Abstract
Hydroxytyrosol (HT) is a phenolic compound with proven biological properties present in a limited number of foods such as table olives, virgin olive oil (VOO) and wines. The present work aims to evaluate the dietary intake of HT in the European (EU) population by compiling scattered literature data on its concentration in foods. The consumption of the involved foods was estimated based on the EFSA Comprehensive European Food Consumption Database. The updated average contents of HT are as follows: 629.1, 5.2 and 2.1 µg/g for olives, olive oil and wine, respectively. The HT estimated intake in the European Union (EU) adult population falls within 0.13–6.82 mg/day/person, with table olives and wine being the main contributors. The estimated mean dietary intake of HT in EU countries is 1.97 ± 2.62 mg/day. Greece showed the highest HT intake (6.82 mg/day), while Austria presented the lowest (0.13 mg/day). Moreover, HT is an authorized novel food ingredient in the EU that can be added to different foods. Since the estimated HT intake is substantially low, the use of HT as a food ingredient seems feasible. This opens new possibilities for revalorizing waste products from olive oil and olive production which are rich HT sources.
Keywords: hydroxytyrosol, virgin olive oil, wine, olives, dietary intake, food consumption
1. Introduction
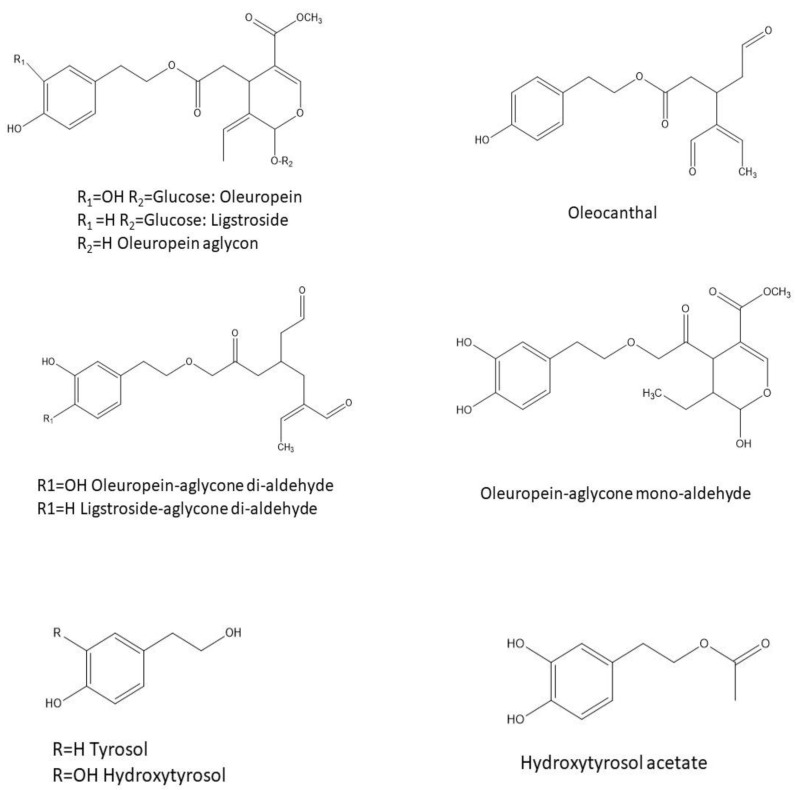
A Mediterranean diet has been associated with a decreased incidence of cancer, cardiovascular and neurodegenerative disorders. Since epidemiological data associate the consumption of certain foods such as olive oil, nuts, fruits and vegetables to a lesser incidence of disease, intense research is conducted to explain how the compounds with biological properties (bioactives) might exert their action. In particular, polyphenolic compounds abundant in plant foods have been extensively studied. This is the case for hydroxytyrosol (HT), present mainly in olives, virgin olive oil and wine, which has attracted scientific interest due to its ortho-dihydroxy conformation in the aromatic ring, a chemical feature related to its antioxidant bioactivity. Indeed, Villaño et al. [1] highlighted that the dihydroxy substitution in the ortho position was related to a higher antioxidant activity when comparing different phenolic compounds (Figure 1). At the same time, Napolitano et al. [2] showed that HT possesses a better H-donor than another simple phenol such as tyrosol (TYR) as well as a greater iron-chelating ability. Thus, HT is more efficient as antioxidant than TYR. HT presents the strongest antioxidant activity among the major olive phenols [3]. Furthermore, its antioxidant effect is not solely due to the capacity of scavenging oxidant chemical species, but to the ability of stimulating the activity and synthesis of antioxidant enzymes [4]. Additionally, HT has shown anti-inflammatory, neuroprotective and anti-angiogenic effects [5,6,7,8,9,10,11,12,13,14,15].
Figure 1.
Structures of the main hydroxytyrosol-related compounds described in olives and olive oil.
All in all, recent literature is gathering evidence that HT may have an important role protecting the most prevalent non-infectious diseases, which are also related to diet. However, it remains difficult to establish the current dietary intake of HT. For this purpose, the first task is to estimate the occurrence of this bioactive compound in foods. With olives, olive oil and wine as the main dietary sources, there are factors to consider such as origin, variety, maturity, food processing, and storage, among others. In this sense, intense efforts have been made to compile food composition data regarding bioactives, HT among them. For example, Phenol Explorer [16] is a database that summarizes scattered data on the phenolic composition of foods in scientific literature. This has proved challenging due to the overwhelming number of papers published each year and the incorporation of new food data since the last Phenol Explorer update (2015). In terms of HT literature, access to Scopus in December 2021 showed a growing trend in the number of articles: 30 papers in 1991, 118 in 2001, and more than 334 in 2021, including different perspectives and scopes.
In regard to food composition data, reliability clearly depends on the analytical methods used to obtain the reported values, thus special attention needs to be paid to the description of these methods in the literature.
In summary, the aim of this work is to evaluate HT dietary intake for the adult population in Europe and to ascertain if its addition to foods as a bioactive ingredient is feasible in the future. In this context, it is important to ensure that the sum of the amount naturally provided by foods and the amount provided as a food ingredient is below any toxic levels, due to safety concerns.
2. Methodology
2.1. Literature Search
A scientific literature search was conducted using the Scopus database until July 2022. The initial search with HT as a keyword was further refined with the food source (Table 1, Table 2 and Table 3). We selected the articles including the analytical determination of HT, TYR, and secoiridoids (oleuropein, oleuropein aglycone, and elenolic acid dialdehydes), since they are HT precursors.
Table 1.
Oleuropein (OLE), tyrosol (TYR), hydroxytyrosol (HT) and hydroxytyrosol acetate (HT-AC) concentration (mg/kg) in table olive samples according to origin, variety, processing and analytical method.
| Origin | Variety | Processing | Concentration (mg/kg) | Method | References | |||
|---|---|---|---|---|---|---|---|---|
| OLE | TYR | HT | HT-AC | |||||
| Spain | Marfil | Greek-style | 1.40 ± 0.31 | 201.2 ± 23.2 | 384.1 ± 81.2 | 2.85 ± 0.71 | LC-ESI-MS/MS | [17] |
| Azeitera | Spanish-style | 105.9 ± 4.7 | 60.2 ± 5.6 | 605.1 ± 10.6 | n.dr. | RP-HPLC-DAD (OLE) RP-HPLC-FLD (TYR, HT) |
[18] | |
| 96.8 ± 6.5 | 54.2 ± 3.9 | 581.2 ± 12.5 | n.dr. | |||||
| Carrasqueña | Spanish-style | 150 ± 10.5 | 75.1 ± 5.2 | 825.3 ± 14.5 | n.dr. | |||
| 138.4 ± 5.9 | 80.2 ± 4.8 | 812.5 ± 11.5 | n.dr. | |||||
| Conserva de Elvas | Spanish-style | 86.4 ± 2.6 | 35.2 ± 5.6 | 415.6 ± 8.5 | n.dr. | |||
| 80.9 ± 4.7 | 39.6 ± 2.1 | 394.1 ± 10.6 | n.dr. | |||||
| Morisca | Spanish-style | 80.5 ± 6.9 | 49.8 ± 4.6 | 398.6 ± 5.8 | n.dr. | |||
| 86.9 ± 2.8 | 45.6 ± 6.4 | 455.9 ± 7.1 | n.dr. | |||||
| Carrasqueña | Spanish-style | 3 ± 1 | 64 ± 10 | 876 ± 82 | n.dr. | RP-HPLC-DAD | [19] | |
| Manzanilla | Spanish-style | 1411.0 ± 452.7 | 78.6 ± 6.4 | 1005.5 ± 25.4 | n.dr. | HPLC-MS | [20] | |
| Hojiblanca | Spanish-style | 96.3 ± 42.8 | 79.1 ± 15.4 | 1133.1 ± 110.6 | n.dr. | |||
| Darkening | n.dr. | 19.5 ± 2.1 | 40.9 ± 6.3 | n.dr. | ||||
| Darkening | n.dr. | 55 ± 3 | 275 ± 10 | n.dr. | HPLC-DAD | [21] | ||
| Italy | Ascolana tenera | Natural-style | 553.4 ± 133 | 97.2 ± 6.5 | 1770.3 ± 324 | n.dr. | RP-HPLC-FLD | [22] |
| Spanish-style | 209.3 ± 8 | 60.7 ± 1.0 | 391.2 ± 8 | n.dr. | ||||
| 156.3 ± 3.9 | 63.1 ± 1.4 | 372 ± 10.1 | n.dr. | |||||
| 89.2 ± 5.2 | 38.5 ± 4.3 | 103.6 ± 8 | n.dr. | |||||
| 77.9 ± 6.7 | 29.7 ± 5.4 | 92.5 ± 11 | n.dr. | |||||
| 225 ± 2.9 | 225.5 ± 13 | 752.5 ± 10 | n.dr. | |||||
| 79.2 ± 2.1 | 71.2 ± 4.2 | 402.1 ± 5.1 | n.dr. | |||||
| 76.8 ± 6.7 | 60.4 ± 8.5 | 324.5 ± 9.4 | n.dr. | |||||
| Leccino | Greek-style | 0.00 | 22.78 ± 6.15 | 150.17 ± 41.20 | n.dr. | UHPLC-DAD | [23] | |
| Bella di Cerignola | Greek-style | n.dr. | 80.5 ± 6.9 | 421.8 ± 30.7 | 26.7 ± 0.9 | HPLC-DAD | [24] | |
| Termite di Bitetto | Greek-style | n.dr. | 28 ± 0.7 | 258.2 ± 12.1 | 0.0 | |||
| Cellina di Nardò | Greek-style | n.dr. | 353.5 ± 26.2 | 1393.3 ± 38 | 0.0 | |||
| Nocellara del Belice | Spanish-style | 35.8 ± 10.3 | 51.5 ± 4.1 | 535.4 ± 37.2 | 62.3 ± 11 | HPLC-MS | [25] | |
| Castelvetrano-style | 49.6 ± 16.5 | 49.0 ± 11.7 | 715.8 ± 79.4 | 26.8 ± 13.8 | ||||
| Portugal | Negrinha de Freixo | California-style | n.dr. | 161.3 ± 15.5 | 672.4 ± 75.4 | n.dr. | RP-HPLC-DAD | [26] |
| Galega | Not mentioned (naturally black) | n.dr. | 139.1 ± 24.0 | 3833.0 ± 180.9 | n.dr. | |||
| California USA | Kalamata | Greek-style | 7.303 | 1.315 | 134.329 | n.dr. | UHPLC-(ESI) MS/MS | [27] |
| Manzanillo | California-style | 0.974 | 0.435 | 19.981 | n.dr. | |||
| Spanish-style | 3.205 | 0.859 | 133.685 | n.dr. | ||||
| Manzanilla | California-style | 36.7 ± 3.1 | n.dr. | 210.0 ± 18.8 | n.dr. | UHPLC-QqQ MS/MS dMRM | [28] | |
| Mission | Dry-salted black olives |
516.2 ± 44.3 | n.dr. | 633.8 ± 55.1 | n.dr. | |||
| Greece | Throuba Thassos | Dry-salted black olives |
1459.5 ± 100.1 | n.dr. | 195.1 ± 7.8 | n.dr. | ||
| Algeria | Azz Sed | Spanish-style | n.dc. | 37.27 ± 0.73 | 105.97 ± 12.2 | n.dr. | RP-HPLC-DAD | [29] |
| Gordal | Spanish-style | n.dc | 43.65 ± 1.09 | 45.68 ± 1.49 | n.dr. | |||
| Sevilla | Spanish-style | n.dc | 106.49 ± 0.26 | 545.42 ± 13.24 | n.dr. | |||
| Sigoise | Spanish-style | 1840.29 ± 49.27 | 35.84 ± 8.76 | 98.80 ± 19.8 | n.dr. | |||
| Teffahi | Spanish-style | n.dc | 24.38 ± 0.00 | 14.49 ± 1.49 | n.dr. | |||
| Bouchouk | Spanish-style | n.dc | 27.60 ± 4.23 | 100.60 ± 1.65 | n.dr. | |||
| Azz Taz | Spanish-style | n.dc | 24.35 ± 0.00 | 38.22 ± 0.00 | n.dr. | |||
| Tunisia | Chétoui | Spanish-style | 307 ± 1.2 | 49 ± 0.2 | 3750 ± 8.3 | n.dr. | HPLC-DAD | [30] |
| 480 ± 2 | 42 ± 0.02 | 2300 ± 9.3 | n.dr. | |||||
| Turkey | Gemlik | Dry-salted black olives |
231 ± 1 | 78 ± 0 | 221 ± 1 | 64 ± 0 | LC-DAD-ESI-MS/MS | [31] |
Non-determined (n.dr.), non-detected (n.dc.).
