Abstract
Older people with chronic pain are at higher risk of developing sarcopenia. Central sensitization (CS) has been implicated in chronic pain among community-dwelling older adults. However, a relationship between CS and chronic pain with sarcopenia has not been established. This cross-sectional study aimed to clarify the relationship between chronic pain with sarcopenia or presarcopenia and CS among community-dwelling older adults. We assessed chronic pain and sarcopenia in 104 older adults participating in community health checks. We defined sarcopenia using the Asian Working Group for Sarcopenia (AWGS) consensus recommendations based on the following outcomes: low muscle mass, low muscle strength, and slow gait speed. Pain-related assessments included pain intensity, the Pain Catastrophizing Scale, the CS Inventory-9, the pressure pain threshold, the Tampa Scale of Kinesiophobia-11, and the EuroQol 5-dimension 5-level (EQ5D-5L). Chronic pain was defined by related symptoms within the month prior to the health check that had continued for ≥ 3 months and corresponded to a numerical rating scale score of ≥ 1 at the site of maximum pain. The prevalence of chronic pain was 43.3%. In addition, the prevalence of chronic pain with sarcopenia or presarcopenia was 29.8%. A logistic regression analysis revealed that the pressure pain threshold (odds ratio: 0.82, 95% CI: 0.95–1.02) and the EQ5D-5L (odds ratio: 0.58, 95% CI: 0.36–0.76) were significantly associated with the presence of chronic pain with sarcopenia or presarcopenia. Chronic pain with sarcopenia or presarcopenia was affected by central sensitization. Therefore, CS should be evaluated in the elderly.
Keywords: central sensitization, chronic pain, pressure pain threshold, sarcopenia
1. Introduction
Sarcopenia is defined as a functional disorder caused by a decline in the quantity or quality of the skeletal muscle.[1] Sarcopenia is often associated with falls, fractures, and disability in individuals with physical frailty.[2–4] The prevalence of sarcopenia is 10% to 26.6% among people aged ≥ 65 years in Japan, and the incidence increases with age.[5,6] Kitamura reported that Japanese community-dwelling older adults with sarcopenia had a roughly 2-fold higher risk of all-cause mortality and the development of disability compared with similar individuals without sarcopenia.[5] Also, older Japanese men and women who meet the Asian criteria for sarcopenia are at increased risk of all-cause mortality and disability.[5]
Sarcopenia has recently been associated with chronic pain, a complex and multidimensional condition affecting a high percentage of older adults[7] and dramatically impacting their functioning and well-being.[8,9] Older Japanese adults with lower-limb pain have poor muscle function and a higher risk of falls and fractures.[10] Furthermore, women with prevalent chronic pain have been described as experiencing greater lower-limb strength declines compared with those without pain.[11] Chronic pain may directly contribute to the progression of sarcopenia in older women. In studies of community-dwelling older adults, the prevalence of chronic pain with sarcopenia was approximately 10%.[12] Moreover, another study showed that older people with chronic pain in Japan are at a higher risk of developing sarcopenia.[6] In Japan, the number of community-dwelling older adults who require long-term care due to physical deterioration has increased as a result of the rapidly aging population. However, there are few reports on the association between chronic pain and sarcopenia or “presarcopenia.”
Imai et al reported an association between chronic pain and community-dwelling older adults with frailty; they also observed that central sensitization (CS) was influenced by chronic pain with frailty.[13] It has been proposed that CS is a physiological phenomenon in which dysregulation in the central nervous system causes neuronal dysregulation and hyperexcitability, resulting in hypersensitivity to both noxious and non-noxious stimuli.[14] Since community-dwelling older adults with chronic pain can find it difficult to ease their pain, we speculated that CS may be involved and that it would thus be important to evaluate CS in these individuals. While CS may also be involved in sarcopenia, this has not yet been reported. We conducted the present cross-sectional study to clarify the relationship between CS and chronic pain with sarcopenia or presarcopenia among community-dwelling older adults.
2. Methods
2.1. Design and setting
Our study was cross-sectional in design and used data from a community health checkup program in Kaizuka City, Japan. The study involved a community-based health check conducted in collaboration with the Osaka Kawasaki Rehabilitation University and Kaizuka City Office. Three community centers in Kaizuka were used to conduct the community health checkup program.
