Abstract
Quaternary climate fluctuations can affect speciation in regional biodiversity assembly in two non-mutually exclusive ways: a glacial species pump, where isolation in glacial refugia accelerates allopatric speciation, and adaptive radiation in underused adaptive zones during ice-free periods. We detected biogeographic and genetic signatures associated with both mechanisms in the assembly of the biota of the European Alps. Age distributions of endemic and widespread species within aquatic and terrestrial taxa (amphipods, fishes, amphibians, butterflies and flowering plants) revealed that endemic fish evolved only in lakes, are highly sympatric, and mainly of Holocene age, consistent with adaptive radiation. Endemic amphipods are ancient, suggesting preglacial radiation with limited range expansion and local Pleistocene survival, perhaps facilitated by a groundwater-dwelling lifestyle. Terrestrial endemics are mostly of Pleistocene age and are thus more consistent with the glacial species pump. The lack of evidence for Holocene adaptive radiation in the terrestrial biome is consistent with faster recolonization through range expansion of these taxa after glacial retreats. More stable and less seasonal ecological conditions in lakes during the Holocene may also have contributed to Holocene speciation in lakes. The high proportion of young, endemic species makes the Alpine biota vulnerable to climate change, but the mechanisms and consequences of species loss will likely differ between biomes because of their distinct evolutionary histories.
Keywords: time for speciation, allopatric speciation, adaptive radiation, Pleistocene refugia, glacial species pump, European Alps
1. Background
Immigration, speciation and extinction are the three main processes underlying the assembly of biodiversity in island-like habitats [1–3]. The relative contribution of these processes depends on size, isolation and fragmentation of the region, ecosystem or habitat. For instance, immigration rates decrease with increasing isolation, extinction rates decrease with increasing area, and rates of in situ speciation increase with area, isolation and fragmentation [4–6]. The occurrence and interaction of these processes over geological history leave strong imprints in the contemporary structure of regional and local species assemblages, including phylogenetic structure and relatedness. The species age distribution (SAD) and the nature and degree of endemism are some of the resulting biodiversity features [7].
Endemism can arise through cladogenetic speciation (when an ancestral species diverges into two or more descendent species within a region), anagenetic speciation (a local or regional population or set of populations diverges from its progenitors outside the region) or through extinction everywhere else [3]. Recent cladogenetic and anagenetic speciation both result in neoendemic species, which are young species with geographically restricted distributions. Therefore, if cladogenetic and anagenetic speciations are important processes behind regional biodiversity assembly, a regional biota can be composed of many relatively young and closely related species. Non-endemic species, in turn, are generally more broadly distributed because they either have immigrated to the focal region from the outside or they have arisen in the focal region and had time to spread beyond it. Such non-endemic species are also expected to be older according to the ‘age and area’ hypothesis [8], which predicts that older species have had more time to disperse and become geographically more widespread. However, the ‘age and area’ hypothesis assumes biome stability (including climatic stability) and does not consider factors other than age that could in fact have strong effects on species range sizes, such as local extinction [9], physical or climatic barriers [10], habitat size, ecological versatility and evolutionary adaptability [11], dispersal ability [12] and ecological interactions [13]. The interaction of these factors can explain, for instance, why some species, despite being old, are geographically narrowly confined in the present time (geographical relicts or palaeoendemics) [14].
Mountain landscapes at lower and intermediate latitudes, such as those of the European Alps (hereafter the Alps), are hotspots of biodiversity and endemism [15,16]. In such environments, endemism and species radiations arise through the interaction of dispersal limitation with steep ecological gradients and often archipelago-like habitat structures [17–20]. In the Alps, multiple terrestrial taxa have undergone local radiations leading to the emergence of endemic clades in several groups such as flowering plants [21–23] and butterflies [24,25]. Some of the largest endemic radiations in European freshwaters also took place in or around the Alps, especially for amphipods [26–28] and fish [29,30]. These radiations occurred in habitats that are geographically isolated from similar habitats elsewhere, but surrounded by less isolated habitats, containing diverse assemblages of widely distributed taxa, such as mountain-tops surrounded by lowlands, or permanently cold, deep lakes isolated from other such lakes by the seasonally warm, shallow rivers.
The Alps began to emerge at the end of the Cretaceous and during the Tertiary due to the collision between the African and European Plates [31], but geological uplift culminated in the Eo-Oligocene [32]. Early biodiversity in the Alps likely assembled through long-distance dispersal from older alpine regions, local range expansions or passive uplift during mountain-building [7,33,34]. Ancient evolutionary radiations are also expected to have taken place, but subsequent geological and climatic processes, such as glaciations, probably erased most of the footprints left by those ancient radiations [28]. The climatic and habitat instability driven by the Quaternary climate fluctuations was one of the most important historical factors shaping the modern biodiversity in the Alps [30,35–40], influencing immigration, speciation and extinction, and reshaping species abundance, range distribution, richness and genetic diversity patterns [41–43]. Neoendemic biodiversity in the Alps may have emerged through two alternative, non-exclusive mechanisms both driven by the succession of glacial–interglacial cycles: (i) fragmentation and isolation during glacial periods, the glacial species pump [44,45] and (ii) adaptive radiation during interglacial periods (hereafter adaptive radiation) [46] (figure 1). The former is a process in which allopatric speciation is accelerated via the isolation of small populations in glacial refugia, operating when the expansion of glaciers makes large areas of a species' range unavailable, but leaves isolated pockets of suitable habitat (figure 1b; [36,45,47,48]). It can therefore be expected that the glacial pump creates assemblages composed of species that originally emerged in allopatry but came into secondary contact more recently (figure 1b–e, butterfly example).
Figure 1.
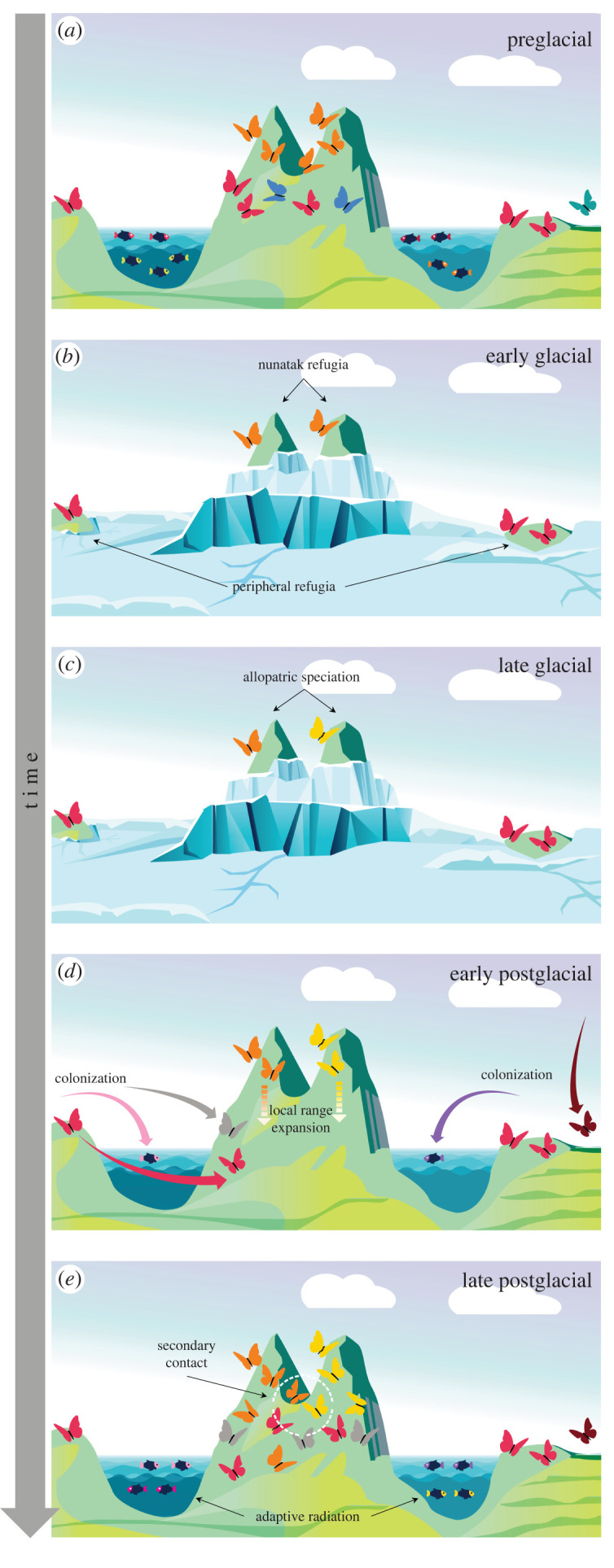
Evolutionary and ecological history of a hypothetical biodiversity assembly in an alpine-like system. (a) Biodiversity in a preglacial phase. (b) Early glacial phase: glacial periods erase freshwater habitats and fragment the terrestrial biome. Some populations survive in refugia and (c) can diverge into distinct species through allopatric speciation. (d) The retreat of glaciers opens up new, unoccupied habitats offering ecological opportunities for colonizers, (e) which undergo adaptive radiation, and niche space is filled up again. (Online version in colour.)
