Abstract
COVID-19 can present with a range of skin manifestations, some of which specific of the pediatric age. The aim of this systematic literature review was to determine the type, prevalence, time of onset, and evolution of cutaneous manifestations associated with COVID-19 in newborns, children, and adolescents, after excluding multisystem inflammatory syndrome in children (MIS-C). PubMed, Tripdatabase, ClinicalTrials, and Cochrane Library databases were searched using an ad hoc string for case reports/series and observational studies, published between December 2019 and February 2022. Study quality was assessed using the STROBE and CARE tools. Seventy-three (49 case reports/series and 24 studies) out of 26,545 identified articles were included in the analysis. Dermatological lesions were highly heterogeneous for clinical presentation, time of onset, and association with other COVID-19 manifestations. Overall, they mainly affected the acral portions, and typically presented a favorable outcome. Pseudo-chilblains were the most common.
Conclusions: Mucocutaneous manifestations could be the only/predominant and early manifestation of COVID-19 that could precede other more severe manifestations by days or weeks. Therefore, physicians of all disciplines should be familiar with them.
|
What is Known: • A variety of cutaneous manifestations have been reported in association with COVID-19. • Urticaria, maculopapular, or vesicular rashes can occur at any age, while chilblains and erythema multiforme are more common in children and young patients. | |
|
What is New: • Skin lesions related to SARS-CoV-2 infection often show a peculiar acral distribution. • Mucocutaneous lesions of various type may be the only/predominant manifestation of COVID-19; they could present in paucisymptomatic and severely ill patients and occur at different stages of the disease. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04585-7.
Keywords: COVID-19, Pseudo-chilblains, Cutaneous acral lesions, Erythema multiforme, Pediatric dermatology
Introduction
The first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), was recognized in China in December 2019 [1]. SARS-CoV-2 infection rapidly spread worldwide, and in March 2020, it was declared a global pandemic by the World Health Organization (WHO). Clinical manifestations of COVID-19 are extremely heterogeneous, ranging from mild symptoms affecting the upper airways and the gastrointestinal tract, to severe respiratory failure and septic shock. Young patients are typically asymptomatic or present with mild symptoms, but a few of them can present with severe COVID-19. More rarely, they develop a peculiar hyperinflammatory disorder, multisystem inflammatory syndrome in children (MIS-C), that typically occurs some weeks after the infection and is characterized by persistent fever, gastrointestinal symptoms, mucocutaneous lesions, and, in severe cases, myocarditis, cardiac dysfunction, and acute kidney injury, leading to hypotension and shock [2]. Finally, the long-COVID-19 syndrome may arise [3].
A variety of cutaneous manifestations has been reported in association with COVID-19. Some, like urticaria, maculopapular, or vesicular rashes, can occur at any age, while some others, like chilblains or erythema multiforme (EM), are more common in the pediatric population [4]. Moreover, different skin manifestations have been described at different stages of the COVID-19 disease, sometimes representing the only, or more prominent, and early manifestation.
This systematic review aimed at providing epidemiological and clinical features typical of dermatological manifestations associated with COVID-19 in newborns, children, and adolescents, excluding those associated with MIS-C.
Methods/literature search
We conducted a systematic literature review following the Preferred Reporting Items for Systematic Reviews (PRISMA) approach [5]. Literature search was performed using PubMed (pubmed.ncbi.nlm.nih.gov), Tripdatabase (tripdatabase.com), ClinicalTrials (clinicaltrials.gov), and Cochrane (cochranelibrary.com) databases, using the string: “(((SARS-CoV-2) OR (COVID19) OR (COVID-19) OR (ncov*) OR (coronavirus)) AND ((Child) OR (children) OR (pediatric) OR (paediatric) OR (infant) OR (adolescent)) AND ((“2019/12/31”[Date—Entry]: “2022/2/28”[Date—Entry]))”.
Articles referring to observational prospective or retrospective studies, case series, and case reports, written in English or in Italian, focusing on skin lesions associated with COVID-19 in people aged < 18 years were initially included. Literature reviews and letters to the Editor; studies of all types in which COVID-19 disease was only clinically suspected but not confirmed biochemically (i.e., molecular or antigenic swab, serology, or RT-PCR on biopsy specimen); and studies not clearly describing clinical presentation and evolution of the dermatological lesions were excluded. Studies involving both pediatric and adult patients were included only if characteristics of people aged < 18 years old were clearly discernible or if they represented a meaningful part of a group/cohort and the mean/median age was < 18 years. The study protocol has been previously published and is available online [6].
Data extraction
Data were extracted by six independent reviewers (AD, DG, FG, GS, LP, MM). Each article retrieved by literature search was then examined by two authors independently, and its eligibility was determined on the basis of the title and the abstract. The inclusion of selected studies in the review was defined according to the information retrieved after examining the full text. A manual search of the bibliography of pertaining articles was finally performed to identify additional studies of interest.
The selected articles were hence tabulated according to study design and setting; sample size; patient gender, age; method used for COVID-19 diagnosis; type and time of onset of skin lesions with respect to COVID-19 diagnosis on skin lesion; associated non-dermatological manifestations, comorbidities/risk factors; treatment for COVID-19; treatment for skin lesion(s); other concurrent treatments; follow-up duration; and patient outcome.
Quality assessment
Three authors (AD, DG, MM) independently and blindly assessed the quality of the included studies using STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) [7], a 22-item tool specifically designed to evaluate observational studies quality, made of 17 standard items and 5 items varying according to the study design (i.e., cohort study, case report, or cross-sectional study). We arbitrarily defined score range for the definition of the study quality: (1) poor, 0–14 points; (2) intermediate, 15–25 points; and (3) good, 26–33 points [8]. Quality of case reports/case series was assessed using CARE (CAse REports) guidelines [9], a 13-item tool specifically designed to evaluate case reports quality. We arbitrarily defined score ranges for the definition of case reports/case series quality: (1) poor, 0–5 points; (2) intermediate, 6–9 points; and (3) good, 10–13 points.
Any disagreement between reviewers was resolved through discussion; whenever not sufficient, a third blind reviewer (IN) was appealed as tie breaker.
Results
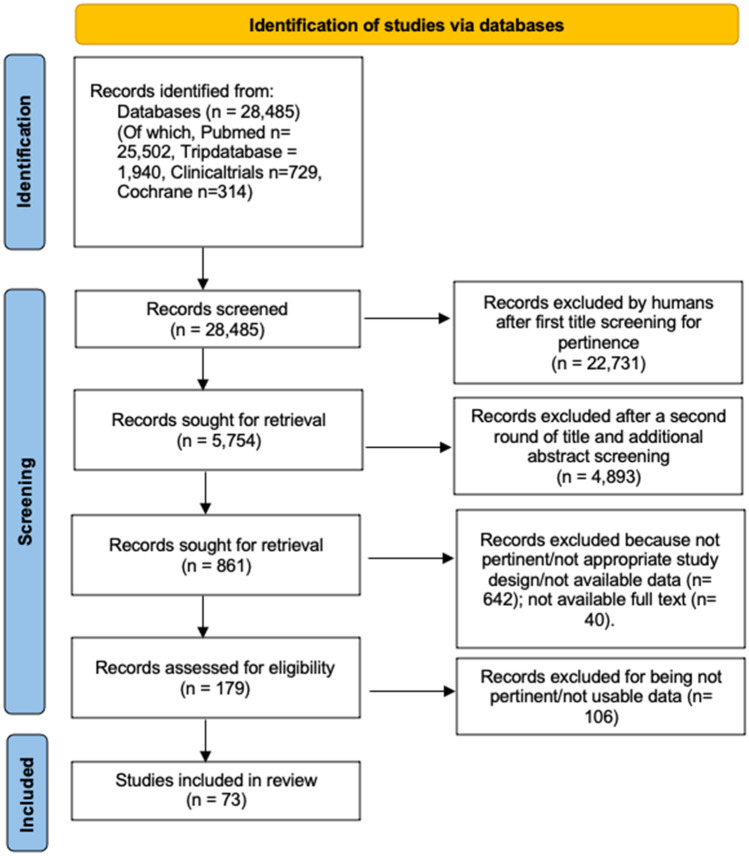
Seventy-three [10–82] out of the 28,485 papers identified by the initial search were evaluated for the features of interest and analyzed (Fig. 1). Twenty-four were observational studies, and 49 were case reports/series.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 flow diagram showing the process for articles selection [5]
Pseudo-chilblains
Chilblains are painful inflammatory skin lesions of the acral sites presenting as erythematous and edematous macules, nodules, or plaques [4]. Since the beginning of the pandemic, infants and children were referred to pediatric dermatology clinics for chilblain-like lesions or pseudo-chilblains more frequently than usual, and efforts have been made to understand their link with COVID-19 [4].
