To the Editor:
During the first two years of the coronavirus disease 2019 (Covid-19) pandemic caused by the spreading of the coronavirus SARS-CoV-2, several publications investigated about the incidence of Covid-19 infection in Philadelphia-negative chronic myeloproliferative neoplasms (MPN), showing an increased risk of thromboembolism in essential thrombocythemia and of mortality in myelofibrosis [1]. In many studies, the risk of SARS-CoV2 infection seemed not elevated [2, 3]; in a large study by the Italian Hematology Alliance, out of 536 patients included, 15% of them were affected by MPN [4]. During the Covid-19 emergency, the GIMEMA group realized two cross-sectional surveys aimed at evaluating the prevalence of infection among MPN patients in Italy [3] and the relative management of these diseases focusing on the administration of ruxolitinib [5]. In the present project, the observation time was extended to the second peak of the pandemic and included the vaccination period. The objectives of this survey were to evaluate: (1) the incidence of SARS-CoV2 infection in MPN patients; (2) the relative percentage of severe and fatal Covid-19 syndromes; (3) treatment changes induced by the pandemic period or by SARS-CoV2 infection; (4) data on vaccination.
Survey data were collected and managed using the REDCap electronic data capture tools hosted at the GIMEMA Foundation [6]. The survey refers to a period starting from January 2020 to June 2021. Thirty-nine centers compiled the survey, referring a whole cohort of 11.276 MPN patients subdivided in 2111 myelofibrosis (MF), 3543 polycythemia vera (PV) and 5622 essential thrombocythemia (ET).
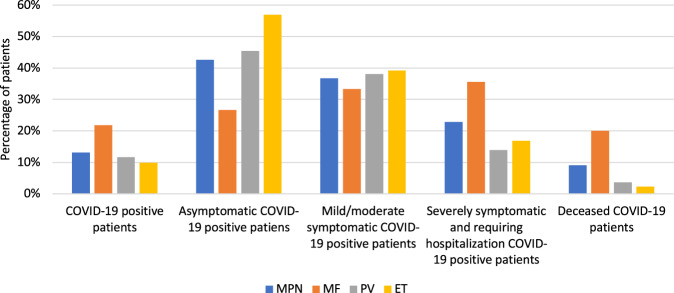
The percentage of patients who acquired the SARS-CoV2 infection was 19.4%, 9.5% and 8.2% in MF, PV and ET, respectively. In MF patients the percentages of patients asymptomatic, mild/moderate symptomatic and severely affected were 27.7%, 33.6% and 38.7%, respectively. In PV patients the same picture was represented by 46.3%, 38% and 15.7%, whereas in ET patients 48.1%, 33.3% and 18.6%. The rate of mortality was 17.5% for MF, 4.6% for PV and 2.3% for ET patients (p < 0.0001) (Fig. 1). Of the whole cohort, 1598 MF patients and 861 PV patients were on treatment with ruxolitinib during the observation time. In MF cohort treated with ruxolitinib in which SARS-CoV2 infection was diagnosed, 29 were asymptomatic and 75 were symptomatic; in PV cohort treated with ruxolitinib, 12 and 14 patients were asymptomatic and symptomatic for Covid-19, respectively.
Fig. 1. Incidence and severity of Covid-19 in MPN patients.
Stratification according to WHO diagnosis in MF, PV, and ET.
Ruxolitinib start was delayed because of pandemic in 25% of MF patients, discontinued due to Covid-19 infection in 3.4% of asymptomatic and in 1.3% of symptomatic patients; indeed, 31% of asymptomatic patients reduced the dose. The same situation was analyzed in PV patients: ruxolitinib start was delayed because of pandemic in 65% of PV patients, discontinued due to infection in 8.3% of asymptomatic and in 71.4% of symptomatic patients; indeed, 8.3% of asymptomatic patients reduced the dose. In MF patients in good response to ruxolitinib who required hospitalization and/or died due to SARS-CoV2 infection, 86% of patients continued the drug and 14% discontinued; in PV patients, the drug was continued in 28% and discontinued in 2% of them, while 70% were treated with conventional cytoreduction.
Anti-SARS-CoV2 vaccination performed within June 2021 was analyzed: 72.3% of MPN patients received mRNA vaccine and only 8% a viral vector vaccine. Among the first category, 77.1% were MF, 74.2% PV and 69.2% ET patients. After vaccination, 1.5% of MPN patients were tested positive for SARS-CoV2 (3.4% MF, 1.2% PV and 1% ET).
After vaccination, 1077 (12.8%) MPN patients were tested for anti-SARS-CoV2 antibodies (347, 20.8% MF; 336, 12.3% PV; 396, 9.8% ET.). Overall, 86.9% developed a humoral response to vaccine. Among them, 72.9% were MF, 85.4% PV and 100% ET. During the vaccination period, 25% of MF and 10.4% of PV patients were in treatment with ruxolitinib.
This survey clearly reports that among MPN diseases, MF is at increased risk of acquiring the SARS-CoV2 infection and of developing severe and fatal Covid-19 syndromes. As stated in literature, the likelihood of SARS-CoV2 infection seems higher in MPNs compared to the healthy population, in particular for ET, whereas MF patients have the highest rate of mortality [7]. The authors also reported that age, male gender, admission to ICU, severity of COVID-19 and ruxolitinib discontinuation at COVID-19 diagnosis were independent risk factors for death [7].
