Abstract
Study Objectives
Sleep is an important dimension in the care of chronic obstructive pulmonary disease (COPD), but its relevance to exacerbations is unclear. We wanted to assess whether sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI) is associated with an increased risk of COPD exacerbations and does this differ by socio-environmental exposures.
Methods
We included 1647 current and former smokers with spirometrically confirmed COPD from the SPIROMICS cohort. We assessed incidence rate ratios for exacerbation using zero-inflated negative binomial regression adjusting for demographics, medical comorbidities, and multiple metrics of disease severity, including respiratory medications, airflow obstruction, and symptom burden. Our final model adjusted for socio-environmental exposures using the Area Deprivation Index, a composite measure of contemporary neighborhood quality, and Adversity–Opportunity Index, a composite measure of individual-level historic and current socioeconomic indicators. We used a pre-determined threshold of 20% missingness to undertake multiple imputation by chained equations. As sensitivity analyses, we repeated models in those with complete data and after controlling for prior exacerbations. As an exploratory analysis, we considered an interaction between socio-environmental condition and sleep quality.
Results
After adjustment for all co-variates, increasing PSQI scores (range 0–21) were associated with a 5% increased risk for exacerbation per point (p = .001) in the imputed dataset. Sensitivity analyses using complete cases and after controlling for prior exacerbation history were similar. Exploratory analysis suggested less effect among those who lived in poor-quality neighborhoods (p-for-interaction = .035).
Conclusions
Poor sleep quality may contribute to future exacerbations among patients with COPD. This represents one target for improving disease control.
Clinical Trial Registration
Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). ClinicalTrials.gov Identifier# NCT01969344. Registry URL: https://clinicaltrials.gov/ct2/show/.
Keywords: sleep quality, PSQI, exacerbations, COPD, health disparities
Statement of Significance.
Exacerbations are one of the important outcomes in chronic obstructive pulmonary disease, but prior evidence about the influence of sleep is conflicting. In this study, which includes both the largest number of participants and the most extensive longitudinal follow-up of any to consider this question, we demonstrate a significant link between current poor sleep quality and future risk of exacerbations.
Introduction
Sleep is increasingly recognized as an important factor in chronic obstructive pulmonary disease (COPD) [1]. Patients may experience significant sleep disturbances, and aspects of their sleep are also thought to influence the disease itself. However, the relationship of sleep to COPD exacerbations remains poorly understood, with major studies offering conflicting evidence [2, 3]. This represents an important gap in the literature, as exacerbations are associated with accelerated lung function decline, worse quality of life, and increased mortality [4]. Due to the marked heterogeneity of their triggers and considerable variance in year-to-year occurrence [5], this question is difficult to study, but may be well addressed through the use of longitudinal cohorts.
A major concern is appropriate adjustment for social and structural determinants of health. In both its incidence and mortality, socio-environmental exposures have a strong gradient effect on COPD [6–8]. Sleep has been similarly characterized. There are marked disparities in sleep metrics by social status in contemporary studies [9], and longer-term evidence that sleep behavior is profoundly influenced by human social structure [10]. Both alterations in sleep quality and adverse socio-environmental exposures are also convergent in postulating increased inflammation as a mechanism of action [2, 11]. To better understand the complicated relationship between these factors that may modify the course of COPD, we studied the relationship between sleep quality and risk of COPD exacerbations, giving particular focus to the potential modifying role of socio-environmental exposures.
Methods
The Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) is a multicenter US longitudinal cohort designed to study subpopulations and intermediate outcome measures in COPD that is described in detail elsewhere [12]. In brief, participants with current and/or former tobacco exposure with and at risk for COPD from 12 clinical centers across the United States had their natural history recorded in a combination of annual in-person visits and bridging phone interviews. All research was conducted in compliance with the Helsinki Principles and under the approval of local institutional review boards.
Exacerbations
We defined exacerbations as short-term increases in symptom severity that resulted in a healthcare visit and required the use of antibiotics or systemic corticosteroids. Events were self-reported by participants during annual follow-up visits and quarterly bridging phone calls with study staff. We also considered whether exacerbation resulted in a hospitalization as a marker of severity of exacerbation. All exacerbations were managed by participants’ local providers without input from SPIROMICS.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) is a self-report survey instrument for measuring sleep quality [13]. It has been widely used in prior studies of COPD and other medical conditions [14]. Possible scores range from 0 to 21, with higher scores representing worse sleep quality and a suggested cut point by Buysse et al. [13] that a total score greater than 5 indicated “poor” sleep. While subscales exist, for comparability to previous studies, we only considered the total PSQI score. We were also influenced by previous work suggesting that meaningfulness of subscales can vary across populations and are not well studied in COPD, limiting their generalizability [15]. As PSQI responses are based on a 1-month recall [13], we included as a co-variate the numerical change on this instrument over time to capture the dynamic nature of this instrument, distinguishing persistently poor sleepers from those whose sleep quality improved or worsened over time. To limit missingness from our rolling recruitment strategy, we assessed only changes between the first and second annual study visit.
Socio-environmental exposures
To interrogate the individual socio-environmental context of our participants, we selected a composite index, which may better capture gestalt effects than individualized metrics of socioeconomic status [16, 17]. Our index was developed for the SPIROMICS cohort by equally weighting five measures impactful to long-term pulmonary health [18]. In utero smoke exposure was record as yes, no, and unsure/declined to answer [19]. Access to fresh food was measured dichotomously as within 1 mile for urban participants or 10 miles for rural participants, per US Department of Agriculture statistics [20–22]. Occupational respiratory exposure was recorded as never, ever, or unsure [23, 24]. Maximal educational attainment [25] was recorded as less than high school diploma, greater than higher school diploma, or high school diploma. Household income was reported as high, low, or unreported, with a threshold set at $50 000/year to approximate 200% of the federal poverty line for a family of four [26, 27]. The overall range of this Adversity–Opportunity Index (AOI) is from 1 to 8, with lower scores representing more adversity and higher scores more favorable circumstances (Table 1). Through this design, we hoped to capture socio-environmental influences over the life course, focused primarily at the individual level. To measure contemporary area-level socio-environmental exposures, we used the Area Deprivation Index (ADI), a composite measure of neighborhood quality previously demonstrated to influence exacerbation risk.[8]
Table 1.
