Abstract
Gastroparesis is characterized by symptoms suggesting retention of food in the stomach with objective evidence of delayed gastric emptying in the absence of mechanical obstruction in the gastric outflow. This condition is increasingly encountered in clinical practice. These guidelines summarize perspectives on the risk factors, diagnosis, and management of gastroparesis in adults (including dietary, pharmacological, device, and interventions directed at the pylorus) and they represent the official practice recommendations of the American College of Gastroenterology. The scientific evidence for these guidelines was assessed using the Grading of Recommendations Assessment, Development and Evaluation process. When the evidence was not appropriate for Grading of Recommendations Assessment, Development and Evaluation, we used expert consensus to develop key concept statements. These guidelines should be considered as preferred but are not the only approaches to these conditions.
INTRODUCTION
Gastroparesis is a motility disorder characterized by symptoms and objective documentation of delayed gastric emptying of solid food without mechanical obstruction, which should be excluded by imaging studies such as upper gastrointestinal endoscopy or radiology (1,2). The chronic symptoms experienced by patients with gastroparesis may be associated with acute exacerbation of symptoms after oral intake of food; the symptoms include postprandial fullness, nausea, vomiting, and upper abdominal pain.
In 2013, the American College of Gastroenterology (ACG) Guideline on Gastroparesis focused on the state of diagnosis and management at the time including assessment and correction of nutritional state, relief of symptoms, improvement of gastric emptying, and, in patients with diabetes, glycemic control.
Patient nutritional state should be managed by oral dietary modifications and, if oral intake is not adequate, by enteral nutrition via jejunostomy tube or rarely parenteral nutrition. Medical treatment detailed the use of prokinetic and antiemetic therapies including metoclopramide, short term use of erythromycin, and gastric electrical stimulation (GES, approved on a humanitarian device exemption), and, in the presence of unmet clinical need, medications used off-label including domperidone, erythromycin (primarily over a short term), and centrally acting antidepressants used as symptom modulators. Second-line approaches include venting gastrostomy or feeding jejunostomy; the latter may be placed directly by percutaneous endoscopic jejunostomy (3). Modifications in percutaneous endoscopic gastrostomy jejunal feeding tubes have reduced likelihood of retrograde displacement of gastrojejunal tubes and reflux of enteral feed back into the duodenal loop and the stomach. These modifications include suture application on the connector and a balloon transgastric jejunal feeding device (4).
Intra-pyloric botulinum toxin injection was not effective in two randomized, controlled trials (5,6). Partial gastrectomy and pyloroplasty should be used rarely, only in carefully selected patients (7). These procedures have been largely replaced by gastric per-oral endoscopic myotomy (G-POEM), which is discussed in detail in this article.
Gastroparesis carries a substantial patient burden (8–10), with a negative correlation observed between symptom severity and patient quality of life. The disease also has wider impacts on healthcare burden such as increased hospitalizations and associated direct and indirect economic consequences. Several publications have demonstrated increased morbidity and mortality in patients with gastroparesis (11–14). While gastroparesis is known to be associated with use of narcotics in pain syndromes, and opioid agents affect gastric as well as pyloric function resulting in retardation of gastric emptying, this was not an objective of the current review, and is covered in a separate, recently published article (15). Nevertheless, it is important to emphasize that potent opioids were associated with worse gastroparesis (16), and pain associated with gastroparesis should not be treated with opioids (including tramadol and tapentadol which retard orocecal transit and gastric emptying respectively) (17,18). The treatment of pain in gastroparesis was not considered in this guideline; there are essentially no clinical trials addressing the treatment of pain in gastroparesis. However, the review addresses the use of central neuromodulators and cannabis in gastroparesis.
In 2021, members of the European Society of Neurogastroenterology and Motility (ESNM) with expertise in gastroparesis and the United European Gastroenterology (UEG) Federation joined forces for developing comprehensive recommendations on gastroparesis (19). This involved a Delphi consensus processes, systematic literature reviews, and grading of the strengths of accepted criteria. An initial North American perspective of those recommendations has been recently published (20) with endorsement or further commentary on the recommendations by the ESNM working group, as well as commentary based on the published evidence base.
The objective of this new guideline is to document, summarize, and update the evidence and develop recommendations for the clinical management of gastroparesis, updating the 2013 ACG guideline on gastroparesis (Figure 1) (1). It is necessary to acknowledge the limitations of guideline recommendations on therapies in the absence of FDA-approved therapies for gastroparesis in the United States and the limitation in duration of prescription to 3 months for the only currently-approved medication, metoclopramide.
Figure 1.
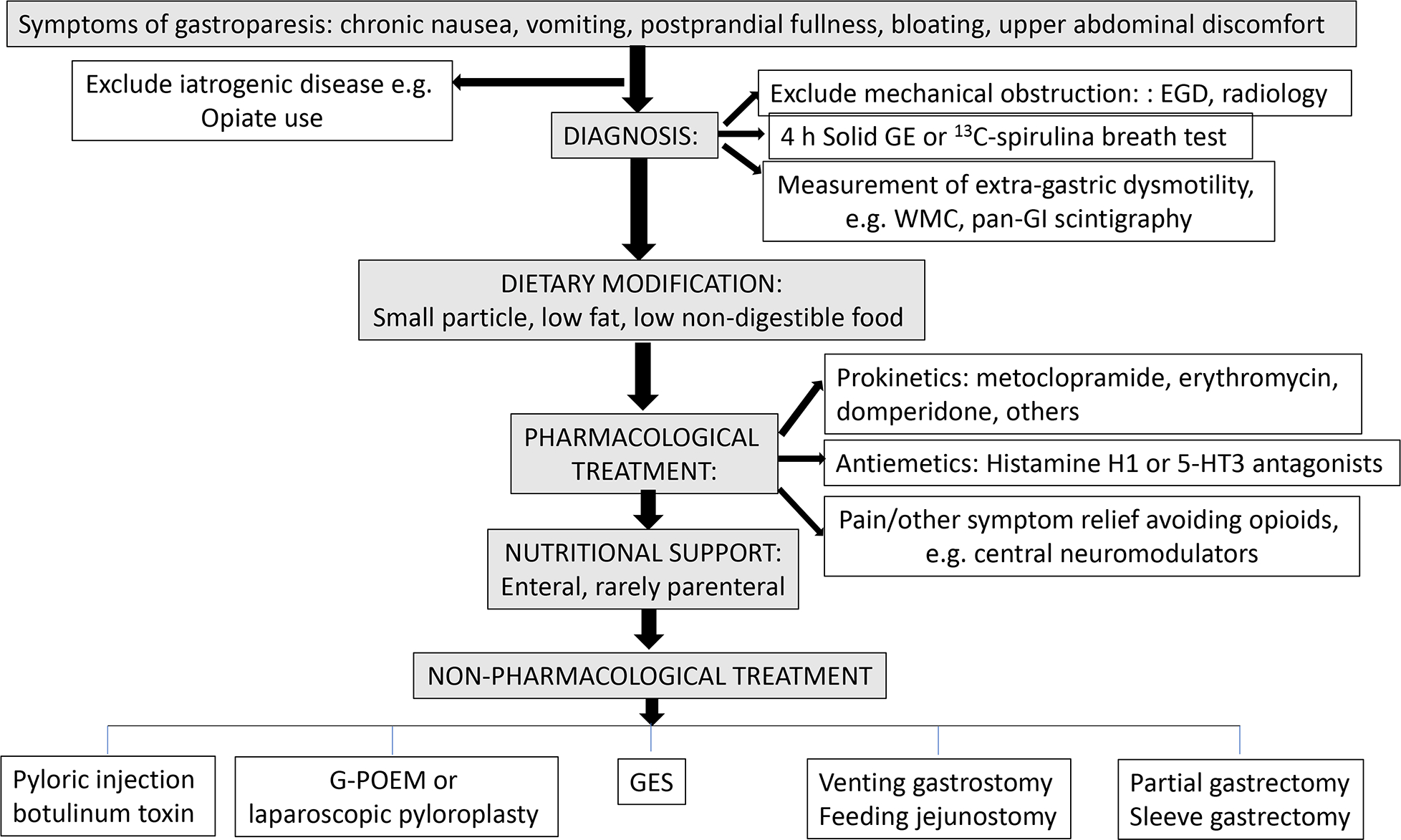
This algorithm updates the algorithm from the 2013 ACG guideline on gastroparesis (1).
ACG guidelines are established to support clinical practice and suggest preferable approaches to a typical patient with a particular medical problem based on the currently available published literature. When exercising clinical judgment, particularly when treatments pose significant risks, health care providers should incorporate this guideline in addition to patient-specific medical comorbidities, health status, and preferences to arrive at a patient-centered care approach.
METHODS
Key Questions
The guideline is framed around several key questions, outlined below. The key questions were developed by the authors and vetted through the ACG leadership. We developed specific questions to address the topics of clinical relevance in the Patient Intervention Comparison and Outcomes (PICO) format (see Supplemental Materials). Emphasis has been placed on having practical recommendations that would be helpful for practicing providers in the US. A broad literature search was conducted to document, by means of detailed tables, information pertaining to the PICO questions, followed by a focused evaluation of the most relevant literature to develop recommendations (Table 1).
Table 1.
Gastroparesis Recommendations
| Recommendation | GRADE Level of Evidence | Strength of Recommendation | |
|---|---|---|---|
| Risk Factors | |||
| 1. | In patients with diabetic gastroparesis, optimal glucose control is suggested to reduce the future risk of aggravation of gastroparesis. | Low | Conditional |
| Diagnostic Testing | |||
| 2. | Scintigraphic gastric emptying assessment is the standard test for the evaluation of gastroparesis in patients with upper GI symptoms. The suggested method of testing includes appraising the emptying of a solid meal over a duration of 3 hours or greater. | Moderate | Strong |
| 3. | Radiopaque markers testing is not suggested for the diagnostic evaluation of gastroparesis in patients with upper GI symptoms. | Very Low | Conditional |
| 4. | Wireless motility capsule testing may be alternative to the scintigraphic gastric emptying assessment for the evaluation of gastroparesis in patients with upper GI symptoms. | Low | Conditional |
| 5. | Stable isotope (13C-spirulina) breath testing is a reliable test for the evaluation of gastroparesis in patients with upper GI symptoms. | Low | Conditional |
| Management | |||
| 6. | Dietary management of gastroparesis should include a small particle diet to increase likelihood of symptom relief and enhanced gastric emptying. | Low | Conditional |
| 7. | In patients with idiopathic and diabetic gastroparesis, pharmacologic treatment should be considered to improve gastric emptying and gastroparesis symptoms, taking into account benefits and risks of treatment. | Low | Conditional |
| 8. | In patients with gastroparesis, we suggest treatment with metoclopramide over no treatment for management of refractory symptoms | Low | Conditional |
| 9. | In patients with gastroparesis where domperidone is approved, we suggest use of domperidone for symptom management | Low | Conditional |
| 10. | In patients with gastroparesis, we suggest use of 5HT4 agonists over no treatment to improve gastric emptying | Low | Conditional |
| 11. | In patients with gastroparesis, use of antiemetic agents is suggested for improved symptom control, however, these medications do not improve gastric emptying. | Low | Conditional |
| 12. | Central neuromodulators are not recommended for management of gastroparesis. | Moderate | Strong |
| 13. | Current data do NOT support the use of ghrelin agonists for management of gastroparesis. | Moderate | Strong |
| 14. | Current data do NOT support the use of haloperidol for treatment of gastroparesis. | Low | Conditional |
| 15. | Gastric electric stimulation (GES) may be considered for control of gastroparesis (GP) symptoms as a humanitarian use device (HUD) | Low | Conditional |
| 16. | Acupuncture alone or acupuncture combined with prokinetic drugs may be beneficial for symptom control in patients with diabetic gastroparesis. Acupuncture cannot be recommended as beneficial for other etiologies of gastroparesis. | Very Low | Conditional |
| 17. | Herbal therapies such as Rikkunshito or STW5 (Iberogast) should NOT be recommended for treatment of gastroparesis. | Low | Conditional |
| 18. | In patients with gastroparesis, EndoFLIP evaluation may have a role in characterizing pyloric function and predicting treatment outcomes following peroral pyloromyotomy. | Very Low | Conditional |
| 19. | Intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized controlled trials. | Moderate | Strong |
| 20. | In patients with gastroparesis with symptoms refractory to medical therapy, we suggest pyloromyotomy over no treatment for symptom control. | Low | Conditional |
Literature Search
In February and March 2019, comprehensive literature searches were conducted by two health sciences librarians (JP and VMV) in PubMed (MEDLINE), EMBASE, and the Cochrane Library databases. Key concepts from the PICO questions were used to develop search terms and translated to appropriate controlled vocabulary for each database; detailed strategies for each section are provided in Appendix 1. Results for all searches were filtered for English language publications, and searches regarding therapeutics were further limited to human populations. Searches were updated in May 2021 using the same criteria to capture literature published during the screening and review process. A hand search of references was conducted, and relevant publications identified by content experts were incorporated for analysis.
Screening
Between February 2019 and July 2021, a team of five content experts (DA, TA, MC, BK, LN) screened a total of 1908 distinct references retrieved by the original and updated searches.
Each reference was screened independently by no fewer than two reviewers, with a third reviewer resolving any conflicts. The inclusion criteria were original research studies on the incidence, diagnosis, and treatment of gastroparesis in adult populations, predominantly based on observational studies and randomized, controlled trials. Open-label and observational studies of treatment modalities were included in the tables. Exclusion criteria were inclusion in the previous ACG guideline (although, where relevant, these were included in tables for completeness of the literature surveyed), theoretical studies using computational models, animal trials, pediatric populations, and publications without original data analysis.
While no restriction was placed on publication dates during the retrieval process, emphasis was placed during screening by content experts on studies published after the searches included in the previous guideline, and tables from the 2013 guideline were updated with more recent evidence from the literature. Similarly, searches were not limited by age range within the databases, but any retrieved studies on an exclusively pediatric population were manually excluded during screening. Review articles, correspondence, and other publications without original data were excluded from analysis, though relevant reviews were retained for hand search of their included references.
After screening, a total of 121 references were identified for inclusion and progressed for evidence appraisal in July 2021.
Assessment
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) process (Table 2) (21) was used to assess the quality of evidence for each question, by two formally trained GRADE methodologists (RHY & KG) to evaluate the quality of the evidence and strength of the recommendations. The quality of evidence is expressed as high (we are confident in the effect estimate to support a particular recommendation), moderate, low, or very low (we have very little confidence in the effect estimate to support a particular recommendation) based on the risk of bias of the studies, evidence of publication bias, heterogeneity among studies, directness of the evidence and precision of the estimate of effect. A strength of recommendation is given as either strong (noted as “recommendations,” and meaning that most patients should receive the recommended course of action) or conditional (noted as “suggestions,” and meaning that many patients should have this recommended course of action, but different choices may be appropriate for some patients) based on the quality of evidence, risks versus benefits, feasibility, and costs, taking into account perceived patient and population-based factors. Furthermore, a narrative evidence summary for each section provides important details for the data supporting the statements. The panel have additionally highlighted “key concepts” that were not included in the GRADE assessment. Key concepts are statements to which the GRADE process has not been applied and often include definitions and epidemiological statements rather than diagnostic or management recommendations.
Table 2.
GRADE quality criteria (GRADE=Grading of Recommendations Assessment, Development and Evaluation) (21)
| Study Design | Quality of Evidence | Reduced Factors | Increased Factors |
|---|---|---|---|
| Randomized trials | High | Risk of bias | Large effect |
| −1 serious | +1 large | ||
| −2 very serious | +2 very large | ||
| Moderate | Inconsistency | Dose response | |
| −1 serious | +1 if gradient | ||
| −2 very serious | |||
| Indirectness | Confounding | ||
| −1 serious | +1 | ||
| −2 very serious | |||
| Observational studies | Low | Imprecision | |
| −1 serious | |||
| −2 very serious | |||
| Very low | Publication bias | ||
| −1 likely | |||
| −2 very likely |
NARRATIVE REVIEW OF EVIDENCE
Risk Factors
Recommendation.
