Key Points
Question
What is the durability of humoral and cellular immune responses in individuals who originally received the BNT162b2 vaccine and were boosted with Ad26.COV2.S or BNT162b2?
Findings
In this cohort study of 68 adults, both Ad26.COV2.S and BNT162b2 were associated with increased humoral and cellular immune responses. Boosting with Ad26.COV2.S was associated with durable antibody and T-cell responses for at least 4 months.
Meaning
A heterologous mix-and-match vaccine strategy was associated with durable antibody and T-cell responses against the SARS-CoV-2 Omicron variant.
This cohort study investigates the immunogenicity and durability of heterologous and homologous prime-boost regimens involving the adenovirus vector vaccine Ad26.COV2.S and the messenger RNA vaccine BNT162b2 among adults previously vaccinated with BNT162b2.
Abstract
Importance
Antibody responses elicited by current messenger RNA (mRNA) COVID-19 vaccines decline rapidly and require repeated boosting.
Objective
To evaluate the immunogenicity and durability of heterologous and homologous prime-boost regimens involving the adenovirus vector vaccine Ad26.COV2.S and the mRNA vaccine BNT162b2.
Design, Setting, and Participants
In this cohort study at a single clinical site in Boston, Massachusetts, 68 individuals who were vaccinated at least 6 months previously with 2 immunizations of BNT162b2 were boosted with either Ad26.COV2.S or BNT162b2. Enrollment of participants occurred from August 12, 2021, to October 25, 2021, and this study involved 4 months of follow-up. Data analysis was performed from November 2021 to February 2022.
Exposures
Participants who were previously vaccinated with BNT162b2 received a boost with either Ad26.COV2.S or BNT162b2.
Main Outcomes and Measures
Humoral immune responses were assessed by neutralizing, binding, and functional antibody responses for 16 weeks following the boost. CD8+ and CD4+ T-cell responses were evaluated by intracellular cytokine staining assays.
Results
Among 68 participants who were originally vaccinated with BNT162b2 and boosted with Ad26.COV2.S (41 participants; median [range] age, 36 [23-84] years) or BNT162b2 (27 participants; median [range] age, 35 [23-76] years), 56 participants (82%) were female, 7 (10%) were Asian, 4 (6%) were Black, 4 (6%) were Hispanic or Latino, 3 (4%) were more than 1 race, and 53 (78%) were White. Both vaccines were found to be associated with increased humoral and cellular immune responses, including against SARS-CoV-2 variants of concern. BNT162b2 boosting was associated with a rapid increase of Omicron neutralizing antibodies that peaked at a median (IQR) titer of 1018 (699-1646) at week 2 and declined by 6.9-fold to a median (IQR) titer of 148 (95-266) by week 16. Ad26.COV2.S boosting was associated with increased Omicron neutralizing antibodies titers that peaked at a median (IQR) of 859 (467-1838) week 4 and declined by 2.1-fold to a median (IQR) of 403 (208-1130) by week 16.
Conclusions and Relevance
Heterologous Ad26.COV2.S boosting was associated with durable humoral and cellular immune responses in individuals who originally received the BNT162b2 vaccine. These data suggest potential benefits of heterologous prime-boost vaccine regimens for SARS-CoV-2.
Introduction
Messenger RNA (mRNA) vaccines for COVID-19 have demonstrated outstanding short-term immunogenicity and protective efficacy.1,2 However, neutralizing antibody (NAb) responses have been reported to wane by 3 to 6 months after primary immunization.3,4,5,6,7 Following a third mRNA dose, Omicron-specific NAbs were induced,8,9,10,11 but these antibody responses and clinical effectiveness also declined after 3 to 6 months.12,13 Following a fourth mRNA dose, protection against infection with SARS-CoV-2 Omicron waned after 4 weeks, although protection against severe disease lasted for at least 6 weeks.14 In contrast with mRNA COVID-19 vaccines, an adenovirus serotype 26 vector-based COVID-19 vaccine induced lower initial NAb titers compared with mRNA vaccines,15,16,17 but these antibody responses and protective efficacy were durable for at least 8 months.7,18,19
Cellular immune responses, and in particular CD8+ T-cell responses, may also contribute to protection, especially long-term protection against severe disease.20,21,22 The SARS-CoV-2 Omicron variant largely escapes from vaccine-elicited NAbs,8,23,24,25 but T-cell responses remain highly cross-reactive against Omicron.26,27,28 T-cell responses are also substantially more durable than serum NAb titers.7,22,29,30
Optimal boosting strategies for induction of Omicron-specific NAbs and CD8+ T-cell responses have not yet been defined and are important for long-term pandemic control. To assess the immunogenicity and durability of heterologous and homologous vaccine boost strategies, we evaluated humoral and cellular immune responses in individuals who were vaccinated at least 6 months previously with 2 immunizations of BNT162b2 (Pfizer-BioNTech) and were then boosted with either Ad26.COV2.S (Janssen) or BNT162b2.
