Abstract
Hedychium coccineum Buch. Ham. ex Sm. is a perennial rhizomatous herb belonging to the family Zingiberaceae. The aim of the present study was to compare the chemical composition and biological activities of H. coccineum rhizome essential oil (HCCRO) and H. coccineum aerial part essential oil (HCCAO). The plant material was subjected to hydro-distillation using Clevenger’s apparatus in order to obtain volatile oil and analyzed for its chemical constituents using GC-MS. The comparative study of the rhizome and aerial part essential oils of H. coccineum displayed that (E)-nerolidol (15.9%), bornyl acetate (13.95%), davanone B (10.9%), spathulenol (8.9%), and 1, 8-cineol (8.5%) contributed majorly to the HCCRO, while 7-hydroxyfarnesen (15.5%), α-farnesene (11.1%), α-pinene (10.9%), spathulenol (7.7%), and β-pinene (6.8%) were present as major constituents in the HCCAO. Both the essential oils were studied for their biological activities, such as nematicidal, insecticidal, herbicidal, antifungal, and antibacterial activities. The essential oils exhibited significant nematicidal activity against Meloidogyne incognita, insecticidal activity against Spodoptera litura, and moderate herbicidal activity against R. raphanistrum sub sp. sativus, and good antifungal activity against Fusarium oxysporum and Curvularialunata. Essential oils were also tested for antibacterial activity against Staphylococcus aureus and Salmonella enterica serotype Typhi. Both oils showed good to moderate activity against the tested pathogens. The significant nematicidal, insecticidal, herbicidal, antifungal, and antibacterial activities of both the essential oils might be helpful for the development of environmentally friendly pesticides that could be an alternative to synthetic pesticides in the future.
Keywords: natural products, bioactive compounds, (E)-nerolidol, 7-hydroxyfarnesen, biological activities
1. Introduction
Zingiberaceae is an important plant family which has 52 genera and 1500 species, including Hedychium coccineum Buch.-Ham. ex Sm. Plants in the Zingiberaceae family are now being investigated extensively for their phytochemistry and pharmacological properties [1]. The genus Hedychium grows as an herb of perennial tuberous rootstocks, with aromatic flowers widely distributed in tropical and subtropical countries [2]. The species Hedychium coccineum Buch.-Ham. ex Sm. is a tall, herbaceous, and perennial herb commonly known as the scarlet ginger lily, scarlet ginger lily, orange ginger lily, and orange bottlebrush ginger, growing at the edge of forests and in mountain grasslands [3]. H. coccineum is intrinsic to the Himalayas of India and Nepal, China, Bangladesh, Myanmar, and Thailand [4]. The rhizome of the H. coccineum is used in the treatment of fever, headache, and body pain, and its flowers’ pulp is used on swollen body parts [5,6]. Indian tribal people believe that wearing the flower behind the ear could be effective against the evil eye and disease [7]. H. coccineum consists of a variety of active compounds and serves as a basis in science for the use of herbs as flavor and fragrance agents, food preservatives, botanical pesticides, nutraceuticals, and pharmaceuticals [8,9,10]. Phytochemical characterization of H. coccineum has been demonstrated to have an important role in recognizing its active principles, and has revealed that it is an important medicinal herb [11,12]. This herb is a source of valuable strong herbal medication and cures for various disorders due to the presence of various bioactive components [13]. H. coccineum rhizome essential oil composition and its biological activities seems to support further studies to describe it as a biopesticides or to develop new chemical compounds and the discovery of new pesticides. Thus, the present research aims to compare the chemical composition and biological activities of essential oils extracted from the rhizome and aerial parts of H. coccineum. This is the first report on chemical analysis and various biological activities of the H. coccineum essential oils from the Indian Himalayan region.
2. Results
2.1. Chemical Compositions of Essential Oils
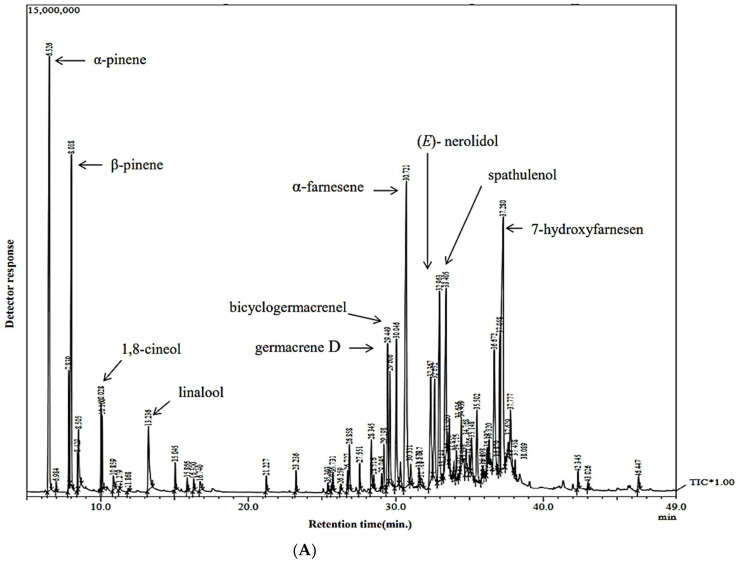
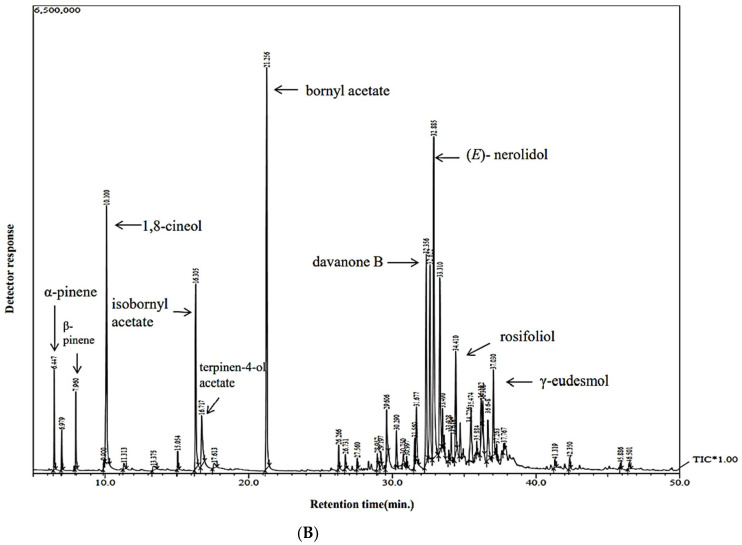
The phytoconstituents present in the HCCAO and HCCRO essential oils were identified, and are presented in Table 1 according to their order of elution on the DB-5 column in GC-MS. This is the first report to present a chemical analysis of the essential oil of Hedychium coccineum from the Indian Himalayan region. Fifty and thirty-two components were identified in HCCAO and HCCRO, contributing to 96.2% and 98.2% of the total volatile oils, respectively. 7-Hydroxyfarnesen (15.5%) and (E)-nerolidol (15.9%) were found to be a major constituent of HCCAO and HCCRO, respectively. Compounds that constituted more than 3.00% were considered main components, whereas compounds that constituted less than 3.00% were considered minor constituents in the essential oils under investigation. The ion-chromatogram of HCCAO and HCCRO essential oil is shown in Figure 1.
Table 1.
Comparative chemical composition of HCCAO and HCCRO with previous work.
| S.N. | Compound Identified | RIc | RIL | Composition % in Present Study (Kausani, Kumaun Region, Uttarakhand, India) |
% Composition of Rhizome Essential Oils in Reported Studies | ||
|---|---|---|---|---|---|---|---|
| HCCAO | HCCRO | Sakhanokho et al. [9] (Mississippi, U.S.) |
Gurib-Fakim et al. [14]. (Pamplemousses, Mauritius) |
||||
| 1. | Artemisia alcohol | 939 | 935 | 3.7 | - | - | - |
| 2. | α-pinene | 948 | 939 | 10.9 | 2.1 | 13.5 | 2.4 |
| 3. | Camphene | 953 | 954 | 0.1 | 0.1 | 2.3 | - |
| 4. | Sulcatone | 968 | 965 | 0.7 | - | - | - |
| 5. | Sabinene | 972 | 975 | 1.9 | - | - | - |
| 6. | β-pinene | 978 | 979 | 6.8 | 6.8 | 7.5 | 1.8 |
| 7. | Myrcene | 991 | 990 | 1.2 | - | - | - |
| 8. | p-cymene | 1024 | 1024 | - | 0.2 | 0.5 | - |
| 9. | Limonene | 1030 | 1029 | 1.5 | - | 1.1 | 0.2 |
| 10. | 1,8-cineol | 1032 | 1031 | 1.1 | 8.5 | 0.1 | - |
| 11. | β-ocimene | 1046 | 1050 | 0.4 | - | - | - |
| 12. | γ-terpinene | 1058 | 1059 | t | 0.3 | - | - |
| 13. | Cis-sabinene hydrate | 1069 | 1070 | t | - | - | - |
| 14. | Cis-linalool oxide (Furanoid) | 1082 | 1072 | - | - | 2.0 | - |
| 15. | Trans-linalool oxide (Furanoid) | 1088 | 1086 | - | - | 1.8 | - |
| 16. | Linalool | 1092 | 1096 | 1.9 | 0.4 | 26.7 | - |
| 17. | Thujol | 1098 | 1095 | 0.3 | - | - | - |
| 18. | Trans-pinocarveol | 1139 | 1139 | - | - | 1.5 | - |
| 19. | Trans- verbenol | 1143 | 1144 | - | - | 0.9 | - |
| 20. | Camphor | 1149 | 1146 | 0.5 | 0.6 | 0.2 | - |
| 21. | Isoborneol | 1165 | 1160 | 0.5 | - | - | - |
| 22. | Borneol | 1169 | 1169 | - | 6.1 | 1.0 | - |
| 23. | Terpinen-4-ol | 1177 | 1178 | 0.3 | 2.6 | 0.1 | - |
| 24. | α-terpineol | 1183 | 1188 | - | 0.4 | 0.6 | 0.6 |
| 25. | Naphthalene | 1189 | 1181 | t | - | - | - |
| 26. | Myrtenol | 1194 | 1195 | - | - | 1.2 | - |
| 27. | Trans-carveol | 1217 | 1216 | - | - | 0.3 | - |
| 28. | α-fenchyl acetate | 1223 | 1220 | - | - | - | 0.2 |
| 29. | Bornyl acetate | 1285 | 1285 | 0.3 | 13.9 | 8.4 | 0.8 |
| 30. | β-elemene | 1390 | 1390 | 0.3 | - | - | - |
| 31. | β-cubebene | 1392 | 1389 | t | - | - | - |
| 32. | β-bourbonene | 1393 | 1388 | 0.1 | - | - | - |
| 33. | α-cis-bergamotene | 1416 | 1412 | 0.8 | 0.6 | - | - |
| 34. | (E)-caryophyllene | 1424 | 1419 | 0.9 | 0.7 | 1.5 | - |
| 35. | γ-elemene | 1432 | 1436 | 0.8 | - | - | - |
| 36. | α-himachalene | 1449 | 1451 | - | 0.9 | - | - |
| 37. | α-humulene | 1461 | 1454 | 0.9 | - | - | - |
