Abstract
As the world’s population ages, diseases predominantly found in the elderly now overlap with diseases that were thought to be the purview of younger patients. This includes chronic liver disease (CLD), which affects more than 2 billion people worldwide1. Due to the obesity epidemic (and associated metabolic diseases), nonalcoholic fatty liver disease has become the most common cause of CLD and cirrhosis2. A major complication of cirrhosis is hepatic encephalopathy (HE), which becomes challenging to diagnose in elderly patients3. HE is usually included in the differential diagnosis of acute delirium, but not of reversible dementias.
To illustrate this point, we present two cases of older patients that were misdiagnosed as having dementia and Parkinson’s disease or a parkinsonian syndrome but had contributions from cirrhosis. Both cognitive impairment and tremor resolved with treatment of HE.
Keywords: Dementia, Parkinson’s disease, Wernicke’s encephalopathy, Alcohol, Lewy Body Dementia, Progressive Supranuclear palsy, EncephalApp
Case 1
A 71-year-old male with hypertension, type 2 diabetes mellitus, non-alcoholic cirrhosis, and thalamic and basal ganglia infarcts presented with tremor, shuffling gait, orthostatic symptoms, and memory issues. On exam, he was alert and oriented, with intact recent and remote memory, but bradyphrenia noted. No signs of asterixis were observed. Working diagnosis was vascular Parkinsonism, versus other parkinsonian syndrome, including multi system atrophy with predominant Parkinsonism, (MSA-P). He was started on a trial of carbidopa/levodopa. At follow-up, patient reported no improvement in symptoms and therefore he was titrated off carbidopa-levodopa. One month later, patient reported worsened memory and onset of visual hallucinations, at that time symptoms did not seem consistent with hepatic encephalopathy given the predominant motor symptoms, intact alertness and orientation and worsened delayed memory.
A few months later, he returned with worsening confusion including putting his clothes on backwards, not recognizing eating utensils, and urinating on bathroom floor. Per caregiver, some days he was better, others worse. Mental status exam revealed that while oriented to name and place, he did not know the month, his age, or date of birth. He also had difficulty following simple commands. Lewy body dementia (LBD) was on top of the differential because of fluctuation of symptoms, vs. vascular dementia. As the patient reported 2–3 bowel movements daily on psyllium and polyethylene glycol, HE was not a major concern.
Two weeks later he presented to the ER with abdominal pain and increased confusion. Labs showed leukocytosis, thrombocytopenia, elevated liver function tests, and ammonia level >100. CT showed cholelithiasis without ductal dilatation. Cultures were negative. He was diagnosed with sepsis possibly due to intra-abdominal infection and acute hepatic encephalopathy. Treatment included lactulose and rifaximin.
Three months later, patient and caregiver reported that his symptoms (memory loss, hallucinations, falls and tremor) resolved after starting lactulose and rifaximin. On exam he was alert and oriented x4, recent and remote memory were intact, attention, concentration and serial 3’s were normal. His wife noted: “He is a different person!”.
Case 2
73-year-old male with hypertension, type 2 diabetes mellitus, anemia, tremor, history of alcohol abuse (quit 2 years prior) and head trauma from boxing presented to ER with history of fever, nausea, vomiting and diarrhea, confusion, and fall resulting in head laceration. He was alert and oriented with normal neurologic exam but had amnesia of the fall and injury. Labs showed normocytic anemia, thrombocytopenia, hyperbilirubinemia, normal AST, ALT and ammonia, and negative urine drug and alcohol screen. Head CT with chronic microvascular ischemic changes frontal lobes. Ultrasound showed cholelithiasis and liver nodularity. He was discharged the next day.
Four months later, patient admitted from an assisted living facility with increased confusion and difficulty ambulating. Labs included elevated creatinine and BUN, hyperbilirubinemia, thrombocytopenia, and prolonged INR. AST and ALT normal. Head CT without acute pathology. CT abdomen showed gallstone and nodular liver. Diagnoses included metabolic encephalopathy, acute kidney injury due to poor oral intake combined with ACEI and HCTZ, urinary retention, possible Parkinson’s disease, and dementia. Treatment included intravenous fluids, tamsulosin, donepezil and carbidopa-levodopa. Patient transferred to skilled nursing facility (SNF).
After 2 months, patient discharged from SNF to move in with family. Three days after moving, he presented to ER with increased confusion and weakness, denied fever, chills, GI, respiratory or urinary symptoms. On exam, disoriented to time and place, without focal neurologic deficits. Labs remarkable for ammonia 114, elevated lactate, and thrombocytopenia. Head CT was unchanged. Ultrasound showed nodular liver and splenomegaly. Asterixis noted on exam the following day. Lactulose started for HE. Mental status improved and patient discharged home. One week later he was readmitted with altered mental status and treated for HE. Lactulose dose increased and he was again discharged home.
At hepatology visit post discharge, mental status exam reported normal and rifaximin started. At follow-up, the caregiver reported that all symptoms including mental clarity have improved since hospitalization and starting lactulose/rifaximin. On exam, he was alert and oriented with recent and remote memory intact. Attention and concentration were also intact and fund of knowledge appropriate. The impression was that his Parkinsonism symptoms were likely due to acute encephalopathy and carbidopa-levodopa was discontinued.