Methods
Study Population
This cohort study was approved by the Beth Israel Deaconess Medical Center (BIDMC) institutional review board. Enrollment of participants occurred from August 12 to October 25, 2021, and this study involved 4 months of follow-up. A specimen biorepository at BIDMC obtained samples from 68 individuals who received the BNT162b2 vaccine at least 6 months prior to boost. Participants either continued follow-up in the biorepository and were boosted with 30 ug BNT162b2 or were enrolled in the COV2008 clinical trial (NCT04999111) and were boosted with 5.0 × 1010, 2.5 × 1010, or 1.0 × 1010 viral particles Ad26.COV2.S. An additional 15 participants were also enrolled in the COV2008 study. The Advarra institutional review board approved the COV2008 study. All participants provided informed consent (verbal consent for the specimen biorepository and written consent for the COV2008 clinical trial). Race and ethnicity data were obtained by self-report. Race and ethnicity are not biological constructs; however, they were assessed for this study given their clinical and public health relevance in the context of disparities in COVID-19 disease outcomes and vaccination and to allow readers to determine generalizability.
Individuals were excluded if they had a history of SARS-CoV-2 infection, received other COVID-19 vaccines, or received immunosuppressive medications. Participants were excluded from the immunologic analysis if they had a positive nucleocapsid serology by electrochemiluminescence assay (ECLA), a confirmed breakthrough COVID-19 infection, or an additional COVID-19 vaccine outside of the study protocol. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Immunogenicity
Humoral immune responses were assessed by pseudovirus NAb assays, live virus NAb assays, enzyme-linked immunosorbent assays, ECLA, antibody-dependent phagocytosis, antibody-dependent neutrophil phagocytosis, and antibody-dependent complement deposition (eMethods in the Supplement). Cellular immune responses were assessed by intracellular cytokine staining assays and receptor-binding domain (RBD)–specific B cell staining (eMethods in the Supplement).
Statistical Analysis
Descriptive statistics were calculated using Prism statistical software version 8.4.3 (GraphPad Software). Data are presented as individual values with medians, or as box-and-whisker plots with medians and ranges. Immune responses were compared with 2-tailed Mann-Whitney tests. P < .05 was considered significant. Data analysis was performed from November 2021 to February 2022.
Results
Study Population
In this cohort study, 68 participants who were originally vaccinated at least 6 months previously with 2 immunizations of BNT162b2 were enrolled and were boosted with either Ad26.COV2.S (41 participants; median [range] age, 36 [23-84] years) or BNT162b2 (27 participants; median [range] age, 35 [23-76] years); 56 participants (82%) were female, 7 (10%) were Asian, 4 (6%) were Black, 4 (6%) were Hispanic or Latino, 3 (4%) were more than 1 race, and 53 (78%) were White. (Table). Participants were excluded if they had a positive nucleocapsid serology by ECLA, a confirmed breakthrough COVID-19 infection, or any additional COVID-19 vaccine. No serious adverse effects were observed following heterologous or homologous boosting in this study.
Table. Characteristics of the Study Population.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Ad26.COV2.S booster (n = 41) | BNT162b2 booster (n = 27) | |
| Age, median (range), y | 36 (23-84) | 35 (23-76) |
| Sex at birth | ||
| Female | 32 (78) | 24 (89) |
| Male | 9 (22) | 3 (11) |
| Race | ||
| White | 30 (73) | 23 (85) |
| Asian | 5 (12) | 2 (7) |
| Black | 3 (7) | 1 (4) |
| >1 Race | 2 (5) | 1 (4) |
| Unknown or othera | 1 (2) | 0 |
| Ethnicity | ||
| Hispanic or Latino | 2 (5) | 2 (7) |
| Non-Hispanic | 39 (95) | 24 (89) |
| Other | 0 | 1 (4) |
| Medical condition | ||
| Obesityb | 9 (22) | 2 (7) |
| Hypertension | 4 (10) | 3 (11) |
| Diabetes | 1 (2) | 2 (7) |
| Stroke | 1 (2) | 0 |
| Pregnantc | 1 (2) | 2 (7) |
| Time from second vaccine to boost, median (IQR), d | 235 (191-245) | 253 (247-257) |
| Time from third vaccine to final collection, median (IQR), d | 121 (108-131) | 119 (113-122) |
This participant self-identified their race as Hispanic.