| 38. | 9-epi-(E)-caryophyllene | 1464 | 1466 | 0.2 | - | - | - |
| 39. | β-acoradiene | 1471 | 1470 | - | 0.3 | - | - |
| 40. | α-curcumene | 1479 | 1480 | 2.4 | 2.2 | 4.1 | - |
| 41. | α-neocallitropsene | 1480 | 1476 | 0.2 | - | - | - |
| 42. | Germacrene D | 1480 | 1481 | 3.0 | - | - | - |
| 43. | β-vetispirene | 1494 | 1493 | 0.4 | - | - | - |
| 44. | Bicyclogermacrene | 1497 | 1500 | 3.8 | - | - | - |
| 45. | α-farnesene | 1502 | 1505 | 11.1 | - | - | 1.9 |
| 46. | β-dihydroagarofuran | 1509 | 1503 | 1.0 | 1.1 | - | - |
| 47. | δ-cadinene | 1518 | 1523 | 0.5 | 0.2 | - | - |
| 48. | Kessane | 1533 | 1530 | 0.3 | 0.7 | - | - |
| 49. | Elemol | 1551 | 1549 | 0.5 | - | - | - |
| 50. | (E)-nerolidol | 1564 | 1563 | 5.3 | 15.9 | 4.6 | 44.4 |
| 51. | Davanone B | 1567 | 1566 | 5.8 | 10.9 | - | - |
| 52. | Spathulenol | 1576 | 1578 | 7.7 | 8.9 | 3.1 | 0.4 |
| 53. | 7-hydroxyfarnesen | 1579 | 1581 | 15.5 | - | - | - |
| 54. | Trans-sesquisabinene hydrate | 1580 | 1579 | - | - | - | 24.2 |
| 55. | γ-turmerol | 1581 | 1582 | - | - | 0.2 | - |
| 56. | Caryophyllene oxide | 1582 | 1583 | - | - | 1.5 | - |
| 57. | Globulol | 1589 | 1590 | - | 0.4 | 0.8 | - |
| 58. | Viridiflorol | 1592 | 1592 | - | 0.3 | 0.5 | - |
| 59. | Salvial-4(14)-en-1-one | 1596 | 1594 | t | - | - | - |
| 60. | Rosifoliol | 1598 | 1601 | 1.0 | - | - | - |
| 61. | Ledol | 1600 | 1602 | t | - | - | - |
| 62. | Guaiol | 1603 | 1600 | 0.5 | 0.9 | - | - |
| 63. | γ-eudesmol | 1632 | 1632 | - | 5.2 | - | - |
| 64. | T-muurolol | 1645 | 1646 | 0.1 | 0.9 | 0.1 | - |
| 65. | α-cadinol | 1648 | 1654 | - | 0.4 | 0.4 | - |
| 66. | Cadin-4-en-10-ol | 1649 | 1647 | 0.3 | - | - | - |
| 67. | Agarospirol | 1649 | 1648 | - | 0.4 | - | - |
| 68. | β-eudesmol | 1652 | 1650 | - | 3.4 | - | - |
| 69. | Bulnesol | 1673 | 1671 | - | 1.9 | - | 0.4 |
| 70. | α-bisabolol | 1686 | 1685 | - | - | - | 0.3 |
| 71. | α-(Z)-bergamotol | 1688 | 1690 | 0.2 | - | - | - |
| 72. | Iso-longifolol | 1725 | 1729 | t | - | - | - |
| 73. | (E)-isovalencenol | 1796 | 1793 | 0.5 | - | - | - |
| Class composition | % Composition | ||||||
| Monoterpene hydrocarbons | 22.8 | 9.5 | 25.2 | 4.4 | |||
| Oxygenated monoterpenes | 9.3 | 32.5 | 44.5 | 1.6 | |||
| Sesquiterpenes hydrocarbons | 25.0 | 4.9 | 5.6 | 1.9 | |||
| Oxygenated sesquiterpenes | 39.1 | 51.3 | 9.0 | 69.7 | |||
| Total (%) | 96.2 | 98.2 | 84.3 | 77.6 | |||
HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome essential oil; “-“—not detected t—trace < 0.1%. RIc—Calculated retention indices value; RIL—Literature retention indices value on a DB-5 MS column in reference Adams, [15].
Figure 1.
(A) Ion-chromatogram of HCCAO. (B) Ion-chromatogram of HCCRO. Relating to the chemical composition of essential oil fractions.
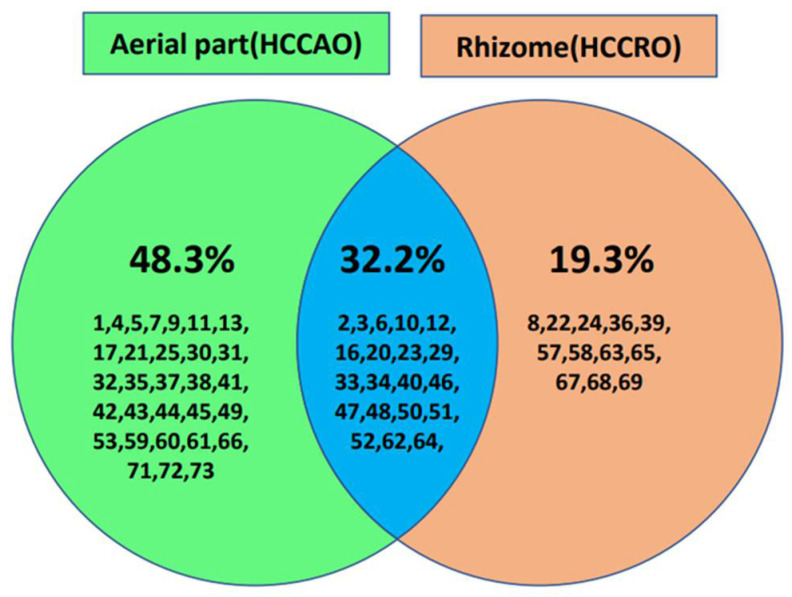
In this study, it was observed that HCCAO was dominated by oxygenated sesquiterpene (39.1%), followed by sesquiterpene hydrocarbon (25.0%), monoterpene hydrocarbon (22.8%), and oxygenated monoterpene (9.3%), while HCCRO was dominated by oxygenated sesquiterpene (51.3%), followed by oxygenated monoterpene (32.5%), monoterpene hydrocarbon (9.5%), and sesquiterpene hydrocarbon (4.9%). Moreover, a Venn diagram (Figure 2) was generated to compare the chemical composition of HCCAO and HCCRO. It is exciting to observe that the essential oil of HCCRO has a remarkably different chemical makeup than HCCAO. Chemical constituents such as α-pinene, camphene, β-pinene, γ-terpinene, 1,8-cineol, linalool, camphor, terpinen-4-ol, bornyl acetate, α-cis-bergamotene, (E)-caryophyllene, α-curcumene, δ-cadinene, β-dihydroagarofuran, kessane, (E)-nerolidol, davanone B, spathulenol, guaiol, and τ-muurolol were present in both samples (HCCRO and HCCAO). However, p-cymene, cis-linalool oxide, trans-linalool oxide, trans-pinocarveol, trans-verbenol, borneol, α-terpineol, α-himachalene, β-acoradiene, globulol, viridiflorol, γ-eudesmolagarospirol, β-eudesmol, α-cadinol, and bulnesol were found to be unique to HCCRO, whereas sabinene, myrcene, artemisia alcohol, sulcatone, cis-sabinene hydrate, thujol, isoborneol, β-bourbonene, β-cubebene, β-elemene, γ-elemene, α–humulene, 9-epi-(E)-caryophyllene, α-neocallitropsene, germacrene D, bicyclogermacrene, α-farnesene, naphthalene, β-vetispirene, elemol, 7-hydroxyfarnesen, salvial-4(14)-en-1-one, rosifoliol, ledol, cadin-4-en-10-ol, α-(Z)-bergamotol, iso-longifolol, (E)-isovalencenol, 2-pentanone, and dodeca-(2E,4E)-dienal were present only in HCCAO, in variable amounts. These variations in the chemical compositions of essential oils obtained from different plant parts were supposed because of the direct contact with sunlight with HCCAO (aerial part), nonavailability of sunlight to HCCRO (rhizome), and different type of physiology in both type of plant parts. In other works, authors have reported that the chemical composition of essential oils can be affected by the presence of light, time of year, rain, dry weather, and circadian rhythm, among other factors [16,17,18]. The GC-MS profile and the detailed comparative chemical composition of HCCAO and HCCRO are represented in Table 1.
Figure 2.
Venn diagram of essential oil composition of HCCAO and HCCRO (the chemical compositions were represented by different colors: HCCAO in green, HCCRO in orange, and common composition of both in blue).
Table 1 represents the detailed comparative chemical composition of HCCAO and HCCRO in previously reported work [9,14]. The essential oil of H. coccineum has previously been reported in Mississippi, (U.S.) [9] and in Pamplemousses, (Mauritius) [14]. Sakhanokho et al. [9] revealed the presence of total 38 components contributing to 84.3% in H. coccineum rhizome part essential oil. The major constituents, such as linalool (26.7%), α-pinene (13.5%), bornyl acetate (8.4%), β-pinene (7.5%), (E)-nerolidol (4.6%), and α-curcumene (4.1%)were identified in the H. coccineum essential oil, whereas Gurib-Fakim et al. [14] revealed the presence of total 12 components, contributing to 77.6% in H. coccineum rhizome part essential oil. (E)-nerolidol (44.4%), trans-sesquisabinene hydrate(24.2%), and α-pinene (2.4%) contributed majorly to the composition of the H. coccineum essential oil sample [9,14].
Comparison of the results of present and previous investigations of H. coccineum rhizome essential oil revealed differences in their oil composition. A total of 50 and 32 components were identified to contribute to the composition of essential oils in the present study for HCCAO (96.2%) and HCCRO (98.2%), respectively. Meanwhile, only 38 and 12 components were identified in previous works [9,14], contributing to a total of 84.3% and 77.6% of the H. coccineum essential oil composition, respectively. Volatiles such as α-pinene, β-pinene, bornyl acetate, spathulenol, and (E)-nerolidol were present in both the samples of the present study (HCCAO and HCCRO) and previous investigations in varying amounts. p-cymene, cis-linalool oxide, trans-linalool oxide, trans-pinocarveol, trans-verbenol,borneol, α-terpineol, α-himachalene, β-acoradiene, globulol, viridiflorol, γ-eudesmolagarospirol, β-eudesmol, α-cadinol, and bulnesol were present only in HCCRO, whereas Sabinene, myrcene, artemisia alcohol, sulcatone, cis-sabinene hydrate, thujol, isoborneol, β-bourbonene, β-cubebene, β-elemene, γ-elemene, α-humulene, 9-epi-(E)-caryophyllene, α-neocallitropsene, germacrene D, bicyclogermacrene, α-farnesene, naphthalene, β-vetispirene, elemol, 7-hydroxyfarnesen, salvial-4(14)-en-1-one, rosifoliol, ledol, cadin-4-en-10-ol, α-(Z)-bergamotol, iso-longifolol, (E)-isovalencenol, and dodeca-(2E,4E)-dienal were found only in HCCAO.
