Abstract
Viruses are ubiquitous intracellular genetic parasites that heavily rely on the infected cell to complete their replication life cycle. This dependency on the host machinery forces viruses to modulate a variety of cellular processes including cell survival and cell death. Viruses are known to activate and block almost all types of programmed cell death (PCD) known so far. Modulating PCD in infected hosts has a variety of direct and indirect effects on viral pathogenesis and antiviral immunity. The mechanisms leading to apoptosis following virus infection is widely studied, but several modalities of PCD, including necroptosis, pyroptosis, ferroptosis, and paraptosis, are relatively understudied. In this review, we cover the mechanisms by which viruses activate and inhibit PCDs and suggest perspectives on how these affect viral pathogenesis and immunity.
Introduction
Programmed cell death (PCD) plays a fundamental role in organismal development, infectious disease pathogenesis, immune regulation, and tissue homeostasis. Dysregulated PCD is associated with a wide variety of diseases, including immunological and developmental disorders, neurodegeneration, and cancer. Hence, understanding the mechanisms of PCD regulation is essential to devise strategies to manipulate PCD to manage several disorders.
PCD is initiated after cells sense genotoxic, metabolic, and infectious insults. During virus infection, sensing of viral-derived nucleic acids can initiate signaling to activate PCD. To a varying degree, viruses utilize the infected cell transcription and translation machinery to complete their life cycle; thus, produce nucleic acid replication intermediates that are identified as non-self by a variety of host-encoded nucleic acid sensors [1]. Detection of nucleic acids activates a plethora of interconnected antiviral pathways including activation of the interferon (IFN)-mediated antiviral state, translational arrest, and PCD. In a concerted approach, these processes restrict virus propagation until the development of virus-specific adaptive immunity [1]. Recently, it has been shown that emission of immunomodulatory secretomes after PCD shapes the development of adaptive immunity. There are several elegant reviews that document the immunogenicity of PCD [2].
While the mechanisms by which pathogen-associated molecular pattern (PAMP) sensing activates antiviral state have been extensively studied, so much remains unknown about how viral sensing regulates new modalities of PCD. Virus-infected cells activate nucleic acid–mediated antiviral responses along with a variety of endoplasmic reticulum (ER) [3], mitochondrial [4] and oxidative stress [5], and translational arrest that can directly or indirectly influence the induction of PCD [6]. In addition, several virus families encode proteins to inhibit the function of nucleic acid sensors [7], various antiviral factors such as IFN-stimulated genes (ISGs), and even proteins involved in the initiation and execution of PCD [8]. Hence, it is still unknown what types of nucleic acid receptors are directly involved in the induction of newly emerging PCD types. In this review, we provide literature on the mechanisms by which viruses activate and inhibit PCD and how that affects viral pathogenesis and host immunity.
Sensing of viral-associated nucleic acids
Availability, localization, and structure of viral-associated nucleic acids in infected cells provide host-encoded sensors to selectively identify non-self-nucleic acids. Viral-associated nucleic acids are sensed by cell surface–expressed (Toll-like receptor 3 (TLR3)), endosome-localized (TLR3, TLR7, TLR8, TLR9), and cytoplasmic sensors (retinoic acid–inducible gene I (RIG-I)/melanoma differentiation–associated gene 5 (MDA5)/nucleotide-binding oligomerization domain-like receptors (NLRPs) such as NLRP1 [9], NLRP6-DHX15 [10], NLRP9-DHX9 [11]/cyclic GMP–AMP synthetase (cGAS) stimulator of IFN genes (STING), absent in melanoma 2 (AIM2). Viral RNA is mainly recognized by TLR3 and RIG-I-like helicases (RLHs) (RIG-I/MDA5), whereas DNA is sensed by cGAS, AIM2-like receptors (ALRs), and TLR9. While most RNA sensors are well established, numerous DNA receptors continue to be discovered, including the human DNA sensor DNA-PK that functions independent of STING [12]. Species and cell-specific effects may account for the plethora of DNA sensors postulated, for instance, deletion of the ALR locus did not affect the IFN response to cytosolic DNA, dispelling the role of ALR in mice [13].
Most nucleic acid receptors, including the TLR family, the RLHs, and cGAS-STING, directly or indirectly induce transcription factors, including IFN-regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), which up-regulate the expression of antiviral effector proteins, chemokines, and cytokines. RLHs and STING-cGAS are major nucleic acid sensors that activate phosphorylation-induced oligomerization of adaptor proteins mitochondrial antiviral-signaling (MAVS) and STING, respectively. Oligomerized MAVS and STING are scaffolding proteins closely associated with the IkB kinase (IKK)-related kinases TANK-binding kinase (TBK1) and IKK-e, which are responsible for phosphorylation of IRF3/7 [14–16], and IKK-b and IKK-a, which phosphorylate NF-kB [17]. IRF3/7 and NF-kB are transcription factors that bind regions in the promoter to up-regulate the expression of IFNs and proinflammatory cytokines, respectively [18,19].
Binding of secreted IFN-a/b to IFN receptors activates the associated Janus kinases (JAKs) tyrosine kinase 2 (TYK2) and JAK1, which phosphorylate both signal transducer and activator of transcription 1 (STAT1) and STAT2. STAT1, STAT2, and IRF9 form the IFN-stimulated gene factor 3 (ISGF3) complex that translocates to the nucleus to bind IFN-stimulated response elements (ISREs) and promote the expression of hundreds of ISGs. ISGs have a variety of functions including antiviral defense and initiation of adaptive immunity and collectively create a nonpermissive antiviral state [20].
Although not a focus of this review, there are also nucleic acid receptors with direct antiviral activity, including double-stranded RNA (dsRNA)-activated protein kinase R, 2′5′oligoadenylate synthetase 1 (OAS1) and adenosine deaminase acting on RNA 1 (ADAR1). OAS1 and ADAR1 recognize foreign nucleic acids to directly act on viral RNA by either inhibiting translation or degradation of the target RNA.
1. Mechanisms of programmed cell death activation after virus infection
In addition to the secretion of proteins with direct antiviral effect, nucleic acid sensing pathways involving RLHs, TLRs, Z-DNA/RNA binding protein 1 (ZBP1)/DNA-dependent activator of IFN-regulatory factors (DAI), NLRPs, and cGAS-STING play a role in the activation of apoptosis, necroptosis, and ferroptosis. Overall, the different types of PCD activated during virus infection involve distinct signaling pathways, some exclusively relying on caspases (apoptosis and pyroptosis) and others depending on kinases (necroptosis) and lipid peroxidation sensors (ferroptosis). In the following sections, we present a concise summary of how viruses activate the various PCD pathways.
Mechanisms of apoptosis induction during virus infection
Apoptosis is one of the most researched viral-induced PCD. In virus-infected cells, apoptosis can be initiated via the extrinsic or intrinsic pathway (Fig 1; [21]). In the extrinsic pathway, apoptosis begins after activation of the death domain receptors by the tumor necrosis factor (TNF) family of proteins including Fas activated by the FasL ligand, TNF-related apoptosis-inducing ligand (TRAIL) receptors 1 and 2 activated by TRAIL, and TNF receptor 1 (TNF-R1) activated by TNF [21,22]. Ligand binding to Fas and TRAIL receptors leads to oligomerization of the death receptors and assembly of the death-inducing signaling complex (DISC) consisting of death receptor, the adaptor molecule FADD (FAS-associated with a death domain), procaspase-8, procaspase-10, and cellular FADD-like interleukin-1β (IL-1β)-converting enzyme (FLICE)-like inhibitory protein (c-FLIP). c-FLIP has 2 isoforms, long and short, which control the activation of caspase cascade that emanates from caspase-8-mediated apoptotic and nonapoptotic signaling. In contrast to Fas and TRAIL death receptors, TNF-R1-mediated apoptotic signaling is complex and recruits TNF-R-associated protein with death domain (TRADD) as an adaptor protein, which subsequently recruits FADD, TNF-associated factor-2 (TRAF-2), receptor-interacting protein (RIP), and RIP-associated IL-1β-converting enzyme homolog (ICH-1)/cell death protein-3 (CED-3)-homologous protein with a death domain (RAIDD). FADD then binds and activates the extrinsic apoptosis initiator caspase-8, which subsequently activates the effector caspases-3/6/7 [23].
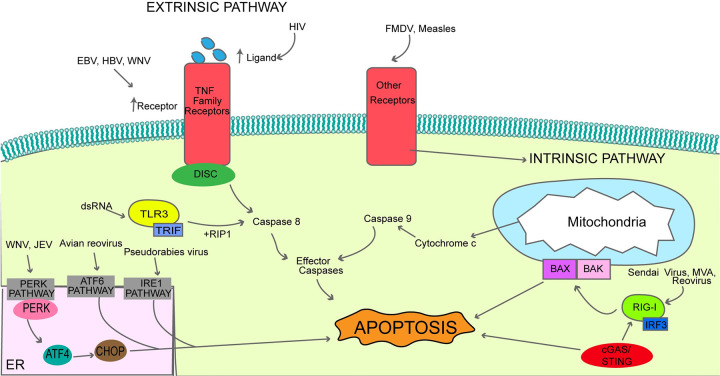
Fig 1. A summary of the major components of the extrinsic and intrinsic pathways of apoptosis induction following viral infection.
In response to viral infection, the extrinsic pathway is initiated by death receptors following stimulation by the TNF proteins, resulting in downstream signaling and caspase-8 activation. The intrinsic pathway of apoptosis arises from proapoptotic stimuli, subsequently initiating mitochondrial membrane permeabilization and activation of caspase-9. Both pathways converge at the terminal activation of caspase-3 that executes apoptosis. ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; BAK, BCL-2 antagonist or killer; BAX, BCL-2-associated X protein; cGAS/STING, cyclic GMP–AMP synthetase/stimulator of IFN genes; CHOP, CCAAT-enhancer-binding protein homologous protein; DISC, death-inducing signaling complex; EBV, Epstein–Barr virus; ER, endoplasmic reticulum; FMDV, Foot-and-mouth disease virus; HBV, hepatitis B virus; IRE1, inositol-requiring enzyme 1; IRF3, IFN-regulatory factor 3; JEV, Japanese encephalitis virus; MVA, modified vaccinia virus; PERK, PKR-like ER kinase; RIG-I, retinoic acid-inducible gene I; TLR3, Toll-like receptor 3; TNF, tumor necrosis factor; TRIF, Toll/IL-1 receptor domain-containing adapter inducing IFN-beta; WNV, West Nile virus.
In the intrinsic pathway, several proapoptotic stimuli result in mitochondrial outer membrane permeabilization [24,25]. Mitochondrial permeabilization permits the release of mitochondrial intermembrane space proteins, such as cytochrome c into the cytosol [24]. This series of events activates the intrinsic apoptosis initiator caspase-9 and downstream effector caspases that are the same as the extrinsic pathway [24]. The B-cell lymphoma 2 (BCL-2) family of proteins regulate the intrinsic pathway at the level of the mitochondria [26]. Some of the BCL-2 family of proteins such as BCL-2 and myeloid leukemia 1 (MCL-1) are antiapoptotic, while others including BCL-2-associated X protein (BAX), BCL-2 antagonist or killer (BAK), and BCL-2 homology domain (BH3)-interacting domain death agonist (BID) are proapoptotic [24]. The antiapoptotic proteins play a role to inhibit proapoptotic proteins BAX/BAK by sequestering BH3-only proteins such as BID [27].
Many viruses can induce apoptosis through one or both of the apoptosis pathways. Receptors are crucial components to activate extrinsic pathway of apoptosis. Some virus families can increase apoptosis by increasing the expression of extrinsic pathway receptors such as Fas and their ligands on the cell surface. Epstein–Barr virus (EBV)-infected B cells express higher levels of the Fas receptor on their surface [28]. Both hepatitis B [29] and West Nile virus infections [30] increase the expression of death receptor–associated genes such as DR5 to activate death receptor–mediated apoptotic pathway. In addition, human immunodeficiency virus (HIV)-infected patients have reduced memory B cells by undergoing the extrinsic pathway of apoptosis involving TRAIL [31]. Vesicular stomatitis virus (VSV) can induce the intrinsic pathway of apoptosis when expressing wild-type viral matrix (M) protein or the extrinsic pathway of apoptosis when expressing a mutant version of the M protein [32]. In the subsequent sections, we have summarized the variety of stages of virus replication that leads to apoptosis.
1.1 Apoptotic induction at the interface of virus–host receptor interaction
Apoptosis can be initiated when a virus particle binds to its receptor on the cell surface prior to initiation of viral genome replication. Measles virus activates the intrinsic pathway of apoptosis when the viral hemagglutinin binds to the cellular signaling lymphocytic activation molecule (SLAM) receptor [33]. Similarly, foot-and-mouth disease virus induces apoptosis via binding to the integrin receptor on immature dendritic cells [34].
1.2 Apoptosis induction after sensing of viral nucleic acid replication intermediates
Many viruses activate apoptosis after starting genome replication in a susceptible cell. After uncoating, viral RNA genomes are detected by cytosolic RIG-I-like helicases (RLH) such as RIG-I, which typically activate IRF3 [35]. IRF3 is well characterized for its function as a transcription factor to stimulate the expression of IFN-mediated antiviral genes [35]. In some instances, IRF3 is also involved in the induction of apoptosis through a pathway commonly referred as the RLR-induced IRF3-mediated pathway of apoptosis (RIPA) [35]. Successful RIPA activation requires IRF3 to interact with BAX via its BH3 and to undergo translocation to the mitochondria, leading to the activation of apoptosis. This process requires additional proteins that are distinct from the antiviral transcription pathway [35]. Activation of the RIPA pathway is reliant on polyubiquitination of 2 specific lysine residues on the IRF3 protein [36]. Additionally, activation of IRF3 through RIG-I can also lead to the transcription of several proapoptotic proteins. Sendai virus, the Ankara strain of modified vaccinia virus (MVA), and reovirus rely on RIG-I-like helicases and IRF3 to activate apoptosis [37–39].
TLR3 is another RNA sensor that activates IRF3. TLR3 signaling and the associated signaling adaptor protein Toll/IL-1 receptor domain-containing adapter inducing IFN-beta (TRIF) can trigger apoptosis through the activation of caspase-8, which also requires receptor-interacting serine/threonine-protein kinase 1 (RIPK-1; [40,41]).
During DNA virus infection, the cytoplasmic DNA sensors cGas/STING can also mediate apoptosis induction. Infection with HSV-1 shows STING-dependent apoptosis at high doses [42]. During human T cell leukemia virus type 1 (HTLV-1) infection, inhibition of reverse transcription creates RNA intermediates that were sensed by STING triggering the IRF3-BAX complex and induction of apoptosis [43]. Without virus infection spontaneous activation of STING after gain of function mutation (N153S) can also activate ER stress and apoptosis [44].
1.3 Apoptosis induction after viral-induced endoplasmic stress and unfolded protein response
Some viruses can impose ER stress through an increase in viral protein synthesis, which translates into the induction of the unfolded protein response (UPR) and, consequently, apoptosis [45]. Depending on the type of virus infection, 3 major UPR response pathways can be activated, namely, PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) [45]. For example, upon infection with Zika virus, trophoblasts activate ER stress response and apoptosis [46]. West Nile virus induces the PERK pathway, which leads to the phosphorylation of eIF2ɑ and the activation of ATF4 [45]. ER stress activates ATF4 protein that subsequently stimulates the expression of the CCAAT-enhancer-binding protein homologous protein (CHOP) to initiate apoptosis [45]. Other viruses such as the Japanese encephalitis virus activate the PERK pathway, as the NS4B protein of Japanese encephalitis virus promotes dimerization of PERK [47]. Pseudorabies virus infection also activates the PERK-ATF4 and the IRE1-mediated apoptosis [48]. During avian reovirus infection, induction of the ATF6 pathway is also crucial for UPR-mediated apoptosis [49].
1.4 Additional miscellaneous pathways leading to viral-mediated apoptosis induction
Viruses can also initiate or enhance infected cell apoptosis by altering cellular proteins and processes such as posttranslational modifications. Influenza virus can alter the functional activities of several host proteins to induce apoptosis. For example, the NS1 protein of influenza A virus (IAV) interacts with heat shock protein Hsp90 mediating the activation of caspase-9 and 3 [50]. Additionally, during influenza virus infection, the cellular protein BAD is phosphorylated to activate the intrinsic apoptosis pathway [51]. Influenza virus [52] and transmissible gastroenteritis virus [53] can activate apoptosis by facilitating the translocation of apoptosis-inducing factor (AIF) to the nucleus.