Obesity is defined as body mass index (calculated as weight in kilograms divided by height in meters squared) greater than or equal to 30.
Pregnant designation reflects status at the time of booster vaccine.
Humoral Immune Responses
NAb responses were assessed following the boost immunization by pseudovirus neutralization assays25,31 and authentic live virus neutralization assays.32 Six months following initial BNT162b2 vaccination, pseudovirus NAb titers were detectable but low against the WA1/2020, Delta, and Beta variants but largely undetectable against Omicron (Figure 1A and 1B). Ad26.COV2.S boosted median (IQR) Omicron BA.1 NAb titers from 21 (20-32) at week 0 to 591 (272-881) at week 2, a peak of 859 (467-1838) at week 4, and a decline of 2.1-fold to 403 (208-1130) at week 16 following the boost (Figure 1A). BNT162b2 boosted median (IQR) Omicron BA.1 NAb titers from 20 (20-27) at week 0 to 1018 (699-1646) at week 2, 345 (230-972) at week 4, and declined by 6.9-fold to 148 (95-266) at week 16 (Figure 1B). At week 16, the median (IQR) BA.1 (403 [208-1130]) and BA.2 (308 [179-1273]) pseudovirus NAb titers were higher following the Ad26.COV2.S boost compared with the BNT162b2 boost (median [IQR], BA.1, 148 [95-266]; BA.2, 146 [96-265]) (Figure 1C and 1D and eFigure1 in the Supplement). Authentic live virus NAb assays showed similar profiles to the pseudovirus NAb assays (Figure 2A and 2B). At week 16, Omicron BA.1 live virus NAb titers were higher following the Ad26.COV2.S boost compared with the BNT162b2 boost (median [IQR], 887 [554-1709] vs 511 [289-838]) (Figure 2C and 2D).
Figure 1. Pseudovirus Neutralizing Antibody (NAb) Responses Following Ad26.COV2.S or BNT162b2 Boosting.