2.2. Participants
This cross-sectional study was conducted in the city of Kaizuka, Japan, from August 7 to September 9, 2021, with recruitment occurring from July 1 to 25, 2021. There were 3 recruitment criteria for participation: (1) living in Kaizuka City, (2) age ≥ 65 years, and (3) no physician-ordered exercise ban. We published a flyer for the community health checkup program in local newspapers and placed the flyer in public offices such as city hall. The inclusion criteria were as follows: age ≥ 65 years, living at home, and able to both walk outdoors and perform activities of daily living independently. Individuals who were unable to respond to questions because of cognitive impairment were excluded.
2.3. Ethical considerations
This study was approved by the Ethics Committee of the Osaka Kawasaki Rehabilitation University (OKRU30-A016; July 9, 2019) in Japan and conformed to the 2008 Declaration of Helsinki Human Rights guidelines. Before the study started, written informed consent to participate was obtained from each subject. In addition, we completed the STROBE checklist for cross-sectional studies and adhered to the guidelines.
2.4. Primary outcome
The Central Sensitization Inventory (CSI) was developed as a screening tool to identify and quantify patients with CS-related symptoms.[14,15] The CSI-9 (a short version of the CSI) is a symptomatological and self-reported questionnaire consisting of 9 items assessing health-related symptoms that are common among individuals with CS syndromes.[16] To assess CS, we evaluated subjects’ pressure pain threshold (PPT). The PPT was determined by a trained physical therapist based on a standardized testing protocol.[17] The PPT was evaluated on the dominant side of the subject’s extensor carpi radialis longus muscle (5 cm distal to the lateral epicondyle). When the PPT is assessed at a distant, healthy site, it reflects systematic altered pain processing, which may be related to CS.[18] The PPT was assessed with an FPX50 algometer with a 1-cm2 probe (Wagner Instruments; Greenwich, CT, USA). The pressure was applied at 1 N/s until the subject verbally indicated when the sensation became painful.[17] The PPT test was repeated 3 times at 30-s intervals, and the mean value was recorded.
2.5. Secondary outcomes
2.5.1. Pain assessments.
The participants completed a numerical rating scale for the assessment of their pain intensity: 0 = no pain and 10 = highest possible degree of pain. We administered the Widespread Pain Index (WPI) to assess the presence of pain at 19 designated body locations (e.g., left upper arm, right lower leg). Each location corresponded to 1 point. All WPI items were summed to yield a total score, with higher scores indicating greater and more widespread pain.
2.5.2. Pain-related assessments.
Pain-related catastrophizing was measured by the Japanese version of the Pain Catastrophizing Scale, which has been confirmed to have internal consistency and criterion validity.[19,20] Kinesiophobia was assessed by the Japanese version of the 11-item Tampa Scale for Kinesiophobia-11 (TSK-11), which shows better internal reliability, identical construction, and known group validity than the 17-item version.[21] Each item of the TSK-11 is scored on a 4-point scale from 1 (strongly disagree) to 4 (strongly agree).
2.5.3. The 5-level EuroQol 5-dimension instrument.
The 5-level version of the EuroQol 5-dimension test (EQ5D-5L) is an instrument that standardizes various diseases commonly used as a complementary assessment for existing health-related quality of life (QOL) measures.[22] It generates a single index that describes the patient’s health state based on a 5-point scale for each parameter. These values are expressed numerically, ranging from 0 (dead) to 1 (full health). The scores can be converted into a weighted index (EQ-5D index) using the EQ-5D-5L Crosswalk Index Value Calculator downloaded from the EuroQol website.[23] The score of the EQ-5D index ranges from 0.111 to 1, where 1 represents preferred health. We used the Japanese version of the EQ5D-5L; the Japanese scoring system has been established as valid and reliable.[24]
2.5.4. Body composition.
Each subject’s body mass index was calculated by dividing their body weight (kg) by their height squared (m2). The appendicular skeletal muscle mass index was derived from the appendicular muscle mass (kg) divided by height squared (m2) using a bioelectrical impedance device (InBody270; InBody, Tokyo). Body fat percentage was also measured using this device. Low muscle mass was defined as a height-adjusted skeletal muscle mass < 7.0 kg/m2 for men and < 5.7 kg/m2 for women.
2.5.5. Muscle strength.
Muscle strength was measured as handgrip strength, which is significantly associated with whole-body muscle strength.[25] The maximum voluntary isometric handgrip strength was measured using a hand dynamometer (Grip-D; Takei, Niigata, Japan) with the subject’s dominant hand while they were in a standing position. Weakness was defined by maximum grip strength cutoff values of <26 kg for men and <18 kg for women.
