Abstract
DNA-DNA interstrand cross-links are the cytotoxic lesions for many chemotherapeutic agents. A plasmid with a single nitrogen mustard (HN2) interstrand cross-link (inter-HN2-pTZSV28) was constructed and transformed into Escherichia coli, and its replication efficiency (RE = [number of transformants from inter-HN2-pTZSV28]/[number of transformants from control]) was determined to be ∼0.6. Previous work showed that RE was high because the cross-link was repaired by a pathway involving nucleotide excision repair (NER) but not recombination. (In fact, recombination was precluded because the cells do not receive lesion-free homologous DNA.) Herein, DNA polymerase II is shown to be in this new pathway, since the replication efficiency (RE) is higher in a polB+ (∼0.6) than in a ΔpolB (∼0.1) strain. Complementation with a polB+-containing plasmid restores RE to wild-type levels, which corroborates this conclusion. In separate experiments, E. coli was treated with HN2, and the relative sensitivity to killing was found to be as follows: wild type < polB < recA < polB recA ∼ uvrA. Because cells deficient in either recombination (recA) or DNA polymerase II (polB) are hypersensitive to nitrogen mustard killing, E. coli appears to have two pathways for cross-link repair: an NER/recombination pathway (which is possible when the cross-links are formed in cells where recombination can occur because there are multiple copies of the genome) and an NER/DNA polymerase II pathway. Furthermore, these results show that some cross-links are uniquely repaired by each pathway. This represents one of the first clearly defined pathway in which DNA polymerase II plays a role in E. coli. It remains to be determined why this new pathway prefers DNA polymerase II and why there are two pathways to repair cross-links.
Interstrand cross-links are likely to be the crucial cytotoxic lesion formed by a variety of classes of effective antitumor agents, including the mustards, of which nitrogen mustard was the first member used and cyclophosphamide is currently the most commonly used (13, 22, 26, 31, 40). Factors that modulate the levels of cytotoxic interstrand cross-links, such as DNA repair, are expected to influence the effectiveness of such agents. It has been known for many years that interstrand cross-links may be repaired by a combination of nucleotide excision repair (NER) and recombination, which has been most extensively studied with psoralens (45, 46, 49). In special cases, other DNA repair pathways may also be important for the repair of intermediates that give rise to interstrand cross-links, for example, with BCNU [1,3-bis-(2-chloroethyl)-1-nitrosourea] (8, 16, 17, 42, 50).
Although some progress on assessing a role for interstrand cross-links in causing cytotoxicity has been made, for example, with HN2 (1, 36, 37), much is still not known, in part because, when cross-linking agents react with DNA, many lesions form, including interstrand, intrastrand, and protein-DNA cross-links, as well monoadducts. This and other factors have made it difficult to dissect definitively the details about how these various lesions are processed and their unique biological effects (30, 43).
To overcome such complications, we developed a general procedure to construct a plasmid with a single DNA-DNA interstrand cross-link at a defined genomic position (21, 38). By these methods, inter-HN2-pTZSV28, a plasmid which contains a single nitrogen mustard interstrand cross-link, was constructed and characterized as described previously (4, 21, 38). Importantly, we showed that more than ∼98% of the plasmids constructed contain a single, intact cross-link. We have shown that the nitrogen mustard moiety is attached to N7-dG in both strands of a 5′-GNC-3′ target sequence in a unique AccI/SalI site (38). The problem of the inherent chemical lability of N7-dG adducts was circumvented by converting them to their corresponding ring-opened, N7-FAPY adducts, which are stable (38).
Cells face a difficult logistical problem in the repair of interstrand cross-links, since both DNA strands are damaged. The commonly accepted pathway to repair interstrand cross-links involves NER to nick the first strand, which is then replaced with DNA from a lesion-free, homologous, sister chromosome by recombination, as demonstrated most clearly for psoralens (45, 46, 49). The second strand is then presumably repaired as a monoadduct by NER.