Literature search retrieved 23 articles demonstrating the occurrence of pseudo-chilblains in young patients with SARS-CoV-2 infection (Table 1). Of these, 6 were case series, 8 case reports, and 9 observational prospective studies. The majority referred to the first COVID-19 wave of spring 2020. Overall, data of 480 patients were available, demonstrating an almost equal gender distribution (males 50.2%). Patient aged ranged from 6 to 17 years; mean age (calculated on the 8 studies in which it was available, N = 307) was 14.7 years.
Table 1.
Studies evaluating the occurrence of pseudo-chilblains in pediatric patients affected by COVID-19 (excluding multisystem inflammatory syndrome in children, MIS-C)
| Author, year | n (M) | Age (mean; range) (yr) |
RT-PCR on oro-/nasopharyngeal swab or endotracheal aspirate (total, positive) |
Serology (Y/N, n) |
RT-PCR on biopsy (Y/N, n) | Contact with COVID-19 patients (n) | SARS-CoV2 detection (Y/N; n) |
|---|---|---|---|---|---|---|---|
| Andina et al. 2020 [10] | 22 (13) |
12 (6–17)* |
19; 1 | N/A | N/A | contact with household of a confirmed COVID-19 case (1); contact with a probable case (12) | Y (1) |
| Carazo-Gallego et al. 2021 [11] | 62 (37) | 10 (N/A) | 36; 0 |
Immunochromatographic assay IgM/IgG (43); CLIA; 61; IgM-/IgG; 7 IgM + /IgG + : 1; IgM + /IgG-: 1 |
N/A | N/A | Y (N/A) |
| Chua et al. 2021 [12] | 1 (NA) | N/A | 1; 1 | N/A | N/A | N/A | Y |
| Colmenero et al. 2020 [13] | 7 (4) | N/A (11–17) | 6; 0 | N/A | N/A (SARS-CoV-2 spike protein in immunohistochemistry) | Contact with probable COVID-19 case (4) | Y (7) |
| Colonna et al. 2020 [14] | 8 (NA) | N/A | 8; 0 |
IgG test spike protein S1/S2 subunit (8) IgG + : 1 |
N/A | N/A | Y (1) |
| El Hachem et al. 2020 [15] | 19 (14) | 14 (11–17) | 19; 0 |
IgG test (19) Ig + : 0 IgG and IgA spike protein S1 subunit test (19) IgG + : 1 IgG borderline: 3 IgA + : 6 IgA borderline: 3 |
Y (3) 0 positive |
Contacts with family member with COVID-19 symptoms 1–2 m before (7) | Y (10) |
| Feito-Rodrıguez et al. 2021 [16] | 37 (17) | 22.1 (N/A); 14* | 37; 3 |
IgG and IgM test (31) IgM + : 3 IgG + : 2 IgM intermediate: 1 IgG e IgM test (13) IgM + : 1 IgG + : 2 2 weeks later IgG e IgM test (12) IgG + : 1 IgG e IgM (25) IgM-/intermediate: 3 IgG + : 1 IgG test (24) IgG + : 1 |
Y (3) Positive 0 |
N/A | Y (N/A) |
| Fertitta et al. 2021 [17] | 17 (10) | 11.2 (1.8–17.3) | 3; 0 |
IgG test (17) IgG + : 1 |
N/A | Contact with a confirmed COVID-19 case (2); contact with a probable case (13) | Y (1) |
| Hubiche et al. 2021 [18] | 103 (55) |
11 (8–15) |
18; 0 |
IgG and IgM test (14) IgG + : 2 IgM + : 0 |
N/A | Contact with a probable COVID-19 case (66) | Y (N/A) |
| Kerber et al. 2020 [19] | 1 (1) | 7 | 1; 0 | IgG + , IgM- | N/A | N/A | Y |
| Ladha et al. 2020 [20] | 1 (0) | 16 | 1; 0 | IgA + IgG + | N/A | N/A | Y |
| Locatelli et al. 2020 [21] | 1 (1) | 16 | 1;1 | N/A | N/A | Concomitant mother COVID-19 positive | Y |
| Magro et al. 2021 [22] | 1 (1) | 16 | 1; 0 | N/A |
Y (rare SARS-CoV-2 RNA + cellS) |
Contact with sibling with fever and cough (not tested for SARS-CoV-2) several weeks earlier | Y |
| Maniaci et al. 2020 [23] | 1 (1) | 15 | 1; 1 | N/A | N/A | Contact with a confirmed COVID-19 case and contact with a probable case | Y |
| Neri et al. 2021 [24] | 1 (0) | 6 | 1; 1 |
IgG test IgG + |
Y (negative) |
N/A | Y |
| Oliva Rodriguez-Pastor et al. 2021 [25] | 34 (20) | 11.4 (8.6–13.1) | 17; 0 |
IgM and IgG test (34) IgG + : 3 IgM + : 1 |
N/A | Contact with a confirmed COVID-19 case (1); contact with a probable case (3) | Y (4) |
| Papa et al. 2021 [26] | 11 (7) |
11 (8–15) |
11; 11 |
IgG test (11) IgG + : 11 |
N/A | N/A | Y (11) |
| Pavone et al. 2021 [27] | 2 (0) | 7–11 | 2; 2 | N/A | N/A | Contact with a confirmed COVID-19 (1) | Y (2) |
| Piccolo et al. 2020 [28] | 63 (30) | 14 (12–16)* | 11; 2 |
Type of test: N/A (6) Positive: 2 |
N/A | Contact with a confirmed COVID-19 case (2); contact with a probable case (6) | Y (2) |
| Quintana-Castanedo et al. 2020 [29] | 1 (1) | 11 | 1; 0 | IgG + IgM- | N/A | No contact | Y |
| Rizzoli et al. 2020 [30] | 12 (4) | 12.3 (9–19) | 12; 0 |
IgM and IgG test IgM—IgG + : 1 |
N/A | Contact with a confirmed COVID-19 cases (2) | Y (1) |
| Rodrıguez-Villa Lario et al. 2020 [31] | 1 (1) | 17 | 1; 0 | IgG + | N/A | Contact with a confirmed COVID-19 case | Y |
| Saenz Aguirre et al. 2021 [32] | 74 (42) | 19.6 (3–100); 14.5* | 11; 1 | N/A | N/A | 25/103 contact with a confirmed or suspected COVID-19 case | Y (1) |
| Author, year | Histology | Electron microscopy | Lesion localization (n) | Latency between symptoms (SARS-CoV-2 infection) and the appearance of pseudo-chilblains | Other symptoms (type, n) | Dermatological lesion treatment (type, n) | Follow-up and outcome of skin lesions |
|---|---|---|---|---|---|---|---|
| (Y/N; n) | (Y/N; n) | ||||||
| Andina et al. 2020 [10] | Y (6) | N/A | Feet (22); hands (3) | 14 (1–28) d before | Cough/rhinitis (9); diarrhea/abdominal pain (2) | Topical corticosteroids (1) | 1–10 d (regression after 3–5 w) |
| Carazo-Gallego et al. 2021 [11] | N/A | N/A | Feet, hands, chest, abdomen (N/A) | N/A | N/A | N/A | N/A |
| Chua et al. 2021 [12] | N/A | N/A | Feet | N/A | N/A | none | N/A (regression after 1 w) |
| Colmenero et al. 2020 [13] | Y (7) | Y (7) | Feet (6), hands and feet (1) | N/A | Respiratory symptoms (5), GI symptoms (1) | N/A | 8 weeks (complete regression) |
| Colonna et al. 2020 [14] | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| El Hachem et al. 2020 [15] | Y (18) | Y (4) | Toes (9), heel (10), sole (9) | 2 months before (4); 1,5 months before (4); 1 month before (1); 1 week after (1) | Fever (5); headache (1); pharyngodynia (2); diarrhea (2); cough (1) | No | 14 d |
| Feito-Rodrıguez et al. 2021 [16] | Y (11) | Y (3) | Toes (15); fingers (10); toes + foot side (7); toes + heel (2); toes + fingers (2); toes + fingers + foot side | 21.8 ± 23 days | N/A | Topical corticosteroids (8); oral corticosteroids (1); pentoxifylline (4) | 2 w (complete regression: 16; partial regression: 14; persistence: 4; worsening:3) |
| Fertitta et al. 2021 [17] | N/A | N/A | Feet (14); hands (2); hands + feet (1) | 22(5–46) d before (6); 19 d after (2); at the same time (2) | Fever (3); flu symptoms (7); respiratory symptoms (7); GI symptoms (3); anosmia (7) | N/A |
41 (11–72) d; resolution in 27 (10–50) d; relapse at 15 d (1) e 45 d (1) |
| Hubiche et al. 2021 [18] | Y (5) | N/A | Hands + feet (71); hands (15); feet (16) | N/A | N/A | N/A | 4 w (complete regression: 36; partial regression: 25; stability: 8; worsening: 2) |
