Abstract
The intracellular parasite Toxoplasma gondii infects nucleated cells in virtually all warm-blooded vertebrates, including one-third of the human population. While immunocompetent hosts do not typically show symptoms of acute infection, parasites are retained in latent tissue cysts that can be reactivated upon immune suppression, potentially damaging key organ systems. Toxoplasma has a multistage life cycle that is intimately linked environmental stresses and host signals. As this protozoan pathogen is transmitted between multiple hosts and tissues, it evaluates these external signals to appropriately differentiate into distinct life cycle stages, such as the transition from its replicative stage (tachyzoite) to the latent stage (bradyzoite) that persists as tissue cysts. Additionally, in the gut of its definitive host, felines, Toxoplasma converts into gametocytes that produce infectious oocysts (sporozoites) that are expelled into the environment. In this review, we highlight recent advances that have illuminated the interfaces between Toxoplasma and host and how these interactions control parasite stage conversion. Mechanisms underlying these stage transitions are important targets for therapeutic intervention aimed at thwarting parasite transmission and pathogenesis.
Introduction
Toxoplasma gondii is an obligate intracellular parasite that can infect any nucleated cell in warm-blooded vertebrates, making it one of the most prevalent parasites in the world (Montoya and Liesenfeld, 2004). The only organisms that support the sexual stage of the parasite are felines; in their intestinal epithelium, Toxoplasma converts into gametocytes that produce sturdy oocysts that are shed in cat feces (Dubey et al., 1970). Upon exposure to oxygen, oocysts undergo a sporulation process and become highly infectious, contaminating water and food supplies (Shapiro et al., 2019). Accidental ingestion of oocysts, which can survive in the environment for 1–2 years, constitutes a major route of Toxoplasma transmission into new hosts (Dabritz and Conrad, 2010). Sporozoites released from ingested oocysts infect the intestinal epithelium and convert into tachyzoites that disseminate throughout the body (Shapiro et al., 2019). Tachyzoites replicate rapidly and asexually within nucleated host cells, contained within a non-fusogenic parasitophorous vacuole (PV) initially formed through host cell membrane invagination during invasion (Håkansson et al., 1999). The PV serves as a protective niche and creates an interface with the host cell cytosol; the PV membrane (PVM) associates with host cell organelles and facilitates acquisition of nutrients and metabolites required for replication (Coppens and Romano, 2018; Schwab et al., 1994). Tachyzoites continue to replicate exponentially until they lyse or exit (egress) the host cell; the extracellular parasites must then invade a new host cell promptly in order to complete another round of this lytic cycle (Blader et al., 2015).
In healthy individuals, the immune system typically controls the initial infection, but parasites are not eradicated from the body. Rather, Toxoplasma converts into bradyzoites, a latent stage of the infection that is characterized by little to no replication and a thickening of the PVM into a tissue cyst wall. The latent tissue cysts, which have a proclivity to form in the brain and heart, do not appear to be efficiently eliminated by the immune response nor are they susceptible to currently approved therapies. In immunocompromised patients, bradyzoites can reconvert into replicating tachyzoites, thereby reactivating the infection and potentially causing serious tissue damage in critical organs. In addition to their clinical relevance, the presence of latent tissue cysts in intermediate hosts constitutes another major route of Toxoplasma transmission through the consumption of raw or undercooked meat (Halonen and Weiss, 2013).
Antifolates (pyrimethamine and sulfadiazine) are the current primary drugs used to treat acute toxoplasmosis. While these drugs are beneficial against replicating parasites, they have adverse side effects and cannot be used to eradicate latent tissue cysts (Halonen and Weiss, 2013). Latent parasites present significant challenges to drug treatment as they are less metabolically active, are encased inside a thick cyst wall within host cells and reside in tissues that are not sufficiently bioavailable for many drugs. A greater understanding of the mechanisms orchestrating Toxoplasma stage transitions will lead to new ways to prevent parasite transmission and pathogenesis.
How Toxoplasma mediates conversion between three major life cycle stages among many different types of host organisms has long been a mystery. Not surprisingly, a common denominator for the transition into each life cycle stage is reprogramming of gene expression that features regulation of the transcriptome and translatome (Hehl et al., 2015; Radke et al., 2018; Ramakrishnan et al., 2019; Sullivan et al., 2009). Furthermore, these regulatory programs interface with that of the host to ensure a safe haven for the parasite and nutrient availability, along with facilitating dissemination of the parasite. This review will highlight recent studies that identified key regulators and mechanisms directing these processes and their interface with the host. We begin with the mode of Toxoplasma infection of host cells and the processes that enable tachyzoites to create a suitable environment and nutrient source to sustain parasite replication. Next, we describe processes that trigger cyst formation and how the differentiated parasites are maintained and protected in host tissues. Finally, we describe formation of Toxoplasma oocysts and the critical roles that the feline host play in this sexual stage.
An unwanted guest: Toxoplasma makes itself at home
Upon infecting an intermediate host, Toxoplasma must proceed to its latent tissue cyst phase to create the opportunity to transmit via predation. The parasite must balance an expansion of its numbers to disseminate throughout the body and maximize cyst formation without killing its host (Kamerkar and Davis, 2012). To achieve these goals, Toxoplasma has developed strategies to maximize its distribution among tissues within host organisms, to ensconce itself within the cytoplasm of host cells, and to ensure appropriate amounts of metabolites are procured from the host to the parasite (Coppens and Romano, 2018).
Toxoplasma has developed many tactics to facilitate its dissemination throughout its host, including the central nervous system (Courret et al., 2006). While it has been shown that tachyzoites can traverse a variety of tissues including the blood-brain-barrier (Konradt et al., 2016), tachyzoites can also induce their infected host cell to migrate. Parasite-induced hypermigratory activity has been proposed to enhance the delivery of parasites into other tissues as a “Trojan Horse” mechanism (Bierly et al., 2008). Early after infection, the parasite rapidly hijacks the migration machinery of its host cell by remodeling the actin cytoskeleton, resulting in dramatic morphological changes (Weidner et al., 2013). The mechanisms underlying this hijacking are complex, involving several proteins secreted by the parasite, including toxofilin, TgWIP, ROP17, and Tg14-3-3, each shown to modulate actin dynamics in the host cell (Delorme-Walker et al., 2012; Drewry et al., 2019; Gonzalez et al., 2009; Sangare et al., 2019; Weidner et al., 2016). We have recently shown that intracellular tachyzoites also alter stress response pathways in the host as a means to initiate hypermigration. Toxoplasma infection activates the host cell’s unfolded protein response (UPR), prompting the ER stress sensor, IRE1, to interact with the actin-binding protein filamin A, which remodels the cytoskeleton to produce hypermigration (Augusto et al., 2020a). Depletion of IRE1 from infected host cells reduced their migration in vitro and significantly hindered parasite dissemination in a mouse model of acute toxoplasmosis. Thus upon infection, Toxoplasma directs reorganization of the cytoskeletal structures of host cells, thereby maximizing propagation of the parasite to multiple organ systems of the infected organism.
