment of hyperkalaemia risk and the time point ranges relevant to the review outcomes.
Members of the WHO Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health for inputs on this review in line with the WHO guideline development process.
Cochrane Public Health supported the authors in the development of this review. The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Julia L Finkelstein, Associate Professor, Division of Nutritional Sciences, and Deputy Director, Affiliate Cochrane Center for Nutrition, Cornell University
Managing Editors (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Colleen Ovelman and Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Anne Lethaby, c/o Cochrane Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Rachael McLean, Department of Preventive and Social Medicine, Dunedin School of Medicine, University of Otago, New Zealand (clinical review); Jessica Rigutto‐Farebrother, Human Nutrition Laboratory, Institute of Food, Nutrition and Health, ETH Zürich, Switzerland (clinical review); Robert Walton, Cochrane UK (summary versions review); Rachel Richardson, Cochrane Evidence Production & Methods Directorate (methods review); Jo Platt, Cochrane GNOC (search review). One additional peer reviewer provided clinical peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Appendix 1. Search strategies
MEDLINE (PubMed)
Searched: 1946 to 18 August 2021
#1 ((((((((randomized controlled trial [pt]) OR controlled clinical trial [pt]) OR randomized [tiab]) OR placebo [tiab]) OR clinical trials as topic [mesh: noexp]) OR randomly [tiab]) OR trial [ti])) NOT ((animals [mh] NOT humans [mh])) #2 "cohort study"[Title/Abstract] OR epidemiologic*[Title/Abstract] OR longitudinal[Title/Abstract] OR "Follow‐up study"[Title/Abstract] OR "Follow up study"[Title/Abstract] OR prospective[Title/Abstract] OR "Observational study"[Title/Abstract] NOT ((animals [mh] NOT humans [mh])) #3 "adverse effects"[MeSH Subheading] OR "complications"[MeSH Subheading] OR "deficiency"[MeSH Subheading] OR "safe"[Title/Abstract] OR "safety"[Title/Abstract] OR "side effect"[Title/Abstract] OR "side effects"[Title/Abstract] OR "undesirable effect"[Title/Abstract] OR "undesirable effects"[Title/Abstract] OR "treatment emergent"[Title/Abstract] OR "tolerability"[Title/Abstract] OR "toxicity"[Title/Abstract] OR "ADRS"[Title/Abstract] OR ("adverse"[Title/Abstract] AND ("effect"[Title/Abstract] OR "effects"[Title/Abstract] OR "reaction"[Title/Abstract] OR "reactions"[Title/Abstract] OR "event"[Title/Abstract] OR "events"[Title/Abstract] OR "outcome"[Title/Abstract] OR "outcomes"[Title/Abstract])) #4 #1 OR #2 OR #3 #5 "salt substitute"[Title/Abstract] OR "salt substitutes"[Title/Abstract] OR "salt substitution"[Title/Abstract] OR "sodium substitute"[Title/Abstract] OR "sodium substitutes"[Title/Abstract] OR "sodium substitution"[Title/Abstract] OR "substituting sodium"[Title/Abstract] OR "sodium chloride substitute"[Title/Abstract] OR "sodium chloride substitutes"[Title/Abstract] OR "sodium chloride substitution"[Title/Abstract] OR "salt alternative"[Title/Abstract] OR "salt alternatives"[Title/Abstract] OR "sodium alternative"[Title/Abstract] OR "low sodium salt"[Title/Abstract] OR "mineral salt"[Title/Abstract] OR "KCl salt"[Title/Abstract] OR "potassium chloride salt"[Title/Abstract] OR "potassium enriched salt"[Title/Abstract] OR "Potassium lactate"[Title/Abstract] OR "magnesium‐enriched salt"[Title/Abstract] OR "magnesium‐enriched salt"[Title/Abstract] OR "sodium replacement"[Title/Abstract] OR "salt replacement"[Title/Abstract] OR "salt replacer"[Title/Abstract] OR "salt replacers"[Title/Abstract] OR "sodium chloride replacement"[Title/Abstract] OR "sodium chloride replacer"[Title/Abstract] #6 "Diet, Sodium‐Restricted"[Mesh] #7 #5 OR #6 #8 #4 AND #7
Embase (Ovid)
Searched: 1947 to 18 August 2021
1 ((salt or sodium) adj1 (substitut* or alternative or replace*)).tw. 2 sodium chloride substitut*.tw. 3 sodium chloride alternative.tw. 4 low* sodium salt.tw. 5 ("mineral salt" or "KCl salt").tw. 6 ("potassium chloride salt" or "potassium enriched salt" or "Potassium lactate").tw. 7 (substitut* or alternative).tw. 8 *sodium chloride/ 9 7 and 8 10 *magnesium salt/ 11 (magnesium chloride salt or magnesium enriched salt).tw. 12 reduced sodium salt.tw. 13 *sodium restriction/ 14 1 or 2 or 3 or 4 or 5 or 6 or 9 or 10 or 11 or 12 or 13 15 (random* or factorial* or crossover* or cross over or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).tw. 16 crossover procedure/ or double blind procedure/ 17 randomized controlled trial/ or single blind procedure/ 18 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset*).ti. and animal experiment/ 19 Animal experiment/ not (human experiment/ or human/) 20 18 or 19 21 15 or 16 or 17 22 21 not 20 23 14 and 22 24 (ae or to).fs. 25 (safe or safety or side effect* or undesirable effect* or treatment emergent or tolerability or toxicity or adrs or (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. 26 24 or 25 27 26 not 20 28 14 and 27 29 23 or 28 30 limit 29 to (conference abstract or conference paper or "conference review") 31 29 not 30 32 limit 30 to yr="2019 ‐Current" 33 31 or 32
CENTRAL, Cochrane Library
Searched: Issue 8 of 12, 2021
#1 MeSH descriptor: [Diet, Sodium‐Restricted] explode all trees #2 ("salt substitute" OR "salt substitutes" OR "salt substitution" OR "sodium substitute" OR "sodium substitutes" OR "sodium substitution" OR "substituting sodium" OR "sodium chloride substitute" OR "sodium chloride substitutes" OR "sodium chloride substitution" OR "salt alternative" OR "salt alternatives" OR "sodium alternative" OR "low sodium salt" OR "mineral salt" OR "KCl salt" OR "potassium chloride salt" OR "potassium enriched salt" OR "Potassium lactate" OR "magnesium‐enriched salt" OR "magnesium enriched salt" OR "sodium replacement" OR "salt replacement" OR "salt replacer" OR "salt replacers" OR "sodium chloride replacement" OR "sodium chloride replacer"):ti,ab,kw #3 #1 OR #2 in Trials
Web of Science Core Collection with Indexes SCI‐Expanded, SSCI, CPCI‐S (Clarivate Analytics)
Searched: 1970 to 18 August 2021
#1 TI=("salt substitute" OR "salt substitutes" OR "salt substitution" OR "sodium substitute" OR "sodium substitutes" OR "sodium substitution" OR "substituting sodium" OR "sodium chloride substitute" OR "sodium chloride substitutes" OR "sodium chloride substitution" OR "salt alternative" OR "salt alternatives" OR "sodium alternative" OR "low sodium salt" OR "mineral salt" OR "KCl salt" OR "potassium chloride salt" OR "potassium enriched salt" OR "Potassium lactate" OR "magnesium‐enriched salt" OR "magnesium‐enriched salt" OR "sodium replacement" OR "salt replacement" OR "salt replacer" OR "salt replacers" OR "sodium chloride replacement" OR "sodium chloride replacer") #2 AB=("salt substitute" OR "salt substitutes" OR "salt substitution" OR "sodium substitute" OR "sodium substitutes" OR "sodium substitution" OR "substituting sodium" OR "sodium chloride substitute" OR "sodium chloride substitutes" OR "sodium chloride substitution" OR "salt alternative" OR "salt alternatives" OR "sodium alternative" OR "low sodium salt" OR "mineral salt" OR "KCl salt" OR "potassium chloride salt" OR "potassium enriched salt" OR "Potassium lactate" OR "magnesium‐enriched salt" OR "magnesium‐enriched salt" OR "sodium replacement" OR "salt replacement" OR "salt replacer" OR "salt replacers" OR "sodium chloride replacement" OR "sodium chloride replacer") #3 #1 OR #2
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost)
Searched: 1937 to 18 August 2021
S1 MW diet, sodium restricted S2 TI "salt substitute" OR "salt substitutes" OR "salt substitution" OR "sodium substitute" OR "sodium substitutes" OR "sodium substitution" OR "substituting sodium" OR "sodium chloride substitute" OR "sodium chloride substitutes" OR "sodium chloride substitution" OR "salt alternative" OR "salt alternatives" OR "sodium alternative" OR "low sodium salt" OR "mineral salt" OR "KCl salt" OR "potassium chloride salt" OR "potassium enriched salt" OR "Potassium lactate" OR "magnesium‐enriched salt" OR "magnesium‐enriched salt" OR "sodium replacement" OR "salt replacement" OR "salt replacer" OR "salt replacers" OR "sodium chloride replacement" OR "sodium chloride replacer" S3 AB "salt substitute" OR "salt substitutes" OR "salt substitution" OR "sodium substitute" OR "sodium substitutes" OR "sodium substitution" OR "substituting sodium" OR "sodium chloride substitute" OR "sodium chloride substitutes" OR "sodium chloride substitution" OR "salt alternative" OR "salt alternatives" OR "sodium alternative" OR "low sodium salt" OR "mineral salt" OR "KCl salt" OR "potassium chloride salt" OR "potassium enriched salt" OR "Potassium lactate" OR "magnesium‐enriched salt" OR "magnesium‐enriched salt" OR "sodium replacement" OR "salt replacement" OR "salt replacer" OR "salt replacers" OR "sodium chloride replacement" OR "sodium chloride replacer" S4 S2 OR S3 S5 S1 OR S4
ClinicalTrials.gov (https://clinicaltrials.gov/)
Searched: 18 August 2021
Studies: All studies Condition or disease: replacement OR replace OR substitute OR substituting OR substitution OR alternative OR reduce OR reduced OR reduction OR lower OR low Other terms: salt OR sodium
WHO International Clinical Trials Registry Platform (ICTRP) (https://trialsearch.who.int/)
Searched: 18 August 2021
salt OR sodium in the title replacement OR replace OR substitute OR substituting OR substitution OR alternative OR reduce OR reduced OR reduction OR lower OR low in the Intervention Recruitment status is ALL
Appendix 2. Appendix 2. Simplified modelling approach to estimate absolute numbers and population impact
The importance of effects of interventions are best understood as absolute numbers rather than relative numbers. Rating certainty of evidence using GRADE in relation to thresholds (other than no effect) with a minimally contextualised approach, requires the use of absolute numbers (Zeng 2021). This required us to estimate the absolute numbers of events prevented or caused for effectiveness outcomes expressed in relative terms. In addition, since changes in blood pressure are surrogate outcomes for cardiovascular health, we were also required to estimate absolute numbers of events prevented or caused by changes in key surrogate outcomes. We used a simplified model for this, making several simplifying assumptions, as detailed below.
Estimating absolute numbers and population impact
To be able to calculate the risk difference, number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) and corresponding number of events prevented or caused, we needed to know the baseline risk of the disease or event that was being measured or estimated. This baseline risk is central to the impact of population‐level interventions, since small changes in a population with a large risk might have considerable impact. As we were investigating the intervention on a global scale, we used baseline risks from the WHO Global Health Estimates 2019 (WHO 2020) and the Global Burden of Disease Study 2016 global incidence (Institute for Health Metrics and Evaluation 2016) to inform our model. While this approach provided information for the global context, this approach did introduce our first simplifying assumption, i.e. that events of interest are homogeneously distributed across the world.
Due to the availability of global baseline risk information and the cardiovascular health focus of this review, the key outcomes to which we applied this approach were: change in diastolic blood pressure (DBP); change in systolic blood pressure (SBP); cardiovascular events: non‐fatal stroke; cardiovascular events: non‐fatal acute coronary syndrome; cardiovascular mortality and stroke mortality. This was only done for adults, since only blood pressure outcomes were reported in children; and age‐specific hazard ratios for blood pressure reductions were not sought for this age group.
1. Approach to estimating population impacts of changes in blood pressure
In order to estimate absolute numbers of events prevented or caused by changes in blood pressure in adults, we first needed to convert changes in blood pressure into corresponding relative risks. This was done by following the approach as described in Verbeek 2021, using age‐specific hazard ratios for stroke and ischaemic heart disease (IHD) in relation to blood pressure reductions (Lewington 2002). Though Lewington 2002 reported hazard ratios for the age categories 40‐49, 50‐59, 60‐69, 70‐79 and 80‐89, we used an average of only the last four categories (i.e. an average of 50‐89); this simple averaging of hazard ratios across age categories was our second simplifying assumption. Averaging hazard ratios for categories starting at 50 years instead of 40 years was necessary as the WHO Global Health Estimates 2019 did not report mortality baseline risk for the 40‐49 years category alone. However, a crude analysis suggested that the contribution of stroke mortality in the 30‐49 cohort was less than 1% of the total events reported for people aged 30‐70+. A third simplifying assumption was the use of stroke hazard ratios rather than IHD hazard ratios from Lewington 2002, as stroke events were a prespecified outcome of this review.
Once corresponding relative risks were calculated, we used the WHO global estimates of stroke mortality, averaged for the 50‐59, 60‐69 and 70+ age categories, as baseline risk to calculate NNTB/NNTH and corresponding estimates of number of stroke deaths prevented or caused by the change in blood pressure. Therefore, our fourth simplifying assumption was that baseline risk of stroke mortality is homogeneously distributed across these age categories.
An example of this approach for the values obtained for change in diastolic blood pressure (DBP) in Comparison 1 (Analysis 1.1) (mean difference (MD) ‐2.43 mmHg, 95% confidence interval (CI) ‐3.50 to ‐1.36) can be seen in Table 12.
8. Worked example of estimated population impact of change in diastolic blood pressure (DBP) in adults.
| Model step | 95% CI (lower) of pooled effect estimate | Pooled effect estimate | 95% CI (upper) of pooled effect estimate |
| Hazard ratio for 10 mmHg reduction in DBP a | 0.46 | 0.46 | 0.46 |
| Observed change from meta‐analysis b | (‐)3.50 | (‐)2.43 | (‐)1.36 |
| Relative risk for the observed reduction c | 0.762 | 0.828 | 0.900 |
| Mortality baseline risk for stroke per 1000 d | 3.51 | 3.51 | 3.51 |
| Risk difference per 1000 e | ‐0.835 | ‐0.604 | ‐0.352 |
| Number needed to treat for an additional beneficial outcome f | 1198 | 1657 | 2843 |
| Number of stroke deaths prevented per 100,000 people aged 50+ years g | 83 | 60 | 35 |
a Source: average of hazard ratios for a 10 mmHg reduction in DBP in four age categories (50‐59, 60‐69, 70‐79, 80‐89) from Lewington 2002 b Source: effect estimate (mean difference and 95% CI limits) obtained in current review c Source: calculated from the first two values using the approach by Verbeek 2021 d Source: WHO Global Health Estimates 2019 (WHO 2020); average of stroke mortality in three age categories (50‐59, 60‐69, 70+) e Source: calculated using the baseline risk and relative risk for the observed reduction using the approach by Verbeek 2021 f Source: calculated from the risk difference g Source: calculated from the number needed to treat for an additional beneficial outcome
Abbreviations: CI: confidence interval DBP: diastolic blood pressure
2. Approach to estimating population impacts of changes in cardiovascular events
We used a similar approach to estimating absolute numbers for relative effects, though we did not need to convert these measures using hazard ratios as for blood pressure. Our source data for baseline risk of non‐fatal outcomes were composite.
We used global incidence from the Global Burden of Disease Study 2016 (Institute for Health Metrics and Evaluation 2016), providing the total number of global stroke and acute coronary syndrome (called ischaemic heart disease in this study) events in 2016. To calculate the number of non‐fatal events, we subtracted the number of corresponding cause‐specific mortality outcomes from the WHO Global Health Estimates 2015 (WHO 2020), and divided by the total population at risk in 2015 (e.g. [total number of strokes in 2016 (GBD 2016) – total number of stroke mortality outcomes in 2015 (WHO 2015)]/[total population in 2015 (WHO 2015)]). The 2015 WHO estimates were used as they map closest to GBD 2016 estimates, representing our fifth simplifying assumption: i.e., that these two datasets are sufficiently comparable to use in this way. In addition, our sixth simplifying assumption was that the numbers estimated for 2015/2016 are still applicable five years later.
An example of this approach for the values obtained for non‐fatal stroke in Comparison 1 (Analysis 1.33) (risk ratio (RR) 0.90 mmHg, 95% CI 0.80 to 1.01) can be seen in Table 13.
9. Worked example of estimated population impact of non‐fatal stroke in adults.
| Model step | 95% CI (lower) of pooled effect estimate | Pooled effect estimate | 95% CI (upper) of pooled effect estimate |
| Observed change from meta‐analysis a | 0.80 | 0.90 | 1.01 |
| Event baseline risk for non‐fatal stroke per 1000 b | 1.06 | 1.06 | 1.06 |
| Risk difference per 1000 c | ‐0.212 | ‐0.106 | 0.011 |
| Number needed to treat for an additional beneficial/harmful outcome (NNTB/NNTH) d | 4717 (NNTB) | 9434 (NNTB) | 94340 (NNTH) |
| Number of non‐fatal strokes prevented or caused per 100,000 people e | 21 prevented | 10 prevented | 1 caused |
a Source: effect estimate (rate ratio and 95% CI limits) obtained in current review b Source: composite baseline risk calculated from Global Burden of Disease Study 2016 estimate (Institute for Health Metrics and Evaluation 2016) and WHO Global Health Estimates 2015 (WHO 2020) c Source: calculated using the baseline risk and relative risk for the observed reduction using the approach by Verbeek 2021 d Source: calculated from the risk difference e Source: calculated from the number needed to treat for an additional beneficial/harmful outcome
Abbreviations: CI: confidence interval NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome
3. Approach to estimating population impacts of changes in cardiovascular mortality
We used a similar approach to estimating absolute numbers for mortality outcomes to the approach detailed for non‐fatal events. The difference was the use of WHO Global Health Estimates 2019 only, as this dataset provided us with cause‐specific mortality data as well as population numbers. Therefore, we simply divided the number of events for a cause‐specific mortality outcome by the total population to estimate a baseline risk.
For example, the dataset reported 17,863,827 cardiovascular mortality outcomes in the world in 2019 and a total global population of 7.708 billion; corresponding to a 17,863,827/7,708,260,547 = 2.32 baseline risk in 2019.
Data and analyses
Comparison 1. Low‐sodium salt substitutes versus regular salt or no active intervention in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in DBP (mmHg) | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.2 Change in DBP (mmHg); subgroup study duration | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.2.1 ≤ 3 months | 8 | 1631 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐3.77, ‐1.31] |
| 1.2.2 > 3 to 12 months | 6 | 995 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐4.79, 0.03] |
| 1.2.3 > 12 months | 5 | 18204 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.24, ‐0.22] |
| 1.3 Change in DBP (mmHg); subgroup age | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.3.1 ≥ 65 years | 3 | 14862 | Mean Difference (IV, Random, 95% CI) | ‐3.16 [‐5.67, ‐0.64] |
| 1.3.2 < 65 years | 14 | 2906 | Mean Difference (IV, Random, 95% CI) | ‐2.36 [‐3.44, ‐1.28] |
| 1.3.3 Unknown | 2 | 3062 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐2.05, 0.94] |
| 1.4 Change in DBP (mmHg); subgroup gender | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.4.1 Mixed | 17 | 17768 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐3.85, ‐1.54] |
| 1.4.2 Unknown | 2 | 3062 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐2.05, 0.94] |
| 1.5 Change in DBP (mmHg); subgroup ethnicity | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.5.1 Asian | 10 | 20115 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐2.64, ‐0.80] |
| 1.5.2 Conducted in Europe (ethnicity unspecified) | 8 | 693 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐4.76, ‐1.79] |
| 1.5.3 Mixed | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐11.87, 2.07] |
| 1.6 Change in DBP (mmHg); subgroup BMI | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.6.1 Normal (18.5 to 24.9 kg/m2 for adult Europids, 18.5 to 22.9 kg/m2 for adult Asians) | 1 | 185 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐7.59, 0.59] |
| 1.6.2 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 9 | 15814 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐4.07, ‐1.09] |
| 1.6.3 Obese (≥ 30 kg/m2 for adult Europids, ≥ 25 kg/m2 for adult Asians) | 3 | 964 | Mean Difference (IV, Random, 95% CI) | ‐2.75 [‐6.60, 1.10] |
| 1.6.4 Unknown | 6 | 3867 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐3.41, ‐0.35] |
| 1.7 Change in DBP (mmHg); subgroup blood pressure status | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐3.40, ‐1.39] |
| 1.7.1 Participants with hypertension | 12 | 1847 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐4.22, ‐1.37] |
| 1.7.2 Participants with normal blood pressure | 4 | 561 | Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐5.93, 0.20] |
| 1.7.3 Unknown | 2 | 3062 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐2.05, 0.94] |
| 1.7.4 Mixed | 3 | 15360 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐4.09, ‐0.05] |
| 1.8 Change in DBP (mmHg); subgroup LSSS implementation | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.8.1 Discretionary only | 16 | 20598 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.00, ‐1.23] |
| 1.8.2 Non‐discretionary only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐2.81, 2.57] |
| 1.8.3 Discretionary and non‐discretionary | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐4.56, ‐3.64] |
| 1.9 Change in DBP (mmHg); subgroup type of LSSS | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.9.1 ≥ 30% KCl | 8 | 16279 | Mean Difference (IV, Random, 95% CI) | ‐2.35 [‐3.86, ‐0.84] |
| 1.9.2 < 30% KCl | 8 | 4285 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐3.01, ‐0.43] |
| 1.9.3 Non‐potassium containing LSSS | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐9.15, ‐2.96] |
| 1.9.4 Unknown | 1 | 185 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐7.59, 0.59] |
| 1.10 Change in DBP (mmHg); subgroup baseline sodium excretion (mmol/24‐h) | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.10.1 High baseline 24‐h sodium excretion (≥ 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 3 | 15087 | Mean Difference (IV, Random, 95% CI) | ‐2.41 [‐4.76, ‐0.05] |
| 1.10.2 Low baseline 24‐h sodium excretion (< 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 8 | 847 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.83, ‐1.32] |
| 1.10.3 Unknown/Not 24‐h | 8 | 4896 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.93, ‐0.52] |
| 1.11 Change in DBP (mmHg); subgroup baseline potassium excretion (mmol/24‐h) | 19 | 20830 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.50, ‐1.36] |
| 1.11.1 High baseline 24‐h potassium excretion (≥ 59 mmol K/24‐h or 2.3 g K/24‐h) | 8 | 693 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐4.76, ‐1.79] |
| 1.11.2 Low baseline 24‐h potassium excretion (< 59 mmol K/24‐h or 2.3 g K/24‐h) | 3 | 15241 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐3.72, ‐0.27] |
| 1.11.3 Unknown/Not 24‐h | 8 | 4896 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.93, ‐0.52] |
| 1.12 Change in DBP (mmHg); sensitivity analysis: study quality | 14 | 19530 | Mean Difference (IV, Random, 95% CI) | ‐2.36 [‐3.37, ‐1.34] |
| 1.13 Change in DBP (mmHg); sensitivity analysis: study design | 12 | 1816 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐4.64, ‐2.25] |
| 1.14 Change in DBP (mmHg) stepped‐wedge trial | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.39, ‐0.13] | |
| 1.15 Change in SBP (mmHg) | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.16 Change in SBP (mmHg); subgroup study duration | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.16.1 ≤ 3 months | 8 | 1631 | Mean Difference (IV, Random, 95% CI) | ‐5.92 [‐8.20, ‐3.64] |
| 1.16.2 > 3 to 12 months | 7 | 1580 | Mean Difference (IV, Random, 95% CI) | ‐4.02 [‐6.45, ‐1.59] |
| 1.16.3 > 12 months | 5 | 18203 | Mean Difference (IV, Random, 95% CI) | ‐4.35 [‐6.68, ‐2.01] |
| 1.17 Change in SBP (mmHg); subgroup age | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.17.1 ≥ 65 years | 3 | 14862 | Mean Difference (IV, Random, 95% CI) | ‐4.66 [‐6.15, ‐3.18] |
| 1.17.2 < 65 years | 15 | 3491 | Mean Difference (IV, Random, 95% CI) | ‐5.08 [‐6.83, ‐3.34] |
| 1.17.3 Unknown | 2 | 3061 | Mean Difference (IV, Random, 95% CI) | ‐4.17 [‐10.91, 2.57] |
| 1.18 Change in SBP (mmHg); subgroup gender | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.18.1 Mixed | 18 | 18353 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐6.20, ‐3.60] |
| 1.18.2 Unknown | 2 | 3061 | Mean Difference (IV, Random, 95% CI) | ‐4.17 [‐10.91, 2.57] |
| 1.19 Change in SBP (mmHg); subgroup ethnicity | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.19.1 Asian | 11 | 20699 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐5.46, ‐2.71] |
| 1.19.2 Conducted in Europe (ethnicity unspecified) | 8 | 693 | Mean Difference (IV, Random, 95% CI) | ‐5.75 [‐8.35, ‐3.15] |
| 1.19.3 Mixed | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐9.18 [‐18.47, 0.11] |
| 1.20 Change in SBP (mmHg); subgroup BMI | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.20.1 Normal (18.5 to 24.9 kg/m2 for adult Europids, 18.5 to 22.9 kg/m2 for adult Asians) | 1 | 185 | Mean Difference (IV, Random, 95% CI) | ‐6.60 [‐11.42, ‐1.78] |
| 1.20.2 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 9 | 15814 | Mean Difference (IV, Random, 95% CI) | ‐4.72 [‐5.97, ‐3.48] |
| 1.20.3 Obese (≥ 30 kg/m2 for adult Europids, ≥ 25 kg/m2 for adult Asians) | 4 | 1549 | Mean Difference (IV, Random, 95% CI) | ‐4.41 [‐7.61, ‐1.21] |
| 1.20.4 Unknown | 6 | 3866 | Mean Difference (IV, Random, 95% CI) | ‐5.01 [‐8.39, ‐1.64] |
| 1.21 Change in SBP (mmHg); subgroup blood pressure status | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.73 [‐5.92, ‐3.55] |
| 1.21.1 Participants with hypertension | 12 | 1847 | Mean Difference (IV, Random, 95% CI) | ‐5.33 [‐6.82, ‐3.83] |
| 1.21.2 Participants with normal blood pressure | 4 | 561 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐9.14, 0.14] |
| 1.21.3 Unknown | 2 | 3061 | Mean Difference (IV, Random, 95% CI) | ‐4.17 [‐10.91, 2.57] |
| 1.21.4 Mixed | 4 | 15945 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐5.34, ‐2.26] |
| 1.22 Change in SBP (mmHg); subgroup LSSS implementation | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.22.1 Discretionary only | 17 | 21182 | Mean Difference (IV, Random, 95% CI) | ‐5.02 [‐6.48, ‐3.55] |
| 1.22.2 Non‐discretionary only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐3.85, 5.31] |
| 1.22.3 Discretionary and non‐discretionary | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐5.12 [‐5.85, ‐4.39] |
| 1.23 Change in SBP (mmHg); subgroup type of LSSS | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.23.1 ≥ 30% KCl | 8 | 16279 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐5.96, ‐3.33] |
| 1.23.2 < 30% KCl | 9 | 4869 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐5.18, ‐1.72] |
| 1.23.3 Non‐potassium containing LSSS | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐9.31 [‐14.23, ‐4.40] |
| 1.23.4 Unknown | 1 | 185 | Mean Difference (IV, Random, 95% CI) | ‐6.60 [‐11.42, ‐1.78] |
| 1.24 Change in SBP (mmHg); subgroup baseline sodium excretion (mmol/24‐h) | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.24.1 High baseline 24‐h sodium excretion (≥ 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 3 | 15087 | Mean Difference (IV, Random, 95% CI) | ‐4.02 [‐5.76, ‐2.29] |
| 1.24.2 Low baseline 24‐h sodium excretion (< 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 8 | 847 | Mean Difference (IV, Random, 95% CI) | ‐5.64 [‐7.65, ‐3.63] |
| 1.24.3 Unknown/Not 24‐h | 9 | 5480 | Mean Difference (IV, Random, 95% CI) | ‐4.16 [‐5.94, ‐2.38] |
| 1.25 Change in SBP (mmHg); subgroup baseline potassium excretion (mmol/24‐h) | 20 | 21414 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐6.01, ‐3.50] |
| 1.25.1 High baseline 24‐h potassium excretion (≥ 59 mmol K/24‐h or 2.3 g K/24‐h) | 8 | 693 | Mean Difference (IV, Random, 95% CI) | ‐5.75 [‐8.35, ‐3.15] |
| 1.25.2 Low baseline 24‐h potassium excretion (< 59 mmol K/24‐h or 2.3 g K/24‐h) | 3 | 15241 | Mean Difference (IV, Random, 95% CI) | ‐4.27 [‐5.65, ‐2.88] |
| 1.25.3 Unknown/Not 24‐h | 9 | 5480 | Mean Difference (IV, Random, 95% CI) | ‐4.16 [‐5.94, ‐2.38] |
| 1.26 Change in SBP (mmHg); sensitivity analysis: study quality | 15 | 20114 | Mean Difference (IV, Random, 95% CI) | ‐4.72 [‐6.21, ‐3.24] |
| 1.27 Change in SBP (mmHg); sensitivity analysis: study design | 13 | 2401 | Mean Difference (IV, Random, 95% CI) | ‐6.03 [‐7.22, ‐4.84] |
| 1.28 Change in SBP (mmHg), stepped‐wedge trial | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐2.17, ‐0.41] | |
| 1.29 Hypertension (as reported, or SBP > 140 mmHg or DBP > 85 mmHg) | 1 | 2566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.03] |
| 1.30 Hypertension (as reported, or SBP > 140 mmHg or DBP > 85 mmHg), stepped‐wedge trial | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.45 [0.31, 0.65] | |
| 1.31 Blood pressure control (achieving blood pressure threshold or 'control' as prespecified) | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.32, 3.41] |
| 1.32 Cardiovascular events: various events | 5 | 982 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.49, 3.04] |
| 1.33 Cardiovascular events: non‐fatal stroke | 3 | 21250 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 1.34 Cardiovascular events: non‐fatal stroke; sensitivity analysis: study quality | 2 | 21220 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.48, 2.20] |
| 1.35 Cardiovascular events: non‐fatal stroke; sensitivity analysis: study design | 2 | 255 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.13, 12.11] |
| 1.36 Cardiovascular events: non‐fatal acute coronary syndrome | 1 | 20995 | Rate Ratio (IV, Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 1.37 Cardiovascular mortality | 3 | 23200 | Rate Ratio (IV, Random, 95% CI) | 0.77 [0.60, 1.00] |
| 1.38 Cardiovascular mortality; sensitivity analysis: study quality | 2 | 21423 | Rate Ratio (IV, Random, 95% CI) | 0.87 [0.79, 0.95] |
| 1.39 Stroke mortality | 2 | 21423 | Rate Ratio (IV, Random, 95% CI) | 0.64 [0.33, 1.25] |
| 1.40 Change in blood potassium (mmol/L) | 6 | 784 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.07, 0.18] |
| 1.41 Change in blood potassium (mmol/L); subgroup risk of hyperkalaemia | 6 | 784 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.07, 0.18] |
| 1.41.1 Adults not at risk of hyperkalaemia | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.06, 0.26] |
| 1.41.2 Adults at possible risk of hyperkalaemia | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.01, 0.25] |
| 1.41.3 Adults at unclear risk of hyperkalaemia | 1 | 555 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.02, 0.18] |
| 1.42 Change in blood potassium (mmol/L); sensitivity analysis: study quality | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.06, 0.26] |
| 1.43 Change in blood potassium (mmol/L); sensitivity analysis: study design | 5 | 229 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.07, 0.22] |
| 1.44 Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors) | 5 | 22849 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.46, 2.38] |
| 1.45 Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); subgroup risk of hyperkalaemia | 5 | 22849 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.46, 2.38] |
| 1.45.1 Adults not at risk of hyperkalaemia | 2 | 823 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.45.2 Adults at possible risk of hyperkalaemia | 2 | 21471 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.97] |
| 1.45.3 Adults at unclear risk of hyperkalaemia | 1 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.44, 2.49] |
| 1.46 Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); sensitivity analysis: study quality | 4 | 22294 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.97] |
| 1.47 Hyperkalaemia (> 5.5 mmol/L, or as reported by study authors); sensitivity analysis: study design | 2 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.48 Hypokalaemia (< 3.5 mmol/L, or as reported by study authors) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.49 All‐cause mortality | 5 | 24005 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.83, 0.95] |
| 1.50 Adverse events: other | 8 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.51 Adverse events: other; subgroup risk of hyperkalaemia | 8 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.51.1 Adults not at risk of hyperkalaemia | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.51.2 Adults at possible risk of hyperkalaemia | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.52 Antihypertensive medication use | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.53 Antihypertensive medication use; subgroup study duration | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.53.1 ≤ 3 months | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.06] |
| 1.53.2 > 3 to 12 months | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 1.53.3 > 12 months | 2 | 2895 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.12] |
| 1.54 Antihypertensive medication use; subgroup age | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.54.1 < 65 years | 3 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.93] |
| 1.54.2 Unknown | 1 | 2566 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 1.55 Antihypertensive medication use; subgroup gender | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.55.1 Mixed | 3 | 735 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.93] |
| 1.55.2 Unknown | 1 | 2566 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 1.56 Antihypertensive medication use; subgroup BMI | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.56.1 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.06] |
| 1.56.2 Obese (≥ 30 kg/m2 for adult Europids, ≥ 25 kg/m2 for adult Asians) | 2 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.05] |
| 1.56.3 Unknown | 1 | 2566 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 1.57 Antihypertensive medication use; subgroup blood pressure status | 4 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.57.1 Participants with hypertension | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 1.57.2 Unknown | 1 | 2566 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 1.57.3 Mixed | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.45, 0.80] |
| 1.58 Change in BMI (kg/m2) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.59 Change in serum creatinine (µmol/L) | 3 | 616 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐0.59, 5.71] |
