Abstract
Helicobacter pylori urease, a nickel-requiring metalloenzyme, hydrolyzes urea to NH3 and CO2. We sought to identify H. pylori genes that modulate urease activity by constructing pHP8080, a plasmid which encodes both H. pylori urease and the NixA nickel transporter. Escherichia coli SE5000 and DH5α transformed with pHP8080 resulted in a high-level urease producer and a low-level urease producer, respectively. An H. pylori DNA library was cotransformed into SE5000 (pHP8080) and DH5α (pHP8080) and was screened for cotransformants expressing either lowered or heightened urease activity, respectively. Among the clones carrying urease-enhancing factors, 21 of 23 contained hp0548, a gene that potentially encodes a DNA helicase found within the cag pathogenicity island, and hp0511, a gene that potentially encodes a lipoprotein. Each of these genes, when subcloned, conferred a urease-enhancing activity in E. coli (pHP8080) compared with the vector control. Among clones carrying urease-decreasing factors, 11 of 13 clones contained the flbA (also known as flhA) flagellar biosynthesis/regulatory gene (hp1041), an lcrD homolog. The LcrD protein family is involved in type III secretion and flagellar secretion in pathogenic bacteria. Almost no urease activity was detected in E. coli (pHP8080) containing the subcloned flbA gene. Furthermore, there was significantly reduced synthesis of the urease structural subunits in E. coli (pHP8080) containing the flbA gene, as determined by Western blot analysis with UreA and UreB antiserum. Thus, flagellar biosynthesis and urease activity may be linked in H. pylori. These results suggest that H. pylori genes may modulate urease activity.
Helicobacter pylori, a gram-negative, microaerophilic, motile, spiral-shaped bacterium, has been established as the etiologic agent of chronic gastritis (22, 37, 38, 60). Chronic infection with H. pylori results in gastric and duodenal ulcers (6, 22, 38) and is a risk factor for gastric adenocarcinoma (47). Isolates of H. pylori that contain the cag pathogenicity island may be involved in more severe disease (9).
Urease (urea amidohydrolase [EC 3.5.1.5]), produced in abundance by H. pylori, is central to the pathogenesis of H. pylori infection and disease, as evidenced by the failure of urease-negative mutants to colonize mice and gnotobiotic piglets (12, 13) (reviewed in references 38a and 42). The protein, comprised of six copies each of two structural subunits, UreA and UreB, is a nickel-requiring metalloenzyme that hydrolyzes urea to ammonia and carbon dioxide (reviewed in references 38a, 42, and 44). Urease-generated ammonia neutralizes gastric acid (22), causes damage to gastric epithelial cells (56), and is assimilated into proteins by synthesis of glutamine from ammonia and glutamate catalyzed by glutamine synthetase (19) or by synthesis of glutamate from ammonia and α-ketoglutarate catalyzed by glutamate dehydrogenase (16).
The nickel ions required for urease activity are transported into H. pylori by a high-affinity cytoplasmic membrane nickel transporter protein, NixA, encoded by the nixA gene (43). The nickel ions are incorporated into apourease, presumably by the urease accessory proteins (UreE, UreF, UreG, and UreH), to yield the catalytically active holoenzyme. A detailed structure-function analysis of nixA and NixA has been recently reported (17). The nixA gene was isolated by its ability to enhance urease activity in Escherichia coli carrying pHP808 (43), a plasmid that contains genes that encode the urease structural subunits and accessory proteins from H. pylori (28, 30). nixA mutants of H. pylori have reduced nickel transport and urease activity compared with the wild-type strain, thus confirming that nixA is a urease-enhancing factor (UEF) (5, 43). The nixA mutant of H. pylori still retained some urease activity (58% of that of the wild type) and nickel transport (30% of that of the wild type), suggesting that additional mechanisms of nickel transport may exist in H. pylori.
In contrast with other bacterial urease gene clusters (44), there do not appear to be any known regulatory signals for H. pylori urease, such as induction by urea for Proteus mirabilis urease (33) or induction by low nitrogen concentrations for Klebsiella pneumoniae urease (45). Thus, it has been hypothesized that H. pylori urease is constitutively expressed (16, 30). However, H. pylori urease can account for up to 10% of the total cellular protein (4, 29), a huge energy expenditure for this fastidious organism. Since the gastric mucosal lumen has a pH of 2 and the pH approaches neutrality at the gastric epithelial cell surface to which H. pylori adheres (51), it is conceivable that high levels of urease activity are not necessary during every stage of H. pylori infection (42). However, the regulatory signals for controlling urease levels have not yet been uncovered.
Previously it was observed that, when grown in minimal medium supplemented with 1 μM NiCl2, E. coli containing the urease gene cluster on pHP808 failed to produce urease activity due to the inability to transport sufficient nickel ions for incorporation into apourease (43). Indeed, it has been very difficult to obtain high-level urease activity in E. coli (pHP808) under any growth condition. Urease activity was restored to E. coli (pHP808) only when it was cotransformed with the nixA-containing plasmid (43). Thus, we hypothesized that additional UEFs, as well as potential urease-decreasing factors (UDFs), could be isolated by screening an H. pylori DNA library in E. coli carrying pHP8080, a single plasmid that encodes both urease and NixA and is capable of generating urease activity in E. coli. To our knowledge, no one has reported a study designed to identify UDFs and UEFs from any urease-positive organism. To that end, we constructed pHP8080 and screened an H. pylori library for cotransformants containing potential UEFs or UDFs. Herein, we provide evidence that several H. pylori genes, in addition to nixA, modulate urease activity in E. coli. We isolated several candidate UEFs (hp0511, a gene that potentially encodes a lipoprotein, and hp0548, a gene that potentially encodes a DNA helicase found within the cag pathogenicity island) and a candidate UDF (hp1041, the flbA flagellar biosynthesis/regulatory gene [also known as flhA]).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
H. pylori 26695 was kindly provided by Kate A. Eaton (Ohio State University, Columbus). H. pylori ATCC 43504 was obtained from the American Type Culture Collection (Rockville, Md.). H. pylori was grown on Brucella agar (Becton Dickinson, Cockeysville, Md.) with 10% (wt/vol) defibrinated pooled sheep blood (Waltz Farm, Smithsburg, Md.) in a microaerobic environment by using the CampyPak Plus system (Becton Dickinson). E. coli DH5α [F− supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] (Clontech, Palo Alto, Calif.), SE5000 [F− araD193 Δ(argF lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR recA56] (20), XL1-Blue MRF′ (thi-1 gyrA96 relA1 recA1 endA1 supE44 hsdR17 lac F′ [proAB lacIqZΔM15 Tn10]) (Stratagene, La Jolla, Calif.), and SURE (thi-1 gyrA96 relA1 recJ recB endA1 sbcC lac Δ[mcrCB-hsdMR-mrr]177 uvrC umu::Tn5 F′ [(proAB lacIqZΔM15 Tn10]) (Clontech) were grown in Luria (L) broth and in Luria agar plus appropriate antibiotics (ampicillin [100 μg/ml], chloramphenicol [20 μg/ml], tetracycline [10 μg/ml], and/or kanamycin [50 μg/ml]) for maintenance of plasmids.
