Abstract
Herein, we report a class of distinctive supramolecular nanostructures in situ-generated from the cationic ring-opening polymerization of a particular 2-oxazoline monomer, i.e., 2-(N-tert-butyloxycarbonylaminomethyl)-2-oxazoline (Ox1). Driven by side-chain hydrogen bonding between neighboring molecules and van der Waals interactions, the growing oligomers of Ox1 precipitate in the form of macroscopic platelets when the degree of polymerization reaches 5–7. A similar self-assembly occurred in the block copolymerization of 2-ethyl-2-oxazoline (EtOx) or 2-pentyl-2-oxazoline (PeOx) and Ox1 as the second monomer. These polymeric aggregates were found to disassemble into rod-like nanoparticles under appropriate conditions, and to form stable organogels in some polar solvents like dimethylformamide as well as in natural liquid fragrances such as (R)-carvone, citronellal, and (R)-limonene. Scanning electron microscopy revealed that the morphology of their xerogels was solvent-dependent, mainly with a lamellar or fibrous structure. The rheology measurements confirmed the as-obtained organogels feature an obvious thixotropic character. The storage modulus was about 7–10 times higher than the loss modulus, indicating the physical crosslinking in the gel. The fragrance release profiles showed that the presented supramolecular gel system exhibits good sustained-release effect for the loaded bioactive volatiles.
1. Introduction
Supramolecular organogels have been attracting considerable attention over the last decades owing to their prospective and realized applications in numerous fields such as food,1,2 cosmetics,3 drug delivery,4−6 encapsulation and controlled release of fragrance,7 and advanced materials.8−10 This kind of physical gel is generally formed by immobilizing organic solvents and oils in a three-dimensional network created by the entanglement of noncovalently self-assembled nanofibers.11,12 Among many known gelators, peptidomimetic oligomers and polymers with secondary structures are particularly attractive gel-forming materials due to their intrinsic self-assembly propensity to hierarchically ordered architectures as well as the potential advantages like biocompatibility and bioactivity.13,14 Previous studies have demonstrated that synthetic polypeptides15−21 or peptide-containing block copolymers22−24 exhibited good gelling activity in appropriate organic solvents in which a rigid α-helical or β-sheet motif plays a critical role in the formation of fibrillar networks.15,18,19,22−24
Polypeptides or polypeptide hybrid materials are readily available through ring-opening polymerization (ROP) of N-carboxyanhydrides (NCAs) using amino-functionalized (macro)initiators.22,25,26 However, the organogelators prepared from the ROP of NCAs usually require a higher molecular weight compared with the peptide-based gelling agents from a stepwise strategy.18,20,22,23 In addition, the current synthesis of NCA monomers needs the use of toxic phosgenation reagents, and the monomers suffer from high sensitivity to moisture and a low stability upon storage.26 In this context, it would be desirable to explore other types of peptido-mimetic gelators with relatively easy and low-cost synthesis having the intriguing organizational characteristics of peptides.27
Poly(2-oxazoline)s, tertiary amide analogues of polypeptides, are a synthetic class of polyamides that have an amide structure of which just the nitrogen is incorporated in the polymer backbone.27−29 They can be prepared by living cationic ROP of 2-oxazolines, providing good control over the resulting (co)polymer structure and properties, narrow molecular weight dispersity, and access to well-defined block copolymers by the sequential addition of different monomers.27 Contrary to polypeptides, poly(2-oxazoline)s (POx) usually lack chiral centers in the main chain, hence cannot form distinct secondary structures through hydrogen bonding interaction. However, chirality or functionality can be readily introduced to the polymer backbone via the substituents in the side chains or at the chain ends,28,29 thus opening the possibility to generate biomimetic self-assemblies with a complex or hierarchical structure.
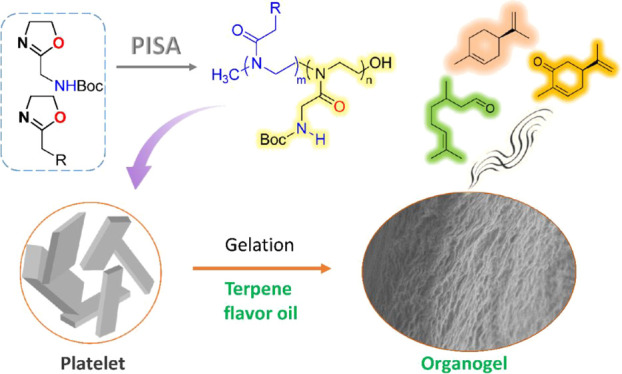
Recently, we serendipitously observed an interesting spontaneously occurring assembly phenomenon during the cationic ROP of a particular 2-oxazoline monomer, namely, 2-(N-tert-butyloxycarbonylaminomethyl)-2-oxazoline (Ox1, Figure 1). Specifically, the polymerization of Ox1 in acetonitrile only yielded the oligomers of 5–7 repeat units, which then aggregated into precipitate insoluble in the reaction medium. Light scattering and microscopy studies revealed that the colloidal suspension of polymeric aggregates displays micrometer-sized lamellar or rod-like morphologies. Such a self-assembly process also took place for the block copolymerization of 2-ethyl-2-oxazoline (EtOx) or 2-pentyl-2-oxazoline (PeOx) and Ox1 as the second monomer, resulting in the corresponding nonspherical micelles with a POx1 core.
Figure 1.
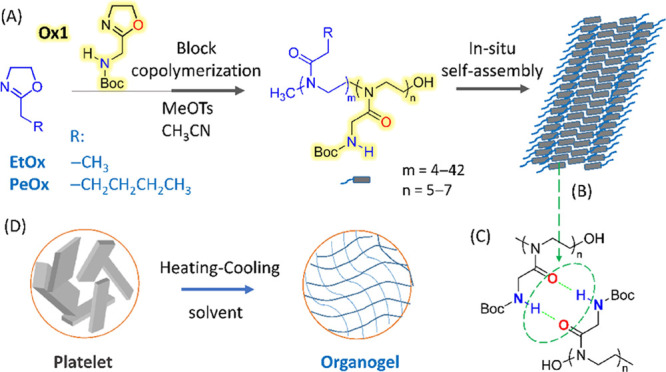
Synthetic route for poly(2-oxazoline)s (A), schematic representation showing (B) the in situ generated supramolecular structures, (C) hydrogen-bonding network linking Ox1 segments, and (D) organogel formation.
