Abstract
Objectives
This study was aimed to evaluating the influences of methotrexate iontophoresis on functional lifestyle disabilities, functional capacity, and pain after a 6-min walking distance (6MWD) test in patients with plantar psoriasis (PP).
Patients and methods
Forty‑five patients (29 females, 16 males; mean age 33.9±6.6 years; range, 20 to 45 years) with hyperkeratotic PP were enrolled in the randomized, placebo-controlled, parallel-groups, double-blinded study. They were assigned randomly into the active methotrexate iontophoresis (MI) group (n=23) and the placebo iontophoresis group (n=22). The patients in both groups were assessed before starting the treatment intervention and after completing eight sessions of the treatment intervention through functional lifestyle disabilities measured on the Arabic version of psoriasis disability index, functional capacity using the 6MWD test, and pain after the 6MWD test.
Results
The pre-treatment measurements of the three variables among the two groups did not reveal statistically significant differences (p>0.05). However, there were statistically significant differences in the post-treatment results between the two groups (p<0.05). Additionally, the pre-and post-treatment values of the three outcome measures revealed statistically significant differences within the MI group (p<0.05). In contrast, there were no significant differences within the placebo group (p>0.05).
Conclusion
Methotrexate iontophoresis is effective in improving functional lifestyle disabilities, enhancing functional capacity, and decreasing pain in patients with PP.
Keywords: Functional capacity, functional lifestyle disabilities, pain, plantar psoriasis
Introduction
Psoriasis is a long-lasting inflammatory disorder that may be recognized by its cutaneous and extra- cutaneous symptoms. Psoriasis can be generalized or localized in various forms, such as plaque, erythrodermic, articular, nail, pustular, guttate, oral, flexural, and ocular.[1] Palmoplantar psoriasis (PPP) is a localized type of psoriasis that emerges on the palms and the soles, and it often appears with psoriasis on any other part of the body.[2] Palmoplantar psoriasis is a chronic, debilitating disorder that influences about 11 to 39% of psoriatic patients.[3] The categorizations of PPP fluctuated from hyperkeratosis and thick plaques with fissuring to pustular lesions.[4]
While PPP may occur on up to five percent of the total body surface area, the consequent disabilities and disfigurements are usually sufficient to produce substantial difficulties in quality of life.[5] Patients with PPP complain about difficulties in walking and a great degree of pain that may lead to an incapability to work.[6] Palmoplantar psoriasis is frequently accompanied by itching, burning sensation, and painful fissuring, with tremendous negative consequences for a variety of functions, cosmetic appearance, and occupation.[7,8]
Corticosteroids are effective topical medications for PPP; however, prolonged use leads to cutaneous side effects such as telangiectasia and atrophy.[9] While systemic medicines such as retinoids, ciclosporin, and methotrexate may be used in severe cases or when the patient is not responding to local medications, the use of these drugs is restricted because of their side effects.[10]
The composition of methotrexate is 4-Amino-N- methyl pteroylglutamic acid, which is similar to that of folic acid. Various topical formulas of methotrexate can be used in the treatment of psoriasis.[11] Methotrexate in combination with a gel formula has a modest effect and continues to be used as an ideal treatment option for moderate to severe psoriatic cases.[12] Its anti- inflammatory effects have established methotrexate in the treatment of PPP.[13]
Iontophoresis, the application of a small electrical current to the affected area of the skin on which the drug is spread, optimizes the penetration of methotrexate through the skin.[14,15]
Painful and itching of the soles resulting from plantar psoriasis (PP) have the anticipated effect of the reduced ambulatory performance. The main idea behind this study is to realize the effects of PP on the feet and its influences on locomotion. There are multiple studies with different treatment modalities for PPP.[16] However, there are few studies of methotrexate iontophoresis (MI) in the treatment of PPP.[17,18] To the best of our knowledge, there are no previous studies on the application of MI in the treatment of PP. Therefore, a rigorous trial has been presented to evaluate the effects of MI on functional lifestyle disabilities, functional capacity, and pain after the 6MWD test in patients with PP.
