Abstract
The clinical translation of stem cells and their extracellular vesicles (EVs)-based therapy for central nervous system (CNS) diseases is booming. Nevertheless, the insufficient CNS delivery and retention together with the invasiveness of current administration routes prevent stem cells or EVs from fully exerting their clinical therapeutic potential. Intranasal (IN) delivery is a possible strategy to solve problems as IN route could circumvent the brain‒blood barrier non-invasively and fit repeated dosage regimens. Herein, we gave an overview of studies and clinical trials involved with IN route and discussed the possibility of employing IN delivery to solve problems in stem cells or EVs-based therapy. We reviewed relevant researches that combining stem cells or EVs-based therapy with IN administration and analyzed benefits brought by IN route. Finally, we proposed possible suggestions to facilitate the development of IN delivery of stem cells or EVs.
Key words: Stem cells, Extracellular vesicles, Central nervous system disorders, Intranasal, Clinical translation, Stroke, Neurodegenerative disease, Glioma
Graphical abstract
Intranasal administration brings multiple benefits to stem cells or extracellular vesicles therapy that could facilitate clinical translation: improve pharmacokinetics, enable non-invasive and flexible treatment regimens and enhance efficacy.
1. Introduction
Because of the therapeutic potential in improving prognosis, stem cells and their extracellular vesicles (EVs)-based therapy is developing rapidly in the research and clinical application of central nervous system (CNS) diseases1, 2, 3, 4, 5, 6. In various CNS diseases, stem cells [e.g., mesenchymal stem cells (MSCs)] are found that can suppress the activation of neuroglial cells, reduce the concentration of inflammatory cytokines and reactive oxygen species (ROS) in the microenvironment, and promote the regeneration of vessels and neurons7, 8, 9, 10, 11. Paracrine is a vital pathway to realize the functions of stem cells. And as an important component of paracrine, EVs of stem cells are found to have similar functions12, 13, 14. Furthermore, stem cells or EVs are observed would actively accumulate in the lesion site of the CNS diseases. Hence, stem cells or EVs can also serve as carriers for drug or gene delivery in CNS disorders treatment15, 16, 17, 18. Moreover, some progenitor cells could differentiate to replenish damaged or missing tissue in the lesion site19,20. However, the blood‒brain barrier (BBB) greatly restricts entry of most cells and vesicles into the brain21, 22, 23. The complex mechanism involved in CNS disorders and the vulnerability of the CNS also increased the difficulties for treatment, the dose of stem cells or EVs is restrained and relapse of diseases is prone to happen24, 25, 26, 27, 28. Therefore, further improvements to stem cells or EVs-based therapy are required to realize the therapeutic potential in clinical CNS disorder management.
The administration route directly influences the pharmacodynamics and pharmacokinetics of therapeutic agents29, 30, 31. Different clinical occasions have additional demands for administration methods32,33. And as active biologics, stem cells or EVs also have more requirements for delivery compared with traditional drugs34,35. Therefore, discussion about the administration route of stem cells or EVs-based therapy in CNS disorder treatments is increasing in the recent five years36, 37, 38, 39. The intranasal (IN) route has a unique nose-to-brain pathway and shows many advantages when delivering biologics like protein and cells34. In the past few years, studies that combined stem cells or EVs-based therapy with the IN route has increased rapidly in various fields of CNS disorder40, 41, 42, 43, 44. However, there is no review available to systematically introduce the up-to-date status of the combination of IN route and stem cells or EVs-based therapy and conclude benefits IN route brought to stem cells or EVs-based therapy. Herein, we analyzed problems in stem cells or EVs-based therapy in clinical trials and the necessity for a new administration approach. We gave an overview of the performances of IN delivering chemical compounds and proteins in preclinical studies and clinical trials. We then discussed the feasibility and possible transportation route of IN delivering stem cells or EVs. We reviewed researches using IN administration for stem cells or EVs delivery and concluded multi-level benefits of the combination of IN route and stem cells or EVs-based therapy. The current limitations of the IN delivery for stem cells or EVs are also analyzed. Finally, we proposed several suggestions to facilitate the development of IN delivering stem cells or EVs.
2. Challenges for stem cells or EVs-based therapy in CNS disorders and opportunities for intranasal route
2.1. Troubles in clinical translation of stem cells or EVs-based therapy
Stem cells or EVs-based therapy has become a hot spot in many research fields due to their unique features45,46. All sorts of stem cells, like MSCs47, neural stem cells7 (NSCs), pluripotent stem cells20,48, and along with EVs12,49,50, have been used in different fields like wound healing51, diabetes52, arthritis53, and especially, CNS diseases. Our previous studies found that engineered MSCs or NSCs could significantly diminish cerebral ischemia volume, reduce mortality rate, and promote motor function recovery in ischemic stroke7,8. Similarly, in a spinal cord injury (SCI) model, we observed that MSCs and EVs promoted angiogenesis and neurogenesis in the lesion site and significantly improved behavioral performance of rats12,54. In other reports, stem cells or EVs-based therapy could restore the deficit memory in Alzheimer's disease (AD) models9, rescue the loss of dopaminergic (DA) neurons in Parkinson's disease (PD) models55, normalize behavior in various psychiatric disorder models11,56,57, and inhibit progress of brain tumors58.
Encouraged by these results in animal models, clinical studies involved with stem cells or EVs also increased in recent years; some of them even proceeded into phase III of clinical trials. Table 114,47,59,60 lists recent representative clinical trials of stem cells or EVs-based therapy.
Table 1.
Representative clinical trials of stem cells or EVs based therapy in CNS diseases.
| Condition | NCT number | Phase | Intervention | Status | Ref. |
|---|---|---|---|---|---|
| Ischemic stroke | NCT03545607 | Phase III | Single intravenous infusion of multipotent adult progenitor cells | Recruiting | 4 |
| NCT03384433 | Phase I/II | Intraparenchymal injection of allogenic MSCs derived exosome | Recruiting | N/A | |
| NCT01716481 | Phase III | Single intravenous administration of autologous MSCs | Unknown | 59 | |
| Alzheimer's disease | NCT02054208 | Phase I/II | Repeated intraventricular administrations of MSCs via an ommaya reservoir at a 4-week interval | Completed | 60 |
| NCT04388982 | Phase I/II | Repeated IN administrations of MSCs-Exos twice a week for 12 weeks | Recruiting | N/A | |
| Parkinson's disease | NCT04506073 | Phase II | Repeated administrated allogeneic MSCs every 3 months for three doses | Recruiting | N/A |
| Multiple sclerosis | NCT04047628 | Phase III | Repeated intrathecal or intravenous administration of autologous MSCs at six-month interval for 2 doses | Completed | 47 |
| Spinal cord injury | NCT02302157 | Phase I/II | Single intralesional administration of oligodendrocyte progenitor cells | Completed | N/A |
| NCT04520373 | Phase II | Single intrathecal administration of autologous MSCs | Recruiting | N/A | |
| Neonatal stroke | NCT03356821 | Phase I/II | Repeated IN administration of allogeneic MSCs twice within the first week of onset | Completed | N/A |
Resource of the clinical trial is from ClinicalTrials.gov.
MSCs, mesenchymal stem cells; IN, intranasal; MSCs-Exos, mesenchymal stem cell derived exosomes; N/A, not applicable.
However, most stem cells or EVs-based treatments are not approved for the clinical treatment of CNS disorders61. Safety is a broad concern, yet most stem cell or EVs therapies did not show more adverse events in existing clinical and preclinical studies, even in the long-term62, 63, 64, 65. Clinical trials of stem cells or EVs-based therapy that were withdrawn or terminated due to safety issues are not significantly higher than other studies61. The source of stem cells or EVs is regarded as another problem, since the isolation and expansion of autologous stem cells commonly used in clinical trials are inconvenient and time-consuming. However, commercial allogeneic stem cells like Stempeucel® began available and used in clinical recently66,67. These commercial cells could be a source in the future.
Lack of efficacy, however, seems to be a big problem for stem cells or EVs-based therapy in clinical translation. Many clinical results showed stem cells or EVs treatment strategies had no or limited effect on different CNS conditions3,4,59. For examples, MSCs intravenous treatment had no or limited effect in ischemic stroke patients. Modified Rankin Scale (mRS), Barthel Index, and National Institute of Health Stroke Scale (NIHSS) showed no significantly improvement in months after MSCs administration. Although all the studies suggested application of MSCs in patients was safe59,65,68. Failures are expected to appear as stem cells or EVs-based therapy is still in its early days of clinical research. We should find the reasons for the unsatisfied efficacy and solutions for the further development of stem cells or EVs-based therapy.
Insufficient lesion site delivery and retention are possible reasons for the unsatisfied efficacy in the clinical trials of stem cells or EVs treatment61. System administration is most often used in both preclinical and clinical studies of CNS diseases. However, many studies have pointed out that less than 1% of total stem cells or EVs could reach the lesion part. Most cells are trapped in small capillaries or retained in organs like the liver and lungs69, 70, 71. The BBB would further prevent stem cells or EVs from entering the brain. Moreover, during the injection, the shear stress in syringe needles could damage stem cells or EVs72,73. The interaction of blood components like complements and lymphocytes with exogenous objects could further influence the viability of stem cells or EVs74. Therefore, stem cells or EVs that reached the lesion site may in a poor state and more vulnerable to the ROS, excitotoxicity, and activated immune cells in the lesion site's harsh microenvironment. As a result, few stem cells or EVs could retain in the lesion site after reaching54. Research showed few days after intralesional administration, a rapid decrease in the number of stem cells happened in the lesion site54. Although even the small percentage of stem cells or EVs remained is enough to treat animal models, patients' primary physiological conditions and regenerative capacity is not as good as model animals and need a higher lesion delivery and retention. Therefore, increasing lesion site delivery efficiency and retention could help to improve the efficacy of stem cells and EV therapy in clinical.
The invasiveness and dose frequency are other possible reasons. A high-invasive administration approach may not be tolerable by CNS patients. Embolism, infection, and trauma could happen during administration75,76. Although intracerebroventricular infusion is regarded as a safe way for long-term administration, infection and other adverse events are still often reported77. Therefore, currently single-dose or doses with long-interval of stem cells or EVs are commonly used to reduce adverse events in patients, Table 1 lists representative stem cells and EVs treatment regimens in clinical trials59,60. But some CNS diseases are progressive or would recurrent, frequent dosage may help to achieve long-term relief24,78. Researches showed that rescue in behavioral performance after single administration could start to diminish in days after administration in some CNS disorder models44,78,79. Therefore, repeated dosage delivered non-invasively may be a better choice for stem cell and EV therapy80,81.
In a word, 1) insufficient lesion site delivery, 2) retention and 3) patient tolerance to multi-dose regimens are serval factors that influence the clinical performance of stem cells or EVs-based therapy. To overcome the above problems, finding a more suitable delivery approach for stem cells or EVs-based therapy should be the most direct and economical solution. A new administration approach that could improve pharmacokinetics and could increase the tolerance of CNS disorder patients to multi-dose may help to better realize the therapeutic potential of stem cells or EVs-based therapy.
2.2. The potential of intranasal administration in CNS disorder treatment
In recent years, IN administration is getting increasing attention due to the unusual transport pattern34,82,83. Special histological features in some nasal cavity regions enable direct access of drugs to the brain84. The olfactory sensory neurons in the olfactory area of the nasal cavity and two branches of the trigeminal nerve in the respiratory region of the nasal cavity have free nerve terminals under the mucosa. Therefore, the drug can pass through the epithelium and reach the olfactory bulb or pons along the axons. Then the drug can enter the perivascular space of the cerebrovascular or spread with the cerebrospinal fluid, and finally, distributed throughout the CNS.
As the IN route could bypass the BBB and avoid the first-pass effect, IN route is used for drugs that have difficulties reaching a therapeutic concentration in the CNS, like protein drugs85, 86, 87. Pharmacokinetic studies have shown that although the nasal epithelium reduces the bioavailability of IN delivery, when the blood drug concentration is the same, the drug concentration in most areas of the CNS after IN administration is ∼10 times higher than that of the systematic injection88, 89, 90. What's more, by increasing the IN-delivery dose, the drug concentration in each part of the CNS would increase proportionally. On the contrary, a study showed that increasing the arterial injection dose by 20-fold can only result in a 1.2-fold increase of the drug concentration in the CNS89. These results indicated that IN route may be more feasible to achieve therapeutic drug concentrations in the CNS compared with systematic injection.
