Abstract
Opioid use disorder (OUD) produces detrimental personal and societal consequences. Astrocytes are a major cell group in the brain that receives little attention in mediating OUD. We determined how astrocytes and the astroglial glutamate transporter, GLT-1, in the nucleus accumbens core adapt and contribute to heroin seeking in rats. Seeking heroin, but not sucrose, produced two transient forms of plasticity in different astroglial subpopulations. Increased morphological proximity to synapses occurred in one subpopulation and increased extrasynaptic GLT-1 expression in another. Augmented synapse proximity by astroglia occurred selectively at D2-dopamine receptor–expressing dendrites, while changes in GLT-1 were not neuron subtype specific. mRNA-targeted antisense inhibition of either morphological or GLT-1 plasticity promoted cue-induced heroin seeking. Thus, we show that heroin cues induce two distinct forms of transient plasticity in separate astroglial subpopulations that dampen heroin relapse.
Different subpopulations of astrocytes engage with accumbens synapses to dampen heroin relapse.
INTRODUCTION
Relapse to opioid use is a leading cause of death in the United States. Decades of research demonstrate the importance of glutamate dysregulation at nucleus accumbens core (NAcore) synapses as a causative factor in relapse-like behavior in animal models (1). Glutamate dysregulation in animal models of addiction is due in large part to changes in NAcore astroglia that express the glutamate transporter GLT-1, which terminates the actions of the bulk of synaptically released glutamate (2, 3). Addictive substances, including alcohol, nicotine, psychostimulants, and opioids, induce an enduring down-regulation of GLT-1 on astrocytes in the NAcore (4). Furthermore, astroglial processes that insulate synapses and take up glutamate during synaptic transmission retract from NAcore synapses after extinction from cocaine, heroin, and methamphetamine use, but not following sucrose self-administration and extinction (5–7). Synaptic retraction of astrocyte processes and down-regulation of GLT-1 disrupt glutamate homeostasis at NAcore synapses, permitting spillover of synaptic glutamate and postsynaptic potentiation required for drug-associated cues to initiate drug seeking (8).
We previously found that synaptic retraction of NAcore astroglia after heroin withdrawal is partially and transiently reversed during cue-induced heroin seeking, a process that involves phosphorylation of the actin-binding protein ezrin (6). Ezrin phosphorylation and astrocyte fine process motility have been linked to signaling through astroglial metabotropic glutamate receptors (mGluRs) in vitro (9), but whether cue-induced astrocyte motility associated with relapse-like behaviors involves astroglial mGluR signaling is not known. Further, whether astrocyte motility initiated by drug-associated cues increases synaptic adjacency of GLT-1 has not been studied. We hypothesize that heroin cue-induced increased proximity of astroglia to synapses increases the synaptic adjacency of GLT-1 on perisynaptic astroglial processes, thereby serving to minimize spillover of synaptically released glutamate that mediates cue-induced drug seeking. We also sought to determine whether synaptic reassociation of astroglial processes stimulated by heroin-associated cues was selective for either of the two main neural subtypes in the NAcore, D1 or D2 receptor–expressing medium spiny neurons (D1- or D2-MSNs) (10), which exert opposing control over drug-seeking behaviors (11–14).
We used confocal microscopy to measure the synaptic coregistration of labeled astrocytes and astroglial GLT-1 in the NAcore after extinction or reinstatement of heroin or sucrose seeking. We found that cued reinstatement of heroin, but not sucrose, seeking produced two transient adaptations that parsed into distinct astroglial subpopulations. In one subpopulation, heroin cues increased synaptic adjacency of astroglial processes without increasing surface-proximal GLT-1, and in the second subpopulation, cued reinstatement elevated surface GLT-1 on astroglia situated more than 250 nm from the synaptic cleft. We independently disrupted the two forms of cue-induced plasticity in each subpopulation of NAcore astroglia by knocking down ezrin, an astroglial-selective actin-binding protein that mediates morphological plasticity in distal astroglial processes (9, 15–18), or by reducing GLT-1 expression. Knockdown of either ezrin or GLT-1 in NAcore astroglia enhanced the capacity of cues to induce heroin seeking. When we assessed whether astrocytes exhibited a bias in their synaptic adjacency with D1- or D2-MSN synapses, we found that astrocytes selectively associated with D1-MSN synapses after extinction of heroin seeking and retracted from D2-MSNs and that this pattern was reversed during cue-reinstated heroin seeking. Moreover, the cue-induced increase in surface GLT-1 was not detected adjacent to either D1- or D2-MSN dendrites during reinstated heroin seeking, supporting a functional role for GLT-1–deficient perisynaptic astroglial protrusions in shaping synaptic activity. In keeping with abundant literature demonstrating suppression of synaptic activity by perisynaptic astroglia (19, 20), we propose that astrocyte insulation of D1-MSN synapses after extinction training suppresses D1-MSN activity to reduce seeking, while retraction from D2-MSNs permits their potentiation. The reversal of this pattern during cue presentation would instead permit D1-MSN potentiation, which is known to drive cued drug seeking (11). In all, our data show that astrocyte morphological plasticity is neuron subtype selective and that two distinct forms of astroglial plasticity are transiently induced by heroin cues in separate subpopulations of astrocytes to dampen cue-induced heroin seeking.
RESULTS
GLT-1 surface expression was transiently elevated during cued heroin seeking
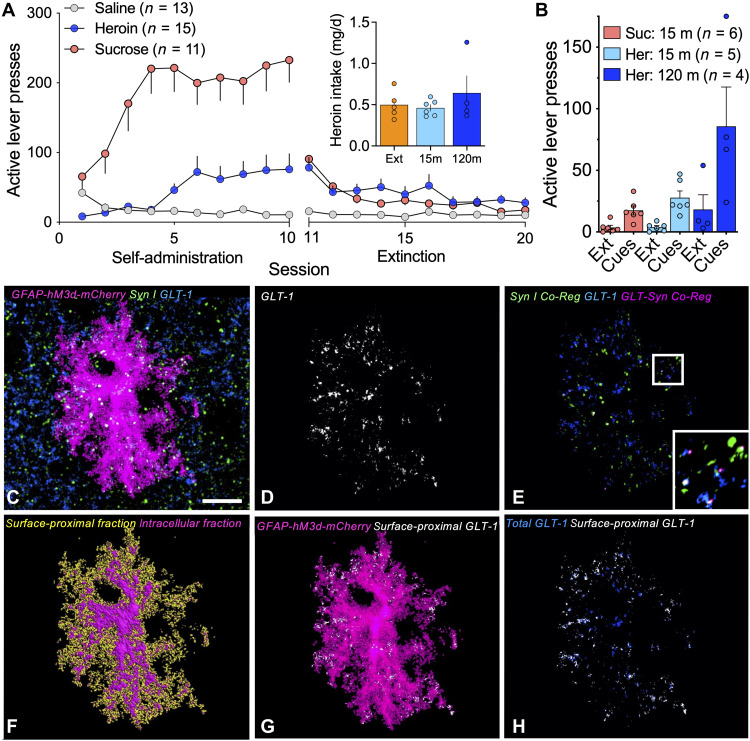
To examine whether enhanced synaptic proximity of NAcore astroglia suppressed cue-induced heroin seeking through changes in synaptic adjacency of GLT-1, NAcore astroglia were selectively labeled with a membrane-bound fluorescent reporter before operant training. Rats were trained to self-administer heroin or sucrose, and reward delivery was paired with light and tone cues (Fig. 1A). Heroin intake was equivalent in rats placed in the extinction, 15-min, or 120-min reinstated groups (Fig. 1A, inset). Animals that received yoked saline delivery and cues served as controls for heroin-trained rats, and animals that received yoked cues were controls for sucrose-trained rats. Operant responding was extinguished by removing both the cues and reinforcers over 10 daily sessions, and brains were extracted from extinguished animals 24 hours after the last extinction session. Remaining rats then underwent reinstatement of heroin or sucrose seeking by restoring conditioned cues without reinforcers to active lever pressing for 15 or 120 min (Fig. 1B). During cue-induced reinstatement of heroin seeking, active lever pressing peaks within the first 30 min of the session (21), and lever pressing within this time frame is considered an indication of craving or seeking, since drug-cue reactivity in human drug users increases with reported craving and is predictive of relapse vulnerability (22–24). After 45 min or more of unrewarded lever pressing, responding for cues is ultimately extinguished during the reinstatement session, a process that we refer to as within-trial extinction or cue extinction (21). Thus, we interpret measurements taken at 15 min of cue exposure as occurring during seeking, and measurements taken at 120 min as occurring after within-trial cue extinction. Rats used to generate the data in Fig. 1 (A and B) were included in a previous study (6), and in the present report, mCherry-transduced astrocytes in tissue slices from these rats were immuno-labeled for the presynaptic marker Synapsin I and GLT-1. After immuno-labeling, NAcore slices from each treatment group (yoked, extinguished, 15-min reinstated, and 120-min reinstated) were imaged using confocal microscopy (Fig. 1C). Total GLT-1, coregistered Synapsin I, and coregistered GLT-1 and Synapsin I were quantified and normalized to the volume of each mCherry-labeled astrocyte (Fig. 1, D and E). To estimate the proportion of surface-proximal GLT-1, we digitally isolated GLT-1 within 250 nm of the astroglial membrane as a proportion of total GLT-1 in each astrocyte (Fig. 1, F to H).
Fig. 1. Workflow used for confocal analysis of astroglial morphology and surface-proximal GLT-1.
