Abstract

Graphene is one of the most promising nanomaterials with many extraordinary properties and numerous exciting applications. In this work, a green, facile, and rapid method was developed to prepare graphene directly from common biomass materials such as banana peels, cantaloupe peels, coconut peels, and orange peels by using concentrated solar radiation. The basic principle of this method is photothermal conversion. On a sunny day, the sunlight was concentrated by a biconvex lens to form a focused light spot with a high temperature above 1000 °C, which can directly convert fruit peels into graphene nanosheets within 2–3 s. The product is named concentrated-solar-induced graphene (CSIG) based on the process employed to generate it. The resulting CSIG was characterized using a range of analytical techniques. The Raman spectrum of the CSIG displayed two distinct peaks corresponding to the D and G bands at ∼1343 and ∼1568 cm–1, respectively. Scanning electron microscopy, transmission electron microscopy, and X-ray diffraction were used to confirm that the CSIG consists of a few layers of turbostratic graphene nanosheets. Atomic force microscopy characterization revealed that the CSIG nanosheets have a thickness of ∼4 nm. The antibacterial potential of the CSIG was also explored. The CSIG had a strong inhibitory effect on the growth of Escherichia coli. This simple, green, and straightforward method for producing graphene may open a new route for turning waste into useful materials: an inexhaustible and pollution-free natural resource can be readily exploited by using a solar tracker-lens system for the large-scale production of graphene materials directly from low-cost biomass materials.
Introduction
Over the past two decades, carbon-based nanomaterials have received tremendous attention because of their unique physical, chemical, and electrical properties.1−5 Among them, graphene has been the most widely studied carbon nanomaterial, which was first discovered in 2004 by Novoselov and Geim.6,7 It is composed of a single layer of carbon atoms with sp2 hybridized bonds that are arranged in a two-dimensional hexagonal lattice nanostructure.1,7 Now, graphene has become a shining star in the field of material science because of its excellent mechanical, thermal, magnetic, optical, and electrical characteristics,1,7 and has been widely applied in a broad range of applications, including biochemical sensing,3 optoelectronics,8 energy storage and conversion,4 photocatalysis,9 and biomedicine.10,11
To date, a number of methods have been developed to prepare graphene, such as mechanical exfoliation,6 ultrasonic exfoliation,12 chemical reduction,13 epitaxial growth,14 chemical vapor deposition (CVD),15−17 physical vapor deposition,18 laser induction,19,20 flash Joule heating,21,22 and carbon-ion implantation.23 However, most of these approaches are expensive and time consuming and require toxic or hazardous chemicals as well as complex and harsh experimental conditions. Furthermore, the growing energy crisis and environmental pollution are driving us to create energy-efficient and environmental-friendly routes for producing graphene on a large scale. Ramaprabhu et al. reported the preparation of graphene by exfoliation of graphene oxide using focused solar radiation.24,25 This method directly employs cost-free, renewable, and inexhaustible solar energy, showing remarkable advantages in contrast to other techniques. However, they use graphene oxide as carbon source, which is a toxic material, thus generating a stumbling block on large-scale production of graphene. Recently, Tour et al. employed low-valued or waste materials, such as cloth, food, wood, and plastic, to easily obtain carbon precursors for the preparation of graphene by laser induction, CVD, or flash Joule heating.21,26−28 Their routes possessed some merits in terms of carbon sources, such as reducing environmental pollution, but still consumed significant energy for the mass production of graphene. Therefore, there is an urgent need to establish an energy-saving and environmentally friendly route for the preparation of graphene.
In this work, we report a green route for the preparation of graphene that is both energy conserving and environmentally friendly. The route uses peels from fruits such as bananas, cantaloupes, coconuts, and oranges as carbon sources to produce graphene by concentrated solar radiation, with the process forming what is called concentrated-solar-induced graphene (CSIG). The technique’s essence is photothermal conversion. By employing a biconvex lens, the normal solar radiation is focused on the surface of fruit peels to scorch the peels at an extremely high temperature (above 1000 °C) spot that can directly convert peels into graphene nanosheets within 2–3 s. Additionally, the CSIG can be easily patterned on a fruit peel. The physical and chemical properties of the resulting CSIG are investigated with spectroscopic techniques and electron microscopy instruments. These results show that the CSIG is composed of 4–5 layers of turbostratic graphene nanosheets with an interlayer space of ∼3.81 Å. Furthermore, the CSIG displays a strong inhibitory effect on the growth of Escherichia coli (E. coli), indicating that it can be used as a potential antibacterial material. Our work has taken a significant step forward in turning waste into useful products while saving energy and protecting the environment.
Results and Discussion
Energy conservation and environmental protection are still the main pillars of the country’s sustainability enhancement program.29 Since the industrial revolution, huge amounts of non-renewable resources, such as fossil fuels, have been mined and consumed, causing unprecedented damage to the environment.30 Currently, a variety of environmental problems, such as global warming, destruction of the ozone layer, acid rain, and hazardous waste, have grown increasingly grim, posing a serious threat to the survival of life on the earth. Therefore, it is imperative for us to develop new energy sources as alternatives to fossil fuels. Obviously, solar energy is one of the most ideal alternative energy sources because it is has significant advantages such as it is clean, relatively cost-free, non-toxic, non-polluting, eco-friendly, renewable, and inexhaustible.31 To date, solar energy has been widely employed to produce usable thermal energy and electricity.31,32 At the same time, it is also necessary to develop some technologies to turn wastes into valuable materials. Recently, some research groups have reported converting waste plastic into graphene nanomaterials by flash Joule heating21,22 and CVD.27,33 These methods for preparing graphene took a step forward in turning waste into valuable materials. However, there remain some drawbacks to these approaches, such as the time required, high energy consumption, and high cost.
To overcome these shortcomings, we develop a route for producing graphene by concentrated solar radiation using fruit peel wastes as carbon sources. The method uses both green energy (sunlight) and eco-friendly materials (fruit peels) to generate graphene at almost zero cost. As depicted in Figure 1, in clear conditions, sunlight (one sun, i.e., 1000 W/m2) is focused on fruit peels through a biconvex lens, and the peels are converted into graphene within 2–3 s. Compared with other methods, the technique of concentrated natural solar radiation shows apparent advantages as it is cost free, chemical free, instantaneous, and energy efficient. Besides, the raw material for the generation of graphene comes from waste fruit peels. Most importantly, in terms of both the technique and the raw material, our route can not only reduce environmental pollution and save energy, but also can turn waste into valuable materials. It’s worth noting that concave mirrors can also be used to concentrate solar energy directly converting into highly intense thermal energy, replacing conventional biconvex lens or Fresnel lens. The concave mirrors collect sunlight from a large area and focus it into a small spot with high temperature by reflection, as in concentrated solar power,31 and it is different from a biconvex lens or a Fresnel lens which focuses light by transmission. With the help of solar power concentrators consisting of concave mirrors, the production of CSIG on a large scale can be easily achieved in the near future by concentrated solar radiation.
