Abstract
Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin in the adult brain and functions as both a primary neurotrophic signal and a neuromodulator. It serves essential roles in neuronal development, maintenance, transmission, and plasticity, thereby influencing aging, cognition, and behavior. Accumulating evidence associates reduced central and peripheral BDNF levels with various neuropsychiatric disorders, supporting its potential utilization as a biomarker of central pathologies. Subsequently, extensive research has been conducted to evaluate restoring, or otherwise augmenting, BDNF transmission as a potential therapeutic approach. Promising results were indeed observed for genetic BDNF upregulation or exogenous administration using a multitude of murine models of neurological and psychiatric diseases. However, varying mechanisms have been proposed to underlie the observed therapeutic effects, and many findings indicate the engagement of disease-specific and other non-specific mechanisms. This is because BDNF essentially affects all aspects of neuronal cellular function through tropomyosin receptor kinase B (TrkB) receptor signaling, the disruptions of which vary between brain regions across different pathologies leading to diversified consequences on cognition and behavior. Herein, we review the neurophysiology of BDNF transmission and signaling and classify the converging and diverging molecular mechanisms underlying its therapeutic potentials in neuropsychiatric disorders. These include neuroprotection, synaptic maintenance, immunomodulation, plasticity facilitation, secondary neuromodulation, and preservation of neurovascular unit integrity and cellular viability. Lastly, we discuss several findings suggesting BDNF as a common mediator of the therapeutic actions of centrally acting pharmacological agents used in the treatment of neurological and psychiatric illness.
Keywords: brain-derived neurotrophic factor, TrkB signaling, neuroprotection, synaptic plasticity, neuromodulation, neurodegeneration, neuroinflammation, oxidative stress
1. Introduction
Various neurotrophic peptides are expressed across the central nervous system (CNS) and primarily function as viability signals. During development, neurotrophins promote neurogenesis in addition to neural differentiation and maturation [1]. In the adult brain, neurotrophins serve to maintain the function and survival of neurons [2]. The most abundant among these is brain-derived neurotrophic factor (BDNF), which is expressed in many organs and by neurons and glial cells, especially astrocytes, throughout the brain [3]. Additional neurophysiological roles for BDNF have been identified, including the modulation of neural activity, synaptic transmission, and plasticity [4] with key roles in cognition [5], sensory function [6,7], motor learning [8], and memory performance [9,10]. The expression level of BDNF is, at least partly, activity-dependent, as observed in the human cerebral cortex [11] as well as murine models, such that enriched environmental experiences and sensory stimulation increase BDNF levels in primary sensory cortices and hippocampus [12,13], while sensory deprivation leads to the opposite [14,15]. However, the synthesis and release of BDNF is heavily regulated by various physiological and pathological stimuli [3,16] and show significant genotype-expression interactions [17] and polymorphic variations [18]. In addition, altered central and peripheral plasma BDNF levels are significantly associated with numerous brain pathologies and, thus, have been described as biomarkers of a wide array of neuropsychiatric disorders [19,20]. Although the association between reduced BDNF levels and CNS pathologies is not necessarily causal, reduced BDNF levels are linked to increased neuronal impairment and degeneration, as observed in Parkinson’s disease patients [21]. Furthermore, reduced levels or hindered transmission of BDNF are expected to alter its corresponding cognitive and behavioral functions. Augmenting BDNF transmission, therefore, has been extensively investigated as a promising therapeutic approach in multiple brain pathologies. In this review, we describe the neurophysiology of BDNF transmission and receptor signaling and discuss the molecular and cellular mechanisms underlying its therapeutic potentials in neuropsychiatric disorders.
2. BDNF Transmission
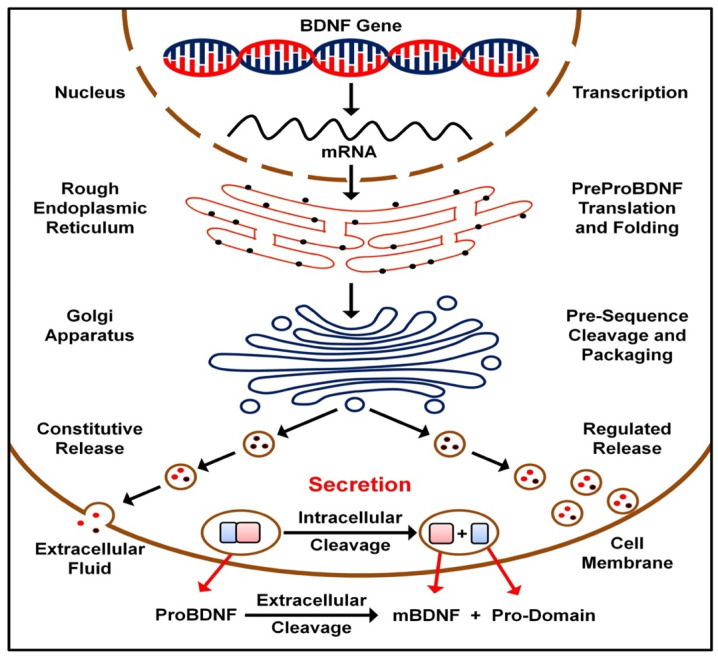
In humans, the proBDNF protein is encoded by the BDNF gene, which includes 11 exons and 9 promoters, with tissue and brain region-specific functionality producing alternatively spliced transcripts and regulated by various non-coding anti-sense ribonucleic acids (RNAs) from the anti-BDNF or BDNFOS gene [22]. Subsequent to translation, folding, and pre-sequence cleavage, the resultant proBDNF becomes packaged into vesicles for either constitutive (spontaneous) or regulated release [23]. The 32 kDa proBDNF can be cleaved by intracellular and extracellular proteases (e.g., plasmin and matrix metalloproteinases) to produce the mature ~14 kDa BDNF (mBDNF) and the propeptide proteins. When intracellularly cleaved, mBDNF and the propeptide can also be stored in dense core vesicles located in excitatory neuronal presynaptic terminals [24]. Therefore, neurons can release both the mature BDNF, as a primary trophic signal or neuromodulator, and the precursor proBDNF forms from presynaptic terminals (Figure 1). However, BDNF can also be released in a retrograde manner from postsynaptic cells to alter presynaptic activity [25] and mediate other specific functions such as synapse elimination in the developing cerebellum [26]. The mature form of BDNF signals primarily via the tropomyosin receptor kinase B (TrkB) receptor, while proBDNF binds to and activates the sortilin and p75 neurotrophin receptor (p75NTR), the latter of which is mainly expressed during development with lesser but maintained levels during adulthood [27]. Contrasting to the TrkB-mediated effects of mBDNF, the activation of sortilin and p75NTR, which is localized in dendritic spines and axon terminals by proBDNF, promotes cell death and attenuation of synaptic transmission through long-term depression (LTD), in addition to increasing anxiety and depression-like behaviors [28,29,30,31]. On the other hand, mBDNF actions mainly involve enhanced neuronal survival, growth, and synaptic activity [32]. However, mBDNF actions mediated via the TrkB receptor are highly diverse and can vary based on multiple factors. The multitude of TrkB-mediated functions is dependent on the specific BDNF mRNA species, pre- versus postsynaptic release, and receptor location, and intrinsic cell-specific interactors with the TrkB receptor [33]. Additionally, differential molecular alterations and cellular functions, such as synaptic activity and plasticity, are observed between acute and gradual activation of the TrkB receptor, and thus TrkB downstream signaling [34]. Similarly, transient activation promotes dendritic growth and spine morphogenesis, while sustained activation facilitates dendritic arborization and spinogenesis [35]. Another source of regulation is the activity-dependent modulation of TrkB receptor trafficking and cell-surface expression and translocation depending on the cell- and potentially synapse-specific degree of activity and BDNF release [36]. Furthermore, glial cells including astrocytes, which regulate BDNF recycling, oligodendrocytes, and microglia are also important targets and sources of BDNF, leading to yet another order of regulation and activity mediation [37,38,39,40]. Lastly, various TrkB receptor isoforms have been identified and mediate different functions, as discussed below. These findings indicate highly and tightly regulated control of endogenous BDNF synthesis, release, and transmission, allowing adequate direction of its diverse functions.
Figure 1.
BDNF synthesis and release.
3. TrkB Receptor Signaling and Cellular Functions
3.1. TrkB Receptor Isoforms
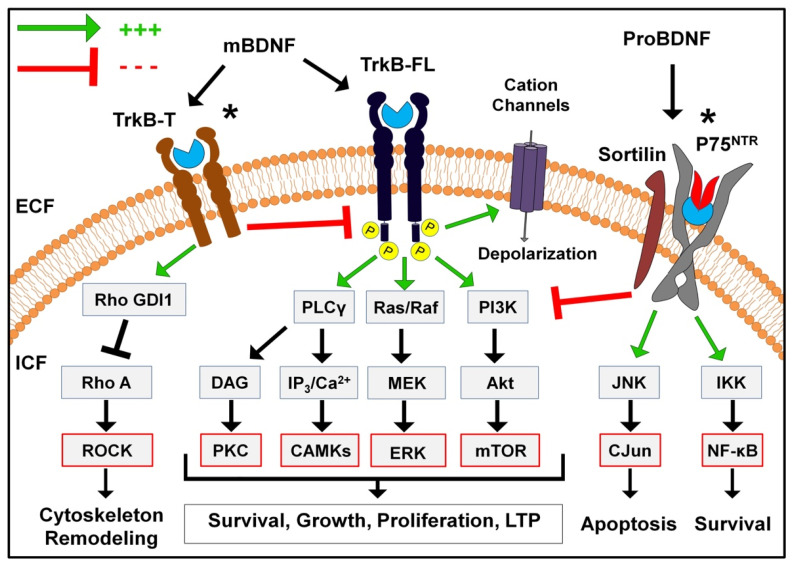
The primary target of mBDNF, or simply BDNF, is the TrkB receptor, which belongs to the Trk family of tyrosine-kinase membrane receptors that mediate neurotrophins’ actions [41]. BDNF, as well as neurotrophin-4/5 peptides, bind the TrkB receptor with high affinity. It is encoded by the human TrkB gene and, under the influence of various promoters and splicing sites, transcribed into three main TrkB receptor splice variants. These include the primary full-length isoform (TrkB-FL), a truncated alternative isoform that lacks the intracellular tyrosine-kinase domain (TrkB-T1), and is hence unable to produce the fast downstream cytoplasmic signals, and another truncated isoform (TrkB-T2 or TrkB-T-Shc), also lacking the catalytic domain but exhibiting an additional Shc binding site [42]. However, evidence indicates that the truncated isoforms can regulate the activity of intracellular kinases [43,44]. Accordingly, differential effects are observed for the activation of TrkB receptor variants [45].
3.2. Truncated TrkB Receptor
The TrkB-T1 isoform exhibits a dominant-negative functionality to inhibit TrkB-FL signaling in neurons and sequester BDNF; however, it is also responsible for other functions such as the regulation of cytoskeletal rearrangement [46]. The loci of expression between the splice variants also differ, and in glial cells TrkB-T1 has more than 100-fold higher expression than TrkB-FL. Additionally, it is found to induce inositol-1,4,5-trisphosphate (IP3)-mediated intracellular calcium signals in astrocytes, indicating key roles in neuroglial communication [47], and mediates astrocyte morphogenesis essential for downstream astrocytic support of neurons and synaptic function [48] as well as energy homeostasis and regulation of glutamate clearance [49]. During gliogenesis, BDNF-induced activation of truncated TrkB stimulates G-protein and protein kinase C (PKC) activation in neural stem cells to form glial progenitors and astrocytes [44]. The TrkB-T1 receptor can also alter cell signaling via Rho protein regulation. Using glioma cells, TrkB-T1 activation is found to dissociate the Rho guanine nucleotide dissociation inhibitor (Rho GDI1), thereby reducing the activity of RhoA, Rho-associated kinase (ROCK), p21-activated kinase (PAK), and extracellular-signal regulated kinase-1/2 (ERK1/2) [50]. Regulation of Rho proteins via TrkB-T1 is also found to inhibit glycine reuptake by astrocytes through endocytosis of glycine transporter [51]. On the other hand, little is known about the signaling mechanisms of TrkB-T1 in neurons, and its knockout mice have normal development in addition to hippocampal morphology, memory, basal transmission, and long-term potentiation (LTP). However, these mice showed increased anxiety and abnormal neurite length and complexity in basolateral amygdala neurons [52]. Very little is known regarding neuronal TrkB-T2 receptor signaling and functions; however, it shares similar properties with TrkB-T1, including the kinetics of signaling [43] and the inhibitory effect on TrkB-FL activation [53]. Nonetheless, these findings indicate that truncated TrkB isoforms are functionally independent receptors that alter cell signaling and balance BDNF-induced cellular functions. In fact, the imbalance between TrkB-FL and truncated TrkB-T1 activity is observed in various models of CNS injury and neuropathic pain, especially via TrkB-T1 upregulation [54].
