ABSTRACT
Spinocerebellar ataxia type 3 (SCA3) is an adult-onset, progressive ataxia. SCA3 presents with ataxia before any gross neuropathology. A feature of many cerebellar ataxias is aberrant cerebellar output that contributes to motor dysfunction. We examined whether abnormal cerebellar output was present in the CMVMJD135 SCA3 mouse model and, if so, whether it correlated with the disease onset and progression. In vivo recordings showed that the activity of deep cerebellar nuclei neurons, the main output of the cerebellum, was altered. The aberrant activity correlated with the onset of ataxia. However, although the severity of ataxia increased with age, the severity of the aberrant cerebellar output was not progressive. The abnormal cerebellar output, however, was accompanied by non-progressive abnormal activity of their upstream synaptic inputs, the Purkinje cells. In vitro recordings indicated that alterations in intrinsic Purkinje cell pacemaking and in their synaptic inputs contributed to abnormal Purkinje cell activity. These findings implicate abnormal cerebellar physiology as an early, consistent contributor to pathophysiology in SCA3, and suggest that the aberrant cerebellar output could be an appropriate therapeutic target in SCA3.
KEY WORDS: Cerebellum, Ataxia, SCA3, Purkinje cells
Summary: In a mouse model of spinocerebellar ataxia type 3 (SCA3), aberrant cerebellar physiology is apparent early in disease, prior to cerebellar neuronal pathology. Aberrant cerebellar output could be a therapeutic target in SCA3.
INTRODUCTION
The cerebellum is involved in motor coordination and maintenance of balance. Dysfunction of the cerebellum can lead to ataxia, or uncoordinated movement. The most common dominantly inherited ataxia is spinocerebellar ataxia type 3 (SCA3), also known as Machado–Joseph disease. SCA3 is caused by a heterozygous (CAG)/polyglutamine expansion of the ataxin-3 gene (Kawaguchi et al., 1994). Patients present with adult-onset progressive ataxia, along with a combination of ophthalmoplegia, spasticity, dystonia, muscular atrophy or other extrapyramidal signs (Coutinho and Andrade, 1978; Barbeau et al., 1984). The most common affected regions in pathology include the cerebellar dentate nucleus, pallidum, substantia nigra, thalamus, subthalamic nuclei, red nuclei and, to a lesser extent, cerebellar cortex (Woods and Schaumburg, 1972; Rosenberg et al., 1976; Romanul et al., 1977; Stefanescu et al., 2015; Hernandez-Castillo et al., 2018; Koeppen, 2018; Wang et al., 2020). Degeneration and other pathological hallmarks, such as protein aggregate formation, occur late in the course of the disease.
In the cerebellum of SCA3 patients, pathology has consistently shown late-onset degeneration of the deep cerebellar nuclei (DCN), the main output of the cerebellum, but little to no degeneration of the Purkinje cells, the main computational unit of the cerebellar cortex (Rosenberg et al., 1976; Haines and Dietrichs, 2012; Koeppen, 2018). It is plausible that cerebellar neuronal dysfunction contributes to the early motor phenotype. The cerebellum encodes movement-related information through rapid increases or decreases in the firing rate of the output nuclei, the DCN (Thach, 1968; Eccles, 1973). The DCN neurons are intrinsically active (Raman et al., 2000), meaning that they fire in the absence of synaptic input and fire with a regular firing pattern (Alviña and Khodakhah, 2008). If the precision of spontaneous activity is altered, the accuracy of encoding synaptic information will be impaired. The DCN receive synaptic input from mossy fibers, climbing fibers and Purkinje cells. Purkinje cells are also intrinsically active neurons (Raman and Bean, 1999) and have a regular firing pattern (Häusser and Clark, 1997). Disruption to the regularity of firing of Purkinje cells and the neurons within the DCN has been found in other mouse models of ataxia (Walter et al., 2006; Alviña and Khodakhah, 2010a,b; Tara et al., 2018). As Purkinje cells are the main computational unit of the cerebellar cortex and converge onto the DCN, disruption to their activity can impact the ability of the output of the cerebellum to accurately encode motor-related events. This raises two questions. Is cerebellar neuronal activity altered in SCA3? If so, is the dysfunction progressive, and does it correlate with the motor phenotype?
A SCA3 mouse model (CMVMJD135) expresses the expanded disease-relevant ataxin-3c isoform under the human cytomegalovirus (CMV) promoter in a heterozygous manner, conferring wide expression at near endogenous levels throughout the nervous system, similar to what is seen in patients (Silva-Fernandes et al., 2014; Teixeira-Castro et al., 2015). Overall, the mouse model is ideal to study the cerebellar contribution of disease as it recapitulates the adult-onset, progressive motor phenotypes seen in SCA3 patients, neuropathological findings and late-in-disease neuronal degeneration profile, with sparing of Purkinje cells.
In the CMVMJD135 mouse model (referred to as SCA3 mice throughout this paper), the DCN had irregular firing in awake, head-restrained mice. This indicated early, non-progressive, cerebellar neuronal alteration in SCA3 mice from either DCN neurons, or from irregular activity upstream of the nuclei. In vivo Purkinje cell activity, which converges onto the DCN, was also irregular, but not progressive, likely contributing to the abnormal cerebellar output. In vitro acute slice recordings allowed investigation of the contributors to Purkinje cell irregular firing. Purkinje cell irregularity had both aberrant intrinsic firing and altered synaptic activity, with a significant synaptic contribution. Altogether, we find that the cerebellum is a site of early dysfunction, before DCN neuronal death or ataxin-3 intranuclear inclusions are detectable, suggesting that Purkinje cell irregular activity may be an early driver of pathophysiology and a potential therapeutic target in SCA3.
RESULTS
SCA3 mice exhibit motor dysfunction early in disease
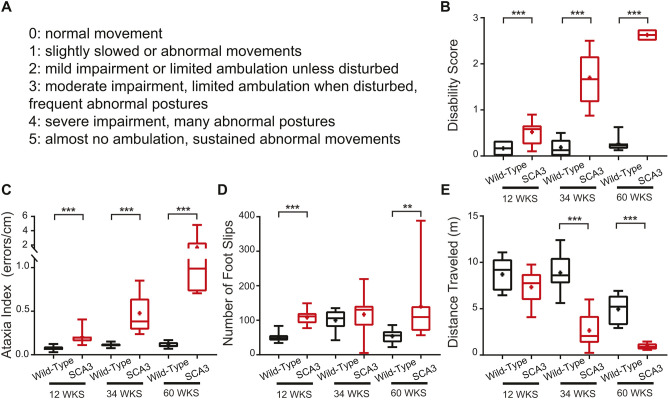
To further characterize the motor phenotype of the SCA3 mice across the course of the disease, mice were assessed using established tests of motor performance not previously applied to this particular disease model at an early, mid and late disease state. The littermate wild types, referred to as wild type throughout this paper, and SCA3 mice were tested on a disability scale for gross motor abnormalities (Weisz et al., 2005; Tara et al., 2018). Using this scale, a mouse with no motor symptoms receives a score of 0, with a score of 1 indicating abnormal gait, and the severity of motor impairment scales up to a score of 5 (Fig. 1A). Based on this assessment, SCA3 mice had motor impairment at 12 weeks of age (wild-type 0.17±0.14, SCA3 0.52±0.28, P=0.0018), with overt impairment at 34 weeks (wild-type 0.19±0.17, SCA3 1.70±0.58, P<0.0001) and severe impairment by 60 weeks (wild-type 0.27±0.16, SCA3 2.63±0.10, P=0.0003) (Fig. 1B). This demonstrates the progressive nature of the disease in SCA3 mice. By 60 weeks, the motor symptoms are a combination of deficits from the central and peripheral nervous systems (Duarte-Silva et al., 2014; Silva-Fernandes et al., 2014; Teixeira-Castro et al., 2015; Esteves et al., 2019).
Fig. 1.
