Abstract
Background
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare form of vasculitis in children. SARS-CoV-2, the virus that causes COVID-19 infection, seems to trigger autoimmunity and new-onset autoimmune disease in pediatric and adult patients. We present a case of new-onset AAV following COVID-19 infection in an adolescent patient, and we review the literature of AAV following COVID-19 infection.
Case presentation
An adolescent female with a history of asthma was diagnosed with mild COVID-19 infection and subsequently developed persistent cough, wheezing, hearing loss, arthralgias, and rash. Her imaging and laboratory workup showed pulmonary nodules and cavitary lesions, elevated inflammatory markers, negative infectious testing, and positive ANCA. She was treated with glucocorticoids, rituximab, and mycophenolate mofetil. At six-month follow-up, she had improvement in her symptoms, pulmonary function tests, imaging findings, and laboratory markers.
Conclusions
We report the second case of new-onset anti-PR3, C-ANCA vasculitis and the fourth case of pediatric-onset AAV following COVID-19 infection. A systematic review of the literature found 6 cases of new-onset AAV in adults after COVID-19 infection. Pediatric and adult patients who develop AAV post COVID-19 infection have few, if any, comorbidities, and show marked radiographic and symptomatic improvement after treatment. There is increasing evidence for COVID-19-induced autoimmunity in children and our case highlights the importance of considering AAV in a child following a recent COVID-19 infection because timely treatment may improve clinical outcomes.
Background
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare form of vasculitis in children with an estimated annual incidence of < 1 per one million children [1–4]. Granulomatosis with polyangiitis (GPA), one of the ANCA-associated vasculitides, is a systemic, necrotizing vasculitis with granulomatous inflammation that typically affects the upper and lower respiratory tract and kidneys [5]. It often presents with nonspecific symptoms including fever, malaise, weight loss, anorexia, myalgias and arthralgias. Although the mechanism of pathogenesis is not fully understood, AAV is thought to be immune-mediated with a chronic and relapsing course [6].
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused significant morbidity and mortality since it was first reported in late 2019 [7]. SARS-CoV-2, similar to other viruses, seems to trigger autoimmunity in both the pediatric and adult populations [8–11]. Acute COVID-19 infection causes less severe symptoms in the pediatric population [12, 13]; however, a small percentage of children subsequently develop immune-mediated disease after COVID-19 infection [8, 9, 14]. We present a case of new onset AAV, most consistent with GPA, following COVID-19 infection in a 16-year-old female, and we review the literature of AAV following COVID-19 infection to highlight another potential SARS-CoV-2 triggered immune-mediated disease in the pediatric population.
Case presentation
A 16-year-old female with a past medical history significant for asthma was referred to the pediatric pulmonology clinic by her pediatrician for persistent cough and wheezing following COVID-19 infection. Her COVID-19 infection consisted of mild upper respiratory symptoms with anosmia and was diagnosed via PCR.
Approximately 1 week after recovering from COVID-19, she developed wheezing and a prominent, non-productive cough. She was initially treated by her pediatrician with albuterol which helped her wheezing but not her cough. A chest x-ray at that time was normal. Over the next month, her symptoms progressed to include sinus pain, serosanguinous ear drainage, and a sensation of fullness in her ears. Her pediatrician treated her with courses of azithromycin, cefdinir, and doxycycline. The antibiotics did not resolve her symptoms and she was trialed on a 5-day course of prednisone (60 mg daily). She saw otolaryngology who diagnosed her with chronic bilateral serous otitis media and recommended tympanostomy tube placement. She underwent tympanostomy tube placement with some relief in her symptoms but reported ongoing bilateral hearing loss. She had a CT scan following tympanostomy tube placement that showed bilateral opacified mastoid air cells consistent with chronic inflammation.
