Abstract
Objectives
This study examines total life expectancies (TLEs) for both healthy and diabetic U.S.-born populations and 2 measures capturing quality of life: (a) the proportion of remaining life to be spent without either other chronic conditions or activities of daily living disabilities (ADLs) and (b) the proportion of remaining life to be spent with ADLs for U.S.-born diabetic populations by race/ethnicity and educational attainment.
Methods
Using the 1998–2014 waves of the Health and Retirement Study (n = 16,983), we apply a Bayesian multistate life table method to calculate these quantities from the constructed life tables.
Results
TLE at age 50 is shorter for diabetic individuals than healthy individuals, for non-Hispanic Blacks than members of other racial/ethnic groups, and for less-educated individuals. Gaps in TLE at age 50 between healthy and diabetic populations range from 6.3 to 8.8 years across sex–race combinations and from 5.6 to 9.2 years across sex–education combinations. Among the diabetic population, those with at least a college degree on average have a higher proportion of remaining life to be spent without either other chronic conditions or ADLs. Hispanics and those without a college degree have a particularly high proportion of remaining life to be spent with ADLs. Although diabetic women on average live longer than men, their quality of life tends to be lower.
Discussion
The impact of diabetes on population health varies across racial/ethnic and educational groups. The findings support targeted interventions for vulnerable groups, such as people of color, women, and less-educated individuals.
Keywords: Diabetes, Education, Health disparities, Life expectancy, Race/ethnicity
In the past several decades, the prevalence of diabetes has increased dramatically in the United States, a trend that parallels the simultaneous rapid increase in obesity (Bhupathiraju & Hu, 2016). Between 1999 and 2002, 9.5% of the population was afflicted with diabetes; less than two decades later, this figure climbed to 12.0% between 2013 and 2016 (Centers for Disease Control Prevention, 2020). The true prevalence may be even higher than what is reported: while 34.2 million people in the United States report having diabetes, an estimated 7.3 million adults may have undiagnosed diabetes (Centers for Disease Control Prevention, 2020). Most common among those aged 65 and older, diabetes is a major cause of death and a risk factor for other health complications including, but not limited to, kidney disease, retinopathy, and cardiovascular disease (Zimmet et al., 2016). In 2016, 7.8 million hospital discharges, which included strokes, lower-extremity amputations, and hyperglycemic crisis, reported diabetes as one of the listed diagnoses. These discharges account for approximately 21.96% of all hospital discharges in 2016 (Centers for Disease Control Prevention, 2020). The economic cost of diabetes in the United States is enormous: costs incurred by diabetic persons accounted for one in four health care dollars spent in the United States in 2017, and the total diabetes-related societal costs are projected to be higher than $622 billion by 2030 (American Diabetes Association, 2018; Rowley et al., 2017).
The public health burden of diabetes varies considerably by race/ethnicity and educational attainment in the United States. Non-Hispanic Blacks (“Blacks” hereafter), Hispanics, and those with lower educational attainments tend to experience greater diabetes incidence (Agardh et al., 2011; Centers for Disease Control Prevention, 2020). Furthermore, Blacks, Hispanics, and those with lower levels of education tend to experience more diabetes-related complications and deaths (Dray-Spira et al., 2010; Dupre et al., 2015; Osborn et al., 2013; Rosenstock et al., 2014; Saydah et al., 2013). Among all diabetes-related complications, other chronic conditions and disability in activities of daily living (ADLs) are two major ones. Prior studies have found that approximately 97.5% of diabetic adults have other chronic conditions (Iglay et al., 2016), and diabetic adults are 1.82 times more likely to develop ADLs compared to those without diabetes (Wong et al., 2013). Racial/ethnic differences in diabetes-related complications vary depending on the type of complications: Diabetic Blacks and Hispanics tend to have lower or equal incidence of cardiovascular disease compared to their non-Hispanic White (“Whites” hereafter) counterparts, but they are more likely to develop microvascular complications and lower limb amputations that can lead to ADLs (Lanting et al., 2005; Spanakis & Golden, 2013). Diabetic adults with lower levels of educational attainment are more likely to develop other chronic conditions and ADLs (Chiu & Wray, 2011; Secrest et al., 2011; Tsai, 2017).
Despite our knowledge about racial/ethnic and educational disparities in incidence and prevalence of diabetes and its downstream health outcomes (e.g., other chronic conditions, disabilities, and death) for diabetic versus nondiabetic persons, few studies have attempted to view the acquisition of diabetes, its evolution toward other chronic conditions and disabilities, and its consequences for survival from a holistic life course perspective. In particular, at the individual level, our lives unfold over time with health at one time being a function of social, economic, and health conditions earlier. In early work in gerontology, Nagi (1976) recognized this evolution in presenting his disablement process. In his theory, Nagi argued that disability was the end of a process of physical deterioration that began with cellular pathology, which then developed into organ system impairments and manifested eventually in physical limitations and ultimately the inability to fulfill social roles. Verbrugge and Jette (1994) extended his theory by arguing that social factors directly affected each stage of the disablement process, either delaying or catalyzing progression toward disability and death. More recently, cumulative inequality theory suggests that poor social and economic conditions accumulate over time to produce poor health outcomes and that poor health outcomes often compound over time (Ferraro et al., 2009). Synthesizing the two lines of theories, poor social and economic conditions may accumulate to accelerate physical deterioration in the disablement process. In the literature on the intersection of age and inequality, race/ethnicity and education constitute two key social dimensions along which health inequalities accumulate (Ferraro, 2011). A large body of research has established that Blacks and Hispanics tend to suffer worse health outcomes than Whites, almost regardless of what health outcome is measured (Hummer et al., 2004; Mays et al., 2007). Similarly, literature that dates back to at least the 1970s has established strong educational gradients in health and mortality (Brown et al., 2012; Kitagawa & Hauser, 1973; Montez et al., 2011). Race and education are closely related: educational attainment is often considered to be a mediator that explains racial/ethnic disparities in health. Due to structural racism, Blacks and Hispanics tend to have greater difficulties in obtaining high educational attainment compared to Whites, and high educational attainment has protective effects on health. Although some may argue that educational gradients in health may vary by racial groupings, we assume, at a minimum, that differences in educational attainment across Whites and non-Whites explain some of the racial differences in health.