Tachyzoites can also directly modulate key signaling events in the host cell that promote pathogenesis. Toxoplasma releases an arsenal of proteins into the host cell to manipulate gene expression and signaling pathways, including those associated with autophagy, immune responses, and metabolism (Bougdour et al., 2013). Toxoplasma also appears to inject parasite proteins into host cells that it does not decide to invade, presumably as part of global strategy to manipulate the host organism (Koshy et al., 2012). Shortly after invasion, Toxoplasma induces rearrangements of host organelles, including gathering of host ER and mitochondria to the PVM. ROP2 directly participates in the recruitment of host ER, and mitochondrial association factor 1 (MAF1) anchors host mitochondria to the PVM (Pernas et al., 2014; Sinai and Joiner, 2001). Furthermore, host endocytic structures, Golgi ministacks, lipid droplets, and transport vesicles are found in proximity to the PV (Coppens and Romano, 2018). Recruitment of these host cell structures is suggested to enhance appropriation of metabolites from the host cell and alter signaling in the infected cells in ways that favor infection.
Upon infection, Toxoplasma secretes a myriad of proteins from an assortment of specialized organelles. Some of the parasite’s dense granule (GRAs) and rhoptry (ROP) proteins are secreted beyond the confines of the PV, making their way into the host cytosol and nucleus (Hakimi et al., 2017). For example, GRA16 travels to the host cell nucleus and can bind at least two host enzymes, including a deubiquitinase and protein phosphatase PP2A that modulate host p53 functions and the cell cycle (Bougdour et al., 2013). GRA16 also contributes to the accumulation of c-Myc protein in infected host cells, potentially by maintaining its phosphorylation at serine 62 through interference with the PP2A complex (Panas and Boothroyd, 2020). GRA24 enhances phosphorylation of host p38α MAP kinase, leading to a proinflammatory response (Braun et al., 2013; Krishnamurthy and Saeij, 2018). A protein secreted by rhoptry organelles, ROP18, phosphorylates immunity-related GTPases (IRGs) in the host cell, promoting parasite survival and virulence (Fentress et al., 2010). Another rhoptry protein, ROP16, modulates host cell function by directly phosphorylating and activating STAT6, a transcription factor that can repress Th1 inflammatory responses (Ong et al., 2010). Another secreted dense granule protein called Inhibitor of STAT Transcription (TgIST) localizes to the host nucleus and alters STAT1 activation by blocking responses to IFN-γ (Matta et al., 2019). Together, the highlighted host target proteins and processes that are directly modulated by secreted Toxoplasma proteins emphasize the different strategies the parasite has developed to control its relationship with the host. For a more comprehensive review of the secreted effectors Toxoplasma releases into host cells, see (Wang et al., 2020).
Parasite replication proceeds exponentially, mandating expansion of the PVM to contain the growing parasite population. The expanding PVM requires phospholipids in addition to the host nutrients and metabolites needed to produce daughter parasites (Gupta et al., 2005). Toxoplasma can manufacture its own lipids to some extent, but still acquires serine, ethanolamine, and choline from host cell to synthesize phospholipids in sufficient amounts (Gupta et al., 2005). The need to substantially increase membrane biogenesis provides another possible explanation why host ER and mitochondria are recruited to the PVM, as these organelles are sites of host cell lipid biosynthesis. Upon infection of a host cell, Toxoplasma induces lipophagy, which is the autophagy of host lipid droplets, as a means to scavenge fatty acids such as FAs oleic acid (Blume and Seeber, 2018; Nolan et al., 2018). The demand for other metabolites appears to outweigh what Toxoplasma can manufacture de novo as tachyzoites rapidly replicate. For example, Toxoplasma can synthesize lipoic acid, a cofactor for vital dehydrogenase complexes, in apicoplast, a non-photosynthetic plastid-like organelle. Nevertheless, tachyzoites still scavenge lipoic acid from the host cell, potentially from host mitochondria recruited to the PV (Crawford et al., 2006).
Recruited mitochondria may also subvert host cell apoptosis (Ghosh et al., 2012) or immune function through the control of cytokine production and regulation of inflammasome pathways (Thakur et al., 2019). Absence of the aforementioned MAF1 protein leads to dissociation of the host mitochondria from the PVM and lower levels of IL-6, IL-10, CXCL1, and CCL-5 in infected cells (Pernas et al., 2014). It should be noted that host cells have been reported to initiate defenses when intracellular parasites pilfer their resources. For example, host mitochondria have been observed to fuse around the PV during Toxoplasma infection in order to curtail the parasite’s uptake of fatty acids (Pernas et al., 2018).
Toxoplasma is auxotrophic for many amino acids, including arginine and tryptophan, mandating their sequestration from host cells (Fox et al., 2004; Pfefferkorn et al., 1986). Of interest, metabolic analysis indicates that there are high levels of tryptophan in host cells throughout Toxoplasma infection (Olson et al., 2020). Given that tryptophan is also an essential amino acid in human cells, this finding suggests that there is enhanced import of this amino acid in host cells infected by the parasite. This idea is bolstered by the finding that expression of LAT1, which transports tryptophan, is significantly enhanced in cells upon infection with Toxoplasma (Olson et al., 2020). Arginine levels are also elevated at 24- and 36-hours post infection, consistent with high expression of arginine succinate synthase 1 (ASS1) found in infected host cells (Olson et al., 2020). In addition, we have recently reported that as Toxoplasma depletes host arginine, the host cell initiates a starvation response through the eIF2 kinase GCN2, leading to increased expression of cationic amino acid transporter 1 (CAT1) to import more arginine into infected host cells (Augusto et al., 2019).
Host microtubules are translocated to the PV as another means to raid the host cell of nutrients. Microtubule-based invaginations of the PVM facilitate the delivery of cargo-filled host endo-lysosomes within the PV (Coppens et al., 2006). In order to break macromolecules down into raw materials the parasite the can use, Toxoplasma is equipped with proteins like cathepsin protease L and B (CPL and CPB), which localize to its lysosome-like vacuolar compartment (VAC) (Di Cristina et al., 2017; Dou et al., 2014; McDonald et al., 2020). Interestingly, the ability to harness nutrients carries over into the latent bradyzoite stage. Bradyzoites lacking CPL contain undigested autophagosomes in the parasite cytosol, demonstrating an unexpected importance for VAC proteolysis in chronic infection (Di Cristina et al., 2017).
Here to stay: Converting to latent tissue cysts
The transition of tachyzoites to bradyzoites has long been associated with stress responses. As one might surmise, dormancy serves as an effective strategy for the parasite to whether storms and survive periods of scare nutrients. Different kinds of stresses have been found to induce differentiation into bradyzoites and formation of tissue cysts in vitro (Skariah et al., 2010). As a general rule, any stress that slows growth of parasites appears to induce some degree of bradyzoite conversion. One of the most common stresses used to induce bradyzoite conversion in the laboratory is exposure to alkaline pH (8.2); differentiation may be further enhanced by combining alkaline pH with CO2 deprivation, which impedes de novo production of pyrimidines. Other insults triggering bradyzoite conversion in the laboratory include nutrient starvation, immune modulators such as IFN-γ, IL-6, and nitric oxide, heat shock, depletion of low-density lipoprotein-derived cholesterol, metabolic inhibitors, sublethal doses of anti-parasitics, and ER stress (Cerutti et al., 2020).