3.3. Full-Length TrkB Receptor
The predominant receptor isoform mediating the neurotrophic pro-survival effects, calcium signaling, and excitatory and inhibitory balance of BDNF in neurons is the non-truncated full-length TrkB-FL receptor exhibiting the tyrosine kinase catalytic subunit [55,56,57]. The binding of BDNF to the TrkB-FL receptor results in receptor phosphorylation, which leads to recruitment of intermediary proteins to bind various docking sites such as Shc adapter protein and activation of phospholipase C (PLC). Three main signaling cascades are subsequently triggered: the Phosphoinositide 3-kinase/Protein kinase B (PI3K/PKB-Akt) pathway, the mitogen activated protein kinase (MAPK) pathway, which is also known as the Ras-Raf-MAPK/ERK kinase (MEK)-ERK pathway, and the PLC pathway, which involves PLC-IP3-Ca2+ and PLC-diacylglycerol (DAG)-PKC signaling [58,59].
3.3.1. The PI3K/Akt Pathway
The PI3K/Akt pathway interacts with the mammalian target of rapamycin (mTOR) and other downstream effectors and promotes protein synthesis, growth, proliferation, and survival [60]. Its upregulation, and subsequent BDNF-dependent activation, in neurons is supported to underlie the attenuation of ischemia-induced apoptosis by quercetin [61] and hippocampal neuronal injury in stroke via heme oxygenase 1 [62] as well as the cognitive enhancing action of L-3-n-butylphthalide in Alzheimer’s mice [63], anti-parkinsonian neuroprotection of curcumin [64], improved recovery from traumatic brain injury (TBI) by simvastatin [65], alleviation of oxidative glutamate toxicity by huperzine A [66], and the antidepressant action of liquiritigenin [67]. Additionally, activating the PI3K/Akt pathway via BDNF leads to increased dendritic translocation of postsynaptic density-95 (PSD-95) protein following N-methyl D-aspartate (NMDA) receptor activation, thus implicating this pathway in activity-dependent synaptic potentiation [68]. Indeed, BDNF-mediated PI3K/Akt signaling prevents the downregulation of synaptic plasticity-associated proteins in hippocampal neurons induced via hyperglycemia [69]. This is consistent with the loss of spine density and impaired synaptic plasticity and cognition concurrently with reduced BDNF levels due to insulin resistance [70], while improved insulin signaling upregulates BDNF, increases Akt phosphorylation, enhances cognition, and halts neuroinflammation and oxidative stress in Alzheimer’s disease [71,72]. Under physiological conditions, the BDNF-mediated activation of the PI3K/Akt/mTOR pathway controls the size of neuronal soma and dendrites; however, concomitant and coordinated activation of the MAPK pathway was found necessary to increase dendritic complexity [73]. These two pathways, both recruited via neurotrophins including BDNF, show bidirectional interactions to promote or inhibit one another, allowing further signal processing refinement [74].
3.3.2. The MAPK Pathway
Upon TrkB stimulation, a cascade of kinases, namely Ras-Raf-MEK-ERK, become activated and trigger the second major signaling pathway. This MAPK pathway is an essential controller of the cell cycle [75], and similar to the PI3K/Akt pathway mediates, particularly via ERK1/2, various anti-apoptotic neuronal processes, both cytoplasmic and transcriptional, following BDNF stimulation [76]. Certain other MAPK subtypes mainly produce pro-apoptotic effects such as C-Jun N-terminal kinase (JNK) and p38-MAPK; however, the different MAPKs show complex interactions and exhibit the ability to promote opposing cellular actions depending on the type, intensity, and duration of cellular stimuli [77]. Apart from neuroprotection, MAPKs play key roles in a wide range of neuronal cellular processes in response to BDNF. These include anterograde dendrite-to-nucleus signaling and induction of immediate early gene expression [78], cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB)-dependent LTP induction and dendritic Arc synthesis-dependent maintenance of LTP [79], and synapsin-dependent axonal growth [80] and presynaptic neurotransmitter release [81]. Additionally, BDNF-mediated MAPK signaling induces α-amino-3-hyroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor trafficking and synaptic delivery [82]. As shown in the nucleus accumbens, however, BDNF effect on AMPA receptor surface expression is bidirectional as it can promote its synaptic delivery, and thus potentiation through ERK, or down-scaling following acute and sustained activation, respectively [83]. Accordingly, various observations support key roles for BDNF in glutamatergic plasticity in the nucleus accumbens and addiction-related activity modulation [84]. The above findings reveal a wide range of cellular actions, both fast cytoplasmic and slow transcriptional, mediated by BDNF-induced MAPK pathway signaling.
3.3.3. The PLC Pathway
The third main signaling pathway of TrkB is triggered through PLC activation, especially PLCγ, which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers DAG and IP3. The latter activates IP3 channel receptors in the endoplasmic reticulum membrane, causing the release of stored Ca2+ into the cytosol [85]. Elevation of neuronal cytosolic Ca2+ concentration leads to a variety of downstream actions, particularly via calmodulin. These include the opening or enhanced permeability of various types of channels such as the membrane transient receptor potential-C (TRPC) cation channels in a store-operated mechanism [86], ligand-gated membrane channels such as NMDA receptors [87], and Ca2+-activated K+ channels, leading to long-lasting currents [88]. On the other hand, PLC-dependent but Ca2+-independent BDNF signaling also modulates Kv7/KCNQ potassium channels, as observed in parvalbumin-positive interneurons [89]. Additional actions include the Ca2+-dependent exocytotic [90], non-exocytotic [91], and reverse transport [92] neurotransmitter release, and activation of protein kinases including Ca2+/calmodulin-dependent protein kinases (CAMKs), which have key roles in BDNF-induced synaptic plasticity [93,94] and alteration of gene transcription via CREB [95]. The other messenger recruited via PLC activation is DAG, which mainly signals through PKC activation. This pathway is implicated in BDNF-mediated neuronal differentiation [96], survival [97], neurite outgrowth of dopaminergic neuron [98], NMDA receptor phosphorylation [99], AMPA receptor phosphorylation [100], γ-aminobutyric acid (GABA) receptor transcription [101], GABA-A receptor internalization during memory consolidation [102], spinal motor potentiation [103], and hippocampal activity integration and plasticity facilitation [104].
3.3.4. Rapid Modulation of Ion Channels
BDNF actions mediated through the above signaling cascades occur mainly over periods of minutes to hours; however, BDNF is also observed to alter neuronal excitability within the second and even millisecond timescales, causing strong depolarization and trains of action potentials at nanomolar concentrations [105]. This rapid neurotransmitter-like BDNF signaling is mediated via direct modulation of membrane channels, especially voltage-gated ion channels [106]. This is observed for tyrosine kinase-dependent BDNF-induced excitation via NaV1.9 sodium channels [107] and calcium transients in dendritic spines via voltage-gated calcium channels, leading to LTP induction [108]. On the other hand, BDNF also modulates NaV1.2 channels through fast inactivation, resulting in reduced inward currents, thereby reducing excitability [109].
The above findings on TrkB-mediated signaling indicate that BDNF essentially affects all aspects of neuronal function, including the cell cycle, synaptic structure, neurotransmitter release, excitability, and plasticity. The diversity of TrkB-mediated cellular processes through its multiple signaling pathways (Figure 2) cross-talk with other cellular stimuli, directly interacting effectors and its bidirectional control nature, and therefore highlight the importance of balanced and coordinated spatiotemporal BDNF release in the maintenance of neuronal homeostasis. Insufficient or imbalanced BDNF transmission can thus have detrimental consequences on neuronal viability and function under certain pathological conditions. Accordingly, restoring or augmenting BDNF transmission and TrkB signaling, ideally in a differential and targeted manner, has emerged as a promising therapeutic approach in the potential management of various neuropsychiatric disorders through the engagement of multiple mechanisms.
Figure 2.
BDNF receptor signaling pathways. (*) different signaling pathways and cellular functions are triggered with heterodimer forms.
4. Therapeutic BDNF Mechanisms
4.1. Neuronal Protection and Survival
BDNF is essential for neuronal survival under physiological conditions, and its mutation or deficiency lead to cell death that is more overt in specific neuronal populations [110,111]. In addition, exogenous supply of BDNF to neuronal cultures promotes survival by preventing apoptosis [112]. These pro-survival roles of BDNF can be through direct anti-apoptotic actions and/or via indirect protection from neuronal injury. Accordingly, BDNF-mediated signaling is able to block hypoxia-induced caspase-3 activation [113] and halt the upregulation of various apoptotic proteins, including phosphorylated C-Jun and cytochrome C due to cortical ablation [114]. The inhibition of p53 tumor suppressor protein and its upregulated modulator of apoptosis (PUMA) is also implicated in BDNF anti-apoptotic action [115]. Additionally, BDNF pre-treatment in neuronal cultures prevents excitotoxicity-induced apoptotic morphology and caspase activity through PI3K and MAPK pathways while increasing B-cell lymphoma 2 (Bcl-2) protein levels [116]. Although BDNF-mediated upregulation of NMDA receptor increases its calcium response and neuronal vulnerability to glutamate-induced necrosis, BDNF is still protected against excitotoxic apoptosis [117]. Furthermore, BDNF blocks caspase-3-independent cell death [118], potentially through the inhibition of apoptosis-inducing factor (AIF) mitochondrio-nuclear translocation, as observed in retinal photoreceptors [119]. Apart from ablative lesions, ischemia, and excitotoxicity, BDNF also exerts protective effects against metabolic stress, such as glucose deprivation-induced apoptotic cell death [120]. Another form of neuroprotection by BDNF involves its anti-oxidative effects, which attenuate neuronal injury under pathological conditions involving oxidative stress. Indeed, BDNF treatment or incubation can lead to reduction in markers of oxidative stress and upregulation of anti-oxidant enzymes such as glutathione reductase, glutathione peroxidase, and superoxide dismutase [121,122,123]. Lastly, BDNF exerts inhibitory effects on autophagy due to mitochondrial dysfunction [124], which is implicated in the multifaceted protective roles of BDNF in neurodegenerative disorders [125].
4.2. Synaptic Maintenence
The synaptic repair therapeutic strategy takes advantage of the dynamic nature of synaptic structure and function, which is the regenerative ability and reversibility of malfunction, and encompasses three main synaptic aspects: transmission, growth, and plasticity [126]. Accordingly, various neurodegenerative disorders are characterized by synaptic loss, which associates with sensory, motor, and cognitive impairments [127]. It is established that BDNF, generally, promotes both excitatory and inhibitory synaptic transmission via different mechanisms [128], induces dendritic growth and branching [129,130], increases synaptic number and density [131], and alters spine morphology and motility [132]. In addition, evidence indicates that BDNF-mediated synaptic modulation is bidirectional and regulates resting synaptic strength and functional plasticity within useful or optimal limits [133,134]. Accordingly, indirect evidence supports targeting BDNF transmission to enhance PKC activation for the prevention of synaptic loss in Alzheimer’s disease [135]. Indeed, more recent findings show that upregulated astrocytic BDNF production, conditioned to astrogliosis, improves cognitive deficits and recovers spine density, morphology, and markers such as PSD-95 in Alzheimer’s mice [136]. Various TrkB activating molecules have also been shown to prevent synaptic loss, such as 7,8-dihydroxyflavone (DHF) [137]. Therefore, the restoration and maintenance of synaptic activity, density, and structure represent a potentially disease-modifying therapeutic strategy in neurodegeneration.