1. In patients with diabetic gastroparesis, optimal glucose control is suggested to reduce the future risk of aggravation of gastroparesis. (conditional recommendation, low level of evidence).
Optimal glucose control reduces the future risk of aggravation of the gastroparesis.
Acute hyperglycemia delays gastric emptying in patients with diabetes and, in the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study, delayed gastric emptying was associated with gastrointestinal symptoms and with measures of early and long-term hyperglycemia (22). However, it was unknown if better glycemic control increases the risk of hypoglycemia or improves hemoglobin A1c levels and gastrointestinal symptoms in diabetic gastroparesis.
Continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) were assessed in 45 poorly controlled type 1 or 2 patients with diabetes and gastroparesis (20). Symptom scores decreased with lower nausea/vomiting, fullness/early satiety, and bloating/distention scores as well as quality-of-life scores, and volumes of liquid nutrient meals tolerated increased at 24 weeks. In conclusion, CSII plus CGM appear to be safe with minimal risk of hypoglycemic events and associated improvements in glycemic control, gastroparesis symptoms, quality-of-life, and meal tolerance in patients with poorly controlled diabetes and gastroparesis. This study supports the safety, feasibility, and potential benefits of improving glycemic control in diabetic gastroparesis (23). On the other hand, after 6 months of intensive therapy which led to decreased levels of glycosylated hemoglobin (from mean 10.6±0.3% to 9±0.4%), gastric emptying (GE) T1/2 did not change (24). Nevertheless, Izzy et al. (25) documented that HbA1C level is significantly associated with the 4-hour retention value on nuclear GE scan.
Diagnostic Testing
After exclusion of mechanical obstruction, diverse tests are available to objectively document the presence of delayed GE. The gold standard is scintigraphic gastric emptying (SGE); this section addresses the diverse methods available for diagnosis of gastroparesis.
Recommendation.
2. Scintigraphic gastric emptying is the standard test for the evaluation of gastroparesis in patients with upper GI symptoms. The suggested method of testing includes appraising the emptying of a solid meal over a duration of 3 hours or greater. (strong recommendation, moderate level of evidence)
Optimal duration of gastric emptying tests.
It is customary to recommend cessation for 48 hours prior to the test of medications including opioids, cannabinoids, prokinetics, antiemetics, and neuromodulators with potential impact on the results of the GE test.
Based on a systematic review and meta-analysis (26) of the literature from 2007 to 2017 that included studies evaluating the association between GE (in 92 studies: 26 breath test, 62 scintigraphy, 1 ultrasound and 3 wireless motility capsule) and nausea, vomiting, early satiety/postprandial fullness, abdominal pain and bloating, 25 studies provided quantitative data for meta-analysis (15 scintigraphy studies enrolling 4056 participants and 10 breath test studies enrolling 2231 participants). Meta-regression demonstrated a significant difference between optimal and suboptimal GE test methods when comparing delayed GE with nausea and vomiting. Studies using optimal GE test methodology (that is solid meal and at least 3 hours of data collection) showed significant associations between GE and nausea (OR: 1.6; 95% CI: 1.4 to 1.8), vomiting (OR: 2.0; 95% CI: 1.6 to 2.7), abdominal pain (OR: 1.5; 95% CI: 1.0 to 2.2), and early satiety/fullness (OR: 1.8; 95% CI: 1.2 to 2.6) for patients with upper gastrointestinal symptoms. Among patients with diabetes, the most significant association with delayed GE was with the symptom of early satiety and fullness, but not with nausea and vomiting (26). Therefore, systematic review and meta-analysis supports an association between optimally measured delayed gastric emptying and upper gastrointestinal symptoms. It is worth noting that scintigraphic assessment should be ideally performed up to 4 hours unless it is documented that more than 90% of the solid meal has emptied at 3 hours (27).
Potential Confounding between Gastroparesis and Functional Dyspepsia
There is increasing attention (28) to the possibility that gastroparesis and functional dyspepsia (FD) may be on a spectrum of gastric dysfunction. Despite generally unaltered symptoms over time, 42% of patients initially diagnosed with gastroparesis and 37% of those diagnosed with FD were reclassified based on presence or absence of GE delay on repeat SGE (28). Degree of impairment of GE may vary over time in patients whose symptoms are generally unaltered over the same time. However, it is also conceivable that part of the overlap of the syndromes reflects the cut–off value of 10% retention at 4 hours that is applied to identify patients with delayed GE based on the ingestion of a 255 kilocalorie, 2% fat Eggbeaters® meal. Further studies are required to appraise the optimal meal composition and cut-off to define normality to address the reported significant overlap between gastroparesis and FD, which may be confounded by the low calorie and fat content of the meal and the use of >10% retention at 4 hours to define delayed gastric emptying. It has been emphasized that the distinction between the two diagnoses is relevant because of the better prognosis of FD in contrast to the persistence of gastroparesis (28).
Diagnosis of gastroparesis using scintigraphy
Recommendation.
3. Radiopaque markers testing is not suggested for the diagnostic evaluation of gastroparesis in patients with upper GI symptoms. (Conditional recommendation, very low level of evidence)
Compared to radiopaque markers (ROM).
There is evidence that GE is accelerated similarly by rectal or oral cisapride when measured by scintigraphy and by ROM (29,30). Several lines of evidence (31,32) suggest that scintigraphy, when compared to ROM, is more accurate in assessing the emptying of the digestible solid food from the stomach. For example, Olausson et al. (32) documented sensitivity and specificity of the ROM test was 34% and 97%, respectively and in contrast to results from scintigraphy which correlate with GI symptom severity, results from ROM test did not. Given that scintigraphy is the gold standard, it is not possible to assess sensitivity and specificity of ROM; however, it is important to acknowledge that the inter-subject coefficients of variation (COVinter) for scintigraphic GE T1/2 were similar in males and females (total 319 healthy controls), overall 24.5% (M 26.0%, F 22.5%), and COVinter for GE at 4 hours was 9.6%. The COVintra in 47 healthy controls for T1/2 and GE at 4 h were 23.8% and 12.6% (33). Similarly, the mean absolute differences in 60 patients with upper GI symptoms undergoing repeat GE studies by scintigraphy an average of 15 days apart were 25 minutes for GE T1/2 and 7% at 1h, 9% at 2h, and 7% at 4h (34).
Recommendation.
4. Wireless motility capsule testing may be an alternative to the scintigraphic gastric emptying assessment for the evaluation of gastroparesis in patients with upper GI symptoms. (conditional recommendation, low quality of evidence)
Compared to wireless motility capsule (WMC).
The results from measurements by SGE and WMC differ. Overall agreement in results between the two methods was 75.7% (kappa=0.42). In subjects without diabetes, the WMC detected a higher proportion of subjects with delayed GE (33.3%) than SGE (17.1%) (P<.001); in contrast, a higher proportion of subjects with diabetes had delayed GE detected by SGE (41.7%) than by WMC (17.1%) (P=.002). Severe delays in GE were observed in a higher proportion of subjects by WMC (13.8%) than by SGE (6.9%) (P=.02). Rapid GE was detected in a higher proportion of subjects by SGE (13.8%) than by WMC (3.3%) (P<.001) (35,36). Research supports WMC testing as an alternative test to SGE for the evaluation of gastroparesis in patients with upper GI symptoms, and one advantage is that it provides a measure of gastric contractile amplitude and this can correspond to the timing of capsule emptying documented by the change in pH measured as the capsule traverses the pylorus.
These features underscore the differences in emptying of a solid meal that could be homogenized in the stomach from the emptying of a solid nondigestible capsule which is greater than 1.5 cm in length and which typically empties from the stomach with the reestablishment of the interdigestive migrating motor complex after the emptying of a meal (37); the capsule is able to provide information about the amplitude of pressure activity in the stomach and small bowel which may be relevant, for example to identify myopathic diseases of the gut or severe antral hypomotility or disorders of motility affecting other regions of the gut such as the small bowel or colon (38). However, overall gastroparesis symptoms and nausea/vomiting, early satiety/fullness, bloating/distention, and upper abdominal pain subscores showed no relation to WMC transit (38).
Transit delays beyond the stomach were found in 45.6% of patients with suspected gastroparesis who underwent WMC testing: 22.8% small bowel, 31.5% colonic and 5.4% global (35). Such extragastric dysmotility may be considered in patients with symptoms of gastroparesis; indeed, up to 64.7% of patients with symptoms of gastroparesis have been found to have slow transit constipation by ROM study (39), and, among 149 patients evaluated at a single tertiary referral center, 77 (52%) had rectal evacuation disorders, and 21 patients (15%) with delayed colonic transit associated with slow ascending colon emptying halftime in 9 and delayed colonic transit due to evacuation disorder in 12 patients (40). The WMC, as with pan-gastrointestinal scintigraphy, provides opportunity to appraise motor function through the entire GI tract (38,41) which may be indicated in patients with gastrointestinal symptoms.
Compared to intra-gastric food identified on upper GI endoscopy.
Retained gastric food (RGF) is frequently identified during esophagogastroduodenoscopy (EGD); however, this should not be deemed to be diagnostic of gastroparesis. In a retrospective study of 85,116 EGDs, 2991 patients without structural abnormalities had undergone SGE using a standard 320kcal 30% fat egg meal. Overall, the positive predictive value (PPV) of RGF for delayed GE was 55%. However, the PPV varied from 32% in patients without risk factors to 79% in patients with type 1 diabetes. Opioids, cardiovascular medications, and acid suppressants were associated with RGF (42). Therefore, the presence of RGF should not be assumed to be diagnostic of gastroparesis, and confounding by medications should be excluded in such patients.
Diagnosis of gastroparesis using stable isotope breath test and comparison with scintigraphy
Recommendation.
5. Stable isotope (13C-spirulina) breath test is a reliable test for the evaluation of gastroparesis in patients with upper GI symptoms. (conditional recommendation, low quality of evidence)
The stable isotope gastric emptying breath test (GEBT) using 13-carbon spirulina has been validated in simultaneous measurements performed with the gold standard scintigraphy and a solid test meal. This has been validated both in patients with upper gastrointestinal symptoms and healthy controls as well as in pharmacologically induced slowing or acceleration of GE (43,44). Though the kappa statistic is not provided, a validation study of 38 healthy volunteers and 129 patients with clinically suspected delayed GE showed that, at 80% specificity, the 45- and 180-minute samples combined were 93% sensitive to identify accelerated GE, and 150- and 180-minute combined were 89% sensitive for delayed GE (43). The test is also approved for use in children.
Additional value of gastric function tests that do not measure emptying, including electrogastrography (EGG)
There are the three types of cutaneous electrogastrography (EGG): 1. Single channel, 2. Low-resolution, and 3. high resolution. They all measure different aspects of gastric electrical activity. In addition, both mucosal and serosal electrical measurements of EGG are also performed. Single channel cutaneous EGG measures only frequency; low resolution EGG measures frequency and amplitude and some measures of propagation; high resolution EGG measures frequency, amplitude, and more precise measures of propagation such as initiation and conduction of gastric electrical signals. The prevalence of 3 cycle per minute (cpm) electrical control activity measured by single channel EEG was more prevalent in patients with gastric outlet obstruction compared to patients with idiopathic gastroparesis (IG) or healthy controls (45). High-amplitude and excessively regular 3 cpm EGG patterns were identified in gastric outlet obstruction, whereas high-amplitude and excessively regular 3 cpm EGG patterns differentiated idiopathic gastroparesis (IG) and healthy controls and were more likely in those with delayed GE (45,46) and in patients with cyclical vomiting and diabetic gastropathy (47) including uremic diabetics and children with diabetes (48,49). In another study, patients with depleted interstitial cells of Cajal (ICC) (50) had significantly more tachygastria and significantly greater total symptom scores compared to those patients whose gastric full-thickness biopsies showed less ICC depletion.
Using high-resolution electrical mapping (256 electrodes; 36 cm2) (51), it was shown that 9 patients with chronic unexplained nausea and vomiting had slow-wave dysrhythmias, with only 1 of 9 controls showing these dysrhythmias. Dysrhythmias included abnormalities of initiation (stable ectopic pacemakers, unstable focal activities) and conduction (retrograde propagation, wavefront collisions, conduction blocks, and re-entry) across slow, normal, or fast frequencies; dysrhythmias also showed velocity anisotropy (mean, 3.3 mm/s longitudinal vs 7.6 mm/s circumferential; P <.01). Such high resolution, spatial mapping is recommended, especially because of the evidence that abnormalities of slow-wave initiation aberrant conduction and low amplitude activity in gastroparesis often occur at normal frequency, which could be missed by tests that lack spatial resolution (52).
In summary, studies suggest a complimentary role of spatial mapping EGG for identification of the pathophysiologic mechanism of gastric function (53). However, at this time, it is unclear that the information is clinically meaningful. Ongoing research of high-resolution EGG should help clarify its clinical role, including its role in patients with FD.
Other Tests for Gastroparesis Based on Full-Thickness Biopsies
The evidence regarding changes at the level of the stomach as identified in histological and molecular studies performed on biopsies taken from patients with gastroparesis are detailed in the Supplement. Similar to the European Society of Neurogastroenterology and Motility (ESNM) Consensus Statement (19), we do not recommend the routine use of full-thickness biopsies. Full-thickness biopsies should be reserved for research purposes to help better understand the causes of gastroparesis, identify biomarkers, guide therapy, and predict outcomes.
MANAGEMENT OF GASTROPARESIS
Small particle diet and nutrition interventions
Recommendation.
6. Dietary management of gastroparesis should include a small particle diet to increase likelihood of symptom relief and enhance GE. (conditional recommendation, low quality of evidence)
Avoidant/restrictive food intake disorder symptoms are frequent in patients with gastroparesis (54), and the ESNM guidelines recommend that eating disorders must be considered in patients with gastroparesis (19).
After the pioneering randomized, controlled trial by Olausson et al. (55) demonstrated efficacy of small particle diet compared to normal diet for relief of symptoms, improving GE and enhancing glycemic control (56) in patients with diabetes, a systematic review (57) of all study types evaluated current evidence-based nutrition interventions involving a total of 15 studies and of 524 subjects, using a stepwise process, progressing from oral nutrition to jejunal nutrition and lastly to parenteral nutrition. Small particle, low-fat diets were significantly better tolerated than the converse, with jejunal nutrition prior to consuming oral food significantly improving oral intake and motility. In more progressive cases, percutaneous endoscopic gastrostomy with jejunal extension nutrition had lower reported symptoms than other enteral routes. Exclusive long-term parenteral nutrition is a feasible option for advanced cases, with a 68% survival rate at 15 years duration, though oral intake plus parenteral nutrition is associated with higher survival rates. The primary role of maintaining or reinstating oral intake was recommended to reduce morbidity and mortality risk.
Pharmacologic agent use in gastroparesis
Recommendation.
7. In patients with idiopathic and diabetic gastroparesis, pharmacologic treatment should be considered to improve GE and gastroparesis symptoms, considering benefits and risks of treatment. (conditional recommendation, low quality of evidence)
8. In patients with gastroparesis, we suggest treatment with metoclopramide over no treatment for management of refractory symptoms. (conditional recommendation, low quality of evidence)
9. In patients with gastroparesis where domperidone is approved, we suggest use of domperidone for symptom management. (conditional recommendation, low quality of evidence)
10. In patients with gastroparesis, we suggest use of 5–HT4 agonists over no treatment to improve gastric emptying. (conditional recommendation, low quality of evidence)
The two medications with the largest number of individual clinical trials for gastroparesis are metoclopramide and domperidone.
Metoclopramide is the only U.S. FDA-approved medication for the treatment of gastroparesis. The FDA placed a Black-Box warning on metoclopramide because of the risk of side effects, including tardive dyskinesia. The efficacy of metoclopramide in the treatment of diabetic gastroparesis (DG) has been assessed in studies that are summarized in Table 3 (58–68) which include newer trials involving the intra-nasal formulation of metoclopramide. The most common adverse effects of metoclopramide nasal spray were dysgeusia (bad, metallic, or bitter taste), headache, and fatigue.
Table 3.