Signaling through secondary messengers in the parasite has also been shown to play a role in stage conversion. Cyclic AMP (cAMP) signaling can induce or suppress bradyzoite differentiation in a dose-dependent manner: a transient increase in cAMP promotes bradyzoite conversion, but a prolonged elevation of cAMP levels impedes this process. Specific inhibitors of the cAMP dependent protein kinase and apicomplexan cGMP dependent protein kinase inhibit tachyzoite replication and induce differentiation (Eaton et al., 2006). Of three protein kinase A (PKA) catalytic subunits in Toxoplasma, the coccidian-specific subunit, TgPKAc3, was demonstrated to be a key factor involved cAMP-dependent tachyzoite maintenance (Sugi et al., 2016).
It is unclear whether parasite stress directly signals a developmental shift or if the consequent delay in growth is the primary driver of bradyzoite differentiation. It has been shown that a slowing of the parasite cell cycle is requisite for progression to the bradyzoite stage (Radke et al., 2003). While fever (heat shock) and immune modulators like IFN-γ have been suggested to be potential stresses that may prompt tachyzoite to bradyzoite conversion in vivo, the type of host cell or its physiological condition may be more relevant factors in triggering formation of tissue cysts (Sullivan et al., 2009). The highest concentrations of tissue cysts found in vivo reside in post-mitotic neuronal and skeletal muscle cells (Remington and Cavanaugh, 1965).
A key question is how these various stress signals are sensed by the parasite to culminate in a coordinated response to differentiate. To address this question, we determined whether apicomplexan parasites utilized an integrated stress response (ISR) that relied on translational control as documented previously in other eukaryotes. The ISR involves a group of eIF2 kinases that recognize stress signals and respond by phosphorylating eIF2α, which governs the rate-limiting step of protein synthesis (Wek et al., 2006). Phosphorylated eIF2α reduces global protein production and promotes the preferential translation of mRNAs that encode factors that remediate the stress. The subset of mRNAs preferentially translated under these conditions tend to have 5’-leader sequences enriched in upstream open reading frames (uORFs) (Young and Wek, 2016). We established that Toxoplasma possesses an ISR and that bradyzoite development is accompanied by enhanced TgIF2α phosphorylation and preferential translation (Holmes et al., 2017; Konrad et al., 2013; Narasimhan et al., 2008).
Four eIF2 kinases have been characterized that sense distinct stresses tachyzoites may encounter. TgIF2K-A is an ER-resident eIF2 kinase that responds to ER stress and controls the unfolded protein response, analogous to PERK in mammalian cells (Joyce et al., 2013; Narasimhan et al., 2008). TgIF2K-B resembles HRI and is activated during oxidative stress (Augusto et al., 2020b). TgIF2K-C and -D are homologues of GCN2 and respond to nutrient deprivation. Interestingly, TgIF2K-D is required to aid survival of extracellular tachyzoites while TgIF2K-C responds to amino acid deprivation in intracellular tachyzoites (Konrad et al., 2011, 2014). Targeted loss of these parasite eIF2 kinases may impair the ability of Toxoplasma to form stress-induced bradyzoites. For example, a pharmacological inhibitor of TgIF2K-A has been shown to reduce the frequency of bradyzoite differentiation in vitro (Augusto et al., 2018). Analysis of the preferentially translated mRNAs during stress-induced differentiation through polysome profiling has also yielded insights into the signaling pathways orchestrating stage conversion. Profiling of ribosomes in polysomes in tachyzoites subjected to ER stress revealed a number of Apetala-2 (AP2) proteins (see below), chromatin remodelers, and the bradyzoite-formation deficient (BFD1) “master regulator” to be preferentially translated (Joyce et al., 2013). Of note, many AP2 factors and BFD1 contain uORFs, suggesting that Toxoplasma uses similar mechanisms to drive preferential translation during developmental changes (Waldman et al., 2020). These results suggest that stressed tachyzoites engage an ISR to preferentially translate mRNAs that encode factors that will reconfigure the expressed genome for bradyzoite conversion.
Transitioning to bradyzoites requires a substantial reprogramming in gene expression, and the consequent use of chromatin remodeling (Jeffers et al., 2018). Curiously, apicomplexan parasites lack key families of transcription factors, such as basic leucine zipper (bZIP) factors, that are preferentially translated in higher eukaryotes to reprogram the genome. Rather, the Apicomplexa deploy a series of factors that harbor a DNA-binding motif related to the Apetala-2 (AP2) domain that was first characterized in plants (Balaji et al., 2005). Toxoplasma contains nearly 70 of these AP2 factors, and the functions of most of them have yet to be elucidated. In the handful that have been characterized to date, it is clear that AP2 factors play an important role in specialized facets of gene expression regulation through cooperation with histone modifying machinery. Different AP2s have been reported to associate with either histone acetyltransferase complexes, which activate gene expression, or histone deacetylase complexes, which repress gene expression (Harris et al., 2019; Saksouk et al., 2005; Wang et al., 2014).
A key AP2 factor associated with stage conversion is AP2IX-9, which restricts the development of bradyzoite tissue cysts (Radke et al., 2013). Overexpression of AP2IX-9 antagonized tissue cyst formation and its genetic ablation increased it, indicating AP2IX-9 serves as a repressor of bradyzoite development. But rather than serving as master regulators, it appears numerous AP2s act to “fine-tune” gene expression, which would provide Toxoplasma with greater flexibility in its developmental commitments. Like AP2IX-9, expression of AP2IV-3 is also upregulated during alkaline pH stress. However, AP2IV-3 activity is more consistent with that of a transcriptional activator, targeting some of the same gene promoters as AP2IX-9. It was proposed that these two AP2s compete to control bradyzoite gene expression, which might allow the parasite to adapt to different host cell backgrounds (Hong et al., 2017).
Consistent with the link between cell cycle progression and developmental switching to bradyzoites, numerous AP2s associated with stage switching also have expression patterns that coincide with distinct phases of the cell cycle (Behnke et al., 2010). AP2IV-4 is expressed in late S phase in tachyzoites and its depletion leads to the expression of a several key bradyzoite proteins (Radke et al., 2018). Without AP2IV-4 suppressing these bradyzoite proteins in tachyzoites, the parasites were cleared by the host immune response and failed to establish chronic infection. Another AP2 factor with enhanced expression during S phase, AP2IX-4, was shown to repress a subset of bradyzoite genes (Huang et al., 2017).
In addition to the contributions made by AP2 factors, the aforementioned “master regulator” transcription factor named BFD1 was recently discovered using a CRISPR/Cas9 screening strategy (Waldman et al., 2020). BFD1 is a Myb-like factor that binds to transcriptional start sites of genes known to be induced during onset of bradyzoite development. Illustrating the complex collaborative efforts to bring about stage conversion, BFD1 was found to regulate the transcription of AP2 factors, including AP2IX-9. Chromatin remodeling enzymes such as the lysine acetyltransferase GCN5a, as well as the lysine deacetylase HDAC3, have also been shown to affect gene expression events critical for stage conversion (Bougdour et al., 2009; Naguleswaran et al., 2010; Saksouk et al., 2005). Precisely how all these various factors interplay to affect developmental transitions is an important question for future research.