HIV is another example where a viral protein can induce apoptosis in a variety of ways. First, the Tat protein has been found to induce apoptosis by altering microtubule dynamics leading to the translocation of proapoptotic protein B cell lymphoma 2–interacting mediator of cell death (BIM) to the mitochondria, inducing mitochondrial membrane permeability and inhibiting cytochrome c oxidase [54,55]. In addition, the protease of HIV can cleave caspase-8 into Casp8p41, which engages BAX/BAK proteins to depolarize the mitochondria and activate caspase-9 [56].
The HPV18 E2 protein can trigger apoptosis by binding to the death effector domain of caspase-8, which induces caspase-8 activation after its oligomerization [57]. In HPV16, the E2 protein leads to the hyperactivation of caspase-8 by suppressing the function of the antiapoptotic protein c-FLIP making the infected cell hypersensitive to extrinsic apoptotic signaling [58]. Hepatitis C virus can initiate apoptosis through its NS4A and NS3A proteins that up-regulate BAX expression to initiate the intrinsic apoptosis [59].
2. Mechanisms of necroptosis induction during virus infection
Necroptosis, a regulated form of necrosis, is often activated after engagement of Toll-like receptors (TLRs), death receptors, and the intracellular sensors Z-DNA/RNA binding protein 1 (ZBP1)/DAI [60,61]. Necroptosis is caspase independent and relies on TNF and Z-DNA/RNA to initiate the signaling cascade and kinases RIPK1/3 and mixed lineage kinase domain-like pseudokinase (MLKL) for execution (Fig 2). The morphological changes associated with necroptosis are distinct from apoptosis; these include organelle swelling and cell lysis [62]. In addition, during necroptosis, RIPK1/3 heterodimers form a complex that activates NF-kB-mediated proinflammatory response to allow the emission of cytokines/chemokines [63–65]. Furthermore, damage-associated molecular patterns (DAMPs), such as ATP and HMGB1, are also profusely secreted during necroptosis [66,67].
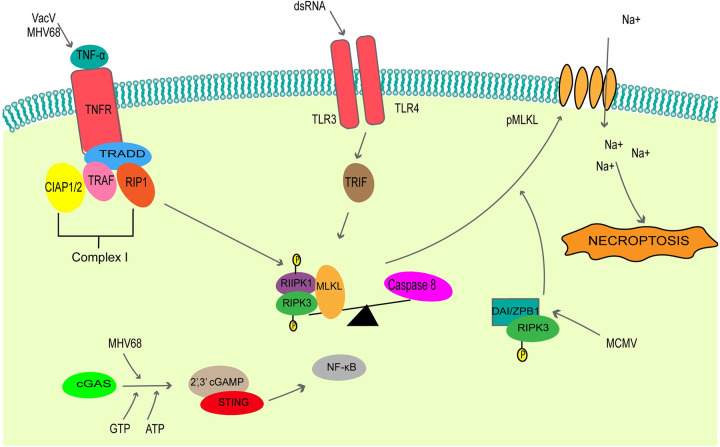
Fig 2. A summary of the major components of the necroptosis induction pathway following viral infection.
Necroptosis is activated during viral infection through Toll-like receptor and TNF receptor activation, in addition to viral nucleic acid recognition by the intracellular sensors DAI/ZPB1. Necroptotic stimuli converge on the activation of the RIPK1/RIPK3 necrosome complex, in turn phosphorylating the effector protein MLKL to initiate cell lysis. cGAS, cyclic GMP–AMP synthetase; CIAP1/2, cellular inhibitor of apoptosis 1/2; DAI/ZPB1, DNA-dependent activator of IFN-regulatory factors/Z-DNA/RNA binding protein 1; dsRNA, double-stranded RNA; MCMV, murine cytomegalovirus; MLKL, mixed lineage kinase domain-like pseudokinase; NF-κB, nuclear factor-κB; pMLKL, phosphorylated MLKL; RIF, Toll/IL-1 receptor domain-containing adapter-inducing IFN-beta; RIP1, receptor-interacting protein 1; RIPK1, receptor-interacting serine/threonine-protein kinase 1; RIPK3, receptor-interacting serine/threonine-protein kinase 3; STING, stimulator of IFN genes; TLR3, Toll-like receptor 3; TLR4, Toll-like receptor 4; TNF, tumor necrosis factor; TNF-α, tumor necrosis factor alpha; TNFR, tumor necrosis factor receptor; TRADD, TNF-R-associated protein with death domain; TRAF, TNF-associated factor; 2′3′ cGAMP, 2′3′ cyclic GMP-AMP.
2.1 Canonical (indirect) activation of necroptosis
The initiation of necroptosis through TNF, also known as the indirect necrosome formation and canonical pathway, leads to the formation of TNF-receptor-associated complex, which independently recruits receptor-interacting protein I (RIPK1) and TRADD through their death domains (DDs) [68]. The expression of TNF is regulated through various mechanisms, including virus infection. The detailed mechanisms for the activation of necroptosis after TNF stimulation is extensively reviewed elsewhere [65,69,70]. Vaccinia virus (VACV) [71] and murine gamma herpesvirus 68 (MHV68; [72]) infected cells utilize TNF-mediated necroptosis. The induction of necroptosis after MHV68 infection can also take place in a STING-dependent manner. The presence of cytosolic DNA in MHV68 [73] and VACV [74] infected cells can trigger the cGAS-STING pathway. cGAS interacts with cytosolic DNA to undergo an active site conformational change for the synthesis of cyclic GMP-AMP (cGAMP) from GTP and ATP [75,76]. cGAMP is a second messenger that binds and activates STING, leading to the activation of the NF-kB pathway, which stimulates TNF expression [77–80]. Secreted TNF binds to the TNFR to induce a signaling cascade that leads to an inflammatory response and necroptosis.
2.2 Direct activation of necroptosis
Direct activation of necroptosis is initiated by the ZBP1/DAI sensors or TRIF adaptor protein [81]. Z-DNA and Z-RNA, nucleic acids with a left-handed conformation of the DNA and RNA structure, are found in actively transcribed regions of the genome and can be bound by Z-DNA binding proteins [82–85]. The Z-DNA binding proteins, upon interaction with Z-DNA, are thought to have roles in gene expression, DNA processing, tumor development, and viral pathogenicity [86–88]. ZBP1/DAI can recognize Z binding domains (ZBDs) on Z-DNAs and Z-RNAs in the cell [88].
During murine cytomegalovirus (MCMV) infection, induction of necroptosis required RNA synthesis but not viral replication since inhibition of RNA transcription but not of viral DNA replication prevented ZBP1-dependent necroptosis [89]. In addition, the binding of ZBDs to ZBP1/DAI are required to induce necroptosis [89]. During influenza A infection, the Z-RNAs generated during virus replication are sensed by ZBP1/DAI [90]. Sensing of Z-RNA leads to dimerization of ZBP1/DAI to interact with RIPK3 via its RHIM domain to initiate necroptosis [91,92].
TLR are activated by several pathogen stimuli to drive a variety of PCD. TLR3 and TLR4 signal through an adaptor protein TRIF when stimulated by either dsRNA [93]. When caspase-8 is inhibited during this stimulation, TLR3 and TLR4 activate necroptosis through TRIF [61]. TRIF is a RHIM-containing protein that forms a noncanonical necrosome by activating RIPK3 [61].
2.3. Execution of necroptosis downstream of RIPK3 activation
Most of the necroptotic pathways converge at RIPK3 recruitment, phosphorylation, and activation. RIPK1 is required for RIPK3 auto-phosphorylation and cross-phosphorylation during indirect canonical necrosome formation [94]. In the noncanonical pathway, the interaction of RIPK3 with DAI or TRIF causes RIPK3 activation and auto-phosphorylation [94]. Activated RIPK3 phosphorylates MLKL, inducing a conformational change that exposes 4 helical bundle domains, which causes oligomerization [95]. MLKL is hypothesized to act in 2 ways. It is suggested that phosphorylated MLKL (P-MLKL) translocates to the plasma membrane via interactions with the amino-terminal of phosphatidylinositol (PIP) and induces membrane rupture [96]. It is also speculated that P-MLKL associates with cation channels, leading to ion influx [97].
3. Mechanisms of pyroptosis induction during virus infection
Pyroptosis plays a crucial role in regulating host immunity against viruses. It is a form of inflammatory cell death that can be initiated after recognition of conserved PAMPs by host pathogen recognition receptors (PRRs) (Fig 3). The molecular response of host cells to pyroptosis is characterized by pore formation, plasma membrane rupture, and the activation and release of proinflammatory cytokines.
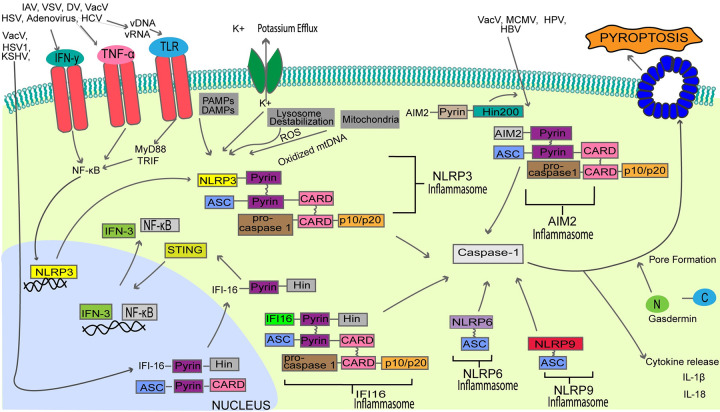
Fig 3. A summary of the major components of the pyroptosis induction pathway following viral infection.
Viral nucleic acids can stimulate pyroptosis through the activation of the NLRP3/6/9 inflammasome, AIM2 inflammasome, and IFI16 inflammasome. Inflammasome activation stimulates the recruitment and activation of pro-caspase-1, resulting in the downstream cleavage of gasdermin proteins into C-terminal and N-terminal portions. The N-terminal gasdermin portion catalyzes pore formation and proinflammatory cytokine release. AIM2, absent in melanoma 2; ASC, Apoptosis-associated speck-like protein containing a CARD; CARD, caspase activation and recruitment domain; DAMP, damage-associated molecular pattern; DV, dengue virus; HBV, hepatitis B virus; HCV, hepatitis c virus; HPV, human papillomavirus; HSV, herpes simplex virus; IAV, influenza A virus; IFI16, IFN-gamma-inducible protein 16; IFN-3, interferon-3; IFN-y, interferon gamma; KSHV, Kaposi sarcoma–associated herpesvirus; MCMV, murine cytomegalovirus; mtDNA, mitochondrial DNA; NF-κB, nuclear factor-κB; NLRP3, Nod-like receptor family pyrin domain containing 3; PAMP, pathogen-associated molecular pattern; ROS, reactive oxygen species; STING, stimulator of IFN genes; TLR, Toll-like receptor; TNF-α, tumor necrosis factor alpha; TRIF, Toll/IL-1 receptor domain-containing adapter inducing IFN-beta; VACV, vaccinia virus; VSV, vesicular stomatitis virus.
A critical step in pyroptosis activation is gasdermin cleavage [98]. Gasdermin proteins are cleaved by caspases into N- and C-terminal fragments. The N-terminal portion of gasdermin functions as the pore-forming domain by inserting itself into the lipid bilayer of the cytoplasm and oligomerizing to form pores in the cell membrane [99]. Pore formation induces osmotic imbalance and ultimately results in cell lysis [99,100]. Like necroptosis, pyroptosis results in the release of a variety of danger molecules such as ATP, HMGB1, and cytokines including IL-18 and IL-1β [101–103].
Overall, pyroptosis is initiated through 2 distinct pathways: the canonical pathway and the noncanonical pathway. The canonical pathway of pyroptosis, otherwise known as caspase-1-dependent PCD, is commonly activated by several viruses. In contrast, the noncanonical pathway that results in the activation of caspase-4, caspase-5, and caspase-11, after sensing of bacterial PAMPs, thus will not be discussed in this review.
3.1. The canonical pathway of pyroptosis activation
The canonical inflammasome sensors are required to induce caspase-1-dependent pyroptosis. Various canonical inflammasomes mediate virus-induced pyroptosis, including the Nod-like receptor family pyrin domain containing 3 (NLRP3), NLRP6, and NLRP9 inflammasomes, AIM2 inflammasome, and the IFN-gamma-inducible protein-16 (IFI16) inflammasome [10,11,104]. Procaspase-1 is a component of the inactive inflammasome with 3 main domains, including 2 catalytic subunits, p10 and p20, and a noncatalytic caspase activation and recruitment domain (CARD). Activation of the inflammasome containing procaspase-1 is required for cleavage of the inactive caspase zymogen [105].
3.1.1. NLRP3/6/9 inflammasome-mediated viral pyroptosis
Many RNA viruses (IAV, VSV, hepatitis C virus, dengue virus (DV)) and some DNA viruses (herpes virus, adenovirus, VACV) are sensed through NLRP3. Unlike AIM2 and IFI16, activation of the NLRP3 inflammasome requires 2 distinct signals. The priming signal is the first one often generated after NF-κB production, which is activated when a variety of ligands stimulate the PRRs (e.g., TLR), TNFR, RIG-I, and IL-1R [106–108]. This up-regulates the transcription of NLRP3 and pro-IL-1β, as well as posttranslational modifications such as NLRP3 phosphorylation and ubiquitination [106,109–112]. Examples of pathways that stimulate NF-kB include IFN-γ and TNF-α [113,114]; MyD88 and TRIF mediated response downstream of TLR stimulation [106], activation of RIG-I-mediated responses after cytosolic viral RNA detection [115].
A second signal is required for the pyrin domain of NLRP3 to bind ASC, causing ASC oligomerization and ASC speck formation [116]. The internal CARD and activation domain recruits and activates caspase-1 [117]. Caspase-1 activation leads to the downstream cleavage of N-terminal gasdermin and pore formation. The exact mechanism for the execution of the second signal is not known.
Two relatively new inflammasomes NLRP6 and NLRP9 can also activate pyroptosis. Activated NLRP6 along with ASC form an inflammasome to activate caspase-1 and caspase-11 [10,11,104]. Furthermore, NLRP9b is one of the 3 mouse paralogs of NLRP9 and recognizes short dsRNA stretches in concert with the RNA helicase Dhx9 to form inflammasome complexes with the adaptor protein ASC to activate caspase-1-mediated gasdermin D (Gsdmd)-induced pyroptosis and the maturation of IL-18 [118–120].
3.1.2. AIM2 inflammasome-mediated viral pyroptosis
The AIM2 inflammasome, a member of the ALR family, consists of a hematopoietic IFN-inducible nuclear (HIN)-200 domain that predominantly binds to a wide range of cytosolic double-stranded (ds)-DNA and, in rare cases, cytosolic RNA [121,122]. Under normal states, the inflammasome maintains an autoinhibited state. However, the interaction of the sugar-phosphate backbone of dsDNA with the HIN-200 domain functionally displaces the pyrin domain from the inhibited state, liberating the pyrin domain to interact with the pyrin domain of ASC [122,123]. The CARD domain of procaspase-1 is recruited through the CARD domain of ASC, allowing for inflammasome assembly and caspase-1 activation. The exact mechanism by which viral DNA is exposed for AIM2 sensing is not precisely known, although several viral dsDNA ligands of VACV, human papillomavirus (HPV), hepatitis B virus, and mouse cytomegalovirus (MCMV) are sensed in an AIM2-dependent manner [124,125].
3.1.3. IFI16 inflammasome-mediated viral pyroptosis
Closely associated with the AIM2 inflammasome is the IFI16 inflammasome, a canonical inflammasome with an ability to bind single and double-stranded DNA, triggering pyroptosis during viral infection. Initially, IFI16 was discovered as a viral DNA sensor of HSV-1, VACV, Kaposi sarcoma–associated herpesvirus (KSHV), and single-stranded DNA from HIV-infected CD4+ T cells [126]. IFI16 is mainly localized within the nucleus; however, it is also known to translocate to the cytoplasm to engage in antiviral immune response [126,127]. As a regulator of pyroptosis, cytoplasmic IFI16 can colocalize with ASC via its PYD domain after viral DNA recognition by the HIN domain [128]. Such interactions allow for caspase-1 activation, leading to cytokine release and pyroptosis.
