Abstract
Brachial-ankle pulse wave velocity (baPWV) is used for predicting the severity of vascular damage and prognosis of atherosclerotic cardiovascular disease (ASCVD) in people with hypertension and diabetes mellitus. This correlation study aimed to compare the baPWV with other risk indicators for identification of subclinical vascular disease for primary prevention and to determine the clinical utility of baPWV-guided therapy in improving prognosis in high-risk subjects.
We included 4881 subjects who underwent voluntary health examination at Mackay Memorial Hospital, Taiwan between 2014 and 2019. Participants were categorized into the low-risk (<5%), borderline-risk (5%–7.4%), intermediate-risk (7.5%–19.9%), and high-risk (≥20%) groups based on the 10-year risk for ASCVD. The predictive risk criteria, that is, the metabolic syndrome score, Framingham Risk Score, estimated glomerular filtration rate, and baPWV were compared among these groups. The chief cause of induced responses and the relationships between parameters were identified using principal component analysis. The participants’ ages, body mass index, systolic, diastolic blood pressure, triglycerides, fasting glucose, hemoglobin A1c, creatinine, neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, metabolic syndrome, Framingham Risk Score, and age-related arterial stiffness (vascular age) increased significantly from the low-risk to high-risk groups (P < .001). The mean estimated glomerular filtration rate decreased significantly from the low- to high-risk groups (P < .001). The predicted vascular age and actual age differed significantly between the intermediate- and high-risk groups (P < .001). High-density lipoprotein levels plummeted significantly among the 4 groups (P < .001). The right and left baPWV and ankle brachial index differed significantly among the 4 groups (all P < .001) and increased from the low-risk to high-risk groups (P < .001). Carotid Doppler ultrasonography revealed a significant increase in plaque formation (23.5%, 35.4%, 46.3%, and 61.5% for the low-, borderline-, intermediate, and high-risk groups, respectively). The total explanatory variation was 61.9% for 2 principal variation factors (baPWV, 36.8% and creatinine, 25.1%). The vascular age predicted using baPWV greatly exceeded the chronological age. Plaque formation was significant even in the low-risk group, and its frequency increased with the predicted ASCVD risk. Risk indicators and baPWV are useful predictors of ASCVD, which in conjunction with conventional pharmacotherapy could be useful for primary prevention of plaque formation in subjects with cardiovascular comorbidities.
Keywords: ankle pulse wave velocity, atherosclerotic cardiovascular disease, brachial
1. Introduction
Cardiovascular disease (CVD) causes tremendous health and economic burdens in the United States and globally. For decades, experts and scholars have been discussing the associated risk factors and have advocated the adoption of a healthy lifestyle as an important factor in preventing CVDs. The 2019 American College of Cardiology/American Heart Association guidelines on the primary prevention of CVD aim to promote the delivery of patient-centered care, which applies to all aspects of clinical practice, for the primary prevention of atherosclerotic cardiovascular disease (ASCVD). As per the 10-year ASCVD risk evaluation for the management of blood cholesterol, adults should be categorized as having low (<5%), borderline (5%–7.4%), intermediate (7.5%–19.9%), or high (≥20%) 10-year risk.[1–5]
Recent studies have indicated that ASCVD is associated with multiple risk factors.[1] A noninvasive method can be used for estimating arterial stiffness, as it is a predictor of the severity of vascular damage and the prognosis of CVD. Moreover, arterial stiffness is implicated in a vicious cycle involving the development and progression of hypertension, diabetes mellitus, and chronic kidney disease. An increase in arterial stiffness is believed to contribute to the development of CVD through pathophysiological abnormalities induced in the heart, brain, kidney, and arteries.[5–8]
Various risk assessment indicators are used to evaluate CVD, such as metabolic syndrome (MS) score, according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III). The Framingham Risk Score (FRS) is used to estimate the 10-year coronary heart disease risk. The estimated glomerular filtration rate (eGFR) risk calculator for pediatric chronic kidney disease suggests the use of inflammatory biomarkers such as C-reactive protein (CRP), high-sensitivity CRP, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) to predict the occurrence of diseases caused by inflammatory reactions.[9–18]
Brachial-ankle pulse wave velocity (baPWV) measurement is a simple tool to predict the severity of vascular damage and the prognosis of CVD in subjects with hypertension and diabetes. The measurement of baPWV has been validated, and the reproducibility of baPWV has been previously reported. We sought to determine whether baPWV could be used as an indicator or predictor in ASCVD, and if CVD risk could be prevented using a simple, noninvasive method for ASCVD risk assessment.[19–25]
The study aimed to investigate whether the ASCVD risk score more effectively identifies subclinical vascular disease for application in primary prevention compared with baPWV and other risk indicators. To further confirm the clinical usefulness of this measure, we examined whether baPWV measurement could improve the prognosis in high-risk subjects.