It has been presumed that elements of the innate immune response generate stresses (e.g. heat shock from fever or reactive oxygen and nitrogen species) that induce bradyzoite formation in vivo, and depletion of IFN-γ will reactivate cysts in mouse models of chronic infection (Gazzinelli et al., 1992). But the frequency of cyst formation varies widely across animal species: cysts are more prevalent in sheep, swine, and goats as opposed to cattle, whereas some species like sea otters, dolphins, and kangaroos often succumb to acute toxoplasmosis (Tenter et al., 2000). Different strains of inbred mice also display varying sensitivity to Toxoplasma that has been traced to differences in major histocompatibility complex (MHC) class II haplotype (Leroux et al., 2015). Adding to the complexity, some host cell backgrounds trigger high frequencies of spontaneous differentiation to bradyzoites (Ferreira da Silva Mda et al., 2008). Primary skeletal muscle cells trigger spontaneous conversion to bradyzoites at higher rates than fibroblasts (Ferreira-da-Silva Mda et al., 2009). Additionally, the proclivity for Toxoplasma to primarily infect neurons and skeletal muscle tissue in vivo meshes with findings that these cell types induce spontaneous differentiation into bradyzoites (Lüder and Rahman, 2017). Together, these findings suggest that various stresses, host cell signatures, and immune modulators can contribute to the induction of bradyzoite differentiation, suggesting that Toxoplasma is equipped with a sophisticated array of sensing mechanisms that can respond to diverse signals.
What’s so special about the cat: Making oocysts
Another key developmental transition in Toxoplasma that is central to parasite transmission is the formation of gametocytes in its definitive hosts, which are restricted to feline species. The sexual stage takes place exclusively in the intestinal epithelium of cats, resulting in the dissemination of infectious oocysts into the environment (Zulpo et al., 2018).
The study of the developmental stages taking place in the cat gut have been stymied by a lack of model systems. Classic studies suggest that upon ingestion, bradyzoites undergo transformation into schizonts and then merozoites, which replicate for 2 to 4 doublings before developing into macrogametes and microgametes that fuse to make diploid oocysts (Dubey and Frenkel, 1972). The lack of convenient experimental models has long stymied study of gametogenesis and fertilization, however recent transcriptomic analyses have revealed a number of genes whose increased expression during these phases likely indicate an important function during these transitions. Study of these genes may lead to a live vaccine capable of blocking parasite transmission by felids. Hapless-2 (HAP2) was identified as a microgametocyte gamete fusion protein in the fellow coccidian parasite Eimeria tenella (Walker et al., 2015). Knockout of HAP2 in Toxoplasma resulted in oocysts that were deformed, fewer in number, and defective in sporulation. Furthermore, inoculation of cats with HAP2-deficient parasites prevented oocyst excretion following infection with wild-type Toxoplasma (Ramakrishnan et al., 2019). Incidentally, antibodies designed to interfere with HAP2 function significantly reduced transmission of multiple Plasmodium species (Angrisano et al., 2017).
Following exposure to the air, parasites within the oocyst mature into sporozoites. Upon ingestion by a host organism, digestive enzymes in the stomach break down the oocyst wall, subsequently releasing sporozoites into the small intestine. Non-replicative sporozoites then invade enterocytes and convert into replicative tachyzoites capable of disseminating the infection throughout the body. Transcriptomic and proteomic studies have shown that, while short-lived, sporozoites express a panel of genes specific to this stage (Fritz et al., 2012a; Fritz et al., 2012b). To address changes in sporozoite-infected host cells, an in vitro model was developed using rat intestinal epithelium cells (Guiton et al., 2017). Initial studies using this model have shown that sporozoites trigger an NF-κB-like response in host cells that largely mirrors what is seen in tachyzoite-infected host cells (Guiton et al., 2017).
The mystery as to why cats were the only known definitive hosts was recently resolved by Di Genova and colleagues (Martorelli Di Genova et al., 2019). Development of cat intestinal organoids allowed the analysis of signaling molecules that might trigger entry into the sexual stage, and it was discovered that linoleic acid prompted more than one-third of the parasites in organoid culture to begin expressing merozoite markers. This finding sparked interest as cats are the only mammal known to lack delta-6-desaturase in their small intestines, an enzyme that converts linoleic acid to arachidonic acid. Consequently, linoleic acid levels are unusually high in felines (MacDonald et al., 1983). Together, these results suggest that the abundance of linoleic acid in the cat gut explains the exclusivity of felines as the definitive host that supports the sexual stage of the Toxoplasma life cycle. Consistent with this idea, the parasites in infected mice that were fed a linoleic acid–rich diet and SC-26196, a delta-6-desaturase inhibitor, displayed the merozoite marker GRA11B and low expression of a tachyzoite-specific marker, SAG1 (Martorelli Di Genova et al., 2019). Moreover, these mice shed infectious oocysts, paving the way for a potentially powerful new model system for the study of sexual stage transitions, including the sporulation of oocysts.
Recent evidence has been presented that implicate a microrchidia (MORC) homologue as instrumental in regulating the changes in gene expression governing the conversion to sexual stages. MORCs are conserved proteins associated with signaling-dependent chromatin remodeling and epigenetic regulation (Li et al., 2013). A MORC homologue from Toxoplasma has been identified that complexes with multiple AP2 transcription factors and the lysine deacetylase HDAC3 to repress sexual stage and oocyst gene expression (Farhat et al., 2020). Parasites lacking MORC displayed significant transcriptional changes that were skewed toward sexual differentiation. It has been proposed that MORC directs the hierarchical expression of secondary AP2 factors, which in turn contributes to the unidirectionality of the parasite life cycle (Farhat et al., 2020). For example, MORC-depleted parasites express AP2IX-9, which was reported to restrict commitment towards bradyzoite differentiation (see above); in this case, MORC’s degradation may induce AP2IX-9 to prevent merozoites from converting back into bradyzoites (Farhat et al., 2020).
Intriguingly, MORC family ATPases have previously been associated with sex-related functions in a number of other diverse organisms. MORC was initially characterized in mice and linked to the control of spermatid formation (Watson et al., 1998). MORC is also more abundant in reproductive tissues and plays a role in sexual development in plants and mammals (Koch et al., 2017). In C. elegans, MORC is essential for transgenerational fertility and acts as an effector of germline-expressed endogenous small interfering RNAs (Weiser et al., 2017). MORC is likely to play similar gene regulatory roles in other apicomplexan parasites as well (Hillier et al., 2019). Recently, a MORC protein was identified in Plasmodium falciparum that binds the promoter region of the major virulence gene family along with the chromatin remodeler ISWI and an AP2 factor (Bryant et al., 2020).
