Abstract
DNA methylation is an epigenetic modification of the genome involved in the regulation of gene expression and modulation of chromatin structure. Plant genomes are widely methylated, and the methylation generally occurs on the cytosine bases through the activity of specific enzymes called DNA methyltransferases. On the other hand, methylated DNA can also undergo demethylation through the action of demethylases. The methylation landscape is finely tuned and assumes a pivotal role in plant development and evolution. This review illustrates different molecular aspects of DNA methylation and some plant physiological processes influenced by this epigenetic modification in model species, crops, and ornamental plants such as orchids. In addition, this review aims to describe the relationship between the changes in plant DNA methylation levels and the response to biotic and abiotic stress. Finally, we discuss the possible evolutionary implications and biotechnological applications of DNA methylation.
Keywords: DNA methylation, plant epigenetics, gene expression, plant genomic imprinting, environmental adaptations
1. Introduction
Epigenetic modifications are heritable chemical changes that do not alter the DNA nucleotide sequence but can influence the phenotype [1,2]. Epigenetics is a fascinating topic in plant biology because epigenome dynamism and plasticity affect plant development and evolution in response to the environment.
The epigenetic marks include chemical modifications of the DNA (i.e., methylation) and numerous post-translational modifications of histone proteins, such as acetylation, methylation, ubiquitination, sumoylation, and phosphorylation. These changes generally occur at the histone N-terminal tails [3,4]. In addition to covalent modifications of DNA and histones, epigenetic mechanisms include the activity of non-coding RNA molecules [5,6,7] and chromatin remodeling factors that control the nucleosome position, assembly, and disassembly [8,9]. These modifications can alter the accessibility of the genomic regions and regulate gene expression, resulting in phenotypic plasticity. The consequent ability to rapidly modulate the responses to environmental changes is crucial for all living organisms, especially for sessile such as plants [10].
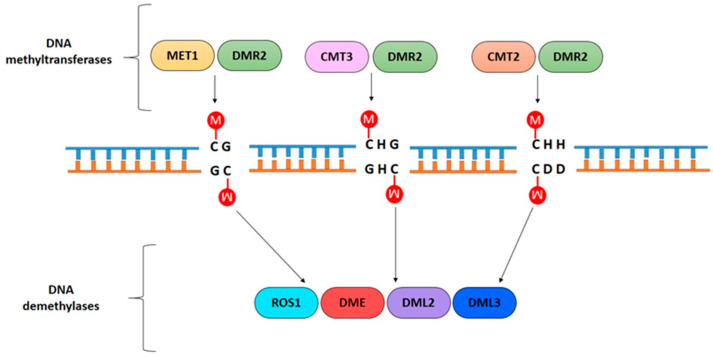
DNA methylation is one of the most studied epigenetic mechanisms [10]. Plant DNA methylation occurs through the link of a methyl group (-CH3) at cytosine in symmetric, CG and CHG; and asymmetric, CHH, contexts (where H is any nucleotide except G), carried out by a family of enzymes called DNA methyltransferases [3,11,12] (Figure 1).
Figure 1.
Specific DNA methyltransferases and demethylases mediate cytosine methylation (red circle) in different sequence contexts. CG, CHG, and CHH methylation are carried out by MET1, CMT3, and CMT2, respectively. DRM2, involved in the RdDM pathway, regulates all sequence context methylation. ROS1, DME, DML2, and DML3 act as demethylases.
In the thale cress (Arabidopsis thaliana), a model species for plant genetics, the CG DNA methylation is maintained by the METHYLTRANSFERASE 1 (MET1) during DNA replication [9], while the CHROMOMETHYLASE 2 and 3 (CMT2 and CMT3) generally methylate the CHG and CHH contexts. In particular, CMT2 acts mainly in the CHH context and CMT3 acts in the CHG context [11,13,14]. In contrast, the DNA methyltransferase DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2) is involved in maintaining the existing methylation landscape in both symmetric and asymmetric contexts, and it is also responsible for de novo DNA methylations. DRM2 is guided to the target sequence by small RNA molecules in a plant-specific pathway known as RNA-directed DNA methylation (RdDM) [3,11,15,16].
In Arabidopsis, RdDM can occur in two different ways: canonical and non-canonical. The canonical pathway is mainly involved in maintaining methylation in heterochromatic regions. During canonical RdDM, the RNA Polymerase IV (Pol IV) interacts with the chromatin remodeler CLASSY 1 (CLSY1) and its interactor SAWADEE HOMEODOMAIN HOMOLOG 1 (SHH1). This complex binds to heterochromatin, and RNA Pol IV transcribes short single-strand RNAs (ssRNAs), approximately 30–45 nucleotides in length. The RNA-directed RNA polymerase RDR2 and RNA Pol IV convert these ssRNAs into double-strand RNAs (dsRNAs). Then, the endoribonuclease DICER-LIKE 3 (DCL3) cleaves the dsRNAs into 24 nucleotides sRNAs. The ARGONAUTE proteins (AGO4, AGO6) convey single strands of the sRNAs toward the complementary RNA transcribed by the plant-specific RNA Polymerase V, recruiting the methyltransferase DRM2 that methylates the neighboring DNA.
The non-canonical pathway is less frequent and includes many variations of the canonical pathway, generally resulting in de novo DNA methylation (for example, methylation of newly transposed mobile elements). In the non-canonical pathway, the biogenesis of sRNAs is not only mediated by Pol IV—RDR2—DCL3, but they can be originated by many other mechanisms such as RNA Polymerase II transcription [17,18,19,20,21,22].
Methylation levels are finely controlled, and the dynamic of DNA methylation/demethylation is a rigorously orchestrated process. In plants, demethylation can be a passive mechanism with the loss of the methylation signal during DNA replication, or an active mechanism mediated by specific enzymes [23] through the DNA base excision repair (BER) [24,25]. Specific plant DNA glycosylases recognize methylated cytosine in any sequence context and break the bond with the deoxyribose sugar, originating an abasic site that will be repaired by DNA polymerase and ligase.
The four DNA demethylases identified in Arabidopsis are the REPRESSOR OF SILENCING 1 (ROS1), DEMETER (DME), and DEMETER-LIKE PROTEIN 2 and 3 (DML2, DML3) [26,27,28,29,30,31] (Figure 1). In mammals, the Ten-Eleven Translocation methylcytosine dioxygenase (TET) enzymes play an important role in demethylation, catalyzing the oxidation of the methylated cytosine [32]. However, plant TET-like enzymes are not yet characterized, even if the existence of the oxidative product during active demethylation suggests the possible involvement of TET-like proteins in this process [33]. Moreover, the overexpression of a human TET protein in Arabidopsis induces significant genome demethylation, further supporting the existence of TET-like proteins in plants [34].
In addition to cytosine methylation, generally associated with transcriptional repression, the plant genome can be dynamically methylated on N6-adenine. The 6-methyl-adenosine (6mA) is associated with expressed genes and seems to be very receptive to environmental stimuli [35,36].
This review will focus on the molecular and cellular functions of DNA methylation in plants, highlighting its role in the plant’s life cycle, response to environmental changes, and evolution.
2. Role of DNA Methylation in Plant Cells
The level of DNA methylation deeply influences the plant genome structure and activity. Numerous and different molecular mechanisms are affected by the action of DNA methylation, underlining the importance of this epigenetic mechanism in the plant cell.
2.1. Gene Expression
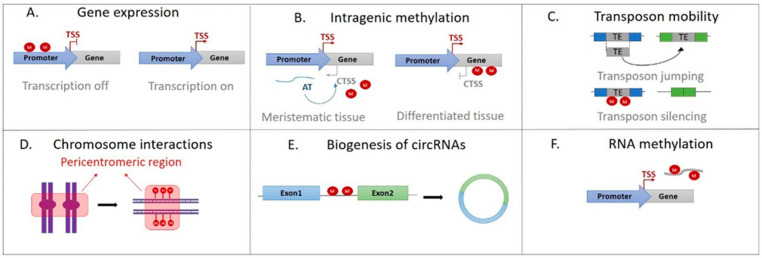
A critical molecular consequence of DNA methylation is the regulation of gene expression (Figure 2A) [17].
Figure 2.
Different roles of DNA methylation in plant cells. (A) The promoter DNA methylation (red circle) represses transcription activity and gene expression; (B) methylation of the coding regions generates an inaccessible chromatin structure suppressing the aberrant transcription start site; (C) DNA methylation affects genome stability by silencing transposons and other DNA repeated sequences; (D) chromosome interactions through pericentromeric regions or heterochromatin islands depend on the methylation of these regions; (E) DNA methylation could be involved in the biogenesis of circRNAs; (F) mRNA methylation controls stability, splicing, and processing of the transcript itself. TSS, transcription start site; CTSS, cryptic transcription start site; AT, aberrant transcript; TE, transposable elements.