During its latent period, KSHV, a dsDNA virus, resides within the nucleus of infected cells, tethered to host cell chromatin. During KSHV infection, oligomers of IFI16 can be seen to colocalize in the nucleus of infected endothelial cells with the KSHV genome in a nonspecific way; this cumulative interaction launches inflammasome formation, which recruits caspase-1 and ASC, inducing pyroptotic cell death and mature inflammatory cytokine release [129]. It is most probable that KSHV binding to IFI16 results in structural changes that allow its interaction with ASC [130].
4. Mechanisms of ferroptosis activation during virus infection
Ferroptosis is a type of PCD that is initiated after sensing free iron accumulation and increased lipid peroxidation. These two are coupled to or caused by the accumulation of intracellular reactive oxygen species (ROS) [131,132]. Lipid peroxidation refers to the result of oxidative attack on polyunsaturated fatty acids by ROS. One of the ways that ferroptosis is induced is via the inhibition of the system Xc− cystine/glutamate antiporter, which is a heterodimer made of solute carrier (SLC) family 7 member (SLC7A11) and SLC3A2. This antiporter functions to import cystine into the cell where it is reduced to cysteine and used for glutathione (GSH) synthesis [133]. GSH works to reduce ROS in the cell via the function of GSH peroxidases such as glutathione peroxidase 4 (GPX4). Many molecules that induce ferroptosis, such as erastin, induce ferroptosis by inhibiting system Xc− or modulating the pathway in some way [132]. Morphologically, ferroptosis mainly manifests as shrinkage of mitochondria with increased membrane density and reduction in or vanishing of mitochondrial cristae, which is a different process from other modes of cell death [132].
Studies elucidating the mechanisms of ferroptosis induction in virus-infected cells are very limited. One study demonstrated that Newcastle disease virus (NDV) can induce ferroptosis [134]. Cells infected by NDV demonstrated an increase in ROS as well as lipid peroxides. The mechanism by which NDV induces ferroptosis is by inhibiting the Xc-GSH-GPX4 pathway and appears to require the p53 protein [134]. Another study highlighted that the expression of the hepatitis A virus 3C protease induced ferroptosis [135]. There is a direct link between the DNA sensor STING and ferroptosis. The classic ferroptosis inducer erastin induces the accumulation of STING in the mitochondria to ROS production and lipid oxidation [136]. Both of these are hallmarks of ferroptosis, and depleting STING1 reduced sensitivity to ferroptosis [136]. In another study, zalcitabine-induced mitochondrial DNA stress activates STING-mediated DNA sensing pathway leading to autophagy dependent ferroptotic cell death via lipid peroxidation, independent of the IFN response [137]. These studies highlight the need for future studies to determine how other viruses activate and inhibit ferroptosis.
5. Mechanisms of paraptosis activation during virus infection
Paraptosis is a type of PCD characterized by cytoplasmic vacuolation, usually involving the swelling of the ER and mitochondria. Paraptosis is often accompanied by an alteration of Ca2+ and redox homeostasis [138], as well as by accumulation of misfolded proteins causing ER stress and cell death [139]. However, these features are not always present in cells undergoing paraptosis. In most cases, paraptosis depends on mitogen-activated protein kinase (MAPK) family members, such as c-Jun N-terminal protein kinase 1 (JNK1), p38, and mitogen-activated protein kinase kinase 2 (MEK-2), and it can be inhibited by the multifunctional adapter protein AIP-1/Alix.
Similar to ferroptosis, there is little research on virus-induced paraptosis. Zika virus infection can activate ER-dependent, large, cytoplasmic vacuoles characteristic of paraptosis. The ability of the virus to create these paraptotic vacuoles was dependent on its ability to replicate within ER-derived membranes [140]. In another study, Singapore grouper iridovirus (SGIV) induced STAT-3-dependent paraptosis of fish cells [141]. Further research is required to understand mechanisms of virus-induced paraptosis.
Inhibition of programmed cell death by viruses
Depending on the type of virus, infection cells attempt to abort the infection by activating PCD. On the other hand, viruses highjack a variety of cellular processes including the initiation and execution of PCD starting early after infection. For example, herpesviruses and poxviruses, which utilize over one-third of their genome to counteract the host antiviral response [142]. In this section, we summarize the various strategies viruses deploy to inhibit apoptosis, necroptosis, and pyroptosis (Tables 1 and 2).
Table 1. Mechanisms of apoptosis inhibition by viruses.
| PCD | Family | Virus | Viral Protein | Proteins Role in Inhibition |
|---|---|---|---|---|
| Apoptosis | Poxviridae | VACV | F1L [145,204] | BCL-2 homolog, sequesters BIM |
| N1L [205] | BCL-2 homolog, NF-kB inhibition | |||
| A46 [206] | ||||
| A49 [207,208] | ||||
| A52 [209] | ||||
| B14 [209] | ||||
| K7 [210] | ||||
| SPI-1/SPI-2/SPI-3 [211–215] | Caspase inhibition | |||
| CrmB/CrmC/CrmE [216] | Mimics TNFR1/2 | |||
| E3 [217] | Inhibits activation of PKR | |||
| D9/D10 [218] | ||||
| M1L [219] | Inhibits apoptosome functions and blocks caspase-9 processing | |||
| VARV | VAR F1L [220] | BCL-2 homolog, sequesters BID, BAK, and BAX | ||
| CrmB [221] | TNFR ½ mimic | |||
| CPXV | CrmA [146,147] | Caspase-8 inhibition | ||
| CrmB [222] | TNFR1/2 mimic | |||
| CrmC [222] | ||||
| CrmD [222] | ||||
| CrmE [156] | ||||
| vCD30 [223] | ||||
| ECTV | EMV025 [224] | BCL-2 homolog, sequesters BAK to inhibit BAK and BAX activity | ||
| vCD30 [225] | TNFR1/2 mimic | |||
| MYXV | M11L [226] | BCL-2 homolog, sequesters BAK and BAX | ||
| SERP1 [227]/SERP2 [228]/SERP3 [229] | Caspase inhibition | |||
| M-T2 [230] | Mimics TNFR1/2 | |||
| M131 [231] | SOD homolog | |||
| M029 [232] | Inhibits activation of PKR | |||
| SFV | T2 [230,233] | TNFR1/2 mimic | ||
| S131 [231] | SOD homolog | |||
| SPV032 [217] | Inhibits PKR activation | |||
| TANV | 16L [234] | BCL-2 homolog | ||
| 2L [235] | TNFR1/2 mimic | |||
| ORFV | ORFV125 [236–239] | BCL-2 homolog | ||
| SPPV | SPPV14 [239,240] | BCL-2 homolog | ||
| DPV | DPV022 [241] | BCL-2 homolog | ||
| FPV | FPV039 [242,243] | BCL-2 homolog, sequester all BH3-only proteins | ||
| CNP | CNP058 [244] | BCL-2 homolog | ||
| MCV | MC163 [245] | SOD homolog | ||
| MC159 [246–250] | - Inhibitor of TNF-α/FasL induced apoptosis - cFLIP homolog, binds FADD - BCL-2 homolog |
|||
| MC160 [246,247,251] | - Inhibitor of TNF-α/ induces NF-kB prosurvival signaling - cFLIP homolog, binds caspase-8 |
|||
| Herpesviridae | HVT | vNr-13 [252] | BCL-2 homolog | |
| HVS | HVS-Bcl-2 [253] | BCL-2 homolog | ||
| ORF-71 [249] | c-FLIP and BCL-2 homolog | |||
| HSV-1 | ICP22 [163,254–257] | Caspase-3 inhibition, antagonizes p53 | ||
| US3 [164,258–261] | Blocks cleavage of procaspase-3 - activates PI3-K/akt pathways, - inhibits NF-kB pathway by hyper-phosphorylating p65, - decreases expression of IL-8, - phosphorylates proapoptotic members of BCL-2 family: BAD and BID |
|||
| HSV | US5 (glycoprotein J) [262,263] | Suppresses caspase-3 and 8 activation, inhibits FoF1 ATP synthase function and ROS formation to suppress apoptosis | ||
| HSV-1 | US6 (glycoprotein D) [264–267] | Inhibits Fas-mediated apoptosis by activating NF-kB pathway | ||
| HSV | US8 (glycoprotein E) [268] | Degrades BIM protein | ||
| HSV 1/2 | ICP6/ICP10 [269] | Inhibits caspase-8 to prevent TNF-induced apoptosis | ||
| MCMV | M36 [270] | Interacts with and inhibits caspase-8 | ||
| HCMV | UL36 [271] | Interacts with and inhibits caspase-8 | ||
| MHV-68 | M11 [272] | Neutralizes proapoptotic BCL-2 family of proteins Inhibits Beclin1-dependent autophagy |
||
| EHV-2 | E8 [249] | c-FLIP homolog, inhibits procaspase-8 activation and Bcl-2 homolog | ||
| HHV-8/KSHV | ORF-71 [249,273–275] | c-FLIP homolog, inhibits CD95 mediated apoptosis and BCL-2 homolog | ||
| K13 [246] | c-FLIP homolog and activates NF-kB | |||
| BHV-4 | ORF-16 [249,276] | c-FLIP homolog, prevents activation of caspase-8 and BCL-2 homolog | ||
| Adenoviridae | E1B 19K | E1B 19K protein [277,278] | Bcl-2 homolog and blocks p53 meditated apoptosis | |
| Baculoviridae | OpMNPV | IAP3 [155] | IAP3 homolog | |
| Anticarsia gemmatalis MNPV | IAP3 [155] | IAP3 homolog | ||
| Helicoverpa armigera NPV | IAP3 [155] | IAP3 homolog | ||
| AmEPV | IAP3 [155] | Carries p35 and IAP3 homolog | ||
| AMViap [279] | Caspase-3 and 9 inhibitor | |||
| CpGV | IAP6 [155] | IAP6 homolog | ||
| Pieris rapae GV | IAP6 [155] | IAP6 homolog | ||
| Cryptophlebia leucotreta GV | IAP6 [155] | IAP6 homolog | ||
| Phthorimaea operculella GV | IAP6 [155] | IAP6 homolog | ||
| Hyphantria cunea NPV | IAP3 [155] | IAP3 homolog | ||
| AcMNPV | IAP1 [155] | Carries p35 and IAP1 homolog |
AcMNPV, Autographa californica multiple nucleopolyhedrovirus; AmEPV, Amsacta moorei entomopoxvirus; ASFV, African swine fever virus; BIR, Baculovirus IAP repeat; CMV, cytomegalovirus; CpGV, Cydia pomonella granulovirus; EHV, equine herpes virus; EV71, enterovirus 71; HHV, human herpes virus; HPIV, human parainfluenza virus; HVS, Herpes virus Saimiri; HVT, herpesvirus of turkeys; IAP, inhibitor of apoptosis protein; ICP, infected cell protein; KSHV, Kaposi sarcoma–associated virus; LAT, latency-associated transcript; MHV-68, murine gammaherpesvirus-68; MV, measles virus; MYXV, myxoma virus; NiV, Nipah virus; OpMNPV, Orgyia pseudotsugata MNPV; PEDV, porcine epidemic diarrhea virus; R1, ribonucleotide reductase; SeV, Sendai virus; SFV, Shope fibroma virus; SVV, Seneca Valley virus; v-FLIP, viral FLICE-like inhibitory proteins; VZC, varicella zoster virus.
Table 2. Mechanisms of necroptosis and pyroptosis inhibition during virus infection.
| PCD | Family | Virus | Viral Protein | Proteins Role in Inhibition |
|---|---|---|---|---|
|
Necroptosis |
Herpesviridae | MCMV | M45 [169,280] | RHIM-dependent amyloid plague formation leading to sequestering of RIPK1, RIPK3, and DAI |
| HSV1 | ICP6 [170] | |||
| HSV2 | ICP10 [269] | |||
| HCMV | UL36 [281,282] | Targeting of MLKL for proteasomal degradation | ||
| HSV1 | ICP6 [169] | IPAM-dependent aggregation and autophagy of RIPK1 | ||
| MCMV | M45 [169] | |||
| EBV | LMP1 | Ubiquitination of RIPK1 causing prosurvival signaling [175] | ||
| Deubiquitination of RIPK3 preventing necrosome formation [175] | ||||
| Hypermethylation of RIPK3 promoter [175] | ||||
| Poxviridae | CPXV | vIRD [176] | RIPK3 ubiquitination and proteasomal degradation | |
| VARV | ||||
| MPXV | ||||
| ECTV | ||||
| Suipoxvirus | E3 [283,284] | Sequestering z-RNA inhibiting PAMP detection | ||
| Caprpoxvirus | ||||
| VARV | ||||
| VACV | ||||
| Carpripoxvirus | vMLKL [284,285] | Sequestering RIPK3 and RIPK1 while lacking the ability to insert into the cell membrane | ||
| Suipoxvirus | ||||
| Avipoxvirus | ||||
| Leporipoxvirus | ||||
| Orthomyxoviridae | IAV | Hemagglutinin of pandemic IAV strains [286] | Inhibition of RIPK3-mediated necroptosis | |
|
Pyroptosis |
Coronavidae | SARS-Cov-2 | NSP5 | Cleavage of Gasdermin D into N-terminal lacking critical residues [182] |
| MARS-Cov | ||||
| PEDV | NSP4 | Cleavage of Gasdermin D into N-terminal lacking critical residues [287] | ||
| TGEV | ||||
| SARS-Cov-2 | NP [288] | Blockage of Gasdermin D cleavage by caspase-1 | ||
| Picoronavidae | EV71 | 3Cpro [289,290] | Cleavage of Gasdermin D into N-terminal lacking critical residues | |
| SVV | ||||
| Arterviridae | EAV | NSP4 [287] | Cleavage of Gasdermin D into N-terminal lacking critical residues | |
| Asfarviridae | ASFV | P5273R [287] | ||
|
Unconfirmed Pyroptosis |
Herpesviridae | EBV | miRNA-BART15 [291] | NLRP3 inflammasome formation |
| KSHV | Orf63 [292] | NLRP1 inflammasome formation | ||
| Orthomyxoviridae | IAV | NS1 [293] | NLRP3-ASC inflammasome interaction | |
| Paramyxoviridae | MV | Protein V [294] | Localizes NLRP3 to peri-nuclear space | |
| SeV | Protein V | NLRP3 Inflammasome-ASC formation [292] | ||
| NiV | NLRP3 Inflammasome-ASC formation [292] | |||
| HPIV | NLRP3 Inflammasome-ASC formation [292] | |||
| Papillomaviridae | HPV | E7 | Ubiquitination and degradation of IFI16 inflammasome [295] | |
| Poxiviridae | SFV | PYD-only Protein | Inflammasome formation [292] | |
| MYXV | M13L [292] | Inflammasome formation |
ASFV, African swine fever virus; EV71, enterovirus 71; HPIV, human parainfluenza virus; MV, measles virus; MYXV, myxoma virus; NiV, Nipah virus; PEDV, porcine epidemic diarrhea virus; SeV, Sendai virus; SFV, Shope fibroma virus; SVV, Seneca Valley virus.
1. Viral-mediated inhibition of apoptosis
Viral BCL-2 (vBCL-2) homologs are among the best studied viral inhibitors of apoptosis. vBCL-2 homologs, widely distributed throughout Poxviridae, Herpesviridae, Asfarviridae, and Iridovirdae, unilaterally possess a hydrophobic BH3 binding domain capable of apoptosis inhibition. Broadly, vBCL-2 homologs either directly inhibit BAX and BAD oligomerization, preventing mitochondrial pore formation and cytochrome c release, or sequester proapoptotic BCL-2 proteins, diminishing proapoptosis signals [143,144]. Although functionally analogous, vBCL-2 homologs interact with diverse and distinct proapoptotic BCL-2 proteins. Within Poxviridae, VACV encodes F1L, which adopts a unique domain swapped BCL-2 topology forming ligand-specific interactions with either BAX or BAK to directly inhibit oligomerization and mitochondrial pore formation, or with BIM, sequestering the proapoptotic signal [145].
Viruses can also block apoptosis by inhibiting a variety of caspases. Expressed during cowpox virus (CPXV) infection, CrmA was the first serpin-like protein found to potently inhibit caspase-8, caspase-1, and caspase-10 [146,147]. Homologs of CrmA in Orthopoxvirus and Chorodopoxvirus display a broad spectrum of caspase inhibition through a pseudo-substrate mechanism [148,149]. Furthermore, viruses can also specifically inhibit caspase-8. Viral inhibitors of caspase-8 (vICA) are distributed throughout Herpesviridae. ICP6 of HSV1 (ICP10 in HSV2), UL36 in human, and M36 in MCMV directly interact with the death effector domains (DEDs) on procaspase-8 inhibiting FADD association and procaspase-8 cleavage, protecting against extrinsic apoptosis [150–152]. Additionally, members of KSHV encode cellular FLIP analogs, termed viral FLIPs (vFLIPs). vFLIPs resemble the short isoform of cellular FLIPs and again completely block caspase-8 proteolytic activity [153,154]. Moreover, large DNA viruses infecting arthropods encode viral inhibitors of apoptosis proteins (vIAPs) containing a baculoviruses IAP repeat (BIR) motifs that binds and inhibit apoptosis mediators Hid, Reaper, or Grim. Moreover, really interesting new gene (RING) domains on vIAPs facilitate the ubiquitination and degradation of these proapoptotic proteins, reinforcing their antiapoptotic effects [155].
