Abstract
Purpose:
This study investigated the efficacy and tolerability of cabozantinib plus nivolumab (CaboNivo) in patients with metastatic urothelial carcinoma (mUC) that progressed on checkpoint inhibition (CPI).
Patients and Methods:
A phase I expansion cohort of patients with mUC who received prior CPI was treated with cabozantinib 40 mg/day and nivolumab 3 mg/kg every 2 weeks until disease progression/unacceptable toxicity. The primary goal was objective response rate (ORR) per RECIST v.1.1. Secondary objectives included progression-free survival (PFS), duration of response (DoR), overall survival (OS), safety, and tolerability.
Results:
Twenty-nine out of 30 patients enrolled were evaluable for efficacy. Median follow-up was 22.2 months. Most patients (86.7%) received prior chemotherapy and all patients received prior CPI (median seven cycles). ORR was 16.0%, with one complete response and three partial responses (PR). Among 4 responders, 2 were primary refractory, 1 had a PR, and 1 had stable disease on prior CPI. Median DoR was 33.5 months [95% confidence interval (CI), 3.7–33.5], median PFS was 3.6 months (95% CI, 2.1–5.5), and median OS was 10.4 months (95% CI, 5.8–19.5). CaboNivo decreased immunosuppressive subsets such as regulatory T cells (Tregs) and increased potential antitumor immune subsets such as nonclassical monocytes and effector T cells. A lower percentage of monocytic myeloid-derived suppressor cells (M-MDSC) and polymorphonuclear MDSCs, lower CTLA-4 and TIM-3 expression on Tregs, and higher effector CD4+ T cells at baseline were associated with better PFS and/or OS.
Conclusions:
CaboNivo was clinically active, well tolerated, and favorably modulated peripheral blood immune subsets in patients with mUC refractory to CPI.
Translational Relevance.
Checkpoint inhibition (CPI) is demonstrating efficacy in earlier states of disease in urothelial carcinoma, including nonmuscle-invasive and muscle-invasive bladder cancer. Developing effective combination strategies with CPI that may overcome resistance post-monotherapy will be crucial to offering patients options after disease progression. This early study of CaboNivo post-CPI offers an exciting signal to pursue in larger studies.
Introduction
Metastatic urothelial carcinoma (mUC), a highly aggressive disease, has a high incidence in the United States (1). Recently, treatment options for mUC have expanded considerably, with the FDA's approval of checkpoint inhibitors (CPI) for patients who have progressed on platinum-based chemotherapy (2), as first-line treatment for cisplatin-ineligible patients with high PD-L1 expression or patients ineligible for any platinum-based chemotherapy (2, 3), and as a maintenance treatment after platinum-based chemotherapy (4). Recently, enfortumab vedotin, sacituzumab govitecan, and erdafitinib have also been approved on the basis of clinical activity in patients with mUC (5–7).
Some patients treated with CPIs may achieve durable responses that are rarely seen with other types of agents (8). This is encouraging for both oncologists and patients; however, most patients will not respond to CPIs or will develop resistance at some point during treatment. Strategies to avoid primary resistance or rescue patients who have progressed on CPIs have been extensively studied. One strategy is to combine a CPI with another agent that can induce alterations in the tumor microenvironment (TME) that ultimately act synergistically to overcome mechanisms of immune evasion.
VEGFR-targeted therapies have been studied as potential synergistic agents with CPIs. Cabozantinib is a tyrosine kinase inhibitor (TKI) targeting MET, VEGFR-2, RET, AXL, KIT, and TIE-2 (8). Preclinical studies have demonstrated that anti-VEGFR agents can induce changes in the TME by reducing immunosuppressive cells, increasing T-cell infiltration, and activating effector T cells (9). Preclinical models have also demonstrated the immunomodulatory effects of cabozantinib (10).
Combining cabozantinib with a CPI has had encouraging results in clinical trials in patients with mUC (11). Our group conducted a phase I trial with expansion cohorts evaluating the safety and clinical activity of cabozantinib and nivolumab (CaboNivo) with or without ipilimumab (CaboNivoIpi) in 54 CPI-naïve patients with metastatic genitourinary tumors. CaboNivo and CaboNivoIpi had an acceptable toxicity profile, and the recommended phase II doses were cabozantinib 40 mg, nivolumab 3 mg/kg, and ipilimumab 1 mg/kg. The overall objective response rate (ORR) was 30.6% for the entire population and 38.5% for the 15 patients with mUC (11). Here we report the results of an expansion cohort of our phase I trial evaluating CaboNivo in patients with mUC refractory to prior CPI therapy.
Patients and Methods
Patient selection
Eligible patients had a histologically confirmed diagnosis of mUC. Patients must have progressed on ≥1 line of standard therapy and on a previous CPI. We considered previous CPI to be treatment with any anti-PD-1, anti-PD-L1, and anti-cytotoxic T-lymphocyte–associated antigen 4 (CTLA4). Responses to previous standard therapy and previous CPI were reviewed and determined by the investigator according to RECIST v.1.1 (12). Other inclusion and exclusion criteria were reported previously (11).
Study design and outcomes
This was a multicenter, open-label, phase I expansion cohort of CaboNivo in patients with mUC who progressed on prior CPI therapy. Planned enrollment was 30 patients. The objective was to determine the clinical activity and safety of CaboNivo in this population. The primary endpoint was ORR, defined as the proportion of patients with either complete response (CR) or partial response (PR), based on investigator-assessed RECIST v.1.1 (12). Secondary endpoints included progression-free survival (PFS), overall survival (OS), duration of response (DoR), disease control rate [DCR; proportion of patients with a confirmed best response of CR, PR, or stable disease (SD)], and safety and toxicity as per the NCI's Common Terminology Criteria for Adverse Events v.5.0. Exploratory analyses included PFS and OS according to peripheral blood immune subsets, inflammatory cytokines, and circulating tumor cells (CTC).
Treatment
Patients received cabozantinib 40 mg orally every day and nivolumab 3 mg/kg intravenously every 2 weeks in 28-day cycles, as described previously (11). After cycle 21, patients received a maintenance dose of nivolumab (480 mg every 4 weeks) and daily cabozantinib. Treatment was continued until disease progression or unacceptable toxicity. Patients had the option of discontinuing therapy after 2 years of confirmed CR or PR. Restaging scans included computed tomography (CT) of chest, abdomen, and pelvis or CT of chest and magnetic resonance imaging of abdomen and pelvis at baseline and every two cycles (every 8 weeks). Patients with bone metastasis had their tumor response assessed by 18F-sodium fluoride PET/CT (13).