As shown in Figure 1A, the POx1 segment features a unique side-chain structure. The pendant groups contain tertiary amide and carbamate moieties linked by a methylene spacer, providing multiple hydrogen bonding sites for the intermolecular association. The macroscopic POx platelets likely formed through a hierarchical oriented organization manner resembling that occurring in natural peptides,30 involving the self-association of POx1 segments driven by intermolecular complementary hydrogen bonding and lateral stacking of the resulting β-sheet-like supramolecular structures (Figure 1B,C). On the other hand, the polypeptide hybrid copolymers with a preassembled secondary structure are prone to undergo sol–gel transition and form nanofibers.22,31,32 Inspired by this, we speculate the self-assembled POx supramolecular nanostructures may also support gelation (Figure 1D). As expected, our results have showed that these in situ formed POx assemblies indeed can gelate a broader range of organic solvents including alcohol, dimethylformamide (DMF), toluene, as well as terpenoid fragrances such as (R)-carvone, citronellal, and (R)-limonene. To the best of our knowledge, POx block copolymers have been extensively investigated as amphiphilic building blocks for spherical micelles.27 Moreover, the fabrication and application of nonspherical POx-related nanostructures have been gaining growing attention in recent years,33−37 but their gelation behavior with different solvents is still scarcely explored, except for an example involving hydrogel preparation36 and one report on gelling properties of poly(2-oxazoline)-grafted chitin nanofibers.38
2. Experimental Section
2.1. Synthesis of Monomers and Polymerization Procedure
The monomers Ox1 and PeOx were prepared according to the reported procedure.39,40 All ROPs were carried out at 80 °C under a nitrogen atmosphere, using MeOTs as the initiator and acetonitrile as a selective solvent for the POx1 segment. The detailed synthesis process and structural characterization data are given in the Supporting Information (Figure S1).
2.2. Gelation Test of Organic Fluids
The mixture of a weighed polymer powder (∼15 wt%) in a specific organic solvent (1 mL) was heated in a screw-capped test tube of 10 mm diameter until the solid was dissolved. Then, the solution was left to cool to r.t. To see whether a gel was obtained or whether the polymer precipitated, the samples were inverted and/or analyzed visually after cooling to r.t. and after storing at r.t. for 24 h.
2.3. Rheology Tests
Rheological measurements were carried out on a TA Instruments DHR-2 rheometer equipped with a Peltier element and a cone plate (40 mm, 1.985°; truncation gap 50 μm). To determine the linear viscoelastic region, a strain sweep was performed on the sample at an angular frequency of 10.0 rad s–1 from 0.01 to 100.0%. G′ and G″ were later obtained using a frequency sweep from 100 to 0.01 rad/s using a fixed strain value of 0.1% for all samples. Each experiment was performed in triplicate. Data were processed using Origin Pro software.
2.4. Fragrance Release Studies under Ambient Conditions
The fragrance release experiments were carried out by the gravimetric method under constant ambient conditions (25 °C, humidity 70%). To be specific, a gel sample (40–50 mg) was placed in a glass vial (50 × 23 mm, height and external diameter), then the release of the fragrance was evaluated by monitoring the weight loss at regular time intervals using a laboratory balance (Metller Toledo MS105DU, precision 0.01 mg). The initial content of fragrance in the gels was determined by thermogravimetric analysis (TGA).
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
With the intention of exploring the gelation potential of the supramolecular nanostructure of POx1, we synthesized diblock copolymers consisting of Ox1 and EtOx or PeOx segments (Figure 1A). EtOx and PeOx were chosen as the monomers for hydrophilic and hydrophobic starting blocks, respectively, since their polymerization in acetonitrile has proved to operate in a homogeneous system,40 allowing for the introduction of POx1 core-forming units in the desired polymers by the sequential polymerization route.
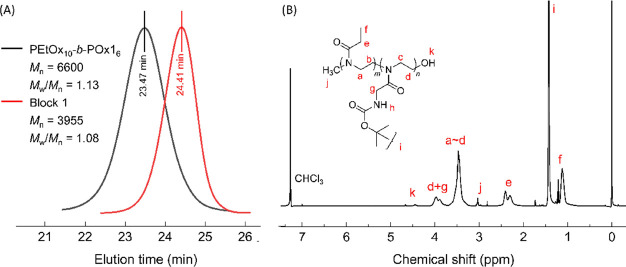
Table 1 summarizes the reaction conditions, yield, and characterization results of polymers. In all experiments, gel permeation chromatography (GPC) analyses of the obtained both first blocks and copolymers gave symmetrical unimodal traces (Figures 2A and S2–S4) and the molecular weight distributions (polydispersity indexes) remained fairly narrow (1.10–1.15). These observations are in accordance with the highly living nature of the cationic ROP of 2-oxazolines.28 The copolymer compositions could be estimated by 1H nuclear magnetic resonance (NMR) spectroscopy according to the procedure reported by Jordan and co-workers.41 Taking the spectrum shown in Figure 2B as an example, the integration of the propionyl peaks on PEtOx (f, ∼1.12 ppm) was compared with the Boc protons of POx1 (i, ∼1.43 ppm), the average number of repeat units of the EtOx and Ox1 blocks was determined to be 9.6 and 6.1, respectively. The polymer is thus denoted as PEtOx10-b-POx16 (entry 3 in Table 1).
Table 1. Results on ROP of 2-Oxazolines Initiated with MeOTs.
| entry | [ROx]:[Ox1]:[I]a | polymerb | yield (%) | DPc (m,n) | Mn,NMR (kDa)c | Mn,GPC (kDa)d | PDId |
|---|---|---|---|---|---|---|---|
| 1 | 0:10:1 | POx1 | 86.0 | 0, 6e | 5.26 | 1.15 | |
| 2 | 5:5:1 | PEtOx4-b-POx15 | 76.1 | 4.2, 4.7 | 1.42 | 4.92 | 1.10 |
| 3 | 10:7:1 | PEtOx10-b-POx16 | 72.3 | 10.4, 6.2 | 2.20 | 6.60 | 1.13 |
| 4 | 42:7:1 | PEtOx42-b-POx17 | 94.6 | 42.4, 7.1 | 5.47 | 9.92 | 1.12 |
| 5 | 10:7:1 | PPeOx10-b-POx16 | 65.8 | 9.7, 5.5 | 2.65 | 5.21 | 1.15 |
Molar ratio in feed, ROx represents EtOx or PeOx; [I] = 2 mol L–1, 80 °C, acetonitrile, the polymerization time for both blocks was 2 h; the conversion of the first monomer (EtOx or PeOx) was more than 98%, estimated from 1H NMR spectra recorded prior to the addition of Ox1.