Previously, we showed that a high yield of progeny plasmids could be derived from inter-HN2-pTZSV28 transformed into Escherichia coli, and this process was dependent on a DNA repair pathway for the interstrand cross-link in inter-HN2-pTZSV28 that included NER (4). However, three lines of evidence argued against a role for recombination in this repair pathway, notably that the yield of progeny plasmids was unaffected in a ΔrecA strain of E. coli. In fact, our experimental approach actually precluded a recombination-dependent repair pathway, since the cells did not receive a lesion-free copy of the pTZSV28 plasmid. We also showed that this recombination-independent pathway did not involve base excision repair (BER) or several 5′→3′ exonuclease activities in E. coli (4). (In previous work we provided arguments for why we concluded that the effects that we have been investigating are by necessity attributable to the nitrogen mustard interstrand cross-link located in inter-HN2-pTZSV28 [4].)
We show here that DNA polymerase II (DNA Pol II) is another component of the recombination-independent, DNA-DNA interstrand cross-link repair pathway. To test the physiological relevance of this new pathway, we describe the treatment of E. coli with nitrogen mustard and conclude that it is likely the NER/DNA Pol II pathway functions in parallel with the NER/recombination pathway in E. coli (i.e., in circumstances where there is a lesion-free homologous chromosome) and that these two pathways are not completely functionally redundant.
MATERIALS AND METHODS
Bacterial strains and plasmids are summarized in Table 1, including several new strains. The uvrA6 derivatives were constructed by transducing strains to mal::Tn10 and then to mal+ uvrA6 by using AB1886 (24) as a donor. ΔrecA derivatives were made by transducing the strains to Δ(recA srlR301::Tn10) (14). Standard genetic techniques were used (32).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype | Source and/or reference |
|---|---|---|
| Strains | ||
| FC40 | Wild type | 10 |
| C600 | Wild type | CSHLa (2) |
| AB1157 | Wild type | S. Boiteux (5) |
| PFB60 | FC40 but with ΔpolB1 | 18 |
| SC301 | C600 but with ΔpolB1 | M. Goodman (18) |
| PFB50 | FC40 but with uvrA6 | This study |
| BH200 | AB1157 but with uvrA::Tn10 | S. Boiteux (15) |
| PFB175 | FC40 but with uvrA6 ΔpolB1 | This study |
| FC348 | FC40 but with ΔrecA | This study |
| PFB98 | FC40 but with ΔrecA ΔpolB1 | This study |
| Plasmids | ||
| pBIP3 | None | 46 |
| pHC206 | pBIP carrying polB+ | M. Goodman (9) |
CSHL, Cold Spring Harbor Laboratory.
All materials and methods were identical to those described previously (4, 21, 38) except as noted below.
In brief, inter-HN2-pTZSV28, which contains a single nitrogen mustard interstrand cross-link, was constructed as follows. A nitrogen mustard interstrand cross-linked duplex oligonucleotide was synthesized (38) and incorporated into the parent plasmid pTZSV28 in a five-step procedure, and closed circular material was isolated by cesium chloride density gradient centrifugation (21). pTZSV28 itself was constructed from pTZ19R (ColE1 and f1 origins, bla for ampicillin resistance and a lacZ′ gene with a polylinker) and a portion of simian virus 40 (simian virus 40 origin and the large T antigen gene). The cross-link is located in a unique AccI/SalI site in the polylinker. Inter-HN2-pTZSV28 was extensively characterized, notably to show that >98% of the material contains a single, intact cross-link (4). Inter-HN2-pTZSV28 or its non-cross-link-containing control (C-pTZSV28) was transformed (via electroporation) in parallel in triplicate into each of the indicated strains (Table 2) and plated in duplicate on ampicillin-containing plates. One day later the numbers of ampicillin-resistant colonies were determined, and the average of the six plates is reported (Table 2).
TABLE 2.