Figure 1.

Schematic diagram of the preparation of graphene derived from banana peels by using concentrated solar radiation.
The detailed mechanism for production of graphene by concentrated solar radiation may be attributed to a photochemical or photothermal process or both. As we know, before picking, fruit peels are exposed to sunlight every day while remain intact. Therefore, photochemical process is hardly participated in converting fruit peels into graphene. Fruit peels are mainly composed of carbon-containing substances such as cellulose, hemicellulose, lignin, sugar, and pectin.34 These carbon-containing materials can be instantaneously converted into graphene at high localized temperatures in ambient atmosphere.26 Biconvex lens is a converging lens that can be used to focus sunlight. The sunlight passing through the lens converges to a focused spot behind the lens. The temperature of the focused spot depends on the size of the biconvex lens. Here, we adopted a diameter of ∼18 cm glass biconvex lens that can focus the sunlight into a high temperature (above 1000 °C) spot with a size of 2–3 mm on sunny days. Such highly intense thermal energy can readily break C=O, C–O, and C–N bonds. These atoms are then recombined and released as gases while the remaining aromatic compounds are graphitized to form graphene nanomaterials. The mechanism is very similar to the photothermal effects used to make infrared laser-induced graphene.19
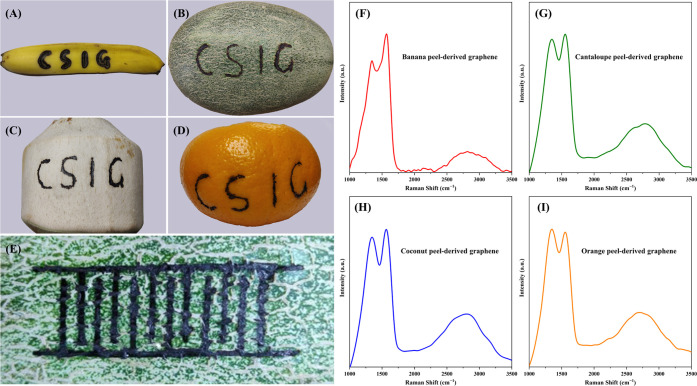
Figure 2A presents a banana peel upon which concentrated solar radiation is used to prepare graphene in the shape of the letters “CSIG”. The areas exposed to the focused spot of light are directly converted into graphene, while the unexposed areas remain unchanged. The same experiment is performed on cantaloupe peels (Figure 2B), coconut peels (Figure 2C), and orange peels (Figure 2D), showing the same results. The size of the focused light spot is about 2–3 mm, and therefore CSIG patterns with millimeter-level resolution can be readily fabricated by direct writing on carbon precursors. As shown in Figure 2E, the CSIG is directly written to 12 interdigital electrodes with line widths of ∼2 mm on cantaloupe peels by concentrated solar radiation. Additionally, interdigital electrodes with ∼3 and ∼5 mm line widths are also fabricated, which are displayed in Figure S1. These interdigitated CSIG electrodes are expected to be used in supercapacitors and sensors in the near future. The fabrication of higher resolution CSIG patterns can be achieved by reducing the size of focused light spot by combining multiple convex lenses. Our method can not only directly produce graphene from fruit peels, but also directly pattern the graphene on fruit peels.
Figure 2.
Photograph of graphene patterned into a shape of the letters “CSIG” by using concentrated solar radiation on (A) banana peel, (B) cantaloupe peel, (C) coconut peel, and (D) orange peel. (E) Photograph of graphene patterned into 12 interdigital electrodes with a line width of ∼2 mm on cantaloupe peel by using concentrated solar radiation. Raman spectra of CSIG were derived from (F) banana peel, (G) cantaloupe peel, (H) coconut peel, and (I) orange peel.
The chemical and physical properties of the CSIG derived from the fruit peels were first investigated with Raman spectroscopy. As shown in Figure 2F, the Raman spectrum of the CSIG derived from banana peels displayed two major peaks: the D peak at ∼1343 cm–1 originating from the defect-induced breathing mode of sp3 hybridized carbon atoms and suggesting the formation of grain boundaries and vacancies because of defects, and the G peak at ∼1568 cm–1 representing the sp2 hybridization of carbon atoms.25,35 The ID/IG ratio was determined to be about 0.8, indicating more domains of sp2 hybridized carbon atoms within the CSIG.35,36 In addition, second-order bands were observed in the range from 2500 to 3300 cm–1, which were assigned to the combination of 2D, D + G, and 2G bands. Similar results were also observed in Raman spectra of graphene prepared by other methods.37,38 The 2D band and 2G band are attributed to the overtone of the D band and G band, respectively. The D + G band is the combined overtone of the D band and G band, which is caused by lattice disorder.38 It’s worth noting that the second-order bands of graphene are highly structure-sensitive. Moreover, the shape of the peak originating from second-order bands could be employed to clearly differentiate monolayer graphene, bilayer graphene, and graphite. Based on previous reports, few-layer graphene nanosheets (2–5 layers) show a broader and symmetrical peak in second-order band, whereas graphene nanosheets with more than 5 layers and graphite display similar features.35,39 It can thus be inferred that the CSIG is composed of few-layer graphene nanosheets. For the carbon precursor (i.e., banana peels), its Raman spectrum (Figure S2) does not show any characteristic peaks of graphene. The result fully demonstrates that banana peels can be converted to graphene nanosheets by employing concentrated solar radiation. The yield of CSIG derived from banana peels was determined to be about 5 mg per minute.
For CSIG from others fruit peels, their Raman spectra (Figure 2G–I) also exhibited characteristic peaks of graphene, which were similar to Figure 2F. In addition to the fruit peels, polyimide (PI) was also chosen as a carbon precursor to further verify the efficiency of the concentrated solar radiation for preparing graphene. As shown in Figure S3, the Raman spectrum of the CSIG derived from PI films also displayed two prominent peaks at ∼1344 and ∼1589 cm–1, corresponding to the D and G bands, respectively. The results agree with the laser-induced graphene using the same carbon source.19 Similar to Figure 2F, a small and broad peak originating from second-order bands was also observed in the range of 2500–3000 cm–1. These Raman results finally confirm that our developed technique can be used for the preparation of graphene nanosheets from common fruit peels and from commercial polymers, and further prove the universality of the technique. In the near future, this approach can be further improved by more advanced sunlight manipulation techniques and can have much broader applications.