Patients and Methods
The randomized, placebo-controlled, parallel- groups, double-blinded study included 45 patients (29 females, 16 males; mean age 33.9±6.6 years; range, 20 to 45 years) with psoriasis, and of these, 36 had PP, and nine had PPP. The patients were referred to the Department of Health and Rehabilitation Sciences, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, between January 2019 and March 2020. The inclusion criteria for the patients were that they should be aged between 20 and 45 years, suffering from hyperkeratotic PP with the affected psoriasis areas exceeding 30% of the soles of the feet, histopathological confirmation of the diagnosis, a body mass index (BMI) of not more than 30, and having had the disease for at least six months. The exclusion criteria were vascular or circulatory disorders, diabetes mellitus, lactating or ongoing pregnancy, malignancy, palmoplantar pustular psoriasis, orthopedic implants in the lower limbs, or any infection in the soles of the feet. The patients who had been on local or systemic medications for psoriasis and been treated with corticosteroids during the first month preceding participation in the study were excluded.
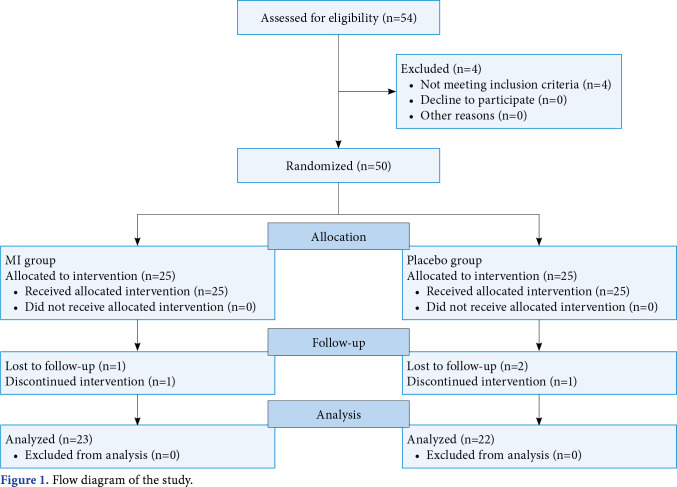
The required sample size was determined based on the functional capacity (as represented by the distance walked during the 6-min walk test). Estimates of means (mean 1=395.40 m; mean 2=363 m) and standard deviations (SD 1=37.85 m; SD 2=43.08) for the walked distance were collected from a pilot study that included 10 patients divided into two equal groups that received the same treatment. Assuming an alpha level of 0.05 and power of 80% in an independent t-test, 21 patients were required per group (total sample of 42 patients). Therefore, the sample was increased to 50 patients to account for a 20% dropout rate. Of these, three were excluded from the study during the treatment since they could not attend the treatment sessions regularly, and two patients withdrew without giving any reason for a total of 45 patients, with 23 in the MI group and 22 in the placebo group (Figure 1).
Figure 1. Flow diagram of the study.
The computer system’s randomization setup that uses a table (Microsoft Excel) was utilized for allocating the subjects randomly to the active MI group or placebo iontophoresis group. The records in the database were updated until the treatments were consistently assigned. Sealed envelopes containing the assigned treatment were presented to the department of physical therapy. A blinded investigator opened every envelope and arranged the patients numerically. The patients remained blinded throughout the conduct of the study. The patients were randomized in a 1:1 ratio into the MI group and the placebo group.
The patients were assessed for functional lifestyle disabilities, functional capacity, and pain after the 6MWD test before the treatment intervention and after completing eight sittings of the treatment intervention. Functional lifestyle disabilities were evaluated by the Arabic version of the psoriasis disability index (PDI), which is a valid and reliable self-reported questionnaire that evaluates and quantifies the influences of psoriasis on the patients’ daily lives. The PDI consisted of 15 clauses that referred to the effects of psoriasis on five domains: activity of daily living (ADL), school or work, personal communications, leisure, and treatment. Every clause had four possible responses according to patients’ self-explanations (0= Not at all, 1= A little, 2= A lot, and 3= Very much). Any clause that was not responded to was assigned a zero. The PDI was then calculated by summating the scores on the 15 clauses. The maximum possible score was 45, and the minimum possible score was 0. Higher values meant greater impairment of the functional lifestyle than lower values.[19,20]
Functional capacity was determined by a 6-min walk distance (6MWD) test, which measures the distance that a patient can walk on a hard and flat path in 6 min. Each patient was instructed to walk on an indoor driveway that was straight, hard, long, and flat. The walking pathway should be typical in all evaluation times for all patients with a distance of 30 m. The distance was measured in meters after finishing the 6MWD test.[21,22]
Pain at the soles of the feet was assessed after completing the 6MWD test by the Visual Analog Scale (VAS). The reported pain intensity was recorded by a single handmade mark placed at a point along the length of a horizontal 10 cm line that represented a continuum between the two ends of VAS. The words “no pain” (0) were written at the left-hand end, and “excruciating pain” (10) was written at the right-hand end of the line. The measurement of pain intensity started from the beginning point (left end = 0) of VAS to the patients’ marks and was recorded in centimeters as pain intensity after the 6MWD test.[22,23]
A direct current (DC) stimulation (Endometer Type Endomed 381 DC; Enraf-Nonius B.V., Echt, Nederlands) was used. Program 6, which provides a Galvanic/Iontophoresis current, was chosen out of 15 possible programs.