Trials of metoclopramide for gastroparesis
| Design | N, Etiology | Dose p.o. | Duration | Results | Reference |
|---|---|---|---|---|---|
| DB, PC, PG RCT | 28 patients: 5 DG, 4 vagotomy and pyloroplasty, and 19 IG | 10mg qid | 3 wk | Symptomatic benefit vs. placebo: mean TSS for metoclopramide:18.4 pre to 7.2 post-study; for placebo, 19.1 pre-to 12.9 post-study | Perkel 1979, ref. 58 |
| DB, PC, PG RCT | 55 patients: 21 vagotomy and drainage, 5 DM, 29 IG delayed GE | 10mg qid | 3 wk | Metoclopramide significantly decreased symptom scores of surgical and idiopathic patients | Perkel 1980, ref. 59 |
| DB, PC, XO, RCT | 10 DM | 10mg qid | 3 wk/arm | Improved symptoms and vomiting; ∼60% acceleration in GE liquid 150kcal meal | Snape 1982, ref. 60 |
| DB, PC, PG, RCT | 28: 5 DG, 4 PS, 19 IG | 10mg qid | 3 wk | Improved symptoms by 29% | Perkel 1979, ref. 58 |
| PC, RCT | 18 DG | 10mg qid | 3 wk | Improved symptom score by 29%, and GE by 25% | McCallum 1983, ref. 61 |
| DB, PC, XO, RCT | 13 DM with GE accelerated by i.m. metoclopramide | 10mg qid | 3 wk/arm | Improved symptoms with mean reduction of 52.6% | Ricci 1985, ref. 62 |
| DB, RCT | 45 diabetic, domperidone-controlled multicenter trial | 10mg qid | 4 wk | Improved symptoms by 39%; similar efficacy with domperidone which had less AEs | Patterson 1999, ref. 63 |
| DB, XO, RCT | 13 DG; erythromycin-controlled | 10mg tid | 3 wk/arm | Both treatments accelerated GE compared to baseline, and improved symptoms score | Erbas 1993, ref. 64 |
| Open | 1 diabetic | 15mg qid | 6 months | Improved symptoms, GE liquids, antral contraction frequency | Longstreth 1977, ref. 65 |
| Open | 10 GI symptomatic T1DM, 6 asymptomatic T1DM, 18 HC | 10mg i.v. | Single dose | Improved GE solids | Loo 1984, ref. 66 |
| Open, PG, RCT | 89 T1DM or T2DM gastroparesis | 10, 20mg spray or 10 mg tab qid | 6 weeks | Nasal 10 and 20 mg had lower TSS compared to oral 10 mg group; More side effects, especially nausea with oral | Parkman 2014, ref. 67 |
| DB, PC, PG, RCT | 285 T1DM 1 or T2DM with delayed GE or nausea and vomiting. | 10 or 14mg nasal spray qid | 4 weeks | Gastroparesis symptom scores were reduced significantly in female subjects, not in males. Adverse effects: dysgeusia, headache, and fatigue. | Parkman 2015, ref. 68 |
(Updated from ref. 1, Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical Guideline: Management of Gastroparesis. Am J Gastroenterol 2013;108:18–37); GE=gastric emptying; T1DM = type 1 diabetes mellitus AE=adverse event; DB=double-blind; DG=diabetic gastroparesis; DM=diabetic; GE=gastric emptying; GI=gastrointestinal; HC=healthy controls; IG=idiopathic gastroparesis; NA=not available; PC=placebo- controlled; PG=parallel group; PS=post-surgical gastroparesis; RCT=randomized controlled trial; T1DM=type 1 diabetes mellitus; T2DM=type 2 diabetes mellitus; TSS=total symptom score; XO=crossover
Regulatory authorities issued restrictions and recommendations regarding long-term use of metoclopramide at oral doses exceeding 10 mg 3–4 times daily because of the risk for development of tardive dyskinesia; the restrictions include use for <12 weeks and age <65 years. Studies in the last decade have addressed the risk of tardive dyskinesia in contrast to reversible involuntary movements on treatment with metoclopramide. First, the relative risk (69) of tardive dyskinesia in metoclopramide users in a VA medical center was not significantly greater than in non-user controls (RR: 1.67; 95% CI: 0.93 to 2.97). Second, it was estimated that the risk of tardive dyskinesia from metoclopramide use is likely to be <1% (70). The most comprehensive assessment (71) showed that the risk of tardive dyskinesia from metoclopramide is in the range of 0.1% per 1000 patient years, below a previously estimated 1%–10% risk suggested in treatment guidelines by regulatory authorities. High-risk groups are elderly females, diabetics, patients with liver or kidney failure, and patients with concomitant antipsychotic drug therapy which reduces the threshold for neurological complications.
The FDA package insert on metoclopramide specifies that restlessness, drowsiness, fatigue, and lassitude occurred in approximately 10% of patients who received 10 mg four times daily. No other quantitative data are provided in the FDA approved insert on the prevalence of other, reversible central nervous system disorders with metoclopramide. One study (72) that documented the epidemiology of extrapyramidal reactions to metoclopramide was studied by examining reports in the Adverse Reactions Register of the Committee on the Safety of Medicines in the United Kingdom in the period 1967–82. Out of an estimated 15.9 million prescriptions, there were 479 reports of extrapyramidal reactions (455 of dystonia-dyskinesia, 20 of parkinsonism, and 4 of tardive dyskinesia). A more recent study of metoclopramide adverse events in the FDA Adverse Event Reporting System (FAERS) for the period 2004–2010 yielded reports of 4,784 neurological reactions and 944 reports were for tardive dyskinesia; the total number of prescriptions was almost 40.5 million (73). These data suggest that 0.1% of prescriptions are associated with non-tardive dyskinesia neurological symptoms, which seem to be low estimates and may reflect the fact that medication cessation with reversal of the neurological symptoms may not be reported to regulatory agencies.
Domperidone is available for treatment of gastroparesis under a special program administered by the Food and Drug Administration. Table 4 provides a summary of clinical trials with domperidone (74–86). Domperidone has been tested in studies that involved patients with IG, DG, or post-surgical gastroparesis (PSG), and it has been associated with symptom improvement manifested as lower overall scores or reduction in frequency and intensity of symptoms of gastroparesis. Four studies have also documented acceleration of GE compared to control or baseline.
Table 4.
Summary of clinical trials with domperidone
| Type of Study | N, etiology | Dose | Duration | Symptom improvement vs. baseline (OPEN) or vs. placebo (RCT) | Δ Gastric emptying | Adverse effects | Reference |
|---|---|---|---|---|---|---|---|
| Open, po | 3 DM | 10mg qid | 1 wk | Yes, not quantified | Improved, not quantified | NA | Watts 1985, ref. 74 |
| Open, po | 12 IG, 3 DM, 2 PS | 20mg qid | 48 mo | 68.3% (P < 0.05) | 34.5% (P <.05) | ↑ prolactin (100%), symptoms (17.6%) | Soykan 1997, ref. 75 |
| Retrospective, p° | 57 DM | Max. dose 80mg/day | 377 days | 70% patients improved | NA | 16% | Kozarek 1990, ref. 76 |
| Open, | 6 DM | 20mg qid | 6 mo | 79.2% (P < 0.01) | 26.9% (NS) | NA | Koch 1989, ref. 77 |
| Open | 12 DM | 20mg tid | Single oral dose 40mg | chronic oral administration 20mg tid (35–51 days) reduced symptoms | ↑ solid and liquid emptying | NA | Horowitz 1985, ref. 78 |
| RCT, PG, PC, withdrawal study | 208 DM | 20mg qid | 4 wk | 53.8% lower overall score with domperidone (P = 0.025) | NA | 2–3% ↑ prolactin, similar to placebo | Silvers 1998 ref. 79 |
| RCT, PC, XO + open label 1yr | 13 DM | NA | 8 wk | ↓ in symptom frequency and intensity (P < 0.03); symptomatic improvement averaging >1y | NA | NA | Braun 1989, ref. 80 |
| RCT, PC, XO | 6 DM | 10mg i.v. | Single | NA | ↑ homogenized solid emptying | NA | Heer 1983, ref. 81 |
| RCT, PC, XO cisapride (C) or DOM (D) | 8 IG; 3 DM | 0.8mg/kg (C) tid or 0.9mg/kg (D) tid | 4 wk | No overall benefit over placebo; 2 of 3 DM improved | NA | Gas pains, skin rash | Franzese 2002, ref. 82 |
| RCT, PC, XO | 11 upper GI distress: 3 DM + severe gastric retention | 10mg qid | 4 wk each Rx | 2/3 diabetics improved with DOM Rx; among total 11 patients, no superiority of DOM over placebo | NA | Abdominal gas pains, skin rash, itching, sweating, dizziness, constipation | Nagler 1981, ref. 83 |
| RCT, PG, DOM vs. metoclopramide | 93 DM | DOM 20mg qid; metoclopramide 10 mg qid | 4 wk | 41.19% improved vs. baseline (NA); NS vs. metoclopramide | NA | Somnolence 49% metoclopramide, 29% DOM | Patterson 1999, ref. 84 |
| RCT, PG, PC in second phase among initial responders over 4weeks | 208 DM responders to initial single-blind treatment with same dose | 20mg domperidone qid | 4 wk | Symptom severity increased in both groups, worse with placebo. For HRQOL (SF-36), improvement in physical component score, borderline in physical functioning, but no difference in 7/8 other HRQOL subscales | NA | Not reported in study | Farup 1998, ref. 85 |
| Cohorts in NIH gastroparesis consortium (63% IG) | 181 in DOM group, 567 in non-DOM group | Not standardized | Up to 96 weeks | DOM patients: moderate but significantly more improvement in gastroparesis outcomes: GCSI, nausea, fullness, upper abdominal pain, GERD scores, and PAGI-QOL | NA | No significant cardiovascular or other DOM-related complications | Sarosiek 2021, ref. 86 |
(Reproduced from ref. 1, Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical Guideline: Management of Gastroparesis. Am J Gastroenterol 2013;108:18–37) DM=diabetic; DOM=domperidone; GCSI=Gastroparesis Cardinal Symptom Index; GERD=gastroesophageal reflux disease; GI=gastrointestinal; HR-QOL=health-related quality of life; IG=idiopathic gastroparesis; NA=not available; NS=not significant; PC=placebo-controlled; po=oral; PAGI-QOL=Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life; PG=parallel-group; PS=post-surgical gastroparesis; RCT=randomized, controlled trial; Rx=treatment; XO=crossover
Table 5 summarizes efficacy of other prokinetic agents (5-HT4 and ghrelin receptor agonists) on symptoms or GE (64,87–100). As a group of medications, prokinetics have the most substantive clinical trials, and overall evidence suggests that they provide symptomatic benefit. For all the medications, the recommendation is conditional for use of treatment over no treatment to improve gastric emptying. The methodological assessment for the 5-HT4 agonists concluded that there was inconsistent data for symptom improvement.
Table 5.
Summary of efficacy of other prokinetic agents (5-HT4 and ghrelin receptor agonists) on symptoms or gastric emptying (GE)
| Medication/trial design | N, Etiology | Dose (p.o.) | Duration | Efficacy | Reference |
|---|---|---|---|---|---|
| 5-HT4 agonists | |||||
| Clebopride PC, DB, RCT | 76 with dyspeptic syndromes and x-ray proven delayed GE | 0.5 mg tid | 3 months | Clebopride was more effective than placebo in reducing or relieving symptoms | Bavestrello 1985, ref. 87 |
| Prucalopride PC, DB, XO, RCT | 13 DM, 2 connective tissue disease | 4mg/day | Two 4-wk treatments with 2 wks washout | GE faster on prucalopride; GCSI scores were lower than baseline but not different between treatment arms. Meal-related symptom scores over time or cumulative score were not significantly different between groups. GE was more rapid in the prucalopride treatment period, | Andrews 2021, ref. 88 |
| Prucalopride PC, DB, XO, RCT | 28 IG, 6 DG | 2mg/day | Two 4-wk treatments with 2 wks washout | Prucalopride significantly improved the total GCSI, subscales of fullness/satiety, nausea/vomiting, and bloating/distention, overall PAC-QOL score and gastric emptying T1/2; also all efficacies were shown only in the idiopathic group | Carbone 2019, ref. 89 |
| Revexepride: PG, DB, PC, stratified, repeated dose RCT | 62 non-DM; 30 DM (55 female, 37 male); gastroparesis symptoms, and slower baseline GEBT T1/2 in placebo group | 0.02, 0.1, or 0.5 mg tid | 4 weeks | Large inter-individual differences in GEBT with no significant treatment effect; GCSI and PAGI-SYM scores decreased at Week 2 and decreased further at Week 4 in all groups including placebo. Quality of life improved in all treatment groups after 4 weeks of treatment. | Tack et al 2016, ref. 90 |
| Velusetrag: DB, PC, RCT; 3-period XO | 18 DG, 16 IG | 5, 15 or 30 mg po daily | 7 days each period | GE T1/2 numerically reduced with all 3 doses of velusetrag vs placebo. Efficacy was similar between subjects with diabetic and idiopathic gastroparesis. | Kuo 2021, ref. 91 |
| Felcisetrag: DB, PC, RCT | 36: 22 IG, 14 DG | 0.1, 0.3 or 1.0mg i.v., daily | 3 days | Felcisetrag significantly accelerated GE, small bowel transit, ascending colon emptying (T1/2) and colonic transit at 48 hours | Chedid 2021, ref. 92 |
| Ghrelin Agonist | |||||
| Relamorelin RCT, PC, XO | 10 T1DM with previous delayed GE | 100 μg SQ | Single dose | Decreased gastric retention of solids at 1h and 2h and decreased GCSI-DD scores and nausea/vomiting/fullness/pain scores | Shin 2013, ref. 93 |
| Relamorelin RCT, PC, PG | 204 DG + moderate to severe symptoms and delayed GE | 10 μg SQ daily or 10 μg SQ bid | 12 weeks | Relamorelin (10 μg bid) significantly accelerated GE and significantly reduced vomiting vs. placebo. Among patients with baseline vomiting, relamorelin accelerated GE, reduced vomiting and improved other symptoms | Lembo 2016, ref. 94 |
| Relamorelin RCT, PC, PG | 393 DM with moderate to severe gastroparesis symptoms | 10 μg, or 30 μg or 100 μg or placebo SQ bid | 12 weeks | 75% reduction in vomiting frequency vs baseline (NS compared with placebo). All 4 symptoms of DG (composite or individual symptoms) significantly reduced over 12-wk in all 3 relamorelin doses and accelerated GE vs. placebo. Adverse effect: impaired glycemic control with relamorelin | Camilleri 2017, ref. 95 |
| Relamorelin and TZP-101 or TZP 102: 6 RCTs in SRMA | DG (N=557) | Diverse doses | Significantly improved overall gastroparesis symptoms (standardized mean difference, −0.34; 95% CI, −0.56 to −0.13) and significantly improved symptoms, including nausea, vomiting, early satiety, and abdominal pain | Hong 2020, ref. 96 | |
| Motilin Agonists | |||||
| Erythromycin RCT, PC, XO | 10 T1DM | 200mg iv; 250mg p.o. tid | 4 weeks | Solid meal retention at 2h: 63±9% with placebo; 4±1% with erythromycin; no effects on the symptoms | Janssens 1990, ref. 97 |
| Erythromycin open trials of i.v. and p.o. | 10 IG and 4 DG; 4 patients dropped out | 6 mg/kg i.v. 500 mg tid-ac and qhs |
Single dose; 4 wk and open 8.4 mo |
Solid meal retention at 2h: 85±11% (SD) at baseline; 20±29% on iv erythromycin (p <0.001); 48±21% after 4 wk of oral therapy (p <0.01). Reduction in total symptom scores and a significant reduction in global assessment scores |
Richards 1993, ref. 98 |
| Erythromycin vs metoclopramide RCT, XO | 13 DG | p.o. 250 mg tid erythromycin; p.o.10 mg tid metoclopramide | 3 weeks each period | Compared with baseline, improved GE parameters after both erythromycin and metoclopramide, with improved total GI symptom scores, more pronounced with erythromycin | Erbas 1993, ref. 64 |
| Erythromycin RCT, PC, XO | 20 IG (functional dyspepsia + delayed GE) | 200mg i.v. | Single dose | Erythromycin accelerated (breath test) solid GE T½=146 (27) vs 72 (7) min, and liquid GE T½=87 (6) vs 63 (5) min; no overall symptom improvement except for bloating | Arts 2005, ref. 99 |
| Erythromyin vs azithromycin retrospective case-control analysis | 120 patients (27 DM) underwent SGE with provocative testing | 250mg i.v. of each drug | Single dose | Both treatments accelerated gastric emptying with no difference between the 2 treatments: erythromyin GE T½=166±68min baseline to 11.9±8.4min; azithromycin GE T½=178±77min baseline to 10.4±7.2min |
Larson 2010, ref. 100 |
DB=double-blind; DM=diabetic; DG=diabetic gastroparesis; GCSI=Gastroparesis Cardinal Symptom Index; GE=gastric emptying; GEBT=gastric emptying breath test; IG=idiopathic gastroparesis; i.v.=intravenous; N=number; NA=not available; PAC-QOL=patient assessment of constipation–quality of life; PAGI-SYM=patient assessment of upper gastrointestinal disorders–symptoms; PC=placebo-controlled; po=oral; PG=parallel-group; p.o.=oral; PSG=post-surgical gastroparesis; RCT=randomized, controlled trial; SGE=GE by scintigraphy; SQ=subcutaneous; SRMA=systematic review and meta-analysis; XO=crossover
Another class of agents is the motilin agonists which are used in the treatment of gastroparesis in adults and children. These medications include erythromycin, clarithromycin, and azithromycin. These medications are generally used in the short term (1–4 weeks) because of development of tachyphylaxis to motilides (101). Based on a systematic review and network meta-analysis of 33 studies and data on 22.6 million subjects, macrolide use was not associated with the risk of arrhythmia or cardiovascular mortality (102).