Outside of the largely conserved mechanisms, virus family-specific mechanisms are extremely diverse. Poxviruses encode TNFR homologs (vTNFRs) capable of binding and depleting proapoptotic TNF along with other immune-modulatory cytokines [156–158]. Additionally, VACV encodes the E3 protein to prevent PKR activation by binding dsRNA [159]. Viral Golgi antiapoptotic protein (vGAAP) of camelpox virus and some strains of VACV have also been shown to inhibit intrinsic apoptosis through a Ca2+-dependent mechanism [160,161]. Varster zooster virus (VZV) of Herpesviridae encodes IE63 capable of inhibiting apoptosis through inhibition of eIF-2α [162,163]. Finally, α-herpesviruses encode US3, adept at phosphorylating BAD and caspase-3, preventing activation [164,165]. Overall, large DNA viruses precisely target apoptosis through distinct and comprehensive mechanisms.
2. Viral-mediated inhibition of necroptosis
Viruses inhibit necroptosis mostly at the level of RIPK1/3 and MLKL. In herpesviruses and poxviruses, inhibiting the kinases RIPK1/3 is a very common process. Both viruses encode various inhibitors of RHIM-dependent functions of RIPK1/3. Multiple herpesviruses encode RHIM-containing proteins that inhibit the formation of amyloid-like filamentous necroptotic signaling complexes; effectively inhibiting the RIPK1-RIPK3 signaling axis and MLKL activation [64,166–168]. The first identified RHIM-dependent inhibitor was the viral ribonuclease reductases subunit (R1) homolog, M45 of murine cytomegalovirus (MCMV) [167,168]. M45 forms homo amyloid-like filamentous structures that bind RHIM domains of RIPK1, RIPK3, and ZBP1/DAI, effectively sequestering RHIM containing necroptotic machinery, inhibiting MLKL activation and cell lysis [167,168]. Additionally, viral UL39 R1 homologs of HSV1 and HSV2, termed ICP6 and ICP10, respectively, form similar RHIM-dependent amyloid-like fibrils to make the kinases dysfunctional [169]. However, underlying mechanisms of dysfunctional amyloid fibril formation differ between HSV and MCMV [170,171]. Furthermore, the RHIM-dependent inhibitor analog of Varicella zoster virus (VZV) ORF20 imposes similar amyloid-like fibrils [172].
Viruses also often facilitate the degradation of RIPK1 and RIPK3. Multiple human herpesviruses, such as herpes simplex virus (HSV), EBV, and KSHV, encode homologs of MCMV M45 protein. These viral inhibitors contain induced protein aggregation motifs (IPAMs) that facilitate insoluble protein aggregation of RIPK1, directing its degradation [173]. IPAM sequences are widely homologous in over 70 herpesviruses, baculoviruses, and giant viruses; however, functional conservation of this mechanism is unconfirmed [173]. EBV also employs LMP1 to manipulate ubiquitination of RIPK1 and RIPK3. LMP1 directly associates with the activation domain of RIPK1 promoting ubiquitination through the E3 ligase TRAF2, restricting RIPK1 to the plasma membrane where it maintains prosurvival signaling [174]. Concurrently, LMP1 facilitates deubiquitylation of RIPK3, preventing necrosome formation [174]. Finally, LMP1 exerts epigenetic control of RIPK3 through 2 axes: LMP1 up-regulates DNMT1 to directly methylate the transcription start site (TSS) of RIPK3 and also down-regulates ten-eleven translocation methylcytosine dioxygenase (TETs) to further methylate promoters of RIPK3 [175].
Poxviruses also target RIPK3 through viral inducers of RIPK3 degradation (vIRD). vIRD analogs are found in CPXV, variola virus (VARV), monkeypox virus (MPXV), and Ectromelia virus (ECTV) and directly bind to RIPK3 through their Ankyrin repeats, while their F-box domains link to host SKP1-Cullin1-F-box machinery. This interaction stimulates ubiquitination of RIPK3 and subsequent proteasomal degradation [176]. Interestingly, VACV, an attenuated virus used to eradicate VARV, lacks a functional vIRD homolog potentially shedding light on its benign and immunogenic nature [176].
Multiple Poxviridae inhibit necroptosis by inhibiting MLKL. Chordopoxvirinae encode E3, a viral protein possessing a z-RNA binding domain. Z-RNA, produced during viral replication, binds to the N-terminal Zα domain of E3, effectively sequestering the viral PAMP inhibiting ZBP1/DAI-dependent necroptosis [177,178]. Suipoxvirus, Carpirpoxvirus, Avipoxvirus, and Lepropoxvirus families also express viral MLKL (vMLKL) analogs [178,179]. vMLKLs retain high homology of the pseudokinase domain, allowing dominant sequestration of RIPK3 from RIPK1; however, lack the 4 helical bundle domains essential for membrane insertion and cell lysis. Human cytomegalovirus (HCMV) also ubiquinates MLKL through the viral protein UL36, causing necroptotic inhibition in human but not murine cell lines [180,181].
In summary, the large diversity and redundancies in viral inhibition of necroptosis support the emerging theory that necroptosis and viruses have coevolved specific virus–host mechanisms to antagonize the other, further highlighting importance of necroptosis in antiviral immunity and viral replication.
3. Viral-mediated inhibition of pyroptosis
Overall, the mechanisms by which viruses inhibit pyroptosis remain largely unknown. However, plus sense RNA viruses, such as Coronaviridae, utilize viral proteases to inhibit pyroptosis [182–184] (Table 2). Highly conserved viral proteases, such as NSP5 in coronaviruses, directly bind and cleave Gasdermin D in porcine and human cells, producing a truncated N-terminal Gasdermin D, lacking essential residues from caspase-1 cleavage [98]. Interestingly, a singular large dsDNA virus, African swine fever virus, has shown a similar mechanism through the viral protease S273R [185].
Additionally, within the Coronaviridae family, SARS-CoV-2 nucleocapsid protein (NP) blocks caspase-1-dependent cleavage of Gasdermin D through unidentified mechanism [186]. Upstream of terminal events, multiple viruses have been implicated in inhibiting PRR interactions, inflammasome formation, and ILb release, these events are summarized in Table 2; however, inhibition of pyroptosis has not been directly confirmed [187–192].
Perspectives and future studies
The complexity of viral sensing pathways, diversity of animal viruses, and the ability of viruses to modulate PCD using their accessory proteins has been challenging to build a universal framework to dissect how viral ligand sensing interacts with the PCD machinery. Most studies we reviewed use live virus infections to study PCD. Hence, it is largely unknown how viral genome sensing in the context of inactivated and replication-defective virus stimulation regulates the PCD machinery. Many viral nucleic acid–like ligands including synthetic dsRNA:Poly I:C [193] and cGAMP [194] can activate cell death, suggesting the likelihood that viral genomes may also directly activate PCD, although similar studies are limited. In relation to this, it remains unknown if viral RNA and DNA genomes engage distinct PCD pathways.
An outcome of nucleic acid sensing is establishment of antiviral state via expression of various ISGs that exert direct antiviral effects in many ways. Although some ISGs have been extensively studied [195], the modes of action of a large majority of ISGs are still unknown [196]. There are more than 15 putative ISGs with proapoptotic functions, although their direct effect on PCD regulation is not known [197–199]. Furthermore, a large-scale proteomics study identified more than 200 novel PCD-associated proteins directly interact with some of the 104 inducibly expressed ISGs [196]. In support of this, IFN-induced transmembrane proteins 3 (IFITM3) directly regulates PCD induction in Zika virus–infected cells [140,200] and overexpression of ISG54 [201] and ISG12b2 [202], independent of IFN stimulation, activate apoptosis. The broader relevance of ISG modulating PCDs in the context of viral infection remains to be investigated.
Much is unknown how PCD influences viral pathogenesis. On one side, activation of PCD in an infected cell can curb virus replication, while some necrotic forms of PCD can assist spreading of virus to the surrounding tissue. In addition, certain PCDs can facilitate the identification of viruses by the adaptive immunity via emission of immunomodulatory secretomes that enhance antigen presentation [203]. To the infected host, activation of certain inflammatory PCDs may exacerbate disease pathogenesis, as these forms of PCD may enhance acute cytokine storms and/or potentially trigger delayed immunopathologies such as autoimmune disorders. Studies investigating how viral induced PCDs may instigate host reactivity against self are currently lacking.
Funding Statement
We thank University of Guelph for Start-up fund. Research in our laboratory is funded by Ontario Cancer Research Institute Joseph and Wolf Lebovic Fellowship Program, Livestock Research Innovation Corporation New Investigator Award, and Pet Trust research awarded to STW. SGV, MJW and JMI are supported by Ontario Graduate Scholarship and Ontario Veterinary College Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Workenhe ST, Rise ML, Kibenge MJ, Kibenge FS. The fight between the teleost fish immune response and aquatic viruses. Mol Immunol. 2010;47(16):2525–36. Epub 2010/08/28. doi: 10.1016/j.molimm.2010.06.009 . [DOI] [PubMed] [Google Scholar]
- 2.Workenhe ST, Mossman KL. Rewiring cancer cell death to enhance oncolytic viro-immunotherapy. Onco Targets Ther. 2013;2(12):e27138–e. Epub 12/09. doi: 10.4161/onci.27138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry G, Fragkoudis R, Ferguson MC, Lulla A, Merits A, Kohl A, et al. Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells. J Virol. 2010;84(14):7369–77. Epub 2010/04/30. doi: 10.1128/JVI.02310-09 ; PubMed Central PMCID: PMC2898233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Gorshkov K, Lee EM, Xu M, Cheng Y-S, Sun N, et al. Zika Virus-Induced Neuronal Apoptosis via Increased Mitochondrial Fragmentation. Front Microbiol. 2020;11(3316). doi: 10.3389/fmicb.2020.598203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reshi ML, Su Y-C, Hong J-R. RNA Viruses: ROS-Mediated Cell Death. Int J Cell Biol. 2014;2014:467452. Epub 2014/05/08. doi: 10.1155/2014/467452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Workenhe ST, Ketela T, Moffat J, Cuddington BP, Mossman KL. Genome-wide lentiviral shRNA screen identifies serine/arginine-rich splicing factor 2 as a determinant of oncolytic virus activity in breast cancer cells. Oncogene. 2015. doi: 10.1038/onc.2015.303 [DOI] [PubMed] [Google Scholar]
- 7.Schulz KS, Mossman KL. Viral Evasion Strategies in Type I IFN Signaling—A Summary of Recent Developments. Front Immunol. 2016;7:498. doi: 10.3389/fimmu.2016.00498 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vloten JP, Workenhe ST, Wootton SK, Mossman KL, Bridle BW. Critical Interactions between Immunogenic Cancer Cell Death, Oncolytic Viruses, and the Immune System Define the Rational Design of Combination Immunotherapies. J Immunol. 2018;200(2):450. doi: 10.4049/jimmunol.1701021 [DOI] [PubMed] [Google Scholar]
- 9.Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021;371(6528). Epub 2020/11/28. doi: 10.1126/science.abd0811 . [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, et al. Nlrp6 regulates intestinal antiviral innate immunity. Science. 2015;350(6262):826–30. Epub 2015/10/24. doi: 10.1126/science.aab3145 ; PubMed Central PMCID: PMC4927078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546(7660):667–70. Epub 2017/06/22. doi: 10.1038/nature22967 ; PubMed Central PMCID: PMC5787375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burleigh K, Maltbaek JH, Cambier S, Green R, Gale M Jr., James RC, et al. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci Immunol. 2020;5(43). Epub 2020/01/26. doi: 10.1126/sciimmunol.aba4219 ; PubMed Central PMCID: PMC7081723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray EE, Winship D, Snyder JM, Child SJ, Geballe AP, Stetson DB. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity. 2016;45(2):255–66. Epub 2016/08/09. doi: 10.1016/j.immuni.2016.06.015 ; PubMed Central PMCID: PMC4988931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–51. Epub 2003/04/19. doi: 10.1126/science.1081315 . [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5(10):1061–8. Epub 2004/09/14. doi: 10.1038/ni1118 . [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227):aaa2630. Epub 2015/02/01. doi: 10.1126/science.aaa2630 . [DOI] [PubMed] [Google Scholar]
- 17.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388(6642):548–54. Epub 1997/08/07. doi: 10.1038/41493 [DOI] [PubMed] [Google Scholar]
- 18.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9(10):899–909. Epub 1995/07/01. . [PubMed] [Google Scholar]
- 19.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17(4):1087–95. Epub 1998/03/28. doi: 10.1093/emboj/17.4.1087 PubMed Central PMCID: PMC1170457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoggins JW. Interferon-Stimulated Genes: What Do They All Do? Annu Rev Virol. 2019;6(1):567–84. Epub 2019/07/10. doi: 10.1146/annurev-virology-092818-015756 . [DOI] [PubMed] [Google Scholar]
- 21.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. Epub 2007/06/15. doi: 10.1080/01926230701320337 ; PubMed Central PMCID: PMC2117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Jiang W, Liu Z, Liu S, Liang X. Virus Infection and Death Receptor-Mediated Apoptosis. Viruses. 2017;9(11). Epub 2017/10/28. doi: 10.3390/v9110316 ; PubMed Central PMCID: PMC5707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imre G. Cell death signalling in virus infection. Cell Signal. 2020;76:109772. Epub 2020/09/16. doi: 10.1016/j.cellsig.2020.109772 ; PubMed Central PMCID: PMC7486881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4(5):e1000018. Epub 2008/06/03. doi: 10.1371/journal.ppat.1000018 ; PubMed Central PMCID: PMC2376094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroemer G, Galluzzi L, Brenner C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006 . [DOI] [PubMed] [Google Scholar]
- 26.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct Activation of Bax by p53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734 [DOI] [PubMed] [Google Scholar]
