Key Points
Question
Is ticagrelor dual antiplatelet therapy (DAPT) for patients undergoing coronary artery bypass graft surgery associated with differences in vein graft failure and bleeding events compared with aspirin?
Findings
In this individual patient data meta-analysis that included 4 randomized clinical trials, 1316 patients and 1668 vein grafts, ticagrelor DAPT compared with aspirin was associated with a significantly lower incidence of vein graft failure (11.2% vs 20.0%) and a significantly higher incidence of Bleeding Academic Research Consortium type 2, 3, or 5 bleeding events (22.1% vs 8.7%).
Meaning
In patients undergoing coronary artery bypass graft surgery, adding ticagrelor to aspirin was associated with a significantly decreased risk of vein graft failure, as well as a significantly increased risk of clinically important bleeding.
Abstract
Importance
The role of ticagrelor with or without aspirin after coronary artery bypass graft surgery remains unclear.
Objective
To compare the risks of vein graft failure and bleeding associated with ticagrelor dual antiplatelet therapy (DAPT) or ticagrelor monotherapy vs aspirin among patients undergoing coronary artery bypass graft surgery.
Data Sources
MEDLINE, Embase, and Cochrane Library databases from inception to June 1, 2022, without language restriction.
Study Selection
Randomized clinical trials (RCTs) comparing the effects of ticagrelor DAPT or ticagrelor monotherapy vs aspirin on saphenous vein graft failure.
Data Extraction and Synthesis
Individual patient data provided by each trial were synthesized into a combined data set for independent analysis. Multilevel logistic regression models were used.
Main Outcomes and Measures
The primary analysis assessed the incidence of saphenous vein graft failure per graft (primary outcome) in RCTs comparing ticagrelor DAPT with aspirin. Secondary outcomes were saphenous vein graft failure per patient and Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events. A supplementary analysis included RCTs comparing ticagrelor monotherapy with aspirin.
Results
A total of 4 RCTs were included in the meta-analysis, involving 1316 patients and 1668 saphenous vein grafts. Of the 871 patients in the primary analysis, 435 received ticagrelor DAPT (median age, 67 years [IQR, 60-72 years]; 65 women [14.9%]; 370 men [85.1%]) and 436 received aspirin (median age, 66 years [IQR, 61-73 years]; 63 women [14.5%]; 373 men [85.5%]). Ticagrelor DAPT was associated with a significantly lower incidence of saphenous vein graft failure (11.2%) per graft than was aspirin (20%; difference, −8.7% [95% CI, −13.5% to −3.9%]; OR, 0.51 [95% CI, 0.35 to 0.74]; P < .001) and was associated with a significantly lower incidence of saphenous vein graft failure per patient (13.2% vs 23.0%, difference, −9.7% [95% CI, −14.9% to −4.4%]; OR, 0.51 [95% CI, 0.35 to 0.74]; P < .001). Ticagrelor DAPT (22.1%) was associated with a significantly higher incidence of BARC type 2, 3, or 5 bleeding events than was aspirin (8.7%; difference, 13.3% [95% CI, 8.6% to 18.0%]; OR, 2.98 [95% CI, 1.99 to 4.47]; P < .001), but not BARC type 3 or 5 bleeding events (1.8% vs 1.8%, difference, 0% [95% CI, −1.8% to 1.8%]; OR, 1.00 [95% CI, 0.37 to 2.69]; P = .99). Compared with aspirin, ticagrelor monotherapy was not significantly associated with saphenous vein graft failure (19.3% vs 21.7%, difference, −2.6% [95% CI, −9.1% to 3.9%]; OR, 0.86 [95% CI, 0.58 to 1.27]; P = .44) or BARC type 2, 3, or 5 bleeding events (8.9% vs 7.3%, difference, 1.7% [95% CI, −2.8% to 6.1%]; OR, 1.25 [95% CI, 0.69 to 2.29]; P = .46).
Conclusions and Relevance
Among patients undergoing coronary artery bypass graft surgery, adding ticagrelor to aspirin was associated with a significantly decreased risk of vein graft failure. However, this was accompanied by a significantly increased risk of clinically important bleeding.
This systematic review and individual patient data meta-analysis of randomized clinical trials compares the effects of ticagrelor dual antiplatelet therapy or ticagrelor monotherapy with aspirin on saphenous vein graft failure among patients undergoing coronary artery bypass graft surgery.
Introduction
Saphenous vein grafts are the most frequently used conduits in coronary artery bypass graft (CABG) surgery, yet as many as 10% to 25% occlude within the first year after surgery.1,2 Early saphenous vein graft failure is mainly due to thrombosis subsequent to endothelial damage or endothelial activation leading to a prothrombotic phenotype.2,3 Inhibition of platelet aggregation with aspirin after CABG surgery has been shown to reduce early saphenous vein graft failure and is endorsed in current practice guidelines.4,5,6 Dual antiplatelet therapy (DAPT), consisting of aspirin and an oral platelet P2Y12 receptor inhibitor, is associated with enhanced platelet inhibitory effects.7 Although DAPT is the guideline-recommended treatment after percutaneous coronary revascularization,7 considerable controversy exists as to the benefit of DAPT for patients after CABG surgery. Studies comparing ticagrelor DAPT with aspirin have yielded conflicting results,8,9,10 and the few studies comparing ticagrelor monotherapy with aspirin9,11 failed to demonstrate an effect of ticagrelor monotherapy on saphenous vein graft failure; however, they were individually underpowered.
A systematic review and individual patient data meta-analysis of all randomized clinical trials (RCTs) comparing the effects of ticagrelor DAPT or ticagrelor monotherapy with aspirin on saphenous vein graft failure among patients undergoing CABG surgery was performed.
Methods
This study design was published a priori on the International Prospective Register of Systematic Reviews (CRD42021291997). The statistical analysis protocol was prespecified to reduce post hoc bias. The analysis was performed in accordance with the Individual Patient Data-Preferred Reporting Items for Systematic Reviews and Meta-Analyses (IPD-PRISMA).12 The PRISMA checklist was followed. Ethics approval and patient consent were obtained locally by each trial team. The Weill Cornell Medicine Institutional Review Board waived the need for ethics approval for the pooled analysis (protocol 22-03024559).