Antiemetics, central neuromodulators in gastroparesis
Recommendation.
11. In patients with gastroparesis, use of antiemetic agents is suggested for improved symptom control; however, these medications do not improve GE. (conditional recommendation, low quality of evidence)
12. Central neuromodulators are not recommended for management of gastroparesis. (strong recommendation, moderate quality of evidence)
13. Current data do NOT support the use of ghrelin agonists for management of gastroparesis. (strong recommendation, moderate quality of evidence)
14. Current data do NOT support the use of haloperidol for treatment of gastroparesis. (conditional recommendation, low quality of evidence)
Table 6 summarizes efficacy of antiemetics and central neuromodulators in gastroparesis (103–109). These are therapies commonly used for symptom relief in gastroparesis. The central neuromodulator studied with the highest level of evidence was the tricyclic antidepressant, nortriptyline, in IG (105). In this randomized, placebo-controlled trial, nortriptyline was no better than placebo in relieving global symptoms of gastroparesis, but some improvement in abdominal pain was noted. In a study of amitriptyline, 50mg/day, there was no retardation of GE in patients with FD (110). Further RCTs are needed to determine the efficacy of other central neuromodulators. Although there are no formal randomized trials, experience with use of haloperidol in emergency room treatment of patients presenting with gastroparesis has led to reduced need for morphine treatment and admission to hospitals (111), rather than documenting effect on gastroparesis symptoms.
Table 6.
Efficacy of antiemetics and central neuromodulators in gastroparesis
| Medication/trial design | N, Etiology | Dose | Duration | Efficacy | Reference |
|---|---|---|---|---|---|
| Aprepitant PC, PG, DB, RCT | 126 pts with at least moderate chronic nausea and vomiting | p.o. 125mg/day | 4-weeks | Aprepitant did not reduce symptoms of nausea (primary outcome measure) but significantly reduced secondary outcomes: in symptom severity for nausea, vomiting and overall symptoms. Adverse events (mild or moderate severity) commoner in aprepitant (35%) vs placebo (17%). | Pasricha 2018, ref. 103 |
| Tradipitant PC, PG, DB, RCT | 152 adults with IG (91) or DG (61) | p.o. 85 mg bid | 4 weeks | Significant decrease in nausea score (reduction of 1.2) at week 4; significant increase in nausea-free days at week 4 with even greater effects in patients with nausea and vomiting at baseline (n = 101). A >1-point improvement in GCSI score in 46.6% on tradipitant compared with 23.5% on placebo. |
Carlin 2021, ref. 104 |
| Nortriptyline PG, PC, DB RCT | 130 IG | dose escalation at 3-week intervals (10, 25, 50, 75 mg) to 75 mg at 12 weeks | 15 weeks | No difference in primary outcome measure (decrease from the patient’s baseline GCSI score of at least 50% on 2 consecutive 3-week GCSI assessments during 15 weeks of treatment); more treatment cessation in nortriptyline group (29%) than placebo group (9%); numbers of adverse events not different. | Parkman 2013, ref. 105 |
| Haloperidol PC, RCT | 33 Emergency Dept. patients with acute exacerbation of diagnosed gastroparesis | 5mg vs. placebo both + conventional therapy (selected by treating physician) | Single dose | One hour after therapy, the mean pain and nausea scores in the haloperidol group were 3.13 and 1.83 compared to 7.17 and 3.39 in the placebo group (symptoms on 10-point scale). No adverse events were reported. |
Roldan 2017, ref. 106 |
| STW5 or STW5-11 vs. cisapride DB, double dummy, RCT | 186 dysmotility type of FD | NA | NA | The lower limit of the confidence interval for both herbal preparations was above the pre-defined lower limit of the equivalence border and hypothesis of non-inferiority was proven for STW 5 & STW 5-II. | Rosch 2002, ref. 107 |
| STW 5 PC, PG, DB, RCT | 103 patients with FD and gastroparesis | 20 drops tid | 4 weeks | Improvement of the GIS (P=0.08) and the proportion of patients with a treatment response (P=0.03) were more pronounced in the STW 5 group compared to placebo. No effect on GEBT. | Braden 2009, ref. 108 |
| Survey questionnaire of treatment of nausea in in clinical practice | 102 patients: gastroparesis 43.1%, FD 27.5%, PSG 8.8%, other 2.0%, undetermined multiple 10.8%. | Patient-reported best treatments were marijuana, ondansetron, and promethazine. Least effective treatments were erythromycin, diphenhydramine, buspirone, gabapentin, pregabalin, acupuncture, and Iberogast. Promethazine was more effective in patients with a higher GCSI. | Zikos 2018, ref. 109 |
DB=double-blind; DG=diabetic gastroparesis; DM=diabetic; FD=functional dyspepsia; GCSI=Gastroparesis Cardinal Symptom Index; GE=gastric emptying; GEBT=gastric emptying breath test; GIS=gastrointestinal symptom; IG=idiopathic gastroparesis; NA=not available; PC=placebo-controlled; p.o.=oral; PG=parallel-group; PSG=post-surgical gastroparesis; RCT=randomized, controlled trial; XO=crossover
Other drug therapies for gastroparesis
A recent study has targeted previously described impaired nitric oxide metabolism and an abnormal tetrahydrobiopterin (BH-4) pathway in gastroparesis patients with diabetes mellitus. This phase II study needs confirmation in other larger controlled studies (112).
A number of other medications are being developed for treatment of gastroparesis. These include 5–HT4 receptor agonists (prucalopride, felcisetrag, and velusetrag) and dopamine D2/D3 receptor antagonists, and the therapeutic trials of these medications are included in Table 5.
Use of pharmacotherapy to reduce the future aggravation of gastroparesis
Based on a referral center experience, predictors of responsiveness to pharmacotherapy (113) were identified. A good response to pharmacological agents can be expected in the viral and dyspeptic subgroups of idiopathics, Parkinson’s disease, and the majority of diabetics; whereas a poorer outcome to prokinetics can be expected in post-vagotomy patients, those with connective tissue disease, a subgroup of diabetics (e.g., with evidence of vagal neuropathy), and the subset of IG dominated by abdominal pain and history of physical and sexual abuse (113). The comprehensive NIH Gastroparesis Consortium database of 748 patients (86) showed 181 (24%) on domperidone and 567 not receiving domperidone; 63% had IG. Compared to patients not receiving domperidone, those patients who were receiving domperidone (median time on domperidone following initiation of 32 weeks, 95% CI: 25–35 weeks) experienced moderate, but significantly more improvement in gastroparesis outcome measures of the Gastroparesis Cardinal Symptom Index (GCSI) total score, nausea and fullness subscales, upper abdominal pain score, gastroesophageal reflux disease (GERD) score, and the patient assessment of upper gastrointestinal disorders – quality of life (PAGI-QOL) score.
In a systematic review (114) of 14 studies that evaluated GE and upper GI symptoms, including IG or DG, and including only studies with optimal GE test methods being evaluated, there was a significant positive association between improvements in GE and upper GI symptoms in response to prokinetic agents.
Immunological therapies
There is insufficient evidence to support routine clinical use of autoimmune therapies in management of gastroparesis. A retrospective analysis of 11 female patients (115) with drug and device resistant gastroparesis with coexisting positive autoimmune profiles who were treated for 8–12 weeks with diverse immunomodulatory treatment showed that total symptom score improved in 6 of 11 patients, with maximum GI symptom improvement with IVIg (2 of the 3 patients treated). In a subsequent open-label study, 14 patients (3 DG, 1 PSG, and 10 IG) with serological and/or tissue evidence of immunological abnormality, IVIg therapy (400 mg/kg infusion weekly for 12 weeks) was associated with significant improvement in symptoms scores for nausea, vomiting, early satiety, and abdominal pain, and 9/14 patients were responders to this open-label treatment (116). This study built upon the retrospective medical record review suggesting a positive experience among 11 patients treated with IVIg or combined mycophenolate mofetil with methylprednisolone, or only mycophenolate mofetil therapy (115).
Non-pharmacological therapy for gastroparesis: gastric electrical stimulation (GES), acupuncture, and herbal medicines
Recommendation.
15. Gastric electric stimulation (GES) may be considered for control of gastroparesis (GP) symptoms as a humanitarian use device (HUD). (conditional recommendation, low quality of evidence)
GES is approved as a humanitarian use (HUD), as defined by the FDA for medically refractory DG or IG. The recommendation includes the use of GES in humanitarian use.
Table 7 shows efficacy of several bioelectric treatments including vagal nerve stimulation, spinal cord stimulation and GES (117–142). A recent randomized, crossover trial of ON vs. OFF GES in patients with medically refractory vomiting with or without delayed GE, GES decreased the vomiting frequency. Severity of nausea and appetite improved while ON compared to OFF. However, there were no differences in GI quality of life, nutritional parameters, or GE (121). Randomized crossover trials of GES for medically refractory DG or IG have shown mixed results which may reflect the variation in trial designs with differing timing of the ON vs. OFF randomization and crossover (120–124). Other modalities of electrostimulation (vagal and spinal cord) appear promising; however, larger randomized, sham-controlled trials are needed to determine the efficacy. However, documented clinical usefulness in both IG and DG (documented in Table 7) suggests there is a role for GES in accordance with its HUD approval.
Table 7.
Efficacy of several bioelectric therapies in gastroparesis
| Device/trial design | Patients | Efficacy | Reference |
|---|---|---|---|
| Vagal Stimulation | |||
| Open-label pilot study: short-term noninvasive cervical vagal nerve stimulation in patients with drug-refractory gastroparesis | 23 patients with gastroparesis for 3 weeks and 7 of these for 6 weeks. | Response rates were 35% at 3 weeks and 43% for 3–6 weeks. Improvements in mean total GCSI and subscales were noted. | Paulon 2017, ref. 117 |
| Open-label pilot study: noninvasive vagal nerve stimulation for 4 wks improves symptoms and gastric emptying in patients with IG | 15 patients with mild to moderate IG | Improvement in total GCSI symptom scores and three subscales, with 40% participants meeting primary endpoint; therapy also associated with a reduction in GE T1/2. | Gottfried-Blackmore 2020, ref. 118 |
| Spinal Cord Stimulation | |||
| Open-label study of spinal stimulation in patients with abdominal pain, with the majority having gastroparesis | 23 patients, 96% Caucasian and 79% women, with gastroparesis in 63% | After 12 months of 10-KHZ spinal cord stimulation, 78% of patients had >50% reduction in pain and 64% remitted in pain. Other outcomes improved in most patients. | Kapural 2020, ref. 119 |
| Controlled Trials in Gastric Electric Stimulation (GES) | |||
| Temporary GES | |||
| RCT, PC, XO trial of two consecutive, 4-day sessions of temporary GES | 58 patients (47 females) with gastroparesis symptoms: 38 IG; 13 DG, 7 PSG | Overall slight, NS daily decrease in average vomiting scores First session was significant, but not significant after XO. Temporary GES may improve symptoms such as vomiting. | Abell T 2011, ref. 120 |
| Permanent GES | |||
| GES reduces refractory vomiting in a randomized, XO trial | 218 patients in 19 centers, 97 with DG and 121 with IG were included and 46 were excluded, thus 172 patients were implanted and analyzed | A randomized, XO trial for 4 months of GES decreased vomiting in DG and IG, irrespective of baseline GE. | Ducrotte 2020, ref. 121 |
| Multicenter, DB, XO, RCT of GES | 17 DG and 16 IG | Self-reported vomiting frequency significantly reduced in the on vs. off period and consistent with the significant patient preference for the on vs. off period; vomiting frequency decreased, and symptom severity and quality of life improved at 6 and 12 months. Once unblinded, the symptom improvement continued at one year. | Abell T 2003, ref. 122 |
| Randomized XO study of GES with all patients turned on for 6 weeks and then with consecutive 3-month XO periods with device on or off | 55 patients with DG | 6 weeks of GES therapy significantly reduced vomiting and gastroparetic symptoms in patients with DG. | McCallum R 2010, ref. 123 |
| Prospective, DB, randomized, XO study of GES with all patients initially having device on for 6 weeks followed by DB consecutive 3-month XO periods with device either on or off. | 32 patients with IG | GES implanted with on stimulation was shown to decrease vomiting symptoms in the initial 6-week on period. NS reduction in vomiting symptoms in on vs. off period. Sustained decrease in vomiting and days of hospitalization at 12 months in the on group. |
McCallum R 2013, ref. 124 |
| Two separate but related studies of the effect of GES on pancreatic function in gastroparesis patients: single-blinded, RCT compared to normal controls | 9 patients with gastroparesis and GES and 9 healthy controls | Pancreatic elastase was significantly different for GES on vs. off: 508 on vs. 378 off. Total GI symptoms were significantly lower on vs. off. Pancreatic polypeptide and heart rate were borderline improved with on vs. off. | Luo 2004, ref. 125 |
| DB, prospective, single-arm, RCT Study of GES in DG | 7 DG patients | No evidence was found for GES-induced modulation of the visceral sensory system and central excitability. Some changes in symptoms noted with GES. | Frokjaer 2009, ref. 126 |
| Propensity score matching. Effect of GES in gastroparesis with prospective data | 319 patients with gastroparesis symptoms, of which, 81 had GES and 231 without GES | Patients treated with GES had clinically significant improvement in gastroparesis symptoms. When adjusted by propensity scoring only nausea remained significant | Abell T 2019, ref. 127 |
| Controlled with medical arm but not randomized study with 1 year of baseline and 3 years of treatment with two groups: GES vs intensive medical therapy | 9 GES patients and 9 similar patients in an outpatient medical program | GES was found to be more effective in improving long-term GI symptoms, decreased costs, and less use of healthcare resources than intensive medical therapy. | Cutts 2005, ref. 128 |
| Meta-analyses Assessing Effectiveness of Gastric Electrical Stimulation | |||
| NICE Guidance on GES for gastroparesis | Several studies reviewed, 2 metanalysis, 2 RCT, XO | Diabetics with severe symptoms may benefit from therapy. | Kong 2015, ref. 129 |
| SRMA 13 studies, 12 lacked controls and 1 blinded and randomized |
13 studies, 12 lacked controls and 1 blinded and randomized | Following GES, improvements in TSS score (3/13 studies), vomiting severity (4/13), nausea severity (4/13), SF-36 physical composite score (4/13), SF-36 mental composite score (4/13), requirement for enteral or parenteral nutrition (8/13), and 4-h gastric emptying (5/13). Weight gain (in 3/13) did not reach overall significance, 3 Device removal or reimplantation rate was 8.3%. Beneficial in improving symptoms in patients with gastroparesis |
O’Grady 2009, ref. 130 |
| SRMA 5 studies randomly allocated patients to periods with or without GES |
5 randomized trials 16 open-label studies |
TSS scores did not differ between these periods with or without GES in randomized trials. Open-label studies showed a significant decrease in TSS scores, which was also shown with medical therapy or placebo arms, or botulinum toxin. Meta-regression analysis showed that significant differences in baseline TSS ratings impacted TSS ratings during treatment. Argues against the use of GES outside of strict clinical trials as viable treatment option. |
Levinthal 2017, ref. 131 |
| SRMA | 21 studies | GES appears to offer significant improvement in symptom control in a subset of patients. | Lal 2015, ref. 132 |
| SRMA | 10 studies | GES is an effective modality for treating gastroparesis refractory to less invasive treatment. | Chu 2012, ref. 133 |
| Selected Open-Label Trials of Gastric Electrical Stimulation | |||
| Multicenter, open-label GES experience in France | 142 patients (60 diabetic, 82 non-diabetic) and medico-economic data were available for 96 patients (36 diabetic, 60 non-diabetic) | 24 months after implantation. GIQLI score increased, with a more significant improvement in non-diabetic than in diabetic patients. Proportion of patients vomiting less than once per month increased by 25.5%. GES decreased mean overall healthcare costs (saving of average $3348/patient/year), with. savings greater for diabetic patients (4096 US$/patient/year). |