4.3. Immunomodulation
Neuroinflammation is often described as a common pathological feature of multiple neurological and psychiatric disorders. While neuroinflammation is indeed central to some disorders, such as stroke, TBI, and infection, the involvement or alteration of certain immunological processes or mediators, which may not necessarily indicate inflammation, is a more accurate description in many others [138]. Accordingly, microglial activation or elevations in common indicators of an immune component, such as cytokines, are observed in a spectrum of CNS disorders, including many neurodegenerative diseases, epilepsy, depression, bipolar disorder, autism spectrum disorder (ASD), and schizophrenia [139,140,141]. BDNF-induced effects on microglial activity, astrocyte reactivity, and CNS cytokines within the context of halting disease progression or pathology will, therefore, be referred to as the immunomodulation mechanism. It should also be stressed that certain immune reactions or mediators may have damaging effects in one disorder while providing beneficial outcomes such as healing and repair in others [138]. Regarding the roles of BDNF and TrkB signaling, opposing actions are observed using different preparations and under different conditions. In microglial cultures, for instance, BDNF exerts activating effects while its sequestration inhibits microglial activation, motility, and production of tumor necrosis factor-α (TNF-α) [142]. Additionally, BDNF promotes astrocytic and microglial activation in the spinal dorsal horn of rats exhibiting cystitis-induced allodynia and upregulates various inflammatory markers such as TNF-α and interleukin-1β (IL-1β), while the opposite is observed upon TrkB blockade [143]. On the other hand, knockdown or pharmacological blockade of hippocampal BDNF increases microglial density, motility, surveillance area, and engulfment of synapses, as observed through in vivo and in vitro experimentation [144]. While many previous studies focused on the alterations of BDNF levels in CNS disorders in regards to their immunological or inflammatory components [145], definitive causal relations cannot be concluded. Nonetheless, recent studies directly investigated the immunomodulatory roles of BDNF showing “anti-inflammatory” actions counteracting brain “neuroinflammation” using various models. Indeed, the overexpression of BDNF blocks hyperglycemia-induced microglial activation and prevents the elevations in TNF-α, IL-1β, and nuclear factor-κB (NF-κB) [146], thereby reducing the central neuroinflammatory components associated with diabetic memory and cognitive impairments [147,148]. Additionally, BDNF supplementation prevents aging-related microglial sensitization and lipopolysaccharide (LPS)-induced dopaminergic neuronal loss and microglial activation, including morphological alterations; proinflammatory cytokine production; and p38-MAPK, JNK, and NF-κB signaling [149]. Similar immunoinhibitory effects attenuating astrocytosis, microcytosis, and cytokine production are observed via BDNF supplementation, together with fibroblast growth factor-2 (FGF-2) in a model of status epilepticus, leading to a significant reduction in seizure frequency [150]. Further supporting evidence for the therapeutic BDNF/TrkB immunomodulation mechanism is observed in models of post-stroke injury [151], depression [152], multiple sclerosis [153], and TBI [154] through anti-inflammatory effects that would protect from the potential damaging consequences of neuroinflammation or signaling disruptions by its mediators [155].
4.4. Plasticity Facilitation
Synaptic plasticity, the activity-dependent modulation of synaptic transmission, encodes environmental experiences, thereby mediating various brain functions including development, learning, and memory, which ultimately shape cognition and behavior [156]. The induction of synaptic plasticity is governed by various rules and signals [157]. Among these is neuromodulatory input, which influences transmission to gate behavior-dependent plasticity induction and thus learning and memory [158]. BDNF acting as a neuromodulator facilitates synaptic plasticity via multiple mechanisms including the modulation of calcium dynamics, which regulate AMPAR trafficking [159] and retrograde signaling [160], attenuation of synaptic fatigue [161], excitability regulation of induction threshold [162], suppression of inhibitory transmission [163], neuronal synchronization by diminishing spike time jitter [164], functional synaptic clustering [165], and suppression of autophagy [166]. In addition, BDNF signaling serves in the synaptic tagging and capture of plasticity-related products [167] and maintenance of LTP [168,169]. Various studies have investigated the effects of reduced BDNF availability on synaptic plasticity using a mouse model (BDNFMet/Met mice) that recapitulates the phenotype of a common human BDNF polymorphism (Val66Met), which leads to reduced activity-dependent BDNF release [170]. Indeed, BDNFMet/Met mice exhibit synaptic plasticity impairments in the hippocampus [171], prefrontal cortex [172], amygdala [173], and striatum [174], thus implicating BDNF deficits in affective and cognitive aspects of neuropsychiatric disorders [175]. On the other hand, augmenting BDNF transmission rescues plasticity impairments in certain brain regions in murine models of Alzheimer’s disease [176], Huntington’s disease [177], fragile X syndrome [178], chronic intermittent hypoxia [179], schizophrenia [180], and aging [181]. As synaptic plasticity is impaired, or otherwise maladaptive, in various neuropsychiatric disorders [182,183] including depression [184], its facilitation by BDNF represents an important potential therapeutic mechanism. However, BDNF-induced spinal LTP of C-fiber synapses [185] can lead to hyperalgesia [186] via central sensitization, which, if it becomes maladaptive, can result in or promote chronic pain [187]. This is not restricted to the spinal dorsal horn as BDNF transmission is also implicated in orofacial neuropathic pain development and hypersensitivity, the treatment of which is challenging with few effective pharmacological therapeutic options [188,189,190,191]. These findings highlight the importance of selective regional targeting of BDNF transmission and TrkB signaling.
4.5. Secondary Neuromodulation
Although BDNF itself exerts neuromodulatory effects on glutamatergic and GABAergic transmission, it also interacts with and affects other neuromodulators. Therefore, the BDNF-induced transmission or signaling alterations of neuromodulators, especially serotonin and dopamine, will be referred to as the secondary neuromodulation mechanism. Early evidence showed that BDNF administration, via midbrain or intracerebroventricular infusion, increases monoamine transmission, particularly serotonin and to a lesser extent dopamine, in various brain regions [192]. In relation to dopamine, BDNF is found to increase dopamine turnover [193] and potentiate its release in the striatum [194] and hippocampus [195]. In addition, BDNF upregulates the D3 receptor, which triggers levodopa sensitization [196] and potentiates the responses to D3 agonists, leading to improvements in motor behavior and recovery of striatal innervation and dendritic spines [197]. Accordingly, augmenting BDNF is a promising therapeutic strategy in Parkinson’s disease, not only by protecting from dopaminergic neuronal degeneration but also via augmenting dopaminergic transmission, as supported by preclinical and clinical evidence [198]. The effects of BDNF on hippocampal serotonin transmission, on the other hand, are mainly mediated by alteration of serotonin reuptake transporter (SERT) activity such that acute single injection of BDNF in the hippocampus leads to higher serotonin reuptake, lower extracellular levels, lower KCl-evoked increase in serotonin, and diminished signal amplitudes triggered by infused serotonin [199]. BDNF downregulation using heterozygous mice leads to opposite effects on serotonin by reduced hippocampal SERT activity, and these effects were observed to be region-specific [200]. Chronic administration of BDNF in the rat dorsal raphe nucleus causes significantly less-regular firing pattern of serotonergic neurons, suggesting that increased serotonin turnover could produce behavioral changes, including antidepressant effects [201]. Indeed, accumulating evidence indicates significant associations between reduced BDNF levels and depression and strong BDNF-mediated anti-depressant actions [202]. Genetic studies revealed interactions between BDNF polymorphisms and serotonin transporter variants on psychiatric functioning [203]. In addition, genetic interactions between BDNF and catechol-o-methyl transferase (COMT), the enzyme responsible for dopamine and norepinephrine degradation, translate into cognitive and behavioral alterations [204] and impact schizophrenia symptoms [205]. Interestingly, mutant mice that lack activity-driven BDNF expression exhibit reduced mRNA levels of 5-hydroxy tryptamine 1b (5-HT1b), 5-HT2a, and 5-HT5b serotonin receptors as well as dopamine D2 receptor subtype and alpha-1a/1d adrenergic receptors but increased levels for dopamine D4 receptor subtype in the frontal cortex. These mice showed depression-like behavior, impaired response inhibition, and inflexible learning, supporting the idea that reduced BDNF may lead to depression and schizophrenia through monoaminergic transmission alterations [206]. BDNF is important for dopamine sensitivity and the expression of dopamine D3 receptor subtype [207], the mRNA of which is decreased in patients with schizophrenia or bipolar disorder and increases following treatment; however, higher D3 levels correlated with negative schizophrenic symptoms [208]. In addition, higher frontal D2/3 binding potential also significantly correlates with positive symptoms [209]. On the other hand, BDNF heterozygous mice exhibit elevated extracellular dopamine levels compared to control but also show impairments in electrically evoked release and uptake of dopamine, suggesting differential roles of BDNF on tonic vs. phasic dopaminergic transmission [210]. Although the relationship between BDNF and schizophrenia is complex [211], these findings suggest that the degree of BDNF transmission can be targeted to alter, and potentially restore, the balance of dopaminergic transmission in schizophrenia [212]. This also applies for the complex BDNF-serotonin interactions in the understanding and treatment of various psychopathologies [213], especially in mood disorders such as anxiety and depression [214]. Interestingly, stress-induced BDNF dysregulation is brain region-specific, as observed in post-traumatic stress disorder (PTSD) in which BDNF mRNA is differentially downregulated in certain brain regions while being upregulated in others [215,216]. This further supports the BDNF stress-sensitivity hypothesis that BDNF dysregulation, as with the Val66Met polymorphism, predicts vulnerability to stress [217]. Indeed, TrkB receptor blockade enhances defeat-induced avoidance and PTSD-like symptoms in models of acute social and single-prolonged stress, which were mitigated by TrkB activation [218,219]. It should be stressed that secondary modulation of dopaminergic and serotonergic transmission by BDNF represents one mechanism affecting anxiety, depression, and schizophrenia as previously described mechanisms such as neuroinflammation, oxidative stress, and plasticity impairments are also involved and modulated by BDNF. Furthermore, the secondary neuromodulation mediated by BDNF could also impact other disorders such as delirium, which is commonly observed in critically ill elderly patients, either drug-induced or following trauma, and characterized by imbalanced neurotransmission including increased dopaminergic activity and altered serotonergic transmission [220,221]. Accordingly, BDNF might be expected to worsen or precipitate delirium by potentiating dopamine activity; however, evidence shows that lower BDNF levels are associated with reduced delirium recovery [222] and higher risk of postoperative delirium occurrence [223]. Interestingly, evidence suggests that corticotropin-releasing hormone-mediated BDNF depletion and spine loss could underlie delirium-like syndrome following trauma [224]. These findings highlight the significant impact of BDNF, via euromodulateon and other mechanisms, on various neuropsychopathologies and associated cognitive and behavioral impairments.
4.6. Preservation of Neurovascular Unit Integrity
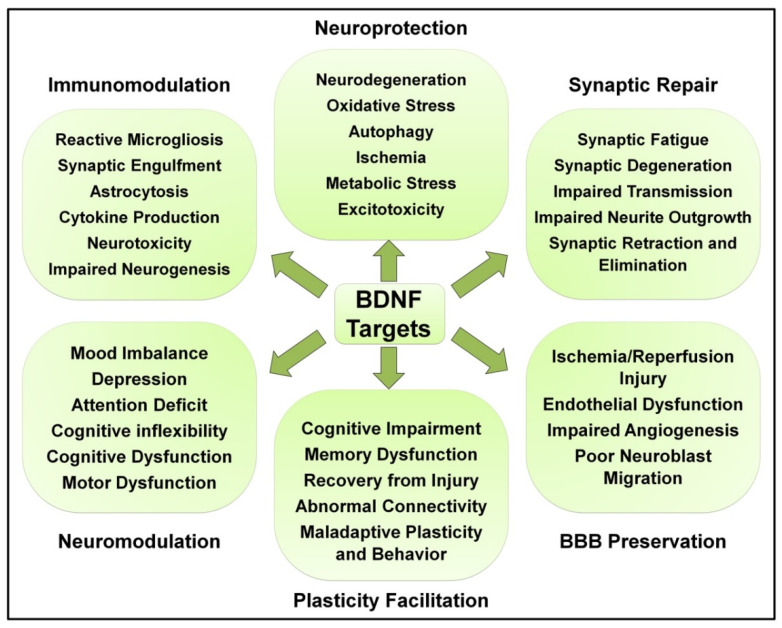
The integrity of the blood brain barrier (BBB) and neurovascular unit’s (NVU) cellular function is essential for CNS homeostasis via the regulation of influx/efflux transport and neurovascular coupling, ensuring adequate cerebral perfusion, while providing a protective barrier against potentially harmful molecules in the peripheral circulation [225]. Various pathological stimuli can lead to disruption or otherwise altered permeability and function of the BBB, such as ischemia, oxidative stress, and inflammation [226]. This is commonly observed in neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s disease as well as multiple sclerosis, leading differentially to microbleeds, leakage, impaired transport function, cellular infiltration, and NVU cellular degeneration [227]. However, multiple other neuropsychiatric disorders have been shown to involve aspects of BBB disruption, including stress disorders such as depression and anxiety [228] as well as ASD [229], epilepsy [230], and schizophrenia [231]. As previously discussed, BDNF levels are reduced in the majority of aforementioned disorders, and its supplementation engages various converging therapeutic mechanisms to counteract associated pathologies that underlie BBB disruptions such as inflammation and oxidative stress. However, accumulating evidence indicates potentially direct roles for BDNF in the preservation of neurovascular integrity and function. The BDNF Val66Met polymorphism, for instance, is associated with poor angiogenic response following stroke through upregulated anti-angiogenic mediators [232]. In addition, BDNF is found to alleviate hyperglycemia-induced endothelial cell injury in the brain microvasculature [233], protect the integrity of the blood-spinal cord barrier following spinal cord injury [234], mediate cholic acid-induced protection of BBB integrity and NVU neuronal viability against hypoglycemic and ischemic injury [235], enhance vasculature-associated migration of neuroblasts towards ischemic lesions [236], facilitate BBB recovery from ischemia following release from astrocytes surrounding blood vessels [237], and trigger robust angiogenesis and promote brain endothelial cell survival [238]. These findings indicate that BDNF has direct protective effects on BBB integrity and NVU cell viability, representing yet another potential therapeutic mechanism in neuropsychiatric disorders. The various pathological processes potentially targeted by BDNF are summarized in Figure 3.