| 1.60 Change in serum creatinine (µmol/L); subgroup risk of hyperkalaemia | 3 | 616 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐0.59, 5.71] |
| 1.60.1 Adults not at risk of hyperkalaemia | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.64 [‐3.90, 7.18] |
| 1.60.2 Adults at unclear risk of hyperkalaemia | 1 | 555 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐0.83, 6.83] |
| 1.61 Microalbuminuria | 2 | 2382 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.53, 0.84] |
| 1.62 Macroalbuminuria | 1 | 1903 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.16, 1.39] |
| 1.63 Change in urinary albumin‐to‐creatinine ratio (uACR) | 1 | 1903 | Mean Difference (IV, Fixed, 95% CI) | ‐1.68 [‐2.87, ‐0.49] |
| 1.64 Change in fasting blood glucose (mmol/L) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.65 Change in blood triglycerides (mmol/L) | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.66 Change in blood triglycerides (mmol/L); subgroup study duration | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.66.1 ≤ 3 months | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.77, 0.08] |
| 1.66.2 > 3 to 12 months | 3 | 357 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.64, 1.39] |
| 1.67 Change in blood triglycerides (mmol/L); subgroup age | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.67.1 ≥ 65 years | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, ‐0.00] |
| 1.67.2 < 65 years | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.18, 1.15] |
| 1.68 Change in blood triglycerides (mmol/L); subgroup ethnicity | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.68.1 Asian | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, ‐0.00] |
| 1.68.2 Conducted in Europe (ethnicity unspecified) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐3.36, 2.54] |
| 1.68.3 Mixed | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.74, 0.66] |
| 1.69 Change in blood triglycerides (mmol/L); subgroup BMI | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.69.1 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 4 | 398 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.26, 0.89] |
| 1.69.2 Obese (≥ 30 kg/m2 for adult Europids, ≥ 25 kg/m2 for adult Asians) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.74, 0.66] |
| 1.70 Change in blood triglycerides (mmol/L); subgroup blood pressure status | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.68, 0.56] |
| 1.70.1 Participants with hypertension | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.76, 0.71] |
| 1.70.2 Participants with normal blood pressure | 3 | 253 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.68, 0.95] |
| 1.71 Change in blood triglycerides (mmol/L); subgroup LSSS implementation | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.71.1 Discretionary only | 3 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.33, 0.57] |
| 1.71.2 Non‐discretionary only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.43, 1.43] |
| 1.71.3 Discretionary and non‐discretionary | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.97, ‐0.03] |
| 1.72 Change in blood triglycerides (mmol/L); subgroup type of LSSS | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.72.1 ≥ 30% KCl | 3 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.33, 0.57] |
| 1.72.2 < 30% KCl | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.19, 1.62] |
| 1.73 Change in blood triglycerides (mmol/L); subgroup baseline sodium excretion (mmol/24‐h) | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.73.1 High baseline 24‐h sodium excretion (≥ 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, ‐0.00] |
| 1.73.2 Low baseline 24‐h sodium excretion (< 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐3.36, 2.54] |
| 1.73.3 Unknown/Not 24‐h | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.74, 0.66] |
| 1.74 Change in blood triglycerides (mmol/L); subgroup baseline potassium excretion (mmol/24‐h) | 5 | 420 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.91, 0.69] |
| 1.74.1 High baseline 24‐h potassium excretion (≥ 59 mmol K/24‐h or 2.3 g K/24‐h) | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐3.36, 2.54] |
| 1.74.2 Low baseline 24‐h potassium excretion (< 59 mmol K/24‐h or 2.3 g K/24‐h) | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.90, ‐0.00] |
| 1.74.3 Unknown/Not 24‐h | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.74, 0.66] |
| 1.75 Change in total blood cholesterol (mmol/L) | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.76 Change in total blood cholesterol (mmol/L); subgroup study duration | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.76.1 ≤ 3 months | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.76, ‐0.05] |
| 1.76.2 > 3 to 12 months | 4 | 446 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.88, 0.32] |
| 1.77 Change in total blood cholesterol (mmol/L); subgroup age | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.77.1 ≥ 65 years | 3 | 378 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.52, 0.52] |
| 1.77.2 < 65 years | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.31, 0.02] |
| 1.78 Change in total blood cholesterol (mmol/L); subgroup ethnicity | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.78.1 Asian | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.58, 0.10] |
| 1.78.2 Conducted in Europe (ethnicity unspecified) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.18, 0.46] |
| 1.78.3 Mixed | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.93, 0.19] |
| 1.79 Change in total blood cholesterol (mmol/L); subgroup BMI | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.79.1 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 5 | 487 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.79, 0.19] |
| 1.79.2 Obese (≥ 30 kg/m2 for adult Europids, ≥ 25 kg/m2 for adult Asians) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.93, 0.19] |
| 1.80 Change in total blood cholesterol (mmol/L); subgroup blood pressure status | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.73, 0.12] |
| 1.80.1 Participants with hypertension | 5 | 274 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.14, 0.34] |
| 1.80.2 Participants with normal blood pressure | 3 | 235 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 1.81 Change in total blood cholesterol (mmol/L); subgroup LSSS implementation | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.81.1 Discretionary only | 3 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.37, 0.17] |
| 1.81.2 Non‐discretionary only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.51, 0.00] |
| 1.81.3 Discretionary and non‐discretionary | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.84, 0.89] |
| 1.82 Change in total blood cholesterol (mmol/L); subgroup type of LSSS | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.82.1 ≥ 30% KCl | 4 | 378 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.05, 0.41] |
| 1.82.2 < 30% KCl | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.52, ‐0.07] |
| 1.83 Change in total blood cholesterol (mmol/L); subgroup baseline sodium excretion (mmol/24‐h) | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.83.1 High baseline 24‐h sodium excretion (≥ 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.58, 0.10] |
| 1.83.2 Low baseline 24‐h sodium excretion (< 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.18, 0.46] |
| 1.83.3 Unknown/Not 24‐h | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.93, 0.19] |
| 1.84 Change in total blood cholesterol (mmol/L); subgroup baseline potassium excretion (mmol/24‐h) | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 1.84.1 High baseline 24‐h potassium excretion (≥ 59 mmol K/24‐h or 2.3 g K/24‐h) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.18, 0.46] |
| 1.84.2 Low baseline 24‐h potassium excretion (< 59 mmol K/24‐h or 2.3 g K/24‐h) | 2 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.58, 0.10] |
| 1.84.3 Unknown/Not 24‐h | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.93, 0.19] |
| 1.85 Change in 24‐h urinary sodium excretion (mmol/24‐h) | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.86 Change in 24‐h urinary sodium excretion (mmol/24‐h) stepped‐wedge trial | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.23, 0.25] | |
| 1.87 Change in 24‐h urinary potassium excretion (mmol/24‐h) | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.88 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup study duration | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.88.1 ≤ 3 months | 4 | 712 | Mean Difference (IV, Random, 95% CI) | 11.18 [4.36, 17.99] |
| 1.88.2 > 3 to 12 months | 5 | 488 | Mean Difference (IV, Random, 95% CI) | 9.47 [0.88, 18.06] |
| 1.88.3 > 12 months | 2 | 2685 | Mean Difference (IV, Random, 95% CI) | 16.03 [‐0.15, 32.21] |
| 1.89 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup age | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.89.1 ≥ 65 years | 4 | 1163 | Mean Difference (IV, Random, 95% CI) | 15.71 [5.88, 25.54] |
| 1.89.2 < 65 years | 6 | 819 | Mean Difference (IV, Random, 95% CI) | 9.22 [2.30, 16.13] |
| 1.89.3 Unknown | 1 | 1903 | Mean Difference (IV, Random, 95% CI) | 8.00 [6.01, 9.99] |
| 1.90 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup gender | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.90.1 Mixed | 10 | 1982 | Mean Difference (IV, Random, 95% CI) | 12.32 [7.28, 17.35] |
| 1.90.2 Unknown | 1 | 1903 | Mean Difference (IV, Random, 95% CI) | 8.00 [6.01, 9.99] |
| 1.91 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup ethnicity | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.91.1 Asian | 5 | 3379 | Mean Difference (IV, Random, 95% CI) | 10.61 [6.37, 14.85] |
| 1.91.2 Conducted in Europe (ethnicity unspecified) | 6 | 506 | Mean Difference (IV, Random, 95% CI) | 13.48 [3.67, 23.28] |
| 1.92 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup BMI | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.92.1 Overweight (25 to 29.9 kg/m2 for adult Europids, 23 to 24.9 kg/m2 for adult Asians) | 8 | 1722 | Mean Difference (IV, Random, 95% CI) | 12.15 [6.33, 17.97] |
| 1.92.2 Unknown | 3 | 2163 | Mean Difference (IV, Random, 95% CI) | 8.82 [6.14, 11.51] |
| 1.93 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup blood pressure status | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 10.99 [7.51, 14.47] |
| 1.93.1 Participants with hypertension | 8 | 965 | Mean Difference (IV, Random, 95% CI) | 11.52 [6.99, 16.04] |
| 1.93.2 Participants with normal blood pressure | 3 | 235 | Mean Difference (IV, Random, 95% CI) | 4.25 [‐5.74, 14.25] |
| 1.93.3 Unknown | 1 | 1903 | Mean Difference (IV, Random, 95% CI) | 8.00 [6.01, 9.99] |
| 1.93.4 Mixed | 1 | 782 | Mean Difference (IV, Random, 95% CI) | 24.52 [18.75, 30.29] |
| 1.94 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup LSSS implementation | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.94.1 Discretionary only | 7 | 3617 | Mean Difference (IV, Random, 95% CI) | 11.10 [7.23, 14.98] |
| 1.94.2 Non‐discretionary only | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐12.49, 5.89] |
| 1.94.3 Discretionary and non‐discretionary | 3 | 178 | Mean Difference (IV, Random, 95% CI) | 18.79 [6.89, 30.68] |
| 1.95 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup type of LSSS | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.95.1 ≥ 30% KCl | 5 | 1546 | Mean Difference (IV, Random, 95% CI) | 13.76 [7.07, 20.45] |
| 1.95.2 < 30% KCl | 6 | 2339 | Mean Difference (IV, Random, 95% CI) | 9.42 [3.55, 15.29] |
| 1.96 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup baseline sodium excretion (mmol/24‐h) | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.96.1 High baseline 24‐h sodium excretion (≥ 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 4 | 1292 | Mean Difference (IV, Random, 95% CI) | 13.48 [5.49, 21.47] |
| 1.96.2 Low baseline 24‐h sodium excretion(< 172 mmol Na/24‐h, 3.95 g Na/24‐h or 9.88 g NaCl/24‐h) | 6 | 690 | Mean Difference (IV, Random, 95% CI) | 11.88 [2.54, 21.21] |
| 1.96.3 Unknown/Not 24‐h | 1 | 1903 | Mean Difference (IV, Random, 95% CI) | 8.00 [6.01, 9.99] |
| 1.97 Change in 24‐h urinary potassium excretion (mmol/24‐h); subgroup baseline potassium excretion (mmol/24‐h) | 11 | 3885 | Mean Difference (IV, Random, 95% CI) | 11.44 [7.62, 15.26] |
| 1.97.1 High baseline 24‐h potassium excretion | 6 | 506 | Mean Difference (IV, Random, 95% CI) | 13.48 [3.67, 23.28] |
| 1.97.2 Low baseline 24‐h potassium excretion | 4 | 1476 | Mean Difference (IV, Random, 95% CI) | 11.69 [5.11, 18.27] |
| 1.97.3 Unknown/Not 24‐h | 1 | 1903 | Mean Difference (IV, Random, 95% CI) | 8.00 [6.01, 9.99] |
| 1.98 Change in 24‐h urinary potassium excretion (mmol/24‐h) stepped‐wedge trial | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.48, 0.78] |
Comparison 2. Low‐sodium salt substitutes versus regular salt or no active intervention in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in DBP (mmHg) at > 3 to 12 months | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐1.56, 4.12] |
| 2.2 Change in SBP (mmHg) at > 3 to 12 months | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐4.41, 4.64] |
| 2.3 Change in BMI (kg/m2) at > 3 to 12 months | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 2.4 Change in 24‐h urinary sodium (mmol/24‐h) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 14.60 [‐11.22, 40.42] |
| 2.5 Change in 24‐h urinary potassium (mmol/24‐h) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐5.13, 13.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allaert 2013.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Cross‐over Country: France Setting: Intervention conducted in households; outcomes measured at 13 centres by community medical practitioners Aim of study: To investigate whether added 'Chitosan' salt (3g/day), when used in conjunction with lifestyle alterations may contribute to a greater reduction in SBP and DBP, compared to standard sea salt. Unit of allocation: individuals Start date: NR End date: NR Relevant study limitations as reported by study authors: Small sample size may not result in optimal assessment of safety (only frequent side effects likely to be observed). Sample size calculation: Yes, "the study was powered to detect a 5 mmHg SBP difference between NaCl salt and chitosan salt at an α of 0.05 (two‐sided) and a β of 0.1." |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: "French‐speaking outpatients of either sex, aged 18 to 85 years, with stage 1 hypertension (SBP 140‐159 mmHg or DBP 90‐99 mmHg) who had never previously received antihypertensive treatment." Exclusion criteria: Participants with allergic reactions to seafood, serious illnesses, or high‐risk cardiovascular profiles were excluded. Pregnant and lactating women, as well as those who were not under effective contraception or had been in menopause for less than two years, were excluded. Pretreatment: none Method of recruitment of participants: Community medical practitioners recruited outpatients Informed consent obtained: yes (written) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: none Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 40 Allocated to LSSS intervention/s: n = 21 (first time period) Allocated to control: n = 19 (first time period) Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): n = 0 Discontinued intervention (LSSS intervention group): n = 1 (second time period) Analysed (LSSS intervention group): n = 21 (first time period); n = 20 (second time period) Excluded from analysis (LSSS intervention group): n = 0 (first time period); n = 0 (second time period) Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 Discontinued intervention (control group): n = 1 (first time period); study authors describe this as "patients's own decision" Analysed (control group): n = 18 (first time period); n = 17 (second time period) Excluded from analysis (control group): n = 1 (second time period) ‐ lack of washout period |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
Integrity of delivery: Added salt intake in g/day (first time period), mean (SD): 3 (1.5) |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: NR Authors name: Francois‐Andre Allaert Institution: University of Burgundy Email: allaert@cenbiotech.com Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article(s) with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study stated it was randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although it was unclear whether the medical practitioners were blinded, the SBP and DBP measurements not likely to be influenced by the lack of blinding (use of automatic BP device) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study used ITT analysis. Missing outcome data were reported in the control group during the first time period 5.3% (1/19). The details of the ITT analysis were not described. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment; low risk of bias for incomplete outcome data |
Allaert 2017.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: France Setting: Intervention conducted at household level; outcomes assessed at a clinical investigation centre Aim of study: To evaluate whether the use of LSSS (Symbiosal) reduces prehypertension more than standard table marine salt (NaCl) Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: Yes, using an estimated reduction of 10 mmHg (SD 10) in SBP in the LSSS group, compared to the control group, at a power of 80% |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Men and women > 18 and < 70 years with systolic pre‐hypertension (SBP > 130 and < 139 mmHg, with or without DBP between 80 and 89 mmHg) on 2 successive visits 7 days apart and on the average daily measurements of participants taken during a 7‐day run‐in period Exclusion criteria: Participants taking antihypertensive medication during the past 12 months, allergic reaction to seafood, or had a high risk cardiovascular profile with at least one of the following comorbidities: heart failure, coronary artery disease, history of stroke or transient ischaemic attack, peripheral arterial disease, kidney failure or kidney disease and diabetes Pretreatment: NR Method of recruitment of participants: NR Informed consent obtained: Yes (written) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: n = 58 Excluded: n = 17 excluded as they did not have a mean SBP of > 130 but < 140mmHg during the run‐in period Randomised: n = 41 Allocated to LSSS intervention/s: n = 22 Allocated to control: n = 19 Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): n = 0 (SBP; DBP); n = 1 (blood potassium): no reason provided Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): n = 22 (SBP; DBP); n = 21 (blood potassium) Excluded from analysis (LSSS intervention group): n = 0 Received allocated control (number): NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 (SBP; DBP); n = 1 (blood potassium): no reason provided Discontinued intervention (control group): n = 0 Analysed (control group): n = 19 (SBP; DBP); n = 18 (blood potassium) Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding source: NR Authors name: Francois‐Andre Allaert Institution: NR Email: allaert@cenbiotech.com Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | Low risk | Study products were pre‐packaged and numbered according to the randomised code and delivered to the clinical investigation centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | LSSS and regular salt were visually comparable and pre‐packaged (not at the investigation centre), therefore both participants and study personnel at the centre were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | SBP, DBP measurements (use of automatic BP device) not likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | SBP and DBP outcomes: no incomplete outcome data for investigator measurements; 9% (2/22) missing data in intervention group and no incomplete data in control group for home BP measurements. Blood potassium reported for 21 out of 22 participants in the intervention group (95.5%), and 18 out of 19 participants (94.7%), respectively. Reasons for missing data not provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Arzilli 1986.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Italy Setting: Intervention conducted in a hospital; outcomes measured in hospital A. Aim of study: To investigate whether a salt high in potassium and low in sodium can reduce blood pressure B. Unit of allocation: adults (aged 18 years or older) C. Start date: NR D. End date: NR E. Relevant study limitations as reported by study authors: NR F. Sample size calculation: NR |
|
| Participants |
Baseline Characteristics Overall
Inclusion criteria: Hospital inpatients with mild hypertension, defined as supine diastolic blood pressure of 95 mmHg after five days in hospital, eating a standard diet containing approximately 20 mmol sodium plus 4 g common salt Exclusion criteria: NR Pretreatment: NR Method of recruitment of participants: NR Informed consent obtained: NR Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 10 Allocated to LSSS intervention/s: n = 5 Allocated to control: n = 5 Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): NR Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): n = 5 Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): NR Discontinued intervention (control group): NR Analysed (control group): n = 5 Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
Integrity of delivery: NR |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Antonio Salvetti Institution: Department of Internal Medicine, University of Pisa Email: a.salvetti@med.unipi.it Possible conflicts of interest (for study authors): NR Sources used for data extraction: Conference abstract about the trial Trial registration details: NR Author correspondence: We contacted Dr. Salvetti 07/07/2020 in an attempt to obtain the full text. Received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study authors reported that the intervention and control salts were administered in 'double‐blind' conditions. Insufficient information available to assess what this meant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were unlikely to be affected by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported in sufficient detail to determine whether any participants were lost. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol or prospective trial registry entry not available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment and incomplete outcome data |
Bernabe‐Ortiz 2014.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised trial Study grouping: Stepped‐wedge Country: Peru Setting: Intervention conducted in semi‐urban villages in Tumbes, northern Peru; outcome measurement conducted at each household Aim of study: To assess the efficacy of a pragmatic salt‐substitution strategy on blood pressure and the incidence of hypertension at population level Unit of allocation: Villages Start date: April 2014 End date: March 2017 Relevant study limitations as reported by study authors: The authors reported absence of dietary assessment of other sources of sodium and potassium, excluding persons with kidney disease and those who were on digoxin, and potential under‐collection of 24‐hour urine at baseline due to lower levels of creatinine at baseline (when compared to follow‐up) as limitations Sample size calculation: Based on an expected difference of 3 mmHg BP between groups (assuming a SD of 20 mmHg of BP within clusters (villages), no. of time periods as 6, a mean cluster size of 300 participants, and an approximation for the ICC of 0.2) using a power of 90% and significance level of 5% |
|
| Participants |
Baseline Characteristics Overall
Inclusion criteria: Villages: mid‐sized villages (350 to 700 people living in 130 to 250 households) in the semi‐urban area of the Tumbes region. Individuals: men and women who are full‐time residents of one of the six randomly selected villages; aged 18 years or older; capable of understanding procedures and providing informed consent Exclusion criteria: Households: any with a family member meeting individual exclusion criteria. Individuals: persons with a history of terminal or severe chronic kidney disease (receiving any form of dialysis); taking digoxin or potassium‐sparing diuretics for heart conditions. Participants with mental illness that impaired their ability of providing consent, were excluded. Pretreatment: The study authors reported that there were baseline differences between the clusters (villages) in terms of age, education, wealth index, BMI, SBP, DBP and the proportion of participants with hypertension. Method of recruitment of participants: Potentially eligible individuals from six villages were identified from the latest census in the area at the time (2010). Engagement with authorities and village leaders was initiated first, through a presentation and explanation of the study at village level. Once the research activities were explained, potential participants were contacted through home visits with the intention of enrolling all members of the family in eligible households. Informed consent obtained: Yes, unclear whether written or oral Clusters: Six clusters (villages) were included in the stepped‐wedge design; comprising n = 536 participants in village A, n = 447 in village B, n = 329 in village C, n = 414 in village D, n = 328 in village E, and n = 322 in village F. The clustering of villages were taken into account in the primary analysis, with a random intercept for cluster used in the modelling of SBP and DBP (standard errors allowed for intra‐class correlation of SBP and DBP). Subgroups planned/measured: The study authors stated the following: "The analysis will compare the information obtained from different subgroups of participants in order to identify and describe the similarities and divergences between men and women, patients from different age groups, and health workers and stakeholders". Subgroups reported: normotensive vs. hypertensive; age < 40 years vs. 40 to 59 years vs. >= 60 years Participant flow Assessed for eligibility: n = 100 clusters (villages); n = 2605 participants from n = 6 randomly selected clusters (villages) Excluded (number with reasons): n = 94 clusters (n = 80 wrong village size; n = 14 randomly excluded); n = 229 participants from n = 6 clusters (reasons NR) Randomised: n = 6 clusters; n = 2376 participants Allocated to LSSS intervention(s): n = 6 clusters; 3605.3 person‐years (n = 536 for 1366.1 person‐years in village A, n = 447 for 883.1 person‐years in village B, n = 329 for 518.3 person‐years in village C, n = 414 for 460.2 person‐years in village D, n = 328 for 256.3 person‐years in village E, and n = 322 for 121.3 person‐years in village F) Allocated to control: n = 6 clusters; 2547.3 person‐years (n = 536 for 1.7 person‐years in village A, n = 447 for 286.9 person‐years in village B, n = 329 for 329.0 person‐years in village C, n = 414 for 542.1 person‐years in village D, n = 328 for 637.0 person‐years in village E, and n = 322 for 750.6 person‐years in village F) Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): Cumulative total: n = 0/6 clusters; n = 950/8987 (10.6%) participants. Step 1: n = 0 clusters; n = 54 in village A. Step 2: n = 76 (n = 0 clusters; n = 46 in village A, and n = 30 in village B). Step 3: n = 0 clusters; n = 115 (n = 56 in village A, n = 51 in village B, and n = 8 in village C). Step 4: n = 0 clusters; n = 116 (n = 44 in village A, n = 34 in village B, n = 27 in village C, and n = 61 in village D). Step 5: n = 0 clusters; n = 224 (n = 57 in village A, n = 43 in village B, n = 27 in village C, n = 68 in village D, and n = 29 in village E). Step 6: n = 0 clusters; n = 315 (n = 77 in village A, n = 62 in village B, n = 29 in village C, n = 84 in village D, n = 28 in village E, and n = 35 in village F). Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): Step 1: n = 1 clusters; n = 482 in village A. Step 2: n = 2 clusters; n = 907 (n = 490 in village A, and n = 417 in village B). Step 3: n = 3 clusters; n = 1177 (n = 480 in village A, n = 396 in village B, and n = 301 in village C). Step 4: n = 4 clusters; n = 1560 (n = 492 in village A, n = 413 in village B, n = 302 in village C, and n = 353 in village D). Step 5: n = 5 clusters; n = 1830 (n = 479 in village A, n = 404 in village B, n = 302 in village C, n = 346 in village D, and n = 299 in village E). Step 6: n = 6 clusters; n = 2011 (n = 459 in village A, n = 385 in village B, n = 300 in village C, n = 330 in village D, n = 300 in village E, and n = 237 in village F) Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): Cumulative total: n = 0/6 clusters; n = 536/5269 (10.2%) participants. Step 1: n = 0 clusters; n = 183 from five villages (B to F). Step 2: n = 0 clusters; n = 146 from four villages (C to F). Step 3: n = 0 clusters; n = 125 from three villages (D to F). Step 4: n = 0 clusters; n = 52 from two villages (E and F). Step 5: n = 0 clusters; n = 30 from one village (F) Discontinued intervention (control group): NR Analysed (control group): Step 1: n = 5 clusters; n = 1657 from five villages (B to F). Step 2: n = 4 clusters; n = 1247 from four villages (C to F). Step 3: n = 3 clusters; n = 939 from three villages (D to F). Step 4: n = 2 clusters; n = 598 from two villages (E and F). Step 5: n = 1 clusters; n = 292 from one village (F) Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study, its authors and the CRONICAS Center of Excellence in Chronic Diseases were supported by the National Heart, Lung, and Blood Institute: the study under the Global Alliance for Chronic Diseases (GACD) programme; the authors and their affiliate centre under the contract Global Health Activities in Developing Countries to Combat Non‐Communicable Chronic Diseases. Investigators were supported by grants from the Wellcome Trust Research Training Fellowship in Public Health and Tropical Medicine and the Dirección de Gestión de la Investigación at the Pontifica Universidad Católica del Perú. Authors name: Antonio Bernabe‐Ortiz; J. Jaime Miranda Institution: CRONICAS Center of Excellence in Chronic Diseases, Universidad Peruana Cayetano Heredia and Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia (both in Lima, Peru) Email: Antonio.Bernabe@upch. pe; jaime.miranda@upch.pe Possible conflicts of interest (for study authors): "The authors declare no competing interests." Sources used for data extraction: Journal article with results of the trial; trial protocol (published; trial registry) Trial registration details: NCT01960972 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated list of random numbers to obtain randomisation sequence of the six villages (clusters) |
| Allocation concealment (selection bias) | Low risk | The allocation of all the villages or clusters was performed by a statistician at the beginning of the study according to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Inhabitants were not blinded to the intervention, but were only informed of the allocation at the moment of implementation in their village (which included a social marketing campaign). The research team were aware of treatment assignment. It was not clear how the lack of blinding may have introduced performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by a separate team of fieldworkers who were blinded to treatment assignment. Primary study outcomes were assessed objectively by standardised techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cumulative loss to follow‐up was n = 950/8987 (10.6%) participants in the intervention and n = 536/5269 (10.2%) participants in the control group. Attrition in the intervention group ranged from 7.7% to 10% at the different time points or steps; with 13.3% at the last time point. For the control group, it ranged between 8% and 10% at the different time points, with 11.7% at the third time point. Reasons for attrition not reported. The study authors stated that a "per‐protocol intention to treat" analysis was conducted. However, no imputation of any data were reported. |
| Selective reporting (reporting bias) | Low risk | The primary outcomes were reported according to those prespecified in the study protocol (SBP, DBP). |
| Other bias | Unclear risk | In some clusters (villages), a reduction in blood pressure was observed before the intervention, and potential explanations for this could be a community‐like white coat effect, which, in the case of rural or semi‐urban areas with limited access to healthcare, can also be present. |
| Recruitment bias (cluster‐RCTs) | Low risk | Recruitment of participants in all selected villages (clusters) conducted before the start of the intervention in the first village (cluster) |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | Results comparable with findings reported by a meta‐analysis of individually randomised RCTs (Peng 2014). "Salt substitutes have been previously tested, mostly in China and mainly on patients with established hypertension, and they show reductions in blood pressure, with a larger effect observed among individuals with hypertension. Similar results have been obtained using home blood pressure measurements." |
| Loss of clusters (cluster‐RCTs) | Low risk | No loss of clusters |
| Baseline imbalance (cluster‐RCTs) | Low risk | Differences were observed between villages for age, education, wealth index, BMI, SBP, DBP and hypertension. Models were adjusted for baseline sex, age, years of education, wealth index and BMI. |
| Incorrect analysis (cluster‐RCTs) | Low risk | The study authors used generalised estimating equations (GEEs) which uses within‐cluster and between‐cluster information to estimate the treatment effect. In addition, they also adjusted the estimates for time (due to the stepped‐wedge design). |
| Overall risk of bias | Unclear risk | Unclear risk of bias for incomplete outcome data; low risk for baseline imbalance, recruitment bias and loss of clusters |