Table 2.
Hydroxytyrosol (HT), tyrosol (TYR), hydroxytyrosol acetate (HT-AC), oleuropein-aglycone di-aldehyde (3,4-DHPEA-EDA), oleuropein-aglycone mono-aldehyde (3,4-DHPEA-EA), ligstroside-aglycone di-aldehyde (p-DHPEA-EDA), oleuropein (OLE) and secoiridoids derivative (S-DER) concentration in olive oil samples according to origin, variety, category, processing and analytical method.
| Origin | Variety | Category | Concentration (mg/kg) | Method | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT | TYR | HT-AC | 3,4-DHPEA-EDA | 3,4-DHPEA-EA | p-HPEA-EDA | p-HPEA-EA | OLE | S-DER | |||||
| Calabria Italy | Frantoio | EVOO | 5.3 ± 0.9 | 5.4 ± 0.7 | n.dr. | 98.4 ± 3.5 | 27.5 ± 1.6 | 52.9 ± 1.7 | 4.4 ± 0.9 | n.dr. | n.dr. | HPLC -DAD |
[32] |
| Calabria Italy | 4.6 ± 0.7 | 2.5 ± 0.3 | 1.9 ± 0.3 | 50.3 ± 2.8 | 22.5 ± 2.2 | 34.9 ± 1.7 | 3.0 ± 0.9 | n.dr. | n.dr. | ||||
| Calabria Italy | 1.2 ± 0.4 | 1.1 ± 0.2 | n.d. | 89.4 ± 2.9 | 19.0 ± 1.7 | 37.9 ± 1.6 | n.d. | n.dr. | n.dr. | ||||
| Praia a mare, Calabria Italy | 1.7 ± 0.1 | 1.7 ± 0.5 | 0.7 ± 0.1 | 56.0 ± 3.1 | 18.9 ± 1.9 | 34.6 ± 2.7 | 1.7 ± 0.9 | n.dr. | n.dr. | ||||
| Italy | Coratina | VOO | 1.97–7.1 | 5.7–16.4 | n.dr. | 65.1–147.7 | 94.6–268.9 | 89.4–153.7 | 25.4–120.8 | n.dr. | n.dr. | IOC Extraction RP-HPLC-DAD | [33] |
| Bosana | 1.9–3.0 | 3.7–5.7 | n.dr. | 71.8–237.2 | 53.4–118.7 | 70.8–155.6 | 16.1–34.7 | n.dr. | n.dr. | ||||
| Semidana | 1.3–4.8 | 2.7–9.2 | n.dr. | 4.5–90.8 | 11.6–66.2 | 17.0–57.5 | 5.5–17.2 | n.dr. | n.dr. | ||||
| Tonda di Cagliari | 0.9–5.3 | 2.2–10.4 | n.dr. | 13.5–138.8 | 13.7–59.9 | 23.2–108.0 | 8.4–26.3 | n.dr. | n.dr. | ||||
| Tuscany Italy |
Multi- varietal |
EVOO | 161.5 ± 4.5 | 122.5 ± 3.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Acid hydrolysis HPLC-DAD | [34] |
| Northern Italy | Frantoio | EVOO | 0.9 ± 0.0 | 4.2 ± 0.6 | 1.3 ± 0.2 | 76.7 ± 55 | 7.9 ± 0.4 | 6.7 ± 0.9 | n.dr. | 18.5 ± 1.6 | n.dr. | LLE HPLC-DAD-MS |
[35] |
| 3.0 ± 0.2 | 4.3 ± 0.3 | 1.9 ± 0.2 | 62.2 ± 4.9 | 2.6 ± 0.3 | 2.2 ± 0.1 | n.dr. | 5.3 ± 0.3 | n.dr. | |||||
| Casaliva | 0.7 ± 0.7 | 3.1 ± 0.4 | 1.8 ± 0.2 | 56.6 ± 4.7 | 3.2 ± 0.1 | 7.4 ± 0.4 | n.dr. | 6.6 ± 0.7 | n.dr. | ||||
| 1.9 ± 0.3 | 4.6 ± 0.4 | 1.5 ± 0.2 | 55.0 ±0.8 | 1.4 ± 0.0 | 2.7 ± 0.2 | n.dr. | 1.8 ± 0.1 | n.dr. | |||||
| Organic Casaliva | 2.3 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.3 | 108.2 ± 9.6 | 9.2 ± 0.9 | 12.3 ± 1.2 | n.dr. | 12.8 ± 1.9 | n.dr. | ||||
| 4.3 ± 0.3 | 4.9 ± 0.8 | 3. ± 0.23 | 98.9 ± 13.3 | 3.5 ± 0.1 | 7.3 ± 0.1 | n.dr. | 2.7 ± 0.2 | n.dr. | |||||
| Multi-varietal | 6.4 ± 0.2 | 2.5 ± 0.4 | 3.4 ± 0.2 | 102.4 ± 4.6 | 5.5 ± 0.1 | 10.9 ± 1.0 | n.dr. | 10.0 ± 1.1 | n.dr. | ||||
| 0.3 ± 0.0 | 1.1 ± 0.1 | 2.9 ± 0.2 | 54.6 ± 3.0 | 6.4 ± 0.6 | 0.9 ± 0.0 | n.dr. | 1.5 ± 0.1 | n.dr. | |||||
| Organic multi-varietal | 11.4 ± 0.6 | 5.6 ± 0.6 | 1.7 ± 0.1 | 106.9 ± 12.6 | 8.8 ± 2.9 | 12.5 ± 0.6 | n.dr. | 15.4 ± 1.5 | n.dr. | ||||
| 0.6 ± 0.0 | 2.8 ± 0.4 | 1.9 ± 0.3 | 89.4 ± 1.3 | 2.2 ± 0.1 | 14.4 ± 1.3 | n.dr. | 9.5 ± 0.7 | n.dr. | |||||
| Salerno Italy | N/D (Cilento PDO) | VOO | 37–41.3 | 23.8–34.6 | n.dr. | n.dr. | 19.9–24.9 | n.dr. | 23.8–35 | 120.4–140 | n.dr. | RP-HPLC-DAD | [36] |
| Abruzzo Italy | Gentile | VOO | 1.6–3.7 | 3.9–5.8 | n.dr. | n.dr. | 20.8–22.0 | n.dr. | n.dr. | n.dr. | 232.8–359.9 | HPLC- DAD HPLC- MS |
[37] |
| 0.8 ± 0.1 | 4.1 ± 0.2 | n.dr. | n.dr. | 15.2 ± 0.3 | n.dr. | n.dr. | n.dr. | 173.8 ± 3.0 | |||||
| Leccino | 2.3 ± 0.1 | 6.1 ± 0.2 | n.dr. | n.dr. | 5.9 ± 0.1 | n.dr. | n.dr. | n.dr. | 91.0 ± 1.6 | ||||
| n.d. | 4.0 ± 0.2 | n.dr. | n.dr. | 5.4 ± 0.2 | n.dr. | n.dr. | n.dr. | 120.1 ± 1.4 | |||||
| Dritta | 2.0–2.8 | 7.4–7.9 | n.dr. | n.dr. | 14.9–30.9 | n.dr. | n.dr. | n.dr. | 189.9–299.1 | ||||
| 3.5 ± 0.1 | 8.8 ± 0.24 | n.dr. | n.dr. | 11.3 ± 0.2 | n.dr. | n.dr. | n.dr. | 310.3 ± 5.3 | |||||
| Southwest Spain | Arbequina | VOO | 1.3 ± 0.4 | 1.9 ± 0.3 | 42.3 ± 3.6 | 75.5 ± 23.9 | 33.7 ± 1.9 | 59.9 ± 6.9 | 16.5 ± 0.9 | n.dr. | n.dr. | SPE RP-HPLC-DAD |
[38] |
| 0.4 ± 0.0 | 0.6 ± 0.0 | 36.8 ± 1.5 | 44.7 ± 1.1 | 104.4 ± 1.2 | 36.8 ± 0.2 | 9.7 ± 1.8 | n.dr. | n.dr. | |||||
| Carrasqueña | 2.8 ± 0.8 | 3.5 ± 0.9 | 7.3 ± 0.3 | 141.1 ± 1.1 | 68.4 ± 14.9 | 73.5 ± 18.9 | 82.9 ± 6.9 | n.dr. | n.dr. | ||||
| 0.6 ± 0.0 | 3.4 ± 0.0 | 6.9 ± 1.4 | 88.7 ± 10.5 | 95.2 ± 33.4 | 36.1 ± 3.1 | 14.3 ± 1.7 | n.dr. | n.dr. | |||||
| Corniche | 2.0 ± 0.8 | 3.1 ± 1.9 | n.d. | 69.0 ± 14.7 | 61.2 ± 16.5 | 101.7 ± 21.5 | 12.4 ± 2.3 | n.dr. | n.dr. | ||||
| 0.9 ± 0.3 | 2.1 ± 1.0 | 20.9 ± 2.5 | 150.5 ± 12.6 | 37.0 ± 9.3 | 116.9 ± 32.4 | 10.5 ± 3.4 | n.dr. | n.dr. | |||||
| Manzanilla Cacereña | 1.5 ± 0.6 | 5.9 ± 1.4 | n.dc. | 56.0 ± 9.6 | 32.8 ± 9.8 | 58.2 ± 7.3 | 73.4 ± 0.9 | n.dr. | n.dr. | ||||
| 0.7 ± 0.3 | 7.8 ± 1.5 | n.dr. | 40.6 ± 4.5 | 48.3 ± 5.9 | 59.4 ± 8.1 | 10.9 ± 3.1 | n.dr. | n.dr. | |||||
| Morisca | 1.3 ± 0.3 | 3.0 ± 0.9 | 6.2 ± 10.0 | 70.6 ± 7.2 | 30.4 ± 4.1 | 41.4 ± 31.6 | 51.0 ± 1.2 | n.dr. | n.dr. | ||||
| 1.4 ± 0.7 | 3.2 ± 1.4 | 43.3 ± 8.3 | 42.5 ± 5.3 | 18.9 ± 0.8 | 40.3 ± 3.6 | 23.9 ± 5.0 | n.dr. | n.dr. | |||||
| Picual | 3.3 ± 1.2 | 5.7 ± 1.3 | n.dc. | 89.3 ± 15.6 | 73.3 ± 16.9 | 44.2 ± 3.5 | 39.8 ± 7.4 | n.dr. | n.dr. | ||||
| 1.6 ± 0.8 | 3.5 ± 2.7 | n.dc. | 71.7 ± 3.9 | 46.9 ± 0.8 | 39.7 ± 7.5 | 68.8 ± 9.6 | n.dr. | n.dr. | |||||
| Verdial de Badajoz | 1.4 ± 0.2 | 6.3 ± 0.6 | n.dr. | 143.8 ± 34.2 | 33.7 ± 5.7 | 166.4 ± 15.0 | 41.6 ± 11.8 | n.dr. | n.dr. | ||||
| 0.8 ± 0.4 | 4.2 ± 1.1 | n.dr. | 71.6 ± 18.8 | 16.5 ± 1.7 | 123.5 ± 19.1 | 6.5 ± 0.5 | n.dr. | n.dr. | |||||
| Jaén, Spain | Picual | VOO | 2.8–7.8 | 4.9–9.9 | n.dr. | 221.4–849.7 | 29.5–929.2 | 19.5–248.9 | 24.4–344.2 | n.dr. | n.dr. | LLE HPLC- DAD |
[39] |
| 2.8–6.2 | 0.9–7.8 | n.dr. | 231.5–788.0 | 26.2–790.7 | 13.5–262.2 | 22.0–269.5 | n.dr. | n.dr. | |||||
| Seville, Spain | Arbequina Hojiblanca Manzanilla Picual N/A | EVOO | 50–200 | 40–180 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Acid hydrolysis HPLC- UV-FL |
[40] |
| N/D | OO | 5-20 | 5–30 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||
| Spain | Picual-Arbequina blend | VOO | 15.7 ± 0.9 | 9.1 ± 0.5 | n.dr. | n.dr. | n.dr. | n.dr. | 38.5 ± 3.8 | n.dr. | n.dr. | SPE HPLC-ESI-TOF/MS |
[41] |
| 14.3 ± 0.2 | 8.9 ± 0.3 | n.dr. | n.dr. | n.dr. | n.dr. | 44.8 ± 0.9 | n.dr. | n.dr. | SPE HPLC-UV |
||||
| Catalonia Spain |
Arbequina | VOO | 2.5 | 3.0 | 1.6 | 152 | 68 | 20 | 42 | n.dr. | 26.4 | LLE UPLC-MS/MS | [42] |
| Spain | Cornicabra | EVOO | 0.9–2.8 | 1.0–2.3 | n.dr. | 396–770 | 136–301 | 228–498 | 39–138 | n.dr. | n.dr. | SPE HPLC-DAD | [43] |
| Messenia, Greece | Koroneiki | EVOO | 4.1 ± 0.1 | 0.4 ± 0.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | Selective ion monitoring GC/MS | [44] |
| 9.3 ± 0.1 | 0.4 ± 0.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||||
| 20.2 ± 1.0 | 0.4 ± 0.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | |||||
| Morocco | Arbequina | VOO | 6.4 ± 2.7 | 11.6 ± 3.3 | 26.6 ± 32.7 | n.dr. | 55.4 ± 6.4 | n.dr. | 53.9 ± 4.4 | n.dr. | n.dr. | LLE LC-ESI- IT-MS |
[45] |
| Arbosana | 1.3 ± 1.9 | 3.3 ± 4.0 | 1.5 ± 1.3 | n.dr. | 21.0 ± 8.4 | n.dr. | 18.9 ± 2.2 | n.dr. | n.dr. | ||||
| Cornicabra | 4.3 ± 0.4 | 6.6 ± 0.4 | 1.4 ± 0.1 | n.dr. | 96.9 ± 14.5 | n.dr. | 93.9 ± 6.4 | n.dr. | n.dr. | ||||
| Frantoio | 0.8 ± 0.4 | 4.1 ± 0.6 | 0.3 ± 0.2 | n.dr. | 24.5 ± 14.6 | n.dr. | 34.8 ± 8.7 | n.dr. | n.dr. | ||||
| Hojiblanca | 0.5 ± 0.3 | 5.4 ± 2.1 | 1.1 ± 0.8 | n.dr. | 35.8 ± 17.1 | n.dr. | 41.1 ± 18.1 | n.dr. | n.dr. | ||||
| Koroneiki | 8.3 ± 2.6 | 7.5 ± 2.4 | 2.2 ± 0.3 | n.dr. | 93.6 ± 9.0 | n.dr. | 66.1 ± 15.8 | n.dr. | n.dr. | ||||
| Manzanilla | 3.8 ± 3.3 | 10.8 ± 5.6 | 1.8 ± 1.5 | n.dr. | 54.0 ± 33.2 | n.dr. | 65.6 ± 37.1 | n.dr. | n.dr. | ||||
| P-Languedoc | 2.2 ± 0.8 | 9.5 ± 1.2 | 0.8 ± 0.2 | n.dr. | 46.6 ± 10.9 | n.dr. | 46.3 ± 14.4 | n.dr. | n.dr. | ||||
| P-Marocaine | 2.2 ± 1.7 | 8.5 ± 1.6 | 0.9 ± 0.3 | n.dr. | 35.2 ± 7.6 | n.dr. | 35.9 ± 3.7 | n.dr. | n.dr. | ||||
| Picual | 4.8 ± 2.7 | 9.2 ± 2.0 | 2.2 ± 1.9 | n.dr. | 51.96 ± 26.81 | n.dr. | 47.4 ± 6.1 | n.dr. | n.dr. | ||||
| Dahbia | 0.2 ± 0.0 | 1.7 ± 0.2 | 0.2 ± 0.0 | n.dr. | 26.1 ± 0.4 | n.dr. | 33.1 ± 0.9 | n.dr. | n.dr. | ||||
| Haouzia | 3.7 ± 1.4 | 7.6 ± 2.0 | 0.5 ± 0.3 | n.dr. | 63.5 ± 12.7 | n.dr. | 51.1 ± 22.8 | n.dr. | n.dr. | ||||
| Menara | 4.1 ± 2.7 | 11.4 ± 2.6 | 0.4 ± 0.2 | n.dr. | 41.6 ± 10.5 | n.dr. | 43.8 ± 5.3 | n.dr. | n.dr. | ||||
| Algeria | Azeradj | EVOO | 2.8–3.0 | 13.3–26.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.2–2.1 | n.dr. | SPE HPLC- UV-vis |
[46] |
| Mekki | 1.1–1.4 | 9.0–11.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Neb djemel | 4.4–7.3 | 15.1–17.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Chemlal | 3.3–4.2 | 13.6–19.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.0–4.4 | n.dr. | ||||