For preparation of urease extracts, strains were grown overnight in M9 minimal medium–1× M9 salts (per liter, 6 g of Na2HPO4, 3 g of KH2PO4, 0.5 g of NaCl, 1 g of NH4Cl), 0.4% glucose, 1 mM MgSO4, 0.1 mM CaCl2, 1.68 μM thiamine-HCl, 0.5% Casamino Acids, plus 1% (vol/vol) L broth, 1 μM NiCl2, and appropriate antibiotics.
Initial screening of UEFs and UDFs was achieved by using modified urea segregation agar, which contained the following per 900 ml: 4 g of yeast extract, 4 g of peptone, 0.34 g of NaH2PO4, 1.03 g of Na2HPO4 · 7H2O, 1 g of gelatin, 5 g of NaCl, 0.90 g of KH2PO4, 1.10 g of K2HPO4, and 15 g of agar. Following autoclaving, a 100-ml filter-sterilized solution of glucose (9 g), urea (6 g), phenol red (0.035 g), 10 μM NiCl2, and appropriate antibiotics was added. Urea segregation agar medium was poured either into standard petri plates or into 96-well microtiter plates (200 μl per well) (Nunc, Roskilde, Denmark) covered with Parafilm. The pH of uninoculated medium was adjusted so that the color was yellow to light orange (∼pH 6.9).
Molecular biology protocols.
Standard molecular biology techniques were used as described (3, 52).
Construction of pHP8080.
To construct pHP8080 (see Fig. 1), the nixA gene and flanking regions (corresponding to nucleotides 1,238 to 2,495 of the sequence with GenBank accession no. Z48742) were PCR amplified from pUEF201 (43), gel purified (Qiaquick Gel Extraction kit; Qiagen, Inc., Valencia, Calif.), and cloned into the NruI and AvaI sites of pHP808, a pACYC184 derivative which carries the urease gene cluster from H. pylori (28, 30). The following primers were used: NixADM-F1 (5′-GCGTCGCGAGCCTTTTTACACCATTCTCC-3′; NruI restriction endonuclease site is underlined), and NixADM-R1 (5′-GGCCTCGAGGCCAAGTTTTTCAAATCAAA-3′; AvaI site is underlined). The PCR conditions were as follows: 94°C for 5 min (first cycle only) and 94°C denaturation for 1 min, 50°C annealing for 90 s, and 72°C annealing for 2 min for a total of 30 cycles, followed by a 5-min extension at 72°C. PCR primers were designed by using a world wide web site (9a) and synthesized on an Applied Biosystems Oligonucleotide Synthesizer by the Biopolymer Core Facility at the University of Maryland, Baltimore. PCRs were carried out on a ThermoCycler (model PTC 150; MJ Research, Waterford, Mass.) by using Pfu DNA polymerase (Stratagene). This construct was confirmed by restriction analyses, by nixA-specific PCR (data not shown), and by qualitative and quantitative determination of the urease activity (see below) in comparison with that of the same E. coli strain carrying pHP808 alone.
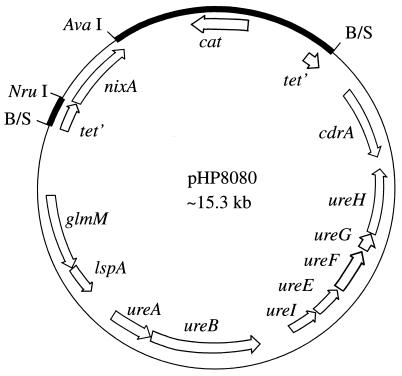
FIG. 1.
Diagram of pHP8080. pHP8080 is a derivative of pHP808 (28, 30); pHP808, a pACYC184 derivative, contains the H. pylori urease gene cluster (ureABIEFGH) cloned into the BamHI site through a Sau3AI partial digestion of H. pylori 43504 genomic DNA (restriction site designated as B/S). The nixA nickel transporter gene was PCR amplified from pUEF201 (43) and cloned into the NruI and AvaI sites in pHP808, which removed the 3′ end of the tetracycline resistance marker. glmM (11) and lspA (58) refer to genes previously designated as ureC and ureD, respectively (10, 35) and are not required for urease activity (10, 44). Thick lines, pACYC184 vector sequences; cat, chloramphenicol resistance marker; tet′, truncated tetracycline resistance marker. Arrows show directions of transcription.
Construction of H. pylori ATCC 43504 library.
Sau3AI partially digested and blunt-ended chromosomal DNA (average size, 6 kb) from H. pylori ATCC 43504 was used for commercial preparation of a λ-ZAPII genomic library (EcoRV site used) (Stratagene) (43). The λ-ZAPII genomic library phage suspension (containing 107 phage particles) was incubated with E. coli XL1-Blue MRF′ (adjusted to optical density at 600 nm [OD600] of 1.0) and 106 ExAssist helper phage particles for 15 min at 37°C. The suspension was then added to 20 ml of L broth and incubated at 37°C for 2 h and then heated at 65°C for 20 min. The suspension was centrifuged (2,500 × g; 10 min, 4°C), and the supernatant was added to a suspension (OD600 = 1.0) of E. coli SURE and incubated at 37°C for 15 min. After incubation, 100 μl was plated onto L agar plates containing ampicillin (200 μg/ml) and incubated for 18 h at 37°C (24). Colonies from 20 plates were pooled and used for a large-scale plasmid (pBluescript library) preparation by column chromatography (Qiagen Midi-Tip 100).
Urease activity determinations on urea segregation agar.
Urease activities of E. coli cotransformants carrying pHP8080 plus the pBluescript library were determined by screening for colonies, grown overnight at 37°C, that changed the medium pH of modified urea segregation agar. As the pH of urea segregation agar rises, presumably due to urease-generated ammonia, the medium changes color from yellow or light orange to red. Cotransformants that were qualitatively confirmed to show enhanced or decreased urease activity were quantitatively assayed for urease activity (see below). E. coli SE5000 (pHP808/pBluescript) and SE5000 (pHP808/pUEF201) served as negative and positive controls, respectively (data not shown).
Urease extract preparations and protein determinations.
E. coli (pHP8080/library) cotransformants were each grown from a single colony in 1.5 ml of M9 minimal medium overnight at 37°C. Bacteria were harvested by centrifugation (12,000 × g; 2 min, 4°C) and washed twice with 50 mM HEPES buffer (pH 7.5). Bacteria were bath sonicated (40% intensity, three pulses for 30 s each) (Cell Disruptor; Ultrasonics, Inc.) on ice to release cytosolic proteins, including urease; >99% of the bacteria were lysed by this method. Following centrifugation (12,000 × g, 2 min, 4°C), supernatants were placed on ice. The pellet fraction (cell debris) was retained for some experiments. The extracts retained the same urease activity after multiple freeze-thaw cycles. Protein determinations of the extracts were conducted by the bicinchoninic acid assay method (Pierce Chemical Company, Rockford, Ill.), according to the manufacturer’s 30-min protocol. Bovine serum albumin was used as the standard.
Urease activity determinations by the phenol-hypochlorite urease assay.
