Abstract
Biological markers, particularly endocrine measurements, are increasingly being integrated into clinical psychological research. We introduce a systematic framework that classifies different functions of such biomarkers. The framework distinguishes between diagnostic biomarkers which add a biological perspective to conventional clinical assessments, prognostic biomarkers that inform about an individual’s risk to develop or maintain a mental health disorder, and intervention-related biomarkers. Regarding interventions, including prevention and treatment, it further distinguishes between prescriptive biomarkers which predict an individual’s response to an intervention, outcome biomarkers which evaluate intervention-related changes on a biological level and indicators of change mechanisms. We demonstrate how to apply the framework by exemplarily classifying and describing previously published systematic reviews and primary empirical studies on endogenous, peripheral cortisol concentrations as a biomarker for posttraumatic stress disorder (PTSD). The evidence on cortisol’s diagnostic and prognostic value is heterogeneous and still sparse regarding parameters based on multiple cortisol measurements, such as the cortisol awakening response. With regard to interventions, most research focused on trauma-focused psychotherapy and cortisol reactivity to trauma reminders. This field of research appears to be growing and very promising due to its potential to optimize PTSD-related interventions. The proposed framework can help in gaining a systematic overview of existing research. It can assist in structuring, comparing, summarizing and evaluating empirical studies, and in identifying research gaps.
Keywords: PTSD, Biomarker, Cortisol, HPA axis, Endocrine
Highlights
-
•
Iagnostic biomarkers can inform about biological alterations in mental disorders.
-
•
Prognostic biomarkers can help to predict the development of a mental disorder.
-
•
Biomarkers can indicate the outcome, differential effects, or mechanisms of change of clinical psychological interventions.
1. A systematic framework to classify biomarkers in clinical psychological research
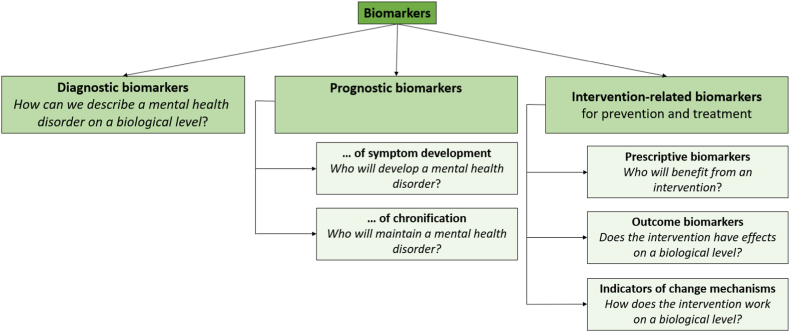
It has long been known that biological and psychological factors interact and jointly contribute to the development and maintenance of mental health disorders [1]. Consequently, clinical psychology aims at describing pathogenesis and psychopathology both at the level of psychological symptoms and through the underlying biological processes. Innovative programs such as RDoC promote this development [2]. Thus, in recent years, clinical psychological assessments have increasingly been complemented by measurements of biological markers. These are objective indicators of normal biological processes, pathogenic processes, or pharmacologic responses to an intervention [3]. Biomarker assessments can include (epi-)genetic, psychophysiological, neuronal, or endocrine data. Including biomarkers into clinical psychological research has great potential, depending on their specific function. Here, we propose a systematic framework of these functions. It distinguishes between diagnostic biomarkers, prognostic biomarkers and intervention-related biomarkers. Intervention-related biomarkers, which can inform both prevention and treatment, are further classified into prescriptive biomarkers, outcome biomarkers and indicators of change mechanisms (see Fig. 1). Together, these biomarkers can help to improve clinical diagnosis, predict the development of a mental health disorder and optimize its prevention and treatment.
Fig. 1.
Systematic framework classifying functions of biomarkers in clinical psychological research.
In the following sections, we define these different functions of biomarkers more precisely and reflect on methodological requirements for their implementation. Furthermore, we exemplarily apply the framework to summarize previous evidence on hypothalamic-pituitary-adrenal (HPA) axis regulation, more specifically, endogenous peripheral cortisol concentrations, in posttraumatic stress disorder (PTSD). If available, we thereby refer to systematic reviews with meta-analyses. Of note, these summarized primary empirical studies of various designs. If meta-research is not available for a specific biomarker function, we describe primary empirical evidence.
2. Application of the systematic framework: classifying research on cortisol as a biomarker for posttraumatic stress disorder (PTSD)
PTSD is a burdensome mental health disorder. It develops after exposure to a traumatic event, i.e., actual or threatened death, serious injury or sexual violence [4]. Defining symptoms of PTSD include intrusions, avoidance of trauma-related content, hyperarousal, and negative alterations of cognition and mood [4]. Worldwide, approximately 70% of the population experience at least one trauma during their lifetime [5,6]. Yet, only a small fraction of trauma exposed individuals subsequently develop a mental health disorder, such as PTSD [7].