| Hamra | 1.1–4.1 | 9.9–20.7 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 8.3–13.1 | n.dr. | ||||
| Blanquette de Guelma | 4.8–8.9 | 19.5–21.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.3–2.2 | n.dr. | ||||
| Limli | 2.9–3.2 | 13.1–18.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Aberkane1 | 1.1–1.2 | 16.7–18.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Aimell | 2.2–6.3 | 18.0–18.3 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Rougette de la mitidja | 4.1–4.6 | 14.6–19.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.2–1.5 | n.dr. | ||||
| Aghenaou | 1.7–2.4 | 14.0–18.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Boughenfas | 2.6–2.8 | 16.6–26.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.6–2.5 | n.dr. | ||||
| Bouichret | 3.1–4.2 | 13.5–14.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Aghenfas | 3.2–3.9 | 18.4–25.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.8–5.2 | n.dr. | ||||
| Bouchouk de Guergour | 3.8–4.2 | 14.5–20.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.3–0.5 | n.dr. | ||||
| X-Aghenfas | 3.5–8.7 | 13.9–36.3 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Bounguergueb | 1.8–3.5 | 13.9–14.0 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dc. | n.dr. | ||||
| Ronde de miliana | 0.0–1.5 | 16.6–20.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 1.0–1.5 | n.dr. | ||||
| Sigoise | 1.4–2.6 | 22.4–27.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.2–0.4 | n.dr. | ||||
| Grosse du Hamma | 9.67-14.93 | 28.1–32.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.4–0.9 | n.dr. | ||||
| Rougette de Guelma | 3.3–3.7 | 14.0–18.5 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | 0.7–1.4 | n.dr. | ||||
| Tunisia | Oueslati | EVOO | 3.8–7.2 | 1.9–3.1 | 0.3–1.3 | n.dr. | 222.6–537.8 | n.dr. | 2.9–19.4 | n.dr. | n.dr. | RRLC- ESI- TOF-MS |
[47] |
| Hatay Turkey | Halhali | VOO | 5.5 ± 0.0 | 10.3 ± 0.2 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | LLE HPLC- DAD |
[48] |
| EVOO | 5.2 ± 0.9 | 14.8 ± 0.4 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Balikesir Turkey | Ayvalik | VOO | 0.1–0.8 | 0.7–1.1 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | HPLC- DAD |
[49] |
| Domat | 0.0–1.2 | 0.2–0.9 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
| Gemlik | 0.2–0.4 | 0.5–1.6 | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | n.dr. | ||||
Non-determined (n.dr.), non-detected (n.dc.), non-declared (N/D).
Table 3.
Tyrosol (TYR) and hydroxytyrosol (HT) content in wine according to origin, variety, and method of analysis.
| Origin | Variety | Year | Concentration (mg/L) | Method | References | |
|---|---|---|---|---|---|---|
| TYR | HT | |||||
| France | Not specified | n.dr. | 0.0092 | LC-MS/MS | [50] | |
| Australia | - | 0.0054 | ||||
| China | 0.0056 | |||||
| China | 0.000071 | |||||
| Salento, Italy | Negroamaro (Red wine) | - | n.dr. | 2.3 ± 0.8 mg/kg | HPLC | [51] |
| Primitivo (Red wine) | - | 2.7 ± 0.7 mg/kg | ||||
| Croatia | Cabernet Sauvignon (Red wine) | 2013 | 44.5 | 2.3 | UV/VIS-HPLC | [52] |
| 2014 | 29.8 | 2.1 | ||||
| 2015 | 46.4 | 1.746 | ||||
| Merlot (Red wine) | 2013 | 40.7 | 2.7 | |||
| 2014 | 41.5 | 3.2 | ||||
| 2015 | 36.9 | 2.6 | ||||
| Plavac mali (Red wine) | 2013 | 41.3 | 2.9 | |||
| 2014 | 31.1 | 2.4 | ||||
| 2015 | 48.3 | 2.6 | ||||
| Teran (Red wines) | 2013 | 40.6 | 3.7 | |||
| 2014 | 21.6 | 4.0 | ||||
| 2015 | 36.5 | 3.4 | ||||
| Jerez de la Frontera, Spain | Corredera (White wine) | - | n.dr. | 0.173 | HPLC | [53] |
| Moscatel (White wine) | 0.159 | |||||
| Chardonnay (White wine) | 0.167 | |||||
| Sauvignon Blanc (White wine) | 0.288 | |||||
| Palomino Fino (White wine) | 0.089 | |||||
| Vijiriega (White wine) | 0.238 | |||||
| Spain | Bach Viña Extrísimo (White wine) | 2016 | 10.4 | 1.3 | LC/MS-MS | [54] |
| Girona, Spain | Jardins Negre (Red wine) | 2017 | 25.30 | 1.80 | LC/MS-MS | [55] |
| Italy | Greco di Tufo (White wine) | 1998 | 1.1 | 2.7 | HPLC | [56] |
| Verdicchio (White wine) | 1998 | 3.0 | 1.6 | |||
| Pinot Grigio (White wine) | 1997 | 2.3 | 1.9 | |||
| Blended | 1998 | 5 | 6.1 | |||
| 1996 | 6 | 5.9 | ||||
| Barbera (Red wine) | - | 5.9 | 9.6 | |||
| Montepulciano (Red wine) | 1998 | 5.9 | 0.5 | |||
| Italy | White wine | 1.42–2.34 | 1.79–2.00 | GC-MS | [57] | |
| Red wine | 3.61–4.80 | 3.81–4.37 | ||||
| Jerez de la Frontera, Spain | Tempranillo (Red wine) | 2009 | 20.51–22.76 | 0.82–1.84 | HPLC-FD | [58] |
| Blasco (Red wine) | 27.38 | 1.55 | ||||
| Cabernet Sauvignon (Red wine) | 31.95–32.57 | 5.02–3.63 | ||||
| Petit Verdot (Red wine) | 40.59–38.50 | 4.12–2.33 | ||||
| Syrah (Red Wine) | 2010 | 40.98–34.11 | 1.11–0.82 | |||
| Merlot (Red Wine) | 44.46 | 1.77 | ||||
| Tintilla de Rota (Red Wine) | 28.91–30.97 | 2.66–1.65 | ||||
| Melonera (Red Wine) | 35.31–36.86 | 0.53–0.45 | ||||
| Tempranillo (Red Wine) | 25.03–44.26 | 1.78 | ||||
| Vitis silvestris (Red Wine) | 20.38–40.20 | 0.28–3.09 | ||||
| Palomino negro (Red Wine) | 29.57 | 1.10 | ||||
| Rome (Red Wine) | 35.41–35.57 | 1.67–1.86 | ||||
| Garnacha (Red Wine) | 26.73–30.15 | 1.02–1.28 | ||||
Non-determined (n.dr.).
2.2. Estimation of Hydroxytyrosol Dietary Intake in Europe
The 2011 EFSA survey, namely the Comprehensive European Food Consumption Database was used [59] to estimate the mean consumption of olive oil, table olives and wine per person in the different European countries, following the previously reported methodology for the intake estimation of other polyphenolic compounds [60]. The food consumption of twenty-two different countries was considered (Austria, Belgium, Croatia, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden and the United Kingdom) since the other countries either did not present data or presented data that did not refer to the adult population. Data regarding the most recent surveys were considered (Table 4). For each food items, the mean consumption data in g/day per person was used, taking into account that all subjects in the surveys were included, not only those consuming the foods under study.
Table 4.
Dietary surveys for the adult population in the European countries used in this study (EFSA Comprehensive European Food Consumption Database).
| Country | Name of the Dietary Survey | Period of Survey | Nº of Subjects |
|---|---|---|---|
| Austria | Austrian Study on Nutritional Status 2010–2012—Adults (ASNS–Adults) | 2010–2012 | 615 |
| Belgium | Belgian National Food consumption survey (NATIONAL-FCS-2014) | 2014–2015 | 2278 |
| Croatia | Croatian food consumption survey on adults (NIPNOP-HAH-2011-2012) | 2011–2012 | 2000 |
| Czech Republic | Czech National Food Consumption Survey (SISP04) | 2003–2004 | 1666 |
| Cyprus | National dietary survey of the adult population of Cyprus (CY 2014-2017-LOT2) | 2014–2017 | 812 |
| Denmark | The Danish National Dietary survey 2005–2008 (DANSDA 2005-08) | 2005–2008 | 1739 |
| Estonia | National Dietary Survey among 11–74 years old individuals in Estonia (DIET-2014-EST-A) | 2013–2015 | 2124 |
| Finland | National FINDIET 2012 Survey (FINDIET2012) | 2012 | 1295 |
| France | The French national dietary survey (INCA3) | 2014–2015 | 1773 |
| Germany | National Nutrition Survey II | 2007 | 10,419 |
| Greece | The EFSA-funded collection of dietary or related data in the general population aged 10–74 years in Greece (GR-EFSA-LOT2 2014-2015) | 2014–2016 | 791 |
| Hungary | National Repr Surv (NATIONAL REPR SURV) | 2003 | 1074 |
| Ireland | National Adult Nutrition Survey (NANS 2012) | 2008–2010 | 1274 |
| Italy | Italian National Food Consumption Survey (INRAN SCAI 2005-06) |
2005–2006 | 2313 |
| Latvia | Latvian National Dietary survey (LATVIA_2014) |
2012–2015 | 1080 |
| Netherlands | Dutch National food consumption survey 2012–2016 (FCS2016_CORE) | 2012–2017 | 4313 |
| Portugal | National Food, Nutrition and Physical Activity Survey of the Portuguese general population (IAN.AF 2015-2016) | 2015–2016 | 3102 |
| Romania | Dietary Pilot Adults | 2012 | 1254 |
| Slovenia | Slovenian national food consumption survey (SI. MENU-2018) | 2017–2018 | 2119 |
| Spain | Spanish National dietary survey in adults, elderly, and pregnant woman (ENALIA2) | 2013–2015 | 669 |
| Sweden | Swedish National Dietary Survey—Riksmaten adults 2010–11 (RIKSMATEN 2010) | 2010–2011 | 1430 |
| United Kingdom | National Diet and Nutrition Survey (NDNS) | 2000–2001 | 1724 |
Concerning food composition, concentration in each food was expressed in ng/g. In the case of wine, the corresponding density was used to convert the intake to ng/g. Finally, HT dietary intake in each country was expressed as the sum of the contribution of all the foods under study in mg/day/person. Statistical analyses were performed using STATISTICA 7® (Palo Alto, CA, USA).