2.5.6. Gait speed.
For assessing gait speed, the subject was instructed to walk 6.4 m (divided into 2 2.0-m zones at each end and a 2.4-m middle zone) at a speed they found comfortable. The time needed (in seconds) to pass the 2.4-m middle zone was measured to calculate gait speed (m/s). The subject could use a cane or walker if they were unable to perform the gait test independently. The average from 5 gait tests was used for the evaluation. Slowness was defined as a walking speed < 1.0 m/s.
2.5.7. Sarcopenia assessment.
We defined sarcopenia using the Asian Working Group for Sarcopenia (AWGS) consensus recommendations based on meeting all of the following outcomes: low muscle mass (<7.0 kg/m2 for men and <5.7 kg/m2 for women), low muscle strength (<26 kg for men and < 18 kg for women), and slow gait speed (<1.0 m/s).[26] Presarcopenia was defined as skeletal muscle mass loss without low physical function.
2.6. Comparisons
First, we divided the subjects into those with chronic pain (the chronic pain group) and those without chronic pain (the nonchronic pain group). We also assessed the presence of chronic pain, which was defined as related symptoms within the month prior that had continued for ≥ 3 months and corresponded to an numerical rating scale score of ≥1 at the site of maximum pain.[27]
To further compare the chronic pain and nonchronic pain groups, we subdivided the proportions of subjects with sarcopenia, presarcopenia, and nonsarcopenia (robust) in each group: chronic pain with sarcopenia (CPS), chronic pain with presarcopenia (CPPS), robust chronic pain (CPR), nonpain with sarcopenia (NCPS), nonpain presarcopenia (NCPPS), and robust nonpain (NCPR). The classification of sarcopenia was as described in the Sarcopenia assessment.
2.7. Statistical analyses
There was an insufficient number of male subjects; therefore, we decided to include only female subjects in the analysis because all the outcome measurements could be affected by unequal numbers of males and females. To account for missing data, our statistical plan was to use multiple imputations. To avert bias caused by missing data, multiple imputations is a procedure used to replace missing values with other plausible values by creating multiple filling-in patterns. It is also recognized as an alternative approach to analyzing incomplete data sets.[28]
We used unpaired t-tests and Mann-Whitney U tests to evaluate significant differences between the chronic pain and nonchronic pain groups. We performed a 1-way analysis of variance (ANOVA) to assess differences in continuous outcome measures between each pair of groups and then applied corrections from Tukey post hoc tests. The other outcomes were examined using Kruskal-Wallis tests. We conducted logistic regression analyses to identify the factors most strongly associated with CPS and CPPS.
To clarify the relationship between CS and chronic pain with sarcopenia or presarcopenia, we conducted the logistic regression analysis. There were 2 regression models: 1 used crude odds ratios (ORs), and the other used adjusted age and body mass index ORs. The presence of CPS and CPPS was the dependent variable in both models. Factors related to CSI with significant differences in ANOVA were included in the logistic regression analysis. The potential EQ5D5L score ranges from 0.111 to 1, and the logistic is usually considered as a single point, but this is difficult to do for the EQ5D5L, so we adjusted the value to 0.1. The significance level was set at P < .05 for all statistical analyses, which were conducted using SPSS ver. 26.0 (IBM, Armonk, NY).
2.8. Sample size
We calculated the required sample size for the logistic linear regression analysis using G*power with a power of 80%, an effect size of 0.15, and a significance level of 0.05. The minimum required sample size was 77.
3. Results
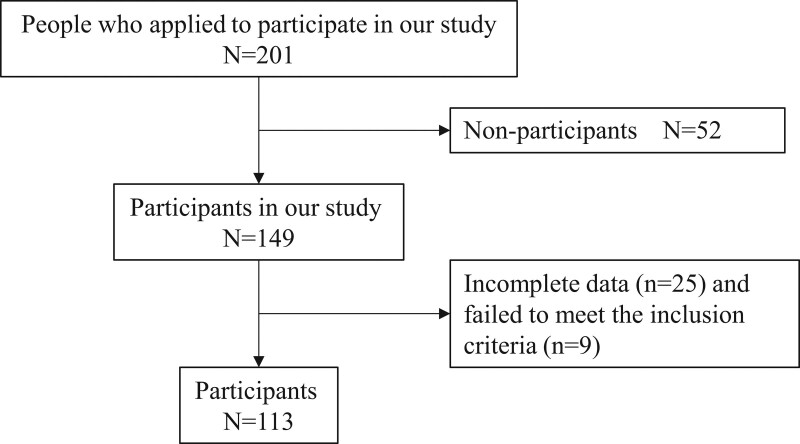
A community health checkup program expected 201 people to attend, but 52 did not; thus, 149 older adults participated in the program. Forty-five subjects did not complete all of the evaluations (n = 25) or failed to meet the inclusion criteria (n = 9). The final number of subjects included in the analyses was 113 older adults (Fig. 1).