In the context of trauma and PTSD, the HPA axis is presumably the most extensively studied neuroendocrine system [8]. Its regulation includes a cascade of hormone releases – with cortisol released from the adrenal gland as its end product – and negative feedback mechanisms [9,10]. Cortisol release follows a diurnal rhythm, characterized by a peak within 30 to 45 minutes after awakening and a subsequent linear decline throughout the day [11]. Several indicators of HPA axis regulation exist, evaluating either HPA axis reactivity in response to acute stressors (e.g. [12]) or basal HPA axis activity (as explained in [13]), which also reflects its specific circadian patterns. The most common indicators of diurnal cortisol secretion include the cortisol awakening response [14,15] – characterized by peaking cortisol concentrations within the first waking hour –, the cortisol decline over the day – often expressed as the diurnal cortisol slope – and the total cortisol output – frequently described by means of area under the curve (AUC) coefficients [13,16]. Each of these indicators requires multiple samplings over one day [17]. Further, cumulative cortisol output can be measured in urine, hair and fingernail samples [[18], [19], [20], [21]]. Single measurements of basal cortisol concentrations in saliva or blood samples are more prone to circadian influences and, as a kind of “snapshot”, are not reliable indicators of HPA axis regulation in PTSD (as discussed in [[22], [23], [24]]). Additionally, the HPA axis’ feedback sensitivity can be measured by pharmacologically blocking glucocorticoid receptors (GR, e.g. using a low dose of dexamethasone) and subsequently measuring circulating cortisol concentrations, which are assumed to decrease with enhanced GR sensitivity [25].
2.1. Diagnostic biomarkers
2.1.1. Definition
In their function as diagnostic biomarker, biological measurements can complement conventional, interview- or questionnaire-based clinical assessments. Thereby, they can help to gain a more comprehensive perspective on mental health disorders, to refine subgroups of patients or determine symptom severity. If biological alterations that are specific for a certain mental health disorder are identifiable, biomarkers might even be used to confirm a diagnosis.
2.1.2. Methodological requirements
Diagnostic biomarkers can be measured within cross-sectional designs, by correlating the biological measurements with self-reported symptom severity, or by testing whether the markers differ between a group of patients and a group of healthy individuals. In the field of psychotraumatology, the mere impact of trauma exposure on the biological system of interest should be specifically considered, by taking potential traumas experienced by the comparison group into account. Thus, diagnostic biomarkers should ideally be compared between PTSD patients, trauma exposed and trauma non-exposed controls.
2.1.3. Previous evidence on cortisol as a diagnostic biomarker for PTSD
Five meta-analyses summarizing evidence on HPA axis regulation in PTSD have been published to date [[22], [23], [24],26,27]. They all indicate PTSD-related dysregulations but are also partly inconsistent. With regard to single cortisol measurements, one meta-analysis reported no differences between PTSD patients and trauma-exposed controls [26], whereas two others detected lower cortisol concentrations in PTSD patients compared with non-exposed controls [22,23]. This suggests that trauma but not trauma-related psychopathology impacts cortisol. One meta-analysis reported no PTSD-related alterations in morning cortisol concentrations [22] whereas two others reported a PTSD-related decrease [23,24]. No group differences in evening cortisol were identified [23,24]. However, as noted before, these findings should be interpreted with caution as single cortisol measurements are not regarded as reliable indicators of HPA axis functioning. Two meta-analyses investigated total daily cortisol output and reported PTSD-related decreases [23,24]. Another meta-analysis focused on 24-h urinary cortisol and revealed lower urinary cortisol concentrations in PTSD patients [27]. Subgroup analysis by control group type identified significant differences between PTSD patients and trauma-exposed controls, but no differences compared with non-exposed controls. This finding was confirmed in one meta-analysis [24], but others did not identify any significant effects [22,26]. Unfortunately, research on PTSD-related alterations in the CAR is still scarce, but one subgroup-meta-analysis suggests that it might be a promising diagnostic biomarker for PTSD [24]. Concerning HPA axis feedback sensitivity, two meta-analyses detected lower post-dexamethasone cortisol concentrations and thus, enhanced GR feedback sensitivity in trauma exposed individuals [23,26]. One of them extended this finding to PTSD patients [23]. However, another meta-analysis which took pre-dexamethasone cortisol concentrations into account failed to confirm these findings [24]. Primary evidence on HPA axis reactivity to social stress exists in adolescents [28,29] and adults [30], even though results are inconsistent and have not been summarized in meta-research yet. Further, there are indications of PTSD-related increased HPA axis reactivity to reminders of trauma, particularly abuse [31,32]. However, other studies failed to detect such a pattern [33,34].