Comparison of the RE values of inter-HN2-pTZSV28 and C-pTZSV28 in different E. coli strains to determine a role for DNA Pol II in interstrand cross-link repaira
| Strainb | Genotypec | Plasmidd | RE in expt no.:
|
RRE (avg)e | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| FC40 | Wild type | 0.64 | 0.51 | 0.57 | 0.60 | 0.59 | 0.67 | 1.00 | |
| C600 | Wild type | 0.56 | 0.58 | 1.00 | |||||
| AB1157 | Wild type | 0.71 | 1.00 | ||||||
| PFB60 | ΔpolB | 0.094 | 0.126 | 0.11 | 0.10 | 0.087 | 0.13 | 0.18 | |
| SC301 | ΔpolB | 0.100 | 0.096 | 0.17 | |||||
| PFB50 | uvrA6 | 0.043 | 0.022 | 0.055 | |||||
| BH200 | uvrA::Tn10 | 0.024 | 0.034 | ||||||
| PFB175 | ΔpolB uvrA6 | 0.017 | 0.028 | ||||||
| FC40 | Wild type | pBIP | 0.56 | 0.58 | 1.00 | ||||
| PFB60 | ΔpolB | pBIP | 0.12 | 0.17 | 0.26 | ||||
| FC40 | Wild type | pHC206 (polB+) | 0.66 | 0.65 | 1.00 | ||||
| PFB60 | ΔpolB | pHC206 (polB+) | 0.57 | 0.71 | 0.98 | ||||
Inter-HN2-pTZSV28 contains a single nitrogen mustard interstrand cross-link. Inter-HN2-pTZSV28 or its non-cross-link-containing control (C-pTZSV28) was transformed (electroporation) in parallel in triplicate into each of the indicated strains and then plated in duplicate on ampicillin-containing plates. One day later the numbers of ampicillin-resistant colonies were determined, and the average of the six plates for each point was calculated. The RE is defined as the ratio of ampicillin-resistant colonies: [inter-HN2-pTZSV28]/[C-pTZSV28].
Strain name (see Table 1).
Relevant genotype (see Table 1). Mutant strains are paired as follows: FC40 and PFB60, PFB50, or PFB175; C600 and SC301; and AB1157 and BH200.
Plasmid name (see Table 1).
RRE is defined as follows: (RE in a repair-deficient strain)/(RE in a repair-proficient strain) (see text). Values for the RRE are based on the average for all of the relevant experiments.
The survival curves after exposure to nitrogen mustard (see Fig. 2) were generated as follows. To 1.0 ml of the indicated E. coli strains (from an overnight culture grown in Luria broth [LB]), nitrogen mustard (stored as the hydrochloride at −80°C in a 1 M stock solution) was added (along with an identical volume of 1 M NaOH) to give the desired final concentrations. After incubation for 1 h at 37°C, the samples were diluted (by a factor of 103 or greater) and plated (in triplicate) on LB plates. After incubation at 37°C for ∼18 h, the colonies were counted to determine the levels of surviving colonies.
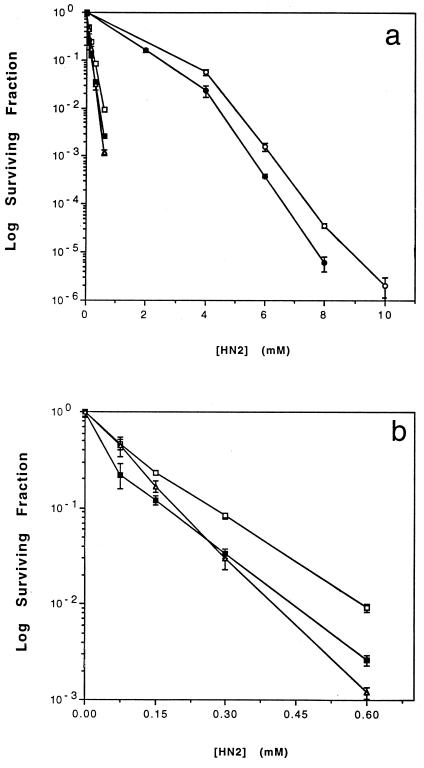
FIG. 2.
Survival curves for various strains of E. coli treated with nitrogen mustard (see Materials and Methods). Strains: FC40 (wild type [○]), PFB60 (ΔpolB [●]), FC348 (ΔrecA [□]), B98 (ΔpolB ΔrecA [■]), and PFB50 (uvrA6 [▵]). Panel a shows all of the data, whereas panel b shows the data for the latter three strains with an expanded abscissa. Strain FC348 is strain FC40 with a ΔrecA allele. Strain B98 is strain FC40 with both ΔpolB and ΔrecA alleles. Strain PFB50 is strain FC40 with a uvrA6 allele. Strains are described in Table 1.