Usually, due to the promoter region methylation, the binding of transcription activators is prevented, and that of transcription repressors is improved, leading to the inactivation or reduction of transcription. Moreover, gene expression regulation results from the cooperation of different epigenetic mechanisms that influence each other, for example, DNA methylation and histone modifications. The N-terminal tails of histones can undergo numerous modifications promoted by specific enzymes. Some of these modifications, such as histone acetylation or H3 lysine 4 monomethylation (H3K4me1), relax the chromatin, thus facilitating the transcription. Generally, regions with a low methylation level also present other modifications that promote transcription, e.g., histone acetylation. However, in some cases, poorly methylated and actively transcribed regions can show histone hypo-acetylation, suggesting that sometimes the loss of the DNA methylation is a mechanism that, alone, can promote the activation of transcription [17,37,38].
Less frequently, DNA methylation can also activate gene transcription, even if the molecular mechanism involved is not yet understood [17].
In addition to the promoter, methylation can also occur within the full-length coding region, generally in the central exons rather than in the transcription start and end regions [17,39]. Unlike promoter methylation, which suppresses or decreases the transcription of the downstream gene, methylation in the coding region inhibits transcript elongation. In this way, the transcription occurs from the transcription start site to the methylation site, with the production of a smaller, possibly inactive transcript [40].
Intragenic methylation plays a relevant regulatory and biological role, preventing the activation of cryptic transcription start sites [36]. In the plant meristem cells, aberrant transcription produces double-stranded RNAs that trigger a feedback methylation mechanism via RdDM and repress the cryptic promoters from which they originated. The methylation of these cryptic transcription start sites is maintained in the differentiated tissues where the chromatin preserves its inaccessible structure, and the aberrant transcription is suppressed [40,41] (Figure 2B).
2.2. Transposon Mobility
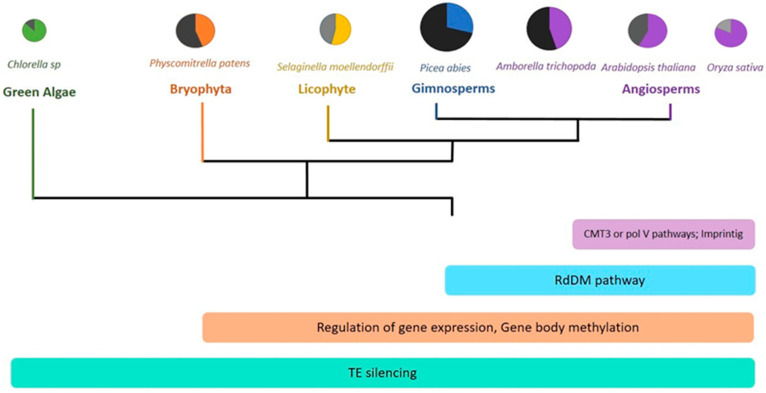
DNA methylation is involved in maintaining plant genome stability by regulating transposon mobility. The methylation of the transposable elements occurs in all sequence contexts and in the genome of all plant species, from mosses to angiosperms (Figure 3) [42,43,44,45,46,47]. In particular, the RdDM pathway maintains the CHH sequence methylation in short and large transposon ends, whereas CMT2 methylates the internal regions of large transposons [14,48].
Figure 3.
DNA methylation during plant evolution. The diameter of the colored circles represents the genome size of selected species; the dark slice represents the percentage of repeated sequences, and the intensity of the gray represents the level of global DNA methylation. Across plant evolution, DNA methylation assumes new roles represented by colored rectangles. The TE silencing constitutes the most conserved function of methylation. More recently, novel regulation pathways have emerged.
The transposon activity within the genome can disrupt the structure of a gene or a regulatory region. Therefore, methylation acts as a cellular defense mechanism by reducing the transposition rate of the mobile elements [17,42] (Figure 2C). Transposon silencing can also cause the spread of methylation to the flanking areas, thus generating differentially methylated regions [49,50]. In the plant genomes, such as maize (Zea mays), the hypermethylated CHH islands maintained by the RdDM mechanism are fundamental to separating the transcriptionally active regions and inactive transposons. In this way, the eventual activation of a silenced transposable element by the positional effect of nearby active euchromatin is unlikely [17,51].
Although its critical function in genome stability, sometimes DNA methylation alone is not sufficient to ensure the silencing of the transposable elements. As mentioned above, additional marks, like histone modifications, are necessary to regulate transposon mobility. For example, dimethylation of H3K9 (H3K9m2) is a significant mark in heterochromatin formation. In particular, in the Arabidopsis genome, there is a correlation between H3K9m2 levels and transposons present in euchromatic chromosome arms, suggesting this modification has a role in the silencing of transposons [52,53,54].
2.3. Chromosome Interactions
Another function of DNA methylation is the regulation of chromosome interactions. The hypermethylation of pericentromeric regions and some interactive heterochromatin islands (IHI) of euchromatic chromosome arms generate an interaction network (Figure 2D). In Arabidopsis, this nuclear complex is called KNOT and is present in all five chromosomes [55,56]. The KNOT complex has a relevant role in maintaining genome integrity, which is threatened by the jumping of transposons [48].
Genomic analyses of Arabidopsis have demonstrated the effect of DNA methylation on chromosome interactions, showing that the genomic regions with a high methylation level are less engaged in long-range chromatin contacts than the low methylated regions [57].
The control of chromosome interactions by DNA methylation is an important mechanism affecting gene expression. Promoters control gene expression in addition to some regulatory regions that can be very distant from the transcription start site (TSS). Although distant enhancers in the plant genomes are still unknown, this type of regulation cannot be excluded. Some speculative models describe how the control of chromatin landscape by methylation level could influence long-range chromosomal interactions and gene expression. In particular, inhibiting the formation of 3D spatial interactions between chromosomes prevents the regulation of gene expression through distant regulatory elements, such as enhancers. These models suggest that RdDM can prevent the binding of the architectural proteins blocking the formation of chromosomal interactions [57].
2.4. Biogenesis of circRNAs
Circular RNAs (circRNAs) are non-coding RNA molecules that have recently been characterized, and much is still unknown about their formation and role. They have been described in different angiosperm genomes such as Arabidopsis [58], rice (Oryza sativa) [59], and maize [60]. The linear mRNA molecules are generated from the canonical splicing mechanism where the upstream (5′) splicing donor site has linked to the downstream (3′) splicing acceptor site. In contrast, circRNAs are produced at the post-transcriptional level by a pre-mRNA back-splicing. The downstream splicing donor site is ligated with an upstream splicing acceptor site, and the 3′ and 5′ ends are covalently closed. circRNAs do not possess a polyadenylated tail and are more stable and resistant to exonuclease degradation than linear RNAs [61,62,63].
Interestingly, recent findings suggest that DNA methylation could also be involved in the biogenesis of circRNAs. For example, circRNA-seq data in the moso bamboo (Phyllostachys edulis) show a high methylation level in flanking intron regions of circRNAs (Figure 2E) [64]. Although the biological role of circRNAs is still unclear, they could be involved in miRNA binding and regulation of gene expression [65,66], generating a new level of epigenetic regulation through DNA methylation.
2.5. RNA Methylation
Methylation is a chemical modification that does not act only on DNA; in fact, it also plays a central role in mRNA stability and splicing, affects mRNA processing by generating alternative polyadenylation sites that cause the production of incomplete and unproductive transcripts, and influences the mRNA migration in the cytoplasm and its translation (Figure 2F) [67,68,69,70,71,72,73,74,75,76,77].
An analysis of plant epitranscriptome reveals that the most common post-transcriptional RNA modifications occur at N6-methyladenosine (m6A) and 5-methylcytidine (m5C) [78]. These two modifications are located in different regions of the transcripts. Near the stop codons and at 3′ untranslated regions (3′ UTR), m6A is abundant, while m5C is more frequent in coding sequences (CDS) [78].
The methylation level of the transcripts is dynamic and changes between tissues and different stages of plant development. This variation is due to the action of specific writer, eraser, and reader enzymes that catalyze epigenetic modifications. The writers generate the chemical modifications, the readers recognize and identify them, and the erasers remove them. The variation of these enzyme levels reflects the complexity of the epigenetic landscape and its dynamism [78,79,80,81,82,83,84].
In Arabidopsis, the m6A modifications on RNA depend on the specific complex that includes the methyltransferase A (MTA) that interacts with the methyltransferase B (MTB) and other factors such as FKBP12 interacting protein 37 (FIP37) [85,86]. The writers TRM4A and TRM4B perform the RNA methylation of m5C: the first is involved in the m5C methylation of tRNAs, and the second acts on mRNAs [87]. Among the erasers, thirteen enzymes have been identified in Arabidopsis. They belong to the ALKBH family and catalyze the removal of the m6A modifications [88]. Three YTHD proteins represent the m6A readers of Arabidopsis so far [89]. The expression of all these enzymes varies under abiotic stress conditions, showing the importance of the regulation of RNA chemical modifications. These enzymes can control the RNA molecule stability and fate by modifying specific nucleotides, thus affecting plant response to environmental conditions [84].
3. DNA Methylation during Plant Development
Variation of DNA methylation in different tissues and during the phases of plant development considerably affects plant physiology [17].