2. Materials and Methods
2.1. Study population and design
All participant information was anonymized and de-identified preceding analysis. The Institutional Review Board of our institution (MacKay Memorial Hospital) approved this retrospective study (IRB No: 20MMHIS175e). All patient information was anonymized and de-identified prior to analysis. Approval to perform retrospective research using secondary data was granted by the Institutional Review Board (20MMHIS175e), and our study was performed in accordance with the relevant guidelines and regulations. We retrospectively collected the data of adult patients (7769) who underwent voluntary health evaluations at the Health Evaluation Center, Mackay Memorial Hospital, Taipei, Taiwan, from 2014 to 2019. Data on demographic characteristics such as age and sex, blood pressure measurement, health questionnaire, physiological consultation, laboratory measurements, baPWV and ankle brachial index (ABI) measurements, and information on color Doppler carotid artery sonography and computed tomography angiography were collected. We excluded participants with incomplete physiological data, as well as those with incomplete anthropometric measurements, laboratory data, and reports. The final cohort comprised 4881 participants who were categorized as low risk (<5%), borderline risk (5%–7.4%), intermediate risk (7.5%–19.9%), and high risk (≥20%) according to the 10-year risk for ASCVD, and compared using other risk prediction criteria such as MS, FRS, and eGFR. Statistical analysis was performed using laboratory data, baPWV measurements, data on carotid artery calcification, and receiver operating characteristic (ROC) curves.
Figure 1 outlines the selection of study participants and subjects excluded from the final analysis.
Figure 1.
Flowchart showing selection of the study participants. ASCVD = atherosclerotic cardiovascular disease.
2.2. Anthropometric measurements
Anthropometric measurements were performed using G-TECH GL-150 (G-Tech Co., Ltd, Gyeonggi-do, Korea) and included height, weight, and body mass index. Systolic (SBP) and diastolic blood pressure (DBP) measurements were obtained using a GE Carescape V100 Vital Signs Monitor (GE, USA), and blood pressure accuracy was determined using American National Standards Institute/Assoc. for the Advancement of Medical Instrumentation standard SP-10:1992 (mean error ≤ 5 mm Hg, standard deviation ≤ 8 mm Hg).
Waist circumference was measured using a measuring tape (KING LIFE 1.5 m–5 ft KP-1508) at the umbilical level, and hip circumference was also measured.
The 10-year risk for ASCVD was categorized as low risk (<5%), borderline risk (5%–7.4%), intermediate risk (7.5%–19.9%), and high risk (≥20%) according to the 2019 American College of Cardiology/American Heart Association Guidelines on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. The subjects had an age range of 40 to 75 years, with low-density lipoprotein cholesterol values in the range of 70 to 190 mg/dL (1.8–4.9 mmol/L), without diabetes mellitus.
MS criteria were defined according to the NCEP ATP III criteria, and included the presence of at least 3 of the following: SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg and/or use of antihypertensive medications; fasting glucose ≥ 100 mg/dL and/or use of antidiabetic medications; hypertriglyceridemia ≥ 150 mg/dL; high-density lipoprotein cholesterol (HDL-C) levels <50 mg/dL for women and <40 mg/dL for men; and waist circumference, women ≥ 80 cm and men, ≥90 cm.
FRSs were calculated based on 6 coronary risk factors, including age, sex, total cholesterol, HDL-C, SBP, and smoking habits. Absolute CVD risk percentage over 10 years was classified as low-risk (<10%), intermediate-risk (10%–20%), and high-risk (>20%). The cutoff values for calculation based on NCEP ATP III criteria 10-year risk percentages were calculated using total points (1 point, 6%; 2 points, 8%; 3 points, 10%; 4 points, 12%; 5 points, 16%; 6 points, 20%; 7 points, 25%; and 10 points or more, >30%). The Modification of Diet in Renal Disease equation was as follow:
eGFR = 186 × Scr–1.154 × Age–0.203 × 0.742 (if female) was used.
2.3. Laboratory measurements
The measurements were performed in a TAF ISO-15189-accredited laboratory. Blood samples were collected after overnight fasting (8–10 hours), and fasting glucose (mg/dL), total cholesterol (mg/dL), triglycerides (mg/dL), HDL-C levels, low-density lipoprotein cholesterol levels, creatinine levels, and CRP levels were analyzed using UniCel DxC 800 Synchron Clinical Systems (Beckman Coulters Corporation, USA).
A high-pressure liquid chromatography machine (Variant II, Bio-Rad, Hercules, CA) was used to measure the hemoglobin A1c (HbA1c) levels. The white blood cell count, platelet count, and leukocyte subtypes were determined using an auto analyzer (Coulter LH 780 Hematology Analyzer, Beckman Coulter Corporation, USA) and urine protein using an analyzer (Siemens Clinitek Novus, Germany). Testing for protein is based on the phenomenon called the “ Protein Error of pH indicators,” and the changes of various colors represent the amount of protein in various concentrations.