Dose reductions for cabozantinib (initially to 20 mg, then to 20 mg every other day) and interruptions of study treatment were specified for the management of adverse events. No dose modification was allowed for nivolumab. Patients could discontinue treatment for disease progression, unacceptable toxicity, withdrawal of consent, or per investigator's clinical judgment. Treatment beyond disease progression was permitted if patients tolerated treatment and the investigator considered that the patient would benefit clinically.
Immune subset analyses
Peripheral immune subsets were analyzed at baseline and before cycle 2 day 1 (C2D1) and C3D1. Peripheral blood samples were collected in Cell Preparation Tubes with sodium citrate (BD Vacutainer CPT Tubes; BD Biosciences). Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation and viably frozen until analysis. Multiparameter flow cytometric analysis was performed on PBMCs as described previously (14). Cells were incubated with Fc receptor blocking agent (Miltenyi Biotec) and stained for 20 to 30 minutes at 4°C in a dark room with mAbs.
The following immunophenotypic markers were used to define immune subsets: classical monocytes: CD14+ CD16−; intermediate monocytes: CD14+ CD16+; nonclassical monocytes: CD14dim CD16+; PMN-MDSC: CD14− CD11b+ CD15+; M-MDSC: CD14+ CD11b+ HLA–DRlow/– CD15–; CD1c+ myeloid DC (mDC): lineage (CD3, CD19, CD56)−HLA-DR+CD11c+CD1c+; CD141+ mDC: lineage−HLA–DR+CD11c+CD141+; CD303+ plasmacytoid DC (pDC): lineage−HLA–DR+CD11c+CD303+; Tregs: CD8−CD4+CD25highFoxp3+; effector Tregs (eTregs): CD8-CD4+CD45RA-Foxp3high; naïve Tregs (nTregs): CD8-CD4+CD45RA+Foxp3+; naïve T cells: CD45RA+CCR7+ CD28+ CD27+; effector T cells: CD45RA+CCR7− CD28− CD27−; EM1 T cells: CD45RA–CCR7− CD28+ CD27+ CD3+ (CD4+ or CD8+); EM2 T cells: CD45RA–CCR7– CD28– CD27+ CD3+ (CD4+ or CD8+); EM3 T cells: CD45RA-CCR7– CD28– CD27– CD3+ (CD4+ or CD8+); EM4 T cells: CD45RA–CCR7− CD28+ CD27− CD3+ (CD4+ or CD8+); and CM T cells: CD45RA–CCR7+ CD28+ CD27+ CD3+ (CD4+ or CD8+). The following mAbs were used: CD14 clone HCD14, CD16 clone 3G8, HLA-DR clone LN3, CD3 clone OKT3, CD56 clone MEM-188, CD19 clone HIB19, CD11b clone ICRF44, CD15 clone W6D3, CD33 clone WM53, CD11c clone Bu15, CD1c clone L161, CD141 clone M80, CD303 clone 201A, CD83 clone HB15e. CD8 clone SK1, CD4 clone RPAT4, CD25 clone BC96, Foxp3 clone 206D, PD-1 clone EH12.2H7, CTLA-4 clone L3D10, TIM-3 clone F38−2E2 ICOS clone C398.4A, CD45RA clone HI100, CCR7 clone G043H7, CD27 clone LG.3A10, and CD28 clone CD28.2 (BioLegend) and Ki67 clone B56 (BD Biosciences).
For analysis of Foxp3 and Ki67 expression, cells were fixed and permeabilized using a Fix/Perm buffer (eBioscience) according to the manufacturer's instructions, then stained with anti-Foxp3 or anti-Ki67 antibody.
Live cells were discriminated by means of LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies) and dead cells were excluded from all analyses. All flow cytometric analyses were performed using a MACSQuant Analyzer (Miltenyi Biotec). Flow cytometric data were quantified either as the median fluorescence intensity or as a percentage of cells, as indicated. Data were analyzed using FlowJo software version 10.6.1. (FlowJo, LLC).
Circulating tumor cell measurement
CTC count per 10 mL of whole blood was assessed as described previously (11, 15).
Cytokine and angiogenic factor analyses
Plasma samples were collected in EDTA plasma tubes at baseline and before C2D1 and C3D1. The samples were processed within 4 hours and stored at −80°C until analysis. The angiogenic cytokines and proinflammatory cytokines were tested using clinically validated ECL immunoassays from Meso-Scale Diagnostics, as described previously (16).
Statistical analysis
A total of 30 patients (15 each in 2 replicate cohorts) were to be enrolled in an expansion cohort of UC patients who had prior CPI therapy. The goal was to determine if the fraction of patients who achieve a response is more likely to be consistent with 25% than with 5%. If 15 patients were to enroll in an expansion cohort, then if ≥ 2 of the 15 experienced a response, the probability of this occurring is 17.1% if the probability of a response was 5%, and the probability of this occurring would be 92.0% if the probability of response was 25%. Thus, obtaining ≥2 patients with a response out of 15 would be adequate to consider the treatment regimen to be successful in this small cohort. At the end of the trial, the actual fraction of responses out of the total evaluable patients enrolled in this cohort was determined along with 80% and 95% two-sided confidence bounds to interpret the results. On the basis of the original cohort of 15 patients and a replicate cohort of 15, the results from the two cohorts were combined and reported together.
Follow-up was calculated as the median of the potential follow-up intervals for each patient from the on-study date until the cutoff date of August 15, 2020. ORR was estimated using a 95% Clopper–Pearson confidence interval (CI). PFS and OS were estimated using the Kaplan−Meier method. PFS was calculated from the on-study date until date of progression, death without progression, or last follow-up. OS was calculated from on-study date until the date of death or last follow-up. DoR was calculated from the date a response was first noted until the date of radiologic progression, clinical progression, or death. The safety and clinical activity of CaboNivo were analyzed in all patients who received at least one dose of the treatment drugs.