Studies that simply investigate the incidence or prevalence of a single health outcome fail to capture a holistic view of the process by which health disparities across race and educational attainment emerge across the life course. Thus, we adopt a methodological approach that involves multiple health outcomes and allows us to estimate the consequences of transitions between states defined by qualitatively distinct health outcomes for years of life to be spent in different stages of the disablement process. Specifically, we model health transitions using a multistate life table (MSLT) method and focus on three quantities for the U.S.-born older population: total life expectancy (TLE), healthy life expectancy, which forecasts years of life disease-free, and disabled life expectancy, which forecasts years of life with ADLs (Katz et al., 1983). Compared to many other population health outcomes such as age-adjusted mortality, life expectancies have the advantage of being easily understood by the general public and policymakers (Silcocks et al., 2001). Therefore, they are particularly useful for policymakers to project future medical care costs and design effective and efficient strategies to meet future needs (Guralnik et al., 2002).
We first differentiate TLE at age 50 between diabetic and nondiabetic populations, by race/ethnicity and educational attainment. Second, we calculate two general measures of population health among diabetic persons, adding a quality-of-life aspect to life expectancy: the proportion of remaining life to be spent at age 50 without either chronic conditions or ADLs (%XLE) and the proportion of remaining life to be spent at age 50 with ADLs (%XDLE). We use a proportion measure rather than a year measure to compensate for differences in TLE across racial/ethnic and educational groups. Finally, we estimate the probabilities that the health of Blacks and Hispanics is poorer than that for Whites, and that the health of those without a college degree is worse than those with a college degree. Based on prior literature, we hypothesize that (a) diabetic populations have lower TLE at age 50 than healthy populations; (b) among the diabetic population, Blacks and Hispanics have worse health outcomes (lower TLE, lower %XLE, and higher %XDLE); and (c) among the diabetic population, those with higher educational levels have better health outcomes. While most existing studies examine the burden of diabetes that different populations are currently facing, our estimates reveal the burden of diabetes that different populations will face over the course of their remaining lives, which may be more relevant for long-term policy and health care budgeting.
Method
Our data come from the Health and Retirement Study (HRS) 1998–2014 waves. The HRS is a biennial, longitudinal, nationally representative panel study that provides health and economic data of American adults older than the age of 50. Respondents in our sample were either interviewed in 1998 or brought into the survey as new cohort HRS members in 2004 or 2010. We include only one person per household to avoid statistical dependency between individuals. Furthermore, given the complexity of the relationship between nativity and race/ethnicity in shaping population health, for simplicity, we exclude individuals who were foreign-born, lived outside of the United States in any wave, or were dropped by the HRS in any wave. Our units of analysis in this study are transitions between health statuses as determined by health measures described below. Transitions are defined based on the observed health states occupied by an individual in adjacent survey waves. After deleting 3,816 transitions (4.54%) due to missing health information, our final sample consists of 80,146 transitions measured on 16,983 respondents. No systematic differences by race/ethnicity or educational attainment are observed between the observations deleted and our sample after controlling for other covariates.
Measures
We consider three categories of health conditions: diabetes, other chronic conditions, and disability. We use individuals’ self-reported diabetes status obtained from the yes/no question: “Has a doctor ever told you that you have diabetes or high blood sugar?” For chronic conditions, we use responses to questions concerning whether the participants had ever been told by a physician that they had heart disease, stroke, cancer, or lung disease, as there is evidence that diabetes is a major risk factor for these chronic conditions (Ehrlich et al., 2010; Wojciechowska et al., 2016). We measure disability using ADLs, which are defined by whether the participant experienced difficulty with at least one of the following activities: bathing, bedding, dressing, eating, toileting, and walking. For older adults, these activities are considered essential for maintaining independence in the community (Katz et al., 1983).
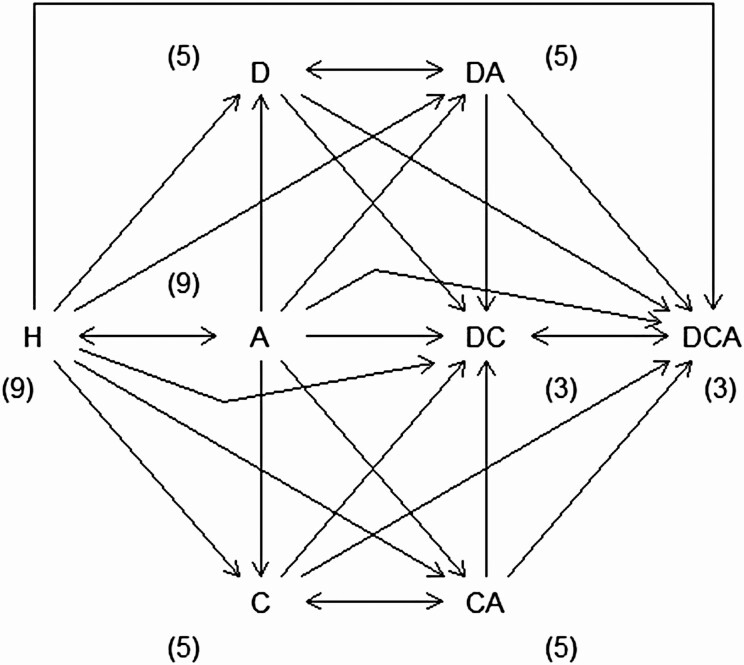
A participant has one of the eight possible health statuses at the start of a 2-year transition interval, as shown in Figure 1: no health problem, any one chronic condition or disability (diabetes, other chronic conditions, ADLs), any pairwise combination, or all three. A participant has a ninth possible outcome at the end of a transition interval: death. These states at the beginning and end of a transition interval allow for 72 possible types of transitions. However, some transitions are impossible (i.e., some states are “transient”). For example, following previous studies, a person cannot transition from being diagnosed with diabetes or other chronic conditions to not being diagnosed (Laditka & Laditka, 2015). Because of these assumptions, we are left with 44 valid transitions. Supplementary Appendix Table A1 presents the frequency of these transitions in a matrix for the full data. Most transitions are on the diagonal of the matrix (i.e., they are nontransitions, or “retentions”): For example, approximately 35% of the recorded transitions are from “healthy” to “healthy.” The next most common transitions are retentions in the “chronic conditions” state, followed by retentions in the “ADL-disabled” state. Among the transitions between states, the most common ones are from “chronic conditions” to “chronic conditions + ADLs,” reflecting health decline probably from disease progression. Due to the instability of coefficient estimates attributable to sparse data in one cell of the matrix, transitions from state A to state DC were combined into the transitions from state A to state DCA.
Figure 1.
State space of interest. Notes: States include (a) being healthy (H), (b) being diabetic (D), (c) having at least one chronic condition (C), (d) having at least one activity of daily living (ADL) disability (A), (e) being diabetic with at least one condition (DC), (f) being diabetic with at least one ADL disability (DA), (g) having at least one condition and one ADL disability (CA), (h) being diabetic with at least one condition and at least one ADL disability (DCA), and (i) death. Death is not shown but is allowed from all states. Retention is not shown but is allowed. The parenthetical numbers next to each state indicate how many transitions are possible from the given state.