Figure 3.
Summary of neuropsychiatric pathological alterations targeted by BDNF.
5. Neuropsychiatric Therapies Converge on BDNF: A Common Mediator
The therapeutic effects of BDNF essentially represent the target actions of various pharmacological agents used in the treatment of neurological and psychiatric illness. Many studies have investigated the effects of a wide range of medications on BDNF expression and its relation to associated therapeutic actions. In this regard, the focus was mainly directed towards certain anti-Alzheimer’s medications, antidepressants, and other miscellaneous synthetic drugs and natural compounds. Numerous pharmacological agents and herbal preparations have been identified and tested for the management of Alzheimer’s disease; however, only a few agents have been approved: the neuroprotective NMDAR antagonist memantine and the acetylcholinesterase inhibitors donepezil, rivastigmine, and galantamine [239,240]. In relation to memantine, it is found to upregulate BDNF expression [241], an effect supported to mediate memantine-induced enhancement of vascularization and recovery from stroke [242]; prevention of plasticity and memory impairment in a model of dopaminergic neurotoxin-induced Parkinson’s disease [243]; and antidepressant action counteracting chronic unpredictable stress-induced mitochondrial dysfunction, excitotoxicity, and oxidative stress [244]. Similarly, donepezil treatment causes a significant elevation of hippocampal BDNF expression in Alzheimer’s rats, mediating its cognitive enhancing action, attenuation of neurodegeneration, and restoration of synapse dendritic spines density [245,246,247]. The upregulation of BDNF is also observed for herbal preparations including gingko biloba, which leads to memory enhancement, prevention of neuronal apoptosis by lead poisoning, and neuroprotection against ischemic stroke [248,249,250,251]. In addition, various findings support the upregulation of BDNF as the mechanism underling the beneficial actions of many other botanical compounds in multiple neurological pathologies [252]. Furthermore, several lines of evidence indicate the upregulation of BDNF as a common transducer of anti-depressant action of many classical antidepressants and other agents such as ketamine [253]. Other medications shown to upregulate BDNF expression include second-generation antipsychotics such as olanzapine and clozapine [254], dopamine agonists such as rotigotine [255], the anesthetic dexmedetomidine, which enhances neuroprotection from cerebral ischemia/reperfusion injury [256,257], and delta opioid receptor agonists, which exert neuroprotective, anxiolytic, and antidepressant actions [258,259]. These findings support the notion that BDNF is potentially a common mediator of the therapeutic efficacy of centrally acting medications across an array of neuropsychiatric disorders. Although this is evident for few medications, definitive conclusions for most cannot be drawn based on associations. Therefore, further direct investigations are still required to evaluate whether attenuating drug-induced BDNF expression changes interfere with the intended therapeutic efficacy and if differences exist between agents of the same pharmacological class.
6. Current Status and Future Directions
Extensive research has been conducted to evaluate the therapeutic potential of BDNF in a wide range of brain pathologies, showing highly promising results. However, many limitations were recognized, especially regarding its delivery and central availability. Nonetheless, novel developments have been made to tackle this issue through various approaches such as gene therapy [260] and carrier-free stabilizing nanoencapsulation [261], which allows intranasal administration [262]. Other approaches include the use of TrkB receptor ligands such as DHF, small-molecule BDNF mimetics, and agonistic antibodies [263,264] as well as compounds boosting BDNF synthesis, transmission, and signaling, including natural products [126,265]. However, these direct and indirect approaches collectively referred to as BDNF-based therapies still face many other challenges [266]. Additionally, further research is still required to elucidate the network-specific functionality of BDNF, decipher how this modulates cognition and behavior, and uncover BDNF transmission disruptions in a disease-specific manner. Other considerations to take into account include systemic side effects, interactions with other disorders in which elevated BDNF levels can have harmful long-term consequences, and the potential impact of different polymorphisms on the efficacy of BDNF-based therapies.
To conclude, BDNF-mediated TrkB signaling controls a manifold of neuronal cellular functions and engages a multitude of converging and diverging molecular mechanisms that counteract multiple pathophysiological processes underpinning key aspects of neuropsychiatric disorders. These include (1) neuroprotection from apoptosis-inducing stimuli and stressors such as ischemia, excitotoxicity, energy imbalance, and oxidative stress; (2) synaptic regeneration and maintenance of activity and structure; (3) immunomodulation against microglial hyperactivity and abnormal production of inflammatory mediators; (4) facilitation and rescue of impaired and maladaptive synaptic plasticity; (5) secondary neuromodulation to alter dopaminergic and serotonergic transmission; and (6) preservation of BBB integrity and NVU cellular viability. Therefore, BDNF-based therapies carry significant therapeutic potentials in various neuropsychiatric disorders, but a set of challenges are still to be tackled.
Abbreviations
5-HT: 5-hydroxy tryptamine; AIF: Apoptosis-inducing factor; AMPA: α-amino-3-hyroxy-5-methyl-4-isoxazole-propionic acid; ASD: Autism spectrum disorder; BBB: Blood brain barrier; Bcl-2: B-cell lymphoma 2; BDNF: Brain-derived neurotrophic factor; CAMK: Ca2+/calmodulin-dependent protein kinase; cAMP: Cyclic adenosine monophosphate; CNS: Central nervous system; COMT: Catechol-o-methyl transferase; CREB: cAMP response element-binding protein; DAG: Diacyl glycerol; DHF: 7,8-dihydroxyflavone; ERK: Extracellular-signal regulated kinase; FGF-2: Fibroblast growth factor-2; GABA: γ-aminobutyric acid; IL-1β: Interleukin-1β; IP3: Inositol-1,4,5-trisphosphate; JNK: C-Jun N-terminal kinase; LPS: Lipopolysaccharide; LTD: Long-term depression; LTP: Long-term potentiation; MAPK: Mitogen activated protein kinase; mBDNF: Mature BDNF; MEK: MAPK/ERK kinase; mTOR: Mammalian target of rapamycin; NF-κB: Nuclear factor-κB; NMDA: N-methyl D-aspartate; NVU: Neurovascular unit; P75NTR: p75 neurotrophin receptor; PAK: P21-activated kinase; PI3K: Phosphoinositide 3-kinase; PIP2: Phosphatidylinositol 4,5-bisphosphate; PKB/Akt: Protein kinase B; PKC: Protein kinase C; PLC: Phospholipase C; PTSD: Post-traumatic stress disorder; PSD-95: Postsynaptic density-95; PUMA: P53 upregulated modulator of apoptosis; Rho GDI1: Rho guanine nucleotide dissociation inhibitor; RNA: Ribonucleic acid; ROCK: Rho-associated kinase; SERT: Serotonin transporter; TBI: Traumatic brain injury; TNF-α: Tumor necrosis factor-α; TrkB: Tropomyosin receptor kinase B; TrkB-FL: Full-length TrkB receptor; TrkB-T: Truncated TrkB receptor; TRPC: Transient receptor potential-C.
Author Contributions
Conceptualization, A.H.B. and F.H.B.; investigation, A.H.B. and F.H.B.; writing—original draft preparation, A.H.B.; writing—review and editing, F.H.B.; project administration, A.H.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sahay A., Kale A., Joshi S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides. 2020;83:102075. doi: 10.1016/j.npep.2020.102075. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky R.H., Marini A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann. N. Y. Acad. Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- 3.Brigadski T., Leßmann V. The physiology of regulated BDNF release. Cell Tissue Res. 2020;382:15–45. doi: 10.1007/s00441-020-03253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman A.S., Yu L., Boyle P.A., Schneider J.A., De Jager P.L., Bennett D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–741. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Šerý O., Šťastný F., Zvolský P., Hlinomazová Z., Balcar V.J. Association between Val66Met polymorphism of Brain-Derived Neurotrophic Factor (BDNF) gene and a deficiency of colour vision in alcohol-dependent male patients. Neurosci. Lett. 2011;499:154–157. doi: 10.1016/j.neulet.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Singer W., Panford-Walsh R., Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014;76:719–728. doi: 10.1016/j.neuropharm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Deveci S.Ş., Matur Z., Kesim Y.Y., Senturk G.G., Sargın-Kurt G.G., Ugur S.A., Öge A.E. Effect of the brain-derived neurotrophic factor gene Val66Met polymorphism on sensory-motor integration during a complex motor learning exercise. Brain Res. 2020;1732:146652. doi: 10.1016/j.brainres.2020.146652. [DOI] [PubMed] [Google Scholar]
- 9.Kambeitz J.P., Bhattacharyya S., Kambeitz-Ilankovic L.M., Valli I., Collier D.A., McGuire P. Effect of BDNF val66met polymorphism on declarative memory and its neural substrate: A meta-analysis. Neurosci. Biobehav. Rev. 2012;36:2165–2177. doi: 10.1016/j.neubiorev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hariri A.R., Goldberg T.E., Mattay V.S., Kolachana B.S., Callicott J.H., Egan M.F., Weinberger D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipovich L., Dachet F., Cai J., Bagla S., Balan K., Jia H., Loeb J.A. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics. 2012;192:1133–1148. doi: 10.1534/genetics.112.145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanda S.A., Mack K.J. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Mol. Brain Res. 2000;78:1–14. doi: 10.1016/S0169-328X(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 13.Valles A., Boender A.J., Gijsbers S., Haast R.A., Martens G.J., de Weerd P. Genomewide analysis of rat barrel cortex reveals time-and layer-specific mRNA expression changes related to experience-dependent plasticity. J. Neurosci. 2011;31:6140–6158. doi: 10.1523/JNEUROSCI.6514-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Pinilla F., Ying Z., Agoncillo T., Frostig R. The influence of naturalistic experience on plasticity markers in somatosensory cortex and hippocampus: Effects of whisker use. Brain Res. 2011;1388:39–47. doi: 10.1016/j.brainres.2011.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpova N.N., Rantamäki T., Di Lieto A., Lindemann L., Hoener M.C., Castrén E. Darkness reduces BDNF expression in the visual cortex and induces repressive chromatin remodeling at the BDNF gene in both hippocampus and visual cortex. Cell. Mol. Neurobiol. 2010;30:1117–1123. doi: 10.1007/s10571-010-9544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg M.E., Xu B., Lu B., Hempstead B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devlin P., Cao X., Stanfill A.G. Genotype-expression interactions for BDNF across human brain regions. BMC Genom. 2021;22:207. doi: 10.1186/s12864-021-07525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen T., You Y., Joseph C., Mirzaei M., Klistorner A., Graham S.L., Gupta V. BDNF polymorphism: A review of its diagnostic and clinical relevance in neurodegenerative disorders. Aging Dis. 2018;9:523–536. doi: 10.14336/AD.2017.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventriglia M., Zanardini R., Bonomini C., Zanetti O., Volpe D., Pasqualetti P., Gennarelli M., Bocchio-Chiavetto L. Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed Res. Int. 2013;2013:901082. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes B.S., Berk M., Turck C.W., Steiner J., Goncalves C.A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: A comparative meta-analysis. Mol. Psychiatry. 2014;19:750–751. doi: 10.1038/mp.2013.172. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Vara J., Sáez-Francàs N., Lorenzo-Bosquet C., Corominas-Roso M., Cuberas-Borròs G., Lucas-Del Pozo S., Carter S., Armengol-Bellapart M., Castell-Conesa J. BDNF levels and nigrostriatal degeneration in “drug naïve” Parkinson’s disease patients. An “in vivo” study using I-123-FP-CIT SPECT. Parkinsonism Relat. Disord. 2020;78:31–35. doi: 10.1016/j.parkreldis.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Pruunsild P., Kazantseva A., Aid T., Palm K., Timmusk T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mowla S.J., Pareek S., Farhadi H.F., Petrecca K., Fawcett J.P., Seidah N.G., Morris S.J., Sossin W.S., Murphy R.A. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieni S., Matsumoto T., Dekkers M., Rauskolb S., Ionescu M.S., Deogracias R., Gundelfinger E.D., Kojima M., Nestel S., Frotscher M., et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magby J.P., Bi C., Chen Z.Y., Lee F.S., Plummer M.R. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J. Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choo M., Miyazaki T., Yamazaki M., Kawamura M., Nakazawa T., Zhang J., Tanimura A., Uesaka N., Watanabe M., Sakimura K., et al. Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 2017;8:195. doi: 10.1038/s41467-017-00260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Siao C.J., Nagappan G., Marinic T., Jing D., McGrath K., Chen Z.Y., Mark W., Tessarollo L., Lee F.S., et al. Neuronal release of proBDNF. Nat. Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng H.K., Teng K.K., Lee R., Wright S., Tevar S., Almeida R.D., Kermani P., Torkin R., Chen Z.Y., Lee F.S., et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo N.H., Teng H.K., Siao C.J., Chiaruttini C., Pang P.T., Milner T.A., Hempstead B.L., Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 30.Bai Y.Y., Ruan C.S., Yang C.R., Li J.Y., Kang Z.L., Zhou L., Liu D., Zeng Y.Q., Wang T.H., Tian C.F., et al. ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology. 2016;41:2882–2892. doi: 10.1038/npp.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong F., Liu L., Wei J.L., Hu Z.L., Li L., Wang S., Xu J.M., Zhou X.F., Li C.Q., Yang Z.Y., et al. Brain-derived neurotrophic factor precursor in the hippocampus regulates both depressive and anxiety-like behaviors in rats. Front. Psychiatry. 2019;9:776. doi: 10.3389/fpsyt.2018.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartrup J.T., Moorman J.M., Newberry N.R. BDNF enhances neuronal growth and synaptic activity in hippocampal cell cultures. Neuroreport. 1997;8:3791–3794. doi: 10.1097/00001756-199712010-00027. [DOI] [PubMed] [Google Scholar]