| Kerber et al. 2020 [19] | N/A | N/A | Toes | 2 m before | N/A | N/A | N/A |
| Ladha et al. 2020 [20] | Y | N/A | Toes | N/A | No | Topical corticosteroids | Regression after 3 w |
| Locatelli et al. 2020 [21] | Y | N/A | Fingers + second right toe | 3 days before | Diarrhea, dysgeusia | No | N/A |
| Magro et al. 2021 [22] | N/A | N/A | Toes | N/A | N/A | N/A | N/A |
| Maniaci et al. 2020 [23] | N/A | N/A | Lower limbs | 3 d before | Fever, asthenia, pharyngodynia, rhinitis | No | 21 d (complete regression) |
| Neri et al. 2021 [24] | Y | N/A | Feet + fingers | 3 w and 2 m before | No | No | N/A (complete regression) |
| Oliva Rodriguez-Pastor et al. 2021 [25] | N/A | N/A | Mostly feet | N/A | Fever (4); cough (2); pharyngodynia (3); abdominal pain (4); diarrhoea (7); vomit (2); myalgia (1); headache (1) | N/A | 1 m (complete regression) |
| Papa et al. 2021 [26] | N/A | N/A | Feet > hands | N/A | N/A | N/A | 2–12 (complete regression) |
| Pavone et al. 2021 [27] | N/A | N/A | Toes | 6 days before—4 days before | Cough (2); fever (1) | Topical corticosteroids (1) | 3 d to 2 w (complete regression) |
| Piccolo et al. 2020 [28] | N/A | N/A | Feet (54); hands + feet (5); hands (4) | N/A | GI symptoms (7); respiratory symptoms (4); fever (3) | N/A |
N/A (stability 50; relapse 9; quick regression 4) |
| Quintana-Castanedo et al. 2020 [29] | N/A | N/A | Toes | N/A | Retinal vasculitis | N/A | N/A |
| Rizzoli et al. 2020 [30] | N/A | N/A | Hands (2); hands + feet (1); feet (9) | N/A | No | N/A | N/A |
| Rodrıguez-Villa Lario et al. 2020 [31] | Y | Y | Toes | N/A | N/A | N/A | N/A |
| Saenz Aguirre et al. 2021 [32] | Y (1) | N/A | Feet (71); hands (6); hands + feet (3) | N/A | N/A | N/A | N/A |
Legend to table: CLIA, chemiluminescence; CS, corticosteroids; d, days; GI, gastrointestinal; m, months; M, males; N, number of patients enrolled in the study; N/A, not available; NC, nucleocapsid; y, years; S, spike; w, weeks; *median
An observational multicenter study by Hubiche et al. [18] reported pseudo-chilblains in almost 80% of the 103 children referred to French dermatology clinics from February to June 2020 for acute acral eruptions with suspected SARS-CoV-2 etiology, of whom 2/103 had a positive serology, and 64% had a positive household contact. SARS-CoV-2 infection was confirmed only in 72 of the 443 (16.3%) tested patients (24/219, 11%, by nasopharyngeal RT-PCR, and 48/224, 21.4%, by serologic tests, IgM, and/or IgG) (Table 1). The rate of test positivity could be underestimated since this information was not reported by Hubiche et al. [18] and Feito Rodrìguez et al. [16] Moreover, SARS-CoV-2 particles were identified in skin biopsy of 8 patients with negative nasopharyngeal swab/serology [13, 14].
Histological examination of the skin lesions was performed in 53 patients from 11 studies (11%) (Table 2) [13–16, 18–22, 24, 31, 32]. Main findings were papillary dermis edema, perivascular and perieccrine lymphocytic infiltrate, lymphocytic vasculitis, and fibrin thrombi.
Table 2.
Histology of SARS-CoV-2-related pseudo-chilblains reported in the selected studies
| Author, year | N patients | Papillary dermis edema | Extravasal erythrocytes | Perivascular and perieccrine lymphocyte infiltrate | Perivascular and perieccrine lymphocyte and neutrophil infiltrate | Lymphocytic vasculitis | Thrombi of fibrin | Spongiosis | Vacuolation of the basal dermis |
|---|---|---|---|---|---|---|---|---|---|
| Andina et al. 2020 [10] | 6 | Yes (6/6) | Yes (N/A) | Yes (6/6) | N/A | Yes (6/6) | Yes (N/A) | N/A | Yes (6/6) |
| Colmenero et al. 2020 [13] | 7 | Yes (7/7) | N/A | Yes (7/7) | N/A | N/A | Yes (4/7) | Yes 7/7 | Yes 7/7 |
| El Hachem et al. 2020 [15] | 18 | Yes (12/18) | Yes (15/18) | Yes (18/18) | N/A | Yes (3/18) | Yes (2/18) | Yes (13/18) | Yes (14/18) |
| Feito-Rodrıguez et al. 2021 [16] | 11 | Yes (4/11) | Yes (6/11) | Yes (9/11) | Yes (2/11) | Yes (11/11) | No (11/11) | N/A | No (11/11) |
| Hubiche et al. 2021 [18] | 5 | N/A | N/A | Yes (5/5) | N/A | Yes (2/5) | N/A | Yes (4/5) | Yes (1/5) |
| Ladha et al. 2020 [20] | 1 | Yes | N/A | Yes | N/A | N/A | N/A | N/A | N/A |
| Locatelli et al. 2020 [21] | 1 | Yes | N/A | Yes | No | N/A | N/A | N/A | N/A |
| Magro et al. 2020 [22] | 1 | Yes | Yes | Yes | No | N/A | Yes | N/A | NA |
| Neri et al. 2021 [24] | 1 | Yes | N/A | Yes | No | N/A | No | N/A | N/A |
| Rodrıguez-Villa Lario et al. 2020 [31] | 1 | Yes | N/A | Yes | N/A | N/A | No | N/A | N/A |
| Saenz Aguirre et al. 2020 [32] | 1 | N/A | N/A | Yes | N/A | N/A | N/A | N/A | N/A |
| Author, year | Exocytosis | Keratinocyte necrosis | Mucin | Acrosirnigia | Lymphocytic eccrine hidradenitis | Vascular ectasia | Hemorrhagic parakeratosis of the stratum corneum | Presence of eosinophils | |
|---|---|---|---|---|---|---|---|---|---|
| Andina et al. 2020 [10] | N/A | N/A | Yes (N/A) | Yes (6/6) | Yes (N/A) | Yes (N/A) | N/A | N/A | |
| Colmenero et al. 2020 [13] | Yes (3/7) | Yes (4/7) | N/A | N/A | N/A | Yes (3/7) | Yes (4/7) | N/A | |
| El Hachem et al. 2020 [15] | Yes (6/18) | N/A | Yes (17/18) | N/A | N/A | N/A | N/A | N/A | |
| Feito-Rodrıguez et al. 2021 [16] | N/A | N/A | Yes (5/11) | N/A | N/A | N/A | N/A | N/A | |
| Hubiche et al. 2021 [18] | N/A | Yes (1/5) | Yes (2/3) | N/A | N/A | N/A | N/A | Yes (2/5) | |
| Ladha et al. 2020 [20] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Locatelli et al. 2020 [21] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Magro et al. 2020 [22] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Neri et al. 2021 [24] | N/A | N/A | N/A | N/A | N/A | Yes | N/A | N/A | |
| Rodrıguez-Villa Lario et al. 2020 [31] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Saenz Aguirre et al. 2020 [32] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Legend to table: N/A, not available
Pseudo-chilblains typically affected feet (291/363; 80.2%) and, more rarely, hands (33/299, 11%). Most of the cases were asymptomatic. When present, the most frequent symptoms were itching and pain. According to the 9 studies reporting this information, pseudo-chilblains presented 3 days to 2 months before the detection of SARS-CoV-2 infection (Table 1). Most of the cases resolved spontaneously; only a few required topic or oral corticosteroids. Feito-Rodrıguez et al. [16] and Papa et al. [26] observed lesion worsening in some patients at follow-up, while Neri et al. [24] recorded relapse at 3 and 6 weeks after SARS-CoV-2 infection.