Discussion:
There is an inadequate recognition of cirrhosis in patients with chronic diseases, especially in elderly populations. This is partly because of a lack of awareness of the demographic shift from younger to older in cirrhosis due to the epidemic of obesity, diabetes, and non-alcoholic fatty liver disease4. A major decompensating event in cirrhosis is the development of hepatic encephalopathy (HE), which is brain dysfunction due to underlying liver disease and portal hypertension3. The staging of HE ranges from covert (includes cognitive impairment called minimal HE or MHE and the erstwhile grade 1) to grade 2–4 which is diagnosed in patients who have disorientation, asterixis and could lead to coma3. While HE has been considered a treatable disease with waxing and waning episodes in the form of a delirious state, the covert stage (CHE), which may predispose patients to chronic cognitive impairment3, 5. Rarely, there may be acquired hepatocerebral degeneration with encephalopathy (a chronic neurological disorder associated with cirrhosis, involves cortical area and basal ganglia manifesting as apathy, lethargy, somnolence, and/or extrapyramidal symptoms)6. In addition, entities such as Parkinson’s disease and alcohol-related cognitive impairments are often co-morbid in this population7, 8. In the aging patient population with cirrhosis, mild cognitive impairment and dementia also become part of the differential diagnosis of cognitive decline.
Mild cognitive impairment (MCI) is a cognitive decline reported by the patient or a caregiver, not due to an acute medical or neurological illness, with deficits that are in one or more cognitive domains and are greater than expected by normal aging, but do not affect daily function9. The patients may perceive more difficulties but is able to continue to be independent with all day-today activities. When cognitive abilities decline to the point to affect function, like managing finances, medications, planning a meal or other activities that were accomplished before, the patient meets criteria of dementia, or “major neurocognitive disorder”, as called by the Diagnostic and Statistical Manual of Mental Disorder, Fifth Edition (DSM-5). Dementias encompass a variety of neurodegenerative and other dementing illnesses of which Alzheimer’s disease (AD) is the most common. AD is characterized by slow progressive decline, in early stages deficits are predominantly of short-term memory while impairment of gait, coordination, swallowing and parkinsonism are usually seen in late stages.
Dementias with parkinsonian features that may overlap include Parkinson Disease dementia (PD first, dementia years later), dementia with Lewy bodies (dementia at onset, early visual hallucinations) and progressive supranuclear palsy (PSP - executive dysfunction, early postural imbalance, axial rigidity, ophthalmoplegia). Vascular dementia (VaD) can also cause parkinsonism if ischemic strokes occur in the basal ganglia (see table 1). Dementia is considered vascular if a patient develops dementia after a stroke or if a patient with dementia has brain imaging that shows vascular injury even without a history of known stroke. Autopsy studies have shown that often patients have mixed dementia, in one study 50% of patients with dementia had multiple diagnosis (pathology consistent with AD, PD/LBD or infarcts) which makes the clinical diagnosis more difficult as symptoms may overlap10.
Table:
Clinical features of overt hepatic encephalopathy versus dementias that may have parkinsonism. Alzheimer’s disease and frontotemporal dementia may also have parkinsonism’s but usually in the late stages.
| Feature | Hepatic Encephalopathy | Normal Pressure Hydrocephalus | Vascular Dementia | Progressive Supranuclear Palsy | Parkinson’s Disease Dementia | Dementia with Lewy Bodies |
|---|---|---|---|---|---|---|
| Onset | Abrupt or subacute | Gradual | Sudden (post stroke) or Gradual | Gradual | Gradual; PD for ≥1 year prior to onset of dementia | Gradual; parkinsonism present at dementia onset |
| Course | Fluctuates | Progressive | Stepwise or Gradual | Progressive | Progressive | Progressive (>PDD) |
| Duration | Hours to days (overt) to months (covert) | Months to Years | Months to Years | Months to years | Months to Years | Months to Years |
| Sleep-Wake Cycle | Disturbed and reversed | Sleep disturbances; OSA common | Nocturnal confusion | Insomnia; REM sleep behavior disorder (RBD) uncommon | RBD, Daytime sleepiness | RBD may be early sign; Daytime drowsiness or naps >2 hours |
| Attention & Alertness | Disturbance in attention early; Alertness may fluctuate | Preserved in earlier stages | Preserved in earlier stages | Preserved in earlier stages | Preserved in earlier stages | Fluctuates |
| Cognitive Symptoms | Impaired addition or subtraction, working memory; Disorientation in overt HE | Fronto-subcortical dysfunction, psychomotor slowing, executive dysfunction | Depends on location of brain injury; Memory loss not as severe as AD, better recognition memory (recall with cues) | Early frontal & executive deficits | Early deficits in executive & visuospatial function (VSF); Memory affected late in course; Testing similar to LBD | Early deficits in attention, executive & VSF; fluctuating symptoms; Memory affected late in course |
| Neuro-psychiatric & Behavioral Symptoms | Disinhibition, euphoria or anxiety, aggressive, agitated | Apathy | Depression, apathy, emotional incontinence-pseudobulbar affect | Apathy, disinhibition, dysphoria, anxiety | Visual hallucinations (VH), paranoid delusions, apathy, depression, anxiety | Early VH, delusions, episodes of bizarre behavior, staring or zoning out; apathy, depression, anxiety |
| Speech | Dysarthria Slurred in overt stages | Preserved | May include aphasia | Perseveration Hoarse, groaning voice | Hypophonia, dysarthria, impaired prosody of speech | Episodes of disorganized speech |