The main predictors of interest are race/ethnicity and educational level. Race/ethnicity includes non-Hispanic White, non-Hispanic Black, and Hispanic. Education is measured as below high school (less than 12 years of education), high school or some college (12–15 years of education), and college or higher (at least 16 years of education). We also control for basic demographic covariates, including age (in years), sex (male = 1), birth cohort (birth year minus 1900), region of birth, and region of residence at time of interview (Northeast, Midwest, South, and West) to adjust for differences in life expectancies due to these characteristics. We do not include any potential mediators through which race/ethnicity or educational attainment may affect life expectancies, such as income, marital status, and access to health care. Descriptive statistics for our covariates are given in Table 1.
Table 1.
Descriptive Statistics
| Variable | Mean (SD) [range] or percent |
|---|---|
| Birth cohort | 35.3 (11.7) [−8, 59] |
| Age | 69.4 (10.7) [50.2, 109.7] |
| Male | 44.4% |
| Race | |
| White (reference) | 79.0% |
| Black | 18.0% |
| Other | 3.0% |
| Hispanic | 4.6% |
| Education level | |
| Below high school (reference) | 58.0% |
| High school and some college | 31.1% |
| College and above | 10.9% |
| Birth region | |
| South | 40.3% |
| Northeast | 20.7% |
| Midwest | 30.0% |
| West | 9.0% |
| Current region | |
| South | 41.4% |
| Northeast | 15.3% |
| Midwest | 26.1% |
| West | 17.2% |
Notes: Data come from 1998 to 2014 waves of the Health and Retirement Survey. Descriptive statistics include all n = 80,146 transition intervals. Cohort is computed as birth year − 1900. Our sample is restricted to the U.S.-born population.
Statistical Methods
We calculate various state expectancies using an MSLT method that extends the Bayesian MSLT method developed by Lynch and Brown (2005) to handle a large number of transitions between living states. The extension of the method can accommodate “transient” states that may not be revisited once they are left. For example, as discussed earlier, once a person has been diagnosed as diabetic, s/he cannot return to a nondiabetic state. Whereas the original method proposed by Lynch and Brown allowed for two living states and modeled these states at a later time as a function of the individual’s state at an earlier time, our approach models the transition itself over time intervals as the outcome using a multinomial logit model with 44 outcomes (less one as the reference outcome).
We first sample parameters from the multinomial logit model predicting transitions with the above-mentioned covariates using Gibbs sampling. We account for sample weighting by directly controlling for major factors that affect the probability of inclusion and nonresponse (e.g., race/ethnicity, sex, and region) in our model (Gelman, 2007). We run two Gibbs samplers in parallel, each producing 2,500 samples of the parameters. The first 500 samples from each sampler are treated as “burn-in” samples and are discarded. To reduce autocorrelation of samples produced by using a Markov chain-based sampling method, we retain every fourth sample from each sampler after discarding the burn-in sample, leaving us with 1,000 samples of the parameters. For each of these samples, we compute predicted transition probability matrices for each age by 2-year increments from ages 50 to 110 (i.e., 50, 52, 54, …, 110) for a total of 31 age-specific transition probability matrices for each posterior sample. The predictors of interest in the study in these computations are set to values to produce sets of transition probability matrices for specific subpopulations of interest (e.g., Whites vs. Blacks, more educated vs. less educated). Control variables are fixed at overall sample means to isolate differences between subpopulations of interest net of compositional differences attributable to the controls. We construct 1,000 life tables for each subpopulation of interest using standard multistate demographic methods applied to the sets of transition probability matrices (Palloni, 2000). Finally, we summarize various state expectancies from the constructed life tables. Interval estimates are computed by using empirically obtained values at desired percentiles from the 1,000 tables (see the work of Lynch and Brown (2005) for more discussion of the general methodology; see Supplementary Appendix for more discussion of our extension).
Status-based life tables are constructed to compare the diabetic population with the nondiabetic population. Tables for the diabetic population are produced by assuming all life table population members are diabetic (but without other conditions or disabilities) at age 50 (i.e., the “radix” population). Tables for the nondiabetic population are produced by assuming all population members are healthy at age 50. State expectancies are compared across and within each sex. Following the frequentist literature showing that comparing 84% confidence intervals is roughly equivalent to hypothesis testing for differences between groups at the α = 0.05 level (under an equal standard error assumption; Payton et al., 2003), we show 84% credible intervals (CIs) for life expectancies in our figures. It is important to note, however, that the interpretation of Bayesian “CIs” is not the same as the interpretation of a classical confidence interval.
We conduct three sets of analyses. First, we compare TLE at age 50 for diabetic and healthy populations to examine the impact of diabetes on population health. TLE is calculated by summing years to be lived in all states (i.e., state expectancies) for each sex–race or sex–education combination. Second, we calculate %XLE and %XDLE to evaluate quality of life for the diabetic population. For diabetic individuals, higher %XLE or lower %XDLE indicates better health. Using diabetic status-based life tables, the former is calculated by dividing the remaining years to be spent without other health problems (XLE) by TLE among diabetic persons, and the latter is calculated by dividing the remaining years to be spent with ADLs (XDLE) by TLE among diabetic persons. Third, we directly calculate the probabilities that people of color have worse health outcomes (i.e., shorter TLE, shorter XLE, lower %XLE, longer XDLE, or higher %XDLE) than Whites and that people without a college degree have worse health outcomes than those with a college degree. Interval estimates and probabilities are computed directly from the 1,000 posterior samples. A detailed description of our method is provided as a Supplementary Appendix. Full results for various state expectancies, including XLE and XDLE, by sex, race, and education are given in (Supplementary Appendix Tables A2-A5).