3. HT Health Benefits and Mechanisms of Action
HT may act in vivo as a strong anti-inflammatory agent, inhibiting lipopolysaccharide (LPS)-mediated expression of inflammatory cytokines, i.e., TNF-α and IL-1β [5]. Indeed, these effects are also related to a neuroprotective role. Microglial activation, expression of NADPH oxidase and MAPKs, production of ROS, and activation of the inflammasome induced by LPS were reduced or prevented by HT. HT was also able to decrease the activation of microglial cells after alpha-synuclein (α-syn) treatment [6], thus demonstrating its capacity to reduce neuroinflammation. Furthermore, it is important to highlight its neuroprotective effect against Parkinson and Alzheimer’s diseases, being able to inhibit the formation of α-synuclein [7,8] and β-amyloid fibrils [9], respectively.
Furthermore, HT exerts antiangiogenic effect by VEGF receptor-2 (VEGFR-2) inhibition [10]. Angiogenesis in adults is involved in the development of cancer and cardiovascular diseases, favoring both tumor development and the development and destabilization of the atheroma plaque [11]. Indeed, tumors express various pro-angiogenic factors, the main one being VEGF, which binds to VEGFR-2 [12]. In addition, HT decreases proliferation in MCF-7 (Michigan Cancer Foundation-7) breast cancer cell model [13]. This effect may be linked to its pro-apoptotic activity [14].
In addition, several studies indicate that HT has an insulin-like effect on target cells including adipocytes, hepatocytes and muscle cells, exerting significant anti-diabetic effects in animals’ models of type 2 diabetes mellitus [61,62].
Certainly, its best-known biological effect is related to blood lipids’ protection from oxidative stress. [15]. In particular, the EU authorized health claim states that “Olive oil polyphenols contribute to the protection of blood lipids from oxidative stress”, based on the protection of LDL particles from oxidative damage. The health claim can be applied to olive oils containing at least 5 mg of HT and its derivatives (e.g., oleuropein complex and TYR) per 20 g of olive oil. Table 5 summarizes the HT mechanisms of action.
Table 5.
Summary of the health benefits and mechanism of action of HT.
| Health Benefit | Mechanism of Action | References |
|---|---|---|
| Anti-inflammatory | Inhibition of LPS mediated expression of TNF-α and IL-1β ↓ Expression of NADPH oxidase and MAPKs ↓ Inflammasome |
[5,6] |
| Neuroprotective | Inhibition of the formation of α-synuclein and β-amyloid fibrils | [7,8,9] |
| Antiangiogenic | Inhibition of VEGF receptor-2 activation | [10] |
| Pro-apoptotic | ↓ Proliferation of MCF-7 | [13,14] |
| Anti-diabetic | Insulin-like effect on target cells | [61,62] |
| Antioxidant | Blood lipids’ protection from oxidative stress | [15] |
4. Determination of HT in Foods
In order to evaluate dietary intake, it is crucial to select reliable data on food composition that largely depends on the performance of the analytical determination. Therefore, it is useful to understand the advantages and drawbacks of the analytical methods used and the possible matrix effect and interferences. HT is present as a minor compound within a complex food matrix, and it is generally determined simultaneously with the phenolic profile. In fact, regarding olive oil, the official method of the International Olive Council (IOC) [63] quantifies HT together with TYR, natural and oxidized oleuropein and ligstroside derivatives, lignans, flavonoids and phenolic acids in olive oil, overall expressed as biophenols.
The first step and most critical point when analyzing HT in olive oil is its extraction from the lipidic food matrix. Solid phase extraction and liquid–liquid (methanol:water 80:20) extraction have been used [64] to extract the polar fraction that contains HT. This last method, which performs acidic hydrolysis before extraction, is expected to become an official method.
Other food matrixes required different pre-treatments. Olive fruits are grinded, followed by extraction with methanol, ethanol or mixtures of both with water. Then, n-hexane is the solvent most frequently used to purify the extract. Optionally, freeze-drying can be performed [65]. In some cases, the extracts are loaded onto SPE cartridges with hexane followed by methanol to eluate HT. For the wine matrix analysis, direct injection of the sample was used with high performance liquid chromatography (HPLC) coupled to fluorescence detectors [58]. Álvarez-Fernández et al. [53] proposed cleaning up the sample with C18 SPE cartridges followed by elution with methanol for subsequent determination by ultrahigh performance liquid chromatography couple to high-resolution mass spectrometry (UHPLC/HRMS). Additionally, the wine extract can be treated after purification with an Amberlite column and separated by high-speed counter current chromatography prior quantification by DAD or HRMS [66].
Liquid chromatography is the most widespread and reliable method for analyzing HT individually in the different foods as displayed in Table 1, Table 2 and Table 3. Reversed-phase, which is based on partition chromatography, is the preferred mode of separation for most polar compounds such as HT in terms of reproducibility of retention time and separation [66]. It separates individual components using a non-polar octade-cylsilane (C18) column as the bonded stationary phase, while the mobile phase is a polar solvent consisting of water acidified with orthophosphoric acid, formic acid, acetic acid, or trichloroacetic acid, and methanol or acetonitrile [27,33,53]. Different detection techniques include the use of a diode array (DAD) detector at 280 nm since phenols possess a strong chromophore system, providing considerable structural information that helps distinguish the type of phenol and the oxidation pattern [67]. Additionally, a fluorescence detector has also been used since HT shows a good response to fluorescence excitation (λex = 279 nm and λem = 631 nm) [68]. However, the most extended powerful analytical tool is the liquid chromatography coupled with mass spectrometry (LC-MS) as a detection system, which provides unequivocal identification based on the molecular masses of the separated compounds obtained through prominent ions [69]. The limit of detection (LOD) and quantitation (LOQ) differ among the techniques being up to 0.09 mg/L and 0.3 mg/L for DAD, and 0.023 mg/L and 0.076 mg/L for fluorescence, [52,58] and 0.5 µg/L and 1 µg/L for LC-MS/MS, respectively [17].
5. Dietary Sources
Despite being a phenolic compound, HT is present in limited food sources. HT has been identified in foods characteristic of the Mediterranean diet such as table olives, olive oil and wine (Table 1, Table 2 and Table 3). HT and its derivates compounds (e.g., oleuro-pein complex and tyrosol, as defined by EFSA [15]) have been only reported in table olives and olive oil (Table 1 and Table 2). Additionally, HT is synthesized by yeast during the alcoholic fermentation and found consequently in wine (Table 3). Solely one study has reported HT in commercial beers at a concentration (ca. 0.03 mg/L) significantly lower than the abovementioned dietary sources [70].
5.1. Olives
Up to 36 phenolic compounds have been identified in olive fruits, with secoiridoids being the most abundant and widespread (Figure 1). Among them, oleuropein and ligstroside are the major main native compounds [71]. Their breakdown products include relevant phenolic constituents of the olive fruit such as HT, oleocanthal, elenolic acid, oleuropein aglycone, and TYR [71]. During olive ripening, storage, and processing, hydrolysis of the secoiridoid compounds yields HT [72]. Generally, as oleuropein decreases, HT increases during maturation, making this substance the main compound in mature olives.
Most table olives worldwide are processed using one of three methods: Spanish-style (green olives), Californian-style (olives darkened by oxidation), or Greek-style (natural darkened olives) processing. Spanish-style includes lye treatment, brining and fermentation. Lye treatment with a NaOH solution (1.5–4.5 w/v) causes the hydrolysis of oleuropein to yield HT and elenolic acid glucoside [73]. Furthermore, yeasts and lactic acid bacteria, naturally present in olives and brine, can metabolize oleuropein in a two-step process. The first step comprises the hydrolysis of the glycosidic linkage of oleuropein by β-glucosidase to form oleuropein-aglycone. Subsequently, this aglycone is hydrolyzed to elenolic acid and HT, probably by an esterase in a second step.
Californian-style black olive processing consists of preserving the fruits in brine or an acidified solution followed by darkening with air under alkaline conditions. The first step causes the diffusion of polyphenols, mainly oleuropein, from the olive flesh into the solution and acid hydrolysis takes place as previously mentioned. Subsequently, the darkening step basically causes ortho-diphenols to be oxidized and polymerized resulting in a decrease in HT. After harvesting, the olives are placed in brine where they are fermented. Similar to the other described processes, the acid hydrolysis of oleuropein and HT glucoside occurs.
Table 1 summarizes data published in the literature on HT and derived compounds present in table olives. HT concentration reported by different authors using the above-mentioned analytical methods does not reflect significant differences (p > 0.05) between Spanish and Greek table olives. Values range from 14.49 to 3750 mg/kg and from 134.33 to 1393.30 mg/kg for Spanish and Greek style, respectively. Values for Californian style olives are in general lower, ranging from 19.98 to 672.40 mg/kg. This observation is in agreement with the results reported by Johnson et al. [27], whose predominant phenolic compound was HT in all three styles of commercial olives with similar concentrations observed for Greek and Spanish olives (134.329 and 133.685 mg/kg, respectively) and significantly lower concentrations for Californian olives (19.981 mg/kg). Romero et al. [74] detected the presence of hydroxytyrosol 4-β-D-glucoside in the olive pulp of the Manzanilla and Picual varieties, pinpointing that not only the hydrolysis of oleuropein produces HT, but it can also come from hydroxytyrosol 4-β-D-glucoside hydrolysis. Indeed, Arroyo López et al. [75] found hydroxytyrosol 4-β-D-glucoside content was greater than that of oleuropein.
As can be seen in Table 1, HT is the main phenolic compound in edible olives followed by oleuropein and TYR. In summary, the processing method is the most determinant factor compared to the variety or geographical origin.
5.2. Olive Oil
Table 2 summarizes the concentration of HT, TYR, hydroxytyrosol acetate (HT-AC), oleuropein-aglycone di-aldehyde (3,4-DHPEA-EDA), oleuropein-aglycone mono-aldehyde (3,4-DHPEA-EA), ligstroside-aglycone di-aldehyde (p-HPEA-EDA), oleuropein (OLE), and secoiridoid derivatives (S-DER) (Figure 1) reported in olive oils from different origins, varieties and categories. As can be seen, the maximum and minimum concentration of HT determined are between 41.3 mg/kg and 0.09 mg/kg, respectively, with noticeable differences according to origin. In particular, the highest mean HT concentration is found in olive oils from Spain (13.31 ± 39.45 mg/kg), followed by Greece (11.17 ± 8.22 mg/kg), and Italy (10.24 ± 29.09 mg/kg). Samples from other origins, such as Tunisia, Algeria, Morocco, and Turkey, present lower concentrations as follows: 5.51 ± 2.36 mg/kg, 3.86 ± 2.73 mg/kg, 3.29 ± 2.44 mg/kg, and 1.89 ± 2.39 mg/kg, respectively.
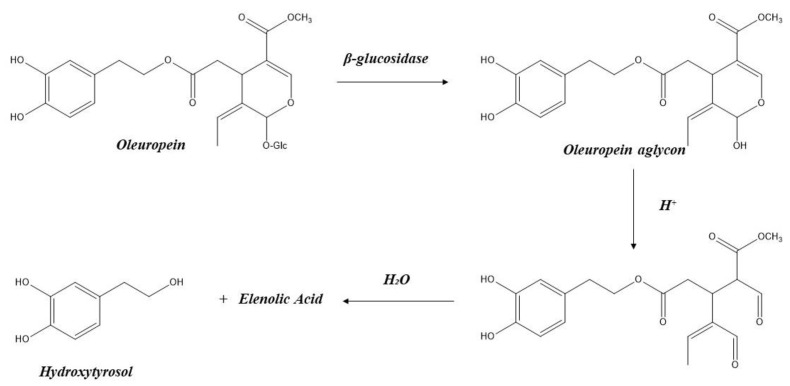
Some analytical procedures include an acid hydrolysis that allows the determination of not only free HT content in olive oil but also the resultant from oleuropein hydrolytic degradation [76] (Figure 2); these values reach 151.5 mg/kg for an oil of Italian origin and 200 mg/kg for an olive oil of Spanish origin. Hence, these values were not considered for the dietary HT intake estimation. It is difficult to ascertain the actual concentrations of oleuropein and HT since different values will be obtained depending on the storage time of the oil prior to analysis. Therefore, as the storage time increases, the concentration of oleuropein decreases and that of HT increases [77]. When we applied analysis of variance (ANOVA) to the HT data displayed in Table 3, no significant differences were observed between the different varieties or between VOO and EVOO (extra virgin olive oil).
Figure 2.