SCA3 motor symptoms progress with age, with onset as early as 12 weeks. (A-E) SCA3 and wild-type mice were tested at 12, 34 and 60 weeks of age on the disability score (A,B) and parallel rod floor test (C-E). SCA3 mice have an increased disability score (A,B) compared to wild types at 12 weeks, and the score progresses with age. SCA3 mice have an increased ataxia index (C) at 12 weeks due to increased foot slips (D), with no change in distance traveled (E). At 34 and 60 weeks of age, the increased ataxia index reflects combined decrease in distance traveled and increased foot slips. Parallel rod floor test: 12 weeks: wild-type N=9, SCA3 N=9. 34 weeks: wild-type N=8, SCA3 N=9. 60 weeks: wild-type N=8, SCA3 N=7. Disability score (all scores are average of two trials): 12 weeks: wild-type N=10, SCA3 N=15. 34 weeks: wild-type N=17, SCA3 N=19. 60 weeks: wild-type N=8, SCA3 N=7. **P<0.01, ***P<0.001. Unpaired, two-tailed Student's t-test (B, 12 weeks; D, 12 weeks, 34 weeks; E, all), Mann–Whitney test (B, 34 weeks, 60 weeks; C, all; D, 60 weeks). In all figures, boxes represent the 25-75th percentiles, and the median is indicated. The mean is indicated by a plus sign. The whiskers indicate the 10th and 90th percentiles.
The second assay, the parallel rod floor test, is more sensitive to motor incoordination and eliminates variability in assessing the disability score due to human error (Kamens et al., 2005; Kamens and Crabbe, 2007). The ataxia index (Fig. 1C), calculated by number of foot slips (Fig. 1D) per distance traveled (cm) (Fig. 1E), was averaged per mouse over two trials. SCA3 mice had a higher ataxia index than wild types as early as 12 weeks (wild-type 0.074±0.029, SCA3 0.202±0.083, P<0.0001), with the ataxia index further increasing at 34 weeks (wild-type 0.112±0.023, SCA3 0.476±0.211, P<0.0001) and 60 weeks (wild-type 0.111±0.035, SCA3 1.645±1.51, P=0.0003) of age (Fig. 1C-E). Interestingly, at 12 weeks, the increased ataxia index was driven by an increased number of foot slips, with no significant decrease in distance traveled, indicating motor incoordination and not an overall change in gross movement as a main contributor to the impairment (Fig. 1D,E). Although the progressive increase in ataxia index was likely due to a combination of increased central and peripheral dysfunction, the parallel rod floor test provides a sensitive measure of motor impairment in SCA3 mice as early as 12 weeks of age.
DCN do not display neuronal cell death at 22 weeks in SCA3 mice
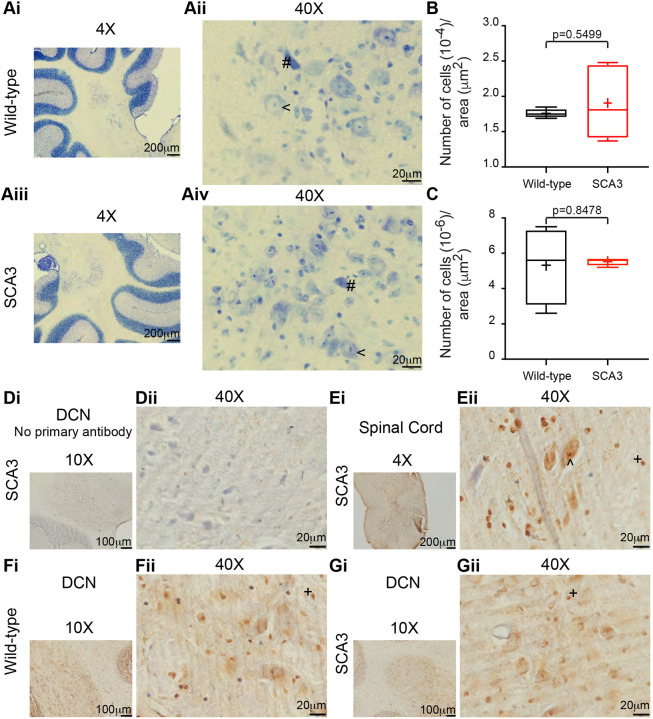
In the SCA3 mice, it has been reported that the DCN neurons have intranuclear ataxin-3 inclusions around 28 weeks, decreased DCN volume around 42 weeks and neuronal cell loss around 56 weeks (Silva-Fernandes et al., 2014; Rodríguez-Cueto et al., 2016; Duarte-Silva et al., 2018; Esteves et al., 2019). Abnormal DCN neuronal pathology or cerebellar neuronal firing changes could contribute to the early cerebellar motor deficits detected here at 12 weeks. To assess DCN neuronal pathology, the number of healthy and pyknotic cells were counted at 22 weeks of age in SCA3 and wild-type mice. The DCN were stained with Cresyl Violet and imaged to visualize the cells (Fig. 2A-C). There was no difference in the number of healthy neurons between wild-type and SCA3 mice (wild-type 1.76×10−4±6.25×10−6, SCA3 1.91×10−4±5.19×10−5, P=0.5499) (Fig. 2B). There was also no difference in the number of pyknotic cells between wild-type and SCA3 mice (wild-type 5.33×10−6±2.22×10−6, SCA3 5.52×10−6±2.17×10−7, P=0.8478) (Fig. 2C). Thus, in agreement with what has previously been demonstrated, increased DCN neuronal loss does not occur in SCA3 mice before 22 weeks of age.
Fig. 2.
Lack of deep cerebellar nuclei (DCN) cell death or ataxin-3 intranuclear inclusions at 22 weeks in SCA3 mice. (Ai-Gii) The DCN were analyzed for SCA3 hallmark pathology at 22 weeks of age. Cresyl Violet staining of the DCN from wild-type (Ai,Aii) and SCA3 (Aiii,Aiv) mice, with an example of a healthy neuron denoted by a ‘<’ and an example of a pyknotic cell denoted by a ‘#’. There was no difference in the number of heathy neurons (B) or the number of pyknotic cells (C) between wild-type and SCA3 mice. Immunohistochemistry for anti-ataxin-3 (1:500, Millipore, 1H9) was used to detect the presence of intranuclear ataxin-3 inclusions. A negative control, without the primary antibody, demonstrated the background signal from the tissue (Di,Dii). A positive control from the spinal cord of a SCA3 mouse at 22 weeks, with an ataxin-3 intranuclear inclusion denoted by a ‘^’ (Ei,Eii). Example images from the DCN of wild-type (Fi,Fii) and SCA3 (Gi,Gii) mice lacked ataxin-3 intranuclear inclusions. Example glial cells are denoted by a ‘+’. Cytoplasmic ataxin-3 was detected in all images. Cresyl Violet staining: wild-type N=4, SCA3 N=5; n=2-8 sections per animal. Ataxin-3 staining: wild-type DCN N=2, n=3 sections per animal; SCA3 DCN N=5, n=3-5 sections per animal. SCA3 spinal cord N=1. Negative control, no primary antibody: SCA3 N=1 for both DCN and spinal cord. Unpaired, two-tailed Student's t-test.
One hallmark of SCA3 is the presence of ataxin-3 intranuclear protein inclusions. In the DCN of SCA3 mice, these inclusions have been reported around 28 weeks (Esteves et al., 2019). We assessed 22-week-old SCA3 mice for DCN ataxin-3 intranuclear inclusions. As expected, when no anti-ataxin-3 primary antibody was used, only background signal was detected in the DCN (Fig. 2D). In the spinal cord (used as a positive control), as expected (Silva-Fernandes et al., 2014; Esteves et al., 2019), ataxin-3 intranuclear inclusions were detected in this group of SCA3 mice (Fig. 2E). In the same animals, where ataxin-3 intranuclear inclusions were detected in the spinal cord, there were no inclusions detected in the DCN of SCA3 mice (Fig. 2G). Thus, we demonstrated early motor impairment at 12 weeks (Fig. 1), an age before cerebellar DCN ataxin-3 nuclear aggregates were detected and when there was no increased cell death, pyknosis or other signs of neuropathology (Fig. 2).
Activity of DCN is altered, but not progressive, in SCA3 mice
We next examined whether there were any alterations in the activity of the cerebellar output nuclei in vivo. To minimize the time-variant information encoded by synaptic activity, we assessed the firing of the neurons in the DCN in stationary animals. Although the cerebellar contribution to actions such as standing remained, the need for motor signals from outside the cerebellum that drive rapid changes in firing was reduced, allowing for a more accurate assessment of the precision of firing. Therefore, in vivo recordings of the DCN neurons were made from awake, head-restrained, stationary mice. The inter-spike interval (ISI) coefficient of variation (CV) was used to measure the regularity of firing (Häusser and Clark, 1997; Walter et al., 2006; Tara et al., 2018). The ISI CV is the standard deviation of the ISI divided by the average ISI. The larger the ISI CV, the more irregular the firing and less precise the activity.