Results
Racial Disparities
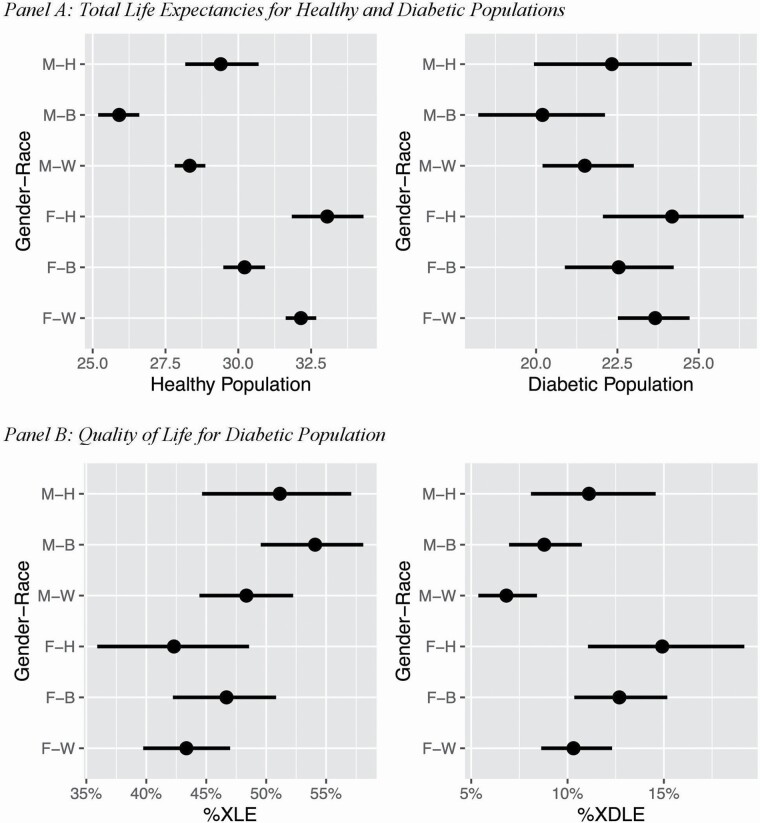
Interval estimates for TLE at age 50 for diabetic and healthy populations are presented for each sex–race combination in Figure 2, Panel A. TLE for the diabetic population ranges from 20.2 to 24.2 years, whereas the TLE for the healthy population ranges from 25.9 to 33.1 years. The difference between these two populations ranges from 5.7 to 8.9 years across sex–race combinations (Supplementary Appendix Table A6). Among the healthy population, the 84% CI for TLE indicates that for any given race, females tend to have longer TLE than males. For both males and females in the healthy population, Whites and Hispanics have longer TLE than Blacks. The median gap between healthy White and Black females is 1.9 years (84% CI, 1.3–2.6), and the corresponding estimate for males is 2.4 (84% CI, 1.7–3.1) years (Supplementary Appendix Table A7). Similar patterns can be seen for the diabetic population, although the estimates are less precise. The median gap between diabetic White and Black females is 1.1 years (84% CI, −0.3 to 2.6), and the estimate for males is 1.3 years (84% CI, –0.2 to 2.9).
Figure 2.
Impact of diabetes on population health by gender–race combinations, with 84% credible intervals. Notes: %XLE refers to the proportion of life to be spent without other chronic conditions or activity of daily living (ADL). %XDLE refers to the proportion of life to be spent with ADL disability. For gender–race combinations, F: female, M: male, W: non-Hispanic White, B: non-Hispanic Black, and H: Hispanic. Estimates only apply to the U.S.-born population.
For diabetic individuals, %XDLE and %XLE are shown in Panel B of Figure 2. In general, for a given race, males have higher %XLE and lower %XDLE than females. For each sex, on average, Blacks tend to have the highest %XLE. Across all sex–race combinations, Black males (54.1 [84% CI, 49.5–58.1]) have longer %XLE compared to Hispanic females (42.3 [84% CI, 35.9–48.6]) and White females (43.3 [84% CI, 39.7–47.0]). White males (6.8 [84% CI, 5.3–8.4]) have the shortest %XDLE, which is substantially different from those for Hispanic males (11.1 [84% CI, 8.1–14.6]), Hispanic females (14.9 [84% CI, 11.1–19.2]), and Black females (12.7 [84% CI, 10.4–15.2]).
Table 2 (Panel A) presents the probability that health for Whites is better (i.e., longer TLE, longer XLE, higher %XLE, shorter XDLE, lower %XDLE) for a given sex–race combination. Among females, Blacks are more likely than Whites (prob = 0.870) to have lower TLE. It is also very likely that Black females have longer XDLE (prob = 0.914) and higher %XDLE (prob = 0.968) than White females. Similarly, Hispanic females have very high probabilities of having longer XDLE (prob = 0.973) and higher %XDLE (prob = 0.973) than White females. In contrast, the probabilities that White females have longer XLE or higher %XLE than Black females are only 0.386 and 0.098, respectively. The patterns for males are similar with those for females, except that the probabilities that Whites have longer XLE or higher %XLE are smaller for Hispanic males compared to Hispanic females.
Table 2.
Posterior Probabilities for Group Comparisons
| Panel A: Probabilities that Whites’ health is better | ||||
|---|---|---|---|---|
| Black female | Hispanic female | Black male | Hispanic male | |
| TLE | 0.870 | 0.375 | 0.894 | 0.308 |
| XLE | 0.386 | 0.505 | 0.294 | 0.234 |
| XDLE (<) | 0.914 | 0.973 | 0.921 | 0.994 |
| %XLE | 0.098 | 0.602 | 0.022 | 0.244 |
| %XDLE (<) | 0.968 | 0.973 | 0.973 | 0.991 |
| Panel B: Probabilities that people without a high school diploma’s health is worse | ||||
| HS female | CH female | HS male | CH male | |
| TLE | 1.000 | 1.000 | 0.999 | 1.000 |
| XLE | 1.000 | 1.000 | 0.999 | 1.000 |
| XDLE (<) | 0.987 | 0.971 | 0.994 | 0.991 |
| %XLE | 0.994 | 0.999 | 0.905 | 0.852 |
| %XDLE (<) | 1.000 | 1.000 | 1.000 | 1.000 |
Notes: TLE = total life expectancy. XLE refers to the life to be spent without other chronic conditions or activities of daily living (ADL). XDLE refers to the life to be spent with ADLs. %XLE and %XDLE stand for the proportion of XLE and XDLE in TLE, respectively. For all outcomes, longer estimates indicate better health, except for XDLE and %XDLE. BH refers to below high school (less than 12 years), HS refers to high school diploma and some college credits (12–15 years), and CH refers to a college degree or higher (more than 15 years). Estimates only apply to the U.S.-born population.