RESULTS
As we had done previously (4), a bacterial transformation assay was used to study the extent to which a single nitrogen mustard interstrand cross-link might inhibit DNA replication. Equal amounts (∼25 pg) of either inter-HN2-pTZSV28, which contains a single cross-link, or C-pTZSV28, which is an identically constructed control plasmid that contains no cross-link, were transformed into cells, and the number of ampicillin-resistant colonies was determined (4). The replication efficiency (RE), i.e., the ratio of colonies from (inter-HN2-pTZSV28/C-pTZSV28), was determined to be ca. 0.3 to 0.6 for cells proficient in all known DNA repair pathways (reference 4 and Table 2). The RE was shown to be significantly lower in several uvrA and uvrB strains compared to corresponding uvr+ strains (reference 4 and Table 2), implicating NER in the repair of the cross-link in HN2-inter-pTZSV28. To simplify comparisons, it is useful to define the relative replication efficiency (RRE) as the ratio of RE for any repair-deficient strain of E. coli versus the RE for its corresponding wild-type strain; e.g., the RRE is 0.055 for uvrA6 versus uvrA+ (Table 2), which is similar to results we obtained previously (4). In previous work, analogous experiments excluded a role for recombination, BER, and several 5′→3′ exonuclease activities in this DNA repair pathway (4).
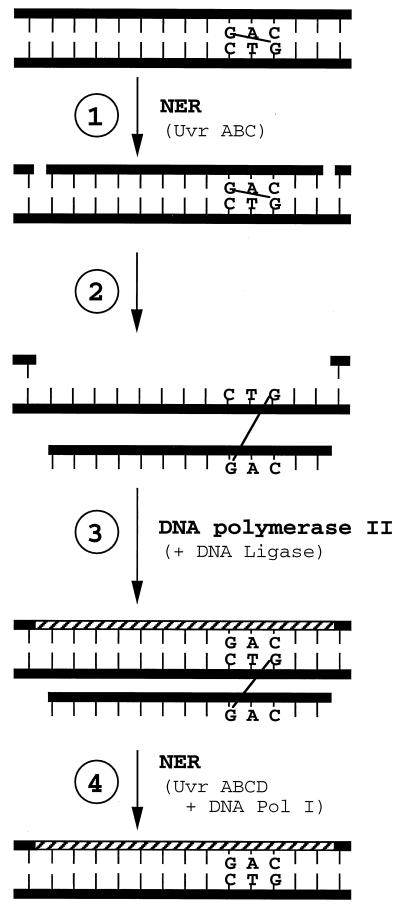
This recombination-independent repair was hypothesized to proceed by UvrABC nicking one strand near the cross-link followed by the action of a DNA polymerase (Fig. 1). A role for DNA Pol II was evaluated, and evidence for its involvement was obtained in that the RRE was ∼0.18 (Table 2) in two different strains of E. coli that contained a deletion of a polB gene, which was originally developed in the laboratory of Myron Goodman (18). The repair defect in the ΔpolB strain was complemented by the cloned polB+ gene (Table 2, compare PFB/pBIP [RRE = 0.26] with PFB/pHC206 [RRE = 0.98]), showing that the deficiency was truly due to the polB allele and not to some other gratuitous genetic change in the cells. No complementation was observed with the plasmid not containing the polB+ gene. In experiment 5 (Table 2), the RE was similar for a uvrA6 (0.022) versus a uvrA ΔpolB (0.017) strain.
FIG. 1.
The NER/DNA Pol II pathway for the repair of a nitrogen mustard interstrand cross-link. Repair of the top strand is proposed to involve NER (step 1), followed by DNA Pol II filling of the gap, including bypass of the lesion and ligation (steps 2 and 3) and finally NER of the resulting monoadduct in the bottom strand (step 4).