| Motor Symptoms | Reverse myoclonus (asterixis); Hypomimia, bradykinesia, tremor, gait disorder possible | Early postural instability; Gait apraxia usually initial symptom & more prominent than dementia & incontinence | Depend on area of ischemia, lower body parkinsonism without tremor if ischemic strokes in the basal ganglia | Early postural instability; Oculomotor findings with limited downward gaze first; Bradykinesia, rigidity axial muscles | Bradykinesia, tremor (more common than in LBD), rigidity, postural instability; PD more likely asymmetric and severe than LBD | Bradykinesia, tremor, rigidity, postural instability; Usually more symmetric than PD |
| Reversible | Usually | Potentially with VP shunt if gait disturbance & mild dementia | Usually Irreversible | Irreversible | Irreversible | Irreversible |
| Neuro-pathology | Alzheimer type 2 astrocytosis microglial activation. Focal loss of neurons may also occur in the basal ganglia, thalamus and cerebellum. | Ventriculomegaly without prominent cortical atrophy | Large vessel infarct or intracerebral hemorrhage or >2 lacunar infarcts, or extensive white matter changes | high density neurofibrillary tangles & neuropil threads in basal ganglia & brain-stem | Lewy bodies | Lewy bodies in the cortex |
Patients with alcohol dependence can develop malnutrition and thiamine deficiency. Wernicke encephalopathy (WE) is the acute form of thiamine deficiency with the triad of encephalopathy, gait ataxia and ocular abnormalities. Not all patients with WE have all three elements of the triad, therefore, to increase the sensitivity and not miss a morbid and easily treatable disease, the Caine criteria require only two of these four criteria to make a diagnosis of WE in patients with alcohol use disorder: 1) Dietary deficiency, 2) Ocular abnormalities, 3) Cerebellar dysfunction and 4) Either altered mental status or mild memory impairment. In some patients with one or more episode of Wernicke encephalopathy, the disease does not resolve completely despite treatment with thiamine and proceeds to the Korsakoff amnestic syndrome, characterized by anterograde and retrograde memory loss, normal alertness, and remote memory. These patients are usually unaware of their deficits and may confabulate. Alcohol toxicity can also contribute to cognitive impairment. A combination of alcohol neurotoxicity and nutritional deficiency can cause alcoholic cerebellar degeneration. It usually develops gradually and causes an ataxic gait similar to acute alcoholic intoxication. The patient is unable to do tandem gait and may have a postural tremor. It does not cause cognitive dysfunction by itself.
The diagnosis of dementia is based on clinical features, review of medications and psychiatric diagnosis (like depression) that could contribute, laboratory tests including CBC, glucose, thyroid function test, renal panel, and liver function tests, and often brain imaging to assess for reversible causes. The American Academy of Neurology (AAN) recommends screening for vitamin B12 deficiency11, while more recently some experts have recommended checking a homocysteine level, which reflects the function of three B vitamins (folate, vitamin B12, B6) and if elevated, treating with all three vitamins, based on the results of limited data (three trials)12, 13. In the VITACOG trial, high dose folic acid (0.8 mg), vitamin B12 (0.5 mg/d) and vitamin B6 (20 mg/d) slowed the rate of brain atrophy in patients with MCI and elevated homocysteine level14. The AAN does not feel there is enough evidence “to support or refute the use of homocysteine-lowering therapies in patients with MCI”15. Tests for syphilis, human immunodeficiency virus (HIV) and Lyme disease should be considered based on clinical history. As part of the evaluation of cognitive impairment, the clinician should rule out the possibility of delirium. Delirium is the hallmark of acute encephalopathy and the term is preferred to synonyms like “altered mental status”, “acute confusional state”, “acute brain failure” or “acute brain dysfunction”16. Delirium is a disorder of attention. Criteria for delirium, based on the Confusion Assessment Method (CAM17) are: 1) Acute change or fluctuating course, 2) Inattention, 3) Disorganized thinking, and/or 4) Altered level of consciousness. To meet criteria of delirium, the patient must have feature 1, 2 and either 3 or 4. Delirium can be hyperactive or hypoactive, hypoactive forms are more likely to be missed in older patients.
The DSM-5 also introduced the possibility of an “attenuated delirium syndrome” (ADS), when a patient does not meet the full criteria of delirium but has one or more of the four CAM criteria. This was previously called “subsyndromal delirium”, it can be present when the patient has evolving or resolving delirium. Among stroke survivors, ADS in the first week post-stroke predicted cognitive decline at 3 months18.
A patient with MHE or covert HE may present with mild symptoms like sleep disturbance, irritability, difficulty concentrating or mild confusion noted by the patient or caregiver, changes that are difficult to diagnose unless the patient undergoes psychometric tests, which show abnormalities in the areas of attention, executive function, visual spatial coordination and reaction times5. While overt HE can occur without the preceding diagnosis of MHE/CHE, overt HE can be predicted by these cognitively impaired patients. However, in elderly patients, if the progression is gradual, the slow progression may lead a clinician to diagnosing dementia rather than considering HE, especially in older patients, where dementia is more prevalent and particularly if underlying cirrhosis is not diagnosed, not connected to these findings, and if competing diagnoses are treated first. In addition to cognitive changes, HE may be associated not only with asterixis (negative myoclonus), but also with extrapyramidal symptoms such as parkinsonism, chorea, hemiballismus or spastic paraparesis which leads the clinician to suspect one of the dementias associated with parkinsonism rather than HE. The fluctuation of attention in addition to the parkinsonism of HE can be easily be misdiagnosed for Lewy body dementia (LBD). Major overlaps and similarities in symptoms are in figure 1.
Figure 1.