Scoring table for the AOI
| Category | Score | |
|---|---|---|
| In utero smoke exposure | 0 | Yes |
| 1 | Unsure/declines | |
| 2 | No | |
| Access to fresh/healthy food | 0 | Not within 1 mile urban or 10 miles rural |
| 2 | Within 1 mile urban or 10 miles rural | |
| Maximum educational attainment | 0 | High school diploma or less |
| 2 | Beyond high school diploma | |
| Job with respiratory exposures | 0 | Yes |
| 1 | Unsure/declines | |
| 2 | No | |
| Household income | 0 | ≤$50 000/year |
| 1 | Unreported | |
| 2 | >50 000/year |
Additional co-variates
We constructed a directed acyclic graph to model exacerbation risk based on literature review to identify factors that exert plausible, significant influence on both sleep quality, and exacerbation risk [28]. Self-identified race [8] was coded as non-Hispanic White, African American, or Other; this last category was aggregated due to small sample size and included 2.9% Latinx, 1.2% Asian, 0.3% Native American, 1.3% Mixed, and 0.6% who declined to self-identify. Biological sex [29] was recorded as male or female. Age [30] was measured continuously. As recommended by the Global Initiative for Obstructive Lung Disease (GOLD), we measured post-bronchodilator percentage predicted forced expiratory volume in one second (FEV1) and COPD Assessment Test (CAT) score to assess COPD [4]. To align with major comparator studies, we included records of bronchodilator prescriptions. Our review identified multiple important medical comorbidities. Most comorbidities were assessed using a previously validated simple count of comorbidities count in COPD as a proxy measure [31] as we were less interested in characterizing their individual effect than the cumulative impact of multiple comorbidities worsening sleep quality. This instrument was specially developed to assess COPD-relevant outcomes and included coronary heart disease, diabetes, congestive heart failure, stroke, or transient ischemic attack (TIA), osteoarthritis, osteoporosis, hypertension, gastro-esophageal reflux disease (GERD), obesity, obstructive sleep apnea, or allergic rhinitis. In SPIROMICS, all these diagnoses were determined by self-report of previous physician diagnosis except body mass index (BMI), which was measured directly. Additionally, we considered self-reported diagnosis of asthma [32], mood symptoms as measured by the Hospital Depression and Anxiety Scale [33], BMI [34], and pack-years smoking history [4]. In addition to studies suggesting its negative impact on COPD exacerbations, sleep apnea has been associated with high PSQI scores, so we separately considered this single component of the comorbidity count for effect modification [35, 36]. Pack year smoking history was also assessed for interaction as it is both fundamental to the underlying disease process in developed economies and can influence sleep quality [4, 37].
Analysis
To account for the high fraction of participants who never reported an exacerbation in their entire period of follow-up [5], we used a zero-inflated negative binomial model to predict exacerbation count. We model the probability of never reporting an exacerbation using CAT scores and post-bronchodilator FEV1. This is consistent with GOLD guidelines for measuring COPD severity and predicting exacerbation risk [4]. To account for the rolling recruitment strategy, years in follow-up was used as an offset. We set a threshold for imputation if overall missingness in the dataset exceeded 20%. We used iterative chained equations for multiple imputation, prespecifying 10 cycles of imputation per previous recommendation [38]. We used 10 cycles for each of the 10 iterations, and the estimated missing values averaged from across all 10 iterations were employed in our regressions [39]. In our analysis, we assumed missingness was at random because the majority of co-variates are about reporting incident diseases; the presence of any one is strongly influenced by other particular pathologies that are present or absent. As sensitivity test, we repeated our analysis controlling for retrospective exacerbation history in the year prior to study enrollment. We only considered co-variate data for imputation. All analyses were performed on Stata Version 16. We set the threshold for significance at α = .05 for all associations.