(A) Rats were trained to self-administer heroin or sucrose over 10 consecutive days of operant training, and reward delivery was paired with light and tone cues. During extinction training, heroin or sucrose and cues were not delivered in response to active lever pressing, and operant responding gradually decreased. Heroin- and sucrose-trained rats differed in active lever pressing during self-administration (two-way ANOVA, F1,124 = 33.44, P < 0.0001). Inset shows that groups of heroin-trained animals took similar amounts of heroin (one-way ANOVA, F2,12 = 0.9187, P = 0.4984). (B) Twenty-four hours after the last extinction session, animals undergoing cue-induced reinstatement of reward seeking were placed in the operant chamber, and cues were restored to the active lever for 15 or 120 min to reinstate seeking (two-way ANOVA session, F1,26 = 16.07, P = 0.0005). (C) Z-series depicting a NAcore astrocyte transfected with AAV5/GFAP-hM3d-mCherry (magenta) and immuno-labeled for Synapsin I (green) and GLT-1 (blue). (D) GLT-1 immunoreactivity coregistered with the mCherry-labeled astrocyte in (C) is shown in white. (E) Coregistration of GLT-1 (blue) with Synapsin I (green) from the region occupied by the astrocyte in (C) is shown in pink. (F) Digital rendering of the astroglial surface (yellow) was used to identify GLT-1 signal within 250 nm of the cell membrane (G and H, white) relative to total GLT-1 from the same astrocyte (H, blue). Bar in (C) = 10 μm. In (A) (inset), Ext, extinguished; 15 m, 15-min cued reinstatement; 120 m, 120-min cued reinstatement. In (B), Suc, sucrose; Her, heroin; Ext, extinction.
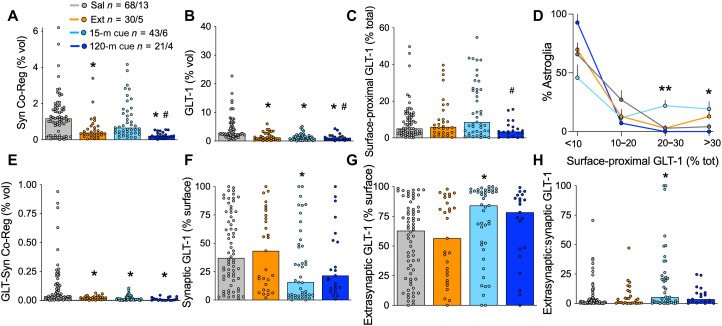
Coregistration of the astroglial membrane with Synapsin I was reduced after extinction of heroin (Fig. 2A), but not extinction following sucrose self-administration (fig. S1A). We also found reductions in total astrocyte GLT-1 expression after extinction from heroin (Fig. 2B), but not sucrose self-administration (fig. S1B). As shown previously (6), exposure to heroin cues for 15 min increased synaptic adjacency of astroglia in heroin-trained, but not sucrose-trained, rats (Fig. 2A versus fig. S1A). Although total levels of GLT-1 were reduced in heroin-extinguished rats, the proportion of GLT-1 on the astroglial surface was unaltered by heroin extinction training (Fig. 2C). Moreover, 15 min of cue exposure transiently increased the proportion of astroglia expressing the highest levels of surface-proximal GLT-1 (Fig. 2D). Both the increases in synapse-proximal astroglia and GLT-1 after 15 min of heroin cues were transient and returned to extinction levels by 120 min after initiating cued reinstatement (Fig. 2, A, C, and D), a time course that parallels the rise and fall of extracellular glutamate during cued relapse (25). The ratio of surface:total GLT-1 was reduced in the NAcore of rats extinguished from sucrose (fig. S1C), but not altered by cued reinstatement (fig. S1, C and D). The coregistration of GLT-1 with Synapsin I was reduced after extinction from heroin self-administration (Fig. 2E), but not sucrose self-administration (fig. S1E). Despite increases in surface GLT-1 and synaptic adjacency by NAcore astroglia, the proximity of GLT-1 to the presynaptic marker Synapsin I was not restored by cued heroin seeking (Fig. 2, E and F). Instead, the increase in surface-proximal GLT-1 in animals that reinstated heroin-seeking for 15 min was targeted extrasynaptically (>250 nm from the synapse) (Fig. 2, G and H). In sucrose-trained rats, levels of synaptic and extrasynaptic GLT-1 were not changed by extinction training or cued reinstatement of sucrose seeking (fig. S1, F to H). Extinction from heroin self-administration did not change total Synapsin I expression in the NAcore (fig. S2).
Fig. 2. Extrasynaptic GLT-1 was transiently elevated during heroin seeking.
We previously found that coregistration of labeled NAcore astroglia with Synapsin I is reduced during extinction from heroin [(A) Kruskal-Wallis = 30.17, P < 0.0001) and that 15 min of cued heroin seeking restores synaptic insulation by astrocytes. (B) Withdrawal from heroin self-administration produced a down-regulation of GLT-1 on NAcore astroglia, whether or not rats underwent cued reinstatement (Kruskal-Wallis = 51.77, P < 0.0001). (C) Surface-proximal GLT-1, shown here as percent of total GLT-1 from each astrocyte, was increased during active seeking (15-m cue) when compared to a time point at which cues no longer evoke seeking (i.e., cue extinction; 120-m cue, Kruskal-Wallis = 9.848, P < 0.05). A greater proportion of astrocytes exhibited high levels of surface-proximal GLT-1 after 15 min of heroin cues compared to yoked saline controls [(D) <10%: Kruskal-Wallis = 6.114, P = 0.1062; 10 to 20%: Kruskal-Wallis = 3.362, P = 0.3391; 20 to 30%: Kruskal-Wallis = 13.42, **P < 0.01 and P < 0.01, 15-min reinstatement versus yoked saline; >30%: Kruskal-Wallis = 9.831, *P < 0.05]. Coregistration of GLT-1 with the presynaptic marker Synapsin I was found to be reduced in heroin-trained rats and was not restored during cued reinstatement [(E) Kruskal-Wallis = 35.48, P < 0.0001]. When synaptic and extrasynaptic fractions of surface GLT-1 were analyzed separately, we found that 15 min of cued heroin seeking decreased synaptic GLT-1 [(F) Kruskal-Wallis = 9.493, P < 0.05] and increased extrasynaptic GLT-1 [(G) Kruskal-Wallis = 9.493, P < 0.05]. The ratio of extrasynaptic and synaptic GLT-1 illustrates the robust increase in extrasynaptic GLT-1 during cued heroin seeking [(H) Kruskal-Wallis = 9.476]. N shown in (A) as cells/animals. In (A) to (C) and (E) to (H), *P < 0.05 compared to yoked control, #P < 0.05 compared to 15 min reinstated using Dunn’s test. Sal, yoked saline; Ext, extinguished; 15-m cue, 15-min cued reinstatement; 120-m cue, 120-min cued reinstatement.
Astrocyte heterogeneity in heroin cue-induced plasticity
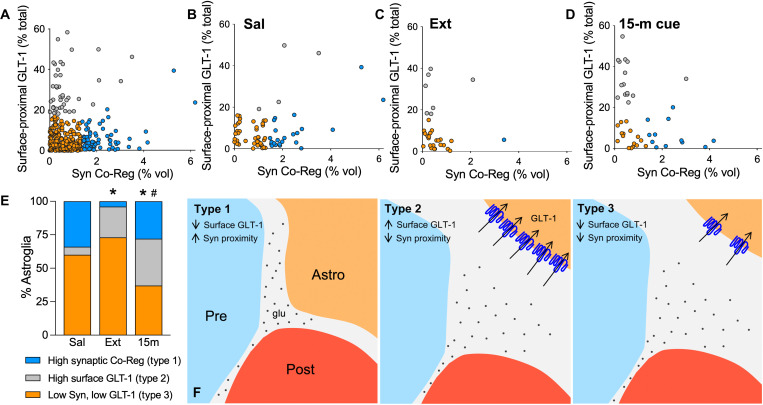
The fact that astrocyte synaptic proximity was transiently increased by 15 min of cued heroin reinstatement, but the increase in surface-proximal GLT-1 did not coregister with Synapsin I indicates that these cue-induced astroglial adaptations may occur in distinct subpopulations of astrocytes. Principal components analysis (PCA; fig. S3) (26) of astroglia from saline-, heroin-, and sucrose-trained rats followed by hierarchical clustering identified three distinct clusters of astroglia we arbitrarily refer to as types 1 to 3: type 1, astrocytes with high synaptic coregistration; type 2, astrocytes with high levels of extrasynaptic GLT-1; and type 3, astrocytes with low-to-moderate synaptic adjacency and surface GLT-1 expression (Fig. 3A). A majority of NAcore astrocytes from yoked saline rats were in the types 1 and 3 subpopulations (Fig. 3, B and E). Following extinction from heroin self-administration, there was a loss of synapse-associated type 1 and an increase in type 2 astrocytes (Fig. 3, C and E). Fifteen minutes of cued heroin reinstatement resulted in a transient restoration of type 1 astrocytes and a further increase in the proportion of type 2 astroglia (Fig. 3, D and E). Because of a collapse of synaptic adjacency and surface-proximal GLT-1 levels after 120 min of heroin cue exposure, type 3 astrocytes were predominant in rats that underwent 120 min of cue-induced heroin seeking (Fig. 2, A and C). In contrast with heroin self-administration and akin to saline rats, all three treatment groups of sucrose-trained rats contained predominately types 1 and 3 astrocytes (fig. S4A). Along with the lack of GLT-1 coregistration with Synapsin I during cued reinstatement (Fig. 2E), these data are consistent with the presence of three distinct astroglial populations in heroin-seeking animals, which is also indicated by the lack of GLT-1 immunostaining in some NAcore astroglia (fig. S5). Figure 3F illustrates the distinct morphology and GLT-1 localization characteristic of each astroglial type.