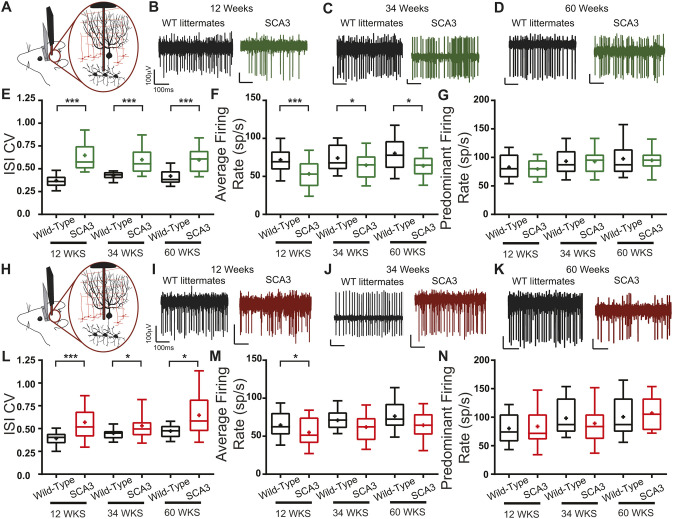
In SCA3 mice, the ISI CV of DCN was increased, but not progressive, at 12, 34 and 60 weeks of age (12 weeks: wild-type 0.371±0.114, SCA3 0.648±0.199, P<0.0001; 34 weeks: wild-type 0.427±0.054, SCA3 0.599±0.170, P<0.0001; 60 weeks: wild-type 0.421±0.121, SCA3 0.599±0.152, P<0.0001) (Fig. 3E). DCN neurons had a decreased average firing rate (the reciprocal of the average ISI) at 12, 34 and 60 weeks of age in SCA3 mice (12 weeks: wild-type 71.9±18.9, SCA3 53.4±21.5, P=0.0002; 34 weeks: wild-type 74.1±19.8, SCA3 64.7±19.8, P=0.0359; 60 weeks: wild-type 80.3±26.0, SCA3 63.9±19.2, P=0.0154) (Fig. 3F). The decrease in average firing rate would be expected to result in a decreased ISI CV if the firing pattern had not changed. Therefore, with a decrease in firing rate, the increased irregularity, as reported by ISI CV, in SCA3 mice was greater than observed. Interestingly, although the ISI CV was increased at all time points, there was no progressive increase in the ISI CV, suggesting extracerebellar contributions to the progressive phenotype of SCA3 mice (Fig. S1A).
Fig. 3.
Cerebellar neuronal dysfunction in SCA3 mice across disease progression. (A,H) In vivo awake, head-fixed cerebellar DCN (A) and Purkinje cell (H) single-unit recordings were performed from SCA3 and wild-type mice at 12, 34 and 60 weeks of age. (B-D) Example DCN firing traces from wild-type (WT; black) and SCA3 (green) mice at 12 (B), 34 (C) and 60 (D) weeks of age. (E-G) DCN inter-spike interval coefficient of variation (ISI CV) (E) is increased in SCA3 mice, while the average firing rate (F) is decreased, with no change in predominant firing rate (G) for all ages tested. (I-K) Example Purkinje cell firing traces from wild-type (black) and SCA3 (red) mice at 12 (I), 34 (J) and 60 (K) weeks of age. (L-N) Purkinje cell ISI CV (L) is increased in SCA3 mice, while the average firing rate (M) is decreased or trends to decrease, with no change in predominant firing rate (N). DCN: 12 weeks: wild-type N=6, n=39, SCA3 N=8, n=33. 34 weeks: wild-type N=12, n=33, SCA3 N=16, n=65. 60 weeks: wild-type N=5, n=24, SCA3 N=3, n=25. Purkinje cells: 12 weeks: wild-type N=6, n=36, SCA3 N=8, n=32. 34 weeks: wild-type N=12, n=29, SCA3 N=16, n=40. 60 weeks: wild-type N=5, n=25, SCA3 N=5, n=25. *P<0.05, ***P<0.001. Unpaired, two-tailed Student's t-test (F, all; G, 12 weeks; M, all; N, 12 weeks, 60 weeks), Mann–Whitney test (E, all; G, 34 weeks, 60 weeks; L, all; N, 34 weeks).
Purkinje cell activity is irregular in SCA3 mice
Abnormal DCN firing can be caused by irregular firing of DCN neurons themselves, and from irregular input from neurons upstream of the output nuclei. Purkinje cells form the sole output of the cerebellar cortex and converge onto the DCN (Sillitoe et al., 2012). To determine whether, in SCA3 mice, Purkinje cell firing is altered, Purkinje cell activity was recorded in vivo in awake, head-fixed, stationary mice. Although it would be surprising to have progressive irregularity in the Purkinje cell activity that was not reflected in the DCN activity, the Purkinje cell activity was assessed for progressive neuronal dysfunction at all stages of disease.
At 12, 34 and 60 weeks, Purkinje cells in SCA3 mice had an increased ISI CV that was not progressive with age (12 weeks: wild-type 0.392±0.113, SCA3 0.570±0.261, P<0.0001; 34 weeks: wild-type 0.448±0.077, SCA3 0.531±0.183, P=0.0330; 60 weeks: wild-type 0.472±0.080, SCA3 0.646±0.262, P=0.0112), indicating irregularity of firing in SCA3 Purkinje cells compared with that of the wild-type mice (Fig. 3L). Similar to the DCN, Purkinje cells in 12-week-old SCA3 mice had a decreased average firing rate compared to wild types, with a trend to decrease at 34 and 60 weeks (12 weeks: wild-type 64.6±19.2, SCA3 54.9±20.5, P=0.0468; 34 weeks: wild-type 71.0±16.3, SCA3 61.9±20.7, P=0.0541; 60 weeks: wild-type 76.3±24.8, SCA3 64.4±21.2, P=0.0741) (Fig. 3M). With the observed decrease in average firing rate, this increased ISI CV likely represents a more pronounced irregularity of Purkinje cells. Taken together, these in vivo data demonstrated that Purkinje cell dysfunction was present at an early age, but not progressive.
Synaptic and intrinsic components contribute to SCA3 Purkinje cell dysfunction
Both the spontaneous intrinsic activity of Purkinje cells and synaptic activity can contribute to the Purkinje cell irregular firing observed in vivo. Although motor-encoding activity was reduced by recordings in standing, stationary animals, synaptic input was still present in vivo. In addition, in vitro synaptic input can affect Purkinje cell firing through the spontaneous activity of neurons. Sensorimotor integration in the cerebellum occurs via the glutamatergic and GABAergic inputs onto Purkinje cells (Sillitoe et al., 2012). Recording Purkinje cell firing in vitro from acute cerebellar slices with synaptic transmission intact or blocked with inhibitors of fast GABAergic and glutamatergic transmission began to disentangle whether the Purkinje cell irregularity observed in vivo was from synaptic or intrinsic sources.
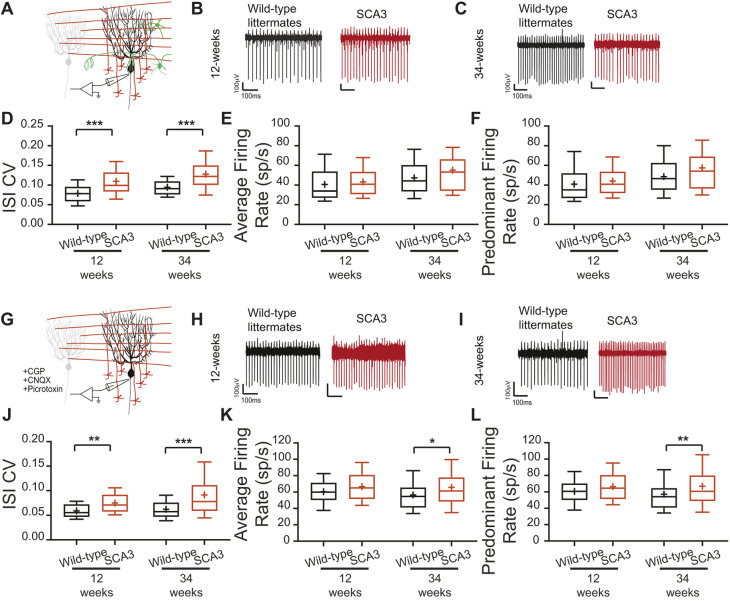
Acute sagittal cerebellar sections were obtained from 12- and 34-week-old mice, and extracellular single-unit recordings were made from visually identified Purkinje cells. Recordings were not made from 60 weeks of age due to technical difficulties maintaining mice to that age. When synaptic transmission was intact, the ISI CV of SCA3 Purkinje cells was significantly increased at both 12 and 34 weeks (12 weeks: wild-type 0.0787±0.0231, SCA3 0.1095±0.0449, P=0.0002; 34 weeks: wild-type 0.0942±0.0239, SCA3 0.1277±0.0439, P<0.0001) (Fig. 4D), whereas the average firing rate did not change (12 weeks: wild-type 40.7±17.7, SCA3 43.1±14.8, P=0.1919; 34 weeks: wild-type 47.3±17.6, SCA3 55.2±26.8, P=0.0743) (Fig. 4E). The increased ISI CV was not progressive, as expected by the in vivo data.