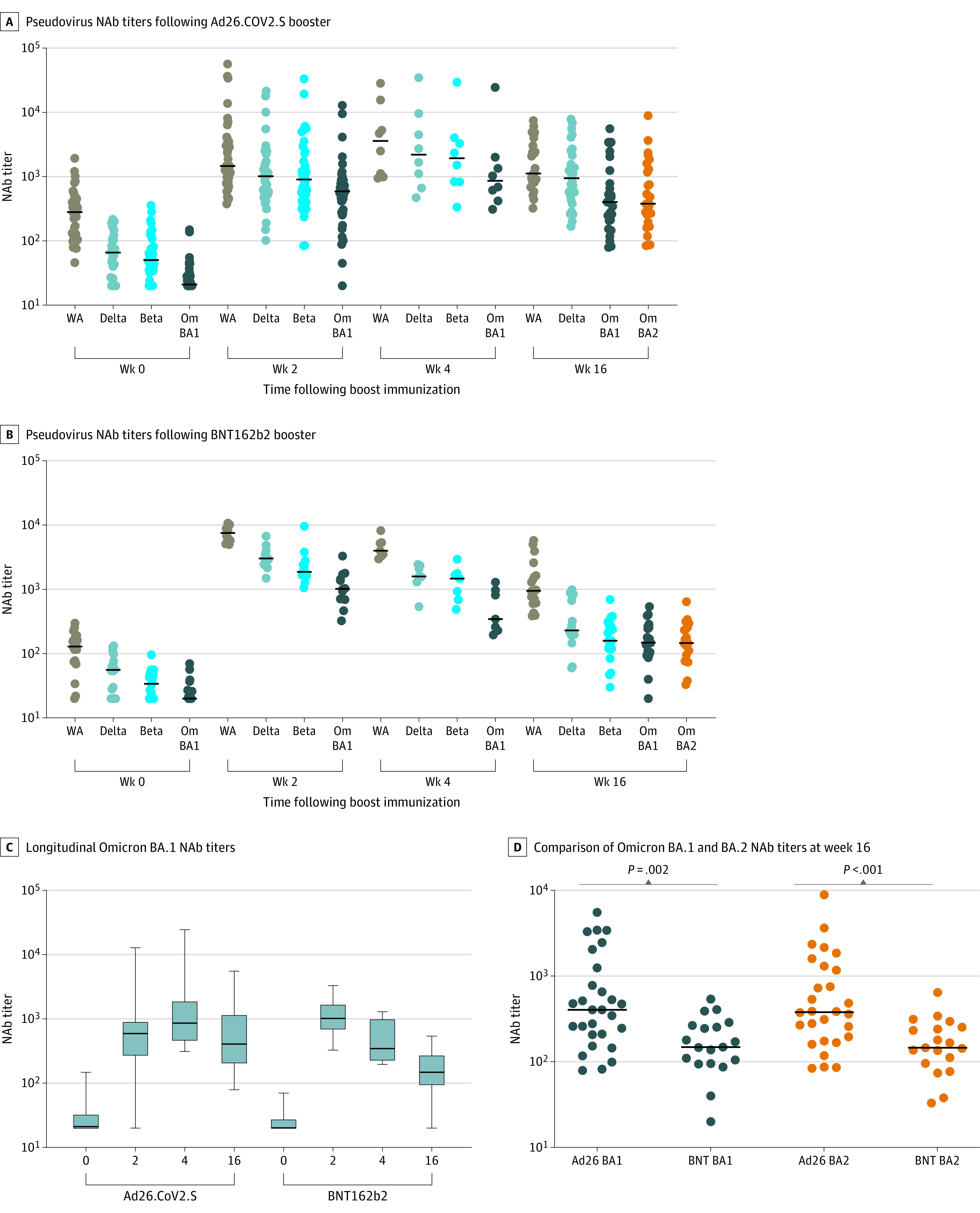
Pseudovirus NAb titers at weeks 0, 2, 4, and 16 following boosting of BNT162b2 vaccinated individuals with Ad26.COV2.S (A) or BNT162b2 (B) are shown. Pseudovirus NAb titers to SARS-CoV-2 W1/2020 (WA), B.1.617.2 (Delta), B.1.351 (Beta), B.1.1.529 (Omicron BA.1; Om BA1), and B.1.1.529 (Omicron BA.2; Om BA2) are shown. Black bars denote medians. Week 4 depicts a subset of samples and includes samples from weeks 4-10. Participants with positive nucleocapsid (N) serology or a history of breakthrough SARS-CoV-2 infection were excluded. Longitudinal Omicron BA.1 NAb titers (C) and comparison of Omicron BA.1 and BA.2 NAb titers at week 16 (D) are shown. P values reflect 2-tailed Mann-Whitney tests.
Figure 2. Live Virus Neutralizing Antibody Responses Following Ad26.COV2.S or BNT162b2 Boosting.
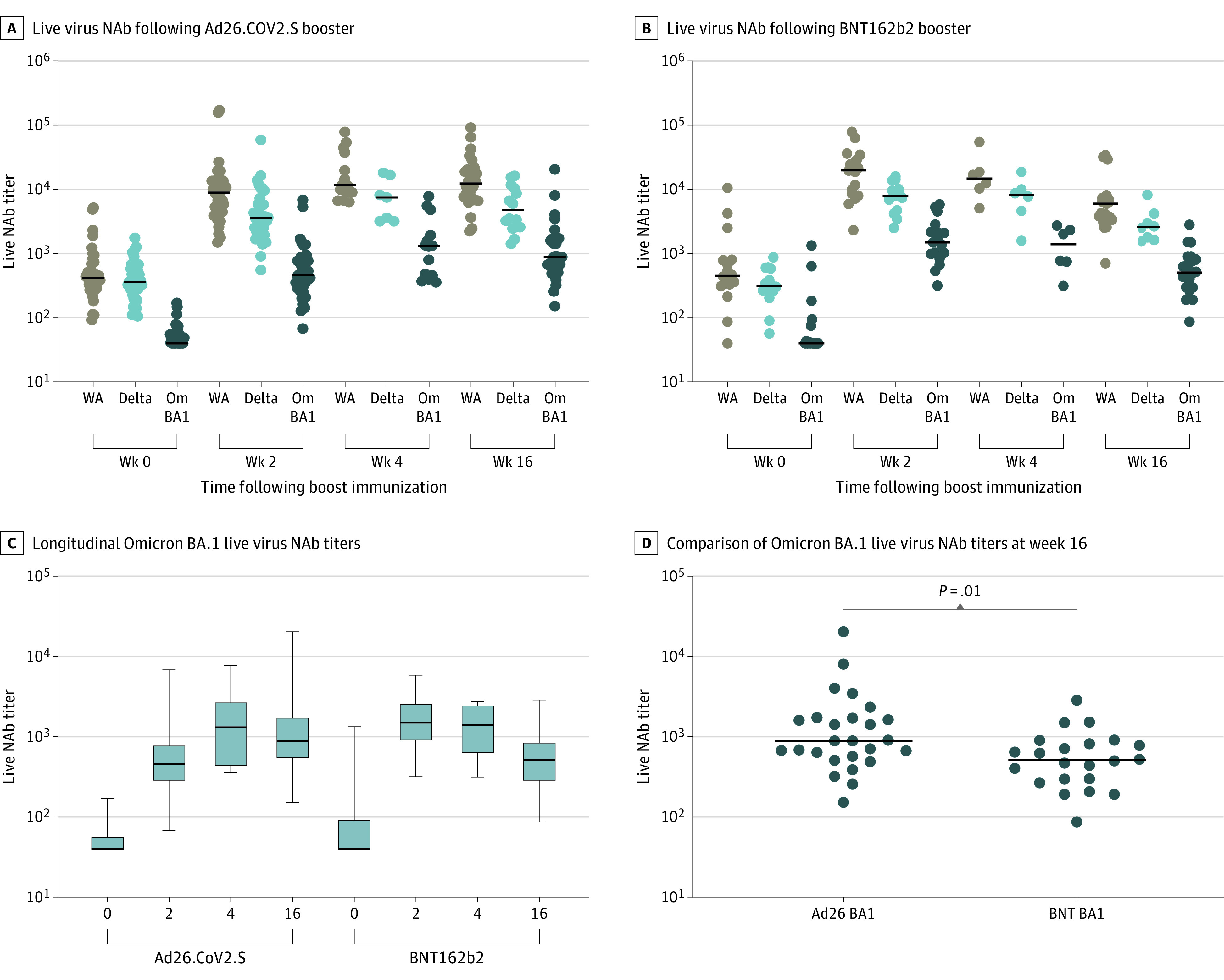
Live virus neutralizing antibody (NAb) titers at weeks 0, 2, 4, and 16 following boosting of BNT162b2 vaccinated individuals with Ad26.COV2.S (A) or BNT162b2 (B). Live virus NAb titers to SARS-CoV-2 WA1/2020 (WA), B.1.617.2 (Delta), and B.1.1.529 (Omicron BA.1; Om BA1). Black bars denote medians. Longitudinal Omicron BA.1 live virus NAb titers (C) and comparison of Omicron BA.1 live virus NAb titers at week 16 (D) are shown. P value reflects a 2-tailed Mann-Whitney test.
Both Ad26.COV2.S and BNT162b2 also boosted RBD-specific binding antibody responses by ECLA33 (eFigure 2 in the Supplement) and enzyme-linked immunosorbent assays (eFigure 3 in the Supplement). Fc functional antibody responses34 were also evaluated given their potential role in protection and were comparable following the Ad26.COV2.S