Figure 1.
Flowchart of inclusion and exclusion criteria for this study.
3.1. Participant characteristics and chronic pain status
Table 1 summarizes the characteristics of the participants (n = 113). In addition, of the 113 participants, 49 (43.4%) had chronic pain. The chronic pain group showed significantly higher Central Sensitization Inventory (CSI)-9 (P = .005) and PPT (P = .011) than the nonchronic pain group. In addition, the chronic pain group showed significantly higher pain intensity (P < .001), WPI (P < .001), and PCS total score (P < .001) than the nonchronic pain group (Table 1). EQ5D5L (P < .001) and gait speed (P = .005) were significantly lower than those of the nonchronic pain group.
Table 1.
Characteristics of the chronic pain and nonchronic pain groups of community-dwelling older adults.
| Characteristic | Total (n = 113) | Chronic pain (n = 50) | Nonchronic pain (n = 63) | P |
|---|---|---|---|---|
| Age (yrs) | 76.3 (5.6) | 77.5 (6.1) | 75.3 (5.1) | .12 |
| BMI (kg/m2) | 22.4 (3.2) | 22.8 (3.1) | 22.1 (3.2) | .201 |
| SMI | 5.6 (0.6) | 5.6 (0.6) | 5.6 (0.6) | .693 |
| MMSE | 28.5 (2.3) | 28.4 (2.5) | 28.5 (2.0) | .672 |
| Education (years) | 11.6 (2.1) | 11.6 (2.2) | 12.0 (2.1) | .348 |
| CSI | 7.0 (5.1) | 8.4 (9.8) | 5.9 (4.6) | .005 |
| PPT | 15.8 (5.3) | 13.9 (4.5) | 17.3 (6.0) | .011 |
| NRS at the site of maximum pain | 2.8 (1.8) | 3.5 (1.5) | 1.7 (1.0) | <.001 |
| WPI | 1.6 (2.3) | 3.2 (2.1) | 0.3 (0.8) | <.001 |
| Site of pain, n (%): | <.001 | |||
| Shoulder or neck | 7 (6.8) | 5 (11.1) | 2 (3.3) | |
| Back | 9 (8.7) | 6 (13.3) | 3 (4.6) | |
| Lower limb | 14 (13.6) | 11 (24.4) | 3 (4.6) | |
| >3 sites | 13 (12.6) | 13 (26.5) | 0 | |
| PCS total score | 12.0 (11.8) | 16.1 (12.6) | 8.9 (9.9) | <.001 |
| TSK-11 | 19.1 (6.4) | 20.3 (6.4) | 18.2 (6.2) | .031 |
| EQ5D5L | 0.8 (0.14) | 0.74 (0.12) | 0.88 (0.13) | <.001 |
| Gait speed (m/s) | 1.36 (0.23) | 1.3 (0.2) | 1.41 (0.2) | .005 |
| Grip strength (kg) | 20.6 (4.2) | 20.0 (4.5) | 21.3 (4.2) | .131 |
3.2. Subject characteristics by chronic pain and sarcopenia status
Table 2 summarizes the proportions of sarcopenia and presarcopenia subjects with and without chronic pain and compares the outcome measures between these groups. The PPT values in the CPS group were significantly lower than those of the NCPS (P = .018) and NCPR (P = .008) groups (Table 2). The CPR group showed significantly higher CSI-9 than the NCPR group (P = .025), but there were no significant differences in the other groups.
Table 2.
Characteristics and comparison of outcome measures among the groups.