Patients in the MI group were placed in a supine, relaxed, and well-supported position. The soles of the feet were cleaned with a sterilized swab to remove any dirt. Any cracks, wounds, or scratches in the soles were covered with a medicated gel before beginning the procedure. A piece of gauze was immersed in a 1 mg/mL solution of methotrexate and placed on the soles of the feet. Afterward, the gauze was covered with aluminum foil that was secured with an adhesive strap and connected with clips to the cathode of the iontophoresis apparatus as an active electrode. The other padded electrode was soaked in water and placed at any location on the posterior aspect of the leg close to the active electrode and fixed with an adhesive strap. The padded electrode was then connected to the anode as a dispersive electrode. The iontophoresis apparatus was turned on, and the intensity of the current was slowly increased up to the limit of the patient’s tolerance. The intensity of the current was 5-10 mA, and the procedure was continued for up to 15 min. The patients felt numbness and mild tingling sensations during the treatment procedure. After the procedure was performed on the sole of one foot, it was repeated on the sole of the other foot.[17,18] The patients in the placebo group were subjected to the same procedure without the application of an electric current. As a placebo treatment, though the iontophoresis device was attached, it was not switched on. The treatment procedures were carried out two times per week for four consecutive weeks (eight sessions per patient) with both groups.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 25.0 software (IBM Corp., Armonk, NY, USA). Data were tested for normality by the Kolmogorov–Smirnov test. The demographic differences at the baseline between groups concerning continuous and categorical variables were assessed using an independent t-test and Fisher exact probability test, respectively. The between- group outcome measures were examined using the independent sample t-test, and within-group measured outcomes were examined using a dependent sample t-test. A p value of <0.05 was considered statistically significant.
Results
The demographics of the two groups were well-balanced. The baseline characteristics were homogenous in both groups in terms of age, sex, BMI, duration of PP. The site of treatment (the sole only/sole and the palm), percentages of the involvement of the sole (right foot and left foot), and sites other than the sole (the dorsum of the foot/the dorsal surface of the hand/the nail/none) were not statistically significant (p>0.05; Table 1).
Table 1. Baseline characteristics of both groups.
| MI group (n=23) | Placebo group (n=22) | p | |||
| n | Mean±SD | n | Mean±SD | ||
| Characteristics | |||||
| Age (year) | 34.3±6.9 | 33.6±6.3 | 0.721† | ||
| Sex | |||||
| Male | 9 | 7 | |||
| Female | 14 | 15 | 0.608‡ | ||
| BMI (kg/m2) | 24.8±2.0 | 25.8±2.7 | 0.165† | ||
| Duration of PP (year) | 3.5±0.8 | 3.3±0.8 | 0.525† | ||
| The site of treatment | |||||
| The sole only | 17 | 19 | |||
| Sole and the palm of the hand | 6 | 3 | 0.296‡ | ||
| Percent of psoriasis areas of the sole of the foot | |||||
| Right side | 60.7±14.8 | 61.3±17.9 | 0.892† | ||
| Left side | 62.3±16.3 | 58.9±14.2 | 0.455† | ||
| Sites other than the sole of the foot | |||||
| The dorsum of the foot | 4 | 3 | |||
| The dorsal surface of the hand | 2 | 3 | |||
| The nail | 1 | 2 | 0.881‡ | ||
| None | 16 | 14 | |||
| MI: Methotrexate iontophoresis; SD: Standard deviation; BMI: Body mass index; PP: Planter psoriasis; Level of significance at p<0.05; † Independent t-test; ‡ Fisher exact test. | |||||
The findings on the PDI (Table 2) revealed that there were no significant inter-group pre-treatment differences in the total PDI scores and the five domains (p>0.05).Thereweresignificantinter-groupdifferences post-treatment in the total PDI scores and the five domains, ADL, work, personal communications, leisure, and treatment (p<0.05). The results of the total PDI scores and the five domains pre-and post-treatment showed significant differences within the MI group (p<0.05). No significant differences were observed in the placebo group (p>0.05). Improvement was detected in the MI group, and there was no improvement in the placebo group.