3.1. Genomic Imprinting
DNA methylation has a critical role in fertilization, the first step of the plant life cycle. During this stage, genomic imprinting can occur at specific loci through differential methylation of the two alleles dependent on their parental origin.
In angiosperms, female gametogenesis occurs in the ovary, where the meiosis of the megasporocyte produces four haploid megaspores. After the degeneration of three megaspores, the only surviving undergoes three sequential mitoses, generating a cell with eight nuclei. Two polar nuclei migrate at the cell center and form the central cell. Three nuclei move upwards, arising antipodal cells, and three migrate at the bottom, generating the two lateral synergid cells and the central egg cell. The pollinic granule is constituted by two spermatic cells and a vegetative cell. The latter forms the pollen tube that directs sperm cells in the ovary during fertilization. One of the two spermatic cells fuses with the egg cell forming the diploid zygote. The other sperm cell combines with the central cell, thus producing the triploid endosperm that nourishes the growing embryo [90]. This kind of megagametophyte is named Polygonum-type and characterizes more than 70% of angiosperm species, including Arabidopsis [91].
Genomic imprinting is a regulatory phenomenon involved principally in the embryo’s growth. It was first described in mammals, where the parental conflict theory explains the functional meaning of genomic imprinting [92]. Applying this theory to plants highlights the different parental contributions to the seed size [93,94]. The growth of the seed depends principally on the maternal food reserves, resulting in higher metabolic costs for maternal tissues than for paternal ones. For this reason, the maternal genes that control the seed size have to ensure a balance between an optimal size, the energy expense of the mother, and the energetic resources that other seeds already in the developing stage need for growth.
In contrast, the paternal tissues do not directly experience the metabolic expense for developing the seed. The only effect of the larger seed suffered by paternal tissue is indirect because there is a lower chance of reproduction due to the presence of fewer ovules to fertilize [92]. Consequently, the genes that repress endosperm growth and reduce the nutrient flow to the embryo are expressed by maternal alleles. On the contrary, the genes that increase endosperm development and stimulate the nutrient flow to the embryo are expressed by paternal alleles [95].
In the maternal central cell, an effective demethylation activity occurs before fertilization. Consequently, in the endosperm, maternal alleles are less methylated and more expressed than paternal ones [96,97,98]. Many imprinted genes, including transcription factors and protein involved in chromatin remodeling, have a significant regulative role in seed development and endosperm proliferation. For example, in the central cell of Arabidopsis, DME demethylates the genes FLOWERING WAGENINGEN (FWA), MEDEA (MEA), and FERTILIZATION-INDEPENDENT SEED 2 (FIS2), while in the spermatic cell, these loci are methylated and silenced. In this way, after fertilization, only the MEA, FIS2, and FWA maternal alleles are expressed in the endosperm [99,100,101].
MEA is a Polycomb group protein (PcG) and is the homolog of Enhancer of zeste of Drosophila [100]. The PcG proteins of Drosophila have a role in maintaining the silenced state of the target genes through chromatin remodeling. The MEA protein of Arabidopsis suppresses central cell proliferation and endosperm development, regulating gene transcription by controlling chromatin accessibility. For this reason, the MEA maternal allele is expressed, as opposed to the paternal one [102]. As MEA, FIS2 is also a PcG protein, the homolog of the Drosophila Suppressor of zeste12 SU(Z)12. In Arabidopsis, FIS2 and MEA function in similar protein complexes playing a role in the transcriptional regulation of target genes [100,103].
In contrast to MEA and FIS2, involved in seed development, the imprinting of the FWA gene is fundamental for flowering. The ectopic expression of FWA and the alteration of the imprinting in fwa mutants are responsible for the plant’s late flowering [99].
In the central and vegetative cells of Arabidopsis [97], rice [104], and maize [105], DME can also demethylate mobile elements. This activates the transcription of small RNA molecules delivered to the egg and sperm cells, reinforcing transposon silencing in the gametes [97,106,107,108,109].
To date, genomic imprinting is described only in species with the Polygonum-type embryo sac, the most common type among angiosperms [110]. Future studies on genomic imprinting in taxa with other types of embryo sacs (e.g., Allium-, Oenothera-, Adoxa-type, etc.) will enhance our knowledge of the epigenetic mechanisms involved in angiosperm fertility.
3.2. Floral Pigmentation, Floral Scent, and Photosynthesis
Besides fertilization, epigenetic modifications are powerful regulators of many other biological processes in plants. For example, DNA methylation can influence the floral pigmentation in the Oncidium orchid.
The labellum of the Oncidium Gower Ramsey (GR) flower has an intense yellow coloration due to the accumulation of carotenoid pigments. The genetic pathway underpinning this pigmentation is known [111], and its variation is responsible for the white flower color in Oncidium White Jade (WJ). The different color patterns of these orchid cultivars depend on the differential expression of carotenoid-related genes in response to their methylation levels. In particular, in Oncidium GR, the OgCCD1 gene is methylated and silenced. On the contrary, in WJ cultivar tissues, the lack of promoter methylation and the consequent active expression of the OgCCD1 gene promotes carotenoid degradation resulting in a white flower [112].
Another characteristic of the pigmentation pattern of the Oncidium GR flower is the presence of a red streak in the perianth, absent in the cultivar Oncidium Honey Dollp (HD). In this case, the different coloration of the orchid floral organs depends on the methylation levels of the genes involved in the anthocyanin-biosynthetic pathway. The methylation of the OgCHS gene promoter in the HD cultivar is responsible for the absence of anthocyanin accumulation. In contrast, the unmethylated gene is effectively expressed in GR [113].
DNA methylation plays an active role in the flower color formation in many other ornamental plants. In the hall crabapple (Malus halliana), petal coloration changes from red to pale pink during development, and this variation is associated with the downregulation of many genes. In particular, the promoter of MhMYB10, a gene of the anthocyanin biosynthesis pathway, is highly methylated, resulting in a reduction of the MhMYB10 gene expression and a decrease in anthocyanin accumulation [114].
The methylation of gene promoters predominantly causes gene expression variation during plant development and growth. The alteration of these marks in genes belonging to the same network indicates the DNA methylation involvement in a specific regulation pathway. For instance, in the Chinese/Japanese plum (Prunus mume), many genes belonging to eight floral scent biosynthesis pathways show different methylation levels during the development of the flower [115]. These genes encode enzymes that are key regulators of floral scent production, such as the Coniferyl Alcohol Acetyltransferase (PmCFAT1a/1c) and the Benzyl Alcohol Acetyltransferase (PmBEAT36/3). The CFAT proteins belong to the acyltransferase family and are fundamental for eugenol synthesis, catalyzing the transformation of the substrate coniferyl alcohol to coniferyl acetate [116]. The BEAT proteins catalyze the transfer of the acetyl group of acetyl-CoA to the carbonyl group of benzyl alcohol, generating the benzyl acetate [117]. Whole-genome bisulfite sequencing in P. mume revealed that most of the differentially methylated genes are involved in different steps of the phenylpropane biosynthesis pathway through which over 90% of the floral volatiles are produced [115].
The dynamic of DNA methylation can vary in space and time, e.g., among different tissues or stages of development. The pineapple (Ananas comosus) leaves are an excellent example of the variation of DNA methylation during the diel (24 h). In the pineapple, the white leaf base is non-photosynthetic tissue. Here, the number of methylated genomic sequences is appreciably reduced across diel time course compared to the photosynthetic tissues, constituted by the green leaf tip. This alteration is particularly evident in the CHH sequences rather than in the CG and CGH context, where significant global differences are absent and occur in both gene and transposon regions. Temporal methylation data reveal that DNA methylation levels are low in green and white leaf tissue in the early morning and then increase during the afternoon (starting at 4 pm). In particular, a differentially methylated profile between green and white leaf tissues during the diel periods is characteristic of the CAM pathway genes, e.g., beta carbonic anhydrase (beta-CA), phosphoenolpyruvate carboxylase (PEPC), phosphoenolpyruvate carboxylase kinase (PPCK), and malate dehydrogenase (MDH). Therefore, DNA methylation can affect CAM photosynthesis in pineapple [118].
4. Methylation, Environment, and Evolution
Plants can respond to environmental stimuli by changing their epigenetic landscape. As proved by methylome analysis of different plant species, biotic or abiotic stress can affect DNA methylation, modifying the expression and regulation of stress-responsive genes [17].
4.1. Abiotic and Biotic Stimuli
In the orchid Dendrobium officinale, the cold and drought stress can influence the expression of the methylase DoC5-MTase and demethylase DodMTase genes. The promoter of these genes contains hormone-, light-, and stress-responsive cis-acting elements, confirming the relationship between the methylase/demethylase expression and the environmental conditions. In particular, the expression of most DoC5MTases is reduced under cold stress while the expression of DodMTases increases. Moreover, the DodMTase and DoC5-MTase gene transcription levels are correlated with the biosynthesis of water-soluble polysaccharides (WSPs). In fact, during the transition from protocorm-like bodies to plantlets in Dendrobium, the WSP content increases, and there is an up-regulation of both the DodMTase genes and the genes involved in WSP biosynthesis. At the same time, the expression of DoC5-MTase decreases. Environmental stresses, such as the cold, can affect the activity of methylase/demethylase transcription. This alteration reflects in a variation of the WSP synthesis and accumulation through regulating the genes involved in this pathway [119] (Table 1).