After each glacial maximum, the retreat of glaciers opens up new, unoccupied habitat in both terrestrial and aquatic ecosystems, which may facilitate early colonists’ in situ diversification through adaptive radiation [49] (figure 1e). Adaptive radiation takes place when an ancestor diversifies ecologically and phenotypically, giving rise to multiple species in short succession [50]. Adaptive radiation can also interact with the glacial pump mechanism as the postglacial expansion of populations may bring lineages together that had previously diverged in Pleistocene refugia [51]. Such secondary contact can facilitate the onset of adaptive radiation, either through ecological character displacement in sympatry occurring in response to competition [50], or through hybridization [52,53].
Although the SAD contains valuable information for investigating biodiversity assembly hypotheses and to understand biogeographic patterns [54], few studies have made use of it [40,55], and fewer even had investigated SADs from a multi-taxon perspective [7,56]. Here, we report on a comparative phylogenetic analysis to quantify SADs and the extent and type of endemism in aquatic and terrestrial ecosystems of the Alps. For terrestrial groups, we predicted that SADs support a scenario with resilience to cold glacial periods and potentially the glacial species pump for the origin of endemism, with species dating to the Pleistocene or before. Aquatic groups' SADs may indicate a prominent role of postglacial adaptive radiation. This is because during glacial maxima, high-altitude ranges in the terrestrial habitats became fragmented but not completely erased, whereas year-round open water bodies were entirely absent, with a few exceptions at the edge of the southern Alps [57]. Regarding non-endemic species, we do not expect differences in age structure among taxa because they tend to be widespread, are probably older and diversified on a wider geographical scale driven by processes that may have been decoupled from the climate dynamics of the Alps. Hence, we predict for all taxa that non-endemic species are older on average than endemics. Because dispersal ability, the distance between sink (new habitats that become available after glacial maxima) and sources of colonization, environmental stability and heterogeneity of habitat occupied vary between the taxa, the magnitude of the difference in SADs between non-endemics and endemics may be taxon dependent.
2. Methods
We focus on the European Alps, following previous delimitations of European high-mountain systems [58] but including the peripheral lowland areas, where the perialpine postglacial lakes are located, many of which emerged as deep fjords carved out by the glaciers. To select the taxonomic groups to be included in this work, we looked for lineages with reliable distribution data, robust dated phylogenetic trees that include most of the diversity of the given lineage, and/or sequence data for most of the recognized species, so that we could estimate and calibrate phylogenetic trees where none existed. We chose five major taxonomic groups to represent the terrestrial and aquatic alpine and pre-alpine biomes: amphipods, fishes, amphibians and butterflies (all nearly completely sampled), and 14 nearly completely sampled monophyletic clades of native flowering plants (Dicotyledons: Asteraceae: Homogyne, Petasites, Tussilago; Campanulaceae: Campanula clades 7–11 and 13–17 sensu [59], Jasione, Physoplexis; Caprifoliaceae: Knautia; Primulaceae: Androsace sect. Aretia, Primula sect. Auricula, Soldanella; Gentianaceae: Gentiana; Saxifragaceae: Saxifraga sect. Saxifraga; Monocotyledons: Cyperaceae Carex sect. Phacocystis; and Poaceae: Festuca sect. Aulaxyper).
To assemble SADs (stem age in million years, Myr), we combined published time-trees and our own estimates. We only used trees containing at least 50% of the valid species in the correspondent clade (electronic supplementary material, table S12). For the majority of butterflies and amphibians, we relied on two recently published super-trees [60,61], but for specific butterfly lineages underrepresented in the super-tree, we used complementary phylogenies [62–65]. To include Alpine species that were absent from these trees, we performed additional calibrations using secondary calibration points. For flowering plants, we used a set of published calibrated trees [66–74]. Amphipod trees were obtained from [28,75–78]. For fishes, we used a partial sequence (approx. 650 bp) of the cytochrome c oxidase subunit I (COI) gene (barcode region). Some of the sequences were generated in the present work, while others were taken from GenBank (electronic supplementary material, table S2). We included all species for which a barcode sequence was available. Calibrations were based on a molecular clock (1.2% genetic distance per Myr [79]). We are aware that this molecular clock derives from a relatively old study and on a biogeographic event (Panama Isthmus closure) that is not related to our target system. Yet, it still remains the most robust molecular clock available for the barcode region of fish. More details are in the electronic supplementary material.
(a) . Endemism and speciation mode
Species were considered endemic if they naturally occur only in the alpine and/or perialpine regions of the Alps. Speciation mode of endemic species was assigned to anagenetic speciation (an Alpine species diverged from its non-Alpine sister-species, but did not undergo in situ diversification) or cladogenetic speciation (Alpine species emerged through in situ diversification, with either the non-endemic sister being native to the region too, or the two or more sister-species all being Alpine endemics), following Rosindell & Phillimore [3] (electronic supplementary material, table S1). Therefore, if an endemic species was nested within a clade composed mainly of species that occur outside of the Alps, and its direct sister-species occurred only outside the Alps, we assumed anagenetic speciation. If the species was nested within a group mostly of species native to the Alps, we assumed cladogenetic speciation.
(b) . Comparisons of species age distributions
We performed permutation tests on the distribution of age estimates to identify differences in the SADs among and within major clades. We asked (i) whether age distributions differed between endemic and non-endemic species, overall and within each taxonomic group independently; (ii) whether SADs differed among taxonomic groups; (iii) whether SADs of non-endemics differed among groups and (iv) whether SADs of endemics differed among groups. When a difference was significant, we performed post hoc pairwise permutations to identify which distribution and taxonomic group (or groups) were distinct from one another after Bonferroni correction. These analyses were performed using the function ‘oneway_test’ of the library ‘coin’ [80] in R v. 4.0.2 1106 [81] with 10 000 resamples with a distribution approximated via Monte Carlo resampling.
(c) . Sensitivity to alternative divergence times
Although our work provides a powerful analysis of biodiversity assembly in the Alps, we are aware of some shortcomings. Our age estimates, by necessity, were assembled from different sources, and therefore no standardized methodology to infer and calibrate the phylogenies over all taxa was possible. For instance, the phylogenies used here can include a different set of markers and calibration strategies (e.g. fossils, secondary calibration or molecular clocks), which may introduce bias in our analyses. To investigate the potential impact that uncertainty in our estimates of species ages may have on our conclusions, we evaluate the 95% highest posterior densities (95% HPD). We first visually compared the minimum and maximum 95% HPD ages estimated for each species with the median values. Furthermore, for each species, we randomly picked an age from the 95% HPD, assuming the range to have a uniform distribution, and replotted the SAD for the group to assess if the patterns obtained with the median age are robust to random resampling (1000 times) from the 95% HPD of each species' ages. Given that fish phylogenies were built from a single short-sequence fragment (the barcode region), we also performed additional calibrations using a constant population tree prior for three lineages (Coregonus, Salvelinus and Cottus) that are known for having recently radiated (electronic supplementary material, Methods).
3. Results
A total of 497 species were included in our analyses: 39 amphipod, 121 fish, 31 amphibian, 158 butterfly and 148 plant species (electronic supplementary material, table S1). Approximately half of the fish and amphipod species were endemic to the European Alps (45% and 49%, respectively), whereas smaller fractions of 13%, 16% and 36% of the amphibians, butterflies and plants, respectively, were found to be endemic (figure 2). Around half of the endemic amphipods, plants and butterflies (53%, 52% and 41%, respectively) and the majority of the endemic amphibians and fish (75% and 98%, respectively) had arisen by cladogenesis (figure 2).
Figure 2.