Search Strategy and Selection Criteria
A medical librarian searched Ovid MEDLINE, Ovid Embase, and the Cochrane Central (Wiley) databases to identify RCTs published between database inception and June 1, 2022, comparing ticagrelor DAPT and/or ticagrelor monotherapy with aspirin in patients undergoing CABG surgery who had follow-up for graft imaging. No language restrictions were imposed. The full search strategy is provided in the Supplement (eMethods 1 in the Supplement). Identification of studies meeting the search criteria was performed by 2 authors (S.S. and K.A.). Conflicts over inclusion were resolved by consultation with a third author (M.G.).
Data Extraction and Quality Assessment
The principal investigators of the eligible trials were contacted and all agreed to share individual patient data. Specifications of core minimum deidentified data requirements were provided to each trial (eMethods 2 in the Supplement). Data received from the individual trial teams by the analysis unit at Weill Cornell Medicine were checked for completeness and consistency with previous publications. Discrepancies were resolved directly with the trial investigators. For harmonization of graft failure definition across trials, occlusion and/or percent stenosis per graft or anastomosis (for sequential grafts) were provided by each trial team. Events were readjudicated centrally. For harmonization of bleeding outcomes, bleeding events were readjudicated by each trial team according to Bleeding Academic Research Consortium (BARC) criteria.13 All analyses were performed independently on the combined data set of individual patient data provided for each trial. The risk of bias was assessed using the Cochrane risk-of-bias tool 214 (eFigure 1 in the Supplement).
Outcomes
The primary outcome was the incidence of saphenous vein graft failure, defined as saphenous vein graft occlusion or stenosis greater than 50% per graft as assessed by either invasive angiography or computed tomographic angiography at the individual trial protocol–defined follow-up. Secondary outcomes were the incidence of saphenous vein graft failure per patient (defined as patients with ≥1 failed saphenous vein graft); the incidence of BARC type 2, 3, or 5 bleeding events; the composite of saphenous vein graft failure or cardiovascular death; and major adverse cardiac and cerebrovascular events (MACCE, defined as the composite of all-cause death, myocardial infarction, stroke, or revascularization). Definitions of events in the individual trials are provided in eTable 1 in the Supplement.
Post hoc outcomes were the incidence of saphenous vein graft occlusion per graft; any graft failure (arterial or saphenous vein grafts); BARC type 2 through 5, 3 through 5, and 3 or 5 bleeding events; the individual components of MACCE; major adverse cardiovascular events (MACE, defined as the composite of cardiovascular death, myocardial infarction, or stroke); and net adverse events (defined as graft failure [arterial or saphenous vein grafts] or BARC type ≥3 bleeding event), net adverse major clinical events (defined as all-cause death, myocardial infarction, stroke, or BARC type ≥3 bleeding event), and overall net adverse events (defined as graft failure, MACCE, or BARC type ≥2 bleeding events).
Data Analysis
Baseline categorical variables are reported as counts and percentages. Continuous variables are reported as medians and IQRs. The primary analysis was performed according to randomization group and compared ticagrelor DAPT with aspirin. The primary analysis set was patients with saphenous vein grafts who were randomized to ticagrelor DAPT or aspirin and for whom protocol-defined imaging was available. The primary outcome was evaluated using a multilevel logistic regression model using the GLIMMIX procedure15 that accounted for clustering of patients within trials and clustering of grafts within patients. Treatment associations are reported as odds ratios (ORs) and 95% CIs.
Secondary outcomes were evaluated using a multilevel logistic regression model with the trial as a random effect (reported as OR and 95% CI) or a Cox proportional hazards frailty model with trial as a random effect (reported as hazard ratio [HR] and 95% CI). Event rates were calculated using the Kaplan-Meier method. The proportional hazards assumption was confirmed for each end point by using Schoenfeld residuals and visual inspection of the Schoenfeld residuals, Kaplan-Meier plots, and log-log plots.
Sensitivity analyses for the primary outcome assessed the incidence of saphenous vein graft failure per anastomosis and in patients who had 1-year imaging. Sensitivity models for the primary outcome were performed in the as-treated population (according to treatment received) and per-protocol population (according to whether treatment was received in compliance with the trial protocol), and after imputation of missing data by multiple imputation (assuming a joint multivariate normal distribution for all variables and imputing 20 data sets). These sensitivity models were adjusted for baseline and procedure-related confounders that included age, sex, clinical presentation, smoking, diabetes, hypertension, hyperlipidemia, prior myocardial infarction, chronic kidney disease, use of cardiopulmonary bypass, endoscopic saphenous vein graft harvesting, and sequential saphenous vein grafting.
Prespecified subgroup analyses for the primary outcome were age, sex, diabetes, smoking, acute coronary syndrome, use of cardiopulmonary bypass, endoscopic saphenous vein graft harvesting, target vessel territory, use of sequential saphenous vein grafts, and treatment duration. For subgroup analyses, an interaction-term between the treatment and the subgroup of interest was included in the logistic regression model.
A supplementary analysis for the primary outcome compared ticagrelor monotherapy with aspirin.
Details on post hoc analyses are provided in eMethods 3 in the Supplement. A post hoc random-effects network meta-analysis was performed to compare the associations of ticagrelor DAPT, ticagrelor monotherapy, and aspirin with saphenous vein graft failure.
A 2-sided P value of <.05 was considered significant for all tests. There was no adjustment for multiplicity. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All analyses were performed using SAS version 9.4 (SAS Institute Inc) except for the network meta-analysis, which was performed using R version 4.1.0.16
Results
Study Selection
The literature search yielded 776 results, of which 557 were screened for eligibility. Four trials meeting the inclusion criteria were included in the analysis: Ticagrelor and Aspirin for the Prevention of Cardiovascular Events after Coronary Artery Bypass Graft Surgery (TAP-CABG),8 Different Antiplatelet Therapy Strategy after Coronary Artery Bypass Graft Surgery (DACAB),9 Effect of Ticagrelor on Saphenous Vein Graft Patency in Patients undergoing Coronary Artery Bypass Grafting Surgery (Popular CABG),10 and Ticagrelor Antiplatelet Therapy to Reduce Graft Events and Thrombosis (TARGET).11 The PRISMA IPD flow diagram is provided in eFigure 2 in the Supplement.