The MORC complex facilitates chromatin remodeling at genes normally expressed during sexual stages, thereby repressing their transcription. MORC may also act on tachyzoite genes to repress them during sexual development, since tachyzoite genes are also repressed upon MORC depletion. MORC may have additional functions at the boundary between other stage transitions, as it was also found in complex with the aforementioned AP2IX-4, which acts as a repressor of a subset of bradyzoite genes (Huang et al., 2017; Srivastava et al., 2020). AP2IX-4 and MORC associate with yet another cell cycle-regulated AP2 factor, AP2XII-2; depletion of AP2XII-2 increases the length of S-phase and enhances frequency of bradyzoite development (Srivastava et al., 2020).
Future Outlook
The ability of Toxoplasma to switch from replicative to latent forms is responsible for pathogenicity and transmission to humans and other animals. Development of latent stages has been historically challenging to study for lack of accessible models. In the case of bradyzoite differentiation, the application of stress to in vitro cultures has allowed substantial discoveries to be made into the transcriptional and translational changes taking place during this developmental process. However, how faithful the in vitro results represent what actually occurs in vivo remains an important concern. Moreover, prolonged passage of Toxoplasma in vitro clearly disrupts developmental competency as the process selects for rapidly growing tachyzoites at the expense of efficient differentiation. The widely used HFF cells are not likely to be representative of what occurs in other cell types, and it is worth noting that neurons are the primary host cells for bradyzoite development (Cabral et al., 2016). A novel method using primary murine neonatal astrocytes and hypoxia conditions has been developed as an improved model for the study of tissue cysts and recrudescence in vitro that may better preserve developmental competency (Goerner et al., 2020). Other cell backgrounds that prompt spontaneous differentiation of tachyzoites into bradyzoites should be compared to those formed in HFF cells to assess similarities and differences in the model systems. Identification of host cell factors that signal to tachyzoites what host cell type they are in is crucial knowledge that is currently lacking.
Many questions remain regarding mechanisms of stress-induced conversion to bradyzoites (Fig. 1). How TgIF2 kinases become activated by various stresses to bring about changes in gene expression remains incompletely characterized. Other aspects of the parasite’s ISR remain unresolved, including mechanisms of preferential translation of mRNAs that contribute to bradyzoite development. Upstream ORFs have been identified in a number of factors involved in bradyzoite conversion, including AP2s and BFD1, but how they function in the context of translation control requires further study. In addition, little is known about how these factors collaborate to bring about changes in the transcriptome that are germane to stage switching. Virtually no work has been done into the signals mediating reactivation of infection, the conversion of bradyzoites back into tachyzoites.
Figure 1. Key host and parasite signaling events during Toxoplasma life cycle stage transitions.
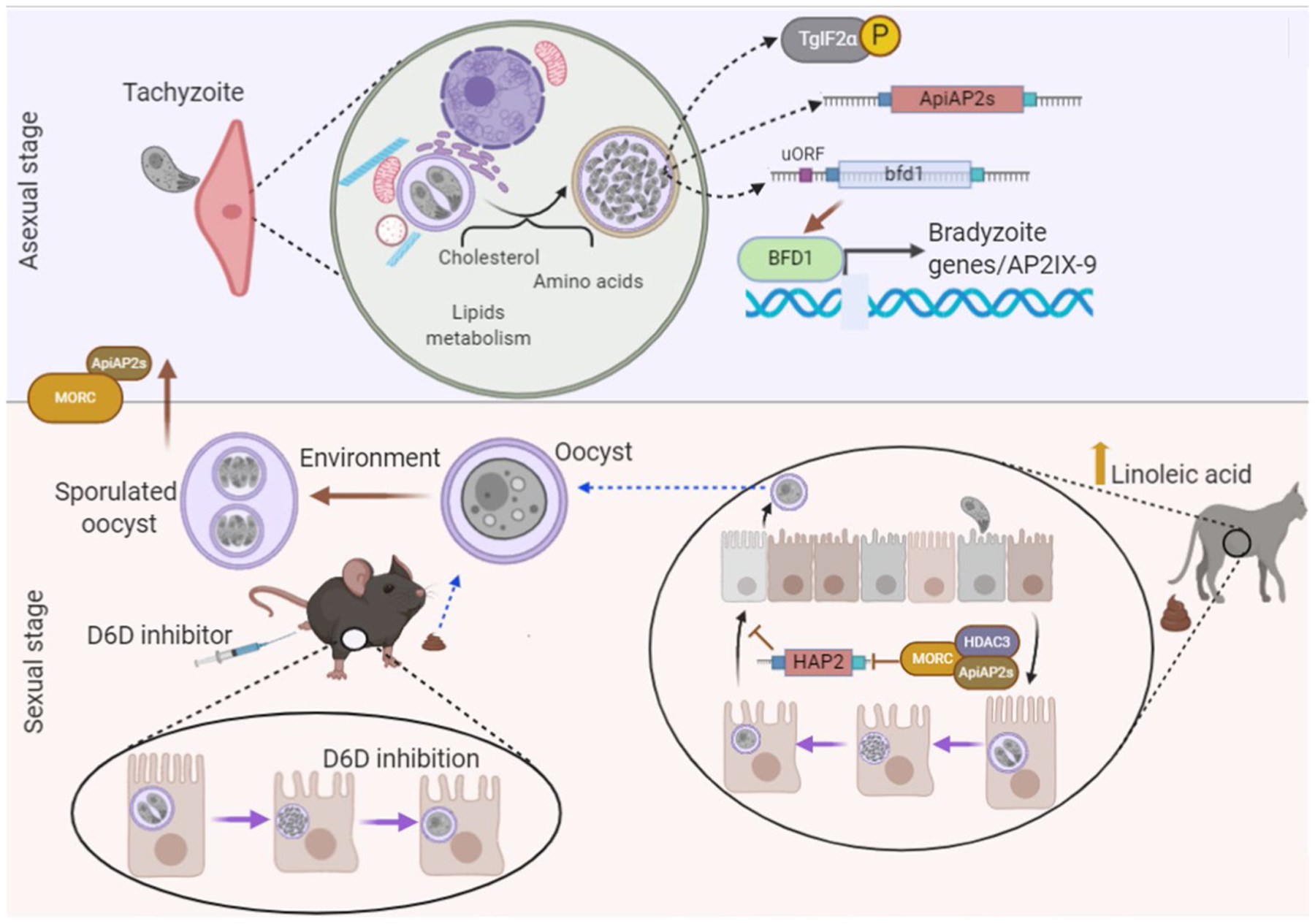
Asexual stage (top): Upon infection, proliferative tachyzoites generate a parasitophorous vacuole (PV) that forms an interface with the host cell. In addition to proteins secreted into the host cell, the PV recruits host cell organelles and microtubules for nutrient acquisition and commandeering of cellular pathways such as overriding apoptosis. In the proper host cell background or in response to stress, tachyzoites convert into latent bradyzoites housed within tissue cysts. Stress-induced differentiation is accompanied by activation of a family of TgIF2 kinases, which direct translational control of select mRNAs (e.g. AP2s and BFD1) via phosphorylation of TgIF2α, culminating in reprogramming of gene expression. Mechanisms of preferential translation caused by TgIF2α phosphorylation may involve unique features in the leader sequences, such as upstream ORFs. Sexual stage (bottom): Felines are the definitive hosts of Toxoplasma capable of supporting the sexual stage that produces transmissible oocysts that sporulate when exposed to environmental oxygen. The MORC complex plays a role in the repression of the sexual stage and oocyst genes such as HAP2. Linoleic acid was found to be a key metabolite in the host that signals Toxoplasma to convert into gametocytes. By administering a delta-6-desaturase (D6D) inhibitor, researchers have been able to isolate oocysts from infected mice, generating a new model system for the study of sexual stage transitions.