To assess whether the NER/DNA Pol II pathway is physiologically significant, the survival of E. coli cells exposed to nitrogen mustard was determined (Fig. 2). Cells deficient in DNA Pol II (ΔpolB) appear to be slightly more sensitive to killing by nitrogen mustard than were wild-type cells. In addition, a ΔpolB ΔrecA strain was more sensitive than a ΔrecA strain.
DISCUSSION
NER and DNA Pol II can be involved in nitrogen mustard interstrand cross-link repair in E. coli based on the RRE values (Table 2) in uvr (0.055) and polB (0.18) strains, respectively, compared to wild-type uvr+ polB+ (1.0) strains. The hypersensitivity of polB E. coli to killing by nitrogen mustard (Fig. 2) suggests that this pathway is physiologically relevant. The fact that the RRE is similar for both uvr and uvr polB strains (Table 2) suggests that NER and DNA Pol II are in the same pathway.
We found evidence for this NER/DNA Pol II pathway only because the commonly accepted pathway for the repair of interstrand cross-links, which involves NER and recombination (45, 46, 49), was precluded since the cells receiving inter-HN2-pTZSV28 in our experiments do not receive a lesion-free piece of DNA that is homologous to the region around the cross-link, thus making recombination impossible. These two pathways for the repair of interstrand cross-links are likely to work in parallel and are not functionally redundant, since cells deficient in either the NER/recombination pathway alone (ΔrecA) or the NER/DNA Pol II pathway alone (ΔpolB) were each hypersensitive to the killing effects of nitrogen mustard (Fig. 2). (It seems most likely that these results are attributable to killing by cross-links rather than to killing by monoadducts, although our results do not definitively distinguish between them.) The NER/Pol II pathway for coping with interstrand cross-links probably serves a role analogous to the role of SOS-dependent, translesion synthesis of bulky monoadducts (20). These pathways are likely to be necessary when lesion tolerance mechanisms (notably, those involving recombinational repair) are impossible, such as when there are closely spaced lesions in both sister chromosomes or when a cell does not have a homologous, sister chromosome (e.g., in late-replicating DNA).
The ΔrecA strain is more sensitive to the killing effects of nitrogen mustard than the ΔpolB strain, suggesting that the NER/recombination pathway probably repairs a greater fraction of cross-links than does the NER/DNA Pol II pathway. While it is difficult to make a rigorous quantitative comparison, we estimate that the NER/DNA Pol II pathway appears to be responsible for repairing ∼12% of the cross-links, based upon the differences in the dose of nitrogen mustard required to kill a particular fraction of wild-type versus polB versus recA cells (Fig. 2). {The 12% value was calculated as follows. The number of cross-links formed in the cells is assumed to be approximately proportional to the dose of nitrogen mustard. It is also assumed that the same number of cross-links are responsible for reducing cell survival to the 1% level in wild-type, polB, and recA cells, which occurs at nitrogen mustard concentrations of 5.0, 4.4, and 0.6 mM, respectively. The relative fraction of lesions removed by the polB-dependent pathway is estimated as follows: (wild type − polB)/([wild type − recA] + [wild type − polB]) = 0.12.} The fact that the survival curve in the ΔpolB ΔrecA strain is virtually superimposable on the curve for the uvrA strain (Fig. 2) suggests that NER is common to both pathways and that these are the only two NER-dependent pathways that repair interstrand cross-links.
The NER/DNA Pol II pathways appears to be active on other cross-links, such as that with mitomycin C (unpublished observation), which may provide an explanation for an old observation that mutagenesis by certain cross-linking agents (e.g., mitomycin C, as well as malondialdehyde) actually decreases in NER-deficient strains (27, 33, 34), implying that the mutations occur during DNA repair of a lesion, probably a cross-link, perhaps via the NER/DNA Pol II pathway. Interestingly, the NER/DNA Pol II pathway does not appear to be able to act on all cross-links, notably those from psoralens (as discussed in reference 4).