Hydroxytyrosol formation pathway from oleuropein in olive oil.
In addition, secoiridoids are hydrolyzed during oil storage, giving rise to the simple phenolic compounds HT and TYR [77]. In fact, HT and TYR concentrations increased after one year of storage in three olive oil varieties, in the following order from highest to lowest concentration: Picual > Hojiblanca > Arbequina [77]. A recent study [78] confirms those findings, observing very low HT (4.72 µg/g of EVOO) and tyrosol (5.47 µg/g of EVOO) concentrations in fresh EVOOs and higher concentrations in one-year old EVOO (20.18 µg/g of EVOO and 54.51 µg/g of EVOO, respectively). Thus, we can conclude that fresh EVOO has a high content of oleocanthal and oleacein and a low concentration of tyrosol and hydroxytyrosol; the hydrolytic processes during the storage time led to the formation of tyrosol and hydroxytyrosol, from their precursors.
Apart from storage time, it must be considered that olive oil is also consumed after heating such as frying, boiling, and oven cooking, which substantially impact the concentration of phenolic compounds to a different degree, depending on the initial concentration and treatment characteristics (time, temperature, humidity). HT derivatives are the first antioxidants lost during thermal oxidation (up to a peroxide value of 20–30 mEq/kg), and TYR derivatives seem to be the most stable compounds [79].
5.3. Wine
Di Tommaso, Calabrese, & Rotilio [57] found HT for the first time in wines at concentrations ranging from 1.9 mg/L to 4.0 mg/L. Later, HT was identified in different wines worldwide varying from 1.5 to 41.5 mg/L as displayed in Table 3. As can be observed in this table, a high percentage of references (around 83%) report an HT concentration of between 1–4 mg/L, regardless the type of wine, origin or variety. In contrast to olives’ composition, TYR is frequently present in wines at higher concentrations than HT, as displayed in Table 3.
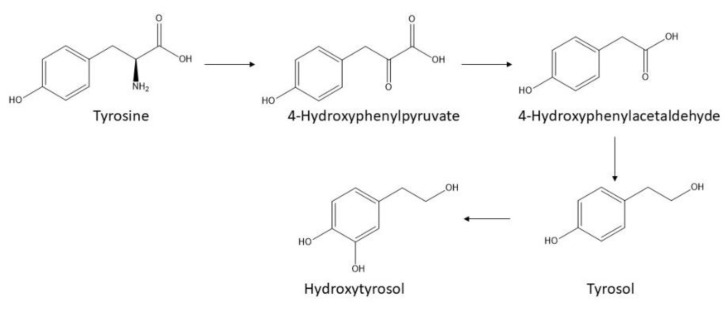
HT occurrence in wine can be related to yeasts’ metabolism. In fact, during alcoholic fermentation, the yeasts metabolize aromatic amino acids by the Ehrlich pathway. As Figure 3 shows, tyrosine can be transformed into p-hydroxyphenylpyruvate by a transamination reaction. Subsequently, decarboxylation of this last compound produces p-hydroxyphenylacetaldehyde, which can be metabolized to form the fusel alcohol [80]. Finally, a tyrosine hydroxylase can generate HT from TYR. Álvarez-Fernández et al. [53] identified HT in the intracellular compartment of wine-making yeast both in Saccharomyces cerevisiae strains and non-Saccharomyces Torulaspora delbrueckii, providing unequivocal proof of the yeast’s role.
Figure 3.
Illustration of the biosynthetic pathway of hydroxytyrosol (3,4-dihydroxyphenethyl alcohol, DOPET, 3-hydroxytyrosol, and homoprotocatechuyl alcohol. The empirical formula of hydroxytyrosol is C8H10O3 and it has a molecular weight of 154.16 g/mol) from tyrosine for different organisms.
In both white and red wines, HT has also been detected at concentrations ranging from 0.28 to 9.6 mg/L. As Table 3 reports, the concentrations determined by different authors do not show a clear difference between white and red wines. Furthermore, no conclusion can be inferred regarding the variety, origin, aging or year of the wine.
6. Estimation of HT Dietary Intake
Different strategies have been proposed to evaluate the dietary intake of micronutrients or bioactive compounds. For most nutrients and bioactives, dietary surveys of food intake, questionnaires, and the use of food composition databases to transform food consumption into nutrient intake can be considered a suitable approach [60].
In this context, the determination of HT in urine has been associated with the intake of foods rich in this phenolic compound such as olives, olive oil, and wine [81]. In particular, it could be proposed as a marker to monitor compliance in the consumption of dietary extra virgin olive oil [27]. Nevertheless, Schröder et al. [82] reported higher urinary recovery of HT than expected after red wine consumption. This fact led researchers to hypothesize that an endogenous synthesis of HT may occur. Thereafter, human intervention studies confirmed higher HT sulfate metabolism after alcohol consumption [83] in a dose-dependent manner. Furthermore, excretion after wine consumption was higher than excretion after alcohol or dealcoholized wine [84]. Considering these data as a whole, it is clear that HT is not only a compound present in foods, but also a human metabolite related to tyramine and dopamine metabolism [85].
Several studies have demonstrated that TYR is converted to HT in vivo [86]. Therefore, these findings imply that TYR intake has to be taken into account when discussing circulating HT levels. Thus, urinary excretion of HT might not be an accurate marker of dietary HT intake. Consequently, another approach should be considered based on the available scientific available data on food composition and food intake.
Table 6 estimates the daily intake of free HT from the consumption of the foods under study (range of means) based on the reported mean free HT content in extra-virgin olive oils (5.2 µg/g), table olives (629.1 µg/g) and wine (2.1 µg/mL) (Table 1, Table 2 and Table 3) and their estimated intake in the EU, as reported in the EFSA Comprehensive Food Consumption Database [59]. These data are based on surveys of more than 45,000 participants (Table 4). Although HT has been reported in beer, it has not been included in the estimation of HT dietary intake given the limited information so far available [70].
Table 6.
Extra Virgin Olive Oil (EVOO)/ Virgin Olive Oil (VOO), table olives and wine (according to levels 3–6 of the FoodEx2 classification system for the adult population) consumption and free hydroxytyrosol daily intake for adult consumers (18–64 years) based on the EFSA Comprehensive European Food Consumption Database.
| Foods under Study | Food Consumption | Free Hydroxytyrosol Daily Intake from Foods under Study | HT from Oleuropein and Aglycone |
|---|---|---|---|
| Range of Means among EU Surveys (g/day) | Range of Means (mg/day) | Range of Means (mg/day) | |
| EVOO/VOO | 0–33.78 | 0–0.18 | 0–1.08 |
| Table olives | 0.02–3.41 | 0.010–2.14 | 0.03–6.42 |
| Wine | 10.65–89.08 | 0.022–0.186 | - |
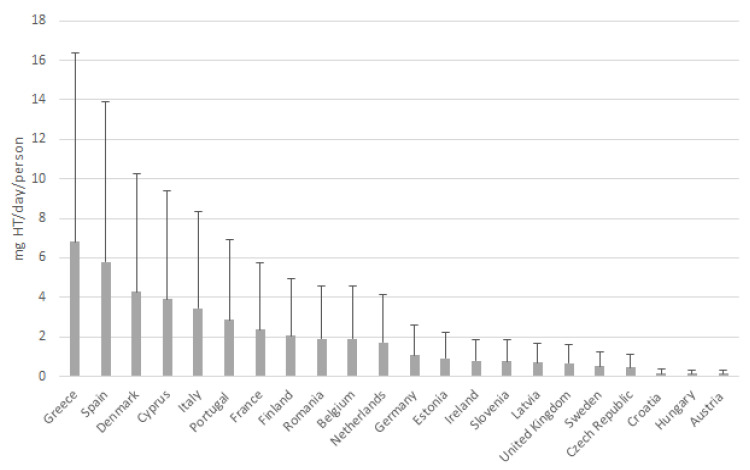
Figure 4 shows the estimation of the mean dietary intake of free HT in the adult population in each of the twenty-two European countries under study. The HT intake in the EU is within the range of 0.13–6.82 mg/day. The country with the highest free HT intake was Greece (6.82 mg/day), followed by Spain (5.79 mg/day), Denmark (4.28 mg/day), Cyprus (3.90 mg/day) and Italy (3.46 mg/day). In contrast, Austria, Hungary, and Croatia showed the lowest intake (0.13, 0.13, and 0.14 mg/day, respectively). The HT intake of the rest of the countries was within 0.46–3.90 mg/day. The estimated mean HT dietary intake in EU countries is 1.97 ± 2.62 mg/day. Only Greece and Spain have mean values that reach an adequate amount to exert beneficial cardiovascular effects of 5 mg/day [15].
Figure 4.
Estimation of the mean free HT dietary intake in the EU adult population.
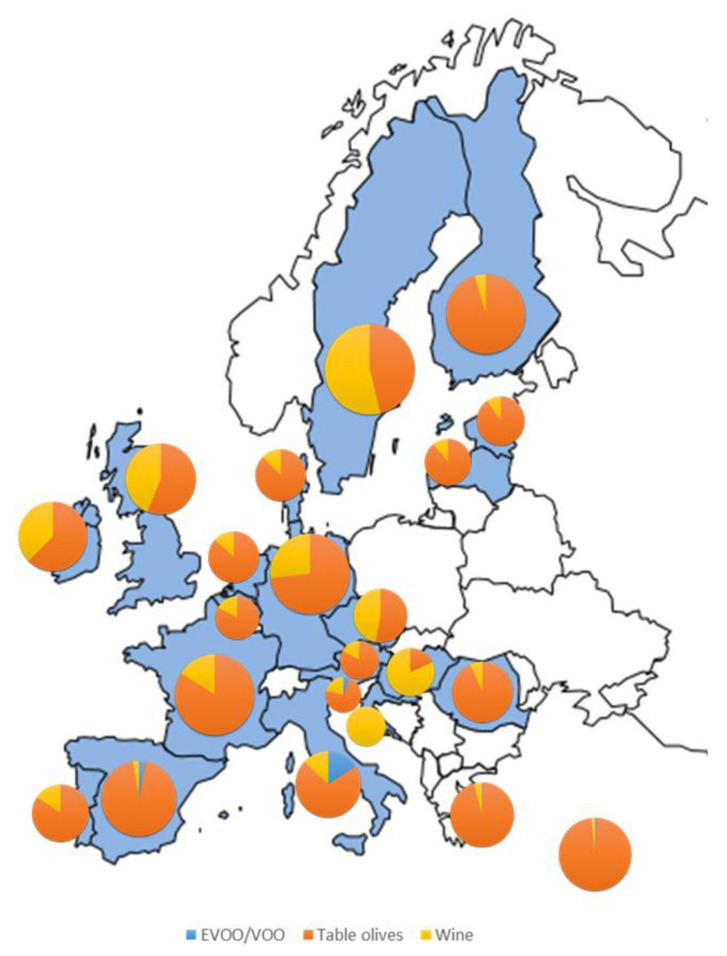
Figure 5 presents the contribution of the different foods under study to HT intake in the different EU countries. As can be seen, table olives and wine are the main contributors to the HT dietary intake in UE countries. Specifically, olives are the principal HT dietary source in all the countries except for Croatia, Hungary, and Sweden, where wine is the biggest contributor one. Although the amount of table olives effectively consumed is not especially relevant, their HT content is the highest among foods. Therefore, we may assume that EU adults that do not consume wine or olives will not consume HT in their diets. Surprisingly, EVOO/VOO significantly contributes to free HT intake only in some of the Mediterranean basin countries (Italy, Spain, Cyprus, and Greece) and in Slovenia (16%, 3%, 0.7%, 0.6% and 6% of the overall HT dietary intake, respectively).
Figure 5.
Relative contribution of EVOO/VOO, olives, and wine to free HT dietary intake in the European adult population by country (percentage %).
The estimation of HT intake was based on free HT. However, EVOO/VOO and table olives also contain oleuropein and oleuropein-aglycone, which can be hydrolyzed to HT as it is bioavailable, thus contributing to total HT intake [87]. Therefore, the actual total dietary HT intake may be higher than the estimated exposure based solely on free HT. Accordingly, the EFSA Scientific Panel assessed that total HT exposure from olives and EVOO/VOO may be estimated to be approximately three and six times higher, respectively, than the exclusive exposure to free HT from these foods [88]. Taking into account these values, total HT intake from EVOO/VOO and table olives would range between 0 and 1.08 mg/day and between 0.03 and 6.42 mg/day, respectively. Therefore, the contribution of EVOO/VOO to total HT intake in the Mediterranean basin countries and in Slovenia would be approximately two times higher (30% in Italy, 6% in Spain, 1% in Cyprus, 1% in Greece, and 13% in Slovenia). Additionally, we should consider the fact that some surveys underestimate EVOO/VOO consumption since they include all the olive oils as one food item and no differentiation is made by the subjects. Moreover, thermal oxidation of EVOO/VOO during frying (180 °C) for 30–60 min causes HT losses between 60% and 90% [89], which in turn would overestimate the HT intake from EVOO/VOO when used for frying.
The EFSA Scientific Panel estimated the intake of dietary HT in the EU population just based solely on the contribution of olive oils and table olives as main food sources [88]. Additionally, the EFSA Panel used the Phenol-Explored database, which was developed with the literature available until 2009, to calculate the mean HT content in these foods (0.007 mg/g in EVOO/VOO and 0.65 mg/g in table olives). However, the present study includes current literature published up to 2022. Additionally, our data show for the first time that wine is one of the main contributors, together with olives, to the dietary HT intake in the EU population. Therefore, wine consumption should be taken into account for the assessment of dietary exposure to this compound to avoid its underestimation.