The subscript denotes the average number of repeating units of the polymer determined by 1H NMR analysis.
Degree of polymerization and molecular weight calculated by 1H NMR integration.
Determined by GPC in TFIP using PMMA calibration, PDI = polydispersity index.
Estimated based on 1H NMR analysis for the diblock copolymers;reliable1H NMR spectra were unavailable for POx1 due to its poor solubility in common deuterated solvents.
Figure 2.
(A) GPC traces (RI detection, TFIP, 0.8 mL min–1, PMMA calibration) for the first block PEtOx (red) and PEtOx10-b-POx16 as an example of block copolymers (black) prepared by a sequential polymerization route (CH3CN, 80 °C). (B) Chemical structure and 1H NMR spectrum (CDCl3) of PEtOx10-b-POx16 (see: Entry 3 in Table 1).
Notably, precipitation or turbidity can also be intuitively observed in the later stage of copolymerizations as in the case of homopolymerization of Ox1. This is because acetonitrile is a good solvent for the starting PEtOx or PPeOx block but a precipitant for the growing POx1 segments of a critical length. As seen from Table 1 and Figures S5–S7, the block lengths of copolymers were very close to the monomer/initiator ratio, and the POx1 segment was always limited in the range of 5–7 monomeric units. In other words, this value could be regarded as the threshold degree of polymerization (DP) of the core-forming block for self-assembly in the present system. The moderate to good isolated yields (66–95%) endow the living ROP of 2-oxazoline-induced self-assembly process with the potential of synthesizing well-defined nano-objects in larger quantities.42 A recent study reported by Liu and coworkers showed that the polymerization of Ox1 affords linear polymers with the DP of 6–40 when using N,N-dimethylacetamide (DMAc) as the reaction medium.39 This result lends indirect support for the supramolecular building blocks proposed based on self-complementary hydrogen-bonding interactions (Figure 1B,C), because acetonitrile, the solvent used here, does not have the powerful ability of DMAc to break the hydrogen bonds linking POx1 segments. The more detailed studies on the mechanism underlying the formation of POx-based nanoparticles will be reported separately.
In addition, the Mn,GPC values are distinctly higher from the those retrieved from NMR (Table 1), a deviation which may impart to the use of PMMA standards for GPC calibration. Nevertheless, this discrepancy also seems to imply that the polymer chains may adopt a rigid rod rather than a random coil conformation as a result of the steric repulsion between the bulky pendant groups on the POx1 skeleton, just as with certain linear polysiloxanes reported in the literature.43 Whereas for rigid-rod-type oligomers or polymers, the calculated Mn based on commonly used standards like polystyrene or PMMA is normally well above their actual molecular weights, typical of the conformational effects.20,44
3.2. Morphology and Gelation Properties of POx Aggregates
The as-obtained POx aggregates are hardly soluble in aqueous and common organic solvents at ambient temperature, but they can be disassembled into smaller size particles by sonication under appropriate conditions. Thus, prior to assessing their gelling capacity we examined the morphologies of the polymeric assemblies. To do so, a 0.5 mg mL–1 suspension of PEtOxm-b-POx1n copolymers in ethanol was prepared by sonication in the presence of ice bath and then the supernatant was taken for transmission electron microscopy (TEM) observation.
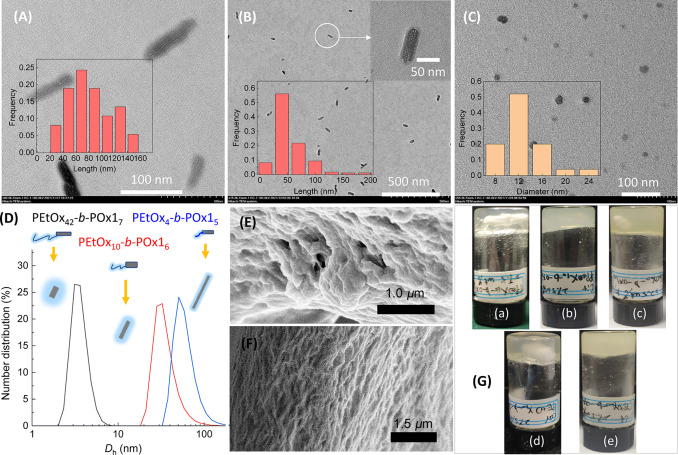
From Figure 3A–C, these copolymers exhibited obvious composition-dependent self-assembly behaviors, as was the case for those peptide-based and POx copolymers reported earlier.22,24,33−35,37 Among them, PEtOx4-b-POx15 and PEtOx10-b-POx16 display a rod-like morphology with the number-average length of 78.5 ± 34.5 and 55.0 ± 29.9 nm, respectively. Furthermore, the nanorods have a discernible layered structure, as indicated by enlarged TEM images (Figure 3A,B). Whereas for PEtOx42-b-POx17, nearly spherical particles with irregular edges were observed, with an average size of 12.7 ± 3.9 nm (Figure 3C). One reasonable explanation is that the increased DP of PEtOx corona presumably interfered with the association of POx1 core-forming segments in the H-bonding direction and subsequent ordered organization, which in turn facilitates the formation of spherical micelles with less regular crystalline cores in the polymerization-induced self-assembly. In fact, the discrepancy in the morphology of assemblies is closely related to the discriminated gelation behavior as discussed in the following section.
Figure 3.
Nanoparticles formed by PEtOxm-b-POx1n copolymers of 0.5 mg mL–1 in ethanol after sonication for 30 min in the presence of ice bath. TEM images of (A) PEtOx4-b-POx15, (B) PEtOx10-b-POx16, and (C) PEtOx42-b-POx17. Insets display the length/size distribution histograms (based on measurements of ∼100 individual particles). (D) DLS measurements for the copolymers in ethanol (0.5 mg mL–1; inset: cartoon representing the size difference in the micelle-like assemblies). Typical SEM images of the xerogels obtained by drying the organogels formed with 15 wt% of (E) PEtOx10-b-POx16 and (F) PPeOx10-b-POx16 in (R)-carvone in an oven (60 °C for 3 days). (G) Photographs of macroscopic gels formed by PPeOx10-b-POx16 in (R)-carvone (a), citronellal (b), and (R)-limonene (c), and by PEtOx10-b-POx16 in (R)-carvone (d) and citronellal (e).