Table 2. Differences of the total scores of PDI and its five domains between both groups.
| MI group (n=23) | Placebo group (n=22) | ||||||
| n | Mean±SD | Sig. | n | Mean±SD | Sig. | Sig. | |
| ADL | |||||||
| Pre-treatment | 12.9±1.0 | 12.8±1.0 | 0.758 | ||||
| Post-treatment | 7.6±3.2 | 12.4±2.7 | < 0.001* | ||||
| Sig. Percent of change Work |
41.4 | <0.001* | 3.6 | 0.909 | |||
| Pre-treatment | 8.0±0.8 | 7.9±0.8 | 0.582 | ||||
| Post-treatment | 5.1±2.5 | 7.7±2.0 | 0.0005* | ||||
| Sig. Percent of change Personal communications |
36.7 | 0.0001* | 2.9 | 0.584 | |||
| Pre-treatment | 4.6±0.5 | 4.8±0.7 | 0.251 | ||||
| Post-treatment | 3.3±1.3 | 4.3±1.5 | 0.022* | ||||
| Sig. Percent of change Leisure |
27.6 | 0.0005* | 10.5 | 0.230 | |||
| Pre-treatment | 10.1±1.4 | 9.9±1.5 | 0.606 | ||||
| Post-treatment | 7.7±3.0 | 9.7±2.2 | 0.015* | ||||
| Sig. Percent of change Treatment |
23.6 | 0.0009* | 1.8 | 0.680 | |||
| Pre-treatment | 2.9±0.3 | 2.9±0.4 | 0.604 | ||||
| Post-treatment | 2.1±0.9 | 2.8±0.6 | 0.005* | ||||
| Sig. Percent of change The total PDI scores |
28.2 | 0.006* | 3.1 | 0.162 | |||
| Pre-treatment | 38.6±2.1 | 38.2±2.1 | 0.546 | ||||
| Post-treatment Sig. Percent of change |
33.1 | 25.8±8.3 | <0.001* | 3.6 | 36.8±8.5 | 0.417 | <0.001* |
| PDI: Psoriasis disability index; MI: Methotrexate iontophoresis; SD: Standard deviation; ADL: Activity of daily living; Sig: Level of significance at p<0.05; * Significant. | |||||||
The values of the 6MWD test (Table 3) revealed no significant inter-group differences pre-treatment (p>0.05); however, significant inter-group differences were identified post-treatment (p<0.05). The recorded values of the 6MWD test pre-and post-treatment showed significant differences in the MI group (p<0.05). However, there were no significant differences within the placebo group (p>0.05). The increase in the distance walked by the patients in the MI group was higher than in the placebo group.
Table 3. Differences of the 6MWD test between both groups.
| MI group (n=23) | Placebo group (n=22) | ||||||
| n | Mean±SD | Sig. | n | Mean±SD | Sig. | Sig. | |
| Pre-treatment | 341.8±18.9 | 348.1±21.4 | 0.306 | ||||
| Post-treatment | 380.5±48.6 | 350.5±28.3 | 0.015* | ||||
| Sig. | 0.0003* | 0.337 | |||||
| Percent of change | 11.3 | 0.7 | |||||
| 6MWD: 6-min walking distance; MI: Methotrexate iontophoresis; SD: Standard deviation; Sig: Level of significance at p<0.05; * Significant. | |||||||
The VAS scores after the 6MWD test (Table 4) proved that there was no significant inter- group difference pre-treatment (p>0.05); however, the post-treatment inter-group differences were significant (p<0.05). The pre-and post-treatment VAS scores after the 6MWD test were significantly different in the MI group (p<0.05). However, the differences within the placebo group were not significant (p>0.05). The scores demonstrated that the pain felt by the patients in the MI group after the 6MWD test was more reduced than in the placebo group.