Total dietary polyphenol intake in the adult population of EU countries has been reported to be between 1706 mg/day (Denmark) and 664 mg/day (Greece) [90]. Considering the mean HT intake for these countries, the relative contribution of the single compound HT to the total polyphenol intake would be around 0.2–1%, respectively. Mean HT intake (1.9 mg/day) is within the magnitude order of the intake of certain individual anthocyanins, such as peonidin and petunidin (1.6 mg/day and 1.13 mg/day, respectively) [91], certain flavonols, such as isorhamnetin (2 mg/day), the flavanone eriodictyol (1 mg/day), and the flavone luteolin (1 mg/day) estimated for the EU population [60].
7. Hydroxytyrosol as a Food Ingredient and Future Trends
The EFSA delivered a positive opinion on the health claim “Olive oil polyphenols contribute to the protection of blood lipids from oxidative stress” for extra virgin olive oil (20 g) containing 5 mg of HT and its derivatives [15]. Moreover, HT is recognized as safe by the European Food Safety Authority [88] and, consequently, the European Commission (2017) [92] authorized the placement of HT in the market as a novel food ingredient under Regulation (EC) Nº 258/97. The food categories authorized for HT addition are fish and vegetable oils (0.215 g/kg) and spreadable fats (0.175 g/kg). According to the dietary data from the EU [88], it is estimated that the mean values for HT consumption in adults are far from the toxicity level, which emphasizes the suitability of the incorporation of HT into other types of products.
The antioxidant and antimicrobial character of HT as well as its bioactive potential is directing the use of HT as a food ingredient. At present, this strategy is almost restricted to the scientific field; however, the promising results obtained may consolidate the addition of HT to foods as a trend in the future. Table 7 presents several formulations, including the amount of HT proposed, its origin, and the food category subject to addition such as yogurts, cookies, blood orange juice and smoothies, increasing the bioactive potential of a diet. Additionally, it may be added to meat-derived products to prevent oxidation in line with consumers’ preferences for more natural ingredients.
Table 7.
Food ingredients and future trends.
| Food | Quantity | Origin | References |
|---|---|---|---|
| Yogurt | 5 mg HT/120 g (1 unit = 120 g) | Olive Pomace Liquid-enriched powder (LOPP) and pulp-enriched powder (POPP) obtained from olive pomace |
[93] |
| Fuet (Dried cured sausage) | 200 mg/kg | Synthetic | [94] |
| Fuet (Dried cured sausage) | 200 mg/kg | Olive vegetation waters of olive | [94] |
| Cookies | 5.25 mg/30 g | Olive oil wastewaters | [95] |
| Smoothies | 5.78 mg HT + OLE/100 g | Olive leaf extracts | [96] |
| Chicken sausage | 50 mg/kg | Olive water/olive leaves | [97] |
| Blood orange juice | 28.8–57.6 mg/L | Olive mill wastewater | [98] |
HT can be obtained by chemical synthesis, produced by biotechnological procedures, or extracted and purified from wastes from food processing, mainly olive oil production and its by-products such as leaves. Indeed, olive oil residues are a natural source of HT in addition to other phenolic compounds. Consequently, different strategies to recover HT from wastes have been evaluated to select the richest waste source and the most efficient and greenest process. The point is to use a polluting product to obtain a natural food ingredient with healthy properties. Lesage-Meessen et al. [99] determined that the extracts obtained by the two-phase olive oil system were significantly higher in HT (1.4 fold) than those obtained for the three-phase method. Allouche, Fki, & Sayadi, [100] obtained a good yield (85.46%) thanks to ethyl acetate used in the batch, allowing HT to be recovered from three-phase olive processing wastewaters. Microfiltration allowed the recovery of HT, which was the main compound in the permeate, representing 54% of the total polyphenols with a HT concentration of 88.7 mg/L [101]. Furthermore, solid phase extraction was evaluated to compare the capacity of different resins to adsorb HT from wastewaters and solvents to desorb it from the resins [102]. The ENV+ resin was able to adsorb almost all the HT and the acidified ethanol mobilized almost all the polyphenols (including HT) adsorbed onto the resins. In addition, ultrasonication has been suggested to improve recovery [103]. Moreover, tangential membrane filtration has been successfully applied to recover phenols from olive oil mill wastewaters, obtaining high concentrations of HT (up to 7203.7 mg/L in the concentrate) [98]. Additionally, enzymatic hydrolysis to release HT has also been evaluated using a culture in Aspergillus niger broth, thus the culture increases the amount of HT obtained [104]. More recently, dried olive mill wastewaters have been obtained using a spray dryer. The resulting phenolic rich dried powder contains, among other phenols, substantial amounts of HT, tyrosol and oleuropein (1.48, 2.04 and 103 mg/kg dry weight, respectively) [105].
A hydrothermal treatment was proposed to obtain HT from solid waste from two-phase olive oil extraction or “alperujo” [106] to provoke an autohydrolysis process which favored subsequent HT extraction. Serrano et al. [107] extracted 1600 mg of HT per 1 kg of olive mill solid waste using a thermal pre-treatment at 170 °C for 60 min. Fernández-Bolaños et al. [108] reported having obtained 4.5–5 kg of HT from approximately 1000 kg of alperujo with 70% humidity, which could be subsequently purified to obtain at least 3 kg of HT at 90–95% of purity.
Moreover, olive leaves represent an agricultural residue that can be used as a source of bioactive compounds [109]. Oleuropein was extracted from the leaves [110] using solid phase extraction. Briante et al. [111] used a thermophilic β-glycosidase immobilized on chitosan that permitted the biotransformation of Olea europaea L. leaf extract, obtaining eluates with high amounts of HT. Similarly, a simple hydrolysis reaction allowed a relatively high amount of purified HT to be obtained from Olea europaea leaf extract (2.3 g per 100 g of fresh olive leaves) [112]. Herrero et al. [113] optimized pressurized liquid extraction, achieving up to 8.542 mg HT/g dried extract using water as an extracting agent, while oleuropein was present in ethanolic extracts (6.156–2.819 mg/g extract). Finally, HT and derivatives can also be obtained in substantial quantities (16.21 mg HT/L residue) from the wastewater generated from table olives production [114].
Summarizing, the current regulatory status for HT within the EU context as an authorized novel food ingredient that can be added to different foods as stated in the Commission Implementing Decision [92] opens possibilities of broadening its use in other foods in the future. Obtaining HT for further uses as a food ingredient from different wastes and byproducts represents a straightforward opportunity for the revalorization of olive oil cultivars, consequently, leading to a more sustainable production.
8. Conclusions
This paper compiles scientific data on HT content in the main food sources. The average content range was as follows: olives > olive oil > wines. The dietary intake in the EU population was estimated based on dietary surveys and updated with current compositional data available in the scientific literature. Greece, Spain, Denmark, Cyprus, and Italy rank as having the largest intake in adult populations. Olives and wine are the main dietary contributors to HT intake in EU. Additionally, the contribution of olive oil to HT dietary intake is relevant in Mediterranean countries (Italy, Spain, Greece, and Cyprus).
Considering HT is present only in food characteristic of the Mediterranean diet, further epidemiological research would be needed to bring to light the role of HT in chronic diseases, other than cardiovascular.
Further research is also necessary to increase the HT concentration in natural sources such as wine, by means of selecting the highest producer microorganisms and fermentation conditions. Additionally, it would be interesting to select the olive production techniques that most preserve HT concentration in the final product, avoiding its losses on the wastewater. Similarly, additional studies should be conducted to select the olive varieties and olive oil production techniques that lead to a higher HT and its precursors’ concentration. Moreover, HT obtained from waste and by-products of the olive industry can be considered a valuable source for HT as a food ingredient to be added to other foods, nutraceuticals, and food supplements, thus increasing the bioactive potential and its dietary intake. Hence, further studies should comprise the selection of efficient and environment friendly HT extraction and purification techniques.
Author Contributions
Conceptualization, A.B.C., A.M.T. and M.C.G.-P.; Formal Analysis, M.G.-F., M.G.-R., A.B.C., A.M.T. and M.C.G.-P.; Investigation, M.G.-F., M.G.-R., A.B.C., A.M.T. and M.C.G.-P.; Data Curation, M.G.-F., M.G.-R., A.B.C., A.M.T. and M.C.G.-P.; Writing—Original Draft Preparation, M.G.-F., M.G.-R., A.B.C., A.M.T. and M.C.G.-P.; Writing—Review and Editing, A.B.C., A.M.T. and M.C.G.-P.; Visualization, M.G.-F., M.G.-R., A.B.C., A.M.T. and M.C.G.-P.; Supervision, A.B.C., A.M.T. and M.C.G.-P.; Project Administration, A.M.T. and M.C.G.-P.; Funding Acquisition, A.M.T. and M.C.G.-P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available from the corresponding author on reasonable request.
Conflicts of Interest
Authors declare no conflicts of interest.
Funding Statement
The authors are very grateful to the Spanish Government for its financial assistance (Project MICINN PID2019-108722RB-C32), the Junta de Andalucía (P18-RT-3098, US-1263469), and the financial support of the Ministry of Science, Innovation and Universities. (M.G.F.’s FPI contract).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villaño D., Fernández-Pachón M.S., Troncoso A.M., García-Parrilla M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta. 2005;538:391–398. doi: 10.1016/j.aca.2005.02.016. [DOI] [Google Scholar]
- 2.Napolitano A., De Lucia M., Panzella L., d’Ischia M. The chemistry of tyrosol and hydroxytyrosol: Implications for oxidative stress. Olives Olive Oil Health Dis. Prev. 2010;134:1225–1232. doi: 10.1016/B978-0-12-374420-3.00134-0. [DOI] [Google Scholar]
- 3.Fitó M., Cladellas M., De La Torre R., Marti J., Alcántara M., Pujadas-Bastardes M., Marrugat J., Bruguera J., López-Sabater M.C., Vila J., et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis. 2005;181:149–158. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez N., Herrera M., Frías L., Provencio M., Pérez-Carrión R., Díaz V., Morse M., Crespo M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019;21:489–498. doi: 10.1007/s12094-018-1950-0. [DOI] [PubMed] [Google Scholar]
- 6.Gallardo-Fernández M., Hornedo-Ortega R., Alonso-Bellido I.M., Rodríguez-Gómez J.A., Troncoso A.M., García-Parrilla M.C., Venero J.L., Espinosa-Oliva A.M., De Pablos R.M. Hydroxytyrosol decreases LPS-and α-synuclein-induced microglial activation in vitro. Antioxidants. 2020;9:36. doi: 10.3390/antiox9010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornedo-Ortega R., Cerezo A.B., Troncoso A.M., Garcia-Parrilla M.C. Protective effects of hydroxytyrosol against α-synuclein toxicity on PC12 cells and fibril formation. Food Chem. Toxicol. 2018;120:41–49. doi: 10.1016/j.fct.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 8.Gallardo-Fernández M., Hornedo-Ortega R., Cerezo A.B., Troncoso A.M., García-Parrilla M.C. Melatonin, protocatechuic acid and hydroxytyrosol effects on vitagenes system against alpha-synuclein toxicity. FCT. 2019;134:110817. doi: 10.1016/j.fct.2019.110817. [DOI] [PubMed] [Google Scholar]
- 9.St-Laurent-Thibault C., Arseneault M., Longpre F., Ramassamy C. Tyrosol and hydroxytyrosol two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011;8:543–551. doi: 10.2174/156720511796391845. [DOI] [PubMed] [Google Scholar]
- 10.Cerezo A.B., Labrador M., Gutiérrez A., Hornedo-Ortega R., Troncoso A.M., Garcia-Parrilla M.C. Anti-VEGF signalling mechanism in HUVECs by melatonin, serotonin, hydroxytyrosol and other bioactive compounds. Nutrients. 2019;11:2421. doi: 10.3390/nu11102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yehya A.H.S., Asif M., Petersen S.H., Subramaniam A.V., Kono K., Majid A.M.S.A., Oon C.E. Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina. 2018;54:8. doi: 10.3390/medicina54010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 13.Chimento A., Casaburi I., Rosano C., Avena P., De Luca A., Campana C., Martire E., Francesca Santolla M., Maggiolini M., Pezzi V., et al. Oleuropein and hydroxytyrosol activate GPER/GPR 30-dependent pathways leading to apoptosis of ER-negative SKBR 3 breast cancer cells. Mol. Nutr. Food Res. 2014;58:478–489. doi: 10.1002/mnfr.201300323. [DOI] [PubMed] [Google Scholar]