Table 1.
Summary of the genes that change the methylation levels in response to abiotic stress.
| Species | Genes | Protein | Phenotype | Stress | Methylation State |
|---|---|---|---|---|---|
| D. officinale | DoC5-MTase | methylase | biosynthesis of WSPs | cold | methylated |
| D. officinale | DodMTase | demethylase | biosynthesis of WSPs | cold | demethylated |
| C. sinensis | C5-MTase | methylase | cold/drought | methylated | |
| C. sinensis | CsdMTase | demethylase | cold/drought | demethylated | |
| B. napus Exagone | LCR | cytochrome P450 monooxygenase | cutin synthesis | salt | methylated |
| B. napus Toccata | LCR | cytochrome P450 monooxygenase | cutin synthesis | salt | unaltered |
| B. napus Exagone | TPS4 | trehalose phosphatase/synthase 4 | biosynthetic pathway of trehalose | salt | demethylated |
| B. napus Toccata | TPS4 | trehalose phosphatase/synthase 4 | biosynthetic pathway of trehalose | salt | methylated |
| S. lycopersicum | Asr2 | putative transcription factor | response to water-stress | drought | demethylated |
Similarly, in the tea plant (Camellia sinensis), the expression of the cytosine-5 DNA methyltransferase (C5-MTase) and DNA demethylase (dMTase) genes is dependent on environmental stimuli. As in Dendrobium, the promoters of CsC5-MTase and CsdMTase have multiple cis-acting elements responsive to light, phytohormones, and stress, suggesting that the relationship between environmental stimuli and DNA methylation is conserved among plants. The expression of the Camellia methylases and demethylases depends on abiotic stress, such as cold and drought. In particular, due to abiotic stress, the transcription of most CsC5-MTases is repressed, and that of all four CsdMTase genes increases [120], showing that the DNA methylation/demethylation activity can be a quick and effective response to environmental changes (Table 1).
There are many other examples where abiotic stress is a critical factor contributing to the modulation of DNA methylation. Cold is a stress factor that controls methylome flexibility in many plant species. Arabidopsis plants grown at two different temperatures that affect flowering, 10 °C and 16 °C, have a significant difference in the methylation in the CHH context. On the contrary, there are no considerable alterations in CG and CHG contexts. The differentially methylated regions (DMR) of the Arabidopsis plants subjected to the two different temperatures are located in transposable elements, according to their association with CHH methylation [121]. Active demethylation is due to low temperature in Z. mays, especially in root tissue [122,123] and in the snapdragon (Antirrhinum majus), where it causes the activation of the Tam3 transposons [123,124,125].
In addition to temperature, other abiotic factors can affect DNA methylation. For example, salt stress induces demethylation in rape (Brassica napus) and O. sativa [123,126,127,128]. In B. napus, two different cultivars have distinct DNA methylation levels under salt stress, resulting in the variation of the expression of two stress-related genes: Lacerata (LCR) and Trehalose Phosphatase/Synthase 4 (TPS4).
The LCR gene encodes a cytochrome P450 monooxygenase involved in the cutin synthesis that controls the water loss. The LCR expression decreases in the salinity-tolerant Exagone cultivar due to increased methylation under high salt conditions. On the contrary, the LCR expression remains unaltered in the salinity-sensitive Toccata cultivar. The TPS4 gene belongs to the biosynthetic pathway of trehalose, a disaccharide involved in response to dehydration. Under salinity stress, the expression of TPS4 increases in Exagone and decreases in Toccata, reflecting its methylation pattern [126] (Table 1).
A diversity of rice varieties respond to salt stress in different ways through DNA methylation changes. For example, under salt stress, the salt-tolerant Pokkali variety promptly removes its DNA methylation marks in comparison to the salt-sensitive variety IR29 [128].
As mentioned before, water deficit is an abiotic stress that affects DNA methylation, for example increasing the demethylation levels in tomato (Solanum lycopersicum) where the drought-responsive gene Asr2 is hypomethylated in roots under drought conditions [123,129] (Table 1).
Salinity and drought stress also affect Z. mays plants, altering cell cycle regulation and chromatin remodeling. In particular, due to the exposure to this abiotic stress, DNA methylation levels are reduced together with different changes in histone modifications [130].
Arsenic toxicity is another abiotic stress affecting DNA methylation, as in the fern Pteris cretica, where arsenic stress reduces DNA methylation in old fronds. Changes in the methylation landscape directed by arsenic exposure alter several physiological parameters of this fern species and reduce the metabolite and water transport between roots and fronds [131].
The heavy metal stress also induces specific DNA methylation changes in the green alga Scenedesmus acutus. Chromium is one of the environment’s most diffused and toxic metals. The methylation pattern of two S. acutus strains with different chromium sensitivity is very different, suggesting that DNA methylation is involved in chromium tolerance in algae [132].
A further interesting example of the abiotic stress effect on methylation is the effective hypermethylation in the Pinus silvestris genome as a protective mechanism and adaptation strategy under the mutagenic ionizing radiations in the Chernobyl area [123,133].
In addition to abiotic factors, biotic stimuli such as the colonization of microorganisms or pathogens can influence DNA methylation levels. For example, infection of nematode cysts stimulates hypomethylation in soybean (Glycine max) and A. thaliana roots [134,135]. Many findings display that the DME mutants of Arabidopsis have an increased susceptibility to infection by pathogenic bacteria and fungi [136], suggesting that the regulation of DNA methylation is related to defense mechanisms against different biotic external agents.
4.2. Environmental Adaptations and Evolution
When environmental stimuli influence the reprogramming of DNA methylation in the germline, these modifications can be transmitted to the next generation [42]. In contrast to animals, where the germline is determined early during development, plants establish the germline later due to environmental signaling and cellular context.
The lack of an early separation between somatic and germline tissues in plants makes transgenerational transmission of epigenetic modifications more probable and straightforward [137,138]. The transgenerational epigenetic inheritance (TEI) in plants is frequently determined by environmental stimuli inducing epimutations that can be inherited across generations [139]. For example, some plants, such as Arabidopsis, acquire a specific methylation profile in response to drought stress. This methylation pattern can be transmitted to the next generations of stressed and unstressed plants. However, a prolonged irrigation period can cause the loss of the epialleles and related phenotype [140]. This suggests low stability of the epimutations, which tends to disappear over time and in the absence of the environmental stimulus.
Despite their instability, variations in DNA methylation and the consequent modification of gene expression can induce phenotypic changes that are evolutionarily advantageous. Natural selection can act on these epimutations, spreading or fixing epialleles that could become adaptive. According to this view, the external environment could contribute to plant evolution [141,142].
An exciting example of the species-specific methylation patterns resulting from the divergent selection driven by eco-environmental variables, such as water availability and temperature, is represented by three closely related allotetraploid orchid species: Dactylorhiza majalis, D. traunsteineri, and D. ebudensis. These orchid species originated from the same diploid parental lineages, D. fuchsii and D. incarnata; however, they appeared at different times during the late Quaternary. Their epigenetic variation, triggered by the doubling of the genome, reflects their evolutionary history, geographical distribution, and ecological niche. In particular, D. majalis shows high resistance to the humidity of the meadows in Western and Central Europe, the Baltic region, and northwestern Russia. D. traunsteineri, widespread in northwestern and central Europe, developed the capability to grow in the marshes. D. ebudensis is distributed principally in northwest Scotland and has poor moisture tolerance. Analyzing the epigenome of the three species, different epiloci related to vapor pressure and temperature show a species-specific methylation profile related to their ecology and evolution [143].
Different methylation patterns can also occur in ecotypes of the same species showing phenotypic divergence due to different environmental conditions. For example, tropical and temperate lotus (Nelumbo nucifera) are adapted to low and high latitudes, respectively, and present different methylation profiles. The rhizome methylome of the temperate lotus is hypermethylated compared to that of the tropical lotus, resulting in the differential expression of many genes related to the differentiation of the root tissue, such as starch-biosynthesis, gibberellin, and brassinosteroid-signaling genes. Due to this different methylation landscape and differential gene expression, the rhizome morphology shows evident differences between the two ecotypes, better adapted to the distinct ecological conditions: the tropical ecotype has thin rhizomes compared to the temperate, characterized by swollen roots [144].
Therefore, epimutations could be considered a significant evolutionary force for plants, and the mutant phenotypes obtained by methylation changes are rapidly exposed to the action of natural selection. In addition, methylated DNA regions have a high mutation rate, and they could represent the first step towards a more stable sequence mutation [138].