For immune subset analysis, we estimated PFS and OS using the Kaplan–Meier method and reported two-tailed, unadjusted log-rank P values. Analyses based on baseline values began at baseline, whereas those based on C3D1 began at C3D1. A cutoff of five CTCs/sample was used, as described previously (14). The statistical significance in changes of cytokines was determined with a paired Wilcoxon t test.
Exploratory analyses were performed to establish an association between immune subsets/functional markers and PFS or OS. The prognostic values of cytokines on PFS and OS were determined with Kaplan−Meier survival analysis for which the P values of log-rank tests were shown. The median levels of each analyte were used as the cutoff values for the high and low groups. Biomarker analyses were exploratory and performed in all patients when a baseline sample was available. Biomarker changes from baseline to C2D1 pre and C3D1 pre were compared using the Wilcoxon signed-rank test.
Data availability statement
The data generated in this study are available upon request from the corresponding author.
Results
Patient characteristics
Thirty patients were enrolled in this study, one of whom never started treatment and therefore was not evaluable for response or safety. At data cutoff, the median follow-up was 22.2 months. Baseline characteristics of the 30 patients are summarized in Table 1.
Table 1.
Baseline characteristics.
| Characteristics | n = 30 |
|---|---|
| Median age (range), years | 64.5 (47–80) |
| Gender, n (%) | |
| Male | 22 (73.3) |
| Female | 8 (26.7) |
| Race, n (%) | |
| White | 28 (93.3) |
| Asian | 2 (6.7) |
| Black/African American | 0 (00) |
| KPS, n (%) | |
| 100% | 2 (6.7) |
| 90% | 14 (46.7) |
| 80% | 13 (43.3) |
| 70% | 1 (3.3) |
| Primary tumor, n (%) | |
| Bladder | 22 (73.3) |
| Upper tract | 8 (26.7) |
| Metastasis location | |
| Lymph node only | 4 (13.8) |
| Viscera | 23 (76.7) |
| Liver | 13 (44.8) |
| Median number of prior treatments (range) | 2 (0–8) |
| Number of prior treatments, n (%) | |
| 0 | 1 (3.3) |
| 1 | 3 (10.0) |
| 2 | 18 (60.0) |
| 3 | 6 (20.0) |
| >3 | 2 (6.7) |
| Prior chemotherapy for metastatic disease, n (%) | |
| Yes | 26 (86.7) |
| No | 4 (13.3) |
| Number of cycles of prior immunotherapy, median (range) | 7 (1–20) |
| Time between prior immunotherapy and start of CaboNivo treatment, median (range), months | 2.25 (1–18) |
| Best response to prior immunotherapy, n (%) | |
| Complete response | 0 |
| Partial response | 1 (3.3) |
| Stable disease | 13 (43.4) |
| Progressive disease | 16 (53.3) |
| Type of prior immunotherapy | |
| Pembrolizumab | 22 (73.3) |
| Atezolizumab | 8 (26.7) |
The median number of prior lines of treatment was 2; 26 patients (86.7%) had ≥2 previous lines of treatment. Most patients (86.7%) had received chemotherapy for metastatic disease and all patients had received prior CPI. One patient received chemotherapy as neoadjuvant treatment and went on off-label adjuvant pembrolizumab and enrolled in our study after disease progression. The median number of cycles of prior immunotherapy was 7 (range, 1–20). Best responses to initial treatment with other CPIs were 0 CR, 1 (3.3%) PR, 13 (43.4%) SD, and 16 (53.3%) with progressive disease (PD). Twenty-two patients (73.3%) had been previously treated with pembrolizumab and 8 (26.7%) had been treated with atezolizumab. Median time between the end of previous CPI therapy and start of CaboNivo was 2.25 months (range, 1–18 months).
Efficacy
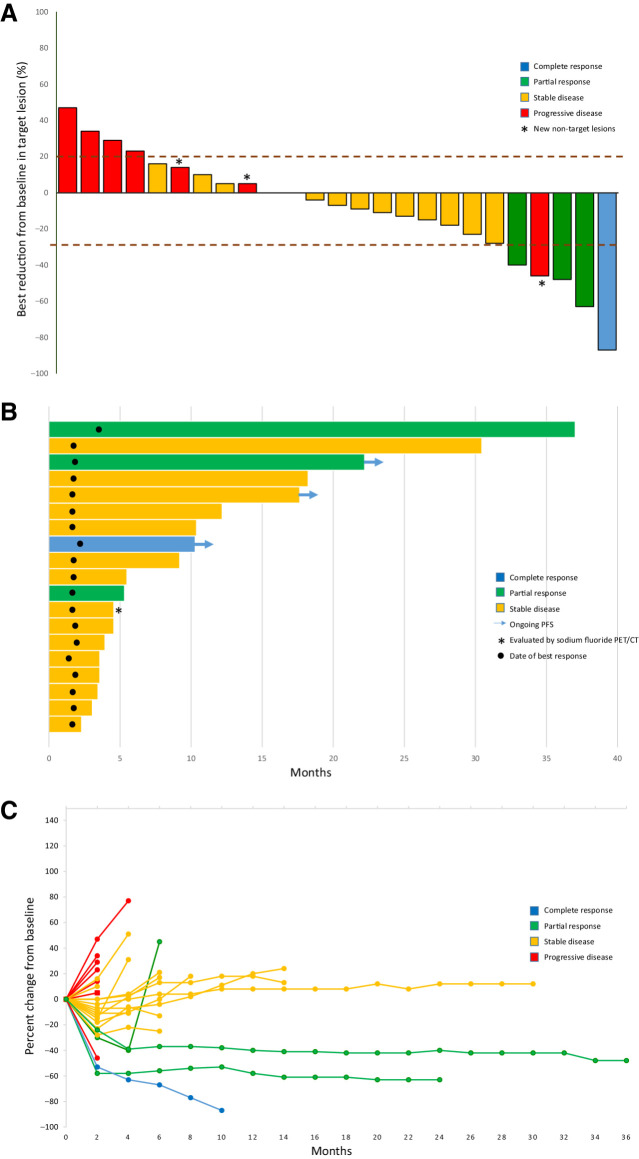
Twenty-nine patients received at least one dose of CaboNivo and were evaluable for efficacy. Four patients (13.8%) were not evaluable for response by RECIST. Two died before the first restaging at 8 weeks, one was off study prior to the first restaging per physician discretion, and another had bone-only disease that was not measurable by RECIST. This last patient was evaluated by sodium fluoride PET/CT (17) and had SD as best response. Among the patients who were evaluable by RECIST v.1.1 (n = 25), the ORR was 16.0% (95% CI, 4.5–36.1). However, conservatively including all 29 patients who received at least one dose of CaboNivo, including those not evaluable by RECIST v.1.1, 4 of the 29 (13.8%) experienced a response (95% CI, 3.9–31.7%). One patient (4.0%) achieved a CR, 3 patients (12.0%) had a PR, 14 patients (56.0%) had SD as best response, and 7 patients (28.0%) had PD (Table 2; Fig. 1A). The DCR was 72% (18/25 patients).