We performed X-ray diffraction (XRD) measurements to investigate the crystalline properties and interlayer spacing (d) of the CSIG, and the results are presented in Figure S4 and Table S1. There was a broad diffraction peak centered at 2θ ≈ 23.3°, corresponding to the (002) lattice planes in the CSIG.37,40 Compared to graphite, the peak was broader and shifted to lower 2θ. The changes in the peak may be attributed to the presence of a small amount of residual oxygen-containing functional groups, corrugated structures, or other structural defects.41,42 The interlayer spacing of the CSIG was calculated using Bragg’s equation, and its crystalline size was obtained by employing the Debye–Scherrer formula.19,37,40,43 As a result, the interlayer spacing of the CSIG was calculated to be ∼3.81 Å, which was slightly larger than the interlayer distance (∼3.35 Å) of graphite.44 The crystalline size along the c axis (Lc) was found to be ∼7.77 Å. These results clearly demonstrate the formation of few-layer stacked graphene nanosheets in the CSIG, agreeing well with the Raman analysis. Additionally, a weak diffraction peak centered at 2θ ≈ 43.8° was attributed to (100) reflections, which are related to an in-plane structure.19,40 These features are typically found in turbostratic graphene nanosheets.22 Based on the (100) diffraction peak, the crystalline size along the a axis (La) was calculated to be ∼27.1 Å by using the Debye–Scherrer formula.19,43
X-ray photoelectron spectroscopy (XPS) was employed to study the surface chemical structure of the prepared CSIG. Figure 3 shows a high-resolution C 1s XPS spectrum of the CSIG, which exhibits a dominant C–C/C=C peak at ∼284.8 eV corresponding to the sp2 hybridization of carbon atoms in graphitic structure12,21 that verifies the successful graphitization of banana peels. The remaining two peaks at ∼286.4 and ∼288.3 eV are assigned to carbon atom in the C–O and C=O functional groups, respectively.12,19 Furthermore, the C–C/C=C peak strongly suppresses C–O and C=O peaks. The relative strength of the C–C/C=C peak implies that the CSIG is dominated by sp2 hybridization of carbon atoms, consistent with the Raman result. The surface chemistry properties of the CSIG were further confirmed with Fourier transform infrared (FT-IR) spectroscopy. As displayed in Figure S5 and Table S2, the FT-IR spectrum of the CSIG exhibits a medium, broad peak at ∼3278.9 cm–1, a medium, sharp peak at ∼2924.5 cm–1, a strong, sharp peak at ∼1602.6 cm–1, a strong, sharp peak at ∼1382.7 cm–1, a medium, sharp peak at ∼1076.6 cm–1, a weak, sharp peak at ∼782.1 cm–1, and a weak, broad peak at ∼675.5 cm–1 corresponding to O–H stretching, C–H stretching, C=C stretching, C–H bending, C–O stretching, C–H out-of-plane ring bending, and ν11 mode of benzene, respectively.37,40,42 The sharp and very strong C=C stretching vibrational peak in the FT-IR spectrum indicates that the CSIG has a large amount of sp2 hybridized carbon, consistent with the results of Raman, XRD, and XPS. Additionally, the oxygen-containing functional groups not only improve the solubility and stability of the CSIG in polar systems, but also promote applications in biology and medicine.
Figure 3.
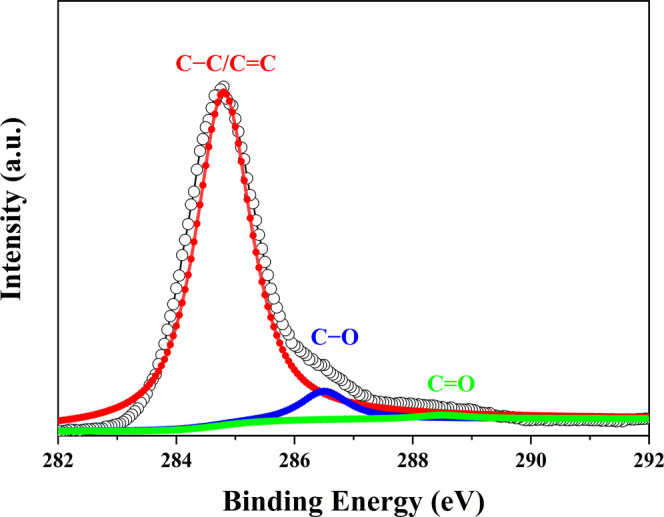
High-resolution C 1s XPS spectrum of CSIG.
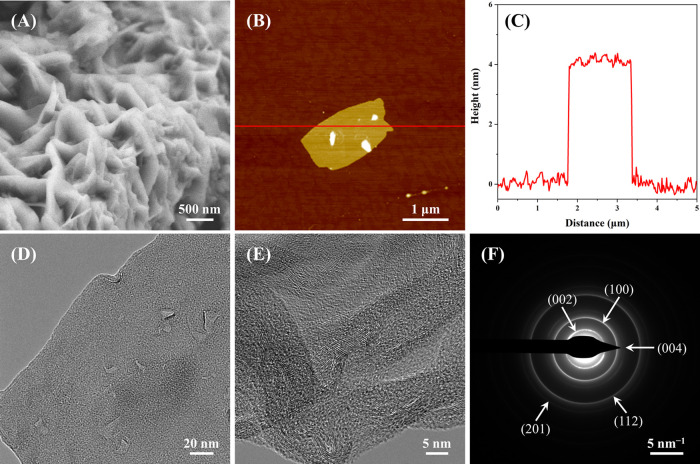
Scanning electron microscopy (SEM) was carried out to characterize the surface morphology of CSIG. As shown in Figures 4A and S6, the SEM image of the CSIG displayed the appearance of typical lamellar structures. It directly demonstrates that the CSIG is composed of stacks of these lamellar structures. However, it is difficult to precisely obtain the number of the layers present within the stacks of the CSIG. Therefore, atomic force microscopy (AFM) was used to further investigate the surface morphology of the CSIG. The CSIG powders were dispersed in ethanol by sonication for 5 min, and then dropped onto a freshly cleaved mica substrate, dried in an oven at 80° for 10 s and immediately used for AFM characterization. Figure 4B presents an AFM image of a 5 × 5 μm2 square of the CSIG. A clear graphene nanosheet structure with a size of 1 × 2 μm2 was observed on the mica substrate, which agreed well with the results of the SEM. The average thickness of the CSIG nanosheets was determined to be ∼4 nm based on the height profile of the AFM image (Figure 4C), which was similar to graphene prepared by other approaches.42,45−48 Furthermore, many AFM results in the literature indicate that the thickness of a single layer of graphene is about 0.8–1 nm.42,49−53 Hence, it can be concluded that the CSIG was composed of 4–5 layers of graphene nanosheets, agreeing with the Raman results.
Figure 4.
(A) SEM image of CSIG nanosheets on a conductive tape. (B) AFM height image of CSIG nanosheet on a mica substrate. (C) The corresponding height profile of the AFM image. (D) Low magnification and (E) high-resolution TEM images of CSIG nanosheets on a carbon-coated copper grid. (F) The SAED pattern of CSIG nanosheets.