Fig. 3. Cue-induced increases in astrocyte motility and GLT-1 surface expression occurred in different astroglial subpopulations.
(A) PCA was used to identify subpopulations of NAcore astroglia according to their synaptic adjacency and levels of surface-proximal GLT-1. Astrocytes with high synaptic adjacency (blue) or high levels of surface-proximal GLT-1 (gray) were identified as separate cell clusters. Distribution of clusters in tissue from yoked saline and extinguished or cue-reinstated rats is shown in (B) to (D). Astrocytes with high surface-proximal GLT-1 but low synaptic coregistration were largely absent from yoked control rats but emerged after extinction of operant heroin training [(E) χ2 = 14.10, *P = 0.0018 Ext. versus Sal). Half of astroglia in cue-reinstated rats exhibited one or the other type of transient plasticity [(E) χ2 = 15.97, *P = 0.0006 versus Sal; χ2 = 11.20, #P = 0.0074 versus Ext). (F) Schematic illustrating three astroglial subtypes identified by PCA and hierarchical clustering. Type 1 astroglia expressed low levels of GLT-1 but exhibited high measures of synaptic adjacency (F, left). Type 2 astroglia expressed high levels of extrasynaptic GLT-1 (blue) and were observed after heroin training but were not abundant in control animals (F, middle). Type 3 astroglia were predominant in control animals and had low-to-moderate GLT-1 expression and synaptic adjacency (F, right).
Gq signaling in NAcore astroglia increased synaptic adjacency, but not surface GLT-1
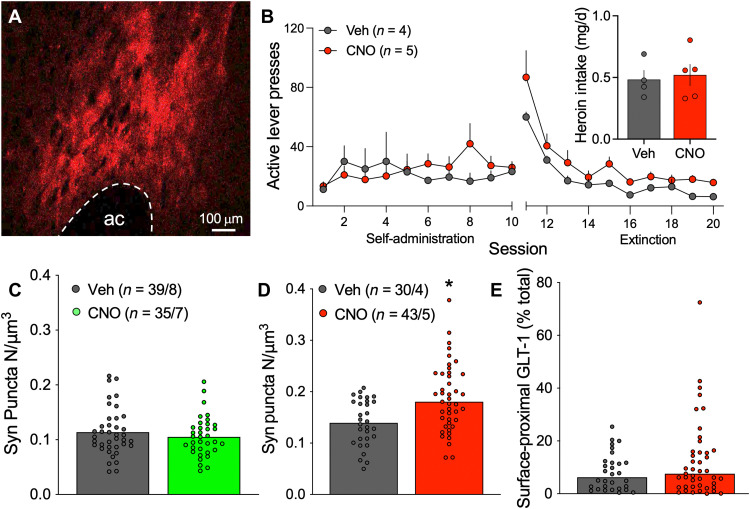
Gq-coupled signaling through metabotropic glutamate receptor mGluR5 on cultured astroglia promotes ezrin-dependent astrocyte fine process motility (9). Moreover, cue-induced heroin seeking is associated with increased ezrin phosphorylation (6) and astrocyte proximity to synapses (Fig. 2A), and increases in synaptic glutamate spillover in NAcore that occur during cued reinstatement of drug seeking (27) could stimulate mGluR5 on astroglial processes (28). To determine whether Gq-type mGluR signaling triggers cue-induced astrocyte process motility, a Gq-coupled designer receptor activated by designer drug (DREADD) was delivered selectively to NAcore astroglia (Fig. 4A). Rats were trained to self-administer heroin before undergoing 10 days of extinction training (Fig. 4B). In lieu of reinstatement, animals were given clozapine N-oxide (CNO) or vehicle, and their brains were removed for analysis of astrocyte synaptic adjacency and surface-proximal GLT-1 expression. In animals expressing a non-DREADD control viral vector, systemic CNO delivery did not affect synaptic adjacency of NAcore astroglia (Fig. 4C). However, in DREADD-expressing animals, CNO delivery increased the coregistration of the astroglial membrane with Synapsin I–positive puncta (Fig. 4D) but did not affect surface-proximal GLT-1 levels (Fig. 4E). These data indicate that Gq signaling in NAcore astroglia triggers morphological plasticity during reinstatement, while the increase in extrasynaptic GLT-1 expression (Fig. 2, G and H) likely involves a different signaling cascade.
Fig. 4. Gq signaling increased synaptic adjacency in NAcore astroglia from heroin-trained rats.
After viral delivery of Gq-DREADD in NAcore astroglia (A, red), rats were trained to self-administer heroin before undergoing extinction training (B). The following day, rats received intraperitoneal injections of vehicle or CNO but did not undergo cued reinstatement. Heroin intake did not differ between vehicle- or CNO-treated rats [(B) inset, t7 = 0.173, P = 0.7602]. (C) CNO delivery did not affect synaptic adjacency of astrocytes labeled with Lck-GFP (Mann-Whitney U = 633, P = 0.5978). (D) Gq signaling increased coregistration of NAcore astroglia with near-adjacent immunolabeled Synapsin I puncta (Mann-Whitney U = 363, P = 0.0013) but did not affect levels of surface-proximal GLT-1 [(E) Mann-Whitney U = 568, P = 0.3934]. In (A), ac, anterior commissure. In (B) to (D), Veh, vehicle. In (C) and (D), N shown as cells/animals.
Reducing cue-induced plasticity in astroglial subpopulations increased heroin seeking
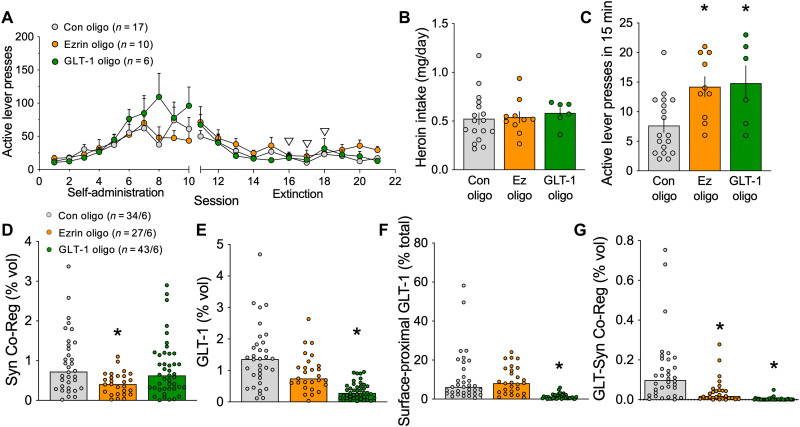
To determine whether cue-induced type 1 and type 2 astroglial plasticity (as shown in Fig. 3F) affected heroin seeking, we implanted cannulae above the NAcore and trained rats to self-administer heroin (Fig. 5A). Rats were divided to maintain equal heroin intake across treatment groups (Fig. 5B) and received bilateral infusions of an inert control vivo-morpholino antisense oligomer, or an oligo targeted to GLT-1 (29) or ezrin (6) on days 6 to 8 of extinction training. Heroin seeking was reinstated in rats by exposure to heroin cues for 15 min, and either ezrin or GLT-1 knockdown potentiated cued seeking (Fig. 5C). Thus, the presence of either type 1 or type 2 astroglial plasticity during cued heroin seeking serves a compensatory function to suppress heroin seeking.
Fig. 5. Impairing cue-induced astrocyte motility or surface GLT-1 elevated heroin seeking.
(A) Rats were trained to self-administer heroin over 10 consecutive days. Starting on day 6 of extinction training for three consecutive days (white arrowheads), animals received NAcore infusions of an ezrin antisense oligomer, a GLT-1 antisense oligomer, or a control oligomer. Animals in each treatment group did not differ in total heroin intake during self-administration. (B) Rats receiving different oligo treatments did not differ in heroin intake (one-way ANOVA, F2,30 = 0.1739, P = 0.8412). Twenty-four hours after the final extinction session, animals were reinstated for 15 min by exposure to light and tone cues previously paired with heroin delivery. (C) Rats that underwent ezrin or GLT-1 knockdown pressed higher on the active lever during the 15-min reinstatement session (one-way ANOVA, F2,30 = 6.230, P = 0.0055, *P = 0.0058 Con versus Ezrin Oligo, *P = 0.0104 Con versus GLT-1 Oligo using Fisher’s test). (D) Astrocytes from rats treated with the ezrin oligo exhibited a significant reduction in synaptic coregistration, consistent with knockdown of astrocyte peripheral process motility (Kruskal-Wallis = 15.85, P = 0.0004, *P = 0.0002 versus Con). The GLT-1 oligo did not affect synaptic coregistration by NAcore astroglia (P = 0.4676 versus Con). (E) GLT-1 levels were unchanged by ezrin antisense oligo delivery (P = 0.3888) but were reduced by treatment with the GLT-1 oligo (Kruskal-Wallis = 54.85, P < 0.0001, *P < 0.0001). (F) Likewise, surface-proximal GLT-1 was reduced after application of the GLT-1 oligo (Kruskal-Wallis = 80.22, P < 0.0001, *P < 0.0001 versus Con), but not the ezrin oligo (P > 0.9999 versus Con). (G) The coregistration between GLT-1 and Synapsin I on astroglia was significantly reduced after ezrin (Kruskal-Wallis = 87.13, P < 0.0001, *P = 0.0147) or GLT-1 knockdown (*P < 0.0001). In (A) to (D), Con, control oligo; Ez, ezrin oligo; GLT-1, GLT-1 oligo. In (D), N shown as cells/animals.