Table 2.
Objective tumor response per RECIST v.1.1.
| Response | n = 25 |
|---|---|
| Overall response rate, n (%) | 4 (16) |
| Best overall response rate, n (%) | |
| Complete response | 1 (4.0) |
| Partial response | 3 (12.0) |
| Stable disease | 14 (56.0) |
| Progressive disease | 7 (28.0) |
Figure 1.
Clinical activity of CaboNivo. A, Plot of confirmed tumor regression from baseline as measured by RECIST in all evaluable patients (n = 25). Upper dotted line represents progression at 20%; lower dotted line represents the RECIST boundary for complete response or partial response at 30%. Red = progressive disease, yellow = stable disease, green = partial response, and blue = complete response. Asterisk represents patients whose scans revealed new nontarget lesions that were considered progression. B, Time to response, duration of treatment, and duration of response to CaboNivo in months for evaluable patients with complete response (blue), partial response (green), and stable disease (yellow; n = 19). Arrows = patients with ongoing response at cutoff date. Circle = date best response was first noted. Asterisk = patient who had no evaluable lesions by RECIST but was considered to have stable disease by sodium fluoride PET/CT. C, Percent change in sum of target lesion diameters from baseline over time for all assessable patients (n = 25), defined as those patients with baseline tumor assessments and at least one post-baseline assessment. Colors represent patients' best response according to RECIST v.1.1. Red = progressive disease as best response, yellow = stable disease, green = partial response, and blue = complete response.
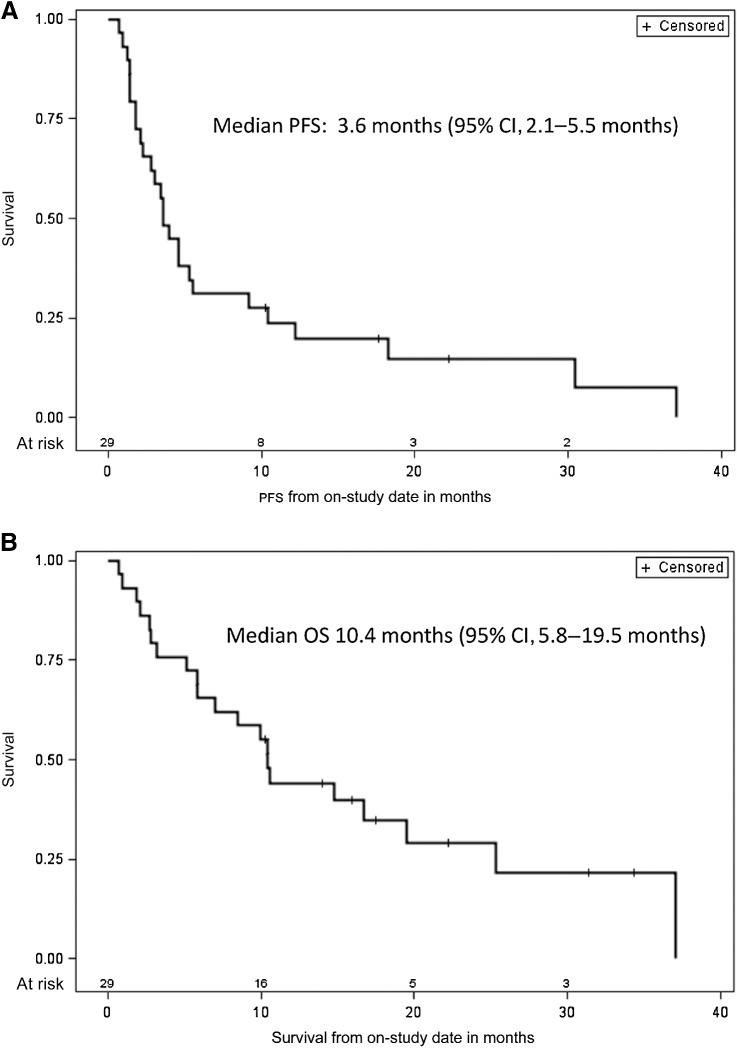
The median PFS was 3.6 months (95% CI, 2.1–5.5 months) and the median OS was 10.4 months (95% CI, 5.8–19.5 months; Fig. 2A and B). Among 15 patients with SD, 6 had PFS for ≥ 6 months, 1 had ongoing PFS at 17.7 months, and 5 had SD that ended at 9.2, 10.4, 12.2, 18.2, and 30.4 months, respectively (Fig. 1B). At data cutoff, the median DoR for 4 patients with a CR or PR was 33.5 months (95% CI, 3.7–33.5 months). Among these 4, one response ended at 3.7 months, 2 responses were still ongoing at 8.1 and 20.4 months, and 1 patient died from a procedure at 33.5 months, without progression (Fig. 1B).
Figure 2.
Kaplan–Meier estimate of overall survival (A) and progression-free survival (B) for overall study population (n = 29). Vertical lines show censored events.
The patient who achieved a CR with CaboNivo previously had SD as best response to CPI. Among the 3 patients who had a PR with CaboNivo, one had a PR and 2 had PD as best response to prior CPI. Responses to CaboNivo in these patients were seen in lung metastasis in 2 patients, liver and bone metastasis in another patient, and 1 patient with lymph node–only metastasis (Supplementary Fig. S1).
Safety and tolerability
Treatment-related adverse events (TRAE) are summarized in Table 3. Twenty-eight patients (97%) had a grade 1 TRAE, 23 (79%) had a grade 2, 15 (52%) had a grade 3, and 1 (3%) had a grade 4 TRAE. There were no grade 5 TRAEs. Five patients (17%) had grade 1 immune-related adverse events, 6 (21%) had a grade 2, and 1 (3%) had a grade 3. Five patients required steroids. Three patients were treated with systemic steroids for rash, hepatitis, and colitis. One was treated with topical steroids (rash) and one received systemic steroids as replacement due to immune-related adrenal insufficiency.