We also used transmission electron microscopy (TEM) to further characterize the fine structure of the irradiated sample with atomic resolution. First, the CSIG powders were dispersed in ethanol by sonication for 5 min, transferred onto a carbon-coated copper grid, and then dried for TEM characterization. As shown in Figures 4D and S7, the CSIG had the typical lamellar nanostructure of graphene on the top of the carbon-coated copper grid, with a few layers of graphene overlap. This result was completely consistent with the results of SEM, AFM, and Raman. Moreover, the high-resolution TEM image (Figure 4E) of CSIG exhibited clear graphene lattice fringes, verifying the crystalline nature of the graphene nanosheets, which were very similar to graphene prepared by flash Joule heating.21 The selected area electron diffraction (SAED) pattern (Figure 4F) displayed five concentric diffraction rings, indicating that the CSIG had polycrystalline structures. From the inside out, these diffraction rings are assigned to the (002), (100), (004), (112), and (201) planes of the hexagonal crystalline graphite (JCPDS, Card No. 41-1487).44 Among them, the diffraction ring corresponding to the (002) plane was the brightest, followed by the (100) plane, which was completely consistent with the XRD data. The signals of the remaining three diffraction rings were relatively weak, and they were also hard to find in the XRD pattern because the second brightest diffraction ring in the SAED pattern indexed to the (100) plane, whereas it corresponded to a very weak peak at 2θ ≈ 43.8° in the XRD pattern. Therefore, we speculate that the signals corresponding to three diffraction rings in XRD were too weak to be detected. In addition, the SAED pattern of CSIG was a set of diffraction rings instead of diffraction spots, which was significantly different from previously reported graphene.12,17,54 The ring pattern suggested that the CSIG is made up of random stacking of the graphene layers. Notably, the laser-induced graphene reported by Tour’s group also showed (002) and (100) diffraction rings instead of diffraction spots in an SAED pattern.19
Previous reports in the literature have cited the strong antibacterial activity of graphene.11,54−57E. coli as a model bacterium was widely used to evaluate the antibacterial properties of graphene as well as graphene derivatives.55−60 To explore the potential applications of the prepared CSIG, E. coli was adopted to assess the antibacterial activity of the CSIG. First, the time dependence of antibacterial behavior on CSIG was studied. The CSIG dispersions with a concentration of 200 μg/mL were incubated with E. coli in Luria-Bertani (LB) broth medium at 37 °C at a shaking speed of 220 rpm. E. coli without CSIG was incubated as a control. Every hour for 6 h E. coli was transferred from the liquid LB medium to a solid LB medium and incubated at 37 °C for 18 h. A classic colony counting method was employed to measure the microbial viability of E. coli treated with CSIG at different incubation times. As shown in Figures 5A and S8, the loss of E. coli viability progressively increased with extending incubation time. In detail, the loss of E. coli viability was 10.6 ± 3.5% after 1 h, and increased to 35.8 ± 3.9, 49.7 ± 3.4, 60.2 ± 4.6, 75.6 ± 3.8, and 91.6 ± 4.2% after 2, 3, 4, 5, and 6 h, respectively. The result shows the time-dependent antibacterial behavior of CSIG, and the majority of E. coli died after incubation with 200 μg/mL CSIG for 6 h.
Figure 5.
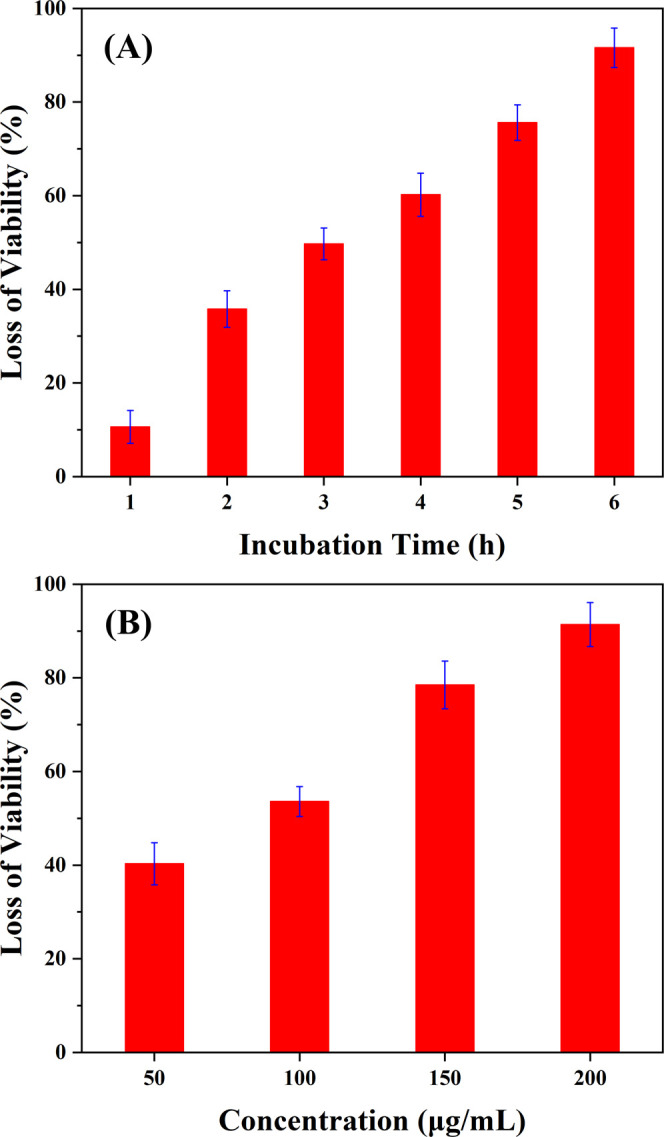
(A) Time-dependent antibacterial activities of CSIG: 200 μg/mL CSIG was incubated with E. coli cells for 6 h at 37 °C; the loss of viability was measured at 1, 2, 3, 4, 5, and 6 h. (B) Concentration-dependent antibacterial activities of CSIG: CSIG (at 50, 100, 150, and 200 μg/mL) was incubated with E. coli cells for 6 h at 37 °C.
Moreover, we also investigated the concentration-dependent antibacterial behavior of CSIG. E. coli was incubated with different concentrations (50, 100, 150, and 200 μg/mL) of CSIG dispersions in LB broth liquid medium at 37 °C for 6 h with a shaking speed of 220 rpm. Similarly, E. coli without CSIG was incubated as a control. After cultivation, all E. coli were immediately transferred to LB solid medium and further incubated at 37 °C for 18 h. The colony counting method was also used to evaluate the microbial viability of E. coli treated with four different concentrations of CSIG. As shown in Figures 5B and S9, the loss of E. coli viability was 40.3 ± 4.5, 53.6 ± 3.2, 78.5 ± 5.1, and 91.4 ± 4.7% for CSIG concentrations of 50, 100, 150, and 200 μg/mL, respectively. The antibacterial ability of CSIG gradually went up with the increase of CSIG concentration, and most of E. coli were killed after incubation with the CSIG concentration of 200 μg/mL. These results indicate that the CSIG has an excellent antibacterial activity.