Urease activity of extracts was determined by measuring the amount of ammonia released from urea in the phenol-hypochlorite urease assay (61). Extracts were added to urease buffer (50 mM HEPES [pH 7.5] plus 25 mM urea) in a 1-ml final volume and were incubated at 37°C for 20 min. The reaction was stopped by removal of an aliquot and addition to a cuvette containing 1.5 ml of solution A (containing [per liter] 10 g of phenol and 50 mg of sodium nitroprusside). An equal volume (1.5 ml) of solution B (NaOH [5 mg/ml]–NaClO [0.044%, vol/vol]) was added, and the contents were mixed well. Following incubation at 37°C for 30 min, the absorbance at 625 nm was measured in a spectrophotometer (Bio-Spec 1601; Shimadzu Scientific Instruments, Inc., Columbia, Md.). A standard ammonium chloride concentration curve was determined to be linear over the range from 10 to 300 nmol of ammonia. A second standard curve, one for jack bean urease (Sigma Chemical Co., St. Louis, Mo.), was shown to be linear with respect to time and amount of urease added (data not shown). Absorbance values were converted to nanomoles of ammonia based on the ammonium chloride standard curve. Data are presented as urease specific activity, defined as nanomoles of NH3/minute/milligram of protein. E. coli SE5000 (pHP808/pBluescript), which lacks nixA, served as the negative control, and the background activity (<10 nmol of NH3/minute/milligram of protein) was subtracted from all values obtained to avoid measuring ammonia generated by urease-independent mechanisms. Statistical analysis of the data was conducted by the alternative Welch’s t test by using InStat 2.03 software (GraphPad Software, San Diego, Calif.).
DNA sequencing and software analysis of the nucleotide sequence.
Plasmids were sequenced by the dideoxy chain termination method (54) by using an Applied Biosystems 373A automated DNA sequencer with the Big Dye Terminator Cycle Sequencing Kit at the University of Maryland, Baltimore, Biopolymer Core Facility, with the T3 and T7 oligonucleotide primers and, when necessary, additional primers based on the nucleotide sequence of the insert. The following software programs were used to analyze and manipulate the sequence: DNASIS version 2.1 (Hitachi Software Engineering Company) and molecular biology world wide web sites (9b, 30a, 30b, 45a, 45b, 45c, 47a, 57a, 57b).
Subcloning of flbA.
The flbA gene plus flanking DNA, corresponding to nucleotides 2,001 to 4,430 in pUDF104 (see the Results section), was subcloned by PCR amplification by using Vent DNA polymerase (New England Biolabs, Beverly, Mass.) and the following primers: FlhA-F1, 5′-GCGCGGATCCGTGGCAAACGCCTTAATGAT-3′ (BamHI restriction endonuclease site is underlined); and FlhA-R1, 5′-GCGCATCGATTGGTAAACTTGCATCATTCTCC-3′ (ClaI site is underlined). The following conditions were used: 94°C for 5 min (first cycle only) and 94°C for 90 s, 50°C for 3 min, and 72°C for 5 min for a total of 30 cycles, followed by a 5-min extension at 72°C. The product of the expected size (2,450 bp) was gel purified, simultaneously digested with BamHI and ClaI, and directionally ligated into pBluescript to yield pBS-flbA. The constructs were confirmed by restriction analysis and by PCR.
Subclones of pUEF1004.
Open reading frames (ORFs) hp0511 (nucleotides 721 to 1,135) and hp0548 (nucleotides 1,360 to 2,409) on pUEF1004 (see Results section) were subcloned by PCR amplification by using Vent DNA polymerase and the following primers: Hel-F1, 5′-GCGCGAATTCCCCTATGATTAGGGACACAGAG-3′ (EcoRI site is underlined); Hel-R1, 5′-GCGCGGATCCTGCAATTTAGGAGCGTTTTG-3′ (BamHI site is underlined); Orf511-F1, 5′-GCGCGGATCCTCTAATTCAAGGAGCCTAACTAAAA-3′ (BamHI site is underlined); and Orf511-R1, 5′-GCGCGAATTCAACCCAATATCAGTTTGATTGC-3′ (EcoRI site is underlined). The following conditions were used: 94°C for 5 min (first cycle only) and 94°C for 60 s, 63°C for 30 s, and 72°C for 2 min for a total of 30 cycles, followed by a 5-min extension at 72°C. The PCR products of the expected sizes (∼415 bp for hp0511 and ∼1,050 bp for hp0548) were gel purified, simultaneously digested with EcoRI and BamHI, and ligated into pBluescript to yield pUEF1004-548 and pUEF1004-511, respectively. To construct pUEF1004-548ΔK, the 339-bp KpnI fragment (including the ∼295-bp 5′ end of hp0548) was removed from pUEF1004-548 and religated. Subclones were confirmed by PCR, restriction, and sequence analyses.
SDS-PAGE and Western blot analysis of urease extracts.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted by the method of Laemmli (36), using a 12% resolving gel and 10 μg of protein loaded per lane. Proteins were transferred to Immobilin-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, Mass.) by using a Bio-Rad Trans-Blot cell transfer apparatus (Bio-Rad, Hercules, Calif.). The membrane was blocked by using 5% nonfat dry milk and washed with Tris-buffered saline containing 0.05% Tween 20 (TBST) (3). Primary antibodies were anti-UreA or anti-UreB (1:100,000 to 1:200,000 dilution), obtained as described previously (30). The secondary antibody was goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma Immunochemical Co.) (1:1,500 to 1:3,000 dilution). Blots were developed by using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate according to the manufacturer’s instructions (Life Technologies). Extracts from E. coli DH5α (pBluescript/pACYC184) and E. coli SE5000 (pBluescript/pACYC184) were used as negative controls. Blots were quantified by using Eagle Eye II software (Stratagene).
Nucleotide sequence accession number.
The 4,582-bp nucleotide sequence of pUDF104 and the 2,693-bp nucleotide sequence of pUEF1004 have been deposited in GenBank under accession no. AF125197 and AF125214, respectively.
RESULTS
E. coli (pHP8080/pBluescript) has urease activity.
Previously, it was observed that E. coli (pHP808), which contains the H. pylori urease gene cluster, had no urease activity when the strain was grown in low nickel concentrations (1 μM); urease activity was obtained when E. coli (pHP808) was cotransformed with the nixA-containing plasmid, pUEF201, thus confirming that nixA is a UEF (5, 43). Further screening of an H. pylori library for other UEFs in E. coli (pHP808) failed to reproducibly yield additional cotransformants with altered urease activity (data not shown), suggesting that it is not possible to obtain additional UEFs unless a gene encoding a high-affinity nickel transporter is also present.
To determine whether we could obtain urease activity from E. coli carrying a single plasmid, we constructed pHP8080, a plasmid that encodes both H. pylori urease and the NixA nickel transporter (Fig. 1; see Materials and Methods section). We observed that E. coli SE5000 (pHP8080/pBluescript) was urease positive on urea segregation agar after overnight incubation (data not shown), as evidenced by a color change of urea segregation agar to red. In contrast, DH5α (pHP8080/pBluescript) failed to produce urease activity on urea segregation agar medium, even after 2 days of incubation at 37°C (data not shown). Urease assays by the phenol-hypochlorite method detected higher urease activity from SE5000 (pHP8080/pBluescript) than from DH5α (pHP8080/pBluescript) (Tables 1 and 2). As a negative control, SE5000 (pHP808/pBluescript), which lacks nixA, had no detectable urease activity (<10 nmol/min/mg). These findings indicate that pHP8080 is sufficient for urease production in E. coli. For comparison, note that the urease activity of H. pylori 43504 averages 30,000 ± 2,000 nmol of ammonia/min/mg of protein.