An overview of the included trials is provided in Table 1. Two trials8,10 compared ticagrelor DAPT with aspirin, 1 trial11 compared ticagrelor monotherapy with aspirin, and 1 trial9 compared ticagrelor DAPT and ticagrelor monotherapy with aspirin. All trials used a 90-mg twice-daily regimen of ticagrelor. A total of 3079 grafts (1668 saphenous vein grafts and 1411 arterial grafts) in 1316 patients were included in the meta-analysis.
Table 1. Trial Characteristics.
| Sourcea | Dates of enrollment | No. of patients | Total No. of graftsb | No. of SVGs | Experimental group | Control group | Treatment duration, mo | Type of graft imaging | Time to graft imaging, mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. of patients with SVGs | No. of SVGs | Treatment | No. ofpatients with SVGs | No. of SVGs | ||||||||
| TAP-CABG,8 2016 | 2011-2014 | 70 | 167 | 76 | Ticagrelor 90 mg 2/d + aspirin 81 mg 1/d | 19 | 34 | Placebo + aspirin 81 mg 1/d | 23 | 42 | 3 | CT angiography | 3 |
| DACAB,9 2018 | 2014-2015 | 500 | 1146 | 712 | Group 1: ticagrelor 90 mg 2/d + aspirin 100 mg 1/d | 167 | 239 | Aspirin 100 mg 1/d | 166 | 242 | 12 | CT or coronary angiography | 12 |
| Group 2: ticagrelor 90 mg 2/d | 166 | 231 | |||||||||||
| TARGET,11 2022 | 2014-2019 | 250 | 688 | 373 | Ticagrelor 90 mg 2/d | 127 | 187 | Aspirin 81 mg 2/d | 123 | 186 | 12 | CT angiography | 12 |
| POPular CABG,10 2020 | 2015-2019 | 496 | 1078 | 507 | Ticagrelor 90 mg 2/d + aspirin 80 mg or 100 mg 1/d | 249 | 254 | Placebo + aspirin 80 mg or 100 mg 1/d | 247 | 253 | 12 | CT angiography | 12 |
Abbreviations: CT, computed tomography; SVG, saphenous vein graft.
See the Methods section for the full names of the studies.
Includes SVGs and arterial grafts (left and/or right internal thoracic artery, and radial artery).
Primary Analysis
The primary analysis included 871 patients from the TAP-CABG,8 DACAB,9 and POPular CABG10 trials. A total of 435 patients (49.9%; 527 saphenous vein grafts) were randomized to ticagrelor DAPT, and 436 patients (50.1%; 537 saphenous vein grafts) were randomized to aspirin (Table 2). The median treatment duration was 365 days (IQR, 307-365 days) for patients in the ticagrelor DAPT group and 364 days (IQR, 315-365 days) for patients in the aspirin group. A total of 394 patients (90.6%) in the ticagrelor DAPT group and 400 patients (91.7%) in the aspirin group underwent protocol-defined imaging (eTables 2-4 in the Supplement). Protocol-defined imaging was performed by computed tomographic angiography in 789 patients and coronary angiography in 5 patients. The median time from CABG surgery to imaging was 369 days (IQR, 364-375 days) in the ticagrelor DAPT group and 370 days (IQR, 364-376 days) in the aspirin group.
Table 2. Baseline Patient and Graft Characteristics Among Patients in the Primary Analysis.
| No. (%) | ||
|---|---|---|
| Ticagrelor DAPT (n = 435) | Aspirin (n = 436) | |
| Patientsa | ||
| Age, median (IQR), y | 67 (60-72) | 66 (61-73) |
| >65 y | 249 (57.2) | 241 (55.3) |
| Sex | ||
| Women | 65 (14.9) | 63 (14.5) |
| Men | 370 (85.1) | 373 (85.5) |
| Medical history | ||
| Hypertensionb | 294 (67.6) | 294 (67.4) |
| Dyslipidemiac | 275 (63.2) | 266 (61.0) |
| ACS at presentationd | 195 (44.8) | 189 (43.3) |
| Diabetes | 143 (32.9) | 143 (32.8) |
| Smokinge | 114 (26.2) | 112 (25.7) |
| Previous myocardial infarction | 92 (21.2) | 91 (20.9) |
| Previous PCI | 50 (11.5) | 65 (14.9) |
| Chronic kidney diseasef | 28 (6.4) | 24 (5.5) |
| LVEF, median (IQR), %g | 58 (51-64) | 60 (51-65) |
| Use of cardiopulmonary bypass | 297 (68.3) | 301 (69.0) |
| Endoscopic saphenous vein graft harvestingh | 19/415 (4.6) | 25/410 (6.1) |
| Sequential saphenous vein grafts | 320 (73.6) | 327 (75.0) |
| Grafts | ||
| No. | 981 | 996 |
| Graft type | ||
| Saphenous vein grafts | 527 (53.7) | 537(53.9) |
| Arterial grafts | 454 (46.3) | 459 (46.1) |
| Graft target | ||
| LAD | 407 (41.5) | 403 (40.5) |
| Saphenous vein grafts | 15 (3.7) | 16 (4.0) |
| Arterial grafts | 392 (96.3) | 387 (96.0) |
| Non-LAD | 574 (58.5) | 593 (59.5) |
| Saphenous vein grafts | 512 (89.2) | 521 (87.9) |
| Arterial grafts | 62 (10.8) | 72 (12.1) |
Abbreviations: ACS, acute coronary syndrome; DAPT, dual antiplatelet therapy; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Includes patients from the TAP-CABG,8 DACAB,9 and POPular CABG10 trials. See the Methods section for the full names of the studies. Definitions and time points of measurement varied by trial.
History of hypertension or systolic blood pressure of at least 140 mm Hg and diastolic blood pressure of at least 90 mm Hg.
Baseline low-density lipoprotein cholesterol of at least 69.5 mg/dL (≥1.8 mmol/L).
ST-elevated myocardial infarction, non–ST-segment elevation ACS, or unstable angina.
Current and former smoking and includes vaping and use of other tobacco products.
Glomerular filtration rate less than 60 mL/min/m2 calculated by the Chronic Kidney Disease Epidemiology Collaboration formula.
The most recent measurement before surgery and varied by trial.
Changes in denominators indicate missing data.
Primary Outcome
The primary outcome of saphenous vein graft failure occurred in 11.2% (54 of 481) of saphenous vein grafts in the ticagrelor DAPT group and in 20.0% (99 of 494) of saphenous vein grafts in the aspirin group (difference, −8.7% [95% CI, −13.5% to −3.9%], OR, 0.51 [95% CI, 0.35 to 0.74]; P < .001; Figure 1A).