The NLR, MLR, and PLR were calculated as follows: NLR = neutrophil count (×109/L)/lymphocyte count (×109/L), MLR = monocyte count (×109/L)/lymphocyte count (×109/L), and PLR = platelet count (×109/L)/lymphocyte count(×109/L).[26]
2.4. baPWV and ABI measurements
A noninvasive vascular screening device was used to evaluate arterial stiffness by measuring the baPWV using an Omron waveform analyzer (VP-1000PLUS Omron Japan); ABI and PWV were measured as an index of arterial wall stiffness; the normal reference range for baPWV is < 1400 cm/s, indicating increased arterial stiffness.
2.5. Color Doppler carotid artery sonography
Color Doppler carotid artery sonography is a noninvasive examination for evaluating the risk of ASCVD (HD15 ultrasound system, Philips, USA). Ultrasonography of the common carotid artery, carotid bifurcation, and internal carotid artery of the left and right carotid arteries was performed using a 7.5-MHz linear-array transducer (ATL Ultra-Mark IV), standard method to measure the intima–media thickness, plaques (localized protruding lesions of ≥ 1.1 mm), and stenosis for evaluating the degree of arteriosclerosis, particularly in the carotid arteries.
2.6. Statistical analysis
1.2.6. Statistical analysis.
Data were collected and analyzed using Excel (Microsoft, WA) and GraphPad Prism 9 software (GraphPad Software, CA). All statistical analyses were conducted using a 2-tailed Student t test or 1-way analysis of variance. All data are expressed as means ± standard deviations. Statistical significance was set at P < .05. For all figures: NS, P > .05; *, P ≤ .05; **, P ≤ .01; ***, P ≤ .001;****, and P ≤ .0001.
2.2.6. Area under the ROCs.
The area under the curve (AUC) and ROC curves were analyzed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA) and were used to determine the optimal cutoff value associated with the maximum sensitivity and specificity for the development of ASCVD.
3.2.6. Principal component analysis.
Principal component analysis was performed using XLSTAT 2014.1 (Addinsoft, NY) to identify the main cause of induced responses and the relationship between these parameters. A biplot was created for both measured parameters and observations.
3. Results
This study involved 4881 adults who underwent health evaluations at a medical center. All subjects were classified into 4 groups according to ASCVD guidelines. Two thousand four hundred forty-nine (50.2%) subjects were categorized as low risk (<5%), 641 (13.1%) subjects were categorized as borderline risk (5%–7.4%), 1404 (28.2%) subjects were categorized as intermediate risk (7.5%–19.9%), and 387 (7.9%) subjects were categorized as high risk (≥20%).
Investigating the anthropometric measurements of the 4 groups, compared with low risk, a significant increase in age (49.5, 55, 59.6, and 68 years) from low risk to high risk was observed. Furthermore, a significant increase in body mass index, SBP, and DBP from low to high risk was observed (P < .001). The percentage of men in each group was 45.1%, 75.4%, 83.1%, and 87.1%, respectively. The percentage of participants who smoked in each group was 5%, 19.7%, 30.8%, and 26.4%, respectively (Table 1; Figure 1, Supplemental Digital Content, http://links.lww.com/MD/G969).
Table 1.
ASCVD risk in 4 categorized of the study population.