Table 3.
Treatment-related AEs.
| n = 29 | ||||
|---|---|---|---|---|
| AEs | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Laboratory | ||||
| Hematology | ||||
| Platelet count decreased | 11 (38) | 1 (3) | 0 | 0 |
| Anemia | 9 (31) | 3 (10) | 0 | 0 |
| White blood cell count decreased | 7 (24) | 5 (17) | 0 | 0 |
| Neutrophil count decreased | 3 (10) | 3 (10) | 0 | 0 |
| Lymphocyte count decreased | 2 (7) | 3 (10) | 3 (10) | 1 (3) |
| Electrolytes | ||||
| Hypomagnesemia | 6 (21) | 2 (7) | 0 | 0 |
| Hypocalcemia | 5 (17) | 1 (3) | 0 | 0 |
| Hyponatremia | 4 (14) | 1 (3) | 0 | 0 |
| Hypophosphatemia | 2 (7) | 8 (28) | 3 (10) | 0 |
| Hypokalemia | 1 (3) | 2 (7) | 0 | 0 |
| Renal | ||||
| Acute kidney injury | 9 (31) | 1 (3) | 1 (3) | 0 |
| Proteinuria | 0 | 2 (7) | 1 (3) | 0 |
| Hepatic | ||||
| Aspartate aminotransferase increased | 7 (24) | 0 | 1 (3) | 0 |
| Alanine aminotransferase increased | 4 (14) | 3 (10) | 0 | 0 |
| Alkaline phosphatase increased | 5 (17) | 1 (3) | 1 (3) | 0 |
| GGT increased | 2 (7) | 2 (7) | 0 | 0 |
| INR increased | 1 (3) | 0 | 0 | 0 |
| Activated partial thromboplastin time prolonged | 2 (7) | 0 | 0 | 0 |
| Pancreatic | ||||
| Lipase increased | 2 (7) | 2 (7) | 1 (3) | 0 |
| Amylase increased | 2 (7) | 0 | 2 (7) | 0 |
| Endocrine | ||||
| Hypothyroidism | 5 (17) | 5 (17) | 0 | 0 |
| Hyperthyroidism | 1 (3) | 0 | 0 | 0 |
| Clinical AEs | ||||
| Diarrhea | 7 (24) | 3 (10) | 2 (7) | 0 |
| Fatigue | 7 (24) | 6 (21) | 3 (10) | 0 |
| Nausea | 7 (24) | 5 (17) | 1 (3) | 0 |
| Weight loss | 7 (24) | 1 (3) | 0 | 0 |
| Anorexia | 6 (21) | 5 (17) | 1 (3) | 0 |
| Dysgeusia | 6 (21) | 3 (10) | 0 | 0 |
| Rash | 4 (14) | 0 | 0 | 0 |
| Dizziness | 3 (10) | 0 | 0 | 0 |
| Headache | 3 (10) | 0 | 0 | 0 |
| Hypertension | 3 (10) | 3 (10) | 2 (7) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 3 (10) | 5 (17) | 0 | 0 |
| Vomiting | 3 (10) | 4 (14) | 1 (3) | 0 |
| Mucositis oral | 2 (7) | 4 (14) | 0 | 0 |
| Paresthesia | 2 (7) | 0 | 0 | 0 |
| Pruritus | 2 (7) | 0 | 0 | 0 |
| Sore throat | 2 (7) | 0 | 0 | 0 |
| Constipation | 1 (3) | 1 (3) | 0 | 0 |
| Dyspnea | 1 (3) | 0 | 1 (3) | 0 |
| Fever | 1 (3) | 0 | 0 | 0 |
| Dehydration | 0 | 1 (3) | 0 | 0 |
| Dyspepsia | 0 | 1 (3) | 0 | 0 |
| Dysphagia | 0 | 1 (3) | 0 | 0 |
| Thromboembolic event | 0 | 0 | 3 (10) | 0 |
| Cardiomyopathy | 0 | 0 | 1 (3) | 0 |
| Immune-related AEs | ||||
| Total | 5 (17) | 6 (21) | 1 (3) | 0 |
| Hepatitis | 3 (10) | 1 (3) | 1 (3) | 0 |
| Maculopapular rash | 2 (7) | 2 (7) | 0 | 0 |
| Diarrhea | 2 (7) | 1 (3) | 0 | 0 |
| Adrenal insufficiency | 0 | 1 (3) | 0 | 0 |
| Colitis | 0 | 1 (3) | 0 | 0 |
Twelve patients (41.4%) had their dose of cabozantinib reduced to 20 mg/day due to TRAEs. Among those, 3 (10.3%) required a further reduction to 20 mg every other day. Four patients (13.8%) had their cycles delayed because of TRAEs. The most common reason for discontinuation of treatment was PD (23 patients, 79%). One patient (3%) discontinued treatment due to physician discretion and one patient (3%) discontinued treatment due to a grade 3 cardiomyopathy and grade 3 thromboembolic event that required hospitalization. Four patients (14%) were still receiving treatment at the cutoff date.
Immune cell subset analysis
Upon CaboNivo treatment, classical monocytes (P = 0.029 at C2D1, P = 0.0042 at C3D1) and intermediate monocytes (P = 0.0023 at C3D1) decreased, whereas nonclassical monocytes (P = 0.046 at C2D1) increased. There was a trend toward a higher percent of baseline nonclassical monocytes among total viable cells being associated with better PFS (P = 0.053; Supplementary Figs. S2A and S2B).