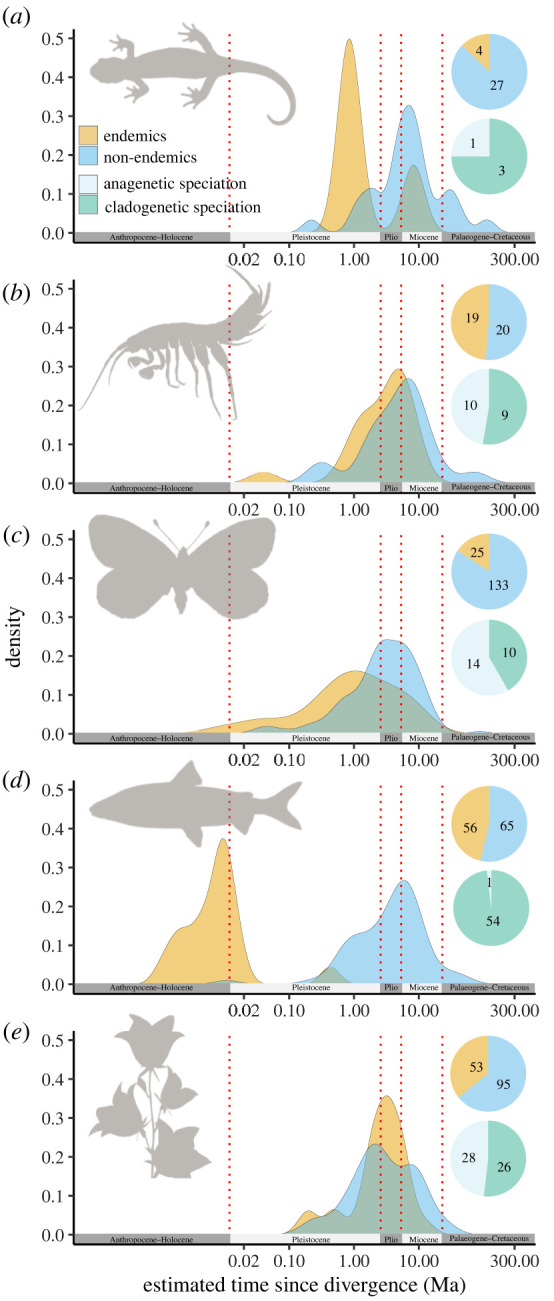
SAD of endemic and non-endemic species of (a) amphibians, (b) amphipods, (c) butterflies, (d) fish and (e) flowering plants. Pie charts show the proportion of endemic and non-endemic species as well as the proportion of endemic species that have emerged through cladogenetic or anagenetic speciation. (Online version in colour.)
Based on the median species age, we found that around 95% of the extant, native species that now occupy the Alps, irrespective of endemism status, had emerged within the past 14 Myr (figure 2). Endemics were overall younger than non-endemics (p-value < 0.0001), and this was also true within each taxonomic group (electronic supplementary material, table S9). We found SAD of non-endemic species to be similar between taxonomic groups (figure 2), with most of the species ages spanning the Late Pleistocene to Early Miocene (90% fell between 0.3 and 17 Ma). The only significant difference between taxa was that non-endemic amphibians were older than non-endemic plants (p < 0.0005; electronic supplementary material, S10). SADs of endemic species were also similar among taxa (90% fell between 0.15 and 8 Ma), except for fish, which are younger than any other group of endemics (90% fell between 1.5 and 114 kyr; p < 0.0001; figure 2; electronic supplementary material, S11). Calibration of the lineages that have diversified after the Last Glacial Maximum (LGM) using a constant population tree prior also supported the postglacial radiation hypothesis (Trees in Data accessibility).
The plots based on random ages picked from the 95% HPD (electronic supplementary material, figure S1) corroborated the patterns. Even only considering the minimum ages, the data would still suggest only a small fraction of postglacial speciation in butterflies and plants. However, if we assumed the maximum ages, the data would imply that most of the endemic fish speciated before the LGM (electronic supplementary material, figure S2).
4. Discussion
We found that most of the non-endemic species of fish, amphipods, amphibians, butterflies and plants of the Alps emerged in the Middle Miocene. This is the time when central and southern Europe had first coalesced into a little continent that was beginning to have faunal exchange with Asia and Northern Europe. It was also the period of maximum geological uplift in the region that culminated with the formation of the Alps [32]. Fossil records from the Miocene indicate that close relatives of many of the modern taxa were already in the region, but many other taxa existed too that later went extinct in Europe but persist until today in Africa, especially among the fish [82,83]. Yet, it is the endemic species that showed the most intriguing patterns. Speciation timing involving endemics was dramatically different among taxa. While most of the Alp's endemics in the terrestrial groups originated in the Pleistocene, most endemic fishes arose after the LGM and re-establishment of permanent open water bodies in the formerly glaciated areas. Although there is some uncertainty around the youngest species age estimates from our calibrated trees for fish, a demographic study in one of the recently diversified lineages (Cottus) showed that the ancestor that gave rise to the modern radiation colonized the region only around 6.6 kyr ago (3.4–10.6 kyr) [39], supporting our results. Given that the vast majority of endemic fish are products of cladogenetic speciation, we suggest that the assembly process of the fish fauna of the Alps is dominated by an interaction between colonization from outside the region and postglacial adaptive radiation in lakes. By contrast, for the terrestrial groups, persistence through glacial climate cycles, the glacial species pump, and recent colonization from outside the region, seem to be the dominant mechanisms, as anagenetic speciation was more important in these taxa, and endemic richness assembled throughout the Pleistocene. Interestingly, we observed some postglacial speciation in butterflies, coinciding with the major mode in fish, but being dwarfed by the much larger Pleistocene mode in butterflies. However, an alternative scenario would be that sister-groups of extant terrestrial species became extinct at some point in time, meaning that extant species should be younger than we observe here, or that the absence of some extant species would also contribute to overestimating species age.
We suggest that common mechanisms underlie these contrasting patterns: (i) Quaternary climate fluctuations initiating allopatric species differentiation during cold stages and creating ecological opportunity for adaptive radiation during interglacial periods, (ii) variation among groups in their dispersal ability and associated rate of range expansion, and (iii) variation among biomes in the predictability and intensity of seasonal access to resources constraining ecological specialization and adaptive radiation.
(a) . Quaternary climate fluctuations
That most endemic fish are of postglacial origin, while endemics in other groups arose in the Pleistocene or earlier, is probably explained by different effects that the Quaternary climate oscillations had on freshwater versus terrestrial habitat availability [57]. Permanent open surface water habitats, as required by fishes, were absent in the glaciated parts of the Alps during the LGM, because lakes and river valleys were filled with glaciers. Therefore, the lack of endemic fish species older than 20 kyr on the northern and western flanks of the Alps is probably due to extirpation of populations across the region during the LGM. With the retreat of glaciers in the course of the Holocene, fish would then have returned to the region from refugia located in downstream sections of large rivers. For most species of temperate climates, these refugia were often far from the Alpine region, especially on the North face of the Alps, i.e. the lower Danube and lower Rhone [84]. This Holocene recolonization explains the large fraction of old, widespread and non-endemic fish species in the northern and western Alpine region.
The first fish to colonize after the LGM were probably species adapted to cold waters, such as salmonids and sculpins, that would have lived nearby in the rivers of the Pleistocene tundra downstream of the Alpine glacier shield, as they exhibit a remarkable ability to establish in postglacial freshwater habitats [85,86]. These fish would have encountered ecological opportunities in the emerging large, deep lakes in response to which they radiated into many distinct species due to strong divergent natural selection [87]. The selective forces driving such radiations are related to vacant niches associated with distinct lacustrine habitats in the deep fjord lakes [29,88,89]. This was likely the main process by which the young endemic species in three lineages, whitefish (Coregonus [88]), chars (Salvelinus [90]) and sculpins (Cottus [30]), diversified in perialpine lakes. The few old, relic endemic fish species in the region that date to prior to the Holocene are the lake herring Alosa agone and the Lake Garda trout Salmo carpio. These species are endemic to lakes in the south of the Alps, a region where probably not all lakes were completely glaciated during the LGM [57]. Therefore, these species may have originated during earlier interglacials, when southern perialpine lakes would have become extensive, and then found refugia during the LGM to persist to the present day [91]. That there are no young postglacial species among the non-endemic fish is perhaps due to insufficient time and connectivity between lakes to allow new species that arose in deep lakes elsewhere in Europe (e.g. in northern Germany and Scandinavia) in the Holocene to expand their range into the Alps or vice-versa.