The partial reversibility of epimutations generates a phenotypic effect more dynamic than DNA sequence changes. One well-known example of gene silencing affecting plant morphology is the methylation of the CYCLOIDEA gene (CYC) in the common toadflax (Linaria vulgaris). In many angiosperms, the CYC gene has a conserved role in establishing bilateral symmetry, promoting the development of the dorsal identity of the flower organs [145]. During the angiosperm evolution, many flower symmetry transitions have occurred [146]. These reversions depend on the modifications of expression profile or mutations in the genes involved in determining flower symmetry. The loss or the reduced expression of the CYC gene in many angiosperms, such as Conandron ramondioides [147] or Plantago lanceolata [148], is associated with the ventralization of the flower [145]. In L. vulgaris, the CYC methylation, and the consequent reduction of its expression, causes the loss of bilateral symmetry, and the flower assumes a peloric mutant phenotype with radial symmetry [149]. The genetic knock-down by hypermethylation can function as the first step of adaptation before the complete loss of the gene function. It guarantees the possibility of implementing a co-adaptation process of other genes that can compensate for the gene loss by generating a functional balance [42,138].
Methylation levels vary among plant species, and this also depends on the genome content of repetitive and mobile DNA, which has high methylation levels [40]. Therefore, DNA methylation is an evolutionary trait related to plant genome size and complexity. However, the relationship between global cytosine methylation and genome size is not linear and is more complex than a simple cause-effect relation [150].
5. Conclusions
DNA methylation has acquired many functions during plant evolution (Figure 3). The ubiquitous presence in all plant species of the methylation of transposable elements demonstrates its very ancient role. The regulation of gene expression is another central function of DNA methylation. This regulatory process is present in all land plants, as well as the methylation of the coding regions. In angiosperms, there is an expansion of the role of methylation. The intervention of PolV in the RdDM pathway could have played a role in reducing genome size and enhancing the diploidization after whole genome duplications [42,151]. Likewise, the maintenance of methylation of non-CG sequences by CMT3 is another angiosperm-specific regulatory mechanism. Finally, genomic imprinting represents a fundamental evolutionary novelty in flowering plants [42].
DNA methylation is one of the best-known epigenetic mechanisms and plays a central role in plant development and evolution. During the plant life cycle, gene expression, transposon mobility, and chromosome interactions depend on the balance between DNA methylation and demethylation. In addition, the environmental stimuli can affect the methylation landscape, resulting in specific adaptation and evolution of traits. However, fully understanding all the functions and mechanisms of mRNA and DNA methylation and its repercussions on the evolution of plant species is still a fascinating challenge.
Up to now, crop improvement has been based on the analysis of genetic variation and the selection of advantageous alleles. In addition to this “classical” approach, the knowledge of epigenetic mechanisms can be applied to develop “epimutagenesis” techniques to modify and improve characters of agronomic interest [34].
In human cells, the application of synthetic epigenetic regulation to control gene expression is already used [79,152]. To date, technologies are being developed to enhance agriculture, manage diseases, and improve crops. One of the most recent strategies is using exogenous dsRNA to induce gene silencing and epigenetic alterations through RNA interference (RNAi) in plants [153].
Currently, advances are being made for locus-specific manipulation of DNA methylation. One of the most exciting approaches is the use of proteins that bind DNA (e.g., zinc finger proteins, dCas9 proteins) fused with RdDM components and TET-like catalytic domains to direct locus-specific DNA demethylation in plant genomes and modify economically and agriculturally significant plant traits [154].
In conclusion, new knowledge of different epigenetic mechanisms will help develop genetic engineering strategies to obtain a synthetic epigenetic regulation that can be an essential tool for plant breeding [79].
Author Contributions
Writing—Original draft preparation, F.L. and M.C.V.; writing—review and editing, F.L. and S.A.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson I.R., Jacobsen S.E. Epigenetic inheritance in plants. Nature. 2007;447:418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- 3.Maeji H., Nishimura T. Epigenetic Mechanisms in Plants. Adv. Bot. Res. 2018;88:21–47. [Google Scholar]
- 4.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Fransz P.F., de Jong J.H. Chromatin dynamics in plants. Curr. Opin. Plant Biol. 2002;5:560–567. doi: 10.1016/S1369-5266(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 6.Zilberman D., Gehring M., Tran R.K., Ballinger T., Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007;39:61–69. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M., Kimatu J.N., Xu K., Liu B. DNA cytosine methylation in plant development. J. Genet. Genom. 2010;37:1–12. doi: 10.1016/S1673-8527(09)60020-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y., Dong A., Shen W.H. Histone variants and chromatin assembly in plant abiotic stress responses. Biochim. Biophys. Acta. 2013;1819:343–348. doi: 10.1016/j.bbagrm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Gentry M., Hennig L. Remodelling chromatin to shape development of plants. Exp. Cell Res. 2014;321:40–46. doi: 10.1016/j.yexcr.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Ashapkin V.V., Kutueva L.I., Aleksandrushkina N.I., Vanyushin B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020;21:7457. doi: 10.3390/ijms21207457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law J.A., Jacobsen S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenbaum Y., Naveh-Many T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981;292:860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- 13.Stroud H., Greenberg M.V.C., Feng S., Bernatavichute Y.V., Jacobsen S.E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152:352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroud H., Do T., Du J., Zhong X., Feng S., Johnson L., Patel D.J., Jacobsen S.E. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat. Struct. Mol. Biol. 2014;21:64–72. doi: 10.1038/nsmb.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X., Jacobsen S.E. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA. 2002;99((Suppl. 4)):16491–16498. doi: 10.1073/pnas.162371599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Jacobsen S.E. Genetic analyses of DNA methyltransferases in Arabidopsis thaliana. Cold Spring Harb. Symp. Quant. Biol. 2006;71:439–447. doi: 10.1101/sqb.2006.71.047. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Lang Z., Zhu J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018;19:489–506. doi: 10.1038/s41580-018-0016-z. [DOI] [PubMed] [Google Scholar]
- 18.Wambui Mbichi R., Wang Q.F., Wan T. RNA directed DNA methylation and seed plant genome evolution. Plant Cell Rep. 2020;39:983–996. doi: 10.1007/s00299-020-02558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matzke M.A., Mosher R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014;15:394–408. doi: 10.1038/nrg3683. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Zhu J.K. RNA-directed DNA methylation. Curr. Opin. Plant Biol. 2011;14:142–147. doi: 10.1016/j.pbi.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pikaard C.S., Haag J.R., Pontes O.M.F., Blevins T., Cocklin R. A transcription fork model for Pol IV and Pol V-dependent RNA-directed DNA methylation. Cold Spring Harb. Symp. Quant. Biol. 2012;77:205–212. doi: 10.1101/sqb.2013.77.014803. [DOI] [PubMed] [Google Scholar]
- 22.Cuerda-Gil D., Slotkin R.K. Non-canonical RNA-directed DNA methylation. Nat. Plants. 2016;2:16163. doi: 10.1038/nplants.2016.163. [DOI] [PubMed] [Google Scholar]