Brains were extracted immediately following the 15-min reinstatement session for analysis of morpholino delivery on presence of astroglial types. Confirming that cannulae placement and morpholino infusions did not affect cue-induced astrocyte plasticity, both type 1 and type 2 adaptations were observed during 15 min of cue-induced heroin seeking in rats treated with the control oligo (compare fig. S4B with Fig. 3D). Ezrin knockdown reduced the synaptic proximity of astroglia in the NAcore (Fig. 5D), and GLT-1 knockdown further reduced total GLT-1 levels and blocked the cue-induced increase in surface-proximal GLT-1 (Fig. 5, E and F). Validating the specificity of the oligos and supporting the presence of distinct subpopulations of astrocytes, the ezrin-targeted oligo selectively eliminated type 1 astrocytes, while the GLT-1–targeted oligo selectively eliminated type 2 astrocytes in rats that reinstated heroin seeking in response to cues for 15 min (fig. S4B). GLT-1 knockdown did not alter synaptic proximity of NAcore astroglia (Fig. 5D), and ezrin knockdown did not affect levels of total GLT-1 or surface-proximal GLT-1 (Fig. 5, E and F). As expected, independent knockdown of either GLT-1 or ezrin reduced GLT-1 coregistration with Synapsin I (Fig. 5G).
Together, these data show that all three types of astroglia are present during cue-induced heroin seeking (Fig. 3F). Akin to extinguished rats, heroin cues are associated with type 2 astroglia having increased surface-proximal expression of GLT-1, and akin to their presence in control saline or sucrose rats, cues induce the presence of type 1 astroglia that have high synaptic proximity but relatively low GLT-1 density on the astroglial surface. The subpopulations are distinct cells and are regulated by distinct signaling pathways. The presence of either type 1 or type 2 subpopulations serves to inhibit cue-induced heroin seeking, since blocking their formation during heroin-cue exposure elevated active lever pressing during the 15-min reinstatement session compared to control-oligo treatment.
NAcore astrocytes differentially associated with D1- and D2-MSNs at baseline and after heroin
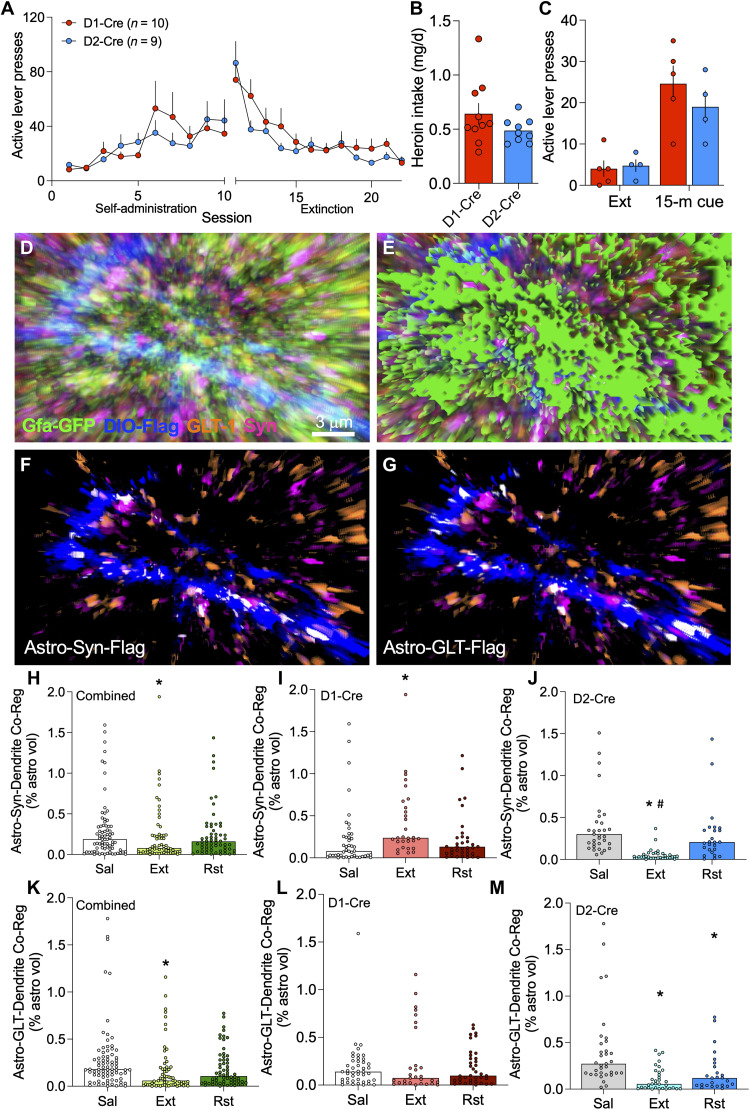
Activity in the two main neuronal subtypes in the NAcore, D1- and D2-MSNs, promotes drug seeking and extinction of seeking, respectively (30, 31), and individual astrocytes in the dorsal striatum signal uniquely with these two neuronal subclasses (19, 32). We hypothesized that the different functional types of astroglia depicted in Fig. 3F would associate uniquely with D1- or D2-MSNs, thereby contributing to their functional roles in reducing cue-induced heroin seeking. To test this hypothesis, we labeled astrocytes and neurons in male and female D1- and D2-Cre transgenic rats before training animals to self-administer heroin for 10 days (Fig. 6, A and B). Rats then underwent 10 to 12 days of extinction training, and brains were extracted from extinguished rats 24 hours after the last extinction session. After extinction training, rats in the reinstatement group underwent exposure to heroin-associated cues for 15 min before brain extraction (Fig. 6C). Isolated astroglia were imaged, and their coregistration with virally labeled D1- or D2-MSN dendrites and the synaptic marker Synapsin I or GLT-1 was quantified (Fig. 6, D to G). Pooling data from D1- and D2-Cre rats revealed the same synaptic retraction by astroglia from NAcore dendrites after extinction and reinsertion of astroglial processes toward synapses during cued reinstatement (Fig. 6H), as was observed in Sprague-Dawley rats (Fig. 2A). When astrocyte-synapse coregistration data were analyzed separately in D1- and D2-Cre rats, the astrocyte association with D1-MSN synapses was lower in yoked saline animals compared with D2-MSN synapses (compare saline-treated groups in Fig. 6, I to J). After extinction training, astrocyte coregistration with Synapsin I was increased at D1-MSN synapses (Fig. 6I) and decreased at D2-MSN synapses (Fig. 6J). Fifteen minutes of exposure to heroin cues restored astrocyte association with both synaptic types to levels observed in yoked saline controls (Fig. 6, I and J).
Fig. 6. Astrocytes increased their adjacency to D1-MSN synapses but retracted from D2-MSN synapses after extinction training.
(A) Male and female D1- and D2-Cre rats were trained to self-administer heroin over 10 days before undergoing extinction training. (B) Heroin intake did not differ between the strains (t17 = 1.449, P = 0.1656). (C) Twenty-four hours after the last extinction session, lever pressing was reinstated by exposure to heroin-conditioned cues for 15 min (two-way ANOVA, time F1,7 = 20.36, P = 0.0028, genotype F1,7 = 0.9944, P = 0.3519). (D) NAcore tissue from these animals was immunolabeled to identify D1- or D2-MSN dendrites (blue), GLT-1 (orange), and Synapsin I (pink). Signal associated with NAcore astroglia (green, D and E) was isolated to quantify the degree of triple coregistration between astroglia and D1- or D2-MSN synapses (F, white) or astroglial GLT-1 and D1- or D2-MSN dendrites (G, white). Synaptic association by astroglia was reduced by extinction training after heroin self-administration when quantified in a cell-nonspecific manner [(H) Kruskal-Wallis = 4.617, P = 0.0994, *P = 0.0380 versus Sal]. When analyzed separately, astrocyte association with D1-MSN synapses was low at baseline and was increased by extinction training [(I) Kruskal-Wallis = 12.35, P = 0.0021, *P = 0.0014 versus Sal). Instead, D2-MSN synapses had high astrocyte insulation at baseline (P < 0.0001 versus D1-Cre, Sal using Dunn’s test) that was reduced by extinction training and recovered during cued reinstatement [(J) Kruskal-Wallis = 45.84, P < 0.0001, *P < 0.0001 versus Sal, and #P < 0.0001 versus Rst). Surface-proximal, dendrite-associated GLT-1 was decreased overall after extinction training in NAcore astrocytes and was restored during 15-min cued reinstatement [(K) Kruskal-Wallis = 15.23, P = 0.0005, *P < 0.05 versus Sal). However, the increase in surface-proximal GLT-1 during cued reinstatement was not associated with dendrites from D1- [(L) Kruskal-Wallis = 0.7498 P = 0.6874) or D2-MSNs [(M) Kruskal-Wallis = 25.49, P < 0.0001, *P < 0.05 versus Sal). Similar to astrocyte-synaptic adjacency, GLT-1 coregistration with D2-dendrites was higher at baseline compared with D1-dendrites (P = 0.0043 using Dunn’s test). In (I), (J), (L), and (M), N = 25–44/4–5 cells/animals per group, and full data spread is reported in table S1. Sal, yoked saline; Ext, extinguished; Rst, 15-min cued reinstatement.