- 14.Han J., Talorete T.P., Yamada P., Isoda H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology. 2009;59:45–53. doi: 10.1007/s10616-009-9191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EFSA Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9:2033. doi: 10.2903/j.efsa.2011.2033. [DOI] [Google Scholar]
- 16.Neveu V., Perez-Jiménez J., Vos F., Crespy V., du Chaffaut L., Mennen L., Knox C., Eisner R., Cruz J., Wishart D., et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database J. Biol. Databases Curation. 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno-González R., Juan M.E., Planas J.M. Table olive polyphenols: A simultaneous determination by liquid chromatography–mass spectrometry. J. Chromatogr. A. 2020;1609:460434. doi: 10.1016/j.chroma.2019.460434. [DOI] [PubMed] [Google Scholar]
- 18.Fernández A., Talaverano M.I., Pérez-Nevado F., Boselli E., Cordeiro A.M., Martillanes S., Foligni R., Martín-Vertedor D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020;44:e14384. doi: 10.1111/jfpp.14384. [DOI] [Google Scholar]
- 19.Schaide T., Cabrera-Bañegil M., Pérez-Nevado F., Esperilla A., Martín-Vertedor D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019;56:3001–3013. doi: 10.1007/s13197-019-03782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García P., Romero C., Brenes M. Bioactive substances in black ripe olives produced in Spain and the USA. J. Food Compos. Anal. 2018;66:193–198. doi: 10.1016/j.jfca.2017.12.022. [DOI] [Google Scholar]
- 21.García P., Romero C., Brenes M. Influence of olive tree irrigation and the preservation system on the fruit characteristics of Hojiblanca black ripe olives. LWT Food Sci. Technol. 2014;55:403–407. doi: 10.1016/j.lwt.2013.06.015. [DOI] [Google Scholar]
- 22.Lodolini E.M., Cabrera-Bañegil M., Fernández A., Delgado-Adámez J., Ramírez R., Martín-Vertedor D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019;286:250–259. doi: 10.1016/j.foodchem.2019.01.191. [DOI] [PubMed] [Google Scholar]
- 23.Caponio F., Difonzo G., Calasso M., Cosmai L., De Angelis M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Int. Food Res. J. 2019;116:1306–1317. doi: 10.1016/j.foodres.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 24.D’Antuono I., Bruno A., Linsalata V., Minervini F., Garbetta A., Tufariello M., Mita G., Logrieco A.F., Bleve G., Cardinali A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018;248:137–145. doi: 10.1016/j.foodchem.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Ambra R., Natella F., Bello C., Lucchetti S., Forte V., Pastore G. Phenolics fate in table olives (Olea europaea L. cv. Nocellara del Belice) debittered using the Spanish and Castelvetrano methods. Int. Food Res. J. 2017;100:369–376. doi: 10.1016/j.foodres.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Pereira J.A., Pereira A.P., Ferreira I.C., Valentão P., Andrade P.B., Seabra R., Estevinho L., Bento A. Table olives from Portugal: Phenolic compounds, antioxidant potential, and antimicrobial activity. J. Agric. Food Chem. 2006;54:8425–8431. doi: 10.1021/jf061769j. [DOI] [PubMed] [Google Scholar]
- 27.Johnson R., Melliou E., Zweigenbaum J., Mitchell A.E. Quantitation of Oleuropein and Related Phenolics in Cured Spanish-Style Green, California-Style Black Ripe, and Greek-Style Natural Fermentation Olives. J. Agric. Food Chem. 2018;66:2121–2128. doi: 10.1021/acs.jafc.7b06025. [DOI] [PubMed] [Google Scholar]
- 28.Melliou E., Zweigenbaum J.A., Mitchell A.E. Ultrahigh-pressure liquid chromatography triple-quadrupole tandem mass spectrometry quantitation of polyphenols and secoiridoids in California-style black ripe olives and dry salt-cured olives. J. Agric. Food Chem. 2015;63:2400–2405. doi: 10.1021/jf506367e. [DOI] [PubMed] [Google Scholar]
- 29.Mettouchi S., Sacchi R., Moussa Z.O., Paduano A., Savarese M., Tamendjari A. Effect of Spanish style processing on the phenolic compounds and antioxidant activity of Algerian green table olives. Grasas y Aceites. 2016;67:e114. doi: 10.3989/gya. [DOI] [Google Scholar]
- 30.Othman N.B., Roblain D., Chammen N., Thonart P., Hamdi M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009;116:662–669. doi: 10.1016/j.foodchem.2009.02.084. [DOI] [Google Scholar]
- 31.Selli S., Kelebek H., Kesen S., Sonmezdag A.S. GC-MS olfactometric and LC-DAD-ESI-MS/MS characterization of key odorants and phenolic compounds in black dry-salted olives. J. Sci. Food Agric. 2018;98:4104–4111. doi: 10.1002/jsfa.8927. [DOI] [PubMed] [Google Scholar]
- 32.Leporini M., Loizzo M.R., Tenuta M.C., Falco T., Sicari V., Pellicanò T.M., Tundis R. Calabrian extra-virgin olive oil from Frantoio cultivar: Chemical composition and health properties. Emir. J. Food Agric. 2018;9:1014. doi: 10.3390/foods9081014. [DOI] [Google Scholar]
- 33.Deiana P., Santona M., Dettori S., Molinu M.G., Dore A., Culeddu N., Azara E., Naziri E., Tsimidou M.Z. Can all the Sardinian varieties support the PDO “Sardegna” virgin olive oil? Eur. J. Lipid Sci. Technol. 2019;121:1800135. doi: 10.1002/ejlt.201800135. [DOI] [Google Scholar]
- 34.Bellumori M., Cecchi L., Innocenti M., Clodoveo M.L., Corbo F., Mulinacci N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules. 2019;24:2179. doi: 10.3390/molecules24112179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trombetta D., Smeriglio A., Marcoccia D., Giofrè S.V., Toscano G., Mazzotti F., Giovanazzi A., Lorenzetti S. Analytical evaluation and antioxidant properties of some secondary metabolites in northern Italian mono-and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int. J. Mol. 2017;18:797. doi: 10.3390/ijms18040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cioffi G., Pesca M.S., De Caprariis P., Braca A., Severino L., De Tommasi N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010;121:105–111. doi: 10.1016/j.foodchem.2009.12.013. [DOI] [Google Scholar]
- 37.Romani A., Pinelli P., Mulinacci N., Galardi C., Vincieri F.F., Liberatore L., Cichelli A. HPLC and HRGC analyses of polyphenols and secoiridoid in olive oil. Chromatographia. 2001;53:279–284. doi: 10.1007/BF02490424. [DOI] [Google Scholar]
- 38.Franco M.N., Galeano-Díaz T., López Ó., Fernández-Bolaños J.G., Sánchez J., De Miguel C., Gil M.V., Martín-Vertedor D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014;163:289–298. doi: 10.1016/j.foodchem.2014.04.091. [DOI] [PubMed] [Google Scholar]
- 39.De Torres A., Espínola F., Moya M., Alcalá S., Vidal A.M., Castro E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT. 2018;90:22–30. doi: 10.1016/j.lwt.2017.12.003. [DOI] [Google Scholar]
- 40.Romero C., Brenes M. Analysis of total contents of hydroxytyrosol and tyrosol in olive oils. J. Agric. Food Chem. 2012;60:9017–9022. doi: 10.1021/jf3026666. [DOI] [PubMed] [Google Scholar]
- 41.García-Villalba R., Carrasco-Pancorbo A., Vázquez-Martín A., Oliveras-Ferraros C., Menéndez J.A., Segura-Carretero A., Fernández-Gutiérrez A. A 2-D-HPLC-CE platform coupled to ESI-TOF-MS to characterize the phenolic fraction in olive oil. Electrophoresis. 2009;30:2688–2701. doi: 10.1002/elps.200800807. [DOI] [PubMed] [Google Scholar]
- 42.Suárez M., Macià A., Romero M.P., Motilva M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A. 2008;1214:90–99. doi: 10.1016/j.chroma.2008.10.098. [DOI] [PubMed] [Google Scholar]
- 43.Gómez-Rico A., Salvador M.D., La Greca M., Fregapane G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006;54:7130–7136. doi: 10.1021/jf060798r. [DOI] [PubMed] [Google Scholar]
- 44.Kaliora A.C., Artemiou A., Giogios I., Kalogeropoulos N. The impact of fruit maturation on bioactive microconstituents, inhibition of serum oxidation and inflammatory markers in stimulated PBMCs and sensory characteristics of Koroneiki virgin olive oils from Messenia, Greece. Food Funct. 2013;4:1185–1194. doi: 10.1039/c3fo60027k. [DOI] [PubMed] [Google Scholar]
- 45.Bajoub A., Medina-Rodríguez S., Gómez-Romero M., Bagur-González M.G., Fernández-Gutiérrez A., Carrasco-Pancorbo A. Assessing the varietal origin of extra-virgin olive oil using liquid chromatography fingerprints of phenolic compound, data fusion and chemometrics. Food Chem. 2017;215:245–255. doi: 10.1016/j.foodchem.2016.07.140. [DOI] [PubMed] [Google Scholar]
- 46.Douzane M., Tamendjari A., Abdi A.K., Daas M.S., Mehdid F., Bellal M.M. Phenolic compounds in mono-cultivar extra virgin olive oils from Algeria. Grasas y Aceites. 2013;64:285–294. doi: 10.3989/gya.072212. [DOI] [Google Scholar]
- 47.Ouni Y., Taamalli A., Gómez-Caravaca A.M., Segura-Carretero A., Fernández-Gutiérrez A., Zarrouk M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC–ESI-TOF MS method. Food Chem. 2011;127:1263–1267. doi: 10.1016/j.foodchem.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 48.Kesen S., Kelebek H., Selli S. Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J. Am. Oil Chem. Soc. 2013;90:1685–1696. doi: 10.1007/s11746-013-2327-8. [DOI] [Google Scholar]
- 49.Dağdelen A., Tümen G., Ozcan M.M., Dündar E. Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalık, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013;136:41–45. doi: 10.1016/j.foodchem.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 50.Wang S.T., Le J., Peng R., Li Y. Efficient extraction and sensitive LC-MS quantification of hydroxytyrosol in wine, oil and plasma. Food Chem. 2020;323:126803. doi: 10.1016/j.foodchem.2020.126803. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 51.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Sparascio F., Maffia M. HPLC analysis of phenols in negroamaro and primitivo red wines from Salento. Foods. 2019;8:45. doi: 10.3390/foods8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Žurga P., Vahčić N., Pasković I., Banović M., Staver M.M. Croatian Wines from Native Grape Varieties Have Higher Distinct Phenolic (Nutraceutic) Profiles than Wines from Non-Native Varieties with the Same Geographic Origin. Chem. Biodivers. 2019;16:e1900218. doi: 10.1002/cbdv.201900218. [DOI] [PubMed] [Google Scholar]
- 53.Álvarez-Fernández M.A., Fernández-Cruz E., Cantos-Villar E., Troncoso A.M., García-Parrilla M.C. Determination of hydroxytyrosol produced by winemaking yeasts during alcoholic fermentation using a validated UHPLC–HRMS method. Food Chem. 2018;242:345–351. doi: 10.1016/j.foodchem.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 54.Boronat Rigol A. Ph.D. Thesis. Universitat Pompeu Fabra; Barcelona, Spain: 2020. [(accessed on 25 June 2022)]. Tyrosol and Its Endogenous Conversion into Hydroxytyrosol in Humans: Dietary Sources, Genetic Modulation and Clinical Effects. Available online: https://hdl.handle.net/10803/668336. [Google Scholar]
- 55.Soldevila-Domenech N., Boronat A., Mateus J., Diaz-Pellicer P., Matilla I., Pérez-Otero M., Aldea-Perona A., de la Torre R. Generation of the Antioxidant Hydroxytyrosol from Tyrosol Present in Beer and Red Wine in a Randomized Clinical Trial. Nutrients. 2019;11:2241. doi: 10.3390/nu11092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minussi R.C., Rossi M., Bologna L., Cordi L., Rotilio D., Pastore G.M., Durán N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003;82:409–416. doi: 10.1016/S0308-8146(02)00590-3. [DOI] [Google Scholar]
- 57.Di Tommaso D., Calabrese R., Rotilio D. Identification and quantitation of hydroxytyrosol in Italian wines. J. High Resolut. Chromatogr. 1998;21:549–553. doi: 10.1002/(SICI)1521-4168(19981001)21:10<549::AID-JHRC549>3.0.CO;2-Z. [DOI] [Google Scholar]
- 58.Piñeiro Z., Cantos-Villar E., Palma M., Puertas B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric. Food Chem. 2011;59:11683–11689. doi: 10.1021/jf202254t. [DOI] [PubMed] [Google Scholar]
- 59.EFSA Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011;9:2097. doi: 10.2903/j.efsa.2011.2097. [DOI] [Google Scholar]
- 60.Vogiatzoglou A., Mulligan A.A., Lentjes M.A.H., Luben R.N., Spencer J.P.E., Schroeter H., Khaw K.T., Kuhnle G.G.C. Flavonoid intake in European adults (18 to 64 Years) PLoS ONE. 2015;10:0128132. doi: 10.1371/journal.pone.0128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamden K., Allouche N., Damak M., Elfeki A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009;180:421–432. doi: 10.1016/j.cbi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Wang N., Liu Y., Ma Y., Wen D. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice. J. Nutr. Biochem. 2018;57:180–188. doi: 10.1016/j.jnutbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 63.IOC . Standards, Methods and Guides. IOC; Lausanne, Switzerland: 2017. COI/T.20/Doc. No 29—Determination of Biophenols in Olive Oils by HPLC. Decision DEC-III-10/106-VI/2017. [Google Scholar]