Phytochemicals such as sabinene, myrcene, β-ocimene, γ-terpinene, artemisia alcohol, sulcatone, cis-sabinene hydrate, thujol, isoborneol, β-bourbonene, β-cubebene, β-elemene, γ-elemene, α-himachalene, α-humulene, 9-epi-(E)-caryophyllene, β-acoradiene, α-neocallitropsene, germacrene D, bicyclogermacrene, δ-cadinene, naphthalene, β-vetispirene, β-dihydroagarofuran, kessane, elemol, davanone B, 7-hydroxyfarnesen, salvial-4(14)-en-1-one, guaiol, rosifoliol, ledol, γ-eudesmol, cadin-4-en-10-ol, agarospirol, β-eudesmol, α-(Z)-bergamotol, iso-longifolol, (E)-isovalencenol, and dodeca-(2E,4E)-dienal were not previously reported, and were identified only in the samples from the present investigation (plants collected from Kausani, Uttarakhand, India). Similarly, compounds such as trans-carveol,cis-linalool oxide (furanoid), trans-linalool oxide (furanoid), trans-pinocarveol, trans-verbenol, myrtenol, α-fenchyl acetate, trans-sesquisabinene hydrate, caryophyllene oxide, ar-turmerol, and bisabolol identified in the previous study were missing in HCCAO and HCCRO. The results were significant in view of chemo-diversity in H. coccineum growing in Himalayan regions and other part of the world. This could be due to variation in their altitudes, environmental circumstance, climatic conditions, geographical distribution, etc.
2.2. Principal Component Analysis
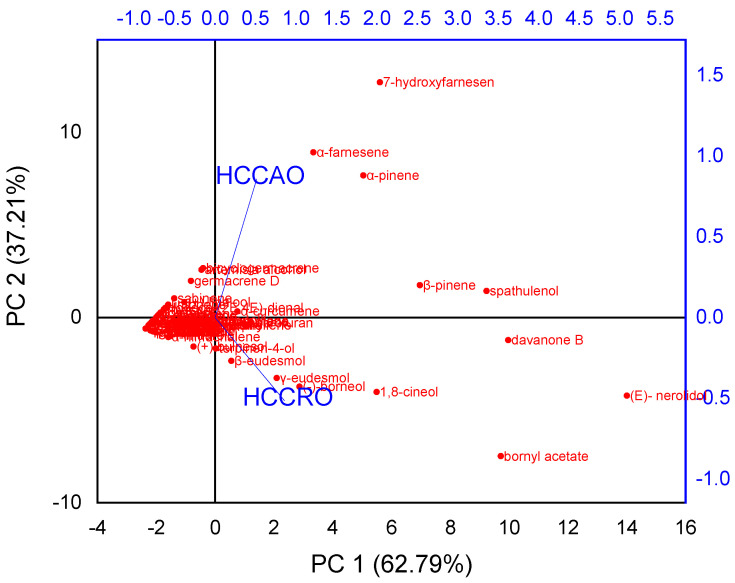
Principal Component Analysis (PCA) is one of the best multivariate statistical methods used to describe most significant aspects of a dataset. PCA pattern recognition of two essential oils was used to evaluate the phytochemical variability due to the type of plant portion from which essential oils were obtained. The collective contribution rate of variance of the first two principal components (PC1 and PC2) obtained from the PCA method was 100% for chemical compositional differences, which describes most of the variance information. Therefore, these two PCs defined the total compositional variability in the essential oils. PC1 contributed 62.79% in the total variance, which was positively correlated with α-famesene, α-pinene, β-pinene, spathulenol, and 7-hydroxyfamesen, whereas contribution of PC2 to the variance was 37.21%, which was positively correlated with β-eudesmol, γ-eudesmol, 1,8-cineol, davanone B, bornyl acetate, and (E)-nerolidol. The Principal Component Analysis (PCA) of HCCAO and HCCRO is shown in Figure 3.
Figure 3.
Principal Component Analysis (PCA) of HCCAO and HCCRO.
2.3. Nematicidal Activity
2.3.1. Effect on Mortality of Second Stage Larvae of M. incognita
The nematicidal activity of HCCAO and HCCRO was applied to second-stage juveniles (J2) of M. incognita for durations of 24, 48, 72, and 96 h. Percent mortality for both the samples was found to increase with an increase, in concentration as well as the incubation time with the essential oils. After 96 h, HCCAO was found to be most effective at 1 µL/mL dose level with 41.33% inhibition in larval mobility, followed by 0.5 µL/mL with 30.66% inhibition. HCCRO was also found to be most effective at 1µL/mL dose level, with 61.66%, inhibition in larval mobility, followed by 0.5 µL/mL with 52.66% inhibition. Silva-Aguayo et al. [19] reported significant nematicidal activity of the essential oil (from Peumusboldus) against Haemonchus contortus at similar levels of concentration (0.25, 0.5, and 1.0 µL/mL). The overall activity of HCCRO for the durations of 24, 48, 72, and 96 h was observed to be higher than HCCAO. HCCAO and HCCRO exhibited significant variation in immobility against M. incognita larvae. The LC50 values of the HCCAO at 24, 48, 72, and 96 h after treatment were 0.26, 0.13, 0.06, and 0.003% and LC50 values of HCCRO were 2.34, 6.92, 2.33, and 0.23%, respectively. The detailed experimental observation of percentage mortality and LC50 values of HCCAO and HCCRO for nematicidal activity against second-stage juveniles of M. incognita has been represented in Table 2 and Table 3, respectively.
Table 2.
Effect of essential oils on second-stage juveniles (J2) of M. incognita at different concentration.
| Treatment (T) | Concentration. (µL/mL) | Percent Mortality and Exposure Time (h.) | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| HCCAO | 0.25 | 17.66 ± 0.57 no | 21.00 ± 1.00 mn | 26.66 ± 1.52 ijk | 29.66 ± 1.52 hij |
| 0.5 | 24.33 ± 0.57 lm | 24.66 ± 0.57 lm | 28.66 ± 1.15 jkl | 30.66 ± 1.15 hi | |
| 1 | 25.66 ± 0.57 kl | 27.33 ± 1.15 ijkl | 33.33 ± 1.52 gh | 41.33 ± 1.15 e | |
| HCCRO | 0.25 | 21.00 ± 1.00 hi | 30.66 ± 1.15 hi | 34.66 ± 0.57 g | 46.66 ± 1.52 d |
| 0.5 | 30.66 ± 1.15 mn | 40.00 ± 1.00 ef | 49.00 ± 1.00 cd | 52.66 ± 1.15 bc | |
| 1 | 36.33 ± 1.52 fg | 46.33 ± 1.52 d | 55.00 ± 1.00 b | 61.66 ± 1.52 a | |
| Control | water | 1.66 ± 2.08 rs | 3.33 ± 1.52 rs | 6.33 ± 1.52 qrs | 14.33 ± 2.08 op |
HCCAO—H. coccineum a aerial part essential oil; HCCRO—H. coccineum rhizome part essential oil; SD—standard deviation. Within a column, mean values followed by the same letter are not significantly different according to Tukey’s test (p < 0.05).
Table 3.
LC50 values of HCCAO and HCCRO for nematicidal activity against second-stage juveniles (J2) of M. incognita.
| Sample | H. | *LC50 (%) | Regression Equation. |
|---|---|---|---|
| HCCAO | 24 | 0.26 | y = 0.007x + 4.06 |
| 48 | 0.13 | y = 0.006x + 4.39 | |
| 72 | 0.06 | y = 0.008x + 4.49 | |
| 96 | 0.03 | y = 0.005x + 4.79 | |
| HCCRO | 24 | 2.34 | y = 0.004x + 4.01 |
| 48 | 6.92 | y = 0.003x + 4.14 | |
| 72 | 2.33 | y = 0.003x + 4.29 | |
| 96 | 0.23 | y = 0.004x + 4.40 |
*LC50—lethal concentration; HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; Reg. eq.—regression equation.
2.3.2. Effect on Egg Hatchability of M. incognita
HCCAO and HCCRO showed a strong inhibitory effect on hatching from eggs in a concentration-dependent manner. The rate of egg hatching was found to be directly proportional to exposure time period and inversely proportional to oil sample concentration. In comparison with HCCAO, HCCRO had a stronger inhibitory effect on M. incognita in terms of egg hatching. After 96 h, the maximum rate of egg hatching in HCCAO (55.00%) and HCCRO (22.66%) was observed at a dose level of 0.25 µL/mL, while the minimum rate of egg hatching in HCCAO (17.66%) and HCCRO (11.33%) was observed at 1 µL/mL. Therefore, maximum egg hatching inhibition was observed in HCCRO at lowest as well as highest concentration levels. It was discovered that increasing the concentration of HCCAO and HCCRO delayed the start of egg hatching. The IC50 values at 24, 48, 72, and 96 h were 2.18, 2.38, 2.48 and 2.72 µL/mL for HCCAO and 1.92, 2.06, 2.19 and 2.07 µL/mL were for HCCRO respectively. The detailed experimental observations of percent egg hatching and IC50 values of HCCAO and HCCRO on the egg hatching of Meloidogyne incognita have been represented in Table 4 and Table 5, respectively.
Table 4.
Nematicidal activity of HCCAO and HCCRO on the egg hatching of Meloidogyne incognita.
| Treatment (T) | Concentration (µL/mL) |
Percent Egg Hatching of Nematodes and Exposure Time (h) | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| HCCAO | 0.25 | 32.33 ± 0.57 j | 43.33 ± 1.52 hi | 45.00 ± 1.73 gh | 55.00 ± 3.00 f |
| 0.5 | 25.00 ± 2.64 klm | 30.00 ± 1.00 jk | 30.00 ± 2.64 jk | 38.33 ± 0.57 i | |
| 1 | 6.66 ± 2.30 t | 14.00 ± 1.73 qrs | 14.00 ± 2.00 qrs | 17.66 ± 2.51 opq | |
| HCCRO | 0.25 | 18.33 ± 0.57 nopq | 23.66 ± 1.52 lmn | 26.66 ± 0.57 kl | 22.66 ± 1.52 lmno |
| 0.5 | 9.00 ± 1.00 st | 18.00 ± 1.00 opq | 19.66 ± 0.57 mnop | 15.33 ± 5.70 opq | |
| 1 | 6.66 ± 1.52 t | 11.13 ± 1.48 rst | 15.97 ± 1.13 pqr | 11.33 ± 0.57 rst | |
| Control | Water | 56.33 ± 4.04 def | 66.33 ± 2.08 cde | 74.33 ± 2.08 b | 92.33 ± 3.21 a |
HCCAO—H. coccineum aerial part essential oil; HCCRO—H. coccineum rhizome essential oil; SD—standard deviation. Within the dataset, mean values with same letter in superscript are not significantly different based on Tukey’s test (p < 0.05).
Table 5.
IC50 values of HCCAO and HCCRO on the egg hatching of Meloidogyne incognita.
| Sample | Time (h) | IC50 (µL/mL) |
|---|---|---|
| HCCAO | 24 | 2.18 |
| 48 | 2.38 | |
| 72 | 2.48 | |
| 96 | 2.72 | |
| HCCRO | 24 | 1.92 |
| 48 | 2.06 | |
| 72 | 2.19 | |
| 96 | 2.07 |
HCCAO—H. coccineum aerial part essential oil; HCCRO—H. coccineum rhizome part essential oil; IC50—half maximal inhibitory concentration.