The drug delivery of IN route is non-invasive. IN-delivery devices like spray, atomization, nose drop, or other devices have relatively simple administrative procedures and would not cause extra harm to the patients91,92. Therefore, patients could tolerant high-frequency IN administration. Repeated IN delivery in a week, even in a day, is commonly found in clinical trials involved with IN administration, as presented in Table 293, 94, 95. And with the assist of administration devices, self-administration is also available for patients, which reduces the influence of the frequent dosage on patient life. Therefore, IN route could enable flexible regimens that have different treatment courses and frequencies for CNS management.
Table 2.
Representative clinical trials using IN administration for CNS disease management.
| Condition | NCT number | Phase | Intervention | Status | Ref. |
|---|---|---|---|---|---|
| Alcoholism | NCT03339024 | Phase III | Repeated IN administration of oxytocin spray twice a day for 30 days | Completed | 93 |
| NCT01829516 | Phase IV | Single IN administration of oxytocin | Completed | N/A | |
| Schizophrenia | NCT00575666 | Phase IV | Repeated IN administration of insulin 4 times a day | Completed | N/A |
| NCT03245437 | Phase IV | Oxytocin nasal spray | Completed | N/A | |
| Alzheimer's disease | NCT01767909 | Phase II/III | Daily IN administration of insulin for 12 months | Completed | 94 |
| Treatment-resistant depression | NCT02497287 | Phase III | Repeated IN self-administration of esketamine twice a week for 4 weeks | Completed | 81 |
| Ischemic stroke | NCT03686163 | Phase IV | Daily IN administration of NGF for 2 weeks | Completed | N/A |
| Epilepsy | NCT01999777 | Phase III | Single IN administrated of midazolam | Completed | 95 |
Resource of the clinical trial is from ClinicalTrials.gov.
IN, intranasal; NGF, nerve growth factor; N/A, not applicable.
The advantages of IN delivery in pharmacokinetics and therapeutic regimen guarantee its benefit in efficacy. IN delivery of nerve growth factors96 (NGF), insulin97, oxytocin98, erythropoietin99, and other drugs have shown promising effects in various CNS disease models. Clinical trials using the IN route for drugs delivery are also emerging in recent years, Table 2 lists the representative clinical trials using IN delivery for CNS disorder treatment81,93, 94, 95. The IN delivery is applied on various clinical occasions and could be used for the elderly or children in clinical trials. Better prognosis and long-term relief were also observed in many clinical trials compared with intravenous administration.
Overall, IN delivering drugs could improve pharmacokinetic, increase patient tolerance and enable flexible multi-dose therapeutic regimens. However, there is less discussion about IN delivery of stem cells or EVs. If these advantages of the IN pathway can also be found when delivering stem cells or EVs, it may compensate for the problems in stem cells or EVs-based therapy. IN pathway would be a possible way to facilitate the clinical translation of stem cell and EV therapy.
2.3. The transportation process of stem cells or EVs in the intranasal pathway
IN route mostly was used for the delivery of drugs or proteins with a relatively small size34 (less than tens of nanometers). The transport capacity of IN route for larger particles has been found only recently100,101. Epithelium in the olfactory and respiratory region is the first barrier for large particles transportation. Researches showed in the olfactory epithelium, the resident basal cell would differentiate and replace sustentacular cells or olfactory sensory neurons. The ciliated and goblet cells in the respiratory epithelium also would undergo similar replacement102. The process of turnover of cells in the nasal epithelium results in discontinuous tight junctions and a higher permeability84,103. Furthermore, lipid nanoparticles were found could transiently open the tight junctions in the nasal epithelium, which means nasal epithelium is more permeable to particles with lipid surfaces104. Besides paracellular bypass the epithelial barrier, nanoparticles including lipid nanoparticles also could cross the epithelium through transcytosis105,106. EVs of stem cells have similar size and surface properties as lipid nanoparticles, a similar transport pattern to cross epithelium may be adapted to EVs. The inflammation tropism ability of stem cells or EVs is also important in the trans-epithelial migration. Stem cells or EVs could interact with epithelial cells and cause a cytoskeletal reorganization of epithelial cells, which makes stem cells or EVs capable to overcome the barrier107. This process is mediated by chemokine gradients and could be augmented under inflammation states. Researchers found the presence of stem cells or EVs in the submucous space after IN delivery84, the number of cells or EVs would increase under pathological conditions108. These findings support the inflammation tropism of stem cells or EVs that could facilitate trans-epithelial migration.
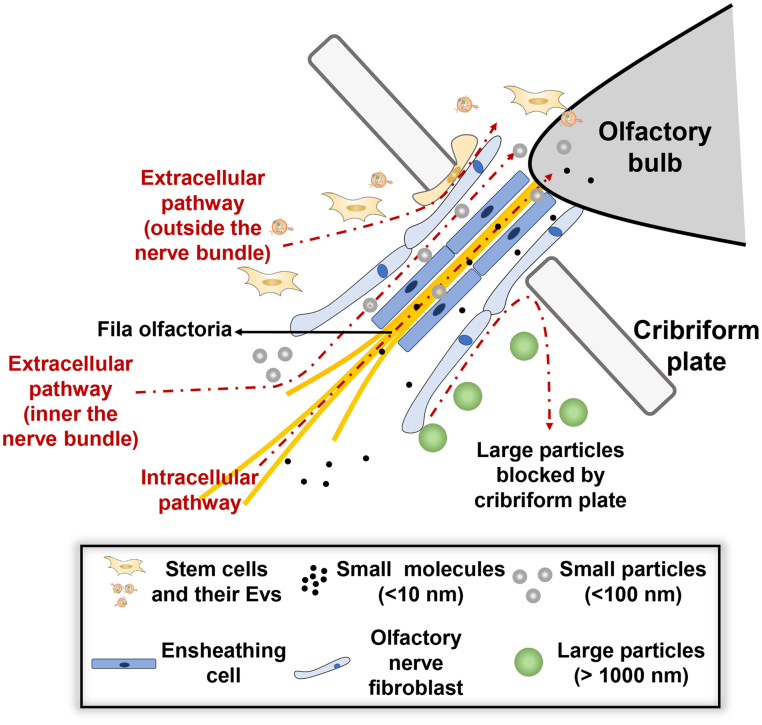
The cribriform plate is another barrier for nose-to-brain transportation through the olfactory nerve pathway84. The small molecular could cross the cribriform plate intracellularly by the axonal transport of olfactory sensory neurons (Fig. 1). And olfactory ensheathing cells in the olfactory nerve bundle maintain continuous open spaces for the regrowth of olfactory nerve fibers, creating an extracellular path (within the nerve bundle) to cross the cribriform plate34 (Fig. 1). However, constrained to the diameter of the olfactory sensory neurons (∼500 nm), both the intracellular and extracellular route seems not applicable to large particles. Researches showed intact polycaprolactone nanoparticles or chitosan-coated nanoemulsions with a size of ∼100 nm could not cross the cribriform plate through the olfactory pathway109,110. Only loaded cargos could be released and enter the brain region110. Intact nanoparticles with a size of ∼100 nm could be detected in the trigeminal nerve tract, and these nanoparticles could reach the brain stem through the trigeminal nerve pathway109. But as the size increases to ∼1000 nm, intact nanoparticles are no longer able to reach the brain stem through the trigeminal nerve pathway in a short time110. Therefore, it seems hard for stem cells (10–20 μm) and EVs (100–1000 nm) to reach the brain especially through olfactory nerve pathways. Yet a quick localization in the olfactory bulb of stem cells or EVs after IN administration was confirmed by many studies111,112. Using MRI tracking, a dynamic transfer from the nasal region to the olfactory bulb could also be observed18,41,111,113. These articles suggest that stem cells or EVs appear to travel along with the olfactory nerve bundles (Fig. 1). Detailed analysis showed the signal of stem cells is just adjacent to, but not within, the olfactory nerve bundles, which is different from the known olfactory nerve pathway84 (Fig. 1). These findings imply stem cells or EVs may employ a novel route crossing the cribriform plate. Immune cells like T cells and monocytes could be observed crossing the cribriform plate from the CNS to the nasal region. When crossing the cribriform plate, immune cells are also found adjacent to the olfactory nerve yet not within the nerve bundle, just like stem cells entering the CNS114, 115, 116. Therefore, there is a hypothesis that stem cells or EVs may enter the CNS following the reverse route used by immune cells. The cytokine concentration gradients may drive the trans-cribriform plate movement of stem cells or EVs. Researches showed that increasing the concentration of SDF-1 (ligand of CXCR4) in the brain could increase the number of stem cells entering the brain for serval-fold, which supports the hypothesis108,117. The cerebrospinal fluid also exits from the brain through the cribriform plate and enters the nasal area unidirectionally, suggesting the hypothesis is histologically feasible118. Yet the pressure gradient between the nasal cavity and the CNS could be a barrier, the stem cells or EVs need to overcome the relatively higher pressure in the cerebral. Hence active migration ability may be necessary to enter the CNS through this hypothesis pathway, which explains why nanoparticles cannot enter the brain through this hypothetical pathway.
Figure 1.
Possible pathways for stem cells, EVs, and particles of different sizes to pass through the cribriform plate. In the olfactory pathway, small molecular drugs could be endocytosed by the neuron and across the cribriform plate through intraneural transport. Small molecules also could enter the gaps in the olfactory nerve bundle and be extracellularly transported to the olfactory bulb. Although small particles (<100 nm) also could be endocytosed by the neuron, the extracellular pathway is more important for transportation, and the trigeminal nerve pathway (not shown) could be dominant. Large particles (>500 nm) could be found near the olfactory nerve bundle, yet intact particles could not reach the olfactory bulb, cribriform blocked the entry of large particles. But stem cells or EVs could cross the cribriform plate through an extracellular pathway independent of the olfactory bundle, the position where stem cells or EVs crossing the cribriform plate is adjacent to the olfactory bundle, the inflammation tropism ability of stem cells or EVs may play an important role.
In a word, the inflammation tropism of stem cells or EVs play an important role in crossing the epithelium and cribriform plate barrier. Stem cells or EVs may have a unique transport pattern to reach the brain compared with small molecules and nanoparticles. The detailed mechanism of the transport route of stem cells or EVs still needs further exploration.
3. Benefits of combining stem cells or EVs-based therapy with intranasal route
3.1. Intranasal route increased lesion site distribution of stem cells or EVs
Since insufficient lesion site delivery is a problem for stem cells or EVs-based therapy, there are growing studies trying to improve the targeting ability of stem cells or EVs. Modification of stem cells or EVs such as hypoxic treatment119, iron oxide nanoparticle stimulation8,120, and C-X-C chemokine receptor type 4 (CXCR4) overexpression121 was employed to enhance the CNS lesion site targeting and improve treatment effect in preclinical studies. Our study had shown that iron oxide nanoparticle stimulation could increase MSCs distribution in the cerebral ischemia site. Reduced mortality rate and improved motor recovery were observed in MCAO mice8. But the engineering of stem cells or EVs would increase the difficulties in quality control and the complexity in manufacture. In contrast, the IN route is a more straightforward solution. Table 3122, 123, 124, 125, 126, 127, 128, 129, 130, 131 lists the representative pharmacokinetic data of IN administration of stem cells or EVs.
Table 3.