Unlike fish, endemic amphipods, the second fully aquatic taxon in our data, emerged during or above all before the Pleistocene. Amphipod radiations in Europe are known for being rather ancient, predating the Pleistocene [28,92,93], while the youngest radiations probably are from the Pleistocene [94]. This corresponds to the paucity of postglacial speciation noticed here for this group across the Alps. The presence of many relatively old endemic amphipods in this region could be because, surviving in smaller water bodies than those required by fish, they may have persisted under ice cover, in glacier forefields [95], or in subterranean refugia, such as caves or groundwaters [26,28,94], habitats that were not completely erased by the Pleistocene glaciations.
Endemic species in the terrestrial groups are also older than endemic fish species, dating mostly to the Pleistocene or before, even if we consider the minimum age from the 95% HPD. Nunataks (mountain peaks that have never been glaciated [96]) and peripheral refugia surrounding the Alps are some of the types of Pleistocene refugia where many terrestrial taxa may have survived glaciations [40,48,97–105], such that extinctions during glacial cycles did not entirely wipe out the terrestrial biodiversity in the region. Although amphibians are in general semi-aquatic organisms, most species only require shallow open water bodies during spring and summer, and are terrestrial for the remainder of the year, while some lack an aquatic life stage altogether [106]. Therefore, amphibians likely found refugia within the Alpine region during glacial maxima [103,104]. In this way, fragmentation of the terrestrial habitat and consequently of populations during the Pleistocene might have accelerated allopatric speciation (Pleistocene species pump) in terrestrial organisms, explaining part of the endemic diversity in this realm. A similar phenomenon likely drove Pleistocene speciation in amphipods, as recently proposed [94]. Therefore, differential impacts of Quaternary climate fluctuations and the resulting glaciations on different biomes and taxa go a long way in helping to explain extant patterns of diversity and endemism in the region.
(b) . Dispersal ability
Dispersal ability often correlates negatively with rates of niche evolution and speciation [107–109]. Therefore, the large number of cases of postglacial speciation in fully aquatic taxa and its rareness in terrestrial taxa could be related to the dispersal rates imposed by the environments. Terrestrial environments, in general, offer less resistance to dispersal and range expansion than dendritic freshwater environments, both because of the higher dimensionality of the terrestrial landscape and as many terrestrial species have acquired adaptations for aerial dispersal, such as active flight in butterflies [110,111] or passive airborne propagation in plants [112]. Conversely, freshwater-bound taxa need to navigate the dendritic landscapes to disperse, making it more difficult to reach isolated habitats [113–115]. Given that, postglacial range expansion may have happened at a slower pace in entirely aquatic than in most terrestrial taxa. Faster recolonization by terrestrial taxa after glacial retreat was likely additionally facilitated by the proximity of refugia to the Alps (including inner-alpine refugia), as can also today be observed in contemporary glacier retreats [116]. Recent studies have shown indeed that many plant species rapidly and substantially expanded their range during postglacial periods [117–119]. Faster filling of emerging terrestrial habitats through colonization and range expansion left fewer opportunities and/or less time for the first colonizing species to undergo ecological speciation and adaptive radiation in response to ecological opportunity and selective forces. To test the relative importance of dispersal limitation versus other aquatic/terrestrial differences, future work could investigate aquatic taxa with strong aerial dispersal abilities, such as Odonata and other insects that spent most of their life cycle in freshwater and have short but highly dispersive terrestrial adult phases.
(c) . Seasonal and inter-annual environmental variation
Habitat stability and predictability at ecological time scales may have been additional factors explaining the larger number of Holocene speciation events only in lacustrine fish. Theory and models suggest that environmental fluctuations and stochasticity can reduce or inhibit ecological speciation because rapid variation in environmental conditions, both seasonal and inter-annual, makes specialization difficult and ecological speciation nearly impossible, especially when predictability is low [120,121].
Environmental conditions in terrestrial ecosystems at higher latitudes are highly seasonal and the onset and length of seasons vary dramatically from year to year, especially in high-mountain environments [122,123]. Aquatic ecosystems, on the other hand, are less seasonal [124,125]. This is especially true for the deeper zones of large and deep lakes, habitats that lack seasonality and year-to-year variation almost entirely and are nearly constant through the year [126,127]. Despite its low productivity, the longer growing and reproductive season and the more stable environment in deep lakes may create favourable conditions for individual specialization and ecological speciation. Moreover, in spite of greater temporal stability, deep lakes have steeper environmental gradients because pressure, light and temperature change faster with depth in water than with elevation in the terrestrial realm. This combination of unique properties of water may facilitate divergent selection leading to speciation and helps to explain the high frequency of ecological speciation in deep lakes, with sister-species being spatially very close to each other but occupying different water depths [89].
5. Final considerations
We suggest that the formation of the unique biota of the European Alps was driven by interacting mechanisms: non-random variation in Pleistocene population persistence, postglacial immigration, vicariant speciation during glacial maxima and adaptive radiation in the postglacial. These interacting mechanisms left distinct imprints on the species age structure of regional assemblages in the different biomes and their associated taxa. Historical factors (Quaternary climate fluctuations and Pleistocene refuge availability) impacted freshwater and terrestrial biomes in different ways, and contemporary ecological factors such as environmental stochasticity and dispersal limitations also vary between these biomes, shaping them very differently through ecological and evolutionary processes: high survival, fast recolonization but slow diversification in terrestrial, versus low survival, slow recolonization but fast diversification in aquatic systems. In the future, a broader taxonomic sampling, including other insect groups, mammals, birds and reptiles, as well as sampling alpine ecosystems in other parts of the world, may shed additional light on the generality of our findings.
Knowing the history of biodiversity formation is crucial for establishing strategies for conservation [128]. Our results confirm that the Alps is an endemism hotspot and improve our understanding of how this endemism emerged. Endemic species are range-restricted, show limited population size and are hence more vulnerable to climate change and other environmental changes than non-endemic species [129]. Because of that, they are of high concern for conservation. Even a comparatively small and transient disturbance of an ecosystem can lead to the extinction of young endemic species that evolved in adaptation to specific ecological conditions [130]. The sharp increase in extinction rates driven by human activity threatens the biodiversity of the Alps, and especially that of endemic species [131]. Therefore, this region, and alpine regions around the world, deserves greater attention to conserve both the regional biodiversity, as well as the eco-evolutionary processes that gave rise to it and that are required to continue operating if biodiversity is to be safeguarded in the longer term.
Acknowledgements
We thank Salome Mwaiko and Rosi Siber for laboratory and GIS support, respectively, and Timothy Alexander for his work in the execution of the Projet Lac. Info Fauna provided distribution data of Swiss fishes. We thank Natalia Tkach, Adrien Favre, Józef Mitka, Carmen Benitez-Benitez, Markus Dillenberger, Andrew Crowl, Gerard Talavera and Božo Frajman for sharing published phylogenetic trees; Kay Lucek, Hanna ten Brink and Catalina Chaparro for thoughtful discussion; and the two reviewers and editor for suggestions and constructive criticisms.
Data accessibility
Newly generated COI-sequences: GenBank accessions ON934039–ON934153. Phylogenetic data and DNA sequence alignments: available at Mendeley Data, https://dx.doi.org/10.17632/95n8kx8h54.1 [132].
The data are provided in the electronic supplementary material [133].
Authors' contributions
L.J.Q.: conceptualization, data curation, formal analysis, investigation, methodology, visualization and writing—original draft; C.J.D.: data curation and writing—review and editing; F.A.: data curation, funding acquisition, investigation, resources and writing—review and editing; R.A.: data curation, investigation, resources and writing—review and editing; Š.B.: data curation, investigation, resources and writing—review and editing; J.B.: data curation, funding acquisition, investigation, resources and writing—review and editing; M.M.G.: funding acquisition, resources and writing—review and editing; C.H.G.: funding acquisition, resources and writing—review and editing; B.M.: funding acquisition, resources and writing—review and editing; I.R.M.: writing—review and editing; L.P.: funding acquisition, resources and writing—review and editing; T.S.: data curation, investigation, resources and writing—review and editing; O.M.S.: data curation, investigation, resources and writing—review and editing; S.V.: data curation and writing—review and editing; L.R.: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision and writing—review and editing; N.E.Z.: conceptualization, funding acquisition, project administration, resources, supervision and writing—review and editing; O.S.: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Funding from the ETH Board through the Blue-Green Biodiversity (BGB) Initiative (BGB 2020) is acknowledged. L.J.Q. was also supported by an Eawag's internal grant (Academic Transition Grant; 5221.00979.008.05 ATG21), and Š.B. by the Slovenian Research Agency through funding programme P1-0184, project J1-2464 and PhD grant.