Erythema multiforme and other acral lesions
Erythema multiforme (EM) and other acral lesions different from pseudo-chilblains were often reported in infants and children with COVID-19 (Table 3).
Table 3.
Studies evaluating the appearance of erythema multiforme (EM) and other acral lesions in pediatric patients affected by COVID-19 (excluding multisystem inflammatory syndrome in children, MIS-C)
| Author, year | Lesion type | n (M) | Age | RT-PCR on oro-/nasopharyngeal swab or endotracheal aspirate (total; positive) | Serology (Y/N, n) | RT-PCR on biopsy (Y/N, n) | Electronic microscopy, histology | SARS-CoV-2 detection (Y/N; n) | Dermatological lesion description | Latency between symptoms (SARS-CoV-2 infection) and the appearance of skin lesions | Other symptoms (type, n) | Dermatological lesion treatment | Follow-up and outcome of skin lesions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andina et al. 2021 [33] | acral purpura | 1 (0) | 2 m | 1; 0 | N | N/A (SARS-CoV-2 spike protein at immunohistochemistry) | Dilated superficial dermal vessels lined by swollen endothelial cells; significant red cell extravasation | Y; 1 | Reticulated purpura on both soles | 3 w | Nasal congestion | None | Regression after 2 w |
| Andina-Martinez et al. Pediatr Dermatol 2021 [34] | acral peeling | 6 (4) | 5–13 y | 3; 3 | N | N/A | N/A | Y; 5 (2/6 antigenic test; 1/6 symptoms and household contact) | Peeling of fingertips and toe, mild erythema | 3–21 d | Headache (2), fever (2), cough (2), GI (1), anosmia (1), dysgeusia (1), myalgia (1) | None | Regression |
| Hubiche et al. 2021 [18] | Acral lesions (chilblain 79.6%, EM 2.9% and others) | 103 (55) | mean 11.1 ± 5.2 y—median 13 (8–15) y | 18; 0 | 14; 2 (IgG + , IgM-) | N/A |
5/103; direct immunofluorescence: IgM deposition; histology: dermal perivascular lymphocytic infiltrate, spongiosis, keratinocyte necrosis, eosinophils, mucin deposition, basal layer vacuolization |
Y; 2 (66/103 household contact) | Chilblain, vesicles, palmar/plantar erythema, purpura, acrocyanosis, teleangectasia, acral edema, EM, papules | N/A | N/A | N/A | 71/103 1-m follow-up: 35/71 total recovery, 25/71 partially regressed, 8/71 stable, 2/71 worsened |
| Janah et al. 2020 [35] | EM | 1 (1) | 17 y | N/A | N | N/A | N/A | Y | Erythematous maculopapular atypical targetoid eruption of palms | 15 d | Mild COVID-19 | N/A | N/A |
| Klimach et al. 2020 [36] | Acral erythematous eruption | 1 (1) | 13 y | 1; 1 | N | N/A | N/A | Y | 1-cm erythematous papules on plantar surface and erythematous macules + petechiae in distal lower extremities | Concomitant | Flu-like symptoms | N/A | resolution in 10–14 d |
| Kumar et al. 2021 [37] | Acral purpura | 1 (1) | 13 y | 1; 1 | N | N/A | No IgA deposits (excludes HSP); superficial epidermal necrosis with intraepidermal pustules and small vessel neutrophilic vasculitis | Y | Palpable purpuric-petechial rash on both feet spreading to ankles and lower legs (HSP excluded by histology) | 4 w | None | None | Slow improvement—still some lesions after 4 w |
| Labè et al. 2020 [38] | EM | 1 (1) | 6 y | 1; 1 | N | N/A | N/A | Y | Targetoid elements on cheek, hands and feet | N/A | Fever, painful cheilitis, conjunctivitis | N/A | Discharged in 2 w |
| Ozsurekci et al. 2021 [39] | Acral edema | 22 (15) | median 12 y (range 0–17) | 22; 22 | N | N/A | N/A | Y | acral oedema 2/22, rash 1/22, conjunctivitis 1/22 | NA | Severe COVID-19 infection | None | N/A |
| Rotulo et al. 2021 [40] | Acral peeling, urticaria | 1 (0) | 6 y | 1; 1 | N | N/A | N/A | Y | giant urticaria and acral peeling | 1 d before (urticaria) and 2 d after (acral peeling) | fever, sore throat | antihistamines for symptomatic relief | resolution in 4 d |
| Torrelo et al. 2020 [41] | EM | 4 (3) | 11–17 y | 4; 1 | N | N/A 2/4 (SARS-CoV-2 spike protein at immunohistochemistry)) | 2/4 histology: deep perivascular and perieccrine infiltrate; absence of necrosis of keratinocytes | Y; 3 | EM—4/4 associated pseudo-chilblains | N/A | Respiratory or GI symptoms, itch, pain | 1/4 oral CS, 1/4 topical CS, 2/4 none | Complete recovery in 1–3 w |
| Wolf et al. 2021 [42] | Beau lines | 2 (NA) | 2 and 5 y | 2; 2 | N | N/A | N/A | Y | Beau lines of all fingernails | 3 w | Fever, GI | None | Complete regression after 4 m |
Legend to table: CS corticosteroids, d days, EM erythema multiforme, GI gastrointestinal, HSP Henoch-Schonlein purpura, m months, MIS-C multisystem inflammatory syndrome in children, N no, N/A, not available, w weeks, y years, Y yes
EM is an immune-mediated reaction triggered by infections or drugs that involves the skin, typically of the distal extremities, and, less frequently, the mucosa, and consists of a polymorphous eruption of macules, papules, and characteristic “target” lesions. EM-like eruptions have been observed more frequent than usual during the SARS-CoV-2 pandemic, in both children and adults [83].
In a cohort of 103 French children with acute acral eruptions likely related to SARS-CoV-2 infection, Hubiche et al. [18] identified EM in 2.9%, and other acral lesions, i.e., palmar/plantar erythema, acral vesicles, acral edema, and acrocyanosis, in 40.8%, 18.4%, 13.6%, and 12.6% of the patients, respectively.
Other 4 cases of EM were reported [41]. Histopathology was performed only in 2 of them and did not demonstrate specific nor typical features of classical EM.
Moreover, acral peeling was reported in 7 patients by 2 studies [34, 40]. Acral edema, erythematous papules and purpura, and nail Beau lines were rarely detected (Table 3).
Lesions manifested from 3 days up to 4 weeks after SARS-CoV-2 diagnosis, although this information was reported only in a minority of patients. Prognosis was good in all cases.
Rash, urticaria, and other mucocutaneous manifestations
Various types or rash, urticaria, and other mucocutaneous lesions were reported in children of any age with SARS-CoV-2 infection (Supplementary Table 1). Literature search identified 25 case reports/case series, 12 retrospective observational studies, and 6 prospective studies.