When she presented to pediatric pulmonology 6 weeks after the onset of her symptoms, she was still wheezing and had a daily paroxysmal cough with occasional post-tussive emesis. She also reported chest tightness and difficulty breathing. She had a chest x-ray which showed patchy airspace disease of the upper lungs, concerning for a multifocal infectious or inflammatory process. Her symptoms suggested an exacerbation of asthma for which she was prescribed another 5-day course of prednisone (40 mg twice daily), albuterol, and she was started on inhaled corticosteroids in combination with a long-acting beta agonist. Her symptoms improved initially but returned as soon as she stopped taking systemic corticosteroids. Over the next 5 weeks, the patient’s cough became productive of green sputum with worsening wheezing that was no longer responsive to bronchodilators. She was treated with a 28-day course of cefdinir for protracted bacterial bronchitis. When she returned for follow up three months later, she continued to complain of cough and wheezing with new onset myalgias. She underwent pulmonary function testing which showed moderate post-bronchodilation small airway obstruction.
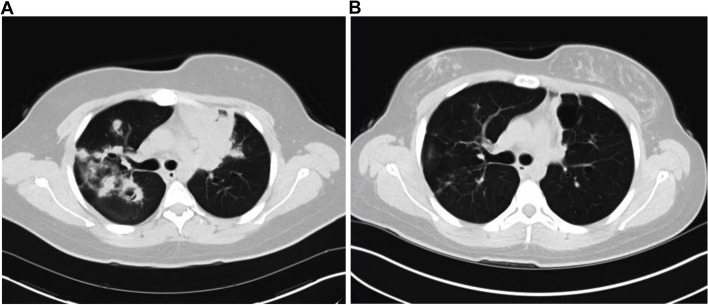
Chest x-ray revealed perihilar and bilateral upper lobe consolidations that represented a significant progression from her previous x-ray 3 months prior. High resolution chest CT demonstrated extensive multifocal pulmonary nodules and regions of consolidation with multiple areas of cavitation and central bronchiectasis with diffuse bronchial wall thickening as well as reactive mediastinal and hilar adenopathy (Fig. 1A). CT findings were concerning for allergic bronchopulmonary aspergillosis or chronic pulmonary aspergillosis. However, the patient underwent aspergillus antibody studies which were negative. (1–3) Beta-D-Glucan (Fungitell) assay was negative. No IFN-gamma response to M tuberculosis antigens was detected (Quantiferon assay). Induced sputum culture was negative for bacteria, fungal organisms, and acid-fast bacilli. Other laboratory parameters were notable for elevated inflammatory markers with a C-reactive protein of 12 mg/L (0.0–10.0 mg/L) and an erythrocyte sedimentation rate of 30 mm/h (0.0–20.0 mm/h). Her ANCA screen was positive: her C-ANCA was positive with a titer of 1:40 (< 1:20). P-ANCA was negative. Proteinase 3 antibody (anti-PR3) was positive with an antibody index (AI) of 1.4 (0.0–0.9 AI). Antinuclear antibody (ANA) screen was positive with a titer of 1:40 in a speckled pattern. Anticardiolipin antibodies, myeloperoxidase antibodies and anti-glomerular basement membrane antibodies were negative. Beta 2 Glycoprotein 1 IgM and IgG were negative; Complement C3 was 165 mg/dL (88–193 mg/dL) and Complement C4 was 23 mg/dL (15–57 mg/dL). A complete blood count (CBC) was notable for a white blood cell count (WBC) of 11.3 × 10^9/L (3.5–11.0 × 10^9/L), hemoglobin of 13.5 g/dL (11.4–15.4 g/dL), hematocrit of 41.6% (34.2–46.2%), platelet count of 464 × 10^9/L (150–400 × 10^9/L). Serum creatinine and urinalysis were normal. Initial pulmonary function testing revealed a moderate obstructive pattern. She was referred to pediatric rheumatology for concern of systemic vasculitis.
Fig. 1.
A High resolution CT chest taken four months after the onset of symptoms shows extensive multifocal pulmonary nodules and regions of consolidation with areas of cavitation and central bronchiectasis. B Repeat high resolution CT chest taken 7 months after initial imaging (A) shows an overall improvement in the extent of previously seen multifocal consolidation, nodularity, and cavitation in the lungs, with scattered regions of scarring and several persistent but much smaller nodules. Patient had successfully completed systemic corticosteroid and rituximab treatment at the time of imaging
When she presented to pediatric rheumatology clinic, her review of systems was noteworthy for a 45-pound weight loss; intermittent conjunctivitis; sinus congestion, purulent nasal discharge, bilateral hearing loss, ear drainage, sinus headaches, recurrent nosebleeds; cough, wheezing and shortness of breath; progressively worsening bilateral arthralgias in her knees, feet and elbows that improved with activity; intermittent rashes, and sun sensitivity.