Fig. 4.
Synaptic and intrinsic components to Purkinje cell irregularity. (A-L) In vitro recordings were performed at 12 and 34 weeks of age, with synaptic transmission intact (A-F) and synaptic transmission blocked (G-L). Example Purkinje cell firing traces from wild-type (black) and SCA3 (red) mice at 12 weeks (B,H) and 34 weeks (C,I) of age. When synaptic transmission is intact (A-F), Purkinje cell ISI CV (C) is increased in SCA3 mice at both time points, with no change in average (E) and predominant (F) firing rate. When synaptic transmission is blocked (G-L), Purkinje cell ISI CV (J) is increased in SCA3 mice at both time points, with no change in average (K) and predominant (L) firing rate at 12 weeks, but an increase in both at 34 weeks. Synaptic transmission intact: 12 weeks: wild-type N=4, n=38; SCA3 N=4, n=50. 34 weeks: wild-type N=16, n=89; SCA3 N=17, n=91. Synaptic transmission blocked: 12 weeks: wild-type N=3, n=22; SCA3 N=3, n=29. 34 weeks: wild-type N=13, n=92; SCA3 N=15, n=121. *P<0.05, **P<0.01, ***P<0.001. Unpaired, two-tailed Student's t-test (K, 12 weeks; L, 12 weeks), Mann–Whitney test (D, all; E, all; J, all; K, 34 weeks; L, 34 weeks).
To determine whether intrinsic Purkinje cell dysfunction contributed to the increased ISI CV in vitro, fast synaptic transmission was blocked with GABAA, GABAB and AMPA (also known as GRIA)/kainate inhibitors. With synaptic transmission blocked, the ISI CV of Purkinje cells from SCA3 mice remained increased, but was not progressive, at 12 and 34 weeks (12 weeks: wild-type 0.0589±0.016, SCA3 0.0750±0.021, P=0.0040; 34 weeks: wild-type 0.0623±0.022, SCA3 0.0914±0.482, P<0.0001) (Fig. 4). The average firing rate of intrinsic Purkinje cell activity had a trend to increase at 12 weeks and was significantly increased at 34 weeks (12 weeks: wild-type 60.3±14.6, SCA3 66.5±19.0, P=0.2057; 34 weeks: wild-type 56.5±20.6, SCA3 65.6±28.0, P=0.0113) (Fig. 4D). The increased average firing rate suggested that the firing rate contributed to the increased ISI CV. Therefore, a difference in ISI CV when synaptic transmission was intact suggested that although both synaptic and intrinsic dysfunction occurred, the synaptic component was greater.
Location dependence of SCA3 Purkinje cell irregularity
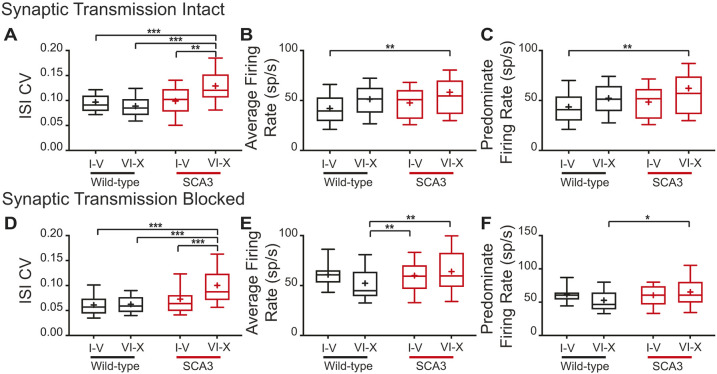
Purkinje cells are not a homogenous population, with differential gene expression in stripes throughout the cerebellum (Cerminara et al., 2015). One differentially expressed gene, zebrin II (also known as Aldoc), is typically used as a guide for this heterogeneity, with lobules I-V being predominantly zebrin II negative, and lobules VI-X being predominantly zebrin II positive (Cerminara et al., 2015). With this rough generalization for gene expression, the Purkinje cell data from 34-week-old animals was sorted by the lobule in which it was located. In this preliminary analysis, SCA3 Purkinje cells in lobules I-V had no change in ISI CV (synaptic transmission intact: wild-type 0.097±0.023, SCA3 0.099±0.029, P>0.9999; synaptic transmission blocked: wild-type 0.061±0.024, SCA3 0.073±0.041, P>0.9999) (Fig. 5A,D). Nor was there a change in average firing rate in lobules I-V in SCA3 mice (synaptic transmission intact: wild-type 42.1±17.4, SCA3 47.7±15.8, P>0.9999; synaptic transmission blocked: wild-type 60.8±14.3, SCA3 60.0±17.5, P>0.9999) (Fig. 5B,E). In contrast, we found that SCA3 Purkinje cells in lobules VI-X had an increased ISI CV when synaptic transmission was intact (synaptic transmission intact: wild-type 0.089±0.025, SCA3 0.129±0.037, P<0.0001) (Fig. 5A) and when synaptic transmission was blocked (synaptic transmission blocked: wild-type 0.062±0.020, SCA3 0.100±0.044, P<0.0001) (Fig. 5D). In lobules VI-X, there was an increased average firing rate only when synaptic transmission was blocked (synaptic transmission intact: wild-type 51.3±15.9, SCA3 58.3±30.4, P>0.9999; synaptic transmission blocked: wild-type 52.3±21.8, SCA3 64.0±24.2, P=0.0076) (Fig. 5B,E). Thus, the aberrant synaptic and irregular intrinsic Purkinje cell activity in SCA3 mice is posterior lobule dependent.
Fig. 5.
SCA3 Purkinje cell dysfunction is heterogeneous. (A-F) The in vitro Purkinje cell recordings from 34-week-old animals demonstrated dependence on location of recording. When synaptic transmission was intact (A-C), Purkinje cell ISI CV (A) was increased, with a small increase in average (B) and predominant (C) firing rates only in SCA3 lobules VI-X. This held true when synaptic transmission was blocked (D-F). The increased ISI CV (D), increased average firing rate (E) and increased predominant firing rate is specific to SCA3 lobules VI-X. Synaptic transmission intact: wild-type I-V: N=10, n=43; wild-type VI-X: N=10, n=33. SCA3 I-V: N=5, n=14; SCA3 VI-X: N=13, n=54. Synaptic transmission blocked: wild-type I-V: N=7, n=29; wild-type VI-X: N=12, n=59. SCA3 I-V: N=11, n=43; SCA3 VI-X: N=14, n=59. *P<0.05, **P<0.01, ***P<0.001. One-way ANOVA with Bonferroni correction for multiple comparisons.
DISCUSSION
Here, we show that, early in disease, the activity of neurons in the DCN of SCA3 mice was irregular, with irregular Purkinje cell activity likely to be a contributing factor. The irregularity in the output of the cerebellum, the DCN, was noted in mice as early as 12 weeks postnatal; however, this irregularity was not progressive with age. Cerebellar Purkinje cells were also irregular in vivo as early as 12 weeks, but the irregularity was again not progressive with age. In in vitro experiments, we found that, in SCA3, both aberrant intrinsic firing and synaptic activity likely contribute to Purkinje cells dysfunction. The irregular firing in the DCN and Purkinje cells results in a decreased ability to accurately encode motor-related information. Therefore, despite the irregular firing not progressing with motor impairment, the early dysfunction, before any detectable DCN ataxin-3 intranuclear inclusion or increased neuronal death, suggested early and consistent cerebellar neuronal dysfunction likely driving the early motor phenotypes in SCA3. In patients, this early motor impairment can impact many aspects of their quality of life, as simple things such as dressing and eating require motor coordination. Therefore, with the goal of improving the quality of life of patients, this early Purkinje cell dysfunction could be a good therapeutic target for SCA3 patients.