Results
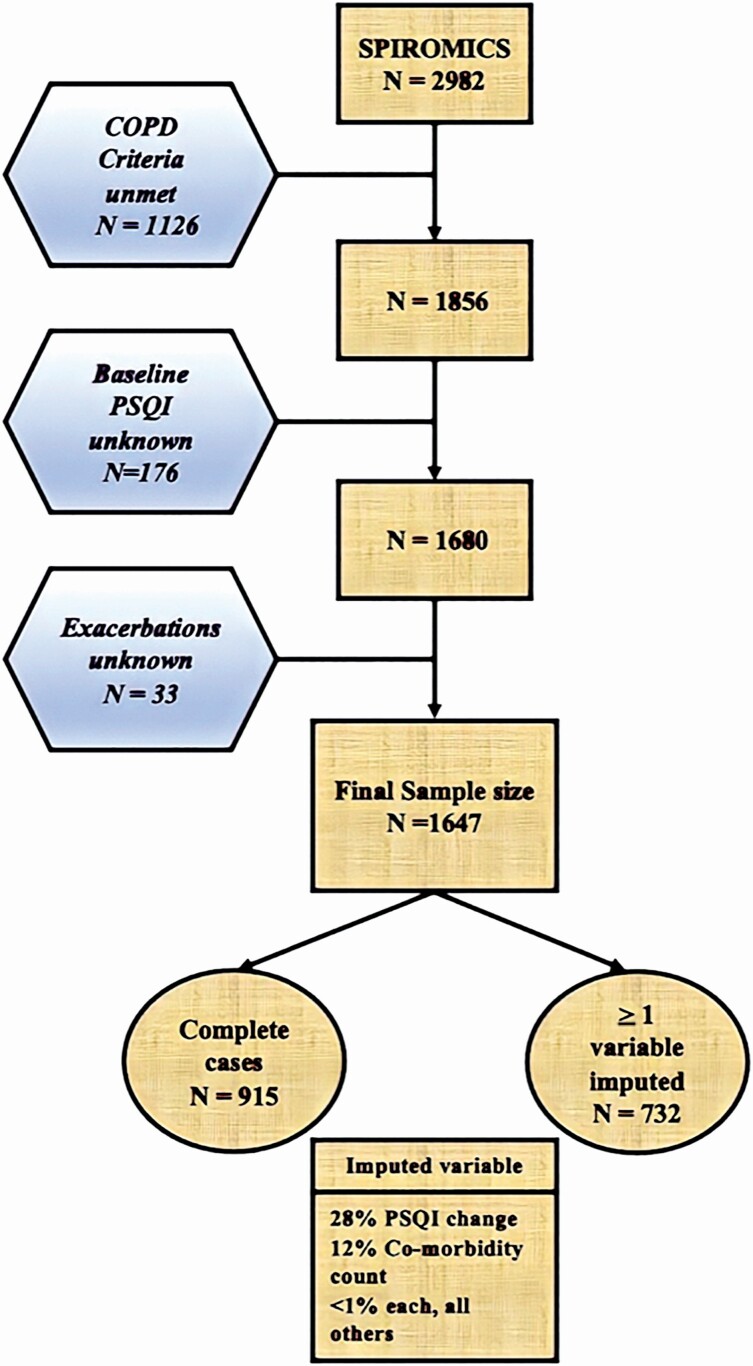
45% of SPIROMICS participants eligible for our analysis were considered to have missing data because they had spirometrically confirmed COPD, a recorded baseline PSQI score, and a reported count of exacerbations from their time in the study but were missing data for at least one co-variate. The greatest source of missingness was missing data for inter-visit change in PSQI score, which was unavailable for 28% of eligible participants (Figure 1). This was followed by diagnosis of sleep apnea or hay fever (both components of the comorbidity count), with 12.2% of eligible participants missing data. The majority of remaining missingness in our eligible dataset had a variety of patterns, each one totaling no more than 1%. In comparison to participants without missing data (complete cases), excluded individuals had higher mean CAT scores (16.9 vs 14, p < .001), more adverse socio-environmental conditions as measured by the AOI (5.2 vs 5.3, p = .03) and poorer sleep quality (7.1 vs 6.2, p < .001), but not a higher comorbidity count (2.35 vs 2.34, p = .95). The missingness in our dataset triggered our use of imputation.
Figure 1.
CONSORT flow diagram demonstrating the differences between the total SPIROMICS cohort and the subset of participants whose data were utilized in the present analysis.
As a group, our participants were predominantly male, reported moderate airway obstruction, heavy COPD symptom burden, poor sleep quality, and reported scores on the AOI within that scale’s interquartile range (Table 2). 54% of the study population met or exceeded the “poor” sleep quality threshold of ≤5 set by Buysse. The highest observed value of the PSQI was 19 (Median 6/Standard deviation 3.9/IQR 5) and subscales were not normally distributed.
Table 2.
Baseline characteristics of participants with spirometrically confirmed COPD in SPIROMICS
| All | ||
|---|---|---|
| Total n | 1647 | |
| Age, mean ± SD | 65.2 ± 8.0 | |
| Male sex, n (%) | 942 (57.2%) | |
| Race/ethnicity, n (%) | Non-Hispanic White | 1314 (79.8%) |
| African American | 234 (14.4%) | |
| Other | 47 (5.8%) | |
| BMI (kg/m2), mean ± SD | 27.4 ± 5.2 | |
| Obstructive sleep apnea, n (%) | 289 (18.8%) | |
| Ever diagnosed with asthma, n (%) | No | 1177 (73.1%) |
| Yes | 367 (22.8%) | |
| Unsure | 66 (4.1%) | |
| Hospital Depression Scale score, mean ± SD | 4.5 ± 3.4 | |
| Hospital Anxiety Scale score, mean ± SD | 5.5 ± 3.7 | |
| COPD Assessment Test (CAT) score, mean ± SD | 15.2 ± 7.9 | |
| Percentage predicted post-bronchodilator FEV1, mean ± SD | 61.4 ± 23.0 | |
| LAMA usage, n (%) | 615 (38.4%) | |
| LABA-ICS usage, n (%) | 689 (43.1%) | |
| Household income, n (%) | <$50k/year | 808 (49.5%) |
| Unreported | 295 (18.1%) | |
| >$50k/year | 530 (32.5%) | |
| Maximal educational attainment, n (%) | Less than high school | 209 (12.7%) |
| High school diploma | 412 (25.1%) | |
| Any post-high school | 1020 (62.2%) | |
| In utero smoke exposure, n (%) | Yes | 827 (51.7%) |
| Uncertain/not disclosed | 494 (30.9%) | |
| No | 277 (17.3%) | |
| Insufficient access to fresh food, n (%) | 536 (33.1%) | |
| Occupational respiratory exposure, n (%) | Yes | 702 (42.7%) |
| Uncertain | 164 (10.0%) | |
| No | 778 (47.3%) | |
| Comorbidity count, mean ± SD | 2.4 ± 1.6 | |
| Adversity–Opportunity Index (AOI), mean ± SD | 5.4 ± 2.0 | |
| Area Deprivation Index (ADI), mean ± SD | 4.8 ± 2.9 | |
| Pittsburgh Sleep Quality Index (PSQI), mean ± SD | 6.6 ± 3.9 | |
| Time in follow-up (years), mean ± SD | 4.1 ± 1.7 | |
| COPD exacerbations per follow-up year, mean ± SD | 0.5 ± 0.9 | |
| COPD exacerbations requiring hospitalization per follow-up year, mean ± SD | 0.2 ± 0.5 | |
Bolded values significant at α < .05. Insufficient Access to fresh food is grocer at 1 mile in urban area or 10 miles in rural area from known residence. AOI is a composite metric of individual-level historic and current socio-environmental indicators.