2.1.4. Conclusion
There are some indications of HPA axis dysregulation in individuals with PTSD. However, inconsistencies in the cumulative evidence suggest that cortisol concentrations cannot be considered as a specific biomarker for PTSD. Notably, most studies relied on single cortisol measurements. Evidence using multi-measurement-based and, thus, more reliable parameters is still scarce.
2.2. Prognostic biomarkers
2.2.1. Definition
Biomarkers can also predict future psychopathological symptoms or mental health disorders. This function is referred to as prognostic biomarker. Two types of prognostic biomarkers can be further distinguished: First, prognostic biomarkers of symptom development that predict the onset and severity of psychopathological symptoms or mental health disorders. Second, prognostic biomarkers that predict the course of symptoms and whether they result in remission or chronification of the mental health disorder.
2.2.2. Methodological requirements
To assess prognostic biomarkers, it is necessary to conduct longitudinal studies. Prognostic biomarkers of symptom development need to be assessed before symptoms evolve. In the field of psychotraumatology, this would be either shortly post-trauma or, in prospective studies, even pre-trauma, for example in high-risk populations for trauma exposure, such as first responders or military personnel. Prognostic biomarkers of remission or chronification of mental health disorders can also be assessed after the onset of symptoms, since they predict the course of the disorder. Either way, the biomarker assessment needs to take place before the follow-up assessment of the psychopathological symptoms or disorders of interest. The association between biomarkers and symptoms or disorders can then be determined with any statistical analysis methods suitable to detect the predictive value of an independent variable (i.e., cortisol concentrations) on a dependent variable (i.e., PTSD symptoms).
2.2.3. Previous evidence on cortisol as a prognostic biomarker for PTSD
2.2.3.1. PTSD development
Prognostic biomarkers, as assessed pre- or shortly post-trauma, can be used to estimate an individual’s risk to develop PTSD. Given the high number of individuals who remain resilient after trauma [[35], [36], [37]], it is necessary to identify such at increased risk for PTSD symptom development to provide them with targeted (preventive) interventions [38]. There is a considerable body of empirical evidence on cortisol’s potential as a prognostic biomarker for PTSD development, as summarized in several reviews [[39], [40], [41], [42]] and one meta-analysis [43]. The latter revealed no significant prognostic effect, based on k = 8 studies which related single or cumulative cortisol measurements, as assessed within 72 h post-trauma, to subsequent PTSD symptoms [43]. Most of the systematic overviews focused on cortisol assessments that took place post-trauma [[39], [40], [41], [42]]. They suggest that lower cortisol concentrations predict PTSD, especially if measured in adults and acutely post-trauma [39,41,42]. Yet, the overall evidence is considerably heterogeneous and as this observation was not confirmed quantitatively, the interpretation must be noted with caution. Although most evidence was based on single cortisol measurements, some primary studies also used multi-measurement-based parameters, such as diurnal cortisol profiles [[44], [45], [46]]. However, these have not been systematically summarized yet, so no final conclusions regarding the prognostic value of indicators of diurnal cortisol secretion can be made. One systematic review reported on the prognostic value of pre-trauma cortisol concentrations [40]. The authors particularly emphasized the potential of cortisol reactivity to stress tasks in predicting PTSD development, while at the same time noting that the evidence on this matter is still sparse.
2.2.3.2. PTSD remission vs. chronification
In individuals who suffer from PTSD, prognostic biomarkers can indicate the chance for remission or, vice versa, risk of chronification. To our knowledge, there are no studies that focus on the prognostic value of cortisol for PTSD remission versus chronification, without including any intervention. Nevertheless, it appears promising to conduct such studies in the future, as they might guide treatment recommendations for individuals at high risk for chronic PTSD.
2.2.4. Conclusion
Compared with the evidence on cortisol’s diagnostic value, evidence on its potential as a prognostic biomarker is still relatively sparse, possibly due to higher methodological requirements. The existing evidence on cortisol’s potential to predict PTSD symptom development is not conclusive. Regarding cortisol’s potential to predict PTSD remission vs. chronification, primary research is still lacking.
2.3. Intervention-related biomarkers
2.3.1. Prescriptive biomarkers
2.3.1.1. Definition
In intervention studies, biological measurements can serve as prescriptive markers, if they were assessed before intervention onset and interact with the intervention’s effects in predicting post-intervention psychopathological symptoms. Clinical interventions can include both, psychotherapeutic or pharmacological interventions, as well as both, prevention or treatment. Prescriptive biomarkers can enable the identification of subgroups who benefit especially well from an intervention or, vice versa, have a higher risk of not responding [47].