Early cerebellar dysfunction contributes to the SCA3 phenotype
Although the motor phenotype in SCA3 mice is progressive, the neuronal dysfunction in DCN and Purkinje cells appeared to be consistent from 12 to 60 weeks of age (Fig. S1). If increased cerebellar dysfunction was the primary factor in causing the progressive ataxia in SCA3, one might expect that, as the motor symptoms progressed, the neuronal dysfunction, or ISI CV, would also increase and correspond to the increased symptom severity (Tara et al., 2018). However, in SCA3, unlike pure cerebellar ataxias, the brainstem, spinal cord, muscles and other brain regions, such as the substantia nigra, are all affected (Woods and Schaumburg, 1972; Rosenberg et al., 1976; Romanul et al., 1977; Stefanescu et al., 2015; Hernandez-Castillo et al., 2018; Koeppen, 2018; Wang et al., 2020). Indeed, in this SCA3 mouse model, it is a combination of these factors that contribute to the presentation of motor symptoms. SCA3 mice have motor incoordination as early as 12 weeks of age and strength-related behavioral phenotypes that were previously shown as early as 6 weeks. By 60 weeks of age, mice are unable to properly support their body weight, often pulling their body to move instead of using proper ambulation (Teixeira-Castro et al., 2015). A potentially consistent cerebellar contribution to SCA3 ataxia has also been proposed based on studies using a different mouse model of SCA3 (Bushart et al., 2021). Additional studies will be needed to determine whether the dysfunctional cerebellum is driving other sites of dysfunction in the central nervous system, or whether these other areas undergo independent dysregulation. Given the ubiquitous expression of ataxin-3 in the nervous system of this mouse model (as in human patients), it might also be that the spinal cord/peripheral nervous system and striatum/substantia nigra-related mechanisms of dysfunction arise separately from the cerebellar mechanisms.
Despite the non-progressive nature of cerebellar irregular firing, the early initial dysfunction could be an appropriate therapeutic target. The cerebellum relies on the regular, spontaneous activity of the DCN to accurately encode movement-related information through rapid changes, up or down, in the firing rate of the DCN (Eccles, 1967; Thach, 1968). When the spontaneous activity of the cerebellum is no longer precise, the encoding of information is impaired, a feature apparent in other cerebellar ataxias (Walter et al., 2006; Alviña and Khodakhah, 2010b; Tara et al., 2018). The SCA3 mice have aberrant spontaneous and synaptic dysfunction of Purkinje cells, likely contributing to the altered DCN activity at an early stage of disease when motor incoordination is present. Targeting this neuronal activity therapeutically might provide a consistent, albeit partial, reduction in the severity of ataxia throughout the disease. In another SCA3 mouse model, when the mutant ataxin-3 was silenced with the use of antisense oligonucleotides, there was an improvement in the cerebellar deficits, and motor performance, of the SCA3 mice (Shakkottai et al., 2011; McLoughlin et al., 2018). This work reinforces the potential for cerebellar dysfunction to contribute to, and be a therapeutic target for, the motor dysfunction in SCA3. For patients, motor incoordination impacts most aspects of daily life. In fact, several components of the scale for assessment and rating of ataxia (SARA) depend on coordination, such as gait, finger-to-nose test, fast-alternating hand movements and finger chase (Schmitz-Hubsch et al., 2006; Jacobi et al., 2020). All of these measurements will affect daily tasks, such as dressing, grooming and eating. As SCA3 patients have a decreased quality of life early in disease (Bolzan et al., 2021), targeting this early dysfunction could alleviate some of these factors, potentially improving their quality of life.
Purkinje cell dysfunction as one potential mechanism of SCA3 pathophysiology
In SCA3 patients, Purkinje cells show limited degeneration, if at all (Woods and Schaumburg, 1972; Rosenberg et al., 1976; Seidel et al., 2012a,b). Here, in a mouse model that does not show Purkinje cell ataxin-3 nuclear inclusions nor degeneration of Purkinje cells even at late disease stages (Rodríguez-Cueto et al., 2016), we found that Purkinje cells have irregular firing starting from the first stages of disease. One interesting feature of this irregularity was the dependence on location of the Purkinje cells within the cerebellum (Fig. 5). Expression of zebrin II correlates with expression of several genes that have the potential to influence the physiological properties of Purkinje cells, including EAAT4 (also known as Slc1a6), GABABr2 (also known as Gabbr2) and mGluR1b (also known as Grm1), among others (Brochu et al., 1990; Dehnes et al., 1998; Mateos et al., 2000; Larouche et al., 2006; Sarna et al., 2006; Chung et al., 2008; Sillitoe et al., 2009; Cerminara et al., 2015). Using zebrin II-linked gene expression patterns, Purkinje cells can be divided based on the modular organization of the cerebellum. The cerebellar vermis is divided anatomically into ten lobules, numbered I-X from anterior to posterior. Lobules I-V have Purkinje cells that are predominantly zebrin II negative, whereas lobules VI-X have more zebrin II positive Purkinje cells (Arancillo et al., 2015; Cerminara et al., 2015). Several different patterns of Purkinje cell degeneration have been noted in many models of cerebellar ataxia (Cerminara et al., 2015; Toscano Márquez et al., 2021). However, it is unclear how Purkinje cells are differentially affected by the different mutations leading to cerebellar ataxia. Further experiments are needed to directly correlate the changes in the firing pattern of Purkinje cells with zebrin II expression, and to delineate the reasons for this difference.
There are a few theories underlying SCA3 pathophysiology, ranging from the toxicity of protein aggregates/intranuclear inclusions to extensive cell death. One of the most consistently affected regions in pathological analysis of SCA3 patients is the DCN (Woods and Schaumburg, 1972; Rosenberg et al., 1976; Seidel et al., 2012a,b). In the SCA3 mouse model used here, the DCN show intranuclear aggregates without neuronal loss by 28 weeks, DCN volume loss by 42 weeks and DCN neuronal loss by 56 weeks (Silva-Fernandes et al., 2014; Rodríguez-Cueto et al., 2016; Duarte-Silva et al., 2018; Esteves et al., 2019). Here, we showed that SCA3 mice did not display intranuclear ataxin-3 inclusions or cell death in the DCN at 22 weeks of age, thus excluding the presence of these disease hallmarks at earlier ages, namely at 12 weeks, when we performed our analyses. Despite there being no pathology at 12 weeks, the DCN neurons in vivo had irregular activity. Although the possibility that this irregular activity is partially driven by DCN neurons themselves remains to be examined, similar to some other cerebellar ataxias, Purkinje cells in SCA3 mice have irregular firing. It is interested to note that erratic firing of Purkinje cells can lead to DCN degeneration, as in other mouse models of movement disorders, DCN pathology can be seen at the late stages of disease even when the causal mutation is isolated to Purkinje cells, or largely drives Purkinje cell specific irregularity (Todorov et al., 2012; Fremont et al., 2015). Together, this suggests the Purkinje cell dysfunction might not only be a contributor to the DCN irregularity but might lead to the DCN pathology observed later in disease. Additional studies are required to understand the mechanisms that cause the aberrant synaptic and intrinsic dysfunction of Purkinje cells. Understanding the contributors to cerebellar dysfunction might inform potential mechanisms driving all neuronal dysfunction in SCA3, which would allow the development of a multifaceted approach for treating this complex disease.