Representative pharmacokinetic data of IN administration of stem cells or EVs in different models.
| Model | Pharmacokinetic data | Ref. |
|---|---|---|
| AD model mice | EVs were detected in the brainstem and olfactory bulb firstly at 0.25 h and reaching a peak at 1 h. | 41 |
| PD mice | Stem cells were detected in the whole brain area 7 days after administration. The brainstem and olfactory bulb contain the most cells (∼20 %, respectively). | 79 |
| PD model rats | Stem cells were detected in the whole brain region and most distributed in the striatum and substantia nigra 2 months after administration. | 122 |
| AD model mice | Stem cells were detected in the olfactory bulb, hippocampus, ventral and dorsal cortex, brain splits, thalamus, and cerebellum 4 months after administrations. | 123 |
| Neonatal brain injury model rat | 24 h after administration, stem cells were detected in the corpus callosum, cerebral cortex, olfactory bulb, and hippocampus. | 124 |
| SCI model of rats | Stem cells were detected in the lesion site of the spinal cord 2 and 4 weeks after the administration. Compared with 2 weeks ago, the number of MSCs in the lesion site even increased 4 weeks after administration. | 125 |
| SCI model rats | Exosomes were mainly detected in the lesion part of the spinal cord at 24 h after administration. The number of exosomes in the spinal cord even exceeds the brain. | 126 |
| Neonatal brain injury model mice | Stem cells were mainly detected in the brain at 12 h after administration. | 127 |
| Stroke model mice | Exosomes were mostly detected in the lesion hemisphere 1 or 24 h after administration. Number of exosomes in the ischemic part increasing overtime. | 128 |
| Glioblastoma model mice | Stem cells were mostly detected at the tumor site at 6 h after administration. The number of stem cells reached a peak at 24 h and remained steady in 5 days. | 111 |
| Glioma model mice | Stem cells were detected in the tumor area at 1 h, and the number of stem cells significantly decreased on day 5 but remained steady by Day 11; distribution of stem cells in the brain of irradiated animals was 2.8 times higher. | 117 |
| Glioma model mice | Stem cells were detected in the brain at 2h, reached a peak at day 1, and slightly decreased by Day 6. | 129 |
| Glioma model mice | Stem cells were detected in the tumor area at 24 h but reached a peak at 120 h after administration when treated with methimazole and fibrin glue. | 18 |
| Btbr T+Itpr3tf/J (Btbr) mice | Exosomes were detected in the brain at 24 h after administration. | 130 |
| EAE model mice | Secretome of amnion-derived multipotent progenitor cells (ST266) was selectively accumulated in the optic nerve and vitreous at 30 min after administration. | 44 |
| Chronic alcohol consumption model rats | Exosomes were detected in the brain at 2 h and gradually increased within 24 h after administration. | 131 |
| Status epilepticus model mice | Exosomes were detected in the cortical and hippocampal at 6 h after administration. | 14 |
AD, Alzheimer's disease; EVs, extracellular vesicles; PD Parkinson's disease; MSCs, mesenchymal stem cells; SCI, spinal cord injury; EAE, experimental autoimmune encephalomyelitis.
Gliomas treatment have difficulties in realizing enough drug concentration at the tumor site132,133. IN delivery of engineered MSCs or NSCs both showed a selective accumulation at the tumor site and better tumor penetration (Table 3). The high distribution of engineered MSCs or NSCs at tumor site also helped to extend survival time in primary tumor tissue grafted rats16,117,134,135. Pretreated nasal cavity with methimazole, drugs could delay the nasal clearance and enhance epithelium penetration, further promoting tumor site delivery of stem cells. Studies have shown that methimazole treatment increased the oncolytic virus-loaded NSC distribution at the tumor site after IN delivery to 20% and prolonged the survival time of rats18. The above studies showed that IN administration was effective to increased tumor accumulation and to boost the efficacy of stem cells or EVs treatment in brain tumors.
In an aged αSyn transgenic PD model, seven days after administration, MSCs were found that accumulated in the striatum where the loss of dopaminergic (DA) neurons happened136. In a 6-OHDA induced PD model rats. IN delivery of human olfactory ecto-mesenchymal stem cells (OE-MSCs) show a comparable grafted rate and even better therapeutic effect in DAergic markers rescue and motor function improvement compared with direct injection into the striatum137,138. These findings proved IN administration could also achieve a high CNS delivery efficiency in PD models.
For other diseases without a specific lesion part like AD. After IN administration, EVs are distributed in various brain areas, not limited to the olfactory bulb41. The EVs seem firstly migrate to the immature olfactory sensory neurons and then migrate along with the axon to the CNS. The intrinsic inflammation tropism (homing) ability of EVs may promote EVs to pass through the epithelium and increase the delivery efficiency. The high graft rate of EVs in the brain help to achieve robust neuroprotection, remarkable neurogenesis, and rescue of memory deficits in App/Ps1 transgenic mice41,79,139,140. Furthermore, IN administration of EVs also showed efficacy in aged App/Ps1 AD mice, whereas intravenous delivery failed to ameliorate symptoms79. The poor basic physical conditions account for the lack of efficacy in elderly mice, which is also one of the reasons for the failure of stem cells or EVs treatment in elderly patients. The better effectiveness of IN delivery in elderly mice suggests that IN route could better exhibit the curative effect of EVs-based therapy.
In cases of acute neuroinflammation disease where barrier function of BBB partly breaks down, stem cells or EVs could be detected in the lesion part within an hour after IN administration (Table 3)122, 123, 124, 125, 126, 127, 128, 129, 130, 131. In a stroke model, the fast delivery of stem cells or EVs ameliorated post-ischemic events, a reduced infarct area and inflammation were observed, and junctions of vascular were well persevered after treatment141. In perinatal brain injury models, a similar fast delivery to the brain was also observed in rat pups. NSCs or exosomes of MSC reached the brain in hours124,142. The immediate intervention significantly rescued motor and cognitive development of pups who received stem cells or EVs-based therapy13,142,143. Even in spinal cord injury models where trauma is out of the brain, IN delivery stem cells or EVs still realized highly efficient lesion part delivery in 24 h, and comparable or even better efficacy as an intralesional injection could be achieved using IN delivery125,126. The quick entry of stem cells or EVs into the CNS enabled by IN administration could help to seize the treatment window144, which is quite meaningful for the prognosis of acute CNS disease like stroke. As IN administration is relatively simple to conduct, IN delivery of stem cells or EVs may serve as a strategy in first-aid, though the storage of stem cells or EVs is a problem.
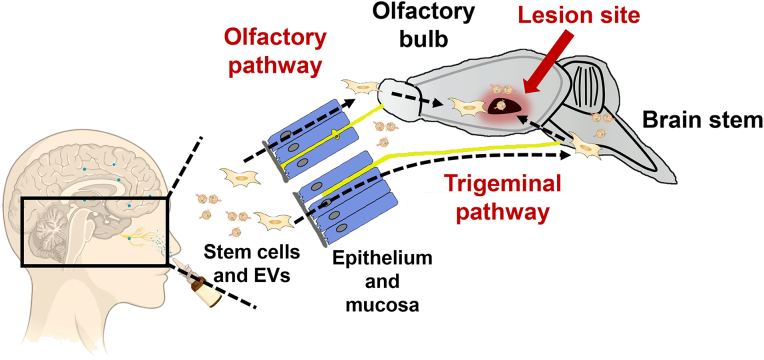
In a word, IN route provide a direct entry to the brain IN for stem cells or EVs130 as shown in Fig. 2. The inflammation tropism ability of stem cells or EVs could facilitate epithelium penetration and further increased CNS delivery. These findings suggest that compared with delivering chemical compounds or protein drugs, IN route showed even better ability to improve biodistribution when delivering stem cells or EVs.
Figure 2.
Lesion site targeting of stem cells and EVs after IN administration. Stem cells or EVs migrate along the olfactory sensory neurons or trigeminal nerve and directly enter the olfactory bulb or brainstem. More stem cells or EVs could reach the CNS as these routes could bypass the BBB. Then they would gradually migrate to the lesion site in the CNS, achieving a higher distribution here.
3.2. Intranasal route prolonged retention of stem cells or EVs in the lesion site
Embed stem cells or EVs in the scaffold is an effective way to protect the viability and prolong retention of stem cells or EVs. Our previous research12 showed scaffold could significantly prolonged the retention of MSCs and EVs in the lesion site of SCI models. But the transplantation of scaffold needs surgery and is not suitable for disease without a specific lesion site. Interestingly, stem cells or EVs grafted by IN route seemed to have longer retention in the lesion site18, as shown in (Table 3)122, 123, 124, 125, 126, 127, 128, 129, 130, 131. IN route may also be a simple solution for the lack of retention.
IN route could prevent the damage to stem cells or EVs caused in syringe needles69,70,73,145. And stem cells or EVs would be directly transported into the brain but not immediately into the lesion site after IN administration, which also helps maintain the viability. In glioma models the number of stem cells in tumor site could remain steady or with mild decrease for in a week111,117,129. Methimazole treatment before administration, the signal of NSCs was even increase in 120 h after administration18.
Combination with radiotherapy also showed improved distribution and retention of MSCs, the number of MSCs at tumor site remained steady from Day 5 to Day 11 after administration108. As irradiation could upregulate the CXCL12 expression in tumor site, the inflammation tropism ability of stem cells may also involve in the improved retention. Similarly, in experimental autoimmune encephalomyelitis (EAE) models, IN delivery showed a superior in lesion site retention compared with intraperitoneal or intravenous injection and CXCR4 overexpression further enhanced retention of MSCs78,146. In 6-OHDA-induced PD model, stem cells or EVs were found to gradually accumulate to the striatum and substantia nigra147, stem cells or EVs could still be observed months after administration138. Meanwhile, the stem cells or EVs in other brain regions, like the olfactory bulb or brainstem, were constantly decreased after administration136. These findings proved that stem cells or EVs would continuously migrate from other brain regions (mainly the olfactory bulb) to the lesion site even weeks after administration. In SCI models, though the lesion site is far from the brain, the migration of stem cells is still found 4 weeks after administration125.
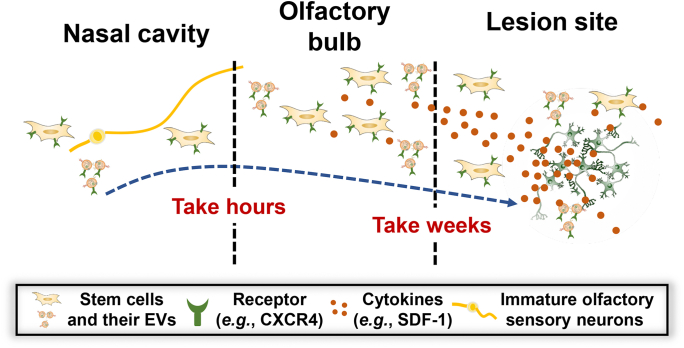
Therefore, the olfactory bulb may act as a reservoir for stem cells or EVs, as the olfactory bulb is the first stop for a major part of stem cell IN delivered. Stem cells or EVs in the olfactory bulb are slowly migrate to the lesion part in the CNS that helps maintain the number of cells or EVs in the lesion part111. And the inflammation tropism ability of stem cells or EVs seems to be the main driving force for the migration. In healthy mice or rats, stem cells or EVs delivered through IN route were found to linger in the olfactory bulb for a longer time and would have universal distribution in brain, lungs and liver, which also proved the reservoir function of the olfactory bulb126,130. These findings suggest the special transport pattern of IN route contribute to the longer retention (Fig. 3).
Figure 3.
Migration track and timeline of stem cells and EVs. Most stem cells or EVs migrate adjacent to the immature olfactory neurons to the brain. Within a day, stem cells or EVs would mostly accumulate in the olfactory bulb. The cytokines in the lesion site (e.g., CXCL12) would gradually attract stem cells or EVs to the lesion site. The migration from the olfactory bulb to the lesion site could continue for weeks until most stem cells or EVs are concentrated in the lesion site. And the gradual migration to the lesion site could help to maintain the number of stem cells or EVs in the lesion site.
In a word, IN route better preserved the viability of stem cells or EVs, and the olfactory bulb may serve as a reservoir that could sustain release of stem cells or EVs and maintain the number of stem cell and EVs in the lesion site. Hence, IN route could also be an answer for the insufficient lesion site retention in stem cells or EVs-based therapy.
3.3. Non-invasive procedures of intranasal route enable multi-dose and flexible regimen
There is a dilemma for CNS treatment: the less invasive intravenous route has unsatisfying lesion delivery; while high transplant efficient topical CNS injection is not tolerable by patients especially when repeated administration. In contrast, IN route could balance the delivery efficiency and patient tolerance. Therefore, multi-dosage of IN administration of stem cells or EVs could be the answer to the conflict, and it may be more effective and safer for CNS disease treatment.
Repeated IN delivery of MSCs and EVs caused no inflammation in the short-term; nor was there any behavioral abnormalities or histological changes in the long term148,149. Mouse148, rat43, and pig150 pups of neonatal brain injury model showed good tolerance to IN administration of stem cells or EVs. A clinical trial also has used two doses of IN administration of 5 × 107 MSCs to treat neonates suffering from perinatal arterial ischemic stroke (NCT03356821). Therefore, IN delivery of stem cells or EVs is patient-friendly and is tolerable even for infants.