References
- 1.MacArthur RH, Wilson EO. 1963. An equilibrium theory of insular zoogeography. Evolution 17, 373-387. ( 10.2307/2407089) [DOI] [Google Scholar]
- 2.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton Univ. Press. [Google Scholar]
- 3.Rosindell J, Phillimore AB. 2011. A unified model of island biogeography sheds light on the zone of radiation. Ecol. Lett. 14, 552-560. ( 10.1111/j.1461-0248.2011.01617.x) [DOI] [PubMed] [Google Scholar]
- 4.Losos JB, Schluter D. 2000. Analysis of an evolutionary species–area relationship. Nature 408, 847-850. ( 10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig ML. 2001. Loss of speciation rate will impoverish future diversity. Proc. Natl Acad. Sci. USA 98, 5404-5410. ( 10.1073/pnas.101092798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valente L, et al. 2020. A simple dynamic model explains the diversity of island birds worldwide. Nature 579, 92-96. ( 10.1038/s41586-020-2022-5) [DOI] [PubMed] [Google Scholar]
- 7.Merckx VSFT, et al. 2015. Evolution of endemism on a young tropical mountain. Nature 524, 347-350. ( 10.1038/nature14949) [DOI] [PubMed] [Google Scholar]
- 8.Willis JC. 1918. The age and area hypothesis. Science 47, 626-628. ( 10.1126/science.47.1226.626) [DOI] [PubMed] [Google Scholar]
- 9.Gaston KJ. 1998. Species-range size distributions: products of speciation, extinction and transformation. Phil. Trans. R. Soc. Lond. B 353, 219-230. ( 10.1098/rstb.1998.0204) [DOI] [Google Scholar]
- 10.Ribas CC, Aleixo A, Nogueira ACR, Miyaki CY, Cracraft J. 2012. A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proc. R. Soc. B 279, 681-689. ( 10.1098/rspb.2011.1120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JH. 1984. On the relationship between abundance and distribution of species. Am. Nat. 124, 255-279. ( 10.1086/284267) [DOI] [Google Scholar]
- 12.Smith BT, et al. 2014. The drivers of tropical speciation. Nature 515, 406-409. ( 10.1038/nature13687) [DOI] [PubMed] [Google Scholar]
- 13.Louthan AM, Doak DF, Angert AL. 2015. Where and when do species interactions set range limits? Trends Ecol. Evol. 30, 780-792. ( 10.1016/j.tree.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 14.Grandcolas P, Nattier R, Trewick S. 2014. Relict species: a relict concept? Trends Ecol. Evol. 29, 655-663. ( 10.1016/j.tree.2014.10.002) [DOI] [PubMed] [Google Scholar]
- 15.Körner C. 2004. Mountain biodiversity, its causes and function. Ambi 33, 11-17. ( 10.1007/0044-7447-33.sp13.11) [DOI] [PubMed] [Google Scholar]
- 16.Rahbek C, et al. 2019. Building mountain biodiversity: geological and evolutionary processes. Science 365, 1114-1119. ( 10.1126/science.aax0151) [DOI] [PubMed] [Google Scholar]
- 17.Hughes C, Eastwood R. 2006. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA 103, 10 334-10 339. ( 10.1073/pnas.0601928103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes CE, Atchison GW. 2015. The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. New Phytol. 207, 275-282. ( 10.1111/nph.13230) [DOI] [PubMed] [Google Scholar]
- 19.Bertuzzo E, Carrara F, Mari L, Altermatt F, Rodriguez-Iturbe I, Rinaldo A. 2016. Geomorphic controls on elevational gradients of species richness. Proc. Natl Acad. Sci. USA 113, 1737-1742. ( 10.1073/pnas.1518922113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie RG, et al. 2020. Comparing adaptive radiations across space, time, and taxa. J. Hered. 111, 1-20. ( 10.1093/jhered/esz064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comes HP, Kadereit JW. 2003. Spatial and temporal patterns in the evolution of the flora of the European Alpine System. TAXON 52, 451-462. ( 10.2307/3647382) [DOI] [Google Scholar]
- 22.Roquet C, Boucher FC, Thuiller W, Lavergne S. 2013. Replicated radiations of the alpine genus Androsace (Primulaceae) driven by range expansion and convergent key innovations. J. Biogeogr. 40, 1874-1886. ( 10.1111/jbi.12135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LB, Peter Comes H, Kadereit WJ. 2015. The temporal course of Quaternary diversification in the European high mountain endemic Primula sect. Auricula (Primulaceae). Int. J. Plant Sci. 165, 191–207. ( 10.1086/380747) [DOI] [Google Scholar]
- 24.Peña C, Witthauer H, Klečková I, Fric Z, Wahlberg N. 2015. Adaptive radiations in butterflies: evolutionary history of the genus Erebia (Nymphalidae: Satyrinae). Biol. J. Linn. Soc. 116, 449-467. ( 10.1111/bij.12597) [DOI] [Google Scholar]
- 25.Pitteloud C, et al. 2017. Climatic niche evolution is faster in sympatric than allopatric lineages of the butterfly genus Pyrgus. Proc. R. Soc. B 284, 20170208. ( 10.1098/rspb.2017.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altermatt F, Alther R, Fišer C, Jokela J, Konec M, Küry D, Mächler E, Stucki P, Westram AM. 2014. Diversity and distribution of freshwater amphipod species in Switzerland (Crustacea: Amphipoda). PLoS ONE 9, e110328. ( 10.1371/journal.pone.0110328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fišer C, Delić T, Luštrik R, Zagmajster M, Altermatt F. 2019. Niches within a niche: ecological differentiation of subterranean amphipods across Europe's interstitial waters. Ecography 42, 1212-1223. ( 10.1111/ecog.03983) [DOI] [Google Scholar]
- 28.Borko Š, Trontelj P, Seehausen O, Moškrič A, Fišer C. 2021. A subterranean adaptive radiation of amphipods in Europe. Nat. Commun. 12, 3688. ( 10.1038/s41467-021-24023-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson AG, Vonlanthen P, Seehausen O. 2011. Rapid parallel adaptive radiations from a single hybridogenic ancestral population. Proc. R. Soc. B 278, 58-66. ( 10.1098/rspb.2010.0925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucek K, Keller I, Nolte AW, Seehausen O. 2018. Distinct colonization waves underlie the diversification of the freshwater sculpin (Cottus gobio) in the Central European Alpine region. J. Evol. Biol. 31, 1254-1267. ( 10.1111/jeb.13339) [DOI] [PubMed] [Google Scholar]
- 31.Fitzsimons SJ, Veit H. 2001. Geology and geomorphology of the European Alps and the Southern Alps of New Zealand. mred 21, 340-349. ( 10.1659/0276-4741(2001)021[0340:GAGOTE]2.0.CO;2) [DOI] [Google Scholar]
- 32.Fauquette S, et al. 2015. Quantifying the Eocene to Pleistocene topographic evolution of the southwestern Alps, France and Italy. Earth Planet. Sci. Lett. 412, 220-234. ( 10.1016/j.epsl.2014.12.036) [DOI] [Google Scholar]
- 33.Heads M. 2019. Passive uplift of plant and animal populations during mountain-building. Cladistics 35, 550-572. ( 10.1111/cla.12368) [DOI] [PubMed] [Google Scholar]
- 34.Ding WN, Ree RH, Spicer RA, Xing YW. 2020. Ancient orogenic and monsoon-driven assembly of the world's richest temperate alpine flora. Science 369, 578-581. ( 10.1126/science.abb4484) [DOI] [PubMed] [Google Scholar]
- 35.Hewitt GM. 1999. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 68, 87-112. ( 10.1111/j.1095-8312.1999.tb01160.x) [DOI] [Google Scholar]
- 36.Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405, 907-913. ( 10.1038/35016000) [DOI] [PubMed] [Google Scholar]
- 37.Hänfling B, Hellemans B, Volckaert FAM, Carvalho GR. 2002. Late glacial history of the cold-adapted freshwater fish Cottus gobio, revealed by microsatellites. Mol. Ecol. 11, 1717-1729. ( 10.1046/j.1365-294X.2002.01563.x) [DOI] [PubMed] [Google Scholar]