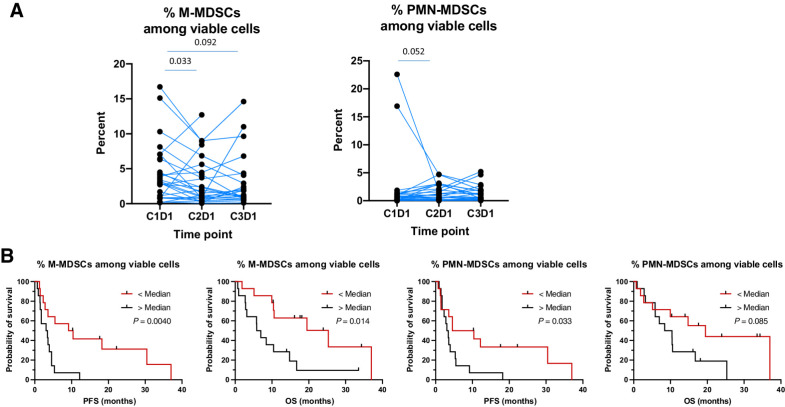
MDSCs, which represent an immature suppressive myeloid population, are composed of two major subsets, M-MDSCs and PMN-MDSCs (18, 19). M-MDSCs decreased after one cycle of CaboNivo. Fewer baseline M-MDSCs and PMN-MDSCs were associated with better PFS (P = 0.004 and 0.033); fewer baseline M-MDSCs were associated with better OS (P = 0.014; Fig. 3).
Figure 3.
Analysis of monocytic myeloid-derived suppressor cells (M-MDSC) and polymorphonuclear MDSCs (PMN-MDSC). A, Percent decrease of M-MDSCs and PMN-MDSCs with treatment. B, Kaplan–Meier curves of PFS and OS according to the percentage of viable M-MDSCs and PMN-MDSCs at baseline.
All three dendritic cell (DC) subsets [CD1c+ myeloid DCs (mDCs), CD141+ mDCs, and CD303+ plasmacytoid DCs (pDCs)] decreased, but expression of the DC maturation marker CD83 increased on all three DC subsets after one cycle of treatment (Supplementary Fig. S3A). CD83 expression on CD141+ DCs was associated with improved PFS (P = 0.033) and OS (P = 0.014; Supplementary Fig. S3B).
Tregs decreased after two cycles of treatment (P = 0.0025; Supplementary Fig. S4). Lower baseline TIM-3 expression on Tregs was associated with better PFS (P = 0.021) and a trend to better OS (P = 0.057; Supplementary Fig. S5). High expression of Ki67–PD1+CD4+ T cells and Ki67–PD1+CD8+ T cells at baseline was associated with better PFS (P = 0.015 and 0.03, respectively) and OS (P = 0.015 and 0.026, respectively; Supplementary Fig. S6). CTLA-4 expression on CD45RA+CD4+ T cells (P = 0.014), CD45RA–CD4+ T cells (P < 0.0001), CD45RA+CD8+ T cells (P < 0.0001), and CD45RA–CD8+ T cells (P < 0.0001) increased at C2D1 (Supplementary Fig. S7). Among Ki67+ subsets, Ki67+ICOS+ (P = 0.019), Ki67+ICOS– (P = 0.008), Ki67+HLA–DR+ (P = 0.030), and Ki67+ HLA–DR– (P = 0.014) decreased among CD4+ T cells after 2 cycles of treatment (Supplementary Fig. S8). Effector CD4+ T cells (P = 0.041) and effector memory 2 (EM2) subsets (20) of both CD4+ T cells (P = 0.023) and CD8+ T cells (P < 0.0001) increased, and naïve CD8+ T cells decreased (P = 0.046) after two cycles of treatment. A higher percentage of effector CD4+ T cells at baseline (P = 0.028) and after two cycles of treatment (P = 0.0009) was associated with better PFS (Supplementary Figs. S9A and S9B).
Measurement of circulating tumor cells
There was a trend of <5 EpCAM+ CTCs per 10 cc of peripheral blood at baseline being associated with better PFS (P = 0.071) and OS (P = 0.057; Supplementary Fig. S10).
Angiogenic and inflammatory cytokines
We evaluated whether CaboNivo induced changes in a panel of angiogenic and proinflammatory cytokines at the end of the first and second cycles (Supplementary Table S1). The results revealed that the most significant and sustained change was the induction of PlGF1 (Supplementary Table S1; Supplementary Fig. S11). The induction of VEGF, on the other hand, was more transient (Supplementary Fig. S11). The correlative analyses of baseline angiogenic cytokines and proinflammatory cytokines showed that a lower IL8 level was associated with better PFS (P = 0.017), with a potential trend toward better OS (P = 0.065; Supplementary Fig. S12).
Discussion
This study showed that CaboNivo is clinically active in patients with mUC who progressed on prior immunotherapy and chemotherapy, with an ORR of 16.0% and DCR of 72%. Our results provide evidence that CaboNivo induced durable responses in certain patients who had already received and progressed on prior CPI therapy. Three of the 4 patients who achieved a CR or PR had ongoing responses, although one died of a procedure at 33.5 months, and 6 patients experienced prolonged SD of 9.2–28.7 months. Most patients in this cohort (86.7%) received CaboNivo as third-line therapy post–platinum combination chemotherapy (86.7%) and CPI (100%), with a median survival of 10.4 months with CaboNivo similar to second-line pembrolizumab (10.3 months; ref. 21) and numerically better than second-line nivolumab (8.7 months; ref. 22).
Our data suggest that cabozantinib may help to prime an effective immune response to CPI even after prior CPI failure. Our preclinical and clinical data suggest that a central component of this immunomodulatory activity of cabozantinib is the consistent decrease in Tregs. Although many mechanisms are possible and may differ between the TME and peripheral immune sites, we have preclinical evidence of two potential pathways. One is our observation, supported by the literature, that a subset of monocytes isolated from peripheral blood can express the hepatocyte growth factor (HGF) receptor MET (23). Furthermore, the level of HGF in plasma in patients with cancer can be elevated, and HGF/MET interaction promotes expression of IL10 (23). The well-documented ability of IL10 to increase Treg levels could, to some extent, be downstream of an autocrine HGF/MET interaction in immune cells. Furthermore, we found that cabozantinib failed to induce IL10 (Supplementary Table S1; Supplementary Fig. S13). A second observation is that when testing the ability of cabozantinib to affect gene expression of the master transcriptional regulators of CD4+ T-cell polarization (Tbet, GATA-3, RORγT, and Foxp3), incubation of cabozantinib with healthy donor PBMCs resulted in no effect or a very slight increase in Tbet, GATA-3, and RORγT gene expression and a dramatic decrease in Foxp3 expression (16). These and other mechanistic studies await further examination.