Chang 2006.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: Taiwan Setting: Intervention conducted in a veteran's retirement home in the northern region; setting where mortality outcomes were recorded was the Department of Health Aim of study: To examine the effects of using potassium‐sparing salt on cardiovascular disease mortality and medical expenditure in elderly veterans Unit of allocation: Retirement home kitchens Start date: October 1995 End date: June 1999 Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Kitchens of a veteran's retirement home. Veterans registering into the retired home during the study period, as well as those already resident in the home Exclusion criteria: Kitchen preparing meals for a squad of bedridden residents, among others; residents with abnormal kidney function as assessed by serum creatinine concentrations of ≥ 3.5 mg/dL (>= 309 µmol/L) Pretreatment: None reported Method of recruitment of participants: Potential participants included veterans already resident in the retired home, as well as those who registered into the retired home during the study period. At baseline, resident participants from 10 squads were screened for eligibility before randomisation of the clusters (kitchens). Informed consent obtained: Yes, unclear whether written or oral Clusters: 5 clusters (Intervention group n = 395; n = 373; Control group n = 390; n = 410; n = 413). ICC calculation and outcome: NR Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: n = 6 clusters; n = 1553 participants from n = 5 included clusters Excluded (number with reasons): n = 1 cluster (preparing meals for a squad of bedridden participants, among others); participants excluded through cluster exclusion NR, n = 2 excluded from other clusters (kidney function did not meet serum creatinine < 3.5 mg/dL) Randomised: n = 5 clusters with n = 1551 participants Allocated to LSSS intervention(s): Clusters n = 2; participants n = 768 (n = 635 at baseline; n = 133 during the study period) Allocated to control: Clusters n = 3; participants n = 1213 (n = 916 at baseline; n = 297 during the study period) Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 0 clusters; n = 265 (died n = 189; institutionalised n = 30; moved out n = 46) Discontinued intervention (LSSS intervention group): n = 0 clusters; n = 0 participants Analysed (LSSS intervention group): n = 2 clusters with n = 692 participants Excluded from analysis (LSSS intervention group): n = 0 clusters; n = 16 participants excluded from overall group (n = 1 invalid date of birth, n = 15 dates missing on death certificates; trial arm distribution not reported) Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 clusters; n = 435 (died n = 307; institutionalised n = 50; moved out n = 78) Discontinued intervention (control group): n = 0 clusters; n = 0 participants Analysed (control group): n = 3 clusters with n = 1085 participants Excluded from analysis (control group): n = 0 clusters; n = 16 participants excluded from overall group (n = 1 invalid date of birth, n = 15 dates missing on death certificates; trial arm distribution not reported) |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: Taiwan Salt Work Company; Academia Sinica Authors name: Hsing‐Yi Chang; Wen‐Harn Pan Institution: Institute of Biomedical Sciences, Academia Sinica, Taipei Email: pan@ibms.sinica.edu.tw Possible conflicts of interest (for study authors): "None of the authors had any conflict of interest or any financial relations with the funding agent." Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors reported that only participants were blinded. Thus, there may be a possibility for performance bias for study personnel e.g. cooks, research personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Official coders of the Department of Health used the International Classification of Diseases 9th revision to assign cause of death. Good agreement between these judgements and those of four physicians. Unlikely that the coders would be aware of treatment allocation; the outcomes were also not likely to be influenced by unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 76/768 (9.9%) in the intervention and 128/1213 (10.6%) in the control group. No difference in reasons for dropout |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Unclear risk | There was a considerable risk of contamination in the intervention group: "Other condiments and spices such as soy sauce and monosodium glutamate were not limited, because reasonably priced low‐sodium soy sauce and monosodium glutamate were not available at the time of the trial." These condiments accounted for approx. 30% of total sodium intake in a subsample of participants during the first three months of the study. New participants joined the study as they moved into the home; it was not clear whether these persons were assessed for renal function. |
| Recruitment bias (cluster‐RCTs) | High risk | A significant number of participants were recruited after the study clusters (kitchens) were randomised (LSSS intervention group n = 133; Control group n = 295). |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | "Recently, Cook et al (31) presented the long‐term effects of sodium reduction on CVD based on the data from the Trials of Hypertension Prevention." |
| Loss of clusters (cluster‐RCTs) | Low risk | No loss of clusters reported |
| Baseline imbalance (cluster‐RCTs) | Unclear risk | Baseline characteristics were reported as not significantly different, however, height, weight, BMI, blood pressure, hypertension as well as sodium‐ and potassium‐to‐creatinine ratios were determined in a subsample of participants; 248/768 (32.3%) in the intervention and 391/1213 (32.2%) in the control group. Presence of comorbidities not reported. No information was provided on whether this subsample was randomly selected. |
| Incorrect analysis (cluster‐RCTs) | Low risk | Cluster effects were accounted for in the survival curves. |
| Overall risk of bias | High risk | Low risk of bias for incomplete outcome data and loss of clusters, unclear risk of bias for baseline imbalance, high risk of recruitment bias |
CSSS Collaborative Group 2007.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: China Setting: Intervention conducted in rural households in northern region; outcomes measured in six regional coordinating centres (Heilongjiang, Tianjin, Liaoning, Shanxi and two centres in rural/suburban Beijing) Aim of study: To test the effect of a salt substitute on blood pressure among rural Chinese individuals at high‐risk from cardiovascular disease Unit of allocation: adults (aged 18 years or older) Start date: May 2004 End date: August 2005 Relevant study limitations as reported by study authors: The authors reported that they could not know by how much sodium intake was reduced (since 24‐hour urine collections were not conducted), and not performing 24‐hour BP measurements (may increase the ability to detect smaller BP differences). F. Sample size calculation: The study was designed to provide 90% power at P = 0.05 to detect a 3.1/1.7 mmHg or greater difference between randomised groups in mean casual follow‐up blood pressure levels. |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Adults (>= 18 years), judged by a doctor's diagnosis, to be at high risk of future vascular disease, defined as a history of any of the following: stroke or transient ischaemic attack; hospitalisation for management of any acute coronary syndrome; surgery or angioplasty for peripheral vascular disease; diabetes and >= 55 years, or SBP > 160 mmHg. In addition, participants were required to have an estimated sodium intake of 260 mmol/24hrs or the expectation that at least 50% of daily dietary salt intake could be replaced with the LSSS (estimated via interview of the potential participant and the individual responsible for daily food preparation (if this was not the patient) using a structured questionnaire). Exclusion criteria: Participants were excluded if they were on potassium‐sparing medication, or if there was a history of significant renal impairment. Persons with physician‐determined abnormal blood tests for serum creatinine and potassium before and after run‐in on the salt substitute; any family member in the household of otherwise eligible participants with a contraindication to the intervention or control, as assessed by a physician Pretreatment: Age, anticoagulant use and BMI were reported as showing 'chance baseline imbalance' (no formal hypothesis tests reported). Method of recruitment of participants: All potential participants attended regional co‐ordinating centres. Informed consent obtained: Yes, unclear whether written or oral Clusters: n/a Subgroups planned/measured: Based on baseline characteristics (not specified) Subgroups reported: NR Participant flow Assessed for eligibility: n = 752 Excluded (number with reasons): n = 144 in total (n = 52 withdrew before run‐in period; reasons not recorded); n = 92 during run‐in period (died n = 2; serious event n = 2; reluctant to continue with follow‐up visits n = 57; other n = 31) Randomised: n = 608 Allocated to LSSS intervention(s): n = 306 Allocated to control: n = 302 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 14 (died n = 4; withdrew n = 1; hospitalised n = 3; lost contact n = 6) Discontinued intervention (LSSS intervention group): n = 1 Analysed (LSSS intervention group): n = 292 (blood pressure); n = 287 (urinary measurements) Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 9 (died n = 4; hospitalised n = 3; lost contact n = 2) Discontinued intervention (control group): n = 0 Analysed (control group): n = 293 (blood pressure); n = 290 (urinary measurements) Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study received financial support from The George Institute for International Health (Australia), The Clinical Trials Research Unit (New Zealand), The Capital Medical Science Development Fund (China) and the National Health and Medical Research Council of Australia (grand ID 358395). Study staff received grants from the High Blood Pressure Research Council of Australia, the University of Sydney, the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. China Salt Substitute Study (CSSS) Authors name: Bruce Neal, Wu Yangfeng, on behalf of The China Salt Substitute Study Collaborative Group Institution: The George Institute for International Health, University of Sydney; The George Institute for International Health, China Email: bneal @george.org.au; ywu@george.org.cn Possible conflicts of interest (for study authors): "None of the collaborative group has any conflict to declare in regard to this work." Sources used for data extraction: Journal articles with results of the trial Trial registration details: NCT00145756 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number sequence generation with stratification within each centre by baseline SBP (3 strata) and use of antihypertensive therapy |
| Allocation concealment (selection bias) | Low risk | Central computerised randomisation, accessed by study physicians via the study website (with back‐up phone and fax services) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Centre physicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was low, with 14/306 (4.6%) in the intervention and 9/302 (3.0%) in the control group. Intention‐to‐treat analyses with no imputation were conducted. |
| Selective reporting (reporting bias) | High risk | All primary and secondary outcomes reported according to prespecified outcomes in the study protocol (NCT 00145756). However, the study protocol was registered after completion of the study. A substudy reported on primary outcomes such as central systolic and diastolic blood pressure (CSP; CDP), central pulse pressure (CPP), reflection time (RT), AUG and AIx that were not prespecified in the protocol. |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Geleijnse 1994.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: The Netherlands Setting: Intervention conducted in households in Rotterdam; outcomes measured at a university study centre Aim of study: To decrease blood pressure in older subjects with mild to moderate hypertension by reducing sodium and increasing potassium and magnesium intake Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: Urinary excretion of magnesium did not reflect a higher level of intake in the intervention group when compared to control (even though differences in sodium and potassium were observed). Blood pressure was measured at follow‐up, but no data on urinary electrolytes were collected post‐intervention. The authors acknowledged that an older study population, manipulating three minerals at the same time, as well as the characteristics of the intervention salt (such as double salt structures), may have led to larger blood pressure reductions than what was observed in other studies. Sample size calculation: The study was powered to detect a difference of 5 mmHg systolic blood pressure and 4 mmHg diastolic blood pressure, with 90% power and alpha of 5% using a two‐tailed test of significance. |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Non‐hospitalised men and women who were aged 55 to 75 years; not on antihypertensive medication; and had a systolic blood pressure between 140 and 200 mmHg as well as diastolic blood pressure between 85 and 110 mmHg at two measurements taken a week apart Exclusion criteria: Participants with systolic blood pressure below 130 mmHg or diastolic blood pressure below 70 mmHg; a history of myocardial infarction, angina pectoris, diabetes mellitus or impaired renal function (defined as serum creatinine > 200 µmol/L); or on a salt‐restricted diet on medical advice Pretreatment: None Method of recruitment of participants: Potentially eligible participants were identified as hypertensive from a population‐based cohort study (the Rotterdam study) measuring their blood pressure. They were invited, either by letter or telephone, for remeasurement of their blood pressure for this study. Informed consent obtained: Yes, written Clusters: n/a Subgroups planned/measured: Sex and age Subgroups reported: Sex and age (narratively) Participant flow Assessed for eligibility: n = 419 Excluded (number with reasons): n = 319 (n = 30 could not be contacted; n = 56 showed no interest; n = 69 met one of the exclusion criteria; n = 17 did not fulfil blood pressure criteria; n = 40 started antihypertensive treatment; n = 55 met other exclusion criteria; n = 52 refused the trial) Randomised: n = 100 Allocated to LSSS intervention(s): n = 49 Allocated to control: n = 51 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 1 Discontinued intervention (LSSS intervention group): n = 1 (disliked intervention foods) Analysed (LSSS intervention group): n = 49 Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 2 Discontinued intervention (control group): n = 2 (n = 2 admitted to hospital for unrelated complaints) Analysed (control group): n = 51 Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study was funded by Akzo Nobel (the provider of the intervention salt) and the Rotterdam Medical Research Foundation (ROMERES). Authors name: J.M. Geleijnse Institution: Department of Epidemiology and Biostatistics, Erasmus University Medical School Email: Marianne.Geleijnse@wur.nl Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal articles with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computerised randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Salt and foods looked identical and were provided based on a double‐blind coding system. Outcomes were unlikely to have been influenced by a lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not reported whether outcome assessors were blinded to allocation. Blood pressure was measured by two investigators, which would minimise any potential detection bias. Blood electrolytes and lipids were done using standard laboratory procedures. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was very low, with 1/49 (2%) participants in the intervention and 2/51 (4%) participants in the control group withdrawing. Missing data was considerably higher for blood potassium (4/49 (8%) participants in the intervention and 8/51 (16%) participants in the control group not contributing data) and total cholesterol (3/49 (6%) participants in the intervention and 8/51 (16%) participants in the control group not contributing data). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | High risk | Unclear risk of bias for allocation concealment; high risk of bias for incomplete outcome data |
Gilleran 1996.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: United Kingdom Setting: Intervention conducted in households in Birmingham; outcomes measured at a diabetic outpatient clinic Aim of study: To assess the effect of dietary sodium reduction, and supplementation of potassium and magnesium, on blood pressure and metabolic parameters of hypertensive type 2 diabetics Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: Biochemical parameters, both in serum and urine, did not change to reflect changes in potassium and magnesium intake, and the authors ascribed this to the possibility that changes in dietary intake were not large enough to detect a difference. It was not possible to determine from this trial which of the three mineral interventions (sodium reduction, potassium increase, or magnesium increase) reduced blood pressure. Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Established diabetic patients with hypertension, defined as three consecutive blood pressure readings of > 160 mmHg for systolic and > 95 mmHg for diastolic blood pressure. Participants who were on antihypertensives were included if they had ceased medication at least one month prior to the trial and met hypertensive criteria. Exclusion criteria: Persons with poor or unstable diabetic control; on antihypertensive medication at the start of the study; who are treated with insulin; who had evidence of diabetic or hypertensive nephropathy, defined as persistent proteinuria on Albustix or elevated serum creatinine of 130 µmol/L; who have cardiac failure, are pregnant, or are already on a low‐sodium diet were excluded. Pretreatment: None. Method of recruitment of participants: Recruited from a diabetic clinic at a hospital in Birmingham Informed consent obtained: Yes (written) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 40 Allocated to LSSS intervention(s): n = 20 Allocated to control: n = 20 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 9 (n = 4 due to hypertensive criteria; n = 5 for other reasons) Discontinued intervention (LSSS intervention group): n = 9 (n = 4 due to hypertensive criteria; n = 5 for other reasons) Analysed (LSSS intervention group): n = 11 Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 12 (n = 10 due to hypertensive criteria; n = 2 for other reasons) Discontinued intervention (control group): n = 12 (n = 10 due to hypertensive criteria; n = 2 for other reasons) Analysed (control group): n = 8 Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: P.M. Dodson Institution: Department of Diabetes and Chemical Pathology, Birmingham Heartlands Hospital, Email: paul.dodson@heartofengland.nhs.uk Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article(s) with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were assigned to the intervention and control in a blinded manner. The intervention and control salts were provided in unlabelled packs, and participants found the LSSS and unlabelled salt to be palatable and indistinguishable from their salt intake prior to the intervention. Outcomes were unlikely to be affected by a lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blood pressure was measured by an observer blinded to treatment allocation using standard methods. It was not reported whether triglycerides and cholesterol were assessed blindly, but blood lipids are laboratory‐assessed outcomes unlikely to be influenced by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was 5/20 (25%) in the intervention and 2/20 (10%) in the control group, all for 'other reasons'. These reasons were not explained in sufficient detail to determine whether withdrawal was differential by reason. An additional 4/20 (20%) in the intervention and 10/20 (50%) in the control group were withdrawn due to hypertensive criteria, and did not contribute further data to blood pressure and blood lipid outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | High risk | Unclear risk of bias for allocation concealment; high risk of bias for incomplete outcome data |
Hu 2018.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in rural households in northern region; outcomes measured at community health centres in Beijing Aim of study: "... to examine the home BP response to a salt substitute by age, baseline BP and gender subgroup among 220 households including 220 patients with hypertension and 380 families aged 18 years or older who participated in the China Salt Substitute Study." Unit of allocation: Households/families Start date: September 2005 End date: NR Relevant study limitations as reported by study authors: Use of morning mid‐stream urine sample instead of a 24‐hour urine sample or conduct dietary recalls to obtain more reliable salt consumption data; seasonal BP changes; duration of intervention too short; limited generalisability of study findings to other areas Sample size calculation: Calculated using an expected difference of 5.0 mmHg SBP (based on the data from the China Salt Substitute study), with a SD of 15 mmHg, power of 90% and confidence level of 5% |
|
| Participants |
Baseline Characteristics LSSS intervention (participants with hypertension)
Control (participants with hypertension)
LSSS intervention (family members)
Control (family members)
Inclusion criteria: Households or families with men or women aged 18 years and older, with a SBP > 140 mm Hg or a DBP > 90 mm Hg at two measurements. Men and women with hypertension and their family members aged >= 18 years had to agree to replace at least half of their dietary salt with the LSSS intervention. Exclusion criteria: Households or families with contra‐indication to the use of a LSSS, such as the use of a potassium‐sparing medication or significant renal impairment (serum creatinine > 177 µmmol/L; eGFR for females aged 45 years: 29mL/min; eGFR for males aged 45 years: 39 mL/min). Households were excluded if they relocated during the study period. Pretreatment: None Method of recruitment of participants: Participants were referred from either general‐practitioner referrals or volunteers at two sites in Beijing. They were invited by letter or telephone for a re‐measurement of BP. Informed consent obtained: yes (written) Clusters: n = 220 households (1 participant with hypertension per household (n = 220 in total); mean no. of family members per household NR (n = 380 in total) Subgroups planned/measured: Age, gender and baseline BP Subgroups reported: Participants with hypertension: < 60 years vs. >= 60 years; male vs. female; stage 1 hypertension vs. stage 2 hypertension; family members: < 60 years vs. >= 60 years; male vs. female; participants with normal blood pressure vs. participants with hypertension Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 220 Allocated to LSSS intervention/s: n = 110 households (n = 110 participants with hypertension; n = 191 family members) Allocated to control: n = 110 households (n = 110 participants with hypertension; n = 189 family members) Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): n = 0 participants with hypertension, n = 17 family members Discontinued intervention (LSSS intervention group): n = 2 households (participants with hypertension: n = 2 disliked the salt); n = 5 family members withdrew due to loss of participants with hypertension Analysed (LSSS intervention group): 110 participants with hypertension; 187 family members. The study authors reported that all participants who had complete data and a valid home blood pressure measurement at baseline were included in an ITT analysis (baseline home BP values were used in the case of incomplete follow‐up data). Excluded from analysis (LSSS intervention group): n = 6 family members excluded due to invalid BP measurements Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 5 households (n = 5 participants with hypertension: n = 1 dislike the salt; n = 1 stroke; n = 1 appendicitis; n = 1 nephritis; n = 1 AMI; n = 14 family members: n = 5 withdrawal due to loss of participants with hypertension; n = 1 go to college; n = 2 marriage/divorce; n = 6 NR) Discontinued intervention (control group): n = 1 households (participants with hypertension: n = 1 disliked the salt; n = 5 family members withdrew due to loss of participants with hypertension) Analysed (control group): 110 participants with hypertension; 186 family members. The study authors reported that all participants who had complete data and a valid home blood pressure measurement at baseline were included in an ITT analysis (baseline home BP values were used in the case of incomplete follow‐up data). Excluded from analysis (control group): n = 6 family members excluded due to invalid BP measurements |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: Danone Nutrition Fund, People’s Republic of China; Beijing Salt Industrial Company (LSSS and regular salt) Authors name: Jihong Hu; Yangfeng Wu Institution: Department of Epidemiology, Cardiovascular Institute and Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and Public Health School, Gansu University of Chinese Medicine Email: hujihonghappy@163.com; wuyf@bjmu.edu.cn Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: The study authors cited NCT00145756 (China Salt Substitute Study). Author correspondence: Dr Wu (wuyf@bjmu.edu.cn) was contacted on 27/05/2020 to clarify whether Hu 2018 was part of the China Salt Substitute Study (CSSS). Received a reply from him on 28/05/2020 confirming that Hu 2018 was a different study from CSSS. Dr. Hu (hujihonghappy@163.com) was contacted on 10/07/2020 to request outcome data (mean changes in DBP, SBP). Received data from author on 17/07/2020. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator used |
| Allocation concealment (selection bias) | Low risk | Interventions were packaged and labelled with randomised codes at central allocation. Sequentially numbered envelopes used for allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interventions were packaged and labelled with randomised codes at central allocation. Sequentially numbered envelopes used for allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants (performing home‐based BP monitoring) and investigators (assessing compliance, adverse events) were blinded until the study base was unlocked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete BP outcome data reported among participants with hypertension in both groups: 7.2% (8/110) in LSSS intervention group vs 3.6 % (4/110) in control group. However, there was differential attrition present among family members: 17.2 % (33/191) in LSSS intervention group vs. 9.5% (18/189) in control group. |
| Selective reporting (reporting bias) | Unclear risk | The study authors reported the trial registration number of another trial (China Salt Substitute study; NCT00145756). Therefore, study protocol not available |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Low risk | Individuals recruited before randomisation of clusters |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | A previous RCT has investigated home BP response to a salt substitute, finding a significant reduction in home SBP among hypertensive patients (Hu 2014). |
| Loss of clusters (cluster‐RCTs) | Low risk | Attrition of households was low (7.2% (8/110) in LSSS intervention group vs 4.5 % (5/110) in control group). ITT analysis performed |
| Baseline imbalance (cluster‐RCTs) | Low risk | The study reported no significant differences in gender, age, BMI, home SBP and DBP, hypertension rate and salt consumption of participants with hypertension and family members. There was a difference in the proportion of family member who were hypertensive (31.6% in the intervention group vs. 24.2% in the control group). |
| Incorrect analysis (cluster‐RCTs) | High risk | Adjustment to account for the effect of clustering was not reported in the statistical analysis or results section for family members and combined data with participants who have hypertension. |
| Overall risk of bias | High risk | Low risk of bias for baseline imbalance, recruitment bias and loss of clusters; high risk of bias for incomplete outcome data |
Kawasaki 1998.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Japan Setting: Intervention conducted in households; setting where outcomes were measured not reported Aim of study: To explore the antihypertensive effects of mineral salt by performing a trial of mild dietary sodium reduction with mild potassium and magnesium supplementation in normotensive and hypertensive middle‐aged and elderly subjects Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
LSSS intervention (participants with normal blood pressure)
Control (participants with normal blood pressure)
LSSS intervention (participants with hypertension)
Control (participants with hypertension)
Inclusion criteria: Clinically healthy middle‐aged and elderly volunteers with ordinary lifestyles Exclusion criteria: Participants who planned to travel during study period, or frequently dined away from home, or had a high risk of noncompliance with study protocol Pretreatment: No significant differences between groups at baseline Method of recruitment of participants: Questionnaire Informed consent obtained: Yes (written) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: participants with normal blood pressure; participants with hypertension Participant flow Assessed for eligibility: "More than 50" Excluded (number with reasons): NR Randomised: n = 41 Allocated to LSSS intervention(s): n = 21 Allocated to control: n = 20 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 0 Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): n = 21 Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 Discontinued intervention (control group): n = 0 Analysed (control group): n = 20 Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The LSSS, described as 'mineral' salt (Pansalt), was supplied by Time Associates Co. Ltd, Tokyo, Japan. Authors name: Terukazu Kawasaki Institution: Institute of Health Science, Kyushu University Email: itohK@k7.dion.ne.jp Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article(s) with results of the trial Trial registration details: NR Author correspondence: Dr. Itoh (co‐author) was contacted on 26/06/2020 to obtain relevant outcome data for control group (SBP, DBP). Received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Taste tests showed no differences between LSSS and control seasonings for savouriness or palatability. It was not clear whether control participants received study seasonings, or whether they used their own. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding not reported. Objective study outcomes: blood pressure (using a standardised procedure and the use of an automatic sphygmanometer) and biochemical assays of blood and urine samples |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment; low risk of bias for incomplete outcome data |
Li 2014.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in local residents' family; outcomes measured at their homes Aim of study: To explore the effects of low‐sodium salt on blood pressure and to find out an economical, effective and easy‐to‐implement method to reduce the blood pressure Unit of allocation: households Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: At least 2 members in each family meet the following conditions: (1) Family members ≥ 50 years old. (2) Eating at home every week > 5 d.(3) Can participate in the whole project, without long‐term arrangement Exclusion criteria: Exclude serious kidney diseases (not defined by study authors) and potassium‐preserving diuretic treatment Pretreatment: Baseline gender, age, SBP and DBP were not significantly different between the intervention group and the control group. In addition, other factors such as television, newspapers and other important information sources, as well as some common food sources of salt were similar between the groups. Method of recruitment of participants: Quote: "66 journalists working for a local newspaper were responsible for recommending participating families and family members. Each reporter was responsible for recommending four families." Informed consent obtained: NR Clusters: n = 66 clusters (n = 253 participants in LLLS group, n = 63 participants in control group) Subgroups planned/measured: NR Subgroups reported: male vs. female; age (50‐59 years vs. 60‐69 years vs. > 70 years) Participant flow Assessed for eligibility: 78 journalists Excluded (number with reasons): 12 journalists (declined to participate) Randomised: n = 264 families recommended by 66 journalists (n = 528 participants) Allocated to LSSS intervention(s): n = 258 participants; n = 129 (clusters) Allocated to control: n = 270 participants; n = 135 (clusters) Received allocated LSSS intervention(s): All participants Did not receive allocated LSSS intervention(s): n = 0 Lost to follow‐up (LSSS intervention group): n = 3 (n = 1 hospitalisation; n = 2 ‘went out’) Discontinued intervention (LSSS intervention group): n = 2 Analysed (LSSS intervention group): n = 253 participants from 129 clusters Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: All participants Did not receive allocated control: n = 0 Lost to follow‐up (control group): n = 3 (n = 3 ‘went out’) Discontinued intervention (control group): n = 4 Analysed (control group): n = 263 participants from 133 clusters Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: China International Center for Chronic Disease Prevention Authors name: Xiang‐Xian, Feng Institution: Department of Preventive Medicine, Changzhi Medical College Email: xfeng66@163.com Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NCT03074851 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants not blinded; BP not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors not blinded, but SBP and DBP measurements conducted with an automatic device |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total attrition was minimal. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registry entry available, but not prospectively registered |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Low risk | Journalists recruited all participants before randomisation. |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | Comparability of the effect estimates of RCTs in the same comparison |
| Loss of clusters (cluster‐RCTs) | Low risk | Loss of two clusters in the control arm; however minimal effect on attrition |
| Baseline imbalance (cluster‐RCTs) | Low risk | No baseline imbalances reported between intervention and control participants |
| Incorrect analysis (cluster‐RCTs) | High risk | No adjustment for clustering in data analysis |
| Overall risk of bias | Low risk | Low risk of bias for incomplete outcome data, baseline imbalance, recruitment bias and loss of clusters |
Li 2016.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in townships/villages from five northern provinces Hebei, Liaoning, Ningxia, Shanxi and Shaanxi; outcomes measured at village level Aim of study: To define the effects of a community‐based sodium reduction strategy on the average sodium intake Unit of allocation: Villages Start date: May 2011 End date: November 2012 Relevant study limitations as reported by study authors: Lack of baseline survey data resulted in less statistical power to reliably detect any effect on blood pressure of the observed changes in sodium consumption; did not systematically collect tolerability data Sample size calculation: To detect a difference in mean 24‐hour excretion of sodium of at least 11 mmol/day (0.65g/day salt) between intervention and control clusters with 90% power (with a two‐sided alpha = 0.05), assuming a standard deviation of 24‐hour sodium excretion of 60 mmol/day, an ICC of 0.05, and a sample of 2400 individuals drawn from 120 clusters randomised equally between intervention and control groups |
|
| Participants |
Baseline Characteristics LSSS intervention (with price subsidy)
LSSS intervention (without price subsidy)
LSSS intervention
Control