To reveal how CSIG kills E. coli, SEM was used to examine the interactions between bacteria and CSIG. As displayed in Figure 6A, E. coli cells maintained membrane integrity with a normal rod-shaped morphology. After exposure to the CSIG dispersions, many E. coli cells lost the integrity of their membrane and released intracellular contents (Figure 6B), which agreed well with previous reports.54−57 It shows that direct contact of E. coli cells with CSIG leads to irreversible cell membrane damage. As we know, graphene nanosheets have a sharp edge that can create significant membrane stress. The results of SEM, AFM, and TEM all reveal that the CSIG nanosheets possess sharp edges, as displayed in Figure 4A,B,D. These nanosheets act as incisive “nanoknife” to destroy cell membranes, resulting in cytoplasmic leakage, and ultimately cell death.11 Oxidative stress also serves as a key factor in killing bacteria. Based on the literature, bacterial lipids and proteins can be oxidized by graphene.11,56 Therefore, we speculate its antibacterial mechanism: CSIG nanosheets directly interact with bacterial cells and disrupt cell membranes, then oxidative stress further damages the cell, finally leading to cell death.
Figure 6.
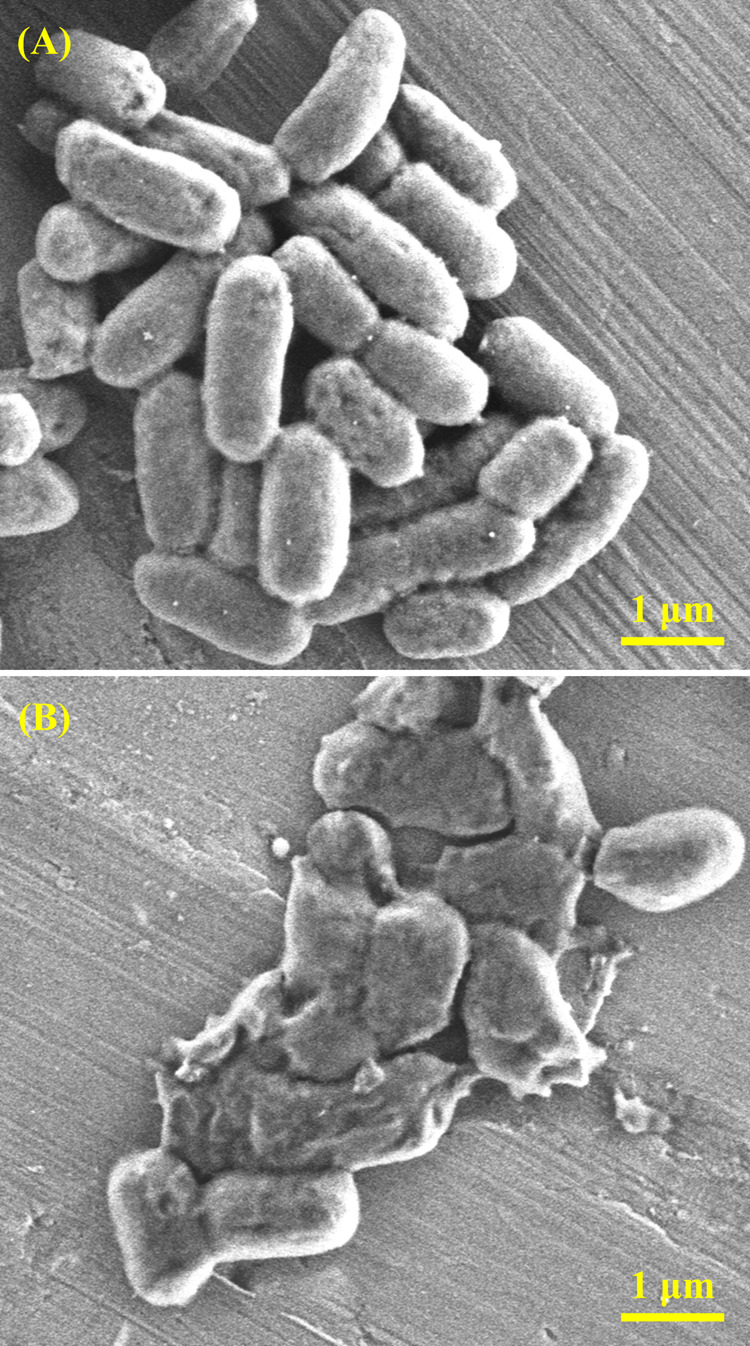
(A) SEM image of E. coli cells after incubation for 6 h at 37 °C without CSIG. (B) SEM image of E. coli cells after incubation with CSIG dispersions at the concentration of 200 μg/mL for 6 h at 37 °C.
Conclusions
We have successfully developed an energy-saving and environmentally friendly approach for the preparation of graphene from fruit peel wastes by using concentrated solar radiation in an ambient atmosphere. The basic principle of the technique is utilizing a biconvex lens to convert normal sunlight into intense heat energy that can directly convert fruit peels into graphene nanosheets within 2–3 s. The resulting CSIG has a thickness of ∼4 nm, which was made of a few layers of turbostratic graphene nanosheets. In addition, the CSIG can be easily patterned into desired structures with millimeter-level resolution. Furthermore, the CSIG presented a strong inhibitory effect on the growth of E. coli, suggesting that it can be used as an antibacterial material. This work has taken a significant and meaningful step forward in turning waste into useful materials in an energy-saving and environmentally friendly way. In the future, with the aid of a solar tracker-lens system, cost-free, pollution-free, and inexhaustible solar energy can be easily exploited for mass-producing graphene materials from wastes.
Experimental Section
Materials
Fresh bananas, cantaloupes, coconuts, and oranges were purchased from a local market. Ethanol (C2H5OH, 75%) was acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used as received. The ultrapurified and distilled-deionized water was obtained from the laboratory of the School of Microelectronics.
Preparation of CSIG
Fruit peels were directly obtained from the purchased fresh fruits, which served as raw materials to produce graphene without any further treatment. On sunny days at 10–15 o’clock, the sunlight (one sun, i.e., 1000 W/m2) was concentrated by a biconvex lens with a diameter of ∼18 cm, forming a high temperature (above 1000 °C) focused light spot with a size of 2–3 mm on the fruit peels, as measured by a digital thermometer (UNI-T UT325, China). Highly intense focused solar radiation directly converted fruit peels into graphene nanosheets within 2–3 s of exposure. These products were collected and manually ground in an agate mortar, washed several times with deionized water followed by washing with 75% ethanol, and finally dried to yield CSIG powders. The powders were stored in a drying cabinet for subsequent characterization and antibacterial experiments.