It has been reported that β-dihydroagarofuran, kessane, elemol, (E)-nerolidol, davanone B, spathulenol, 7-hydroxyfarnesen, rosifoliol, T-muurolol, linalool, and E-isovalencenol were among the most oxygenated sesquiterpenoids observed as main components in plant essential oils, and showed egg-hatching and nematicidal activity in terms of mortality against the root knot nematode, M. Incognita [20]. Oxygenated sesquiterpenoid (E)-nerolidol, davanone B, spathulenol, 7-hydroxyfarnesen, globulol, and τ-muurolol) have been reported to efficiently inhibit the nematode eggs hatching and mortality, which indicates that essential oils with a high content of these compounds could be useful as natural nematicides for the control of M. incognita. The presence of one of the single major compounds or synergetic effects of major and minor constituents of essential oil might be responsible for the nematicidal activity of HCCAO and HCCRO towards the egg hatching and immobility of second-stage larvae of M. incognita [21,22].
2.4. Insecticidal Activity
The insecticidal activity of essential oils from rhizome and the aerial part of H. coccineum was estimated against Spodoptera litura (cotton cutworm) insects using the leaf-dip method. Fourth instar larvae of S. litura were used for different concentrations of essential oils to test the activity. The experiment was conducted in triplicate, and the total number of test insects per treatment was five. Tween-20 (1.0%) water solution was taken as control. The results showed that HCCRO was more effective than HCCAO and showed good mortality in a concentration-dependent manner (Table 6). During the experiment, no mortality was observed after 72 h. The mortality percentage of S. litura insect, treated with the essential oils of rhizome and aerial part of H. coccineum, is presented in Table 6. The LC50 values of HCCAO were 0.007, 0.006, and 0.005%, and the values of HCCRO were 0.007, 0.006, and 0.005% at 24, 48, and 72 h, respectively. The LC30, LC50, and LC90 value of essential oils from rhizome and the aerial part of H. coccineum are presented in Table 7. Significant insecticidal activity was reported for the essential oil (Mentha pulegium) at concentrations similar to the present investigation (10–100 µL) in fumigation conditions against Bruchus rufimanus [23].
Table 6.
Mortality percentage of S. litura insect treated with HCCRO and HCCAO.
| Essential Oil | Concentration (µL/mL) | No. of Insects Used | No. of Insects Dead | % of Average Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |||
| HCCAO | 10 | 5 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 | 0.00 | 0.00 |
| 25 | 5 | 1.00 ± 0.00 cd | 1.66 ± 0.57 bc | 2.00 ± 1.00 bc | 20.00 | 33.33 | 40.00 | |
| 50 | 5 | 1.66 ± 0.57 bc | 2.33 ± 1.57 abc | 3.00 ± 0.00 ab | 33.33 | 46.66 | 60.00 | |
| 100 | 5 | 2.33 ± 0.57 abc | 2.66 ± 0.57 ab | 3.66 ± 0.57 a | 46.66 | 53.33 | 73.33 | |
| HCCRO | 10 | 5 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 | 0.00 | 0.00 |
| 25 | 5 | 0.66 ± 0.57 cd | 1.33 ± 0.57 bcd | 1.66 ± 0.57 abc | 13.33 | 26.66 | 33.33 | |
| 50 | 5 | 1.66 ± 0.57 bcd | 2.00 ± 1.00 abcd | 3.00 ± 1.00 ab | 33.33 | 40.00 | 60.00 | |
| 100 | 5 | 2.00 ± 1.00 abcd | 2.66 ± 0.57 abc | 4.00 ± 0.00 a | 40.00 | 53.33 | 80.00 | |
| Control | water | 5 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 | 0.00 | 0.00 |
HCCAO—H. coccineum aerial part essential oil; HCCRO—H. coccineum rhizome part essential oil; SD—standard deviation. Within the dataset, mean values with same letter in superscript are not significantly different, based on Tukey’s test (p < 0.05).
Table 7.
LC30, LC50, and LC90 value for insecticidal activity of HCCRO and HCCAO against S. litura.
| Sample | Time (h) | LC30 (%) | LC50 (%) | LC90 (%) | Chi-Squared | Regression Equation |
|---|---|---|---|---|---|---|
| HCCAO | 24 | 0.005 | 0.007 | 0.01 | 0.80 | y = 0.06x + 0.38 |
| 48 | 0.004 | 0.006 | 0.01 | 0.83 | y = 0.06x + 0.51 | |
| 72 | 0.004 | 0.005 | 0.009 | 0.88 | y = 0.06x + 0.43 | |
| HCCRO | 24 | 0.006 | 0.007 | 0.01 | 0.82 | y = 0.005x + 0.30 |
| 48 | 0.005 | 0.006 | 0.01 | 0.74 | y = 0.062x + 0.42 | |
| 72 | 0.004 | 0.005 | 0.008 | 0.80 | y = 0.07x + 0.27 |
LC—Lethal concentration; HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil.
The insecticidal efficacy of H. coccineum rhizome essential oil has also been reported against three insects, Stephanitis pyrioides, Aedes aegypti, and Solenopsisinvicta [9]. The toxicity of essential oils against test insect might be due to the presence of various terpenoids found in the essential oils, or even may be due to the interaction of the major and the minor components present in the botanicals.
2.5. Herbicidal Activity
2.5.1. Inhibition of Seed Germination
The mean percent seed germination inhibition of essential oils from aerial part and rhizome of H. coccineum at different concentrations (50–200 µL/mL) has been depicted in Table 8. The essential oils possess moderate herbicidal activity in a dose-dependent manner. The herbicidal activity of rhizome and aerial part essential oil of H. coccineum at the highest concentration (200 µL/mL) was found in the order of HCCRO (96%) > HCCAO (92.00%). Essential oils from Limnophila indica have also been reported to have significant herbicidal activity at similar levels of treatment concentrations (50–200 µL/mL) [24]. IC50 was calculated at the time when 100% germination was achieved in the control and is used to compare the relative herbicidal activities of all the samples, as the lower the herbicidal activity, the higher its IC50 values. The order in which the activity was observed in terms of LC50 was as follows: HCCRO (62.78 ± 5.86 µL/mL) > HCCAO (88.09 ± 3.42 µL/mL) in Table 9.
Table 8.
Mean percent seed germination inhibition of HCCAO and HCCRO.
| S. No. | Sample Name | % Inhibition of Seed Germination | |||
|---|---|---|---|---|---|
| Essential Oil | 50 µL/mL | 100 µL/mL | 150 µL/mL | 200 µL/mL | |
| 1. | HCCAO | 36.00 ± 2.00 ab | 51.66 ± 0.57 c | 78.66 ± 1.52 fg | 92.00 ± 2.00 h |
| 2. | HCCRO | 47.66 ± 2.51 b | 61 ± 1.00 d | 73.33 ± 2.08 ef | 96.33 ± 1.52 h |
| 3. | Pendimethalin * | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
*—Standard herbicide; HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; values are means of three replicates ± SD; SD—standard deviation. Within a column, mean values followed by the same letter are not significantly different according to Tukey’s test (p < 0.05).
Table 9.
IC50 values of seed germination inhibition of HCCAO and HCCRO.
| S. No. | Sample Name | IC50 Values (µL/mL) in Triplicates | Mean IC50 Values (µL/mL) ± SD | ||
|---|---|---|---|---|---|
| Essential Oil | I | II | III | ||
| 1 | HCCAO | 90.54 | 84.18 | 89.55 | 88.09 ± 3.42 |
| 2 | HCCRO | 57.29 | 62.10 | 68.96 | 62.78 ± 5.86 |
HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; IC50—half maximal inhibitory concentration.
It was observed that HCCRO exhibited more herbicidal activity than HCCAO. Herbicidal activity of the Hedychium spicatum rhizome essential oil has also been reported against Radish (Raphanus raphanistrum) seeds in a previous study [25]. It was inferred that the herbicidal activity was due to the presence of various bioactive components such as camphor, 1,8-cineole, isoborneol, and linalool in the essential oil, or might be a possible synergistic effect of the minor as well as major compounds present in the H. coccineum rhizome and aerial part essential oils.
2.5.2. Inhibition of Root Length
The inhibition of root length was assessed as the measure of herbicidal activity. The percent root length inhibition of seeds germinated was calculated when 100% germination was achieved at various concentration ranges of 50, 100, 150, and 200 µL/mL. In the case of HCCRO, the percent inhibition of root length was recorded as 34.44%, 53.33%, 67.77%, and 84.07% from lowest to highest concentrations, while in the case of HCCAO, the percent inhibition was measured as 27.03%, 56.29%, 73.33%, and 90.37%, respectively, from lower to higher concentrations, as represented in Table 10. IC50 was calculated when 100% germination was achieved in the control, and was used to compare the relative herbicidal activities in terms of inhibition of root growth of all the samples, as the lower the herbicidal activity, the higher its IC50 values. The order in which the activity was observed was as follows: HCCRO (94.68 ± 2.74 µL/mL) > HCCAO (96.85 ± 0.38 µL/mL) (Table 11).
Table 10.
Mean percent inhibition of root length of HCCAO and HCCRO.
| S. No. | Sample Name | % Inhibition of Root Length | |||
|---|---|---|---|---|---|
| Essential Oil | 50 µL/mL | 100 µL/mL | 150 µL/mL | 200 µL/mL | |
| 1. | HCCAO | 27.03 ± 0.64 a | 56.29 ± 0.64 d | 73.33 ± 1.11 f | 90.37 ± 0.64 h |
| 2. | HCCRO | 34.44 ± 1.11 a | 53.33 ± 1.11 d | 67.77 ± 1.11 e | 84.07 ± 0.64 g |
| 3. | Pendimethalin * | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ±0.00 |
*—Standard herbicide, HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil. Values are means of three replicates ± SD; SD—standard deviation. Within a column, mean values followed by the same letter are not significantly different according to Tukey’s test (p < 0.05).
Table 11.
IC50 value of inhibition of root length of HCCAO and HCCRO.
| S. No. | Sample Name | IC50 Values (µL/mL) in Triplicates | Mean IC50 Values (µL/mL) ± SD | ||
|---|---|---|---|---|---|
| Essential Oil | I | II | III | ||
| 1 | HCCAO | 96.69 | 96.57 | 97.29 | 96.85 ± 0.38 |
| 2 | HCCRO | 93.1 | 97.85 | 93.1 | 94.68 ± 2.74 |
HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; IC50—half maximal inhibitory concentration.
2.5.3. Inhibition of Shoot Length
The inhibition of shoot length was also assessed as the measure of herbicidal activity. The percent shoot length inhibition was calculated when 100% germination was achieved at various concentrations ranging between 50, 100, 150, and 200 µL/mL. In case of HCCRO, the percent inhibition of shoot length was recorded as 40%, 47.77%, 74.44%, and 99.62% from lowest to highest concentrations, while in case of HCCAO, the percent inhibition was measured as 34.44%, 52.22%, 66.66%, and 81.11%, respectively, from lower to higher concentrations, and represented in Table 12. IC50 was calculated when 100% germination was achieved in the control, and was used to compare the relative herbicidal activities in terms of inhibition of root growth of all the samples, as the lower the herbicidal activity, the higher its IC50 values. The order in which the activity was observed in terms of IC50 values was as follows: HCCRO (87.44 ± 2.98 µL/mL) > HCCAO (133.06 ± 17.22 µL/mL) (Table 13).
Table 12.
Mean percent inhibition of shoot length of HCCAO and HCCRO.
| S. No. | Sample Name | % Inhibition of Shoot Length | |||
|---|---|---|---|---|---|
| Essential Oil | 50 µL/mL | 100 µL/mL | 150 µL/mL | 200 µL/mL | |
| 1. | HCCAO | 34.44 ± 1.11 b | 52.22 ± 4.00 d | 66.66 ± 2.22 e | 81.11 ± 2.93 g |
| 2. | HCCRO | 40.00 ± 1.11 b | 47.77 ± 1.92 c | 74.44 ± 1.11 f | 99.62 ± 0.64 h |
| 3. | Pendimethalin * | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
*—Standard herbicide; HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; values are means of three replicates ± SD; SD—standard deviation. Within the dataset, mean values with the same letter in superscript are not significantly different, based on Tukey’s test (p < 0.05).