DLS measurements also showed a trend in the particle size with the copolymer composition, similar to that observed from TEM. The number-average hydrodynamic diameter (Dh) of POx assemblies dispersed in various solvents decreased in the order PEtOx4-b-POx15 > PEtOx10-b-POx16 > PEtOx42-b-POx17, being opposite with the trend of increasing PEtOx block length (Figure S8). By way of example, the Dh value of PEtOx4-b-POx15 micelles in ethanol was 60.0 ± 8.1 nm, roughly two times larger than that of PEtOx10-b-POx16 (32.7 ± 2.5) and 17-times for PEtOx42-b-POx17 (3.5 ± 0.5 nm), as shown in Figure 3D. The particle size by TEM is slightly larger than that determined by DLS, which may be related to the agglomeration during sample preparation for TEM.
To study the gelation ability of the as-prepared poly(2-oxazoline)s, the vial inversion tests45 were conducted using 1 mL of solvent and 15 wt% of polymer for the initial screening. As can be seen from Table 2, these polymers exhibited relatively poor ability to gelate both the polar and protic organic solvents. In contrast, they were moderate gelators for some organic liquids such as toluene as well as highly volatile fragrance compounds, including linalool, geraniol, citronellal, (R)-carvone, and (R)-limonene (Lim.). In these low-polarity fluids, PEtOx10-b-POx16 formed strong gels in most cases, while PEtOx4-b-POx15 and the Ox1 oligomer (POx1) provide mostly weak ones. As for PEtOx42-b-POx16, no gelation was observed in all cases. Moreover, PPeOx10-b-POx16 proved to be more efficient in gelating (R)-limonene as compared to PEtOx10-b-POx16. In the vial inversion tests, the former provided gels for limonene with higher mechanical stability than the latter. Also, PPeOx10-b-POx16 was the only gelator in acetone where the others were soluble or insoluble.
Table 2. Gelation Properties of POx’s in Different Solventsa.
| solvents | POx1 | PEtOx4-b-POx15 | PEtOx10-b-POx16 | PEtOx42-b-POx17 | PPeOx10-b-POx16 |
|---|---|---|---|---|---|
| H2O | I | I | I | S | I |
| methanol | I | G(w) | G(w) | S | G(w) |
| ethanol | I | G(s) | G(w) | S | G(s) |
| acetone | I | I | I | S | G(w) |
| butyl acetate | I | I | I | S(p) | I |
| chloroform | I | S | S | S | S |
| DMSO | S | S | G(w) | S | G(w) |
| DMF | G(s) | G(w) | G(s) | S | G(s) |
| toluene | G(w) | G(w) | G(s) | S | G(s) |
| linalool | G(w) | G(w) | G(s) | S | G(s) |
| geraniol | G(w) | G(w) | G(s) | S | G(s) |
| citronellal | G(w) | G(w) | G(s) | S | G(s) |
| (R)-carvone | G(w) | G(s) | G(s) | S | G(s) |
| (R)-limonene | G(w) | G(w) | G(p) | S | G(s) |
The POx-solvent mixture was heated and then cooled to room temperature; by visual inspection, gels formed within several minutes. The formation of a strong gel was confirmed when it did not flow under its own weight upon vial inversion. I: insoluble/recrystallization/precipitate, S: soluble upon heating, S(p): partially soluble; G(s): strong gel, G(w): weak gel, G(p): partially gel.
It is noteworthy that with these poly(2-oxazoline)s gelation also occurred in hydrogen-bonding destructive solvents, such as methanol, ethanol, and DMF. Similarly, the oligomer POx1 was able to gelate DMF as well form a strong gel (Figure S10). This is often the case with gelling agents based on multiple hydrogen bonding interaction.46−48 The above observations demonstrated that the poly(2-oxazoline) gelators feature a strong hydrogen-bonding network responsible for the gel formation. The preassembled nanostructure of POx1 segments remained intact upon gelation in the solvents tested here, as evidenced by infrared analysis (Figure S9). Meanwhile, the hydrophobicity of longer alkyl side chains and the dense Boc groups combined with the unique polar tertiary amide backbone enhanced its organogelation ability, for example through solubility improvement or/and van der Waals interactions.49 Such strong self-complementary intermolecular hydrogen-bonding interactions do not allow POx1 to exhibit enough solubility in some solvents so as to provide gels; this is the case for ethanol, where it is fully insoluble while PEtOx4-b-POx15 and PPeOx10-b-POx16 afford the corresponding effective organogels. Therefore, although the presence of strong unidirectional intermolecular interactions such as H-bonding has been identified as a molecular requirement for gelation ability,50 the gel formation in a particular solvent should be the result of a balance of multiple noncovalent forces.48
The as-prepared platelet gels were opaque (Figures 3G and S10), indicating the presence of large aggregates with a size comparable with the wavelengths of visible light.50 Scanning electron microscopy (SEM) performed on the xerogels showed that these organogels had a fibrillar or lamellar nature (Figures 3E,F and S11), similar to some supramolecular nanosheet gels.51−53
3.3. Viscoelastic Properties of the Gels
In order to get more reliable, quantitative data for the stability of the gels, rheology measurements were made for the fragrance gels with the oligomers. In a typical measurement, a sweep of strain from 0.1 to 100% was carried out with a logarithmic variation of deformation and the storage modulus (G′) and the loss modulus (G″) were determined. First, we established the linear viscoelastic domains of these gels in the dynamic oscillating mode (Figures S12 and S13). The results show that the linearity domain was not followed when strains above ca. 1.0% were applied. In the following measurements, the strain applied to the gels was fixed at 0.1%.
