Abstract
A Clostridium histolyticum 116-kDa collagenase has an H415EXXH motif but not the third zinc ligand, as found in already characterized zinc metalloproteinases. To identify its catalytic site, we mutated the codons corresponding to the three conserved residues in the motif to other amino acid residues. The mutation affecting His415 or His419 abolished catalytic activity and zinc binding, while that affecting Glu416 did the former but not the latter. These results suggest that the motif forms the catalytic site. We also mutated the codons corresponding to other amino acid residues that are likely zinc ligands. The mutation affecting Glu447 decreased markedly both the enzymatic activity and the zinc content, while that affecting Glu446 or Glu451 had smaller effects on activity and zinc binding. These mutations caused a decrease in kcat but no significant change in Km. These results are consistent with the hypothesis that Glu447 is the third zinc ligand. The spacing of the three zinc ligands is the same in all known clostridial collagenases but not in other known gluzincins, indicating that they form a new gluzincin subfamily. The effects of mutations affecting Glu446 and Glu451 suggest that the two residues are also involved in catalysis, possibly through an interaction with the two zinc-binding histidine residues.
The Clostridium histolyticum collagenase has been widely used for the disintegration of connective tissue and separation of tissue culture cells, because of its broad substrate specificity and its abundance in culture filtrates (31). However, at least six different forms with molecular masses ranging from 68 to 125 kDa are present in a commercial preparation (6, 7). The difficulty in separating individual enzymes and the lot-to-lot variation of commercial collagenase preparations limit its practical use. Cloning, nucleotide sequence analysis, and recombinant DNA technology of the corresponding gene(s) would facilitate the purification of the C. histolyticum collagenase and also increase our understanding of this enzyme.
In a previous study we have cloned and sequenced a colH gene encoding the 116-kDa collagenase (35). We have identified a collagen-binding domain at the C terminus, which binds to insoluble type I collagen in vitro (22) and also to collagen fibers in vivo (25). Recently we have identified a colG gene encoding another 116-kDa collagenase and presented evidence supporting the prediction (8) that multiple forms of the C. histolyticum collagenase are produced by different genes that have evolved from one another by gene duplication (21). The enzymatic properties of the C. histolyticum collagenase and its isoforms have been extensively studied by other workers (24). The enzymes cleave peptide bonds on the amino side of the glycine residue in the PXGP sequence, like other bacterial collagenases (27). Studies by atomic absorption spectrophotometry and metal replacement with chelators have shown that all isoforms contain one catalytically essential zinc atom per molecule (2, 6), so they are considered to be zinc metalloproteinases. Chemical modification studies have demonstrated that all the forms share functionally essential aspartate or glutamate and tyrosine residues (5).
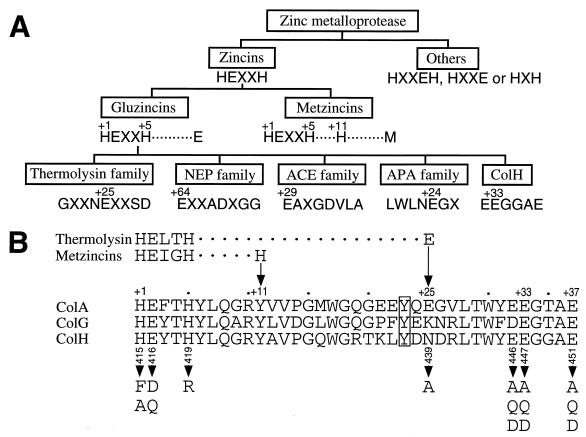
In a previous study we have shown that the N-terminal 80-kDa domain of ColH degrades the water-soluble substrates, gelatin, and Pz peptide (4-phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-d-Arg) (Sigma Chemical Co., St. Louis, Mo.), suggesting that it contains the catalytic site (22). This peptide contains the sequence HEXXH, the zincin consensus motif, which is present in most zinc metalloproteinases (zincins) except for some enzymes containing the HXXEH, HXXE, or HXH sequence (Fig. 1A). The motif is conserved in three clostridial collagenases, ColH, ColG (21), and ColA (23), as shown in Fig. 1B. Thus, these clostridial collagenases seem to belong to the zincin superfamily, which includes the vertebrate collagenases (matrix metalloproteinases [MMPs] 1, 8, and 13) as the matrixin subfamily (Fig. 1A). Although a three-dimensional structure has been well defined for the N-terminal catalytic domains of these vertebrate collagenases and the full-length MMP-1 (4, 12, 20, 28), these MMPs do not show significant homology to the clostridial collagenases. Moreover, the clostridial collagenases do not share the third zinc-binding residue conserved in the metalloproteinases of the metzincin family, a histidine residue at position +11, or that in the gluzincin family, a glutamate residue at position +24, +25, +29, or +64 (note that these coordinates are numbered in respect to the first zinc-binding histidine residue [Fig. 1A]). The alignment of the amino acid sequences of the three collagenases reveals that they conserve the sequence E446(D in the case of ColG)E447XXXE451 C-terminal to the zincin motif (Fig. 1B). This leads us to suspect that any one of the glutamate residues could be the third zinc ligand. Furthermore, the spacing between the latter two glutamate residues is comparable to that between the glutamate and aspartate residues in the EXXXD sequence of thermolysin, which is located C-terminal to the zincin motif with a gap of 19 residues.