She underwent lung tissue biopsy which showed non-specific findings with focal areas of parenchymal atelectasis as well as scattered intra-alveolar macrophages. There was no evidence of acute or granulomatous inflammation or vasculitis identified in the biopsied specimen. There were no yeast or fungal elements seen.
Our patient’s clinical presentation and positive serology is most consistent with GPA. To induce remission, she was started on rituximab infusions which she tolerated well. She completed a glucocorticoid taper and was started on mycophenolate mofetil as maintenance therapy. At her most recent follow up, she reported a marked improvement in her symptoms although she continues to have bilateral conductive hearing loss necessitating hearing aids. Repeat CT imaging showed an overall improvement in the extent of previously seen multifocal consolidation, nodularity, and cavitation in her lungs (Fig. 1B). She demonstrated significant improvement in her pulmonary function tests (Table 1), and her ANCA and PR3 antibodies have remained negative to date.
Table 1.
Pulmonary function tests before and after AAV treatment
| Pulmonary function tests prior to treatment | Pulmonary function tests after treatment | |||
|---|---|---|---|---|
| % Predicted | % Predicted | |||
| FVC (L) | 2.62 | 75 | 3.88 | 96 |
| FEV1 (L) | 1.95 | 63 | 3.04 | 86 |
| FEV1/FVC (%) | 74.6 | 85 | 68.0 | 78 |
| FEF 25–75% (L/Second) | 1.82 | 1.82 | 2.80 | 68 |
| PEF (L/Second) | 2.27 | 30 | 5.15 | 73 |
FEV1 Forced expiratory volume in one second, FVC, Forced vital capacity FEV1/FVC ratio, Percentage of the FVC expired in one second FEF 25–75%, Forced expiratory flow over the middle one-half of the FVC PEF, Peak expiratory flow
Discussion
Here we report the second case of new-onset anti-PR3, C-ANCA vasculitis and the fourth case of AAV following COVID-19 infection in the pediatric population. The gold standard for diagnosing childhood vasculitis is with either histopathology from tissue biopsy or by characteristic lesions detected by imaging studies. Multiple studies in adults with AAV have found that less than half of all biopsies show the characteristic inflammatory and vasculitic changes to confirm the diagnosis [15–17], and sampling difficulty is often a limitation which could have explained our patient’s biopsy findings. Different classification criteria have been proposed to diagnose GPA in children [5, 18–20]. The mostly commonly used classification system in pediatrics is the European League Against Rheumatism/Pediatric Rheumatology International Trials Organization/Pediatric Rheumatology European Society (EULAR/PRINTO/PRES) classification scheme; it requires three of the following six features to diagnose GPA: abnormal urinalysis with hematuria or proteinuria; upper airway involvement (chronic purulent or bloody nasal discharge, nasal septum perforation or saddle nose deformity, chronic or recurrent sinus inflammation); granulomatous inflammation on biopsy; subglottic, tracheal or endobronchial stenosis; abnormal chest x-ray or CT; ANCA positivity (MPO-ANCA, PR3-ANCA or no specificity) [5, 18, 21]. Most patients (> 80%) have a positive ANCA, and GPA is primarily associated with PR3-ANCA [22, 23]. Approximately 90% of patients with GPA have ear, nose and throat (ENT) manifestations such as rhinosinusitis, otitis media, earache, conductive and/or sensorineural hearing loss, persistent rhinorrhea, purulent or bloody nasal discharge [24–26]. Our patient meets criteria for AAV and her clinical presentation—upper airway and middle ear involvement with saddle-nose deformity, recurrent nosebleeds, bilateral chronic serous otitis media and conductive hearing loss, abnormal CT showing cavitary lesions, and PR3 and C-ANCA positivity—is most consistent with GPA [18].