Incident rate ratio of COPD exacerbation
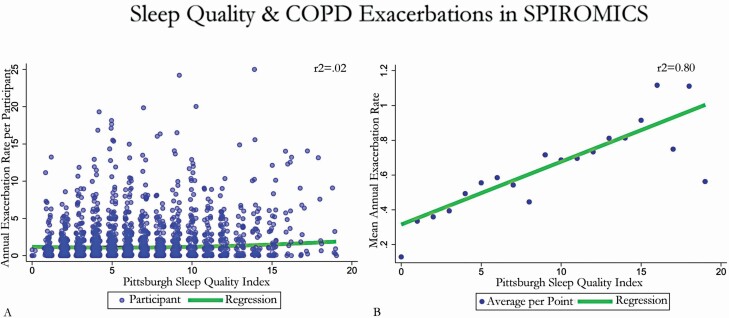
We observed a significant association between PSQI score and total and mean exacerbations in the unadjusted analysis (incidence rate ratios [IRR] 1.09, 95% confidence interval [CI] 1.05–1.13, Figure 2). This association was similar in effect size and significance after adjustment for demographics, medical comorbidities, disease severity, medication usage, and socio-environmental exposures (IRR 1.08, 95% CI: 1.03–1.13). In this multivariable model, increased pack-years, previous diagnosis of asthma, long-acting muscarinic antagonist (LAMA) or long-acting beta-2 agonist with inhaled corticosteroid (LABA-ICS) prescription, a greater number of comorbidities, and increasing CAT or change in serial PSQI score were also associated with an increased risk of exacerbation (Table 3). Other self-identified race, as well as increasing FEV1, AOI, and BMI were associated with a decreased risk of exacerbation (Table 3). Sensitivity testing by complete case analysis and after controlling for prior exacerbation history yielded similar results (Table 3).
Figure 2.
Association between Pittsburgh Sleep Quality Index (PSQI) score and total and mean annualized exacerbations in the unadjusted analysis. Scatter plot of exacerbations during study participation versus baseline PSQI score. (A) Demonstrates total annualized exacerbations, while (B) displays mean per 1-point PSQI score. A linear regression of the trend of B and zero-inflated negative binomial regression of A is super-imposed.
Table 3.
Change in annual rate of COPD exacerbation per 1-point increase in PSQI and co-variates in SPIROMICS, final models
| Complete cases model (n = 915) | p | Complete cases model, controlling for exacerbation history (n = 915) | p | Imputed model (n = 1647) | p | Imputed model, controlling for exacerbation history (n = 1647) | p | |
|---|---|---|---|---|---|---|---|---|
| Pittsburgh Sleep Quality Index (PSQI) | 1.08 (1.03–1.13) | .001 | 1.06 (1.01–1.11) | .01 | 1.05 (1.01–1.09) | .006 | 1.04 (1.01–1.08) | .02 |
| Inter-visit change in PSQI | 1.06 (1.01–1.11) | .01 | 1.04 (1.00–1.09) | .07 | 1.05 (1.01–1.09) | .02 | 1.03 (1.00–1.08) | .08 |
| Age | 0.83 (0.71–0.97) | .01 | 0.86 (0.74–1.00) | .048 | 0.91(0.82–1.02) | .14 | 0.93 (0.84–1.04) | 0.23 |
| Sex | 1.04 (0.78–1.37) | .79 | 0.96 (0.72–1.26) | .75 | 1.24 (1.00–1.53) | .03 | 1.14 (0.92–1.40) | .22 |
| BMI | 0.78 (0.67–0.91) | .002 | 0.78 (0.67–0.91) | .002 | 0.83 (0.74–0.94) | .001 | 0.83 (0.74–0.93) | .001 |
| Race | ||||||||
| Non-Hispanic White | Referent | Referent | Referent | Referent | ||||
| Black | 1.36 (0.87–2.11) | .18 | 1.37 (0.88–2.12) | .16 | 1.02 (0.75–1.38) | .98 | 1.04 (0.76–1.41) | .79 |
| Other | 0.44 (0.22–0.90) | .03 | 0.46 (0.23–0.94) | .03 | 0.55 (0.34–0.9) | .01 | 0.57 (0.35–0.91) | .02 |
| Asthma | ||||||||
| No | Referent | Referent | Referent | Referent | ||||
| Uncertain | 1.62 (0.81–3.26) | .17 | 1.60 (1.16–2.21) | .35 | 1.03 (0.63–1.68) | .92 | 0.93 (0.57–1.51) | .76 |
| Yes | 1.66 (1.20–2.30) | .002 | 1.39 (0.70–2.75) | .004 | 1.48 (1.17–1.89) | .001 | 1.37 (1.08–1.74) | .001 |
| Hospital Depression score | 1.01 (0.96–1.07) | .59 | 1.01 (0.96–1.07) | .63 | 1.00 (0.97–1.04) | 0.97 | 1.00 (0.97–1.04) | 1 |
| Hospital Anxiety score | 1.01 (0.96–1.06) | .71 | 1.01 (0.96–1.05) | .78 | 1.00 (0.96–1.03) | 0.97 | 0.99 (0.95–1.02) | .47 |
| Smoking pack-years | 1.19 (1.02–1.40) | .003 | 1.19 (1.02–1.38) | .03 | 1.05 (0.94–1.18) | 0.43 | 1.06 (0.95–1.19) | 1 |
| Percentage predicted post- bronchodilator FEV1 | 0.64 (0.55–0.76) | <.001 | 0.68 (0.57–0.80) | <.001 | 0.62 (0.55–0.71) | <.001 | 0.65 (0.58–0.75) | <.001 |
| COPD Assessment Test score | 1.03 (1.01–1.05) | .01 | 1.02 (1.00–1.05) | .04 | 1.04 (1.02–1.06) | <.001 | 1.04 (1.02–1.06) | <.001 |
| LAMA usage | 1.44 (1.07–1.93) | .02 | 1.26 (0.94–1.70) | .11 | 1.28 (1.02–1.59) | .06 | 1.17 (0.94–1.45) | .16 |
| LABA-ICS usage | 2.14 (1.60–2.87) | <.001 | 1.87 (1.40–2.50) | <.001 | 1.51 (1.21–1.89) | <.001 | 1.42 (1.14–1.77) | .002 |
| Comorbidity count | 1.20 (1.03–1.40) | .02 | 1.20 (1.03–1.39) | .02 | 1.17 (1.03–1.33) | .01 | 1.18 (1.04–1.34) | .01 |
| Exacerbation in year prior–enrollment | NA | 1.50 (1.28–1.76) | <.001 | NA | 1.45 (1.31–1.61) | <.001 | ||
| Area Deprivation Index (ADI) | 0.95 (0.82–1.12) | .56 | 0.99 (0.85–1.15) | .90 | 1.07 (0.95–1.20) | .24 | 1.09 (0.97–1.22) | .13 |
| Adversity–Opportunity Index | 0.97 (0.91–1.04) | .46 | 0.98 (0.92–1.05) | .58 | 0.94 (0.89–0.99) | .03 | 0.96 (0.91–1.01) | .12 |
This model suggests that for every 1-point increase in baseline Pittsburgh Sleep Quality Index (PSQI) score, holding all other values equal, the risk of prospective COPD exacerbation is increased by 5% in the follow-up year. Or for every additional 1 standard deviation change in post-bronchodilator FEV1 at baseline, the risk of exacerbation is decreased by 36% in the follow-up year.