Table 4. Differences of the VAS after 6MWD test between both groups.
| MI group (n=23) | Placebo group (n=22) | Sig. | |||||
| n | Mean±SD | Sig. | n | Mean±SD | Sig. | ||
| Pre-treatment | 8.5±0.8 | 8.4±0.8 | 0.586 | ||||
| Post-treatment | 4.6±1.6 | 7.9±1.5 | <0.001 | ||||
| Sig. | <0.001* | 0.159 | |||||
| % of change | 45.8 | 6.3 | |||||
| VAS: Visual Analog Scale; 6MWD: 6-min walk distance; MI: Methotrexate iontophoresis; SD: Standard deviation; Sig: Level of significance at p<0.05; * Significant. | |||||||
Discussion
Palmoplantar psoriasis is accompanied by highly negative influences on quality of life, self-care, and normal activity, as well as significant dependence on local treatments.[24,25] This study aimed to assess the influence of MI in functional lifestyle disabilities, functional capacity, and pain after the 6MWD test in patients with PP.
The results of this study showed that there were satisfactory enhancements to the total PDI scores with improvement in all five domains. In addition, there were marked increases in the distance walked and decreases in VAS scores after the 6MWD test in patients in the MI group. Moreover, there were no apparent changes in all outcome measures with patients in the placebo group. The findings of the MI group were expressive due to the improvement in the PDI in addition to reduced intensity of pain, which was accompanied by an increase in the distance walked.
In an analog study that compared MI and coal tar ointment in the treatment of PPP, there were improvements in both groups.[17] However, the improvements were greater in the MI group than in the other group that was treated with coal tar ointment. The findings from another study did not reveal any significant differences between MI and local corticosteroid in the management of palmar psoriasis. However, in these studies and the one presented here, positive and significant improvements were experienced by groups subjected to MI, and it was as effective and potent as the local corticosteroid application for palmar psoriasis in the last-mentioned study.[18]
The local application of methotrexate was a very effective therapeutic choice for PPP with minimum side effects. In a study that included 16 PPP patients, the patients’ conditions had improved by 80% in palmar psoriasis cases and 64% in plantar psoriasis cases after topical application of methotrexate over the psoriatic areas for 30 min twice a day.[26] Another study discovered that 0.25% methotrexate in hydrophilic gel provided an acceptable result in the management of PPP.[27] Opposingly, a study showed the minimal impacts of local methotrexate on PPP.[28] This may have resulted from the use of inappropriate vehicles, lower concentration of methotrexate, or inability to deeply infiltrate the dermis.
Iontophoresis is one of the best and most advantageous means for drug delivery. It has been verified that it causes increased ion penetration through the skin even with a small quantity of drug under external stimulation with a low electrical current.[29,30] Iontophoresis is a valid, non-invasive, localized, easily applicable, appropriate, and fast modality for transdermal drug delivery of ionized medications and water-soluble drugs through the dermal layers.[31]
In this study, the sole was covered by gauze immersed in methotrexate and connected to the cathode (methotrexate is negatively charged)[32] as an active electrode, while the other water-soaked padded electrode was connected to the anode. The electromotive energy of iontophoresis enhanced the penetration of the ions through the skin; thus, iontophoresis considerably improved the penetration of methotrexate through the skin. Methotrexate is an anti-inflammatory, anti-proliferative, and immunosuppressant drug. It is responsible for the inhibition of dihydrofolate reductase and hence the stimulation of folic acid formation. Folic acid inhibits the action of thymidylate synthase, which is an essential constituent for the production of pyrimidines and purines and, consequently, for the synthesis of deoxyribonucleic acid (DNA).[33] Through the inhibition of DNA synthesis, methotrexate decreases the hyperplasia of the epithelium, promotes the apoptosis of activated T cells, and inhibits the chemotaxis of neutrophils. Furthermore, it can reduce the synthesis of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1.[34]
Alvarez-Figueroa et al.[32] evaluated MI as a technique for transdermal drug delivery through boars’ skin in 2001 and recommended that iontophoresis may increase the influence of local methotrexate in psoriasis. In a case study, an individual with palmar psoriasis was subjected to MI as treatment.[29] The results were exceptional, with improvement exceeding about 75% after four weeks of treatment compared to the control.
The strengths of the present study are that it demonstrated MI as a safe, non-invasive, and efficient transdermal drug delivery method for the treatment of PP. In addition to using more than one method of assessment, it used the Arabic version of PDI, a valid and reliable assessment tool, the 6MDW test, an objective method for the evaluation of the functional capacity, and VAS, a subjective scale and
a valid and reliable tool for the assessment of pain intensity. This study is a leading trial of MI on PP and functional capacity. Eight sessions of MI proved beneficial for the patients with PP in improving their functional lifestyle disabilities, functional capacity, and reducing pain.