- 38.Stehlik I. 2003. Resistance or emigration? Response of alpine plants to the ice ages. TAXON 52, 499-510. ( 10.2307/3647448) [DOI] [Google Scholar]
- 39.Neuenschwander S, Largiadèr CR, Ray N, Currat M, Vonlanthen P, Excoffier L. 2008. Colonization history of the Swiss Rhine basin by the bullhead (Cottus gobio): inference under a Bayesian spatially explicit framework. Mol. Ecol. 17, 757-772. ( 10.1111/j.1365-294X.2007.03621.x) [DOI] [PubMed] [Google Scholar]
- 40.Menchetti M, et al. 2021. Two ways to be endemic. Alps and Apennines are different functional refugia during climatic cycles. Mol. Ecol. 30, 1297-1310. ( 10.1111/mec.15795) [DOI] [PubMed] [Google Scholar]
- 41.Médail F, Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 36, 1333-1345. ( 10.1111/j.1365-2699.2008.02051.x) [DOI] [Google Scholar]
- 42.Holm SR, Svenning JC. 2014. 180,000 years of climate change in Europe: avifaunal responses and vegetation implications. PLoS ONE 9, e94021. ( 10.1371/journal.pone.0094021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordonez A, Svenning JC. 2017. Consistent role of Quaternary climate change in shaping current plant functional diversity patterns across European plant orders. Sci. Rep. 7, 42988. ( 10.1038/srep42988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoville SD, Roderick GK, Kavanaugh DH. 2012. Testing the ‘Pleistocene species pump’ in alpine habitats: lineage diversification of flightless ground beetles (Coleoptera: Carabidae: Nebria) in relation to altitudinal zonation. Biol. J. Linn. Soc. 107, 95-111. ( 10.1111/j.1095-8312.2012.01911.x) [DOI] [Google Scholar]
- 45.April J, Hanner RH, Dion-Côté AM, Bernatchez L. 2013. Glacial cycles as an allopatric speciation pump in north-eastern American freshwater fishes. Mol. Ecol. 22, 409-422. ( 10.1111/mec.12116) [DOI] [PubMed] [Google Scholar]
- 46.Schluter D, Rambaut A, Clarke BC, Grant PR. 1996. Ecological speciation in postglacial fishes. Phil. Trans. R. Soc. Lond. B 351, 807-814. ( 10.1098/rstb.1996.0075) [DOI] [Google Scholar]
- 47.Hewitt GM. 2004. Genetic consequences of climatic oscillations in the Quaternary. Phil. Trans. R. Soc. Lond. B 359, 183-195. ( 10.1098/rstb.2003.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holderegger R, Thiel-Egenter C. 2009. A discussion of different types of glacial refugia used in mountain biogeography and phylogeography. J. Biogeogr. 36, 476-480. ( 10.1111/j.1365-2699.2008.02027.x) [DOI] [Google Scholar]
- 49.Stroud JT, Losos JB. 2016. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst 47, 507-532. ( 10.1146/annurev-ecolsys-121415-032254) [DOI] [Google Scholar]
- 50.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: OUP. [Google Scholar]
- 51.Kangas VM, Kvist L, Kholodova M, Nygrén T, Danilov P, Panchenko D, Fraimout A, Aspi J. 2015. Evidence of post-glacial secondary contact and subsequent anthropogenic influence on the genetic composition of Fennoscandian moose (Alces alces). J. Biogeogr. 42, 2197-2208. ( 10.1111/jbi.12582) [DOI] [Google Scholar]
- 52.Seehausen O. 2004. Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198-207. ( 10.1016/j.tree.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 53.Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8, 14363. ( 10.1038/ncomms14363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg EE, Roy K, Lande R, Jablonski D. 2005. Diversity, endemism, and age distributions in macroevolutionary sources and sinks. Am. Nat. 165, 623-633. ( 10.1086/430012) [DOI] [PubMed] [Google Scholar]
- 55.Pellissier L, et al. 2014. Quaternary coral reef refugia preserved fish diversity. Science 344, 1016-1019. ( 10.1126/science.1249853) [DOI] [PubMed] [Google Scholar]
- 56.Rull V. 2008. Speciation timing and neotropical biodiversity: the tertiary–Quaternary debate in the light of molecular phylogenetic evidence. Mol. Ecol. 17, 2722-2729. ( 10.1111/j.1365-294X.2008.03789.x) [DOI] [PubMed] [Google Scholar]
- 57.Seguinot J, Ivy-Ochs S, Jouvet G, Huss M, Funk M, Preusser F. 2018. Modelling last glacial cycle ice dynamics in the Alps. The Cryosphere 12, 3265-3285. ( 10.5194/tc-12-3265-2018) [DOI] [Google Scholar]
- 58.Schmitt T. 2017. Molecular biogeography of the high mountain systems of Europe: an overview. In High mountain conservation in a changing world (eds Catalan J, Ninot JM, Aniz MM), pp. 63-74. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 59.Mansion G, et al. 2012. How to handle speciose clades? Mass Taxon-sampling as a strategy towards illuminating the natural history of Campanula (Campanuloideae). PLoS ONE 7, e50076. ( 10.1371/journal.pone.0050076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiemers M, Chazot N, Wheat CW, Schweiger O, Wahlberg N. 2020. A complete time-calibrated multi-gene phylogeny of the European butterflies. ZooKeys 938, 97-124. ( 10.3897/zookeys.938.50878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jetz W, Pyron RA. 2018. The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat. Ecol. Evol. 2, 850-858. ( 10.1038/s41559-018-0515-5) [DOI] [PubMed] [Google Scholar]
- 62.Fric ZF, Maresova J, Kadlec T, Tropek R, Pyrcz TW, Wiemers M. 2019. World travellers: phylogeny and biogeography of the butterfly genus Leptotes (Lepidoptera: Lycaenidae). Syst. Entomol. 44, 652-665. ( 10.1111/syen.12349) [DOI] [Google Scholar]
- 63.Ebdon S, Laetsch DR, Dapporto L, Hayward A, Ritchie MG, Dincǎ V, Vila R, Lohse K. 2021. The Pleistocene species pump past its prime: evidence from European butterfly sister species. Mol. Ecol. 30, 3575-3589. ( 10.1111/mec.15981) [DOI] [PubMed] [Google Scholar]
- 64.Talavera G, Lukhtanov V, Pierce NE, Vila R. 2022. DNA barcodes combined with multilocus data of representative taxa can generate reliable higher-level phylogenies. Syst. Biol. 71, 382-395. ( 10.1093/sysbio/syab038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, He B, Tao R, Su C, Ma J, Hao J, Yang Q. 2022. Phylogeny and biogeographic history of Parnassius butterflies (Papilionidae: Parnassiinae) reveal their origin and deep diversification in West China. Insects 13, 406. ( 10.3390/insects13050406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inda LA, Segarra-Moragues JG, Müller J, Peterson PM, Catalán P. 2008. Dated historical biogeography of the temperate Loliinae (Poaceae, Pooideae) grasses in the northern and southern hemispheres. Mol. Phylogenet. Evol. 46, 932-957. ( 10.1016/j.ympev.2007.11.022) [DOI] [PubMed] [Google Scholar]
- 67.Boucher FC, Thuiller W, Roquet C, Douzet R, Aubert S, Alvarez N, Lavergne S. 2012. Reconstructing the origins of high-alpine niches and cushion life form in the genus Androsace S.l. (Primulaceae). Evolution 66, 1255-1268. ( 10.1111/j.1558-5646.2011.01483.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boucher FC, Zimmermann NE, Conti E. 2016. Allopatric speciation with little niche divergence is common among alpine Primulaceae. J. Biogeogr. 43, 591-602. ( 10.1111/jbi.12652) [DOI] [Google Scholar]
- 69.Crowl AA, Mavrodiev E, Mansion G, Haberle R, Pistarino A, Kamari G, Phitos D, Borsch T, Cellinese N. 2014. Phylogeny of campanuloideae (Campanulaceae) with emphasis on the utility of nuclear pentatricopeptide repeat (PPR) genes. PLoS ONE 9, e94199. ( 10.1371/journal.pone.0094199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Favre A, et al. 2016. Out-of-Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae). J. Biogeogr. 43, 1967-1978. ( 10.1111/jbi.12840) [DOI] [Google Scholar]