The involvement of DNA Pol II in this pathway is of interest for several reasons. Although the polB gene for DNA Pol II was first identified in 1972 (11, 23), the pathway depicted in Fig. 1 represents one of the few cases where there is good evidence of a concrete role for DNA Pol II in E. coli. It has been known for some time that DNA Pol II is damage inducible as part of the SOS response, implying that it plays some role in damage management and DNA repair (6, 7, 25). However, reports have indicated that DNA Pol II appears not to participate in UV resistance (25), UV mutagenesis (25), cyclobutane dimer mutagenesis (28), repair gap UV mutagenesis in cells (12) (although it can function in vitro [48]), Weigle reactivation (28), thymine glycol mutagenesis (28), mismatch repair (28), and UVM mutagenesis (39). There is indirect evidence for DNA Pol II involvement in abasic site mutagenesis (47), although direct evidence suggests otherwise (28). Studies have shown that DNA Pol II can substitute for DNA Pol III in some aspects of E. coli replication (41), that it is important for adaptive mutagenesis (18, 19), and that it is involved in protecting cells from H2O2 toxicity (18), although its exact role in each of these processes is unclear. In summary, DNA Pol II seems not to play a role in a variety of replication and repair processes, and where there is some evidence for its involvement, the components and the details of the pathway have not been delineated.
The results presented in Table 2 clearly indicate that DNA Pol II can be involved in the pathway depicted in Fig. 1. However, the RRE in polB cells (∼0.18) is higher than in uvr cells (∼0.055). This implies that some polymerase in addition to DNA Pol II either is responsible for a minor fraction of the repair or can substitute for DNA Pol II, albeit less efficiently (i.e., <20%). Nevertheless, it does appear that DNA Pol II is preferred, raising the question: why does this pathway not use either of E. coli’s other two DNA polymerases (i.e., Pol I or Pol III), which participate in other DNA repair pathways (20). Perhaps DNA Pol I is too error prone in the bypass of lesions (as discussed in references 29 and 30), whereas DNA Pol III, which is able to bypass lesions reasonably accurately, at least in some cases (e.g., see references 3 and 35), is too large a complex to bypass the extremely bulky lesion depicted in step 3 in Fig. 1.
ACKNOWLEDGMENTS
We gratefully acknowledge Myron Goodman for having provided numerous strains and plasmids.
This work was supported by NIH grants to E.L.L. (CA49198 and CA63396) and to P.L.F. (GM54084).
REFERENCES
- 1.Aida T, Bodell W J. Cellular resistance to chloroethylnitrosoureas, nitrogen mustard, and cis-diamminedichloroplatinum(II) in human glial-derived cell lines. Cancer Res. 1987;47:1361–1366. [PubMed] [Google Scholar]
- 2.Appleyard R K. Segregation of new lysogenic types during growth of a doubly lysogenic strain derived from Escherichia coli K12. Genetics. 1954;39:440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey E A, Iyer R, Stone M P, Harris T M, Essigmann J M. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc Natl Acad Sci USA. 1996;93:1535–1539. doi: 10.1073/pnas.93.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardini M, Mackay W, Loechler E L. A site-specific study of a plasmid containing single nitrogen mustard interstrand cross-link: evidence for a second, recombination-independent pathway for the DNA repair of interstrand cross-links. Biochemistry. 1997;36:306–313. doi: 10.1021/bi962778w. [DOI] [PubMed] [Google Scholar]
- 5.Boiteux S, Huisman O. Isolation of a formamidopyrimidine-DNA glycosylase (fpg) mutant of Escherichia coli K12. Mol Gen Genet. 1989;215:300–305. doi: 10.1007/BF00339732. [DOI] [PubMed] [Google Scholar]
- 6.Bonner C A, Randall S K, Rayssiguier C, Radman M, Eritja R, Kaplan B E, McEntee K, Goodman M F. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988;263:18946–18952. [PubMed] [Google Scholar]
- 7.Bonner C A, Hays S, McEntee K, Goodman M F. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:7663–7667. doi: 10.1073/pnas.87.19.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennard J, Margison G P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci USA. 1986;83:6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H. Ph.D. thesis. Los Angeles, Calif: University of Southern California; 1995. [Google Scholar]