The study of sexual stages has been even more intractable, but the 2019 landmark study by Di Genova et al. promises to open new avenues to study Toxoplasma gametogenesis and oocyst formation (Martorelli Di Genova et al., 2019). The demonstration that oocysts can be generated in cat intestinal organoids and in a mouse model through administration of a delta-6-desaturase (D6D) inhibitor provides unprecedented opportunities to study how Toxoplasma signals forms oocysts (Fig. 1). Moreover, continued characterization of the MORC complex as a regulator of sexual stage genes should reveal insights into gene networks deployed during this transition.
Only a handful of the secreted proteins ejected into host cells have been investigated to date, and the full scope of their activities remains an outstanding question. A variety of mechanisms have been implicated in the initiation of hypermigratory activity in certain host cells; how these activities interplay is an open question. Finally, the mechanisms and purpose of host organelle recruitment to the PV remain poorly understood. Shedding light on these processes will not only advance our understanding of host-parasite interaction but will also uncover new potential drug targets aimed at better controlling toxoplasmosis.
Acknowledgements
Research in our laboratories is supported by a research grants from National Institutes of Health (AI124723 to W.J.S. and R.C.W.; AI152583 and AI116496 to W.J.S). R.C.W. has received grant support from Eli Lilly and Company and is a scientific advisor to HiberCell.
Reference
- Angrisano F, Sala KA, Da DF, Liu Y, Pei J, Grishin NV, Snell WJ, and Blagborough AM (2017). Targeting the Conserved Fusion Loop of HAP2 Inhibits the Transmission of Plasmodium berghei and falciparum. Cell Rep 21, 2868–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto L, Amin PH, Wek RC, and Sullivan WJ Jr. (2019). Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii. PLoS pathogens 15, e1007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto L, Martynowicz J, Amin PH, Alakhras NS, Kaplan MH, Wek RC, and Sullivan WJ Jr. (2020a). Toxoplasma gondii Co-opts the Unfolded Protein Response To Enhance Migration and Dissemination of Infected Host Cells. mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto L, Martynowicz J, Amin PH, Carlson KR, Wek RC, and Sullivan WJ (2020b). TgIF2K-B is an eIF2α kinase in Toxoplasma gondii that responds to oxidative stress and optimizes pathogenicity. bioRxiv, 2020.2009.2024.312744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto L, Martynowicz J, Staschke KA, Wek RC, and Sullivan WJ Jr. (2018). Effects of PERK eIF2α Kinase Inhibitor against Toxoplasma gondii. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Babu MM, Iyer LM, and Aravind L (2005). Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res 33, 3994–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, Sibley LD, and White MW (2010). Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One 5, e12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli AE, and Denkers EY (2008). Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. Journal of immunology (Baltimore, Md. : 1950) 181, 8485–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader IJ, Coleman BI, Chen CT, and Gubbels MJ (2015). Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu Rev Microbiol 69, 463–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume M, and Seeber F (2018). Metabolic interactions between Toxoplasma gondii and its host. F1000Research 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, et al. (2013). Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13, 489–500. [DOI] [PubMed] [Google Scholar]
- Bougdour A, Maubon D, Baldacci P, Ortet P, Bastien O, Bouillon A, Barale JC, Pelloux H, Ménard R, and Hakimi MA (2009). Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J Exp Med 206, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, et al. (2013). A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med 210, 2071–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JM, Baumgarten S, Dingli F, Loew D, Sinha A, Claës A, Preiser PR, Dedon PC, and Scherf A (2020). Exploring the virulence gene interactome with CRISPR/dCas9 in the human malaria parasite. Mol Syst Biol 16, e9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, and Koshy AA (2016). Neurons are the Primary Target Cell for the Brain-Tropic Intracellular Parasite Toxoplasma gondii. PLoS Pathog 12, e1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Blanchard N, and Besteiro S (2020). The Bradyzoite: A Key Developmental Stage for the Persistence and Pathogenesis of Toxoplasmosis. Pathogens (Basel, Switzerland) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, and Joiner KA (2006). Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125, 261–274. [DOI] [PubMed] [Google Scholar]
- Coppens I, and Romano JD (2018). Hostile intruder: Toxoplasma holds host organelles captive. PLoS Pathog 14, e1006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, and Tardieux I (2006). CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, and Seeber F (2006). Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. Embo j 25, 3214–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabritz HA, and Conrad PA (2010). Cats and Toxoplasma: implications for public health. Zoonoses and public health 57, 34–52. [DOI] [PubMed] [Google Scholar]
- Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, Gonzalez V, Page C, Chauvet J, Ochoa W, Volkmann N, et al. (2012). Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci 125, 4333–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristina M, Dou Z, Lunghi M, Kannan G, Huynh MH, McGovern OL, Schultz TL, Schultz AJ, Miller AJ, Hayes BM, et al. (2017). Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nat Microbiol 2, 17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, McGovern OL, Di Cristina M, and Carruthers VB (2014). Toxoplasma gondii ingests and digests host cytosolic proteins. MBio 5, e01188–01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, and Sibley LD (2019). The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, and Frenkel JK (1972). Cyst-induced toxoplasmosis in cats. The Journal of protozoology 19, 155–177. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Miller NL, and Frenkel JK (1970). The Toxoplasma gondii oocyst from cat feces. J Exp Med 132, 636–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton MS, Weiss LM, and Kim K (2006). Cyclic nucleotide kinases and tachyzoite-bradyzoite transition in Toxoplasma gondii. Int J Parasitol 36, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat DC, Swale C, Dard C, Cannella D, Ortet P, Barakat M, Sindikubwabo F, Belmudes L, De Bock PJ, Couté Y, et al. (2020). A MORC-driven transcriptional switch controls Toxoplasma developmental trajectories and sexual commitment. Nat Microbiol 5, 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, et al. (2010). Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8, 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-da-Silva Mda F, Takács AC, Barbosa HS, Gross U, and Lüder CG (2009). Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite-to-bradyzoite conversion at higher rates than fibroblasts. International journal of medical microbiology : IJMM 299, 381–388. [DOI] [PubMed] [Google Scholar]
- Ferreira da Silva Mda F, Barbosa HS, Gross U, and Lüder CG (2008). Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Molecular bioSystems 4, 824–834. [DOI] [PubMed] [Google Scholar]
- Fox BA, Gigley JP, and Bzik DJ (2004). Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol 34, 323–331. [DOI] [PubMed] [Google Scholar]