- 64.Tasioula-Margari M., Tsabolatidou E. Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants. 2015;4:548–562. doi: 10.3390/antiox4030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gómez-Caravaca A.M., Lozano-Sánchez J., Gámez M.C., Carretero A.S., Taamalli A. Bioactive phenolic compounds from Olea europaea: A challenge for analytical chemistry. In: Boskou D., editor. Olive and Olive Oil Bioactive Constituents. 1st ed. AOCS Press; Amsterdam, The Netherlands: 2015. pp. 261–298. [DOI] [Google Scholar]
- 66.Schwarz M., Weber F., Durán-Guerrero E., Castro R., Rodríguez-Dodero M.D.C., García-Moreno M.V., Winterhalter P., Guillén-Sánchez D. Hplc-dad-ms and antioxidant profile of fractions from amontillado sherry wine obtained using high-speed counter-current chromatography. Foods. 2021;10:131. doi: 10.3390/foods10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrasco-Pancorbo A., Cerretani L., Bendini A., Segura-Carretero A., Gallina-Toschi T., Fernández-Gutierrez A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005;28:837–858. doi: 10.1002/jssc.200500032. [DOI] [PubMed] [Google Scholar]
- 68.Brenes M., García A., Ríos P., García J.J., Garrido A. Use of 1-acetoxypinoresinol to authenticate Picual olive oils. Int. J. Food Sci. Technol. 2002;37:615–625. doi: 10.1046/j.1365-2621.2002.00591.x. [DOI] [Google Scholar]
- 69.Olmo-García L., Carrasco-Pancorbo A. Chromatography-MS based metabolomics applied to the study of virgin olive oil bioactive compounds: Characterization studies, agro-technological investigations, and assessment of healthy properties. Trends Analyt. Chem. 2021;135:116153. doi: 10.1016/j.trac.2020.116153. [DOI] [Google Scholar]
- 70.Boronat A., Soldevila-Domenech N., Rodríguez-Morató J., Martínez-Huélamo M., Lamuela-Raventós R.M., De la Torre R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules. 2020;25:2582. doi: 10.3390/molecules25112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenes M., De Castro A. Transformation of oleuropein and its hydrolysis products during Spanish-style green olive processing. J. Sci. Food Agric. 1998;77:353–358. doi: 10.1002/(SICI)1097-0010(199807)77:3<353::AID-JSFA50>3.0.CO;2-G. [DOI] [Google Scholar]
- 72.Robles-Almazan M., Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Rodriguez-Garcia C., Quiles J.L., Ramirez-Tortosa M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018;105:654–667. doi: 10.1016/j.foodres.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 73.Brenes M., Rejano L., García P., Sánchez A.H., Garrido A. Biochemical Changes in Phenolic Compounds during Spanish-Style Green Olive Processing. J. Agric. Food Chem. 1995;43:2702–2706. doi: 10.1021/jf00058a028. [DOI] [Google Scholar]
- 74.Romero C., Brenes M., García P., Garrido A. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits and derived products. Food Res. Int. 2002;50:3835–3839. doi: 10.1021/jf011485t. [DOI] [PubMed] [Google Scholar]
- 75.Arroyo López F.N., Romero C., Durán Quintana M.D.C., López A.L., García P.G., Fernández A.G. Kinetic study of the physicochemical and microbiological changes in “seasoned” olives during the shelf-life period. J. Agric. Food Chem. 2005;53:5285–5292. doi: 10.1021/jf050501+. [DOI] [PubMed] [Google Scholar]
- 76.Romero-Segura C., García-Rodríguez R., Sánchez-Ortiz A., Sanz C., Pérez A.G. The role of olive β-glucosidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2012;45:191–196. doi: 10.1016/j.foodres.2011.10.024. [DOI] [Google Scholar]
- 77.Brenes M., Garcia A., Garcia P., Garrido A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001;49:5609–5614. doi: 10.1021/jf0107860. [DOI] [PubMed] [Google Scholar]
- 78.Esposito Salsano J., Digiacomo M., Cuffaro D., Bertini S., Macchia M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods. 2022;11:1354. doi: 10.3390/foods11091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nissiotis M., Tasioula-Margari M. Changes in antioxidant concentration of virgin olive oil during thermal oxidation. Food Chem. 2002;77:371–376. doi: 10.1016/S0308-8146(02)00113-9. [DOI] [Google Scholar]
- 80.Mas A., Guillamon J.M., Torija M.J., Beltran G., Cerezo A.B., Troncoso A.M., Garcia-Parrilla M.C. Bioactive compounds derived from the yeast metabolism of aromatic amino acids during alcoholic fermentation. BioMed. Res. Int. 2014;2014:898045. doi: 10.1155/2014/898045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noh H., Freisling H., Assi N., Zamora-Ros R., Achaintre D., Affret A., Mancini F., Boutron-Ruault M.C., Flögles A., Boeing H., et al. Identification of urinary polyphenol metabolite patterns associated with polyphenol-rich food intake in adults from four European Countries. Nutrients. 2017;9:796. doi: 10.3390/nu9080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schröder H., De La Torre R., Estruch R., Corella D., Martínez-González M.A., Salas-Salvadó J., Ros E., Arós F., Flores G., Civit E., et al. Alcohol consumption is associated with high concentrations of urinary hydroxytyrosol. Am. J. Clin. Nutr. 2009;90:1329–1335. doi: 10.3945/ajcn.2009.27718. [DOI] [PubMed] [Google Scholar]
- 83.Pérez-Mañá C., Farré M., Pujadas M., Mustata C., Menoyo E., Pastor A., Langohr K., De La Torre R. Ethanol induces hydroxytyrosol formation in humans. Pharmacol. Res. 2015;95–96:27–33. doi: 10.1016/j.phrs.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Pérez-Mañá C., Farré M., Rodríguez-Morató J., Papaseit E., Pujadas M., Fitó M., Robledo P., Covas M.I., Cheynier V., Meudec E., et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015;59:1213–1216. doi: 10.1002/mnfr.201400842. [DOI] [PubMed] [Google Scholar]
- 85.Rodríguez-Morató J., Boronat A., Kotronoulas A., Pujadas M., Pastor A., Olesti E., Pérez-Mañá C., Khymenets O., Fitó M., Farré M., et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016;48:218–236. doi: 10.1080/03602532.2016.1179754. [DOI] [PubMed] [Google Scholar]
- 86.Boronat Rigol A., Mateus J., Soldevila-Domenech N., Guerra M., Rodríguez-Morató J., Varon C., Muñoz D., Barbosa F., Morales J.C., Gaedigk A., et al. Data on the endogenous conversion of tyrosol into hydroxytyrosol in humans. Data Br. 2019;27:104787. doi: 10.1016/j.dib.2019.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De La Torre R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology. 2008;16:245–247. doi: 10.1007/s10787-008-8029-4. [DOI] [PubMed] [Google Scholar]
- 88.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017;15:4728. doi: 10.2903/j.efsa.2017.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Attya M., Benabdelkamel H., Perri E., Russo A., Sindona G. Effects of conventional heating on the stability of major olive oil phenolic compounds by tandem mass spectrometry and isotope dilution assay. Molecules. 2010;15:8734–8746. doi: 10.3390/molecules15128734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ziauddeen N., Rosi A., Del Rio D., Amoutzopoulos B., Nicholson S., Page P., Scazzina F., Brighenti F., Sumantra R., Mena P. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014) Eur. J. Nutr. 2019;58:3183–3198. doi: 10.1007/s00394-018-1862-3. [DOI] [PubMed] [Google Scholar]
- 91.Zamora-Ros R., Knaze V., Luján-Barroso L., Slimani N., Romieu I., Touillaud M., Kaaks R., Teucher B., Mattiello A., Grioni S., et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011;106:1090–1099. doi: 10.1017/S0007114511001437. [DOI] [PubMed] [Google Scholar]
- 92.European Commission Commission Implementing Decision (EU) 2017/2373 of 14 December 2017 authorising the placing on the market of hydroxytyrosol as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union. 2017;L 337:56–59. [Google Scholar]
- 93.Ribeiro T.B., Bonifácio-Lopes T., Morais P., Miranda A., Nunes J., Vicente A.A., Pintado M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021;297:110476. doi: 10.1016/j.jfoodeng.2021.110476. [DOI] [Google Scholar]
- 94.Martínez-Zamora L., Peñalver R., Ros G., Nieto G. Innovative natural functional ingredients from olive and citrus extracts in spanish-type dry-cured sausage “fuet”. Antioxidants. 2021;10:180. doi: 10.3390/antiox10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mateos R., Martínez-López S., Baeza Arévalo G., Amigo-Benavent M., Sarriá B., Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016;205:248–256. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Kranz P., Braun N., Schulze N., Kunz B. Sensory quality of functional beverages: Bitterness perception and bitter masking of olive leaf extract fortified fruit smoothies. J. Food Sci. 2010;75:S308–S311. doi: 10.1111/j.1750-3841.2010.01698.x. [DOI] [PubMed] [Google Scholar]
- 97.Nieto G., Martínez L., Castillo J., Ros G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017;97:3761–3771. doi: 10.1002/jsfa.8240. [DOI] [PubMed] [Google Scholar]
- 98.Foti P., Occhipinti P.S., Romeo F.V., Timpanaro N., Musumeci T., Randazzo C.L., Caggia C. Phenols recovered from olive mill wastewater as natural booster to fortify blood orange juice. Food Chem. 2022;393:133428. doi: 10.1016/j.foodchem.2022.133428. [DOI] [PubMed] [Google Scholar]
- 99.Lesage-Meessen L., Navarro D., Maunier S., Sigoillot J.C., Lorquin J., Delattre M., Simon J.-L., Asther M., Labat M. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001;75:501–507. doi: 10.1016/S0308-8146(01)00227-8. [DOI] [Google Scholar]
- 100.Allouche N., Fki I., Sayadi S. Toward a High Yield Recovery of Antioxidants and Purified Hydroxytyrosol from Olive Mill Wastewaters. J. Agric. Food Chem. 2004;52:267–273. doi: 10.1021/jf034944u. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Castello E., Cassano A., Criscuoli A., Conidi C., Drioli E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010;44:3883–3892. doi: 10.1016/j.watres.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Bertin L., Ferri F., Scoma A., Marchetti L., Fava F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011;171:1287–1293. doi: 10.1016/j.cej.2011.05.056. [DOI] [Google Scholar]
- 103.Jerman Klen T., Mozetič Vodopivec B. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with conventional methods. J. Agric. Food Chem. 2011;59:12725–12731. doi: 10.1021/jf202800n. [DOI] [PubMed] [Google Scholar]
- 104.Khoufi S., Hamza M., Sayadi S. Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 2011;102:9050–9058. doi: 10.1016/j.biortech.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 105.Benincasa C., Pellegrino M., Romano E., Claps S., Fallara C., Perri E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2022;8:782693. doi: 10.3389/fnut.2021.782693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernández-Bolaños J., Rodríguez G., Gómez E., Guillén R., Jiménez A., Heredia A., Rodríguez R. Total recovery of the waste of two-phase olive oil processing: Isolation of added-value compounds. J. Agric. Food Chem. 2004;52:5849–5855. doi: 10.1021/jf030821y. [DOI] [PubMed] [Google Scholar]
- 107.Serrano A., Fermoso F.G., Alonso-Fariñas B., Rodríguez-Gutierrez G., Fernandez-Bolaños J., Borja R. Olive mill solid waste biorefinery: High-temperature thermal pre-treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017;148:314–323. doi: 10.1016/j.jclepro.2017.01.152. [DOI] [Google Scholar]
- 108.Fernández-Bolaños J., Rodríguez G., Rodríguez R., Heredia A., Guillén R., Jiménez A. Production in large quantities of highly purified hydroxytyrosol from liquid− solid waste of two-phase olive oil processing or “Alperujo”. J. Agric. Food Chem. 2002;50:6804–6811. doi: 10.1021/jf011712r. [DOI] [PubMed] [Google Scholar]
- 109.Silva S., Gomes L., Leitão F., Coelho A.V., Boas L.V. Phenolic compounds and antioxidant activity of Olea europaea L. Fruits and leaves. Food Sci. Technol. Int. 2006;12:385–396. doi: 10.1177/1082013206070166. [DOI] [Google Scholar]
- 110.Guinda Á., Castellano J.M., Santos-Lozano J.M., Delgado-Hervás T., Gutiérrez-Adánez P., Rada M. Determination of major bioactive compounds from olive leaf. Food Sci. Technol. 2015;64:431–438. doi: 10.1016/j.lwt.2015.05.001. [DOI] [Google Scholar]
- 111.Briante R., Patumi M., Terenziani S., Bismuto E., Febbraio F., Nucci R. Olea europaea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002;50:4934–4940. doi: 10.1021/jf025540p. [DOI] [PubMed] [Google Scholar]
- 112.Bouaziz M., Sayadi S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005;107:497–504. doi: 10.1002/ejlt.200501166. [DOI] [Google Scholar]
- 113.Herrero M., Temirzoda T.N., Segura-Carretero A., Quirantes R., Plaza M., Ibañez E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A. 2011;1218:7511–7520. doi: 10.1016/j.chroma.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 114.Huertas-Alonso A.J., Gonzalez-Serrano D.J., Hadidi M., Salgado-Ramos M., Orellana-Palacios J.C., Sánchez-Verdú M.P., Xia Q., Simirgiotis M.J., Barba F.J., Nabi Dar B., et al. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation. 2022;8:215. doi: 10.3390/fermentation8050215. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available from the corresponding author on reasonable request.