In this trial, we found the immunomodulatory effects of CaboNivo very similar to those previously seen in our cabozantinib monotherapy trial (14), that is, (i) decreased protumorigenic classical monocytes, (ii) increased antitumor nonclassical monocytes, and (iii) decreased immunosuppressive M-MDSCs and Tregs. In addition, we observed that enhanced survival was associated with a higher percent of nonclassical monocytes, a lower percent of M-MDSCs and PMN-MDSCs, higher expression of the DC maturation marker CD83 on CD141+ mDCs, lower TIM-3 expression on Tregs, and a higher percent of effector cells among CD4+ T cells. Several reports are consistent with our observation that a lower-than-median baseline level of M-MDSCs appears to be related to the response to anti-PD-1 blockade (24, 25). Previously, we reported that increased CD83 on CD141+ mDCs was associated with better PFS in patients with prostate cancer in response to durvalumab in combination with olaparib (26).
Previous studies revealed an increase in Ki67+PD-1+ T cells after anti-PD-1 therapy, suggesting that these cells may represent reinvigorated T cells (27–29). We found that, among both responders and nonresponders, Ki67+ subsets decreased among CD4+ T cells after one cycle of treatment. The previous studies were in CPI-naïve patients, whereas all patients in this trial were refractory to CPI therapy. Taken together, the data from these patients treated with prior CPI suggest that cabozantinib may play an important role in modulating both innate and adaptive immune cells in the direction of enhanced antitumoral immunity in CPI-refractory patients.
Lower baseline CTC counts showed a trend to better survival (PFS and OS), which is consistent with our previous CTC data reported for cabozantinib monotherapy (14) and CaboNivo therapy (11). Our results confirm that cabozantinib-mediated inhibition of VEGFR1 and VEGFR2 leads to increased PlGF and VEGF in blood. Previous studies revealed that VEGF inhibits the differentiation of monocytes into DCs and their maturation and promotes the accumulation and proliferation of immunosuppressive cells such as MDSCs and Tregs (30). Cabozantinib may impede these immunosuppressive activities by blocking VEGFR1 and VEGFR2 signaling.
The immunosuppressive cytokine IL8, produced by tumor cells, some myeloid cells, and cancer-associated fibroblasts, also promotes inflammation and angiogenesis. Thus, lower levels of IL8 would contribute to a less immunosuppressive environment. Recent studies have shown that increased baseline IL8 was associated with worse OS in various cancers, including patients with mUC on checkpoint therapies (31–33). An IL8 inhibitor has been evaluated in clinical trials (34). Our study adds to the existing body of knowledge by demonstrating an association between lower IL8 and better PFS.
Our trial was limited by the small sample size and lack of randomization that would be needed to prove that the clinical effect is due to combination therapy and not to cabozantinib or nivolumab alone. In fact, our previous work showed that cabozantinib alone achieved an ORR of 19.1% (95% CI, 8.6–34.1; ref. 14), which is similar to the response rate obtained in this cohort. However, the durable responses demonstrated in this study and the data obtained in the correlative studies show that CaboNivo may be a potential option for patients with mUC who progressed on prior CPI. Larger trials with this combination in mUC are needed to confirm the clinical benefit of CaboNivo.
Immunotherapy with CPI is now part of the standard of care for patients with mUC and many other types of cancer. Strategies to overcome immune evasion and/or to rescue patients who have progressed on prior CPI have been extensively studied, and other clinical trials are exploring the combination of CPI and other therapies in genitourinary tumors and mUC. Enfortumab vedotin is an antibody–drug conjugate that demonstrated significantly prolonged OS compared with chemotherapy in patients with mUC who were previously treated with chemotherapy and CPI (6). Enfortumab vedotin is also being evaluated in combination with pembrolizumab as first-line treatment in patients with mUC, with promising results showing an ORR of 73.3% (35). Other combinations of CPI and targeted therapies are being studied. The ICONIC trial (NCT03866382) is a phase II trial evaluating the combination of cabozantinib, nivolumab, and ipilimumab in rare genitourinary tumors, including rare bladder histologies such as plasmacytoid, micropapillary, and sarcomatoid. Sitravatinib, a TKI that targets VEFR2, c-MET, c-KIT, and the TAM receptors, has shown clinical activity in combination with nivolumab for patients with mUC that progressed after platinum-based chemotherapy but who were CPI naïve, with an ORR of 31% (36). Moreover, the Alliance group is developing a clinical trial of cabozantinib associated with avelumab as a maintenance therapy after first-line platinum-based chemotherapy.
Ethics approval and consent to participate
The study protocol (NCT02496208) was approved by institutional review boards at all participating institutions. The study was conducted in accordance with the laws and regulations for the conduct of clinical trials in the United States and in accordance with the International Conference on Harmonization and Good Clinical Practice. All patients provided written informed consent before study entry.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH, and by a Cooperative Research and Development Agreement between Cancer Therapy Evaluation Program and the NCI. We thank the patients involved in this study and their families. We express appreciation to the nurses, medical oncology fellows, and consultation services at the NIH Clinical Center for their excellent patient care. We thank Bonnie L. Casey from the NCI for editorial assistance. Exelixis provided cabozantinib and Bristol Myers Squibb provided nivolumab.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
D.M. Girardi reports other support from Merck and personal fees from Pfizer outside the submitted work. A. Mortazavi reports other support from NIH during the conduct of the study, as well as other support from Seattle Genetics, Pfizer, Debiopharm Group, Acerta Pharma, Genentech, Roche, Merck, Novartis, Seattle Genetics, Astellas Pharma, Mirati Therapeutics, Bristol Myers Squibb, and Debiopharm Group outside the submitted work. S.K. Pal reports personal fees from F. Hoffman-La Roche outside the submitted work, as well as research funding to his institution from Eisai, Genentech, Roche, Exelixis, and Pfizer. B. Saraiya reports personal fees from Eisai, as well as grants from Merck and Advaxis outside the submitted work. D.P. Bottaro reports a patent for US 10,035,833 issued, a patent for US 9,550,818 and related international WO/2013/163606 issued, a patent for US 8,617,831 issued, a patent for US 7,964,365 and related international WO/2007/056523 issued, a patent for US 8,304,199 issued, a patent for US 8,569,360 and related international WO/2009/124024 and WO/2009/124013 issued, and a patent for US 7,871,981 and related international WO/2001/028577 issued. No disclosures were reported by the other authors.