2.3.1.2. Methodological requirements
To examine prescriptive biomarkers in intervention studies, the biological assessment should be conducted before intervention onset to ensure that the biological system of interest is not (yet) influenced by the intervention. Psychopathological symptom assessments should be conducted at least pre- and post-intervention. Thereby, the biomarker, as assessed pre-intervention, can predict post-intervention psychopathological symptoms while controlling for pre-intervention symptom load. Further, the biomarker’s main (prognostic) effect should be distinguishable from its interaction with the intervention (prescriptive effect, see [48]). To this end, some variation in the intervention variable is required. This can be operationalized by varying the intensity of intervention provision or by comparing an active intervention condition (e.g. psychotherapy) to a comparison condition (e.g. waitlist or placebo). Accordingly, the statistical analyses should reflect this distinction, for example by testing both, the main effect of the biomarker, as well as the interaction effect with the intervention. However, it must be acknowledged that, for ethical or practical reasons, implementing intervention variation might not always be feasible. Additionally, in contrast to prognostic effects that explain psychopathological symptoms after the mere passage of time, in intervention studies, the biomarker’s predictive value is not independent of the intervention effect. Therefore, we will also consider such designs when discussing previous evidence on cortisol as a prescriptive biomarker, even though with a disclaimer that these effects are not truly prescriptive, as time and intervention effects cannot truly be disentangled.
2.3.1.3. Previous evidence on cortisol as a prescriptive biomarker
One meta-analysis investigated cortisol assessment in studies on psychotherapeutic treatment for PTSD [49]. Of the k = 12 included studies, only k = 5 used pre-intervention cortisol levels to predict post-intervention clinical outcomes [[50], [51], [52], [53], [54]]. Schumacher et al. [49] refrained from meta-analytically summarizing them. However, most of the single effects that were calculated were non-significant. Most included studies (k = 4) investigated combat-related PTSD in military personnel and all studies investigated the effects of trauma-focused psychotherapy, including exposure. The cortisol parameters investigated were the CAR [50,52,53], CAR after the dexamethasone suppression test [50] and cortisol reactivity to trauma reminders [51,53]. As reviewed by Schumacher et al. (2018) [49], these analyses yielded mixed results regarding prescriptive effects of cortisol: Rapcencu et al. (2017) [52] found that the CAR predicted greater decrease of PTSD symptoms. In contrast, Nijdam et al. (2015) [50] reported that greater CAR suppression by dexamethasone, but not natural CAR, predicted PTSD symptom decrease. Norrholm et al. (2016) [51] found a prescriptive effect of cortisol reactivity to trauma reminders, but not baseline cortisol, in patients who underwent virtual exposure therapy enhanced with alprazolam, but not in such who received d-cycloserine enhancement or a placebo. Likewise, Rauch et al. (2015) [53] found prescriptive effects of the CAR and cortisol reactivity to trauma reminders only in patients who received prolonged exposure instead of present-centered therapy. A more recent investigation not included in the meta-analysis found that total cortisol output during exposure to a trauma reminder, but not cortisol reactivity to the reminder, predicted treatment response in youths who underwent trauma-focused psychotherapy [55]. All these studies serve as examples of how biological measurements can be implemented into clinical trials to predict psychotherapeutic treatment success. Pre-intervention cortisol can further be used to evaluate potential prescriptive effects in PTSD prevention. To date, only one study tested how different cortisol parameters interacted with a pharmacological preventive intervention. However, neither the natural, nor suppressed CAR, nor single cortisol measurements interacted with the effects of repeated intranasal oxytocin administration [56].
2.3.1.4. Conclusion
So far, only few studies have evaluated cortisol’s potential as a prescriptive biomarker in trauma-focused psychotherapy. It is to be considered positive that mainly methodologically sophisticated designs and multi-measurement-based cortisol parameters, such as the CAR or cortisol reactivity to trauma reminders, have been investigated.
2.3.2. Outcome biomarkers
2.3.2.1. Definition
Next to their prescriptive value, biological measurements can also serve as an outcome variable in intervention studies. A prerequisite for this approach is the assumption that the mental health disorder is associated with dysregulations in the respective biological system of interest. Consequently, successful interventions should alleviate or even reverse this dysregulation. Accordingly, a biological marker which is assumed to act as a surrogate for the system can be selected to investigate intervention-induced changes on both a biological and psychological level. Hence, biological measurements can be regarded as outcome biomarkers if a change from pre-to post-intervention is related to changes in psychopathological symptoms.