Table 13.
IC50 value of inhibition of shoot length of HCCAO and HCCRO.
| S. No. | Sample Name | IC50 Values (µL/mL) in Triplicates | Mean IC50 Values (µL/mL) ± SD | ||
|---|---|---|---|---|---|
| Essential Oil | I | II | III | ||
| 1. | HCCAO | 133.76 | 149.93 | 115.5 | 133.06 ± 17.22 |
| 2. | HCCRO | 86.2 | 90.85 | 85.27 | 87.44 ± 2.98 |
HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil IC50—half maximal inhibitory concentration.
2.6. Antifungal Activity
The antifungal activity of HCCAO and HCCRO was evaluated against two phytopathogenic fungi (Fusarium oxysporum and Curvularialunata) at varied doses (50–750 µL/mL). The antifungal activity of the essential oils is shown in Table 14. The essential oils exhibited good antifungal activity by inhibiting the mycelial growth of pathogenic fungi. HCCRO (88.1%) had the maximum antifungal activity against F. oxysporum, followed by HCCAO (83.3%), while HCCAO (84.1%), followed by HCCRO (74.8%), had the strongest antifungal activity against C. lunata at higher concentrations (750 µL/mL). The antifungal activity of HCCAO and HCCRO was significantly lower compared to standard fungicide Carbendazim (100%), even at a higher concentration (750 µL/mL) against both the tested fungi. Antifungal activity was also demonstrated for the essential oil at 50–500 µL/mL in a previous study [26].
Table 14.
Percent mycelial growth inhibition of F. oxysporum, and C. lunata by HCCAO and HCCRO.
| Percent Mycelial Growth Inhibition | ||||
|---|---|---|---|---|
| Concentration (µL/mL) |
Fusarium oxysporum | Curvularia lunata | ||
| HCCAO | HCCRO | HCCAO | HCCRO | |
| 50 | 15.9 ± 0.64 a | 38.1 ± 0.64 c | 27.0 ± 0.64 b | 18.5 ± 0.67 a |
| 100 | 32.9 ± 0.64 b | 52.6 ± 0.61 d | 32.9 ± 0.64 c | 32.4 ± 0.29 c |
| 250 | 54.0 ± 1.69 d | 66.7 ± 0.12 e | 57.0 ± 0.64 d | 42.9 ± 1.69 e |
| 500 | 69.9 ± 0.57 e | 72.7 ± 0.55 e | 72.2 ± 1.05 f | 58.5 ± 0.57 e |
| 750 | 83.3 ± 1.11 f | 88.1 ±1.28 f | 84.1 ± 0.57 h | 74.8 ± 0.64 g |
| Carbendazim * | 100 ± 00 | 100 ± 00 | 100 ± 00 | 100 ± 00 |
*—Standard pesticide; HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; SD—Standard deviation. Within the dataset, mean values with same letter in superscript are not significantly different based on Tukey’s test (p < 0.05).
Several biologically active compounds, such as (E)-nerolidol, davanone B, spathulenol, limonene, (E)-caryophyllene, bicyclogermacrene, and 7-hydroxyfarnesen have been reported to possess the antifungal properties of the essential oils tested against Colletotrichum acutatum, C. fragariae, and C. gloeosporioides [9]. Studies have confirmed that the Hedychium essential oil, which is rich in (E)-nerolidol, α-farnesene, α-pinene, and β-pinene, shows potential antifungal activity against Candida albicans and Fusarium oxysporum [27]. The presence of individual major compounds or the synergetic effect of major/minor constituents of essential oil might be responsible for the antifungal activity of HCCAO and HCCRO towards F. oxysporum and C. lunata.
2.7. Antibacterial Activity
The emerging antibiotic resistance in bacteria and the high cost of developing novel antimicrobial drugs has encouraged researchers to search for novel effective and economically viable broad-spectrum natural products with different modes of action. Essential oils and their chemical constituents in pure form have been reported to have effective action against resistant microbial strains [28,29,30]. Therefore, in this study, we have explored the antibacterial activity of HCCRO and HCCAO using zones of inhibition assay against Gram-positive bacteria, Staphylococcus aureus, and Gram-negative bacteria, Salmonella enterica serovar Typhi. The spot diffusion method confirmed that both HCCAO and HCCRO showed antibacterial activity against both the bacterial pathogens. However, HCCRO showed a higher zone of inhibition against both Gram-positive and Gram-negative pathogens. Of these strains, Gram-positive Staphylococcus aureus was more susceptible to HCCRO than Gram-negative Salmonella enterica serovar Typhi, with average zones of inhibition of 25 mm and 6 mm, respectively. Staphylococcus aureus is a Gram-positive opportunistic pathogenic bacterium which causes nosocomial and community infections such as bloodstream infections, pneumonia, skin and soft tissue infections, and bone and joint infections [31]. Salmonella enterica serovar Typhi is a common and clinically significant Gram-negative pathogenic bacterium that causes gastroenteritis and typhoid fever in humans, affecting over 20 million people worldwide and killing 220,000 people each year [32,33]. Results showed that HCCRO had potential antibacterial activity against both bacterial pathogens. The colony farming unit (CFL/mL) of Staphylococcus aureus and Salmonella enterica serovar Typhi by essential oils from the aerial and rhizome part of H. coccineum is represented in Table 15.
Table 15.
Colony-forming unit (CFL/mL) of Staphylococcus aureus and Salmonella enterica serovar Typhi by essential oils from the aerial and rhizome part of H. coccineum.
| Concentration (μL/100 μL) |
Staphylococcus aureus (log10CFU/mL ± SD) |
Salmonella enterica serovar Typhi (log10CFU/mL ± SD) |
||
|---|---|---|---|---|
| HCCAO | HCCRO | HCCAO | HCCRO | |
| 5 | 1 ± 0 g | 1 ± 0 h | 6.97 ± 0.41 f | 6.34 ± 0.22 h |
| 2.5 | 2.67 ± 0.11 e | 2.079 ± 0.12 f | 7.17 ± 0.33 e | 6.97 ± 0.37 g |
| 1.25 | 5.38 ± 0.22 d | 5.16 ± 0.34 e | 8.00 ± 0.48 d | 7.83 ± 0.55 e |
| 0.625 | 7.38 ± 0.33 c | 6.28 ± 0.2 5 c | 9.15 ± 0.36 c | 9.12 ± 0.39 c |
| Untreated cells | 8.57 ± 0.31 a | 8.57 ± 0.31 b | 9.16 ± 0.58 a | 9.16 ± 0.58 b |
HCCAO—Hedychium coccineum aerial part essential oil; HCCRO—Hedychium coccineum rhizome part essential oil; SD—standard deviation. Within the dataset, mean values with same letter in superscript are not significantly different based on Tukey’s test (p < 0.05). CFL—Colony forming unit.
Determination of Minimum Inhibitory (MIC) Concentration and Minimum Bactericidal Concentration (MBC)
The minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values were determined using the broth dilution method to evaluate the effectiveness in controlling bacterial pathogens. The results revealed that in the presence of HCCRO (2.5 μL/100 μL) and HCCAO (2.5 μL/100 μL), 6.5 and 6 Log CFU/mL, respectively, reductions in the growth of Staphylococcus aureus were observed, while the growth was completely inhibited at higher concentration (5 μL/100 μL). The MIC and MBC values of HCCRO against Staphylococcus aureus were 2.5 μL/100 μL and 5 μL/100 μL, respectively. Meanwhile, in the case of Salmonella enterica serovar typhi, 3 and 2.3 log reductions in the CFU were observed in the presence of HCCRO and HCCAO, respectively. Changes in bacterial cell suppression by essential oils could be attributed to chemical components and the volatile nature of their components, or differences in the composition of Gram-positive and Gram-negative bacterial membranes [34,35].
It has been observed that HCCRO exhibits more antibacterial efficacy than HCCAO against Staphylococcus aureus and Salmonella enterica serovar Typhi. Studies have confirmed that the essential oil rich in α-farnesene, α-pinene and (E)-nerolidol shows potential antibacterial activity against Staphylococcus aureus, Penicillium chrysogenum, Bacillus subtilis, Escherichia coli, and Saccharomyces cerevisiae [36]. The essential oils of H. venustum, H. spicatum, H. coronarium, and H. flavescens have also been reported for their antibacterial activity against Salmonella typhi and Escherichia coli [37]. Antibacterial efficacy of HCCAO and HCCRO might be due to the presence of the main constituents such as 7-hydroxyfarnesen, bicyclogermacrene, germacrene D, α-farnesene, (E)-caryophyllene, α-farnesene (11.1%), α-pinene (10.9%), (E)-nerolidol (15.9%), bornyl acetate (13.9%), davanone B (10.9%), and spathulenol (8.9%), or might be a possible synergistic effect of the major/minor compounds present in the H. coccineum rhizome and aerial part essential oils.
2.8. In Silico PASS Prediction of HCCAO and HCCRO
In silico PASS predictions for antibacterial, antifungal, and nematicidal activity of selected phytochemical compounds from HCCAO and HCCRO are reported in Table 16. Among the identified compounds, davanone B, α-farnesene, davanone B, α-curcumene, germacrene D, and (E)-caryophyllene were observed to exhibit acceptable Pa/Pi values. However, other compounds were observed to exhibit negligible nematicidal activity as per PASS prediction. These data support the in vitro nematicidal activity for HCCRO and HCCAO performed in the present investigation. From the PASS prediction data, it can be inferred that the nematicidal activity of these essential oils is governed by one of the above-mentioned compounds having acceptable Pa/Pi values or the result of the synergistic effect of more than one component present in essential oil. Volatile compounds exhibited a good Pa/Pi range, (0.45 > 0.02). Among the identified compounds, 7-hydroxyfarnesen, bicyclogermacrene, germacrene D, α-farnesene, (E)-caryophyllene, and (E)-nerolidol were found to exhibit acceptable antibacterial effects (in terms of Pa/Pi values). However, some other major compounds, such as β-pinene, 1,8-cineol, borneol, γ-eudesmol, α-curcumene, and β-dihydroagarofuran were predicted to have comparatively low antibacterial activities. Overall, the PASS prediction supported the antibacterial activity of HCCAO and HCCRO compounds. The Pa/Pi value of major compounds such as (E)-nerolidol, linalool, α-farnesene, davanone B,limonene, (E)-caryophyllene, bicyclogermacrene, 7-hydroxyfarnesen, and spathulenol for the antifungal potential was higher than that of these compounds for antibacterial activity. The other predicted compounds also exhibited superior antifungal activity. Hence, the PASS prediction supports the present high antifungal activities of HCCAO and HCCRO. Therefore, it is supposed that these biological activities of HCCAO and HCCRO are governed by the compounds showing a higher Pa/Pi ratio, or it may be a combined effect of more than one compound.
Table 16.