Figure 1. Individual and Pooled Estimates for Saphenous Vein Graft Failure.
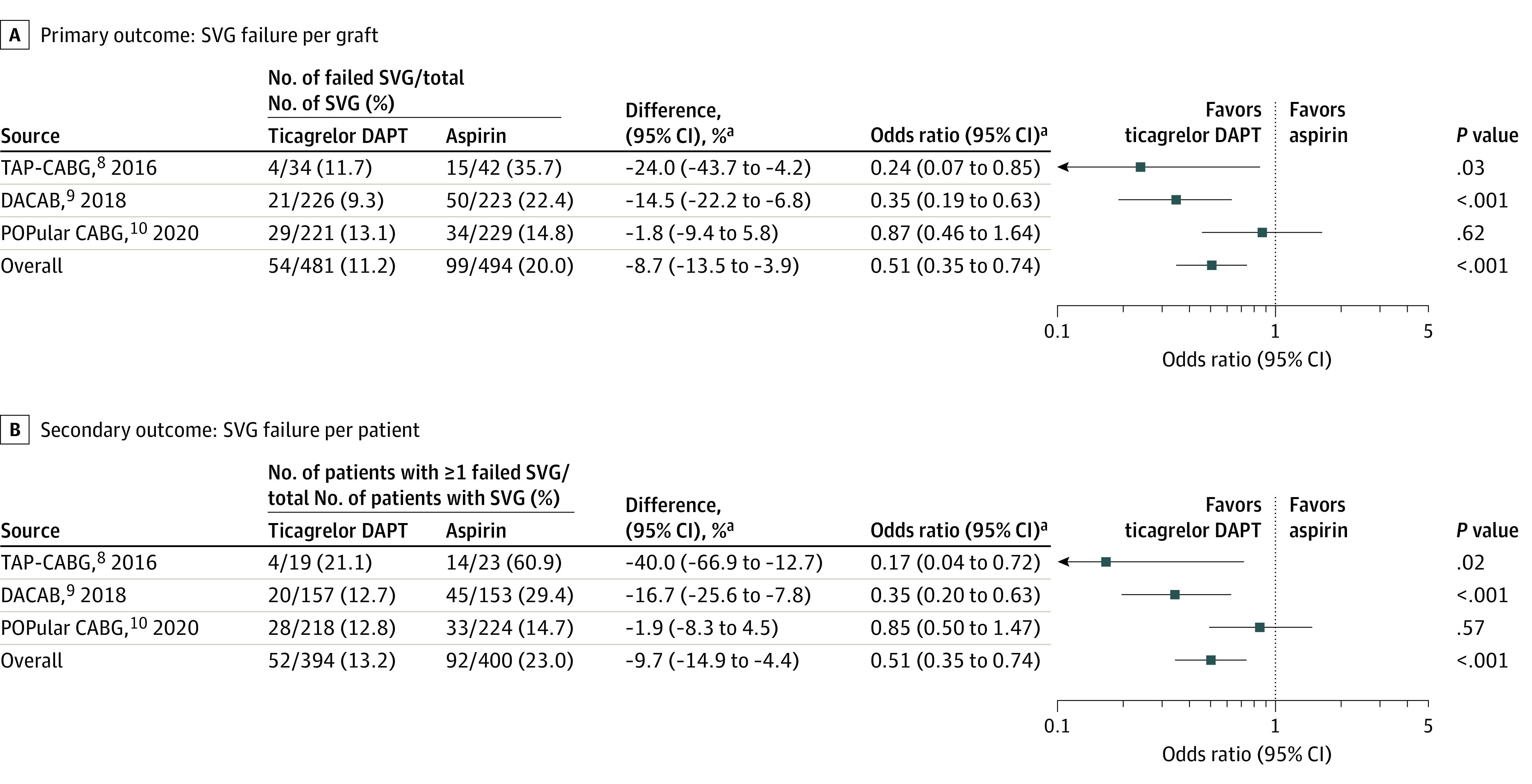
aAdjusted by trial.
DAPT indicates dual antiplatelet therapy; SVG, saphenous vein graft.
Secondary Outcomes
When assessed per patient, saphenous vein graft failure occurred in 13.2% (52 of 394) of patients in the ticagrelor DAPT group and 23.0% (92 of 400) of patients in the aspirin group (difference, −9.7% [95% CI, −14.9% to −4.4%]; OR, 0.51 [95% CI, 0.35 to 0.74]; P < .001; Figure 1B).
Ticagrelor DAPT was associated with a significantly higher risk of BARC type 2, 3, or 5 bleeding events compared with aspirin (22.1% vs 8.7%, difference; 13.3% [95% CI, 8.6% to 18.0%]; OR, 2.98 [95% CI, 1.99 to 4.47]; P < .001) (Figure 2A; eFigure 3 in the Supplement). Ticagrelor DAPT was associated with a significantly lower risk of the composite of saphenous vein graft failure or cardiovascular death compared with aspirin (13.9% vs 23.4%: difference, −9.4% [95% CI, −14.7% to −4.1%]; OR, 0.52 [95% CI, 0.36 to 0.76]; P < .001) but was not significantly associated with lower risk of MACCE (6.7% vs 5.5%; difference, 1.2% [95% CI, −2.0% to 4.3%]; HR, 1.21 [95% CI, 0.70 to 2.08]; P = .50; Figure 2B).
Figure 2. Pooled Estimates for Bleeding Events and Cardiovascular Events.