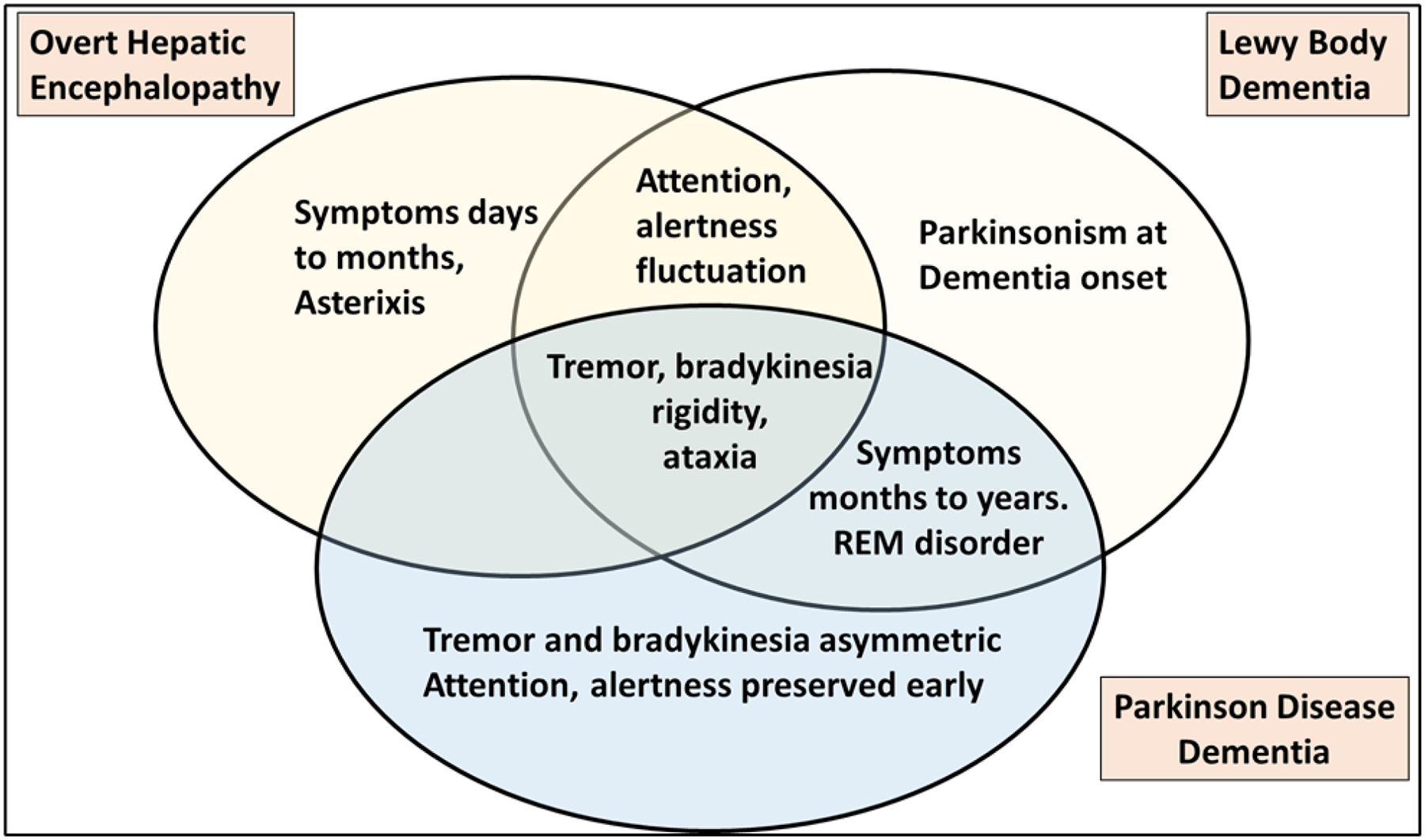
Venn diagram of major symptoms of overt hepatic encephalopathy, Lewy Body dementia and Parkinsonian Disease Dementia
These cases are reflective of challenges faced in taking care of elderly patients who have a major burden of concomitant illnesses. There are challenges in communications between specialties, reduced awareness of overlaps, reduced awareness of cirrhosis in specialties other than gastroenterology and ultimately delay in appropriate therapy. However, a greater concern is the lack of adequate diagnosis of cirrhosis or inadequate diagnostic tests to rule in HE, especially in the context of concomitant disease processes. These two aspects are critical in determining the optimal management of patients in whom either the diagnosis of cirrhosis may be missed or HE as the major cause of cognitive impairment may not be completely recognized. This can impact the treatment and delay the appropriate therapy.
In both cases above, HE-related medical treatment was both diagnostic and therapeutic and on hindsight relieved several symptoms that were presumed to be due to PD, dementia or MSA. However, the treatment was delayed until the patient reached the overtly delirious stage and the long lag period before that was not used for (1) recognizing cirrhosis and HE as a potential etiology for the chronic symptom change and (2) questioning the lack of response to therapy in those who do not respond to diagnoses made by other specialties. These could have saved months of debility, admission to specialized nursing facilities, and burden on the system, family, and patients.
Solutions to this issue:
There are two broad areas that could relieve this burden:
- Awareness of cirrhosis and HE: this is critical in specialties that do not usually treat patients with liver disease to increase surveillance using easy lab tests such as low platelet count (<150/mm3), FIB-4 (Fibrosis-4, >2.67) and APRI (AST-to-Platelet Ratio Index, >1.0) to screen for underlying cirrhosis in patients who are high risk in inpatients and outpatient settings especially in neurology, geriatric, psychiatric, and substance abuse clinics19. Patient at high risk may have:
- Alcohol-related complications and history of past or current alcohol use disorder
- Diabetes mellitus and features of metabolic syndrome
- Unexplained alteration in mental status or neurological symptoms not responding to first-line treatment
- Unusual presentations of Parkinson’s disease (predominant gait problems, rapid progression, lack of “pill rolling” tremor)
- Worsening of underlying course i.e., development of delirium in an otherwise stable patient with dementia or mild cognitive impairment
- Patient with mild cognitive impairment with predominant non-amnestic changes
- Specific testing strategies to differentiate between entities or confirm the overlap of HE with other disease processes.