In silico PASS prediction for antibacterial, antifungal, and nematicidal activity of selected phytochemical compounds from HCCAO and HCCRO.
| Pass (Pa > Pi) | ||||
|---|---|---|---|---|
| S.No. | Compounds Name | Antibacterial | Antifungal | Nematicidal |
| 1 | β-pinene | 0.23 > 0.09 | 0.22 > 0.12 | 0.24 > 0.16 |
| 2 | 1,8-cineol | 0.29 > 0.06 | 0.24 > 0.12 | 0.28 > 0.12 |
| 3 | borneol | 0.26 > 0.07 | 0.34 > 0.06 | 0.26 > 0.05 |
| 4 | (E)-nerolidol | 0.43 > 0.02 | 0.61 > 0.01 | 0.36 > 0.02 |
| 5 | (E)-caryophyllene | 0.44 > 0.02 | 0.58 > 0.02 | 0.48 > 0.01 |
| 6 | linalool | 0.38 > 0.03 | 0.59 > 0.01 | 0.37 > 0.02 |
| 7 | α- pinene | 0.32 >0.05 | 0.43 > 0.04 | 0.35 > 0.06 |
| 8 | α-farnesene | 0.41 > 0.02 | 0.60 > 0.01 | 0.45 > 0.01 |
| 9 | limonene | 0.40 > 0.02 | 0.58 > 0.02 | 0.59 > 0.00 |
| 10 | terpinen-4-ol | 0.32 > 0.05 | 0.46 > 0.03 | 0.46 > 0.02 |
| 11 | spathulenol | 0.40 > 0.02 | 0.51 > 0.02 | - |
| 12 | davanone B | 0.45 > 0.02 | 0.59 > 0.01 | 0.45 > 0.01 |
| 13 | γ-eudesmol | 0.26 > 0.07 | 0.28 > 0.08 | 0.26 > 0.15 |
| 14 | bulnesol | 0.32 > 0.05 | 0.19 > 0.03 | 0.21 > 0.19 |
| 15 | β–eudesmol | 0.30 > 0.05 | 0.40 > 0.04 | 0.22 > 0.06 |
| 16 | α-curcumene | 0.29 > 0.06 | 0.44 > 0.04 | 0.41 > 0.01 |
| 17 | germacrene D | 0.42 > 0.02 | 0.57 > 0.02 | 0.45 > 0.00 |
| 18 | bicyclogermacrene | 0.42 > 0.02 | 0.53 > 0.02 | 0.63> 0.00 |
| 19 | 7-hydroxyfarnesen | 0.44 > 0.02 | 0.62 > 0.01 | 0.34 > 0.02 |
| 20 | β dihydroagarofuran | 0.21 > 0.10 | 0.17 > 0.05 | 0.32 > 0.09 |
PASS—prediction of activity spectra for substance; Pa—probable activity; Pi—probable inactivity.
3. Materials and Methods
3.1. Plant Material
H. coccineum plant material was collected in August 2021 from Kausani (Altitude-1672 m, Latitude 29.8445° N, and Longitude 79.6039° E), Bageshwar, Uttarakhand, India. Dr. D.S. Rawat (Plant Taxonomist), Department of Biological Sciences, College of Basic Science and Humanities, G.B.P.U.A.T, Pantnagar, recognized the plant material and submitted the herbarium (specimen no. GBPUH-1040) to the Department of Biological Sciences.
3.2. Essential Oil Isolation
The essential oils from the aerial part and rhizome of H. coccineum were extracted using the hydro distillation method by subjecting the fresh plant materials (1.2 kg of arial part and 0.9 kg rhizome) to the Clevenger-type apparatus for about 3 h [38,39,40]. The obtained essential oils were dried over anhydrous sodium sulphate before being filtered and stored in dark glass vials at 4 °C for further use.
3.3. GC-MS Analysis
The phytochemical composition of both essential oils was analyzed using gas chromatography-mass spectrometry (GC-MS) analysis (A.I.R.F. (J.N.U), New Delhi, India) with a GCMS-QP 2010 Ultra DB-5 and GCMS-QP 2010 Ultra Rtx-5MS column (30 m × 0.25 mm i.d., 0.25 µm). Helium was employed as a carrier gas at a flow rate of 1.21 mL/min, with a split ratio of 10.0. The GC oven temperature program was 50–280 °C with a temperature gradient of 3 °C/min up to 210 °C (isotherm for 2 min), then 6 °C/min up to 280 °C. The constituents of essential oils were identified by comparing their mass spectrum fragmentation patterns and their relative retention index (RI) values with the MS library (NIST14.lib, FFNSC2.lib, WILEY8.LIB), as well as comparing the spectra with literature data [15].
3.4. Nematicidal Activity
3.4.1. Nematode Population Collection
Meloidogyne incognita eggs were collected from nematode-infected tomato (Solanum lycopersicum) roots collected from the Crop Research Center, G. B. P.U.A.T, Pantnagar, in a glasshouse, maintained at 25 ± 2 °C. The sample was collected on the basis of the visual symptoms of root knots or galls formed in the plant. Hand-picked matured egg masses from infected tomato roots were cultured in distilled water in a growth chamber at 25 °C. For future use, emerged juveniles were collected and preserved at 5 °C [41,42].
In Vitro Mortality Assay on Second Stage Larvae of M. incognita
For in vitro mortality assay, second-stage juveniles (100 in number) collected from hatched eggs within 48 h were placed on gridded Petri dishes with stock solution and 1.0 mL of distilled water. There were three different doses, i.e., 0.25, 0.5, and 1 µL/mL of essential oils in a 1.0% Tween-20 water solution. The treatments were performed in triplicate and arranged in randomized order. The juveniles immersed in Tween-20 (1.0%) water solution were used as a control group. The number of dead juveniles was counted using a stereo-binocular microscope throughout time periods of 24, 48, 72, and 96 h. Totally motionless (dead larvae) nematodes were picked out of the Petri dish and placed in distilled water. Percent mortality was calculated using Abbott’s formula [43].
where, Nt = Mortality in treatment; Nc = Mortality in control.
Effect of Essential Oils on Egg Hatchability Test of M. incognita
Two egg masses of M. incognita were suspended in 0.25, 0.5, and 1 µL/mL conc. of HCCAO and HCCRO in gridded Petri dishes. The egg masses suspended in a Tween-20 (1.0%) water solution were used as a control. All of the treatments were set up in triplicate and in a completely random order in the BOD incubator at a constant temperature of 27 ± 1 °C. Observations on percent egg hatching were made at time intervals of 24, 48, 72, and 96 h. The counting of the number of eggs hatched was performed under a microscope at a magnification of 4×. Percent egg hatching was computed using Abbott’s formula [44].
| (1) |
where, Nt = egg hatching in treatment; NC = egg hatching in control.
3.5. Insecticidal Activity
3.5.1. Test Insect
Insecticidal activity of HCCAO and HCCRO were tested against cotton cut worm (Spodoptera litura belongs to family: Noctuidae and order: Lapidoptera), which is a serious polyphagous pest in Asia, Oceania, and the Indian subcontinent. Although it is a harmful pest in tobacco, it also attacks cole crops, castor, cotton, chilli peppers, tomato, etc.
3.5.2. Collection of Larvae and Maintenance
Initial culture of S. litura as egg mass was collected from wild castor (Ricinus communis) plant from CRC (Crop Research Center), G.B.P.U.A&T., Pantnagar, Uttarakhand, India. The test insects were reared in a clean plastic container covered with muslin cloth in ideal laboratory conditions, with the temperature kept at 27 °C, and humidity kept at 75–80%. Test insects were served fresh castor leaf every day until they reached the fourth instar larval stage. Finally, fourth instar larvae were starved for 12 to 24 h before being used in insecticidal activity.
3.5.3. Bioassay of Insecticidal Activity
The leaf dip method was used to assess the insecticidal activity of rhizome and aerial part essential oils of H. coccineum [45]. For evaluating the insecticidal activity, different concentrations of essential oils (10, 25, 50 and 100 µL/mL) were prepared in Tween-20 (1.0%) solution in distilled water. The castor leaves were cleaned and washed in distilled water before being air dried for an hour. Each castor leaf was sliced into a 25 sq.cm section and immersed in various concentrations of essential oils. The leaf discs were slanted on blotting paper for 2–3 min before being placed in the tray to drain excess solution for 2 h at room temperature. Four instar adult five larvae were released in individual Petri dishes after being starved for 12–24 h. Blotting paper was placed at the bottom of each plate. For 72 h, these Petri plates were monitored for any insecticidal activity. This activity took place in ideal laboratory conditions, with a temperature of 27 °C and a relative humidity of 75–80%. The mortality (%) was calculated after 24, 48, and 72 h of the treatment using Abbott’s formula [43]. LC50 values were analyzed using Probit analysis [46].
where, T = Mortality in treatment; C = Mortality in control.
3.6. Herbicidal Activity
3.6.1. Evaluation of Herbicidal Activity
The herbicidal action of essential oils was assessed based on various parameters such as inhibition of seed germination, inhibition of shoot length, and inhibition of root length against R. raphanistrum subsp. Sativus (Radish) seeds.
3.6.2. Herbicidal Bioassay
The herbicidal activity of essential oils was evaluated using the method reported by [47,48,49,50]. Raphanus raphanistrum subsp. Sativus (L.) (Radish) seeds were obtained from the VRC (Vegetable Research Centre), G.B.P.U.A.T. Pantnagar. To evaluate the seed germination inhibition, various conc. of essential oils were prepared in Tween-20 (1.0%) aqueous solution. Prior to usage, R. raphanistrum subsp. sativus seeds were surface sterilized for 15 min in a 5% sodium hypochlorite solution. Ten sterilized seeds of R. raphanistrum sub sp. sativus were placed on the Petri plates, which were coated with regular filter papers. Then, 2 mL of various concentrations of the tested sample were put onto the plates and left to germinate at 25 ± 1 °C for 12 h in an incubator. Pendimethalin was used as a standard herbicide. Tween-20 (1.0%) solution in sterilized distilled water was taken as a control for essential oils. Percent inhibition of seed germination and inhibition of root and shoot length were measured after 5 days of incubation. The formulae used for determination of inhibition of seed germination, inhibition of shoot length, and inhibition of root length are as follows.
Inhibition of seed germination
where, Gt—no. of seeds germination in treatment;
Gc—No. of seeds germination in control.
-
b.
Inhibition of shoot length
where, Ct –shoot length in treatment;
Cc—shoot length in control.
-
c.
Inhibition of root length
where, Rt—root length in treatment;
Rc—root length in control.
3.7. Antifungal Activity
Fusarium oxysporum and Curvularia lunata, two phytopathogenic fungi, were provided by the Department of Plant Pathology, College of Agriculture, G.B.P.U.A.T, Pantnagar, India. HCCRO and HCCAO were tested against the test fungus using the poisoned food technique developed by [51]. The phytopathogenic fungi were revived and grown by placing the fungal colonies aseptically on the Petri plates containing the Potato Dextrose Agar (PDA) media. The Petri plates were incubated for one week at 26 ± 2 °C. The assay discs (diameter = 5 mm) of a 7-day-old culture of the test fungus were inoculated aseptically, with the prepared plates containing varied conc. of essential oils (50–750 µL/mL) prepared in Tween-20 (1.0%) water solution. A control devoid of essential oils was prepared under the same conditions. The control plate was cultured for 7 days until the growth reached the plate’s edge. The percent inhibition of radial growth of each fungal strain was calculated in comparison with the control. Antifungal activity was detected by clear zones of mycelia growth inhibition surrounding the Petri plate, which were measured in millimeters. Carbendazim (50% WP) was employed as the standard fungicide, and percent inhibition was calculated using McKinney’s formula [46].
where, X = Radial growth in control, Y = Radial growth in treatment.