- Fritz HM, Bowyer PW, Bogyo M, Conrad PA, and Boothroyd JC (2012a). Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS One 7, e29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HM, Buchholz KR, Chen X, Durbin-Johnson B, Rocke DM, Conrad PA, and Boothroyd JC (2012b). Transcriptomic analysis of toxoplasma development reveals many novel functions and structures specific to sporozoites and oocysts. PLoS One 7, e29998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R, Xu Y, Hieny S, Cheever A, and Sher A (1992). Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. Journal of immunology (Baltimore, Md. : 1950) 149, 175–180. [PubMed] [Google Scholar]
- Ghosh D, Walton JL, Roepe PD, and Sinai AP (2012). Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol 14, 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerner AL, Vizcarra EA, Hong DD, Bergersen KV, Alvarez CA, Talavera MA, Wilson EH, and White MW (2020). An ex vivo model of Toxoplasma recrudescence. bioRxiv, 2020.2005.2018.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, Leroy C, Blazquez S, Menard R, and Tardieux I (2009). Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe 5, 259–272. [DOI] [PubMed] [Google Scholar]
- Guiton PS, Sagawa JM, Fritz HM, and Boothroyd JC (2017). An in vitro model of intestinal infection reveals a developmentally regulated transcriptome of Toxoplasma sporozoites and a NF-κB-like signature in infected host cells. PLoS One 12, e0173018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Zahn MM, Coppens I, Joiner KA, and Voelker DR (2005). Selective disruption of phosphatidylcholine metabolism of the intracellular parasite Toxoplasma gondii arrests its growth. J Biol Chem 280, 16345–16353. [DOI] [PubMed] [Google Scholar]
- Håkansson S, Morisaki H, Heuser J, and Sibley LD (1999). Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell 10, 3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi MA, Olias P, and Sibley LD (2017). Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin Microbiol Rev 30, 615–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen SK, and Weiss LM (2013). Toxoplasmosis. Handb Clin Neurol 114, 125–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MT, Jeffers V, Martynowicz J, True JD, Mosley AL, and Sullivan WJ Jr. (2019). A novel GCN5b lysine acetyltransferase complex associates with distinct transcription factors in the protozoan parasite Toxoplasma gondii. Mol Biochem Parasitol 232, 111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl AB, Basso WU, Lippuner C, Ramakrishnan C, Okoniewski M, Walker RA, Grigg ME, Smith NC, and Deplazes P (2015). Asexual expansion of Toxoplasma gondii merozoites is distinct from tachyzoites and entails expression of non-overlapping gene families to attach, invade, and replicate within feline enterocytes. BMC genomics 16, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier C, Pardo M, Yu L, Bushell E, Sanderson T, Metcalf T, Herd C, Anar B, Rayner JC, Billker O, et al. (2019). Landscape of the Plasmodium Interactome Reveals Both Conserved and Species-Specific Functionality. Cell Rep 28, 1635–1647.e1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MJ, Augusto LDS, Zhang M, Wek RC, and Sullivan WJ Jr. (2017). Translational Control in the Latency of Apicomplexan Parasites. Trends Parasitol 33, 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DP, Radke JB, and White MW (2017). Opposing Transcriptional Mechanisms Regulate Toxoplasma Development. mSphere 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Holmes MJ, Radke JB, Hong DP, Liu TK, White MW, and Sullivan WJ Jr. (2017). Toxoplasma gondii AP2IX-4 Regulates Gene Expression during Bradyzoite Development. mSphere 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers V, Tampaki Z, Kim K, and Sullivan WJ Jr. (2018). A latent ability to persist: differentiation in Toxoplasma gondii. Cell Mol Life Sci 75, 2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce BR, Tampaki Z, Kim K, Wek RC, and Sullivan WJ Jr. (2013). The unfolded protein response in the protozoan parasite Toxoplasma gondii features translational and transcriptional control. Eukaryot Cell 12, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S, and Davis PH (2012). Toxoplasma on the brain: understanding host-pathogen interactions in chronic CNS infection. Journal of parasitology research 2012, 589295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Kang HG, Steinbrenner J, Dempsey DA, Klessig DF, and Kogel KH (2017). MORC Proteins: Novel Players in Plant and Animal Health. Frontiers in plant science 8, 1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Queener SF, Wek RC, and Sullivan WJ Jr. (2013). Inhibitors of eIF2α dephosphorylation slow replication and stabilize latency in Toxoplasma gondii. Antimicrob Agents Chemother 57, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Wek RC, and Sullivan WJ Jr. (2011). A GCN2-like eukaryotic initiation factor 2 kinase increases the viability of extracellular Toxoplasma gondii parasites. Eukaryot Cell 10, 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Wek RC, and Sullivan WJ Jr. (2014). GCN2-like eIF2alpha kinase manages the amino acid starvation response in Toxoplasma gondii. Int J Parasitol 44, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, et al. (2016). Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1, 16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, and Boothroyd JC (2012). Toxoplasma co-opts host cells it does not invade. PLoS Pathog 8, e1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, and Saeij JPJ (2018). Toxoplasma Does Not Secrete the GRA16 and GRA24 Effectors Beyond the Parasitophorous Vacuole Membrane of Tissue Cysts. Frontiers in cellular and infection microbiology 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux LP, Nishi M, El-Hage S, Fox BA, Bzik DJ, and Dzierszinski FS (2015). Parasite Manipulation of the Invariant Chain and the Peptide Editor H2-DM Affects Major Histocompatibility Complex Class II Antigen Presentation during Toxoplasma gondii Infection. Infect Immun 83, 3865–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Nair SS, and Kumar R (2013). The MORC family: new epigenetic regulators of transcription and DNA damage response. Epigenetics 8, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüder CGK, and Rahman T (2017). Impact of the host on Toxoplasma stage differentiation. Microbial cell (Graz, Austria) 4, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald ML, Rogers QR, and Morris JG (1983). Role of linoleate as an essential fatty acid for the cat independent of arachidonate synthesis. The Journal of nutrition 113, 1422–1433. [DOI] [PubMed] [Google Scholar]
- Martorelli Di Genova B, Wilson SK, Dubey JP, and Knoll LJ (2019). Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol 17, e3000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SK, Olias P, Huang Z, Wang Q, Park E, Yokoyama WM, and Sibley LD (2019). Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc Natl Acad Sci U S A 116, 17480–17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Smith D, Di Cristina M, Kannan G, Dou Z, and Carruthers VB (2020). Toxoplasma Cathepsin Protease B and Aspartyl Protease 1 Are Dispensable for Endolysosomal Protein Digestion. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JG, and Liesenfeld O (2004). Toxoplasmosis. Lancet 363, 1965–1976. [DOI] [PubMed] [Google Scholar]