- Risk factors, time-course, and features
- Cognitive testing, including orientation, attention, short-term and delayed memory assessment
- Brain imaging (investigational)
- Gut-brain axis changes (investigational)
Increasing knowledge of cirrhosis and HE in geriatric populations and differentiating between these and concomitant disorders:
Given the increasing incidence of obesity and non-alcoholic fatty liver disease (NAFLD) that can progress to cirrhosis, it becomes more important for providers to be aware of overlap of symptoms due to HE with other causes of delirium and of dementia.
For patients who would otherwise be at a high risk of cirrhosis including diabetes, metabolic syndrome, prior or current alcohol misuse, and with atypical Parkinsonian features, the first step is to look for a high FIB-4 index, or thrombocytopenia that accompanies cirrhosis19. This can be then further evaluated using imaging, transient elastography, and/or referral to gastroenterology. After diagnosing cirrhosis, then a judgement call needs to be made to determine if this neuropsychiatric disorder is only HE or overlaps with another disease process. These decisions need to be aided by first-line testing, questioning of caregivers and family, then with further tests that determine the presence of cirrhosis and HE.
Cirrhosis is formally diagnosed using liver biopsy, or a combination of laboratory and radiological investigations including transient elastography, low platelet count and high AST/ALT ratio in a patient with risk factors for chronic liver disease19. Other signs, such as ascites, varices on imaging or endoscopy, can also be used to bolster the case for diagnosis of cirrhosis. Since most HE cases occur in the background of cirrhosis, the diagnosis of cirrhosis is essential before moving onto the next set of investigations that evaluate whether the process is solely HE or an overlap with other disease processes.
HE is predominantly associated with subcortical changes that reflect in the cognitive alterations. Metabolic studies of HE reveals intracellular dysregulation involving glutamate, myoinositol, and choline, as well as enhanced sensitization to inflammatory cytokines, and increased permeability of the blood brain barrier. The functional changes associated with HE reveal a propensity for HE to impact, particularly in the early stages of the disorder, subcortical region integrity20–22.
Although it is well-known that pathological changes of neurodegenerative disorders are not limited exclusively to either cortical or subcortical brain regions, the cortical-subcortical distinction serves as a useful model for describing the pattern of neuropsychological deficits that are observed in these patients. While cortical dementias are often associated with visual-spatial deficit, agnosia, aphasia, apraxia, executive dysfunction, and learning/memory deficits, the two cases described in the current report illustrate the challenge for clinicians to differentiate hepatic encephalopathy from subcortical dementias. In both Parkinson’s disease (PD) and HE the associated neuropathology impacts, particularly in the early stages of the disease process, subcortical brain regions. PD is characterized by loss of pigmented cells in the substantia nigra pars compacta resulting in a major depletion of dopamine; and the presence of Lewy bodies in the substantia nigra, locus coeruleus, dorsal motor nucleus of the vagus, and the substantia innominata23–25. The classic motor-symptom of PD includes asymmetric resting Tremor, Rigidity, and Akinesia/bradykinesia, Postural instability (mnemonic TRAP) with associated postural stooping, gait disturbance, masked facies, dysarthria, and monotoned speech (impaired prosody)26. Non-motor symptoms of PD include emotional apathy, depression, anxiety, olfactory dysfunction, constipation, REM sleep disorder, cognitive dysfunction, and psychosis. Dementia occurs later in the course of PD, by definition at least one year after the onset of motor symptoms and is called Parkinson Disease with (Lewy body) Dementia (PDD). If cognitive impairment starts before or at the same time of parkinsonism, the patient is diagnosed with Dementia with Lewy body (DLB). The term “Lewy body dementia” includes both PDD and DLB. DLB is the most common neurodegenerative dementia after Alzheimer’s disease. In addition to cognitive impairment, patients with DLB often have fluctuation of cognitive function, visual hallucinations early in the disease, dysautonomia and sleep disorders. Studies using dopamine transporter (DaT) imaging (DaTscan) with either positron emission tomography (PET) or single photon emission computed tomography (SPECT) have been able to differentiate LBD from AD by demonstrating decreased dopaminergic activity in the nigrostriatal pathway in patients with LBD, while DaTscan is normal in patients with AD27. DaTscan cannot differentiate PD from other disorders of nigrostriatal degeneration (Progressive supranuclear palsy (PSP), multi-system atrophy, corticobasal degeneration) as it is abnormal in these disorders too. In addition to AD, DaTscan is normal in vascular parkinsonism, drug-induced parkinsonism, and essential tremor. In a case series of patients with cirrhosis and parkinsonism, as expected DaTscan was abnormal in two patients who had clinical features of idiopathic Parkinson disease: hemiparkinsonism, resting tremor, without early gait disturbance or postural instability while it was normal in two patients with atypical features: symmetric parkinsonism, early gait disturbances and postural instability and no resting tremor28. DaTscan was approved by the FDA in 2011 to differentiate PD from essential tremor. Most cases of PD can be diagnosed clinically, an important criterion is dramatic response to dopaminergic agents.