| ASCVD risk | Low | Borderline | Intermediate | High | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <5% | 5%–7.4% | 7.5%–19.9% | ≥20% | ||||||||
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | P value | Mean ± SD | 95% CI | P value | Mean ± SD | 95% CI | P value | |
| Anthropometric measurements | |||||||||||
| Subjects, n (%) | 2449 (50.2%) | 641 (13.1%) | 1404 (28.8%) | 387 (7.9%) | |||||||
| Age (yr) | 49.5 ± 6.2 | 49.2–49.7 | 55 ± 6.6 | 54.5–55.5 | <.001 | 59.6 ± 7 | 59.2–60 | <.001 | 68 ± 6.1 | 67.4–68.7 | <.001 |
| Gender, male | 1104 (45.1%) | 483 (75.4%) | 1167 (83.1%) | 337 (87.1%) | |||||||
| Smoking (%) | 123 (5%) | 126 (19.7%) | 433 (30.8%) | 102 (26.4%) | |||||||
| Body mass index (kg/m2) | 24 ± 3.6 | 23.8–24.1 | 25.2 ± 3.3 | 24.9–25.4 | <.001 | 25.2 ± 3.2 | 25–25.4 | <.001 | 25.6 ± 3.2 | 25.3–26 | <.001 |
| Systolic blood pressure (mm Hg) | 121.9 ± 15.9 | 121.2–122.5 | 129.3 ± 17.1 | 128–130.7 | <.001 | 134.4 ± 16.8 | 133.5–135.2 | <.001 | 144.4 ± 15.9 | 142.9–146 | <.001 |
| Diastolic blood pressure (mm Hg) | 74.5 ± 11.2 | 74.1–75 | 79.8 ± 11.1 | 78.9–80.7 | <.001 | 81.3 ± 10.8 | 80.8–81.9 | <.001 | 83.4 ± 10.7 | 82.3–84.5 | <.001 |
| Biochemical parameters | |||||||||||
| Neutrophil-to-lymphocyte ratio | 1.93 ± 0.77 | 1.9–1.96 | 1.88 ± 0.78 | 1.82–1.94 | .47 | 1.95 ± 0.77 | 1.91–1.99 | .90 | 2.1 ± 0.89 | 2–2.18 | <.001 |
| Monocyte-to-lymphocyte ratio | 0.24 ± 0.09 | 0.23–0.24 | 0.24 ± 0.1 | 0.24–0.25 | .22 | 0.25 ± 0.09 | 0.25–0.26 | <.001 | 0.27 ± 0.1 | 0.25–0.28 | <.001 |
| Platelet-to-lymphocyte ratio | 149 ± 53.9 | 146.8–151.1 | 144.2 ± 54.4 | 139.9–148.4 | .20 | 142.2 ± 54.6 | 139.4–145.1 | .001 | 153.8 ± 66.8 | 147.1–160.5 | .38 |
| Cholesterol (mg/dL) | 202.6 ± 35.8 | 201.2–204 | 203 ± 36.3 | 200.2–205.9 | .99 | 204.9 ± 37.1 | 203–206.9 | .23 | 201 ± 38.2 | 197.1–204.9 | .86 |
| Triglycerides (mg/dL) | 112.3 ± 65.4 | 109.8–114.9 | 142 ± 83.2 | 135.5–148.4 | <.001 | 148.6 ± 89.5 | 143.9–153.3 | <.001 | 149 ± 88.9 | 140–157.9 | <.001 |
| HDL (mg/dL) | 56.5 ± 14.9 | 55.9–57 | 49.1 ± 12.6 | 48.2–50.1 | <.001 | 47.4 ± 12.6 | 46.7–48 | <.001 | 45.8 ± 11.8 | 44.6–47 | <.001 |
| LDL (mg/dL) | 127.3 ± 32.1 | 126.1–128.6 | 131.4 ± 34 | 128.7–134 | .03 | 132 ± 33.9 | 130.2–133.7 | <.001 | 129.6 ± 35.6 | 126–133.3 | .60 |
| Fasting glucose (mg/dL) | 96.5 ± 14.9 | 95.9–97 | 102.5 ± 19.4 | 101–104 | <.001 | 104.8 ± 22.4 | 103.6–106 | <.001 | 113.7 ± 29.4 | 110.8–116.7 | <.001 |
| HbA1c (%) | 5.5 ± 0.5 | 5.4–5.5 | 5.7 ± 0.76 | 5.7–5.8 | <.001 | 5.9 ± 0.9 | 5.8–5.9 | <.001 | 6.3 ± 1 | 6.2–6.4 | <.001 |
| Creatinine (mg/dL) | 0.81 ± 0.2 | 0.8–0.82 | 0.90 ± 0.19 | 0.88–0.91 | <.001 | 0.94 ± 0.2 | 0.93–0.95 | <.001 | 1 ± 0.29 | 0.97–1.03 | <.001 |
| Urine protein | 0.09 ± 0.26 | 0.08–0.10 | 0.13 ± 0.37 | 0.11–0.16 | <.001 | 0.16 ± 0.42 | 0.14–0.18 | <.001 | 0.28 ± 0.60 | 0.22–0.34 | <.001 |
| baPWV and ABI measurements | |||||||||||
| RbaPWV (cm/s) | 1340 ± 218 | 1331–1348 | 1467 ± 314 | 1446–1488 | <.001 | 1567 ± 314 | 1551–1584 | <.001 | 1811 ± 387 | 1772–1849 | <.001 |
| LbaPWV (cm/s) | 1351 ± 224 | 1342–1360 | 1474 ± 266 | 1453–1494 | <.001 | 1580 ± 319 | 1563–1597 | <.001 | 1831 ± 401 | 1790–1871 | <.001 |