- 23.Penterman J., Uzawa R., Fischer R.L. Genetic Interactions between DNA Demethylation and Methylation in Arabidopsis. Plant Physiol. 2007;145:1549–1557. doi: 10.1104/pp.107.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Zhu J.K. Active DNA demethylation in plants and animals. Cold Spring Harb. Symp. Quant. Biol. 2012;77:161–173. doi: 10.1101/sqb.2012.77.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega-Galisteo A.P., Morales-Ruiz T., Ariza R.R., Roldán-Arjona T. Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol. Biol. 2008;67:671–681. doi: 10.1007/s11103-008-9346-0. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Macias M.I., Qian W., Miki D., Pontes O., Liu Y., Tang K., Liu R., Morales-Ruiz T., Ariza R.R., Roldán-Arjona T., et al. A DNA 3′ phosphatase functions in active DNA demethylation in Arabidopsis. Mol. Cell. 2012;45:357–370. doi: 10.1016/j.molcel.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J., Jang H., Shin H., Choi W.L., Mok Y.G., Huh J.H. AP endonucleases process 5-methylcytosine excision intermediates during active DNA demethylation in Arabidopsis. Nucleic Acids Res. 2014;42:11408–11418. doi: 10.1093/nar/gku834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Cordoba-Canero D., Qian W., Zhu X., Tang K., Zhang H., Ariza R.R., Roldan-Arjona T., Zhu J.K. An AP endonuclease functions in active DNA demethylation and gene imprinting in Arabidopsis [corrected] PLoS Genet. 2015;11:e1004905. doi: 10.1371/journal.pgen.1004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales-Ruiz T., Ortega-Galisteo A.P., Ponferrada-Marín M.I., Martínez-Macías M.I., Ariza R.R., Roldán-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl. Acad. Sci. USA. 2006;103:6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penterman J., Zilberman D., Huh J.H., Ballinger T., Henikoff S., Fischer R.L. DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. USA. 2007;104:6752–6757. doi: 10.1073/pnas.0701861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J., Kapoor A., Sridhar V.V., Agius F., Zhu J.K. The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr. Biol. 2007;17:54–59. doi: 10.1016/j.cub.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 32.Wu X., Zhang Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 33.Mahmood A.M., Dunwell J.M. Evidence for novel epigenetic marks within plants. AIMS Genet. 2019;6:70–87. doi: 10.3934/genet.2019.4.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji L., Jordan W.T., Shi X., Hu L., He C., Schmitz R.J. TET-mediated epimutagenesis of the Arabidopsis thaliana methylome. Nat. Commun. 2018;9:895. doi: 10.1038/s41467-018-03289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang Z., Shen L., Cui X., Bao S., Geng Y., Yu G., Liang F., Xie S., Lu T., Gu X., et al. DNA N(6)-Adenine Methylation in Arabidopsis thaliana. Dev. Cell. 2018;45:406–416.e3. doi: 10.1016/j.devcel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Takuno S., Gaut B.S. Body-methylated genes in Arabidopsis thaliana are functionally important and evolve slowly. Mol. Biol. Evol. 2012;29:219–227. doi: 10.1093/molbev/msr188. [DOI] [PubMed] [Google Scholar]
- 37.Domcke S., Bardet A.F., Ginno P.A., Hartl D., Schübeler L.B., Schübeler D. Competition between DNA methylation and transcription factors determines binding of NRF1. Nature. 2015;528:575–579. doi: 10.1038/nature16462. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H., Wang G., Qian J. Transcription factors as readers and effectors of DNA methylation. Nat. Rev. Genet. 2016;17:551–565. doi: 10.1038/nrg.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takuno S., Gaut B. Gene body methylation is conserved between plant orthologs and is of evolutionary consequence. Proc. Natl. Acad. Sci. USA. 2013;110:1797–1802. doi: 10.1073/pnas.1215380110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gehring M., Henikoff S. DNA methylation dynamics in plant genomes. Biochim. Biophys. Acta. 2007;1769:276–286. doi: 10.1016/j.bbaexp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Tran R.K., Henikoff J.G., Zilberman D., Ditt R.F., Jacobsen S.E., Henikoff S. DNA methylation profiling identifies CG methylation clusters in Arabidopsis genes. Curr. Biol. 2005;15:154–159. doi: 10.1016/j.cub.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Brautigam K., Cronk Q. DNA Methylation and the Evolution of Developmental Complexity in Plants. Front. Plant Sci. 2018;9:1447. doi: 10.3389/fpls.2018.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan A.P., Melake-Berhan A., O’Brien K., Buckley S., Quan H., Chen D., Lewis M., Banks J.A., Rabinowicz P.D. The highest-copy repeats are methylated in the small genome of the early divergent vascular plant Selaginella moellendorffii. BMC Genom. 2008;9:282. doi: 10.1186/1471-2164-9-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng S., Cokus S.J., Zhang X., Chen P.Y., Bostick M., Goll M.G., Hetzel J., Jain J., Strauss S.H., Halpern M.E., et al. Conservation and divergence of methylation patterning in plants and animals. Proc. Natl. Acad. Sci. USA. 2010;107:8689–8694. doi: 10.1073/pnas.1002720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zemach A., McDaniel I.E., Silva P., Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–919. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- 46.Niederhuth C.E., Bewick A.J., Ji L., Alabady M.S., Kim K.D., Li Q., Rohr N.A., Rambani A., Burke J.M., Udall J.A., et al. Widespread natural variation of DNA methylation within angiosperms. Genome Biol. 2016;17:194. doi: 10.1186/s13059-016-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang S.Y., Xiao R.J., Gu L., Guo Y.G., Wen R., Wan L.J. Interfacial Mechanism in Lithium-Sulfur Batteries: How Salts Mediate the Structure Evolution and Dynamics. J. Am. Chem. Soc. 2018;140:8147–8155. doi: 10.1021/jacs.8b02057. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S., Mohapatra T. Dynamics of DNA Methylation and Its Functions in Plant Growth and Development. Front. Plant Sci. 2021;12:596236. doi: 10.3389/fpls.2021.596236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollister J.D., Gaut B.S. Epigenetic silencing of transposable elements: A trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Res. 2009;19:1419–1428. doi: 10.1101/gr.091678.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed I., Sarazin A., Bowler C., Colot V., Quesneville H. Genome-wide evidence for local DNA methylation spreading from small RNA-targeted sequences in Arabidopsis. Nucleic Acids Res. 2011;39:6919–6931. doi: 10.1093/nar/gkr324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q., Gent J.I., Zynda G., Song J., Makarevitch I., Hirsch C.D., Hirsch C.N., Dawe R.K., Madzima T.F., McGinnis K.M., et al. RNA-directed DNA methylation enforces boundaries between heterochromatin and euchromatin in the maize genome. Proc. Natl. Acad. Sci. USA. 2015;112:14728–14733. doi: 10.1073/pnas.1514680112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cokus S.J., Feng S., Zhang X., Chen Z., Merriman B., Haudenschild C.D., Pradhan S., Nelson S.F., Pellegrini M., Jacobsen S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roudier F., Ahmed I., Bérard C., Sarazin A., Mary-Huard T., Cortijo S., Bouyer D., Caillieux E., Duvernois-Berthet E., Al-Shikhley L., et al. Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J. 2011;30:1928–1938. doi: 10.1038/emboj.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernatavichute Y.V., Zhang X., Cokus S., Pellegrini M., Jacobsen S.E. Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS ONE. 2008;3:e3156. doi: 10.1371/journal.pone.0003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grob S., Schmid M.W., Grossniklaus U. Hi-C analysis in Arabidopsis identifies the KNOT, a structure with similarities to the flamenco locus of Drosophila. Mol. Cell. 2014;55:678–693. doi: 10.1016/j.molcel.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Feng S., Cokus S.J., Schubert V., Zhai J., Pellegrini M., Jacobsen S.E. Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol. Cell. 2014;55:694–707. doi: 10.1016/j.molcel.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rowley M.J., Rothi M.H., Böhmdorfer G., Kuciński J., Wierzbicki A.T. Long-range control of gene expression via RNA-directed DNA methylation. PLoS Genet. 2017;13:e1006749. doi: 10.1371/journal.pgen.1006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G., Cui J., Wang L., Zhu Y., Lu Z., Jin B. Genome-Wide Identification of Circular RNAs in Arabidopsis thaliana. Front. Plant Sci. 2017;8:1678. doi: 10.3389/fpls.2017.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu T., Cui L., Zhou Y., Zhu C., Fan D., Gong H., Zhao Q., Zhou C., Zhao Y., Lu D., et al. Transcriptome-wide investigation of circular RNAs in rice. RNA. 2015;21:2076–2087. doi: 10.1261/rna.052282.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L., Zhang P., Fan Y., Lu Q., Li Q., Yan J., Muehlbauer G.J., Schnable P.S., Dai M., Li L. Circular RNAs mediated by transposons are associated with transcriptomic and phenotypic variation in maize. New Phytol. 2018;217:1292–1306. doi: 10.1111/nph.14901. [DOI] [PubMed] [Google Scholar]
- 61.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki H., Tsukahara T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int. J. Mol. Sci. 2014;15:9331–9342. doi: 10.3390/ijms15069331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L.L., Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z., Wang H., Wang Y., Xi F., Wang H., Kohnen M.V., Gao P., Wei W., Chen K., Liu X., et al. Whole-genome characterization of chronological age-associated changes in methylome and circular RNAs in moso bamboo (Phyllostachys edulis) from vegetative to floral growth. Plant J. 2021;106:435–453. doi: 10.1111/tpj.15174. [DOI] [PubMed] [Google Scholar]