| Chronic pain | Nonchronic pain | P | |||||
|---|---|---|---|---|---|---|---|
| Sarcopenia (n = 11) | Presarcopenia (n = 23) | Robust (n = 16) | Sarcopenia (n = 11) | Presarcopenia (n = 22) | Robust (n = 30) | ||
| Age | 79.3 (5.6) | 76.3 (5.8) | 75.6 (4.7) | 77.9 (7.0) | 74.8 (3.6) | 74.9 (5.2) | .231 |
| BMI (kg/m2) | 21.6 (1.9) | 21.7 (2.4) | 25.3 (3.5)*,† | 21.5 (3.0)‡ | 20.3 (2.1)‡ | 23.6 (3.1)† | <.001 |
| SMI | 5.2 (0.2) | 5.3 (0.2) | 6.3 (0.6)*,† | 4.9 (0.4)‡ | 5.2 (0.3)‡ | 6.1 (0.4)*,† | <.001 |
| MMSE | 28.2 (1.8) | 28.7 (1.9) | 28.2 (2.2) | 27.3 (5.1) | 28.8 (1.4) | 29.1 (1.3) | .283 |
| Educatiuon period (year) | 10.9 (2.0) | 11.9 (2.6) | 11.7 (1.8) | 11.3 (1.6) | 11.8 (.4) | 12.5 (2.4) | .391 |
| NRS at the site of maixmum pain | 4.1 (1.8) | 3.0 (1.4) | 3.0 (1.3) | 2.2 (2.2) | 1.7 (1.4)*,‡ | 1.6 (2.1)*,†,‡ | .001 |
| WPI | 4.1 (2.8) | 2.5 (1.0) | 2.3 (1.5)* | 0 (0)*,†,‡ | 0.4 (1.0)*,†,‡ | 0.2 (0.8)*,†,‡ | <.001 |
| Site of pain, n (%) | |||||||
| Shoulder or Neck | 6 (5.3) | 6 (5.3) | 0 (0) | 1 (0.9) | 3 (2.7) | 0 (0) | |
| Back | 0 (0) | 5 (4.4) | 2 (1.8) | 0 (0) | 1 (0.9) | 1 (0.9) | |
| Lower limb | 6 (5.3) | 2 (1.8) | 5 (4.4) | 0 (0) | 0 (0) | 3 (2.7) | |
| More 3 site | 7 (1.8) | 5 (4.4) | 2 (6.2) | 0 (0) | 0 (0) | 0 (0) | |
| PCS total score | 12.5 (11.8) | 13.4 (12.3) | 22.7 (12.4)† | 6.3 (8.6)‡ | 11.7 (9.7)‡ | 7.8 (0.4)*,‡ | .001 |
| CSI | 7.8 (6.6) | 7.3 (5.8) | 10.1 (4.5) | 6.4 (5.7) | 6.6 (5.0) | 5.2 (3.6)‡ | .051 |
| TSK-11 | 19.7 (6.3) | 19.9 (6.4) | 21.4 (7.0) | 17.4 (6.3) | 19.2 (6.1) | 17.6 (6.4) | .354 |
| Pressure pain threshold | 12.4 (3.2) | 13.2 (4.6) | 16.0 (4.6) | 17.6 (4.9)* | 16.1 (4.7) | 18.9 (7.1)*,† | .007 |
| EQ5D5L | 0.76 (0.1) | 0.76 (0.11) | 0.71 (0.13) | 0.86 (0.15)†,‡ | 0.84 (0.13)*,†,‡ | 0.91 (0.1)*,†,‡ | <.001 |
| Gait speed (m/s) | 1.23 (0.16) | 1.34 (0.3) | 1.26 (0.2) | 1.34 (0.2) | 1.47 (0.21)* | 1.41 (0.2)* | .016 |
| Grip strength (kg) | 16.6 (1.3) | 21.1 (2.1)* | 20.1 (6.9)* | 16.6 (2.9)† | 21.4 (2.7)* | 22.7 (4.4)* | <.001 |
In the pain-related assessments, the CPS group showed significantly higher pain intensity than the NCPPS (P = .009) and NCPR groups (P = .007), and pain intensity in the CCPS group was significantly higher than that in the NCPR group (P = .024). The EQ5D5L values in the CPS group were significantly higher than those of the NSPPS (P = .027), and NCPR group (P = .011). The EQ5D5L values in the CPPS group were significantly higher than those of the NCPS (P = .008), NCPPS (P = .041), and NCPR group (P = .001). The EQ5D5L values in the CPR group were significantly higher than those of the NCPS (P = .021), NSPPS (P = .003), and NCPR group (P < .001). In addition, the CPR group’s PCS total score was significantly higher than that of the NCPS (P = .008), NSPPS (P = .031), and NCPR group (P < .001).
In the physical assessments, gait speed in the CPS groups was significantly slower compared to the NCPPS (P = .038) and NCPR groups (P = .044). In addition, grip strength in the CPS group was significantly lower compared to the NCPPS (P = .011) and NCPR group (P < .001) (Table 2). Grip strength in the NCPS group was significantly lower compared to the NCPPS (P < .001) and NCPR group (P < .001).