- 33.De Vincenti A.P., Ríos A.S., Paratcha G., Ledda F. Mechanisms that modulate and diversify BDNF functions: Implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 2019;13:135. doi: 10.3389/fncel.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y., Lu Y., Yang F., Shen W., Tang T.T., Feng L., Duan S., Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo W., Nagappan G., Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 2018;78:647–659. doi: 10.1002/dneu.22592. [DOI] [PubMed] [Google Scholar]
- 36.Nagappan G., Lu B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Jang M., Gould E., Xu J., Kim E.J., Kim J.H. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. eLife. 2019;8:e42156. doi: 10.7554/eLife.42156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrini F., De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergami M., Santi S., Formaggio E., Cagnoli C., Verderio C., Blum R., Berninger B., Matteoli M., Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J. Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalo U., Bogdanov A., Moss G.W., Pankratov Y. Astroglia-derived BDNF and MSK-1 mediate experience-and diet-dependent synaptic plasticity. Brain Sci. 2020;10:462. doi: 10.3390/brainsci10070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patapoutian A., Reichardt L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11:272–280. doi: 10.1016/S0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 42.Stoilov P., Castren E., Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002;290:1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- 43.Baxter G.T., Radeke M.J., Kuo R.C., Makrides V., Hinkle B., Hoang R., Medina-Selby A., Coit D., Valenzuela P., Feinstein S.C. Signal transduction mediated by the truncated trkB receptor isoforms, trkB. T1 and trkB. T2. J. Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng A., Coksaygan T., Tang H., Khatri R., Balice-Gordon R.J., Rao M.S., Mattson M.P. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J. Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- 45.Yacoubian T.A., Lo D.C. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 46.Fenner B.M. Truncated TrkB: Beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24. doi: 10.1016/j.cytogfr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Rose C.R., Blum R., Pichler B., Lepier A., Kafitz K.W., Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 48.Holt L.M., Hernandez R.D., Pacheco N.L., Ceja B.T., Hossain M., Olsen M.L. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB. T1. eLife. 2019;8:e44667. doi: 10.7554/eLife.44667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ameroso D., Meng A., Chen S., Felsted J., Dulla C.G., Rios M. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat. Metab. 2022;4:627–643. doi: 10.1038/s42255-022-00566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohira K., Homma K.J., Hirai H., Nakamura S., Hayashi M. TrkB-T1 regulates the RhoA signaling and actin cytoskeleton in glioma cells. Biochem. Biophys. Res. Commun. 2006;342:867–874. doi: 10.1016/j.bbrc.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Aroeira R.I., Sebastião A.M., Valente C.A. BDNF, via truncated TrkB receptor, modulates GlyT1 and GlyT2 in astrocytes. Glia. 2015;63:2181–2197. doi: 10.1002/glia.22884. [DOI] [PubMed] [Google Scholar]
- 52.Carim-Todd L., Bath K.G., Fulgenzi G., Yanpallewar S., Jing D., Barrick C.A., Becker J., Buckley H., Dorsey S.G., Lee F.S., et al. Endogenous truncated TrkB. T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J. Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eide F.F., Vining E.R., Eide B.L., Zang K., Wang X.Y., Reichardt L.F. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J. Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao T., Matyas J.J., Renn C.L., Faden A.I., Dorsey S.G., Wu J. Function and mechanisms of truncated BDNF receptor TrkB. T1 in neuropathic pain. Cells. 2020;9:1194. doi: 10.3390/cells9051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ninkina N., Adu J., Fischer A., Pinon L.G., Buchman V.L., Davies A.M. Expression and function of TrkB variants in developing sensory neurons. EMBO J. 1996;15:6385–6393. doi: 10.1002/j.1460-2075.1996.tb01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He J., Gong H., Luo Q. BDNF acutely modulates synaptic transmission and calcium signalling in developing cortical neurons. Cell. Physiol. Biochem. 2005;16:69–76. doi: 10.1159/000087733. [DOI] [PubMed] [Google Scholar]
- 57.Elmariah S.B., Crumling M.A., Parsons T.D., Balice-Gordon R.J. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J. Neurosci. 2004;24:2380–2393. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan D.R., Miller F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 59.Gupta V.K., You Y., Gupta V.B., Klistorner A., Graham S.L. TrkB receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013;14:10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemmings B.A., Restuccia D.F. Pi3k-pkb/akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao R.Q., Qi D.S., Yu H.L., Liu J., Yang L.H., Wu X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF–TrkB–PI3K/Akt signaling pathway. Neurochem. Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 62.Qi D., Ouyang C., Wang Y., Zhang S., Ma X., Song Y., Yu H., Tang J., Fu W., Sheng L., et al. HO-1 attenuates hippocampal neurons injury via the activation of BDNF–TrkB–PI3K/Akt signaling pathway in stroke. Brain Res. 2014;1577:69–76. doi: 10.1016/j.brainres.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Xiang J., Pan J., Chen F., Zheng L., Chen Y., Zhang S., Feng W. L-3-n-butylphthalide improves cognitive impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int. J. Clin. Exp. Med. 2014;7:1706–1713. [PMC free article] [PubMed] [Google Scholar]
- 64.Jin T., Zhang Y., Benson O.A., Zhang J., Fan R., Zhang Y., Liu X. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food. Chem. Toxicol. 2022;164:113091. doi: 10.1016/j.fct.2022.113091. [DOI] [PubMed] [Google Scholar]
- 65.Wu H., Lu D., Jiang H., Xiong Y., Qu C., Li B., Mahmood A., Zhou D., Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma. 2008;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 66.Mao X.Y., Zhou H.H., Li X., Liu Z.Q. Huperzine A alleviates oxidative glutamate toxicity in hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cell. Mol. Neurobiol. 2016;36:915–925. doi: 10.1007/s10571-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao W., Dong Y., Su Q., Wang H., Chen Y., Xue W., Chen C., Xia B., Duan J., Chen G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016;308:177–186. doi: 10.1016/j.bbr.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 68.Yoshii A., Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 69.Zhong Y., Zhu Y., He T., Li W., Li Q., Miao Y. Brain-derived neurotrophic factor inhibits hyperglycemia-induced apoptosis and downregulation of synaptic plasticity-related proteins in hippocampal neurons via the PI3K/Akt pathway. Int. J. Mol. Med. 2019;43:294–304. doi: 10.3892/ijmm.2018.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stranahan A.M., Norman E.D., Lee K., Cutler R.G., Telljohann R.S., Egan J.M., Mattson M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bazzari F.H., Abdallah D.M., El-Abhar H.S. Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules. 2019;24:1992. doi: 10.3390/molecules24101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bazzari F.H., Abdallah D.M., El-Abhar H.S. Chenodeoxycholic acid reduces neuroinflammation and oxidative stress in aluminium chloride-induced rat model of Alzheimer’s disease. Br. J. Pharmacol. 2021;178:458. [Google Scholar]
- 73.Kumar V., Zhang M.X., Swank M.W., Kunz J., Wu G.Y. Regulation of dendritic morphogenesis by Ras–PI3K–Akt–mTOR and Ras–MAPK signaling pathways. J. Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aksamitiene E., Kiyatkin A., Kholodenko B.N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: A fine balance. Biochem. Soc. Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson M.G., Millar J.B. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J. 2000;14:2147–2157. doi: 10.1096/fj.00-0102rev. [DOI] [PubMed] [Google Scholar]
- 76.Bonni A., Brunet A., West A.E., Datta S.R., Takasu M.A., Greenberg M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 77.Yue J., López J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020;21:2346. doi: 10.3390/ijms21072346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen M.S., Orth C.B., Kim H.J., Jeon N.L., Jaffrey S.R. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc. Natl. Acad. Sci. USA. 2011;20108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ying S.W., Futter M., Rosenblum K., Webber M.J., Hunt S.P., Bliss T.V., Bramham C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marte A., Messa M., Benfenati F., Onofri F. Synapsins are downstream players of the BDNF-mediated axonal growth. Mol. Neurobiol. 2017;54:484–494. doi: 10.1007/s12035-015-9659-3. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Q., Song S.H., Augustine G.J. Calcium-dependent and synapsin-dependent pathways for the presynaptic actions of BDNF. Front. Cell. Neurosci. 2017;11:75. doi: 10.3389/fncel.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W., Keifer J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol. Learn. Mem. 2009;91:243–249. doi: 10.1016/j.nlm.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reimers J.M., Loweth J.A., Wolf M.E. BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons. Eur. J. Neurosci. 2014;39:1159–1169. doi: 10.1111/ejn.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quintero G.C. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr. Dis. Treat. 2013;9:1499–1512. doi: 10.2147/NDT.S45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H.S., Xu X.Z., Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/S0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 87.Crozier R.A., Black I.B., Plummer M.R. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn. Mem. 1999;6:257–266. doi: 10.1101/lm.6.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mizoguchi Y., Monji A., Nabekura J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur. J. Neurosci. 2002;16:1417–1424. doi: 10.1046/j.1460-9568.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- 89.Nieto-Gonzalez J.L., Jensen K. BDNF depresses excitability of parvalbumin-positive interneurons through an M-like current in rat dentate gyrus. PLoS ONE. 2013;8:e67318. doi: 10.1371/journal.pone.0067318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsumoto T., Numakawa T., Adachi N., Yokomaku D., Yamagishi S., Takei N., Hatanaka H. Brain-derived neurotrophic factor enhances depolarization-evoked glutamate release in cultured cortical neurons. J. Neurochem. 2001;79:522–530. doi: 10.1046/j.1471-4159.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- 91.Takei N., Numakawa T., Kozaki S., Sakai N., Endo Y., Takahashi M., Hatanaka H. Brain-derived neurotrophic factor induces rapid and transient release of glutamate through the non-exocytotic pathway from cortical neurons. J. Biol. Chem. 1998;273:27620–27624. doi: 10.1074/jbc.273.42.27620. [DOI] [PubMed] [Google Scholar]
- 92.Numakawa T., Matsumoto T., Adachi N., Yokomaku D., Kojima M., Takei N., Hatanaka H. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca2+ and Na+ in cultured cerebellar neurons. J. Neurosci. Res. 2001;66:96–108. doi: 10.1002/jnr.1201. [DOI] [PubMed] [Google Scholar]