Characterization
A HORIBA Jobin Yvon Raman spectrometer (LabRAM HR Evolution, France) using 633 nm laser excitation at room temperature was employed to obtain Raman spectra of CSIG and carbon sources. The surface morphology of CSIG was characterized by a field-emission SEM (Zeiss GeminiSEM 300, Germany) with an accelerating voltage of 5 kV and a working distance of ∼4.5 mm. The topography of CSIG was investigated with an AFM (Park NX10, Korea). The crystallographic states of CSIG were studied with XRD (Bruker D8, Germany) with Cu Kα radiation (λ = 1.54 Å) in the 2θ range of 10–90°, while TEM (Thermo Scientific Talos F200X G2, USA) was used to obtain the fine structures. XPS (Thermo Scientific Escalab250Xi, USA) was adopted for the measurement of elemental composition of CSIG. FT-IR spectroscopy (PerkinElmer Spectrum BX, Germany) was carried out to investigate the surface functional groups of CSIG.
Test of Antibacterial Properties
The CSIG powder was sterilized under ultraviolet light for 30 min. E. coli (DH5α) was diluted to 106 colony-forming units (CFU) per mL with LB broth liquid medium. The sterilized CSIG powder was added to the LB liquid medium to form a suspension. The CSIG suspension was diluted to different concentrations. Then, identical amounts of E. coli liquid medium were added to different concentrations of CSIG suspensions, and incubated for 6 h at 37 °C with a shaking speed of 220 rpm. E. coli without the CSIG dispersion was incubated as a control. After incubation, these E. coli liquid mediums were diluted 10-fold with LB liquid medium, with a final E. coli concentration of 107 CFU per mL, then transferred to LB solid medium, and further incubated at 37 °C for 18 h. The culture plates with active E. coli were photographed according to the National Standard of China GB 4789.2-2016 protocol. Colonies were recorded and compared with those on control plates to obtain the loss of E. coli viability. For time-dependent antibacterial experiments, 200 μg/mL CSIG were incubated with E. coli in LB broth liquid medium at 37 °C under the shaking speed of 220 rpm. Similarly, E. coli without CSIG dispersion was incubated as control. After 1, 2, 3, 4, 5, and 6 h, E. coli was transferred from liquid medium to solid medium and further incubated at 37 °C for 18 h. After incubation, the culture plates with active bacteria were photographed, and E. coli colonies were counted and compared to those on control plates for measuring the loss of bacteria viability. To ensure the validity of the results, the tests were repeated three times.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 61974030).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02159.
Photograph of graphene patterned into a shape of the interdigital electrodes on cantaloupe peels (Figure S1); Raman spectra of four different carbon sources (Figure S2); Raman spectrum of CSIG from PI film (Figure S3); XRD pattern of CSIG from banana peels (Figure S4); Gauss fitting parameter of the XRD pattern (Table S1); FT-IR spectrum of CSIG from banana peels (Figure S5); assignment of the peaks in the FT-IR spectrum (Table S2); SEM images of CSIG from banana peels at different magnifications (Figure S6); TEM images of CSIG from banana peel at different magnifications (Figure S7); photographs showing the bacterial culture plates of E. coli incubated without CSIG, and with 200 μg/mL CSIG exposure for 1, 2, 3, 4, 5, and 6 h (Figure S8); photographs showing the bacterial culture plates of E. coli upon a 6 h exposure to the control (without CSIG) and four different concentrations of CSIG dispersion (Figure S9) (PDF)
Author Contributions
† X.-H.H. and R.Z. contributed equally to this work. X.-H.H. proposed the initial idea of this work and designed the whole experiments. X.-H.H. and R.Z. were responsible for the preparation, characterization, and application of CSIG. Z.W. was responsible for SEM characterization of CSIG. The manuscript was prepared and written by X.-H.H. and S.X. and was approved by all coauthors.
The authors declare no competing financial interest.
Supplementary Material
References
- Allen M. J.; Tung V. C.; Kaner R. B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- Singh V.; Joung D.; Zhai L.; Das S.; Khondaker S. I.; Seal S. Graphene Based Materials: Past, Present and Future. Prog. Mater. Sci. 2011, 56, 1178–1271. 10.1016/j.pmatsci.2011.03.003. [DOI] [Google Scholar]
- Liu Y.; Dong X.; Chen P. Biological and Chemical Sensors based on Graphene Materials. Chem. Soc. Rev. 2012, 41, 2283–2307. 10.1039/C1CS15270J. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhi L. Graphene Hybridization for Energy Storage Applications. Chem. Soc. Rev. 2018, 47, 3189–3216. 10.1039/C7CS00871F. [DOI] [PubMed] [Google Scholar]
- Hu X.-H.; An X.; Li L. Easy Synthesis of Highly Fluorescent Carbon Dots from Albumin and Their Photoluminescent Mechanism and Biological Imaging Applications. Mater. Sci. Eng., C 2016, 58, 730–736. 10.1016/j.msec.2015.09.066. [DOI] [PubMed] [Google Scholar]
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Geim A. K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- Bonaccorso F.; Sun Z.; Hasan T.; Ferrari A. C. Graphene Photonics and Optoelectronics. Nat. Photon. 2010, 4, 611–622. 10.1038/nphoton.2010.186. [DOI] [Google Scholar]
- Li X.; Yu J.; Wageh S.; Al-Ghamdi A. A.; Xie J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. 10.1002/smll.201600382. [DOI] [PubMed] [Google Scholar]
- Yang K.; Feng L.; Shi X.; Liu Z. Nano-Graphene in Biomedicine: Theranostic Applications. Chem. Soc. Rev. 2013, 42, 530–547. 10.1039/C2CS35342C. [DOI] [PubMed] [Google Scholar]