TABLE 1.
Summary of UEFs in DH5α (pHP8080)
| Plasmid name | Insert size (kb)a | Candidate gene(s) | Mean urease activity ± SDb | nc | P valued | No. of similar clones |
|---|---|---|---|---|---|---|
| pBS | None | None | 778 ± 628 | 33 | NAe | NA |
| pUEF1004 | 2.7 | helAB, lpp | 6,518 ± 2,993 | 16 | <0.0001 | 21f |
| pUEF1004-511 | 0.4 | lpp | 2,505 ± 428 | 8 | 0.0003 | NA |
| pUEF1004-548 | 1.1 | helAB | 2,598 ± 838 | 9 | <0.0001 | NA |
| pUEF1004-548ΔK | 0.7 | helB | 4,161 ± 816 | 7 | 0.0001 | NA |
| pUEF1014 | 7.1 | hp0839–0846 | 2,917 ± 2,000 | 7 | 0.0335 | 1 |
| pUEF1023w | 8.4 | hp1400–1403 | 10,236 ± 4,449 | 10 | <0.0001 | 1 |
Size was determined based on the results of restriction analysis, PCR, and sequencing.
Urease activity in DH5α (pHP8080) plus each of the plasmids listed. Data are expressed as specific activity (nanomoles of NH3/minute/milligram of protein) (mean ± standard deviation).
n, number of experiments.
P value for urease activity as compared with urease activity from DH5α (pHP8080/pBluescript).
NA, not applicable.
Other clones determined to contain helAB (hp0548) and lpp (hp0511) based on the results of restriction analysis, PCR, and sequencing were as follows: pUEF1002, -1005, -1006, -1007, -1008, -1010, -1012, -1015, -1018, -1020, -1026, -1027, -1028, -1030, -1031, -1033, -1034, -1035, -1036, and -1038. Insert sizes varied from 2.7 to 12.3 kb.
TABLE 2.
Summary of UDFs in SE5000 (pHP8080)
| Plasmid, extract | Insert size (kb)a | Candidate gene(s) | Mean urease activity ± SDb | nc | P valued | No. of similar clonese |
|---|---|---|---|---|---|---|
| pBS, cytosolicf | None | None | 1,542 ± 786 | 41 | NAg | NA |
| pBS, pellet | None | None | 409 ± 203 | 5 | NA | NA |
| pBS, supernatant | None | None | 0 ± 0 | 3 | NA | NA |
| pUDF104, cytosolic | 4.7 | hp1038–1042 | 450 ± 219 | 10 | <0.0001 | 11 |
| pBS-flbA, cytosolic | 2.4 | flbA | 95 ± 73 | 11 | <0.0001 | NA |
| pBS-flbA, pellet | 2.4 | flbA | 27 ± 19 | 5 | <0.0001 | NA |
| pBS-flbA, supernatant | 2.4 | flbA | 0 ± 0 | 3 | <0.0001 | NA |
| pUDF140, cytosolic | 4.0 | hp554–558 | 0 ± 0 | 8 | <0.0001 | 1 |
Size was determined based on the results of restriction analysis, PCR, and sequencing.
Urease activity in SE5000 (pHP8080) plus each of the plasmids listed. Data are expressed as specific activity (nanomoles of NH3/minute/milligram of protein) (mean ± standard deviation).
n, number of experiments.
P value for urease activity as compared with urease activity from SE5000 (pHP8080/pBluescript).
Other clones determined to contain flbA (hp1041) based on the results of restriction analysis, PCR, and sequencing were as follows: pUDF109, -119, -137, -138, -141, -145, -149, -153, -156, and -158.
Cytosolic, pellet, and supernatant refer to crude extracts of the cytosol, the cell debris, and the extracellular supernatant, respectively.
NA, not applicable.
Urease activities of pUEF- and pUDF-containing cotransformants on urea segregation agar.
To investigate genes that modulate urease activity in E. coli in addition to nixA, we transformed the low-level urease-producing E. coli strain DH5α (pHP8080) and the high-level urease-producing E. coli strain SE5000 (pHP8080) with a pBluescript library of H. pylori. Cotransformants were screened on urea segregation agar for enhanced urease activity or decreased urease activity, respectively. Among ∼5,700 DH5α (pHP8080/library) cotransformants screened, 30 isolates (0.5%) turned urea segregation agar red after overnight growth (these are designated as pUEFs). Among ∼1,400 SE5000 (pHP8080/library) cotransformants screened, 34 isolates (2%) showed delay in their ability to turn urea segregation agar red (these are designated as pUDFs), relative to that of the control, SE5000 (pHP8080/pBluescript) (data not shown).
Urease activities of cotransformants containing pUEFs and pUDFs determined by the phenol-hypochlorite urease assay.
Urease activity was quantified for all pUDF and pUEF E. coli (pHP8080) cotransformants. Of the 30 potential UEFs obtained, 23 DH5α (pHP8080/pUEF) library cotransformants (77%) had urease specific activities 3- to 13-fold higher than that of the control, DH5α (pHP8080/pBluescript) (Table 1; representative examples are shown). Indeed, most of the UEFs were so active that the extracts had to be diluted 1:10 to avoid saturating the assay. The majority of the pUEFs (21 of 23 isolates) had common restriction fragments (XbaI and HindIII double digest). There were also two unique restriction patterns observed (pUEF1014 and pUEF1023w) (Table 1).
Of the 34 potential UDFs obtained, 13 SE5000 (pHP8080/pUDF) library cotransformants (38%) had urease specific activities 3- to 750-fold lower than that of the control, SE5000 (pHP8080/pBluescript) (Table 2; representative examples are shown). The majority of the pUDFs (11 of 13 isolates) had common restriction patterns (XbaI and HindIII double digest). There were also two unique restriction patterns (pUDF123 and pUDF140). These results confirmed that H. pylori genes could modulate urease activity in E. coli (pHP8080). Furthermore, only a few genes are potentially involved in this modulation.
To minimize artifacts, we isolated plasmid DNA from con-firmed DH5α (pHP8080/pUEFs) and SE5000 (pHP8080/ pUDFs) and electroporated the plasmids into both DH5α and SE5000. Ampicillin-resistant, chloramphenicol-susceptible colonies (i.e., colonies of isolates lacking pHP8080) were retransformed with pHP8080 and reconfirmed by the phenol-hypochlorite urease assay. Thus, E. coli (pHP8080/pUEFs) or E. coli (pHP8080/pUDFs) had a urease-enhancing or urease-decreasing phenotype, respectively, regardless of the strain of E. coli used (data not shown). The only exception to this was pUDF123, which had no urease activity due to an E. coli chromosomal mutation in the original isolate (data not shown). This clone was not analyzed further.
Sequence analysis of pUDFs.