When dendrite-associated GLT-1 was analyzed in pooled D1- and D2-Cre animals, we observed a reduction in dendrite-associated GLT-1 after extinction training that was restored to saline control levels after 15-min exposure to heroin cues (Fig. 6K), akin to the increase in surface-proximal GLT-1 during 15 min of cue-induced heroin seeking in Sprague-Dawley rats (Fig. 2D). When dendritic adjacency of GLT-1 was examined separately in D1- and D2-Cre rats, levels of GLT-1 adjacent to D2-dendrites were higher when compared with D1 dendrites (compare saline-treated groups in Fig. 6, L and M). The extinction-associated reduction in the pooled data occurred at D2-dendrites, not D1-dendrites (Fig. 6, L and M). However, the increase in surface-proximal GLT-1 after 15 min of cue exposure did not coregister with either D1- or D2-dendrites (Fig. 6, L and M). Both the morphological plasticity and GLT-1 dynamics in NAcore astroglia from D1- and D2-Cre animals were similar in male and female animals, except for the D1-dendritic association of GLT-1, which was lower in extinguished males, compared to females (fig. S6).
Together, these data show that the cue-induced increase in type 1 astroglia during heroin seeking (Fig. 3, D and E) can be linked to morphological plasticity of astroglia surrounding D2-dendrites (Fig. 6, H and J), but the cue-induced increase in type 2 astroglia exhibiting high levels of extrasynaptic GLT-1 (Fig. 3, D and E) are not associated with either D1- or D2-dendrites (Fig. 6, K and M). A summary schematic illustrating subcircuit-selective astrocyte adaptations after extinction of heroin seeking is shown in fig. S7.
DISCUSSION
Synaptic glutamate spillover from prefrontal cortical synapses in NAcore is produced by drug-associated cues and is necessary for drug cues to induce synaptic adaptations that regulate the intensity of drug seeking. Astroglia have the capacity to strongly regulate glutamate spillover from synapses by their morphological proximity to synapses (25) and their expression of GLT-1, especially in perisynaptic astroglial processes (33–36). We show that during cue-induced heroin seeking, astroglia use both of these mechanisms to dampen the intensity of seeking. Unexpectedly, cue-induced increases in synaptic proximity and surface GLT-1 expression occurred in separate astroglial subpopulations and were triggered by different signaling cascades, with astroglial Gq-signaling selectively activating astrocyte fine process motility with no impact on surface GLT-1 expression. Type 1 astroglia had greater proximity to synapses in NAcore, while type 2 astroglia showed increased surface expression of GLT-1. The functional relevance of cues transiently inducing these astroglial subpopulations was shown by selectively eliminating either type 1 or type 2 astroglial plasticity and potentiating cue-induced heroin seeking. Last, we demonstrated that the type 1 adaptation was selective for D2-MSNs, not D1-MSNs, during seeking, while the cue-induced type 2 adaptation was not significantly associated with either D1- or D2-MSNs. Together, our data reveal at least two mechanisms whereby astroglia negatively regulate cue-induced motivation to seek heroin, but not sucrose. At least part of this action may arise from Gq-coupled stimulation of astroglial processes toward D2-MSN synapses.
Distinct subpopulations of astroglia during cue-induced heroin seeking
During cue-induced heroin seeking, three distinct subpopulations of astroglia were present, type 1 showing increased synaptic proximity, type 2 showing up-regulated surface GLT-1, and type 3 having relatively lower levels of both synaptic adjacency and surface GLT-1. Unbiased PCA and hierarchical clustering revealed that type 1 and type 2 adaptations were in distinct subpopulations of astroglia. The appearance of type 1 and type 2 subpopulations induced by cued seeking was detected during 15 min of heroin seeking but absent after 120 min of unrewarded seeking, akin to increases in extracellular glutamate and postsynaptic potentiation of D1-MSNs, both of which occur transiently during seeking initiated by drug-conditioned cues (25, 31, 37).
Control saline or sucrose rats showed largely type 1 and 3 astroglia. Type 2 astroglia emerged, while type 1 astroligia were eliminated in heroin-extinguished rats. Cue-induced heroin seeking was accompanied by transient return of type 1 astroglia to levels present in control rats and a transient elevation in the proportion of astroglia exhibiting the type 2 adaptation. The increases in synaptic adjacency and surface-proximal GLT-1 were both abolished after 120 min of heroin cue exposure, when cue-induced increases in active lever pressing (6) and extracellular glutamate (25) are both diminished. In sum, our data clearly show that the appearance and disappearance of different types of astroglial adaptations depend on whether the animal is actively seeking or has extinguished seeking, and reveal remarkable morphological and signaling plasticity in astroglia to negatively regulate cue-induced heroin seeking. Whether and how heroin intake, which accompanies relapse in human drug users but was withheld during our cue-induced relapse test, affects emergence of astroglial types is a remaining question that merits further investigation.
Type 1 astroglia: Cue-induced morphological plasticity with no increase in surface GLT-1
A portion of mature synapses throughout the brain are insulated by astroglia (38), and the perisynaptic astroglial membrane expresses the highest density of GLT-1, glutamate receptors, and other proteins, including actin binding proteins such as ezrin, which together contribute to modulating glutamatergic synaptic activity. Perisynaptic astroglia modulate synaptic glutamate homeostasis in part through dynamic morphological plasticity in response to synaptic neurotransmission (33, 38, 39). In the hippocampus and cerebellum, the astroglial sheath is biased toward the postsynapse (40), permitting access of synaptically released glutamate onto presynaptic mGluR2/3 autoreceptors that negatively regulate release probability (41). Notably, dense expression of GLT-1 proximal to the synapse may prevent recruitment of this autoinhibitory mechanism, by retrieving glutamate from the synapse and thus reducing glutamate concentration at presynaptic autoreceptors. In this way, the GLT-1–deficient astroglial processes present in the type 1 subpopulation can promote autoinhibitory regulation of excitatory transmission in the NAcore by sterically guiding synaptically released glutamate toward presynaptic mGluR2/3 in the absence of glutamate uptake. The importance of guiding glutamate to presynaptic mGluR2/3 to negatively regulate relapse is supported by the fact that stimulating mGluR2/3 in the accumbens inhibits drug seeking (42, 43).
Astrocyte fine process motility is increased by activating Gq-coupled mGluR5 (9, 16). Also, stimulating Gq-DREADD in NAcore astroglia reduces reinstated cocaine seeking by promoting astroglial glutamate release onto presynaptic mGluR2/3 (44). Our findings complement this work by showing that activating astroglial Gq-DREADD in the NAcore increases the number of synaptic puncta with near-adjacent astroglial processes, although the resolution limit of confocal microscopy does not permit us to determine whether astroglial processes were oriented toward the pre- or postsynapse after DREADD stimulation. Increasing Gq signaling in NAcore astroglia did not affect surface-proximal GLT-1 levels, supporting discrete signaling mechanisms for heroin cue-induced formation of type 1 and type 2 astroglial plasticity.
In addition to potentially guiding synaptically released glutamate toward mGluR2/3 autoreceptors, the synaptic reassociation induced by heroin cues in type 1 astroglia may limit access of synaptic glutamate (i.e., glutamate spillover) to perisynaptic N-methyl-d-aspartate (NMDA) glutamate receptors containing the 2B subunit (NR2B) that must be stimulated for cues to initiate heroin seeking and for the synaptic potentiation that accompanies heroin seeking (37). Last, not only would synaptic insulation by astroglial processes be more likely to engage presynaptic autoinhibition and block access to NR2B necessary for synaptic potentiation, but synaptic adjacency of astroglial processes reduces the possibility of synaptic recruitment that occurs via transmitter spillover to neighboring synapses. This is supported by the fact that synaptic retraction of astroglial processes after LTP induction promotes synaptic cross-talk (45).
Type 2 astroglia: Cue-induced increases in surface-proximal GLT-1 with no change in synaptic proximity
In a portion of astroglia, heroin use and extinction increased levels of surface-proximal GLT-1, and this adaptation was transiently accentuated by heroin cues. However, the increase in GLT-1 did not coregister with Synapsin I in either treatment group, indicating that surface GLT-1 in extinguished and reinstated animals was distant (>250 nm) from the synapse (i.e., extrasynaptic). Moreover, the increase in surface-proximal GLT-1 during cue-induced heroin seeking was not associated with either D1- or D2-dendrites. We hypothesize that type 2 astroglia do not guide glutamate spillover to presynaptic autoreceptors or sterically shield extrasynaptic high-affinity glutamate receptors from synaptic glutamate, as discussed above for type 1 astroglia. However, diffusion of glutamate to more distal sites would be reduced by increased extrasynaptic GLT-1 (27). Thus, the primary impact of type 2 astroglia may be to deny access of synaptic glutamate to neighboring synapses, thereby preventing synaptic recruitment. Notably, by inhibiting glutamate access to neuronal nitric oxide synthase (nNOS) interneurons where mGluR5 stimulation promotes NO-induced synaptic potentiation in D1-MSNs (46) or reducing NR2B stimulation on adjacent synapses (37), extrasynaptic GLT-1 could dampen cued heroin seeking and the associated synaptic potentiation (47).
While some studies demonstrate that the bulk of GLT-1 is on the astroglial surface (35), there are notable differences between hippocampal or cultured astrocytes and striatal astrocytes studied here (48). Our data indicate that a proportion of NAcore astroglia express relatively high levels of surface-proximal GLT-1 and that these cells target their GLT-1 extrasynaptically during relapse. Heterogeneity in GLT-1 expression by NAcore astroglia is depicted in fig. S5 and is further supported by the non-Gaussian distribution of GLT-1 expression quantified on astrocytes from yoked saline control animals (Fig. 2, B and C). Notably, type 2 astrocytes that exhibit high levels of surface GLT-1 but low synaptic proximity were not abundant in control saline or sucrose groups but emerged after extinction from heroin self-administration. Whether this adaptation was a consequence of heroin, heroin withdrawal or extinction training during withdrawal is a remaining question. However, it is noteworthy that extinction from sucrose self-administration did not increase the presence of type 2 astroglia, indicating that extinction training per se is not sufficient to produce this adaptation.