3.3. Logistic regression analysis of chronic pain with sarcopenia or presarcopenia
The results of the logistic regression analysis are presented in Table 3. CSI and PPT were used to assess CS, but the ANOVA results showed no significant difference in CSI. In addition, the PPT, PCS, and EQ-5D-5L demonstrating significant differences in ANOVA were designated as independent variables. Based on this analysis, the PPT (OR 0.82, 95% CI: 0.95–1.02, P < .001) and the EQ5D5L (OR 0.53, 95% CI: 0.36–0.76, P = .001) were identified as significant factors associated with the presence of chronic pain with sarcopenia or presarcopenia.
Table 3.
Logistic regression analysis identifying the factors associated with the presence of chronic pain with sarcopenia or presarcopenia.
| Independent variables | Crude OR | 95% CI | P | aOR* | 95% CI | P |
|---|---|---|---|---|---|---|
| PCS, total score | 0.99 | 0.95–1.03 | .65 | 0.98 | 0.95–1.03 | .825 |
| EQ5D5L index | 0.52 | 0.36–0.77 | .001 | 0.53 | 0.37–0.77 | .001 |
| PPT | 0.81 | 0.72–0.89 | <.001 | 0.82 | 0.73–0.92 | <.001 |
4. Discussion
We sought to reveal the relationship between chronic pain and sarcopenia or presarcopenia among community-dwelling older adults. The prevalences of CPS and CPPS were 9.7% and 21.4%, respectively. Compared with the CPS and CPPS groups, the NCPS, NCPPS, and NCPR subjects differed in the PPT and EQ5D5L. However, there were no significant differences for the CSI-9, although the outcome was higher in all of the chronic pain groups. In addition, the PPT and EQ5D-5L were significantly associated with the presence of chronic pain with sarcopenia or presarcopenia. Chronic pain with sarcopenia or presarcopenia was affected by CS, and thus CS should be evaluated in the elderly.
The prevalence of CPS among community-dwelling older adults in the present investigation (9.7%) was similar to a previous study.[12] However, the rate increased to 19.5% when including chronic pain with presarcopenia. There have been many investigations of pain and frailty in Japan, but to the best of our knowledge, there are few reports on the prevalence of sarcopenia or presarcopenia and chronic pain.[12,29] In addition, there are no reports of an association between chronic pain with sarcopenia or presarcopenia and central sensitization; our present findings thus provide a useful starting point for this topic.
The PPT, a type of quantitative sensory test (QST), is currently the standard for identifying pain sensitization.[30] Peripheral sensitization can be measured when the PPT of an affected area is evaluated. On the other hand, CS can be evaluated when the PPT is measured outside the affected area. In the present study, the PPT was measured after confirming there was no pain at the measurement site. Thus, CS was likely involved in the pain of the CPS and CPPS subjects in this study. CS results in a state of nervous system hypersensitivity to pain,[31] facilitation, central amplification of nociception, and decreased pain inhibition.[32] This can result in secondary hyperalgesia at unaffected tissue sites and widespread hyperalgesia at multiple sites on the body.[32] The reality that community-dwelling older adults with chronic pain can find it difficult to ease their pain suggests that CS may be involved. Therefore, it is important to evaluate CS in these individuals.
Central sensitization may also augment or amplify symptom intensity in patients with musculoskeletal disorders, such as chronic lower back, knee, or shoulder pain.[29,33,34] Imai et al[35] reported that community-dwelling older adults with chronic pain had significantly greater CSI values compared to those who did not have chronic pain. However, in this study, CSI was not significantly associated with the presence of chronic pain with sarcopenia or presarcopenia. The possible reason for this result is that a different method and comprehensive screening tool were used for assessing CS syndromes, and thus CS and CS-related syndromes are not the same. In addition, it can be difficult for community-dwelling older adults to answer the CSI questionnaire. Imai et al[35] showed that chronic pain with prefrailty was associated with the CSI, but there are similarities between frailty and CSI questions.
It has been reported that CS contributes to persistent pain and health-related QOL.[13] The EQ5D5L is a subjective questionnaire, and while subjects can be aware of their pain, few people are aware of their sarcopenia. An individual’s health-related QOL may be more closely involved in chronic pain and CS than their sarcopenia status.
There are several limitations to the present study. First, the sample size was small (n = 113), but as the survey was conducted during a pandemic, it was difficult to recruit a greater sample size. Second, the study’s cross-sectional design precluded the assumption of a causal relationship between CS symptoms and chronic pain with sarcopenia. Third, study data were obtained from a single city, which limits the generalizability of the results, as meaningful activities in daily life are also affected by geographic location. Therefore, sampling bias cannot be ruled out. Forth, because the number of factors that can be collected in this study was limited, we were not able to investigate socioeconomic factors. Finally, compared to previous studies, subjects in this study had less pain. Thus, compared to all individuals with chronic pain, our subjects’ symptoms might not have been as severe.