Gourcerol 2020, ref. 134 |
| Open-label GES study | 16 patients with PSG refractory to medical therapy | Severity and frequency of all 6 upper GI symptoms, TSS, physical composite score, and mental composite score significantly improved after 6 months and sustained at 12 months; 4/7 stopped jejunal feeding; mean number of hospitalization days significantly reduced by a mean 25 days compared with prior year. No effect on GE. |
McCallum 2005, ref. 135 |
| Open-label GES study | 37 gastroparesis patients preop. and 1y post-GES implant | 8/27 off prokinetics; 9/26 off antiemetics at 1y; mean TSS significantly reduced, overall SF-36 scores (HR-QOL) significantly improved, and hospitalizations decreased from 50 ± 10 days for the year prior to GES therapy to 14 ± 3 days. GE was not significantly improved. | Lin 2005, ref. 136 |
| Open-label GES study | 55 patients with gastroparesis with follow-up information for over 3y | Of the 55 patients, 10 died of unrelated complications, 6 had devices removed and 2 could not be reached. 37 patients had activated GES for mean 45 months: TSS, hospitalization days and the use of medications all significantly reduced at 1 and 3 y. Among 15/37 patients requiring nutritional support, only 5 continued beyond 3y. Mean HbA1c in diabetics reduced from 9.5 to 7.9% at 3y. |
Lin 2006, ref. 137 |
| Open-label GES study | 15 patients with gastroparesis | Four patients (4 idiopathic) failed to improve more than 20% on multiple assessments after a year of therapy. All diabetic patients experienced a durable symptomatic improvement with GES. GES non-responders had less severe vomiting preoperatively. | Musunuru 2010, ref. 138 |
| Open-label GES clinical experience | 221 patients with gastroparesis: 142 (64%) DG, 48 (21%) IG, 31 (14%) PSG | At follow-up of at least 1 year, there was association of symptom improvement with improved GE in DG, not in IG. Patient age, gender, baseline TSS score, and baseline gastric retention had no significant effect on clinical improvement in response to GES. | Hou 2012, ref. 139 |
| Open-label experience | 4 patients with gastroparesis | Mean length of hospital stay in the year pre-GES was 81.75 days and 62.25 days in the year post-GES; also no improvement in glycemic control following GES. | Hannon 2011, ref. 140 |
| Open-label follow-up study of GES after successful initial temporary GES | IG 9, DG 3 with long duration symptoms (7.3 years) | Short-term: improved TSS, body weight, BMI, and serum albumin by 3 to 6 months. Intermediate (1 to 2 years) and long-term (5 year) data: continued improvement in TSS, weekly vomiting frequency score, QOL measures, and maintained weight gain. | Abell T 2003, ref. 141 |
| Open-label GES study | Refractory gastroparesis: DG 39, PSG 9, IG 7 | TSS and the physical and mental composite scores of QOL improved significantly; GE did not change; BMI and body weight increased; days spent in hospital admissions significantly decreased. | Forster 2003, ref. 142 |
DG=diabetic gastroparesis; DM=diabetic; GCSI=Gastroparesis Cardinal Symptom Index; GE=gastric emptying; GES=gastric electrical stimulation; GIQLI=Gastrointestinal quality of life; HR-QOL=health-related quality of life; IG=idiopathic gastroparesis; NA=not available; NS=not significant; PC=placebo-controlled; po=oral; PG=parallel-group; PSG=post-surgical gastroparesis; RCT=randomized, controlled trial; TSS=total symptom severity; XO=crossover
Recommendation.
16. Acupuncture alone or acupuncture combined with prokinetic drugs may be beneficial for symptom control in patients with DG. Acupuncture cannot be recommended as beneficial for other etiologies of gastroparesis. (conditional recommendation, very low quality of evidence)
17. Herbal therapies such as Rikkunshito or STW5 (Iberogast) should NOT be recommended for treatment of gastroparesis. (conditional recommendation, low quality of evidence)
Table 8 summarizes information on effects of electro-acupuncture, acupuncture, and herbal medicines in gastroparesis (143–154). The evidence available does not support their use in clinical practice.
Table 8.
Effect of electro-acupuncture, acupuncture, and herbal medicines in gastroparesis
| Electro-acupuncture | |||
| Device/trial design | Patients | Efficacy | Reference |
| Multicenter sham-controlled, XO, 4-week RCT of transcutaneous electroacupuncture (TEA) via surface ECG electrodes at acupoints PC6 and ST36. | 26 DG patients, 18 completed study; TEA performed using pulse trains self-applied for 2 hrs. post-lunch/dinner | 4-wk TEA, not sham-TEA, significantly improved 5 of 9 gastroparesis symptoms: nausea by 29.7%, vomiting by 39.3%, abdominal fullness by 21.4%, bloating by 20.6%, and retching by 31.1%. A significant change in pain was also noted with TEA. | Xu 2015, ref. 143 |
| Acupuncture | |||
| Device/trial design | Patients | Efficacy | Reference |
| Single-blind, RCT, XO trial of acupuncture for 1 week vs sham acupuncture with 1-month washout period | 25 DG patients | Real acupuncture was associated with significantly greater reductions in gastric retention at 2h and 4h and in GCSI score with no differences in fasting blood glucose or HbA1c | Li 2015, ref. 144 |
| Single-center, DG comparison of acupuncture to control | Acupuncture treatment group (n=16 (5M/11F), 5 times per week 40 minutes each for 10 days, and a control group (n=16 (7M/9F). | Compared to control group, acupuncture resulted in the clinically significant improvement of the severity of symptoms and the GCSI nausea by 68,4%, retching by 76,8%, vomiting by 86,7%, stomach fullness by 62,5%, not able to finish a normal-sized meal by 21,2%, stomach visibly larger by 13,4%, loss of appetite by 12,8%, feeling excessively full after meals by 64,7% and bloating by 22.5% | Kostitska 2016, ref. 145 |
| Single-center, RCT of acupuncture applied to Zusanii once per day and other acupoints compared to metoclopramide 20mg tid i.m. | Acute PSG in 63 patients | Significant differences in gastric drainage volume, cure rate and number of treatments with cure rate was 90.6% with acupuncture and 32.3% with metoclopramide | Sun 2010, ref. 146 |
| Single-center comparison of 6-day Rx with acupoint stimulation (bilateral TEA) at Neiguan, PC-6 or prokinetic (metoclopramide, cisapride, erythromycin) | 30 mechanically-ventilated neurosurgical ICU patients with delayed GE [gastric residual volume (GRV) >500 mL for ≥2 days] | After 5 days of treatment, 80% of patients in the acupoint group successfully developed feeding tolerance (GRV <200mL/24h) versus 60% in the prokinetic group; benefit was documented from day 1 of treatment. Similarly, feeding balance improved significantly on all days of treatment with acupoint vs. prokinetic therapy. | Pfab 2011, ref. 147 |
| Single-center, open-label treatment with needleless TEA | 11 patients with DG evaluated with visual stimulation (VS) to evoke nausea and EEG | TEA improves gastric dysrhythmia and ameliorates nausea. TEA treatment of nausea provoked by VS resulted in a change of dominance from right to left inferior frontal lobe activity on EEG. | Sarosiek 2017, ref. 148 |
| RCT of acupuncture points: group A Zhongwan (CV 12) and Zusanli (ST 36); group B, Neiguan (PC 6) and Zusanli (ST 36); group C, non-acupoint and Zusanli (ST 36). | 99 patients with gastroparesis at 3 clinical centers | Treatment was performed for 30 minutes every day, 5 days as a course of treatment. GCSI scores of each group after treatment and at follow-up were significantly lower than those before treatment (P <0.01), and the reduction in group A (Zhongwan (CV 12) and Zusanli (ST 36)) was greater than that of groups B and C (P <0.01). SF36 scores similar in the three groups. | Xuefen 2020, ref. 149 |
| SRMA of acupuncture either manually stimulated (24 studies) or electrically stimulated (8 studies). | 32 studies with a total of 2601 participants: DG (31 studies) or PSG (1 study) | There was low-certainty evidence that symptom scores of participants receiving acupuncture did not differ from those receiving sham acupuncture at 3 months when measured by a validated scale. There was very low-certainty evidence that acupuncture had ‘improved’ symptoms compared to gastrokinetic medication (4–12 weeks) (12 studies; 963 participants). | Kim 2018, ref. 150 |
| SRMA of 14 RCTs of acupuncture | 14 RCTs of DG | Acupuncture treatment had a higher response rate than controls (RR, 1.20 [95% confidence interval (CI), 1.12 to 1.29], P < 0.00001), and significantly improved dyspeptic symptoms compared with the control group. | Yang 2013, ref. 151 |
| Open-label treatment with behavioral technique, autonomic training with directed imagery (verbal instructions) | 26 patients with chronic nausea and vomiting | Gastrointestinal symptoms decreased by >30% in 58% of the treated patients; responders manifested mild to moderate delay in baseline GE; the sympathetic adrenergic measure (change in the foot cutaneous blood flow in response to cold stress) predicted improvement in autonomic training outcome. | Rashed 2002, ref. 152 |
| Chinese Herbal Medicine | |||
| SRMA Banxiaxiexin decoction for DG | 16 RCTs involving 1302 patients | Effect of Banxiaxiexin decoction (BXXD) for DG was superior to the control group (n = 1302, RR 1.23, 95% CI 1.17 to 1.29). Methodological quality of included studies was low, and long-term efficacy and safety are still uncertain. |
Tian 2013, ref. 153 |
| SRMA in comparison to conventional treatment (Western medicine treatment [metoclopramide, mosapride, cisapride, domperidone]), placebo, and no treatment (blank) for DG | Ten RCTs involving 867 patients (441 in the experimental groups [herbs alone], and 426 in the control groups [all prokinetic]) | Effects of Xiangshaliujunzi Decoction (XSLJZD) for the treatment of DG were superior to the control group (n=867, RR=1.33, 95% CI: 1.24–1.42) based on symptoms and gastric emptying. Evidence remains weak due to the poor methodological quality of the included studies. |
Tian 2014, ref. 154 |
DG=diabetic gastroparesis; GCSI=Gastroparesis Cardinal Symptom Index; GE=gastric emptying; PSG=post-surgical gastroparesis; RCT=randomized, controlled trial; SRMA=systematic review and meta-analysis; TEA= transcutaneous electroacupuncture; XO=crossover
Pyloric Interventions: Diagnostic and Therapeutic
Recommendation.
18. In patients with gastroparesis, EndoFLIP evaluation may have a role in characterizing pyloric function and predicting treatment outcomes following peroral pyloromyotomy. (conditional recommendation, very low quality of evidence)
19. Intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized, controlled trials. (strong recommendation, moderate quality of evidence)
20. In patients with gastroparesis with symptoms refractory to medical therapy, we suggest pyloromyotomy over no treatment for symptom control. (conditional recommendation, low quality of evidence)
Table 9 shows results of EndoFLIP for selection of patients for pyloromyotomy or pyloric botulinum toxin injection (155–161). Current evidence suggests that such measurements of pyloric diameter and distensibility index or compliance are associated with greater gastric retention, and that the measurements may predict response to therapy, particularly, significant enlargement of the post-G-POEM pyloric diameter (159). It is reasonable to consider such pyloric interventions in a clinical trial and to include assessments of pyloric physiology to appraise the impact of pyloric dysfunctions on outcomes. Thus, whereas intrapyloric injection of botulinum toxin is not recommended for patients with gastroparesis based on randomized, controlled trials (162), a recent large multicenter study from France documents the efficacy of botulinum toxin injection, particularly for the relief of vomiting, when patients are selected based on measurements of pyloric distensibility (161).
Table 9.
EndoFLIP for Selection of Patients for Pyloromyotomy or Pyloric Botulinum Toxin Injection
| Patients | Measurement | Results | Reference |
|---|---|---|---|
| 21 HC, 27 patients with gastroparesis and 5 patients with esophagectomy | Fasting pyloric pressure and compliance | Fasting pyloric compliance 25.2±2.4 mm/mmHg in HV, 16.9±2.1 mm/mmHg in gastroparesis (P <0.05) and 10.9±2.9 mm/mmHg in patients with esophagectomy (; P <0.05). Pyloric dilation in 10 gastroparesis patients with low fasting pyloric compliance increased compliance from 7.4±0.4 to 20.1±4.9 mm/mmHg (P <0.01) and improved the GIQLI score. | Gourcerol 2015, ref. 155 |
| 54 patients (39 IG, 15 DG) | Fasting pyloric diameter, CSA, pressure, length, DI | Wide range seen in diameter (5.6–22.1 mm) and distensibility (1–55 mm2/mmHg) of the pylorus. Symptoms of early satiety and postprandial fullness were inversely correlated with pyloric sphincter diameter and CSA. | Malik 2015, ref. 156 |
| 47 DG patients and 67 IG patients with nausea and vomiting | Sleeve manometry and EndoFLIP performed sequentially during the same endoscopy | Basal pyloric pressure was elevated (>10 mmHg) in 34 patients (42% of patients with delayed emptying); significant decrease in distensibility in patients with gastric retention (>20% at 4 h) compared with patients with normal gastric retention (<10%). | Snape 2016, ref. 157 |
| 30 IG patients and 14 DG patients | Fasting pyloric diameter, CSA, and DI | Greater gastric retention tended to correlate with decreased CSA and pyloric DI. Greater pyloric compliance at baseline correlated with greater improvement in early satiety and nausea at 8 weeks and greater pyloric DI correlated with improvement in upper abdominal pain. | Saadi 2018, ref. 158 |
| 37 patients with refractory gastroparesis | Fasting CSA, balloon pressure, and DI | Post-G-POEM CSA and DI were significantly higher in the clinical success group and improvement in gastric emptying. | Vosoughi 2020, ref. 159 |
| 20 patients with refractory gastroparesis | Fasting pyloric diameter and DI before and after G-POEM | G-POEM increased mean and maximum pyloric diameters and mean and maximum pyloric DI on 50 mL EndoFLIP inflation; therapy enhances pyloric opening but may not impair pyloric closure. The clinical success of G-POEM using EndoFLIP inflated to 50mL had specificity of 100% and sensitivity of 72.2% (area under the curve 0.72) at a distensibility threshold of 9.2 mm2/mmHg. | Watts 2020, ref. 160 |
| 35 patients with gastroparesis: 11 DG, 6 PSG, 17 IG | Fasting pyloric diameter and distensibility before BOTOX | 19/35 patients with reduced (<10 mm2/mm Hg) pyloric distensibility) had benefits: TSS decreased at 3 months and gastric fullness, bloating and GIQLI score and gastric emptying T1/2 all improved; no such benefit in those with normal distensibility. | Desprez 2019, ref. 161 |
(CSA=cross-sectional area; DI =distensibility index; DG=diabetic gastroparesis; GIQLI=Gastrointestinal Quality of Life Index; HC=healthy controls; IG=idiopathic gastroparesis; NA=not available; PSG=post-surgical gastroparesis; TSS=total symptom score
Efficacy of G-POEM for gastroparesis based on open-label studies
Table 10 shows efficacy of G-POEM for gastroparesis based on open-label studies (163–181). Overall, these open-label studies suggest there is benefit in terms of symptom improvement and improved GE, though most studies were of only 3–6 months’ duration. A 12-month study showed 56% patients improved at 1 year (173). Symptom control after endoscopic pyloromyotomy is comparable to surgical myotomy; however, endoscopic myotomy has been associated with fewer post–procedural complications and shorter length of hospital stay. A recent study has identified benefit in relief of symptoms as well as improved GE with G-POEM procedure followed for 6 months in a sham-controlled study (174). Other pylorus-directed procedures are also available such as surgical pyloroplasty, though there is more evidence on G-POEM. Heineke-Mikulicz pyloroplasty involves longitudinal incision across the pylorus, which is then closed transversely, and this results in division of both longitudinal and circular muscle layers. In 177 patients with gastroparesis, laparoscopic pyloroplasty achieved improved GE in 90% of patients and induced short-term improvement of nausea, vomiting, bloating, and abdominal pain. However, morbidity rate was 6.8%, with problems such as confirmed leaks or further surgical interventions including jejunostomy and subtotal gastrectomy (182).