FIG. 1.
Families of zinc metallopeptidases and alignment of amino acid sequences around the putative zinc-binding residues of clostridial collagenases. (A) The families of the zinc metallopeptidases and their interrelationships are based on the sequence around the zinc binding residues (15). The zinc-binding amino acid residues are numbered on the top. Numbers refer to the position relative to the first zinc-binding amino acid residue. (B) Amino acid sequence alignment of three clostridial collagenases, ColA (23), ColG (21), and ColH (35). The boxed residue indicates tyrosine conserved in the three collagenases, which is suggested for thermolysin to stabilize the catalytic intermediate (see Discussion). Arrows indicate the positions of the third zinc ligands in thermolysin and metzincins. Arrowheads indicate the amino acid residues of ColH that are changed, and the positions relative to the N-terminal residue are numbered underneath the ColH sequence. NEP, neutral endopeptidase 24.11; ACE, angiotensin I-converting enzyme; APA, aminopeptidase A.
Identifying and characterizing the catalytic center of ColH is a prerequisite for the elucidation of the mechanism underlying the hydrolysis of triple-helical collagen molecules by this enzyme. Thus, we constructed various ColH mutants with mutations in residues presumed to form the active site and examined the effects of the mutations on zinc retention and enzymatic activity. First, we characterized His415, Glu416, and His419 mutants to examine whether the HEXXH motif forms the catalytic center. Second, we constructed mutants with mutations in candidates for the third zinc ligand, one asparagine and three glutamate residues at positions 439, 446, 447, and 451, respectively (Fig. 1B). In this paper, we propose a new subfamily of gluzincins and discuss a possible role for the glutamate residues around the third zinc ligand in the formation of the catalytic center of clostridial collagenases.
MATERIALS AND METHODS
Bacterial strains and plasmid.
Escherichia coli DH5α (3, 13) and the plasmid pBluescript II KS(+) (1) were used as the host and vector for the construction of recombinant plasmids, respectively. To express wild-type and mutated colH genes, we used an E. coli-Bacillus subtilis shuttle vector, pAT19 (32), and the hosts E. coli DH5α and B. subtilis DB104 (his nprR2 nprE18 aprD3) (17).
Construction of plasmid encoding wild-type ColH.
A 4-kb BssHII fragment containing a full-length colH gene was isolated from plasmid pCHC208 (16), filled in with Klenow enzyme, and inserted into the SmaI site of pAT19. The resultant plasmid, which contained the colH gene in the same direction as the lacZα gene, was designated pJCM600. This plasmid was used for the preparation of wild-type ColH and also for the construction of plasmids expressing mutant colH genes mutated at codons corresponding to the HEXXH motif.
Site-directed mutagenesis.
Mutagenesis was performed by using the Transformer site-directed mutagenesis kit (CLONTECH Laboratories, Inc., Palo Alto, Calif.) according to the instructions of the manufacturer. Plasmid pCHC201, which bears a 2.8-kb HaeIII-PstI fragment encoding segments 1 and 2a of ColH in pBluescript II KS(+) (22), was used as the template for mutagenesis. The mutagenic primers used in this study are listed in Table 1, and 5′-GTGACTGGTGAGGCCTCAACCAAGTC-3′ was used as the selection primer. Mutations were identified by nucleotide sequencing, which was performed by using the ABI Prism BigDye terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer, Foster City, Calif.) and a synthetic primer, 5′-ACAACAGTCCCGAAGAAT-3′, on a Perkin-Elmer 377 DNA sequencer.
TABLE 1.
Oligonucleotide sequences used for site-directed mutagenesis of colH
| Enzymea | Sequenceb |
|---|---|
| Wild type | 1648GAAGAATTATTTAGACATGAATATACACATTATTTGCAAGGAAG1691 |
| H415F | 1648GAAGAATTATTTAGATTTGAATATACACATTATTTG |
| H415A | 1648GAAGAATTATTTAGAGCTGAATATACACATTATTTG |
| E416D | 1651GAATTATTTAGACATGATTATACACATTATTTGC |
| E416Q | 1651GAATTATTTAGACATCAATATACACATTATTTGC |
| H419R | 1661GACATGAATATACACGTTATTTGCAAGGAAG |
| Wild type | 1723AAACTTTATGACAATGATAGATTAACTTGGTATGAAGAAGGTGGAGCAGAATTATTTGCAGGTTCTACT1791 |
| N439A | 1723AAACTTTATGACGCTGATAGATTAACTTGGTATGAA |
| E446Q | 1741AGATTAACTTGGTATCAAGAAGGTGGAGCAGAATTA |
| E446A | 1741AGATTAACTTGGTATGCAGAAGGTGGAGCAGAATTA |
| E446D | 1741AGATTAACTTGGTATGACGAAGGTGGAGCAGAATTA |
| E447Q | 1744TTAACTTGGTATGAACAAGGTGGAGCAGAATTA |
| E447A | 1744TTAACTTGGTATGAAGCAGGTGGAGCAGAATTATTT |
| E447D | 1744TTAACTTGGTATGAAGACGGTGGAGCAGAATTATTT |
| E451Q | 1756GAAGAAGGTGGAGCACAATTATTTGCAGGTTCTACT |
| E451A | 1756GAAGAAGGTGGAGCAGCATTATTTGCAGGTTCTACT |
| E451D | 1756GAAGAAGGTGGAGCAGACTTATTTGCAGGTTCTACT |
Mutant enzymes are designated by the amino acid residue replaced, its position, and the amino acid that was substituted for it.