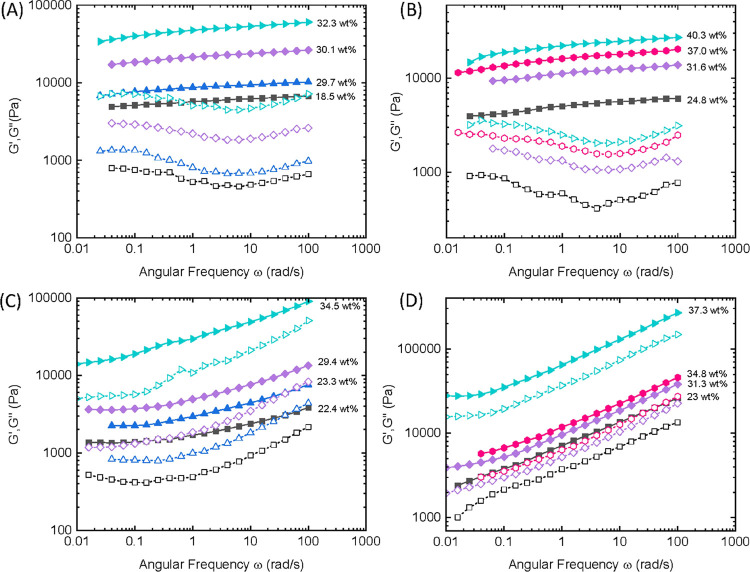
Figure 4 depicts the frequency-dependent storage G′ and loss moduli G″ of the fragrance gels formed in either (R)-carvone or citronellal by PPeOx10-b-POx16 and PEtOx10-b-POx16 with different polymer contents. In all cases, the G′ values are several times up to about one order of magnitude higher than G″ values over the whole frequency range (ω = 0.01–100 rad s–1). This is a typical characteristic of gels with a high degree of crosslinks.51,54 Strong gels with G′ > 3000 Pa could be obtained at the polymer content of 15∼23 wt%, including the limonene gel by PPeOx10-b-POx16 (Figure S14).
Figure 4.
Frequency-dependent storage (G′) and loss moduli (G″) of supramolecular organogels with different polymer contents performed at 0.1% strain, 25 °C. Gels formed by PPeOx10-b-POx16 (the upper panel) in (R)-carvone (A) and citronellal (B), by PEtOx10-b-POx16 (the lower panel) in (R)-carvone (C) and citronellal (D).
A comparison among the storage modulus-frequency curves reveals that the G′ values increase more steadily and slowly with increasing frequency for PPeOx10-b-POx16 gels than the corresponding PEtOx10-b-POx16 ones (Figure 4A vs C and B vs D). This difference implies that PPeOx10-b-POx16 molecular stacking is more loosened by the solvents due to the relatively high affinity of longer alkyl side chains of the gelator toward the terpene compounds, thus leading to the more permeated and diffused fibers.50,55 In such instances, PPeOx10-b-POx16 presented a more homogeneous fibril network and more stable gels compared to its analogue PEtOx10-b-POx16. Furthermore, on closer inspection of the G′-ω curves from (R)-carvone and (R)-limonene gels of PPeOx10-b-POx16 (Figures 4A vs S14), it was found that the latter showed a slightly steeper slope than the former. Likely, the limonene gel network structure was more heterogeneous at the microscale, because the driving force of the monocyclic terpene molecule permeation into the fibers is not as strong as that of its carbonyl analogues. This speculation is consistent with the fact that the limonene gel is more opaque in appearance than the carvone one (see: a and c in Figure 3G).
Step-strain measurements were then carried out to examine the recovery of material properties following network rupture at high strains.8,55,56 A high magnitude strain (ε = 100% for 30 s) was applied to break the gel structure followed by a low magnitude strain (ε = 0.1%) to probe the rate and extent of recovery of bulk properties. As depicted in Figure 5, the organogel formed with PPeOx10-b-POx16 exhibited exceptionally fast and complete recovery of properties within seconds following stress-induced flow. Furthermore, the rate and extent of recovery remained virtually unchanged for three cycles of breaking and reforming, indicating the reversible and robust nature of the noncovalently crosslinked organogel structure. Such a thixotropic behavior may be explained by the specific nature that the gel fibers are extremely thin, extended, and stable, and have low crystallinity.56 For the gel from PEtOx10-b-POx16 the moduli G′ and G″ can only restore to 60–70% of the initial values.
Figure 5.
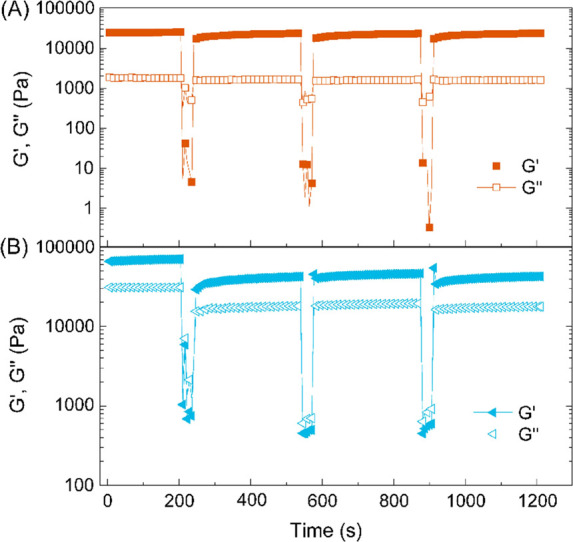
Step-strain measurements of organogels formed with 27.5 wt% of either PPeOx10-b-POx16 or PEtOx10-b-POx16 in (R)-carvone over three cycles (ω = 10 rad s–1, 25 °C).
3.4. Fragrance Release Behavior
Finally, we investigated the release behavior of three terpene fragrances from the organogels at ambient conditions by the gravimetric method. It is evident from Figure 6A that the pure fragrance liquids as a control fully evaporated within 2 days, whereas the release rate of fragrances from the organogels was significantly retarded. The seven-day total fraction of (R)-carvone (Car.) and citronellal (Cit.) released from the PPeOx10-b-POx16 matrix was approximately 20% and 35% with respect to the initial loading contents, respectively. For the gels formed by PEtOx10-b-POx16, the corresponding released amounts were 40 and 30%. However, the release rate of (R)-limonene (Lim.) was much greater than the other two carbonyl compounds, and the two-day cumulative released amount reached about 95%. Thus, the release of fragrances from a particular gel matrix follows the order of Car. < Cit. < Lim., being consistent with the increasing trend of their saturated vapor pressure (Car. 7.16 Pa/20 °C,57 Cit. 87.99 Pa/25 °C,58 Lim. 134.27 Pa/20 °C59).
Figure 6.
(A) Histogram of cumulative release percentages of fragrance organogels over a week, as measured by weighting samples under ambient conditions (25 °C, 75% humidity). Note: the release time was 2 days for the pure fragrances used as a control (3 mL). (B) Fragrance release curves (symbol) from different gel matrices. Dashed lines represent the corresponding fits with the Weibull equation. Gels were formed with 17.5 wt% of polymers in the corresponding liquid fragrances, and the initial loading contents were determined by TGA (see Figure S15).