The limitations of this study were possible blinding issues and the lack of short-and long-term follow-up. Applying various concentrations of methotrexate, measuring the percentage of the area of the sole covered by psoriasis after the treatment, and using objective methods of evaluation, such as histopathological examination, would have provided more accurate results. Although iontophoresis is used for treating several conditions, there is a lack of standard procedures for its application for treating various conditions.
In conclusion, MI may be an appropriate, effective, and valuable treatment option for patients with plantar psoriasis. Overall, MI effectively improves functional lifestyle disabilities, enhances functional capacity, and decreases pain, as demonstrated by the 6MWD test of patients with plantar psoriasis.
Footnotes
Ethics Committee Approval: The study protocol was approved by the College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Alkarj, Saudi Arabia (No: RHPT/18/096). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Study conception and design: N.M.; Data collection: A.S.; Analysis and interpretation of results, draft manuscript preparation: N.M., A.S.; All authors reviewed the results and approved the final version of the manuscript.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each participant.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: A phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024–1031. doi: 10.1016/j.jaad.2008.11.910. [DOI] [PubMed] [Google Scholar]
- 3.Pettey AA, Balkrishnan R, Rapp SR, Fleischer AB, Feldman SR. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: Implications for clinical practice. J Am Acad Dermatol. 2003;49:271–275. doi: 10.1067/s0190-9622(03)01479-8. [DOI] [PubMed] [Google Scholar]
- 4.Adişen E, Tekin O, Gülekon A, Gürer MA. A retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patients. J Eur Acad Dermatol Venereol. 2009;23:814–819. doi: 10.1111/j.1468-3083.2009.03197.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung J, Callis Duffin K, Takeshita J, Shin DB, Krueger GG, Robertson AD, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71:623–632. doi: 10.1016/j.jaad.2014.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E, Zarei M, LaSenna C, Villada G, Romanelli P. Psoriasis targeted therapy: Characterization of interleukin 17A expression in subtypes of psoriasis. J Drugs Dermatol. 2015;14:1133–1136. [PubMed] [Google Scholar]
- 7.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: Results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- 8.Khandpur S, Singhal V, Sharma VK. Palmoplantar involvement in psoriasis: A clinical study. Indian J Dermatol Venereol Leprol. 2011;77:625–625. doi: 10.4103/0378-6323.84071. [DOI] [PubMed] [Google Scholar]
- 9.Mehta BH, Amladi ST. Evaluation of topical 0. 1% tazarotene cream in the treatment of palmoplantar psoriasis: An observer-blinded randomized controlled study. Indian J Dermatol. 2011;56:40–43. doi: 10.4103/0019-5154.77550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: A prospective, randomized study. e384-9J Eur Acad Dermatol Venereol. 2013;27 doi: 10.1111/jdv.12004. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmi PK, Devi GS, Bhaskaran S, Sacchidanand S. Niosomal methotrexate gel in the treatment of localized psoriasis: Phase I and phase II studies. Indian J Dermatol Venereol Leprol. 2007;73:157–161. doi: 10.4103/0378-6323.32709. [DOI] [PubMed] [Google Scholar]
- 12.Eskicirak B, Zemheri E, Cerkezoglu A. The treatment of psoriasis vulgaris: 1% topical methotrexate gel. Int J Dermatol. 2006;45:965–969. doi: 10.1111/j.1365-4632.2006.02911.x. [DOI] [PubMed] [Google Scholar]
- 13.Belgi G, Friedmann PS. Traditional therapies: Glucocorticoids, azathioprine, methotrexate, hydroxyurea. Clin Exp Dermatol. 2002;27:546–554. doi: 10.1046/j.1365-2230.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- 14.Wong TW, Zhao YL, Sen A, Hui SW. Pilot study of topical delivery of methotrexate by electroporation. Br J Dermatol. 2005;152:524–530. doi: 10.1111/j.1365-2133.2005.06455.x. [DOI] [PubMed] [Google Scholar]