2.3.2.2. Methodological requirements
Concerning methodological requirements, at least one pre- and another one post-intervention assessment for both, the biological and psychopathological symptom measurement should be conducted. The circumstances of data collection should be held as constant as possible between pre- and post-intervention. This concerns aspects like the time of day of sampling or sampling during the week vs. the weekend. For practical issues, it is seldomly possible to keep season of the year constant, but it might be worthwhile to control for this influence. Many statistical techniques are suitable to test how the intervention’s psychological effects accompany potential changes in cortisol as a dependent variable. One possibility is a responder analysis. Hence, patients who show a significant symptom reduction following the intervention can be compared to patients who do not show a response, using the (residual) change in the biological measurement as outcome.
2.3.2.3. Previous evidence on cortisol as an outcome biomarker
In the aforementioned meta-analysis by Schumacher et al. (2018) [49], k = 8 of the k = 12 included studies used a cortisol parameter as an outcome variable to test the biological effects of psychotherapeutic treatment. While most of these studies also focused on exposure-based therapy approaches [[52], [53], [54],[57], [58], [59], [60]], one study tested the effects of mindfulness or meditation interventions on PTSD symptoms and cortisol parameters [61]. Summarizing the evidence for different cortisol parameters, including single measurements and the CAR, Schumacher et al. [49] identified no changes from pre-to post-treatment, neither in analyses including all patients, nor in responder analyses.
2.3.2.4. Conclusion
Outcome biomarkers have the potential to bridge the gap between psychological and biological intervention effects. If measured properly, they can provide valuable insights due to their potential to identify individuals that may respond differently based not only on demographic or psychological characteristics, but biological systems. However, to date, no cortisol parameters that are clearly indicative for PTSD-treatment associated changes on a biological level have been identified.
2.3.3. Indicators of change mechanisms
2.3.3.1. Definition
Lastly, the regulation a biological system during (a specific part or technique of) an intervention can be decisive for its effectiveness. Therefore, biomarkers can provide insights into the mechanisms of change underlying effective interventions. Thereby, biological indicators of change mechanisms can help to understand how exactly an intervention exerts its effects and to identify its active ingredients. Promoting these active ingredients can ultimately contribute to optimizing intervention efficacy and effectiveness, by speeding up remission and increasing response rates [62].
2.3.3.2. Methodological requirements
On a content level, prior knowledge about the intervention and its psychological mechanisms of change are required. On a methodological level, coordinated measurements of these known psychological and the assumed associated biological mechanisms of change are required, ideally at least once during the intervention, at the point in time at which the psychological change mechanisms take their effects.
2.3.3.3. Previous evidence on cortisol as an indicator of change mechanisms
Only few previous studies investigated cortisol as an indicator of change mechanisms, all focusing on trauma-focused psychotherapies, including exposure. Their results have not been summarized in systematic reviews, yet. Consequently, these primary empirical studies are only exemplarily presented here, with the disclaimer that no final conclusions regarding cortisol’s potential as an indicator of change mechanisms can be made at this point in time. A study by van Gelderen et al. (2020) [63] demonstrated that higher cortisol concentrations before and after exposure sessions were associated with a greater psychotherapy response. This is in line with findings by Rauch et al. (2015) [53] who investigated the CAR and cortisol reactivity to trauma cues as a potential biological underpinning of trauma-focused psychotherapies. In both, prolonged exposure and present centered psychotherapy, cortisol concentrations, as measured before and several times after trauma-related imagery tasks before, during and after the intervention, were higher in treatment responders than in non-responders. However, with an increasing number of completed therapy sessions, cortisol reactivity to trauma-related imagery showed stronger increases in non-responders than in responders [64].
2.3.3.4. Conclusion
As noted, the evidence on cortisol as an indicator of change mechanisms in (exposure-based) interventions is still sparse, but it might be very promising. The rationale of these investigations is built upon a more basic theory regarding the influence of cortisol on extinction learning and memory consolidation [65]. Therefore, exciting studies on this topic can certainly be expected in the future, especially since one can already notice the practical implications of such investigations, for example in the application of hydrocortisone in PTSD prevention [66,67].
3. Summary and integration
As this paper demonstrated, using the example of cortisol, the proposed systematic framework can be helpful in gaining a clear, precise, and comprehensive overview of studies on selected biomarkers in clinical psychological research, depending on the respective scientific question and, accordingly, biomarker function of interest. Such an overview is warranted, as on the one hand, clinical psychology has already benefited from translational approaches that enhanced our understanding of the biological mechanisms underlying PTSD [68]. On the other hand, it has to be acknowledged that despite considerable efforts, “we’re still waiting” [69] for specific biomarkers that provide precise, reliable and valid diagnoses. Further, it can be challenging to keep up with, structure, compare, summarize and evaluate the growing number of studies that is being published every year. Additionally, the proposed framework can be helpful to identify gaps and potentials in this growing field of research. We hope to provide researchers who plan to perform biomarker studies in the future with a proposal for an accurate nomenclature, with reflections on methodological requirements, and, regarding the example of cortisol, an initial overview on the current state of research. Lastly, we would like to note that, although here, we focused on cortisol as a biomarker for PTSD, this framework can of course also be applied to other mental health disorders or other biomarkers.