Nucleotide positions are numbered as in reference 35. Nucleotide substitutions are underlined.
Construction of plasmids encoding mutant ColH proteins.
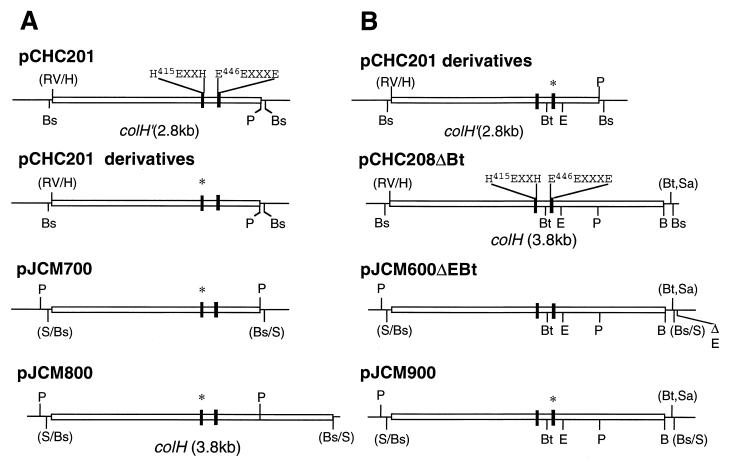
Plasmid derivatives to produce the enzymes with H415A, H415F, E416D, E416Q, and H419R mutations (Fig. 1) were constructed as follows (Fig. 2A). A 3-kb BssHII fragment containing the colH gene truncated at the 3′-terminal side was isolated from each pCHC201 mutant derivative, filled in, and ligated into the SmaI site of plasmid pAT19. These plasmids were designated the pJCM700 series. A 3-kb PstI fragment was excised from each of these plasmids and used to replace the PstI fragment of pJCM600. The resultant plasmids were designated the pJCM800 series.
FIG. 2.
Schematic overview of plasmids used for production of mutant ColH enzymes. (A) Plasmid derivatives for construction of mutant ColH enzymes, in which amino acid residues within the HEXXH motif were replaced with other amino acid residues. (B) Plasmid derivatives for construction of mutant ColH enzymes, in which the putative third zinc-ligating residue was replaced with other amino acid residues. Shown are plasmid DNA (thin bar), the colH gene (open box), a region encoding the putative zinc-ligating residues (solid box), and a region with a point mutation (∗). B, BamHI; Bs, BssHII; Bt, BstXI; E, EcoRI; RV, EcoRV; H, HaeIII; P, PstI; S, SmaI; Sa, SacII.
In order to facilitate the construction of the other mutant enzymes (with N439A, E446Q, E446A, E446D, E447Q, E447A, E447D, E451Q, E451A, and E451D mutations), a 200-bp BstXI-EcoRI fragment was used as a mutation cassette (Fig. 2B). Plasmid pCHC208 was digested with SacII, followed by removal of the 3′ overhangs with T4 DNA polymerase and by religation, which resulted in the removal of a BstXI site that overlapped the SacII site in the vector. The resultant plasmid, named pCHC208ΔBt, has a BstXI site within the colH gene. A 4-kb BssHII fragment isolated from pCHC208ΔBt was inserted into the SmaI site of pAT19ΔE, a pAT19-derived plasmid lacking the unique EcoRI site. The resulting plasmid, named pJCM600ΔEBt, contained only one BstXI site and one EcoRI site in the colH gene. A 200-bp BstXI-EcoRI fragment of pJCM600ΔEBt was replaced with the corresponding fragment isolated from each of the pCHC201 mutant derivatives, and the resulting plasmids were designated the pJCM900 series. After all the plasmids of the pJCM800 and pJCM900 series had been examined for the correct construction by nucleotide sequencing, they were used to transform B. subtilis DB104 as described previously (16).
DNA manipulations.
Restriction endonucleases were purchased from Takara Shuzo (Kyoto, Japan), Toyobo (Osaka, Japan), and New England Biolabs (Beverly, Mass.). The DNA ligation kit and DNA blunting kit were products of Takara Shuzo. All recombinant DNA procedures were carried out as described by Sambrook et al. (30).
Purification of wild-type and mutant ColH proteins.
Wild-type and mutant ColH enzymes were purified from cultures of recombinant strains of either B. subtilis DB104 or E. coli DH5α. All recombinant B. subtilis strains were grown in MLSE8 broth (10 g of Bactotrypton [Difco Laboratories, Detroit, Mich.] per liter, 5 g of yeast extract [Difco] per liter, 5 g of NaCl per liter, 2 g of glucose per liter, 135 g of sodium succinate hexahydrate per liter, and 0.008 g of erythromycin per liter). Cultures were started by inoculating 1 ml of a preculture into each of four 500-ml flasks containing 200 ml of broth. After incubating for 7 h at 37°C with shaking at 100 rpm, cells were removed by centrifugation at 10,000 × g for 30 min at 4°C. The supernatant was subjected to ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography as described previously (16), except that a linear gradient from 0 to 0.5 M NaCl was used for ion-exchange chromatography. All recombinant E. coli strains were cultured in two 3-liter flasks, each containing 1 liter of Luria-Bertani medium supplemented with 150 μg of erythromycin per ml. Cultures were started with a 1% inoculum of an overnight preculture and grown for 16 h at 37°C with shaking at 150 rpm. The cells were collected by centrifugation at 10,000 × g for 20 min at 4°C and resuspended in 200 ml of phosphate-buffered saline. The suspension was treated with polymyxin B to obtain a periplasmic fraction (26). Fifty milliliters of a phosphate-buffered saline solution containing 10,000 U of polymyxin B (Taito Pfizer Ltd., Tokyo, Japan) per ml was added to the suspension. After being incubated for 30 min at 37°C, the suspension was centrifuged at 10,000 × g for 30 min at 4°C. The supernatant (the periplasmic fraction) was used to purify recombinant ColH enzymes. The subsequent purification procedures were essentially the same as those from the recombinant B. subtilis strains described above.