- Karahan H. E.; Wiraja C.; Xu C.; Wei J.; Wang Y.; Wang L.; Liu F.; Chen Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthcare Mater. 2018, 7, 1701406 10.1002/adhm.201701406. [DOI] [PubMed] [Google Scholar]
- Hernandez Y.; Nicolosi V.; Lotya M.; Blighe F. M.; Sun Z.; De S.; McGovern I. T.; Holland B.; Byrne M.; Gun’Ko Y. K.; Boland J. J.; Niraj P.; Duesberg G.; Krishnamurthy S.; Goodhue R.; Hutchison J.; Scardaci V.; Ferrari A. C.; Coleman J. N. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- Stankovich S.; Dikin D. A.; Piner R. D.; Kohlhaas K. A.; Kleinhammes A.; Jia Y.; Wu Y.; Nguyen S. T.; Ruoff R. S. Synthesis of Graphene-based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- Yang W.; Chen G.; Shi Z.; Liu C.-C.; Zhang L.; Xie G.; Cheng M.; Wang D.; Yang R.; Shi D.; Watanabe K.; Taniguchi T.; Yao Y.; Zhang Y.; Zhang G. Epitaxial Growth of Single-domain Graphene on Hexagonal Boron Nitride. Nat. Mater. 2013, 12, 792–797. 10.1038/nmat3695. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Lee E. K.; Joo W.-J.; Jang Y.; Kim B.-S.; Lim J. Y.; Choi S.-H.; Ahn S. J.; Ahn J. R.; Park M.-H.; Yang C.-W.; Choi B. L.; Hwang S.-W.; Whang D. Wafer-Scale Growth of Single-Crystal Monolayer Graphene on Reusable Hydrogen-Terminated Germanium. Science 2014, 344, 286–289. 10.1126/science.1252268. [DOI] [PubMed] [Google Scholar]
- Mattevi C.; Kim H.; Chhowalla M. A Review of Chemical Vapour Deposition of Graphene on Copper. J. Mater. Chem. 2011, 21, 3324–3334. 10.1039/C0JM02126A. [DOI] [Google Scholar]
- Li J.; Chen M.; Samad A.; Dong H.; Ray A.; Zhang J.; Jiang X.; Schwingenschlögl U.; Domke J.; Chen C.; Han Y.; Fritz T.; Ruoff R. S.; Tian B.; Zhang X. Wafer-Scale Single-Crystal Monolayer Graphene Grown on Sapphire Substrate. Nat. Mater. 2022, 21, 740–747. 10.1038/s41563-021-01174-1. [DOI] [PubMed] [Google Scholar]
- Garlow J. A.; Barrett L. K.; Wu L.; Kisslinger K.; Zhu Y.; Pulecio J. F. Large-Area Growth of Turbostratic Graphene on Ni(111) via Physical Vapor Deposition. Sci. Rep. 2016, 6, 19804 10.1038/srep19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Peng Z.; Liu Y.; Ruiz-Zepeda F.; Ye R.; Samuel E. L. G.; Yacaman M. J.; Yakobson B. I.; Tour J. M. Laser-induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714 10.1038/ncomms6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy L. X.; Peng Z.; Li Y.; Zhang J.; Ji Y.; Tour J. M. Laser-Induced Graphene Fibers. Carbon 2018, 126, 472–479. 10.1016/j.carbon.2017.10.036. [DOI] [Google Scholar]
- Algozeeb W. A.; Savas P. E.; Luong D. X.; Chen W.; Kittrell C.; Bhat M.; Shahsavari R.; Tour J. M. Flash Graphene from Plastic Waste. ACS Nano 2020, 14, 15595–15604. 10.1021/acsnano.0c06328. [DOI] [PubMed] [Google Scholar]
- Stanford M. G.; Bets K. V.; Luong D. X.; Advincula P. A.; Chen W.; Li J. T.; Wang Z.; McHugh E. A.; Algozeeb W. A.; Yakobson B. I.; Tour J. M. Flash Graphene Morphologies. ACS Nano 2020, 14, 13691–13699. 10.1021/acsnano.0c05900. [DOI] [PubMed] [Google Scholar]
- Kim J.; Lee G.; Kim J. Wafer-scale Synthesis of Multi-layer Graphene by High-temperature Carbon Ion Implantation. Appl. Phys. Lett. 2015, 107, 033104 10.1063/1.4926605. [DOI] [Google Scholar]
- Eswaraiah V.; Sankaranarayanan V.; Ramaprabhu S. Graphene-Based Engine Oil Nanofluids for Tribological Applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. 10.1021/am200851z. [DOI] [PubMed] [Google Scholar]
- Eswaraiah V.; Jyothirmayee Aravind S. S.; Ramaprabhu S. Top Down Method for Synthesis of Highly Conducting Graphene by Exfoliation of Graphite Oxide Using Focused Solar Radiation. J. Mater. Chem. 2011, 21, 6800–6803. 10.1039/c1jm10808e. [DOI] [Google Scholar]
- Chyan Y.; Ye R.; Li Y.; Singh S. P.; Arnusch C. J.; Tour J. M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. 10.1021/acsnano.7b08539. [DOI] [PubMed] [Google Scholar]
- Ruan G.; Sun Z.; Peng Z.; Tour J. M. Growth of Graphene from Food, Insects, and Waste. ACS Nano 2011, 5, 7601–7607. 10.1021/nn202625c. [DOI] [PubMed] [Google Scholar]
- Ye R.; Chyan Y.; Zhang J.; Li Y.; Han X.; Kittrell C.; Tour J. M. Laser-Induced Graphene Formation on Wood. Adv. Mater. 2017, 29, 1702211 10.1002/adma.201702211. [DOI] [PubMed] [Google Scholar]
- Kou L. Application of Environmental Protection and Energy Saving Technology in Building Decoration Engineering. J. Phys.: Conf. Ser. 2022, 2152, 012042 10.1088/1742-6596/2152/1/012042. [DOI] [Google Scholar]
- Stearns P. N.The Industrial Revolution in World History, 5th ed.; Routledge: New York, 2020. [Google Scholar]
- Weinstein L. A.; Loomis J.; Bhatia B.; Bierman D. M.; Wang E. N.; Chen G. Concentrating Solar Power. Chem. Rev. 2015, 115, 12797–12838. 10.1021/acs.chemrev.5b00397. [DOI] [PubMed] [Google Scholar]
- Kannan N.; Vakeesan D. Solar Energy for Future World: A review. Renewable Sustainable Energy Rev. 2016, 62, 1092–1105. 10.1016/j.rser.2016.05.022. [DOI] [Google Scholar]
- Sharma S.; Kalita G.; Hirano R.; Shinde S. M.; Papon R.; Ohtani H.; Tanemura M. Synthesis of Graphene Crystals from Solid Waste Plastic by Chemical Vapor Deposition. Carbon 2014, 72, 66–73. 10.1016/j.carbon.2014.01.051. [DOI] [Google Scholar]
- Pathak P. D.; Mandavgane S. A.; Kulkarni B. D. Fruit Peel Waste: Characterization and Its Potential Uses. Curr. Sci. 2017, 113, 444–454. 10.18520/cs/v113/i03/444-454. [DOI] [Google Scholar]
- Ferrari A. C.; Basko D. M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. 10.1038/nnano.2013.46. [DOI] [PubMed] [Google Scholar]
- Dervishi E.; Ji Z.; Htoon H.; Sykora M.; Doorn S. K. Raman Spectroscopy of Bottom-Up Synthesized Graphene Quantum Dots: Size and Structure Dependence. Nanoscale 2019, 11, 16571–16581. 10.1039/C9NR05345J. [DOI] [PubMed] [Google Scholar]