Table 10.
Efficacy of G-POEM for gastroparesis based on open-label studies.
| # Pts | Types of gastroparesis pts | Changes in GE | Changes in symptoms | Duration follow up | Adverse events | Ref. # |
|---|---|---|---|---|---|---|
| 29 | DG=7 IG= 15 PSG=5 scleroderma=2 |
70% Normalized | 79% at 3 months; 69% at 6 months. GCSI improved from 3.5 to 0.9 at 3 months | 3 and 6 months | 17% (2/12) Pneumoperitoneum requiring decompression | Gonzalez 2017, ref. 163 |
| 16 | DG=9 IG=5 PSG=1 post-infectious = 1 |
75% normalized, 25% improved | 81% improvement. GCSI improved from baseline of 3.4 to 1.46 12 months later | 12 months | None | Dacha 2017, ref. 164 |
| 47 | DG=12 IG=27 PSG=8 |
4h retention improved: from 37.2 to 20.4% | GCSI improved from 4.6 to 3.3 | 3 months (follow-up in 31/47 pts) | 1 death (unrelated) | Rodriguez 2017, ref. 165 |
| 30 | DG=11 IG=7 PSG=12 |
47% Normalized | No validated outcome measure available | 6 months | 2/30 (6%): 1 pre-pyloric ulcer and 1 capnoperitoneum | Khashab 2017, ref. 166 |
| 13 | DG=1 IG=4 PSG=8 |
4/6 improved; % retention at 4h improved from 49 to 33% | In 11: 4 considerably better, 4 somewhat better, 1 no Δ, 2 worse | 3 months | 3 accidental mucosotomy closed with clips; 1 pulmonary embolism | Malik 2018, ref. 167 |
| 16 | DG=3 PSG=13 |
Mean % retention (radiolabeled bread) at 2h from 69.3% to 33.4% | Mean total symptom score from 24.25 to 6.37; 13/16 substantial improvement | 3 months | 1 pyloric stenosis at day 45 | Xu 2018, ref. 168 |
| 20 | DG=10 non-diabetic=10 |
% retention at 4h improved from 57.5 to 15%; and 30% normalized | GCSI improved from 3.5 to 1.3; QOL improved | 3 months | 3 mild hemorrhage, 3 gastric perforation, 1 moderate dyspepsia | Jacques 2019, ref. 169 |
| 40 | DG=15 Nondiabetic=25 (of which 18 were IG) |
% retention at 4h reduced by 41.7% | Improved GCSI, nausea/vomiting, not bloating | median 15 months | 1 tension capnoperitoneum, 1 exacerbation of COPD; 1 (Ehlers-Danlos syndrome) disrupted mucosotomy + ulcer | Mekaroonkamol 2019, ref. 170 |
| 22 | DG=8, IG=14, all with GES and most with diverse other procedures | In 7/11 with post-G-POEM, GE was normal | GCSI improved (reduction 1.63 points); improved all sub-scores | 1 and 3 months | 1 laparoscopy for pain due to capnoperitoneum and adhesions | Strong 2019, ref. 171 |
| 38 | PSG (76% for fundoplication or hiatal hernia repair) | % retention at 4h improved from 46.4 to 17.9%; 50% normalized | GCSI improved (mean reduction 1.29 points); improved all subscores | 1 month | 2 readmissions: 1 melena; 1 dehydration | Strong 2019, ref. 172 |
| 80 | IG (41.3%), PSG (35%) and DG (23.8%). | GE scintigraphy improvement in 64.2% and normalized in 47.2% (of 53 cases with test) at 3 months | Decrease in total GCSI >1 + >25% decrease in at least two of the subscales in 66.6% at 12 months | 3 months GES, 12 months clinical | 3 symptomatic capnoperitoneum, 1 mucosotomy; 1 thermal mucosal injury | Vosoughi 2021, ref. 173 |
| 9 | 5 PSG, 2 DG, 1 IG, and 1 PSG and diabetic | Mean GSCI decreased from 3.16 to 0.86 (3 months), 0.74 (6 months), 1.07 (12 months) and 1.31 (24 months [ns]) after the procedure. GIQLI improved from baseline at 12 months; not significant at 24 months | median follow-up was 23 (range 12–31) months | 1 delayed bleeding from gastric ulcer | Hustak 2020, ref. 174 | |
| 76 | Gastroparesis with median duration 48 months; median gastric retention at 4h 45% and median GCSI 3.6 | High rate of gastric retention at 4h was significantly associated with clinical failure | Clinical success in 65.8% of patients at 1 year, with median of reduction in GCSI score of 41 %; high preop GCSI satiety score predicted clinical success | At least 1 y | Ragi 2021, ref. 175 | |
| SRMA | 14 studies with total 276 patients | Pooled GE scintigraphy normalization rate was 61.3% (95% CI, 51.5–70.8%) | Clinical symptom improvement rate was 88.2% (95% CI, 83.6–93.1%). Mean GCSI score improvement rate: 90.2% at 1 month, 83.3% at 3 months, 70.3% at 6months, 52.4% at 12 months and 57.1% at 18 months. |
Up to 18 months | Intra-operative complications were found in about 3.2% and postoperative adverse events in 2.1% | Zhang 2019, ref. 176 |
| SRMA | 6 studies | GE scintigraphy not improved | Improvement in GCSI score after 3 months of G-POEM as compared with pre-G-POEM GCSI scores. | 3 months | Pooled rate of total adverse events was 9% (95% C.I. 2.7–25.9). | Garg 2020, ref. 177 |
| SRMA | 272 patients in 8 studies | The pooled results of 4h GE scintigraphy were 41.89% (95% CI, 32.75–51.03%) pre-G-POEM and 16.48% (95% CI, 9.83–23.14%) post-G-POEM | Pooled rates of GCSI were 3.25 (95% CI, 2.75–3.75) pre-procedure, 1.80 (95% CI, 1.10–2.49) at 1–3 months, 1.56 (95% CI, 0.45–2.68) at 6 months, and 1.10 (95% CI, 0.75–1.45) at 12 months | 1, 3, 6, and 12 months | Pooled adverse events rate was 12% (95% CI, 6–19%) | Li 2021, ref. 178 |
| SRMA | 10 studies, 292 patients | GE scintigraphy, significant decrease of the residual percentage at 2 and 4 hours | Significant symptomatic improvement was achieved after 83.9% of procedures | Mean follow-up, 7.8 ± 5.5 months). | The overall adverse events rate was 6.8%. | Spadaccini 2020, ref. 179 |
| Laparoscopic pyloroplasty compared to G-POEM procedure | ||||||
| 60 | Retrospective comparison lap pyloroplasty (LP) vs. G-POEM, Single-center, 30 per group (19 IG, 6 PSG, 5 DG), matched by propensity scoring | LP and G-POEM both resulted in similar, significant improvements in GCSI scores (overall and each of 3 subscales) with no differences between treatment groups | LP and G-POEM both resulted in similar, significant improvements in objective GE with no differences between treatment groups | 1-month outcome (28 G-POEM, 22 LP) 3-month outcome (25 G-POEM, 21 LP) |
Longer length of stay, operative time, more estimated blood loss and complications in the LP group (surgical site infection, pneumonia, and unplanned ICU admission | Landreneau 2019, ref. 180 |
| SRMA | G-POEM (332 in 11 studies) vs. surgical pyloroplasty (375 in 7 studies | 4h GE scintigraphy success results: G-POEM 85.1% (95% CI 68.9–93.7) and surgical pyloroplasty 84% (95% CI 64.493.8) with no significant difference | Clinical success, based on GCSI score: G-POEM 75.8% (95% CI 68.1–82.1) and surgical pyloroplasty 77.3% (95% CI 66.4–85.4), with no significant difference |
Overall adverse events were comparable | Mohan 2020, ref. 181 | |
DG=diabetic gastroparesis; GCSI=Gastroparesis Cardinal Symptom Index; GE=gastric emptying; GIQLI=Gastrointestinal Quality of Life Index; IG=idiopathic gastroparesis; LP=laparoscopic pyloroplasty; PSG=post-surgical gastroparesis; QOL=quality of life; SRMA=systematic review and meta-analysis; TSS=total symptom score; XO=crossover
CONCLUSION AND A LOOK TO THE FUTURE
This guideline has focused on the diagnosis and treatment of gastroparesis in adults (including dietary, pharmacological, device, and interventions directed at the pylorus). The recommendations made are guided by assessment using GRADE methodology. Nevertheless, this is an area with considerable ongoing innovation, validation, and research that is likely to impact future iterations of these guidelines. In particular, the following have potential future impact on the management of gastroparesis: the diagnostic value of wireless motility capsule for gastroparesis and for measurements of pan-gastrointestinal transit and pressure profiles, and autonomic nervous system dysfunction are under investigation. Similarly, studies are exploring the optimal approaches to select and individualize patients for treatments including documentation of circulating antibodies, measurements of the pylorus and high resolution antropyloroduodenal manometry, extensive surface electrogastrography (high-resolution electrical mapping), and full-thickness antral and pyloric biopsies. Such advances should clarify the role of immunotherapies, novel pharmacological agents, pyloric interventions, bioelectric therapy, and surgical approaches for gastroparesis.
Supplementary Material
Acknowledgements:
The authors thank Dr. David Armstrong (McMaster University, Hamilton, Ontario, Canada) for participation in the initial literature reviews, Dr. Prateek Mathur (University of Louisville, Kentucky) for assistance with some of the tables in the manuscript, and Mrs. Cindy Stanislav for excellent secretarial assistance.
Financial support:
MC receives NIH funding for studies on gastroparesis (R01-DK122280 and R01-DK125680). TA receives NIH funding for gastroparesis (7U01-DK074007-07 and R56DK126935)
BK receives NIH funding for gastroparesis (R21-DK116029, U01-DK112193); NIDDK Diabetic Complications Consortium (DK076169, DK115255).
LN receives support for research by a philanthropic gift from Colleen and Robert D. Hass.