Enzyme assay and protein determination.
The collagenase activity was assayed by the Pz peptide (Sigma Chemical Co.) hydrolyzing method (34). One unit of enzyme activity is defined as the amount of enzyme which causes an increase of 0.1 A320 unit per min. For kinetics studies, the assay was carried out with varying concentrations (0.05 to 0.32 mM) of the substrate. The activity unit was converted on the basis of an observed value of the molecular extinction coefficient at 320 nm of phenylazobenzyloxycarbonyl-Phe-Leu (Bachem, Budendorf, Switzerland) in ethylacetate (ɛ = 20.67 cm/mM), and the data were displayed as a Lineweaver-Burk plot, from which the Km and kcat values were calculated by the least-squares method. The collagenase activities of some ColH mutants were also assayed by using collagen from bovine achilles tendon (CLSPA; Worthington Biochemical Co., Freehold, N.J.) according to the instruction manual from the supplier. Briefly, 25 mg of collagen and 5 ml of a reaction buffer [50 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid–NaOH buffer (pH 7.5) containing 0.36 mM CaCl2] were placed into each test tube, and collagen was swollen by incubating at 15°C for 15 min. One unit of enzyme activity equals 1 μmol of l-leucine equivalents liberated from collagen in 5 h at 37°C (pH 7.5) under the specified conditions. Protein concentrations were determined by the method of Bradford (9) with the Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, Calif.) and bovine serum albumin as a standard. All the assays were carried out in triplicate.
SDS-PAGE and N-terminal amino acid sequencing.
The purities of wild-type and mutant ColH enzymes were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were separated on a 7.5% polyacrylamide gel and stained with Coomassie brilliant Blue R (18). The N-terminal amino acid sequence of purified recombinant wild-type ColH was determined on an automatic protein sequencer (model 492; Perkin-Elmer) as described previously (22).
Determination of zinc content in wild-type and mutant ColH proteins.
The amount of zinc was determined with an atomic absorption spectrophotometer (model Z-8200; Hitachi, Tokyo, Japan) and a zinc standard solution (Wako Pure Chemicals, Osaka, Japan). The results obtained from each batch of wild-type or mutant enzyme were averages of two determinations. All solutions were prepared in plasticware with Nanopure water (Barnstead, Dubuque, Iowa).
RESULTS
Mutagenesis of colH and purification of mutant enzymes.
We employed a shuttle vector plasmid, pAT19, and the B. subtilis host for the production of wild-type ColH. The recombinant enzyme with an apparent molecular mass of 116 kDa was purified to homogeneity based on SDS-PAGE analysis (data not shown). The specific activity of the purified recombinant ColH was 1,238 ± 45 (the mean ± standard deviation) U/mg of protein. Some of the plasmid constructs carrying the mutant colH genes were successfully introduced into B. subtilis DB104, and the transformants were used for the purification of enzymes with H415F, E416D, H419R, E446Q, E447Q, and E451Q mutations. They were also purified to apparent homogeneity based on SDS-PAGE analysis (data not shown). The transformation of B. subtilis DB104 with plasmids encoding the other mutant enzymes (with H415A, E416Q, N439A, E446A, E446D, E447A, E447D, E451A, and E451D mutations) was unsuccessful. Therefore, we purified these mutant ColH enzymes as well as the wild type from the periplasmic fractions of E. coli transformants. The specific activity of wild-type ColH from the recombinant E. coli was 1,011 ± 34 U/mg of protein. SDS-PAGE analysis revealed that both the wild-type and mutant recombinant E. coli enzymes were purified to homogeneity (data not shown). The N-terminal amino acid sequence of the wild-type enzyme obtained from E. coli, AVDKNNATA AVQNESKRYTV, was identical to that from B. subtilis. Furthermore, no Pz peptidase activity was found in the periplasmic fraction from the E. coli host strain, and no proteinase activity other than ColH was detected in the periplasmic fraction from the recombinant E. coli strain carrying the wild-type colH gene by gelatin zymography, a sensitive method for the detection of contaminating proteinase activity.
Identification of the HEXXH sequence as the active site.
The HEXXH (amino acids 415 to 419) zinc metalloproteinase consensus motif in ColH predicts that the two histidine residues coordinate a zinc ion and that the glutamate residue acts as a catalytic base. To test this, we constructed colH mutants in which the codons corresponding to the histidine and glutamate residues were individually mutated. The histidine residue at position 415 was replaced with an alanine residue which lacks an imidazole ring (H415A). The histidine residue at position 419 was replaced with an arginine residue which has a positive charge (H419R). The glutamate residue at position 416 was replaced with an aspartate residue, in which one methylene group is removed to shorten the side chain (E416D).