Educational Disparities
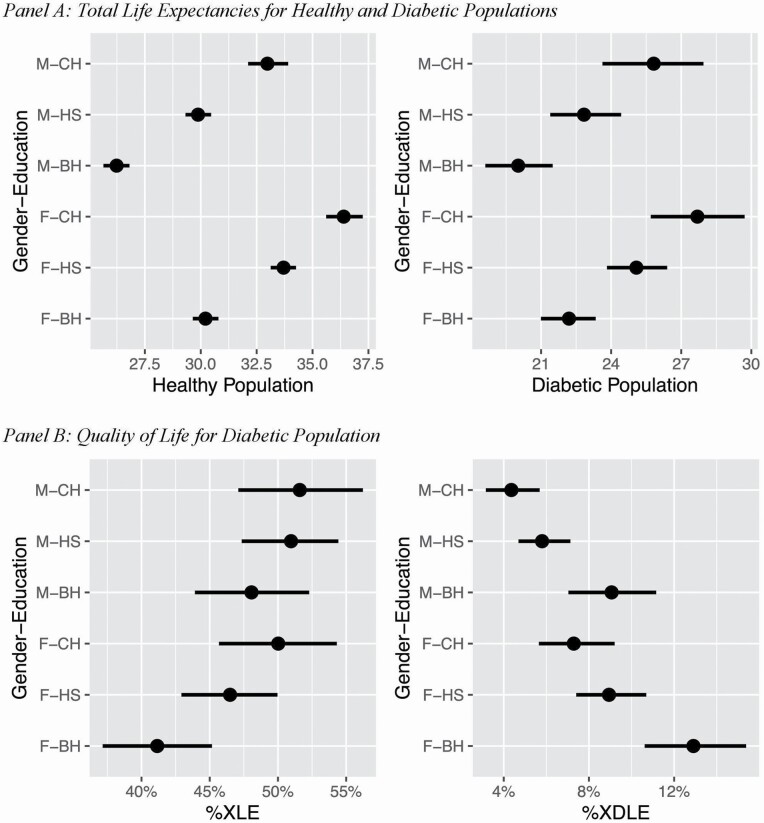
As shown in Panel A of Figure 3, the mean TLE for the healthy population ranges from 26.2 to 36.4 years, whereas the range for the diabetic population is from 20.0 to 27.7 years. The gap in TLE between these two populations ranges from 6.2 to 8.7 years across sex–education combinations (Supplementary Appendix Table A6). Among the healthy population, for a given education level, females have longer TLE than males. For both sexes, we observe a clear educational gradient—those with a college degree have longer TLE, followed by high school graduates. Among the healthy population, the median gap between those with and without a college degree is 2.9 years (84% CI, 2.1–3.6; Supplementary Appendix Table A8). The corresponding estimate for the gap between those with and without a high school diploma is 3.5 years (84% CI, 3.0–4.1). A similar educational gradient can be seen in the diabetic population. Particularly, diabetic individuals with a college degree have the longest TLE, which is substantially different from those of their less-educated counterparts. Among the diabetic population, the median gap between those with and without a college degree is 2.9 years (84% CI, 0.9–4.6). The corresponding estimates for the gap between those with and without a high school diploma are 2.8 years (84% CI, 1.4–4.2).
Figure 3.
Impact of diabetes on population health by educational attainments, with 84% credible intervals. Notes: %XLE refers to the proportion of life to be spent without other chronic conditions or activity of daily living (ADL). %XDLE refers to the proportion of life to be spent with ADL disability. For gender–education combinations, F: female, M: male, BH: below high school (less than 12 years), HS: high school diploma and some college credits (12–15 years), and CH: college degree or higher (more than 15 years). Estimates only apply to the U.S.-born population.
For diabetic individuals, %XDLE and %XLE are shown for each sex–education combination in Figure 3, Panel B. %XDLE and %XLE range from 4.3% to 12.9% and 41.2% to 51.6%, respectively. On average, for a given sex, those with less than a high school education have lower %XLE and higher %XDLE. Across sex–education combinations, male high school graduates have higher %XLE (51.0 [84% CI, 47.3–54.4]) in comparison to the group with the lowest mean %XLE—females with less than a high school education (41.2 [84% CI, 37.1–45.2]). Males with a college degree (4.4 [84% CI, 3.2–5.7]) and male high school graduates (5.8 [84% CI, 4.7–7.1]) both have lower %XDLE in comparison to females with less than a high school education (12.9 [84% CI, 10.6–15.4]). Males with a college degree also have lower %XDLE than female high school graduates (8.9 [84% CI, 7.4–10.7]).
Table 2 (Panel B) presents the probability that a person’s health without a high school diploma is worse than that of persons with a diploma. Females with at least a high school diploma have high probabilities (prob >95%) of having longer TLE, longer XLE, higher %XLE, shorter XDLE, and lower %XDLE than those without a high school diploma. Similar patterns are observed for males. However, the probability that males without a high school diploma have lower %XLE is 0.905 when compared to high school graduates and is 0.852 when compared to college degree holders. These probabilities are relatively smaller than the corresponding probabilities for females.
Discussion
Using population-based survey data and an innovative life table method, this study is the first to document racial/ethnic and educational disparities in not only TLE but also quantities capturing healthy life expectancy among U.S.-born diabetic older adults. We found marked disparities in health outcomes by race/ethnicity and educational level in both healthy and diabetic populations, after adjusting for a variety of demographic characteristics such as region and birth cohort. Consistent with our hypothesis, TLE at age 50 is shorter for the diabetic population compared to the healthy population. The gap in TLE between these two populations ranges from 5.7 to 8.9 years across sex–race combinations and from 6.2 to 8.7 years across sex–education combinations. Our estimates of TLE among healthy and diabetic populations at age 50 are highly consistent with national estimates from the Framingham Heart Study (Franco et al., 2007; Jonker et al., 2006) and from other studies using the HRS (Díaz-Venegas et al., 2017). We also estimate the proportions of remaining life to be spent without other health problems among diabetic individuals to be 42.3%–54.1% across race–sex combinations and 41.2%–51.6% across education–sex combinations. The proportions of remaining life to be spent with ADLs among diabetic individuals are estimated to be 6.8%–14.9% across race–sex combinations and 4.4%–12.9% across education–sex combinations.
Our findings tell a complex story about race/ethnicity. On the one hand, our findings on TLE confirm that both healthy and diabetic people of color experience poorer health outcomes than their White counterparts (Harris et al., 1998; Osborn et al., 2013; Resnick et al., 2004). We find that the TLE for White females is around 0.9 years longer than that for Black females in the healthy population and 1.2 years longer in the diabetic population. The corresponding numbers for males are 2.4 years in the healthy population and 1.3 years in the diabetic population. Among the diabetic population, White males also have the lowest %XDLE. Cumulative inequality theory can help explain these disparities. Culturally and socioeconomically advantaged individuals often have biased views about people of color, those with low socioeconomic status (SES), women, and older adults, and these views are reflected in social institutions such as aging policies (Putnam, 2002). People of color face structural racism in the United States and are more likely than Whites to have poor social and economic conditions in childhood, and thus lower SES in adulthood, and fewer resources as older adults. These adversities accumulate over the life course, leading people of color to experience faster health deterioration in the disablement process. On the other hand, Blacks have higher %XLE than Whites and Hispanics. Prior studies show that although Black diabetic adults have equal or lower incidences of cardiovascular diseases than Whites, their mortality rate caused by cardiovascular diseases is higher (Spanakis & Golden, 2013). Because our measure of other chronic conditions disproportionately captures cardiovascular diseases (e.g., heart diseases and stroke), this may explain the observed higher %XLE among Blacks: Diabetic Blacks are on average less likely to have cardiovascular diseases, but once they have, they die faster than Whites. This result suggests that for Blacks, cumulative disadvantages may affect the progression of cardiovascular diseases more compared to the onset of the diseases.