- 27.Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NS, et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017;24(5):878–88. Epub 2017/04/01. doi: 10.1038/cdd.2017.30 ; PubMed Central PMCID: PMC5423112 institute receives milestone payments for the development of BH3 mimetic drugs for therapy from Genentech and AbbVie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Incrocci R, Hussain S, Stone A, Bieging K, Alt LA, Fay MJ, et al. Epstein-Barr virus Latent Membrane Protein 2A (LMP2A)-mediated changes in Fas expression and Fas-dependent apoptosis: Role of Lyn/Syk activation. Cell Immunol. 2015;297(2):108–19. Epub 2015/08/11. doi: 10.1016/j.cellimm.2015.08.001 ; PubMed Central PMCID: PMC4618070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong F, You H, Zhao J, Liu W, Hu L, Luo W, et al. The enhanced expression of death receptor 5 (DR5) mediated by HBV X protein through NF-kappaB pathway is associated with cell apoptosis induced by (TNF-α related apoptosis inducing ligand) TRAIL in hepatoma cells. Virol J. 2015;12:192. Epub 2015/11/19. doi: 10.1186/s12985-015-0416-z ; PubMed Central PMCID: PMC4650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke P, Leser JS, Quick ED, Dionne KR, Beckham JD, Tyler KL. Death receptor-mediated apoptotic signaling is activated in the brain following infection with West Nile virus in the absence of a peripheral immune response. J Virol. 2014;88(2):1080–9. Epub 2013/11/08. doi: 10.1128/JVI.02944-13 ; PubMed Central PMCID: PMC3911655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Grevenynghe J, Cubas RA, Noto A, DaFonseca S, He Z, Peretz Y, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J Clin Invest. 2011;121(10):3877–88. Epub 2011/09/20. doi: 10.1172/JCI59211 ; PubMed Central PMCID: PMC3195482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaddy DF, Lyles DS. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol. 2005;79(7):4170–9. Epub 2005/03/16. doi: 10.1128/JVI.79.7.4170-4179.2005 ; PubMed Central PMCID: PMC1061557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasa T, Suga S, Qi L, Komada Y. Apoptosis of human peripheral blood mononuclear cells by wild-type measles virus infection is induced by interaction of hemagglutinin protein and cellular receptor, SLAM via caspase-dependent pathway. Microbiol Immunol. 2010;54(7):405–16. Epub 2010/07/14. doi: 10.1111/j.1348-0421.2010.00231.x . [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Xiao C, Zhao G, Du X, Yu Y, Kang Y, et al. Induction of immature dendritic cell apoptosis by foot and mouth disease virus is an integrin receptor mediated event before viral infection. J Cell Biochem. 2007;102(4):980–91. Epub 2007/04/13. doi: 10.1002/jcb.21332 ; PubMed Central PMCID: PMC7166979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29(10):1762–73. Epub 2010/04/03. doi: 10.1038/emboj.2010.50 ; PubMed Central PMCID: PMC2876960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chattopadhyay S, Kuzmanovic T, Zhang Y, Wetzel JL, Sen GC. Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis. Immunity. 2016;44(5):1151–61. Epub 2016/05/15. doi: 10.1016/j.immuni.2016.04.009 ; PubMed Central PMCID: PMC4991351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acrani GO, Gomes R, Proença-Módena JL, da Silva AF, Carminati PO, Silva ML, et al. Apoptosis induced by Oropouche virus infection in HeLa cells is dependent on virus protein expression. Virus Res. 2010;149(1):56–63. Epub 2010/01/19. doi: 10.1016/j.virusres.2009.12.013 . [DOI] [PubMed] [Google Scholar]
- 38.Eitz Ferrer P, Potthoff S, Kirschnek S, Gasteiger G, Kastenmüller W, Ludwig H, et al. Induction of Noxa-mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF-3/IFN-β-dependent induction of pro-apoptotic Noxa. PLoS Pathog. 2011;7(6):e1002083. Epub 2011/06/24. doi: 10.1371/journal.ppat.1002083 ; PubMed Central PMCID: PMC3116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowlton JJ, Dermody TS, Holm GH. Apoptosis induced by mammalian reovirus is beta interferon (IFN) independent and enhanced by IFN regulatory factor 3- and NF-κB-dependent expression of Noxa. J Virol. 2012;86(3):1650–60. Epub 2011/11/18. doi: 10.1128/JVI.05924-11 ; PubMed Central PMCID: PMC3264342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, et al. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19(9):1482–94. Epub 2012/03/17. doi: 10.1038/cdd.2012.22 ; PubMed Central PMCID: PMC3422471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Häcker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17(6):942–51. Epub 2009/12/19. doi: 10.1038/cdd.2009.190 . [DOI] [PubMed] [Google Scholar]
- 42.Reinert LS, Rashidi AS, Tran DN, Katzilieris-Petras G, Hvidt AK, Gohr M, et al. Brain immune cells undergo cGAS/STING-dependent apoptosis during herpes simplex virus type 1 infection to limit type I IFN production. J Clin Invest. 2021;131(1). Epub 2020/09/30. doi: 10.1172/JCI136824 ; PubMed Central PMCID: PMC7773356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sze A, Belgnaoui SM, Olagnier D, Lin R, Hiscott J, van Grevenynghe J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe. 2013;14(4):422–34. Epub 2013/10/22. doi: 10.1016/j.chom.2013.09.009 . [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Chen Y-J, Dobbs N, Sakai T, Liou J, Miner JJ, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216(4):867–883. doi: 10.1084/jem.20182192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medigeshi GR, Lancaster AM, Hirsch AJ, Briese T, Lipkin WI, Defilippis V, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81(20):10849–60. Epub 2007/08/10. doi: 10.1128/JVI.01151-07 ; PubMed Central PMCID: PMC2045561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthuraj PG, Sahoo PK, Kraus M, Bruett T, Annamalai AS, Pattnaik A, et al. Zika virus infection induces endoplasmic reticulum stress and apoptosis in placental trophoblasts. Cell Death Dis. 2021;7(1):24. Epub 2021/01/28. doi: 10.1038/s41420-020-00379-8 ; PubMed Central PMCID: PMC7838309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, Xin X, Wang T, Wan J, Ou Y, Yang Z, et al. Japanese Encephalitis Virus Induces Apoptosis and Encephalitis by Activating the PERK Pathway. J Virol. 2019;93(17). Epub 2019/06/14. doi: 10.1128/JVI.00887-19 ; PubMed Central PMCID: PMC6694805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S, Zhu J, Zhou X, Wang H, Li X, Zhao A. Induction of the unfolded protein response (UPR) during pseudorabies virus infection. Vet Microbiol. 2019;239:108485. Epub 2019/11/27. doi: 10.1016/j.vetmic.2019.108485 . [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Hu J, Wang X, Wang Y, Guo M, Zhang X, et al. Avian reovirus infection activate the cellular unfold protein response and induced apoptosis via ATF6-dependent mechanism. Virus Res. 2021;297:198346. Epub 2021/03/21. doi: 10.1016/j.virusres.2021.198346 . [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Yang Y, Zhou X, Yang Z, Liu X, Cao Z, et al. The NS1 protein of influenza A virus interacts with heat shock protein Hsp90 in human alveolar basal epithelial cells: implication for virus-induced apoptosis. Virol J. 2011;8:181. Epub 20110419. doi: 10.1186/1743-422X-8-181 ; PubMed Central PMCID: PMC3098181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran AT, Cortens JP, Du Q, Wilkins JA, Coombs KM. Influenza virus induces apoptosis via BAD-mediated mitochondrial dysregulation. J Virol. 2013;87(2):1049–60. Epub 2012/11/09. doi: 10.1128/JVI.02017-12 ; PubMed Central PMCID: PMC3554053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu X, Ding X, Duan M, Yang J, Lin R, Zhou Z, et al. Influenza virus infection induces translocation of apoptosis-inducing factor (AIF) in A549 cells: role of AIF in apoptosis and viral propagation. Arch Virol. 2017;162(3):669–75. Epub 2016/11/18. doi: 10.1007/s00705-016-3151-x . [DOI] [PubMed] [Google Scholar]
- 53.Ding L, Li J, Li W, Fang Z, Li N, Wu S, et al. p53- and ROS-mediated AIF pathway involved in TGEV-induced apoptosis. J Vet Med Sci. 2018;80(11):1775–81. Epub 2018/09/27. doi: 10.1292/jvms.18-0104 ; PubMed Central PMCID: PMC6261820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lecoeur H, Borgne-Sanchez A, Chaloin O, El-Khoury R, Brabant M, Langonné A, et al. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis. 2012;3(3):e282. Epub 2012/03/16. doi: 10.1038/cddis.2012.21 ; PubMed Central PMCID: PMC3317353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo L, Li D, Sun L, Liu M, Shi X, Sun X, et al. Tat acetylation regulates its actions on microtubule dynamics and apoptosis in T lymphocytes. J Pathol. 2011;223(1):28–36. Epub 2010/09/08. doi: 10.1002/path.2768 . [DOI] [PubMed] [Google Scholar]
- 56.Sainski AM, Natesampillai S, Cummins NW, Bren GD, Taylor J, Saenz DT, et al. The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak. J Virol. 2011;85(16):7965–75. Epub 2011/06/10. doi: 10.1128/JVI.02515-10 ; PubMed Central PMCID: PMC3147983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thierry F, Demeret C. Direct activation of caspase 8 by the proapoptotic E2 protein of HPV18 independent of adaptor proteins. Cell Death Differ. 2008;15(9):1356–63. Epub 2008/04/19. doi: 10.1038/cdd.2008.53 . [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Fang Y, Sima N, Li Y, Li W, Li L, et al. Triggering of death receptor apoptotic signaling by human papillomavirus 16 E2 protein in cervical cancer cell lines is mediated by interaction with c-FLIP. Apoptosis. 2011;16(1):55–66. Epub 2010/10/01. doi: 10.1007/s10495-010-0543-3 . [DOI] [PubMed] [Google Scholar]
- 59.Javed F, Manzoor S. HCV non-structural NS4A protein of genotype 3a induces mitochondria mediated death by activating Bax and the caspase cascade. Microb Pathog. 2018;Epub 2018/09/05 (124):346–355. doi: 10.1016/j.micpath.2018.08.065 . [DOI] [PubMed] [Google Scholar]
- 60.Jeffries AM, Suptela AJ, Marriott I. Z-DNA binding protein 1 mediates necroptotic and apoptotic cell death pathways in murine astrocytes following herpes simplex virus-1 infection. J Neuroinflammation. 2022;19(1):109. Epub 20220513. doi: 10.1186/s12974-022-02469-z ; PubMed Central PMCID: PMC9103380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–79. Epub 20130909. doi: 10.1074/jbc.M113.462341 ; PubMed Central PMCID: PMC3829437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rello S, Stockert JC, Moreno V, Gámez A, Pacheco M, Juarranz A, et al. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis. 2005;10(1):201–208. doi: 10.1007/s10495-005-6075-6 . [DOI] [PubMed] [Google Scholar]
- 63.Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Núñez G, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114(6):E961–e9. Epub 2017/01/18. doi: 10.1073/pnas.1613305114 ; PubMed Central PMCID: PMC5307433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15(1):199. Epub 2018/07/08. doi: 10.1186/s12974-018-1235-0 ; PubMed Central PMCID: PMC6035417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amin P, Florez M, Najafov A, Pan H, Geng J, Ofengeim D, et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis. Proc Natl Acad Sci U S A. 2018;115(26):E5944–e53. Epub 2018/06/13. doi: 10.1073/pnas.1806973115 ; PubMed Central PMCID: PMC6042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858 . [DOI] [PubMed] [Google Scholar]
- 67.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388–93. Epub 20091116. doi: 10.1073/pnas.0908698106 ; PubMed Central PMCID: PMC2787135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x . [DOI] [PubMed] [Google Scholar]
- 69.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2(11):e230. Epub 2011/11/18. doi: 10.1038/cddis.2011.111 ; PubMed Central PMCID: PMC3223695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kearney CJ, Martin SJ. An Inflammatory Perspective on Necroptosis. Mol Cell. 2017;65(6):965–73. Epub 2017/03/18. doi: 10.1016/j.molcel.2017.02.024 . [DOI] [PubMed] [Google Scholar]
- 71.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23. Epub 2009/06/16. doi: 10.1016/j.cell.2009.05.037 ; PubMed Central PMCID: PMC2727676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schock SN, Chandra NV, Sun Y, Irie T, Kitagawa Y, Gotoh B, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24(4):615–25. Epub 20170106. doi: 10.1038/cdd.2016.153 ; PubMed Central PMCID: PMC5384020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular Nucleic Acid Sensing Triggers Necroptosis through Synergistic Type I IFN and TNF Signaling. J Immunol. 2018;200(8):2748–56. Epub 2018/03/16. doi: 10.4049/jimmunol.1701492 ; PubMed Central PMCID: PMC5893403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whilding LM, Archibald KM, Kulbe H, Balkwill FR, Öberg D, McNeish IA. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol Ther. 2013;21(11):2074–86. Epub 2013/08/30. doi: 10.1038/mt.2013.195 ; PubMed Central PMCID: PMC3831043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Wu J, Du F, Xu H, Sun L, Chen Z, et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 2014;6(3):421–30. Epub 20140123. doi: 10.1016/j.celrep.2014.01.003 ; PubMed Central PMCID: PMC3969844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39(6):1019–1031. doi: 10.1016/j.immuni.2013.10.019 ; PubMed Central PMCID: PMC3886715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Guan W. STING Signaling Promotes Apoptosis, Necrosis, and Cell Death: An Overview and Update. Mediators Inflamm. 2018;2018:1202797. Epub 2019/01/01. doi: 10.1155/2018/1202797 ; PubMed Central PMCID: PMC6286756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet. 2019;20(11):657–74. Epub 2019/07/31. doi: 10.1038/s41576-019-0151-1 . [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–35. Epub 20130606. doi: 10.1016/j.molcel.2013.05.022 ; PubMed Central PMCID: PMC3808999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yum S, Li M, Fang Y, Chen ZJ. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc Natl Acad Sci U S A. 2021;118(14). doi: 10.1073/pnas.2100225118 ; PubMed Central PMCID: PMC8040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. Cell Mol Life Sci. 2016;73(11–12):2137–52. Epub 2016/04/08. doi: 10.1007/s00018-016-2189-y ; PubMed Central PMCID: PMC4887535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbert A. Z-DNA and Z-RNA in human disease. Commun Biol. 2019;2:7. Epub 2019/02/08. doi: 10.1038/s42003-018-0237-x ; PubMed Central PMCID: PMC6323056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang G, Vasquez KM. Z-DNA, an active element in the genome. Front Biosci. 2007;12:4424–38. Epub 2007/05/09. doi: 10.2741/2399 . [DOI] [PubMed] [Google Scholar]
- 84.Wang AH, Quigley GJ, Kolpak FJ, Crawford JL, van Boom JH, van der Marel G, et al. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979;282(5740):680–686. doi: 10.1038/282680a0 [DOI] [PubMed] [Google Scholar]
- 85.Shin SI, Ham S, Park J, Seo SH, Lim CH, Jeon H, et al. Z-DNA-forming sites identified by ChIP-Seq are associated with actively transcribed regions in the human genome. DNA Res. 2016;23(5):477–486. doi: 10.1093/dnares/dsw031 ; PubMed Central PMCID: PMC5066173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boehm T, Mengle-Gaw L, Kees UR, Spurr N, Lavenir I, Forster A, et al. Alternating purine-pyrimidine tracts may promote chromosomal translocations seen in a variety of human lymphoid tumours. EMBO J. 1989;8(9):2621–2631. doi: 10.1002/j.1460-2075.1989.tb08402.x PubMed Central PMCID: PMC401268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh DB, Kim YG, Rich A. Z-DNA-binding proteins can act as potent effectors of gene expression in vivo. Proc Natl Acad Sci U S A. 2002;99(26):16666–71. Epub 20021216. doi: 10.1073/pnas.262672699 ; PubMed Central PMCID: PMC139201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A. 2003;100(12):6974–9. Epub 20030530. doi: 10.1073/pnas.0431131100 ; PubMed Central PMCID: PMC165815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36(17):2529–43. Epub 2017/07/19. doi: 10.15252/embj.201796476 ; PubMed Central PMCID: PMC5579359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, Shubina M, et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell. 2020;180(6):1115–29.e13. Epub 2020/03/24. doi: 10.1016/j.cell.2020.02.050 ; PubMed Central PMCID: PMC7153753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–7. Epub 2012/03/20. doi: 10.1016/j.chom.2012.01.016 ; PubMed Central PMCID: PMC3531981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181(9):6427–34. Epub 2008/10/23. doi: 10.4049/jimmunol.181.9.6427 ; PubMed Central PMCID: PMC3104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108(50):20054–9. Epub 2011/11/30. doi: 10.1073/pnas.1116302108 ; PubMed Central PMCID: PMC3250173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019 ; PubMed Central PMCID: PMC3664196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A. 2014;111(42):15072–7. Epub 20141006. doi: 10.1073/pnas.1408987111 ; PubMed Central PMCID: PMC4210347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, Rodriguez DA, et al. Sequential Engagement of Distinct MLKL Phosphatidylinositol-Binding Sites Executes Necroptosis. Mol Cell. 2016;61(4):589–601. Epub 20160204. doi: 10.1016/j.molcel.2016.01.011 ; PubMed Central PMCID: PMC4769881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. Epub 20131208. doi: 10.1038/ncb2883 ; PubMed Central PMCID: PMC8369836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5. Epub 2015/09/17. doi: 10.1038/nature15514 . [DOI] [PubMed] [Google Scholar]
- 99.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–78. Epub 20160714. doi: 10.15252/embj.201694696 ; PubMed Central PMCID: PMC5010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–25. Epub 20060704. doi: 10.1111/j.1462-5822.2006.00751.x . [DOI] [PubMed] [Google Scholar]