Search strategy and literature review
The authors conducted a systematic review of the literature from December 1, 2019 to January 1, 2022, in PubMed/MEDLINE, combining the MeSH search terms (COVID-19) AND (ANCA-associated vasculitis), filtered by age (birth-18) and date (Jan. 1, 2019-Jan. 1, 2022); ((Adolescent) AND (COVID-19) AND (ANCA-associated vasculitis)); ((Pediatric) AND (COVID-19) AND (ANCA-associated vasculitis)). Two authors (MCB, AY) independently screened titles, abstracts, and full texts of all relevant articles. All articles reporting ANCA-associated vasculitis after COVID-19 infection in the pediatric population were included. From each article, the authors collected data on publication year, age, gender, comorbidities, chronicity with COVID-19 infection, laboratory tests, serological profile, lung pathology, kidney pathology, AAV therapy, and outcomes.
Results from the literature review
A review of the literature found three cases of PAAV following COVID-19 infection, and, with the present report, four cases were included in this review [27–29]. Of the four patients (including ours), two were male [27–29]. Two patients had no prior comorbidities while two had pre-existing asthma [29]. Immunological tests showed C-ANCA positivity in two cases [27] and P-ANCA positivity in two [28, 29]. Two patients had anti-PR3 antibodies [27], and two patients had anti-MPO antibodies [28, 29]. Pulmonary imaging studies showed multifocal cavitary pulmonary nodules in two patients [27]; diffuse alveolar hemorrhage in two patients [28, 29]; dense patchy infiltrates in two patients [28, 29]. Two patients had normal kidney function at the time of presentation [27] while two patients had necrotizing glomerulonephritis on renal biopsy [28, 29]. Neither required hemodialysis [30–33]. All four patients were treated with glucocorticoids which are the standard of care for chronic vasculitis in children [34, 35]. Three patients received rituximab therapy [27, 28] and one patient received plasmapheresis [29]; for maintenance therapy, two were treated with cyclophosphamide [28, 29], one was treated with mycophenolate mofetil, and one did not continue therapy [27]. All four patients had symptomatic and radiographic improvement at follow up (Table 2) [27–29].
Table 2.
Summary of clinical findings, demographics, and treatment strategies of pediatric-onset AAV after COVID-19 infection
| Bryant et al | Powell et al | Fireizen et al | Reiff et al | |
|---|---|---|---|---|
| Age, years | 16 | 12 | 17 | 17 |
| Sex | Female | Female | Male | Male |
| Comorbidities | Asthma | None | Asthma and Obesity | None |
| Chronology with COVID-19 | 1–2 weeks following infection | 2 weeks following infection | 2 months following infection | Concurrent |
| Positive serology | Anti-PR3 and C-ANCA | Anti-MPO and ANCA | Anti-MPO and P-ANCA | Anti-PR3 and C-ANCA |
| Lung involvement at presentation | Extensive multifocal pulmonary nodules and regions of consolidation with multiple areas of cavitation and central bronchiectasis with diffuse bronchial wall thickening | Dense consolidation in the left lower lobe and patchy infiltrate in the right middle and upper lobes without ground-glass opacities; diffuse alveolar hemorrhage | Extensive heterogeneous infiltrates in both lungs with an unusual fluffy central distribution concerning for diffuse alveolar hemorrhage | Multiple bilateral cavitary lung lesions |
| Kidney involvement at presentation | Normal kidney function (Cr. 0.79 mg/dL) | Pauci-immune necrotizing and crescentic glomerulonephritis | Renal biopsy showed necrotizing glomerulonephritis with limited immune complex deposition | Normal kidney function (Cr. 0.74 mg/dL) |
| AAV treatment | Prednisone, rituximab, mycophenolate mofetil | Methylprednisolone, rituximab, cyclophosphamide | Methylprednisolone, plasmapheresis, cyclophosphamide | Methylprednisolone, rituximab, not on maintenance therapy |
| Antibody titers at presentation | 1:40 and a proteinase 3 antibody (PR3) level of 1.4 (normal < 1.0) | 1:640 and a perinuclear pattern | Not available | 1:640 and a proteinase 3 antibody (PR3) level of 251.9 (normal < 1.0) |
| Outcomes | Marked improvement of multifocal consolidation, nodularity, and cavitation on CT | Improvement in clinical symptoms | Resolution of DAH and AKI, not requiring outpatient dialysis | Clinically asymptomatic with marked improvement of cavitary lung nodules on CT |
There have been six reported cases of adults developing AAV following COVID-19 infection and a table summarizing these cases is well documented in an article by Izci Duran et al. [30] Similar to our findings, half of the adult patients had C-ANCA vasculitis with anti-PR3 antibodies. The most common lung findings were bilateral cavitary lesions, pulmonary infiltrates, and alveolar hemorrhage which were similar to the findings in children. Kidney involvement was more severe in the adults who developed AAV [30–33]. All adult patients had either crescentic or necrotizing glomerulonephritis on renal biopsy, active urinary sediment, and high creatinine levels. Two patients had kidney failure that required hemodialysis. Studies from the NIH report that glomerulonephritis is present in only 18% of patients at presentation [24]. However, 77 to 85% of patients subsequently develop glomerulonephritis, usually within the first two years of disease onset [24, 36].