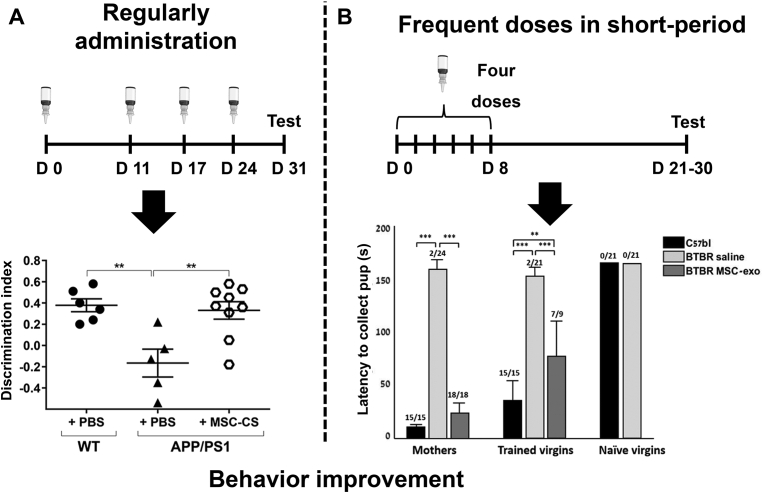
The more frequent dosage enabled by IN route appears to be beneficial for CNS disorder treatment (Table 4)151, 152, 153. In a perinatal asphyxia model, the additional dosage of MSCs secretome seven days after modeling dose seems further improve the locomotor activity, recognition memory, and anxiety of mouse pups152. In chronic diseases like neurodegeneration diseases, multi-dosage is more often used. The research found that AD model mice were well tolerated to weekly or even daily IN administration79,139. A single administration secretome of MSCs exposed to App/Ps1 AD mouse brain homogenates (MSC-CS) through both IV or IN routes could reduce neuroinflammation in the brain and partly restore damaged memory. Yet, these functional recoveries are transient and have much less effect in 25-month-old aged mice. In contrast, weekly IN administration secretome of MSC-CS for four weeks led to a more long-lasting improvement in memory for both young and aged AD mice. Increased neuronal density and prolonged lifespan were also observed in aged mice after multi-dose treatment79. However, the memory in treated mice could get impaired again after stop treatment for a month, which emphasized the importance of sustained and long-term intervention. Weekly administration of NSCs or repeated administration EVs of MSCs also showed long-term behavioral improvement in App/Ps1 AD mice during treatment41,123,139,140. Therefore, for progressive diseases like AD, regularly IN administration of stem cells or EVs seems to be essential for long-term efficacy in AD treatment and the non-invasive IN route may be the best choice (Fig. 4A)79.
Table 4.
Representative treatment regimens using IN administration of stem cells or EVs based therapy.
| Model | Treatment regimen | Pharmacodynamic data | Ref. |
|---|---|---|---|
| AD model mice | Once a day for 3 weeks | Pathology changes were ameliorated 2 days after treatment that is like a single intravenous injection of MSCs. | 139 |
| AD model mice | Once a week for 4 weeks | 7 days after treatment, the memory was fully restored in aged mice after repeated IN administration. Single IN or intravenous administration fails to rescue memory fully. | 79 |
| AD model mice | Every two days for 2 weeks | Pathology changes were ameliorated and behavioral performances were improved at the end of treatment. | 41 |
| AD model mice | Once a week for 4 weeks | Behavioral performances were significantly improved 2 months after administration, and pathology changes were ameliorated 3 months after treatment. | 123 |
| PD model rats | Single administration | Behavioral performances were improved one week after administration and the improvement remained to 4 weeks. | 147 |
| Ischemic stroke and refusion model mice | 1 h after modeling, twice a day for 7 days | Inflammation was inhibited and histological structure was restored at end of treatment. Behavioral performances were continuously improved during 7-days-treatment. | 141 |
| Neonatal brain injury model mice | A dose of MSCs administered 3, 10, or 17 days after modeling | Cognitive function improvement was achieved when administration at 3 or 10 but not 17 days after modeling. | 151 |
| Perinatal asphyxia model rats | Two doses of exosomes administered 2 h and 7 days after modeling | Inflammation was inhibited, and motor function was improved after the first dose; further improvement in behavioral performances was found after the second dose. | 152 |
| Neonatal brain injury model mice | Single dose administered immediately after modeling | Inflammation and brain tissue volume loss were inhibited, and behavioral performances were improved 2 days after administration. | 143 |
| SCI model rats | A single dose administered 24 h after modeling | Significant behavioral performances improvement was only observed at day 7 after modeling. | 125 |
| SCI model rats | 2–3 days after modeling, once a day for five days | Behavioral performances were improved starting from 2 weeks after administration and a significant benefit was maintained to 8 weeks. Intralesional injection fails to improve behavioral performance. | 126 |
| Glioma model mice | Irradiation for 5 days combined with IN delivery of MSCs once a week for 4 weeks | The survival of mice was improved. Combination with irradiation could further enhance the efficacy of stem cell transplantation. | 117 |
| Glioma model mice | A single dose of NSCs administered after treating with methimazole and fibrin glue followed | The survival of mice was improved after stem cell transplantation. Methimazole and fibrin glue treatment could further enhance efficacy. | 18 |
| Chronic alcohol consumption model rats | Once a week for 5 weeks | Improvement in behavioral performances was achieved both after a single dose of IN or intracerebral exosomes, only repeated IN administration resulted in long-term relief. | 131 |
| Demyelination model mice | Once a week for 12 weeks | Pathology changes were ameliorated and behavioral performances improved 30 days after treatment. | 146 |
| Btbr mice | Every two days for 8 days | Behavioral performances were improved 2 weeks after treatment. | 130 |
| Schizophrenia model mice | Once a day for 14 days | Behavioral performances were improved 2–3 weeks after treatment. | 153 |
| EAE model mice | Daily administration for 4 weeks | Pathology changes were ameliorated, and behavioral performances were improved during treatment. Efficacy was diminished when treatment stopped. | 44 |
| SE model mice | Two hours after modeling IN deliver of two doses of exosomes within 18 h | Inflammation was inhibited, and long-term protection of memory and cognitive function were achieved. | 14 |
AD, Alzheimer's disease; EVs, extracellular vesicles; PD Parkinson's disease; MSCs, mesenchymal stem cells; NSCs, neural stem cells; SCI, spinal cord injury; EAE, experimental autoimmune encephalomyelitis; SE, status epilepticus; IN, intranasal.
Figure 4.
Different treatment strategies of IN administration. IN route could make flexible multi-dose treatment regimens possible. (A) Regular administration for long-term could maintain the behavior benefits in AD mice, the novel object recognition memory test showed the memory of aged AD mice fully recovered after treatment. Data are presented as mean ± SEM (WT + PBS n = 6; APP/PS1 + PBS n = 5; APP/PS1 + MSC-CS n = 9); ∗∗P < 0.01, One-way ANOVA. Reprinted with the permission from Ref. 79. Copyright © 2020, The author (s). (B) Frequent doses in a short period also could bring a long relief. Improvement in learning of maternal pup retrieval behaviors and maternal pup retrieval were observed in autism spectrum disorder mice weeks after last administration. Data are presented as mean ± SEM (n = 7); ∗∗P < 0.01. ∗∗∗P < 0.001, One-way ANOVA. Reprinted with the permission from Ref. 130. Copyright © 2018, The author (s).
For PD treatment, pluripotent stem cells transplantation has been used to supplement the loss of DA neurons in substantia nigra pars compacta63 (SNpc). Yet after a single administration, “on‒off” reactions in treatment were still reported in some studies154,155. Multi-dose stem cell transplantation through the IN route may help to prolong the duration of the therapeutic effect, though there is no report on the use of IN for the administration of pluripotent stem cells. Besides pluripotent stem cells, researches showed that weekly IN administration of MSCs or EVs could reduce neuron loss and improve rat behavioral performance156, 157, 158. These reports support the feasibility of repeated IN administration of stem cells for PD treatment.
Psychiatric disorder patients are also needed long-term intervention. IN administration of MSC exosomes every other day for 8 days showed a long-lasting benefit in the social interaction of Btbr T+Itprtf/J (Btbr) autism spectrum disorder (ASD) mice130. The research also suggested that the multi-dosage of IN delivered exosomes had similar efficacy as a single intracranial injection of MSCs159. Therefore, frequent repeated IN administration in a short period could also have a long-term effect, as indicated in (Fig. 4B)130. In the chronic alcohol intake model, the inhibition effect in ethanol intake began to decline 24 h after a single IV administration of MSCs-derived exosomes. Although single IN administration also only had a transient treatment effect, repeated IN administration within a month showed a more effective and long-lasting reverse in alcohol intake than single IV administration131.
In summary, long-term regularly administration of stem cells or EVs or shot-term multiple IN administration of them both showed an impressive effect in different animal models (Fig. 4)79,130. Since chronic CNS disease patients need long-term intervention, using IN administration would cause less pain and more negligible influence in patients’ lives. The flexible treatment regimens provided by the non-invasive IN route also could solve problems in invasiveness and regimen of stem cells or EVs-based therapy.
4. Future perspective for IN administration of stem cells or EVs-based therapy
As we discussed above, IN route showed many advantages over current used administration route: the IV route has a poor lesion site transplantation rate69, 70, 71; the topical injection is highly invasive and the frequency of dosage in topical injection was constrained77,145,160; other administration routes like intra-arterial (IA) or intraperitoneal injection (IP) also fail to realize high CNS delivery efficiency, and both are relatively invasive161,162. On the contrary, IN route could provide a direct nose-to-brain delivery and a high brain graft rate for stem cells or EVs. The non-invasive trait of IN route also makes it more suitable for CNS disorder patients who need long-term intervention. Table 5 shows the comparison among different administration routes.
Table 5.
Comparison among different administration routes for stem cells or EVs transplantation.
| Route | Invasiveness | Delivery efficiency | Process complexity | Bioavailability | Dosage frequency |
|---|---|---|---|---|---|
| IV | ++ | + | + | +++ | ++ |
| IC | ++++ | ++++ | ++++ | +++ | + |
| IA | +++ | ++ | ++++ | +++ | + |
| IP | ++ | + | ++ | +++ | ++ |
| IN | – | +++ | + | + | ++++ |
IV, intravenous; IC,intracranial; IA, intra-artery; IP, intraperitoneal; IN, intranasal.
In recent years, several clinical trials have used IN administration of stem cells or EVs to treat CNS diseases, including PD, stroke, and neonatal brain injury. Table 6163 lists representative clinical trials of stem cells or EVs delivered by IN route in recent years.
Table 6.
Representative clinical trials of IN delivering stem cells or EVs for the treatment of CNS diseases.
| Condition | NCT numbers | Phase | Intervention | Status | Ref. |
|---|---|---|---|---|---|
| Ischemic stroke | NCT05008588 | Phase I/II | IN delivery of MSC's condition medium | Not yet recruiting | N/A |
| Perinatal arterial ischemic stroke | NCT03356821 | Phase I/II | IN administration of MSCs at confirmation of the stroke and within the first week of onset | Completed | N/A |
| Alzheimer's disease | NCT03724136 | N/A | IN administration of MSCs | Recruiting | N/A |
| Parkinson's disease | NCT04146519 | Phase II/III | Tandem (IN + intravenous) injections of MSCs | Recruiting | 163 |
| Multiple CNS diseases | NCT03899298 | Phase I | IN delivery of amniotic and umbilical cord tissue | Recruiting | N/A |
Resource the clinical trial is from ClinicalTrials.gov.
MSCs, mesenchymal stem cells; CNS, central nerve system; IN, intranasal; N/A, not applicable.
Nevertheless, the research on IN delivery of stem cells or EVs is still in its initial stage, and related researches still face some critical problems. The biggest problem is the relatively low bioavailability of IN route164,165. Currently, nose drops are primarily used in both preclinical and clinical for stem cells or EVs delivery. Stem cells or EVs would be cleared out of the nasal cavity before across the epithelium. The effective area for direct nose-to-brain delivery only takes up 3%‒10% of the surface area of the nasal cavity, stem cells or EVs may enter circulation rather than enter CNS after crossing the epithelium. Therefore, only a part of stem cells or EVs could directly enter the brain.