3.8. Antibacterial Activity
3.8.1. Diffusion Agar Antibacterial Assay
The antibacterial activity of the essential oils was investigated qualitatively via diffusion assay. Briefly, the overnight grown bacterial cultures (Staphylococcus aureus and Salmonella enterica serovar typhi) were sub-cultured in Luria Bertani (LB) broth and grown till OD600nm reached 0.2. Next, 100 µL of the above culture of each bacterial cell was spread plated on an LB agar plate. Then, 10 µL of rhizome and aerial essential oils was spotted onto the LB agar plates separately and incubated at 37 °C for 24 h. Upon incubation, the inhibition zone diameter of the inoculated plate was measured.
3.8.2. Determination of Minimum Inhibitory Concentration
The susceptibility of both Gram-positive (Staphylococcus aureus) and Gram-negative (Salmonella enterica serovar Typhi) bacterial cells to essential oils was estimated by the micro broth dilution method as per clinical and laboratory standards institute (CLSI) guidelines in brain heart infusion (BHI) and MH broth, respectively [52,53,54]. Briefly, the overnight grown bacterial cells were sub-cultured in respective broths and grown till the mid log phase (OD reached 0.4). After that, each bacterial cell suspension was diluted 1000-fold to attain an inoculum of 105 colony forming units (105CFU/100 μL) and mixed with an equal volume (100 μL:100 μL) of 2-fold-diluted essential oils. The growth of bacterial cells was assessed by enumerating CFU in the agar plate after incubating the bacterial cells for 12 h under a static condition in a humidity-controlled incubator at 37 °C. The MIC of a plant extract is the lowest concentration that inhibits observable microorganism growth. The experiments were repeated three times, with two replicates in each dish.
3.9. In Silico PASS Prediction of Biological Activities
The biological activities of 20 major compounds present in the HCCAO and HCCRO essential oils were predicted using PASS (prediction of activity spectra for substances) software [55,56]. PASS is a free online cheminformatic software that assesses the biological activities of chemical compounds based on structural similarities to a large library of active compounds. Pa or Pi readings were used to calculate the bioactivity score. If the Pa value (chances to be active) was greater than the Pi value (chances to be inactive), the projected compound was likely to be active. HCCAO and HCCRO were predicted to exhibit diverse bioactivities (Pa > Pi).
3.10. Statistical Analysis
All of the experiments were carried out in three replicates, with the results represented as mean ± standard deviation (SD). A two-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test was performed to test the differences in the means of treatment using RStudio2021.09.2. OriginPro 2021 version 9.8.0.200 was used to perform Principal Component Analysis (PCA) on the chemical composition of the essential oils under investigation to identify the most significant feature in the dataset.
4. Conclusions
According to the present study, it can be observed that GC-MS analysis of aerial part and rhizome essential oils (HCCAO and HCCRO) of H. coccineum showed the presence of 50 and 32 compounds, respectively. The tested essential oils possessed significant antibacterial (S. aureus and S. typhi) and antifungal (against F. oxysporum and C. lunata) activities and moderate nematicidal (against M. Incognita), insecticidal (against S. litura), and herbicidal (against R. raphanistrum subsp. sativus) activity in a tested concentration, which can be used to create a highly effective botanical pesticide. The antimicrobial action of H. coccineum essential oil on bacterial and fungal strains demonstrated the plants’ potential as a source of natural antimicrobial agents. Nematicidal activity of the essential oils might be a good source of more selective, biodegradable, and environmentally friendly natural nematicides, acting as a substitute to synthetic nematicides and a good source of herbal nutraceuticals and phytochemicals. The herbicidal activity results were also validated by IC50 values, as the higher the IC50 value, the lower the herbicidal activity. The order in which the samples exhibited herbicidal potential in terms of percent seed germination inhibition was found HCCRO (62.78 ± 5.86 µL/mL) > HCCAO (88.09 ± 3.42 µL/mL). Herbicidal potential in terms of root length inhibition was found in the following order: HCCRO (94.68 ± 2.74 µL/mL) > HCCAO (96.85 ± 0.38 µL/mL), while herbicidal potential in terms of shoot length inhibition was found in the following order: HCCAO (133.06 ± 17.22 µL/mL) > HCCAO (87.44 ± 2.98 µL/mL), respectively.
Acknowledgments
The authors would like to express gratitude to Dharmendra Singh Rawat, Department of Biological Sciences, C.B.S.H., G.B.P.U.A&T., Pantnagar for the identification of the plant species and A.I.R.F. (J.N.U) New Delhi for providing a facility for GC-MS analysis. The author Mozaniel de Oliveira, thanks PCI-MCTIC/MPEG, as well as CNPq for the process number: [300983/2022-0].
Author Contributions
S.A.: Performed the experiment, literature search, and manuscript writing; R.K.: experiment design, conceptualization, and editing of the manuscript; O.P.: manuscript editing; S.K.: experiment design and manuscript editing; S.K.M.: manuscript writing; S.C.: manuscript editing; P.K.: experiment design and manuscript writing; R.M.S.: experiment design; M.S.d.O.: manuscript editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Sample: Hedychium coccineum Buch.-Ham. ex Sm of the compounds is available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fournet J., Sastre C. Progrès Récents Dans La Connaissance de La Flore de Guadeloupe et de Martinique. Acta Bot. Gall. 2002;149:481–500. doi: 10.1080/12538078.2002.10515977. [DOI] [Google Scholar]
- 2.Govaerts R. World Checklist of Selected Plant Families. 2011 ed. Royal Botanic Gardens; Londo, UK: 2009. [Google Scholar]
- 3.Wongsuwan P., Picheansoonthon C. Taxonomic Revision of the Genus Hedychium J. Koenig (Zingiberaceae) in India. J. R. Inst. Thail. 2011;III:126–149. [Google Scholar]
- 4.Jiangyun G., Chunling S., Shuxia Y. Adaptive Significance of Mass-Flowering in Hedychium Coccineum (Zingiberaceae) Biodivers. Sci. 2013;20:376–385. doi: 10.3724/SP.J.1003.2012.10034. [DOI] [Google Scholar]
- 5.Quattrocchi U. In: CRC World Dictionary of Medicinal and Poisonous Plants. 1st ed. Quattrocchi U., editor. CRC Press; New York, NY, USA: 2016. [Google Scholar]
- 6.Tushar S., Basak S., Sarma G.C., Rangan L. Ethnomedical Uses of Zingiberaceous Plants of Northeast India. J. Ethnopharmacol. 2010;132:286–296. doi: 10.1016/j.jep.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Johnson T. In: CRC Ethnobotany Desk Reference. 1st ed. Johnson T., editor. CRC Press; New York, NY, USA: 2019. [Google Scholar]
- 8.Basak S., Ramesh A.M., Kesari V., Parida A., Mitra S., Rangan L. Genetic Diversity and Relationship of Hedychium from Northeast India as Dissected Using PCA Analysis and Hierarchical Clustering. Meta Gene. 2014;2:459–468. doi: 10.1016/j.mgene.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakhanokho H., Sampson B., Tabanca N., Wedge D., Demirci B., Baser K., Bernier U., Tsikolia M., Agramonte N., Becnel J., et al. Chemical Composition, Antifungal and Insecticidal Activities of Hedychium Essential Oils. Molecules. 2013;18:4308–4327. doi: 10.3390/molecules18044308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira M.S. In: de Essential Oils—Applications and Trends in Food Science and Technology. 1st ed. Santana de Oliveira M., editor. Springer International Publishing; Cham, Switzerland: 2022. [Google Scholar]
- 11.Shifah F., Tareq A., Sayeed M., Islam M., Emran T., Ullah M., Mukit M., Ullah A. Antidiarrheal, Cytotoxic and Thrombolytic Activities of Methanolic Extract of Hedychium Coccineum Leaves. J. Adv. Biotechnol. Exp. Ther. 2020;3:77. doi: 10.5455/jabet.2020.d110. [DOI] [Google Scholar]
- 12.Nishi S.I., Barua N., Sayeed M.A., Tareq A.M., Mina S.B., Emran T.B., Dhama K. In vivo and in vitro evaluation of pharmacological activities of hedychium coccineum rhizomes extract. J. Exp. Biol. Agric. Sci. 2021;9:335–342. doi: 10.18006/2021.9(3).335.342. [DOI] [Google Scholar]
- 13.Rawat A., Prakash O., Kumar R., Arya S., Srivastava R.M. Hedychium Spicatum Sm.: Chemical Composition with Biological Activities of Methanolic and Ethylacetate Oleoresins from Rhizomes. J. Biol. Act. Prod. from Nat. 2021;11:269–288. doi: 10.1080/22311866.2021.1923572. [DOI] [Google Scholar]
- 14.Gurib-Fakim A., Maudarbaccus N., Leach D., Doimo L., Wohlmuth H. Essential Oil Composition of Zingiberaceae Species from Mauritius. J. Essent. Oil Res. 2002;14:271–273. doi: 10.1080/10412905.2002.9699850. [DOI] [Google Scholar]
- 15.Adams R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th Edition. Illinois USA Allured Publ. Corp. Carol Stream. 2007:804–806. [Google Scholar]
- 16.Rodrigues T.L.M., Castro G.L.S., Viana R.G., Gurgel E.S.C., Silva S.G., de Oliveira M.S., Andrade E.H. Physiological Performance and Chemical Compositions of the Eryngium Foetidum L. (Apiaceae) Essential Oil Cultivated with Different Fertilizer Sources. Nat. Prod. Res. 2020;34:1–5. doi: 10.1080/14786419.2020.1795653. [DOI] [PubMed] [Google Scholar]
- 17.Mesquita K.D.S.M., Feitosa B.D.S., Cruz J.N., Ferreira O.O., Franco C.D.J.P., Cascaes M.M., Oliveira M.S.D., Andrade E.H.D.A. Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia Circinnata Link Var. Circinnata. (Piperaceae) in Artemia Salina Leach. Molecules. 2021;26:7359. doi: 10.3390/molecules26237359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Silva Júnior O.S., Franco C.D.J.P., de Moraes Â.A.B., Pastore M., Cascaes M.M., do Nascimento L.D., de Oliveira M.S., de Aguiar Andrade E.H. Chemical Variability of Volatile Concentrate from Two Ipomoea L. Species within a Seasonal Gradient. Nat. Prod. Res. 2022:1–8. doi: 10.1080/14786419.2022.2070618. [DOI] [PubMed] [Google Scholar]