Bolded values statistically significant at α < 0.05. Co-variates are presented per 1-point change for scaled instruments, and per 1 standard deviation change for continuous variables. All results are presented as “point estimate (95% CI)”.
Risk of hospitalization from COPD exacerbation
PSQI score was independently associated with an increased risk of hospitalization (Table 4) and remained significant in the full model with a 7% increase in risk of hospitalization with each 1-point increase in PSQI.
Table 4.
Change in annual rate of COPD exacerbation resulting in hospitalization per 1-point increase in PSQI and co-variates in SPIROMICS, full models
| Model A: complete cases (n = 915) | p | Model B: complete cases, controlling for exacerbation history (n = 915) | p | Model C: imputed model (n = 1647) | Model D: imputed model, controlling for exacerbation history (n = 1647) | |||
|---|---|---|---|---|---|---|---|---|
| Pittsburgh Sleep Quality Index (PSQI) | 1.08 (1.01–1.16) | .03 | 1.07 (1.00–1.15) | .05 | 1.07 (1.02–1.13) | .01 | 1.06 (1.01–1.12) | .02 |
| Inter-visit change in PSQI | 1.03 (0.96–1.11) | .34 | 1.02 (0.95–1.09) | .65 | 1.04 (0.98–1.11) | .14 | 1.03 (0.97–1.09) | 0.34 |
| Age | 0.94 (0.75–1.17) | .57 | 1.02 (0.82–1.27) | .89 | 0.91 (0.77–1.08) | .29 | 0.95 (0.81–1.12) | .55 |
| Sex | 1.05 (0.69–1.61) | .81 | 1.04 (0.69–1.58) | .84 | 1.12 (0.82–1.53) | .49 | 1.06 (0.78–1.44) | .73 |
| BMI | 0.79 (0.62–1.01) | .06 | 0.80 (0.63–1.01) | .06 | 0.84 (0.71–1.00) | .055 | 0.84 (0.70–1.00) | .05 |
| Race | ||||||||
| Non-Hispanic White | Referent | Referent | Referent | Referent | ||||
| Black | 3.49 (1.81–6.73) | <.001 | 3.55 (1.84–6.85) | <.001 | 2.13 (1.37–3.31) | .001 | 2.29 (1.47–3.56) | <.001 |
| Other | 0.60 (0.21–1.74) | .35 | 0.65 (0.23–1.82) | .42 | 0.66 (0.33–1.31) | .24 | 0.69 (0.35–1.35) | .28 |
| Asthma | ||||||||
| No | Referent | Referent | Referent | Referent | ||||
| Uncertain | 0.26 (0.08–0.89) | .03 | 0.28 (0.09–0.87) | .03 | 0.44 (0.20–0.96) | .04 | 0.44 (0.20–0.95) | .04 |
| Yes | 1.07 (0.65–1.76) | .79 | 1.02 (0.63–1.66) | .93 | 1.51 (1.07–2.15) | .02 | 1.36 (0.96–1.93) | .08 |
| Hospital Depression Scale score | 0.98 (0.90–1.07) | .71 | 1.00 (0.92–1.08) | .92 | 1.01 (0.96–1.07) | .63 | 1.03 (0.97–1.09) | 0.32 |
| Hospital Anxiety Scale score | 1.03 (0.96–1.11) | .41 | 1.02 (0.95–1.09) | .61 | 0.99 (0.94–1.05) | .76 | 0.98 (0.93–1.03) | 0.41 |
| Smoking pack-years | 1.25 (0.97–1.62) | .08 | 1.26 (0.98–1.60) | .07 | 1.18(0.99–1.42) | .07 | 1.19(1.00–1.42) | .05 |
| Percentage predicted post- bronchodilator FEV1 | 0.62 (0.47–0.82) | .001 | 0.64 (0.48–0.84) | .002 | 0.57 (0.46–0.70) | <.001 | 0.59 (0.48–0.73) | <.001 |
| COPD Assessment Test score | 1.03 (0.99–1.07) | .15 | 1.02 (0.99–1.06) | .21 | 1.02 (0.99–1.05) | .15 | 1.02 (0.99–1.05) | .17 |
| LAMA usage | 1.19 (0.77–1.84) | .43 | 0.96 (0.62–1.49) | .87 | 1.13 (0.81–1.55) | .47 | 1.03 (0.75–1.42) | .86 |
| LABA-ICS usage | 2.79 (1.79–4.43) | <.001 | 2.43 (1.57–3.77) | <.001 | 1.50 (1.07–2.10) | .02 | 1.39 (1.00–1.94) | .049 |
| Comorbidity count | 1.40 (1.12–1.76) | .003 | 1.39 (1.11–1.) | .004 | 1.22 (1.01–1.47) | .04 | 1.23 (1.02–1.48) | .03 |
| Exacerbations in year prior to enrollment | NA | 1.64 (1.31–2.05) | <.001 | NA | 1.43 (1.24–1.66) | <.001 | ||
| Area Deprivation Index (ADI) | 0.90 (0.72–1.14) | .39 | 1.00 (0.93–1.08) | .93 | 0.98 (0.83–1.16) | .85 | 1.02 (0.86–1.20) | |
| Adversity–Opportunity Index | 0.98 (0.87–1.09) | .70 | 0.99 (0.88–1.10) | .82 | 0.94 (0.86–1.03) | .15 | 0.96 (0.88–1.04) | .34 |
This model suggests that for every 1-point increase in baseline Pittsburgh Sleep Quality Index (PSQI) score, holding all other values equal, the risk of prospective COPD exacerbation is increased by 7% in the follow-up year. Or for every additional standard deviation change in post-bronchodilator FEV1 at baseline, the risk of exacerbation is decreased by 38% in the follow-up year.