Inclusion criteria: Villages within each township (total of 120 included townships) from that were closest to the geographic centre of the township. If the village declined to participate, the next most geographically central village was asked to participate. Exclusion criteria: Villages where the township healthcare centre was situated Pretreatment: The study presenting the main results of the trial stated the following: "Baseline survey data were not available because of resource constraints." During the end‐line survey, no group differences were reported in terms of age, gender, education, smoking and drinking history and BMI (Li 2016). Method of recruitment of participants: Two counties from each province were selected on the basis of their interest in and willingness to participate, their proximity to the local research team, and their representativeness of the socioeconomic development level of counties in each province. In each county, 12 townships were selected primarily through discussion with the leadership of the local county Bureau of Health to identify willing and accessible partners. Finally, within each township one village was selected, typically the village closest to the geographic centre of the township. Informed consent obtained: yes (verbal consent from village chiefs; written consent from participants) Clusters: n = 120 villages (n = 60 intervention villages [n = 30 with price subsidy; n = 30 without price subsidy]; n = 60 control villages); average population size of villages: 1867 individuals living in 512 households Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: 10 to 20 villages per township (n = 12) Excluded (number with reasons): NR Randomised: n = 120 villages Allocated to LSSS intervention(s): n = 60 villages [n = 30 villages and n = 1268 participants (LSSS intervention with price subsidy) and n = 30 villages and n = 1253 participants (LSSS intervention without price subsidy)] Allocated to control: n = 60 villages Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 0 Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): SBP, DBP outcomes: n = 650 individuals (LSSS intervention with price subsidy); n = 644 (LSSS intervention without price subsidy); urinary creatinine ratio; microalbuminuria outcomes: n = 528 individuals (LSSS intervention with price subsidy); n = 447 (LSSS intervention without price subsidy) Excluded from analysis (LSSS intervention group): n = 122 individuals (LSSS intervention with price subsidy); n = 197 individuals (LSSS intervention without price subsidy) (ineligible urine samples) Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 1 village Discontinued intervention (control group): n = 1 village (due to urbanisation of the village and relocation of participants) Analysed (control group): SBP, DBP outcomes: n = 1272 individuals; urinary creatinine ratio; microalbuminuria outcomes: n = 928 individuals Excluded from analysis (control group): n = 344 individuals (ineligible urine samples) |
|
| Interventions |
Intervention Characteristics LSSS intervention (with price subsidy)
LSSS intervention (without price subsidy)
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, and National Center for Chronic Disease Prevention and Health Promotion (CDC). Additional support from the United Health Group Chronic Disease Initiative. Study authors supported by fellowships from an Australian Research Council; National Health and Medical Research Council; MRC‐PHE Centre for Environment and Health, Imperial College London, and by the National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London The China Rural Health Initiative Sodium Reduction Study (CHRS‐SRS) Authors name: Nicole Li; Lijing Yan; Yangfeng Wu Institution: The George Institute for Global Health at Peking University Health Science Center, China; the George Institute for Global Health, Sydney, Australia; Sydney Medical School, the University of Sydney, Australia Email: ywu@georgeinstitute.org.cn; lyan@georgeinstitute.org.cn (LY) Possible conflicts of interest (for study authors): "Bruce Neal is the Chair of Australian Division of World Action on Salt and Health." Sources used for data extraction: Journal articles with results of the trial; trial protocol (published); conference abstracts Trial registration details: NCT01259700 (China Rural Health Initiative: High cardiovascular risk management and salt reduction in rural villages in China ‐ two parallel large cluster‐randomised controlled trials) Author correspondence: Drs. Yan and Wu were contacted to clarify the baseline survey characteristics, as reported by Li 2013. Received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised sequence generation |
| Allocation concealment (selection bias) | Low risk | Central computerised randomisation, stratified by country to intervention or control group. LSSS intervention villages further assigned at random to receive subsidisation of the price of LSSS |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants or personnel, such as study staff, township health educators, the village council and village doctors. Outcomes were objective measures unlikely to be prone to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Endline survey was carried out by a study team independent of those involved in the intervention implementation, including village doctors, local project officers, intervention trainers and officials in the county Bureau of Health. The study authors stated: "Since blinding of data collection to the randomized allocation of the villages may be difficult to maintain, there will be a strong focus on training staff to apply rigorously standardized evaluation techniques for every outcome assessed ". Some objective study outcomes, such as BP measurements, were measured using automated devices. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We assessed the incompleteness of outcome data collected from participants who participated in the endline survey (with no baseline data). Complete data was collected for antihypertensive medication use; minimal incomplete data for SBP (n = 1 in LSSS with price subsidy intervention group) and DBP (n = 1 in LSSS without price subsidy group). However, incomplete urinary outcome data (urinary creatinine:albumin ratio; albuminuria): 19% in the LSSS with price subsidy intervention group; 31% in the LSSS without price subsidy intervention group, and 27% in the control group. In a sensitivity analysis of the urinary outcomes, the study authors included data from all urine samples and reported the effect estimates as unchanged (data not shown) (see Jardine 2019). |
| Selective reporting (reporting bias) | High risk | The primary outcomes cited in the trial registry protocol for the overall study differed from those reported by the published study protocol (see Li 2013). For example, SBP is listed as a primary outcome in the trial registry protocol, but only as a secondary outcome in the published study protocol. In addition, a substudy (see Jardine 2019) stated that their primary outcome (urinary creatinine‐albumin ratio) was prespecified; however this outcome was not described in the trial register study protocol. |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Low risk | Individual participants from all the selected clusters (villages) participated in a baseline survey before any interventions were implemented. |
| Comparability with individually randomised trials (cluster‐RCTs) | Unclear risk | "Eight RCTs of LSSS interventions report reductions or trends towards lowering sodium excretion and SBP, however these trials achieved much larger reductions in sodium excretion (and thus intake of sodium), compared to the present study." |
| Loss of clusters (cluster‐RCTs) | Low risk | One cluster in the control group was lost to follow‐up (due to relocation of all village inhabitants). No clusters were lost to follow‐up in the intervention group. |
| Baseline imbalance (cluster‐RCTs) | Unclear risk | The paper with the main results of the trial (Li 2016) stated the following: "Baseline survey data were not available because of resource constraints." Therefore, we have not evaluated the baseline data presented in the study protocol publication (see Li 2013). |
| Incorrect analysis (cluster‐RCTs) | Low risk | All statistical analyses were adjusted for the effects of clustering. |
| Overall risk of bias | Unclear risk | Unclear risk of bias for incomplete outcome data and baseline imbalance, low risk of bias for loss of clusters and recruitment bias |
Mu 2003.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: China Setting: Intervention conducted in households; outcomes measured at research site, Hanzhong city Aim of study: To observe the effect of long‐term supplementation of potassium and calcium on the blood pressure and sodium metabolism of adolescents by adding potassium and calcium to salt used by the family Unit of allocation: adults (aged 18 years or older) Start date: March 1997 End date: March 1999 Relevant study limitations as reported by study authors: Low follow‐up rate (80%) may have resulted in loss of statistical power. Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Adults, aged 18 to 22 years with high blood pressure (above 90th percentile for age and gender) at multiple follow‐up visits during the previous 8‐year period Exclusion criteria: NR Pretreatment: None Method of recruitment of participants: Recruited from 4623 adolescents in the cardiovascular disease prevention and treatment area of Hanzhong city Informed consent obtained: NR Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 220 Allocated to LSSS intervention(s): n = 110 Allocated to control: n = 110 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 22 (no reasons reported) Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): n = 88 Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 13 (no reasons reported) Discontinued intervention (control group): NR Analysed (control group): n = 97 Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Mu Jian‐jun Institution: The First Hospital of Xi of Jiaotong University Email: NR Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reported to be randomised but insufficient information on method reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors referred to the study as 'single‐blind'; unclear what this meant. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study only reported 'single blinding'. The study authors reported the use of standardised BP measurements; however, it was unclear if an automated device was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was greater than 10% in both groups: 20% (22/110) intervention group and 11.8% (13/110) in the control group. Reasons for lost to follow‐up data were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol or prospective trial registry entry not available |
| Other bias | Low risk | None identified |
| Overall risk of bias | High risk | Unclear risk of bias for allocation concealment; high risk of bias for incomplete outcome data |
Mu 2009.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention was conducted in rural villages in Hanzhong; setting where outcomes were measured: NR Aim of study: To observe the effects of dietary salt with added potassium and calcium on the BP of adolescents and their family members Unit of allocation: families or households Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention (participants with hypertension)
Control (participants with hypertension)
LSSS intervention (family members)
Control (family members)
Inclusion criteria: Families with young adults with high BP (defined as SBP = 90th percentile by age and sex) and other family members who were prepared to eat all dinners at home throughout the study period Exclusion criteria: Families with any family member who reported the use of a potassium‐sparing medication or with significant renal impairment (defined by study authors and confirmed by responsible physician as an abnormal serum creatinine concentration) or hyperkalaemia Pretreatment: There were more family members included in the LSSS intervention, compared to the control group (334 vs. 254) at baseline. Method of recruitment of participants: Adolescents who had been followed up for 10 years were screened (recruitment method not described) Informed consent obtained: Yes (unclear whether verbal or written) Clusters: families or households Subgroups planned/measured: NR Subgroups reported: Young adults with hypertension vs. family members; salt‐sensitive (SS) subjects vs. non‐salt‐sensitive (NSS) group Participant flow Assessed for eligibility: n = 350 families with young adults with hypertension Excluded (number with reasons): n = 25 families with young adults with hypertension (inability to have their dinners at home all or nearly all the time throughout the study) Randomised: n = 325 families; n = 325 young adults with hypertension; (n = 101 LSSS intervention group; n = 110 salt‐restricted intervention group; n = 114 control group and n = 334 family members) and n = 978 family members (n = 334 LSSS intervention group; n = 338 salt‐restricted intervention group; n = 114 control group) Allocated to LSSS intervention/s: n = 101 families; n = 110 young adults with hypertension (n = 334 family members) Allocated to control: n = 114 families; n = 114 young adults with hypertension (n = 254 family members) Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): NR [Young adults with hypertension: Total loss to follow‐up reported for 2 intervention groups and control group as 9.8% (32/325): n = 18 moved away; n = 4 joined the army; n = 8 remote places to work; n = 2 other reasons; family members: total loss to follow‐up reported as 14.3%] Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): NR Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): NR [Young adults with hypertension: Total loss to follow‐up reported for 2 intervention groups and control group as 9.8% (32/325): n = 18 moved away; n = 4 joined the army; n = 8 remote places to work; n = 2 other reasons; family members: total loss to follow‐up reported as 14.3 Discontinued intervention (control group): NR Analysed (control group): NR Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention (total)
Control (total)
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: National Health Ministry of China Authors name: Jianjun Mu Institution: Department of Cardiology, First Affiliated Hospital of Medical school of Xi’an Jiaotong University, Xi’an, People’s Republic of China Email: mujjun@163.com Possible conflicts of interest (for study authors): "The authors declared no conflict of interest." Sources used for data extraction: Journal article with results of the trial Trial registration details: NR Author correspondence: Dr. Mu was contacted on 23/07/2020 to provide us with outcome data (mean changes in SBP, DBP). Received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by coin toss to either intervention or control. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was described as 'single‐blinded' ‐ unclear what this meant. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study only reported 'single‐blinded' but no detail on who was blinded. BP was not measured with an automatic device. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported per treatment group. Overall attrition for hypertensive young adults was reported as 9.8%, and 14.3% overall for family members. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Unclear risk | It was unclear whether all the eligible family members within families were identified before randomisation of the families. More family members were included in the LSSS intervention, compared to the control group (334 vs. 254) at baseline, suggesting differential selection bias. |
| Comparability with individually randomised trials (cluster‐RCTs) | Unclear risk | The China Salt Substitute study (RCT) demonstrated a lowering effect on SBP in high‐risk adults with the use of a LSSS. A RCT (Liu 1996) among school children showed that calcium and potassium supplements (capsules) may limit the rise of BP with age; however, this was not a LSSS intervention. |
| Loss of clusters (cluster‐RCTs) | Unclear risk | Insufficient information provided on the number of families or households that were lost to follow‐up |
| Baseline imbalance (cluster‐RCTs) | Unclear risk | The baseline characteristics of the hypertensive young adults were similar between the 3 groups. However, the characteristics of the family members were not reported. |
| Incorrect analysis (cluster‐RCTs) | Low risk | Family codes were included as a random factor in the general linear modelling analysis. |
| Overall risk of bias | Unclear risk | Unclear risk of bias for incomplete outcome data, baseline imbalance, recruitment bias and loss of clusters |
Neal 2021.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in villages from five provinces Hebei, Liaoning, Ningxia, Shanxi and Shaanxi in northern region. NR where outcomes were measured Comments: SSaSS: Salt Substitute and Stroke Study Aim of study: The primary objective of the SSaSS is to determine the effects of sodium reduction achieved through the use of a reduced‐sodium, added potassium salt substitute on the risk of fatal or non‐fatal stroke. The secondary objectives are to determine the effects of the intervention on (1) major vascular events, a composite of non‐fatal stroke, non‐fatal acute coronary syndrome, and vascular death, and (2) total mortality. Unit of allocation: Villages Start date: April 2014 End date: March 2021 Relevant study limitations as reported by study authors: Neal 2021: No serial assessments of blood potassium, therefore, hyperkalaemia may have been missed in some participants. Dose‐response analysis could not be conducted since only one type of LSSS was used. No concealment of intervention delivery; however, objective primary and secondary outcomes were reported. In addition, systematic searches of routinely collected health data and verification by an independent end‐point adjudication committee, the members of which were unaware of the trial group assignments, was conducted, but authors stated that "Information that could be used for adjudication of outcome events was limited, and definitive assignment of causation was difficult in many cases.” No evidence was found to suggest certainty of adjudications had any substantive implications for the primary conclusions of the trial. Huang 2020: Hyperkalaemia risk in the study did not include vulnerable populations (e.g. CKD). Increased uncertainty of the effect estimates for intermediate outcomes due to the cluster‐randomised trial design (compared to an individual‐randomised trial). Incomplete participation of invited participants and incomplete collection of 24‐hour urine collections in interim surveys (however: those that participated were broadly representative of overall study population); and absence of 24‐hour urine for two provinces at baseline Sample size calculation: Estimated sample size of 21,000 participants in 600 clusters (n = 300 in the LSSS intervention group; n = 300 in control group, with 35 participants in each cluster) to detect a >= 13% relative risk reduction for stroke in the intervention villages compared with control villages, with 90% statistical power and 95% confidence level (two‐sided). The estimate assumed a primary outcome event rate of 3.5% per year, a systolic blood pressure difference of 3.0 mmHg between randomised groups, and an ICC of 0.04. |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Individuals in the selected villages with an elevated risk of stroke, defined as a history of stroke (regardless of type or knowledge of aetiology) and/or age 60 years or greater with uncontrolled high blood pressure (systolic blood pressure >= 140 mm Hg at visit if on blood pressure–lowering medication or systolic blood pressure >= 160 mm Hg if not on blood pressure–lowering medication). Participants were also required to be contactable by telephone and be able to nominate a friend or relative through which they could also be contacted, if necessary. Exclusion criteria: Participant or family members were excluded if they: (1) or someone living in their household had a potential contraindication to the salt substitute used in the trial; contraindications included use of a potassium‐sparing diuretic, use of a potassium supplement, or known serious kidney disease; (2) had serious renal impairment; (3) were taking potassium‐sparing diuretic; (4) were taking potassium supplement;(5) had concerns about using salt substitute; (6) ate most meals outside home; (7) were expected to live less than 6 months from date of baseline assessment by village doctors Pretreatment: No group differences Method of recruitment of participants: Two counties per province were selected by the research team based upon their willingness to participate, their proximity to the local research team and being representative of the socioeconomic level of each province. Sixty villages in each of the counties were recruited through a consent process involving the leadership of the local county Bureaus of Health (identified villages that were willing and accessible). About 35 individuals at elevated risk of stroke were recruited in each village. Informed consent obtained: Yes (written) Clusters: n = 600; cluster size: n = 35 individuals Subgroups planned/measured: NR Subgroups reported: Age > 65 years vs. age <= 65 years; female vs. male; less educated vs. more educated; cerebrovascular disease vs. no cerebrovascular disease; diabetes vs. no diabetes; hypertensive vs. non‐hypertensive; using antihypertensive medication vs. not using antihypertensive medication; SBP > 153 mmHg vs. SBP <= 153 mmHg; DBP > 89 mmHg vs. <= 89 mmHg and BMI > 24.6 kg/m2 vs. BMI <= 24.6 kg/m2 Participant flow Assessed for eligibility: NR Excluded (number with reasons): n = 1 individual (death) Randomised: n = 600 villages (n = 20,995 individuals) Allocated to LSSS intervention(s): n = 300 villages (n = 10,504 individuals) Allocated to control: n = 300 villages (n = 10,491 individuals) Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 1969 individuals (death) Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): n = 10,504 (cardiovascular outcomes ‐ fatal or non‐fatal stroke; major cardiovascular outcomes; all‐cause mortality) n = 786 at 12 months (DBP, SBP); n = 1412 at 24 months (DBP, SBP); n = 584 at 36 months (DBP, SBP); n = 587 at 48 months (DBP, SBP); n = 7436 (60 months) (DBP, SBP) Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): 2203 individuals (death) Discontinued intervention (control group): NR Analysed (control group): n = 10,491 (cardiovascular outcomes ‐ fatal or non‐fatal stroke; major cardiovascular outcomes; all‐cause mortality) n = 658 at 12 months (DBP, SBP); n = 1374 at 24 months (DBP, SBP); n = 584 at 36 months (DBP, SBP); n = 559 at 48 months (DBP, SBP); n = 7081 (60 months) (DBP, SBP) Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: Supported by research grants (APP1164206 and APP1049417) and an investigator grant (APP1197709, to Dr. Neal) from the National Health and Medical Research Council of Australia. Dr J HY Wu is supported by a UNSW Scientia Fellowship. Salt substitute donated by Jiangsu Sinokone Technology for years 3 to 4 of the study Authors name: Bruce Neal Institution: Sydney School of Public Health, University of Sydney, Sydney, Australia. Email: bneal@georgeinstitute.org.au Possible conflicts of interest (for study authors): Paul Elliott declared various grants from non‐profit organisations and is an unpaid member of 'Action on Salt and health; World Action on Salt, Sugar and health'; Dr. Yangfeng Wu received a research grant from Health Source (Chongqing) Cardiovascular Health Technology Co. (a pilot study on the effect of very low‐sodium salt substitute among patients with hypertension); other study authors declared no conflict of interest. Sources used for data extraction: Journal article(s) with results of the trial; trial protocol (published; trial registry); conference abstract Trial registration details: NCT02092090 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors stated that county‐stratified random assignment occurred through a central computerised process, done by an independent biostatistician. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by an independent biostatistician through a central computerised process. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although participants and personnel involved with study implementation, e.g. village doctors, were not blinded, the risk of bias was minimised by the measurement of objective outcomes such as clinical events or measurements such as blood pressure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial registry indicated that investigators and outcomes assessors were blinded to allocation, measurement of process indicators were intended to be measured separately to avoid unblinding, and it appeared as though an analysis plan was finalised before data unblinding. However, Huang and colleagues 2020 reported that ‘the trial has an unavoidable open design and biases consequent upon the unblinded design cannot be entirely excluded’. Endpoint adjudication committees were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mortality: Both groups had complete follow‐up outcome data. Cardiovascular events: Non‐fatal events were assessed among the participants for 99,473 of 99,522 person‐years (> 99.9%) of scheduled follow‐up. SBP and DBP: Death was the main reason for the attrition in both groups at 60‐months follow‐up. Reported attrition, other than death, was 10.5% (1099/10504) in the intervention vs. 11.5% (1207/10491) in the control group. Data for mortality were complete, and data for events could also be found for all surviving participants (albeit from sources with different quality and risk for misclassification). Attrition for blood pressure measurements in participants surviving at 60 months was 1099/8535 (13%) in the intervention and 1207/8288 (15%) in the control arm, however, there appeared to be no difference in the reasons for most of the missingness (followed up through nominated contact, followed up through record linkage, followed up at previous visit). Blood pressure measurements for participants followed up in person remained unaccounted for in 286/7722 (4%) participants in the intervention and 327/7408 (4%) participants in the control arm. |
| Selective reporting (reporting bias) | Unclear risk | Some prespecified outcomes e.g. total major vascular events (as a composite outcome) were not reported. The study authors reported subgroup analyses, which was not prespecified. |
| Other bias | High risk | Misclassification bias: The study authors reported that information used for adjudication of outcome events was limited, and definitive assignment of causation was difficult in many cases. However, they reported no evidence of a potential effect of misclassification of clinical end‐points on effect estimates. Further, the authors stated that the lack of serial monitoring of blood potassium in the study may have resulted in missing participants with hyperkalaemia. Definite and probable hyperkalaemia events were defined on the basis of medical reports documenting a serum potassium > 5.5 mmol/L or ECG or enzyme changes. A post hoc decision was taken to classify participants with possible hyperkalaemia (based on self‐report; not requiring any supporting documentation). |
| Recruitment bias (cluster‐RCTs) | Low risk | All participants in the selected villages were recruited before villages were allocated to the intervention or control group. |
| Comparability with individually randomised trials (cluster‐RCTs) | Unclear risk | There were no RCTs that reported on long‐term cardiovascular clinical outcomes, following LSSS interventions. To date, some RCTs have reported short term benefits in cardiovascular risk factors, such as reductions in blood pressure (CSSS Collaborative Group 2007; Zhao 2014), while others have not (Gilleran 1996; Mu 2003; Sarkkinen 2011; Suppa 1988). |
| Loss of clusters (cluster‐RCTs) | Low risk | The complete data on vital status for all participants indicated that it was very unlikely that entire clusters were lost from the study. |
| Baseline imbalance (cluster‐RCTs) | Low risk | No baseline imbalances reported between the intervention and control groups in terms of demographic characteristics or comorbidity risk |
| Incorrect analysis (cluster‐RCTs) | Low risk | Adjustment for clustering was made with the use of a hierarchical Poisson regression model. |
| Overall risk of bias | Low risk | Low risk of bias for incomplete outcome data, baseline imbalance, recruitment bias and loss of clusters |
Omvik 1995.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Norway Setting: Intervention conducted in households; outcomes measured at an outpatient clinic in Bergen Aim of study: To examine whether reductions in intake of dietary sodium, combined with low‐sodium high‐potassium salt substitute instead of standard table salt, would result in clinically meaningful blood pressure reduction among people with hypertension. The study also aimed to determine whether this combination would reduce total peripheral resistance. Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Men and women with mild or moderate essential hypertension, according to World Health Organization stages I and II, which were untreated at the time of recruitment were included, but it is not clear whether these were inclusion criteria per se. Exclusion criteria: Serum creatinine outside normal limits and secondary hypertension were determined by routine clinical examination, concentration of serum electrolytes, urine analysis, chest X‐ray and, on specific indications, hormonal analysis, radio‐isotope pyelography and renal arteriography. Both were excluded in all participants, but it was not clear whether these were exclusion criteria per se. Pretreatment: None Method of recruitment of participants: Patients were recruited from an outpatient clinic at the cardiology department of Haukeland Hospital. Informed consent obtained: Yes, unclear whether written or oral Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 40 Allocated to LSSS intervention(s): n = 20 Allocated to control: n = 20 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 1 (newly developed angina pectoris) Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): n = 19 Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 Discontinued intervention (control group): n = 0 Analysed (control group): n = 20 Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study was supported by The Norwegian National Health Association, The Norwegian Council for Cardiovascular Disease. Pansalt was sponsored by Reformi‐Keskus Oriola Oy, Espoo, Finland and Searle Norge A/S Oslo Norway. Authors name: Per Omvik Institution: Department of Cardiology, Haukeland Hospital Email: Per.Omvik@uib.no Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was reported as 'double‐blind'. Intervention and control salts were pre‐numbered, according to the participants' randomisation numbers, and identically wrapped. It was not explicitly stated who were blinded, but outcomes were unlikely to have been affected by any potential lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was reported as 'double‐blind'. It was not clear whether outcome assessors were blinded; however, blood pressure measurements were taken using a standard technique and automated monitor and serum values were laboratory‐determined. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was low, with 1/20 (5%) in the intervention and 0/20 (0%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment; low risk of bias for incomplete outcome data |
Pan 2017.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Taiwan Setting: Intervention conducted in households; outcomes measured at 8 clinical centres Aim of study: To evaluate the effects of potassium‐ and magnesium‐enriched salts on the neurologic performance of stroke patients Unit of allocation: Adults (age 45 years and older) Start date: December 2009 End date: May 2013 Relevant study limitations as reported by study authors: Lack of statistical power due to small sample size; synergistic effects of magnesium and potassium may be present; large dropout rate; under‐consumption of intervention salt and collection of first morning urine samples (instead of 24‐hour urine samples) Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention (≥ 30% KCl)
LSSS intervention (≥ 30% KCl)
Control
Inclusion criteria: Adults, aged 45 years and older, who were hospitalised for <= 1 month because of cerebral infarction or haemorrhage, with a modified Rankin scale (mRS) score of <= 4 at the time of discharge, who agreed to prepare foods with salt provided by the study Exclusion criteria: Patients with poor kidney function (GFR < 60 mL/min), secondary hypertension, cancer, or liver diseases, those with eating disorders; those taking potassium‐sparing medicines, or consuming salt substitutes Pretreatment: None reported at baseline. However, the proportion of participants with hypertension in the control group (50.5%) was lower compared to the intervention groups, especially the < 50% KCl group (68.4%). Method of recruitment of participants: Medical practitioners at 8 participating hospitals identified potentially eligible patients. Informed consent obtained: yes (written) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 291 Allocated to LSSS intervention/s: n = 97 (LSSS 50% KCl); n = 95 (LSSS < 50% KCl) Allocated to control: n = 99 Received allocated LSSS intervention/s: NR Did not receive allocated LSSS intervention/s: NR Lost to follow‐up (LSSS intervention group): At 3 months: n = 21 (LSSS 50% KCl) ‐ Reasons: Moved away (n = 2), hospital transfer (n = 1), left study (n = 15), lost to follow‐up (n = 3)n = 20; (LSSS < 50% KCl)‐ Reasons: Suicidal death (n = 1), hospital transfer (n = 2), left study (n = 16), lost to follow‐up (n = 1) At 6 months: n = 14 (LSSS 50% KCl)‐ Reasons: Hospital transfer (n = 3), left study (n = 9), lost to follow‐up (n = 2) n = 11; (LSSS < 50% KCl) ‐ Reasons: Hospital transfer (n = 1), left study (n = 8), lost to follow‐up (n = 2) Discontinued intervention (LSSS intervention group): At 3 months: n = 15 (LSSS 50% KCl) n = 16 (LSSS < 50% KCl) At 6 months: n = 9 (LSSS 50% KCl) n = 8 (LSSS < 50% KCl) Analysed (LSSS intervention group): n = 97 (LSSS 50% KCl); n = 95 (LSSS < 50% KCl) ‐ ITT analysis was performed (the last‐observation‐carried‐forward method) Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): At 3 months: n = 15 Reasons ‐ Suicidal death (n = 1), hospital transfer (n = 1), left study (n = 12), lost to follow‐up (n = 1) At 6 months: n = 13 Reasons ‐ Hospital transfer (n = 1), left study (n = 10), lost to follow‐up (n = 2) Discontinued intervention (control group): At 3 months: n = 12 At 6 months: n = 10 Analysed (control group): n = 99 ‐ ITT analysis was performed (the last‐observation‐carried‐forward method) Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention (50% KCl)
LSSS intervention (< 50% KCl)
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes
|
|
| Notes |
Funding source: Supported by grants from the Institute of Biomedical Sciences, Academia Sinica, and from the Ministry of Health and Welfare (DOH098‐TD‐F‐113‐098030). Authors name: Wen‐Harn Pan Institution: Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan Email: pan@ibms.sinica.edu.tw Possible conflicts of interest (for study authors): None. Sources used for data extraction: Journal article with results of the trial Trial registration details: NCT02910427 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation sequence generation. A stratified randomisation method was used according to a clinical history of ischaemic or haemorrhagic stroke, diabetes or no diabetes, and the use of magnesium‐containing medication or not. |