ABBREVIATIONS USED
- AE
adverse event
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- DB
double blind
- DM
diabetic
- DG
diabetic gastroparesis
- EEG
electroencephalogram
- EGG
electrogastrography
- ESNM
European Society of Neurogastroenterology and Motility
- FD
functional dyspepsia
- GCSI
Gastroparesis Cardinal Symptom Index
- GCSI-DD
Gastroparesis Cardinal Symptom Index-Daily Diary
- GE
gastric emptying
- GEBT
gastric emptying breath test
- GERD
gastroesophageal reflux disease
- GES
gastric electrical stimulation
- GCSI
gastroparesis cardinal symptom index
- GI
gastrointestinal
- GIQLI
gastrointestinal quality of life index
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- G-POEM
gastric per oral endoscopic myotomy
- HC
healthy control
- HV
healthy volunteer
- HR-QOL
health-related quality of life
- HUD
humanitarian use device
- IG
idiopathic gastroparesis
- IV
intravenous
- LP
laparoscopic pyloroplasty
- NICE
National Institute for Health and Care Excellence
- NA
not available
- NS
not significant
- PAC-QOL
patient assessment of constipation – quality of life
- PAGI-QOL
patient assessment of upper gastrointestinal disorders – quality of life
- PAGI-SYM
patient assessment of upper gastrointestinal disorders – symptoms
- PC
placebo-controlled
- PG
parallel-group
- PICO
Patient Intervention Comparison and Outcomes
- po
oral
- PSG
post-surgical gastroparesis
- RCT
randomized controlled trial
- ROM
radiopaque marker
- Rx
treatment
- SGE
gastric emptying by scintigraphy
- SRMA
systematic review and meta-analysis
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TEA
transcutaneous electrical acupuncture
- TSS
total symptom score
- WMC
wireless motility capsule
- XO
crossover
Footnotes
Potential conflicts of interest:
MC: Single-center research studies: Allergan, Takeda, and Vanda; Consulting with compensation to his employer: Takeda, Alpha Sigma Wasserman
TA: Investigator: Censa, Clndome, Vanda, Allergan, Neurogastrix; Consultant: Censa, Nuvaira, Takeda, Medtronic; Speaker: Takeda, Medtronic; Reviewer: UpToDate; Writer: Med Study; Editorial: Neuromodulation, Wikistim; ADEPT-GI: IP for autonomic/enteric diagnosis and therapies
BK: Clinical trials with Takeda, Vanda, Alpha Sigma Wasserman, GSK; Consulting with Takeda, Cindome, Neurogastrix; Speaking for Medtronic
LN: Investigator: Allergan, Vanda; Consultant: Abbvie, Ironwood, Alnylam, Eli Lilly, Gemelli, Neurogastrx, Pendulum, Phathom, RosVivo, Salix, Takeda. Scientific Advisory Board: Gemelli, RosVivo
VMV: Nothing to disclose
JP: Nothing to disclose
KG: Nothing to disclose
RY: Consultant through Institutional Agreement: Medtronic, Ironwood Pharmaceuticals, Diversatek, StatLinkMD; Research support: Ironwood Pharmaceuticals; Advisory Board: Phathom Pharmaceuticals; RJS Mediagnostix
REFERENCES
- 1.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol 2013;108:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1592–1622. [DOI] [PubMed] [Google Scholar]
- 3.Nishiwaki S, Kurobe T, Baba A, et al. Prognostic outcomes after direct percutaneous endoscopic jejunostomy in elderly patients: comparison with percutaneous endoscopic gastrostomy. Gastrointest Endosc 2021;94:48–56. [DOI] [PubMed] [Google Scholar]
- 4.Tan Y-W, Yan Ting Chua A, Ng Yin K, et al. Optimal management of gastrojejunal tube in the ENFit era - Interventions that changed practice. J Pediatr Surg 2021; 6:1430–1435. [DOI] [PubMed] [Google Scholar]
- 5.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–23 [DOI] [PubMed] [Google Scholar]
- 6.Arts J, Holvoet L, Caenepeel P, Bisschops R, Sifrim D, Verbeke K, Janssens J, Tack J. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007. Nov 1;26(9):1251–8. [DOI] [PubMed] [Google Scholar]
- 7.Zoll B, Zhao H, Edwards MA, Petrov R, Schey R, Parkman HP. Outcomes of surgical intervention for refractory gastroparesis: a systematic review.J Surg Res. 2018. Nov;231:263–269 [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Ramsey FV, Norton WF, et al. The burdens, concerns, and quality of life of patients with gastroparesis. Dig Dis Sci 2017;62:879–893. [DOI] [PubMed] [Google Scholar]
- 9.Wadhwa V, Mehta D, Jobanputra Y, et al. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol 2017;23:4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy BE, Crowell MD, Mathis C, et al. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol 2018;52:20–24. [DOI] [PubMed] [Google Scholar]
- 11.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology 2009;137:445–452. [DOI] [PubMed] [Google Scholar]
- 12.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y, Jiang B, Manne S, et al. Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut 2021;70:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y, Yin Y, Huh SY, et al. Epidemiology and etiology of gastroparesis in the USA: real-world evidence from a large national claims database. Gastroenterology 2022;162:109–121.e5. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Sanders KM. Commentary: Opiates, the pylorus, and gastroparesis. Gastroenterology 2020;159:414–421. [DOI] [PubMed] [Google Scholar]
- 16.Hasler WL, Wilson LA, Nguyen LA, et al. Opioid use and potency are associated with clinical features, quality of life, and use of resources in patients with gastroparesis. Clin Gastroenterol Hepatol 2019;17:1285–1294.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer AH, Krevsky B, Knight LC, et al. Opioid and opioid-like drug effects on whole-gut transit measured by scintigraphy. J Nucl Med 1996;37:818–822. [PubMed] [Google Scholar]
- 18.Jeong ID, Camilleri M, Shin A, et al. A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther 2012;35:1088–1096. [DOI] [PubMed] [Google Scholar]
- 19.Schol J, Wauters L, Dickman R, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United European Gastroenterol J 2021;9:287–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilleri M, Dilmaghani S, Vosoughi K, et al. A North American perspective on the ESNM consensus statement on gastroparesis. Neurogastroenterol Motil 2021. May 17:e14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharucha AE, Batey-Schaefer B, Cleary PA, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology 2015;149:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calles-Escandon J, Koch KL, Hasler WL, et al. Glucose sensor-augmented continuous subcutaneous insulin infusion in patients with diabetic gastroparesis: An open-label pilot prospective study. PLoS One 2018;13:e0194759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015;13:466–476.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izzy M, Lee M, Johns-Keating K, et al. Glycosylated hemoglobin level may predict the severity of gastroparesis in diabetic patients. Diabetes Res Clin Pract 2018;135:45–49. [DOI] [PubMed] [Google Scholar]
- 26.Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: A systematic review and meta-analysis. Gut 2019;68:804–813. [DOI] [PubMed] [Google Scholar]
- 27.Abell TL, Camilleri M, Donohoe K, et al. American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753–763. [DOI] [PubMed] [Google Scholar]
- 28.Pasricha PJ, Grover M, Yates KP, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology 2021;160:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann JF, Chassany O, Guillausseau PJ, et al. Simultaneous noninvasive evaluation of gastric emptying and orocaecal transit times. Use in studying the actions of cisapride in diabetic patients. Eur J Clin Pharmacol 1992;43:121–124. [DOI] [PubMed] [Google Scholar]
- 30.Brummer RJ, Schoenmakers EA, Kemerink GJ, et al. The effect of a single rectal dose of cisapride on delayed gastric emptying. Aliment Pharmacol Ther 1997;11:781–785. [DOI] [PubMed] [Google Scholar]
- 31.Chang CS, Chen GH, Kao CH, et al. Correlation between patterns of antral contractility and gastric emptying of radiopaque markers. Am J Gastroenterol 1997;92:830–834. [PubMed] [Google Scholar]
- 32.Olausson EA, Brock C, Drewes AM, et al. Measurement of gastric emptying by radiopaque markers in patients with diabetes: correlation with scintigraphy and upper gastrointestinal symptoms. Neurogastroenterol Motil 2013;25:e224–232. [DOI] [PubMed] [Google Scholar]
- 33.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012;24:1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai A, O’Connor M, Neja B, et al. Reproducibility of gastric emptying assessed with scintigraphy in patients with upper GI symptoms. Neurogastroenterol Motil 2018;30:e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AA, Rao S, Nguyen LA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol 2019;17:1770–1779.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee A, Wilding G, Kuo B. Variable abnormal physiological motility in the proximal upper gastrointestinal tract in gastroparesis. Neurogastroenterol Motil 2012;24:652–657.e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008;20:311–319. [DOI] [PubMed] [Google Scholar]
- 38.Hasler WL, May KP, Wilson LA, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil 2018. Feb;30(2): 10.1111/nmo.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zikos TA, Kamal AN, Neshatian L, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil 2019;25:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolar GJ, Camilleri M, Burton D, et al. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil 2014;26:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkman HP, Sharkey E, McCallum RW, et al. Constipation in patients with symptoms of gastroparesis: analysis of symptoms and gastrointestinal transit. Clin Gastroenterol Hepatol 2020. Oct 28;S1542-3565(20)31503-2. doi: 10.1016/j.cgh.2020.10.045. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi D, Choi C, League J, et al. Food residue during esophagogastroduodenoscopy is commonly encountered and is not pathognomonic of delayed gastric emptying. Dig Dis Sci 2021;66:3951–3959. [DOI] [PubMed] [Google Scholar]
- 43.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol 2008;6:635–643.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viramontes BE, Kim DY, Camilleri M, et al. Validation of a stable isotope gastric emptying test for normal, accelerated or delayed gastric emptying. Neurogastroenterol Motil 2001;13:567–574. [DOI] [PubMed] [Google Scholar]
- 45.Brzana RJ, Koch KL, Bingaman S. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am J Gastroenterol 1998;93:1803–1809. [DOI] [PubMed] [Google Scholar]
- 46.Chen JD, Lin Z, Pan J, et al. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci 1996;41:1538–1545. [DOI] [PubMed] [Google Scholar]
- 47.Christensen CJ, Johnson WD, Abell TL. Patients with cyclic vomiting pattern and diabetic gastropathy have more migraines, abnormal electrogastrograms, and gastric emptying. Scand J Gastroenterol 2008;43:1076–1081. [DOI] [PubMed] [Google Scholar]
- 48.Gaber AO, Oxley D, Karas J, et al. Changes in gastric emptying in recipients of successful combined pancreas-kidney transplants. Dig Dis 1991;9:437–443. [DOI] [PubMed] [Google Scholar]
- 49.Cucchiara S, Franzese A, Salvia G, et al. Gastric emptying delay and gastric electrical derangement in IDDM. Diabetes Care 1998;21:438–443. [DOI] [PubMed] [Google Scholar]
- 50.Forster J, Damjanov I, Lin Z, et al. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg 2005;9:102–108. [DOI] [PubMed] [Google Scholar]
- 51.Angeli TR, Cheng LK, Du P, et al. Loss of interstitial cells of Cajal and patterns of gastric dysrhythmia in patients with chronic unexplained nausea and vomiting. Gastroenterology 2015;149:56–66.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Grady G, Angeli TR, Du P, et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 2012;143:589–598.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carson DA, O’Grady G, Du P, Gharibans AA, Andrews CN. Body surface mapping of the stomach: New directions for clinically evaluating gastric electrical activity. Neurogastroenterol Motil. 2021. Mar;33(3):e14048. [DOI] [PubMed] [Google Scholar]
- 54.Burton Murray H, Jehangir A, Silvernale CJ, et al. Avoidant/restrictive food intake disorder symptoms are frequent in patients presenting for symptoms of gastroparesis. Neurogastroenterol Motil 2020;32:e13931. [DOI] [PubMed] [Google Scholar]
- 55.Olausson EA, Störsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol 2014;109:375–385. [DOI] [PubMed] [Google Scholar]
- 56.Olausson EA, Alpsten M, Larsson A, et al. Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res Clin Pract 2008;80:231–237. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann S, Ferrie S, Carey S. Nutrition management in patients with chronic gastrointestinal motility disorders: a systematic literature review. Nutr Clin Pract 2020;35:219–230. [DOI] [PubMed] [Google Scholar]
- 58.Perkel MS, Moore C, Hersh T, et al. Metoclopramide therapy in patients with delayed gastric emptying: a randomized, double-blind study. Dig Dis Sci 1979;24:662–666. [DOI] [PubMed] [Google Scholar]
- 59.Perkel MS, Hersh T, Moore C, et al. Metoclopramide therapy in fifty-five patients with delayed gastric emptying. Am J Gastroenterol 1980;74:231–236. [PubMed] [Google Scholar]
- 60.Snape WJ Jr, Battle WM, Schwartz SS, et al. Metoclopramide to treat gastroparesis due to diabetes mellitus: a double-blind, controlled trial. Ann Intern Med 1982;96:444–446. [DOI] [PubMed] [Google Scholar]
- 61.McCallum RW, Ricci DA, Rakatansky H, et al. A multicenter placebo-controlled clinical trial of oral metoclopramide in diabetic gastroparesis. Diabetes Care 1983;6:463–467. [DOI] [PubMed] [Google Scholar]
- 62.Ricci DA, Saltzman MB, Meyer C, et al. Effect of metoclopramide in diabetic gastroparesis. J Clin Gastroenterol 1985;7:25–32. [DOI] [PubMed] [Google Scholar]
- 63.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol 1999;94:1230–1234. [DOI] [PubMed] [Google Scholar]
- 64.Erbas T, Varoglu E, Erbas B, et al. Comparison of metoclopramide and erythromycin in the treatment of diabetic gastroparesis. Diabetes Care 1993;16:1511–1514. [DOI] [PubMed] [Google Scholar]
- 65.Longstreth GF, Malagelada JR, Kelly KA. Metoclopramide stimulation of gastric motility and emptying in diabetic gastroparesis. Ann Intern Med 1977;86:195–196. [DOI] [PubMed] [Google Scholar]
- 66.Loo FD, Palmer DW, Soergel KH, et al. Gastric emptying in patients with diabetes mellitus. Gastroenterology 1984;86:485–494. [PubMed] [Google Scholar]
- 67.Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil 2014;26:521–528. [DOI] [PubMed] [Google Scholar]
- 68.Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: Results of a phase 2b randomized study. Clin Gastroenterol Hepatol 2015;13:1256–1263. [DOI] [PubMed] [Google Scholar]
- 69.Ganzini L, Casey DE, Hoffman WF, et al. The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med 1993;153:1469–1475. [PubMed] [Google Scholar]
- 70.Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther 2010;31:11–19. [DOI] [PubMed] [Google Scholar]
- 71.Al-Saffar A, Lennernäs H, Hellström PM. Gastroparesis, metoclopramide, and tardive dyskinesia: Risk revisited. Neurogastroenterol Motil 2019;31:e13617. [DOI] [PubMed] [Google Scholar]
- 72.Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. Br Med J (Clin Res Ed) 1985;291:930–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehrenpreis ED, Deepak P, Sifuentes H, et al. The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. Am J Gastroenterol 2013;108:866–872. [DOI] [PubMed] [Google Scholar]
- 74.Watts GF, Armitage M, Sinclair J, et al. Treatment of diabetic gastroparesis with oral domperidone. Diabet Med 1985;2:491–492. [DOI] [PubMed] [Google Scholar]
- 75.Soykan I, Sarosiek I, McCallum RW. The effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesis Am J Gastroenterol 1997;92:976–980. [PubMed] [Google Scholar]
- 76.Kozarek R Domperidone for symptomatic management of diabetic gastroparesis in metoclopramide treatment failures. Adv Ther 1990;7:61–68. [Google Scholar]
- 77.Koch KL, Stern RM, Stewart WR, et al. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment Am J Gastroenterol 1989;84:1069–1075. [PubMed] [Google Scholar]
- 78.Horowitz M, Harding PE, Chatterton BE, et al. Acute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathy. Dig Dis Sci 1985;30:1–9. [DOI] [PubMed] [Google Scholar]
- 79.Silvers M, Kipnes V, Broadstone A, et al. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. Clin Ther 1998;20:438–453. [DOI] [PubMed] [Google Scholar]
- 80.Braun AP. Domperidone in the treatment of symptoms of delayed gastric emptying in diabetic patients Adv Ther 1989;6:51–62. [Google Scholar]
- 81.Heer M, Müller-Duysing W, Benes I, et al. Diabetic gastroparesis: treatment with domperidone—a double-blind, placebo-controlled trial. Digestion 1983;27:214–217. [DOI] [PubMed] [Google Scholar]
- 82.Franzese A, Borrelli O, Corrado G, et al. Domperidone is more effective than cisapride in children with diabetic gastroparesis Aliment Pharmacol Ther 2002;16:951–957. [DOI] [PubMed] [Google Scholar]
- 83.Nagler J, Miskovitz P. Clinical evaluation of domperidone in the treatment of chronic postprandial idiopathic upper gastrointestinal distress. Am J Gastroenterol 1981;76:495–499. [PubMed] [Google Scholar]
- 84.Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol 1999;94:1230–1234. [DOI] [PubMed] [Google Scholar]
- 85.Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care 1998;21:1699–1706. [DOI] [PubMed] [Google Scholar]
- 86.Sarosiek I, Van Natta M, Parkman HP, et al. Effect of domperidone therapy on gastroparesis symptoms: results of a dynamic cohort study by NIDDK Gastroparesis Consortium. Clin Gastroenterol Hepatol 2021. Jun 2;S1542-3565(21)00602-9. doi: 10.1016/j.cgh.2021.05.063. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bavestrello L, Caimi L, Barbera A. A double-blind comparison of clebopride and placeb in dyspepsia secondary to delayed gastric emptying. Clin Ther 1985;7:468–473. [PubMed] [Google Scholar]
- 88.Andrews CN, Woo M, Buresi M, et al. Prucalopride in diabetic and connective tissue disease-related gastroparesis: Randomized placebo-controlled crossover pilot trial” Neurogastroenterol Motil 2021;33:e13958. [DOI] [PubMed] [Google Scholar]