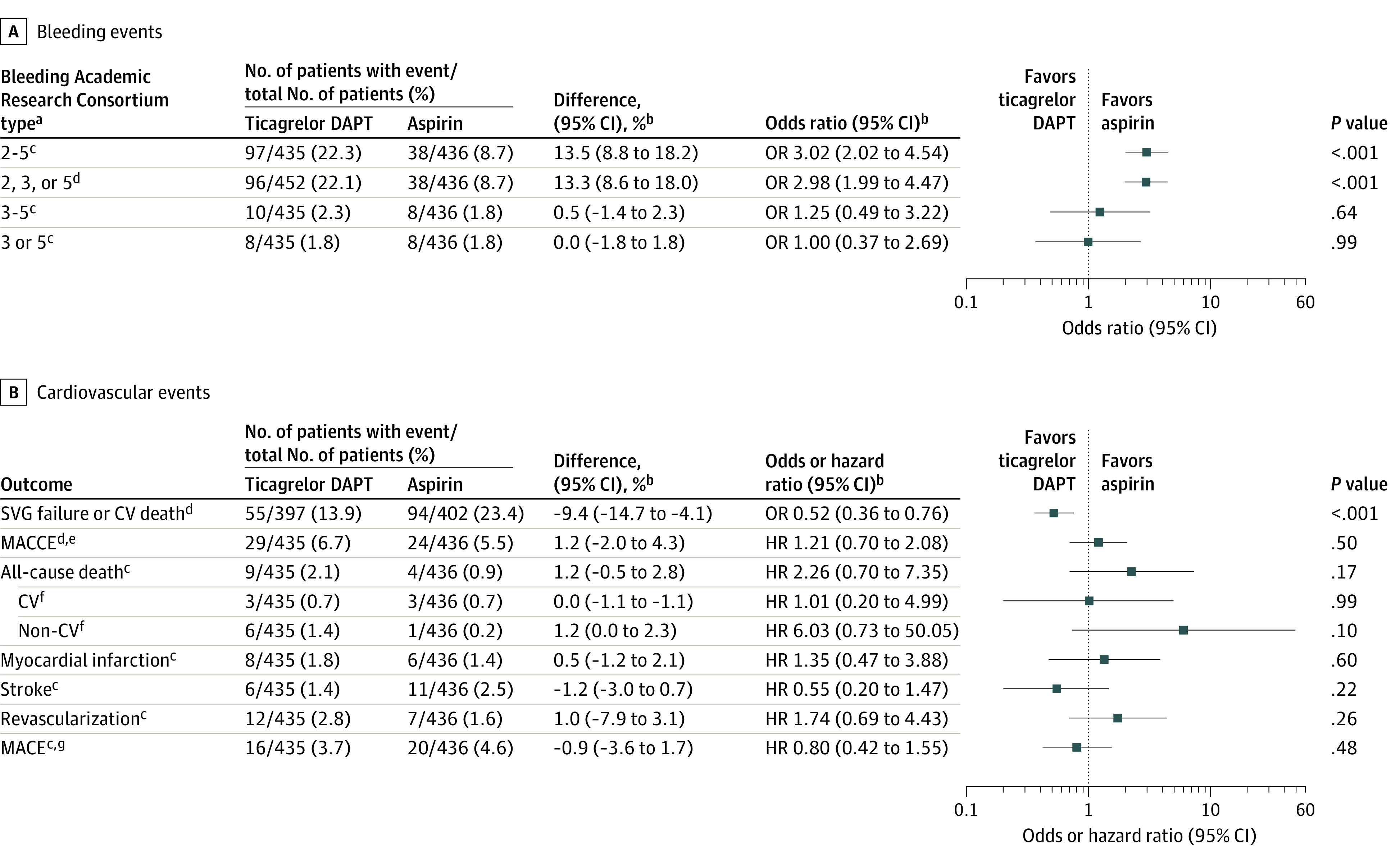
aType 0 indicates no bleeding; type 1, bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment; type 2, any overt, actionable sign of hemorrhage (eg, bleeding that does not fit type 3, 4, or 5 criteria but meets at least 1 of the following: [1] requires nonsurgical medical intervention, [2] leads to hospitalization or increased level of care, or [3] prompts evaluation); type 3a, overt bleeding plus hemoglobin drop of 3 to 5 g/dL (provided hemoglobin drop is related to the bleeding event) or any transfusion with overt bleeding; 3b, overt bleeding plus hemoglobin drop of 5 g/dL (provided hemoglobin drop is related to the bleeding event), cardiac tamponade, bleeding requiring surgical intervention for control (excluding dental, nasal, skin, or hemorrhoid), and bleeding requiring intravenous vasoactive agents; 3c, intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation, does include intraspinal), subcategories confirmed by autopsy or imaging or lumbar puncture, intraocular bleed compromising vision; type 4, coronary artery bypass graft surgery–related bleeding; and type 5, fatal bleeding.
bAdjusted by trial.
cPost hoc outcomes.
dSecondary outcome.
eDefined as the composite of all-cause death, myocardial infarction, stroke, or revascularization.
fAdditional outcomes.
gDefined as the composite of cardiovascular death, myocardial infarction, or stroke.
Includes patients from the TAP-CABG,8 DACAB,9 and POPular CABG10 trials. See the Methods section for the full names of the studies.
CV indicates cardiovascular; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular event; MACE, major adverse cardiovascular event; OR, odds ratio; SVG, saphenous vein graft.
Prespecified Sensitivity and Subgroup Analyses
The results for the primary outcome were confirmed in the sensitivity analysis for saphenous vein graft failure per anastomosis (ticagrelor DAPT vs aspirin, 8.6% vs 14.6%; difference, −6.1% [95% CI, −9.9% to −2.4%]; OR, 0.54 [95% CI, 0.37 to 0.80]; P < .001; eFigure 4 in the Supplement) and in the sensitivity analysis that included only patients with protocol-defined imaging at 1 year (eFigure 5 in the Supplement). Sensitivity analyses for the primary outcome in the as-treated and per-protocol populations and after imputation of missing data were also consistent with the main analysis (eTable 5 in the Supplement).
The association of ticagrelor DAPT with the risk of saphenous vein graft failure was consistent across all prespecified subgroups (Figure 3).
Figure 3. Saphenous Vein Graft Failure in Subgroups.