Overall, 0.5 to 15% of the patients presented with rash, typically maculopapular, and 0.4 to 0.9% with urticaria. A study by Feldstein et al. [52] performed in 577 children and adolescents affected by severe acute COVID-19 reported a 10.2% incidence of mucocutaneous lesions. Of note, the study by Gale et al. [54] analyzed a cohort of 62 newborns (mean age 9.5 days) with SARS-CoV-2 and reported the presence of a rash in 1 of them (2%).
Purpuric lesions (i.e., thrombocytopenic purpura, Henoch-Schonlein purpura (HSP); n = 8), reactive infectious mucocutaneous eruptions (n = 2), eczema (n = 1), Gianotti-Crosti dermatitis (n = 1), oral mucosa lesions (n = 15), erythema nodosum (n = 2), and severe cutaneous adverse reactions (SCARs; n = 5) were also reported. More details can be found at Supplementary Table 1.
Timing of lesion appearance in relation with SARS-CoV-2 infection was reported by 21 studies and ranged from 3 weeks before to 5 weeks after the occurrence of other COVID-19 symptoms, being concomitant in most of the cases. Lesions typically resolved spontaneously; few others were treated with topical or oral corticosteroids and/or oral antihistamines. Prognosis was good, although outcome was reported in a minority of the studies. Kari et al. [63], in a study performed in 88 children, identified an association between rash and higher risk of death; however, about 1/3 of the patients presented with one or more severe comorbidities (Supplementary Table 1).
Quality assessment
Based on the STROBE evaluation performed in the 24 observational studies retrieved, 12 were classified as low, 5 as intermediate, and 7 as high quality (Supplementary Table 2). Based on the CARE evaluation performed in the 49 case reports/series retrieved, 19 were classified as low, 20 as intermediate, and 12 as high quality (Supplementary Table 3).
Discussion
We present the results of a systematic literature review showing an overall high prevalence and wide heterogeneity of mucocutaneous lesions associated with SARS-CoV-2 infection in newborns, children, and adolescents.
Overall, skin lesions presented a peculiar acral distribution, a characteristic common to other viruses, in particular Parvovirus B19, presenting with purpuric exanthems of the limbs and arms, i.e., “gloves and socks syndrome” and “acropetechial syndrome” [84, 85]. Although the exact underlying pathogenic mechanism(s) remain to be determined, direct endothelial injury induced by the virus has been suggested [85]. This could be particularly true for SARS-CoV-2 for its ability to induce vascular damage by infecting endothelial cells through the ACE2 receptors [86]. Other risk factors could be represented by the characteristics of the microcirculation in these sites, as well as the immune response to the virus.
Pseudo-chilblains may be considered one of the most peculiar skin manifestation associated with pediatric SARS-CoV-2 infection, and their etiology has been thoroughly debated. However, our review highlighted that SARS-CoV-2 infection was ascertained only in a minority of the cases. This could depend on methodological study limitations (i.e., data collected by telemedicine; limited availability of diagnostic tests, mainly during the first pandemic wave), diagnostic test precision, but also on the high number of children that remained untested because pauci-/asymptomatic for typical COVID-19, as well as the extremely variable time of onset of pseudo-chilblains with regard to SARS-CoV-2 infection, and, finally, on the absence of a humoral response (due to the activation of the type-I interferon, IFN-1, pathway) [87, 88].
On the other hand, albeit rare, the retrieval of SARS-CoV-2 RNA and other components in cells from pseudo-chilblain biopsies supports its pathogenic role [13, 22, 89]. Other elements supporting pseudo-chilblains as a COVID-19 manifestation are their rapid increase concomitant to the SARS-CoV-2 pandemic; the co-occurrence of mild respiratory or gastrointestinal symptoms in a non-negligible portion of children; a frequent history of contact with suspected/confirmed COVID-19 cases; and the onset of similar skin lesions in siblings, together with the occasional virus detection by RT-PCR, serological test, or electron microscopy within endothelial cells of vessels [90]. In this respect, Andina et al. hypothesized some possible pathogenetic mechanisms [4]: (1) an early, strong interferon type I response against the viral infection that might attenuate viral replication, but induce microangiopathic changes producing a chilblain-like eruption (virus-induced type I interferonopathy hypothesis); (2) chilblains as a manifestation of thrombosis/coagulopathy, as increased risk of thromboembolism with high levels of D-dimer and acral ischemia have been clearly demonstrated in patients with COVID-19, and microthrombi have been observed in chilblains; (3) chilblains as a specific microvascular pathology induced by SARS-CoV-2 (vasculitis hypothesis), in which pericytes endothelial cells play a major role, due to their expression of high levels of ACE2 receptor [91]; (4) change in patient habits secondary to SARS-CoV-2 lockdown, i.e., reduced physical activity and physical and mental stress. Moreover, even if this topic is out of the scope of the present review, pseudo-chilblains have also been reported following the administration of COVID-19 vaccines [92–94], further supporting the link between SARS-CoV-2 and these skin manifestations.
Among the various mucocutaneous lesions reported in association with pediatric SARS-CoV-2 infection, five authors describe the occurrence of HSP [43, 47, 59, 62, 73] (Supplementary Table 1). SARS-CoV-2 has been suggested as trigger for HSP in children [95], but its pathogenic role beyond incidental co-infection remains to be demonstrated. The case described by Borocco et al. [47] of SARS-CoV-2 associated with Epstein Barr virus (EBV), that is reported to act as a trigger in 4.2% of childhood HSP cases [96], further questions this association.
Mucocutaneous lesions of various type can present at any age (newborn to adolescent) in paucisymptomatic as well as in severely ill patients, or they may be the only manifestation of COVID-19. They can appear at different stages of the disease, i.e., some days/weeks before, in concomitance with or some weeks after the resolution of the other more typical COVID-19 manifestations and/or serological negativization [26]. The late appearance can be one possible explanation for negative RT-PCR test in most of the cases [90, 97].
Before SARS-CoV-2 pandemic, chilblains typically occurred in children affected by type 1 interferonopathies (i.e., systemic lupus erythematosus) [98] as an expression of the associated microangiopathy. The interferon type 1 response developed by pediatric patients, in which the immune response induces skin lesions, but downregulates the release of other cytokines, thus preventing the “cytokine storm,” might be the reason why children with chilblains present with mild forms of COVID-19 [26].
Main strengths of the present review are the comprehensive literature assessment, which covers the entire time period of SARS-CoV-2 pandemic, from the first wave up to the most recently reported cases (indeed, another systematic review on this topic referred to the first pandemic wave only had been previously published [99]) and the systematic evaluation of the quality of both observational and case studies included in the analysis. On the other hand, results remain inconclusive due to the important methodological limitations of the primary studies: properly designed studies were few and heterogeneous in terms of clinical evaluations, study setting, sample size, and follow-up duration, as well as the methods used for the assessment of Sars-CoV-2 infection. Indeed, in a non-negligible number of studies, the presence of COVID-19 disease was established on the basis of patient symptoms, and/or detection of the virus RNA/particles in mucocutaneous lesion, and/or in patient siblings/contacts, and not on the positivity to serological/molecular tests.