| RABI | 1.14 ± 0.08 | 1.13–1.14 | 1.15 ± 0.08 | 1.15–1.16 | <.001 | 1.16 ± 0.08 | 1.15–1.16 | <.001 | 1.16 ± 0.08 | 1.15–1.17 | <.001 |
| LABI | 1.14 ± 0.08 | 1.13–1.14 | 1.16 ± 0.08 | 1.15–1.16 | <.001 | 1.16 ± 0.08 | 1.15–1.16 | <.001 | 1.17 ± 0.08 | 1.16–1.17 | <.001 |
| Risk assessment indicators | |||||||||||
| MS | 1.5 ± 1.1 | 1.4–1.6 | 2 ± 1.2 | 1.9–2.1 | <.001 | 2.3 ± 1.2 | 2.2–2.3 | <.001 | 2.7 ± 1.2 | 2.6–2.9 | <.001 |
| FRS | 2.5 ± 2.1 | 2.4–2.6 | 6.8 ± 3.3 | 6.5–7 | <.001 | 11.5 ± 5.4 | 11.2–11.8 | <.001 | 16.3 ± 6.1 | 15.7–17 | <.001 |
| eGFR (mL/min/1.73 m2) | 90.6 ± 19.5 | 89.8–91.3 | 85.9 ± 17.6 | 84.5–87.3 | <.001 | 82 ± 17.7 | 81–82.9 | <.001 | 76.3 ± 17.9 | 74.5–78.1 | <.001 |
| Carotid plaque (%) | 576 (23.5%) | 227 (35.4%) | 650 (46.3%) | 238 (61.5%) | |||||||
| Age (yr) | 49.5 ± 6.2 | 49.2–49.7 | 55 ± 6.6 | 54.5–55.5 | <.001 | 59.6 ± 7 | 59.2–60 | <.001 | 68 ± 6.1 | 67.4–68.7 | <.001 |
| Vascular age (yr) | 54.9 ± 11 | 54.5–55.3 | 62.3 ± 12.9 | 61.3–63.3 | <.001 | 68.3 ± 14.9 | 67.5–69.1 | <.001 | 81.2 ± 18.2 | 80–83.7 | <.001 |
| Vascular age – age (yr) | 5.4 ± 9.1 | 5.1–5.8 | 7.2 ± 11.1 | 6.4–8.1 | .002 | 8.8 ± 13.3 | 8.1–9.4 | <.001 | 14 ± 17.3 | 12.3–15.8 | <.001 |
| Hazard ratio | 1.11 ± 0.19 | 1.11–1.12 | 1.13 ± 0.21 | 1.12–1-15 | .07 | 1.15 ± 0.23 | 1.14–1.16 | <.001 | 1.21 ± 0.26 | 1.18–1.24 | <.001 |
| Carotid plaque (%) | 576 (23.5%) | 227 (35.4%) | 650 (46.3%) | 238 (61.5%) | |||||||
For the biochemical parameters, significant increases in triglycerides, fasting glucose, HbA1c, creatinine, and urine protein levels from low to high risk were observed (P < .001). The NLR was significantly increased only in the low-risk and high-risk groups, whereas the MLR was significantly increased in intermediate-risk and high-risk groups (P < .001). HDL levels were significantly decreased among the 4 groups (P < .001; Table 1; Figure 1, Supplemental Digital Content, http://links.lww.com/MD/G969).
baPWV and 4 variable ABI factors RbaPWV, LbaPWV, right ABI, and left ABI were measured for the 4 groups. Mean RbaPWV values were obtained for the 4 groups, respectively (1340 [95% CI: 1331–1348], 1467 [95% CI: 1446–1488], 1567 [95% CI: 1551–1584), and 1811 [95% CI: 1772–1849]; P < .001). Mean LbaPWV values were 1351 (95% CI: 1342–1360), 1474 (95% CI: 1453–1494), 1580 (95% CI: 1563–1597), and 1831 (95% CI: 1790–1871), respectively (P < .001). Right ABI and left ABI significantly increased from low- to high-risk groups (P < .001; Table 1; Figure 2, Supplemental Digital Content, http://links.lww.com/MD/G969).
Regarding risk assessment indicators, MS, FRS, and eGFR, of the 4 groups, compared with the low-risk group, a significant increase in mean MS (1.5 [95% CI: 1.4–1.6], 2 [95% CI: 1.9–2.1], 2.3 [95% CI: 2.2–2.3], and 2.7 [95% CI: 2.6–2.9]; P < .001) for all groups and mean FRS (2.5 [95% CI: 2.4–2.6], 6.8 [95% CI: 6.5–7], 11.5 [95% CI: 11.2–11.8], and 16.3 [95% CI: 15.7–17]; P < .001) was noted. The mean eGFR values (90.6 [95% CI: 89.8–91.3], 85.9 [95% CI: 84.5–87.3], 82 [95% CI: 81–82.9], and 76.3 [95% CI: 74.5–78.1]) were significantly decreased among the 4 groups (P < .001; Table 1; Figure 2, Supplemental Digital Content, http://links.lww.com/MD/G969).