- 71.Frajman B, Rešetnik I, Niketić M, Ehrendorfer F, Schönswetter P. 2016. Patterns of rapid diversification in heteroploid Knautia sect. Trichera (Caprifoliaceae, Dipsacoideae), one of the most intricate taxa of the European flora. BMC Evol. Biol. 16, 204. ( 10.1186/s12862-016-0773-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steffen S, Dillenberger MS, Kadereit JW. 2016. Of dwarfs and giants: phylogeny of the Petasites-clade (Asteraceae-Senecioneae) and evolution of miniaturization in Arctic-Alpine environments. Plant Syst. Evol. 302, 545-559. ( 10.1007/s00606-016-1282-x) [DOI] [Google Scholar]
- 73.Tkach N, Röser M, Suchan T, Cieślak E, Schönswetter P, Ronikier M. 2019. Contrasting evolutionary origins of two mountain endemics: Saxifraga wahlenbergii (Western Carpathians) and S. styriaca (Eastern Alps). BMC Evol. Biol. 19, 18. ( 10.1186/s12862-019-1355-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benítez-Benítez C, et al. 2021. Geographical vs. ecological diversification in Carex section Phacocystis (Cyperaceae): patterns hidden behind a twisted taxonomy. J. Syst. Evol. 59, 642-667. ( 10.1111/jse.12731) [DOI] [Google Scholar]
- 75.Mamos T, Wattier R, Burzyński A, Grabowski M. 2016. The legacy of a vanished sea: a high level of diversification within a European freshwater amphipod species complex driven by 15 My of Paratethys regression. Mol. Ecol. 25, 795-810. ( 10.1111/mec.13499) [DOI] [PubMed] [Google Scholar]
- 76.Davolos D, Matthaeis ED, Latella L, Tarocco M, Özbek M, Vonk R. 2018. On the molecular and morphological evolution of continental and insular Cryptorchestia species, with an additional description of C. garbinii (Talitridae). ZooKeys 783, 37-54. ( 10.3897/zookeys.783.26179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sket B, Hou Z. 2018. Family Gammaridae (Crustacea: Amphipoda), mainly its Echinogammarus clade in SW Europe. Further elucidation of its phylogeny and taxonomy. Acta Biol. Slov. 61, 93-102. [Google Scholar]
- 78.Copilaş-Ciocianu D, Sidorov D, Gontcharov A. 2019. Adrift across tectonic plates: molecular phylogenetics supports the ancient Laurasian origin of old limnic crangonyctid amphipods. Org. Divers. Evol. 19, 191-207. ( 10.1007/s13127-019-00401-7) [DOI] [Google Scholar]
- 79.Bermingham E, McCafferty SS, Martin AP. 1997. Fish biogeography and molecular clocks: perspectives from the Panamanian Isthmus. In Molecular systematics of fishes (eds Kocher TD, Stepien CA), pp. 113-128. San Diego, CA: Academic Press. [Google Scholar]
- 80.Hothorn T, Hornik K, van de Wiel MA, Zeileis A.. 2008. Implementing a class of permutation tests: the coin package. J. Stat. Softw. 28, 1-23. ( 10.18637/jss.v028.i08)27774042 [DOI] [Google Scholar]
- 81.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 82.Jost J, Kälin D, Börner S, Vasilyan D, Lawver D, Reichenbacher B. 2015. Vertebrate microfossils from the Upper Freshwater Molasse in the Swiss Molasse Basin: implications for the evolution of the North Alpine Foreland Basin during the miocene climate optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 426, 22-33. ( 10.1016/j.palaeo.2015.02.028) [DOI] [Google Scholar]
- 83.Keith P, Poulet N, Denys G, Changeux T, Feunteun E, Persat H. 2020. Les poissons d'eau douce de France, 2nd edn. Mèze, France: BIOTOPE. [Google Scholar]
- 84.Seifertová M, Bryja J, Vyskočilová M, Martínková N, Šimková A. 2012. Multiple Pleistocene refugia and post-glacial colonization in the European chub (Squalius cephalus) revealed by combined use of nuclear and mitochondrial markers. J. Biogeogr. 39, 1024-1040. ( 10.1111/j.1365-2699.2011.02661.x) [DOI] [Google Scholar]
- 85.Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O'Connell MF, Mortensen E. 2003. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshw. Fish 12, 1-59. ( 10.1034/j.1600-0633.2003.00010.x) [DOI] [Google Scholar]
- 86.Salisbury SJ, Ruzzante DE. 2022. Genetic causes and consequences of sympatric morph divergence in Salmonidae: a search for mechanisms. Ann. Rev. Anim. Biosci. 10, 81-106. ( 10.1146/annurev-animal-051021-080709) [DOI] [PubMed] [Google Scholar]
- 87.Doenz CJ, Bittner D, Vonlanthen P, Wagner CE, Seehausen O. 2018. Rapid buildup of sympatric species diversity in Alpine whitefish. Ecol. Evol. 8, 9398-9412. ( 10.1002/ece3.4375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hudson AG, Lundsgaard-Hansen B, Lucek K, Vonlanthen P, Seehausen O. 2017. Managing cryptic biodiversity: fine-scale intralacustrine speciation along a benthic gradient in Alpine whitefish (Coregonus spp.). Evol. Appl. 10, 251-266. ( 10.1111/eva.12446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seehausen O, Wagner C. 2014. Speciation in freshwater fishes. Ann. Rev. Ecol. Evol. Syst. 45, 621-651. ( 10.1146/annurev-ecolsys-120213-091818) [DOI] [Google Scholar]
- 90.Freyhof J, Kottelat M. 2007. Handbook of European freshwater fishes. See https://portals.iucn.org/library/node/9068.
- 91.Splendiani A, Ruggeri P, Giovannotti M, Pesaresi S, Occhipinti G, Fioravanti T, Lorenzoni M, Nisi Cerioni P, Caputo Barucchi V. 2016. Alien brown trout invasion of the Italian peninsula: the role of geological, climate and anthropogenic factors. Biol. Invasions 18, 2029-2044. ( 10.1007/s10530-016-1149-7) [DOI] [Google Scholar]
- 92.Hou Z, Sket B, Fišer C, Li S. 2011. Eocene habitat shift from saline to freshwater promoted Tethyan amphipod diversification. Proc. Natl Acad. Sci. USA 108, 14 533-14 538. ( 10.1073/pnas.1104636108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Copilaş-Ciocianu D, Borko Š, Fišer C. 2020. The late blooming amphipods: global change promoted post-Jurassic ecological radiation despite Palaeozoic origin. Mol. Phylogenet. Evol. 143, 106664. ( 10.1016/j.ympev.2019.106664) [DOI] [PubMed] [Google Scholar]
- 94.Borko Š, Altermatt F, Zagmajster M, Fišer C. 2022. A hotspot of groundwater amphipod diversity on a crossroad of evolutionary radiations. Divers. Distrib. 13, 1–13. ( 10.1111/ddi.13500) [DOI] [Google Scholar]
- 95.Vader W, Tandberg AHS. 2019. Gammarid amphipods (Crustacea) in Norway, with a key to the species. Fauna Norvegica 39, 12-25. ( 10.5324/fn.v39i0.2873) [DOI] [Google Scholar]
- 96.Dahl E. 1987. The nunatak theory reconsidered. Ecol. Bullet. 38, 77-94. [Google Scholar]
- 97.Nordal I. 1987. Tabula rasa after all? Botanical evidence for ice-free refugia in Scandinavia reviewed. J. Biogeogr. 14, 377-388. ( 10.2307/2844945) [DOI] [Google Scholar]
- 98.Schönswetter P, Stehlik I, Holderegger R, Tribsch A. 2005. Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol. Ecol. 14, 3547-3555. ( 10.1111/j.1365-294X.2005.02683.x) [DOI] [PubMed] [Google Scholar]
- 99.Szövényi P, Arroyo K, Guggisberg A, Conti E. 2009. Effects of Pleistocene glaciations on the genetic structure of Saxifraga florulenta (Saxifragaceae), a rare endemic of the Maritime Alps. TAXON 58, 532-543. ( 10.1002/tax.582017) [DOI] [Google Scholar]
- 100.Schneeweiss GM, Schönswetter P. 2011. A re-appraisal of nunatak survival in arctic-alpine phylogeography. Mol. Ecol. 20, 190-192. ( 10.1111/j.1365-294X.2010.04927.x) [DOI] [PubMed] [Google Scholar]