- 93.Kanhema T., Ying S.W., Nairn A.C., Bramham C.R. BDNF-induced long-term potentiation in the dentate gyrus in vivo is associated with phosphorylation of elongation factor-2. Soc. Neurosci. Abstr. 2001;27:920.17. [Google Scholar]
- 94.Caldeira M.V., Melo C.V., Pereira D.B., Carvalho R., Correia S.S., Backos D.S., Carvalho A.L., Esteban J.A., Duarte C.B. Brain-derived neurotrophic factor regulates the Expression and synaptic delivery ofα-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- 95.Blanquet P.R., Mariani J., Derer P. A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience. 2003;18:477–490. doi: 10.1016/S0306-4522(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 96.Hellmann J., Rommelspacher H., Wernicke C. Long-term ethanol exposure impairs neuronal differentiation of human neuroblastoma cells involving neurotrophin-mediated intracellular signaling and in particular protein kinase C. Alcohol. Clin. Exp. Res. 2009;33:538–550. doi: 10.1111/j.1530-0277.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 97.Zirrgiebel U., Ohga Y., Carter B., Berninger B., Inagaki N., Thoenen H., Lindholm D. Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: Involvement of protein kinase C in neuronal survival. J. Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]
- 98.Sotogaku N., Tully S.E., Gama C.I., Higashi H., Tanaka M., Hsieh-Wilson L.C., Nishi A. Activation of phospholipase C pathways by a synthetic chondroitin sulfate-E tetrasaccharide promotes neurite outgrowth of dopaminergic neurons. J. Neurochem. 2007;103:749–760. doi: 10.1111/j.1471-4159.2007.04849.x. [DOI] [PubMed] [Google Scholar]
- 99.Liu M., Kay J.C., Shen S., Qiao L.Y. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J. Neuroinflamm. 2015;12:151. doi: 10.1186/s12974-015-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tao W., Chen Q., Zhou W., Wang Y., Wang L., Zhang Z. Persistent inflammation-induced up-regulation of brain-derived neurotrophic factor (BDNF) promotes synaptic delivery of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor GluA1 subunits in descending pain modulatory circuits. J. Biol. Chem. 2014;289:22196–22204. doi: 10.1074/jbc.M114.580381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roberts D.S., Hu Y., Lund I.V., Brooks-Kayal A.R., Russek S.J. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type a GABA Receptorα4 subunits in hippocampal neurons. J. Biol. Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 102.Mou L., Heldt S.A., Ressler K. Rapid BDNF-dependent sequestration of amygdala and hippocampal GABAA receptors via different TrkB-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agosto-Marlin I.M., Mitchell G.S. Spinal BDNF-induced phrenic motor facilitation requires PKCθ activity. J. Neurophysiol. 2017;118:2755–2762. doi: 10.1152/jn.00945.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colgan L.A., Hu M., Misler J.A., Parra-Bueno P., Moran C.M., Leitges M., Yasuda R. PKCα integrates spatiotemporally distinct Ca2+ and autocrine BDNF signaling to facilitate synaptic plasticity. Nat. Neurosci. 2018;21:1027–1037. doi: 10.1038/s41593-018-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kafitz K.W., Rose C.R., Thoenen H., Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 106.Kovalchuk Y., Holthoff K., Konnerth A. Neurotrophin action on a rapid timescale. Curr. Opin. Neurobiol. 2004;14:558–563. doi: 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 107.Blum R., Kafitz K.W., Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel NaV1. 9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- 108.Kovalchuk Y., Hanse E., Kafitz K.W., Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- 109.Ahn M., Beacham D., Westenbroek R.E., Scheuer T., Catterall W.A. Regulation of NaV1. 2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J. Neurosci. 2007;27:11533–11542. doi: 10.1523/JNEUROSCI.5005-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Linnarsson S., Willson C.A., Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Mol. Brain Res. 2000;75:61–69. doi: 10.1016/S0169-328X(99)00295-8. [DOI] [PubMed] [Google Scholar]
- 111.Acheson A., Conover J.C., Fandl J.P., DeChiara T.M., Russell M., Thadani A., Squinto S.P., Yancopoulos G.D., Lindsay R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 112.Kubo T., Nonomura T., Enokido Y., Hatanaka H. Brain-derived neurotrophic factor (BDNF) can prevent apoptosis of rat cerebellar granule neurons in culture. Dev. Brain Res. 1995;85:249–258. doi: 10.1016/0165-3806(94)00220-T. [DOI] [PubMed] [Google Scholar]
- 113.Han B.H., D’Costa A., Back S.A., Parsadanian M., Patel S., Shah A.R., Gidday J.M., Srinivasan A., Deshmukh M., Holtzman D.M. BDNF blocks caspase-3 activation in neonatal hypoxia–ischemia. Neurobiol. Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- 114.Madeddu F., Naska S., Bozzi Y. BDNF down-regulates the caspase 3 pathway in injured geniculo-cortical neurones. Neuroreport. 2004;15:2045–2049. doi: 10.1097/00001756-200409150-00010. [DOI] [PubMed] [Google Scholar]
- 115.Hua Z., Zhan Y., Zhang S., Dong Y., Jiang M., Tan F., Liu Z., Thiele C.J., Li Z. P53/PUMA are potential targets that mediate the protection of brain-derived neurotrophic factor (BDNF)/TrkB from etoposide-induced cell death in neuroblastoma (NB) Apoptosis. 2018;23:408–419. doi: 10.1007/s10495-018-1467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Almeida R.D., Manadas B.J., Melo C.V., Gomes J.R., Mendes C.S., Graos M.M., Carvalho R.F., Carvalho A.P., Duarte C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death. Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 117.Glazner G.W., Mattson M.P. Differential effects of BDNF, ADNF9, and TNFα on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp. Neurol. 2000;161:442–452. doi: 10.1006/exnr.1999.7242. [DOI] [PubMed] [Google Scholar]
- 118.Bifrare Y.D., Kummer J., Joss P., Täuber M.G., Leib S.L. Brain-derived neurotrophic factor protects against multiple forms of brain injury in bacterial meningitis. J. Infect. Dis. 2005;191:40–45. doi: 10.1086/426399. [DOI] [PubMed] [Google Scholar]
- 119.Hisatomi T., Sakamoto T., Murata T., Yamanaka I., Oshima Y., Hata Y., Ishibashi T., Inomata H., Susin S.A., Kroemer G. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am. J. Pathol. 2001;158:1271–1278. doi: 10.1016/S0002-9440(10)64078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tong L., Perez-Polo R. Brain-derived neurotrophic factor (BDNF) protects cultured rat cerebellar granule neurons against glucose deprivation-induced apoptosis. J. Neural Transm. 1998;105:905–914. doi: 10.1007/s007020050101. [DOI] [PubMed] [Google Scholar]
- 121.Spina M.B., Squinto S.P., Miller J., Lindsay R.M., Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: Involvement of the glutathione system. J. Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 122.Mattson M.P., Lovell M.A., Furukawa K., Markesbery W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 123.Ikeda O., Murakami M., Ino H., Yamazaki M., Koda M., Nakayama C., Moriya H. Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J. Neuropathol. Exp. Neurol. 2002;61:142–153. doi: 10.1093/jnen/61.2.142. [DOI] [PubMed] [Google Scholar]
- 124.Wu C.L., Chen C.H., Hwang C.S., Chen S.D., Hwang W.C., Yang D.I. Roles of p62 in BDNF-dependent autophagy suppression and neuroprotection against mitochondrial dysfunction in rat cortical neurons. J. Neurochem. 2017;140:845–861. doi: 10.1111/jnc.13937. [DOI] [PubMed] [Google Scholar]
- 125.Chen S.D., Wu C.L., Hwang W.C., Yang D.I. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int. J. Mol. Sci. 2017;18:545. doi: 10.3390/ijms18030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu B., Nagappan G., Guan X., Nathan P.J., Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 127.Subramanian J., Tremblay M.È. Synaptic Loss and Neurodegeneration. Front. Cell. Neurosci. 2021;15:681029. doi: 10.3389/fncel.2021.681029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bolton M.M., Pittman A.J., Lo D.C. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J. Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jin X., Hu H., Mathers P.H., Agmon A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J. Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lazo O.M., Gonzalez A., Ascano M., Kuruvilla R., Couve A., Bronfman F.C. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J. Neurosci. 2013;33:6112–6122. doi: 10.1523/JNEUROSCI.4630-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bamji S.X., Rico B., Kimes N., Reichardt L.F. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–β-catenin interactions. J. Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Horch H.W. Local effects of BDNF on dendritic growth. Rev. Neurosci. 2004;15:117–130. doi: 10.1515/REVNEURO.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- 133.Horvath P.M., Chanaday N.L., Alten B., Kavalali E.T., Monteggia L.M. A subthreshold synaptic mechanism regulating BDNF expression and resting synaptic strength. Cell Rep. 2021;36:109467. doi: 10.1016/j.celrep.2021.109467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dean C., Liu H., Mark Dunning F., Chang P.Y., Jackson M.B., Chapman E.R. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat. Neurosci. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hongpaisan J., Sun M.K., Alkon D.L. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Pins B., Cifuentes-Díaz C., Farah A.T., López-Molina L., Montalban E., Sancho-Balsells A., López A., Ginés S., Delgado-García J.M., Alberch J., et al. Conditional BDNF delivery from astrocytes rescues memory deficits, spine density, and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J. Neurosci. 2019;39:2441–2458. doi: 10.1523/JNEUROSCI.2121-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Z., Liu X., Schroeder J.P., Chan C.B., Song M., Yu S.P., Weinshenker D., Ye K. 7, 8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Estes M.L., McAllister A.K. Alterations in immune cells and mediators in the brain: It’s not always neuroinflammation! Brain Pathol. 2014;24:623–630. doi: 10.1111/bpa.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hong H., Kim B.S., Im H.I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016;20:S2–S7. doi: 10.5213/inj.1632604.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guzman-Martinez L., Maccioni R.B., Andrade V., Navarrete L.P., Pastor M.G., Ramos-Escobar N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019;10:1008. doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gilhus N.E., Deuschl G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019;15:429–430. doi: 10.1038/s41582-019-0227-8. [DOI] [PubMed] [Google Scholar]
- 142.Zhang X., Zeng L., Yu T., Xu Y., Pu S., Du D., Jiang W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell. Physiol. Biochem. 2014;34:715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- 143.Ding H., Chen J., Su M., Lin Z., Zhan H., Yang F., Li W., Xie J., Huang Y., Liu X., et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 2020;17:19. doi: 10.1186/s12974-020-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Onodera J., Nagata H., Nakashima A., Ikegaya Y., Koyama R. Neuronal brain-derived neurotrophic factor manipulates microglial dynamics. Glia. 2021;69:890–904. doi: 10.1002/glia.23934. [DOI] [PubMed] [Google Scholar]
- 145.Lima Giacobbo B., Doorduin J., Klein H.C., Dierckx R.A., Bromberg E., de Vries E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han R., Liu Z., Sun N., Liu S., Li L., Shen Y., Xiu J., Xu Q. BDNF alleviates neuroinflammation in the hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-κB pathway. Aging Dis. 2019;10:611. doi: 10.14336/AD.2018.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rom S., Zuluaga-Ramirez V., Gajghate S., Seliga A., Winfield M., Heldt N.A., Kolpakov M.A., Bashkirova Y.V., Sabri A.K., Persidsky Y. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019;56:1883–1896. doi: 10.1007/s12035-018-1195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim O.Y., Song J. The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol. Res. 2020;160:105083. doi: 10.1016/j.phrs.2020.105083. [DOI] [PubMed] [Google Scholar]
- 149.Wu S.Y., Pan B.S., Tsai S.F., Chiang Y.T., Huang B.M., Mo F.E., Kuo Y.M. BDNF reverses aging-related microglial activation. J. Neuroinflamm. 2020;17:210. doi: 10.1186/s12974-020-01887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bovolenta R., Zucchini S., Paradiso B., Rodi D., Merigo F., Mora G.N., Osculati F., Berto E., Marconi P., Marzola A., et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J. Neuroinflamm. 2010;7:81. doi: 10.1186/1742-2094-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsu C.C., Kuo T.W., Liu W.P., Chang C.P., Lin H.J. Calycosin preserves BDNF/TrkB signaling and reduces post-stroke neurological injury after cerebral ischemia by reducing accumulation of hypertrophic and TNF-α-containing microglia in rats. J. Neuroimmune Pharmacol. 2020;15:326–339. doi: 10.1007/s11481-019-09903-9. [DOI] [PubMed] [Google Scholar]