- 10.Cairns J, Foster P L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991;128:695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell J L, Soll L, Richardson C C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci USA. 1972;69:2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Fix O, Livneh Z. In vitro UV mutagenesis associated with nucleotide excision-repair gaps in Escherichia coli. J Biol Chem. 1994;269:4953–4958. [PubMed] [Google Scholar]
- 13.Colvin M. The alkylating agents. In: Chabner B, editor. Pharmacological principles of cancer treatment. W. B. Philadelphia, Pa: Saunders Co.; 1982. pp. 276–308. [Google Scholar]
- 14.Csonka L N, Clark A J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979;93:321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czeczot H, Tudek B, Lambert B, Laval J, Boiteux S. Escherichia coli Fpg and UvrABC endonuclease repair damage induced by methylene blue plus visible light in vivo and in vitro. J Bacteriol. 1991;173:3419–3424. doi: 10.1128/jb.173.11.3419-3424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan M E, Young G S, Pegg A E. Effect of O-alkylguanine pretreatment on the sensitivity of human colon tumor cells to the cytotoxic effects of chloroethylating agents. Cancer Res. 1986;46:4500–4504. [PubMed] [Google Scholar]
- 17.Erickson L C, Laurent G, Sharkey N A, Kohn K W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumor cells. Nature. 1980;288:727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- 18.Escarceller M, Hicks J, Gudmundsson G, Trump G, Touati D, Lovett S, Foster P L, McEntee K, Goodman M F. Involvement of Escherichia coli DNA polymerase II in response to oxidative damage and adaptive mutation. J Bacteriol. 1994;176:6221–6228. doi: 10.1128/jb.176.20.6221-6228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster P L, Gudmundsson G, Trimarchi J M, Cai H, Goodman M F. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:7951–7955. doi: 10.1073/pnas.92.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 21.Grueneberg D A, Ojwang J O, Benasutti M, Hartman S, Loechler E L. Construction of a human shuttle vector containing a single nitrogen mustard interstrand, DNA-DNA cross-link at a unique plasmid location. Cancer Res. 1991;51:2273–2279. [PubMed] [Google Scholar]
- 22.Hemminki K, Ludlum D B. Covalent modification of DNA by antineoplastic agents. J Natl Cancer Inst. 1984;73:1021–1028. [PubMed] [Google Scholar]
- 23.Hirota Y M, Gefter M, Mindich L. A mutant of Escherichia coli defective in DNA polymerase II. Proc Natl Acad Sci USA. 1972;69:3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard-Flanders P, Boyce R P, Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966;53:1137–1150. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki H, Nakata A, Walker G C, Shinagawa H. The Escherichia coli polB gene, which encodes DNA polymerase II, is regulated by the SOS system. J Bacteriol. 1990;172:6268–6273. doi: 10.1128/jb.172.11.6268-6273.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohn K W, Gibson N W. DNA cross-linking by chloroethylating agents. In: Singer B, Bartsch H, editors. The role of cyclic nucleic acid adducts in carcinogenesis and mutagenesis. Lyon, France: IARC; 1986. pp. 155–162. [Google Scholar]
- 27.Kondo S, Ichikawa H, Iwo K, Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970;66:187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kow Y W, Faundez G, Hays S, Bonner C A, Goodman M F, Wallace S S. Absence of a role for DNA polymerase II in SOS-induced translesion bypass of Φχ174. J Bacteriol. 1993;175:561–564. doi: 10.1128/jb.175.2.561-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loechler E L. Mechanism by which aflatoxins and other bulky carcinogens induce mutations. In: Eaton D L, Groopman J D, editors. The toxicology of aflatoxins: human health, veterinary, and agricultural significance. Orlando, Fla: Academic Press, Inc.; 1994. pp. 149–178. [Google Scholar]
- 30.Loechler E L. Commentary: the role of adduct-site-specific mutagenesis in understanding how carcinogen DNA adducts cause mutations: perspective, prospects and problems. Carcinogenesis. 1996;17:895–902. doi: 10.1093/carcin/17.5.895. [DOI] [PubMed] [Google Scholar]