and BNT162b2 boosts, with relatively preserved antibody-dependent cellular phagocytosis but reduced antibody-dependent neutrophil phagocytosis and antibody-dependent complement deposition responses against Omicron BA.1 compared with WA1.2020 (eFigure 4 in the Supplement).
Cellular Immune Responses
SARS-CoV-2–specific CD8+ and CD4+ T-cell responses were assessed by pooled peptide Spike-specific intracellular cytokine staining assays.27 Ad26.COV2.S boosted median Omicron BA.1 Spike-specific interferon (IFN)–γ CD8+ T-cell responses from medians (IQRs) of 0.017% (0.009%-0.030%) at week 0 to 0.093% (0.045%-0.181%) at week 2 and 0.081% (0.042%-0.200%) at week 16 (Figure 3A) and IFN-γ CD4+ T cell responses from medians (IQRs) of 0.030% (0.011%-0.047%) at week 0 to 0.092% (0.046%-0.123%) at week 2 and 0.065% (0.027%-0.110%) at week 16 (Figure 3B). BNT162b2 boosted median Omicron BA.1 Spike-specific IFN-γ CD8+ T-cell responses from medians (IQRs) of 0.023% (0.010%-0.050%) at week 0 to 0.033% (0.018%-0.094%) at week 2 and 0.024% (0.007%-0.063%) at week 16 (Figure 3A) and IFN-γ CD4+ T-cell responses from medians (IQRs) of 0.027% (0.015%-0.070%) at week 0 to 0.039% (0.028%-0.090%) at week 2 and 0.027% (0.015%-0.063%) at week 16 (Figure 3B). At week 16, median (IQR) Omicron T cell responses following the Ad26.COV2.S boost (CD8, 0.081% [0.042%-0.200%]; CD4, 0.065% [0.027%-0.110%]) were higher compared with the BNT162b2 boost (CD8, 0.024% [0.007%-0.063%]); CD4, 0.027% [0.015%-0.063%]) (Figure 3C). CD8+ and CD4+ T-cell responses were comparable for Omicron BA.1 and WA1/2020, consistent with prior studies.26,27
Figure 3. Cellular Immune Responses Following Ad26.COV2.S or BNT162b2 Boosting.