The treatment strategy was similar in adults and children with all patients receiving systemic corticosteroids [27–33]. The adult patients received either rituximab or cyclophosphamide for induction therapy [30]. Cyclophosphamide is often added for patients with more severe disease [37]. Other immunosuppressive medications, such as methotrexate and azathioprine, as well as biologic agents (e.g., TNF-inhibitors, rituximab, and tocilizumab) have been used to treat adults with AAV and are increasingly being used to treat chronic vasculitis in the pediatric population [35, 38].
The clinical outcomes following treatment were generally favorable in both adult and pediatric patients: all patients had symptomatic and radiographic improvement; the patients with renal involvement had improvement or resolution of their hematuria, proteinuria and creatinine levels at follow-up; none required long-term dialysis; Two patients (one child and one adult) had bilateral hearing loss as part of their initial presentation [30]. Unfortunately, both continued to have profound hearing loss with the need for hearing aids or cochlear implants [30].
Conclusion
Our case presentation and review of the pediatric and adult literature show that AAV may be another autoimmune-mediated sequela of COVID-19. We report the tenth case of AAV following COVID-19 infection. In general, patients who develop AAV after COVID-19 infection have few, if any, comorbidities, and show marked radiographic and symptomatic improvement after treatment.
Autoimmunity may be generated by a combination of genetic, hormonal, and environmental factors in susceptible people [39, 40]. One such environmental trigger is viral illness. Studies have suggested a causal relationship between viral infections and the onset of autoimmune disease with molecular mimicry, hyperstimulation, dysregulation of the immune system, and complement activation being proposed mechanisms [10, 41]. Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus are examples of viruses that have an established association to multiple autoimmune diseases [42–44]. There is increasing evidence that SARS-CoV-2 is another virus that can lead to dysregulation of the immune system and the development of autoimmune disease in children and adults [11]. A recent study found that children with a history of COVID-19 infection were at an increased risk of developing type 1 diabetes mellitus than those without a history of COVID-19 [9]. Antecedent viral infections have been implicated in the development of PAAV including GPA [45]. Our report and review of the literature suggest that COVID-19 may be another viral trigger for the development of AAV in children and adults. One proposed mechanism for the development of autoantibodies including ANCA in the context of COVID-19 infection is the presence of extensive neutrophil infiltration at sites of tissue necrosis and immunothrombosis that could contribute to tissue tolerance failure with antibody production [46]. More research is needed to better understand how SARS-CoV-2 may act as a precipitating trigger of autoimmunity and autoimmune disease in children. Our case highlights the importance of considering AAV in a pediatric patient presenting with pulmonary and renal disease following a recent COVID-19 infection because timely treatment may improve clinical outcomes [45].
Acknowledgements
The authors would like to acknowledge David W. Swenson, MD, Assistant Professor of diagnostic imaging at The Warren Alpert Medical School of Brown University, for his expertise in interpreting our patient’s diagnostic imaging studies.
Abbreviations
- ANCA
Antineutrophil cytoplasmic antibody
- AAV
ANCA-associated vasculitis
- COVID-19
Coronavirus disease 2019
- GPA
Granulomatosis with polyangiitis
- PAAV
Pediatric-onset ANCA-associated vasculitis
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Authors’ contributions
A.Y. and L.T.S carried out the patient’s medical treatment. M.C.B and A.Y carried out the review of literature. M.C.B drafted the manuscript. All authors proof-read and approved the final version of the manuscript.