Clinicians can rely on demographic variables and neuropsychological testing to distinguish these disorders. While in most neuropsychiatric disorders, the five demographic variables: age at illness onset, gender, family history, treatment response, and illness trajectory are critical predictors of the underlying process. A second important strategy for diagnostic clarification reflects an awareness that the neuropathological processes associated with PD and HE differ, and that these differences result in a unique pattern of cognitive impairment. The pattern of cognitive impairment associated with HE, particularly in the earlier stages of the disorder, includes psychomotor slowing, reduced vigilance, and working memory. Patients with PDD typically exhibit motor disordered symptoms (i.e., shuffling gait, micrographia, hypophonia, dysarthria, speech prosody impairment, resting tremor, rigidity, and bradykinesia), as well as deficiencies in planning, and visual perceptual/constructional deficits. Neuropsychological evaluation uses reliable and valid measures to identify the individual patients’ neurobehavioral strengths and weaknesses. In summary, the combination of careful history taking, observation of present behavior, along with neuropsychological evaluation, can aid in the differential diagnosis of PDD and HE, despite both being predominantly subcortical in nature.
Complicating matters is the entity of Parkinsonian features that co-exist with cirrhosis in the endstage. These differ from idiopathic PD by demonstrating a more rapid progression, symmetric changes, predominant gait alterations, and lower likelihood of tremors. These patients often have diagnosed HE but this is why “atypical” PD should be in the differential for cirrhosis-related changes.
Cognitive testing
Cognitive testing focused on delayed memory versus visuo-spatial and attention are also useful in differentiating or defining overlap between HE and MCI. In prior studies there was a significant overlap of patients deemed to be MCI and MHE based on cognitive testing in single and multi-center studies of those who were more than 65 years of age29. The likelihood of both MCI and MHE coexistence is higher in those with cirrhosis, even without prior HE and is characterized by deficits in long-term memory and other “cortical” impairments compared to MHE only patients, where visuo-spatial, attention, and psycho-motor speed changes predominate. These data also showed that combined MCI and MHE worsened the healthrelated quality of life in stable outpatients.
In the same study, we demonstrated that the Attentional Index of the RBANS was able to differentiate MHE from MCI: while it was normal in patients with MCI, it was significantly lower in those with MHE (p<0.05). The 2 subtests that make up the Attentional Index of the RBANS are the Digit Span and Coding subtests. Of the 2 subtests, the Coding subtest (similar to the digit symbol substitution test) emphasizes the subcortical attentional network (impaired cortical tone and arousal), while Digit Span interrogates the higher cortical attentional network.
The Attentional Index would be a very good screening test to follow older patients with or without MCI and cirrhosis, a deterioration of score could suggest MHE or other form of delirium. The RBANS and its subtests are copyrighted, so other screening test for attention should be considered for use in the clinical setting.
A simple test to screen for MHE is the EncephalApp Stroop test (www.encephalapp.com) this test has the advantage of being free30, 31. This has been shown to be relatively specific for MHE when compared to MCI+MHE in a multi-center study29. The EncephalApp tests psychomotor speed and cognitive flexibility by measuring the users’ response rate in identifying the color of printed text and has an even shorter version called “QuickStroop”. Clinicians could familiarize themselves and download the app on their smartphone. However, this and other screening tests such as the animal naming test may not be completely specific and other data and clinical inputs are also needed32.
In older patients with a diagnosis or suspicion of cirrhosis, clinicians, even nongastroenterologists, or psychologists, need to monitor cognitive function and suspect HE especially if there is a decline in the areas of orientation and/or attention. Patients with MHE meet the criteria of overt HE when they have temporal disorientation or asterixis. Disturbance of attention could be due to MHE, overt HE, other form of delirium or worsening of dementia. Examples of cognitive screening tests that are commonly used in geriatrics and that include both orientation and attention questions are the Montreal Cognitive Assessment (MoCA) and the St. Louis University Mental Status Exam (SLUMS). While the MoCA may be used without permission for clinical use, training, and certification to administer and score the MoCA accurately have become mandatory. The SLUMS has the advantage that is not copyrighted and does not require formal training to administer. It includes two attention questions: reverse digit span (the patient is asked to repeat two, three, and then four numbers in reverse) and the one minute animal naming test (ANT1,). The ANT1 is a semantic fluency test where the patient is asked to name as many animals as possible within one minute. The ANT1 by itself has been validated as a simple test to assess for MHE33. The results are influenced by education (<8 years) and age (>80 years). The simplified ANT1 (S-ANT1) adjusts the results by adding 3 animals for patients with < 8 years of education and 3 animals for age > 80, so for example, if an 81 year old patient with 7 years of education is able to list only 9 animals in 1 minute, 6 animals are added and the adjusted number of animals would be 15. The probability that a person with S-ANT1 ≥ 15 does not have HE is at least 80%. The optimal cutoffs of the S-ANT1 are: ≥ 15 for normal, between 10 and <15 for MHE, and < 10 for overt HE. The S-ANT1 performance depends not only on sustained attention but also other neuropsychological domains (executive function, working memory) and can be affected by other forms of cognitive impairment, so it should be considered just a quick screening test that requires further evaluation if abnormal. Further evaluation may include a history from a caregiver on the cognitive symptoms and any change in function in addition to a more detailed cognitive test like the full SLUMS to determine need the to work up possible delirium versus dementia and, when repeated at each visit, its progression with time.