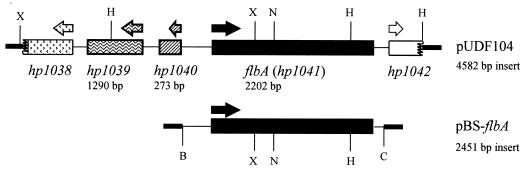
Of the 11 pUDFs that gave similar restriction enzyme patterns, we chose pUDF104 for further analysis. Sequence analysis of both DNA strands of the 4,582-bp insert of pUDF104 revealed three complete ORFs (Fig. 2, top), corresponding to hp1039, hp1040, and hp1041 in sections 89 and 90 of the complete genome sequence of H. pylori (58) (corresponding to nucleotides 8,634 to 10,782 and 59 to 2,497 of the sequences with accession no. AE000611 and AE000612, respectively). The flanking regions of pUDF104 consisted of truncated hp1038 at the left junction (T3 primer of pBluescript) and hp1042 at the right junction (T7 primer of pBluescript). Since hp1039 encodes a predicted hypothetical protein and hp1040 encodes a predicted small ribosomal protein (RpsL5), we focused our attention on hp1041, which contains the flbA gene (2,202 bp). The FlbA protein is involved in secretion, assembly, and regulation of the expression of flagellar proteins in H. pylori (55). There is a predicted ς28 promoter (consensus sequence is TAAAN15GCCGAT(A/T); flbA sequence is TAAGN14ACCGAAAT), upstream of the flbA gene.
FIG. 2.
Schematic maps of pUDF104 and pBS-flbA. The numbering of the ORFs of the genes in pUDF104 is based on the system developed by Tomb et al. (58). The size of each complete gene and the size of the total insert are shown. The flbA gene plus flanking DNA was subcloned into the BamHI (B) and ClaI (C) sites in pBluescript by PCR as described in the Materials and Methods section to yield pBS-flbA. Truncated genes are denoted by jagged edges. H, HindIII; X, XbaI; N, NheI. Thick lines, pBluescript vector sequences. Arrows show directions of transcription.
The flbA gene encodes an 80.9-kDa cytoplasmic membrane protein (FlbA) that has seven predicted transmembrane domains within the amino-terminal half of the protein (55). The carboxy-terminal half of FlbA is hydrophilic and is likely located in the cytosol. There is no apparent signal sequence in FlbA. Comparison of the H. pylori FlbA predicted amino acid sequence with the FlbA and FlhA proteins of other strains of H. pylori (15, 55, 58) revealed a high degree of sequence conservation (>98% identity). The H. pylori FlbA was most similar with the FlbA protein from Campylobacter jejuni (55) (51.4% identity and 80.2% similarity).
The LcrD family is comprised of two subfamilies: FlhA and LcrD. H. pylori FlbA has a higher degree of amino acid homology with the FlhA subfamily (range, 34.6 to 51.4% amino acid sequence identity and 68.3 to 80.2% amino acid sequence similarity) than with the LcrD subfamily (range, 29.1 to 34.8% identity). At 733 amino acids, the H. pylori FlbA is the largest member of the FlhA subfamily.
The vector-insert junctions of four additional pUDFs were sequenced. pUDF109 was found to have sequence identical to that of pUDF104. Similarly, pUDF119 and pUDF141 were sequenced across the vector-insert junctions and found to have the same insert as pUDF104, except that both had the insert in the opposite orientation. Plasmid pUDF140, which has a unique restriction pattern, was sequenced across the junctions and was found to contain truncated genes hp0554 and hp0558. Based on the size of the insert (4.0 kb) and the similar size of this region in the H. pylori genome, this clone probably contains genes hp0554 to hp0558. This clone will not be discussed further in this study.
flbA is a UDF.
Since the flbA gene appeared to be the best candidate gene among the three complete predicted ORFs in pUDF104, we subcloned flbA (hp1041) by PCR (see Materials and Methods section) to yield pBS-flbA (Fig. 2, bottom). When pBS-flbA was cotransformed into SE5000 with pHP8080, urease specific activity was reduced by 15-fold in comparison with that of the control, SE5000 (pBluescript/pHP8080) (Table 2; see data for cytosolic fraction). These results confirm that flbA is a UDF in E. coli (pHP8080). Except for pUDF123 and pUDF140, all the other pUDFs were positive for the 2.4-kb flbA gene by PCR or by inference based on restriction analysis.
Given the potential role of FlbA as a component of the flagellar secretion apparatus (55), we investigated whether urease activity is directed to a different cellular compartment using crude extracts of the cytosol, the resulting pellet cell debris, or extracellular supernatants. We found that negligible urease activity was found in the pellets of E. coli (pBS-flbA/pHP8080) (Table 2), and no urease activity was found in extracellular supernatants of either E. coli (pBS-flbA/pHP8080) or E. coli (pBS/pHP8080). These results suggest that FlbA does not change the location of urease activity to another cellular compartment. Additionally, about 75% of the total urease activity is found in cytosolic extracts [compare data for cytosolic and pellet fractions of E. coli (pBS/pHP8080) given in Table 2]).
Sequence analysis of UEFs.
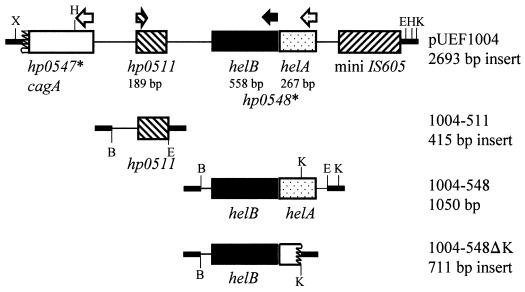
One confirmed UEF-bearing plasmid, pUEF1004, was sequenced and contained the genes encoding a potential lipoprotein (hp0511; lpp) (58), a potential DNA helicase (hp0548), and a portion of cagA, as well as a fragment of IS605 (Fig. 3). hp0511 encodes a predicted 62-amino-acid polypeptide that is larger than the corresponding protein in H. pylori 26695, which is only 38 amino acids in length (58). hp0511 or Hp0511 has no homology at the nucleotide or amino acid level with any other gene or protein in the database. The cagA gene and hp0548 are found near the right junction of the cag pathogenicity island (1, 9, 58). In all cag pathogenicity islands investigated to date, the hp0548 gene has a frameshift, presumably resulting in a nonfunctional protein (1, 9, 58). However, in the case of H. pylori 43504, used in this study, hp0548 was not frameshifted but instead had a stop codon about one third of the way into the gene. Further analysis of hp0548 suggested the potential for two ORFs, designated here as helA and helB.
FIG. 3.
Schematic maps of pUEF1004 and subclones. Subclones were constructed as described in the Materials and Methods section. E, EcoRI; K, KpnI; B, BamHI. ∗, genes in the cag pathogenicity island. Other abbreviations and notations are as described in the legend to Fig. 2.
Clones pUEF1010, pUEF1012, and pUEF1038 were sequenced and found to also contain hp0548 and hp0511 (Table 1). Except for clones pUEF1014 and pUEF1023w, all the remaining UEF-containing clones were positive for both hp0548 and hp0511 by PCR or by inference based on restriction analysis (Table 1).