That Gq signaling did not stimulate plasticity in type 2 astroglia raises the possibility that levels and/or patterns of Gq-coupled receptor expression may distinguish type 1 from type 2 astroglia. In keeping with this hypothesis, astroglial mGluR5 signaling stimulates peripheral process motility (9) and down-regulates GLT-1 (49). Such a dual role for mGluR5 signaling would contribute to the relatively low levels of surface GLT-1 in type 1 astroglia. Alternatively, stimulation of astroglial mGluR3 has been linked to increased GLT-1 expression (49), raising the possibility that cue-induced glutamate spillover may act via receptors differentially expressed on types 1 and 2 astroglia.
Astroglial adaptations are differentially regulated around D1- and D2-MSNs
D1- and D2-MSNs together constitute 90 to 95% of all NAcore neurons (10), and different astroglial subpopulations modulate synaptic transmission selectively on one or the other neuronal subtype (19, 32). Consistent with astroglia associating selectively with one or the other, but not both types of MSN, astroglia were more proximal to D2- than D1-MSN dendrites in yoked saline control animals. The finding that astroglia increased their proximity to D1-MSN synapses after extinction training, but retracted from D2-MSNs, is consistent with the fact that synaptic adaptations associated with extinction from addictive drug self-administration are found primarily on D2-MSNs, while adaptations produced by drug cues are predominant on D1-MSNs (31, 50–52). We predict that astrocyte insulation of D1-MSNs after extinction training would reduce synaptic glutamate spillover, extrasynaptic receptor simulation, and, ultimately, synaptic potentiation of D1-synapses during extinction, relative to D2-MSNs (31, 52). Moreover, since astrocytes can trigger long-term depression at D1-MSN synapses via mGluR5-dependent ATP/adenosine signaling in the dorsal striatum (19), an increase in astrocyte association with D1-MSN synapses after heroin extinction may serve to suppress synaptic activity at D1-neurons and to thereby dampen seeking. Conversely, retraction of astrocytes from D2-MSNs after extinction training is expected to be permissive of synaptic potentiation at D2-MSN synapses via glutamate spillover. A number of findings are consistent with this interpretation, including the following: Extinction from cocaine is associated with synaptic potentiation at D2-MSN, but not D1-MSN, excitatory synapses (31); matrix metalloprotease-2 activity is elevated in heroin-extinguished rats only around NAcore D2-MSN dendritic spines (50); and chemogenetically activating D2-MSNs suppresses drug seeking (11).
That astrocyte processes surrounding D1-MSNs retracted during heroin-cue exposure is consistent with permitting glutamate spillover and potentiating synaptic activity at D1-synapses, as has been reported for both glutamate transmission and spine morphology (i.e., increased spine head diameter and/or spine density) during cued drug seeking (31, 51). Since astrocytes dampen synaptic potentiation via a number of mechanisms (20, 44), we hypothesize that increased astrocyte insulation of D2-MSNs during cue-reinstated heroin seeking may reduce D2-MSN potentiation relative to the extinguished context (31, 45). However, a number of reports indicate that subpopulations of D2-MSNs in the striatum promote, rather than inhibit, motivated behaviors (53–56). Moreover, cortical inputs linked to cued reinstatement (57) innervate D2-MSNs to a greater extent than D1-MSNs (58). For this reason, the possibility remains that increased astrocyte insulation on D2-MSNs during cued reinstatement may serve a compensatory function by triggering autoinhibition at cortical terminals that release glutamate in response to heroin-conditioned cues. In this way, astrocyte insertion onto D2-MSNs may contribute to reducing glutamate spillover onto both D2-MSNs, as well as D1-MSNs, that receive less direct innervation by prefrontal terminals (58) but are significantly engaged during cued reinstatement to drive seeking (31).
Akin to astroglial synaptic proximity, surface GLT-1 was lower adjacent to D1-dendrites compared with D2-dendrites in saline rats. However, the overall dynamic in type 2 astroglia after extinction and cued reinstatement observed in wild-type rats was not recapitulated in either D1- or D2-MSNs. This could result from increased surface GLT-1 in type 2 astroglia targeting nondendritic portions of D1- and D2-MSNs or targeting subpopulations of interneurons or other astroglia that were not labeled in D1- and D2-Cre rats.
Astroglial plasticity during sucrose versus heroin seeking
Unlike extinction training after heroin self-administration, extinction training after sucrose self-administration did not down-regulate GLT-1 or produce changes in synaptic proximity by NAcore astroglia in any treatment group (6, 59). The relative lack of changes in astroglial subpopulation plasticity after extinction from sucrose self-administration and cued reinstatement supports the likelihood that changes in the proportion of astroglial subtypes in heroin-trained rats may be selective for addictive drugs and not natural rewards. Although we found no change in coregistration of GLT-1 with Synapsin I in sucrose-trained or sucrose cue-reinstated animals, we observed reduced surface expression of GLT-1 after extinction from sucrose self-administration compared to yoked controls, further reducing the proportion of type 2 astroglia in this group. As described above, we predict that synaptic adjacency of astroglial processes deficient in GLT-1 would favor presynaptic autoinhibition upon transmitter release (40). Consistent with this hypothesis, sucrose-trained rats exhibit more rapid extinction training and less perseverative reward seeking compared with drug-trained animals (21, 60), even though sucrose self-administration produced more active lever presses than heroin self-administration. Last, while the present experiments do not test whether extinction training following self-administration, rather than self-administration alone, is necessary for the adaptations observed in astroglia from extinguished animals trained to self-administer either sucrose or heroin, studies on astroglia in different brain regions demonstrate that extinction training is capable of inducing robust alterations in astroglial morphology and transporter expression relative to self-administration alone or withdrawal without extinction training (21, 61).
Implications for treating substance use disorders
We found that astroglia in the NAcore exhibited two forms of plasticity capable of shaping cue-induced heroin seeking that occurred in separate astroglial subpopulations and were regulated by distinct signaling mechanisms. Moreover, both increasing astroglial proximity to synapses and glutamate transport involve proteins selectively expressed in astroglia (ezrin and GLT-1, respectively). The fact that none of the plasticity produced in NAcore astroglia from heroin-trained rats was recapitulated by sucrose training supports the potential that selective interventions in one or the other astroglial dynamic may be therapeutically beneficial in treating substance use disorder. Pharmacological treatments such as ceftriaxone and N-acetylcysteine that elevate GLT-1 are effective at reducing drug seeking in rodent models of relapse (62–64) and reducing drug-cue reactivity in humans (65). Unfortunately, while reducing craving induced by drug cues, N-acetylcysteine has proven only marginally effective at reducing relapse in human trials (66–68). Given that astroglia engage two distinct processes for dampening cue-induced drug seeking and that increasing synaptic adjacency of astroglial processes in the absence of GLT-1 reduces the intensity of relapse, the poor efficacy in relapse prevention by drugs restoring GLT-1 might be improved in combination with drugs that promote synaptic adjacency of NAcore astroglia. Since ceftriaxone has been shown to both increase GLT-1 expression in NAcore astroglia and restore synaptic adjacency of astroglial processes (4, 5), this and other similar therapeutics may serve as promising candidates for treatment of relapse in substance use disorders.
MATERIALS AND METHODS
Experimental design and statistical analyses
Experimental procedures involving animals were conducted in accordance with guidelines established by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. All experiments included groups with ≥4 animals each to minimize variability due to animal behavior. All immunohistochemical and imaging procedures using tissue from experimental groups (i.e., extinguished, reinstated) were conducted alongside yoked controls to minimize the impact of experimental variability on independent groups. Data were analyzed using GraphPad Prism and a D’Agostino-Pearson normality test followed by Kruskal-Wallis or Mann-Whitney tests when one or more groups were not normally distributed. For non-Gaussian datasets, including all astroglial measures, no outliers were removed, and data were plotted with values for each cell depicted individually and a bar to indicate the group median. Dunn’s test was used for post hoc comparisons. Normally distributed datasets, including all behavioral data, were analyzed using a one- or two-way analysis of variance (ANOVA) or a Student’s t test. To more clearly show median differences in Fig. 6, y axes show data ≤2.0% coregistration. Full datasets for Fig. 6 are reported in table S1. Cumulative distributions were analyzed using Kolmogorov-Smirnov or chi-square, and a Bonferroni correction was applied for multiple comparisons. In all cases, P < 0.05 was considered significant.
Self-administration
Operant training was conducted as previously described (6). Briefly, male Sprague-Dawley rats or male and female Long Evans rats (200 to 250 g) were anesthetized with intramuscular ketamine (100 mg/kg) and xylazine (7 mg/kg) and fitted with intrajugular catheters. Rats were trained to self-administer heroin during 3-hour sessions for 10 days, and presses on an active lever were paired with light and tone cues and intravenous heroin infusion. Animals trained to self-administer sucrose did not undergo catheter implantation and received sucrose (45 mg, Bio-Serv) in place of heroin along with cues during self-administration (2 hours/day). Yoked controls were played cues when a paired rat received heroin or sucrose. Rats yoked to heroin self-administering animals also received intravenous saline infusions. After self-administration, animals underwent 10 to 12 days of extinction training (3 hours/day after heroin, 2 hours/day after sucrose) where active lever presses yielded no reward or cues. Extinguished rats and yoked controls were euthanized 24 hours after the final extinction session. Reinstated animals were placed in the operant chamber for 15 or 120 min, and cues were restored to the active lever, but no reward was delivered. At the start of each reinstatement session, animals were exposed to a single noncontingent delivery of the compound cue to initiate the reinstatement session, which lasted for 15 or 120 min from the initial cue exposure. Additional cue exposures were self-administered during the reinstatement session, and lever pressing plotted for each animal reflects the number of self-administered cues over the 15- or 120-min session.