Age-related arterial stiffness (vascular age; 54.9, 62.3, 68.3, and 81.2) from low to high risk was observed (P < .001). Age-related arterial stiffness (vascular age), actual age (5.4, 6.4, 8.8, and 14), hazard ratio (1.11, 1.13, 1.15, and 1.19; P < .001 for all groups). Color Doppler carotid artery sonography was used to investigate the 4 groups; a significant increase in plaques (localized protruding lesions of ≥ 1.1 mm; 576 [23.5%], 227 [35.4%], 650 [46.3%], and 238 [61.5%]) from low- to high-risk groups was observed (Table 1; Figure 3, Supplemental Digital Content, http://links.lww.com/MD/G969).
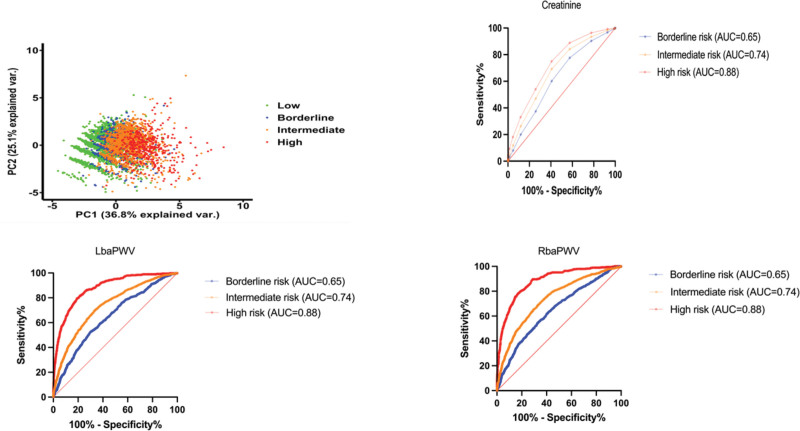
A total of 14 factors were analyzed using principal component analysis. When 2 principal variation factors were selected, the total explanatory variation was 61.9%. The first and second principal components accumulated at 36.8% and 25.1%, respectively. We observed that through the analysis of these 2 main components, the low-risk group of ASCVD and high-risk group of ASCVD gradually separated into different groups (Fig. 2). After deducting the original factors used to calculate ASCVD, these 2 factors were the main variances and can be used to predict in which risk of ASCVD each factor should be.
Figure 2.
The PC, baPWV, and creatinine ROC curve. AUC = area under the curve, baPWV = brachial-ankle pulse wave velocity, PC = principal component, ROC = receiver operating characteristic.
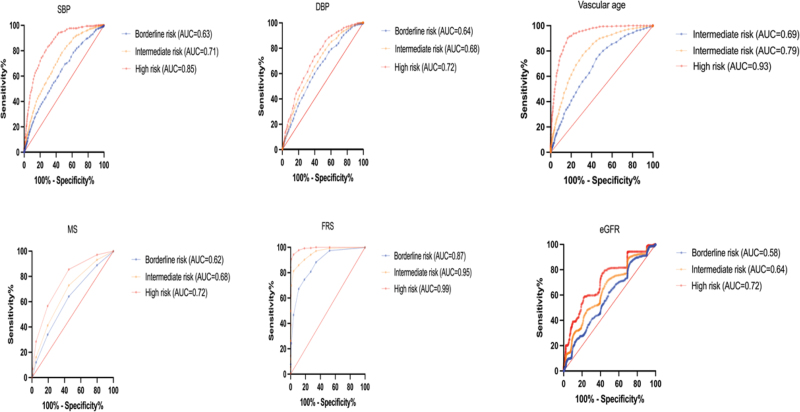
ROC curve analysis was used to compare the screening values between ASCVD and baPWV, MS, FRS, and eGFR risk indicators, and biochemical parameter values were significantly increased from low-risk to high-risk groups (AUC > 0.72–0.99; Fig. 3).
Figure 3.
SBP, DBP, vascular age, MS, FRS, eGFR, and ROC curve. AUC = area under the curve, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FRS = Framingham Risk Score, MS = metabolic syndrome, ROC = receiver operating characteristic, SBP = systolic blood pressure.