- 101.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat Commun. 2020;11(1):4561. Epub 20200911. doi: 10.1038/s41467-020-18443-3 ; PubMed Central PMCID: PMC7486936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–98. Epub 20151127. doi: 10.1038/cr.2015.139 ; PubMed Central PMCID: PMC4670995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol. 2013;25(6):363–72. Epub 20130226. doi: 10.1093/intimm/dxs161 . [DOI] [PubMed] [Google Scholar]
- 104.Shen C, Li R, Negro R, Cheng J, Vora SM, Fu TM, et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell. 2021;184(23):5759–74.e20. Epub 2021/10/23. doi: 10.1016/j.cell.2021.09.032 ; PubMed Central PMCID: PMC8643277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elliott JM, Rouge L, Wiesmann C, Scheer JM. Crystal structure of procaspase-1 zymogen domain reveals insight into inflammatory caspase autoactivation. J Biol Chem. 2009;284(10):6546–53. Epub 20081230. doi: 10.1074/jbc.M806121200 ; PubMed Central PMCID: PMC2649088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–91. Epub 20090701. doi: 10.4049/jimmunol.0901363 ; PubMed Central PMCID: PMC2824855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xing Y, Yao X, Li H, Xue G, Guo Q, Yang G, et al. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling-Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J Immunol. 2017;199(5):1561–6. Epub 20170724. doi: 10.4049/jimmunol.1700175 . [DOI] [PubMed] [Google Scholar]
- 108.Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, et al. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 2013;9(4):e1003256. Epub 20130411. doi: 10.1371/journal.ppat.1003256 ; PubMed Central PMCID: PMC3623797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20(13). Epub 2019/07/10. doi: 10.3390/ijms20133328 ; PubMed Central PMCID: PMC6651423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. Epub 2015/06/30. doi: 10.1038/nm.3893 ; PubMed Central PMCID: PMC4519035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, et al. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14(12):1247–55. Epub 20131103. doi: 10.1038/ni.2749 ; PubMed Central PMCID: PMC4813763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodgers MA, Bowman JW, Fujita H, Orazio N, Shi M, Liang Q, et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014;211(7):1333–47. Epub 20140623. doi: 10.1084/jem.20132486 ; PubMed Central PMCID: PMC4076580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang CH, Murti A, Pfeffer SR, Basu L, Kim JG, Pfeffer LM. IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc Natl Acad Sci U S A. 2000;97(25):13631–13636. doi: 10.1073/pnas.250477397 ; PubMed Central PMCID: PMC17627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336 PubMed Central PMCID: PMC286907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, et al. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178(6):3368–3372. doi: 10.4049/jimmunol.178.6.3368 . [DOI] [PubMed] [Google Scholar]
- 116.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277(24):21119–22. Epub 20020419. doi: 10.1074/jbc.C200179200 . [DOI] [PubMed] [Google Scholar]
- 117.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–604. Epub 20070629. doi: 10.1038/sj.cdd.4402194 ; PubMed Central PMCID: PMC3345951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. Epub 20100328. doi: 10.1038/ni.1864 ; PubMed Central PMCID: PMC2887480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, et al. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 2013;305(8):723–32. Epub 20130614. doi: 10.1007/s00403-013-1375-0 . [DOI] [PubMed] [Google Scholar]
- 120.Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X, et al. AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1β, and IL-18. Mediators Inflamm. 2014;2014:190860. Epub 20140220. doi: 10.1155/2014/190860 ; PubMed Central PMCID: PMC3950499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–13. Epub 20090121. doi: 10.1038/nature07710 ; PubMed Central PMCID: PMC2862225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–8. Epub 20090121. doi: 10.1038/nature07725 ; PubMed Central PMCID: PMC2726264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36(4):561–71. Epub 20120405. doi: 10.1016/j.immuni.2012.02.014 ; PubMed Central PMCID: PMC3334467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu F, Lan Z, Xin Z, He C, Guo Z, Xia X, et al. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2020;235(4):3207–21. Epub 2019/10/18. doi: 10.1002/jcp.29268 . [DOI] [PubMed] [Google Scholar]
- 125.Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269–80. Epub 2015/12/03. doi: 10.1002/eji.201545839 ; PubMed Central PMCID: PMC4758349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang Z, Wei F, Zhang Y, Wang T, Gao W, Yu S, et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat Microbiol. 2021;6(7):932–45. Epub 20210513. doi: 10.1038/s41564-021-00907-x . [DOI] [PubMed] [Google Scholar]
- 127.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. Epub 20101003. doi: 10.1038/ni.1932 ; PubMed Central PMCID: PMC3142795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coulon PG, Dhanushkodi N, Prakash S, Srivastava R, Roy S, Alomari NI, et al. NLRP3, NLRP12, and IFI16 Inflammasomes Induction and Caspase-1 Activation Triggered by Virulent HSV-1 Strains Are Associated With Severe Corneal Inflammatory Herpetic Disease. Front Immunol. 2019;10:1631. Epub 2019/08/02. doi: 10.3389/fimmu.2019.01631 ; PubMed Central PMCID: PMC6644090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, et al. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol. 2013;87(8):4417–31. Epub 2013/02/08. doi: 10.1128/JVI.03282-12 ; PubMed Central PMCID: PMC3624349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9(5):363–75. Epub 2011/05/18. doi: 10.1016/j.chom.2011.04.008 ; PubMed Central PMCID: PMC3113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11–12):2195–209. Epub 2016/04/07. doi: 10.1007/s00018-016-2194-1 ; PubMed Central PMCID: PMC4887533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042 ; PubMed Central PMCID: PMC3367386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27(11):1618–28. Epub 20070910. doi: 10.1038/sj.onc.1210796 . [DOI] [PubMed] [Google Scholar]
- 134.Kan X, Yin Y, Song C, Tan L, Qiu X, Liao Y, et al. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience. 2021;24(8):102837. Epub 2021/08/10. doi: 10.1016/j.isci.2021.102837 ; PubMed Central PMCID: PMC8326413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Komissarov AA, Karaseva MA, Roschina MP, Shubin AV, Lunina NA, Kostrov SV, et al. Individual Expression of Hepatitis A Virus 3C Protease Induces Ferroptosis in Human Cells In Vitro. Int J Mol Sci. 2021;22(15). Epub 2021/08/08. doi: 10.3390/ijms22157906 ; PubMed Central PMCID: PMC8348068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C, Liu J, Hou W, Kang R, Tang D. STING1 Promotes Ferroptosis Through MFN1/2-Dependent Mitochondrial Fusion. Front Cell Dev Biol. 2021;9:698679. Epub 2021/07/02. doi: 10.3389/fcell.2021.698679 ; PubMed Central PMCID: PMC8236825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17(4):948–60. Epub 2020/03/19. doi: 10.1080/15548627.2020.1739447 ; PubMed Central PMCID: PMC8078708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoon MJ, Lee AR, Jeong SA, Kim YS, Kim JY, Kwon YJ, et al. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget. 2014;5(16):6816–31. Epub 2014/08/26. doi: 10.18632/oncotarget.2256 ; PubMed Central PMCID: PMC4196165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kessel D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem Photobiol. 2019;95(1):119–25. Epub 2018/06/09. doi: 10.1111/php.12952 ; PubMed Central PMCID: PMC6286706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Monel B, Compton AA, Bruel T, Amraoui S, Burlaud-Gaillard J, Roy N, et al. Zika virus induces massive cytoplasmic vacuolization and paraptosis-like death in infected cells. EMBO J. 2017;36(12):1653–68. Epub 2017/05/06. doi: 10.15252/embj.201695597 ; PubMed Central PMCID: PMC5470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang X, Huang Y, Yang Y, Wei S, Qin Q. Involvement of fish signal transducer and activator of transcription 3 (STAT3) in SGIV replication and virus induced paraptosis. Fish Shellfish Immunol. 2014;41(2):308–16. Epub 2014/09/18. doi: 10.1016/j.fsi.2014.09.011 . [DOI] [PubMed] [Google Scholar]
- 142.Smith GL, Benfield CTO, Maluquer de Motes C, Mazzon M, Ember SWJ, Ferguson BJ, et al. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94(Pt 11):2367–92. Epub 2013/09/04. doi: 10.1099/vir.0.055921-0 . [DOI] [PubMed] [Google Scholar]
- 143.Banjara S, Suraweera CD, Hinds MG, Kvansakul M. The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms. Biomolecules. 2020;10(1). Epub 2020/01/17. doi: 10.3390/biom10010128 ; PubMed Central PMCID: PMC7022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor JM, Quilty D, Banadyga L, Barry M. The vaccinia virus protein F1L interacts with Bim and inhibits activation of the pro-apoptotic protein Bax. J Biol Chem. 2006;281(51):39728–39. Epub 2006/11/01. doi: 10.1074/jbc.M607465200 . [DOI] [PubMed] [Google Scholar]
- 145.Campbell S, Thibault J, Mehta N, Colman PM, Barry M, Kvansakul M. Structural insight into BH3 domain binding of vaccinia virus antiapoptotic F1L. J Virol. 2014;88(15):8667–77. Epub 2014/05/23. doi: 10.1128/JVI.01092-14 ; PubMed Central PMCID: PMC4135939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, et al. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69(4):597–604. Epub 1992/05/15. doi: 10.1016/0092-8674(92)90223-y . [DOI] [PubMed] [Google Scholar]
- 147.Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272(12):7797–800. Epub 1997/03/21. doi: 10.1074/jbc.272.12.7797 . [DOI] [PubMed] [Google Scholar]
- 148.Suraweera CD, Hinds MG, Kvansakul M. Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens. 2020;10(1). Epub 2020/12/31. doi: 10.3390/pathogens10010006 ; PubMed Central PMCID: PMC7823800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bloomer DT, Kitevska-Ilioski T, Pantaki-Eimany D, Ji Y, Miles MA, Heras B, et al. CrmA orthologs from diverse poxviruses potently inhibit caspases-1 and -8, yet cleavage site mutagenesis frequently produces caspase-1-specific variants. Biochem J. 2019;476(9):1335–57. Epub 2019/04/18. doi: 10.1042/BCJ20190202 . [DOI] [PubMed] [Google Scholar]
- 150.Daley-Bauer LP, Roback L, Crosby LN, McCormick AL, Feng Y, Kaiser WJ, et al. Mouse cytomegalovirus M36 and M45 death suppressors cooperate to prevent inflammation resulting from antiviral programmed cell death pathways. Proc Natl Acad Sci U S A. 2017;114(13):E2786–e95. Epub 2017/03/16. doi: 10.1073/pnas.1616829114 ; PubMed Central PMCID: PMC5380087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98(14):7829–34. Epub 2001/06/28. doi: 10.1073/pnas.141108798 ; PubMed Central PMCID: PMC35427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dufour F, Sasseville AM, Chabaud S, Massie B, Siegel RM, Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis. 2011;16(3):256–71. Epub 2010/11/26. doi: 10.1007/s10495-010-0560-2 . [DOI] [PubMed] [Google Scholar]
- 153.Ruder B, Günther C, Stürzl M, Neurath MF, Cesarman E, Ballon G, et al. Viral FLIP blocks Caspase-8 driven apoptosis in the gut in vivo. PLoS ONE. 2020;15(1):e0228441. Epub 2020/01/31. doi: 10.1371/journal.pone.0228441 ; PubMed Central PMCID: PMC6992192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bélanger C, Gravel A, Tomoiu A, Janelle ME, Gosselin J, Tremblay MJ, et al. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J Hum Virol. 2001;4(2):62–73. Epub 2001/07/05. . [PubMed] [Google Scholar]
- 155.Clem RJ. Viral IAPs, then and now. Semin Cell Dev Biol. 2015;39:72–9. Epub 2015/02/06. doi: 10.1016/j.semcdb.2015.01.011 . [DOI] [PubMed] [Google Scholar]
- 156.Graham SC, Bahar MW, Abrescia NG, Smith GL, Stuart DI, Grimes JM. Structure of CrmE, a virus-encoded tumour necrosis factor receptor. J Mol Biol. 2007;372(3):660–71. Epub 2007/08/08. doi: 10.1016/j.jmb.2007.06.082 . [DOI] [PubMed] [Google Scholar]
- 157.Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol. 2003;3(1):36–50. Epub 2003/01/04. doi: 10.1038/nri980 . [DOI] [PubMed] [Google Scholar]
- 158.Xu X, Nash P, McFadden G. Myxoma virus expresses a TNF receptor homolog with two distinct functions. Virus Genes. 2000;21(1–2):97–109. Epub 2000/10/07. . [PubMed] [Google Scholar]
- 159.Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89(11):4825–9. Epub 1992/06/01. doi: 10.1073/pnas.89.11.4825 PubMed Central PMCID: PMC49180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gubser C, Bergamaschi D, Hollinshead M, Lu X, van Kuppeveld FJ, Smith GL. A new inhibitor of apoptosis from vaccinia virus and eukaryotes. PLoS Pathog. 2007;3(2):e17. Epub 2007/02/27. doi: 10.1371/journal.ppat.0030017 ; PubMed Central PMCID: PMC1803007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veyer DL, Maluquer de Motes C, Sumner RP, Ludwig L, Johnson BF, Smith GL. Analysis of the anti-apoptotic activity of four vaccinia virus proteins demonstrates that B13 is the most potent inhibitor in isolation and during viral infection. J Gen Virol. 2014;95(Pt 12):2757–68. Epub 2014/08/06. doi: 10.1099/vir.0.068833-0 ; PubMed Central PMCID: PMC4233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kinchington PR, Leger AJ, Guedon JM, Hendricks RL. Herpes simplex virus and varicella zoster virus, the house guests who never leave. Herpes. 2012;3(1):5. Epub 2012/06/14. doi: 10.1186/2042-4280-3-5 ; PubMed Central PMCID: PMC3541251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ambagala AP, Cohen JI. Varicella-Zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81(15):7844–51. Epub 2007/05/18. doi: 10.1128/JVI.00325-07 ; PubMed Central PMCID: PMC1951283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Benetti L, Roizman B. In transduced cells, the US3 protein kinase of herpes simplex virus 1 precludes activation and induction of apoptosis by transfected procaspase 3. J Virol. 2007;81(19):10242–8. Epub 2007/07/20. doi: 10.1128/JVI.00820-07 ; PubMed Central PMCID: PMC2045497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Benetti L, Munger J, Roizman B. The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J Virol. 2003;77(11):6567–73. Epub 2003/05/14. doi: 10.1128/jvi.77.11.6567-6573.2003 ; PubMed Central PMCID: PMC155029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Seo J, Nam YW, Kim S, Oh DB, Song J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp Mol Med. 2021;53(6):1007–17. Epub 2021/06/03. doi: 10.1038/s12276-021-00634-7 ; PubMed Central PMCID: PMC8166896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–13. Epub 2010/04/24. doi: 10.1016/j.chom.2010.03.006 ; PubMed Central PMCID: PMC4279434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Upton JW, Kaiser WJ. DAI Another Way: Necroptotic Control of Viral Infection. Cell Host Microbe. 2017;21(3):290–3. Epub 2017/03/11. doi: 10.1016/j.chom.2017.01.016 . [DOI] [PubMed] [Google Scholar]
- 169.Muscolino E, Schmitz R, Loroch S, Caragliano E, Schneider C, Rizzato M, et al. Herpesviruses induce aggregation and selective autophagy of host signalling proteins NEMO and RIPK1 as an immune-evasion mechanism. Nat Microbiol. 2020;5(2):331–42. Epub 2019/12/18. doi: 10.1038/s41564-019-0624-1 . [DOI] [PubMed] [Google Scholar]
- 170.Shanmugam N, Baker M, Sanz-Hernandez M, Sierecki E, Gambin Y, Steain M, et al. Herpes simplex virus encoded ICP6 protein forms functional amyloid assemblies with necroptosis-associated host proteins. Biophys Chem. 2021;269:106524. Epub 2020/12/22. doi: 10.1016/j.bpc.2020.106524 . [DOI] [PubMed] [Google Scholar]