As tests used to measure attention like the S-ANT1, the digit span, or months of the year backwards also require executive function and working memory, recognizing delirium superimposed on dementia (DSD) is particularly challenging34. Even the “letters attention test” a simple test of attention used as part of the CAM for ICU patients (CAM-ICU), had a high rate of false positives in patients with dementia (predominantly AD) without delirium, with a fail rate of 16% in mild, 56% in moderate and 72% in severe dementia35. As delirium is a disorder of both attention and arousal, an alternative way to detect delirium is to assess the level of arousal, which is usually preserved until advanced dementia. In this regard a helpful tool is the Richmond Agitation and Sedation Scale (RASS), a quick assessment of alertness based on observation of the patient which was initially created to evaluate level of arousal in the ICU setting36. It was then modified to be used on the wards (m-RASS)37. The RASS is also used in the CAM-ICU to assess level of consciousness. The m-RASS has scores ranging from +4 to −5 with 0 denoting normal alertness (calm and alert patient), positive scores indicates agitation from +1 (restless, slightly distractable) to +4 (combative with no attention). Negative scores indicate increased sedation from −1 slightly drowsy to −5 unarousable. Among older patients with dementia, a mRASS score other than 0 (normal) had a sensitivity of 71% and specificity of 85% to detect delirium. The mRASS may be a useful tool to detect HE in patients with DSD.
Therefore, in patients who are >65 years and have cirrhosis or an unexplained cognitive change pattern vis-à-vis the disease ascribed to them, a diagnosis of MHE should be considered. This can then be confirmed on detailed cognitive testing.
Studies have also shown changes in gut-brain axis and imaging in patients where the MCI and MHE was diagnosed by a neuropsychologist blinded to the cirrhosis or not status of the patients29. Groups were divided into (1) unimpaired (2) MCI only (3) MHE only, and (4) MCI+MHE overlap. More patients with cirrhosis were in the MHE ±MCI groups than the others. On brain imaging, the MCI-only subjects had lower white matter, gray matter and total brain volume as well as hippocampal and left thalamic volume compared to the other two impaired subgroups. In addition, right thalamic volumes in MCI-only group were only lower than the MCI+MHE group.
A specific signature of MHE and MCI was also seen on MR spectroscopic brain imaging. In patients with cirrhosis, due to hyperammonemia, there is an increase in osmotically active glutamine in astrocytes. To counteract this osmotic activity, myo-inositol (mI) and choline are extruded to reduce the chance of cerebral edema. N-acetyl aspartate and N-acetyl aspartate glutamate (NAA+NAAG) are neuronal markers that are often unimpaired in cirrhosis. In the evaluation of MHE vs MCI vs both, patients with cirrhosis had lower creatine ratios of myo-inositol and NAA + NAAG, and increased ratio of Glutamate/Glutamine to creatine in the anterior cingulate cortex. Using the neuropsychological classification, MCI+MHE patients had lower mI and NAA+NAAG than MCI alone, which was also found compared to unimpaired patients. When gut microbiota of these patients was analyzed, the MCI+MHE patients had progressively lower commensals that produce beneficial short-chain fatty acids (Faecalibacterium and Butyricoccus) compared to unimpaired and groups only with MHE or MCI rather than both impairments. Oral-origin taxa such as Porphyromonadaceae were higher in those with MCI, which in the past have been associated with cirrhosis and dementia.
The study was done in patients without prior overt HE to mimic circumstances where these could be used as potential tools to differentiate groups. These data provide proof-of-concept but neither specialized brain imaging nor microbial profiling is currently ready for clinical practice.
Cases:
Both patients described had parkinsonism and cognitive decline. The first case had a history of cirrhosis but because he had parkinsonism, orthostasis and basal ganglia infarct, vascular parkinsonism or MSA-P seemed the most likely diagnosis. MSA-P is characterized by akinetic/bradykinetic parkinsonism with postural instability and early dysautonomia (urinary dysfunction, orthostatic hypotension) but usually the average age of onset is younger (54–60, range 31–78) and does not cause dementia. PD was unlikely as he did not respond to a trial of carbidopa/levodopa. When he presented with worsening confusion and fluctuation of mental status, DLB was added to the differential. The similarities of hepatic encephalopathy with DLB (fluctuation and attention and alertness) made the transitional change into HE difficult to diagnose until he had further worsening of his cognitive function. In the second case, the diagnosis of cirrhosis was missed, or HE was not considered in the differential until the patient was referred to a second hospital that re-evaluated the delirious changes.
However, the hope is that with greater education and engagement, we can diagnose MHE and initiate therapy before overt delirium and HE set in.
Summary:
In summary, we describe two cases that presented with parkinsonism and cognitive decline. While parkinsonism suggested PD versus a subcortical dementia, motor symptoms resolved, and the caregivers felt that the patients went back to their baseline mental status once they were adequately treated for HE.
The lack of awareness that hepatic encephalopathy does not always present as an acute delirium but may present initially as an attenuated delirium with mild deficit of attention, sleep disturbance and irritability, and that patients with cirrhosis may have parkinsonism, together with the high prevalence of dementias with aging, may cause delay in diagnosis and management of HE in the older population. HE should be in the differential in older patients with cirrhosis who presents with even mild changes in cognitive function. If the patient does not have a history of cirrhosis but has the risk factors described, the clinician should review the record and, if needed, order labs and imaging to rule out cirrhosis.