- 101.Westergaard KB, Alsos IG, Popp M, Engelskjøn T, Flatberg KI, Brochmann C. 2011. Glacial survival may matter after all: nunatak signatures in the rare European populations of two west-arctic species. Mol. Ecol. 20, 376-393. ( 10.1111/j.1365-294X.2010.04928.x) [DOI] [PubMed] [Google Scholar]
- 102.Schmitt T, Louy D, Zimmermann E, Habel JC. 2016. Species radiation in the Alps: multiple range shifts caused diversification in Ringlet butterflies in the European high mountains. Org. Divers. Evol. 16, 791-808. ( 10.1007/s13127-016-0282-6) [DOI] [Google Scholar]
- 103.Vörös J, Mikulíček P, Major Á, Recuero E, Arntzen JW. 2016. Phylogeographic analysis reveals northerly refugia for the riverine amphibian Triturus dobrogicus (Caudata: Salamandridae). Biol. J. Linn. Soc. 119, 974-991. ( 10.1111/bij.12866) [DOI] [Google Scholar]
- 104.Lucati F, et al. 2020. Multiple glacial refugia and contemporary dispersal shape the genetic structure of an endemic amphibian from the Pyrenees. Mol. Ecol. 29, 2904-2921. ( 10.1111/mec.15521) [DOI] [PubMed] [Google Scholar]
- 105.Pan D, Hülber K, Willner W, Schneeweiss GM. 2020. An explicit test of Pleistocene survival in peripheral versus nunatak refugia in two high mountain plant species. Mol. Ecol. 29, 172-183. ( 10.1111/mec.15316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wells KD. 2010. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 107.Claramunt S, Derryberry EP, Remsen JV, Brumfield RT. 2012. High dispersal ability inhibits speciation in a continental radiation of passerine birds. Proc. R. Soc. B 279, 1567-1574. ( 10.1098/rspb.2011.1922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riginos C, Buckley YM, Blomberg SP, Treml EA. 2014. Dispersal capacity predicts both population genetic structure and species richness in reef fishes. Am. Nat. 184, 52-64. ( 10.1086/676505) [DOI] [PubMed] [Google Scholar]
- 109.Fernández-Palacios JM, Otto R, Borregaard MK, Kreft H, Price JP, Steinbauer MJ, Weigelt P, Whittaker RJ. 2021. Evolutionary winners are ecological losers among oceanic island plants. J. Biogeogr. 48, 2186-2198. ( 10.1111/jbi.14143) [DOI] [Google Scholar]
- 110.Stevens VM, Turlure C, Baguette M. 2010. A meta-analysis of dispersal in butterflies. Biol. Rev. 85, 625-642. ( 10.1111/j.1469-185X.2009.00119.x) [DOI] [PubMed] [Google Scholar]
- 111.Stevens VM, Trochet A, Dyck HV, Clobert J, Baguette M. 2012. How is dispersal integrated in life histories: a quantitative analysis using butterflies. Ecol. Lett. 15, 74-86. ( 10.1111/j.1461-0248.2011.01709.x) [DOI] [PubMed] [Google Scholar]
- 112.Frei T. 1997. Pollen distribution at high elevation in Switzerland: evidence for medium range transport. Grana 36, 34-38. ( 10.1080/00173139709362587) [DOI] [Google Scholar]
- 113.Brown BL, Swan CM. 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. J. Anim. Ecol. 79, 571-580. ( 10.1111/j.1365-2656.2010.01668.x) [DOI] [PubMed] [Google Scholar]
- 114.Altermatt F. 2013. Diversity in riverine metacommunities: a network perspective. Aquat. Ecol. 47, 365-377. ( 10.1007/s10452-013-9450-3) [DOI] [Google Scholar]
- 115.Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C. 2013. Individual dispersal, landscape connectivity and ecological networks. Biol. Rev. 88, 310-326. ( 10.1111/brv.12000) [DOI] [PubMed] [Google Scholar]
- 116.Losapio G, Cerabolini BEL, Maffioletti C, Tampucci D, Gobbi M, Caccianiga M. 2021. The consequences of Glacier retreat are uneven between plant species. Front. Ecol. Evol. 8, 616562. ( 10.3389/fevo.2020.616562) [DOI] [Google Scholar]
- 117.Magri D, et al. 2006. A new scenario for the Quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol. 171, 199-221. ( 10.1111/j.1469-8137.2006.01740.x) [DOI] [PubMed] [Google Scholar]
- 118.Tinner W, Lotter AF. 2006. Holocene expansions of Fagus silvatica and Abies alba in Central Europe: where are we after eight decades of debate? Quat. Sci. Rev. 25, 526-549. ( 10.1016/j.quascirev.2005.03.017) [DOI] [Google Scholar]
- 119.Magri D. 2008. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 35, 450-463. ( 10.1111/j.1365-2699.2007.01803.x) [DOI] [Google Scholar]
- 120.Johansson J, Ripa J. 2006. Will sympatric speciation fail due to stochastic competitive exclusion? Am. Nat. 168, 572-578. ( 10.1086/507996) [DOI] [PubMed] [Google Scholar]
- 121.Ripa J, Dieckmann U. 2013. Mutant invasions and adaptive dynamics in variable environments. Evolution 67, 1279-1290. ( 10.1111/evo.12046) [DOI] [PubMed] [Google Scholar]
- 122.Häder DP, Barnes PW. 2019. Comparing the impacts of climate change on the responses and linkages between terrestrial and aquatic ecosystems. Sci. Total Environ. 682, 239-246. ( 10.1016/j.scitotenv.2019.05.024) [DOI] [PubMed] [Google Scholar]
- 123.Liu J, Liu J, Linderholm HW, Chen D, Yu Q, Wu D, Haginoya S. 2012. Observation and calculation of the solar radiation on the Tibetan Plateau. Energy Convers. Manage. 57, 23-32. ( 10.1016/j.enconman.2011.12.007) [DOI] [Google Scholar]
- 124.Halley JM. 2005. Comparing aquatic and terrestrial variability: at what scale do ecologists communicate? Mar. Ecol. Prog. Ser. 304, 274-280. [Google Scholar]
- 125.Steele JH, Brink KH, Scott BE. 2019. Comparison of marine and terrestrial ecosystems: suggestions of an evolutionary perspective influenced by environmental variation. ICES J. Mar. Sci. 76, 50-59. ( 10.1093/icesjms/fsy149) [DOI] [Google Scholar]
- 126.Fenocchi A, Rogora M, Sibilla S, Dresti C. 2017. Relevance of inflows on the thermodynamic structure and on the modeling of a deep subalpine lake (Lake Maggiore, Northern Italy/Southern Switzerland). Limnologica 63, 42-56. ( 10.1016/j.limno.2017.01.006) [DOI] [Google Scholar]
- 127.Arai T. 1981. Attenuation of incident solar radiation in Lake Water. Jap. J. Limniol. 42, 92-99. ( 10.3739/rikusui.42.92) [DOI] [Google Scholar]
- 128.Posadas P, Esquivel DRM, Crisci JV. 2001. Using phylogenetic diversity measures to set priorities in conservation: an example from southern South America. Conserv. Biol. 15, 1325-1334. ( 10.1111/j.1523-1739.2001.99404.x) [DOI] [Google Scholar]
- 129.Manes S, et al. 2021. Endemism increases species' climate change risk in areas of global biodiversity importance. Biol. Conserv. 257, 109070. ( 10.1016/j.biocon.2021.109070) [DOI] [Google Scholar]
- 130.Vonlanthen P, et al. 2012. Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482, 357-362. ( 10.1038/nature10824) [DOI] [PubMed] [Google Scholar]
- 131.Johnson CN, Balmford A, Brook BW, Buettel JC, Galetti M, Guangchun L, Wilmshurst JM. 2017. Biodiversity losses and conservation responses in the Anthropocene. Science 356, 270-275. ( 10.1126/science.aam9317) [DOI] [PubMed] [Google Scholar]
- 132.Jardim de Queiroz L, et al. 2022. Data from: climate, immigration and speciation shape terrestrial and aquatic biodiversity in the European Alps. Mendeley Data ( 10.17632/95n8kx8h54.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jardim de Queiroz L, et al. 2022. Climate, immigration and speciation shape terrestrial and aquatic biodiversity in the European Alps. FigShare. ( 10.6084/m9.figshare.c.6124007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Newly generated COI-sequences: GenBank accessions ON934039–ON934153. Phylogenetic data and DNA sequence alignments: available at Mendeley Data, https://dx.doi.org/10.17632/95n8kx8h54.1 [132].
The data are provided in the electronic supplementary material [133].