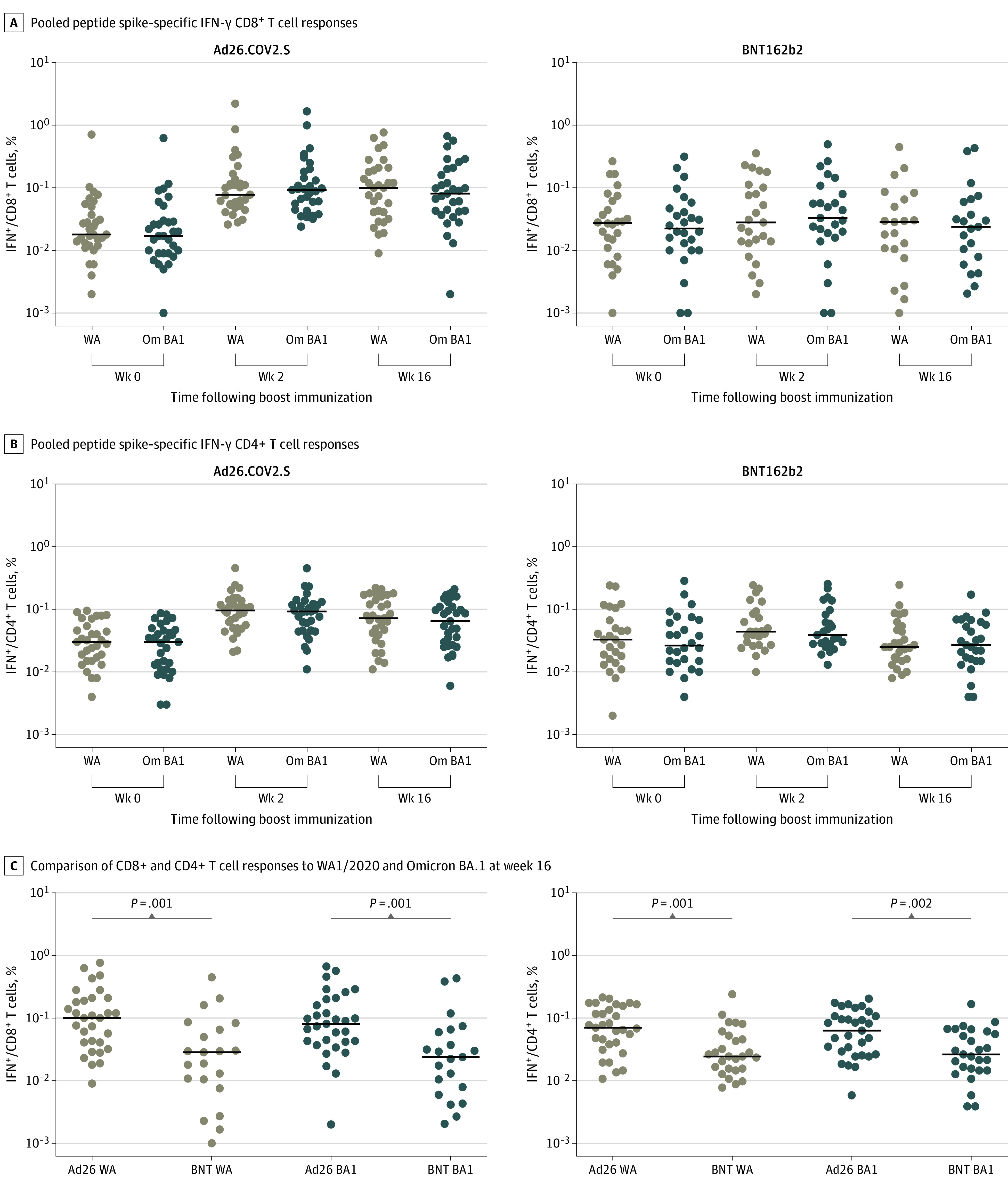
T-cell responses at weeks 0, 2, and 16 following boosting of BNT162b2 vaccinated individuals with Ad26.COV2.S or BNT162b2. Pooled peptide Spike-specific interferon (IFN)–γ CD8+ T-cell responses (A) and CD4+ T-cell responses (B) by intracellular cytokine staining assays. Ad26.COV2.S (left) and BNT162b2 (right) boosting are displayed on separate plots. C, Comparison of CD8+ and CD4+ T cell responses to WA1/2020 and Omicron BA.1 at week 16 are shown. Black bars denote medians. P values reflect 2-tailed Mann-Whitney tests.