- 89.Carbone F, Van den Houte K, Clevers E, et al. Prucalopride in gastroparesis: a randomized placebo-controlled crossover study. Am J Gastroenterol 2019;114:1265–1274. [DOI] [PubMed] [Google Scholar]
- 90.Tack J, Rotondo A, Meulemans A, et al. Randomized clinical trial: a controlled pilot trial of the 5-HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis. Neurogastroenterol Motil 2016;28:487–497. [DOI] [PubMed] [Google Scholar]
- 91.Kuo B, Barnes CN, Nguyen DD, et al. Velusetrag accelerates gastric emptying in subjects with gastroparesis: a multicentre, double-blind, randomised, placebo-controlled, phase 2 study. Aliment Pharmacol Ther 2021;53:1090–1097. [DOI] [PubMed] [Google Scholar]
- 92.Chedid V, Brandler J, Arndt K, et al. Randomised study: effects of the 5-HT(4) receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis. Aliment Pharmacol Ther 2021;53:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol 2013;11:1453–1459.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology 2016;151:87–96.e6. [DOI] [PubMed] [Google Scholar]
- 95.Camilleri M, McCallum RW, Tack J, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology 2017;153:1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong SW, Chun J, Kim J, et al. Efficacy and safety of ghrelin agonists in patients with diabetic gastroparesis: A systematic review and meta-analysis. Gut Liver 2020;14:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 1990;322:1028–1031. [DOI] [PubMed] [Google Scholar]
- 98.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol 1993;88:203–207. [PubMed] [Google Scholar]
- 99.Arts J, Caenepeel P, Verbeke K, et al. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut 2005;54:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larson JM, Tavakkoli A, Drane WE, et al. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil 2010;16:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thielemans L, Depoortere I, Perret J, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther 2005;313:1397–1405. [DOI] [PubMed] [Google Scholar]
- 102.Gorelik E, Masarwa R, Perlman A, et al. Systematic Review, Meta-analysis, and network meta-analysis of the cardiovascular safety of macrolides. Antimicrob Agents Chemother 2018;62:e00438–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology 2018;154:65–76.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carlin JL, Lieberman VR, Dahal A, et al. Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Gastroenterology 2021;160:76–87. [DOI] [PubMed] [Google Scholar]
- 105.Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: The NORIG randomized clinical trial. JAMA 2013;310:2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roldan CJ, Chambers KA, Paniagua L, et al. Randomized controlled double-blind trial comparing haloperidol combined with conventional therapy to conventional therapy alone in patients with symptomatic gastroparesis. Acad Emerg Med 2017;24:1307–1314. [DOI] [PubMed] [Google Scholar]
- 107.Rosch W, Vinson B, Sassin I. A randomised clinical trial comparing the efficacy of a herbal preparation STW 5 with the prokinetic drug cisapride in patients with dysmotility type of functional dyspepsia. Z Gastroenterol 2002;40:401–408. [DOI] [PubMed] [Google Scholar]
- 108.Braden B, Caspary W, Börner N, et al. Clinical effects of STW 5 (Iberogast) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil 2009;21:632–638.e25. [DOI] [PubMed] [Google Scholar]
- 109.Zikos TA, Nguyen L, Kamal A, et al. Marijuana, ondansetron, and promethazine are perceived as most effective treatments for gastrointestinal nausea. Dig Dis Sci 2020;65:3280–3286. [DOI] [PubMed] [Google Scholar]
- 110.Lacy BE, Saito YA, Camilleri M, et al. Effects of antidepressants on gastric function in patients with functional dyspepsia. Am J Gastroenterol 2018;113:216–224. [DOI] [PubMed] [Google Scholar]
- 111.Ramirez R, Stalcup P, Croft B, et al. Haloperidol undermining gastroparesis symptoms (HUGS) in the emergency department. Am J Emerg Med 2017;35:1118–1120. [DOI] [PubMed] [Google Scholar]
- 112.Abell TL, Garcia LM, Wiener GJ, et al. Effect of oral CNSA-001 (sepiapterin, PTC923) on gastric accommodation in women with diabetic gastroparesis: a randomized, placebo-controlled, Phase 2 trial. J Diabetes Complications 2021;35:107961. [DOI] [PubMed] [Google Scholar]
- 113.Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci 1998;43:2398–2404. [DOI] [PubMed] [Google Scholar]
- 114.Vijayvargiya P, Camilleri M, Chedid V, et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 2019;156:1650–1660. [DOI] [PubMed] [Google Scholar]
- 115.Soota K, Kedar A, Nikitina Y, et al. Immunomodulation for treatment of drug and device refractory gastroparesis. Results Immunol 2016;6:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashat M, Lewis A, Liaquat H, et al. Intravenous immunoglobulin in drug and device refractory patients with the symptoms of gastroparesis-an open-label study. Neurogastroenterol Motil 2018. Mar;30(3). doi: 10.1111/nmo.13256. [DOI] [PubMed] [Google Scholar]
- 117.Paulon E, Nastou D, Jaboli F, et al. Proof of concept: short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis. Frontline Gastroenterol 2017;8:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gottfried-Blackmore A, Adler EP, Fernandez-Becker N, et al. Open-label pilot study: Non-invasive vagal nerve stimulation improves symptoms and gastric emptying in patients with idiopathic gastroparesis. Neurogastroenterol Motil 2020;32:e13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapural L, Brown BK, Harandi S, et al. Effects of spinal cord stimulation in patients with chronic nausea, vomiting, and refractory abdominal pain. Dig Dis Sci 2021. Feb 23. doi: 10.1007/s10620-021-06896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc 2011;74:496–503.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ducrotte P, Coffin B, Bonaz B, et al. Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Gastroenterology 2020;158:506–514.e2. [DOI] [PubMed] [Google Scholar]
- 122.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:421–428. [DOI] [PubMed] [Google Scholar]
- 123.McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol 2010;8:947–954. [DOI] [PubMed] [Google Scholar]
- 124.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil 2013;25:815–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo J, Al-Juburi A, Rashed H, et al. Gastric electrical stimulation is associated with improvement in pancreatic exocrine function in humans. Pancreas 2004;29:e41–e44. [DOI] [PubMed] [Google Scholar]
- 126.Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol 2008;43:1066–1075. [DOI] [PubMed] [Google Scholar]
- 127.Abell TL, Yamada G, McCallum RW, et al. Effectiveness of gastric electrical stimulation in gastroparesis: Results from a large prospectively collected database of national gastroparesis registries. Neurogastroenterol Motil 2019;31:e13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil 2005;17:35–43. [DOI] [PubMed] [Google Scholar]
- 129.Kong M-F. NICE guidance on gastroelectrical stimulation for gastroparesis. Br J Diab Vasc Dis 2015; 15. 10.15277/bjdvd.2015.001. [DOI] [Google Scholar]
- 130.O’Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg 2009;33:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Levinthal DJ, Bielefeldt K. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci 2017;202:45–55. [DOI] [PubMed] [Google Scholar]
- 132.Lal N, Livemore S, Dunne D, et al. Gastric electrical stimulation with the Enterra System: a systematic review. Gastroenterol Res Pract 2015;2015:762972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chu H, Lin Z, Zhong L, et al. Treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol 2012;27:1017–1026. [DOI] [PubMed] [Google Scholar]
- 134.Gourcerol G, Coffin B, Bonaz B, et al. Impact of gastric electrical stimulation on economic burden of refractory vomiting: a French nationwide multicentre study. Clin Gastroenterol Hepatol 2020. Nov 13;S1542-3565(20)31544-5. doi: 10.1016/j.cgh.2020.11.011. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol 2005;3:49–54. [DOI] [PubMed] [Google Scholar]
- 136.Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci 2005;50:1328–1334. [DOI] [PubMed] [Google Scholar]
- 137.Lin Z, Sarosiek I, Forster J, et al. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil 2006;18:18–27. [DOI] [PubMed] [Google Scholar]
- 138.Musunuru S, Beverstein G, Gould J. Preoperative predictors of significant symptomatic response after 1 year of gastric electrical stimulation for gastroparesis. World J Surg 2010;34:1853–1858. [DOI] [PubMed] [Google Scholar]
- 139.Hou Q, Lin Z, Mayo MS, et al. Is symptom relief associated with reduction in gastric retention after gastric electrical stimulation treatment in patients with gastroparesis? A sensitivity analysis with logistic regression models. Neurogastroenterol Motil 2012;24:639–645.e274. [DOI] [PubMed] [Google Scholar]
- 140.Hannon MJ, Dinneen S, Yousif O, et al. Gastric pacing for diabetic gastroparesis--does it work? Ir Med J 2011;104:135–137. [PubMed] [Google Scholar]
- 141.Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. J Parenter Enteral Nutr 2003;27:277–281. [DOI] [PubMed] [Google Scholar]
- 142.Forster J, Sarosiek I, Lin Z, et al. Further experience with gastric stimulation to treat drug refractory gastroparesis. Am J Surg 2003;186:690–695. [DOI] [PubMed] [Google Scholar]
- 143.Xu F, Tan Y, Huang Z, et al. Ameliorating effect of transcutaneous electroacupuncture on impaired gastric accommodation in patients with postprandial distress syndrome-predominant functional dyspepsia: a pilot study. Evid Based Complement Alternat Med 2015;2015:168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li G, Huang C, Zhang X, et al. The short-term effects of acupuncture on patients with diabetic gastroparesis: a randomised crossover study. Acupunct Med 2015;33:204–209. [DOI] [PubMed] [Google Scholar]
- 145.Kostitska IO. Efficacy of acupuncture in the treatment of patients with diabetic gastroparesis. Diabetologia 2016;59:S464. [Google Scholar]
- 146.Sun BM, Luo M, Wu S-B, et al. Acupuncture versus metoclopramide in treatment of postoperative gastroparesis syndrome in abdominal surgical patients: A randomized controlled trial. Chin J Integr Med 2010;8:641–644. [DOI] [PubMed] [Google Scholar]
- 147.Pfab F, Winhard M, Nowak-Machen M, et al. Acupuncture in critically ill patients improves delayed gastric emptying: A randomized controlled trial. Anesth Analg 2011;112:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarosiek I, Song G, Sun Y, et al. Central and peripheral effects of transcutaneous acupuncture treatment for nausea in patients with diabetic gastroparesis. J Neurogastroenterol Motil 2017;23:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xuefen W, Ping L, Li L, et al. A clinical randomized controlled trial of acupuncture treatment of gastroparesis using different acupoints. Pain Res Manag 2020. Apr 25;2020:8751958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim KH, Lee MS, Choi T-Y, et al. Acupuncture for symptomatic gastroparesis. Cochrane Database Syst Rev 2018. Dec 18;12(12):CD009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang M, Li X, Liu S, et al. Meta-analysis of acupuncture for relieving non-organic dyspeptic symptoms suggestive of diabetic gastroparesis. BMC Complement Altern Med 2013;13:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rashed H, Cutts T, Abell T, et al. Predictors of response to a behavioral treatment in patients with chronic gastric motility disorders. Dig Dis Sci 2002;47:1020–1026. [DOI] [PubMed] [Google Scholar]
- 153.Tian J, Li M, Liao J, et al. Chinese herbal medicine banxiaxiexin decoction treating diabetic gastroparesis: A systematic review of randomized controlled trials. Evid Based Complement Alternat Med 2013;2013:749495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tian JX, Li M, Liao J-Q, et al. Xiangshaliujunzi Decoction for the treatment of diabetic gastroparesis: A systematic review. World J Gastroenterol 2014;20:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gourcerol G, Tissier F, Melchio C, et al. Impaired fasting pyloric compliance in gastroparesis and the therapeutic response to pyloric dilatation. Aliment Pharmacol Ther 2015;41:360–367. [DOI] [PubMed] [Google Scholar]
- 156.Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil 2015;27:524–531. [DOI] [PubMed] [Google Scholar]
- 157.Snape WJ, Lin MS, Agarwal N, et al. Evaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomiting. Neurogastroenterol Motil 2016;28:758–764. [DOI] [PubMed] [Google Scholar]
- 158.Saadi M, Yu D, Malik Z, et al. Pyloric sphincter characteristics using EndoFLIP((R)) in gastroparesis. Rev Gastroenterol Mex 2018;83:375–384. [DOI] [PubMed] [Google Scholar]
- 159.Vosoughi K, Ichkhanian Y, Jacques J, et al. Role of endoscopic functional luminal imaging probe in predicting the outcome of gastric peroral endoscopic pyloromyotomy. Gastrointest Endosc 2020;91:1289–1299. [DOI] [PubMed] [Google Scholar]
- 160.Watts LS, Baker JR, Lee AA, et al. Impact of gastric per-oral endoscopic myotomy on static and dynamic pyloric function in gastroparesis patients Neurogastroenterol Motil 2020;32:e13892. [DOI] [PubMed] [Google Scholar]
- 161.Desprez C, Melchior C, Wuestenberghs F, et al. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc 2019;90:754–760.e1. [DOI] [PubMed] [Google Scholar]
- 162.Bai Y, Xu MJ, Yang X, et al. A systematic review on intrapyloric botulinum toxin injection for gastroparesis. Digestion 2010;81:27–34. [DOI] [PubMed] [Google Scholar]
- 163.Gonzalez JM, Benezech A, Vitton V, et al. G-POEM with antro-pyloromyotomy for the treatment of refractory gastroparesis: mid-term follow-up and factors predicting outcome. Aliment Pharmacol Ther 2017;46:364–370. [DOI] [PubMed] [Google Scholar]
- 164.Dacha S, Mekaroonkamol P, Li L, et al. Outcomes and quality-of-life assessment after gastric per-oral endoscopic pyloromyotomy (with video). Gastrointest Endosc 2017;86:282–289. [DOI] [PubMed] [Google Scholar]
- 165.Rodriguez JH, Haskins IN, Strong AT, et al. Per oral endoscopic pyloromyotomy for refractory gastroparesis: initial results from a single institution. Surg Endosc 2017;31:5381–5388. [DOI] [PubMed] [Google Scholar]
- 166.Khashab MA, Ngamruengphong S, Carr-Locke D, et al. Gastric per-oral endoscopic myotomy for refractory gastroparesis: results from the first multicenter study on endoscopic pyloromyotomy (with video). Gastrointest Endosc 2017;85:123–128. [DOI] [PubMed] [Google Scholar]
- 167.Malik Z, Kataria R, Modayil R, et al. Gastric per oral endoscopic myotomy (G-POEM) for the treatment of refractory gastroparesis: early experience. Dig Dis Sci 2018;63:2405–2412. [DOI] [PubMed] [Google Scholar]
- 168.Xu J, Chen T, Elkholy S, et al. Gastric peroral endoscopic myotomy (G-POEM) as a treatment for refractory gastroparesis: long-term outcomes. Can J Gastroenterol Hepatol 2018;2018:6409698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jacques J, Pagnon L, Hure F, et al. Peroral endoscopic pyloromyotomy is efficacious and safe for refractory gastroparesis: prospective trial with assessment of pyloric function. Endoscopy 2019;51:40–49. [DOI] [PubMed] [Google Scholar]
- 170.Mekaroonkamol P, Patel V, Shah R, et al. Association between duration or etiology of gastroparesis and clinical response after gastric per-oral endoscopic pyloromyotomy. Gastrointest Endosc 2019;89:969–976. [DOI] [PubMed] [Google Scholar]
- 171.Strong AT, Rodriguez J, Kroh M, et al. Safety and feasibility of per-oral pyloromyotomy as augmentative therapy after prior gastric electrical stimulation for gastroparesis. J Am Coll Surg 2019;229:589–595. [DOI] [PubMed] [Google Scholar]
- 172.Strong AT, Landreneau JP, Cline M, et al. Per-oral pyloromyotomy (POP) for medically refractory post-surgical gastroparesis. J Gastrointest Surg 2019;23:1095–1103. [DOI] [PubMed] [Google Scholar]
- 173.Vosoughi K, Ichkhanian Y, Benias P, et al. Gastric per-oral endoscopic myotomy (G-POEM) for refractory gastroparesis: results from an international prospective trial. Gut 2021. Mar 19;gutjnl-2020-322756. doi: 10.1136/gutjnl-2020-322756. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 174.Hustak R, Mares J, Vackova1 Z, et al. Endoscopic pyloromyotomy (G-POEM) improves symptoms in patients with refractory gastroparesis – a randomized sham controlled trial. UEG Journal 2021; 9:10–11 (abstract) [Google Scholar]
- 175.Ragi O, Jacques J, Branche J, et al. One-year results of gastric peroral endoscopic myotomy for refractory gastroparesis: a French multicenter study. Endoscopy 2021;53:480–490. [DOI] [PubMed] [Google Scholar]
- 176.Zhang H, Zhang J, Jiang A, et al. Gastric peroral endoscopic myotomy for gastroparesis: A systematic review of efficacy and safety. Gastroenterol Hepatol 2019;42:413–422. [DOI] [PubMed] [Google Scholar]
- 177.Garg R, Mohan BP, Aggarwal M, et al. Peroral pyloromytomy is effective and safe for postsurgical gastroparesis. J Gastrointest Surg 2020;24:1417–1420. [DOI] [PubMed] [Google Scholar]
- 178.Li P, Ma B, Gong S, et al. Gastric per-oral endoscopic myotomy for refractory gastroparesis: a meta-analysis. J Gastrointest Surg 2021;25:1108–1116. [DOI] [PubMed] [Google Scholar]
- 179.Spadaccini M, Maselli R, Chandrasekar VT, et al. Gastric peroral endoscopic pyloromyotomy for refractory gastroparesis: a systematic review of early outcomes with pooled analysis. Gastrointest Endosc 2020;91:746–752.e5. [DOI] [PubMed] [Google Scholar]
- 180.Landreneau JP, Strong AT, El-Hayek K, et al. Laparoscopic pyloroplasty versus endoscopic per-oral pyloromyotomy for the treatment of gastroparesis. Surg Endosc 2019;33:773–781. [DOI] [PubMed] [Google Scholar]
- 181.Mohan BP, Chandan S, Jha LK, et al. Clinical efficacy of gastric per-oral endoscopic myotomy (G-POEM) in the treatment of refractory gastroparesis and predictors of outcomes: a systematic review and meta-analysis using surgical pyloroplasty as a comparator group. Surg Endosc 2020;34:3352–3367. [DOI] [PubMed] [Google Scholar]
- 182.Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc 2016;30:1326–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