MATERIALS AND METHODS
Animals
Experiments were performed on 12- to 60-week-old CMVMJD135 (SCA3) mice with 140±10 CAG repeats, bred and genotyped at the Life and Health Science Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal – ICVS/3B's – PT Government Associate Laboratory. Mice were housed at weaning in groups of five to six animals in filter-topped polysulfone cages 267×207×140 mm (370 cm2 floor area) (Tecniplast, Buguggiate, Italy), with corncob bedding (Scobis Due, Mucedola SRL, Settimo Milanese, Italy) in a specific pathogen-free animal facility. All animals were maintained under standard laboratory conditions: an artificial 12 h light/dark cycle (lights on from 08:00 to 20:00), with a room temperature of 21±1°C and a relative humidity of 50-60%. Mice were given a standard diet (4RF25 during the gestation and postnatal periods, and 4RF21 after weaning) (Mucedola SRL, Settimo Milanese, Italy) and water ad libitum. Health monitoring was performed according to Federation of European Laboratory Animal Science Association (FELASA) guidelines, confirming the specified pathogens status of sentinel animals maintained in the same animal room. All procedures were conducted in accordance with European regulations (European Union Directive 86/609/EEC). Animal facilities and the people directly involved in vertebrate animal experiments (A.N.-C.) as well as coordinating the research (P.M.) were certified by the Portuguese regulatory entity [Direcção Geral de Alimentação e Veterinária (DGAV)]. All protocols performed were approved by the Animal Ethics Committee of the Life and Health Sciences Research Institute, University of Minho and by the DGAV (reference 020317).
Mice were shipped to Albert Einstein College of Medicine and allowed at least 2 weeks of recovery before any experiments were conducted. All experiments were conducted in accordance with the guidelines set by the Institute of Animal Safety and Institute of at Albert Einstein College of Medicine under the senior investigator's (K.K.) Institutional Animal Care and Use Committee approved protocol. Mice were housed on a 12:12 h reversed light/dark cycle. Data were analyzed to examine any effect of repeat length on the electrophysiology, with no effect detected. As there have been no reported sex differences in these mice (Silva-Fernandes et al., 2010, 2014; Teixeira-Castro et al., 2011, 2015; Duarte-Silva et al., 2014, 2018; Esteves et al., 2015, 2019), only female mice were used for Figs 1 and 3, with all other figures including both male and female mice, with no difference between sex detected. For the 12-week time point, mice were 12-15 weeks of age. For the 34-week time point, mice were 34-40 weeks of age. For the 60-week time point, mice were 50-60 weeks of age. Mice were used for behavior first, followed by either in vivo or in vitro experiments.
Behavior
All experiments were performed during the mouse's dark cycle. Mice were acclimated to the behavior room for at least 1 h before the start of all experiments. For all behavior experiments, the experimenter was blinded to the genotype of the mice.
Parallel rod floor test
Mice were assessed on the parallel floor rod test for 10-min sessions, once a day for 2 days (Kamens et al., 2005; Kamens and Crabbe, 2007). The trials were averaged to obtain total distance traveled and number of foot slips per animal. Ataxia ratio is defined as the number of foot slips (errors) divided by distance traveled (cm).
Disability score
Mice were assessed using the previously published disability score as follows: 0, normal motor behavior; 1, slightly slowed or abnormal movements; 2, mild impairments, limited ambulation unless disturbed; 3, moderate impairment, limited ambulation even when disturbed, frequent abnormal postures; 4, severe impairment, almost no ambulation, sustained abnormal postures; 5, prolonged immobility in abnormal postures (Weisz et al., 2005; Tara et al., 2018). To assess the disability score, mice were individually placed in an open field for 30 min. The videos were blinded, and a 30-s segment was selected in which the mouse was ambulating. The cropped video was sent to four viewers trained on the disability scale and blind to the genotype of the animal. These four scores were averaged to produce a score for each trial per mouse. The final score for each mouse was reported as the average of at least two trials from separate days.
Neuropathology analysis
SCA3 and wild-type littermate mice (aged 22 weeks) were deeply anesthetized and perfused with PBS followed by 4% paraformaldehyde (PFA) in PBS. Brains were harvested and post-fixed overnight in a fixative solution. The following day, brains were transferred to sucrose 30% solution and further sliced in a vibratome. Free-floating sections (40 µm thick) were stained with Cresyl Violet or processed for immunohistochemistry with mouse anti-ataxin-3 (1:500, Millipore, 1H9, #MAB5360). Immunohistochemistry was performed using an ACUITYAdvanced Biotin Free Polymer DAB Kit (BioLegend, #931001) following the manufacturer's instructions. Pyknotic and healthy cells were quantified in the DCN (100% coverage; N=5 animals/group; n=2-8 slices/animal) and normalized for the total area (μm2) using a BX51 stereological microscope (Olympus) and Visiopharma integrator system software (Visiopharm). Representative staining images were acquired using optical microscopy (Olympus Widefield Upright Microscope BX61). All the samples were codified immediately after euthanasia (using numeric codes) and the quantifications were performed blindly.
Electrophysiology
In vivo
Mice were implanted with a titanium bracket fixed to the skull with optibond (Kerr Dental) and charisma (Kulzer). A recording chamber was formed on the skull above the cerebellum with dental cement. Craniotomies for recordings were made at the following locations (anterior/posterior, medial/lateral): −6.2, ±1.75; and −7, ±0.25. Until time of recording, craniotomies were covered with silicone adhesive (KWIK-SIL, WPI). Surgery was performed on day 1. Recordings were performed on days 2-6. Mice were habituated to the head-fixed apparatus for at least 1 h before the recordings began each day. Recordings were only performed if the mouse was not moving in the apparatus. The same mouse was recorded from for a maximum of 3 days. From awake, head-fixed, non-moving mice, extracellular single-unit activity was recorded by advancing a tungsten electrode (Thomas Recordings, 2-3 MΩ) until Purkinje cell layers or DCN were detected. Purkinje cells were identified by location, presence of complex spikes and characteristic firing rate. DCN were identified by location and characteristic firing rate. Signals were filtered (200 Hz-20 kHz) and amplified (2000×) using a custom-built amplifier, and then digitized (20 kHz) using a National Instruments card (PCI-MIO-16-XE) with custom-written software in Labview. Waveforms were sorted offline using amplitude, energy and principal component analysis (Plexon offline sorter).
In vitro
All experiments were conducted blinded to the genotype of the mouse. Mice were anesthetized with isoflurane and rapidly decapitated. The brain was rapidly removed and placed in warm (35±2°C) artificial cerebral spinal fluid (ACSF): NaCl 125 mM, KCl 2.5 mM, NaHCO3 26 mM, NaH2PO4 1.25 mM, MgCl2 1 mM, CaCl2 2 mM, glucose 11 mM, pH 7.4 when gassed with 5% CO2:95% O2. The cerebellum was dissected, and 300 µm thick sagittal sections were made using a Campden Instruments 7000sz vibratome. Slices were kept in oxygenated ACSF at 35±2°C for 1 h and then kept at room temperature until use (up to 4 h). At the time of recording, slices were placed in a recording chamber perfused with warm (35±2°C) ACSF at 1.5 ml/min. Single-unit extracellular recordings were obtained from visually identified Purkinje cells with an upright microscope (Zeiss) using a home-made differential amplifier and glass pipette back-filled with ACSF. Where indicated, to isolate Purkinje cell intrinsic activity, perfusion ACSF contained 10 μM picrotoxin, 1 μM CGP55845 and 10 μM cyanquixaline (CNQX) to block GABAA, GABAB and AMPA/kainate receptors, respectively. Signals were digitized (10 kHz) using a National Instruments card (PCI-MIO-16-XE) with custom-written software in Labview. Waveforms were sorted offline using amplitude, energy and principal component analysis (Plexon offline sorter), and output was analyzed using custom-written Labview software. For all experiments, each cell was held for a minimum of 5 min.
Statistics
Data were graphed using 10-90 box plots, with the mean indicated by the plus sign. Unpaired, two-tailed Student's t-tests were used for all pairwise comparisons. All data were assessed for normalcy with the Shapiro–Wilk test, and non-parametric tests (Mann–Whitney) were conducted for all datasets that were not normally distributed. All data are reported in the text as ±s.d. For Fig. S1, nonlinear regression was performed with least squares regression, no weighting and a confidence interval of 95%. ‘N’ refers to number of mice; ‘n’ refers to number of cells. P<0.05 was considered significant.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.M.-P., A.N.-C., P.M., K.K.; Methodology: K.M.-P., A.N.-C., P.M., K.K.; Formal analysis: K.M.-P.; Investigation: K.M.-P., A.N.-C.; Resources: P.M., K.K.; Data curation: S.D.-S., D.M.-F.; Writing - original draft: K.M.-P., K.K.; Writing - review & editing: K.M.-P., A.N.-C., P.M., K.K.; Visualization: K.M.-P.; Supervision: P.M., K.K.; Project administration: K.K.; Funding acquisition: K.M.-P., A.N.-C.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke [R01NS050808 to K.K.; F31NS105406 to K.M.-P.]; National funds through the Fundação para a Ciência e a Tecnologia (FCT) – project UIDB/50026/2020 and UIDP/50026/2020 [SFRH/BPD/118779/2016 to A.N.-C.; CEECIND/00685/2020 to S.D.-S.; SFRH/BD/147947/2019 to D.M.-F.]; and the ICVS Scientific Microscopy Platform, a member of the national infrastructure Portuguese Platform of Bioimaging [PPBI-POCI-01-0145-FEDER-022122]. Open Access funding provided by National Institute of Neurological Disorders and Stroke [R01NS050808 to K.K.]. Deposited in PMC for immediate release.