Omicron BA.1 RBD-specific memory B-cell responses were also assessed by flow cytometry.35 At week 16, BA.1 RBD-specific memory B cells were 0.053% after the Ad26.COV2.S boost and 0.047% after the BNT162b2 boost (eFigure 5 in the Supplement). Omicron BA.1 memory B cells were lower than WA1/2020 memory B cells (eFigure 5 in the Supplement). Memory B cells showed primarily an activated memory phenotype at week 2 following the boost but transitioned back to a resting memory phenotype by week 16 (eFigure 6 in the Supplement).
Discussion
Evaluations of COVID-19 vaccine boosters have to date largely focused on early NAb responses shortly after boosting. Multiple studies have shown that a third mRNA vaccine effectively induces Omicron-specific NAb titers.8,9,10,11 However, serum antibody responses and clinical effectiveness have been reported to wane by 4 months following an mRNA vaccine boost.12,13 Following a fourth mRNA vaccine, protective efficacy against infection with SARS-CoV-2 Omicron has been shown to wane even more quickly.14 Such rapid waning of immunity has led to recommendations for frequent mRNA vaccine boosting, which may be challenging to sustain as a long-term policy in the developed world and difficult to implement in the developing world. The development of boosting strategies with improved durability would, therefore, be desirable.
In the present study, both heterologous Ad26.COV2.S and homologous BNT162b2 boosting were associated with increased Omicron-specific humoral and cellular immune responses in individuals who were vaccinated at least 6 months previously with BNT162b2. BNT162b2 boosting was associated with a rapid increase of Omicron NAbs that peaked at week 2 and declined 6.9-fold by week 16. In contrast, Ad26.COV2.S boosting was associated with increased Omicron NAb titers that peaked later at week 4 but only declined 2.1-fold by week 16, suggesting improved durability of the heterologous boosting approach (Figure 1B). We speculate that the differences in the kinetics of the immune responses may be related to differences in the kinetics of immunogen expression in vivo. These observations are consistent with recent reports of the durability of immune responses and protection following initial Ad26.COV2.S vaccination.7,18 These data also extend the results of a prior mix-and-match study, which evaluated a shorter boosting interval of 3 to 4 months, although this prior study only reported responses at 2 to 4 weeks following the boost.36
An Ad26.COV2.S booster dose was also associated with increased Omicron-specific CD8+ T-cell responses following the boost immunization. Preclinical studies have suggested that CD8+ T-cell responses contribute to protection against SARS-CoV-2, particularly when antibody responses are subprotective.20 Moreover, cellular immune responses have shown greater durability and more cross-reactivity against SARS-CoV-2 variants than serum NAb responses,7,15,29,30 suggesting their importance for protection against virus variants such as Omicron that largely escape NAb responses. Consistent with these data are recent studies that have reported that BNT162b2 and Ad26.COV2.S provided 70% and 85% efficacy, respectively, against hospitalization with the SARS-CoV-2 Omicron variant in South Africa37,38 in the absence of high levels of Omicron-specific NAbs, suggesting the importance of other immune responses in protection against severe disease.
Limitations
This study has several limitations. First, the small size of the study, lack of randomization, and female predominance of the study population suggest that larger studies should be performed. Second, participants were enrolled at a single site in Boston, and, thus, generalizability will require further study. Third, immunogenicity data are shown for 4 months following immunization, and, thus, additional follow-up time will be required to assess long-term durability.