On the other hand, the release profiles are related in some way to the properties of the corresponding supramolecular network. As shown in Figure 6B, the gels formed with PPeOx10-b-POx16 (solid signs) provide slower release rates for both (R)-carvone and citronellal compared to those with PEtOx10-b-POx16 (hollow signs). This may be ascribed to the fact that the gel fibril homogeneity of PPeOx10-b-POx16 was superior to that of PEtOx10-b-POx16observed in rheological studies above. Apparently, the more homogeneous or more stable the gel network, the more efficient the aroma entrapment and the slower the release, as would be the case for some low-molecular-weight organogel systems.7
From Figure 6B, the evaporation of carbonyl compounds occurred in a slow and steady manner without any burst release. Four mathematic models were employed to fit the obtained release data, including zero-order (r = kt), first-order (r = 1 – e–kt), Higuchi (r = kt0.5), and Weibull (r = 1 – e–(kt)n) models. Overall, the release kinetics indicated that the release of fragrances from the organogel matrices was in agreement with the Weibull equation (Figure 6B and Table S1), following a combined mechanism of Fickian diffusion and case II transport.60
4. Conclusions
In summary, we have demonstrated a class of supramolecular assemblies composed of β-sheet-like packing motifs in situ generated in the cationic ROP of a particularly designed 2-oxazoline monomer Ox1. The oligomer of Ox1 (POx1), in particular, the diblock copolymers prepared through the copolymerization of EtOx or PeOx with Ox1, was found to have the ability to form organogels in bioactive terpene flavor oils. The rheology measurements revealed the storage modulus was several times up to about one order of magnitude higher than the loss modulus, indicating the physical crosslinking in the organogel. Furthermore, these polymer gels exhibited significant self-healing properties and allowed the release of the loaded bioactive compounds in a slow and steady manner through the gel matrix. Clearly, this kind of oligo(2-oxazoline) self-assembling gelator is not as well-defined as traditional low-molecular-weight gelators, but the ease of synthesis and high loading capacity are highly attractive, expected to find potential applications in medicine, food, fragrance, and related areas.
Acknowledgments
This work is supported by the National Key Research and Development Program of China (2016YFA0200301).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02905.
Synthesis of 2-oxazoline monomers Ox1 and PeOx and their 1H NMR and MS(ESI-TOF) spectra; general procedure of ROP; GPC traces for POx1 and its block copolymers with EtOx or PeOx; 1H NMR spectra of the copolymers PEtOxm-b-POx1n and PPeOxm-b-POx1n; histograms of the number-average hydrodynamic diameters obtained by DLS measurements for the copolymers in various media; Fourier transform infrared spectra of the monomer, polymer, and the corresponding gels; representative photographs and SEM images of organogels; amplitude sweeps for representative organogels formed in (R)-carvone, citronellal, and (R)-limonene; fragrance loading capacity of gelators determined by TGA; release kinetic parameters for fragrance gels obtained by mathematical models (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pinto T. C.; Martins A. J.; Pastrana L.; Pereira M. C.; Cerqueira M. A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2021, 7, 86. 10.3390/gels7030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.; Lu Y.; Cui M.; Miao S.; Gao Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. 10.1080/10408398.2019.1587737. [DOI] [PubMed] [Google Scholar]
- Mosquera Narvaez L. E.; Ferreira L. M. D. M. C.; Sanches S.; Alesa Gyles D.; Silva-Júnior J. O. C.; Ribeiro Costa R. M. A review of potential use of amazonian oils in the synthesis of organogels for cosmetic application. Molecules 2022, 27, 2733. 10.3390/molecules27092733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr J.; Saldías C.; Díaz D. D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. 10.1039/c7cs00515f. [DOI] [PubMed] [Google Scholar]
- Skilling K. J.; Citossi F.; Bradshaw T. D.; Ashford M.; Kellam B.; Marlow M. Insights into low molecular mass organic gelators: a focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. 10.1039/c3sm52244j. [DOI] [PubMed] [Google Scholar]
- Kaplan S.; Colak M.; Hosgorwn H.; Pirinccioglu N. Design of L-lysine-based organogelators and their applications in drug release processes. ACS Omega 2019, 4, 12342–12356. 10.1021/acsomega.9b01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls A.; Castillo A.; Porcar R.; Hietala S.; Altava B.; García-Verdugo E.; Luis S. V. Urea-based low-molecular-weight pseudopeptidic organogelators for the encapsulation and slow release of (R)-limonene. J. Agric. Food Chem. 2020, 68, 7051–7061. 10.1021/acs.jafc.0c01184. [DOI] [PubMed] [Google Scholar]
- Babu S. S.; Praveen V. K.; Ajayaghosh A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. 10.1021/cr400195e. [DOI] [PubMed] [Google Scholar]
- Malviya N.; Sonkar C.; Kundu B. K.; Mukhopadhyay S. Discotic organogelators in ion sensing, metallogel formation, and bioinspired catalysis. Langmuir 2018, 34, 11575–11585. 10.1021/acs.langmuir.8b02352. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Lin X.; Li P.; Liu F. Q.; Guo H.; Li W. H. Recent advances of organogels: from fabrications and functions to applications. Prog. Org. Coat. 2021, 159, 106417 10.1016/j.porgcoat.2021.106417. [DOI] [Google Scholar]
- George M.; Weiss R. G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res. 2006, 39, 489–497. 10.1021/ar0500923. [DOI] [PubMed] [Google Scholar]