Bolded values statistically significant at α < 0.05. All results are presented as “point estimate (95% CI)”. Co-variates are presented as per 1-point change for scaled instruments, and per 1 standard deviation change for continuous variables.
Interaction testing
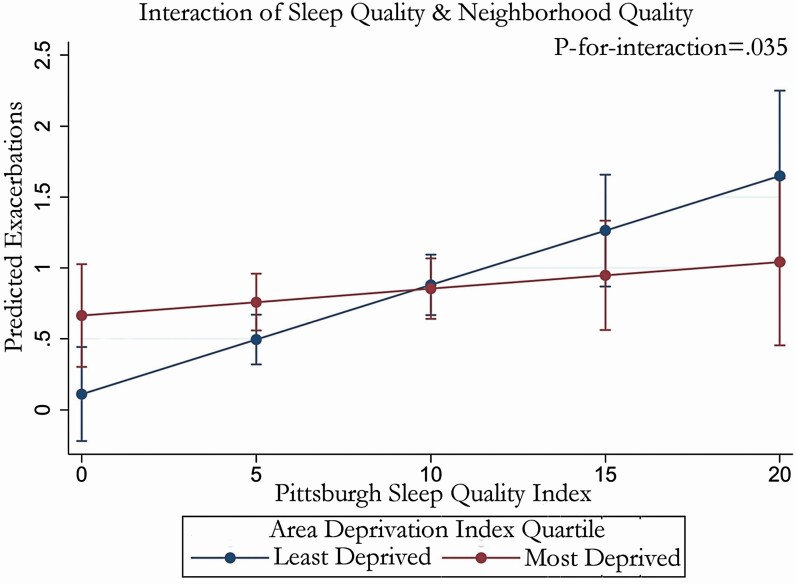
Our exploratory analysis did not suggest a uniform impact of PSQI on the risk of exacerbation across levels of the ADI. Instead, in the presence of high neighborhood deprivation as indicated by a high ADI, baseline sleep bore little relationship to risk for exacerbations (Figure 3). By contrast, in the presence of more favorable levels of ADI (i.e., a low ADI), exacerbation risk increased as PSQI increased (p-for-interaction = .035). There was no evidence of effect modification for AOI (p-for-interaction = .34), smoking pack-years (p-for-interaction = .70), or diagnosis of obstructive sleep apnea (p-for-interaction = .32). Sensitivity analyses were similar (data not shown).
Figure 3.
In the presence of worse neighborhood deprivation (in those in the highest quartile of the Area Deprivation Index [ADI]), baseline sleep bore little relationship to risk for exacerbations. Shown is a marginal effects plot demonstrating the predicted exacerbation count across the spectrum of Pittsburgh Sleep Quality Index (PSQI) scores at various, baseline ADI scores.
Discussion
In a multicenter cohort of 1647 participants with spirometrically confirmed COPD, we found sleep quality as measured by the PSQI to be an important risk factor independently associated with both total exacerbations, and the subset resulting in hospitalization. Our study contributes to the literature by better outlining the nuances in which sleep quality is a predictor of COPD exacerbation.
These results align with two previous reports identifying poor sleep quality as measured by the PSQI as associated with an increased exacerbation risk [2, 40]. The earlier of these was notable for its consideration of socioeconomic status but limited by small sample size, use of now-outdated metrics for COPD severity, and limited consideration of comorbidities [40]. Shorofsky et al. [2] addressed many of these concerns in a cohort of 480 individuals with COPD in a multicenter Canadian cohort, where he found a decreased time to first exacerbation with reports of poor sleep after adjustment for demographics, COPD severity, comorbidities, and medication usage. By contrast, a secondary analysis of 1117 participants in a study on the utility of azithromycin for frequent COPD exacerbations found that unadjusted associations between sleep quality and exacerbation rate were largely explained by comorbidities and medication usage [3]. This last study had several similarities to our own, including overall sample size, event definition, and statistical model employed. However, their population of interest was enriched for frequent exacerbators: the reported annual exacerbation rate among the original trial’s placebo group is several times our own (1.83/yr vs 0.5/yr).
Our study has several strengths, including that it is one of few studies [40] to collect more than 1 year of follow-up data. The lack of serial measurements in prior studies may have contributed to misestimate of the association between PSQI score and exacerbation risk. Additionally, we, similar to Omachi et al. [40], include metrics of socio-environmental condition. This is an important consideration, as important influences in longitudinal sleep quality have been documented in both adults and adolescents [41, 42].
Consistent with past literature, we found that socio-environmental exposures were an important factor in characterizing participants’ experience of COPD [43]. In the present study, more favorable exposures appeared to be associated with reduced exacerbation risk. Previous work demonstrates a longitudinal association of improved sleep quality with proximity to built features of the neighborhood environment that improve livability and investment value [44]. Contemporary neighborhood exposures have also been identified as an important contributor to outcome disparities in COPD [8]. Multiple mechanisms are likely at work. Adverse neighborhood conditions can create barriers to needed care through diminished social capital, limiting local information networks, and negatively influencing individuals’ sense of self-efficacy [45–47]. These would understandably yield worse outcomes. While these social dynamics represent an urgent priority for the healthcare system, mechanisms for direct biological impact on disease outcome are increasingly described: one of the most widely studied in this regard is allostatic load. This approach aims to quantify the effect of prolonged exposure to heightened levels of stress-related biomarkers [48]. Allostatic load is a framework that has previously been used to capture the physiologic consequences of environmental stressors like poverty and minority/marginalized status [49]. Redline et al. [50] previously reported that sleep problems were associated with increased allostatic load, independent of socioeconomic status. At the same time, neighborhood poverty as measured by the ADI is associated with high allostatic load [51]. We might therefore postulate that while worsened sleep quality as represented by increased PSQI increases the allostatic load for all individuals, its relative effect is dependent on background conditions. Where there are other reasons for high allostatic load, such as socio-environmental adversity, the impact of poor sleep is relatively smaller because there is already a strong propensity toward exacerbation. By contrast, in those with better living conditions, this contribution might be more influential in that it is more likely to push them across a threshold to exacerbation. This interaction also adds nuance to previously reported findings that social conditions can influence PSQI score [52, 53]. Overall, these points highlight the importance of factors outside of medical care in determining disease outcomes.