Conclusions
Our findings show that both heterologous and homologous COVID-19 vaccines boost Omicron-specific antibody and T-cell responses in BNT162b2-vaccinated individuals. NAb and CD8+ T-cell responses were higher following the Ad26.COV2.S boost compared with the BNT162b2 boost at week 16. These data suggest potential immunologic benefits of mix-and-match heterologous COVID-19 vaccine regimens and emphasizes the importance of durability for COVID-19 vaccine boosting strategies. Future studies could explore reduced booster doses as well as Omicron-containing boosters.
eMethods. Supplemental Methods
eReferences
eFigure 1. Pseudovirus Neutralizing Antibody Responses Following Ad26.COV2.S by Dose or BNT162b2 Boosting
eFigure 2. ECLA Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 3. ELISA Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 4. Fc Functional Antibody Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 5. RBD-Specific Memory B Cell Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 6. Phenotype of RBD-Specific Memory B Cells Following Ad26.COV2.S Boosting
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Frenck RW Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627-1629. doi: 10.1056/NEJMc2113468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. doi: 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria-Rose N, Suthar MS, Makowski M, et al. ; mRNA-1273 Study Group . Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259-2261. doi: 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372-1377. doi: 10.1126/science.abj4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier AY, Yu J, McMahan K, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. 2021;385(21):2010-2012. doi: 10.1056/NEJMc2115596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2021;602(7898):676-681. doi: 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 9.Carreno JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602(7898):682-688. doi: 10.1038/s41586-022-04399-5 [DOI] [PubMed] [Google Scholar]
- 10.Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med. 2022;386(5):492-494. doi: 10.1101/2021.12.13.21267670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. medRxiv. Preprint posted online December 13, 2021. doi: 10.1101/2021.12.12.21267646 [DOI] [Google Scholar]
- 12.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386(11):1088-1091. doi: 10.1056/NEJMc2119912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255-263. doi: 10.15585/mmwr.mm7107e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712-1720. doi: 10.1056/NEJMoa2201570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268-272. doi: 10.1038/s41586-021-03681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group . Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325(15):1535-1544. doi: 10.1001/jama.2021.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polinski JM, Weckstein AR, Batech M, et al. Durability of the single-dose Ad26.COV2.S vaccine in the prevention of COVID-19 infections and hospitalizations in the US before and during the Delta variant surge. JAMA Netw Open. 2022;5(3):e222959. doi: 10.1001/jamanetworkopen.2022.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385(10):951-953. doi: 10.1056/NEJMc2108829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630-634. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489-1501.e15. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861-880. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654-656. doi: 10.1038/s41586-021-04387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553-556. doi: 10.1038/s41586-022-04594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386(16):1579-1580. doi: 10.1056/NEJMc2201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488-492. doi: 10.1038/s41586-022-04460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603(7901):493-496. doi: 10.1038/s41586-022-04465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847-859.e11. doi: 10.1016/j.cell.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel RR, Painter MM, Apostolidis SA, et al. ; UPenn COVID Processing Unit . mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572):abm0829. doi: 10.1126/science.abm0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Li Z, He X, et al. Deletion of the SARS-CoV-2 spike cytoplasmic tail increases infectivity in pseudovirus neutralization assays. J Virol. 2021;95(11):e00044-21. doi: 10.1128/JVI.00044-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez DR, Schäfer A, Leist SR, et al. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science. 2021;373(6558):991-998. doi: 10.1126/science.abi4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob-Dolan C, Feldman J, McMahan K, et al. Coronavirus-specific antibody cross reactivity in rhesus macaques following SARS-CoV-2 vaccination and infection. J Virol. 2021;95(11):e00117-21. doi: 10.1128/JVI.00117-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung AW, Kumar MP, Arnold KB, et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163(4):988-998. doi: 10.1016/j.cell.2015.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X, Chandrashekar A, Zahn R, et al. Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques. Cell. 2021;184(13):3467-3473.e11. doi: 10.1016/j.cell.2021.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atmar RL, Lyke KE, Deming ME, et al. ; DMID 21-0012 Study Group . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046-1057. doi: 10.1056/NEJMoa2116414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med. 2022;386(5):494-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray GE, Collie S, Garrett N, et al. Vaccine effectiveness against hospital admission in South African health care workers who received a homologous booster of Ad26.COV2 during an Omicron COVID19 wave: preliminary results of the Sisonke 2 Study. medRxiv. Preprint posted online December 29, 2021. doi: 10.1101/2021.12.28.21268436 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eReferences
eFigure 1. Pseudovirus Neutralizing Antibody Responses Following Ad26.COV2.S by Dose or BNT162b2 Boosting
eFigure 2. ECLA Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 3. ELISA Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 4. Fc Functional Antibody Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 5. RBD-Specific Memory B Cell Responses Following Ad26.COV2.S or BNT162b2 Boosting
eFigure 6. Phenotype of RBD-Specific Memory B Cells Following Ad26.COV2.S Boosting