Conclusions
The comprehensive assessment of literature focusing on COVID-19 manifestations all along the pandemic period has demonstrated that dermatological lesions of different types frequently occur in affected newborns, children, and adolescents, independently from the presence and severity of other more typical signs and symptoms. Skin lesions typically present with a peculiar acral distribution and include pseudo-chilblains, EM, or acral purpura, peeling, or edema and, more rarely, HSP and reactive infectious mucocutaneous eruption. Mucocutaneous lesions usually subside spontaneously, and only a minority persist/worsen so to require topic/systemic therapy. However, it is fundamental for physicians of all specialties to be aware and promptly recognize them, not only for a timely and adequate management, but, even more important, since they can precede other more typical COVID-19 manifestations, to adopt all required measures to prevent disease diffusion. On the other hand, underlying pathogenic mechanisms have only been hypothesized, and the actual role of SARS-CoV-2 remains to be ascertained. Finally, our work highlights that most of the evidence available up to now is low-to-medium quality, so that further well-designed studies should be conducted to clarify the association of SARS-CoV-2 with dermatological lesions in children.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- COVID-19
Coronavirus disease 2019
- EM
Erythema multiforme
- IFN-1
Type-I interferon
- MIS-C
Multisystem inflammatory syndrome in children
- PRISMA
Preferred Reporting Items for Systematic Reviews
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- CARE
CAse REport guidelines
- WHO
World Health Organization
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Arianna Dondi, Giacomo Sperti, Lorenza Parini, Davide Gori, Marco Montalti, and Federica Guaraldi. The first draft of the manuscript was written by Arianna Dondi, Giacomo Sperti, and Lorenza Parini, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marcello Lanari and Iria Neri equally contributed to this paper.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP et al (2021) Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc Heal [Internet] 5(5):323–31. Available from: 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed]
- 3.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr Int J Paediatr. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andina D, Belloni-Fortina A, Bodemer C, Bonifazi E, Chiriac A, Colmenero I, et al. Skin manifestations of COVID-19 in children: Part 1. Clin Exp Dermatol. 2021;46(3):444–450. doi: 10.1111/ced.14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondi A, Sperti G, Gori D, Montalti M, Parini L, Guaraldi F et al (2022) A systematic review about skin lesions in children affected by coronavirus disease 2019 (excluding multisystem inflammatory syndrome in children): study protocol. medRxiv [Internet]. Available from: http://medrxiv.org/content/early/2022/03/07/2022.03.04.22271888.abstract
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2014) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg [Internet] 12(12):1495–1499. Available from: 10.1016/j.ijsu.2014.07.013
- 8.Biagi C, Di Nunzio M, Bordoni A, Gori D, Lanari M. Effect of adherence to Mediterranean diet during pregnancy on children’s health: A systematic review. Nutrients. 2019;11(5):1–25. doi: 10.3390/nu11050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, CARE Group The CARE guidelines: consensus‐based clinical case reporting guideline development. Headache: The Journal of Head and Face Pain. 2013;53(10):1541–1547. doi: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 10.Andina D, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristán J, Alonso-Cadenas JA, Escalada-Pellitero S, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carazo Gallego B, Martín Pedraz L, Galindo Zavala R, Rivera Cuello M, Mediavilla Gradolph C, Núñez CE. Skin lesions in children during the first wave of the SARS-CoV-2 pandemic. Med Clin (Barc) 2021;157(1):33–37. doi: 10.1016/j.medcli.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua GT, Wong JSC, Lam I, Ho PPK, Chan WH, Yau FYS et al (2021) Clinical characteristics and transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open 1–11 [DOI] [PMC free article] [PubMed]
- 13.Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonna C, Spinelli F, Monzani NA, Ceriotti F, Gelmetti C. Chilblains in children in the time of COVID-19: New evidence with serology assay. Pediatr Dermatol. 2020;37(5):1000–1001. doi: 10.1111/pde.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Hachem M, Diociaiuti A, Concato C, Carsetti R, Carnevale C, Ciofi Degli Atti M, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620–2629. doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feito-Rodríguez M, Mayor-Ibarguren A, Cámara-Hijón C, Montero-Vega D, Servera-Negre G, Ruiz-Bravo E, et al. Chilblain-like lesions and COVID-19 infection: A prospective observational study at Spain’s ground zero. J Am Acad Dermatol. 2021;84(2):507–509. doi: 10.1016/j.jaad.2020.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fertitta L, Welfringer-Morin A, Ouedrani A, Polivka L, Chhun S, Chatenoud L, et al. Immunological and virological profile of children with chilblain-like lesions and SARS-CoV-2. J Eur Acad Derma Venereol. 2021;35(3):e164–e167. doi: 10.1111/jdv.16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubiche T, Phan A, Leducq S, Rapp J, Fertitta L, Aubert H, et al. Acute acral eruptions in children during the COVID-19 pandemic: characteristics of 103 children and their family clusters. Ann Dermatol Venereol. 2021;148(2):94–100. doi: 10.1016/j.annder.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerber AA, Soma DB, Youssef MJ. Chilblains-like dermatologic manifestation of COVID-19 diagnosed by serology via multidisciplinary virtual care. Int J Dermatol. 2020;59(8):1024–1025. doi: 10.1111/ijd.14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladha MA, Dupuis EC. SARS-CoV-2-related chilblains. CMAJ. 2020;192(28):E804. doi: 10.1503/cmaj.201348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locatelli AG, Robustelli Test E, Vezzoli P, Carugno A, Moggio E, Consonni L, et al. Histologic features of long-lasting chilblain-like lesions in a paediatric COVID-19 patient. J Eur Acad Derma Venereol. 2020;34(8):e365–e368. doi: 10.1111/jdv.16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro CM, Mulvey JJ, Laurence J, Sanders S, Crowson AN, Grossman M, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: A case series. Br J Dermatol. 2021;184(1):141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniaci A, Iannella G, Vicini C, Pavone P, Nunnari G, Falsaperla R, et al. A case of COVID-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep. 2020;21:1–6. doi: 10.12659/AJCR.925813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neri I, Conti F, Virdi A, Guglielmo A, Leonardi L, Corsini I, et al. Chilblains in a child with confirmed SARS-CoV-2 infection: a red flag for late-onset skin manifestation in previously infected individuals. J Eur Acad Derma Venereol. 2021;35(6):e357–e359. doi: 10.1111/jdv.17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliva Rodríguez-Pastor S, Martín Pedraz L, Carazo Gallego B, Galindo Zavala R, Lozano Sánchez G, de Toro PI, et al. Skin manifestations during the COVID-19 pandemic in the pediatric emergency department. Pediatr Int. 2021;63(9):1033–1037. doi: 10.1111/ped.14568. [DOI] [PubMed] [Google Scholar]
- 26.Papa A, Salzano AM, Di Dato MT, Bianco G Lo, Tedesco M, Salzano A et al (2021) COVID-19 related acro-ischemic neuropathic-like painful lesions in pediatric patients: A case series. Anesthesiol Pain Med 11(2) [DOI] [PMC free article] [PubMed]
- 27.Pavone P, Marino S, Marino L, Cacciaguerra G, Guarneri C, Nunnari G, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34(1):2–6. doi: 10.1111/dth.14502. [DOI] [PubMed] [Google Scholar]