The thermosensitive hydrogel could form in situ hydrogel after administration, reduce the clearance of the nasal cavity, fix stem cells or EVs in the olfactory region, and help increase the bioavailability of IN administration166,167. Currently, chitosan168,169 and poloxamer166,170 are commonly used to prepare thermosensitive hydrogel. These materials show good biocompatibility and have been used in clinics. Hence the two materials could possibly aid the IN delivery of stem cells or EVs. Absorption enhancer is another strategy. There are a variety of compounds that could facilitate the absorption of IN administration. Among them, surfactants are an important category of absorption enhancers171,172. It is worth noting that both chitosan and poloxamer have been reported to enhance the permeability of epithelium173,174. Hence, chitosan or poloxamer thermosensitive hydrogel may also promote stem cell cells or EVs migration through the epithelium. Apart from surfactants, researches showed that pretreated nasal cavity with fibrin glue could inhibit cilia's movement and prevent the clearance of stem cells from the nasal cavity175. Hyaluronidase is another commonly used penetration enhancer for stem cells or EVs delivery. Hyaluronidase could degrade hyaluronic acid in the extracellular matrix (ECM) and loosening the epithelium, which help stem cells or EVs cross the epithelium barrier.
Another problem is that stem cells or EVs are unsuitable for the administration devices of IN delivery. IN-delivery devices can reduce drug loss during administration, promote deposition in correct region of nasal cavity, and enable self-administration. Currently, powder formulations or liquid formulations are primarily used in various IN administration devices176. For example, the Bi-Directional™ Breath Powered® device of OptiNose company is used for drugs like oxytocin powder delivery (NCT02414503), LMA MAD Nasal™ of Teleflex company is used for dexmedetomidine liquid delivery (NCT02955732). Yet, stem cells or EVs may lose their biological activity during preparation, storage, or delivery of formulations. The current formulation preparation process needs to be modified, or new formulation are required to help stem cells or EVs adapt to the IN-delivery devices. Biomimetic mineralization is a possible strategy to protect stem cells. Artificial mineral coats would form a rigid and degradable inorganic shell on the cell surface after mineralization and increased the resistance of cells to the adverse environment177. This technology may enable the preservation of stem cells in powders. Prepared stem cells into spheroids also could prolong the storage time. The research found that MSCs in spheroids could remain >90 % viability under ambient conditions and still showed therapeutic effects in mouse colitis models178. Since EVs of stem cells are not living cells, they may be easier to be prepared into powder formulations. Lyophilization with cryoprotectants could avoid aggregation of EVs and extend the storage time of EVs179. Mannitol180 and trehalose181,182 were effective cryoprotectants for EVs and may enable EVs to adapt to powder IN-delivery devices.
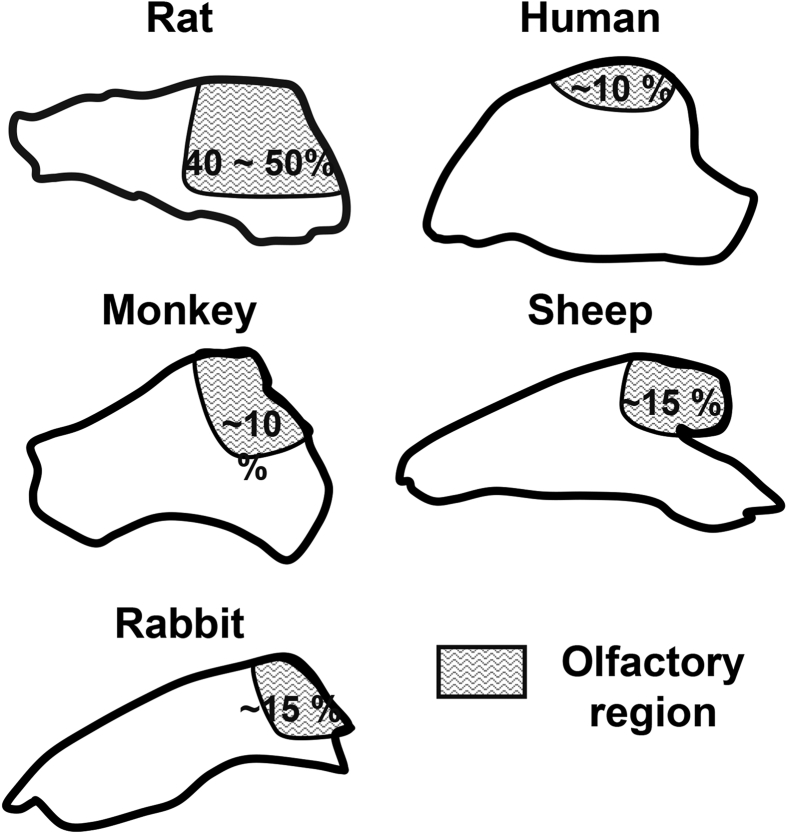
The in vivo animal models for the studies of intranasal delivery is also a problem. For future research on stem cells or EVs formulations or administration devices, evaluation of pharmacodynamics and pharmacokinetics in animal models is necessary. Rodents like rats and mice are the most commonly used animals for experiments, as rats and mice are genetically similar to humans and have similar pathological mechanisms to humans in many diseases. However, the histological structure of the rodent nasal cavity is very different from that of humans34 (Fig. 5). Rats and mice are obligate nasal breathers and have a more acute sense of smell. Therefore, the structure of the nasal passages of rats and mice is more complex and has a significantly higher surface area to volume ratio. The olfactory area of rats and mice accounts for 40%–50% of the total nasal surface area, while the human olfactory area accounts for only 10 % of the nasal surface area183,184 (Fig. 5). Mucociliary clearance is also different between rodents and human. The direction of mucociliary movement in human is backward towards the nasopharynx, opposite to the direction of mucociliary movement in rodents185. Furthermore, rodents have small nostrils, making the IN-delivery process cumbersome, and it's also difficult for rodents to adapt to IN-delivery devices. These facts make the pharmacokinetics of drugs delivered through IN route quite different between rodents and human. Therefore, data from large animals are crucial for preclinical studies and IN formulation and device development. Monkeys have a nasal cavity structure closer to humans34,186. Administration devices such as atomizers are also have been used in monkeys187. But the experimental monkey is very difficult to obtain, and the cost could be unaffordable. Rabbit may be an ideal substitute for large animals. The ratio of rabbit olfactory region in total nasal cavity surface is ∼15%188 (Fig. 5), which is close to human. Current research shows that compared with other animals such as sheep or mice, the bioavailability and absorption rate of the drug in rabbits after IN administration is closer to that of humans189,190. Therefore, in addition to research on small animals, it is also recommended to conduct preclinical research on the IN delivery of stem cells or EVs in large animals such as rabbits. Due to the vast difference in the nasal cavity structure between rodents and humans, the data obtained on large animals will have more reference value for clinical translation.
Figure 5.
The nasal cavity structure of different animals and humans. The shaded area represents the olfactory region. The rodents like rats have a much larger olfactory region compared with human (50% vs. 10%). Nasal cavity of monkey is the one most similar to human. Other large animals like sheep and rabbit also have an olfactory region ratio similar to human.
5. Conclusions
Stem cells or EVs-based therapy brings hope to the treatment of various CNS diseases. Yet the clinical translation of stem cells or EVs is facing many challenges. From the recent clinical trials involved with stem cells or EVs-based therapy, we conclude 1) insufficient lesion site delivery, 2) retention and 3) patients’ tolerance to multi-dose regimens are three problems in stem cells or EVs-based therapy. We suggest that utilizing IN route for the administration of stem cells or EVs could be a simple but effective solution. The feasibility of delivering stem cells or EVs through IN path have been proved in many articles, though the detailed mechanism is not entirely clear. In current in vivo preclinical studies, we summarized that IN route could improve the biodistribution and retention of stem cells or EVs in the lesion site of the CNS. A more frequent and flexible IN treatment regime also enhances the efficacy of stem cells or EVs.
Yet current preclinical works are mainly focused on verifying the effect of IN delivery on the stem cell and EVs-based therapy for the CNS treatment. Future studies may also need to pay attention to the development of stem cells or EVs formulation for IN delivery. Integrate stem cells or EVs in formulation like hydrogel could prolong mucus retention and increase bioavailability. And these formulations may also help stem cells or EVs to fit IN-delivery devices, which could further simplify the administration procedure and improve delivery efficiency. And for the clinical translation, more experiments in large animals would be necessary, especially considering the vast difference between humans and rodents in the nasal cavity. Experiments on large animals would help understand the influence of formulation on the pharmacokinetics, as the data from large animal is closer to humans. Large animals are also more suitable for IN administration devices. Hence there is still a lot to be improved for the IN delivery of stem cells or EVs. Anyway, despite the intranasal route is still a new approach for the delivery of stem cells or EVs, we believe that it shows great potential and may become a critical factor in promoting the clinical translation of stem cells and EVs-based therapy.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81973252, 82003668), China Postdoctoral Science Foundation (2020M671771), Ten-thousand Talents Program of Zhejiang Province (2018R52049, China), Natural Science Foundation of Zhejiang Province (LQ21H300002, China), Fundamental Research Funds for the Central Universities (2021QNA7021, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Yaosheng Li collected related research article and wrote the manuscript. Honghui Wu revised the manuscript. Xinchi Jiang revised the manuscript. Yunfei Dong revised the manuscript. Juanjuan Zhen revised the manuscript. Jianqing Gao provided the idea and revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Lindvall O., Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z.G., Buller B., Chopp M. Exosomes-beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 3.Muir K.W., Bulters D., Willmot M., Sprigg N., Dixit A., Ward N., et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2) J Neurol Neurosurg Psychiatry. 2020;91:396–401. doi: 10.1136/jnnp-2019-322515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess D.C., Wechsler L.R., Clark W.M., Savitz S.I., Ford G.A., Chiu D., et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 5.Madrazo I., Kopyov O., Ávila-Rodríguez M.A., Ostrosky F., Carrasco H., Kopyov A., et al. Transplantation of human neural progenitor cells (NPC) into putamina of Parkinsonian patients: a case series study, safety and efficacy four years after surgery. Cell Transplant. 2019;28:269–285. doi: 10.1177/0963689718820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B., Jiang X.C., Zhang T.Y., Hu Y.L., Tabata Y., Chen Z., et al. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. Int J Pharm. 2017;531:90–100. doi: 10.1016/j.ijpharm.2017.08.073. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X.C., Xiang J.J., Wu H.H., Zhang T.Y., Zhang D.P., Xu Q.H., et al. Neural stem cells transfected with reactive oxygen species-responsive polyplexes for effective treatment of ischemic stroke. Adv Mater. 2019;31 doi: 10.1002/adma.201807591. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T.Y., Li F.Y., Xu Q.H., Wang Q.Y., Jiang X.C., Liang Z.Y., et al. Ferrimagnetic nanochains-based mesenchymal stem cell engineering for highly efficient post-stroke recovery. Adv Funct Mater. 2019;29:1900603. [Google Scholar]
- 9.Jia Y.L., Cao N., Zhai J.L., Zeng Q., Zheng P., Su R.Y., et al. HGF mediates clinical-grade human umbilical cord-derived mesenchymal stem cells improved functional recovery in a senescence-accelerated mouse model of Alzheimer's disease. Adv Sci (Weinh) 2020;7:1903809. doi: 10.1002/advs.201903809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald C.A., Djuliannisaa Z., Petraki M., Paton M.C.B., Penny T.R., Sutherland A.E., et al. Intranasal delivery of mesenchymal stromal cells protects against neonatal hypoxic-ischemic brain injury. Int J Mol Sci. 2019;20:2449. doi: 10.3390/ijms20102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha S., Park H., Mahmood U., Ra J.C., Suh Y.H., Chang K.A. Human adipose-derived stem cells ameliorate repetitive behavior, social deficit and anxiety in a VPA-induced autism mouse model. Behav Brain Res. 2017;317:479–484. doi: 10.1016/j.bbr.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Li L.M., Zhang Y., Mu J.F., Chen J.C., Zhang C.Y., Cao H.C., et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 13.Thomi G., Surbek D., Haesler V., Joerger-Messerli M., Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. 2019;10:105. doi: 10.1186/s13287-019-1207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long Q.F., Upadhya D., Hattiangady B., Kim D.K., An S.Y., Shuai B., et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114:e3536–e3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 16.Mangraviti A., Tzeng S.Y., Gullotti D., Kozielski K.L., Kim J.E., Seng M., et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander J.F., Seua A.V., Arroyo L.D., Ray P.R., Heiβ-Lücckemann L., Heimann L., et al. Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics. 2021;11:3109–3130. doi: 10.7150/thno.53474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer D., Yu D., Morshed R.A., Li G., Pituch K.C., Gao D.X., et al. Pharmacologic modulation of nasal epithelium augments neural stem cell targeting of glioblastoma. Theranostics. 2019;9:2071–2083. doi: 10.7150/thno.29581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweitzer J.S., Song B., Herrington T.M., Park T.Y., Lee N., Ko S., et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 2020;382:1926–1932. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi D., Magotani H., Kikuchi T., Ikeda M., Hiramatsu S., Yoshida K., et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun. 2020;11:3369. doi: 10.1038/s41467-020-17165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X.Y., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., et al. Blood‒brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163‒164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H.M., Mu Q.H., Bao Z.S., Chen Y.Y., Liu Y.W., Chen J., et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell. 2018;175:1665–1678. doi: 10.1016/j.cell.2018.09.038. e18. [DOI] [PubMed] [Google Scholar]