Individuals with a college degree unsurprisingly have the longest TLE, followed closely by high school graduates. In the healthy population, the median difference in TLE between individuals who hold a college degree and those who do not is 2.7 years for females and 3.1 years for males. This gap is about the same among the diabetic population, with the median gap being 2.6 years for females and 3.0 years for males. Additionally, for those with diabetes, individuals with higher educational levels also have better health outcomes. These patterns are consistent with our hypothesis and findings from prior research documenting that diabetes-related mortality is inversely related to education (Saydah et al., 2013; Vandenheede et al., 2015). Lower educational attainments may lead to lower levels of income and wealth and thus a lower ability to afford high-quality health care (Hahn & Truman, 2015). These adversities may accumulate over the life course to produce the observed educational disparities among older adults. Beyond these economic factors, one potential explanation for the observed educational disparities is that low educational levels are associated with nonattainment of goals relating to diabetes care (Heltberg et al., 2017), which leads to a faster health deterioration in the disablement process. Thus, if education serves as a protective factor against diabetes-related complications, it is imperative that health care providers take the backgrounds of individuals with diabetes into account when monitoring compliance with diabetic care.
Limitations
There are several limitations to this study. First, our life table method uses a discrete-time Markov chain approach, with the assumption that only one net transition can happen for each respondent within each 2-year transition interval in the HRS. This assumption is not ideal, considering that older adults may experience rapid disease accumulation and may experience more than one net health transition within a 2-year interval. Thus, we may overestimate the years individuals will spend healthy. Although alternative assumptions can be made regarding (possible) unobserved transitions, no method performs particularly well without highly frequent measurement occasions (Wolf & Gill, 2009).
Second, life table estimates are based on a hypothetical cohort, that is, transition probabilities are stationary. Although we include birth cohort as a covariate in our models, which allows for some relaxation of the stationarity assumption, our estimates cannot anticipate dramatic changes in transition probabilities that may occur outside the time frame of our data, such as those that could be attributed to coronavirus disease 2019 or other significant period changes, such as the adoption of the Affordable Care Act. This is, however, a limitation of any data source and any method.
Third, the use of self-reported measures of diabetes and other chronic conditions is a limitation. In particular, among people of color and individuals with low educational attainment, diabetes may be underdiagnosed and therefore underreported. However, Heiss et al. (2017) found that self-reported diabetes and diabetes “diagnosis” based on biomarkers in the HRS are consistent for 85% of the observations, and the proportion of undiagnosed cases is around 3%–4%. In addition, no evidence shows that the probability of being undiagnosed is related to race/ethnicity or educational attainment (Heiss et al., 2017). Future studies are needed to combine self-reported measures, information from biomarkers, and information from insurance claims to construct high-quality measures of chronic conditions (St Clair et al., 2017; Heiss et al., 2017). Relatedly, we only focused on ADLs as our measure of disability in this study. Most studies of disabled life expectancy, including the original study by Katz et al. (1983), have exclusively used ADLs. However, other types of measures of disability, such as those based on physical functioning (e.g., the ability to climb a flight of stairs or lift a bag of flour) or those based on more complicated tasks (e.g., those measured with instrumental activities of daily living), are precursors to ADLs and are also affected by diabetes. Future studies may consider these types of disabilities.
Fourth, we only focused on the U.S.-born population in this study. Considering the large proportion of foreign-born Hispanics in the United States and the health differences between U.S.- and foreign-born Hispanics (Palloni & Arias, 2004; Riosmena et al., 2013), our estimates for Hispanics cannot be generalized to Hispanics in general. Future studies are needed to examine the complex interaction between race/ethnicity and nativity when examining health disparities among diabetic older adults. Relatedly, the intersectionality theory also highlights the importance of examining health disparities by both race/ethnicity and SES (Brown et al., 2016). Future studies should also examine interactions between race/ethnicity and SES because returns to education may differ by race/ethnicity.
Public Health Implications
To improve current diabetic care, understanding how factors such as race/ethnicity and education are associated with health outcomes over the life course is crucial. Our findings have relevance in how health care resources can be deployed more effectively to the most vulnerable populations. In all health outcomes we examined, the most vulnerable populations tend to be people of color and those with less schooling. Clinicians should also be aware of the management of comorbidities in certain diabetic individuals, which may lead to a greater proportion of remaining life to be spent with ADLs. A number of underlying mechanisms, including persons with diabetes, providers, and overall health care system factors, may help explain the disparities seen in this study. Thus, prospective studies on differing provider care among these vulnerable populations are also warranted to improve overall diabetes care in the United States. The life expectancy quantities provided in this study are also useful for policymakers when making health care budgets and estimating health care burden in the coming years.
Supplementary Material
Contributor Information
Emma Zang, Department of Sociology, Yale University, New Haven, Connecticut, USA.
Scott M Lynch, Department of Sociology, Duke University, Durham, North Carolina, USA; Duke University Population Research Institute, Duke University, Durham, North Carolina, USA.
Chen Liu, Department of Biostatistics, Yale University, New Haven, Connecticut, USA.
Nancy Lu, Harvard Medical School, Harvard University, Boston, Massachusetts, USA.
Julia Banas, Department of Epidemiology, Yale University, New Haven, Connecticut, USA.
Funding
This work was supported by the Research Education Core of the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine sponsored by the National Institute on Aging (P30AG021342) to E. Zang; the Panel Study of Income Dynamics Small Grant is part of the bigger grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for Research Using Data from CDS and TAS (R25-HD083146) to E. Zang; and the National Institute on Aging (R01AG040199) to S. M. Lynch.
Conflict of Interest
None declared.
Author Contributions
E. Zang planned the study, supervised the data analysis, and wrote the article. S. M. Lynch helped to plan the study, including the design of the statistical model and data cleaning, and to revise the manuscript. C. Liu performed all statistical analyses and contributed to revising the article. N. Lu and J. Banas contributed to the first drafts of the manuscript.