The Working Group on Hepatic Encephalopathy recommends several psychometric tests to diagnosis hepatic encephalopathy, including the PHES and RBANS, but these tests are time consuming, most are copyrighted and not practical in a primary care practice38. A simple and quick screening test is the EncephalApp Stroop test (www.encephalapp.com). Another simple test that does not require equipment and is easy to administer is the S-ANT1, If the S-ANT1 is abnormal it can be followed by the complete SLUMS or other cognitive testing to aid in the diagnosis of HE versus other forms of delirium, dementia, or worsening dementia. For patients with known dementia the m-RASS is a simple assessment of alertness, that can help determine whether the patient may have HE or other form of delirium superimposed to dementia. Further studies are needed to delineate the best screening tests and to develop an algorithm to aid in diagnosing HE in older patients and in particularly in those with underlying dementia.
Funding:
Partly supported by VA Merit Review 2I0CX001076 and NCATS R21TR003095 to JSB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None for any author
References:
- 1.Asrani SK, Kouznetsova M, Ogola G, et al. Increasing Health Care Burden of Chronic Liver Disease Compared With Other Chronic Diseases, 2004–2013. Gastroenterology 2018;155:719–729 e4. [DOI] [PubMed] [Google Scholar]
- 2.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 4.Baki JA, Tapper EB. Contemporary Epidemiology of Cirrhosis. Curr Treat Options Gastroenterol 2019;17:244–253. [DOI] [PubMed] [Google Scholar]
- 5.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol 2012;10:1208–19. [DOI] [PubMed] [Google Scholar]
- 6.Stracciari A, Guarino M, Pazzaglia P, et al. Acquired hepatocerebral degeneration: full recovery after liver transplantation. J Neurol Neurosurg Psychiatry 2001;70:136–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetauerova D, Hildebrand P, Scala S, et al. A Prospective Study of the Prevalence of Parkinsonism in Patients With Liver Cirrhosis. Hepatol Commun 2021;5:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019;16:235–246. [DOI] [PubMed] [Google Scholar]
- 9.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204. [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1143–53. [DOI] [PubMed] [Google Scholar]
- 12.Smith AD, Refsum H, Bottiglieri T, et al. Homocysteine and Dementia: An International Consensus Statement. J Alzheimers Dis 2018;62:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jager CA, Oulhaj A, Jacoby R, et al. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 2012;27:592–600. [DOI] [PubMed] [Google Scholar]
- 14.Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 2010;5:e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattison MLP. Delirium. Ann Intern Med 2020;173:ITC49–ITC64. [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 18.Ojagbemi A, Bello T, Owolabi M, et al. Cognitive, Functional, and Mortality Outcomes of Attenuated Delirium Syndrome in Stroke Survivors. J Geriatr Psychiatry Neurol 2021;34:606–612. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guideline P, Chair, et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659–689. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia V, Betrapally NS, Hylemon PB, et al. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep 2016;6:26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahluwalia V, Wade JB, Moeller FG, et al. The etiology of cirrhosis is a strong determinant of brain reserve: A multimodal magnetic resonance imaging study. Liver Transpl 2015;21:1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, Ahluwalia V, Steinberg JL, et al. Elderly patients have an altered gut-brain axis regardless of the presence of cirrhosis. Sci Rep 2016;6:38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen LA, Galasko D. Lewy body disease. Curr Opin Neurol Neurosurg 1992;5:889–94. [PubMed] [Google Scholar]
- 24.K. J. The pathology of parkinsonism. In: Marsden CDFS, ed. Neurology, Vol 7: Movement Disorders London: Buttersworths, 1987:124–165. [Google Scholar]
- 25.Jankovic J. Pathophysiology and clinical assessment of parkinsonian symptoms and signs. Neurological disease and therapy 2003;59:71–108. [Google Scholar]
- 26.Marsden CD. Parkinson’s disease. Lancet 1990;335:948–52. [DOI] [PubMed] [Google Scholar]
- 27.McCleery J, Morgan S, Bradley KM, et al. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev 2015;1:CD010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang HJ, Park SH, Seo M, et al. (18)F-FP-CIT dopamine transporter PET findings in cirrhotic patients with parkinsonism. Neurotoxicology 2018;64:78–84. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj JS, Duarte-Rojo A, Xie JJ, et al. Minimal Hepatic Encephalopathy and Mild Cognitive Impairment Worsen Quality of Life in Elderly Patients With Cirrhosis. Clin Gastroenterol Hepatol 2020;18:3008–3016 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acharya C, Shaw J, Duong N, et al. QuickStroop, a Shortened Version of EncephalApp, Detects Covert Hepatic Encephalopathy with Similar Accuracy within One Minute. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allampati S, Duarte-Rojo A, Thacker LR, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol 2016;111:78–86. [DOI] [PubMed] [Google Scholar]
- 32.Tapper EB, Kenney B, Nikirk S, et al. Animal Naming Test Is Associated With Poor Patient-Reported Outcomes and Frailty in People With and Without Cirrhosis: A Prospective Cohort Study. Clin Transl Gastroenterol 2022;13:e00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campagna F, Montagnese S, Ridola L, et al. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology 2017;66:198–208. [DOI] [PubMed] [Google Scholar]
- 34.Morandi A, Davis D, Bellelli G, et al. The Diagnosis of Delirium Superimposed on Dementia: An Emerging Challenge. J Am Med Dir Assoc 2017;18:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oudewortel L, Joling KJ, Hertogh C, et al. Performance on bedside tests of attention and organized thinking in patients with dementia free from delirium. Int Psychogeriatr 2019;31:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. [DOI] [PubMed] [Google Scholar]
- 37.Chester JG, Beth Harrington M, Rudolph JL, et al. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med 2012;7:450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randolph C, Hilsabeck R, Kato A, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int 2009;29:629–35. [DOI] [PubMed] [Google Scholar]