Regarding data availability, our research is not publicly deposited, so please contact the authors if you need information.
5. Conclusions
We investigated the relationship between CS and chronic pain considering sarcopenia or presarcopenia status among community-dwelling older adults in a city in Japan. The PPT and EQ5D5L index were significantly associated with the presence of chronic pain with sarcopenia or presarcopenia. Chronic pain with sarcopenia or presarcopenia was affected by central sensitization, so CS should be evaluated in the elderly. In the future, we would like to investigate the causal relationship between chronic pain with sarcopenia and CS. We intend to conduct this investigation as an intervention and prevention study against CS.
Acknowledgments
We thank the participants in Kaizuka City and the volunteer staff for their contributions to data collection. This research was supported by a Grant-in-Aid for Young Scientists in the Japan Society for the Promotion of Science [KAKENHI(20K19364)].
Author contributions
Conceptualization: RI, MI, MH, MN
Data curation: All authors
Formal analysis: RI, MI
Investigation: All authors
Methodology: RI, MI, MH
Project administration: MI, MN
Resources: MI, MN
Supervision: RI, MI, MN
Visualization:RI, MI
Writing—original :draft RI
Writing—review & editing: All authors
Abbreviations:
- ANOVA =
- analysis of variance
- AWGS =
- Asian Working Group for Sarcopenia
- BMI =
- body mass index
- CPPS =
- chronic pain with presarcopenia
- CPR =
- chronic pain,
- CPS =
- chronic pain with sarcopenia
- CS =
- central sensitization
- CSI =
- Central Sensitization Inventory
- EQ5D-5L =
- The EuroQol 5-dimension 5-level
- MMSE =
- Mini-Mental State Examination
- NCPPS =
- nonpain with presarcopenia
- NCPR =
- Nonpain,
- NCPS =
- nonpain with sarcopenia
- NRS =
- numerical rating scale
- PCS =
- Pain Catastrophizing Scale
- PPT =
- pressure pain threshold
- QOL =
- quality of life
- SMI =
- skeletal muscle mass index
- TSK-11 =
- Tampa Scale for Kinesiophobia-11
- WPI =
- Widespread Pain Index.
The authors declare no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Imai R, Imaoka M, Nakao H, Hida M, Tazaki F, Inoue T, Orui J, Nakamura M. Association between chronic pain with presarcopenia and central sensitization in Japanese community-dwelling older adults: A cross-sectional study. Medicine 2022;101:32(e29998).
Data are mean (SD) or count (%).
BMI = body mass index, CSI = Central Sensitization Inventory, EQ5D5L = five-level EuroQol 5-dimension, MMSE = Mini-Mental State Examination, NRS = numerical rating scale, ns = nonsignificant, PCS = Pain Catastrophizing Scale, PPT = Pressure pain threshold, SMI = skeletal muscle mass index, TSK-11 = Tampa Scale for Kinesiophobia, WPI = Widespread Pain Index.
P < .05 vs chronic pain with sarcopenia.
P < .05 vs chronic pain with presarcopenia.
P < .05 vs robust chronic pain.
Data are mean (SD) or count (%).
BMI = body mass index; CSI = Central Sensitization Inventory; EQ5D5L = five-level EuroQol 5-dimension; MMSE = Mini-Mental State Examination; NRS = numerical rating scale; PCS = Pain Catastrophizing Scale; PPT = Pressure pain threshold; SMI = skeletal muscle mass index; TSK-11 = Tampa Scale for Kinesiophobia; WPI = Widespread Pain Index.
Adjusted for age and body mass index (BMI).
EQ5D5L = EuroQol 5-dimension 5-level, PCS = Pain Catastrophizing Scale, PPT = pressure pain threshold.
Contributor Information
Masakazu Imaoka, Email: imaokam@kawasakigakuen.ac.jp.
Hidetoshi Nakao, Email: nakaoh@kawasakigakuen.ac.jp.
Mitsumasa Hida, Email: hidam@kawasakigakuen.ac.jp.
Fumie Tazaki, Email: tazakif@kawasakigakuen.ac.jp.
Takao Inoue, Email: inouet@kawasakigakuen.ac.jp.
Junya Orui, Email: orui@kawasakigakuen.ac.jp.
Misa Nakamura, Email: nakamuram@kawasakigakuen.ac.jp.