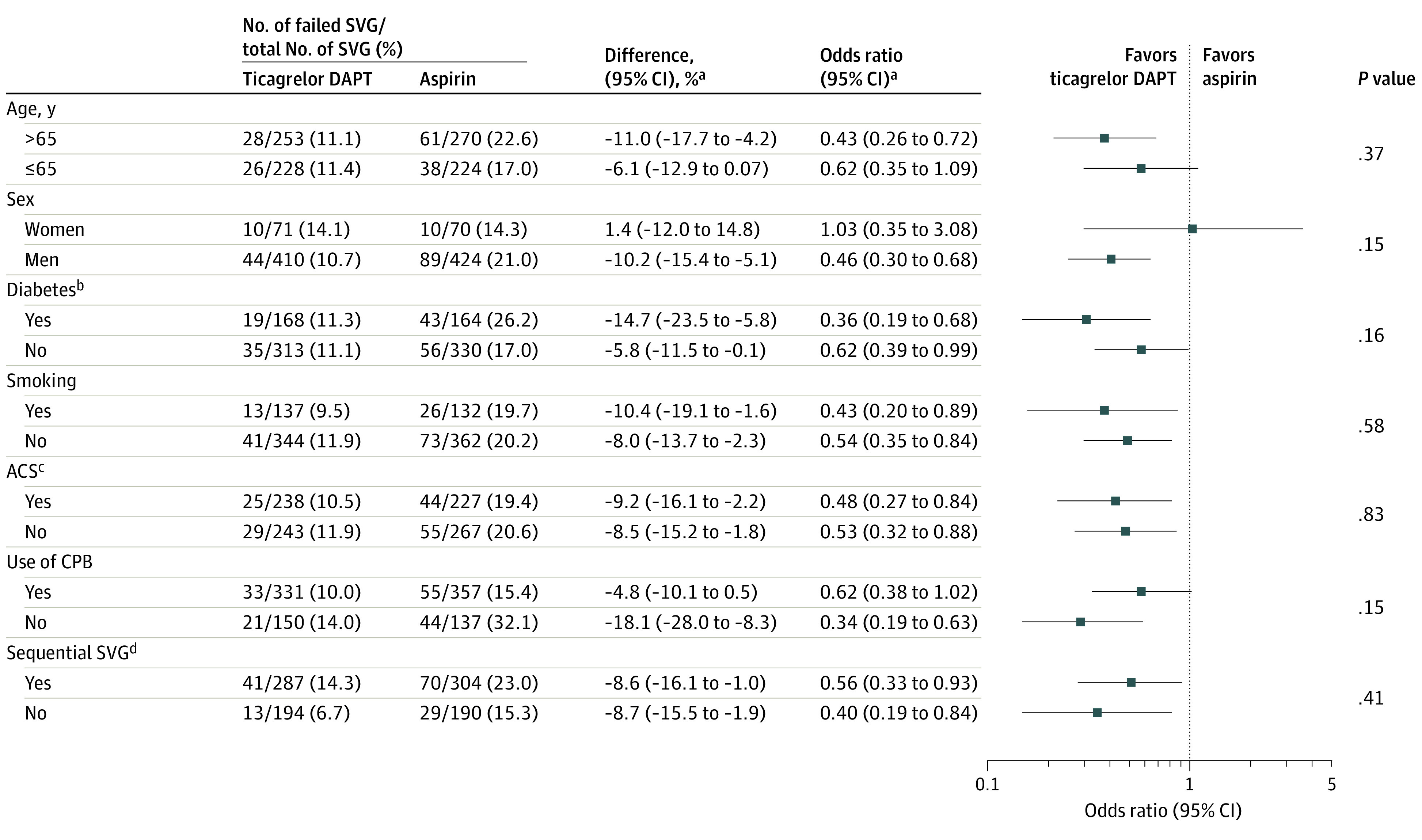
Subgroup analyses for harvesting technique and target vessel territory could not be performed due to the limited number of events in the endoscopic-harvesting group and left anterior descending coronary artery territory group. Treatment duration varied in 1 trial only.
aAdjusted by trial.
bSelf-reported diagnosis, elevated hemoglobin A1c levels, or active therapy. Definitions varied by trial.
cIncludes ST-segment elevation myocardial infarction, non–ST-segment elevation ACS, or unstable angina. Definitions varied by trial.
dDefined as saphenous vein grafts with more than 1 anastomosis.
Includes patients from the TAP-CABG,8 DACAB,9 and POPular CABG10 trials. See the Methods section for the full names of the studies.
ACS indicates acute coronary syndrome; CPB, cardiopulmonary bypass; DAPT, dual antiplatelet therapy; SVG, saphenous vein graft.
Post Hoc Outcomes
Ticagrelor DAPT was associated with a significantly lower risk of saphenous vein graft occlusion than was aspirin (9.6% vs 16.2%; difference, −6.6% [95% CI, −11.0% to −2.2%]; OR, 0.55 [95% CI, 0.37 to 0.82]; P = .003) (eFigure 6 in the Supplement).
The results of the post hoc analyses for any graft failure were consistent with the main analysis (eTable 6 in the Supplement). The association of ticagrelor DAPT with any graft failure remained consistent when stratified by graft type and target vessel territory (eFigure 7 in the Supplement).
The association of ticagrelor DAPT with bleeding events is shown in Figure 2 and eFigure 3 in the Supplement. There were no instances of BARC type 5 bleeding events. The association of ticagrelor DAPT with the risk of BARC type 2, 3, or 5 bleeding events was consistent across important clinical subgroups (eFigure 8 in the Supplement). Ticagrelor DAPT was not associated with significant differences in the individual components of MACCE or MACE (Figure 2).
Ticagrelor DAPT was associated with a significantly lower risk of net adverse events than was aspirin (17.0% vs 27.8%; difference, −10.6% [95% CI, −16.3% to −4.9%]; OR, 0.53 [95% CI, 0.38 to 0.75]; P < .001) but was not associated with significant differences in net adverse major clinical events or overall net adverse events (eTable 7 in the Supplement).
In the network meta-analysis with aspirin as the reference group, ticagrelor DAPT was associated with a significantly lower risk of saphenous vein graft failure per graft (OR, 0.49 [95% CI, 0.27 to 0.87]) whereas ticagrelor monotherapy was not (OR, 0.94 [95% CI, 0.51 to 1.74]; I2 = 55.4%; eFigure 9 in the Supplement).
Supplementary Analysis
Ticagrelor monotherapy was not associated with a significant difference in saphenous vein graft failure compared with aspirin per graft (19.3% vs 21.7%; difference, −2.6% [95% CI, −9.1% to 3.9%]; OR, 0.86 [95% CI, 0.58 to 1.27]; P = .44) or per patient (25.2% vs 29.3%; difference, −4.1% [95% CI, −11.9% to 3.7%]; OR, 0.81 [95% CI, 0.55 to 1.20]; P = .30; eTable 8 in the Supplement). There was no significant difference between ticagrelor monotherapy and aspirin in the association with BARC type 2, 3, or 5 bleeding events (8.9% vs 7.3%; difference, 1.7%; [95% CI, −2.8% to 6.1%]; OR, 1.25 [95% CI, 0.69 to 2.29]; P = .46; eTable 9 in the Supplement).