- Naguleswaran A, Elias EV, McClintick J, Edenberg HJ, and Sullivan WJ Jr. (2010). Toxoplasma gondii lysine acetyltransferase GCN5-A functions in the cellular response to alkaline stress and expression of cyst genes. PLoS Pathog 6, e1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, Dixon SE, Coppens I, Wek RC, and Sullivan WJ Jr. (2008). Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem 283, 16591–16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan SJ, Romano JD, Kline JT, and Coppens I (2018). Novel Approaches To Kill Toxoplasma gondii by Exploiting the Uncontrolled Uptake of Unsaturated Fatty Acids and Vulnerability to Lipid Storage Inhibition of the Parasite. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson WJ, Martorelli Di Genova B, Gallego-Lopez G, Dawson AR, Stevenson D, Amador-Noguez D, and Knoll LJ (2020). Dual metabolomic profiling uncovers Toxoplasma manipulation of the host metabolome and the discovery of a novel parasite metabolic capability. PLoS Pathog 16, e1008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong YC, Reese ML, and Boothroyd JC (2010). Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem 285, 28731–28740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas MW, and Boothroyd JC (2020). Toxoplasma Uses GRA16 To Upregulate Host c-Myc. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, Boyle JP, and Boothroyd JC (2014). Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol 12, e1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, Bean C, Boothroyd JC, and Scorrano L (2018). Mitochondria Restrict Growth of the Intracellular Parasite Toxoplasma gondii by Limiting Its Uptake of Fatty Acids. Cell Metab 27, 886–897 e884. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Eckel M, and Rebhun S (1986). Interferon-gamma suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol 20, 215–224. [DOI] [PubMed] [Google Scholar]
- Radke JB, Lucas O, De Silva EK, Ma Y, Sullivan WJ Jr., Weiss LM, Llinas M, and White MW (2013). ApiAP2 transcription factor restricts development of the Toxoplasma tissue cyst. Proc Natl Acad Sci U S A 110, 6871–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JB, Worth D, Hong D, Huang S, Sullivan WJ Jr., Wilson EH, and White MW (2018). Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLoS Pathog 14, e1007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Guerini MN, Jerome M, and White MW (2003). A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol 131, 119–127. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C, Maier S, Walker RA, Rehrauer H, Joekel DE, Winiger RR, Basso WU, Grigg ME, Hehl AB, Deplazes P, et al. (2019). An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Sci Rep 9, 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington JS, and Cavanaugh EN (1965). Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. The New England journal of medicine 273, 1308–1310. [DOI] [PubMed] [Google Scholar]
- Saksouk N, Bhatti MM, Kieffer S, Smith AT, Musset K, Garin J, Sullivan WJ Jr., Cesbron-Delauw MF, and Hakimi MA (2005). Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol 25, 10301–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare LO, Olafsson EB, Wang Y, Yang N, Julien L, Camejo A, Pesavento P, Sidik SM, Lourido S, Barragan A, et al. (2019). In Vivo CRISPR Screen Identifies TgWIP as a Toxoplasma Modulator of Dendritic Cell Migration. Cell Host Microbe 26, 478–492.e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JC, Beckers CJ, and Joiner KA (1994). The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci U S A 91, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Bahia-Oliveira L, Dixon B, Dumètre A, de Wit LA, VanWormer E, and Villena I (2019). Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food and waterborne parasitology 15, e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai AP, and Joiner KA (2001). The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol 154, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skariah S, McIntyre MK, and Mordue DG (2010). Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol Res 107, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, White MW, and Sullivan WJ (2020). Toxoplasma gondii AP2XII-2 contributes to proper progression through S-phase of the cell cycle. bioRxiv, 2020.2006.2009.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T, Ma YF, Tomita T, Murakoshi F, Eaton MS, Yakubu R, Han B, Tu V, Kato K, Kawazu S, et al. (2016). Toxoplasma gondii Cyclic AMP-Dependent Protein Kinase Subunit 3 Is Involved in the Switch from Tachyzoite to Bradyzoite Development. MBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan WJ Jr., Smith AT, and Joyce BR (2009). Understanding mechanisms and the role of differentiation in pathogenesis of Toxoplasma gondii: a review. Mem Inst Oswaldo Cruz 104, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, and Weiss LM (2000). Toxoplasma gondii: from animals to humans. Int J Parasitol 30, 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Mikkelsen H, and Jungersen G (2019). Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. Journal of immunology research 2019, 1356540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman BS, Schwarz D, Wadsworth MH, 2nd, Saeij JP, Shalek AK, and Lourido S (2020). Identification of a Master Regulator of Differentiation in Toxoplasma. Cell 180, 359–372.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA, Sharman PA, Miller CM, Lippuner C, Okoniewski M, Eichenberger RM, Ramakrishnan C, Brossier F, Deplazes P, Hehl AB, et al. (2015). RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC genomics 16, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dixon SE, Ting LM, Liu TK, Jeffers V, Croken MM, Calloway M, Cannella D, Hakimi MA, Kim K, et al. (2014). Lysine acetyltransferase GCN5b interacts with AP2 factors and is required for Toxoplasma gondii proliferation. PLoS Pathog 10, e1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sangaré LO, Paredes-Santos TC, and Saeij JPJ (2020). Toxoplasma Mechanisms for Delivery of Proteins and Uptake of Nutrients Across the Host-Pathogen Interface. Annu Rev Microbiol 74, 567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ML, Zinn AR, Inoue N, Hess KD, Cobb J, Handel MA, Halaban R, Duchene CC, Albright GM, and Moreadith RW (1998). Identification of morc (microrchidia), a mutation that results in arrest of spermatogenesis at an early meiotic stage in the mouse. Proc Natl Acad Sci U S A 95, 14361–14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner JM, Kanatani S, Hernandez-Castaneda MA, Fuks JM, Rethi B, Wallin RP, and Barragan A (2013). Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell Microbiol 15, 1735–1752. [DOI] [PubMed] [Google Scholar]
- Weidner JM, Kanatani S, Uchtenhagen H, Varas-Godoy M, Schulte T, Engelberg K, Gubbels MJ, Sun HS, Harrison RE, Achour A, et al. (2016). Migratory activation of parasitized dendritic cells by the protozoan Toxoplasma gondii 14-3-3 protein. Cell Microbiol 18, 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser NE, Yang DX, Feng S, Kalinava N, Brown KC, Khanikar J, Freeberg MA, Snyder MJ, Csankovszki G, Chan RC, et al. (2017). MORC-1 Integrates Nuclear RNAi and Transgenerational Chromatin Architecture to Promote Germline Immortality. Developmental cell 41, 408–423.e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, and Anthony TG (2006). Coping with stress: eIF2 kinases and translational control. Biochemical Society transactions 34, 7–11. [DOI] [PubMed] [Google Scholar]
- Young SK, and Wek RC (2016). Upstream Open Reading Frames Differentially Regulate Gene-specific Translation in the Integrated Stress Response. J Biol Chem 291, 16927–16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulpo DL, Sammi AS, Dos Santos JR, Sasse JP, Martins TA, Minutti AF, Cardim ST, de Barros LD, Navarro IT, and Garcia JL (2018). Toxoplasma gondii: A study of oocyst re-shedding in domestic cats. Veterinary parasitology 249, 17–20. [DOI] [PubMed] [Google Scholar]