References
- Alviña, K. and Khodakhah, K. (2008). Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats. J. Physiol. 586, 2523-2538. 10.1113/jphysiol.2007.148197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alviña, K. and Khodakhah, K. (2010a). KCa channels as therapeutic targets in episodic ataxia type-2. J. Neurosci. 30, 7249-7257. 10.1523/JNEUROSCI.6341-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alviña, K. and Khodakhah, K. (2010b). The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J. Neurosci. 30, 7258-7268. 10.1523/JNEUROSCI.3582-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancillo, M., White, J. J., Lin, T., Stay, T. L. and Sillitoe, R. V. (2015). In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J. Neurophysiol. 113, 578-591. 10.1152/jn.00586.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau, A., Roy, M., Cunha, L., de Vincente, A. N., Rosenberg, R. N., Nyhan, W. L., MacLeod, P. L., Chazot, G., Langston, L. B. and Dawson, D. M. (1984). The natural history of Machado-Joseph disease: an analysis of 138 personally examined cases. Can. J. Neurol. Sci. 11, 510-525. 10.1017/S0317167100034983 [DOI] [PubMed] [Google Scholar]
- Bolzan, G., Leotti, V. B., de Oliveira, C. M., Ecco, G., Cappelli, A. H., Rocha, A. G., Kersting, N., Rieck, M., de Sena, L. S., Martins, A. C.et al. (2021). Quality of life since pre-ataxic phases of spinocerebellar ataxia type 3/Machado-Joseph Disease. Cerebellum 21, 297-305. 10.1007/s12311-021-01299-8 [DOI] [PubMed] [Google Scholar]
- Brochu, G., Maler, L. and Hawkes, R. (1990). Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol. 291, 538-552. 10.1002/cne.902910405 [DOI] [PubMed] [Google Scholar]
- Bushart, D. D., Zalon, A. J., Zhang, H., Morrison, L. M., Guan, Y., Paulson, H. L., Shakkottai, V. G. and McLoughlin, H. S. (2021). Antisense oligonucleotide therapy targeted against ATXN3 improves potassium channel-mediated purkinje neuron dysfunction in spinocerebellar ataxia type 3. Cerebellum 20, 41-53. 10.1007/s12311-020-01179-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerminara, N. L., Lang, E. J., Sillitoe, R. V. and Apps, R. (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 16, 79-93. 10.1038/nrn3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S.-H., Kim, C.-T. and Hawkes, R. (2008). Compartmentation of GABA B receptor2 expression in the mouse cerebellar cortex. Cerebellum 7, 295-303. 10.1007/s12311-008-0030-3 [DOI] [PubMed] [Google Scholar]
- Coutinho, P. and Andrade, C. (1978). Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology 28, 703-709. 10.1212/WNL.28.7.703 [DOI] [PubMed] [Google Scholar]
- Dehnes, Y., Chaudhry, F. A., Ullensvang, K., Lehre, K. P., Storm-Mathisen, J. and Danbolt, N. C. (1998). The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18, 3606-3619. 10.1523/JNEUROSCI.18-10-03606.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Silva, S., Neves-Carvalho, A., Soares-Cunha, C., Teixeira-Castro, A., Oliveira, P., Silva-Fernandes, A. and Maciel, P. (2014). Lithium chloride therapy fails to improve motor function in a transgenic mouse model of Machado-Joseph disease. Cerebellum 13, 713-727. 10.1007/s12311-014-0589-9 [DOI] [PubMed] [Google Scholar]
- Duarte-Silva, S., Neves-Carvalho, A., Soares-Cunha, C., Silva, J. M., Teixeira-Castro, A., Vieira, R., Silva-Fernandes, A. and Maciel, P. (2018). Neuroprotective effects of creatine in the CMVMJD135 mouse model of spinocerebellar ataxia type 3. Mov. Disord. 33, 815-826. 10.1002/mds.27292 [DOI] [PubMed] [Google Scholar]
- Eccles, J. C. (1967). Circuits in the cerebellar control of movement. Proc. Natl. Acad. Sci. USA 58, 336-343. 10.1073/pnas.58.1.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, J. C. (1973). The cerebellum as a computer: patterns in space and time. J. Physiol. 229, 1-32. 10.1113/jphysiol.1973.sp010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves, S., Duarte-Silva, S., Naia, L., Neves-Carvalho, A., Teixeira-Castro, A., Rego, A. C., Silva-Fernandes, A. and Maciel, P. (2015). Limited effect of chronic valproic acid treatment in a mouse model of Machado-Joseph disease. PLoS ONE 10, e0141610. 10.1371/journal.pone.0141610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves, S., Oliveira, S., Duarte-Silva, S., Cunha-Garcia, D., Teixeira-Castro, A. and Maciel, P. (2019). Preclinical evidence supporting early initiation of citalopram treatment in Machado-Joseph disease. Mol. Neurobiol. 56, 3626-3637. 10.1007/s12035-018-1332-1 [DOI] [PubMed] [Google Scholar]
- Fremont, R., Tewari, A. and Khodakhah, K. (2015). Aberrant Purkinje cell activity is the cause of dystonia in a shRNA-based mouse model of Rapid Onset Dystonia-Parkinsonism. Neurobiol. Dis. 82, 200-212. 10.1016/j.nbd.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines, D. E. and Dietrichs, E. (2012). Chapter 1 - The cerebellum – structure and connections. In Handbook of Clinical Neurology (ed. Sankara H. S. and Alexandra D.), pp. 3-36. Elsevier. [DOI] [PubMed] [Google Scholar]
- Häusser, M. and Clark, B. A. (1997). Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665-678. 10.1016/S0896-6273(00)80379-7 [DOI] [PubMed] [Google Scholar]
- Hernandez-Castillo, C. R., King, M., Diedrichsen, J. and Fernandez-Ruiz, J. (2018). Unique degeneration signatures in the cerebellar cortex for spinocerebellar ataxias 2, 3, and 7. Neuroimage Clin. 20, 931-938. 10.1016/j.nicl.2018.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi, H., du Montcel, S. T., Romanzetti, S., Harmuth, F., Mariotti, C., Nanetti, L., Rakowicz, M., Makowicz, G., Durr, A., Monin, M.-L.et al. (2020). Conversion of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 to manifest ataxia (RISCA): a longitudinal cohort study. Lancet Neurol. 19, 738-747. 10.1016/S1474-4422(20)30235-0 [DOI] [PubMed] [Google Scholar]
- Kamens, H. M. and Crabbe, J. C. (2007). The parallel rod floor test: a measure of ataxia in mice. Nat. Protoc. 2, 277-281. 10.1038/nprot.2007.19 [DOI] [PubMed] [Google Scholar]
- Kamens, H. M., Phillips, T. J., Holstein, S. E. and Crabbe, J. C. (2005). Characterization of the parallel rod floor apparatus to test motor incoordination in mice. Genes Brain Behav. 4, 253-266. 10.1111/j.1601-183X.2004.00100.x [DOI] [PubMed] [Google Scholar]
- Kawaguchi, Y., Okamoto, T., Taniwaki, M., Aizawa, M., Inoue, M., Katayama, S., Kawakami, H., Nakamura, S., Nishimura, M., Akiguchi, I.et al. (1994). CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 8, 221-228. 10.1038/ng1194-221 [DOI] [PubMed] [Google Scholar]
- Koeppen, A. H. (2018). The Neuropathology of Spinocerebellar Ataxia Type 3/Machado-Joseph Disease. Adv. Exp. Med. Biol. 1049, 233-241. 10.1007/978-3-319-71779-1_11 [DOI] [PubMed] [Google Scholar]
- Larouche, M., Che, P. M. and Hawkes, R. (2006). Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J. Comp. Neurol. 494, 215-227. 10.1002/cne.20791 [DOI] [PubMed] [Google Scholar]
- Mateos, J. M., Benítez, R., Elezgarai, I., Azkue, J. J., Lázaro, E., Osorio, A., Bilbao, A., Doñate, F., Sarría, R., Conquet, F.et al. (2000). Immunolocalization of the mGluR1b splice variant of the metabotropic glutamate receptor 1 at parallel fiber-Purkinje cell synapses in the rat cerebellar cortex. J. Neurochem. 74, 1301-1309. 10.1046/j.1471-4159.2000.741301.x [DOI] [PubMed] [Google Scholar]