- 65.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 66.Zhao W., Chu S., Jiao Y. Present Scenario of Circular RNAs (circRNAs) in Plants. Front. Plant Sci. 2019;10:379. doi: 10.3389/fpls.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M., Ma J., Wu L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mauer J., Luo X., Blanjoie A., Jiao X., Grozhik A.V., Patil D.P., Linder B., Pickering B.F., Vasseur J.J., Chen Q., et al. Reversible methylation of m(6)Am in the 5′ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao B.S., Wang X., Beadell A.V., Lu Z., Shi H., Kuuspalu A., Ho R.K., He C. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nat. Plants. 2017;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L.H., Song P., Wang Y., Lu Z., Tang Q., Yu Q., Xiao Y., Zhang X., Duan H.C., Jia G. The m(6)A Reader ECT2 Controls Trichome Morphology by Affecting mRNA Stability in Arabidopsis. Plant Cell. 2018;30:968–985. doi: 10.1105/tpc.17.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X., Yang Y., Sun B.F., Shi Y., Yang X., Xiao W., Hao Y.J., Ping X.L., Chen Y.S., Wang W.J., et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haussmann I.U., Bodi Z., Sanchez-Moran E., Mongan N.P., Archer N., Fray R.G., Soller M. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 73.Xiao W., Adhikari S., Dahal U., Chen Y.S., Hao Y.J., Sun B.F., Sun H.Y., Li A., Ping X.L., Lai W.-Y., et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Fustin J.M., Doi M., Yamaguchi Y., Hida H., Nishimura S., Yoshida M., Isagawa T., Morioka M.S., Kakeya H., Manabe I., et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 75.Zheng G., Dahl J., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer K.D., Patil D.P., Zhou J., Zinoviev A., Skabkin M.A., Elemento O., Pestova T.V., Qian S.B., Jaffrey S.R. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H., Weng X., Chen K., Shi H., He C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi J., Leong K.W., Demirci H., Chen J., Petrov A., Prabhakar A., O’Leary S.E., Dominissini D., Rechavi G., Soltis S.M. N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol. 2016;23:110–115. doi: 10.1038/nsmb.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Z., Riaz A., Chachar S., Ding Y., Hai Du H., Gu X. Epigenetic Modifications of mRNA and DNA in Plants. Mol. Plant. 2020;13:14–30. doi: 10.1016/j.molp.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Zhong S., Li H., Bodi Z., Button J., Vespa L., Herzog M., Fraya R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen L., Liang Z., Gu X., Chen Y., Teo Z.W.N., Hou X., Cai W.M., Dedon P.C., Liu L., Yu H. N6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev. Cell. 2016;38:186–200. doi: 10.1016/j.devcel.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui X., Liang Z., Shen L., Zhang Q., Bao S., Geng Y., Zhang B., Leo V., Vardy L.A., Lu T., et al. 5-Methylcytosine RNA Methylation in Arabidopsis thaliana. Mol. Plant. 2017;10:1387–1399. doi: 10.1016/j.molp.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Biswas S., Rao C.M. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur. J. Pharmacol. 2018;837:8–24. doi: 10.1016/j.ejphar.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 84.Hu J., Manduzio S., Kang H. Epitranscriptomic RNA Methylation in Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2019;10:500. doi: 10.3389/fpls.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruzicka K., Zhang M., Campilho A., Bodi Z., Kashif M., Saleh M., Eeckhout D., El-Showk S., Li H., Zhong S., et al. Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–172. doi: 10.1111/nph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bodi Z., Zhong S., Mehra S., Song J., Graham N., Li H., May S., Fray R.G. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3’ End and Reduced Levels Cause Developmental Defects. Front. Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.David R., Burgess A., Parker B., Li J., Pulsford K., Sibbritt T., Preiss T., Searle I.R. Transcriptome-Wide Mapping of RNA 5-Methylcytosine in Arabidopsis mRNAs and Noncoding RNAs. Plant Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mielecki D., Zugaj D.Ł., Muszewska A., Piwowarski J., Chojnacka A., Mielecki M., Nieminuszczy J., Grynberg M., Grzesiuk E. Novel AlkB dioxygenases —Alternative models for in silico and in vivo studies. PLoS ONE. 2012;7:e30588. doi: 10.1371/journal.pone.0030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arribas-Hernandez L., Bressendorff S., Hansen M.H., Poulsen C., Erdmann S., Brodersen P. An m(6)A-YTH Module Controls Developmental Timing and Morphogenesis in Arabidopsis. Plant Cell. 2018;30:952–967. doi: 10.1105/tpc.17.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamamura Y., Nagahara S., Higashiyama T. Double fertilization on the move. Curr. Opin. Plant Biol. 2012;15:70–77. doi: 10.1016/j.pbi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Rudall P.J. How many nuclei make an embryo sac in flowering plants? BioEssays. 2006;28:1067–1071. doi: 10.1002/bies.20488. [DOI] [PubMed] [Google Scholar]
- 92.Haig D., Westoby M. Genomic imprinting in endosperm: Its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philos. Trans. R. Soc. Lond. 1991;333:1–13. [Google Scholar]
- 93.Scott R.J., Spielman M., Bailey J., Dickinson H.G. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- 94.Ikeda Y. Plant imprinted genes identified by genome-wide approaches and their regulatory mechanisms. Plant Cell Physiol. 2012;53:809–816. doi: 10.1093/pcp/pcs049. [DOI] [PubMed] [Google Scholar]
- 95.Kinoshita T., Yadegari R., Harada J.J., Goldberg R.B., Fischer R.L. Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gehring M., Bubb K.L., Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsieh T.F., Ibarra C.A., Silva P., Zemach A., Eshed-Williams L., Fischer R.L., Zilberman D. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klosinska M., Picard C.L., Gehring M. Conserved imprinting associated with unique epigenetic signatures in the Arabidopsis genus. Nat. Plants. 2016;2:16145. doi: 10.1038/nplants.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kinoshita T., Miura A., Choi Y., Kinoshita Y., Cao X., Jacobsen S.E., Fischer R.L. KakutOne-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 2004;303:521–523. doi: 10.1126/science.1089835. [DOI] [PubMed] [Google Scholar]
- 100.Luo M., Bilodeau P., Dennis E.S., Peacock W.J., Chaudhury A. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc. Natl. Acad. Sci. USA. 2000;97:10637–10642. doi: 10.1073/pnas.170292997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jullien P.E., Kinoshita T., Ohad N., Berger F. Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell. 2006;18:1360–1372. doi: 10.1105/tpc.106.041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiyosue T., Ohad N., Yadegari R., Hannon M., Dinneny J., Wells D., Katz A., Margossian L., Harada J.J., Goldberg R.B., et al. Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kohler C., Hennig L., Spillane C., Pien S., Gruissem W., Grossniklaus U. The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 2003;17:1540–1553. doi: 10.1101/gad.257403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zemach A., Kim M.Y., Silva P., Rodrigues J.A., Dotson B., Brooks M.D., Zilberman D. Local DNA hypomethylation activates genes in rice endosperm. Proc. Natl. Acad. Sci. USA. 2010;107:18729–18734. doi: 10.1073/pnas.1009695107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu X., Wang W., Ren W., Chai Z., Guo W., Chen R., Wang L., Zhao J., Lang Z., Fan Y., et al. Genome-Wide Epigenetic Regulation of Gene Transcription in Maize Seeds. PLoS ONE. 2015;10:e0139582. doi: 10.1371/journal.pone.0139582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slotkin R.K., Vaughn M., Borges F., Tanurdzić M., Becker J.D., Feijó J.A., Martienssen R.A. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–472. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoft V.K., Chumak N., Choi Y., Hannon M., Garcia-Aguilar M., Machlicova A., Slusarz L., Mosiolek M., Park J.S., Park G.T., et al. Function of the DEMETER DNA glycosylase in the Arabidopsis thaliana male gametophyte. Proc. Natl. Acad. Sci. USA. 2011;108:8042–8047. doi: 10.1073/pnas.1105117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calarco J.P., Borges F., Donoghue M.T.A., Van Ex F., Jullien P.E., Lopes L., Gardner R., Berger F., Feijó J.A., Becker J.D., et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151:194–205. doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibarra C.A., Feng X., Schoft V.K., Hsieh T.F., Uzawa R., Rodrigues J.A., Zemach A., Chumak N., Machlicova A., Nishimura T., et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science. 2012;337:1360–1364. doi: 10.1126/science.1224839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kordyum E.L., Mosyakin S.L. Endosperm of Angiosperms and Genomic Imprinting. Life. 2020;10:104. doi: 10.3390/life10070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hieber A.D., Mudalige-Jayawickrama R.G., Kuehnle A.R. Color genes in the orchid Oncidium Gower Ramsey: Identification, expression, and potential genetic instability in an interspecific cross. Planta. 2006;223:521–531. doi: 10.1007/s00425-005-0113-z. [DOI] [PubMed] [Google Scholar]
- 112.Chiou C.Y., Pan H.A., Chuang Y.N., Yeh K.W. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in floral tissues of Oncidium cultivars. Planta. 2010;232:937–948. doi: 10.1007/s00425-010-1222-x. [DOI] [PubMed] [Google Scholar]
- 113.Liu X.J., Chuang Y.N., Chiou C.Y., Chin D.C., Shen F.Q., Yeh K.W. Methylation effect on chalcone synthase gene expression determines anthocyanin pigmentation in floral tissues of two Oncidium orchid cultivars. Planta. 2012;236:401–409. doi: 10.1007/s00425-012-1616-z. [DOI] [PubMed] [Google Scholar]