Funding
No financial support was received for this case report.
Availability of data and materials
All data regarding this study has been reported in the manuscript. Please contact the corresponding author if you are interested in any further information.
Declarations
Ethics approval and consent to participate
The present study was exempt from review by the Institutional Review Board of The Warren Alpert Medical School of Brown University and Lifespan Health System.
Consent for publication
Written informed consent was obtained from the patient’s parents regarding the publication of this case report. The purpose of this research was completely explained to the parents and the patient, and they were assured that their information would be kept confidential by the researchers.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dolezalová P, Telekesová P, Nemcová D, Hoza J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J Rheumatol. 2004;31(11):2295–2299. [PubMed] [Google Scholar]
- 2.Koldingsnes W, Nossent H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheum. 2000;43(11):2481–2487. doi: 10.1002/1529-0131(200011)43:11<2481::AID-ANR15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Stegmayr BG, Gothefors L, Malmer B, Müller Wiefel DE, Nilsson K, Sundelin B. Wegener granulomatosis in children and young adults. A case study of ten patients. Pediatr Nephrol Berl Ger. 2000;14(3):208–213. 10.1007/s004670050043 [DOI] [PubMed]
- 4.Sacri AS, Chambaraud T, Ranchin B, et al. Clinical characteristics and outcomes of childhood-onset ANCA-associated vasculitis: a French nationwide study. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2015;30(Suppl 1):i104–112. doi: 10.1093/ndt/gfv011. [DOI] [PubMed] [Google Scholar]
- 5.Cabral DA, Uribe AG, Benseler S, et al. Classification, presentation, and initial treatment of Wegener’s granulomatosis in childhood. Arthritis Rheum. 2009;60(11):3413–3424. doi: 10.1002/art.24876. [DOI] [PubMed] [Google Scholar]
- 6.Ewert BH, Jennette JC, Falk RJ. The pathogenic role of antineutrophil cytoplasmic autoantibodies. Am J Kidney Dis Off J Natl Kidney Found. 1991;18(2):188–195. doi: 10.1016/s0272-6386(12)80879-1. [DOI] [PubMed] [Google Scholar]
- 7.CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Published March 28, 2020. https://covid.cdc.gov/covid-data-tracker. Accessed 30 Jan 2022.
- 8.Consiglio CR, Cotugno N, Sardh F, et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020;183(4):968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett CE. Risk for Newly Diagnosed Diabetes 30 Days After SARS-CoV-2 Infection Among Persons Aged 18 Years — United States, March 1, 2020–June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71.10.15585/mmwr.mm7102e2 [DOI] [PMC free article] [PubMed]
- 10.Damoiseaux J, Dotan A, Fritzler MJ, et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun Rev. 2022;21(3):103012. doi: 10.1016/j.autrev.2021.103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 13.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J Pediatr. 2020;226:45–54.e1. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masiak A, Zdrojewski Z, Pęksa R, et al. The usefulness of histopathological examinations of non-renal biopsies in the diagnosis of granulomatosis with polyangiitis. Reumatologia. 2017;55(5):230–236. doi: 10.5114/reum.2017.71638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lie JT. Biopsy diagnosis of systemic vasculitis. Baillieres Clin Rheumatol. 1997;11(2):219–236. doi: 10.1016/s0950-3579(97)80044-1. [DOI] [PubMed] [Google Scholar]
- 17.Takwoingi YM, Dempster JH. Wegener’s granulomatosis: an analysis of 33 patients seen over a 10-year period. Clin Otolaryngol Allied Sci. 2003;28(3):187–194. doi: 10.1046/j.1365-2273.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65(7):936–941. doi: 10.1136/ard.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunder GG, Arend WP, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction Arthritis Rheum. 1990;33(8):1065–1067. doi: 10.1002/art.1780330802. [DOI] [PubMed] [Google Scholar]
- 20.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 21.Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806. 10.1136/ard.2009.116657 [DOI] [PubMed]