Author contributions
SE, HK, SL and CK performed the literature search and drafted the manuscript. All authors revised the manuscript for important intellectual content.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Sinha Engel, Email: s.engel@fu-berlin.de.
Hannah Klusmann, Email: h.klusmann@fu-berlin.de.
Sebastian Laufer, Email: Sebastian.laufer@fu-berlin.de.
Claudia Kapp, Email: claudia.kapp@outlook.de.
Sarah Schumacher, Email: sarah.schumacher@health-and-medical-university.de.
Christine Knaevelsrud, Email: christine.knaevelsrud@fu-berlin.de.
References
- 1.Engel G.L. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 2.Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Aust. J. Pharm. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 3.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 4.Psychiatric Association American. fifth ed. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. [DOI] [Google Scholar]
- 5.Benjet C., Bromet E., Karam E.G., Kessler R.C., McLaughlin K.A., Ruscio A.M., et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med. 2016;46:327–343. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G., et al. Trauma and PTSD in the WHO world mental health surveys. Eur. J. Psychotraumatol. 2017;8 doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkonigg A., Kessler R.C., Storz S., Wittchen H.-U. Traumatic events and post-traumatic stress disorder in the community: prevalence,risk factors and comorbidity: post-traumatic stress disorder in the community. Acta Psychiatr. Scand. 2000;101:46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- 8.Olff M., van Zuiden M. Neuroendocrine and neuroimmune markers in PTSD: pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Curr. Opin.Psychol. 2017;14:132–137. doi: 10.1016/j.copsyc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Andrews J., Ali N., Pruessner J.C. Reflections on the interaction of psychogenic stress systems in humans: the stress coherence/compensation model. Psychoneuroendocrinology. 2013;38:947–961. doi: 10.1016/j.psyneuen.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 11.Pruessner J.C., Wolf O.T., Hellhammer D.H., Buske-Kirschbaum A., von Auer K., Jobst S., et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/S0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 12.Kirschbaum C., Pirke K.-M., Hellhammer D.H. The ‘trier social stress test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 13.Adam E.K., Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Skoluda N., Linnemann A., Nater U.M. The role of week(end)-day and awakening time on cortisol and alpha-amylase awakening responses. Stress. 2016;19:333–338. doi: 10.1080/10253890.2016.1174850. [DOI] [PubMed] [Google Scholar]
- 15.Stalder T., Kirschbaum C., Kudielka B.M., Adam E.K., Pruessner J.C., Wüst S., et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 17.Ross K.M., Murphy M.L.M., Adam E.K., Chen E., Miller G.E. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burch W.M. Urine free-cortisol determination: a useful tool in the management of chronic hypoadrenal states. JAMA. 1982;247 doi: 10.1001/jama.1982.03320390064047. 2002–4. [DOI] [PubMed] [Google Scholar]
- 19.Contreras L.N., Hane S., Tyrrell J.B. Urinary cortisol in the assessment of pituitary-adrenal function: utility of 24-hour and spot determinations. J. Clin. Endocrinol. Metab. 1986;62:965–969. doi: 10.1210/jcem-62-5-965. [DOI] [PubMed] [Google Scholar]
- 20.Fischer S., Schumacher S., Skoluda N., Strahler J. Fingernail cortisol - state of research and future directions. Front. Neuroendocrinol. 2020;58 doi: 10.1016/j.yfrne.2020.100855. [DOI] [PubMed] [Google Scholar]
- 21.Stalder T., Kirschbaum C. Analysis of cortisol in hair--State of the art and future directions. Brain Behav. Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Meewisse M.-L., Reitsma J.B., De Vries G.-J., Gersons B.P.R., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 23.Morris M.C., Compas B.E., Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher S., Niemeyer H., Engel S., Cwik J.C., Laufer S., Klusmann H., et al. HPA axis regulation in posttraumatic stress disorder: a meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 2019;100:35–57. doi: 10.1016/j.neubiorev.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Barton C., March S., Wittert G.A. The low dose dexamethasone suppression test: effect of time of administration and dose. J. Endocrinol. Invest. 2002;25:RC10–R12. doi: 10.1007/BF03344008. [DOI] [PubMed] [Google Scholar]