| Allocation concealment (selection bias) | Low risk | Salts were labelled by numbers 1, 2, 3 during manufacturing. Allocation was performed by software once the participant's details were entered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study authors reported that participants and personnel, such as the medical practitioners involved in the salt distribution, were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The medical practitioners performing the patient evaluations were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data reported at 6 months were 34% (33/97)(LSSS 50% KCl) and 34.7% (33/95) (LSSS < 50% KCl), compared to 28.3% (28/99) in the control group. The study authors stated that no significant difference was found in baseline characteristics between participants who remained in the study at month 3 and at month 6, compared to those who did not. The study authors conducted an ITT analysis (last‐observation‐carried‐forward). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported according to those pre‐specified in the study protocol (NCT02910427) |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Pereira 2005.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Brazil Setting: Intervention conducted in households in São Paulo; outcomes measured at the outpatient clinic of a federal university Aim of study: To evaluate the effect of potassium supplementation of table salt, weight reduction through caloric restriction as well as increased physical activity on blood pressure and insulin resistance in people who are overweight, have hypertension and are treated with diuretics Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: Limited adherence to the LSSS resulted in sodium chloride replacement of approximately 30%, presumably as some participants did not eat all their meals at home. Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Men and women who are overweight or living with obesity, and have hypertension, aged 20 to 70 years; exclusively using diuretics (hydrochlorothiazide or chlorthalidone) for at least three weeks; systolic blood pressure > 140 mmHg and < 160 mmHg and diastolic blood pressure > 90 mmHg and < 105 mmHg; with BMI of 27 kg/m2 calculated during medical consultation; and central body fat distribution, defined as waist‐to‐hip ratio of 0.85 for women and 0.95 for men Exclusion criteria: Participants with clinical or laboratory evidence of heart disease, stroke blood dyscrasias, kidney or liver disease, diabetes mellitus; serum potassium concentrations of 5.0 or < 3.0 mEq/L; or use of any antihypertensives other than diuretics Pretreatment: None Method of recruitment of participants: Patients were recruited from a hypertension outpatient clinic and diabetes hospital which they attended. Informed consent obtained: Yes, written Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 28 Allocated to LSSS intervention(s): n = 15 Allocated to control: n = 13 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): Total group n = 4 (needed to change medication); distribution by trial arm not reported Discontinued intervention (LSSS intervention group): Total group n = 4 (needed to change medication); distribution by trial arm not reported Analysed (LSSS intervention group): n = 12 Excluded from analysis (LSSS intervention group): Total group n = 2 (lack of adherence to treatment); distribution by trial arm not reported Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): Total group n = 4 (needed to change medication); distribution by trial arm not reported Discontinued intervention (control group): Total group n = 4 (needed to change medication); distribution by trial arm not reported Analysed (control group): n = 10 Excluded from analysis (control group): Total group n = 2 (lack of adherence to treatment); distribution by trial arm not reported |
|
| Interventions |
Intervention Characteristics LSSS intervention Theoretical basis: Reduction of dietary sodium and increases in potassium intake may lower blood pressure, while the latter may also increase plasma potassium and decrease mortality from stroke and heart disease. Increases in serum potassium have been linked to decreased blood pressure and decreased mortality from stroke cerebral and heart disease via reduced neural adrenergic activity, decreased levels of plasma renin, inhibition of the formation of free radicals and increased enzyme activity Na + / K + ‐ATPase, increasing cell uptake of potassium and reducing intracellular sodium. Description: Potassium chloride salt (50% NaCl; 50% KCl) LSSS category: ≥ 30% KCl Contains fortificant: NR Delivery: discretionary use Duration of run‐in period: none Duration of active intervention: 12 weeks Duration of follow‐up (as reported): none Total duration of study (as reported): 12 weeks Timing: LSSS to be used throughout the study as table and cooking salt Implementation: Packs, similar in appearance to control salt, of 1 kg LSSS were used; frequency of distribution NR Providers: NR Co‐interventions: Individualised hypocaloric diet using body composition to calculate basal metabolic rate with an activity factor to determine energy needs with 1000 Kcal deficit (min prescription 1200 kCal); increased physical activity advised Resource requirements: NR Integrity of delivery: Urine collections were done at all study visits to assess adherence to the salt intervention. Participants were asked to prepare all meals at home and to take home‐cooked meals to work. Two participants were excluded from the total group due to a lack of adherence. Control Theoretical basis: To enable comparison of LSSS intervention with standard practice. Description: normal salt (100% NaCl) LSSS category: n/a Contains fortificant: NR Delivery: discretionary use Duration of run‐in period: none Duration of active intervention: 12 weeks Duration of follow‐up (as reported): none Total duration of study (as reported): 12 weeks Timing: Normal salt to be used throughout the study as table and cooking salt Implementation: Packs, similar in appearance to LSSS, of 1 kg control salt were used; frequency of distribution NR Providers: NR Co‐interventions: Individualised hypocaloric diet using body composition to calculate basal metabolic rate with an activity factor to determine energy needs with 1000 Kcal deficit (min prescription 1200 kCal) ; increased physical activity advised Resource requirements: NR Integrity of delivery: Urine collections were done at all study visits to assess adherence to the salt intervention. Participants were asked to prepare all meals at home and to take home‐cooked meals to work. Two participants were excluded from the total group due to a lack of adherence. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Maria Alice de Gouveia Pereira Institution: Disciplines of Nutrition and Metabolism, Kidney and Hypertension Hospital, Federal University of São Paulo Email: alicegouveia@ig.com.br Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors reported that the study was 'double‐blind', and intervention and control salts were packaged to look the same. It was not explicitly stated who was blinded, but outcomes were unlikely to have been affected by any potential lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study reported 'double‐blinding', but it was not clear whether outcome assessors were blinded. Blood pressure was determined using the auscultatory method, which may be more prone to detection bias than automated techniques. Other outcomes were laboratory‐determined measures unlikely to be affected by a lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall attrition was 3/15 (20%) in the intervention and 3/13 (23%) in the control group. Reasons for attrition (exclusion due to non‐adherence and change in antihypertensive medication) were not reported per trial arm. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment and incomplete outcome data |
Sarkkinen 2011.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Finland Setting: Intervention conducted in households in Kuopio; outcomes measured at Food and Health Research Centre, University of Eastern Finland Aim of study: To determine the feasibility of replacing sodium chloride with a novel mineral salt (50% sodium chloride, 25% potassium chloride and 25% magnesium ammonium potassium chloride hydrate); and the effect of replacement on blood pressure Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: Small sample size Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Subjects aged between 25 and 75 years; with systolic blood pressure between 130 and 159 mmHg and/or diastolic blood pressure between 85 and 99 mmHg; with BMI between 23 and 40 kg/m2, and stable body weight Exclusion criteria: Subjects on antihypertensive medication, nonsteroidal anti‐inflammatory drugs, cyclosporine or tacrolimus; with secondary hypertension, type 1 or 2 diabetes, history of active heart disease or cancer, abnormal electrolyte concentrations, proteinuria; with abnormal liver, kidney or thyroid function; currently following a low salt diet (six points or fewer on the salt intake test of the Finnish Heart Association); abusing drugs or alcohol; who are pregnant, or lactating Pretreatment: None Method of recruitment of participants: Recruitment of subjects was done through announcements in local newspapers and from the volunteer register of Oy Foodfiles (affiliation of the first author), after which potential participants were screened by telephone. Informed consent obtained: Yes, written Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: n = 168 Excluded (number with reasons): n = 118 (reasons NR) Randomised: n = 50 Allocated to LSSS intervention(s): n = 25 Allocated to control: n = 25 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 3 (reasons NR) Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): n = 22 Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 2 (reasons NR) Discontinued intervention (control group): NR Analysed (control group): n = 23 Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study was supported by SmartSalt® Inc, California, USA. Authors name: Essi S. Sarkkinen Institution: Oy Foodfiles Ltd, CRO in the field of nutrition Email: essi.sarkkinen@foodfiles.com Possible conflicts of interest (for study authors): "The authors declare that they have no competing interests." Sources used for data extraction: Journal article with results of the trial; non‐commercial trial registry record Trial registration details: ISRCTN01739816 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was reported as 'double‐blind', but it was not clear whether participants and personnel were blinded (outcome assessors were reported as blinded). Given that the intervention involved test foods in addition to salt intervention, leading to minimal potential lifestyle modification, it was unlikely that outcomes of interest would be affected by a lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was reported that the study nurse was unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 3/25 (12%) in the intervention and 2/25 (8%) in the control group, with no reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment; low risk of bias for incomplete outcome data |
Suppa 1988.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Italy Setting: Intervention conducted in households; outcomes measured at 31 clinical centres Aim of study: To evaluate the effects of a low‐sodium/high potassium salt as an additional treatment in patients with mild and moderate hypertension on beta‐blockers Unit of allocation: adults (aged 18 years or older) Start date: NR End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Participants with supine diastolic BP greater or equal to 95 mmHg only taking metoprolol 200 mg daily Exclusion criteria: Patients with contraindications for β‐blockers; secondary hypertension; renal failure (serum creatinine >= 1.5 mg/dL or 133 µmol/L; eGFR females, mean age 47 years: 41mL/min; males, mean age 47 years: 54 mL/min) or other major diseases, and women of childbearing potential Pretreatment: None Method of recruitment of participants: NR Informed consent obtained: NR Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: n = 358 Excluded (number with reasons): n = 36 (n = 6 due to undesirable side effects such as asthenia, insomnia, hypotension and headaches, n = 4 due to severe hypertension, n = 26 for poor compliance mainly to collecting urine samples) Randomised: n = 322 Allocated to LSSS intervention(s): n = 163 Allocated to control: n = 159 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 0 Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): n = 163 Excluded from analysis (LSSS intervention group): n = 0 (DBP; SBP) n = 50; (urinary sodium and potassium): Study authors only analysed data from participants if 3 urine samples were correctly collected. Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 0 Discontinued intervention (control group): n = 0 Analysed (control group): n = 159 Excluded from analysis (control group): n = 0 (DBP, SBP) n = 51 (urinary sodium and potassium): Study authors only analysed data from participants who collected 3 urine samples correctly. |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes | Outcomes Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Giuseppe Suppa Institution: The Italian Group for the Prevention and Care of Arterial Hypertension Email: NR Possible conflicts of interest (for study authors): NR Sources used for data extraction: Journal article with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was reported as 'double‐blinded' but it was unclear what this meant. The salt was packaged in identical packets; therefore it was likely that participants were blinded. However, it was unclear whether the personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was reported as 'double blinded', but it was unclear what this meant. BP measurements were conducted with a non‐automated conventional device and therefore detection bias was possible if outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data reported for SBP, DBP outcomes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol or prospective trial registry entry not available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for allocation concealment; low risk of bias for incomplete outcome data |
Toft 2020.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: Denmark Setting: Intervention conducted in households from five municipalities ((Albertslund, Ballerup, Egedal, Glostrup and Rødovre) in the Southwestern part of the Capital Region; outcomes measured at research centre Comments: SalT Reduction InterVEntion (STRIVE) study Aim of study: To examine the effects of two different salt reduction strategies on selected cardiovascular risk factors Unit of allocation: Families Start date: February 2018 End date: June 2018 Relevant study limitations as reported by study authors: Low statistical power due to a lower number of participants than planned in power calculations, low level of salt reduction in the intervention groups making it difficult to investigate the potential adverse effect of larger salt reduction; a confounding factor in these analyses could be changes in other dietary factors. This paper did not include dietary data. Sample size calculation: Estimated sample size of 25 families (100 participants) in each of the three groups, at a 5% confidence level and 80% power, based on expected reductions of 1.25 g salt (0.5 g sodium) per day in the intervention group receiving low‐sodium bread and 3 g salt (1.2 g sodium) per day in the intervention group receiving low‐sodium bread combined with dietary counselling. ICC within families = 0.33 |
|
| Participants |
Baseline Characteristics LSSS intervention (adults)
LSSS intervention (children)
Control (adults)
Control (children)
Overall
Overall (children)
Inclusion criteria: Families with at least one child (3–17 years) and one parent (18–65 years) and a daily bread consumption among adults. Children of divorced parents had to stay more than half the time with the participating parent, if both parents were not participating. Exclusion criteria: Antihypertensive and lipid‐lowering treatment, pregnancy, diabetes, coronary heart disease and urine albumin > 300 mg/day Pretreatment: The study authors reported differences in BMI, activity levels and alcohol consumption between the groups, but did not confirm these differences with the use of statistical analysis. Method of recruitment of participants: Families were recruited through social media at schools, kindergartens and large companies, word‐of‐mouth and posters in the local area during the period January–February 2018. Informed consent obtained: Yes (written) Clusters: Families [family size: LSSS Intervention 3.2 (0.8); control 3.5 (1.1)] Subgroups planned/measured: NR Subgroups reported: Participants in the intervention groups that decreased their estimated daily salt intake by at least 3 g; or the sodium to potassium ratio by at least 20% from baseline to 4‐month follow‐up Participant flow Assessed for eligibility: n = 152 families (581 individuals) Excluded (number with reasons): n = 63 (n = 272 individuals): withdrew or did not fulfil eligibility criteria Randomised: n = 89 families (n = 309 individuals) Allocated to LSSS intervention(s): Salt‐reduced bread group: n = 25 families (n = 81 individuals) Allocated to control: Standard bread group: n = 29 families (n = 101 individuals) Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 7 individuals (n = 2 families); Reasons ‐ Lack of time: n = 3 individuals (1 family); Reason for not attending follow‐up not reported: n = 4 individuals (1 family) Discontinued intervention (LSSS intervention group): n = 0 Analysed (LSSS intervention group): Multiple imputation analysis: n = 41 adults; n = 40 children Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 4 individuals (2 families); Reasons ‐ Family issues (n = 3 individuals from 1 family); Moved (n = 1 individual) Discontinued intervention (control group): n = 2 individuals (n = 1 family) Analysed (control group): Multiple imputation analysis: n = 49 adults; n = 52 children Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: Danish Heart Foundation (2016‐R107‐A6700‐22998¸2018‐R124‐A8520‐22098), The Research Fond of the Capital Region of Denmark, The Toyota Foundation, Ministry of Environment and Food of Denmark, Axel Muusfeldt’s Foundation and Sofus Friis’ Foundation Authors name: Ulla Toft Institution: Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital; Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen Email: ulla.toft@regionh.dk Possible conflicts of interest (for study authors): The authors declared no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results. Sources used for data extraction: Journal article with results of the trial; unpublished trial protocol and trial registry entry Trial registration details: NCT03810885 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence of random group assignment |
| Allocation concealment (selection bias) | Low risk | Study authors reported the use of a computer‐generated sequence of random group assignment, and that the secretary who screened and enrolled participants was blinded with respect to group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was achieved through colour‐coding of bread packaging. Study personnel were not blinded and the reduction in the salt content of the intervention bread may have resulted in taste changes in the intervention groups, However, the outcomes measured were objective clinical outcomes such as BP and blood concentrations e.g. blood lipids. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (nurses) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total attrition was low, 20/309 (6.5%), with no differential attrition between any group (9% in intervention A and 6% each in intervention B and the control group). Reasons for attrition were similar. Missing data were imputed using a multiple imputation approach. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes listed in the trial registry (NCT03810885), such as hip and waist circumference, baseline urine albumin and change in urinary creatinine, were not reported in the main results. |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Low risk | Baseline assessments were done before randomisation. |
| Comparability with individually randomised trials (cluster‐RCTs) | Unclear risk | The comparability of this study with other RCTs with similar LSSS interventions (i.e. non‐discretionary LSSS use), or at similar time points, was unclear. |
| Loss of clusters (cluster‐RCTs) | Low risk | Loss of clusters was low and similar between the groups (LSSS intervention group n = 2 families; control group n = 3 families). |
| Baseline imbalance (cluster‐RCTs) | Unclear risk | The study authors reported differences in BMI, activity levels and alcohol consumption between the groups, but did not confirm these differences with the use of statistical analysis. |
| Incorrect analysis (cluster‐RCTs) | Low risk | Missing data at baseline and follow‐up were imputed using multiple imputation with 100 samples and taking into account cluster effects. Linear mixed modelling was used with the cardiovascular risk factors at follow‐up as outcome variables, treatment groups and baseline measures of the risk factor as fixed‐effects and family ID as random‐effects. |
| Overall risk of bias | Unclear risk | Low risk of bias for incomplete outcome data, loss of clusters and recruitment bias; unclear risk of bias for baseline imbalance |
Yu 2021.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: India Setting: Intervention conducted in villages in northern region of the Indian state of Telangana; outcomes measured during face‐to‐face follow‐up visits of households Comments: Salt Substitute in India Study (SSiIS) Aim of study: The primary aim of SSiIS is to evaluate the effect of a 3‐month reduced‐sodium, added‐potassium salt substitute intervention on SBP among patients with hypertension in rural India. The secondary aims are to determine the effects of the salt substitute on DBP, urinary sodium and potassium excretion, as well as to assess dietary sources of sodium and the acceptability of the salt substitute. Unit of allocation: Adults (age 18 years and older) Start date: November 2019 End date: April 2020 Relevant study limitations as reported by study authors: Short intervention duration; findings may not be generalisable to urban Indian hypertensive populations; exclusion of hypertensive patients with kidney disease; cannot rule out presence of undetected hyperkalaemia in some participants; no collection of 24‐hour urine biomarkers at one month follow‐up to determine trajectory of changes in urinary sodium and potassium over the study period, and did not assess the completeness of 24‐hour urine collections by administration of para‐aminobenzoic acid Sample size calculation: Estimated sample size was 498 participants, at 80% power and a significance level of 5% (one‐sided test). The power estimate assumed a mean baseline SBP of 140 mmHg (SD 20 mmHg) and a 20% loss to follow‐up. The study was powered to detect a 5 mmHg or greater difference in mean SBP between groups. |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Adults aged 18 years or over with a history of hypertension diagnosed by a health professional (hypertension may be self‐reported and antihypertensive drugs may or may not be used for management) who eat most of their meals at home. Exclusion criteria: Participants or any of their household members with self‐reported acute or chronic kidney disease (according to standard diagnostic clinical criteria; verified by study physician at baseline), who used potassium‐sparing diuretics, potassium supplements, who were not expected to live longer than 6 months from the baseline assessment, or where another household member was already enrolled in the study Pretreatment: Baseline clinical and demographic characteristics of participants were similar between the intervention and the control group. Method of recruitment of participants: Villages were purposively selected based on their willingness to be involved and their proximity to the infrastructure necessary for the study, including delivery of the intervention salts and being accessible to study personnel. After we approached the households, each household decided for themselves which of the adult members of the household (who also met eligibility criteria) would be the study participant. Informed consent obtained: Yes (written consent) Clusters: n/a Subgroups planned/measured: NR Subgroups reported: Age < 65 years vs. >= 65 years; male vs. female; diabetes vs. no diabetes Participant flow Assessed for eligibility: n = 1923 Excluded (number with reasons): n = 1421 (n = 286 eat most meals outside the home; n = 21 withdrew consent; n = 350 had concerns about use of LSSS; n = 206 used a potassium‐sparing diuretic; n = 193 used potassium supplements; n = 117 had kidney disease; n = 132 another household member already enrolled; n = 101 not interested) Randomised: n = 502 Allocated to LSSS intervention(s): n = 252 Allocated to control: n = 252 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 3 at 1 month (n = 2 lost contact; n = 1 death); n = 6 at 3 months (n = 4 lost contact; n = 2 hospitalised) Discontinued intervention (LSSS intervention group): n = 1 at 3 months Analysed (LSSS intervention group): SBP; DBP: n = 242; urinary sodium and potassium (24‐hour urine): n = 238; questionnaire: n = 242 Excluded from analysis (LSSS intervention group): Urinary sodium and potassium: n = 26 excluded due to incomplete urine samples Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 3 at 1 month (n = 3 lost contact); n = 7 at 3 months (n = 7 lost contact) Discontinued intervention (control group): n = 3 at 1 month; n = 3 at 3 months Analysed (control group): SBP, DBP: n = 234; urinary sodium and potassium (24‐hour urine): 224; questionnaire: 234 Excluded from analysis (control group): Urinary sodium and potassium: n = 29 excluded due to incomplete urine samples |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The George Institute for Global Health Seed Grant (grant number 0141030). Regular salt used in the study was provided free of charge by Siddharth Starch Pvt. Ltd. company. Authors name: Jie Yu Institution: The George Institute for Global Health, University of New South Wales, Sydney, New South Wales, Australia and Department of Cardiology, Peking University Third Hospital, Beijing, China Email: jyu1@georgeinstitute.org.au Possible conflicts of interest (for study authors): The authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors stated that random assignment occurred through a central computerised process, done by an independent biostatistician. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated to either group according to a prepared random allocation sequence list, drawn up by an independent biostatistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded. LSSS and regular salt were provided as masked, identical packages, with only the unique identification number on each pack. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel who collected outcome data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | BP data were available for 96 % (242/252) and 92.6% (234/250) of intervention and control participants, respectively. In addition, the study authors demonstrated that there were no differences in demographic and clinical characteristics of participants who completed the study, compared to those who did not (supplementary data). |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes reported according to the published study protocol. Pulse rate and medication use were indicated as outcomes at one and three months, but were not reported. Dietary recall (24 h) was also indicated as an outcome at three months, but was not reported. The authors reported a subgroup analysis which was not prespecified. |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Zhang 2015.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in nursing houses in northern China; outcomes measured in these nursing houses Aim of study: To observe the long‐term effect of consuming enriched potassium salt (KCl:NaCl = 1:1 by weight) on blood pressure, all‐cause mortality, target organ damage and safety in persons living in Chinese nursing houses. Unit of allocation: Nursing houses Start date: 2011 to 2012 End date: NR Relevant study limitations as reported by study authors: NR Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Overall
Inclusion criteria: Clusters: nursing houses in the northern regions of China. Participants: inhabitants of nursing houses Exclusion criteria: None reported Pretreatment: No apparent imbalance Method of recruitment of participants: NR Informed consent obtained: NR Clusters: n = 30 clusters (number of participants NR); no details on whether analyses were adjusted for clustering Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: NR Excluded (number with reasons): NR Randomised: n = 30 clusters; number of participants NR Allocated to LSSS intervention(s): NR Allocated to control: NR Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): NR Discontinued intervention (LSSS intervention group): NR Analysed (LSSS intervention group): n = 30 clusters at 3 to 5 months and 1 to 1.5 years, n = 22 clusters at 3 years, and n = 28 clusters at 4 years in total group (trial arm distribution NR); n = 1105 participants at 3 to 5 months and 1 to 1.5 years, n = 1032 at 3 years, and number of participants NR at 4 years Excluded from analysis (LSSS intervention group): NR Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): NR Discontinued intervention (control group): NR Analysed (control group): n = 30 clusters at 3 to 5 months and 1 to 1.5 years, n = 22 clusters at 3 years, and n = 28 clusters at 4 years in total group (trial arm distribution NR); n = 947 participants at 3 to 5 months and 1 to 1.5 years, n = 807 at 3 years, and number of participants NR at 4 years Excluded from analysis (control group): NR |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Hongye Zhang Institution: Beijing Hypertension League Institute, Beijing, China Email: NR Possible conflicts of interest (for study authors): NR Sources used for data extraction: Conference abstract about the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no information available on whether participants and people delivering the intervention were blinded. Outcomes are objective and not likely to be affected by a lack of blinding, but modifications in behaviour due to awareness of assignment may have resulted in some performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospective trial registry entry available |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Unclear risk | Insufficient information reported on whether clusters were identified before randomisation |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | A previous RCT investigated the use of mineral salt with 10% potassium in middle‐aged and elderly Japanese and also found it to be a safe alternative to regular salt (Kawasaki 1998). This study also found a significant decrease in blood pressure in those consuming the mineral salt. Another RCT in older Dutch people found that replacing regular salt with low‐sodium high‐potassium salt led to blood pressure reduction in people with mild to moderate hypertension (Geleijnse 1994). A parallel‐design study (Zhou 2009) also reported similar reduction of blood pressure. |
| Loss of clusters (cluster‐RCTs) | High risk | A total of 27% (8/30) of clusters were not included in the analysis at year 3, and 7% (2/30) of clusters were not included in the analysis at year 4. |
| Baseline imbalance (cluster‐RCTs) | Unclear risk | Based on five baseline characteristics reported, there appeared to be no serious baseline imbalance initially; however, due to in‐ and out‐migration to and from the nursing homes, it was not clear whether baseline equivalence was maintained over time. |
| Incorrect analysis (cluster‐RCTs) | Unclear risk | Insufficient information on statistical analysis and adjustment for clustering in conference abstracts (full text not available) |
| Overall risk of bias | High risk | Unclear risk of bias for incomplete outcome data, recruitment bias and baseline imbalance; high risk for loss of clusters |
Zhao 2014.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: Tibet Setting: Intervention conducted in households in two townships (Yangbajing and Gongtangxiang); setting where outcomes were measured was not reported. Aim of study: To evaluate the effect of low‐sodium, high‐potassium salt substitute in blood pressure reduction in Tibetans living at high altitudes Unit of allocation: adults (aged 18 years or older) Start date: February 2009 End date: May 2009 Relevant study limitations as reported by study authors: A single follow‐up was done and urinary potassium and sodium were not measured, due to financial constraints and difficult sampling conditions. In addition, baseline information on education, occupation, smoking and alcohol use were only collected for a subsample of participants. Salt consumption data collected did not include sodium intake from sodium naturally occurring in food and was also only done for a small subsample. The study definition of hypertension excluded isolated diastolic hypertension, limiting findings. The mean blood pressure in the control group dropped significantly, which the authors attributed to either seasonal effects or regression to the mean (which should have taken into account the 'white coat hypertension' effect). Sample size calculation: The study was powered to detect a difference of 5.0 mmHg in systolic blood pressure at 90% power using a one‐tailed test and alpha of 0.05. |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Participants 40 years or older in the Gongtangxian, Yangbajing and Gongtangxiang townships; systolic blood pressure was confirmed greater or equal to 140 mmHg regardless use of antihypertensive medications Exclusion criteria: Participants were excluded for current use of potassium supplements, previously diagnosed kidney disease or gout (or if they lived in a household with someone who does), if their physician judged that they could not take a salt substitute, or if they intended to use salt outside the assigned treatment salt. Pretreatment: None Method of recruitment of participants: Potentially eligible participants were identified in a community‐based survey; they were first invited to a hypertension screening, and those who had a systolic blood pressure ≥ 140 mmHg were invited to the trial three months later. If the person's systolic blood pressure was again confirmed as ≥ 140 mmHg they were invited to participate in the trial, regardless of their antihypertensive medication use. Informed consent obtained: Yes, unclear whether written or oral. Provided by participants and family patriarchs Clusters: n/a Subgroups planned/measured: NR Subgroups reported: NR Participant flow Assessed for eligibility: n = 287 Excluded (number with reasons): n = 5 (n = 1 too old and weak to travel, n = 1 withdrew after informed consent, n = 3 excluded due to living too far thereby making follow‐up too difficult) Randomised: n = 282 Allocated to LSSS intervention(s): n = 141 Allocated to control: n = 141 Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 42 (n = 14 lost to follow‐up, n = 1 fatal cerebral haemorrhage, n = 27 discontinued intervention) Discontinued intervention (LSSS intervention group): n = 27 (n = 25 use study salt and local salt alternately, n = 1 abdominal distension, n = 1 stomach ache) Analysed (LSSS intervention group): n = 99 per protocol; n = 141 intention‐to‐treat Excluded from analysis (LSSS intervention group): n = 42 (n = 14 lost to follow‐up, n = 1 fatal cerebral haemorrhage, n = 27 discontinued intervention) Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 27 (n = 17 lost to follow‐up, n = 1 fatal cerebral haemorrhage, n = 1 fatal liver cancer, n = 8 discontinued intervention) Discontinued intervention (control group): n = 8 (n = 7 use study salt and local salt alternately, n = 1 complained of poor taste) Analysed (control group): n = 114 per protocol; n = 141 intention‐to‐treat Excluded from analysis (control group): n = 27 (n = 17 lost to follow‐up, n = 1 fatal cerebral haemorrhage, n = 1 fatal liver cancer, n = 8 discontinued intervention) |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Outcomes Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: The study was funded by the Bureau of Science and Technology of Tibet Autonomous Region Grant; the United States National Heart, Lung, and Blood Institute (National Institutes of Health); the United Health Group Chronic Disease Initiative; and the People's Republic of China Ministry of Science and Technology. China Salt Substitute Study in Tibet (CSSS‐Tibet) Authors name: Xingshan Zhao and Yangfeng Wu Institution: Department of Cardiology, Beijing Jishuitan Hospital, Peking University (X. Zhao); The George Institute for Global Health at Peking University Health Science Center and Department of Epidemiology and Biostatistics, Peking University (Y. Wu) Email: ywu@georgeinstitute.org.cn Possible conflicts of interest (for study authors): "There [are] no competing interests there and the funding does not alter the authors' adherence to PLOS One policies on sharing data and materials." Sources used for data extraction: Journal article with results of the trial, trial protocol published with journal article, non‐commercial trial registry record Trial registration details: NCT01429246 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator and computer‐generated randomisation list was used to assign participants following stratification by township, gender and dichotomised baseline SBP. |