- 28.Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Derma Venereol. 2020;34(7):e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana-Castanedo L, Feito-Rodríguez M, Fernández-Alcalde C, Granados-Fernández M, Montero-Vega D, Mayor-Ibarguren A, et al. Concurrent chilblains and retinal vasculitis in a child with COVID-19. J Eur Acad Derma Venereol. 2020;34(12):e764–e766. doi: 10.1111/jdv.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzoli L, Collini L, Magnano M, Termine S, Barcelli R, Infusino SD, et al. Chilblain-like lesions during the COVID-19 pandemic: A serological study on a case series. Br J Dermatol. 2020;183(4):782–784. doi: 10.1111/bjd.19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Villa Lario A, Vega-Díez D, González-Cañete M, Gómez-Zubiaur A, Pérez-Mesonero R, Bandini M, et al. Histological findings in chilblain lupus-like COVID lesions: in search of an answer to understand their aetiology. J Eur Acad Derma Venereol. 2020;34(10):e572–e574. doi: 10.1111/jdv.16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saenz Aguirre A, De la Torre Gomar FJ, Rosés-Gibert P, Gimeno Castillo J, de Lagrán Martinez, Alvarez de Arcaya Z, Gonzalez-Perez R. Novel outbreak of acral lesions in times of COVID-19: A description of 74 cases from a tertiary university hospital in Spain. Clin Exp Dermatol. 2020;45(8):1065–1067. doi: 10.1111/ced.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andina D, Colmenero I, Santonja C, Muñoz de León I, Noguera-Morel L, Hernández-Martín A, et al. Suspected COVID-19-related reticulated purpura of the soles in an infant. Pediatr Dermatol. 2021;38(1):301–303. doi: 10.1111/pde.14409. [DOI] [PubMed] [Google Scholar]
- 34.Andina-Martínez D, Villaizán-Perez C, Pavo-García MR, Suárez-Gómez O, Monzón-Bueno AI, Sanchez-Prieto I, et al. Acral peeling as the sole skin manifestation of COVID-19 in children. Pediatr Dermatol. 2021;38(3):664–666. doi: 10.1111/pde.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars-Cov-2. J Eur Acad Derma Venereol. 2020;34(8):e373–e375. doi: 10.1111/jdv.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klimach A, Evans J, Stevens J, Creasey N. Rash as a presenting complaint in a child with COVID-19. Pediatr Dermatol. 2020;37(5):966–967. doi: 10.1111/pde.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar G, Pillai S, Norwick P, Bukulmez H (2021) Leucocytoclastic vasculitis secondary to COVID-19 infection in a young child. BMJ Case Rep 14(4) [DOI] [PMC free article] [PubMed]
- 38.Labé P, Ly A, Sin C, Nasser M, Chapelon-Fromont E, Ben Saïd P, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Derma Venereol. 2020;34(10):e539–e541. doi: 10.1111/jdv.16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozsurekci Y, Gürlevik S, Kesici S, Akca UK, Oygar PD, Aykac K, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic in Turkey: First report from the Eastern Mediterranean. Clin Rheumatol. 2021;40(8):3227–3237. doi: 10.1007/s10067-021-05631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotulo GA, Signa S, Rosina S, Pastorino C, Bondi E, Maghnie M. Giant urticaria and acral peeling in a child with coronavirus disease 2019. J Pediatr. 2021;230:261–263. doi: 10.1016/j.jpeds.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torrelo A, Andina D, Santonja C, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristán J, et al. Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol. 2020;37(3):442–446. doi: 10.1111/pde.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf GK, French LE. Beau-Lines of the fingernails in association with pediatric SARS-CoV-2 infections. JDDG - J Ger Soc Derma. 2021;19(5):744–745. doi: 10.1111/ddg.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alghoozi DA, Alkhayyat HM. A child with Henoch-Schonlein purpura secondary to a COVID-19 infection. BMJ Case Rep. 2021;14(1):2020–2022. doi: 10.1136/bcr-2020-239910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alnajjar AA, Dohain AM, Abdelmohsen GA, Alahmadi TS, Zaher ZF, Abdelgalil AA. Clinical characteristics and outcomes of children with COVID-19 in Saudi Arabia. Saudi Med J. 2021;42(4):391–398. doi: 10.15537/smj.2021.42.4.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andina-Martinez D, Nieto-Moro M, Alonso-Cadenas JA, Añon-Hidalgo J, Hernandez-Martin A, Perez-Suarez E, et al. Mucocutaneous manifestations in children hospitalized with COVID-19. J Am Acad Dermatol. 2021;85(1):88–94. doi: 10.1016/j.jaad.2021.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bardellini E, Bondioni MP, Amadori F, Veneri F, Lougaris V, Meini A, et al. Non-specific oral and cutaneous manifestations of coronavirus disease 2019 in children. Med Oral Patol Oral y Cir Bucal. 2021;26(5):e549–e553. doi: 10.4317/medoral.24461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borocco C, Lafay C, Plantard I, Gottlieb J, Koné-Paut I, Galeotti C. SARS-CoV-2-associated Henoch-Schönlein purpura in a 13-year-old girl. Arch Pediatr. 2021;28(7):573–575. doi: 10.1016/j.arcped.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowe S, O’Connor C, Gleeson C, Murphy M. Reactive infectious mucocutaneous eruption in children diagnosed with COVID-19. Pediatr Dermatol. 2021;38(5):1385–1386. doi: 10.1111/pde.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cairoli H, Raiden S, Chiolo MJ, Di Lalla S, Ferrero F. Patients assisted at the department of medicine of a pediatric hospital at the beginning of the COVID-19 pandemic in buenos aires, argentina. Arch Argent Pediatr. 2020;118(6):423–426. doi: 10.5546/aap.2020.eng.423. [DOI] [PubMed] [Google Scholar]
- 50.Chen V, Escandon Brehm J, Bellodi SF. Acute urticaria preceding other COVID-19–associated manifestations—a case report. Pediatr Dermatol. 2021;38(2):455–457. doi: 10.1111/pde.14505. [DOI] [PubMed] [Google Scholar]
- 51.El-Assaad I, Hood-Pishchany MI, Kheir J, Mistry K, Dixit A, Halyabar O, Mah DY, Meyer-Macaulay CCH. Complete heart block, severe ventricular dysfunction, and myocardial inflammation in a child with COVID-19 infection. JACC Case Rep. 2020;2(9):1351–1355. doi: 10.1016/j.jaccas.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman M, Gaietto K, DiCicco L, Rauenswinter S, Squire J, Aldewereld Z et al (2020) A comprehensive clinical description of pediatric SARS-CoV-2 infection in Western Pennsylvania. medRxiv Prepr Serv Heal Sci [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/33354687/
- 54.Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES et al (2021) Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc Heal [Internet] 5(2):113–121. Available from: 10.1016/S2352-4642(20)30342-4 [DOI] [PMC free article] [PubMed]
- 55.Genovese G, Colonna C, Marzano AV. Varicella-like exanthem associated with COVID-19 in an 8-year-old girl: a diagnostic clue? Pediatr Dermatol. 2020;37(3):435–436. doi: 10.1111/pde.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gianotti R, Restano L, Cutrone M, Colonna C, Fellegara G, Debernardi I, et al. Papulo-purpuric dermatitis of childhood: A distinct PLEVA-like eruption associated to SARS-CoV-2 infection. Clinical, histopathological and immunohistochemical study of 10 cases. Pediatr Dermatol. 2021;38(5):1185–1190. doi: 10.1111/pde.14777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He M, Wang C, Xu L, Zhang H, Liu Y, Zhao Y, et al. Epidemiological and clinical characteristics of 35 children with COVID-19 in Beijing. China Pediatr Investig. 2020;4(4):230–235. doi: 10.1002/ped4.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holcomb ZE, Hussain S, Huang JTDS. Reactive infectious mucocutaneous eruption associated with SARS-CoV-2 infection. JAMA Dermatol. 2021;157(5):603–605. doi: 10.1001/jamadermatol.2021.0385. [DOI] [PubMed] [Google Scholar]
- 59.Hoskins B, Keeven N, Dang M, Keller E, Nagpal R. A child with COVID-19 and immunoglobulin a vasculitis. Pediatr Ann. 2021;50(1):e44–e48. doi: 10.3928/19382359-20201211-01. [DOI] [PubMed] [Google Scholar]
- 60.Houshmand H, Abounoori M, Ghaemi R, Bayat S, Houshmand G. Ten-year-old boy with atypical COVID-19 symptom presentation: A case report. Clin Case Rep. 2021;9(1):304–308. doi: 10.1002/ccr3.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ISARIC Clinical Characterisation Group COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. 2021;49(5):889–905. doi: 10.1007/s15010-021-01599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobi M, Lancrei HM, Brosh-Nissimov T, Yeshayahu Y. Purpurona: a novel report of COVID-19-related Henoch-Schonlein purpura in a child. Pediatr Infect Dis J. 2021;40(2):E93–E94. doi: 10.1097/INF.0000000000003001. [DOI] [PubMed] [Google Scholar]
- 63.Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Sukkar SA, MohamedNur HAH et al (2021) Coronavirus disease in children: A multicentre study from the Kingdom of Saudi Arabia. J Infect Public Health [Internet] 14(4):543–549. Available from: 10.1016/j.jiph.2021.01.011 [DOI] [PMC free article] [PubMed]