- 171.Hu H, Wu X, Wu G, Nan N, Zhang J, Zhu X, et al. RIP3-mediated necroptosis is regulated by inter-filament assembly of RIP homotypic interaction motif. Cell Death Differ. 2021;28(1):251–66. Epub 2020/08/02. doi: 10.1038/s41418-020-0598-9 ; PubMed Central PMCID: PMC7853141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Steain M, Baker M, Pham CLL, Shanmugam N, Gambin Y, Sierecki E, et al. Varicella zoster virus encodes a viral decoy RHIM to inhibit cell death. PLoS Pathog. 2020;16(7):e1008473. Epub 2020/07/11. doi: 10.1371/journal.ppat.1008473 ; PubMed Central PMCID: PMC7375649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Muscolino E, Luoto L-M, Brune W. Viral Induced Protein Aggregation: A Mechanism of Immune Evasion. Int J Mol Sci. 2021;22(17):9624. doi: 10.3390/ijms22179624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu X, Li Y, Peng S, Yu X, Li W, Shi F, et al. Epstein-Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination. Cell Death Dis. 2018;9(2):53. doi: 10.1038/s41419-017-0081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shi F, Zhou M, Shang L, Du Q, Li Y, Xie L, et al. EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter. Theranostics. 2019;9(9):2424–38. Epub 2019/05/28. doi: 10.7150/thno.30941 ; PubMed Central PMCID: PMC6525991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Z, Nailwal H, Rector J, Rahman MM, Sam R, McFadden G, et al. A class of viral inducer of degradation of the necroptosis adaptor RIPK3 regulates virus-induced inflammation. Immunity. 2021;54(2):247–58.e7. Epub 2021/01/15. doi: 10.1016/j.immuni.2020.11.020 ; PubMed Central PMCID: PMC7878414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koehler H, Cotsmire S, Zhang T, Balachandran S, Upton JW, Langland J, et al. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe. 2021;29(8):1266–76.e5. Epub 2021/07/01. doi: 10.1016/j.chom.2021.05.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koehler HS, Jacobs BL. Subversion of Programed Cell Death by Poxviruses. Curr Top Microbiol Immunol. 2021. Epub 2021/01/29. doi: 10.1007/82_2020_229 ; PubMed Central PMCID: PMC8319220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Petrie EJ, Sandow JJ, Lehmann WIL, Liang LY, Coursier D, Young SN, et al. Viral MLKL Homologs Subvert Necroptotic Cell Death by Sequestering Cellular RIPK3. Cell Rep. 2019;28(13):3309–19.e5. Epub 2019/09/26. doi: 10.1016/j.celrep.2019.08.055 . [DOI] [PubMed] [Google Scholar]
- 180.Muscolino E, Castiglioni C, Brixel R, Frascaroli G, Brune W. Species-Specific Inhibition of Necroptosis by HCMV UL36. Viruses. 2021;13(11). Epub 2021/11/28. doi: 10.3390/v13112134 ; PubMed Central PMCID: PMC8621378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fletcher-Etherington A, Nobre L, Nightingale K, Antrobus R, Nichols J, Davison AJ, et al. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc Natl Acad Sci U S A. 2020;117(31):18771–9. Epub 2020/07/22. doi: 10.1073/pnas.2001887117 ; PubMed Central PMCID: PMC7414183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shi F, Lv Q, Wang T, Xu J, Xu W, Shi Y, et al. Coronaviruses Nsp5 Antagonizes Porcine Gasdermin D-Mediated Pyroptosis by Cleaving Pore-Forming p30 Fragment. MBio. 2022;13(1):e0273921. Epub 2022/01/12. doi: 10.1128/mbio.02739-21 ; PubMed Central PMCID: PMC8749417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wen W, Li X, Wang H, Zhao Q, Yin M, Liu W, et al. Seneca Valley Virus 3C Protease Induces Pyroptosis by Directly Cleaving Porcine Gasdermin D. J Immunol. 2021;207(1):189–99. Epub 2021/06/30. doi: 10.4049/jimmunol.2001030 . [DOI] [PubMed] [Google Scholar]
- 184.Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J. Enterovirus 71 Inhibits Pyroptosis through Cleavage of Gasdermin D. J Virol. 2017;91(18). Epub 2017/07/07. doi: 10.1128/JVI.01069-17 ; PubMed Central PMCID: PMC5571240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao G, Li T, Liu X, Zhang T, Zhang Z, Kang L, et al. African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D. J Biol Chem. 2022;298(1):101480. Epub 2021/12/11. doi: 10.1016/j.jbc.2021.101480 ; PubMed Central PMCID: PMC8728581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma J, Zhu F, Zhao M, Shao F, Yu D, Ma J, et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking Gasdermin D cleavage. EMBO J. 2021;40(18):e108249. Epub 2021/07/24. doi: 10.15252/embj.2021108249 ; PubMed Central PMCID: PMC8420271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang H, Lei X, Xiao X, Yang C, Lu W, Huang Z, et al. Reciprocal Regulation between Enterovirus 71 and the NLRP3 Inflammasome. Cell Rep. 2015;12(1):42–8. Epub 2015/06/30. doi: 10.1016/j.celrep.2015.05.047 . [DOI] [PubMed] [Google Scholar]
- 188.Komatsu T, Tanaka Y, Kitagawa Y, Koide N, Naiki Y, Morita N, et al. Sendai Virus V Protein Inhibits the Secretion of Interleukin-1β by Preventing NLRP3 Inflammasome Assembly. J Virol. 2018;92(19). Epub 2018/07/20. doi: 10.1128/JVI.00842-18 ; PubMed Central PMCID: PMC6146803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dorfleutner A, Talbott SJ, Bryan NB, Funya KN, Rellick SL, Reed JC, et al. A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes. 2007;35(3):685–94. Epub 2007/08/07. doi: 10.1007/s11262-007-0141-9 ; PubMed Central PMCID: PMC4257706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. 2012;189(8):3795–9. Epub 2012/09/18. doi: 10.4049/jimmunol.1200312 . [DOI] [PubMed] [Google Scholar]
- 191.Moriyama M, Chen IY, Kawaguchi A, Koshiba T, Nagata K, Takeyama H, et al. The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1β Secretion. J Virol. 2016;90(8):4105–14. Epub 2016/02/13. doi: 10.1128/JVI.00120-16 ; PubMed Central PMCID: PMC4810543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Komune N, Ichinohe T, Ito M, Yanagi Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J Virol. 2011;85(24):13019–26. Epub 2011/10/14. doi: 10.1128/JVI.05942-11 ; PubMed Central PMCID: PMC3233149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bianchi F, Pretto S, Tagliabue E, Balsari A, Sfondrini L. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol Ther. 2017;18(10):747–56. Epub 2017/09/07. doi: 10.1080/15384047.2017.1373220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang-Bishop L, Wehbe M, Shae D, James J, Hacker BC, Garland K, et al. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J Immunother Cancer. 2020;8(1). Epub 2020/03/15. doi: 10.1136/jitc-2019-000282 ; PubMed Central PMCID: PMC7069313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89(6):812–8. doi: 10.1016/j.biochi.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 196.Hubel P, Urban C, Bergant V, Schneider WM, Knauer B, Stukalov A, et al. A protein-interaction network of interferon-stimulated genes extends the innate immune system landscape. Nat Immunol. 2019;20(4):493–502. doi: 10.1038/s41590-019-0323-3 [DOI] [PubMed] [Google Scholar]
- 197.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69(6):912–20. Epub 2001/06/19. . [PubMed] [Google Scholar]
- 198.Der SD, Zhou A, Williams BRG, Silverman RH. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc Natl Acad Sci. 1998;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/a:1023668705040 [DOI] [PubMed] [Google Scholar]
- 200.Savidis G, Perreira Jill M, Portmann Jocelyn M, Meraner P, Guo Z, Green S, et al. The IFITMs Inhibit Zika Virus Replication. Cell Rep. 2016;15(11):2323–30. doi: 10.1016/j.celrep.2016.05.074 [DOI] [PubMed] [Google Scholar]
- 201.Stawowczyk M, Van Scoy S, Kumar KP, Reich NC. The interferon stimulated gene 54 promotes apoptosis. J Biol Chem. 2011;286(9):7257–66. Epub 2010/12/31. doi: 10.1074/jbc.M110.207068 ; PubMed Central PMCID: PMC3044982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu MY, Liao F. Interferon-stimulated gene ISG12b2 is localized to the inner mitochondrial membrane and mediates virus-induced cell death. Cell Death Differ. 2011;18(6):925–936. doi: 10.1038/cdd.2010.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol Ther. 2014;22(2):251–256. doi: 10.1038/mt.2013.220 ; PubMed Central PMCID: PMC3916036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15(10):1564–71. Epub 2008/06/14. doi: 10.1038/cdd.2008.83 . [DOI] [PubMed] [Google Scholar]
- 205.Maluquer de Motes C, Cooray S, Ren H, Almeida GM, McGourty K, Bahar MW, et al. Inhibition of apoptosis and NF-κB activation by vaccinia protein N1 occur via distinct binding surfaces and make different contributions to virulence. PLoS Pathog. 2011;7(12):e1002430. Epub 2011/12/24. doi: 10.1371/journal.ppat.1002430 ; PubMed Central PMCID: PMC3240604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fedosyuk S, Grishkovskaya I, de Almeida Ribeiro E, Skern T Jr, Characterization and structure of the vaccinia virus NF-κB antagonist A46. J Biol Chem. 2014;289(6):3749–62. Epub 2013/12/21. doi: 10.1074/jbc.M113.512756 ; PubMed Central PMCID: PMC3916572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Neidel S, Ren H, Torres AA, Smith GL. NF-κB activation is a turn on for vaccinia virus phosphoprotein A49 to turn off NF-κB activation. Proc Natl Acad Sci U S A. 2019;116(12):5699–704. Epub 2019/03/02. doi: 10.1073/pnas.1813504116 ; PubMed Central PMCID: PMC6431142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neidel S, Maluquer de Motes C, Mansur DS, Strnadova P, Smith GL, Graham SC. Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family. J Biol Chem. 2015;290(10):5991–6002. Epub 2015/01/22. doi: 10.1074/jbc.M114.624650 ; PubMed Central PMCID: PMC4358236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Graham SC, Bahar MW, Cooray S, Chen RA, Whalen DM, Abrescia NG, et al. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 2008;4(8):e1000128. Epub 2008/08/16. doi: 10.1371/journal.ppat.1000128 ; PubMed Central PMCID: PMC2494871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Di Pilato M, Mejías-Pérez E, Sorzano COS, Esteban M. Distinct Roles of Vaccinia Virus NF-κB Inhibitor Proteins A52, B15, and K7 in the Immune Response. J Virol. 2017;91(13). Epub 2017/04/21. doi: 10.1128/jvi.00575-17 ; PubMed Central PMCID: PMC5469261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kettle S, Alcamí A, Khanna A, Ehret R, Jassoy C, Smith GL. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78 (Pt 3):677–85. Epub 1997/03/01. doi: 10.1099/0022-1317-78-3-677 [DOI] [PubMed] [Google Scholar]
- 212.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70(9):6479–85. Epub 1996/09/01. doi: 10.1128/jvi.70.9.6479-6485.1996 ; PubMed Central PMCID: PMC190684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kettle S, Blake NW, Law KM, Smith GL. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206(1):136–47. Epub 1995/01/10. doi: 10.1016/s0042-6822(95)80028-x . [DOI] [PubMed] [Google Scholar]
- 214.Shisler JL, Isaacs SN, Moss B. Vaccinia Virus Serpin-1 Deletion Mutant Exhibits a Host Range Defect Characterized by Low Levels of Intermediate and Late mRNAs. Virology. 1999;262(2):298–311. doi: 10.1006/viro.1999.9884 [DOI] [PubMed] [Google Scholar]
- 215.Turner PC, Moyer RW. Orthopoxvirus fusion inhibitor glycoprotein SPI-3 (open reading frame K2L) contains motifs characteristic of serine proteinase inhibitors that are not required for control of cell fusion. J Virol. 1995;69(10):5978–5987. doi: 10.1128/JVI.69.10.5978-5987.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reading PC, Khanna A, Smith GL. Vaccinia virus CrmE encodes a soluble and cell surface tumor necrosis factor receptor that contributes to virus virulence. Virology. 2002;292(2):285–98. Epub 2002/03/07. doi: 10.1006/viro.2001.1236 . [DOI] [PubMed] [Google Scholar]
- 217.Myskiw C, Arsenio J, Hammett C, van Bruggen R, Deschambault Y, Beausoleil N, et al. Comparative analysis of poxvirus orthologues of the vaccinia virus E3 protein: modulation of protein kinase R activity, cytokine responses, and virus pathogenicity. J Virol. 2011;85(23):12280–91. Epub 2011/09/16. doi: 10.1128/JVI.05505-11 ; PubMed Central PMCID: PMC3209343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu SW, Katsafanas GC, Liu R, Wyatt LS, Moss B. Poxvirus decapping enzymes enhance virulence by preventing the accumulation of dsRNA and the induction of innate antiviral responses. Cell Host Microbe. 2015;17(3):320–31. Epub 2015/03/15. doi: 10.1016/j.chom.2015.02.002 ; PubMed Central PMCID: PMC4359750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ryerson MR, Richards MM, Kvansakul M, Hawkins CJ, Shisler JL. Vaccinia Virus Encodes a Novel Inhibitor of Apoptosis That Associates with the Apoptosome. J Virol. 2017;91(23). Epub 2017/09/15. doi: 10.1128/JVI.01385-17 ; PubMed Central PMCID: PMC5686757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marshall B, Puthalakath H, Caria S, Chugh S, Doerflinger M, Colman PM, et al. Variola virus F1L is a Bcl-2-like protein that unlike its vaccinia virus counterpart inhibits apoptosis independent of Bim. Cell Death Dis. 2015;6(3):e1680. Epub 2015/03/15. doi: 10.1038/cddis.2015.52 ; PubMed Central PMCID: PMC4385930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pontejo SM, Alejo A, Alcami A. Comparative Biochemical and Functional Analysis of Viral and Human Secreted Tumor Necrosis Factor (TNF) Decoy Receptors. J Biol Chem. 2015;290(26):15973–84. Epub 2015/05/06. doi: 10.1074/jbc.M115.650119 ; PubMed Central PMCID: PMC4481203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gileva IP, Nepomnyashchikh TS, Antonets DV, Lebedev LR, Kochneva GV, Grazhdantseva AV, et al. Properties of the recombinant TNF-binding proteins from variola, monkeypox, and cowpox viruses are different. Biochim Biophys Acta. 2006;1764(11):1710–8. Epub 2006/10/31. doi: 10.1016/j.bbapap.2006.09.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Panus JF, Smith CA, Ray CA, Smith TD, Patel DD, Pickup DJ. Cowpox virus encodes a fifth member of the tumor necrosis factor receptor family: a soluble, secreted CD30 homologue. Proc Natl Acad Sci U S A. 2002;99(12):8348–53. Epub 2002/05/30. doi: 10.1073/pnas.122238599 ; PubMed Central PMCID: PMC123070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mehta N, Taylor J, Quilty D, Barry M. Ectromelia virus encodes an anti-apoptotic protein that regulates cell death. Virology. 2015;475:74–87. Epub 2014/12/03. doi: 10.1016/j.virol.2014.10.023 . [DOI] [PubMed] [Google Scholar]
- 225.Saraiva M, Smith P, Fallon PG, Alcami A. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med. 2002;196(6):829–39. Epub 2002/09/18. doi: 10.1084/jem.20020319 ; PubMed Central PMCID: PMC2194064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kvansakul M, van Delft MF, Lee EF, Gulbis JM, Fairlie WD, Huang DC, et al. A structural viral mimic of prosurvival Bcl-2: a pivotal role for sequestering proapoptotic Bax and Bak. Mol Cell. 2007;25(6):933–42. Epub 2007/03/28. doi: 10.1016/j.molcel.2007.02.004 . [DOI] [PubMed] [Google Scholar]
- 227.Macen JL, Upton C, Nation N, McFadden G. SERP1, a serine proteinase inhibitor encoded by myxoma virus, is a secreted glycoprotein that interferes with inflammation. Virology. 1993;195(2):348–63. Epub 1993/08/01. doi: 10.1006/viro.1993.1385 . [DOI] [PubMed] [Google Scholar]
- 228.Turner PC, Sancho MC, Thoennes SR, Caputo A, Bleackley RC, Moyer RW. Myxoma virus Serp2 is a weak inhibitor of granzyme B and interleukin-1beta-converting enzyme in vitro and unlike CrmA cannot block apoptosis in cowpox virus-infected cells. J Virol. 1999;73(8):6394–404. Epub 1999/07/10. doi: 10.1128/JVI.73.8.6394-6404.1999 ; PubMed Central PMCID: PMC112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guerin JL, Gelfi J, Camus C, Delverdier M, Whisstock JC, Amardeihl MF, et al. Characterization and functional analysis of Serp3: a novel myxoma virus-encoded serpin involved in virulence. J Gen Virol. 2001;82(Pt 6):1407–17. Epub 2001/05/23. doi: 10.1099/0022-1317-82-6-1407 . [DOI] [PubMed] [Google Scholar]
- 230.Schreiber M, Rajarathnam K, McFadden G. Myxoma virus T2 protein, a tumor necrosis factor (TNF) receptor homolog, is secreted as a monomer and dimer that each bind rabbit TNFalpha, but the dimer is a more potent TNF inhibitor. J Biol Chem. 1996;271(23):13333–41. Epub 1996/06/07. doi: 10.1074/jbc.271.23.13333 . [DOI] [PubMed] [Google Scholar]