The activities of three enzymes with H415A, E416D, and H419R mutations against type I insoluble collagen and Pz peptide were determined and compared with those of wild-type ColH. The activities of these mutant enzymes were reduced to 0.33% ± 0.57%, 3.52% ± 1.76%, and 1.96% ± 0.59% (means ± standard deviations), respectively, of that of wild-type ColH when determined on collagen. Their activities on the Pz peptide were 0.08% ± 0.02%, 0.23% ± 0.14%, and 0.13% ± 0.06%, respectively, of that of wild-type ColH. Since the activities of these mutant enzymes toward the soluble substrate paralleled those toward the insoluble substrate, we used Pz peptide as the substrate for subsequent enzyme assays. Two other enzymes with H415F and H416Q mutations, in which the histidine residue at position 415 and the glutamine residue at position 416 were replaced with phenylalanine and glutamine residues, respectively, were constructed. The Pz peptidase activities of these enzymes were 0.25% ± 0.03% and 0.23% ± 0.09% (means ± standard deviations), respectively, of that of wild-type ColH. The zinc contents of the enzymes with E416D and E416Q mutations were almost the same as that of the wild-type enzyme, which contained approximately one atom of zinc per protein, whereas those of the enzymes with H415A, H415F, and H419R mutations decreased markedly to less than 50% of that of the wild-type enzyme (Table 2). These results indicate that the two histidine and the glutamate residues in the HEXXH motif likely play a crucial role in catalysis, as observed for other zincins, and suggest that the two histidine residues coordinate a zinc ion.
TABLE 2.
Zinc contents of the wild-type and mutant ColH enzymes
| Enzyme | Zinc content (mol/mol of protein)a |
|---|---|
| Wild type | 0.92 |
| H415F | 0.10 |
| H415A | 0.45 |
| E416D | 0.88 |
| E416Q | 1.15 |
| H419R | 0.17 |
| N439A | 0.92 |
| E446Q | 0.94 |
| E446A | 0.79 |
| E446D | 0.89 |
| E447Q | 0.39 |
| E447A | 0.32 |
| E447D | 0.18 |
| E451Q | 0.64 |
| E451A | 0.80 |
| E451D | 0.97 |
Values are averages for duplicate determinations with <10% variation.
Putative identification of the third zinc ligand.
In the enzymes in the thermolysin family, a glutamate residue (position +25) serves as the third zinc-binding residue. To examine the possibility that an asparagine residue (position +25) is the third zinc ligand in ColH, we constructed an enzyme with an N439A mutation, in which the side chain of Asn439 is replaced with a hydrogen, and the enzymatic activity and zinc content of this mutant enzyme were determined (Table 2 and Table 3). Neither were affected by this point mutation. The most likely candidate for the third zinc ligand is one of three glutamate residues at positions 446, 447, and 451, all of which are conserved in ColH and ColA and two of which are conserved in all three clostridial collagenases. We generated nine mutations of the codons corresponding to these three glutamate residues to identify the third zinc ligand: Glu446, Glu447, and Glu451 were changed to glutamine (E446Q, E447Q, and E451Q), alanine (E446A, E447A, and E451A), or aspartate (E446D, E447D, and E451D). The effects of these mutations on enzymatic activity and zinc coordination were examined (Table 2 and Table 3). The replacement of Glu447 with an alanine or glutamine residue reduced markedly both the enzymatic activity and the zinc content. Even when only one methylene group was removed from the residue (E447D), both the enzyme activity and zinc coordination were severely affected. On the other hand, the replacement of Glu451 with an alanine residue did not affect the zinc content, although the glutamine residue did slightly. Surprisingly, these mutations also caused a marked decrease in enzymatic activity, though not as much as mutations of Glu447 (Table 3). Mutations affecting Glu446 caused a significant but less prominent decrease in the enzymatic activity, but they did not affect the zinc content except for E446A, because of which the zinc content decreased slightly. Taken together, it is suggested that Glu447 is a zinc ligand, and it seems likely that Glu446 and Glu451 do not coordinate a zinc ion directly but are important for catalytic activity.
TABLE 3.
Enzymatic activities of ColH mutant enzymes
| Enzymea | % (± SD %) of wild-type ColH activityb |
|---|---|
| Wild type | 100 |
| N439A | 108.43 ± 6.99 |
| E446Q | 14.75 ± 0.99 |
| E446A | 13.72 ± 0.73 |
| E446D | 49.00 ± 1.87 |
| E447Q | 5.13 ± 0.07 |
| E447A | 0.70 ± 0.15 |
| E447D | 0.80 ± 0.11 |
| E451Q | 5.55 ± 0.55 |
| E451A | 5.40 ± 0.11 |
| E451D | 12.31 ± 0.44 |
The enzymes with E446A, E446D, E447A, E447D, E451A, and E451D mutations were purified from recombinant E. coli strains, while the rest of the mutant enzymes were from recombinant B. subtilis strains.