- 152.Li W., Ali T., Zheng C., He K., Liu Z., Shah F.A., Li N., Yu Z.J., Li S. Anti-depressive-like behaviors of APN KO mice involve Trkb/BDNF signaling related neuroinflammatory changes. Mol. Psychiatry. 2022;27:1047–1058. doi: 10.1038/s41380-021-01327-3. [DOI] [PubMed] [Google Scholar]
- 153.Makar T.K., Nimmagadda V.K., Singh I.S., Lam K., Mubariz F., Judge S.I., Trisler D., Bever C.T., Jr. TrkB agonist, 7,8-dihydroxyflavone, reduces the clinical and pathological severity of a murine model of multiple sclerosis. J. Neuroimmunol. 2016;292:9–20. doi: 10.1016/j.jneuroim.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Yin R., Zhao S., Qiu C. Brain-derived neurotrophic factor fused with a collagen-binding domain inhibits neuroinflammation and promotes neurological recovery of traumatic brain injury mice via TrkB signalling. J. Pharm. Pharmacol. 2020;72:539–550. doi: 10.1111/jphp.13233. [DOI] [PubMed] [Google Scholar]
- 155.Lyman M., Lloyd D.G., Ji X., Vizcaychipi M.P., Ma D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 156.Citri A., Malenka R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 157.Magee J.C., Grienberger C. Synaptic plasticity forms and functions. Ann. Rev. Neurosci. 2020;43:95–117. doi: 10.1146/annurev-neuro-090919-022842. [DOI] [PubMed] [Google Scholar]
- 158.Bazzari A.H., Parri H.R. Neuromodulators and long-term synaptic plasticity in learning and memory: A steered-glutamatergic perspective. Brain Sci. 2019;9:300. doi: 10.3390/brainsci9110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakata H., Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 160.Gangarossa G., Perez S., Dembitskaya Y., Prokin I., Berry H., Venance L. BDNF controls bidirectional endocannabinoid plasticity at corticostriatal synapses. Cereb. Cortex. 2020;30:197–214. doi: 10.1093/cercor/bhz081. [DOI] [PubMed] [Google Scholar]
- 161.Gottschalk W., Pozzo-Miller L.D., Figurov A., Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kramár E.A., Lin B., Lin C.Y., Arai A.C., Gall C.M., Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J. Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu H., Cheng P.L., Lim B.K., Khoshnevisrad N., Poo M.M. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fujisawa S., Yamada M.K., Nishiyama N., Matsuki N., Ikegaya Y. BDNF boosts spike fidelity in chaotic neural oscillations. Biophys. J. 2004;86:1820–1828. doi: 10.1016/S0006-3495(04)74249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Niculescu D., Michaelsen-Preusse K., Güner Ü., van Dorland R., Wierenga C.J., Lohmann C. A BDNF-mediated push-pull plasticity mechanism for synaptic clustering. Cell Rep. 2018;24:2063–2074. doi: 10.1016/j.celrep.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 166.Nikoletopoulou V., Sidiropoulou K., Kallergi E., Dalezios Y., Tavernarakis N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 2017;26:230–242. doi: 10.1016/j.cmet.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 167.Bramham C.R., Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 168.Mei F., Nagappan G., Ke Y., Sacktor T.C., Lu B. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMζ. PLoS ONE. 2011;6:e21568. doi: 10.1371/journal.pone.0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Panja D., Kenney J.W., D’Andrea L., Zalfa F., Vedeler A., Wibrand K., Fukunaga R., Bagni C., Proud C.G., Bramham C.R. Two-stage translational control of dentate gyrus LTP consolidation is mediated by sustained BDNF-TrkB signaling to MNK. Cell Rep. 2014;9:1430–1445. doi: 10.1016/j.celrep.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 170.Dincheva I., Glatt C.E., Lee F.S. Impact of the BDNF Val66Met polymorphism on cognition: Implications for behavioral genetics. Neuroscientist. 2012;18:439–451. doi: 10.1177/1073858411431646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ninan I., Bath K.G., Dagar K., Perez-Castro R., Plummer M.R., Lee F.S., Chao M.V. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pattwell S.S., Bath K.G., Perez-Castro R., Lee F.S., Chao M.V., Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J. Neurosci. 2012;32:2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Galvin C., Lee F.S., Ninan I. Alteration of the centromedial amygdala glutamatergic synapses by the BDNF Val66Met polymorphism. Neuropsychopharmacology. 2015;40:2269–2277. doi: 10.1038/npp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jing D., Lee F.S., Ninan I. The BDNF Val66Met polymorphism enhances glutamatergic transmission but diminishes activity-dependent synaptic plasticity in the dorsolateral striatum. Neuropharmacology. 2017;112:84–93. doi: 10.1016/j.neuropharm.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu B.D., Nagappan G., Lu Y.B. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 176.Criscuolo C., Fabiani C., Bonadonna C., Origlia N., Domenici L. BDNF prevents amyloid-dependent impairment of LTP in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol. Aging. 2015;36:1303–1309. doi: 10.1016/j.neurobiolaging.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 177.Simmons D.A., Rex C.S., Palmer L., Pandyarajan V., Fedulov V., Gall C.M., Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc. Natl. Acad. Sci. USA. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lauterborn J.C., Rex C.S., Kramár E., Chen L.Y., Pandyarajan V., Lynch G., Gall C.M. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J. Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie H., Leung K.L., Chen L., Chan Y.S., Ng P.C., Fok T.F., Wing Y.K., Ke Y., Li A.M., Yung W.H. Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol. Dis. 2010;40:155–162. doi: 10.1016/j.nbd.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 180.Yang Y.J., Li Y.K., Wang W., Wan J.G., Yu B., Wang M.Z., Hu B. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol. Biochem. Behav. 2014;122:30–36. doi: 10.1016/j.pbb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 181.Li M., Dai F.R., Du X.P., Yang Q.D., Zhang X., Chen Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav. Brain Res. 2012;231:146–153. doi: 10.1016/j.bbr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 182.Dhuriya Y.K., Sharma D. Neuronal plasticity: Neuronal organization is associated with neurological disorders. J. Mol. Neurosci. 2020;70:1684–1701. doi: 10.1007/s12031-020-01555-2. [DOI] [PubMed] [Google Scholar]
- 183.Pittenger C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin. Neurosci. 2013;15:455–463. doi: 10.31887/DCNS.2013.15.4/cpittenger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vose L.R., Stanton P.K. Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 2017;15:71–86. doi: 10.2174/1570159X14666160202121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou L.J., Zhong Y., Ren W.J., Li Y.Y., Zhang T., Liu X.G. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp. Neurol. 2008;212:507–514. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 186.Groth R., Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/S0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 187.Bazzari A.H., Bazzari F.H. Advances in targeting central sensitization and brain plasticity in chronic pain. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022;58:38. doi: 10.1186/s41983-022-00472-y. [DOI] [Google Scholar]
- 188.Wang H., Wei Y., Pu Y., Jiang D., Jiang X., Zhang Y., Tao J. Brain-derived neurotrophic factor stimulation of T-type Ca2+ channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci. Signal. 2019;12:eaaw2300. doi: 10.1126/scisignal.aaw2300. [DOI] [PubMed] [Google Scholar]
- 189.Luo D., Luo L., Lin R., Lin L., Lin Q. Brain-derived neurotrophic factor and Glial cell line-derived neurotrophic factor expressions in the trigeminal root entry zone and trigeminal ganglion neurons of a trigeminal neuralgia rat model. Anat. Rec. 2020;303:3014–3023. doi: 10.1002/ar.24364. [DOI] [PubMed] [Google Scholar]
- 190.Takeda M., Takahashi M., Kitagawa J., Kanazawa T., Nasu M., Matsumoto S. Brain-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masseter muscle inflammation. Mol. Pain. 2013;9:49. doi: 10.1186/1744-8069-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bazzari F.H., Bazzari A.H. Orofacial neuropathic pain: A pharmacological approach. S. Afr. Pharm. J. 2019;86:23–28. [Google Scholar]
- 192.Siuciak J.A., Boylan C., Fritsche M., Altar C.A., Lindsay R.M. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 193.Altar C.A., Boylan C.B., Fritsche M., Jackson C., Hyman C., Lindsay R.M. The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp. Neurol. 1994;130:31–40. doi: 10.1006/exnr.1994.1182. [DOI] [PubMed] [Google Scholar]
- 194.Goggi J., Pullar I.A., Carney S.L., Bradford H.F. Signalling pathways involved in the short-term potentiation of dopamine release by BDNF. Brain Res. 2003;968:156–161. doi: 10.1016/S0006-8993(03)02234-0. [DOI] [PubMed] [Google Scholar]
- 195.Paredes D., Granholm A.C., Bickford P.C. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain. Res. 2007;1141:56–64. doi: 10.1016/j.brainres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guillin O., Griffon N., Bezard E., Leriche L., Diaz J., Gross C., Sokoloff P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: Therapeutic implications in Parkinson’s disease. Eur. J. Pharmacol. 2003;480:89–95. doi: 10.1016/j.ejphar.2003.08.096. [DOI] [PubMed] [Google Scholar]
- 197.Razgado-Hernandez L.F., Espadas-Alvarez A.J., Reyna-Velazquez P., Sierra-Sanchez A., Anaya-Martinez V., Jimenez-Estrada I., Bannon M.J., Martinez-Fong D., Aceves-Ruiz J. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS ONE. 2015;10:e0117391. doi: 10.1371/journal.pone.0117391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Palasz E., Wysocka A., Gasiorowska A., Chalimoniuk M., Niewiadomski W., Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020;21:1170. doi: 10.3390/ijms21031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Benmansour S., Deltheil T., Piotrowski J., Nicolas L., Reperant C., Gardier A.M., Frazer A., David D.J. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur. J. Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 200.Deltheil T., Guiard B.P., Guilloux J.P., Nicolas L., Deloménie C., Repérant C., Le Maitre E., Leroux-Nicollet I., Benmansour S., Coudoré F., et al. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: Studies in adult mice hippocampus. Pharmacol. Biochem. Behav. 2008;90:174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 201.Celada P., Siuciak J.A., Tran T.M., Altar C.A., Tepper J.M. Local infusion of brain-derived neurotrophic factor modifies the firing pattern of dorsal raphe serotonergic neurons. Brain Res. 1996;712:293–298. doi: 10.1016/0006-8993(95)01469-1. [DOI] [PubMed] [Google Scholar]
- 202.Castrén E., Monteggia L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry. 2021;90:128–136. doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 203.Nestor P.G., O’Donovan K., Lapp H.E., Hasler V.C., Boodai S.B., Hunter R. Risk and protective effects of serotonin and BDNF genes on stress-related adult psychiatric symptoms. Neurobiol. Stress. 2019;11:100186. doi: 10.1016/j.ynstr.2019.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Witte A.V., Kürten J., Jansen S., Schirmacher A., Brand E., Sommer J., Flöel A. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J. Neurosci. 2012;32:4553–4561. doi: 10.1523/JNEUROSCI.6010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Han D.H., Park D.B., Choi T.Y., Joo S.Y., Lee M.K., Park B.R., Nishimura R., Chu C.C., Renshaw P.F. Effects of brain-derived neurotrophic factor–catecholamine-O-methyltransferase gene interaction on schizophrenic symptoms. Neuroreport. 2008;19:1155–1158. doi: 10.1097/WNR.0b013e32830867ad. [DOI] [PubMed] [Google Scholar]
- 206.Sakata K., Duke S.M. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260:265–275. doi: 10.1016/j.neuroscience.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 207.Guillin O., Diaz J., Carroll P., Griffon N., Schwartz J.C., Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- 208.Vogel M., Pfeifer S., Schaub R.T., Grabe H.J., Barnow S., Freyberger H.J., Cascorbi I. Decreased levels of dopamine D3 receptor mRNA in schizophrenic and bipolar patients. Neuropsychobiology. 2004;50:305–310. doi: 10.1159/000080958. [DOI] [PubMed] [Google Scholar]
- 209.Glenthoj B.Y., Mackeprang T., Svarer C., Rasmussen H., Pinborg L.H., Friberg L., Baaré W., Hemmingsen R., Videbaek C. Frontal dopamine D2/3 receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol. Psychiatry. 2006;60:621–629. doi: 10.1016/j.biopsych.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 210.Bosse K.E., Maina F.K., Birbeck J.A., France M.M., Roberts J.J., Colombo M.L., Mathews T.A. Aberrant striatal dopamine transmitter dynamics in brain-derived neurotrophic factor-deficient mice. J. Neurochem. 2012;120:385–395. doi: 10.1111/j.1471-4159.2011.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Di Carlo P., Punzi G., Ursini G. BDNF and schizophrenia. Psychiatr. Genet. 2019;29:200–210. doi: 10.1097/YPG.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guillin O., Demily C., Thibaut F. Brain-derived neurotrophic factor in schizophrenia and its relation with dopamine. Int. Rev. Neurobiol. 2007;78:377–395. doi: 10.1016/S0074-7742(06)78012-6. [DOI] [PubMed] [Google Scholar]