- 31.Ludlum D B. Formation of cyclic adducts in nucleic acids by the haloethylnitrosoureas. In: Singer B, Bartsch H, editors. The role of cyclic nucleic acid adducts in carcinogenesis and mutagenesis. Lyon, France: IARC; 1986. pp. 137–146. [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 33.Mukai F H, Goldstein B D. Mutagenicity of malondialdehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science. 1976;191:868–869. doi: 10.1126/science.766187. [DOI] [PubMed] [Google Scholar]
- 34.Murayama I, Otsuji N. Mutation by mitomycins in the ultraviolet light-sensitive mutant of Escherichia coli. Mutat Res. 1973;18:117–119. doi: 10.1016/0027-5107(73)90027-4. [DOI] [PubMed] [Google Scholar]
- 35.Naser L J, Pinto A L, Lippard S J, Essigmann J M. Chemical and biological studies of the major DNA adduct of cis-diamminedichloroplatinum(II), cis-[Pt(NH3)2{d(GpG)}], built into a specific site in a viral genome. Biochemistry. 1988;27:4357–4367. doi: 10.1021/bi00412a024. [DOI] [PubMed] [Google Scholar]
- 36.O’Conner P M, Kohn K W. Comparative pharmacokinetics of DNA lesion formation and removal following treatment of L1210 cells with nitrogen mustard. Cancer Commun. 1990;2:387–394. doi: 10.3727/095535490820873949. [DOI] [PubMed] [Google Scholar]
- 37.O’Conner P M, Wassermann K, Sarang M, Magrath I, Bohr V A, Kohn K W. Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt’s lymphoma cell lines differing in sensitive nitrogen mustard. Cancer Res. 1991;51:6550–6557. [PubMed] [Google Scholar]
- 38.Ojwang J O, Grueneberg D A, Loechler E L. Synthesis of a duplex oligonucleotide containing a nitrogen mustard interstrand cross-link. Cancer Res. 1989;49:6529–6537. [PubMed] [Google Scholar]
- 39.Palewala V A, Wang G E, Murphy H S, Humayun M Z. Functional recA, lexA, umuD, umuC, polA, and polB are not required for the Escherichia coli UVM response. J Bacteriol. 1995;177:6041–6048. doi: 10.1128/jb.177.21.6041-6048.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt W B, Ruddon R W, Ensminger W D, Maybaum J. The anticancer drugs. 2nd ed. New York, N.Y: Oxford University Press, Inc.; 1994. [Google Scholar]
- 41.Rangarajan S, Gudmundsson G, Qiu Z, Foster P L, Goodman M F. Escherichia coli DNA polymerase II catalyzes chromosomal and episomal DNA synthesis in vivo. Proc Natl Acad Sci USA. 1997;94:946–951. doi: 10.1073/pnas.94.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samson L, Derfler B, Waldstein E A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci USA. 1986;83:5607–5611. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer B, Essigmann J M. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991;12:949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- 44.Sladek F M, Melian A, Howard-Flanders P. Incision by UvrABC is a step in the path to mutagenesis by psoralen crosslinks in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:3982–3986. doi: 10.1073/pnas.86.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sladek F M, Munn M M, Rupp W D, Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC and the 5′-exonuclease of DNA polymerase I. J Biol Chem. 1989;264:6755–6765. [PubMed] [Google Scholar]
- 46.Slater S C, Maurer R. Simple phagemid-based system for generating allele replacements in Escherichia coli. J Bacteriol. 1993;175:4260–4262. doi: 10.1128/jb.175.13.4260-4262.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessman I, Kennedy M A. DNA polymerase II of Escherichia coli in the bypass of abasic sites in vivo. Genetics. 1994;136:439–448. doi: 10.1093/genetics/136.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomer G, Cohen-Fix O, O’Donnell M, Goodman M, Livneh Z. Reconstitution of repair-gap UV mutagenesis with purified proteins from Escherichia coli: a role for DNA polymerases III and II. Proc Natl Acad Sci USA. 1996;93:1376–1380. doi: 10.1073/pnas.93.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Houten B, Gamper H, Holbrook S R, Hearst J E, Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci USA. 1986;83:8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlotogorski C, Erickson L C. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand cross-links not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1984;4:759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]