Viral labeling
After catheter implantation or 5 days before starting sucrose self-administration, rats received microinjections (1 μl per hemisphere, 0.15 μl/min, 5-min diffusion) of a virus driving expression of membrane-targeted mCherry under control of the glial fibrillary acidic protein (GFAP) promoter [AAV5/GFAP-hM3dq-mCherry, University of Zurich (Figs. 1 to 5) or AAV5/GfaABC1D-Lck–green fluorescent protein (GFP), Addgene (Figs. 4C and 6)] in the NAcore [+1.5 mm anteroposterior (AP), ±1.8 mm mediolateral (ML), and −7.0 mm dorsoventral (DV)]. To label D1- and D2-MSN dendrites, AAV1/CAG-Flex-Ruby2sm-Flag (Addgene) was coinjected in D1- or D2-Cre rats along with virus used to label astroglia. Virus incubation occurred over the course of operant training.
Confocal imaging and image analysis
Animals were anesthetized with an overdose of pentobarbital [20 mg, intravenously, or 100 mg, intraperitoneally (ip)] and perfused transcardially with 4% paraformaldehyde (PFA). Brains were incubated overnight in 4% PFA and sliced at 100 μm using a vibratome (Thermo Fisher Scientific). Slices containing the NAcore were permeabilized in PBS with 2% Triton X-100 for 1 hour at room temperature. Nonspecific epitope binding was blocked by incubation in PBS with 0.2% Triton X-100 (PBST) and 2% normal goat serum (block) for 1 hour at room temperature before incubation in primary antibodies (rabbit anti–Synapsin I, ab64581, Abcam; guinea pig anti–GLT-1, ab1783, EMD Millipore; mouse anti-FLAG, F1804, Sigma-Aldrich) at 1:1000 in block for 48 hours. After washing in PBST, tissue was incubated overnight in biotinylated anti–guinea pig antibody (BA-7000, Vector Laboratories) in PBST and then overnight in fluorescently labeled antibodies (Thermo Fisher Scientific) in PBST after washing. Tissue was mounted onto glass slides before imaging with a Leica SP5 laser scanning confocal microscope. All images were acquired at 63× using an oil immersion objective lens, 1024 × 1024 frame size, 12-bit resolution, four-frame averaging, and a 1-μm step size. Z-stacks were iteratively deconvolved 10 times (Autoquant), and digital analysis of astroglial mCherry or GFP signal intensity relative to background was used to generate a digital model of each astrocyte (Bitplane Imaris). Rendered astrocytes were used to mask Synapsin I, GLT-1, and Flag signal that was not coregistered with the astroglial volume. Coregistration (astrocyte with Synapsin I, astrocyte with GLT-1, astrocyte with GLT-1 and Synapsin I, astrocyte with Flag and Synapsin I, and astrocyte with Flag and GLT-1) was determined on the basis of thresholded signal intensity in each channel. Voxels containing signal intensity greater than noise in each channel were determined empirically using the colocalization module and were used to build a colocalization channel. The surface module was then used to determine the net volume of coregistered signal. All measures, with the exception of surface-proximal GLT-1 and synaptic and extrasynaptic GLT-1, were normalized to the volume of the astrocyte from which they were generated to control for changes in astroglial volume (6) and to permit inclusion of astrocytes that were cropped along the z axis during imaging. Surface-proximal GLT-1 was determined by excluding coregistered signal that was within the astrocyte volume but >250 nm (a distance roughly equivalent to the lateral resolution of the microscope used for imaging) from the membrane and was normalized to total GLT-1 from the astroglial volume. Synaptic and extrasynaptic GLT-1 values were normalized to surface GLT-1 levels from each astrocyte. In all cases, imaging and analyses were conducted blind to animal treatment.
PCA and hierarchical clustering
To identify astroglial clusters according to synaptic coregistration as percentage of astrocyte volume and surface-proximal GLT-1 expression as percentage of total GLT-1 expression, hierarchical clustering on principal components was computed using R software (R Core Team, 2020) according to (26). PCA was first applied to provide common scaling across dimensions, despite no need for dimension reduction. Ward’s criterion was then applied on selected principal components, a process focused on minimizing variance within clusters, resulting in a total of three clusters as shown in fig. S3.
Gq-DREADD stimulation
AAV5/GFAP-hM3dq-mCherry (University of Zurich) was diluted 1:10 in sterile saline and delivered to the NAcore (+1.5 mm AP, ±1.8 mm ML, and −7.0 mm DV) while rats were under anesthesia for jugular catheter placement as in (44). This dilution was used because it produced sufficient astroglial labeling with little off-target labeling in neurons (Fig. 4A). To test for the effect of systemic CNO on astrocyte morphology, control animals received NAcore infusions of undiluted AAV5/GfaABC1D-Lck-GFP (Addgene). Viral incubation occurred over the course of operant training. Twenty-four hours after the last extinction session, rats received CNO (3 mg/kg, ip; Abcam) or vehicle (5% dimethyl sulfoxide in sterile saline) 30 min before perfusion and brain extraction, since intraperitoneal CNO 90 and 30 min before cue-induced reinstatement attenuates active lever pressing induced by drug-associated cues (44).
Vivo-morpholino knockdown
After catheter placement, animals were fitted with bilateral cannulae above the NAcore (+1.5 mm AP, ±1.8 mm ML, and −5.5 mm DV). Starting on day 6 of extinction training, animals received infusions of an ezrin antisense vivo-morpholino oligo (6), a GLT-1 antisense vivo-morpholino oligo (29), or a control oligo (Gene Tools Inc.) 1.5 mm beyond the base of the guide cannulae (50 μM, 1 μl per hemisphere, and 0.5 μl/min) for three consecutive days. After three additional days of extinction training, rats underwent cued reinstatement for 15 min before euthanasia.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (DA007288 and DA044782 to A.K.; DA003906 and DA012513 to P.W.K.) and the National Science Foundation (OIA 1539034, P.W.K.).
Author contributions: A.K. and P.W.K. designed and interpreted the study and wrote the paper. A.K. conducted experiments with assistance from A.A. and C.G.-K. H.L. performed PCA on astrocyte data generated by A.K.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Table S1
REFERENCES AND NOTES
- 1.Kalivas P. W., The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 10, 561–572 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Lehre K. P., Danbolt N. C., The number of glutamate transporter subtype molecules at glutamatergic synapses: Chemical and stereological quantification in young adult rat brain. J. Neurosci. 18, 8751–8757 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danbolt N. C., Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Roberts-Wolfe D. J., Kalivas P. W., Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol. Disord. Drug Targets 14, 745–756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scofield M. D., Li H., Siemsen B. M., Healey K. L., Tran P. K., Woronoff N., Boger H. A., Kalivas P. W., Reissner K. J., Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biol. Psychiatry 80, 207–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruyer A., Scofield M. D., Wood D., Reissner K. J., Kalivas P. W., Heroin cue-evoked astrocytic structural plasticity at nucleus accumbens synapses inhibits heroin seeking. Biol. Psychiatry 86, 811–819 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siemsen B. M., Reichel C. M., Leong K. C., Garcia-Keller C., Gipson C. D., Spencer S., McFaddin J. A., Hooker K. N., Kalivas P. W., Scofield M. D., Effects of methamphetamine self-administration and extinction on astrocyte structure and function in the nucleus accumbens core. Neuroscience 406, 528–541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gipson C. D., Kupchik Y. M., Kalivas P. W., Rapid, transient synaptic plasticity in addiction. Neuropharmacology 76 Pt B, 276–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavialle M., Aumann G., Anlauf E., Pröls F., Arpin M., Derouiche A., Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 12915–12919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerfen C. R., Surmeier D. J., Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinsbroek J. A., Neuhofer D. N., Griffin W. C. III, Siegel G. S., Bobadilla A. C., Kupchik Y. M., Kalivas P. W., Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J. Neurosci. 37, 757–767 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobadilla A. C., Dereschewitz E., Vaccaro L., Heinsbroek J. A., Scofield M. D., Kalivas P. W., Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol. Psychiatry 25, 3150–3163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuber G. D., Sparta D. R., Stamatakis A. M., van Leeuwen W. A., Hardjoprajitno J. E., Cho S., Tye K. M., Kempadoo K. A., Zhang F., Deisseroth K., Bonci A., Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kravitz A. V., Tye L. D., Kreitzer A. C., Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi K., Yoshida S., Hatano R., Asano S., Pathophysiological roles of ezrin/radixin/moesin proteins. Biol. Pharm. Bull. 40, 381–390 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Derouiche A., Geiger K. D., Perspectives for ezrin and radixin in astrocytes: Kinases, functions and pathology. Int. J. Mol. Sci. 20, 3776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautreau A., Louvard D., Arpin M., Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J. Cell Biol. 150, 193–204 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derouiche A., Frotscher M., Peripheral astrocyte processes: Monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36, 330–341 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Cavaccini A., Durkee C., Kofuji P., Tonini R., Araque A., Astrocyte signaling gates long-term depression at corticostriatal synapses of the direct pathway. J. Neurosci. 40, 5757–5768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durkee C., Kofuji P., Navarrete M., Araque A., Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 44, 837–848 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruyer A., Dixon D., Angelis A., Amato D., Kalivas P. W., Astrocytes in the ventral pallidum extinguish heroin seeking through GAT-3 upregulation and morphological plasticity at D1-MSN terminals. Mol. Psychiatry 27, 855–864 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter B. L., Tiffany S. T., Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340 (1999). [PubMed] [Google Scholar]
- 23.Back S. E., Hartwell K., DeSantis S. M., Saladin M., McRae-Clark A. L., Price K. L., Moran-Santa Maria M. M., Baker N. L., Spratt E., Kreek M. J., Brady K. T., Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 106, 21–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Li W., Wang H., Wang Y., Zhang Y., Zhu J., Zheng Y., Zhang D., Wang L., Li Y., Yan X., Chang H., Fan M., Li Z., Tian J., Gold M. S., Wang W., Liu Y., Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: An event-related functional magnetic resonance imaging study. Addict. Biol. 20, 968–978 (2015). [DOI] [PubMed] [Google Scholar]
- 25.LaLumiere R. T., Kalivas P. W., Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J. Neurosci. 28, 3170–3177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.F. Husson, J. Josse, J. Pages, Principal Component Methods-Hierarchical Clustering-Partitional Clustering: Why Would We Need to Choose for Visualizing Data (Applied Mathematics Department, 2010), vol. 17.