- 26.Klaassens E.R., Giltay E.J., Cuijpers P., van Veen T., Zitman F.G. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37:317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Pan X., Kaminga A.C., Wen S.W., Wang Z., Wu X., Liu A. The 24-hour urinary cortisol in post-traumatic stress disorder: a meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilberdink C.E., van Zuiden M., Schrantee A., Korosi A., Kaiser A., Zhutovsky P., et al. Dysregulated functional brain connectivity in response to acute social-evaluative stress in adolescents with PTSD symptoms. Eur. J. Psychotraumatol. 2021;12 doi: 10.1080/20008198.2021.1880727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman A., Halligan S., Skeen S., Morgan B., Fraser A., Fearon P., et al. PTSD symptoms and cortisol stress reactivity in adolescence: findings from a high adversity cohort in South Africa. Psychoneuroendocrinology. 2020;121 doi: 10.1016/j.psyneuen.2020.104846. [DOI] [PubMed] [Google Scholar]
- 30.Wichmann S., Kirschbaum C., Boehme C., Petrowski K. Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology. 2017;83:135–141. doi: 10.1016/j.psyneuen.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Elzinga B.M., Schmahl C.G., Vermetten E., van Dyck R., Bremner J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 32.Gola H., Engler H., Schauer M., Adenauer H., Riether C., Kolassa S., et al. Victims of rape show increased cortisol responses to trauma reminders: a study in individuals with war- and torture-related PTSD. Psychoneuroendocrinology. 2012;37:213–220. doi: 10.1016/j.psyneuen.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Kolassa I.-T., Eckart C., Ruf M., Neuner F., de Quervain D.J., Elbert T. Lack of cortisol response in patients with posttraumatic stress disorder (PTSD) undergoing a diagnostic interview. BMC Psychiatr. 2007;7:54. doi: 10.1186/1471-244X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberzon I., Abelson J.L., Flagel S.B., Raz J., Young E.A. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 35.Bonanno G.A. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Galatzer-Levy I.R., Huang S.H., Bonanno G.A. Trajectories of resilience and dysfunction following potential trauma: a review and statistical evaluation. Clin. Psychol. Rev. 2018;63:41–55. doi: 10.1016/j.cpr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S.R., Ratanatharathorn A., Lai B.S., van der Mei W., Barbano A.C., Bryant R.A., et al. Posttraumatic stress disorder symptom trajectories within the first year following emergency department admissions: pooled results from the International Consortium to predict PTSD. Psychol. Med. 2020:1–11. doi: 10.1017/S0033291719004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalev A.Y., Barbano A.C. PTSD: risk assessment and early management. Psychiatr. Ann. 2019;49:299–306. doi: 10.3928/00485713-20190605-01. [DOI] [Google Scholar]
- 39.Delahanty D.L. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann. N. Y. Acad. Sci. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 40.Dunlop B.W., Wong A. The hypothalamic-pituitary-adrenal axis in PTSD: pathophysiology and treatment interventions. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2019;89:361–379. doi: 10.1016/j.pnpbp.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Marshall R.D., Garakani A. Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatr. Clin. 2002;25:385–395. doi: 10.1016/S0193-953X(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 42.Morris M.C., Rao U. Psychobiology of PTSD in the acute aftermath of trauma: integrating research on coping. HPA Function and Sympathetic Nervous System Activity. 2014:41. doi: 10.1016/j.ajp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris M.C., Hellman N., Abelson J.L., Rao U. Vol. 35. 2017. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: a systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cieslak R., Benight C.C., Luszczynska A., Laudenslager M.L. Longitudinal relationships between self-efficacy, post-traumatic distress and salivary cortisol among motor vehicle accident survivors: cortisol, Self-efficacy and PTSD. Stress Health. 2011;27:e261–e268. doi: 10.1002/smi.1379. [DOI] [Google Scholar]
- 45.Pervanidou P., Kolaitis G., Charitaki S., Lazaropoulou C., Papassotiriou I., Hindmarsh P., et al. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biol. Psychiatr. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Pervanidou P., Kolaitis G., Charitaki S., Margeli A., Ferentinos S., Bakoula C., et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 48.Fournier J.C., DeRubeis R.J., Shelton R.C., Hollon S.D., Amsterdam J.D., Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J. Consult. Clin. Psychol. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumacher S., Niemeyer H., Engel S., Cwik J.C., Knaevelsrud C. Psychotherapeutic treatment and HPA axis regulation in posttraumatic stress disorder: a systematic review and meta-analysis. Psychoneuroendocrinology. 2018;98:186–201. doi: 10.1016/j.psyneuen.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Nijdam M.J., van Amsterdam J.G.C., Gersons B.P.R., Olff M. Dexamethasone-suppressed cortisol awakening response predicts treatment outcome in posttraumatic stress disorder. J. Affect. Disord. 