| Allocation concealment (selection bias) | Low risk | A random number generator provided a treatment allocation identification number to each patient, and assignment was secured in a password‐protected encrypted digital registry. Salt was provided in indistinguishable containers with only the study allocation number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only participants were blinded to treatment allocation; the registry also reported that this was a single‐blinded trial. Intervention and control salts and containers were indistinguishable in appearance, and 70 to 80% of taste testers could not tell the difference between the two. The authors reported successful participant blinding as one control participant left the study due to 'poor taste' of the salt. Outcomes were objective in nature and it was unlikely that a lack of personnel blinding would have resulted in performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not blinded, but blood pressure and mortality outcomes are highly objective and not prone to detection bias. In the case of medication use and adverse events, participants were the outcome assessors; and they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was high, with 42/141 (30%) in the intervention and 27/141 (19%) in the control group. Of these, 27/141 (19%) intervention and 8/141 (6%) control participants discontinued use of the salts. Intention‐to‐treat analyses using multiple imputation were performed for the primary outcomes; with no change in nominal significance for these variables. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol available (published concurrently with journal article) confirmed three‐month follow‐up, and indicated that six‐month follow‐up (with minor alterations to intervention) was intended. Intended outcomes were not explicitly reported. Trial was registered retrospectively. |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Zhou 2009.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Country: China Setting: Intervention conducted in rural households in the Hedong district, Tianjin; outcomes measured at participants' homes Aim of study: To evaluate the safety and efficacy of Compound Ion Salt on blood pressure control in an aged population with high dietary salt intake Unit of allocation: adults (aged 18 years or older) Start date: September 2003 End date: May 2004 Relevant study limitations as reported by study authors: The single‐blinded design of the study may have resulted in systematic bias in blood pressure measurement; only aged people were included in the study due to their regularised work‐rest schedule and them taking most meals at home, thereby limiting generalisability; quantitative determination of salty flavour perception was not done, only flavour tests in a small population which found no difference in perceived taste between the intervention and control. Sample size calculation: The sample size was calculated to detect a ≥ 8 mmHg difference in systolic blood pressure with 80% power and significance of 5% using a two‐tailed test. |
|
| Participants |
Baseline Characteristics LSSS intervention (participants with normal blood pressure)
Control (participants with normal blood pressure)
LSSS intervention (participants with hypertension)
Control (participants with hypertension)
Inclusion criteria: Men and women, aged between 50 and 80, willing to undertake long‐term use of the intervention salt; with serum potassium < 5.5 mmol/L, and net increase of < 1.0 mmol/L for serum potassium at the end of the run‐in period Exclusion criteria: Participants who ate more than one meal per week outside their homes; currently used potassium‐sparing medication/diuretics; already had a family member enrolled in the study; had a history of heart attack or stroke in the past six months, current angina pectoris, or congestive heart failure; had diabetes mellitus, serious mental or physical illnesses, malignancy, or secondary hypertension; or had impaired renal function Pretreatment: None Method of recruitment of participants: Han people from ten communities in the Hedong District, Tianjin were recruited. Informed consent obtained: Yes, unclear whether written or oral Clusters: n/a Subgroups planned/measured: Participants with normal blood pressure and participants with hypertension Subgroups reported: Participants with normal blood pressure and participants with hypertension Participant flow Assessed for eligibility: n = 264 Excluded (number with reasons): n = 16 (met exclusion criteria, < 5.5 mmol/L serum potassium or net change of <1.0 mmol/L serum potassium following run‐in) Randomised: n = 248 Allocated to LSSS intervention(s): n = 119 (n = 62 participants with hypertension; n = 57 participants with normal blood pressure) Allocated to control: n = 129 (n = 64 participants with hypertension; n = 65 participants with normal blood pressure) Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 13 (n = 10 (n = 8 participants with hypertension; n = 2 participants with normal blood pressure) withdrew; n = 3 participants with normal blood pressure lost to contact) Discontinued intervention (LSSS intervention group): n = 10 (n = 8 participants with hypertension; n = 2 participants with normal blood pressure) Analysed (LSSS intervention group): n = 119 (intention‐to‐treat with baseline values carried forward); n = 62 (participants with hypertension) n = 57 (participants with normal blood pressure) Excluded from analysis (LSSS intervention group): n = 0 Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 9 (n = 8 (n = 6 participants with hypertension; n = 2 participants with normal blood pressure) withdrew; n = 1 participants with normal blood pressure lost to contact) Discontinued intervention (control group): n = 8 (n = 6 participants with hypertension; n = 2 participants with normal blood pressure) Analysed (control group): n = 129 (intention‐to‐treat with baseline values carried forward) Excluded from analysis (control group): n = 0 |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: NR Authors name: Xin Zhou and Yu‐Ming Li Institution: Institute of Cardiovascular Disease and Division of Cardiology, Pingjin Hospital, Medical College of the Chinese People's Armed Police Forces, Tianjin Email: cardiolab@gmail.com Possible conflicts of interest (for study authors): None Sources used for data extraction: Journal article of meeting report, with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence numbers were placed in sealed envelopes. Intervention and control bags were manufactured by a supplier and only identified with the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to assignment; no information on whether people delivering the intervention were blinded. Given that participants were blinded and minimal interventionist involvement was reported, the study is not likely to be prone to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not blinded. Outcomes which may be prone to detection bias, e.g. auscultatory determination of blood pressure, were done by two experienced physicians. Other outcomes were not prone to detection bias as they were laboratory‐determined, or self‐reported by blinded participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 22/248 (9%), with 13/248 (5%) in the intervention and 9/248 (4%) in the control group; and similar reasons for dropouts reported per group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or prospectively trial registry entry available |
| Other bias | Low risk | None identified |
| Overall risk of bias | Low risk | Low risk of bias for allocation concealment and incomplete outcome data |
Zhou 2013.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised controlled trial Country: China Setting: Intervention conducted in families from five villages in Liaoning, rural North China; setting where outcomes were measured not reported, but presumably at participants' homes Aim of study: To investigate the effect of the long‐term use of low‐sodium salt substitutes, to reduce dietary sodium intake, on the blood pressure of people with normal blood pressure and people who are moderately hypertensive Unit of allocation: Families Start date: NR End date: NR Relevant study limitations as reported by study authors: Sample sizes were too small to adequately explore the effect of LSSS on blood pressure in various age subgroups; variations in blood pressure were observed throughout the year, which the authors attributed to seasonal changes; baseline blood pressure was higher in the intervention group than in the control group; first morning urine instead of 24‐hour urine samples were collected at 24‐month follow‐up as a previous study reported good safety, and no significant difference in urinary electrolytes at this time point should be interpreted with caution. Sample size calculation: NR |
|
| Participants |
Baseline Characteristics LSSS intervention
Control
Inclusion criteria: Families: At least one family member is a patient with hypertension, with systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg and with an estimated daily sodium intake of ≥ 260 mmol/day Participants: Aged at least 18 years Exclusion criteria: Families: Member of the family with hypertension moving away during the study period. Participants: Family members with significant renal impairment or other indication for using potassium‐sparing medication, normotensive family members who move away during the study period Pretreatment: Participants in the LSSS intervention group had higher alcohol consumption (P < 0.01) and were more likely to have a family history of hypertension (P < 0.01) compared to those in the control group. Method of recruitment of participants: NR Informed consent obtained: yes (written) Clusters: n = 200 clusters with n = 462 participants; average cluster size (number of family members) not reported; no adjustment for clustering Subgroups planned/measured: Age and gender Subgroups reported: Age (<= 40 years vs. 40 to 50 years vs. 50 to 60 years vs. 60 to 70 years vs. > 70 years) and gender Participant flow Assessed for eligibility: n = 223 clusters with n = 543 participants Excluded (number with reasons): n = 23 clusters with n = 81 participants (n = 3 clusters with n = 12 people refused to participate; n = 20 clusters with n = 69 people did not meet inclusion criteria; with n = 1 cluster having member(s) using potassium‐sparing medication, n = 7 clusters having members with renal disease and n = 12 clusters not resident) Randomised: n = 200 clusters with n = 462 participants Allocated to LSSS intervention(s): n = 100 clusters with n = 224 participants Allocated to control: n = 100 clusters with n = 238 participants Received allocated LSSS intervention(s): NR Did not receive allocated LSSS intervention(s): NR Lost to follow‐up (LSSS intervention group): n = 11 clusters with n = 41 participants (n = 7 died, n = 15 lost contact, n = 10 reluctant to follow up, and n = 9 withdrew; distribution by cluster not reported) at 2 years. At 3 years: n = 0 (n = 183 individuals completed follow‐up) Discontinued intervention (LSSS intervention group): At 2 years: n = 9 withdrew Analysed (LSSS intervention group): At 2 years: n = 224 participants (ITT analysis; ITT method not described) Excluded from analysis (LSSS intervention group): n = 0 participants Received allocated control: NR Did not receive allocated control: NR Lost to follow‐up (control group): n = 7 clusters with n = 49 participants (n = 8 died, n = 23 lost contact, and n = 18 withdrew; distribution by cluster not reported). At 3 years: n = 1 (n = 188 individuals completed follow‐up) Discontinued intervention (control group): At 2 years: n = 18 withdrew Analysed (control group): At 2 years: n = 238 participants (ITT analysis; ITT method not described) Excluded from analysis (control group): n = 0 participants |
|
| Interventions |
Intervention Characteristics LSSS intervention
Control
|
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Funding source: Programme of the National Natural Science Foundation of China Authors name: Bo Zhou and Jing‐Pu Shi Institution: Department Cardiovascular of Clinical Epidemiology, The First Hospital of China Medical University and Institute of China Medical University, China Medical University, Shenyang, China Email: sjp56@sina.com; sjp562013@163.com Possible conflicts of interest (for study authors): "The authors declare no conflict of interest." Sources used for data extraction: Journal articles with results of the trial Trial registration details: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were randomised based on a computer‐generated randomisation scheme. |
| Allocation concealment (selection bias) | Unclear risk | Intervention and control salts were packaged in identical bags and were only identified by a three‐digit code; they were packaged and labelled by an external salt manufacturing company, but insufficient information on how the randomisation sequence was protected |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to treatment allocation ‐ LSSS and control salt packaged in identical bags with three‐digit codes (unlocked at the end of the study). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear whether outcome assessors were blinded to allocation, but the clinical and mortality outcomes of the study were objective and unlikely to be influenced by a lack of blinding. Blood pressure was measured with an automatic sphygmomanometer. Any self‐reported outcomes were assessed by participants ‐ not clear whether they were still blinded at 36 months. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was high in both groups at 24‐month follow‐up: LSSS intervention group 18.3% (41/22) vs. control group 20.6% (49/238). However, the study authors reported minimal attrition at the 36‐month follow‐up (LSSS intervention group 18.3% (41/224) vs. control group 20.6% (50/238)). Blood pressure outcomes were assessed using an intention‐to‐treat approach with mixed linear models; it was not clear what the ITT approach was for other outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol or prospective trial registry entry not available |
| Other bias | Low risk | None identified |
| Recruitment bias (cluster‐RCTs) | Unclear risk | It was not clear whether all the individual participants were identified before the randomisation of the families. |
| Comparability with individually randomised trials (cluster‐RCTs) | Low risk | The China Salt Substitute Study also found no change in DBP during a one‐year follow‐up; another RCT by Little 2004 found no decrease in SBP and DBP in persons randomised to LSSS and lifestyle interventions at six months follow‐up; the authors report interpreted these findings as being reflected by DBP only changing after 18 months in the present study. |
| Loss of clusters (cluster‐RCTs) | Unclear risk | Loss of clusters (families) was similar for both groups (LSSS intervention group 11/100; control 7/100). Although the study authors stated that the main reason for the loss of clusters was that participants withdrew from the study, they did not comment on any differences in baseline characteristics of families who were lost to follow‐up, compared to those who remained in the study. |
| Baseline imbalance (cluster‐RCTs) | Low risk | Baseline imbalances were found for family history of hypertension and alcohol consumption, both of which were significantly higher in the intervention group (P = 0.007 and P = 0.012, respectively). Analyses were adjusted for these covariates and were reported as showing that these imbalances were due to chance. |
| Incorrect analysis (cluster‐RCTs) | High risk | Adjustment to account for the effect of clustering was not reported in the statistical analysis or results section. |
| Overall risk of bias | Unclear risk | Low risk of bias for incomplete outcome data and baseline imbalance; unclear risk of bias for loss of clusters and recruitment bias |
ACE: angiotensin‐converting enzyme AE: adverse event Alx: aortic pressure augmentation index AMI: acute myocardial infarction ARB: angiotensin receptor blocker AUG: aortic pressure augmentation BMI: body mass index BP: blood pressure Ca: calcium CDP: central diastolic pressure CPP: central pulse pressure CSP: central systolic pressure CSSS: China Salt Substitute Study CVD: cardiovascular disease DBP: diastolic blood pressure DM: diabetes mellitus (e)GFR: (estimated) glomerular filtration rate FFQ: food frequency questionnaire GEE: generalised estimating equation ICC: intra‐cluster correlation coefficient ICD‐9/10: International Classification of Diseases (ninth revision/tenth revision) IQR: interquartile range ITT: intention‐to‐treat Kcal: kilocalorie K(Cl): potassium (chloride) LSSS: low‐sodium salt substitute MAPA: ambulatory monitoring of arterial pressure/ambulatory blood pressure monitoring MgSO4: magnesium sulfate mmHg: millimeters of mercury MSG: monosodium glutamate n/a: not applicable Na(CI): sodium (chloride) NR: not reported NSS: non‐salt‐sensitive PEN: Peruvian Sol pm: post meridiem RCT: randomised controlled trial RT: reflection time SBP: systolic blood pressure SD: standard deviation SS: salt‐sensitive SSaSS: Salt Substitute and Stroke Study SSiIS: Salt Substitute in India Study uACR: urinary albumin‐to‐creatinine ratio WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allaert 2015 | Wrong study design (single‐arm trial) |
| Baek 2015 | Wrong comparator (sodium intake identical between LSSS and control group) |
| Barros 2013 | Wrong study design (quasi‐randomised controlled trial) |
| Barros 2015 | Wrong study design (quasi‐randomised controlled trial) |
| ChiCTR1800016804 | Wrong type of intervention (salt reduction intervention strategy) |
| ChiCTR1800018119 | Multifactorial intervention ‐ could not isolate LSSS effect |
| ChiCTR1800019727 | Case series |
| ChiCTR2000033349 | Multifactorial intervention ‐ could not isolate LSSS effect |
| Dent 2011 | Wrong study design |
| Doorenbos 2003 | Wrong study design (case study) |
| Farrand 2019 | Wrong study design (commentary) |
| He 2020 | Wrong study design |
| Hueston 1989 | Wrong study design (case series) |
| Itoh 1997 | Wrong study design |
| Janda 2017 | Wrong study design (non‐randomised trial) |
| Jeffrey 1984 | Wrong comparator (low sodium high potassium diet) |
| JPRN‐UMIN000012560 | Wrong outcomes (sensory/organoleptic outcomes) |
| Karppanen 1984 | Wrong study design (cross‐over study but first‐phase data unavailable) |
| Katz 1999 | Wrong study design (single‐arm trial) |
| Lambert 2019 | Wrong study design (letter in response to excluded review by Farrand 2019) |
| Little 2004 | Multifactorial intervention ‐ could not isolate LSSS effect |
| Maleki 2016 | Wrong outcomes (sensory/organoleptic outcomes) |
| Maruya 2020 | Wrong outcomes (sensory/organoleptic outcomes) |
| Matlou 1986 | Wrong type of intervention (potassium chloride salt administered as a supplement; not instead of sodium chloride) |
| Mu 2020 | Wrong study design (single‐arm trial) |
| Nakano 2016 | Wrong type of intervention (salt‐restriction education) |
| NCT02105727 | Wrong study design (single‐arm trial) (NCT02105727) |
| Pietinen 1981 | Wrong study design (non‐randomised trial) |
| Robare 2010 | Wrong type of intervention (dietary sodium reduction intervention) |
| Salvetti 1988 | Wrong comparator (metoprolol treatment) |
| Sciarrone 1992 | Wrong type of intervention (low‐sodium low‐fat high fibre diet) |
| Zoccali 1985 | Wrong comparator (lactose placebo) |
LSSS: low‐sodium salt substitute
Characteristics of studies awaiting classification [ordered by study ID]
Jones 2019.
| Methods | Design: Proposed RCT Country: USA Setting: NR |
| Participants | Inclusion criteria: age > 50 years with at least one of the following: presence of clinical or subclinical cardiovascular disease (pooled Cohort Equation Risk Score for 10‐year cardiovascular disease risk > 15%) or chronic kidney disease (defined as eGFR 20–59 mL/min/1.73 m2) or age > 70 years Exclusion criteria: NR |
| Interventions | Combination intervention of diet education, primarily focussed on increased dietary potassium, and the use of a potassium‐based salt substitute for food preparation Comparator: Traditional diet |
| Outcomes | Acute coronary syndrome (ACS), stroke, congestive heart failure (CHF), cardiovascular (CV) death, and a 15% change in eGFR |
| Notes |
Neutel 1996.
| Methods | Design: Double‐blind, placebo‐controlled clinical study Country: NR Setting: NR |
| Participants | Inclusion criteria: Patients with mild to moderate hypertension Exclusion criteria: NR |
| Interventions | Intervention: Reduced sodium salt containing potassium and magnesium Comparator: Regular salt |
| Outcomes | Lowering blood pressure |
| Notes |
Voloshyna 2017.
| Methods | Design: RCT Country: Ukraine Setting: NR |
| Participants | Inclusion criteria: Symptomatic patients with chronic heart failure aged 66 to 80 years on stable treatment with three or more drugs and baseline serum potassium levels 3.9 to 4.7 mmol/L Exclusion criteria: NR |
| Interventions | Intervention: 5 to 6 g/day of potassium‐enriched salt (30% potassium; 5% magnesium) Comparator: Moderately salt‐restricted diet (5 to 6 g salt/day) |
| Outcomes | Physical function assessment, quality of life, health status, daily activity |
| Notes |
ACS: acute coronary syndrome CHF: congestive heart failure CV: cardiovascular eGFR: estimated glomerular filtration rate NR: not reported RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619000352101.
| Study name | Salt ALTernatives Study (SALTS): a smartphone app and dietary alternative salt to lower blood pressure for adults with high blood pressure |
| Methods | Design: RCT Country: New Zealand Setting: Household and supermarkets, Auckland |
| Participants | Inclusion criteria: >= 18 years or older who have a SBP >= 140 mmHg and/or DBP >= 85 mmHg Exclusion criteria: Severe kidney disease; use of a potassium‐sparing diuretic, furosemide, NSAIDs, prednisone; history of a stroke or cardiovascular event (hospitalisation for heart attack, coronary artery revascularisation (CABG or stenting), stroke or heart failure) in the previous 6 months; diagnosed heart failure |
| Interventions | Intervention: Consists of two components: (1) a smartphone application (app) called SaltSwitch (helps users to select lower salt packaged foods by enabling them to scan the barcode of a packaged food and receive an immediate, interpretive traffic light nutrition label on‐screen plus suggestions for lower salt alternatives), and (2) a dietary alternative salt (74.5% potassium chloride and 24.5% sodium chloride and ~1% silicon dioxide) Comparator: Generic information about heart‐healthy eating from the Heart Foundation of New Zealand |
| Outcomes | Primary: SBP (week 12) Secondary: SBP (week 6); urinary sodium; sodium content of packaged food purchases; BP control (135/85 mmHg); use of SaltSwitch app; use of dietary alternative salt |
| Starting date | May 2019 |
| Contact information | Contact person(s): Helen Eyles Email: h.eyles@auckland.ac.nz |
| Notes | Sponsors and Collaborators: Health Research Council of New Zealand |
ChiCTR08000296.
| Study name | China Salt trial |
| Methods | Design: Cluster‐randomised trial Country: China Setting: Families/households, Shandong province |
| Participants | Inclusion criteria: families with adults > 18 years with treated or untreated hypertension who has an estimated salt intake > 200 mmol/d with 50% of the salt added in the cooking or at the table (estimated through an interview with the participant) Exclusion criteria: Chronic renal disease with renal impairment, e.g. nephritis, nephrotic syndrome, nephrolithiasis, renal cysts; plasma creatinine > 1.7 mg/dL or eGFR < 40 mL/min; plasma K > 4.5 mmol/L at start or > 5.0 mmol/L at end of 1 month run‐in; use of potassium retaining drugs or NSAIDs; malignancy during the past 5 years; any other serious life‐threatening illness that required regular medical treatment; psychiatric disease; pregnancy or intent to become pregnant during the study |
| Interventions | Intervention: Supply of table salt containing equal amounts by weight of NaCl and KCl to be used in cooking and at the table or with education materials and with appropriate items to reduce their NaCl intake to 6 g NaCl/day Comparator: Supply of normal salt from market to be used in cooking and at the table |
| Outcomes | Blood pressure, urine Na and K concentration, microalbuminuria, plasma renin activity, plasma aldosterone |
| Starting date | May 2009 |
| Contact information | Contact person(s): Jichun Chen; Lisheng Liu, Dongfeng Gu Email: jcch70@sina.com; lshypt@yahoo.com.cn |
| Notes | Sponsors and Collaborators: Fuwai Hospital |
ChiCTR09000538.
| Study name | The study on pathogeny of hypertension and the salt intervention on diet in the population of Zhangwu County |
| Methods | Design: RCT Country: China Setting: Families or households |
| Participants | Inclusion criteria: Families with at least one adult (aged 18 to 70 years) with hypertension (SBP > 140 mmHg or DBP > 90 mmHg). Diagnosis of stroke, TIA and coronary heart disease made by the hospital of the county or higher level. Exclusion criteria: Liver or kidney disease |
| Interventions | Interventions: Salt with lower sodium (Mg 10%, K 25%, Na 65%) Comparator: Common iodine salt |
| Outcomes | Primary: Blood pressure Secondary: Salty feeling tendency, urinary sodium and potassium |
| Starting date | 01 June 2005 |
| Contact information | Contact person(s): Gang Lv, Jingpu Shi Email: sjp56@yahoo.com |
| Notes | Sponsors and Collaborators:; Science and technology projects of Liaoning Province; the First Hospital of China Medical University |
ChiCTR2000029017.
| Study name | Study on the application of low‐sodium formula salt in hypertensive patients with diabetes |
| Methods | Design: RCT Country: China Setting: Chongqing Nan'an District People's Hospital |
| Participants | Inclusion criteria: 35 to 75 years with diabetes and hypertension, currently taking medications to lower blood sugar and blood pressure (the type and dose remain unchanged for > 3 months) with baseline untreated systolic blood pressure between 130‐159 mmHg, and diastolic blood pressure between 80‐100 mmHg Exclusion criteria: Malignancy, acute myocardial infarction and acute stroke within three months or expected survival time is < 1 year; secondary hypertension (hypercortisolism or hyperaldosteronism); acute diseases; deafness, dementia and other disabilities, as well as severe depression or other mental disorders; abnormal liver function, renal dysfunction (stage 4 and 5 chronic kidney disease); serum potassium < 3.5 mmol/L or > 5.5 mmol/L; use of potassium diuretics; pregnant women, or those with other serious chronic diseases |
| Interventions | Intervention: DASH diet and 65% low‐sodium salt; DASH diet and 18% low‐sodium salt Comparator: DASH diet and common salt |
| Outcomes | Primary: Blood pressure, fasting blood glucose, 2‐hour postprandial glucose |
| Starting date | February 2020 (not yet recruiting) |
| Contact information | Contact person(s): Huakun Rao; Lihong Mu Email: 931575032@qq.com; 1097123703@qq.com |
| Notes | Sponsors and Collaborators: Chongqing Science and Technology Bureau |
IRCT2016103130572N1.
| Study name | The effects of a salt substitute on an Iranian population |
| Methods | Design: RCT Country: Iran Setting: Preventive & Health Promoting Clinic, Baharlou Hospital, Tehran |
| Participants | Inclusion criteria: 25‐65 years; SBP > 140 mmHg or DBP > 90 mmHg in three measurements in two settings or consuming an antihypertensive drug Exclusion criteria: Abnormal serum creatinine or urea; consuming medications: digoxin, lithium, K‐sparing diuretics; acute or severe cardiovascular disease or psychotic disorders |
| Interventions | Intervention: Low‐sodium salt (80 percent sodium chloride and 20 percent potassium chloride) Comparator: Regular salt (100 percent sodium chloride) |
| Outcomes | Primary: SBP; DBP; 24‐hour urinary sodium and potassium |
| Starting date | December 2016 |
| Contact information | Contact person(s): Mohammad Mohammadi Email: mohammadi.mo@razi.tums.ac.ir |
| Notes | Sponsors and Collaborators: Research Deputy of Tehran University of Medical Sciences; Shournamak Yazd Company |
NCT02016404.
| Study name | The effect of a low sodium‐high potassium salt on blood pressure in Vietnamese adults |
| Methods | Design: RCT Country: Vietnam Setting: Hai Ba Trung district, Hanoi |
| Participants | Inclusion criteria: Adults aged between 45 and 64 years with untreated prehypertension or mild hypertension (systolic BP between 130 and 160 mmHg and/or diastolic BP between 85 and 100 mmHg) based on the mean of two measurements with a 1‐week interval Exclusion criteria: Diagnosed or self‐reported cardiovascular or kidney disease |
| Interventions | Intervention: Iodised low‐sodium, high‐potassium botcanh (a traditional mixture of salt, MSG, sugar and herbs) plus and iodised low‐sodium, high‐potassium salt for home food preparation Comparator: Regular iodised high‐sodium botcanh (a traditional mixture of salt, MSG, sugar and herbs) and high‐sodium salt |
| Outcomes | Primary: SBP, DBP Secondary: 24‐hour urinary sodium, potassium, calcium, creatinine, protein, iodine; body weight; heart rate |
| Starting date | June 2013 |
| Contact information | Contact person(s): Ha TP Do Email: NR |
| Notes | Sponsors and Collaborators: Wageningen University; National Institute of Nutrition, Vietnam |
NCT02021435.
| Study name | Tibet salt reduction study |
| Methods | Design: Cluster‐randomised trial Country: Tibet Setting: Villages from Damxung and Maizhokunggar county |
| Participants |
|
| Interventions | Intervention: Salt substitute provided to households free of charge. To receive health education on salt reduction Comparator: Buy own normal salt. To receive health education on salt reduction |
| Outcomes | Primary: Blood pressure Secondary: Total mortality, cardiovascular mortality, life expectancy, serum cholesterol and random blood glucose levels (> 60 years) |
| Starting date | April 2014 |
| Contact information | Contact person(s): Xingshan Zhao Email: xingshanzh@gmail.com |
| Notes | Sponsors and Collaborators: Beijing Jishuitan Hospital; the George Institute for Global Health at PUHSC; the George Institute for Global Health, Australia; Science and Technology Department of Ningxia; People's Hospital of Tibet; People's Hospital of Maizhokunggar; Tibet University; Peking University; People's Hospital of Damxung County, Tibet |
NCT03290716.
| Study name | Diet, exercise and cardiovascular health ‐ effect of salt substitute and stepwise salt supply control in reducing blood pressure in the elderly in nursing homes in China |
| Methods | Design: Cluster‐randomised trial Country: China Setting: Nursing Homes, Jincheng, Shanxi |
| Participants |
|
| Interventions | Intervention: Salt substitute only: Replace regular salt with market available potassium‐enriched salt substitute in kitchens of nursing homes Stepwise salt supply control only: A stepwise approach to reduce salt used in the kitchen of nursing homes by controlling the supply of salt; or Salt substitute plus stepwise salt supply control: combination of both interventions Comparator: No intervention |
| Outcomes | Primary: SBP Secondary: Hyperkalaemia; hyponatremia; 24‐hour urinary sodium and potassium; DBP; cardiovascular events; total mortality; incremental cost‐effectiveness ratio |
| Starting date | 25 September 2017 |
| Contact information | Contact person(s): Yangfeng Wu Email: NR |
| Notes | Sponsors and Collaborators: Peking University; Changzhi Medical College; Xian Jiaotong University; Hohhot Center for Disease Control and Prevention; Yangcheng Ophthalmology Hospital |
BP: blood pressure CABG: coronary artery bypass graft DASH: Dietary Approaches to Stop Hypertension DBP: diastolic blood pressure eGFR: estimated glomerular filtration rate K(Cl): potassium (chloride) Mg: magnesium MSG: monosodium glutamate Na(Cl): sodium (chloride) NSAID: non‐steroidal anti‐inflammatory drug RCT: randomised controlled trial SBP: systolic blood pressure TIA: transient ischaemic attack
Differences between protocol and review
Populations at risk of hyperkalaemia: The PICO was initially conceptualised into six comparisons by the WHO NUGAG Subgroup on Diet and Health, such that effects of LSSS in populations at possible risk of hyperkalaemia would be explored by stratifying the data into three comparisons in each of the subpopulations, namely adults, children, pregnant women possibly at risk of hyperkalaemia. During the guideline development process, the WHO NUGAG requested that effects of LSSS in populations at possible risk of hyperkalaemia rather be explored by subgroup analysis instead of by this stratification, and only for the safety outcomes (change in blood potassium, hyperkalaemia, hypokalaemia, adverse events, renal function and hyponatraemia).