- 27.Shen H. W., Scofield M. D., Boger H., Hensley M., Kalivas P. W., Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J. Neurosci. 34, 5649–5657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Ascenzo M., Fellin T., Terunuma M., Revilla-Sanchez R., Meaney D. F., Auberson Y. P., Moss S. J., Haydon P. G., mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 104, 1995–2000 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reissner K. J., Sartor G. C., Vazey E. M., Dunn T. E., Aston-Jones G., Kalivas P. W., Use of vivo-morpholinos for control of protein expression in the adult rat brain. J. Neurosci. Methods 203, 354–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nall R. W., Heinsbroek J. A., Nentwig T. B., Kalivas P. W., Bobadilla A. C., Circuit selectivity in drug versus natural reward seeking behaviors. J. Neurochem. 157, 1450–1472 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts-Wolfe D., Bobadilla A. C., Heinsbroek J. A., Neuhofer D., Kalivas P. W., Drug refraining and seeking potentiate synapses on distinct populations of accumbens medium spiny neurons. J. Neurosci. 38, 7100–7107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin R., Bajo-Graneras R., Moratalla R., Perea G., Araque A., Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Cholet N., Pellerin L., Magistretti P. J., Hamel E., Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb. Cortex 12, 515–525 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Minelli A., Barbaresi P., Reimer R. J., Edwards R. H., Conti F., The glial glutamate transporter GLT-1 is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience 108, 51–59 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Michaluk P., Heller J. P., Rusakov D. A., Rapid recycling of glutamate transporters on the astroglial surface. eLife 10, e64714 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy-Royal C., Dupuis J. P., Varela J. A., Panatier A., Pinson B., Baufreton J., Groc L., Oliet S. H. R., Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 18, 219–226 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Shen H., Moussawi K., Zhou W., Toda S., Kalivas P. W., Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 19407–19412 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heller J. P., Rusakov D. A., Morphological plasticity of astroglia: Understanding synaptic microenvironment. Glia 63, 2133–2151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dvorzhak A., Helassa N., Torok K., Schmitz D., Grantyn R., Single synapse indicators of impaired glutamate clearance derived from fast iGlu u imaging of cortical afferents in the striatum of normal and huntington (Q175) mice. J. Neurosci. 39, 3970–3982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehre K. P., Rusakov D. A., Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys. J. 83, 125–134 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietrich D., Kral T., Clusmann H., Friedl M., Schramm J., Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology 42, 297–305 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Bossert J. M., Gray S. M., Lu L., Shaham Y., Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31, 2197–2209 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters J., Kalivas P. W., The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 186, 143–149 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Scofield M. D., Boger H. A., Smith R. J., Li H., Haydon P. G., Kalivas P. W., Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol. Psychiatry 78, 441–451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henneberger C., Bard L., Panatier A., Reynolds J. P., Kopach O., Medvedev N. I., Minge D., Herde M. K., Anders S., Kraev I., Heller J. P., Rama S., Zheng K., Jensen T. P., Sanchez-Romero I., Jackson C. J., Janovjak H., Ottersen O. P., Nagelhus E. A., Oliet S. H. R., Stewart M. G., Nägerl U. V., Rusakov D. A., LTP induction boosts glutamate spillover by driving withdrawal of perisynaptic astroglia. Neuron 108, 919–936.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith A. C. W., Kupchik Y. M., Scofield M. D., Gipson C. D., Wiggins A., Thomas C. A., Kalivas P. W., Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat. Neurosci. 17, 1655–1657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith A. C. W., Scofield M. D., Heinsbroek J. A., Gipson C. D., Neuhofer D., Roberts-Wolfe D. J., Spencer S., Garcia-Keller C., Stankeviciute N. M., Smith R. J., Allen N. P., Lorang M. R., Griffin W. C. III, Boger H. A., Kalivas P. W., Accumbens nNOS interneurons regulate cocaine relapse. J. Neurosci. 37, 742–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai H., Diaz-Castro B., Shigetomi E., Monte E., Octeau J. C., Yu X., Cohn W., Rajendran P. S., Vondriska T. M., Whitelegge J. P., Coppola G., Khakh B. S., Neural circuit-specialized astrocytes: Transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aronica E., Gorter J. A., Ijlst-Keizers H., Rozemuller A. J., Yankaya B., Leenstra S., Troost D., Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: Opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 17, 2106–2118 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Chioma V. C., Kruyer A., Bobadilla A.-C., Angelis A., Ellison Z., Hodebourg R., Scofield M. D., Kalivas P. W., Heroin seeking and extinction from seeking activate matrix metalloproteinases at synapses on distinct subpopulations of accumbens cells. Biol. Psychiatry 89, 947–958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Keller C., Scofield M. D., Neuhofer D., Varanasi S., Reeves M. T., Hughes B., Anderson E., Richie C. T., Mejias-Aponte C., Pickel J., Hope B. T., Harvey B. K., Cowan C. W., Kalivas P. W., Relapse-associated transient synaptic potentiation requires integrin-mediated activation of focal adhesion kinase and cofilin in D1-expressing neurons. J. Neurosci. 40, 8463–8477 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bobadilla A. C., Heinsbroek J. A., Gipson C. D., Griffin W. C., Fowler C. D., Kenny P. J., Kalivas P. W., Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Prog. Brain Res. 235, 93–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruyer A., Parrilla-Carrero J., Powell C., Brandt L., Gutwinski S., Angelis A., Chalhoub R. M., Jhou T. C., Kalivas P. W., Amato D., Accumbens D2-MSN hyperactivity drives antipsychotic-induced behavioral supersensitivity. Mol. Psychiatry 26, 6159–6169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soares-Cunha C., Coimbra B., David-Pereira A., Borges S., Pinto L., Costa P., Sousa N., Rodrigues A. J., Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 7, 11829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares-Cunha C., de Vasconcelos N. A. P., Coimbra B., Domingues A. V., Silva J. M., Loureiro-Campos E., Gaspar R., Sotiropoulos I., Sousa N., Rodrigues A. J., Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatry 25, 3241–3255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Y., Gao G., Liu K., Shi X., Cheng M., Xiong Y., Song S., Projections from D2 neurons in different subregions of nucleus accumbens shell to ventral pallidum play distinct roles in reward and aversion. Neurosci. Bull. 37, 623–640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalivas P. W., Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox. Res. 14, 185–189 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Deroche M. A., Lassalle O., Castell L., Valjent E., Manzoni O. J., Cell-type- and endocannabinoid-specific synapse connectivity in the adult nucleus accumbens core. J. Neurosci. 40, 1028–1041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alasmari F., Bell R. L., Rao P. S. S., Hammad A. M., Sari Y., Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol. Biochem. Behav. 170, 44–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Fardon R., Weiss F., Perseveration of craving: Effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict. Biol. 22, 923–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Testen A., Sepulveda-Orengo M. T., Gaines C. H., Reissner K. J., Region-specific reductions in morphometric properties and synaptic colocalization of astrocytes following cocaine self-administration and extinction. Front. Cell. Neurosci. 12, 246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knackstedt L. A., Melendez R. I., Kalivas P. W., Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry 67, 81–84 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reissner K. J., Gipson C. D., Tran P. K., Knackstedt L. A., Scofield M. D., Kalivas P. W., Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 20, 316–323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou W., Kalivas P. W., N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry 63, 338–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duailibi M. S., Cordeiro Q., Brietzke E., Ribeiro M., LaRowe S., Berk M., Trevizol A. P., N-acetylcysteine in the treatment of craving in substance use disorders: Systematic review and meta-analysis. Am. J. Addict. 26, 660–666 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Woodcock E. A., Lundahl L. H., Khatib D., Stanley J. A., Greenwald M. K., N-acetylcysteine reduces cocaine-seeking behavior and anterior cingulate glutamate/glutamine levels among cocaine-dependent individuals. Addict. Biol. 26, e12900 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray K. M., Carpenter M. J., Baker N. L., DeSantis S. M., Kryway E., Hartwell K. J., McRae-Clark A. L., Brady K. T., A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am. J. Psychiatry 169, 805–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amen S. L., Piacentine L. B., Ahmad M. E., Li S. J., Mantsch J. R., Risinger R. C., Baker D. A., Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36, 871–878 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Table S1