- 23.Yang A.C., Stevens M.Y., Chen M.B., Lee D.P., Stähli D., Gate D., et al. Physiological blood‒brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583:425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangialasche F., Solomon A., Winblad B., Mecocci P., Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Che T., Levit A., Shoichet B.K., Wacker D., Roth B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature. 2018;555:269–273. doi: 10.1038/nature25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A.C., et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36:3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathys H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., et al. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daly E.J., Singh J.B., Fedgchin M., Cooper K., Lim P., Shelton R.C., et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatr. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lofwall M.R., Walsh S.L., Nunes E.V., Bailey G.L., Sigmon S.C., Kampman K.M., et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs. daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178:764–773. doi: 10.1001/jamainternmed.2018.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plavina T., Fox E.J., Lucas N., Muralidharan K.K., Mikol D. A randomized trial evaluating various administration routes of natalizumab in multiple sclerosis. J Clin Pharmacol. 2016;56:1254–1262. doi: 10.1002/jcph.707. [DOI] [PubMed] [Google Scholar]
- 32.Blair J.C., McKay A., Ridyard C., Thornborough K., Bedson E., Peak M., et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomised controlled trial and economic evaluation. Br Med J. 2019;365:l1226. doi: 10.1136/bmj.l1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borlaug B.A., Melenovsky V., Koepp K.E. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–886. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lochhead J.J., Thorne R.G. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 36.Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M.I., et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8:215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K., Xue Y., Lee J., Kim H.J., Liu Y., Tebon P., et al. A patch of detachable hybrid microneedle depot for localized delivery of mesenchymal stem cells in regeneration therapy. Adv Funct Mater. 2020;30:2000086. doi: 10.1002/adfm.202000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D.Y., Choi S.H., Lee J.S., Kim H.J., Kim H.N., Lee J.E., et al. Feasibility and efficacy of intra-arterial administration of embryonic stem cell derived-mesenchymal stem cells in animal model of Alzheimer's disease. J Alzheimers Dis. 2020;76:1281–1296. doi: 10.3233/JAD-200026. [DOI] [PubMed] [Google Scholar]
- 40.Fathollahi A., Hashemi S.M., Hoseini M.H.M., Tavakoli S., Farahani E., Yeganeh F. Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int Immunopharm. 2021;90:107207. doi: 10.1016/j.intimp.2020.107207. [DOI] [PubMed] [Google Scholar]
- 41.Ma X.Y., Huang M., Zheng M.N., Dai C.X., Song Q.X., Zhang Q., et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J Control Release. 2020;327:688–702. doi: 10.1016/j.jconrel.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Vilaça-Faria H., Salgado A.J., Teixeira F.G. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson's disease?. Cells. 2019;8:118. doi: 10.3390/cells8020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larpthaveesarp A., Pathipati P., Ostrin S., Rajah A., Ferriero D., Gonzalez F.F. Enhanced mesenchymal stromal cells or erythropoietin provide long-term functional benefit after neonatal stroke. Stroke. 2021;52:284–293. doi: 10.1161/STROKEAHA.120.031191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan R.S., Dine K., Bauman B., Lorentsen M., Lin L., Brown H., et al. Intranasal delivery of a novel amnion cell secretome prevents neuronal damage and preserves function in a mouse multiple sclerosis model. Sci Rep. 2017;7:41768. doi: 10.1038/srep41768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch T., Rothoeft T., Teig N., Bauer J.W., Pellegrini G., De Rosa L., et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–332. doi: 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrou P., Kassis I., Levin N., Paul F., Backner Y., Benoliel T., et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574–3588. doi: 10.1093/brain/awaa333. [DOI] [PubMed] [Google Scholar]
- 48.Upadhya D., Hattiangady B., Castro O.W., Shuai B., Kodali M., Attaluri S., et al. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci U S A. 2019;116:287–296. doi: 10.1073/pnas.1814185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adamiak M., Cheng G.M., Bobis-Wozowicz S., Zhao L., Kedracka-Krok S., Samanta A., et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res. 2018;122:296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazini L., Rochette L., Admou B., Amal S., Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21:1306. doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sneddon J.B., Tang Q.Z., Stock P., Bluestone J.A., Roy S., Desai T., et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell. 2018;22:810–823. doi: 10.1016/j.stem.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L.M., Huang S.G., Li S.M., Li M., Shi J., Bai W., et al. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Dev Ther. 2019;13:4331–4340. doi: 10.2147/DDDT.S225613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L.M., Xiao B., Mu J.F., Zhang Y., Zhang C.Y., Cao H.C., et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano. 2019;13:14283–14293. doi: 10.1021/acsnano.9b07598. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y.L., Liu X.S., Wang S.S., Xue P., Zeng Z.L., Yang X.P., et al. Curcumin-activated mesenchymal stem cells derived from human umbilical cord and their effects on MPTP-mouse model of Parkinson's disease: a new bological therapy for Parkinson's disease. Stem Cells Int. 2020;2020:4636397. doi: 10.1155/2020/4636397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You M.J., Bang M., Park H.S., Yang B., Jang K.B., Yoo J., et al. Human umbilical cord-derived mesenchymal stem cells alleviate schizophrenia-relevant behaviors in amphetamine-sensitized mice by inhibiting neuroinflammation. Transl Psychiatry. 2020;10:123. doi: 10.1038/s41398-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang X., Fei G.Q., Liu W.J., Ding J., Wang Y., Wang H., et al. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-κB signaling pathways. Acta Pharmacol Sin. 2020;41:612–619. doi: 10.1038/s41401-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li A., Zhang T.Y., Huang T., Lin R.Y., Mu J.F., Su Y.Q., et al. Iron oxide nanoparticles promote Cx43-overexpression of mesenchymal stem cells for efficient suicide gene therapy during glioma treatment. Theranostics. 2021;11:8254–8269. doi: 10.7150/thno.60160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung J.W., Chang W.H., Bang O.Y., Moon G.J., Kim S.J., Kim S.K., et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. 2021;96:e1012–e1023. doi: 10.1212/WNL.0000000000011440. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.J., Cho K.R., Jang H., Lee N.K., Jung Y.H., Kim J.P., et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase I clinical trial. Alzheimer's Res Ther. 2021;13:154. doi: 10.1186/s13195-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Hallett P.J., Cooper O., Sadi D., Robertson H., Mendez I., Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 2014;7:1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kefalopoulou Z., Politis M., Piccini P., Mencacci N., Bhatia K., Jahanshahi M., et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang J., Zhang H.Y., Kong W., Deng W., Wang D.D., Feng X.B., et al. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9:312. doi: 10.1186/s13287-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Díez-Tejedor E., Gutiérrez-Fernández M., Martínez-Sánchez P., Rodríguez-Frutos B., Ruiz-Ares G., Lara M.L., et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23:2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Thej C., Balasubramanian S., Rengasamy M., Walvekar A., Swamynathan P., Raj S.S., et al. Human bone marrow-derived, pooled, allogeneic mesenchymal stromal cells manufactured from multiple donors at different times show comparable biological functions in vitro, and in vivo to repair limb ischemia. Stem Cell Res Ther. 2021;12:279. doi: 10.1186/s13287-021-02330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta P.K., Dutta S., Kala S., Nekkanti M., Desai S.C., Mahapatra S.S., et al. Phase IV postmarketing surveillance study shows continued efficacy and safety of stempeucel® in patients with critical limb ischemia due to Buerger's disease. Stem Cells Transl Med. 2021;10:1602–1613. doi: 10.1002/sctm.21-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaillard A., Hommel M., Moisan A., Zeffiro T.A., Favre-Wiki I.M., Barbieux-Guillot M., et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11:910–923. doi: 10.1007/s12975-020-00787-z. [DOI] [PubMed] [Google Scholar]
- 69.Zhang S., Lachance B.B., Moiz B., Jia X.F. Optimizing stem cell therapy after ischemic brain injury. J Stroke. 2020;22:286–305. doi: 10.5853/jos.2019.03048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodríguez-Frutos B., Otero-Ortega L., Gutiérrez-Fernández M., Fuentes B., Ramos-Cejudo J., Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7:378–387. doi: 10.1007/s12975-016-0482-6. [DOI] [PubMed] [Google Scholar]
- 71.Schmuck E.G., Koch J.M., Centanni J.M., Hacker T.A., Braun R.K., Eldridge M., et al. Biodistribution and clearance of human mesenchymal stem cells by quantitative three-dimensional cryo-imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl Med. 2016;5:1668–1675. doi: 10.5966/sctm.2015-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aguado B.A., Mulyasasmita W., Su J., Lampe K.J., Heilshorn S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mamidi M.K., Singh G., Husin J.M., Nathan K.G., Sasidharan G., Zakaria Z., et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J Transl Med. 2012;10:229. doi: 10.1186/1479-5876-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Fung J., Lin F. Local inhibition of complement improves mesenchymal stem cell viability and function after administration. Mol Ther. 2016;24:1665–1674. doi: 10.1038/mt.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tahara M., Oeda T., Okada K., Kiriyama T., Ochi K., Maruyama H., et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:298–306. doi: 10.1016/S1474-4422(20)30066-1. [DOI] [PubMed] [Google Scholar]
- 76.Wilson J.G., Liu K.D., Zhuo H.J., Caballero L., McMillan M., Fang X.H., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pels H., Schmidt-Wolf I.G.H., Glasmacher A., Schulz H., Engert A., Diehl V., et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 78.Fransson M., Piras E., Wang H., Burman J., Duprez I., Harris R.A., et al. Intranasal delivery of central nervous system-retargeted human mesenchymal stromal cells prolongs treatment efficacy of experimental autoimmune encephalomyelitis. Immunology. 2014;142:431–441. doi: 10.1111/imm.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santamaria G., Brandi E., Vitola P.L., Grandi F., Ferrara G., Pischiutta F., et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer's mice. Cell Death Differ. 2021;28:203–218. doi: 10.1038/s41418-020-0592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coles L.D., Tuite P.J., Öz G., Mishra U.R., Kartha R.V., Sullivan K.M., et al. Repeated-dose oral N-acetylcysteine in Parkinson's disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol. 2018;58:158–167. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wajs E., Aluisio L., Holder R., Daly E.J., Lane R., Lim P., et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2) J Clin Psychiatry. 2020;81:19m12891. doi: 10.4088/JCP.19m12891. [DOI] [PubMed] [Google Scholar]