- 231.Cao JX, Teoh ML, Moon M, McFadden G, Evans DH. Leporipoxvirus Cu-Zn superoxide dismutase homologs inhibit cellular superoxide dismutase, but are not essential for virus replication or virulence. Virology. 2002;296(1):125–35. Epub 2002/05/31. doi: 10.1006/viro.2002.1383 . [DOI] [PubMed] [Google Scholar]
- 232.Rahman MM, Liu J, Chan WM, Rothenburg S, McFadden G. Myxoma virus protein M029 is a dual function immunomodulator that inhibits PKR and also conscripts RHA/DHX9 to promote expanded host tropism and viral replication. PLoS Pathog. 2013;9(7):e1003465. Epub 2013/07/16. doi: 10.1371/journal.ppat.1003465 ; PubMed Central PMCID: PMC3701710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, et al. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248(4958):1019–23. Epub 1990/05/25. doi: 10.1126/science.2160731 . [DOI] [PubMed] [Google Scholar]
- 234.Suraweera CD, Anasir MI, Chugh S, Javorsky A, Impey RE, Hasan Zadeh M, et al. Structural insight into tanapoxvirus-mediated inhibition of apoptosis. FEBS J. 2020;287(17):3733–50. Epub 2020/05/16. doi: 10.1111/febs.15365 . [DOI] [PubMed] [Google Scholar]
- 235.Yang Z, West AP Jr., Bjorkman PJ. Crystal structure of TNFalpha complexed with a poxvirus MHC-related TNF binding protein. Nat Struct Mol Biol. 2009;16(11):1189–91. Epub 20091018. doi: 10.1038/nsmb.1683 ; PubMed Central PMCID: PMC2819277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Westphal D, Ledgerwood EC, Hibma MH, Fleming SB, Whelan EM, Mercer AA. A novel Bcl-2-like inhibitor of apoptosis is encoded by the parapoxvirus ORF virus. J Virol. 2007;81(13):7178–88. Epub 20070502. doi: 10.1128/JVI.00404-07 ; PubMed Central PMCID: PMC1933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Westphal D, Ledgerwood EC, Tyndall JD, Hibma MH, Ueda N, Fleming SB, et al. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis. 2009;14(11):1317–1330. doi: 10.1007/s10495-009-0403-1 . [DOI] [PubMed] [Google Scholar]
- 238.Beckham JD, Tuttle KD, Tyler KL. Caspase-3 activation is required for reovirus-induced encephalitis in vivo. J Neurovirol. 2010;16(4):306–317. doi: 10.3109/13550284.2010.499890 ; PubMed Central PMCID: PMC3023174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Suraweera CD, Hinds MG, Kvansakul M. Crystal structures of ORFV125 provide insight into orf virus-mediated inhibition of apoptosis. Biochem J. 2020;477(23):4527–4541. doi: 10.1042/BCJ20200776 ; PubMed Central PMCID: PMC7719400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Okamoto T, Campbell S, Mehta N, Thibault J, Colman PM, Barry M, et al. Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins. J Virol. 2012;86(21):11501–11. Epub 20120815. doi: 10.1128/JVI.01115-12 ; PubMed Central PMCID: PMC3486325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burton DR, Caria S, Marshall B, Barry M, Kvansakul M. Structural basis of Deerpox virus-mediated inhibition of apoptosis. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 8):1593–603. Epub 20150728. doi: 10.1107/S1399004715009402 . [DOI] [PubMed] [Google Scholar]
- 242.Banadyga L, Veugelers K, Campbell S, Barry M. The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis. J Virol. 2009;83(14):7085–98. Epub 20090513. doi: 10.1128/JVI.00437-09 ; PubMed Central PMCID: PMC2704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Anasir MI, Caria S, Skinner MA, Kvansakul M. Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039. J Biol Chem. 2017;292(22):9010–21. Epub 20170414. doi: 10.1074/jbc.M116.768879 ; PubMed Central PMCID: PMC5454088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Anasir MI, Baxter AA, Poon IKH, Hulett MD, Kvansakul M. Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses. 2017;9(10). Epub 20171020. doi: 10.3390/v9100305 ; PubMed Central PMCID: PMC5691656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Coutu J, Ryerson MR, Bugert J, Nichols DB. The Molluscum Contagiosum Virus protein MC163 localizes to the mitochondria and dampens mitochondrial mediated apoptotic responses. Virology. 2017;505:91–101. Epub 20170221. doi: 10.1016/j.virol.2017.02.017 . [DOI] [PubMed] [Google Scholar]
- 246.Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201–52. Epub 20150113. doi: 10.1016/bs.aivir.2014.11.004 . [DOI] [PubMed] [Google Scholar]
- 247.Murao LE, Shisler JL. The MCV MC159 protein inhibits late, but not early, events of TNF-alpha-induced NF-kappaB activation. Virology. 2005;340(2):255–264. doi: 10.1016/j.virol.2005.06.036 . [DOI] [PubMed] [Google Scholar]
- 248.Gil J, Rullas J, Alcami J, Esteban M. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-kappaB activation and apoptosis induced by PKR. J Gen Virol. 2001;82(Pt 12):3027–3034. doi: 10.1099/0022-1317-82-12-3027 . [DOI] [PubMed] [Google Scholar]
- 249.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386(6624):517–521. doi: 10.1038/386517a0 . [DOI] [PubMed] [Google Scholar]
- 250.Senkevich TG, Bugert JJ, Sisler JR, Koonin EV, Darai G, Moss B. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science. 1996;273(5276):813–816. doi: 10.1126/science.273.5276.813 . [DOI] [PubMed] [Google Scholar]
- 251.Martsevich S, Metelitsa VI, Kozyreva MP, Blagodatskikh SV, Simkhaev LS, Piotrovskii VK. New drug forms of isosorbide dinitrate: the problem of an objective evaluation in patients with ischemic heart disease. Farmakol Toksikol. 1991;54(3):53–56. . [PubMed] [Google Scholar]
- 252.Reddy V, Sadigh Y, Tang N, Yao Y, Nair V. Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition. J Virol. 2020;94(10). Epub 20200504. doi: 10.1128/JVI.02049-19 ; PubMed Central PMCID: PMC7199394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Derfuss T, Fickenscher H, Kraft MS, Henning G, Lengenfelder D, Fleckenstein B, et al. Antiapoptotic activity of the herpesvirus saimiri-encoded Bcl-2 homolog: stabilization of mitochondria and inhibition of caspase-3-like activity. J Virol. 1998;72(7):5897–5904. doi: 10.1128/JVI.72.7.5897-5904.1998 PubMed Central PMCID: PMC110393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27(50):6507–6521. doi: 10.1038/onc.2008.315 ; PubMed Central PMCID: PMC2657599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Munger J, Hagglund R, Roizman B. Infected cell protein No. 22 is subject to proteolytic cleavage by caspases activated by a mutant that induces apoptosis. Virology. 2003;305(2):364–370. doi: 10.1006/viro.2002.1728 . [DOI] [PubMed] [Google Scholar]
- 256.Gerada C, Steain M, McSharry BP, Slobedman B, Abendroth A. Varicella-Zoster Virus ORF63 Protects Human Neuronal and Keratinocyte Cell Lines from Apoptosis and Changes Its Localization upon Apoptosis Induction. J Virol. 2018;92(12). Epub 20180529. doi: 10.1128/JVI.00338-18 ; PubMed Central PMCID: PMC5974485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Maruzuru Y, Fujii H, Oyama M, Kozuka-Hata H, Kato A, Kawaguchi Y. Roles of p53 in herpes simplex virus 1 replication. J Virol. 2013;87(16):9323–32. Epub 20130619. doi: 10.1128/JVI.01581-13 ; PubMed Central PMCID: PMC3754062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chang CD, Lin PY, Liao MH, Chang CI, Hsu JL, Yu FL, et al. Suppression of apoptosis by pseudorabies virus Us3 protein kinase through the activation of PI3-K/Akt and NF-kappaB pathways. Res Vet Sci. 2013;95(2):764–74. Epub 20130705. doi: 10.1016/j.rvsc.2013.06.003 . [DOI] [PubMed] [Google Scholar]
- 259.Wang K, Ni L, Wang S, Zheng C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-kappaB activation. J Virol. 2014;88(14):7941–51. Epub 20140507. doi: 10.1128/JVI.03394-13 ; PubMed Central PMCID: PMC4097809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang X, Patenode C, Roizman B. US3 protein kinase of HSV-1 cycles between the cytoplasm and nucleus and interacts with programmed cell death protein 4 (PDCD4) to block apoptosis. Proc Natl Acad Sci U S A. 2011;108:14632–14636. doi: 10.1073/pnas.1111942108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Brzozowska A, Lipińska AD, Derewońko N, Lesiak D, Rychłowski M, Rąbalski Ł, et al. Inhibition of apoptosis in BHV-1-infected cells depends on Us3 serine/threonine kinase and its enzymatic activity. Virology. 2018;513:136–145. doi: 10.1016/j.virol.2017.09.029 . [DOI] [PubMed] [Google Scholar]
- 262.Aubert M, Chen Z, Lang R, Dang CH, Fowler C, Sloan DD, et al. The antiapoptotic herpes simplex virus glycoprotein J localizes to multiple cellular organelles and induces reactive oxygen species formation. J Virol. 2008;82:617–629. doi: 10.1128/JVI.01341-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jerome KR, Fox R, Chen Z, Sears AE, Lee H-y, Corey L. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J Virol. 1999;73:8950–8957. doi: 10.1128/JVI.73.11.8950-8957.1999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffrè-Cuculletto M, Venuti A, et al. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem Pharmacol. 2008;76:1522–1532. doi: 10.1016/j.bcp.2008.07.030 . [DOI] [PubMed] [Google Scholar]
- 265.Marino-Merlo F, Papaianni E, Medici MA, Macchi B, Grelli S, Mosca C, et al. HSV-1-induced activation of NF-κB protects U937 monocytic cells against both virus replication and apoptosis. Cell Death Dis. 2016;7. doi: 10.1038/cddis.2016.250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhou G, Avitabile E, Campadelli-Fiume G, Roizman B. The domains of glycoprotein D required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1. J Virol. 2003;77:3759–3767. doi: 10.1128/jvi.77.6.3759-3767.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J Biol Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200 . [DOI] [PubMed] [Google Scholar]
- 268.Pontes MS, Van Waesberghe C, Nauwynck H, Verhasselt B, Favoreel HW. Pseudorabies virus glycoprotein gE triggers ERK1/2 phosphorylation and degradation of the pro-apoptotic protein Bim in epithelial cells. Virus Res. 2016;213:214–218. doi: 10.1016/j.virusres.2015.12.008 . [DOI] [PubMed] [Google Scholar]
- 269.Guo H, Kaiser WJ, Mocarski ES. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol. 2015;204:439–448. doi: 10.1007/s00430-015-0410-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chaudhry MZ, Kasmapour B, Plaza-Sirvent C, Bajagic M, Garduño RC, Borkner L, et al. UL36 Rescues Apoptosis Inhibition and In vivo Replication of a Chimeric MCMV Lacking the M36 Gene. Front Cell Infect Microbiol. 2017;7. doi: 10.3389/FCIMB.2017.00312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang GH, Garvey TL, Cohen JI. The murine gammaherpesvirus-68 M11 protein inhibits Fas- and TNF-induced apoptosis. J Gen Virol. 1999;80(Pt 10):2737–2740. doi: 10.1099/0022-1317-80-10-2737 . [DOI] [PubMed] [Google Scholar]
- 273.Bélanger C, Gravel A, Tomoiu A, Janelle M, Tremblay M, Flamand L. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation—PubMed. J Hum Virol. 2001;4:62–73. . [PubMed] [Google Scholar]
- 274.Baratchian M, Davis CA, Shimizu A, Escors D, Bagnéris C, Barrett T, et al. Distinct Activation Mechanisms of NF-κB Regulator Inhibitor of NF-κB Kinase (IKK) by Isoforms of the Cell Death Regulator Cellular FLICE-like Inhibitory Protein (cFLIP). J Biol Chem. 2016;291:7608–7620. doi: 10.1074/jbc.M116.718122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu Rev Microbiol. 2003;57:609–639. doi: 10.1146/annurev.micro.57.030502.090824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Morán P, Manrique J, Pérez S, Romeo F, Odeón A, Jones L, et al. Analysis of the anti-apoptotic v-Bcl2 and v-Flip genes and effect on in vitro programmed cell death of Argentinean isolates of bovine gammaherpesvirus 4 (BoHV-4). Microb Pathog. 2020;144. doi: 10.1016/j.micpath.2020.104170 . [DOI] [PubMed] [Google Scholar]
- 277.Chiou SK, Tseng CC, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/JVI.68.10.6553-6566.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4. doi: 10.1371/journal.ppat.1000018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li Q, Liston P, Moyer RW. Functional analysis of the inhibitor of apoptosis (iap) gene carried by the entomopoxvirus of Amsacta moorei. J Virol. 2005;79:2335–2345. doi: 10.1128/JVI.79.4.2335-2345.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chugani S, Greenberg EP. LuxR homolog-independent gene regulation by acyl-homoserine lactones in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2010;107:10673–10678. doi: 10.1073/pnas.1005909107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Muscolino E, Castiglioni C, Brixel R, Frascaroli G, Brune W. Species-Specific Inhibition of Necroptosis by HCMV UL36. Viruses. 2021;13. doi: 10.3390/V13112134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fletcher-Etherington A, Nobre L, Nightingale K, Antrobus R, Nichols J, Davison AJ, et al. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc Natl Acad Sci U S A. 2020;117:18771–18779. doi: 10.1073/pnas.2001887117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Koehler H, Cotsmire S, Zhang T, Balachandran S, Upton JW, Langland J, et al. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe. 2021;29:1266–76.e5. doi: 10.1016/j.chom.2021.05.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Koehler HS, Jacobs BL. Subversion of Programed Cell Death by Poxviruses. Current topics in microbiology and immunology. 2021. doi: 10.1007/82_2020_229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Petrie EJ, Sandow JJ, Lehmann WIL, Liang LY, Coursier D, Young SN, et al. Viral MLKL Homologs Subvert Necroptotic Cell Death by Sequestering Cellular RIPK3. Cell Rep. 2019;28:3309–19.e5. doi: 10.1016/j.celrep.2019.08.055 . [DOI] [PubMed] [Google Scholar]
- 286.Hartmann BM, Albrecht RA, Zaslavsky E, Nudelman G, Pincas H, Marjanovic N, et al. Pandemic H1N1 influenza A viruses suppress immunogenic RIPK3-driven dendritic cell death. Nat Commun. 2017;8. doi: 10.1038/S41467-017-02035-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhao G, Li T, Liu X, Zhang T, Zhang Z, Kang L, et al. African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D. J Biol Chem. 2022;298. doi: 10.1016/j.jbc.2021.101480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ma J, Zhu F, Zhao M, Shao F, Yu D, Ma J, et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking Gasdermin D cleavage. EMBO J. 2021;40. doi: 10.15252/embj.2021108249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J. Enterovirus 71 Inhibits Pyroptosis through Cleavage of Gasdermin D. J Virol. 2017;91. doi: 10.1128/JVI.01069-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wen W, Li X, Wang H, Zhao Q, Yin M, Liu W, et al. Seneca Valley Virus 3C Protease Induces Pyroptosis by Directly Cleaving Porcine Gasdermin D. J Immunol. 2021;207:189–199. doi: 10.4049/jimmunol.2001030 . [DOI] [PubMed] [Google Scholar]
- 291.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey A-A, Pich D, McInnes IB, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312 . [DOI] [PubMed] [Google Scholar]
- 292.Komatsu T, Tanaka Y, Kitagawa Y, Koide N, Naiki Y, Morita N, et al. Sendai Virus V Protein Inhibits the Secretion of Interleukin-1β by Preventing NLRP3 Inflammasome Assembly. J Virol. 2018;92. doi: 10.1128/JVI.00842-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Moriyama M, Chen I-Y, Kawaguchi A, Koshiba T, Nagata K, Takeyama H, et al. The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1β Secretion. J Virol. 2016;90:4105–4114. doi: 10.1128/JVI.00120-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Komune N, Ichinohe T, Ito M, Yanagi Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J Virol. 2011;85:13019–13026. doi: 10.1128/JVI.05942-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Song Y, Wu X, Xu Y, Zhu J, Li J, Zou Z, et al. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int J Biol Sci. 2020;16:2924–2937. doi: 10.7150/ijbs.50074 . [DOI] [PMC free article] [PubMed] [Google Scholar]