- 114.Han M.L., Yin J., Zhao Y.H., Sun X.W., Meng J.X., Zhou J., Shen T., Li H.H., Zhang F. How the Color Fades from Malus halliana Flowers: Transcriptome Sequencing and DNA Methylation Analysis. Front. Plant Sci. 2020;11:576054. doi: 10.3389/fpls.2020.576054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan X., Ma K., Zhang M., Wang J., Zhang Q. Integration of Transcriptome and Methylome Analyses Provides Insight Into the Pathway of Floral Scent Biosynthesis in Prunus mume. Front. Genet. 2021;12:779557. doi: 10.3389/fgene.2021.779557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang T., Huo T., Ding A., Hao R., Wang J., Cheng T., Bao F., Zhang Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS ONE. 2019;14:e0223974. doi: 10.1371/journal.pone.0223974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bao F., Ding A., Zhang T., Luo L., Wang J., Cheng T., Zhang Q. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic. Res. 2019;6:24. doi: 10.1038/s41438-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi Y., Zhang X., Chang X., Yan M., Zhao H., Qin Y., Wang H. Integrated analysis of DNA methylome and transcriptome reveals epigenetic regulation of CAM photosynthesis in pineapple. BMC Plant Biol. 2021;21:19. doi: 10.1186/s12870-020-02814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu Z., Zhang G., Teixeira da Silva J.A., Li M., Zhao C., He C., Si C., Zhang M., Duan J. Genome-wide identification and analysis of DNA methyltransferase and demethylase gene families in Dendrobium officinale reveal their potential functions in polysaccharide accumulation. BMC Plant Biol. 2021;21:21. doi: 10.1186/s12870-020-02811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu C., Zhang S., Zhou C., Chen L., Fu H., Li X., Lin Y., Lai Z., Guo Y. Genome-wide investigation and transcriptional analysis of cytosine-5 DNA methyltransferase and DNA demethylase gene families in tea plant (Camellia sinensis) under abiotic stress and withering processing. PeerJ. 2020;8:e8432. doi: 10.7717/peerj.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dubin M.J., Zhang P., Meng D., Remigereau M.S., Osborne E.J., Casale P.F., Drewe P., Kahles A., Jean G., Vilhjálmsson B., et al. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. eLife. 2015;4:e05255. doi: 10.7554/eLife.05255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steward N., Ito M., Yamaguchi Y., Koizumi N., Sano H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 2002;277:37741–37746. doi: 10.1074/jbc.M204050200. [DOI] [PubMed] [Google Scholar]
- 123.Santos A.P., Ferreira L.J., Oliveira M.M. Concerted Flexibility of Chromatin Structure, Methylome, and Histone Modifications along with Plant Stress Responses. Biology. 2017;6:3. doi: 10.3390/biology6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coen E.S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986;47:285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- 125.Hashida S.N., Kitamura K., Mikami T., Kishima Y. Temperature shift coordinately changes the activity and the methylation state of transposon Tam3 in Antirrhinum majus. Plant Physiol. 2003;132:1207–1216. doi: 10.1104/pp.102.017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marconi G., Pace R., Traini A., Raggi L., Lutts S., Chiusano M., Guiducci M., Falcinelli M., Benincasa P., Albertini E. Use of MSAP markers to analyse the effects of salt stress on DNA methylation in rapeseed (Brassica napus var. oleifera) PLoS ONE. 2013;8:e75597. doi: 10.1371/journal.pone.0075597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karan R., DeLeon T., Biradar H., Subudhi P.K. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE. 2012;7:e40203. doi: 10.1371/journal.pone.0040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferreira L.J., Azevedo V., Maroco J., Oliveira M.M., Santos A.P. Salt Tolerant and Sensitive Rice Varieties Display Differential Methylome Flexibility under Salt Stress. PLoS ONE. 2015;10:e0124060. doi: 10.1371/journal.pone.0124060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gonzalez R.M., Ricardi M.M., Iusem N.D. Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics. 2013;8:864–872. doi: 10.4161/epi.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kamal K.Y., Khodaeiaminjan M., Yahya G., El-Tantawy A.A., Abdel El-Moneim D., El-Esawi M.A., Abd-Elaziz M.A.A., Nassrallah A.A. Modulation of cell cycle progression and chromatin dynamic as tolerance mechanisms to salinity and drought stress in maize. Physiol. Plant. 2021;172:684–695. doi: 10.1111/ppl.13260. [DOI] [PubMed] [Google Scholar]
- 131.Zemanova V., Popov M., Pavlíková D., Kotrba P., Hnilička F., Česká J., Pavlík M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020;20:130. doi: 10.1186/s12870-020-2325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferrari M., Torelli A., Marieschi M., Cozza R. Role of DNA methylation in the chromium tolerance of Scenedesmus acutus (Chlorophyceae) and its impact on the sulfate pathway regulation. Plant Sci. 2020;301:110680. doi: 10.1016/j.plantsci.2020.110680. [DOI] [PubMed] [Google Scholar]
- 133.Kovalchuk O., Burke P., Arkhipov A., Kuchma N., James S.J., Kovalchuk I., Pogribny I. Genome hypermethylation in Pinus silvestris of Chernobyl—A mechanism for radiation adaptation? Mutat. Res. 2003;529:13–20. doi: 10.1016/S0027-5107(03)00103-9. [DOI] [PubMed] [Google Scholar]
- 134.Rambani A., Rice J.H., Liu J., Lane T., Ranjan P., Mazarei M., Pantalone V., Stewart C.N., Jr., Staton M., Hewezi T. The Methylome of Soybean Roots during the Compatible Interaction with the Soybean Cyst Nematode. Plant Physiol. 2015;168:1364–1377. doi: 10.1104/pp.15.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hewezi T., Lane T., Piya S., Rambani A., Rice J.H., Staton M. Cyst Nematode Parasitism Induces Dynamic Changes in the Root Epigenome. Plant Physiol. 2017;174:405–420. doi: 10.1104/pp.16.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zeng W., Huang H., Lin X., Zhu C., Kosami K.I., Huang C., Zhang H., Duan C.G., Zhu J.K., Miki D. Roles of DEMETER in regulating DNA methylation in vegetative tissues and pathogen resistance. J. Integr. Plant Biol. 2021;63:691–706. doi: 10.1111/jipb.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Whipple C. Plant science. Defining the plant germ line—Nature or nurture? Science. 2012;337:301–302. doi: 10.1126/science.1224362. [DOI] [PubMed] [Google Scholar]
- 138.Cronk Q.C. Plant evolution and development in a post-genomic context. Nat. Rev. Genet. 2001;2:607–619. doi: 10.1038/35084556. [DOI] [PubMed] [Google Scholar]
- 139.Paszkowski J., Grossniklaus U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr. Opin. Plant Biol. 2011;14:195–203. doi: 10.1016/j.pbi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 140.Sadhukhan A., Prasad S., Mitra J., Siddiqui N., Sahoo L., Kobayashi Y., Koyama H. How do plants remember drought? Planta. 2022;256:7. doi: 10.1007/s00425-022-03924-0. [DOI] [PubMed] [Google Scholar]
- 141.Turner B.M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:3403–3418. doi: 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richards E.J. Inherited epigenetic variation—Revisiting soft inheritance. Nat. Rev. Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 143.Paun O., Bateman R.M., Fay M.F., Hedrén M., Civeyrel L., Chase M.W. Stable epigenetic effects impact adaptation in allopolyploid orchids (Dactylorhiza: Orchidaceae) Mol. Biol. Evol. 2010;27:2465–2473. doi: 10.1093/molbev/msq150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li H., Yang X., Wang Q., Chen J., Shi T. Distinct methylome patterns contribute to ecotypic differentiation in the growth of the storage organ of a flowering plant (sacred lotus) Mol. Ecol. 2021;30:2831–2845. doi: 10.1111/mec.15933. [DOI] [PubMed] [Google Scholar]
- 145.Lucibelli F., Valoroso M.C., Aceto S. Radial or Bilateral? The Molecular Basis of Floral Symmetry. Genes. 2020;11:395. doi: 10.3390/genes11040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Citerne H.J., Jabbour F., Nadot S., Dameval C. The evolution of floral symmetry. Adv. Bot. Res. 2010;54:85–137. [Google Scholar]
- 147.Hsin K.T., Wang C.N. Expression shifts of floral symmetry genes correlate to flower actinomorphy in East Asia endemic Conandron ramondioides (Gesneriaceae) Bot. Stud. 2018;59:24. doi: 10.1186/s40529-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reardon W., Gallagher P., Nolan K.M., Wright H., Cardeñosa-Rubio M.C., Bragalini C., Lee C.S., Fitzpatrick D.A., Corcoran K., Wolff K., et al. Different outcomes for the MYB floral symmetry genes DIVARICATA and RADIALIS during the evolution of derived actinomorphy in Plantago. New Phytol. 2014;202:716–725. doi: 10.1111/nph.12682. [DOI] [PubMed] [Google Scholar]
- 149.Cubas P., Vincent C., Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- 150.Alonso C., Pérez R., Bazaga P., Herrera C.M. Global DNA cytosine methylation as an evolving trait: Phylogenetic signal and correlated evolution with genome size in angiosperms. Front. Genet. 2015;6:4. doi: 10.3389/fgene.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matzke M.A., Kanno T., Matzke A.J. RNA-Directed DNA Methylation: The Evolution of a Complex Epigenetic Pathway in Flowering Plants. Annu. Rev. Plant. Biol. 2015;66:243–267. doi: 10.1146/annurev-arplant-043014-114633. [DOI] [PubMed] [Google Scholar]
- 152.Park M., Patel N., Keung A.J., Khalil A.S. Engineering Epigenetic Regulation Using Synthetic Read-Write Modules. Cell. 2019;176:227–238.e20. doi: 10.1016/j.cell.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Das P.R., Sherif S.M. Application of Exogenous dsRNAs-induced RNAi in Agriculture: Challenges and Triumphs. Front. Plant. Sci. 2020;11:946. doi: 10.3389/fpls.2020.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gallego-Bartolome J. DNA methylation in plants: Mechanisms and tools for targeted manipulation. New Phytol. 2020;227:38–44. doi: 10.1111/nph.16529. [DOI] [PubMed] [Google Scholar]