- 82.Le Guellec S., Ehrmann S., Vecellio L. In vitro–in vivo correlation of intranasal drug deposition. Adv Drug Deliv Rev. 2021;170:340–352. doi: 10.1016/j.addr.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Scherer T., Sakamoto K., Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17:468–483. doi: 10.1038/s41574-021-00498-x. [DOI] [PubMed] [Google Scholar]
- 84.Galeano C., Qiu Z.F., Mishra A., Farnsworth S.L., Hemmi J.J., Moreira A., et al. The route by which intranasally delivered stem cells enter the central nervous system. Cell Transplant. 2018;27:501–514. doi: 10.1177/0963689718754561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salzman R., Manson J.E., Griffing G.T., Kimmerle R., Ruderman N., McCall A., et al. Intranasal aerosolized insulin–mixed-meal studies and long-term use in type I diabetes. N Engl J Med. 1985;312:1078–1084. doi: 10.1056/NEJM198504253121702. [DOI] [PubMed] [Google Scholar]
- 86.Pietrowsky R., Strüben C., Mölle M., Fehm H.L., Born J. Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatr. 1996;39:332–340. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 87.Bohnen N.I., Twijnstra A., Jolles J. A controlled trial with vasopressin analogue (DGAVP) on cognitive recovery immediately after head trauma. Neurology. 1993;43:103–106. doi: 10.1212/wnl.43.1_part_1.103. [DOI] [PubMed] [Google Scholar]
- 88.Kozlovskaya L., Abou-Kaoud M., Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 89.Kumar N.N., Lochhead J.J., Pizzo M.E., Nehra G., Boroumand S., Greene G., et al. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: distribution, dose-response, and mechanisms of delivery. J Control Release. 2018;286:467–484. doi: 10.1016/j.jconrel.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Q.W., Peng S.H., Hu Y., Wong C.H., Kwan K.M., Chan H.Y.E., et al. Efficient brain uptake and distribution of an expanded CAG RNA inhibitor DB213 via intranasal administration. Eur J Pharm Sci. 2019;127:240–251. doi: 10.1016/j.ejps.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 91.Lapidus K.A.B., Levitch C.F., Perez A.M., Brallier J.W., Parides M.K., Soleimani L., et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatr. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reger M.A., Watson G.S., Green P.S., Wilkinson C.W., Baker L.D., Cholerton B., et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 93.Melby K., Fasmer O.B., Henriksen T.E., Gråwe R.W., Aamo T.O., Spigset O. Actigraphy assessment of motor activity and sleep in patients with alcohol withdrawal syndrome and the effects of intranasal oxytocin. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Craft S., Raman R., Chow T.W., Rafii M.S., Sun C.K., Rissman R.A., et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77:1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Detyniecki K., Van Ess P.J., Sequeira D.J., Wheless J.W., Meng T.C., Pullman W.E. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters-a randomized, double-blind, placebo-controlled trial. Epilepsia. 2019;60:1797–1808. doi: 10.1111/epi.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lei J.A., Feng F., Duan Y.Y., Xu F., Liu Z.G., Lian L.F., et al. Intranasal nerve growth factor attenuating the seizure onset via p75R/Caspase pathway in the experimental epilepsy. Brain Res Bull. 2017;134:79–84. doi: 10.1016/j.brainresbull.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Frazier H.N., Ghoweri A.O., Sudkamp E., Johnson E.S., Anderson K.L., Fox G., et al. Long-term intranasal insulin aspart: a profile of gene expression, memory, and insulin receptors in aged F344 rats. J Gerontol A Biol Sci Med Sci. 2020;75:1021–1030. doi: 10.1093/gerona/glz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tunstall B.J., Kirson D., Zallar L.J., McConnell S.A., Vendruscolo J.C.M., Ho C.P., et al. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.2006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larpthaveesarp A., Pathipati P., Ostrin S., Rajah A., Ferriero D., Gonzalez F.F. Enhanced mesenchymal stromal cells or erythropoietin provide long-term functional benefit after neonatal stroke. Stroke. 2021;52:284–293. doi: 10.1161/STROKEAHA.120.031191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Velthoven C.T., Kavelaars A., van Bel F., Heijnen C.J. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 101.Gao X.L., Tao W.X., Lu W., Zhang Q.Z., Zhang Y., Jiang X.G., et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27:3482–3490. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 102.Altner H., Altner-Kolnberger I. Freeze-fracture and tracer experiments on the permeability of the zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974;154:51–59. doi: 10.1007/BF00221071. [DOI] [PubMed] [Google Scholar]
- 103.Agrawal M., Saraf S., Saraf S., Dubey S.K., Puri A., Gupta U., et al. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J Control Release. 2020;327:235–265. doi: 10.1016/j.jconrel.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 104.Sabir F., Ismail R., Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discov Today. 2020;25:185–194. doi: 10.1016/j.drudis.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Cunha S., Amaral M.H., Lobo J.M.S., Silva A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit Rev Ther Drug Carrier Syst. 2017;34:257–282. doi: 10.1615/CritRevTherDrugCarrierSyst.2017018693. [DOI] [PubMed] [Google Scholar]
- 106.Battaglia L., Panciani P.P., Muntoni E., Capucchio M.T., Biasibetti E., De Bonis P., et al. Lipid nanoparticles for intranasal administration: application to nose-to-brain delivery. Expert Opin Drug Deliv. 2018;15:369–378. doi: 10.1080/17425247.2018.1429401. [DOI] [PubMed] [Google Scholar]
- 107.Wu H.H., Zhou Y., Tabata Y., Gao J.Q. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J Control Release. 2019;294:102–113. doi: 10.1016/j.jconrel.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 108.Dey M., Yu D., Kanojia D., Li G., Sukhanova M., Spencer D.A., et al. Intranasal oncolytic virotherapy with CXCR4-enhanced stem cells extends survival in mouse model of glioma. Stem Cell Rep. 2016;7:471–482. doi: 10.1016/j.stemcr.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y., Wang C.L., Zong S.Y., Qi J.P., Dong X.C., Zhao W.L., et al. The trigeminal pathway dominates the nose-to-brain transportation of intact polymeric manoparticles: evidence from aggregation-caused quenching probes. J Biomed Nanotechnol. 2019;15:686–702. doi: 10.1166/jbn.2019.2724. [DOI] [PubMed] [Google Scholar]
- 110.Ahmad E., Feng Y.H., Qi J.P., Fan W.F., Ma Y.H., He H.S., et al. Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale. 2017;9:1174–1183. doi: 10.1039/c6nr07581a. [DOI] [PubMed] [Google Scholar]
- 111.Reitz M., Demestre M., Sedlacik J., Meissner H., Fiehler J., Kim S.U., et al. Intranasal delivery of neural stem/progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl Med. 2012;1:866–873. doi: 10.5966/sctm.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhuang X.Y., Xiang X.Y., Grizzle W., Sun D.M., Zhang S.Q., Axtell R.C., et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herman S., Fishel I., Offen D. Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cell. 2021;39:1589–1600. doi: 10.1002/stem.3456. [DOI] [PubMed] [Google Scholar]
- 114.Kaminski M., Bechmann I., Kiwit J., Glumm J. Migration of monocytes after intracerebral injection. Cell Adhes Migrat. 2012;6:164–167. doi: 10.4161/cam.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldmann J., Kwidzinski E., Brandt C., Mahlo J., Richter D., Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 116.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balyasnikova I.V., Prasol M.S., Ferguson S.D., Han Y., Ahmed A.U., Gutova M., et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol Ther. 2014;22:140–148. doi: 10.1038/mt.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buchowicz B., Chen B.S., Bidot S., Bruce B.B., Newman N.J., Saindane A.M., et al. Prediction of postoperative risk of raised intracranial pressure after spontaneous skull base cerebrospinal fluid leak repair. J Neuro Ophthalmol. 2021;41:e490–e497. doi: 10.1097/WNO.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei L., Fraser J.L., Lu Z.Y., Hu X.Y., Yu S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim H.Y., Kim T.J., Kang L., Kim Y.J., Kang M.K., Kim J., et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- 121.Park S.A., Ryu C.H., Kim S.M., Lim J.Y., Park S.I., Jeong C.H., et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int J Oncol. 2011;38:97–103. [PubMed] [Google Scholar]
- 122.Simorgh S., Alizadeh R., Shabani R., Karimzadeh F., Seidkhani E., Majidpoor J., et al. Olfactory mucosa stem cells delivery via nasal route: a simple way for the treatment of Parkinson disease. Neurotox Res. 2021;39:598–608. doi: 10.1007/s12640-020-00290-1. [DOI] [PubMed] [Google Scholar]
- 123.Lu M.H., Ji W.L., Chen H., Sun Y.Y., Zhao X.Y., Wang F., et al. Intranasal transplantation of human neural stem cells ameliorates Alzheimer's disease-like pathology in a mouse model. Front Aging Neurosci. 2021;13:650103. doi: 10.3389/fnagi.2021.650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji G., Liu M., Zhao X.F., Liu X.Y., Guo Q.L., Guan Z.F., et al. NF-κB signaling is involved in the effects of intranasally engrafted human neural stem cells on neurofunctional improvements in neonatal rat hypoxic-ischemic encephalopathy. CNS Neurosci Ther. 2015;21:926–935. doi: 10.1111/cns.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ninomiya K., Iwatsuki K., Ohnishi Y., Ohkawa T., Yoshimine T. Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J Neurosurg Spine. 2015;23:111–119. doi: 10.3171/2014.10.SPINE14690. [DOI] [PubMed] [Google Scholar]
- 126.Guo S.W., Perets N., Betzer O., Ben-Shaul S., Sheinin A., Michaelevski I., et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 127.Vaes J.E.G., van Kammen C.M., Trayford C., van der Toorn A., Ruhwedel T., Benders M.J.N.L., et al. Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity. Glia. 2021;69:655–680. doi: 10.1002/glia.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Betzer O., Perets N., Angel A., Motiei M., Sadan T., Yadid G., et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 129.Soria B., Martin-Montalvo A., Aguilera Y., Mellado-Damas N., López-Beas J., Herrera-Herrera I., et al. Human mesenchymal stem cells prevent neurological complications of radiotherapy. Front Cell Neurosci. 2019;13:204. doi: 10.3389/fncel.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perets N., Hertz S., London M., Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of Btbr mice. Mol Autism. 2018;9:57. doi: 10.1186/s13229-018-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ezquer F., Quintanilla M.E., Morales P., Santapau D., Ezquer M., Kogan M.J., et al. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict Biol. 2019;24:994–1007. doi: 10.1111/adb.12675. [DOI] [PubMed] [Google Scholar]
- 132.Tang W., Fan W.P., Lau J., Deng L.M., Shen Z.Y., Chen X.Y. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48:2967–3014. doi: 10.1039/c8cs00805a. [DOI] [PubMed] [Google Scholar]
- 133.Aldape K., Brindle K.M., Chesler L., Chopra R., Gajjar A., Gilbert M.R., et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16:509–520. doi: 10.1038/s41571-019-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clavreul A., Pourbaghi-Masouleh M., Roger E., Lautram N., Montero-Menei C.N., Menei P. Human mesenchymal stromal cells as cellular drug-delivery vectors for glioblastoma therapy: a good deal?. J Exp Clin Cancer Res. 2017;36:135. doi: 10.1186/s13046-017-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu D., Li G., Lesniak M.S., Balyasnikova I.V. Intranasal delivery of therapeutic stem cells to glioblastoma in a mouse model. J Vis Exp. 2017:55845. doi: 10.3791/55845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Danielyan L., Beer-Hammer S., Stolzing A., Schäfer R., Siegel G., Fabian C., et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer's and Parkinson's disease. Cell Transplant. 2014;23(Suppl 1):S123–S139. doi: 10.3727/096368914X684970. [DOI] [PubMed] [Google Scholar]
- 137.Nandakumar S., Shahani P., Datta I., Pal R. Interventional strategies for Parkinson disease: can neural precursor cells forge a path ahead?. ACS Chem Neurosci. 2021;12:3785–3794. doi: 10.1021/acschemneuro.1c00525. [DOI] [PubMed] [Google Scholar]
- 138.Alizadeh R., Boroujeni M.E., Kamrava S.K., Tehrani A.M., Bagher Z., Heidari F., et al. From transcriptome to behavior: intranasal injection of late passage human olfactory stem cells displays potential in a rat model of Parkinson's disease. ACS Chem Neurosci. 2021;12:2209–2217. doi: 10.1021/acschemneuro.1c00225. [DOI] [PubMed] [Google Scholar]