References
- [1].Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s. [DOI] [PubMed] [Google Scholar]
- [2].Chalhoub D, Cawthon PM, Ensrud KE, et al. Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc. 2015;63:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harada A. [Locomotive syndrome and frailty. Frailty in patients with fall & fall-related fracture]. Clin Calcium. 2012;22:27–33. [PubMed] [Google Scholar]
- [4].Oliveira A, Vaz C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol. 2015;34:1673–80. [DOI] [PubMed] [Google Scholar]
- [5].Kitamura A, Seino S, Abe T, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sakai Y, Matsui H, Ito S, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia. 2017;3:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zimmer Z, Zajacova A, Grol-Prokopczyk H. Trends in pain prevalence among adults aged 50 and older across Europe, 2004 to 2015. J Aging Health. 2020;32:1419–32. [DOI] [PubMed] [Google Scholar]
- [8].Peng X, Bao X, Xie Y, et al. The mediating effect of pain on the association between multimorbidity and disability and impaired physical performance among community-dwelling older adults in southern China. Aging Clin Exp Res. 2020;32:1327–34. [DOI] [PubMed] [Google Scholar]
- [9].Welsh TP, Yang AE, Makris UE. Musculoskeletal pain in older adults: a clinical review. Med Clin North Am. 2020;104:855–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maruya K, Fujita H, Arai T, et al. Sarcopenia and lower limb pain are additively related to motor function and a history of falls and fracture in community-dwelling elderly people. Osteoporos Sarcopenia. 2019;5:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scott D, Blizzard L, Fell J, et al. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res (Hoboken). 2012;64:30–7. [DOI] [PubMed] [Google Scholar]
- [12].Sit RWS, Zhang D, Wang B, et al. Sarcopenia and chronic musculoskeletal pain in 729 community-dwelling chinese older adults with multimorbidity. J Am Med Dir Assoc. 2019;20:1349–50. [DOI] [PubMed] [Google Scholar]
- [13].Ide K, Yasuda T, Hasegawa T, et al. Evaluation of the Central Sensitization Inventory Score in elderly adults with musculoskeletal examination. Mod Rheumatol. 2021;31:885–9. [DOI] [PubMed] [Google Scholar]
- [14].Neblett R, Cohen H, Choi Y, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nishigami T, Tanaka K, Mibu A, et al. Development and psychometric properties of short form of central sensitization inventory in participants with musculoskeletal pain: a cross-sectional study. PLoS One. 2018;13:e0200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [18].Neogi T, Frey-Law L, Scholz J, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matsuoka H, Sakano Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of pain catastrophizing scale. Jpn J Psychosom Med. 2007;47:95–102. [Google Scholar]
- [20].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- [21].Kikuchi N, Matsudaira K, Sawada T, et al. Psychometric properties of the Japanese version of the Tampa Scale for Kinesiophobia (TSK-J) in patients with whiplash neck injury pain and/or low back pain. J Orthop Sci. 2015;20:985–92. [DOI] [PubMed] [Google Scholar]
- [22].Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- [23].EQ5D5L Crosswalk Index Value Calculator. Available at: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
- [24].Shiroiwa T, Ikeda S, Noto S, et al. Comparison of value set based on DCE and/or TTO data: scoring for EQ-5D-5L health states in Japan. Value Health. 2016;19:648–54. [DOI] [PubMed] [Google Scholar]
- [25].Makizako H, Shimada H, Doi T, et al. Age-dependent changes in physical performance and body composition in community-dwelling Japanese older adults. J Cachexia Sarcopenia Muscle. 2017;8:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. [DOI] [PubMed] [Google Scholar]
- [27].Eggermont LH, Leveille SG, Shi L, et al. Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc. 2014;62:1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–98. [DOI] [PubMed] [Google Scholar]
- [29].Lluch E, Torres R, Nijs J, et al. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. 2014;18:1367–75. [DOI] [PubMed] [Google Scholar]
- [30].Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain. 2016;17(9 Suppl):T50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hassan H, Walsh DA. Central pain processing in osteoarthritis: implications for treatment. Pain Manag. 2014;4:45–56. [DOI] [PubMed] [Google Scholar]
- [33].Correa JB, Costa LO, de Oliveira NT, et al. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case-control study. Exp Brain Res. 2015;233:2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roussel NA, Nijs J, Meeus M, et al. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain. 2013;29:625–38. [DOI] [PubMed] [Google Scholar]
- [35].Imai R, Imaoka M, Nakao H, et al. Association between chronic pain and pre-frailty in Japanese community-dwelling older adults: a cross-sectional study. PLoS One. 2020;15:e0236111. [DOI] [PMC free article] [PubMed] [Google Scholar]