Authors' Contributions
D.M. Girardi: Data curation, formal analysis, investigation, writing–original draft, writing–review and editing. S.A. Niglio: Data curation, formal analysis, investigation, writing–review and editing. A. Mortazavi: Data curation, investigation, writing–review and editing. R. Nadal: Data curation, formal analysis, investigation, writing–review and editing. P. Lara: Data curation, investigation, writing–review and editing. S.K. Pal: Data curation, investigation, writing–review and editing. B. Saraiya: Data curation, investigation, writing–original draft. L. Cordes: Data curation, investigation, writing–review and editing. L. Ley: Data curation, investigation, writing–review and editing. O.S. Ortiz: Data curation, investigation, writing–review and editing. J. Cadena: Investigation, writing–review and editing. C. Diaz: Investigation, writing–review and editing. H. Bagheri: Data curation, investigation, writing–review and editing. B. Redd: Data curation, investigation, writing–review and editing. S.M. Steinberg: Conceptualization, formal analysis, writing–review and editing. R. Costello: Data curation, investigation, writing–review and editing. K.S. Chan: Formal analysis, writing–review and editing. M.-J. Lee: Formal analysis, writing–review and editing. S. Lee: Formal analysis, writing–original draft. Y. Yu: Formal analysis, writing–review and editing. S. Gurram: Investigation, writing–review and editing. H.J. Chalfin: Investigation, writing–review and editing. V. Valera: Investigation, writing–review and editing. W.D. Figg: Conceptualization, data curation, formal analysis, supervision, investigation, methodology, writing–review and editing. M. Merino: Investigation, writing–review and editing. A. Toubaji: Investigation, writing–review and editing. H. Streicher: Conceptualization, methodology, writing–review and editing. J.J. Wright: Conceptualization, methodology, writing–review and editing. E. Sharon: Writing–review and editing. H.L. Parnes: Investigation, writing–review and editing. Y.-M. Ning: Investigation, writing–review and editing. D.P. Bottaro: Conceptualization, data curation, formal analysis, investigation, methodology, writing–review and editing. L. Cao: Conceptualization, data curation, formal analysis, investigation, methodology, writing–review and editing. J.B. Trepel: Conceptualization, data curation, formal analysis, supervision, investigation, methodology, writing–review and editing. A.B. Apolo: Conceptualization, resources, data curation, formal analysis, supervision, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev 2019;76:10–21. [DOI] [PubMed] [Google Scholar]
- 3. Galsky MD, Arija JA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57. [DOI] [PubMed] [Google Scholar]
- 4. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–30. [DOI] [PubMed] [Google Scholar]
- 5. Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48. [DOI] [PubMed] [Google Scholar]
- 6. Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021;384:1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tagawa ST, Faltas B, Lam E, Saylor P, Bardia A, Hajdenberg J, et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): Results from a phase I/II study. J Clin Oncol 2019;37:abstr 354. [Google Scholar]
- 8. Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019;30:385–96. [DOI] [PubMed] [Google Scholar]
- 9. Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol 2015;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergerot P, Lamb P, Wang E, Pal SK. Cabozantinib in combination with immunotherapy for advanced renal cell carcinoma and urothelial carcinoma: rationale and clinical evidence. Mol Cancer Ther 2019;18:2185–93. [DOI] [PubMed] [Google Scholar]
- 11. Apolo AB, Nadal R, Girardi DM, Niglio SA, Ley L, Cordes LM, et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol 2020;38:3672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 13. Lin C, Bradshaw T, Perk T, Harmon S, Eickhoff J, Jallow N, et al. Repeatability of quantitative 18F-NaF PET: a multicenter study. J Nucl Med 2016;57:1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apolo AB, Nadal R, Tomita Y, Davarpanah NN, Cordes LM, Steinberg SM, et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: an open-label, single-centre, phase 2 trial. Lancet Oncol 2020;21:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Apolo AB, Karzai FH, Trepel JB, Alarcon S, Lee S, Lee MJ, et al. A phase II clinical trial of TRC105 (anti-endoglin antibody) in adults with advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer 2017;15:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apolo AB, Tomita Y, Lee M, Lee S, Frosch A, Steinberg SM, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma. J Clin Oncol 2014;32:abstr 4501. [Google Scholar]
- 17. Lim I, Lindenberg ML, Mena E, Verdini N, Shih JH, Mayfield C, et al. (18)F-Sodium fluoride PET/CT predicts overall survival in patients with advanced genitourinary malignancies treated with cabozantinib and nivolumab with or without ipilimumab. Eur J Nucl Med Mol Imaging 2020;47:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karin N. The development and homing of myeloid-derived suppressor cells: From a two-stage model to a multistep narrative. Front Immunol 2020;11:557586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 2007;178:4112–9. [DOI] [PubMed] [Google Scholar]
- 21. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22. [DOI] [PubMed] [Google Scholar]
- 23. Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol 2014;96:295–303. [DOI] [PubMed] [Google Scholar]
- 24. Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res 2016;4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Limagne E, Richard C, Thibaudin M, Fumet JD, Truntzer C, Lagrange A, et al. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology 2019;8:e1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer 2018;6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hellmann MD, Nabet BY, Rizvi H, Chaudhuri AA, Wells DK, Dunphy MPS, et al. Circulating tumor DNA analysis to assess risk of progression after long-term response to PD-(L)1 blockade in NSCLC. Clin Cancer Res 2020;26:2849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol 2012;2012:492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 2020;26:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 2020;26:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harshman LC, Wang VX, Hamid AA, Santone G, Drake CG, Carducci MA, et al. Impact of baseline serum IL-8 on metastatic hormone-sensitive prostate cancer outcomes in the Phase 3 CHAARTED trial (E3805). Prostate 2020;80:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer 2019;7:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedlander T, Milowsky M, Bilen M, Srinivas S, McKay R, Flaig T, et al. Study EV-103: update on durability results and long-term outcome of enfortumab vedotin + pembrolizumab in first line locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol 2021;39:4528. [Google Scholar]
- 36. Msaouel P, Siefker-Radtke A, Sweis R, Mao S, Rosenberg J, Vaishampayan U, et al. 705MO Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann Oncol 2020;31:S556. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.