Mechanistically, these findings complement a larger body of work demonstrating the negative health consequences of impaired sleep. Animal models suggest the lung is especially sensitive to such insults, demonstrating the most extensive DNA damage of multiple organ systems after a trial of partial sleep deprivation in rats [54]. Multiple studies have demonstrated sleep disturbance, including those measuring sleep quality with the PSQI, to be correlated with a variety of deranged immunologic functions including a pro-inflammatory cytokine environment and vaccination response [55–58]. These immunologic perturbations may be particularly impactful given the importance of infectious triggers in COPD exacerbation. They also rationalize the diminished impact of sleep quality among those we would expect to have high background inflammation.
This study has several important limitations. Measures of subjective sleep quality do not necessarily correlate with objective measures and could produce different results. As a group, our Other self-identified race participants were too heterogeneous to allow generalization from these data. Imputation is a well-validated approach in data analysis, but a complete dataset would yield the most accurate estimates. Though we compared baseline sleep quality to prospective exacerbations, we cannot entirely exclude the possibility that our reported association between PSQI and exacerbation risk was a reflection of the known negative impact of respiratory symptoms on sleep quality. Alternative study designs could have more firmly established the direction of causality. While we controlled for respiratory medications, previous reports noted associations to non-respiratory medications unavailable in our dataset including anti-psychotics and opiates, which might have influenced results. Though we accounted for medication prescriptions, data on usage or compliance were not available. Similarly, adjustment for severity rather than mere presence of obstructive sleep apnea might also yield additional insights. The ADI captures some important dimensions of neighborhood quality but is far from comprehensive [59]. In particular, it does not address several that might impact sleep quality including ambient light, noise pollution, and violent crime rate. More generally, we encourage future research in sleep medicine to give greater emphasis to social conditions, which can have a demonstrably powerful influence. Though the current analysis suggests their importance, dedicated analyses designed to produce unconfounded estimates of impact of social conditions would yield higher quality estimates [60]. More generally, the significance of all co-variates should be interpreted with caution, given the possible issue of multiple comparison in addition to possible confounding. Like previous studies of the relationship between sleep quality and COPD exacerbations, we did not control for healthcare access. However, together with prior literature, there are now positive results across both Canadian and American samples, despite very different roles for governmental payers [61]. By contrast, disease severity or treatment compliance are likely important considerations for comorbidities like asthma and obstructive sleep apnea, but we were unable to account for this level of nuance in our analysis. Treatment adherence for COPD itself presents a similar challenge.
Conclusion
In this prospective, longitudinal, multicenter cohort of 1647 participants with spirometrically confirmed COPD, worse sleep quality was associated with an increased risk of both overall COPD exacerbations and the subset of exacerbations resulting in hospitalization. These findings highlight the importance of life circumstances in influencing disease control.
Acknowledgments
Zaid Alawi, BS assisted with graphics and visualization. The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R Graham Barr, MD, DrPH; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN.
Contributor Information
Aaron Baugh, Department of Medicine, University of California San Francisco, San Francisco, CA, USA.
Russell G Buhr, Department of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Pedro Quibrera, Collaborative Studies Coordination Center, University of North Carolina Gillings School of Global Public Health, Chapel Hill, NC, USA.
Igor Barjaktarevic, Department of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
R Graham Barr, Department of Medicine, Columbia University, New York, NY, USA.
Russell Bowler, Department of Medicine, National Jewish Health, Denver, CO, USA.
Meilan King Han, Department of Medicine, University of Michigan, Ann Arbor, MI, USA.
Joel D Kaufman, Department of Medicine, University of Washington, Seattle, WA, USA.
Abigail L Koch, Department of Medicine, Veterans Administration Miami Healthcare, Miami, FL, USA.
Jerry Krishnan, Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Wassim Labaki, Department of Medicine, University of Michigan, Ann Arbor, MI, USA.
Fernando J Martinez, Department of Medicine, Cornell University, Ithaca, NY, USA.
Takudzwa Mkorombindo, Department of Medicine, University of Alabama, Birmingham, AL, USA.
Andrew Namen, Department of Medicine, Wake Forest Baptist Health, Winston-Salem, NC, USA.
Victor Ortega, Department of Medicine, Mayo Clinic, Phoenix, AZ, USA.
Robert Paine, Department of Medicine, University of Utah, Salt Lake City, UA, USA.
Stephen P Peters, Department of Medicine, Wake Forest Baptist Health, Winston-Salem, NC, USA.
Helena Schotland, Department of Medicine, University of Michigan, Ann Arbor, MI, USA.
Krishna Sundar, Department of Medicine, University of Utah, Salt Lake City, UA, USA.
Michelle R Zeidler, Department of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Nadia N Hansel, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Prescott G Woodruff, Department of Medicine, University of California San Francisco, San Francisco, CA, USA.
Neeta Thakur, Department of Medicine, University of California San Francisco, San Francisco, CA, USA.
Disclosure Statement
Non-financial disclosures: Dr. Buhr, Dr. Koch, and Dr. Zeidler are employees of the Veterans Health Administration. The findings in this report do not necessarily reflect the positions of the Department of Veterans Affairs or US Government.
Financial disclosures: SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan. SPIROMICS AIR was additionally supported by NIEHS grant R01ES023500. Dr. Buhr is additionally supported by NIH/NCATS grant KL2TR001882 and NIH/NHLBI grant L30HL134025.