- Draper E. R.; Adams D. J. Low-molecular-weight gels: the state of the art. Chem 2017, 3, 390–410. 10.1016/j.chempr.2017.07.012. [DOI] [Google Scholar]
- Song Z.; Fu H.; Wang R.; Pacheco L. A.; Wang X.; Lin Y.; Cheng J. Secondary structures in synthetic polypeptides from N-carboxyanhydrides: design, modulation, association, and material applications. Chem. Soc. Rev. 2018, 47, 7401–7425. 10.1039/c8cs00095f. [DOI] [PubMed] [Google Scholar]
- Hartlieb M.; Mansfield E. D. H.; Perrier S. A guide to supramolecular polymerizations. Polym. Chem. 2020, 11, 1083–1110. 10.1039/C9PY01342C. [DOI] [Google Scholar]
- Vacogne C. D.; Schopferer M.; Schlaad H. Physical gelation of α-helical copolypeptides. Biomacromolecules 2016, 17, 2384–2391. 10.1021/acs.biomac.6b00427. [DOI] [PubMed] [Google Scholar]
- Murphy R. D.; Bobbi E.; de Oliveira F. C. S.; Cryan S.-A.; Heise A. Gelating polypeptide matrices based on the difunctional N-carboxyanhydride diaminopimelic acid cross-linker. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 1209–1215. 10.1002/pola.29376. [DOI] [Google Scholar]
- Chen C.; Wu D.; Fu W.; Li Z. Tunable organogelator from alkyl-polypeptide diblock prepared by ring-opening polymerization. Aust. J. Chem. 2014, 67, 59–65. 10.1071/CH13349. [DOI] [Google Scholar]
- Gibson M. I.; Cameron N. R. Organogelation of sheet–helix diblock copolypeptides. Angew. Chem., Int. Ed. 2008, 47, 5160–5162. 10.1002/anie.200801056. [DOI] [PubMed] [Google Scholar]
- Tadmor R.; Khalfin R. L.; Cohen Y. Reversible gelation in isotropic solutions of the helical polypeptide poly(γ-benzyl-L-glutamate): kinetics and formation mechanism of the fibrillar network. Langmuir 2002, 18, 7146–7150. 10.1021/la0256026. [DOI] [Google Scholar]
- Kim K. T.; Park C.; Kim C.; Winnik M. A.; Manners I. Self-assembly of dendron-helical polypeptide copolymers: organogels and lyotropic liquid crystals. Chem. Commun. 2006, 1372–1374. 10.1039/B516625J. [DOI] [PubMed] [Google Scholar]
- Kim K. T.; Winnik M. A.; Manners I. Synthesis and self-assembly of dendritic-helical block copolypeptides. Soft Matter 2006, 2, 957–965. 10.1039/b606272e. [DOI] [PubMed] [Google Scholar]
- Hermes F.; Otte K.; Brandt J.; Gräwert M.; Börner H. G.; Schlaad H. Polypeptide-based organogelators: effects of secondary structure. Macromolecules 2011, 44, 7489–7492. 10.1021/ma201232a. [DOI] [Google Scholar]
- Kim K. T.; Park C.; van der Meulen G. W. M.; Rider D. A.; Kim C.; Winnik M. A.; Manners I. Gelation of helical polypeptide–random coil diblock copolymers by a nanoribbon mechanism. Angew. Chem., Int. Ed. 2005, 44, 7964–7968. 10.1002/anie.200502809. [DOI] [PubMed] [Google Scholar]
- Choi Y. Y.; Jeong Y.; Joo M. K.; Jeong B. Reverse thermal organogelation of poly(ethylene glycol)-polypeptide diblock copolymers in chloroform. Macromol. Biosci. 2009, 9, 869–874. 10.1002/mabi.200900095. [DOI] [PubMed] [Google Scholar]
- Deming T. J. Polypeptide Materials: New synthetic methods and applications. Adv. Mater. 1997, 9, 299–311. 10.1002/adma.19970090404. [DOI] [Google Scholar]
- Kricheldorf H. R. Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew. Chem., Int. Ed. 2006, 45, 5752–5784. 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- Hoogenboom R.; Schlaad H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. 10.1039/C6PY01320A. [DOI] [Google Scholar]
- Wilson P.; Ke P. C.; Davis T. P.; Kempe K. Poly(2-oxazoline)-based micro- and nanoparticles: A review. Eur. Polym. J. 2017, 88, 486–515. 10.1016/j.eurpolymj.2016.09.011. [DOI] [Google Scholar]
- Hoogenboom T. Poly(2-oxazoline)s: a polymer class with numerous potential applications. Angew. Chem., Int. Ed. 2009, 48, 7978–7994. 10.1002/anie.200901607. [DOI] [PubMed] [Google Scholar]
- Aggeli A.; Nyrkova I. A.; Bell M.; Harding R.; Carrick L.; McLeish T. C. B.; Semenov A. N.; Boden N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide β-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 11857. 10.1073/pnas.191250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. Y.; Joo M. K.; Sohn Y. S.; Jeong B. Significance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymers. Soft Matter 2008, 4, 2383–2387. 10.1039/b809116a. [DOI] [Google Scholar]
- Oh H. J.; Joo M. K.; Sohn Y. S.; Jeong B. Secondary structure effect of polypeptide on reverse thermal gelation and degradation of l/dl-poly(alanine)–poloxamer–l/dl-poly(alanine) copolymers. Macromolecules 2008, 41, 8204–8209. 10.1021/ma8014504. [DOI] [Google Scholar]
- Finnegan J. R.; Pilkington E. H.; Alt K.; Rahim M. A.; Kent S. J.; Davis T. P.; Kempe K. Stealth nanorods via the aqueous living crystallisation-driven self-assembly of poly(2-oxazoline)s. Chem. Sci. 2021, 12, 7350–7360. 10.1039/d1sc00938a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabiyan A.; Biehl P.; Schacher F. H. Crystallization vs metal chelation: solution self-assembly of dual responsive block copolymers. Macromolecules 2020, 53, 5056–5067. 10.1021/acs.macromol.0c00792. [DOI] [Google Scholar]
- Finnegan J. R.; Davis T. P.; Kempe K. Heat-Induced Living Crystallization-Driven Self-Assembly: The Effect of Temperature and Polymer Composition on the Assembly and Disassembly of Poly(2-oxazoline) Nanorods. Macromolecules 2022, 55, 3650–3660. 10.1021/acs.macromol.2c00298. [DOI] [Google Scholar]
- Nishimura T.; Sumi N.; Mukai S.; Sasaki Y.; Akiyoshi K. Supramacromolecular injectable hydrogels by crystallization-driven self-assembly of carbohydrateconjugated poly(2-isopropyloxazoline)s for biomedical applications. J. Mater. Chem. B 2019, 7, 6362–6369. 10.1039/C9TB00918C. [DOI] [PubMed] [Google Scholar]
- Legros C.; De Pauw-Gillet M.-C.; Tam K. C.; Taton D.; Lecommandoux S. Crystallisation-driven self-assembly of poly(2-isopropyl-2-oxazoline)-block-poly(2-methyl-2-oxazoline) above the LCST. Soft Matter 2015, 11, 3354–3359. 10.1039/c5sm00313j. [DOI] [PubMed] [Google Scholar]