- McLoughlin, H. S., Moore, L. R., Chopra, R., Komlo, R., McKenzie, M., Blumenstein, K. G., Zhao, H., Kordasiewicz, H. B., Shakkottai, V. G. and Paulson, H. L. (2018). Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice. Ann. Neurol. 84, 64-77. 10.1002/ana.25264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, I. M. and Bean, B. P. (1999). Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J. Neurosci. 19, 1663-1674. 10.1523/JNEUROSCI.19-05-01663.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman, I. M., Gustafson, A. E. and Padgett, D. (2000). Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J. Neurosci. 20, 9004-9016. 10.1523/JNEUROSCI.20-24-09004.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cueto, C., Hernández-Gálvez, M., Hillard, C. J., Maciel, P., García-García, L., Valdeolivas, S., Pozo, M. A., Ramos, J. A., Gómez-Ruiz, M. and Fernández-Ruiz, J. (2016). Dysregulation of the endocannabinoid signaling system in the cerebellum and brainstem in a transgenic mouse model of spinocerebellar ataxia type-3. Neuroscience 339, 191-209. 10.1016/j.neuroscience.2016.09.046 [DOI] [PubMed] [Google Scholar]
- Romanul, F. C. A., Fowler, H. L., Radvany, J., Feldman, R. G. and Feingold, M. (1977). Azorean disease of the nervous system. N. Engl. J. Med. 296, 1505-1508. 10.1056/NEJM197706302962606 [DOI] [PubMed] [Google Scholar]
- Rosenberg, R. N., Nyhan, W. L., Bay, C. and Shore, P. (1976). Autosomal dominant striatonigral degeneration. A clinical, pathologic, and biochemical study of a new genetic disorder. Neurology 26, 703-714. 10.1212/WNL.26.8.703 [DOI] [PubMed] [Google Scholar]
- Sarna, J. R., Marzban, H., Watanabe, M. and Hawkes, R. (2006). Complementary stripes of phospholipase Cβ3 and Cβ4 expression by Purkinje cell subsets in the mouse cerebellum. J. Comp. Neurol. 496, 303-313. 10.1002/cne.20912 [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch, T., du Montcel, S. T., Baliko, L., Berciano, J., Boesch, S., Depondt, C., Giunti, P., Globas, C., Infante, J., Kang, J.-S.et al. (2006). Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66, 1717-1720. 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- Seidel, K., Meister, M., Dugbartey, G. J., Zijlstra, M. P., Vinet, J., Brunt, E. R. P., van Leeuwen, F. W., Rub, U., Kampinga, H. H. and den Dunnen, W. F. (2012a). Cellular protein quality control and the evolution of aggregates in spinocerebellar ataxia type 3 (SCA3). Neuropathol. Appl. Neurobiol. 38, 548-558. 10.1111/j.1365-2990.2011.01220.x [DOI] [PubMed] [Google Scholar]
- Seidel, K., Siswanto, S., Brunt, E. R. P., den Dunnen, W., Korf, H.-W. and Rüb, U. (2012b). Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 124, 1-21. 10.1007/s00401-012-1000-x [DOI] [PubMed] [Google Scholar]
- Shakkottai, V. G., do Carmo Costa, M., Dell'Orco, J. M., Sankaranarayanan, A., Wulff, H. and Paulson, H. L. (2011). Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J. Neurosci. 31, 13002-13014. 10.1523/JNEUROSCI.2789-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe, R. V., Gopal, N. and Joyner, A. L. (2009). Embryonic origins of ZebrinII parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience 162, 574-588. 10.1016/j.neuroscience.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe, R. V., Fu, Y. and Watson, C. (2012). Chapter 11 – Cerebellum. In The Mouse Nervous System (ed. C. Watson, G. Paxinos, L. Puelles), pp. 360-397. Academic Press. [Google Scholar]
- Silva-Fernandes, A., Costa, M. C., Duarte-Silva, S., Oliveira, P., Botelho, C. M., Martins, L., Mariz, J. A., Ferreira, T., Ribeiro, F., Correia-Neves, M.et al. (2010). Motor uncoordination and neuropathology in a transgenic mouse model of Machado-Joseph disease lacking intranuclear inclusions and ataxin-3 cleavage products. Neurobiol. Dis. 40, 163-176. 10.1016/j.nbd.2010.05.021 [DOI] [PubMed] [Google Scholar]
- Silva-Fernandes, A., Duarte-Silva, S., Neves-Carvalho, A., Amorim, M., Soares-Cunha, C., Oliveira, P., Thirstrup, K., Teixeira-Castro, A. and Maciel, P. (2014). Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph disease. Neurotherapeutics 11, 433-449. 10.1007/s13311-013-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanescu, M. R., Dohnalek, M., Maderwald, S., Thurling, M., Minnerop, M., Beck, A., Schlamann, M., Diedrichsen, J., Ladd, M. E. and Timmann, D. (2015). Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich's ataxia. Brain 138, 1182-1197. 10.1093/brain/awv064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tara, E., Vitenzon, A., Hess, E. and Khodakhah, K. (2018). Aberrant cerebellar Purkinje cell activity as the cause of motor attacks in a mouse model of episodic ataxia type 2. Dis. Model. Mech. 11, dmm034181. 10.1242/dmm.034181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Castro, A., Ailion, M., Jalles, A., Brignull, H. R., Vilaça, J. L., Dias, N., Rodrigues, P., Oliveira, J. F., Neves-Carvalho, A., Morimoto, R. I.et al. (2011). Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum. Mol. Genet. 20, 2996-3009. 10.1093/hmg/ddr203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Castro, A., Jalles, A., Esteves, S., Kang, S., da Silva Santos, L., Silva-Fernandes, A., Neto, M. F., Brielmann, R. M., Bessa, C., Duarte-Silva, S.et al. (2015). Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain 138, 3221-3237. 10.1093/brain/awv262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach, W. T. (1968). Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J. Neurophysiol. 31, 785-797. 10.1152/jn.1968.31.5.785 [DOI] [PubMed] [Google Scholar]
- Todorov, B., Kros, L., Shyti, R., Plak, P., Haasdijk, E. D., Raike, R. S., Frants, R. R., Hess, E. J., Hoebeek, F. E., De Zeeuw, C. I.et al. (2012). Purkinje cell-specific ablation of Cav2.1 channels is sufficient to cause cerebellar ataxia in mice. Cerebellum 11, 246-258. 10.1007/s12311-011-0302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano Márquez, B., Cook, A. A., Rice, M., Smileski, A., Vieira-Lomasney, K., Charron, F., McKinney, R. A. and Watt, A. J. (2021). Molecular identity and location influence Purkinje cell vulnerability in autosomal-recessive spastic ataxia of charlevoix-saguenay mice. Front. Cell Neurosci. 15, 707857. 10.3389/fncel.2021.707857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, J. T., Alviña, K., Womack, M. D., Chevez, C. and Khodakhah, K. (2006). Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 9, 389-397. 10.1038/nn1648 [DOI] [PubMed] [Google Scholar]
- Wang, P.-S., Wu, Y.-T., Wang, T.-Y., Wu, H.-M., Soong, B.-W. and Jao, C.-W. (2020). Supratentorial and infratentorial lesions in spinocerebellar ataxia type 3. Front. Neurol. 11, 124. 10.3389/fneur.2020.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz, C. J. C., Raike, R. S., Soria-Jasso, L. E. and Hess, E. J. (2005). Potassium channel blockers inhibit the triggers of attacks in the calcium channel mouse mutant tottering. J. Neurosci. 25, 4141-4145. 10.1523/JNEUROSCI.0098-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, B. T. and Schaumburg, H. H. (1972). Nigro-spino-dentatal degeneration with nuclear ophthalmoplegia. A unique and partially treatable clinico-pathological entity. J. Neurol. Sci. 17, 149-166. 10.1016/0022-510X(72)90137-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.