- 64.Krajcar N, Marić LS, Šurina A, Filipović SK, Trkulja V, Roglić S, et al. Epidemiological and clinical features of Croatian children and adolescents with a PCR-confirmed coronavirus disease 2019: differences between the first and second epidemic wave. Croat Med J. 2020;61(6):491–500. doi: 10.3325/cmj.2020.61.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Marques HH, Pereira MFB, Dos Santos AC, Fink TT, de Paula CSY, Litvinov N, et al. Differences in children and adolescents with SARS-CoV-2 infection: A cohort study in a Brazilian tertiary referral hospital. Clinics. 2021;76(4):1–8. doi: 10.6061/clinics/2021/e3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metbulut AP, Özkaya Parlakay A, Bayhan Gİ, Kanık Yüksek S, Gülhan B, Şengül Emeksiz Z, et al. Evaluation of cutaneous symptoms in children infected with COVID-19. Pediatr Allergy Immunol. 2021;32(5):1120–1125. doi: 10.1111/pai.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morey-Olivé M, Espiau M, Mercadal-Hally M, Lera-Carballo EG-PV. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019) An Pediatr (Engl Ed) 2020;92(6):374–375. doi: 10.1016/j.anpede.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panetta L, Proulx C, Drouin O, Autmizguine J, Luu TM, Quach C, et al. Clinical characteristics and disease severity among infants with SARS-CoV-2 infection in Montreal, Quebec. Canada JAMA Netw Open. 2020;3(12):10–14. doi: 10.1001/jamanetworkopen.2020.30470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel PA, Chandrakasan S, Mickells GE, Yildirim I, Kao CM, Bennett CM (2020) Severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics 146(1) [DOI] [PMC free article] [PubMed]
- 70.Proietti I, Mambrin A, Bernardini N, Tolino E, Balduzzi V, Maddalena P, et al. Urticaria in an infant with SARS-CoV-2 positivity. Dermatol Ther. 2020;33(6):2–3. doi: 10.1111/dth.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabha AC, Oliveira FI De, Oliveira TA De, Cesar RG, Fongaro G, Mariano RF et al (2021) Clinical manifestations of children and adolescents with COVID-19: Report of the first 115 cases from Sabará Hospital Infantil. Rev Paul Pediatr 39 [DOI] [PMC free article] [PubMed]
- 72.Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Garg A (2021) Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID-19 and multisystem inflammatory syndrome in children. J Am Acad Dermatol [Internet] 84(2):408–414. Available from: 10.1016/j.jaad.2020.10.060 [DOI] [PMC free article] [PubMed]
- 73.Riscassi S, Kalapurackal MA, Battisti L, Eisendle K, Raffeiner B, Mercolini F (2021) Vasculitis in a child with COVID-19: A novel presentation of Henoch-Schönlein purpura. Klin Padiatr 7–9 [DOI] [PubMed]
- 74.Salih AF, Hamasalih K, Rahman HS, Mohammed GA (2022) Pediatric COVID-19 infection in Sulaimaniyah Governorate, Iraq. Am J Otolaryngol - Head Neck Med Surg [Internet] 43(1):103199. Available from: 10.1016/j.amjoto.2021.103199 [DOI] [PMC free article] [PubMed]
- 75.Shahbaznejad L, Rouhanizadeh H, Navaeifar MR, Hosseinzadeh F, Movahedi FS, Rezai MS. Clinical characteristics and outcomes of COVID-19 in children in Northern Iran. Int J Pediatr (United Kingdom) 2021;2021:10–15. doi: 10.1155/2021/5558287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swali RN, Lee EB, Adams JL. Gianotti-crosti syndrome in the setting of recent coronavirus disease-19 infection. Pediatr Dermatol. 2021;38(3):629–631. doi: 10.1111/pde.14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tammaro A, Karim D, Adebanjo GAR, Parisella FR, Chello C, Salih AF et al (2021) COVID-19 cutaneous manifestations in pediatric patients: 24 multisystem inflammatory syndrome in children and six Kawasaki disease cases. J Eur Acad Dermatol Venereol 5–7 [DOI] [PMC free article] [PubMed]
- 78.Tsao HS, Chason HM, Fearon DM (2020) Immune thrombocytopenia (ITP) in a pediatric patient positive for SARS-CoV-2. Pediatrics 146(2) [DOI] [PubMed]
- 79.Van Der Zalm MM, Lishman J, Verhagen LM, Redfern A, Smit L, Barday M, et al. Clinical experience with severe acute respiratory syndrome coronavirus 2-related illness in children: hospital experience in Cape Town. South Africa Clin Infect Dis. 2021;72(12):E938–E944. doi: 10.1093/cid/ciaa1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52.e5. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zawadka K, Oldakowska A. Urticaria multiforme in a child with SARS-CoV-2 infection. Dermatol Rep. 2021;13(2):2–3. doi: 10.4081/dr.2021.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou L, Song X, Lu H, Mao Y, Liu C, Yuan Y, et al. Clinical analysis of seven pediatric patients with coronavirus disease 2019 (COVID-19) in Jingzhou, Hubei, China: A retrospective study. Transl Pediatr. 2021;10(3):616–624. doi: 10.21037/tp-21-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andina D, Belloni-Fortina A, Bodemer C, Bonifazi E, Chiriac A, Colmenero I, et al. Skin manifestations of COVID-19 in children: Part 2. Clin Exp Dermatol. 2021;46(3):451–461. doi: 10.1111/ced.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sève P, Ferry T, Charhon A, Calvet A, Broussolle C. Systemic manifestations of Parvovirus B19 infections. Rev Med Interne. 2004;25(10):740–751. doi: 10.1016/j.revmed.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Harel L, Straussberg I, Zeharia A, Praiss D, Amir J. Papular purpuric rash due to parvovirus B19 with distribution on the distal extremities and the face. Clin Infect Dis. 2002;35(12):1558–1561. doi: 10.1086/344773. [DOI] [PubMed] [Google Scholar]
- 86.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet [Internet] 395(10234):1417–1418. Available from: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed]
- 87.Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K, et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1(4):1–23. doi: 10.1126/sciimmunol.aah6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, Ferbas KG, Tobin NH, Aldrovandi GMYO. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolivras A, Thompson C, Pastushenko I, Mathieu M, Bruderer P, de Vicq M, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49(1):17–28. doi: 10.1111/cup.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khalili M, Iranmanesh B, Mohammadi S, Aflatoonian M (2021) Cutaneous and histopathological features of coronavirus disease 2019 in pediatrics: A review article. Dermatol Ther 34(1) [DOI] [PMC free article] [PubMed]
- 91.He L, Mäe MA, Muhl L, Sun Y, Pietilä R, Nahar K et al (2020) Pericyte-Specific vascular expression of SARS-CoV-2 receptor ACE2 – implications for microvascular inflammation and hypercoagulopathy in COVID-19. bioRxiv
- 92.Pileri A, Guglielmo A, Raone B, Patrizi A. Chilblain lesions after COVID-19 mRNA vaccine. Br J Dermatol. 2021;185(1):e3. doi: 10.1111/bjd.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davido B, Mascitti H, Fortier-Beaulieu M, Jaffal K, de Truchis P. “Blue toes” following vaccination with the BNT162b2 mRNA COVID-19 vaccine. J Travel Med. 2021;28(4):1–2. doi: 10.1093/jtm/taab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Piccolo V, Bassi A, Argenziano G, Mazzatenta C, Cutrone M, Neri I, et al. BNT162b2 mRNA COVID-19 vaccine-induced chilblain-like lesions reinforces the hypothesis of their relationship with SARS-CoV-2. J Eur Acad Derma Venereol. 2021;35(8):e493–e494. doi: 10.1111/jdv.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batu ED, Sener S, Ozen S. COVID-19 associated pediatric vasculitis: A systematic review and detailed analysis of the pathogenesis. Semin Arthritis Rheum. 2022;55:152047. doi: 10.1016/j.semarthrit.2022.152047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu HB, Wu JG, Cheng Y, Li JJ. Epidemiology and clinical characteristics of Henoch-Schönlein purpura associated with Epstein-Barr virus infection. Mediterr J Hematol Infect Dis. 2021;13(1):e2021064. doi: 10.4084/MJHID.2021.064.PMID:34804438;PMCID:PMC8577555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shams S, Rathore SS, Anvekar P, Sondhi M, Kancherla N, Tousif S, et al. Maculopapular skin eruptions associated with COVID-19: A systematic review. Dermatol Ther. 2021;34(2):1–14. doi: 10.1111/dth.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.d’Angelo DM, Di Filippo P, Breda L, Chiarelli F. Type I interferonopathies in children: An overview. Front Pediatr. 2021;9:1–16. doi: 10.3389/fped.2021.631329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah S, Akhade K, Ganguly S, Nanda R, Mohapatra EGA. Cutaneous manifestations associated with COVID-19 in children: A systematic review. J Fam Med Prim Care. 2021;10(1):93–101. doi: 10.4103/jfmpc.jfmpc_1389_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.