- 22.Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis Nat Rev Dis Primer. 2020;6(1):71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 23.Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–430. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 25.Seo P, Stone JH. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. 2004;117(1):39–50. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337(21):1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 27.Reiff DD, Meyer CG, Marlin B, Mannion ML. New onset ANCA-associated vasculitis in an adolescent during an acute COVID-19 infection: a case report. BMC Pediatr. 2021;21(1):333. doi: 10.1186/s12887-021-02812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell WT, Campbell JA, Ross F, Peña Jiménez P, Rudzinski ER, Dickerson JA. Acute ANCA Vasculitis and Asymptomatic COVID-19. Pediatrics. 2021;147(4):e2020033092. doi: 10.1542/peds.2020-033092. [DOI] [PubMed] [Google Scholar]
- 29.Fireizen Y, Shahriary C, Imperial ME, Randhawa I, Nianiaris N, Ovunc B. Pediatric P-ANCA vasculitis following COVID-19. Pediatr Pulmonol. 2021;56(10):3422–3424. doi: 10.1002/ppul.25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izci Duran T, Turkmen E, Dilek M, Sayarlioglu H, Arik N. ANCA-associated vasculitis after COVID-19. Rheumatol Int. 2021;41(8):1523–1529. doi: 10.1007/s00296-021-04914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uppal NN, Kello N, Shah HH, et al. De Novo ANCA-Associated Vasculitis With Glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5(11):2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moeinzadeh F, Dezfouli M, Naimi A, Shahidi S, Moradi H. Newly Diagnosed Glomerulonephritis During COVID-19 Infection Undergoing Immunosuppression Therapy, a Case Report. Iran J Kidney Dis. 2020;14(3):239–242. [PubMed] [Google Scholar]
- 33.Jalalzadeh M, Valencia-Manrique JC, Boma N, Chaudhari A, Chaudhari S. Antineutrophil Cytoplasmic Antibody-Associated Glomerulonephritis in a Case of Scleroderma After Recent Diagnosis With COVID-19. Cureus. 2021;13(1):e12485. doi: 10.7759/cureus.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Graeff N, Groot N, Brogan P, et al. European consensus-based recommendations for the diagnosis and treatment of rare paediatric vasculitides - the SHARE initiative. Rheumatol Oxf Engl. 2019;58(4):656–671. doi: 10.1093/rheumatology/key322. [DOI] [PubMed] [Google Scholar]
- 35.Morishita KA, Wagner-Weiner L, Yen EY, et al. Consensus Treatment Plans for Severe Pediatric Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res. Published online March 6, 2021. doi:10.1002/acr.24590 [DOI] [PubMed]
- 36.Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98(1):76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 37.Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003;114(6):463–469. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 38.Morishita K, Brown K, Cabral D. Pediatric vasculitis: advances in treatment. Curr Opin Rheumatol. 2015;27(5):493–499. doi: 10.1097/BOR.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 39.Shoenfeld Y, Gilburd B, Abu-Shakra M, et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases–2008. Isr Med Assoc J IMAJ. 2008;10(1):3–7. [PubMed] [Google Scholar]
- 40.Shoenfeld Y, Zandman-Goddard G, Stojanovich L, et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases–2008. Isr Med Assoc J IMAJ. 2008;10(1):8–12. [PubMed] [Google Scholar]
- 41.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toussirot E, Roudier J. Epstein-Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol. 2008;22(5):883–896. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 44.Muller S, Richalet P, Laurent-Crawford A, et al. Autoantibodies typical of non-organ-specific autoimmune diseases in HIV-seropositive patients. AIDS Lond Engl. 1992;6(9):933–942. doi: 10.1097/00002030-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Weiss PF. Pediatric Vasculitis. Pediatr Clin North Am. 2012;59(2):407–423. doi: 10.1016/j.pcl.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giryes S, Bragazzi NL, Bridgewood C, De Marco G, McGonagle D. COVID-19 Vasculitis and vasculopathy-Distinct immunopathology emerging from the close juxtaposition of Type II Pneumocytes and Pulmonary Endothelial Cells. Semin Immunopathol. 2022;44(3):375–390. doi: 10.1007/s00281-022-00928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data regarding this study has been reported in the manuscript. Please contact the corresponding author if you are interested in any further information.