2015;184:205–208. doi: 10.1016/j.jad.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 51.Norrholm S.D., Jovanovic T., Gerardi M., Breazeale K.G., Price M., Davis M., et al. Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behav. Res. Ther. 2016;82:28–37. doi: 10.1016/j.brat.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rapcencu A.E., Gorter R., Kennis M., van Rooij S.J.H., Geuze E. Pre-treatment cortisol awakening response predicts symptom reduction in posttraumatic stress disorder after treatment. Psychoneuroendocrinology. 2017;82:1–8. doi: 10.1016/j.psyneuen.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Rauch S.A.M., King A.P., Abelson J., Tuerk P.W., Smith E., Rothbaum B.O., et al. Biological and symptom changes in posttraumatic stress disorder treatment: a randomized clinical trial. Depress. Anxiety. 2015;32:204–212. doi: 10.1002/da.22331. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda R., Pratchett L.C., Elmes M.W., Lehrner A., Daskalakis N.P., Koch E., et al. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus. 2014;4 doi: 10.1098/rsfs.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zantvoord J.B., Ensink J.B.M., Op den Kelder R., Wessel A.M.A., Lok A., Lindauer R.J.L. Pretreatment cortisol predicts trauma-focused psychotherapy response in youth with (partial) posttraumatic stress disorder. Psychoneuroendocrinology. 2019;109 doi: 10.1016/j.psyneuen.2019.104380. [DOI] [PubMed] [Google Scholar]
- 56.Engel S., van Zuiden M., JessieL Frijling, Koch S.B.J., Nawijn L., Yildiz R.L.W., et al. Early posttraumatic autonomic and endocrine markers to predict posttraumatic stress symptoms after a preventive intervention with oxytocin. Eur. J. Psychotraumatol. 2020;11 doi: 10.1080/20008198.2020.1761622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerardi M., Rothbaum B.O., Astin M.C., Kelley M. Cortisol response following exposure treatment for PTSD in rape victims. J. Aggress. Maltreat. Trauma. 2010;19:349–356. doi: 10.1080/10926771003781297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason J.W., Wang S., Yehuda R., Lubin H., Johnson D., Bremner J.D., et al. Marked lability in urinary cortisol levels in subgroups of combat veterans with posttraumatic stress disorder during an intensive exposure treatment program. Psychosom. Med. 2002;64:238–246. doi: 10.1097/00006842-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Olff M., de Vries G.-J., Güzelcan Y., Assies J., Gersons B.P.R. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology. 2007;32:619–626. doi: 10.1016/j.psyneuen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Pacella M.L., Feeny N., Zoellner L., Delahanty D.L. The impact of PTSD treatment on the cortisol awakening response. Depress. Anxiety. 2014;31:862–869. doi: 10.1002/da.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahbeh H., Goodrich E., Goy E., Oken B.S. Mechanistic pathways of mindfulness meditation in combat veterans with posttraumatic stress disorder. J. Clin. Psychol. 2016;72:365–383. doi: 10.1002/jclp.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stojek M.M., McSweeney L.B., Rauch S.A.M. Neuroscience informed prolonged exposure practice: increasing efficiency and efficacy through mechanisms. Front. Behav. Neurosci. 2018;12:281. doi: 10.3389/fnbeh.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Gelderen M., Nijdam M., Vries F., Meijer O., Vermetten E. Exposure-related cortisol predicts outcome of psychotherapy in veterans with treatment-resistant posttraumatic stress disorder. J. Psychiatr. Res. 2020;130 doi: 10.1016/j.jpsychires.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Rauch S.A.M., King A.P., Liberzon I., Sripada R.K. Changes in salivary cortisol during psychotherapy for posttraumatic stress disorder: a pilot study in 30 veterans. J. Clin. Psychiatr. 2017;78:599–603. doi: 10.4088/JCP.15m10596. [DOI] [PubMed] [Google Scholar]
- 65.de Quervain D., Schwabe L., Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017;18:7–19. doi: 10.1038/nrn.2016.155. [DOI] [PubMed] [Google Scholar]
- 66.Frijling J., Olff M., van Z.M. Pharmacological prevention of PTSD: current evidence for clinical practice. Psychiatr. Ann. 2019;49:307–313. doi: 10.3928/00485713-20190604-01. [DOI] [Google Scholar]
- 67.Sijbrandij M., Kleiboer A., Bisson J.I., Barbui C., Cuijpers P. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatr. 2015;2:413–421. doi: 10.1016/S2215-0366(14)00121-7. [DOI] [PubMed] [Google Scholar]
- 68.Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kupfer D. 2013. Chair of DSM-5 Task Force Discusses Future of Mental Health Research.https://www.madinamerica.com/wp-content/uploads/2013/05/Statement-from-dsm-chair-david-kupfer-md.pdf [Google Scholar]