Clones pUEF1014 and pUEF1023w were sequenced across the vector-insert junctions and were found to contain H. pylori genes hp0839 through hp0846 and hp1400 through hp1403, respectively (Table 1), and will not be discussed further in this study.
helB and lpp are UEFs.
To determine which gene(s) in pUEF1004 was responsible for urease-enhancing activity, we subcloned the hp0511 (lpp) and hp0548 (helAB) genes (Fig. 3). Surprisingly, both genes separately conferred urease-enhancing activities (Table 1), although these activities were not as great as that of the clone that contains both genes (pUEF1004). When the 5′ end of the helA gene and the IS605 fragment were deleted (construct pUEF1004-548ΔK in Fig. 3), urease activity still remained elevated (Table 1), suggesting that helB is the gene responsible for the urease-enhancing activity.
Urease protein levels in extracts from E. coli (pHP8080) carrying pUDFs or pUEFs.
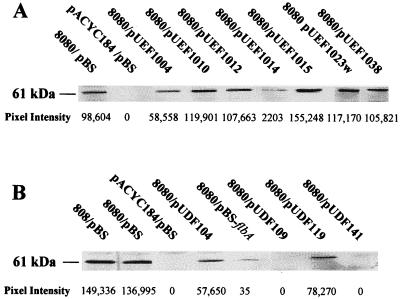
To determine whether genes on pUDFs or pUEFs affected the amounts of the urease structural proteins in E. coli (pHP8080), extracts from E. coli (pHP8080/pUDFs) or E. coli (pHP8080/pUEFs) were analyzed by SDS-PAGE and Western blot analysis by using anti-UreB antiserum. As expected, extracts from DH5α (pBluescript/pACYC184) and SE5000 (pBluescript/pACYC184), which have neither urease activity nor the urease genes, lacked the 61-kDa UreB polypeptide (large subunit of urease) as assayed by Western blotting (Fig. 4A and B). Also, SE5000 (pBluescript/pHP808), which has no detectable urease activity, was still able to synthesize UreB (Fig. 4A and B), as expected from previous studies in our laboratory (30). We found that extracts from DH5α (pHP8080/pUEFs) generally did not have an increase in the amount of UreB compared with the positive control, DH5α (pHP8080/pBluescript) (Fig. 4A). A similar finding was observed with extracts from subclones DH5α (pHP8080/pUEF1004-511) and DH5α (pHP8080/pUEF1004-548) (data not shown). In contrast, extracts from SE5000 (pHP8080/pUDFs) had a two- to threefold drop in the amount of UreB (for pUDF104 and pUDF119) or no detectable UreB (for pUDF109 and pUDF141) compared with the positive control, SE5000 (pHP8080/pBluescript) (Fig. 4B). Furthermore, extracts from SE5000 (pHP8080/pBS-flbA), which contains the subcloned flbA flagellar biosynthesis/regulatory gene and has greatly reduced urease specific activity (Table 1), had a dramatic drop in the amount of UreB (Fig. 4B). No UreB was detectable in pellets or culture supernatants of SE5000 (pHP8080/pBS-flbA) (data not shown). Comparable results for UEFs and UDFs were also obtained by using UreA (small subunit of urease)-specific antiserum (data not shown). These results suggest that flbA dramatically reduces the amounts of the urease structural subunits, UreA and UreB.
FIG. 4.
Western blot analysis of urease extracts by using anti-UreB. Cytosolic protein extracts (10 μg) were electrophoresed through an SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with anti-UreB as described in the Materials and Methods section. (A) Extracts from DH5α (pHP8080/pBluescript) and DH5α (pHP8080/pUEFs). (B) Extracts from SE5000 (pHP8080/pBluescript) and SE5000 (pHP8080/pUDFs). Density values are shown below individual blots. Results are representative of three experiments performed by using extracts prepared on 3 separate days. pBS, pBluescript; 808, pHP808; 8080, pHP8080.
DISCUSSION
Our study suggests that H. pylori may potentially modulate urease levels in the cell, in contrast with previous reports (16, 30). This is supported by our discovery of both UEFs and UDFs that alter urease activity in E. coli strains expressing both H. pylori urease and the NixA nickel transporter on plasmid pHP8080. E. coli (pHP8080) is capable of generating urease activity, in contrast with E. coli (pHP808), which expresses the H. pylori urease structural and accessory genes but lacks nixA and thus has urease that is catalytically inactive due to lack of nickel ions in the urease active site. This observation indicates that expression of both the NixA nickel transporter and the urease structural and accessory proteins is necessary for high-level urease activity in E. coli. Most other bacterial ureases have been successfully expressed in E. coli in the absence of a nickel transporter-containing plasmid (reviewed in reference 44). As E. coli has a low-affinity nickel transporter, NikD (46), H. pylori apourease may have a lower affinity for nickel than other bacterial apoureases, which may be compensated by the high-affinity nickel transporter, NixA. At low nickel concentrations or under nickel-chelating conditions, little or no urease activity was observed in E. coli (pHP808) lacking nixA (28, 30, 43).
We used the high-level urease-producing strain, SE5000 (pHP8080/pBluescript), to obtain UDFs, and the low-level urease-producing strain, DH5α (pHP8080/pBluescript), to obtain UEFs. The urease activity difference between these E. coli strains is probably due to uncharacterized genetic differences in strain backgrounds. Our ability to uncover UEFs and UDFs was greatly facilitated by using urea segregation agar as a screening tool. Many cotransformants that exhibited UEF or UDF activity by the urea segregation agar method were subsequently confirmed by the phenol-hypochlorite urease assay (77% of UEFs and 38% of UDFs). Since the urea segregation agar screen relies on a pH indicator, it is possible that some UEFs or UDFs not subsequently confirmed by the phenol-hypochlorite urease assay could contain gene products that alter the medium pH rather than modulate urease activity. Nevertheless, our results suggest that the use of urea segregation agar as a screening tool is a highly effective method to obtain UEFs and UDFs.
UEFs and UDFs may act on urease activity in a direct or indirect fashion. For example, UEFs or UDFs may act upon the nickel transporter gene or the corresponding protein, which would affect the amount of urease activity observed by increasing or decreasing the amount of nickel ions available for incorporation into apourease. Also, UEFs or UDFs could affect the expression of the urease structural or accessory genes or the corresponding proteins.
Of the 13 UDFs obtained, 11 contained the flbA flagellar biosynthesis/regulatory gene. The flbA gene is a UDF, since SE5000 (pHP8080) containing the subcloned flbA gene (pBS-flbA) has almost no detectable urease activity (Table 1). Furthermore, SE5000 (pHP8080/pBS-flbA) had almost no UreB (Fig. 4B) or UreA (data not shown) by Western blot analysis. That the amounts of both structural subunits are reduced is not surprising since ureA and ureB are likely cotranscribed from the same promoter (35). As UreA and UreB are each essential for urease activity, loss of either one of these subunits would result in failure to produce catalytically active urease (44). FlbA does not redirect urease activity or an inactive urease protein to another cellular compartment, as little urease activity was observed in other compartments in crude extracts of E. coli (pBS-flbA/pHP8080) (Table 1) and no urease protein was detectable in other cellular compartments (data not shown).