References
- Agardh, E., Allebeck, P., Hallqvist, J., Moradi, T., & Sidorchuk, A. (2011). Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. International Journal of Epidemiology, 40(3), 804–818. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . (2018). Economic costs of diabetes in the US in 2017. Diabetes Care, 41(5), 917–928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathiraju, S. N., & Hu, F. B. (2016). Epidemiology of obesity and diabetes and their cardiovascular complications. Circulation Research, 118(11), 1723–1735. doi: 10.1161/CIRCRESAHA.115.306825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. C., Hayward, M. D., Montez, J. K., Hummer, R. A., Chiu, C. T., & Hidajat, M. M. (2012). The significance of education for mortality compression in the United States. Demography, 49(3), 819–840. doi: 10.1007/s13524-012-0104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T. H., Richardson, L. J., Hargrove, T. W., & Thomas, C. S. (2016). Using multiple-hierarchy stratification and life course approaches to understand health inequalities: The intersecting consequences of race, gender, SES, and age. Journal of Health and Social Behavior, 57(2), 200–222. doi: 10.1177/0022146516645165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention . (2020). National diabetes statistics report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services. [Google Scholar]
- Chiu, C. J., & Wray, L. A. (2011). Physical disability trajectories in older Americans with and without diabetes: The role of age, gender, race or ethnicity, and education. The Gerontologist, 51(1), 51–63. doi: 10.1093/geront/gnq069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Venegas, C., Schneider, D. C., Myrskylä, M., & Mehta, N. K. (2017). Life expectancy with and without cognitive impairment by diabetes status among older Americans. PLoS One, 12(12), e0190488. doi: 10.1371/journal.pone.0190488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray-Spira, R., Gary-Webb, T. L., & Brancati, F. L. (2010). Educational disparities in mortality among adults with diabetes in the U.S. Diabetes Care, 33(6), 1200–1205. doi: 10.2337/dc09-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre, M. E., Silberberg, M., Willis, J. M., & Feinglos, M. N. (2015). Education, glucose control, and mortality risks among U.S. older adults with diabetes. Diabetes Research and Clinical Practice, 107(3), 392–399. doi: 10.1016/j.diabres.2014.12.013 [DOI] [PubMed] [Google Scholar]
- Ehrlich, S. F., Quesenberry, C. P.Jr, Van Den Eeden, S. K., Shan, J., & Ferrara, A. (2010). Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care, 33(1), 55–60. doi: 10.2337/dc09-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, K. F. (2011). Health and aging: Early origins, persist inequalities? In Settersten R. A. & Angel J. J. L. (Eds.), Handbook of sociology of aging (pp. 465–475). Springer. [Google Scholar]
- Ferraro, K. F., Shippee, T. P., & Schafer, M. H. (2009). Cumulative inequality theory for research on aging and the life course. In Bengtson V. L., Gans D., Putney N., & Silverstein M. (Eds.), Handbook of theories of aging (pp. 413–433). Springer Publishing Company. [Google Scholar]
- Franco, O. H., Steyerberg, E. W., Hu, F. B., Mackenbach, J., & Nusselder, W. (2007). Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Archives of Internal Medicine, 167(11), 1145–1151. doi: 10.1001/archinte.167.11.1145 [DOI] [PubMed] [Google Scholar]
- Gelman, A. (2007). Struggles with survey weighting and regression modeling. Statistical Science, 22(2), 153–164. doi: 10.1214/088342306000000691 [DOI] [Google Scholar]
- Guralnik, J. M., Alecxih, L., Branch, L. G., & Wiener, J. M. (2002). Medical and long-term care costs when older persons become more dependent. American Journal of Public Health, 92(8), 1244–1245. doi: 10.2105/ajph.92.8.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, R. A., & Truman, B. I. (2015). Education improves public health and promotes health equity. International Journal of Health Services: Planning, Administration, Evaluation, 45(4), 657–678. doi: 10.1177/0020731415585986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M. I., Klein, R., Cowie, C. C., Rowland, M., & Byrd-Holt, D. D. (1998). Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care, 21(8), 1230–1235. doi: 10.2337/diacare.21.8.1230 [DOI] [PubMed] [Google Scholar]
- Heiss, F., McFadden, D., Winter, J., Wuppermann, A., & Zhu, Y. (2017). Measuring disease prevalence in surveys. In D. A. WISE (Eds.), Insights in the economics of aging, Vol. 227. University Of Chicago Press. https://www.nber.org/books-and-chapters/insights-economics-aging/measuring-disease-prevalence-surveys-comparison-diabetes-self-reports-biomarkers-and-linked [Google Scholar]
- Heltberg, A., Andersen, J. S., Kragstrup, J., Siersma, V., Sandholdt, H., & Ellervik, C. (2017). Social disparities in diabetes care: A general population study in Denmark. Scandinavian Journal of Primary Health Care, 35(1), 54–63. doi: 10.1080/02813432.2017.1288702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer, R. A., Benjamins, M. R., & Rogers, R. G. (2004). Racial and ethnic disparities in health and mortality among the US elderly population. In Anderson N. B., Bulatao R. A., & Cohen B. (Eds.), Critical perspectives on racial and ethnic differences in health in late life (pp. 53–94). National Academies Press. [PubMed] [Google Scholar]
- Iglay, K., Hannachi, H., Joseph Howie, P., Xu, J., Li, X., Engel, S. S., Moore, L. M., & Rajpathak, S. (2016). Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Current Medical Research and Opinion, 32(7), 1243–1252. doi: 10.1185/03007995.2016.1168291 [DOI] [PubMed] [Google Scholar]
- Jonker, J. T., De Laet, C., Franco, O. H., Peeters, A., Mackenbach, J., & Nusselder, W. J. (2006). Physical activity and life expectancy with and without diabetes: Life table analysis of the Framingham Heart Study. Diabetes Care, 29(1), 38–43. doi: 10.2337/diacare.29.01.06.dc05-0985 [DOI] [PubMed] [Google Scholar]
- Katz, S., Branch, L. G., Branson, M. H., Papsidero, J. A., Beck, J. C., & Greer, D. S. (1983). Active life expectancy. The New England Journal of Medicine, 309(20), 1218–1224. doi: 10.1056/NEJM198311173092005 [DOI] [PubMed] [Google Scholar]
- Kitagawa, E. M., & Hauser, P. M. (1973). Differential mortality in the United States. Harvard University Press. [Google Scholar]
- Laditka, S. B., & Laditka, J. N. (2015). Active life expectancy of Americans with diabetes: Risks of heart disease, obesity, and inactivity. Diabetes Research and Clinical Practice, 107(1), 37–45. doi: 10.1016/j.diabres.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Lanting, L. C., Joung, I. M., Mackenbach, J. P., Lamberts, S. W., & Bootsma, A. H. (2005). Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: A review. Diabetes Care, 28(9), 2280–2288. doi: 10.2337/diacare.28.9.2280 [DOI] [PubMed] [Google Scholar]