- 19.Silva-Aguayo G., Aguilar-Marcelino L., Cuevas-Padilla E., Loyola-Zapata P., Rodríguez-Maciel J.C., Castañeda-Ramírez G., Figueroa-Cares I. Essential Oil of Peumus Boldus Molina against the Nematode Haemonchus Contortus (L3) and Three Stored Cereal Insect Pests. Chil. J. Agric. Res. 2021;81:390–397. doi: 10.4067/S0718-58392021000300390. [DOI] [Google Scholar]
- 20.Kalsi P.S., Bajaj K.L., Mahajan R., Singh P. Nematicidal Activity of Some Sesquiterpenoids against Rootknot Nematode (Meloidogyne Incognita) Nematologica. 1986;32:119–123. doi: 10.1163/187529286X00101. [DOI] [Google Scholar]
- 21.Ntalli N.G., Ferrari F., Giannakou I., Menkissoglu-Spiroudi U. Phytochemistry and Nematicidal Activity of the Essential Oils from 8 Greek Lamiaceae Aromatic Plants and 13 Terpene Components. J. Agric. Food Chem. 2010;58:7856–7863. doi: 10.1021/jf100797m. [DOI] [PubMed] [Google Scholar]
- 22.Lahlou M. Methods to Study the Phytochemistry and Bioactivity of Essential Oils. Phyther. Res. 2004;18:435–448. doi: 10.1002/ptr.1465. [DOI] [PubMed] [Google Scholar]
- 23.Amzouar S., Boughdad A., Maatoui A., Allam L. Comparison of the Chemical Composition and the Insecticidal Activity of Essential Oils of Mentha Pulegium L. Collected from Two Different Regions of Morocco, against Bruchus Rufimanus (Bohman) (Coleoptera: Chrysomelidae) Adv. Environ. Biol. 2016;10:199–207. [Google Scholar]
- 24.Kumar R., Kumar R., Prakash O. Evaluation of In-Vitro Herbicidal Efficacy of Essential Oil and Chloroform Extract of Limnophila Indica. Pharma Innov. 2021;10:386–390. [Google Scholar]
- 25.Rawat A., Thapa P., Prakash O., Kumar R., Pant A.K., Srivastava R.M., Rawat D.S. Chemical Composition, Herbicidal, Antifeedant and Cytotoxic Activity of Hedychium Spicatum Sm.: A Zingiberaceous Herb. Trends Phytochem. Res. 2019;43:123–136. [Google Scholar]
- 26.Joshi A., Prakash O., Pant A.K., Kumar R., Szczepaniak L., Kucharska-Ambrożej K. Methyl Eugenol, 1,8-Cineole and Nerolidol Rich Essential Oils with Their Biological Activities from Three Melaleuca Species Growing in Tarai Region of North India. Brazilian Arch. Biol. Technol. 2021;64 doi: 10.1590/1678-4324-2021210186. [DOI] [Google Scholar]
- 27.Ray A., Jena S., Dash B., Kar B., Halder T., Chatterjee T., Ghosh B., Panda P.C., Nayak S., Mahapatra N. Chemical Diversity, Antioxidant and Antimicrobial Activities of the Essential Oils from Indian Populations of Hedychium Coronarium Koen. Ind. Crops Prod. 2018;112:353–362. doi: 10.1016/j.indcrop.2017.12.033. [DOI] [Google Scholar]
- 28.Spengler G., Gajdács M., Donadu M.G., Usai M., Marchetti M., Ferrari M., Mazzarello V., Zanetti S., Nagy F., Kovács R. Evaluation of the Antimicrobial and Antivirulent Potential of Essential Oils Isolated from Juniperus Oxycedrus L. Ssp. Macrocarpa Aerial Parts. Microorganisms. 2022;10:758. doi: 10.3390/microorganisms10040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L., Long N., Qiu M., Liu Y., Sun F., Dai M. The Inhibitory Efficiencies of Geraniol as an Anti-Inflammatory, Antioxidant, and Antibacterial, Natural Agent Against Methicillin-Resistant Staphylococcus Aureus Infection in Vivo. Infect. Drug Resist. 2021;14:2991–3000. doi: 10.2147/IDR.S318989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.do Vale J.P.C., de Freitas Ribeiro L.H., de Vasconcelos M.A., Sá-Firmino N.C., Pereira A.L., do Nascimento M.F., Rodrigues T.H.S., da Silva P.T., de Sousa K.C., da Silva R.B., et al. Chemical Composition, Antioxidant, Antimicrobial and Antibiofilm Activities of Vitex Gardneriana Schauer Leaves’s Essential Oil. Microb. Pathog. 2019;135:103608. doi: 10.1016/j.micpath.2019.103608. [DOI] [PubMed] [Google Scholar]
- 31.Tong S.Y.C., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Näsström E., Vu Thieu N.T., Dongol S., Karkey A., Voong Vinh P., Ha Thanh T., Johansson A., Arjyal A., Thwaites G., Dolecek C., et al. Salmonella Typhi and Salmonella Paratyphi A Elaborate Distinct Systemic Metabolite Signatures during Enteric Fever. Elife. 2014;3:e03100. doi: 10.7554/eLife.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur R., Oh H., Zhang D., Park S.-G., Seo J., Koblansky A., Hayden M.S., Ghosh S. A Mouse Model of Salmonella Typhi Infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epand R.M., Walker C., Epand R.F., Magarvey N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta-Biomembr. 2016;1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Tao N., Liu Y., Zhang M. Chemical Composition and Antimicrobial Activities of Essential Oil from the Peel of Bingtang Sweet Orange ( Citrus Sinensis Osbeck) Int. J. Food Sci. Technol. 2009;44:1281–1285. doi: 10.1111/j.1365-2621.2009.01947.x. [DOI] [Google Scholar]
- 37.Sabulal B., George V., Dan M., Pradeep N.S. Chemical Composition and Antimicrobial Activities of the Essential Oils from the Rhizomes of Four Hedychium Species from South India. J. Essent. Oil Res. 2007;19:93–97. doi: 10.1080/10412905.2007.9699237. [DOI] [Google Scholar]
- 38.Clevenger J.F. Apparatus for the Determination of Volatile Oil. J. Am. Pharm. Assoc. 1928;17:345–349. doi: 10.1002/jps.3080170407. [DOI] [Google Scholar]
- 39.Franco C.D.J.P., Ferreira O.O., Antônio Barbosa de Moraes Â., Varela E.L.P., Nascimento L.D.D., Percário S., de Oliveira M.S., Andrade E.H.D.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia Patrisii Vahl, E. Punicifolia (Kunth) DC., and Myrcia Tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules. 2021;26:3292. doi: 10.3390/molecules26113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira O.O., da Cruz J.N., Franco C.D.J.P., Silva S.G., da Costa W.A., de Oliveira M.S., Andrade E.H.D.A. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia Eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules. 2020;25:783. doi: 10.3390/molecules25040783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenback J.D. Diagnostic Characters Useful in the Identification of the Four Most Common Species of Root-Knot Nematodes (Meloidogyne Spp.) In: Sasser J.N., Carter C., editors. An Advanced Treatise on Meloidogyne. University Graphics; Raleigh, NC, USA: 1985. pp. 95–112. [Google Scholar]
- 42.Latif R., Abbasi M.W., Zaki M.J., Khan D. Nemticidal Activity of Bark of Some Tree Species against Root-Knot Nematode Meloidogyne Javanica (Treub) Chitwood. FUUAST J. Biol. 2014;4:247–251. [Google Scholar]
- 43.Dawidar A.E.M., Mortada M.M., Raghib H.M., Abdel-Mogib M. Molluscicidal Activity of Balanites Aegyptiaca against Monacha Cartusiana. Pharm. Biol. 2012;50:1326–1329. doi: 10.3109/13880209.2012.674950. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira Barros A., Paulo Campos V., Lopes de Paula L., Alaís Pedroso L., de Jesus Silva F., Carlos Pereira da Silva J., Ferreira de Oliveira D., Humberto Silva G. The Role of Cinnamomum Zeylanicum Essential Oil, (E)-Cinnamaldehyde and (E)-Cinnamaldehyde Oxime in the Control of Meloidogyne Incognita. J. Phytopathol. 2021;169:229–238. doi: 10.1111/jph.12979. [DOI] [Google Scholar]
- 45.Tabashnic T., Cushing N.L. Leaf Residue vs. Topical Bioassays for Assessing Insecticide Resistance in the Diamond-Back Moth, Plutella Xylostella L. FAO Plant Prot. Bull. 1987;35:12. [Google Scholar]
- 46.Eloff J. Which Extractant Should Be Used for the Screening and Isolation of Antimicrobial Components from Plants? J. Ethnopharmacol. 1998;60:1–8. doi: 10.1016/S0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 47.Sahu A., Devkota A. Allelopathic Effects of Aqueous Extract of Leaves of Mikania Micrantha H.B.K. on Seed Germination and Seedling Growht of Oryza Sativa L. and Raphanus Sativus L. Sci. World. 2013;11:91–93. doi: 10.3126/sw.v11i11.8559. [DOI] [Google Scholar]
- 48.Batista C.D.C.R., de Oliveira M.S., Araújo M.E., Rodrigues A.M., Botelho J.R.S., da Silva Souza Filho A.P., Machado N.T., Junior R.N.C. Supercritical CO2 Extraction of Açaí (Euterpe Oleracea) Berry Oil: Global Yield, Fatty Acids, Allelopathic Activities, and Determination of Phenolic and Anthocyanins Total Compounds in the Residual Pulp. J. Supercrit. Fluids. 2015;107:364–369. doi: 10.1016/j.supflu.2015.10.006. [DOI] [Google Scholar]
- 49.de Oliveira M.S., da Costa W.A., Pereira D.S., Botelho J.R.S., de Alencar Menezes T.O., de Aguiar Andrade E.H., da Silva S.H.M., da Silva Sousa Filho A.P., de Carvalho R.N. Chemical Composition and Phytotoxic Activity of Clove (Syzygium Aromaticum) Essential Oil Obtained with Supercritical CO2. J. Supercrit. Fluids. 2016;118:185–193. doi: 10.1016/j.supflu.2016.08.010. [DOI] [Google Scholar]
- 50.Gurgel E.S.C., de Oliveira M.S., Souza M.C., da Silva S.G., de Mendonça M.S., da Silva Souza Filho A.P. Chemical Compositions and Herbicidal (Phytotoxic) Activity of Essential Oils of Three Copaifera Species (Leguminosae-Caesalpinoideae) from Amazon-Brazil. Ind. Crops Prod. 2019;142 doi: 10.1016/j.indcrop.2019.111850. [DOI] [Google Scholar]
- 51.Goswami S., Kanyal J., Prakash O., Kumar R., Rawat D.S., Srivastava R.M., Pant A.K. Chemical Composition, Antioxidant, Antifungal and Antifeedant Activity of the Salvia Reflexa Hornem. Essential Oil. Asian J. Appl. Sci. 2019;12:185–191. doi: 10.3923/ajaps.2019.185.191. [DOI] [Google Scholar]
- 52.(CLSI) Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Volume 2. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2006. Approved Standard—Seventh Edition. [Google Scholar]
- 53.Ferreira O.O., da Silva S.H.M., de Oliveira M.S., de Andrade E.H.A. Chemical Composition and Antifungal Activity of Myrcia Multiflora and Eugenia Florida Essential Oils. Molecules. 2021;26:7259. doi: 10.3390/molecules26237259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mesomo M.C., Corazza M.L., Ndiaye P.M., Dalla Santa O.R., Cardozo L., Scheer A.D.P. Supercritical CO2 extracts and Essential Oil of Ginger (Zingiber Officinale R.): Chemical Composition and Antibacterial Activity. J. Supercrit. Fluids. 2013;80:44–49. doi: 10.1016/j.supflu.2013.03.031. [DOI] [Google Scholar]
- 55.Filimonov D.A., Lagunin A.A., Gloriozova T.A., Rudik A.V., Druzhilovskii D.S., Pogodin P.V., Poroikov V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014;50:444–457. doi: 10.1007/s10593-014-1496-1. [DOI] [Google Scholar]
- 56.Concato J., Hartigan J.A. P Values: From Suggestion to Superstition. J. Investig. Med. 2016;64:1166–1171. doi: 10.1136/jim-2016-000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.