The WHO NUGAG decided to exclude the effectiveness outcomes (change in DBP, change in SBP, hypertension, blood pressure control, cardiovascular events, cardiovascular mortality, all‐cause mortality, antihypertensive medication use, change in fasting blood glucose, change in blood triglycerides, change in total blood cholesterol, change in 24‐h urinary sodium and potassium excretion as well as diagnosis of diabetes mellitus and change in BMI (for adults) and growth changes, bone densitometry and bone health (for children)) from this subgrouping, since there are no clinical justifications to expect differences in effectiveness outcomes in the ‘at risk’ populations, and their ‘at risk status’ is related specifically to the safety outcomes, which are primarily linked to potassium metabolism. The sodium in most of the low‐sodium salt substitutes is partially replaced by potassium resulting in an increase in potassium intake with their use.
Change to original title: We removed 'renal health' from the original title to ensure the title more accurately reflected the focus of the primary cardiovascular outcomes of the review, since renal outcomes were only included as secondary outcomes.
Screening: The protocol stated that 'an initial electronic title screen using keywords to remove records that are obviously irrelevant' would be conducted. We replaced this with a full duplicate and independent screening process of all records yielded by the searches.
Measures of treatment effect: The search update in August 2022 resulted in the inclusion of a trial reporting event rates. In addition, a stepped‐wedge trial reporting hazard ratios for incident hypertension was included in the review. The analytical approaches to meta‐analysis of these data were added to the Methods section.
Additional outcomes: Two additional outcomes (change in 24‐hour urinary sodium and potassium excretion) were added by WHO NUGAG following the guideline development process, and were consequently incorporated into the review. A third outcome in children (all‐cause mortality) was erroneously omitted from the protocol and was included in the review.
Subgroups: During the guideline development process, it was decided that the subgroups total potassium intake and total sodium intake were not important to include, and were therefore excluded. The subgroup listed as duration of intervention in the protocol is included in the review as duration of study.
Sensitivity analyses: The protocol stated that 'We will also consider other potential sources of heterogeneity, such as methodological sources (using sensitivity analysis)', but did not specify the exact sensitivity analyses that were planned. Sensitivity analyses investigating the effect of excluding trials at high risk of bias and excluding trials with clusters as the unit of allocation on primary outcomes were reported in the methods conducted in the review.
Contributions of authors
The protocol for the review was drafted by CN, AB, MV and AS, in line with the PICO question developed by the World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health. The protocol was approved by WHO, and the review was prospectively registered on the international prospective register of systematic reviews (PROSPERO 2020 CRD42020180162).
AB and CN drafted the review and MV and AS provided inputs to finalise the review. All authors approved the final manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
World Health Organization, Other
The World Health Organization (WHO) provided funding to Stellenbosch University towards the cost of carrying out this systematic review.
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
-
Research, Evidence and Development Initiative (READ‐It), UK
READ‐It (project number 300342‐104) is funded by UK aid from the UK government.
Declarations of interest
AB: partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies; partial support paid to my institution for a scoping review on total fat intake and health outcomes other than measures of unhealthy weight gain (2020); a systematic review on low sodium salt substitutes and cardiovascular health (2020‐2021); rapid scoping reviews on coconut and palm oil intake and cardiovascular health (2021); a scoping review on the health effects of tropical oil consumption (2022).
MV: partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies; partial support paid to my institution for a scoping review on total fat intake and health outcomes other than measures of unhealthy weight gain (2020); a systematic review on low sodium salt substitutes and cardiovascular health (2020‐2021); rapid scoping reviews on coconut and palm oil intake and cardiovascular health (2021); a scoping review on health effects of tropical oil consumption (2022).
AS: partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies.
CN: partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies; partial support paid to my institution for a scoping review on total fat intake and health outcomes other than measures of unhealthy weight gain; a systematic review on low sodium salt substitutes and cardiovascular health; rapid scoping reviews on coconut and palm oil intake and cardiovascular health; a scoping review on the health effects of tropical oil consumption.
*CN is Co‐director of Cochrane Nutrition, and AB and MV are members of the Cochrane Nutrition local coordination team. These authors had no involvement in the editorial process for this review.
New
References
References to studies included in this review
Allaert 2013 {published data only}
- Allaert FA. Double-blind, randomized, crossover, controlled clinical trial of NaCl + Chitosan 3% versus NaCl on mild or moderate high blood pressure during the diet and lifestyle improvement period before possible prescription of an antihypertensive treatment. International Angiology 2013;32(1):94-101. [PubMed] [Google Scholar]
Allaert 2017 {published data only}
- Allaert FA. Double blind, randomized, controlled clinical trial of NaCl + Chitosan 3% vs. NaCl on the decrease of blood pressure induced by a low salt diet in healthy prehypertensive volunteers. Archives of Cardiovascular Diseases Supplements 2019;11(Suppl.3):e372. [DOI: 10.1016/j.acvdsp.2019.05.098] [DOI] [Google Scholar]
- Allaert FA. Effect of NaCl + Chitosan 3% vs. NaCl on high blood pressure parameters of healthy volunteers with prehypertension. Minerva Cardioangiologica 2017;65(6):563-76. [DOI: 10.23736/S0026-4725.17.04451-6] [DOI] [PubMed] [Google Scholar]
Arzilli 1986 {published data only}
- Arzilli F, Taddei S, Graziadei L, Bichisao E, Giovannetti R, Salvetti A. Potassium-rich and sodium-poor salt reduces blood pressure in hospitalized patients. Journal of Hypertension 1986;4(5 Suppl 1):S347-50. [PubMed] [Google Scholar]
Bernabe‐Ortiz 2014 {published data only}
- Bernabe-Ortiz A, Diez-Canseco F, Gilman RH, Cárdenas MK, Sacksteder KA, Miranda JJ. Launching a salt substitute to reduce blood pressure at the population level: a cluster randomized stepped wedge trial in Peru. Trials 2014;15:93. [DOI: 10.1186/1745-6215-15-93] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe-Ortiz A, Sal y Rosas VG, Ponce-Lucero V, Cárdenas MK, Carrillo-Larco RM, Diez-Canseco F, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nature Medicine 2020;26:374-8. [DOI: 10.1038/s41591-020-0754-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01960972. Launching a salt substitute to reduce blood pressure at the population level in Peru. clinicaltrials.gov/ct2/show/NCT01960972 (first received 11 October 2013).
Chang 2006 {published data only}
- Chang H-Y, Hu Y-W, Yue C-S, Wen Y-W, Yeh W-T, Hsu L-S, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. American Journal of Clinical Nutrition 2006;83(6):1289-96. [DOI: 10.1093/ajcn/83.6.1289] [DOI] [PubMed] [Google Scholar]
- Chang H-Y, Pan W-H. Reply to CK Chow. American Journal of Clinical Nutrition 2006;84(6):1553-4. [DOI: 10.1093/ajcn/84.6.1553] [DOI] [PubMed] [Google Scholar]
- Chow CK. Does potassium-enriched salt or sodium reduction reduce cardiovascular mortality and medical expenses? American Journal of Clinical Nutrition 2006;84(6):1552-3. [DOI: 10.1093/ajcn/84.6.1552] [DOI] [PubMed] [Google Scholar]
CSSS Collaborative Group 2007 {published data only}
- China Salt Substitute Study Collaborative Group. Salt substitution: a low-cost strategy for blood pressure control among rural Chinese. A randomized, controlled trial. Journal of Hypertension 2007;25(10):2011-8. [DOI: 10.1097/HJH.0b013e3282b9714b] [DOI] [PubMed] [Google Scholar]
- He FJ, MacGregor GA. Can a low-sodium, high-potassium salt substitute reduce blood pressure in rural Chinese people? Nature Reviews Cardiology 2008;5(4):186-7. [DOI: 10.1038/ncpcardio1122] [DOI] [PubMed] [Google Scholar]
- Hu J, Jiang X, Li N, Yu X, Perkovic V, Chen B, et al. Effects of salt substitute on pulse wave analysis among individuals at high cardiovascular risk in rural China: a randomized controlled trial. Hypertension Research 2009;32(4):282-8. [DOI: 10.1038/hr.2009.7] [DOI] [PubMed] [Google Scholar]
- Jiang X, Hu J, Li N, Wu Y. Effects of a reduced sodium increased potassium salt substitute on periphral and central blood pressure among high risk individuals in rural China: a randomised controlled trial. Journal of Hypertension 2009;27 Suppl 4:S276. [Google Scholar]
- Li N, Prescott J, Wu Y, Barzi F, Yu X, Zhao L, et al, China Salt Substitute Study Collaborative Group. The effects of a reduced-sodium, high-potassium salt substitute on food taste and acceptability in rural northern China. British Journal of Nutrition 2009;101(7):1088-93. [DOI: 10.1017/S0007114508042360] [DOI] [PubMed] [Google Scholar]
- Li NY, Wu Y, Barzi F, Yu X, Zhao L, Neal BC. A low sodium, high potassium salt substitute substantially lowers blood pressure levels among high-risk individuals in rural northern China – the China Salt Substitute Study. Journal of Hypertension. Supplement: Official Journal of the International Society of Hypertension 2006;24(4):S150-1. [Google Scholar]
- NCT00145756. China Salt Substitute Study [China Salt Substitute Study - a randomised trial to determine the long-term effects of a low sodium, high potassium salt substitute on blood pressure among high-risk individuals in Northern China]. clinicaltrials.gov/ct2/show/NCT00145756 (first received 5 September 2005).
- Wang H, Yang Y, Shi J, Li H, Zhou B, Fu L. A randomized double controlled trial: the effect of salt substitution on blood pressure for hypertension patients among rural people in north of China. Zhongguo Shiyong Neike Za Zhi [Chinese Journal of Practical Internal Medicine] 2010;30(6):552-3. [Google Scholar]
Geleijnse 1994 {published data only}
- Geleijnse JM, Witteman JC, Bak AA, Den Breeijen JH, Grobbee DE. Long-term moderate sodium restriction does not adversely affect the serum HDL/total cholesterol ratio. Journal of Human Hypertension 1995;9(12):975-9. [PubMed] [Google Scholar]
- Geleijnse JM, Witteman JC, Bak AA, Den Breeijen JH, Grobbee DE. Reducing blood pressure by use of a low sodium high potassium, high magnesium mineral salt in older persons with mild to moderate hypertension [Verlaging van de bloeddruk door gebruik van een mineraalzout met een verlaagd natriumgehalte en een verhoogd kalium- en magnesiumgehalte bij ouderen met milde tot matige hypertensie]. Nederlands Tijdschrift voor Geneeskunde 1995;139:512-8. [Google Scholar]
- Geleijnse JM, Witteman JC, Bak AA, Den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ (Clinical research ed.) 1994;309(6952):436-40. [DOI: 10.1136/bmj.309.6952.436.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilleran 1996 {published data only}
- Gilleran G, O'Leary M, Bartlett WA, Vinall H, Jones AF, Dodson PM. Effects of dietary sodium substitution with potassium and magnesium in hypertensive type II diabetics: a randomised blind controlled parallel study. Journal of Human Hypertension 1996;10(8):517-21. [PubMed] [Google Scholar]
Hu 2018 {published and unpublished data}
- Hu J, Zhao L, Thompson B, Zhang Y, Wu Y. Effects of salt substitute on home blood pressure differs according to age and degree of blood pressure in hypertensive patients and their families. Clinical and Experimental Hypertension 2018;40(7):664-72. [DOI: 10.1080/10641963.2018.1425415] [DOI] [PubMed] [Google Scholar]
- Hu J-H, Zhao L-C, Li X, Wu Y-F. Effects of salt substitution on blood pressure using home measurements in essential hypertensive patients: a double-blinded, randomized controlled trial. Chinese Journal of Hypertension 2014;22(1):42-6. [Google Scholar]
- Visser ME. Low sodium salt Substitutes (LSSS) systematic review: request for outcome data [personal communication]. Email to: J Hu 10 July 2020.
Kawasaki 1998 {published data only}
- Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium-reduced, potassium- and magnesium-enriched mineral salt in subjects with mild essential hypertension. Hypertension Research 1998;21(4):235-43. [DOI: 10.1291/hypres.21.235] [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li Z, Feng X, Wu T, Yan L, Elliott P, Wu Y. Randomised trial on effect of involving media reporters in salt reduction programme to increase media reports and the public's knowledge, belief and behaviors on salt and health: Changzhi reporters trial. PLoS One 2021;16(7):e0252989. [DOI: 10.1371/journal.pone.0252989] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yang H, Wu Y, Elliott P, Du S, X Feng, et al. Relationship of the dietary sodium substitution with low sodium and high potassium salt with the blood pressure among middle aged and elderly people: a randomized controlled study [低钠富钾替代盐与中老年人群血压关系的 随机对照研究]. Chinese Journal of Geriatrics 2014;33(4):365-7. [Google Scholar]
Li 2016 {published data only}
- Chu H, Zhang J, Fetters MD, Niu W, Li H, Li N, et al. A mixed methods process evaluation of a clustered-randomized controlled trial to determine the effects of community-based dietary sodium reduction in rural China. Frontiers in Medicine 2021;8:646576. [DOI: 10.3389/fmed.2021.646576] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine M, Li N, Ninomiya T, Feng X, Zhang J, Shi J, et al. Dietary sodium reduction reduced albuminuria in 1,903 rural Chinese: a cluster randomised trial. Nephrology 2014;19 Suppl 4:28. [Google Scholar]
- Jardine M, Li N, Ninomiya T, Feng X, Zhang J, Shi J, et al. Dietary sodium reduction reduces albuminuria: a cluster randomized trial. Journal of Renal Nutrition 2019;29(4):276-84. [DOI: 10.1053/j.jrn.2018.10.009] [DOI] [PubMed] [Google Scholar]
- Li N, Yan L, Niu W, Yao C, Feng X, Zhang J, et al. China Rural Health Initiative - Sodium Reduction Study: the effects of a community-based sodium reduction program on 24hr urinary sodium and blood pressure in rural China. Circulation 2013;128(24):2707. [Google Scholar]
- Li N, Yan LL, Niu W, Labarthe D, Feng X, Shi J. A large-scale cluster randomized trial to determine the effects of community-based dietary sodium reduction - the China Rural Health Initiative Sodium Reduction Study. American Heart Journal 2013;166(5):815-22. [DOI: 10.1016/j.ahj.2013.07.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yan LL, Niu W, Yao C, Feng X, Zhang J, et al. The effects of a community-based sodium reduction program in rural China – a cluster-randomized trial. PLOS One 2016;11(12):e0166620. [DOI: 10.1371/journal.pone.0166620] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01259700. High cardiovascular risk management and salt reduction in rural villages in China [China Rural Health Initiative: high cardiovascular risk management and salt reduction in rural villages in China - two parallel large cluster randomized controlled trials]. clinicaltrials.gov/ct2/show/NCT01259700 (first received 14 December 2010).
- Wang X, Li X, Vaartjes I, Neal B, Bots ML, Hoes AW, et al. Does education level affect the efficacy of a community based salt reduction program? - a post-hoc analysis of the China Rural Health Initiative Sodium Reduction Study (CRHI-SRS). BMC Public Health 2016;16(1):759. [DOI: 10.1186/s12889-016-3454-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LL, Li N, Niu W, Chu H, Zhang J, Hao Z, et al. China Rural Health Initiative - Sodium Reduction Study: a cluster randomized trial on a community-based dietary sodium reduction program. Annals of Nutrition and Metabolism 2013;63 Suppl 1:48. [Google Scholar]
Mu 2003 {published data only}
- Mu J-J, Liu Z-Q, Yang J, Liang Y-M, Zhy D-J, Wang Y-X, et al. Long term observation in effects of potassium and calcium supplementation on arterial blood pressure and sodium metabolism in adolescents with higher blood pressure. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2003;37(2):90-2. [PubMed] [Google Scholar]
Mu 2009 {published data only}
- Mu J, Liu Z, Liu F, Xu X, Liang Y, Zhu D. Family-based randomized trial to detect effects on blood pressure of a salt substitute containing potassium and calcium in hypertensive adolescents. American Journal of Hypertension 2009;22(9):943-7. [DOI: 10.1038/ajh.2009.136] [DOI] [PubMed] [Google Scholar]
Neal 2021 {published data only}
- Huang L, Tian M, Li N, Elliott P, Yan L, Labarthe D, et al. Interim effects of salt substitution on urinary electrolytes and blood pressure in the China Salt Substitute and Stroke Study (SSaSS). Journal of Hypertension 2018;36(Suppl.3):e279. [DOI: 10.1097/01.hjh.0000549138.27560.0d] [DOI] [PubMed] [Google Scholar]
- Huang L, Tian M, Yu J, Li Q, Liu Y, Yin X, et al. Interim effects of salt substitution on urinary electrolytes and blood pressure in the China Salt Substitute and Stroke Study (SSaSS). American Heart Journal 2020;221:136-45. [DOI: 10.1016/j.ahj.2019.12.020] [DOI] [PubMed] [Google Scholar]
- Huang L, Woodward M, Stepien S, Tian M, Yin X, Hao Z, et al. Spot urine samples compared with 24-h urine samples for estimating changes in urinary sodium and potassium excretion in the China Salt Substitute and Stroke Study. International Journal of Epidemiology 2018;47(6):1811-20. [DOI: 10.1093/ije/dyy206] [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR. Can salt substitution save at-risk persons from stroke? New England Journal of Medicine 2021;385(12):1137-8. [DOI: 10.1056/NEJMe2112857] [DOI] [PubMed] [Google Scholar]
- NCT02092090. China Salt Substitute and Stroke Study (SSaSS) [A large-scale cluster randomized trial to determine the effects of sodium reduction on stroke: the China Salt Substitute and Stroke Study (SSaSS)]. clinicaltrials.gov/ct2/show/NCT02092090 (first received 19 March 2014).
- Neal B, Tian M, Li N, Elliott P, Yan LL, Labarthe DR, et al. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS)-a large-scale cluster randomized controlled trial. American Heart Journal 2017;188:109-17. [DOI: 10.1016/j.ahj.2017.02.033] [DOI] [PubMed] [Google Scholar]
- Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, et al. Effect of salt substitution on cardiovascular events and death. New England Journal of Medicine 2021;385(12):1067-77. [DOI: 10.1056/NEJMoa2105675] [DOI] [PubMed] [Google Scholar]
- Zhang T, Chang X, Liu W, Li X, Wang F, Huang L, et al. Comparison of sodium, potassium, calcium, magnesium, zinc, copper and iron concentrations of elements in 24-h urine and spot urine in hypertensive patients with healthy renal function. Journal of Trace Elements in Medicine and Biology 2017;44:104-8. [DOI: 10.1016/j.jtemb.2017.06.006] [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu WL, Liu S, Li XX, Yin T, Liu XY, et al. Estimating 24-h urinary sodium excretion from casual spot urine specimen among hypertensive patients in Northwest China: the Salt Substitute and Stroke Study. Public Health Nutrition 2021;24(4):604-10. [DOI: 10.1017/S1368980019005019] [DOI] [PubMed] [Google Scholar]
Omvik 1995 {published data only}
- Omvik P, Myking OL. Unchanged central hemodynamics after six months of moderate sodium restriction with or without potassium supplement in essential hypertension. Blood Pressure 1995;4(1):32-41. [DOI: 10.3109/08037059509077565] [DOI] [PubMed] [Google Scholar]
Pan 2017 {published data only}
- Lai YH, Chen JR, Jeng JS, Chen CM, Bai CH, Lin RT, et al. The effect of intervention with potassium and magnesium-enriched salt on neurological performance in stroke patients. Cerebrovascular Diseases 2014;38(Suppl.1):98. [DOI: 10.1159/000367674] [DOI] [Google Scholar]
- NCT02910427. The effect of intervention with potassium and/or magnesium-enriched salt on neurological performance of stroke patients. https://clinicaltrials.gov/ct2/show/NCT02910427 (first received 22 September 2016).
- Pan W-H, Lai Y-H, Yeh W-T, Chen J-R, Jeng J-S, Bai C-H, et al. Intake of potassium- and magnesium-enriched salt improves functional outcome after stroke: a randomized, multicenter, double-blind controlled trial. American Journal of Clinical Nutrition 2017;106(5):1267-73. [DOI: 10.3945/ajcn.116.148536] [DOI] [PubMed] [Google Scholar]
Pereira 2005 {published data only}
- Pereira MA, Galvão R, Zanella MT. Effects of potassium supplementation by salt on arterial blood pressure and insulin resistance in hypertensive obese patients on diuretic therapy [Efeitos da suplementação de potássio via sal de cozinha sobre a pressão arterial e a resistência à insulina em pacientes obesos hipertensos em uso de diuréticos]. Revista de Nutrição [Brazilian Journal of Nutrition] 2005;18(1):5-17. [DOI: 10.1590/S1415-52732005000100001] [DOI] [Google Scholar]
Sarkkinen 2011 {published data only}
- ISRCTN01739816. The effect of replacing regular salt with SmartSalt® mineral salt on blood pressure in middle-aged subjects with high blood pressure or with mild hypertension: a randomised controlled two-arm human study. doi.org/10.1186/ISRCTN01739816 (first received 11 November 2009).
- Sarkkinen ES, Kastarinen MJ, Niskanen TH, Karjalainen PH, Venäläinen TM, Udani JK, et al. Feasibility and antihypertensive effect of replacing regular salt with mineral salt - rich in magnesium and potassium - in subjects with mildly elevated blood pressure . Nutrition Journal 2011;10(1):88. [DOI: 10.1186/1475-2891-10-88] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suppa 1988 {published data only}
- Suppa G, Pollavini G, Alberti D, Savonitto S. Effects of a low-sodium high-potassium salt in hypertensive patients treated with metoprolol: a multicentre study. Journal of Hypertension 1988;6(10):787-90. [PubMed] [Google Scholar]
Toft 2020 {published data only}
- Bjoernsbo KS, Riis NL, Andreasen AH, Petersen J, Lassen AD, Trolle E, et al. Salt reduction intervention in families investigating metabolic, behavioral and health effects of targeted intake reductions: study protocol for a four months three-armed, randomized, controlled “real-life” trial. International Journal of Environmental Research and Public Health 2019;16(19):3532. [DOI: 10.3390/ijerph16193532] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis NL, Bjoernsbo KS, Toft U, Trolle E, Hyldig G, Hartley IE, et al. Impact of salt reduction interventions on salt taste sensitivity and liking, a cluster randomized controlled trial. Food Quality and Preference 2021;87:104059. [DOI: 10.1016/j.foodqual.2020.104059] [DOI] [Google Scholar]
- Toft U, Riis NL, Lassen AD, Trolle E, Andreasen AH, Frederiksen AK, et al. The effects of two intervention strategies to reduce the intake of salt and the sodium-to-potassium ratio on cardiovascular risk factors. A 4-month randomised controlled study among healthy families. Nutrients 2020;12(5):1467. [DOI: 10.3390/nu12051467] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME. Request for information on the composition of Viva salt [personal communication]. Email to: A Woitrin 22 September 2021.
Yu 2021 {published data only}
- NCT03909659. The effects on blood pressure of salt substitute among adults with hypertension in India [A randomized-controlled trial to determine the effects on blood pressure of a reduced-sodium added-potassium salt substitute among adults with hypertension in India]. https://clinicaltrials.gov/ct2/show/NCT03909659 (first received 10 April 2019).
- Thout SR, Yu J, Tian M, Huffman MD, Arnott C, Li Q, et al. Rationale, design, and baseline characteristics of the Salt Substitute in India Study (SSiIS): the protocol for a double-blinded, randomized-controlled trial. Journal of Clinical Hypertension 2020;22(8):1504-12. [DOI: 10.1111/jch.13947] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visaria A, Shahani J, Shah M, Modak A, Chilakapati R. Maximizing the potential of the Salt Substitute in India Study. Journal of Clinical Hypertension 2021;23(1):197-8. [DOI: 10.1111/jch.14109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Thout SR, Li Q, Tian M, Marklund M, Arnott C, et al. Effects of a reduced-sodium added-potassium salt substitute on blood pressure in rural Indian hypertensive patients: a randomized, double-blind, controlled trial. American Journal of Clinical Nutrition 2021;114(1):185-93. [DOI: 10.1093/ajcn/nqab054.] [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang H, Li DF, Li WW, Wang Q, Brunner HR, Zhang BQ, et al. Safety of long-term enriched potassium salt consumption and effect on blood pressure in Chinese. Journal of Hypertension 2015;33 e-Suppl 1:e148-9. [Google Scholar]
- Zhang H, Wang Q, Brunner HR, Li W, Zhang B, Dong Y, et al. Effects of long-term enriched potassium salt consumption on premature death in Chinese living in nursing houses - preliminary analysis. Journal of Hypertension 2018;36 e-Suppl 3:e286-7. [Google Scholar]
- Zhang H, Wang Q, Guo Y, Li D, Zhang B, Dong Y, et al. Effect of long-term enriched potassium salt consumption on all causes mortality in Chinese living in nursing houses - a preliminary analysis. Journal of Hypertension 2016;34 e-Suppl 1:e52-3. [Google Scholar]
Zhao 2014 {published data only}
- NCT01429246. China Salt Substitute Study in Tibet (CSSS-Tibet) [China Salt Substitute Study in Tibet: efficacy of salt substitute in reducing blood pressure in hypertensive adults]. clinicaltrials.gov/ct2/show/NCT01429246 (first received 7 September 2011).