- Lynch, S. M., & Brown, J. S.(2005). A new approach to estimating life tables with covariates and constructing interval estimates of life table quantities. Sociological Methodology, 35(1), 177–225. [Google Scholar]
- Mays, V. M., Cochran, S. D., & Barnes, N. W. (2007). Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology, 58, 201–225. doi: 10.1146/annurev.psych.57.102904.190212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez, J. K., Hummer, R. A., Hayward, M. D., Woo, H., & Rogers, R. G. (2011). Trends in the educational gradient of U.S. adult mortality from 1986 to 2006 by race, gender, and age group. Research on Aging, 33(2), 145–171. doi: 10.1177/0164027510392388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi, S. Z. (1976). An epidemiology of disability among adults in the United States. The Milbank Memorial Fund Quarterly. Health and Society, 54(4), 439–467. doi: 10.2307/3349677 [DOI] [PubMed] [Google Scholar]
- Osborn, C. Y., de Groot, M., & Wagner, J. A. (2013). Racial and ethnic disparities in diabetes complications in the northeastern United States: The role of socioeconomic status. Journal of the National Medical Association, 105(1), 51–58. doi: 10.1016/s0027-9684(15)30085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palloni, A. (2000). Increment–decrement life tables. In Preston S., Heuveline P., & Guillot M. (Eds.), Demography: Measuring and modeling population processes (pp. 256–272). Blackwell. [Google Scholar]
- Palloni, A., & Arias, E. (2004). Paradox lost: Explaining the Hispanic adult mortality advantage. Demography, 41(3), 385–415. doi: 10.1353/dem.2004.0024 [DOI] [PubMed] [Google Scholar]
- Payton, M. E., Greenstone, M. H., & Schenker, N. (2003). Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? Journal of Insect Science (Online), 3, 34. doi: 10.1093/jis/3.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, M. (2002). Linking aging theory and disability models: Increasing the potential to explore aging with physical impairment. The Gerontologist, 42(6), 799–806. doi: 10.1093/geront/42.6.799 [DOI] [PubMed] [Google Scholar]
- Resnick, H. E., Carter, E. A., Sosenko, J. M., Henly, S. J., Fabsitz, R. R., Ness, F. K., Welty, T. K., Lee, E. T., & Howard, B. V.; Strong Heart Study . (2004). Incidence of lower-extremity amputation in American Indians: The Strong Heart Study. Diabetes Care, 27(8), 1885–1891. doi: 10.2337/diacare.27.8.1885 [DOI] [PubMed] [Google Scholar]
- Riosmena, F., Wong, R., & Palloni, A. (2013). Migration selection, protection, and acculturation in health: A binational perspective on older adults. Demography, 50(3), 1039–1064. doi: 10.1007/s13524-012-0178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock, S., Whitman, S., West, J. F., & Balkin, M. (2014). Racial disparities in diabetes mortality in the 50 most populous US cities. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 91(5), 873–885. doi: 10.1007/s11524-013-9861-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley, W. R., Bezold, C., Arikan, Y., Byrne, E., & Krohe, S. (2017). Diabetes 2030: Insights from yesterday, today, and future trends. Population Health Management, 20(1), 6–12. doi: 10.1089/pop.2015.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydah, S. H., Imperatore, G., & Beckles, G. L. (2013). Socioeconomic status and mortality: Contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care, 36(1), 49–55. doi: 10.2337/dc11-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrest, A. M., Costacou, T., Gutelius, B., Miller, R. G., Songer, T. J., & Orchard, T. J. (2011). Associations between socioeconomic status and major complications in type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complication (EDC) study. Annals of Epidemiology, 21(5), 374–381. doi: 10.1016/j.annepidem.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcocks, P. B., Jenner, D. A., & Reza, R. (2001). Life expectancy as a summary of mortality in a population: Statistical considerations and suitability for use by health authorities. Journal of Epidemiology and Community Health, 55(1), 38–43. doi: 10.1136/jech.55.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis, E. K., & Golden, S. H. (2013). Race/ethnic difference in diabetes and diabetic complications. Current Diabetes Reports, 13(6), 814–823. doi: 10.1007/s11892-013-0421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair, P., Gaudette, É., Zhao, H., Tysinger, B., Seyedin, R., & Goldman, D. P. (2017). Using self-reports or claims to assess disease prevalence: It’s complicated. Medical Care, 55(8), 782–788. doi: 10.1097/MLR.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, Y. (2017). Education and disability trends of older Americans, 2000–2014. Journal of Public Health, 39(3), 447–454. doi: 10.1093/pubmed/fdw082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenheede, H., Deboosere, P., Espelt, A., Bopp, M., Borrell, C., Costa, G., Eikemo, T. A., Gnavi, R., Hoffmann, R., Kulhanova, I., Kulik, M., Leinsalu, M., Martikainen, P., Menvielle, G., Rodriguez-Sanz, M., Rychtarikova, J., & Mackenbach, J. P. (2015). Educational inequalities in diabetes mortality across Europe in the 2000s: The interaction with gender. International Journal of Public Health, 60(4), 401–410. doi: 10.1007/s00038-015-0669-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge, L. M., & Jette, A. M. (1994). The disablement process. Social Science & Medicine (1982), 38(1), 1–14. doi: 10.1016/0277-9536(94)90294-1 [DOI] [PubMed] [Google Scholar]
- Wojciechowska, J., Krajewski, W., Bolanowski, M., Kręcicki, T., & Zatoński, T. (2016). Diabetes and cancer: A review of current knowledge. Experimental and Clinical Endocrinology & Diabetes, 124(5), 263–275. doi: 10.1055/s-0042-100910 [DOI] [PubMed] [Google Scholar]
- Wolf, D. A., & Gill, T. M. (2009). Modeling transition rates using panel current-status data: How serious is the bias? Demography, 46(2), 371–386. doi: 10.1353/dem.0.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, E., Backholer, K., Gearon, E., Harding, J., Freak-Poli, R., Stevenson, C., & Peeters, A. (2013). Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. The Lancet. Diabetes & Endocrinology, 1(2), 106–114. doi: 10.1016/S2213-8587(13)70046-9 [DOI] [PubMed] [Google Scholar]
- Zimmet, P., Alberti, K. G., Magliano, D. J., & Bennett, P. H. (2016). Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nature Reviews. Endocrinology, 12(10), 616–622. doi: 10.1038/nrendo.2016.105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.