We propose four possibilities whereby flbA may cause a reduction in the urease activity. First, FlbA may increase the turnover of either the urease structural subunits UreA and UreB or the mRNA for ureA and ureB. Second, FlbA may repress transcription of the urease genes. However, there are no DNA-binding motifs or motifs suggestive of a two-component regulatory system in FlbA or in any member of the LcrD family (40, 53, 57). Thus, the protein could be acting as a signal transducer through another protein (2, 8, 18, 48, 50). Third, FlbA could titrate ς28 away from activation of the urease gene cluster; it is unclear which sigma factor transcribes the H. pylori urease gene cluster. Finally, FlbA may decrease assembly of the very large (550 kDa) urease apoenzyme. Future experiments will center on determining the mechanism behind the decreased urease activity and decreased amounts of urease structural subunits produced by FlbA.
A mutation of H. pylori in the flbA gene has pleiotropic effects (55), as is the case for other members of the LcrD family (8, 23, 49). The H. pylori flbA mutant is nonmotile, has no flagella, and has reduced synthesis of the products of the flaA, flaB, and flgE flagellar biosynthesis genes. Notably, the flbA mutant has slightly increased levels of urease activity measured by a semiquantitative method (55), which supports our finding that flbA is a UDF in E. coli.
The FlbA protein is homologous with members of the LcrD family which are involved in secretion, assembly, and/or regulation of the expression of virulence-related proteins, and are structural constituents of a secretion apparatus (reviewed in references 26 and 27). The LcrD family is comprised of two distinct but evolutionarily related subfamilies: LcrD and FlhA. The LcrD subfamily is involved in type III secretion of virulence-related proteins (e.g., LcrD of Yersinia spp., MxiA of Shigella flexneri, InvA of Salmonella spp., and SepA/EscV of enteropathogenic E. coli) (2, 18, 31, 48, 49, 59). There does not appear to be a type III secretion system in H. pylori (58). The FlhA subfamily is involved in secretion and external localization of flagellar proteins (8, 19, 23, 40, 41, 50, 53, 55).
We also observed, based upon restriction, PCR, and sequence analyses, that 21 of the 23 UEFs have the hp0511 and hp0548 genes in common. These genes are adjacent to each other on all of our clones and thus do not appear to be chromosomal fragments ligated together from separate parts of the genome. Instead, it is likely that H. pylori 43504 has a different gene order than strain 26695, as has been widely found in H. pylori (7, 32). An intriguing finding is that the gene encoding the putative DNA helicase, hp0548, is found in the cag pathogenicity island and, instead of being frameshifted (i.e., inactive) as is found for other H. pylori strains (1, 9, 58), appears to be two ORFs, helA and helB. Our data suggest that the gene responsible for the urease-enhancing activity is helB (Table 1). Future experiments will center on understanding the mechanism of how helB causes urease-enhancing activity and whether the helicase produced can regulate urease gene transcription.
Other H. pylori genes have been shown to affect urease activity. Specifically, an H. pylori ATP-binding cassette (ABC) transporter gene cluster, abcABCD, enhances urease activity; this activity is reduced by 88% in an H. pylori abcD mutant (25). A nixA abcC double mutant of H. pylori also has almost no urease activity. AbcABCD may be involved in energy-driven transport of nickel ions into the cell (25). Other gene products that appear to affect urease activity are a P-type ATPase (39), a heat shock protein A (HspA) (34), and histidine-rich protein Hpn (21).
In summary, we have successfully obtained urease activity in E. coli carrying a single plasmid, pHP8080, that expresses both H. pylori urease and the NixA nickel transporter. Using this plasmid, we found candidate genes, UEFs and UDFs, from H. pylori that can modulate urease activity in E. coli. One confirmed UDF is the flbA flagellar biosynthesis/regulatory gene, which dramatically decreases urease activity and reduces the amounts of urease structural subunits detected on Western blot analysis for E. coli (pHP8080/pBS-flbA). Thus, flagellar biosynthesis and urease activity may be linked in H. pylori. We also identified two confirmed UEFs, hp0511 and helB. helB is a potential component of a gene encoding a putative DNA helicase (hp0548) that is located in the cag pathogenicity island. Our findings should lead to a better understanding of the regulation of H. pylori urease. This may in turn lead to novel anti-urease therapies against H. pylori and other urease-producing bacteria.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Public Health Service grants AI25567 (to H.L.T.M.) and AI10098-01 (to D.J.M.).
We thank Christopher Coker and Susan R. Heimer for helpful suggestions and critical reading of the manuscript. We thank Kristen M. Ruley and Magdalene Spence for expert technical assistance and Lisa Sadzewicz for DNA sequence determinations.
REFERENCES
- 1.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews G P, Maurelli A T. mxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homolog of the low-calcium response protein, LcrD, of Yersinia pestis. Infect Immun. 1992;60:3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 4.Bauerfeind P, Garner R M, Dunn B E, Mobley H L T. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut. 1997;40:25–30. doi: 10.1136/gut.40.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauerfeind P, Garner R M, Mobley H L T. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect Immun. 1996;64:2877–2880. doi: 10.1128/iai.64.7.2877-2880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck G E, Gourley W K, Lee W K, Subramanyan J M. Relation of Campylobacter pyloridis to gastritis and peptic ulcers. J Infect Dis. 1986;153:664–669. doi: 10.1093/infdis/153.4.664. [DOI] [PubMed] [Google Scholar]
- 7.Bukanov N O, Berg D E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994;11:509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter P B, Ordal G W. Bacillus subtilis FlhA: a flagellar protein related to a new family of signal-transducing receptors. Mol Microbiol. 1993;7:735–743. doi: 10.1111/j.1365-2958.1993.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type-I specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Center for Genome Research. Primer 3. [Online.] http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi.
- 9b.Centre National de la Recherche Scientifique. Align query. [Online.] http://vega.igh.cnrs.fr/bin/align-guess.cgi.
- 10.Cussac V, Ferrero R L, Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992;174:2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Reuse H, Labigne A, Mengin-Lecreulx D. The Helicobacter pylori ureC gene codes for a phosphoglucosamine mutase. J Bacteriol. 1997;179:3488–3493. doi: 10.1128/jb.179.11.3488-3493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauconnier A, Allaoui A, Campos A, Van Elsen A, Cornelis G R, Bollen A. Flagellar flhA, flhB and flhE genes, organized in an operon, cluster upstream from the inv locus in Yersinia enterocolitica. Microbiology. 1997;143:3461–3471. doi: 10.1099/00221287-143-11-3461. [DOI] [PubMed] [Google Scholar]
- 15.Fauconnier, A., A. Lage, A. Bollen, and E. Godfroid. 1997. Unpublished observations.