Discussion
In this individual patient data meta-analysis of 4 RCTs including 1316 patients and 1668 saphenous vein grafts, ticagrelor DAPT was associated with a significantly lower risk of saphenous vein graft failure and a significantly higher risk of clinically important bleeding events than was aspirin.
CABG surgery is the treatment of choice for patients with high-complexity coronary artery disease and those with reduced left ventricular ejection fraction.6 In the US alone approximately 300 000 patients undergo CABG surgery annually.17 The saphenous vein is used in more than 90% of CABG procedures.18
RCTs investigating the effect of ticagrelor DAPT vs aspirin on saphenous vein graft failure have reported conflicting results. In the DACAB trial,9 ticagrelor DAPT significantly increased saphenous vein graft patency 1 year after CABG. In contrast, in the POPular CABG trial,10 ticagrelor DAPT did not significantly reduce saphenous vein graft occlusion 1 year after CABG surgery. The TAP-CABG trial8 also did not find a significant difference in the absolute risk of saphenous vein graft occlusion between ticagrelor DAPT and aspirin 3 months after CABG.
In addition, the published RCTs were all individually underpowered to detect even moderate differences in bleeding outcomes. Although they did not individually report an increase in major bleeding events with ticagrelor DAPT,8,9,10 a solid estimate of the risk to benefit ratio of ticagrelor DAPT after CABG surgery was not possible.
An aggregate network meta-analysis of 20 RCTs that assessed the effects of oral antithrombotic drugs and included 2 RCTs (203 patients) comparing ticagrelor DAPT with aspirin did not show a significant difference in major bleeding events between the groups (OR, 1.93 [95% CI, 0.30-12.4]).19 In another meta-analysis of 5 RCTs and 3996 patients, there was no significant difference in the risk of bleeding between ticagrelor–based antiplatelet therapy vs aspirin and/or clopidogrel (relative risk, 1.04 [95% CI, 0.95-1.14]; P = .41).20 However, individual studies used different bleeding definitions and this greatly limits the clinical relevance of the results from trial-level meta-analyses.
In the present work, graft failure and bleeding events were readjudicated using a common definition before pooling, allowing generation of homogeneous pooled estimates of the association of ticagrelor DAPT with saphenous vein graft failure and bleeding events.
This comprehensive synthesis of all RCTs with angiographic follow-up provides solid evidence that ticagrelor DAPT is associated with a significantly lower risk of saphenous vein graft failure 1 year after CABG surgery. The association of ticagrelor DAPT with the risk of saphenous vein graft failure was consistent across subgroups. However, compared with aspirin, ticagrelor DAPT was also associated with a significantly higher risk of BARC type 2 or higher bleeding events. There was also an absolute increase in the risk of all-cause death in the ticagrelor DAPT group, although not statistically significant. Taken together, the present analysis suggests that a patient’s individual risk of graft failure, ischemic events, and bleeding needs to be weighed carefully when deciding whether to add ticagrelor to aspirin after CABG surgery. Longer-term follow-up is required to fully evaluate a potential benefit of ticagrelor DAPT on clinical events.21
Individual RCTs investigating the effect of ticagrelor monotherapy vs aspirin after CABG surgery have not shown an effect on saphenous vein graft failure but have all been limited by the small sample size.9,11 In this meta-analysis, ticagrelor monotherapy was not associated with a significant difference in the risk of saphenous vein graft failure compared with aspirin, but the pooled estimate was compatible with a potential benefit of ticagrelor monotherapy. Because ticagrelor monotherapy was not associated with a significant difference in the risk of bleeding events compared with aspirin, its role as a treatment option after CABG surgery requires further investigation.
Limitations
This study has several limitations. First, the pooled analysis is subject to the limitations of the original RCTs, including the open-label treatment allocation in 1 of them, although outcome adjudication was blinded in all RCTs. Second, there was heterogeneity in surgical technique and postoperative management across the included RCTs. Third, the duration of ticagrelor DAPT and timing of follow-up ranged from 3 to 12 months across the included RCTs. Fourth, the analysis was not designed to evaluate differences in clinical outcomes between groups. Lastly, protocol-directed imaging was missing in 9.1% of patients. However, patient characteristics were balanced across randomization groups for patients who underwent imaging, and the results for the primary outcome were consistent after multiple imputation of missing data.
Conclusions
In patients undergoing coronary artery bypass graft surgery, adding ticagrelor to aspirin was associated with a significantly decreased risk of vein graft failure. However, this was accompanied by a significantly increased risk of clinically important bleeding.