References
- 1. Agusti A, et al. . Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shorofsky M, et al. . Impaired sleep quality in COPD is associated with exacerbations: the CanCOLD cohort study. Chest. 2019;156(5):852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geiger-Brown J, et al. . Self-reported sleep quality and acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogelmeier CF, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 5. Han MLK, et al. . Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prescott E, et al. . Social position and mortality from respiratory diseases in males and females. Eur Respir J. 2003;21(5):821–826. [DOI] [PubMed] [Google Scholar]
- 7. Shin S, et al. . Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based cohort study. Am J Respir Crit Care Med. 2021;203(9):1138–1148. [DOI] [PubMed] [Google Scholar]
- 8. Ejike CO, et al. . Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021;203(8):987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel NP, et al. . “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ekirch AR. Sleep we have lost: pre-industrial slumber in the British Isles. Am Hist Rev. 2001;106(2):343–386. [PubMed] [Google Scholar]
- 11. Anda RF, et al. . Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34(5):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Couper D, et al. . Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 2014;69(5):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buysse DJ, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 14. Garrow A, et al. . Systematic literature review of patient-reported outcome measures used in assessment and measurement of sleep disorders in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mollayeva T, et al. . The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 16. Thakur N, et al. . Acculturation is associated with asthma burden and pulmonary function in Latino youth: the GALA II study. J Allergy Clin Immunol. 2019;143(5):1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su JG, et al. . An index for assessing demographic inequalities in cumulative environmental hazards with application to Los Angeles, California. Environ Sci Technol. 2009;43(20):7626–7634. [DOI] [PubMed] [Google Scholar]
- 18. Baugh AD, et al. . Reconsidering the utility of race-specific lung function prediction equations. Am J Respir Crit Care Med. 2022;205(7):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaakkola JJK, et al. . Prenatal and postnatal tobacco smoke exposure and respiratory health in Russian children. Respir Res. 2006;7(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. USDA ERS. Documentation. https://www.ers.usda.gov/data-products/food-access-research-atlas/documentation/. Accessed November 1, 2020.
- 21. Moughames E, et al. . Disparities in access to food and chronic obstructive pulmonary disease (COPD)-related outcomes: a cross-sectional analysis. BMC Pulm Med. 2021;21(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keranis E, et al. . Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. [DOI] [PubMed] [Google Scholar]
- 23. Blanc PD, et al. . Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paulin LM, et al. . Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(5):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krzyżanowska M, et al. . Do biomarkers vary by social class, education and region and is migration important? Evidence from a cohort of British adults. J Biosoc Sci. 2019;51(1):95–117. [DOI] [PubMed] [Google Scholar]
- 26. Guzman GG. Household Income: 2017. www.census.gov/programs. Accessed August 10, 2020.
- 27. Amemiya A, et al. . Association of low family income with lung function among children and adolescents: results of the J-SHINE study. J Epidemiol. 2019;29(2):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenland S, et al. . Causal Diagrams. Wiley StatsRef: Statistics Reference Online. Published online November 15, 2017:1–10. doi: 10.1002/9781118445112.stat03732.pub2. [DOI]
- 29. Grabicki M, et al. . COPD course and comorbidities: are there gender differences? In: Pokorski M, ed. Advances in Experimental Medicine and Biology. Vol. 1113. New York, NY: Springer LLC; 2019: 43–51. [DOI] [PubMed] [Google Scholar]
- 30. Müllerova H, et al. . Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. [DOI] [PubMed] [Google Scholar]
- 31. Putcha N, et al. . A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9(12):e114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menezes AMB, et al. . Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. [DOI] [PubMed] [Google Scholar]
- 33. Pooler A, et al. . Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jo YS, et al. . Impact of BMI on exacerbation and medical care expenses in subjects with mild to moderate airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2018;13:2261–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lusic Kalcina L, et al. . Good and poor sleepers among OSA patients: sleep quality and overnight polysomnography findings. Neurol Sci. 2017;38(7):1299–1306. [DOI] [PubMed] [Google Scholar]
- 36. Naranjo M, et al. . Undiagnosed OSA may significantly affect outcomes in adults admitted for COPD in an inner-city hospital. Chest. 2020;158(3):1198–1207. [DOI] [PubMed] [Google Scholar]
- 37. Conway SG, et al. . Effect of smoking habits on sleep. Braz J Med Biol Res. 2008;41(8):722–727. [DOI] [PubMed] [Google Scholar]
- 38. Raghunathan TE, et al. . IVEware: imputation and variance estimation software user guide. Published 2002. http://www.isr.umich.edu/src/smp/ive/. Accessed July 6, 2021.
- 39. Azur MJ, et al. . Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Omachi TA, et al. . Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saini EK, et al. . Socioeconomic status and sleep among couples. Behav Sleep Med. 2021;19(2):159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthews KA, et al. . Socioeconomic status in childhood predicts sleep continuity in adult Black and White men. Sleep Health. 2018;4(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miravitlles M, et al. . Socioeconomic status and health-related quality of life of patients with chronic obstructive pulmonary disease. Respiration. 2011;82(5):402–408. [DOI] [PubMed] [Google Scholar]
- 44. Dubowitz T, et al. . Does investing in low-income urban neighborhoods improve sleep? Sleep. 2021;44(6):1–8. doi: 10.1093/sleep/zsaa292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kind AJ, et al. . Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Ann Intern Med. 2014;161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong MS, et al. . Relationship of neighborhood social determinants of health on racial/ethnic mortality disparities in US veterans—mediation and moderating effects. Health Serv Res. 2020;55(Suppl 2):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prentice JC. Neighborhood effects on primary care access in Los Angeles. Soc Sci Med. 2006;62(5):1291–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Juster RP, et al. . Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. [DOI] [PubMed] [Google Scholar]
- 49. Barry LE, et al. . Association between asthma, corticosteroids and allostatic load biomarkers: a cross-sectional study. Thorax. 2020;75(10):835–841. [DOI] [PubMed] [Google Scholar]
- 50. Chen X, et al. . Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24(8):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schulz AJ, et al. . Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. 2012;102(9):1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Friedman EM, et al. . Socioeconomic status predicts objective and subjective sleep quality in aging women. Psychosom Med. 2007;69(7):682–691. [DOI] [PubMed] [Google Scholar]
- 53. Moazzami K, et al. . Racial disparities in sleep disturbances among patients with and without coronary artery disease: the role of clinical and socioeconomic factors. Sleep Health. 2020;6(5):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Everson CA, et al. . Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37(12):1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Irwin MR, et al. . Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spiegel K, et al. . Effect of sleep deprivation on response to immunizaton. J Am Med Assoc. 2002;288(12):1471–1472. [DOI] [PubMed] [Google Scholar]
- 57. Taylor DJ, et al. . Is insomnia a risk factor for decreased influenza vaccine response? Behav Sleep Med. 2017;15(4):270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prather AA, et al. . Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diez Roux AV, et al. . Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 60. Westreich D, et al. . The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ridic G, et al. . Comparisons of health care systems in the United States, Germany and Canada. Mater Sociomed. 2012;24(2):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]