- Pandey S.; Kumar A.; Karakoti M.; Garg K. K.; Rana A.; Tatrari G.; Bohra B. S.; Yadav P.; Singh R. K.; Sahoo N. G. 3D Graphene Nanosheets from Plastic Waste for Highly Efficient HTM Free Perovskite Solar Cells. Nanoscale Adv. 2021, 3, 4726–4738. 10.1039/D1NA00183C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.; Rodriguez R. D.; Ruban A.; Pavlov S.; Sheremet E. The Correlation Between Electrical Conductivity and Second-order Raman Modes of Laser-Reduced Graphene Oxide. Phys. Chem. Chem. Phys. 2019, 21, 10125–10134. 10.1039/C9CP00093C. [DOI] [PubMed] [Google Scholar]
- Ferrari A. C.; Meyer J. C.; Scardaci V.; Casiraghi C.; Lazzeri M.; Mauri F.; Piscanec S.; Jiang D.; Novoselov K. S.; Roth S.; Geim A. K. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401 10.1103/PhysRevLett.97.187401. [DOI] [PubMed] [Google Scholar]
- Pandey S.; Karakoti M.; Surana K.; Dhapola P. S.; SanthiBhushan B.; Ganguly S.; Singh P. K.; Abbas A.; Srivastava A.; Sahoo N. G. Graphene Nanosheets Derived from Plastic Waste for the Application of DSSCs and Supercapacitors. Sci. Rep. 2021, 11, 3916 10.1038/s41598-021-83483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Li D.; Too C. O.; Wallace G. G. Electrochemical Properties of Graphene Paper Electrodes Used in Lithium Batteries. Chem. Mater. 2009, 21, 2604–2606. 10.1021/cm900764n. [DOI] [Google Scholar]
- Sahoo S. K.; Mallik A. Simple, Fast and Cost-Effective Electrochemical Synthesis of Few Layer Graphene Nanosheets. Nano 2015, 10, 1550019 10.1142/S1793292015500198. [DOI] [Google Scholar]
- Warren B. E. X-Ray Diffraction in Random Layer Lattices. Phys. Rev. 1941, 59, 693–698. 10.1103/PhysRev.59.693. [DOI] [Google Scholar]
- Matassa R.; Orlanducci S.; Tamburri E.; Guglielmotti V.; Sordi D.; Terranova M. L.; Passeri D.; Rossi M. Characterization of Carbon Structures Produced by Graphene Self-Assembly. J. Appl. Crystallogr. 2014, 47, 222–227. 10.1107/S1600576713029488. [DOI] [Google Scholar]
- Biswas S.; Drzal L. T. A Novel Approach to Create a Highly Ordered Monolayer Film of Graphene Nanosheets at the Liquid–Liquid Interface. Nano Lett. 2009, 9, 167–172. 10.1021/nl802724f. [DOI] [PubMed] [Google Scholar]
- Robinson J. T.; Zalalutdinov M.; Baldwin J. W.; Snow E. S.; Wei Z.; Sheehan P.; Houston B. H. Wafer-scale Reduced Graphene Oxide Films for Nanomechanical Devices. Nano Lett. 2008, 8, 3441–3445. 10.1021/nl8023092. [DOI] [PubMed] [Google Scholar]
- Ma J.; Meng Q.; Zaman I.; Zhu S.; Michelmore A.; Kawashima N.; Wang C. H.; Kuan H.-C. Development of Polymer Composites using Modified, High-Structural Integrity Graphene Platelets. Compos. Sci. Technol. 2014, 91, 82–90. 10.1016/j.compscitech.2013.11.017. [DOI] [Google Scholar]
- Lu Z.; Ma L.; Tan J.; Wang H.; Ding X. Transparent Multi-Layer Graphene/Polyethylene Terephthalate Structures with Excellent Microwave Absorption and Electromagnetic Interference Shielding Performance. Nanoscale 2016, 8, 16684–16693. 10.1039/C6NR02619B. [DOI] [PubMed] [Google Scholar]
- Lotya M.; Hernandez Y.; King P. J.; Smith R. J.; Nicolosi V.; Karlsson L. S.; Blighe F. M.; De S.; Wang Z.; McGovern I. T.; Duesberg G. S.; Coleman J. N. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. 10.1021/ja807449u. [DOI] [PubMed] [Google Scholar]
- Liao K.-H.; Mittal A.; Bose S.; Leighton C.; Mkhoyan K. A.; Macosko C. W. Aqueous Only Route toward Graphene from Graphite Oxide. ACS Nano 2011, 5, 1253–1258. 10.1021/nn1028967. [DOI] [PubMed] [Google Scholar]
- Su C.-Y.; Lu A.-Y.; Xu Y.; Chen F.-R.; Khlobystov A. N.; Li L.-J. High-Quality Thin Graphene Films from Fast Electrochemical Exfoliation. ACS Nano 2011, 5, 2332–2339. 10.1021/nn200025p. [DOI] [PubMed] [Google Scholar]
- Nemes-Incze P.; Osváth Z.; Kamarás K.; Biró L. P. Anomalies in Thickness Measurements of Graphene and Few Layer Graphite Crystals by Tapping Mode Atomic Force Microscopy. Carbon 2008, 46, 1435–1442. 10.1016/j.carbon.2008.06.022. [DOI] [Google Scholar]
- Wang G.; Wang B.; Park J.; Wang Y.; Sun B.; Yao J. Highly Efficient and Large-Scale Synthesis of Graphene by Electrolytic Exfoliation. Carbon 2009, 47, 3242–3246. 10.1016/j.carbon.2009.07.040. [DOI] [Google Scholar]
- Li J.; Wang G.; Zhu H.; Zhang M.; Zheng X.; Di Z.; Liu X.; Wang X. Antibacterial Activity of Large-Area Monolayer Graphene Film Manipulated by Charge Transfer. Sci. Rep. 2015, 4, 4359 10.1038/srep04359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.; Peng C.; Luo W.; Lv M.; Li X.; Li D.; Huang Q.; Fan C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zeng T. H.; Hofmann M.; Burcombe E.; Wei J.; Jiang R.; Kong J.; Chen Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy K.; Veerapandian M.; Zhang L.-H.; Yun K.; Kim S. J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. 10.1021/jp3047054. [DOI] [Google Scholar]
- Xiong K.-r.; Liang Y.-r.; Ou-yang Y.; Wu D.-c.; Fu R.-w. Nanohybrids of Silver Nanoparticles Grown in-situ on a Graphene Oxide Silver Ion Salt: Simple Synthesis and Their Enhanced Antibacterial Activity. New Carbon Mater. 2019, 34, 426–433. 10.1016/S1872-5805(19)60024-7. [DOI] [Google Scholar]
- Wang X.-d.; Zhou N.-l.; Wang W.-y.; Tang Y.-d.; Zhang J.; Shen J. The Antimicrobial Properties of Carboxylated Graphene Oxide Decorated with La Particles. New Carbon Mater. 2012, 27, 385–392. [Google Scholar]
- Ma J.; Zhang J.; Xiong Z.; Yong Y.; Zhao X. S. Preparation, Characterization and Antibacterial Properties of Silver-modified Graphene Oxide. J. Mater. Chem. 2011, 21, 3350–3352. 10.1039/C0JM02806A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.