eMethods 1. Search strategy
eMethods 2. Combined dataset
eMethods 3. Post hoc analyses
eTable 1. Definition of major adverse cardiac and cerebrovascular events in randomized trials included in the IPD meta-analysis
eTable 2. Patient characteristics for patients with saphenous vein grafts who did versus did not have per-protocol imaging
eTable 3. Patient characteristics for patients with saphenous vein grafts who underwent per-protocol imaging, by randomization group
eTable 4. Patient characteristics for patients with saphenous vein grafts who did not undergo per-protocol imaging, by randomization group
eTable 5. Sensitivity analyses for the primary outcome
eTable 6. Post hoc analyses for any graft failure
eTable 7. Net adverse events in patients receiving saphenous vein grafts
eTable 8. Comparison of outcomes for saphenous vein graft failure among patients randomized to ticagrelor monotherapy or aspirin
eTable 9. Comparison of bleeding events among patients randomized to ticagrelor monotherapy or aspirin
eFigure 1. Risk of bias in the included trials as assessed by the Cochrane risk of bias assessment tool 2
eFigure 2. PRISMA IPD Flow Diagram
eFigure 3. Individual and pooled estimates for bleeding events in the primary analysis
eFigure 4. Sensitivity analysis for saphenous vein graft failure per anastomosis
eFigure 5. Sensitivity analysis for the primary outcome in patients with 1-year imaging
eFigure 6. Post hoc analysis for saphenous vein graft occlusion
eFigure 7. Treatment-by-subgroup interaction for any graft failure
eFigure 8. BARC type 2, 3, or 5 bleeding in subgroups
eFigure 9. Forest plot for saphenous vein graft failure for aspirin, ticagrelor dual antiplatelet therapy, and ticagrelor monotherapy in the post hoc network meta-analysis
eReferences
References
- 1.Antonopoulos AS, Odutayo A, Oikonomou EK, et al. ; SAFINOUS-CABG (Saphenous Vein Graft Failure—An Outcomes Study in Coronary Artery Bypass Grafting) group . Development of a risk score for early saphenous vein graft failure: an individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2020;160(1):116-127.e4. doi: 10.1016/j.jtcvs.2019.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xenogiannis I, Zenati M, Bhatt DL, et al. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. 2021;144(9):728-745. doi: 10.1161/CIRCULATIONAHA.120.052163 [DOI] [PubMed] [Google Scholar]
- 3.Gaudino M, Antoniades C, Benedetto U, et al. ; ATLANTIC (Arterial Grafting International Consortium) Alliance . Mechanisms, consequences, and prevention of coronary graft failure. Circulation. 2017;136(18):1749-1764. doi: 10.1161/CIRCULATIONAHA.117.027597 [DOI] [PubMed] [Google Scholar]
- 4.Chakos A, Jbara D, Singh K, Yan TD, Tian DH. Network meta-analysis of antiplatelet therapy following coronary artery bypass grafting (CABG): none versus one versus two antiplatelet agents. Ann Cardiothorac Surg. 2018;7(5):577-585. doi: 10.21037/acs.2018.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa-Uva M, Neumann FJ, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55(1):4-90. doi: 10.1093/ejcts/ezy289 [DOI] [PubMed] [Google Scholar]
- 6.Lawton JS, Tamis-Holland JE, Bangalore S, et al. ; Writing Committee Members . 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi: 10.1016/j.jacc.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371-e1396. doi: 10.4244/EIJ-D-21-00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saw J, Wong GC, Mayo J, et al. Ticagrelor and aspirin for the prevention of cardiovascular events after coronary artery bypass graft surgery. Heart. 2016;102(10):763-769. doi: 10.1136/heartjnl-2015-308691 [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Zhu Y, Xu Z, et al. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA. 2018;319(16):1677-1686. doi: 10.1001/jama.2018.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willemsen LM, Janssen PWA, Peper J, et al. Effect of adding ticagrelor to standard aspirin on saphenous vein graft patency in Patients Undergoing Coronary Artery Bypass Grafting (POPular CABG): a randomized, double-blind, placebo-controlled trial. Circulation. 2020;142(19):1799-1807. doi: 10.1161/CIRCULATIONAHA.120.050749 [DOI] [PubMed] [Google Scholar]
- 11.Kulik A, Abreu AM, Boronat V, Kouchoukos NT, Ruel M. Ticagrelor versus aspirin and vein graft patency after coronary bypass: a randomized trial. J Card Surg. 2022;37(3):563-570. doi: 10.1111/jocs.16189 [DOI] [PubMed] [Google Scholar]
- 12.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 13.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 15.SAS/STAT 14.3 User’s Guide: the GLIMMIX Procedure. SAS Institute Inc; 2017. Accessed June 14, 2022. https://documentation.sas.com/api/collections/pgmsascdc/9.4_3.3/docsets/statug/content/glimmix.pdf?locale=en#nameddest=statug_glimmix_syntax01
- 16.Network Meta-Analysis Using Frequentist Methods. R version 2.5-0; July 11, 2022. Accessed June 14, 2022. https://cran.r-project.org/web/packages/netmeta/netmeta.pdf
- 17.Bowdish ME, D’Agostino RS, Thourani VH, et al. STS adult cardiac surgery database: 2021 update on outcomes, quality, and research. Ann Thorac Surg. 2021;111(6):1770-1780. doi: 10.1016/j.athoracsur.2021.03.043 [DOI] [PubMed] [Google Scholar]
- 18.Schwann TA, Habib RH, Wallace A, et al. Operative outcomes of multiple-arterial versus single-arterial coronary bypass grafting. Ann Thorac Surg. 2018;105(4):1109-1119. doi: 10.1016/j.athoracsur.2017.10.058 [DOI] [PubMed] [Google Scholar]
- 19.Solo K, Lavi S, Kabali C, et al. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ. 2019;367:l5476. doi: 10.1136/bmj.l5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Scheidt M, Bongiovanni D, Tebbe U, et al. Ticagrelor-based antiplatelet regimens in patients treated with coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2020;57(3):520-528. doi: 10.1093/ejcts/ezz260 [DOI] [PubMed] [Google Scholar]
- 21.Steg PG, Bhatt DL, Simon T, et al. ; THEMIS Steering Committee and Investigators . Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309-1320. doi: 10.1056/NEJMoa1908077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search strategy
eMethods 2. Combined dataset
eMethods 3. Post hoc analyses
eTable 1. Definition of major adverse cardiac and cerebrovascular events in randomized trials included in the IPD meta-analysis
eTable 2. Patient characteristics for patients with saphenous vein grafts who did versus did not have per-protocol imaging
eTable 3. Patient characteristics for patients with saphenous vein grafts who underwent per-protocol imaging, by randomization group
eTable 4. Patient characteristics for patients with saphenous vein grafts who did not undergo per-protocol imaging, by randomization group
eTable 5. Sensitivity analyses for the primary outcome
eTable 6. Post hoc analyses for any graft failure
eTable 7. Net adverse events in patients receiving saphenous vein grafts
eTable 8. Comparison of outcomes for saphenous vein graft failure among patients randomized to ticagrelor monotherapy or aspirin
eTable 9. Comparison of bleeding events among patients randomized to ticagrelor monotherapy or aspirin
eFigure 1. Risk of bias in the included trials as assessed by the Cochrane risk of bias assessment tool 2
eFigure 2. PRISMA IPD Flow Diagram
eFigure 3. Individual and pooled estimates for bleeding events in the primary analysis
eFigure 4. Sensitivity analysis for saphenous vein graft failure per anastomosis
eFigure 5. Sensitivity analysis for the primary outcome in patients with 1-year imaging
eFigure 6. Post hoc analysis for saphenous vein graft occlusion
eFigure 7. Treatment-by-subgroup interaction for any graft failure
eFigure 8. BARC type 2, 3, or 5 bleeding in subgroups
eFigure 9. Forest plot for saphenous vein graft failure for aspirin, ticagrelor dual antiplatelet therapy, and ticagrelor monotherapy in the post hoc network meta-analysis
eReferences