- Zhao X, Ke L, Li S, Li N, Yan LL, Ba S, et al. Effects on blood pressure of a low-sodium, high-potassium salt substitute among Tibetan Chinese: a randomized controlled trial. Circulation 2010;122(2):e353. [Google Scholar]
- Zhao X, Yin X, Li X, Yan LL, Lam CT, Li S, et al. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient-blinded randomized controlled trial. PLOS One 2014;9(10):e110131. [DOI: 10.1371/journal.pone.0110131] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2009 {published data only}
- Yang G-H, Zhou X, Ji W-J, Liu J-X, Sun J, Shi R, et al. Effects of a low salt diet on isolated systolic hypertension: a community-based population study. Medicine 2018;97(14):e0342. [DOI: 10.1097/MD.0000000000010342] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GH, Zhou X, Ji W-J, Liu J-X, Sun J, Shi R, et al. Effects of low salt diet on isolated systolic hypertension: a community-based population study. Journal of Hypertension 2018;36 e-Suppl 3:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liu J-X, Shi R, Yang N, Song D-L, Pang W, et al. Compound ion salt, a novel low-sodium salt substitute: from animal study to community-based population trial. American Journal of Hypertension 2009;22(9):934-42. [DOI: 10.1038/ajh.2009.135] [DOI] [PubMed] [Google Scholar]
Zhou 2013 {published data only}
- Sun H, Ma B, Wu X, Wang H, Zhou B. Long-term effect of salt substitute on all-cause and cardiovascular disease mortality: an exploratory follow-up of a randomized controlled trial. Frontiers in Cardiovascular Medicine 2021;8:645902. [DOI: 10.3389/fcvm.2021.645902] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang H-L, Wang W-L, Wu X-M, Fu L-Y, Shi J-P. Long-term effects of salt substitution on blood pressure in a rural north Chinese population. Journal of Human Hypertension 2013;27(7):427-33. [DOI: 10.1038/jhh.2012.63.] [DOI] [PubMed] [Google Scholar]
- Zhou B, Webster J, Fu L-Y, Wang H-L, Wu X-M, Wang W-L, et al. Intake of low sodium salt substitute for 3 years attenuates the increase in blood pressure in a rural population of North China - a randomized controlled trial. International Journal of Cardiology 2016;215:377-82. [DOI: 10.1016/j.ijcard.2016.04.073] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allaert 2015 {published data only}
- Allaert F, Melero C. CO-42: Observational study of the effect of substituting NaCl with NaCl+ chitosan 3% (Symbiosal®) in the diet of elderly subjects on their blood pressure values [Étude observationnelle de l‘effet d‘une substitution du NaCl par du NaCl + chitosan 3% (Symbiosal®) dans l‘alimentation des personnes âgées sur leurs paramètres tensionnels]. Annales de Cardiologie et d'Angéiologie 2015;64(Supplement 1):S19. [Google Scholar]
- Allaert FA. Observational study of the effect of substituting NaCl with NaCl+Chitosan 3% (Symbiosal) in the diet of elderly subjects on their blood pressure values. Minerva Cardioangiologica 2015;63(6):515-23. [PubMed] [Google Scholar]
Baek 2015 {published data only}
- Baek SH, Ahn JW, Lee HR, Cho SH, Kim JH. Anti-hypertensive effect of a solar salt diet in elderly hypertensive patients: a preliminary randomized, double-blind clinical trial. Korean Journal of Health Promotion 2015;15(3):98-107. [Google Scholar]
Barros 2013 {published data only}
- Barros CL. Substitution of Regular Salt with Light Salt: Impact on Blood Pressure of Hypertensive Patients (Impacto da Substituição do Sal Convencional Pelo Sal Light sobre a Pressão Arterial de Hipertensos [Master's thesis]. Goiânia (Brazil): Universidade Federal de Goiás Faculdade de Nutrição Programa de Pós-Graduação em Nutrição e Saúde, 2013. [Google Scholar]
Barros 2015 {published data only}
- Barros CL, Sousa AL, Chinem BM, Rodrigues RB, Jardim TS, Carneiro SB, et al. Impact of light salt substitution for regular salt on blood pressure of hypertensive patients. Arquivos Brasileiros de Cardiologia 2015;104(2):128-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1800016804 {unpublished data only}
- ChiCTR1800016804. Home cook intervention to reduce salt intake: in China - a cluster randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=25400 (first received 25 June 2018).
ChiCTR1800018119 {published data only}
- ChiCTR1800018119. A community-based comprehensive intervention to reduce salt intake in the Chinese population: a cluster randomized controlled trial. www.chictr.org.cn/com/25/showproj.aspx?proj=30631 (first received 31 August 2018).
ChiCTR1800019727 {published data only}
- ChiCTR1800019727. Effects of salt substitution on blood pressure using home blood pressure, ambulatory blood pressure and clinical blood pressure in essential hypertensive patients: a randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=31036 (first received 25 November 2018).
ChiCTR2000033349 {published data only}
- ChiCTR2000033349. Zhejiang province pilot of intervention evaluation project of salt reduction and prevention and control of hypertension: a cluster randomized controlled trial. www.chictr.org.cn/hvshowproject.aspx?id=36685 (first received 29 May 2020).
Dent 2011 {published data only}
- Dent A, Walmsley D, Dhandapani S. Hyperkalaemia is a risk with low sodium salt in vulnerable patients. British Medical Journal 2011;343:2. [DOI] [PubMed] [Google Scholar]
Doorenbos 2003 {published data only}
- Doorenbos CJ, Vermeij CG. Lesson of the week: danger of salt substitutes that contain potassium in patients with renal failure. British Medical Journal (International Edition) 2003;326(7379):35-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrand 2019 {published data only}
- Farrand C, MacGregor G, Campbell NR, Webster J. Potential use of salt substitutes to reduce blood pressure. Journal of Clinical Hypertension 2019;21(3):350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2020 {published data only}
- He FJ, Tan MN, Song J, MacGregor GA. Salt substitution to lower population blood pressure. Nature Medicine 2020;26(3):313-4. [DOI] [PubMed] [Google Scholar]
Hueston 1989 {published data only}
- Hueston WJ. Use of salt substitutes in the treatment of diuretic-induced hypokalemia. Journal of Family Practice 1989;29(6):623-6. [PubMed] [Google Scholar]
Itoh 1997 {published data only}
- Itoh K, Kawasaki T. Clinical and nutritional study of low sodium, high potassium and high magnesium salt on sensory test and electrolyte balance. Japanese Journal of Nutrition 1997;55:263-72. [Google Scholar]
Janda 2017 {published data only}
- Janda J, Veleminsky M, Sulakova T, Prochazka B, Eliasek J, Stransky P, et al. Effect of the DASH-diet and salt Kardisal on blood pressure in adolescents with prehypertension (cooperative multicentre interventional study). Neuroendocrinology Letters 2017;38(8):544-8. [PubMed] [Google Scholar]
Jeffrey 1984 {published data only}
- Jeffery RW, Pirie PL, Elmer PJ, Bjornson-Benson WM, Mullenbach VA, Kurth CL, et al. Low-sodium, high-potassium diet: feasibility and acceptability in a normotensive population. American Journal of Public Health 1984;74(5):492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
JPRN‐UMIN000012560 {published data only}
- JPRN-UMIN000012560. Effect of using low sodium seasonings (miso and soy sauce) or monitoring salt concentration of dishes prepared at home for 12 weeks on the reduction of sodium intake among healthy middle-aged Japanese individuals: a randomized, controlled, crossover trial. upload.umin.ac.jp/cgi-open-bin/icdr_e/ctr_his_list.cgi?recptno=R000013246 (first received 12 December 2013).
Karppanen 1984 {published data only}
- Karppanen H, Tanskanen A, Tuomilehto J, Puska P, Vuori J, Jantti V, et al. Safety and effects of potassium-containing and magnesium-containing low sodium-salt mixtures . Journal of Cardiovascular Pharmacology 1984;6:S236-43. [DOI] [PubMed] [Google Scholar]
Katz 1999 {published data only}
- Katz A, Rosenthal T, Maoz C, Peleg E, Zeidenstein R, Levi Y. Effect of a mineral salt diet on 24-h blood pressure monitoring in elderly hypertensive patients. Journal of Human Hypertension 1999;13(11):777-80. [DOI] [PubMed] [Google Scholar]
Lambert 2019 {published data only}
- Lambert K, Conley M, Dumont R, Montgomery R, Noble S, Notaras S, et al. Letter to the editor on "Potential use of salt substitutes to reduce blood pressure". Journal of Clinical Hypertension 2019;21(10):1609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2004 {published data only}
- Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ 2004;328(7447):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME. Request for study data [personal communication]. Email to: P Little 10 September 2021.
Maleki 2016 {published data only}
- Maleki A, Soltanian AR, Zeraati F, Sheikh V, Poorolajal J. The flavor and acceptability of six different potassium-enriched (sodium reduced) iodized salts: a single-blind, randomized, crossover design. Clinical Hypertension 2016;22(18):eCollection 2016. [DOI: 10.1186/s40885-016-0054-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruya 2020 {published data only}
- Maruya S, Takachi R, Kanda M, Nakadate M, Ishihara J. Short-term effects of salt restriction via home dishes do not persist in the long term: a randomized control study. Nutrients 2020;12(10):3034. [DOI: 10.3390/nu12103034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matlou 1986 {published data only}
- Matlou SM, Isles CG, Higgs A, Milne FJ, Murray GD, Schultz E, et al. Potassium supplementation in blacks with mild to moderate essential hypertension. Journal of Hypertension 1986;4(1):61-4. [DOI] [PubMed] [Google Scholar]
Mu 2020 {published data only}
- Mu L, Li C, Liu T, Xie W, Li G, Wang M, et al. A pilot study on efficacy and safety of a new salt substitute with very low sodium among hypertension patients on regular treatment. Medicine 2020;99(8):e19263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakano 2016 {published data only}
- Nakano M, Eguchi K, Sato T, Onoguchi A, Hoshide S, Kario K. Effect of intensive salt-restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3-month education period. Journal of Clinical Hypertension 2016;18(5):385-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02105727 {published data only}
- NCT02105727. Changing population salt consumption in Lithgow, Australia [A before after comparison of the effectiveness of a community-based salt reduction program done in Lithgow, Australia]. clinicaltrials.gov/ct2/show/NCT02105727 (first received 7 April 2014).
Pietinen 1981 {published data only}
- Pietinen P, Ruotsalainen P, Tanskanen A, Puska P. Sodium intake reduction in volunteer families by using a salt substitute and nutrition counselling. Annals of Nutrition & Metabolism 1981;25(6):371-80. [DOI] [PubMed] [Google Scholar]
Robare 2010 {published data only}
- Robare JF, Milas NC, Bayles CM, Williams K, Newman AB, Lovalekar MT, et al. The key to life nutrition program: results from a community-based dietary sodium reduction trial. Public Health Nutrition 2010;13(5):606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvetti 1988 {published data only}
- Salvetti A, Bichisao E, Caiazza A, Bartolomei G, Cagianelli MA, Federighi G, et al. The combination of a low-Na/high-K salt with metoprolol in the treatment of mild-moderate hypertension. A multicenter study. American Journal of Hypertension 1988;1(3 Pt 3):201s-5s. [DOI] [PubMed] [Google Scholar]
Sciarrone 1992 {published data only}
- Sciarrone SE, Beilin LJ, Rouse IL, Rogers PB. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. Journal of Hypertension 1992;10(3):287-98. [DOI] [PubMed] [Google Scholar]
Zoccali 1985 {published data only}
- Zoccali C, Cumming AM, Hutcheson MJ, Barnett P, Semple PF. Effects of potassium on sodium balance, renin, noradrenaline and arterial pressure. Journal of Hypertension 1985;3(1):67-72. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jones 2019 {published data only}
- Jones D. Dietary sodium and potassium intake: an event-based randomized clinical trial. Journal of Hypertension 2019;37(Supplement 1):e252. [Google Scholar]
Neutel 1996 {published data only}
- Neutel J. Replacing regular salt with sodium-reduced, potassium- and magnesium-enriched mineral salt may offer non-pharmacological approach to lowering blood pressure. American Journal of Hypertension 1996;9:94A. [Google Scholar]
Voloshyna 2017 {published data only}
- Voloshyna I, Vizir VA, Voloshyn MA, Krivenko VI, Ponomarenko VI. Effect of potassium/magnesium enriched salt on functional capacity and quality of life in symptomatic patients with chronic heart failure. European Journal of Heart Failure 2017;19(Supplement 1):53. [Google Scholar]
References to ongoing studies
ACTRN12619000352101 {published data only}
- ACTRN12619000352101. Salt ALTernatives Study (SALTS): a smartphone app and dietary alternative salt to lower blood pressure for adults with high blood pressure. trialsearch.who.int/?TrialID=ACTRN12619000352101 (first received 06 March 2019).
ChiCTR08000296 {published data only}
- ChiCTR-TRC-08000296. China Salt trial. www.chictr.org.cn/showproj.aspx?proj=9234 (first received 30 December 2008).
ChiCTR09000538 {published data only}
- ChiCTR-TRC-09000538. The study on pathogeny of hypertension and the salt intervention on diet in the population of Zhangwu County. www.chictr.org.cn/showproj.aspx?proj=8997 (first received 11 September 2009).
ChiCTR2000029017 {published data only}
- ChiCTR2000029017. Study on the application of low-sodium formula salt in hypertensive patients with diabetes. www.chictr.org.cn/showproj.aspx?proj=47080 (first received 01 November 2020).
IRCT2016103130572N1 {published data only}
- IRCT2016103130572N1. The effects of a salt substitute on an Iranian population. trialsearch.who.int/?TrialID=IRCT2016103130572N1 (first received 08 December 2016).
NCT02016404 {published data only}
- NCT02016404. The effect of a low sodium-high potassium salt on blood pressure in Vietnamese adults. clinicaltrials.gov/ct2/show/NCT02016404?term=NCT+02016404&draw=2&rank=1 (first received 20 December 2013).
NCT02021435 {published data only}
- NCT02021435. Tibet salt reduction study. clinicaltrials.gov/ct2/show/NCT02021435?term=NCT02021435&draw=2&rank=1 (first received 27 December 2013).
NCT03290716 {published data only}
- Jin A, Liu K, Labarthe DR, Feng X, Zhang R, Wang H, et al. Impact of salt substitute and stepwise reduction of salt supply on blood pressure in residents in senior residential facilities: design and rationale of the DECIDE-Salt trial. American Heart Journal 2020;226:198-205. [DOI: 10.1016/j.ahj.2020.05.013] [DOI] [PubMed] [Google Scholar]
- NCT03290716. Diet, exercise and cardiovascular health - effect of salt substitute and stepwise salt supply control in reducing blood pressure in the elderly in nursing homes in China. clinicaltrials.gov/ct2/show/NCT03290716?term=NCT03290716&draw=2&rank=1 (first received 25 September 2017).
Additional references
Ahee 2000
- Ahee P, Crowe AV. The management of hyperkalaemia in the emergency department. Journal of Accident & Emergency Medicine 2000;17(3):188-91. [DOI: 10.1136/emj.17.3.188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnett 2019
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2019;74(10):e177-232. [DOI: 10.1016/j.jacc.2019.03.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banegas 2017
- Banegas JR, Ruilope LM, De la Sierra A, Vinyoles E, Gorostidi M, De la Cruz JJ, et al. Clinic versus daytime ambulatory blood pressure difference in hypertensive patients: the impact of age and clinic blood pressure. Hypertension 2017;69(2):211-9. [DOI: 10.1161/HYPERTENSIONAHA.116.08567] [DOI] [PubMed] [Google Scholar]
Bilano 2014
- Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLOS One 2014;9(3):e91198. [DOI: 10.1371/journal.pone.0091198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boston University School of Public Health 2018
- Boston University School of Public Health. Comparing frequencies: rate ratios. sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717_ComparingFrequencies/PH717_ComparingFrequencies9.html (accessed 8 October 2021).
Burkard 2018
- Burkard T, Mayr M, Winterhalder C, Leonardi L, Eckstein J, Vischer AS. Reliability of single office blood pressure measurements. Heart 2018;104(14):1173-9. [DOI: 10.1136/heartjnl-2017-312523] [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cepanec 2017
- Cepanec K, Vugrinec S, Cvetković T, Ranilović J. Potassium chloride-based salt substitutes: a critical review with a focus on the patent literature. Comprehensive Reviews in Food Science and Food Safety 2017;16(5):881-94. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cnossen 2008
- Cnossen JS, Vollebregt KC, De Vrieze N, Ter Riet G, Mol BW, Franx A, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ 2008;336(7653):1117-20. [DOI: 10.1136/bmj.39540.522049.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohn 2000
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Archives of Internal Medicine 2000;160(16):2429-36. [DOI: 10.1001/archinte.160.16.2429] [DOI] [PubMed] [Google Scholar]
Couser 2011
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney International 2011;80(12):1258-70. [DOI: 10.1038/ki.2011.368] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed prior to 4 November 2021. Melbourne, Australia: Veritas Health Innovation, 2017. Available at covidence.org.
Cuijpers 2021
- Cuijpers P, Griffin JW, Furukawa TA. The lack of statistical power of subgroup analyses in meta-analyses: a cautionary note. Epidemiology and Psychiatric Sciences 2021;30:e78. [DOI: 10.1017/S2045796021000664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook .
De Moraes 2014
- De Moraes AC, Lacerda MB, Moreno LA, Horta BL, Carvalho HB. Prevalence of high blood pressure in 122,053 adolescents: a systematic review and meta-regression. Medicine 2014;93(27):e232. [DOI: 10.1097/MD.0000000000000232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ettehad 2016
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387(10022):957-67. [DOI: 10.1016/S0140-6736(15)01225-8] [DOI] [PubMed] [Google Scholar]
Filippatos 2017
- Filippatos TD, Makri A, Elisaf MS, Liamis G. Hyponatremia in the elderly: challenges and solutions. Clinical Interventions in Aging 2017;12:1957-65. [DOI: 10.2147/CIA.S138535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippini 2017
- Filippini T, Violi F, D'Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. International Journal of Cardiology 2017;230:127-35. [DOI: 10.1016/j.ijcard.2016.12.048.] [DOI] [PubMed] [Google Scholar]
Forouzanfar 2017
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317(2):165-82. [DOI: 10.1001/jama.2016.19043] [DOI] [PubMed] [Google Scholar]
Franklin 2011
- Franklin SS, Levy D. Aging, blood pressure, and heart failure: what are the connections? Hypertension 2011;58(5):760-2. [DOI: 10.1161/HYPERTENSIONAHA.111.179119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2009
- Giles TD, Materson BJ, Cohn JN, Kostis JB. Definition and classification of hypertension: an update. Journal of Clinical Hypertension 2009;11(11):611-4. [DOI: 10.1111/j.1751-7176.2009.00179.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Global Dietary Database 2022
- Gerald J, Dorothy R Friedman School of Nutrition Science and Policy at Tufts University. Global Dietary Database 2018 Final Estimates. www.globaldietarydatabase.org/data-download (accessed 13 March 2022).
Golder 2006
- Golder S, McIntosh HM, Duffy S, Glanville J, Centre for Reviews and Dissemination and UK Cochrane Centre Search Filters Design Group. Developing efficient search strategies to identify reports of adverse effects in MEDLINE and EMBASE. Health Information and Libraries Journal 2006;23(1):3-12. [DOI: 10.1111/j.1471-1842.2006.00634.x.] [DOI] [PubMed] [Google Scholar]
Golder 2012
- Golder S, Loke YK. The performance of adverse effects search filters in MEDLINE and EMBASE. Health Information and Libraries Journal 2012;29(2):141-51. [DOI: 10.1111/j.1471-1842.2012.00980.x] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 4 November 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Greer 2020
- Greer RC, Marklund M, Anderson CA, Cobb LK, Dalcin AT, Henry M, et al . Potassium-enriched salt substitutes as a means to lower blood pressure: benefits and risks. Hypertension 2020;75(2):266-74. [DOI: 10.1161/HYPERTENSIONAHA.119.13241] [DOI] [PubMed] [Google Scholar]
Guang Wang 2005
- Guang Wang J, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension 2005;45(5):907-13. [DOI: 10.1161/01.HYP.0000165020.14745.79.] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI: 10.1016/j.jclinepi.2011.01.012] [DOI] [PubMed] [Google Scholar]
Hernandez 2019
- Hernandez AV, Emonds EE, Chen BA, Zavala-Loayza AJ, Thota P, Pasupuleti V, et al. Effect of low-sodium salt substitutes on blood pressure, detected hypertension, stroke and mortality. Heart 2019;105(12):953-60. [DOI: 10.1136/heartjnl-2018-314036.] [DOI] [PubMed] [Google Scholar]
Herttua 2013
- Herttua K, Tabák AG, Martikainen P, Vahtera J, Kivimäki M. Adherence to antihypertensive therapy prior to the first presentation of stroke in hypertensive adults: population-based study. European Heart Journal 2013;34(38):2933-9. [DOI: 10.1093/eurheartj/eht219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s) . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/ .
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Higgins 2020a
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Higgins 2020b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Hollander‐Rodriguez 2006
- Hollander-Rodriguez JC, Calvert Jr JF. Hyperkalemia. American Family Physician 2006;73(2):283-90. [PMID: ] [PubMed] [Google Scholar]
Hu 2014
- Hu JH, Zhao LC, Li X, Wu YF. Effects of salt substitution on blood pressure using home measurements in essential hypertensive patients: a double-blinded, randomized controlled trial. Chinese Journal of Hypertension 2014;22(1):42-6. [Google Scholar]
Huang 2020
- Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NR, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ 2020;368:m315. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI: 10.1016/j.jclinepi.2017.05.006.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Institute for Health Metrics and Evaluation 2016
- Institute for Health Metrics and Evaluation . The Global Burden of Disease Study 2016: Global prevalence, incidence, and YLDs for 2016, percentage change of YLD counts, and percentage change of age-standardised YLD rates between 2006 and 2016 for all causes and nine impairments. ghdx.healthdata.org/record/ihme-data/gbd-2016-incidence-prevalence-and-ylds-1990-2016 (accessed 4 November 2021).
Jafarnejad 2020
- Jafarnejad S, Mirzaei H, Clark CC, Taghizadeh M, Ebrahimzadeh A. The hypotensive effect of salt substitutes in stage 2 hypertension: a systematic review and meta-analysis. BMC Cardiovascular Disorders 2020;20(1):98. [DOI: 10.1186/s12872-020-01347-x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2020
- Jin A, Xie W, Wu Y. Effect of salt reduction interventions in lowering blood pressure in Chinese populations: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2020;10(2):e032941. [DOI: 10.1136/bmjopen-2019-032941.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovesdy 2018
- Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. European Heart Journal 2018;39(17):1535-42. [DOI: 10.1093/eurheartj/ehy100.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010
- Lee H-Y, Oh B-H. Aging and arterial stiffness. Circulation Journal 2010;74(11):2257-62. [DOI: 10.1253/circj.cj-10-0910] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Levey 2013
- Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. American Journal of Kidney Diseases 2013;61(5):686-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903-13. [DOI: 10.1016/s0140-6736(02)11911-8] [DOI] [PubMed] [Google Scholar]
Li 2019
- Li L, Smith HE, Atun R, Tudor Car L. Search strategies to identify observational studies in MEDLINE and Embase. Cochrane Database of Systematic Reviews 2019;3(3):MR000041. [DOI: 10.1002/14651858.MR000041.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 1996
- Liu ZQ, Mu JJ, Liu WM, Liang YM, Yang DY, Zhu DJ, et al. Reduction of blood pressure with calcium and potassium supplementation in children: a randomized double-blind placebo controlled trial of two years. Chinese Circulation Journal 1996;11:534-6. [DOI] [PubMed] [Google Scholar]
Mirmiran 2021
- Mirmiran P, Bahadoran Z, Gaeini Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. International Journal of Endocrinology and Metabolism 2021;19(3):e108170. [DOI: 10.5812/ijem.108170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naude 2018
- Naude CE, Visser ME, Nguyen KA, Durão S, Schoonees A. Effects of total fat intake on bodyweight in children. Cochrane Database of Systematic Reviews 2018;2(2):CD012960. [DOI: 10.1002/14651858.CD012960.] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCD Risk Factor Collaboration 2021
- NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021;398(10304):957-80. [DOI: 10.1016/S0140-6736(21)01330-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neal 2017
- Neal B, Tian M, Li N, Elliott P, Yan LL, Labarthe DR, et al. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS) - a large-scale cluster randomized controlled trial. American Heart Journal 2017;188:109-17. [DOI: 10.1016/j.ahj.2017.02.033.] [DOI] [PubMed] [Google Scholar]
Noubiap 2017
- Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta-analysis. Lancet Public Health 2017;2(8):E375-86. [DOI: 10.1016/S2468-2667(17)30123-8] [DOI] [PubMed] [Google Scholar]
Palei 2013
- Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiologica 2013;208(3):224-33. [DOI: 10.1111/apha.12106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2014
- Peng YG, Li W, Wen XX, Li Y, Hu JH, Zhao LC. Effects of salt substitutes on blood pressure: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2014;100(6):1148-54. [DOI: 10.3945/ajcn.114.089235] [DOI] [PubMed] [Google Scholar]
Powles 2013
- Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013;3(12):e003733. [DOI: 10.1136/bmjopen-2013-003733.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahimi 2021
- Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan R, et al, Blood Pressure Lowering Treatment Trialists' Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021;397(10285):1625-36. [DOI: 10.1016/S0140-6736(21)00590-0.] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 3.12.1. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Sahay 2014
- Sahay M, Sahay R. Hyponatremia: a practical approach. Indian Journal of Endocrinology and Metabolism 2014;18(6):760-71. [DOI: 10.4103/2230-8210.141320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook .
Shahbabu 2016
- Shahbabu B, Dasgupta A, Sarkar K, Sahoo SK. Which is more accurate in measuring the blood pressure? A digital or an aneroid sphygmomanometer. Journal of Clinical and Diagnostic Research 2016;10(3):LC11-4. [DOI: 10.7860/JCDR/2016/14351.7458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Staessen 2000
- Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355(9207):865-72. [DOI: 10.1016/s0140-6736(99)07330-4] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I. Chapter 10: Addressing reporting biases. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Thangaratinam 2011
- Thangaratinam S, Langenveld J, Mol BW, Khan KS. Prediction and primary prevention of pre-eclampsia. Best Practice & Research: Clinical Obstetrics & Gynaecology 2011;25(4):419-33. [DOI: 10.1016/j.bpobgyn.2011.02.008] [DOI] [PubMed] [Google Scholar]
Thomopoulos 2017
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence. 12. Effects in individuals with high-normal and normal blood pressure: overview and meta-analyses of randomized trials. Journal of Hypertension 2017;35(11):2150-60. [DOI: 10.1097/HJH.0000000000001547] [DOI] [PubMed] [Google Scholar]
Tsirimiagkou 2022
- Tsirimiagkou C, Karatzi K, Basdeki ED, Argyris AA, Papaioannou TG, Yannakoulia M. Dietary sodium estimation methods: accuracy and limitations of old and new methods in individuals at high cardiovascular risk. Public Health Nutrition 2022;25(4):866-78. [DOI: 10.1017/S1368980021004390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Upadhyay 2009
- Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Seminars in Nephrology 2009;29(3):227-38. [DOI: 10.1016/j.semnephrol.2009.03.004] [DOI] [PubMed] [Google Scholar]
Verbeek 2021
- Verbeek J, Hoving J, Boschman J, Chong L-Y, Livingstone-Banks J, Bero L. Systematic reviews should consider effects from both the population and the individual perspective. American Journal of Public Health 2021;111(5):820-5. [DOI: 10.2105/AJPH.2020.306147.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinceti 2016
- Vinceti M, Filippini T, Crippa A, De Sesmaisons A, Wise LA, Orsini N. Meta-analysis of potassium intake and the risk of stroke. Journal of the American Heart Association 2016;5(10):e004210. [DOI: 10.1161/JAHA.116.004210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018
- Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in China: results from the China Hypertension Survey, 2012–2015. Circulation 2018;137:2344-56. [DOI: 10.1161/CIRCULATIONAHA.117.032380] [DOI] [PubMed] [Google Scholar]
Wang 2019
- Wang L, Song L, Liu B, Zhang L, Wu M, Cao Z, et al. Trends and status of the prevalence of elevated blood pressure in children and adolescents in China: a systematic review and meta-analysis. Current Hypertension Reports 2019;21(11):88. [DOI: 10.1007/s11906-019-0992-1] [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment; February 2000. apps.who.int/iris/handle/10665/206936 (accessed 4 November 2021).
WHO 2012a
- World Health Organization. Guideline: Sodium intake for adults and children; December 2012. www.who.int/publications/i/item/9789241504836 (accessed 4 November 2021).
WHO 2012b
- World Health Organization. Guideline: Potassium intake for adults and children; December 2012. www.who.int/publications/i/item/9789241504829 (accessed 4 November 2021).
WHO 2020
- World Health Organization. Global health estimates 2019: deaths by cause, age, sex, by country and by region, 2000-2019. www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed 4 November 2021).
Yan 2014
- Yan LL, Fang W, Delong E, Neal B, Peterson ED, Huang Y, et al. Population impact of a high cardiovascular risk management program delivered by village doctors in rural China: design and rationale of a large, cluster-randomized controlled trial. BMC Public Health 2014;14:345. [DOI: 10.1186/1471-2458-14-345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaxley 2015
- Yaxley JP, Thambar SV. Resistant hypertension: an approach to management in primary care. Journal of Family Medicine and Primary Care 2015;4(2):193-9. [DOI: 10.4103/2249-4863.154630] [DOI] [PMC free article] [PubMed] [Google Scholar]
Younge 2015
- Younge JO, Kouwenhoven-Pasmooij TA, Freak-Poli R, Roos-Hesselink JW, Hunink MM. Randomized study designs for lifestyle interventions: a tutorial. International Journal of Epidemiology 2015;44(6):2006-19. [DOI: 10.1093/ije/dyv183.] [DOI] [PubMed] [Google Scholar]
Zeng 2021
- Zeng L, Brignardello-Petersen R, Guyatt G. When applying GRADE, how do we decide the target of certainty of evidence rating? Evidence-Based Mental Health 2021;24:121-3. [DOI: 10.1136/ebmental-2020-300170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019
- Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the GRADE approach. Research Synthesis Methods 2019;10(3):312-29. [DOI: 10.1002/jrsm.1313] [DOI] [PubMed] [Google Scholar]