- 15.Dardas A, Bae GH, Yule A, Wright J, Straughn N, Day CS. Acetic acid iontophoresis for recalcitrant scarring in post- operative hand patients. J Hand Ther. 2014;27:44–48. doi: 10.1016/j.jht.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Sarma N. Evidence and suggested therapeutic approach in psoriasis of difficult-to-treat areas: Palmoplantar psoriasis, nail psoriasis, scalp psoriasis, and intertriginous psoriasis. Indian J Dermatol. 2017;62:113–122. doi: 10.4103/ijd.IJD_539_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haseena K, George S, Riyaz N, Sasidharanpillai S, Puthussery PV. Methotrexate iontophoresis versus coal tar ointment in palmoplantar psoriasis: A pilot study. Indian J Dermatol Venereol Leprol. 2017;83:569–573. doi: 10.4103/ijdvl.IJDVL_185_16. [DOI] [PubMed] [Google Scholar]
- 18.Andanooru Chandrappa NK, Channakeshavaiah Ravikumar B, Rangegowda SM. Iontophoretic delivery of methotrexate in the treatment of palmar psoriasis: A randomised controlled study. Australas J Dermatol. 2020;61:140–146. doi: 10.1111/ajd.13228. [DOI] [PubMed] [Google Scholar]
- 19.Finlay AY, Khan GK, Luscombe DK, Salek MS. Validation of sickness impact profile and psoriasis disability index in psoriasis. Br J Dermatol. 1990;123:751–756. doi: 10.1111/j.1365-2133.1990.tb04192.x. [DOI] [PubMed] [Google Scholar]
- 20.Zedan M, Gaber H, Ibrahim AK, Refaa EZ. Reliability and validity of the Arabic version of the Psoriasis Disability Index questionnaire. Journal of the Egyptian Women's Dermatologic Society. 2016;13:143–150. [Google Scholar]
- 21.Elnaggar RK, Mohammed RA, Abdelhalim NM, Samhan AF. A rehabilitation program recommendation for children with juvenile psoriatic arthritis. International Journal of Therapies and Rehabilitation Research. 2015;4:1–8. [Google Scholar]
- 22.Akınoğlu B, Köse N. A comparison of the acute effects of radial extracorporeal shockwave therapy, ultrasound therapy, and exercise therapy in plantar fasciitis. J Exerc Rehabil. 2018;14:306–312. doi: 10.12965/jer.1836048.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira HA, Jones A, Moreira E, Jennings F, Natour J. Effectiveness of total contact insoles in patients with plantar fasciitis. J Rheumatol. 2015;42:870–878. doi: 10.3899/jrheum.140429. [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1–11. doi: 10.2165/00128071-200708010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis: A study of 3065 patients. Acta Derm Venereol. 2002;82:192–195. doi: 10.1080/00015550260132488. [DOI] [PubMed] [Google Scholar]
- 26.Ravi Kumar BC, Kaur I, Kumar B. Topical methotrexate therapy in palmoplantar psoriasis. Indian J Dermatol Venereol Leprol. 1999;65:270–272. [PubMed] [Google Scholar]
- 27.Kumar B, Sandhu K, Kaur I. Topical 0. 25% methotrexate gel in a hydrogel base for palmoplantar psoriasis. J Dermatol. 2004;31:798–801. doi: 10.1111/j.1346-8138.2004.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari SB, Kumar BC, Udupa N, Balachandran C. Topical methotrexate delivered by iontophoresis in the treatment of recalcitrant psoriais--a case report. Int J Dermatol. 2003;42:157–159. doi: 10.1046/j.1365-4362.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 29.Green PG, Flanagan M, Shroot B, Guy RH. In: Drugs and the pharmaceutical sciences. Walters KA, Hadgraft J, editors. New York: Marcel Dekker Inc.; 1993. In physical skin penetration enhancement; pp. 311–333. [Google Scholar]
- 30.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis - an approach for controlled drug delivery: A review. Curr Drug Deliv. 2007;4:1–10. doi: 10.2174/1567201810704010001. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Figueroa MJ, Delgado-Charro MB, Blanco-Méndez J. Passive and iontophoretic transdermal penetration of methotrexate. Int J Pharm. 2001;212:101–107. doi: 10.1016/s0378-5173(00)00599-8. [DOI] [PubMed] [Google Scholar]
- 33.Cronstein BN, Naime D, Ostad E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv Exp Med Biol. 1994;370:411–416. doi: 10.1007/978-1-4615-2584-4_89. [DOI] [PubMed] [Google Scholar]
- 34.Elango T, Dayalan H, Gnanaraj P, Malligarjunan H, Subramanian S. Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin Exp Med. 2014;14:431–437. doi: 10.1007/s10238-013-0252-7. [DOI] [PubMed] [Google Scholar]