We analyzed the population of MF and PV patients in treatment with ruxolitinib and among MF cohort an increased rate of symptomatic patients was reported. The start of ruxolitinib was delayed due to SARS-CoV2 in the majority of PV patients: in this disease, physicians discontinued the drug during infection while in MF, the drug was discontinued only in few patients. This behavior reflects the absence of urgencies in the treatment of PV in which the dosage was reduced even in asymptomatic patients. Indeed, in MF patients the drug was continued even in patients that required hospitalization for severe SARS-CoV2 form. Interesting data were reported in this survey: about 75% of MPN patients received vaccination and the whole except for few cases with mRNA vaccine. After the vaccination, among the MPN patients tested for anti-spike antibodies, it seems that a seroconversion was achieved in all ET patients, in more than 80% of PV patients, while 72.9% of MF patients achieved a response. It has been reported that MPN patients may have lower response to vaccination due to immunological competence [1, 8, 9]. As suggested by other reports, the results of the survey confirmed that among MPN a reduced seroconversion should be considered in MF: the negative role of ruxolitinib for the possible seroconversion has been reported in different small cohorts of patients [10–13], suggesting the need for a full vaccination course.
In conclusions, the results of the survey indicated that MPNs, and particularly MF patients, may be at increased risk of severe SARS-CoV-2 infection. Patients in treatment with ruxolitinib, in particular MF, should be candidate to mRNA full vaccination cycle, monitored for seroconversion and possible implementation with new monoclonal antibodies for SARS-CoV2 prevention.
Acknowledgements
The authors wish to thank all the GIMEMA centers that completed the survey.
Author contributions
MB analyzed data and wrote manuscript; MM, AP, SS, PF, MV analyzed data and revised the manuscript; VDS, MBe, AI, BM, SS, FA, BM, PG and FP collected the data, revised, and accepted the final version.
Competing interests
MB received honoraria by Novartis, Pfizer, Incyte, BMS. FP received honoraria by Novartis, Celgene, AOP, Sierra Oncology and CTI. VDS received grants from Amgen, Celgene, Novartis.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palandri F, Breccia M, De Stefano V, Passamonti F. Philadelphia-negative chronic myeloproliferative neoplasms during the COVID-19 pandemic: challenges and future scenarios. Cancers. 2021;13:4750. doi: 10.3390/cancers13194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson-Poirier K, Harnois M, Olney HJ, Sirhan S, Gratton MO, Assouline S, et al. Risk of infection in MPN patients in the era of Covid-19: A prospective multicenter study of 257 patients from the CML-MPN Quebec Research Group. Am J Hematol. 2021;96:E200–e203. doi: 10.1002/ajh.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breccia M, Piciocchi A, De Stefano V, Finazzi G, Iurlo A, Fazi P, et al. COVID-19 in Philadelphia-negative myeloproliferative disorders: a GIMEMA survey. Leukemia. 2020;34:2813–4. doi: 10.1038/s41375-020-01032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palandri F, Piciocchi A, De Stefano V, Breccia M, Finazzi G, Iurlo A, et al. How the coronavirus pandemic has affected the clinical management of Philadelphia-negative chronic myeloproliferative neoplasms in Italy-a GIMEMA MPN WP survey. Leukemia. 2020;34:2805–8. doi: 10.1038/s41375-020-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium, The REDCap consortium: Building an international community of software partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed]
- 7.Barbui T, Vannucchi AM, Alvarez-Larran A, Iurlo A, Masciulli A, Carobbio A, et al. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021;35:485–93. doi: 10.1038/s41375-020-01107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J Hematol Oncol. 2021;14:119. doi: 10.1186/s13045-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auteri G, Bartoletti D, Di Pietro C, Sutto E, Mazzoni C, Romagnoli AD, et al. Longer-term response to SARS-CoV-2 vaccine in MPN patients: role of ruxolitinib and disease severity. Leuk Res. 2022;116:106819. doi: 10.1016/j.leukres.2022.106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maneikis K, Sablauskas K, Ringeleviciutè U, Vaitekenaite V, Cekauskiene R, Kryzauskaité L, et al. Immunogenicity of the BNT162B2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:583–92. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorino F, Sicuranza A, Ciabattini A, Santoni A, Pastore G, Simoncelli M, et al. The slower antibody response in myelofibrosis patients after two doses of mRNA SARS-CoV2 vaccine calls for a third dose. Biomedicines. 2021;9:1480. doi: 10.3390/biomedicines9101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo D, Bucelli C, Cavallaro F, Consonni D, Iurlo A. Impact of diagnosis and treatment on response to COVID-19 vaccine in patients with BCR-ABL1-negative myeloproliferative neoplasms. A single-center experience. Blood Cancer J. 2021;11:185. doi: 10.1038/s41408-021-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglielmelli P, Mazzoni A, Maggi L, Kiros ST, Zammarchi L, Pilerci S, et al. Impaired response to first SARS-CoV-2 dose vaccination in myeloproliferative neoplasm patients receiving ruxolitinib. Am J Hematol. 2021;96:E408–E410. doi: 10.1002/ajh.26305. [DOI] [PMC free article] [PubMed] [Google Scholar]