- 139.Harach T., Jammes F., Muller C., Duthilleul N., Cheatham V., Zufferey V., et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer's disease. Neurobiol Aging. 2017;51:83–96. doi: 10.1016/j.neurobiolaging.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 140.Losurdo M., Pedrazzoli M., D'Agostino C., Elia C.A., Massenzio F., Lonati E., et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xtg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068–1084. doi: 10.1002/sctm.19-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalani A., Chaturvedi P., Kamat P.K., Maldonado C., Bauer P., Joshua I.G., et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomi G., Joerger-Messerli M., Haesler V., Muri L., Surbek D., Schoeberlein A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells. 2019;8:855. doi: 10.3390/cells8080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sisa C., Kholia S., Naylor J., Herrera Sanchez M.B., Bruno S., Deregibus M.C., et al. Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front Physiol. 2019;10:282. doi: 10.3389/fphys.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emberson J., Lees K.R., Lyden P., Blackwell L., Albers G., Bluhmki E., et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iwami K., Momota H., Natsume A., Kinjo S., Nagatani T., Wakabayashi T. A novel method of intracranial injection via the postglenoid foramen for brain tumor mouse models. J Neurosurg. 2012;116:630–635. doi: 10.3171/2011.10.JNS11852. [DOI] [PubMed] [Google Scholar]
- 146.Beigi Boroujeni F., Pasbakhsh P., Mortezaee K., Pirhajati V., Alizadeh R., Aryanpour R., et al. Intranasal delivery of SDF-1α-preconditioned bone marrow mesenchymal cells improves remyelination in the cuprizone-induced mouse model of multiple sclerosis. Cell Biol Int. 2020;44:499–511. doi: 10.1002/cbin.11250. [DOI] [PubMed] [Google Scholar]
- 147.Simorgh S., Bagher Z., Farhadi M., Kamrava S.K., Boroujeni M.E., Namjoo Z., et al. Magnetic targeting of human olfactory mucosa stem cells following intranasal administration: a novel approach to Parkinson's disease treatment. Mol Neurobiol. 2021;58:3835–3847. doi: 10.1007/s12035-021-02392-z. [DOI] [PubMed] [Google Scholar]
- 148.Donega V., Nijboer C.H., van Velthoven C.T., Youssef S.A., de Bruin A., van Bel F., et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78:520–526. doi: 10.1038/pr.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bahadur S., Sachan N., Harwansh R.K., Deshmukh R. Nanoparticlized system: promising approach for the management of Alzheimer's disease through intranasal delivery. Curr Pharmaceut Des. 2020;26:1331–1344. doi: 10.2174/1381612826666200311131658. [DOI] [PubMed] [Google Scholar]
- 150.Robertson N.J., Meehan C., Martinello K.A., Avdic-Belltheus A., Boggini T., Mutshiya T., et al. Human umbilical cord mesenchymal stromal cells as an adjunct therapy with therapeutic hypothermia in a piglet model of perinatal asphyxia. Cytotherapy. 2021;23:521–535. doi: 10.1016/j.jcyt.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donega V., van Velthoven C.T., Nijboer C.H., van Bel F., Kas M.J., Kavelaars A., et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8 doi: 10.1371/journal.pone.0051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Farfán N., Carril J., Redel M., Zamorano M., Araya M., Monzón E., et al. Intranasal administration of mesenchymal stem cell secretome reduces hippocampal oxidative stress, neuroinflammation and cell death, improving the behavioral outcome following perinatal asphyxia. Int J Mol Sci. 2020;21:7800. doi: 10.3390/ijms21207800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsivion-Visbord H., Perets N., Sofer T., Bikovski L., Goldshmit Y., Ruban A., et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10:305. doi: 10.1038/s41398-020-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Y.K., Zhu W.W., Wu M.H., Wu Y.H., Liu Z.X., Liang L.M., et al. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson's disease. Stem Cell Rep. 2018;11:171–182. doi: 10.1016/j.stemcr.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Venkataramana N.K., Kumar S.K., Balaraju S., Radhakrishnan R.C., Bansal A., Dixit A., et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 156.Salama M., Sobh M., Emam M., Abdalla A., Sabry D., El-Gamal M., et al. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson's disease. Exp Ther Med. 2017;13:976–982. doi: 10.3892/etm.2017.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Narbute K., Piļipenko V., Pupure J., Dzirkale Z., Jonavičė U., Tunaitis V., et al. Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-Hydroxydopamine-treated rats. Stem Cells Transl Med. 2019;8:490–499. doi: 10.1002/sctm.18-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Narbute K., Pilipenko V., Pupure J., Klinovičs T., Auders J., Jonavičė U., et al. Time-dependent memory and gait improvement by intranasally-administered extracellular vesicles in Parkinson's disease model rats. Cell Mol Neurobiol. 2021;41:605–613. doi: 10.1007/s10571-020-00865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Segal-Gavish H., Karvat G., Barak N., Barzilay R., Ganz J., Edry L., et al. Mesenchymal stem cell transplantation promotes neurogenesis and ameliorates autism related behaviors in Btbr mice. Autism Res. 2016;9:17–32. doi: 10.1002/aur.1530. [DOI] [PubMed] [Google Scholar]
- 160.Cohen-Pfeffer J.L., Gururangan S., Lester T., Lim D.A., Shaywitz A.J., Westphal M., et al. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 161.Spiliopoulos S., Festas G., Reppas L., Brountzos E. Intra-arterial administration of cell-based biological agents for ischemic stroke therapy. Expert Opin Biol Ther. 2019;19:249–259. doi: 10.1080/14712598.2019.1566454. [DOI] [PubMed] [Google Scholar]
- 162.Gordon D., Pavlovska G., Glover C.P., Uney J.B., Wraith D., Scolding N.J. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci Lett. 2008;448:71–73. doi: 10.1016/j.neulet.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boika A., Aleinikava N., Chyzhyk V., Zafranskaya M., Nizheharodava D., Ponomarev V. Mesenchymal stem cells in Parkinson's disease: motor and nonmotor symptoms in the early posttransplant period. Surg Neurol Int. 2020;11:380. doi: 10.25259/SNI_233_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hogan R.E., Gidal B.E., Koplowitz B., Koplowitz L.P., Lowenthal R.E., Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61:455–464. doi: 10.1111/epi.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Paudel K.S., Hammell D.C., Agu R.U., Valiveti S., Stinchcomb A.L. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36:1088–1097. doi: 10.3109/03639041003657295. [DOI] [PubMed] [Google Scholar]
- 166.Xia H.M., Jin H.L., Cheng Y.F., Cheng Z.Q., Xu Y.X. The controlled release and anti-inflammatory activity of a tetramethylpyrazine-loaded thermosensitive poloxamer hydrogel. Pharm Res (N Y) 2019;36:52. doi: 10.1007/s11095-019-2580-0. [DOI] [PubMed] [Google Scholar]
- 167.Ma J., Wang C., Sun Y., Pang L., Zhu S., Liu Y., et al. Comparative study of oral and intranasal puerarin for prevention of brain injury induced by acute high-altitude hypoxia. Int J Pharm. 2020;591:120002. doi: 10.1016/j.ijpharm.2020.120002. [DOI] [PubMed] [Google Scholar]
- 168.Gholizadeh H., Cheng S., Pozzoli M., Messerotti E., Traini D., Young P., et al. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin Drug Deliv. 2019;16:453–466. doi: 10.1080/17425247.2019.1597051. [DOI] [PubMed] [Google Scholar]
- 169.Li Y., He J.T., Lyu X.L., Yuan Y., Wang G.Z., Zhao B.H. Chitosan-based thermosensitive hydrogel for nasal delivery of exenatide: effect of magnesium chloride. Int J Pharm. 2018;553:375–385. doi: 10.1016/j.ijpharm.2018.10.071. [DOI] [PubMed] [Google Scholar]
- 170.Xu X.F., Shen Y., Wang W., Sun C.M., Li C., Xiong Y.R., et al. Preparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochloride. Eur J Pharm Biopharm. 2014;88:998–1004. doi: 10.1016/j.ejpb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 171.Li Y., Li J.F., Zhang X., Ding J.J., Mao S.R. Non-ionic surfactants as novel intranasal absorption enhancers: in vitro and in vivo characterization. Drug Deliv. 2016;23:2272–2279. doi: 10.3109/10717544.2014.971196. [DOI] [PubMed] [Google Scholar]
- 172.Xia Y.J., Li L., Huang X.W., Wang Z.M., Zhang H., Gao J., et al. Performance and toxicity of different absorption enhancers used in the preparation of Poloxamer thermosensitive in situ gels for ketamine nasal administration. Drug Dev Ind Pharm. 2020;46:697–705. doi: 10.1080/03639045.2020.1750625. [DOI] [PubMed] [Google Scholar]
- 173.Shah B., Khunt D., Misra M., Padh H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: formulation, physicochemical and pharmacokinetic consideration. Eur J Pharm Sci. 2016;91:196–207. doi: 10.1016/j.ejps.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 174.Na L.D., Mao S.R., Wang J., Sun W. Comparison of different absorption enhancers on the intranasal absorption of isosorbide dinitrate in rats. Int J Pharm. 2010;397:59–66. doi: 10.1016/j.ijpharm.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 175.Li Y.H., Yu J.W., Xi J.Y., Yu W.B., Liu J.C., Wang Q., et al. Fasudil enhances therapeutic efficacy of neural stem cells in the mouse model of MPTP-induced Parkinson's disease. Mol Neurobiol. 2017;54:5400–5413. doi: 10.1007/s12035-016-0027-8. [DOI] [PubMed] [Google Scholar]
- 176.Tiozzo Fasiolo L., Manniello M.D., Tratta E., Buttini F., Rossi A., Sonvico F., et al. Opportunity and challenges of nasal powders: drug formulation and delivery. Eur J Pharm Sci. 2018;113:2–17. doi: 10.1016/j.ejps.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 177.Wang B., Liu P., Jiang W.G., Pan H.H., Xu X.R., Tang R.K. Yeast cells with an artificial mineral shell: protection and modification of living cells by biomimetic mineralization. Angew Chem Int Ed Engl. 2008;47:3560–3564. doi: 10.1002/anie.200704718. [DOI] [PubMed] [Google Scholar]
- 178.Jiang B., Yan L., Miao Z., Li E., Wong K.H., Xu R.H. Spheroidal formation preserves human stem cells for prolonged time under ambient conditions for facile storage and transportation. Biomaterials. 2017;133:275–286. doi: 10.1016/j.biomaterials.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 179.Akers J.C., Ramakrishnan V., Yang I., Hua W., Mao Y., Carter B.S., et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 2016;17:125–132. doi: 10.3233/CBM-160609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mocchi M., Bari E., Marrubini G., Bonda A.F., Perteghella S., Tartara F., et al. Freeze-dried mesenchymal stem cell-secretome pharmaceuticalization: optimization of formulation and manufacturing process robustness. Pharmaceutics. 2021;13:1129. doi: 10.3390/pharmaceutics13081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Charoenviriyakul C., Takahashi Y., Nishikawa M., Takakura Y. Preservation of exosomes at room temperature using lyophilization. Int J Pharm. 2018;553:1–7. doi: 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 182.El Baradie K.B.Y., Nouh M., O'Brien F., Iii, Liu Y.T., Fulzele S., Eroglu A., et al. Freeze-dried extracellular vesicles from adipose-derived stem cells prevent hypoxia-induced muscle cell injury. Front Cell Dev Biol. 2020;8:181. doi: 10.3389/fcell.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ali J., Ali M., Baboota S., Sahani J.K., Ramassamy C., Dao L., et al. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharmaceut Des. 2010;16:1644–1653. doi: 10.2174/138161210791164108. [DOI] [PubMed] [Google Scholar]
- 184.Graff C.L., Pollack G.M. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 185.DeSesso J.M. The relevance to humans of animal models for inhalation studies of cancer in the nose and upper airways. Qual Assur. 1993;2:213–231. [PubMed] [Google Scholar]
- 186.Harkema J.R. Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ Health Perspect. 1990;85:231–238. doi: 10.1289/ehp.85-1568334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saccone P.A., Lindsey A.M., Koeppe R.A., Zelenock K.A., Shao X., Sherman P., et al. Intranasal opioid administration in rhesus monkeys: PET imaging and antinociception. J Pharmacol Exp Ther. 2016;359:366–373. doi: 10.1124/jpet.116.235192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johnson-Delaney C.A., Orosz S.E. Rabbit respiratory system: clinical anatomy, physiology and disease. Vet Clin North Am Exot Anim Pract. 2011;14:257–266. doi: 10.1016/j.cvex.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 189.Lindhardt K., Bagger M., Andreasen K.H., Bechgaard E. Intranasal bioavailability of buprenorphine in rabbit correlated to sheep and man. Int J Pharm. 2001;217:121–126. doi: 10.1016/s0378-5173(01)00591-9. [DOI] [PubMed] [Google Scholar]
- 190.Weiland L.C., Kluge K., Kutter A.P.N., Kronen P.W. Clinical evaluation of intranasal medetomidine-ketamine and medetomidine-S(+)-ketamine for induction of anaesthesia in rabbits in two centres with two different administration techniques. Vet Anaesth Analg. 2017;44:98–105. doi: 10.1111/vaa.12408. [DOI] [PubMed] [Google Scholar]