- Kitasono S.; Yamamoto K.; Kadokawa J. Preparation and gelation behaviors of poly(2-oxazoline)-grafted chitin nanofibers. Carbohydr. Polym. 2021, 259, 117709 10.1016/j.carbpol.2021.117709. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Qian Y.; Xie J.; Zhang W.; Jiang W.; Xiao X.; Chen S.; Dai C.; Cong Z.; Ji Z.; Shao N.; Liu L.; Wu Y.; Liu R. Poly(2-oxazoline)-based functional peptide mimics: eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angew. Chem., Int. Ed. 2020, 59, 6412–6419. 10.1002/anie.202000505. [DOI] [PubMed] [Google Scholar]
- Hu F.; Du G.; Ye L.; Zhu J.; Wang Y.; Jiang L. Novel amphiphilic poly(2-oxazoline)s bearing L-prolinamide moieties as the pendants: Synthesis, micellization and catalytic activity in aqueous aldol reaction. Polymer 2016, 102, 33–42. 10.1016/j.polymer.2016.08.089. [DOI] [Google Scholar]
- Cesana S.; Auernheimer J.; Jordan R.; Kessler H.; Nuyken O. First poly(2-oxazoline)s with pendant amino groups. Macromol. Chem. Phys. 2006, 207, 183–192. 10.1002/macp.200500495. [DOI] [Google Scholar]
- Penfold N. J. W.; Yeow J.; Boyer C.; Armes S. P. Emerging trends in polymerization-induced self-assembly. ACS Macro Lett. 2019, 8, 1029–1054. 10.1021/acsmacrolett.9b00464. [DOI] [PubMed] [Google Scholar]
- Villegas J. A.; Olayo R.; Cervantes J. Effect of side groups on the conformation of a series of polysiloxanes in solution. J. Inorg. Organomet. Polym. 2003, 13, 205–222. 10.1023/A:1026191926742. [DOI] [Google Scholar]
- Gettinger C. L.; Heeger A. J. A photoluminescence study of poly(phenylene vinylene) derivatives: The effect of intrinsic persistence length. J. Chem. Phys. 1994, 101, 1673–1678. 10.1063/1.468438. [DOI] [Google Scholar]
- Cai W.; Wang G. T.; Du P.; Wang R. X.; Jiang X. K.; Li Z. T. Foldamer organogels: A circular dichroism study of glucose-mediated dynamic helicity induction and amplification. J. Am. Chem. Soc. 2008, 130, 13450–13459. 10.1021/ja8043322. [DOI] [PubMed] [Google Scholar]
- Sreenivasachary N.; Lehn J.-M. Gelation-driven component selection in the generation of constitutional dynamic hydrogels based on guanine-quartet formation. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 5938–5943. 10.1073/pnas.0501663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchs B.; Fieber W.; Vigouroux-Elie F.; Sreenivasachary N.; Lehn J.-M.; Herrmann H. Release of bioactive volatiles from supramolecular hydrogels: influence of reversible acylhydrazone formation on gel stability and volatile compound evaporation. Org. Biomol. Chem. 2011, 9, 2906–2919. 10.1039/c0ob01139h. [DOI] [PubMed] [Google Scholar]
- Ortuño R. M. Carbocycle-based organogelators: influence of chirality and structural features on their supramolecular arrangements and properties. Gels 2021, 7, 54. 10.3390/gels7020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst A. R.; Coates I. A.; Boucheteau T. R.; Miravet J. F.; Escuder B.; Castelletto V.; Hamley I. W.; Smith D. K. Low-molecular-weight gelators: elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J. Am. Chem. Soc. 2008, 130, 9113–9121. 10.1021/ja801804c. [DOI] [PubMed] [Google Scholar]
- Jeong Y.; Hanabusa K.; Masunaga H.; Akiba I.; Miyoshi K.; Sakurai S.; Sakurai K. Solvent/gelator interactions and supramolecular structure of gel fibers in cyclic bis-urea/primary alcohol organogels. Langmuir 2005, 21, 586–594. 10.1021/la047538t. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Wang D.; Cui J.; Mezger M.; Auernhammer G. K.; Koynov K.; Butt H.-J.; Ikeda T. Macromol. Rapid Commun. 2018, 39, 1800282 10.1002/marc.201800282. [DOI] [PubMed] [Google Scholar]
- Davis R.; Berger R.; Zentel R. Two-dimensional aggregation of organogelators induced by biaxial hydrogen-bonding gives supramolecular nanosheets. Adv. Mater. 2007, 19, 3878–3881. 10.1002/adma.200701057. [DOI] [Google Scholar]
- Hirst A. R.; Smith D. K.; Harrington J. P. Unique nanoscale morphologies underpinning organic gel-phase materials. Chem. – Eur. J. 2005, 11, 6552–6559. 10.1002/chem.200500501. [DOI] [PubMed] [Google Scholar]
- Gumtya M.; Mondal S.; Kumar S.; Ibukun O. J.; Haldar D. A peptidomimetic-based thixotropic organogel showing syneresis-induced anti-adhesion against water and ice. New J. Chem. 2022, 46, 1105–1110. 10.1039/D1NJ04647K. [DOI] [Google Scholar]
- Zhu Y. D.; Li D.; Wang L.-J. Dynamic rheological properties of peanut protein isolate and aggregation suspension and acid-induced gel. Powder Technol. 2019, 358, 95–102. 10.1016/j.powtec.2018.08.052. [DOI] [Google Scholar]
- Shirakawa M.; Fujita N.; Shinkai S. A stable single piece of unimolecularly π-stacked porphyrin aggregate in a thixotropic low molecular weight gel: A one-dimensional molecular template for polydiacetylene wiring up to several tens of micrometers in length. J. Am. Chem. Soc. 2005, 127, 4164–4165. 10.1021/ja042869d. [DOI] [PubMed] [Google Scholar]
- Widegren J. A.; Bruno T. J. Vapor pressure measurements on low-volatility terpenoid compounds by the concatenated gas saturation method. Environ. Sci. Technol. 2010, 44, 388–393. 10.1021/es9026216. [DOI] [PubMed] [Google Scholar]
- Sarkaria D. S.; Brown A. W. A. Studies on the responses of the female aëdes mosquito. Part II.—The action of liquid repellent compounds. Bull. Entomol. Res. 1951, 42, 115–122. 10.1017/S0007485300025190. [DOI] [Google Scholar]
- Štejfa V.; Fulem M.; Růžička K.; Červinka C. Thermodynamic study of selected monoterpenes III. J. Chem. Thermodyn. 2014, 79, 280–289. 10.1016/j.jct.2014.04.022. [DOI] [Google Scholar]
- Papadopoulou V.; Kosmidis K.; Vlachou M.; Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.