4. Discussion
The use of ASCVD risk factors to predict 10-year risk is an option for primary prevention. However, these results should be communicated during patient risk discussions to decide how to implement preventive measures, particularly to initiate medical therapy.[1–3]
In our study, we investigated the main components of ASCVD, except for the factors calculated in the risk formula. A total of 14 factors were analyzed using principal component analysis. When 2 principal variation factors were selected, the total explanatory variation was 61.9%. The main principal component 1 (36.8%) was baPWV, and component 2 (25.1%) was creatinine. The ROC curve AUC from borderline risk and intermediate risk to high risk was the baPWV (0.65, 0.74, and 0.88) and creatinine (0.65, 0.74, and 0.88). The AUC index of baPWV and creatinine in intermediate risk represents acceptable discrimination of ASCVD (0.7 ≦ AUC ≦ 0.8), and the AUC index of baPWV and creatinine in high risk represents excellent discrimination of ASCVD (0.8 ≦ AUC ≦ 0.9). Manjunath et al reported the level of kidney function and serum creatinine (1.1 mg/dL with a range of 1.0–5.0 mg/dL) as a risk factor for ASCVD; our result in high-risk subjects was 1 + 0.29 (95% CI: 0.97–1.03, P < .001).[27]
The biochemical parameters, NLR and MLR, were significantly increased in the high-risk group (P < .001 and P < .001, respectively), but PLR was not significantly increased, which is different from the results of other studies and requires further exploration. Other risk factors for ASCVD, particularly advanced age and high blood pressure, HbA1c, and creatinine levels have been reported to be associated with an increase in arterial stiffness.[16–18]
Subjects with hypertension, diabetes, MS, chronic kidney disease, as well as aging exhibited increased baPWV.[28] Table 1 shows the comparison between vascular age predicted by baPWV analysis and actual age for the 4 groups of participants. It can be observed that the difference between the predicted vascular age and actual age was statistically significant, with a P value of <.001 for the intermediate- and high-risk groups and hazard ratios of 1.11 + 0.19, 1.13 + 0.21, 1.15 + 0.23, and 1.21 + 0.26, respectively. These results demonstrate that vascular age predicted using baPWV was considerably higher than the actual age, with a highly significant statistical difference.
When the 4 groups underwent carotid artery sonography to detect carotid plaques, we observed plaque formation in 576 (23.5%), 227 (35.4%), 650 (46.3%), and 238 (61.5%) subjects, respectively. Therefore, there was considerable plaque formation even in the low-risk group (23.5%) and the proportion of subjects with carotid plaques increased with the predicted ASCVD risk.
5. Conclusion
For several decades, countries around the world have placed great emphasis on disease prevention methods and the implementation of healthy lifestyle changes to combat CVD. In particular, lifelong adoption of a healthy lifestyle is the most important method for the prevention of ASCVD, heart failure, and atrial fibrillation. Risk indicators and baPWV are useful predictors of ASCVD, which in conjunction with conventional pharmacotherapy could be useful for primary prevention of plaque formation in subjects with cardiovascular comorbidities.
6. Limitations
This was a retrospective observational study that only included subjects from relatively high-income groups with elevated health awareness, which may not represent the general population. The limitations of our study are that the study subjects underwent a health examination and may or may not have been diagnosed with CVD. However, our results showed that a ROC curve analysis was used to compare the screening values between ASCVD and baPWV, MS, FRS, and eGFR.
Although this study involved prospective patient enrollment and follow-up, the study design was observational in nature and subject to limitations, including selection bias, and uncorrected confounding.
Acknowledgments
This work was supported by the Health Evaluation Center, Mackay Memorial Hospital, and was accomplished with the courtesy of all our colleagues at the center.
Author contributions
C-CL contributed to study conception, design, and coordination, and contributed to drafting the manuscript.
H-JK, K-CH, and P-JH performed the statistical analysis and data curation.
C-LH and L-YY performed the experiments, and reviewed and edited the manuscript.
S-CS and Y-CH were responsible for project administration.
All authors read and approved the manuscript.
Supplementary Material
Abbreviations:
- ABI =
- ankle brachial index
- ASCVD =
- atherosclerotic cardiovascular disease
- CI =
- confidence interval
- CRP =
- C-reactive protein
- CVD =
- cardiovascular disease
- baPWV =
- brachial-ankle pulse wave velocity
- DBP =
- diastolic blood pressure
- DM =
- diabetes mellitus
- eGFR =
- estimated Glomerular filtration rate
- FRS =
- Framingham Risk Score
- HbA1c =
- hemoglobin A1c
- HDL-cholesterol =
- high-density lipoprotein cholesterol
- HR =
- hazard ratio
- HsCRP =
- high-sensitivity C-reactive protein
- LDL-cholesterol =
- low-density lipoprotein cholesterol
- MLR =
- monocyte-to-lymphocyte ratio
- MS =
- metabolic syndrome
- NLR =
- neutrophil-to-lymphocyte ratio
- PLR =
- platelet-to-lymphocyte ratio
- ROC =
- receiver operating characteristic
- SBP =
- systolic blood pressure.
How to cite this article: Ko H-J, Liu C-C, Hsu P-J, Hu K-C, Hung C-L, Yu L-Y, Huang Y-C, Shih S-C. Risk assessment indicators and brachial-ankle pulse wave velocity to predict atherosclerotic cardiovascular disease. Medicine 2022;101:32(e29609).
Supplemental Digital Content is available for this article.
The authors have no conflict of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to “MacKay Memorial Hospital” Institutional Data Access/Ethics Committee for researchers (Institutional Review Board Contact information: MacKay Memorial Hospital. Address: No. 92, Sec. 2, Zhongshan N. Rd., Taipei City 10449, Taiwan. TEL: 02-25433535#3486~3488, e-mail: mmhirb82@gmail.com).