- 16.Ferrero R L, Hazell S L, Lee A. The urease enzymes of Campylobacter pylori and a related bacterium. J Med Microbiol. 1988;27:33–40. doi: 10.1099/00222615-27-1-33. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson J F, Jr, Garner R M, Mobley H L T. Conserved residues and motifs in the NixA protein of Helicobacter pylori are critical for the high affinity transport for nickel ions. J Biol Chem. 1998;273:235–241. doi: 10.1074/jbc.273.1.235. [DOI] [PubMed] [Google Scholar]
- 18.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner R M, Fulkerson J F, Jr, Mobley H L T. Helicobacter pylori glutamine synthetase lacks features associated with transcriptional and posttranslational regulation. Infect Immun. 1998;66:1839–1847. doi: 10.1128/iai.66.5.1839-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gherardini F C, Hobbs M M, Stamm L V, Bassford P J., Jr Complementation of an Escherichia coli proC mutation by a gene cloned from Treponema pallidum. J Bacteriol. 1990;172:2996–3002. doi: 10.1128/jb.172.6.2996-3002.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert J V, Ramakrishna J, Sunderman F W, Jr, Wright A, Plaut A G. Protein Hpn: cloning and characterization of a histidine-rich metal-binding polypeptide in Helicobacter pylori and Helicobacter mustelae. Infect Immun. 1995;63:2682–2688. doi: 10.1128/iai.63.7.2682-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin C S, Armstrong J A, Marshall B J. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986;39:353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gygi D, Bailey M J, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 24.Hay B, Short J M. ExAssist helper phage and SOLR cells for Lambda ZAP II excisions. Strategies Mol Cell Biol. 1992;5:16–18. [Google Scholar]
- 25.Hendricks J K, Mobley H L T. Helicobacter pylori ABC transporter: effect of allelic exchange mutagenesis on urease activity. J Bacteriol. 1997;179:5892–5902. doi: 10.1128/jb.179.18.5892-5902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He S Y. Hrp-controlled interkingdom protein transport: learning from flagellar assembly. Trends Microbiol. 1997;5:489–495. doi: 10.1016/S0966-842X(97)01163-3. [DOI] [PubMed] [Google Scholar]
- 27.Hueck C J. Type III secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu L-T, Foxall P A, Russell R, Mobley H L T. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992;60:2657–2666. doi: 10.1128/iai.60.7.2657-2666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L-T, Mobley H L T. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990;58:992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu L-T, Mobley H L T. Expression of catalytically active recombinant Helicobacter pylori urease at wild-type levels in Escherichia coli. Infect Immun. 1993;61:2563–2569. doi: 10.1128/iai.61.6.2563-2569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Human Genome Center. 13 October 1998, last date modified. Multiple sequence alignments. [Online.] Baylor College of Medicine. http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html.
- 30b.Institute for Genome Research. 29 September 1998, last date modified. The Helicobacter pylori genome database. [Online.] http://www.tigr.org/tdb/mdb/hpdb/hpdb.html.
- 31.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones B D, Mobley H L T. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988;170:3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kansau I, Labigne A. Heat shock proteins of Helicobacter pylori. Alimentary Pharmacol Ther. 1996;10(Suppl. 1):51–56. doi: 10.1046/j.1365-2036.1996.22164005.x. [DOI] [PubMed] [Google Scholar]
- 35.Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991;173:1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Marshall B J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 38.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 38a.McGee D J, Mobley H L T. Mechanisms of Helicobacter pylori infection: bacterial factors. In: Westblom T U, Czinn S J, Nedrud J G, editors. Current topics in microbiology and immunology. Gastroduodenal disease and Helicobacter pylori: pathophysiology, diagnosis and treatment. Berlin, Germany: Springer-Verlag; 1999. pp. 155–180. [DOI] [PubMed] [Google Scholar]
- 39.Melchers K, Weitzenegger T, Buhmann A, Steinhilber W, Sachs G, Schäfer K P. Cloning and membrane topology of a P type ATPase from Helicobacter pylori. J Biol Chem. 1996;271:446–457. doi: 10.1074/jbc.271.1.446. [DOI] [PubMed] [Google Scholar]
- 40.Miller S, Pesci E C, Pickett C L. A Campylobacter jejuni homolog of the LcrD/FlbF family of proteins is necessary for flagellar biogenesis. Infect Immun. 1993;61:2930–2936. doi: 10.1128/iai.61.7.2930-2936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minamino T, Iino T, Kutsukake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mobley H L T. Structure and function of Helicobacter pylori urease. In: Ernst P B, Michetti P, Smith P D, editors. Immunobiology of H. pylori from pathogenesis to prevention. New York, N.Y: Lippincott-Raven Publishers; 1997. pp. 59–73. [Google Scholar]
- 43.Mobley H L T, Garner R M, Bauerfeind P. Helicobacter pylori nickel transport gene nixA: synthesis of catalytically active urease in Escherichia coli independent of growth conditions. Mol Microbiol. 1995;16:97–109. doi: 10.1111/j.1365-2958.1995.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 44.Mobley H L T, Island M D, Hausinger R P. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulrooney S B, Pankratz H S, Hausinger R P. Regulation of gene expression and cellular localization of cloned Klebsiella aerogenes (Klebsiella pneumoniae) urease. J Gen Microbiol. 1989;135:1769–1776. doi: 10.1099/00221287-135-6-1769. [DOI] [PubMed] [Google Scholar]
- 45a.Nakai, K. 18 February 1998, last date modified. PSORT. [Online.] http://psort.nibb.ac.jp:8800/.
- 45b.National Center for Biotechnology Information. BLAST. [Online.] National Library of Medicine, National Institutes of Health. http://www.ncbi.nlm.nih.govBLAST.
- 45c.National Center for Biotechnology Information. Entrez. [Online.] National Library of Medicine, National Institutes of Health. http://www.ncbi.nlm.nih.gov/Entrez.
- 46.Navarro C, Wu L-F, Mandrand-Berthelot M-A. The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport system for nickel. Mol Microbiol. 1993;9:1181–1191. doi: 10.1111/j.1365-2958.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 47.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 47a.Pedro. 15 June 1996, last date modified. Pedro’s Molecular Biology Tools and web sites therein. [Online.] http://www.public.iastate.edu/∼pedro/rt_1.html.
- 48.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramakrishnan G, Zhao J-L, Newton A. The cell cycle-regulated flagellar gene flbF of Caulobacter crescentus is homologous to a virulence locus (lcrD) of Yersinia pestis. J Bacteriol. 1991;173:7283–7292. doi: 10.1128/jb.173.22.7283-7292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawlings J W, Danesh B J, Lucas M L, Morgan R J, Main A N, Russell R I. Gastroduodenal mucosal surface and luminal pH in gastric ulcer. Dig Dis Sci. 1991;36:1543–1549. doi: 10.1007/BF01296395. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 53.Sanders L A, van Way S, Mullin D A. Characterization of the Caulobacter crescentus flbF promoter and identification of the inferred FlbF product as a homolog of LcrD protein from a Yersinia enterocolitica virulence plasmid. J Bacteriol. 1992;174:857–866. doi: 10.1128/jb.174.3.857-866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smoot D T, Mobley H L T, Chippendale G R, Lewison J F, Resau J H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990;58:1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 57a.Swiss Institute for Experimental Cancer Research. 22 June 1997, last date modified. TMRED prediction of transmembrane regions and orientation. [Online.] http://www.isrec.isb-sib.ch/software/TMPRED_form.html.
- 57b.Swiss Institute of Bioinformatics. ExPASy molecular biology server and web sites therein. [Online.] http://www.expasy.ch/.
- 58.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 59.Viitanen A-M, Toivanen P, Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990;172:3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warren J R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 61.Weatherburn M W. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]