The relative enzyme activity was expressed as a percentage of the relevant recombinant wild-type ColH. Pz peptide-hydrolyzing activities of the wild-type ColH enzymes from the recombinant B. subtilis and E. coli strains were 1,238 ± 45 and 1,011 ± 34 (the mean ± standard deviation) U/mg of protein, respectively. The results are shown as means ± standard deviations of triplicate determinations.
Kinetic analysis of the enzymes with Glu446, Glu447, and Glu451 mutations.
To characterize more precisely the functions of these three mutants, we performed kinetics studies of the wild-type enzyme and the enzymes with E446A, E447Q, and E451A mutations (Table 4). Since the enzyme activity of E447A was too low, E447Q was used in place of E447A. The Km values of E446A, E447Q, and E451A calculated from Lineweaver-Burk plots were similar to that of the wild-type enzyme, as shown in Table 4. In contrast, the kcat values of E447Q and E451A were decreased 20- and 15-fold, respectively, and their specificity constants (kcat/Km) were reduced to less than 10% of that of the wild-type enzyme. The effect of mutating Glu446 on the catalytic activity was smaller with the kcat values reduced about fourfold, and the corresponding specificity constant (kcat/Km) decreased to about 20% of that of the wild-type enzyme.
TABLE 4.
Kinetic parameters of the wild-type and mutant ColH enzymesa
| Enzyme | Km (mM) | kcat (s−1) | kcat / Km (mM−1 s−1) |
|---|---|---|---|
| Wild type | 0.88 ± 0.21 | 0.11 ± 0.019 | 0.13 |
| E446A | 0.88 ± 0.13 | 0.026 ± 0.0039 | 0.030 |
| E447Q | 0.65 ± 0.10 | 0.0055 ± 0.00054 | 0.0085 |
| E451A | 0.80 ± 0.39 | 0.0072 ± 0.0024 | 0.0090 |
The activities of the wild-type and mutant ColH enzymes were determined by using Pz peptide as a substrate. Km and kcat values were obtained by Lineweaver-Burk plot analysis. Values are means ± standard deviations of triplicate measurements.
DISCUSSION
In order to purify and characterize the various mutant ColH enzymes, we attempted to find an expression system which produces them in quantity. To solve the problem encountered previously (an E. coli-B. subtilis shuttle vector, pHY300PLK, with the colH gene was unstable [16]), we used another shuttle vector, pAT19, which replicates in the theta mode. Unfortunately, the efficiency of transformation of B. subtilis was too low to obtain cells containing some of the plasmids. Therefore, these enzymes were obtained from E. coli, although the yield was approximately half of that from B. subtilis. The purities of the enzyme preparations were the same for both organisms, and they had the same N-terminal amino acid sequence, suggesting that they are synthesized in a similar way. The recovery of the mutant enzymes was almost the same as that of the wild-type enzyme in each system, suggesting that the mutations do not affect the biosynthesis, the folding, or the stability of the mutant enzymes.
The mutation affecting the histidine residues in the HEXXH motif abolished the enzymatic activity and reduced the zinc binding. The replacement of Glu416 with a glutamine or an aspartate residue abolished enzymatic activity. On the basis of crystallographic studies in related systems, the modification of Glu to an aspartate residue is expected to move the carboxyl group 1.4 Å further from the zinc, which would reduce the polarization of the zinc-coordinated water molecule (33), so it would not be sufficiently activated to initiate a nucleophilic attack on the substrate carbonyl group (14). These results indicate that the HEXXH motif likely forms the catalytic center in ColH, as in other zinc metalloproteinases which belong to the metzincin and gluzincin families.
Asn439 (at position +25) is unlikely to be the third zinc ligand, since the N439A mutant exhibited the same enzymatic activity and zinc coordination as the wild type. The glutamate residues at positions 446, 447, and 451 were replaced with a glutamine residue, a weaker nucleophile; an alanine residue, a residue unable to make a coordinate bond or a hydrogen bond; and an aspartate residue, a similar nucleophile with a shorter side chain. All the mutations decreased enzymatic activity, with changes of Glu447 exhibiting the most prominent decrease. The zinc content was reduced in the Glu447 mutants but less so in the other mutants. These results indicate that Glu447 is likely to be the third zinc ligand. The replacement of Glu447 with an alanine residue decreased the specific activity to 0.7%. Changing the residue to an aspartate residue (mutant E447D), thus restoring a carboxyl group in the side chain, did not restore enzymatic activity (0.8%). On the other hand, the mutation of Glu447 to a glutamine residue (mutant E447Q) restored activity to 5.1% of that of the wild-type enzyme. Of the three Glu447 mutants the zinc content is highest in the enzyme with the E447Q mutation. This restoration may be due to the carbamoyl group in the glutamine residue which can act as a weak zinc coordinator.