ABI = ankle brachial index, ASCVD = atherosclerotic cardiovascular disease, CI = confidence interval, baPWV = brachial-ankle pulse wave velocity, eGFR = estimated glomerular filtration rate, FRS = Framingham Risk Score, HbA1c = hemoglobin A1c, HDL = high-density lipoprotein, LABI = left ankle brachial index, LbaPWI = left brachial-ankle PWV, LDL = low-density lipoprotein, MS = metabolic syndrome, RbaPWI = right brachial-ankle PWV, RABI = right ankle brachial index, SD = standard deviation.
Contributor Information
Hung-Ju Ko, Email: bonnie@mmh.org.tw.
Po-Jui Hsu, Email: ray60115@yahoo.com.tw.
Kuang-Chun Hu, Email: mimiandbear2001@yahoo.com.tw.
Chung-Lieh Hung, Email: jotaro3791@gmail.com.
Lo-Yip Yu, Email: benny7190@gmail.com.
Yun-Chieh Huang, Email: hungyunnjye@gmail.com.
Shou-Chuan Shih, Email: shihshou@gmail.com.
References
- [1].Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2020;141:e59. [DOI] [PubMed] [Google Scholar]
- [2].Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
- [3].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare subjects: the million hearts longitudinal ASCVD risk assessment tool: a special report from the American Heart Association and American College of Cardiology. Circulation. 2017;135:e793–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ashraf T, Mengal MN, Muhammad AS, et al. Ten years risk assessment of atherosclerotic cardiovascular disease using Astro-CHARM and pooled cohort equation in a south Asian sub-population. BMC Public Health. 2020;20:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ren H, Zhao L, Zou Y, et al. Association between atherosclerotic cardiovascular diseases risk and renal outcome in subjects with type 2 diabetes mellitus. Ren Fail. 2021;43:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- [10].Grundy SM, Brewer HBJ, Cleeman JI, et al. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–8. [DOI] [PubMed] [Google Scholar]
- [11].Assmann G, Guerra R, Fox G, et al. Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the adult treatment panel III and the international diabetes federation in United States American and European populations. Am J Cardiol. 2007;99:541–8. [DOI] [PubMed] [Google Scholar]
- [12].Dommermuth R, Ewing K. Metabolic syndrome: systems thinking in heart disease. Prim Care. 2018;45:109–29. [DOI] [PubMed] [Google Scholar]
- [13].Meysamie A, Salarvand F, Khorasanizadeh M, et al. Cardiovascular risk assessment by FRS and SCORE in Iranian adult population. J Diabetes Metab Disord. 2017;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trusinskis K, Lapsovs M, Paeglite S, et al. Plasma circulating microRNAs in subjects with stable coronary artery disease—impact of different cardiovascular risk profiles and glomerular filtration rates. J Clin Transl Res. 2021;7:270–6. [PMC free article] [PubMed] [Google Scholar]
- [15].Liu C-C, Ko H-J, Liu W-S, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine (Baltimore). 2019;98:e17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li Y, Chen X, Huang L, et al. Association between neutrophil–lymphocyte ratio and arterial stiffness in subjects with acute coronary syndrome. Biosci Rep. 2019;39:BSR20190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park SY, Chin SO, Rhee SY, et al. Cardio-ankle vascular index as a surrogate marker of early atherosclerotic cardiovascular disease in Koreans with type 2 diabetes mellitus. Diabetes Metab J. 2018;42:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive subjects. Hypertension. 2001;37:1236–41. [DOI] [PubMed] [Google Scholar]
- [19].Tomiyama H, Shiina K. State of the art review: brachial-ankle PWV. J Atheroscler Thromb. 2020;27:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. [DOI] [PubMed] [Google Scholar]
- [21].Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement: a survey of 12517 subjects. Atherosclerosis. 2003;166:303–9. [DOI] [PubMed] [Google Scholar]
- [22].Kim BH, Jang JS, Kwon YS, et al. High brachial ankle pulse wave velocity as a marker for predicting coronary artery stenosis in subjects with type 2 diabetes. Endocrinol Metab (Seoul). 2018;33:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim H-L, Kim S-H. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ato D, Sawayama T. Factors associated with high brachial–ankle pulse wave velocity in non-hypertensive and appropriately treated hypertensive subjects with atherosclerotic risk factors. Vasc Health Risk Manag. 2017;13:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Satomi A, Murakami S, Ishida K, et al. Significance of increased neutrophils in subjects with advanced colorectal cancer. Acta Oncol. 1995;34:69–73. [DOI] [PubMed] [Google Scholar]
- [27].Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. [DOI] [PubMed] [Google Scholar]
- [28].Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse (Basel). 2016;3:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.