- 213.Homberg J.R., Molteni R., Calabrese F., Riva M.A. The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014;43:35–47. doi: 10.1016/j.neubiorev.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 214.Martinowich K., Lu B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 215.Maurel O.M., Torrisi S.A., Barbagallo C., Purrello M., Salomone S., Drago F., Ragusa M., Leggio G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int. J. Mol. Sci. 2021;22:5157. doi: 10.3390/ijms22105157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Torrisi S.A., Lavanco G., Maurel O.M., Gulisano W., Laudani S., Geraci F., Grasso M., Barbagallo C., Caraci F., Bucolo C., et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress. 2021;14:100286. doi: 10.1016/j.ynstr.2020.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Notaras M., van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry. 2020;25:2251–2274. doi: 10.1038/s41380-019-0639-2. [DOI] [PubMed] [Google Scholar]
- 218.Rosenhauer A.M., Beach L.Q., Jeffress E.C., Thompson B.M., McCann K.E., Partrick K.A., Diaz B., Norvelle A., Choi D.C., Huhman K.L. Brain-derived neurotrophic factor signaling mitigates the impact of acute social stress. Neuropharmacology. 2019;148:40–49. doi: 10.1016/j.neuropharm.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang J., Gao F., Cui S., Yang S., Gao F., Wang X., Zhu G. Utility of 7, 8-dihydroxyflavone in preventing astrocytic and synaptic deficits in the hippocampus elicited by PTSD. Pharmacol. Res. 2022;176:106079. doi: 10.1016/j.phrs.2022.106079. [DOI] [PubMed] [Google Scholar]
- 220.Bazzari F.H., Bazzari A.H. Drug-Induced Delirium: A Mini Review. BMH. Med. J. 2018;5:51–56. [Google Scholar]
- 221.Soysal P., Isik A.T. Pathogenesis of Delirium. In: Isik A., Grossberg G., editors. Delirium in Elderly Patients. Springer; Cham, Switzerland: 2018. [Google Scholar]
- 222.Williams J., Finn K., Melvin V., Meagher D., McCarthy G., Adamis D. The association of serum levels of brain-derived neurotrophic factor with the occurrence of and recovery from delirium in older medical inpatients. BioMed Res. Int. 2017;2017:5271395. doi: 10.1155/2017/5271395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wyrobek J., LaFlam A., Max L., Tian J., Neufeld K.J., Kebaish K.M., Walston J.D., Hogue C.W., Riley L.H., Everett A.D., et al. Association of intraoperative changes in brain-derived neurotrophic factor and postoperative delirium in older adults. Br. J. Anaesth. 2017;119:324–332. doi: 10.1093/bja/aex103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cursano S., Battaglia C.R., Urrutia-Ruiz C., Grabrucker S., Schön M., Bockmann J., Braumüller S., Radermacher P., Roselli F., Huber-Lang M., et al. A CRHR1 antagonist prevents synaptic loss and memory deficits in a trauma-induced delirium-like syndrome. Mol. Psychiatry. 2021;26:3778–3794. doi: 10.1038/s41380-020-0659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 226.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Welcome M.O., Mastorakis N.E. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020;157:104769. doi: 10.1016/j.phrs.2020.104769. [DOI] [PubMed] [Google Scholar]
- 229.Fiorentino M., Sapone A., Senger S., Camhi S.S., Kadzielski S.M., Buie T.M., Kelly D.L., Cascella N., Fasano A. Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism. 2016;7:49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Van Vliet E.A., Aronica E., Gorter J.A. Blood–brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015;38:26–34. doi: 10.1016/j.semcdb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 231.Najjar S., Pahlajani S., De Sanctis V., Stern J.N., Najjar A., Chong D. Neurovascular unit dysfunction and blood–brain barrier hyperpermeability contribute to schizophrenia neurobiology: A theoretical integration of clinical and experimental evidence. Front. Psychiatry. 2017;8:83. doi: 10.3389/fpsyt.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Qin L., Kim E., Ratan R., Lee F.S., Cho S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J. Neurosci. 2011;31:775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jin H., Zhu Y., Li Y., Ding X., Ma W., Han X., Wang B. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis. 2019;24:511–528. doi: 10.1007/s10495-019-01535-x. [DOI] [PubMed] [Google Scholar]
- 234.Ying X., Xie Q., Yu X., Li S., Wu Q., Chen X., Yue J., Zhou K., Tu W., Jiang S. Water treadmill training protects the integrity of the blood-spinal cord barrier following SCI via the BDNF/TrkB-CREB signalling pathway. Neurochem. Int. 2021;143:104945. doi: 10.1016/j.neuint.2020.104945. [DOI] [PubMed] [Google Scholar]
- 235.Li C., Wang X., Yan J., Cheng F., Ma X., Chen C., Wang W., Wang Q. Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway. Oxid. Med. Cell. Longev. 2020;2020:1201624. doi: 10.1155/2020/1201624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Grade S., Weng Y.C., Snapyan M., Kriz J., Malva J.O., Saghatelyan A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS ONE. 2013;8:e55039. doi: 10.1371/journal.pone.0055039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Becerra-Calixto A., Posada-Duque R., Cardona-Gómez G.P. Recovery of neurovascular unit integrity by CDK5-KD astrocyte transplantation in a global cerebral ischemia model. Mol. Neurobiol. 2018;55:8563–8585. doi: 10.1007/s12035-018-0992-1. [DOI] [PubMed] [Google Scholar]
- 238.Kim H., Li Q., Hempstead B.L., Madri J.A. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J. Biol. Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 239.Bazzari F.H., Abdallah D.M., El-Abhar H.S. Pharmacological interventions to attenuate Alzheimer’s disease progression: The story so far. Curr. Alzheimer Res. 2019;16:261–277. doi: 10.2174/1567205016666190301111120. [DOI] [PubMed] [Google Scholar]
- 240.Bazzari A.H., Bazzari F.H. Medicinal plants for Alzheimer’s disease: An updated review. J. Med. Plant. Stud. 2018;6:81–85. [Google Scholar]
- 241.Marvanová M., Lakso M., Pirhonen J., Nawa H., Wong G., Castrén E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell. Neurosci. 2001;18:247–258. doi: 10.1006/mcne.2001.1027. [DOI] [PubMed] [Google Scholar]
- 242.López-Valdés H.E., Clarkson A.N., Ao Y., Charles A.C., Carmichael S.T., Sofroniew M.V., Brennan K.C. Memantine enhances recovery from stroke. Stroke. 2014;45:2093–2100. doi: 10.1161/STROKEAHA.113.004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhu G., Li J., He L., Wang X., Hong X. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br. J. Pharmacol. 2015;172:2354–2368. doi: 10.1111/bph.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mishra S.K., Hidau M.K., Rai S. Memantine treatment exerts an antidepressant-like effect by preventing hippocampal mitochondrial dysfunction and memory impairment via upregulation of CREB/BDNF signaling in the rat model of chronic unpredictable stress-induced depression. Neurochem. Int. 2021;142:104932. doi: 10.1016/j.neuint.2020.104932. [DOI] [PubMed] [Google Scholar]
- 245.Mohseni I., Peeri M., Azarbayjani M.A. Improvement of Learning and Memory Deficits with Aerobic Training and Donepezil Co-therapy in Amyloid-β beta Injected Male Rats Through the CREB and BDNF Signaling Pathway. Pharm. Biomed. Res. 2020;6:181–190. doi: 10.18502/pbr.v6i3.4644. [DOI] [Google Scholar]
- 246.Zheng H., Niu S., Zhao H., Li S., Jiao J. Donepezil improves the cognitive impairment in a tree shrew model of Alzheimer’s disease induced by amyloid-β1–40 via activating the BDNF/TrkB signal pathway. Metab. Brain Dis. 2018;33:1961–1974. doi: 10.1007/s11011-018-0303-6. [DOI] [PubMed] [Google Scholar]
- 247.Jian W.X., Zhang Z., Zhan J.H., Chu S.F., Peng Y., Zhao M., Wang Q., Chen N.H. Donepezil attenuates vascular dementia in rats through increasing BDNF induced by reducing HDAC6 nuclear translocation. Acta Pharmacol. Sin. 2020;41:588–598. doi: 10.1038/s41401-019-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muratori B.G., Zamberlam C.R., Mendes T.B., Nozima B.H., Cerutti J.M., Cerutti S.M. BDNF as a Putative Target for Standardized Extract of Ginkgo biloba-Induced Persistence of Object Recognition Memory. Molecules. 2021;26:3326. doi: 10.3390/molecules26113326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hamdan M., AS N., Rahayu M., Machfoed M.H. Influence of ginkgo biloba (Egb) extracts in apoptosis index and number of neurons at Rattus novergicus with lead (pb) exposure. Res. J. Pharm. Technol. 2019;12:5883–5887. doi: 10.5958/0974-360X.2019.01020.5. [DOI] [Google Scholar]
- 250.Ben-Azu B., Adebayo O.G., Wopara I., Aduema W., Onyeleonu I., Umoren E.B., Kolawole T.A., Ebo O.T., Akpotu A.E., Ajibo D.N., et al. Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: Reversal effects of Gingko biloba supplement. J. Trace Elem. Med. Biol. 2022;71:126919. doi: 10.1016/j.jtemb.2021.126919. [DOI] [PubMed] [Google Scholar]
- 251.Zhang Z., Peng D., Zhu H., Wang X. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012;87:193–198. doi: 10.1016/j.brainresbull.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 252.Sangiovanni E., Brivio P., Dell’Agli M., Calabrese F. Botanicals as modulators of neuroplasticity: Focus on BDNF. Neural Plast. 2017;2017:5965371. doi: 10.1155/2017/5965371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Björkholm C., Monteggia L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bai O., Chlan-Fourney J., Bowen R., Keegan D., Li X.M. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J. Neurosci. Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 255.Adachi N., Yoshimura A., Chiba S., Ogawa S., Kunugi H. Rotigotine, a dopamine receptor agonist, increased BDNF protein levels in the rat cortex and hippocampus. Neurosci. Lett. 2018;62:44–50. doi: 10.1016/j.neulet.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 256.Yang L., Xu J.M., Jiang X., Ruan W., Cui Y., He L. Effect of dexmedetomidine on plasma brain-derived neurotrophic factor: A double-blind, randomized and placebo-controlled study. Upsala J. Med. Sci. 2013;118:235–239. doi: 10.3109/03009734.2013.808295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Li Z.C., Jia Y.P., Wang Y., Qi J.L., Han X.P. Effects of dexmedetomidine post-treatment on BDNF and VEGF expression following cerebral ischemia/reperfusion injury in rats. Mol. Med. Rep. 2018;17:6033–6037. doi: 10.3892/mmr.2018.8597. [DOI] [PubMed] [Google Scholar]
- 258.Sheng S., Huang J., Ren Y., Zhi F., Tian X., Wen G., Ding G., Xia T.C., Hua F., Xia Y. Neuroprotection against hypoxic/ischemic injury: δ-opioid receptors and BDNF-TrkB pathway. Cell. Physiol. Biochem. 2018;47:302–315. doi: 10.1159/000489808. [DOI] [PubMed] [Google Scholar]
- 259.Bazzari A.H., Bazzari F.H. Promising Therapeutic Agents: Delta Opioid Receptor Agonists. Int. Ann. Med. 2017;1:1–5. doi: 10.24087/IAM.2017.1.8.252. [DOI] [Google Scholar]
- 260.Fukui H., Wong H.T., Beyer L.A., Case B.G., Swiderski D.L., Di Polo A., Ryan A.F., Raphael Y. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci. Rep. 2012;2:838. doi: 10.1038/srep00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dąbkowska M., Łuczkowska K., Rogińska D., Sobuś A., Wasilewska M., Ulańczyk Z., Machaliński B. Novel design of (PEG-ylated) PAMAM-based nanoparticles for sustained delivery of BDNF to neurotoxin-injured differentiated neuroblastoma cells. J. Nanobiotechnol. 2020;18:120. doi: 10.1186/s12951-020-00673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jiang Y., Fay J.M., Poon C.D., Vinod N., Zhao Y., Bullock K., Qin S., Manickam D.S., Yi X., Banks W.A., et al. Nanoformulation of Brain-Derived Neurotrophic Factor with Target Receptor-Triggered-Release in the Central Nervous System. Adv. Funct. Mater. 2018;28:1703982. doi: 10.1002/adfm.201703982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang S., Yao H., Xu Y., Hao R., Zhang W., Liu H., Huang Y., Guo W., Lu B. Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics. 2020;10:6854–6874. doi: 10.7150/thno.44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bawari S., Tewari D., Argüelles S., Sah A.N., Nabavi S.F., Xu S., Vacca R.A., Nabavi S.M., Shirooie S. Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 2019;148:104458. doi: 10.1016/j.phrs.2019.104458. [DOI] [PubMed] [Google Scholar]
- 265.Gudasheva T.A., Povarnina P., Tarasiuk A.V., Seredenin S.B. The low molecular weight brain-derived neurotrophic factor mimetics with antidepressant-like activity. Curr. Pharm. Des. 2019;25:729–737. doi: 10.2174/1381612825666190329122852. [DOI] [PubMed] [Google Scholar]
- 266.Miranda-Lourenço C., Ribeiro-Rodrigues L., Fonseca-Gomes J., Tanqueiro S.R., Belo R.F., Ferreira C.B., Rei N., Ferreira-Manso M., de Almeida-Borlido C., Costa-Coelho T., et al. Challenges of BDNF-based therapies: From common to rare diseases. Pharmacol. Res. 2020;162:105281. doi: 10.1016/j.phrs.2020.105281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.