The fact that the Glu446 and Glu451 mutants exhibited decreased enzymatic activities suggests a functional role for their carboxyethyl side chains in the catalytic mechanism. The Km values of enzymes with E446A and E451A mutations, which decreased enzymatic activity the most of all the Glu446 and Glu451 mutants, were not changed significantly (P, 0.957 and 0.791, respectively), indicating that their substrate binding is not impaired. Therefore, it seems unlikely that the mutations alter the global three-dimensional structure of the enzyme. On the other hand, their kcat values decreased significantly, indicating that the catalysis was impaired. Some enzymes in the gluzincin family, such as thermolysin, neutral endopeptidase 24.11, angiotensin I-converting enzyme, and lactococcal endopeptidase, have an aspartate residue on the carboxyl side of the zinc-binding glutamate residue with a gap of three residues (EXXXD) (19, 29, 33). The role of the aspartate residue in thermolysin has been identified from the crystal structure of the enzyme, which reveals that the side chain forms a salt link with the imidazole ring of the first histidine residue in the HEXXH motif (11). It was also pointed out that an asparagine residue located on the amino side of the EXXXD sequence of thermolysin forms a carbonyl-histidine-zinc triad along with the second histidine residue in the HEXXH motif (10). The EEXXXE sequence in ColH, in which the second E is probably the third zinc ligand, is comparable to the NEXXXD sequence in thermolysin, in which E is the third zinc ligand. Thus, the other two glutamate residues in the ColH sequence could form two carboxylate-histidine-zinc triads.
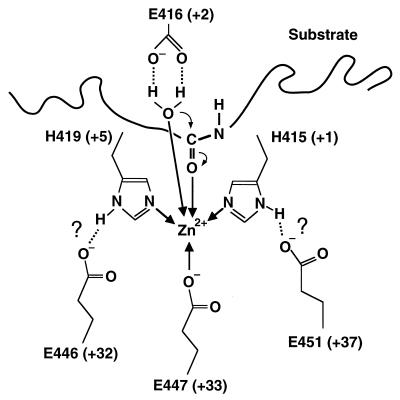
The tyrosine residue(s) has been shown to be essential for the C. histolyticum collagenase by chemical modification studies (5). A tyrosine residue, which is separated by seven residues from the NEXXXD sequence of thermolysin and is proposed to interact with a carbonyl oxygen in the tetrahedral intermediate (14), is also conserved in all of the clostridial collagenases at eight residues from the EEXXXE sequence (Fig. 1) near the catalytic site. This reinforces the structural similarity between thermolysin and ColH near the catalytic site. Based on this similarity and the results from the mutational analysis, we predict that the catalytic zinc ion in ColH is coordinated by two glutamate-histidine-zinc triads, one made by Glu446 and His419 and the other made by Glu451 and His415, both of which play a critical role in the catalytic mechanism (Fig. 3).
FIG. 3.
Proposed structure of the catalytic center of ColH. The zinc-coordinating bonds and hydrogen bonds are indicated by thick arrows and dotted lines, respectively. Thin arrows represent the first steps of the mechanism of action.
The present study has presented evidence that ColH constitutes a new subfamily of gluzincin along with the other clostridial collagenases, ColG and ColA. They do not show any significant similarity to the vertebrate collagenases in the amino acid sequences of the whole peptides, catalytic site, or C-terminal domain. The structural difference can also be predicted from the fact that ζ-collagenase corresponding to ColH possesses five Ca atoms per molecule (24), unlike MMP-1, which contains two Ca atoms per molecule (20). Therefore, we expect that the two types of collagenases differ in overall structure. A crystallographic study, which is now in progress, is a prerequisite to prove our hypothesis. In addition, it would provide insights into the similarity and dissimilarity of the two types of collagenases.
ACKNOWLEDGMENTS
We thank David B. Wilson (Section of Biochemistry, Molecular and Cell Biology, Cornell University, Ithaca, N.Y.) for invaluable discussion and assistance in preparing the manuscript. We also thank Patrice Courvalin (Unité des Agents Antibactériens, Institut Pasteur, Paris, France) for providing us with plasmid vector pAT19. We are indebted to Masahiro Nagahama (Department of Microbiology, Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Tokushima, Japan) for the determination of the zinc content of ColH and its mutant derivatives.
REFERENCES
- 1.Alting-Mees M A, Short J M. pBluescript II: gene mapping vectors. Nucleic Acids Res. 1989;17:9494. doi: 10.1093/nar/17.22.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angleton E L, Van Wart H E. Preparation and reconstitution with divalent metal ions of class I and class II Clostridium histolyticum apocollagenases. Biochemistry. 1988;27:7406–7412. doi: 10.1021/bi00419a035. [DOI] [PubMed] [Google Scholar]
- 3.Bethesda Research Laboratories. BRL pUC host: E. coli DH5α competent cells. Focus. 1986;8:9. [Google Scholar]
- 4.Bode W, Reinemer P, Huber R, Kleine T, Schnierer S, Tschesche H. The X-ray crystal structure of the catalytic domain of human neutrophil collagenase inhibited by a substrate analogue reveals the essentials for catalysis and specificity. EMBO J. 1994;13:1263–1269. doi: 10.1002/j.1460-2075.1994.tb06378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond M D, Steinbrink D R, Van Wart H E. Identification of essential amino acid residues in Clostridium histolyticum collagenase using chemical modification reactions. Biochem Biophys Res Commun. 1981;102:243–249. doi: 10.1016/0006-291x(81)91513-8. [DOI] [PubMed] [Google Scholar]
- 6.Bond M D, Van Wart H E. Characterization of the individual collagenases from Clostridium histolyticum. Biochemistry. 1984;23:3085–3091. doi: 10.1021/bi00308a036. [DOI] [PubMed] [Google Scholar]
- 7.Bond M D, Van Wart H E. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984;23:3077–3085. doi: 10.1021/bi00308a035. [DOI] [PubMed] [Google Scholar]
- 8.Bond M D, Van Wart H E. Relationship between the individual collagenases of Clostridium histolyticum: evidence for evolution by gene duplication. Biochemistry. 1984;23:3092–3099. doi: 10.1021/bi00308a037. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Christianson D W, Alexander R S. Carboxylate-His-zinc interactions in protein structure and function. J Am Chem Soc. 1989;111:6412–6419. [Google Scholar]
- 11.Colman P M, Jansonius J N, Matthews B W. The structure of thermolysin: an electron density map at 2.3 Å resolution. J Mol Biol. 1972;70:701–724. doi: 10.1016/0022-2836(72)90569-4. [DOI] [PubMed] [Google Scholar]
- 12.Gomis-Ruth F X, Gohlke U, Betz M, Knauper V, Murphy G, Lopez-Otin C, Bode W. The helping hand of collagenase-3 (MMP-13): 2.7 Å crystal structure of its C-terminal haemopexin-like domain. J Mol Biol. 1996;264:556–566. doi: 10.1006/jmbi.1996.0661. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning: a practical approach. Vol. 1. Oxford, England: IRL Press; 1985. pp. 109–135. [Google Scholar]
- 14.Holden H M, Tronrud D E, Monzingo A F, Weaver L H, Matthews B W. Slow- and fast-binding inhibitors of thermolysin display different modes of binding: crystallographic analysis of extended phosphonamidate transition-state analogues. Biochemistry. 1987;26:8542–8553. doi: 10.1021/bi00400a008. [DOI] [PubMed] [Google Scholar]
- 15.Hooper N M. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- 16.Jung C-M, Matsushita O, Katayama S, Minami J, Ohhira I, Okabe A. Expression of the colH gene encoding Clostridium histolyticum collagenase in Bacillus subtilis and its application to enzyme purification. Microbiol Immunol. 1996;40:923–929. doi: 10.1111/j.1348-0421.1996.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura F, Doi R H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral protease. J Bacteriol. 1984;160:442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Le Moual H, Dion N, Roques B P, Crine P, Boileau G. Asp650 is crucial for catalytic activity of neutral endopeptidase 24-11. Eur J Biochem. 1994;221:475–480. doi: 10.1111/j.1432-1033.1994.tb18760.x. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Brick P, O’Hare M C, Skarzynski T, Lloyd L F, Curry V A, Clark I M, Bigg H F, Hazleman B L, Cawston T E, Blow D M. Structure of full-length porcine synovial collagenase reveals a C-terminal domain containing a calcium-linked, four-bladed beta-propeller. Structure. 1995;3:541–549. doi: 10.1016/s0969-2126(01)00188-5. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita O, Jung C-M, Katayama S, Minami J, Takahashi Y, Okabe A. Gene duplication and multiplicity of collagenases in Clostridium histolyticum. J Bacteriol. 1999;181:923–933. doi: 10.1128/jb.181.3.923-933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita O, Jung C-M, Minami J, Katayama S, Nishi N, Okabe A. A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J Biol Chem. 1998;273:3643–3648. doi: 10.1074/jbc.273.6.3643. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita O, Yoshihara K, Katayama S-I, Minami J, Okabe A. Purification and characterization of a Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene. J Bacteriol. 1994;176:149–156. doi: 10.1128/jb.176.1.149-156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mookhtiar K A, Van Wart H E. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix. 1992;1(Suppl.):116–126. [PubMed] [Google Scholar]
- 25.Nishi N, Matsushita O, Yuube K, Miyanaka H, Okabe A, Wada F. Collagen-binding growth factors: production and characterization of functional fusion proteins having a collagen-binding domain. Proc Natl Acad Sci USA. 1998;95:7018–7023. doi: 10.1073/pnas.95.12.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabe A, Matsushita O, Katayama S, Hayashi H. Lincomycin stimulates synthesis of TEM-2 β-lactamase by Escherichia coli. Antimicrob Agents Chemother. 1986;30:82–87. doi: 10.1128/aac.30.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterkofsky B. Bacterial collagenase. Methods Enzymol. 1982;82:453–471. [Google Scholar]
- 28.Reinemer P, Grams F, Huber R, Kleine T, Schnierer S, Piper M, Tschesche H, Bode W. Structural implications for the role of the N terminus in the ‘superactivation’ of collagenases. A crystallographic study. FEBS Lett. 1994;338:227–233. doi: 10.1016/0014-5793(94)80370-6. [DOI] [PubMed] [Google Scholar]
- 29.Roques B P, Noble F, Dauge V, Fournié-Zaluski M C, Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–145. [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Seglen P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 32.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991;102:99–104. doi: 10.1016/0378-1119(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 33.Vazeux G, Wang J, Corvol P, Llorens-Cortes C. Identification of glutamate residues essential for catalytic activity and zinc coordination in aminopeptidase A. J Biol Chem. 1996;271:9069–9074. doi: 10.1074/jbc.271.15.9069. [DOI] [PubMed] [Google Scholar]
- 34.Wünsch E, Heidrich H-G. Zur quantitativen Bestimmung der Kollagenase. Hoppe-Seyler’s Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- 35.Yoshihara K, Matsushita O, Minami J, Okabe A. Cloning and nucleotide sequence analysis of the colH gene from Clostridium histolyticum encoding a collagenase and a gelatinase. J Bacteriol. 1994;176:6489–6496. doi: 10.1128/jb.176.21.6489-6496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]